Abstract
Regulatory T (Treg) cells are critical in preventing aberrant immune responses. Posttranscriptional control of gene expression by microRNA (miRNA) has recently emerged as an essential genetic element for Treg cell function. Here, we report that mice with Treg cell–specific ablation of miR-142 (hereafter Foxp3CremiR-142fl/fl mice) developed a fatal systemic autoimmune disorder due to a breakdown in peripheral T-cell tolerance. Foxp3CremiR-142fl/fl mice displayed a significant decrease in the abundance and suppressive capacity of Treg cells. Expression profiling of miR-142–deficient Treg cells revealed an up-regulation of multiple genes in the interferon gamma (IFNγ) signaling network. We identified several of these IFNγ-associated genes as direct miR-142-3p targets and observed excessive IFNγ production and signaling in miR-142–deficient Treg cells. Ifng ablation rescued the Treg cell homeostatic defect and alleviated development of autoimmunity in Foxp3CremiR-142fl/fl mice. Thus, our findings implicate miR-142 as an indispensable regulator of Treg cell homeostasis that exerts its function by attenuating IFNγ responses.
This study identifies microRNA-142 as a central regulator of the development, homeostasis and function of regulatory T cells. Mechanistically, the miR-142-3p isoform controls regulatory T cell homeostasis and immune tolerance by attenuating IFNγ responses.
Introduction
Regulatory T (Treg) cells are vital in maintaining immune self-tolerance and restraining aberrant immune responses against infections [1,2]. Foxp3, an X chromosome–linked member of the forkhead box/winged helix family of transcription factors, is a master regulator of the genetic program that governs development and suppressive activity of Treg cells. Humans and mice that carry loss-of-function Foxp3 mutations develop a fatal autoimmune disease due to impaired Treg cell activity [3–7]. The majority of Treg cells are generated in the thymus (tTreg cells) through a selection process that favors cells with a strong functional T cell receptor (TCR) avidity toward self-antigens. In contrast, peripheral Treg (pTreg) cells arise from naive CD4+ T cells upon encounter of non–self-antigens in the context of appropriate cytokine stimulation [8–10]. Harnessing the power of Treg cells to control immunological responses has a great potential for human therapy because, on one hand, Treg cells can promote transplantation tolerance, but on the other, can hinder antitumor immunity.
Posttranscriptional regulation of gene expression by microRNA (miRNA), a class of small (approximately 22 nucleotides) noncoding RNA, recently emerged as a critical genetic element that is essential for Treg cell function. Treg cell–specific knockouts (KOs) of either Drosha or Dicer genes, encoding 2 endonucleases required for mature miRNA generation from precursor transcripts, phenocopy mice with Foxp3 ablation, and develop severe systemic autoimmunity because of a defect in Treg cell activity [11–13]. Furthermore, deletion of Dicer at the double positive (DP) thymocyte stage in mice significantly diminished the frequency of tTreg cells, suggesting that miRNA-dependent gene control is also required for normal development of Treg cells [11]. Thus, the current pressing challenge in the field is to determine how specific miRNA gene(s) exert control of the Treg cell genetic program. The present report addresses this goal by examining the role of miR-142 in Treg cell development and function.
miR-142 is predominantly expressed in cells of hematopoietic origin and encodes 2 abundant mature miRNA molecules—miR-142-5p and miR-142-3p—which arise from the opposite strands of the hairpin-like miR-142 precursor. Using genetic loss-of-function studies, miR-142 was previously implicated in the regulation of ontogenesis and function of several immune cell types. Our earlier report determined that deletion of this miRNA gene in mice results in aberrant B lymphopoiesis and impaired humoral immunity [14]. In addition, miR-142–deficient mice develop thrombocytopenia stemming from defective megakaryocyte maturation [15] and exhibit dysregulation of dendritic cell (DC) function [16]. Disruption of 2 miR-142 paralog genes in zebra danio using zinc-finger nucleases was reported to cause aberrant neutrophil differentiation [17]. In the T-cell compartment, miR-142 is required for the homeostasis of peripheral T effector (Teff) cells, but is apparently dispensable for conventional T (Tconv) cell development in the thymus [14,18,19]. A recent study by Anandagoda and colleagues has demonstrated that miR-142 is essential for the immunosuppressive activity of Treg cells, but failed to reveal a significant role for this miRNA in Treg cell development and homeostasis [20]. The authors suggest that posttranscriptional repression of the cAMP-hydrolyzing enzyme Pde3b by miR-142-5p isoform plays a key role in the regulation of the Treg cell suppressive function.
Here, we show that mice with a conditional deletion of miR-142 in Treg cells develop severe autoimmune disease due to a profound defect in Treg cell homeostasis and function. In addition, our findings suggest that miR-142 plays an important role in tTreg cell development. We have determined that the miR-142-3p isoform and its capacity to silence multiple interferon gamma (IFNγ)-associated genes play a critical role in mediating the regulatory activity of miR-142 in Treg cells. Global ablation of IFNγ rescues the Treg cell defect and autoimmunity in Foxp3CremiR-142fl/fl mice, thus providing further evidence for the essential role of the miR-142-IFNγ signaling pathway in the regulation of Treg cell homeostasis and function.
Results
miR-142 is dynamically expressed in T cells, and its global ablation results in a Treg cell defect
Our efforts to define the role of miR-142 in the regulation of T-cell tolerance stem from an unexpected observation that global miR-142 ablation results in a marked Treg cell defect. We found that germline miR-142 KO mice display a significant drop in Treg cell numbers in both thymus and secondary lymphoid organs (S1A–S1C Fig), indicating that miR-142 plays an important role in Treg cell development and homeostasis. The observed defect was specific to Treg cells, because thymic development of Tconv cells was largely normal in the germline miR-142 KO mice [14,18,19]. Our expression profiling experiments revealed that the mature miR-142-3p isoform is abundantly expressed in thymic and pTreg cells, whereas the mature miR-142-5p isoform is present in significantly lower amounts (S1D Fig). The level of miR-142-3p expression in Treg cells is roughly comparable to the expression of this miRNA in naive and activated Teff cells. Of note, miR-142 is dynamically expressed during T-cell development: its abundance gradually increases following T-cell maturation in the thymus and reaches a peak at the single positive (SP) thymocyte stage; however, mature T cells that egress from the thymus into periphery display a somewhat reduced miR-142 expression in comparison to SP thymocytes (S1D Fig).
Lethal autoimmune disease in mice with Treg cell–specific miR-142 deletion
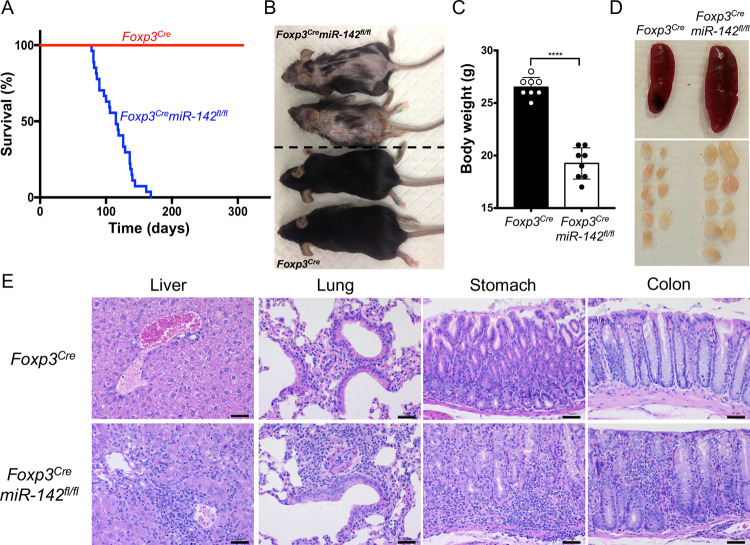
To determine the role of miR-142 in Treg cell function, we created mice with a Treg cell–specific ablation of this miRNA (hereafter Foxp3CremiR-142fl/fl mice). Foxp3Cre deleter/reporter mice [21] that were used to generate the Treg cell–specific excision of miR-142 conditional allele (miR-142fl/fl) express Cre recombinase as a fusion protein with yellow fluorescent protein (YFP) under the control of endogenous Foxp3 promoter, providing an effective way to purify Treg cells by flow cytometry. As expected, CD4+YFP+ Treg cells isolated from Foxp3CremiR-142fl/fl mice were virtually devoid of mature miR-142-3p, whereas expression of this miRNA in CD4+ Tconv cells remained unchanged (S1E Fig). Despite appearing normal at birth, Foxp3CremiR-142fl/fl mice exhibited an apparent failure to thrive: they had a very short life span (Fig 1A), were smaller in size (Fig 1B), and had significantly lower body weight than wild-type (WT; Foxp3Cre) littermates (Fig 1C). Gross morphological analysis revealed that Foxp3CremiR-142fl/fl mice developed a systemic lymphoproliferative autoimmune disorder that was characterized by significant lymphadenopathy (Fig 1D and S1J Fig), mild splenomegaly (Fig 1D, S1F and S1H Fig), thymic involution (S1G and S1I Fig), dermatitis (Fig 1B), and massive immune cell infiltration into various peripheral organs (Fig 1E). Taken together, the phenotypic findings from Foxp3CremiR-142fl/fl mice resemble the autoimmune pathology observed in mice with severe Treg cell defects (e.g., scurfy mutants or Foxp3 KO mice [3–7]), suggesting that miR-142 is involved in the regulation of Treg cell homeostasis or stability.
Fig 1. Fatal autoimmune disorder in mice with Treg cell–specific disruption of miR-142.
(A) Kaplan–Meier survival curves of Foxp3Cre and Foxp3CremiR-142fl/fl mice (n = 27 per group) (P < 0.0001). (B) Photograph of 14-week-old female Foxp3Cre (lower 2) and Foxp3CremiR-142fl/fl (upper 2) mice. Note smaller body size and severe dermatitis in Foxp3CremiR-142fl/fl mice. (C) Body weight comparison of 8- to 10-week-old male Foxp3Cre and Foxp3CremiR-142fl/fl mice (n = 8 per group). (D) Representative images of spleen and peripheral lymph nodes from Foxp3Cre (left) and Foxp3CremiR-142fl/fl (right) mice. (E) HE staining of liver, lung, stomach, and colon tissue sections from Foxp3Cre and Foxp3CremiR-142fl/fl mice. Note massive accumulation of leukocytes in Foxp3CremiR-142fl/fl tissues. Scale bar, 50 μm. Results are shown as mean ± SD. P values were determined by log-rank test (A) or 2-tailed Student t test (C); ****, P < 0.0001. The underlying raw data can be found in S1 Data file. HE, hematoxylin–eosin; SD, standard deviation; Treg, regulatory T.
Treg cell defect and aberrant T-cell activation in Foxp3CremiR-142fl/fl mice
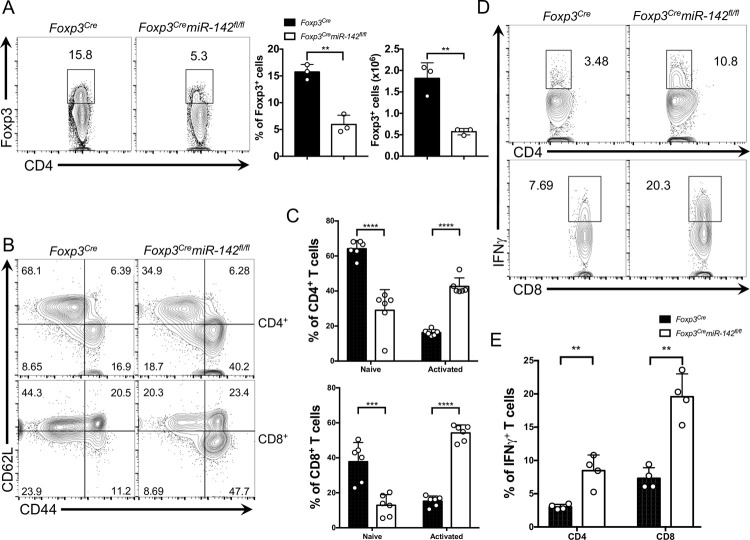
In agreement with this notion, we observed a marked reduction in Treg cell population in the Foxp3CremiR-142fl/fl spleen (Fig 2A). Furthermore, the absolute number of thymic Treg cells in Foxp3CremiR-142fl/fl mice was also significantly lower, despite a slight increase in the frequency of Treg cells in the atrophied thymus (S2A and S2B Fig). Because Treg cells play a crucial role in suppressing self-destructive responses elicited by autoreactive T cells, we examined the activation status of peripheral T cells in Foxp3CremiR-142fl/fl mice by flow cytometry. In comparison to WT mice, the number of CD4+ and CD8+ T cells with memory/effector phenotype (CD44hiCD62Llo) was significantly higher in the spleen and lymph nodes of Foxp3CremiR-142fl/fl mice, whereas the pool of naive (CD44loCD62Lhi) CD4+ and CD8+ T cells in the KO animals diminished considerably (Fig 2B and 2C, S2C and S2D Fig). Foxp3CremiR-142fl/fl mice exhibited an expansion of peripheral CD4+ and CD8+ T-cell compartments (S2G Fig), most likely due to a failure of miR-142–deficient Treg cells to suppress proliferation of hyperactivated T lymphocytes. In addition, both CD4+ and CD8+ T cells isolated from Foxp3CremiR-142fl/fl mice displayed a sharp increase in production of IFNγ (Fig 2D and 2E, S2E and S2F Fig), a pro-inflammatory cytokine that promotes type 1 T helper cell 1 (Th1) responses. In contrast, no obvious changes in interleukin (IL)-4 (secreted by Th2 cells) or IL-17 (secreted by Th17 cells) production by splenic CD4+ T cells were observed in Foxp3CremiR-142fl/fl animals (S2H Fig). Thus, our findings suggest that conditional miR-142 deletion impairs Treg cell function and subsequently drives severe dysregulation of Teff responses.
