Abstract
Acute neurological alterations have been associated with SARS-CoV-2 infection. Additionally, it is becoming clear that coronavirus disease 2019 (COVID-19) survivors may experience long-term neurological abnormalities, including cognitive deficits and mood alterations. The mechanisms underlying acute and long-term impacts of COVID-19 in the brain are being actively investigated. Due to the heterogeneous manifestations of neurological outcomes, it is possible that different mechanisms operate following SARS-CoV-2 infection, which may include direct brain infection by SARS-CoV-2, mechanisms resulting from hyperinflammatory systemic disease, or a combination of both. Inflammation is a core feature of COVID-19, and both central and systemic inflammation are known to lead to acute and persistent neurological alterations in other diseases. Here, we review evidence indicating that COVID-19 is associated with neuroinflammation, along with blood-brain barrier dysfunction. Similar neuroinflammatory signatures have been associated with Alzheimer's disease and major depressive disorder. Current evidence demonstrates that patients with pre-existing cognitive and neuropsychiatric deficits show worse outcomes upon infection by SARS-CoV-2 and, conversely, COVID-19 survivors may be at increased risk of developing dementia and mood disorders. Considering the high prevalence of COVID-19 patients that recovered from infection in the world and the alarming projections for the prevalence of dementia and depression, investigation of possible molecular similarities between those diseases may shed light on mechanisms leading to long-term neurological abnormalities in COVID-19 survivors.
This article is part of the special Issue on ‘Cross Talk between Periphery and the Brain’.
Keywords: SARS-CoV-2, Cytokine, Blood-brain barrier, Neuro-infectious diseases, Memory, Mood disorders
Abbreviations
- ACE2
Angiotensin-Converting Enzyme 2
- AD
Alzheimer's disease
- BBB
blood-brain barrier
- C1q
complement component 1q
- CART
Combination Antiretroviral Therapy
- CNS
central nervous system
- COVID-19
coronavirus disease 2019
- CSF
cerebrospinal fluid
- EBV
Epstein-Barr Virus
- gp130
glycoprotein 130
- HAD
HIV-associated dementia
- HAND
HIV-associated neurocognitive disorder
- HCoV
Human Coronavirus
- HIV
human immunodeficiency virus
- HSE
herpes simplex encephalitis
- HSV
herpes simplex virus
- LPS
lipopolysaccharide
- IL-1α
interleukin-1alpha
- IL-6
interleukin-6
- IL-6R
IL-6 receptor
- iPSCs
induced Pluripotency Stem Cells
- JAK
Janus kinase
- JEV
Japanese Encephalitis Virus
- MDD
major depressive disorder
- SARS
Severe Acute Respiratory Syndrome
- SARS-CoV-1
Severe Acute Respiratory Syndrome Coronavirus 1
- SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus 2
- sIL-6R
soluble isoform of IL-6- receptor
- TBEV
Tick Born Encephalitis Virus
- TLR
Toll-Like Receptor
- TNF-⍺
tumor necrosis factor-alpha
- TNF-R
TNF receptors
- WHO
World Health Organization
- WNV
West Nile virus
- ZIKV
Zika virus
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic is one of modern society's most significant health crises. According to the World Health Organization (WHO), as of February 2022, over 430 million people have been infected by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), with almost 6 million confirmed deaths worldwide. Beyond causing a prominent respiratory distress syndrome, COVID-19 is a systemic disease that affects multiple organs (Wadman et al., 2020). Severe patients develop peripheral hyper-inflammation, coagulopathy, hypoxia, hepatic dysfunction, sepsis, and acute kidney failure. Neurological disturbances are frequent in COVID-19 patients and range from relatively mild symptoms, such as headache, anosmia, and ageusia, to severe complications, including encephalopathy, stroke, delirium, and coma (Chen et al., 2020a; Mao et al., 2020; Helms et al., 2020a; Romero-Sánchez et al., 2020; Lechien et al., 2020; Oxley et al., 2020; Poyiadji et al., 2020; Benussi et al., 2020; Chou et al., 2021).
Follow-up studies carried out thus far indicate that a significant proportion of COVID-19 survivors experience persistent neuropsychological alterations, including anxiety, depression, and cognitive impairment (Nath, 2020; Crunfli et al., 2020; Mazza et al., 2021, Taquet et al., 2021b Taquet et al., 2021a, Taquet et al., 2021b. Both the manifestation of neurological symptoms during hospitalization and the existence of prior neurological diseases, including dementia and neuropsychiatric disorders, are associated with higher mortality by COVID-19 (Chou et al., 2021; Taquet et al., 2021a; Tahira et al., 2021; Romagnolo et al., 2021; Nemani et al., 2021). Likewise, age, diabetes, and maladaptive immune response are risk factors for COVID-19 severity and mortality (Lucas et al., 2020; Webb et al., 2020; Tay et al., 2020). However, the mechanisms by which COVID-19 triggers neurological impairments and its long-term consequences remain to be determined. We and others have shown that depression, dementia, and diabetes share pathophysiological mechanisms leading to brain dysfunction in animal models (Ledo et al., 2013; Neves et al., 2018; Batista et al., 2018; Lourenco et al., 2013). Here, we examine the available evidence on brain infection by SARS-CoV-2, the induction of systemic inflammation, coagulopathy, and blood-brain barrier (BBB) dysfunction as possible mechanisms underlying neurological impairments in COVID-19. Considering the sky-rocketing number of COVID-19 survivors worldwide, it is of utmost importance to understand factors leading to persistent or late-onset neurological outcomes to inform public health policies and develop preventative and treatment strategies for those patients.
2. Viral infections and human neurological diseases
As alarming as it may seem, the fact that COVID-19 induces neurological complications should not come as a surprise. Viruses from diverse families exhibit tropism for the central nervous system (CNS) and have been implicated in acute and persistent neurological alterations in humans.
Herpes Simplex Viruses (HSV-1 and HSV-2) are the leading identified cause of encephalitis in the United States of America, causing a condition termed HSV encephalitis (HSE) (Gnann and Whitley, 2017). HSV belongs to the herpesviridae family (Whitley and Roizman, 2001), causes mild mucocutaneous infections in humans and establishes latent infections in sensory ganglia (Whitley and Roizman, 2001). While innate immune responses are important to halt viral replication, HSV-induced CNS pathology results from a combination of viral-driven cytolysis and inflammation-mediated effects (Gnann and Whitley, 2017; Marcocci et al., 2020). HSE survivors frequently experience long-term neuropsychological sequelae and are at risk of autoimmune encephalitis relapses (Gnann and Whitley, 2017). Recurrent HSV infections have been proposed to be a long-term pathogenic mechanism linked to the development of Alzheimer's disease (AD) (Marcocci et al., 2020). Similarly, infection by Epstein-Barr virus (EBV) has been linked to promoting of neuronal damage in late-onset multiple sclerosis (Bjornevik et al., 2022).
The human immunodeficiency virus (HIV) can also invade the CNS and become latent. Persistent CNS infection, systemic and central inflammation can cause HIV-associated neurocognitive disorder (HAND) (Saylor et al., 2016). Due to the advent of combination antiretroviral therapy (CART) to halt HIV replication, progression of HAND to the most severe HIV-associated dementia (HAD) is now uncommon, occurring just in a subset of HIV+ patients (Saylor et al., 2016).
The Flaviviridae family of arboviruses (arthropod-borne virus) includes several members capable of inducing neurological disorders and long-term neurological sequelae, such as Japanese Encephalitis Virus (JEV), tick-borne encephalitis virus (TBEV), and West Nile virus (WNV) (Maximova and Pletnev, 2018; Turtle and Solomon, 2018). A recent Zika virus (ZIKV) outbreak in the Americas led the WHO (2016) to declare a public health emergency of international concern due to neurological disorders associated with the infection. ZIKV infection during pregnancy may cause a complex congenital syndrome, including microcephaly (Souza et al., 2019). In adults, infection may lead to encephalitis, myelitis, and Guillain-Barre Syndrome cases (Souza et al., 2019). Long-term neurological consequences of ZIKV infection, however, are still largely unknown. Animal studies suggest that direct viral cytolysis and neuroinflammation play important roles in flavivirus-induced neurological damage (Maximova and Pletnev, 2018; Turtle and Solomon, 2018; Figueiredo et al., 2019; Souza et al., 2018).
