Abstract
Borrelia burgdorferi sensu lato, the spirochete that causes human Lyme borreliosis (LB), is a genetically and phenotypically divergent species. In the past several years, various molecular approaches have been developed and used to determine the phenotypic and genetic heterogeneity within the LB-related spirochetes and their potential association with distinct clinical syndromes. These methods include serotyping, multilocus enzyme electrophoresis, DNA-DNA reassociation analysis, rRNA gene restriction analysis (ribotyping), pulsed-field gel electrophoresis, plasmid fingerprinting, randomly amplified polymorphic DNA fingerprinting analysis, species-specific PCR and PCR-based restriction fragment length polymorphism (RFLP) analysis, and sequence analysis of 16S rRNA and other conserved genes. On the basis of DNA-DNA reassociation analysis, 10 different Borrelia species have been described within the B. burgdorferi sensu lato complex: B. burgdorferi sensu stricto, Borrelia garinii, Borrelia afzelii, Borrelia japonica, Borrelia andersonii, Borrelia valaisiana, Borrelia lusitaniae, Borrelia tanukii, Borrelia turdi, and Borrelia bissettii sp. nov. To date, only B. burgdorferi sensu stricto, B. garinii, and B. afzelii are well known to be responsible for causing human disease. Different Borrelia species have been associated with distinct clinical manifestations of LB. In addition, Borrelia species are differentially distributed worldwide and may be maintained through different transmission cycles in nature. In this paper, the molecular methods used for typing of B. burgdorferi sensu lato are reviewed. The current taxonomic status of B. burgdorferi sensu lato and its epidemiological and clinical implications, especiallly correlation between the variable clinical presentations and the infecting Borrelia species, are discussed in detail.
Lyme borreliosis (LB), or Lyme disease, represents a new global public health problem (16). It is now the most common vector-borne disease in North America (41, 237) and Eurasia (172, 291). In the United States, more than 100,000 LB cases have been reported from 47 states in 1982 through 1996, with more than 16,000 cases in 1996 (41). It is estimated that annually about 50,000 cases occur in Europe (172).
In 1982, the bacterium that causes LB was first isolated by Willy Burgdorfer and colleagues from the hard tick Ixodes dammini (now Ixodes scapularis [175]) collected on Long Island, N.Y. (32). The isolate was subsequently identified as a new species of the genus Borrelia and was named Borrelia burgdorferi in 1984 (111). Since then, hundreds of B. burgdorferi isolates have been cultured worldwide from various geographic regions and biological sources, including Ixodes ticks, their reservoir hosts, and specimens from patients with different clinical syndromes. Molecular analysis has indicated that these B. burgdorferi isolates are genetically and phenotypically divergent. A closely related cluster containing several tick-borne Borrelia species and genomic groups associated with LB has been defined (14, 35, 68, 111, 116, 125, 143, 201, 267). The term “B. burgdorferi sensu lato” is now collectively used to refer to all Borrelia isolates within this cluster and to distinguish it from the species “B. burgdorferi sensu stricto” (strict sense of B. burgdorferi) (14).
Like other Borrelia species, B. burgdorferi sensu lato is a spiral-shaped, gram-negative bacterium with 7 to 11 periplasmic flagella. It varies from 10 to 30 μm in length and 0.2 to 0.5 μm in width (18). The genome of the type strain B. burgdorferi sensu stricto B31 contains a linear chromosome of 910,725 bp, with an average G+C content of 28.6%, and 21 plasmids (9 circular and 12 linear) with a combined size of more than 613,000 bp (38, 65). The G+C content of individual plasmids ranges from 23.1 to 32.3% (65).
Human infection due to B. burgdorferi sensu lato may involve multiple organs or tissues, resulting in skin, cardiac, neurological and musculoskeletal disorders. Lyme disease (236) was described as a new clinical entity in 1977 because of a geographic clustering of children with rheumatoid-like arthritis in Lyme, Conn. (239, 240, 241). Retrospective analysis revealed that many of the clinical manifestations of LB had been separately recorded by European clinicians since the end of 19th century (270). Table 1 lists the spectrum of the major clinical manifestations of human LB in North America and Europe. It has been shown that multiple erythema migrans (EM) and Lyme arthritis are more common in the United States than in Europe, whereas neuroborreliosis has more frequently occurred in European patients, especially in children with LB (23, 43, 44, 79, 178, 259). Borrelial lymphocytoma and acrodermatitis chronica atrophicans (ACA) are well documented in European LB patients but are rarely recognized among LB patients in the United States (194, 235). As a result of its protean clinical manifestations, LB was described as the new “great imitator” of various human diseases (179).
TABLE 1.
Major clinical manifestations of Lyme borreliosis in North America and Europe
| Stagea | Clinical featureb | Incidence (reference) in:
|
|
|---|---|---|---|
| North Americac | Europed | ||
| I | Early local infection | ||
| EM | Common (60–90%) (44, 79) | Common (∼77%) (23) | |
| Tick bite recalled | 25% (245) | 64% (245) | |
| Central clearing of EM | 35% (245) | 68% (245) | |
| Systemic symptoms | 50–69% (161, 245) | 38–51% (245, 246) | |
| II | Early disseminated infection | ||
| Multiple EM | Common (>18%) (161)e | Unusual (6%) (246)e | |
| Neuroborreliosis | Common (10–20%) (44, 79) | Common (16–80%) (23, 43) | |
| Meningoradiculitis | 3–21% (44, 79)f | 37–61% (92, 178)f | |
| Meningitis | 2–17% (44, 79)f | 4–27% (23, 43, 178)f | |
| Carditis | 0.5–10% (44, 79)f | 0.5–4% (46, 178) | |
| Borrelial lymphocytoma | Rare | Well documented (3%) (23) | |
| III | Late LB | ||
| Lyme arthritis | Common (51–57%) (44, 242)g | Uncommon (∼7%) (23) | |
| ACA | Rare (194) | Well documented (3%) (23) | |
| Peripheral neuropathy | 30–70% late NB (44, 91)f | 40–63% ACA patients (120) | |
| CNS involvement | Well documented (90) | <9% NB (178)f | |
| Encephalomyelitis | Rare (0.1%) (91) | 4–6% (92, 178)f | |
| Meninggencephalitis | 9% (44)f | 0.5–4% (92, 178)f | |
Stages of the clinical features are those of Steere (236).
NB, neuroborreliosis; CNS, central nervous system.
Data were based mainly on an earlier report by the Centers for Disease Control and Prevention on LB surveillance from 1984 to 1986 (44) and a population-based study in children in Southern Connecticut (79), except for those indicated specifically.
Data were based mainly on a population-based study in Southern Sweden (23), except for those indicated specifically.
Numerous studies indicate that the B. burgdorferi sensu lato population is genetically highly divergent (1, 12, 14, 26, 71, 109, 130, 132, 144, 145, 147, 155, 168, 197, 259, 268). Different Borrelia species may be associated with distinct clinical manifestations of LB (9, 11, 14, 35, 56, 173, 186, 194, 259). In this paper, various molecular methods used for the identification and typing of B. burgdorferi sensu lato are reviewed. The current taxonomic status of B. burgdorferi sensu lato and the epidemiological and clinical implications of typing of B. burgdorferi sensu lato, especially the correlation between the various clinical presentations of LB and the infecting Borrelia species, are discussed.
PHENOTYPIC METHODS FOR TYPING OF B. BURGDORFERI SENSU LATO
Molecular techniques used for the identification and typing of microorganisms can be categorized as either phenotypic or genetic on the basis of the macromolecular targets used for analysis (249). For B. burgdorferi sensu lato, phenotypic typing systems such as biotyping, phage typing, and antibiotic susceptibility analysis, which are used for various bacterial species, are not feasible. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis protein (187, 257, 276) and fatty acid profile analysis (135, 136) have been used for typing of B. burgdorferi sensu lato, but the conclusions based on both of these phenotypic characteristics are not always reliable. Therefore, serotyping represents the most commonly used phenotypic method for B. burgdorferi sensu lato.
Serotyping
The protein profiles of B. burgdorferi sensu lato isolates are heterogeneous (19, 276, 279, 280). Currently, two serotyping systems, based on the heterogeneity of outer surface protein A (OspA) and outer surface protein C (OspC) of B. burgdorferi sensu lato, are well established.
OspA serotyping.
OspA is one of the major outer membrane lipoproteins of B. burgdorferi and has been used for serological diagnosis as well as for vaccine development (97, 119). The molecular mass of OspA ranges from 31 to 34 kDa among the different species of B. burgdorferi sensu lato (267, 280). Based on the differential reactivities of 112 European and 24 North American B. burgdorferi sensu lato isolates with eight OspA-specific monoclonal antibodies (MAbs), seven different OspA serotypes (serotype 1 to 7) were defined by Wilske et al. in 1993 (280). Later, an additional OspA serotype 8 was reported (277). Among Japanese B. burgdorferi sensu lato isolates, OspA serotypes J1 to J11 were recognized (152, 287).
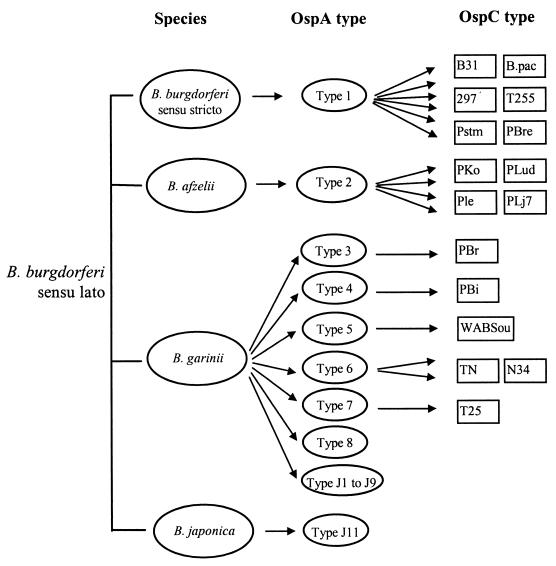
Analysis of phenotypic characteristics in parallel with genetic markers showed that OspA serotypes correlated well with the current classification of B. burgdorferi sensu lato (280). As shown in Fig. 1, OspA serotypes 1, 2, and J11 correspond to B. burgdorferi sensu stricto, B. afzelii, and B. japonica, respectively, and serotypes 3 to 8 (types J1 to J9 in Japan) correspond to B. garinii (278, 287). OspA serotypes are likely to be differentially distributed among tick and human isolates and have been associated with distinct manifestations of LB (268, 277, 278, 280). In a report of 201 European B. burgdorferi sensu lato isolates, 53% of the 90 tick isolates were OspA serotype 6 but only 9% were OspA serotype 2. In contrast, 56% of the 111 isolates from patients with LB were OspA serotype 2 whereas only 13% were OspA serotype 6 (278). Although OspA serotype 4 has often been detected from cerebrospinal fluid CSF specimens of patients with Lyme neuroborreliosis (277), this serotype has not been observed among more than 90 tick isolates from various regions in Europe (278).
FIG. 1.
Relationship between species and OspA and OspC serotypes of B. burgdorferi sensu lato. OspA serotype J10 of Japanese isolates corresponds to the European B. afzelii strains of serotype 2. Data from references 152 and 279 are included. Adapted from reference 278 with permission of the publisher.
OspC serotyping.
OspC is the predominant seroreactive antigen in the early stage of human infection due to B. burgdorferi sensu lato (2, 56, 74, 185). The molecular mass of OspC among the different species of B. burgdorferi sensu lato varies from 20 to 25 kDa (180, 279). Similar to OspA serotyping, analysis of the reactivity of B. burgdorferi sensu lato with a set of MAbs specific for OspC has led to the definition of 16 different OspC serotypes for European and North American isolates (278, 279). Comparison of OspA and OspC serotypes of B. burgdorferi sensu lato suggests that OspC is much more heterogeneous than OspA, especially for B. burgdorferi sensu stricto and B. afzelii strains. While only one OspA serotype for each of these species is known, six corresponding OspC serotypes for B. burgdorferi sensu stricto and four OspC serotypes for B. afzelii were identified (278). The relationships among OspA and OspC serotypes and the delineated Borrelia species are summarized in Fig. 1.
OspA and OspC serotyping provides a simple, straightforward approach for the analysis of phenotypic characteristics among B. burgdorferi sensu lato at the species and subspecies level. Identification of the major B. burgdorferi sensu lato species can be made based on the reactivity of Borrelia isolates with the well-characterized species-specific MAbs (Table 2). However, the utility of both serotyping systems may be hampered due to the lack of OspA or OspC, the aberrant expression of these proteins, and possibly their variation during growth in vitro as well as in vivo (100, 244, 280).
TABLE 2.
Reactivity of B. burgdorferi sensu lato species with different species-specific MAbs
| Borrelia species | Reactivity with MAba:
|
Reference(s) | |||||
|---|---|---|---|---|---|---|---|
| H9724 | H5332 | H3TS | D6 | I17.3 | O1141b | ||
| B. burgdorferi sensu stricto | + | + | + | − | − | − | 14, 148 |
| B. garinii | + | + or − | − | + | − | − | 14, 148 |
| B. afzelii | + | − | − | − | + | − | 35, 148 |
| B. japonica | + | + | − | − | − | + | 148 |
The reactivity is referred to as positive (+) and negative (−).
Multilocus Enzyme Electrophoresis
Multilocus enzyme electrophoresis (MLEE) is a protein-based typing method whose results can be directly correlated with the genotype. MLEE is accepted as a promising method to elucidate the population genetics of bacteria (254). For this method, bacterial lysates are subjected to electrophoresis under nondenaturing conditions and the electrophoretic mobility of metabolic enzymes is determined after specific staining. Each electromorph is equated with an allele at the corresponding enzyme genetic locus. Thus, by associating each isolate with an electrophoretic type (ET), MLEE allows the differentiation of isolates by marking them with significant characteristics.
MLEE was used to analyze the population genetics of B. burgdorferi sensu lato in 1992 (26). In this study, 50 B. burgdorferi sensu lato isolates were grouped into 35 ETs, constituting three main divisions separated at a genetic distance greater than 0.75. Each of these three divisions corresponded to either B. burgdorferi sensu stricto, B. garinii, or B. afzelii (26). Later, this method was used by Balmelli and Piffaretti (12) to determine the overall genetic polymorphism of 54 B. burgdorferi sensu lato isolates from different regions of the world. A total of 12 genetic loci were characterized, and 50 ETs were distinguished. Cluster analysis of a matrix of genetic distances for pairs of ETs revealed 11 divisions separated from each other at a genetic distance greater than 0.65. Of these 11 divisions, 10 corresponded to B. burgdorferi sensu stricto (division I), B. garinii (divisions II and III), B. afzelii (division VII), B. japonica (division VIII), B. valaisiana (division V), B. lusitaniae (division IV), B. andersonii (division XI), and B. bissettii sp. nov. (divisions IX and X). Division VI contained only one isolate (CA2). Previously, this isolate had been placed into B. bissettii sp. nov., formerly genomic group DN127 (197, 201). Additional studies are needed to determine whether this strain constitutes a distinct species.
MLEE provides an estimate of the overall genetic relatedness and genetic diversity of B. burgdorferi sensu lato population. Almost all Borrelia isolates analyzed can be classified at the species level, and these assignments agree well with the results obtained from various genetic typing techniques (12, 26). However, this method is labor-intensive, mainly because large quantities of spirochetes have to be grown to obtain enough lysate for MLEE analysis. Therefore, MLEE is now used mainly to elucidate the population genetics of B. burgdorferi sensu lato.
GENOTYPIC METHODS FOR TYPING OF B. BURGDORFERI SENSU LATO
Molecular typing based on the genetic characteristics of microorganisms can provide more precise information on the diversity of pathogenic bacteria. During the past several years, a number of genotyping methods have been used to assess the genetic relationships among B. burgdorferi sensu lato species.
DNA-DNA Reassociation Analysis
DNA-DNA reassociation analysis is acknowledged to be a superior method for examining relationships between closely related taxa and represents the best applicable procedure for bacterial taxonomy at present (231). As recommended by the Ad Hoc Committee on reconciliation of approaches to bacterial systematics, the phylogenetic definition of a species generally would include strains with approximately 70% or more DNA-DNA relatedness and with a ΔTm of 5°C or less (269).
The DNA reassociation value among strains in the genus Borrelia varies, ranging from 30 to 100% (18, 111). It is reported that the level of DNA homology between Borrelia species causing relapsing fever and those causing LB is about 30 to 44% (18). Among different B. burgdorferi sensu lato species, the level of DNA relatedness is about 48 to 70% (14, 111, 197). For example, the type strain B. afzelii VS461 has 48, 65, 54, 64, and 58% DNA homology to the type strains B. burgdorferi sensu stricto B31, B. garinii 20047 (14), B. japonica HO14 (116), B. valaisiana VS116, and B. lusitaniae PotiB2, respectively (197).
Because plasmid DNA may account for one third of the total genome of B. burgdorferi sensu lato, the results of DNA-DNA hybridization will also be affected by differences in the plasmid contents between strains. It is expected that plasmid loss occurring during in vitro cultivation (222) may result in a DNA-DNA relatedness below 100% between an isolate and its subcultured variants or among certain highly related strains. However, no data is currently available on this issue, and Borrelia isolates showing less than 70% homology to each other in DNA-DNA reassociation analysis do belong to different genospecies when other genetic techniques such as sequencing of the chromosomal genes are used for typing (14, 57, 127).
DNA hybridization is currently used as the reference method for species delineation of B. burgdorferi sensu lato (14, 116, 197). On the basis of DNA-DNA relatedness, several different Borrelia species or genomic groups associated with LB have been identified (see “Taxonomy of B. burgdorferi sensu lato,” below, for details).
rRNA Restriction Analysis (Ribotyping)
Ribotyping has been frequently used for both taxonomic purposes and subgroup characterization of microorganisms belonging to different genera and species (195). Grouping of bacteria by ribotyping is based on the profiles obtained by restriction fragment patterns of chromosomal DNA digested with appropriate restriction enzymes and hybridized with a probe derived from a highly conserved rRNA. The restriction enzymes EcoRI, EcoRV, PstI, HincII, HpaI, and HindIII and probes from 16S + 23S rRNA of Escherichia coli (14, 267) or from 16S rRNA (144), 23S rRNA (71), or 5S rRNA (132, 225) of the LB spirochete have been successfully used to identify B. burgdorferi sensu lato strains at the species level. An example of the restriction patterns obtained after EcoRV digestion of genomic DNAs from isolates belonging to different Borrelia species is summarized by Postic et al. (196). It is noteworthy that almost all of the LB-related spirochetes from a 3.2-kb EcoRV (14, 71), a 3.2-kb HpaI (132, 225), or a 2.2-kb HindIII (259) restriction fragment. Therefore, these bands can be used as genetic markers for the identification of B. burgdorferi sensu lato species. In addition, species-specific HindIII fragments were recognized by ribotyping for B. burgdorferi sensu stricto (1.45 kb), B. garinii (1.2 kb), and B. afzelii (4.0 and 1.45 kb) (259). These species-specific bands can be used to distinguish the three human pathogenic B. burgdorferi sensu lato species from each other.
A large number of B. burgdorferi sensu lato isolates from different sources have been analyzed by ribotyping (14, 71, 130, 164, 168, 259). In these studies, the restriction patterns of B. burgdorferi sensu stricto and B. afzelii appear very homogeneous whereas B. garinii is more heterogeneous. In a study including 51 Borrelia isolates (21 from Far Eastern Russia, Japan, and China; 20 from Europe; and 10 from North America), the 18 B. burgdorferi sensu stricto isolates belonged to only one ribotype while the 10 B. afzelii isolates showed three ribotypes and the 23 B. garinii isolates exhibited nine ribotypes (71). Thus, Borrelia strains can be distinguished by ribotyping at both species and subspecies levels. The result of ribotyping is rather reproducible. A relative drawback of this method is that more spirochetal DNAs are required than for other PCR-based typing approaches.
Pulsed-Field Gel Electrophoresis
Pulsed-field gel electrophoresis (PFGE) was first described in 1984 as a tool for examining the chromosomal DNA profiles of eukaryotic organisms (224). Subsequently, it has proven to be a highly effective molecular typing technique for many bacterial species (250). For this method, the bacterial genomic DNA is separated by PFGE after digestion with a restriction enzyme having relatively few recognition sites. Discrimination of the species and among strains is based on the large restriction fragment length polymorphism (LRFLP) of the chromosomal DNAs.
