Abstract
Mesenchymal stem cells (MSCs) have been repeatedly shown to be a valuable source for cell-based therapy in regenerative medicine, including bony tissue repair. However, engraftment at the injury site is poor. Recently, it has been suggested that MSCs and other cells act through a paracrine signaling mechanism. Exosomes are nanostructures that have been implicated in this process. They carry DNA, RNA, proteins, and lipids and play an important role in cell-to-cell communication directly modulating their target cell at a transcriptional level. In a bone microenvironment, they have been shown to increase osteogenesis and osteogenic differentiation in vivo and in vitro. In the following review, we will discuss the most advanced and significant knowledge of biological functions of exosomes in bone regeneration and their clinical applications in osseous diseases.
Impact statement
Mesenchymal stem cells have been shown to be a promising tool in bone tissue engineering. Recently, it has been suggested that they secrete exosomes containing messenger RNA, proteins, and lipids, thus acting through paracrine signaling mechanisms. Considering that exosomes are nonteratogenic and have low immunogenic potential, they could potentially replace stem-cell based therapy and thus eradicate the risk of neoplastic transformation associated with cell transplantations in bone regeneration.
Keywords: exosomes, mesenchymal stromal cells, bone tissue engineering, bone defect, regenerative medicine
Introduction
Bone defects and their surgical reconstruction represent a major financial burden for worldwide health care systems.1–4 Tumors, trauma, surgery, or inflammatory diseases can lead to critical-sized bone defects, necessitating extensive surgical reconstruction. Bone has limited self-regenerating capabilities. Similarly, alternative therapeutic options for bone repair, such as autologous or allogenic transplantation or prosthetic material, come with considerable drawbacks. For instance, implantation of prosthetic material is expensive, and postoperative physical rehabilitation is time consuming and difficult for patients. Bone autografts also cause donor site morbidity, are limited in their availability, and have long operating times. Bone allografts are also disfavored as they lead to an increased immunogenic response, are expensive, and often lead to nonunion.1–4
Taking into account our rapidly aging population and an increase in obesity-related diseases, bone defects and consecutively the cost for repair and reconstruction are only going to rise in the coming years.3,5 Thus, alternative approaches to repair bone are highly sought after.
Bone repair using culture-expanded mesenchymal stem cells (MSCs) has been shown to be highly effective in bone regeneration.6–10 It was initially thought that the MSC's differentiation potential would be crucial in tissue engineering using MSCs. However, MSC engraftment and differentiation at the injury site are poor and do not correlate with the good clinical results.4,11,12 Thus, it has been proposed that MSC-derived paracrine signaling may be responsible for the tissue regeneration and not the MSCs themselves.4,11,12 In a bone regeneration environment, Osugi et al.13 showed increased expression of osteogenic markers in vitro and enhanced bone formation in calvarial defects in vivo using bone marrow stem cell (BMSC)-derived conditioned medium (CM), further supporting a paracrine signaling mechanism of MSCs. Recently, exosomes released by MSCs have been implicated to play an important role as key signaling mediators between cells.
Exosomes are biomolecular nanostructures released from a variety of different cells that play an important role in cell-to-cell communication by delivering functional biomolecules, for example, microRNA (miRNA), messenger RNA (mRNA), proteins, and lipids. Exosomes can directly modulate the function of the target cell by triggering gene transcription and therefore protein levels and are thus involved in a vast amount of physiological processes, which makes them an interesting target for clinical application and modulation of diseases.14–17 As such, applications of exosomes have shown promising results in neurodegenerative diseases,18–21 in modulating the immune response,16,18,22,23 as vehicles for antigen delivery,18,24,25 in cardiac and pulmonary disease,16,26–29 and acute kidney injury.30,31 In contrast, tumor-derived exosomes are also implicated in promoting tumor progression and metastasis16,25,32–35 and the spread of infectious diseases.14,36,37
In a bone microenvironment, exosome treatment leads to increased osteogenic potential in vitro and in vivo and has been successfully applied in a variety of osseous diseases.4,38–40 This review aims to provide an overview over the current knowledge of exosomes in the context of bone regeneration.
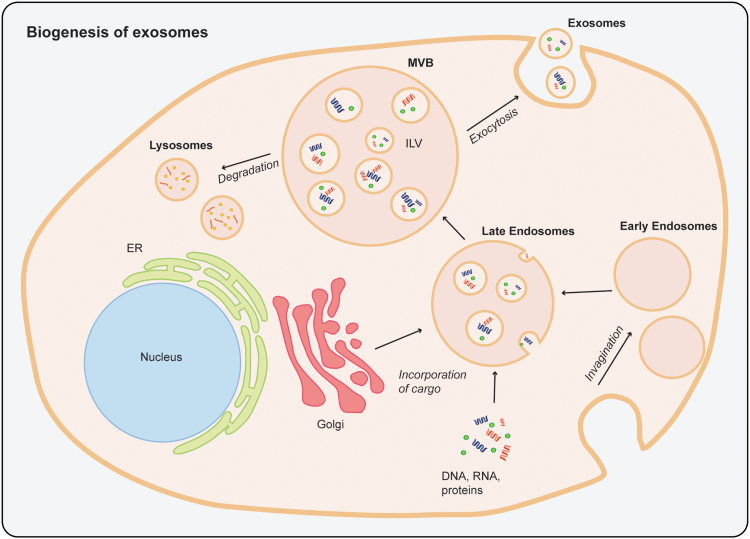
Biogenesis of Exosomes
During biogenesis of exosomes, endosomal vesicles are formed by invagination of the plasma membrane. Early endosomes develop into late endosomes and accumulate intraluminal vesicles (ILVs). These multivesicular bodies fuse with the plasma membrane and release ILVs, now exosomes, or merge with lysosomes for degradation16,41–43 (Fig. 1). After exocytosis, exosomes communicate with other cells by fusion with the plasma membrane of the target cells and release their cargo to regulate cellular processes.41 Exosomes can be isolated using a variety of different methods, most commonly differential ultracentrifugation. Characterization of exosomes can be achieved based on detection of surface protein markers, determination of size and morphology, or size distribution. Exosome biogenesis, isolation, and characterization have been described in detail elsewhere.41,44–46
FIG. 1.
Schematic of the biogenesis of exosomes. Invagination of the plasma membrane to form early endosomes. Involving the Golgi apparatus, DNA, RNA, proteins, and lipids are incorporated by invagination of the endosomal membrane to form late endosomes. A multivesicular body is formed containing multiple intraluminal vesicles. The multivesicular body is either processed by lysosomes for degradation or fuses with the cell plasma membrane to release the intraluminal vesicles, now called exosomes, into the extracellular space. This process is called exocytosis. ER, endoplasmatic reticulum; ILV, intraluminal vesicle; MVB, multivesicular body.
Exosomes in Cell-to-Cell Communication
The cargo of exosomes contains DNA, miRNA, mRNA, proteins, and lipids (Fig. 1).41,42 By delivering their cargo, exosomes are able to modify gene expression and signaling pathways of the recipient cell and are thus involved in regulation of physiological processes of the target cells, such as activation, migration, differentiation, apoptosis, or necrosis.14,16,42,47
Exosomal proteins and lipids are mainly involved in biogenesis and trafficking of exosomes and fusion with target cells but have also been shown to directly activate signaling processes in target cells.48–50
However, exosomal miRNA may be the most important regulator of cell–cell communication. MiRNAs are small noncoding RNAs that act as negative regulators of post-transcriptional gene expression. They bind to complementary sequences located in the 3′ untranslated and the 5′ end “seed” region of mRNAs.51,52 The “seed” region spans from nucleotide position 2 to 7 and plays an important role in target recognition.52 This leads to destabilization and consequent degradation of mRNA and thus suppression of expression of their target genes.51,53 It has been suggested that a majority of protein-coding genes may be controlled by miRNAs.52,54 Packaged in exosomes, miRNAs are protected from degradation by serum ribonucleases.55
Exosomes in Bone Tissue Engineering
Exosomes can be isolated from a variety of different cell sources, and exosome cargo has been shown to be reflective of function of their parent cells.16,38,40,47,55–58 Below, we will provide an overview over MSC-derived exosomes and exosomes from other parent cell sources (Tables 1 and 2).
Table 1.
