Abstract
Aging is by far the most prominent risk factor for Alzheimer's disease (AD), and both aging and AD are associated with apparent metabolic alterations. As developing effective therapeutic interventions to treat AD is clearly in urgent need, the impact of modulating whole-body and intracellular metabolism in preclinical models and in human patients, on disease pathogenesis, have been explored. There is also an increasing awareness of differential risk and potential targeting strategies related to biological sex, microbiome, and circadian regulation. As a major part of intracellular metabolism, mitochondrial bioenergetics, mitochondrial quality-control mechanisms, and mitochondria-linked inflammatory responses have been considered for AD therapeutic interventions. This review summarizes and highlights these efforts.
Key words: Mitochondrial DNA, Mitochondrial electron transport chain, Mitochondrial quality control, Reactive species, DAMPs, Hexokinase biosynthesis pathway, Diabetes, Circadian regulation, Microbiome
Abbreviations: αkG, alpha-ketoglutarate; Aβ, amyloid β; AD, Alzheimer's disease; ADP, adenosine diphosphate; ADRD, AD-related dementias; ACE2, angiotensin I converting enzyme (peptidyl-dipeptidase A) 2; cAMP, cyclic adenosine monophosphate; cGAS, cyclic GMP/AMP synthase; CSF, cerebrospinal fluid; DAMPs, damage-associated molecular patterns; ER, estrogen receptor; ETC, electron transport chain; FCCP, trifluoromethoxy carbonylcyanide phenylhydrazone; PET, fluorodeoxyglucose (FDG)-positron emission tomography; FPR-1, formyl peptide receptor 1; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide-1; HBP, hexoamine biosynthesis pathway; HTRA, high temperature requirement A; hAPP, human amyloid precursor protein; hPREP, human presequence protease; I3A, indole-3-carboxaldehyde; IRF-3, interferon regulatory factor 3; i.p., intraperitoneal; LC3, microtubule associated protein light chain 3; LRR, leucine-rich repeat; LPS, lipopolysaccharide; MCI, mild cognitive impairment; MAVS, mitochondrial anti-viral signaling; Mdivi-1, mitochondrial division inhibitor 1; mtDNA, mitochondrial DNA; MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy; mTOR, mechanistic target of rapamycin; NeuN, neuronal nuclear protein; NLRP3, leucine-rich repeat (LRR)-containing protein (NLR)-like receptor family pyrin domain containing 3; NOD, nucleotide-binding oligomerization domain; PKA, protein kinase A; POLβ, the base-excision repair enzyme DNA polymerase β; ROS, reactive oxygen species; SAMP8, senescence-accelerated mice; SCFAs, short-chain fatty acids; SIRT3, NAD-dependent deacetylase sirtuin-3; SkQ1, plastoquinonyldecyltriphenylphosphonium; STING, stimulator of interferon genes; STZ, streptozotocin; T2D, type 2 diabetes; TCA, Tricarboxylic acid; TLR9, toll-like receptor 9; TP, tricyclic pyrone; TRF, time-restricted feeding; TMAO, trimethylamine N-oxide
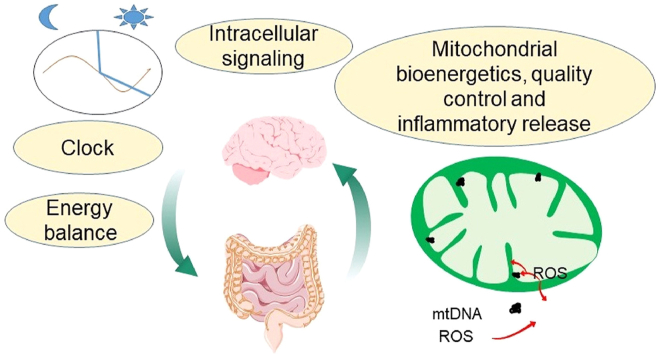
Graphical abstract
This review summarizes and highlights the increasing awareness of whole body metabolism, sex differences, microbiome, circadian regulation, as well as mitochondrial bioenergetics, mitochondrial quality control and mitochondrial-linked inflammatory responses in the context of AD therapeutic considerations.
1. Introduction
Alzheimer's disease (AD) is an age-dependent progressive neurodegenerative disorder and the most common cause of dementia. Pathologically, AD postmortem brains exhibit protein aggregation, mitochondrial dysfunction, and neuroinflammation1,2. Many studies have indicated that aging is the greatest risk factor for most chronic, non-communicable diseases, including AD. Specifically, the per capita death rate from AD at ages 75–85 is 70 times higher than at ages 55–65, and 700 times higher than at ages 45–553. Metabolic perturbations have been linked to AD both in the whole body and at the cellular level in the brain1. Indeed, impaired insulin sensitivity is observed in the majority of people 65 years old or older4, and type two diabetes (T2D) increases the risk of AD5, 6, 7. Epidemiology studies as well as investigations of clinical manifestations and mechanisms indicate common links between diabetes and AD5, 6, 7, 8, 9, 10, 11, 12, 13. While metabolic pathways contributing to energy production are essential for normal neuronal function, decreased glucose metabolism occurs in AD brains7,9,10. In rodent models that recapitulate aspects of AD, for example, APP/PS1 mice, impaired glucose tolerance and insulin sensitivity have been observed14. Streptozotocin (STZ) not only induces diabetes by disrupting pancreatic β cell function, but also affects learning/memory and exacerbates pathology in AD models15,16. Although the molecular mechanisms that underlie the connection between AD and diabetes are not yet clear17,18, these observations led to the idea that anti-diabetic drugs may have benefit in AD18, 19, 20, 21. Regulation of glucose metabolism including glycolysis and the hexosamine biosynthesis pathway have also been discussed in the context of AD pathogenesis and as the basis of intervention strategies22, 23, 24, 25, 26.
Glycolysis and mitochondrial energy production are closely linked in nearly all cell types and tissues, and together they contribute to whole body and cellular metabolism. Perturbation of glucose metabolism and mitochondrial dysfunction are a common feature of both diabetes and AD6, 7, 8, 9, 10, 11, 12, 13,27. It is clear that mitochondrial DNA mutations, deficiencies in electron transport chain activities, and deficits in glucose and lipid metabolism take place in AD28, 29, 30, 31, 32, 33, 34, 35. The “mitochondrial cascade hypothesis”33,36, 37, 38 has proposed that mitochondrial dysfunction plays an important role in the etiology of AD. Because of the crucial role of mitochondria in bioenergetics, metabolites homeostasis, electron transport chain related redox signaling, and neuroinflammation phenotypes, targeting the mitochondrial function, homeostasis, and mitochondrial quality-control mechanisms has emerged as a therapeutic strategy for age-related neurodegenerative diseases, including AD27,34,35,37,39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53. This review will summarize and highlight recent efforts to target metabolism and mitochondrial function, as well as inflammation, as a strategy for AD therapy. With the recent increasing awareness of sex differences, microbiome, and circadian regulation of metabolism, we will also discuss renewed interest in targeting these pathways.
