Abstract
Since Helicobacter pylori was first cultivated from human gastric biopsy specimens in 1982, it has become apparent that many related species can often be found colonizing the mucosal surfaces of humans and other animals. These other Helicobacter species can be broadly grouped according to whether they colonize the gastric or enterohepatic niche. Gastric Helicobacter species are widely distributed in mammalian hosts and are often nearly universally prevalent. In many cases they cause an inflammatory response resembling that seen with H. pylori in humans. Although usually not pathogenic in their natural host, these organisms serve as models of human disease. Enterohepatic Helicobacter species are an equally diverse group of organisms that have been identified in the intestinal tract and the liver of humans, other mammals, and birds. In many cases they have been linked with inflammation or malignant transformation in immunocompetent hosts and with more severe clinical disease in immunocompromised humans and animals. The purpose of this review is to describe these other Helicobacter species, characterize their role in the pathogenesis of gastrointestinal and enterohepatic disease, and discuss their implications for our understanding of H. pylori infection in humans.
It was long believed that the normal human stomach was sterile or colonized only with small numbers of bacteria. The mechanism of what was referred to as the “gastric bactericidal barrier” was debated in the early part of this century 22, but most authors then, as well as more recently 171, concluded that the predominant effect was due to gastric acid. However, the cultivation of a novel bacterium from gastric mucosa in 1982 marked a turning point in our understanding of gastrointestinal microbial ecology and disease. Marshall and Warren 265 described spiral or curved bacilli in histologic sections from 58 of 100 consecutive biopsy specimens of human gastric antral mucosa, 11 of which were culture positive for a gram-negative, microaerophilic bacterium. The organism was thought originally to be a member of the genus Campylobacter and was named Campylobacter pyloridis, later corrected to Campylobacter pylori. Because subsequent 16S rRNA sequence analysis showed that the distance between the true campylobacters and C. pylori was sufficient to exclude it from the Campylobacter genus 336, it was renamed Helicobacter pylori 180, the first member of the new genus Helicobacter.
The early proposal of Marshall and Warren 265 that the newly described bacterium caused gastritis and peptic ulcer proved correct. Furthermore, there is now overwhelming evidence that H. pylori is linked to gastric adenocarcinoma 208, 301, 317, the second most common cause of cancer morbidity and mortality worldwide, and to the development of gastric non-Hodgkin's lymphoma 318, 435. The clinical significance of this bacterium has recently been emphasized by a National Institutes of Health consensus panel that recommended antibiotic therapy for the large majority of peptic ulcer patients who are infected with H. pylori 12 and by classification of H. pylori as a class I (definite) carcinogen by the World Health Organization 13. The vast literature on H. pylori has been reviewed recently in this journal 93, and the complete genome sequence of two strains is now available 6, 82, 260, 395.
Nevertheless, Marshall and Warren were not the first to detect gastric spiral bacteria. Spiral organisms were first seen in human gastric mucosa beginning early in the 20th century 235 and were subsequently described by several investigators (reviewed in reference 264. The bacteria were often seen in malignant or ulcerated gastric tissue 165, and the possibility of an infectious cause of peptic ulcer disease was considered 19. Some even specifically proposed that a search be made for an organism “thriving in hydrochloric acid medium … as a possible factor of chronicity, if not an etiological factor, in peptic ulcer” (see the discussion following reference 165. The suggestion that ulcers might be caused by infection was not new at that time, although an authoritative review published in 1950 concluded that the evidence did not support infection as a cause of peptic ulcer in humans 216. Even before the early observations of gastric spiral bacteria in humans, similar organisms were seen in animals. In his 1881 thesis submitted to the Faculty of Medicine, Rappin described spiral bacteria in gastric scrapings from dogs 330. This observation was later confirmed by Bizzozero 30 and Salomon 344, who performed experimental inoculations with gastric scrapings to transmit infection to mice. Gastric spiral bacteria were subsequently seen in cats 255, rhesus macaques 81, and, more recently, in a variety of other animals.
The cultivation of H. pylori and the recognition of its clinical significance served to renew interest in bacteria associated with the gastrointestinal and hepatobiliary tracts of humans and other animals, many of which have now been identified as novel species of Helicobacter. These organisms are of interest both because of their pathogenic role in humans and animals and because of their value as models of human disease. Other bacteria have also been newly identified, or in some cases reclassified, as novel Helicobacter species that infect humans. The purpose of this review is to describe these other Helicobacter species, characterize their role in the pathogenesis of gastrointestinal and enterohepatic diseases, and discuss their implications for our understanding of H. pylori infection in humans. We conclude with a discussion of an ecological perspective on Helicobacter pathogenesis and recommendations for future work.
GASTRIC HELICOBACTER SPECIES
To date, eight cultivated Helicobacter species have been found in the stomach of humans and other animals, as well as two uncultivated organisms (Table 1). Occasionally a species such as Helicobacter muridarum, which typically colonizes the rodent bowel, is found in the stomach. These organisms are considered later along with other enterohepatic Helicobacter species.
TABLE 1.
Gastric Helicobacter taxa
| Taxon | Natural host | Strain or clone | GenBank 16S rRNA accession no. | Reference |
|---|---|---|---|---|
| H. acinonychis | Cheetah | ATCC 51101T | M88148 | 97 |
| H. bizzozeroni | Dog | ATCC 700030T | Y09404 | 195 |
| Candidatus Helicobacter bovis | Cattle | Clone R2XA | AF127027 | 71 |
| H. felis | Cat, dog | ATCC 49179T | M37642 | 319 |
| “H. heilmannii”a | Human, nonhuman primatea | Clone G1A1 | L10079 | 371 |
| Candidatus Helicobacter suis | Pig | Clone V2BXA | AF127028 | 70 |
| H. mustelae | Ferret | ATCC 43772T | M35048 | 180 |
| H. nemestrinaeb | Pigtailed macaque | ATCC 49396T | X67854 | 42 |
| H. pylori | Human, rhesus macaque | ATCC 43504T | M88157 | 180 |
| H. salomonis | Dog | CCUG37845T | U89351 | 219 |
| “H. suncus” | House musk shrew | Kaz-1 | AB006147 | 182 |
Probably identical to Candidatus Helicobacter suis. Bacteria with the 16S rRNA sequence present in “H. heilmannii” type 1 have to date been identified in humans, pigs, and nonhuman primates, although the host range is probably more extensive. Other hosts have organisms with the “H. heilmannii” morphology that may represent H. bizzozeronii or other Helicobacter species not yet identified.
Unpublished data suggest that H. nemestrinae may be identical to H. pylori (see the text).
Early Morphological Observations
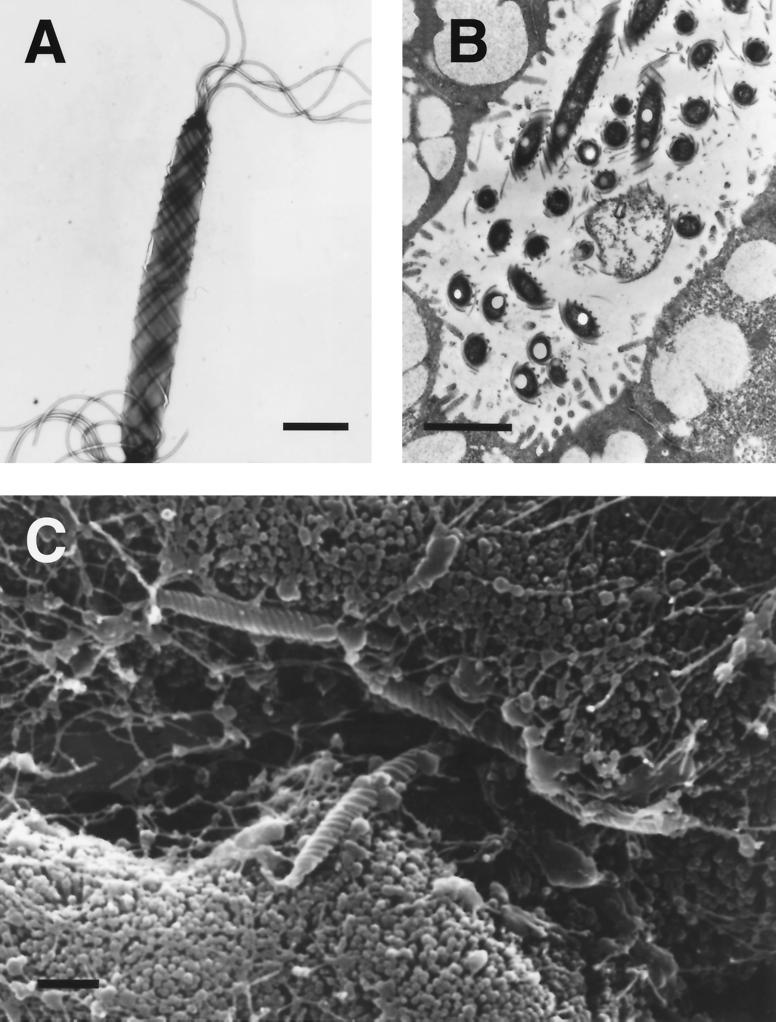
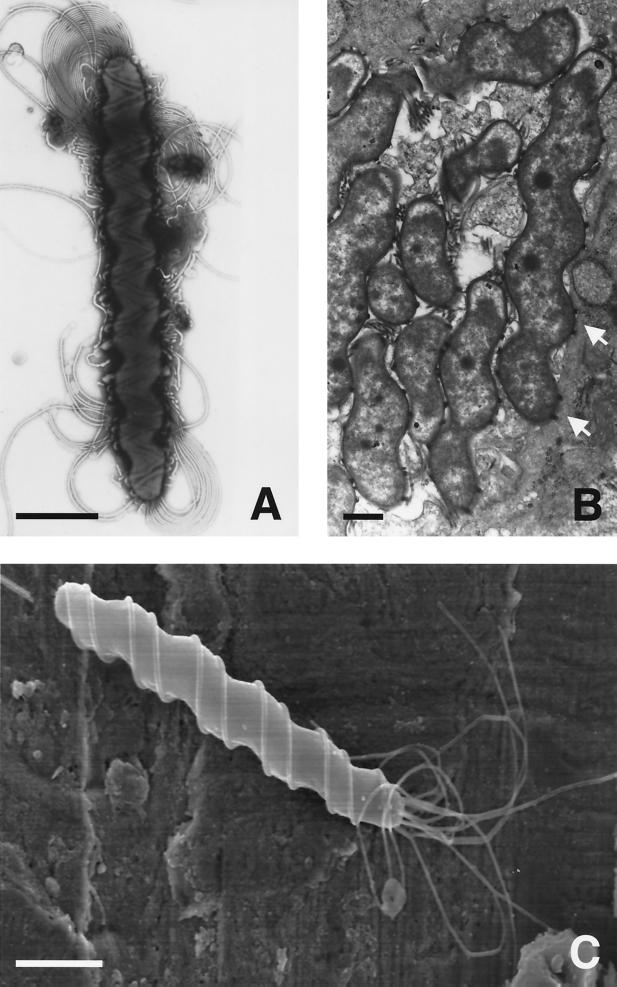
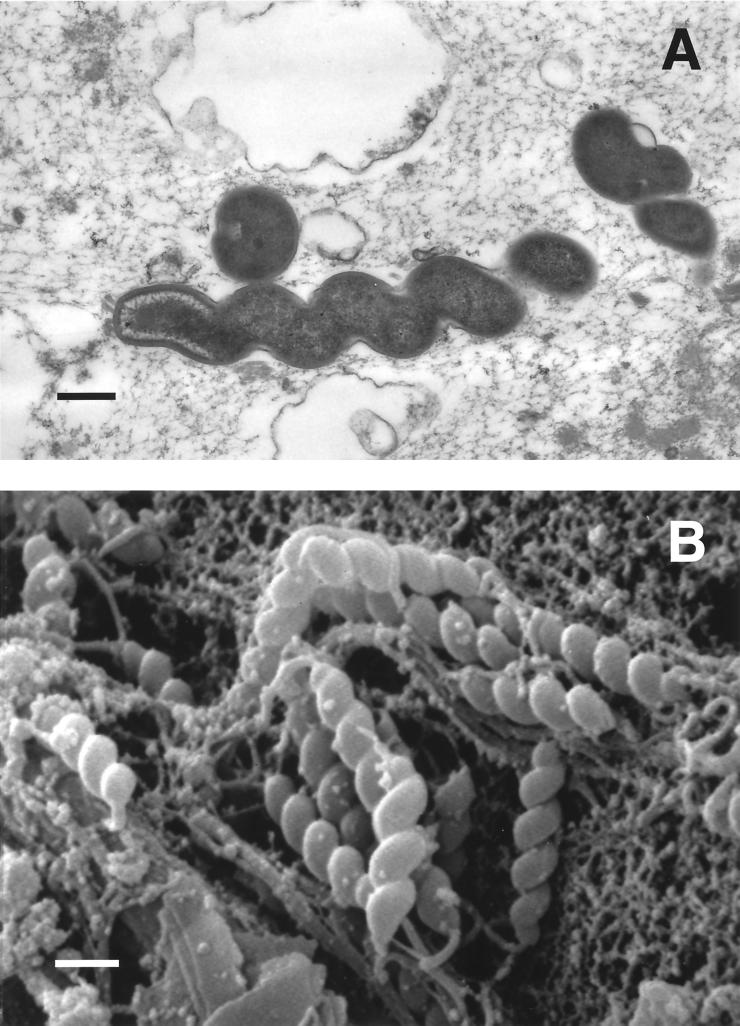
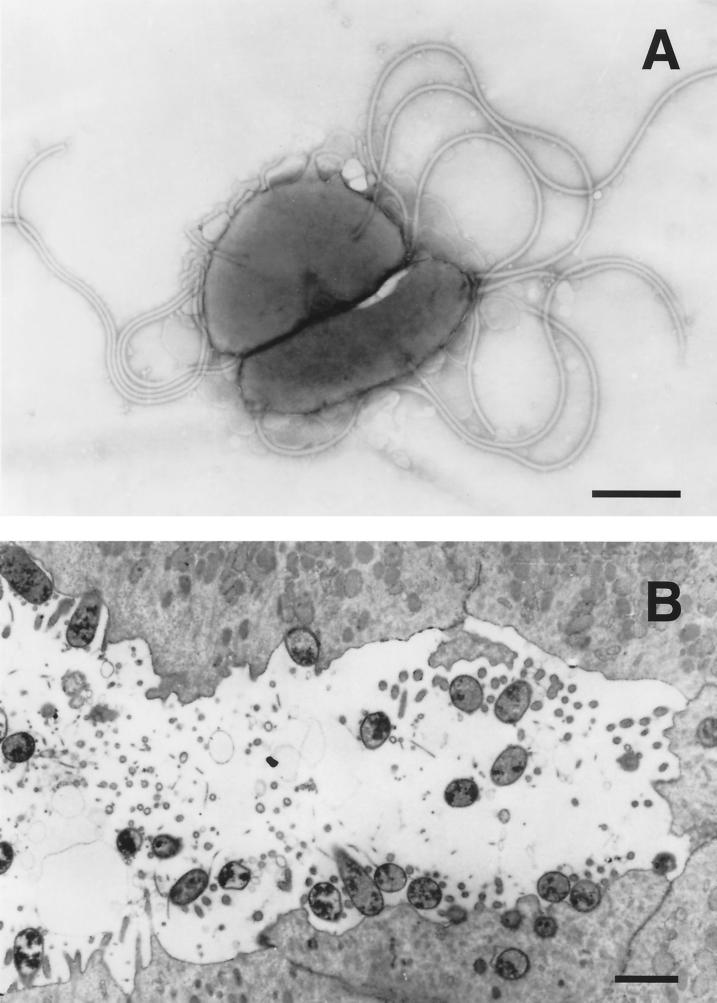
Most of the early observations on gastric spiral bacteria were made in dogs and cats. When the first electron micrograph of these bacteria was published, it was immediately apparent that more than one morphological form could be found 418. Lockard and Boler 257 provided the first high-quality electron micrographs of what are now called Lockard type 1, 2, and 3 bacteria 150, 151; all are now known to represent Helicobacter species. Lockard type 1, which is representative of Helicobacter sp. flexispira, Helicobacter bilis, and others (see “Enterohepatic Helicobacter species” below), has a fusiform to slightly spiral morphology with tapered ends. Multiple periplasmic fibers appear to cover the entire surface of the bacterium (Fig. 1). Lockard type 2 is spiral rather than cylindrical and has periplasmic fibers that are more sparsely distributed and can appear singly or in groups of two, three, and occasionally four (Fig. 2). This organism is the typical morphology of Helicobacter felis. Lockard type 3, which resembles type 2 but is somewhat more tightly coiled and does not have periplasmic fibers, is typical of Helicobacter bizzozeronii and the uncultivated “Helicobacter heilmannii” (Fig. 3). A fourth type, similar to Lockard type 3 but thicker and with fewer coils, was described in the original electron micrographs published by Weber and Schmittdiel 418 but not by Lockard and Boler. This organism may represent Helicobacter salomonis, recently cultivated from dogs 219. The morphology of gastric Helicobacter species isolated from hosts other than dogs and cats is sometimes distinctive (e.g., Helicobacter mustelae [Fig. 4]) and in other cases resembles H. pylori (eg., H. acinonychis).
FIG. 1.
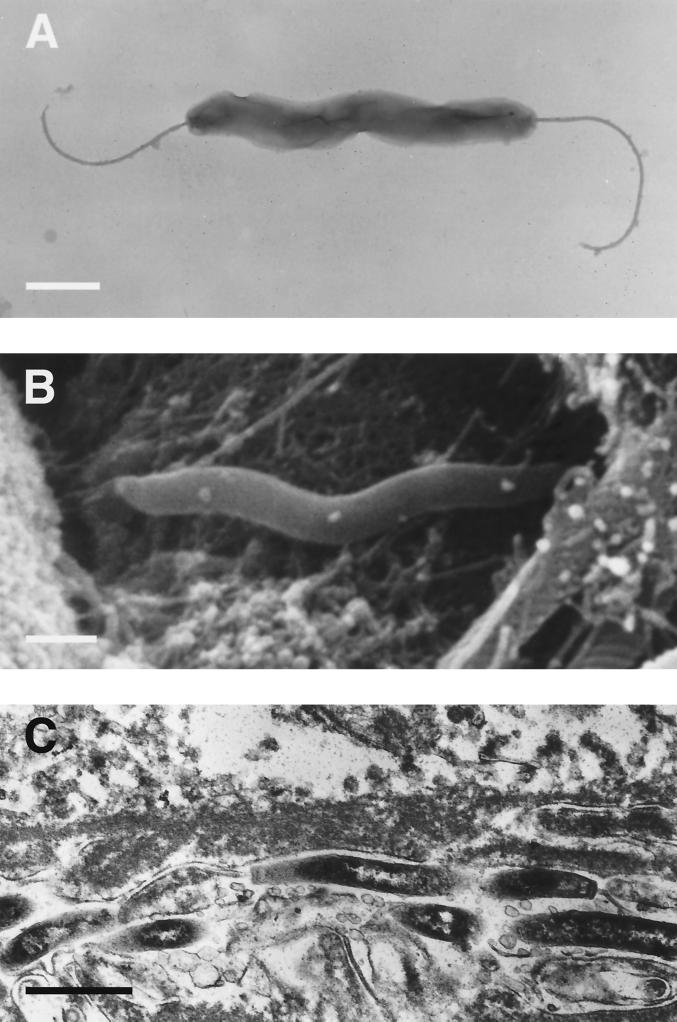
(A and B) Transmission electron micrographs of Helicobacter sp. flexispira (Lockard type 1) taken from a pure culture (A) and from an intestinal crypt in a mouse (B). Bipolar flagella and periplasmic fibers are apparent. (C) Scanning electron micrograph of Helicobacter sp. flexispira shows the organisms among intestinal microvilli in a mouse. Bars, 1 μm. Reprinted from reference 346 with permission from the publisher.
FIG. 2.
(A) Negatively stained preparation of H. felis (Lockard type 2) isolated from the gastric mucosa of an adult cat shows the multiple bipolar flagella. Bar, 1 μm. (C) Although faintly seen in Panel A, the characteristic periplasmic fibers are better visualized on a scanning electron micrograph. Bar, 1 μm. (B) Transmission electron micrograph of H. felis in the gastric mucosa of a germ-free mouse shows the characteristic spiral morphology. Pairs of periplasmic fibers can be seen en face (arrows). Bar, 0.5 μm. Photos courtesy of Adrian Lee, Jani O'Rourke, and Lucinda Thompson.
FIG. 3.
Transmission (A) and scanning (B) electron micrographs of bacteria in the gastric mucosa of a healthy pet cat. The helical morphology without periplasmic fibers (Lockard type 3) is characteristic of “H. heilmannii” and H. bizzozeronii. Bars, 0.5 μm. (A) Reprinted from reference 303 with permission from the American Society for Microbiology. (B) Photo courtesy of Robert Munn.
FIG. 4.
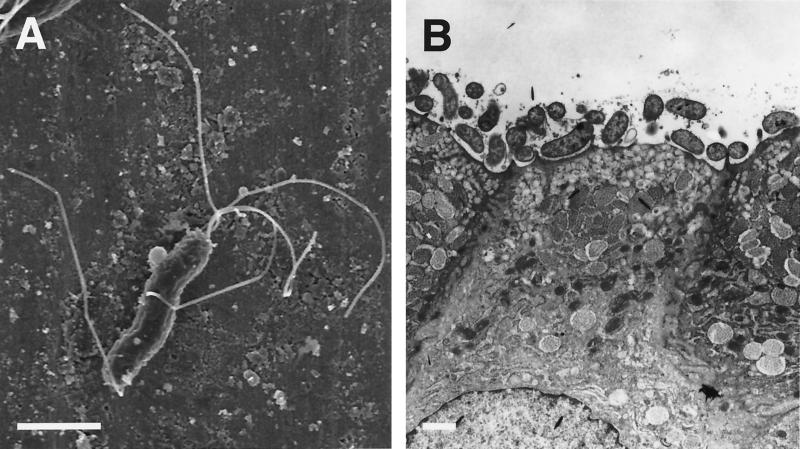
(A) Negatively stained preparation of H. mustelae shows the bipolar and lateral flagella (bar, 0.5 μm). (B) Thin section of a ferret antral gastric pit shows the intimate association of the bacterium with the epithelial surface, including the apparent formation of adhesion pedestals, Bar, 1 μm. Reprinted from reference 309 with permission from the publisher.
Helicobacter mustelae
The ferret (Mustela putorius) stomach has anatomical and physiological similarities to that of humans 135 and is known to experience naturally occurring gastritis and gastric ulcers 191. Shortly after the publication in 1984 of the seminal observations of Marshall and Warren, a Campylobacter-like organism was isolated by Fox et al. from gastric tissue of one ferret with a gastric ulcer and from two others with normal gastric mucosa 148. This observation was quickly confirmed by others 331, 396. The ferret organism was morphologically and biochemically very similar to what was then called C. pylori, and it was originally designated C. pylori subsp. mustelae 139. However, DNA relatedness and 16S rRNA sequence analyses showed that the organism isolated from ferrets was a novel species, which was named C. mustelae 141 and later renamed H. mustelae 180.
Microbiology and phylogeny.
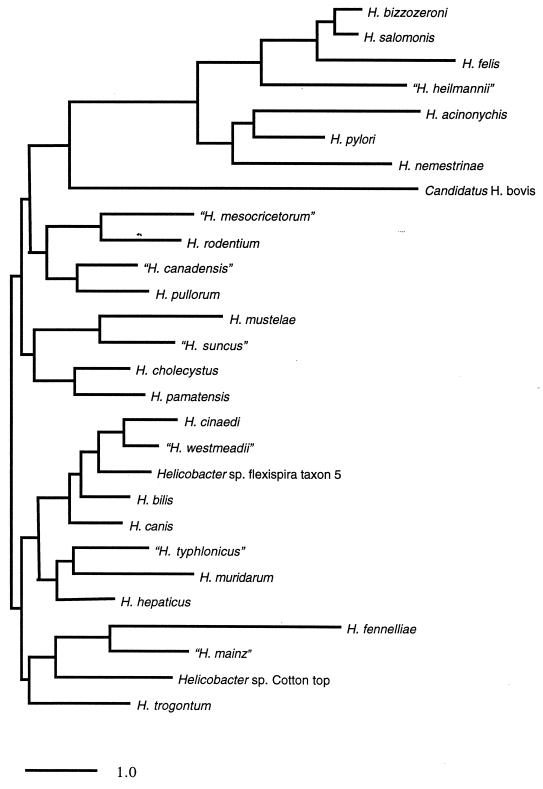
Compared with H. pylori (Fig. 5), H. mustelae is a small rod (0.5 by 2 μm), sometimes slightly curved, with multiple sheathed flagella located at both poles as well as laterally (Fig. 4). Like all gastric Helicobacter species, H. mustelae hydrolyzes urea, although it has other distinctive characteristics such as susceptibility to nalidixic acid (Table 2). The fatty acid composition 379 and protein profiles 287 of H. mustelae are also distinct from those of H. pylori. A phylogenetic tree (Fig. 6) based on a 16S rRNA similarity matrix (Table 3) places H. mustelae closer to Helicobacter species that infect the colon or the hepatobiliary system, particularly H. pametensis and H. cholecystus, than to other gastric Helicobacter species. H. mustelae has a preponderance of hexadecanoic fatty acids, which is characteristic of enteric Helicobacter species and unusual among species that infect the stomach 198. The genome size of H. mustelae is approximately 1.7 Mb 387, which is nearly the same as that determined by sequencing the H. pylori genome 395. Interestingly, there appears to be significant genomic conservation among isolates of H. mustelae 286, 387. This is in marked contrast to the heterogeneity seen among isolates of H. pylori, which are nearly always genetically unique unless derived from the same or related persons 3, 174.
FIG. 5.
(A) Scanning electron micrograph of H. pylori shows the gently curved morphology, multiple bipolar flagella, and absence of periplasmic fibers. Bar, 1 μm. Photo courtesy of Adrian Lee, Jani O'Rourke, and Lucinda Thompson. (B) Transmission electron micrograph of human gastric epithelium with large numbers of H. pylori intimately attached to the surface. Bar, 1 μm. Reprinted from reference 248a with permission from the publisher.
TABLE 2.
| Taxon | Catalase production | Nitrate reduction | Alkaline phosphatase hydrolysis | Urease production | Indoxyl acetate hydrolysis | γ-Glutamyl transferase production | Growth at 42°C | Growth with 1% glycine | Susceptibility to:
|
Periplasmic fibers | No. of flagella | Distribution of flagella | G+C content (mol%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nalidixic acid (30-μg disk) | Cephalothin (30-μg disk) | |||||||||||||
| Gastric | ||||||||||||||
| H. mustelae | + | + | + | + | + | + | + | − | S | R | − | 4–8 | Peritrichous | 36 |
| H. pylori | + | − | + | + | − | + | − | − | R | S | − | 4–8 | Bipolar | 39 |
| H. bizzozeronii | + | + | + | + | + | + | + | − | R | S | − | 10–20 | Bipolar | ND |
| H. felis | + | + | + | + | − | + | + | − | R | S | + | 14–20 | Bipolar | 42 |
| H. acinonychis | + | − | + | + | − | + | − | − | R | S | − | 2–5 | Bipolar | 30 |
| H. nemestrinaec | + | − | + | + | − | ND | + | − | R | S | − | 4–8 | Bipolar | 24 |
| H. salomonis | + | + | + | + | + | ND | − | ND | R | S | − | 10–23 | Bipolar | ND |
| H. suncus | + | + | + | + | − | − | ND | ND | R | R | − | 2 | Bipolar | ND |
| Enterohepatic | ||||||||||||||
| H. rodentium | + | + | − | − | − | − | + | + | R | R | − | 2 | Bipolar | ND |
| H. pullorum | + | + | − | − | − | ND | + | ND | R | S | − | 1 | Monopolar | 34–35 |
| “H. canadensis” | + | V | − | − | + | − | + | + | R | R | − | 1–2 | Bipolar | ND |
| H. fennelliae | + | − | + | − | + | − | − | + | S | S | − | 2 | Bipolar | 35 |
| H. trogontum | + | + | − | + | ND | + | + | ND | R | R | + | 5–7 | Bipolar | ND |
| H. muridarum | + | − | + | + | + | + | − | − | R | R | + | 10–14 | Bipolar | 34 |
| H. hepaticus | + | + | ND | + | + | ND | − | + | R | R | − | 2 | Bipolar | ND |
| H. canis | − | − | + | − | + | ND | + | ND | S | I | − | 2 | Bipolar | 48 |
| H. bilis | + | + | ND | + | − | ND | + | + | R | R | + | 3–14 | Bipolar | ND |
| H. cinaedi | + | + | − | − | − | − | − | + | S | I | − | 1–2 | Bipolar | 37–38 |
| “Helicobacter sp. flexispira” | + | − | − | + | ND | + | + | − | R | R | + | 10–20 | Bipolar | 34 |
| H. cholecystus | + | + | + | − | − | − | + | + | I | R | − | 1–3 | Monopolar | ND |
| “H. typhlonicus” | + | + | − | − | − | − | − | + | ND | ND | − | 2 | Bipolar | ND |
| “H. mesocricetorum” | + | + | + | − | ND | − | + | − | S | R | − | 1 | Bipolar | ND |
| H. pametensis | + | + | + | − | − | − | + | + | S | S | − | 2 | Bipolar | 38 |
| “Helicobacter westmeadii” | + | + | + | + | ND | ND | − | ND | S | R | − | 1 | Monopolar | ND |
| Helicobacter sp. cotton top | + | + | + | + | − | + | + | + | S | R | − | 2 | Bipolar | 31 |
| “Helicobacter mainz” | + | − | − | − | − | ND | − | ND | R | S | ND | ND | ND | ND |
Adapted from reference 151 with permission from the publisher.
+, positive reaction; −, negative reaction; S, susceptible; R, resistant; I, intermediate; ND, not determined; V, variable.
Unpublished data suggest that H. nemestrinae may be identical to H. pylori (see the text).
FIG. 6.
Phylogenetic tree for 19 validated Helicobacter species and 9 additional provisional species, based on 16S rRNA sequence. The scale bar represents a 1% difference in nucleotide sequence as determined by measuring the lengths of horizontal lines connecting any two species. The tree was constructed from the differences matrix (Table 3) using TREEVIEW (314a).
TABLE 3.
