Purpose:
The aim of this study was to demonstrate the safety and effectiveness of a single TearCare procedure compared with a single LipiFlow procedure in treatment of the dry eye disease associated with meibomian gland dysfunction.
Methods:
In a multicenter, masked, randomized controlled trial, 135 subjects received a single TearCare (TC) treatment (n = 67) or a single LipiFlow (LF) treatment (n = 68) at baseline and were followed up for 1 month posttreatment. Tear film breakup time, meibomian gland function, and corneal and conjunctival staining scores were assessed as dry eye signs at baseline, 2 weeks, and 1 month; dry eye symptoms were assessed using the Ocular Surface Disease Index, Symptom Assessment in Dry Eye, and eye dryness questionnaires at baseline and 1 month.
Results:
At 1 month posttreatment, both groups demonstrated significant improvements (P < 0.0001) in mean tear film breakup time and meibomian gland secretion score to 3.0 ± 4.4 and 11.2 ± 11.1 in the TC group and 2.6 ± 3.3 and 11.0 ± 10.4 in the LF group, respectively. The mean eye dryness, Symptom Assessment in Dry Eye, and Ocular Surface Disease Index scores were significantly reduced (P < 0.0001) by 35.4 ± 34.1, 38.2 ± 31.0, and 27.9 ± 20.5 in the TC group and 34.9 ± 26.9, 38.0 ± 25.9, and 23.4 ± 17.7 in the LF group, respectively. There were no statistically significant differences for any result between the groups. However, the TC group demonstrated numerically greater improvements consistently in all signs and symptoms. Device-related ocular adverse events were reported in 3 patients in the TC group (superficial punctate keratitis, chalazion, and blepharitis) and 4 patients in the LF group (blepharitis, 2 cases of foreign body sensation, and severe eye dryness).
Conclusions:
A single TearCare treatment significantly alleviates the signs and symptoms of dry eye disease in patients with meibomian gland dysfunction and is equivalent in its safety and effectiveness profile to LipiFlow treatment as shown in this 1-month follow-up study.
Key Words: dry eye disease, meibomian gland dysfunction, TearCare procedure
Dry eye disease (DED) is a chronic eye condition that underscores a multitude of symptoms in patients including redness, irritation/ocular discomfort, fluctuating vision, and, often, substantially decreased quality of life. 1,2 DED continues to present a challenge because symptoms and prevalence tend to increase with age, environmental factors, and with screen usage. 3 The overall financial burden of DED is also considerable, with an estimated direct cost of medical treatment of US$3.84 billion annually for all patients seeking medical treatment for DED in the United States. 4 However, the indirect societal costs to the US society are estimated to be much greater, with a cost of US$55.4 billion per year through loss of workplace productivity. 4
Of the 2 major types of DED, evaporative DED is the most common and is characterized by a vicious cycle of impaired tear film quality, primarily due to meibomian gland dysfunction (MGD). 5 Although healthy and functioning meibomian glands are vital to ocular surface health, MGD is characterized by chronic glandular inflammation, thickening of the meibum, obstruction of gland channel terminal ducts, and glandular atrophy. 6 These changes result in altered delivery of meibomian gland secretions and further lead to decreased tear film stability. 6 This causes accelerated tear evaporation and a variety of symptoms and can act as an inductor of the multifactorial pathological changes leading to DED.
It is estimated that MGD underscores 86% of DED cases seen in the clinic and population-based studies because of the disruption of the lipid layer of tears. 7–9 The alterations in the glandular environment are accompanied by changes in the structure of meibum and an increase in the phase-transition temperature. 10 The treatment of MGD is often focused on applying heat to soften or liquefy thickened meibum, promote secretion from the meibomian glands, and increase the output of meibum for providing relief of MGD-associated evaporative DED. 11–13 Self-treatments such as warm compresses, eyelid massaging, and eyelid hygiene serve as a first-line treatment; however, temperature levels, lasting improvement, and compliance are often low. 14,15 The in-office treatments that provide lasting relief of signs and symptoms by treating meibomian gland obstructions optimally and effectively have shown promise to treat the root cause of MGD-related DED. 16
This study evaluated the TearCare system (Sight Sciences, Inc, Menlo Park, CA) in comparison with the LipiFlow Thermal Pulsation System (Johnson & Johnson Vision, Milpitas, CA) in patients with MGD and DED. The TearCare system is a blink-assisted device that applies heat to the eyelids to provide relief for MGD, DED, or blepharitis patients. The LipiFlow system is indicated for the treatment of MGD through localized heat and pressure therapy in adult patients with chronic MGD. The objective of this study was to demonstrate the safety and effectiveness of a single TearCare procedure compared with a single LipiFlow procedure in the treatment of the signs and symptoms of DED in adult patients with MGD.
MATERIALS AND METHODS
Study Devices
The TearCare system is a class II-exempt device that is currently listed and commercially available in the United States. The system is designed to conform to the eyelids externally to deliver controlled, precise heat to the tarsal plates and underlying meibomian glands of the eyelids for 15 minutes. 17,18 The wearable SmartLid devices are affixed to the patient's eyelids using a medical-grade adhesive such that the patient's native blinking is unobstructed. The meibum-melting session of TearCare is initiated by activation of the SmartHub controller, which gradually increases the temperature of the 4 SmartLids attached to patients' eyelids to the therapeutically optimal temperature of 45°C at the outer surface of 4 eyelids. Maintaining an outer eyelid temperature of 45°C is necessary to achieve the therapeutically optimal meibum-melting temperature of 41°C within the meibomian glands at the posterior eyelid. After the thermal treatment, the treating physician uses the gland clearance device to further evacuate the meibomian glands manually under direct visualization by using either slit lamp biomicroscope or surgical loupes.