Fig 2. miR-142 is required for maintenance of Treg cell–mediated immune tolerance.
(A) Left panel, FACS analysis of splenic lymphocytes from 12-week-old Foxp3Cre and Foxp3CremiR-142fl/fl mice with anti-CD4 and anti-Foxp3 specific antibodies. Numbers indicate percentage of CD4+Foxp3+ Treg cells in the gate. Right panel, frequency and absolute number of Treg cells in Foxp3Cre and Foxp3CremiR-142fl/fl spleens (n = 3 per group). (B) FACS analysis of splenic CD4+ and CD8+ T cells from 8- to 10-week-old Foxp3Cre and Foxp3CremiR-142fl/fl mice with anti-CD44 and anti-CD62L specific antibodies. Numbers indicate percentage of cells in the quadrants. (C) Frequency of naive (CD44−CD62L+) and activated (CD44+CD62L−) splenic CD4+ (top) and CD8+ (bottom) T lymphocytes isolated from 8- to 10-week-old Foxp3Cre and Foxp3CremiR-142fl/fl mice (n = 6 per group). (D) Intracellular FACS analysis of IFNγ production in splenic CD4+ (top) and CD8+ (bottom) T cells. Numbers indicate percentage of IFNγ+ T cells in the gate. (E) Frequency of IFNγ expressing splenic CD4+ and CD8+ T cells isolated from 8- to 10-week-old Foxp3Cre and Foxp3CremiR-142fl/fl mice (n = 4 per group). Results are shown as mean ± SD. P values were calculated using 2-tailed Student t test. **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. The underlying numerical raw data can be found in S1 Data file. The underlying flow cytometry raw data can be found at the Figshare repository. FACS, fluorescence activated cell sorting; IFNγ, interferon gamma; SD, standard deviation; Treg, regulatory T.
miR-142 is essential for the homeostasis and suppressive activity of Treg cells
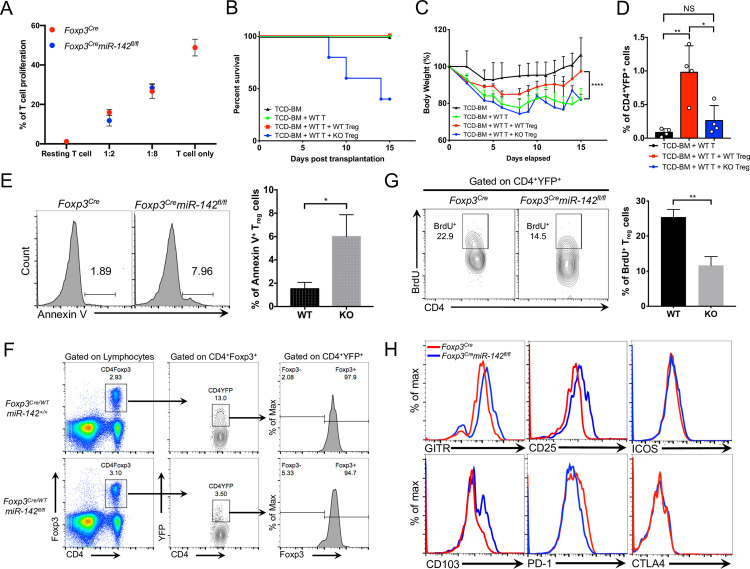
To determine how miR-142 ablation affects suppressive function of Treg cells, we cocultured purified miR-142–deficient and miR-142–sufficient Treg cells together with WT Tconv cells that were induced to proliferate by antigen receptor stimulation. We found that miR-142–deficient Treg cells exhibited a comparable capacity to restrain the proliferation of activated Tconv cells as miR-142–sufficient Treg cells in this classical in vitro Treg cell suppression assay (Fig 3A, S3A Fig). Similar in vitro findings were previously reported for miR-146a and miR-181a/b-1, two other miRNAs that are implicated in the regulation of Treg cell activity [22,23]. Nevertheless, adoptive transfer of miR-142–deficient Treg cells failed to attenuate development of systemic inflammatory syndrome in a mouse model of acute graft-versus-host disease (aGVHD). Lethally irradiated BALB/c mice (H2d) transplanted with allogeneic CD4+ Tconv cells from C57BL/6 donors (H2b) rapidly developed sublethal aGVHD that was manifested by hunched posture, severe diarrhea, and significant weight loss in the host mice (Fig 3B and 3C). The concomitant transfer of WT Treg cells together with allogeneic donor T cells reduced the severity of aGVHD symptoms, as indicated by diminished diarrhea and less pronounced body weight loss in the host mice (Fig 3C). In contrast, adoptive transfer of miR-142–deficient Treg cells failed to protect the host mice from the development of aGVHD and resulted in mortality, perhaps by exacerbating the inflammatory response (Fig 3B and 3C). The failure of miR-142–deficient Treg cells to suppress aGVHD indicated an essential role of miR-142 in the regulation of Treg cell suppressive activity in vivo. In addition, a significantly lower frequency of donor miR-142–deficient Treg cells in the spleens of host mice 17 days posttransplantation (Fig 3D) suggested the survival or homeostatic defect of miR-142–deficient Treg cells.
Fig 3. miR-142 ablation impairs suppressive function and homeostasis of Treg cells.
(A) in vitro Treg suppression assay. Purified CD4+ Tconv cells were loaded with CTV dye and incubated with FACS-sorted Treg cells from Foxp3Cre (red dots) and Foxp3CremiR-142fl/fl (blue dots) spleens (n = 3) in the presence of beads coated with anti-CD3 and anti-CD28 specific antibodies. After 3 days, the rate of Tconv cell proliferation was determined by flow cytometry as dilution of the CTV dye. Several Treg to Tconv cell ratios were analyzed as indicated in the graph. Unstimulated Tconv cells were used as control. (B) Survival of mice in aGVHD model. Lethally irradiated BALB/c recipient mice were transplanted with T-cell–depleted bone marrow from C57BL/6J mice (TCD-BM), Thy1.2+CD4+ T cells from C57BL/6J spleens (WT T), and CD4+YFP+ Treg cells derived from either Foxp3Cre (WT) or Foxp3CremiR-142fl/fl (KO) spleens (P = 0.0096; n = 5 per group). (C) Body weight analysis of mice in aGVHD model. Mouse grouping as in B (n = 5 per group). (D) Frequency of donor Foxp3Cre (WT) or Foxp3CremiR-142fl/fl (KO) CD4+YFP+ Treg cells in spleens of BALB/c recipient mice on day 17 after BMT (n = 4 for all groups). (E) Left panel, FACS analysis of activation-induced cell death in splenic Foxp3Cre and Foxp3CremiR-142fl/fl Treg cells. Apoptotic Treg cells were detected by Annexin V staining upon stimulation with anti-CD3 (1 μg/ml) specific antibodies for 48 hours. Numbers indicate percentage of Annexin V+ cells. Right panel, the frequency of Annexin V+ Foxp3Cre (WT) and Foxp3CremiR-142fl/fl (KO) Treg cells (n = 3 per group). (F) FACS analysis of CD4+Foxp3+ Treg cells (left panels), YFP-Cre expression in CD4+Foxp3+ Treg cells (middle panels), and Foxp3 expression in CD4+YFP+ miR-142–sufficient and miR-142–deficient Treg cells (right panels) from female Foxp3Cre/WTmiR-142+/+ and Foxp3Cre/WTmiR-142fl/fl mice. (G) Left panel, intracellular FACS analysis of BrdU incorporation into Treg cells isolated from Foxp3Cre and Foxp3CremiR-142fl/fl spleens. FACS plots were pregated on CD4+YFP+ Treg cells, and numbers indicate percentage of cells in the gate. Right panel, the frequency of BrdU+ Treg cells in Foxp3Cre (WT) and Foxp3CremiR-142fl/fl (KO) spleens 16 hours after BrdU injection (1mg i.p.) (n = 3 per group). (H) FACS analysis of cell surface markers on Treg cells isolated from Foxp3Cre (red) and Foxp3CremiR-142fl/fl (blue) spleens with anti-GITR, anti-CD25, anti-CD103, anti-PD-1, anti-CTLA-4, and anti-ICOS specific antibodies. Histograms were pregated on CD4+YFP+ Treg cells. Results are shown as mean ± SD. P values were calculated using log-rank test (B), 1-way ANOVA (C), and 2-tailed Student t test (D, E, G). *, P < 0.05; **, P < 0.01; ****, P < 0.0001; NS, not significant. The underlying numerical raw data can be found in S1 Data file. The underlying flow cytometry raw data can be found at the Figshare repository. aGVHD, acute graft-versus-host disease; BMT, bone marrow transplantation; CTV, cell trace violet; FACS, fluorescence activated cell sorting; KO, knockout; SD, standard deviation; Treg, regulatory T; YFP, yellow fluorescent protein; WT, wild-type.
To test whether miR-142 ablation affects survival of Treg cells, we compared induction of apoptosis in miR-142–sufficient and miR-142–deficient Treg cells in response to TCR stimulation. We found that purified splenic miR-142 KO Treg cells were more prone to activation-induced cell death (Fig 3E), although resting Treg cells from Foxp3CremiR-142fl/fl spleen displayed no obvious difference in Annexin V staining (S3B Fig). To further establish the cell-intrinsic role of miR-142 in Treg cell homeostasis, we enumerated miR-142–deficient Treg cells in Foxp3Cre/WTmiR-142fl/fl heterozygous female mice. Due to random X chromosome inactivation of Foxp3Cre allele, female Foxp3Cre/WTmiR-142fl/fl mice generate both YFP− miR-142–sufficient and YFP+ miR-142–deficient Treg cells. Our analysis revealed a marked reduction in the frequency of splenic YFP+ miR-142–deficient Treg cells in the mosaic mice (Fig 3F, S3C Fig), indicating that miR-142 plays a cell-autonomous role in Treg cell homeostasis. In addition, in vivo labeling experiments using 5-bromo-2-deoxyuridine (BrdU) revealed that miR-142–deficient Treg cells have almost 2-fold lower proliferation capacity than WT Treg cells (Fig 3G). Collectively, these findings implicate miR-142 in the regulation of homeostatic maintenance and proliferation of Treg cells.
Analysis of cell surface markers on pTreg cells from Foxp3CremiR-142fl/fl mice by flow cytometry suggested that miR-142–deficient Treg cells display an activated/effector phenotype [24]. Consistent with this notion, miR-142–deficient Treg cells exhibited an up-regulation of several T-cell activation markers, including glucocorticoid-induced tumor necrosis factor receptor family-related gene (GITR), interleukin-2 receptor α (CD25), and integrin αE (CD103) (Fig 3H, S3D Fig). On the other hand, the levels of programmed death-1 (PD-1) receptor, which protects Treg cells from apoptosis [25], were significantly lower on miR-142–deficient Treg cells (Fig 3H, S3D Fig), although expression of other immune checkpoint molecules such as cytotoxic T-lymphocyte associated protein 4 (CTLA4) and inducible T-cell co-stimulator (ICOS) was not affected.
