Abstract
Arthropod-borne virus (arbovirus) infections cause a number of emerging and resurgent human and veterinary infectious diseases. Traditional means of controlling arbovirus diseases include vaccination of susceptible vertebrates and mosquito control, but in many cases these have been unavailable or ineffective, and so novel strategies for disease control are needed. One possibility is genetic manipulation of mosquito vectors to render them unable to transmit arboviruses. This review describes recent work to test the concept of pathogen-derived resistance in arthropods by expression of viral genes in mosquito cell cultures and mosquitoes. Sense and antisense genome sequences from La Crosse virus (LAC) (a member of the Bunyaviridae) and dengue viruses serotypes 1 to 4 (DEN-1 to DEN-4) (members of the Flaviviridae) were expressed in mosquito cells from double-subgenomic and replicon vectors based on Sindbis virus (a member of the Togaviridae). The cells were then challenged with homologous or related viruses. For LAC, expression of antisense sequences from the small (S) genome segment, particularly full-length antisense S RNA, effectively interfered with replication of challenge virus, whereas expression of either antisense or sense RNA from the medium (M) segment was completely ineffective in LAC inhibition. Expression of sense and antisense RNA derived from certain regions of the DEN genome also blocked homologous virus replication more effectively than did RNA from other regions. Other parameters of RNA-mediated interference have been defined, such as the time when replication is blocked and the minimum size of effector RNA. The mechanism of RNA inhibition has not been determined, although it resembles double-stranded RNA interference in other nonvertebrate systems. Prospects for application of molecular strategies to control arbovirus diseases are briefly reviewed.
ARBOVIRUS DISEASES AND THEIR CONTROL
Arbovirus Diseases Are Emerging and Resurgent Public Health Problems
Arboviruses cause some of the most devastating diseases known to human and veterinary medicine. There are over 500 identified arboviruses, most of which are members of the families Togaviridae, Flaviviridae, Bunyaviridae, Rhabdoviridae, and Reoviridae. Arboviruses are maintained in natural cycles in which they multiply in a hematophagous arthropod, such as a mosquito or tick, and are transmitted in saliva to a vertebrate host when the arthropod takes a blood meal. Replication in the principal vertebrate host causes a viremia of sufficient titer and duration to permit infection of another blood-feeding arthropod. The virus is transmitted by the arthropod to a new vertebrate host only after amplification and dissemination from the midgut to various other tissues, in particular the salivary glands. The extrinsic incubation period in the mosquito usually is 7 to 14 days, although more rapid spread of virus to other tissues has been observed (19, 84). The vertebrate infection is generally acute and self-limited, whereas the arthropod is persistently infected for life. In most of these natural cycles, neither the arthropod nor the vertebrate suffers apparent adverse effects from the infection. Interestingly, in the only two of these cycles in which humans are the principal vertebrate hosts, the urban cycles of dengue and yellow fever viruses, serious human disease may result from infection. More usually, human arbovirus disease occurs when a vector chooses a human alternative to its preferred vertebrate host. The result is frequently a dead end for virus transmission, since the human is not the preferred host for the blood-feeding insect or may develop a short-lived or low-titer viremia, but the outcome for the human may be a syndrome with significant morbidity and/or mortality such as encephalitis or hemorrhagic fever.
Arboviruses occur throughout the world, with each virus having a geographic distribution determined largely by the range of its arthropod vector(s). Occurrence of disease tends to be seasonal; arthropod vectors are generally most active during warm periods in temperate zones and rainy seasons in the tropics. During the last two decades, the incidence of a number of arbovirus diseases that were previously unknown or thought to be controlled has increased dramatically. The emergence of arbovirus diseases as a serious public health problem can be attributed to a number of factors such as the growth of human populations (97), increased urbanization, incursion of human activity into new ecosystems, increased global travel, climatic changes, and collapse of vector control and public health programs (33, 60). This increased disease incidence due to changing conditions points to the need for new approaches to disease control.
The Example of Dengue Virus and Its Diseases
One major focus of our research at the Arthropod-Borne and Infectious Diseases Laboratory (AIDL) is on dengue viruses (DEN), etiologic agents of both a long-recognized human febrile disease and a more recently described hemorrhagic disease, both of which now occur epidemically around the world (33). DEN is a member of the genus Flavivirus with four antigenically distinct serotypes (DEN-1 through DEN-4). The viruses are transmitted primarily by the anthropophilic mosquito, Aedes aegypti, which is also the principal urban vector of yellow fever virus (YFV). An epidemic illness thought to be dengue fever (DF) was described in North America as well as Africa and Asia as early as 1779 (33). Classic DF is an acute febrile disease with rash and joint and muscle pain; it can result from infection by any one of the four DEN serotypes. It is debilitating, but recovery is inevitable (60). Infrequent epidemics occurred on three continents from 1780 to 1940, and individual viral serotypes probably became endemic in tropical population centers during this period. During and immediately following World War II, DF epidemics became more frequent and more widely distributed and hyperendemicity (cocirculation of multiple serotypes) developed in Southeast Asia. Although hemorrhagic signs associated with cases of DF had been described occasionally, the first known epidemic of dengue hemorrhagic fever (DHF), a more severe consequence of DEN infection, occurred in the Philippines in 1953 to 1954 (33). DHF has an acute onset like DF but proceeds to hemorrhagic manifestations and may have a case fatality rate of 1 to 20%, depending on the level of supportive treatment. It occurs most frequently in individuals who have had a previous heterotypic DEN infection and has been attributed to immune enhancement of infection (60). During the 20 years following the Philippine outbreak, epidemics of DHF occurred throughout Southeast Asia and then in other parts of Asia and in the South and Central Pacific, but epidemic DHF was not reported in the Americas until an extensive Cuban epidemic in 1981 (33). Temporary sparing of the Western Hemisphere from DEN and DHF epidemics was largely due to a widespread A. aegypti eradication program that was begun in the 1950s but discontinued in the early 1970s (33). During the 10 years following the 1981 Cuban epidemic, DHF was reported in 11 additional countries in Central, South, and North America. It is estimated that currently as many as 100 million cases of DF occur worldwide each year, and whereas the mean annual incidence of DHF from 1956 to 1980 was 30,000 cases, this number has now risen to 250,000 to 500,000 notified cases per year (60).
Traditional Strategies for Arbovirus Disease Control
Historically, two methods have been used to control human and veterinary arbovirus diseases. The first is elimination of the arthropod vector. As mentioned above, A. aegypti control programs effectively delayed the reemergence of DEN in the Western Hemisphere until they were discontinued in the 1970s. Chemical control measures are subject to several problems, however, such as lack of program sustainability, especially where cooperation between political entities is required; high cost; adverse effects on nontarget organisms and other environmental damage due to residual pesticides; and development by vectors of genetic resistance to insecticides.
The second conventional control method is the development and use of vaccines to protect humans and domestic animals from infection. For example, a safe, effective attenuated virus vaccine for the prototype Flavivirus YFV (17D) was developed by Theiler and Smith in 1937 (93), although variable vaccine coverage in regions of endemic infection has resulted in failure to prevent resurgent epidemics, particularly in Africa (61). In contrast, the development of a safe, effective DEN vaccine has been an elusive goal, and current knowledge that DHF appears to be due to immune enhancement of infection by a secondary, heterologous DEN virus serotype suggests that a safe vaccine must simultaneously elicit immunity to all four serotypes.
Novel Strategies: Genetic Manipulation of Mosquitoes and Pathogen-Derived Resistance
Although ongoing efforts to develop and improve conventional methods for control of arbovirus diseases have not been abandoned, novel strategies are needed. An approach we are pursuing at AIDL is the genetic manipulation of mosquito vectors to render them unable to be infected by and to transmit arboviruses (24). This could hypothetically be done in one of two ways: (i) identification of naturally occurring genes conditioning vector competence and selection for increased dispersal and expression of the alleles that confer refractoriness; or (ii) introduction and expression in mosquitoes of new genetic material that alters vector competence. The most desirable outcome of either strategy would be that a given arthropod vector is rendered refractory to all the distinct viral pathogens it is capable of transmitting.
Mosquito competence to transmit arboviruses appears to be genetically determined. For example, several studies have shown that subspecies and strains of A. aegypti vary phenotypically in their susceptibility to DEN (34, 35) and that competence to transmit both DEN and YFV appears to have a genetic basis (57, 90). Bosio et al. (13) recently used a quantitative genetic approach to show that at least two genes or sets of genes underlie the differing abilities of two subspecies of A. aegypti to be infected by and transmit DEN-2. In addition to the influence of multiple genes on vector competence, they observed a large variance in virus infection and dissemination rates due to environmental effects. This information was used in mapping the quantitative trait loci that condition vector competence (12).
Mosquito refractoriness to arbovirus infection can also be acquired. Natural interference to superinfection by both the homologous virus and heterologous but related arboviruses has been demonstrated in mosquito cells. Condreay and Brown (22) found that cultured mosquito cells infected by Sindbis virus (SIN) (Togaviridae) developed resistance to superinfection by homologous virus within 10 h after the initial infection. Cultured Aedes albopictus cells infected with DEN-1 developed complete resistance to infection by a heterotypic DEN (DEN-3) between 8 and 24 h after infection. The development of interference required protein synthesis (26). Beaty and colleagues showed that Aedes triseriatus mosquitoes that had been orally infected with La Crosse virus (LAC) (Bunyaviridae) began to exhibit interference to oral superinfection with homologous or closely related viruses by 24 h after infection. Interference correlated with the level of initial virus replication and antigen expression in the midgut (10, 11, 89).
