Abstract
Introduction
Rotavirus (RV) is the most common cause of acute gastroenteritis in children <5 years of age worldwide, and vaccination reduces the disease burden. Evidence from postmarketing surveillance studies suggested an increased risk of intussusception (IS) in infants post-RV vaccination. An overall positive benefit–risk balance for the human RV vaccine (HRV) Rotarix (GlaxoSmithKline [GSK], Belgium) has been established and recent findings indicate an indirect effect of reduced IS over the long term.
Objective
The aim of this study was to discuss spontaneous data from the GSK worldwide safety database on IS post-Rotarix administration.
Methods
The database was reviewed for all spontaneous IS cases from 2004 to 2020. Additionally, an observed versus expected (O/E) analysis was done for adverse events attributed to IS. Data were reviewed as overall worldwide and stratified by region (Europe/USA/Japan) and dose.
Results
A male predominance of IS patients was observed, consistent with earlier reports. The most frequently reported events in confirmed IS cases (Brighton Collaboration Working Group [BCWG] level 1) with time to onset ≤ 30 days post-vaccination were vomiting (55.8%), haematochezia (47.2%), and crying (21.1%). The observations from the IS spontaneous cases review and results of the O/E analysis are consistent with the known IS safety profile of RV vaccines: a transient increased incidence of IS post-vaccination (primarily in Europe/Japan/worldwide), mostly within 7 days postdose 1.
Conclusion
Since the outcomes of early IS management are favourable over delayed management, healthcare professionals should inform parents about the importance of seeking immediate medical advice in case of unusual behaviour of the vaccinated infant. GSK continues to monitor the IS risk post-Rotarix administration through routine pharmacovigilance activities.
Graphic abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s40264-021-01141-4.
Plain language summary
Rotavirus (RV) is the most common cause of acute gastroenteritis and a major cause of death in young children worldwide. Vaccination has been instrumental in reducing the impact of RV disease. Real-world evidence suggests an increased risk of intussusception (an infrequent type of bowel obstruction) in infants following RV vaccination. We reviewed IS cases reported spontaneously worldwide in children following a two-dose vaccination with the human RV vaccine (Rotarix, GlaxoSmithKline [GSK]) since its launch in 2004. We observed that (1) IS occurred more frequently 7 days after the first dose and, to a lesser extent, after the second dose; (2) boys were more frequently affected than girls (56.3%); (3) of 862 confirmed reported cases, 557 required hospitalisation; and (4) surgical intervention was required for 294 of 557 hospitalised cases. We used statistical analysis to assess whether the number of cases observed would be higher or lower than the natural occurrence of IS (irrespective of vaccination). These results were in line with the known RV vaccine safety profile. It is important to constantly monitor the real-world safety profile of RV vaccines in the postmarketing setting. Since the outcomes of early management of IS are favourable compared with delayed management, healthcare professionals should inform parents to seek immediate medical advice if they observe unusual behaviour in their vaccinated child. In conclusion, our analyses on data of a large patient pool for this rare event reinforce the favourable safety profile of human RV vaccine and the benefits of vaccination in young children.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40264-021-01141-4.
Key Points
| The human rotavirus vaccine (HRV) was first approved in 2004 for the prevention of rotavirus infection in young children. |
| As part of routine pharmacovigilance activities, we are monitoring the incidence of intussusception (IS) following vaccination with HRV, based on spontaneous reports. |
| Spontaneous reports collected since 2004 indicate a transient increased incidence of IS after vaccination with HRV, mostly within 7 days after the first dose and, to a lesser extent, after the second dose, which is in line with published literature. |
| Our analysis highlights the importance of early management of IS since this resulted in more favourable outcomes compared with delayed treatment. |
Introduction
Rotavirus (RV) infection represents the most common cause of severe, dehydrating gastroenteritis in infants and children below the age of 5 years worldwide [1–3]. However, implementation of RV vaccines has substantially decreased hospitalisations from RV as well as from all-cause acute gastroenteritis [4].
In Europe, most cases of RV infection occur in children under 2 years of age, with a peak incidence between 6 and 24 months of age. Primary infection after 3 months of age usually causes the most severe disease, while subsequent infections typically manifest with much milder symptoms [5, 6]. In developing countries, gastroenteritis due to RV infection remains a major cause of childhood death despite the introduction of RV vaccines. Globally, the number of deaths in children under 5 years of age caused by RV in 2013 was estimated at 215,000 [7, 8].
Intussusception (IS), a type of bowel obstruction, occurs in 0.5–4.3 per 1000 live births in Europe, North America, and Australia [9], and estimates from Latin America show similar rates to those identified in industrialised countries [10, 11]. While the aetiology of paediatric IS is typically idiopathic, several predisposing factors (mostly anatomic or infectious) have been identified [12, 13]. For instance, children with a medical history of IS, with uncorrected congenital malformation (such as Meckel’s diverticulum) of the gastrointestinal tract, or antecedent viral illness are prone to IS [9, 12]. Generally, IS is most common among children under 1 year of age [9, 14] and a male predominance has been noted worldwide [9].
An association between IS as a post-immunisation adverse event (AE) and RV vaccines was first identified for a tetravalent RV vaccine, RotaShield (Wyeth Lederle Laboratories), which was subsequently withdrawn from the market in 1999. As a potential causal link, the replication of the RV in the intestinal epithelium may trigger an inflammatory reaction and predispose to IS [15, 16].
Currently, four oral, live attenuated vaccines against RV are available internationally and are World Health Organization (WHO) prequalified: Rotarix (hereafter referred to as human RV vaccine [HRV], GSK, Belgium; liquid formulation prequalified in 2009); RotaTeq (referred to as human-bovine reassortant rotavirus vaccine [HBRV], Merck&Co., Inc., USA; prequalified in 2008); Rotavac (Bharat Biotech, Hyderabad, India; prequalified in 2018); and Rotasiil (Serum Institute of India PVT. Ltd, Pune, India; prequalified in 2018) [17]. All four vaccines are considered to be well tolerated and effective in reducing the burden of gastrointestinal disease caused by RV [18]. The WHO recommends including vaccination of infants against RV in all routine immunisation programmes (with the first dose of RV vaccine administered as soon as possible after 6 weeks of age, along with the diphtheria-tetanus-pertussis vaccination), especially in countries with high mortality caused by RV infection [7].
HRV contains the human RV strain RIX4414 (genotype G1P [8]) [19] and was first licensed in Mexico on 12 July 2004. Currently, HRV is approved in the US, all member states of the European Economic area/UK, Japan, and several other countries (vaccine introduction current/planned in more than 90 countries globally [20]), with an indication for active immunisation of infants aged 6–24 weeks to reduce the burden of gastroenteritis due to RV infection.
The risk of IS following vaccination with HRV has been evaluated in a large-scale post-approval safety study sponsored by GSK and conducted in Mexico, in collaboration with the Mexican Institute of Social Security [21]. Episodes of IS were identified according to the case definition developed by the Brighton Collaboration Working Group (BCWG) for Intussusception [22]. Clustering of IS within 7 days of vaccination was observed postdose 1. An attributable risk of three to four additional cases of IS per 100,000 vaccinated infants was estimated [21]. In addition, various postmarketing surveillance studies were conducted providing risk estimates of IS after vaccination with HRV and HBRV [23–29]. The analytical period of these studies ranged from about 1.5–7 years. While some studies claim no increased risk of IS or IS-related hospital admission following vaccination [24, 30–32], there is a growing body of evidence for a slightly increased risk of IS post-vaccination (notably postdose 1) [25, 33–35] that translates into an increased risk of hospitalisation [36]. A meta-analysis of the available data up to 2013 was published to provide an overall estimate of the IS risk after vaccination with HRV and HBRV when considering the heterogeneity among the studies [25]. This meta-analysis of postmarketing surveillance data showed an increased risk of IS during the first 7 days after administration of dose 1 and, to a lesser extent, of dose 2. The overall (fixed-effect) estimate of IS risk during the 7 days after vaccination with HRV was 5.4 (95% confidence interval [CI] 3.9–7.4) after dose 1 and 1.8 (95% CI 1.3–2.5) after dose 2 [25]. Of note, as recently proposed by Vetter et al., the increased risk of IS following vaccination should be weighed against the risk of IS caused by naturally occurring RV infection [37]. A favourable benefit–risk balance for HRV has been suggested in various studies [35, 38–40]. However, the concern of IS occurring in temporal association with immunisation subsequent to the findings in 1999, among other reasons, has led to some hesitancy in the implementation of the RV vaccines in national immunisation programmes [41]. Continuous postmarketing surveillance is important for monitoring this safety concern, especially for the newly introduced RV vaccines, to provide real-time safety data for such less frequent events that are difficult to observe in prelicensure clinical trials or postmarketing data with a small proportion of the exposed population analysed.