Specific deletion of miR-142 in Treg cells results in global derepression of miR-142-3p targets
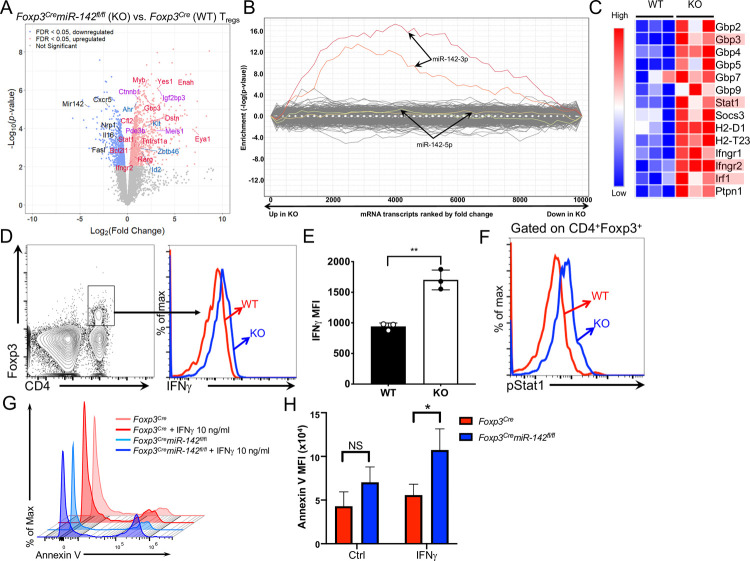
To uncover the molecular mechanism by which miR-142 controls Treg cell function, we performed global transcriptome analysis of splenic CD4+YFP+ miR-142–sufficient and miR-142–deficient Treg cells using RNA sequencing (RNA-seq). We identified a total of 1,520 genes showing statistically significant change in expression due to miR-142 ablation, 988 of which were up-regulated and 532 of which were down-regulated in miR-142–deficient Treg cells (Fig 4A, S1 Table). Analysis of differentially expressed genes in miR-142–deficient Treg cells with the SylArray and ToppFun software algorithms [26,27] revealed a significant enrichment of miR-142-3p (85 out of 429 annotated target genes), but not miR-142-5p, direct targets among the up-regulated genes (Fig 4B), suggesting that impaired homeostasis and function of Treg cells in Foxp3CremiR-142fl/fl mice are most likely driven by the loss of mature miR-142-3p expression. This conclusion aligns well with significantly more abundant expression of miR-142-3p in Treg cells (S1D Fig) and a large number of previously published observations from a broad spectrum of miR-142–deficient immune cell types [14–18,28–30]. Nevertheless, the possibility that miR-142-5p contributes to the regulation of Treg cell biology (as was recently proposed by Anandagoda and colleagues [20]) either by controlling the expression of a limited number of target mRNAs or by exerting its regulatory effect entirely at the translational level cannot be excluded, although our results suggest that the Treg cell defect in Foxp3CremiR-142fl/fl mice is primarily mediated by miR-142-3p.
Fig 4. Global derepression of miR-142-3p targets and dysregulated IFNγ signaling in miR-142–deficient Treg cells.
(A) Volcano plot showing statistical significance versus magnitude of change for visualizing the distribution of differentially expressed genes. Selected miR-142 targets (red for -3p, blue for -5p, and purple for both) and other genes of functional interest (black; including miR-142) are labeled. (B) Global derepression of miR-142-3p target genes in miR-142–deficient Treg cells. SylArray analysis of 3′ UTRs of differentially expressed transcripts in miR-142–deficient Treg cells for miRNA SCSs. Enrichment P values for each miRNA SCS are plotted on the y-axis against the ranked gene list on the x-axis (the most up-regulated genes in miR-142–deficient Treg cells are toward the left, while the most down-regulated genes are toward the right). Plots corresponding to two 7-mer seeds of miR-142-3p are highlighted in red and orange, and two 7-mer seeds of miR-142-5p are depicted in green and yellow. (C) Heat map of expression profiles of IFNγ-associated genes in Foxp3Cre (WT) and Foxp3CremiR-142fl/fl (KO) Treg cells (n = 3 per group). Genes highlighted in red are putative targets of miR-142-3p. (D–F) Intracellular FACS analysis of IFNγ production (D) and Stat1(Y701) phosphorylation (F) in Foxp3Cre (red line) and Foxp3CremiR-142fl/fl (blue line) CD4+Foxp3+ Treg cells. (E) MFI of IFNγ expression in Foxp3Cre (WT) and Foxp3CremiR-142fl/fl (KO) Treg cells (n = 3 per group). (G) FACS analysis of Annexin V-stained splenic CD4+YFP+ Treg cells from Foxp3Cre (WT) or Foxp3CremiR-142fl/fl (KO) mice. Splenocytes were incubated for 24 hours in cell culture in the presence or absence of IFNγ (10 ng/ml) prior to flow cytometry analysis. (H) MFI of Annexin V staining on Treg cells from Foxp3Cre (WT) or Foxp3CremiR-142fl/fl (KO) spleens with or without IFNγ treatment (10 ng/ml). Results are shown as mean ± SD. P values were calculated using 2-tailed Student t test. *, P < 0.05; **, P < 0.01; NS, not significant. The underlying numerical raw data can be found in S1 Table and S1 Data file. The underlying flow cytometry raw data can be found at the Figshare repository. FACS, fluorescence activated cell sorting; IFNγ, interferon gamma; KO, knockout; MFI, mean fluorescence intensity; SCS, seed complementary sequence; SD, standard deviation; Treg, regulatory T; WT, wild-type.
Pathway enrichment analysis of the differentially expressed genes identified significant overrepresentation of biological processes that are associated with innate and adaptive immune responses (S2 Table). In line with these findings, miR-142–deficient Treg cells display an up-regulated expression of diverse cytokine, chemokine, and immune receptor genes (S4A Fig). One potential caveat of this finding is that the aberrant inflammation observed in Foxp3CremiR-142fl/fl mice can conceivably induce pro-inflammatory changes in the gene expression of Treg cells. In addition, expression of several Treg cell signature genes was altered by the loss of miR-142 (S4B Fig). Interestingly, the transcripts up-regulated in miR-142–deficient Treg cells were enriched for genes from the IFNγ signaling network according to the Enrichr software algorithm [31] and the Gene Set Enrichment Analysis (GSEA) [32] (Fig 4C, S4C Fig, S2 Table). In agreement with our bioinformatic findings, we found that miR-142–deficient Treg cells produced significantly more IFNγ in comparison with WT cells (Fig 4D and 4E, S4D Fig) and displayed dysregulation of IFNγ signaling, manifested by a substantial increase in Stat1 activation (Fig 4F, S4E Fig). Because miR-142 deletion impairs Treg cell homeostasis, we examined the effect of IFNγ stimulation on Treg cell survival and found that IFNγ treatment significantly enhanced apoptosis of miR-142–deficient Treg cells in vitro (Fig 4G and 4H).
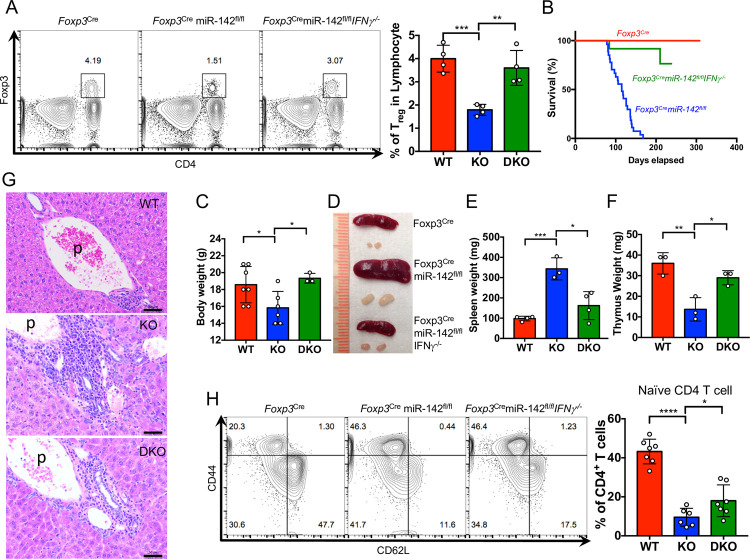
Ifng ablation rescues the Treg homeostatic defect and alleviates development of the systemic autoimmune disorder in Foxp3CremiR-142fl/fl mice
To determine how dysregulated IFNγ signaling impacts the homeostasis of miR-142–deficient Treg cells, we generated Foxp3CremiR-142fl/flIfng−/− double KO mice by crossing Foxp3CremiR-142fl/fl mice with Ifng−/− mice. Intriguingly, our analysis revealed a complete rescue of the Treg homeostatic defect in the double KO mice (Fig 5A). Furthermore, Ifng deletion alleviated development of the systemic lymphoproliferative and fatal autoimmune disease in Foxp3CremiR-142fl/fl mice. This conclusion was supported by observations of improved survival (Fig 5B), body weight normalization (Fig 5C) and marked rescue of the lymphadenopathy (Fig 5D), splenomegaly (Fig 5D and 5E), and thymic involution (Fig 5F) defects in Foxp3CremiR-142fl/flIfng−/− mice. Of note, the double KO mice displayed an abatement of tissue inflammation, evidenced by a significant decrease in the amount of immune cell infiltration into peripheral tissues (Fig 5G). These morphological changes correlated with a modest increase in the number of naive Tconv cells in the periphery of Foxp3CremiR-142fl/flIfng−/− mice (Fig 5H), suggesting that the miR-142-IFNγ signaling axis is primarily important for maintaining Treg cell homeostasis. Despite the restoration of Treg cell frequency in Foxp3CremiR-142fl/flIfng−/− mice, the remaining hyperactivation of CD4+ Tconv cells in the double KO mice implies a potential role for miR-142 in the regulation of Treg cell immunosuppressive activity.
Fig 5. Blockade of IFNγ production rescues the Treg cell homeostatic defect and alleviates systemic autoimmunity in Foxp3CremiR-142fl/fl mice.
(A) Left panel, FACS analysis of splenic Treg cells from 12- to 15-week-old Foxp3Cre (WT), Foxp3CremiR-142fl/fl (KO), and Foxp3CremiR-142fl/flIfng−/− (DKO) mice (n = 4 per group). Foxp3+CD4+ Treg cells are gated and numbers indicate the percentage of cells in the gate. Right panel, frequency of CD4+Foxp3+ Treg cells in Foxp3Cre (WT), Foxp3CremiR-142fl/fl (KO), and Foxp3CremiR-142fl/flIfng−/− (DKO) spleens. (B) Kaplan–Meier survival curves for Foxp3Cre (red line; n = 27), Foxp3CremiR-142fl/fl (blue line; n = 27), and Foxp3CremiR-142fl/flIfng−/− (green line; n = 12) mice. (C) Body weight comparison of 7- to 9-week-old female Foxp3Cre (WT, red bar, n = 7), Foxp3CremiR-142fl/fl (KO, blue bar, n = 6), and Foxp3CremiR-142fl/flIfng−/− (DKO, green bar, n = 3) mice. (D) Representative images of spleen and inguinal lymph nodes from 14-week-old Foxp3Cre, Foxp3CremiR-142fl/fl, and Foxp3CremiR-142fl/flIfng−/− mice. (E) Spleen weights in 12- to 15-week-old Foxp3Cre (WT, red bar, n = 4), Foxp3CremiR-142fl/fl (KO, blue bar, n = 3), and Foxp3CremiR-142fl/flIfng−/− (DKO, green bar, n = 4) mice. (F) Thymus weights (1 lobe) in 6- to 8-week-old Foxp3Cre (WT, red bar), Foxp3CremiR-142fl/fl (KO, blue bar), and Foxp3CremiR-142fl/flIfng−/− (DKO, green bar) mice (n = 3 per group). (G) HE staining of liver tissue sections from Foxp3Cre (WT), Foxp3CremiR-142fl/fl (KO), and Foxp3CremiR-142fl/flIfng−/− (DKO) mice. Note diminished infiltration of leukocytes into liver tissue around portal vein area in DKO mice. Scale bar, 50 μm; p, portal vein. (H) Left panel, FACS analysis of CD44 and CD62L expression in splenic CD4+ T cells from 7- to 15-week-old Foxp3Cre, Foxp3CremiR-142fl/fl, and Foxp3CremiR-142fl/flIfng−/− mice. Numbers indicate percentage of cells in the quadrants. Right panel, frequency of CD44−CD62L+ naive CD4+ T cell in Foxp3Cre (WT, n = 7), Foxp3CremiR-142fl/fl (KO, n = 6), and Foxp3CremiR-142fl/flIfng−/− (DKO, n = 7) spleens. Results are shown as mean ± SD. P values were calculated using 2-tailed Student t test. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. WT, Foxp3Cre; KO, Foxp3CremiR-142fl/fl; DKO, Foxp3CremiR-142fl/flIfng−/−. The underlying numerical raw data can be found in S1 Data file. The underlying flow cytometry raw data can be found at the Figshare repository. DKO, double knockout; FACS, fluorescence activated cell sorting; HE, hematoxylin–eosin; IFNγ, interferon gamma; KO, knockout; SD, standard deviation; WT, wild-type.
miR-142-3p targets multiple IFNγ-associated genes
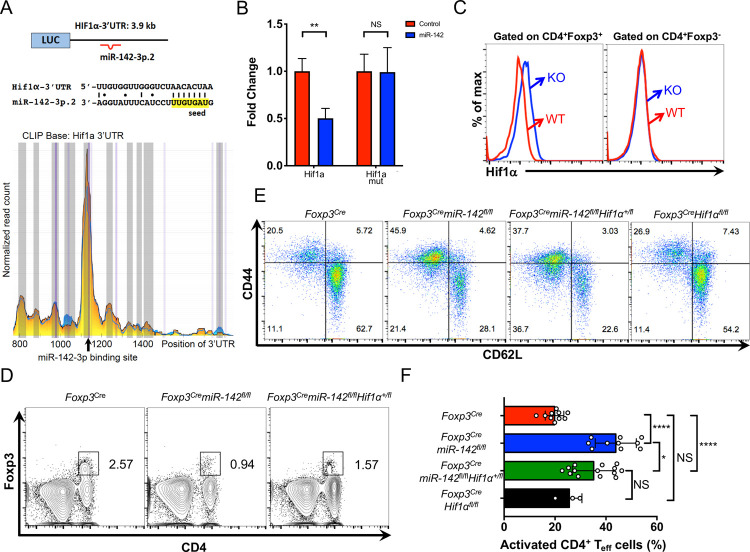
Several of the IFNγ-associated genes that were derepressed in miR-142–deficient Treg cells, including Ifngr2, Stat1, Irf1, and Gbp3, are predicted by the TargetScan algorithm [33] as putative direct targets of miR-142-3p (Fig 4C, S5A Fig). We validated these bioinformatic predictions by examining the high-throughput sequencing of RNAs isolated by cross-linking immunoprecipitation (HITS-CLIP) database that was previously compiled by Rudensky and colleagues [34]. In activated CD4+ T cells, 2 of the 4 (Ifngr2 and Gbp3) target genes showed detectible peaks of Ago2 binding activity in their respective 3′ UTRs, corresponding to predicted binding sites for miR-142-3p (S5A Fig). An extended analysis of the HITS-CLIP database revealed hypoxia-induced factor 1 alpha (Hif1a) as a potential miR-142-3p target (Fig 6A). Our interest in this transcription factor was prompted by a previous report that implicated Hif1a in the regulation of Treg cell homeostasis and suppressive function through the control of IFNγ expression [35]. Using 3′ UTR luciferase reporter assay, we confirmed the capacity of miR-142-3p to attenuate Hif1a and Ifngr2 expression by binding directly to evolutionary conserved seed sequences in their respective 3′ UTRs (Fig 6B, S5B Fig). In line with these findings, we detected a significant increase in Hif1α and IFNγR2 protein levels in miR-142–deficient Treg cells, whereas Hif1α expression in CD4+ Tconv cells remained unchanged (Fig 6C, S5C and S5D Fig). Of note, we did not observe changes in Hif1a mRNA expression in Treg cells lacking miR-142 expression.
