This randomized clinical trial evaluates whether 1 to 2 months of dual antiplatelet therapy (DAPT) followed by clopidogrel monotherapy is noninferior to 12 months of DAPT with aspirin and clopidogrel for patients with acute coronary syndromes.
Key Points
Question
Is 1 to 2 months of dual antiplatelet therapy (DAPT) followed by clopidogrel monotherapy noninferior to 12 months of DAPT with aspirin and clopidogrel for patients with acute coronary syndromes?
Findings
In this randomized clinical trial enrolling 4136 patients, the 1-year incidence rate of the primary end point comprising cardiovascular and bleeding events was 3.2% in those in the 1- to 2-month DAPT group and 2.8% in the 12-month DAPT group, which did not meet the noninferiority of the 1- to 2-month DAPT group.
Meaning
The effectiveness of clopidogrel monotherapy after 1 to 2 months of DAPT is inconclusive, and further investigation is needed to define the optimal therapy for patients with acute coronary syndromes.
Abstract
Importance
Clopidogrel monotherapy after short dual antiplatelet therapy (DAPT) after percutaneous coronary intervention (PCI) has not yet been fully investigated in patients with acute coronary syndrome (ACS).
Objective
To test the hypothesis of noninferiority of 1 to 2 months of DAPT compared with 12 months of DAPT for a composite end point of cardiovascular and bleeding events in patients with ACS.
Design, Setting, and Participants
This multicenter, open-label, randomized clinical trial enrolled 4169 patients with ACS who underwent successful PCI using cobalt-chromium everolimus-eluting stents at 96 centers in Japan from December 2015 through June 2020. These data were analyzed from June to July 2021.
Interventions
Patients were randomized either to 1 to 2 months of DAPT followed by clopidogrel monotherapy (n = 2078) or to 12 months of DAPT with aspirin and clopidogrel (n = 2091).
Main Outcomes and Measures
The primary end point was a composite of cardiovascular (cardiovascular death, myocardial infarction [MI], any stroke, or definite stent thrombosis) or bleeding (Thrombolysis in MI major or minor bleeding) events at 12 months, with a noninferiority margin of 50% on the hazard ratio (HR) scale. The major secondary end points were cardiovascular and bleeding components of the primary end point.
Results
Among 4169 randomized patients, 33 withdrew consent. Of the 4136 included patients, the mean (SD) age was 66.8 (11.9) years, and 856 (21%) were women, 2324 (56%) had ST-segment elevation MI, and 826 (20%) had non–ST-segment elevation MI. A total of 4107 patients (99.3%) completed the 1-year follow-up in June 2021. One to 2 months of DAPT was not noninferior to 12 months of DAPT for the primary end point, which occurred in 65 of 2058 patients (3.2%) in the 1- to 2-month DAPT group and in 58 of 2057 patients (2.8%) in the 12-month DAPT group (absolute difference, 0.37% [95% CI, −0.68% to 1.42%]; HR, 1.14 [95% CI, 0.80-1.62]; P for noninferiority = .06). The major secondary cardiovascular end point occurred in 56 patients (2.8%) in the 1- to 2-month DAPT group and in 38 patients (1.9%) in the 12-month DAPT group (absolute difference, 0.90% [95% CI, −0.02% to 1.82%]; HR, 1.50 [95% CI, 0.99-2.26]). The major secondary bleeding end point occurred in 11 patients (0.5%) in the 1- to 2-month DAPT group and 24 patients (1.2%) in the 12-month DAPT group (absolute difference, −0.63% [95% CI, −1.20% to −0.06%]; HR, 0.46 [95% CI, 0.23-0.94]).
Conclusions and Relevance
In patients with ACS with successful PCI, clopidogrel monotherapy after 1 to 2 months of DAPT failed to attest noninferiority to standard 12 months of DAPT for the net clinical benefit with a numerical increase in cardiovascular events despite reduction in bleeding events. The directionally different efficacy and safety outcomes indicate the need for further clinical trials.
Trial Registration
ClinicalTrials.gov Identifiers: NCT02619760 and NCT03462498
Introduction
Patients with acute coronary syndrome (ACS) have been regarded as having higher long-term risk of cardiovascular events after percutaneous coronary intervention (PCI) compared with patients with chronic coronary syndrome (CCS). Therefore, the recommended duration of dual antiplatelet therapy (DAPT) after PCI was longer in patients with ACS than in patients with CCS. In the latest US and European guidelines, the recommended duration of DAPT after PCI is basically 12 months in patients with ACS, while it is 6 months in patients with CCS.1,2 The rationale for the specific DAPT duration of 12 months in patients with ACS was derived from a trial conducted in the late 1990s.3,4 However, more and more concerns have been raised on the increase in bleeding events associated with prolonged DAPT. Abbreviated DAPT durations have been already recommended, even in patients with ACS, if they have high bleeding risk.5,6,7 Recently, 5 clinical trials8,9,10,11,12 enrolling a total of more than 30 000 patients have suggested benefit of very short (1 to 3 months) DAPT with subsequent P2Y12 inhibitor monotherapy after PCI in reducing bleeding events without increasing cardiovascular events compared with prolonged DAPT (12 to 15 months) both in patients with ACS and CCS.8,9,10,11,12,13,14 In these trials, most patients with ACS were treated with ticagrelor monotherapy after stopping DAPT at 1 to 3 months. However, despite trials showing the superiority of ticagrelor and prasugrel over clopidogrel in the ACS setting,15,16 use of clopidogrel remains high across the globe, while clopidogrel monotherapy after very short DAPT has not yet been fully investigated in patients with ACS.9,10 Therefore, we sought to explore the safety and efficacy of clopidogrel monotherapy after DAPT for 1 to 2 months compared with continued DAPT with aspirin and clopidogrel in the ACS population.
Methods
Study Design and Population
We previously reported the STOPDAPT-2 (Short and Optimal Duration of Dual Antiplatelet Therapy After Everolimus-Eluting Cobalt-Chromium Stent-2) trial, which compared 1-month DAPT with 12-month DAPT in 3009 patients (CCS, 1861 patients; ACS, 1148 patients) who underwent PCI using cobalt-chromium everolimus-eluting stents (CoCr-EES; Abbott Laboratories).9 Before the end of follow-up for the STOPDAPT-2 trial in December 2017, the steering committee decided to enroll additional patients with ACS as the STOPDAPT-2 ACS trial in an attempt to have a powered analytic population of patients with ACS pooled with the patients with ACS in the STOPDAPT-2 trial. The study protocol for the STOPDAPT-2 ACS trial was identical to that of the STOPDAPT-2 trial except for the exclusive enrollment of patients with ACS and can be found in Supplement 1.9 ACS was defined as ST-segment elevation myocardial infarction (STEMI), non-STEMI, or unstable angina based on the previous guidelines.17 Key exclusion criteria are limited to continued use of oral anticoagulants and previous history of hemorrhagic stroke. We screened all the patients with ACS who were eligible for the study and compared the baseline characteristics between the enrolled and nonenrolled patients. The enrolled patients were randomly assigned to either the 1- to 2-month DAPT group or 12-month DAPT group in a 1-to-1 fashion, stratified only by centers before discharge from the index hospitalization. If scheduled staged PCI was needed after the initial PCI for ACS, we recommended the staged PCI to be performed during the same index hospitalization. Randomization was performed after the final PCI (index PCI) during the index hospitalization for ACS. Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines were used.
The study-group assignments were blinded to the statistician, members of the independent clinical event committee, steering committee, and the sponsor (Abbott Medical). Complete lists of the study organization, participating centers, and investigators are available in eAppendix 1 and 2 in Supplement 2. The study protocol was approved by the central review board, Kyoto University Certified Review Board, based on the enforcement of the Clinical Trials Act in Japan.18 Written informed consent was provided from all enrolled patients.
Antiplatelet Regimen
Within the first month after the index PCI, patients in both groups were to receive DAPT with aspirin (doses determined by sites) and a P2Y12 inhibitor (clopidogrel, 75 mg per day, or prasugrel, 3.75 mg per day, at the discretion of the attending physicians). At 30 to 59 days after the index PCI, patients in the 1- to 2-month DAPT group were to stop aspirin and to receive clopidogrel monotherapy, while patients in the 12-month DAPT group were to receive DAPT with aspirin and clopidogrel for up to 12 months. In patients who had received prasugrel, it was switched to clopidogrel at 1 to 2 months in both groups. We collected data for discontinuation, change, or restart of antithrombotic therapy, including anticoagulation, on a daily basis. Persistent DAPT discontinuation was defined as stopping of either aspirin or P2Y12 inhibitor by the study protocol or stopping treatment for more than 60 days for any reason.
