Abstract
Malaria remains one of the world's worst health problems with 1.5 to 2.7 million deaths annually; these deaths are primarily among children under 5 years of age and pregnant women in sub-Saharan Africa. Of significance, more people are dying from malaria today than 30 years ago. This review considers the factors which have contributed to this gloomy picture, including those which relate to the vector, the female anopheline mosquito; to human activity such as creating new mosquito breeding sites, the impact of increased numbers of people, and how their migratory behavior can increase the incidence and spread of malaria; and the problems of drug resistance by the parasites to almost all currently available antimalarial drugs. In a selective manner, this review describes what is being done to ameliorate this situation both in terms of applying existing methods in a useful or even crucial role in control and prevention and in terms of new additions to the antimalarial armory that are being developed. Topics covered include biological control of mosquitoes, the use of insecticide-impregnated bed nets, transgenic mosquitoes manipulated for resistance to malaria parasites, old and new antimalarial drugs, drug resistance and how best to maintain the useful life of antimalarials, immunity to malaria and the search for antimalarial vaccines, and the malaria genome project and the potential benefits to accrue from it.
“Malaria disaster in Africa” heads the letter from Kevin Marsh to the Lancet in September 1998 107, a disaster, he states, which is not just on its way but is already happening. This warning was prompted by a report from Trape and colleagues 188 on changing mortality patterns in three areas of Senegal over an 11-year period, areas representing three different transmission levels. In two areas, high-transmission and low-to-moderate-transmission areas, the risk of death had more than doubled, a significant rise in itself, but in the third area, of moderate transmission, the risk of deaths in children less than 5 years of age, the group at most risk, had risen by eightfold. The principal reason for this dramatic increase in deaths from malaria in this part of Senegal is attributed to the spread to West Africa of resistance to the first-line antimalarial chloroquine.
The deteriorating situation in Senegal is repeated in other malarious areas. More people are dying each year from malaria than 30 years ago, and malaria is returning to areas from which it had been eradicated and entering new areas such as Eastern Europe and Central Asia (Malaria Foundation International [http://www.malaria.org]). It is perceived as the world's worst health problem, but as the areas of the world which suffer the greatest burden of mortality in early childhood and clinical disease have the least developed systems of health reporting, the (repeatedly) quoted figures for annual deaths and clinical cases are best guesses 177). Thus, global figures for deaths from malaria range from 1.5 to 2.7 million each year, most of whom are children under 5 years of age and pregnant women 160, 180, 212). Almost all these deaths are caused by Plasmodium falciparum, one of the four species of malaria parasites in humans. The others are Plasmodium vivax, Plasmodium malariae, and Plasmodium ovale. A child is estimated to die from malaria every 12 or so seconds. This burden of mortality is not equally shared, falling most heavily on sub-Saharan Africa, where >90% of these deaths occur and 5% of children die from the disease before reaching 5 years; among the newborns of Africa an estimated 3 million suffer complications from low birth weight, including death, arising from malaria infection during pregnancy. Malaria is responsible globally for 500 million cases of clinical disease and presents a public health problem for 2.4 billion people, representing over 40% of the world's population in over 90 countries (Malaria Foundation International). Almost 10% of the world's population will suffer a clinical attack of malaria each year. Fortunately, most will survive after an illness lasting 10 to 20 days, but during the clinical illness, they will be unable to attend school or work, diminishing educational attainment and productivity. Why malaria is severe and life threatening in only a small proportion of cases is considered by Greenwood, Marsh, and Snow 66) and is thought to be determined by the interaction of a number of factors: these include the size of the infective dose of sporozoites, nutritional status of the host, level of acquired immunity, host genetic factors such as the presence of sickle cell hemoglobin, parasite features such as growth rate and drug resistance, and socioeconomic factors as basic as the availability of health care and education. It has been calculated that 76% more productive life years are lost from malaria than from all cancers in all economically developed countries, while funding of cancer research can be 10- to 50-fold greater (Malaria Foundation International).
The several factors which contribute to this gloomy picture are briefly summarized to set the scene and will be expanded later in the review. Since malaria is transmitted by the female anopheline mosquito (see below), a major strategy of control is to attack the vector with insecticides. Extended use, however, has led and continues to lead to the emergence of insecticide-resistant mosquitoes. The cost of control programs has forced their reduction or total abandonment in some regions. New breeding sites for mosquitoes are created by road building, deforestation, mining (especially open cast mining), irrigation projects, and new agricultural practices, all environmental changes which might be expected to be of economic benefit. Brazil provides a good illustration 27). In the 1950s in Brazil, control programs had reduced the 5 million malaria cases annually by 100-fold. Twenty years later, there were major but largely unplanned developments in the Amazon region; cattlemen, laborers, prospectors, and others migrated into the area, many of them having no immunity to malaria. In 1991, over 700,000 cases were reported 44. The steep rises in population in some malarious areas, together with migration from rural to more densely populated urban areas, have led to higher rates of transmission: as a consequence, some people with little or no immunity will be exposed to higher rates of infection, for example, in Karachi (Pakistan), Bombay (India), Lagos (Nigeria), Kinshasa (Democratic Republic of Congo), and Dar-es-Salaam (Tanzania) 21. Immigration of people from malarious areas to areas freed from the disease can lead to serious reintroductions. For example, development of gem mines in Cambodia brought large concentrations of miners into a jungle location with few medical services, and malaria extracted a heavy price for the gems in a fatality rate for the miners working for 2 to 4 weeks of 10 to 15%. Worse, miners returning from the mines to Thailand and Burma introduced malaria into areas free of malaria. Drug resistance, alluded to above in the context of chloroquine, a cheap and safe drug and the drug of choice in times past for treating acute malaria, is a spreading and growing problem in South America, Asia, and Africa. Indeed, in some parts of Asia there are parasites resistant to all the principal antimalarial drugs, creating major clinical challenges. For the major pharmaceutical companies, developing new antimalarials does not make economic sense—it is said that not a single major Western pharmaceutical company is currently developing new antimalarials. So the picture is not good, but nonetheless, there are a number of initiatives aimed at preventing the situation from becoming worse and then at improving it. These will be reviewed.
BACKGROUND
In 1998, malariologists celebrated the centenary of the demonstration that the female mosquito transmits malaria. Ronald Ross reported in August 1897 that the parasite could infect the female mosquito. The following year, he showed that the parasite completed its developmental cycle in the mosquito and, when that mosquito took another blood meal, it passed on the parasite (see reference 133 for a summary of the discovery of the life cycle of the malaria parasite). He later wrote that “the discovery was, perhaps, as really important as the discovery of America” 152. His discovery allowed a line of attack on the disease other than using quinine, an antimalarial. Notwithstanding the huge strides in biology and medicine in the 100 years after his discovery, which Ross could never have predicted, he might be surprised if alive today to discover how serious a problem malaria remains.
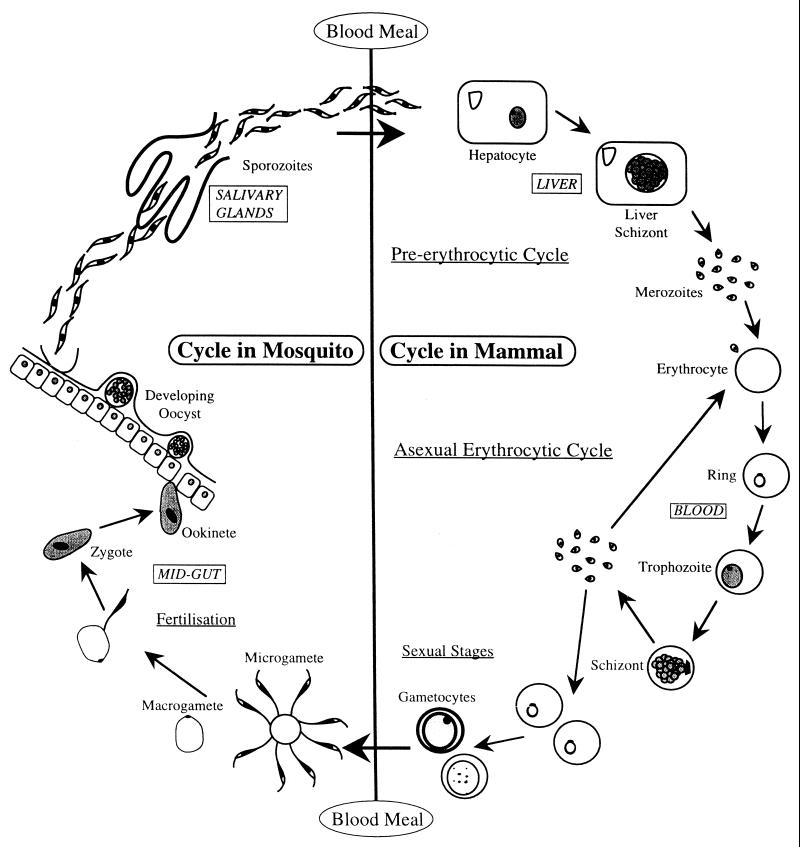
Briefly, the life cycle is as follows (Fig. 1). Sporozoites, thought to be less than 100 on each occasion 151, are released from the female mosquito's salivary glands, in her saliva, into the circulating blood of the host and within 30 to 45 min have entered hepatocytes. It is not clear how sporozoites squeeze through the sinusoid lining into the space of Disse (or, as might be the case, pass through Kupffer cells on the walls of the sinusoids 193) to reach the hepatocytes or the precise nature of the sporozoite-hepatocyte ligand-receptor interaction which enables the parasite to recognize the host cell. Peptides forming part of the major surface protein on the sporozoite, the circumsporozoite protein (CSP), have been suggested to interact with receptors on the hepatocyte 29, 167. Growth and division in the liver for the human malaria parasites take from approximately 6 to 15 days depending on the species: approximately 6, 10, and 15 days for P. falciparum, P. vivax, and P. ovale and P. malariae, respectively. At the end of the preerythrocytic cycle, thousands of merozoites are released into the blood flowing through the sinusoids and, within 15 to 20 s, attach to and invade erythrocytes. Recognition and attachment are via a receptor-ligand interaction, and at least for P. vivax and P. falciparum, the host and parasite molecules involved are different (reviewed in reference 60). In P. vivax and P. ovale, some of the sporozoites appear to develop for about 24 h before becoming dormant as a hypnozoite stage; this form can remain as such for months and even years until reactivated to complete the liver cycle, releasing merozoites into the blood to precipitate a relapse infection.
FIG. 1.
The life cycle of the malaria parasite in mammals.
The asexual erythrocytic cycle produces more merozoites that are released with the destruction of the red blood cell after 48 or 72 h for the human malaria parasites, depending on the species, and which then immediately invade additional erythrocytes. The asexual cycle usually continues until controlled by the immune response or chemotherapy or until the patient dies (in the case of P. falciparum). Most malaria parasites developing in the host's red blood cells grow in synchrony with one another, for at least some animal species apparently tuning into the host's circadian rhythms 73. There is no compelling evidence as yet that this is the case for human malaria parasites 73. Consequently, they complete schizogony together at the end of the asexual cycle, releasing pyrogenic materials which induce the characteristic fever spike and clinical symptoms. The morbidity and mortality associated with malaria are derived solely from the erythrocytic stages. A full description of all aspects of severe malaria is given in reference 210.
After invading red blood cells, eventually some merozoites differentiate into sexual forms (gametocytes) and, following ingestion by another female mosquito, will mature to male and female gametes in the blood meal. After fertilization, the resulting zygote matures within 24 h to the motile ookinete, which burrows through the midgut wall to encyst on the basal lamina, the extracellular matrix layer separating the hemocoel from the midgut. Within the developing oocysts, there are many mitotic divisions resulting in oocysts full of sporozoites. Rupture of the oocysts releases the sporozoites, which migrate through the hemocoel to the salivary glands to complete the cycle approximately 7 to 18 days after gametocyte ingestion, depending on host-parasite combination and external environmental conditions. All stages in the life cycle are through to be haploid, apart from the diploid zygote, which immediately after fertilization undergoes a two-step meiotic division, the resulting cell containing a nucleus with four haploid genomes 14. The sexual process and meiotic division following fertilization allow genetic recombination to occur, which is reflected in the genetic makeup of the sporozoites and together with mutations provides the raw material upon which selective pressures such as antimalarial drugs can work.
DIAGNOSIS
Conventional diagnosis still uses the skilled but laborious and time-consuming microscopic examination of thin and thick blood films stained with Giemsa's or Field's stain. Newly developed tests include the quantitative buffy coat method (Becton Dickinson, Sparks, Md.) for the fluorescent staining of parasites after an enrichment step for the infected erythrocyte (reported to be as good as thick films for P. falciparum but not for the other species 6; the ParaSight F (Becton Dickinson 157 and the Malaquick tests 91 (ICT Diagnostics, Sydney, Australia, based on the immunological capture of the P. falciparum histidine-rich protein 2 in whole blood; and the OptiMal (Flow Laboratories, Portland, Oreg.) assay, which is antibody-based detection of parasite lactate dehydrogenase 102. These antibody-based dipstick tests are still being evaluated. PCR-based diagnostic tests for human malarias have been developed 118, but these are more applicable to large-scale surveys than to clinical diagnosis. PCR has been especially effective at detecting submicroscopic levels of parasitemia. (See references 101 and 208 for an evaluation of these methods.)
MALARIA CONTROL AND PREVENTION
The world's malaria situations can be divided into three categories 211. First, there are areas where malaria transmission is intense and which to date have largely been unaffected by vector control programs, such as tropical Africa. The second category represents the malaria situation in most malarious countries in Asia and the Americas, where large-scale vector control programs are or have been operating—interruption of these programs or a gradual breakdown in the commitment to them leads to outbreaks of the disease. Into the third category are placed areas of rapid economic development and countries seriously affected by social disruption, both of which can lead to environmental disturbances, population movements, and the absence of health care infrastructure.
Malaria is a focal disease which differs in its characteristics from country to country and even within the same country. No single strategy is applicable for all situations, and implementing any of these may be problematic in an area: there has to be a regular assessment of each country's malaria situation. There are a variety of factors to take into account, including (i) the biological, anthropological, cultural, and social characteristics of the population; (ii) the intensity and periodicity of malaria transmission; (iii) the species of malaria parasites and their sensitivity to antimalarial drugs; (iv) the species of the mosquito vector, their behavior, and their susceptibility to insecticides (nearly 70 different species of mosquitoes are thought to be capable of malaria transmission, each with its own ecological requirements and behavioral characteristics); (v) the presence of social and ecological change; and (vi) the characteristics of the existing health services.
Before considering what is new in malaria control, it is worth briefly reviewing the long-established and much-used practices which form the backbone of antimalarial measures 24, 162. History shows that, given proper organization, sufficient funds, and the proper ecological context, e.g., Boston and Rome versus Democratic Republic of Congo, these can give good results in malaria control. The World Health Organization 209 suggests that there are three essential elements of malaria control. First is the selective application of vector control by, for example, reduction of the numbers of vector mosquitoes either by eliminating, where feasible, or reducing mosquito breeding sites; destroying larval, pupal, and adult mosquitoes; and reducing human-mosquito contact. Second are early diagnosis and effective and prompt treatment of malarial disease (which also reduce a source of parasites for infection of mosquitoes as well as reducing morbidity and mortality, in all areas where people are at risk, whatever the economic and social circumstances. The third element is early detection or forecasting of epidemics and rapid application of control measures.
While the value of environmental management in the form of filling in ditches; covering water containers; flushing irrigation channels; clearing ponds of weed growth, which allows the introduction into ponds of fish which eat mosquito eggs and larvae; and other measures to reduce breeding sites and mosquito breeding success is recognized, at the same time there might be major environmental changes which run counter to these efforts. Mining, logging, and land clearance for planting are three such operations which can have a rapid impact on the tropical environment. Extensive borrow pits which hold water are dug alongside new roads constructed for access to these kinds of developments, and the new roads often obstruct existing drainage, causing water to accumulate. Open cast mining by the nature of the operation creates new water catchment areas. Clear felling exposes thin fragile topsoil, which quickly erodes, creating water catchment sites, and in addition, the eroded topsoil can cause siltation in local rivers, leading to shallower river margins. Thus, new mosquito breeding sites emerge, and at the same time a workforce may be introduced from an area where malaria may be absent or infrequent and hence may lack an effective level of acquired immunity. In addition, the increased level of malaria transmission in the locality brought about by the changes can impact on the indigenous population. This is well illustrated by the 70-fold increase in the incidence of malaria in the Yanomami Indians in the Amazon with the introduction of logging and mining into their traditional home region.