As part of routine pharmacovigilance activities, GSK is monitoring the incidence of IS following RV vaccination since HRV launch and reports are being collected in a worldwide safety database. The objective of this article is to discuss the spontaneous cases of IS after HRV administration from the worldwide safety database received over the last 15 years.
Methodology
Spontaneous Report Data
Spontaneous report data were collated from unsolicited communications describing the event of IS with or without other AEs that occurred in infants who had received RV vaccination. These communications (referred to as ‘spontaneous reports’) were either submitted to GSK directly—voluntarily from individual reporters (who might be reporting for themselves or others) via local reception/call centres—or were collected by GSK from interactive digital media (i.e., social media or other such platforms). Follow-up questionnaires on IS were referred to for most of the scientific case evaluations. Individual reporters included healthcare professionals (HCPs), regulatory authorities, consumers, and others. All reported AEs were coded using the International Conference on Harmonisation (ICH) Medical Dictionary for Regulatory Activities (MedDRA®) [42]. One spontaneous report could contain more than one AE reported by the same individual. AEs were classified as serious if meeting the ICH regulatory definition: any untoward medical occurrence that results in death, is life-threatening, requires hospitalisation or prolongation of existing hospitalisation, results in disability/incapacity, is a congenital anomaly/birth defect in the offspring, or is a medically important event [43]. Reported AEs, while temporally associated with the product’s use, may not necessarily be causally associated with it [44].
Data Extraction and Selection Criteria
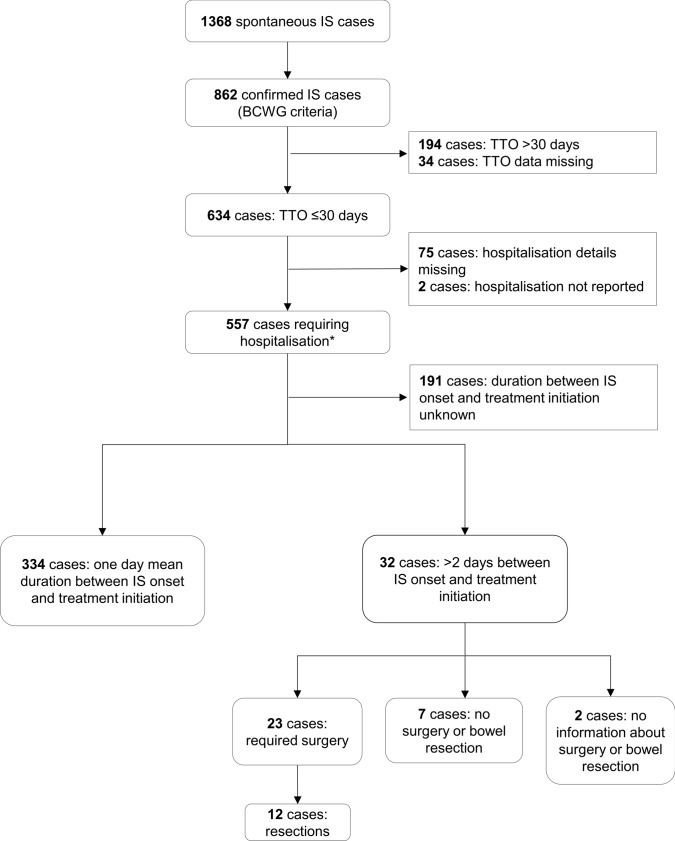
For this review and all analyses, spontaneous report data were extracted from the GSK worldwide safety database (Oracle Argus) for the period 12 July 2004 (international launch date for Rotarix) until 11 July 2020 using an automated search for Rotarix in combination with the MedDRA® preferred term ‘intussusception’. All identified reports were medically reviewed at GSK and were assessed according to BCWG criteria to determine the level of diagnostic certainty (as described by Bines et al. [22]). Reports that could be categorised as fulfilling BCWG criteria level I were classified as confirmed. Time to onset (TTO) for the event of IS was calculated as the occurrence of the first sign/symptoms related to IS since the date of vaccination with any RV vaccine dose (day 0), for reports containing this information. Thus, the risk period calculation was based on the TTO (when the event occurred after vaccine administration) and not on when the initial report was received. Analyses were conducted worldwide, in Europe, the US, and Japan, and for two risk periods after each vaccine dose: 7 days (day 0 to day 6) and 30 days (day 0 to day 29), in line with previous analyses [45–47]. In a descriptive analysis, for all confirmed IS cases with a TTO ≤ 30 days postvaccination, we described events reported in at least 5% of the cases, hospitalisation requirements, and duration between IS onset and initiation of treatment (i.e., ‘time to treatment initiation’). To assess the impact of a delayed treatment initiation, we described the surgical management of cases requiring hospitalisation, in which there was a delay of > 2 days between TTO and treatment initiation. Medical assessment was performed for confirmed IS cases with TTO ≤ 30 days (please refer to the algorithm in Fig. 1).
Fig. 1.
Overview of the time to onset of intussusception, reported hospitalisation, duration between intussusception onset and treatment start, and required surgery with or without resection for all spontaneous intussusception cases. *Duration of hospitalisation for these cases is detailed in electronic supplementary material 2. BCWG Brighton Collaboration Working Group, IS intussusception, TTO time to onset
Postmarketing collection and evaluation of safety data on an ongoing basis is part of GSK’s systematic approach for the identification of potential safety signals. As information on the actual number of infants exposed to HRV worldwide is not available, patient exposure was approximated by the number of doses distributed from launch until 30 April 2020, which represents the most reliable data source for patient exposure to a vaccine in a postapproval setting. Of note, the sales database is subject to updates and corrections depending on information provided by GSK local country subsidiaries (e.g., vaccine doses may be returned by subsidiaries to the central warehouse). These constant updates may result in discrepancies between consecutive queries of the database. The time lag between an actual distribution to a country and recording in the database is 2 months on average. In order to minimise the risk of inaccuracy and to better reflect the exposure data, the sales information was retrieved with a data lock point (11 July 2020) minus 2 months (30 April 2020).
Observed versus Expected Analysis
The observed versus expected (O/E) analysis was performed to determine whether the observed number of AEs attributed to IS corresponded to the number of events expected to occur within a predefined risk period, under the null hypothesis of no association with HRV vaccination. The ratio of O/E number of IS cases was calculated for the period from 12 July 2004 until 11 July 2020. Background incidence rates were defined by the number of incident reports of a condition or event occurring naturally in the population, expressed in person-time [48]. These age and country-stratified background estimates were obtained from the literature [49–55], considering populations with similar characteristics as the RV target population. For the O/E analysis, only cases occurring in the predefined risk period were counted. All IS cases with BCWG level 1–3 that occurred within 7 and 30 days after each vaccine dose in infants under 1 year of age were considered. Conservatively, all infant IS case levels 1–4 BCWG occurring in the same post-vaccination periods were also considered for this O/E analysis. If TTO was missing, cases were considered as having occurred in the risk period for the respective analysis (7 or 30 days). When unknown, the dose number was imputed according to the dose distribution of the other cases in the same region. Cases with unknown age at vaccination were considered as under 1 year of age and included in the O/E analysis. The cases were analysed worldwide, in Europe, the US, and Japan. The exposures are all the sales of lyophilised and liquid HRV vaccine from the international birth date (12 July 2004) until 30 April 2020.