Distance matrix based on 16S rRNA comparisons
| Taxon | % Differencea
|
|||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H. acinonychis | H. pylori | H. nemestrinae | H. bizzozeronii | H. salomonis | H. felis | “H. heilmannii” | H. mustelae | “H. suncus” | “H. mesocricetorum” | H. rodentium | H. cholecystus | H. pamatensis | “H. canadensis” | H. pullorum | H. cinaedi | “H. westmeadii” | “Helicobacter sp. flexispira” | H. bilis | H. canis | H. hepaticus | “H. typhlonicus” | H. trogontum | H. muridarum | H. fennelliae | “H. mainz” | “Helicobacter sp. cottontop” | Candidatus H. bovis | |
| H. acinonychis | 4.2 | 6.7 | 5.8 | 5.7 | 6.2 | 7.6 | 9.9 | 9.6 | 9.2 | 9.5 | 8.8 | 8.4 | 8.8 | 8.4 | 10.5 | 10.4 | 10.5 | 10.2 | 9.2 | 9.2 | 10.3 | 10.1 | 9.6 | 12.3 | 10.0 | 9.1 | 12.6 | |
| H. pylori | 3.8 | 5.6 | 5.6 | 6.7 | 6.3 | 8.3 | 8.0 | 6.9 | 7.3 | 7.3 | 6.8 | 7.2 | 6.6 | 9.2 | 9.0 | 8.7 | 8.2 | 7.4 | 7.8 | 8.8 | 8.4 | 8.5 | 10.4 | 8.0 | 7.2 | 11.1 | ||
| H. nemestrinaeb | 6.3 | 6.3 | 8.1 | 7.3 | 9.7 | 8.3 | 8.8 | 8.8 | 8.1 | 8.5 | 8.6 | 8.2 | 10.1 | 9.5 | 9.6 | 9.1 | 8.6 | 8.8 | 9.5 | 9.5 | 10.4 | 12.1 | 9.4 | 8.5 | 11.6 | |||
| H. bizzozeronii | 0.9 | 3.3 | 3.9 | 9.4 | 8.7 | 9.2 | 8.6 | 7.3 | 8.0 | 8.1 | 7.2 | 9.5 | 9.3 | 9.3 | 9.0 | 8.4 | 8.1 | 8.9 | 8.6 | 9.5 | 10.7 | 9.1 | 7.0 | 10.8 | ||||
| H. salomonis | 3.0 | 3.9 | 9.2 | 8.5 | 9.1 | 8.7 | 7.1 | 7.8 | 8.1 | 7.0 | 9.5 | 9.1 | 9.3 | 9.1 | 8.1 | 8.1 | 8.9 | 8.5 | 9.8 | 10.6 | 9.0 | 7.0 | 11.0 | |||||
| H. felis | 6.3 | 10.2 | 10.5 | 11.1 | 10.6 | 9.3 | 9.2 | 9.8 | 9.0 | 11.1 | 11.1 | 10.8 | 11.0 | 10.1 | 9.9 | 11.0 | 10.4 | 10.4 | 11.8 | 10.8 | 9.0 | 13.1 | ||||||
| “H. heilmannii” | 10.7 | 9.6 | 9.3 | 9.0 | 9.1 | 9.5 | 8.5 | 8.0 | 10.0 | 9.8 | 9.5 | 9.5 | 8.9 | 8.9 | 9.7 | 9.6 | 10.4 | 10.9 | 9.6 | 8.0 | 11.6 | |||||||
| H. mustelae | 3.4 | 6.7 | 7.0 | 5.1 | 4.5 | 6.2 | 6.7 | 7.1 | 6.8 | 5.9 | 6.0 | 5.5 | 5.5 | 6.8 | 5.3 | 6.2 | 9.4 | 6.5 | 6.5 | 9.7 | ||||||||
| “H. suncus” | 6.0 | 5.6 | 4.4 | 4.5 | 4.6 | 5.0 | 5.9 | 5.6 | 5.6 | 4.9 | 4.5 | 4.3 | 5.4 | 4.0 | 6.0 | 10.0 | 6.2 | 5.7 | 9.1 | |||||||||
| “H. mesocricetorum” | 3.0 | 5.2 | 4.3 | 4.3 | 4.1 | 5.8 | 5.0 | 5.7 | 5.3 | 5.4 | 5.6 | 6.1 | 4.6 | 6.9 | 7.9 | 5.0 | 5.8 | 9.8 | ||||||||||
| H. rodentium | 4.6 | 4.9 | 3.7 | 4.3 | 5.4 | 5.0 | 5.1 | 4.6 | 4.9 | 5.0 | 5.8 | 4.9 | 6.5 | 8.4 | 6.0 | 5.9 | 9.1 | |||||||||||
| H. cholecystus | 2.2 | 3.6 | 3.7 | 5.1 | 4.5 | 4.1 | 3.6 | 3.6 | 3.9 | 4.5 | 4.6 | 5.0 | 7.9 | 5.6 | 5.2 | 8.7 | ||||||||||||
| H. pamatensis | 3.7 | 3.6 | 5.8 | 5.2 | 4.5 | 4.2 | 4.5 | 4.4 | 5.5 | 4.6 | 5.0 | 7.8 | 5.5 | 5.8 | 9.3 | |||||||||||||
| “H. canadensis” | 2.2 | 5.2 | 4.9 | 4.0 | 4.0 | 3.9 | 2.8 | 4.1 | 4.6 | 4.6 | 8.3 | 5.6 | 5.5 | 8.6 | ||||||||||||||
| H. pullorum | 5.2 | 4.9 | 4.6 | 4.3 | 3.9 | 4.4 | 4.7 | 4.2 | 6.0 | 7.5 | 4.9 | 4.7 | 9.2 | |||||||||||||||
| H. cinaedi | 1.5 | 2.0 | 2.6 | 3.0 | 3.8 | 3.1 | 4.5 | 5.6 | 7.6 | 4.8 | 4.9 | 10.3 | ||||||||||||||||
| “H. westmeadii” | 2.2 | 2.4 | 2.6 | 3.6 | 3.0 | 3.9 | 5.5 | 7.2 | 4.3 | 4.5 | 10.0 | |||||||||||||||||
| “Helicobacter sp. flexispira”c | 2.0 | 2.6 | 3.0 | 3.2 | 4.9 | 5.1 | 7.6 | 5.2 | 5.1 | 9.5 | ||||||||||||||||||
| H. bilis | 1.7 | 2.9 | 2.8 | 4.1 | 4.9 | 7.6 | 4.8 | 4.4 | 9.2 | |||||||||||||||||||
| H. canis | 3.0 | 2.7 | 4.0 | 4.6 | 7.9 | 4.5 | 3.6 | 9.2 | ||||||||||||||||||||
| H. hepaticus | 2.5 | 3.1 | 3.3 | 7.6 | 4.7 | 4.4 | 8.1 | |||||||||||||||||||||
| “H. typhlonicus” | 3.7 | 3.4 | 7.6 | 4.7 | 4.3 | 9.8 | ||||||||||||||||||||||
| H. trogontum | 4.8 | 7.1 | 4.3 | 4.4 | 9.5 | |||||||||||||||||||||||
| H. muridarum | 8.5 | 5.8 | 5.4 | 10.1 | ||||||||||||||||||||||||
| H. fennelliae | 5.4 | 6.6 | 13.6 | |||||||||||||||||||||||||
| “H. mainz” | 4.7 | 11.2 | ||||||||||||||||||||||||||
| “Helicobacter sp. cottontop” | 10.1 | |||||||||||||||||||||||||||
| Candidatus H. bovis | ||||||||||||||||||||||||||||
Sequences from strains shown in Tables 1 and 2 were aligned with PILEUP and compared with DISTANCES (Wisconsin Sequence Analysis Package; Genetics Computer Group). Only sequence positions for which data were available for at least 90% of strains were included in the analysis (E. coli positions 28 to 1473). Intervening sequences were removed from H. bilis, H. typhlonicus, and Helicobacter sp. cottontop. Percentages of difference were corrected for multiple base changes by the method of Jukes and Cantor.
Unpublished data sugggest that H. nemestrinae may be identical to H. pylori.
Taxon 5.
Epizootiology.
In the original report, 3 (18%) of 17 ferrets aged 9 to 10 months were found to be infected with H. mustelae when examined at necropsy 148. Subsequent studies showed that the prevalence of H. mustelae infection increases with age, from 0% in kits less than 1 month of age to 100% in adults over 1 year 139, 156. This relationship of prevalence to age mimics the seroepidemiology of H. pylori in humans, particularly in developing countries 21, as well as the seroepizootiology of H. pylori in nonhuman primates 89, 369. Like H. pylori, infection with H. mustelae is apparently persistent. H. mustelae is widespread among colonies of laboratory ferrets 180, 331, 396 and has also been seen in ferrets kept as pets 156. Although examination of adult ferrets from one New Zealand pelt farm failed to find any evidence of infection 290, more recently H. mustelae has been isolated from captive and wild ferrets in New Zealand 131. It seems likely that H. mustelae is a member of the resident flora of the ferret stomach that infects virtually all animals by adulthood, much as is true for human H. pylori infection in most of the world.
Transmission.
Direct person-to-person transmission of H. pylori is supported by the clustering of cases in families 86, the similarity of H. pylori genotypes that is sometimes found among isolates from related persons 409, and the failure to find evidence of an environmental reservoir, although transmission in developing countries by contaminated food or water remains possible 209, 232. Nevertheless, the mechanism by which H. pylori moves from the stomach of one host to that of another remains an enigma. Fox and colleagues have offered a series of observations which suggest that transmission of H. mustelae may occur by the fecal-oral route and that it is promoted by hypochlorhydria. Fecal cultures from 9-week-old ferrets were positive for H. mustelae in 8 (31%) of 26 animals, but cultures from the same ferrets at 20 weeks of age were negative 158. The authors hypothesized that the animals were naturally infected at 5 to 6 weeks of age and were hyphochlorhydric when sampled at 9 weeks of age, a time course which roughly corresponds to the period of transient hypochlorhydria seen in experimentally inoculated animals 157. The increased gastric pH may have permitted greater numbers of bacteria to exit the stomach and enter the lower gastrointestinal tract, where they could be cultivated from feces and possibly serve as a mechanism for transmission. However, the gastric pH was not measured, nor was the timing of acute infection documented, although subsequent rising titers suggested that it was recent. Furthermore, fecal cultures from three (75%) of four ferrets that were 8 months old, and probably not in the window of transient hypochlorhydria, were also positive for H. mustelae. When adult ferrets chronically infected with H. mustelae were treated with a proton pump inhibitor (omeprazole) to raise their gastric pH, recovery of H. mustelae in fecal cultures was increased compared to that before treatment, although in one animal the fecal cultures were positive even though the gastric pH failed to rise 138. On balance, these experiments suggest a role for raised gastric pH in transmission of H. mustelae by the fecal-oral route, although they do not directly demonstrate fecal-oral transmission. Replication of these results by an alternate method of raising the gastric pH, such as blockade of histamine 2 receptors, might exclude the possibility that the effect of omeprazole was through a mechanism other than alteration of gastric pH. Future studies on transmission may find it useful to exploit the nonhuman primate model of H. pylori 87, 88, 369 to extend the provocative work by Fox et al.
Development of the ferret model.
Early attempts to develop a small-animal model of H. pylori were unsuccessful. Although small laboratory animals have subsequently been infected with H. pylori 261, 361, 424, none of these animals is naturally infected. H. mustelae infection of ferrets remains the best-studied animal model of a gastric Helicobacter in its natural host and provides the opportunity to study the relationship between infection and disease.
(i) Gastritis.
Ferrets naturally infected with H. mustelae have a predominantly mononuclear gastritis composed of lymphocytes and plasma cells, with only occasional eosinophilic and polymorphonuclear leukocytes 142, 183. The near absence of an active (polymorphonuclear) component to the inflammation distinguishes the gastritis from that often seen in adults infected with H. pylori, although children and most other animals infected with a gastric Helicobacter strain tend also to have a mononuclear infiltrate. In the corpus of the ferret stomach, the gastritis is minimal or only superficial. However, in the antrum, where the bacteria predominate, the inflammatory infiltrate may occupy the full thickness of the mucosa, similar to the diffuse antral gastritis seen in humans infected with H. pylori. Gastric glands may also be affected, with evidence of both atrophy and regeneration. Experimental inoculation of ferrets with H. mustelae produces elevated immunoglobulin G (IgG) titers to H. mustelae and a histologic gastritis 157 which resembles that seen in naturally infected animals 157. These changes are also accompanied by an elevation in meal-stimulated gastrin levels similar to what is seen in humans infected with H. pylori 320. Surprisingly, very minimal gastritis was seen in H. mustelae-infected ferrets in England 396. This observation has not been repeated, and it is unknown whether it was due to differences in the host, the pathogen, or both.
(ii) Ulcers.
Unlike gastritis, which is seen in all cases, most patients infected with H. pylori do not develop peptic ulcer disease. While the association between H. pylori and peptic ulcer disease is undisputed—the current standard of care is to use antibiotics to treat patients with peptic ulcers and H. pylori infection—the evidence for this association is nonetheless indirect. It is based primarily on the demonstration that ulcer recurrence in patients treated with antibiotics, with or without acid suppression, is markedly lower than in patients treated with acid suppression alone 184. Limited case-control data also show that preexisting H. pylori infection increases the risk for subsequent development of duodenal and gastric ulcers 302. In principle, H. mustelae infection in the ferret offers the opportunity to study experimentally the association between Helicobacter infection and peptic ulcer. Ferrets sometimes develop gastric ulcers and hemorrhagic gastric erosions 191, with a prevalence of 35% in a series of 31 ferrets in England 9 but only 1.4% in a large postmortem study of 350 animals performed by the same author 8. Gastric ulcers have also been found in related fur-bearing animals 26 and other laboratory species 269. However, there are no studies which provide convincing evidence that H. mustelae infection in the ferret causes peptic ulcer disease, although H. mustelae has occasionally been seen in ferrets with gastric ulcer. Such a demonstration would require long-term observation of experimentally infected and pathogen-free ferrets, which has not been done. Nevertheless, since H. mustelae-infected ferrets may develop duodenal ulcer or gastric ulcer, the latter of which may be associated with atrophic gastritis, dysplasia, and gastric adenocarcinoma, the model mimics in many respects the relationship between human hosts and infection with H. pylori.
(iii) Gastric malignancy.
H. mustelae also provides an opportunity to study Helicobacter infection and gastric tumorigenesis, but, as with ulcer disease, the role of H. mustelae in gastric tumors of the ferret has not yet been clearly demonstrated. Gastric adenocarcinoma 333 and gastric mucosa-associated lymphoid tissue (MALT) lymphoma 110 have been found occasionally in the ferret, sometimes in association with H. mustelae infection 143. However, since ferrets are routinely infected with H. mustelae, infection alone must be insufficient to produce malignancy with any frequency, at least during the first few years of the animal's life, when most studies are performed. Treatment of H. mustelae-infected ferrets with N-methyl-N-nitro-N′-nitrosoguanidine (MNNG), a gastric carcinogen in several animal species, produced adenocarcinoma in 9 of 10 animals 159. Unfortunately, uninfected animals were not studied, and MMNG alone produces gastric adenocarcinoma in many species 166, 381, 382. H. mustelae-infected ferrets do have an increase in gastric epithelial-cell proliferation, especially in the antrum, where colonization is heaviest. This cell proliferation might promote the development of carcinoma in the setting of an appropriate initiation event 434. Although definitive evidence for the role of H. mustelae in gastric malignancy and peptic ulcer disease is lacking, the model remains a valuable tool for the study of gastritis and epithelial proliferation. Future studies will probably clarify the role of H. mustelae in the development of peptic ulcer disease and gastric cancer.
(iv) Therapy.
Limited studies of antibiotic therapy for H. mustelae indicate that it is sensitive in vitro to many of the agents effective against H. pylori. The MIC of amoxicillin is 1 to 2 log units higher than for H. pylori 4, 221, 313, and therefore combination treatment containing amoxicillin may be less effective in ferrets than in humans 4, 313. The combination of ranitidine bismuth citrate and clarithromycin is effective therapy for H. mustelae infection in ferrets 263. Both these drugs are often used to treat H. pylori infection in humans, although this combination is not a generally recommended treatment regimen.
Virulence determinants.
(i) Urease.
H. mustelae produces a urease, composed of two subunits, that is similar in stoichiometry (hexameric 1:1), molecular mass (564 kDa), Km (0.45 mM), and percentage of total cellular protein (2%) to that from H. pylori and other gastric Helicobacter species 94, 401, 402. Partial DNA sequencing of the H. mustelae urease genes showed that the predicted proteins were 67 to 68% identical to other Helicobacter ureases for UreA and 79% identical for UreB 370. The ureases of H. pylori, H. felis, and “H. heilmannii” are more closely related to one another than they are to the urease of H. mustelae. This finding is similar to the results of a phylogenetic study based on 16S rRNA sequence as well as G+C content 370, demonstrating that the urease structural genes can be used as a basis for phylogenetic analysis. An isogenic urease-negative mutant of H. mustelae does not colonize the ferret 11, much the same as has been found for H. pylori in the pig and mouse models 98, 101, 400. The mechanism for this failure is unknown, but it probably involves more than buffering of gastric acid, since an isogenic urease-negative H. pylori strain will not colonize the pig even if the gastric pH is raised pharmacologically 100. H. mustelae appears to have reduced survival at pH 6.0 if physiological concentrations of urea are present, which has been attributed to the accumulation of ammonia and its metabolism by the cell 423. However, a similar observation made with H. pylori appears to result from a rise in the pH of the medium rather than from ammonia accumulation 54. The apparent requirement of urease for colonization has led to an investigation of the therapeutic potential of fluorofamide, a potent urease inhibitor. Although fluorofamide markedly inhibited H. mustelae urease in vitro and in vivo 270, 324 and reduced bacterial numbers, it failed to eradicate H. mustelae, even when administered with amoxicillin. This may be due to inadequate drug delivery or to residual intracellular urease. It may also suggest that in vivo some bacteria are not completely dependent on urease for survival 324. H. mustelae has a gene identical to the H. pylori hpn locus, which codes for a protein with 47% histidine residues that binds nickel but is not required for urease activity 172.
(ii) Flagella.
Flagellar organization in H. mustelae (and H. pylori) resembles that seen in Campylobacter, where the mature flagella are assembled from two flagellin proteins, FlaA and FlaB. The flaB gene has a sigma 54-type promoter that is functionally active, which suggests that it may be environmentally regulated. FlaA and FlaB of H. mustelae have 80% amino acid identity to the corresponding proteins in H. pylori. Unlike in Campylobacter, the Helicobacter flaA and flaB genes are not linked on the chromosome, nor are they closely related to one another 225, 380. Many other genes have been identified in the H. pylori genome that are probably involved in secretion, regulation, and assembly of flagella 395. One of these, the flagellar hook gene (flgE), has also been identified in H. mustelae 312, and it is likely that others will be found. A flaA isogenic mutant of H. mustelae has markedly diminished motility, while the motility of a flaB mutant is diminished 30 to 40% 225. Single-gene mutations in flaA or flaB reduce the density of colonization, whereas the nonmotile flaA flaB double mutant is unable to colonize the ferret 10. These results clearly indicate that motility is an important virulence factor for colonization in the H. mustelae ferret model.
(iii) Attachment.
H. mustelae is found almost exclusively in intimate association with ferret gastric epithelial cells (Fig. 4), with few if any bacteria in the overlying mucus gel 309. Occasionally, bacteria are actually endocytosed into the gastric epithelial cells. Studies of attachment in Helicobacter have sometimes been plagued by a discrepancy between the striking host and tissue specificity seen in vivo and the sometimes nonspecific attachment seen in vitro. When examined by flow cytometry, H. pylori but not H. mustelae attached well to AGS cells, a human gastric carcinoma cell line. This observation suggests some specificity for the appropriate host cell 258, but H. mustelae adhered to explants of stomach from pigs, rats, and rabbits, as well as to pig duodenum and urinary bladder 279. H. pylori attachment to gastric epithelial cell lines causes pedestal formation similar to that seen with enteropathogenic Escherichia coli, accompanied by cytoskeletal rearrangements and tyrosine phosphorylation of host cell proteins 350. Similar adhesion pedestals have been seen with H. mustelae 309, but recruitment of filamentous actin was not apparent when examined in tissue culture by electron microscopy 176. The alpAB locus, which codes for outer membrane proteins that are required for in vitro attachment of H. pylori to gastric epithelium, is not present in H. mustelae or in H. felis 306.
The mechanism by which H. mustelae attaches to gastric epithelium is unknown. Most strains of H. mustelae agglutinate red blood cells derived from various hosts 389. Although hemagglutination is inhibited by pronase, heat, and trypsin, thereby implying the presence of a protein ligand, it is also sometimes partially inhibited by blocking with fetuin or neuraminidase. H. mustelae and H. pylori appear to bind common lipid receptors, particularly phosphatidylethanolamine (PE), and adhesion to intact eukaryotic cells correlates with the amount of PE present 176, 178. Attachment of H. mustelae to PE can be partially inhibited by bovine and human colostrum 29. This may result from inhibition by colostral PE or PE derivatives, but the importance of other constituents of colostrum cannot yet be excluded. Nonspecific cell surface properties such as hydrophobicity may also contribute to H. mustelae binding to gastric epithelium 177. Estimates of the relative hydrophobicity of H. mustelae depend on the assay used, and there may also be local differences at different places on the bacterial cell membrane. However, H. mustelae is thought to be predominantly hydrophilic. Inflammation induced by H. mustelae is associated with a reduction in mucosal hydrophobicity, which may promote nonspecific attachment. Similar findings have been reported with H. pylori 175. Attachment based on surface characteristics may explain the apparent nonspecific binding that is sometimes observed 279 in vitro but that contrasts strikingly with the host and tissue specificity of H. mustelae. It is likely that attachment of H. mustelae to gastric epithelium is dependent on more than a single ligand-receptor interaction.
The outer membrane of H. mustelae is studded with an array of 8.5-nm-diameter rings that are composed of a 150-kDa protein designated Hsr (for “Helicobacter surface ring”) 310. The C-terminal portion of Hsr has limited homology to a cleaved portion of SepA, the major extracellular protein of Shigella flexneri 25. Cross-reactive proteins are present in three strains of H. mustelae, but the protein and the hsr gene are absent in H. pylori. Determination whether Hsr contributes to colonization, either as an adhesin or perhaps by inhibition of complement-mediated killing as is the case for the S-layer of Campylobacter fetus 37, will require examination of isogenic hsr mutants in the ferret model.
(iv) Lipopolysaccharide.
H. pylori lipopolysaccharide (LPS) has relatively low biological activity compared to that from the family Enterobacteriaceae 294, but it is of particular interest because of evidence that it expresses human Lewis (Le) antigens that are also present on the gastric epithelium 358. The relatively inactive LPS, combined with host antigens on its surface, may be a mechanism for H. pylori to down regulate and evade the host inflammatory response and thereby favor long-term colonization 36. Autoantibodies against the bacterial Le antigens may also be important in the pathogenesis of gastroduodenal pathology 15. H. mustelae LPS does not express Le antigens, nor are they expressed on ferret gastric epithelial cells. However, both ferret gastric epithelium and H. mustelae LPS express blood group antigen A, which may be a mechanism of molecular mimicry similar to expression of Le antigens by H. pylori 285, 305.
Helicobacter felis
Spiral bacteria have long been seen on histologic sections of gastric mucosa from cats and dogs. In 1988 Lee et al. reported for the first time the culture of one of these organisms from the cat stomach 246. A similar organism was found in dogs, and both were designated H. felis 319.
Microbiology and phylogeny.
H. felis is biochemically similar to other gastric Helicobacter species (Table 2). It has a helical morphology (Fig. 2), rather than the curved or loosely spiral appearance of H. pylori. H. felis is also characterized by periplasmic fibers, usually in pairs, that wind around the organism and have been used to distinguish it microscopically from the morphologically very similar but uncultivated “H. heilmannii.” However, these fibers may not be present on all strains of H. felis and may disappear on subculture 96. Their function and genetic basis of expression are unknown. The sequence of the 16S rRNA gene from several cat and dog isolates of H. felis shows that they differ by less than 1% 96 and are most similar to other gastric Helicobacter species (Fig. 6; Table 3). The cat isolates are more closely related to one another, as are the dog isolates, but these differences are subtle. The fatty acid composition of H. felis is typical of other gastric Helicobacter species, with a predominance of 19-carbon cyclopropane fatty acid and tetradecanoic acid 198.
Epizootiology.
It is now apparent that the many descriptions of pleomorphic spiral bacteria in the stomachs of dogs and cats 92, 255, 257, 353, 404, 417, 418 represent multiple species that often can be distinguished by 16S rRNA sequence analysis or DNA-DNA hybridization. These include, as well as H. felis, several other organisms discussed below such as H. bizzozeronii, H. salomonis, and “H. heilmannii.” Still other isolates that may be novel species remain unnamed 96. The combination of enzyme-linked immunosorbent assay and immunoblotting detected Helicobacter infection with 95.6% sensitivity and 79.8% specificity in dogs 378, but efforts to specifically detect H. felis were less successful 351. Therefore, there are no seroepidemiologic studies which examine the prevalence of H. felis. Nevertheless, despite its name, H. felis is apparently not the most common Helicobacter species in cats and dogs. In six studies that collectively cultured gastric biopsy specimens from 147 cats and 85 dogs, one H. felis strain was isolated from a cat and only four strains were identified in dogs 96, 197, 298, 303, 315, 427. Even with modified culture conditions that appear more effective than those used previously, H. felis was cultivated from only 3 of 22 cats and 8 of 95 dogs 220. Most animals were colonized by organisms that resembled “H. heilmannii” and could not be cultivated. Human infection with H. felis has been reported rarely 168, 237, apparently as a zoonosis, but no environmental or other host reservoir is known.
Surprisingly, H. pylori has also been isolated from domestic cats obtained from a single commercial breeder 193. The gastric mucosa of the infected cats was characterized histologically by moderate to severe lymphoplasmacytic follicular infiltrates, predominantly in the gastric antrum, where bacterial colonization was greatest 194. The histology of naturally infected cats was reproduced by experimental inoculation of specific-pathogen-free animals with feline- and human-derived H. pylori 137. Although initially these data raised the possibility that H. pylori in humans may be a zoonosis, this conclusion is not supported by seroepidemiologic data 14, 416 or by studies of gastric Helicobacter species in domestic pet cats 14, 298, 303, 416. Natural H. pylori infection in cats appears to be restricted to animals obtained from a particular commercial breeder, and infection was probably acquired as an anthroponosis.
Clinical significance.
Much as was true of the early descriptions of H. pylori infection in humans, the clinical significance of gastric Helicobacter infection in dogs and cats has been difficult to determine. Most studies find that between 50 and 100% of cats and dogs are infected with Helicobacter. There is no convincing evidence that infection is associated with clinical symptoms such as chronic vomiting or inappetence, nor is there typically a clear association between infection and histologic gastritis 96, 116, 169, 197, 200, 202, 203, 298, 303, 314, 315, 321, 353, 377, 417, 418, 427. However, in many studies the animals are incompletely described, there are no controls, and the bacteria are only characterized by urease assay or routine histologic testing. Since animals may be infected with more than one species of Helicobacter that are urease positive and that are indistinguishable on light microscopy, the pathogenic role of H. felis is difficult to determine from these observational studies. Experimental infection of H. felis in 7-day-old gnotobiotic dogs resulted in seroconversion and large numbers of lymphoid nodules throughout the gastric mucosa, as well as a mild diffuse lymphocytic infiltrate 247. Similar nodules described previously as components of the normal microscopic anatomy of the dog stomach may also have been a result of H. felis infection 1. Control animals had no evidence of infection or lymphoid nodules at necropsy. However, when 4-month-old specific-pathogen-free dogs were infected with the same strain of H. felis, the results were very different 363. Mild gastritis and lymphoid follicles were found in both infected and uninfected dogs. There was no correlation between the number of organisms and the intensity of inflammation, nor did infection produce alterations in gastric pH, acid output, or plasma gastrin. The different results in these two studies probably reflect host differences, such as alterations in gastric acidity or immune response with age, or differences between specific-pathogen-free and gnotobiotic animals. Experimental inoculation of specific-pathogen-free cats with H. felis induced lymphoid follicular hyperplasia but only mild gastritis 364. There was no accompanying up regulation of antral mucosal interleukin-1α (IL-1α), IL-1β, or tumor necrosis factor alpha, nor were there changes in gastric secretory function.
Virulence determinants and other characterized genes.
The H. felis urease is composed of A and B subunits, which are 73.5 and 88.2% identical, respectively, to the corresponding polypeptides from H. pylori 117, 181. One presumes that urease is required for H. felis colonization, but efforts at genetic manipulation have been largely unsuccessful. Recently, the flagellin genes from H. felis (flaA and flaB) were cloned and isogenic mutants were generated by electroporation 224. Transformation efficiency was low relative to what is typically seen with H. pylori or H. mustelae, and mutants could be obtained only using plate-grown bacteria. Both flaA and flaB mutants showed truncated flagella and were poorly motile in vitro, and the flaA mutant was unable to colonize gastric mucosa in the mouse model. This result differs from that with H. mustelae, in which mutation in a single flagellin gene reduces but does not abolish colonization. Unlike H. mustelae, H. felis is found exclusively in the mucus layer and is not attached to the gastric epithelium 348; it has been speculated that any impairment of motility may therefore eliminate colonization 224.
The cagA and vacA genes appear to be absent from H. felis 283. The only other sequences from H. felis that have been published to date are for a P-type ATPase encoded by the copAP operon 24 and for a nearby open reading frame with homology to the E. coli ftsH gene encoding an ATP-dependent metalloprotease 273. Both have closely related homologs in H. pylori.
Development of the rodent model.
H. felis readily colonizes mice, with the same gastric tropism seen with H. pylori in humans 77, 134, 244. Infection typically predominates in the gastric antrum but, interestingly, is accompanied by a largely mononuclear cell inflammatory response that is more prominent in the corpus 134, 281, 341. The difference in the anatomic locations of the infection and the inflammation has led to the suggestion that H. felis in mice may stimulate an autoimmune response to gastric tissue 341. A similar mechanism has been proposed for H. pylori gastritis in humans based on molecular mimicry between bacterial LPS and host Le antigens 15. As inflammation progresses into atrophic gastritis, substantial reduction in bacterial colonization of the gastric antrum may develop 341. This can also be produced, along with increased colonization of the corpus, by treatment of H. felis-infected mice with the proton pump inhibitor omeprazole 64. These findings suggest that local acid production may be important in the distribution of H. felis in the mouse stomach. Unlike H. pylori in humans, H. felis in the mouse does not attach intimately to gastric epithelial cells, nor does it produce a prominent polymorphonuclear infiltrate.
H. felis infection of the rat has also been used as a model of H. pylori infection. Although limited reagents and lack of genetically characterized strains make the rat less attractive than the mouse in general, the similarity in gastric physiology between the rat and human may make it more useful for some studies. H. felis infection in the gnotobiotic rat is localized to the gastric antrum, causes a predominantly mononuclear cell infiltrate, and is accompanied by a transient IgM and sustained IgG antibody response 152. In one study with conventional rats, neither H. felis nor “H. heilmannii” (see below) induced significant gastritis 63. Since these rats also showed no changes in gastrin and acid output, it was concluded that the inflammatory response, rather than direct bacterial effects, is probably responsible for changes in gastric physiology induced by H. pylori in humans. A more direct test of this hypothesis might utilize isogenic strains of H. felis or H. pylori that differ in the extent of the inflammatory response, in order to avoid comparisons across different animal hosts and bacterial species.
With the successful introduction of H. pylori into mice 261 and the availability of the H. pylori genome 395, future work will probably rely more on the H. pylori mouse model than on H. felis. Nevertheless, the H. felis rodent model has produced significant results that further our understanding of Helicobacter pathogenesis.
(i) Atrophic gastritis, lymphoma, and gastric cancer.
Atrophic gastritis (thought to be the histologic precursor lesion to gastric adenocarcinoma) and gastric MALT lymphoma associated with H. pylori infection in humans 56, 435 have also been observed in mice infected with H. felis 239. In an initial study of conventional mice experimentally infected with H. felis CS1, atrophic changes in the gastric corpus developed 48 weeks after inoculation and progressively increased over the subsequent 24 weeks 241. Uninoculated animals developed less extensive atrophy, but the results were difficult to interpret because animals in both groups developed coinfection with Helicobacter muridarum, an organism that normally colonizes the small and large bowel of rodents. However, subsequent inoculation of specific-pathogen-free mice with H. felis confirmed the development of corpus atrophy without coinfection by H. muridarum 341. Long-term infection of specific-pathogen-free mice with H. felis can also produce lesions that resemble human gastric B-cell MALT lymphoma 109. Antibiotic treatment of H. felis in chronically infected mice reduces the development of gastric MALT lymphoma 108. This suggests that the lymphoma is antigen dependent, at least while it remains low grade, in much the same fashion as H. pylori-associated gastric MALT lymphoma in humans 211, 335. Elegant studies with insulin-gastrin (INS-GAS) transgenic mice also suggest that H. felis infection acts synergistically with chronic hypergastrinemia to produce parietal cell loss and development of gastric cancer 410. Further studies with transgenic mice, as well as the recently described H. pylori gerbil model 383, will probably contribute significantly to our understanding of the relationship between Helicobacter infection and gastric malignancy.
(ii) Therapy.
The H. felis mouse model has been promoted as a preclinical tool for evaluation of novel antimicrobial therapy for H. pylori infection. In general, the results obtained by treatment of H. felis in the mouse model mimic the outcome of human clinical trials investigating the treatment of H. pylori 78, 206, 233, 367. However, given the relative ease of performing human clinical studies, hundreds of which have now been completed 403, and the development of the H. pylori mouse model, it seems unlikely that the H. felis mouse model will play an important role in efficacy studies of novel H. pylori therapies.
(iii) Mechanisms of inflammation and atrophy.
The nature of the host response is an important factor in Helicobacter-associated diseases. Infection with H. felis in several inbred strains of mice (C57BL/6, C3H/He, and SJL) produces severe gastritis, while in other strains (BALB/c, CBA) the inflammatory response is much less marked 283, 341. F1 hybrids of the CBA mouse crossed with strains that develop severe gastritis maintained the mild inflammation phenotype of the CBA parent 384. Thus, low inflammation was a dominant response which might in part reflect immune suppression rather than a genetic defect. It has also been proposed that increased proliferation and apoptosis seen in the C57BL/6 mice may be related to the lack of secretory phospholipase A2 (sPLA2), which is encoded by the Mom1 locus responsible for variability in the number of polyps in mice with multiple intestinal neoplasia 411. SV129 mice are also sPLA2−/− and show similarly severe pathologic changes in response to H. felis infection, while BALB/c and C3H/HeJ mice are sPLA2+/+ and have less inflammation. The decrease in gastric sPLA2 levels after infection of C57BL/6 mice with H. felis is also consistent with the role of sPLA2 in maintaining gastric differentiation 254. However, since these studies were not performed on congenic strains, other genes may also be involved. For example, C3H/HeJ mice have a defect in responsiveness to LPS that also contributes to reduced atrophic gastritis 342. Genes of the major histocompatibility complex probably also contribute to individual differences described in mouse strains 283.
Genetically well-characterized mice have recently been exploited to extend our understanding of Helicobacter-induced gastric inflammation. H. felis infection in C.B-17 mice with severe combined immunodeficiency (SCID mice) produces an inflammatory response that does not differ from that seen in immunocompetent controls 31. However, infection of T-cell-deficient C57BL/6 mice with H. felis results in minimal gastritis compared with infection of B-cell-deficient and wild-type C57BL/6 mice 338. The difference between these results is probably due to the host strain, since the C.B-17 SCID mouse is in a BALB/c background, which does not develop extensive gastric pathologic changes. Host adaptive immunity is therefore likely to be involved in the development of Helicobacter gastritis 106. IL-6-deficient mice do not show any difference in the mucosal IgA response to H. felis infection or to local immunization, which is unexpected in view of the presumed role of IL-6 in the terminal differentiation of IgA-producing B cells 41. Presumably the IL-6 defect can be compensated by production of other cytokines. Inoculation of H. felis into mice deficient in the anti-inflammatory cytokine IL-10 results in a markedly increased mononuclear cell inflammation that progresses to loss of parietal and chief cells 28. This severe gastritis is probably an exaggeration of the normal Th1-type immune response that occurs after infection with H. pylori and H. felis 18, 266, 281. It may result from loss of IL-10 regulation of the host response to LPS 27 and is consistent with the observation that C3H/HeJ mice, which are not responsive to LPS, do not develop atrophic gastritis with chronic H. felis infection 342. IL-10 knockout mice infected with H. felis also show increased gastric epithelial cell proliferation and loss of normal differentiation, which is seen in a milder form in p53 hemizygous transgenic mice 153. Surprisingly, H. felis infection of mice with truncation of the Apc gene (a tumor suppressor involved in colorectal carcinogenesis) causes decreases in inflammation, serum IgG levels, and epithelial cell proliferation 144.
(iv) Vaccine development.
The limitations of current H. pylori therapies have prompted interest in the development of vaccines for both treatment and primary prevention. Although natural infection with H. pylori induces specific IgG and IgA that do not prevent initial colonization or subsequent reinfection, the results of considerable work suggest that it may be possible to prevent Helicobacter infection by immunization. Much of this work has utilized the H. felis mouse model. Orogastric immunization of mice with an H. felis sonic extract together with cholera toxin produces a significant antibody response in serum and gastric secretions 62 that is associated with protection after challenge with H. felis, often with 90 to 100% efficacy 33, 51, 61, 118–120, 240, 316, 328, 352. H. pylori sonic extract plus cholera toxin also protects against challenge with H. felis, although less effectively 240, 280. Studies of particular antigens suggest that protection against H. felis infection in the mouse model can be achieved with H. pylori urease holoenzyme or its B subunit 112, 118, 250, 280, 297, 316, catalase 329, and the GroES and GroEL homologs, HspA and HspB, respectively 119. Similar vaccine efficacy has also been described in the H. pylori mouse model by immunization with H. pylori cytotoxin (VacA), a protein associated with cytotoxin expression (CagA), catalase, and urease 58, 170, 179, 261, 262, 329.