The LipiFlow system uses vectored thermal pulsation technology to gently heat and massage the inner and outer eyelids. 19 The LipiFlow activators are designed to be placed under and over the eyelid and are specifically contoured to avoid contact with the ocular surface. With a single 12-minute LipiFlow procedure, maximum results are typically experienced 2 to 4 weeks posttreatment. 20
Study Design
This study was conducted in compliance with the Declaration of Helsinki for the protection of human subjects in medical research and under the approval of the Aspire International Review Board. This was a randomized, masked, multicenter, controlled trial to evaluate the safety and effectiveness of the TearCare System compared with LipiFlow in the treatment of the signs and symptoms of DED associated with MGD. Eligible subjects, who provided informed consent, were treated at baseline and reassessed at 2 weeks and 1 month posttreatment. Subjects randomized to the TearCare arm received one in-office TearCare treatment on study day 0, whereas subjects randomized to the control arm received one in-office LipiFlow procedure on study day 0. After each procedure, the subjects in both arms received identical follow-up instructions for the duration of the study to refrain from use of artificial tears or other type of dry eye treatment [including other medications (eg, Restasis and Xiidra), warm compress and lid massage, or TrueTear device treatment]. Subjects who felt they required rescue therapy to relieve their symptoms were allowed to use only the same type of tear drops or lubricants they were using before the study and to record any use in the prescribed Dry Eye Drop Log.
Study Population
Subjects were included in the study if they reported dry eye symptoms within the past 3 months and used artificial tear lubricants regularly over the past month. To be enrolled in the study, subjects were required to have Ocular Surface Disease Index (OSDI) scores of 23 to 79 at baseline, tear film breakup time (TBUT) of ≤7 seconds in both eyes, meibomian gland secretion score (MGSS) ≤12 in both eyes, and at least 15 expressible glands in the lower lid.
Subjects were excluded from enrollment if they had demonstrated use of Restasis or Xiidra within 60 days, antihistamines within 10 days, antiglaucoma and nonsteroidal antiinflammatory medications within 30 days, and systemic medications known to cause dry eye, including Accutane and antibiotics, within the 30 days. Subjects were ineligible if they had intense pulsed light or LipiFlow within 12 months, meibomian gland expression within 6 months, microblepharoexfoliation or other debridement within 3 months, punctal occlusion within 30 days, or used the TrueTear device within 2 weeks. Any subject with an active or recurring eye or eyelid infection or ocular surface abrasion was excluded. Subjects with current use of Latisse and Retin-A or permanent eyelid cosmetic work were excluded. In addition, any subjects with preexisting diseases causing dry eye (eg, autoimmune diseases such as Sjogren syndrome, rheumatoid arthritis, lupus, Graves disease, and sarcoidosis), ocular trauma, or allergy to silicone adhesives used in TearCare were excluded. Eligible subjects were randomized at the baseline visit into either the TearCare or the LipiFlow treatment group using a random number generator with subjects enrolled according to a predetermined list. After randomization, the subject underwent the assigned treatment procedure.
Endpoint Assessments
The primary effectiveness endpoints were defined as change from baseline to 1 month for TBUT and total MGSS. Secondary effectiveness endpoints included meibomian gland assessment, corneal and conjunctival staining scores, and assessment of dry eye symptoms using validated questionnaires. For each outcome measure, results from the subjects receiving the single TearCare treatment were compared with the results from the subjects receiving the single LipiFlow treatment. All endpoints assessing the DED signs were collected by the masked assessor, and the symptoms were self-reported by each subject at each visit.
The TBUT assessment was performed using fluorescein solution prepared per the method by Gyau et al. 21 Fluorescein-impregnated portion of 3 1.0-mg sodium fluorescein ophthalmic strips were cut using sterile scissors into 3 pieces, each resulting in 9 pieces in a disposable microcentrifuge tube. The study staff used 200 μL of sterile saline to soak the fluorescein pieces for approximately 10 minutes. Using a micropipette, 5 μL of the fluorescein solution was instilled into the lower conjunctival fornix. TBUT was measured quickly after instilling the fluorescein using the cobalt blue illumination of the slit lamp and a Wratten filter number 12. The subjects blinked 3 times and then held their eye open, and using a stopwatch, the time required for appearance of the first dry spot (negative staining) was recorded as the time when the tear film breaks up, that is, TBUT. The average of 3 such measurements was recorded as TBUT for the eye.
The MGSS assessment to evaluate the quality of the secretions produced by the meibomian glands in the lower eyelids was performed using the Meibomian Gland Evaluator (TearScience, Inc). The quality of secretions in 5 central glands in the lateral, central, and temporal thirds of the lower eyelids was graded, for a total of 15 glands per eye as described by Korb and Blackie. 22 The part of the instrument's contact surface was placed onto the skin immediately inferior to the eyelashes of the lower eyelid so that the long dimension is parallel to the eyelid margin. Once full contact was achieved between the instrument and the skin immediately below the lash line of the lower lid, the shaft of the instrument was rotated downward approximately 15 to 45 degrees. Then, the shaft was depressed midway (∼3 mm) and the lower eyelid margin rolled slightly outward, avoiding contact with the ocular surface. The instrument was held in place over each third of the lid for a minimum of 10 and a maximum of 15 seconds while grading the quality of secretion of the 5 glands in the center of the instrument (15 glands total per eye). The quality of the secretions was graded per the following scale described by Lane et al 19 : 0 = nothing, 1 = toothpaste, 2 = cloudy, and 3 = clear. Total MGSS was calculated as the sum of the grade (0–3) for each of the 15 glands ranging from 0 to 45. Count of the meibomian glands yielding clear liquid secretions and count of the glands secreting any liquid (clear or cloudy) was also recorded, ranging from 0 to 15 as one of the secondary endpoints.
Corneal and conjunctival staining scores were measured using National Eye Institute/Industry Grading System 23 as secondary endpoint. Corneal staining was scored within 1–4 minutes of installation of fluorescein dye to prevent diffusion of dye into stroma, under moderate illumination on the slit lamp using a cobalt blue filter 3 mm width, ×10 magnification, and a yellow Wratten filter (number 12). Corneal staining was scored per the following scale for 5 corneal sectors (superior, temporal, inferior, nasal, and central): grade 0, no staining; grade 1, scattered, micropunctate staining; grade 2, grouped, micropunctate staining; and grade 3, diffuse micropunctate or macropunctate staining. Total corneal staining score was calculated as the sum of the grade (0–3) for each of the 5 sectors, ranging from 0 to 15.