Chemicals continue as the mainstay of mosquito control and broadly fall into five groups. Petroleum oils and derivatives sprayed onto water, forming a film, prevent larvae and pupae from breathing through the surface of the water. Introduced in 1921, Paris green (copper acetoarsenite) is another larvicide 24 which, as very small particles, is filtered out by the feeding larvae, which are poisoned. Natural constituents of the flowers of pyrethrum, pyrethrins, and later synthetic derivatives, the pyrethroids, form another group. Organochlorines, which include dichlorodiphenyltrichloroethane (DDT) and dieldrin; the organophosphates, such as malathion and temephos; and carbamates, such as propoxur, constitute the three remaining groups. The last two groups are relatively dangerous in handling, requiring specialist equipment for their use 24. The rationale for use of these chemicals is described in detail elsewhere 209, but in short the basis is indoor spraying with a persistent insecticide, i.e., it remains active on the sprayed surface for weeks or even months to kill or at least repel the adult female mosquito. The problems of resistance are well documented 24, 209 and have increased with time and use, from 2 species of mosquito being resistant to DDT in 1946 to 55 or more species being resistant to one or more insecticides 50 years later; some very important malaria vectors are included among this list. It is worth remembering that, where mosquitoes are resistant, they frequently still find DDT an irritant when they land on it, a sufficient irritant to deflect them from biting a person.
Not only can exposure to insecticides lead to resistant mosquitoes, but it may also result in modifications in mosquito behavior whereby mosquitoes and insecticide have fleeting if any contact 105, 207. Malaria control in the Amazon region is predominantly based on insecticide use inside dwellings (mainly of DDT) together with active searching for and treatment of cases with antimalarials (chloroquine, Fansidar, and quinine) (see below), but the results have been less than expected 105. Drug-resistant strains of malaria parasites seem to be widespread, and the normally endophilic (resting inside the dwelling after taking a blood meal) Anopheles darlingi is exophilic and rests inside for only a few minutes before and after feeding. Endophilic mosquitoes are thereby exposed to the insecticide by resting habits whereas exophilic mosquitoes are not exposed. In the coastal regions of Venezuela and Surinam, A. darlingi remains endophilic and DDT is effective 105. DDT remains in use as a residual spray in homes (it is not used for outdoor spraying) in Africa, Asia, and the Americas. Undoubtedly, DDT has saved hundreds of thousands of lives, but 120 countries are currently negotiating a treaty to ban the production and use of DDT by 2007. This ban is felt by some to be unjustified where there are inadequate alternative malaria control methods available at an affordable cost to poor tropical countries (see the discussion compiled by Taverne 186).
Recently, evidence has been gathered to show that malaria parasites play an important role in regulating the feeding behavior of their mosquito hosts to the enhancement of transmission 20, 147. For example, in mosquitoes infected with the bird malaria parasite, Plasmodium gallinaceum, duration of probing for blood was increased and their biting rate on a host doubled 153. In Papua New Guinea, Anopheles punctulatus mosquitoes infected with P. vivax or P. falciparum were more tenacious in their blood feeding behavior, were less likely to respond to disturbance, and had increased multiple feedings 89, 90. As biting rate is one of the principal entomological variables determining the rate of transmission 53, such change in mosquito behavior can impact on the epidemiology of the disease. Further, it has been found that mosquitoes located blood faster in mice infected with Plasmodium berghei than in control mice 154.
Antimalarial drugs form an important element in control programs in treating cases to remove a source of infection for feeding mosquitoes. Antimalarials are also used prophylactically. Drug resistance has emerged and is spreading, not least the resistance to chloroquine, the mainstay for treatment of P. falciparum. I shall deal with the current position with antimalarials below.
WHAT'S NEW IN MALARIA CONTROL?
Notwithstanding that the situation is very serious, some positive aspects can be identified in malaria control over recent years 24, 68, 174. According to the work of Greenwood 68, the consensus is that the most efficient malaria control comes from a team effort where malaria control is just one of the public health activities which make up primary health care programs within a region or country. The concern with insecticide resistance and the threat of the withdrawal of DDT have emphasized the need for the search for other control measures 174. Biological control of mosquito larvae with naturally occurring bacteria that synthesize potent larvicidal toxins has received less attention than might be expected, given that these bacteria (Bacillus sphaericus and Bacillus thuringiensis) have been used safely in the field for many years 140, 144. During sporulation, the bacteria produce as crystals protoxins which are toxic to some insect larvae after ingestion as food. The protoxins are solubilized in the alkaline pH of the larval midgut, where they are proteolytically activated and bind to specific receptors on epithelial cells, leading to cell lysis, and the larva stops feeding and dies. These are safe for animals and the environment. A good example of their use is the very successful control of the larvae of blackflies, the vector of the filarial parasite Onchocerca volvulus, which causes river blindness, with B. thuringiensis subsp. israelensis 81, 144. Although B. sphaericus and B. thuringiensis have the potential for controlling the mosquitoes Culex quinquefasciatus, Culex pipiens, and Aedes aegypti, their toxicity to Anopheles has been found to be marginal and variable 144. Currently, these mosquitocidal toxins have not had very widespread commercial use for a variety of reasons. First, the spores of the bacilli sediment rapidly from the larval feeding zones, a particular problem in the case of Anopheles larvae, which feed at the surface. Second, the spore crystal is sensitive to UV light. Third, the rate of killing with spores is relatively low compared with that by chemical insecticides. Finally, the toxins can be relatively specific to mosquito species, but most importantly, the formulations are up to three times more expensive than are chemical insecticides. The cloning of mosquitocidal toxin genes from B. thuringiensis and B. sphaericus has allowed the re-expression of a combination of toxins from both species in one recombinant cell in an attempt to broaden the target range of mosquitoes of the toxins and possibly produce a synergistic effect 96. Given the current public resistance to use of genetically modified organisms, obtaining permission for release of such modified bacteria into the environment may not be easy, notwithstanding the worthiness of the objectives.
As noted above, control programs have a central structure of antimosquito measures combined with identification and treatment of infected individuals. Treatment has to be readily available and accessible. Such an approach has been very successful in Southeast Asia in reducing the incidence of severe disease, but less so in Africa 209. Almost all antimalarials are primarily targeted at the asexual erythrocytic stages, which cause the morbidity and mortality of malaria. Sexual stages, gametocytes, if they are also present, will not be affected by some antimalarials, and hence transmission from these patients might occur for some weeks after treatment. For example, where treatment is in the form of a combination of chloroquine and Fansidar (pyrimethamine-sulfadoxine), which have little action on gametocytes, transmission may not be much affected. A new class of antimalarials (see below), the artemisinin family, not only controls the asexual erythrocytic stages which cause pathology but also significantly reduces the numbers of gametocytes and hence also reduces opportunities for transmission from the patients 143, 174. A trial in Thailand in which the prevalence of gametocytemia (prevalence of gametocytes in the peripheral blood) was assessed in children after treatment with either mefloquine, halofantrine, or artemesinin showed an average duration of gametocytemia to be 34.1 weeks with mefloquine, 16.7 weeks with halofantrine, and 3.9 weeks with artemisinin.
Protection of the individual from mosquitoes can be achieved through a number of methods including mosquito repellents, protective clothing, insect screens, siting of dwellings away from breeding areas, improvement of the design and construction of dwellings, and bed nets. One of the most encouraging recent developments in malaria control has been the finding that impregnation of bed nets and curtains with insecticides can significantly reduce morbidity and mortality 94. Choi et al. 34 concluded that studies in Asia and Africa in different epidemiological situations show that insecticide-impregnated bed nets reduce the incidence of clinical attacks by 50%. A trial in the Gambia 2 showed that insecticide-impregnated bed nets (the pyrethroid permethrin was used) reduced overall deaths by >50%. Further trials in other countries after the successful study in the Gambia did not show such a beneficial effect on mortality rates, but nonetheless, there was a reduction in overall child mortality of 17 to 33% 120, 176. Beneficial features of bed nets are that they are readily accepted by the population and that they have the high usage rate of 86%, even in the absence of expensive promotional programs. There is an argument that governments willing to invest money in residual insecticide spraying might be willing to redirect some of this expenditure into bed nets 120. Successful trials were conducted with free nets and insecticide at the village level 2, while other studies have shown that, where nets and insecticide are not free, the cost of reimpregnating the nets—an essential requirement—or even repair of nets, never mind replacing worn or torn nets, is often beyond the means of the population 30. In the Gambia, when a charge of $0.50 for insecticide was introduced, usage declined from >70 to <20% 30. In some communities, the concept of paying for a health delivery system may be new and not readily accepted, and therefore a sustained and monitored bed net program will require some level of subsidy 30. If the nets and/or insecticide is not manufactured locally, purchase uses valuable foreign exchange.
The long-term impact of bed net programs is a matter of some debate 189. Undoubtedly, the reduced level of exposure through bed net intervention is likely to have an adverse effect on the acquisition of immunity (see below), and any beneficial outcome is likely to be transitory in areas of high transmission 98. Greenwood has summarized the arguments 67, 68. The incidence of malaria rises with an increase in entomological inoculation rate (EIR), i.e., the number of bites from an infective mosquito per person per year, but only to a maximum rate, after which incidence levels off or even falls even though the EIR may continue to rise. Thus, vector control may have a measurable effect on morbidity and mortality only where the curve of EIR against mortality is rising, but if EIR is at the plateau, even a substantial reduction of EIR may not measurably reduce malaria. Trape and Rogier 189 conclude that it is premature to invest so much expectation in bed net programs in tropical Africa. In this region, where the EIR is high, it is unlikely that a sufficient reduction in EIR can be maintained long enough to reduce the burden of malaria on the whole community. On the other hand, Greenwood 67, 68 believes that it is premature to draw such pessimistic conclusions, arguing that there should be immediate beneficial effects and that this may be a long enough window for other control measures to be developed and introduced.
Another concern about the use of impregnated bed nets has been their impact on the development of acquired immunity in the population. Acquired immunity (see below) against clinical malaria is thought to develop gradually with time and to be a function of the frequency of infections. There might, therefore, be a concern that the period during which a child is at risk from clinical malaria might increase where bed nets are used 173, 189. If a child was protected by bed nets but later these were no longer provided or were not used, there might be a rebound effect of clinical disease when the child was exposed to highly infectious mosquitoes. There is some evidence that a reduced risk from clinical malaria is associated with the presence of multiple concurrent infections with P. falciparum of different genotypes 12. Thus, bed nets might reduce exposure and consequently the multiplicity of infections with a corresponding delay of acquired immunity. A study in Tanzania 58, 185 compared children sleeping with and those sleeping without impregnated bed nets. Although bed nets reduced anemia, the multiplicity of genotypes was not changed between the with-bed net and without-bed net groups; this suggested that the development of acquired immunity in these particular epidemiological conditions was not compromised by the nets.
It has been known for many years that mosquito numbers depend on climate 62, 63 and that monitoring meteorological variables may be helpful in predicting malaria epidemics. Through a better understanding of how the variables of rainfall, temperature, and humidity can influence mosquito populations, it is possible to make better-informed predictions of transmission rates 190. In addition, many mosquito species lay their eggs in specific aquatic habitats which often are characterized by an easily identifiable plant community 119, 149. Remote sensing techniques, such as local aerial photography and satellite-borne sensors to identify mosquito breeding sites, have considerable potential for bringing together climatic and vegetative variables for making predictions about future malaria epidemics and, in time, for antimalarial measures to be planned and implemented to good effect 74. The impact of global warming on malaria transmission is another area for concern 97. Small increases in temperature in temperate and subtropical areas where malaria is unstable or currently absent might markedly increase transmission, whereas stable-transmission areas might be little affected by a temperature rise.
TRANSGENIC MOSQUITOES
There is a developing interest in the possibility of using molecular genetics to manipulate the genomes of insect vectors such that they are no longer capable of acting as a vector. A principal focus of this approach is Anopheles gambiae, together with Anopheles arabiensis and Anopheles funestus, three of the most efficient malaria vectors in the world. The ambitious aim is to replace natural vector populations of mosquitoes with populations which are unable to support complete development of the malaria parasite. The strategy depends on progress in three areas 38: the identification of parasite-inhibiting genes, the availability of techniques for introducing such genes into the mosquito genome, and strategies for spreading these genes through the natural populations of the vectors. Inbred strains of A. gambiae that are refractory to infection by malaria parasites have been selected. Several different mechanisms whereby the mosquito inhibits establishment or development of the malaria parasite have been detected. There are good prospects for identifying and cloning genes controlling these antiparasite processes. In addition, a number of mobile genetic elements which could act as gene vectors have been identified and isolated 126. However, the response of the general public to the release of genetically modified mosquitoes into the environment might be adverse.
ANTIMALARIAL DRUGS AND RESISTANCE
Reviews of antimalarials, their history, their mode of action, and the development of resistance have been provided by a number of authors 56, 130, 145. In general, malaria is a curable disease, and if everyone has access to early treatment, nobody should die from it. For the past 50 years, there have been two main classes of antimalarial agents in use, the antifolates and the cinchona alkaloids or the quinoline-containing drugs. In most cases, these agents are targeted at the asexual erythrocytic stage of the parasite. The antifolates include the diaminopyrimidines, such as pyrimethamine and trimethoprim; the biguanides, represented by proguanil (cycloguanil) and chlorproguanil; and the sulfa drugs, including the sulfonamides and the sulfones. In the 1960s, it was discovered that the latter two drug types have synergistic activity with pyrimethamine or proguanil, the most frequently used combinations being pyrimethamine-sulfadoxine (Fansidar) and pyrimethamine-dapsone (Maloprim) 145. The quinoline-containing drugs include the cinchona alkaloids, quinine and quinidine, and the aminoalcohol quinine analogues mefloquine 203 (a 4-quinoline methanol) and halofantrine (a 9-phenathrene methanol), which are recent introductions. There are also the 8-aminoquinoline primaquine, which is used for its gametocidal effect and its action on the liver stage of P. vivax, and the 4-aminoquinolines, chloroquine and its relative amodiaquine.
In addition to mefloquine and halofantrine, newer therapies in development include antibiotics such as tetracyclines, doxycycline, and azithromycin, which is closely related to erythromycin; atovaquone, which is a hydroxynaphthoquinone 11; pyronaridine, which was developed in China 20 years ago as a derivative of mepacrine; and derivatives 59 of artemisinin (qinghaosu), the active antimalarial principle in the leaves of the wormwood plant which was isolated in 1972 26. Artemisinin has been used in traditional Chinese medicine for centuries for the treatment of falciparum and vivax malaria. Artemisinin itself is poorly absorbed, and therefore a number of derivatives have been prepared and evaluated: three semisynthetic derivatives are in use, water-soluble artesunate and two oil-soluble compounds, artemether and arteether. These compounds are widely used in China and Southeast Asia. Artemisinin derivatives are the fastest-acting antimalarials available 202. When they are used by themselves, there is a relatively high recrudescence rate, but in combination with other antimalarials, particularly mefloquine, complete cures have been recorded 205. The main concern with the lipid-soluble derivatives is related to their toxicity in animals 22, but similar toxicity has not become an issue with humans.
With the possible exception of artemisinin derivatives, resistance to all antimalarials has been recorded 201, 204 but has developed at different rates for different compounds. Quinine, the active ingredient from the cinchona bark, introduced into Europe in the 17th century from South America and isolated by the French chemists Caventou and Pellitier in 1820, has had the longest period of effective use, but resistance has been reported 201. Nonetheless, it remains an important member of the antimalarial armory. Chloroquine, originally synthesized by German chemists in the mid-1930s, after the Second World War rapidly became the drug of choice both for treating acute falciparum malaria and for prophylactic purposes. It is cheap, at around $0.08 per treatment, and in 1994 was said to be the second or third most widely consumed drug in the world after aspirin and paracetamol 57. Chloroquine too has had a reasonable period of effective deployment, albeit the first reports of chloroquine resistance appeared in 1957 and more significant reports appeared in 1961 199. Initially spreading through South America and Southeast Asia, chloroquine resistance by P. falciparum reached East Africa, from the east, in 1978 and had crossed the African continent by 1985 15. Chloroquine resistance has also been found in P. vivax 159. Undoubtedly, the low cost of chloroquine and its ready availability contributed to the development of resistance (see below). Resistance to the antifolates (dihydrofolate reductase [DHFR] inhibitors) developed more quickly after their introduction, although the combination of pyrimethamine with sulfa drugs delayed the spread of resistance to pyrimethamine. Resistance to mefloquine was reported even before the drug was brought into routine use in Southeast Asia, and its usefulness in this area is now severely compromised.
The mechanisms of resistance in the antifolates such as pyrimethamine and the sulfa drugs are well known. Malaria parasites meet their folate needs by de novo synthesis. The two main targets of the antifolates in the folate synthesis pathway are the enzymes DHFR and dihydropteroate synthase 56. Pyrimethamine and proguanil target plasmodial DHFR, while the sulfur drugs target dihydropteroate synthase, hence their synergistic activity when used in combination. Resistance to the antifolate drugs is the result of point mutations in the substrate binding site of the target enzymes 42, 139. At the present time, there are seven identified point mutations occurring in the DHFR gene which are associated with reduced drug binding capacity in resistant DHFR strains of P. falciparum. For example, a serine-to-asparagine change at codon 108 is the critical mutation found in all pyrimethamine-resistant isolates 41, 131.