Sanford and Johnston (81) coined the term “pathogen-derived resistance” (PDR) in proposing that artificial expression of pathogen gene sequences by a potential host organism could render that organism resistant to the pathogen. PDR has been demonstrated in various transgenic plants in which, depending on the virus involved, the expression of protein gene products (68), untranslatable mRNA (52), or antisense RNA (38) derived from a plant viral genome was shown to prevent infection by the homologous virus. Induction of PDR has rarely been attempted in animals. Although external administration of antisense oligonucleotides has been tested extensively as a potential antiviral therapy in animal cell culture (see, e.g., references 20, 36, 73, and 94) and less frequently in vertebrate animals (64), few attempts to engineer intracellular expression of animal virus gene sequences as an interference strategy have been reported (82).
Observations that mosquito cells and mosquitoes develop resistance to arbovirus superinfection due to expression of viral gene products suggested to us that PDR may be used to induce interference in vectors. Although colleagues are mapping the endogenous genes that render mosquitoes refractory to virus transmission, in this review we focus on the introduction of viral genetic sequences into mosquitoes to test the concept that PDR has the potential to interrupt the transmission of bunyaviruses and flaviviruses. Fully achieving the goal of developing molecular strategies for interrupting arbovirus transmission by mosquitoes requires the accomplishment of several objectives: (i) finding an efficient system for the introduction and expression of exogenous genes in mosquitoes; (ii) identifying genes or gene products that are effective as agents of PDR; (iii) establishing stable, heritable maintenance and expression of the identified genes in the vector; and (iv) driving these genes into a mosquito population. Here we emphasize the identification of molecular effectors for inhibiting viral transmission by arthropods. Furthermore, we indicate that our initial narrow focus on inhibition of virus replication now appears to have revealed a fundamental mechanism of gene silencing in mosquito cells. Potential systems for stable introduction of exogenous genetic material into mosquitoes are described, and the significant questions connected with field application such as safety, environmental impacts, regulation, and public perception are briefly mentioned, since they are discussed in detail elsewhere (7, 41, 42, 86) and are beyond the scope of this review.
SYSTEMS FOR INTRODUCTION AND EXPRESSION OF EXOGENOUS GENES IN MOSQUITOES
Transformation and Introduction of DNA into the Germ Line
In 1982, Rubin and Spradling (80) discovered that the naturally occurring transposable P-element of Drosophila melanogaster can be manipulated to introduce heterologous genetic material into its natural host. This method of transformation has become a routine means of creating transgenic insects in studies of Drosophila genetics and development. The same approach was initially used in attempts to transform mosquitoes; marker DNA embedded in the D. melanogaster P-element transposon was microinjected into preblastoderm embryos (56, 58, 62). Although a very low rate of integration of foreign DNA into the mosquito chromosome was observed, it did not result from action of the P-element transposase. Demonstration that the Drosophila P-element would not reliably transform mosquitoes led to a 10-year search for transposable elements in mosquitoes and other insects that could be used efficiently and predictably to integrate new genetic material into the mosquito chromosome. Tony James's research group demonstrated transposition and stable transformation of A. aegypti by two-component systems constructed from both the Hermes element from the housefly, Musca domestica (44), and the mariner element MOSI from Drosophila mauritiana (21). These systems have the advantages that the transposon with inserted (marker) genes can be remobilized by introduction of functional transposase but is unlikely to be repressed by endogenous elements (6).
Another strategy for stable introduction of exogenous genes into mosquito chromosomes is the pantropic retroviral vector developed by Jane Burns and colleagues (17). Pseudotyping the retroviral genome with the vesicular stomatitis virus envelope glycoprotein gives this vector a universal host range, and 10 to 13 kb of inserted heterologous DNA can be integrated into the host genome by retrotransposition. Matsubara et al. (55) demonstrated the ability of the retroviral vector to integrate and express exogenous genes in cultured cells of the malaria mosquito, Anopheles gambiae. Insect parvoviruses of the genus Densovirus also are potential gene transfer vectors for mosquitoes (2). The Aedes densonucleosis virus is being developed and examined by Jonathan Carlson and colleagues (3, 23) at AIDL.
Extrachromosomal (paratransgenic) expression of exogenous genes in arthropods by bacterial symbionts is another possible means of genetic alteration. Scott O'Neill's group showed that the obligate intracellular bacteria Wolbachia will infect a variety of insect tissues and suggested that genetically modified Wolbachia could be used to transduce mosquitoes with transmission-blocking genes (87). Ben Beard's group has demonstrated that expression of the antimicrobial peptide cecropin A in the midgut of triatomine bugs by the nutritional mutualist bacterium Rhodococcus rhodnii renders a large proportion of the bugs refractory to infection by Trypanosoma cruzi (9).
Transient Gene Expression: Sindbis Virus-Based Systems
Despite the development of several promising mosquito transformation systems, the construction of transgenic mosquitoes is still very labor-intensive and inefficient, and we have thus used transient gene expression systems to test the effect of introducing heterologous genetic material into mosquitoes and mosquito cell cultures. A SIN-based expression system developed by Charles Rice and colleagues (96) has provided an ideal means of testing the concept of PDR in mosquitoes. SIN is an arbovirus and the prototype of the genus Alphavirus in the family Togaviridae. The natural cycle of SIN involves birds and Culex mosquitoes (92). SIN also will infect Aedes and Anopheles mosquitoes (43) and has a broad host range in vertebrate animal cells. Of particular importance for our work, SIN replicates efficiently in many cells and tissues of Aedes mosquitoes (74), causing a persistent, noncytocidal infection.
The molecular biology of SIN is well studied (88). SIN has a positive-sense RNA genome 11,703 nucleotides (nt) in length. Nonstructural proteins which comprise the RNA-dependent RNA polymerase (RDRP) and other replicative functions are encoded in and translated from the 5′ two-thirds of the genome. The structural proteins are encoded in the 3′ one-third of the RNA and are translated from a subgenomic mRNA that is transcribed intracellularly by the RDRP from an internal promoter (88). A complete cDNA copy of the genome, from which infectious viral RNA can be transcribed in vitro (an infectious clone), was produced by Rice et al. in 1987 (78).
Two types of expression vectors that have been derived from the infectious clone have been of particular use in identifying successful viral interference strategies (65). The double-subgenomic SIN (dsSIN) expression vector TE/3′J has a duplicated internal promoter from which inserted heterologous genetic material is expressed (37). The dsSIN recombinant RNA genome is infectious and can be efficiently packaged into transducing virions. Recombinant virus will express the inserted gene in a broad range of host cells and spread to surrounding cells and tissues (67).
A second expression system, the SIN replicon, consists of the SIN infectious clone with the structural protein genes deleted and replaced by up to 6,000 bp of heterologous genetic material that is expressed from the native internal promoter (48). The replicon is capable of intracellular self-amplification but can be packaged into infectious virions only in cells that are coinfected with a helper virus expressing the viral structural genes (96). Packaged replicons efficiently infect cells but do not spread from the initially infected cell in the absence of helper.
USE OF THE SINDBIS VIRUS DOUBLE SUBGENOMIC AND REPLICON EXPRESSION SYSTEMS TO IDENTIFY EFFECTORS OF VIRAL INTERFERENCE
Effectiveness in Mosquito Cells and Mosquitoes
To test the level, duration, and fidelity of expression of heterologous genes from the dsSIN vector in mosquitoes, a recombinant dsSIN virus was constructed to express bacterial chloramphenicol acetyltransferase (CAT) from the second subgenomic promoter. When cultured A. albopictus C6/36 cells were infected with recombinant virus at a multiplicity of infection (MOI) of >20, 100% of the cells expressed CAT within 24 h, as detected by immunofluorescence with an anti-CAT antibody. An assay of the enzyme in cell lysates showed that 8.3 × 105 CAT polypeptides per cell were expressed (67). After intrathoracic inoculation of A. triseriatus mosquitoes with approximately 104 50% tissue culture infectious doses (TCID50) of the recombinant dsSIN virus TE/3′2J/CAT, viral titers of >106 TCID50 per mosquito were detected within 4 days. CAT enzymatic activity peaked at 4 × 10−3 units (7.2 × 1011 polypeptides) per mosquito at 6 days after inoculation and remained at peak levels to day 20 postinoculation (67). CAT protein expressed from both recombinant dsSIN in A. triseriatus and from a SIN replicon virus in Culex pipiens mosquitoes was detected in neural tissues, midguts, and salivary glands (47, 48, 67).
These results demonstrated several properties of the SIN expression systems that made them ideal for testing the concept of PDR in mosquitoes. (i) Recombinant SIN caused a persistent, noncytocidal infection in a variety of mosquito cells and tissues, including the salivary glands, which play a crucial role in transmission of arboviruses. (ii) The heterologous gene or gene product was expressed in the cytoplasm, thus obviating the need for nuclear regulation, processing, or transport of the transcript. (iii) The heterologous gene product was expressed abundantly and persisted for an extended period. The last two points suggested that any DNA-based system that ultimately deploys antiviral genes identified by expression with the SIN systems may require a similar high level of expression in the cytoplasm for efficacy.