2. Whole-organism energetics and potential targets for AD therapeutics
2.1. Glucose metabolism and insulin signaling
Multiple clinical observations have revealed a relationship between AD and AD-related dementias (ADRD) and energy balance. Energy balance is most simply defined in biological systems as the difference between energy intake (dietary calories) and energy expenditure (metabolic rate) over a set period of time54. These energetic imbalances are representative of both systemic and localized changes in metabolism within the body. Both obesity and weight loss have been suggested to be risk factors or signs of AD development55, 56, 57. Mid-life obesity has generally been associated with earlier onset and severity of AD/ADRD (albeit with exception)58, 59, 60. Energy imbalance as observed with weight loss in midlife or late life is associated with increased dementia risk61, 62, 63. Thus the contribution of energy imbalance to AD onset or progression and the complexity of the relationship based on the timing of the energy balance assessment (pre-versus post-AD diagnosis) need to be fully understood; similarly, clinical studies may need to consider both BMI and energy balance in their design (Fig. 1)64,65.
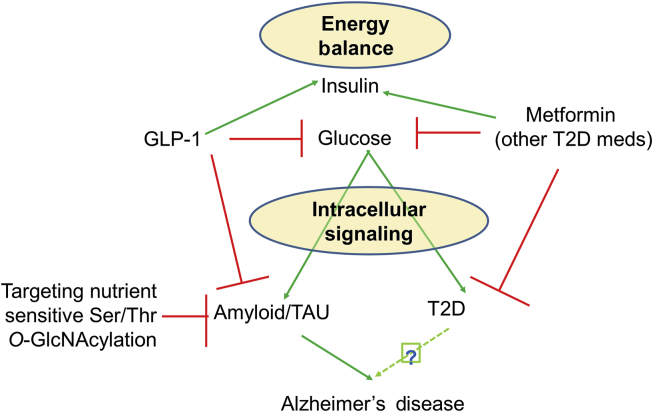
Figure 1.
Relationships among energy balance, insulin/glucose, intracellular insulin signaling, targeting protein Ser/Thr O-GlcNAcylation, type two diabetes (T2D), and AD risk. Green arrows indicate “stimulation” and red lines “inhibition”.
Multiple methods have been used to perform energy-expenditure assessments following AD diagnosis. Studies that used the doubly labeled water technique for daily energy expenditure and measurements of body composition via dual-energy X-ray absorptiometry in subjects with and without AD revealed that, in older subjects with AD (~70 years of age), daily energy expenditure was significantly lower than in healthy controls (~14% lower), as were levels of physical activity. However, this difference in energy expenditure was representative of the difference in body composition between the groups66,67, resulting in no difference in energy expenditure when included in the statistical models. In contrast, resting energy expenditure measured by indirect calorimetry is reported to be either slightly elevated or to have no significant difference with AD68,69. Assessments of energy intake have generally reported no differences between subjects with and without AD, thus raising further questions regarding the temporal pattern of weight loss and energy expenditure. These results contrast with multiple pre-clinical models of AD where a hypermetabolic state has been shown with elevated food intake but decreased body weight gain, reflective of elevated energy expenditure70,71.
Alongside whole-body measures of energy expenditure, tissue-specific and substrate-specific metabolism have been measured in multiple AD models and clinical states. Pre-clinical models of both diabetic and non-diabetic rats with increased β-amyloid exposure in the hippocampus show significant decreases in energy intake, activity, and fat oxidation but increases in carbohydrate oxidation and energy expenditure (resulting in negative energy balance and weight loss)72. Assessments of in vivo metabolism through various indirect methods have shown that, in patients with AD, cerebrospinal fluid (CSF) concentrations of lactate and pyruvate were higher, while succinate, fumarate, and glutamine were lower than controls, suggesting an impairment of mitochondrial oxidative metabolism of glucose73, 74, 75. A proposed deficit in cerebral glucose metabolism is further confirmed by in vivo imaging [e.g., FDG-PET and magnetic resonance imaging (MRI)] of tissue atrophy and lower glucose metabolism, including specific metabolic deficits in the precuneus, posterior cingulate, and temporal-parietal, as well as the hippocampus and default mode network brain regions, which vary by age at onset and disease progression74,76, 77, 78, 79, 80, 81. Complementary methodologies using magnetic resonance spectroscopy measures of glucose and related metabolite levels in brains of subjects with AD support a reduced glucose metabolism, including elevations in the glucose:creatine ratio82,83.
These systemic and localized deficits of carbohydrate metabolism are reminiscent of known risk factors for AD, which include both T2D and metabolic syndrome84,85. While a unified model of insulin resistance in contribution to AD onset or progression is yet to be fully verified, hyperglycemia and insulin resistance appear to precede and have predictive significance for AD-related biomarkers in both pre-clinical and clinical studies16,86, 87, 88. Cellular defects related to the metabolic stress present with both T2D and metabolic syndrome highlight the significance of insulin and insulin-like growth factor in peripheral and central metabolism, with further focus being placed on cell-specific insulin sensitivity in cerebral tissues86,89,90. Whether and how insulin sensitivity itself or in combination with pathological alterations accompanying insulin resistance (e.g., lipid metabolism, inflammation, misfolded protein stress, oxidative stress) contribute to AD risk and development remain to be fully dissected. However, a growing body of evidence suggests that targeting insulin resistance may have beneficial or protective effects despite a lack of clarity regarding the underlying mechanisms19,20.
One of the most-used diabetes drugs is metformin, which improves insulin sensitivity with effects including but not limited to the increasing peripheral uptake of glucose and suppression of hepatic glucose production91. Metformin's potential beneficial effect in AD is being explored92,93. An ongoing clinical trial NCT03733132 (double-blind, placebo-controlled, randomized design) will recruit 40 participants (>65 years with fasting glucose between 100 and 140 mg/dL and abdominal girth of >102 cm in men and >88 cm in women) to determine brain ATP production by 31P-MRS, brain structure and blood flow by functional MRI, muscle mitochondrial respiration, and cognitive function, after 40 weeks of metformin. This study may help provide evidence of the effects of metformin on cognition and bioenergetic function. As will be discussed later in “Section 3 Targeting mitochondrial bioenergetics and mitochondrial quality control”, the effect of metformin on mitochondrial complex I activity was demonstrated first in 2000 in permeabilized hepatocytes and isolated mitochondria94,95 and then reported in many other cell types. Note that high concentrations of metformin have been used in these studies, and a lower concentration was found to be required to activate AMPK and inhibit mitochondrial glycerophosphate dehydrogenase96. Whether metformin's inhibition of mitochondrial complex I is a prerequisite for its beneficial effects for diabetes or AD is still being debated.
Based on the observation that insulin signaling can regulate second messengers including AKT and MTOR97, and that perturbation of insulin signaling occurs in AD patient brains18, insulin, glucagon-like peptide-1 (GLP-1), and glucose-dependent insulinotropic polypeptide (GIP) receptor agonists have been used in animal models to test their effects on AD-related phenotypes. In clinical trials for mild cognitive impairment (MCI)/AD patients, nasal delivery of insulin was used, since systemic administration of insulin may lower blood sugar levels in people who are not diabetic. In non-AD young adults, 8 weeks of intranasal administration of insulin improved memory and mood in a double-blind, between-subject comparison98. Memory improvement was later found to occur in memory-impaired older adults and early AD patients99, 100, 101 and was also shown in several pilot clinical trials102. Because larger scale clinical trials have had mixed results, there is still a need for further investigations on if there are yet unknown factors that influence the outcome of intranasal insulin administration103,104.