Conjunctival staining score was measured using lissamine green solution prepared per Gyau et al 21 as described in fluorescein dye preparation earlier. For scoring conjunctival staining, 5 μL of lissamine green solution was instilled into the lower conjunctival fornix, and grading was performed 1 minute after and within 4 minutes of instilling the solution. Starting on a low setting, the level of illumination was increased until the lissamine green staining was most visible. Conjunctival staining was scored per the following scale for 3 conjunctival sectors in nasal (superior, inferior, and nasal) and temporal (superior, inferior, and temporal) parts of the interpalpebral fissure: grade 0, no staining; grade 1, scattered, micropunctate staining; grade 2, grouped, micropunctate staining; and grade 3, diffuse micropunctate or macropunctate staining. Total conjunctival staining score was calculated as the sum of the grade (0–3) for each of the 6 sectors, ranging from 0 to 18.
The Eye Dryness Score (EDS) was derived from the visual analog scale (VAS), which measured subject's level of discomfort related to eye dryness ranging from no discomfort to maximal discomfort. The EDS ranging from 0 to 100 is the distance (in mm) between the left end of the scale and the subject's response. The Symptom Assessment in Dry Eye (SANDE) is a dry eye instrument containing 2 items measuring the frequency and severity of symptoms, with each assessed on a 100-mm VAS ranging from never/very comfortable to all the time/very severe and scored from 0 to 100. A SANDE total score was calculated as the square root of the product of the frequency and severity scores, which ranged from 0 to 100. The OSDI is a 12-question tool, and based on the answers provided by the subject, the overall OSDI score was calculated, ranging from 0 to 100. Based on the recommended cutoffs for OSDI score, the severity of the subject's dry eye symptoms was categorized as follows: normal category, 0–12; mild category, 13–22; moderate category, 23–32; and severe category: 33 or higher. In addition, the use of dry eye drops or lubricants as rescue therapy to relieve dry eye symptoms during the follow-up period was recorded using a drop log.
For the study population, safety endpoints were assessed as the frequency and nature of adverse events, discomfort/pain during treatment, change in best-corrected visual acuity (BCVA) as measured by the Early Treatment for Diabetic Retinopathy Scale (ETDRS Chart), and change in intraocular pressure (IOP).
Statistical Analyses
The sample size was calculated to provide more than 90% power to meet both the TBUT and MGSS effectiveness endpoints and sufficient precision around adverse event (AE) estimates. The primary analysis population was the per-protocol (PP) population that included all subjects who completed the study and had no major protocol deviations, specifically related to use of dry eye medications such as Restasis and Xiidra, medications interfering with the ocular surface, or other medications interfering with outcome measures. The primary and secondary endpoint analyses were conducted on the primary analysis population.
Outcomes measured on a per-eye basis were analyzed using data from both eyes. Least squares means were estimated as the change from baseline by treatment arm from a linear mixed-effects model with a random effect for subjects and fixed effect for treatment and baseline measure. The random-effects model adjusts the standard error (SE) and the confidence interval (CI) for within-person correlation between eyes. All outcome measures, IOP measures, and use of dry eye lubricants were tabulated by visit and treatment group. The secondary effectiveness outcomes for the PP population were measured on either a per-eye basis (corneal staining, conjunctival staining, and meibomian gland scores) or per-subject basis (OSDI score, SANDE scores, and EDS), as appropriate. The per-eye secondary endpoints were analyzed using a linear mixed-effects modeling approach similar to that use for the primary endpoint analysis. Per-subject endpoints were analyzed using a paired t-test to evaluate change from baseline to 1 month posttreatment. Subjects' use of eye drops as rescue therapy was analyzed as an exploratory endpoint to understand improvements to patient's quality of life. The proportion of subjects achieving OSDI improvement at least by one category (eg, number of subjects changing from severe category at baseline at least to moderate category at 1 month) was calculated for each group. Analysis of binary endpoints, including analyses of subjects achieving OSDI improvement at least by one category, was conducted using Fisher exact test with α = 0.05.
Noninferiority margins of the TearCare treatment to the LipiFlow control were TBUT measures within 3 seconds and MGSS scores within 5 units. Meeting of both endpoints was required to meet the study success. Both hypotheses were tested at a 1-sided α = 0.05. Because both primary effectiveness hypotheses were met, the secondary effectiveness endpoints were sequentially tested, and adjustments for multiplicity were necessary.
Clinically Significant Effects
Criteria for clinical relevance were used to establish the noninferiority margins for change from baseline and comparisons of study arms. A 5-point difference in MGSS, indicating a change in MGD severity, was the criterion for clinical relevance. 24 Because criteria for clinically relevant improvements in TBUT have not been determined, clinical relevance was based on the labeling of the dry eye test strips. The difference between dry and normal tear stability was 5 seconds; thus, a moderate change of more than 50% defines a TBUT difference of 3 seconds as clinically relevant.
RESULTS
Subject Demographics
A total of 141 subjects were enrolled and randomized 1:1 to receive either a single TearCare treatment or a single LipiFlow treatment; 69 subjects were assigned into the TearCare treatment group, whereas 72 subjects were assigned into the LipiFlow treatment group. Six subjects were excluded from the primary analysis PP population because the 1-month follow-up visits were missed (2 in the TearCare group and 4 in the LipiFlow group). In total, efficacy and noninferiority of TearCare compared with LipiFlow was assessed on the PP population of 135 subjects, with 67 subjects receiving TearCare treatment and 68 subjects receiving LipiFlow treatment. The demographics data of all study subjects were similar between the treatment groups (Table 1).
TABLE 1.
