Abstract
Human infections with Plasmodium falciparum may result in severe forms of malaria. The widespread and rapid development of drug resistance in P. falciparum and the resistance of the disease-transmitting mosquitoes to insecticides make it urgent to understand the molecular background of the pathogenesis of malaria to enable the development of novel approaches to combat the disease. This review focuses on the molecular mechanisms of severe malaria caused by the P. falciparum parasite. The nature of severe malaria and the deleterious effects of parasite-derived toxins and host-induced cytokines are introduced. Sequestration, brought about by cytoadherence and rosetting, is linked to severe malaria and is mediated by multiple receptors on the endothelium and red blood cells. P. falciparum erythrocyte membrane protein 1 (PfEMP1) is the ligand responsible for a majority of binding interactions, and the multiply adhesive features of this sticky molecule are presented. Antigenic variation is also a major feature of PfEMP1 and of the surface of the P. falciparum-infected erythrocyte. Possible mechanisms of P. falciparum antigenic variation in asexual stages are further discussed. We conclude this review with a perspective and suggestions of important aspects for future investigations.
Malaria, one of the most health-threatening diseases in the world, claims millions of lives each year. Plasmodium falciparum is the malaria parasite that causes the most severe disease (86, 129, 132). Within minutes after being inoculated into the human host by blood-sucking mosquitoes, the parasites (in the form of sporozoites) target and invade hepatocytes (112), where they propagate rapidly. Thousands of merozoites are subsequently released from the liver and invade red blood cells (RBCs). Severe malaria occurs after parasite proliferation inside erythrocytes and the consequent binding of infected RBCs to the vascular endothelium (cytoadherence) and to noninfected erythrocytes (rosetting). These bindings will eventually lead to the accumulation of parasitized cells in the local postcapillary microvasculature and block the blood flow, limiting the local oxygen supply. The most common clinical symptoms of severe malaria are high fever, progressing anemia, multiorgan dysfunction, and unconsciousness, i.e., coma, which is a sign of cerebral malaria and one of the causes of death (86).
Severe malaria is a complicated syndrome, which is determined by factors from both the parasite and the human host. To survive inside the human body, P. falciparum has developed several mechanisms through which it may escape the host immune system as well as antimalarial agents. While these survival strategies provide for temporary parasitization, they often cause death to their host in the end. The most notorious survival mechanism of the malaria parasite is its ability to undergo almost unlimited antigenic variation through changing the antigens on the infected erythrocyte surface. Some of these erythrocyte antigens are specific receptor-adhesive ligands mediating adhesion of the infected erythrocyte in the intravascular microenvironments, especially the postcapillary regions. In addition, by sequestration, the parasite escapes nonspecific clearance in the spleen, which may improve its ability to reinvade and proliferate.
Tremendous progress has been made in the past decade in understanding the molecular background of severe malaria. The identification and characterization of the var genes (12, 115, 116) encoding P. falciparum erythrocyte membrane protein 1 (PfEMP1), the rosettin/rif multigene family, and the encoded rosettin and rifin proteins on the infected erythrocyte surface (20, 46, 51, 72) represent major breakthroughs in malaria research. Undoubtedly, they will lead to the discovery of novel therapeutic agents and immune prevention approaches for severe malaria. The malaria genome project joined by many laboratories around the world will provide more genetic data for malariologists (20, 39, 48, 51) to better understand the organism and carry out further investigations. Recent progress in molecular aspects of severe malaria is summarized in this review.
ERYTHROCYTE MEMBRANE MODIFICATIONS
The differences between parasite-infected and uninfected RBCs are great. First, the mechanical properties of the parasitized RBCs (pRBCs) are changed dramatically. The membrane is less flexible, which makes it difficult for the cell to pass through the microvasculature. Parasite nutrients, such as carbohydrates, amino acids, and purine bases, are transported into the cells through special channels or pores created by the parasites in the erythrocyte membrane (42). Furthermore, some pRBC membrane components (both proteoglycans and proteins) are digested or modified. Most interestingly, several parasite-derived polypeptides, including PfEMP1 and rosettins/rifins, are inserted into and then protrude from the erythrocyte membrane. All these parasite-derived polypeptides significantly change the nature of the RBC membrane. The most prominent ultrastructural change in the pRBC is the appearance of electron-dense protrusions with a diameter of ≈100 μm produced by the parasite, called knobs (77). These structures are only found on the infected RBC surface and are thought to be composed of several polypeptides (one of which is PfEMP1) synthesized and transported from the intracellular parasite, as seen by transmission electron microscopy (TEM) (for details, see reference 50). Many studies by TEM and scanning electron microscopy have confirmed that knobs are the sites where pRBCs bind to other cell surfaces, including the surface of normal RBCs (e.g., in a rosette). Knobs were also determined to be the sites of attachment of pRBCs to brain endothelium of patients who died of severe malaria (1, 2, 79). However, knobs are not absolutely essential for erythrocyte binding. Knobless pRBCs may still be very adhesive, and some parasites indeed express PfEMP1, rosettin/rifin, and other proteins which are transported to the surface of the infected RBC, where they may mediate binding to multiple receptors (31, 45).
The flexibility of uninfected erythrocytes is reduced extensively in malaria, and this has been proposed to be one of the important parameters for prediction of the severity of disease (43). The unparasitized erythrocytes from severe malarial cases showed significant reduction in deformation compared with RBCs from patients with moderate disease. Rigid erythrocytes in severe patients are likely to both participate in sequestration in the capillaries and contribute to impaired microcirculatory flow and fatal outcome. Two factors are thought to cause this reduction in RBC deformability: (i) severe blood acidosis due to anaerobic glycolysis, and (ii) parasite-derived antigens adsorbed onto the membrane of uninfected erythrocytes. It has also been suggested that P. falciparum-secreted lipid antigens may be inserted into the uninfected erythrocyte membrane, which by yet another mechanism might contribute to the rigidity of the cells (88). The reduction in RBC deformability is one consequence of malaria infection that may contribute to disease severity.
CYTOKINES AND TOXINS
Malaria infections are complicated syndromes involving many inflammatory responses which may enhance cell-to-cell interactions (cytoadherence) and cell stimulation involving both malaria-derived antigens (or toxins) and host-derived factors such as cytokines. Malaria toxins include parasite-derived molecules which are secreted or released from parasites at late stages (trophozoite and especially the schizont stage) and are contained among the glycosylphosphatidylinositol (GPI)-anchored proteins. Parasite products either directly damage host tissues or, more important, stimulate the overproduction of host cytokines (67, 108). Moderate amounts of cytokines such as tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), and interleukin-1 (IL-1) are necessary for the human host to fight invading microorganisms, but the overinduction of host cytokines can also be very harmful to the host. TNF-α can cause fever and disturbance of the immune system. But TNF-α and IFN-γ also upregulate endothelial receptors such as ICAM-1 and probably redistribute other receptors, such as CD31 on endothelium, which may enhance sequestration. Clinically, it is crucial to reverse the effects of both toxins and cytokines to prevent further complications of malarial disease.
Cytokine Contributions
The importance of cytokines, especially TNF-α and IFN-γ, and their contribution to severe malaria have been extensively reviewed (38, 61, 67, 86). Malaria parasites, by producing diverse elements, stimulate the human host and thereby result in excessive production of cytokines, which will have an adverse effect on disease progression. For example, high circulating levels of TNF-α and IFN-γ are more often found in patients with severe malaria than in uncomplicated cases (70) (Fig. 1). Extensive deposition of TNF-α, IFN-γ, and IL-1 in organs with massive sequestration (especially in the brain) is more frequently seen in patients who died of cerebral malaria (123). Elevated expression of relevant cytokine mRNAs was also detected in the affected organs of these patients. The reason for cytokine deposition during sequestration is not clear, yet it is possible, as in trypanosomiasis, in which overproduced immunoglobulins (Igs), especially IgM, are not functional, that cytokines contribute to the disease process and are not protective. The proposed contributions of cytokines to severe malaria include (i) upregulation of endothelial receptor expression and redistribution on the endothelial surface; (ii) physical disturbances of the host, such as high fever; (iii) upregulation of nitric oxide production, which may cause local damage at sequestered loci (4, 54); and (iv) suppression of erythrocyte production in the bone marrow. Reversing the deleterious effect of overproduced cytokines might be important for the clinical treatment of severe malaria. However, infusion of an anti-TNF-α monoclonal antibody (MAb) into comatose children with malaria infection did not affect the outcome of the disease, although fever was abated (69). Thus, it might be important to further uncover the property of parasite antigens that is responsible for cytokine stimulation in order to understand the disease and design novel preventive measures.
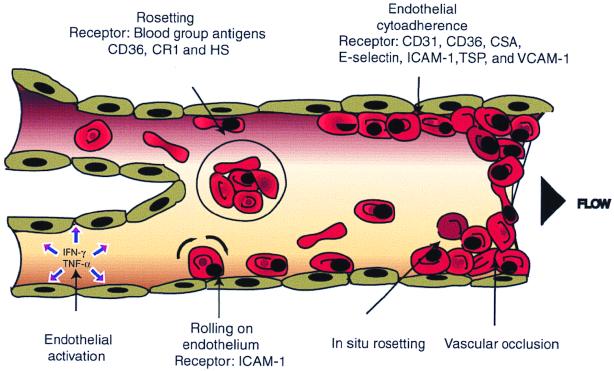
FIG. 1.
Cytoadherence and rosetting in postcapillary vasculature. P. falciparum-infected RBCs bind to the postcapillary endothelial lines and to noninfected RBCs. Both phenomena are thought to contribute to the occlusion of blood flow and consequent severe disease. Parasite antigens could stimulate IFN-γ and TNF-α release, which upregulates receptor expression (such as ICAM-1) and redistribution (such as CD31) on the endothelium. ICAM-1 is suggested to mediate pRBC rolling on endothelium, while CD36, CD31, and other receptors are responsible for more stable binding. Sequestration could be augmented by the ability of spontaneous rosetting (in situ rosetting) and cytoadherence of the parasite.
Malaria Toxins
Malaria toxins are parasite-derived molecules which induce the human host to overproduce serum-bound factors. Supernatants of P. falciparum cultures contain such elements stimulating the secretion of TNF-α, IL-1, and other cytokines from various host cells (13, 127). The immune responses to these molecules are crucial for disease resistance, as the cytokines, among other effects, upregulate endothelial receptors and cause fever.
GPI molecules are widely used as anchors of transmembrane proteins in protozoa such as Trypanosoma brucei as well as in mammalian cells (44, 128). They are attached to the carboxy terminus of proteins, where they serve a variety of functions in addition to that of being membrane anchors, such as in the maturation and intracellular transport of proteins and in cell signal transduction processes; importantly, they are at least in part the toxins of the malaria parasite (93, 108). Most malaria toxins are resistant to protease treatment and depend on a phospholipid structure (14). Phosphatidylinositol was suggested to be the main component of toxin molecules, since specific antibodies to it could neutralize the toxic effect, but the exact origin of most of the toxins is still not known. Furthermore, the GPI-anchored components of merozoite surface antigens, like MSP-1 and MSP-2, were found to induce TNF-α and IL-1 (108). Pf155 (ring-stage erythrocyte surface antigen [RESA]) antigens have also been reported to induce TNF-α production (93). During the process of merozoite invasion of P. falciparum, the MSP precursors are cleaved by enzymes released from rhoptries into several parts. The N-terminal fragments, which are released from the merozoite surface, might serve as cytokine inducers. Recent studies of P. falciparum suggest that GPI-anchored antigens are well presented at the late stages and are the most common form of glycosylation (52, 53). GPI anchors are thought to be essential for parasite survival, but they also induce the expression of inflammatory cytokines and nitric oxide synthase. This might augment the sequestration of pRBCs in the postcapillary vasculature and other pathogenic responses of the host (53).