Zoonotic viruses can occasionally cause disease in humans. The most prominent example of a zoonotic virus causing neurological damage is rabies virus (Hemachudha et al., 2013). Another example is Borna disease virus, which causes fatal encephalitis in rare human spill-over infections in endemic areas (Rubbenstroth et al., 2019). Members of the coronavirus family have been introduced to humans from a zoonotic origin and have also been implicated in CNS damage (Li et al., 2020; de Felice et al., 2020; Zubair et al., 2020). Infection by the Severe Acute Respiratory Syndrome (SARS) Coronavirus (SARS-CoV-1), closely related to SARS-CoV-2, has been associated with myopathy, polyneuropathy, seizures, and stroke (Tsai et al., 2005; Lau et al., 2004). SARS-CoV-1 protein and genetic material have been found in patient cerebrospinal fluid (CSF) and post-mortem brains (Lau et al., 2004; Ding et al., 2004; Gu et al., 2005; Xu et al., 2005). Similarly, human coronavirus OC43 (HCoV-OC43) has been reported to invade the brain parenchyma and induce fatal encephalitis (Morfopoulou et al., 2016; Nilsson et al., 2020). HCoV-OC43 and HCoV-229E have been implicated in long-term neurological disease, including a possible role in multiple sclerosis (Desforges et al., 2019). However, as discussed in the next section, coronaviruses, including SARS-CoV-2, are not primarily neurotropic viruses. Instead, they attack airways and lungs while retaining the capacity of infecting diverse cell types, including CNS cells or endothelia (Puelles et al., 2020; Liu et al., 2021).
3. Evidence of brain infection by SARS-CoV-2
SARS-CoV-2 is a single-stranded RNA virus, a member of the Betacoronavirus genus, of the Coronaviridae family within the Riboviria realm (Coronaviridae Study Group, 2020). Each virion contains four types of structural proteins, named spike (S), envelope (E), membrane (M), and nucleocapsid (N) (Mariano et al., 2020). Infection by SARS-CoV-2 begins with the interaction of the viral outer-membrane spike protein with angiotensin-converting enzyme 2 (ACE2) at the plasma membrane of host cells. Physiologically, peripheral ACE2 is involved in the conversion of the hormone angiotensin that regulates blood pressure (Alenina and Bader, 2019). In the brain, ACE2 appears to be involved in brain injury recovery, stress response, and memory function (Alenina and Bader, 2019).
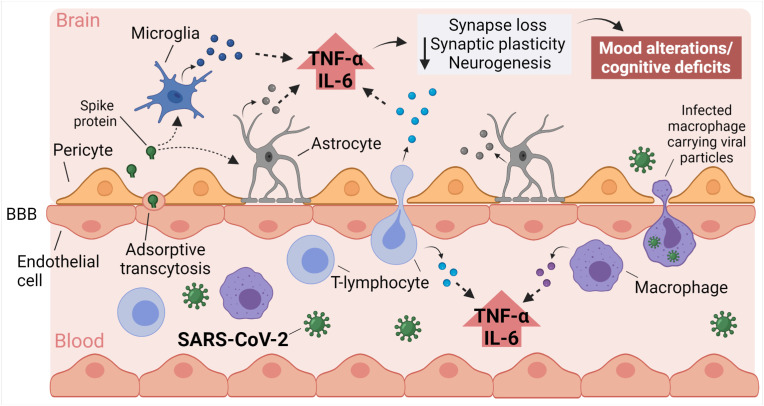
Analysis of post-mortem COVID-19 patient brains provided initial evidence of neuroinvasion by SARS-CoV-2 (Crunfli et al., 2020; Puelles et al., 2020; Song et al., 2021; Meinhardt et al., 2021; Matschke et al., 2020). Different routes have been proposed for CNS infection by human coronaviruses, including retrograde transport across peripheral nerves and the intranasal pathway leading to the olfactory bulb (Desforges et al., 2019; Meinhardt et al., 2021; Cooper et al., 2020). Several studies indicate that, although the sustentacular cells of the olfactory epithelium express ACE2, this protein is not present in olfactory receptor neurons (Butowt et al., 2021; Brann et al., 2020; Chen et al., 2020b; Klingenstein et al., 2020). Combined with weak evidence supporting the presence of viral particles in olfactory neurons and bulb, these data indicate that this is not a probable route for CNS infection (Butowt et al., 2021). Alternatively, after reaching the bloodstream, viruses may enter the brain by transport across the BBB via endothelial cells or infected leukocytes, a process referred to as a “Trojan horse mechanism” (Fig. 1 ), or may reach the CSF by crossing epithelial cells in the choroid plexus (blood-CSF barrier) (Pezzini and Padovani, 2020).
Fig. 1.
Proposed mechanisms underlying neurological impact in COVID-19. Systemic inflammation induced by COVID-19, as well as brain invasion by viral proteins or SARS-CoV-2 can add up to cause blood-brain barrier (BBB) dysfunction, brain microvascular damage, and brain inflammation. Activation of toll-like receptors (TLRs) and accumulation of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) in the brain, either by local production or from the periphery, triggers synaptic damage, leading to depressive and cognitive symptoms in COVID-19 patients. Created with BioRender.com.
Brain infection and neurotropism has been demonstrated following intranasal administration of SARS-CoV-2 in mice that overexpress the human isoform of ACE2 (Song et al., 2021). In wild-type mice, the S1 subunit of the spike protein crosses the BBB and reaches the brain parenchyma via a vesicular-dependent transport mechanism known as adsorptive transcytosis (Fig. 1). (Rhea et al., 2020) Due to the limitations of animal models, several groups have used brain organoids derived from human induced pluripotency stem cells (iPSCs) to evaluate the impact of SARS-CoV-2 infection (Crunfli et al., 2020; Song et al., 2021; Ramani et al., 2020, 2021; Zhang et al., 2020; Pedrosa et al., 2021). Although findings diverge, those studies suggest that SARS-CoV-2 could infect human neurons or astrocytes, and lead to inflammation and molecular alterations related to neurodegeneration. Collectively, results support the idea that brain invasion by SARS-CoV-2 or by viral proteins could, at least in part, trigger neurological deficits in COVID-19.
On the other hand, the search for molecular traces of SARS-CoV-2 in post-mortem patient brains and CSF samples has produced inconsistent results, raising the possibility that this is not a primary neuropathogenic mechanism in COVID-19 (Espíndola et al., 2020; Thakur et al., 2021; Yang et al., 2021; Lee et al., 2020). Indeed, unlike the case in HSE, the SARS-CoV-2 genome is rarely found in the CSF of COVID-19 patients presenting neurological symptoms (Espíndola et al., 2020). In two independent studies, Yang et al. (2021) and Lee et al. (2020) found no evidence of SARS-CoV-2 RNA or protein in systematic analyses of post-mortem brain samples (Yang et al., 2021; Lee et al., 2020). Thakur et al. (2021) found low but detectable levels of viral mRNA at multiple brain sites using quantitative real-time PCR (Thakur et al., 2021). Nonetheless, RNA-scope and immunohistochemistry assays targeting nucleocapsid and spike proteins failed to confirm those results. Further, sites of possible infection did not coincide with neuropathology and microgliosis (Thakur et al., 2021). The discrepant results reported in different studies may reflect (1) poor or transient viral invasion of the CNS with fast clearance, leading to low viral levels that approach technical detection limits; (2) methodological issues, such as tissue conservation, blood contamination, and antibody specificity; and (3) positive signals arising from infection of vascular or meningeal cells, rather than in the brain parenchyma.
4. Brain inflammation in infectious diseases
CNS infections cause cognitive, mood, and motor deficits that may persist beyond the acute phase of the disease. In some cases, neurological sequelae may result from irreversible damage and neuronal death triggered by pathogens (van den Pol, 2009). Alternatively, infection-driven inflammation can disturb neuronal function and cause long-term deficits (Figueiredo et al., 2019; Vasek et al., 2016; Frost et al., 2019; De Sousa et al., 2021). Dysregulation of inflammatory responses may cause brain dysfunction and behavioral alterations via multiple mechanisms (Figueiredo et al., 2019; Klein et al., 2017; Garber et al., 2019).
Microglia are the primary neuroimmune cells and perform vital functions in brain homeostasis and defense against pathogens, but they can also contribute to disease processes (reviewed in Hickman et al., 2018; Xie et al., 2019). In response to infection, microglia increase in number, migrate to the primary site of infection and acquire an activated phagocytic phenotype associated with pro-inflammatory cytokine release (Frost et al., 2019; De Sousa et al., 2021; Hickman et al., 2018). Microglia contribute to memory deficits in mouse models of CNS viral infection by actively attacking and eliminating synapses (Figueiredo et al., 2019; Vasek et al., 2016). Once activated, microglia may respond with greater sensitivity to subsequent stimuli (a mechanism known as priming), resulting in exaggerated inflammatory responses and increasing susceptibility to neurodegenerative processes (Frost et al., 2019; De Sousa et al., 2021).