Fig 6. Lowering the Hif1a gene dose partially restores the normal size of Treg population and attenuates hyperactivation of peripheral T cells in Foxp3CremiR-142fl/fl mice.
(A) Diagram (top panel) and sequence alignment (middle panel) of miR-142-3p putative binding site in the Hif1a 3′ UTR. HITS-CLIP analysis (bottom panel) of Ago2 binding sites in the Hif1a 3′ UTR in activated CD4+ T cells (blue plot, WT; yellow plot, miR-155 KO). Note the peak of Ago2 binding activity that corresponds to the predicted miR-142-3p binding site (depicted by arrow). (B) Validation of Hif1a as direct miR-142-3p target by the 3′ UTR luciferase reporter assay. Relative expression of WT and miR-142-3p seed mutated Hif1a 3′ UTR reporter constructs upon cotransfection with either miR-142 precursor expressing plasmid or empty vector control. Expression of WT Hif1a 3′ UTR reporter in the presence of empty vector was arbitrarily set to 1. (C) Intracellular FACS analysis of Hif1α expression in CD4+Foxp3+ and CD4+Foxp3- T cells from Foxp3Cre (red line) and Foxp3CremiR-142fl/fl (blue line) spleens. (D) FACS analysis of splenic lymphocytes from 12- to 18-week-old Foxp3Cre, Foxp3CremiR-142fl/fl, and Foxp3CremiR-142fl/flHif1a+/fl mice with anti-CD4 and anti-Foxp3 specific antibodies. Foxp3+CD4+ Treg cells are gated, and numbers indicate the percentage of cells in the gate. (E) FACS analysis of CD44 and CD62L expression in splenic CD4+ T cells from Foxp3Cre, Foxp3CremiR-142fl/fl, Foxp3CremiR-142fl/flHif1a+/fl, and Foxp3CreHif1afl/fl mice. Numbers indicate percentage of cells in the quadrants. (F) Frequency of activated (CD44+CD62L−) splenic CD4+ T cells in Foxp3Cre (n = 10), Foxp3CremiR-142fl/fl (n = 10), Foxp3CremiR-142fl/flHif1a+/fl (n = 14), and Foxp3CreHif1afl/fl (n = 3) mice. Results are shown as mean ± SD. P values were calculated using 2-tailed Student t test. *, P < 0.05;**, P < 0.01; ****, P < 0.0001; NS, not significant. WT, Foxp3Cre; KO, Foxp3CremiR-142fl/fl. The underlying numerical raw data can be found in S1 Data file. The underlying flow cytometry raw data can be found at the Figshare repository. FACS, fluorescence activated cell sorting; Hif1a, hypoxia-induced factor 1 alpha; KO, knockout; SD, standard deviation; WT, wild-type.
In addition, we examined the capacity of miR-142-3p to regulate the expression of phosphodiesterase 3b (Pde3b), which was recently identified as a critical miR-142-5p target gene modulating Treg cell activity [20]. In agreement with the previously published results, we detected an approximately 3-fold increase of Pde3b mRNA expression in miR-142–deficient Treg cells (S1 Table). Analysis of the Pde3b 3′ UTR by the TargetScan algorithm revealed 2 putative poorly conserved miR-142-5p binding sites and a single predicted poorly conserved miR-142-3p binding site (S5E Fig). Using the 3′ UTR luciferase reporter assay, we determined that overexpression of miR-142 precursor can modestly attenuate Pde3b expression. Interestingly, we observed that mutation of the miR-142-3p binding site in the Pde3b 3′ UTR can completely negate the silencing effect of miR-142 on the Pde3b reporter (S5B and S5F Fig). Thus, our findings have uncovered Pde3b as a novel miR-142-3p target gene, providing additional evidence for the critical role of miR-142-3p isoform in the regulation of Treg cell biology.
Hif1a haploinsufficiency partially rescues the Treg cell homeostasis defect in Foxp3CremiR-142fl/fl mice
Next, we sought to determine the contribution of the miR-142-Hif1a axis in Treg cell homeostasis and function. We lowered the Hif1a gene dose in miR-142–deficient Treg cells by crossing Foxp3CremiR-142fl/fl mice with mice carrying a conditional Hif1a allele (Hif1afl/fl). The resultant Foxp3CremiR-142fl/flHif1a+/fl mice expressed normal levels of Hif1α protein in Treg cells (S6A Fig) and displayed a partial rescue of Treg cell abundance (Fig 6D, S6B Fig). Furthermore, Foxp3CremiR-142fl/flHif1a+/fl mice exhibited a partial reduction in the frequency of activated/effector CD4+ T cells in the periphery (Fig 6E and 6F). Nevertheless, Hif1a haploinsufficiency in Foxp3CremiR-142fl/fl mice failed to ameliorate the lethal autoimmunity (S6C Fig), nor did Hif1a deletion restore the suppressive capacity (S6F and S6G Fig) of miR-142-deficient Treg cells and attenuate the peripheral Teff cell hyperactivation (S6H Fig), perhaps because of a failure to effectively rescue aberrant IFNγ production and signaling in Treg cells (S6D and S6E Fig).
Discussion
Although miRNA-mediated posttranscriptional control of gene expression is recognized as crucial for Treg cell development and function [11–13], our understanding of how specific miRNA genes govern Treg cell responses is incomplete. Here, we report that mice with Treg cell–specific miR-142 deletion develop a fatal systemic autoimmune disease due to a severe defect in Treg cell homeostasis and suppressive activity. Furthermore, we found that constitutive miR-142 ablation results in aberrant thymic Treg cell development. Thus, our findings have uncovered an indispensable role for miR-142 in the control of Treg cell–mediated immunological tolerance. We propose that miR-142 plays a dominant role among miRNAs involved in the regulation of Treg cell activity because the phenotype of Foxp3CremiR-142fl/fl mice closely resembles the autoimmune pathology observed in mice with global disruption of miRNA biogenesis in Treg cells [11–13]. This notion should be confirmed by further conditional KO studies of several miRNA genes that were previously implicated in the regulation of Treg cell function, including miR-146a, miR-155, and miR-27 [22,36,37].
The role of miR-142 in Treg cell function was previously examined by Anandagoda and colleagues using a conditional KO mouse model [20]. In contrast with the findings from this report, we determined that deletion of miR-142-3p and not miR-142-5p (as proposed by Anandagoda and colleagues) is the major driver of the Treg cell defect and subsequent systemic autoimmunity in Foxp3CremiR-142fl/fl mice. This conclusion is strongly supported by the observed global derepression of miR-142-3p target genes in miR-142–deficient Treg cells, whereas the levels of the majority of miR-142-5p targets did not significantly change. Our inference of a critical role for miR-142-3p in Treg cells is well aligned with the fact that miR-142-3p is more abundantly expressed in Treg cells than miR-142-5p and a large body of literature that assigns the main regulatory role in immune cells to miR-142-3p [14–18,28–30]. Our data revealing that miR-142-3p can directly bind and regulate the phosphodiesterase Pde3b gene, through which, as Anandagoda and colleagues suggest [20], miR-142 controls Treg cell immunosuppressive activity, further validates miR-142-3p as the key miR-142 isoform in Treg cells.
Our findings indicate that miR-142-3p controls Treg cell homeostasis and function by attenuating IFNγ production and signaling. Interestingly, dysregulation of IFNγ responses is increasingly recognized as a critical factor that negatively impacts Treg cell activity. For example, excessive IFNγ production was reported to drive functional “fragility” of Treg cells in the context of antitumor immunity [38]. Additionally, conditional deletion of the E3 ubiquitin ligase von Hippel–Lindau (Vhl) gene was shown to impair Treg cell function through IFNγ dysregulation [35]. Moreover, unrestrained Stat1 activation and subsequent IFNγ production by Socs1-deficient Treg cells was linked to a severe failure of immunological tolerance [22]. Finally, a loss of functional activity by Dicer-deficient Treg cells was associated with excessive IFNγ production [13].
miRNAs, despite eliciting a moderate effect on the expression of their target genes, often have a significant impact on cellular physiology through coordinated and coherent targeting of multiple key molecules in a signaling cascade [39]. In agreement with this notion, we found that miR-142 is predicted to control expression of several IFNγ-associated genes, including Ifngr2, Gbp3, Stat1, Irf1, and Hif1a and validated some of these as bona fide miR-142-3p targets. We propose that coordinated derepression of these target genes in miR-142–deficient Treg cells drives the observed dysregulation of IFNγ responses. In support of this hypothesis, we found that genetic blockade of IFNγ production rescues the homeostatic defect in miR-142–deficient Treg cells and prevents development of systemic lymphoproliferative and autoimmune disorder in Foxp3CremiR-142fl/fl mice. Despite a complete restoration of normal Treg cell frequency in Foxp3CremiR-142fl/flIfng−/− mice, the partial rescue of peripheral Teff cell hyperactivation in these mice suggests a possibility that miR-142–mediated control of Treg cell function is not limited to the attenuation of IFNγ signaling and likely involves additional molecular targets. Another caveat of our genetic epistasis experiments using Foxp3CremiR-142fl/flIfng−/− mice is a potential non–cell-autonomous effect of IFNγ deletion on miR-142–deficient Treg cells. Because we found a significant IFNγ up-regulation in both miR-142–deficient Treg cells and miR-142–sufficient Teff cells in Foxp3CremiR-142fl/fl mice, the global IFNγ ablation might potentially impact miR-142–deficient Treg cell function in cell-extrinsic manner via changes in their IFNγ-rich inflammatory environment. Future analysis of miR-142–sufficient and miR-142–deficient Treg cells from animals that lack IFNγ expression such as Foxp3CremiR-142fl/flIfng−/− and female Foxp3Cre/+miR-142fl/fl mice will be required to corroborate the intrinsic effect of IFNγ on miR-142-deficient Treg cells and uncover the additional signaling pathways through which miR-142 mediates its regulatory functions in Treg cells.
Our investigation of Hif1a, a validated miR-142-3p target, revealed an important role for the miR-142-Hif1a axis in the regulation of Treg cell homeostasis. We observed that lowering the Hif1a gene dose in miR-142–deficient Treg cells partially rescued the Treg homeostatic defect and modestly reduced the hyperactivation of peripheral Teff cells in Foxp3CremiR-142fl/fl mice. The role suggested by our findings for Hif1a as a negative regulator of Treg cell homeostasis is consistent with the conclusions of 2 previous reports [35,38]. However, Hif1a haploinsufficiency had little impact on the dysregulated IFNγ production in miR-142–deficient Treg cells and failed to prevent development of fatal autoimmunity in Foxp3CremiR-142fl/fl mice. This outcome is not surprising given the fact that the Treg cell number in Foxp3CremiR-142fl/flHif1a+/fl mice is only partially restored. The failure of Hif1a haploinsufficiency to fully rescue the Treg cell defect in Foxp3CremiR-142fl/fl mice is probably linked to the abundance of miR-142-3p target genes in the IFNγ signaling pathway. The existence of multiple miR-142-3p targets likely makes the dysregulated state of IFNγ signaling in miR-142–deficient Treg cells refractory to changes in the expression of a single target. This conclusion is supported by studies in miR-142-3p-deficient zebra danio, which display impaired myelopoiesis due to an aberrant activation of the IFNγ signaling pathway [17]. This developmental defect in miR-142-3p-deficient zebra danio could be rescued by a compound knockdown of stat1a and irf1b genes, whereas silencing of either factor alone was insufficient to restore normal neutrophil differentiation. Of note, based on the observations of dysregulated IFNγ signaling in miR-142–deficient immune cells from zebra danio and rodents, the role of miR-142 in suppressing IFNγ signaling appears to be evolutionary conserved. In contrast, the function of miR-142-Pde3b signaling axis is unlikely to be evolutionary preserved, because miR-142-3p and miR-142-5p binding sites in the mouse Pde3b 3′ UTR are poorly conserved.