After digestion of the genome with MluI, the LRFLPs of B. burgdorferi sensu lato are both species and strain specific. As described by Belfaiza et al. (20) and confirmed by others, all three LB-causing B. burgdorferi sensu lato species are characterized by one to three species-specific fragments: a band at 135 kb for B. burgdorferi sensu stricto, two bands at 220 and 80 kb for B. garinii, and three bands at 460, 320, and 90 kb for B. afzelii isolates (33, 34, 72, 155, 191, 192). The PFGE method also allows the discrimination between strains within each Borrelia species. For example, 20 B. burgdorferi sensu stricto isolates were separated into 10 MluI LRFLPs, while 24 B. garinii and 6 B. japonica isolates exhibited 4 and 2 LRFLPs, respectively (20, 198, 199). Strikingly, only a single MluI pattern was observed among the 20 B. afzelii isolates (20, 198). In addition, PFGE can be used to construct physical maps of the genomes and to define different groups of B. burgdorferi sensu lato by combination of the chromosomal LRFLPs resulting from digestion of a set of restriction enzymes, e.g., MluI, SacI, BssHII, EagI, SmaI, ApaI, CspI, and SgraAI (36, 37, 51, 174).
PFGE represents a reproducible and highly discriminative typing method for B. burgdorferi sensu lato. Characterization of isolates by PFGE usually correlates well with species designation of B. burgdorferi sensu lato by other methods (20, 72). The findings from studies using this method have both taxonomic and clinical implications (20, 155). Mathiesen et al. (155) reported that B. burgdorferi sensu lato isolates from patients in the United States, irrespective of their geographic region, belonged to a single rDNA cluster. In this study, results obtained from PFGE and ospA sequence analysis were in accordance.
Plasmid Fingerprinting
B. burgdorferi sensu lato strains have unusual double-stranded linear plasmids, in addition to the typical supercoiled circular plasmids (17, 65, 98, 107). Usually, plasmids are present at a low copy number of approximately one to two per chromosome equivalent (37, 98). There may be as many as 21 or more different plasmids within one spirochete cell (38, 65). Since both the number and the size of plasmids can vary among strains, plasmid profile analysis may be used for strain and species identification of B. burgdorferi sensu lato.
Xu and Johnson (285) studied 40 B. burgdorferi sensu lato isolates from diverse biological sources and geographical locations by plasmid fingerprinting. The number of plasmids in the B. burgdorferi sensu lato isolates ranged from 4 to 10, and the size of these plasmids ranged from 13.3 to 57.7 kb. The overall plasmid profiles might correlate with the species of B. burgdorferi sensu lato, although definite species-specific plasmids could not be identified in the three Borrelia species studied. Nevertheless, only linear plasmids, but not circular plasmids, are well separated in plasmid fingerprinting (285). The usefulness of plasmid fingerprinting for the classification of B. burgdorferi sensu lato seems to be limited since the results could be affected simply by the loss of plasmids during in vitro cultivation (15, 222, 286) or by inter- and intraplasmidic recombination (38, 212).
Randomly Amplified Polymorphic DNA
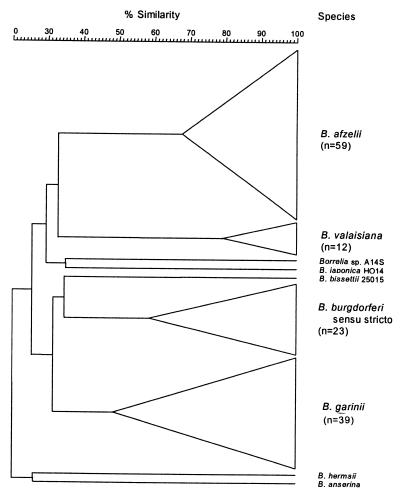
There is an increasing interest in the molecular typing of bacteria by using randomly amplified polymorphic DNA (RAPD) fingerprinting (275), or arbitrarily primed PCR (AP-PCR) (271). Both of these techniques use low-stringency PCR amplification with a single primer with an arbitrary sequence to generate strain-specific arrays of anonymous DNA fragments. In an early AP-PCR study, 29 B. burgdorferi sensu lato isolates were divided into three genospecies-specific groups (272). Later, AP-PCR was used to investigate the evolution of B. burgdorferi sensu stricto (64). More recently, this approach was evaluated by typing a large collection of B. burgdorferi sensu lato isolates from various biological and geographical sources (268). In this study, a total of 136 B. burgdorferi sensu lato strains were divided into seven genetic clusters, based on the DNA fingerprints generated with four arbitrary primers (Fig. 2). Six clusters contained 135 isolates corresponding to the well-defined species. One isolate did not belong to any of the known LB-related Borrelia species. Furthermore, pathogenic subgroups of B. garinii from LB patients with disseminated infections were identified by this analysis (268). Since RAPD is a highly discriminatory method and is easy to perform, this method is now a powerful tool to distinguish the different Borrelia species from each other as well as to recognize Borrelia strains within each of the species (268).
FIG. 2.
Simplified dendrogram of 136 Lyme disease spirochete isolates from different B. burgdorferi sensu lato species based on their RAPD fingerprints. The numbers of isolates for each B. burgdorferi sensu lato species studied is indicated in parenthesis. The size of the vertical bars of the triangles for the four major B. burgdorferi sensu lato species represents the number of isolates studied, while the position of the left angle of these triangles is representative of the percent similarity within each of these species. Modified from reference 268.
PCR and PCR-Based Restriction Fragment Length Polymorphism Analysis
Species-specific PCR.
PCR amplification with species-specific primers, in which the conserved 16S rRNA gene (131, 141) or species-specific plasmid gene loci (159, 160) are targeted, can be used directly for species identification of the LB spirochetes. The former approach has been used to differentiate the three human pathogenic species, B. burgdorferi sensu stricto, B. garinii, and B. afzelii (121, 141), as well as B. valaisiana (131), from each other. However, cross-amplification between Borrelia species may occur when using primers derived from the species-specific plasmid sequences (159).
rDNA PCR-RFLP analysis.
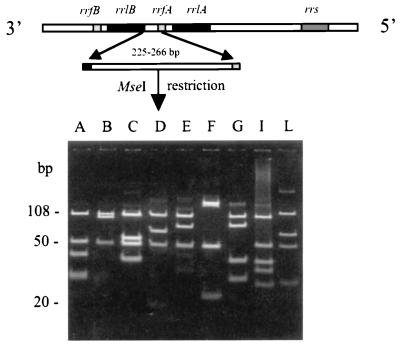
The rrn cluster of most B. burgdorferi sensu lato strains contains a single copy of 16S rRNA (rrs) and tandem repeated 23S rRNA (rrlA and rrlB) and 5S rRNA (rrfA and rrfB) (Fig. 3) (73, 77, 174, 225). The rDNA gene cluster is located at the center of the linear chromosome and is arranged in the following order: rrs-rrlA-rrfA-rrlB-rrfB. Three different PCR-RFLP approaches have been designed for B. burgdorferi sensu lato, based on the unique rRNA structure of these microorganisms, i.e., rrs, rrfA-rrlB spacer, and rrs-rrlA spacer.
FIG. 3.
Typing of B. burgdorferi sensu lato isolates by using the rrfA-rrlB intergenic spacer PCR-RFLP analysis. The rrfA-rrlB intergenic spacer was amplified by PCR, and this was followed by the analysis of MseI restriction polymorphism of PCR products. The eight B. burgdorferi sensu lato species included are B. burgdorferi sensu stricto (pattern A), B. garinii (patterns B and C), B. afzelii (pattern D), B. japonica (pattern E), B. valaisiana (pattern F), B. lusitaniae (pattern G), B. bissettii sp. nov. (pattern I), and B. andersonii (pattern L). Modified from reference 196 with permission of the publisher.
DNA sequence analysis of rrs gene is able to discriminate various B. burgdorferi sensu lato species (see the following section). Several species of B. burgdorferi sensu lato can also be distinguished from each other on the basis of their BfaI restriction patterns of PCR-amplified rrs (127, 203) or by single-strand conformational polymorphism–PCR analysis of the rrs (273). However, RFLP analysis of the PCR-amplified DNA from spacers between the ribosomal genes shows more discriminatory power.
rrfA-rrlB spacer PCR-RFLP analysis is a widely used typing system for B. burgdorferi sensu lato (197). Development and application of this method led to the description of eight different species or genomic groups within B. burgdorferi sensu lato in 1994 by Postic et al. (197). PCR amplification of the highly variable rrfA-rrlB intergenic spacer usually yields a 225- to 266-bp amplicon among strains from different species of B. burgdorferi sensu lato (149, 197, 265). No amplification occurred with the relapsing-fever borreliae. Digestion of amplicons with MseI resulted in different restriction fragments with species-differentiating characteristics (Fig. 3) (197). Table 3 summarizes the MseI restriction patterns of B. burgdorferi sensu lato identified to date. The results based on the MseI restriction patterns are generally in accordance with those of DNA-DNA hybridization (197).
TABLE 3.
MseI restriction fragments of the 5S-23S rRNA (rrfA-rrlB) intergenic spacer of B. burgdorferi sensu lato
| Species | Strain | Amplicon size (bp) | RFLP pattern | MseI restriction fragment size (bp) | Reference |
|---|---|---|---|---|---|
| B. burgdorferi sensu stricto | B31 | 254 | A | 108, 51, 38, 29, 28 | 197 |
| B. garinii | 20047 | 253 | B | 108, 95, 50 | 197 |
| NT29 | 253 | C | 108, 57, 50, 38 | 197 | |
| B. afzelii | VS461 | 246 | D | 108, 68, 50, 20 | 197 |
| B. japonica | HO14 | 236 | E | 108, 78, 50 | 197 |
| B. vailaisiana | VS116 | 255 | F | 175, 50, 23, 7 | 267 |
| Am501 | 249 | Q | 169, 51, 23, 6 | 149 | |
| B. lusitaniae | PotiB2 | 257 | G | 108, 81, 39, 29 | 197 |
| PotiB3 | 255 | H | 108, 79, 52, 16 | 197 | |
| B. bissettii | DN127 | 257 | I | 108, 51, 38, 33, 27 | 197 |
| CA | 226 | J | 108, 51, 38, 29 | 197 | |
| 25015 | 253 | K | 108, 51, 34, 27, 17, 12, 4 | 197 | |
| B. andersonii | 19857 | 266 | L | 120, 67, 51, 28 | 197 |
| CA2 | 255 | M | 91, 50, 40, 28, 22, 17, 7 | 197 | |
| B. tanukii | Hk501 | 245 | O | 174, 51, 20 | 149 |
| B. turdi | Ya501 | 248 | P | 107, 51, 38, 21, 16, 8, 7 | 149 |
| Borrelia sp. | A14S | 225 | R | 108, 66, 51 | 265 |
The rrs-rrlA intergenic spacer is about 3.2 kb in B. burgdorferi sensu stricto and 5 kb in B. garinii and B. afzelii (77, 174, 225). Liveris et al. reported that amplification of the partial rrs-rrlA spacer, followed by digestion with HinfI and MseI, produced both species- and subspecies-specific RFLP patterns (132, 134). This method has been applied to typing of B. burgdorferi directly in human tissue specimens and field-collected ticks, thus obviating the need for culture isolation (133, 134). These studies demonstrated that a significant proportion of human EM lesions contained mixtures of different B. burgdorferi genotypes. Furthermore, typing of B. burgdorferi isolates by this method may allow the differentiation of isolates with different degrees of pathogenic potential (134, 284).
Ribosomal spacer DNA RFLP analysis of B. burgdorferi sensu lato has a number of advantages. It is a rather simple and useful tool for rapid screening and species identification of large collections of B. burgdorferi sensu lato isolates. Furthermore, it can be used for typing uncultured samples collected from ticks and patients with LB. This facilitates epidemiological monitoring of the distribution of the different B. burgdorferi sensu lato species in Ixodes ticks and reservoir hosts. By using reverse line blotting of the rrfA-rrlB spacer which was amplified by PCR and subsequently hybridized with species-specific oligonucleotide probes, Rijpkema et al. were able to identify four different Borrelia species in ticks collected in the Netherlands (207, 208). The prevalence of B. burgdorferi sensu lato infection in ticks and various reservoir hosts in several European countries was also investigated successfully by reverse line blotting (47, 118, 206).
DNA Sequence Analysis
Whole DNA-DNA reassociation analysis is the most robust approach by which B. burgdorferi sensu lato can be classified based on phylogenetic relationships since the results are ultimately based on the entire genome sequence of the organism. Generally, this method is useful for the study of bacterial genetics, evolution, taxonomy, and epidemiology (231). However, DNA sequence analysis of some highly conserved gene loci can be used as a suitable alternative method. For example, rrs, fla, and ospA have been used for this purpose with B. burgdorferi sensu lato.
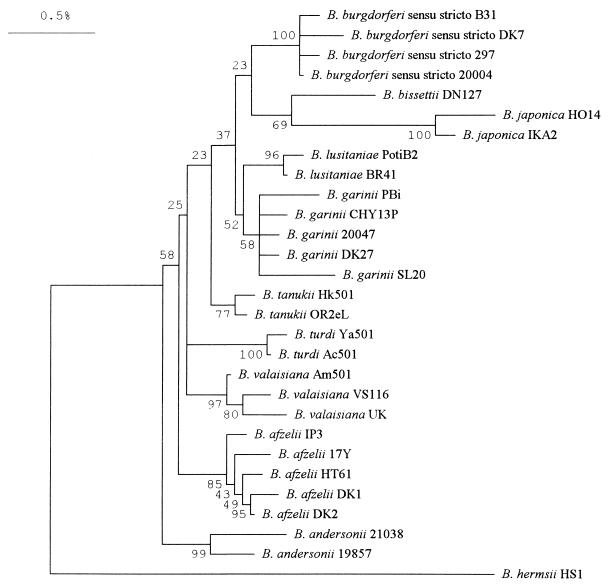
Sequence analysis of bacterial rrs has been widely used for assessing the evolutionary history and species identification of B. burgdorferi sensu lato (68, 127, 142, 143, 201, 267). More than 200 rrs sequences from various B. burgdorferi sensu lato species have been determined (many of them partial sequences) and are now available from the GenBank database (22). The DNA sequences of rrs among strains from different B. burgdorferi sensu lato species are highly homologous, ranging from 95.3 to 99.6% (127). By using a 1,368-bp portion of the rrs gene from representative isolates of each Borrelia species obtained from diverse geographic regions and various biological sources (Table 4), a phylogenetic tree was constructed by the neighbour-joining method and is presented in Fig. 4. Borrelia isolates belonging to distinct species fall into individual clusters in the phylogenetic tree. Thus, sequence analysis of the 16S rRNA gene represents a reliable method for inferring the taxa of B. burgdorferi sensu lato (68, 127, 142, 201, 267).
TABLE 4.
B. burgdorferi sensu lato strains used for phylogenetic analysis in Fig. 4
| Species and strain | Biological source | Geographic region | Accession no. of rrs | Reference(s) |
|---|---|---|---|---|
| B. burgdorferi sensu stricto | ||||
| B31 | Ixodes scapularis | United States | U03309 | 225 |
| 297 | Human, CSF | United States | X85204 | 251 |
| 20004 | Ixodes ricinus | France | M64310 | 141 |
| DK7 | Human skin, ACA | Denmark | X89195 | 251 |
| B. garinii | ||||
| 20047 | Ixodes ricinus | France | D67018 | 67 |
| PBi | Human, CSF | Germany | X85199 | 251 |
| DK27 | Human shin, EM | Denmark | X85193 | 251 |
| JL20 | Human, CSF | Sweden | X95198 | 251 |
| ChY13p | Ixodes persulatus | China | AB007450 | 129 |
| B. afzelii | ||||
| DK1 | Human skin, EM | Denmark | X85109 | 251 |
| DK2 | Human skin, ACA | Denmark | X85198 | 251 |
| R-IP3 | Ixodes persulatus | Russia | L46697 | 141 |
| HT61 | Ixodes persulatus | Japan | D67019 | 67 |
| 17Y | Ixodes persulatus | Korea | U44939 | GenBank |
| B. japonica | ||||
| HO14 | Ixodes ovatus | Japan | L40597 | 67 |
| IKA2 | Ixodes ovatus | Japan | L40598 | 67 |
| B. valaisiana | ||||
| VS116 | Ixodes ricinus | Switzerland | X98232 | 265 |
| UK | Ixodes ricinus | England | X98233 | 265 |
| Am501 | Ixodes columnae | Japan | D67021 | 67 |
| B. lusitaniae | ||||
| PotiB2 | Ixodes ricinus | Portugal | X98228 | 127 |
| BR41 | Ixodes ricinus | Czech Republic | X98231 | 127 |
| B. andersonii | ||||
| 19857 | Rabbit | United States | L46688 | 67, 143 |
| 21038 | Ixodes dentanus | United States | L46701 | 67, 143 |
| B. tanukii | ||||
| Hk501 | Ixodes tanuki | Japan | D67023 | 67, 68 |
| OR2eL | Ixodes tanuki | Japan | D67020 | 67, 68 |
| B. turdi | ||||
| Ya501 | Ixodes turdus | Japan | D67022 | 67, 68 |
| Ac502 | Ixodes turdus | Japan | D67024 | 67, 68 |
| B. bissettii sp. nov. | ||||
| DN127 | Ixodes pacificus | United States | L40596 | 67, 197 |
| B. hermsii | ||||
| HS1 | Ornithodoros hermsii | United States | U42292 | 76 |
FIG. 4.
Phylogeny of Lyme disease spirochete isolates as inferred from 16S rRNA gene sequence analysis. The phylogenetic tree was constructed by using the neighbor-joining method in the MEGA program as described in reference 267. A total of 28 B. burgdorferi sensu lato isolates representing 10 different Borrelia species were included in this analysis. B. hermsii HS1 was used as the outgroup. The sources of each isolate are given in Table 4. Numbers at the branch nodes indicate the results of bootstrap analysis. The bar represents 0.5% sequence divergence.
The fla gene (76), now known as flaB, encodes a 41-kDa flagellin protein or FlaB (78) and is located on the linear chromosome (65). By phylogenetic analysis based on fla gene sequences, the relapsing-fever borreliae can be separated from the LB-related borreliae and different B. burgdorferi sensu lato species can also be distinguished from each other (69, 70).
The ospA gene, located on a 49- to 57-kb linear plasmid (24, 217), is present in almost all B. burgdorferi sensu lato isolates (266, 285). Sequence analysis of ospA genes showed homogeneity within B. burgdorferi sensu stricto and B. afzelii but revealed major subgroups within the B. garinii species (274, 280). Although lateral gene transfer and recombination of ospA between different Borrelia species may occur, the frequency of such events is rather low (57, 212). Thus, cluster analysis of the ospA gene could provide useful phylogenetic information. Usually, the clustering of Borrelia strains in the phylogenetic tree based on the sequence of ospA and its predicted amino acid sequence is in agreement with the classification based on sequence analysis of conserved chromosomal genes such as rrs (155), p93 (57), and fla (69), as well as with data obtained by PFGE (33, 155) and RAPD fingerprinting (64, 268). However, a recent study of eight B. valaisiana isolates showed that ospA sequence analysis might not be appropriate for species identification of B. burgdorferi sensu lato (266).
Comparative analysis of the deduced amino acids sequences from B. burgdorferi sensu lato revealed a species-specific motif in the conserved amino-terminal of the OspC protein (66, 67, 109, 137). Although lateral transfer of ospC genes between species has been described (137, 205, 264), the species-specific motif region had not been transferred except for a few B. valaisiana isolates, which possibly obtained a complete ospC gene from B. afzelii or B. garinii (264). However, it is usually inconclusive for assignment of an isolate to a specific Borrelia species because of the overall high variability of ospC sequences (109, 252, 281) and lateral transfer of ospC gene between species (137, 205, 264).
Sequence analysis of other gene loci such as p93 (57), hbb (258), p39 (211), hsp60 (263), and hsp70 (263) can provide additional information for species identification of B. burgdorferi sensu lato.