Astrocytes are an essential component of the neurovascular unit and provide a link between neurons and blood vessels. In homeostasis, astrocytes support and maintain BBB integrity, function as well as neuronal connectivity (Matias et al., 2019). In response to infections, astrocytes undergo morphological, molecular, and functional changes that can have beneficial consequences (e.g., pathogen elimination and tissue repair) or detrimental effects (Matias et al., 2019; Escartin et al., 2021). Microglia-released factors, namely interleukin-1α (IL-1α), tumor necrosis factor α (TNFα), and complement component 1q (C1q) induce a functional shift of astrocytes to the so-called A1 phenotype, a subtype of astrocytes that lose their physiological roles in neuronal and synaptic maintenance (Liddelow et al., 2017). A1 astrocytes are observed in CNS diseases and injuries, and trigger neuronal and oligodendrocyte loss (Liddelow et al., 2017; Liddelow and Barres, 2017). Astrocytes are involved with both acute and chronic neurological effects, as well as long-term sequelae in neuro-infectious diseases, as several viruses can infect and activate astrocytes, altering BBB permeability and neuronal function (reviewed in Soung and Klein, 2018).
As a consequence of infection or other injuries, monocytes differentiate into macrophages recruited to affected tissues (Wynn et al., 2013). In the CNS, macrophages are present at perivascular spaces, meninges, and the choroid plexus, where they act as scavengers and physiological modulators (Xie et al., 2019). While macrophages are critical for innate immune response, T-lymphocytes are the primary effectors of adaptive immunity. T-lymphocytes also play a role in the CNS, patrolling the meningeal spaces and contributing to normal behavior and cognition. In CNS infections, macrophages and lymphocytes infiltrate the brain parenchyma and contribute to local cytokine release (Klein et al., 2017; Garber et al., 2019; Cusick et al., 2013).
Cytokines play important roles in synaptic plasticity and neuronal physiology (Stellwagen and Malenka, 2006; Cunningham et al., 1996; Habbas et al., 2015; Creed Pettigrew et al., 2016; Hosseini et al., 2020; Ross et al., 2003; Li et al., 1997; D'Arcangelo et al., 2000), but aberrant upregulation of cytokine production and secretion in the CNS interferes with neuronal function and with neurotransmitter-mediated pathways (Klein et al., 2017). As aforementioned, COVID-19 patients experience a hyper-inflammatory syndrome, with increased circulating levels of several cytokines, including TNF-⍺ and interleukin-6 (IL-6) (Lucas et al., 2020; Webb et al., 2020; Tay et al., 2020). TNF-α is a homotrimer that signals through TNF receptors (TNF-R) 1 and 2 and activates cellular stress response pathways (Wajant et al., 2003; Vanamee and Faustman, 2018). With a broad spectrum of activity, TNF-α may be regarded as a significant pro-inflammatory mediator of the innate immune system (for review, see Wajant et al., 2003 and Vanamee and Faustman, 2018). In brain homeostasis, glial TNF-α contributes to the regulation of synaptic connectivity (Stellwagen and Malenka, 2006). However, upregulation of brain TNF-α impairs synaptic plasticity, motor control, memory and induces microglial and astrocyte activation (Figueiredo et al., 2019; Souza et al., 2018; Cunningham et al., 1996; Habbas et al., 2015; Creed Pettigrew et al., 2016).
Under physiological conditions, IL-6 is present at low levels in the brain and has known roles in neurogenesis, gliogenesis, and regeneration of peripheral nerves (for a review, see Rothaug et al., 2016). IL-6 signaling requires two co-receptors, the IL-6-binding receptor (IL-6R) and the signal-transducing protein, glycoprotein 130 (gp130). Classical IL-6 signaling involves binding IL-6 to IL-6R followed by activation of gp130 and recruitment of Janus kinase (JAK) to initiate intracellular signaling (Rothaug et al., 2016; Erta et al., 2012). Interestingly, neurons do not appear to express relevant amounts of the membrane-bound IL-6R isoform; instead, they express a soluble isoform of the receptor (sIL-6R) (März et al., 1998), which binds circulating IL-6 and activates membrane-bound gp130. This process, known as trans-signaling (Rothaug et al., 2016; Erta et al., 2012), has been implicated in cognitive dysfunction caused by lipopolysaccharide (LPS) in aged mice (Burton and Johnson, 2012).
5. Inflammation as a potential driver of cognitive decline and depressive symptoms in COVID-19
Delirium and encephalopathy are commonly reported in COVID-19 hospitalized patients; however, estimates of the incidence of delirium and encephalopathy diverge largely among different cohorts (Chou et al., 2021; Helms et al., 2020b; Ellul et al., 2020). A retrospective cohort study of electronic health records of 236,379 COVID-19 survivors found that one-third of the patients developed neurological or psychiatric conditions within six months of infection (Taquet et al., 2021b; Mahase, 2021). Those rates were higher in severe COVID-19 cases (e.g., individuals admitted to intensive care units) and were higher for SARS-CoV-2 than for influenza infections. Post-mortem analysis of COVID-19 patient brains revealed local inflammation, microglial activation, immune cell infiltration, signs of hypoxia, infarcts, and microvascular damage (Crunfli et al., 2020; Song et al., 2021; Thakur et al., 2021; Yang et al., 2021; Lee et al., 2020). Additionally, COVID-19 patients show elevated blood and CSF biomarkers of inflammation, neuronal damage, and astrocytic activation, associated with neurological symptoms and disease severity (Sutter et al., 2020; Virhammar et al., 2021; Kanberg et al., 2020; Espíndola et al., 2021). Molecular and metabolic changes triggered by hypoxia and inflammation may induce encephalopathy and long-term neurological dysfunction independently of CNS infection in critically ill patients (Gofton and Bryan Young, 2012; Sasannejad et al., 2019; Solomon, 2021). Considering that recent findings do not support the presence of SARS-CoV-2 viral material in the brain as the primary source of COVID-19 neuroinflammation (Thakur et al., 2021; Yang et al., 2021), an alternative hypothesis implicates systemic factors as mediators of CNS pathology.
IL-6 and TNF-⍺ are critical mediators of the inflammatory response in COVID-19. Blood levels of IL-6 predict disease progression and correlate with COVID-19 severity and mortality (Santa Cruz et al., 2021; Zeng et al., 2020). Blood TNF-α is high in critically ill COVID-19 patients (Zeng et al., 2020). Evidence supporting the involvement of IL-6 and TNF-⍺ in the progression of COVID-19 has led to ongoing research aimed at inhibiting these cytokines as acute treatment options for severe disease cases (Rubin et al., 2021; Robinson et al., 2020; Angriman et al., 2021). In August 2021, the WHO announced a clinical trial to test three COVID-19 candidate drugs. One of them was Infliximab, a monoclonal antibody that blocks TNF-α signaling (World Health Organization, 2021) and found to alleviate cognitive deficits in mouse models of brain infection by Zika virus (Figueiredo et al., 2019) and of AD. (Lourenco et al., 2013).
While microglia and macrophages express ACE2, this protein is not abundantly expressed in most blood-derived immune cells (Song et al., 2020a), suggesting that other receptors, including toll-like receptors (TLRs), might be involved in the inflammatory response to SARS-CoV-2. Exposure to Spike protein in microglia, peripheral blood mononuclear cell, and macrophage cultures elicits TNF-⍺ and IL-6 production (Olajide et al., 2021, 2022; Zhao et al., 2021; Shirato and Kizaki, 2021). Interestingly, TLR4 blockage or knockdown decreases the overall immune response, including TNF-⍺ and IL-6 production caused by the Spike protein (Zhao et al., 2021; Shirato and Kizaki, 2021; Olajide et al., 2022), suggesting that TLR4 is a mediator of neuroinflammation promoted by SARS-CoV-2 (Fig. 1). TLR2 activation mediates cytokine production in HSE (Gnann and Whitley, 2017; Marcocci et al., 2020). Similarly, SARS-CoV-2's envelope may activate TLR2 and induce cytokine release (Zheng et al., 2021).
Elevated cytokine levels associated with systemic inflammation may also play a role in long-term brain damage in COVID-19. Mazza et al. (2021) prospectively evaluated psychological and cognitive status in a cohort of 226 COVID-19 survivors in Milan (Italy) for up to three months following hospital discharge. Remarkably, 78% of the survivors performed poorly in at least one cognitive domain, with the most significant impact on executive functions and motor coordination (Mazza et al., 2021). In addition, 36% of the patients self-reported psychopathological symptoms, including persistent depressive traits. Significantly, the persistence of depressive symptoms was associated with systemic inflammation biomarkers during acute infection and in the follow-up visit (Mazza et al., 2021).