Mesenchymal Stem Cell-Derived Exosomes in Bone Regeneration
| Parent cells | Target cells | Isolation | Characterization | Preconditioning of parent cells | In vitro | Animal models | In vivo |
|---|---|---|---|---|---|---|---|
| hBMSCs58–64 mBMSCs65 rBMSCs66–71,75 hBMSCs from the jaw72 Rabbit BMSCs73 hBMSCs from traumatic ONFH patients74 |
hBMSCs58,59,62,64,72 mBMSCs (MRL/lpr)65 Human osteoblasts60,75 mouse Osteoblasts70 BMSCs from SFHN rat66 rBMSCs67,69 irradiated rBMSCs71 hUVECs63,70,74 |
ExoQuick-TC58,59,62,66 UC60,61,63–65,68–73,75 Total Exosome Isolation Kit67,74 UF69,70 |
TEM58,60,62–64,66–74 DLS59,69 Atomic force microscopy59 Laser Doppler micro-electrophoresis59 Flow cytometry59,60,69 WB61–67,70–74 FTE61 NTA62–64,67,69,70,72–74 TRPS68 |
Osteogenic induction58,59,62 HIF-1α-adenovirus transfection67 Dimethyloxaloylglycine-stimulation63 miR-122-5p transfection73 |
Increased expression of osteogenic proteins/genes58–60,62,64–69,72 Increased mineralization60,64,66–70,72 Decreased adipogenesis66 Increased angiogenesis63,64,70,74 Increased proliferation68,70,75 Increased migration70 Reduced oxidative stress, accelerated DNA repair, attenuated inhibition of proliferation, and osteogenic differentiation potential in irradiated BMSCs71 |
Implantation in the back of immunocompromised mice58,62 IV injection in a mouse model of SLE65 Calvarial bone defect in rats60,63,64and mice72 Femur fracture in mice 61 and rats69,70 ONFH model in rats67,74 and rabbits73 Tibial distraction model in rats68 Radiation-induced bone loss in rats71 |
Increased vascularization58,63,64,70,73,74 Increased bone formation58,60,61,63–74 Increased expression of osteogenic proteins/genes58,62,65,69,70 Increased expression of angiogenic proteins/genes58,70 Reduced oxidative stress71 Prevention of bone mineral loss67,73,74 |
| hASCs76,77 | hBMSC76,77 | UF76 UC76,77 |
TEM76,77 NTA76,77 WB76,77 |
Osteogenic differentiation76 miR-375 lentiviral transfection77 |
Increased proliferation76 Increased migration76 Increased mineralization76 Increased expression of osteogenic proteins/genes76 |
Calvarial bone defects in mice76 and rats77 | Increased bone formation76,77 Increased expression of osteogenic proteins/genes76 |
| Synovial-derived MSCs78 | hBMSCs Treated and untreated with steroids78 |
UF78 UC78 |
DLS78 TEM78 WB78 |
Increased proliferation in untreated BMSCs78 Inhibition of decreased proliferation of steroid treated BMSCs78 Inhibition of apoptotic effects78 |
ONFH model in rats78 | Prevention of bone mineral loss and cystic degeneration78 Increased bone formation78 |
|
| hiPSC-MSCs79–81 | rBMSC-OVX80 hBMSCs81 hUVECs79 |
UF80,81 UC79–81 |
TRPS80,81 WB79–81 TEM79,81 NTA79 |
Increased proliferation79–81 Increased mineralization80,81 Increased expression of osteogenic proteins/genes80,81 Increased migration79 Increased tube formation79 |
Calvarial bone defects in OVX rats80 and rats81 ONFH model in rats79 |
Increased bone formation80,81 Increased expression of osteogenic proteins/genes80,81 Increased vascularization79,80 Increased expression of angiogenic proteins/genes80 Prevention of bone mineral loss79 |
|
| human Wharton's jelly of umbilical cord MSCs82 | Mouse osteocyte-like cells treated with steroids82 | exoEasy Maxi Kit82 | TEM82 DLS82 WB82 |
Inhibition of decreased proliferation of steroid-treated cells82 Inhibition of apoptotic effects82 |
ONFH model in rats82 | Prevention of bone mineral loss82 | |
| hucMSCs83–87 | hUVECs83,84 human Osteoblasts83 mouse Osteoblasts84 rBMSCs from DOP model87 |
UC83–87 UF83,84,86 |
TEM83–87 WB83–87 NTA83,87 DLS84 |
Hypoxic conditions83 | Increased expression of angiogenic proteins/genes83,84 Increased proliferation83,84 Increased migration83,84 Increased tube formation83,84 Inhibition of decreased proliferation in DOP87 Inhibition of apoptotic effects in DOP87 |
ONFH model in rats85 Femur fracture in mice83 and rats84,86 DOP model in rats87 |
Increased bone formation83–87 Inhibition of apoptotic effects85,87 Increased vascularization84,85 Increased expression of osteogenic proteins/genes86 |
| hMSCs51 | hMSCs51 | UC51 UF51 |
TEM51 NTA51 |
Osteogenic induction51 | Increased mineralization51 |
ASC, adipose tissue-derived MSC; BMSC, bone marrow stem cell; DLS, dynamic light scattering; DOP, disuse osteoporosis; FTE, high-resolution frequency transmission electric-field imaging; h, human; HIF-1α, hypoxia-inducible factor-1 alpha; hiPSC, human induced pluripotent stem cell; hucMSC, human umbilical cord-derived MSC; hUVEC, human umbilical vein endothelial cell; IV, intravenous; m mouse; MRL, Murphy Roths Large; MSC, mesenchymal stem cell; NTA, nanoparticle tracking analysis; ONFH osteonecrosis of the femoral head; OVX, ovariectomy; r, rat; SLE, systemic lupus erythematosus; TEM, transmission electron microscopy; TRPS, tunable resistive pulse sensing; UC, ultracentrifugation; UF, ultrafiltration; WB, western blot.
Table 2.
Exosomes Derived from Other Parent Cell Sources in Bone Regeneration
| Parent cells | Target cells | Isolation | Characterization | Preconditioning of parent cells | In vitro | Animal models | In vivo |
|---|---|---|---|---|---|---|---|
| hMSC-derived adipocytes96 | hMSC-derived osteocytes96 | UC96 | TEM96 | Transfer of adipogenic mRNAs96 Increased expression of adipogenic genes96 |
|||
| Mouse dendritic cells | UC96 UF96 |
WB96 NTA96 TEM96 |
Treatment with TGF-β1 and IL1096 | Inhibition of inflammatory alveolar bone loss97 | |||
| Osteoclasts from miR-214 knock-in mouse92,93 Osteoclasts from osteoporotic patients and mice92 Mouse osteoclasts89,92,93 Mouse osteoclast precursors89 |
Mouse osteoblasts92,93 Osteoblasts from OVX-mice92 1,25(OH)2D3-stimulated mouse marrow89 |
UC92,93 ExoQuick-TC89 |
WB89,93 NTA93 FACS92 DLS92 TEM89 |
miR214-mimic and antagomir transfection92,93 | Osteoclast-derived miR-214 inhibits osteoblast activity92,93 Exosomes from osteoclast precursors stimulate osteoclastogenesis, Exosomes from osteoclasts inhibit osteoclastogenesis89 |
IV-injection in WT mouse93 OVX-induced osteoporotic mouse model92,93 |
Osteoclast-derived miR-214-3p inhibits bone formation and expression of osteogenic markers in WT mice93 Inhibition of osteoclast-derived miR-214-3p promotes bone formation in OVX mice92,93 Inhibition of exosome release in osteoclasts from OVX mice leads to increased bone formation and promotes osteoblast activity92 |
| Mouse osteoblasts88,90,91 | Mouse osteoclasts88 mBMSCs90 Mouse macrophages91 |
UC88 Exoquick-TC90 |
WB88 TEM88 SEM90 NTA91 |
PTH88 Imipramine91 |
Enhanced RANK-mediated osteoclast precursor differentiation88 Increased expression of osteogenic proteins/genes90 Increased mineralization90 Inhibition of osteoclast differentiation and activation91 |
OVX-induced osteoporotic mouse model91 | Inhibition of decrease of bone mineral density91 Increased bone volume91 |
| Mouse osteocytes94 | Mouse osteoblasts94 | UC94 | WB94 TEM94 |
Myostatin94 | Decreased expression of osteogenic proteins/genes94 Inhibition of osteoblastic differentiation and activity94 |
||
| Human monocytes95 | hBMSCs95 | UC95 | Flow cytometry95 WB95 |
Increased expression of osteogenic proteins/genes95 |
FACS, fluorescence-activated cell sorting; IL10, interleukin 10; mRNA, messenger RNA; PTH, parathormon; SEM, scanning electron microscopy; TGF, transforming growth factor; WT, wild type.