Because high circulating levels of TNF-α and high fever occur at the rupture of schizonts, antigens from schizonts might have potential toxic effects (68). Pichyangkul et al. partially purified a soluble antigen predominantly associated with schizonts that was not found in other stages of infection (93). This schizont-associated antigen (SAA) was able to specifically stimulate γδ T cells in secreting IFN-γ. The effect was enhanced by IL-1, IL-10, and IL-12. This SAA was further characterized as being resistant to protease digestion, heating, and pH changes and containing a phosphate group(s), which was believed to be crucial for its toxic activities. These antigens are all parasite-derived products of which the GPI anchor and phosphate groups are suggested to be important for their biological activities.
The metabolites of P. falciparum are themselves toxic. During the asexual stages of proliferation in erythrocytes, P. falciparum consumes hemoglobin as an energy source and produces hemozoin as the main breakdown product, which is deposited in the cell compartment as pigment. This pigment, released immediately after schizont rupture, has been suggested to induce IL-1 production (94). Finally, since the deleterious effects of toxins are immediate and serious to malaria patients, a reduction of their harmfulness would be the first step after patient admission. Yet treatment would be more effective if the biological nature of the malaria toxins were better understood.
SEQUESTRATION AND SEVERE MALARIA
Sequestration is the removal of infected RBCs from the peripheral circulation by binding to the vascular endothelium, predominantly in postcapillary venules of the deep tissues, often accompanied by uninfected erythrocytes. Dysfunction of affected organs may occur with excessive binding due to the occlusion of blood flow that causes impaired oxygen delivery. Massive sequestration in the brain is believed to be the underlying cause of coma in cerebral malaria. The reason for the parasites to sequester is unknown. However, it is speculated that they grow better in an oxygen-depleted environment than in ambient air, and binding to the endothelium is also a way to circumvent spleen-dependent destruction.
Endothelial Receptors for Adhesion
CD36 and thrombospondin (TSP) were the first described endothelial receptors that bound pRBCs (5, 6, 98). MAbs specific to CD36 and soluble CD36 blocked the binding of pRBCs to melanoma cells and CD36-expressing COS cells (5, 6, 63). Furthermore, most wild isolates studied also adhered to CD36 (90). By using similar approaches, P. falciparum receptors VCAM-1, ICAM-1, and E-selectin were later identified (15, 91).
The affinity of P. falciparum for binding to endothelial cell receptors is diverse, as is their role in sequestration. ICAM-1 appears to be important, since isolates from patients with severe malaria bound to this receptor and it can be upregulated by TNF-α, an important cytokine believed to contribute to severe malaria. Yet the difference in the level of binding of infected RBCs to this receptor between patients with mild and severe malaria is not statistically significant (90). In addition, the low affinity of most malaria parasites for this receptor indicates that ICAM-1 only mediates pRBC rolling on the endothelial lining and that stable binding most likely occurs synergistically with other receptors such as CD36 (36, 84). CD36 and TSP receptors are not well distributed on brain endothelium, and their expression is not sensitive to cytokine (IFN-γ or TNF-α) stimulation. PECAM-1 (platelet/endothelial cell adhesion molecule 1), also called CD31, is a highly glycosylated 130-kDa polypeptide mainly located on the rim of endothelial cells, and it was recently identified as a P. falciparum receptor (119). Antibodies to domains 1 and 2 of CD31 showed specific inhibition of pRBC binding to both CD31-transfected L cells and endothelial cells. Parasite binding to CD31 was also upregulated by IFN-γ. In a recent study performed in Kenya, we found that CD31-binding parasites were almost as frequent as CD36-binding ones and that parasites from patients with severe symptoms had a greater tendency to bind soluble CD31 (A. Heddini, J. Obiero, A. Barragan, Q. Chen, O. Kai, M. Wahlgren, and K. Marsh, submitted for publication). Thus, CD31 may be one of the virulence-associated receptors of P. falciparum. An interesting phenomenon found with both laboratory strains and wild isolates of P. falciparum is that they may bind to several receptors simultaneously. This is shown by an experiment in which MAb to either CD36 or ICAM-1 blocked only some of the parasite binding to endothelial cells coexpressing these two molecules (84), yet a mixture of both antibodies inhibited binding (see the next section also).
Negatively charged glycosaminoglycans (GAGs) such as heparan sulfate (HS) and chondroitin sulfate A (CSA) are well distributed on the endothelial cell surface. The potential participation of these GAGs in the merozoite invasion, cytoadherence, and rosetting processes has drawn special attention from malaria researchers (8, 33, 60, 113). Heparin and HS were found to inhibit P. falciparum merozoite invasion of erythrocytes and to disrupt malaria rosetting (26, 33). CSA is particularly well expressed on the surface of syncytiotrophoblasts in the placenta, and it can be used to flush out sequestered pRBCs from this organ. Such parasites were found to bind avidly to CSA. Thus, CSA seems to be an important receptor for P. falciparum-infected RBCs, and a role in sequestration in the placenta and in maternal malaria has been suggested (22, 49, 96, 97, 100, 101).
Multiadhesion of pRBCs
Although several receptors for both cytoadherence and rosetting have been identified, one question concerning the ability of singly infected RBCs or single isolates to bind to these receptors has not been extensively addressed. Because cloned parasites have been reported to bind to both CD36 and ICAM-1, two receptors could synergistically enforce the attachment of parasites to endothelial cells (84). However, panning parasites on one or two receptors might not completely mirror the binding that occurs in vivo, where all the receptors are simultaneously available. A recent report provided evidence that some parasites indeed display a multiply or panadhesive phenotype (45). The FCR3S1.2 clone, selected for excessive rosetting, also autoagglutinated pRBCs and avidly bound to a large number of receptors, including blood group antigen A, heparin-like GAGs, IgM, CD36, and CD31 (31, 45, 119) (Fig. 2). This parasite also binds somewhat to IgG, ICAM-1, and blood group B antigen. Clinically, the multiadhesive phenotype might be better correlated to parasite virulence than other adhesive phenotypes, since isolates from patients suffering from severe disease, at least in one study, tended to frequently adhere to several receptors (Heddini et al., submitted). In vivo, adhesion is likely to be the collective interaction of a parasite ligand(s) and most endothelial cell and RBC receptors (34). It could be that parasites take advantage of binding with broad specificity to several receptors. The molecular basis behind the multiadhesive phenotypes is discussed below.
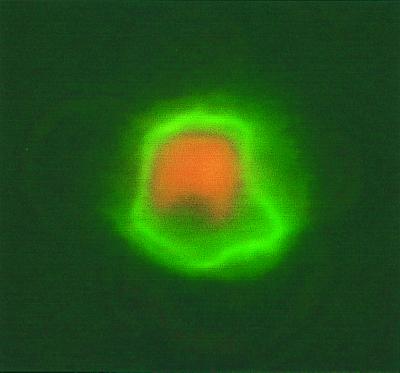
FIG. 2.
Soluble CD36 binds to FCR3S1.2-infected RBC surface. Fluorescence (Alexa 488; Bioprobe)-labeled soluble CD36 directly binds to the live infected RBC surface.
ROSETTING
The spontaneous binding of normal RBCs to malaria-infected RBCs was frequently found in P. falciparum cultures and is termed malaria rosetting (41, 57, 122, 124). Its association with severe malaria has been extensively studied both clinically and experimentally by several research groups.
Rosetting Associated with Severe Malaria
Rosetting was first discovered after the establishment of in vitro cultivation and the use of “live staining” of malaria parasites, in which the binding of uninfected RBCs to infected RBCs, stained with either ethidium bromide or acridine orange, could be visualized by fluorescence microscopy. The presence of the phenomenon in in vitro-grown parasites motivated us to investigate whether it was found in wild isolates as well. Studies in most malaria-endemic areas (Africa, South America, and Asia) revealed that about 50% of parasites form rosettes when cultured in vitro (125). When the rosetting capacity of parasites from cases of cerebral malaria and uncomplicated malaria was subsequently compared (27), the difference between the two groups was quite obvious. The parasites causing severe malaria displayed much higher rosetting rates than those causing uncomplicated disease (27, 103, 120; Heddini et al., submitted). It was also found that serum from patients with severe malaria usually contains a low titer of antirosette-specific antibodies (especially predominant in children) (9, 66, 102), while patients with uncomplicated malaria have a higher titer of antirosette antibodies (9, 27, 120). These observations in African cases indicate that rosetting is a phenotype which is associated with the severity of malarial disease and that antirosette immunity is an important factor in disease outcome.
Multiple Rosetting Receptors on RBC Surface
To date, five rosetting receptors have been identified on RBCs: blood group antigens A and B, CD36, complement receptor 1 (CR1), and HS-like GAGs. CD36 was suggested as a rosetting receptor by Handunetti et al. (56). Since the copy number of CD36 molecules on mature erythrocytes is low, its contribution to the rosetting phenomenon may be limited to immature RBCs. In contrast, blood group antigens A and B are widely distributed on the surface of non-O RBCs. Their function as rosetting receptors has been systematically investigated (29). All isolates tested showed a high preference for either A or B blood group RBCs. A and B trisaccharides blocked rosette reformation by the respective parasite strain, and in vitro tests have shown that rosettes formed in type A or B blood are always larger, tighter, and stronger than those formed in type O blood (29). Furthermore, individuals of the blood group A antigen phenotype were more frequently affected by severe malaria and coma than those of other blood groups (47). Thus A and B blood group antigens are important malaria-rosetting receptors.
HS and HS-like GAGs are also used by P. falciparum as rosetting receptors. The high sensitivity of P. falciparum rosettes in type O blood to heparin and N-sulfated glycans has been seen in both laboratory strains and wild isolates (7, 26, 29). The rosettes of most wild isolates can be disrupted by at least one GAG if not by heparin (7, 26). These observations suggest that malaria parasites use a GAG on the surface of RBCs as a rosetting receptor. When the FCR3S1.2 clone was grown in type O blood pretreated with heparinase, it was unable to form rosettes (7, 31). Both heparin and HS disrupted rosettes of FCR3S1 parasites and blocked rosette reformation, whereas CSA, a similar GAG, had little or no effect on rosette formation. Furthermore, heparin analogs with sulfate groups of the same molarity but at different positions on the sugar backbone display widely different rosette disruption activities (7). These data suggested that the antirosette effect of heparin is mainly due to the specific sulfate groups on the molecule. HS or an HS-like GAG is likely to be a rosetting receptor for this parasite.