In addition to insulin, other diabetic drugs that modulate insulin signaling have been tested in animal models of AD. Daily intraperitoneal (i.p.) injection for 8 weeks of the GLP-1 analogue liraglutide in APP/PS1 mice has been shown to decrease β-amyloid plaque, decrease numbers of activated microglia, increase dentate gyrus young neurons, and improve synaptic plasticity105. A 12-month multicenter, randomized, double-blind, placebo-controlled phase IIb trial is ongoing with 206 patients106. In addition, dual GLP-1/GIP receptor agonists DA5-CH and DA4-JC also attenuated memory loss in APP/PS1107. The pancreatic hormone amylin has been targeted by using its synthetic analogue as a potential therapy for AD, perhaps due to its plaque-forming potential and its effects on cAMP and PKA signaling108. Neuropeptides that may increase neuronal glucose transport are also considered to be potential drug targets in AD prevention and therapy109.
Downstream of glucose phosphorylation by hexokinase, the hexoamine biosynthesis pathway (HBP) generates O-GlcNAc to enable post-translational modification of proteins on Ser/Thr residues110. This post-translational modification is sensitive to nutrient availability and cellular stress26,110, 111, 112, 113, 114, 115, 116, 117. It has been observed that pharmacological inhibition of the enzyme that removes the O-GlcNAc from proteins resulted in more preserved tau O-GlcNAcylation, and decreased tau phosphorylation and aggregation22,118, 119, 120, 121. Thus, there has been significant effort put forth to improve the brain penetration and efficacy of these pharmacological agents to aid in developing suitable potential drugs for clinical trials22. However, there are still concerns regarding targeting this pathway based on studies in cell culture, which demonstrated potential inhibition of autophagy113,122,123, and in animal models, which demonstrated a potential detrimental effect on learning and memory124. A better understanding of the functional consequences of O-GlcNAc modifications of specific proteins and development of approaches to change the O-GlcNAcylation status of specific proteins are needed.
2.2. The gut microbiota and AD
AD has long been considered solely as a brain disease, with no apparent relationship to other distal tissues. This view has begun to change over the last 20 years, beginning with the Rotterdam study in 1999125, suggesting that metabolic inflexibility (the inability to shift substrate utilization) is a major contributor to the development of AD and related pathologies. As discussed above, conceptually, there is a strong correlation of pathological mechanisms of AD and metabolic disorders such as obesity, metabolic syndrome, and diabetes, with hallmarks including dysregulated glucose homeostasis and insulin resistance126. This metabolic inflexibility increases with age, the greatest known risk factor for the development of AD; however, in retrospective analyses, metabolic disturbances in even relatively young individuals predict future diagnosis of AD61,127, 128, 129, 130, 131, 132, 133.
A common link between metabolic dysregulation and AD is dysbiosis of the human intestinal tract or “gut”—commonly defined as a disturbance of or change in the density and/or composition of gut microbiota. Indeed, the human gut is inhabited by over 100 trillion microorganisms; including but not limited to over 1000 known species of bacteria134, 135, 136. As early as 1908137, a link between the gut and senility was proposed, but until the last 20 years, this connection was mainly overlooked in the context of human health and disease. However, gut microorganisms encode >150 times more genes than the human genome and are highly involved in numerous metabolic reactions. Most gut bacteria can be classified as either harmless (commensal) or beneficial (symbiont), with a very few classified as pathogenic (pathobiont)138. Still, pathogenic bacteria are often suppressed by a healthy balance among all gut bacteria to maintain gut homeostasis139,140.
Gut dysbiosis has been linked to a wide variety of metabolic-related diseases, including obesity and diabetes141. The two most dominant phyla in the human and mouse gut are Firmicutes and Bacteroides, representing more than 90% of the total bacterial community142,143. Many early studies suggested that metabolic conditions, including metabolic syndrome and diabetes, are associated with a lower Bacteroidetes/Firmicutes ratio144, 145, 146—although this measure has become more controversial in the last few years143. In line with this reasoning, there is growing literature demonstrating that the microbiota are critical to several functions of the central nervous system that occur in the context of AD147, including myelination, neuroinflammation, and regulation of blood–brain barrier integrity, as well as regulation of neurogenesis and accumulation of α-synuclein148. This communication occurs via the “gut–brain axis,” which includes the central nervous system, the autonomic and the enteric nervous system, and the peripheral nerves149, to influence host health- and lifespan. Studies of the gut microbiota profile in patients with AD indicate that this profile is markedly different from that of persons without AD150,151. As is the case in the metabolic diseases described above, one study has shown that patients with AD showed decreased microbiota richness with a decrease in the ratio of Firmicutes to Bacteroidetes151. However, inconsistent results have been noted both in human patients and in animal studies, including mouse, rat, and fly models of AD148. Although these studies support the interaction between gut microbiota and AD pathogenesis, the reason for the discrepancy in the changed composition of gut microbiota is not clear. To be sure, levels of these two phyla depend on many things, including natural variations in host relative abundance (which may be strain and husbandry dependent in the case of preclinical studies), host diet, host physical activity levels, and potentially gut regionality changes in these bacteria. In addition, changes to the relative abundance of more pathobionts may play a larger role in both metabolic disorders and AD discussed here145,148.
Recent studies of the contribution of bacterial metabolism and their metabolites and how they influence metabolic alterations in the host aim to explain AD pathogenesis in the context of metabolic disruption. For example, a hallmark of insulin resistance in humans is an increase in the gut-microbe-derived metabolite trimethylamine N-oxide (TMAO), which is also associated with several metabolic disorders152. Studies have shown elevated TMAO levels, correlating more with p-tau than Aβ levels, in the CSF of AD patients153. Bacteria-derived secondary bile acids, that are known to impact metabolic health and disease through modulation of cholesterol metabolism and clearance154, are elevated in patients with AD compared to non-afflicted controls155, 156, 157, associated with cognitive dysfunction—although the exact mechanism is unknown. Short-chain fatty acids (SCFAs), which are essential to lipid/protein metabolic process and influence glycemic control in a variety of metabolic conditions158, may have a neuroprotective role in AD. This protection may occur by enhancing energy substrates to the brain or regulating microglial maturation and function159. Tryptophan is also known to influence metabolic health, perhaps through its downstream derivative, indole160. Indole derivatives can have beneficial or toxic effects, and the regulation of derivative production is dependent upon the composition of the intestinal microbiota. Meanwhile, the vast majority of tryptophan is metabolized to the neuro-regulatory kynurenine pathway—a key link between gut dysbiosis and neurodegenerative conditions including AD161, 162, 163, 164, 165, 166. Notably, members of the genus Lactobacilli promote the production of indole-3-carboxaldehyde (I3A), which promotes the production of interleukin 22 and the maintenance of mucosal reactivity167. Mechanistically, angiotensin I converting enzyme (peptidyl-dipeptidase A) 2 (ACE2) is essential to intestinal absorption of tryptophan168,169, regulates intestinal immune function and gut microbiota ecology, and attenuates intestinal inflammation suffered in response to epithelial damage168, 169, 170, 171.
How the gut microbiome affects whole-body metabolism and mitochondrial bioenergetics both in the brain and in the circulation is unclear. Whether changes to the gut microbiome are a consequence of or a contributing factor to the pathogenesis of AD still remains to be addressed. Overall, the gut–brain axis is an important integrative system that modulates metabolic balance, and hence is a potential target for managing AD (Fig. 2). Recent clinical studies found that remodeling the gut microbiome with GV-971, a sodium oligomannate, improved cognitive function in a phase III, double-blind, placebo-controlled trial with 818 mild to moderate AD patients in as little as 4 weeks, perhaps through decreasing neuroinflammation172, 173, 174, 175, 176. A small clinical trial (10 participants, ClinicalTrials.gov Identifier: NCT03856359, Bausch Health and Duke University) is being conducted to investigate the effect of rifaximin, an antibiotic that alters gut microbiota, on cognition and serum pro-inflammatory markers, as well as gut microbiota. How specific dietary modulation and pro/prebiotic supplementation affect gut microbiome as well as whole-body and neuronal energetics still needs to be critically evaluated.