End Points
The end points were the same as those in the STOPDAPT-2 trial. The primary end point was a composite of cardiovascular outcomes (cardiovascular death, myocardial infarction, definite stent thrombosis, or any stroke) or bleeding outcomes defined as Thrombolysis in Myocardial Infarction (TIMI) major or minor criteria.19 We chose a noninferiority design because 12-month DAPT was the standard of care in patients with ACS. The major secondary end points were the cardiovascular and bleeding components of the primary end point. Other secondary end points were exploratory and described in eAppendix 3 in Supplement 2. Follow-up was commenced at randomization, while the time interval was indicated from the index PCI. All the end points were assessed at 12 months (335 to 394 days), but were censored at 366 days. All the clinical events comprising the primary end point were adjudicated in the independent clinical event committee in a blinded fashion to the assigned groups (eAppendix 1 in the Supplement 2).
Statistical Analysis
The study hypothesis was that the experimental arm (1- to 2-month DAPT group) is noninferior to the control arm (12-month DAPT group) in terms of the primary end point at 12 months. The initial trial design calculated a sample size of 2676 patients including 1148 patients in the STOPDAPT-2 trial, assuming a 5.5% estimated event rate based on the previous studies and setting a relative noninferiority margin of 50% on the hazard ratio (HR) scale with power of 80% and 1-sided α of .025.20,21 The relative noninferiority margin of 50% was chosen considering the feasibility of patient enrollment and the margins adopted in previous major trials and the STOPDAPT-2 trial.22,23 However, the actual rate of the primary end point in patients with ACS in the STOPDAPT-2 trial was lower than anticipated (4.0% at 12 months). Therefore, we amended the protocol in August 2019 and recalculated a sample size of 4036 patients, assuming a 4.0% event rate with power of 90% and 1-sided α of .025. For the major secondary end points, the estimated sample size would provide 80% power for noninferiority on the cardiovascular end point (3.0% assumed event rate) and 81% power for superiority on the bleeding end point (1.5% assumed event rate and 60% relative risk reduction). For the primary and major secondary end points, hierarchical testing was predefined in the following order: (1) noninferiority test on the primary end point; (2) noninferiority test for the major secondary cardiovascular composite end point; (3) superiority test for the major secondary bleeding end point; and (4) superiority test for the primary end point. The main results were described in the intention-to-treat population. Sensitivity analyses for the primary end point were also performed in the per-protocol population, as-treated population, worst-case scenario, and landmark analyses at 30 and 60 days as defined in the statistical analysis plan (Supplement 1). We also conducted the post hoc landmark analysis at the day of modifying the antiplatelet regimen at 1 to 2 months because of the considerable variation of the day of modification. Moreover, we estimated the cumulative incidence of the primary end point in the 1- to 2-month DAPT group comparing between the 2 groups of patients who discontinued aspirin within and beyond the median days after index PCI. For the prespecified subgroups, the interaction tests were made to confirm the consistency of the treatment effect on the primary end point (eMethods in Supplement 2). As the exploratory analyses, we compared the effects of 1 to 2 months of DAPT with 12 months of DAPT for the primary and major secondary end points between patients enrolled in the STOPDAPT-2 trial and patients enrolled in the STOPDAPT-2 ACS trial.
Categorical variables were presented as numbers and percentages and continuous variables as mean with SDs or medians with IQR. The cumulative incidence of event was estimated by the Kaplan-Meier method. The treatment effects were presented as HR and 95% CIs calculated from the Wald statistics by the Cox proportional hazard model.
A physician (Hirotoshi Watanabe) and a statistician (T. M.) performed all statistical analyses with the use of JMP version 15.2.0 (SAS Institute) and SAS version 9.4 (SAS Institute). In the noninferiority testing, a 1-sided P value <.025 was considered statistically significant.
Results
Patients Recruitment and Assignment
In the STOPDAPT-2 ACS trial, we randomized 3008 patients at 74 centers in Japan from March 2018 to June 2020. Including 1161 patients with ACS enrolled in the STOPDAPT-2 trial at 75 centers in Japan from December 2015 to December 2020, 4169 patients with ACS from 96 centers were randomized to either the 1- to 2-month DAPT group or 12-month DAPT group in the present pooled study population. Excluding 33 patients who withdrew consent, there were 4136 patients in the intention-to-treat population (mean [SD], age 66.8 years [11.9]; 856 women [21%]), 2058 in the 1- to 2-month DAPT group, and 2078 in the 12-month DAPT group (Figure 1). Randomization was performed at a median (IQR) of 5 (2-9) days after the index PCI. Among those patients who were eligible for the study, the enrolled patients were younger and less often had comorbidities than the nonenrolled patients (eTable 1 in Supplement 2).
Figure 1. Study Flowchart.
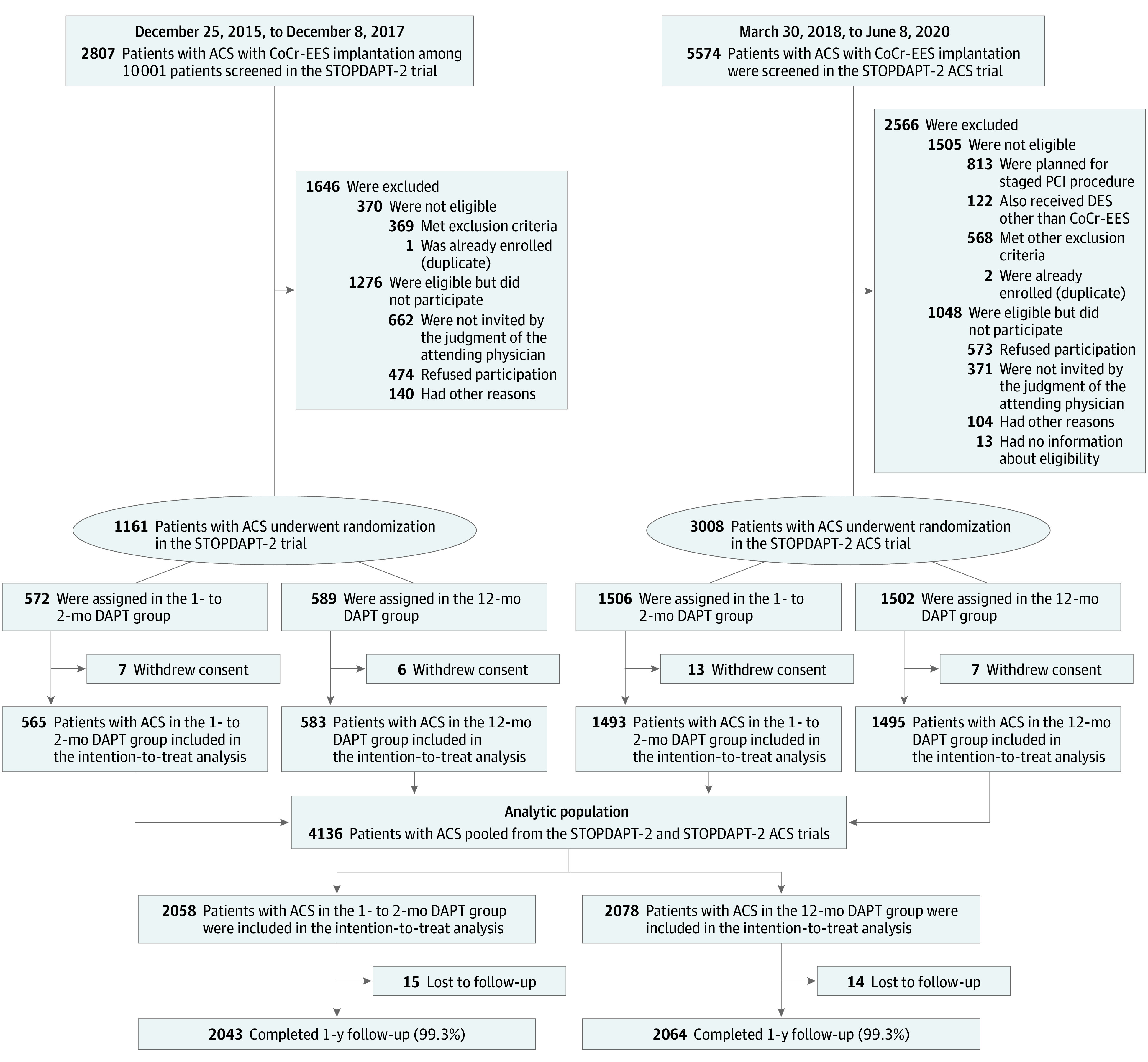
In the Short and Optimal Duration of Dual Antiplatelet Therapy After Everolimus-Eluting Cobalt-Chromium Stent-2 (STOPDAPT-2) trial, 1161 patients with acute coronary syndrome (ACS) were enrolled and randomized at 75 centers in Japan from December 2015 to December 2017, while in the STOPDAPT-2 ACS trial, 3008 patients with ACS were enrolled and randomized at 74 centers in Japan from March 2018 to June 2020. Substantial proportions of the eligible patients were not enrolled in the study mainly by the judgment of the attending physicians or by the patients’ refusal. Excluding 33 patients who withdrew consent, 4136 patients were included in the intention-to-treat population, 2058 patients in the 1- to 2-month dual antiplatelet therapy group (DAPT) and 2078 patients in the 12-month DAPT group. Randomization was performed at a median (IQR) of 5 (2-9) days after the index percutaneous coronary intervention. CoCr-EES indicates cobalt-chromium everolimus-eluting stent and DES indicates drug-eluting stent.