However, some recent studies with mice and with more relevant animal models such as the pig and rhesus monkey suggest that when careful quantitative colony counts are performed, current vaccine formulations yield 1 to 2 log unit reductions in H. pylori colonies but do not provide sterilizing immunity 107, 188, 231, 249. The clinical significance of such quantitative reductions is unknown. Furthermore, protection may be more difficult to achieve in animals naturally infected with Helicobacter. When ferrets naturally infected with H. mustelae were immunized with H. pylori urease, colonization was eliminated in only 30% of animals 60. Immunization of uninfected ferret kits with H. mustelae lysate, together with the adjuvant muramyl dipeptide, was also ineffective at primary prevention 422. These disappointing results may be attributed to many variables in addition to the fact that H. mustelae naturally infects the ferret. For example, there are limited data on optimal adjuvant and dosage conditions in the ferret, and there may be significant differences between the important epitopes on H. mustelae and H. pylori urease. However, a study with rhesus monkeys found that after immunization with H. pylori urease plus E. coli heat-labile enterotoxin, an estimated 31% of animals were protected from natural infection with H. pylori, compared with 7% protected by administration of placebo plus heat-labile enterotoxin 90. Although the difference between the groups was statistically significant, the results are difficult to interpret because the initial absence of H. pylori infection was documented only by serologic testing, which is not sensitive for the detection of recent infection. More recently, a study with specific-pathogen-free rhesus monkeys found no evidence that urease vaccination could prevent H. pylori infection or reduce bacterial colony counts after experimental challenge 368. These results raise concern that protection of an animal from a Helicobacter species with which it is naturally colonized may be more difficult than protection from an ecologically irrelevant species.
The availability of the H. pylori genome 6, 395 now permits the evaluation of numerous recombinant vaccine candidates, which should probably first be evaluated in the H. pylori mouse model and then, if effective, be studied in nonhuman primates. Vaccines that are effective in primary prevention may also be useful for therapeutic immunization 57, 170, which may be increasingly important as antibiotic-resistant strains of H. pylori become more prevalent. H. pylori and H. felis in the mouse model will also continue to be useful in our attempts to better understand mechanisms of immunity. Some evidence suggests that the pathologic changes associated with natural infection may be due predominantly to a Th1 cell-mediated immune response while protection following immunization may be associated with a Th2 response 119, 281, 282, 343. However, this may be an oversimplification. The density of H. felis colonization in mice can be substantially reduced by infecting them with a replication-defective adenovirus. This effect was dependent on the presence of gamma interferon and IL-12, which are Th1 cytokines 222. Others have also recently shown that protection from H. pylori colonization in the mouse model can be achieved by systemic vaccination that induces either a Th1- or Th2-type cytokine response 32. The relative importance of IgG and IgA antibodies remains controversial 120, 250, although recent work with B-cell knockout mice suggests that antibody responses to urease are not required for protection in the mouse model 113.
Helicobacter bizzozeronii and Helicobacter salomonis
Large gastric spiral bacteria, which resemble those seen by early investigators and described most clearly by Lockard and Boler 257, were recently cultivated 195, 219 and named after Bizzozero 30 and Salomon 344. Both organisms were isolated from dogs by using culture techniques that differed only modestly from those used by other investigators, particularly in using brain heart infusion rather than brucella broth, cultivation for up to 12 days, careful attention to keeping plates moist, and cultivation only of biopsy specimens that were rapidly urease positive. H. bizzozeronii, which is indistinguishable morphologically from the Lockard type 3 bacterium and from “H. heilmannii,” is typically 5 to 10 μm long and 0.3 μm wide, with bipolar sheathed flagella (Fig. 3). H. salomonis is usually somewhat smaller (5 to 7 by 0.8 to 1.2 μm) and less tightly coiled, a morphology similar to that originally published by Weber and Schmittdiel 418 but not described by Lockard and Boler. The 16S rRNA sequences from these bacteria are approximately 99% similar to one another and to that from the closely related H. felis. Restriction fragment length polymorphism (RFLP) analysis suggests that the 23S rDNA genes are also closely related 218. However, distinct species occasionally have 16S rRNA genes that are virtually identical 133. In this case, DNA-DNA hybridization studies show clearly that H. bizzozeronii and H. salomonis are each genetically homogeneous and distinct from one another, as well as from other canine and feline gastric Helicobacter species. Pulsed-field gel electrophoresis suggests that there is more heterogeneity among strains of H. bizzozeronii than among strains of H. salomonis 196, although this result will require replication with a larger number of strains.
Cultivation of Helicobacter species from dogs and cats has been typically unsuccessful, despite microscopic and DNA evidence of Helicobacter 96, 298, 303, 427. When the culture methods used originally to isolate H. bizzozeronii and H. salomonis were applied to specimens from a group of dogs and cats, the results showed that the seemingly subtle modifications in culture methods were probably important 220. Cultures of specimens from 48 (51%) of 95 dogs and 3 (14%) of 22 cats were positive, which was considerably greater than the 0 to 10% that has recently been reported 96, 298, 303, 427. Approximately half the positive cultures from dogs yielded H. bizzozeronii. The remainder were about evenly divided between H. felis and H. salomonis, with “Helicobacter sp. flexispira” (see “Enterohepatic Helicobacter species” below) cultivated in two dogs. The three positive cultures in cats were all H. felis. H. bizzozeronii, H. salomonis, and H. felis were difficult to distinguish unequivocally by using morphology, bacteriology, routine biochemistry, or 16S rRNA sequence analysis. However, numerical analysis of whole-cell-protein electrophoresis results and calculation of percent similarity yielded clusters that best corresponded to the three different species.
“Helicobacter heilmannii”
In 1987 a novel gram-negative spiral bacterium was found in gastric biopsy specimens from three patients, although in retrospect the organism may have been the same as that described in humans many years earlier 235. It was easily distinguished from H. pylori by virtue of its larger size, more tightly coiled morphology, and failure to grow in microaerobic culture 72. Although it was recognized early that the organism closely resembled bacteria seen in a variety of other mammals, it was nevertheless tentatively assigned a new genus and species designation, “Gastrospirillum hominis,” which reflected its occurrence in humans 271. The initial observation was quickly confirmed by numerous other case reports that described similar organisms in small numbers of patients 2, 5, 38, 85, 95, 121, 122, 124, 204, 213, 284, 289, 307, 385, 426, 428, 430; L. Mazzucchelli, Letter, Dig. Dis. Sci. 40:1463, 1995).
Taxonomy and nomenclature.
In vitro cultivation of this large gastric spiral bacterium remains elusive, but inoculation of mice and rats with gastric homogenates from infected humans and nonhuman primates permitted cultures to be maintained in vivo 77, 243. Mouse gastric tissue infected with organisms from two human patients was then used as a DNA template to amplify and sequence bacterial 16S rRNA genes, a technology that is now commonplace for the identification of uncultivated bacteria 347. The results confirmed early speculation based on antibody cross-reactivity 242 that the large gastric spiral organism originally designated “Gastrospirillum hominis” was actually a new species of Helicobacter that is most closely related to H. felis 371. The bacterium was tentatively designated “Helicobacter heilmannii” 371, after Konrad Heilmann, a German histopathologist who at the time had described the largest series of cases and who died prematurely shortly after its publication 201.
Unexpectedly, however, the 16S rRNA sequences from two different patient isolates of the large gastric spiral bacterium differed by 3.5%, which is sufficient to consider these organisms different species. We originally referred to these as “Gastrospirillum hominis” (“H. heilmannii” 1) and “Gastrospirillum hominis” (“H. heilmannii” 2) 371. The 16S rRNA sequence from what was recently described as the first culturable example of “H. heilmannii” 7, 205 is between 98.2 and 98.4% identical to sequences from H. felis, H. bizzozeronii, and H. salomonis. Since this organism was cultivated, it should be possible to perform DNA-DNA hybridization to confirm its species identity, but it is probably not the same as “H. heilmannii” 1. We have also reported recently one nearly complete and nine partial 16S rRNA sequences that were amplified from bacteria in the stomachs of healthy cats 303. These sequences are very similar but not identical to sequences from H. felis, H. salomonis, H. bizzozeronii, and “H. heilmannii” 2, all of which are closely related by 16S rRNA sequence analysis. Sequence comparison using urease or other genes may provide additional discrimination beyond 16S rRNA 50.
What, then, should we call uncultivated gastric bacteria that are urease positive and have the morphology of gastrospirillum? Both “Gastrospirillum” and “hominis” are inappropriate because these bacteria clearly belong in the Helicobacter genus and because humans are not typically the natural host. A recent proposal addressed the confusion created by the proliferation of new taxa invoked for uncultivated bacteria that are defined by limited data, such as 16S rRNA sequence. It was suggested that the usual binomial species designations be replaced with a new category, Candidatus (L., a candidate), followed by a descriptive epithet 295, 296. Following this suggestion, the designation “Candidatus Helicobacter suis” was proposed based on the identification in swine of a gastrospirillum which had a 16S rRNA gene that was 99.5% identical to that from “H. heilmannii” type 1 67, 326. However, organisms with virtually identical 16S rRNA sequences have also been commonly found in rhesus monkeys and other nonhuman primates, as well as in additional human patients (288; J. V. Solnick and J. O'Rourke, unpublished observations). Since the host range of this organism appears broad, the epithet “suis” may not be appropriate. The designation “H. heilmannii” is in common use, appropriately pays tribute to an early worker in the field, and avoids the implication that the organism has a restricted host range. Although current published data do not yet satisfy the criteria recently proposed for assignment of “H. heilmannii” to Candidatus status 75, ongoing experiments are likely to do so. We therefore propose that the designation “H. heilmannii” be applied to what was previously called “H. heilmannii” type 1 and to closely related bacteria. Newly observed uncultivated bacteria with a gastrospirillum morphology, but with a 16S rRNA sequence that is about 98% or more similar to H. felis, might be referred to as a member of the “H. felis species group” 303 or as “H. felis-like.” This would apply to what we have previously called “H. heilmannii” type 2. “H. heilmannii”-like may also be used descriptively in the absence of genetic information, with the understanding that it may obscure species differences that will be apparent when 16S rRNA sequence or better cultivation methods are available.
Microbiology.
The morphology of “H. heilmannii” is similar to that of H. felis, but periplasmic fibers are absent. The organism is 4 to 10 μm in length and 0.5 to 0.8 μm in diameter and has four to eight tight spirals (Fig. 3). There are typically 6 to 10 tufts of bipolar flagella. “H. heilmannii” is well visualized and distinguished from H. pylori by light microscopic examination of paraffin sections stained with hematoxylin and eosin or Warthin-Starry silver stain. Surprisingly, a recent report found that H. pylori can be induced to assume the morphology of “H. heilmannii” by being grown in brucella broth with 1% cyclodextrin rather than on blood agar 115. Since 16S rDNA sequences amplified from tissue infected with an “H. heilmannii”-like organism do not typically resemble H. pylori, it is unlikely that this observation is often relevant in vivo, but further study is warranted.
Several case reports indicated that a urease assay on tissue infected with “H. heilmannii” was negative, or slow to develop, which suggested that the urease of “H. heilmannii” might be quite different from that of other gastric Helicobacter species. However, PCR and DNA sequencing using degenerate primers based on H. pylori sequences showed that the “H. heilmannii” urease is composed of two subunits of approximately 26 and 62 kDa, which are 82 and 92% identical at the amino acid level to the corresponding UreA and UreB, respectively, from H. felis 372. The urea breath test has been used in animals to detect “H. heilmannii” 299, 369, and it is likely that a small percentage of humans with a positive urea breath test are infected with “H. heilmannii” and not H. pylori. Immunization with recombinant “H. heilmannii” urease has recently been shown to protect mice from infection with “H. heilmannii” and H. felis, but protection was accompanied by increased corpus atrophy. The possible role of residual undetected infection in promoting atrophy in the immunized animals 112 could be addressed by studying the effects of antibiotic therapy on atrophy in immunized animals. Beyond the ultrastructure and presence of the typical Helicobacter urease, little is known about the microbiology of this uncultivated organism.
Host range and epidemiology.
The prevalence of infection with “H. heilmannii”-like organisms in humans is less than 0.5% among patients presenting for upper gastrointestinal endoscopy 124, 201, 271, 289, although it is reportedly as high as 6% in China and Thailand 425, 429. This latter observation requires confirmation by studies in other developing countries. In contrast to the low prevalence in humans, infection is very common in dogs 96, 427, cats 298, 303, 427, pigs 20, 326, and nonhuman primates 88, 91, 369. Recent studies also suggest that “H. heilmannii”-like organisms may infect wild urban rats 173 and both small and large exotic felids 104, 217, 227, 228. Thus, unlike the host range restriction of many Helicobacter species, such as H. pylori in humans and other primates, H. felis in cats and dogs, and H. mustelae in ferrets, “H. heilmannii”-like organisms are widely distributed in the animal kingdom. To date, “H. heilmannii” infection has been confirmed by 16S rDNA sequence analysis (rather than morphology alone) only in humans 371, pigs 70, 326, and nonhuman primates (Solnick and O'Rourke, unpublished), although it seems likely that it will be identified in other hosts.
Since “H. heilmannii” is common in animals, it has often been suggested that “H. heilmannii” infection in humans may be a zoonosis. “H. heilmannii” observed in a child morphologically resembled bacteria which were seen in the stomachs of her pet dogs 394. A human patient and his pet cat have also been documented to harbor “H. heilmannii” strains, which may have been the same organism since they had 580 bp of identical sequence from the ureB gene 79. Neither of these observations conclusively documents zoonotic transmission, since “H. heilmannii”-like bacteria are common in dogs and cats and the ureB gene is highly conserved. However, these findings are consistent with recent data which suggest that compared to patients infected with H. pylori, those infected with “H. heilmannii” are significantly more likely to report contact with a variety of animals, particularly dogs, cats, pigs, and cattle 272. If, as seems likely, “H. heilmannii” infection in humans is a zoonosis, the organism may be poorly adapted to the human gastric environment, since infection of humans is rare despite frequent contact with domestic animals. Alternatively, H. pylori may be simply better adapted to humans and may also protect against subsequent infection with “H. heilmannii,” since dual infections are rarely seen.
Histopathology.
The histopathology of “H. heilmannii”-like infection in humans was described for 39 cases in the first large series reported by Heilmann 201 and more recently in a study that compared 202 patients with “H. heilmannii” infection to 202 matched controls infected with H. pylori 376. Compared with H. pylori, “H. heilmannii” infection in humans is more often focal, with fewer organisms, and is often restricted to the gastric antrum. “H. heilmannii” is typically found in the mucus layer above surface epithelial cells and does not show the intimate adherence and pedestal formation often seen with H. pylori. Organisms may also be found deep in the lumen of the gastric glands and within parietal cells. Gastritis, while present, is much less marked in patients infected with “H. heilmannii” than in those infected with H. pylori 376, 425. The relatively mild inflammatory response to natural infection with “H. heilmannii”-like organisms has also been found in cats 303, dogs 96, and nonhuman primates 91, 369. However, the elevation of acid output in rhesus monkeys infected with “H. heilmannii” suggests that, despite the minimal inflammatory response, the organism does alter the host gastric physiology 91.
Domestic swine may also be naturally infected with “H. heilmannii,” with a prevalence of about 10% when evaluated by histologic testing 185, 277, 278, 327 but as high as 50 to 60% when evaluated by the more sensitive mouse inoculation assay 276, 326. Infection is associated with mononuclear inflammation and lymphoid follicles in the pylorus. Natural infection is also associated with gastric ulcer of the pars esophagea, a nonglandular area of stratified squamous epithelium that extends from the esophagus into the stomach 326, 431. When examined by mouse inoculation of gastric contents from swine, “H. heilmannii” was found in 20 (100%) of 20 pigs with ulcers and in 27 (90%) of 30 pigs with preulcer lesions but in only 7 (35%) of 35 pigs with macroscopically normal pars esophagea. This association is potentially important, since gastric ulcers in farmed pigs cause up to 2.5% of animals to die of gastrointestinal hemorrhage before slaughter. However, recent experimental inoculation of gnotobiotic piglets with “H. heilmannii,” originally isolated from cheetahs and maintained in vivo in the mouse, did not reproduce the gastroesophageal ulcers seen in naturally infected animals 234. Furthermore, while gastritis was seen in experimentally inoculated animals, it occurred only in the gastric fundus, while in naturally infected animals gastritis has been observed in the pylorus. Since “H. heilmannii” is poorly characterized, these discrepancies may be due to strain differences or perhaps to host variables, such as the age at which infection occurs. It may also simply be that gastric ulcers in swine are not caused by “H. heilmannii,” which is not the first microorganism to be found in association with ulceration of the pars esophagea 386.
Although laboratory rodents are not naturally colonized with “H. heilmannii,” they can be readily infected experimentally. Human-derived “H. heilmannii” in the conventional Quackenbush Swiss mouse produced a mononuclear and polymorphonuclear gastritis, which progressed to gastric atrophy beginning 48 weeks after infection 241. Inoculation of “H. heilmannii” from pigs into Wistar rats or CFW(LOB) axenic mice resulted in a mild to moderate mononuclear infiltrate, predominantly in the gastric antrum 274, 292. In contrast, BALB/c mice infected with “H. heilmannii” derived from cheetahs with severe gastritis developed an initially mild lymphocytic and lymphofollicular inflammation, which progressed to severe gastritis with ulceration after 6 months 102. Kittens could also be infected with cheetah-derived “H. heilmannii,” but the inflammation was mild and did not progress over an 11-month observation period 103. Thus, while the differences in histopathologic changes seen with experimental “H. heilmannii” infection may be partially related to bacterial differences, particularly since the inoculum is poorly characterized, host factors are also clearly important.
Clinical considerations.
Although inflammation in humans infected with “H. heilmannii” is less severe than in humans infected with H. pylori, gastritis is nevertheless always present. Furthermore, human infection with “H. heilmannii” has been documented in association with acute and chronic gastrointestinal symptoms 2, 4, 85, 204, 289, 307, 385, 428, which in some cases have resolved with effective therapy, and in association with gastric pathologic responses, including ulcer, adenocarcinoma, and lymphoma 38, 68, 332, 430. Recently, remission of gastric MALT lymphoma was described after treatment of “H. heilmannii” infection in five patients, a phenomenon that is well known in H. pylori-associated gastric MALT lymphoma 288. Two of the five patients from whom bacterial 16S rRNA sequence was available were infected with an organism that was 99.3% identical to “H. heilmannii” (type 1), although only 172 bp were sequenced and the region of the 16S rRNA gene was not specified. Not surprisingly, “H. heilmannii” infection also occurs in asymptomatic individuals 268, which is also the case for, and in fact is typical of, infection with H. pylori 83. Whether “H. heilmannii” infection causes gastrointestinal symptoms and gastric pathologic changes is difficult to determine because of its low prevalence. However, it is probably prudent to use the same guidelines for when and how to treat “H. heilmannii” infection that have been proposed for infection with H. pylori 13, 93. Touch cytologic testing performed by rolling a gastric biopsy specimen onto a glass slide and staining it with modified Giemsa or Gram stain may be more sensitive than biopsy 67, 69. Treatment of “H. heilmannii”-like organisms in dogs and cats appears to be relatively ineffective, which may be consistent with their role as members of the normal flora 55, 299.
Helicobacter acinonychis
Chronic gastritis is widespread among captive cheetahs in zoological parks and is a significant clinical problem 293. Investigation of cheetahs with chronic vomiting revealed two kinds of gastric spiral bacteria 105. One could not be cultivated and resembled “H. heilmannii,” although periplasmic fibers were sometimes present. The other was morphologically and biochemically similar to H. pylori but somewhat smaller (0.3 by 1.5 to 2.0 μm) and with a G+C content of 30% rather than the 39% that is typical for H. pylori. Analysis of the 16S rRNA gene from the cultivated organism showed that it was most closely related to H. pylori (97.4% similar), and the name H. acinonyx was proposed 97, recently changed to H. acinonychis 399. Bacteria isolated from two Sumatran tigers killed because of age-related disability also appears to be H. acinonychis 349.
H. acinonychis appears to be more closely associated with gastritis in cheetahs than does the “H. heilmannii”-like organism. Animals infected with H. acinonychis with or without the “H. heilmannii”-like organism typically have severe lymphoplasmacytic gastritis with scattered neutrophils and lymphoid follicles, sometimes accompanied by gross thickening of the gastric rugae, erosions, and punctate hemorrhages. In contrast, animals infected with the “H. heilmannii”-like organisms alone sometimes have only minimal gastritis 104, 408. However, the pathogenic role of H. acinonychis in cheetahs is unproven. Antibiotic treatment of H. acinonychis was reported to provide symptomatic relief from vomiting, anorexia, and weight loss in three cheetahs without clearing the infection 408. Interestingly, the gastritis seen in captive cheetahs is not found in wild cheetahs, although infection with gastric spiral organisms is nearly always present in both 392. Whether this is related to differences in the bacterial species infecting captive and wild animals or to host variables such as stress of captivity remains to be determined. Further studies of therapy and experimental inoculation will be required to determine if H. acinonychis plays a role in clinical and histologic gastritis in cheetahs, although such studies face obvious logistical difficulties.
Little is known about possible virulence genes in H. acinonychis. One presumes that urease, flagella, and perhaps other documented virulence factors in other Helicobacter species are important. The H. acinonychis homologue (hxaA) of a putative H. pylori adhesin (hpaA) is probably not an adhesin, since later work has shown that HpaA is in fact a flagellar sheath protein that is not involved in attachment 114, 223, 311.
Helicobacter nemestrinae
An organism isolated from the pigtailed macaque (Macaca nemestrina) was reported to differ from H. pylori by virtue of its growth at 42°C and its cellular fatty acid profile 42. Studies of DNA-DNA hybridization and 16S rDNA also suggested that it was a novel species most closely related to H. pylori. Surprisingly, the G+C content was only 24%, much lower than those of all known Helicobacter species, which prompted a reappraisal by two groups. The results suggest that H. nemestrinae (ATCC 49396) may in fact be identical to H. pylori. Repeat determination of the 16S rDNA sequence (F. Dewhirst, personal communication) showed that it is 99.5% identical to human H. pylori and 100% identical to H. pylori isolated from the rhesus macaque 84. Repeat analysis of DNA base composition indicates that H. nemestrinae (ATCC 49396) is 39% G+C (identical to H. pylori) and that the protein profile is also consistent with H. pylori (P. Vandamme, personal communication). Confirmation must await publication of these results.
“Helicobacter suncus”
Cultures from the stomach of house musk shrews (Suncus murinus) with chronic gastritis yielded a gram-negative, urease-positive bacterium whose 16S rRNA sequence is most closely related to that from a Helicobacter organism isolated from birds. The name “H. suncus” has been proposed 182 but not validated. The organism has been described as having an interesting tendency to occur predominantly in a coccoid or coccobacillary form in vitro, with about 1% of cells appearing fusiform, while in vivo only fusiform bacteria are seen.
Candidatus Helicobacter bovis
Urease-positive bacteria resembling Helicobacter have been observed in histologic sections of the bovine abomasum 40, 187, 199, which is the true glandular stomach in ruminants. Infection may be associated with a lymphocytic and plasmacytic infiltrate. Although efforts to cultivate these organisms have been unsuccessful, sequence analysis of 16S rDNA amplified from abomasal tissue suggests that they represent a novel Helicobacter species (Table 3; Fig. 6), which has recently been designated Candidatus Helicobacter bovis 71. It is unknown whether Candidatus H. bovis infection is related to abomasal ulcers in cattle that were previously attributed to diet and stress due to illness or weather 186.
Other Gastric Bacteria
With the exception of occasional transient colonization by enteric bacteria, only Helicobacter species are generally known to infect the human stomach. However, a urease-positive coccoid organism has recently been cultivated from gastric biopsy specimens obtained from Korean patients 252. Biochemical analysis of the urease from this bacterium showed that kinetic parameters, relative abundance, and subunit composition are similar to those of Helicobacter urease 251. Preliminary taxonomic classification by fatty acid composition and biochemical analysis suggested that the organism was related to Staphylococcus. The 16S rDNA sequence from this organism is essentially identical to that from S. saprophyticus and also very similar (<1% different) to those from the closely related species S. xylosus and S. cohnii. (D. H. Calhoun, personal communication). The prevalence and clinical significance of gastric colonization with this bacterium are unknown.
ENTEROHEPATIC HELICOBACTER SPECIES
In addition to the gastric spiral-shaped bacteria, there is an equally diverse group of Helicobacter species that have been identified in the intestinal tract and/or the liver of humans, other mammals, and birds (Table 4). These enterohepatic Helicobacter species (EHS) do not normally colonize the gastric mucosa but do have features of ultrastructure and physiology in common with the gastric Helicobacter species (Table 2). The EHS were first recognized in laboratory rodents, where they are highly prevalent in most inbred strains and outbred stocks. Consequently, the EHS have been considered a component of the resident microbiota, or normal flora. It is now clear that some, and perhaps all, of the EHS have the ability to cause disease in normal, immunocompetent rodents. A growing number of EHS have also been reported to be associated with gastroenteritis, hepatitis, and other disease states in humans and in other animal species. The significance of the EHS in human disease and the true prevalence of these organisms in human populations remain to be determined. Nonetheless, the potential importance of these emerging pathogens cannot be overlooked.
TABLE 4.
Enterohepatic Helicobacter taxa
| Taxon | Natural host | Strain | GenBank 16S rRNA accession no. | Reference |
|---|---|---|---|---|
| H. bilis | Mouse, dog, human | ATCC 51630T | U18766 | 161 |
| H. canis | Dog, human | ATCC 5140T | L13464 | 375 |
| H. cinaedi | Human, hamster | ATCC 35683T | M88150 | 397 |
| H. cholecystus | Hamster | ATCC 700242T | U46129 | 162 |
| H. fennelliae | Human | ATCC 35684T | M88154 | 397 |
| H. hepaticus | Mouse | FRED1 | L39122 | 23 |
| H. muridarum | Mouse, rat | ATCC 49282T | M80205 | 248 |
| H. pamntenis | Bird, swine | ATCC 51478T | M88147 | 76 |
| H. pullorum | Chicken, human | ATCC 51801T | L36141 | 374 |
| “H. canadensis” | Human | NLEP 16143 | AF262037 | 140 |
| H. rodentium | Mouse | ATCC 700285T | U96296 | 356 |
| H. trogontum | Rat | ATCC 700114T | U65103 | 275 |
| “H. typhlonicus” | Mouse | MU96-1T | AF061104 | 164 |
| “H. mesocricetorum” | Hamster | MU97-1514T | AF072471 | 362 |
| “Helicobacter sp. flexispira” taxon 5 | Sheep, dog, human, mouse | ATCC 43966 | M88137 | 74 |
| “H. mainz” | Human | ATCC 51800 | X81028 | 210 |
| “H. westmeadii” | Human | NAa | U44756 | 398 |
| Helicobacter sp. cottontop | Cottontop tamarin | NA | AF107494 | 345 |
NA, not available.
Early Morphologic Observations
The surface mucus gel layer and the contiguous mucus deep in the crypts represents the interface between the host epithelium and the lumen of the gastrointestinal tract. Early studies characterizing the resident microbiota in the gut of laboratory rodents led to the discovery of a diverse population of spiral-shaped bacteria uniquely adapted to thrive in this transitional zone. These early studies, which utilized transmission electron microscopy rather than culture and isolation, described two morphologic types of organisms, both of which are now known to be EHS. Members of the first group superficially resemble Campylobacter species but are longer and have a single polar flagellum at each end (Fig. 7). Members of the second group, which includes the Lockard type 1 organism, have periplasmic fibers that wrap helically around the body of the bacterium as well as bipolar tufts of sheathed flagella (Fig. 1). Because of the periplasmic fibers, these organisms have a cross-hatched appearance in negative-stain preparations (Fig. 1A) but appear barber pole-like in longitudinal sections and scalloped in oblique sections in transmission electron micrographs (Fig. 1B). In studies that characterized the patterns of bacterial colonization of the large intestines of laboratory rodents, Davis et al. identified both morphologic types of organisms in the mucus of the cecum and colon 65, 66. The bacteria could be found during the first week of life, and they remained on the apical surface of the intestinal epithelium and packed deep in the crypts throughout the life of the animals. Bacteria seen by electron microscopy in early studies of “intestinal spirochetosis” in dogs and rats were also probably Helicobacter species 238.
FIG. 7.
(A and B) Negatively stained preparation (A) and transmission electron micrograph (B) of H. hepaticus show the absence of periplasmic fibers and the single bipolar flagella. (C) Transmission electron micrograph of a liver section in a mouse reveals H. hepaticus in a bile canaliculus. Panel C is reprinted from reference 253 with permission from the American Society for Microbiology. Bars, 0.5 μm.
Perhaps because their ultrastructure is less remarkable, the early literature contains fewer reports of the simple spiral-shaped organisms than of the organisms with periplasmic fibers. Nonetheless, the simple spiral-shaped, organisms have been isolated from a variety of mammals, including humans (H. cinaedi, H. fennelliae, and H. canis), and rodents (H. hepaticus, H. rodentium, and H. cholecystus), as well as from birds (H. pametensis and H. pullorum). The distinction between these organisms and the organisms with periplasmic fibers has a morphologic basis only; no comparable phylogenetic dichotomy has been recognized (Fig. 6). On the other hand, the presence of periplasmic fibers has facilitated the recognition of members of the second group of EHS in a variety of locations. Spiral-shaped bacteria with periplasmic fibers were observed free in the cytoplasm of enterocytes as well as deeper in the lamina propria of mice following treatment with nitrogen mustard 192. Such treatment results in a generalized loss of epithelial integrity, but it is interesting that the EHS were the only organisms found to invade under these conditions. The abundance of these organisms in the mucus deep in the crypts of the ileum and their proximity to the apical surface of the epithelial cells lining the crypts may account, at least in part, for these observations. Erlandsen and Chase exploited the ultrastructural characteristics of these organisms to ascertain the fate of bacteria following phagocytosis from the crypts by differentiated enterocytes in the ileum of untreated rats 111. Davis et al. also noted the occasional penetration of EHS into the epithelium of the rat cecum 66. More recently, invasion into the lamina propria of the cecum by EHS in mice following challenge with the spirochete Serpulina hyodysenteriae has been reported 212. The significance of cell entry and/or tissue invasion by EHS and the conditions under which these events take place have not been fully elucidated. Tissue invasion may be a prerequisite for or a consequence of Helicobacter-associated disease in the gastrointestinal tract. It may also play a role in bacterial translocation to the liver and/or into the systemic circulation, either as a primary event or secondary to other disease states.
The fact that many investigators have encountered EHS with periplasmic fibers in the gastrointestinal tracts of laboratory rodents no doubt reflects the frequency with which these animals are used in biomedical research. Bacteria with the same morphology have also been isolated from the gastrointestinal tracts of other mammals, including dogs, cats, nonhuman primates, and humans (“Helicobacter sp. flexispira” and H. bilis). The complete range of host species from which these organisms can be isolated is not known. It may be that such bacteria can flourish wherever a mucus-rich interface between epithelial cells and the lumen of an alimentary tract is found. Certainly, the observation of bacteria that appear morphologically indistinguishable from EHS in the hindgut of Periplaneta americana, the American cockroach 39, suggests that the distribution of these microbes is very wide indeed.
Helicobacter hepaticus
Historical perspective.
In autumn 1992, pathologists at the National Cancer Institute-Fredrick Cancer Research and Development Center recognized that male A/JCr mice serving as saline-injected controls in a long-term chemical carcinogenesis assay had a higher than expected incidence of liver tumors 415. Historically, hepatocellular tumors were found in male mice of this inbred strain with an incidence of approximately 1% at 15 months of age or older. However, evaluation of tissue from animals euthanized between August and October 1992 revealed liver tumors in 3 of 6 mice, and in December 1992 liver tumors were found in 11 of 12 mice 415. All of the animals with liver tumors also had chronic active hepatitis. At the time, contamination with an environmental chemical was suspected as the cause of the hepatocellular tumors and the hepatitis, but extensive analyses of food, bedding, and water proved negative 415. An environmental toxin seemed even less likely when it was discovered that mice in the breeding colony, housed in a separate facility, also had hepatic lesions. Hepatitis was worse in males than in females and was documented in A/JCr, C3H/HeNCr, SJL/NCr, BALB/cAnNCr, and SCID/NCr mice but not in C57BL/6NCr mice 415. A/J mice without hepatitis were obtained from a commercial vendor, and when the disease was shown to be transmissible by inoculating these animals with homogenates of liver from affected A/JCr mice, the search for an infectious agent was intensified. Using Steiner's silver impregnation as a special stain, spiral-shaped bacteria were found in bile canaliculi and gallbladders of affected mice 415. It was then that microaerobic culture and isolation was performed on liver tissue and on cecal and colonic mucosal scrapings 146.
Culture and isolation.
Fox et al. succeeded in isolating H. hepaticus from the liver and cecal and colonic mucosa of mice with chronic active hepatitis 146. After a 3- to 7-day incubation under anaerobic or microaerobic conditions, but not in ambient O2, the organism grew as a spreading film on solid media supplemented with serum or blood. The organism is a simple spiral 1.5 to 5 μm long and 0.2 to 0.3 μm in diameter, with a single-sheathed flagellum at each end but without periplasmic fibers (Fig. 7). H. hepaticus exhibits catalase, urease, and oxidase activities, and it grows at 37°C but not at 42 or 25°C (Table 2). Nucleotide sequence determination of the 16S rRNA gene shows that H. hepaticus is a distinct species of Helicobacter most closely related to H. muridarum 23, 146. H. hepaticus is probably the best characterized of the EHS. It is now known to cause chronic active hepatitis and typhlitis in many susceptible strains and stocks of mice 154, 412, 415, hepatocellular tumors in male A/J 154, 415 and B6C3F1 189 mice, and inflammatory bowel disease in a variety of mouse lines with altered immune functions 48, 236, 253, 407, 413. Infection with H. hepaticus is highly prevalent in laboratory mouse colonies 355, but the organism has not been isolated from other host species to date. In immunocompetent mice, there are no clinical signs of disease and no obvious reduction in breeding efficiency. Thus, many investigators may not be aware that their mice are infected with H. hepaticus.