MSC-derived exosomes
BMSC-derived exosomes
In bone regeneration, the most widely used source of exosome parent cells is BMSCs,58–74 including human BMSCs (hBMSC),58–64,72,74 mouse BMSCs,65 rat BMSCs,66–71,75 and rabbit BMSCs.73 Target cells for in vitro experiments include hBMSCs,58,59,62,64,72 mouse BMSCs (mBMSC),65 rat BMSCs,61,66,67,69 human60,75 and mouse70 osteoblasts, and human umbilical vein endothelial cells (HUVECs).63,70,74
Treatment with BMSC-derived exosomes led to increased expression levels of osteogenic growth factors and bone-related proteins, such as Osteopontin (OPN), Runt-related transcription factor 2 (RUNX2), collagen type 1 (COL1), transcription factors, for example, RUNX2, bone morphogenetic protein (BMP) 9, transforming growth factor (TGF)-β1, alkaline phosphatase (ALP), collagen type 1 alpha 1 (COL1A1), and extracellular matrix molecules,58–60,62,64–69,72 as well as increased calcium deposition and matrix mineralization60,64,66–70,72 in vitro. Moreover, three studies indicated increased proliferation68,70,75 and migration70 after exosome treatment in vitro. BMSC-derived exosomes have also been applied in several in vivo experiments. Increased bone formation and enhanced expression levels of osteogenic markers have been shown after implantation of BMSC-derived exosomes on the back of immunocompromised mice,58,62 in rat60,63,64 and mice72 calvarial defects, in a mouse61 and rat69,70 femur fracture, in a model of osteonecrosis of the femoral head (ONFH) in rats67,74 and rabbits,73 in a tibial distraction model in rats,68 in a rat model of radiation-induced bone loss,71 and after intravenous injection in a mouse model of systemic lupus erythematosus (SLE).65
Adipose tissue-derived exosomes (ASC)
Li et al.76 harvested exosomes from osteogenically induced hASCs and showed increased ALP staining and activity and cell matrix mineralization and enhanced expression of osteoblastogenesis-related genes (RUNX2, ALP, COL1A1) in osteogenically induced hBMSCs. Respectively, they showed increased bone formation and enhanced expression of key osteogenic markers in a mouse calvarial defect treated with a PGLA scaffold with hASC-derived exosomes. After lentiviral transfection of hASCs, Chen et al.77 isolated miR-375-overexpressing exosomes and were also able to show increased osteogenic potential in hBMSC-target cells in vitro and enhanced bone formation in rat calvarial defects in vivo compared to an untreated control group.77
Other MSC-derived exosomes
Imitating steroid-induced bone loss, Guo et al.78 could show inhibition of decreased proliferation and reduced apoptotic effects in hBMSCs after treatment with exosomes from synovial-derived MSCs. In a rat model of steroid induced ONFH, treatment with exosomes led to reduced ONFH.78
Liu et al.79 confirmed these findings in a similar animal model using human induced pluripotent stem cell (hiPSC)–MSC-derived exosomes and also showed increased tube formation in HUVECs in vitro and increased vascularization in vivo. hiPSCs are reprogrammed cells that can be used to create case-specific embryonic stem cells (ESCs) and can differentiate into every cell type. Moreover, they display lower immunogenic potential and do not raise ethical concerns as the use of ESCs does,80 which makes them an ideal parent cell for isolation of exosomes. Treatment with hiPSC-MSC-derived exosomes led to increased proliferation, ALP activity, and matrix mineralization, as well as enhanced expression of osteogenic genes and proteins in BMSCs from ovariectomized rats80 and hBMSCs81 in vitro and increased bone formation and angiogenesis in rat calvarial defects in vivo.
Exosomes can also be isolated from human umbilical cord-derived MSCs (hucMSCs). Kuang et al.82 isolated exosomes from Wharton's jelly of hucMSCs and were able to attenuate decreased proliferation and apoptotic effects in steroid-treated murine osteocyte like cells. In a rat model of steroid-induced ONFH, treatment with exosomes led to reversed bone loss and prevented osteonecrosis.82 In HUVECs, treatment with hucMSC-derived exosomes led to increased expression of angiogenic markers, increased migration, and tube formation, but did not lead to changes in expression of osteogenic marker genes in osteoblasts.83,84 In a steroid-induced model of ONFH in rats85 and a femoral fracture model in mice83 and rats,84,86 hucMSC-exosome treatment led to increased neovascularization and enhanced bone regeneration in vivo. Yang et al.87 created a rat model of disuse osteoporosis by hind limb unloading. In hence isolated rat BMSCs, they demonstrated a decrease of apoptosis-related proteins and a rescue of decreased proliferation in vitro and an increase in bone volume in vivo after treatment with hucMSC-derived exosomes.87
Wang et al.51 isolated MSC-derived exosomes in different stages of osteogenic induction and demonstrated increased ALP activity, increased calcium and phosphate deposition, and enhanced matrix mineralization in hMSCs after exosome treatment in vitro; however, they did not further specify the parent cell source.
Exosomes from other parent cell sources
The osteoblast–osteoclast interaction is very important in maintenance of bone homeostasis. Receptor activator of nuclear factor kappa-B ligand (RANKL) is a transmembrane protein, expressed by osteoblasts, that binds to the receptor RANK on monocytes to initiate their differentiation into osteoclasts.38,88,89 Mature osteoclasts continuously require RANKL stimulation to resorb bone.89 Deng et al.88 demonstrated that osteoblasts release microvesicles containing RANKL protein, thus stimulating osteoclast differentiation in vitro. Cui et al.90 showed an upregulation of osteogenic marker genes and enhanced matrix mineralization in mBMSCs after treatment with exosomes derived from mineralizing osteoblasts. Deng et al.91 decreased generation of osteoblast-derived exosomes and thus serum levels of RANKL through imipramine and thus were able to inhibit osteoclast differentiation and activation in vitro and attenuate decreased bone mineral density and show increased bone volume in ovariectomized mice in vivo.
In contrast, exosomes derived from osteoclasts containing miR-214 have been shown to inhibit osteoblast activity in vitro92,93 and decreased bone formation and expression of osteogenic marker genes in vivo.93 Respectively, inhibition of exosome release from osteoclasts led to increased bone mineral density and upregulated osteoblast activity in ovariectomized mice.92 Huynh et al.89 demonstrated that exosomes derived from osteoclast precursors stimulated osteoclastogenesis, whereas exosomes derived from mature osteoclasts inhibited osteoclastogenesis.
Similarly, exosomes derived from osteocytes led to inhibition of osteoblast differentiation and activity in osteoblasts in vitro,94 whereas exosomes derived from human monocytes led to increased expression of osteogenic marker genes in hBMSCs in vitro.95
Martin et al.96 investigated the interaction between adipocytes and osteoblasts and the paracrine mechanisms influencing MSC differentiation. They showed that hMSC-derived adipocytes produce extracellular vesicles containing adipogenic miRNAs decreasing osteoblast marker expression in osteocytes.96 This is important in the context of bone loss in osteoporosis and the accompanying accumulation of adipocytes in the bone marrow, leading to an increased fracture risk.96
Other promising cell sources for isolation of exosomes and application in a bone microenvironment include dendritic cells. Dendritic cells are major regulators of the immune response, including direct modulation of T cells.97 Elashiry et al.97 loaded dendritic cell-derived exosomes with TGF-β1 and interleukin 10 (IL10), which were taken up by dendritic cells and T cells in vivo. Exosome treatment led to inhibition of maturation of dendritic cells and suppression of Th17 effector cells and thus stimulation of regulatory T cells, ultimately leading to inhibition of bone loss in inflammatory alveolar bone loss.97
The impact of tissue origin of exosomes
Interestingly, exosomes derived from naive MSCs can influence osteogenesis in target cells in different ways depending on their parent cell and can induce lineage-specific changes in naive MSCs in vitro, as well as in vivo.58,60,72,98 Narayanan et al.58 investigated whether exosomes derived from osteogenic MSCs were able to induce osteogenic differentiation of naive BMSCs. Thus, they compared the gene expression profiles in BMSCs treated with exosomes from noninduced BMSCs and with exosomes derived from BMSCs upon osteogenic differentiation. They were able to show that both regular and osteogenically induced exosomes led to a significant upregulation in expression levels of BMP9 and TGF-β1 in vitro and increased calcium phosphate nucleation in vivo, which was however more pronounced in the induced exosomes.58 Qin et al.60 have also shown upregulation of three important miRNAs in BMSC-derived extracellular vesicles and have identified miR-196a as a key player in osteoblastic differentiation and expression of osteogenic genes. Baglio et al.98 characterized the small RNA content of hASC- and hBMSC-derived exosomes, investigating whether they differ respective to their parent cell. Interestingly, they found that the exosomal RNA content is not reflective of RNA content of parent cells, while miRNAs and transfer RNAs indicative of the parent cell origin were selectively packaged into exosomes.98 Interestingly, in a recent study, Li et al.72 demonstrated that exosomes isolated from neural crest-derived BMSCs from the jaw enhanced osteogenic differentiation of mesoderm-derived BMSCs from the ilium in a coculture in vitro. In a calvarial defect in vivo, they were able to show increased bone formation upon treatment with iliac BMSCs cultured with jaw BMSC exosomes compared to BMSCs cultured with iliac BMSC exosomes, thus supporting packaging of cell-specific signaling molecules into exosomes.72
In addition to undifferentiated MSC-derived exosomes displaying certain characteristics of their parent cells, multiple studies have shown increased osteogenic effects in vitro and in vivo after treatment with exosomes derived from osteogenically differentiating hBMSCs53,58,59,62 and hASCs.76
Besides the osteogenic lineage commitment, previous studies have also shown specific alterations in exosomal cargo upon induction of adipogenic and chondrogenic differentiation in hBMSCs.62,96
Thus, exosomal cargo is highly dependent on tissue origin of parent cells and can induce lineage-specific changes in target cells.