Baseline Demographics and Measurements of Study Endpoints for DED Signs and Symptoms in the Primary Analysis Population
| Parameter | TearCare Group | LipiFlow Group |
| Demographics | ||
| N (subjects) | 67 | 68 |
| Mean age [yr, (SD)] | 56.1 (13.7) | 52.3 (15.1) |
| Sex (%) | ||
| Women | 49 (73.1) | 42 (61.8) |
| Men | 17 (25.4) | 26 (38.2) |
| Race (%) | ||
| American Indian/Alaska Native | 0 (0.0) | 0 (0.0) |
| Asian | 2 (3.0) | 5 (7.4) |
| Black or African American | 3 (4.5) | 4 (5.9) |
| Indian | 0 (0.0) | 0 (0.0) |
| Iranian | 1 (1.5) | 0 (0.0) |
| Middle Eastern | 0 (0.0) | 1 (1.5) |
| Spanish | 1 (1.5) | 0 (0.0) |
| White | 59 (88.1) | 58 (85.3) |
| Dry eye sign endpoints | ||
| N (eyes) | 134 | 136 |
| Mean TBUT* (SD) | 4.6 (1.2) | 4.5 (1.0) |
| Mean total meibomian gland secretion score† (SD) | 6.5 (3.1) | 6.3 (2.7) |
| Mean corneal staining score total‡ (SD) | 2.5 (2.1) | 2.5 (2.3) |
| Mean conjunctival staining score total‡ (SD) | 4.1 (3.3) | 4.8 (3.1) |
| Mean # meibomian glands yielding any liquid (SD) | 5.6 (2.8) | 5.4 (2.6) |
| Mean # meibomian glands yielding clear liquid (SD) | 0.0 (0.2) | 0.0 (0.0) |
| Dry eye symptom endpoints | ||
| N (subjects) | 67 | 8 |
| Mean OSDI score§ (SD) | 52.0 (14.4) | 51.1 (16.1) |
| Mean SANDE score‖ (SD) | 68.4 (20.3) | 73.2 (16.1) |
| Mean eye dryness score¶ (SD) | 68.9 (22.1) | 68.9 (18.8) |
TBUT is the number of seconds between a blink and the appearance of a first dry spot or negative staining in the tear film.
Total Meibomian Gland Secretion Score is a weighted sum of the number of glands producing secretions, weighted by secretion quality.
Corneal and conjunctival staining scores are the totals across all ocular regions.
Overall score from OSDI questionnaire.
VAS from SANDE questionnaire. The SANDE score is calculated as the geometric mean of the SANDE severity VAS and SANDE frequency VAS.
VAS measured subject's level of discomfort related to eye dryness, ranging from no discomfort to maximal discomfort.
Baseline Disease Characteristics
Per the inclusion criteria, all subjects had TBUT <7 seconds with a mean TBUT of 4.6 ± 1.2 seconds and 4.5 ± 1.0 seconds for the TearCare group and LipiFlow group, respectively. Baseline MGSS was similar for both groups with scores of 6.5 ± 3.1 and 6.3 ±2.7 for the TearCare and LipiFlow groups, respectively. Similarly, there were no notable differences at baseline between the study groups for any of the secondary effectiveness measures (corneal and conjunctival staining scores, the numbers of meibomian glands yielding any or clear liquid, OSDI scores, SANDE scores, and eye dryness scores) assessed (Table 1).
Effectiveness Results
The primary effectiveness endpoints of TBUT and MGSS in subjects treated with both devices demonstrated a statistically significant increase (P < 0.0001) in the mean TBUT and mean MGSS in both groups at all follow-up time points (2 weeks and 1 month).
For subjects treated with TearCare, TBUT increased by 2.74 ± 4.0 seconds and by 3.02 ± 4.4 seconds from baseline at 2 weeks and 1 month postprocedure, respectively. In comparison, subjects treated with LipiFlow experienced an increase of 2.19 ± 2.46 and of 2.58 ± 3.2 seconds from baseline TBUT, respectively (Fig. 1A). In direct comparison, TearCare enabled an increase of 0.49 ± 0.63 seconds in TBUT than that enabled by LipiFlow, with a 90% CI (90% CITBUT) = (− 0.56 to 1.54) at 1 month. Because the noninferiority criteria for TearCare was established PP as a lower 90% CI limit greater than −3.0 for mean TBUT, TearCare established noninferiority with LipiFlow for mean TBUT at 1 month postprocedure (Fig. 1A). The change in TBUT from baseline in the TearCare group was consistently numerically better compared with that of the LipiFlow group at both 2 weeks and 1 month.
FIGURE 1.
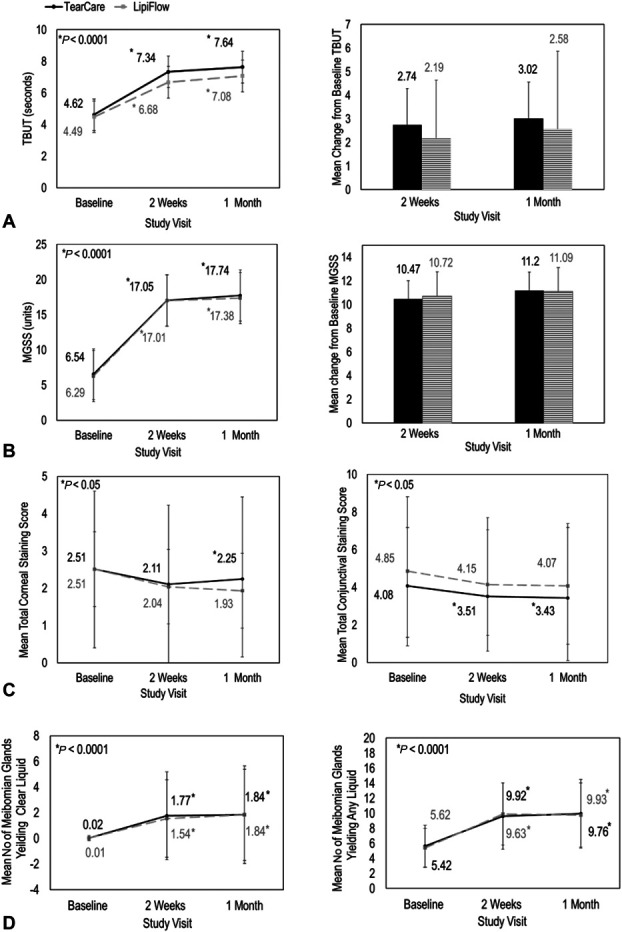
A single TearCare treatment (BLACK, solid) and LipiFlow treatment (GRAY, dashed) were equivalent for the improvement in DED signs, TBUT, MGSS, corneal and conjunctival staining, and meibomian gland health. Panel A, TBUT (seconds) improved for both TearCare and LipiFlow at all study visits. Change from baseline TBUT was similar for both treatment groups. Panel B, MGSS improved for both TearCare and LipiFlow at all study visits. Change from baseline MGSS was similar for both treatment groups. Error bars represent standard deviation. Panel C, Mean total corneal staining (seconds) and mean conjunctival staining improved for both TearCare and LipiFlow at all study visits. Panel D, TearCare enabled equivalent improvements to overall meibomian gland health as measured by the number of meibomian glands yielding clear liquid and the number or meibomian glands yielding any liquid on manual expression of the glands.