In summary, our results have established miR-142 as a central regulator of Treg cell development, homeostasis, and suppressive activity that mediates its function in Treg cells in part by limiting IFNγ production and responsiveness. Besides advancing our understanding of the Treg cell biology, these novel insights may open a new avenue for targeted pharmacological manipulation of Treg cell activity in cancer immunotherapy and autoimmune disease settings.
Materials and methods
Mice
C57BL/6J (stock#000664), B6/CD45.1 (stock#002014), Hif1afl/fl (stock#007561), and Foxp3YFP-Cre (stock#016959) mice were purchased from the Jackson Laboratory (Bar Harbor, Maine, USA). BALB/c mice were obtained from Charles River Laboratories (Wilmington, Massachusetts, USA). miR-142fl/fl mice were described previously [14]. Foxp3CremiR-142fl/fl mice were generated by crossing miR-142fl/fl mice with Foxp3YFP-Cre deleter/reporter mice [21]. Mice were kept in a specific pathogen-free facility at the City of Hope Animal Resource Center, and all animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the City of Hope. Our approved animal protocols that are relevant for this work are the following: IACUC#13021, IACUC#13020, and IACUC#03008.
Flow cytometry
For surface marker analysis, single cell suspensions from thymus, spleen, and peripheral lymph nodes (axillary, brachial, inguinal, and cervical) were treated with red blood cell (RBC) lysis buffer (BioLegend, San Diego, California, USA) to eliminate mature erythrocytes and then blocked with anti-CD16/CD32 antibody (Ab) to prevent nonspecific binding. Cells were stained with monoclonal fluorophore-conjugated antibodies against specific cell surface markers, such as anti-CD4 (clone RM4-5), anti-CD8α (clone 53–6.7), anti-CD25 (clone PC61), anti-CD44 (clone IM7), anti-CD62L (clone MEL-14), anti-CD45.1 (clone A20), anti-ICOS (clone 7E.17G9), anti-GITR (clone DTA-1), anti-CTLA-4 (clone UC10-4B9), anti-PD-1 (clone 29F.1A12), and anti-CD103 (clone 2E7) antibodies (all from BioLegend). Intracellular staining with anti-Foxp3 (clone FJK-16s; eBioscience, Santa Clara, California, USA) and anti-Hif1α (clone 241812; R&D Systems, Minneapolis, Minnesota, USA) antibodies was performed using fixed and permeabilized cells following the manufacturer’s protocol. For detection of phosphorylated Stat1 (pStat1), purified CD4+ T cells were first stimulated with IFNγ (100 ng/mL) for 24 hours, fixed with Cytofix buffer and permeabilized with Phosflow Perm Buffer III, and subsequently stained with anti-pStat1 (Y701; clone 4a; BD Biosciences, San Jose, California, USA) antibodies. For intracellular cytokine staining, T cells derived from spleen and lymph nodes or purified Treg cells were first stimulated with phorbol 12-myristate 13-acetate (PMA; 50 ng/mL) and ionomycin (500 ng/mL) for 4 hours in the presence of monensin (2 μM) and then fixed, permeabilized, and stained following the manufacturer’s protocol (BioLegend). Data were acquired on Accuri C6 flow cytometer (BD Biosciences) and analyzed with FlowJo software BD Biosciences (San Jose, California, USA). Cell sorting for the analysis of miR-142 expression in T-cell lineage was performed using FACSAriaIII (BD Biosciences) instrument.
All flow cytometry raw data generated in this study can be found at the Figshare data repository (https://figshare.com/projects/microRNA-142_guards_against_autoimmunity_by_controlling_Treg_cell_homeostasis_and_function/128960).
Global transcriptome profiling by RNA-seq
CD4+YFP+ Treg cells were sorted using BD FACSAria II machine from single-cell splenocyte suspensions derived from 8- to 11-week-old Foxp3Cre and Foxp3CremiR-142fl/fl female mice (n = 3 per group). Total RNA from purified WT and miR-142–deficient Treg cells was isolated using miRNeasy kit (QIAGEN, Hilden, Germany) and subjected to RNA-seq. RNA quality was determined with an Agilent Bioanalyzer (RNA integrity number (RIN) > 7.5 for all samples). Library was prepared according to the manufacturer’s protocol using Illumina TruSeq RNA Library Prep Kit v2 (San Diego, Califonia, USA) and subsequently loaded on an Illumina HiSeq 2500 for parallel sequencing. The 51 base pair single-ended sequence reads were mapped to the mouse reference genome (mm10) using the alignment program HISAT (https://daehwankimlab.github.io/hisat2). Gene expression was measured from alignment bam files by read counting function featureCounts in the Bioconductor package Rsubread (http://bioconductor.org/packages/Rsubread). The unstranded raw counts were then normalized using a trimmed mean of M values (TMM) method implemented in the Bioconductor package edgeR (https://bioconductor.org/packages/edgeR). A total of 11,658 genes having counts per million (CPM) values higher than 1 in at least 3 samples were included in the downstream differential expression analysis. Differentially expressed genes were tested using 3 statistical methods in edgeR, including the generalized linear model (GLM) quasi-likelihood F (QLF) test, the likelihood ratio (LR) test, and the exact test based on quantile-adjusted conditional maximum likelihood (qCML) methods. A complete list of genes and the statistical test results (miR-142–deficient (KO) versus miR-142–sufficient (WT) Treg cells) are shown in S1 Table. Statistical P values were adjusted by Benjamini–Hochberg method for false discovery rate (FDR) controls. The volcano plot visualizing the distribution of differentially expressed genes was based on QLF test results. The RNA-seq raw data were deposited in the Gene Expression Omnibus under the accession number GSE190192.
Pathway and GSEA
Differentially expressed genes passing the criterion of FDR lower than 0.05 for all 3 statistical methods mentioned above were subjected for pathway and GSEA using Enrichr (http://amp.pharm.mssm.edu/Enrichr) software algorithm [31]. Top ranked pathways across major databases, including Panther (http://www.pantherdb.org), Reactome (https://reactome.org), KEGG (https://www.genome.jp/kegg), and WikiPathways (https://www.wikipathways.org) were identified and are listed in S2 Table. In addition, GSEA analysis (http://software.broadinstitute.org/gsea/index.jsp)) [32] was performed with the CPM values from the 11,658 expressed genes rank-ordered by Signal2Noise metric. Gene ontology gene sets from Molecular Signatures Database (MSigDB) v7.1 were evaluated for the enrichment.
Sylamer analysis
Analysis of miRNA seed enrichment in the 3′ UTRs of genes that are differentially expressed in miR-142 KO Treg cells was performed by the Web-based SylArray software algorithm (http://www.ebi.ac.uk/enright-srv/sylarray)) [26].
In vitro Treg cell suppression assay
CD4+ T cells were isolated from Foxp3Cre, Foxp3CremiR-142fl/fl, and Foxp3CremiR-142fl/flHif1afl/fl spleens using anti-CD4-biotin (clone GK1.5), anti-Biotin MicroBeads (Miltenyi Bergisch, Gladbach, North Rhine-Westphalia, Germany), LS columns (Miltenyi), and a MiniMACS Separator (Miltenyi). Enriched CD4+ T cells were sorted for YFP+(Treg) and YFP−(Tcon) cells using BD FACS Fusion. Moreover, 105 Foxp3Cre CD4+YFP− Tcon cells were labeled with 4μM CellTrace Violet (CTV) (Thermo Fisher, Waltham, Massachusetts, USA) at 37°C for 7.5 minutes and cultured with either Foxp3CremiR-142fl/fl or Foxp3CremiR-142fl/flHif1afl/fl CD4+YFP+ Treg cells at ratios of 1:0.5 and 1:0.125 in the presence of CD3ɛ/CD28-conjugated MACSiBead Particles (mouse T Cell Activation/Expansion Kit, Miltenyi) at a bead-to-cell ratio of 2:1. After 3 days of culturing, proliferation of Foxp3Cre CD4+YFP− Tcon cells was measured by flow cytometry as CTV dilution.
In vitro Treg survival assay
CD4+ T cells isolated from Foxp3Cre and Foxp3CremiR-142fl/fl spleens by EasySep Mouse CD4+ T Cell Isolation Kit (STEMCELL Technologies, Vancouver, British Columbia, Canada) were sorted for YFP expressing cells using BD FACSAria II instrument. CD4+YFP+ Treg cells were stimulated with anti-CD3 (1 μg/mL) specific antibodies for 48 hours, stained with Annexin V-PE (BioLegend) and analyzed by flow cytometry. For the IFNγ-induced apoptosis of Treg cells assay, splenocytes were cultured for 24 hours in the presence or absence of IFNγ (10 ng/mL) and then harvested and stained with Annexin V-PE for fluorescence activated cell sorting (FACS) analysis.
In vivo labeling of Treg cells with BrdU
Foxp3Cre and Foxp3CremiR-142fl/fl mice were injected intraperitoneally with 1 mg of BrdU and splenic BrdU+ Treg cells (CD4+YFP+) were quantified by flow cytometry 16 hours postinjection. Intracellular staining with anti-BrdU specific antibodies was performed using BrdU Flow Kit (BD Biosciences).
aGVHD mouse model
BALB/c recipient mice were lethally irradiated (850 cGy) 8 to 10 hours prior to bone marrow transplantation (BMT) and subsequently transplanted via intravenous injection with T-cell–depleted bone marrow from C57BL/6J mice (TCD-BM, 2.5 × 106 cells), Thy1.2+CD4+ T cells from C57BL/6J spleens (0.5 to 0.6 × 106 cells), and CD4+YFP+ Treg cells derived from either Foxp3Cre or Foxp3CremiR-142fl/fl spleens (0.1 to 0.12 × 106 cells). Body weight and severity of diarrhea in host mice were monitored and recorded for 15 days after BMT. Donor Treg cell frequency in the host spleen was assessed by FACS on day 17 after transplantation.
miRNA qRT-PCR
Total RNA was isolated using miRNeasy kit (QIAGEN) and reverse transcribed using the TaqMan MicroRNA Reverse Transcription Kit (Life Technologies, Carlsbad, California, USA). Mature miR-142-3p expression was assessed by TaqMan Real-Time miRNA assay (Life Technologies) and normalized to snoRNA234 levels.
3′ UTR luciferase reporter assays
DNA fragments encompassing WT and miR-142-3p seed mutated 3′ UTRs of mouse Hif1a, Ifngr2, and Pde3b genes were synthesized and cloned into pMIR-Report vector (Ambion, Austin, Texas, USA). The sequence complementary to the miR-142-3p.2 seed sequence (8-mer) in the Ifngr2 3′ UTR was mutated from 5′-AACACTAA-3′ to 5′-ACGTACCA-3′. The sequence complementary to the miR-142-3p.2 seed sequence (8-mer) in the Hif1a 3′ UTR was mutated from 5′- AACACTAA-3′ to 5′- ACGTACCA-3′. The sequence complementary to the miR-142-3p.1 seed sequence (6-mer) in the Pde3b 3′ UTR was mutated from 5′- CACTAC-3′ to 5′- CCAGCC-3′. To perform the 3′ UTR reporter assays, 105 293T cells in 24-well plates were transiently transfected using calcium phosphate with 10 ng of pMIR-Report-3′ UTR firefly luciferase plasmid, 20 ng of Renilla luciferase reporter plasmid (pRL-SV40; Promega, Madison, Wisconsin, USA), and 450 ng of either pMDH1-miR-142 or control pMDH1 vector (Addgene, Watertown, Massachusetts, USA). Cells were lysed 48 hours posttransfection, and the luciferase activities were assessed using Dual Luciferase Reporter assay (Promega).
IFNγ ELISA
CD4+ T cells purified from Foxp3Cre and Foxp3CremiR-142fl/fl splenocytes with the help of EasySep Mouse CD4+ T Cell Isolation Kit (STEMCELL Technologies) were sorted for YFP expressing cells using BD FACSAria II instrument. CD4+YFP+ Treg cells (106/mL) were stimulated with anti-CD3 (5 μg/mL) and anti-CD28 (2 μg/mL) specific antibodies for 48 hours in the presence of IL-2 (50 ng/mL) and cell culture supernatants were assessed for IFNγ production by sandwich ELISA. Briefly, 96-well flat-bottom Maxisorp (Thermo Fisher) plates were coated with anti-mouse IFNγ-specific capture antibodies (eBioscience, clone XMG1.2), and the captured mouse IFNγ was detected with biotin labeled anti-mouse IFNγ-specific antibodies (eBioscience, clone R4-6A2). Mouse recombinant IFNγ (eBioscience) was used as a standard to quantify the results.
Histopathology
For histological sectioning, mouse tissues were collected and placed into 10% formalin, fixed for 24 hours, washed, and transferred to 70% ethanol before standard paraffin embedding. Tissue sections were stained with hematoxylin and eosin and examined by an experienced veterinary pathologist.