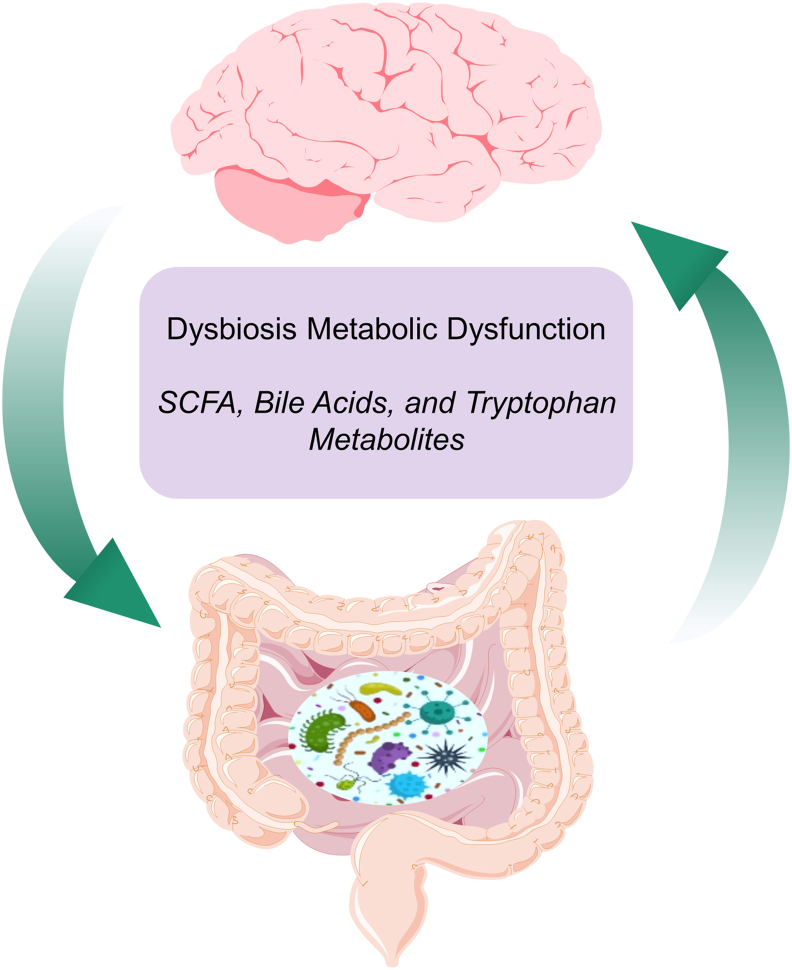
Figure 2.
A common link between metabolic dysregulation and AD is dysbiosis of the human intestinal tract or “gut”—commonly defined as a disturbance of or change in the density and/or composition of gut microbiota. Bacterial metabolites (purple box), may influence metabolism to directly or indirectly impact neuronal function. Overall, the gut–brain axis (the brain affects the gut through neuronal and hormonal signals, and the gut microbiome affects the brain) is an important integrative system that modulates metabolic balance and, hence, is a potential target for managing AD.
2.3. Targeting circadian regulation in diabetes treatment and implication in AD
Disturbances in the sleep/wake cycle are common in the elderly and in AD patients, with fragmented nighttime sleep, increased nocturnal activity, and daytime sleepiness, which are considered among the most disruptive symptoms of AD177, 178, 179, 180, 181. Metabolism, mitochondrial function, and the cellular protein/organelle quality-control mechanisms (including autophagy and mitophagy) have all been shown to be regulated by central and peripheral circadian clocks115,182, 183, 184, 185. In mouse models, disruptions of circadian-regulating transcription factors result in impaired learning and memory186, 187, 188, 189, 190, 191, 192, 193, 194.
Therapeutic strategies targeting the sleep/wake cycle or the circadian clock have been explored. In a 2008 report of a trial in group care facilities that lasted more than 12 months, 189 residents were with a randomized assignment to whole-day bright versus dim light. Furthermore, they were double-blind and placebo-controlled for evening 2.5 mg melatonin. The study has shown that light treatment attenuated cognitive decline, and attenuated melatonin use was associated with withdrawn behavior195. In a 2014 multicenter study of 73 mild and moderate AD patients, in addition to standard therapy of acetylcholinesterase inhibitors ± memantine, patients were given a placebo for 2 weeks and then randomized to receive a placebo or 2 mg prolonged-release melatonin nightly for 24 weeks, before receiving 2 weeks of a placebo. The melatonin group had improved cognition and a similar adverse event profile as the placebo group196. A recent study of 37 nursing home residents 70–93 years of age treated with bright light for 90 min in the morning for 5 days also showed cognitive improvement197. How cognitive improvement is associated with other elements of aging in response to light and/or melatonin therapy, and whether it involves metabolic and redox-related mechanisms, will need further investigation.
Coffee consumption has been used in humans to improve alertness during day time activities; caffeine has also been reported to decrease AD risk in humans and decrease pathological and cognitive impairments in AD mouse models198. However, a 2018 meta-analysis found no change in risk of AD with coffee consumption199, suggesting that the current data are insufficient as evidence of the utility of coffee consumption as an AD prevention strategy. Time-restricted feeding (TRF) is another intervention being explored for metabolite modulation, healthy aging, and potential AD therapy184,200, 201, 202, 203. TRF applies the idea that restricting the daily feeding period without decreasing caloric intake by aligning feeding/fasting activities with the intrinsic and extrinsic circadian clock is better for overall health in general and for alleviating neurodegenerative disease in particular. TRF in mice changes gene expression and metabolite abundance in multiple tissues, with the most extensive studies focusing on the liver204. In a human study with 11 overweight adults, early TRF (8 a.m.–2 p.m.) decreased mean 24-h glucose level, increased before-breakfast ketones, cholesterol, and the expression of SIRT1 and LC3A in the white blood cells, and increased evening MTOR gene expression in the white blood cells, compared to the control (8 a.m.–8 p.m.)203. A randomized pilot study in 24 healthy people with 10 people feeding normally for 6 weeks and then feeding within 8 h/day for 6 weeks and 14 people feeding within 8 h/day for 6 weeks before normal feeding for 6 weeks did not find that TRF changed lean mass, bone density, nutrient intake, or cardiovascular function205. A short-term (5-day) crossover study with 8-h versus 15-h TRF in 11 obese people found changed lipid and amino acid metabolites in serum and muscle200. Patients with T2D (19 completed) in a TRF (within 9 h/day) study for 4 weeks did not find a consistent decrease in body mass or a consistent improvement of cognition, but the regimen had various barriers to adherence206. These studies have presented both potential benefits and challenges to using this strategy in humans.