The observed number of cases was compared with the estimated number of cases computed from background incidence rates. Exact lower and upper 95% CIs were calculated around the number of observed cases. The small number of observed cases were considered as the largest source of statistical uncertainty, while exposure to the vaccine and background incidence rates were considered fixed numbers or with an uncertainty that was negligible compared with the uncertainty from the observed numbers. The number of observed cases and associated CIs were calculated from an exact Poisson distribution [56], which depends only on one parameter, i.e., the number of observed cases. Data were stratified worldwide and by region (Europe, the US, and Japan), and dose (first, second, or any dose [in a few instances, a third dose was administered]). A range of expected number of IS cases based on varying background incidence rates was calculated.
The reported numbers of cases and their 95% CIs were rescaled according to varying levels of reported fraction (RF) to obtain the observed number of cases. The RF is the proportion of cases reported among all those that occurred in the vaccinated population within the risk period, regardless of causality. Graphs of the observed numbers of cases and their 95% CI (y-axis) according to a range of RFs (x-axis) were developed for each analysis. The age-adjusted expected number of cases corresponding to selected background incidence rates were presented as horizontal reference lines. These reference lines allowed to perform an exploratory comparison of the observed number of cases to the expected number of cases: (1) higher than expected if the lower limit of the 95% CI computed from the observed is above the reference line of the expected; and (2) lower than expected if the upper limit of the 95% CI computed from the observed is below the reference line of the expected.
The number of cases expected to occur within a particular risk period following vaccination (Ne) was adjusted from the following simplified formula for the distribution of age (in months) at administration (Eq. 1):
| 1 |
where is the identifier of the age classes by month during the first year of age; is the incidence of intussusception in the first year of life (expressed as incidence per day); and is the proportion of intussusception occurring in the ith month of age.
Regional age-specific distributions of IS were used. For Europe, these data were taken from surveillance in Switzerland (2003–2006) [57], and worldwide, US and Japan data were used [51, 58, 59].
Note: is the age-specific incidence rate of IS, assuming that the monthly age classes are balanced in the first year of age in the population; is the number of doses of HRV used in the ith month of age, which is based on country-level sales data and an estimate of the age at vaccine administration based on the distribution of age at vaccination reported in spontaneous Rotarix cases available in the safety database (stratified by region and dose); and is the risk period after dose administration (7 or 30 days)
Background Incidence Data
The range of IS background incidence rates was based on studies listed in electronic supplementary material (ESM) 1. The maximum (i.e., ‘high ref’ on figures in the ESM) and minimum (i.e., ‘low ref’ on figures in the ESM) values of the IS background ranges used for Europe refer to an Irish [49] and German (unpublished work) study (24.2 to 96.7/100,000 person years), respectively. The minimum and maximum incidence range values used for the US were based on two US studies (33–54/100,000 person years) [54, 55], the values for Japan stemmed from two Japanese studies [50, 52, 53] (143.5–191/100,000 person years), and the lowest and highest values of the worldwide expected range (3.8–105.3/100,000 person years) was based on Latin American studies [51] since 32% of HRV doses are sold to Latin American countries.
Results
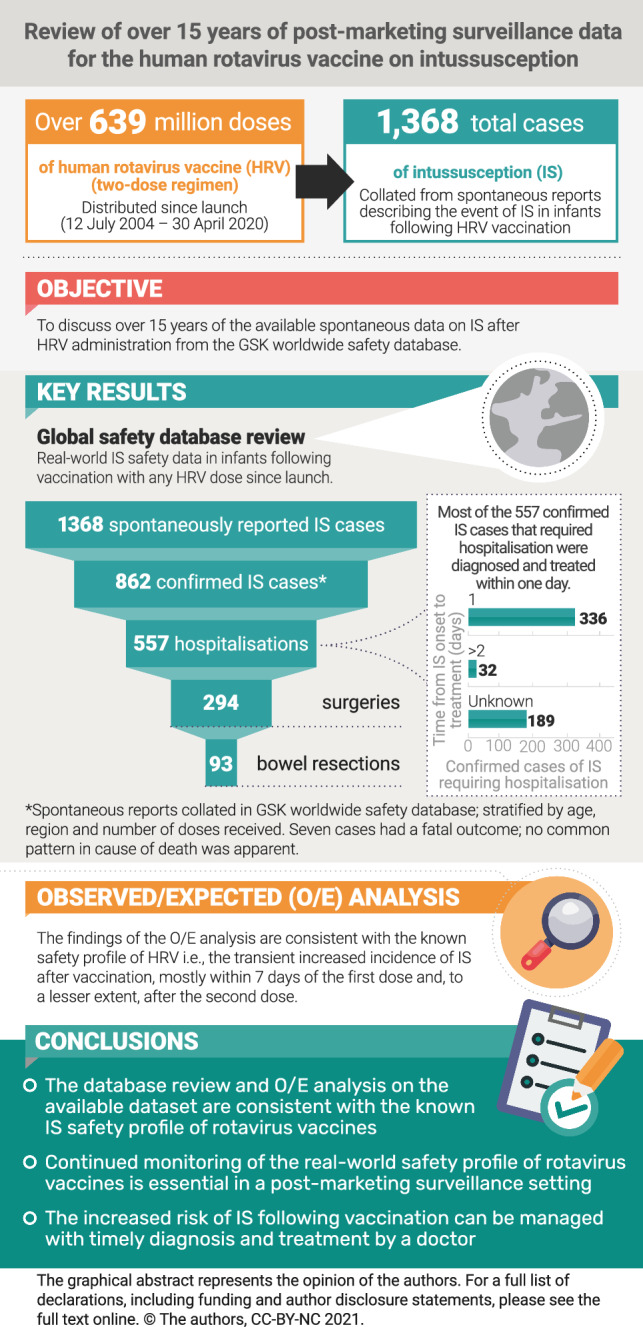
From launch until 30 April 2020, an estimated 639,391,442 doses of Rotarix were distributed. As an infant could receive one and two doses, depending on compliance with the vaccination schedule, the estimated number of infants exposed from launch until 30 April 2020 ranged between 319,695,721 and 639,391,442.
Overall Dataset of Spontaneous Adverse Event Reports
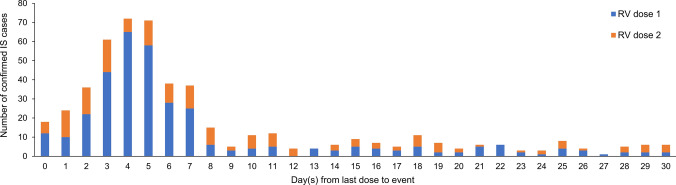
A total of 1368 spontaneous IS reports were retrieved from the GSK worldwide safety database for the analytical period, of which 862 were confirmed IS cases according to BCWG criteria, with IS occurring more frequently in males (56.4%) than in females (40.6%); for the remaining three percent, the sex was unknown. Mean age at IS onset was 4.2 months (Table 1). The occurrence of confirmed IS following HRV vaccination was more frequently observed after the first dose of HRV (in almost half of the confirmed cases) and relatively less frequently after the second dose (in about one-third of the confirmed cases) [Table 1; Fig. 2]. For approximately 20% of cases, the dose after which confirmed IS occurred was not detailed in the report (Table 1).
Table 1.
Patient characteristics of the confirmed intussusception cases
| Confirmed IS cases [N = 862] |
|
|---|---|
| Age at onset, months | |
| Mean ± SD | 4.17 ± 2.47 |
| Median (minimum; maximum) | 3.84 (0; 32.04) |
| Sex | |
| Male | 486 (56.4) |
| Female | 350 (40.6) |
| Unknown | 26 (3.0) |
| Dose | |
| First | 404 (46.9) |
| Second | 290 (33.6) |
| Anya | 696 (80.7) |
| Unknown | 166 (19.3) |
Data are expressed as number (%) of cases in each category unless otherwise specified
IS intussusception, N number of confirmed cases, SD standard deviation
aNote that an extra third dose was administered in a few instances
Fig. 2.