The mechanisms of MSC-derived exosomes in bone regeneration
The pro-osteogenic effect of MSC-derived exosomes on bone regeneration may be due to (1) direct modulation of the osteogenic differentiation process of the neighboring target cells53,60,61,99,100 and (2) stimulation of angiogenesis and thus optimizing the microenvironment to create ideal conditions for bone regeneration.13,64,101–103
Exosomes modulate the osteogenic differentiation process of target cells
Exosomes have been shown to carry specific osteogenic proteins, extracellular matrix proteins, and bone-related miRNAs.53,61,67 Parent cells can be modified to express specific proteins and miRNAs, which allow targeted delivery of stimulating factors to neighboring cells.63,67,73,77,83,88,92–94,97
Xu et al.53 demonstrated significant upregulation of seven miRNAs and downregulation of five miRNAs in BMSC-derived exosomes upon osteogenic induction that play important roles in modulating osteogenesis in target MSCs. For example, miR-218 enhances Wnt signaling by downregulating Wnt antagonists DKK2 and SFRP2 in hASCs thus leading to increased bone formation.99 Furuta et al.61 have demonstrated reduced fracture healing and delayed callus formation due to impaired endochondral ossification in CD9−/− mice that are known for decreased exosome production compared to wild-type mice. They were able to rescue this effect by injection of BMSC-derived exosomes and also found increased bone union rates in exosome-treated fractures in wild-type mice compared to untreated fractures.61 Interestingly, levels of cytokines monocyte chemoattractant protein (MCP)-1, MCP-3, and stromal cell derived factor (SDF)-1 promoting osteogenesis and angiogenic factors were lower in MSC-derived exosomes than in CM. However they could show enhanced levels of miR-21 in both CM, as well as exosomes.61 miR-21 promotes osteogenic differentiation and increases matrix mineralization by directly repressing expression of Smad7 by inhibition of translation.61,104
Parent cells can also be genetically modified or medically or environmentally stimulated to manipulate cargo load of exosomes: hypoxia-inducible factor-1α (HIF-1α) is an important factor regulating osteogenesis and angiogenesis under hypoxic conditions by promoting expression of osteogenic and angiogenic marker genes.67 Treatment with exosomes secreted by BMSCs overexpressing HIF-1α after adenovirus transfection led to increased expression of osteogenic markers, such as osteocalcin (OCN) and ALP, in vitro and enhanced trabecular density in a steroid-induced ONFH model in vivo.67 Similarly, Liu et al.83 demonstrated increased bone fracture healing in mice after treatment with exosomes secreted in hypoxic conditions through HIF-1α activation and subsequent packaging of miR-126 into exosomes.
Further studies have shown increased osteogenic effects in vitro and in vivo in gain-of-function experiments with parent cells overexpressing miR-122-5p73 or miR-375.77 On the contrary, overexpression of miR-214 in osteoclasts led to downregulation of osteoblast activity in vitro and decreased bone formation in vivo.92,93
Treatment of dendritic cells with immunomodulatory TGF-β1 and IL10 led to selective packaging into exosomes and resulted in an inhibition of inflammatory alveolar bone loss.97 Treatment of osteoblasts with parathormon (PTH) resulted into an exosome-mediated enhancement of osteoclast precursor differentiation,88 whereas treatment of osteocytes with Myostatin led to decreased osteoblastic differentiation potential and activity through downregulation of miR-218 in exosomes.94
In conclusion, exosomal miRNAs regulate osteogenic differentiation by activating or deactivating different cellular signaling pathways and thus modulating gene transcription and expression of their target proteins. They also function as vehicles for delivery of important signaling molecules by protecting them from degradation.
Stimulation of angiogenesis by exosomes in bone formation
Bone regeneration is highly dependent on angiogenesis. A sufficient blood supply is essential for bone growth, but also vascular endothelial growth factor (VEGF) acts directly on vascular endothelial cells, which in turn stimulates osteoblast activity and maturation.64 MSC-derived exosomes and conditioned media contain angiogenesis-enhancing factors, thus creating an optimal niche for bone regeneration.40,63,64,67,69,70,73,74,79,80,83,101–103
It is well known that hypoxic conditions lead to stimulation of angiogenesis through activation of the HIF-1α complex and subsequent translocation in the nucleus and transcription of angiogenic marker genes.63 Thus, multiple studies have shown that treatment with exosomes derived from cells in hypoxic conditions or hypoxia-simulated conditions led to increased tube formation, increased proliferation and migration in HUVECs, and increased neovascularization in animal models in vivo.63,67,83 Moreover, several authors showed increased expression of osteogenic and angiogenic factors in exosome-treated BMSCs,64 increased proliferation and migration in exosome-treated HUVECs70,74,79 in vitro, and increased bone formation and neovascularization64,69,70,79,80 in vivo. Takeuchi et al.64 further supported their findings by reversing the pro-angiogenic effect of BMSC-derived exosomes by adding a VEGF inhibitor. Xu et al.69 identified miR-224-3p as a key regulator in angiogenesis in bone formation and were able to demonstrate increased angiogenesis by downregulation of miR-224-3p through inhibition of downregulation of target gene FIP200 (focal adhesion kinase family interacting protein of 200 kDa).
Exosomes in Osseous Diseases
Exosomes have been shown to be effective in the treatment of various skeletal diseases involving bone tissue, such as osteomyelitis,105 SLE,65 ONFH,66,67,73,74,78,79,82,85 bone fractures,61,69,70,83,84,86 osteoporosis,80,87,91,92 and radiation-induced bone loss.71
Osteomyelitis is an inflammatory process that ultimately leads to bone destruction.106 The most common microorganism causing this disease is Staphylococcus aureus.106 Besides its extracellular localization, S. aureus can also be found intracellularly colocalizing with lysosomes. Due to this intracellular localization, therapeutic efficacy of antibiotic treatment has been shown to be limited.105 Yang et al. showed that by incorporating linezolid into macrophage-derived exosomes, they could enhance the efficacy of the antibiotic in an intracellular environment in vitro and in vivo.105
SLE is an autoimmune disease that, among others, affects bone tissue and can cause osteopenia. Liu et al.65 demonstrated rescue of impaired osteogenic differentiation in BMSCs derived from a mouse model of SLE after coculture with wild-type BMSC, through alteration of miR-29b and Notch1 levels. They were able to show a reversal of this rescue effect after knockdown rab27a, thus blocking exosome secretion, in vitro and in vivo.65 Respectively, they confirmed their results after direct treatment of SLE-BMSCs in vitro and in vivo with BMSC-derived exosomes, showing reduced levels of miR-29b, downregulation of Notch, increased osteogenic differentiation, and bone formation.65 Thus, they identified exosomes as an important target for pharmaceutical approach to improve osteopenia in SLE.65
Long-term steroid use, osteoporosis, or trauma can ultimately lead to reduced blood supply to the femoral head and thus cause death of osteocytes resulting in collapse of the femoral head and hip joint pain and dysfunction.66,73,74 Resulting ONFH represents a major burden for the health care system resulting in total hip arthroplasty in 65% of patients.66,73 Multiple authors were able to demonstrate decreased fatty degeneration of the bone marrow, reduced apoptotic effects, increased osteogenic differentiation, and increased tube formation, proliferation, and migration in vitro66,67,73,74,78,79,82 and increased bone regeneration and neovascularization in vivo67,73,74,78,79,82,85 after exosomal treatment.