Pharmacological reagents that target circadian-regulating transcription factors have also been explored in AD animal models. As transcriptional repressors that are part of the circadian clock, REV-ERBs have ligand-binding domains that bind heme and have been found to be regulated by synthetic agonists and antagonists207, 208, 209, 210. On one hand, activation of REV-ERB by SR9009 suppressed lipopolysaccharide (LPS)-induced hippocampal neuroinflammation211, decreased Aβ in the cortex, and reversed cognitive dysfunction of aged SAMP8 mice212, which seems to be consistent with the finding that loss of REV-ERBα resulted in impaired memory193,194. However, pharmacological inhibition of REV-ERBs (with antagonist SR8278) or knockdown of REV-ERBs has been found to accelerate microglial uptake of fibrillary Aβ and increase transcription of BMAL1213. Furthermore, REV-ERBα knockout decreased plaque number and size and prevented an increase in the microglial proteins TREM2, CD45, and CLEC7A in 5XFAD mice213. The lack of consistency between these observations may be due to the differences in the mouse models—LPS and SAMP8 models compared to 5xFAD models—or because both activation and suppression of REV-ERBs can stimulate intracellular processes, which is protective against neuroinflammation and proteotoxicity (Fig. 3).
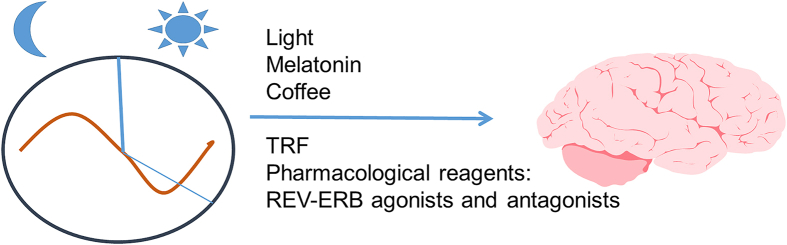
Figure 3.
The circadian clock regulates metabolism and mitochondrial function in various model systems. Disturbances in the sleep/wake cycle in aging people and AD patients were prominent disruptive symptoms. Thus, restricted light, restricted food intake, and reagents that modulate the circadian clock, such as melatonin, coffee, and REV-ERB agonists and antagonists, are being considered for targeting whole-body and mitochondrial metabolism in the context of AD therapy.
2.4. Sex differences and AD
Sex differences in the incidence and progression of chronic diseases of aging, such as AD, are ubiquitous. In the United States, men die at higher age-adjusted rates from eight of the 10 top causes of death. The two exceptions are stroke, for which there is no sex difference, and AD of which women die at a consistently higher age-adjusted rate. AD is the only major cause of death from which women suffer and succumb at a higher rate than men3. This sex bias in AD prevalence and deaths is not confined to the U.S. Although the reported prevalence of AD varies widely across the globe, the female sex bias is reported in national vital statistics from the EU, UK, Japan, and, in fact, all countries that maintain reliable health records214, 215, 216. Neurodegenerative diseases more generally are either not sex biased or are biased toward men. For instance, men have substantially higher incidences of Amyloid Lateral Sclerosis, Parkinson's disease, and vascular dementia215,217.
Almost two-thirds of people currently under clinical care for AD are women. That number is influenced by both women's greater age-independent incidence and the fact that women live longer than men. Given that men also display more of some prominent AD risk factors, such as greater prevalence of metabolic diseases, the female bias becomes even more puzzling. One intriguing hypothesis posits that survivor bias is largely responsible. That is, men at the highest risk for AD die from cardiovascular events before they can develop AD1. Another hypothesis relates to the educational advantage that men had for most of the 20th century. Educational attainment is a well-known protective factor for AD218. People of an age to be most susceptible to AD now were educated at a time when women had less access to education for a variety of reasons. As the mechanistic link between educational achievement and AD is as obscure as the pattern is clear, we will not pursue it further.
Focusing on possible biological causes for women's increased susceptibility to AD, we are left with some alluring hypotheses and provocative but not compelling evidence. As alluded to previously, the evidence for some mitochondrial role in the development and progression of AD is now compelling38. It is the link between sex differences in mitochondrial function, and sex differences in AD, that requires clarification, as the evidence from mouse models has been complex and sometimes conflicting. There seems to be a consensus that males and females differ in mitochondrial substrate utilization, particularly under nutrient stress219. However, there is little consensus on anything else. This is likely due to variation among the more than 100 mouse models of AD220 and the range of genetic backgrounds on which they are expressed. Also, most neurological sex differences are likely to be cell-type, possibly cell-compartment, and brain-region specific. For instance, AD patients showed a deficiency in the base-excision repair enzyme DNA polymerase beta (POLdβ), and that important repair enzyme was present in mitochondria in the brain, but not the liver. Other recent work observed mitochondrial complex I—specific impairment in cortical synaptic brain mitochondria in female, but not male, 3xTg mice, a common mouse model of AD221. On the other hand, in the same model, females, but not males, displayed enhancement of complex II-dependent respiration in non-synaptic, glial-enriched mitochondria from the cortex and hippocampus222. The challenges in interpretation of these complex findings are significant.
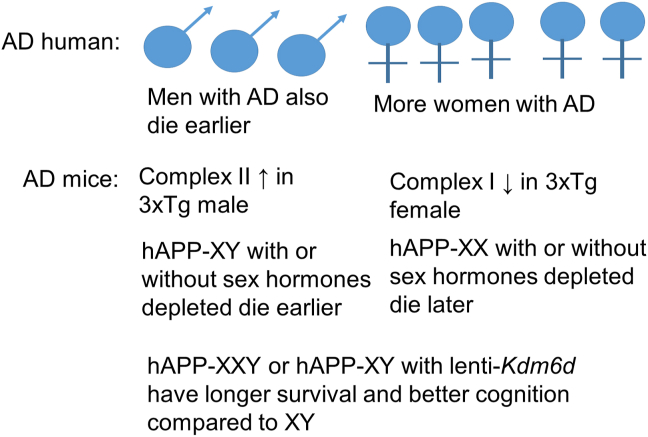
Arnold has divided mechanisms of sex differences into three categories223. The first category is organizational differences due to differential hormone exposure during development. Briefly, the SRY region of the Y chromosome directs testis formation in the developing embryo, and these hormones produced during fetal life have permanent structural effects on the brain. These organizational factors impact the structure, cell number, and cell types in the developing mouse brain224. Second, activational differences are due to the adult hormonal milieu, and they can be modulated by gonadectomy and hormone supplementation. The third is chromosomal differences, due either to genes expressed on the Y chromosome or those expressed differentially by incompletely inactivated X chromosomes. A number of genetic mouse models have been generated in order to study these categories separately225. One observation is that men with AD die earlier than women with AD. Using mice genetically modified to express the human amyloid precursor protein (hAPP) and to develop either testes or ovaries independent of X and Y chromosome dosage, it was shown that hAPP-XY mice died earlier and showed greater cognitive deficits regardless of whether they had testes or ovaries compared with hAPP-XX mice, regardless of whether they had testes or ovaries. In other words, an extra X chromosome was beneficial regardless of gonadal sex. Furthermore, a candidate gene, Kdm6a, encoding a histone demethylase, has been identified for the resilience accompanying a second X chromosome that is not inactivated226. It will be interesting to see whether this candidate gene bears out as a main contributor to this effect, whether it changes whole-body and mitochondrial metabolism, and whether enhancing its function or the function of its downstream targets can be of therapeutic value (Fig. 4).
Figure 4.
Currently, more women than men have AD. However, this may be due to socioeconomic or lifespan differences between men and women. It has been shown that men with AD die earlier than women with AD. Even though there is no difference in lifespan between sexes, mitochondrial complex I and II activities are different between sexes in the 3xTg AD model. In the hAPP model, the X chromosome has been shown to extend survival and enhance cognition.