Identification of La Crosse Virus Genes or Gene Products That Can Be Expressed to Inhibit Virus Replication
Plant researchers have used both transgenesis and transient expression by RNA virus transduction systems to demonstrate that interference to virus replication can be mediated by expression of the viral coat protein (68), its mRNA (52), or its antisense complement (8, 38). Therefore, Powers, Kamrud, et al. (46, 69–71) tested the concept of PDR to LAC in mosquito cells by transient expression of viral structural protein genes by dsSIN TE/3′2J. LAC is a member of the genus Bunyavirus in the family Bunyaviridae. Its principal vector is A. triseriatus in a natural cycle involving small mammals such as chipmunks and squirrels. Humans may be tangential hosts for LAC infection when they are bitten by infected A. triseriatus, and LAC infection is an important cause of pediatric encephalitis in the United States (95). LAC has a tripartite, single-stranded, negative-sense RNA genome. The genes for the major virion structural proteins are on the small (S) RNA segment, which encodes the nucleocapsid protein (N) as well as a nonstructural protein (NSS), and the medium (M) RNA segment, which encodes the two glycoproteins (G1 and G2) and another nonstructural protein (NSM) (83). Regions of these two genome segments expressed as cDNA ranging in size from 374 to 1,300 bp were cloned into pTE/3′2J for expression of either sense or antisense transcripts in mosquito cells (Table 1) (46, 69–71). Portions of the S segment that were inserted for expression by recombinant viruses included the entire S cDNA, the NSS coding sequence, and the conserved complementary terminal sequences (69, 71). The M segment cDNA was divided into four regions to give fragments from nt 1 to 1040 (the G2 gene), 1021 to 2453 (encompassing the NSM and 5′ portion of the G1 gene), 2454 to 3625 (the center of the G1 gene), and 3621 to 4495 (the 3′ portion of the G1 gene) (46, 70).
TABLE 1.
Interference with LAC replication due to dsSIN-expressed sequencesa
| dsSIN insertb | Mean LAC titer (SD) (log10 TCID50/ml)c at:
|
|||
|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | |
| None (TE/3′2J) | 4.5 (0.3) | 5.6 (0.3) | 5.9 (0.3) | 5.9 (0.3) |
| LAC M segment RNA inserts | ||||
| nt 1–1040 (G2 gene) | 4.3 (0.4) | 5.9 (0.3) | 5.6 (0.3) | 5.9 (0.3) |
| Antisense 1–1040 | 3.2 (0.6) | 5.0 (0.5) | 5.2 (0.6) | 5.3 (0.3) |
| nt 1021–2453 (NSm and 5′ G1 genes) | 4.9 (0.3) | 5.9 (0.3) | 5.9 (0.3) | 6.3 (0.0) |
| nt 2454–3625 (G1 gene center) | 4.3 (0.0) | 4.3 (0.3) | 5.3 (0.3) | 4.9 (0.3) |
| Antisense 2454–3625 | 4.6 (0.3) | 5.3 (0.4) | 4.9 (0.3) | 5.3 (0.4) |
| nt 3621–4495 (3′ G1 gene) | 5.3 (0.4) | 5.6 (0.3) | 6.3 (0.0) | 5.9 (0.3) |
| Antisense 3621–4495 | 2.7 (0.3) | 6.0 (0.5) | 6.3 (0.3) | 6.2 (0.6) |
| LAC S segment RNA inserts | ||||
| Full-length S | 1.9 (1.7) | 2.3 (1.9) | 2.8 (2.0) | 2.7 (2.3) |
| Antisense S | 0.9 (0.9) | 1.3 (1.4) | 0.9 (1.2) | 0.6 (1.8) |
| Antisense complementary termini | 1.7 (0.8) | 3.5 (0.9) | 4.3 (0.4) | 4.3 (0.8) |
| Nonstructural gene | 3.7 (0.3) | 4.8 (0.4) | 5.1 (0.8) | 5.2 (0.8) |
| Antisense nonsructural gene | 1.3 (0.6) | 3.3 (0.3) | 4.2 (0.6) | 4.2 (0.6) |
A. albopictus C6/36 mosquito cells were infected with recombinant dsSIN containing the insert shown at a MOI of 20 to 50. After 48 h, cell cultures were challenged with LAC at a MOI of 0.01.
The infectious LAC titer was determined in medium removed every 24 h. Boldface numbers indicate LAC titers >1.7 log10 lower than the LAC titer in nonrecombinant SIN (TE/3′2J)-infected control cultures at the same time point and are considered to indicate significant interference.
Cultured mosquito (C6/36) cells were infected with one of the recombinant viruses at a MOI of 20 to 50 and challenged with LAC virus (at a MOI of 0.01 to 0.1) 48 h later when all the cells were shown to be infected with dsSIN. An enzyme-linked immunosorbent assay was used to measure progeny LAC released from the cells. Infection with four of the five dsSIN strains expressing the LAC S segment sequences significantly reduced (by >50-fold) LAC titers in the medium of cultured mosquito cells (Table 1) (69). TE/3′2J/αS, expressing the full-length S antisense RNA, was most effective; it virtually eliminated LAC replication. In contrast, cells infected with dsSIN strains expressing all sequences from the LAC M segment were permissive for LAC replication (Table 1) (46, 70).
The interference was shown in all cases to be RNA rather than protein mediated since it was manifested when the positive-sense S segment transcript from TE/3′2J/S was rendered nontranslatable by site-directed mutagenesis (70). To determine the degree of cross-protection to heterologous virus afforded by expression of LAC S antisense RNA, C6/36 cells infected with TE/3′2J/αS were challenged with the related bunyaviruses snowshoe hare virus (SSH), Tahyna virus (TAH), or trivittatus virus (TVT) or the unrelated flavivirus YFV. Interference with the replication of SSH and TAH was similar to that for LAC; however, by 96 h postchallenge, 50% of cells expressing LAC antisense S RNA were permissive for TVT and no interference with YFV was observed. The S segments of SSH and TAH have >80% sequence identity to LAC, but TVT has only 62% S segment sequence identity (4, 14). A high degree of sequence relatedness also was shown to be necessary to effect RNA-mediated interference with heterologous viruses in transgenic plants (25).
Interference was tested in adult A. triseriatus mosquitoes by providing them with a blood meal containing 107 TCID50 of LAC per ml and then inoculating them intrathoracically with TE/3′2J/αS. After 12 to 18 days, the mosquitoes were assayed for infectious LAC or viral envelope antigens. Inoculation with the recombinant virus resulted in a 10- to 100-fold reduction in LAC titers compared to those in mosquitoes inoculated with nonrecombinant SIN and challenged with LAC (70).
These studies showed that expression in mosquito cells and adult mosquitoes of certain regions of LAC RNA, particularly those from the S RNA segment with antisense polarity, rendered the cells resistant to infection by the homologous and closely related viruses. The resistance was RNA mediated and was dependent on a high degree of sequence identity between the effector RNA and its target.
Testing the Principle of PDR with Structural Genes from Dengue and Yellow Fever Viruses
Our initial attempts to inhibit DEN replication also used the expression of genes encoding structural proteins. Flaviviruses have a nonsegmented, positive-sense RNA genome about 11,000 nt in length. The genome acts as the only mRNA, encoding a single polyprotein that is co- and posttranslationally cleaved to give three structural and seven nonstructural polypeptides. The gene order is 5′-C-prM-E-NS1-NS2a-NS2b-NS3-NS4a-NS4b-NS5-3′, with the three structural proteins encoded in the 5′ one-quarter of the genome and the nonstructural proteins encoded in the remainder (18, 77).
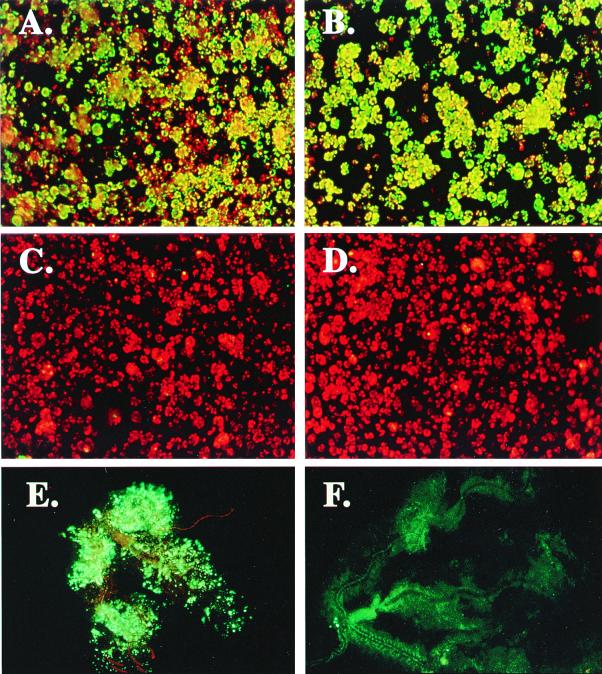
The full-length premembrane (prM) protein-coding region (567 nt) of the DEN-2 genome was expressed in C6/36 cells in either the sense or antisense orientation by infection with the recombinant dsSIN strains D2prMs or D2prMa, respectively, at a MOI of 50. When cells infected with either recombinant were challenged 48 h later with DEN-2 at a MOI of 0.1, they were completely resistant to homologous DEN infection, whereas cells infected with a nonrecombinant dsSIN strain supported high levels of DEN-2 replication (Fig. 1A to D) (31). Mosquito cells expressing untranslatable prM sense RNA were resistant to challenge at a MOI of 10, whereas cells expressing prM protein exhibited some breakthrough of DEN-2 replication at a high-MOI challenge, indicating that interference was RNA and not protein mediated. No interference was seen in cells expressing DEN-2 prM sequences and challenged with DEN-3 or DEN-4, which have 68 and 66% sequence identity, respectively, to DEN-2 in their prM genes (31). Similar experiments with YFV showed that the threshold for effective interference was ∼80% sequence identity (39).