Inflammation is a point of convergence between neurodegenerative diseases and mood disorders, particularly major depressive disorder (MDD) (van den Ameele et al., 2017; Johnson et al., 2017). MDD patients have upregulation of inflammatory pathways (Miller and Raison, 2015), and psychosocial stress can increase inflammatory response (Pace et al., 2006; Aschbacher et al., 2012). Investigation of the impact of inflammation on brain circuits involved in mood control has elucidated processes underlying resistance to conventional anti-depressants (Miller and Raison, 2015), and has inspired clinical trials using anti-inflammatory strategies to treat subgroups of depressive patients (Köhler et al., 2014). In animals, infections induce a stereotyped and conserved “sickness behavior”, characterized by lethargy, anorexia, anhedonia, and depressive-like behavior (Hart, 1988), which has been shown to result from elevated circulating cytokines (Klein et al., 2017; Dantzer et al., 2008). Although it is challenging to mimic the complexity of depression in rodents, work with animal models has helped increase our understanding of the role of pro-inflammatory molecules in MDD (Dantzer et al., 2008; Cheng et al., 2018; Konsman et al., 2008). For example, intracranial administration of IL-6 or TNF-⍺ triggers depressive-like behavior in mice (Sukoff Rizzo et al., 2012; Kaster et al., 2012), and TNF-⍺ blockade rescued memory impairment in a stress-induced rat model of depression (Şahin et al., 2015).
Inflammation is also thought to be a fundamental component of neurodegenerative processes (Heneka et al., 2015; Selles et al., 2018). Plasma TNF-⍺ and soluble TNF-α receptors are typically reported as upregulated in severe AD cases (Zuliani et al., 2007; Ledo et al., 2016; Swardfager et al., 2010; Bonotis et al., 2008; Baranowska-Bik et al., 2008; Álvarez et al., 2007; Lai et al., 2017), and elevated production of TNF-⍺ by peripheral blood mononuclear cells is associated with an increased risk of developing AD. (Tan et al., 2007) Circulating IL-6 levels are elevated in AD patients (Lai et al., 2017; Lyra e Silva et al., 2021), predict cognitive decline later in life (Singh-Manoux et al., 2014), and show a negative correlation with memory scores (Lai et al., 2017; Lyra e Silva et al., 2021). In vivo and in vitro studies show that both cytokines trigger stress response mechanisms and disrupt synaptic plasticity, memory formation, and hippocampal neurogenesis (Tancredi et al., 1992; Balschun et al., 2004; Monje et al., 2003). In mouse models of AD, blockage of TNF-α and IL-6 signaling pathways rescues memory (Lourenco et al., 2013; Lyra e Silva et al., 2021; Escrig et al., 2019). Collectively, data indicate that aberrant signaling by TNF-α and IL-6 disturbs memory-related mechanisms and promotes cognitive decline.
Systemic inflammation associated with infections contributes to persistent molecular alterations and functional deficits in the CNS. Sepsis survivors frequently experience neurological sequelae and present an increased risk for developing dementia (Kao et al., 2015; Iwashyna et al., 2010). In mice, sepsis enhances brain amyloid pathology, induces brain metabolic changes and TNF-⍺ upregulation, analogous to what is found in animal models of AD. (Neves et al., 2018; Frost et al., 2019; De Sousa et al., 2021; Basak et al., 2021) Furthermore, sepsis-surviving mice exhibit increased susceptibility to toxicity induced by soluble amyloid-β oligomers, with excessive neuroinflammatory responses and IL-6 upregulation (Frost et al., 2019; De Sousa et al., 2021). Aberrant microglial activation appears to play a central role in sepsis-induced late cognitive impairment (Frost et al., 2019; De Sousa et al., 2021). Similarly, infection by ZIKV induces synapse damage and persistent memory deficits via microglia-mediated synapse engulfment and elimination (Figueiredo et al., 2019). ZIKV infection also induces upregulation of TNF-α, which, in this case, appears to be upstream of microglial activation. Importantly, blockage of TNF-α signaling rescues acute and persistent brain pathology, as well as synaptic plasticity and memory in ZIKV-infected mice (Figueiredo et al., 2019; Souza et al., 2018).
In summary, TNF-α and IL-6 are two major cytokines upregulated in COVID-19 that directly affect brain physiology. Their roles in MDD and AD strongly suggest that these cytokines may drive dysfunctional stress-related responses, mood alterations, and cognitive impairments observed in COVID-19 survivors.
6. BBB dysfunction as a possible mediator of neurological damage in COVID-19
The brain is mainly protected from circulating inflammation via the selectivity of the BBB. Under certain pathological conditions, however, defective BBB function may expose the brain to molecules and cells involved in peripheral inflammation (Galea, 2021). For example, available evidence indicates that BBB alterations in AD allows brain infiltration by immune cells, increases permeability to molecular signals from the periphery, and exacerbates neuroinflammation (Nation et al., 2019; Sweeney et al., 2018). Sepsis and the associated cytokine storm also contribute to vascular damage, coagulopathy, and BBB instability (Cheng et al., 2018; Sharshar et al., 2007). In mice, BBB disruption and TNF-⍺ mediated inflammation contribute to depressive-like behavior (Cheng et al., 2018).
Intriguingly, COVID-19 patients with neurological symptoms frequently present anti-SARS-CoV-2 antibodies in their CSF (Alexopoulos et al., 2020; Garcia et al., 2021; Bernard-Valnet et al., 2021; Song et al., 2020b; Benameur et al., 2020). Possible explanations for this include BBB (Fig. 1) and/or blood-CSF barrier leakage. Although viral particles and RNA have not been detected consistently in CSF and brain tissue from patients (Espíndola et al., 2020; Thakur et al., 2021; Yang et al., 2021; Lee et al., 2020), SARS-CoV-2 spike protein was found to cross the BBB in a rodent model, and to induce functional changes in an in vitro model, possibly via a pro-inflammatory response from endothelial cells (Rhea et al., 2020; Buzhdygan et al., 2020). Consistent with this finding, CSF/serum albumin ratio measurement revealed BBB dysfunction in 40% of the individuals in a French cohort of COVID-19 patients with neurological manifestations (Lersy et al., 2020). On the other hand, elevated inflammatory markers in the CSF are verified only in a subset of COVID-19 patients with neurological symptoms, and are independent of circulating cytokine levels (Espíndola et al., 2021; Garcia et al., 2021; Bernard-Valnet et al., 2021).
7. Concluding remarks
Understanding the neurological consequences of COVID-19 poses several challenges. First, substantial variability is observed regarding the types and frequencies of neurological symptoms. Moreover, the biological impact of COVID-19 on mental health may be confounded by adverse effects associated with social isolation, job/financial insecurity, and other social impacts of the pandemic. From a molecular/cellular standpoint, post-mortem brain analysis is generally limited to severe cases of COVID-19, and pathogenic mechanisms revealed in such studies may differ significantly from mechanisms operating in the brains of COVID-19 survivors. As the COVID-19 pandemic continues to ravage the world in its third year, leaving an unprecedented number of infected survivors, possible late neurological outcomes pose a significant future threat to patients and public health systems. Hopefully, shared efforts by scientists from multiple disciplines to tackle this problem and to anticipate late neurological outcomes of COVID-19 will contribute to a better understanding of disease mechanisms so that treatments can be suggested for this emerging global health problem.
Funding
Work in the authors' laboratories has been supported by grants from Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), National Institute for Translational Neurosciences (INNT/Brazil) and Canadian Institutes of Health Research (CIHR). The sponsors had no involvement in the article's design, writing, or decision to submit it for publication.
Authors contributions
Natalia M. Lyra e Silva: Writing-Original draft preparation, Writing-Reviewing, and Editing, Figure preparation. Fernanda G. Q. Barros-Aragão: Writing-Original draft preparation, Writing-Reviewing, and Editing. Fernanda G. De Felice: Writing-Reviewing and Editing. Sergio T. Ferreira: Writing-Reviewing and Editing.
Declaration of competing interest
The authors have nothing to disclose.