At baseline, subjects had poor meibomian gland secretions, with scores of 6.4 on a scale from 0 to 45 (higher number indicates more normal meibomian gland activity). Similar and statistically significant improvements in MGSS were observed for subjects treated with both TearCare and LipiFlow (Fig. 1B). At 2 weeks and 1 month posttreatment, the change from baseline MGSS for subjects receiving a single TearCare treatment (change in MGSS = 10.47 ± 10.89 and 11.20 ± 11.13, respectively) was nearly equivalent to those of subject receiving a single LipiFlow Treatment (change in MGSS = 10.72 ± 9.76 and 11.09 ± 10.41, respectively). In direct comparison, TearCare provided an increase of 0.29 ± 1.88 units in therapeutic benefit in comparison with LipiFlow, with a 90% CIMGSS = (−2.83 to 3.40) at 1 month. Because the noninferiority criteria for TearCare were established per-protocol as a lower 90% CI limit greater than −5.0 for mean MGSS, TearCare established noninferiority with LipiFlow for mean MGSS at 1 month posttreatment (Fig. 1B).
Decrease in the mean corneal staining compared with baseline was statistically significant (P < 0.05) in the TearCare-treated subjects at 1 month postprocedure (Fig. 1C). Furthermore, decreases (P < 0.05) in the mean conjunctival staining were statistically significant compared with baseline at both 2 weeks and 1 month postprocedure for subjects treated with TearCare (Fig. 1C). There was a decrease in corneal and conjunctival staining at 2 weeks and 1 month in the LipiFlow group, but it was not statistically significantly different from baseline. Corneal and conjunctival staining scores showed similar reductions for both devices at 2 weeks and 1 month postprocedure (Fig. 1C). At 2 weeks postprocedure, both TearCare and LipiFlow decreased the mean corneal staining score by approximately 0.4 units [mean reduction from baseline corneal staining score (ΔCSS)TC, 2 weeks = 0.4 ± 1.98, 95% CI = (0.74, 0.06); ΔCSSLF, 2 weeks = 0.46 ± 2.24, 95% CI = (0.84, 0.08)] and at 1 month posttreatment by at least 0.25 units from baseline [ΔCSSTC, 1 month = 0.25 ± 1.98, 95% CI = (0.59, 0.08); CSSLF, 1 month = 0.57 ± 2.01, 95% CI = (0.91, 0.23)]. At 2 weeks postprocedure, both TearCare and LipiFlow decreased the mean conjunctival staining score by approximately 0.5 units [mean reduction from baseline conjunctival staining score (ΔConjSS)C, 2 weeks = 0.57 ± 2.06, 95% CI = (0.92, 0.21); ΔConjSSLF, 2 weeks = 0.69 ± 2.68, 95% CI = (1.15, 0.24)] and at 1 month posttreatment by at least 0.6 units from baseline [ΔConjSSTC, 1 month = 0.66 ± 1.00, 95% CI = (1.04, 0.27); ConjSSLF, 1 month = 0.78 ± 3.18, 95% CI = (1.32, 0.24)].
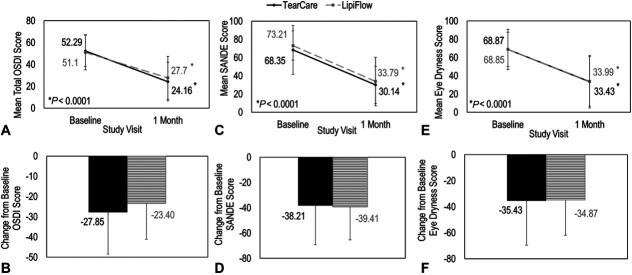
Further analysis of the symptom endpoints demonstrated that both TearCare and LipiFlow procedures resulted in statistically significant improvements compared with baseline in MGD-related symptoms of DED as assessed by OSDI, Eye Dryness, and SANDE questionnaires at 1 month postprocedure (P < 0.0001). There was no difference in the improvement of these assessments between TearCare and LipiFlow (Figs. 2B, D, F). For both devices, the change from baseline OSDI scores was similar and statistically significant with a score of 27.9 ± 20.5 for subjects treated with TearCare and a score of 23.4 ± 17.7 for subjects treated with LipiFlow (Fig. 2A). The proportion of subjects with OSDI improvement of at least 1 severity category was 72% for TearCare and 59% for LipiFlow (Fig. 3A). This analysis revealed that a significantly greater proportion (P < 0.05) of patients in the TearCare group experienced symptomatic relief compared with that of the LipiFlow group. The improvement in MGD-associated DED symptoms was also consistent across assessments of SANDE (Fig. 2C) and Eye Dryness (Fig. 2E) questionnaires. At 1 month postbaseline, subjects treated with TearCare and LipiFlow demonstrated improvements from baseline in mean SANDE scores of 38.2 ± 31.0 and 38.0 ± 25.9, respectively. Similarly, subjects in both treatment groups demonstrated improvements in EDS at 1 month postbaseline. On average, subjects treated with TearCare improved by 35.4 ± 34.1 and subjects treated with LipiFlow improved by 34.9 ± 26.9.
FIGURE 2.
A single TearCare treatment (BLACK, solid) and LipiFlow treatment (GRAY, dashed) were equivalent for the improvement in DED symptoms as assessed by OSDI, SANDE, and EDS. Panel A, OSDI scores improved for both TearCare and LipiFlow at 1 month. Panel B, Changes from baseline OSDI score was similar for both treatment groups. Panel C, SANDE scores improved for both TearCare and LipiFlow at 1 month. Panel D, Changes from baseline SANDE score was similar for both treatment groups. Panel E, SANDE scores improved for both TearCare and LipiFlow at 1 month. Panel F, Changes from baseline SANDE score was similar for both treatment groups. Error bars represent standard deviation.