We want to emphasize that, for all the wonderful biology that can be performed with genetically modified mice, there is a possibility that this is simply not the right model with which to explore human sex differences in AD, both in terms of understanding AD pathogenesis and with regard to testing therapeutic interventions. Despite the wealth of genetically engineered mouse AD models available, there have been no therapeutic treatments that showed promise in mice and have translated to humans. Mice, unlike humans, do not have consistent differences in longevity between the sexes227, so it is conceivable that other sex differences apparent in humans may not be replicated even in the most “humanized” mouse. Alternative models are becoming available, such as transgenic rats, which seem to replicate the human AD phenotype more closely228.
3. Targeting mitochondrial bioenergetics and mitochondrial quality control
The major source of energy comes from the mitochondria. Furthermore, mitochondria provide key metabolites for biosynthesis and signaling molecules that sense and respond to the environment and modulate cellular function. Mitochondrial dysfunction has been proposed to play an important role in AD36. The link between mitochondrial and metabolic dysfunction in AD has been demonstrated with the identification of mitochondrial DNA mutations, impairment of electron transport chain activities, and deficits in glucose and lipid metabolism28, 29, 30, 31, 32, 33, 34, 35,229. Both coupled (with ADP) and uncoupled (with FCCP) oxygen consumption rate in the presence of complex I substrates in freshly isolated mitochondria from the cortex and hippocampus were decreased in the 3xTg mice, earlier in females than males, and before a detectable decrease of representative proteins in the electron transport chain complexes230,231. In contrast to complexes I and IV33,232, evidence for deficiencies of complex II, III, or V activities in AD were not as pronounced. Nonetheless, decreased complex II activity has been reported in the APP/PS1 mouse model233, and levels of representative proteins of all the five electron transport chain complexes were decreased in the cortical piriform and insular regions of the 3xTg mice before the onset of detectable plaques231. Targeting the mitochondrial bioenergetics and quality-control mechanisms, including mitochondrial dynamics, mitochondrial movement, and mitophagy, has been increasingly explored for AD therapeutics development34,35,37,39,40. First, we will discuss targeting mitochondrial respiratory chains and then mitochondrial quality control (Fig. 5).
Figure 5.
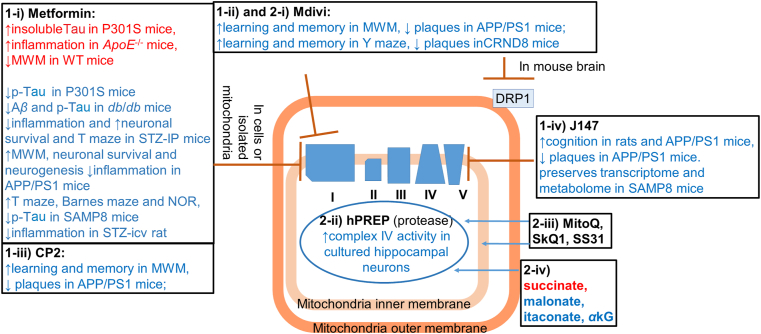
Compounds or peptides that target mitochondrial complex I, complex V, mitochondrial fission protein DRP1; and means to deliver mitochondrial protease, and TCA cycle substrates have been tested in preserving/improving mitochondrial function or in AD models.
3.1. Targeting mitochondrial electron transport chain (ETC) proteins
Because of the observation that there is a decline in glucose metabolism, an obvious approach would be to attempt to supplement glucose. However, decreased glucose metabolism is not associated with decreased circulatory glucose; on the contrary, it is often associated with increased blood glucose, as with diabetes. Hence, not surprisingly, supplementation of glucose alone does not seem to be a viable strategy for AD treatment. Enhancing mitochondrial bioenergetics has been considered. Interestingly, however, inhibition of mitochondrial respiration in many cases turned out to enhance lifespan and protect against tissue damage, a phenomenon likely attributable either to decreased mitochondrial production of reactive species or to an adaptive mitohormesis response to selective stress234, 235, 236, 237, 238, 239, 240, 241, 242, 243, 244, 245, 246, 247. For example, inhibition of ETC complex I increased lifespan in non-mammalian species248, and several compounds that have been found to be beneficial in animal models of AD have mild complex I inhibitory activities.
3.1.1. Metformin
As described earlier in “Section 2 Whole-organism energetics and potential targets for AD therapeutics”, metformin is a compound that has been discovered to have an anti-diabetic effect and was later reported to reversibly inhibit complex I (IC50 ~20 mmol/L)91,94,95,249. In primary neurons from wildtype and human tau transgenic mice, metformin decreases tau phosphorylation, consistent with a beneficial effect250. However, metformin increases Aβ generation in neuroblastoma cells251, activates AMPA/BACE1 in wildtype mice251, increases insoluble Tau, and exacerbates hindlimb atrophy and hyperactivity in P301S Tau transgenic mice, despite the decrease in p-Tau252. In ApoE–/– mice, metformin treatment for 18 weeks led to increased p-Tau, decreased NeuN and PSD95 positive cells, and increased neuroinflammation253. In wildtype C57BL/6J mice, metformin in drinking water for 4+ weeks led to improved motor coordination but worse performance in the Morris water maze254. These observations argue against using it alone as a therapeutic strategy. In a study with diabetic db/db male mice, metformin administration for 4.5 months alleviated db/db-associated increase of Aβ and p-Tau, as well as db/db-associated decrease of synaptophysin, but did not change db/db-associated poor performance in Barnes maze and fear conditioning learning and memory test255. In mice with diabetes induced by i.p. injection of streptozotocin, metformin twice-daily gavage for 21 days improved diabetes-associated learning and memory performance on a T-maze, decreased neuroinflammation, and increased neuronal survival256. Metformin i.p. injection starting at 26 weeks of age for 14 days in APP/PS1 mice led to improved performance in the Morris water maze learning and memory test, decreased neuroinflammation, and increased neuronal survival and neurogenesis257. Metformin also decreased neuroinflammation and Aβ in APP/PS1 mice treated at 7 months of age for 8 weeks258. Daily injection of metformin to the senescence-accelerated mice (SAMP8) starting at 11 months of age for 8 weeks led to improved performance in the T-maze, novel object recognition, and Barnes maze and decreased p-Tau259. In rats with intracerebroventricular injection of streptozotocin, metformin administration improved neuroinflammation and cognition both via oral gavage260 and via nanoliposomes261. In mice 8–10 months of age, daily i.p. injection of metformin for 14 days increased neurogenesis, and 3xTg mice of similar age and treatment had improved learning and memory, as assessed by the Morris water maze test262. Thus, despite its use in diabetes patients and the idea that diabetes and AD share several common mechanisms, including perturbation of insulin signaling, in rodents, metformin's effects on neuropathology and cognition vary with the specific animal model263,264. Furthermore, the effects of metformin administration on mitochondrial bioenergetics in vivo in AD models have not been thoroughly investigated.
Epidemiological data regarding metformin's positive effect on cognition is also mixed, likely due to the diversity of the clinical populations263, 264, 265. In a recent meta-analysis of 10 studies, it was concluded that metformin significantly decreased cognitive deficits in patients with T2D, and significant improvements are evident in patients in the Americas and Europe but not in Asia266. A study of all community-dwelling AD patients in Finland diagnosed from 2005 to 2011 with diabetes diagnosed ≥ 3 years before AD, in which a total of 7225 cases and 14,528 controls received metformin at least once, found that the adjusted odds of AD were lower among people dispensed metformin for ≥10 years267. Whether these positive effects occur in the non-diabetic population is not known. Thus far, no studies have investigated the impact of metformin on brain metabolism in human AD patients.