Mechanisms of resistance to chloroquine have been much more difficult to unravel, and indeed the mode of action of this antimalarial is not beyond debate. Chloroquine is thought to accumulate to high levels in the food vacuole of the asexual erythrocytic malaria parasite, where it interferes with the polymerization of heme. The parasite derives a substantial part of its nutrition from the digestion of the host cell's globin in hemoglobin, leaving heme as a by-product. Since heme is toxic, the parasite polymerizes it to the harmless hemozoin or malaria pigment. Wellems and colleagues 181, examining a cross between a chloroquine-sensitive and a resistant strain of P. falciparum, have found two genes on chromosome 7, cg1 and cg2, which have a complex polymorphic mutation pattern when the chloroquine-sensitive (HB3) and the chloroquine-resistant (Dd2) clones of P. falciparum are compared. cg2 codes for a transmembrane protein. The chloroquine-resistant Dd2-type polymorphisms in cg1 and especially in cg2 were significantly associated with chloroquine resistance when tested against chloroquine-resistant parasites from Africa and Asia but not when tested against chloroquine-sensitive parasites from these two continents. Immunoelectron microscopy indicates that the localization of CG2 protein is not inconsistent with its being a transporter protein. It would not be unreasonable to speculate that chloroquine resistance is associated with a transporter protein which prevents the antimalarial from reaching lethal concentrations in the cytoplasm or food vacuole of the parasite. For a full discussion on the possible mechanisms of chloroquine action and resistance, see the work of Ginsburg and colleagues 64.
The widespread and in some cases rapid development of drug resistance requires that measures be taken to prevent such being the fate of the remaining effective compounds and any new compounds which might be produced in the future. White and Olliaro 204 review the scenarios which might promote or delay or prevent emergence of drug resistance. Ideally, drugs should become available only when needed and should be given to a patient who is compliant with respect to the treatment regimen. A number of factors have been identified as playing a role in the emergence of drug resistance. The pharmacodynamic and pharmacokinetic properties of the antimalarials are important 196. Drugs which have a long elimination half-life are potentially more likely to select for drug resistance than those which are rapidly eliminated 196. In the former situation, some parasites are likely to be exposed to the drug at suboptimal levels for extended periods of time; these suboptimal levels are sufficient to inhibit but not to kill the parasite, thereby providing conditions which may promote selection of resistant parasites. Ironically, antimalarial drugs with long half-lives were seen as desirable because they could be used prophylactically and taken on a weekly basis rather than daily and when used for treatment could be given in a single dose 196. Most of the commonly used drugs such as chloroquine, pyrimethamine, the sulfur compounds, and mefloquine fall into this category. Drugs which are rapidly eliminated (and therefore require multiple treatments over a period of time) will exert minimal selection pressures, as they remain at subtherapeutic antimalarial levels for a short period of time 196; these are conditions where resistance will develop more slowly.
Another factor determining the rate at which resistance may develop is the nature of the mechanism of resistance. Where resistance is conferred by a single mutation, as was noted for resistance to the antifolate drugs, then resistance may develop very suddenly. For example, resistance of P. falciparum in Thailand to the synergistic combination of pyrimethamine and sulfadoxine took only 5 years to develop 132. Frequency of reinfection, i.e., intensity of transmission, can influence the rate at which resistance emerges 204. Thus, if a patient is reinfected after recently being successfully treated, but when subcurative levels of the drug remain in the blood, conditions prevail which can exert selective pressure on the reinfection parasites. Mutations resulting in resistance are at low frequency, and therefore the chance that a resistant parasite will emerge from the reinfection will generally be higher where the parasite load is higher.
With this information, how can the life of existing antimalarials be extended? In the choice of drug combinations, the drugs should have similar pharmacokinetics and pharmacodynamics and not interact in any adverse way such that additional problems of toxicity might occur. It is preferable that known drug resistance mechanisms in each of the drugs being combined are not linked. A number of drug combinations have been explored 200. The combination of artemisinin derivatives with mefloquine has been very successful in southeast Asia 84, 142. Artemisinins are so potent that a single dose can reduce the parasite load by a factor of 104, and over a 3-day course the parasite numbers can be reduced by 108, leaving an average of 103 and a maximum of 105 parasites. Consequently, there will be a relatively low parasite load remaining for the second drug, mefloquine, to deal with when at its maximum therapeutic level. These conditions reduce the chances that a drug-resistant mutant will escape. In Thailand, the use of this combination therapy against P. falciparum has not only helped with the serious problem of mefloquine resistance in the parasite but has apparently led to a drop in the level of mefloquine resistance in the area (N. J. White, personal communication).
Particularly in the context of chloroquine resistance in P. falciparum, the spread of resistance from Southeast Asia to the eastern seaboard of Africa and then its remorseless westward move to the west coast of the African continent have been described elsewhere 199. Spread occurs via the mosquito vector; two aspects of this should be briefly mentioned. First, most patients are infected with mixtures of genetically distinct clones in areas where malaria is highly endemic. When mosquitoes take blood from such patients, there is a strong possibility that cross-mating will occur between such clones. The process has been demonstrated both in crossing experiments in the laboratory and in naturally infected wild-caught mosquitoes in the field 3, 4, 194. Following such cross-mating, recombination generates parasites with genotypes different from those of the parental populations. Thus, because there is frequent recombination in nature, parasites with the potential to exhibit a range of responses to different drugs will be generated.
The second aspect to consider is whether resistance to antimalarial drugs might bring with it other changes in the biology of the parasite which might promote or diminish its survival in the host and transmission to another host. Resistance to antimalarials was low before their widespread use, suggesting that resistant mutants were usually eliminated in the absence of the selection pressure of antimalarials, i.e., they were at a selective disadvantage compared with the drug-sensitive parasites. This suggestion is supported by the observation that, in a few cases where drug pressure in the field has decreased, there is some evidence that drug resistance has also decreased 88. These observations could indicate that resistant parasites are less well transmitted than are sensitive parasites 88. Laboratory studies, however, with rodent malaria parasites, such as Plasmodium yoelii nigeriensis 82, and recent field studies have found that infectiousness to mosquitoes was higher in chloroquine-resistant parasites than in sensitive parasites 70. Increased infectiousness of gametocytes to a mosquito does not automatically mean that the parasite will have an increased chance of being transmitted to another host. In order to be transmitted to another host, the mosquito must survive long enough for the parasite to complete its life cycle and produce viable sporozoites. There is, however, evidence that malaria parasites increase the mortality of mosquitoes and that the increase is commensurate with the number of malaria parasites 32. Therefore, drug resistance may increase infectiousness to the vector but this infection may also lead to premature death of some mosquitoes before they can transmit. Which of these conflicting pressures is more important and determines whether drug resistance promotes transmission of the parasite compared with drug-sensitive strains is not certain, but the fact that, when drug pressure is withdrawn, the incidence of drug resistance can diminish suggests that drug resistance carries a cost rather than a benefit (see the work of Koella 88).
Notwithstanding the major problems with drug resistance briefly discussed above, and the continued importance of malaria across the tropics, the fact is that no major pharmaceutical company deems it worth their while to invest in the development of new antimalarial compounds. The potential market is, of course, very large, comprising almost half the world's population living in areas of endemicity together with a sizable market of 20 to 30 million people living in Europe and North America who visit malarious areas on business or for pleasure 57. As already noted, chloroquine, which can be used both for treatment, as it is rapidly acting, and prophylactically, has the advantage of being cheap. Any replacement compound has to be equally cheap, otherwise it will be limited to use for travelers or as a second-line drug in a limited number of hospitals. The relatively recent introductions, such as mefloquine and halofantrine, are too expensive. For example, mefloquine costs about 10 times as much as chloroquine, and halofantrine costs 20 to 30 times as much. Regrettably, the major stimulus for development of new antimalarials has been war in tropical or subtropical regions of the world, in particular the Second World War and the conflict in Vietnam.
VACCINES AGAINST MALARIA
The foregoing gloomy picture of malaria and the armory of methods to combat its ravages, mainly in indigenous populations, has led some commentators to conclude that vaccination against P. falciparum and P. vivax is the method of intervention with the greatest potential to reduce the morbidity and mortality associated with severe malaria in areas of intense transmission 112 and even contribute to eradicating the disease, which is not an impossibility 67: the success of early vector control programs suggests that an optimistic view is not unreasonable. As with antimalarials, there is little commercial incentive for industry to invest in development of vaccines against any human parasite, including malaria.
A malaria vaccine represents a major challenge, even with unlimited resources to devote to the task. In this section, I will outline the nature of this challenge, the steps forward (and backward) toward meeting the challenge, and some of the current endeavors and favored strategies. In this discussion, I will be referring to P. falciparum unless stated otherwise, because almost all of the effort is directed to this, the most dangerous of human malaria parasites. As previously mentioned, most residents living in areas of endemicity and exposed to P. falciparum malaria on a regular basis do develop an acquired immunity to malaria, and with few exceptions, the process of acquisition of immunity starts in babies and infants and is sustained into later life. Most of these young people survive infection with P. falciparum, but in about 1 to 2% of infections, severe malaria develops and can be fatal 66. It would be a very tall order to produce a vaccine against a parasite for which natural exposure does not stimulate a protective immune response. Although protective immunity does develop against P. falciparum, such protection is not easily won 136. In areas of endemicity, the pattern is for babies and infants up to the age of about 5 years to be at risk of dying from the disease, but parasite prevalence, parasite density, and the number of clinical episodes do decline progressively with increasing age 69, 109, 110. Even in adulthood, however, immunity is never complete 109, 110, and during the transmission season, it is not unusual for the occasional clinical episode to occur and for parasites to be detectable in the blood even when the patient is clinically well. It might be that, after first infections in adulthood, an effective immunity develops more quickly 5, 7, 83. As a result of government policy of economic development in Indonesia, there has been a massive movement of people from heavily populated areas, often free of malaria, to the outer islands, where malaria is endemic. Many of these transplanted peoples have set up agricultural schemes involving irrigation, creating ideal breeding sites for mosquitoes. Consequently, malaria exploded among these peoples, for whom it was observed that among the adults there were no fatalities after 2 years of residence, suggesting that lifesaving immunity developed in 2 years in adults compared with 5 years in small children 5, 83. Current thinking is that natural acquired immunity is normally specific for a species of malaria parasite. People who move out of an area of endemicity for an extended period appear to lose their immunity, suggesting that repeated exposure is necessary to maintain resistance 110. Immunity is apparently, therefore, invariably incomplete, and to think in terms of a vaccine which gives total protection, we are looking for a level of immunity never achieved by natural exposure. If natural immunity could be mimicked, then vaccination would prevent severe malaria and malaria-related deaths but would not give complete protection.
A vaccine to provide immunity comparable to that naturally acquired in areas of endemicity would have to be targeted at the asexual intraerythrocytic stage of the infection, because it is this stage which is responsible for the pathology and clinical symptoms of the disease: prolonged protection by this vaccine would depend on regular boosting by reinfection. (In general, there is a correlation between high parasitemia and disease severity.) Such a vaccine may be of little value to the traveler to a malarious area. He or she would ideally require complete protection, and that would be obtained by inducing protection which prevented the parasite from either gaining entry to the hepatocyte or completing its development in the liver. Nonetheless, a partially effective preerythrocytic vaccine may be of some value to residents of areas of endemicity insofar as a reduced number of merozoites emanating from the liver will extend the period before the asexual parasites in the blood build up to numbers causing pathology, giving the host an additional period in which to mobilize the immune response. Thus, there are two principal targets for a malarial vaccine—the preerythrocytic stage (sporozoite and hepatic stages) and the asexual erythrocytic parasites. There is a third target which some investigators are pursuing, and that is the sexual or transmission stages, with the aim of producing a vaccine which would prevent sexual stages taken up by a feeding female mosquito from completing their development in the mosquito and thereby prevent the parasite being transmitted to another host, limiting the spread of the parasite 109. Since this transmission-blocking vaccine is an unusual vaccine in that it does not directly protect the vaccinee, it has been called an altruistic vaccine.
The search for vaccine formulations has, to some extent, gone hand in hand with attempts to understand better the mechanisms of protective immunity induced by infection. Such knowledge might indicate those protective mechanisms which a vaccine might preferentially induce and also indicate which are the major antigens for consideration as vaccine candidates. Further, it is clear that some of the pathology associated with acute malaria has an immunological basis, and therefore a vaccine should not induce pathological reactions directly during the vaccination process or leave the vaccinee at a greater risk from such harmful responses if a natural challenge infection follows vaccination. A difficulty of investigating immune mechanisms in human malaria is that the human parasites are largely host specific and will infect only people and a few nonhuman primates and monkeys. The availability, expense, and ethical considerations of using nonhuman primates require that much of our knowledge comes from rodent malarias. A look at the life cycle of the parasite might suggest particularly vulnerable points for immune attack. Extracellular stages would be more accessible to immune attack than would intracellular stages: these are the sporozoites introduced into the blood by the feeding mosquito and which circulate for up to 30 to 40 min, the merozoites released from hepatocytes and erythrocytes at the end of the preerythrocytic and asexual erythrocytic cycles but thought to be extracellular for only 15 to 20 s before attaching to and invading erythrocytes, and the male and female gametes which are released when the gametocytes mature in the midgut of the mosquito after a blood meal. Changes to host cell surfaces as a result of parasitization could make them recognizable by the host's immune response 109. Little is known about changes to the surface of hepatocytes following parasitization, but there is some evidence that parasite-derived peptides might be located in or on the cell surface. For example, cytotoxic T cells have been identified which recognize a peptide from the CSP on malaria-infected hepatocytes (see “Preerythrocytic Vaccines” below) 198. CSP is a major protein on the surface of sporozoites.
Changes to the red blood cell surface after parasitization have been reported previously 13, 25. For example, in P. falciparum and a few other species, in the last third of each asexual cycle the infected erythrocytes bind (cytoadhere) to endothelial cells lining postcapillary venules and as a result stop circulating in the peripheral blood. This process is known as sequestration. The infected erythrocyte remains bound until schizogony is completed, whereupon the released merozoites rapidly invade more red blood cells. A major parasite-derived molecule or antigen becomes detectable on the surface of the infected erythrocyte as the asexual parasite develops, and this molecule mediates binding of the infected erythrocyte to receptors on the endothelial cells 13. (There are likely to be other cytoadherent molecules as yet not characterized. In P. falciparum, the cytoadherent molecule is known as PfEMP1 (P. falciparum erythrocyte membrane protein 1). There are a number of host cell receptors involved (intercellular adhesion molecule 1, CD36, thrombospondin, vascular cell adhesion molecule 1 [reviewed in reference 13), and different populations of P. falciparum may use different combinations of these receptors. The immune response to PfEMP1 is discussed below. Gametocytes of this species also are sequestered during their extended period of development of 9 to 10 days, and this too appears to be mediated by PfEMP1 150. A surface antigen is also exposed on the red cell surface in other species, for example, in the monkey malaria parasite Plasmodium knowlesi, where sequestration does not occur; the function of such a molecule in these is not known 1. Other prospective targets of immune attack would be molecules involved in the recognition of, attachment to, and penetration of the host cell.
Acquired Immunity
A brief resume of protective immune mechanisms to the malaria parasite following infection and reinfection is as follows. There is considerable uncertainty about mechanisms in vivo in humans.
Ironically, understanding of the immune mechanisms directed against the preerythrocytic stages has come from vaccination experiments, initially with animals and later with humans 125, rather than from observations of natural or experimental infections. Very strong resistance to a challenge with viable sporozoites is induced with irradiated sporozoites of P. berghei 124 and P. yoelii 197 in mice and of P. falciparum 36 and P. vivax 36 in humans. The irradiated sporozoites are able to invade hepatocytes but do not complete their development in the liver 146, and it is thought that this period, albeit shortened, of metabolic activity is important for mobilizing protective immunity. In these murine systems, the degree of immunogenicity of the irradiated sporozoites varied with the inbred strain of mouse and species of rodent malaria agent (reviewed in reference 183). The protection mechanisms induced by irradiated sporozoites were investigated, and initially the focus was on the role of protective antibody. Serum from immunized mice passively protected mice against a sporozoite challenge, probably by blocking invasion of the hepatocytes by the sporozoites 141. Subsequently, it was found that mice lacking B cells but not T cells 33 could be immunized with irradiated P. berghei sporozoites, which demonstrated that the antibody-independent (cellular) as well as antibody-dependent immune mechanisms are induced by the irradiated sporozoites. Much work has gone into investigating the nature of the antibody-independent responses. It has been found that primarily CD8+ T cells but also CD4+ T cells mediated the protection, by cytotoxic activity directed at the infected hepatocyte, and by secreting gamma interferon which, in turn, induces nitric oxide-dependent killing of the parasite within the hepatocyte 111, 121.