Statistical analysis
All statistical analyses were performed using Prism 6 (GraphPad, San Diego, California, USA) software. Statistical analyses were performed using 2-tailed Student t test or ANOVA. Results were considered significant when P ≤ 0.05.
Supporting information
(A) Impaired Treg cell development in miR-142−/− mice. Left panel, FACS analysis of WT and miR-142−/− thymocytes with anti-CD4 and anti-Foxp3 antibodies. Foxp3+CD4+ Treg cells are gated and numbers indicate the percentage of cells in the gate. Right panel, frequency of CD4+Foxp3+ Treg cells in WT and miR-142−/− thymi (n = 3 per group). (B, C) Treg cell defect in the periphery of miR-142−/− mice. FACS analysis of Treg cells in spleen (B) and MLNs (C) from WT and miR-142−/− mice. Right panel, frequency of CD4+Foxp3+ Treg cells in WT and miR-142−/− spleens and MLNs (n = 4 per group). (D) qRT-PCR analysis of mature miR-142-3p and miR-142-5p expression in different T-cell subsets purified from Foxp3Cre mice (n = 2). DN, double negative CD4-CD8- thymocytes; DP, double positive CD4+CD8+ thymocytes; SP, single positive CD4+YFP− thymocytes; Treg, CD4+YFP+ Treg cells from thymus and spleen, respectively; TNaive, naive CD4+YFP−CD62L+CD44− splenic T cells; TActivated, activated CD4+YFP−CD62L−CD44+ splenic T cells. Expression level of miR-142-3p in DN population was arbitrarily set to 1. snoRNA234 levels were used for normalization. (E) qRT-PCR analysis of mature miR-142-3p expression in CD4+YFP+ Treg and CD4+YFP− Teff cells purified from Foxp3Cre and Foxp3CremiR-142fl/fl spleens. Expression level of miR-142-3p in CD4+YFP+ Treg cells isolated from Foxp3CremiR-142fl/fl spleen was arbitrarily set to 1. snoRNA234 levels were used for normalization. Spleen (F) and thymus (G) weights in 8- to 11-week-old male Foxp3Cre and Foxp3CremiR-142fl/fl mice (n ≥ 7 per group). Absolute cell counts in spleen (H), thymus (I), and peripheral lymph nodes (J) from Foxp3Cre and Foxp3CremiR-142fl/fl mice (n = 6 per group). Results are shown as mean ± SD. P values were calculated using 2-tailed Student t test. **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; NS, not significant. The underlying numerical raw data can be found in S1 Data file. The underlying flow cytometry raw data can be found at the Figshare repository. FACS, fluorescence activated cell sorting; KO, knockout; MLN, mesenteric lymph node; PLN, peripheral lymph node; SD, standard deviation; SP, spleen; Treg, regulatory T; WT, wild-type; YFP, yellow fluorescent protein.
(PDF)
(A) FACS analysis of lymphocytes from PLNs (left panel) and thymus (right panel) of 12-week-old Foxp3Creand Foxp3CremiR-142fl/fl mice with anti-CD4 and anti-Foxp3 specific antibodies. Foxp3+CD4+ Treg cells are gated and numbers indicate the percentage of cells in the gate. (B) Frequency (left panel) and total number (right panel) of Treg cells in PLNs and thymi of 12-week-old Foxp3Cre (red bars) and Foxp3CremiR-142fl/fl (blue bars) mice (n = 3 per group). (C) FACS analysis of CD44 and CD62L expression on CD4+ (upper panel) and CD8+ (bottom panel) T cells from Foxp3Cre and Foxp3CremiR-142fl/fl PLNs. Numbers indicate percentage of cells in the quadrants. (D) Frequency of CD44−CD62L+ (naive) and CD44+CD62L− (activated) CD4+ (left panel) and CD8+ (right panel) T cells in Foxp3Cre and Foxp3CremiR-142fl/fl PLNs (n = 6 per group). (E) Intracellular FACS analysis of IFNγ production by CD4+ (upper panel) and CD8+ (bottom panel) T cells from Foxp3Cre and Foxp3CremiR-142fl/fl PLNs. IFNγ+ T cells are gated and numbers indicate percentage of cells in the gate. (F) Frequency of IFNγ-expressing CD4+ and CD8+ T cells in Foxp3Cre (filled bars) and Foxp3CremiR-142fl/fl (open bars) PLNs (n = 4 per group). (G) Total CD4+ and CD8+ T cell counts in Foxp3Cre (filled bars) and Foxp3CremiR-142fl/fl (open bars) PLNs (n = 6 per group). (H) Frequencies of IFNγ-, IL-4-, and IL-17-expressing CD4+ T cells isolated from Foxp3Cre (filled bars) and Foxp3CremiR-142fl/fl (open bars) spleens (n ≥ 3 per group). Results are shown as mean ± SD. P values were calculated using 2-tailed Student t test. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; NS, not significant. The underlying numerical raw data can be found in S1 Data file. The underlying flow cytometry raw data can be found at the Figshare repository. FACS, fluorescence activated cell sorting; IFNγ, interferon gamma; IL, interleukin; PLN, peripheral lymph node; SD, standard deviation; Treg, regulatory T.
(PDF)
(A) FACS analysis of Treg suppression activity in vitro. Purified CD4+ Tconv cells were loaded with CTV dye and incubated with FACS-sorted Treg cells from Foxp3Cre (red line) and Foxp3CremiR-142fl/fl (blue line) spleens in the presence of beads coated with anti-CD3 and anti-CD28 specific antibodies. Several Treg to Tconv cell ratios were shown as indicated. (B) Left panel, FACS analysis of Annexin V-stained CD4+YFP+ Treg cells from Foxp3Cre (WT) or Foxp3CremiR-142fl/fl (KO) spleens. Right panel, MFI of Annexin V staining on Treg cells from Foxp3Cre (WT) or Foxp3CremiR-142fl/fl (KO) spleens. (C) Frequency of splenic YFP+ Treg cells in female Foxp3Cre/WTmiR-142+/+ and Foxp3Cre/WTmiR-142fl/fl mice. (D) MFI of GITR, CD25, CD103, PD-1, CTLA-4, and ICOS expression on splenic CD4+YFP+ Treg cells from Foxp3Cre (WT) or Foxp3CremiR-142fl/fl (KO) mice. Results are shown as mean ± SD. P values were calculated using 2-tailed Student t test *, P < 0.05; **, P < 0.01; NS, not significant. The underlying numerical raw data can be found in S1 Data file. The underlying flow cytometry raw data can be found at the Figshare repository. CTV, cell trace violet; FACS, fluorescence activated cell sorting; KO, knockout; MFI, mean fluorescence intensity; Treg, regulatory T; WT, wild-type; YFP, yellow fluorescent protein.
(PDF)
Heatmap visualization of differentially expressed cytokine, chemokine, and immune receptor genes (A) and Treg cell signature genes (B). Genes highlighted in red are putative miR-142-3p targets. (C) Standard enrichment plot demonstrating the enrichment of up-regulated genes in miR-142–deficient Treg cells from gene ontology term “regulation of response to interferon gamma” using the GSEA tool. (D) ELISA analysis of IFNγ production by Foxp3Cre (WT) and Foxp3CremiR-142fl/fl (KO) Treg cells (n = 4 per group). Purified CD4+YFP+ Treg cells (106/mL) were stimulated with anti-CD3 (5 μg/ml) and anti-CD28 (2 μg/ml) antibodies in the presence of IL-2 (50 ng/ml) for 48 hours. (E) MFI of phospho-Stat1(Y701) levels in Foxp3Cre (WT; n = 6) and Foxp3CremiR-142fl/fl (KO; n = 5) Treg cells. P values were calculated using 2-tailed Student t test. *, P < 0.05; **, P < 0.01. The underlying raw data can be found in S1 Data file. GSEA, Gene Set Enrichment Analysis; IFNγ, interferon gamma; IL, interleukin; KO, knockout; MFI, mean fluorescence intensity; Treg, regulatory T; WT, wild-type; YFP, yellow fluorescent protein.
(PDF)
(A) Diagrams (top left) and sequence alignments (top right) of putative miR-142-3p binding sites in the 3′ UTRs of Stat1, Ifngr2, Irf1, Gbp3, and Pde3b genes. HITS-CLIP analysis of Ago2 binding to the 3′ UTRs of Ifngr2 (bottom-left) and Gbp3 (bottom-right) genes in WT (blue plot) and miR-155 KO (yellow plot) activated CD4+ T cells. Sequences corresponding to miR-142-3p binding sites are labeled by arrows. (B) Validation of Pde3b and Ifngr2 as direct miR-142-3p targets by the 3′ UTR luciferase reporter assay (n = 2). Relative expression of WT and miR-142-3p seed mutated Pde3b and Ifngr2 3′ UTR reporter constructs upon cotransfection with either miR-142 precursor expressing plasmid or empty vector control. Expression of WT Pde3b and Ifngr2 3′ UTR reporters in the presence of empty vector were set to 1. (C) MFI of Hif1α in Foxp3Cre (WT; red bars) and Foxp3CremiR-142fl/fl (KO; blue bars) CD4+Foxp3+ Treg and CD4+Foxp3- Tconv cells (n = 6 per group). (D) Left panel, FACS analysis of IFNγR2 expression in CD4+YFP+ T cells from Foxp3Cre (red line) and Foxp3CremiR-142fl/fl (blue line) spleens; right panel, MFI of IFNγR2 in Foxp3Cre (WT; red bars) and Foxp3CremiR-142fl/fl (KO; blue bars) CD4+YFP+ Treg cells. (E) Schematic diagram and sequence conservation of 2 miR-142-5p and one miR-142-3p binding sites in the 3′ UTR of mouse Pde3b gene as determined by the TargetScan algorithm. (F) Sequence alignment of miR-142-3p binding sites in mouse Pde3b-WT and Pde3b-MUT 3′ UTR reporter constructs. Results are shown as mean ± SD. P values were calculated using 2-tailed Student t test. *, P < 0.05; **, P < 0.01; ***, P < 0.001; NS, not significant. The underlying numerical raw data can be found in S1 Data file. The underlying flow cytometry raw data can be found at the Figshare repository. FACS, fluorescence activated cell sorting; IFNγ, interferon gamma; KO, knockout; MFI, mean fluorescence intensity; SD, standard deviation; Treg, regulatory T; WT, wild-type; YFP, yellow fluorescent protein.
(PDF)
(A) Relative Hif1α protein expression in Foxp3Cre (WT; red bar), Foxp3CremiR-142fl/fl (KO; blue bar), and Foxp3CremiR-142fl/flHif1a+/fl (HET; green bar) Treg cells. The Hif1α levels in WT Treg cells were arbitrarily set to 1. (B) Frequency of CD4+Foxp3+ Treg cells in splenic lymphocytes from Foxp3Cre (WT; red bar; n = 7), Foxp3CremiR-142fl/fl (KO; blue bar; n = 5) and Foxp3CremiR-142fl/flHif1a+/fl (HET; green bar; n = 3) mice. (C) Kaplan–Meier survival curves for Foxp3Cre (red line; n = 27), Foxp3CremiR-142fl/fl (blue line; n = 27), and Foxp3CremiR-142fl/flHif1a+/fl (green line; n = 8) mice. Analysis of IFNγ production (D) and Stat1 activation (E) in Treg cells from Foxp3CremiR-142fl/flHif1a+/fl mice. Left panels, intracellular FACS analysis of splenic CD4+Foxp3+ Treg cells from Foxp3Cre (red line; WT), Foxp3CremiR-142fl/fl (blue line; KO) and Foxp3CremiR-142fl/flHif1a+/fl (green line; HET) mice with anti-IFNγ (D) and anti-pStat1 (Y701) (E) antibodies. Right panels, MFI of IFNγ and phospho-Stat1 (pSTAT1) in Foxp3Cre (WT; red bar), Foxp3CremiR-142fl/fl (KO; blue bar) and Foxp3CremiR-142fl/flHif1a+/fl (HET; green bar) Treg cells. (F, G) FACS analysis of immunosuppressive activity of Treg cells derived from Foxp3Cre (WT; red dot), Foxp3CremiR-142fl/fl (KO; blue dot) and Foxp3CremiR-142fl/flHif1afl/fl (DKO; green dot) mice (n = 3) in vitro. Several Treg to Tconv cell ratios were analyzed as indicated in the graph. Unstimulated Tconv cells were used as control. Representative FACS plot analysis is shown in G. (H) FACS analysis of CD44 and CD62L expression in splenic CD4+ T cells from Foxp3Cre, Foxp3CremiR-142fl/flHif1a+/fl, and Foxp3CremiR-142fl/flHif1afl/fl mice. Numbers indicate percentage of cells in the quadrants. Results are shown as mean ± SD. P values were calculated using 2-tailed Student t test. *, P < 0.05; **, P < 0.01; ****, P < 0.0001; NS, not significant. The underlying numerical raw data can be found in S1 Data file. The underlying flow cytometry raw data can be found at the Figshare repository. DKO, double knockout; FACS, fluorescence activated cell sorting; IFNγ, interferon gamma; KO, knockout; MFI, mean fluorescence intensity; pStat1, phosphorylated Stat1; SD, standard deviation; Treg, regulatory T; WT, wild-type.