FIGURE 3.
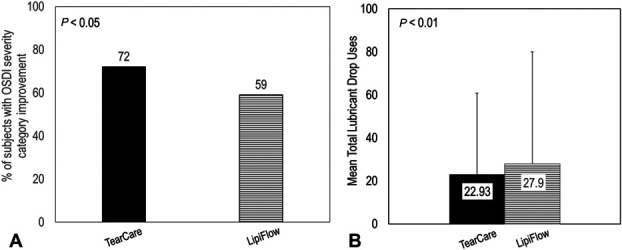
TearCare (BLACK, solid) achieved significantly greater proportion (P < 0.05) of patient experiencing OSDI improvement of at least one severity category and greater decrease in total lubricant drops use (P < 0.01) compared with LipiFlow (GRAY, dashed). Panel A, The proportion of subjects with OSDI severity category improvement was 72% for TearCare and 59% for the LipiFlow treatment group. Panel B, TearCare enabled a greater decrease in subject mean total lubricant drop usage compared with LipiFlow (22.93 ± 37.75 for the TearCare group and 27.9 ± −52.02 for the LipiFlow group). Error bars represent standard deviation.
Similarly, significant and comparable improvements in meibomian gland health as assessed by the number of meibomian glands yielding any liquid (Fig. 1D) or the number of meibomian glands yielding clear liquid (Fig. 1D) were seen in both groups at 2 weeks and at 1 month posttreatment (P < 0.0001). At baseline, subjects in the TearCare and LipiFlow groups had, on average, less than 1 gland yielding clear liquid (nGlands, Clear, TC = 0.02 ± 0.19 glands; nGlands, Clear, LF = 0.01± 0.01 glands) and less than 6 glands yielding any liquid (nGlands, Any, TC = 5.62 ± 2.82 glands; nGlands, Any, LF = 5.42 ± 2.57 glands). At 2 weeks posttreatment, greater than 1.5 glands in both groups yielded clear liquid (nGlands, Clear, TC = 1.77 ± 3.43 glands; nGlands, Clear, LF = 1.54 ± 3.01 glands) and, approximately, 10 glands yielded any liquid (nGlands, Any, TC = 9.63 ± 4.40 glands; nGlands, Any, LF = 9.92 ± 4.13 glands). At 1 month posttreatment, greater than 1.5 glands in both groups yielded clear liquid (nGlands, Clear, TC = 1.74 ± 3.41 glands; nGlands, Clear, LF = 1.53 ± 3.02 glands) and, approximately, 10 glands yielded any liquid (nGlands, Any, TC = 9.93 ± 4.56 glands; nGlands, Any, LF = 9.76 ± 4.27 glands).
Exploratory analyses were conducted to understand the effectiveness of TearCare in reducing the need for lubricant drop use per day compared with LipiFlow and the total lubricant drop uses (Fig. 3B) by subjects during the 1-month follow-up period. The average lubricant drop uses per day was 0.85 ± 1.4 for the TearCare group and 1.07 ± 2.04 for the LipiFlow group. The total number of lubricant drop uses was 22.93 ± 37.75 for the TearCare group [95% CI = (13.72, 32.13)] and 27.9 ± −52.02 [95% CI = (15.31, 40.49)] for the LipiFlow group. A single TearCare treatment resulted in a significantly greater reduction (22% more; P < 0.01) in lubricant drop use compared with a single LipiFlow treatment (Fig. 3B).
Safety Assessments
The TearCare procedure was demonstrated to be as safe as the LipiFlow procedure. The frequency of AEs reported was similar between TearCare and LipiFlow groups, with 7 subjects (6.1%) treated with TearCare experiencing any AE and 8 subjects (6.7%) treated with LipiFlow experiencing any AE. No SAEs were reported in either treatment group. Two severe AEs were reported in the LipiFlow treatment group, both of which (melanoma and hernia) were unrelated to the procedure. Of the 7 AEs experienced by subjects in the TearCare treatment group, 3 AEs were graded as procedure related—one AE of chalazion and graded as definitely related, 1 AE of superficial punctate keratitis and graded as probably related, and 1 AE of blepharitis and graded as possibly related. In comparison, of the 8 AEs reported in the LipiFlow group, 4 AEs were graded as possibly related to the procedure (blepharitis, 2 cases of foreign body sensation, and severe eye dryness).
DISCUSSION
From the results of this study, it is evident that a single treatment of TearCare safely and successfully treats the signs and symptoms of DED in adult patients with MGD. Both TearCare and the active-control device, LipiFlow, resulted in statistically and clinically significant improvements of all signs of DED assessed. Furthermore, the difference between treatment groups was not influenced by sex, severity of DED, or clinical site. Both primary effectiveness endpoints, TBUT and MGSS, improved significantly from baseline in both groups, at both follow-up visits, and in both eyes. These results further confirmed previously reported effectiveness and safety of TearCare for DED treatment. 17,18,25 In a previous exploratory study, improvements in both DED signs (TBUT, MGSS, and corneal and conjunctival staining) and symptoms (OSDI) were observed at the 1-week and 1-month time points for TearCare in comparison with pretreatment baseline. 17 TearCare also performed better than warm compress in previous studies by significantly improving DED signs and symptoms measurements, and such effects were maintained up to 12 months. 18,25 In this study, the 1-month improvement in TBUT was greater than the previously reported clinically meaningful level of 2.5 seconds. 26 Similarly, the 1-month improvement of the MGSS was substantially greater than the established 5-point threshold for clinical relevance. 24 In addition, statistically significant improvements were observed in all symptoms from baseline in both groups. A significantly greater number of subjects treated with TearCare experienced symptomatic relief that reflected in the improvement of OSDI category compared with subjects treated with LipiFlow. Similarly, a significantly smaller number of subjects treated with TearCare required rescue therapy in the form of lubricant drops, through the duration of study compared with subjects treated with LipiFlow.