Distribution of the time to onset of intussusception after administration of the rotavirus vaccine for the confirmed cases with a time to onset of ≤ 30 days (N = 634). Cases with a reported time to onset of ‘less than one a day’ or ‘same day’ are represented as 0 days from the last dose; cases with a reported time to onset of ‘2–3 days’ are represented as 2 days from the last dose; cases with a reported time to onset of ‘< 7 days’ are represented as 6 days from the last dose; and cases with a reported TTO of ‘1 week’ are represented as 7 days from the last dose. IS intussusception, N total number of confirmed cases, RV rotavirus vaccine
Of the 862 confirmed IS cases, 634 had a TTO ≤ 30 days, 194 had an extended TTO (> 30 days), which makes a link with vaccination less likely, and 34 cases had an unknown TTO. Worldwide, the most frequently reported events in the 634 confirmed cases with a TTO of ≤ 30 days were vomiting (55.8%) and haematochezia (47.2%), followed by crying (21.1%) and abdominal pain (15.9%) [Table 2; region-wise data are available in this table]. The events listed in Table 2 were reported in at least 5% of the 634 confirmed cases that had a TTO of ≤30 days.
Table 2.
Frequently reported events in confirmed intussusception cases with a time to onset of ≤30 days [N = 634]
| Co-reported events | n (%) | ||||
|---|---|---|---|---|---|
| Europea | Japan | US | Rest of the worldb | Worldwide | |
| Vomitingc | 96 (15.14) | 125 (19.72) | 6 (0.95) | 127 (20.03) | 354 (55.84) |
| Haematochezia | 76 (11.99) | 127 (20.03) | 2 (0.32) | 94 (14.83) | 299 (47.16) |
| Cryingd | 56 (8.83) | 41 (6.47) | 2 (0.32) | 35 (5.52) | 134 (21.14) |
| Abdominal paine | 39 (6.15) | 13 (2.05) | 1 (0.16) | 48 (7.57) | 101 (15.93) |
| Altered mood | 0 (0) | 73 (11.51) | 0 (0) | 0 (0) | 73 (11.51) |
| Diarrhoeaf | 26 (4.10) | 27 (4.26) | 1 (0.16) | 30 (4.73) | 84 (13.25) |
| Abdominal mass | 0 (0) | 55 (8.68) | 0 (0) | 10 (1.58) | 65 (10.25) |
| Pallor | 33 (5.21) | 14 (2.21) | 0 (0) | 14 (2.21) | 61 (9.62) |
| Pyrexiag | 18 (2.84) | 14 (2.21) | 1 (0.16) | 21 (3.31) | 54 (8.52) |
| Abdominal distension | 11 (1.74) | 6 (0.95) | 0 (0) | 36 (5.68) | 53 (8.36) |
| Irritability | 6 (0.95) | 1 (0.16) | 2 (0.32) | 28 (4.42) | 37 (5.84) |
| Decreased appetite | 20 (3.15) | 4 (0.63) | 1 (0.16) | 12 (1.89) | 37 (5.84) |
Data are expressed as number (%) of cases for respective events represented region-wise. Events reported in < 5% of the worldwide confirmed cases with a time to onset ≤ 30 days are not reported in this table
N total number of cases used for calculating the percentages
aEurope includes Austria, Belgium, Cyprus, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Lithuania, Luxemburg, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden, Turkey, and the UK
bRest of the world excluding Europe, Japan, and the US
cCount includes projectile vomiting
dCount includes high-pitched crying
eCount includes both upper and lower abdominal pain
fCount includes haemorrhagic diarrhoea
gCount includes hyperpyrexia
Most IS cases occurred in the first 7 days after infants received the RV vaccine dose (Fig. 2).
Among the 634 confirmed IS cases with a TTO of ≤ 30 days, hospitalisation occurred in 557 cases (Fig. 1) and there was no hospitalisations reported for two cases. For the remaining 75 cases, no details about hospitalisation were available. Of the abovementioned 557 IS cases with hospitalisation, 366 included details that allowed the calculation of the time between onset of IS and the treatment initiation. In these cases, the mean duration between the onset of IS and treatment initiation was 1 day. A delay of more than 2 days between onset of IS and treatment was observed for 32 cases. Surgery was required in 23 of these 32 cases, and in 12 of those cases with surgery, the patients underwent resection. Of the remaining 9 cases, 7 did not require any surgery or bowel resection, and for 2 cases, it was unknown if surgery or resection was performed (Fig. 1).
For approximately one-third of the 557 confirmed cases with hospitalisation, the duration of hospitalisation was 4 days or fewer (67 cases, ≤1 day; 52 cases, 2 days; 41 cases, 3 days; 37 cases, 4 days; duration of hospitalisation was unknown in 271 cases) [ESM 2]. In 18 of the 89 cases that required hospitalisation for more than 4 days, the delay in IS diagnosis or treatment could not be calculated due to missing information in the reports. In 62 of these 89 cases, the extended period of hospitalisation was unlikely to be due to a delay in IS diagnosis or treatment initiation because the period between IS onset and IS treatment start was 2 days or fewer. For 9 of these 89 cases, the delay in IS diagnosis or treatment initiation was at least 3 days, and in 4 of these 9 cases, patients underwent surgery with resection, 2 patients had surgery without resection, and it was unknown if resection was performed for the remaining 3 patients (ESM 3).
Of all the confirmed cases that required hospitalisation (n = 557), 294 required surgery, of whom 93 required bowel resections. In 31 of these 93 cases, hospitalisation occurred for more than 4 days (ESM 4a). The need for bowel resection in these 93 cases could not be easily attributed to a delay in IS diagnosis or treatment because the period between IS onset and IS treatment was 2 days or fewer in 52 cases, 3 days in 7 cases, 4 days in 4 cases, 17 days in one case, and unknown in 29 cases of bowel resection (ESM 4b).
Seven of the 862 confirmed IS cases had a fatal outcome. The deaths in these seven cases were reported to have historically occurred between 2006 and 2014. One death per year occurred from 2006 to 2011 and one occurred in 2014. The TTO of the first sign/symptom that was known for five cases varied from 3 to 7 days after patients received the first or second dose of HRV. In four fatal cases, sufficient information for adequate medical assessment was lacking, including two cases for which the TTO of the events was unknown. In two of the seven fatal cases, patients had an underlying condition and/or a medical history as a confounding factor. In one of the fatal cases, the physician advised the infant’s parents to hospitalise the infant on the basis of a preliminary IS diagnosis 3 days after receiving the RV vaccine. The parents refused to comply with the doctor’s advice and the infant died the next day. No common pattern in the cause of death was apparent from the analysis of these fatal cases.
Observed versus Expected Analysis
With an RF of 50%, the observed cases within 30 days after the second dose were far below the lowest estimate of expected number of cases for all geographic locations (ESM 5). Likewise, with an RF of 50%, the observed cases within 7 days after the second dose were below the lowest estimate of the expected number of cases in the US and Japan (ESM 6).
Worldwide and in Europe, the observed cases (all BCWG levels) within 7 days after the second dose were significantly higher than the lowest expected number of cases for RF less than 68% and 59%, respectively (ESM 7). Worldwide, the numbers of observed cases following any dose and dose 1 were within or below the expected range regardless of the risk period, the BCWG diagnostic levels, or the RF (Table 3, ESM 8). In Europe, the numbers of observed cases within 30 days following any dose and dose 1 were within or below the expected range whatever diagnostic levels were considered (Table 3). For the worst-case safety scenario that included unconfirmed cases with a 7-day risk period following dose 1 in Europe, the number of observed cases was above the expected range. For RF lower than 73%, the observed cases were above the highest expected cases for BCWG level 1 cases (ESM 9). In the US, the numbers of observed cases following any dose and dose 1 were below the expected range irrespective of the risk period or the diagnostic levels considered, and for a large range of RFs (Table 3, ESM 10). In Japan, the numbers of observed cases following any dose and dose 1 were below or within the expected range for the 30-day risk period whatever the diagnostic levels considered (Table 3, ESM 11a and b). Considering a 7-day risk period following any dose or dose 1, the observed numbers of cases were above the expected range irrespective of the diagnostic levels considered (ESM 11c and d).