(PDF)
(XLSX)
Treg, regulatory T.
(XLSX)
(XLSX)
Acknowledgments
We would like to thank Dr. Zuoming Sun and members of the Boldin laboratory at the City of Hope for helpful discussions and suggestions regarding the manuscript. We thank the City of Hope Animal Research Center for their help and care in breeding and maintaining our mouse colonies.
Abbreviations
- Ab
antibody
- aGVHD
acute graft-versus-host disease
- BMT
bone marrow transplantation
- BrdU
5-bromo-2-deoxyuridine
- CPM
counts per million
- CTLA4
cytotoxic T-lymphocyte associated protein 4
- CTV
CellTrace Violet
- DC
dendritic cell
- DP
double positive
- FACS
fluorescence activated cell sorting
- FDR
false discovery rate
- GITR
glucocorticoid-induced tumor necrosis factor receptor family-related gene
- GLM
generalized linear model
- GSEA
Gene Set Enrichment Analysis
- Hif1a
hypoxia-induced factor 1 alpha
- HITS-CLIP
high-throughput sequencing of RNAs isolated by cross-linking immunoprecipitation
- IACUC
Institutional Animal Care and Use Committee
- ICOS
inducible T-cell co-stimulator
- IFNγ
interferon gamma
- IL
interleukin
- KO
knockout
- LR
likelihood ratio
- miRNA
microRNA
- PD-1
programmed death-1
- PMA
phorbol 12-myristate 13-acetate
- pStat1
phosphorylated Stat1
- pTreg
peripheral Treg
- qCML
quantile-adjusted conditional maximum likelihood
- QLF
quasi-likelihood F
- RBC
red blood cell
- RIN
RNA integrity number
- RNA-seq
RNA sequencing
- SP
single positive
- Tconv
conventional T
- TCR
T cell receptor
- Teff
T effector
- Th1
T helper cell 1
- Treg
regulatory T
- TMM
trimmed mean of M values
- VHL
von Hippel–Lindau
- WT
wild-type
- YFP
yellow fluorescent protein
Data Availability
The underlying raw data can be found within the paper and its Supporting Information files. The RNA-seq data used in the paper are publicly available from the GEO database (accession number GSE190192). The underlying flow cytometry raw data can be found at the Figshare data repository (https://figshare.com/projects/microRNA-142_guards_against_autoimmunity_by_controlling_Treg_cell_homeostasis_and_function/128960).
Funding Statement
This study was funded in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health R01 AI125615 (to M. P. B.) and R01 AI146313 (to M. P. B.) grants and the American Association of Immunologists Careers in Immunology Fellowship (to M. P. B. and W. L. W.). The work in the Zeng lab was supported by the National Cancer Institute of the National Institutes of Health R01 CA228465 grant (to D. Z.). Research reported in this publication includes work performed in the Analytical Cytometry, Integrative Genomics and Veterinary Pathology Cores supported by the National Cancer Institute of the National Institutes of Health under P30 CA033572 award (to City of Hope Comprehensive Cancer Center). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–64. doi: 10.1146/annurev.immunol.25.022106.141623 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–87. doi: 10.1016/j.cell.2008.05.009 . [DOI] [PubMed] [Google Scholar]
- 3.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27(1):20–1. doi: 10.1038/83713 . [DOI] [PubMed] [Google Scholar]
- 4.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–6. doi: 10.1038/ni904 . [DOI] [PubMed] [Google Scholar]
- 5.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–61. doi: 10.1126/science.1079490 . [DOI] [PubMed] [Google Scholar]
- 6.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4(4):337–42. doi: 10.1038/ni909 . [DOI] [PubMed] [Google Scholar]
- 7.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27(1):18–20. doi: 10.1038/83707 . [DOI] [PubMed] [Google Scholar]
- 8.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198(12):1875–86. doi: 10.1084/jem.20030152 ; PubMed Central PMCID: PMC2194145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidson TS, DiPaolo RJ, Andersson J, Shevach EM. Cutting Edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3+ T regulatory cells. J Immunol. 2007;178(7):4022–6. doi: 10.4049/jimmunol.178.7.4022 . [DOI] [PubMed] [Google Scholar]
- 10.Haribhai D, Williams JB, Jia S, Nickerson D, Schmitt EG, Edwards B, et al. A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity. 2011;35(1):109–22. doi: 10.1016/j.immuni.2011.03.029 ; PubMed Central PMCID: PMC3295638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chong MM, Rasmussen JP, Rudensky AY, Littman DR. The RNAseIII enzyme Drosha is critical in T cells for preventing lethal inflammatory disease. J Exp Med. 2008;205(9):2005–17. doi: 10.1084/jem.20081219 ; PubMed Central PMCID: PMC2526196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liston A, Lu LF, O’Carroll D, Tarakhovsky A, Rudensky AY. Dicer-dependent microRNA pathway safeguards regulatory T cell function. J Exp Med. 2008;205(9):1993–2004. doi: 10.1084/jem.20081062 ; PubMed Central PMCID: PMC2526195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou X, Jeker LT, Fife BT, Zhu S, Anderson MS, McManus MT, et al. Selective miRNA disruption in T reg cells leads to uncontrolled autoimmunity. J Exp Med. 2008;205(9):1983–91. doi: 10.1084/jem.20080707 ; PubMed Central PMCID: PMC2526194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kramer NJ, Wang WL, Reyes EY, Kumar B, Chen CC, Ramakrishna C, et al. Altered lymphopoiesis and immunodeficiency in miR-142 null mice. Blood. 2015;125(24):3720–30. Epub 2015/05/02. doi: 10.1182/blood-2014-10-603951 . [DOI] [PubMed] [Google Scholar]
- 15.Chapnik E, Rivkin N, Mildner A, Beck G, Pasvolsky R, Metzl-Raz E, et al. miR-142 orchestrates a network of actin cytoskeleton regulators during megakaryopoiesis. Elife. 2014;3:e01964. Epub 2014/05/27. doi: 10.7554/eLife.01964 ; PubMed Central PMCID: PMC4067751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mildner A, Chapnik E, Manor O, Yona S, Kim KW, Aychek T, et al. Mononuclear phagocyte miRNome analysis identifies miR-142 as critical regulator of murine dendritic cell homeostasis. Blood. 2013;121(6):1016–27. doi: 10.1182/blood-2012-07-445999 . [DOI] [PubMed] [Google Scholar]
- 17.Fan HB, Liu YJ, Wang L, Du TT, Dong M, Gao L, et al. miR-142-3p acts as an essential modulator of neutrophil development in zebrafish. Blood. 2014;124(8):1320–30. doi: 10.1182/blood-2013-12-545012 . [DOI] [PubMed] [Google Scholar]
- 18.Mildner A, Chapnik E, Varol D, Aychek T, Lampl N, Rivkin N, et al. MicroRNA-142 controls thymocyte proliferation. Eur J Immunol. 2017;47(7):1142–52. doi: 10.1002/eji.201746987 . [DOI] [PubMed] [Google Scholar]
- 19.Sun Y, Oravecz-Wilson K, Mathewson N, Wang Y, McEachin R, Liu C, et al. Mature T cell responses are controlled by microRNA-142. J Clin Invest. 2015;125(7):2825–40. doi: 10.1172/JCI78753 ; PubMed Central PMCID: PMC4563679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anandagoda N, Willis JC, Hertweck A, Roberts LB, Jackson I, Gokmen MR, et al. microRNA-142-mediated repression of phosphodiesterase 3B critically regulates peripheral immune tolerance. J Clin Invest. 2019;129(3):1257–71. Epub 2019/02/12. doi: 10.1172/JCI124725 ; PubMed Central PMCID: PMC6391082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28(4):546–58. doi: 10.1016/j.immuni.2008.02.017 . [DOI] [PubMed] [Google Scholar]
- 22.Lu LF, Boldin MP, Chaudhry A, Lin LL, Taganov KD, Hanada T, et al. Function of miR-146a in controlling Treg cell-mediated regulation of Th1 responses. Cell. 2010;142(6):914–29. Epub 2010/09/21. doi: 10.1016/j.cell.2010.08.012 ; PubMed Central PMCID: PMC3049116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyszkiewicz M, Winter SJ, Witzlau K, Fohse L, Brownlie R, Puchalka J, et al. miR-181a/b-1 controls thymic selection of Treg cells and tunes their suppressive capacity. PLoS Biol. 2019;17(3):e2006716. Epub 2019/03/12. doi: 10.1371/journal.pbio.2006716 ; PubMed Central PMCID: PMC6428341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cretney E, Kallies A, Nutt SL. Differentiation and function of Foxp3(+) effector regulatory T cells. Trends Immunol. 2013;34(2):74–80. doi: 10.1016/j.it.2012.11.002 . [DOI] [PubMed] [Google Scholar]
- 25.Asano T, Meguri Y, Yoshioka T, Kishi Y, Iwamoto M, Nakamura M, et al. PD-1 modulates regulatory T-cell homeostasis during low-dose interleukin-2 therapy. Blood. 2017;129(15):2186–97. doi: 10.1182/blood-2016-09-741629 ; PubMed Central PMCID: PMC5391624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bartonicek N, Enright AJ. SylArray: a web server for automated detection of miRNA effects from expression data. Bioinformatics. 2010;26(22):2900–1. doi: 10.1093/bioinformatics/btq545 . [DOI] [PubMed] [Google Scholar]
- 27.Chen J, Bardes EE, Aronow BJ, Jegga AG. ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res. 2009;37(Web Server issue):W305–11. doi: 10.1093/nar/gkp427 ; PubMed Central PMCID: PMC2703978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao J, Gu J, Pan X, Gan X, Ju Z, Zhang S, et al. Blockade of miR-142-3p promotes anti-apoptotic and suppressive function by inducing KDM6A-mediated H3K27me3 demethylation in induced regulatory T cells. Cell Death Dis. 2019;10(5):332. Epub 2019/04/17. doi: 10.1038/s41419-019-1565-6 ; PubMed Central PMCID: PMC6465300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang B, Zhao J, Lei Z, Shen S, Li D, Shen GX, et al. miR-142-3p restricts cAMP production in CD4+CD25- T cells and CD4+CD25+ TREG cells by targeting AC9 mRNA. EMBO Rep. 2009;10(2):180–5. Epub 2008/12/23. doi: 10.1038/embor.2008.224 ; PubMed Central PMCID: PMC2637310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu Y, Gao J, Zhang S, Gu J, Lu H, Xia Y, et al. miR-142-3p regulates autophagy by targeting ATG16L1 in thymic-derived regulatory T cell (tTreg). Cell Death Dis. 2018;9(3):290. Epub 2018/02/21. doi: 10.1038/s41419-018-0298-2 ; PubMed Central PMCID: PMC5833855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics. 2013;14:128. Epub 2013/04/17. doi: 10.1186/1471-2105-14-128 ; PubMed Central PMCID: PMC3637064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545–50. doi: 10.1073/pnas.0506580102 ; PubMed Central PMCID: PMC1239896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015;4. Epub 2015/08/13. doi: 10.7554/eLife.05005 ; PubMed Central PMCID: PMC4532895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loeb GB, Khan AA, Canner D, Hiatt JB, Shendure J, Darnell RB, et al. Transcriptome-wide miR-155 binding map reveals widespread noncanonical microRNA targeting. Mol Cell. 2012;48(5):760–70. doi: 10.1016/j.molcel.2012.10.002 ; PubMed Central PMCID: PMC3562697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JH, Elly C, Park Y, Liu YC. E3 Ubiquitin Ligase VHL Regulates Hypoxia-Inducible Factor-1alpha to Maintain Regulatory T Cell Stability and Suppressive Capacity. Immunity. 2015;42(6):1062–74. doi: 10.1016/j.immuni.2015.05.016 ; PubMed Central PMCID: PMC4498255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cruz LO, Hashemifar SS, Wu CJ, Cho S, Nguyen DT, Lin LL, et al. Excessive expression of miR-27 impairs Treg-mediated immunological tolerance. J Clin Invest. 2017;127(2):530–42. doi: 10.1172/JCI88415 ; PubMed Central PMCID: PMC5272185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu LF, Thai TH, Calado DP, Chaudhry A, Kubo M, Tanaka K, et al. Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein. Immunity. 2009;30(1):80–91. doi: 10.1016/j.immuni.2008.11.010 ; PubMed Central PMCID: PMC2654249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Overacre-Delgoffe AE, Chikina M, Dadey RE, Yano H, Brunazzi EA, Shayan G, et al. Interferon-gamma Drives Treg Fragility to Promote Anti-tumor Immunity. Cell 2017;169(6):1130–41 e11. doi: 10.1016/j.cell.2017.05.005 ; PubMed Central PMCID: PMC5509332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inui M, Martello G, Piccolo S. MicroRNA control of signal transduction. Nat Rev Mol Cell Biol. 2010;11(4):252–63. doi: 10.1038/nrm2868 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Impaired Treg cell development in miR-142−/− mice. Left panel, FACS analysis of WT and miR-142−/− thymocytes with anti-CD4 and anti-Foxp3 antibodies. Foxp3+CD4+ Treg cells are gated and numbers indicate the percentage of cells in the gate. Right panel, frequency of CD4+Foxp3+ Treg cells in WT and miR-142−/− thymi (n = 3 per group). (B, C) Treg cell defect in the periphery of miR-142−/− mice. FACS analysis of Treg cells in spleen (B) and MLNs (C) from WT and miR-142−/− mice. Right panel, frequency of CD4+Foxp3+ Treg cells in WT and miR-142−/− spleens and MLNs (n = 4 per group). (D) qRT-PCR analysis of mature miR-142-3p and miR-142-5p expression in different T-cell subsets purified from Foxp3Cre mice (n = 2). DN, double negative CD4-CD8- thymocytes; DP, double positive CD4+CD8+ thymocytes; SP, single positive CD4+YFP− thymocytes; Treg, CD4+YFP+ Treg cells from thymus and spleen, respectively; TNaive, naive CD4+YFP−CD62L+CD44− splenic T cells; TActivated, activated CD4+YFP−CD62L−CD44+ splenic T cells. Expression level of miR-142-3p in DN population was arbitrarily set to 1. snoRNA234 levels were used for normalization. (E) qRT-PCR analysis of mature miR-142-3p expression in CD4+YFP+ Treg and CD4+YFP− Teff cells purified from Foxp3Cre and Foxp3CremiR-142fl/fl spleens. Expression level of miR-142-3p in CD4+YFP+ Treg cells isolated from Foxp3CremiR-142fl/fl spleen was arbitrarily set to 1. snoRNA234 levels were used for normalization. Spleen (F) and thymus (G) weights in 8- to 11-week-old male Foxp3Cre and Foxp3CremiR-142fl/fl mice (n ≥ 7 per group). Absolute cell counts in spleen (H), thymus (I), and peripheral lymph nodes (J) from Foxp3Cre and Foxp3CremiR-142fl/fl mice (n = 6 per group). Results are shown as mean ± SD. P values were calculated using 2-tailed Student t test. **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; NS, not significant. The underlying numerical raw data can be found in S1 Data file. The underlying flow cytometry raw data can be found at the Figshare repository. FACS, fluorescence activated cell sorting; KO, knockout; MLN, mesenteric lymph node; PLN, peripheral lymph node; SD, standard deviation; SP, spleen; Treg, regulatory T; WT, wild-type; YFP, yellow fluorescent protein.