FIG. 1.
Use of the indirect-immunofluorescence assay to detect the inhibition of DEN replication in mosquito cells and tissues. Cultured mosquito (C6/36) cells were uninfected (A), infected with nonrecombinant dsSIN (TE3′/2J) (B), or infected with recombinant dsSIN expressing the full-length premembrane (prM) protein coding region (567 nt) of the DEN-2 genome in either sense (D2prMs), (C) or antisense (D2prMa) (D) orientation at a MOI of 50. All cells were challenged 48 h later with DEN-2 at a MOI of 0.1. DEN-2 replication was detected 5 days postchallenge by the indirect-immunofluorescence assay. Adult female A. aegypti mosquitoes were injected intrathoracically with DEN-2 alone (E), (magnification ×200) or coinjected with D2prMa (105 TCID50) and DEN-2 (103 TCID50) (F) (magnification, ×400) and maintained at 28°C for 11 days. Salivary glands were removed and subjected to IFA with the use of anti-DEN E monoclonal primary antibodies and a biotinylated sheep anti-mouse secondary antibody. Fluorescence produced by bound fluorescein-streptavidin (Amersham, Arlington Heights, Ill.) was viewed with an Olympus BH-2 epifluorescence microscope. Cell cultures were counterstained with Evans blue. Cells and tissues positive for DEN-2 E antigen were considered positive for DEN-2 replication.
Expression in C6/36 cells of the full-length antisense capsid (C) coding region (375 nt) from DEN-2 by dsSIN D2Ca virus also conferred complete resistance to homologous challenge. Interestingly, expression of the full-length C protein was toxic to mosquito cells (31). A truncated C protein, lacking the 24 carboxy-terminal hydrophobic amino acids that serve as a signal sequence for the prM protein (77), could be expressed at high levels without killing the mosquito cells (30).
D2prMa virus was used to transduce mosquitoes in experiments in which both recombinant dsSIN and challenge DEN-2 were simultaneously inoculated into the thoraxes of female A. aegypti mosquitoes at doses of 105 and 103 TCID50, respectively. After 11 days, the midguts and salivary glands were removed by dissection and examined by immunofluorescence for presence of SIN envelope and DEN-2 envelope proteins. Surprisingly, abundant DEN antigen was observed in midgut epithelial cells; however, DEN-2 replication was dramatically inhibited in all tissues of the salivary glands (Fig. 1E and F). SIN envelope antigen was seen only in the nerve and muscle tissues of the midgut but in all tissues of the salivary gland. Therefore, we concluded that the midgut was not resistant to DEN-2 replication, because dsSIN virus expressing the anti-DEN effector RNA and target DEN had different tissue tropisms (66). In subsequent studies, we have identified a SIN isolate (MRE16) that readily infects A. aegypti midgut epithelial cells and have used its structural proteins to construct chimeric SIN strains with broader mosquito tissue tropisms (85).
The ultimate test of effective interference is interruption of DEN transmission in A. aegypti saliva. When saliva of the mosquitoes coinoculated with dsSIN D2prMa and DEN-2 was assayed for infectious DEN-2, only 1 of 26 mosquitoes was weakly positive (66). Thus, the expression of antisense DEN RNA in epidemiologically relevant mosquito tissues can block homologous virus transmission. In related experiments, A. aegypti mosquitoes were coinoculated with YFV and dsSIN expressing either antisense prM RNA or antisense RNA from a 1,000-nt region of the NS5 gene from 17D YFV. Neither mosquito group transmitted the closely related West African YFV, and both only poorly transmitted a more distantly related South American strain of YFV. Mosquitoes transduced with D2prMa, however, were fully competent to transmit YFV (39).
Nonstructural Protein Genes and Gene Products as Potential Mediators of Broadly Based Interference
The experiments described above demonstrated the very high degree (>80%) of sequence identity required between target and effector RNA molecules for successful RNA-mediated interference. In an attempt to identify a more broadly based interference strategy that could be targeted to a heterologous group of viruses with a common vector mosquito, we explored the possibility of interference mediated by conserved, nonstructural proteins. In plant virus interference studies, expression of certain domains or truncated versions of viral RDRP proteins conferred resistance to both homologous virus and a variety of related viruses (27, 32). The NS5 gene of flaviviruses is thought to encode the RDRP, since its protein product contains a GDD (Gly-Asp-Asp) amino acid motif, which is the active site for RNA elongation (59), and has RNA-polymerizing activity in vitro (91). We have expressed from the dsSIN and SIN replicons, respectively, a truncated version (335 amino acids from the C-terminal half, containing the GDD motif) of the 17D YFV NS5 gene product and a full-length gene product (913 amino acids) that reacts with anti-NS5 monoclonal antibodies and have tested their abilities to interfere with homologous West African YFV and more distantly related South American YFV and DEN-2. For both truncated and full-length protein expression, interference with the replication of YFV but not DEN-2 was observed in cultured mosquito cells (75). Furthermore, the level of interference directly correlated with the degree of sequence identity, and antisense RNA to the GDD-encompassing coding region was more effective than protein-expressing sense RNA, suggesting RNA-mediated interference (39). In fact, expression of the truncated RDRP protein in mosquitoes completely failed to interfere with the replication of YFV (75). Most reports of RDRP-mediated plant virus interference involved the expression of a truncated, GDD-containing domain of the enzyme (32) or a protein with site-directed mutations in the putative RNA-binding site (5, 15, 53). As with interference studies involving structural genes and proteins, the RDRP-mediated flavivirus interference appeared to be due to expression of RNA rather than of protein. Since RNA-mediated interference is highly sequence specific, it did not broaden the range of target viruses. Other approaches such as expression of RDRP protein with site-directed mutations should perhaps be pursued because they have the potential to inhibit a broader range of viruses.
PARAMETERS OF RNA-MEDIATED INTERFERENCE
The experiments described above demonstrated the feasibility of PDR in mosquitoes and defined the following limits: (i) to date, genetically engineered resistance to virus infection is RNA rather than protein mediated; (ii) effector RNAs require >80% sequence identity to the viral RNA target; (iii) effective RNA inhibitors must be expressed in or introduced into cells, and possibly into specific cellular compartments, where target virus replicates; and (iv) the minimum effective intracellular concentration of antisense RNA is not known. The dsSIN system was shown initially to express RNA at a very high level (>105 copies/cell [30]), although transcript levels decline after 6 to 8 days. Any DNA-based system must be engineered to achieve initial high expression levels, possibly by transformation with an entire cDNA copy of a recombinant SIN replicon genome (which would then self-amplify its subgenomic RNA in the cytoplasm).
With the continuing goal of identifying an effector that will confer interference to several distinct viruses when expressed in transgenic mosquitoes, we embarked on a series of experiments to further define the parameters of RNA-mediated interference by asking the following questions. Are all regions of the genome equally vulnerable targets for antisense RNA effectors? What is the minimum size requirement of an antisense effector? If the minimum size requirement is >50 but <250 nt, can several effectors, each specific for a different DEN serotype, be expressed in tandem to create broad-based interference? By what mechanism does antisense RNA interfere?
Genome Region
We prepared a series of recombinant dsSIN TE/3′2J strains, each designed to express a 180- to 290-nt insert that was complementary (antisense) to the 5′ region of the gene encoding either the C, prM, NS3, or NS5 protein from a single DEN serotype. Each of these was tested in mosquito cell culture for its ability to inhibit the replication of superinfecting homologous virus (Table 2). Antisense sequences derived from the prM and C genes for all four serotypes exhibited >95% homologous interference. In contrast, although the expression of 240-nt RNA complementary to the region surrounding either the DEN-1 or DEN-3 GDD-coding sequence of the NS5 gene also gave >95% homologous interference, a 240-nt fragment complementary to the 5′ end of the same gene from DEN-1 was totally ineffective as a replication inhibitor. Similarly, the complement to the 5′ end of the DEN-3 NS3 gene exhibited only 85% interference with the homologous virus. Recombinant viruses that elicited a high level of interference in cell culture were also tested in mosquitoes. In most cases, interference in cell culture was an accurate predictor of effectiveness in the vector (Table 2). Although the reason(s) for the variability in interference effectiveness is unknown, it could be due to the position on the genome (e.g., the distance from the 5′ or 3′ end) or to the local secondary structure of either target or effector RNA (Z. N. Adelman, K. E. Olson, S. Higgs, B. J. Beaty, and C. D. Blair, unpublished data).
TABLE 2.