Table 3.
Comparison of the number of spontaneous intussusception reports received and number expected to have occurred by chance in 30 days and 7 days after vaccination (worldwide, Europe, US, and Japan)
| Risk period (days) | Dose | Total number of doses | Expected number of cases (range) | Observed number of cases (95% CI) | ||
|---|---|---|---|---|---|---|
| BCWG L1a | BCWG L1–3a | BCWG L1–4a | ||||
| Worldwide | ||||||
| 30 | First | 319,695,721.12 | 614.4–17,025.2 | 440 (399.8–483.1) | 469 (427.5–513.4) | 741 (688.6–796.3) |
| 30 | Second | 319,695,721.12 | 1,164.8–32,277.9 | 217 (189.1–247.9) | 230 (201.2–261.7) | 364 (327.6–403.4) |
| 30 | Anyb | 639,391,442.23 | 1,598.5–44,295.2 | 659 (609.6–711.3) | 701 (650.1–754.9) | 1108 (1043.7–1175.2) |
| 7 | First | 319,695,721.12 | 143.4–3,972.5 | 321 (286.8–358.1) | 342 (306.7–380.2) | 559 (513.6–607.3) |
| 7 | Second | 319,695,721.12 | 271.8–7,531.5 | 114 (94.0–136.9) | 121 (100.4–144.6) | 213 (185.4–243.6) |
| 7 | Anyb | 639,391,442.23 | 373.0–10,335.6 | 436 (396.0–478.9) | 464 (422.7–508.2) | 774 (720.4–830.5) |
| Europe | ||||||
| 30 | First | 16,029,101 | 170.2–680.2 | 187 (161.2–215.8) | 199 (172.3–228.7) | 325 (290.6–362.3) |
| 30 | Second | 16,029,101 | 277.3–1,108.1 | 44 (32.0–59.1) | 46 (33.7–61.4) | 98 (79.6–119.4) |
| 30 | Anyb | 32,058,202 | 427.6–1,708.6 | 231 (202.2–262.8) | 245 (215.3–277.7) | 423 (383.6–465.3) |
| 7 | First | 16,029,101 | 39.7–158.7 | 138 (115.9–163.0) | 148 (125.1–173.9) | 249 (219.0–281.9) |
| 7 | Second | 16,029,101 | 64.7–258.5 | 22 (13.8–33.3) | 23 (14.6–34.5) | 51 (38.0–67.1) |
| 7 | Anyb | 32,058,202 | 99.8–398.7 | 160 (136.2–186.8) | 171 (146.3–198.6) | 300 (267.0–335.9) |
| US | ||||||
| 30 | First | 10,945,330 | 250.5–410.0 | 6 (2.2–13.1) | 8 (3.5–15.8) | 19 (11.4–29.7) |
| 30 | Second | 10,945,330 | 307.2–502.7 | 6 (2.2–13.1) | 6 (2.2–13.1) | 13 (6.9–22.2) |
| 30 | Anyb | 21,890,660 | 576.4–943.3 | 13 (6.9–22.2) | 15 (8.4–24.7) | 34 (23.5–47.5) |
| 7 | First | 10,945,330 | 58.5–95.7 | 5 (1.6–11.7) | 6 (2.2–13.1) | 14 (7.7–23.5) |
| 7 | Second | 10,945,330 | 71.7–117.3 | 5 (1.6–11.7) | 5 (1.6–11.7) | 9 (4.1–17.1) |
| 7 | Anyb | 21,890,660 | 134.5–220.1 | 10 (4.8–18.4) | 11 (5.5–19.7) | 24 (15.4–35.7) |
| Japan | ||||||
| 30 | First | 3,406,093 | 109.5–145.8 | 105 (85.9–127.1) | 110 (90.4–132.6) | 140 (117.8–165.2) |
| 30 | Second | 3,406,093 | 398.1–529.9 | 67 (51.9–85.1) | 70 (54.6–88.4) | 78 (61.7–97.3) |
| 30 | Anyb | 6,812,186 | 437.6–582.4 | 172 (147.3–199.7) | 180 (154.7–208.3) | 218 (190.0–248.9) |
| 7 | First | 3,406,093 | 25.6–34.0 | 78 (61.7–97.3) | 80 (63.4–99.6) | 103 (84.1–124.9) |
| 7 | Second | 3,406,093 | 92.9–123.6 | 36 (25.2–49.8) | 37 (26.1–51.0) | 43 (31.1–57.9) |
| 7 | Anyb | 6,812,186 | 102.1–135.9 | 114 (94.0–136.9) | 117 (96.8–140.2) | 146 (123.3–171.7) |
BCWG L1, Brighton Collaboration Working Group level 1, BCWG L1–3 Brighton Collaboration Working Group levels 1–3, BCWG L1–4 Brighton Collaboration Working Group levels 1–4, CI confidence interval
aAs defined by Bines et al. [22]
bNote that an extra third dose was admin in a few instances
Discussion
Since its 2004 launch, over 639 million doses of HRV have been distributed worldwide. HRV has currently active marketing authorisation in more than 100 countries worldwide [60] and is prequalified by the WHO. HRV has a long-established safety and efficacy/effectiveness profile that has not altered after many years on the market, and a large proportion of the population has been vaccinated [18, 61–64]. Since evidence from postsurveillance studies pointed towards an increased risk of IS in infants following RV vaccination, GSK monitored the incidence of IS following RV vaccination as part of its routine pharmacovigilance activities. This risk of IS is also included in the packaging insert for HRV. Review of all confirmed IS cases retrieved from GSK’s safety database showed that IS is more frequently observed in males than in females. Our findings are in line with earlier reports demonstrating a male predominance of IS patients [22].
A meta-analysis on data from postmarketing surveillance studies to further evaluate the association between RV vaccination and IS was performed in 2013 by GSK [25]. The conclusion of this analysis was that postmarketing safety studies indicated a transient increased incidence of IS after vaccination with HRV, mostly within 7 days after the first dose and, to a lesser extent, after the second dose, which is in line with our findings. As recently postulated, the low-level IS risk observed for HRV and HBRV may relate to the class effect of replicating RV vaccines [65, 66].
The benefits of RV vaccines have been well documented in prelicensure clinical efficacy studies and numerous postlicensure vaccine effectiveness and impact studies conducted in different parts of the world [41]. These benefits include impact on diarrhoea-related deaths, hospitalisations, and other health outcomes associated with RV gastroenteritis and may also go beyond reducing RV gastroenteritis-related mortality and morbidity (e.g., reduced incidence of childhood seizures [67] and improved hospital quality of care [68]). Additionally, while a short-term increased IS risk may exist following vaccination, this may not hold true for the long term [30, 67]. When taking into account the role of natural RV infection as an independent risk factor for IS, the benefit–risk profile of RV vaccination may in fact be even more advantageous than previously anticipated [37].
The current review of GSK’s safety database has shown a relatively low number of confirmed reports in which the infants underwent surgery, making it difficult to confirm a correlation between time-to-treatment and the risk of surgery or resection.
The O/E analysis suggested that regardless of the region or risk period considered, the number of observed cases of IS following dose 2 are below the range of expected IS cases, even when considering the worst-case safety scenario (i.e., inclusion of unconfirmed cases). The findings of the O/E analysis are also consistent with the known IS safety profile of the vaccine, i.e., the transient increased incidence of IS after vaccination, mostly within 7 days of the first dose and, to a lesser extent, after the second dose.