Pathology.
In naturally infected A/JCr mice, H. hepaticus causes focal nonsuppurative necrotizing hepatitis that progresses to chronic active hepatitis characterized by oval cell hyperplasia, cholangitis, and minimal necrosis 154, 412. Males have liver lesions that develop earlier and are more severe than those in females. The basis for the increased susceptibility in males is not understood. Most animals have no gross pathologic lesions in the liver, but severely affected mice may have yellow to white foci and/or a prominent reticular pattern in one or more liver lobes 154, 412. Microscopically, focal lesions can be seen as early as 1 month of age 412. These areas of hepatic necrosis and inflammation become multifocal and may coalesce by 3 to 6 months of age 154. With time, oval cell hyperplasia and lymphoplasmacytic infiltration of surrounding bile ducts and portal veins become prominent 154, 412. The most chronic lesions, seen after 8 months of age, include bile duct hyperplasia in many portal areas, cytomegaly and karyomegaly of hepatocytes, and intranuclear pseudoinclusions 154, 412. Hepatocellular necrosis becomes less prominent at this stage. Between 12 and 18 months of age, most male mice go on to develop preneoplastic nodular hyperplasia and hepatocellular tumors 154, 412. Chronic active hepatitis leading to hepatocellular neoplasia was also observed in germ-free female Swiss Webster mice following experimental infection with H. hepaticus, clearly establishing the organism as a murine pathogen 160.
Carcinogenesis and a confounding factor in risk assessment.
More recently, H. hepaticus infection has also been shown to be associated with hepatic neoplasia in B6C3F1 mice used for carcinogenesis testing by the National Toxicology Program 155, 189. Unfortunately, several 2-year carcinogenesis studies were confounded by the presence of hepatocellular tumors and hepatic hemangiosarcoma in control male mice. It is of interest that genetic susceptibility to Helicobacter hepatitis and hepatic neoplasia appears to have a dominant pattern of inheritance, since B6C3F1 mice are produced by interbreeding susceptible C3H and resistant C57BL/6 strains of mice. A genetic role in susceptibility to inflammation from H. hepaticus is also suggested by studies with recombinant inbred AXB mice that are derived from matings between C57BL/6 and susceptible A/J mice 214. The mechanism by which H. hepaticus infection leads to hepatic neoplasia remains poorly understood. No evidence of mutations in ras or in the p53 tumor suppressor gene was found in liver tumors taken from A/JCr mice infected with H. hepaticus 366. The absence of p53 mutations is consistent with earlier findings, but activating mutations in the H-ras oncogene are characteristic of chemically initiated murine liver tumors. The absence of mutations in H. hepaticus-associated liver tumors suggests that the mechanism of H. hepaticus carcinogenesis may not involve genotoxic damage 49. Instead, the enhanced rate of hepatocyte proliferation and apoptosis seen in affected mice may influence hepatocarcinogenesis by acting as a tumor promoter 80, 154, 304. H. hepaticus infection also results in oxidative stress in the liver, as evidenced by elevated levels of 8-hydroxydeoxyguanosine (8-oxo-dG) found in affected male A/JCr mice 365. Reactive oxygen species and reactive nitrogen species may also contribute to hepatocarcinogenesis in H. hepaticus infection.
Inflammatory bowel disease.
H. hepaticus organisms seen with Steiner stain in the liver are present in bile canaliculi but not within hepatocytes. This was confirmed by transmission electron microscopy 154, 412 (Fig. 7C). Bacteria are also found in the gallbladder and in large numbers on the surface epithelium in the intestine and deep in the crypts, particularly in the cecum 154, 415. Indeed, H. hepaticus is found earlier and more consistently in the intestine than in the liver, indicating that this is the primary site of colonization 154. In the intestines of immunocompetent mice, H. hepaticus infection causes mild inflammation and epithelial hyperplasia that is typically seen as a relatively late change 160, 421. In contrast, H. hepaticus infection in immunodeficient nude and SCID mice is associated with marked typhlitis, colitis, and proctitis, often with a high incidence of rectal prolapse 253, 339, 413. Similar lesions have been associated with H. hepaticus infection in lines of targeted gene mutant mice (knockout mice) that have been used as models of idiopathic inflammatory bowel disease (IBD) 128. Furthermore, experimental infection with H. hepaticus has been shown to be sufficient to induce IBD-like lesions in SCID mice reconstituted with naive CD4+ CD45RBhigh T cells 48, as well as in IL-10-deficient mice 236 and Rag-2-deficient mice 407. Some controversy remains about the exact role of H. hepaticus infection in the etiopathogenesis of IBD in various knockout mouse models. Nonetheless, it is now clear that H. hepaticus infection can cause severe intestinal inflammation that resembles Crohn's disease and ulcerative colitis in knockout mice that have a dysregulated immune response.
Bacterial pathogenesis.
The mechanism by which H. hepaticus causes hepatic and intestinal disease remains poorly understood. Infected mice develop persistent humoral and mucosal immune responses that are not protective 154, 256, 412, 421. Both chronic active hepatitis in A/JCr mice and IBD in IL-10 knockout and Rag-2 knockout mice are associated with a Th1 immune response, which is characterized by high levels of gamma interferon and the presence of activated macrophages 236, 407, 421. H. hepaticus and HSP70 share cross-reactive epitopes, and it has been suggested that autoimmunity could play a role in disease pathogenesis 414. A novel toxin activity has been identified in H. hepaticus that causes vacuole formation in a murine liver cell line, resulting in a granular appearance of the affected cells 388. This granulating cytotoxin is a heat-labile secreted protein with a native molecular mass of >100 kDa that is distinct from the vacuolating cytotoxin of H. pylori. More recently, Young et al. have identified three genes encoding cytolethal distending toxin (CDT) and CDT activity in H. hepaticus 433. H. hepaticus CDT causes cell cycle arrest in HeLa cells and is closely related to the CDT of Campylobacter species 323. Genetic and phenotypic evidence of CDT has also been found in, H. bilis, H. canis, and H. pullorum 52, 432. The role of these toxins in H. hepaticus pathogenesis remains to be determined.
Diagnosis and treatment.
H. hepaticus can be isolated from the livers of animals with hepatitis 146. However, it is more consistently recovered from the intestinal tract 154. This is particularly true in C57BL/6 mice that are resistant to hepatic disease. Sensitive isolation procedures typically include incubation on a selective medium and/or passage through a 0.45-μm-pore-size filter to enrich for H. hepaticus organisms 355. Although reliable in the hands of an experienced microbiologist, these procedures are tedious and require days to weeks for successful culture and isolation. PCR provides more rapid results and greater sensitivity. Several PCR methods have been described, including H. hepaticus-specific amplification with primers complementary to regions in the 16S rRNA gene 355 and a PCR-RFLP that also amplifies a portion of the 16S rRNA gene 334. Shen et al. have described a second PCR-RFLP method that specifically amplifies a portion of the H. hepaticus urease structural genes ureAB and then allows genotyping to be performed 357. Serodiagnosis of H. hepaticus infection by enzyme-linked immunosorbent assay using a membrane preparation as antigen has also been described 256. With regard to treatment, several antibiotic regimens have been described for eradication of H. hepaticus 129, 130, 339, but treatment failures do occur. Rederivation of genetically characterized mice by constructing the strain in Helicobacter-free recipient mice may be a more reliable method for the elimination of this pervasive murine pathogen.
Helicobacter cinaedi and Helicobacter fennelliae
First identified as Campylobacter species, H. cinaedi and H. fennelliae are simple spiral-shaped organisms that resemble H. hepaticus morphologically (Fig. 7) but do not produce urease. Totten et al. isolated these organisms from rectal swabs taken from homosexual men 397. Over 30 isolates of H. cinaedi (from the Latin for “of a homosexual”) were recovered from asymptomatic individuals and individuals with proctitis, proctocolitis, or enteritis. Another six isolates, all recovered from patients with clinical signs, were shown to comprise a distinct species and were named H. fennelliae after Cynthia Fennell, the technologist who first isolated the organism. A lone isolate from a symptomatic individual was designated Campylobacter-like organism 3 (CLO-3) and has still not been named 397. Additional isolates of H. cinaedi from dogs, cats, and Syrian hamsters were shown by DNA-DNA hybridization to belong to a single species 226. Although the hamsters from which H. cinaedi was isolated appeared healthy 167, experimental inoculation of infant pigtailed macaques (M. nemestrina) with H. cinaedi or H. fennelliae caused diarrhea and bacteremia 125. Indeed, although H. cinaedi has been documented as a cause of acute diarrhea in otherwise healthy individuals 390, bacteremia without gastroenteritis has been more frequently associated with H. cinaedi and H. fennelliae infection in patients immunocompromised due to AIDS 53, 259, 300, 340 or other underlying conditions 207. These infections can also manifest as cellulitis or septic arthritis 45. Interestingly, H. cinaedi has also been isolated from the blood of immunocompetent children and adults with and without diarrhea 405 and in one case from a neonate with bacteremia and meningitis whose mother had cared for pet hamsters during pregnancy 308. Thus, these organisms—like Campylobacter fetus and C. hyointestinalis, which are typically associated with disease in farm animals—cause invasive disease as well as gastroenteritis in humans, particularly those with underlying immunosuppression such as that caused by AIDS.
Helicobacter canis
A separate group of isolates originally designated CLOs were recovered from the feces of dogs with or without diarrhea. These isolates morphologically resemble H. hepaticus but grow at 42 as well as 37°C and do not exhibit urease activity. On the basis of DNA-DNA hybridization and 16S rRNA sequencing, these strains were shown to comprise a distinct species that was named H. canis 375. H. canis has also been isolated from the feces of a 5 1/2-year-old boy with gastroenteritis 47 and from the liver of a 2-month-old puppy suffering from multifocal necrotizing hepatitis 147. More recently, H. canis has been isolated from Asian leopard cat-domestic cat hybrids (Bengal cats) with a 6-month history of episodic diarrhea 126. However, because the cats were infected with other potential enteric pathogens, including Campylobacter helveticus, and because H. canis was also isolated from cats without diarrhea, the pathogenic potential of this organism in cats remains unclear. Further studies are needed to determine whether H. canis is a cause of hepatic disease as well as gastroenteritis in carnivores such as dogs and cats, as well as in humans.
Helicobacter pametensis, Helicobacter pullorum, and “Helicobacter canadensis”
H. pametensis and H. pullorum both infect birds and mammals. H. pametensis was first isolated from the feces of wild birds and a domestic pig near the Pamet River on Cape Cod, Mass. 354. Six isolates of H. pametensis from gulls, terns, and the pig were characterized by 16S rRNA gene sequencing and found to represent a single, distinct species 76. In addition, other Helicobacter species were isolated from terns and from a house sparrow. These Helicobacter species were designated Helicobacter sp. Bird-B and Helicobacter sp. Bird-C, respectively 76. They have not yet been named. H. pametensis has also been isolated from the stomach of a cat coinfected with “H. heilmannii” 298. H. pullorum was designated a separate species on the basis of 16S rRNA gene sequencing 374. Isolates were recovered from the ceca of subclinically infected broiler chickens, from the livers and intestinal contents of laying hens with vibrionic hepatitis, and from humans with gastroenteritis 374. One individual, in addition to having diarrhea, developed elevated liver enzyme levels and hepatomegaly 46. There is clearly a potential for zoonotic food-borne transmission of H. pullorum to humans, as is known to occur with Campylobacter species. While both H. pametensis and H. pullorum are urease negative and grow readily at 42 as well as 37°C, they can be distinguished by the fact that H. pametensis has sheathed flagella but H. pullorum does not 374. Importantly, H. pullorum does not grow on campylobacter selective medium containing polymyxin B; it grows well on blood agar supplemented with cefoperazone, vancomycin, and amphotericin B 17, 374. Recently, four isolates cultured from Canadian patients with diarrhea, which were originally classified as H. pullorum, were shown by 16S rRNA sequence to represent a novel species, designated “H. canadensis” 140. Whether “H. canadensis” has an avian reservoir and is acquired by humans as a zoonosis has not yet been determined.
Helicobacter cholecystus
Franklin et al. isolated a novel Helicobacter species from the gallbladder of Syrian hamsters affected with cholangiofibrosis and centrilobular pancreatitis 162. This organism is called H. cholecystus, and it is somewhat morphologically distinct from the other simple spiral-shaped Helicobacter species. H. cholecystus has a rod-shaped protoplasmic cylinder and a single polar, sheathed flagellum, but it does not have periplasmic fibers. It is urease negative and grows at 42°C.
Helicobacter rodentium
H. rodentium is also a urease-negative, spiral-shaped organism that grows well at 42 as well as 37°C 356. Like H. pullorum, H. rodentium has unsheathed flagella. This organism was first isolated from subclinically infected laboratory mice. Subsequently, Shomer et al. reported an outbreak of diarrhea in a colony of SCID mice carrying mutations in the p53 tumor suppressor gene that were coinfected with H. rodentium and H. bilis 359. The true pathogenic potential of H. rodentium in immunodeficient and immunocompetent mice remains to be determined.
“Helicobacter mesocricetorum”
The most recently described Helicobacter species, “H. mesocricetorum,” is a urease-negative bacterium 362 isolated from fecal pellets of asymptomatic Syrian hamsters (Mesocricetus auratus). It is closely related to H. rodentium and H. pullorum, which share the somewhat unusual property among Helicobacter species of having unsheathed flagella. Histologic evaluation of the gastrointestinal tracts of “H. mesocricetorum”-infected hamsters revealed no pathologic changes, and thus this bacterium is probably commensal. Whether “H. mesocricetorum” can be transmitted to humans as a zoonosis, like H. cholecystis and H. cinaedi, which also infect hamsters, is unknown.
“Helicobacter typhlonicus”
Fox et al. isolated a novel species from H. hepaticus-free IL-10-deficient mice with IBD 149. IL-10-deficient mice experimentally infected with this organism developed typhlocolitis and proctitis by 4 months postinoculation, demonstrating the ability of this EHS to cause disease. Although the infected IL-10-deficient mice had focal hepatic granulomatous inflammation and mild cholangitis, no bacterial organisms were seen in the liver. In SCID mice experimentally infected with this organism, there was mild to moderate proliferative typhlitis at 4 months postinoculation, while experimentally infected A/JCr mice had minimal to mild typhlitis 6 months postinoculation. Shortly after this publication appeared, Franklin et al. cultivated from the feces of BALB/c mice an EHS that was identical to that described by Fox et al., and the designation “H. typhlonicus” was proposed 164. Pathologic changes similar to those reported by Fox et al. were seen in SCID mice experimentally inoculated with “H. typhlonicus” 164.
Other Urease-Negative Helicobacter Species without Periplasmic Fibers
In addition to these 11 formally or provisionally named species, other EHS without periplasmic fibers have been described. “H. mainz” was isolated from the blood and joint fluid of an AIDS patient with septic arthritis 210 and from the blood of two other AIDS patients with bacteremia 123. Provisionally named after Mainz, Germany, the city where they were first isolated, these isolates have a 16S rRNA gene sequence that is 97.7% similar to H. fennelliae. Thus, it is not clear if “H. mainz” can truly be considered a distinct species. Likewise, “H. westmeadii” was isolated from the blood of two AIDS patients with bacteremia and provisionally named after Westmead, New South Wales, Australia 398. Because these isolates have a 16S rRNA gene sequence that is very similar to that of H. cinaedi (Table 3), they, too, may not represent distinct species. More recently, a similar organism was isolated by Weir et al. from the blood of an AIDS patient 420. The definitive classification of this isolate also remains to be determined.
Foley et al. have described an organism that was identified in the intestine of a kitten with diarrhea 127. This organism was provisionally named “H. colifelis” and was found to have a 16S rRNA gene sequence 98.3% similar to H. canis. Because the organism was not successfully cultured, it is difficult to determine if it represents a novel EHS. Its association with diarrhea is also not proven, because inoculation of infected feces did not produce diarrhea in specific-pathogen-free cats, although the feces was positive by PCR using primers specific for the Helicobacter genus.
Helicobacter muridarum
Historical perspective.
Phillips and Lee succeeded in isolating the first EHS in 1983 by inoculating blood- or serum-enriched media with mucosal scrapings from the intestines of conventional Wistar rats and BALB/c mice almost 10 years before it would be formally named as a Helicobacter species 322. The isolates grew slowly, spreading as a thin film after a few days of incubation at 37°C, even on media containing 3% agar. The growth requirements of the isolates were reminiscent of the in vivo niche in which they thrived. Like Campylobacter species, they required a reduced partial pressure of O2 and an increased partial pressure of CO2. These microaerobic conditions could be produced by equilibrating evacuated jars with a bottled gas mixture or with anaerobic gas generator envelopes, without the use of catalyst so as not to remove residual O2. The isolates also required media with surface moisture for optimal growth. Plates that had been predried to remove surface condensation did not generally support growth, but biphasic conditions, with broth over a layer of solid medium, were well suited for culture of these organisms. In pure culture, the isolates were found to be spiral shaped and gram negative. Like the organisms seen by Davis et al. 66 and Erlandsen and Chase 111 by electron microscopy, the isolates were 0.5 to 0.6 μm in diameter and 3.5 to 5 μm long. They had two to three spiral turns, periplasmic fibers, and bipolar tufts of sheathed flagella. Nucleotide sequence determination of 16S rRNA eventually confirmed what growth requirements, ultrastructure, and biochemical characteristics had suggested: H. muridarum is a separate species in the genus Helicobacter 248.
Epidemiology.
Based on microscopic observations of H. muridarum in the intestinal crypts of conventional rats and mice, Phillips and Lee found that the ileum had a higher density of colonization than did the cecum or the colon 322. However, when gnotobiotic animals were experimentally inoculated with pure cultures of the organism, few of the ileal crypts in rats and none of the ileal crypts in mice contained spirals. Instead, the cecum, and to a lesser extent the colon, was found to contain the highest density of organisms. This suggests that in the absence of competing microbiota, the large intestine, and the cecum in particular, is the primary site of colonization by H. muridarum. In something of a contrast to what was found by Erlandsen and Chase in normal rats 111, Phillips and Lee observed bacteria free in the cytoplasm of epithelial cells lining the crypts of the gnotobiotic rats and mice but not in the conventional animals. The cells containing these bacteria appeared damaged, containing numerous vacuoles, swollen mitochondria, and diffuse cytoplasm 322. It is apparent that H. muridarum can invade the intestinal mucosa of rodents, and its presence there is associated with cellular degeneration. The circumstances under which this tissue invasion takes place, and the ultimate fate of the invading bacteria, have still not been elucidated.
Pathology.
It has also been noted that H. muridarum can colonize the stomachs of mice, where it is associated with inflammatory lesions. Queiroz et al. described gastritis, characterized by a mixed-cell infiltrate, that varied from mild to severe in 6- to 8-week-old BALB/c mice 325. H. muridarum infection in the stomach and the associated gastritis were found in over half of the mouse colonies examined. However, the prevalence within a given colony varied from 5 to 100%. All of the mice examined by Queiroz et al. were found to have H. muridarum in the cecum, and most of the animals had the bacteria in the ileum as well, although no inflammation was observed at these sites. In some mouse colonies, occasional gastric colonization with H. muridarum occurs spontaneously in older animals, presumably as a consequence of reduced parietal cell mass 136. It has also been reported that when mice enzootically infected with H. muridarum in the lower bowel are experimentally challenged with gastric Helicobacter species, or simply as the mice age, H. muridarum can take advantage of the altered gastric milieu, displace the gastric spirals, and persistently colonize the stomach 241. In the study by Queiroz et al., the mice were young and had not been experimentally inoculated with another Helicobacter species. Details of the process by which hypochlorhydria and/or perturbations of the indigenous gastric microbiota lead to colonization of the stomach by EHS remain to be determined. However, the potential confounding influence of the EHS on in vivo studies of gastric Helicobacter species should not be ignored.
Helicobacter sp. flexispira (“Flexispira rappini”)
Nomenclature.
A heterogeneous group of organisms that are all ultrastructurally identical to the Lockard type 1 organism has been given the provisional name “Flexispira rappini” 43. This name has never been validated 215 and is therefore parenthetical. The species name is eponymous for Rappin, who in 1881 described spiral organisms in mucosal scrapings taken from the gastric mucosa of dogs. The generic name Flexispira should be abandoned in favor of Helicobacter, since several studies have shown that these organisms are members of this taxon 136, 145, 161, 248, 346. Recent data suggest that what has been called “Flexispira rappini” represents at least 10 different Helicobacter taxa 74, including the two named species H. bilis 161 and H. trogontum 275. Thus, as is the case for “H. heilmannii,” multiple different species are morphologically indistinguishable. Members of the remaining eight species have been isolated from many sources (Table 5), including aborted sheep fetuses 59, 230, humans with or without diarrhea 337, 373, 391, 420, dogs 99, 220, 337, and apparently healthy laboratory mice 346. Most recently, they have been isolated from patients with bacteremia and from cottontop tamarins with colitis 345, 346. However, none of the flexispira taxa contain enough phenotypically and genotypically characterized strains to be formally named “Helicobacter rappini.” Therefore, we have adopted the suggestion of Dewhirst et al. that these organisms be collectively referred to as Helicobacter sp. flexispira (or as flexispira-like for brevity), with a specific taxon designation number applied when appropriate 74.
TABLE 5.
Examples of isolates comprising Helicobacter sp. flexispira
| Source | Strain | Other designations | GenBank 16S rRNA accession no. | Reference |
|---|---|---|---|---|
| 47-year-old-man with diarrhea (case 1) | ATCC 43879 | CCUG 23435, SLH-38264, LMG 8738, NADC 1937 | M88138 | 337 |
| 16-yr-old asymptomatic girl (case 1) | ATCC 49309 | NADC 1939 | NAa | 337 |
| 5-mo-old puppy (case 1) | ATCC 49308 | NADC 1938 | NA | 337 |
| 40-yr-old man with diarrhea (case 2) | ATCC 43880 | SLH-14020, LMG 8457 | NA | 337 |
| Aborted sheep fetus | ATCC 43966 | NADC 1893 | M88137 | 230 |
| Canine feces | ATCC 49317 | NADC2016 | AF047851 | C.E. Gates and C. A. Kirkbride, unpublished |
| Mouse feces | DBS59 | L12765 | 346 | |
| 9-yr-old girl with bacteremia and pneumonia | FH 9702248 | AF034135 | 391 | |
| 65-yr-old immunocompromised man with bacteremia | H1353 | AF118017 | 373 | |
| 36-yr-old immunocompromised man with recurrent bacteremia | CDC-H69 | AF118807 | 420 |
NA, not available.
Historical perspective.
Organisms fitting the description of Helicobacter sp. flexispira were first isolated from late-term-aborted sheep fetuses by Kirkbride et al. 230. The fetal lambs had focal hepatic necrosis that was suggestive of Campylobacter infection. However, culture of fetal liver, lung, and abomasal contents yielded Helicobacter sp. flexispira after 1 week of incubation on blood agar under an atmosphere of 80% N2, 10% CO2, and 10% H2 at 37°C 230. Koch's postulates were fulfilled by producing abortion in a small percentage of pregnant ewes inoculated intravenously with Helicobacter sp. flexispira 229. Bryner et al. went on to show that intraperitoneal inoculation of pregnant guinea pigs caused abortion featuring suppurative placentitis and splenitis 44. The organism was cultured from heart blood at necropsy of the guinea pigs 11 days after inoculation, suggesting persistent bacteremia. Characterization of this isolate demonstrated that it was positive for catalase, oxidase, and urease activities 16. Pure cultures of isolates that were catalase, oxidase, and urease negative were recovered from the placenta, liver, and abomasal contents of aborted lambs in Britain 59, but these isolates were not extensively characterized.
Human infection and disease.
Two cases of chronic diarrhea apparently caused by Helicobacter sp. flexispira in adults have also been described by Romero et al. 337. The first was in a 47-year-old man with a 1-month history of nonbloody diarrhea, fever, headache, and lower abdominal pain. The organism was also recovered from his asymptomatic 16-year-old daughter and from a subclinically infected 5-month-old female puppy in the household. The second case was in a 40-year-old man with a 2-month history of nonbloody diarrhea without fever. This individual had no known contact with animals. Both patients were successfully treated with erythromycin. These strains were shown to differ from Kirkbride's ovine isolate by the lack of catalase activity and by exhibiting a more rapid urease reaction 16. An essentially identical strain was isolated from the ileum and cecum of healthy outbred mice 346. The nucleotide sequence of a partial 16S rRNA gene fragment from the murine isolate was shown to be over 99% similar to that of the human isolate and that of the ovine isolate 346, indicating that these organisms are all closely related. They also appear to have the potential to cause zoonotic infections.
Two cases of bacteremia caused by Helicobacter sp. flexispira have also been reported. The first was in a 9-year-old girl with a 5-day history of fever, productive cough, and malaise 391. A blood sample obtained at the time she was diagnosed with pneumonia yielded a pure culture of a strain that was oxidase positive but catalase and urease negative. This isolate was shown by 16S rRNA nucleotide sequence determination to be most closely related to an ovine isolate of Helicobacter sp. flexispira. The second case was in a 65-year-old man undergoing hemodialysis for end-stage renal failure 373. The patient also had a history of pancreatitis due to alcoholism with secondary diabetes mellitus and severe peripheral vascular disease. A few days after initiation of hemodialysis through a Hickman catheter, he developed bacteremia. A strain that was oxidase and urease positive but catalase negative was isolated from a blood culture. The patient was treated successfully with intravenous vancomycin and amikacin, but 3 weeks later he developed recurrent fever, dyspnea, and a productive cough. The same strain was again isolated from blood cultures and was shown to have a 16S rRNA gene sequence that was 99.6% similar to that of the urease-negative strain isolated from the pediatric patient 391. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of total bacterial proteins from this isolate also showed a pattern virtually indistinguishable from that of the Helicobacter sp. flexispira isolate described by Romero et al. 337. More recently, a flexispira-like organism was isolated from the blood of a patient with X-linked agammaglobulinemia suffering from persistent bacteremia 419. The organism was positive for catalase, oxidase, and urease and was found to be closely related to Romero's human isolate and Kirkbride's ovine isolate by 16S rRNA gene sequence determination. Surprisingly, DNA-DNA hybridization studies indicated that these organisms were only 24 to 37% related, and conclusive identification of this isolate remains to be performed 419.
Other host species.
Other examples of flexispira-like organisms have been isolated from the stomachs of healthy dogs from a commercial supplier of random-source animals 96 and the stomachs of pet dogs which presented for gastrointestinal signs or for euthanasia 220. In the study by Eaton et al. 96, pure cultures of gastric Helicobacter species were isolated from 6 of 54 dogs. Two of the isolates had a flexispira-like morphology and were urease positive and catalase negative. The nucleotide sequence of the 16S rRNA gene from one of these isolates indicated that it was a distinct species, while the other isolate was an H. bilis strain (see below). In the study by Jalava et al. 220, gastric Helicobacter species were isolated from 48 of 95 dogs. Two of the isolates were reported to be Helicobacter sp. flexispira based on their morphology. The strains were both catalase positive, and one had urease activity while the other was urease negative. Total bacterial protein profiles indicated that the two strains were similar to one another but sufficiently different from the isolate described by Romero et al. to be members of a distinct species 220. More recently, Saunders et al. reported that a novel Helicobacter species could be isolated from cottontop tamarins, a species of New World monkey that develops ulcerative colitis and colon cancer 345. This Helicobacter species is morphologically flexispira-like and exhibits oxidase and catalase activities but is urease negative. Nucleotide sequence determination of the 16S rRNA gene from this organism indicated that it is a separate species.
Helicobacter bilis
Mice.
First identified in aged, inbred mice with chronic hepatitis, H. bilis is a distinct species that also has an “H. rappini”-like, or Lockard type 1, ultrastructure 161. Fox et al. recovered H. bilis from the livers and intestines of subclinically infected C57BL/6, CBA/CA, DBA/2, and BALB/c mice between 19 and 27 months of age. The isolates grew at 42 as well as 37°C and exhibited catalase, oxidase, and urease activities (Table 2). Growth was observed in the presence of bile at concentrations of up to 20%. Nucleotide sequence determination of the 16S rRNA genes from these isolates unambiguously identified H. bilis as a separate species 161. Infection with H. bilis has also been associated with typhlocolitis and diarrhea in immunodeficient rodents, The first case report described dual infection with H. bilis and H. rodentium, as described in an earlier section of this review 359. The affected animals were SCID mice and had germ line mutations in the p53 tumor suppressor gene. These animals were a combination of C57BL/6 and 129/Sv crossed with a C.B-17 genetic background, and they developed epizootic diarrhea associated with hyperplastic typhlocolitis. Younger animals, in particular, had marked thickening of the colon with dramatic crypt elongation and bloody mucoid diarrhea 359. The only liver lesions seen in these young animals were consistent with septicemia. One adult female SCID mouse also developed diarrhea and rectal prolapse. It is not clear if the high morbidity described in this case report was due to the dual infection, to the virulence of the H. bilis strain, or to some other predisposing factor(s).
Two groups have fulfilled Koch's postulates with H. bilis in SCID mice. Shomer et al. used intraperitoneal injection to experimentally inoculate defined-flora outbred ICR SCID mice with the H. bilis type strain 360. Franklin et al. orally inoculated inbred C.B-17 SCID mice with a different strain of H. bilis 163. Defined-flora SCID mice, which have a microbiota composed entirely of eight species of anaerobic bacteria (the altered Schaedler's flora) 73, developed a hyperplastic typhlocolitis. Some of the experimentally inoculated mice developed diarrhea, but at 7 weeks postinoculation, which was the conclusion of the study, there was no evidence of hepatitis 360. Conversely, the orally inoculated C.B-17 SCID mice with a conventional microbiota did not exhibit clinical signs of disease. By 3 months postinoculation, the animals had hyperplastic typhlitis and more mild lesions in the proximal colon 163. Male mice also developed chronic active hepatitis by 3 months postinoculation; liver lesions were seen in the female mice at 6 and 9 months postinoculation.
Rats.
H. bilis has also been isolated from outbred athymic nude rats with typhlitis 190. These animals, some of which exhibited mild diarrhea, had hyperplastic typhlitis with or without colonic inflammation. The 5- to 8-month-old male rats did not have any significant lesions in the stomach or in the liver. The H. bilis isolate was inoculated by intraperitoneal injection into Helicobacter-free 2-month-old male outbred nude rats 190. These animals lost weight, and some developed watery diarrhea 2 to 3 months postinoculation. All of the experimentally inoculated rats developed hyperplastic typhlocolitis that was seen as early as 1 month postinoculation. None of these animals developed any significant lesions in the stomach or in the liver.
Host range.
Like Helicobacter sp. flexispira, H. bilis may be transmitted between host species and cause zoonotic infections. As mentioned above, a Helicobacter isolate from the stomach of a random-source laboratory dog was identified as H. bilis by 16S rRNA gene sequencing 96. Sequencing of PCR-amplified 16S rRNA gene fragments has also been used to show that H. bilis can infect the gallbladders of humans with chronic cholecystitis 145. A total of 9 of 23 gallbladders and 13 of 23 bile samples taken from Chilean patients undergoing cholecystectomy were PCR positive for Helicobacter species 145. Although culture and isolation was also attempted, no Helicobacter organisms were recovered from the samples. The complete nucleotide sequences of eight of the amplicons were determined. Five of these were found to be H. bilis 145. Two of the amplicons were Helicobacter sp. flexispira with a high degree of similarity to the 16S rRNA gene sequence of the isolates described by Romero et al. 337. One additional amplicon was found to be H. pullorum. Establishing a causal relationship between H. bilis infection and human diseases, including chronic cholecystitis and biliary cancer—for which the Chilean population is at high risk—will require further studies. Nonetheless, it seems likely that H. bilis-associated diseases are not limited to laboratory rats and mice. In unpublished studies mentioned previously 145, H. bilis has also been isolated from the stomach and the cecum of gerbils and from the feces of cats.
Helicobacter trogontum
A Helicobacter species that seemed essentially identical to H. bilis was isolated from the colonic mucosa of Holtzman and Wistar rats by Mendes et al. 275. Like H. bilis, H. trogontum grew at 42 as well as 37°C but not at 25°C. The isolates were also positive for urease, catalase, and oxidase activities (Table 2). Despite these phenotypic similarities, nucleotide sequence determination of 16S rRNA gene fragments from H. trogontum showed that it differed from that of H. bilis by 3.9% and from that of Kirkbride's ovine Helicobacter sp. rappini isolate by 4.3% 275. Thus, H. trogontum is a distinct species. Experimental inoculation of gnotobiotic outbred mice resulted in primarily cecal colonization, with fewer organisms in the colon, at 3 weeks postinoculation 291. Transmission electron microscopy revealed that, like H. muridarum, H. trogontum invaded enterocytes in the cecum of gnotobiotic mice, where it was found free in the cytoplasm. An organism with an ultrastructure indistinguishable from H. trogontum was seen in the common bile ducts of rats experimentally inoculated with the liver fluke, Fasciola hepatica 132. Since the organism was not cultured, it is not clear if these rats were infected with H. trogontum, H. bilis, or another member of the Helicobacter sp. rappini group. It remains to be determined if H. trogontum can colonize the liver of rats and if rats are susceptible to Helicobacter-associated hepatitis.
HELICOBACTER PATHOGENESIS: AN ECOLOGICAL PERSPECTIVE
Bacterial colonization of the gastrointestinal tract of humans and other animals has been a subject of study for decades. It has long been known that the colon and, to a lesser extent, the small bowel are populated with a complex microbial ecosystem made up largely of anaerobic bacteria which thrive in the highly reduced environment of the bowel. What has become clear over the last 20 years is that the stomachs of humans and a wide range of other animals also have a microbial ecology that consists primarily of one or more urease-producing Helicobacter species. Furthermore, Helicobacter species are commonly present as a part of the enteric and hepatobiliary biota in humans and a variety of other animals. In short, members of the Helicobacter genus are ubiquitous colonizers of the enteric mucosal surface, which forms a critical interface between an organism and its environment.