3.1.2. Mitochondrial division inhibitor 1 (Mdivi-1)
One compound, Mdivi-1, which was discovered to be a mitochondrial fission inhibitor through screening a chemical compound library, was later found to be an inhibitor of mitochondrial electron transport chain complex I activity at ~50 μmol/L concentration in multiple types of cells tested268. Since Mdivi-1 can pass the blood–brain barrier, 10 and 40 mg/kg of Mdivi-1 was administered by daily oral injection for 1 month in control and APP/PS1 mice and shown to alleviate learning and memory deficits in Morris water maze tests and decrease plaque in APP/PS1 mice269. In CRND8 and wildtype mice, i.p. injection of Mdivi-1 at 1.5 mg/30 g once every 2 weeks for 3 months resulted in enhanced learning and memory in the Y-maze test, decreased plaques, increased mitochondrial movement, length, neurite location, and protein content, and increased heme oxygenase-1 protein levels in the hippocampus270. The effects of Mdivi-1 administration in vivo in animal models on mitochondrial bioenergetics have not been thoroughly investigated.
3.1.3. CP2
The compound CP2 has been found to be a tricyclic pyrone (TP) molecule that attenuated cell death in MC65 cells in response to Aβ oligomers and inhibits Aβ aggregation in vitro271. CP2 has been shown to be an inhibitor of Acyl-CoA:cholesterol acyltransferase as well as a mild inhibitor of mitochondrial ETC complex I activities (by 20%–45% at 1–50 μmol/L assayed with isolated mitochondria; at 50 μmol/L can reach 80% inhibition with isolated mitochondria)272, 273, 274. CP2 (2 μmol/L, 24 h) decreased basal, ATP-linked, leak-linked, and non-mitochondrial oxygen consumption rate and stimulated AMPK in cultured primary neurons, decreased ATP, and increased AMP and the AMP:ATP ratio in APP/PS1 brains after 2 months272,273. Further related to metabolic regulation, SIRT3, PGC1α, TFAM, GLUT3, and GLUT4 were increased and phospho-PDH:total PDH ratio was decreased 24 h after gavage of CP2 at 25 mg/kg in APP/PS1 mice274. Chronic administration (2–14.5 months) improved cognition in the APP/PS1 and 3xTg mouse models of AD and decreased amyloid plaques in APP/PS1 mice after 4 months272, 273, 274, 275.
3.1.4. J147
J147 is a compound derivative from curcumin that exhibits neuroprotective features and low EC50s in multiple cell culture-based assays276. It has been shown to improve multiple cognitive tests in rats and the APP/PS1 mouse model of AD, as well as decrease pathology in APP/PS1 mice276,277. With a drug-affinity-responsive-target-stability identification approach, it was found that J147 targets ATP5A (a subunit of the mitochondrial complex V). Following its target identification, it has been found that J147 inhibits ATP synthase activity from isolated bovine heart mitochondria, localizes to mitochondria, and increases mitochondrial membrane potential in HT22 cells, as well as elevates total ATP in fly heads278. In the SAMP8 premature aging mice, J147 treatment (oral daily administration starting at 3 month of age) preserves a more youthful transcriptional profile in the hippocampus and a more youthful metabolomics profile in the plasma and brain, comparing three and 10 months of age278. It is yet to be shown whether J147 affects mitochondrial bioenergetics in the brain. Nonetheless, because of its bioavailability, penetration of the blood–brain barrier, and apparent lack of toxicity in mice, it is now being used in a phase I randomized, double-blind, placebo-controlled clinical trial with a single ascending oral dose in 64 healthy young and elderly volunteers (NCT03838185).
3.1.5. S-Equol
Connected to the previous section discussing sex differences in AD, estrogen or estrogen receptor (ER) agonists have been investigated in AD animal models as well as human patients. One recent study administered S-equol (an ERβ agonist produced by intestinal bacteria metabolism of a soy isoflavone component, 10 mg twice a day for 2 weeks) to 15 women with AD. Mitochondrial complex IV activity was measured in protein extracts from the platelets 2 weeks before treatment and 2 weeks after stopping the treatment. Although limited by the number of participating patients, this was the first study that directly measured drug effects on bioenergetics in human AD patients and no doubt will lead to more studies in the future279. Furthermore, it is still unclear the extent to which estrogen agonists affect mitochondrial bioenergetics by directly regulating mitochondrial ETC activities or by indirectly regulating mitochondrial quality control; thus, the mechanisms underlying estrogen agonists action remain to be determined.
3.2. Targeting mitochondrial quality control
In addition to the regulation of mitochondrial function in cellular metabolism, mitochondria number and metabolic function are regulated by fission and fusion, biogenesis, protease activities, and degradation by the lysosomes. Approaches targeting these processes have been explored in the context of AD therapeutics development.
3.2.1. Fission
Above, we discussed Mdivi-1, which inhibits mitochondrial ETC complex I activity and mitochondrial fission, and plays a neuroprotective role in AD animal models. In addition to Mdivi-1, a peptide inhibitor of fission protein DRP1, P110, has been developed based on binding to regions of homology between human DRP1 and FIS1280. P110 has been shown to attenuate the decrease of mitochondrial membrane potential in response to neurotoxins in cell models and to attenuate cell death in cell lines and animal models of Parkinson's disease, Huntington's disease, and amyloid lateral sclerosis280, 281, 282, 283. P110 also enhances mitochondrial bioenergetics in isolated cardiac mitochondria from post-myocardial infarction animals, as well as human iPSC-derived cardiomyocytes expressing toxic proteins284,285. Relevant to AD, in N2a cells expressing APPSwe mutant protein, in SH-SY5Y cells exposed to Aβ42, and in human AD patient-derived fibroblasts, P110 attenuates disease model-associated decrease of oxygen consumption rate, citrate synthase activity, levels of selective mitochondrial proteins tested, mitochondrial elongation, and cell death282. However, in human skin fibroblasts, both P110 and Mdivi-1 abolished circadian-dependent oscillation of total cellular ATP286, suggesting that there may be potential detrimental effects of blockade of mitochondrial fission. DA1 is another peptide that was developed based on sequence alignment between DRP1 and its interacting protein, mitochondrial nucleoid protein ATAD3A287. There are more ATAD3A pulled down together with DRP1 in Huntington's disease patient iPS cells and HD mouse brains, and DA1 decreases DRP1–ATAD3A interaction and suppresses mitochondrial fragmentation and bioenergetic deficits287. Whether DA1 peptide can attenuate neurological and neuropathological defects in AD models has not been tested. Mitochondrial fission can also be enhanced through AMPK activation, resulting in removal of mitochondria288. The strategies have not been investigated in preclinical or clinical models of AD.
3.2.2. hPreP
Mitochondrial misfolded, oxidized, or damaged proteins can be degraded by some of the mitochondrial proteases, including LONP, ATP-dependent CLP protease, human presequence protease (hPREP), and high temperature requirement A (HTRA)289. The observation that transgenic increase of neuronal PREP activity decreased Aβ accumulation, attenuated neuroinflammation and improved cognition in AD mouse models, and increased mitochondrial complex IV activity in cultured hippocampal neurons suggests that hPREP agonists may be neuroprotective in AD290,291.