Baseline Characteristics and Medications
The clinical presentations of ACS were STEMI in 2324 of 4136 patients (56%), non-STEMI in 826 of 4136 patients (20%), and unstable angina in 986 patients (24%). Most patients had low/intermediate thrombotic and bleeding risk based on both CREDO-Kyoto (Coronary Revascularization Demonstrating Outcome Study in Kyoto) and PARIS (Patterns of Non-Adherence to Anti-Platelet Regimen in Stented Patients) risk scores.24,25 Radial approach was used in 3695 patients (89%), and intracoronary imaging guidance was performed in 4023 patients (97%). Statins were prescribed in 3989 patients (96%) and high-intensity statins were prescribed in 1407 patients (34%). Proton pump inhibitors were prescribed in 3808 patients (92%) (Table 1; eTable 2 in Supplement 2). Compared with patients in the STOPDAPT-2 trial, patients in the STOPDAPT-2 ACS trial more often had clinical presentation of acute myocardial infarction, heart failure, emergent procedure, radial approach, left main coronary artery target, and treatment with guideline-directed medications, high-intensity statins in particular (eTable 3 in Supplement 2). The baseline characteristics and medications were well balanced between the 1- to 2-month and 12-month DAPT groups (Table 1; eTable 2 in Supplement 2).
Table 1. Patient, Lesion, and Procedural Characteristics and Medicationsa.
| Characteristic | No. (%) | |
|---|---|---|
| 1- to 2-mo DAPT (n = 2058) | 12-mo DAPT (n = 2078) | |
| Demographic characteristics | ||
| Age, y | ||
| Mean, (SD) | 67.0 (11.9) | 66.6 (11.9) |
| ≥75 | 585 (28.4) | 598 (28.8) |
| Men | 1631 (79.3) | 1649 (79.4) |
| Women | 427 (20.8) | 429 (20.6) |
| Body mass indexb | 24.1 (3.7) | 24.2 (3.5) |
| Mean, (SD) | ||
| <25 | 1301 (63.2) | 1298 (62.5) |
| Clinical presentation | ||
| Acute myocardial infarction | 1578 (76.7) | 1572 (75.7) |
| STEMI | 1179 (74.7) | 1145 (72.8) |
| Non-STEMI | 399 (25.3) | 427 (27.2) |
| Treated ≤24 h | 1386 (87.8) | 1348 (85.8) |
| >24 h | 192 (12.2) | 224 (14.3) |
| Onset to arrival time at hospital, median (IQR), h | 3.0 (1.3-9.4) | 3.0 (1.3-10.1) |
| Door to wire crossing time in patients with STEMI within 24 h, median (IQR), min | 60 (44-81) | 60 (44-77) |
| Killip class | ||
| 1 | 1351 (85.6) | 1366 (87.1) |
| 2 | 140 (8.9) | 123 (7.8) |
| 3 | 34 (2.2) | 32 (2.0) |
| 4 | 53 (3.4) | 48 (3.1) |
| Location of STEMIc | ||
| Anterior | 628 (53.3) | 613 (53.6) |
| Inferior | 466 (39.6) | 459 (40.2) |
| Posterolateral | 154 (13.1) | 132 (11.6) |
| Peak CK/ULN, median (IQR) | 6.5 (2.4-13.2) | 6.3 (2.2-13.4) |
| Peak CK-MB/ULN, median (IQR) | 8.6 (2.3-22.1) | 8.2 (2.2-21.3) |
| Unstable anginad | 480 (23.3) | 506 (24.4) |
| Braunwald class | ||
| I | 206 (42.9) | 205 (40.5) |
| II | 75 (15.6) | 62 (12.3) |
| III | 199 (41.5) | 239 (47.2) |
| Culprit vessels, No./total No. (%)e | ||
| Left anterior descending coronary artery | 1108/2052 (54.0) | 1100/2068 (53.2) |
| Left circumflex coronary artery | 284/2052 (13.8) | 270/2068 (13.1) |
| Right coronary artery | 632/2052 (30.8) | 677/2068 (32.7) |
| Left main coronary artery | 27/2052 (1.3) | 19/2068 (0.9) |
| Saphenous vein graft | 1/2052 (0.1) | 2/2068 (0.1) |
| CPAOA | 22 (1.1) | 18 (0.9) |
| ECMO use | 7 (0.3) | 6 (0.3) |
| Impella use | 2 (0.1) | 1 (0.1) |
| IABP use | 84 (4.1) | 64 (3.1) |
| History and comorbidities | ||
| Prior percutaneous coronary intervention | 225 (10.9) | 202 (9.7) |
| Prior first-generation DES | 43 (2.1) | 32 (1.5) |
| Prior CABG | 9 (0.4) | 18 (0.9) |
| Prior myocardial infarction | 135 (6.6) | 109 (5.3) |
| Prior stroke | 98 (4.8) | 95 (4.6) |
| Prior bleeding events | 18 (0.9) | 14 (0.7) |
| Heart failure | 157 (7.6) | 151 (7.3) |
| Atrial fibrillation | 35 (1.7) | 16 (0.8) |
| Anemiaf | 117 (5.7) | 130 (6.3) |
| Thrombocytopeniag | 9 (0.4) | 12 (0.6) |
| Chronic obstructive pulmonary disease | 34 (1.7) | 53 (2.6) |
| Cirrhosis | 5 (0.2) | 5 (0.2) |
| Cancer | 135 (6.6) | 137 (6.6) |
| Peripheral artery disease | 40 (1.9) | 42 (2.0) |
| Severe chronic kidney diseaseh | 68 (3.3) | 70 (3.4) |
| eGFR <30 mL/min/1.73 m2 not receiving dialysis | 42 (2.0) | 47 (2.3) |
| Dialysis | 26 (1.3) | 23 (1.1) |
| Hypertension | 1396 (67.8) | 1414 (68.1) |
| Hyperlipidemia | 1373 (66.7) | 1391 (66.9) |
| Diabetes | 608 (29.5) | 621 (29.9) |
| Diabetes with insulin | 51 (2.5) | 74 (3.6) |
| Current smoker | 718 (34.9) | 702 (33.8) |
| Left ventricular ejection fraction, %i | ||
| Mean (SD) | 56.7 (10.6) | 56.9 (10.5) |
| <40%, No./total No. (%) | 95/1903 (5.0) | 76/1921 (4.0) |
| Risk scores | ||
| PARIS thrombotic risk score, median (IQR) | 3 (2-4) | 3 (2-4) |
| High (≥5) | 347 (16.9) | 338 (16.3) |
| Intermediate (3-4) | 1076 (52.3) | 1051 (50.6) |
| Low (0-2) | 635 (30.9) | 689 (33.2) |
| PARIS bleeding risk score, median (IQR) | 5 (3-7) | 5 (3-7) |
| High (≥8) | 380 (18.5) | 367 (17.7) |
| Intermediate (4-7) | 1071 (52.0) | 1067 (51.4) |
| Low (0-3) | 607 (29.5) | 644 (31.0) |
| CREDO-Kyoto thrombotic risk score, median (IQR) | 1 (0-1) | 1 (0-1) |
| High (≥4) | 78 (3.8) | 93 (4.5) |
| Intermediate (2-3) | 355 (17.3) | 339 (16.3) |
| Low (0-1) | 1625 (79.0) | 1646 (79.2) |
| CREDO-Kyoto bleeding risk score, median (IQR) | 0 (0-0) | 0 (0-0) |
| High (≥3) | 71 (3.5) | 61 (2.9) |
| Intermediate (1-2) | 402 (19.5) | 398 (19.2) |
| Low (0) | 1585 (77.0) | 1619 (77.9) |
| Procedural characteristics | ||
| Radial approach | 1832 (89.0) | 1863 (89.7) |
| Invasive fractional flow reserve | 60 (2.9) | 77 (3.7) |
| Staged procedurej | 280 (13.6) | 317 (15.3) |
| No. of procedures, mean (SD) | 1.15 (0.39) | 1.17 (0.41) |
| No. of target lesions, mean (SD)j | 1.27 (0.60) | 1.28 (0.59) |
| Target lesion location | ||
| Left main coronary artery | 52 (2.5) | 58 (2.8) |
| Left anterior descending coronary artery | 1242 (60.4) | 1255 (60.4) |