The present consideration of multiple Helicobacter species in a wide range of hosts provides a broad view of the question that has been repeatedly asked: Are these bacteria pathogens? Even as the National Institutes of Health consensus statement was published in 1994, recommending antibiotic therapy for patients with H. pylori infection and peptic ulcer 12, it was recognized that H. pylori was a “slow bacterium” 36, “almost normal flora” 245, and that “in a world of black and white Helicobacter pylori is gray” 35. Emerging evidence suggests that while H. pylori infection can cause duodenal ulcer and gastric cancer, it may also protect against diseases such as adenocarcinoma of the proximal stomach and lower esophagus, which suggests a commensal or in fact symbiotic host-parasite interaction. Thus, the relationship between H. pylori and human health and disease is probably complex and dynamic, as described recently in a thoughtful review by Blaser 34.
Examination of the broader range of Helicobacter infections emphasizes the perspective that these bacteria often do not have a clearly pathogenic relationship with their host. However, there are important caveats. First, there are exceptions, such as H. cinaedi and H. fennelliae, which clearly produce disease in a primate model and have been found to be associated with human disease. Second, clinical disease can be absent while pathologic changes are profound. For example, H. hepaticus causes a chronic active hepatitis and hepatocellular tumors in many strains of immunocompetent mice, even though the animals appear to be clinically fit. In contrast, “H. heilmannii” appears to cause minimal inflammation in nonhuman primates and other natural hosts, as do the multiple Helicobacter species that infect dogs and cats. Third, we have very limited knowledge of the prevalence and natural history of many Helicobacter infections as they occur in nature in nonhumans. Fourth, it should be remembered that the association between disease and H. pylori infection in humans is based on long-term case-control and treatment studies, which utilized very large sample sizes and long-term follow-up that are often impractical in other hosts. If experimental inoculation of H. pylori were performed in humans, it would probably be difficult to show clinical disease without decades of observation on large numbers of individuals. We suspect that the occurrence of disease in a minority of humans infected with H. pylori is not unique but, rather, represents a “an overstepping of the line by one side or the other, a biological misinterpretation of borders” 393 that can sometimes be found in other interactions between Helicobacter species and their natural hosts. Finally, the relationship between disease and Helicobacter infection is often host specific. This pertains not only to an immunocompromised host, such as IBD in SCID or IL-10-deficient mice infected with H. hepaticus, but also to infection with a Helicobacter species not normally present in a given host. The potential importance of this should not be underestimated, since many emerging human infectious diseases represent transmission of agents that are harmless and enzootic in their natural host, such as Borrelia burgdorferi, the agent of Lyme disease. We should keep in mind that enteric infections such as acute diarrheal disease are commonly due to unrecognized pathogens. Since Helicobacter probably would not be identified by the cultivation methods routinely applied to cases of human diarrheal disease, its contribution remains unknown. Regardless of whether these Helicobacter species cause disease in their natural host, they provide an important resource to better understand gastric and enterohepatic diseases in humans.
RECOMMENDATIONS AND CONCLUSIONS
Currently there are 20 formally named species comprising the genus Helicobacter (one of which, Candidatus Helicobacter suis, is likely to be changed to Candidatus Helicobacter heilmannii). Additional species have been provisionally named but not yet validated, and still others have been isolated but not yet named. The genus Helicobacter will continue to grow at a brisk pace, and it would not surprise us if novel species not discussed here are described before this article goes to press. Consequently, some basic recommendations are in order. First, it bears repeating that identification—and thus, the ability to recognize Helicobacter species in the etiopathogenesis of disease—is dependent on good classification. Minimal standards for the description of new Helicobacter species are still in development 75. Nonetheless, the Subcommittee on the Taxonomy of Campylobacter and Related Bacteria (of the International Committee on Systematic Bacteriology) did agree that such minimal standards should be based on a minimum number of 5 to 10 strains per taxon 215. Along these lines, there are several good examples of Helicobacter species that have not been named due to insufficient numbers of isolates but have been sufficiently well described in the literature to permit their recognition in a clinical setting. Certainly not every novel Helicobacter species should be named, even provisionally, when first isolated. Sufficient characterization should be performed to ensure the validity of the taxon. For the cultured Helicobacter species, this should include a combination of 16S rDNA sequencing, DNA-DNA hybridization, protein profiling, cellular fatty acid profiling, and biochemical characterization. Characterization of uncultivated species is obviously more limited and relies largely on 16S rDNA sequence comparison, which can sometimes be misleading when sequences are very closely related. While not every method can or should be used for every novel Helicobacter species, a polyphasic approach is clearly in order 406.
For the uncultured Helicobacter species, important advances have been made by using PCR-based methods and by conducting monoassociation studies with gnotobiotic rodents. However, successful culture and isolation of these organisms will confer a degree of clarity to their taxonomy that currently is out of reach. While some groups are achieving greater and greater success in culturing these fastidious organisms, even the more hardy Helicobacter species can pose a challenge to the microbiologist whose only experience with the genus is H. pylori. These Helicobacter species require special conditions for growth. They require a microaerobic environment, which can be achieved with a commercial gas generator envelope, but many grow better under an atmosphere of 5 to 10% hydrogen (such as is provided in a bottle gas mixture), and in fact some of the EHS will not grow at all with commercial gas generator systems. These Helicobacter species also grow slowly, and contaminating enteric microbiota that are present in the lower bowel or transiently in the gastric compartment can overgrow them. Worse still, thermophilic Campylobacter species may be inadvertently cocultured with these Helicobacter species, and they are similar enough to make isolation quite difficult. Selective media can be used, but it is important to keep in mind that some Helicobacter species are sensitive to the common antimicrobial agents incorporated into commercial Campylobacter media. Finally, some of these Helicobacter species seem to require surface moisture on solid media for growth. Freshly poured plates are typically better than plates that have been predried, and—contrary to typical good microbiologic technique—the plates should be incubated with the lid uppermost. Some of these organisms simply fail to grow as discrete colonies but, rather, form a fine spreading film that may go unrecognized as bacterial growth by the casual observer. Additional recommendations about culture technique for Helicobacter species can be found in the excellent review by Fox and Lee 151.
The true extent of ecological niches occupied by species of Helicobacter is not yet known. Neither has the full spectrum of disease syndromes that are associated with Helicobacter infections been defined. Only by developing new and improved molecular diagnostic techniques and by optimizing culture and isolation methods will we develop a more complete understanding of the Helicobacter genus. As progress is made in this area, it will also become important to increase our understanding of the mechanisms of pathogenesis that characterize these organisms. Certainly, some features of H. pylori pathogenesis will be shared by the other gastric Helicobacter species, as well as by the enterohepatic Helicobacter species. However, there will be some important differences as well. It seems likely that future studies on H. pylori and the other members of the Helicobacter genus will benefit from comparative analyses of these organisms. This is all the more true, given the genomic database that has become available for H. pylori. Similar advances in genomic sequencing of Campylobacter species will also provide valuable insights into the biology of spiral-shaped bacteria that inhabit mucus gel layers. Certainly, our understanding and our appreciation of H. pylori and its role in our gastric microenvironment have grown in the past decade. By taking advantage of these genomic resources and some of the recently developed tools for genetic manipulation of Helicobacter species in the coming years, we may also gain new perspectives on the intricate coexistence that we and our diverse spiral-shaped microbiota maintain in health and in disease.
ACKNOWLEDGMENTS
We thank Jim Fox and Adrian Lee, friends and colleagues, whose enthusiasm and contributions to the study of Helicobacter pathogenesis have motivated and inspired us.
Helicobacter research in our laboratories is funded by grants AI42081 (J.V.S.) and DK52413 (D.B.S.) from the National Institutes of Health.
REFERENCES
- 1.Adam W S, Calhoun M L, Smith E M, Stinson A W. Microscopic anatomy of the dog. Springfield, Il: Charles C Thomas; 1970. [Google Scholar]
- 2.Akin O Y, Tsou V M, Werner A L. Gastrospirillum hominis-associated chronic active gastritis. Pediatr Pathol Lab Med. 1995;15:429–435. doi: 10.3109/15513819509026978. [DOI] [PubMed] [Google Scholar]
- 3.Akopyants N, Bukanov N O, Westblom T U, Kresovich S, Berg D E. DNA diversity among clinical isolates of Helicobacter pylori detected by PCR-based RAPD fingerprinting. Nucleic Acids Res. 1992;20:5137–5142. doi: 10.1093/nar/20.19.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alder J D, Ewing P J, Mitten M J, Oleksijew A, Tanaka S K. Relevance of the ferret model of Helicobacter-induced gastritis to evaluation of antibacterial therapies. Am J Gastroenterol. 1996;91:2347–2354. [PubMed] [Google Scholar]
- 5.al-Himyary A J, Zabaneh R I, Zabaneh S S, Barnett S. Gastrospirillum hominis in acute gastric erosion. South Med J. 1994;87:1147–1150. doi: 10.1097/00007611-199411000-00019. [DOI] [PubMed] [Google Scholar]
- 6.Alm R A, Ling L L, Moir D T, King B L, Brown E D, Doig P C, Smith D R, Noonan B, Guild B C, deJonge B L, Carmel G, Tummino P J, Caruso A, Uria-Nickelsen M, Mills D M, Ives C, Gibson R, Merberg D, Mills S D, Jiang Q, Taylor D E, Vovis G F, Trust T J. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;397:176–180. doi: 10.1038/16495. [DOI] [PubMed] [Google Scholar]
- 7.Andersen L P, Boye K, Blom J, Holck S, Norgaard A, Elsborg L. Characterization of a culturable “Gastrospirillum hominis” (Helicobacter heilmannii) strain isolated from human gastric mucosa. J Clin Microbiol. 1999;37:1069–1076. doi: 10.1128/jcm.37.4.1069-1076.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrews P L, Illman O, Mellersh A. Some observations of anatomical abnormalities and disease states in a population of 350 ferrets (Mustela furo L.) Z Verstierkd. 1979;21:346–353. [PubMed] [Google Scholar]
- 9.Andrews P L, Illman O, Wynne R D. Gastric ulceration in the ferret (Mustela furo L.) Z Verstierkd. 1976;18:285–291. [PubMed] [Google Scholar]
- 10.Andrutis K A, Fox J G, Schauer D B, Marini R P, Li X, Yan L, Josenhans C, Suerbaum S. Infection of the ferret stomach by isogenic flagellar mutant strains of Helicobacter mustelae. Infect Immun. 1997;65:1962–1966. doi: 10.1128/iai.65.5.1962-1966.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrutis K A, Fox J G, Schauer D B, Marini R P, Murphy J C, Yan L, Solnick J V. Inability of an isogenic urease-negative mutant strain of Helicobacter mustelae to colonize the ferret stomach. Infect Immun. 1995;63:3722–3725. doi: 10.1128/iai.63.9.3722-3725.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anonymous. Helicobacter pylori in peptic ulcer disease. JAMA. 1994;272:65–69. [PubMed] [Google Scholar]
- 13.Anonymous. Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monogr Eval Carcinog Risks Hum. 1994;61(5):1–241. [PMC free article] [PubMed] [Google Scholar]
- 14.Ansorg R, Von Heinegg E H, Von Recklinghausen G. Cat owners' risk of acquiring a Helicobacter pylori infection. Z Bakteriol. 1995;283:122–126. doi: 10.1016/s0934-8840(11)80898-4. [DOI] [PubMed] [Google Scholar]
- 15.Appelmelk B J, Simoons-Smit I, Negrini R, Moran A P, Aspinall G O, Forte J G, De Vries T, Quan H, Verboom T, Maaskant J J, Ghiara P, Kuipers E J, Bloemena E, Tadema T M, Townsend R R, Tyagarajan K, Crothers J M, Jr, Monteiro M A, Savio A, De Graaff J. Potential role of molecular mimicry between Helicobacter pylori lipopolysaccharide and host Lewis blood group antigens in autoimmunity. Infect Immun. 1996;64:2031–2040. doi: 10.1128/iai.64.6.2031-2040.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Archer J R, Romero S, Ritchie A E, Hamacher M E, Steiner B M, Bryner J H, Schell R F. Characterization of an unclassified microaerophilic bacterium associated with gastroenteritis. J Clin Microbiol. 1988;26:101–105. doi: 10.1128/jcm.26.1.101-105.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atabay H I, Corry J E, On S L. Identification of unusual Campylobacter-like isolates from poultry products as Helicobacter pullorum. J Appl Microbiol. 1998;84:1017–1024. doi: 10.1046/j.1365-2672.1998.00438.x. [DOI] [PubMed] [Google Scholar]
- 18.Bamford K B, Fan X, Crowe S E, Leary J F, Gourley W K, Luthra G K, Brooks E G, Graham D Y, Reyes V E, Ernst P B. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998;114:482–492. doi: 10.1016/s0016-5085(98)70531-1. [DOI] [PubMed] [Google Scholar]
- 19.Barber M, Franklin R H. Bacteriology of stomach and duodenum in cases of peptic ulcer and gastric carcinoma. Br Med J. 1946;1:951–953. [PubMed] [Google Scholar]
- 20.Barbosa A J, Silva J C, Nogueira A M, Paulino Júnior E, Miranda C R. Higher incidence of Gastrospirillum sp. in swine with gastric ulcer of the pars oesophagea. Vet Pathol. 1995;32:134–139. doi: 10.1177/030098589503200206. [DOI] [PubMed] [Google Scholar]
- 21.Bardhan P K. Epidemiological features of Helicobacter pylori in developing countries. Clin Infect Dis. 1998;25:973–978. doi: 10.1086/516067. [DOI] [PubMed] [Google Scholar]
- 22.Bartle H J, Harkins M J. The gastric secretion: its bactericidal value to man. Am J Med Sci. 1925;169:373–388. [Google Scholar]
- 23.Battles J K, Williamson J C, Pike K M, Gorelick P L, Ward J M, Gonda M A. Diagnostic assay for Helicobacter hepaticus based on nucleotide sequence of its 16S rRNA gene. J Clin Microbiol. 1995;33:1344–1347. doi: 10.1128/jcm.33.5.1344-1347.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bayle D, Wängler S, Weitzenegger T, Steinhilber W, Volz J, Przybylski M, Schäfer K P, Sachs G, Melchers K. Properties of the P-type ATPases encoded by the copAP operons of Helicobacter pylori and Helicobacter felis. J Bacteriol. 1998;180:317–329. doi: 10.1128/jb.180.2.317-329.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benjelloun-Touimi Z, Sansonetti P J, Parsot C. SepA, the major extracellular protein of Shigella flexneri: autonomous secretion and involvement in tissue invasion. Mol Microbiol. 1995;17:123–135. doi: 10.1111/j.1365-2958.1995.mmi_17010123.x. [DOI] [PubMed] [Google Scholar]
- 26.Berestov V A. Noninfectious diseases of fur-bearing animals. New Delhi, India: Oxonian Press; 1984. [Google Scholar]
- 27.Berg D J, Kühn R, Rajewsky K, Müller W, Menon S, Davidson N, Grünig G, Rennick D. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Shwartzman reaction but not endotoxin tolerance. J Clin Investig. 1995;96:2339–2347. doi: 10.1172/JCI118290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berg D J, Lynch N A, Lynch R G, Lauricella D M. Rapid development of severe hyperplastic gastritis with gastric epithelial dedifferentiation in Helicobacter felis-infected IL-10(−/−) mice. Am J Pathol. 1998;152:1377–1386. [PMC free article] [PubMed] [Google Scholar]
- 29.Bitzan M M, Gold B D, Philpott D J, Huesca M, Sherman P M, Karch H, Lissner R, Lingwood C A, Karmali M A. Inhibition of Helicobacter pylori and Helicobacter mustelae binding to lipid receptors by bovine colostrum. J Infect Dis. 1998;177:955–961. doi: 10.1086/515256. [DOI] [PubMed] [Google Scholar]
- 30.Bizzozero G. Sulle ghiandole tubulari del tube gastroenterico e sui rapporti del loro coll'epitelio de rivestimento della mucosa. Atti R Accad Sci Torino. 1892;28:233–251. [Google Scholar]
- 31.Blanchard T G, Czinn S J, Nedrud J G, Redline R W. Helicobacter-associated gastritis in SCID mice. Infect Immun. 1995;63:1113–1115. doi: 10.1128/iai.63.3.1113-1115.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blanchard T G, Gottwein J M, Targoni O S, Eisenberg J C, Zagorski B M, Trezza R P, Redline R, Nedrud J G, Tary-Lehman M, Lehman P V, Czinn S J. Systemic vaccination inducing either Th1 or Th2 immunity protects mice from challenge with H. pylori. Gastroenterology. 1999;116:A695. [Google Scholar]
- 33.Blanchard T G, Lycke N, Czinn S J, Nedrud J G. Recombinant cholera toxin B subunit is not an effective mucosal adjuvant for oral immunization of mice against Helicobacter felis. Immunology. 1998;94:22–27. doi: 10.1046/j.1365-2567.1998.00482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blaser M J. Hypothesis: the changing relationships of Helicobacter pylori and humans: implications for health and disease. J Infect Dis. 1999;179:1523–1530. doi: 10.1086/314785. [DOI] [PubMed] [Google Scholar]
- 35.Blaser M J. In a world of black and white, Helicobacter pylori is gray. Ann Intern Med. 1999;130:695–697. doi: 10.7326/0003-4819-130-8-199904200-00019. [DOI] [PubMed] [Google Scholar]
- 36.Blaser M J, Parsonnet J. Parasitism by the “slow” bacterium Helicobacter pylori leads to altered gastric homeostasis and neoplasia. J Clin Investig. 1994;94:4–8. doi: 10.1172/JCI117336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blaser M J, Smith P F, Repine J E, Joiner K A. Pathogenesis of Campylobacter fetus infections. Failure of encapsulated Campylobacter fetus to bind C3b explains serum and phagocytosis resistance. J Clin Investig. 1988;81:1434–1444. doi: 10.1172/JCI113474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borody T J, Brandl S, Andrews P, Jankiewicz E, Ostapowicz N. Helicobacter pylori-negative gastric ulcer. Am J Gastroenterol. 1992;87:1403–1406. [PubMed] [Google Scholar]
- 39.Bracke J W, Cruden D L, Markovetz A J. Intestinal microbial flora of the American cockroach, Periplaneta americana L. Appl Environ Microbiol. 1979;38:945–955. doi: 10.1128/aem.38.5.945-955.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Braun U, Anliker H, Corboz L, Ossent P. Untersuchungen über das vorkommen von spiralförmigen bakterien im labmagen das rindes. Schweiz Arch Tierheilkd. 1997;139:507–516. [PubMed] [Google Scholar]
- 41.Bromander A K, Ekman L, Kopf M, Nedrud J G, Lycke N Y. IL-6-deficient mice exhibit normal mucosal IgA responses to local immunizations and Helicobacter felis infection. J Immunol. 1996;156:4290–4297. [PubMed] [Google Scholar]
- 42.Brondson M, Goodwin C, Sly L, Chilvers T, Schoenknecht F. Helicobacter nemestrinae sp. nov., a spiral bacterium found in the stomach of a pigtailed macaque (Macaca nemestrina) Int J Syst Bacteriol. 1991;41:148–153. doi: 10.1099/00207713-41-1-148. [DOI] [PubMed] [Google Scholar]
- 43.Bryner J H. Flexispira rappini, gen. nov., sp. nov. A motile, urease-producing rod similar to Campylobacter pyloridis. In: Kaijser B, Falsen E, editors. Campylobacter IV. Kungalv, Sweden: Goterna; 1988. p. 440. [Google Scholar]
- 44.Bryner J H, Ritchie A E, Pollet L, Kirkbride C A, Collins J E. Experimental infection and abortion of pregnant guinea pigs with a unique spirillum-like bacterium isolated from aborted ovine fetuses. Am J Vet Res. 1987;48:91–95. [PubMed] [Google Scholar]
- 45.Burman W J, Cohn D L, Reves R R, Wilson M L. Multifocal cellulitis and monoarticular arthritis as manifestations of Helicobacter cinaedi bacteremia. Clin Infect Dis. 1995;20:564–570. doi: 10.1093/clinids/20.3.564. [DOI] [PubMed] [Google Scholar]
- 46.Burnens A P, Stanley J, Morgenstern R, Nicolet J. Gastroenteritis associated with Helicobacter pullorum. Lancet. 1994;344:1569–1570. doi: 10.1016/s0140-6736(94)90376-x. [DOI] [PubMed] [Google Scholar]
- 47.Burnens A P, Stanley J, Schaad U B, Nicolet J. Novel Campylobacter-like organism resembling Helicobacter fennelliae isolated from a boy with gastroenteritis and from dogs. J Clin Microbiol. 1993;31:1916–1917. doi: 10.1128/jcm.31.7.1916-1917.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cahill R J, Foltz C J, Fox J G, Dangler C A, Powrie F, Schauer D B. Inflammatory bowel disease: an immunity-mediated condition triggered by bacterial infection with Helicobacter hepaticus. Infect Immun. 1997;65:3126–3131. doi: 10.1128/iai.65.8.3126-3131.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Canella K A, Diwan B A, Gorelick P L, Donovan P J, Sipowicz M A, Kasprzak K S, Weghorst C M, Snyderwine E G, Davis C D, Keefer L K, Kyrtopoulos S A, Hecht S S, Wang M, Anderson L M, Rice J M. Liver tumorigenesis by Helicobacter hepaticus: considerations of mechanism. In Vivo. 1996;10:285–292. [PubMed] [Google Scholar]
- 50.Cattoli G, van Vugt R, Sanguinetti V, Vandenbroucke-Grauls C M J E, Kusters J G. Differentiation of gastrospirillum-like organisms by a ureAB based PCR. Gastroenterology. 1999;116:A133. [Google Scholar]
- 51.Chen M, Lee A, Hazell S, Hu P, Li Y. Immunisation against gastric infection with Helicobacter species: first step in the prophylaxis of gastric cancer? Int J Med Microbiol Virol Parasitol Infect Dis. 1993;280:155–165. doi: 10.1016/s0934-8840(11)80952-7. [DOI] [PubMed] [Google Scholar]
- 52.Chien C C, Taylor N S, Ge Z, Schauer D B, Young V B, Fox J G. Identification of cdtB homologues and cytolethal distending toxin activity in enterohepatic Helicobacter spp. J Med Microbiol. 2000;49:525–534. doi: 10.1099/0022-1317-49-6-525. [DOI] [PubMed] [Google Scholar]
- 53.Cimolai N, Gill M J, Jones A, Flores B, Stamm W E, Laurie W, Madden B, Shahrabadi M S. “Campylobacter cinaedi” bacteremia: case report and laboratory findings. J Clin Microbiol. 1987;25:942–943. doi: 10.1128/jcm.25.5.942-943.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clyne M, Labigne A, Drumm B. Helicobacter pylori requires an acidic environment to survive in the presence of urea. Infect Immun. 1995;63:1669–1673. doi: 10.1128/iai.63.5.1669-1673.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cornetta A M, Simpson K W, Strauss-Ayali D, McDonough P L, Gleed R D. Use of a [13C]urea breath test for detection of gastric infection with Helicobacter spp in dogs. Am J Vet Res. 1998;59:1364–1369. [PubMed] [Google Scholar]
- 56.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process. First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- 57.Corthésy-Theulaz I, Porta N, Glauser M, Saraga E, Vaney A C, Haas R, Kraehenbuhl J P, Blum A L, Michetti P. Oral immunization with Helicobacter pylori urease B subunit as a treatment against Helicobacter infection in mice. Gastroenterology. 1995;109:115–121. doi: 10.1016/0016-5085(95)90275-9. [DOI] [PubMed] [Google Scholar]
- 58.Corthésy-Theulaz I E, Hopkins S, Bachmann D, Saldinger P F, Porta N, Haas R, Zheng-Xin Y, Meyer T, Bouzourène H, Blum A L, Kraehenbuhl J P. Mice are protected from Helicobacter pylori infection by nasal immunization with attenuated Salmonella typhimurium phoPc expressing urease A and B subunits. Infect Immun. 1998;66:581–586. doi: 10.1128/iai.66.2.581-586.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crawshaw T R, Fuller H E. Flexispira rappini suspected in ovine abortion. Vet Rec. 1994;134:507. doi: 10.1136/vr.134.19.507-b. [DOI] [PubMed] [Google Scholar]
- 60.Cuenca R, Blanchard T G, Czinn S J, Nedrud J G, Monath T P, Lee C K, Redline R W. Therapeutic immunization against Helicobacter mustelae in naturally infected ferrets. Gastroenterology. 1996;110:1770–1775. doi: 10.1053/gast.1996.v110.pm8964402. [DOI] [PubMed] [Google Scholar]
- 61.Czinn S J, Cai A, Nedrud J G. Protection of germ-free mice from infection by Helicobacter felis after active oral or passive IgA immunization. Vaccine. 1993;11:637–642. doi: 10.1016/0264-410x(93)90309-l. [DOI] [PubMed] [Google Scholar]
- 62.Czinn S J, Nedrud J G. Oral immunization against Helicobacter pylori. Infect Immun. 1991;59:2359–2363. doi: 10.1128/iai.59.7.2359-2363.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Danon S J, Moss N D, Larsson H, Arvidsson S, Ottosson S, Dixon M F, Lee A. Gastrin release and gastric acid secretion in the rat infected with either Helicobacter felis or Helicobacter heilmannii. J Gastroenterol Hepatol. 1998;13:95–103. doi: 10.1111/j.1440-1746.1998.tb00552.x. [DOI] [PubMed] [Google Scholar]
- 64.Danon S J, O'Rourke J L, Moss N D, Lee A. The importance of local acid production in the distribution of Helicobacter felis in the mouse stomach. Gastroenterology. 1995;108:1386–1395. doi: 10.1016/0016-5085(95)90686-x. [DOI] [PubMed] [Google Scholar]
- 65.Davis C P, McAllister J S, Savage D C. Microbial colonization of the intestinal epithelium in suckling mice. Infect Immun. 1973;7:666–672. doi: 10.1128/iai.7.4.666-672.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Davis C P, Mulcahy D, Takeuchi A, Savage D C. Location and description of spiral-shaped microorganisms in the normal rat cecum. Infect Immun. 1972;6:184–192. doi: 10.1128/iai.6.2.184-192.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Debongnie J G, Donnay M, Mairesse J. Gastrospirillum hominis (“Helicobacter heilmanii”): a cause of gastritis, sometimes transient, better diagnosed by touch cytology? Am J Gastroenterol. 1995;90:411–416. [PubMed] [Google Scholar]
- 68.Debongnie J C, Donnay M, Mairesse J, Lamy V, Dekoninck X, Ramdani B. Gastric ulcers and Helicobacter heilmannii. Eur J Gastroenterol Hepatol. 1998;10:251–254. doi: 10.1097/00042737-199803000-00011. [DOI] [PubMed] [Google Scholar]
- 69.Debongnie J C, Mairesse J, Donnay M, Dekoninck X. Touch cytology. A quick, simple, sensitive screening test in the diagnosis of infections of the gastrointestinal mucosa. Arch Pathol Lab Med. 1994;118:1115–1118. [PubMed] [Google Scholar]
- 70.De Groote D, van Doorn L-J, Ducatelle R, Verschuuren A, Haesebrouck F, Quint W G V, Jalava K, Vandamme P. ‘Candidatus Helicobacter suis’, a gastric helicobacter from pigs, and its phylogenetic relatedness to other gastrospiralla. Int J Syst Bacteriol. 1999;49:1769–1777. doi: 10.1099/00207713-49-4-1769. [DOI] [PubMed] [Google Scholar]
- 71.De Groote D, van Doorn L J, Ducatelle R, Verschuuren A, Tilmant K, Quint W G V, Haesebrouck F, Vandamme P. Phylogenetic characterization of ‘Candidatus Helicobacter bovis’, a new gastric helicobacter in cattle. Int J Syst Bacteriol. 1999;49:1707–1715. doi: 10.1099/00207713-49-4-1707. [DOI] [PubMed] [Google Scholar]
- 72.Dent J C, McNulty C A M, Uff J C, Wilkinson S P, Gear M W L. Spiral organisms in the gastric antrum. Lancet. 1987;ii:96. doi: 10.1016/s0140-6736(87)92754-1. [DOI] [PubMed] [Google Scholar]
- 73.Dewhirst F E, Chien C C, Paster B J, Ericson R L, Orcutt R P, Schauer D B, Fox J G. Phylogeny of the defined murine microbiota: altered Schaedler flora. Appl Environ Microbiol. 1999;65:3287–3292. doi: 10.1128/aem.65.8.3287-3292.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dewhirst, F. E., J. G. Fox, E. N. Mendes, B. J. Paster, C. E. Gates, C. A. Kirkbride, and K. A. Eaton. “Flexispira rappini” strains represent at least ten Helicobacter taxa. Int. J. Syst. Evol. Microbiol., in press. [DOI] [PubMed]
- 75.Dewhirst, F. E., J. G. Fox, and S. L. W. On. Proposal of minimal standards for describing new species of the genus Helicobacter. Int. J. Syst. Evol. Microbiol., in press. [DOI] [PubMed]
- 76.Dewhirst F E, Seymour C, Fraser G J, Paster B J, Fox J G. Phylogeny of Helicobacter isolates from bird and swine feces and description of Helicobacter pametensis sp. nov. Int J Syst Bacteriol. 1994;44:553–560. doi: 10.1099/00207713-44-3-553. [DOI] [PubMed] [Google Scholar]
- 77.Dick E, Lee A, Watson G, O'Rourke J. Use of the mouse for the isolation and investigation of stomach-associated, spiral-helical bacteria from man and other animals. J Med Microbiol. 1989;29:55–62. doi: 10.1099/00222615-29-1-55. [DOI] [PubMed] [Google Scholar]
- 78.Dick-Hegedus E, Lee A. Use of a mouse model to examine anti-Helicobacter pylori agents. Scand J Gastroenterol. 1991;26:909–915. doi: 10.3109/00365529108996241. [DOI] [PubMed] [Google Scholar]
- 79.Dieterich C, Wiesel P, Neiger R, Blum A, Corthésy-Theulaz I. Presence of multiple “Helicobacter heilmannii” strains in an individual suffering from ulcers and in his two cats. J Clin Microbiol. 1998;36:1366–1370. doi: 10.1128/jcm.36.5.1366-1370.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Diwan B A, Ward J M, Ramljak D, Anderson L M. Promotion by Helicobacter hepaticus-induced hepatitis of hepatic tumors initiated by N-nitrosodimethylamine in male A/JCr mice. Toxicol Pathol. 1997;25:597–605. doi: 10.1177/019262339702500610. [DOI] [PubMed] [Google Scholar]
- 81.Doenges J L. Spirochetes in the gastric glands of cacacus rhesus and of man without related disease. Arch Pathol. 1939;25:469–477. [Google Scholar]
- 82.Doig P, de Jonge B L, Alm R A, Brown E D, Uria-Nickelsen M, Noonan B, Mills S D, Tummino P, Carmel G, Guild B C, Moir D T, Vovis G F, Trust T J. Helicobacter pylori physiology predicted from genomic comparisons of two strains. Microbiol Mol Biol Rev. 1999;63:675–707. doi: 10.1128/mmbr.63.3.675-707.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dooley C P, Cohen H, Fitzgibbons P L, Bauer M, Appleman M D, Perez-Perez G I, Blaser M J. Prevalence of Helicobacter pylori infection and histologic gastritis in asymptomatic persons. N Engl J Med. 1989;321:1562–1566. doi: 10.1056/NEJM198912073212302. [DOI] [PubMed] [Google Scholar]
- 84.Drazek E S, Dubois A, Holmes R K. Characterization and presumptive identification of Helicobacter pylori isolates from rhesus monkeys. J Clin Microbiol. 1994;32:1799–1804. doi: 10.1128/jcm.32.7.1799-1804.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Drewitz D J, Shub M D, Ramirez F C. Gastrospirillum hominis gastritis in a child with celiac sprue. Dig Dis Sci. 1997;42:1083–1086. doi: 10.1023/a:1018861708647. [DOI] [PubMed] [Google Scholar]
- 86.Drumm B, Perez-Perez G I, Blaser M J, Sherman P M. Intrafamilial clustering of Helicobacter pylori infection. N Engl J Med. 1990;322:359–363. doi: 10.1056/NEJM199002083220603. [DOI] [PubMed] [Google Scholar]
- 87.Dubois A, Berg D E, Incecik E T, Fiala N, Heman-Ackah L M, Perez-Perez G I, Blaser M J. Transient and persistent experimental infection of nonhuman primates with Helicobacter pylori: implications for human disease. Infect Immun. 1996;64:2885–2891. doi: 10.1128/iai.64.8.2885-2891.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dubois A, Fiala N, Heman-Ackah L M, Drazek E S, Tarnawski A, Fishbein W N, Perez-Perez G I, Blaser M J. Natural gastric infection with Helicobacter pylori in monkeys: a model for spiral bacteria infection in humans. Gastroenterology. 1994;106:1405–1417. doi: 10.1016/0016-5085(94)90392-1. [DOI] [PubMed] [Google Scholar]
- 89.Dubois A, Fiala N, Weichbrod R H, Ward G S, Nix M, Mehlman P, Taub D M, Perez-Perez G I, Blaser M J. Seroepizootiology of Helicobacter pylori gastric infection in nonhuman primates housed in social environments. J Clin Microbiol. 1995;33:1492–1495. doi: 10.1128/jcm.33.6.1492-1495.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dubois A, Lee C K, Fiala N, Kleanthous H, Mehlman P T, Monath T. Immunization against natural Helicobacter pylori infection in nonhuman primates. Infect Immun. 1998;66:4340–4346. doi: 10.1128/iai.66.9.4340-4346.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dubois A, Tarnawski A, Newel D G, Fiala N, Dabros W, Stachura J, Krivan H, Heman-Ackah L M. Gastric injury and invasion of parietal cells by spiral bacteria in Rhesus monkeys. Gastroenterology. 1991;100:884–889. doi: 10.1016/0016-5085(91)90260-r. [DOI] [PubMed] [Google Scholar]
- 92.Dubosq O, Lebailly C. Spirella canis, n.g., n.sp., spirille de l'estomac du chien. C R Acad Sci. 1912;154:835–837. [Google Scholar]
- 93.Dunn B E, Cohen H, Blaser M J. Helicobacter pylori. Clin Microbiol Rev. 1997;10:720–741. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dunn B E, Sung C-C, Taylor N S, Fox J G. Purification and characterization of Helicobacter mustelae urease. Infect Immun. 1991;59:3343–3345. doi: 10.1128/iai.59.9.3343-3345.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dye K R, Marshall B J, Frierson H F, Guerrant R L, McCallum R W. Ultrastructure of another spiral organism associated with human gastritis. Dig Dis Sci. 1989;34:1787–1791. doi: 10.1007/BF01540059. [DOI] [PubMed] [Google Scholar]
- 96.Eaton K, Dewhirst F, Paster B, Tzellas N, Coleman B, Paola J, Sherding A. Prevalence and varieties of Helicobacter species in dogs from random sources and pet dogs: animal and public health implications. J Clin Microbiol. 1996;34:3165–3170. doi: 10.1128/jcm.34.12.3165-3170.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Eaton K, Dewhirst F, Radin M. Helicobacter acinonyx sp. nov., isolated from cheetahs with gastritis. Int J Syst Bacteriol. 1993;43:99–106. doi: 10.1099/00207713-43-1-99. [DOI] [PubMed] [Google Scholar]
- 98.Eaton K A, Brooks C L, Morgan D R, Krakowka S. Essential role of urease in pathogenesis of gastritis induced by Helicobacter pylori in gnotobiotic piglets. Infect Immun. 1991;59:2470–2475. doi: 10.1128/iai.59.7.2470-2475.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Reference deleted.