References
- Alenina N., Bader M. ACE2 in brain physiology and pathophysiology: evidence from transgenic animal models. Neurochem. Res. 2019;44:1323–1329. doi: 10.1007/s11064-018-2679-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos H., Magira E., Bitzogli K., et al. Anti-SARS-CoV-2 antibodies in the CSF, blood-brain barrier dysfunction, and neurological outcome: studies in 8 stuporous and comatose patients. Neurology: Neuroimmunol. Neuroinflammation. 2020;7 doi: 10.1212/NXI.0000000000000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Álvarez A., Cacabelos R., Sanpedro C., García-Fantini M., Aleixandre M. Serum TNF-alpha levels are increased and correlate negatively with free IGF-I in Alzheimer disease. Neurobiol. Aging. 2007;28:533–536. doi: 10.1016/j.neurobiolaging.2006.02.012. [DOI] [PubMed] [Google Scholar]
- van den Ameele S., Coppens V., Schuermans J., et al. Neurotrophic and inflammatory markers in bipolar disorder: a prospective study. Psychoneuroendocrinology. 2017;84:143–150. doi: 10.1016/j.psyneuen.2017.07.003. [DOI] [PubMed] [Google Scholar]
- Angriman F., Ferreyo B., Burry L., Fan E. Interleukin-6 receptor blockade in patients with COVID-19: placing clinical trials into context. Lancet Respir. Med. 2021;9:655–664. doi: 10.1016/S2213-2600(21)00139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschbacher K., Epel E., Wolkowitz O.M., Prather A.A., Puterman E., Dhabhar F.S. Maintenance of a positive outlook during acute stress protects against pro-inflammatory reactivity and future depressive symptoms. Brain Behav. Immun. 2012;26:346–352. doi: 10.1016/j.bbi.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balschun D., Wetzel W., Rey A., et al. Interleukin‐6: a cytokine to forget. Faseb. J. 2004;18:1788–1790. doi: 10.1096/fj.04-1625fje. [DOI] [PubMed] [Google Scholar]
- Baranowska-Bik A., Bik W., Wolinska-Witort E., et al. Plasma beta amyloid and cytokine profile in women with Alzheimer's disease. Neuroendocrinol. Lett. 2008;29:75–79. [PubMed] [Google Scholar]
- Basak J.M., Ferreiro A., Cohen L.S., et al. Bacterial sepsis increases hippocampal fibrillar amyloid plaque load and neuroinflammation in a mouse model of Alzheimer's disease. Neurobiol. Dis. 2021:152. doi: 10.1016/j.nbd.2021.105292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista A.F., Forny-Germano L., Clarke J.R., et al. The diabetes drug liraglutide reverses cognitive impairment in mice and attenuates insulin receptor and synaptic pathology in a non-human primate model of Alzheimer's disease. J. Pathol. 2018;245:85–100. doi: 10.1002/path.5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benameur K., Agarwal A., Auld S.C., et al. Encephalopathy and encephalitis associated with cerebrospinal fluid cytokine alterations and coronavirus disease, Atlanta, Georgia, USA, 2020. Emerg. Infect. Dis. 2020;26:2016. doi: 10.3201/eid2609.202122. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benussi A., Pilotto A., Premi E., et al. Clinical characteristics and outcomes of inpatients with neurologic disease and COVID-19 in Brescia, Lombardy, Italy. American Academy of Neurology. 2020 doi: 10.1212/WNL.0000000000009848. [DOI] [PubMed] [Google Scholar]
- Bernard-Valnet R., Perriot S., Canales M., et al. Encephalopathies Associated With Severe COVID-19 Present Neurovascular Unit Alterations Without Evidence for Strong Neuroinflammation. Neurol. Neuroimmunol. Neuroinflamm. 2021;8(5):e1029. doi: 10.1212/NXI.0000000000001029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornevik K., Cortese M., Healy B.C., et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science (New York, N.Y.) 2022;375:296–301. doi: 10.1126/science.abj8222. [DOI] [PubMed] [Google Scholar]
- Bonotis K., Krikki E., Holeva V., Aggouridaki C., Costa V., Baloyannis S. Systemic immune aberrations in Alzheimer's disease patients. J. Neuroimmunol. 2008;193:183–187. doi: 10.1016/j.jneuroim.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Brann D.H., Tsukahara T., Weinreb C., et al. Non-neural expression of SARS-CoV-2 entry genes in the olfactory epithelium suggests mechanisms underlying anosmia COVID-19-associated anosmia. Sci. Adv. 2020;6(31):eabc5801. doi: 10.1126/sciadv.abc5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton M.D., Johnson R.W. Interleukin-6 trans-signaling in the senescent mouse brain is involved in infection-related deficits in contextual fear conditioning. Brain Behav. Immun. 2012;26:732–738. doi: 10.1016/j.bbi.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butowt R., Meunier N., Bryche B., von Bartheld C.S. The olfactory nerve is not a likely route to brain infection in COVID-19: a critical review of data from humans and animal models. Acta Neuropathol. 2021;141:809–822. doi: 10.1007/s00401-021-02314-2. 2021 141:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzhdygan T.P., DeOre B.J., Baldwin-Leclair A., et al. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood–brain barrier. Neurobiol. Dis. 2020:146. doi: 10.1016/j.nbd.2020.105131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Wu D., Chen H., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020:368. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Shen W., Rowan N.R., et al. Elevated ACE-2 expression in the olfactory neuroepithelium: implications for anosmia and upper respiratory SARS-CoV-2 entry and replication. Eur. Respir. J. 2020;56 doi: 10.1183/13993003.01948-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Desse S., Martinez A., Worthen R.J., Jope R.S., Beurel E. TNFα disrupts blood brain barrier integrity to maintain prolonged depressive-like behavior in mice. Brain Behav. Immun. 2018;69:556–567. doi: 10.1016/j.bbi.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S.H.-Y., Beghi E., Helbok R., et al. Global incidence of neurological manifestations among patients hospitalized with COVID-19-A report for the GCS-NeuroCOVID consortium and the ENERGY consortium. JAMA Neurol. 2021;4 doi: 10.1001/jamanetworkopen.2021.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper K.W., Brann D.H., Farruggia M.C., et al. COVID-19 and the chemical senses: supporting players take center stage. Neuron. 2020;107:219–233. doi: 10.1016/j.neuron.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nature Microbiology. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed Pettigrew L., Kryscio R.J., Norris C.M. 2016. The TNFα-Transgenic Rat: Hippocampal Synaptic Integrity, Cognition, Function, and Post-Ischemic Cell Loss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunfli F., Corasolla Carregari V., Protasio Veras F., et al. SARS-CoV-2 infects brain astrocytes of COVID-19 patients and impairs neuronal viability. medRxiv. 2020:19. 10.09.2020. [Google Scholar]
- Cunningham A.J., Murray C.A., O'Neill L.A.J., Lynch M.A., O'Connor J.J. Interleukin-1β (IL-1β) and tumour necrosis factor (TNF) inhibit long-term potentiation in the rat dentate gyrus in vitro. Neurosci. Lett. 1996;203:17–20. doi: 10.1016/0304-3940(95)12252-4. [DOI] [PubMed] [Google Scholar]
- Cusick M.F., Libbey J.E., Patel D.C., Doty D.J., Fujinami R.S. Infiltrating macrophages are key to the development of seizures following virus infection. J. Virol. 2013;87:1849–1860. doi: 10.1128/JVI.02747-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R., O'Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desforges M., Le Coupanec A., Dubeau P., et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12 doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., He L., Zhang Q., et al. Organ distribution of severe acute respiratory syndrome(SARS) associated coronavirus(SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J. Pathol. 2004;203:622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Arcangelo G., Tancredi V., Onofri F., D'Antuono M., Giovedì S., Benfenati F. Interleukin-6 inhibits neurotransmitter release and the spread of excitation in the rat cerebral cortex. Eur. J. Neurosci. 2000;12:1241–1252. doi: 10.1046/j.1460-9568.2000.00011.x. [DOI] [PubMed] [Google Scholar]
- Ellul M.A., Benjamin L., Singh B., et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19:767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erta M., Quintana A., Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int. J. Biol. Sci. 2012;8:1254–1266. doi: 10.7150/ijbs.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escartin C., Galea E., Lakatos A., et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021;24:312–325. doi: 10.1038/s41593-020-00783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escrig A., Canal C., Sanchis P., et al. IL-6 trans-signaling in the brain influences the behavioral and physio-pathological phenotype of the Tg2576 and 3xTgAD mouse models of Alzheimer's disease. Brain Behav. Immun. 2019;82:145–159. doi: 10.1016/j.bbi.2019.08.005. [DOI] [PubMed] [Google Scholar]