Comparison and Selection of Typing Methods
Molecular typing systems can be characterized in terms of typeability, reproducibility, discriminatory power, ease of performance, and ease of interpretation (6, 249). The choice between various methods depends on a number of factors, such as the objectives of the study, the level of discriminatory power required, the kinds of DNA preparations and laboratory conditions available, and the technical skills of laboratory personnel. At this point, we recommend the ribosomal spacer PCR-RFLP as the method for Borrelia species identification during molecular epidemiological studies of B. burgdorferi sensu lato infection in Ixodes ticks, reservoir hosts, and patients. The discriminatory power of PFGE and RAPD is high, and both methods can be used to evaluate the genetic heterogeneity within Borrelia species. RAPD is easier to perform than PFGE and can be used to monitor large numbers of isolates. DNA-DNA reassociation analysis is currently the reference method for species delineation, although it is a time-consuming and labor-intensive method. Therefore, this method is used mainly for confirmation of the taxonomic status of isolates that are not identifiable or typeable by ribosomal spacer RFLP and rrs sequence analysis, as well as for the identification of newly recognized Borrelia species or genomic groups.
TAXONOMY OF B. BURGDORFERI SENSU LATO
Taxonomy and Evolution of B. burgdorferi Sensu Lato
The spirochetes are one of the few major bacterial groups whose natural phylogenetic relationships are evident at the level of gross phenotypic characteristics. These organisms form a coherent taxon composed of six major groups as indicated by phylogenetic analysis (183, 282). The taxonomy of the order Spirochetae is out of the range of this review and can be found in two references (183, 233). Our interest focuses on the taxonomy of the genus Borrelia, especially the Borrelia species within the B. burgdorferi sensu lato complex.
The genus Borrelia represents a tight phylogenetic cluster, which is differentiated from other spirochetal phylogenetic groups by base signature analysis of rrs (183). More than 20 species have been identified within the genus so far (18, 223). These Borrelia species are usually categorized into two major categories, the relapsing-fever borreliae and the LB borreliae, on the basis of the differences between their ecological and genetic characteristics (18).
MLEE analysis has revealed a clonal population structure of B. burgdorferi sensu lato based upon the linkage disequilibrium of allele distributions (26), indicating that in the absence of recombination, all genes in strains belonging to the same species have a common evolutionary history. This conclusion has also been drawn from RAPD fingerprinting (13) and comparison of the sequences of the highly conserved chromosomal genes fla and p93 (57). In addition, clonal populations were evidenced by the conserved chromosomal gene order in different B. burgdorferi sensu lato species (36, 174). It is not clear whether such populations are strictly clonal, since localized horizontal gene transfer of the outer membrane protein-encoding genes has been found in B. burgdorferi sensu lato (137, 145, 146, 205, 212, 264). For example, a close examination of the highly variable ospC gene indicated that lateral gene transfer might have occurred quite frequently, not only between members of the same species but also between strains from different species (137). As a result of such gene transfer and subsequent recombination, the linkage disequilibrium among different species might be disturbed. This would lead to a different topology of phylogenetic relationship based on ospC sequence analysis compared to that based on rrs gene analysis. Nevertheless, in general, ospC genes from strains of the same species appear to be more closely related to each other than to ospC genes from different species. Furthermore, species-specific motifs recognized in the conserved amino terminal of the OspC proteins (67, 109, 137) indicate that ospC is still clonally inherited.
It is assumed that speciation among B. burgdorferi sensu lato is only a recent phenomenon, since divergence of the rrs gene sequences among representatives of different Borrelia species may not exceed 1% (127). Assuming a clonal evolution of B. burgdorferi sensu lato, one may predict that the greater the genetic diversity of the isolates within a given species, the longer the period available for evolution. Since the diversity of B. burgdorferi sensu lato in Eurasia is much greater than in North America, it is likely that this complex originated in Eurasia. Furthermore, B. garinii, which shows the greatest genetic heterogeneity by most analytical methods, is most likely the species which is closest to the common ancestor of B. burgdorferi sensu lato, as was recently proposed (64, 205).
Species within the B. burgdorferi Sensu Lato Complex
Different Borrelia species can be distinguished from each other by analysis of their phenotypic features, such as reactivity with species-specific MAbs (Table 2), or by genetic typing as outlined in the previous section. Since the discovery of B. burgdorferi in 1982, 10 different Borrelia species or genomic groups within the B. burgdorferi sensu lato complex have been identified. Nine are separated at the species level and were designated B. burgdorferi sensu stricto, B. garinii (14), B. afzelii (14, 35), B. japonica (116), B. valaisiana (267), B. lusitaniae (127), B. andersonii (143), B. tanukii (68), and B. turdi (68) (the name has been corrected from B. turdae [108]). More recently, the 10th genomic group, characterized by isolate DN127 (197), has been reevaluated and proposed as a new species, B. bissettii sp. nov. (201). The major ecological and human pathogenic characteristics of the LB-related B. burgdorferi sensu lato species, as well as their geographic distributions, are summarized in Table 5.
TABLE 5.
Borrelia species associated with LB, their ecological and pathogenic characteristics, and their geographic distribution
| Taxon | Vector | Animal host | Human diseasea | Distribution | Reference(s) |
|---|---|---|---|---|---|
| B. burgdorferi sensu lato | I. scapularis | Mammals, birds | EM, arthritis, carditis, neuroborreliosis | United States | 14, 111 |
| I. pacificus | |||||
| I. ricinus | Europe | 14 | |||
| I. persulatus? | Asia? | 130 | |||
| B. garinii | I. ricinus | Birds, small mammals | EM, arthritis, neuroborreliosis | Europe | 14 |
| I. persulatus | Asia | ||||
| B. afzelii | I. ricinus | Small mammals | EM, arthritis, neuroborreliosis, ACA | Europe | 14, 35 |
| I. persulatus | Asia | ||||
| B. japonica | I. ovatus | Small mammals | No | Japan | 116 |
| B. valaisiana | I. ricinus | Birds | Unclear | Europe | 267 |
| I. granulatus | Asia | ||||
| I. columnae | |||||
| B. lusitaniae | I. ricinus | Unknown | No | Central Europe | 127 |
| B. andersonii | I. dentatus | Rabbit | No | United States | 143 |
| B. bissettii sp. nov. | I. pacificus | Rodents, birds | Unclear | United States | 197, 201 |
| I. neotomae | |||||
| I. scapularis | |||||
| I. ricinus | EM, lymphocytoma | Slovenia | 247 | ||
| B. takunii | I. takunus | Small mammals | No | Japan | 68 |
| B. turdib | I. turdus | Small mammals | No | Japan | 68 |
A number of atypical Borrelia isolates, not clearly belonging to one of the described species, have been cultured from North America (36, 155, 191, 201), Europe (193, 268), and China (130, 291). One of these was cultured from a patient with LB in Europe (265, 268). It is reasonable to expect that more Borrelia species will be recognized as more isolates are recovered from divergent biological and geographic sources and are studied by various molecular typing methods.
Borrelia Species Pathogenic to Humans
Not all strains from the described Borrelia species or genomic groups are pathogenic for humans. Currently, only B. burgdorferi sensu stricto, B. garinii, and B. afzelii have been cultured frequently from patients with LB (33, 134, 155, 161, 246, 259, 280). These three major species appear to be responsible for causing different clinical syndromes of human LB (11, 35, 56, 173, 259). A recent study of nine B. burgdorferi sensu lato isolates recovered from patients with LB in Slovenia revealed that they were most closely related to the North American isolate 25015, which belongs to B. bissettii sp. nov. (193, 247). Thus, B. bissettii sp. nov. isolates may represent the fourth Borrelia species that could cause human LB. Interestingly, B. bissettii sp. nov. is the second reported B. burgdorferi sensu lato species (B. burgdorferi sensu stricto is the other) to be present in both the Old World and New World (193, 247). B. valaisiana is widely distributed in European countries as well as in Asia (267). It might be pathogenic for humans, since DNA specific for this species has been detected by PCR from patients with LB (209).
Although it is uncertain whether the other LB-related Borrelia species cause human disease, their pathogenic role is far less important than that of the three major Borrelia species. For example, B. japonica was only weakly pathogenic in mice (114). Although I. ovatus ticks, the specific vector for B. japonica, are distributed widely in Japan and frequently bite humans, no LB patients with a confirmed I. ovatus tick bite have been reported, except for one case suspected on the basis of the serological findings (153).
EPIDEMIOLOGICAL IMPLICATIONS
Geographic Distribution of Different Borrelia Species
LB is a globally distributed zoonosis (21, 221). Human cases occur predominantly in the northern hemisphere (162, 221). Three LB-associated Borrelia species, B. burgdorferi sensu stricto (111), B. andersonii (143), and B. bissettii sp. nov. (201), are recognized in North America. B. burgdorferi sensu stricto is widely distributed in the northeast, midwest, and western regions of the United States (41). It is the only Borrelia species cultured from patients with LB in North America. B. andersonii and B. bissettii sp. nov. were cultured mainly from ticks and small mammals from New York and California, respectively (4, 28, 201).
B. burgdorferi sensu stricto, B. garinii, B. afzelii, B. valaisiana, B. lusitaniae, and B. bissettii sp. nov. have been documented in Europe. The geographic distribution of these species on the European continent has been reviewed recently based on a total of 1,263 records (738 isolates and 525 molecular DNA detections) from 26 countries (101). B. garinii and B. afzelii are the most frequently cultured species in Europe. B. burgdorferi sensu stricto is distributed mainly in western European countries but is rarely recognized in eastern areas of Europe. B. valaisiana has been cultured or detected in ticks or avian reservoirs from at least eight European countries, including the United Kingdom (123), Ireland (117, 118), The Netherlands (207, 267), Switzerland (105, 187, 188), Germany (131, 216), Italy (48), Croatia (86, 206), and the Czech Republic (200). This species seems abundantly present in ticks in Ireland, Germany, and Croatia (118, 131, 206). Only a few B. lusitaniae strains have been isolated in Portugal (197), the Czech Republic, Moldova, and Ukraine (200). Nine isolates with genotypic and phenotypic similarity to B. bissettii sp. nov. have been cultured in Slovenia (193, 247).
Six B. burgdorferi sensu lato species have been reported in Far Eastern Russia and Asian countries. B. garinii and B. afzelii are largely found throughout the range of I. persulcatus ticks in China (129, 248, 290), Japan (72, 164), Korea (181), and Far Eastern Russia (147, 200, 218). B. japonica, B. tanukii, and B. turdi are limited to Japan (68, 116, 149). One isolate from an I. columnae tick in Japan was classified as B. valaisiana (267). The presence of B. burgdorferi sensu stricto in Asia is controversial. Although most reports suggest that B. burgdorferi sensu stricto is absent (129, 147, 164, 200, 248), two recent studies indicated the presence of this Borrelia species in the mainland of China (130) and in Taiwan (226).
LB cases in the southern hemisphere including South America (10), Africa (158, 219), and Australia (157) have been reported. However, these cases were based on serology only. B. burgdorferi sensu lato has not been isolated from local Ixodes ticks or any other suspected vectors or patients (31, 213). Recently, B. garinii was recovered from a patient with LB in Australia; however, the infection may have been acquired in Europe (103).
Vectors and Borrelia Species Specificity
The principal vectors of B. burgdorferi sensu lato are ticks of the I. ricinus complex (31). These include I. scapularis (I. dammini) and I. pacificus in the United States, I. ricinus in Europe, and I. persulcatus in Asian Russia, China and Japan (3, 31, 53, 164, 290). These vectors for B. burgdorferi sensu lato are not strictly species specific, and the specificity displayed by many of the relapsing-fever borreliae for a single tick species is probably not applicable to the LB spirochetes. For example, the European sheep tick I. ricinus has been recognized as a vector of all three human pathogenic Borrelia species, B. burgdorferi sensu stricto, B. garinii, and B. afzelii (80, 189). I. scapularis and I. pacificus in the United States and I. ricinus in Europe are vectors of B. burgdorferi sensu stricto (31, 208, 236).
On the other hand, B. japonica is found mainly in I. ovatus in Japan, and B. andersonii appears to be restricted to I. dentatus in the United States. Furthermore, B. burgdorferi sensu stricto, the most widely distributed Borrelia species in I. scapularis in North America and I. ricinus in Europe, is rarely found in the I. persulcatus regions in Far Eastern Asia (129, 164, 200). These findings suggest that some particular vector species will harbor and transmit only one specific Borrelia species. A recent comparative study of the three main clusters of European I. ricinus indicated significant differences in their susceptibility to B. afzelii (60). This finding may explain, in part, the uneven distributions of Borrelia species in Europe.
Both I. ricinus and I. persulatus can be infected with B. garinii and B. afzelii (102, 164, 200, 291). Other Borrelia species may also be transmitted by several vector species of the I. ricinus group (e.g., B. bissettii sp. nov. strains have been isolated from I. neotomae [now I. spinipalpis] [171], I. scapularis, and I. pacificus in the United States [197], as well as from I. ricinus in Europe [247]). Although B. valaisiana and B. lusitaniae are cultured mainly from European I. ricinus ticks, the former species has also been cultured from I. columnae ticks in Japan (67, 127, 267).
Additional tick vectors as well as hematophagous insects may play a role in maintenance of B. burgdorferi sensu lato in enzootic cycles in nature and occasionally in transmitting B. burgdorferi sensu lato to humans (31). These include I. uriae, Dermacentor variabilis, D. parumapertus, Amblyomma americanum, Rhipicephalus sanguineus, and Haemaphysalis leporislustris in North America (31); I. hexagonus, I. uriae, I. trianguliceps, I. acuminatus, I. frontalis, D. reticulatus, and H. punctata in Europe (80, 229); I. tanuki and I. turdus in Japan (67); and I. granulatus, H. bispinosa, H. concinna, and H. longicornis in southern China (290, 291). The relationship between these possible tick vectors and various B. burgdorferi sensu lato species remains unknown.
Mixed infections of multiple B. burgdorferi sensu lato species have been found in ticks (131, 160, 164, 190, 200, 206), reservoir hosts (123, 163, 164, 200), and in patients with LB (52, 133, 209). The prevalence of mixed infections in ticks varies from 5 to 40% in different geographic regions (131, 160, 190, 200). Such mixed infections may result directly from feeding on a host infected with multiple Borrelia species or through cofeeding transmission, by which various spirochetes may be exchanged among ticks infesting a single host (84, 165, 184, 204). Alternatively, ticks may acquire different Borrelia species from different individual hosts during their three life cycle stages, since the spirochetes can survive through the molts and are present in all subsequent stages of the vectors (3, 31).
Hosts and Borrelia Species Association
B. burgdorferi sensu stricto in the United States and B. garinii in Eurasia have been isolated from a large diversity of mammalian hosts and birds (3, 81, 166, 176). In contrast, B. afzelii in Eurasia and B. burgdorferi sensu stricto in Europe were isolated mainly from rodents (81, 83). Many species of mammals are hosts of B. japonica in Japan (150, 151). However, birds are assumed to be the only hosts for B. valaisiana in Europe, since no B. valaisiana isolate has been cultured or detected from mammals or rodents to date (105, 123, 267). Only a few B. lusitaniae strains were isolated from I. ricinus in southern, central, and eastern Europe (127, 200). Data for hosts of this species are currently not available. B. andersonii and B. bissettii sp. nov. in North America seem to involve particular and narrow host spectra, i.e., cottontail rabbits (Sylvilagus floridanus) (4) and wood mice (Neotomae fuscipes) (28), respectively, although further studies are necessary to clarify these unique relationships.
Transmission Cycles of Different Borrelia Species in Nature
Increasing data indicate that two specific enzootic transmission cycles (rodent-tick and bird-tick) are involved in maintaining different B. burgdorferi sensu lato species in nature, as suggested by Nakao et al. (164) and Humair et al. (104). Reservoir hosts infected by various B. burgdorferi sensu lato species may not transmit them to ticks with equal efficiency (55, 122, 123, 154, 214). Hosts acting as filters seem to be predisposed to mainly transmitting one species to ticks, to the detriment of other genomic groups (123). The mechanisms underlying the apparent differential transmission of B. burgdorferi sensu lato by various mammalian and avian hosts remain to be determined, although a recent study revealed that differences in serum complement sensitivity among Borrelia species could be a key factor in LB ecology (124).
Different B. burgdorferi sensu lato species may be maintained through distinct transmission cycles in natural foci (83, 104, 105, 106, 123, 164). In North America, B. burgdorferi sensu stricto is maintained mainly by the rodent (white-foot mice, Peromyscus leucopus)-tick cycle, while the tick-infested resident and migrant birds are potentially important in regional spread and in dissemination of the spirochetes (3, 166, 230, 232). B. andersonii and B. bissettii sp. nov. have their own distinct enzootic cycles involving other tick vectors and vertebrate hosts (4, 28, 201). In Eurasia, the major species, B. afzelii, is circulated mainly between the reservoirs, particular Apodemus mice and Clethrionomys voles, and Ixodes ticks (81, 104, 106, 164) whereas B. garinii may be maintained predominantly through the bird-tick transmission cycle, especially in northern Europe (176, 177). B. valaisiana has been detected in various avian reservoirs but never in rodent hosts (105, 123, 267). Therefore, this species may not survive and persist in small mammals and most probably circulates between its avian reservoirs and ticks in natural foci (123).
IMPLICATIONS FOR CLINICAL DISEASE, DIAGNOSIS, AND VACCINE DEVELOPMENT
Given the genetic diversity of B. burgdorferi sensu lato and the protean disease manifestations of LB, the use of molecular typing provides valuable data for possible elucidation of the association of particular Borrelia species with the distinct clinical manifestations of LB (259). Molecular typing may also contribute to establishing a correlation between the spirochetal subtypes and the occurrence and severity of particular clinical manifestations of LB (133, 268, 284). Such data are important for the clinical and laboratory diagnosis of LB as well as for vaccine development.
Association of Clinical Disease and Infecting Borrelia Species
The difficulty in culturing B. burgdorferi sensu lato from clinical specimens, other than skin, has hampered assessments of the association between Borrelia species and distinct clinical entities. However, there is increasing evidence supporting such an association. First, numerous Borrelia isolates recovered worldwide from LB patients with different clinical syndromes have been subjected to molecular typing analysis. We reviewed these reports on isolates from LB patients present in the Medline database from 1992 through October 1998. Only cultured isolates that had been subjected to molecular typing analysis and assigned properly to their taxon, and whose clinical source was indicated, were included in the analysis. The distribution of Borrelia species of 472 B. burgdorferi sensu lato isolates recovered from patients is summarized in Table 6. Different species of B. burgdorferi sensu lato are associated with distinct clinical manifestations of LB: Lyme arthritis is associated with B. burgdorferi sensu stricto infection, neuroborreliosis is associated with B. garinii infection, and ACA is associated with B. afzelii infection (11, 14, 35, 155, 173, 186, 194, 197, 259, 273, 280). In a recent study, Wormser et al. demonstrated a significant association between the infecting genotype of B. burgdorferi in an EM lesion and the presence of spirochetemia or multiple EM lesions, suggesting that hematogenous dissemination is related to the B. burgdorferi genotype (284). A limitation of this analysis may be the differences in culture recovery for different B. burgdorferi sensu lato species or genotypes. Two reports suggest that the distribution of genotypes in tick or human tissue specimens, as measured by sequencing or PCR-RFLP analysis, is different from that obtained by culture (133, 170). Another confounding factor may be publication bias (87). Nevertheless, molecular analysis clearly points to a correlation between genotype and clinical manifestation. Second, a number of European studies showed that the antibody responses in LB patients to the three B. burgdorferi sensu lato pathogens varied with disease manifestation (5, 8, 9, 56, 169). For example, Assous et al. studied sera from 67 LB patients and found that sera from 55% of the 20 patients with Lyme arthritis had preferential reactivity (a serum sample showed preferential reactivity when it detected two or more protein bands with a bacterial suspension of a given species compared to suspensions of the other Borrelia species) with a B. burgdorferi sensu stricto isolate, sera from 47% of the 23 patients with neuroborreliosis had more reactivity with a B. garinii strain, and sera from 100% of the 8 patients with ACA had greater reactivity with a B. afzelii strain in Western blot analysis (8, 9). Third, in vitro experiments in which the tissue tropism of Borrelia species was evaluated by incubation of various cultured Borrelia species with human brain tissue have provided evidence that B. garinii invaded brain tissue more efficiently than did B. burgdorferi sensu stricto and B. afzelii (289).
TABLE 6.