It is very uncertain to what extent the mechanisms of protection against the preerythrocytic stages induced by vaccination with irradiated sporozoites are induced by natural infection. In areas of endemicity, an antibody to sporozoites is detectable in residents from about 10 years of age 122, and it is reported that serum containing such antibodies can inhibit invasion of hepatoma cells by sporozoites 121. Similarly, using in vitro systems there is evidence of cytotoxic CD8+ T cells 50, 76 in the peripheral blood of immune adults.
Immunity to the asexual erythrocytic stages following infection is multifactorial. It is uncertain to what extent the wealth of information derived from animal models and in vitro assays can be applied to malarias in people. Antibody has an important role. Numerous experiments with animal models showed that passive protection with serum was possible. Cohen, McGregor, and Carrington 37 and later Druihle and colleagues 17, 18 controlled a patent P. falciparum parasitemia by passive transfer of immunoglobulin G from immune adults. The question is how is the protective antibody exerting its protective effect? In different host-parasite combinations and at different times after infection or reinfection, the mechanism might be different. Thus, roles reported include blocking invasion of red cells by merozoites, opsonization of merozoites and infected erythrocytes promoting their uptake by phagocytic cells, antibody-dependent cellular cytotoxicity, agglutination of merozoites, preventing sequestration of P. falciparum, and neutralizing malaria toxins 100, 136. As some of these proposals are based solely on in vitro observations, it cannot be assumed that they have a similar role in vivo.
Cellular antibody-independent mechanisms of immunity to the asexual erythrocytic parasites are also reported elsewhere 95. Cytokines such as tumor necrosis factor alpha and most importantly gamma interferon play a major role. In a rodent model, there is a switch from antibody-independent immune mechanisms during the acute blood parasitemia to antibody-dependent mechanisms as the infection becomes chronic 95, 134, 187, 192, but it is not clear if this progression occurs in human infections. Exactly how the parasite is affected by the immune response, either within the red cell or as the merozoite passes from one red cell to another, again is not certain.
Finally, protective immunity to the sexual stages is thought to be induced by infection; again, there is evidence from different host-parasite combinations that both antibody-dependent and antibody-independent mechanisms can play a role 76. These anti-sexual-stage protective factors come into effect in the blood meal in the mosquito midgut and may prevent fertilization from occurring.
The foregoing summary indicates the complexity of the immune response to malaria parasites. There are a number of other features of malaria parasites which make vaccine development particularly difficult. Unlike, for example, most viral vaccines, malaria vaccines almost certainly must be subunit vaccines because, although it is possible to grow all stages of the malaria life cycle in vitro, it is not feasible presently to envisage growing any stage of the life cycle on a mass scale to provide material for a whole-parasite vaccine. Protozoa are relatively complex, and in malaria parasites, it is estimated that there are 5,000 to 7,000 proteins. Immunity to each stage of the life cycle is largely specific, probably because each stage produces its own novel antigens. Therefore, selecting targets for vaccine-induced immunity and the corresponding peptides with which to induce that immunity has been extremely difficult. Many of the immunodominant antigens in natural infections have not been shown to be targets of protective immunity 79. Many antigens recognized by the immune response show allelic polymorphisms 136. Antigenic diversity is compounded by the fact that at least one antigen, PfEMP1, and its possible homologue or analogue in a small number of other species such as P. knowlesi and Plasmodium chabaudi undergo clonal variation during the course of infection and contribute to the parasite's immune evasion mechanisms 135, 156, 172. Thus, where the antigen included in the subunit vaccine shows allelic polymorphisms or clonal variation, the conserved regions will be selected as being parts of the molecule concerned with a vital biological function and where variation would be unlikely. It is assumed that the optimally effective vaccine will induce appropriate humoral and/or cellular responses against several key parasite antigens expressed during various stages of the life cycle. There are two important additional complicating factors which hinder progress. The first is that animal models are not ideal for evaluating vaccine candidates to be used in humans, albeit valuable information has come from animal models. Second, there is no suitable in vitro assay for measuring levels of protective immunity in vivo 113, for example, being able to relate levels of antibody to a specific antigen to the level of protection in vivo. Therefore, the only way of determining, at present, the efficacy of a vaccine candidate is to set up human trials, involving natural or experimental challenge, which are very expensive and take a long time to complete and evaluate.
It is worth repeating that severe malaria, predominantly manifest as anemia and cerebral malaria, can have an immunological component, something which a vaccine should not induce 114. The pattern of disease in African children, usually in children under 5 years of age, is linked to the levels of transmission, as well as genetic factors and probably virulence of the infecting populations of P. falciparum 106, 175. In areas where transmission rates are low (<15 bites/individual per year), severe anemia occurs in children around 2 years old and cerebral malaria occurs in children 3 to 4 years old. This suggests that there might be a developmental process of susceptibility to cerebral malaria, linked to low transmission rates and a corresponding slow buildup of immunity. In high-transmission areas (>100 bites/individual per year), severe anemia occurs earlier and cerebral malaria is very infrequent 106, 112, possibly because the children become immune before the age at which cerebral malaria occurs. Therefore, assumptions that a vaccine will limit the incidence of severe malaria by limiting parasitemia may be incorrect—some complications might be worsened by vaccination. Malaria vaccines, like other malaria control measures, have the potential for upsetting a balance between the malaria parasite and its host for the worse.
Once the target antigens have been identified, with the coding genes being cloned and sequenced, safe and potent delivery systems will be required. Adjuvants for use in humans are an issue. In experimental animals, very powerful antimalarial immunity has been induced, for example, with crude or purified blood-stage antigens with complete Freund's adjuvant 23, 115. Complete Freund's adjuvant is not acceptable for human use: the only licensed adjuvant for use in disease control is alum, and to date this, in the context of candidate malarial vaccines, has not induced the level of response thought to be required for consistent protection. A number of novel adjuvants and delivery systems are under investigation 54. There has been considerable promising progress in nucleic acid vaccines using plasmid DNA encoding various malaria genes 54.
Preerythrocytic Vaccines
The first effective preerythrocytic vaccines used radiation-attenuated sporozoites. Introduced by allowing the infected and irradiated mosquitoes to feed on volunteers, irradiated sporozoites of P. vivax and P. falciparum induced complete protection 36. The protection was species specific but appeared to be independent of the parasite strain 36. This vaccine would be ideal for travelers to malarious areas because it completely prevents disease. It is completely impractical, however, because vaccinating each individual requires thousands of infected mosquitoes. From human vaccination studies and similar animal studies 125, a protein on the surface of the sporozoite was identified as a target for vaccine-induced immunity. The coding gene in P. falciparum was cloned and sequenced in 1984 46. The protein, known as the CSP, is the dominant protein on the sporozoite and contains, in the center portion of the molecule in P. falciparum, the 4-amino-acid sequence NANP (Asn-Ala-Asn-Pro) repeated more than 40 times. The repetitive region is conserved within species but varies among species. Synthetic and recombinant vaccines based on the B-cell epitope NANP have been tested in phase I and II trials with limited success 54. In the early trials, however, with alum as adjuvant, two individuals with the highest antibody responses were completely protected, indicating CSP to be a viable vaccine candidate. Increasing the complexity of the immunogen combined with novel adjuvants protected six of seven volunteers against sporozoite challenge 179. The antigen, RTS, S, is a hybrid containing the central NANP repeats and most of the C terminus of CSP fused to the hepatitis B virus surface (S) antigen in a complex adjuvant mixture based on monophosphoryl lipid A and QS21 (SBAS4). Field trials with this combination in the Gambia have shown that the formulation gives good short-term protection (G. A. T. Targett, personal communication). A vaccine targeting the immune response to the sporozoite ligand which binds to a receptor on the hepatocytes is being pursued. CSPs across different Plasmodium species have two stretches of amino acids which are highly conserved, named region I and region II 168, 169, and hence these might be predicted to be possible parasite ligands for binding to hepatocytes. Some evidence suggests that this is the case and that they might be vaccine targets 168. A synthetic peptide representing region I bound specifically to hepatoma cells in vitro, and antibodies to the region inhibited invasion of hepatoma cells by sporozoites 167, 169. Thus, a sequence in the CSP has been identified as a binding ligand and is being examined along with a second sporozoite surface protein (SSP2) which has sequences homologous to region II in CSP. Region II contains a well-known cell-adhesive motif, and peptides representing region II inhibited both CSP binding to hepatoma cells and sporozoite invasion.
The protective response following immunization with irradiated sporozoites, as noted above, included antibodies and protective CD4+ and CD8+ T cells exerting their effect as cytotoxic cells and releasing cytokines. Low levels of circulating malaria-specific CD8+ T cells in humans suggest that boosting of these CD8+ T cells with a vaccine might enhance natural immunity 138. Efforts continue to identify the peptides recognized on the infected hepatocytes by the cytotoxic cells as potential vaccine candidates. Results to date indicate that protective T cells may recognize epitopes from the CSP, SSP2, and other liver-stage-specific antigens such as liver-stage antigen 1 51. Murine models, such as P. yoelii, have indicated the potential of liver-stage antigens in a multicomponent vaccine, and the goal will be to develop a vaccine containing sporozoite surface proteins and the proteins expressed early in the development of the liver stage. Naked DNA is being developed as a delivery strategy for this stage vaccine because it might be a particularly useful means of inducing CD8+ T cells 71, 72, 195. For example, immunization of mice with a plasmid carrying DNA coding for the CSP of P. yoelii induced extremely high levels of anti-P. yoelii CSP antibodies and cytotoxic T lymphocytes 161. This immunity protected up to 83% of mice challenged with P. yoelii sporozoites and was abolished by removal of CD8+ T cells, by treatment with antibody to gamma interferon, and by prevention of nitric oxide production. Hill and colleagues have now shown that priming a mouse with DNA coding for a preerythrocytic-stage antigen and boosting with a recombinant poxvirus expressing the same malaria antigen (so-called prime-booster strategy) resulted in much higher levels of cytotoxic T lymphocytes 158. This approach is currently being tested in humans.
Blood-Stage Vaccines
It has long been known that crude blood-stage vaccines can induce strong resistance in animal models; for example, in the monkey malaria parasite P. knowlesi in an abnormal and very susceptible host, the rhesus monkey, a crude merozoite vaccine in complete Freund's adjuvant gave very good protection against both homologous and heterologous strain challenges 117. The challenge now, as outlined above, is to identify within these crude complex mixtures those antigens essential for induction of protection. A number of candidate antigens are under evaluation, a proportion of which have been found using monoclonal antibodies 80. Monoclonal antibodies raised to rodent malaria and human malaria parasites, especially P. falciparum, were tested for protective activity. In the case of the rodent malaria agents, this was in protection tests of passive transfer of the antibody into naive mice, and in the case of P. falciparum, those showing growth-inhibitory activity in vitro were deemed to be protective, but as noted above, antibodies active in vitro may not be so in vivo. The molecules recognized by these protective antibodies were determined, and in a number of cases the coding genes were cloned and sequenced as described below.
One of the front-runners for a blood-stage vaccine is merozoite surface protein 1 (MSP-1), and this was initially described with a protective monoclonal antibody to P. yoelii 80. The affinity-purified protein induced some protection in mice. Homologous proteins were identified in other species with molecular masses of between 190 and 230 kDa. The candidacy of this molecule in P. falciparum was established when the native protein was shown to protect squirrel monkeys completely 165. MSP-1, the major merozoite surface protein, is synthesized by the intracellular schizont as a 185- to 205-kDa precursor protein. On the released merozoite surface, it is present in the form of a complex of polypeptides following the proteolytic processing of the precursor 16, 40, 49; only the carboxy-terminal 19 kDa (MSP-119) remains on the surface and is carried into the newly infected red blood cell 16. This fragment is a conserved region of MSP-1, has two cysteine-rich epidermal growth factor-like domains, and is thought to play a crucial role in the process of invading the red blood cell. A monoclonal antibody which inhibits the growth of P. falciparum in vitro targets the first of the epidermal growth factor domains 31. Immunization experiments with mice and Aotus monkeys with recombinant proteins from the MSP-119 of P. yoelii and P. falciparum, respectively, were successful, and the native form was also successful in Aotus monkeys 92. Investigations with mice indicate that immunization is successful through the induction of protective antibody 84.
Other leading vaccine candidates 136 for P. falciparum include apical membrane antigen 1 (AMA-1), one of the proteins found in the rhoptry organelles which are involved in invasion of the red blood cell; erythrocyte-binding antigen 175 (EBA-175); and serine-rich antigen (SERA). The first evidence that AMA-1 was a vaccine candidate came from studies with the monkey parasite P. knowlesi 48. A monoclonal antibody which inhibited invasion of P. knowlesi in vitro reacted with a 66-kDa protein located initially in the merozoite rhoptries but which was partially transferred to the merozoite surface at about the time of schizont release. Later, AMA-1 was found in other species including P. falciparum. The P. falciparum protein has been expressed as a full-length protein and has been used in seroepidemiological studies and immunization experiments 39. There are a number of factors which determine whether a merozoite can invade a red blood cell, and these include the presence of particular molecules of the erythrocyte and the age of the erythrocyte 116. Different species or even clones of species can have different erythrocyte specificities. Binding of parasite ligands to specific receptors on the surface of the erythrocyte is an integral part of the invasion process, and antibodies to these parasite ligands might have neutralizing activity. A concern is that they may be exposed on the merozoite surface only for a very short time. Of those identified to date 155, the best-characterized receptors are glycophorin A for P. falciparum and the Duffy blood group antigen for P. vivax and P. knowlesi. Sialic acid residues are the binding specificity on glycophorin A 166. EBA-175 is a microneme protein (micronemes are part of the organelles making up the apical complex at the anterior end of the merozoite thought to be the invasion apparatus), and binds to sialic acid, and presumably it is released when the merozoite and the erythrocyte come into contact. The gene for EBA-175 has been cloned and sequenced 166, and the erythrocyte-binding domain has been located 47. Antibodies to EBA-175 can inhibit growth of P. falciparum in vitro, and hence it is considered an important vaccine candidate. SERA is a soluble protein synthesized by late-erythrocytic-stage parasites and secreted into the parasitophorous vacuole and may be a protease. Evidence that SERA is a vaccine candidate comes from direct immunization experiments with monkeys 129.
The only malaria vaccine to undergo extensive field trials in Latin America, Africa, and Southeast Asia is SPf66 128, a synthetic peptide cocktail developed in Colombia by Patarroyo and colleagues. Three peptides, which induced some protection in Aotus monkeys against a blood-stage challenge, were synthesized as a single 66-amino-acid peptide, polymerized, and bound to aluminum hydroxide 127. In these field trials, the vaccine, under conditions of high as well as low malaria transmission, gave partial protection in some studies 191 and none in others 45. An analysis of all published trials to date in various age groups and epidemiologies concluded that SPf66 had a combined estimate of efficacy of 23% 45, 148.
A major obstacle to developing a malaria vaccine, as noted above, is that of antigenic diversity and immune evasion. In P. falciparum, one antigen in which clonal antigenic variation occurs in PfEMP1, and a major advance was the cloning and sequencing of the corresponding gene 9, 170, 182. In fact, there is a family of 50 or more var genes encoding PfEMP1 which occur on multiple parasite chromosomes. The importance of this molecule in P. falciparum is that it appears to be the major molecule on the surface of the infected erythrocyte, mediating the cytoadherence of infected erythrocytes to the endothelial cells lining the postcapillary venules. Binding leads to sequestration, a process which contributes to pathology. The molecule is exposed on the red cell for about 30 of the 48 h of the asexual cycle. As antibody develops to one antigenic type and parasites are destroyed, new antigenic types are constantly reappearing to replace them. Many field studies have attested to the extreme variability in parasite-induced surface antigens on the infected erythrocyte 93; however, the biological function of PfEMP1 might require a domain within this otherwise very variable molecule which is conserved 184. The serological response to different variants in the field indicates that the conserved adhesive domain 10, 123 for some reason is either weakly immunogenic, cryptic, or unrecognized by the host's immune response. Nonetheless, it may be possible to develop an immunogen of this domain that induces antibody, with an appropriate adjuvant perhaps, to block endothelial attachment.
Transmission-Blocking Vaccines
Currently, there are three target antigens of transmission-blocking immunity 86: (i) prefertilization antigens expressed either completely or predominantly in gametocytes, (ii) postfertilization antigens expressed solely or predominantly on zygotes or ookinetes, and (iii) late-midgut-stage antigens such as parasite-produced chitinase required for the ookinete to penetrate through the peritrophic membrane.