Impaired fracture healing due to reduced blood supply, insufficient immobilization, infection, and other causes leads to nonunion or delayed union in about 10% of cases with numbers increasing with an aging population.61,69,107,108 Thus, new pharmacological treatment approaches are much needed. Furuta et al.61 were able to identify MSC-derived exosomes as important regulators in tissue repair process by showing reduced fracture healing in mice secreting reduced levels of exosomes. Xu et al.69 demonstrated significantly reduced fracture healing in exosomes derived from MSCs from aged rats and were able to attenuate this effect by treatment with an miR-128-3p inhibitor. Multiple authors were able to show increased fracture healing treated with exosomes derived from naive MSCs70,84,86 and hypoxic MSCs.83,108
Osteoporosis, one of the most problematic challenges in orthopedic surgery, is common in our aging society, but can also be caused by trauma, infection, skeletal defects, or tumors.80,107 Ovariectomy (OVX) in mice or rats represents a very commonly used model for osteoporosis in research. Qi et al.80 were able to show enhanced cell proliferation and increased expression of osteogenic markers after treatment of OVX-BMSCs with hiPSC-MSC-derived exosomes. In vivo, they demonstrated increased bone regeneration and angiogenesis in a calvarial defect model.80 Sun et al.92 identified exosomes as vehicles for transfer of miR-214 and were able to attenuate impaired osteoblast activity in ovariectomized rats by blocking the secretion of osteoclast-derived exosomes through rab27, thus blocking the transfer of miR-214. Yang et al.87 demonstrated rescue of decreased proliferation and decreased apoptosis-related proteins in rat BMSCs from a disuse osteoporosis model in vitro and attenuated decreased trabecular thickness and increased bone volume in vivo after treatment with hucMSC-derived exosomes. Deng et al.91 demonstrated that treatment with imipramine led to reduced production of osteoblast-derived exosomes and thus decreased serum levels of RANKL, which ultimately protected against bone loss in OVX mice.
Similar to changes in osteoporosis, radiation-induced bone loss is also characterized by fatty marrow and is a common cause for bone fractures in cancer patients.71 It has been hypothesized that radiation causes damage of the DNA, increases reactive oxygen species, and promotes cell aging of BMSCs, thus reducing the proliferative and differentiative capacity of BMSCs.71 Zuo et al.71 were able to attenuate radiation-induced bone loss after treatment with BMSC-derived exosomes, thus reducing oxidative stress, accelerating DNA repair, and restoring impaired proliferation differentiation in irradiated BMSCs in vitro and reducing bone loss and increasing bone volume in vivo.
Exosomes in Cartilage Repair
Osteoarthritis (OA) is a chronic rheumatic joint disease affecting about 250 million people worldwide.109–111 It mainly occurs in the hip and knee joint and is characterized by cartilage degeneration, bone sclerosis, formation of osteophytes, synovial inflammation, and calcification of menisci and ligaments.109,110,112 Current treatment is essentially directed at relieving symptoms, such as anti-inflammatory pain medication; however there is no treatment available to repair the damaged cartilage tissue.109,110,112 Recently, MSC-derived exosomes have shown promise in cartilage repair: In a recent review, Tan et al.111 identified 13 studies describing chondroprotective effects and increased cartilage regeneration after treatment with MSC-derived exosomes in animal models of OA and osteochondral defects.112–124 In addition, Wong et al.125 further improved the efficacy of exosome treatment in a rabbit osteochondral defect by adding hyaluronic acid. In a model of temporomandibular joint OA, Zhang et al.126 demonstrated an early suppression of inflammation followed by a proliferative phase with increased matrix synthesis after exosome treatment. Toh et al.109 propose that MSC-derived exosomes restore cartilage by modulating the immune response, increasing the anabolic activity and matrix synthesis of cells, and regulating homeostasis in bioenergetics in target cells. Moreover, exosomes carry a unique set of miRNAs that are also known to be potent regulators of chondrogenesis, such as miR-23b, miR-92a, miR-125b, and miR-320.109,127 A recent review has further described potential applications of exosomes in OA.107
Exosomes in Tendon Repair
Like cartilage, tendon also shows limited regeneration abilities and typically exhibits fibrotic healing after injury, thus restricting movement and bearing a high risk of re-tear.128–131 Tendinopathy or tendon injuries are, however, very common disorders often affecting professional athletes and other people frequently undergoing physical activities.128,131 Several studies demonstrated increased expression of tenogenic marker genes and increased proliferation and migration in vitro and/or increased tendon injury healing in vivo after treatment with tendon stem cell-derived exosomes,128,132 tenocyte derived exosomes,133 ASC-derived exosomes,134 and BMSC-derived exosomes.135,136 The positive effect of exosome treatment in tendon injury has also been ascribed to a reduced pro-inflammatory response in the early stages of tendon repair.132,136–138 Cui et al.130 recently showed that the fibrotic healing response in tendon injury is mainly mediated by macrophage-derived exosomes containing miR-21-5p. They were able to reduce the peritendinous fibrosis after injury in macrophage-depleted mice, identifying exosomes as an important potential target for pharmacological approaches to improve tendon healing.130 Treatment with hucMSC-derived exosomes also led to reduced fibrosis after tendon injury, which was ascribed to a decreased expression of miR-21a-3p in the exosomal cargo.138 A recently published review further analyzes and describes the important mechanism leading to increased tendon healing after exosomal treatment.131
Future Perspectives
The adaptability of exosomes in their interaction with target cells makes them a promising tool for translational research purposes. Especially, exosomal mRNA and miRNA display major reprogramming capacity for modulation of the function of recipient cells.18
Most importantly, exosomes can be applied in targeted drug delivery, for example, manipulation of exosomes allows enhancement of the bodily response to fight tumor or infection by direct loading of antigenic peptide.18,25,139,140 Furthermore, exosomes can be loaded with specific miRNAs to modulate the response of target cells on a transcriptional level. This could be applied in a regenerative environment, such as enhancement of proliferation, migration, or differentiation of recipient cells. In bone regeneration, it is hypothesized that many key players regulating osteogenesis are targets of bone-derived exosomes57,141 and can thus be altered in such a way to promote ossification.
Given their complex biochemical activities, exosomes represent an appealing tool for clinical applications. Considering that they are nonteratogenic and have low immunogenic potential, exosomes could potentially replace stem-cell based therapy and thus eradicate the risk of neoplastic transformation associated with cell transplantations.
In a bone microenvironment, exosomes can be modified to trigger specific changes in the transcriptomic profile of target cells and deliver important growth factors, thus creating an optimized niche to allow bone regeneration.
However, to make the step of standardizing the use of exosomes in a large-scale clinical setting, a uniform method to isolate a highly pure population of exosomes, that is easy and quick to handle, as well as standardized characterization and identification of exosomes, needs to be developed.
Authors' Contributions
J.H. performed literature research and writing of the article. M.F.G., M.T.L., and N.Q. contributed to editing and writing of the article. All authors who participated in the creation of this article are listed.
Disclosure Statement
The authors have no conflict of interest to declare.
Funding Information
This work was supported by the German Research Foundation (HU 2817/1-1) to J.H. and NIH (R01 DE026730; R01 DE027323) to M.T.L.