As with all vaccines, immediate medical advice should be sought in case of any reaction or event following the vaccine administration. As indicated in the summary of product characteristics for HRV, it is advisable that vaccination should be preceded by a review of the medical history (especially with regard to the contraindications) and a clinical examination [19]. Since the outcomes of early management of IS are favourable compared with cases with delayed medical attention [69, 70], the HCP should inform the parents about the importance of seeking immediate medical advice in case they observe any unusual behaviour of the vaccinated infant. Given the evidence of a 7-day IS risk period following RV vaccination, parents/guardians are advised to promptly report any symptom indicative of IS (severe abdominal pain, persistent vomiting, bloody stools, abdominal bloating, and/or high fever), allowing HCPs to promptly follow-up on these symptoms [19].
While prelicensure clinical studies assess the vaccine’s safety profile, continued monitoring of the vaccine’s real-world safety profile in the postlicensure setting is essential. One of the main sources of safety information is the worldwide routine postmarketing surveillance of AEs reported spontaneously. One of the strengths of the passive safety surveillance is the rapid collection of data following real-world use of the product in the general population, including individuals with concurrent illnesses, concomitant medications, and rare or less frequent events difficult to observe in prelicensure clinical trials. Limitations of postmarketing passive surveillance that relies on spontaneous reports include reporting bias (which can be due to country-specific reporting environment, influences of media, or length of time the product has been on the market), underreporting, missing information such as lack of denominator data, misclassification or incorrect information, and the absence of an adequate comparator group [71]. Such limitations may have hindered full assessment and medical review of the reported IS cases. Additionally, a reporting bias may have artificially skewed the reported data towards specific regions or patient groups.
While interpreting the O/E analysis, it should be reminded that this is a signal strengthening tool aiming to support signal evaluation, and that it is only one method among other data sources and quantitative methods available for pharmacovigilance. O/E analyses are not suited to perform specific hypothesis testing or to measure the strength of associations between AEs and vaccines. Furthermore, it needs to be considered that there are specific factors impacting this presented analysis, pertaining to (1) the estimation of the background rate; (2) the uncertainty on the distribution of the age at which children are vaccinated (dose 1, dose 2, and any dose); (3) estimation of the exposure based on sales data; (4) underreporting of cases for spontaneous reports; (5) lack of gender stratification in background incidence rates; (6) some background incidence rates based on a population that includes vaccinated children; (7) uncertainty on the proportion of the disease by month of the first year of life; and (8) CIs did not take all uncertainty into account (i.e., coverage, precision of background incidence rates and RF). In addition, multiplicity of tests was ignored. The limitation of the current approach based on sales figures has been recognised, and to what extent the use of alternative data sources may provide additional information on the actual number of infants exposed will be investigated. However, we can already anticipate some difficulties linked to the complexity of the approach. Actually, several elements should be taken into consideration, such as availability and heterogeneity of data sources (between and within countries), multiple national immunisation schedules, specific mass vaccination campaign, incidence of the disease, and catch-up and outbreak containment. As detailed in the Methodology section, a time lag of 2 months was chosen to minimise the risk of inaccuracy due to constant updates/corrections of the database and to consider the time lag between dose shipment and dose administration. However, despite these limitations, the spontaneous and the O/E data analyses provide reassurance of a favourable safety risk profile for HRV with respect to IS.
Conclusions
Despite the known inherent limitations of postmarketing passive surveillance that relies on spontaneous AE reports, the presented review based on postmarketing spontaneous data on IS retrieved from a database and reported over 15 years since the launch of HRV is consistent with the known IS safety profile of the HRV vaccine. The presented data have thus not altered GSK’s position regarding the overall positive benefit–risk balance of HRV vaccination for infants. The findings of the O/E analysis are also consistent with the known IS safety profile of the vaccine, i.e., postmarketing safety studies indicate a transient increased incidence of IS after vaccination, mostly within 7 days of the first dose and, to a lesser extent, after the second dose. GSK continues to monitor the safety concern of IS following administration of HRV through its ongoing pharmacovigilance activities.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Dan Bi, Brigitte Cheuvart, and Priya Pereira for their critical review of the manuscript. Moreover, the authors would like to thank Hubert Buyse and Paola Pirrotta for their past contribution to this study. The authors also thank Anne Henze (Modis c/o GSK) for medical writing support and Manuel Zocco (Modis c/o GSK) for editorial support and manuscript coordination.
Declarations
Funding
This work was supported by GlaxoSmithKline Biologicals SA, which was the funding source and was involved in all stages of the study conduct and analysis. GlaxoSmithKline Biologicals SA also took responsibility for all costs associated with the development and publishing of the present manuscript.
Conflict of interest
Tina Singh, Frédérique Delannois, François Haguinet, and Lifeter Yenwo Molo are employees of the GSK group of companies. Frédérique Delannois, François Haguinet, and Tina Singh hold shares in the GSK group of companies as part of their employee remuneration. The authors declare no other financial or non-financial conflicts of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Trademark statements
Rotarix is a trademark owned by the GSK group of companies; RotaTeq is a registered trademark of Merck & Co., Inc.; Rotavac is a registered trademark of Bharat Biotech; and Rotasiil is a registered trademark of the Serum Institute of India Ltd.
Availability of data and material
The data that support the findings of this work are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Author contributions
All authors participated in the design, implementation, analysis and interpretation of the study, as well as the development of this manuscript. All authors had full access to the data and granted their final approval of the paper before submission.
Footnotes
The original online version of this article was revised due to a retrospective Open Access order.
Change history
3/24/2022
A Correction to this paper has been published: 10.1007/s40264-022-01167-2
References
- 1.Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis. 2003;9(5):565–572. doi: 10.3201/eid0905.020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cunliffe NA, Kilgore PE, Bresee JS, Steele AD, Luo N, Hart CA, et al. Epidemiology of rotavirus diarrhoea in Africa: a review to assess the need for rotavirus immunization. Bull World Health Organ. 1998;76:525–537. [PMC free article] [PubMed] [Google Scholar]
- 3.Kane EM, Turcios RM, Arvay ML, Garcia S, Bresee JS, Glass RI. The epidemiology of rotavirus diarrhea in Latin America. Anticipating rotavirus vaccines. Rev Panam Salud Publ. 2004;16(6):371–377. doi: 10.1590/s1020-49892004001200002. [DOI] [PubMed] [Google Scholar]
- 4.Burnett E, Jonesteller CL, Tate JE, Yen C, Parashar UD. Global impact of rotavirus vaccination on childhood hospitalizations and mortality from diarrhea. J Infect Dis. 2017;215(11):1666–1672. doi: 10.1093/infdis/jix186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehlken B, Laubereau B, Karmaus W, Petersen G, Rohwedder A, Forster J. Prospective population-based study on rotavirus disease in Germany. Acta Paediatr. 2002;91(7):769–775. doi: 10.1080/08035250213227. [DOI] [PubMed] [Google Scholar]
- 6.Ruggeri FM, Declich S. Rotavirus infection among children with diarrhoea in Italy. Acta Paediatr Suppl. 1999;88(426):66–71. doi: 10.1111/j.1651-2227.1999.tb14329.x. [DOI] [PubMed] [Google Scholar]
- 7.Rotavirus vaccines. WHO position paper—January 2013. Wkly Epidemiol Rec. 2013;88:49-64 [PubMed]
- 8.Tate JE, Burton AH, Boschi-Pinto C, Parashar UD. Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000–2013. Clin Infect Dis. 2016;62(Suppl 2):S96–S105. doi: 10.1093/cid/civ1013. [DOI] [PubMed] [Google Scholar]
- 9.Bines JE, Ivanoff B. Acute intusussception in infants and children. Incidence, clinical presentation and management: a global perspective. 2002. http://apps.who.int/iris/bitstream/10665/67720/1/WHO_V-B_02.19_eng.pdf. Accessed 29 Nov 2021.