Distribution of B. burgdorferi sensu lato species in 472 isolates cultured from LB patients with different clinical syndromesa
| Specimen | Total no. |
B. burgdorferi sensu stricto
|
B. garinii
|
B. afzelii
|
Otherf
|
|---|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | No. (%) | ||
| Skin, EMb | 308 | 96 (31.2) | 50 (16.2) | 156 (50.7) | 6 (1.9) |
| CSFc | 68 | 9 (13.2) | 46 (67.7) | 10 (14.7) | 3 (4.4) |
| Skin, ACAd | 74 | 3 (3.0) | 5 (6.8) | 66 (89.2) | 0 (0) |
| Otherse | 22 | 16 (72.8) | 3 (13.6) | 2 (9.1) | 1 (4.5) |
| Total | 472 | 124 (26.3) | 104 (22.0) | 234 (49.6) | 10 (2.1) |
Only isolates with indication of clinical source and belonging to Borrelia species in published papers were included for analysis. These isolates were recovered from patients from 18 countries including Germany (n = 123), United States (n = 92), The Netherlands (n = 74), Slovenia (n = 63), Austria (n = 45), Denmark (n = 21), Japan (n = 12), Sweden (n = 10), Russia (n = 7), Switzerland (n = 6), Czech Republic (n = 6), China (n = 4), France (n = 3), Italy (n = 2), Poland (n = 1), Croatia (n = 1), Lithuania (n = 1), and Australia (n = 1). Isolates with mixed infections of two species were excluded. A detailed list of Borrelia isolates included in our analysis is available upon request.
Isolates are from references 1, 20, 34, 64, 70, 85, 132, 137, 155, 168, 194, 197, 200, 251, 259, 268, 273, 281, and 285.
The mechanisms by which infection with different Borrelia species may lead to distinct clinical syndromes of LB are unclear. Different serum susceptibility (27, 260) and tissue tropism among Borrelia species may in part account for such differences (49, 128, 182, 253, 289). Although each of the three human pathogenic B. burgdorferi sensu lato species has been associated with different clinical syndromes of LB, the clinical spectrum caused by these species can largely overlap.
Erythema migrans.
EM is an expanding red or bluish-red rash, often with central clearing, which occurs in up to 90% of patients with objective evidence of LB (162). Usually, EM appears at the early stage of B. burgdorferi sensu lato infection. All three species of B. burgdorferi sensu lato pathogenic to humans have been repeatedly cultured from skin biopsy specimens from patients with EM (Table 6). No significant difference between the occurrence of EM and infection with a particular Borrelia species has been reported. This is to be expected since all infections with B. burgdorferi causing LB result from an initial tick bite. However, it was noted that EM patients infected with B. burgdorferi sensu stricto in North America complained more frequently of systemic symptoms than did EM patients in Eurasia (161, 245, 246), where EM is caused predominantly by infection due to B. afzelii (216, 259).
Lyme carditis.
Lyme carditis is a well-known clinical manifestation in both North American and European patients with LB (46, 238, 261). Early studies showed that the frequency of this syndrome was considerably higher in North America, where about 4 to 10% of untreated LB patients presented with cardiac abnormalities (44, 236), than in Europe, where only 0.5 to 4% of untreated LB patients presented with similar abnormalities (46, 162, 178). However, the incidence has been lower in two recent population-based studies in the United States and in Europe (23, 79). The only Borrelia isolate recovered from a myocardial specimen of an Austrian patient in Europe to date was typed as B. burgdorferi sensu stricto (216, 234).
Lyme arthritis and B. burgdorferi sensu stricto infection.
Lyme arthritis is the most common musculoskeletal symptom resulting from B. burgdorferi sensu stricto infection in North America. It occurs either at the early disseminated stage or as a more chronic manifestation. About 60% of untreated patients with EM experienced brief attacks of monoarticular or oligoarticular arthritis in the United States (44, 236, 242). In contrast, only 3 to 15% of patients suffered from arthritis in Europe (23, 45, 178), where B. garinii and B. afzelii are more frequently recovered than B. burgdorferi sensu stricto (101, 216). This unequal distribution of Borrelia species may directly be related to the disparity in the reported incidence of Lyme arthritis between North America and European countries. Although different B. burgdorferi sensu lato species pathogenic to humans can be detected or recovered from the joint or synovial tissue in animal experimental studies (220, 288) and in patients with Lyme arthritis (59, 167, 262), the only three Borrelia isolates recovered from the synovial fluids of patients with Lyme arthritis were typed as B. burgdorferi sensu stricto (12, 216).
Neuroborreliosis and B. garinii infection.
Neuroborreliosis is the most frequent manifestation of disseminated infection in Europe (43, 75, 113) and is a common symptom in North American LB patients as well (44, 91). Both the central and peripheral nervous system can be involved (90, 120). All three species, B. burgdorferi sensu stricto, B. garinii, and B. afzelii, are known to cause Lyme neuroborreliosis. In European patients, B. garinii constituted up to 72% (n = 55) of the 76 Borrelia isolates or DNAs detected in human CSF samples, whereas 8% (n = 6) and 20% (n = 15) of the these specimens were identified as B. burgdorferi sensu stricto and B. afzelii, respectively (101). Of 36 CSF isolates mainly recovered from patients with neuroborreliosis in Southern Germany, B. garinii, B. afzelii, and B. burgdorferi sensu stricto accounted for 58, 28, and 11%, respectively (33). These findings in European patients suggest an association between infection of B. garinii and the development of Lyme neuroborreliosis (11, 125, 186, 259, 277). This is not strictly the case, since obviously, B. burgdorferi sensu stricto is responsible for human neuroborreliosis in the United States. It may be noteworthy that the incidence of lymphocytic meningoradiculitis (Bannwarth syndrome) in LB patients is relatively lower in the United States (75, 89) than in European countries. This also suggests differences in pathogenicity between B. burgdorferi sensu stricto and B. garinii.
Acrodermatitis chronica atrophicans and B. afzelii infection.
ACA is a late cutaneous manifestation of LB characterized by chronic and long-lasting progressive red and bluish-red lesions, usually on the extensor surface of the extremities (235). It was described as early as 1883 (270) and is widely encountered throughout Europe (7, 45, 235); there have been few reports from the United States (58, 115, 155). Molecular studies of ACA isolates from patients in several European countries have provided evidence suggesting an exclusive association of ACA and B. afzelii infection (9, 11, 259, 273). However, a Danish isolate, DK7, derived from an ACA lesion was classified as B. burgdorferi sensu stricto by OspA serotyping (280) as well as by 16S rRNA and ospC gene sequence analysis (251). In a recent study, 22 B. burgdorferi sensu lato isolates from Slovenian patients with ACA were subjected to PFGE and species-specific PCR analysis (194). Results showed that infection was due to B. afzelii in 17 cases, to B. garinii in 4 cases, to B. burgdorferi sensu stricto in 1 case. One American isolate from a patient with ACA was also typed as B. burgdorferi sensu stricto (155). These studies indicate that B. afzelii is the predominant but not the exclusive etiologic agent of ACA.
Implication for Serological Diagnosis
The diagnosis of LB is primarily clinical, but serological tests can provide very useful supporting evidence, especially in patients with disseminated or chronic infection. The serological tests usually used to assess B. burgdorferi antibodies include enzyme-linked immunosorbent assay (ELISA), immunofluorescence assays, and Western blotting. ELISA and immunofluorescence assays are the most widely used but have low specificity (99, 256). Cross-reactive antibodies can produce a false-positive test in patients with other illnesses, e.g., other spirochetal infections, nonspirochetal bacterial endocarditis, Epstein-Barr virus infection, rheumatoid arthritis, and systemic lupus erythematosus (139, 140). In the United States, a two-step protocol for the evaluation of the B. burgdorferi antibodies in sera was recommended by the Centers for Disease Control and Prevention (40). The performance of this protocol, as well as its simplified approach (255), may improve the specificity of serodiagnosis for LB (50, 110, 126), but the high percentage of seronegativity in 20 to 50% of patients, probably dependent on the stage of LB, remains a problem and limits the value of serological tests (2, 95, 99, 246). In addition, the antigenic heterogeneity of B. burgdorferi sensu lato may influence the sensitivity and specificity of serological tests for LB, especially on the European continent, where three pathogenic species of B. burgdorferi sensu lato and at least eight OspA serotypes are well documented (277, 280). Differences in the regional distributions of borrelial species may also affect preferential reactivities of sera from patients with LB (30, 169). Several studies have shown differences in the reactivity patterns in Western blot analysis, depending on the species, strain, or serotype used as the source of antigen (56, 94, 95, 156, 169). This issue should be readdressed, since molecular typing results based on hundreds of isolates have indicated that the genetic diversity of B. burgdorferi sensu lato is much greater than was previously thought, even in North America (134, 155, 197, 287). In a recent study, the influence of interspecies variability of B. burgdorferi sensu lato on the serodiagnosis of LB was evaluated by using immunoglobulin M and immunoglobulin G ELISA with antigens prepared from representative isolates of B. burgdorferi sensu stricto (isolates B31 and PKa2), B. garinii (PBi), and B. afzelii (PKo). Variations resulting from the use of different strains for antigenic preparations were noted in sera from 222 patients with clinically defined LB of all stages, 133 blood donors, and 458 forest workers (94). Such differences were also noted in ELISA or Western blotting by using whole-cell antigenic preparation (95), recombinant OspC (156), internal flagellin fragment (96), and P39 protein (211) from different species of B. burgdorferi sensu lato. Therefore, serological tests involving a combination of antigens derived from two or more strains (from different species in Europe) might be most informative as a response to the impact of strain heterogeneity, especially in Eurasia.
Implication for Vaccine Development
In the past years, different types of vaccines, including a whole-cell vaccine (112), live attenuated vaccine from an aflagellar mutant (215), recombinant OspA (61), OspC subunit vaccines (292), and OspA-based DNA vaccines (138, 228), have been developed (see also reference 283). Recombinant OspA vaccine has been highly successful in protecting laboratory animals against challenge by homologous strains of B. burgdorferi sensu lato (61). Recently, the efficacy of this recombinant OspA vaccine was evaluated in two large clinical trials involving more than 21,000 people from areas of the United States where LB is endemic (227, 243). The efficacy of these OspA vaccines was 76 to 92% in the second year after a booster dose (227, 243). Vaccine failure appears to be due to a low OspA antibody level. In December 1998, one of the OspA-based vaccine was approved for human use by the Food and Drug Administration in the United States (42). A recent study might raise questions about the safety of these OspA vaccines, since it seems theoretically possible that the OspA protein could provoke autoimmunity in susceptible individuals (88). The efficacy of this OspA-based vaccine in Europe may be considerably lower, given the substantial variations displayed in OspA proteins of B. burgdorferi sensu lato, as revealed by molecular typing analysis. Immunization with recombinant OspA derived from any one isolate may fail to protect against the heterogeneous population of organisms present in the natural focus. OspA immunization may protect mice from tick-borne infection with heterogeneous B. burgdorferi sensu lato from different geographic regions by blocking spirochetal transmission from the vector to the host (54, 63). However, it is not clear whether the specific antibody in persons who are immunized with an OspA vaccine derived from one isolate can also provide coverage against a wide range of strains. Passive and active immunization against OspC can serve in protective immunity. Recently, passive immunization with a high dose of anti-OspC immune sera appeared to be therapeutic for chronic LB in mice (292). However, the OspC-mediated protective immunity is probably strain specific (25). Thus, the general applicability of this approach may be limited by the well-documented heterogeneity of OspC among members of the species B. burgdorferi sensu lato (137, 279). One strategy to circumvent OspA and OspC heterogeneity is the use of vaccines made of a cocktail of various recombinant OspA and/or OspC gene products derived from different strains. In Europe, a multiple-subunit vaccine is expected to yield protective immunity against infections of different species of B. burgdorferi sensu lato. Gern et al. (82) reported that a polyvalent OspA vaccine prepared from three isolates of different species efficiently protected mice against infection due to either B. burgdorferi sensu stricto, B. garinii, or B. afzelii.
Other potential candidates for vaccines include decorin binding protein A (DbpA) (39, 93, 210), P35 (62), and P66 (29, 202). Further information on the distribution of various Borrelia species in their natural reservoir hosts and vectors and knowledge of the Borrelia antigenic compositions is needed to find representative immunogenic antigens for vaccine development.
CONCLUSIONS
Since different Borrelia strains may intrinsically be associated with distinct pathogenic properties, application of molecular typing methods to the classification of B. burgdorferi sensu lato will provide the framework for a systematic approach to characterize differences in infectivity as well as in pathogenicity between strains. Among the typing methods available for B. burgdorferi sensu lato, OspA serotyping can be used to analyze the phenotypic characteristics. Both PFGE and RAPD fingerprinting analysis are powerful tools in elucidating the genetic relationship between closely related Borrelia isolates within a species. rDNA spacer RFLP analysis is simple and can be applied to uncultured specimens; it is therefore ideally suited for molecular epidemiological and population genetic studies of B. burgdorferi sensu lato. Phylogenetic analysis based on 16S rRNA gene sequence may represent a potential method for inferring the taxa of B. burgdorferi sensu lato (68, 127). However, DNA-DNA reassociation data are generally required for designation of a new species because of the high degree of sequence similarity of the 16S rRNA genes between closely related Borrelia species (127, 267). In general, there is excellent concordance in the typing results obtained by different molecular approaches at the species level, with the exception of DNA sequence analysis of ospC.
Ten B. burgdorferi sensu lato species have been identified. The pathogenicity of three of these species is well documented in humans. Distribution of Borrelia species and clinical syndromes of LB are different in North America and Eurasia, possibly due to the different pathogenicities of Borrelia species. Current data indicate that B. burgdorferi sensu stricto infection is associated with articular disorders, B. garinii infection is associated with neuroborreliosis, and B. afzelii infection is associated with ACA. Since the recovery of Borrelia from clinical specimens of patients with LB by culture is low, in particular in patients with Lyme arthritis and carditis, assessment of the correlation between infecting species and clinical manifestations will require other approaches. Appropriate serological tests capable of differentiating the antibody response of patients infected with different Borrelia species may be helpful in this regard. In addition, sensitive molecular typing techniques which do not require large amounts of patient material or cultivation of the spirochete will play an increasingly important role in elucidation of the pathogenic potential of different B. burgdorferi genotypes. Ultimately, the development of suitable animal models for investigation of the tissue tropisms of different B. burgdorferi sensu lato species will provide more direct evidence for the correlation between Borrelia species and clinical symptoms of LB.
ACKNOWLEDGMENTS
We thank D. Postic and D. A. Mathiesen for providing further information on the B. burgdorferi sensu lato isolates included in Table 6 and E. Fikrig, G. Wormser, and J. M. Leong for comments on the manuscript.