Interference with DEN replication by recombinant dsSIN viruses with single and multiple tandem antisense RNA insertsab
| dsSIN insertc | Insert size (nt) | % Inhibition in C6/36 cells infected with:
|
% Inhibition in A. aegypti infected with:
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| DEN-1 | DEN-2 | DEN-3 | DEN-4 | DEN-1 | DEN-2 | DEN-3 | DEN-4 | ||
| Single inserts (−RNA) | |||||||||
| D1/prM | 249 | >99 | 100 | ||||||
| D1/NS5/GDD | 240 | >99 | 100 | ||||||
| D1/NS5/5′ | 180 | 0 | NDd | ||||||
| D2/prM | 290 | >99 | 100 | ||||||
| D3/prM | 286 | >99 | 100 | ||||||
| D3/NS3/5′ | 216 | 85 | ND | ||||||
| D4/C | 240 | >99 | 100 | ||||||
| Double inserts (5′-3′) | |||||||||
| D1/prM/D3/prM | 540 | 97 | 97 | 99 | 97 | ||||
| D1/GDD/D3/GDD | 490 | 98 | 90 | 91 | 92 | ||||
| D4/C/D1/GDD | 480 | >99 | 80 | ND | ND | ||||
| Triple inserts (5′-3′) | |||||||||
| D1/GDD/D4/C/D2/prM | 770 | >99 | 0 | 70 | ND | ND | ND | ||
| Variable-size inserts | |||||||||
| D1/GDD | 240 | >99 | 50 | 100 | |||||
| D1/GDDFsh | 160 | >99 | 50 | 100 | |||||
| D1/GDDRsh | 183 | 98 | 50 | 89 | |||||
| D1/GDDFshRsh | 105 | 85 | 0 | 70 | |||||
Adapted from Adelman et al., unpublished.
Assays of interference with DEN replication in C6/36 mosquito cell cultures were performed as described in Table 1. Challenge with DEN was at a MOI of 0.1. The proportion of cells escaping interference was determined, 7 days after DEN challenge, by the indirect-immunofluorescence assay using a primary monoclonal antibody specific for DEN E protein. For interference assays in A. aegypti, adult female mosquitoes were inoculated intrathoracically simultaneously with 105 TCID50 recombinant dsSIN plus 3 × 103 PFU of DEN of the indicated serotype. The mosquitoes were held for 14 days at 28°C, and then the heads were removed and examined by the indirect-immunofluorescence assay for the presence of the DEN E protein, indicating escape from interference.
Inserts expressed from the subgenomic dsSIN promoter are designated by the DEN virus serotype and genome region from which the antisense RNA was derived.
ND, not determined.
Effector Size and “Multivalent” Effectors
To determine the minimal size requirements for effectors of antisense RNA-mediated interference, inserts were truncated in the virus designated D1/GDD. The recombinant virus D1/GDD expressed a 240-nt antisense RNA targeted to the 3′ half of the NS5 gene of DEN-1. As noted above and in Table 2, infection of cultured mosquito cells with D1/GDD resulted in complete replication inhibition of challenge DEN-1. The size of the DEN-1-specific insert in D1/GDD was shortened to 160, 183, or 105 nt by truncation at either its 5′ or 3′ end or both ends, respectively, and the resulting recombinant viruses were tested for their ability to inhibit DEN-1 replication. As shown in Table 2, the 160- and 183-nt antisense RNAs retained complete ability to interfere with homologous virus replication, but reduction of the insert size to 105 nt significantly diminished the degree of interference. Similarly, truncation of the insert size reduced its ability to interfere in mosquitoes (Adelman et al., unpublished).
With knowledge of specific sequence and minimal size constraints for effector RNA, recombinant SIN strains were constructed with tandem 240- to 290-nt antisense RNA inserts complementary to the C, prM, or GDD regions of two or three different DEN serotype genomes and were tested separately for interference with each homologous virus (Table 2). The D1/prM/D3/prM and D1/GDD/D3/GDD recombinant viruses effectively inhibited both DEN serotypes. Subsequently, other dsSIN viruses with chimeric inserts have exhibited interference with replication of two DEN serotypes, although interference results were unpredictable with regard to the origin of the effector sequence or its position in the chimera (Adelman et al., unpublished).
All our results suggest that the induction of RNA-mediated interference requires the presence of sequence-specific double-stranded RNA. The reduction in interference effectiveness observed on either decreasing the length or arraying antisense effectors in tandem could be the result of a reduction in the ability to form RNA-RNA duplexes. Although it has been shown that antisense RNAs as small as 60 nt can bind stably to their targets (79), the heterologous RNA transcribed from dsSIN virus templates is embedded within a larger RNA expressed from the second subgenomic promoter. SIN-specific RNAs comprising at least 50 nt of 5′ leader RNA and 400 nt of 3′ untranslated RNA surround the DEN-specific sequence. It is difficult to estimate the effect of these extraneous sequences on the formation of double-stranded RNA (Adelman et al., unpublished).
An alternative explanation for the lack of interference by the shorter effector RNA sequences is the proliferation of escape mutants. Bull et al. (16) described RNA bacteriophage mutants with only 3 or 4 base substitutions that escaped interference by a 240-nt antisense RNA. Since RNA viruses such as DEN exhibit sequence variation, even in a single isolate (40), it is possible that nucleotide substitutions in the genome were sufficient to disrupt stable RNA duplex formation with shorter effectors. The generation of escape mutants does not explain the decrease in interference sometimes seen when individually effective antisense sequences are tandemly arrayed, however. Further exploration of the mechanism of antisense RNA interference will be required to better understand the parameters for construction of “multivalent” effectors.
Understanding the Mechanism of Inhibition
Several mechanisms have been proposed by which the expression of sense or antisense RNA or the presence of double-stranded RNA in eukaryotic cells results in interference with superinfecting homologous virus. A straightforward possibility for the mechanism of RNA-RNA hybrid inhibition of virus replication involves physical hindrance of access or procession of translational machinery on the mRNA, especially if the hybrid is formed near the 5′ end. Hybrid formation may also block viral RDRP binding or movement for transcription. In vertebrate animal cells, double-stranded RNA induces the synthesis of alpha/beta interferon. The presence of double-stranded RNA in cells that have been exposed to interferon leads to a cascade of events resulting in the inhibition of translation by phosphorylation of an initiation factor and degradation of mRNA by induced RNase L (51). Furthermore, double-stranded RNA itself can elicit antiviral effects, even in the absence of interferon (51).
Interferon-related double-stranded RNA-mediated interference has not been reported to occur in invertebrate animals. Nevertheless, double-stranded RNA appears to play a role in complex regulatory mechanisms in a number of nonvertebrate systems. Evidence has been presented for degradation, in plant cells, of mRNA that is part of an RNA-RNA hybrid by a cellular RNase that, along with other induced enzymes, is involved in posttranscriptional regulation of gene expression and recovery from plant virus infection (8, 72). Fire et al. (29) observed specific interference with gene expression in Caenorhabditis elegans after injection of homologous double-stranded RNA. The interference was due to a decrease or elimination of the endogenous mRNA. Double-stranded RNA has recently been shown to trigger sequence-specific gene silencing in a number of other organisms (28), including Trypanosoma brucei (63) and D. melanogaster (49). Further pursuit of the mechanism of RNA-mediated virus interference should lead to new insights into mosquito cell gene regulation.
Preliminary studies in our laboratory indicate that antisense RNA-mediated interference with DEN replication in mosquito cells occurs within the first 8 h after infection (Adelman et al., unpublished). Furthermore, in mosquito cells expressing effector RNA and challenged with homologous DEN, transcripts of the DEN genome fail to accumulate as they would in an uninhibited DEN infection (Adelman et al., unpublished). This could result from degradation of template RNA, from direct blockage of RNA synthesis, or indirectly from inhibition of translation of the RNA polymerase complex. It is possible that more than one of these mechanisms is operating with different effector RNAs or even with a single effector. Further studies are required to determine the possible role of double-stranded RNA, the level of RNA expression required, and the best way to target the cellular compartment to most effectively interrupt infection.
CAN THESE STRATEGIES BE APPLIED IN THE FIELD?
Although we do not fully understand the mechanism of RNA-mediated interference, we have shown that expression of effector RNA in mosquito cells is an effective molecular strategy for interrupting specific Bunyavirus and Flavivirus replication in mosquitoes. The SIN expression systems have the potential not only to test PDR as a strategy for interrupting arbovirus transmission but also to lead to a better understanding of the regulation of mosquito and viral gene expression and virus-vector interactions (45, 54). However, two additional objectives stated earlier in this review must be accomplished in order to apply RNA-mediated interference in the field: (i) DNA-based, heritable expression will be necessary, and (ii) a means of driving transgenes into mosquito populations must be found.
Although the SIN expression systems have been invaluable in establishing these principles, direct use of SIN is not an option for long-term expression of an antiviral agent in mosquito field populations. As mentioned above, several methods to introduce stable, DNA-based exogenous genetic material into mosquitoes are under active development. The introduced DNA could contain a single gene or gene segment or an entire cDNA copy of a recombinant SIN replicon genome, preferably under the control of an inducible promoter.