- 10.O'Ryan M, Lucero Y, Pena A, Valenzuela MT. Two year review of intestinal intussusception in six large public hospitals of Santiago, Chile. Pediatr Infect Dis J. 2003;22(8):717–721. doi: 10.1097/01.inf.0000078374.82903.e8. [DOI] [PubMed] [Google Scholar]
- 11.Perez-Schael I, Escalona M, Salinas B, Materan M, Perez ME, Gonzalez G. Intussusception-associated hospitalization among Venezuelan infants during 1998 through 2001: anticipating rotavirus vaccines. Pediatr Infect Dis J. 2003 doi: 10.1097/01.inf.0000055064.76457.f3. [DOI] [PubMed] [Google Scholar]
- 12.Marsicovetere P, Ivatury SJ, White B, Holubar SD. Intestinal intussusception: etiology, diagnosis, and treatment. Clin Colon Rectal Surg. 2017 doi: 10.1055/s-0036-1593429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burnett E, Kabir F, Van Trang N, Rayamajhi A, Satter SM, Liu J, et al. Infectious etiologies of intussusception among children < 2 years old in 4 Asian Countries. J Infect Dis. 2020 doi: 10.1093/infdis/jiz621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuppermann N, O'Dea T, Pinckney L, Hoecker C. Predictors of intussusception in young children. Arch Pediatr Adolesc Med. 2000 doi: 10.1001/archpedi.154.3.250. [DOI] [PubMed] [Google Scholar]
- 15.Huppertz HI, Soriano-Gabarro M, Grimprel E, Franco E, Mezner Z, Desselberger U, et al. Intussusception among young children in Europe. Pediatr Infect Dis J. 2006 doi: 10.1097/01.inf.0000197713.32880.46. [DOI] [PubMed] [Google Scholar]
- 16.Peter G, Myers MG. Intussusception, rotavirus, and oral vaccines: summary of a workshop. Pediatrics. 2002 doi: 10.1542/peds.110.6.e67. [DOI] [PubMed] [Google Scholar]
- 17.WHO Prequalified Vaccines. WHO. https://extranet.who.int/gavi/PQ_Web/Default.aspx?nav=1. Accessed 23 Jul 2020.
- 18.Bergman H, Henschke N, Hungerford D, Pitan F, Ndwandwe D, Cunliffe N, et al. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database Syst Rev. 2021 doi: 10.1002/14651858.CD008521.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rotarix Summary of Product Characteristics. 2020. https://www.ema.europa.eu/en/documents/product-information/rotarix-epar-product-information_en.pdf. Accessed 29 Nov 2021.
- 20.International Vaccine Access Center (IVAC). Johns Hopkins Bloomberg School of Public Health. https://view-hub.org/. Accessed 23 July 2020.
- 21.Velazquez FR, Colindres RE, Grajales C, Hernandez MT, Mercadillo MG, Torres FJ, et al. Postmarketing surveillance of intussusception following mass introduction of the attenuated human rotavirus vaccine in Mexico. Pediatr Infect Dis J. 2012 doi: 10.1097/INF.0b013e318253add3. [DOI] [PubMed] [Google Scholar]
- 22.Bines JE, Kohl KS, Forster J, Zanardi LR, Davis RL, Hansen J, et al. Acute intussusception in infants and children as an adverse event following immunization: case definition and guidelines of data collection, analysis, and presentation. Vaccine. 2004 doi: 10.1016/j.vaccine.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 23.Buttery JP, Danchin MH, Lee KJ, Carlin JB, McIntyre PB, Elliott EJ, et al. Intussusception following rotavirus vaccine administration: post-marketing surveillance in the National Immunization Program in Australia. Vaccine. 2011 doi: 10.1016/j.vaccine.2011.01.088. [DOI] [PubMed] [Google Scholar]
- 24.Tate JE, Mwenda JM, Armah G, Jani B, Omore R, Ademe A, et al. Evaluation of intussusception after monovalent rotavirus vaccination in Africa. N Engl J Med. 2018 doi: 10.1056/NEJMoa1713909. [DOI] [PubMed] [Google Scholar]
- 25.Rosillon D, Buyse H, Friedland LR, Ng SP, Velazquez FR, Breuer T. Risk of intussusception after rotavirus vaccination: meta-analysis of postlicensure studies. Pediatr Infect Dis J. 2015 doi: 10.1097/inf.0000000000000715. [DOI] [PubMed] [Google Scholar]
- 26.Haber P, Patel M, Pan Y, Baggs J, Haber M, Museru O, et al. Intussusception after rotavirus vaccines reported to US VAERS, 2006–2012. Pediatrics. 2013 doi: 10.1542/peds.2012-2554. [DOI] [PubMed] [Google Scholar]
- 27.Patel MM, López-Collada VR, Bulhões MM, De Oliveira LH, Bautista Márquez A, Flannery B, et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N Engl J Med. 2011 doi: 10.1056/NEJMoa1012952. [DOI] [PubMed] [Google Scholar]
- 28.Yih WK, Lieu TA, Kulldorff M, Martin D, McMahill-Walraven CN, Platt R, et al. Intussusception risk after rotavirus vaccination in US infants. N Engl J Med. 2014 doi: 10.1056/NEJMoa1303164. [DOI] [PubMed] [Google Scholar]
- 29.Carlin JB, Macartney KK, Lee KJ, Quinn HE, Buttery J, Lopert R, et al. Intussusception risk and disease prevention associated with rotavirus vaccines in Australia's National Immunization Program. Clin Infect Dis. 2013 doi: 10.1093/cid/cit520. [DOI] [PubMed] [Google Scholar]
- 30.Hawken S, Ducharme R, Rosella LC, Benchimol EI, Langley JM, Wilson K, et al. Assessing the risk of intussusception and rotavirus vaccine safety in Canada. Hum Vaccin Immunother. 2017 doi: 10.1080/21645515.2016.1240846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nam H, Lim KI, Tchah H, Ryoo E, Sun Y, Cho H. Occurrence pattern of intussusception according to the introduction of rotavirus vaccine: an observational study at a university hospital. Pediatr Infect Vaccine. 2016 doi: 10.14776/piv.2016.23.3.202. [DOI] [Google Scholar]
- 32.Yen C, Shih S-M, Tate JE, Wu F-T, Huang Y-C, Parashar UD, et al. Intussusception-related hospitalizations among infants before and after private market licensure of rotavirus vaccines in Taiwan, 2001–2013. Pediatr Infect Dis J. 2017 doi: 10.1097/INF.0000000000001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koch J, Harder T, von Kries R, Wichmann O. Risk of intussusception after rotavirus vaccination. Dtsch Arztebl Int. 2017 doi: 10.3238/arztebl.2017.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kassim P, Eslick GD. Risk of intussusception following rotavirus vaccination: an evidence based meta-analysis of cohort and case-control studies. Vaccine. 2017 doi: 10.1016/j.vaccine.2017.05.064. [DOI] [PubMed] [Google Scholar]
- 35.Stowe J, Andrews N, Ladhani S, Miller E. The risk of intussusception following monovalent rotavirus vaccination in England: a self-controlled case-series evaluation. Vaccine. 2016 doi: 10.1016/j.vaccine.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 36.Tate JE, Yen C, Steiner CA, Cortese MM, Parashar UD. Intussusception rates before and after the introduction of rotavirus vaccine. Pediatrics. 2016 doi: 10.1542/peds.2016-1082. [DOI] [PubMed] [Google Scholar]
- 37.Vetter V, Pereira P, Benninghoff B. Rotavirus vaccination and intussusception: a paradigm shift? Hum Vaccin Immunother. 2021;17(1):278–282. doi: 10.1080/21645515.2020.1770035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ledent E, Lieftucht A, Buyse H, Sugiyama K, McKenna M, Holl K. Post-marketing benefit-risk assessment of rotavirus vaccination in Japan: a simulation and modelling analysis. Drug Saf. 2016 doi: 10.1007/s40264-015-0376-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clark A, Tate J, Parashar U, Jit M, Hasso-Agopsowicz M, Henschke N, et al. Mortality reduction benefits and intussusception risks of rotavirus vaccination in 135 low-income and middle-income countries: a modelling analysis of current and alternative schedules. Lancet Glob Health. 2019 doi: 10.1016/s2214-109x(19)30412-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arlegui H, Nachbaur G, Praet N, Bégaud B. Quantitative benefit-risk models used for rotavirus vaccination: a systematic review. Open Forum Infect Dis. 2020 doi: 10.1093/ofid/ofaa087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pereira P, Vetter V, Standaert B, Benninghoff B. Fifteen years of experience with the oral live-attenuated human rotavirus vaccine: reflections on lessons learned. Expert Rev Vaccines. 2020 doi: 10.1080/14760584.2020.1800459. [DOI] [PubMed] [Google Scholar]
- 42.Medical Dictionary for Regulatory Activities Terminology (MedDRA). Version 23.0. http://www.meddra.org/. Accessed 28 Jul 2020.