REFERENCES
- 1.Adam T, Gassmann G S, Rasiah C, Gobel U B. Phenotypic and genotypic analysis of Borrelia burgdorferi isolates from various sources. Infect Immun. 1991;59:2579–2585. doi: 10.1128/iai.59.8.2579-2585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguero-Rosenfeld M E, Nowakowski J, Bittker S, Cooper D, Nadelman R B, Wormser G P. Evolution of the serologic response to Borrelia burgdorferi in treated patients with culture-confirmed erythema migrans. J Clin Microbiol. 1996;34:1–9. doi: 10.1128/jcm.34.1.1-9.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson J F. Epizootiology of Lyme borreliosis. Scand J Infect Dis Suppl. 1991;77:23–34. [PubMed] [Google Scholar]
- 4.Anderson J F, Magnarelli L A, LeFebvre R B, Andreadis T G, McAninch J B, Perng G C, Johnson R C. Antigenically variable Borrelia burgdorferi isolated from cottontail rabbits and Ixodes dentatus in rural and urban areas. J Clin Microbiol. 1989;27:13–20. doi: 10.1128/jcm.27.1.13-20.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anthonissen F M, De Kesel M, Hoet P P, Bigaignon G H. Evidence for the involvement of different genospecies of Borrelia in the clinical outcome of Lyme disease in Belgium. Res Microbiol. 1994;145:327–331. doi: 10.1016/0923-2508(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 6.Arbeit R D. Laboratory procedures for the epidemiologic analysis of microorganisms. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C.: ASM Press; 1995. pp. 190–208. [Google Scholar]
- 7.Asbrink E, Hovmark A, Hederstedt B. The spirochetal etiology of acrodermatitis chronica atrophicans Herxheimer. Acta Dermatol Venereol. 1984;64:506–512. [PubMed] [Google Scholar]
- 8.Assous M, Postic D, Baranton G. Clinical and epidemiological implications of Borrelia burgdorferi sensu lato taxonomy. In: Yanagihara Y, Masuzawa T, editors. Proceeding of the International Symposium on Lyme Disease in Japan 1994. 1994. pp. 148–162. [Google Scholar]
- 9.Assous M V, Postic D, Paul G, Nevot P, Baranton G. Western blot analysis of sera from Lyme borreliosis patients according to the genomic species of the Borrelia strains used as antigens. Eur J Clin Microbiol Infect Dis. 1993;12:261–268. doi: 10.1007/BF01967256. [DOI] [PubMed] [Google Scholar]
- 10.Azulay R D, Azulay-Abulafia L, Sodre C T, Azulay D R, Azulay M M. Lyme disease in Rio de Janeiro, Brazil. Int J Dermatol. 1991;30:569–571. doi: 10.1111/j.1365-4362.1991.tb02642.x. [DOI] [PubMed] [Google Scholar]
- 11.Balmelli T, Piffaretti J C. Association between different clinical manifestations of Lyme disease and different species of Borrelia burgdorferi sensu lato. Res Microbiol. 1995;146:329–340. doi: 10.1016/0923-2508(96)81056-4. [DOI] [PubMed] [Google Scholar]
- 12.Balmelli T, Piffaretti J C. Analysis of the genetic polymorphism of Borrelia burgdorferi sensu lato by multilocus enzyme electrophoresis. Int J Syst Bacteriol. 1996;46:167–172. doi: 10.1099/00207713-46-1-167. [DOI] [PubMed] [Google Scholar]
- 13.Bandi C, Sironi M, Sambri V, Fabbi M, Solari-Basano F, Genchi C. Molecular characterisation of Lyme disease borreliae using RAPD analysis and 16S rDNA sequencing. Ann Ist Super Sanita. 1997;33:225–229. [PubMed] [Google Scholar]
- 14.Baranton G, Postic D, Saint Girons I, Boerlin P, Piffaretti J C, Assous M, Grimont P A. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int J Syst Bacteriol. 1992;42:378–383. doi: 10.1099/00207713-42-3-378. [DOI] [PubMed] [Google Scholar]
- 15.Barbour A G. Plasmid analysis of Borrelia burgdorferi, the Lyme disease agent. J Clin Microbiol. 1988;26:475–478. doi: 10.1128/jcm.26.3.475-478.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barbour A G, Fish D. The biological and social phenomenon of Lyme disease. Science. 1993;260:1610–1616. doi: 10.1126/science.8503006. [DOI] [PubMed] [Google Scholar]
- 17.Barbour A G, Garon C F. Linear plasmids of the bacterium Borrelia burgdorferi have covalently closed ends. Science. 1987;237:409–411. doi: 10.1126/science.3603026. [DOI] [PubMed] [Google Scholar]
- 18.Barbour A G, Hayes S F. Biology of Borrelia species. Microbiol Rev. 1986;50:381–400. doi: 10.1128/mr.50.4.381-400.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barbour A G, Heiland R A, Howe T R. Heterogeneity of major proteins in Lyme disease borreliae: a molecular analysis of North American and European isolates. J Infect Dis. 1985;152:478–484. doi: 10.1093/infdis/152.3.478. [DOI] [PubMed] [Google Scholar]
- 20.Belfaiza J, Postic D, Bellenger E, Baranton G, Saint Girons I. Genomic fingerprinting of Borrelia burgdorferi sensu lato by pulsed-field gel electrophoresis. J Clin Microbiol. 1993;31:2873–2877. doi: 10.1128/jcm.31.11.2873-2877.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennett C E. Ticks and Lyme disease. Adv Parasitol. 1995;36:343–405. doi: 10.1016/s0065-308x(08)60494-7. [DOI] [PubMed] [Google Scholar]
- 22.Benson D A, Boguski M S, Lipman D J, Ostell J, Ouellette B F. GenBank. Nucleic Acids Res. 1998;26:1–7. doi: 10.1093/nar/26.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berglund J, Eitrem R, Ornstein K, Lindberg A, Ringer A, Elmrud H, Carlsson M, Runehagen A, Svanborg C, Norrby R. An epidemiologic study of Lyme disease in southern Sweden. N Engl J Med. 1995;333:1319–1327. doi: 10.1056/NEJM199511163332004. [DOI] [PubMed] [Google Scholar]
- 24.Bergstrom S, Bundoc V G, Barbour A G. Molecular analysis of linear plasmid-encoded major surface proteins, OspA and OspB, of the Lyme disease spirochaete Borrelia burgdorferi. Mol Microbiol. 1989;3:479–486. doi: 10.1111/j.1365-2958.1989.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 25.Bockenstedt L K, Hodzic E, Feng S, Bourrel K W, de Silva A, Montgomery R R, Fikrig E, Radolf J D, Barthold S W. Borrelia burgdorferi strain-specific OspC-mediated immunity in mice. Infect Immun. 1997;65:4661–4667. doi: 10.1128/iai.65.11.4661-4667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boerlin P, Peter O, Bretz A-G, Postic D, Baranton G, Piffaretti J-C. Population genetic analysis of Borrelia burgdorferi isolates by multilocus enzyme electrophoresis. Infect Immun. 1992;60:1677–1683. doi: 10.1128/iai.60.4.1677-1683.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Breitner-Ruddock S, Wurzner R, Schulze J, Brade V. Heterogeneity in the complement-dependent bacteriolysis within the species of Borrelia burgdorferi. Med Microbiol Immunol (Berlin) 1997;185:253–260. doi: 10.1007/s004300050038. [DOI] [PubMed] [Google Scholar]
- 28.Brown R N, Lane R S. Lyme disease in California: a novel enzootic transmission cycle of Borrelia burgdorferi. Science. 1992;256:1439–1442. doi: 10.1126/science.1604318. [DOI] [PubMed] [Google Scholar]
- 29.Bunikis J, Noppa L, Ostberg Y, Barbour A G, Bergstrom S. Surface exposure and species specificity of an immunoreactive domain of a 66-kilodalton outer membrane protein (P66) of the Borrelia spp. that cause Lyme disease. Infect Immun. 1996;64:5111–5116. doi: 10.1128/iai.64.12.5111-5116.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bunikis J, Olsen B, Westman G, Bergstroom S. Variable serum immunoglobulin responses against different Borrelia burgdorferi sensu lato species in a population at risk for and patients with Lyme disease. J Clin Microbiol. 1995;33:1473–1478. doi: 10.1128/jcm.33.6.1473-1478.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burgdorfer W, Anderson J F, Gern L, Lane R S, Piesman J, Spielman A. Relationship of Borrelia burgdorferi to its arthropod vectors. Scand J Infect Dis Suppl. 1991;77:35–40. [PubMed] [Google Scholar]
- 32.Burgdorfer W, Barbour A G, Hayes S F, Benach J L, Grunwaldt E, Davis J P. Lyme disease—a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 33.Busch U, Hizo-Teufel C, Boehmer R, Fingerle V, Nitschko H, Wilske B, Preac-Mursic V. Three species of Borrelia burgdorferi sensu lato (B. burgdorferi sensu stricto, B. afzelii, and B. garinii) identified from cerebrospinal fluid isolates by pulsed-field gel electrophoresis and PCR. J Clin Microbiol. 1996;34:1072–1078. doi: 10.1128/jcm.34.5.1072-1078.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Busch U, Hizo-Teufel C, Bohmer R, Fingerle V, Rossler D, Wilske B, Preac-Mursic V. Borrelia burgdorferi sensu lato strains isolated from cutaneous Lyme borreliosis biopsies differentiated by pulsed-field gel electrophoresis. Scand J Infect Dis. 1996;28:583–589. doi: 10.3109/00365549609037965. [DOI] [PubMed] [Google Scholar]
- 35.Canica M M, Nato F, du Merle L, Mazie J C, Baranton G, Postic D. Monoclonal antibodies for identification of Borrelia afzelii sp. nov. associated with late cutaneous manifestations of Lyme borreliosis. Scand J Infect Dis. 1993;25:441–448. doi: 10.3109/00365549309008525. [DOI] [PubMed] [Google Scholar]
- 36.Casjens S, Delange M, Ley III H L, Rosa P, Huang W M. Linear chromosomes of Lyme disease agent spirochetes: genetic diversity and conservation of gene order. J Bacteriol. 1995;177:2769–2780. doi: 10.1128/jb.177.10.2769-2780.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Casjens S, Huang W M. Linear chromosomal physical and genetic map of Borrelia burgdorferi, the Lyme disease agent. Mol Microbiol. 1993;8:967–980. doi: 10.1111/j.1365-2958.1993.tb01641.x. [DOI] [PubMed] [Google Scholar]
- 38.Casjens S, van Vugt R, Tilly K, Rosa P A, Stevenson B. Homology throughout the multiple 32-kilobase circular plasmids present in Lyme disease spirochetes. J Bacteriol. 1997;179:217–227. doi: 10.1128/jb.179.1.217-227.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cassatt D R, Patel N K, Ulbrandt N D, Hanson M S. DbpA, but not OspA, is expressed by Borrelia burgdorferi during spirochetemia and is a target for protective antibodies. Infect Immun. 1998;66:5379–5387. doi: 10.1128/iai.66.11.5379-5387.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the second national conference on serologic diagnosis of Lyme disease. Morbid Mortal Weekly Rep. 1995;44:590–591. [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention. Lyme disease—United States, 1996. Morbid Mortal Weekly Rep. 1997;46:531–535. [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention. Availability of Lyme disease vaccine. Morbid Mortal Weekly Rep. 1999;48:35–36. , 43. [Google Scholar]
- 43.Christen H J, Hanefeld F, Eiffert H, Thomssen R. Epidemiology and clinical manifestations of Lyme borreliosis in childhood. A prospective multicentre study with special regard to neuroborreliosis. Acta Paediatr Suppl. 1993;386:1–75. doi: 10.1111/j.1651-2227.1993.tb18082.x. [DOI] [PubMed] [Google Scholar]
- 44.Ciesielski C A, Markowitz L E, Horsley R, Hightower A W, Russell H, Broome C V. Lyme disease surveillance in the United States, 1983–1986. Rev Infect Dis. 1989;11(Suppl. 6):S1435–S1441. [PubMed] [Google Scholar]
- 45.Cimmino M, Granstrom M, Gray J S, Guy E C, O’Connell S, Stanek G. European Lyme borreliosis clinical spectrum. Zentbl Bakteriol. 1998;287:248–252. doi: 10.1016/s0934-8840(98)80126-6. [DOI] [PubMed] [Google Scholar]
- 46.Cimmino M A. Relative frequency of Lyme borreliosis of its clinical manifestations in Europe. Infection. 1998;26:298–300. doi: 10.1007/BF02962251. [DOI] [PubMed] [Google Scholar]
- 47.Cinco M, Padovan D, Murgia R, Frusteri L, Maroli M, van de Pol I, Verbeek-De K N, Rijpkema S G, Taggi F. Prevalence of Borrelia burgdorferi infection in Ixodes ricinus in central Italy. Eur J Clin Microbiol Infect Dis. 1998;17:134–135. doi: 10.1007/BF01682174. [DOI] [PubMed] [Google Scholar]
- 48.Cinco M, Padovan D, Murgia R, Poldini L, Frusteri L, van de Pol I, Verbeek-De K N, Rijpkema S G, Maroli M. Rate of infection of Ixodes ricinus ticks with Borrelia burgdorferi sensu stricto, Borrelia garinii, Borrelia afzelii and group VS116 in an endemic focus of Lyme disease in Italy. Eur J Clin Microbiol Infect Dis. 1998;17:90–94. doi: 10.1007/BF01682162. [DOI] [PubMed] [Google Scholar]
- 49.Coburn J, Barthold S W, Leong J M. Diverse Lyme disease spirochetes bind integrin alpha IIb beta 3 on human platelets. Infect Immun. 1994;62:5559–5567. doi: 10.1128/iai.62.12.5559-5567.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Craven R B, Quan T J, Bailey R E, Dattwyler R, Ryan R W, Sigal L H, Steere A C, Sullivan B, Johnson B J, Dennis D T, Gubler D J. Improved serodiagnostic testing for Lyme disease: results of a multicenter serologic evaluation. Emerg Infect Dis. 1996;2:136–140. doi: 10.3201/eid0202.960211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davidson B E, MacDougall J, Saint Girons I. Physical map of the linear chromosome of the bacterium Borrelia burgdorferi 212, a causative agent of Lyme disease, and localization of rRNA genes. J Bacteriol. 1992;174:3766–3774. doi: 10.1128/jb.174.11.3766-3774.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Demaerschalck I, Ben Messaoud A, De Kesel M, Hoyois B, Lobet Y, Hoet P P, Bigaignon G, Bollen A, Godfroid E. Simultaneous presence of different Borrelia burgdorferi genospecies in biological fluids of Lyme disease patients. J Clin Microbiol. 1995;33:602–608. doi: 10.1128/jcm.33.3.602-608.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dennis D T, Nekomoto T S, Victor J C, Paul W S, Piesman J. Reported distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the United States. J Med Entomol. 1998;35:629–638. doi: 10.1093/jmedent/35.5.629. [DOI] [PubMed] [Google Scholar]
- 54.de Silva A M, Telford S R 3, Brunet L R, Barthold S W, Fikrig E. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996;183:271–275. doi: 10.1084/jem.183.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Donahue J G, Piesman J, Spielman A. Reservoir competence of white-footed mice for Lyme disease spirochetes. Am J Trop Med Hyg. 1987;36:92–96. doi: 10.4269/ajtmh.1987.36.92. [DOI] [PubMed] [Google Scholar]
- 56.Dressler F, Ackermann R, Steere A C. Antibody responses to the three genomic groups of Borrelia burgdorferi in European Lyme borreliosis. J Infect Dis. 1994;169:313–318. doi: 10.1093/infdis/169.2.313. [DOI] [PubMed] [Google Scholar]
- 57.Dykhuizen D E, Polin D S, Dunn J J, Wilske B, Preac-Mursic V, Dattwyler R J, Luft B J. Borrelia burgdorferi is clonal: implications for taxonomy and vaccine development. Proc Natl Acad Sci USA. 1993;90:10163–10167. doi: 10.1073/pnas.90.21.10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Edwards L, Hoshaw R A, Burgdorf W H. Acrodermatitis chronica atrophicans. Arch Dermatol. 1992;128:858–860. [PubMed] [Google Scholar]
- 59.Eiffert H, Karsten A, Thomssen R, Christen H J. Characterization of Borrelia burgdorferi strains in Lyme arthritis. Scand J Infect Dis. 1998;30:265–268. doi: 10.1080/00365549850160918. [DOI] [PubMed] [Google Scholar]
- 60.Estrada-Pena A, Daniel M, Frandsen F, Gern L, Gettinby G, Gray J S, Jaenson T G, Jongejan F, Kahl O, Korenberg E, Mehl R, Nuttall P A. Ixodes ricinus strains in Europe. Zentbl Bakteriol. 1998;287:185–189. doi: 10.1016/s0934-8840(98)80119-9. [DOI] [PubMed] [Google Scholar]
- 61.Fikrig E, Barthold S W, Kantor F S, Flavell R A. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science. 1990;250:553–556. doi: 10.1126/science.2237407. [DOI] [PubMed] [Google Scholar]
- 62.Fikrig E, Barthold S W, Sun W, Feng W, Telford S R, Flavell R A. Borrelia burgdorferi P35 and P37 proteins, expressed in vivo, elicit protective immunity. Immunity. 1997;6:531–539. doi: 10.1016/s1074-7613(00)80341-6. [DOI] [PubMed] [Google Scholar]
- 63.Fikrig E, Telford S R, Wallich R, Chen M, Lobet Y, Matuschka F R, Kimsey R B, Kantor F S, Barthold S W, Spielman A. Vaccination against Lyme disease caused by diverse Borrelia burgdorferi. J Exp Med. 1995;181:215–221. doi: 10.1084/jem.181.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Foretz M, Postic D, Baranton G. Phylogenetic analysis of Borrelia burgdorferi sensu stricto by arbitrarily primed PCR and pulsed-field gel electrophoresis. Int J Syst Bacteriol. 1997;47:11–18. doi: 10.1099/00207713-47-1-11. [DOI] [PubMed] [Google Scholar]
- 65.Fraser C M, Casjens S, Huang W M, Sutton G G, Clayton R, Lathigra R, White O, Ketchum K A, Dodson R, Hickey E K, Gwinn M, Dougherty B, Tomb J F, Fleischmann R D, Richardson D, Peterson J, Kerlavage A R, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams M D, Gocayne J, Venter J C. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 66.Fukunaga M, Hamase A. Outer surface protein C gene sequence analysis of Borrelia burgdorferi sensu lato isolates from Japan. J Clin Microbiol. 1995;33:2415–2420. doi: 10.1128/jcm.33.9.2415-2420.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fukunaga M, Hamase A, Okada K, Inoue H, Tsuruta Y, Miyamoto K, Nakao M. Characterization of spirochetes isolated from ticks (Ixodes tanuki, Ixodes turdus, and Ixodes columnae) and comparison of the sequences with those of Borrelia burgdorferi sensu lato strains. Appl Environ Microbiol. 1996;62:2338–2344. doi: 10.1128/aem.62.7.2338-2344.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fukunaga M, Hamase A, Okada K, Nakao M. Borrelia tanukii sp. nov. and Borrelia turdae sp. nov. found from ixodid ticks in Japan: rapid species identification by 16S rRNA gene-targeted PCR analysis. Microbiol Immunol. 1996;40:877–881. doi: 10.1111/j.1348-0421.1996.tb01154.x. [DOI] [PubMed] [Google Scholar]
- 69.Fukunaga M, Koreki Y. A phylogenetic analysis of Borrelia burgdorferi sensu lato isolates associated with Lyme disease in Japan by flagellin gene sequence determination. Int J Syst Bacteriol. 1996;46:416–421. doi: 10.1099/00207713-46-2-416. [DOI] [PubMed] [Google Scholar]
- 70.Fukunaga M, Okada K, Nakao M, Konishi T, Sato Y. Phylogenetic analysis of Borrelia species based on flagellin gene sequences and its application for molecular typing of Lyme disease borreliae. Int J Syst Bacteriol. 1996;46:898–905. doi: 10.1099/00207713-46-4-898. [DOI] [PubMed] [Google Scholar]
- 71.Fukunaga M, Sohnaka M, Yanagihara Y. Analysis of Borrelia species associated with Lyme disease by rRNA gene restriction fragment length polymorphism. J Gen Microbiol. 1993;139:1141–1146. doi: 10.1099/00221287-139-6-1141. [DOI] [PubMed] [Google Scholar]
- 72.Fukunaga M, Takahashi Y. Pulsed field gel electrophoresis analysis of Borrelia burgdorferi sensu lato isolated in Japan and taxonomic implications with Lyme disease spirochetes. Microbiol Immunol. 1994;38:747–751. doi: 10.1111/j.1348-0421.1994.tb01851.x. [DOI] [PubMed] [Google Scholar]
- 73.Fukunaga M, Yanagihara Y, Sohnaka M. The 23S/5S ribosomal RNA genes (rrl/rrf) are separate from the 16S ribosomal RNA gene (rrs) in Borrelia burgdorferi, the aetiological agent of Lyme disease. J Gen Microbiol. 1992;138:871–877. doi: 10.1099/00221287-138-5-871. [DOI] [PubMed] [Google Scholar]
- 74.Fung B P, McHugh G L, Leong J M, Steere A C. Humoral immune response to outer surface protein C of Borrelia burgdorferi in Lyme disease: role of the immunoglobulin M response in the serodiagnosis of early infection. Infect Immun. 1994;62:3213–3221. doi: 10.1128/iai.62.8.3213-3221.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garcia-Monco J C, Benach J L. Lyme neuroborreliosis. Ann Neurol. 1995;37:691–702. doi: 10.1002/ana.410370602. [DOI] [PubMed] [Google Scholar]