CR1 is also widely distributed on the RBC surface. CR1 seems to be an important rosetting receptor, as the S1(a−) CR1 blood group occurs in about 30% of African-Americans, who might thus be protected from malaria (104). Some parasites cultured in CR1-deficient RBCs lose their capacity to form rosettes (104). The conclusion was further strengthened by the ability of soluble CR1 to disrupt rosettes and block rosette reformation by some parasite strains. Interestingly, CR1 is also glycosylated, and this part of the molecule could potentially contribute to the binding, but this remains to be investigated.
Serum Proteins Involved in Rosette Formation
The binding of Igs to P. falciparum pRBCs occurred during incubation of pRBCs with immune or normal serum (78, 82). Why nonimmune Igs bind to the pRBC surface remains to be explained. In several subsequent investigations, it was found that a majority of rosetting parasites engage in Ig-binding activities, but some nonrosetting parasites also bind IgM and/or IgG (109, 110, 118). Some pRBCs bind only one class of Ig (IgM or IgG), while most bind both classes of Igs. TEM showed the bound Ig to be associated with knobs, where fibrillar structures are formed between infected and uninfected RBCs (109). Ig deposition on the affected vascular endothelium in cerebral malaria is also quite common (1), but the reason for local Ig accumulation is not known. Luzzi et al. investigated the cellular mechanism by which alpha-thalassemia might protect against P. falciparum infection (78). They found that infected alpha-thalassemic cells bound more Ig from serum obtained from individuals living in malaria-endemic areas than normal RBC controls in vitro and that the binding increased exponentially during parasite maturation. Furthermore, they also observed an increase in Ig binding from nonimmune serum to infected thalassemic RBCs during parasite maturation. These observations indicated that parasite-derived antigens presented on pRBCs bound both specific and nonspecific Ig. The Ig-binding ligand in the novel fibrillar structures observed by Scholander et al. (109) could be parasite-derived proteins, e.g., PfEMP1 (see next section). Why does the parasite bind Ig? It could be speculated that in the infected human host, nonspecific Ig could somehow hinder the access of specific antibodies to pRBCs and thus prevent immune recognition of infected cells. The parasite may also use Ig molecules as a bridge for binding to other receptors on the endothelium or the RBC membrane, in, for example, the formation of rosettes (109, 110, 121).
In addition to IgG and IgM, several other serum proteins play a role in rosetting. Purified serum proteins, primarily fibrinogen and albumin and other proteins, were found to promote rosetting (121). Importantly, as for Ig binding, the prorosetting effect of fibrinogen could be reversed with antifibrinogen antibodies, and fibrinogen could be detected on the pRBC surface of some parasite strains by TEM (121). Furthermore, in cultures without serum proteins, almost 100% of rosettes could be restored by the addition of IgM, fibrinogen, and albumin to the culture medium. These serum proteins participate in rouleaux formation, the binding of uninfected RBCs to each other, suggesting that parasites induce local rouleaux formation at the infected RBC surface (121). This could be an effect of direct binding of Igs, fibrinogen, or albumin to the natural rouleaux receptors present on the uninfected RBC as well as to PfEMP1, which has been localized at knobs of infected RBCs.
PFEMP1 IS AN ADHESIVE LIGAND
PfEMP1 is a polypeptide of high molecular mass (200 to 400 kDa) which is trypsin sensitive, insoluble in nonionic detergents, and readily labeled by conventional surface iodination techniques. PfEMP1 is purported to be composed of several extracellular Duffy binding-like domains (DBL 1 to 5), with one to two cysteine-rich interdomain regions (CIDRs) distributed in between the DBLs, a transmembrane (TM) region, and an intracellular acidic segment (ATS) (Fig. 3) (115, 116). PfEMP1 is a variable polypeptide encoded by the multiple var gene family (see below). The most conserved regions are located in both the N- and C-terminal domains. DBL-1α together with CIDR1α is thought to form a semiconserved head structure (116) in which at least six regions in DBL-1α and several cysteine residues in CIDR1α are almost 100% conserved. The common structures harbored in different PfEMP1 molecules indicate that they mediate similar binding activities, yet their multiple and polymorphic nature also suggests an effective role in immune evasion. When using different receptors (CD36, TSP, and ICAM-1), only PfEMP1 could be precipitated from the sodium dodecyl sulfate-soluble fraction of pRBCs, suggesting that PfEMP1 is indeed the adhesive ligand involved in binding to those receptors (10). Attempts to map the binding site of receptors on PfEMP1 have been successful only with CD36, for which binding has conclusively been mapped to the semiconserved CIDR1α domain (10–12, 114).
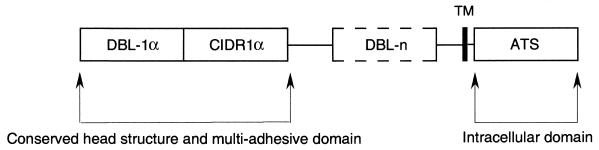
FIG. 3.
General structure of var PfEMP1. The semiconserved DBL-1α domain and CIDR1α are present in all var genes sequenced to date, and they are the most conserved regions among different sequences. Downstream from the first DBL and CIDR domains, there are a variable number of less-conserved DBL structures. A putative transmembrane domain (TM) is followed by the highly conserved acidic terminal sequence (ATS), which is presumably cytoplasmically located.
Binding to Multiple Endothelial Cell Receptors
Even though seven cytoadherent receptors have been identified (probably there are more) on the endothelium used by the malaria parasite (see above and Table 1), the only ligand derived from the parasite that has until now been proven to mediate adhesion is PfEMP1, the first parasite-derived molecule identified on the infected RBC surface (64, 75). Other surface-located oligopeptides, e.g., the rosettins/rifins, sequestrins, and even modified band 3, have also been suggested to participate in binding (30, 45, 46, 73, 87), but their importance in cytoadherence remains to be further studied.
TABLE 1.
Host cell receptors and ligands for the adhesion of P. falciparum-infected RBCs
| Host receptor(s) | Receptor location | Parasite ligand |
|---|---|---|
| HS-like GAGs | RBC | PfEMP1 (DBL-1α) |
| CD35 (CR1) | RBC | PfEMP1 (DBL-1α) |
| Blood group antigen A and B | RBC | PfEMP1? |
| TSP | Serum, endothelium | PfEMP1? |
| CD36 | Endothelium, RBC | PfEMP1 (CIDR1α) |
| ICAM-1 | Endothelium | PfEMP1 (DBL-2β) |
| CD31 | Endothelium | PfEMP1? |
| VCAM-1 | Endothelium | ? |
| E-selectin | Endothelium | ? |
| CSA | Endothelium | PfEMP1 (DBL-3) |
| IgM and IgG | Serum | PfEMP1? |
How can one parasite bind to more than one receptor? One explanation is that the parasite expresses only one adhesin and that different domains within this molecule might mediate various adhesive events. This is most likely the case, as concluded from experiments in which the pRBCs of the multiadhesive parasite FCR3S1.2 were carefully treated with increasing concentrations of trypsin. There was a striking correlation between the adhesion of pRBCs to several receptors and the presence of PfEMP1 (45). It was also found that the DBL-1α domain mediates rosetting and the CIDR1α domain binds to CD36 (11, 31, 104). It could be that PfEMP1 alone mediates binding to receptors for both rosetting and cytoadherence. Further evidence for the latter case is the finding that a cloned multiadhesive parasite expresses only one kind of PfEMP1 mRNA in the late stages (31, 32, 107) and that only one PfEMP1 polypeptide could be immunoprecipitated from this parasite clone with broadly reactive anti-PfEMP1 serum. Both surface trypsinization of infected RBCs and immunoprecipitation of PfEMP1 (10) with different receptors also favor this hypothesis. It is quite possible that one PfEMP1 can bind to several (or at least more than one) receptors. The available data also suggest that it is likely that an individual domain or region of PfEMP1 mediates independent functions, as has been found to be the case with other large multidomain proteins in which domains are folded independently and each domain displays a distinct function (37). The multiadhesive ligand may contribute to the broad specificity in the binding of malaria parasites to diverse receptors and might indeed be an essential survival strategy to allow parasitization of a broad range of human hosts independent of race, age, sex, or the activation status of the microvasculature's endothelium. Potential anticytoadherence vaccines should thus be designed to reverse multivalent interactions and sequestration in vivo.
Binding to Multiple RBC Receptors
The molecular background to rosetting has been elucidated in several studies (7, 26, 28, 31, 104). The rosettins, now known to be at least in part encoded by the rif gene family, were first proposed to act as rosetting ligands from their association with the rosetting phenotype in P. falciparum parasites. Current evidence indicates that it is PfEMP1 that mediates rosetting. By comparing var transcripts from rosetting and nonrosetting parasites, Rowe et al. identified a var mRNA that is unique to rosetting parasites (104). A study on the expression and characterization of recombinant DBL-1α, CIDR1α, DBL-2β, and DBL-3γ concluded that DBL-1α was the rosetting ligand of the R29 clone (104). In our experiments, we directly amplified the var gene mRNA from single-rosetting trophozoites (31). The idea was that if PfEMP1 mediates malaria rosetting, the var mRNA amplified directly from single-rosetting parasites must encode the rosetting ligand. Based on the amplified sequence, the full-length cDNA of the rosetting var gene was cloned. The high sensitivity of rosettes to heparin in this strain led to the analysis of the deduced amino acid sequence for potential GAG-binding motifs composed of basic amino acids (25, 26, 31). Multiple motifs were indeed found in the N-terminal region of this rosetting PfEMP1 sequence (31). Recombinant DBL-1α, which contained eight heparin-binding motifs, bound directly to heparin both in solution and on a solid matrix. Furthermore, this DBL-1α fusion protein of FCR3S1.2 var1 disrupted naturally formed rosettes and directly adhered to the uninfected RBC surface; this binding was abated by pretreating the RBCs with heparinase. These findings strongly indicated that the DBL-1α region of FCR3S1.2 var1 is the rosetting ligand. GAG-binding motifs are also located in other regions of PfEMP1 (CIDR1α and DBL-2δ); their interaction with RBC receptors cannot be ruled out, although the binding might be weak (Q. Chen, unpublished data). The pool of var genes in the wild is vast (71), and therefore there may be many rosetting ligands, and there is a possibility that the rosetting ligands might bind to similar receptors on vascular endothelium. PfEMP1, in particular the DBL-1α domain, could be the molecular link between the rosetting phenotype and the virulence of the parasite in vivo.
ANTIGENIC VARIATION
In malaria infections, especially in chronic cases, the peaks of parasitemia may fluctuate over time (21, 86). Recrudescence with the appearance of different antigenic parasites is an essential strategy for malaria parasite survival. Both merozoite- and late-stage-trophozoite- or schizont-infected RBCs undergo antigenic variation, which makes the host immune response frequently inefficient, leading to prolonged infection. Malaria parasites challenge the human immune system in at least two ways: through genetic recombination in mosquitoes, a process which results in unlimited changes of the malaria genomic pool in the wild, and through the existence of several variable antigenic gene families (the var family, the rosettin/rif family, and the Pf60 family) in the genome of the parasites. The large reservoir of variant antigens gives the parasite the ability to avoid specific and unspecific immune clearance by the host, thereby ensuring transmission.