| Left circumflex coronary artery | 408 (19.8) | 417 (20.1) |
| Right coronary artery | 719 (34.9) | 767 (36.9) |
| Bypass graft | 1 (0.1) | 2 (0.1) |
| Chronic total occlusion | 66 (3.2) | 62 (3.0) |
| Bifurcation lesion | 552 (26.8) | 549 (26.4) |
| Target of 2 vessels or more | 344 (16.7) | 390 (18.8) |
| Use of intravascular imaging | 2916 (97.6) | 1107 (96.4) |
| Use of intravascular ultrasonography | 1796 (87.3) | 1792 (86.2) |
| Use of optical coherence tomography | 279 (13.6) | 310 (14.9) |
| No. of implanted stents, mean (SD) | 1.40 (0.77) | 1.41 (0.79) |
| Minimal stent diameter, mm | ||
| Mean (SD) | 3.01 (0.51) | 3.02 (0.50) |
| <3.0 | 817 (39.7) | 782 (37.6) |
| Total stent length, mm | ||
| Mean (SD) | 34.3 (22.6) | 34.6 (23.5) |
| ≥28 | 1111 (54.0) | 1127 (54.2) |
| Length of hospital stay, median (IQR), d | ||
| Admission to discharge | 9 (6-12) | 9 (5-12) |
| Index PCI to discharge | 8 (3-11) | 7 (3-11) |
| Admission to staged PCI | 7 (4-11) | 7 (3-11) |
| Medication at discharge | ||
| Aspirin | 2055 (99.9) | 2076 (99.9) |
| 200 mg/d | 1 (0.1) | 1 (0.1) |
| 100 mg/d | 2027 (98.6) | 2043 (98.4) |
| 81 mg/d | 27 (1.3) | 32 (1.5) |
| P2Y12 inhibitors | 2055 (99.9) | 2076 (99.9) |
| Clopidogrel | 1062 (51.6) | 1108 (53.3) |
| Prasugrel | 994 (48.3) | 968 (46.6) |
| Anticoagulantsk | 10 (0.5) | 13 (0.6) |
| ACE-I/ARB | 1552 (75.4) | 1573 (75.7) |
| β-Blockers | 1246 (60.5) | 1190 (57.3) |
| Statins | 1981 (96.3) | 2008 (96.6) |
| High-intensity statin therapyl | 710 (34.5) | 697 (33.6) |
| Proton pump inhibitors | 1875 (91.1) | 1933 (93.0) |
Abbreviations: ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin-2 receptor blocker; CABG, coronary artery bypass grafting; CK, creatine kinase; CK-MB, creatine kinase–MB fraction; CPAOA, cardiopulmonary arrest on arrival; CREDO-Kyoto, Coronary Revascularization Demonstrating Outcome Study in Kyoto; DAPT, dual antiplatelet therapy; DES, drug-eluting stent; ECMO, extracorporeal membrane oxygenation; eGFR, estimated glomerular filtration rate; IABP, intra-aortic balloon pumping; PARIS, Patterns of Non-Adherence to Anti-Platelet Regimen in Stented Patients trial; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; ULN, upper limit of normal.
Acute coronary syndrome was defined as myocardial infarction within 7 days or unstable angina.
Calculated as weight in kilograms divided by height in meters squared.
Some patients had 2 or more locations of myocardial infarction.
Unstable angina was defined as Braunwald classification I to III, without confirmation of any biomarker elevation.
The culprit vessels are missing in 6 patients in 1- to 2-month DAPT group and 10 patients in 12-month DAPT group.
Anemia was defined as hemoglobin less than 11 g/dL. Hemoglobin values were missing in 3 patients, who were included in the no anemia group.
Thrombocytopenia was defined as platelet counts less than 100×109/L. Platelet counts were missing in 12 patients, who were included in the no thrombocytopenia group.
Severe chronic kidney disease is defined as eGFR less than 30 mL/min/1.73 m2 or maintenance dialysis therapy. Preprocedural creatinine values were missing in 11 patients. One of these patients receiving dialysis was included in severe chronic kidney disease, while the remaining 10 patients were regarded as not having severe chronic kidney disease.
Left ventricular ejection fraction was missing in 312 patients, who were excluded for the calculation of left ventricular ejection fraction less than 40%.
Lesions treated at the staged procedure(s) preceding the index PCI procedure were included as the target lesions. Clinical diagnosis and baseline characteristics were defined based on the findings at the time of first PCI for the index acute coronary syndrome event.
Concomitant oral anticoagulant use was one of the exclusion criteria, but some patients started anticoagulation after enrollment (eg, new onset of atrial fibrillation or venous thrombosis).
High-intensity statin therapy was defined by the maximum approved dose of strong statin in Japan (eg, rosuvastatin, 10 mg, atorvastatin, 20 mg, or pitavastatin, 4 mg).
Antiplatelet Therapy
During DAPT treatment within 1 to 2 months, the selected P2Y12 inhibitor was clopidogrel in 2170 of 4136 patients (52%)and prasugrel in 1962 of 4136 patients (47%). The rates of DAPT discontinuation were 96.1% at 60 days and 98.7% at 335 days in the 1- to 2-month DAPT group and 1.6% at 60 days and 8.8% at 335 days in the 12-month DAPT group (eFigure 1 in Supplement 2). In the 1- to 2-month DAPT group, aspirin was actually discontinued at a median (IQR) of 39 (34-46) days after PCI.
One-Year Clinical Outcomes
Final 1-year clinical follow-up was completed in June 2021. Complete 1-year clinical follow-up was achieved in 4107 of 4136 patients (99.3%) (Figure 1). The primary end point occurred in 65 patients (3.2%) in the 1- to 2-month DAPT group and in 58 patients (2.8%) in the 12-month DAPT group. One to 2 months of DAPT group did not meet criteria for noninferiority compared with 12 months of DAPT for the primary end point (absolute difference, 0.37% [95% CI, −0.68% to 1.42%]; HR, 1.14 [95% CI, 0.80-1.62]; P = .06 for noninferiority) (Figure 2A; Table 2). Results for the primary end point were consistent in the per-protocol and as-treated populations as well as in the worst-case scenario and the landmark analyses (eTable 4 and eFigures 2-7 in Supplement 2). The cumulative 1-year incidence of the primary and major secondary cardiovascular and bleeding end points in the 1- to 2-month DAPT group was not different between the 2 groups of patients who discontinued aspirin within and beyond the median of 39 days after index PCI (eFigure 8 in Supplement 2).
Figure 2. Time-to-Event Curves for the Primary and Secondary End Points.