- 76.Gassmann G S, Jacobs E, Deutzmann R, Gobel U B. Analysis of the Borrelia burgdorferi GeHo fla gene and antigenic characterization of its gene product. J Bacteriol. 1991;173:1452–1459. doi: 10.1128/jb.173.4.1452-1459.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gazumyan A, Schwartz J J, Liveris D, Schwartz I. Sequence analysis of the ribosomal RNA operon of the Lyme disease spirochete, Borrelia burgdorferi. Gene. 1994;146:57–65. doi: 10.1016/0378-1119(94)90833-8. [DOI] [PubMed] [Google Scholar]
- 78.Ge Y, Old I G, Saint Girons I, Charon N W. Molecular characterization of a large Borrelia burgdorferi motility operon which is initiated by a consensus sigma70 promoter. J Bacteriol. 1997;179:2289–2299. doi: 10.1128/jb.179.7.2289-2299.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gerber M A, Shapiro E D, Burke G S, Parcells V J, Bell G L. Lyme disease in children in southeastern Connecticut. N Engl J Med. 1996;335:1270–1274. doi: 10.1056/NEJM199610243351703. [DOI] [PubMed] [Google Scholar]
- 80.Gern L, Burgdorfer W, Aeschlimann A, Krampitz H E. The ecology of Lyme borreliosis in Europe. In: Weber K, Burgdorfer W, editors. Aspects of Lyme borreliosis. Berlin, Germany: Springer-Verlag KG; 1993. pp. 59–69. [Google Scholar]
- 81.Gern L, Estrada-Pena A, Frandsen F, Gray J S, Jaenson T G, Jongejan F, Kahl O, Korenberg E, Mehl R, Nuttall P A. European reservoir hosts of Borrelia burgdorferi sensu lato. Zentbl Bakteriol. 1998;287:196–204. doi: 10.1016/s0934-8840(98)80121-7. [DOI] [PubMed] [Google Scholar]
- 82.Gern L, Hu C M, Voet P, Hauser P, Lobet Y. Immunization with a polyvalent OspA vaccine protects mice against Ixodes ricinus tick bites infected by Borrelia burgdorferi ss, Borrelia garinii and Borrelia afzelii. Vaccine. 1997;15:1551–1557. doi: 10.1016/s0264-410x(97)00066-2. [DOI] [PubMed] [Google Scholar]
- 83.Gern L, Humair P F. Natural history of Borrelia burgdorferi sensu lato. Wien Klin Wochenschr. 1998;110:856–858. [PubMed] [Google Scholar]
- 84.Gern L, Rais O. Efficient transmission of Borrelia burgdorferi between cofeeding Ixodes ricinus ticks (Acari: Ixodidae) J Med Entomol. 1996;33:189–192. doi: 10.1093/jmedent/33.1.189. [DOI] [PubMed] [Google Scholar]
- 85.Golde W T, Piesman J, Dolan M C, Kramer M, Hauser P, Lobet Y, Capiau C X, Desmons P, Voet P, Dearwester D, Frantz J C. Reactivity with a specific epitope of outer surface protein A predicts protection from infection with the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1997;65:882–889. doi: 10.1128/iai.65.3.882-889.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Golubic D, Rijpkema S G, Tkalec-Makovec N, Ruzic E. Epidemiologic, ecologic and clinical characteristics of Lyme borreliosis in northwest Croatia. Acta Med Croat. 1998;52:7–13. [PubMed] [Google Scholar]
- 87.Gregoire G, Derderian F, Le Lorier J. Selecting the language of the publications included in a meta-analysis: is there a Tower of Babel bias? J Clin Epidemiol. 1995;48:159–163. doi: 10.1016/0895-4356(94)00098-b. [DOI] [PubMed] [Google Scholar]
- 88.Gross D M, Forsthuber T, Tary-Lehmann M, Etling C, Ito K, Nagy Z A, Field J A, Steere A C, Huber B T. Identification of LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science. 1998;281:703–706. doi: 10.1126/science.281.5377.703. [DOI] [PubMed] [Google Scholar]
- 89.Halperin J J. North American Lyme neuroborreliosis. Scand J Infect Dis Suppl. 1991;77:74–80. [PubMed] [Google Scholar]
- 90.Halperin J J. Neuroborreliosis: central nervous system involvement. Semin Neurol. 1997;17:19–24. doi: 10.1055/s-2008-1040908. [DOI] [PubMed] [Google Scholar]
- 91.Halperin J J, Logigian E L, Finkel M F, Pearl R A. Practice parameters for the diagnosis of patients with nervous system Lyme borreliosis. Neurology. 1996;46:619–627. [PubMed] [Google Scholar]
- 92.Hansen K, Lebech A M. The clinical and epidemiological profile of Lyme neuroborreliosis in Denmark 1985–1990. A prospective study of 187 patients with Borrelia burgdorferi specific intrathecal antibody production. Brain. 1992;115:399–423. doi: 10.1093/brain/115.2.399. [DOI] [PubMed] [Google Scholar]
- 93.Hanson M S, Cassatt D R, Guo B P, Patel N K, McCarthy M P, Dorward D W, Hook M. Active and passive immunity against Borrelia burgdorferi decorin binding protein A (DbpA) protects against infection. Infect Immun. 1998;66:2143–2153. doi: 10.1128/iai.66.5.2143-2153.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hauser U, Krahl H, Peters H, Fingerle V, Wilske B. Impact of strain heterogeneity on Lyme disease serology in Europe: comparison of enzyme-linked immunosorbent assays using different species of Borrelia burgdorferi sensu lato. J Clin Microbiol. 1998;36:427–436. doi: 10.1128/jcm.36.2.427-436.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hauser U, Lehnert G, Lobentanzer R, Wilske B. Interpretation criteria for standardized Western blots for three European species of Borrelia burgdorferi sensu lato. J Clin Microbiol. 1997;35:1433–1444. doi: 10.1128/jcm.35.6.1433-1444.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hauser U, Wilske B. Enzyme-linked immunosorbent assays with recombinant internal flagellin fragments derived from different species of Borrelia burgdorferi sensu lato for the serodiagnosis of Lyme neuroborreliosis. Med Microbiol Immunol (Berlin) 1997;186:145–151. doi: 10.1007/s004300050057. [DOI] [PubMed] [Google Scholar]
- 97.Hilton E, Devoti J, Sood S. Recommendation to include OspA and OspB in the new immunoblotting criteria for serodiagnosis of Lyme disease. J Clin Microbiol. 1996;34:1353–1354. doi: 10.1128/jcm.34.6.1353-1354.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hinnebusch J, Barbour A G. Linear- and circular-plasmid copy numbers in Borrelia burgdorferi. J Bacteriol. 1992;174:5251–5257. doi: 10.1128/jb.174.16.5251-5257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hofmann H. Lyme borreliosis—problems of serological diagnosis. Infection. 1996;24:470–472. doi: 10.1007/BF01713052. [DOI] [PubMed] [Google Scholar]
- 100.Hu C M, Gern L, Aeschlimann A. Changes in the protein profile and antigenicity of different Borrelia burgdorferi strains after reintroduction to Ixodes ricinus ticks. Parasite Immunol. 1992;14:415–427. doi: 10.1111/j.1365-3024.1992.tb00016.x. [DOI] [PubMed] [Google Scholar]
- 101.Hubalek Z, Halouzka J. Distribution of Borrelia burgdorferi sensu lato genomic groups in Europe, a review. Eur J Epidemiol. 1997;13:951–957. doi: 10.1023/a:1007426304900. [DOI] [PubMed] [Google Scholar]
- 102.Hubalek Z, Halouzka J. Prevalence rates of Borrelia burgdorferi sensu lato in host-seeking Ixodes ricinus ticks in Europe. Parasitol Res. 1998;84:167–172. doi: 10.1007/s004360050378. [DOI] [PubMed] [Google Scholar]
- 103.Hudson B J, Stewart M, Lennox V A, Fukunaga M, Yabuki M, Macorison H, Kitchener-Smith J. Culture-positive Lyme borreliosis. Med J Aust. 1998;168:500–502. doi: 10.5694/j.1326-5377.1998.tb141415.x. [DOI] [PubMed] [Google Scholar]
- 104.Humair P F, Peter O, Wallich R, Gern L. Strain variation of Lyme disease spirochetes isolated from Ixodes ricinus ticks and rodents collected in two endemic areas in Switzerland. J Med Entomol. 1995;32:433–438. doi: 10.1093/jmedent/32.4.433. [DOI] [PubMed] [Google Scholar]
- 105.Humair P F, Postic D, Wallich R, Gern L. An avian reservoir (Turdus merula) of the Lyme borreliosis spirochetes. Zentbl Bakteriol. 1998;287:521–538. [PubMed] [Google Scholar]
- 106.Humair P F, Rais O, Gern L. Transmission of Borrelia afzelii from Apodemus mice and Clethrionomys voles to Ixodes ricinus ticks: differential transmission pattern and overwintering maintenance. Parasitology. 1999;118:33–42. doi: 10.1017/s0031182098003564. [DOI] [PubMed] [Google Scholar]
- 107.Hyde F W, Johnson R C. Characterization of a circular plasmid from Borrelia burgdorferi, etiologic agent of Lyme disease. J Clin Microbiol. 1988;26:2203–2205. doi: 10.1128/jcm.26.10.2203-2205.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.International Union of Microbiological Societies. Validation of the publication of new names and new combinations previously effectively published outside the IJSB. Int J Syst Bacteriol. 1998;47:1274. doi: 10.1099/00207713-47-4-1274. [DOI] [PubMed] [Google Scholar]
- 109.Jauris-Heipke S, Liegl G, Preac-Mursic V, Rössler D, Schwab E, Soutschek E, Will G, Wilske B. Molecular analysis of genes encoding outer surface protein C (OspC) of Borrelia burgdorferi sensu lato: Relationship to ospA genotype and evidence of lateral gene exchange of ospC. J Clin Microbiol. 1995;33:1860–1866. doi: 10.1128/jcm.33.7.1860-1866.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Johnson B J, Robbins K E, Bailey R E, Cao B L, Sviat S L, Craven R B, Mayer L W, Dennis D T. Serodiagnosis of Lyme disease: accuracy of a two-step approach using a flagella-based ELISA and immunoblotting. J Infect Dis. 1996;174:346–353. doi: 10.1093/infdis/174.2.346. [DOI] [PubMed] [Google Scholar]
- 111.Johnson R C. Borrelia burgdorferi sp. nov.: etiological agent of Lyme disease. Int J Syst Bacteriol. 1984;34:496–497. [Google Scholar]
- 112.Johnson R C, Kodner C, Russell M. Active immunization of hamsters against experimental infection with Borrelia burgdorferi. Infect Immun. 1986;54:897–898. doi: 10.1128/iai.54.3.897-898.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kaiser R. Variable CSF findings in early and late Lyme neuroborreliosis: a follow-up study in 47 patients. J Neurol. 1994;242:26–36. doi: 10.1007/BF00920571. [DOI] [PubMed] [Google Scholar]
- 114.Kaneda K, Masuzawa T, Simon M M, Isogai E, Isogai H, Yasugami K, Suzuki T, Suzuki Y, Yanagihara Y. Infectivity and arthritis induction of Borrelia japonica on SCID mice and immune competent mice: possible role of galactosylceramide binding activity on initiation of infection. Microbiol Immunol. 1998;42:171–175. doi: 10.1111/j.1348-0421.1998.tb02268.x. [DOI] [PubMed] [Google Scholar]
- 115.Kaufman L D, Gruber B L, Phillips M E, Benach J L. Late cutaneous Lyme disease: acrodermatitis chronica atrophicans. Am J Med. 1989;86:828–830. doi: 10.1016/0002-9343(89)90482-8. [DOI] [PubMed] [Google Scholar]
- 116.Kawabata H, Masuzawa T, Yanagihara Y. Genomic analysis of Borrelia japonica sp. nov. isolated from Ixodes ovatus in Japan. Microbiol Immunol. 1993;37:843–848. doi: 10.1111/j.1348-0421.1993.tb01714.x. [DOI] [PubMed] [Google Scholar]
- 117.Kirstein F, Rijpkema S G, Molkenboer M, Gray J S. Local variations in the distribution and prevalence of Borrelia burgdorferi sensu lato genomospecies in Ixodes ricinus ticks. Appl Environ Microbiol. 1997;63:1102–1106. doi: 10.1128/aem.63.3.1102-1106.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kirstein F, Rijpkema S G, Molkenboer M, Gray J S. The distribution and prevalence of B. burgdorferi genomospecies in Ixodes ricinus ticks in Ireland. Eur J Epidemiol. 1997;13:67–72. doi: 10.1023/a:1007360422975. [DOI] [PubMed] [Google Scholar]
- 119.Kramer M D, Wallich R, Simon M M. The outer surface protein A (OspA) of Borrelia burgdorferi: a vaccine candidate and bioactive mediator. Infection. 1996;24:190–194. doi: 10.1007/BF01713338. [DOI] [PubMed] [Google Scholar]
- 120.Kristoferitsch W. Chronic peripheral neuropathy. In: Weber K, Burgdorfer W, editors. Aspects of Lyme borreliosis. Berlin, Germany: Springer-Verlag KG; 1993. pp. 205–218. [Google Scholar]
- 121.Kuiper H, van Dam A P, Spanjaard L, de Jongh B M, Widjojokusumo A, Ramselaar T C, Cairo I, Vos K, Dankert J. Isolation of Borrelia burgdorferi from biopsy specimens taken from healthy-looking skin of patients with Lyme borreliosis. J Clin Microbiol. 1994;32:715–720. doi: 10.1128/jcm.32.3.715-720.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kurtenbach K, Dizij A, Seitz H M, Margos G, Moter S E, Kramer M D, Wallich R, Schaible U E, Simon M M. Differential immune responses to Borrelia burgdorferi in European wild rodent species influence spirochete transmission to Ixodes ricinus L. (Acari: Ixodidae) Infect Immun. 1994;62:5344–5352. doi: 10.1128/iai.62.12.5344-5352.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kurtenbach K, Peacey M, Rijpkema S G, Hoodless A N, Nuttall P A, Randolph S E. Differential transmission of the genospecies of Borrelia burgdorferi sensu lato by game birds and small rodents in England. Appl Environ Microbiol. 1998;64:1169–1174. doi: 10.1128/aem.64.4.1169-1174.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kurtenbach K, Sewell H S, Ogden N H, Randolph S E, Nuttall P A. Serum complement sensitivity as a key factor in Lyme disease ecology. Infect Immun. 1998;66:1248–1251. doi: 10.1128/iai.66.3.1248-1251.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lebech A M, Hansen K, Wilske B, Theisen M. Taxonomic classification of 29 Borrelia burgdorferi strains isolated from patients with Lyme borreliosis: a comparison of five different phenotypic and genotypic typing schemes. Med Microbiol Immunol (Berlin) 1994;183:325–341. doi: 10.1007/BF00196683. [DOI] [PubMed] [Google Scholar]
- 126.Ledue T B, Collins M F, Craig W Y. New laboratory guidelines for serologic diagnosis of Lyme disease: evaluation of the two-test protocol. J Clin Microbiol. 1996;34:2343–2350. doi: 10.1128/jcm.34.10.2343-2350.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Le Fleche A, Postic D, Girardet K, Peter O, Baranton G. Characterization of Borrelia lusitaniae sp. nov. by 16S ribosomal DNA sequence analysis. Int J Syst Bacteriol. 1997;47:921–925. doi: 10.1099/00207713-47-4-921. [DOI] [PubMed] [Google Scholar]
- 128.Leong J M, Wang H, Magoun L, Field J A, Morrissey P E, Robbins D, Tatro J B, Coburn J, Parveen N. Different classes of proteoglycans contribute to the attachment of Borrelia burgdorferi to cultured endothelial and brain cells. Infect Immun. 1998;66:994–999. doi: 10.1128/iai.66.3.994-999.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li M, Masuzawa T, Takada N, Ishiguro F, Fujita H, Iwaki A, Wang H, Wang J, Kawabata M, Yanagihara Y. Lyme disease Borrelia species in northeastern China resemble those isolated from far eastern Russia and Japan. Appl Environ Microbiol. 1998;64:2705–2709. doi: 10.1128/aem.64.7.2705-2709.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liang J G, Zhang Z F. Analysis of rRNA gene restriction fragment length polymorphism of B. burgdorferi sensu lato isolated in China. Chin J Microbiol Immunol. 1996;16:359–362. [Google Scholar]
- 131.Liebisch G, Sohns B, Bautsch W. Detection and typing of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks attached to human skin by PCR. J Clin Microbiol. 1998;36:3355–3358. doi: 10.1128/jcm.36.11.3355-3358.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liveris D, Gazumyan A, Schwartz I. Molecular typing of Borrelia burgdorferi sensu lato by PCR-restriction fragment length polymorphism analysis. J Clin Microbiol. 1995;33:589–595. doi: 10.1128/jcm.33.3.589-595.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liveris D, Varde S, Iyer R, Koenig S, Bittker S, Cooper D, McKenna D, Nowakowski J, Nadelman R B, Wormser G P, Schwartz I. Genetic diversity of Borrelia burgdorferi in Lyme disease patients as determined by culture versus direct PCR with clinical specimens. J Clin Microbiol. 1999;37:565–569. doi: 10.1128/jcm.37.3.565-569.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liveris D, Wormser G P, Nowakowski J, Nadelman R, Bittker S, Cooper D, Varde S, Moy F H, Forseter G, Pavia C S, Schwartz I. Molecular typing of Borrelia burgdorferi from Lyme disease patients by PCR-restriction fragment length polymorphism analysis. J Clin Microbiol. 1996;34:1306–1309. doi: 10.1128/jcm.34.5.1306-1309.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Livesley M A, Thompson I P, Gern L, Nuttall P A. Analysis of intra-specific variation in the fatty acid profiles of Borrelia burgdorferi. J Gen Microbiol. 1993;139:2197–2201. doi: 10.1099/00221287-139-9-2197. [DOI] [PubMed] [Google Scholar]
- 136.Livesley M A, Thompson I P, Rainey P B, Nuttall P A. Comparison of Borrelia isolated from UK foci of Lyme disease. FEMS Microbiol Lett. 1995;130:151–157. doi: 10.1111/j.1574-6968.1995.tb07712.x. [DOI] [PubMed] [Google Scholar]
- 137.Livey I, Gibbs C P, Schuster R, Dorner F. Evidence for lateral transfer and recombination in OspC variation in Lyme disease Borrelia. Mol Microbiol. 1995;18:257–269. doi: 10.1111/j.1365-2958.1995.mmi_18020257.x. [DOI] [PubMed] [Google Scholar]
- 138.Luke C J, Carner K, Liang X, Barbour A G. An OspA-based DNA vaccine protects mice against infection with Borrelia burgdorferi. J Infect Dis. 1997;175:91–97. doi: 10.1093/infdis/175.1.91. [DOI] [PubMed] [Google Scholar]
- 139.Magnarelli L A. Current status of laboratory diagnosis for Lyme disease. Am J Med. 1995;98:10S–12S. doi: 10.1016/s0002-9343(99)80039-4. [DOI] [PubMed] [Google Scholar]
- 140.Magnarelli L A, Miller J N, Anderson J F, Riviere G R. Cross-reactivity of nonspecific treponemal antibody in serologic tests for Lyme disease. J Clin Microbiol. 1990;28:1276–1279. doi: 10.1128/jcm.28.6.1276-1279.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Marconi R T, Garon C F. Development of polymerase chain reaction primer sets for diagnosis of Lyme disease and for species-specific identification of Lyme disease isolates by 16S rRNA signature nucleotide analysis. J Clin Microbiol. 1992a;30:2830–2834. doi: 10.1128/jcm.30.11.2830-2834.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Marconi R T, Garon C F. Identification of a third genomic group of Borrelia burgdorferi through signature nucleotide analysis and 16S rRNA sequence determination. J Gen Microbiol. 1992b;138:533–536. doi: 10.1099/00221287-138-3-533. [DOI] [PubMed] [Google Scholar]
- 143.Marconi R T, Liveris D, Schwartz I. Identification of novel insertion elements, restriction fragment length polymorphism patterns, and discontinuous 23S rRNA in Lyme disease spirochetes: phylogenetic analyses of rRNA genes and their intergenic spacers in Borrelia japonica sp. nov. and genomic group 21038 (Borrelia andersonii sp. nov.) isolates. J Clin Microbiol. 1995;33:2427–2434. doi: 10.1128/jcm.33.9.2427-2434.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Marconi R T, Lubke L, Hauglum W, Garon C F. Species-specific identification of and distinction between Borrelia burgdorferi genomic groups by using 16S rRNA-directed oligonucleotide probes. J Clin Microbiol. 1992;30:628–632. doi: 10.1128/jcm.30.3.628-632.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Marconi R T, Samuels D S, Landry R K, Garon C F. Analysis of the distribution and molecular heterogeneity of the ospD gene among the Lyme disease spirochetes: evidence for lateral gene exchange. J Bacteriol. 1994;176:4572–4582. doi: 10.1128/jb.176.15.4572-4582.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Marconi R T, Sung S Y, Hughes C A, Carlyon J A. Molecular and evolutionary analyses of a variable series of genes in Borrelia burgdorferi that are related to ospE and ospF, constitute a gene family, and share a common upstream homology box. J Bacteriol. 1997;178:5615–5626. doi: 10.1128/jb.178.19.5615-5626.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Masuzawa T, Iwaki A, Sato Y, Miyamoto K, Korenberg E I, Yanagihara Y. Genetic diversity of Borrelia burgdorferi sensu lato isolated in far eastern Russia. Microbiol Immunol. 1997;41:595–600. doi: 10.1111/j.1348-0421.1997.tb01897.x. [DOI] [PubMed] [Google Scholar]