More than half a dozen prefertilization target antigens have been discovered to date. Monoclonal antibodies to a number of them, for example, Pf230, Pf48/45, and Pf11.1, have transmission-blocking activity in vitro, but results of studies to demonstrate whether similar antibodies occur naturally in the field have given mixed results. For example, Graves et al. 65 reported that the presence of anti-Pfs230 antibodies but not anti-Pfs48/45 antibodies in the sera of humans in areas of endemicity in Papua New Guinea was strongly associated with transmission-blocking activity. This and other studies suggest that antibodies to Pfs230 may be complement dependent, and therefore inclusion of such an antigen in a vaccine may require an appropriate adjuvant to induce antibodies of the right isotype. A caveat is that in P. vivax transmission-blocking antibodies, produced as a result of natural infection, may enhance transmission when present in low dilution 137, 171.
Postfertilization antigens are antigens not expressed by the parasite when it is in the vertebrate host. The major disadvantage associated with these antigens is that antibody responses would not be boosted after natural infection. On the other hand, because these antigens have not been exposed to the immune response over thousands of years, they will not have been exposed to selection of immune evasion mechanisms. Two postfertilization antigens are being explored for their vaccine potential: P25 8 (Pfs25 in P. falciparum and its analogues in other species) and P28 52 (Pfs28 in P. falciparum and its analogues in other species). Pfs25 was the first malaria sexual-stage antigen gene to be cloned, and hence there is more known about this molecule than about any other sexual-stage constituent (see references 85 and 86. Although detectable in maturing gametocytes, Pfs25 is mainly expressed after release of the gametes and fertilization and is the major surface protein on the zygote and ookinete, where it is continually expressed. In a number of parasite isolates from different malarious areas, Pfs25 showed little antigenic diversity. Recombinant Pfs25 has been prepared in mammalian expression systems and in yeast cells and has the capacity to induce transmission-blocking antibody in mice and monkeys 8, 87. Pfs25 has recently gone into phase I trials 86. Work on P28 is less advanced, but antibodies to Pfs28 and its homologue in the avian malaria parasite P. gallinaceum have transmission-blocking activity 52.
In the mosquito vector, the developing parasite spends most of its time associated with the midgut, beginning to develop almost immediately after ingestion, and 1 day later the ookinete reaches the midgut epithelium prior to attaching itself to the hemocoel side of the midgut. The midgut provides the nutrients and signals to support the parasite's development but also exposes the parasite to a number of biochemical and physical barriers 164. Following ingestion of the blood meal into the midgut of the mosquito, the peritrophic membrane-matrix, a chitinous layer, is formed around it, thereby presenting the parasite with a barrier in its passage from the blood meal through the epithelial cells of the midgut wall to the outside of the midgut where the oocyst develops. There is good evidence that the ookinete releases a chitinase from its apical end (possibly activated by host midgut proteases) which allows the ookinete to digest its way through the peritrophic membrane 52. Subsequently, it was found that a chitinase inhibitor blocked both parasite infectivity in the mosquito and transmission 163. The ookinete interacts with the midgut in two distinct steps, recognition of the midgut epithelium followed by adhesion to the midgut surface. Receptors for both events are being sought as potential targets for blocking malarial transmission. Further investigations of this site in the mosquito as a source of antigen(s) for anti-mosquito antigen vaccines, which might block a wide range of vector-borne diseases, are ongoing.
Multistage, Multivalent Vaccines
There is a good case that malaria vaccines should be multistage and multivalent 54. We have already noted that there are antigens specific for each life cycle stage. A vaccine, therefore, directed against one stage, if not fully effective, has no activity against later stages in the life cycle. Hence, a vaccine should be more effective if it contains antigens against more than one stage. The immune response has a genetic element in its control 75. Thus, within a population there will be individuals unable to generate CD8+ or CD4+ T-cell or B-cell responses (in the latter case through lack of T-cell help) to specific epitopes on different parasite populations of different Plasmodium spp.: a vaccine with multiple epitopes should reduce or eliminate this difficulty. Further, the potential problems from antigenic diversity and antigenic variation by malaria parasites have been covered above, and this might be alleviated by including a selection of the more frequently occurring allelic variants of the protective polymorphic antigens. NYVAC-Pf7 is a genetically engineered, attenuated vaccinia virus, multistage, multicomponent P. falciparum vaccine that includes the transmission-blocking Pfs25; the pre-erythrocytic antigens CSP, SSP2/TRAP, and liver-stage antigen 1; and the asexual blood-stage antigens MSP-1, AMA-1, and SERA. This recombinant vaccine has been through phase I-IIa safety and efficacy studies, but the results in terms of protection were disappointing 178.
MALARIA GENOME PROJECT
It is clear that new targets for drugs and vaccines are essential and that a better understanding of the biology of the parasite is prerequisite, but this is hampered by the complexity of the parasite's life cycle. The sequencing of the Plasmodium genome should contribute to our knowledge of the parasite, and a combined effort of several groups, the Malaria Genome Sequencing Consortium 28, 55, 77, is currently sequencing the genome of P. falciparum. (Some 16 microbial genomes have been sequenced in the last 3 years: a bacterial genome is approximately the same size as a single Plasmodium chromosome.) The genome of P. falciparum is approximately 30 Mb, contains 14 chromosomes ranging in size from 0.65 to 3.4 Mb, and has a base composition of 82% A+T. The biased nucleotide composition presents technical difficulties for the sequencing project. Chromosomes from different field isolates show extensive size polymorphisms. In P. falciparum, there are also two organellar genomes. The mitochondrial genome is a 5.9-kb tandemly repeated DNA molecule, and there is also a 35-kb circular DNA molecule carrying genes that are normally associated with plastid genomes 206. Chromosome 2, containing about 209 protein-encoding genes, was sequenced in 1998 61, and chromosome 3, which carries 215 genes, was sequenced in 1999 19. These two chromosomes together make up approximately 7% of the genome, and it is estimated that the total genome codes for approximately 6,500 genes. Some interesting patterns have emerged from these two chromosomes. Genes encoding proteins produced at similar stages in the parasite's life cycle or proteins with similar functions appear to be grouped in particular chromosomes or chromosomal locations. For example, there are several genes on chromosome 2 encoding membrane-associated proteins and serine repeat antigens and also genes encoding proteins found on the surface of the merozoite. The genes for variable antigens on the surface of the infected erythrocyte, the var and rif genes, are found close to the telomeres of both chromosome 2 and chromosome 3, each telomere being flanked by one var gene and several members of the rif family. It is estimated that there are 200 rif genes per genome, which makes it the largest gene family in P. falciparum. As var and rif are located close to one another, it is possible that they are coexpressed and coregulated. Bowman and colleagues 19, in their analysis of chromosome 3, have discovered additional gene families such as the clag (cytoadherence-linked asexual gene) and CTP (conserved telomeric protein) families, located in the subtelomeric regions of chromosome 3. The role of the clag genes is unknown, but the clag gene products might be expressed on the surface of the infected erythrocyte and have a biologically important function.
The Malaria Genome Sequencing Consortium aims to generate almost all of the P. falciparum genome sequence in a rough form in the year 2000, although a fully annotated representation of the genome may take another 2 to 3 years. Almost all of the parasite's genes will, therefore, become available for other malaria researchers, and from the genes vaccine and drug targets may be identified. It will be no easy task to screen the thousands of new proteins and then identify those with potential as vaccine candidates. As Hoffman and colleagues 78 note, the current gene-by-gene and protein-by-protein approach will hardly scratch the surface. They propose a scheme for DNA “vaccinomics” which will allow screening of all open reading frames for their potential as coding for proteins relevant to the protective immune response.
The postgenomics agenda for functional analysis of P. falciparum has been reviewed previously 43. What are the functions of the thousands of parasite proteins? The recently developed transfection system for P. falciparum which allows specific knockout of a gene provides a powerful tool for understanding the biological function of proteins and also their contribution in some cases to the pathophysiology of infection.
CONCLUDING REMARKS
At the start of the new millennium, malaria still poses the greatest threat of all parasites to human health, and one would guess that this represents no change from 100 years ago. There are substantial parts of the world, however, which were malarious 100 years ago but are now malaria free. Thus, there has been substantial progress, although how much of this is due to application of control programs and how much is due to improvements in living conditions in general may be debatable. There must have been optimism in the early years of the 20th century that a knowledge of the parasite's life cycle, methods available for controlling the vector, and an effective antimalarial in the form of quinine might lead to the eradication of the disease, an aim which was boosted in the 1940s by the introduction of DDT and chloroquine. The fact that this has not been achieved should only reinforce the resolve that early in the 21st century this aim should be fulfilled. The recent rapid and spectacular developments in molecular biology and allied biological sciences raise hopes that new antimalarials and antimalarial vaccines will be developed, but the underlying problem remains one of financing, initially the necessary research and development work and secondly the cost of providing vaccines and antimalarials to communities that currently would not have the means to pay for them.
The driving force for malaria control early in the last century was the pursuit of economic and commercial gain, be it the building of the Panama Canal, for example, or the development of agricultural schemes for fruit, rubber, and other crops 99. The discovery of new antimalarials was in part driven by wars. The former incentive applies only on a local basis, and I hope that the latter will no longer be a factor. Hence is seen the importance of the recent initiative of the Roll Back Malaria Programme in which the World Health Organization will lead a partnership of a number of agencies and interested bodies in order to coordinate and focus activities and agree on priorities in the pursuit of the control of malaria and to alleviate the suffering that the disease causes. The international commitment to alleviating the suffering from malaria will be a window on the commitment that the richer nations have to dealing with poverty, the use and abuse of the world's natural resources, and damage to the environment.
This review has necessarily been selective and has not touched in any detail on many exciting areas of malaria research. Thus, the fact that life-threatening malaria can develop so rapidly has prompted detailed investigations into the pathogenic processes (grossly seen as neurological disturbances and respiratory distress), and from this knowledge, it may be possible to plan more effective intervention strategies 35, 104, 108. Research into how mosquitoes find their host and the recognition that this can be a nonrandom choice of host by the mosquito influenced by factors such as the bacterial skin flora of the host have extended the epidemiology of malaria into the microepidemiology of the disease 20. Exciting times in malaria research are ahead, but history has taught us that this excitement has to be tempered with realism and that progression from the laboratory bench to a benefit in the field can be a slow and tortuous path.
ACKNOWLEDGMENT
I thank Mulu Gedle for her invaluable help in preparing the manuscript.
REFERENCES
- 1.Al-Khedery B, Barnwell J W, Galinski M R. Antigenic variation in malaria: a 3′ genomic alteration associated with the expression of a Plasmodium knowlesi variant antigen. Mol Cell. 1999;3:131–141. doi: 10.1016/s1097-2765(00)80304-4. [DOI] [PubMed] [Google Scholar]
- 2.Alonso P L, Lindsay S W, Armstrong J R M, Conteh M, Hill A G, David P H, Fegan G, de Francisco A, Hall A J, Shenton F C, Cham K, Greenwood B M. The effect of insecticide-treated bed nets on mortality of Gambian children. Lancet. 1991;337:1499–1502. doi: 10.1016/0140-6736(91)93194-e. [DOI] [PubMed] [Google Scholar]
- 3.Babiker H A, Walliker D. Current views on the population structure of Plasmodium falciparum: implications for control. Parasitol Today. 1997;13:262–267. doi: 10.1016/s0169-4758(97)01075-2. [DOI] [PubMed] [Google Scholar]
- 4.Babiker H A, Ranford-Cartwright L C, Currie D, Charlwood J, Billingsley P, Teuscher T, Walliker D. Random mating in natural populations of the malaria parasite Plasmodium falciparum. Parasitology. 1994;109:413–421. doi: 10.1017/s0031182000080665. [DOI] [PubMed] [Google Scholar]
- 5.Baird J K. Host age as a determinant of naturally acquired immunity to Plasmodium falciparum. Parasitol Today. 1995;11:105–111. doi: 10.1016/0169-4758(95)80167-7. [DOI] [PubMed] [Google Scholar]
- 6.Baird J K, Purnomo, Jones T R. Diagnosis of malaria in the field by fluorescence microscopy of QRC capillary tubes. Trans R Soc Trop Med Hyg. 1992;86:3–5. doi: 10.1016/0035-9203(92)90412-6. [DOI] [PubMed] [Google Scholar]
- 7.Baird J K, Jones T R, Danudirgo E W, Annis B A, Bangs M J, Basri H, Purnomo, Marbar S. Age-dependent acquired protection against Plasmodium falciparum in people having two years exposure to hyperendemic malaria. Am J Trop Med Hyg. 1991;45:65–76. doi: 10.4269/ajtmh.1991.45.65. [DOI] [PubMed] [Google Scholar]