- 100.Eaton K A, Krakowka S. Effect of gastric pH on urease-dependent colonization of gnotobiotic piglets by Helicobacter pylori. Infect Immun. 1994;62:3604–3607. doi: 10.1128/iai.62.9.3604-3607.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Eaton K A, Krakowka S. The pathogenesis of urease-dependent colonization of gnotobiotic piglets by Helicobacter pylori. Ir J Med Sci. 1992;161(Suppl. 10):8. [Google Scholar]
- 102.Eaton K A, Radin M J, Krakowka S. An animal model of gastric ulcer due to bacterial gastritis in mice. Vet Pathol. 1995;32:489–497. doi: 10.1177/030098589503200506. [DOI] [PubMed] [Google Scholar]
- 103.Eaton K A, Radin M J, Krakowka S. Animal models of bacterial gastritis: the role of host, bacterial species and duration of infection on severity of gastritis. Int J Med Microbiol Virol Parasitol Infect Dis. 1993;280:28–37. doi: 10.1016/s0934-8840(11)80938-2. [DOI] [PubMed] [Google Scholar]
- 104.Eaton K A, Radin M J, Kramer L, Wack R, Sherding R, Krakowka S, Fox J G, Morgan D R. Epizootic gastritis associated with gastric spiral bacilli in cheetahs (Acinonyx jubatus) Vet Pathol. 1993;30:55–63. doi: 10.1177/030098589303000107. [DOI] [PubMed] [Google Scholar]
- 105.Eaton K A, Radin M J, Kramer L, Wack R, Sherding R, Krakowka S, Morgan D R. Gastric spiral bacilli in captive cheetahs. Scand J Gastroenterol. 1991;26(Suppl. 181):38–42. doi: 10.3109/00365529109093206. [DOI] [PubMed] [Google Scholar]
- 106.Eaton K A, Ringler S R, Danon S J. Murine splenocytes induce severe gastritis and delayed-type hypersensitivity and suppress bacterial colonization in Helicobacter pylori-infected SCID mice. Infect Immun. 1999;67:4594–4602. doi: 10.1128/iai.67.9.4594-4602.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Eaton K A, Ringler S S, Krakowka S. Vaccination of gnotobiotic piglets against Helicobacter pylori. J Infect Dis. 1998;178:1399–1405. doi: 10.1086/314463. [DOI] [PubMed] [Google Scholar]
- 108.Enno A, O'Rourke J, Braye S, Howlett R, Lee A. Antigen-dependent, progression of mucosa-associated lymphoid tissue (MALT)-type lymphoma in the stomach. Effects of antimicrobial therapy on gastric MALT lymphoma in mice. Am J Pathol. 1998;152:1625–1632. [PMC free article] [PubMed] [Google Scholar]
- 109.Enno A, O'Rourke J L, Howlett C R, Jack A, Dixon M F, Lee A. MALToma-like lesions in the murine gastric mucosa after long-term infection with Helicobacter felis. A mouse model of Helicobacter pylori-induced gastric lymphoma. Am J Pathol. 1995;147:217–222. [PMC free article] [PubMed] [Google Scholar]
- 110.Erdman S E, Correa P, Coleman L A, Schrenzel M D, Li X, Fox J G. Helicobacter mustelae-associated gastric MALT lymphoma in ferrets. Am J Pathol. 1997;151:273–280. [PMC free article] [PubMed] [Google Scholar]
- 111.Erlandsen S L, Chase D G. Paneth cell function: phagocytosis and intracellular digestion of intestinal microorganisms. II. Spiral microorganism. J Ultrastruct Res. 1972;41:319–333. doi: 10.1016/s0022-5320(72)90072-x. [DOI] [PubMed] [Google Scholar]
- 112.Ermak T H, Ding R, Ekstein B, Hill J, Myers G A, Lee C K, Pappo J, Kleanthous H K, Monath T P. Gastritis in urease-immunized mice after Helicobacter felis challenge may be due to residual bacteria. Gastroenterology. 1997;113:1118–1128. doi: 10.1053/gast.1997.v113.pm9322506. [DOI] [PubMed] [Google Scholar]
- 113.Ermak T H, Giannasca P J, Nichols R, Myers G A, Nedrud J, Weltzin R, Lee C K, Kleanthous H, Monath T P. Immunization of mice with urease vaccine affords protection against Helicobacter pylori infection in the absence of antibodies and is mediated by MHC class II-restricted responses. J Exp Med. 1998;188:2277–2288. doi: 10.1084/jem.188.12.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Evans D G, Lampert H C, Nakano H, Eaton K A, Burnens A P, Bronsdon M A, Evans D J., Jr Genetic evidence for host specificity in the adhesin-encoding genes hxaA of Helicobacter acinonyx, hnaA of H. nemestrinae and hpaA of H. pylori. Gene. 1995;163:97–102. doi: 10.1016/0378-1119(95)00404-t. [DOI] [PubMed] [Google Scholar]
- 115.Fawcett P T, Gibney K M, Vinette K M. Helicobacter pylori can be induced to assume the morphology of Helicobacter heilmannii. J Clin Microbiol. 1999;37:1045–1048. doi: 10.1128/jcm.37.4.1045-1048.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Feinstein R E, Olsson E. Chronic gastroenterocolitis in nine cats. J Vet Diagn Investig. 1992;4:293–298. doi: 10.1177/104063879200400311. [DOI] [PubMed] [Google Scholar]
- 117.Ferrero R L, Labigne A. Cloning, expression and sequencing of Helicobacter felis urease genes. Mol Microbiol. 1993;9:323–333. doi: 10.1111/j.1365-2958.1993.tb01693.x. [DOI] [PubMed] [Google Scholar]
- 118.Ferrero R L, Thiberge J-M, Huerre M, Labigne A. Recombinant antigens prepared from the urease subunits of Helicobacter spp.: evidence of protection in a mouse model of gastric infection. Infect Immun. 1994;62:4981–4989. doi: 10.1128/iai.62.11.4981-4989.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ferrero R L, Thiberge J-M, Kansau I, Wuscher N, Huerre M, Labigne A. The GroES homolog of Helicobacter pylori confers protective immunity against mucosal infection in mice. Proc Natl Acad Sci USA. 1995;92:6499–6503. doi: 10.1073/pnas.92.14.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ferrero R L, Thiberge J M, Labigne A. Local immunoglobulin G antibodies in the stomach may contribute to immunity against Helicobacter infection in mice. Gastroenterology. 1997;113:185–194. doi: 10.1016/s0016-5085(97)70094-5. [DOI] [PubMed] [Google Scholar]
- 121.Figura N, Guglielmetti P, Quaranta S. Spiral shaped bacteria in gastric mucosa. J Clin Pathol. 1990;43:173. doi: 10.1136/jcp.43.2.173-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fischer R, Samisch W. “Gastrospirillum hominis”: another four cases. Lancet. 1990;i:59. doi: 10.1016/0140-6736(90)90195-b. [DOI] [PubMed] [Google Scholar]
- 123.Fleisch F, Burnens A, Weber R, Zbinden R. Helicobacter species strain Mainz isolated from cultures of blood from two patients with AIDS. Clin Infect Dis. 1998;26:526–527. doi: 10.1086/517110. [DOI] [PubMed] [Google Scholar]
- 124.Flejou J-F, Diomande I, Molas G, Goldfain D, Rotenberg A, Florent M, Potet F. Gastrite chronique associee chez l'homme a la presence de germes spirales non-Helicobacter pyori (“Gastrospirillum hominis”) Gastroenterol Clin Biol. 1990;14:806–810. [PubMed] [Google Scholar]
- 125.Flores B M, Fennell C L, Kuller L, Bronsdon M A, Morton W R, Stamm W E. Experimental infection of pig-tailed macaques (Macaca nemestrina) with Campylobacter cinaedi and Campylobacter fennelliae. Infect Immun. 1990;58:3947–3953. doi: 10.1128/iai.58.12.3947-3953.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Foley J E, Marks S L, Munson L, Melli A, Dewhirst F E, Yu S, Shen Z, Fox J G. Isolation of Helicobacter canis from a colony of bengal cats with endemic diarrhea. J Clin Microbiol. 1999;37:3271–3275. doi: 10.1128/jcm.37.10.3271-3275.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Foley J E, Solnick J V, Lapointe J M, Jang S, Pedersen N C. Identification of a novel enteric Helicobacter species in a kitten with severe diarrhea. J Clin Microbiol. 1998;36:908–912. doi: 10.1128/jcm.36.4.908-912.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Foltz C J, Fox J G, Cahill R, Murphy J C, Yan L, Shames B, Schauer D B. Spontaneous inflammatory bowel disease in multiple mutant mouse lines: association with colonization by Helicobacter hepaticus. Helicobacter. 1998;3:69–78. doi: 10.1046/j.1523-5378.1998.08006.x. [DOI] [PubMed] [Google Scholar]
- 129.Foltz C J, Fox J G, Yan L, Shames B. Evaluation of antibiotic therapies for eradication of Helicobacter hepaticus. Antimicrob Agents Chemother. 1995;39:1292–1294. doi: 10.1128/aac.39.6.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Foltz C J, Fox J G, Yan L, Shames B. Evaluation of various oral antimicrobial formulations for eradication of Helicobacter hepaticus. Lab Anim Sci. 1996;46:193–197. [PubMed] [Google Scholar]
- 131.Forester N T, Parton K, Lumsden J S, O'Toole P W O. Isolation of Helicobacter mustelae from ferrets in New Zealand. N Z Vet J. 2000;48:65–69. doi: 10.1080/00480169.2000.36161. [DOI] [PubMed] [Google Scholar]
- 132.Foster J R. Bacterial infection of the common bile duct in chronic fascioliasis in the rat. J Comp Med. 1984;94:175–181. doi: 10.1016/0021-9975(84)90038-0. [DOI] [PubMed] [Google Scholar]
- 133.Fox G E, Wisotzkey J D, Jurtshuk P J. How close is close: 16S rRNA sequence identity may not be sufficient to guarantee species identity. Int J Syst Bacteriol. 1992;42:166–170. doi: 10.1099/00207713-42-1-166. [DOI] [PubMed] [Google Scholar]
- 134.Fox J, Blanco M, Murphy J, Taylor N, Lee A, Kabok Z, Pappo J. Local and systemic immune responses in murine Helicobacter felis active chronic gastritis. Infect Immun. 1993;61:2309–2315. doi: 10.1128/iai.61.6.2309-2315.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fox J G. Anatomy of the ferret. In: Fox J G, editor. Biology and diseases of the ferret. Baltimore, Md: The Williams & Wilkins Co.; 1998. pp. 19–69. [Google Scholar]
- 136.Fox J G. The expanding genus of Helicobacter: pathogenic and zoonotic potential. Semin Gastrointest Dis. 1997;8:124–141. [PubMed] [Google Scholar]
- 137.Fox J G, Batchelder M, Marini R, Yan L, Handt L, Li X, Shames B, Hayward A, Campbell J, Murphy J C. Helicobacter pylori-induced gastritis in the domestic cat. Infect Immun. 1995;63:2674–2681. doi: 10.1128/iai.63.7.2674-2681.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fox J G, Blanco M C, Yan L, Shames B, Polidoro D, Dewhirst F E, Paster B J. Role of gastric pH in isolation of Helicobacter mustelae from the feces of ferrets. Gastroenterology. 1993;104:86–92. doi: 10.1016/0016-5085(93)90839-5. [DOI] [PubMed] [Google Scholar]
- 139.Fox J G, Cabot E B, Taylor N S, Laraway R. Gastric colonization by Campylobacter pylori subsp. mustelae in ferrets. Infect Immun. 1988;56:2994–2996. doi: 10.1128/iai.56.11.2994-2996.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fox J G, Chien C C, Dewhirst F E, Paster B J, Shen Z, Melito P L, Woodward D L, Rodgers F G. Helicobacter canadensis sp. nov. isolated from humans with diarrhea as an example of an emerging pathogen. J Clin Microbiol. 2000;38:2546–2549. doi: 10.1128/jcm.38.7.2546-2549.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fox J G, Chilvers T, Goodwin C S, Taylor N S, Edmonds P, Sly L I, Brenner D. Campylobacter mustelae, a new species resulting from the elevation of Campylobacter pylori subsp. mustelae to species status. Int J Syst Bacteriol. 1989;39:301–303. [Google Scholar]
- 142.Fox J G, Correa P, Taylor N S, Lee A, Otto G, Murphy J C, Rose R. Helicobacter mustelae-associated gastritis in ferrets: an animal model of Helicobacter pylori gastritis in humans. Gastroenterology. 1990;99:352–361. doi: 10.1016/0016-5085(90)91016-y. [DOI] [PubMed] [Google Scholar]
- 143.Fox J G, Dangler C A, Sager W, Borkowski R, Gliatto J M. Helicobacter mustelae-associated gastric adenocarcinoma in ferrets (Mustela putorius furo) Vet Pathol. 1997;34:225–229. doi: 10.1177/030098589703400308. [DOI] [PubMed] [Google Scholar]
- 144.Fox J G, Dangler C A, Whary M T, Edelman W, Kucherlapati R, Wang T C. Mice carrying a truncated Apc gene have diminished gastric epithelial proliferation, gastric inflammation, and humoral immunity in response to Helicobacter felis infection. Cancer Res. 1997;57:3972–3978. [PubMed] [Google Scholar]
- 145.Fox J G, Dewhirst F E, Shen Z, Feng Y, Taylor N S, Paster B J, Ericson R L, Lau C N, Correa P, Araya J C, Roa I. Hepatic Helicobacter species identified in bile and gallbladder tissue from Chileans with chronic cholecystitis. Gastroenterology, 1998;114:755–763. doi: 10.1016/s0016-5085(98)70589-x. [DOI] [PubMed] [Google Scholar]
- 146.Fox J G, Dewhirst F E, Tully J G, Paster B J, Yan L, Taylor N S, Collins M J, Jr, Gorelick P L, Ward J M. Helicobacter hepaticus sp. nov., a microaerophilic bacterium isolated from livers and intestinal mucosal scrapings from mice. J Clin Microbiol. 1994;32:1238–1245. doi: 10.1128/jcm.32.5.1238-1245.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fox J G, Drolet R, Higgins R, Messier S, Yan L, Coleman B E, Paster B J, Dewhirst F E. Helicobacter canis isolated from a dog liver with multifocal necrotizing hepatitis. J Clin Microbiol. 1996;34:2479–2482. doi: 10.1128/jcm.34.10.2479-2482.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fox J G, Edrise B M, Cabot E B, Beaucage C, Murphy J C, Prostak K S. Campylobacter-like organisms isolated from gastric mucosa in ferrets. Am J Vet Res. 1986;47:236–239. [PubMed] [Google Scholar]
- 149.Fox J G, Gorelick P L, Kullberg M C, Ge Z, Dewhirst F E, Ward J M. A novel urease-negative Helicobacter species associated with colitis and typhlitis in IL-10-deficient mice. Infect Immun. 1999;67:1757–1762. doi: 10.1128/iai.67.4.1757-1762.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fox J G, Lee A. Gastric Campylobacter-like organisms: their role in gastric disease of laboratory animals. Lab Anim Sci. 1989;39:543–553. [PubMed] [Google Scholar]
- 151.Fox J G, Lee A. The role of Helicobacter species in newly recognized gastrointestinal tract diseases of animals. Lab Anim Sci. 1997;47:222–255. [PubMed] [Google Scholar]
- 152.Fox J G, Lee A, Otto G, Taylor N S, Murphy J C. Helicobacter felis gastritis in gnotobiotic rats: an animal model of Helicobacter pylori gastritis. Infect Immun. 1991;59:785–791. doi: 10.1128/iai.59.3.785-791.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fox J G, Li X, Cahill R J, Andrutis K, Rustgi A K, Odze R, Wang T C. Hypertrophic gastropathy in Helicobacter felis-infected wild-type C57BL/6 mice and p53 hemizygous transgenic mice. Gastroenterology. 1996;110:155–166. doi: 10.1053/gast.1996.v110.pm8536852. [DOI] [PubMed] [Google Scholar]
- 154.Fox J G, Li X, Yan L, Cahill R J, Hurley R, Lewis R, Murphy J C. Chronic proliferative hepatitis in A/JCr mice associated with persistent Helicobacter hepaticus infection: a model of helicobacter-induced carcinogenesis. Infect Immun. 1996;64:1548–1558. doi: 10.1128/iai.64.5.1548-1558.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fox J G, MacGregor J A, Shen Z, Li X, Lewis R, Dangler C A. Comparison of methods of identifying Helicobacter hepaticus in B6C3F1 mice used in a carcinogenesis bioassay. J Clin Microbiol. 1998;36:1382–1387. doi: 10.1128/jcm.36.5.1382-1387.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fox J G, Otto G, Murphy J C, Taylor N S, Lee A. Gastric colonization of the ferret with Helicobacter species: natural and experimental infections. Rev Infect Dis. 1991;13(Suppl. 8):S671–S680. doi: 10.1093/clinids/13.supplement_8.s671. [DOI] [PubMed] [Google Scholar]
- 157.Fox J G, Otto G, Taylor N S, Rosenblad W, Murphy J C. Helicobacter mustelae-induced gastritis and elevated gastric pH in the ferret (Mustela putorius furo) Infect Immun. 1991;59:1875–1880. doi: 10.1128/iai.59.6.1875-1880.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fox J G, Paster B J, Dewhirst F E, Taylor N S, Yan L-L, Macuch P J, Chmura L M. Helicobacter mustelae isolation from feces of ferrets: evidence to support fecal-oral transmission of gastric Helicobacter. Infect Immun. 1992;60:606–611. doi: 10.1128/iai.60.2.606-611.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fox J G, Wishnok J S, Murphy J C, Tannenbaum S R, Correa P. MNNG-induced gastric carcinoma in ferrets infected with Helicobacter mustelae. Carcinogen. 1993;14:1957–1961. doi: 10.1093/carcin/14.9.1957. [DOI] [PubMed] [Google Scholar]
- 160.Fox J G, Yan L, Shames B, Campbell J, Murphy J C, Li X. Persistent hepatitis and enterocolitis in germfree mice infected with Helicobacter hepaticus. Infect Immun. 1996;64:3673–3681. doi: 10.1128/iai.64.9.3673-3681.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fox J G, Yan L-L, Dewhirst F E, Paster B J, Shames B, Murphy J C, Hayward A, Belcher J C, Mendes E N. Helicobacter bilis sp. nov., a novel Helicobacter species isolated from bile, livers, and intestines of aged, inbred mice. J Clin Microbiol. 1995;33:445–454. doi: 10.1128/jcm.33.2.445-454.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Franklin C L, Beckwith C S, Livingston R S, Riley L K, Gibson S V, Besch-Williford C L, Hook R R., Jr Isolation of a novel Helicobacter species, Helicobacter cholecystus sp. nov., from the gallbladders of Syrian hamsters with cholangiofibrosis and centrilobular pancreatitis. J Clin Microbiol. 1996;34:2952–2958. doi: 10.1128/jcm.34.12.2952-2958.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Franklin C L, Riley L K, Livingston R S, Beckwith C S, Besch-Williford C L, Hook R R., Jr Enterohepatic lesions in SCID mice infected with Helicobacter bilis. Lab Anim Sci. 1998;48:334–339. [PubMed] [Google Scholar]
- 164.Franklin C L, Riley L K, Livingston R S, Beckwith C S, Hook R R, Jr, Besch-Williford C L, Hunziker R, Gorelick P L. Enteric lesions in SCID mice infected with “Helicobacter typhlonicus,” a novel urease-negative Helicobacter species. Lab Anim Sci. 1999;49:496–505. [PubMed] [Google Scholar]
- 165.Freedberg A S, Barron L E. The presence of spirochaetes in human gastric mucosa. Am J Dig Dis. 1940;38:443–445. [Google Scholar]
- 166.Fujimura S, Kogure K, Oboshi S, Sugimura T. Production of tumors in glandular stomach of hamsters by N-methyl-N′-nitro-N-nitrosoguanidine. Cancer Res. 1970;30:1444–1448. [PubMed] [Google Scholar]
- 167.Gebhart C J, Fennell C L, Murtaugh M P, Stamm W E. Campylobacter cinaedi is normal intestinal flora in hamsters. J Clin Microbiol. 1989;27:1692–1694. doi: 10.1128/jcm.27.7.1692-1694.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Germani Y, Dauga C, Duval P, Huerre M, Levy M, Pialoux G, Sansonetti P, Grimont P A. Strategy for the detection of Helicobacter species by amplification of 16S rRNA genes and identification of H. felis in a human gastric biopsy. Res Microbiol. 1997;148:315–326. doi: 10.1016/S0923-2508(97)81587-2. [DOI] [PubMed] [Google Scholar]
- 169.Geyer C, Colbatzky F, Lechner J, Hermanns W. Occurrence of spiral-shaped bacteria in gastric biopsies of dogs and cats. Vet Rec. 1993;133:18–19. doi: 10.1136/vr.133.1.18. [DOI] [PubMed] [Google Scholar]
- 170.Ghiara P, Rossi M, Marchetti M, Di Tommaso A, Vindigni C, Ciampolini F, Covacci A, Telford J L, De Magistris M T, Pizza M, Rappuoli R, Del Giudice G. Therapeutic intragastric vaccination against Helicobacter pylori in mice eradicates an otherwise chronic infection and confers protection against reinfection. Infect Immun. 1997;65:4996–5002. doi: 10.1128/iai.65.12.4996-5002.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Giannella R A, Broitman S A, Zamcheck N. Gastric acid barrier to ingested microorganisms in man: studies in vivo and in vitro. Gut. 1972;13:251–256. doi: 10.1136/gut.13.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gilbert J V, Ramakrishna J, Sunderman F W, Jr, Wright A, Plaut A G. Protein Hpn: cloning and characterization of a histidine-rich metal-binding polypeptide in Helicobacter pylori and Helicobacter mustelae. Infect Immun. 1995;63:2682–2688. doi: 10.1128/iai.63.7.2682-2688.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Giusti A M, Crippa L, Bellini O, Luini M, Scanziani E. Gastric spiral bacteria in wild rats from Italy. J Wildl Dis. 1998;34:168–172. doi: 10.7589/0090-3558-34.1.168. [DOI] [PubMed] [Google Scholar]
- 174.Go M F, Kapur V, Graham D Y, Musser J M. Population genetic analysis of Helicobacter pylori by multilocus enzyme electrophoresis: extensive allelic diversity and recombinational population structure. J Bacteriol. 1996;178:3934–3938. doi: 10.1128/jb.178.13.3934-3938.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Goggin P M, Marrero J M, Spychal R T, Jackson P A, Corbishley C M, Northfield T C. Surface hydrophobicity of gastric mucosa in Helicobacter pylori infection: effect of clearance and eradication. Gastroenterology. 1992;103:1486–1490. doi: 10.1016/0016-5085(92)91168-4. [DOI] [PubMed] [Google Scholar]
- 176.Gold B D, Dytoc M, Huesca M, Philpott D, Kuksis A, Czinn S, Lingwood C A, Sherman P M. Comparison of Helicobacter mustelae and Helicobacter pylori adhesion to eukaryotic cells in vitro. Gastroenterology. 1995;109:692–700. doi: 10.1016/0016-5085(95)90375-5. [DOI] [PubMed] [Google Scholar]
- 177.Gold B D, Islur P, Policova Z, Czinn S, Neumann A W, Sherman P M. Surface properties of Helicobacter mustelae and ferret gastrointestinal mucosa. Clin Investig Med. 1996;19:92–100. [PubMed] [Google Scholar]
- 178.Gold B D, Sherman P M, Lingwood C A. Helicobacter mustelae and Helicobacter pylori bind to common lipid receptors in vitro. Infect Immun. 1993;61:2632–2638. doi: 10.1128/iai.61.6.2632-2638.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gómez-Duarte O G, Lucas B, Yan Z X, Panthel K, Haas R, Meyer T F. Protection of mice against gastric colonization by Helicobacter pylori by single oral dose immunization with attenuated Salmonella typhimurium producing urease subunits A and B. Vaccine. 1998;16:460–471. doi: 10.1016/s0264-410x(97)00247-8. [DOI] [PubMed] [Google Scholar]
- 180.Goodwin C S, Armstrong J A, Chilvers T, Peters M, Colins M D, Sly L, McConnel W, Harper W E S. Transfer of Campylobacter pylori and Campylobacter mustelae to Helicobacter gen. nov. as Helicobacter pylori comb. nov. and Helicobacter mustelae comb. nov., respectively. Int J Syst Bacteriol. 1989;39:397–405. [Google Scholar]
- 181.Gootz T D, Perez-Perez G I, Clancy J, Martin B A, Tait-Kamradt A, Blaser M J. Immunological and molecular characterization of Helicobacter felis urease. Infect Immun. 1994;62:793–798. doi: 10.1128/iai.62.3.793-798.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Goto K, Ohashi H, Ebukuro S, Itoh K, Tohma Y, Takakura A, Wakana S, Ito M, Itoh T. Isolation and characterization of Helicobacter species from the stomach of the house musk shrew (Suncus murinus) with chronic gastritis. Curr Microbiol. 1998;37:44–51. doi: 10.1007/s002849900335. [DOI] [PubMed] [Google Scholar]
- 183.Gottfried M R, Washington K, Harrell L J. Helicobacter pylori-like microorganisms and chronic active gastritis in ferrets. Am J Gastroenterol. 1990;85:813–818. [PubMed] [Google Scholar]
- 184.Graham D Y, Lew G M, Klein P D, Evans D G, Evans D J, Jr, Saeed Z A, Malaty H M. Effect of treatment of Helicobacter pylori infection on the long-term recurrence of gastric or duodenal ulcer. Ann Intern Med. 1992;116:705–708. doi: 10.7326/0003-4819-116-9-705. [DOI] [PubMed] [Google Scholar]
- 185.Grasso G M, Ripabelli G, Sammarco M L, Ruberto A, Iannitto G. Prevalence of Helicobacter-like organisms in porcine gastric mucosa: a study of swine slaughtered in Italy. Comp Immunol Microbiol Infect Dis. 1996;19:213–217. doi: 10.1016/0147-9571(96)00007-0. [DOI] [PubMed] [Google Scholar]
- 186.Guard C. Abomasal ulcers. In: Smith B P, editor. Large animal internal medicine: diseases of horses, cattle, sheep, and goats. 2nd ed. St. Louis, Mo: C. V. Mosby Co.; 1996. pp. 874–877. [Google Scholar]
- 187.Günther H, Schulze F. Histologische Untersuchungen zum Vorkommen von Campylobacter-ähnlich Geformten keimen im Labmagen von Kälbern. Zentbl Veterinaermed Ser B. 1992;39:737–745. [PubMed] [Google Scholar]
- 188.Guy B, Hessler C, Fourage S, Haensler J, Vialon-Lafay E, Rokbi B, Millet M J. Systemic immunization with urease protects mice against Helicobacter pylori infection. Vaccine. 1998;16:850–856. doi: 10.1016/s0264-410x(97)00258-2. [DOI] [PubMed] [Google Scholar]
- 189.Hailey J R, Haseman J K, Bucher J R, Radovsky A E, Malarkey D E, Miller R T, Nyska A, Maronpot R R. Impact of Helicobacter hepaticus infection in B6C3F1 mice from twelve national toxicology program two-year carcinogenesis studies. Toxicol Pathol. 1998;26:602–611. doi: 10.1177/019262339802600503. [DOI] [PubMed] [Google Scholar]
- 190.Haines D C, Gorelick P L, Battles J K, Pike K M, Anderson R J, Fox J G, Taylor N S, Shen Z, Dewhirst F E, Anver M R, Ward J M. Inflammatory large bowel disease in immunodeficient rats naturally and experimentally infected with Helicobacter bilis. Vet Pathol. 1998;35:202–208. doi: 10.1177/030098589803500305. [DOI] [PubMed] [Google Scholar]
- 191.Hammond J, Jr, Chesterman F C UFAW, editors. The Universities Federation for Animal Welfare handbook on the care and management of laboratory animals. London, United Kingdom: Churchill Livingstone; 1972. The ferret; pp. 354–363. [Google Scholar]
- 192.Hampton J C. The effects of nitrogen mustard on the intestinal epithelium of the mouse. Radiat Res. 1967;30:576–589. [PubMed] [Google Scholar]
- 193.Handt L K, Fox J G, Dewhirst F E, Fraser G J, Paster B J, Yan L L, Rozmiarek H, Rufo R, Stalis I H. Helicobacter pylori isolated from the domestic cat: public health implications. Infect Immun. 1994;62:2367–2374. doi: 10.1128/iai.62.6.2367-2374.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Handt L K, Fox J G, Stalis I H, Rosemarie R, Lee G, Linn J, Li X, Kleanthous H. Characterization of feline Helicobacter pylori strains and associated gastritis in a colony of domestic cats. J Clin Microbiol. 1995;33:2280–2289. doi: 10.1128/jcm.33.9.2280-2289.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hänninen M-L, Happonen I, Saari S, Jalava K. Culture and characteristics of Helicobacter bizzozeronii, a new canine gastric Helicobacter sp. Int J Syst Bacteriol. 1996;46:160–166. doi: 10.1099/00207713-46-1-160. [DOI] [PubMed] [Google Scholar]
- 196.Hänninen M L, Hirvi U. Genetic diversity of canine gastric helicobacters, Helicobacter bizzozeronii and H. salomonis studied by pulsed-field gel electrophoresis. J Med Microbiol. 1999;48:341–347. doi: 10.1099/00222615-48-4-341. [DOI] [PubMed] [Google Scholar]
- 197.Happonen I, Saari S, Castren L, Tyni O, Hänninen M L, Westermarck E. Occurrence and topographical mapping of gastric Helicobacter-like organisms and their association with histological changes in apparently healthy dogs and cats. Zentbl Veterinaermed Ser A. 1996;43:305–315. doi: 10.1111/j.1439-0442.1996.tb00457.x. [DOI] [PubMed] [Google Scholar]
- 198.Haque M, Hirai Y, Yokota K, Mori N, Jahan I, Ito H, Hotta H, Yano I, Kanemasa Y, Oguma K. Lipid profile of Helicobacter spp.: presence of cholesteryl glucoside as a characteristic feature. J Bacteriol. 1996;178:2065–2070. doi: 10.1128/jb.178.7.2065-2070.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Haringsma P C, Mouwen J M. Mogelijke betekenis van spirilvormige bacteriën bij het onstaan van lebmaagzweren bih het volwassen rund. Tijdschr Diergeneeskd. 1992;117:485–487. [PubMed] [Google Scholar]
- 200.Haziroglu R, Diker K S, Guvenc T, Kul O. Canine gastritis associated with Helicobacter felis. Dtsch Tieraerztl Wochenschr. 1995;102:474–476. [PubMed] [Google Scholar]
- 201.Heilmann K L, Borchard F. Gastritis due to spiral shaped bacteria other than Helicobacter pylori: clinical, histological, and ultrastructural findings. Gut. 1991;32:137–140. doi: 10.1136/gut.32.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Henry G A, Long P H, Burns J L, Charbonneau D L. Gastric spirillosis in beagles. Am J Vet Res. 1987;48:831–836. [PubMed] [Google Scholar]
- 203.Hermanns W, Kregel K, Breuer W, Lechner J. Helicobacter-like organisms: histological examination of gastric biopsies from dogs and cats. J Comp Pathol. 1995;112:307–318. doi: 10.1016/s0021-9975(05)80083-0. [DOI] [PubMed] [Google Scholar]
- 204.Hilzenrat N, Lamoureux E, Weintrub I, Alpert E, Lichter M, Alpert L. Helicobacter heilmannii-like spiral bacteria in gastric mucosal biopsies. Prevalence and clinical significance. Arch Pathol Lab Med. 1995;119:1149–1153. [PubMed] [Google Scholar]
- 205.Holck S, Ingeholm P, Blom J, Nørgaard A, Elsborg L, Adamsen S, Andersen L P. The histopathology of human gastric mucosa inhabited by Helicobacter heilnannii-like (Gastrospirillum hominis) organisms, including the first culturable case. Apmis. 1997;105:746–756. doi: 10.1111/j.1699-0463.1997.tb05080.x. [DOI] [PubMed] [Google Scholar]
- 206.Höök-Nikanne J, Aho P, Kärkkäinen P, Kosunen T U, Salaspuro M. The Helicobacter felis mouse model in assessing anti-Helicobacter therapies and gastric mucosal prostaglandin E2 levels. Scand J Gastroenterol. 1996;31:334–338. doi: 10.3109/00365529609006406. [DOI] [PubMed] [Google Scholar]
- 207.Hsueh P R, Teng L J, Hung C C, Chen Y C, Yang P C, Ho S W, Luh K T. Septic shock due to Helicobacter fennelliae in a non-human immunodeficiency virus-infected heterosexual patient. J Clin Microbiol. 1999;37:2084–2086. doi: 10.1128/jcm.37.6.2084-2086.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Huang J Q, Sridhar S, Chen Y, Hunt R H. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology. 1998;114:1169–1179. doi: 10.1016/s0016-5085(98)70422-6. [DOI] [PubMed] [Google Scholar]
- 209.Hulten K, Han S W, Enroth H, Klein P D, Opekun A R, Gilman R H, Evans D G, Engstrand L, Graham D Y, El-Zaatari F A. Helicobacter pylori in the drinking water in Peru. Gastroenterology. 1996;110:1031–1035. doi: 10.1053/gast.1996.v110.pm8612990. [DOI] [PubMed] [Google Scholar]