Merozoite Surface Antigens
Living within the human bloodstream means that the parasites are under continuous pressure from the host immune system. During their short extracellular presence in the blood, merozoites should be easily recognized by specific antibodies, but the merozoite surface also varies among parasites. Parasites expose different amino acid sequences of merozoite surface protein 1 (MSP-1) to the immune system. Within the MSP-1 peptide sequence, only the C-terminal 19-kDa fragment, which is carried with the parasite during invasion, is relatively conserved. The rest of the peptide, which is released upon invasion, is antigenically variable. Furthermore, four other MSP antigens (MSP-2, -3, -4, and -5) have been found on the P. falciparum merozoite surface (51, 83). Like the surface antigens of the Toxoplasma gondii SAG family, blocking the activity of one SAG protein will not efficiently hinder parasite invasion. The RBC membrane-binding protein (EBA-175) is another surface-located polypeptide which mediates merozoite attachment to glycophorin of human RBCs (111). It is encoded by a single-copy gene, but its sequence is still not completely conserved among different isolates. This means that the same functional EBA-175 from different P. falciparum parasites is antigenically variant.
Recently, another group of antigens named Pf60 were identified on the P. falciparum merozoite. These antigens were initially identified with a cross-reactive antiserum to a Babesia divergens rhoptry-located protein (23, 24, 55), where the antibodies specifically labeled P. falciparum merozoites both as free merozoites and within well-differentiated schizonts. The proteins were clearly located in the apex of the merozoites and could be detected after 42 h postinvasion in the schizonts. Evidence indicated that this polypeptide is located in the rhoptries and is released and deposited by invading merozoites onto the surface of RBCs during attachment. Interestingly, when attempting to isolate the gene encoding this polypeptide, Carcy et al. found that it was encoded by a large gene family with about 140 copies per haploid genome (23). The deduced amino acid sequences of several clones showed high homologies to several parasite proteins. First, they had homologous sequences with a Babesia rhoptry-located protein. Second, the Pf60 sequences showed high similarity to the PfEMP1 ATS, the conserved intracellular domain.
The Pf60 multigene family was the first large gene family ever found in P. falciparum. It is still not known how many of these genes are expressed by a single merozoite, and the biological function of these proteins is also not yet clear. Their location in the rhoptry and deposition on the RBC surface during invasion indicate that they are involved in merozoite attachment to the RBCs and/or are directly involved in invasion. Genetic rearrangement or modification of the Pf60 genes also seems to occur in the parasite genome (17, 76). The high sequence similarity of Pf60 to the var family suggests that Pf60 may play an important role in antigenic variation of the merozoite surface. The genes of the var and Pf60 families are both in part localized to the subtelomeric region of chromosomes and are both found on chromosomes 2 and 3 (51). Even though Pf60 sequences were termed var pseudogenes (20, 51), the encoded proteins were identified in the parasite, indicating that these genes encode polypeptides which are biologically significant to the parasite (23, 24). Several members of a related family of Py235 merozoite polypeptides in Plasmodium yoelii were recently discovered to be simultaneously expressed in schizonts, but only one family member per merozoite. More than 10 multicopy Py235 genes encoding the P. yoelii rhoptry protein Py235 were found (95). With the gene sequences at hand, functional and genetic characterization of these merozoite proteins in the generation of variability is merely a matter of time.
PfEMP1
A phenomenon of malaria is that the immune response in patients is never able to eradicate the infection. Even though some do not suffer much from the disease, parasites can persist for a long period in their human hosts. The periodic recrudescence of parasitemia accompanied by serum agglutination of homologous pRBCs was first found in Plasmodium knowlesi infections and was suggested to be due to antigenic variation of the infected RBC surface (21). Howard et al. identified a variable high-molecular-weight polypeptide on the P. knowlesi-infected RBC surface which was recently found to be encoded by the schizont-infected cell agglutination (SICA) var multigene family (3, 64). Homologous polypeptides with a molecular mass of about 250 to 300 kDa present on the surface of P. falciparum-infected RBCs were also described in 1984, and the protein was named P. falciparum erythrocyte membrane protein 1 (PfEMP1) (75). Several studies have since demonstrated that PfEMP1 is expressed on the surface of infected RBCs and that it is one of the main variant antigens which is comodulated with the adherent phenotype of pRBCs (16, 65, 80). Roberts et al. subsequently studied the antigenic switching of the pRBC surface with different clones from the same parental parasite (99). Even in in vitro culture without any selective pressure, the parasites showed rapid clonal antigenic switching, with a rate of 2.4% per generation, but this is low in other parasites. Micromanipulation-generated offspring clones selected with a micropipette from the same parental parasite strain showed diverse binding phenotypes and corresponding antigenic differences at the pRBC surface. The rate of antigenic switching of the P. falciparum-infected RBC surface in patients is still not known, but clinical studies have confirmed that human antibody responses to the surface antigen are predominantly variant specific (81, 89).
PfEMP1 is one of the two variant antigen families expressed by P. falciparum on the RBC surface; the other is the rosettin/rifin family. How is one PfEMP1 exchanged for another, and what is the genetic background of this antigenic variation? For a long time, due to the small amount of PfEMP1 present on the pRBC surface, these questions have intrigued malariologists and were not answered until the relatively recent identification of the var genes encoding PfEMP1.
var gene family.
In their search for the chloroquine-associated resistance genes, Su et al. encountered a group of genes with large open reading frames (ORFs) which encoded polypeptides with molecular masses of 200 to 350 kDa (116). Since the sequences of these genes showed considerable variability or polymorphism, they were collectively named var genes. The var genes are composed of two exons separated by an approximately 1-kb intron (Fig. 3). The deduced amino acid sequences of these genes showed high similarity to the sequences simultaneously cloned by two other groups (12, 115). By combining their data, these groups concluded that the identified sequences encoded proteins of the same family, i.e., PfEMP1. Alignment of the sequences with those in the database revealed that the deduced amino acid sequences contained conserved stretches of high similarity to the cysteine-rich domains of the Duffy antigen-binding proteins (DABP) of Plasmodium vivax and P. knowlesi and EBA-175 of P. falciparum (92). Those semiconserved regions of PfEMP1 were therefore named Duffy-binding-like domains (DBL). The number of var genes in the P. falciparum haploid genome is estimated to be between 40 and 50 (32). The var genes appear to be scattered on all chromosomes, as Rubio et al. hybridized the var-c (exon II) probe to all 14 chromosomes (105), while one var gene is found at each subtelomeric region. However, var genes are not evenly distributed among the chromosomes, with chromosomes 4, 7, and 12 harboring more var genes than the other chromosomes.
Polymorphic genome and universal var genes.
One feature of malaria parasites is the high polymorphism of their genome. By using the pulsed-field gel electrophoresis (PFGE) technique, all the chromosomes can now be separated from each other (105). Although 14 chromosomes are found in P. falciparum, the size of any given chromosome in different strains and isolates varies greatly. Most of the genomic polymorphism of the malaria parasite is determined during the transient diploid stage in the mosquito gut, where genomic crossover events occur (74). Even within the haploid genome, interchromosomal crossovers have been found (117), suggesting that genes with the same function could be located on different chromosomes.
The var genes have been found to exist in all P. falciparum parasites (115–117). In their studies on var transcription in different parasite clones generated from the same parental parasite, A4, Smith et al. used different var transcripts as probes to hybridize to genomic DNA digested with several restriction enzymes (115). The results showed that all cDNA probes detected similar genes in different clones of A4. In our recent studies, a specific var cDNA was used to probe for the genes in the genome of the following FCR3S1 cloned parasites: FCR3S1 R+, FCR3S1.2 R+, FCR3S1.6 R−, and FCR3S/a R− (R indicates the rosetting phenotype). Even though the mRNA transcript was not found in either FCR3S1.6 or FCR3S/a (rosetting negative), the gene FCR3S1.2 var1 was targeted to chromosome 4 in all those parasites independently of their phenotypes (32). While mapping the var genes on different chromosomes, Thompson et al. found that, unlike the polymorphic var genes localized in the subtelomeric regions, the ones harbored in the central part of the chromosomes are quite conserved (117). A reason for the parasite to have a relatively large reservoir of var genes is to vary the antigenic profile in order to avoid immune clearance by the human host. Without the ability to change the PfEMP1 expressed on the infected RBC surface, most parasites perhaps could not survive for more than a few generations.
var genes are universal to all P. falciparum parasites. Studies on both wild isolates and laboratory-adapted strains are quite disappointing, since the var gene profile of strains from different malaria-endemic areas and even among different strains does not overlap. In our recent experiments, the gene locations of four different var transcripts, cloned from the FCR3S1.2 parasite, were in all cases on different chromosomes (32). Interestingly, these sequences never hybridized to any of the chromosomes of 3D7 parasites, suggesting that the 3D7 genome has a very different var gene repertoire from that of FCR3 parasites (32). Similar results were obtained with wild isolates (71). Thus, all P. falciparum strains have var genes, but individual strains or isolates seem to harbor unique sets of var gene variants. Although conserved regions and amino acid residues are indeed found in PfEMP1 (DBL-1α and CIDR1α), whether cross-reactive antibodies to these regions can be induced remains to be tested. In any case, the huge var diversity makes immunoprevention based on var PfEMP1 sequences challenging.
Chromosomal location of var genes.
After identification of the var genes, their location and expression sites on chromosomes were extensively studied. var gene locations on chromosome 12 of the 3D7 parasite were first reported. With both a universal var probe (var-C/exon II) and a subtelomere-specific probe (rep20), Rubio et al. found var genes in both subtelomeric regions as well as in the central part of the chromosome (105). Studies on other chromosomes of 3D7 using two-dimensional PFGE made them conclude that most var genes are located in subtelomeric regions of most chromosomes. Furthermore, the expressed var genes are also likely to be found at subtelomeric regions, even though some centrally localized var genes could also be expressed (107). Most interestingly, several var genes at the subtelomeric regions of the same chromosome hybridized with a specific var probe, suggesting that those var genes had very homologous sequences or most likely were of the same origin. The centrally located var genes did not show this property. Thus, some var genes located in the subtelomeric regions are probably closely related, and the occurrence of recombination between heterologous chromosomes is thus also possible. In P. falciparum, exchanges of large subtelomeric fragments (>100 kb) between heterologous chromosomes, duplication and divergence of gene-containing regions, and spontaneous deletion events are known to lead to phenotypic variability of the genome (35, 74, 106, 107). Even though the exact contribution of var genes to chromosome subtelomeric variability has not been solved, the antigenic variation and cytoadhesive phenotypic switching due to subtelomeric recombination are in part implicated in the sequestration of pRBCs with novel PfEMP1 molecules displayed at the surface (106).
var gene activation and switching mechanisms.