The ideal transgene should be able to spread unaided and become fixed in natural mosquito populations. One possible mechanism for such spread is introduction in a transposon. Kidwell has presented evidence that the transposable P-element, despite a possible fitness reduction in carriers, has recently and rapidly spread worldwide in D. melanogaster populations (50). Since a transposable element introduced by one parental genome during mating may integrate at many sites in the genome of the other (wild-type) parent, the element can eventually spread to all chromosomes. Ribeiro and Kidwell (76) have developed a model suggesting that engineered transposons may be used to drive genes, such as those for parasite resistance, into wild vector populations. Much remains to be learned, however, about the stability of transgenes introduced by various means, the effects of genome insertion position, appropriate promoters, and possible silencing effects of multiple gene copies.
In addition, a number of other aspects of the eventual release of genetically modified arthropod vectors of human disease must be considered. These include potential environmental hazards, public health risks, and public perceptions. The National Institutes of Health and the Centers for Disease Control and Prevention have developed standards for the classification of risk and conditions for work with human disease agents. The U.S. Department of Agriculture Animal and Plant Health Inspection Service (USDA-APHIS) has established guidelines and an approval process for the release of transgenic arthropod plant pests. While none of these directly addresses the release of genetically modified human disease vectors, they establish precedents for risk assessment and regulation (41, 42, 86). Individual and public health risks must be assessed in both laboratory and pilot field studies, and accurate information must be provided to the public at the earliest possible time (7). As scientists, we have the responsibility not only to be sure that the disease control strategies we develop are effective but also that risks to health and environment do not outweigh the risks of the diseases being targeted.
CONCLUSIONS
We have demonstrated a potential molecular strategy for interrupting mosquito transmission of certain arbovirus infections. We have shown, by use of an efficient transient-expression system, that the presence in the mosquito salivary gland cell cytoplasm of high levels of sense or antisense RNA molecules (of established minimum size and derived from certain viral genes) effectively blocks the replication and transmission of the homologous infecting virus. This interference strategy appears to require the presence of stable RNA-RNA hybrids between the effector and the viral RNA, and it results in a lack of accumulation of viral genome transcripts in infected mosquito cells. The mechanism of interference is unknown and could involve steric hindrance of translation or transcription or double-stranded RNA-induced suppression of viral gene expression. Other interference strategies, such as intracellular expression of mutated viral RDRP or of ribozymes or single-chain antibodies, remain to be explored. Application of this interference strategy will require the development of DNA-based, heritable expression of the transgene, a mechanism to drive the gene into mosquito populations, and assurances that the strategy is safe and effective.
ACKNOWLEDGMENTS
Work at the Arthropod-borne and Infectious Diseases Laboratory was supported by National Institutes of Health grants AI34014 and AI25629 and the John D. and Catherine T. MacArthur Foundation.
REFERENCES
- 1.Reference deleted.
- 2.Afanasiev B N, Kozlov Y V, Carlson J O, Beaty B J. Densovirus of Aedes aegypti as an expression vector in mosquito cells. Exp Parasitol. 1994;79:322–339. doi: 10.1006/expr.1994.1095. [DOI] [PubMed] [Google Scholar]
- 3.Afanasiev B N, Ward T W, Beaty B J, Carlson J O. Transduction of Aedes aegypti mosquitoes with vectors derived from Aedes densovirus. Virology. 1999;257:62–72. doi: 10.1006/viro.1999.9621. [DOI] [PubMed] [Google Scholar]
- 4.Akashi H, Bishop D H. Comparison of the sequences and coding of La Crosse and snowshoe hare bunyavirus S RNA species. J Virol. 1983;45:1155–1158. doi: 10.1128/jvi.45.3.1155-1158.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson J M, Palukaitis P, Zaitlin M. A defective replicase gene induces resistance to cucumber mosaic virus in transgenic tobacco plants. Proc Natl Acad Sci USA. 1992;89:8759–8763. doi: 10.1073/pnas.89.18.8759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashburner M, Hoy M A, Peloquin J J. Prospects for the genetic transformation of arthropods. Insect Mol Biol. 1998;7:201–213. doi: 10.1046/j.1365-2583.1998.00084.x. [DOI] [PubMed] [Google Scholar]
- 7.Asner M. Public relations: the scientist and the public, the government, and the media. In: Purchase H G, MacKenzie J, editors. Agricultural biotechnology. Introduction to field testing. Washington, D.C.: Office of Agricultural Biotechnology, U.S. Department of Agriculture; 1990. p. 35. [Google Scholar]
- 8.Baulcombe D C. Mechanisms of pathogen-derived resistance to viruses in transgenic plants. Plant Cell. 1996;8:1833–1844. doi: 10.1105/tpc.8.10.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beard C B, Durvasula R V, Richards F F. Bacterial symbiosis in arthropods and the control of disease transmission. Emerg Infect Dis. 1998;4:581–591. doi: 10.3201/eid0404.980408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beaty B J, Bishop D H, Gay M, Fuller F. Interference between bunyaviruses in Aedes triseriatus mosquitoes. Virology. 1983;127:83–90. doi: 10.1016/0042-6822(83)90373-2. [DOI] [PubMed] [Google Scholar]
- 11.Beaty B J, Sundin D R, Chandler L J, Bishop D H. Evolution of bunyaviruses by genome reassortment in dually infected mosquitoes (Aedes triseriatus) Science. 1985;230:548–550. doi: 10.1126/science.4048949. [DOI] [PubMed] [Google Scholar]
- 12.Bosio C F. Ph.D. dissertation. Fort Collins: Colorado State University; 1999. [Google Scholar]
- 13.Bosio C F, Beaty B J, Black W C., IV Quantitative genetics of vector competence for dengue-2 virus in Aedes aegypti. Am J Trop Med Hyg. 1998;59:965–970. doi: 10.4269/ajtmh.1998.59.965. [DOI] [PubMed] [Google Scholar]
- 14.Bowen M D, Jackson A O, Bruns T D, Hacker D L, Hardy J L. Determination and comparative analysis of the small RNA genomic sequences of California encephalitis, Jamestown Canyon, Jerry Slough, Melao, Keystone and Trivittatus viruses (Bunyaviridae, genus Bunyavirus, California serogroup) J Gen Virol. 1995;76:559–572. doi: 10.1099/0022-1317-76-3-559. [DOI] [PubMed] [Google Scholar]
- 15.Braun C J, Hemenway C L. Expression of amino-terminal portions or full-length viral replicase genes in transgenic plants confers resistance to potato virus X infection. Plant Cell. 1992;4:735–744. doi: 10.1105/tpc.4.6.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bull J J, Jacobson A, Badgett M R, Molineux I J. Viral escape from antisense RNA. Mol Microbiol. 1998;28:835–846. doi: 10.1046/j.1365-2958.1998.00847.x. [DOI] [PubMed] [Google Scholar]
- 17.Burns J C, Friedmann T, Driever W, Burrascano M, Yee J K. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chambers T J, Hahn C S, Galler R, Rice C M. Flavivirus genome organization, expression, and replication. Annu Rev Microbiol. 1990;44:649–688. doi: 10.1146/annurev.mi.44.100190.003245. [DOI] [PubMed] [Google Scholar]
- 19.Chandler L J, Blair C D, Beaty B J. La Crosse virus infection of Aedes triseriatus (Diptera: Culicidae) ovaries before dissemination of virus from the midgut. J Med Entomol. 1998;35:567–572. doi: 10.1093/jmedent/35.4.567. [DOI] [PubMed] [Google Scholar]
- 20.Chang L J, Stoltzfus C M. Inhibition of Rous sarcoma virus replication by antisense RNA. J Virol. 1987;61:921–924. doi: 10.1128/jvi.61.3.921-924.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coates C J, Jasinskiene N, Miyashiro L, James A A. Mariner transposition and transformation of the yellow fever mosquito, Aedes aegypti. Proc Natl Acad Sci USA. 1998;95:3748–3751. doi: 10.1073/pnas.95.7.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Condreay L D, Brown D T. Exclusion of superinfecting homologous virus by Sindbis virus-infected Aedes albopictus (mosquito) cells. J Virol. 1986;58:81–86. doi: 10.1128/jvi.58.1.81-86.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corsini J, Afanasiev B, Maxwell I H, Carlson J O. Autonomous parvovirus and densovirus gene vectors. Adv Virus Res. 1996;47:303–351. doi: 10.1016/s0065-3527(08)60738-1. [DOI] [PubMed] [Google Scholar]