- Espíndola O., Siqueira M., Soares C., et al. Patients with COVID-19 and neurological manifestations show undetectable SARS-CoV-2 RNA levels in the cerebrospinal fluid. Int. J. Infect. Dis. 2020;96:567–569. doi: 10.1016/j.ijid.2020.05.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espíndola O.M., Gomes Y.C.P., Brandão C.O., et al. Inflammatory cytokine patterns associated with neurological diseases in COVID-19. Ann. Neurol. 2021 doi: 10.1002/ana.26041. 00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Felice F.G., Tovar-Moll F., Moll J., Munoz D.P., Ferreira S.T. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the central nervous system. Trends Neurosci. 2020 doi: 10.1016/j.tins.2020.04.004. published online April. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo C.P., Barros-Aragão F.G.Q., Neris R.L.S., et al. Zika virus replicates in adult human brain tissue and impairs synapses and memory in mice. Nat. Commun. 2019;10 doi: 10.1038/s41467-019-11866-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost P.S., Barros-Aragão F., da Silva R.T., et al. Neonatal infection leads to increased susceptibility to Aβ oligomer-induced brain inflammation, synapse loss and cognitive impairment in mice. Cell Death Dis. 2019;10:323. doi: 10.1038/s41419-019-1529-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea I. The blood–brain barrier in systemic infection and inflammation. Cell. Mol. Immunol. 2021;18:2489–2501. doi: 10.1038/s41423-021-00757-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber C., Soung A., Vollmer L.L., et al. T cells promote microglia-mediated synaptic elimination and cognitive dysfunction during recovery from neuropathogenic flaviviruses. Nat. Neurosci. 2019;22:1276–1288. doi: 10.1038/s41593-019-0427-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M.A., Barreras P.V., Lewis A., et al. Cerebrospinal fluid in COVID-19 neurological complications: no cytokine storm or neuroinflammation. medRxiv. 2021 doi: 10.1101/2021.01.10.20249014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnann J.W., Whitley R.J. Herpes simplex encephalitis: an update. Curr. Infect. Dis. Rep. 2017;19 doi: 10.1007/S11908-017-0568-7. [DOI] [PubMed] [Google Scholar]
- Gofton T.E., Bryan Young G. Sepsis-associated encephalopathy. Nat. Rev. Neurol. 2012;8:557–566. doi: 10.1038/nrneurol.2012.183. [DOI] [PubMed] [Google Scholar]
- Gu J., Gong E., Zhang B., et al. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 2005;202:415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habbas S., Santello M., Becker D., et al. Neuroinflammatory TNFα impairs memory via astrocyte signaling. Cell. 2015;163:1730–1741. doi: 10.1016/j.cell.2015.11.023. [DOI] [PubMed] [Google Scholar]
- Hart B.L. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H., et al. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020 doi: 10.1056/nejmc2008597. published online April 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H., et al. Delirium and encephalopathy in severe COVID-19: a cohort analysis of ICU patients. Crit. Care. 2020;24:491. doi: 10.1186/s13054-020-03200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemachudha T., Ugolini G., Wacharapluesadee S., Sungkarat W., Shuangshoti S., Laothamatas J. Human rabies: neuropathogenesis, diagnosis, and management. Lancet Neurol. 2013;12:498–513. doi: 10.1016/S1474-4422(13)70038-3. [DOI] [PubMed] [Google Scholar]
- Heneka M.T., Carson M.J., Khoury J El, et al. Neuroinflammation in Alzheimer's disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman S., Izzy S., Sen P., Morsett L., El Khoury J. Microglia in neurodegeneration. Nat. Neurosci. 2018;21:1359–1369. doi: 10.1038/s41593-018-0242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini S., Michaelsen-Preusse K., Grigoryan G., Chhatbar C., Kalinke U., Korte M. Type I interferon receptor signaling in astrocytes regulates hippocampal synaptic plasticity and cognitive function of the healthy CNS. Cell Rep. 2020:31. doi: 10.1016/J.CELREP.2020.107666. [DOI] [PubMed] [Google Scholar]
- Iwashyna T.J., Ely E.W., Smith D.M., Langa K.M. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L.A., Edwards M., Gamboa A., Hall J., Robinson M., O'Bryant S.E. Depression, inflammation, and memory loss among Mexican Americans: analysis of the HABLE cohort. Int. Psychogeriatr. 2017;29:1693–1699. doi: 10.1017/S1041610217001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanberg N., Ashton N.J., Andersson L.-M., et al. Neurochemical evidence of astrocytic and neuronal injury commonly found in COVID-19. Neurology. 2020 doi: 10.1212/WNL.0000000000010111. [DOI] [PubMed] [Google Scholar]
- Kao L.T., Sheu J.J., Lin H.C., Tsai M.C., Chung S.D. Association between sepsis and dementia. J. Clin. Neurosci. 2015;22:1430–1433. doi: 10.1016/j.jocn.2015.02.035. [DOI] [PubMed] [Google Scholar]
- Kaster M.P., Gadotti V.M., Calixto J.B., Santos A.R.S., Rodrigues A.L.S. Neuropharmacology. Pergamon; 2012. Depressive-like behavior induced by tumor necrosis factor-α in mice; pp. 419–426. [DOI] [PubMed] [Google Scholar]
- Klein R.S., Garber C., Howard N. Infectious immunity in the central nervous system and brain function. Nat. Immunol. 2017;18:132–141. doi: 10.1038/ni.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenstein M., Klingenstein S., Neckel P.H., et al. Evidence of SARS-CoV2 entry protein ACE2 in the human nose and olfactory bulb. Cells Tissues Organs. 2020;209:155–164. doi: 10.1159/000513040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler O., Benros M.E., Nordentoft M., et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatr. 2014;71:1381–1391. doi: 10.1001/jamapsychiatry.2014.1611. [DOI] [PubMed] [Google Scholar]
- Konsman J.P., Veeneman J., Combe C., Poole S., Luheshi G.N., Dantzer R. Central nervous action of interleukin-1 mediates activation of limbic structures and behavioural depression in response to peripheral administration of bacterial lipopolysaccharide. Eur. J. Neurosci. 2008;28:2499–2510. doi: 10.1111/j.1460-9568.2008.06549.x. [DOI] [PubMed] [Google Scholar]
- Lai K.S.P., Liu C.S., Rau A., et al. Peripheral inflammatory markers in Alzheimer's disease: a systematic review and meta-analysis of 175 studies. J. Neurol. Neurosurg. Psychiatr. 2017;88:876–882. doi: 10.1136/jnnp-2017-316201. [DOI] [PubMed] [Google Scholar]
- Lau K.K., Yu W.C., Chu C.M., Lau S.T., Sheng B., Yuen K.Y. Possible central nervous system infection by SARS coronavirus. Emerg. Infect. Dis. 2004;10:342–344. doi: 10.3201/eid1002.030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechien J.R., Chiesa-Estomba C.M., De Siati D.R., et al. European Archives of Oto-Rhino-Laryngology; 2020. Olfactory and Gustatory Dysfunctions as a Clinical Presentation of Mild-To-Moderate Forms of the Coronavirus Disease (COVID-19): a Multicenter European Study; pp. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledo J.H., Azevedo E.P., Clarke J.R., et al. Amyloid-β oligomers link depressive-like behavior and cognitive deficits in mice. Mol. Psychiatr. 2013;18:1053–1054. doi: 10.1038/mp.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledo J.H., Azevedo E.P., Beckman D., et al. Cross talk between brain innate immunity and serotonin signaling underlies depressive-like behavior induced by Alzheimer's amyloid-β oligomers in mice. J. Neurosci. 2016;36:12106–12116. doi: 10.1523/JNEUROSCI.1269-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.-H., Perl D.P., Nair G., et al. Microvascular injury in the brains of patients with covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2033369. published online Dec 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lersy F., Benotmane I., Helms J., et al. Cerebrospinal fluid features in COVID-19 patients with neurologic manifestations: correlation with brain MRI findings in 58 patients. J. Infect. Dis. 2020 doi: 10.1093/infdis/jiaa745. published online Nov 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A.J., Katafuchi T., Oda S., Hori T., Oomura Y. Interleukin-6 inhibits long-term potentiation in rat hippocampal slices. Brain Res. 1997;748:30–38. doi: 10.1016/s0006-8993(96)01283-8. [DOI] [PubMed] [Google Scholar]
- Li Y., Bai W., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020;92(6):552–555. doi: 10.1002/jmv.25728. jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow S.A., Barres B.A. Reactive astrocytes: production, function, and therapeutic potential. Immunity. 2017;46:957–967. doi: 10.1016/j.immuni.2017.06.006. [DOI] [PubMed] [Google Scholar]
- Liddelow S.A., Guttenplan K.A., Clarke L.E., et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Li Y., Liu Q., et al. SARS-CoV-2 cell tropism and multiorgan infection. Cell Discovery. 2021;7:1–4. doi: 10.1038/s41421-021-00249-2. 2021 7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenco M.V., Clarke J.R., Frozza R.L., et al. TNF-α mediates PKR-dependent memory impairment and brain IRS-1 inhibition induced by Alzheimer's β-amyloid oligomers in mice and monkeys. Cell Metabol. 2013;18:831–843. doi: 10.1016/j.cmet.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Lucas C., Wong P., Klein J., et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584:463–469. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyra e Silva N.M., Gonçalves R.A., Pascoal T.A., et al. Pro-inflammatory interleukin-6 signaling links cognitive impairments and peripheral metabolic alterations in Alzheimer's disease. Transl. Psychiatry. 2021;11:251. doi: 10.1038/s41398-021-01349-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahase E. Covid-19: one in three has neurological or psychiatric condition diagnosed after covid infection, study finds. BMJ. 2021;373:n908. doi: 10.1136/bmj.n908. [DOI] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcocci M.E., Napoletani G., Protto V., et al. Herpes simplex virus-1 in the brain: the dark side of a sneaky infection. Trends Microbiol. 2020;28:808–820. doi: 10.1016/j.tim.2020.03.003. [DOI] [PubMed] [Google Scholar]