References
- 1. O'Keefe, R.J., and Mao, J.. Bone tissue engineering and regeneration: from discovery to the clinic—an overview. Tissue Eng Part B Rev 17, 389, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. González Díaz, E.C., Shih, Y.V., Nakasaki, M., Liu, M., and Varghese, S.. Mineralized biomaterials mediated repair of bone defects through endogenous cells. Tissue Eng A 24, 1148, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amini, A.R., Laurencin, C.T., and Nukavarapu, S.P.. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng 40, 363, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tan, S.H.S., Wong, J.R.Y., Sim, S.J.Y., et al. Mesenchymal stem cell exosomes in bone regenerative strategies-a systematic review of preclinical studies. Mater Today Bio 7, 100067, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baroli, B. From natural bone grafts to tissue engineering therapeutics: brainstorming on pharmaceutical formulative requirements and challenges. J Pharm Sci 98, 1317, 2009. [DOI] [PubMed] [Google Scholar]
- 6. Wallner, C., Abraham, S., Wagner, J.M., et al. Local application of isogenic adipose-derived stem cells restores bone healing capacity in a type 2 diabetes model. Stem Cells Transl Med 5, 836, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Im, G.I., Shin, Y.W., and Lee, K.B.. Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthr Cartil 13, 845, 2005. [DOI] [PubMed] [Google Scholar]
- 8. Liu, T.M., Martina, M., Hutmacher, D.W., Hui, J.H., Lee, E.H., and Lim, B.. Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells 25, 750, 2007. [DOI] [PubMed] [Google Scholar]
- 9. Hosseini, S., Shamekhi, M.A., Jahangir, S., Bagheri, F., and Eslaminejad, M.B.. The robust potential of mesenchymal stem cell-loaded constructs for hard tissue regeneration after cancer removal. Adv Exp Med Biol 1084, 17, 2019. [DOI] [PubMed] [Google Scholar]
- 10. Murray, I.R., West, C.C., Hardy, W.R., et al. Natural history of mesenchymal stem cells, from vessel walls to culture vessels. CMLS 71, 1353, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Katsha, A.M., Ohkouchi, S., Xin, H., et al. Paracrine factors of multipotent stromal cells ameliorate lung injury in an elastase-induced emphysema model. Mol Ther 19, 196, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Caplan, A.I., and Dennis, J.E.. Mesenchymal stem cells as trophic mediators. J Cell Biochem 98, 1076, 2006. [DOI] [PubMed] [Google Scholar]
- 13. Osugi, M., Katagiri, W., Yoshimi, R., Inukai, T., Hibi, H., and Ueda, M.. Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng Part A 18, 1479, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lai, R.C., Yeo, R.W., and Lim, S.K.. Mesenchymal stem cell exosomes. Semin Cell Dev Biol 40, 82, 2015. [DOI] [PubMed] [Google Scholar]
- 15. Yu, B., Zhang, X., and Li, X.. Exosomes derived from mesenchymal stem cells. Int J Mol Sci 15, 4142, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kourembanas, S. Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu Rev Physiol 77, 13, 2015. [DOI] [PubMed] [Google Scholar]
- 17. Raposo, G., and Stoorvogel, W.. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200, 373, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pitt, J.M., Kroemer, G., and Zitvogel, L.. Extracellular vesicles: masters of intercellular communication and potential clinical interventions. J Clin Invest 126, 1139, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zappulli, V., Friis, K.P., Fitzpatrick, Z., Maguire, C.A., and Breakefield, X.O.. Extracellular vesicles and intercellular communication within the nervous system. J Clin Invest 126, 1198, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goldie, B.J., Dun, M.D., Lin, M., et al. Activity-associated miRNA are packaged in Map1b-enriched exosomes released from depolarized neurons. Nucleic Acids Res 42, 9195, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chivet, M., Hemming, F., Pernet-Gallay, K., Fraboulet, S., and Sadoul, R.. Emerging role of neuronal exosomes in the central nervous system. Front Physiol 3, 145, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Raposo, G., Nijman, H.W., Stoorvogel, W., et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med 183, 1161, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thery, C., Regnault, A., Garin, J., et al. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol 147, 599, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Testa, J.S., Apcher, G.S., Comber, J.D., and Eisenlohr, L.C.. Exosome-driven antigen transfer for MHC class II presentation facilitated by the receptor binding activity of influenza hemagglutinin. J Immunol (Baltimore, MD: 1950) 185, 6608, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Robbins, P.D., Dorronsoro, A., and Booker, C.N.. Regulation of chronic inflammatory and immune processes by extracellular vesicles. J Clin Invest 126, 1173, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mackie, A.R., Klyachko, E., Thorne, T., et al. Sonic hedgehog-modified human CD34+ cells preserve cardiac function after acute myocardial infarction. Circ Res 111, 312, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sahoo, S., Klychko, E., Thorne, T., et al. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circ Res 109, 724, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee, C., Mitsialis, S.A., Aslam, M., et al. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation 126, 2601, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liang, O.D., Mitsialis, S.A., Chang, M.S., et al. Mesenchymal stromal cells expressing heme oxygenase-1 reverse pulmonary hypertension. Stem Cells 29, 99, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bruno, S., Grange, C., Deregibus, M.C., et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol 20, 1053, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhou, Y., Xu, H., Xu, W., et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther 4, 34, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Taylor, D.D., Gercel-Taylor, C., Lyons, K.S., Stanson, J., and Whiteside, T.L.. T-cell apoptosis and suppression of T-cell receptor/CD3-zeta by Fas ligand-containing membrane vesicles shed from ovarian tumors. Clin Cancer Res 9, 5113, 2003. [PubMed] [Google Scholar]
- 33. Costa-Silva, B., Aiello, N.M., Ocean, A.J., et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol 17, 816, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zomer, A., Maynard, C., Verweij, F.J., et al. In Vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell 161, 1046, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vakhshiteh, F., Atyabi, F., and Ostad, S.N.. Mesenchymal stem cell exosomes: a two-edged sword in cancer therapy. Int J Nanomed 14, 2847, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bukong, T.N., Momen-Heravi, F., Kodys, K., Bala, S., and Szabo, G.. Exosomes from hepatitis C infected patients transmit HCV infection and contain replication competent viral RNA in complex with Ago2-miR122-HSP90. PLoS Pathogens 10, e1004424, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fevrier, B., Vilette, D., Laude, H., and Raposo, G.. Exosomes: a bubble ride for prions? Traffic (Copenhagen, Denmark) 6, 10, 2005. [DOI] [PubMed] [Google Scholar]
- 38. Liu, J., Li, D., Wu, X., Dang, L., Lu, A., and Zhang, G.. Bone-derived exosomes. Curr Opin Pharmacol 34, 64, 2017. [DOI] [PubMed] [Google Scholar]
- 39. Liu, M., Sun, Y., and Zhang, Q.. Emerging role of extracellular vesicles in bone remodeling. J Dent Res 97, 859, 2018. [DOI] [PubMed] [Google Scholar]
- 40. Lyu, H., Xiao, Y., Guo, Q., Huang, Y., and Luo, X.. The role of bone-derived exosomes in regulating skeletal metabolism and extraosseous diseases. Front Cell Dev Biol 8, 89, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gurunathan, S., Kang, M.H., Jeyaraj, M., Qasim, M., and Kim, J.H.. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells 8, 307, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Casado-Díaz, A., Quesada-Gómez, J.M., and Dorado, G.. Extracellular vesicles derived from mesenchymal stem cells (MSC) in regenerative medicine: applications in skin wound healing. Front Bioeng Biotechnol 8, 146, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Colombo, M., Raposo, G., and Thery, C.. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30, 255, 2014. [DOI] [PubMed] [Google Scholar]
- 44. Liga, A., Vliegenthart, A.D., Oosthuyzen, W., Dear, J.W., and Kersaudy-Kerhoas, M.. Exosome isolation: a microfluidic road-map. Lab Chip 15, 2388, 2015. [DOI] [PubMed] [Google Scholar]
- 45. Chen, B.Y., Sung, C.W., Chen, C., et al. Advances in exosomes technology. Clin Chim Acta 493, 14, 2019. [DOI] [PubMed] [Google Scholar]
- 46. Zhang, Y., Liu, Y., Liu, H., and Tang, W.H.. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci 9, 19, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mittelbrunn, M., and Sanchez-Madrid, F.. Intercellular communication: diverse structures for exchange of genetic information. Nat Rev Mol Cell Biol 13, 328, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang, B., Wu, X., Zhang, X., et al. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/beta-catenin pathway. Stem Cells Transl Med 4, 513, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Katsuda, T., Tsuchiya, R., Kosaka, N., et al. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci Rep 3, 1197, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang, B., Wang, M., Gong, A., et al. HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells 33, 2158, 2015. [DOI] [PubMed] [Google Scholar]
- 51. Wang, X., Omar, O., Vazirisani, F., Thomsen, P., and Ekstrom, K.. Mesenchymal stem cell-derived exosomes have altered microRNA profiles and induce osteogenic differentiation depending on the stage of differentiation. PLoS One 13, e0193059, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ha, M., and Kim, V.N.. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol 15, 509, 2014. [DOI] [PubMed] [Google Scholar]