- 24.Crampton J M, Warren A, Lycett G J, Hughes M A, Comley I P, Eggleston P. Genetic manipulation of insect vectors as a strategy for the control of vector-borne disease. Ann Trop Med Parasitol. 1994;88:3–12. doi: 10.1080/00034983.1994.11812828. [DOI] [PubMed] [Google Scholar]
- 25.de Haan P, Gielen J J L, Prins M, Wijkamp I G, Van Schepen A, Peters D, Van Grinsven M Q J M, Goldbach R. Characterization of RNA-mediated resistance to tomato spotted wilt virus in transgenic tobacco. Bio/Technology. 1992;10:1133–1137. doi: 10.1038/nbt1092-1133. [DOI] [PubMed] [Google Scholar]
- 26.Dittmar D, Castro A, Haines H. Demonstration of interference between dengue virus types in cultured mosquito cells using monoclonal antibody probes. J Gen Virol. 1982;59:273–282. doi: 10.1099/0022-1317-59-2-273. [DOI] [PubMed] [Google Scholar]
- 27.Donson J, Kearney C M, Turpen T H, Khan I A, Kurath G, Turpen A M, Jones G E, Dawson W O, Lewandowski D J. Broad resistance to tobamoviruses is mediated by a modified tobacco mosaic virus replicase transgene. Mol Plant-Microbe Interact. 1993;6:635–642. doi: 10.1094/mpmi-6-635. [DOI] [PubMed] [Google Scholar]
- 28.Fire A. RNA-triggered gene silencing. Trends Genet. 1999;15:358–363. doi: 10.1016/s0168-9525(99)01818-1. [DOI] [PubMed] [Google Scholar]
- 29.Fire A, Xu S, Montgomery M K, Kostas S A, Driver S E, Mello C C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 30.Gaines P J. Interference with dengue-2 virus replication in mosquito cells by expression of dengue-2 C and prM genes from recombinant Sindbis virus. M.S. thesis. Fort Collins: Colorado State University; 1996. [Google Scholar]
- 31.Gaines P J, Olson K E, Higgs S, Powers A M, Beaty B J, Blair C D. Pathogen-derived resistance to dengue type 2 virus in mosquito cells by expression of the premembrane coding region of the viral genome. J Virol. 1996;70:2132–2137. doi: 10.1128/jvi.70.4.2132-2137.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Golemboski D B, Lomonossoff G P, Zaitlin M. Plants transformed with a tobacco mosaic virus nonstructural gene sequence are resistant to the virus. Proc Natl Acad Sci USA. 1990;87:6311–6315. doi: 10.1073/pnas.87.16.6311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gubler D J. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gubler D J, Nalim S, Tan R, Saipan H, Sulianti Saroso J. Variation in susceptibility to oral infection with dengue viruses among geographic strains of Aedes aegypti. Am J Trop Med Hyg. 1979;28:1045–1052. doi: 10.4269/ajtmh.1979.28.1045. [DOI] [PubMed] [Google Scholar]
- 35.Gubler D J, Rosen L. Variation among geographic strains of Aedes albopictus in susceptibility to infection with dengue viruses. Am J Trop Med Hyg. 1976;25:318–325. doi: 10.4269/ajtmh.1976.25.318. [DOI] [PubMed] [Google Scholar]
- 36.Gutierrez A, Martinez-Salas E, Pintado B, Sobrino F. Specific inhibition of aphthovirus infection by RNAs transcribed from both the 5′ and the 3′ noncoding regions. J Virol. 1994;68:7426–7432. doi: 10.1128/jvi.68.11.7426-7432.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hahn C S, Hahn Y S, Braciale T J, Rice C M. Infectious Sindbis virus transient expression vectors for studying antigen processing and presentation. Proc Natl Acad Sci USA. 1992;89:2679–2683. doi: 10.1073/pnas.89.7.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hemenway C, Fang R X, Kaniewski W K, Chua N H, Tumer N E. Analysis of the mechanism of protection in transgenic plants expressing the potato virus X coat protein or its antisense RNA. EMBO J. 1988;7:1273–1280. doi: 10.1002/j.1460-2075.1988.tb02941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Higgs S, Rayner J O, Olson K E, Davis B S, Beaty B J, Blair C D. Engineered resistance in Aedes aegypti to a West African and a South American strain of yellow fever virus. Am J Trop Med Hyg. 1998;58:663–670. doi: 10.4269/ajtmh.1998.58.663. [DOI] [PubMed] [Google Scholar]
- 40.Holland J, Spindler K, Horodyski F, Grabau E, Nichol S, VandePol S. Rapid evolution of RNA genomes. Science. 1982;215:1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- 41.Hollander A K. Environmental impacts of genetically engineered microbial and viral biocontrol agents. In: Maramorosch K, editor. Biotechnology for biological control of pests and vectors. Boca Raton, Fla: CRC Press, Inc.; 1991. pp. 251–266. [Google Scholar]
- 42.Hoy M A. Impact of risk analyses on pest-management programs employing transgenic arthropods. Parasitol Today. 1995;11:229–232. [Google Scholar]
- 43.Hurlbut H, Thomas J. The experimental host range of the arthropod-borne animal viruses in arthropods. Virology. 1960;12:391. doi: 10.1016/0042-6822(60)90162-8. [DOI] [PubMed] [Google Scholar]
- 44.Jasinskiene N, Coates C J, Benedict M Q, Cornel A J, Rafferty C S, James A A, Collins F H. Stable transformation of the yellow fever mosquito, Aedes aegypti, with the Hermes element from the housefly. Proc Natl Acad Sci USA. 1998;95:3743–3747. doi: 10.1073/pnas.95.7.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson B W, Olson K E, Allen-Miura T, Rayms-Keller A, Carlson J O, Coates C J, Jasinskiene N, James A A, Beaty B J, Higgs S. Inhibition of luciferase expression in transgenic Aedes aegypti mosquitoes by Sindbis virus expression of antisense luciferase RNA. Proc Natl Acad Sci USA. 1999;96:13399–13403. doi: 10.1073/pnas.96.23.13399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kamrud K I. Characterization and use of Sindbis virus expression vectors in mosquitoes and mosquito cells. Ph.D. dissertation. Fort Collins: Colorado State University; 1996. [Google Scholar]
- 47.Kamrud K I, Olson K E, Higgs S, Powers A M, Carlson J O, Beaty B J. Detection of expressed chloramphenicol acetyltransferase in the saliva of Culex pipiens mosquitoes. Insect Biochem Mol Biol. 1997;27:423–429. doi: 10.1016/s0965-1748(97)00014-3. [DOI] [PubMed] [Google Scholar]
- 48.Kamrud K I, Powers A M, Higgs S, Olson K E, Blair C D, Carlson J O, Beaty B J. The expression of chloramphenicol acetyltransferase in mosquitoes and mosquito cells using a packaged Sindbis replicon system. Exp Parasitol. 1995;81:394–403. doi: 10.1006/expr.1995.1130. [DOI] [PubMed] [Google Scholar]
- 49.Kennerdell J R, Carthew R W. Use of dsRNA-mediated genetic interference to demonstrate that frizzled and frizzled 2 act in the wingless pathway. Cell. 1998;95:1017–1026. doi: 10.1016/s0092-8674(00)81725-0. [DOI] [PubMed] [Google Scholar]
- 50.Kidwell M G. Evolution of hybrid dysgenesis determinants in Drosophila melanogaster. Proc Natl Acad Sci USA. 1983;80:1655–1659. doi: 10.1073/pnas.80.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar M, Carmichael G G. Antisense RNA: function and fate of duplex RNA in cells of higher eukaryotes. Microbiol Mol Biol Rev. 1998;62:1415–1434. doi: 10.1128/mmbr.62.4.1415-1434.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lindbo J A, Dougherty W G. Untranslatable transcripts of the tobacco etch virus coat protein gene sequence can interfere with tobacco etch virus replication in transgenic plants and protoplasts. Virology. 1992;189:725–733. doi: 10.1016/0042-6822(92)90595-g. [DOI] [PubMed] [Google Scholar]
- 53.MacFarlane S A, Davies J W. Plants transformed with a region of the 201-kilodalton replicase gene from pea early browning virus RNA1 are resistant to virus infection. Proc Natl Acad Sci USA. 1992;89:5829–5833. doi: 10.1073/pnas.89.13.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marshall A. The insects are coming. Nat Biotechnol. 1998;16:530–533. doi: 10.1038/nbt0698-530. [DOI] [PubMed] [Google Scholar]
- 55.Matsubara T, Beeman R W, Shike H, Besansky N J, Mukabayire O, Higgs S, James A A, Burns J C. Pantropic retroviral vectors integrate and express in cells of the malaria mosquito, Anopheles gambiae. Proc Natl Acad Sci USA. 1996;93:6181–6185. doi: 10.1073/pnas.93.12.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McGrane V, Carlson J O, Miller B R, Beaty B J. Microinjection of DNA into Aedes triseriatus ova and detection of integration. Am J Trop Med Hyg. 1988;39:502–510. doi: 10.4269/ajtmh.1988.39.502. [DOI] [PubMed] [Google Scholar]
- 57.Miller B R, Mitchell C J. Genetic selection of a flavivirus-refractory strain of the yellow fever mosquito Aedes aegypti. Am J Trop Med Hyg. 1991;45:399–407. doi: 10.4269/ajtmh.1991.45.399. [DOI] [PubMed] [Google Scholar]
- 58.Miller L H, Sakai R K, Romans P, Gwadz R W, Kantoff P, Coon H G. Stable integration and expression of a bacterial gene in the mosquito Anopheles gambiae. Science. 1987;237:779–781. doi: 10.1126/science.3039658. [DOI] [PubMed] [Google Scholar]
- 59.Mills D R, Priano C, DiMauro P, Binderow B D. Q beta replicase: mapping the functional domains of an RNA-dependent RNA polymerase. J Mol Biol. 1989;205:751–764. doi: 10.1016/0022-2836(89)90319-7. [DOI] [PubMed] [Google Scholar]