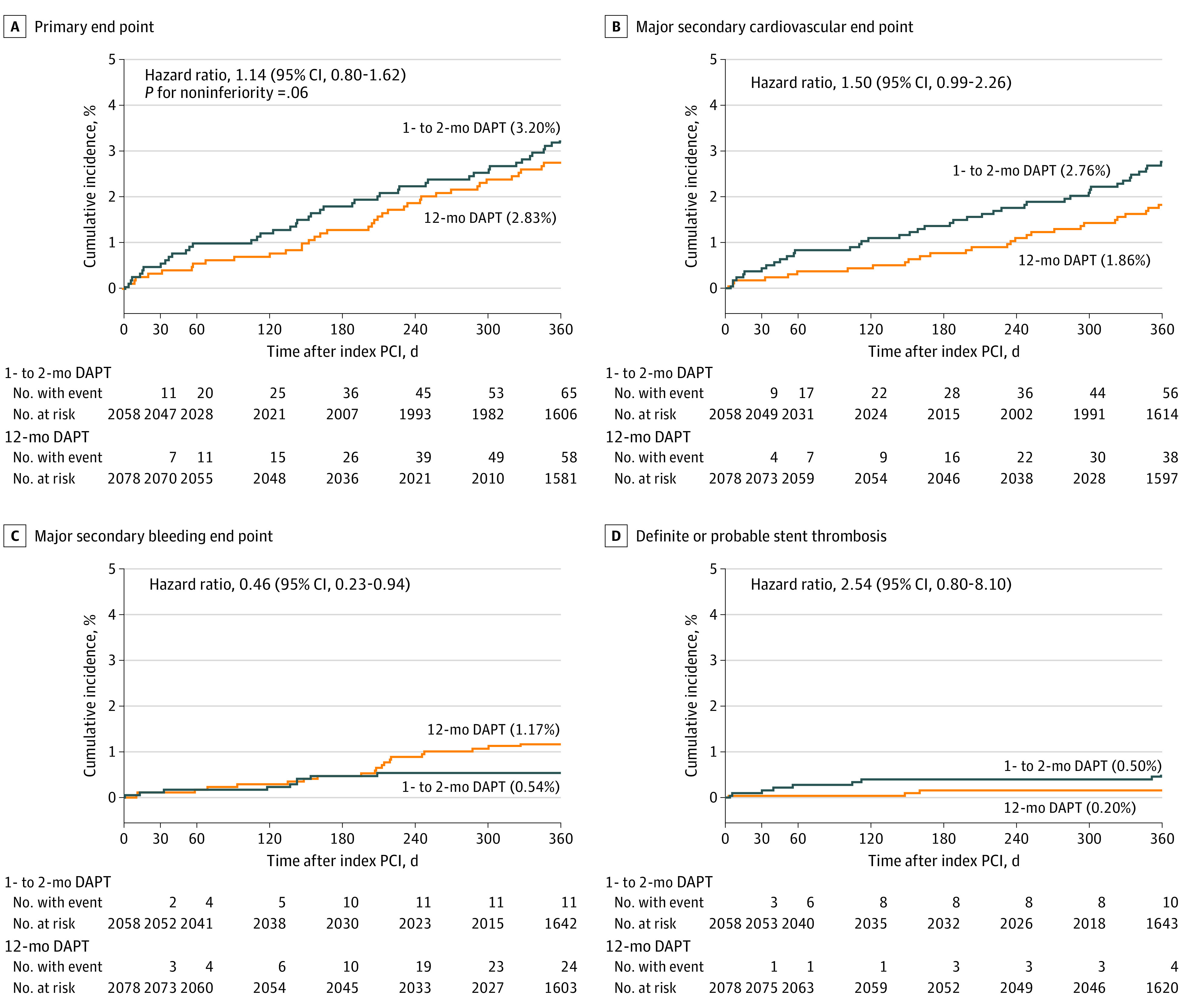
Time-to-event curves during 1 year after index percutaneous coronary intervention (PCI) for the primary end point (a composite of cardiovascular death, myocardial infarction, definite stent thrombosis, any stroke, or Thrombolysis in Myocardial Infarction major/minor bleeding) (A), the major secondary cardiovascular end point (a composite of cardiovascular death, myocardial infarction, definite stent thrombosis, or any stroke) (B),the major secondary bleeding end point (Thrombolysis in Myocardial Infarction major or minor bleeding) (C), and definite or probable stent thrombosis (D). DAPT indicates dual antiplatelet therapy.
Table 2. Clinical Outcomes at 1 Year.
| Outcomea | Patients with event, No. (%)b | Hazard ratio (95% CI) | P value for noninferiorityc | |
|---|---|---|---|---|
| 1- to 2-mo DAPT (n = 2058) | 12-mo DAPT (n = 2078) | |||
| Primary end point | ||||
| A composite of cardiovascular death, myocardial infarction, definite stent thrombosis, ischemic/hemorrhagic stroke, or TIMI major/minor bleeding | 65 (3.2) | 58 (2.8) | 1.14 (0.80-1.62) | .06 |
| Major secondary end points | ||||
| Cardiovascular end point: a composite of cardiovascular death, myocardial infarction, definite stent thrombosis, or ischemic/hemorrhagic stroke | 56 (2.7) | 38 (1.9) | 1.50 (0.99-2.26) | NA |
| Bleeding end point: TIMI major/minor bleeding | 11 (0.5) | 24 (1.2) | 0.46 (0.23-0.94) | NA |
| Other secondary end points | ||||
| Death | 28 (1.4) | 19 (0.9) | 1.49 (0.83-2.67) | NA |
| Death from cardiac causes | 9 (0.4) | 7 (0.3) | 1.30 (0.48-3.49) | NA |
| Death from cardiovascular causes | 10 (0.5) | 10 (0.5) | 1.01 (0.42-2.43) | NA |
| Sudden cardiac death | 3 (0.2) | 3 (0.2) | 1.01 (0.20-5.02) | NA |
| Death from noncardiovascular causes | 18 (0.9) | 9 (0.4) | 2.02 (0.91-4.50) | NA |
| Myocardial infarction | 32 (1.6) | 17 (0.9) | 1.91 (1.06-3.44) | NA |
| Large MI (CK-MB ≥ 10×ULN) | 6 (0.3) | 4 (0.2) | 1.53 (0.43-5.43) | NA |
| Small MI (CK-MB) < 10×ULN) | 17 (0.8) | 8 (0.4) | 2.65 (0.93-5.00) | NA |
| MI without CK-MB elevation | 9 (0.5) | 4 (0.2) | 2.28 (0.70-7.41) | NA |
| MI without measurement of CK-MB | 0 | 1 (0.1) | NA | NA |
| Spontaneous MI | 30 (1.5) | 15 (0.8) | 2.03 (1.09-3.78) | NA |
| Procedural MI | 2 (0.1) | 2 (0.1) | 1.01 (0.14-7.20) | NA |
| MI related to the target lesion | 13 (0.7) | 10 (0.5) | 1.32 (0.58-3.01) | NA |
| Definite stent thrombosis | 9 (0.5) | 4 (0.2) | 2.29 (0.70-7.42) | NA |
| Definite or probable stent thrombosis | 10 (0.5) | 4 (0.2) | 2.54 (0.80-8.10) | NA |
| Stroke | 15 (0.7) | 11 (0.5) | 1.38 (0.63-3.00) | NA |
| Ischemic | 13 (0.6) | 10 (0.5) | 1.32 (0.58-3.00) | NA |
| Hemorrhagic | 2 (0.1) | 1 (0.1) | 2.02 (0.18-22.28) | NA |
| Bleeding | ||||
| TIMI major | 7 (0.3) | 13 (0.6) | 0.54 (0.22-1.36) | NA |
| TIMI minor | 4 (0.2) | 13 (0.6) | 0.31 (0.10-0.95) | NA |
| BARC 3/5 | 11 (0.5) | 27 (1.3) | 0.41 (0.20-0.83) | NA |
| BARC 5 | 1 (0.1) | 0 | NA | NA |
| BARC 3 | 10 (0.5) | 27 (1.3) | 0.37 (0.18-0.77) | NA |
| GUSTO moderate/severe | 10 (0.5) | 24 (1.2) | 0.42 (0.20-0.88) | NA |
| GUSTO severe | 7 (0.3) | 12 (0.6) | 0.59 (0.23-1.50) | NA |
| GUSTO moderate | 3 (0.2) | 13 (0.6) | 0.23 (0.07-0.82) | NA |
| Intracranial bleeding | 5 (0.2) | 3 (0.2) | 1.68 (0.40-7.04) | NA |
| Gastrointestinal bleeding | 5 (0.2) | 19 (0.9) | 0.21 (0.07-0.62) | NA |
| Any coronary revascularization | 114 (5.7) | 74 (3.7) | 1.58 (1.18-2.13) | NA |
| TLR | 46 (2.3) | 32 (1.6) | 1.46 (0.93-2.30) | NA |
| Clinically driven TLR | 38 (1.9) | 26 (1.3) | 1.49 (0.90-2.45) | NA |
| Non-TLR | 81 (4.1) | 50 (2.5) | 1.67 (1.17-2.38) | NA |
| CABG | 7 (0.4) | 3 (0.2) | 2.36 (0.61-9.12) | NA |
| Death or myocardial infarction | 60 (3.0) | 36 (1.8) | 1.69 (1.12-2.56) | NA |
| Cardiovascular death or myocardial infarction | 42 (2.1) | 27 (1.3) | 1.58 (0.97-2.56) | NA |
| Major adverse cardiac eventsd | 64 (3.1) | 40 (2.0) | 1.63 (1.10-2.42) | NA |
Abbreviations: BARC, Bleeding Academic Research Consortium; CABG, coronary artery bypass grafting; CK-MB, creatine kinase–MB fraction; DAPT, dual antiplatelet therapy; GUSTO, Global Use of Strategies to Open Occluded Arteries; MI, myocardial infarction, NA, not applicable; TIMI, Thrombolysis in Myocardial Infarction; TLR, target-lesion revascularization; ULN, upper limit of normal.
Definitions of the end points were described in eAppendix 3 in Supplement 2.
Percentages are Kaplan-Meier estimates at 365 days.
P for noninferiority was derived from Cox hazard model with prespecified relative 50% margin of noninferiority in the hazard ratio scale.
Major adverse cardiac events were defined as composite of cardiac death, myocardial infarction, and clinically driven TLR.