Due to the important contribution of PfEMP1 to malaria pathogenesis, further characterization of the activation and switching mechanism is still an attractive theme in malaria research (18, 62, 126). It is still a mystery how var gene transcription is switched on and off. In eukaryotic cells, the subtelomeric regions are supposedly very unstable; large deletions have indeed been found in malaria parasites (74). Since the var genes are harbored in these regions, some var genes could be transposed with the movement of chromosome fragments, but recombination or transposition events have not been found to be a major mechanism of var gene activation (106). Studies with in vitro-cloned parasites showed that at least 2% of the parasites switch antigens each generation (99). One PfEMP1 had been exchanged with another (115). An interesting result from Smith et al. was that multiple chromosome sites were probably activated during var gene transcription (115). Recent studies (31, 107) further confirmed that phenotypically similar parasites could activate many different var loci after RBC invasion. Rowe et al. first found that in the same R29 clonal population, many different var transcripts could be detected if the parasites were not separated by developmental stage (104). While the Scherf group (107) compared the transcription of var genes from ring and trophozoite stages of FCR3 P. falciparum with a defined phenotype, for example, CSA binding, they found that at the early stage, most of the var transcripts could be detected (at least the DBL-1α sequences of those genes). Surprisingly, at the late trophozoite stage, only one var mRNA was found (107). When we extended the studies to single-infected RBCs with either ring or trophozoite stage parasites, it was quite clear that at the ring stage there is massive var gene activation (32). Several var transcripts could be detected by reverse transcription-PCR and subsequent hybridization to the var gene array, while only one var mRNA and one PfEMP1 polypeptide were found in the late-stage parasite (32). Furthermore, several distinct transcripts from the same cell hybridized to different chromosomes, indicating that genes located on different chromosomes were transcribed simultaneously (32). The data suggested that the parasite activates several of its var genes at an early stage of RBC infection, while the expression of only one var gene is maintained at the late stage. It is not known how this kind of activation happens or whether all the transcripts are full length, but it is quite clear that the mechanism is different from that of variant surface glycoprotein (VSG) gene activation in African trypanosomes, where only one of the VSG genes is transcribed at one specific subtelomeric transcription site (19). The initiation of transcription of each malaria var gene is therefore most likely controlled by its own promoter, but unique promoters to transcribed var genes have not been found yet. On the other hand, methylation of genomic DNA may be important in var gene regulation. Chromatin structure modification contributing to var gene regulation has also been hypothesized, but supporting experimental data are still missing (40, 126, 131). Finally, all these data were obtained from studies on parasites under long-term in vitro growth. Whether they truly reflect the nature of var gene switching by parasites within the human host needs to be verified.
rif Multigene Family
Parasite-derived, low-molecular-weight surface polypeptides, collectively named rosettins or rifins, are frequently seen at the infected RBC surface of both laboratory-adapted strains and wild isolates (28, 46, 59). Antibodies from malaria patients can immunoprecipitate several different proteins (PfEMP1 and rifins) from pRBCs. This indicated that rifins, like PfEMP1, are the targets of host immune response. Through the malaria genome project, several rif genes were recently found on chromosomes 2 and 3 of the 3D7 parasite (20, 51). Rifin mRNA was also identified in the parasite. The identification of a signal peptide sequence at the N terminus as well as a transmembrane region in the deduced amino acid sequence indicated that the protein might be exposed on the RBC surface. The deduced rifin polypeptides are much smaller than PfEMP1 but very similar to rosettins. Are rosettins thus rifins? The immunoprecipitation of rosettins with rifin-specific antibodies bridged for the first time the gap between a protein with an unknown gene and a gene family with unknown proteins (46). Because only low-molecular-weight polypeptides were precipitated with antirifin antibodies, this indicated that some of the rosettins are indeed rifins and that they are encoded by the rif multigene family (46).
From their distribution on chromosomes 2 and 3, the total number of rif genes in the malaria genome was predicted to be about 200 (20, 51). If this is true, the rif gene family might be the largest multigene family in the malaria parasite. Chromosome mapping clearly showed that rif genes are located in the subtelomeric regions of chromosomes directly adjacent to a var gene (20, 51). As in the case of PfEMP1, the rosettins and rifins are also clonally antigenically variable (46). Due to their abundance at the pRBC surface, the role of rosettins and rifins in antigenic variation could be significant.
The sequence of rifins differs from that of PfEMP1, indicating that they are biologically different proteins. Do rosettins and rifins adhere to host receptors? With the sequences now available, the immunological and biological significance of this group of small polypeptides can be investigated.
PERSPECTIVES
Malaria has plagued mankind with a myriad of mechanisms for avoiding natural as well as induced immune responses. Campaigns against both the parasite and the disease at the molecular level are crucial. Now, although the mystery of the var genes and the function of their encoded proteins has in part been uncovered, the PfEMP1 molecules and the exact mechanism of var gene switching and regulation must be further characterized. If all the var genes can be activated simultaneously, when and how is the selection for continued expression of one mRNA accomplished? With more var gene sequences available, the technique of DNA array hybridization might answer this question (85, 130). As no signal sequence was found in the N-terminal part of PfEMP1 molecules, the question is whether the signal is harbored in the internal part of PfEMP1. Chemicals that block the intracellular transport of PfEMP1 to its final destination could be a potential therapeutic and preventive medicine for malaria. Will PfEMP1 be a potential candidate for an antimalaria vaccine? Concerning its biological and pathological significance, it is natural to think that to intervene in the process of sequestration, the cytoadherent and rosetting functions of PfEMP1 may need to be blocked when the pRBCs are bound in the microvasculature. Receptor analogs which disrupt or destabilize the PfEMP1-host interaction might allow reperfusion of clogged vessels. Since so many PfEMP1s may perform the same or similar binding functions, the common structure of the molecules should be elucidated. Moreover, like PfEMP1, rifins are surface located and are even more variable in sequence than PfEMP1. Functional analysis is most likely the next step in the investigation of this interesting family of polypeptides. Like other variable proteins, rifins might have important functions such as receptor-ligand interactions, or they may function as transporters of molecules from the RBC surface. Finally, malaria molecular biology is progressing rapidly, but much still remains to be unveiled.
ACKNOWLEDGMENTS
Some parts of the work discussed here were supported by grants from the Swedish Cancer Society (Cancerfonden), the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR), an INCO-DC grant from the European Commission, and the Swedish Medical Research Council.
REFERENCES
- 1.Aikawa M. Human cerebral malaria. Am J Trop Med Hyg. 1988;39:3–10. doi: 10.4269/ajtmh.1988.39.3. [DOI] [PubMed] [Google Scholar]
- 2.Aley S B, Sherwood J A, Howard R J. Knob-positive and knob-negative Plasmodium falciparum differ in expression of a strain-specific malarial antigen on the surface of infected erythrocytes. J Exp Med. 1984;160:1585–1590. doi: 10.1084/jem.160.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Khedery B, Barnwell J W, Galinski M R. Antigenic variation in malaria: a 3′ genomic alteration associated with the expression of a P. knowlesi variant antigen. Mol Cell. 1999;3:131–141. doi: 10.1016/s1097-2765(00)80304-4. [DOI] [PubMed] [Google Scholar]
- 4.Anstey N M, Weinberg J B, Hassanali M Y, Mwaikambo E D, Manyenga D, Misukonis M A, Arnelle D R, Hollis D, McDonald M I, Granger D L. Nitric oxide in Tanzanian children with malaria: inverse relationship between malaria severity and nitric oxide production/nitric oxide synthase type 2 expression. J Exp Med. 1996;184:557–567. doi: 10.1084/jem.184.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnwell J W, Asch A S, Nachman R L, Yamaya M, Aikawa M, Ingravallo P. A human 88-kD membrane glycoprotein (CD36) functions in vitro as a receptor for a cytoadherence ligand on Plasmodium falciparum-infected erythrocytes. J Clin Investig. 1989;84:765–772. doi: 10.1172/JCI114234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnwell J W, Ockenhouse C F, Knowles D M. Monoclonal antibody OKM5 inhibits the in vitro binding of Plasmodium falciparum-infected erythrocytes to monocytes, endothelial, and C32 melanoma cells. J Immunol. 1985;135:3494–3497. [PubMed] [Google Scholar]
- 7.Barragan A, Spillmann D, Wahlgren M, Carlson J. Erythrocyte glucans as Plasmodium falciparum rosetting receptors: molecular background of strain specific rosette disruption by glycosaminoglycans and sulfated glycoconjugates. Exp Parasitol. 1998;91:133–143. doi: 10.1006/expr.1998.4349. [DOI] [PubMed] [Google Scholar]
- 8.Barragan A, Spillmann D, Carlson J, Wahlgren M. The role of glycans in Plasmodium falciparum infection. Biochem Soc Trans. 1999;27:487–493. doi: 10.1042/bst0270487. [DOI] [PubMed] [Google Scholar]
- 9.Barragan A, Kremsner P G, Weiss W, Wahlgren M, Carlson J. Age-related buildup of humoral immunity against epitopes for rosette formation and agglutination in African areas of malaria endemicity. Infect Immun. 1998;66:4783–4787. doi: 10.1128/iai.66.10.4783-4787.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baruch D I, Gormley J A, Ma C, Howard R J, Pasloske B L. Plasmodium falciparum membrane protein 1 is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin, and intercellular adhesion molecule 1. Proc Natl Acad Sci USA. 1996;93:3497–3502. doi: 10.1073/pnas.93.8.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baruch D I, Ma X C, Singh H B, Bi X, Pasloske B L, Howard R J. Identification of a region of PfEMP1 that mediates adherence of Plasmodium falciparum infected erythrocytes to CD36: conserved function with variant sequence. Blood. 1997;90:3766–3775. [PubMed] [Google Scholar]