- Mariano G., Farthing R.J., Lale-Farjat S.L.M., Bergeron J.R.C. Structural characterization of SARS-CoV-2: where we are, and where we need to Be. Frontiers in Molecular Biosciences. 2020;7:344. doi: 10.3389/fmolb.2020.605236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- März P., Cheng J.G., Gadient R.A., et al. Sympathetic neurons can produce and respond to interleukin 6. Proc. Natl. Acad. Sci. Unit. States Am. 1998;95:3251–3256. doi: 10.1073/pnas.95.6.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias I., Morgado J., Gomes F.C.A. Astrocyte heterogeneity: impact to brain aging and disease. Front. Aging Neurosci. 2019;11 doi: 10.3389/FNAGI.2019.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matschke J., Lütgehetmann M., Hagel C., et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19:919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximova O.A., Pletnev A.G. Flaviviruses and the central nervous system: revisiting neuropathological concepts. Annual Review of Virology. 2018;5:255–272. doi: 10.1146/annurev-virology-092917-043439. [DOI] [PubMed] [Google Scholar]
- Mazza M.G., Mariagrazia P., de Lorenzo R., et al. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: effect of inflammatory biomarkers at three-month follow-up. Brain Behav. Immun. 2021 doi: 10.1016/j.bbi.2021.02.021. published online Feb 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt J., Radke J., Dittmayer C., et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021;24:168–175. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- Miller A.H., Raison C.L. 2015. The Role of Inflammation in Depression: from Evolutionary Imperative to Modern Treatment Target. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje M.L., Toda H., Palmer T.D. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Morfopoulou S., Brown J.R., Davies E.G., et al. Human coronavirus OC43 associated with fatal encephalitis. N. Engl. J. Med. 2016;375:497–498. doi: 10.1056/NEJMc1509458. [DOI] [PubMed] [Google Scholar]
- Nath A. Long-haul COVID. Neurology. 2020;95:559–560. doi: 10.1212/WNL.0000000000010640. [DOI] [PubMed] [Google Scholar]
- Nation D.A., Sweeney M.D., Montagne A., et al. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 2019;25:270–276. doi: 10.1038/s41591-018-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemani K., Li C., Olfson M., et al. Association of psychiatric disorders with mortality among patients with COVID-19. JAMA Psychiatr. 2021;78:380–386. doi: 10.1001/jamapsychiatry.2020.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves F.S., Marques P.T., Barros-Aragão F., et al. Brain-defective insulin signaling is associated to late cognitive impairment in post-septic mice. Mol. Neurobiol. 2018;55:435–444. doi: 10.1007/s12035-016-0307-3. [DOI] [PubMed] [Google Scholar]
- Nilsson A., Edner N., Albert J., Ternhag A. Infectious Diseases Fatal encephalitis associated with coronavirus OC43 in an immunocompromised child. Infectious Diseases. 2020;52:419–422. doi: 10.1080/23744235.2020.1729403. [DOI] [PubMed] [Google Scholar]
- Olajide O.A., Iwuanyanwu V.U., Lepiarz-Raba I., Al-Hindawi A.A. Induction of exaggerated cytokine production in human peripheral blood mononuclear cells by a recombinant SARS-CoV-2 spike glycoprotein S1 and its inhibition by dexamethasone. Inflammation. 2021;1–13 doi: 10.1007/s10753-021-01464-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olajide O.A., Iwuanyanwu V.U., Adegbola O.D., Al-Hindawi A.A. SARS-CoV-2 spike glycoprotein S1 induces neuroinflammation in BV-2 microglia. Mol. Neurobiol. 2022;59:445–458. doi: 10.1007/s12035-021-02593-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxley T.J., Mocco J., Majidi S., et al. Large-vessel stroke as a presenting feature of covid-19 in the Young. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace T.W.W., Mletzko T.C., Alagbe O., et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am. J. Psychiatr. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Pedrosa C. da SG., Goto-Silva L., Temerozo J.R., et al. Non-permissive SARS-CoV-2 infection in human neurospheres. Stem Cell Res. 2021;54 doi: 10.1016/J.SCR.2021.102436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzini A., Padovani A. Lifting the mask on neurological manifestations of COVID-19. Nat. Rev. Neurol. 2020;16:636–644. doi: 10.1038/s41582-020-0398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol A.N. Viral infection leading to brain dysfunction: more prevalent than appreciated? Neuron. 2009;64:17–20. doi: 10.1016/j.neuron.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19–associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020 doi: 10.1148/radiol.2020201187. published online March 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puelles V.G., Lütgehetmann M., Lindenmeyer M.T., et al. Multiorgan and renal tropism of SARS-CoV-2. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2011400. published online May 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani A., Müller L., Ostermann P.N., et al. SARS‐CoV‐2 targets neurons of 3D human brain organoids. EMBO J. 2020;39 doi: 10.15252/embj.2020106230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani A., Pranty A.-I., Gopalakrishnan J. Neurotropic effects of SARS-CoV-2 modeled by the human brain organoids. Stem Cell Reports. 2021;16:373–384. doi: 10.1016/j.stemcr.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhea E.M., Logsdon A.F., Hansen K.M., et al. The S1 protein of SARS-CoV-2 crosses the blood–brain barrier in mice. Nat. Neurosci. 2020 doi: 10.1038/s41593-020-00771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson P.C., Richards D., Tanner H.L., Feldmann M. Accumulating evidence suggests anti-TNF therapy needs to be given trial priority in COVID-19 treatment. The Lancet Rheumatology. 2020;2:e653–e655. doi: 10.1016/S2665-9913(20)30309-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnolo A., Balestrino R., Imbalzano G., et al. Neurological comorbidity and severity of COVID-19. J. Neurol. 2021;268:762–769. doi: 10.1007/s00415-020-10123-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Sánchez C.M., Díaz-Maroto I., Fernández-Díaz E., et al. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020 doi: 10.1212/wnl.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross F.M., Allan S.M., Rothwell N.J., Verkhratsky A. A dual role for interleukin-1 in LTP in mouse hippocampal slices. J. Neuroimmunol. 2003;144:61–67. doi: 10.1016/j.jneuroim.2003.08.030. [DOI] [PubMed] [Google Scholar]
- Rothaug M., Becker-Pauly C., Rose-John S. The role of interleukin-6 signaling in nervous tissue. Biochim. Biophys. Acta. 2016;1863:1218–1227. doi: 10.1016/j.bbamcr.2016.03.018. [DOI] [PubMed] [Google Scholar]
- Rubbenstroth D., Schlottau K., Schwemmle M., Rissland J., Beer M. Human bornavirus research: back on track. PLoS Pathog. 2019;15 doi: 10.1371/journal.ppat.1007873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin E.J., Longo D.L., Baden L.R. Interleukin-6 receptor inhibition in covid-19 - cooling the inflammatory soup. N. Engl. J. Med. 2021 doi: 10.1056/NEJMe2103108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Şahin T.D., Karson A., Balci F., Yazir Y., Bayramgürler D., Utkan T. TNF-alpha inhibition prevents cognitive decline and maintains hippocampal BDNF levels in the unpredictable chronic mild stress rat model of depression. Behav. Brain Res. 2015;292:233–240. doi: 10.1016/j.bbr.2015.05.062. [DOI] [PubMed] [Google Scholar]
- Santa Cruz A., Mendes-Frias A., Oliveira A.I., et al. Interleukin-6 is a biomarker for the development of fatal severe acute respiratory syndrome coronavirus 2 pneumonia. Front. Immunol. 2021:12. doi: 10.3389/FIMMU.2021.613422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasannejad C., Ely E.W., Lahiri S. Long-term cognitive impairment after acute respiratory distress syndrome: a review of clinical impact and pathophysiological mechanisms. Crit. Care. 2019;23:1–12. doi: 10.1186/s13054-019-2626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor D., Dickens A.M., Sacktor N., et al. HIV-associated neurocognitive disorder — pathogenesis and prospects for treatment. Nat. Rev. Neurol. 2016;12:234–248. doi: 10.1038/nrneurol.2016.27. 2016 12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selles M.C., Oliveira M.M., Ferreira S.T. Brain inflammation connects cognitive and non-cognitive symptoms in Alzheimer's disease. J. Alzheim. Dis. 2018;64:S313–S327. doi: 10.3233/JAD-179925. [DOI] [PubMed] [Google Scholar]