The incidence of the major secondary cardiovascular end point was numerically higher in the 1- to 2-month DAPT group than in the 12-month DAPT group (2.76% vs 1.86%; absolute difference, 0.90% [95% CI, −0.02% to 1.82%]; HR, 1.50 [95% CI, 0.99-2.26]) (Figure 2B; Table 2). The incidence of the major secondary bleeding end point was lower in the 1- to 2-month DAPT group than in the 12-month DAPT group (0.54% vs 1.17%; absolute difference, −0.63% [95% CI, −1.20% to −0.06%]; HR, 0.46 [95% CI, 0.23-0.94]) (Figure 2C; Table 2). There was no difference in mortality between the 2 groups (1.4% and 0.90%). The incidence of myocardial infarction was higher in the 1- to 2-month DAPT group than in the 12-month DAPT group (1.59% vs 0.85%; absolute difference, 0.74% [95% CI, 0.07%-1.41%]; HR, 1.91; [95% CI, 1.06-3.44]), while the incidence of large myocardial infarction with creatine kinase–MB fraction more than 10 times the upper limit of normal was very low in both groups (0.31% vs 0.20%). The incidence of definite or probable stent thrombosis was very low, but was numerically higher in the 1- to 2-month DAPT group than in the 12-month DAPT group. For the 1- to 2 month DAPT group, throughout 1 year, 10 patients (0.5%) and 4 patients (0.2%) experienced stent thrombosis; and beyond the day of modifying the antiplatelet regimen: 7 patients (0.4%) and 3 patients (0.2%) experienced stent thrombosis for the 12-month DAPT group (Figure 2D; Table 2; eTable 5 in Supplement 2).
Subgroup Analyses
In the subgroup analyses, there was no treatment by subgroup interaction for the primary end point and major secondary end points in all the subgroups except for the subgroups stratified by the PARIS bleeding risk score on primary end point (eFigure 9 in Supplement 2). In the exploratory analyses stratified by the STOPDAPT-2 trial and STOPDAPT-2 ACS trial, the excess risk of 1 to 2 months of DAPT relative to 12 months of DAPT was significant for the major secondary cardiovascular end point in the STOPDAPT-2 ACS trial, but not in the STOPDAPT-2 trial, although there were no interactions for all the end points (Figure 3; eFigure 10 in Supplement 2).
Figure 3. Subgroup Analysis by Study Cohorts for Primary and Major Secondary End Points.
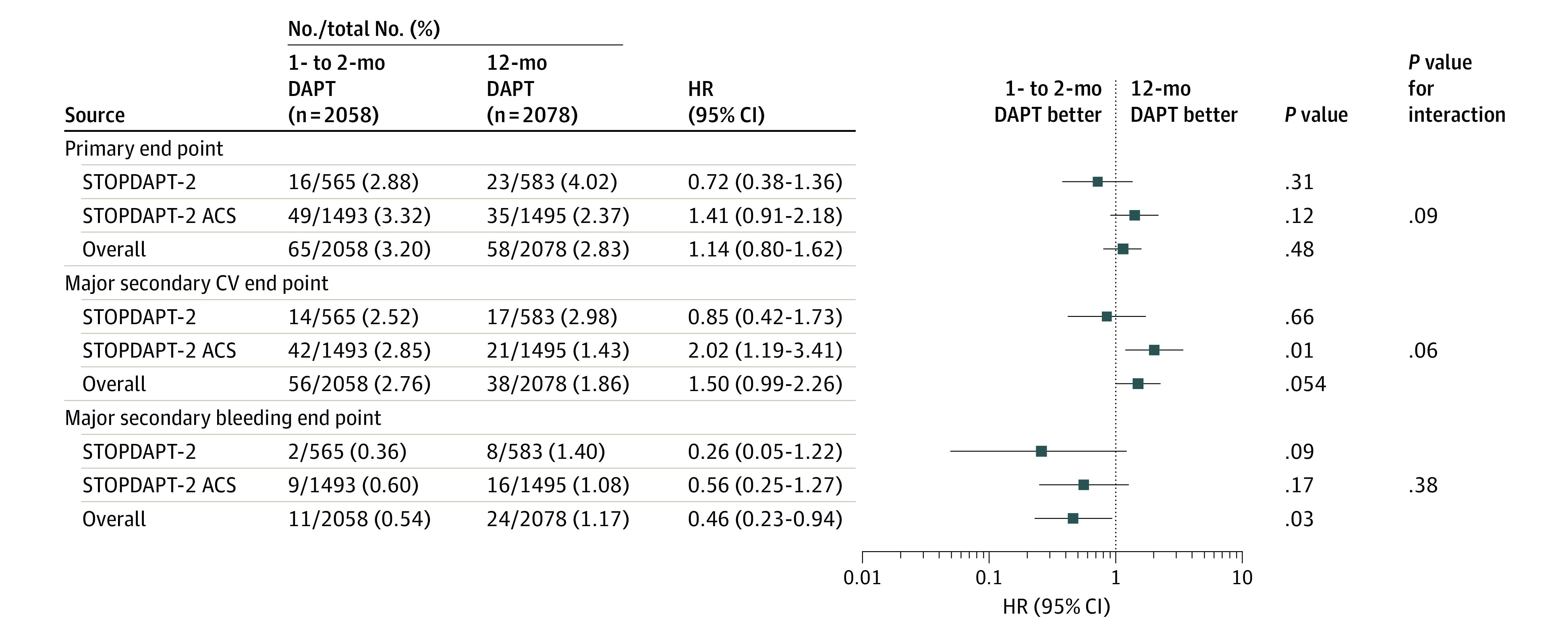
The subgroup analysis of the effect of 1 to 2 months of dual antiplatelet therapy (DAPT) relative to 12 months of dual antiplatelet therapy for the primary and major secondary end points in the 1148 patients with acute coronary syndrome enrolled in the Short and Optimal Duration of Dual Antiplatelet Therapy After Everolimus-Eluting Cobalt-Chromium Stent-2 (STOPDAPT-2) trial and the additional 2988 patients enrolled in the Short and Optimal Duration of Dual Antiplatelet Therapy After Everolimus-Eluting Cobalt-Chromium Stent-2 trial. CV indicates cardiovascular; HR, hazard ratio.
Discussion
The main findings of the present study were the following: (1) in patients with ACS who underwent PCI using CoCr-EES, clopidogrel monotherapy after 1 to 2 months of DAPT failed to attest noninferiority to 12 months of DAPT with aspirin and clopidogrel for a composite of cardiovascular or bleeding events; (2) clopidogrel monotherapy after 1 to 2 months of DAPT compared with 12 months of DAPT with aspirin and clopidogrel was associated with a reduction in major bleeding events, but with a numerical increase in cardiovascular events. Globally, the standard regimen after PCI in patients with ACS is 12 months of DAPT with aspirin and a newer P2Y12 inhibitor, such as ticagrelor or prasugrel.1,2,15,16 However, thrombotic risk of patients with ACS is known to attenuate over time.26 Moreover, the prevalence of patients with high bleeding risk is much higher in real clinical practice than in clinical trials.27,28,29 Therefore, de-escalation of antithrombotic therapy in parallel with time-dependent attenuation of thrombotic risk might be a reasonable approach in patients with ACS. Among 5 clinical trials exploring very short DAPT duration after PCI, 3 trials12,30,31enrolling nearly 15 000 patients with ACS have convincingly demonstrated that ticagrelor monotherapy after 3 months or shorter of DAPT was associated with substantial reduction in bleeding events without increase in cardiovascular events compared with 12 months of DAPT with aspirin and ticagrelor.12,14,30,31 In a substudy of patients with ACS from the GLOBAL LEADERS trial, ticagrelor monotherapy even after 1 month of DAPT compared with 12 months of DAPT with aspirin and ticagrelor was associated with a significant reduction in major bleeding events without an increase in cardiovascular events.30 In the STOPDAPT-2 trial including patients with both CCS and ACS, clopidogrel monotherapy after 1 to 2 months of DAPT was superior in bleeding outcome and noninferior in cardiovascular outcome to 12 months of DAPT with aspirin and clopidogrel.9 In the present study analyzing a larger number of patients with ACS, clopidogrel monotherapy after 1 to 2 months of DAPT failed to attest noninferiority to 12 months of DAPT with aspirin and clopidogrel for the primary end point evaluating the net clinical benefit, although it was associated with reduction in major bleeding. The present study would be inconclusive, given the failure of noninferiority testing for the primary end point. One might argue that we should be cautious in adopting clopidogrel monotherapy after 1 to 2 months of DAPT in patients with ACS, because it was associated with a numerical increase in cardiovascular events compared with 12 months of DAPT with aspirin and clopidogrel. Others might argue that clopidogrel monotherapy after 1 to 2 months of DAPT remains a viable option even in patients with ACS, because it was associated with significant reduction in major bleeding without meaningful difference in cardiovascular death, large myocardial infarction, and stroke compared with 12 months of DAPT with aspirin and clopidogrel. Clopidogrel monotherapy might not be the optimal antithrombotic regimen after very short DAPT in patients with ACS who underwent successful PCI. Several previous studies suggested that aspirin monotherapy was safe and effective after a 3-month course of DAPT in patients with ACS or CCS, although the number of patients studied was limited, particularly in patients with ACS.32,33,34 Which antiplatelet agent is optimal for monotherapy after very short DAPT is still the moving target. Further studies are warranted to define the optimal antithrombotic regimen after PCI in patients with ACS.