- 210.Husmann M, Gries C, Jehnichen P, Woelfel T, Gerken G, Ludwig W, Bhakdi S. Helicobacter sp. strain Mainz isolated from an AIDS patient with septic arthritis: case report and nonradioactive analysis of 16S rRNA sequence. J Clin Microbiol. 1994;32:3037–3039. doi: 10.1128/jcm.32.12.3037-3039.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hussell T, Isaacson P G, Crabtree J E, Spencer J. Helicobacter pylori-specific tumour-infiltrating T cells provide contact dependent help for the growth of malignant B cells in low-grade gastric lymphoma of mucosa-associated lymphoid tissue. J Pathol. 1996;178:122–127. doi: 10.1002/(SICI)1096-9896(199602)178:2<122::AID-PATH486>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 212.Hutto D L, Wannemuehler M J. A comparison of the morphologic effects of Serpulina hyodysenteriae or its beta-hemolysin on the murine cecal mucosa. Vet Pathol. 1999;36:37–47. doi: 10.1354/vp.36-5-412. [DOI] [PubMed] [Google Scholar]
- 213.Ierardi E, Monno R, Mongelli A, Allegretta L, Milone E, Rizzi S, Panza P, Coppolecchia P, Francavilla A. Gastrospirillum hominis associated chronic active gastritis: the first report from Italy. Ital J Gastroenterol. 1991;23:86–87. [PubMed] [Google Scholar]
- 214.Ihrig M, Schrenzel M D, Fox J G. Differential susceptibility to hepatic inflammation and proliferation in AXB recombinant inbred mice chronically infected with Helicobacter hepaticus. Am J Pathol. 1999;155:571–582. doi: 10.1016/S0002-9440(10)65152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.International Committee on Systematic Bacteriology, Subcommittee on the Taxonomy of Campylobacter and Related Bacteria. Minutes of the meetings, 18 and 19 September 1997, Cape Town, South Africa. Int J Syst Bacteriol. 1998;48:619–620. [Google Scholar]
- 216.Ivy A C, Grossman M I, Bachrach W H. Peptic ulcer. Philadelphia, Pa: The Blakiston Company; 1950. pp. 266–272. [Google Scholar]
- 217.Jakob W, Stolte M, Valentin A, Schröder H D. Demonstration of Helicobacter pylori-like organisms in the gastric mucosa of captive exotic carnivores. J Comp Pathol. 1997;116:21–33. doi: 10.1016/s0021-9975(97)80040-0. [DOI] [PubMed] [Google Scholar]
- 218.Jalava K, Hielm S, Hirvi U, Hänninen M L. Evaluation of a molecular identification scheme based on 23S rRNA gene polymorphisms for differentiating canine and feline gastric Helicobacter spp. Lett Appl Microbiol. 1999;28:269–274. doi: 10.1046/j.1365-2672.1999.00527.x. [DOI] [PubMed] [Google Scholar]
- 219.Jalava K, Kaartinen M, Utriainen M, Happonen I, Hänninen M-L. Helicobacter salomonis sp. nov., a canine gastric Helicobacter sp. related to Helicobacter felis and Helicobacter bizzozeronii. Int J Syst Bacteriol. 1997;47:975–982. doi: 10.1099/00207713-47-4-975. [DOI] [PubMed] [Google Scholar]
- 220.Jalava K, On S L W, Vandamme P A, Happonen I, Sukura A, Hänninen M L. Isolation and identification of Helicobacter spp. from canine and feline gastric mucosa. Appl Environ Microbiol. 1998;64:3998–4006. doi: 10.1128/aem.64.10.3998-4006.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Jeffries L, Buckley D E, Blower P R, Plumb J N. Comparative sensitivities to antimicrobial agents of Campylobacter pylori and the gastric campylobacter like organism from the ferret. J Clin Pathol. 1987;40:1265–1267. doi: 10.1136/jcp.40.10.1265-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Jiang B, Jordana M, Xing Z, Smaill F, Snider D P, Borojevic R, Steele-Norwood D, Hunt R H, Croitoru K. Replication-defective adenovirus infection reduces Helicobacter felis colonization in the mouse in a gamma interferon- and interleukin-12-dependent manner. Infect Immun. 1999;67:4539–4544. doi: 10.1128/iai.67.9.4539-4544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Jones A C, Logan R P, Foynes S, Cockayne A, Wren B W, Penn C W. A flagellar sheath protein of Helicobacter pylori is identical to HpaA, a putative N-acetylneuraminyllactose-binding hemagglutinin, but is not an adhesin for AGS cells. J Bacteriol. 1997;179:5643–5647. doi: 10.1128/jb.179.17.5643-5647.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Josenhans C, Ferrero R L, Labigne A, Suerbaum S. Cloning and allelic exchange mutagenesis of two flagellin genes of Helicobacter felis. Mol Microbiol. 1999;33:350–362. doi: 10.1046/j.1365-2958.1999.01478.x. [DOI] [PubMed] [Google Scholar]
- 225.Josenhans C, Labigne A, Suerbaum S. Comparative ultrastructural and functional studies of Helicobacter pylori and Helicobacter mustelae flagellin mutants: both flagellin subunits, FlaA and FlaB, are necessary for full motility in Helicobacter species. J Bacteriol. 1995;177:3010–3020. doi: 10.1128/jb.177.11.3010-3020.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kiehlbauch J A, Brenner D J, Cameron D N, Steigerwalt A G, Makowski J M, Baker C N, Patton C M, Wachsmuth I K. Genotypic and phenotypic characterization of Helicobacter cinaedi and Helicobacter fennelliae strains isolated from humans and animals. J Clin Microbiol. 1995;33:2940–2947. doi: 10.1128/jcm.33.11.2940-2947.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kinsel M J, Briggs M B, Venzke K, Forge O, Murnane R D. Gastric spiral bacteria and intramuscular sarcocysts in African lions from Namibia. J Wildl Dis. 1998;34:317–324. doi: 10.7589/0090-3558-34.2.317. [DOI] [PubMed] [Google Scholar]
- 228.Kinsel M J, Kovarik P, Murnane R D. Gastric spiral bacteria in small felids. J Zoo Wildl Med. 1998;29:214–220. [PubMed] [Google Scholar]
- 229.Kirkbride C A, Gates C E, Collins J E. Abortion in sheep caused by a nonclassified, anaerobic, flagellated bacterium. Am J Vet Res. 1986;47:259–262. [PubMed] [Google Scholar]
- 230.Kirkbride C A, Gates C E, Collins J E, Ritchie A E. Ovine abortion associated with an anaerobic bacterium. J Am Vet Med Assoc. 1985;186:789–791. [PubMed] [Google Scholar]
- 231.Kleanthous H, Myers G A, Georgakopoulos K M, Tibbitts T J, Ingrassia J W, Gray H L, Ding R, Zhang Z Z, Lei W, Nichols R, Lee C K, Ermak T H, Monath T P. Rectal and intranasal immunizations with recombinant urease induce distinct local and serum immune responses in mice and protect against Helicobacter pylori infection. Infect Immun. 1998;66:2879–2886. doi: 10.1128/iai.66.6.2879-2886.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Klein P D, Graham D Y, Gaillour A, Opekun A R, Smith E O B. Water source as risk factor for Helicobacter pylori infection in Peruvian children. Lancet. 1991;i:1503–1506. doi: 10.1016/0140-6736(91)93196-g. [DOI] [PubMed] [Google Scholar]
- 233.Kong L, Smith J G, Bramhill D, Abruzzo G K, Bonfiglio C, Cioffe C, Flattery A M, Gill C J, Lynch L, Scott P M, Silver L, Thompson C, Kropp H, Bartizal K. A sensitive and specific PCR method to detect Helicobacter felis in a conventional mouse model. Clin Diagn Lab Immunol. 1996;3:73–78. doi: 10.1128/cdli.3.1.73-78.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Krakowka S, Eaton K A, Rings D M, Argenzio R A. Production of gastroesophageal erosions and ulcers (GEU) in gnotobiotic swine monoinfected with fermentative commensal bacteria and fed high-carbohydrate diet. Vet Pathol. 1998;35:274–282. doi: 10.1177/030098589803500406. [DOI] [PubMed] [Google Scholar]
- 235.Krienitz W. Auftreten von spirochäten verschiedener Form im Mageninhalt bei Carcinoma ventriculi. Dtsch Med Wochenschr. 1906;28:872–889. [Google Scholar]
- 236.Kullberg M C, Ward J M, Gorelick P L, Caspar P, Hieny S, Cheever A, Jankovic D, Sher A. Helicobacter hepaticus triggers colitis in specific-pathogen-free interleukin-10 (IL-10)-deficient-mice through an IL-12- and gamma interferon-dependent mechanism. Infect Immun. 1998;66:5157–5166. doi: 10.1128/iai.66.11.5157-5166.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Lavelle J P, Landas S, Mitros F A, Conklin J L. Acute gastritis associated with spiral organisms from cats. Dig Dis Sci. 1994;39:744–750. doi: 10.1007/BF02087417. [DOI] [PubMed] [Google Scholar]
- 238.Leach W D, Lee A, Stubbs R P. Localization of bacteria in the gastrointestinal tract: a possible explanation of intestinal spirochaetosis. Infect Immun. 1973;7:961–972. doi: 10.1128/iai.7.6.961-972.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Lee A. The use of a mouse model in the study of Helicobacter sp.- associated gastric cancer. Eur J Gastroenterol Hepatol. 1994;6(Suppl. 1):S67–S71. [PubMed] [Google Scholar]
- 240.Lee A, Chen M. Successful immunization against gastric infection with Helicobacter species: use of a cholera toxin B-subunit-whole-cell vaccine. Infect Immun. 1994;62:3594–3597. doi: 10.1128/iai.62.8.3594-3597.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lee A, Chen M, Coltro N, O'Rourke J, Hazell S, Hu P, Li Y. Long term infection of the gastric mucosa with Helicobacter species does induce atrophic gastritis in an animal model of Helicobacter pylori infection. Zentbl Bakteriol. 1993;280:38–50. doi: 10.1016/s0934-8840(11)80939-4. [DOI] [PubMed] [Google Scholar]
- 242.Lee A, Dent J, Hazell S, McNulty C. Origin of spiral organisms in human gastric antrum. Lancet. 1988;i:300–301. doi: 10.1016/s0140-6736(88)90382-0. [DOI] [PubMed] [Google Scholar]
- 243.Lee A, Eckstein R P, Fevre D I, Dick E, Kellow J E. Non Campylobacter pylori spiral organisms in the gastric antrum. Aust N Z J Med. 1989;19:156–158. doi: 10.1111/j.1445-5994.1989.tb00232.x. [DOI] [PubMed] [Google Scholar]
- 244.Lee A, Fox J G, Otto G, Murphy J. A small animal model of human Helicobacter pylori active chronic gastritis. Gastroenterology. 1990;99:1315–1323. doi: 10.1016/0016-5085(90)91156-z. [DOI] [PubMed] [Google Scholar]
- 245.Lee A, Hazell S L. Campylobacter pylori in health and disease: an ecological perspective. Microb Ecol Health Dis. 1988;1:1–16. [Google Scholar]
- 246.Lee A, Hazell S L, O'Rourke J, Kouprach S. Isolation of a spiral-shaped bacterium from the cat stomach. Infect Immun. 1988;56:2843–2850. doi: 10.1128/iai.56.11.2843-2850.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Lee A, Krakowka S, Fox J G, Otto G, Eaton K A, Murphy J C. Role of Helicobacter felis in chronic canine gastritis. Vet Pathol. 1992;29:487–494. doi: 10.1177/030098589202900601. [DOI] [PubMed] [Google Scholar]
- 248.Lee A, Phillips M W, O'Rourke J L, Paster B J, Dewhirst F E, Fraser G J, Fox J G, Sly L I, Romaniuk P J, Trust T J, et al. Helicobacter muridarum sp. nov., a microaerophilic helical bacterium with a novel ultrastructure isolated from the intestinal mucosa of rodents. Int J Syst Bacteriol. 1992;42:27–36. doi: 10.1099/00207713-42-1-27. [DOI] [PubMed] [Google Scholar]
- 248a.Lee A, O'Rourke J L. Ultrastructure of Helicobacter organisms and possible relevance for pathogenesis. In: Goodwin C S, Worsley B W, editors. Helicobacter pylori: biology and clinical practice. Boca Raton, Fla: CRC Press, Inc.; 1993. p. 30. [Google Scholar]
- 249.Lee C K, Soike K, Hill J, Georgakopoulos K, Tibbitts T, Ingrassia J, Gray H, Boden J, Kleanthous H, Giannasca P, Ermak T, Weltzin R, Blanchard J, Monath T P. Immunization with recombinant Helicobacter pylori urease decreases colonization levels following experimental infection of rhesus monkeys. Vaccine. 1999;17:1493–1505. doi: 10.1016/s0264-410x(98)00365-x. [DOI] [PubMed] [Google Scholar]
- 250.Lee C K, Weltzin R, Thomas W D, Jr, Kleanthous H, Ermak T H, Soman G, Hill J E, Ackerman S K, Monath T P. Oral immunization with recombinant Helicobacter pylori urease induces secretory IgA antibodies and protects mice from challenge with Helicobacter felis. J Infect Dis. 1995;172:161–172. doi: 10.1093/infdis/172.1.161. [DOI] [PubMed] [Google Scholar]
- 251.Lee S G, Calhoun D H. Urease from a potentially pathogenic coccoid isolate: purification, characterization, and comparison to other microbial ureases. Infect Immun. 1997;65:3991–3996. doi: 10.1128/iai.65.10.3991-3996.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Lee S G, Kim C, Ha Y C. Successful cultivation of a potentially pathogenic coccoid organism with trophism for gastric mucin. Infect Immun. 1997;65:49–54. doi: 10.1128/iai.65.1.49-54.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Li X, Fox J G, Whary M T, Yan L, Shames B, Zhao Z. SCID/NCr mice naturally infected with Helicobacter hepaticus develop progressive hepatitis, proliferative typhlitis, and colitis. Infect Immun. 1998;66:5477–5484. doi: 10.1128/iai.66.11.5477-5484.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Lichtenberger L M, Dial E J, Ottlecz A, Romero J J, Lechago J, Fox J G. Attenuation of hydrophobic phospholipid barrier is an early event in Helicobacter felis-induced gastritis in mice. Dig Dis Sci. 1999;44:108–115. doi: 10.1023/a:1026610418663. [DOI] [PubMed] [Google Scholar]
- 255.Lim R K S. A parasitic spiral organism in the stomach of the cat. Parasitology. 1920;12:108–113. [Google Scholar]
- 256.Livingston R S, Riley L K, Steffen E K, Besch-Williford C L, Hook R R, Jr, Franklin C L. Serodiagnosis of Helicobacter hepaticus infection in mice by an enzyme-linked immunosorbent assay. J Clin Microbiol. 1997;35:1236–1238. doi: 10.1128/jcm.35.5.1236-1238.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Lockard V G, Boler R K. Ultrastructure of spiraled microorganism in the gastric mucosa of dogs. Am J Vet Res. 1970;31:1453–1462. [PubMed] [Google Scholar]
- 258.Logan R P, Robins A, Turner G A, Cockayne A, Borriello S P, Hawkey C J. A novel flow cytometric assay for quantitating adherence of Helicobacter pylori to gastric epithelial cells. J Immunol Methods. 1998;213:19–30. doi: 10.1016/s0022-1759(98)00018-0. [DOI] [PubMed] [Google Scholar]
- 259.Mammen M P, Jr, Aronson N E, Edenfield W J, Endy T P. Recurrent Helicobacter cinaedi bacteremia in a patient infected with human immunodeficiency virus: case report. Clin Infect Dis. 1995;21:1055. doi: 10.1093/clinids/21.4.1055. [DOI] [PubMed] [Google Scholar]
- 260.Marais A, Mendz G L, Hazell S L, Mégraud F. Metabolism and genetics of Helicobacter pylori: the genome era. Microbiol Mol Biol Rev. 1999;63:642–674. doi: 10.1128/mmbr.63.3.642-674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Marchetti M, Arico B, Burroni D, Figura N, Rappuoli R, Ghiara P. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science. 1995;267:1655–1658. doi: 10.1126/science.7886456. [DOI] [PubMed] [Google Scholar]
- 262.Marchetti M, Rossi M, Giannelli V, Giuliani M M, Pizza M, Censini S, Covacci A, Massari P, Pagliaccia C, Manetti R, Telford J L, Douce G, Dougan G, Rappuoli R, Ghiara P. Protection against Helicobacter pylori infection in mice by intragastric vaccination with H. pylori antigens is achieved using a non-toxic mutant of E. coli heat-labile enterotoxin (LT) as adjuvant. Vaccine. 1998;16:33–37. doi: 10.1016/s0264-410x(97)00153-9. [DOI] [PubMed] [Google Scholar]
- 263.Marini R P, Fox J G, Taylor N S, Yan L, McColm A A, Williamson R. Ranitidine bismuth citrate and clarithromycin, alone or in combination, for eradication of Helicobacter mustelae infection in ferrets. Am J Vet Res. 1999;60:1280–1286. [PubMed] [Google Scholar]
- 264.Marshall B J. History of the discovery of C. pylori. In: Blaser M J, editor. Campylobacter pylori in gastritis and peptic ulcer disease. New York, N.Y: Igaku-Shoin; 1989. pp. 7–23. [Google Scholar]
- 265.Marshall B J, Warren J R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;i:1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 266.Mattapallil J J, Dandekar S, Canfield D R, Solnick J V. A predominant Th-1 type of immune response is induced early during acute Helicobacter pylori infection in the rhesus monkey. Gastroenterology. 2000;118:307–315. doi: 10.1016/s0016-5085(00)70213-7. [DOI] [PubMed] [Google Scholar]
- 267.Reference deleted.
- 268.Mazzucchelli L, Wilder-Smith C H, Ruchti C, Meyer-Wyss B, Merki H S. Gastrospirillum hominis in asymptomatic, healthy individuals. Dig Dis Sci. 1993;38:2087–2089. doi: 10.1007/BF01297089. [DOI] [PubMed] [Google Scholar]
- 269.McClure H M, Chapman W L J, Hooper B E, Smith F G, Fletcher O J. The digestive system. In: Jones T C, Anderson M P, Benirschke K, Garner F M, editors. Pathology of laboratory animals. New York, N.Y: Springer-Verlag; 1978. pp. 195–230. [Google Scholar]
- 270.McColm A A, Bagshaw J A, O'Malley C F O. Development of a 14C-urea breath test in ferrets colonised with Helicobacter mustelae: effects of treatment with bismuth, antibiotics, and urease inhibitors. Gut. 1993;34:181–186. doi: 10.1136/gut.34.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.McNulty C A, Dent J C, Curry A, Uff J S, Ford G A, Gear M W, Wilkinson S P. New spiral bacterium in gastric mucosa. J Clin Pathol. 1989;42:585–591. doi: 10.1136/jcp.42.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Meining A, Kroher G, Stolte M. Animal reservoirs in the transmission of Helicobacter heilmannii. Results of a questionnaire-based study. Scand J Gastroenterol. 1998;33:795–798. doi: 10.1080/00365529850171422. [DOI] [PubMed] [Google Scholar]
- 273.Melchers K, Wiegert T, Buhmann A, Postius S, Schäfer K P, Schumann W. The Helicobacter felis ftsH gene encoding an ATP-dependent metalloprotease can replace the Escherichia coli homologue for growth and phage lambda lysogenization. Arch Microbiol. 1998;169:393–396. doi: 10.1007/s002030050588. [DOI] [PubMed] [Google Scholar]
- 274.Mendes E N, Queiroz D M, Coimbra R S, Moura S B, Barbosa A J, Rocha G A. Experimental infection of Wistar rats with “Gastrospirillum suis”. J Med Microbiol. 1996;44:105–109. doi: 10.1099/00222615-44-2-105. [DOI] [PubMed] [Google Scholar]
- 275.Mendes E N, Queiroz D M, Dewhirst F E, Paster B J, Moura S B, Fox J G. Helicobacter trogontum sp. nov., isolated from the rat intestine. Int J Syst Bacteriol. 1996;46:916–921. doi: 10.1099/00207713-46-4-916. [DOI] [PubMed] [Google Scholar]
- 276.Mendes E N, Queiroz D M, Moura S B, Rocha G A. Mouse inoculation for the detection of non-cultivable gastric tightly spiralled bacteria. Braz J Med Biol Res. 1998;31:373–376. doi: 10.1590/s0100-879x1998000300007. [DOI] [PubMed] [Google Scholar]
- 277.Mendes E N, Queiroz D M M, Rocha G A, Moura S B, Leite V H R, Fonseca M E F. Ultrastructure of a spiral micro-organism from pig gastric mucosa (“Gastrospirillum suis”) J Med Microbiol. 1990;33:61–66. doi: 10.1099/00222615-33-1-61. [DOI] [PubMed] [Google Scholar]
- 278.Mendes E N, Queiroz D M M, Rocha G A, Nogueira A M M F, Carbalho A C T, Lage A P, Barbosa A J A. Histopathological study of porcine gastric mucosa with and without a spiral bacterium (“Gastrospirillum suis”) J Med Microbiol. 1991;35:345–348. doi: 10.1099/00222615-35-6-345. [DOI] [PubMed] [Google Scholar]
- 279.Meyer-Rosberg K, Berglindh T. Helicobacter colonization of biopsy specimens cultured in vitro is dependent on both mucosal type and bacterial strain. Scand J Gastroenterol. 1996;31:434–441. doi: 10.3109/00365529609006761. [DOI] [PubMed] [Google Scholar]
- 280.Michetti P, Corthésy-Theulaz I, Davin C, Haas R, Vaney A C, Heitz M, Bille J, Kraehenbuhl J P, Saraga E, Blum A L. Immunization of BALB/c mice against Helicobacter felis infection with Helicobacter pylori urease. Gastroenterology. 1994;107:1002–1011. doi: 10.1016/0016-5085(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 281.Mohammadi M, Czinn S, Redline R, Nedrud J. Helicobacter-specific cell-mediated immune responses display a predominant Th1 phenotype and promote a delayed-type hypersensitivity response in the stomachs of mice. J Immunol. 1996;156:4729–4738. [PubMed] [Google Scholar]
- 282.Mohammadi M, Nedrud J, Redline R, Lycke N, Czinn S J. Murine CD4 T-cell response to Helicobacter infection: TH1 cells enhance gastritis and TH2 cells reduce bacterial load. Gastroenterology. 1997;113:1848–1857. doi: 10.1016/s0016-5085(97)70004-0. [DOI] [PubMed] [Google Scholar]
- 283.Mohammadi M, Redline R, Nedrud J, Czinn S. Role of the host in pathogenesis of Helicobacter-associated gastritis: H. felis infection of inbred and congenic mouse strains. Infect Immun. 1996;64:238–245. doi: 10.1128/iai.64.1.238-245.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Monno R, Ierardi E, Valenza M A, Campanale A, Francavilla A, Fumarola L. Gastrospirillum hominis and human chronic gastritis. New Microbiol. 1995;18:441–444. [PubMed] [Google Scholar]
- 285.Monteiro M A, Zheng P Y, Appelmelk B J, Perry M B. The lipopolysaccharide of Helicobacter mustelae type strain ATCC 43772 expresses the monofucosyl A type 1 histo-blood group epitope. FEMS Microbiol Lett. 1997;154:103–109. doi: 10.1111/j.1574-6968.1997.tb12630.x. [DOI] [PubMed] [Google Scholar]
- 286.Morgan D D, Owen R J. Use of DNA restriction endonuclease digest and ribosomal RNA gene probe patterns to fingerprint Helicobacter pylori and Helicobacter mustelae isolated from human and animal hosts. Mol Cell Probes. 1990;4:321–334. doi: 10.1016/0890-8508(90)90023-s. [DOI] [PubMed] [Google Scholar]
- 287.Morgan D R, Fox J G, Leunk R D. Comparison of isolates of Helicobacter pylori and Helicobacter mustelae. J Clin Microbiol. 1991;29:395–397. doi: 10.1128/jcm.29.2.395-397.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Morgner A, Lehn N, Andersen L P, Thiede C, Bennedsen M, Trebesius K, Neubauer B, Neubauer A, Stolte M, Bayerdörffer E. Helicobacter heilmannii-associated primary gastric low-grade MALT lymphoma: complete remission after curing the infection. Gastroenterology. 2000;118:821–828. doi: 10.1016/s0016-5085(00)70167-3. [DOI] [PubMed] [Google Scholar]
- 289.Morris A, Ali M R, Thomsen L, Hollis B. Tightly spiral shaped bacteria in the human stomach: another cause of active chronic gastritis? Gut. 1990;31:139–143. doi: 10.1136/gut.31.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Morris A, Thomasen L, Tasman-Jones C, Nicholson G. Failure to detect gastric campylobacter-like organisms in a group of ferrets in New Zealand. N Z Med J. 1988;101:275. [PubMed] [Google Scholar]
- 291.Moura S B, Mendes E N, Queiroz D M, Camargos E R, Evangelina M, Fonseca F, Rocha G A, Nicoli J R. Ultrastructure of Helicobacter trogontum in culture and in the gastrointestinal tract of gnotobiotic mice. J Med Microbiol. 1998;47:513–520. doi: 10.1099/00222615-47-6-513. [DOI] [PubMed] [Google Scholar]
- 292.Moura S B, Queiroz D M, Mendes E N, Nogueira A M, Rocha G A. The inflammatory response of the gastric mucosa of mice experimentally infected with “Gastrospirillum suis.”. J Med Microbiol. 1993;39:64–68. doi: 10.1099/00222615-39-1-64. [DOI] [PubMed] [Google Scholar]
- 293.Munson L. Diseases of captive cheetahs (Acinonyx jubatus): results of the Cheetah Research Council pathology survey, 1989–1992. Zoo Biol. 1993;12:105–124. [Google Scholar]
- 294.Muotiala A, Helander I M, Pyhälä L, Kosunen T U, Moran A P. Low biological activity of Helicobacter pylori lipopolysaccharide. Infect Immun. 1992;60:1714–1716. doi: 10.1128/iai.60.4.1714-1716.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Murray R G, Stackebrandt E. Taxonomic note: implementation of the provisional status Candidatus for incompletely described procaryotes. Int J Syst Bacteriol. 1995;45:186–187. doi: 10.1099/00207713-45-1-186. [DOI] [PubMed] [Google Scholar]
- 296.Murray R G E, Schleifer K H. Taxonomic notes: a proposal for recording the properties of putative taxa of prokaryotes. Int J Syst Bacteriol. 1994;44:174–176. doi: 10.1099/00207713-44-1-174. [DOI] [PubMed] [Google Scholar]
- 297.Myers G A, Ermak T H, Georgakopoulos K, Tibbitts T, Ingrassia J, Gray H, Kleanthous H, Lee C K, Monath T P. Oral immunization with recombinant Helicobacter pylori urease confers long-lasting immunity against Helicobacter felis infection. Vaccine. 1999;17:1394–1403. doi: 10.1016/s0264-410x(98)00387-9. [DOI] [PubMed] [Google Scholar]
- 298.Neiger R, Dieterich C, Burnens A, Waldvogel A, Corthesy-Theulaz I, Halter F, Lauterburg B, Schmassmann A. Detection and prevalence of Helicobacter infection in pet cats. J Clin Microbiol. 1998;36:634–637. doi: 10.1128/jcm.36.3.634-637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Neiger R, Seiler G, Schmassmann A. Use of a urea breath test to evaluate short-term treatments for cats naturally infected with Helicobacter heilmannii. Am J Vet Res. 1999;60:880–883. [PubMed] [Google Scholar]
- 300.Ng V L, Hadley W K, Fennell C L, Flores B M, Stamm W E. Successive bacteremias with “Campylobacter cinaedi” and “Campylobacter fennelliae” in a bisexual male. J Clin Microbiol. 1987;25:2008–2009. doi: 10.1128/jcm.25.10.2008-2009.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Nomura A, Stemmerman G N, Chyou P-H, Kato I, Perez-Perez G I, Blaser M J. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991;325:1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- 302.Nomura A, Stemmermann G N, Chyou P H, Perez-Perez G I, Blaser M J. Helicobacter pylori infection and the risk for duodenal and gastric ulceration. Ann Intern Med. 1994;120:977–981. doi: 10.7326/0003-4819-120-12-199406150-00001. [DOI] [PubMed] [Google Scholar]
- 303.Norris C R, Marks S L, Eaton K A, Torabian S Z, Munn R J, Solnick J V. Healthy cats are commonly colonized with “Helicobacter heilmannii” that is associated with minimal gastritis. J Clin Microbiol. 1999;37:189–194. doi: 10.1128/jcm.37.1.189-194.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Nyska A, Maronpot R R, Eldridge S R, Haseman J K, Hailey J R. Alteration in cell kinetics in control B6C3F1 mice infected with Helicobacter hepaticus. Toxicol Pathol. 1997;25:591–596. doi: 10.1177/019262339702500609. [DOI] [PubMed] [Google Scholar]
- 305.O. Cróinín T, Clyne M, Drumm B. Molecular mimicry of ferret gastric epithelial blood group antigen A by Helicobacter mustelae. Gastroenterology. 1998;114:690–696. doi: 10.1016/s0016-5085(98)70582-7. [DOI] [PubMed] [Google Scholar]
- 306.Odenbreit S, Till M, Hofreuter D, Faller G, Haas R. Genetic and functional characterization of the alpAB gene locus essential for the adhesion of Helicobacter pylori to human gastric tissue. Mol Microbiol. 1999;31:1537–1548. doi: 10.1046/j.1365-2958.1999.01300.x. [DOI] [PubMed] [Google Scholar]
- 307.Oliva M M, Lazenby A J, Perman J A. Gastritis associated with Gastrospirillum hominis in children. Comparison with Helicobacter pylori and review of the literature. Mod Pathol. 1993;6:513–515. [PubMed] [Google Scholar]
- 308.Orlicek S L, Welch D F, Kuhls T L. Septicemia and meningitis caused by Helicobacter cinaedi in a neonate. J Clin Microbiol. 1993;31:569–571. doi: 10.1128/jcm.31.3.569-571.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.O'Rourke J, Lee A, Fox J G. An ultrastructural study of Helicobacter mustelae and evidence of a specific association with gastric mucosa. J Med Microbiol. 1992;36:420–427. doi: 10.1099/00222615-36-6-420. [DOI] [PubMed] [Google Scholar]
- 310.O'Toole P W, Austin J W, Trust T J. Identification and molecular characterization of a major ring-forming surface protein from the gastric pathogen Helicobacter mustelae. Mol Microbiol. 1994;11:349–361. doi: 10.1111/j.1365-2958.1994.tb00315.x. [DOI] [PubMed] [Google Scholar]
- 311.O'Toole P W, Janzon L, Doig P, Huang J, Kostrzynska M, Trust T J. The putative neuraminyllactose-binding hemagglutinin HpaA of Helicobacter pylori CCUG 17874 is a lipoprotein. J Bacteriol. 1995;177:6049–6057. doi: 10.1128/jb.177.21.6049-6057.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.O'Toole P W, Kostrzynska M, Trust T J. Non-motile mutants of Helicobacter pylori and Helicobacter mustelae defective in flagellar hook production. Mol Microbiol. 1994;14:691–703. doi: 10.1111/j.1365-2958.1994.tb01307.x. [DOI] [PubMed] [Google Scholar]
- 313.Otto G, Fox J G, Wu P Y, Taylor N S. Eradication of Helicobacter mustelae from the ferret stomach: an animal model of Helicobacter (Campylobacter) pylori chemotherapy. Antimicrob Agents Chemother. 1990;34:1232–1236. doi: 10.1128/aac.34.6.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Otto G, Hazell S H, Fox J G, Howlett C R, Murphy J C, O'Rourke J L, Lee A. Animal and public health implications of gastric colonization of cats by Helicobacter-like organisms. J Clin Microbiol. 1994;32:1043–1049. doi: 10.1128/jcm.32.4.1043-1049.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314a.Page R D M. TREEVIEW: an application to display phylogenetic trees on personal computers. Comput Appl Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- 315.Papasouliotis K, Gruffydd-Jones T J, Werrett G, Brown P J, Pearson G R. Occurrence of ‘gastric Helicobacter-like organisms’ in cats. Vet Rec. 1997;140:369–370. doi: 10.1136/vr.140.14.369. [DOI] [PubMed] [Google Scholar]
- 316.Pappo J, Thomas W D, Jr, Kabok Z, Taylor N S, Murphy J C, Fox J G. Effect of oral immunization with recombinant urease on murine Helicobacter felis gastritis. Infect Immun. 1995;63:1246–1252. doi: 10.1128/iai.63.4.1246-1252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Parsonnet J, Friedman G D, Vandersteen D P, Chang Y, Vogelman J H, Orentreich N, Sibley R K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1136. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 318.Parsonnet J, Hansen S, Rodriguez L, Gelb A B, Warnke R A, Jellum E, Orentreich N, Vogelman J H, Friedman G D. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267–1271. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 319.Paster B J, Lee A, Fox J G, Dewhirst F E, Tordoff L A, Fraser G J, O'Rourke J L, Taylor N S, Ferrero R. Phylogeny of Helicobacter felis sp. nov., Helicobacter mustelae, and related bacteria. Int J Syst Bacteriol. 1991;41:31–38. doi: 10.1099/00207713-41-1-31. [DOI] [PubMed] [Google Scholar]
- 320.Perkins S E, Fox J G, Walsh J H. Helicobacter mustelae-associated hypergastrinemia in ferrets (Mustela putorius furo) Am J Vet Res. 1996;57:147–150. [PubMed] [Google Scholar]
- 321.Peyrol S, Lecoindre P, Berger I, Deleforge J, Chevallier M. Differential pathogenic effect of two Helicobacter-like organisms in dog gastric mucosa. J Submicrosc Cytol Pathol. 1998;30:425–433. [PubMed] [Google Scholar]