- 53. Xu, J.F., Yang, G.H., Pan, X.H., et al. Altered microRNA expression profile in exosomes during osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. PLoS One 9, e114627, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Friedman, R.C., Farh, K.K., Burge, C.B., and Bartel, D.P.. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19, 92, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mittelbrunn, M., Gutierrez-Vazquez, C., Villarroya-Beltri, C., et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun 2, 282, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Montecalvo, A., Larregina, A.T., Shufesky, W.J., et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood 119, 756, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Xie, Y., Chen, Y., Zhang, L., Ge, W., and Tang, P.. The roles of bone-derived exosomes and exosomal microRNAs in regulating bone remodelling. J Cell Mol Med 21, 1033, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Narayanan, R., Huang, C.C., and Ravindran, S.. Hijacking the cellular mail: exosome mediated differentiation of mesenchymal stem cells. Stem Cells Int 2016, 3808674, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Martins, M., Ribeiro, D., Martins, A., Reis, R.L., and Neves, N.M.. Extracellular vesicles derived from osteogenically induced human bone marrow mesenchymal stem cells can modulate lineage commitment. Stem Cell Rep 6, 284, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Qin, Y., Wang, L., Gao, Z., Chen, G., and Zhang, C.. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci Rep 6, 21961, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Furuta, T., Miyaki, S., Ishitobi, H., et al. Mesenchymal stem cell-derived exosomes promote fracture healing in a mouse model. Stem Cells Transl Med 5, 1620, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Huang, C.C., Kang, M., Narayanan, R., et al. Evaluating the endocytosis and lineage-specification properties of mesenchymal stem cell derived extracellular vesicles for targeted therapeutic applications. Front Pharmacol 11, 163, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liang, B., Liang, J.M., Ding, J.N., Xu, J., Xu, J.G., and Chai, Y.M.. Dimethyloxaloylglycine-stimulated human bone marrow mesenchymal stem cell-derived exosomes enhance bone regeneration through angiogenesis by targeting the AKT/mTOR pathway. Stem Cell Res Ther 10, 335, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Takeuchi, R., Katagiri, W., Endo, S., and Kobayashi, T.. Exosomes from conditioned media of bone marrow-derived mesenchymal stem cells promote bone regeneration by enhancing angiogenesis. PLoS One 14, e0225472, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liu, S., Liu, D., Chen, C., et al. MSC transplantation improves osteopenia via epigenetic regulation of notch signaling in lupus. Cell Metab 22, 606, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fang, S., Li, Y., and Chen, P.. Osteogenic effect of bone marrow mesenchymal stem cell-derived exosomes on steroid-induced osteonecrosis of the femoral head. Drug Des Devel Ther 13, 45, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Li, H., Liu, D., Li, C., et al. Exosomes secreted from mutant-HIF-1α-modified bone-marrow-derived mesenchymal stem cells attenuate early steroid-induced avascular necrosis of femoral head in rabbit. Cell Biol Int 41, 1379, 2017. [DOI] [PubMed] [Google Scholar]
- 68. Jia, Y., Qiu, S., Xu, J., Kang, Q., and Chai, Y.. Exosomes secreted by young mesenchymal stem cells promote new bone formation during distraction osteogenesis in older rats. Calcified Tissue Int 106, 509, 2020. [DOI] [PubMed] [Google Scholar]
- 69. Xu, T., Luo, Y., Wang, J., et al. Exosomal miRNA-128-3p from mesenchymal stem cells of aged rats regulates osteogenesis and bone fracture healing by targeting Smad5. J Nanobiotechnol 18, 47, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhang, L., Jiao, G., Ren, S., et al. Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Res Ther 11, 38, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zuo, R., Liu, M., Wang, Y., et al. BM-MSC-derived exosomes alleviate radiation-induced bone loss by restoring the function of recipient BM-MSCs and activating Wnt/β-catenin signaling. Stem Cell Res Ther 10, 30, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Li, X., Zheng, Y., Hou, L., et al. Exosomes derived from maxillary BMSCs enhanced the osteogenesis in iliac BMSCs. Oral Dis 26, 131, 2020. [DOI] [PubMed] [Google Scholar]
- 73. Liao, W., Ning, Y., Xu, H.J., et al. BMSC-derived exosomes carrying microRNA-122-5p promote proliferation of osteoblasts in osteonecrosis of the femoral head. Clin Sci (Lond) 133, 1955, 2019. [DOI] [PubMed] [Google Scholar]
- 74. Xu, H.J., Liao, W., Liu, X.Z., et al. Down-regulation of exosomal microRNA-224-3p derived from bone marrow-derived mesenchymal stem cells potentiates angiogenesis in traumatic osteonecrosis of the femoral head. Faseb J 33, 8055, 2019. [DOI] [PubMed] [Google Scholar]
- 75. Zhao, P., Xiao, L., Peng, J., Qian, Y.Q., and Huang, C.C.. Exosomes derived from bone marrow mesenchymal stem cells improve osteoporosis through promoting osteoblast proliferation via MAPK pathway. Eur Rev Med Pharmacol Sci 22, 3962, 2018. [DOI] [PubMed] [Google Scholar]
- 76. Li, W., Liu, Y., Zhang, P., et al. Tissue-engineered bone immobilized with human adipose stem cells-derived exosomes promotes bone regeneration. ACS Appl Mater Interfaces 10, 5240, 2018. [DOI] [PubMed] [Google Scholar]
- 77. Chen, S., Tang, Y., Liu, Y., et al. Exosomes derived from miR-375-overexpressing human adipose mesenchymal stem cells promote bone regeneration. Cell Prolif 52, e12669, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Guo, S.C., Tao, S.C., Yin, W.J., Qi, X., Sheng, J.G., and Zhang. C.Q. Exosomes from human synovial-derived mesenchymal stem cells prevent glucocorticoid-induced osteonecrosis of the femoral head in the rat. Int J Biol Sci 12, 1262, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Liu, X., Li, Q., Niu, X., et al. Exosomes secreted from human-induced pluripotent stem cell-derived mesenchymal stem cells prevent osteonecrosis of the femoral head by promoting angiogenesis. Int J Biol Sci 13, 232, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Qi, X., Zhang, J., Yuan, H., et al. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int J Biol Sci 12, 836, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhang, J., Liu, X., Li, H., et al. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res Ther 7, 136, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kuang, M.J., Huang, Y., Zhao, X.G., et al. Exosomes derived from Wharton's jelly of human umbilical cord mesenchymal stem cells reduce osteocyte apoptosis in glucocorticoid-induced osteonecrosis of the femoral head in rats via the miR-21-PTEN-AKT signalling pathway. Int J Biol Sci 15, 1861, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Liu, W., Li, L., Rong, Y., et al. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater 103, 196, 2020. [DOI] [PubMed] [Google Scholar]
- 84. Zhang, Y., Hao, Z., Wang, P., et al. Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF-1α-mediated promotion of angiogenesis in a rat model of stabilized fracture. Cell Prolif 52, e12570, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Li, R., Chen, C., Zheng, R.Q., Zou, L., Hao, G.L., and Zhang, G.C.. Influences of hucMSC-exosomes on VEGF and BMP-2 expression in SNFH rats. Eur Rev Med Pharmacol Sci 23, 2935, 2019. [DOI] [PubMed] [Google Scholar]
- 86. Zhou, J., Liu, H.X., Li, S.H., et al. Effects of human umbilical cord mesenchymal stem cells-derived exosomes on fracture healing in rats through the Wnt signaling pathway. Eur Rev Med Pharmacol Sci 23, 4954, 2019. [DOI] [PubMed] [Google Scholar]
- 87. Yang, B.C., Kuang, M.J., Kang, J.Y., Zhao, J., Ma, J.X., and Ma, X.L.. Human umbilical cord mesenchymal stem cell-derived exosomes act via the miR-1263/Mob1/Hippo signaling pathway to prevent apoptosis in disuse osteoporosis. Biochem Biophys Res Commun 524, 883, 2020. [DOI] [PubMed] [Google Scholar]
- 88. Deng, L., Wang, Y., Peng, Y., et al. Osteoblast-derived microvesicles: a novel mechanism for communication between osteoblasts and osteoclasts. Bone 79, 37, 2015. [DOI] [PubMed] [Google Scholar]
- 89. Huynh, N., VonMoss, L., Smith, D., et al. Characterization of regulatory extracellular vesicles from osteoclasts. J Dent Res 95, 673, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cui, Y., Luan, J., Li, H., Zhou, X., and Han, J.. Exosomes derived from mineralizing osteoblasts promote ST2 cell osteogenic differentiation by alteration of microRNA expression. FEBS Lett 590, 185, 2016. [DOI] [PubMed] [Google Scholar]
- 91. Deng, L., Peng, Y., Jiang, Y., et al. Imipramine protects against bone loss by inhibition of osteoblast-derived microvesicles. Int J Mol Sci 18, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sun, W., Zhao, C., Li, Y., et al. Osteoclast-derived microRNA-containing exosomes selectively inhibit osteoblast activity. Cell Discov 2, 16015, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Li, D., Liu, J., Guo, B., et al. Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation. Nat Commun 7, 10872, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Qin, Y., Peng, Y., Zhao, W., et al. Myostatin inhibits osteoblastic differentiation by suppressing osteocyte-derived exosomal microRNA-218: a novel mechanism in muscle-bone communication. J Biol Chem 292, 11021, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ekström K, Omar, O., Granéli, C., Wang, X., Vazirisani, F., and Thomsen, P.. Monocyte exosomes stimulate the osteogenic gene expression of mesenchymal stem cells. PLoS One 8, e75227, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Martin, P.J., Haren, N., Ghali, O., et al. Adipogenic RNAs are transferred in osteoblasts via bone marrow adipocytes-derived extracellular vesicles (EVs). BMC Cell Biol 16, 10, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Elashiry, M., Elashiry, M.M., Elsayed, R., et al. Dendritic cell derived exosomes loaded with immunoregulatory cargo reprogram local immune responses and inhibit degenerative bone disease in vivo. J Extracell Vesicles 9, 1795362, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Baglio, S.R., Rooijers, K., Koppers-Lalic, D., et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther 6, 127, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zhang, W.B., Zhong, W.J., and Wang, L.. A signal-amplification circuit between miR-218 and Wnt/beta-catenin signal promotes human adipose tissue-derived stem cells osteogenic differentiation. Bone 58, 59, 2014. [DOI] [PubMed] [Google Scholar]