Limitations
The present study has several important limitations. First, we pooled the study patients from the 2 different trials (STOPDAPT-2 and STOPDAPT-2 ACS), which might confound the interpretation of data. The characteristics of the 2 subgroups of patients were different in several aspects, such as clinical presentation of acute myocardial infarction, heart failure, emergent procedure, radial approach, left main coronary artery target, and treatment with guideline-directed medications. Indeed, in the exploratory analyses stratified by the STOPDAPT-2 tria and STOPDAPT-2 ACS trial, the excess risk of 1 to 2 months of DAPT relative to 12 months of DAPT was significant for the major secondary cardiovascular end point in the STOPDAPT-2 ACS trial, but not in the STOPDAPT-2 trial. A larger trial that included only these patients as enrolled in STOPDAPT-2 ACS trial might show significant harm of clopidogrel monotherapy after very short DAPT in terms of cardiovascular end point. Second, we used a composite end point for both cardiovascular and bleeding outcomes as the primary end point to evaluate net clinical benefit. However, the validity of evaluating net clinical benefit has not yet been established, particularly when a given intervention affects the components of the primary end point in opposite directions. Nevertheless, net clinical benefit might be a clinically relevant measure for comparing a given antithrombotic regimen with another. Third, open-label trial design might have resulted in the differences in the ascertainment of outcomes, although the components of the primary end points in the present study might not so much be affected by the open-label trial design. Fourth, randomization was not performed at 1 to 2 months when the antiplatelet regimen was actually modified according to the protocol. Thus, the nonrandomized treatment period for 1 to 2 month was included for comparison, rendering the noninferiority comparison toward null. Fifth, as in the many other short DAPT studies, randomization was performed after successful PCI, and thus excluded those patients with cardiovascular and bleeding events after PCI. Also, among the eligible patients for the study, enrolled patients were younger and less often had comorbidities than nonenrolled patients. Therefore, the current study patients would have represented a substantially lower-risk population than the all-comers population with ACS undergoing PCI in the real clinical practice. Sixth, in the 1- to 2-month DAPT group, aspirin was discontinued not exactly at 1 month, but at 30 to 59 days after index PCI. Seventh, the incidence of the primary end point in the 12-month DAPT group was remarkably lower than anticipated, rendering the present study substantially underpowered. We could not deny the possibility of play of chance for the very low event rate in the 12-month DAPT group. Eighth, we did not assess the influence of clopidogrel resistance owing to CYP2C19 variations on clinical outcomes. It is well known that the prevalence of clopidogrel resistance is high in Japanese patients, which might be one of the reasons for the numerically higher rate of cardiovascular events in the 1- to 2-month DAPT group than in the 12-month DAPT group in the present study.
Conclusions
In patients with ACS who underwent successful PCI using CoCr-EES, clopidogrel monotherapy after 1 to 2 months of DAPT failed to attest noninferiority to 12 months of DAPT with aspirin and clopidogrel for the net clinical benefit with a numerical increase in cardiovascular events despite reduction in major bleeding events. The directionally different efficacy and safety outcomes indicate the need for further clinical trials.
Trial protocol.
eAppendix 1. STOPDAPT-2 ACS Study Organization.
eAppendix 2. Participating centers in the STOPDAPT-2 ACS.
eAppendix 3. Definition of end points.
eMethods.
eTable 1. Clinical and procedural characteristics compared between enrolled patients and eligible but non-enrolled patients among overall ACS patients, ACS patients in STOPDAPT-2, and patients in STOPDAPT-2 ACS.
eTable 2. Complete baseline characteristics.
eTable 3. Comparison of baseline characteristics between STOPDAPT-2 and STOPDAPT-2 ACS.
eTable 4. Per-protocol and as-treated population according to the mode of antithrombotic therapy at 60-day.
eTable 5. Details of cases with definite or probable stent thrombosis.
eFigure 1. The rate of persistent DAPT discontinuation rate.
eFigure 2. Per-protocol analysis for the primary end point.
eFigure 3. As-treated analysis for the primary end point.
eFigure 4. Worst-case scenario for the primary end point.
eFigure 5. Landmark analysis at 30 days.
eFigure 6. Landmark analysis at 60 days.
eFigure 7. Landmark analysis at the day of drug modification.
eFigure 8. Time-to-event curves for the primary and major secondary cardiovascular and bleeding end points in the 1-2 month DAPT group stratified by the median value of DAPT duration.
eFigure 9. Subgroup analyses for the relative effect of 1-2 month DAPT on the primary and major secondary end points.
eFigure 10. Time-to-event curves for the primary and major secondary cardiovascular and bleeding end points stratified by the study (patients in STOPDAPT-2 and STOPDAPT-2 ACS.
The STOPDAPT-2 ACS Investigators.
Data sharing statement.
References
- 1.Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease, 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation. 2016;134(10):e123-e155. [DOI] [PubMed] [Google Scholar]
- 2.Valgimigli M, Bueno H, Byrne RA, et al. ; ESC Scientific Document Group; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies . 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for Dual Antiplatelet Therapy in Coronary Artery Disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39(3):213-260. doi: 10.1093/eurheartj/ehx419 [DOI] [PubMed] [Google Scholar]
- 3.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators . Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345(7):494-502. doi: 10.1056/NEJMoa010746 [DOI] [PubMed] [Google Scholar]
- 4.Mehta SR, Yusuf S, Peters RJ, et al. ; Clopidogrel in Unstable angina to prevent Recurrent Events trial (CURE) Investigators . Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001;358(9281):527-533. doi: 10.1016/S0140-6736(01)05701-4 [DOI] [PubMed] [Google Scholar]
- 5.Urban P, Mehran R, Colleran R, et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention. Circulation. 2019;140(3):240-261. doi: 10.1161/CIRCULATIONAHA.119.040167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakamura M, Kimura K, Kimura T, et al. JCS 2020 guideline focused update on antithrombotic therapy in patients with coronary artery disease. Circ J. 2020;84(5):831-865. doi: 10.1253/circj.CJ-19-1109 [DOI] [PubMed] [Google Scholar]
- 7.Collet J-P, Thiele H, Barbato E, et al. ; ESC Scientific Document Group . 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42(14):1289-1367. doi: 10.1093/eurheartj/ehaa575 [DOI] [PubMed] [Google Scholar]
- 8.Vranckx P, Valgimigli M, Jüni P, et al. ; GLOBAL LEADERS Investigators . Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-label, randomised superiority trial. Lancet. 2018;392(10151):940-949. doi: 10.1016/S0140-6736(18)31858-0 [DOI] [PubMed] [Google Scholar]
- 9.Watanabe H, Domei T, Morimoto T, et al. ; STOPDAPT-2 Investigators . Effect of 1-month dual antiplatelet therapy followed by clopidogrel vs 12-month dual antiplatelet therapy on cardiovascular and bleeding events in patients receiving PCI: the STOPDAPT-2 randomized clinical trial. JAMA. 2019;321(24):2414-2427. doi: 10.1001/jama.2019.8145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hahn J-Y, Song YB, Oh JH, et al. ; SMART-CHOICE Investigators . Effect of P2Y12 inhibitor monotherapy vs dual antiplatelet therapy on cardiovascular events in patients undergoing percutaneous coronary intervention: the SMART-CHOICE randomized clinical trial. JAMA. 2019;321(24):2428-2437. doi: 10.1001/jama.2019.8146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehran R, Baber U, Sharma SK, et al. Ticagrelor with or without aspirin in high-risk patients after PCI. N Engl J Med. 2019;381(21):2032-2042. doi: 10.1056/NEJMoa1908419 [DOI] [PubMed] [Google Scholar]