- 148.Masuzawa T, Kawabata H, Beppu Y, Miyamoto K, Nakao M, Sato N, Muramatsu K, Johnson R C, Yanagihara Y. Characterization of monoclonal antibodies for identification of Borrelia japonica, isolates from Ixodes ovatus. Microbiol Immunol. 1994;38:393–398. doi: 10.1111/j.1348-0421.1994.tb01797.x. [DOI] [PubMed] [Google Scholar]
- 149.Masuzawa T, Komikado T, Iwaki A, Suzuki H, Kaneda K, Yanagihara Y. Characterization of Borrelia sp. isolated from Ixodes tanuki, I. turdus, and I. columnae in Japan by restriction fragment length polymorphism of rrf(5S)-rrl (23S) intergenic spacer amplicons. FEMS Microbiol Lett. 1996;142:77–83. doi: 10.1111/j.1574-6968.1996.tb08411.x. [DOI] [PubMed] [Google Scholar]
- 150.Masuzawa T, Komikado T, Kaneda K, Fukui T, Sawaki K, Yanagihara Y. Homogeneity of Borrelia japonica and heterogeneity of Borrelia afzelii and ‘Borrelia tanukii’ isolated in Japan, determined from ospC gene sequences. FEMS Microbiol Lett. 1997;153:287–293. doi: 10.1111/j.1574-6968.1997.tb12587.x. [DOI] [PubMed] [Google Scholar]
- 151.Masuzawa T, Suzuki H, Kawabata H, Ishiguro F, Takada N, Yano Y, Yanagihara Y. Identification of spirochetes isolated from wild rodents in Japan as Borrelia japonica. J Clin Microbiol. 1995;33:1392–1394. doi: 10.1128/jcm.33.5.1392-1394.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Masuzawa T, Wilske B, Komikado T, Suzuki H, Kawabata H, Sato N X M K, Sato N, Isogai E, Isogai H, Johnson R C, Yanagihara Y. Comparison of OspA serotypes for Borrelia burgdorferi sensu lato from Japan, Europe and North America. Microbiol Immunol. 1996;40:539–545. doi: 10.1111/j.1348-0421.1996.tb01106.x. [DOI] [PubMed] [Google Scholar]
- 153.Masuzawa T, Yanagihara Y, Fujita H. A case of Lyme borreliosis which was suspected to be caused by Borrelia japonica infection in Shizuoka, Japan. J Jpn Assoc Infect Dis. 1996;70:264–267. doi: 10.11150/kansenshogakuzasshi1970.70.264. [DOI] [PubMed] [Google Scholar]
- 154.Mather T N, Wilson M L, Moore S I, Ribeiro J M, Spielman A. Comparing the relative potential of rodents as reservoirs of the Lyme disease spirochete (Borrelia burgdorferi) Am J Epidemiol. 1989;130:143–150. doi: 10.1093/oxfordjournals.aje.a115306. [DOI] [PubMed] [Google Scholar]
- 155.Mathiesen D A, Oliver J H, Jr, Kolbert C P, Tullson E D, Johnson B J, Campbell G L, Mitchell P D, Reed K D, Telford S R 3, Anderson J F, Lane R S, Persing D H. Genetic heterogeneity of Borrelia burgdorferi in the United States. J Infect Dis. 1997;175:98–107. doi: 10.1093/infdis/175.1.98. [DOI] [PubMed] [Google Scholar]
- 156.Mathiesen M J, Hansen K, Axelsen N, Halkier-Sorensen L, Theisen M. Analysis of the human antibody response to outer surface protein C (OspC) of Borrelia burgdorferi sensu stricto, B. garinii, and B. afzelii. Med Microbiol Immunol (Berl) 1996;185:121–129. doi: 10.1007/s004300050021. [DOI] [PubMed] [Google Scholar]
- 157.McCrossin I. Lyme disease on the NSW south coast. Med J Aust. 1986;144:724–725. doi: 10.5694/j.1326-5377.1986.tb113715.x. [DOI] [PubMed] [Google Scholar]
- 158.Mhalu F S, Matre R. Serological evidence of Lyme borreliosis in Africa: results from studies in Dar es Salaam, Tanzania. East Afr Med J. 1996;73:583–585. [PubMed] [Google Scholar]
- 159.Misonne M C, Hoet P P. Species-specific plasmid sequences for PCR identification of the three species of Borrelia burgdorferi sensu lato involved in Lyme disease. J Clin Microbiol. 1998;36:269–272. doi: 10.1128/jcm.36.1.269-272.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Misonne M C, van Impe G, Hoet P P. Genetic heterogeneity of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks collected in Belgium. J Clin Microbiol. 1998;36:3352–3354. doi: 10.1128/jcm.36.11.3352-3354.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nadelman R B, Nowakowski J, Forseter G, Goldberg N S, Bittker S, Cooper D, Aguero-Rosenfeld M, Wormser G P. The clinical spectrum of early Lyme borreliosis in patients with culture-confirmed erythema migrans. Am J Med. 1996;100:502–508. doi: 10.1016/s0002-9343(95)99915-9. [DOI] [PubMed] [Google Scholar]
- 162.Nadelman R B, Wormser G P. Lyme borreliosis. Lancet. 1998;352:557–565. doi: 10.1016/S0140-6736(98)01146-5. [DOI] [PubMed] [Google Scholar]
- 163.Nakao M, Miyamoto K. Mixed infection of different Borrelia species among Apodemus speciosus mice in Hokkaido, Japan. J Clin Microbiol. 1995;33:490–492. doi: 10.1128/jcm.33.2.490-492.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nakao M, Miyamoto K, Fukunaga M. Lyme disease spirochetes in Japan: enzootic transmission cycles in birds, rodents, and Ixodes persulcatus ticks. J Infect Dis. 1994;170:878–882. doi: 10.1093/infdis/170.4.878. [DOI] [PubMed] [Google Scholar]
- 165.Nakao M, Sato Y. Refeeding activity of immature ticks of Ixodes persulcatus and transmission of Lyme disease spirochete by partially fed larvae. J Parasitol. 1996;82:669–672. [PubMed] [Google Scholar]
- 166.Nicholls T H, Callister S M. Lyme disease spirochetes in ticks collected from birds in midwestern United States. J Med Entomol. 1996;33:379–384. doi: 10.1093/jmedent/33.3.379. [DOI] [PubMed] [Google Scholar]
- 167.Nocton J J, Dressler F, Rutledge B J, Rys P N, Persing D H, Steere A C. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med. 1994;330:229–234. doi: 10.1056/NEJM199401273300401. [DOI] [PubMed] [Google Scholar]
- 168.Nohlmans L M, de Boer R, van den Bogaard A E, van Boven C P. Genotypic and phenotypic analysis of Borrelia burgdorferi isolates from The Netherlands. J Clin Microbiol. 1995;33:119–125. doi: 10.1128/jcm.33.1.119-125.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Norman G L, Antig J M, Bigaignon G, Hogrefe W R. Serodiagnosis of Lyme borreliosis by Borrelia burgdorferi sensu stricto, B. garinii, and B. afzelii Western blots (immunoblots) J Clin Microbiol. 1996;34:1732–1738. doi: 10.1128/jcm.34.7.1732-1738.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Norris D E, Johnson B J, Piesman J, Maupin G O, Clark J L, Black W C., IV Culturing selects for specific genotypes of Borrelia burgdorferi in an enzootic cycle in Colorado. J Clin Microbiol. 1997;35:2359–2364. doi: 10.1128/jcm.35.9.2359-2364.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Norris D E, Klompen J S H, Keirans J E, Lane R S, Piesman J, Black W C., IV Taxonomic status of Ixodes neotomae and I. spinipalpis (Acari Ixodidae) based on mitochondrial DNA evidence. J Med Entomol. 1997;34:696–703. doi: 10.1093/jmedent/34.6.696. [DOI] [PubMed] [Google Scholar]
- 172.O’Connell S, Granstrom M, Gray J S, Stanek G. Epidemiology of European Lyme borreliosis. Zentbl Bakteriol. 1998;287:229–240. doi: 10.1016/s0934-8840(98)80124-2. [DOI] [PubMed] [Google Scholar]
- 173.Ohlenbusch A, Matuschka F R, Richter D, Christen H J, Thomssen R, Spielman A, Eiffert H. Etiology of the acrodermatitis chronica atrophicans lesion in Lyme disease. J Infect Dis. 1996;174:421–423. doi: 10.1093/infdis/174.2.421. [DOI] [PubMed] [Google Scholar]
- 174.Ojaimi C, Davidson B E, Saint Girons I, Old I G. Conservation of gene arrangement and an unusual organization of rRNA genes in the linear chromosomes of the Lyme disease spirochaetes Borrelia burgdorferi, B. garinii and B. afzelii. Microbiology. 1994;140:2931–2940. doi: 10.1099/13500872-140-11-2931. [DOI] [PubMed] [Google Scholar]
- 175.Oliver J H J, Owsley M R, Hutcheson H J, James A M, Chen C, Irby W S, Dotson E M, McLain D K. Conspecificity of the ticks Ixodes scapularis and I. dammini (Acari: Ixodidae) J Med Entomol. 1993;30:54–63. doi: 10.1093/jmedent/30.1.54. [DOI] [PubMed] [Google Scholar]
- 176.Olsen B, Jaenson T G, Bergstrom S. Prevalence of Borrelia burgdorferi sensu lato-infected ticks on migrating birds. Appl Environ Microbiol. 1995;61:3082–3087. doi: 10.1128/aem.61.8.3082-3087.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Olsen B, Jaenson T G, Noppa L, Bunikis J, Bergstrom S. A Lyme borreliosis cycle in seabirds and Ixodes uriae ticks. Nature. 1993;362:340–342. doi: 10.1038/362340a0. [DOI] [PubMed] [Google Scholar]
- 178.Oschmann P, Dorndorf W, Hornig C, Schafer C, Wellensiek H J, Pflughaupt K W. Stages and syndromes of neuroborreliosis. J Neurol. 1998;245:262–272. doi: 10.1007/s004150050216. [DOI] [PubMed] [Google Scholar]
- 179.Pachner A R. Neurologic manifestations of Lyme disease, the new “great imitator.”. Rev Infect Dis. 1989;11(Suppl. 6):S1482–S1486. [PubMed] [Google Scholar]
- 180.Padula S J, Sampieri A, Dias F, Szczepanski A, Ryan R W. Molecular characterization and expression of p23 (OspC) from a North American strain of Borrelia burgdorferi. Infect Immun. 1993;61:5097–5105. doi: 10.1128/iai.61.12.5097-5105.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Park K H, Chang W H, Schwan T G. Identification and characterization of Lyme disease spirochetes, Borrelia burgdorferi sensu lato, isolated in Korea. J Clin Microbiol. 1993;31:1831–1837. doi: 10.1128/jcm.31.7.1831-1837.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Parveen N, Robbins D, Leong J M. Strain variation in glycosaminoglycan recognition influences cell-type-specific binding by Lyme disease spirochetes. Infect Immun. 1999;67:1743–1749. doi: 10.1128/iai.67.4.1743-1749.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Paster B J, Dewhirst F E, Weisburg W G, Tordoff L A, Fraser G J, Hespell R B, Stanton T B, Zablen L, Mandelco L, Woese C R. Phylogenetic analysis of the spirochetes. J Bacteriol. 1991;173:6101–6109. doi: 10.1128/jb.173.19.6101-6109.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Patrican L A. Acquisition of Lyme disease spirochetes by cofeeding Ixodes scapularis ticks. Am J Trop Med Hyg. 1997;57:589–593. doi: 10.4269/ajtmh.1997.57.589. [DOI] [PubMed] [Google Scholar]
- 185.Pavia C S, Wormser G P, Mormser G L, Norman G L. Activity of sera from patients with Lyme disease against Borrelia burgdorferi. Clin Infect Dis. 1997;25:S25–S30. doi: 10.1086/516168. [DOI] [PubMed] [Google Scholar]
- 186.Peter O, Bretz A-G, Postic D, Dayer E. Association of distinct species of Borrelia burgdorferi sensu lato with neuroborreliosis in Switzerland. Clin Microbiol Infect. 1997;3:423–431. doi: 10.1111/j.1469-0691.1997.tb00278.x. [DOI] [PubMed] [Google Scholar]
- 187.Peter O, Bretz A G. Polymorphism of outer surface proteins of Borrelia burgdorferi as a tool for classification. Zentbl Bakteriol. 1992;277:28–33. doi: 10.1016/s0934-8840(11)80867-4. [DOI] [PubMed] [Google Scholar]
- 188.Peter O, Bretz A G, Bee D. Occurrence of different genospecies of Borrelia burgdorferi sensu lato in ixodid ticks of Valais, Switzerland. Eur J Epidemiol. 1995;11:463–467. doi: 10.1007/BF01721234. [DOI] [PubMed] [Google Scholar]
- 189.Pfister H W, Wilske B, Weber K. Lyme borreliosis: basic science and clinical aspects. Lancet. 1994;343:1013–1016. doi: 10.1016/s0140-6736(94)90130-9. [DOI] [PubMed] [Google Scholar]
- 190.Pichon B, Godfroid E, Hoyois B, Bollen A, Rodhain F, Perez-Eid C. Simultaneous infection of Ixodes ricinus nymphs by two Borrelia burgdorferi sensu lato species: possible implications for clinical manifestations. Emerg Infect Dis. 1995;1:89–90. doi: 10.3201/eid0103.950304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Picken R N, Cheng Y, Han D, Nelson J A, Reddy A G, Hayden M K, Picken M M, Strle F, Bouseman J K, Trenholme G M. Genotypic and phenotypic characterization of Borrelia burgdorferi isolated from ticks and small animals in Illinois. J Clin Microbiol. 1995;33:2304–2315. doi: 10.1128/jcm.33.9.2304-2315.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Picken R N, Cheng Y, Strle F, Cimperman J, Maraspin V, Lotric-Furlan S, Ruzic-Sabljic E, Han D, Nelson J A, Picken M M, Trenholme G M. Molecular characterization of Borrelia burgdorferi sensu lato from Slovenia revealing significant differences between tick and human isolates. Eur J Clin Microbiol Infect Dis. 1996;15:313–323. doi: 10.1007/BF01695664. [DOI] [PubMed] [Google Scholar]
- 193.Picken R N, Cheng Y, Strle F, Picken M M. Patient isolates of Borrelia burgdorferi sensu lato with genotypic and phenotypic similarities of strain 25015. J Infect Dis. 1996;174:1112–1115. doi: 10.1093/infdis/174.5.1112. [DOI] [PubMed] [Google Scholar]
- 194.Picken R N, Strle F, Picken M M, Ruzic-Sabljic E, Maraspin V, Lotric-Furlan S, Cimperman J. Identification of three species of Borrelia burgdorferi sensu lato (B. burgdorferi sensu stricto, B. garinii, and B. afzelii) among isolates from acrodermatitis chronica atrophicans lesions. J Investig Dermatol. 1998;110:211–214. doi: 10.1046/j.1523-1747.1998.00130.x. [DOI] [PubMed] [Google Scholar]
- 195.Popovic T, Bopp C A, Olsvik O, Kiehlbauch J A. Ribotyping in molecular epidemiology. In: Persing D H, Smith T F, Tenover F C, White T J, editors. Diagnostic molecular microbiology. Washington, D.C.: American Society for Microbiology; 1993. pp. 573–589. [Google Scholar]
- 196.Postic D, Assous M, Belfaiza J, Baranton G. Genetic diversity of Borrelia of Lyme borreliosis. Wien Klin, Wochenschr. 1996;108:748–751. [PubMed] [Google Scholar]
- 197.Postic D, Assous M V, Grimont P A D, Baranton G. Diversity of Borrelia burgdorferi sensu lato evidenced by restriction fragment length polymorphism of rrf(5S)-rrl(23S) intergenic spacer amplicons. Int J Syst Bacteriol. 1994;44:743–752. doi: 10.1099/00207713-44-4-743. [DOI] [PubMed] [Google Scholar]
- 198.Postic D, Baranton G. Molecular fingerprinting and phylogeny of Borrelia burgdorferi sensu lato. In: Yanagihara Y, Masuzawa T, editors. Proceeding of the International Symposium on Lyme Disease in Japan 1994. 1994. pp. 133–147. [Google Scholar]
- 199.Postic D, Belfaiza J, Isogai E, Saint Girons I, Grimont P A, Baranton G. A new genomic species in Borrelia burgdorferi sensu lato isolated from Japanese ticks. Res Microbiol. 1993;144:467–473. doi: 10.1016/0923-2508(93)90054-6. [DOI] [PubMed] [Google Scholar]
- 200.Postic D, Korenberg E, Gorelova N, Kovalevski Y V, Bellenger E, Baranton G. Borrelia burgdorferi sensu lato in Russia and neighbouring countries: high incidence of mixed isolates. Res Microbiol. 1997;148:691–702. doi: 10.1016/S0923-2508(99)80068-0. [DOI] [PubMed] [Google Scholar]
- 201.Postic D, Ras N M, Lane R S, Hendson M, Baranton G. Expanded Diversity among California Borrelia isolates and description of Borrelia bissettii sp. nov. (formerly Borrelia group DN127) J Clin Microbiol. 1998;36:3497–3504. doi: 10.1128/jcm.36.12.3497-3504.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Probert W S, Allsup K M, LeFebvre R B. Identification and characterization of a surface-exposed, 66-kilodalton protein from Borrelia burgdorferi. Infect Immun. 1995;63:1933–1939. doi: 10.1128/iai.63.5.1933-1939.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ralph D, Postic D, Baranton G, Pretzman C, McClelland M. Species of Borrelia distinguished by restriction site polymorphisms in 16S rRNA genes. FEMS Microbiol Lett. 1993;111:239–244. doi: 10.1111/j.1574-6968.1993.tb06392.x. [DOI] [PubMed] [Google Scholar]
- 204.Randolph S E, Gern L, Nuttall P A. Co-feeding ticks: epidemiological significance for tick-borne pathogen transmission. Parasitol Today. 1996;12:472–479. doi: 10.1016/s0169-4758(96)10072-7. [DOI] [PubMed] [Google Scholar]
- 205.Ras N M, Postic D, Foretz M, Baranton G. Borrelia burgdorferi sensu stricto, a bacterial species “made in the U.S.A.”? Int J Syst Bacteriol. 1997;47:1112–1117. doi: 10.1099/00207713-47-4-1112. [DOI] [PubMed] [Google Scholar]
- 206.Rijpkema S G, Golubic D, Molkenboer M, Verbeek-De Kruif N, Schellekens J F. Identification of four genomic groups of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks collected in a Lyme borreliosis endemic region of northern Croatia. Exp Appl Acarol. 1996;20:23–30. doi: 10.1007/BF00051474. [DOI] [PubMed] [Google Scholar]
- 207.Rijpkema S G, Herbes R G, Verbeek-De Kruif N, Schellekens J F. Detection of four species of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks collected from roe deer (Capreolus capreolus) in The Netherlands. Epidemiol Infect. 1996;117:563–566. doi: 10.1017/s0950268800059252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Rijpkema S G, Molkenboer M J, Schouls L M, Jongejan F, Schellekens J F. Simultaneous detection and genotyping of three genomic groups of Borrelia burgdorferi sensu lato in Dutch Ixodes ricinus ticks by characterization of the amplified intergenic spacer region between 5S and 23S rRNA genes. J Clin Microbiol. 1995;33:3091–3095. doi: 10.1128/jcm.33.12.3091-3095.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Rijpkema S G, Tazelaar D J, Molkenboer M, Noordhoek G T, Plantinga G, Schouls L M, Schellekens J F. Detection of Borrelia afzelii, Borrelia burgdorferi sensu stricto, Borrelia garinii and group VS116 by PCR in skin biopsies of patients with erythema migrans and acrodermitis chronica atrophicans. Clin Microbiol Infect. 1997;3:109–116. doi: 10.1111/j.1469-0691.1997.tb00259.x. [DOI] [PubMed] [Google Scholar]
- 210.Roberts W C, Mullikin B A, Lathigra R, Hanson M S. Molecular analysis of sequence heterogeneity among genes encoding decorin binding proteins A and B of Borrelia burgdorferi sensu lato. Infect Immun. 1998;66:5275–5285. doi: 10.1128/iai.66.11.5275-5285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Roessler D, Hauser U, Wilske B. Heterogeneity of BmpA (P39) among European isolates of Borrelia burgdorferi sensu lato and influence of interspecies variability on serodiagnosis. J Clin Microbiol. 1997;35:2752–2758. doi: 10.1128/jcm.35.11.2752-2758.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Rosa P A, Schwan T, Hogan D. Recombination between genes encoding major outer surface proteins A and B of Borrelia burgdorferi. Mol Microbiol. 1992;6:3031–3040. doi: 10.1111/j.1365-2958.1992.tb01761.x. [DOI] [PubMed] [Google Scholar]
- 213.Russell R C. Lyme disease in Australia—still to be proven. Emerg Infect Dis. 1995;1:29–31. doi: 10.3201/eid0101.950106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ryan J R, Levine J F, Apperson C S, Lubke L, Wirtz R A, Spears P A, Orndorff P E. An experimental chain of infection reveals that distinct Borrelia burgdorferi populations are selected in arthropod and mammalian hosts. Mol Microbiol. 1998;30:365–379. doi: 10.1046/j.1365-2958.1998.01071.x. [DOI] [PubMed] [Google Scholar]
- 215.Sadziene A, Thompson P A, Barbour A G. A flagella-less mutant of Borrelia burgdorferi as a live attenuated vaccine in the murine model of Lyme disease. J Infect Dis. 1996;173:1184–1193. doi: 10.1093/infdis/173.5.1184. [DOI] [PubMed] [Google Scholar]
- 216.Saint G I, Gern L, Gray J S, Guy E C, Korenberg E, Nuttall P A, Rijpkema S G, Schonberg A, Stanek G, Postic D. Identification of Borrelia burgdorferi sensu lato species in Europe. Zentbl Bakteriol. 1998;287:190–195. doi: 10.1016/s0934-8840(98)80120-5. [DOI] [PubMed] [Google Scholar]
- 217.Samuels D S, Marconi R T, Garon C F. Variation in the size of the ospA-containing linear plasmid, but not the linear chromosome, among the three Borrelia species associated with Lyme disease. J Gen Microbiol. 1993;139:2445–2449. doi: 10.1099/00221287-139-10-2445. [DOI] [PubMed] [Google Scholar]