- Sharshar T., Carlier R., Bernard F., et al. Brain lesions in septic shock: a magnetic resonance imaging study. Intensive Care Med. 2007;33:798–806. doi: 10.1007/s00134-007-0598-y. [DOI] [PubMed] [Google Scholar]
- Shirato K., Kizaki T. SARS-CoV-2 spike protein S1 subunit induces pro-inflammatory responses via toll-like receptor 4 signaling in murine and human macrophages. Heliyon. 2021;7 doi: 10.1016/J.HELIYON.2021.E06187/ATTACHMENT/0EE7C716-C2F3-443E-AEB0-DB4C34CE1A85/MMC2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Manoux A., Dugravot A., Brunner E., et al. Interleukin-6 and C-reactive protein as predictors of cognitive decline in late midlife. Neurology. 2014;83:486–493. doi: 10.1212/WNL.0000000000000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon T. Neurological infection with SARS-CoV-2 — the story so far. Nat. Rev. Neurol. 2021;17:65–66. doi: 10.1038/s41582-020-00453-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X., Hu W., Yu H., et al. Little to no expression of angiotensin-converting enzyme-2 on most human peripheral blood immune cells but highly expressed on tissue macrophages. Cytometry. 2020 doi: 10.1002/CYTO.A.24285. [DOI] [PubMed] [Google Scholar]
- Song E., Bartley C.M., Chow R.D., et al. Divergent and self-reactive immune responses in the CNS of COVID-19 patients with neurological symptoms. Cell Rep Med. 2021;2(5):100288. doi: 10.1016/j.xcrm.2021.100288. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E., Zhang C., Israelow B., et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J. Exp. Med. 2021:218. doi: 10.1084/jem.20202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soung A., Klein R.S. Viral encephalitis and neurologic diseases: focus on astrocytes. Trends Mol. Med. 2018;24:950–962. doi: 10.1016/j.molmed.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sousa V.L., Araújo S.B., Antonio L.M., et al. Innate immune memory mediates increased susceptibility to Alzheimeŕs disease-like pathology in sepsis surviving mice. Brain Behav. Immun. 2021 doi: 10.1016/j.bbi.2021.04.001. published online April. [DOI] [PubMed] [Google Scholar]
- Souza IN. de O., Frost P.S., França J v, et al. Acute and chronic neurological consequences of early-life Zika virus infection in mice. Sci. Transl. Med. 2018;10:eaar2749. doi: 10.1126/scitranslmed.aar2749. [DOI] [PubMed] [Google Scholar]
- Souza I.N.O., Barros-Aragão F.G.Q., Frost P.S., Figueiredo C.P., Clarke J.R. Late neurological consequences of Zika virus infection: risk factors and pharmaceutical approaches. Pharmaceuticals. 2019;12 doi: 10.3390/ph12020060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D., Malenka R.C. Synaptic scaling mediated by glial TNF-α. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Sukoff Rizzo S.J., Neal S.J., Hughes Z.A., et al. Evidence for sustained elevation of IL-6 in the CNS as a key contributor of depressive-like phenotypes. Transl. Psychiatry. 2012;2 doi: 10.1038/tp.2012.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter R., Hert L., De Marchis G.M., et al. Serum neurofilament light chain levels in the intensive care unit: comparison between severely ill patients with and without Coronavirus Disease 2019. Ann. Neurol. 2020;89:610–616. doi: 10.1002/ana.26004. [DOI] [PubMed] [Google Scholar]
- Swardfager W., Lanctot K., Rothenburg L., Wong A., Cappell J., Herrmann N. A meta-analysis of cytokines in Alzheimer's disease. Biol. Psychiatr. 2010;68:930–941. doi: 10.1016/j.biopsych.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Sweeney M.D., Sagare A.P., Zlokovic B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018;14:133–150. doi: 10.1038/nrneurol.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahira A.C., Verjovski‐Almeida S., Ferreira S.T. Dementia is an age‐independent risk factor for severity and death in COVID‐19 inpatients. Alzheimer's Dementia. 2021:1–14. doi: 10.1002/alz.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Z.S., Beiser A.S., Vasan R.S., et al. Inflammatory markers and the risk of Alzheimer disease. Neurology. 2007;68:1902–1908. doi: 10.1212/01.wnl.0000263217.36439.da. [DOI] [PubMed] [Google Scholar]
- Tancredi V., D'Arcangelo G., Grassi F., et al. Tumor necrosis factor alters synaptic transmission in rat hippocampal slices. Neurosci. Lett. 1992;146:176–178. doi: 10.1016/0304-3940(92)90071-e. [DOI] [PubMed] [Google Scholar]
- Taquet M., Luciano S., Geddes J.R., Harrison P.J. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatr. 2021;8:130–140. doi: 10.1016/S2215-0366(20)30462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taquet M., Geddes J.R., Husain M., Luciano S., Harrison P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatr. 2021 doi: 10.1016/S2215-0366(21)00084-5. published online April 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur K.T., Miller E.H., Glendinning M.D., et al. COVID-19 neuropathology at columbia university irving medical center/New York presbyterian hospital. Brain. 2021 doi: 10.1093/BRAIN/AWAB148. published online April 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai L.-K., Hsieh S.-T., Chang Y.-C. Neurological manifestations in severe acute respiratory syndrome. Acta Neurol. Taiwan. 2005;14:113–119. [PubMed] [Google Scholar]
- Turtle L., Solomon T. Japanese encephalitis — the prospects for new treatments. Nat. Rev. Neurol. 2018;14:298–313. doi: 10.1038/nrneurol.2018.30. 2018 14:5. [DOI] [PubMed] [Google Scholar]
- Vanamee É.S., Faustman D.L. Structural principles of tumor necrosis factor superfamily signaling. Sci. Signal. 2018;11:4910. doi: 10.1126/scisignal.aao4910. [DOI] [PubMed] [Google Scholar]
- Vasek M.J., Garber C., Dorsey D., et al. A complement–microglial axis drives synapse loss during virus-induced memory impairment. Nature. 2016;534:538–543. doi: 10.1038/nature18283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virhammar J., Nääs A., Fällmar D., et al. Biomarkers for CNS injury in CSF are elevated in COVID‐19 and associated with neurological symptoms and disease severity. Eur. J. Neurol. 2021;(28):3324–3331. doi: 10.1111/ene.14703. ene.14703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadman M., Couzin-Frankel J., Kaiser J., Matacic C. How does coronavirus kill? Clinicians trace a ferocious rampage through the body, from brain to toes. Science. 2020 doi: 10.1126/science.abc3208. published online April 17. [DOI] [PubMed] [Google Scholar]
- Wajant H., Pfizenmaier K., Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- Webb B.J., Peltan I.D., Jensen P., et al. Clinical criteria for COVID-19-associated hyperinflammatory syndrome: a cohort study. The Lancet Rheumatology. 2020;2:e754–e763. doi: 10.1016/S2665-9913(20)30343-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley R.J., Roizman B. Herpes simplex virus infections. Lancet. 2001;357:1513–1518. doi: 10.1016/S0140-6736(00)04638-9. [DOI] [PubMed] [Google Scholar]
- World Health Organization W. Solidarity trial plus: an international randomized trial of additional treatments for COVID-19 in hospitalized patients who are all receiving the local standard of care. ISRCTN. 2021 doi: 10.1186/ISRCTN18066414. [DOI] [Google Scholar]
- Wynn T.A., Chawla A., Pollard J.W. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie D., He M., Hu X. Microglia/macrophage diversities in central nervous system physiology and pathology. CNS Neurosci. Ther. 2019;25:1287–1289. doi: 10.1111/cns.13257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Zhong S., Liu J., et al. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin. Infect. Dis. 2005;41:1089–1096. doi: 10.1086/444461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A.C., Kern F., Losada P.M., et al. Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature. 2021;595:565–571. doi: 10.1038/s41586-021-03710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z., Yu H., Chen H., et al. Longitudinal changes of inflammatory parameters and their correlation with disease severity and outcomes in patients with COVID-19 from Wuhan, China. Crit. Care. 2020;1(24):1–12. doi: 10.1186/s13054-020-03255-0. 2020 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B.Z., Chu H., Han S., et al. SARS-CoV-2 infects human neural progenitor cells and brain organoids. Cell Res. 2020;30:928–931. doi: 10.1038/s41422-020-0390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Kuang M., Li J., et al. SARS-CoV-2 spike protein interacts with and activates TLR41. Cell Res. 2021;31:818–820. doi: 10.1038/s41422-021-00495-9. 2021 31:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M., Karki R., Williams E.P., et al. TLR2 senses the SARS-CoV-2 envelope protein to produce inflammatory cytokines. Nat. Immunol. 2021;22:829–838. doi: 10.1038/s41590-021-00937-x. 2021 22:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubair A.S., McAlpine L.S., Gardin T., Farhadian S., Kuruvilla D.E., Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020:E1–E10. doi: 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuliani G., Ranzini M., Guerra G., et al. Plasma cytokines profile in older subjects with late onset Alzheimer's disease or vascular dementia. J. Psychiatr. Res. 2007;41:686–693. doi: 10.1016/j.jpsychires.2006.02.008. [DOI] [PubMed] [Google Scholar]