- 8.Barr P J, Green K M, Gibson H L, Bathurst I C, Quakyi I A, Kaslow D C. Recombinant Pfs25 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in experimental animals. J Exp Med. 1991;174:1203–1208. doi: 10.1084/jem.174.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baruch D I, Pasloske B L, Singh H B, Bi X, Ma X C, Feldman M, Taraschi T F, Howard R J. Cloning the Plasmodium falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–87. doi: 10.1016/0092-8674(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 10.Baruch D I, Ma X C, Singh H B, Bi X, Pasloske B L, Howard R J. Identification of a region of PfEMP1 that mediates adherence of Plasmodium falciparum infected erythrocytes to CD36: conserved function with variant sequence. Blood. 1997;90:3766–3775. [PubMed] [Google Scholar]
- 11.Basco L K, Ramiliarisoa O, Le Bras J. In vitro activity of atovaquone against the African isolates and clones of Plasmodium falciparum. Am J Trop Med Hyg. 1995;53:381–391. doi: 10.4269/ajtmh.1995.53.388. [DOI] [PubMed] [Google Scholar]
- 12.Beck H-P, Felger I, Huber W, Steiger S, Smith T, Weiss N, Alonso P, Tanner M. Analysis of multiple Plasmodium falciparum infections in Tanzanian children during the phase III trial of the malaria vaccine SPf66. J Infect Dis. 1997;175:921–926. doi: 10.1086/513991. [DOI] [PubMed] [Google Scholar]
- 13.Berendt A R, Ferguson D J P, Newbold C J. Sequestration in Plasmodium falciparum malaria: sticky cells and sticky problems. Parasitol Today. 1990;6:247–254. doi: 10.1016/0169-4758(90)90184-6. [DOI] [PubMed] [Google Scholar]
- 14.Billingsley P F, Sinden R E. Determinants of malaria-mosquito specificity. Parasitol Today. 1997;13:297–301. [Google Scholar]
- 15.Bjorkman A, Phillips-Howard P A. The epidemiology of drug-resistant malaria. Trans R Soc Trop Med Hyg. 1990;84:177–180. doi: 10.1016/0035-9203(90)90246-b. [DOI] [PubMed] [Google Scholar]
- 16.Blackman M J, Chappel J A, Shai S, Holder A A. A conserved parasite serine protease processes the Plasmodium falciparum merozoite surface protein-1. Mol Biochem Parasitol. 1993;62:103–114. doi: 10.1016/0166-6851(93)90182-w. [DOI] [PubMed] [Google Scholar]
- 17.Bouharoun-Tayoun H, Oeuvray C, Lunel F, Druihle P. Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J Exp Med. 1995;182:409–418. doi: 10.1084/jem.182.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouharoun-Tayoun H, Attanath P, Sabchareon A, Chongsuphajaisiddhi T, Druihle P. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J Exp Med. 1990;172:1633–1641. doi: 10.1084/jem.172.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bowman S, Lawson D, Basham D, Brown D, Chillingworth T, Churcher C M, Craig A, Davies R M, Devlin K, Feltwell T, Gentles S, Gwilliam R, Hamlin N, Harris D, Holroyd S, Hornsby T, Horrocks P, Jagels K, Jassal B, Kyes S, McLean J, Moule S, Mungall K, Murphy L, Oliver K, Quail M A, Rajandream M-A, Rutter S, Skelton J, Squares R, Squares S, Sulston J E, Whitehead S, Woodward J R, Newbold C, Barrell B G. The complete nucleotide sequence of chromosome 3 of Plasmodium falciparum. Nature. 1999;400:532–538. doi: 10.1038/22964. [DOI] [PubMed] [Google Scholar]
- 20.Braks M A H, Anderson R A, Knot B C J. Infochemicals in mosquito host selection: human skin microflora and Plasmodium parasites. Parasitol Today. 1999;15:409–413. doi: 10.1016/s0169-4758(99)01514-8. [DOI] [PubMed] [Google Scholar]
- 21.Breman J G, Campbell C C. Combating severe malaria in African children. Bull W H O. 1988;66:611–616. [PMC free article] [PubMed] [Google Scholar]
- 22.Brewer T G, Peggins J O, Grate S J, Petras J M, Levine B S, Weina P G, Swearengen J, Heiffer M H, Schuster B G. Neurotoxicity in animals due to artemether and arteether. Trans R Soc Trop Med Hyg. 1994;88(Suppl. 1):33–36. doi: 10.1016/0035-9203(94)90469-3. [DOI] [PubMed] [Google Scholar]
- 23.Brown K N, Brown I N, Hills L A. Immunity to malaria. I. Protection against Plasmodium knowlesi shown by monkeys sensitized with drug-suppressed infection or by dead parasites in Freund's adjuvant. Exp Parasitol. 1970;28:304–317. doi: 10.1016/0014-4894(70)90101-3. [DOI] [PubMed] [Google Scholar]
- 24.Bruce-Chwatt L. In: Essential malariology. 3rd ed. Gillies H M, Warrall D A, editors. London, United Kingdom: Edward Arnold; 1993. [Google Scholar]
- 25.Bull P C, Lowe B S, Kortok M, Molyneux C S, Newbold C I, Marsh K. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat Med. 1998;4:358–360. doi: 10.1038/nm0398-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butler A R, Wu Y L. Artemisinin (qinghaosu): a new type of antimalarial drug. Chem Soc Rev. 1992;21:85–90. [Google Scholar]
- 27.Campbell C C. Malaria: an emerging and re-emerging global plague. FEMS Immunol Med Microbiol. 1997;18:325–331. doi: 10.1111/j.1574-695X.1997.tb01063.x. [DOI] [PubMed] [Google Scholar]
- 28.Carucci D J, Gardner M J, Tettelin H, Cummings L M, Smith H O, Adams M D, Venter J C, Hoffman S L. Sequencing the genome of Plasmodium falciparum. Curr Opin Infect Dis. 1998;11:531–534. doi: 10.1097/00001432-199810000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Cerami C, Frevert U, Sinnis P, Takacs B, Clavijo P, Santos M J, Nussenzweig V. The basolateral domain of the hepatocyte plasma membrane bears receptors for the circumsporozoite protein of Plasmodium falciparum sporozoites. Cell. 1992;70:1021–1033. doi: 10.1016/0092-8674(92)90251-7. [DOI] [PubMed] [Google Scholar]
- 30.Cham M K, Olayeye B, D'Alessandro U, Aikins M, Cham B, Maine N, Williams L A, Mills A, Greenwood B M. The impact of charging for insecticide on the Gambian national impregnated bed-net programme. Health Policy Planning. 1997;12:240–247. doi: 10.1093/heapol/12.3.240. [DOI] [PubMed] [Google Scholar]
- 31.Chappel J A, Holder A A. Monoclonal antibodies that inhibit Plasmodium falciparum invasion in vitro recognise the first growth factor-like domain of merozoite surface protein-1. Mol Biochem Parasitol. 1993;60:303–312. doi: 10.1016/0166-6851(93)90141-j. [DOI] [PubMed] [Google Scholar]
- 32.Chege G M M, Beier J C. Effect of Plasmodium falciparum on the survival of naturally infected afrotropical Anopheles (Diptera: Culcidae) J Med Entomol. 1990;27:454–458. doi: 10.1093/jmedent/27.4.454. [DOI] [PubMed] [Google Scholar]
- 33.Chen D H, Tigelaar R E, Weinbaum F I. Immunity to sporozoite-induced malaria infection in mice. I. The effect of immunization of T and B cell-deficient mice. J Immunol. 1977;118:1322–1327. [PubMed] [Google Scholar]
- 34.Choi H W, Breman J G, Teutsch S M, Liu S, Hightower A W, Sexton J D. The effectiveness of insecticide-impregnated bed nets in reducing cases of malaria infection: a meta-analysis of published results. Am J Trop Med Hyg. 1995;52:377–382. doi: 10.4269/ajtmh.1995.52.377. [DOI] [PubMed] [Google Scholar]
- 35.Clark I A, Cowden W B. Why is the pathology of falciparum worse than that of vivax malaria? Parasitol Today. 1999;15:458–461. doi: 10.1016/s0169-4758(99)01535-5. [DOI] [PubMed] [Google Scholar]
- 36.Clyde D F. Immunity to falciparum and vivax malaria induced by irradiated sporozoites: a review of the University of Maryland studies 1971–1975. Bull W H O. 1990;68(Suppl.):9–12. [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen S, McGregor I A, Carrington S S. Gamma globulin and acquired immunity to human malaria. Nature. 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 38.Collins F H, Besansky N J. Vector biology and the control of malaria in Africa. Science. 1994;264:1874–1875. doi: 10.1126/science.8009215. [DOI] [PubMed] [Google Scholar]
- 39.Collins W E, Pye D, Crewther P E, Vandenberg K L, Galland G G, Sulzer A J, Kemp D J, Edwards S J, Coppel R L, Sullivan J S, Morris C L, Anders R F. Protective immunity induced in squirrel monkeys with recombinant apical membrane antigen-1 of Plasmodium fragile. Am J Trop Med Hyg. 1994;51:711–719. doi: 10.4269/ajtmh.1994.51.711. [DOI] [PubMed] [Google Scholar]
- 40.Cooper J A. Merozoite surface-antigen of Plasmodium falciparum. Parasitol Today. 1993;9:50–54. doi: 10.1016/0169-4758(93)90031-a. [DOI] [PubMed] [Google Scholar]
- 41.Cortese J F, Plowe C V. Antifolate resistance due to new and known Plasmodium falciparum dihydrofolate reductase mutations expressed in yeast. Mol Biochem Parasitol. 1998;94:205–214. doi: 10.1016/s0166-6851(98)00075-9. [DOI] [PubMed] [Google Scholar]
- 42.Cowman A F, Lew A M. Chromosomal rearrangements and point mutations in the DHFR-TS gene of Plasmodium chabaudi under antifolate selection. Mol Biochem Parasitol. 1990;42:21–29. doi: 10.1016/0166-6851(90)90109-y. [DOI] [PubMed] [Google Scholar]
- 43.Craig A A, Walters A P, Ridley R G. Malaria genome project task force: a first genomic agenda for functional analysis. Parasitol Today. 1999;15:211–214. doi: 10.1016/s0169-4758(99)01461-1. [DOI] [PubMed] [Google Scholar]
- 44.Cruz M A. Human migration and the spread of malaria in Brazil. Parasitol Today. 1987;3:166–170. doi: 10.1016/0169-4758(87)90170-0. [DOI] [PubMed] [Google Scholar]
- 45.D'Alessandro U, Leach A, Drakeley C J, Bennett S, Olaleye B O, Fegan G W, Jawara M, Langerock P, George M O, Targett G A, Greenwood B M. Efficacy trial of malaria vaccine SPf66 in Gambian infants. Lancet. 1995;346:462–467. doi: 10.1016/s0140-6736(95)91321-1. [DOI] [PubMed] [Google Scholar]
- 46.Dame J B, Williams J L, McCutchan T F, Weber J L, Wirtz R A, Hockmeyer W T, Malay W L, Haynes J D, Schneider I, Roberts D, Sanders G S, Reddy E P, Diggs C L, Miller L H. Structure of the gene encoding the immunodominant surface antigen on the sporozoite of the human malaria parasite Plasmodium falciparum. Science. 1984;225:593–599. doi: 10.1126/science.6204383. [DOI] [PubMed] [Google Scholar]
- 47.Daugherty J R, Murphy C I, Doros-Richert L A, Barbosa A, Kashala L O, Ballou W R, Snellings N J, Ockenhouse C F, Lanar D E. Baculovirus-mediated expression of Plasmodium falciparum erythrocyte binding antigen 175 polypeptides and their recognition by human antibodies. Infect Immun. 1997;65:3631–3637. doi: 10.1128/iai.65.9.3631-3637.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deans J A, Knight A M, Jean W C, Waters A P, Cohen S, Mitchell G H. Vaccination trials in rhesus monkeys with a minor, invariant, Plasmodium knowlesi 66kD merozoite antigen. Parasite Immunol. 1988;10:535–552. doi: 10.1111/j.1365-3024.1988.tb00241.x. [DOI] [PubMed] [Google Scholar]
- 49.Diggs C L, Ballou W R, Miller L H. The major merozoite surface protein as a malaria vaccine target. Parasitol Today. 1993;9:300–302. doi: 10.1016/0169-4758(93)90130-8. [DOI] [PubMed] [Google Scholar]
- 50.Doolan D L, Hoffman S L. Multi-gene vaccination against malaria: a multi-stage, multi-immune response approach. Parasitol Today. 1997;13:171–178. doi: 10.1016/s0169-4758(97)01040-5. [DOI] [PubMed] [Google Scholar]
- 51.Doolan D L, Hoffman S L. Pre-erythrocytic-stage immune effector mechanisms in Plasmodium spp infections. Phil Trans R Soc Lond B Biol Sci. 1997;352:1361–1367. doi: 10.1098/rstb.1997.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duffy D E, Pimenta P, Kaslow D C. Pgs28 belongs to a family of epidermal growth factor-like antigens that are targets of malaria transmission-blocking antibodies. J Exp Med. 1993;177:505–510. doi: 10.1084/jem.177.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dye C. The analysis of parasite transmission by bloodsucking insects. Annu Rev Entomol. 1992;37:1–19. doi: 10.1146/annurev.en.37.010192.000245. [DOI] [PubMed] [Google Scholar]
- 54.Engers H D, Godal T. Malaria vaccine development: current status. Parasitol Today. 1998;14:56–64. doi: 10.1016/s0169-4758(97)01184-8. [DOI] [PubMed] [Google Scholar]
- 55.Fletcher C. The Plasmodium falciparum genome project. Parasitol Today. 1998;14:342–344. doi: 10.1016/s0169-4758(98)01300-3. [DOI] [PubMed] [Google Scholar]
- 56.Foote S J, Cowman A F. The mode of action and the mechanism of resistance to antimalarial drugs. Acta Trop. 1994;56:157–171. doi: 10.1016/0001-706x(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 57.Foster S. Economic prospects for a new antimalarial drug. Trans R Soc Trop Med Hyg. 1994;88(Suppl. 1):55–56. doi: 10.1016/0035-9203(94)90476-6. [DOI] [PubMed] [Google Scholar]
- 58.Fraser-Hurt N, Felger I, Edoh D, Steiger S, Mashaka M, Masanja H, Smith T, Mbena F, Beck H-P. Effect of insecticide-treated bed nets on haemoglobin values, prevalence and multiplicity of infection with Plasmodium falciparum in a randomized controlled trial in Tanzania. Trans R Soc Trop Med Hyg. 1999;93(Suppl. 1):S1/47–S1/52. doi: 10.1016/s0035-9203(99)90327-9. [DOI] [PubMed] [Google Scholar]
- 59.Fu S, Xiao S H. Pyronaridine: a new antimalarial drug. Parasitol Today. 1991;7:310–313. doi: 10.1016/0169-4758(91)90267-r. [DOI] [PubMed] [Google Scholar]
- 60.Galinski M R, Barnwell J W. Plasmodium vivax: merozoites, invasion of reticulocytes and considerations for malaria vaccine development. Parasitol Today. 1996;12:20–29. doi: 10.1016/0169-4758(96)80641-7. [DOI] [PubMed] [Google Scholar]
- 61.Gardner M J, Tettelin H, Carneci D J, Cummings L M, Aravind L, Koemin E V, Shallom S, Mason T, Yu K, Fujii C, Pederson J, Shen K, Jing J, Aston C, Lai Z, Schwartz D C, Pertea M, Salzberg S, Zhou L, Sutton G G, Clayton R, White O, Smith H V, Fraser C M, Adams M D, Venter J C, Hoffman S L. Chromosome 2 sequence of the human malaria parasite Plasmodium falciparum. Science. 1998;282:1126–1132. doi: 10.1126/science.282.5391.1126. [DOI] [PubMed] [Google Scholar]
- 62.Gill C A. The role of meteorology in malaria. Indian J Med Res. 1921;8:633–693. [Google Scholar]
- 63.Gill C A. The prediction of malaria epidemics. Indian J Med Res. 1923;10:1136–1143. [Google Scholar]
- 64.Ginsburg H, Ward S A, Brag P G. An integrated model of chloroquine action. Parasitol Today. 1999;15:357–360. doi: 10.1016/s0169-4758(99)01502-1. [DOI] [PubMed] [Google Scholar]
- 65.Graves P M, Carter R, Burkot T R, Quakyi I A, Kumar N. Antibodies to Plasmodium falciparum gamete surface antigens in Papua New Guinea sera. Parasite Immunol. 1988;10:209–218. doi: 10.1111/j.1365-3024.1988.tb00215.x. [DOI] [PubMed] [Google Scholar]
- 66.Greenwood B, Marsh K, Snow R W. Why do some African children develop severe malaria? Parasitol Today. 1991;7:277–281. doi: 10.1016/0169-4758(91)90096-7. [DOI] [PubMed] [Google Scholar]
- 67.Greenwood B M. Malaria transmission and control. Parasitol Today. 1997;13:90–92. doi: 10.1016/s0169-4758(97)01002-8. [DOI] [PubMed] [Google Scholar]
- 68.Greenwood B M. What's new in malaria control? Ann Trop Med Parasitol. 1997;91:523–531. doi: 10.1080/00034989760897. [DOI] [PubMed] [Google Scholar]
- 69.Greenwood B M, Bradley A K, Greenwood A M, Byass P, Jammeh K, Marsh K, Tulloch S, Oldfield F S J, Hayes R. Mortality and morbidity from malaria among children in a rural area of The Gambia, West Africa. Trans R Soc Trop Med Hyg. 1986;81:478–486. doi: 10.1016/0035-9203(87)90170-2. [DOI] [PubMed] [Google Scholar]
- 70.Handunnetti S M, Gunewardena D M, Pathirana P P, Ekanaayake K, Weerasinghe S, Mendis K N. Features of recrudescent chloroquine-resistant Plasmodium falciparum infections confer a survival advantage on parasites and have implications for disease control. Trans R Soc Trop Med Hyg. 1996;90:563–567. doi: 10.1016/s0035-9203(96)90325-9. [DOI] [PubMed] [Google Scholar]
- 71.Hanke T, McMichael A. Pre-clinical development of a multi-CTL epitope-based DNA prime MVA boost vaccine for AIDS. Immunol Lett. 1999;66:177–181. doi: 10.1016/s0165-2478(98)00164-3. [DOI] [PubMed] [Google Scholar]
- 72.Hanke T, Schneider J, Gilbert S C, Hill A V S, McMichael A. DNA multi-CTL epitope vaccines for HIV and Plasmodium falciparum: immunogenicity in mice. Vaccine. 1998;16:426–435. doi: 10.1016/s0264-410x(97)00296-x. [DOI] [PubMed] [Google Scholar]