- 43.International Conference on Harmonisation. Topic E2A: Clinical safety data management: definitions and standards for expedited reporting. 1994. https://database.ich.org/sites/default/files/E2A_Guideline.pdf. Accessed 29 Nov 2021.
- 44.Halsey NA. The science of evaluation of adverse events associated with vaccination. Semin Pediatr Infect Dis. 2002 doi: 10.1053/spid.2002.125864. [DOI] [PubMed] [Google Scholar]
- 45.Escolano S, Farrington CP, Hill C, Tubert-Bitter P. Intussusception after rotavirus vaccination–spontaneous reports. N Engl J Med. 2011 doi: 10.1056/NEJMc1107771. [DOI] [PubMed] [Google Scholar]
- 46.Shui IM, Baggs J, Patel M, Parashar UD, Rett M, Belongia EA, et al. Risk of intussusception following administration of a pentavalent rotavirus vaccine in US infants. JAMA. 2012 doi: 10.1001/jama.2012.97. [DOI] [PubMed] [Google Scholar]
- 47.Weintraub ES, Baggs J, Duffy J, Vellozzi C, Belongia EA, Irving S, et al. Risk of intussusception after monovalent rotavirus vaccination. N Engl J Med. 2014 doi: 10.1056/NEJMoa1311738. [DOI] [PubMed] [Google Scholar]
- 48.Mahaux O, Bauchau V, Van Holle L. Pharmacoepidemiological considerations in observed-to-expected analyses for vaccines. Pharmacoepidemiol Drug Saf. 2016 doi: 10.1002/pds.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samad L, Cortina-Borja M, Bashir HE, Sutcliffe AG, Marven S, Cameron JC, et al. Intussusception incidence among infants in the UK and Republic of Ireland: a pre-rotavirus vaccine prospective surveillance study. Vaccine. 2013 doi: 10.1016/j.vaccine.2013.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takeuchi M, Osamura T, Yasunaga H, Horiguchi H, Hashimoto H, Matsuda S. Intussusception among Japanese children: an epidemiologic study using an administrative database. BMC Pediatr. 2012 doi: 10.1186/1471-2431-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sáez-Llorens X, Velázquez FR, Lopez P, Espinoza F, Linhares AC, Abate H, et al. A multi-country study of intussusception in children under 2 years of age in Latin America: analysis of prospective surveillance data. BMC Gastroenterol. 2013 doi: 10.1186/1471-230x-13-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.ClinicalTrials.gov. Study on the incidence of intussusception (IS) in children aged < 12 months in Japan. 2012. https://clinicaltrials.gov/ct2/show/NCT01479491. Accessed 29 Nov 2021.
- 53.GSK study register. Study on the incidence of intussusception (IS) in children aged <12 months in Japan. Available at: https://www.gsk-studyregister.com/en/trial-details/?id=116259. Accessed 29 Nov 2021.
- 54.Eng PM, Mast TC, Loughlin J, Clifford CR, Wong J, Seeger JD. Incidence of intussusception among infants in a large commercially insured population in the United States. Pediatr Infect Dis J. 2012 doi: 10.1097/inf.0b013e31824213b1. [DOI] [PubMed] [Google Scholar]
- 55.Chang HG, Smith PF, Ackelsberg J, Morse DL, Glass RI. Intussusception, rotavirus diarrhea, and rotavirus vaccine use among children in New York state. Pediatrics. 2001 doi: 10.1542/peds.108.1.54. [DOI] [PubMed] [Google Scholar]
- 56.Garwood F. (i) Fiducial limits for the Poisson distribution. Biometrika. 1936 doi: 10.1093/biomet/28.3-4.437. [DOI] [Google Scholar]
- 57.Buettcher M, Baer G, Bonhoeffer J, Schaad UB, Heininger U. Three-year surveillance of intussusception in children in Switzerland. Pediatrics. 2007 doi: 10.1542/peds.2007-0035. [DOI] [PubMed] [Google Scholar]
- 58.Parashar UD, Holman RC, Cummings KC, Staggs NW, Curns AT, Zimmerman CM, et al. Trends in intussusception-associated hospitalizations and deaths among US infants. Pediatrics. 2000 doi: 10.1542/peds.106.6.1413. [DOI] [PubMed] [Google Scholar]
- 59.Noguchi A, Nakagomi T, Kimura S, Takahashi Y, Matsuno K, Koizumi H, et al. Incidence of intussusception as studied from a hospital-based retrospective survey over a 10-year period (2001–2010) in Akita Prefecture, Japan. Jpn J Infect Dis. 2012 doi: 10.7883/yoken.65.301. [DOI] [PubMed] [Google Scholar]
- 60.World Health Organization. WHO vaccine-preventable diseases: monitoring system. 2019 global summary. Immunization schedule for 1 disease (Rotavirus). 2020. http://apps.who.int/immunization_monitoring/globalsummary/diseases. Accessed 19 Jan 2021.
- 61.Bonaldo G, Noseda R, Ceschi A, Vaccheri A, Motola D. Evaluation of the safety profile of rotavirus vaccines: a pharmacovigilance analysis on American and European data. Sci Rep. 2020 doi: 10.1038/s41598-020-70653-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hallowell BD, Tate J, Parashar U. An overview of rotavirus vaccination programs in developing countries. Expert Rev Vaccines. 2020 doi: 10.1080/14760584.2020.1775079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jonesteller CL, Burnett E, Yen C, Tate JE, Parashar UD. Effectiveness of rotavirus vaccination: a systematic review of the first decade of global postlicensure data, 2006–2016. Clin Infect Dis. 2017 doi: 10.1093/cid/cix369. [DOI] [PubMed] [Google Scholar]
- 64.Haber P, Tate J, Marquez PL, Moro PL, Parashar U. Safety profile of rotavirus vaccines among individuals aged ≥8months of age, United States, vaccine adverse event reporting system (VAERS), 2006–2019. Vaccine. 2021 doi: 10.1016/j.vaccine.2020.11.026. [DOI] [PubMed] [Google Scholar]
- 65.Aliabadi N, Tate JE, Parashar UD. Potential safety issues and other factors that may affect the introduction and uptake of rotavirus vaccines. Clin Microbiol Infect. 2016 doi: 10.1016/j.cmi.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O'Ryan M, Lopman BA. Parenteral protein-based rotavirus vaccine. Lancet Infect Dis. 2017 doi: 10.1016/S1473-3099(17)30244-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burke RM, Tate JE, Dahl RM, Aliabadi N, Parashar UD. Does rotavirus vaccination affect longer-term intussusception risk in US infants? J Pediatric Infect Dis Soc. 2020 doi: 10.1093/jpids/piz035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Standaert B, Alwan A, Strens D, Raes M, Postma MJ. Improvement in hospital Quality of Care (QoC) after the introduction of rotavirus vaccination: An evaluation study in Belgium. Hum Vaccin Immunother. 2015 doi: 10.1080/21645515.2015.1029212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.DiFiore JW. Intussusception. Semin Pediatr Surg. 1999 doi: 10.1016/s1055-8586(99)70029-6. [DOI] [PubMed] [Google Scholar]
- 70.Ogundoyin OO, Olulana DI, Lawal TA. Childhood intussusception: Impact of delay in presentation in a developing country. Afr J Paediatr Surg. 2016 doi: 10.4103/0189-6725.194665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goldman SA. Limitations and strengths of spontaneous reports data. Clin Ther. 1998 doi: 10.1016/s0149-2918(98)80007-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.