- 100. Taipaleenmaki, H., Bjerre Hokland, L., Chen, L., Kauppinen, S., and Kassem, M.. Mechanisms in endocrinology: micro-RNAs: targets for enhancing osteoblast differentiation and bone formation. Eur J Endocrinol 166, 359, 2012. [DOI] [PubMed] [Google Scholar]
- 101. Kawai, T., Katagiri, W., Osugi, M., Sugimura, Y., Hibi, H., and Ueda, M.. Secretomes from bone marrow-derived mesenchymal stromal cells enhance periodontal tissue regeneration. Cytotherapy 17, 369, 2015. [DOI] [PubMed] [Google Scholar]
- 102. Katagiri, W., Kawai, T., Osugi, M., et al. Angiogenesis in newly regenerated bone by secretomes of human mesenchymal stem cells. Maxillofac Plast Reconstr Surg 39, 8, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Anderson, J.D., Johansson, H.J., Graham, C.S., et al. Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis via nuclear factor-KappaB signaling. Stem Cells 34, 601, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Li, H., Yang, F., Wang, Z., Fu, Q., and Liang, A.. MicroRNA-21 promotes osteogenic differentiation by targeting small mothers against decapentaplegic 7. Mol Med Rep 12, 1561, 2018. [DOI] [PubMed] [Google Scholar]
- 105. Yang, X., Shi, G., Guo, J., Wang, C., and He, Y.. Exosome-encapsulated antibiotic against intracellular infections of methicillin-resistant Staphylococcus aureus. Int J Nanomed 13, 8095, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lew, D.P., and Waldvogel, F.A.. Osteomyelitis. Lancet (London, England) 364, 369, 2004. [DOI] [PubMed] [Google Scholar]
- 107. Ni, Z., Zhou, S., Li, S., et al. Exosomes: roles and therapeutic potential in osteoarthritis. Bone Res 8, 25, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lu, J., Wang, Q.Y., and Sheng, J.G.. Exosomes in the repair of bone defects: next-generation therapeutic tools for the treatment of nonunion. Biomed Res Int 2019, 1983131, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Toh, W.S., Lai, R.C., Hui, J.H.P., and Lim, S.K.. MSC exosome as a cell-free MSC therapy for cartilage regeneration: implications for osteoarthritis treatment. Semin Cell Dev Biol 67, 56, 2017. [DOI] [PubMed] [Google Scholar]
- 110. Pourakbari, R., Khodadadi, M., Aghebati-Maleki, A., Aghebati-Maleki, L., and Yousefi, M.. The potential of exosomes in the therapy of the cartilage and bone complications; emphasis on osteoarthritis. Life Sci 236, 116861, 2019. [DOI] [PubMed] [Google Scholar]
- 111. Tan, S.S.H., Tjio, C.K.E., Wong, J.R.Y., et al. Mesenchymal stem cell exosomes for cartilage regeneration: a systematic review of preclinical in vivo studies. Tissue Eng Part B Rev 27, 1, 2021. [DOI] [PubMed] [Google Scholar]
- 112. Cosenza, S., Ruiz, M., Toupet, K., Jorgensen, C., and Noël, D.. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep 7, 16214, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Chen, P., Zheng, L., Wang, Y., et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics 9, 2439, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Chen, Y., Xue, K., Zhang, X., Zheng, Z., and Liu, K.. Exosomes derived from mature chondrocytes facilitate subcutaneous stable ectopic chondrogenesis of cartilage progenitor cells. Stem Cell Res Ther 9, 318, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Liu, X., Yang, Y., Li, Y., et al. Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale 9, 4430, 2017. [DOI] [PubMed] [Google Scholar]
- 116. Liu, Y., Zou, R., Wang, Z., Wen, C., Zhang, F., and Lin, F.. Exosomal KLF3-AS1 from hMSCs promoted cartilage repair and chondrocyte proliferation in osteoarthritis. Biochem J 475, 3629, 2018. [DOI] [PubMed] [Google Scholar]
- 117. Mao, G., Zhang, Z., Hu, S., et al. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res Ther 9, 247, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Tao, S.C., Yuan, T., Zhang, Y.L., Yin, W.J., Guo, S.C., and Zhang, C.Q.. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics 7, 180, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wang, Y., Yu, D., Liu, Z., et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res Ther 8, 189, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wang, R., Xu, B., and Xu, H.. TGF-β1 promoted chondrocyte proliferation by regulating Sp1 through MSC-exosomes derived miR-135b. Cell Cycle 17, 2756, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Wu, J., Kuang, L., Chen, C., et al. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials 206, 87, 2019. [DOI] [PubMed] [Google Scholar]
- 122. Zhang, S., Chu, W.C., Lai, R.C., Lim, S.K., Hui, J.H., and Toh, W.S.. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis Cartilage 24, 2135, 2016. [DOI] [PubMed] [Google Scholar]
- 123. Zhang, S., Chuah, S.J., Lai, R.C., Hui, J.H.P., Lim, S.K., and Toh, W.S.. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials 156, 16, 2018. [DOI] [PubMed] [Google Scholar]
- 124. Zhu, Y., Wang, Y., Zhao, B., et al. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res Ther 8, 64, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wong, K.L., Zhang, S., Wang, M., et al. Intra-articular injections of mesenchymal stem cell exosomes and hyaluronic acid improve structural and mechanical properties of repaired cartilage in a rabbit model. Arthroscopy 36, 2215.e2212, 2020. [DOI] [PubMed] [Google Scholar]
- 126. Zhang, S., Teo KYW, Chuah, S.J., Lai, R.C., Lim, S.K., and Toh, W.S.. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials 200, 35, 2019. [DOI] [PubMed] [Google Scholar]
- 127. Ham, O., Song, B.W., Lee, S.Y., et al. The role of microRNA-23b in the differentiation of MSC into chondrocyte by targeting protein kinase A signaling. Biomaterials 33, 4500, 2012. [DOI] [PubMed] [Google Scholar]
- 128. Wang, Y., He, G., Guo, Y., et al. Exosomes from tendon stem cells promote injury tendon healing through balancing synthesis and degradation of the tendon extracellular matrix. J Cell Mol Med 23, 5475, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Connor, D.E., Paulus, J.A., Dabestani, P.J., et al. Therapeutic potential of exosomes in rotator cuff tendon healing. J Bone Miner Metab 37, 759, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Cui, H., He, Y., Chen, S., Zhang, D., Yu, Y., and Fan, C.. Macrophage-derived miRNA-containing exosomes induce peritendinous fibrosis after tendon injury through the miR-21-5p/Smad7 pathway. Mol Ther Nucleic Acids 14, 114, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Lui, P.P.Y. Mesenchymal stem cell-derived extracellular vesicles for the promotion of tendon repair—an update of literature. Stem Cell Rev Rep 2020. DOI: 10.1007/s12015-020-10023-8. [DOI] [PubMed] [Google Scholar]
- 132. Zhang, M., Liu, H., Cui, Q., et al. Tendon stem cell-derived exosomes regulate inflammation and promote the high-quality healing of injured tendon. Stem Cell Res Ther 11, 402, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Xu, T., Xu, M., Bai, J., et al. Tenocyte-derived exosomes induce the tenogenic differentiation of mesenchymal stem cells through TGF-β. Cytotechnology 71, 57, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Wang, C., Hu, Q., Song, W., Yu, W., and He, Y.. Adipose stem cell-derived exosomes decrease fatty infiltration and enhance rotator cuff healing in a rabbit model of chronic tears. Am J Sports Med 48, 1456, 2020. [DOI] [PubMed] [Google Scholar]
- 135. Yu, H., Cheng, J., Shi, W., et al. Bone marrow mesenchymal stem cell-derived exosomes promote tendon regeneration by facilitating the proliferation and migration of endogenous tendon stem/progenitor cells. Acta Biomater 106, 328, 2020. [DOI] [PubMed] [Google Scholar]
- 136. Shi, Y., Kang, X., Wang, Y., et al. Exosomes derived from bone marrow stromal cells (BMSCs) enhance tendon-bone healing by regulating macrophage polarization. Med Sci Monit 26, e923328, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Shen, H., Yoneda, S., Abu-Amer, Y., Guilak, F., and Gelberman, R.H.. Stem cell-derived extracellular vesicles attenuate the early inflammatory response after tendon injury and repair. J Orthop Res 38, 117, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Yao, Z., Li, J., Wang, X., et al. MicroRNA-21-3p engineered umbilical cord stem cell-derived exosomes inhibit tendon adhesion. J Inflamm Res 13, 303, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Hsu, D.H., Paz, P., Villaflor, G., et al. Exosomes as a tumor vaccine: enhancing potency through direct loading of antigenic peptides. J Immunother (Hagerstown, Md: 1997) 26, 440, 2003. [DOI] [PubMed] [Google Scholar]
- 140. Wolfers, J., Lozier, A., Raposo, G., et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med 7, 297, 2001. [DOI] [PubMed] [Google Scholar]
- 141. Qin, Y., Sun, R., Wu, C., Wang, L., and Zhang, C.. Exosome: a novel approach to stimulate bone regeneration through regulation of osteogenesis and angiogenesis. Int J Mol Sci 17, 712, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]