- 73.Hawking F, Worms M J, Gammage K. 24- and 48-hour cycles of malaria parasites in the blood; their purpose, production and control. Trans R Soc Trop Med Hyg. 1968;62:731–760. doi: 10.1016/0035-9203(68)90001-1. [DOI] [PubMed] [Google Scholar]
- 74.Hay S I, Snow R W, Rogers D J. From predicting mosquito habitat to malaria seasons using remotely sensed data: practice, problems and perspectives. Parasitol Today. 1998;14:306–313. doi: 10.1016/s0169-4758(98)01285-x. [DOI] [PubMed] [Google Scholar]
- 75.Hill A V, Allsopp C E, Kwiatkowski D, Anstey N M, Twamasi P, Rowe P R, Bennett S, Brewster D, McMichael A J, Greenwood B M. Common West African HLA antigens are associated with protection from severe malaria. Nature. 1991;352:596–600. doi: 10.1038/352595a0. [DOI] [PubMed] [Google Scholar]
- 76.Hoffman S L, editor. Malaria vaccine development: a multi-immune response approach. Washington, D.C.: ASM Press; 1996. [Google Scholar]
- 77.Hoffman S L, Bancroft W H, Gottlieb M, James S L, Burroughs E C, Stephenson J R, Morgan M J. Funding for the malaria genome project. Nature. 1997;387:647. doi: 10.1038/42571. [DOI] [PubMed] [Google Scholar]
- 78.Hoffman S L, Rogers W O, Carucci D J, Venter J C. From genomics to vaccines: malaria as a model system. Nat Med. 1998;4:1351–1353. doi: 10.1038/3934. [DOI] [PubMed] [Google Scholar]
- 79.Holder A A. Preventing merozoite invasion of erythrocytes. In: Hoffman S L, editor. Malaria vaccine development: a multi-immune response approach. Washington, D.C.: ASM Press; 1996. pp. 77–104. [Google Scholar]
- 80.Holder A A, Freeman R R. Immunization against blood-stage rodent malaria using purified antigens. Nature. 1981;294:361–364. doi: 10.1038/294361a0. [DOI] [PubMed] [Google Scholar]
- 81.Hougard J M, Back C. Perspectives on the bacterial control of vectors in the tropics. Parasitol Today. 1992;11:364–366. doi: 10.1016/0169-4758(92)90168-2. [DOI] [PubMed] [Google Scholar]
- 82.Ichimori K, Curtis C F, Targett G A T. The effects of chloroquine on the infectivity of chloroquine-sensitive and -resistant populations of Plasmodium yoelii nigeriensis to mosquitoes. Parasitology. 1990;100:377–381. doi: 10.1017/s0031182000078641. [DOI] [PubMed] [Google Scholar]
- 83.Jones T R, Baird J K, Basri H, Purnomo, Danudirgo E W. Prevalence of malaria in native and transmigrant populations. Effects of age and history of exposure. Trop Geogr Med. 1991;43:1–6. [PubMed] [Google Scholar]
- 84.Karbwang J, Na-Bangchang K, Thanavibul A, Dittar-in M, Harinasuta T. A comparative clinical trial of two different regimens of artemether plus mefloquine in multi-drug resistant falciparum malaria. Trans R Soc Trop Med Hyg. 1995;89:296–298. doi: 10.1016/0035-9203(95)90549-9. [DOI] [PubMed] [Google Scholar]
- 85.Kaslow D C. Transmission-blocking immunity against malaria and other vector-borne diseases. Curr Opin Immunol. 1993;5:557–565. doi: 10.1016/0952-7915(93)90037-s. [DOI] [PubMed] [Google Scholar]
- 86.Kaslow D C. Transmission blocking vaccines: uses and current status of development. Int J Parasitol. 1997;27:183–189. doi: 10.1016/s0020-7519(96)00148-8. [DOI] [PubMed] [Google Scholar]
- 87.Kaslow D C, Shiloach J. Production, purification and immunogenicity of a malaria transmission-blocking vaccine candidate-Tbv25H expressed in yeast and purified using Ni-VTA agarose. Bio/Technology. 1994;12:494–499. doi: 10.1038/nbt0594-494. [DOI] [PubMed] [Google Scholar]
- 88.Koella J C. Costs and benefits of resistance against antimalarial drugs. Parasitol Today. 1998;14:360–364. doi: 10.1016/s0169-4758(98)01297-6. [DOI] [PubMed] [Google Scholar]
- 89.Koella J C, Packer M J. Malaria parasites enhance blood-feeding of their naturally infected vector Anopheles punctulatus. Parasitology. 1996;113:105–109. doi: 10.1017/s0031182000066348. [DOI] [PubMed] [Google Scholar]
- 90.Koella J C, Sorensen T L, Anderson R A. The malaria parasite Plasmodium falciparum increases the frequency of multiple feeding of its mosquito vector, Anopheles gambiae. Proc R Soc Lond Ser B. 1998;265:763–768. doi: 10.1098/rspb.1998.0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kumar A, Sharma V P, Tharaselam D, Sumodan P K. Clinical trials of a new immunochromatographic test for diagnosis of Plasmodium falciparum malaria in Goa. Indian J Malariol. 1996;33:166–172. [PubMed] [Google Scholar]
- 92.Kumar S, Yadavam A, Keister D B, Tian J H, Ohl M, Perdue-Greenfield K A, Miller L H, Kaslow D C. Immunogenicity and in vivo efficacy of recombinant Plasmodium falciparum merozoite surface protein-1 in Aotus monkeys. Mol Med. 1995;1:325–332. [PMC free article] [PubMed] [Google Scholar]
- 93.Kyes S, Taylor H, Craig A, Marsh K, Newbold C. Genomic representation of var gene sequences in Plasmodium falciparum field isolates from different geographic regions. Mol Biochem Parasitol. 1997;87:235–238. doi: 10.1016/s0166-6851(97)00071-6. [DOI] [PubMed] [Google Scholar]
- 94.Langeler C, Smith T A, Schellenberg J A. Focus on the effects of bed nets on malaria morbidity and mortality. Parasitol Today. 1997;13:123–124. doi: 10.1016/s0169-4758(97)84870-3. [DOI] [PubMed] [Google Scholar]
- 95.Langhorne J. The role of CD4+ T-cells in the immune response to Plasmodium chabaudi. Parasitol Today. 1989;5:362–364. doi: 10.1016/0169-4758(89)90113-0. [DOI] [PubMed] [Google Scholar]
- 96.Lereclus D, Vallade M, Chaufaux J, Arantes O, Rambaud S. Expansion of insecticidal host range of Bacillus thuringiensis by in vivo genetic recombination. Bio/Technology. 1992;10:418–421. doi: 10.1038/nbt0492-418. [DOI] [PubMed] [Google Scholar]
- 97.Lindsay S W, Birley M H. Climate change and malaria transmission. Ann Trop Med Parasitol. 1996;90:573–588. doi: 10.1080/00034983.1996.11813087. [DOI] [PubMed] [Google Scholar]
- 98.Lines J, Armstrong J R M. For a few parasites more: inoculum size, vector control and strain-specific immunity to malaria. Parasitol Today. 1992;8:381–383. doi: 10.1016/0169-4758(92)90176-3. [DOI] [PubMed] [Google Scholar]
- 99.Litsios S. The tomorrow of malaria. Wellington, New Zealand: Pacific Press; 1996. [Google Scholar]
- 100.Long C A. Immunity to blood stages of malaria. Curr Opin Immunol. 1993;5:548–556. doi: 10.1016/0952-7915(93)90036-r. [DOI] [PubMed] [Google Scholar]
- 101.Makler M T, Palmer C J, Ager A L. A review of practical techniques for the diagnosis. Ann Trop Med Parasitol. 1998;192:419–433. doi: 10.1080/00034989859401. [DOI] [PubMed] [Google Scholar]
- 102.Makler M T, Piper R C, Milhous W K. Lactate dehydrogenase and the diagnosis of malaria. Parasitol Today. 1998;14:376–377. doi: 10.1016/s0169-4758(98)01284-8. [DOI] [PubMed] [Google Scholar]
- 103.Malaria Foundation International at http://www.malaria.org.
- 104.Mandis K N, Carter R. Clinical disease and pathogenesis in malaria. Parasitol Today. 1995;11:PTI2–PTI15. doi: 10.1016/0169-4758(95)80143-x. [DOI] [PubMed] [Google Scholar]
- 105.Marques A C. Human migration and the spread of malaria in Brazil. Parasitol Today. 1987;3:166–170. doi: 10.1016/0169-4758(87)90170-0. [DOI] [PubMed] [Google Scholar]
- 106.Marsh K. Malaria—a neglected disease? Parasitology. 1992;104:S53–S69. doi: 10.1017/s0031182000075247. [DOI] [PubMed] [Google Scholar]
- 107.Marsh K. Malaria disaster in Africa. Lancet. 1998;352:924. doi: 10.1016/S0140-6736(05)61510-3. [DOI] [PubMed] [Google Scholar]
- 108.Marsh K, English M, Crawley J. The pathogenesis of severe malaria in African children. Ann Trop Med Parasitol. 1996;90:395–402. doi: 10.1080/00034983.1996.11813068. [DOI] [PubMed] [Google Scholar]
- 109.McGregor I A. Studies in the acquisition of immunity to Plasmodium falciparum infection in Africa. Trans R Soc Trop Med Hyg. 1964;58:80–87. doi: 10.1016/0035-9203(64)90073-2. [DOI] [PubMed] [Google Scholar]
- 110.McGregor I A. The development and maintenance of immunity to malaria in highly endemic areas. Clin Trop Med Commun Dis. 1986;1:29–53. [Google Scholar]
- 111.Mellouk S, Green S J, Nacy C A, Hoffman S L. IFN gamma inhibits development of Plasmodium berghei exoerythrocytic stages in hepatocytes by an L-arginine-dependent effector mechanism. J Immunol. 1991;146:3971–3976. [PubMed] [Google Scholar]
- 112.Miller L H, Hoffman S L. Research toward vaccines against malaria. Nat Med Vaccine Suppl. 1998;4:520–524. doi: 10.1038/nm0598supp-520. [DOI] [PubMed] [Google Scholar]
- 113.Miller L H, Good M F, Kaslow D S. The need for assays predictive of protection in development of malaria blood stage vaccines. Parasitol Today. 1997;13:46–47. doi: 10.1016/s0169-4758(96)20063-8. [DOI] [PubMed] [Google Scholar]
- 114.Miller L H, Good M F, Milton G. Malaria pathogenesis. Science. 1994;264:1878–1883. doi: 10.1126/science.8009217. [DOI] [PubMed] [Google Scholar]
- 115.Mitchell G H. An update on candidate malaria vaccines. Parasitology. 1989;98:S29–S47. doi: 10.1017/s0031182000072231. [DOI] [PubMed] [Google Scholar]
- 116.Mitchell G H, Bannister L H. Malaria parasite invasion: interactions with the red cell membrane. Crit Rev Oncol Hematol. 1988;8:255–310. doi: 10.1016/s1040-8428(88)80011-8. [DOI] [PubMed] [Google Scholar]
- 117.Mitchell G H, Butcher G A, Cohen S. Merozoite vaccination against Plasmodium knowlesi malaria. Immunology. 1975;29:397–407. [PMC free article] [PubMed] [Google Scholar]
- 118.Morgan U M, Thompson R L A. Molecular detection of parasitic protozoa. Parasitology. 1998;117:S73–S85. doi: 10.1017/s0031182099004102. [DOI] [PubMed] [Google Scholar]
- 119.Muir D A. Anopheline mosquitoes: vector reproduction, life cycle and biotape. In: Wernsdorfer W H, McGregor I A, editors. Malaria: principles and practice of malariology. Vol. 1. Edinburgh, United Kingdom: Churchill Livingstone; 1988. pp. 431–451. [Google Scholar]
- 120.Mutambu S, Shiff C. Implementing and sustaining community-based mosquito net interventions in Africa. Parasitol Today. 1997;13:204–206. [Google Scholar]
- 121.Nardin E H, Nussenzweig R S. T-cell responses to pre-erythrocytic stages of malaria: role in protection and vaccine development against pre-erythrocytic stages. Annu Rev Immunol. 1993;11:687–727. doi: 10.1146/annurev.iy.11.040193.003351. [DOI] [PubMed] [Google Scholar]
- 122.Nardin E H, Nussenzweig R S, McGregor I A, Bryan J H. Antibodies to sporozoites: their frequent occurrence in individuals living in an area of hyperendemic malaria. Science. 1979;237:597–599. doi: 10.1126/science.386511. [DOI] [PubMed] [Google Scholar]
- 123.Newbold C I, Craig A G, Kyes S, Berendt A R, Snow R W, Peshu N, Marsh K. PfEMP1, polymorphism and pathogenesis. Ann Trop Med Parasitol. 1997;91:551–557. doi: 10.1080/00034989760923. [DOI] [PubMed] [Google Scholar]
- 124.Nussenzweig R S, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of X-irradiated sporozoites of Plasmodium berghei. Nature. 1967;216:1160–1162. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- 125.Nussenzweig V, Nussenzweig R S. Rationale for the development of an engineered sporozoite malaria vaccine. Adv Immunol. 1989;45:283–334. doi: 10.1016/s0065-2776(08)60695-1. [DOI] [PubMed] [Google Scholar]
- 126.O'Brochta D A, Atkinson P W. Recent developments in transgenic insect technology. Parasitol Today. 1997;13:99–104. doi: 10.1016/s0169-4758(97)01006-5. [DOI] [PubMed] [Google Scholar]
- 127.Patarroyo M E, Romero P, Torres M L, Clarijo P, Moreno A, Martinez A, Rodriguez R, Guzman F, Cabezas E. Induction of protective immunity against experimental infection with malaria using synthetic peptides. Nature. 1987;328:629–632. doi: 10.1038/328629a0. [DOI] [PubMed] [Google Scholar]
- 128.Patarroyo M E, Amedor R, Claijo P, Moreno A, Guzman F, Romero P, Tascan R, Franco A, Murillo L A, Ponton G, Trujillo G. A synthetic vaccine protects humans against challenge with asexual blood stages of Plasmodium falciparum malaria. Nature. 1988;332:158–161. doi: 10.1038/332158a0. [DOI] [PubMed] [Google Scholar]
- 129.Perrin L H, Merkli B, Loche M, Chizzolini C, Smart J, Richle R. Antimalarial immunity in Saimiri monkeys J. Exp Med. 1984;160:441–451. doi: 10.1084/jem.160.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Peters W. Chemotherapy and drug resistance in malaria. 2nd ed. London, United Kingdom: Academic Press; 1987. [Google Scholar]
- 131.Peterson D S, Di Santi S M, Povoa M, Calvosa V S, Do Rosario V E, Wellems T E. Prevalence of the DHFR Asn-108 mutation as the basis for pyrimethamine resistant falciparum malaria in the Brazilian Amazon. Am J Trop Med Hyg. 1991;45:492–497. doi: 10.4269/ajtmh.1991.45.492. [DOI] [PubMed] [Google Scholar]
- 132.Peterson D S, Milhous W K, Wellems T E. Molecular basis of a differential resistance to cycloguanil and pyrimethamine in Plasmodium falciparum malaria. Proc Natl Acad Sci USA. 1990;87:3018–3022. doi: 10.1073/pnas.87.8.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Phillips R S. Studies in biology, no. 152. Malaria. London, United Kingdom: Edward Arnold Publishers; 1983. [Google Scholar]
- 134.Phillips R S, Mathers K E, Taylor-Robinson A W. T-cells in immunity to Plasmodium chabaudi chabaudi: operation and regulation of different pathways of protection. Res Immunol. 1994;145:406–412. doi: 10.1016/s0923-2494(94)80169-x. [DOI] [PubMed] [Google Scholar]
- 135.Phillips R S, Brannan L R, Neuville P. Antigenic variation during malaria infection—the contribution from the murine parasite Plasmodium chabaudi. Parasite Immunol. 1997;19:427–434. doi: 10.1046/j.1365-3024.1997.d01-239.x. [DOI] [PubMed] [Google Scholar]
- 136.Phillips R S. Malaria vaccines—a problem solved or simply a promising start. Protozool Abstr. 1994;18:458–486. [Google Scholar]
- 137.Pieris J S, Premawansa S, Ranawaka M B R, Udagama P V, Musasinghe Y D, Nanayakkara M V, Gamage C P, Carter R, David P H, Mendis K N. Monoclonal and polyclonal antibodies both block and enhance transmission of human Plasmodium vivax malaria. Am J Trop Med Hyg. 1988;39:26–32. doi: 10.4269/ajtmh.1988.39.26. [DOI] [PubMed] [Google Scholar]
- 138.Plebanski M, Aidoo M, Whittle H C, Hill A V. Precursor frequency analysis of cytotoxic T lymphocytes to pre-erythrocytic antigens of Plasmodium falciparum in West Africa. J Immunol. 1997;158:2849–2855. [PubMed] [Google Scholar]
- 139.Plowe C V, Cortese J F, Djimde A, Okey C, Winstanley P A, Doumbo C K. Mutations in Plasmodium falciparum dihydrofolate reductase and dihydropterate synthase and epidemiological patterns of pyrimethamine-sulfodoxine use and resistance. J Infect Dis. 1997;176:1590–1596. doi: 10.1086/514159. [DOI] [PubMed] [Google Scholar]
- 140.Porter A G. Mosquitocidal toxins, genes and bacteria: the hit squad. Parasitol Today. 1996;12:175–179. doi: 10.1016/0169-4758(96)10013-2. [DOI] [PubMed] [Google Scholar]
- 141.Potocnjak P, Yoshiba N, Nussenzweig R S, Nussenzweig V. Monovalent fragments (Fab) of monoclonal antibodies to a sporozoite surface antigen (Pb44) protect mice against malaria infection. J Exp Med. 1980;151:1504–1513. doi: 10.1084/jem.151.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Price R N, Nosten F, Luxemburger C, van Vugt M, Phaipun L, Chongsuphajaisiddhi T, White N J. Artesunate/mefloquine treatment of multi-drug resistant falciparum malaria. Trans R Soc Trop Med Hyg. 1997;91:574–577. doi: 10.1016/s0035-9203(97)90032-8. [DOI] [PubMed] [Google Scholar]