- 322.Phillips M W, Lee A. Isolation and characterization of a spiral bacterium from the crypts of rodent gastrointestinal tracts. Appl Environ Microbiol. 1983;45:675–683. doi: 10.1128/aem.45.2.675-683.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Pickett C L, Whitehouse C A. The cytolethal distending toxin family. Trends Microbiol. 1999;7:292–297. doi: 10.1016/s0966-842x(99)01537-1. [DOI] [PubMed] [Google Scholar]
- 324.Pope A J, Toseland C D, Rushant B, Richardson S, McVey M, Hills J. Effect of potent urease inhibitor, fluorofamide, on Helicobacter sp. in vivo and in vitro. Dig Dis Sci. 1998;43:109–119. doi: 10.1023/a:1018884322973. [DOI] [PubMed] [Google Scholar]
- 325.Queiroz D M, Contigli C, Coimbra R S, Nogueira A M, Mendes E N, Rocha G A, Moura S B. Spiral bacterium associated with gastric, ileal and caecal mucosa of mice. Lab Anim. 1992;26:288–294. doi: 10.1258/002367792780745760. [DOI] [PubMed] [Google Scholar]
- 326.Queiroz D M, Rocha G A, Mendes E N, De Moura S B, De Oliveira A M, Miranda D. Association between Helicobacter and gastric ulcer disease of the pars esophagea in swine. Gastroenterology. 1996;111:19–27. doi: 10.1053/gast.1996.v111.pm8698198. [DOI] [PubMed] [Google Scholar]
- 327.Queiroz D M, Rocha G A, Mendes E N, Lage A P, Carvalho A C, Barbosa A J. A spiral microorganism in the stomach of pigs. Vet Microbiol. 1990;24:199–204. doi: 10.1016/0378-1135(90)90067-6. [DOI] [PubMed] [Google Scholar]
- 328.Radcliff F J, Chen M, Lee A. Protective immunization against Helicobacter stimulates long-term immunity. Vaccine. 1996;14:780–784. doi: 10.1016/0264-410x(95)00246-w. [DOI] [PubMed] [Google Scholar]
- 329.Radcliff F J, Hazell S L, Kolesnikow T, Doidge C, Lee A. Catalase, a novel antigen for Helicobacter pylori vaccination. Infect Immun. 1997;65:4668–4674. doi: 10.1128/iai.65.11.4668-4674.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Rappin G. Contribution a l'étude bactéries de la bouche a l'état normal et dans la fievre typhoid. Paris, France: A. Parent; 1881. [Google Scholar]
- 331.Rathbone B J, West A P, Wyatt J I, Johnson A W, Tompkins D S, Heatley R V. Campylobacter pyloridis, urease, and gastric ulcers. Lancet. 1986;ii:400–401. doi: 10.1016/s0140-6736(86)90089-9. [DOI] [PubMed] [Google Scholar]
- 332.Regimbeau C, Karsenti D, Durand V, D'Alteroche L, Copie-Bergman C, Metman E H, Machet M C. Low-grade gastric MALT lymphoma and Helicobacter heilmannii (Gastrospirillum hominis) Gastroenterol Clin Biol. 1998;22:720–723. [PubMed] [Google Scholar]
- 333.Rice L E, Stahl S J, McLeod C G., Jr Pyloric adenocarcinoma in a ferret. J Am Vet Med Assoc. 1992;200:1117–1118. [PubMed] [Google Scholar]
- 334.Riley L K, Franklin C L, Hook R R, Jr, Besch-Williford C. Identification of murine helicobacters by PCR and restriction enzyme analyses. J Clin Microbiol. 1996;34:942–946. doi: 10.1128/jcm.34.4.942-946.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Roggero E, Zucca E, Pinotti G, Pascarella A, Capella C, Savio A, Pedrinis E, Paterlini A, Venco A, Cavalli F. Eradication of Helicobacter pylori infection in primary low-grade gastric lymphoma of mucosa-associated lymphoid tissue. Ann Intern Med. 1995;122:767–769. doi: 10.7326/0003-4819-122-10-199505150-00006. [DOI] [PubMed] [Google Scholar]
- 336.Romaniuk P J, Zoltowska B, Trust T J, Lane D J, Olsen G J, Pace N R, Stahl D A. Campylobacter pylori, the spiral bacterium associated with human gastritis, is not a true Campylobacter sp. J Bacteriol. 1987;169:2137–2141. doi: 10.1128/jb.169.5.2137-2141.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Romero S, Archer J R, Hamacher M E, Bologna S M, Schell R F. Case report of an unclassified microaerophilic bacterium associated with gastroenteritis. J Clin Microbiol. 1988;26:142–143. doi: 10.1128/jcm.26.1.142-143.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Roth K A, Kapadia S B, Martin S M, Lorenz R G. Cellular immune responses are essential for the development of Helicobacter felis-associated gastric pathology. J Immunol. 1999;163:1490–1497. [PubMed] [Google Scholar]
- 339.Russell R J, Haines D C, Anver M R, Battles J K, Gorelick P L, Blumenauer L L, Gonda M A, Ward J M. Use of antibiotics to prevent hepatitis and typhlitis in male scid mice spontaneously infected with Helicobacter hepaticus. Lab Anim Sci. 1995;45:373–378. [PubMed] [Google Scholar]
- 340.Sacks L V, Labriola A M, Gill V J, Gordin F M. Use of ciprofloxacin for successful eradication of bacteremia due to Campylobacter cinaedi in a human immunodeficiency virus-infected person. Rev Infect Dis. 1991;13:1066–1068. doi: 10.1093/clinids/13.6.1066. [DOI] [PubMed] [Google Scholar]
- 341.Sakagami T, Dixon M, O'Rourke J, Howlett R, Alderuccio F, Vella J, Shimoyama T, Lee A. Atrophic gastric changes in both Helicobacter felis and Helicobacter pylori infected mice are host dependent and separate from antral gastritis. Gut. 1996;39:639–648. doi: 10.1136/gut.39.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Sakagami T, Vella J, Dixon M F, O'Rourke J, Radcliff F, Sutton P, Shimoyama T, Beagley K, Lee A. The endotoxin of Helicobacter pylori is a modulator of host-dependent gastritis. Infect Immun. 1997;65:3310–3316. doi: 10.1128/iai.65.8.3310-3316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Saldinger P F, Porta N, Launois P, Louis J A, Waanders G A, Bouzouréne H, Michetti P, Blum A L, Corthésy-Theulaz I E. Immunization of BALB/c mice with Helicobacter urease B induces a T helper 2 response absent in Helicobacter infection. Gastroenterology. 1998;115:891–897. doi: 10.1016/s0016-5085(98)70261-6. [DOI] [PubMed] [Google Scholar]
- 344.Salomon H. Uber das Spirillum des Saugetiermagens und sein Verhalten zu den Belegzellen. Zentbl Bakteriol. 1896;19:433–442. [Google Scholar]
- 345.Saunders K E, Shen Z, Dewhirst F E, Paster B J, Dangler C A, Fox J G. Novel intestinal Helicobacter species isolated from cotton-top tamarins (Saguinus oedipus) with chronic colitis. J Clin Microbiol. 1999;37:146–151. doi: 10.1128/jcm.37.1.146-151.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Schauer D B, Ghori N, Falkow S. Isolation and characterization of “Flexispira rappini” from laboratory mice. J Clin Microbiol. 1993;31:2709–2714. doi: 10.1128/jcm.31.10.2709-2714.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Schmidt T M, Relman D A. Phylogenetic identification of uncultured pathogens using ribosomal RNA sequences. Methods Enzymol. 1994;235:205–222. doi: 10.1016/0076-6879(94)35142-2. [DOI] [PubMed] [Google Scholar]
- 348.Schreiber S, Stüben M, Josenhans C, Scheid P, Suerbaum S. In vivo distribution of Helicobacter felis in the gastric mucus of the mouse: experimental method and results. Infect Immun. 1999;67:5151–5156. doi: 10.1128/iai.67.10.5151-5156.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Schröder H D, Ludwig C, Jakob W, Reischl U, Stolte M, Lehn N. Chronic gastritis in tigers associated with Helicobacter acinonyx. J Comp Pathol. 1998;119:67–73. doi: 10.1016/s0021-9975(98)80072-8. [DOI] [PubMed] [Google Scholar]
- 350.Segal E D, Falkow S, Tompkins L S. Helicobacter pylori attachment to gastric cells induces cytoskeletal rearrangements and tyrosine phosphorylation of host proteins. Proc Natl Acad Sci USA. 1996;93:1259–1264. doi: 10.1073/pnas.93.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Seidel K E, Stolte M, Lehn N, Bauer J. Antibodies against Helicobacter felis in sera of cats and dogs. Zentbl Veterinaermed Ser B. 1999;46:181–188. doi: 10.1046/j.1439-0450.1999.00219.x. [DOI] [PubMed] [Google Scholar]
- 352.Sellman S, Blanchard T G, Nedrud J G, Czinn S J. Vaccine strategies for prevention of Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 1995;7(Suppl. 1):S1–S6. [PubMed] [Google Scholar]
- 353.Serna J H, Genta R M, Lichtenberger L M, Graham D Y, el-Zaatari F A. Invasive Helicobacter-like organisms in feline gastric mucosa. Helicobacter. 1997;2:40–43. doi: 10.1111/j.1523-5378.1997.tb00056.x. [DOI] [PubMed] [Google Scholar]
- 354.Seymour C, Lewis R G, Kim M, Gagnon D F, Fox J G, Dewhirst F E, Paster B J. Isolation of Helicobacter strains from wild bird and swine feces. Appl Environ Microbiol. 1994;60:1025–1028. doi: 10.1128/aem.60.3.1025-1028.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Shames B, Fox J G, Dewhirst F, Yan L, Shen Z, Taylor N S. Identification of widespread Helicobacter hepaticus infection in feces in commercial mouse colonies by culture and PCR assay. J Clin Microbiol. 1995;33:2968–2972. doi: 10.1128/jcm.33.11.2968-2972.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Shen Z, Fox J G, Dewhirst F E, Paster B J, Foltz C J, Yan L, Shames B, Perry L. Helicobacter rodentium sp. nov., a urease-negative Helicobacter species isolated from laboratory mice. Int J Syst Bacteriol. 1997;47:627–634. doi: 10.1099/00207713-47-3-627. [DOI] [PubMed] [Google Scholar]
- 357.Shen Z, Schauer D B, Mobley H L, Fox J G. Development of a PCR-restriction fragment length polymorphism assay using the nucleotide sequence of the Helicobacter hepaticus urease structural genes ureAB. J Clin Microbiol. 1998;36:2447–2453. doi: 10.1128/jcm.36.9.2447-2453.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Sherburne R, Taylor D E. Helicobacter pylori expresses a complex surface carbohydrate, Lewis X. Infect Immun. 1995;63:4564–4568. doi: 10.1128/iai.63.12.4564-4568.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Shomer N H, Dangler C A, Marini R P, Fox J G. Helicobacter bilis/Helicobacter rodentium co-infection associated with diarrhea in a colony of scid mice. Lab Anim Sci. 1998;48:455–459. [PubMed] [Google Scholar]
- 360.Shomer N H, Dangler C A, Schrenzel M D, Fox J G. Helicobacter bilis-induced inflammatory bowel disease in scid mice with defined flora. Infect Immun. 1997;65:4858–4864. doi: 10.1128/iai.65.11.4858-4864.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Shomer N H, Dangler C A, Whary M T, Fox J G. Experimental Helicobacter pylori infection induces antral gastritis and gastric mucosa-associated lymphoid tissue in guinea pigs. Infect Immun. 1998;66:2614–2618. doi: 10.1128/iai.66.6.2614-2618.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Simmons J H, Riley L K, Besch-Williford C L, Franklin C L. Helicobacter mesocricetorum sp. nov., a novel Helicobacter isolated from the feces of syrian hamsters. J Clin Microbiol. 2000;38:1811–1817. doi: 10.1128/jcm.38.5.1811-1817.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Simpson K W, McDonough P L, Strauss-Ayali D, Chang Y F, Harpending P, Valentine B A. Helicobacter felis infection in dogs: effect on gastric structure and function. Vet Pathol. 1999;36:237–248. doi: 10.1354/vp.36-3-237. [DOI] [PubMed] [Google Scholar]
- 364.Simpson K W, Strauss-Ayali D, Scanziani E, Straubinger R K, McDonough P L, Straubinger A F, Chang Y F, Domeneghini C, Arebi N, Calam J. Helicobacter felis infection is associated with lymphoid follicular hyperplasia and mild gastritis but normal gastric secretory function in cats. Infect Immun. 2000;68:779–790. doi: 10.1128/iai.68.2.779-790.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Sipowicz M A, Chomarat P, Diwan B A, Anver M A, Awasthi Y C, Ward J M, Rice J M, Kasprzak K S, Wild C P, Anderson L M. Increased oxidative DNA damage and hepatocyte overexpression of specific cytochrome P450 isoforms in hepatitis of mice infected with Helicobacter hepaticus. Am J Pathol. 1997;151:933–941. [PMC free article] [PubMed] [Google Scholar]
- 366.Sipowicz M A, Weghorst C M, Shiao Y H, Buzard G S, Calvert R J, Anver M R, Anderson L M, Rice J M. Lack of p53 and ras mutations in Helicobacter hepaticus-induced liver tumors in A/JCr mice. Carcinogenesis. 1997;18:233–236. doi: 10.1093/carcin/18.1.233. [DOI] [PubMed] [Google Scholar]
- 367.Smith J G, Kong L, Abruzzo G K, Gill C J, Flattery A M, Scott P M, Silver L, Kropp H, Bartizal K. Evaluation of experimental therapeutics in a new mouse model of Helicobacter felis utilizing 16S rRNA polymerase chain reaction for detection. Scand J Gastroenterol. 1997;32:297–302. doi: 10.3109/00365529709007675. [DOI] [PubMed] [Google Scholar]
- 368.Solnick J V, Canfield D R, Hansen L M, Torabian S Z. Immunization with recombinant Helicobacter pylori urease in specific-pathogen-free rhesus monkeys (Macaca mulatta) Infect Immun. 2000;68:2560–2565. doi: 10.1128/iai.68.5.2560-2565.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Solnick J V, Canfield D R, Yang S, Parsonnet J. The rhesus monkey (Macaca mulatta) model of Helicobacter pylori: noninvasive detection and derivation of specific pathogen free monkeys. Lab Anim Sci. 1999;49:197–201. [PubMed] [Google Scholar]
- 370.Solnick J V, Josenhans C, Suerbaum S, Tompkins L S, Labigne A. Construction and characterization of an isogenic urease-negative mutant of Helicobacter mustelae. Infect Immun. 1995;63:3718–3722. doi: 10.1128/iai.63.9.3718-3721.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Solnick J V, O'Rourke J, Lee A, Paster B, Dewhirst F E, Tompkins L S. An uncultured gastric spiral organism is a newly identified Helicobacter in humans. J Infect Dis. 1993;168:379–385. doi: 10.1093/infdis/168.2.379. [DOI] [PubMed] [Google Scholar]
- 372.Solnick J V, O'Rourke J, Lee A, Tompkins L S. Molecular analysis of urease genes from a newly identified uncultured species of Helicobacter. Infect Immun. 1994;62:1631–1638. doi: 10.1128/iai.62.5.1631-1638.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Sorlin P, Vandamme P, Nortier J, Hoste B, Rossi C, Pavlof S, Struelens M J. Recurrent “Flexispira rappini” bacteremia in an adult patient undergoing hemodialysis: case report. J Clin Microbiol. 1999;37:1319–1323. doi: 10.1128/jcm.37.5.1319-1323.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Stanley J, Linton D, Burnens A P, Dewhirst F E, On S L, Porter A, Owen R J, Costas M. Helicobacter pullorum sp. nov.—genotype and phenotype of a new species isolated from poultry and from human patients with gastroenteritis. Microbiology. 1994;140:3441–3449. doi: 10.1099/13500872-140-12-3441. [DOI] [PubMed] [Google Scholar]
- 375.Stanley J, Linton D, Burnens A P, Dewhirst F E, Owen R J, Porter A, On S L, Costas M. Helicobacter canis sp. nov., a new species from dogs: an integrated study of phenotype and genotype. J Gen Microbiol. 1993;139:2495–2504. doi: 10.1099/00221287-139-10-2495. [DOI] [PubMed] [Google Scholar]
- 376.Stolte M, Kroher G, Meining A, Morgner A, Bayerdörffer E, Bethke B. A comparison of Helicobacter pylori and H. heilmannii gastritis. A matched control study involving 404 patients. Scand J Gastroenterol. 1997;32:28–33. doi: 10.3109/00365529709025059. [DOI] [PubMed] [Google Scholar]
- 377.Strauss-Ayali D, Simpson K W. Gastric Helicobacter infection in dogs. Vet Clin North Am Small Anim Pract. 1999;29:397–414. , vi. [PubMed] [Google Scholar]
- 378.Strauss-Ayali D, Simpson K W, Schein A H, McDonough P L, Jacobson R H, Valentine B A, Peacock J. Serological discrimination of dogs infected with gastric Helicobacter spp. and uninfected dogs. J Clin Microbiol. 1999;37:1280–1287. doi: 10.1128/jcm.37.5.1280-1287.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Suerbaum S, Geis G, Josenhans C, Opferkuch W. Biochemical studies of Helicobacter mustelae fatty acid composition and flagella. Infect Immun. 1992;60:1695–1698. doi: 10.1128/iai.60.4.1695-1698.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Suerbaum S, Josenhans C, Labigne A. Cloning and genetic characterization of the Helicobacter pylori and Helicobacter mustelae flaB flagellin genes and construction of H. pylori flaA- and flaB-negative mutants by electroporation-mediated allelic exchange. J Bacteriol. 1993;175:3278–3288. doi: 10.1128/jb.175.11.3278-3288.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Sugimura T, Fujimura S. Tumour production in glandular stomach of rat by N-methyl-N′-nitro-N-nitrosoguanidine. Nature. 1967;216:943–944. doi: 10.1038/216943a0. [DOI] [PubMed] [Google Scholar]
- 382.Sugimura T, Tanaka N, Kawachi T, Kogure K, Fujimura S. Production of stomach cancer in dogs by N-methyl-N'-nitro-N-nitrosoguanidine. Gann. 1971;62:67. [PubMed] [Google Scholar]
- 383.Sugiyama A, Maruta F, Ikeno T, Ishida K, Kawasaki S, Katsuyama T, Shimizu N, Tatematsu M. Helicobacter pylori infection enhances N-methyl-N-nitrosourea-induced stomach carcinogenesis in the Mongolian gerbil. Cancer Res. 1998;58:2067–2069. [PubMed] [Google Scholar]
- 384.Sutton P, Wilson J, Genta R, Torrey D, Savinainen A, Pappo J, Lee A. A genetic basis for atrophy: dominant non-responsiveness and helicobacter induced gastritis in F(1) hybrid mice. Gut. 1999;45:335–340. doi: 10.1136/gut.45.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Tanaka M, Saitoh A, Narita T, Hizawa Y, Nakazawa H, Narita N, Kudo H. Gastrospirillum hominis-associated gastritis: the first reported case in Japan. J Gastroenterol. 1994;29:199–202. doi: 10.1007/BF02358683. [DOI] [PubMed] [Google Scholar]
- 386.Tannock G W, Smith J M. The microflora of the pig stomach and its possible relationship to ulceration of the pars oesophagea. J Comp Pathol. 1970;80:359–367. doi: 10.1016/0021-9975(70)90066-6. [DOI] [PubMed] [Google Scholar]
- 387.Taylor D E, Chang N, Taylor N S, Fox J G. Genome conservation in Helicobacter mustelae as determined by pulsed-field gel electrophoresis. FEMS Microbiol Lett. 1994;118:31–36. doi: 10.1111/j.1574-6968.1994.tb06799.x. [DOI] [PubMed] [Google Scholar]
- 388.Taylor N S, Fox J G, Yan L. In-vitro hepatotoxic factor in Helicobacter hepaticus, H. pylori and other Helicobacter species. J Med Microbiol. 1995;42:48–52. doi: 10.1099/00222615-42-1-48. [DOI] [PubMed] [Google Scholar]
- 389.Taylor N S, Hasubski A T, Fox J G, Lee A. Haemagglutination profiles of Helicobacter species that cause gastritis in man and animals. J Med Microbiol. 1992;37:299–303. doi: 10.1099/00222615-37-5-299. [DOI] [PubMed] [Google Scholar]
- 390.Tee W, Anderson B N, Ross B C, Dwyer B. Atypical campylobacters associated with gastroenteritis. J Clin Microbiol. 1987;25:1248–1252. doi: 10.1128/jcm.25.7.1248-1252.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Tee W, Leder K, Karroum E, Dyall-Smith M. “Flexispira rappini” bacteremia in a child with pneumonia. J Clin Microbiol. 1998;36:1679–1682. doi: 10.1128/jcm.36.6.1679-1682.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Terio K, Munson L, Solnick J V. Chronic Helicobacter associated gastritis in cheetahs. Vet Pathol. 1999;36:5. doi: 10.1177/0300985811412620. [DOI] [PubMed] [Google Scholar]
- 393.Thomas L. The lives of a cell: notes of a biology watcher. New York, N.Y: Viking Press; 1974. [Google Scholar]
- 394.Thomson M A, Storey P, Greer R, Cleghorn G J. Canine-human transmission of Gastrospirillum hominis. Lancet. 1994;343:1605–1607. doi: 10.1016/s0140-6736(94)93060-0. [DOI] [PubMed] [Google Scholar]
- 395.Tomb J F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, Nelson K, Quackenbush J, Zhou L, Kirkness E F, Peterson S, Loftus B, Richardson D, Dodson R, Khalak H G, Glodek A, McKenney K, Fitzegerald L M, Lee N, Adams M D, Venter J C, et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 396.Tompkins D S, Wyatt J I, Rathbone B J, West A P. The characterization and pathological significance of gastric Campylobacter-like organisms in the ferret: a model for chronic gastritis? Epidemiol Infect. 1988;101:269–278. doi: 10.1017/s0950268800054182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Totten P A, Fennell C L, Tenover F C, Wezenberg J M, Perine P L, Stamm W E, Holmes K K. Campylobacter cinaedi (sp. nov.) and Campylobacter fennelliae (sp. nov.): two new Campylobacter species associated with enteric disease in homosexual men. J Infect Dis. 1985;151:131–139. doi: 10.1093/infdis/151.1.131. [DOI] [PubMed] [Google Scholar]
- 398.Trivett-Moore N L, Rawlinson W D, Yuen M, Gilbert G L. Helicobacter westmeadii sp. nov., a new species isolated from blood cultures of two AIDS patients. J Clin Microbiol. 1997;35:1144–1150. doi: 10.1128/jcm.35.5.1144-1150.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Trüper H G, DeClari L. Taxonomic note. Necessary correction of specific epithets formed as substantives (nouns) “in apposition.”. Int J Syst Bacteriol. 1997;47:908–909. [Google Scholar]
- 400.Tsuda M, Karita M, Morshed M G, Okita K, Nakazawa T. A urease-negative mutant of Helicobacter pylori constructed by allelic exchange mutagenesis lacks the ability to colonize the nude mouse stomach. Infect Immun. 1994;62:3586–3589. doi: 10.1128/iai.62.8.3586-3589.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Turbett G R, Høj P B, Horne R, Mee B J. Purification and characterization of the urease enzymes of Helicobacter species from humans and animals. Infect Immun. 1992;60:5259–5266. doi: 10.1128/iai.60.12.5259-5266.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Turbett G R, Nandapalan N, Campbell I G, Nikoletti S M, Mee B J. Characterisation of the urease from Helicobacter pylori and comparison with the ureases from related spiral gastric bacteria. FEMS Microbiol Immunol. 1991;3:19–24. doi: 10.1111/j.1574-6968.1991.tb04158.x. [DOI] [PubMed] [Google Scholar]
- 403.Unge P. What other regimens are under investigation to treat Helicobacter pylori infection? Gastroenterology. 1997;113(Suppl. 6):S131–S48. doi: 10.1016/s0016-5085(97)80027-3. [DOI] [PubMed] [Google Scholar]
- 404.Utriainen M, Jalava K, Sukura A, Hänninen M L. Morphological diversity of cultured canine gastric Helicobacter spp. Comp Immunol Microbiol Infect Dis. 1997;20:285–297. doi: 10.1016/s0147-9571(97)00009-x. [DOI] [PubMed] [Google Scholar]
- 405.Vandamme P, Falsen E, Pot B, Kersters K, De Ley J. Identification of Campylobacter cinaedi isolated from blood and feces of children and adult females. J Clin Microbiol. 1990;28:1016–1020. doi: 10.1128/jcm.28.5.1016-1020.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Vandamme P, Pot B, Gillis M, de Vos P, Kersters K, Swings J. Polyphasic taxonomy, a consensus approach to bacterial systematics. Microbiol Rev. 1996;60:407–438. doi: 10.1128/mr.60.2.407-438.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.von Freeden-Jeffry U, Davidson N, Wiler R, Fort M, Burdach S, Murray R. IL-7 deficiency prevents development of a non-T cell non-B cell-mediated colitis. J Immunol. 1998;161:5673–5680. [PubMed] [Google Scholar]
- 408.Wack R F, Eaton K A, Kramer L W. Treatment of gastritis in cheetahs (Acinonyx jubatus) J Zoo Wildl Med. 1997;28:260–266. [PubMed] [Google Scholar]
- 409.Wang J T, Sheu J C, Lin J T, Wang T H, Wu M S. Direct DNA amplification and restriction pattern analysis of Helicobacter pylori in patients with duodenal ulcer and their families. J Infect Dis. 1993;168:1544–1548. doi: 10.1093/infdis/168.6.1544. [DOI] [PubMed] [Google Scholar]
- 410.Wang T C, Dangler C A, Chen D, Goldenring J R, Koh T, Raychowdhury R, Coffey R J, Ito S, Varro A, Dockray G J, Fox J G. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology. 2000;118:36–47. doi: 10.1016/s0016-5085(00)70412-4. [DOI] [PubMed] [Google Scholar]
- 411.Wang T C, Goldenring J R, Dangler C, Ito S, Mueller A, Jeon W K, Koh T J, Fox J G. Mice lacking secretory-phospholipase A2 show altered apoptosis and differentiation with Helicobacter felis infection. Gastroenterology. 1998;114:675–689. doi: 10.1016/s0016-5085(98)70581-5. [DOI] [PubMed] [Google Scholar]
- 412.Ward J M, Anver M R, Haines D C, Benveniste R E. Chronic active hepatitis in mice caused by Helicobacter hepaticus. Am J Pathol. 1994;145:959–968. [PMC free article] [PubMed] [Google Scholar]
- 413.Ward J M, Anver M R, Haines D C, Melhorn J M, Gorelick P, Yan L, Fox J G. Inflammatory large bowel disease in immunodeficient mice naturally infected with Helicobacter hepaticus. Lab Anim Sci. 1996;46:15–20. [PubMed] [Google Scholar]
- 414.Ward J M, Benveniste R E, Fox C H, Battles J K, Gonda M A, Tully J G. Autoimmunity in chronic active Helicobacter hepatitis of mice. Serum antibodies and expression of heat shock protein 70 in liver. Am J Pathol. 1996;148:509–517. [PMC free article] [PubMed] [Google Scholar]
- 415.Ward J M, Fox J G, Anver M R, Haines D C, George C V, Collins M J, Jr, Gorelick P L, Nagashima K, Gonda M A, Gilden R V, Tully J G, Russell R J, Benveniste R E, Paster B J, Dewhirst F E, Donovan J C, Anderson L M, Rice J M. Chronic active hepatitis and associated liver tumors in mice caused by a persistent bacterial infection with a novel Helicobacter species. J Natl Cancer Inst. 1994;86:1222–1227. doi: 10.1093/jnci/86.16.1222. [DOI] [PubMed] [Google Scholar]
- 416.Webb P M, Knight T, Elder J B, Newell D G, Forman D. Is Helicobacter pylori transmitted from cats to humans? Helicobacter. 1996;1:79–81. doi: 10.1111/j.1523-5378.1996.tb00014.x. [DOI] [PubMed] [Google Scholar]
- 417.Weber A F, Hasa O, Sautter J H. Some observations concerning the presence of spirilla in the fundic glands of dogs and cats. Am J Vet Res. 1958;19:677–680. [PubMed] [Google Scholar]
- 418.Weber A F, Schmittdiel E F. Electron microscopic and bacteriologic studies of spirilla isolated from the fundic stomachs of cats and dogs. Am J Vet Res. 1962;23:422–427. [PubMed] [Google Scholar]
- 419.Weir S, Cuccherini B, Whitney A M, Ray M L, MacGregor J P, Steigerwalt A, Daneshvar M I, Weyant R, Wray B, Steele J, Strober W, Gill V J. Recurrent bacteremia caused by a “Flexispira”-like organism in a patient with X-linked (Bruton's) agammaglobulinemia. J Clin Microbiol. 1999;37:2439–2445. doi: 10.1128/jcm.37.8.2439-2445.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Weir S C, Gibert C L, Gordin F M, Fischer S H, Gill V J. An uncommon Helicobacter isolate from blood: evidence of a group of Helicobacter spp. pathogenic in AIDS patients. J Clin Microbiol. 1999;37:2729–2733. doi: 10.1128/jcm.37.8.2729-2733.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Whary M T, Morgan T J, Dangler C A, Gaudes K J, Taylor N S, Fox J G. Chronic active hepatitis induced by Helicobacter hepaticus in the A/JCr mouse is associated with a Th1 cell-mediated immune response. Infect Immun. 1998;66:3142–3148. doi: 10.1128/iai.66.7.3142-3148.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Whary M T, Palley L S, Batchelder M, Murphy J C, Yan L, Taylor N S, Fox J G. Promotion of ulcerative duodenitis in young ferrets by oral immunization with Helicobacter mustelae and muramyl dipeptide. Helicobacter. 1997;2:65–77. doi: 10.1111/j.1523-5378.1997.tb00061.x. [DOI] [PubMed] [Google Scholar]
- 423.Williams C, Neithercut W D, Hossack M, Hair J, McColl K E. Urease-mediated destruction of bacteria is specific for Helicobacter urease and results in total cellular disruption. FEMS Immunol Med Microbiol. 1994;9:273–280. doi: 10.1111/j.1574-695X.1994.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 424.Wirth H-P, Beins M H, Yang M, Tham K T, Blaser M J. Experimental infection of mongolian gerbils with wild-type and mutant Helicobacter pylori strains. Infect Immun. 1998;66:4856–4866. doi: 10.1128/iai.66.10.4856-4866.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Yali Z, Yamada N, Wen M, Matsuhisa T, Miki M. Gastrospirillum hominis and Helicobacter pylori infection in Thai individuals: comparison of hlstopathological changes of gastric mucosa. Pathol Int. 1998;48:507–511. doi: 10.1111/j.1440-1827.1998.tb03941.x. [DOI] [PubMed] [Google Scholar]
- 426.Yamamoto T, Matsumoto J, Shiota K, Kitajima S, Goto M, Imaizumi M, Arima T. Helicobacter heilmannii associated erosive gastritis. Intern Med. 1999;38:240–243. doi: 10.2169/internalmedicine.38.240. [DOI] [PubMed] [Google Scholar]
- 427.Yamasaki K, Suematsu H, Takahashi T. Comparison of gastric lesions in dogs and cats with and without gastric spiral organisms. J Am Vet Assoc. 1998;212:529–533. [PubMed] [Google Scholar]
- 428.Yang H, Dixon M F, Li X, Xu Z, Zhou D, Blum A L. Acute gastritis associated with infection of large spiral-shaped bacteria. Am J Gastroenterol. 1995;90:307–309. [PubMed] [Google Scholar]
- 429.Yang H, Goliger J A, Song M, Zhou D. High prevalence of Helicobacter heilmannii infection in China. Dig Dis Sci. 1998;43:1493. doi: 10.1023/a:1018854513056. [DOI] [PubMed] [Google Scholar]
- 430.Yang H, Li X, Xu Z, Zhou D. “Helicobacter heilmannii” infection in a patient with gastric cancer. Dig Dis Sci. 1995;40:1013–1014. doi: 10.1007/BF02064190. [DOI] [PubMed] [Google Scholar]
- 431.Yeomans N D, Kolt S D. Helicobacter heilmannii (formerly Gastrospirillum): association with pig and human gastric pathology. Gastroenterology. 1996;111:244–247. doi: 10.1053/gast.1996.v111.agast961110244. [DOI] [PubMed] [Google Scholar]
- 432.Young V B, Chien C-C, Knox K A, Taylor N S, Schauer D B, Fox J G. Cytolethal distending toxin in avian and human isolates of Helicobacter pullorum. J Infect Dis. 2000;182:620–623. doi: 10.1086/315705. [DOI] [PubMed] [Google Scholar]
- 433.Young V B, Knox K A, Schauer D B. Cytolethal distending toxin sequence and activity in the enterohepatic pathogen Helicobacter hepaticus. Infect Immun. 2000;68:184–191. doi: 10.1128/iai.68.1.184-191.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Yu J, Russell R M, Salomon R N, Murphy J C, Palley L S, Fox J G. Effect of Helicobacter mustelae infection on ferret gastric epithelial cell proliferation. Carcinogenesis. 1995;16:1927–1931. doi: 10.1093/carcin/16.8.1927. [DOI] [PubMed] [Google Scholar]
- 435.Zucca E, Bertoni F, Rogerro E, Bosshard G, Cazzaniga G, Pedrinis E, Biondi A, Cavalli V. Molecular analysis of the progression from Helicobacter pylori-associated chronic gastritis to mucosa-associated lymphoid tissue lymphoma of the stomach. N Eng J Med. 1998;338:804–810. doi: 10.1056/NEJM199803193381205. [DOI] [PubMed] [Google Scholar]