- 12.Kim B-K, Hong S-J, Cho Y-H, et al. ; TICO Investigators . Effect of ticagrelor monotherapy vs ticagrelor with aspirin on major bleeding and cardiovascular events in patients with acute coronary syndrome: the TICO randomized clinical trial. JAMA. 2020;323(23):2407-2416. doi: 10.1001/jama.2020.7580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giacoppo D, Matsuda Y, Fovino LN, et al. Short dual antiplatelet therapy followed by P2Y12 inhibitor monotherapy vs. prolonged dual antiplatelet therapy after percutaneous coronary intervention with second-generation drug-eluting stents: a systematic review and meta-analysis of randomized clinical trials. Eur Heart J. 2021;42(4):308-319. doi: 10.1093/eurheartj/ehaa739 [DOI] [PubMed] [Google Scholar]
- 14.Valgimigli M, Gragnano F, Branca M, et al. P2Y12 inhibitor monotherapy or dual antiplatelet therapy after coronary revascularisation: individual patient level meta-analysis of randomised controlled trials. BMJ. 2021;373(n1332):n1332. doi: 10.1136/bmj.n1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiviott SD, Braunwald E, McCabe CH, et al. ; TRITON-TIMI 38 Investigators . Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001-2015. doi: 10.1056/NEJMoa0706482 [DOI] [PubMed] [Google Scholar]
- 16.Wallentin L, Becker RC, Budaj A, et al. ; PLATO Investigators . Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045-1057. doi: 10.1056/NEJMoa0904327 [DOI] [PubMed] [Google Scholar]
- 17.Thygesen K, Alpert JS, Jaffe AS, et al. ; Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction . Third universal definition of myocardial infarction. Circulation. 2012;126(16):2020-2035. doi: 10.1161/CIR.0b013e31826e1058 [DOI] [PubMed] [Google Scholar]
- 18.Ministry of Health , Labour and Welfare. Clinical Trials Act (Act No. 16 of April 14, 2017). Accessed July 8, 2012. https://www.mhlw.go.jp/file/06-Seisakujouhou-10800000-Iseikyoku/0000213334.pdf
- 19.Rao AK, Pratt C, Berke A, et al. Thrombolysis in Myocardial Infarction (TIMI) Trial—phase I: hemorrhagic manifestations and changes in plasma fibrinogen and the fibrinolytic system in patients treated with recombinant tissue plasminogen activator and streptokinase. J Am Coll Cardiol. 1988;11(1):1-11. doi: 10.1016/0735-1097(88)90158-1 [DOI] [PubMed] [Google Scholar]
- 20.Kimura T, Morimoto T, Natsuaki M, et al. ; RESET Investigators . Comparison of everolimus-eluting and sirolimus-eluting coronary stents: 1-year outcomes from the Randomized Evaluation of Sirolimus-Eluting Versus Everolimus-Eluting Stent Trial (RESET). Circulation. 2012;126(10):1225-1236. doi: 10.1161/CIRCULATIONAHA.112.104059 [DOI] [PubMed] [Google Scholar]
- 21.Natsuaki M, Kozuma K, Morimoto T, et al. ; NEXT Investigators . Biodegradable polymer biolimus-eluting stent versus durable polymer everolimus-eluting stent: a randomized, controlled, noninferiority trial. J Am Coll Cardiol. 2013;62(3):181-190. doi: 10.1016/j.jacc.2013.04.045 [DOI] [PubMed] [Google Scholar]
- 22.Serruys PW, Morice MC, Kappetein AP, et al. ; SYNTAX Investigators . Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360(10):961-972. doi: 10.1056/NEJMoa0804626 [DOI] [PubMed] [Google Scholar]
- 23.Park SJ, Kim YH, Park DW, et al. Randomized trial of stents versus bypass surgery for left main coronary artery disease. N Engl J Med. 2011;364(18):1718-1727. doi: 10.1056/NEJMoa1100452 [DOI] [PubMed] [Google Scholar]
- 24.Natsuaki M, Morimoto T, Yamaji K, et al. ; CREDO‐Kyoto PCI/CABG Registry Cohort 2, RESET, and NEXT trial investigators . Prediction of thrombotic and bleeding events after percutaneous coronary intervention: CREDO-Kyoto thrombotic and bleeding risk scores. J Am Heart Assoc. 2018;7(11):e008708. doi: 10.1161/JAHA.118.008708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baber U, Mehran R, Giustino G, et al. Coronary thrombosis and major bleeding after PCI with drug-eluting stents: risk scores from PARIS. J Am Coll Cardiol. 2016;67(19):2224-2234. doi: 10.1016/j.jacc.2016.02.064 [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez F, Harrington RA. Management of antithrombotic therapy after acute coronary syndromes. N Engl J Med. 2021;384(5):452-460. doi: 10.1056/NEJMra1607714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Natsuaki M, Morimoto T, Shiomi H, et al. Application of the Academic Research Consortium High Bleeding Risk Criteria in an all-comers registry of percutaneous coronary intervention. Circ Cardiovasc Interv. 2019;12(11):e008307. doi: 10.1161/CIRCINTERVENTIONS.119.008307 [DOI] [PubMed] [Google Scholar]
- 28.Natsuaki M, Morimoto T, Shiomi H, et al. ; CREDO-Kyoto PCI/CABG Registry Cohort-3 Investigators . Application of the modified high bleeding risk criteria for Japanese patients in an all-comers registry of percutaneous coronary intervention—from the CREDO-Kyoto Registry Cohort-3. Circ J. 2021;85(6):769-781. doi: 10.1253/circj.CJ-20-0836 [DOI] [PubMed] [Google Scholar]
- 29.Kanenawa K, Yamaji K, Tashiro H, et al. Patient selection and clinical outcomes in the STOPDAPT-2 trial: an all-comer single-center registry during the enrollment period of the STOPDAPT-2 randomized controlled trial. Circ Cardiovasc Interv. 2021;14(2):e010007. doi: 10.1161/CIRCINTERVENTIONS.120.010007 [DOI] [PubMed] [Google Scholar]
- 30.Tomaniak M, Chichareon P, Onuma Y, et al. ; GLOBAL LEADERS Trial Investigators . Benefit and risks of aspirin in addition to ticagrelor in acute coronary syndromes: a post hoc analysis of the randomized GLOBAL LEADERS trial. JAMA Cardiol. 2019;4(11):1092-1101. doi: 10.1001/jamacardio.2019.3355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baber U, Dangas G, Angiolillo DJ, et al. Ticagrelor alone vs. ticagrelor plus aspirin following percutaneous coronary intervention in patients with non-ST-segment elevation acute coronary syndromes: TWILIGHT-ACS. Eur Heart J. 2020;41(37):3533-3545. doi: 10.1093/eurheartj/ehaa670 [DOI] [PubMed] [Google Scholar]
- 32.Feres F, Costa RA, Abizaid A, et al. ; OPTIMIZE Trial Investigators . Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA. 2013;310(23):2510-2522. doi: 10.1001/jama.2013.282183 [DOI] [PubMed] [Google Scholar]
- 33.Kim B-K, Hong MK, Shin D-H, et al. ; RESET Investigators . A new strategy for discontinuation of dual antiplatelet therapy: the RESET trial (real safety and efficacy of 3-month dual antiplatelet therapy following endeavor zotarolimus-eluting stent implantation). J Am Coll Cardiol. 2012;60(15):1340-1348. doi: 10.1016/j.jacc.2012.06.043 [DOI] [PubMed] [Google Scholar]
- 34.Valgimigli M, Campo G, Monti M, et al. ; Prolonging Dual Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia Study (PRODIGY) Investigators . Short-versus long-term duration of dual-antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation. 2012;125(16):2015-2026. doi: 10.1161/CIRCULATIONAHA.111.071589 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol.
eAppendix 1. STOPDAPT-2 ACS Study Organization.
eAppendix 2. Participating centers in the STOPDAPT-2 ACS.
eAppendix 3. Definition of end points.
eMethods.
eTable 1. Clinical and procedural characteristics compared between enrolled patients and eligible but non-enrolled patients among overall ACS patients, ACS patients in STOPDAPT-2, and patients in STOPDAPT-2 ACS.
eTable 2. Complete baseline characteristics.
eTable 3. Comparison of baseline characteristics between STOPDAPT-2 and STOPDAPT-2 ACS.
eTable 4. Per-protocol and as-treated population according to the mode of antithrombotic therapy at 60-day.
eTable 5. Details of cases with definite or probable stent thrombosis.
eFigure 1. The rate of persistent DAPT discontinuation rate.
eFigure 2. Per-protocol analysis for the primary end point.
eFigure 3. As-treated analysis for the primary end point.
eFigure 4. Worst-case scenario for the primary end point.
eFigure 5. Landmark analysis at 30 days.
eFigure 6. Landmark analysis at 60 days.
eFigure 7. Landmark analysis at the day of drug modification.
eFigure 8. Time-to-event curves for the primary and major secondary cardiovascular and bleeding end points in the 1-2 month DAPT group stratified by the median value of DAPT duration.
eFigure 9. Subgroup analyses for the relative effect of 1-2 month DAPT on the primary and major secondary end points.
eFigure 10. Time-to-event curves for the primary and major secondary cardiovascular and bleeding end points stratified by the study (patients in STOPDAPT-2 and STOPDAPT-2 ACS.
The STOPDAPT-2 ACS Investigators.
Data sharing statement.