(PDF)
(A) FACS analysis of lymphocytes from PLNs (left panel) and thymus (right panel) of 12-week-old Foxp3Creand Foxp3CremiR-142fl/fl mice with anti-CD4 and anti-Foxp3 specific antibodies. Foxp3+CD4+ Treg cells are gated and numbers indicate the percentage of cells in the gate. (B) Frequency (left panel) and total number (right panel) of Treg cells in PLNs and thymi of 12-week-old Foxp3Cre (red bars) and Foxp3CremiR-142fl/fl (blue bars) mice (n = 3 per group). (C) FACS analysis of CD44 and CD62L expression on CD4+ (upper panel) and CD8+ (bottom panel) T cells from Foxp3Cre and Foxp3CremiR-142fl/fl PLNs. Numbers indicate percentage of cells in the quadrants. (D) Frequency of CD44−CD62L+ (naive) and CD44+CD62L− (activated) CD4+ (left panel) and CD8+ (right panel) T cells in Foxp3Cre and Foxp3CremiR-142fl/fl PLNs (n = 6 per group). (E) Intracellular FACS analysis of IFNγ production by CD4+ (upper panel) and CD8+ (bottom panel) T cells from Foxp3Cre and Foxp3CremiR-142fl/fl PLNs. IFNγ+ T cells are gated and numbers indicate percentage of cells in the gate. (F) Frequency of IFNγ-expressing CD4+ and CD8+ T cells in Foxp3Cre (filled bars) and Foxp3CremiR-142fl/fl (open bars) PLNs (n = 4 per group). (G) Total CD4+ and CD8+ T cell counts in Foxp3Cre (filled bars) and Foxp3CremiR-142fl/fl (open bars) PLNs (n = 6 per group). (H) Frequencies of IFNγ-, IL-4-, and IL-17-expressing CD4+ T cells isolated from Foxp3Cre (filled bars) and Foxp3CremiR-142fl/fl (open bars) spleens (n ≥ 3 per group). Results are shown as mean ± SD. P values were calculated using 2-tailed Student t test. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; NS, not significant. The underlying numerical raw data can be found in S1 Data file. The underlying flow cytometry raw data can be found at the Figshare repository. FACS, fluorescence activated cell sorting; IFNγ, interferon gamma; IL, interleukin; PLN, peripheral lymph node; SD, standard deviation; Treg, regulatory T.
(PDF)
(A) FACS analysis of Treg suppression activity in vitro. Purified CD4+ Tconv cells were loaded with CTV dye and incubated with FACS-sorted Treg cells from Foxp3Cre (red line) and Foxp3CremiR-142fl/fl (blue line) spleens in the presence of beads coated with anti-CD3 and anti-CD28 specific antibodies. Several Treg to Tconv cell ratios were shown as indicated. (B) Left panel, FACS analysis of Annexin V-stained CD4+YFP+ Treg cells from Foxp3Cre (WT) or Foxp3CremiR-142fl/fl (KO) spleens. Right panel, MFI of Annexin V staining on Treg cells from Foxp3Cre (WT) or Foxp3CremiR-142fl/fl (KO) spleens. (C) Frequency of splenic YFP+ Treg cells in female Foxp3Cre/WTmiR-142+/+ and Foxp3Cre/WTmiR-142fl/fl mice. (D) MFI of GITR, CD25, CD103, PD-1, CTLA-4, and ICOS expression on splenic CD4+YFP+ Treg cells from Foxp3Cre (WT) or Foxp3CremiR-142fl/fl (KO) mice. Results are shown as mean ± SD. P values were calculated using 2-tailed Student t test *, P < 0.05; **, P < 0.01; NS, not significant. The underlying numerical raw data can be found in S1 Data file. The underlying flow cytometry raw data can be found at the Figshare repository. CTV, cell trace violet; FACS, fluorescence activated cell sorting; KO, knockout; MFI, mean fluorescence intensity; Treg, regulatory T; WT, wild-type; YFP, yellow fluorescent protein.
(PDF)
Heatmap visualization of differentially expressed cytokine, chemokine, and immune receptor genes (A) and Treg cell signature genes (B). Genes highlighted in red are putative miR-142-3p targets. (C) Standard enrichment plot demonstrating the enrichment of up-regulated genes in miR-142–deficient Treg cells from gene ontology term “regulation of response to interferon gamma” using the GSEA tool. (D) ELISA analysis of IFNγ production by Foxp3Cre (WT) and Foxp3CremiR-142fl/fl (KO) Treg cells (n = 4 per group). Purified CD4+YFP+ Treg cells (106/mL) were stimulated with anti-CD3 (5 μg/ml) and anti-CD28 (2 μg/ml) antibodies in the presence of IL-2 (50 ng/ml) for 48 hours. (E) MFI of phospho-Stat1(Y701) levels in Foxp3Cre (WT; n = 6) and Foxp3CremiR-142fl/fl (KO; n = 5) Treg cells. P values were calculated using 2-tailed Student t test. *, P < 0.05; **, P < 0.01. The underlying raw data can be found in S1 Data file. GSEA, Gene Set Enrichment Analysis; IFNγ, interferon gamma; IL, interleukin; KO, knockout; MFI, mean fluorescence intensity; Treg, regulatory T; WT, wild-type; YFP, yellow fluorescent protein.
(PDF)
(A) Diagrams (top left) and sequence alignments (top right) of putative miR-142-3p binding sites in the 3′ UTRs of Stat1, Ifngr2, Irf1, Gbp3, and Pde3b genes. HITS-CLIP analysis of Ago2 binding to the 3′ UTRs of Ifngr2 (bottom-left) and Gbp3 (bottom-right) genes in WT (blue plot) and miR-155 KO (yellow plot) activated CD4+ T cells. Sequences corresponding to miR-142-3p binding sites are labeled by arrows. (B) Validation of Pde3b and Ifngr2 as direct miR-142-3p targets by the 3′ UTR luciferase reporter assay (n = 2). Relative expression of WT and miR-142-3p seed mutated Pde3b and Ifngr2 3′ UTR reporter constructs upon cotransfection with either miR-142 precursor expressing plasmid or empty vector control. Expression of WT Pde3b and Ifngr2 3′ UTR reporters in the presence of empty vector were set to 1. (C) MFI of Hif1α in Foxp3Cre (WT; red bars) and Foxp3CremiR-142fl/fl (KO; blue bars) CD4+Foxp3+ Treg and CD4+Foxp3- Tconv cells (n = 6 per group). (D) Left panel, FACS analysis of IFNγR2 expression in CD4+YFP+ T cells from Foxp3Cre (red line) and Foxp3CremiR-142fl/fl (blue line) spleens; right panel, MFI of IFNγR2 in Foxp3Cre (WT; red bars) and Foxp3CremiR-142fl/fl (KO; blue bars) CD4+YFP+ Treg cells. (E) Schematic diagram and sequence conservation of 2 miR-142-5p and one miR-142-3p binding sites in the 3′ UTR of mouse Pde3b gene as determined by the TargetScan algorithm. (F) Sequence alignment of miR-142-3p binding sites in mouse Pde3b-WT and Pde3b-MUT 3′ UTR reporter constructs. Results are shown as mean ± SD. P values were calculated using 2-tailed Student t test. *, P < 0.05; **, P < 0.01; ***, P < 0.001; NS, not significant. The underlying numerical raw data can be found in S1 Data file. The underlying flow cytometry raw data can be found at the Figshare repository. FACS, fluorescence activated cell sorting; IFNγ, interferon gamma; KO, knockout; MFI, mean fluorescence intensity; SD, standard deviation; Treg, regulatory T; WT, wild-type; YFP, yellow fluorescent protein.
(PDF)
(A) Relative Hif1α protein expression in Foxp3Cre (WT; red bar), Foxp3CremiR-142fl/fl (KO; blue bar), and Foxp3CremiR-142fl/flHif1a+/fl (HET; green bar) Treg cells. The Hif1α levels in WT Treg cells were arbitrarily set to 1. (B) Frequency of CD4+Foxp3+ Treg cells in splenic lymphocytes from Foxp3Cre (WT; red bar; n = 7), Foxp3CremiR-142fl/fl (KO; blue bar; n = 5) and Foxp3CremiR-142fl/flHif1a+/fl (HET; green bar; n = 3) mice. (C) Kaplan–Meier survival curves for Foxp3Cre (red line; n = 27), Foxp3CremiR-142fl/fl (blue line; n = 27), and Foxp3CremiR-142fl/flHif1a+/fl (green line; n = 8) mice. Analysis of IFNγ production (D) and Stat1 activation (E) in Treg cells from Foxp3CremiR-142fl/flHif1a+/fl mice. Left panels, intracellular FACS analysis of splenic CD4+Foxp3+ Treg cells from Foxp3Cre (red line; WT), Foxp3CremiR-142fl/fl (blue line; KO) and Foxp3CremiR-142fl/flHif1a+/fl (green line; HET) mice with anti-IFNγ (D) and anti-pStat1 (Y701) (E) antibodies. Right panels, MFI of IFNγ and phospho-Stat1 (pSTAT1) in Foxp3Cre (WT; red bar), Foxp3CremiR-142fl/fl (KO; blue bar) and Foxp3CremiR-142fl/flHif1a+/fl (HET; green bar) Treg cells. (F, G) FACS analysis of immunosuppressive activity of Treg cells derived from Foxp3Cre (WT; red dot), Foxp3CremiR-142fl/fl (KO; blue dot) and Foxp3CremiR-142fl/flHif1afl/fl (DKO; green dot) mice (n = 3) in vitro. Several Treg to Tconv cell ratios were analyzed as indicated in the graph. Unstimulated Tconv cells were used as control. Representative FACS plot analysis is shown in G. (H) FACS analysis of CD44 and CD62L expression in splenic CD4+ T cells from Foxp3Cre, Foxp3CremiR-142fl/flHif1a+/fl, and Foxp3CremiR-142fl/flHif1afl/fl mice. Numbers indicate percentage of cells in the quadrants. Results are shown as mean ± SD. P values were calculated using 2-tailed Student t test. *, P < 0.05; **, P < 0.01; ****, P < 0.0001; NS, not significant. The underlying numerical raw data can be found in S1 Data file. The underlying flow cytometry raw data can be found at the Figshare repository. DKO, double knockout; FACS, fluorescence activated cell sorting; IFNγ, interferon gamma; KO, knockout; MFI, mean fluorescence intensity; pStat1, phosphorylated Stat1; SD, standard deviation; Treg, regulatory T; WT, wild-type.
(PDF)
(XLSX)
Treg, regulatory T.
(XLSX)
(XLSX)
Data Availability Statement
The underlying raw data can be found within the paper and its Supporting Information files. The RNA-seq data used in the paper are publicly available from the GEO database (accession number GSE190192). The underlying flow cytometry raw data can be found at the Figshare data repository (https://figshare.com/projects/microRNA-142_guards_against_autoimmunity_by_controlling_Treg_cell_homeostasis_and_function/128960).