- 60.Monath T P. Dengue: the risk to developed and developing countries. Proc Natl Acad Sci USA. 1994;91:2395–2400. doi: 10.1073/pnas.91.7.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Monath T P, Heinz F X. Flaviviruses. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 961–1034. [Google Scholar]
- 62.Morris A C, Eggleston P, Crampton J M. Genetic transformation of the mosquito Aedes aegypti by micro-injection of DNA. Med Vet Entomol. 1989;3:1–7. doi: 10.1111/j.1365-2915.1989.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 63.Ngo H, Tschudi C, Gull K, Ullu E. Double-stranded RNA induces mRNA degradation in Trypanosoma brucei. Proc Natl Acad Sci USA. 1998;95:14687–14692. doi: 10.1073/pnas.95.25.14687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Offensperger W B, Offensperger S, Walter E, Teubner K, Igloi G, Blum H E, Gerok W. In vivo inhibition of duck hepatitis B virus replication and gene expression by phosphorothioate modified antisense oligodeoxynucleotides. EMBO J. 1993;12:1257–1262. doi: 10.1002/j.1460-2075.1993.tb05767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Olson K, Beaty B, Higgs S. Sindbis virus expression systems for the manipulation of insect vectors. In: Miller L K, Ball L A, editors. The insect viruses. New York, N.Y: Plenum Publishing Corp.; 1998. pp. 371–404. [Google Scholar]
- 66.Olson K E, Higgs S, Gaines P J, Powers A M, Davis B S, Kamrud K I, Carlson J O, Blair C D, Beaty B J. Genetically engineered resistance to dengue-2 virus transmission in mosquitoes. Science. 1996;272:884–886. doi: 10.1126/science.272.5263.884. [DOI] [PubMed] [Google Scholar]
- 67.Olson K E, Higgs S, Hahn C S, Rice C M, Carlson J O, Beaty B J. The expression of chloramphenicol acetyltransferase in Aedes albopictus (C6/36) cells and Aedes triseriatus mosquitoes using a double subgenomic recombinant Sindbis virus. Insect Biochem Mol Biol. 1994;24:39–48. doi: 10.1016/0965-1748(94)90121-x. [DOI] [PubMed] [Google Scholar]
- 68.Powell-Abel P, Nelson R S, De B, Hoffman N, Rogers S G, Beachy R N. Delay of disease development in transgenic plants that express the tobacco mosaic virus coat protein gene. Science. 1986;232:738–743. doi: 10.1126/science.3457472. [DOI] [PubMed] [Google Scholar]
- 69.Powers A M. Genetically engineered resistance to Bunyavirus infection in mosquito cells. Ph.D. dissertation. Fort Collins: Colorado State University; 1995. [Google Scholar]
- 70.Powers A M, Kamrud K I, Olson K E, Higgs S, Carlson J O, Beaty B J. Molecularly engineered resistance to California serogroup virus replication in mosquito cells and mosquitoes. Proc Natl Acad Sci USA. 1996;93:4187–4191. doi: 10.1073/pnas.93.9.4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Powers A M, Olson K E, Higgs S, Carlson J O, Beaty B J. Intracellular immunization of mosquito cells to LaCrosse virus using a recombinant Sindbis virus vector. Virus Res. 1994;32:57–67. doi: 10.1016/0168-1702(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 72.Prins M, Goldbach R. RNA-mediated virus resistance in transgenic plants. Arch Virol. 1996;141:2259–2276. doi: 10.1007/BF01718629. [DOI] [PubMed] [Google Scholar]
- 73.Raviprakash K, Liu K, Matteucci M, Wagner R, Riffenburgh R, Carl M. Inhibition of dengue virus by novel, modified antisense oligonucleotides. J Virol. 1995;69:69–74. doi: 10.1128/jvi.69.1.69-74.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rayms-Keller A, Powers A M, Higgs S, Olson K E, Kamrud K I, Carlson J O, Beaty B J. Replication and expression of a recombinant Sindbis virus in mosquitoes. Insect Mol Biol. 1995;4:245–251. doi: 10.1111/j.1365-2583.1995.tb00030.x. [DOI] [PubMed] [Google Scholar]
- 75.Rayner J O. Interference with yellow fever virus infection using strategies based on the NS5 gene. Ph.D. dissertation. Fort Collins: Colorado State University; 1998. [Google Scholar]
- 76.Ribeiro J M, Kidwell M G. Transposable elements as population drive mechanisms: specification of critical parameter values. J Med Entomol. 1994;31:10–16. doi: 10.1093/jmedent/31.1.10. [DOI] [PubMed] [Google Scholar]
- 77.Rice C M. Flaviviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 931–959. [Google Scholar]
- 78.Rice C M, Levis R, Strauss J H, Huang H V. Production of infectious RNA transcripts from Sindbis virus cDNA clones: mapping of lethal mutations, rescue of a temperature-sensitive marker, and in vitro mutagenesis to generate defined mutants. J Virol. 1987;61:3809–3819. doi: 10.1128/jvi.61.12.3809-3819.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rittner K, Burmester C, Sczakiel G. In vitro selection of fast-hybridizing and effective antisense RNAs directed against the human immunodeficiency virus type 1. Nucleic Acids Res. 1993;21:1381–1387. doi: 10.1093/nar/21.6.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rubin G M, Spradling A C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982;218:348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- 81.Sanford J C, Johnston S A. The concept of pathogen derived resistance—deriving resistance genes from the parasite's own genome. J Theor Biol. 1985;113:395–405. [Google Scholar]
- 82.Sasaki N, Hayashi M, Aoyama S, Yamashita T, Miyoshi I, Kasai N, Namioka S. Transgenic mice with antisense RNA against the nucleocapsid protein mRNA of mouse hepatitis virus. J Vet Med Sci. 1993;55:549–554. doi: 10.1292/jvms.55.549. [DOI] [PubMed] [Google Scholar]
- 83.Schmaljohn C S. Bunyaviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1447–1471. [Google Scholar]
- 84.Scott T W, Burrage T G. Rapid infection of salivary glands in Culiseta melanura with eastern equine encephalitis virus: an electron microscopic study. Am J Trop Med Hyg. 1984;33:961–964. doi: 10.4269/ajtmh.1984.33.961. [DOI] [PubMed] [Google Scholar]
- 85.Seabaugh R C, Olson K E, Higgs S, Carlson J O, Beaty B J. Development of a chimeric Sindbis virus with enhanced per os infection of Aedes aegypti. Virology. 1998;243:99–112. doi: 10.1006/viro.1998.9034. [DOI] [PubMed] [Google Scholar]
- 86.Simonsen L, Levin B R. Evaluating the risk of releasing genetically engineered organisms. Tree. 1988;3:27. doi: 10.1016/0169-5347(88)90135-8. [DOI] [PubMed] [Google Scholar]
- 87.Sinkins S P, Curtis C F, O'Neill S L. The potential application of inherited symbiont systems to pest control. In: O'Neill S L, Hoffman A, Werren J, editors. Influential passengers. Oxford, United Kingdom: Oxford University Press; 1997. pp. 155–175. [Google Scholar]
- 88.Strauss J H, Strauss E G. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sundin D R, Beaty B J. Interference to oral superinfection of Aedes triseriatus infected with La Crosse virus. Am J Trop Med Hyg. 1988;38:428–432. doi: 10.4269/ajtmh.1988.38.428. [DOI] [PubMed] [Google Scholar]
- 90.Tabachnick W J, Wallis G P, Aitken T H, Miller B R, Amato G D, Lorenz L, Powell J R, Beaty B J. Oral infection of Aedes aegypti with yellow fever virus: geographic variation and genetic considerations. Am J Trop Med Hyg. 1985;34:1219–1224. doi: 10.4269/ajtmh.1985.34.1219. [DOI] [PubMed] [Google Scholar]
- 91.Tan B H, Fu J, Sugrue R J, Yap E H, Chan Y C, Tan Y H. Recombinant dengue type 1 virus NS5 protein expressed in Escherichia coli exhibits RNA-dependent RNA polymerase activity. Virology. 1996;216:317–325. doi: 10.1006/viro.1996.0067. [DOI] [PubMed] [Google Scholar]
- 92.Taylor R M, Hurlbut H S, Work T H, Klingston J R, Frothingham T E. Sindbis virus: A newly recognized arthropod-transmitted virus. Am J Trop Med Hyg. 1955;4:844. doi: 10.4269/ajtmh.1955.4.844. [DOI] [PubMed] [Google Scholar]
- 93.Theiler M, Smith H H. The use of yellow fever virus modified by in vitro cultivation for human immunization. J Exp Med. 1937;65:787–800. doi: 10.1084/jem.65.6.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.To R Y, Booth S C, Neiman P E. Inhibition of retroviral replication by anti-sense RNA. Mol Cell Biol. 1986;6:4758–4762. doi: 10.1128/mcb.6.12.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tsai T F. Arboviral infections in the United States. Infect Dis Clin North Am. 1991;5:73–102. [PubMed] [Google Scholar]
- 96.Xiong C, Levis R, Shen P, Schlesinger S, Rice C M, Huang H V. Sindbis virus: an efficient, broad host range vector for gene expression in animal cells. Science. 1989;243:1188–1191. doi: 10.1126/science.2922607. [DOI] [PubMed] [Google Scholar]
- 97.Zanotto P M, Gould E A, Gao G F, Harvey P H, Holmes E C. Population dynamics of flaviviruses revealed by molecular phylogenies. Proc Natl Acad Sci USA. 1996;93:548–553. doi: 10.1073/pnas.93.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]