- 218.Sato Y, Miyamoto K, Iwaki A, Masuzawa T, Yanagihara Y, Korenberg E I, Gorelova N B, Volkov V I, Ivanov L I, Liberova R N. Prevalence of Lyme disease spirochetes in Ixodes persulcatus and wild rodents in far eastern Russia. Appl Environ Microbiol. 1996;62:3887–3889. doi: 10.1128/aem.62.10.3887-3889.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Schafrank S N, Kurban A K, Martone G. Lyme disease acquired in southeast Africa. Arch Dermatol. 1990;126:685–686. [PubMed] [Google Scholar]
- 220.Schaible U E, Kramer M D, Museteanu C, Zimmer G, Mossmann H, Simon M M. The severe combined immunodeficiency (scid) mouse. A laboratory model for the analysis of Lyme arthritis and carditis. J Exp Med. 1989;170:1427–1432. doi: 10.1084/jem.170.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Schmid G P. The global distribution of Lyme disease. Rev Infect Dis. 1985;7:41–50. doi: 10.1093/clinids/7.1.41. [DOI] [PubMed] [Google Scholar]
- 222.Schwan T G, Burgdorfer W, Garon C F. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect Immun. 1988;56:1831–1836. doi: 10.1128/iai.56.8.1831-1836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Schwan T G, Burgdorfer W, Rosa P A. Borrelia. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C.: American Society for Microbiology; 1995. pp. 626–635. [Google Scholar]
- 224.Schwartz D C, Cantor C R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984;37:67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- 225.Schwartz J J, Gazumyan A, Schwartz I. rRNA gene organization in the Lyme disease spirochete, Borrelia burgdorferi. J Bacteriol. 1992;174:3757–3765. doi: 10.1128/jb.174.11.3757-3765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Shih C M, Chang H M, Chen S L, Chao L L. Genospecies identification and characterization of Lyme disease spirochetes of genospecies Borrelia burgdorferi sensu lato isolated from rodents in Taiwan. J Clin Microbiol. 1998;36:3127–3132. doi: 10.1128/jcm.36.11.3127-3132.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sigal L H, Zahradnik J M, Lavin P, Patella S J, Bryant G, Haselby R, Hilton E, Kunkel M, Adler-Klein D, Doherty T, Evans J, Malawista S E. A vaccine consisting of recombinant Borrelia burgdorferi outer-surface protein A to prevent Lyme disease. N Engl J Med. 1998;339:216–222. doi: 10.1056/NEJM199807233390402. [DOI] [PubMed] [Google Scholar]
- 228.Simon M M, Gern L, Hauser P, Zhong W, Nielsen P J, Kramer M D, Brenner C, Wallich R. Protective immunization with plasmid DNA containing the outer surface lipoprotein A gene of Borrelia burgdorferi is independent of an eukaryotic promoter. Eur J Immunol. 1996;26:2831–2840. doi: 10.1002/eji.1830261206. [DOI] [PubMed] [Google Scholar]
- 229.Smith M, Gettinby G, Granstrom M, Gray J S, Guy E C, Revie C, Robertson J N, Stanek G. The European union concerted action world wide web site for Lyme borreliosis. Zentbl Bakteriol. 1998;287:266–269. doi: 10.1016/s0934-8840(98)80128-x. [DOI] [PubMed] [Google Scholar]
- 230.Smith R P J, Rand P W, Lacombe E H, Morris S R, Holmes D W, Caporale D A. Role of bird migration in the long-distance dispersal of Ixodes dammini, the vector of Lyme disease. J Infect Dis. 1996;174:221–224. doi: 10.1093/infdis/174.1.221. [DOI] [PubMed] [Google Scholar]
- 231.Stackebrandt E, Goebel B M. Taxonomic note: a place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int J Syst Bacteriol. 1994;44:846–849. [Google Scholar]
- 232.Stafford K C, Bladen V C, Magnarelli L A. Ticks (Acari: Ixodidae) infesting wild birds (Aves) and white-footed mice in Lyme, CT. J Med Entomol. 1995;32:453–466. doi: 10.1093/jmedent/32.4.453. [DOI] [PubMed] [Google Scholar]
- 233.Staley J T, Krieg N R, editors. Bergey’s manual of systematic bacteriology. Vol. 3. Baltimore, Md: The Williams & Wilkins Co.; 1989. p. 1601. [Google Scholar]
- 234.Stanek G, Klein J, Bittner R, Glogar D. Isolation of Borrelia burgdorferi from the myocardium of a patient with longstanding cardiomyopathy. N Engl J Med. 1990;322:249–252. doi: 10.1056/NEJM199001253220407. [DOI] [PubMed] [Google Scholar]
- 235.Stanek G, O’Connell S, Cimmino M, Aberer E, Kristoferitsch W, Granstrom M, Guy E, Gray J. European union concerted action on risk assessment in Lyme borreliosis: clinical case definitions for Lyme borreliosis. Wien Klin Wochenschr. 1996;108:741–747. [PubMed] [Google Scholar]
- 236.Steere A C. Lyme disease. N Engl J Med. 1989;263:201–205. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 237.Steere A C. Lyme disease: a growing threat to urban populations. Proc Natl Acad Sci USA. 1994;91:2378–2383. doi: 10.1073/pnas.91.7.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Steere A C, Batsford W P, Weinberg M, Alexander J, Berger H J, Wolfson S, Malawista S E. Lyme carditis: cardiac abnormalities of Lyme disease. Ann Intern Med. 1980;93:8–16. doi: 10.7326/0003-4819-93-1-8. [DOI] [PubMed] [Google Scholar]
- 239.Steere A C, Broderick T F, Malawista S E. Erythema chronicum migrans and Lyme arthritis: epidemiologic evidence for a tick vector. Am J Epidemiol. 1978;108:312–321. doi: 10.1093/oxfordjournals.aje.a112625. [DOI] [PubMed] [Google Scholar]
- 240.Steere A C, Malawista S E, Hardin J A, Ruddy S, Askenase W, Andiman W A. Erythema chronicum migrans and Lyme arthritis: the enlarging clinical spectrum. Ann Intern Med. 1977;86:685–698. doi: 10.7326/0003-4819-86-6-685. [DOI] [PubMed] [Google Scholar]
- 241.Steere A C, Malawista S E, Snydman D R, Shope R E, Andiman W A, Ross M R, Steele F M. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three connecticut communities. Arthritis Rheum. 1977;20:7–17. doi: 10.1002/art.1780200102. [DOI] [PubMed] [Google Scholar]
- 242.Steere A C, Schoen R T, Taylor E. The clinical evolution of Lyme arthritis. Ann Intern Med. 1987;107:725–731. doi: 10.7326/0003-4819-107-5-725. [DOI] [PubMed] [Google Scholar]
- 243.Steere A C, Sikand V K, Meurice F, Parenti D L, Fikrig E, Schoen R T, Nowakowski J, Schmid C H, Laukamp S, Buscarino C, Krause D S. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. N Engl J Med. 1998;339:209–215. doi: 10.1056/NEJM199807233390401. [DOI] [PubMed] [Google Scholar]
- 244.Stevenson B, Barthold S W. Expression and sequence of outer surface protein C among North American isolates of Borrelia burgdorferi. FEMS Microbiol Lett. 1994;124:367–372. doi: 10.1111/j.1574-6968.1994.tb07310.x. [DOI] [PubMed] [Google Scholar]
- 245.Strle F, Nadelman R B, Cimperman J, Nowakowski J, Picken R N, Schwartz I, Maraspin V, Aguero-Rosenfeld M E, Varde S, Lotric-Furlan S, Wormser G P. Comparison of culture-confirmed erythema migrans caused by Borrelia burgdorferi sensu stricto in New York State and by Borrelia afzelii in Slovenia. Ann Intern Med. 1999;130:32–36. doi: 10.7326/0003-4819-130-1-199901050-00006. [DOI] [PubMed] [Google Scholar]
- 246.Strle F, Nelson J A, Ruzic-Sabljic E, Cimperman J, Maraspin V, Lotric-Furlan S, Cheng Y, Picken M M, Trenholme G M, Picken R N. European Lyme borreliosis: 231 culture-confirmed cases involving patients with erythema migrans. Clin Infect Dis. 1996;23:61–65. doi: 10.1093/clinids/23.1.61. [DOI] [PubMed] [Google Scholar]
- 247.Strle F, Picken R N, Cheng Y, Cimperman J, Maraspin V, Lotric-Furlan S, Ruzic-Sabljic E, Picken M M. Clinical findings for patients with Lyme borreliosis causing by Borrelia burgdorferi sensu lato with genotypic and phenotypic similarities to strain 25015. Clin Infect Dis. 1997;25:273–280. doi: 10.1086/514551. [DOI] [PubMed] [Google Scholar]
- 248.Takada N, Ishiguro F, Fujita H, Wang H P, Wang J C, Masuzawa T. Lyme disease spirochetes in ticks from northeastern China. J Parasitol. 1998;84:499–504. [PubMed] [Google Scholar]
- 249.Tenover F C, Arbeit R D, Goering R V. How to select and interpret molecular strain typing methods for epidemiological studies of bacterial infections: a review for healthcare epidemiologists. Infect Control Hosp Epidemiol. 1997;18:426–439. doi: 10.1086/647644. [DOI] [PubMed] [Google Scholar]
- 250.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Theisen M, Borre M, Mathiesen M J, Mikkelsen B, Lebech A M, Hansen K. Evolution of the Borrelia burgdorferi outer surface protein OspC. J Bacteriol. 1995;177:3036–3044. doi: 10.1128/jb.177.11.3036-3044.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Theisen M, Frederiksen B, Lebech A M, Vuust J, Hansen K. Polymorphism in ospC gene of Borrelia burgdorferi and immunoreactivity of OspC protein: implications for taxonomy and for use of OspC protein as a diagnostic antigen. J Clin Microbiol. 1993;31:2570–2576. doi: 10.1128/jcm.31.10.2570-2576.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Thomas D D, Cadavid D, Barbour A G. Differential association of Borrelia species with cultured neural cells. J Infect Dis. 1994;169:445–448. doi: 10.1093/infdis/169.2.445. [DOI] [PubMed] [Google Scholar]
- 254.Tibayrenc M. Population genetics of parasitic protozoa and other microorganisms. Adv Parasitol. 1995;36:47–115. doi: 10.1016/s0065-308x(08)60490-x. [DOI] [PubMed] [Google Scholar]
- 255.Trevejo R T, Krause P J, Sikand V K, Schriefer M E, Ryan R, Lepore T, Porter W, Dennis D T. Evaluation of two-test serodiagnostic method for early Lyme disease in clinical practice. J Infect Dis. 1999;179:931–938. doi: 10.1086/314663. [DOI] [PubMed] [Google Scholar]
- 256.Tugwell P, Dennis D T, Weinstein A, Wells G, Shea B, Nichol G, Hayward R, Lightfoot R, Baker P, Steere A C. Laboratory evaluation in the diagnosis of Lyme disease. Ann Intern Med. 1997;127:1109–1123. doi: 10.7326/0003-4819-127-12-199712150-00011. [DOI] [PubMed] [Google Scholar]
- 257.Tuomi J, Rantamaki L K, Tanskanen R, Junttila J. Characterization of Finnish Borrelia burgdorferi sensu lato isolates by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and with monoclonal antibodies. J Clin Microbiol. 1995;33:1989–1996. doi: 10.1128/jcm.33.8.1989-1996.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Valsangiacomo C, Balmelli T, Piffaretti J C. A phylogenetic analysis of Borrelia burgdorferi sensu lato based on sequence information from the hbb gene, coding for a histone-like protein. Int J Syst Bacteriol. 1997;47:1–10. doi: 10.1099/00207713-47-1-1. [DOI] [PubMed] [Google Scholar]
- 259.van Dam A P, Kuiper H, Vos K, Widjojokusumo A, de Jongh B M, Spanjaard L, Ramselaar A C, Kramer M D, Dankert J. Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin Infect Dis. 1993;17:708–717. doi: 10.1093/clinids/17.4.708. [DOI] [PubMed] [Google Scholar]
- 260.van Dam A P, Oei A, Jaspars R, Fijen C, Wilske B, Spanjaard L, Dankert J. Complement-mediated serum sensitivity among spirochetes that cause Lyme disease. Infect Immun. 1997;65:1228–1236. doi: 10.1128/iai.65.4.1228-1236.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.van der Linde M R. Lyme carditis: clinical characteristics of 105 cases. Scand J Infect Dis Suppl. 1991;77:81–84. [PubMed] [Google Scholar]
- 262.Vasiliu V, Herzer P, Rossler D, Lehnert G, Wilske B. Heterogeneity of Borrelia burgdorferi sensu lato demonstrated by an ospA-type-specific PCR in synovial fluid from patients with Lyme arthritis. Med Microbiol Immunol (Berlin) 1998;187:97–102. doi: 10.1007/s004300050079. [DOI] [PubMed] [Google Scholar]
- 263.Wallich R, Helmes C, Schaible U E, Lobet Y, Moter S E, Kramer M D, Simon M M. Evaluation of genetic divergence among Borrelia burgdorferi isolates by use of OspA, fla, HSP60, and HSP70 gene probes. Infect Immun. 1992;60:4856–4866. doi: 10.1128/iai.60.11.4856-4866.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Wang G, van Dam A P, Dankert J. Evidence for frequent ospC gene transfer between Borrelia valaisiana sp. nov. and other Lyme disease spirochetes. FEMS Microbiol Lett. 1999;177:289–296. doi: 10.1111/j.1574-6968.1999.tb13745.x. [DOI] [PubMed] [Google Scholar]
- 265.Wang G, van Dam A P, Dankert J. Phenotypic and genetic characterization of a novel Borrelia burgdorferi sensu lato isolate from a patient with Lyme borreliosis. J Clin Microbiol. 1999;37:3025–3028. doi: 10.1128/jcm.37.9.3025-3028.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Wang, G., A. P. van Dam, and J. Dankert. Plasmid fingerprinting and sequence analysis of the plasmid-encoded outer membrane protein OspA of B. valaisiana sp. nov. Submitted for publication.
- 267.Wang G, van Dam A P, Le Fleche A, Postic D, Peter O, Baranton G, de Boer R, Spanjaard L, Dankert J. Genetic and phenotypic analysis of Borrelia valaisiana sp. nov. (Borrelia genomic groups VS116 and M19) Int J Syst Bacteriol. 1997;47:926–932. doi: 10.1099/00207713-47-4-926. [DOI] [PubMed] [Google Scholar]
- 268.Wang G, van Dam A P, Spanjaard L, Dankert J. Molecular typing of Borrelia burgdorferi sensu lato by randomly amplified polymorphic DNA fingerprinting analysis. J Clin Microbiol. 1998;36:768–776. doi: 10.1128/jcm.36.3.768-776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Wayne L G, Brenner D J, Colwell R R, Grimont P A D, Kandler O, Krichevsky M I, Moore L H, Moore W E C, Murray R G E, Stackebrandt E, Starr M P, Truper H G. Report of the Ad Hoc Committee on reconciliation of approaches to bacterial systematics. Int J Syst Bacteriol. 1987;37:463–464. [Google Scholar]
- 270.Weber K, Pfister H-W. History of Lyme borreliosis in Europe. In: Weber K, Burgdorfer W, editors. Aspects of Lyme borreliosis. Berlin, Germany: Springer-Verlag KG; 1993. pp. 1–20. [Google Scholar]
- 271.Welsh J, McClelland M. Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Res. 1990;18:7213–7218. doi: 10.1093/nar/18.24.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Welsh J, Pretzman C, Postic D, Saint Girons I, Baranton G, McClelland M. Genomic fingerprinting by arbitrarily primed polymerase chain reaction resolves Borrelia burgdorferi into three distinct phyletic groups. Int J Syst Bacteriol. 1992;42:370–377. doi: 10.1099/00207713-42-3-370. [DOI] [PubMed] [Google Scholar]
- 273.Wienecke R, Zochling N, Neubert U, Schlupen E M, Meurer M, Volkenandt M. Molecular subtyping of Borrelia burgdorferi in erythema migrans and acrodermatitis chronica atrophicans. J Investig Dermatol. 1994;103:19–22. doi: 10.1111/1523-1747.ep12388947. [DOI] [PubMed] [Google Scholar]
- 274.Will G, Jauris-Heipke S, Schwab E, Busch U, Rossler D, Soutschek E X W B, Preac-Mursic V. Sequence analysis of ospA genes shows homogeneity within Borrelia burgdorferi sensu stricto and Borrelia afzelii strains but reveals major subgroups within the Borrelia garinii species. Med Microbiol Immunol (Berlin) 1995;184:73–80. doi: 10.1007/BF00221390. [DOI] [PubMed] [Google Scholar]
- 275.Williams J G, Kubelik A R, Livak K J, Rafalski J A, Tingey S V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990;18:6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Wilske B, Anderson J F, Baranton G, Barbour A G, Hovind-Hougen K, Johnson R C, Preac-Mursic V. Taxonomy of Borrelia spp. Scand J Infect Dis Suppl. 1991;77:108–129. [PubMed] [Google Scholar]
- 277.Wilske B, Busch U, Eiffert H, Fingerle V, Pfister H W, Rossler D, Preac-Mursic V. Diversity of OspA and OspC among cerebrospinal fluid isolates of Borrelia burgdorferi sensu lato from patients with neuroborreliosis in Germany. Med Microbiol Immunol (Berlin) 1996;184:195–201. doi: 10.1007/BF02456135. [DOI] [PubMed] [Google Scholar]
- 278.Wilske B, Busch U, Fingerle V, Jauris-Heipke S, Preac Mursic V, Rossler D, Will G. Immunological and molecular variability of OspA and OspC. Implications for Borrelia vaccine development. Infection. 1996;24:208–212. doi: 10.1007/BF01713341. [DOI] [PubMed] [Google Scholar]
- 279.Wilske B, Jauris-Heipke S, Lobentanzer R, Pradel I, Preac-Mursic V, Rössler D, Soutschek E, Johnson R C. Phenotypic analysis of outer surface protein C (OspC) of Borrelia burgdorferi sensu lato by monoclonal antibodies: relationship to genospecies and OspA serotype. J Clin Microbiol. 1995;33:103–109. doi: 10.1128/jcm.33.1.103-109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Wilske B, Preac-Mursic V, Göbel U B, Graf B, Jauris S, Soutschek E, Schwab E, Zumstein G. An OspA serotyping system for Borrelia burgdorferi based on reactivity with monoclonal antibodies and OspA sequence analysis. J Clin Microbiol. 1993;31:340–350. doi: 10.1128/jcm.31.2.340-350.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Wilske B, Preac-Mursic V, Jauris S, Hofmann A, Pradel I, Soutschek E, Schwab E, Will G, Wanner G. Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein of Borrelia burgdorferi. Infect Immun. 1993;61:2182–2191. doi: 10.1128/iai.61.5.2182-2191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Woese C R. Bacterial evolution. Microbiol Rev. 1987;51:221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Wormser G P. Prospects for a vaccine to prevent Lyme disease in humans. Clin Infect Dis. 1995;21:1267–1274. doi: 10.1093/clinids/21.5.1267. [DOI] [PubMed] [Google Scholar]
- 284.Wormser G P, Liveris D, Nowakowski J, Nadelman R B, Cavaliere F L, McKenna D, Holmgren D, Schwartz I. Association of specific subtypes of Borrelia burgdorferi with hematogenous dissemination in early Lyme disease. J Infect Dis. 1999;180:720–725. doi: 10.1086/314922. [DOI] [PubMed] [Google Scholar]
- 285.Xu Y, Johnson R C. Analysis and comparison of plasmid profiles of Borrelia burgdorferi sensu lato strains. J Clin Microbiol. 1995;33:2679–2685. doi: 10.1128/jcm.33.10.2679-2685.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Xu Y, Kodner C, Coleman L, Johnson R C. Correlation of plasmids with infectivity of Borrelia burgdorferi sensu stricto type strain B31. Infect Immun. 1996;64:3870–3876. doi: 10.1128/iai.64.9.3870-3876.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Yanagihara Y, Masuzawa T. Lyme disease (Lyme borreliosis) FEMS Immunol Med Microbiol. 1997;18:249–261. doi: 10.1111/j.1574-695X.1997.tb01053.x. [DOI] [PubMed] [Google Scholar]
- 288.Yang L, Weis J H, Eichwald E, Kolbert C P, Persing D H, Weis J J. Heritable susceptibility to severe Borrelia burgdorferi-induced arthritis is dominant and is associated with persistence of large numbers of spirochetes in tissues. Infect Immun. 1994;62:492–500. doi: 10.1128/iai.62.2.492-500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Yrjnien Y, Oksi J, Kalimo H, Viljanen M K. Program and Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C.: American Society for Microbiology; 1997. Invasiveness of different genospecies of Borrelia burgdorferi sensu lato in brain tissue; p. 351. [Google Scholar]
- 290.Zhang Z F, Duo G L, Zhang J S, Wan K L, Zhu G F, Zheng L, Wang H Y, Hou X X. Survey on tick vectors of Lyme disease spirochetes in China. Chin J Vectors Biol Control. 1992;1:140–143. [Google Scholar]
- 291.Zhang Z F, Wan K L, Zhang J S. Studies on epidemiology and etiology of Lyme disease in China. Chin J Epidemiol. 1997;18:8–11. [PubMed] [Google Scholar]
- 292.Zhong W, Stehle T, Museteanu C, Siebers A, Gern L, Kramer M, Wallich R, Simon M M. Therapeutic passive vaccination against chronic Lyme disease in mice. Proc Natl Acad Sci USA. 1997;94:12533–12538. doi: 10.1073/pnas.94.23.12533. [DOI] [PMC free article] [PubMed] [Google Scholar]