- 12.Baruch D I, Pasloske B L, Singh H B, Ma X C, Feldman M, Taraschi T F, Howard R J. Cloning the P. falciparum gene encoding PfEMP1, a malaria variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–87. doi: 10.1016/0092-8674(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 13.Bate C A W, Taverne J, Playfair J H L. Soluble malaria antigens are toxic and induce the production of tumor necrosis factor in vivo. Immunology. 1989;66:600–605. [PMC free article] [PubMed] [Google Scholar]
- 14.Bate C A W, Taverne J, Roman E, Moreno C, Playfair J H L. TNF induction by malaria exoantigens depends upon phospholipid. Immunology. 1992;75:129–135. [PMC free article] [PubMed] [Google Scholar]
- 15.Berendt A R, Simmons D L, Tansey J, Newbold C I, Marsh K. Intercellular adhesion molecule-1 is an endothelial cell adhesion receptor for Plasmodium falciparum. Nature. 1989;341:57–59. doi: 10.1038/341057a0. [DOI] [PubMed] [Google Scholar]
- 16.Biggs B, Anders R F, Dillon H E, Davern K M, Martin M, Petersen C, Brown G V. Adherence of infected erythrocytes to venular endothelium selects for antigenic variation of Plasmodium falciparum. J Immunol. 1992;149:2047–2054. [PubMed] [Google Scholar]
- 17.Bonnefoy S, Bischoff E, Guillotte M, Mercereau-Puijalon O. Evidence for distinct prototype sequences with the Plasmodium falciparum Pf60 multigene family. Mol Biochem Parasitol. 1997;87:1–11. doi: 10.1016/s0166-6851(97)00033-9. [DOI] [PubMed] [Google Scholar]
- 18.Borst P, Bitter W, McCulloch R, Leeuwen F V, Rudenko G. Antigenic variation in malaria. Cell. 1995;82:1–4. doi: 10.1016/0092-8674(95)90044-6. [DOI] [PubMed] [Google Scholar]
- 19.Borst P, Rudenko G. Antigenic variation in African trypanosomes. Science. 1994;264:1872–1873. doi: 10.1126/science.7516579. [DOI] [PubMed] [Google Scholar]
- 20.Bowman S, et al. The complete nucleotide sequence of chromosome 3 of Plasmodium falciparum. Nature. 1999;400:532–538. doi: 10.1038/22964. [DOI] [PubMed] [Google Scholar]
- 21.Brown K N, Brown I N. Immunity to malaria: antigenic variation in chronic infections of Plasmodium knowlesi. Nature. 1965;208:1286–1288. doi: 10.1038/2081286a0. [DOI] [PubMed] [Google Scholar]
- 22.Buffet P A, Gamain B, Scheidig C, Baruch D, Smith J D, Hernandez-Rivas R, Pouvelle B, Oishi S, Fujii N, Fusai T, Parzy D, Miller L H, Gysin J, Scherf A. Plasmodium falciparum domain mediating adhesion to chondroitin sulfate A: a receptor for human placental infection. Proc Natl Acad Sci USA. 1999;96:12743–12748. doi: 10.1073/pnas.96.22.12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carcy B, Bonnefog S, Guillotte M, Scarf C L, Grellier P, Schrevel J, Fandeur T, Mercereau-Puijalon O. A large multigene family expressed during the erythrocytic schizogony of Plasmodium falciparum. Mol Biochem Parasitol. 1994;68:221–233. doi: 10.1016/0166-6851(94)90167-8. [DOI] [PubMed] [Google Scholar]
- 24.Carcy B, Precigout E, Valentin A, Gorenflot A, Schrevel J. A 37-kilodalton glycoprotein of Babesia divergens is a major component of a protective fraction containing low-molecular-mass culture-derived exoantigens. Infect Immun. 1995;63:811–817. doi: 10.1128/iai.63.3.811-817.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cardin A D, Weintraub H J R. Molecular modelling of protein-glycosaminoglycan interaction. Arteriosclerosis. 1989;9:21–32. doi: 10.1161/01.atv.9.1.21. [DOI] [PubMed] [Google Scholar]
- 26.Carlson J, Ekre H P, Helmby H, Gysin J, Greenwood B M, Wahlgren M. Disruption of Plasmodium falciparum erythrocyte rosettes by standard heparin and heparin devoid of anticoagulant activity. Am J Trop Med Hyg. 1992;46:595–602. doi: 10.4269/ajtmh.1992.46.595. [DOI] [PubMed] [Google Scholar]
- 27.Carlson J, Helmby H, Hill A V S, Brewster D, Greenwood B M, Wahlgren M. Human cerebral malaria: association with erythrocyte rosetting and lack of anti-rosetting antibodies. Lancet. 1990;336:1457–1460. doi: 10.1016/0140-6736(90)93174-n. [DOI] [PubMed] [Google Scholar]
- 28.Carlson J, Holmquist G, Taylor D W, Perlmann P, Wahlgren M. Antibodies to a histine-rich protein (PfHRP1) disrupt spontaneously formed Plasmodium falciparum erythrocyte rosettes. Proc Natl Acad Sci USA. 1990;87:2511–2515. doi: 10.1073/pnas.87.7.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carlson J, Wahlgren M. Plasmodium falciparum erythrocyte rosetting is mediated by promiscuous lectin-like interactions. J Exp Med. 1992;176:1311–1317. doi: 10.1084/jem.176.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaiyaroj S C, Coppel R L, Novakovic S, Brown G V. Multiple ligands for cytoadherence can be present simultaneously on the surface of Plasmodium falciparum-infected erythrocytes. Proc Natl Acad Sci USA. 1994;91:10805–10808. doi: 10.1073/pnas.91.23.10805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Q, Barragan A, Fernandez V, Sundström A, Schlichtherle M, Sahlén A, Carlson J, Datta S, Wahlgren M. Identification of Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) as the rosetting ligand of the malaria parasite P. falciparum. J Exp Med. 1998;187:15–23. doi: 10.1084/jem.187.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Q, Fernandez V, Sundström A, Schlichtherle M, Datta S, Hagblom P, Wahlgren M. Developmental selection of var gene expression in Plasmodium falciparum. Nature. 1998;394:392–395. doi: 10.1038/28660. [DOI] [PubMed] [Google Scholar]
- 33.Clark D L, Su S, Davidson E A. Saccharide anions as inhibitors of the malaria parasite. Glycoconj J. 1997;14:473–479. doi: 10.1023/a:1018551518610. [DOI] [PubMed] [Google Scholar]
- 34.Coppel L R, Brown G V, Nussenzweig V. Adhesive proteins of the malaria parasite. Curr Opin Microbiol. 1998;1:472–481. doi: 10.1016/s1369-5274(98)80068-4. [DOI] [PubMed] [Google Scholar]
- 35.Corcoran L M, Thompson J K, Walliker D, Kemp D J. Homologous recombination subtelomeric repeat sequences generate chromosome size polymorphism in P. falciparum. Cell. 1988;53:807–813. doi: 10.1016/0092-8674(88)90097-9. [DOI] [PubMed] [Google Scholar]
- 36.Craig A G, Pinches R, Khan S, Roberts D J, Turner G D H, Newbold C I, Berendt A R. Failure to block adhesion of Plasmodium falciparum-infected erythrocytes to ICAM-1 with soluble ICAM-1. Infect Immun. 1997;65:4580–4585. doi: 10.1128/iai.65.11.4580-4585.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Creighton E T. Protein folding. New York, N.Y: W. H. Freeman and Company; 1992. pp. 405–454. [Google Scholar]
- 38.Cubas A B C, Gentilini M, Monjour L. Cytokines and T-cell response in malaria. Biomed Pharmacother. 1994;48:27–33. doi: 10.1016/0753-3322(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 39.Dame J B, et al. Current status of the Plasmodium falciparum genome project. Mol Biochem Parasitol. 1996;79:1–12. doi: 10.1016/0166-6851(96)02641-2. [DOI] [PubMed] [Google Scholar]
- 40.Darkin-Rattray S J, Gurnett A M, Myers R W, Dulski P M, Crumley T M, Allocco J J, Cannova C, Meinke P T, Colletti S L, Bednarek M A, Singh S B, Goetz M A, Dombrowski A W, Polishook J D, Schmatz D M. Apicidin: a novel antiprotozoal agent that inhibits parasite histone deacetylase. Proc Natl Acad Sci USA. 1996;93:13143–13147. doi: 10.1073/pnas.93.23.13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.David P H, Handunnetti S M, Leech J H, Gamage P, Mendis K N. Rosetting: a new cytoadherence property of malaria-infected erythrocytes. Am J Trop Med Hyg. 1988;38:289–297. doi: 10.4269/ajtmh.1988.38.289. [DOI] [PubMed] [Google Scholar]
- 42.Deitsch K W, Wellems T E. Membrane modifications in erythrocytes parasitized by Plasmodium falciparum. Mol Biochem Parasitol. 1996;76:1–10. doi: 10.1016/0166-6851(95)02575-8. [DOI] [PubMed] [Google Scholar]
- 43.Dondorp A M, Angus B J, Hardeman M R, Chotivanich K T, Silamut K, Ruangveerayuth R, Kager P A, White N J, Vreeken J. Prognostic significance of reduced red blood cell deformability in severe falciparum malaria. Am J Trop Med Hyg. 1997;57:507–511. doi: 10.4269/ajtmh.1997.57.507. [DOI] [PubMed] [Google Scholar]
- 44.Ferguson M A, Homans S W, Dwek R A, Rademacher T W. The glycosylphosphatidylinositol membrane anchor of Trypanosoma brucei variant surface glycoprotein. Biochem Soc Trans. 1988;16:265–268. doi: 10.1042/bst0160265. [DOI] [PubMed] [Google Scholar]
- 45.Fernandez V, Treutiger C J, Nash G B, Wahlgren M. Multiple adhesive phenotypes linked to rosetting-binding of erythrocytes in Plasmodium falciparum malaria. Infect Immun. 1998;66:2969–2975. doi: 10.1128/iai.66.6.2969-2975.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fernandez V, Hommel M, Chen Q, Hagblom P, Wahlgren M. Small clonally variant antigens on the surface of the Plasmodium falciparum-infected erythrocyte are encoded by the rif gene family and are the target of human immune responses. J Exp Med. 1999;190:1393–1403. doi: 10.1084/jem.190.10.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fischer P R, Boone P. Short report: severe malaria associated with blood group. Am J Trop Med Hyg. 1998;58:122–123. doi: 10.4269/ajtmh.1998.58.122. [DOI] [PubMed] [Google Scholar]
- 48.Foster F. The Plasmodium falciparum genome project: a resource for researchers. Parasitol Today. 1995;11:1–4. [Google Scholar]
- 49.Fried M, Duffy P E. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272:1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 50.Fujioka H, Aikawa M. The molecular basis of pathogenesis of cerebral malaria. Microb Pathog. 1996;20:63–72. doi: 10.1006/mpat.1996.0006. [DOI] [PubMed] [Google Scholar]
- 51.Gardner J M, et al. Chromosome 2 sequence of the human malaria parasite Plasmodium falciparum. Science. 1998;282:1126–1132. doi: 10.1126/science.282.5391.1126. [DOI] [PubMed] [Google Scholar]
- 52.Gerold P, Dieckmann-Schuppert A, Schwarz R T. Glycosyl-phosphatidylinositols synthesized by asexual erythrocytic stages of the malaria parasite, Plasmodium falciparum, candidates for plasmodial glycosyl-phosphatidylinositol membrane anchor precursors and pathogenicity factors. J Biol Chem. 1994;269:2597–2606. [PubMed] [Google Scholar]
- 53.Gowda D C, Davidson E A. Protein glycosylation in the malaria parasite. Parasitol Today. 1999;15:147–152. doi: 10.1016/s0169-4758(99)01412-x. [DOI] [PubMed] [Google Scholar]
- 54.Green S J, Scheller L F, Marletta M A, Seguin M C, Klotz F W, Slayter M, Nelson B J, Nacy C A. Nitric oxide: cytokine-relation of nitric oxide in host resistance to intracellular pathogens. Immunol Lett. 1994;43:87–94. doi: 10.1016/0165-2478(94)00158-8. [DOI] [PubMed] [Google Scholar]
- 55.Grellier P, Precigout E, Valentin A, Carcy B, Schrevel J. Characterization of a 60 kDa apical protein of Plasmodium falciparum merozoite expressed in late schizogony. Biol Cell. 1994;82:129–138. doi: 10.1016/s0248-4900(94)80015-4. [DOI] [PubMed] [Google Scholar]
- 56.Handunetti S M, van Schravendijk M R, Hasler T, Barnwell J W, Greenwalt D E, Howard R J. Involvement of CD36 on erythrocytes as a rosetting receptors for Plasmodium falciparum-infected erythrocytes. Blood. 1992;80:2097–2104. [PubMed] [Google Scholar]
- 57.Handunnetti S M, David P H, Perera K L, Mendis K N. Uninfected erythrocytes form “rosettes” around Plasmodium falciparum infected erythrocytes. Am J Trop Med Hyg. 1989;40:115–118. doi: 10.4269/ajtmh.1989.40.115. [DOI] [PubMed] [Google Scholar]
- 58.Reference deleted.