All secondary effectiveness signs (corneal and conjunctival staining, number of meibomian glands yielding clear liquid, and number of meibomian glands yielding any liquid) were significantly improved at the 2-week and 1-month follow-up time points in both groups. These improvements further underscore the effectiveness of both devices. Significant reductions in staining reflect improvements to the ocular surface and can possibly be attributed to the improved meibomian gland function as shown by more glands secreting meibum (from approximately 5 secreting glands at baseline to approximately 10 glands secreting meibum at 1 month).
The obstruction of meibomian glands, the main cause of MGD, is associated with alterations in meibum chemistry, leading to hyposecretion of meibum. 6,8,27 These obstructions affect tear film stability causing rapid tear film evaporation and drying, tear film hyperosmolarity, and subsequent desiccation and inflammatory damage of epithelial layer, leading to the unhealthy ocular surface. Classic clinical signs are increased corneal–conjunctival staining, hyposecretory or blocked meibomian glands, and reduced TBUT. These changes result in symptoms such as visual degradation, blurred vision, ocular fatigue, ocular discomfort, and foreign body sensation. A stable and normal functioning tear film is the key to maintaining the health of the ocular surface. As such, the first step in treating MGD-related DED is to ameliorate the dysfunctional homeostasis of the tear film, which cannot be achieved without restoration of the lipid layer. Removing the obstruction of meibomian glands is proven to be the most essential step in the treatment for MGD-associated DED. 10,12 Lid hygiene, intraductal probing, topical or systemic medications, application of heat, and lid expression have been used in the treatment of MGD. 12,28 Debridement alone does not target the deeper gland ducts. 12 Gland expression has also been used, but this can be ineffective in some patients with severe blockages and is often sufficiently uncomfortable for patients in whom comprehensive gland clearance is difficult. 29 Because sustained and sufficient therapeutic temperatures at the level of the meibomian glands are critical for the effective liquefaction and clearance of hardened meibum obstructions, optimization of thermal treatments has been sought.
Medical device innovations designed to deliver controlled, thermal therapy to the eyelids to sufficiently liquefy hardened meibum and clear gland obstructions have been gaining traction. The historical challenge has been to achieve a therapeutically sufficient temperature at the tarsal conjunctiva with a noninvasive externally applied heat source. Achieving the therapeutic temperature level of 41°C at the tarsal conjunctiva requires optimized transtarsal deployment of energy for a sufficient period to effectively penetrate highly vascular eyelid tissue. 10 The TearCare system was designed to address this challenge and to deliver optimal therapeutic temperature at the tarsal conjunctiva in the form of a safe, noninvasive, and effective wearable eyelid technology. As evidenced by the clinical data presented earlier, it is reasonable to speculate that the TearCare system reliably achieves a therapeutically effective temperature at the tarsal conjunctiva without the risk of exceeding safety thresholds for the external surface of the eyelid or to the cornea unlike closed eye techniques. The system's eyelid-worn therapeutic devices, SmartLids, offer conformance and adherence across the entire geography of eyelids to maximize transtarsal thermal energy deployment for targeted heat delivery to the underlying meibomian glands. TearCare software and sensor technology enable the tight control and maintenance of a maximized external eyelid temperature of up to 45°C for a sufficient period (15 minutes). The open eye blink-assisted technology allows patients to blink during the thermal portion of procedure, thus facilitating the natural secretion of meibum after liquefying hardened meibum obstructions and priming the glands for evacuation. The TearCare Clearance Assistant permits practitioners to attempt complete removal of liquefied meibum with a tailored gland-by-gland approach under optimal visualization as opposed to gland clearance in devices using automated and invisible approaches of gland massaging. Ultimately, this safe and effective clearance of meibomian gland obstructions with TearCare breaks the vicious cycle of evaporative DED associated with MGD, thereby restoring the quality of the tear film and reducing the rate of tear film evaporation, tear film osmolarity, and ocular surface damage.
No subject in either group experienced any serious adverse events or device-related adverse event that required further management. Three subjects in the TearCare group and 4 subjects in the LipiFlow group experienced device-related adverse events. All these events were self-limited, transient, and resolved without sequelae. Similar results were reported in a previous LipiFlow study, in which 3 of 138 eyes experienced moderate eyelid pain, one eye had a moderate conjunctival vascular injection, and 2 eyes experienced a 10-letter decrease in BCVA. Previous TearCare studies reported no significant adverse events. In general, there were no between-group differences in pain, surface staining, IOP, and BCVA, confirming that the 2 devices were equally safe.
Limitations of the study include the subjective nature of the outcome measures collected in this study. The objective assessments such as meibography and/or noninvasive tear breakup time could have avoided discrepancy in subjective assessment, which often correlates with the level of clinician's experience. Although the investigators assessing the outcomes were masked from the treatment and a comprehensive training was performed to standardize the clinical examination, variance in interpretation may exist. Another limitation of this study is the lack of long-term follow-up. In previous studies, both TearCare and LipiFlow devices were studied for a longer follow-up time. TearCare effectiveness was shown to last 6 months and at least up to 12 months with a re-treatment. 18,25 LipiFlow effectiveness has been studied up to 3 years with positive results. 16 In this study, both devices were shown to be comparable in their safety and effectiveness profile for up to 1 month.
In summary, this clinical evaluation demonstrated that the TearCare System is substantially equivalent to the LipiFlow Thermal Pulsation system in both efficacy and safety. The noninferiority objective of this study was met by demonstrating improvements in TBUT within 3 seconds and improvements in MGSS within 5 units compared with the LipiFlow. On meeting the noninferiority goal, superiority of TearCare compared with LipiFlow was evaluated for signs and symptoms. TearCare has shown to offer significantly better symptomatic relief as measured by proportion of subjects achieving OSDI improvement by at least one severity category and as reflected in the reduced need for use of lubricating drops compared with LipiFlow. Superiority of TearCare could not be established regarding statistical significance but it is noteworthy that TearCare results are consistently numerically better compared with LipiFlow in most of the outcome measures at all follow-up time points. Assessments by 3 independent tools of DED symptoms (OSDI, eye dryness, and SANDE) indicated consistent, global improvements superior to alleviation of symptoms and led to positive patient experience demonstrated by the large effect size of symptomatic improvement after a single TearCare treatment up to 1 month.