- 143.Price R, Nosten F, Luxemburger C, Ter Kuile F O, Paiphun L, Chongsuphajaisiddhi T, White N J. Effects of artemisinin derivatives on malaria transmissibility. Lancet. 1996;347:1654–1658. doi: 10.1016/s0140-6736(96)91488-9. [DOI] [PubMed] [Google Scholar]
- 144.Priest F G. Biological control of mosquitoes and other biting flies by Bacillus sphaericus and Bacillus thuringiensis. J Appl Bacteriol. 1992;72:357–369. doi: 10.1111/j.1365-2672.1992.tb01847.x. [DOI] [PubMed] [Google Scholar]
- 145.Pudney M. Antimalarials: from quinine to atovaquone. Soc Gen Microbiol. 1994;53:229–247. [Google Scholar]
- 146.Ramsay J M, Beaudoin R L, Hollingdale M R. Nuclear techniques in the study of parasitic infections. Vienna, Austria: International Atomic Energy Agency; 1982. Infection of tissue-culture cells with Co gamma-irradiated malaria sporozoites; pp. 19–25. [Google Scholar]
- 147.Ribeiro J M C. Role of saliva in blood-feeding by arthropods. Annu Rev Entomol. 1987;32:463–478. doi: 10.1146/annurev.en.32.010187.002335. [DOI] [PubMed] [Google Scholar]
- 148.Riley E. Malaria vaccine trials: SPf66 and all that. Curr Opin Immunol. 1995;7:612–616. doi: 10.1016/0952-7915(95)80065-4. [DOI] [PubMed] [Google Scholar]
- 149.Rioux J A. Phyto-ecological basis of mosquito control: cartography of larval biotypes. Mosq News. 1968;28:572–582. [Google Scholar]
- 150.Rogers N J, Daramola O, Targett G A T, Hall B S. CD36 and intercellular adhesion molecule 1 mediate adhesion of developing Plasmodium falciparum gametocytes. Infect Immun. 1996;64:1480–1483. doi: 10.1128/iai.64.4.1480-1483.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rosenberg R, Wirtz R A, Schneider I, Burge R. An estimation of the number of malaria sporozoites ejected by a feeding mosquito. Trans R Soc Trop Med Hyg. 1990;84:209–212. doi: 10.1016/0035-9203(90)90258-g. [DOI] [PubMed] [Google Scholar]
- 152.Ross R. Studies on malaria. London, United Kingdom: John Murray Publishers; 1928. [Google Scholar]
- 153.Rossignol P A, Ribeiro J M C, Spielman A. Increased intradermal probing time in sporozoite-infected mosquitoes. Am J Trop Med Hyg. 1984;33:17–20. doi: 10.4269/ajtmh.1984.33.17. [DOI] [PubMed] [Google Scholar]
- 154.Rossignol P A, Ribeiro J M C, Jungery M, Turrell M J, Spielman A, Bailey C L. Enhanced mosquito blood-finding success on parasitaemic hosts: evidence for vector-parasite mutualism. Proc Natl Acad Sci USA. 1985;82:7725–7727. doi: 10.1073/pnas.82.22.7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sam-Yellowe T Y. Rhoptry organelles of the apicomplexa: their role in host cell invasion and intracellular survival. Parasitol Today. 1996;12:308–316. doi: 10.1016/0169-4758(96)10030-2. [DOI] [PubMed] [Google Scholar]
- 156.Saul A. The role of variant surface antigens on malaria-infected red blood cells. Parasitol Today. 1999;15:455–457. doi: 10.1016/s0169-4758(99)01534-3. [DOI] [PubMed] [Google Scholar]
- 157.Schiff L J, Minjas J, Premji Z. The rapid manual Parasight F test. A new diagnostic tool for Plasmodium falciparum infection. Trans R Soc Trop Med Hyg. 1993;87:646–648. doi: 10.1016/0035-9203(93)90273-s. [DOI] [PubMed] [Google Scholar]
- 158.Schneider J, Gilbert S C, Blanchard T J, Hanka T, Robson K J, Hannon C M, Backer M, Sinden R E, Smith G L, Hill A V. Enhanced immunogenicity for CD8+ T cell induction and complete efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat Med. 1998;4:397–402. doi: 10.1038/nm0498-397. [DOI] [PubMed] [Google Scholar]
- 159.Schuurkamp G J, Spicer P E, Keren R K, Bulungol P K, Rieckmann K H. Chloroquine-resistant Plasmodium vivax in Papua New Guinea. Trans R Soc Trop Med Hyg. 1992;86:121–122. doi: 10.1016/0035-9203(92)90531-g. [DOI] [PubMed] [Google Scholar]
- 160.Schwartlander B. Global burden of disease. Lancet. 1997;350:141–142. doi: 10.1016/S0140-6736(05)61844-2. [DOI] [PubMed] [Google Scholar]
- 161.Sedegah M, Hedstrom R, Hobart P, Hoffman S L. Protection against malaria by immunization with plasmid DNA encoding circumsporozoite protein. Proc Natl Acad Sci USA. 1994;91:9866–9870. doi: 10.1073/pnas.91.21.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Service M W. Medical entomology for students. London, United Kingdom: Chapman and Hall Publishers; 1996. [Google Scholar]
- 163.Shahabuddin M, Toyoshima F J A, Aikawa M, Kaslow D C. Transmission-blocking activity of a chitinase inhibitor and activation of malarial parasite chitinase by mosquito protease. Proc Natl Acad Sci USA. 1993;90:4266–4270. doi: 10.1073/pnas.90.9.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Shahabuddin S, Cociancich S, Zieler H. The search for novel malaria transmission-blocking targets in the mosquito midgut. Parasitol Today. 1998;14:493–497. doi: 10.1016/s0169-4758(98)01348-9. [DOI] [PubMed] [Google Scholar]
- 165.Siddiqui W A, Tam L Q, Kramer K J. Merozoite surface coat precursor protein completely protects Aotus monkeys against Plasmodium falciparum malaria. Proc Natl Acad Sci USA. 1987;84:3014–3018. doi: 10.1073/pnas.84.9.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sim B K L. EBA-175: an erythrocyte binding ligand of Plasmodium falciparum. Parasitol Today. 1995;11:213–217. doi: 10.1016/0169-4758(95)80080-8. [DOI] [PubMed] [Google Scholar]
- 167.Sinnis P, Sim B K L. Cell invasion by the vertebrate stages of Plasmodium. Trends Microbiol. 1997;5:52–58. doi: 10.1016/s0966-842x(97)84657-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sinnis P, Nussenzweig V. Preventing sporozoite invasion of hepotocytes. In: Hoffman S L, editor. Malaria vaccine development: a multi-immune response approach. Washington, D.C.: ASM Press; 1996. pp. 15–33. [Google Scholar]
- 169.Sinnis P, Willow T, Brionas M R S, Herz J, Nussenzweig V. Remnant lipoproteins inhibits malaria sporozoite invasion of hepatocytes. J Exp Med. 1996;184:945–954. doi: 10.1084/jem.184.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Smith J D, Chitnis C E, Craig A G, Roberts D J, Hudson-Taylor D E, Peterson D S, Pinches R A, Newbold C I, Miller L H. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherence phenotypes of infected erythrocytes. Cell. 1995;82:101–110. doi: 10.1016/0092-8674(95)90056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Snewin V A, Premawansa S, Kapilanda G M G, Ratnayaka L, Udagama P V, Mattei D M, Khonsi E, Del Guidice G, Pieris J S M, Mendis K N, David P H. Transmission blocking immunity in Plasmodium vivax malaria: antibodies raised against a peptide block parasite development in the mosquito vector. J Exp Med. 1995;181:357–362. doi: 10.1084/jem.181.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Snounou G, Jarra W, Preiser P R. Malaria multigene families: the price of chronicity. Parasitol Today. 1999;16:28–30. doi: 10.1016/s0169-4758(99)01546-x. [DOI] [PubMed] [Google Scholar]
- 173.Snow R W, Marsh K. Will reducing Plasmodium falciparum transmission alter malaria transmission among African children? Parasitol Today. 1995;11:188–190. doi: 10.1016/0169-4758(95)80025-5. [DOI] [PubMed] [Google Scholar]
- 174.Snow R W, Marsh K. New insights into the epidemiology of malaria relevant for disease control. Br Med Bull. 1998;54:293–309. doi: 10.1093/oxfordjournals.bmb.a011689. [DOI] [PubMed] [Google Scholar]
- 175.Snow R W, Omumbo J A, Lowe B, Molyneux C S, Obiero J O, Palmer A, Weber M W, Pinder M, Nahlen B, Obonyo C, Newbold C, Gupta S, Marsh K. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. Lancet. 1997;349:1650–1654. doi: 10.1016/S0140-6736(97)02038-2. [DOI] [PubMed] [Google Scholar]
- 176.Snow R W, Marsh K, le Sueur D. The needs for maps of transmission intensity to guide malaria control in Africa. Parasitol Today. 1996;12:455–457. [Google Scholar]
- 177.Snow R W, Craig M H, Deichmann U, le Sueur D. A preliminary continental risk map for malaria mortality among African children. Parasitol Today. 1999;15:99–104. doi: 10.1016/s0169-4758(99)01395-2. [DOI] [PubMed] [Google Scholar]
- 178.Stanley S L., Jr Malaria vaccines: are seven antigens better than one. Lancet. 1998;352:1163–1164. doi: 10.1016/S0140-6736(05)60525-9. [DOI] [PubMed] [Google Scholar]
- 179.Stoute J A, Slaoui M, Heppner D G, Momin P, Kester K E, Desmons P, Wellde B T, Garcon N, Krzych U, Marchand M, Ballou W R, Cohen J D. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. N Engl J Med. 1997;336:86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 180.Sturchler D. How much malaria is there worldwide? Parasitol Today. 1989;5:39–40. doi: 10.1016/0169-4758(89)90188-9. [DOI] [PubMed] [Google Scholar]
- 181.Su X, Kirkman L A, Fujioka H, Wellems T E. Complex polymorphisms in an ∼300 kDa protein are linked to chloroquine-resistant Plasmodium falciparum in Southeast Asia and Africa. Cell. 1997;91:593–603. doi: 10.1016/s0092-8674(00)80447-x. [DOI] [PubMed] [Google Scholar]
- 182.Su X-Z, Heatwole Y, Wertheimer S, Guinet F, Herrfeldt J A, Peterson D S, Ravetch J A, Wellems T E. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum infected erythrocytes. Cell. 1995;82:89–100. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 183.Suhrbier A. Immunity to liver stage of malaria. Parasitol Today. 1991;7:160–163. doi: 10.1016/0169-4758(91)90120-d. [DOI] [PubMed] [Google Scholar]
- 184.Sutherland C J. The flip-side of cytoadherence: immune selection, antigenic variation and the var genes of Plasmodium falciparum. Parasitol Today. 1998;14:329–332. doi: 10.1016/s0169-4758(98)01276-9. [DOI] [PubMed] [Google Scholar]
- 185.Tanner M, Beck H-P, Felger I, Smith T. The epidemiology of multiple Plasmodium falciparum infections. 1. General introduction. Trans R Soc Trop Med Hyg. 1999;93(Suppl. 1):S1/1–S1/2. doi: 10.1016/s0035-9203(99)90319-x. [DOI] [PubMed] [Google Scholar]
- 186.Taverne J. DDT—to ban or not to ban? Parasitol Today. 1999;15:180–181. doi: 10.1016/s0169-4758(99)01446-5. [DOI] [PubMed] [Google Scholar]
- 187.Taylor-Robinson A W. Regulation of immunity to malaria: valuable lessons learned from murine models. Parasitol Today. 1995;11:334–342. doi: 10.1016/0169-4758(95)80186-3. [DOI] [PubMed] [Google Scholar]
- 188.Trape J F, Pison G, Prezio M-P, Enel C, du Lou Desgrees A, Delaunay V, Samb B, Lagarde E, Molez J-F, Simondon F. Impact of chloroquine resistance on malaria mortality. C R Acad Sci Ser III. 1998;321:689–697. doi: 10.1016/s0764-4469(98)80009-7. [DOI] [PubMed] [Google Scholar]
- 189.Trape J-F, Rogier C. Combating malaria morbidity and mortality by reducing transmission. Parasitol Today. 1996;12:236–240. doi: 10.1016/0169-4758(96)10015-6. [DOI] [PubMed] [Google Scholar]
- 190.Unori E, Grab B. Indicators for the forecasting of malaria epidemics. Bull W H O. 1980;58:91–98. [PMC free article] [PubMed] [Google Scholar]
- 191.Valero M V, Amador L R, Galindo C, Figueroa J, Bello M S, Manillo L A, Mora A L, Patarroyo G, Rocha C L, Rojas M, Aponte J J, Sarmiento L E, Lozoaki D M, Coronell C G, Ortega N M, Rojas J E, Alonso P L, Patusrago M E. Vaccination with SPf66, a chemically synthesised vaccine against Plasmodium falciparum in Colombia. Lancet. 1993;341:705–710. doi: 10.1016/0140-6736(93)90483-w. [DOI] [PubMed] [Google Scholar]
- 192.Van der Weid T, Langhorne J. The role of cytokines produced in the immune response to the erythrocytic stages of mouse malarias. Immunobiology. 1993;189:392–418. doi: 10.1016/s0171-2985(11)80367-0. [DOI] [PubMed] [Google Scholar]
- 193.Vreden S S. The role of Kupffer cells in the clearance of malaria sporozoites from the circulation. Parasitol Today. 1994;10:304–308. doi: 10.1016/0169-4758(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 194.Walliker D, Quakyi I A, Wellems T E, McCutchan T F, Szarfman A, Carter R. Genetic analysis of the human malaria parasite Plasmodium falciparum. Science. 1987;236:1661–1666. doi: 10.1126/science.3299700. [DOI] [PubMed] [Google Scholar]
- 195.Wang R, Doolan D L, Le T P, Hedstrom R C, Coonan K M, Charoenvit Y, Jones T R, Hobart P, Marglith M, Ng J, Weiss W R, Sedegah M, de Taisne C, Norman J A, Hoffman S L. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malarial DNA vaccine. Science. 1998;282:446–480. doi: 10.1126/science.282.5388.476. [DOI] [PubMed] [Google Scholar]
- 196.Watkins W M, Mosobo M. Treatment of Plasmodium falciparum malaria with PSD: selective pressure for resistance is a function of long elimination half life. Trans R Soc Trop Med Hyg. 1993;87:75–78. doi: 10.1016/0035-9203(93)90431-o. [DOI] [PubMed] [Google Scholar]
- 197.Weiss W R, Sedegah M, Beaudoin R L, Miller L H. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc Natl Acad Sci USA. 1988;85:573–576. doi: 10.1073/pnas.85.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Weiss W R, Mellouk S, Houghten R A, Sedegah M, Kumar S, Good M F, Berzofsky J A, Miller L H, Hoffman S L. Cytotoxic T cells recognize a peptide from circumsporozoite protein on malaria-infected hepatocytes. J Exp Med. 1990;171:763–773. doi: 10.1084/jem.171.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wernsdorfer W H. The development and spread of drug-resistant malaria. Parasitol Today. 1991;7:297–303. doi: 10.1016/0169-4758(91)90262-m. [DOI] [PubMed] [Google Scholar]
- 200.White N J. Combination treatment for falciparum prophylaxis. Lancet. 1987;i:680–681. doi: 10.1016/s0140-6736(87)90438-7. [DOI] [PubMed] [Google Scholar]
- 201.White N J. Antimalarial drug resistance: the pace quickens. J Antimicrob Chemother. 1992;30:S71–S78. doi: 10.1093/jac/30.5.571. [DOI] [PubMed] [Google Scholar]
- 202.White N J. Artemisinin: current status. Trans R Soc Trop Med Hyg. 1994;88(Suppl. 1):3–4. doi: 10.1016/0035-9203(94)90459-6. [DOI] [PubMed] [Google Scholar]
- 203.White N J. Mefloquine in the prophylaxis and treatment of falciparum malaria. Br Med J. 1994;30:286–287. [Google Scholar]
- 204.White N J, Olliaro P L. Strategies for the prevention of antimalarial drug resistance: rationale for combination chemotherapy for malaria. Parasitol Today. 1996;12:399–401. doi: 10.1016/0169-4758(96)10055-7. [DOI] [PubMed] [Google Scholar]
- 205.White N J, Nosten F. Advances in chemotherapy and prophylaxis of malaria. Curr Opin Infect Dis. 1993;6:323–330. [Google Scholar]
- 206.Wilson R J M, Gardner M J, Feagin J E, Williamson D H. Have malaria parasites three genomes? Parasitol Today. 1991;7:134–136. doi: 10.1016/0169-4758(91)90276-t. [DOI] [PubMed] [Google Scholar]
- 207.World Health Organization. Vector resistance to pesticides. 15th report of the WHO Expert Committee on Vector Biology and Control. WHO Tech Rep Ser. 1992;818:1–71. [PubMed] [Google Scholar]
- 208.World Health Organization. Report of a joint WHO/USAID informal consultation. Geneva, Switzerland: World Health Organization; 2000. Malaria diagnosis—new perspectives. [Google Scholar]
- 209.World Health Organization. Vector control for malaria and other mosquito-borne diseases. WHO Tech Rep Ser. 1995;857:1–91. [PubMed] [Google Scholar]
- 210.World Health Organization. Severe falciparum malaria. Trans R Soc Trop Med Hyg. 2000;94(Suppl. 1):S1/1–S1/74. [PubMed] [Google Scholar]
- 211.World Health Organization. A global strategy for malaria. Geneva, Switzerland: World Health Organization; 1993. [Google Scholar]
- 212.World Health Organization. Investing in health research and development. Document TDR/Gen/96.1. Geneva, Switzerland: Ad Hoc Committee of Health Research Relating to Future Intervention Options, World Health Organization; 1996. [Google Scholar]