- 59.Helmby H, Cavelier L, Pettersson U, Wahlgren M. Rosetting Plasmodium falciparum-infected erythrocytes express unique strain-specific antigens on their surface. Infect Immun. 1993;61:284–288. doi: 10.1128/iai.61.1.284-288.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoessli D C, Davidson E A, Schwarz R T, Nasir-Ud-Din Glycobiology of Plasmodium falciparum: an emerging area of research. Glycoconj J. 1996;13:1–3. doi: 10.1007/BF01049673. [DOI] [PubMed] [Google Scholar]
- 61.Hommel M. Physiopathology of symptoms of malaria: role of cytokines, cytoadherence and preimmunity. Presse Med. 1996;25:70–76. [PubMed] [Google Scholar]
- 62.Horrocks P, Dechering K, Lanzer M. Control of gene expression in Plasmodium falciparum. Mol Biochem Parasitol. 1998;95:171–181. doi: 10.1016/s0166-6851(98)00110-8. [DOI] [PubMed] [Google Scholar]
- 63.Howard R J, Gilladoga A D. Molecular studies related to the pathogenesis of cerebral malaria. Blood. 1989;74:2603–2618. [PubMed] [Google Scholar]
- 64.Howard R J, Barnwell J W, Kao Y. Antigenic variation of Plasmodium knowlesi malaria: identification of the variant antigen on infected erythrocytes. Proc Natl Acad Sci USA. 1983;80:4129–4133. doi: 10.1073/pnas.80.13.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Howard R J, Barnwell J W, Rock E P, Janet N, Ofori-Adjei D, Maloy W L, Lyon J A, Saul A. Two approximately 300 kilodalton Plasmodium falciparum proteins at the surface membrane of infected erythrocytes. Mol Biochem Parasitol. 1988;27:207–224. doi: 10.1016/0166-6851(88)90040-0. [DOI] [PubMed] [Google Scholar]
- 66.Imbert P, Sartelet I, Rogier C, Ka S, Baujat G, Candito D. Severe malaria among children in a low seasonal transmission area, Dakar, Senegal: influence of age on clinical presentation. Trans R Soc Trop Med Hyg. 1997;91:22–24. doi: 10.1016/s0035-9203(97)90380-1. [DOI] [PubMed] [Google Scholar]
- 67.Jakobsen P H, Bate C A W, Taverne J, Playfair J H L. Malaria: toxins, cytokines and disease. Parasite Immunol. 1995;17:223–231. doi: 10.1111/j.1365-3024.1995.tb01019.x. [DOI] [PubMed] [Google Scholar]
- 68.Karunaweera N D, Grau G E, Gamage P, Carter R, Mendis K N. Dynamics of fever and serum levels of tumor necrosis factor are closely associated during clinical paroxysms in Plasmodium vivax malaria. Proc Natl Acad Sci USA. 1992;89:3200–3203. doi: 10.1073/pnas.89.8.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kwiatkowski D, Molyneux M E, Stephens S, Curtis N, Klein N, Pointaire P, Smit M, Allan R, Brewster D R, Grau G E, Greenwood B M. Anti-TNF therapy inhibits fever in cerebral malaria. Q J Med. 1993;86:91–98. [PubMed] [Google Scholar]
- 70.Kwiatkowski D, Hill A V, Sambou I, Twumasi P, Castracane J, Manogue K R, Cerami A, Brewster D R, Greenwood B M. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet. 1990;336:1201–1204. doi: 10.1016/0140-6736(90)92827-5. [DOI] [PubMed] [Google Scholar]
- 71.Kyes S, Taylor H, Craig A, Marsh K, Newbold C I. Genomic representation of var gene sequences in Plasmodium falciparum field isolates from different geographic regions. Mol Biochem Parasitol. 1997;87:235–238. doi: 10.1016/s0166-6851(97)00071-6. [DOI] [PubMed] [Google Scholar]
- 72.Kyes S A, Rowe J A, Kriek N, Newbold C I. Rifins: a second family of clonally variant proteins expressed on the surface of red cells infected with Plasmodium falciparum. Proc Natl Acad Sci USA. 1999;96:9333–9338. doi: 10.1073/pnas.96.16.9333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Land K M, Sherman J, Gysin J, Crandall I E. Antiadhesive antibodies and peptides as potential therapeutics for Plasmodium falciparum malaria. Parasitol Today. 1995;11:19–23. [Google Scholar]
- 74.Lanzer M, Fischer K, Le Blancq S M. Parasitism and chromosome dynamics in protozoan parasites: is there a connection? Mol Biochem Parasitol. 1995;70:1–8. doi: 10.1016/0166-6851(95)00021-r. [DOI] [PubMed] [Google Scholar]
- 75.Leech J H, Barnwell J W, Miller L M, Howard R J. Identification of a strain-specific malarial antigen exposed on the surface of Plasmodium falciparum-infected erythrocytes. J Exp Med. 1984;159:1567–1575. doi: 10.1084/jem.159.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Le Scanf C, Carcy B, Bonnefoy S, Fandeur T, Mercereau-Puijalon O. A modification in restriction pattern of the Plasmodium falciparum Pf60 multifamily associated with a specific antigenic variation switch in the Palo Alto line. Behring Inst Mitt. 1997;99:16–24. [PubMed] [Google Scholar]
- 77.Luse S A, Miller L H. Plasmodium falciparum malaria: ultrastructure of parasitized erythrocytes in cardiac vessels. Am J Trop Med Hyg. 1971;20:655–660. [PubMed] [Google Scholar]
- 78.Luzzi G A, Merry A H, Newbold C I, Marsh K, Pasvol G. Protection by alpha-thalassaemia against Plasmodium falciparum malaria: modified surface antigen expression rather than impaired growth or cytoadherence. Immunol Lett. 1991;30:2233–2240. doi: 10.1016/0165-2478(91)90031-5. [DOI] [PubMed] [Google Scholar]
- 79.MacPherson G G, Warrel M L J, White N J, Looareesuwan S, Warrell D A. Human severe malaria: a quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985;119:385–401. [PMC free article] [PubMed] [Google Scholar]
- 80.Magowan C, Wollish W, Anderson L, Leech J. Cytoadherence by Plasmodium falciparum-infected erythrocytes is correlated with the expression of a family of variable proteins on infected erythrocytes. J Exp Med. 1988;168:1307–1320. doi: 10.1084/jem.168.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marsh K, Howard R J. Antigens induced on erythrocytes by Plasmodium falciparum: expression of diverse and conserved determinants. Science. 1986;231:150–153. doi: 10.1126/science.2417315. [DOI] [PubMed] [Google Scholar]
- 82.Marsh K, Sherwood J A, Howard R J. Parasite-infected-cell-agglutination and indirect immunofluorescence assays for detection of human serum antibodies bound to antigens on Plasmodium falciparum-infected erythrocytes. J Immunol Methods. 1986;91:107–115. doi: 10.1016/0022-1759(86)90108-0. [DOI] [PubMed] [Google Scholar]
- 83.Marshall V M, Silva A, Foley M, Cranmer S, Wang L, McColl D J, Kemp D J, Coppel R L. A second merozoite surface protein (MSP-4) of Plasmodium falciparum that contains an epidermal growth factor-like domain. Infect Immun. 1997;65:4460–4467. doi: 10.1128/iai.65.11.4460-4467.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McCormic C J, Craig A, Roberts D, Newbold C I, Berendt A. Intercellular adhesion molecule-1 and CD36 synergies to mediate adherence of Plasmodium falciparum-infected erythrocytes to cultured human microvascular endothelial cells. J Clin Investig. 1997;100:2521–2529. doi: 10.1172/JCI119794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Michael J K, Shah N, Shen N, Yang R, Fucini R, Merigan T C, Richman G D, Morris D, Hubbell E, Chee M, Gingeras T R. Extensive polymorphism observed in HIV clade B protease gene using high-density oligonucleotide arrays. Nat Med. 1996;2:753–759. doi: 10.1038/nm0796-753. [DOI] [PubMed] [Google Scholar]
- 86.Miller H L, Good M F, Milon G. Malaria pathogenesis. Science. 1994;264:1878–1883. doi: 10.1126/science.8009217. [DOI] [PubMed] [Google Scholar]
- 87.Miller L H, Haynes J D, McAuliffe F M, Shiroishi T, Durocher J R, McGinniss M H. Evidence for differences in erythrocyte surface receptors for the malarial parasites, Plasmodium falciparum and Plasmodium knowlesi. J Exp Med. 1977;146:277–281. doi: 10.1084/jem.146.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nauman K M, Jones G L, Saul A, Smith R A P. Plasmodium falciparum exo-antigen alters erythrocyte membrane deformability. FEBS Lett. 1991;292:95–97. doi: 10.1016/0014-5793(91)80842-q. [DOI] [PubMed] [Google Scholar]
- 89.Newbold C I, Pinches R, Roberts D J, Marsh K. Plasmodium falciparum: the human agglutinating antibody response to the infected red cell surface is predominantly variant specific. Exp Parasitol. 1992;75:281–292. doi: 10.1016/0014-4894(92)90213-t. [DOI] [PubMed] [Google Scholar]
- 90.Newbold C I, Warn P, Black G, Berendt A, Craig A, Snow B, Msobo M, Peshu N, Marsh K. Receptor-specific adhesion and clinical disease in Plasmodium falciparum. Am J Trop Med Hyg. 1997;57:389–398. doi: 10.4269/ajtmh.1997.57.389. [DOI] [PubMed] [Google Scholar]
- 91.Ockenhouse C F, Tegoshi T, Maeno Y, Benjamin C, Ho M, Kan K E, Thway Y, Win K, Aikawa M, Lobb R R. Human vascular endothelial cell adhesion receptors for Plasmodium falciparum-infected erythrocytes: roles for endothelial leukocyte adhesion molecule 1 and vascular cell adhesion molecule 1. J Exp Med. 1992;176:1183–1189. doi: 10.1084/jem.176.4.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Peterson D S, Miller L H, Wellems T E. Isolation of multiple sequences from the Plasmodium falciparum genome that encode conserved domains homologous to those in erythrocyte-binding proteins. Proc Natl Acad Sci USA. 1995;92:7100–7104. doi: 10.1073/pnas.92.15.7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pichyangkul S, Saengkrai P, Yongvanitchit K, Stewart A, Heppner D G. Activation of γδ T cells in malaria: interaction of cytokines and a schizont-associated Plasmodium falciparum antigen. J Infect Dis. 1997;176:233–241. doi: 10.1086/514029. [DOI] [PubMed] [Google Scholar]
- 94.Pichyangkul S, Saengkrai P, Webster H K. Plasmodium falciparum pigment induces monocytes to release high levels of tumor necrosis factor-α and interleukin-1. Am J Trop Med Hyg. 1994;51:430–435. [PubMed] [Google Scholar]
- 95.Preiser P R, Jarra W, Capiod T, Snounou G. A rhoptry-protein-associated mechanism of clonal phenotypic variation in rodent malaria. Nature. 1999;398:618–622. doi: 10.1038/19309. [DOI] [PubMed] [Google Scholar]