ACKNOWLEDGMENTS
The authors thank Gerry Gray, PhD, at Clinreg Consulting, Inc, for statistical analyses support and Ora, Inc, for medical writing support.
Footnotes
This study was funded by Sight Sciences, Inc.
K. Dhamdhere is an employee at Sight Sciences, Inc. The other authors have no conflicts of interest to disclose.
Contributor Information
Preeya K. Gupta, Email: preeya.gupta@duke.edu.
Edward J. Holland, Email: eholland@holprovision.com.
John Hovanesian, Email: jhovanesian@researchinsightca.com.
Jennifer Loh, Email: jenniferlohmd@gmail.com.
Mitchell A. Jackson, Email: mjlaserdoc@msn.com.
Paul M. Karpecki, Email: karpecki@karpecki.com.
Kavita Dhamdhere, Email: kdhamdhere@sightsciences.com.
REFERENCES
- 1. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:276–283. [DOI] [PubMed] [Google Scholar]
- 2. McGinnigle S, Naroo SA, Eperjesi F. Evaluation of dry eye. Surv Ophthalmol. 2012;57:293–316. [DOI] [PubMed] [Google Scholar]
- 3. Pflugfelder SC, de Paiva CS. The pathophysiology of dry eye disease: what we know and future directions for research. Ophthalmology. 2017;124:S4–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yu J, Asche CV, Fairchild CJ. The economic burden of dry eye disease in the United States: a decision tree analysis. Cornea. 2011;30:379–387. [DOI] [PubMed] [Google Scholar]
- 5. Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15:438–510. [DOI] [PubMed] [Google Scholar]
- 6. Nelson JD, Shimazaki J, Benitez-del-Castillo JM, et al. The international workshop on meibomian gland dysfunction: report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci. 2011;52:1930–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Geerling G, Tauber J, Baudouin C, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52:2050–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bron AJ, Tiffany JM. The contribution of meibomian disease to dry eye. Ocul Surf. 2004;2:149–165. [DOI] [PubMed] [Google Scholar]
- 9. Lemp MA, Crews LA, Bron AJ, et al. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31:472–478. [DOI] [PubMed] [Google Scholar]
- 10. Borchman D. The optimum temperature for the heat therapy for meibomian gland dysfunction. Ocul Surf. 2019;17:360–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Borchman D, Foulks GN, Yappert MC, et al. Human meibum lipid conformation and thermodynamic changes with meibomian-gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52:3805–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Qiao J, Yan X. Emerging treatment options for meibomian gland dysfunction. Clin Ophthalmol. 2013;7:1797–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maskin SL. Intraductal meibomian gland probing relieves symptoms of obstructive meibomian gland dysfunction. Cornea. 2010;29:1145–1152. [DOI] [PubMed] [Google Scholar]
- 14. Alghamdi YA, Camp A, Feuer W, et al. Compliance and subjective patient responses to eyelid hygiene. Eye Contact Lens. 2017;43:213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bitton E, Lacroix Z, Leger S. In-vivo heat retention comparison of eyelid warming masks. Cont Lens Anterior Eye. 2016;39:311–315. [DOI] [PubMed] [Google Scholar]
- 16. Greiner JV. Long-term (3 year) effects of a single thermal pulsation system treatment on meibomian gland function and dry eye symptoms. Eye Contact Lens. 2016;42:99–107. [DOI] [PubMed] [Google Scholar]
- 17. Karpecki P, Wirta D, Osmanovic S, et al. A prospective, post-market, multicenter trial (CHEETAH) suggested TearCare system as a safe and effective blink-assisted eyelid device for the treatment of dry eye disease. Clin Ophthalmol. 2020;14:4551–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Badawi D. TearCare system extension study: evaluation of the safety, effectiveness, and durability through 12 months of a second TearCare treatment on subjects with dry eye disease. Clin Ophthalmol. 2019;13:189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lane SS, DuBiner HB, Epstein RJ, et al. A new system, the LipiFlow, for the treatment of meibomian gland dysfunction. Cornea. 2012;31:396–404. [DOI] [PubMed] [Google Scholar]
- 20. Marshall LL, Roach JM. Treatment of dry eye disease. Consult Pharm. 2016;31:96–106. [DOI] [PubMed] [Google Scholar]
- 21. Awisi Gyau D, Begley CG, Daniel Nelson J. A simple and cost effective method for preparing FL and LG solutions. Ocul Surf. 2018;16:139–145. [DOI] [PubMed] [Google Scholar]
- 22. Korb DR, Blackie CA. Meibomian gland diagnostic expressibility: correlation with dry eye symptoms and gland location. Cornea. 2008;27:1142–1147. [DOI] [PubMed] [Google Scholar]
- 23. Lemp MA. Report of the national eye institute/industry workshop on clinical trials in dry eyes. CLAO J. 1995;21:221–232. [PubMed] [Google Scholar]
- 24. Tomlinson A, Bron AJ, Korb DR, et al. The international workshop on meibomian gland dysfunction: report of the diagnosis subcommittee. Invest Ophthalmol Vis Sci. 2011;52:2006–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Badawi D. A novel system, TearCare, for the treatment of the signs and symptoms of dry eye disease. Clin Ophthalmol. 2018;12:683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tauber J. A 6-week, prospective, randomized, single-masked study of lifitegrast ophthalmic solution 5% versus thermal pulsation procedure for treatment of inflammatory meibomian gland dysfunction. Cornea. 2020;39:403–407. [DOI] [PubMed] [Google Scholar]
- 27. Knop E, Knop N, Millar T, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011;52:1938–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arita R, Fukuoka S. Non-pharmaceutical treatment options for meibomian gland dysfunction. Clin Exp Optom. 2020;103:742–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Korb D, Blackie C. Meibomian gland therapeutic expression: quantifying the applied pressure and the limitation of resulting pain. Eye Contact Lens. 2011;37:298–301. [DOI] [PubMed] [Google Scholar]