- 96.Reeder J C, Cowman A F, Davern K M, Beeson J G, Thompson J K, Rogerson S J, Brown G V. The adhesion of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate A is mediated by P. falciparum erythrocyte membrane protein 1. Proc Natl Acad Sci USA. 1999;96:5198–5202. doi: 10.1073/pnas.96.9.5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Robert C, Pouvelle B, Meyer P, Muanza K, Fujioka H, Aikawa M, Scherf A, Gysin J. Chondroitin-4-sulfate (proteoglycan), a receptor for Plasmodium falciparum-infected erythrocyte adherence on brain microvascular endothelial cells. Res Immunol. 1995;146:383–393. doi: 10.1016/0923-2494(96)81042-x. [DOI] [PubMed] [Google Scholar]
- 98.Roberts D D, Sherwood J A, Spitalnik S L, Panton L J, Howard R J, Dixit V M, Frazier W A, Miller L H, Ginsburg V. Thrombospondin binds falciparum malaria parasitized erythrocytes and may mediate cytoadherence. Nature. 1985;318:64–66. doi: 10.1038/318064a0. [DOI] [PubMed] [Google Scholar]
- 99.Roberts D J, Craig A G, Berendt A R, Pinches R, Nash G, Marsh K, Newbold C I. Rapid switching to multiple antigenic and adhesive phenotypes in malaria. Nature. 1992;357:689–692. doi: 10.1038/357689a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rogerson S J, Chaiyaroj S C, Ng K, Reeder J C, Brown G. Chondroitin-sulfate A is a cell surface receptor for Plasmodium falciparum-infected erythrocytes. J Exp Med. 1995;182:15–20. doi: 10.1084/jem.182.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rogerson S J, Brown G V. Chondroitin sulfate A as an adherence receptor for Plasmodium falciparum-infected erythrocytes. Parasitol Today. 1997;13:70–75. doi: 10.1016/s0169-4758(96)10081-8. [DOI] [PubMed] [Google Scholar]
- 102.Rogerson S J, Beck H P, Al-Yaman F, Currie B, Alpers M P, Brown G V. Disruption of erythrocyte rosettes and agglutination of erythrocytes infected with Plasmodium falciparum by the sera of Papua New Guineas. Trans R Soc Trop Med Hyg. 1996;90:80–84. doi: 10.1016/s0035-9203(96)90487-3. [DOI] [PubMed] [Google Scholar]
- 103.Rowe A, Obeiro J, Newbold C I, Marsh K. Plasmodium falciparum rosetting is associated with malaria severity in Kenya. Infect Immun. 1995;63:2323–2326. doi: 10.1128/iai.63.6.2323-2326.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rowe J A, Moulds J M, Newbold C I, Miller L H. P. falciparum rosetting mediated by a parasite-variant erythrocyte membrane protein and complement-receptor. Nature. 1997;388:292–295. doi: 10.1038/40888. [DOI] [PubMed] [Google Scholar]
- 105.Rubio P J, Thompson J K, Cowman A F. The var genes of Plasmodium falciparum are located in the subtelomeric region of most chromosomes. EMBO J. 1996;15:4069–4077. [PMC free article] [PubMed] [Google Scholar]
- 106.Scherf A. The malaria genome. In: Wahlgren M, Perlmann P, editors. Malaria: molecular and clinical aspects. The Netherlands: Harwood Academic Publishers; 1999. pp. 153–180. [Google Scholar]
- 107.Scherf A, Hernandez-Rivas R, Buffet P, Bottius E, Benatar C, Pouvelle B, Gysin J, Lanzer M. Antigenic variation in malaria: in situ switching, relaxed and mutually exclusive transcription of var genes during intra-erythrocytic development in Plasmodium falciparum. EMBO J. 1998;17:5418–5426. doi: 10.1093/emboj/17.18.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schofield L, Hackett F. Signal transduction in host cells by a glycosylphosphatidylinositol toxin of malaria parasites. J Exp Med. 1993;177:145–153. doi: 10.1084/jem.177.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Scholander C, Treutiger C J, Hultenby K, Wahlgren M. Novel fibrillar structure confers adhesive property to malaria-infected erythrocytes. Nat Med. 1996;2:204–208. doi: 10.1038/nm0296-204. [DOI] [PubMed] [Google Scholar]
- 110.Scholander C, Carlson J, Kremsner P G, Wahlgren M. Extensive immunoglobin binding of Plasmodium falciparum-infected erythrocytes in a group of children with moderate anemia. Infect Immun. 1998;66:361–363. doi: 10.1128/iai.66.1.361-363.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sim B K L, Chinis C E, Wasniowska K, Hadley T J, Miller L H. Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum. Science. 1994;264:1941–1944. doi: 10.1126/science.8009226. [DOI] [PubMed] [Google Scholar]
- 112.Sinnis P, Clavijo P, Fenyo D, Chait B T, Cerami C, Nussenzweig V. Structural and functional properties of region II-plus of the malaria circumsporozoite protein. J Exp Med. 1994;180:297–306. doi: 10.1084/jem.180.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sjöberg K, Hosein Z, Wahlin B, Carlson J, Wahlgren M, Troye-Blomberg M, Berzins K, Perlmann P. Plasmodium falciparum: an invasion inhibitory human monoclonal antibody is directed against a malarial glycolipid antigen. Exp Parasitol. 1991;73:317–325. doi: 10.1016/0014-4894(91)90103-4. [DOI] [PubMed] [Google Scholar]
- 114.Smith D J, Kyes S, Craig A G, Fagan T, Hudson-Taylor D, Miller L H, Baruch D I, Newbold C I. Analysis of adhesive domains from the A4Var Plasmodium falciparum erythrocyte membrane protein-1 identifies a CD36 binding domain. Mol Biochem Parasitol. 1998;97:133–148. doi: 10.1016/s0166-6851(98)00145-5. [DOI] [PubMed] [Google Scholar]
- 115.Smith J D, Chitnis C E, Craig A G, Roberts D J, Hudson-Taylor D E, Peterson D S, Pinches R, Newbold C I, Miller L H. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell. 1995;82:101–110. doi: 10.1016/0092-8674(95)90056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Su X-Z, Heatwole V M, Wertheimer S P, Guinet F, Herrfelt J A, Peterson D S, Pinches R, Wellems T E. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82:89–99. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 117.Thompson J K, Robio J P, Caruana S, Brockman A, Wickham M E, Cowman A F. The chromosomal organization of the Plasmodium falciparum var gene family is conserved. Mol Biochem Parasitol. 1997;87:49–60. doi: 10.1016/s0166-6851(97)00041-8. [DOI] [PubMed] [Google Scholar]
- 118.Treutiger C J, Carlson J, Scholander C, Wahlgren M. The time course of cytoadhesion, immunoglobulin-binding, rosetting and serum-induced agglutination of Plasmodium falciparum-infected erythrocytes. Am J Trop Med Hyg. 1998;9:202–207. doi: 10.4269/ajtmh.1998.59.202. [DOI] [PubMed] [Google Scholar]
- 119.Treutiger C J, Heddini A, Fernandez V, Muller W A, Wahlgren M. PECAM-1/CD31, an endothelial receptor for binding Plasmodium falciparum-infected erythrocytes. Nat Med. 1997;3:1405–1408. doi: 10.1038/nm1297-1405. [DOI] [PubMed] [Google Scholar]
- 120.Treutiger C J, Hedlund I, Helmby H, Carlson J, Jepson A, Twumasi P, Kwiatkowski D, Greenwood B M, Wahlgren M. Rosette formation in Plasmodium falciparum isolates and anti-rosette activity of sera from Gambians with cerebral or uncomplicated malaria. Am J Trop Med Hyg. 1992;46:503–510. doi: 10.4269/ajtmh.1992.46.503. [DOI] [PubMed] [Google Scholar]
- 121.Treutiger C J, Scholander C, Carlson J, McAdam K P, Raynes J G, Falksveden L, Wahlgren M. Rouleaux-forming serum proteins are involved in the rosetting of Plasmodium falciparum-infected erythrocytes. Exp Parasitol. 1999;93:1–9. doi: 10.1006/expr.1999.4454. [DOI] [PubMed] [Google Scholar]
- 122.Udomsangpetch R, Wahlin B, Carlson J, Berzins K, Torii M, Aikawa M, Perlmann P, Wahlgren M. Plasmodium falciparum-infected erythrocytes form spontaneous erythrocyte rosettes. J Exp Med. 1989;169:1835–1840. doi: 10.1084/jem.169.5.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Udomsangpetch R, Chivapat S, Viriyavejakul P, Riganti M, Wilairatana P, Pongponratin E, Looareesuwan S. Involvement of cytokines in the histopathology of cerebral malaria. Am J Trop Med Hyg. 1997;57:501–506. doi: 10.4269/ajtmh.1997.57.501. [DOI] [PubMed] [Google Scholar]
- 124.Wahlgren M. Antigens and antibodies involved in humoral immunity to Plasmodium falciparum. Stockholm, Sweden: Karolinska Institutet; 1986. [Google Scholar]
- 125.Wahlgren M, Carlson J, Ruangjirachuporn W, Conway D, Helmby H, Martinez A, Patarroyo M E, Riley E. Geographical distribution of Plasmodium falciparum erythrocyte rosetting and frequency of rosetting antibodies in human sera. Am J Trop Med Hyg. 1991;43:333–338. doi: 10.4269/ajtmh.1990.43.333. [DOI] [PubMed] [Google Scholar]
- 126.Wahlgren M, Fernandez V, Chen Q, Svärd S, Hagblom P. Waves of malarial var-iations. Cell. 1999;96:603–606. doi: 10.1016/s0092-8674(00)80569-3. [DOI] [PubMed] [Google Scholar]
- 127.Wahlgren M, Abrams J S, Fernandez V, Bejarano M T, Azuma M, Torii M, Aikawa M, Howard R J. Adhesion of Plasmodium falciparum-infected erythrocytes to human cells and secretion of cytokines (IL-1-beta, IL-1RA, IL-6, IL-8, IL-10, TGF beta, TNF alpha, G-CSF, GM-CSF) Scand J Immunol. 1995;42:626–636. doi: 10.1111/j.1365-3083.1995.tb03705.x. [DOI] [PubMed] [Google Scholar]
- 128.Webster P, Joiner K, Andrews N W. Why do so many surface proteins of trypanosomatids have GPI-anchors? Cell Biol Int Rep. 1991;15:799–813. doi: 10.1016/0309-1651(91)90034-g. [DOI] [PubMed] [Google Scholar]
- 129.White N J, Ho M. The pathophysiology of malaria. Adv Parasitol. 1992;31:83–173. doi: 10.1016/s0065-308x(08)60021-4. [DOI] [PubMed] [Google Scholar]
- 130.Wodicka L, Dong H, Mittman M, Ho M-H, Lockhart D J. Genome-wide expression monitoring in Saccharomyces cerevisiae. Nat Biotechnol. 1997;15:1359–1367. doi: 10.1038/nbt1297-1359. [DOI] [PubMed] [Google Scholar]
- 131.Wolffe A P. Histone deacetylase: a regulator of transcription. Science. 1996;272:371. doi: 10.1126/science.272.5260.371. [DOI] [PubMed] [Google Scholar]
- 132.World Health Organization. The world health report. Geneva, Switzerland: World Health Organization; 1998. pp. 45–48. [Google Scholar]