3.2.3. Other mitochondrial-targeted compounds
A number of mitochondrial-targeted compounds have been tested in preclinical models for attenuating AD-related pathological and neurological deficits. Not all of these have been shown to impact mitochondrial bioenergetics. For example, in a rat model generated based on galactose-rich diet and breeding selection of cataract development with associated accelerated aging in multiple tissues, including declines of cognition (OXYS rat), dietary supplementation with plastoquinonyldecyltriphenylphosphonium (SkQ1) attenuated OXYS-associated decrease of mitochondrial area, density of asymmetric synapses, and neurons in the hippocampus. In addition, SkQ1 decreased Aβ40, Aβ42, total and p-Tau, and improved learning and memory in these rats. Mitochondrial function has not been assessed in SkQ1-treated OXYS rats292, 293, 294, 295. A ubiquinone compound, MitoQ, which targets the mitochondria due to a covalently bound lipophilic cation triphenylphosphonium, has been shown in 3xTg-AD mice to increase learning and memory and synaptophysin level and decrease GFAP, IBA1, and Aβ42, total and p-TAU296,297. A small clinical trial is testing MitoQ effects on carotid artery endothelial function and brain blood flow in mild cognitive impairment patients (ClinicalTrials.gov Identifier: NCT03514875). The mitochondrial-targeted peptide SS-31 associates with cardiolipin in the mitochondrial membrane298. In N2a cells exposed to Aβ, mitochondrial numbers are increased, and neuritic outgrowth and cytochrome oxidase activities are decreased, while MitoQ or SS31 both attenuate these Aβ-induced changes. Neuritic outgrowth in primary neurons of Tg2576 mice is also improved by MitoQ or SS31 treatment299. In a Tg2576 mouse model, SS31 i.p. injection decreased Aβ levels, increased fusion proteins and synaptic proteins, and increased cytochrome c activity300.
3.2.4. Metabolites in the mitochondria
Metabolites from the TCA cycle provide substrates for mitochondrial function as well as signaling and building blocks for other cellular molecules. It has been shown that the levels of the mitochondrial complex II substrate succinate can be modulated by supplementation of malonate and affect ischemia reperfusion injury301,302. Itaconate treatment decreased complex II respiration in primary neurons, while its infusion in vivo protected against traumatic brain injury303. Alpha-ketoglutarate (αkG) has been shown to extend lifespan in mice304. These studies suggest that by changing the relative levels of metabolites important for mitochondrial function, cellular function and survival may be affected.
3.2.5. Mitophagy
Aged, dysfunctional, and damaged mitochondria or mitochondrial fragments can be removed via mitophagy305, 306, 307, 308, 309, 310, 311. Many efforts have been made to enhance mitophagy as an approach for disease therapy (Fig. 6)312. One key pathway is the targeting of PINK1/Parkin to the mitochondria, PINK1 phosphorylation of Parkin and ubiquitin and Parkin ubiquitination of substrates are involved in the process305, 306, 307, 308, 309, 310. PINK1/Parkin independent mitophagy also present in various cells and tissues mediated in part by the mitophagy adaptor proteins305, 306, 307, 308, 309, 310. At present, although compounds that target mitophagy have been identified, some have unknown cellular targets, some have not been shown to impact whole-body or mitochondrial metabolism, and some have not been tested in AD models. In addition, many of the mitophagy adaptors, perhaps including but not limited to NBR1, TAX1BP1, P62, NDP52, FUNDC1, FKBP8, BNIP3, NIX, PGAM5, RHEB, BAX, and BECN, have not been specifically targeted with pharmacological compounds as a mitophagy-enhancement strategy in AD. Some have been targeted using pharmacological agents to enhance or prevent apoptosis or general autophagy, including in AD models. It is conceivable that, in the near future, more compounds targeting these mitophagy adaptors will be developed and tested in potentially different disease models including AD models depending on the mechanisms of action of these adaptor proteins.
Figure 6.
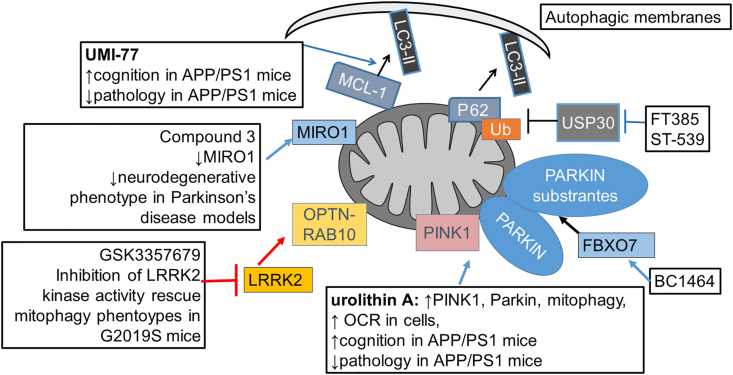
Examples of compounds that enhances mitophagy. Urolithin A and UMI-77 have been shown to improve cognition and decrease pathology in the APP/PS1 model of AD. Urolithin A increases mitophagy by increasing PINK1 and Parkin and improves bioenergetics in cells and worms. UMI-77 enhances MCL-1 and LC3 interaction on mitochondria, and show benefits in attenuating cognitive deficits and pathology in APP/PS1 mice. The impact of other mitophagy enhancement compounds on AD phenotypes is unclear. Compound 3 enables MIRO1 removal and decreases Parkinson's disease-related phenotypes. LRRK2 kinase inhibitors potentially decrease RAB10 phosphorylation, enable OPTN–RAB10 interaction on mitochondria, and restore mitophagy defects caused by LRRK2 mutation. FT385, ST-539, and BC1464 target Parkin-related mitophagy mechanisms, and while their targets are known, their effects on bioenergetics or AD phenotypes are unclear.
As a key protein involved in mitophagy, PINK1 kinase activity has been shown to be enhanced by ATP analogue kinetin triphosphate KTP, kinetin riboside and its ProTides, and mitochondrial uncoupler niclosamide and analogues313, 314, 315. One strategy that increased PINK1 and Parkin by urolithin A supplementation found enhanced mitophagy without inducing general autophagy, improved oxygen consumption rates that were impaired by Aβ and APOE/E4 in worms and cells, and improved cognition and pathology in the APP/PS1 mouse model, supporting the utility of targeting mitophagy for improvement of bioenergetics in AD35. However, the target of urolithin A that mediates the enhancement of mitophagy has not been identified. As a mitochondrial-targeted protein, miro 1 has been shown to be resistant to removal from mitochondria after CCCP in Parkinson's disease fibroblasts but not AD fibroblasts, compared to healthy controls. Using this observation, compound 3 has been identified from a high-throughput screen study and found to decrease Parkinson's disease-related neurodegenerative phenotypes in fibroblasts and fly models. Western blots and immunocytochemistry suggest an impact on mitophagy, although its impact on bioenergetics or AD-related phenotypes is unclear316.
Large scale screening approaches have identified compounds that enhance mitophagy in both PINK1-Parkin-dependent and independent manners305, 306, 307, 308, 309,312. Some target proteins that inhibit PINK1-Parkin-dependent mitophagy, including the FT385 and ST-539 compounds that target USP30317,318 and the BC1464 compound that targets FBXO7319. Other studies use reporters or tags as a drug discovery strategy to identify mitophagy-enhancement compounds, including using mitochondria-targeting AUTAC (autophagy-targeting chimera) to identify AUTAC4 that target TSPO320. Using mitoQC, iron chelators including deferoxamine have been shown to stimulate mitophagy independent of PINK1 and Parkin in SH-SY5Y cells, and time dependently decreased mitochondrial respiration without increasing DCFDA fluorescence as a measure of reactive oxygen species321. It is unclear whether these compounds are protective against AD-related phenotypes. It has also been shown that LRRK2 kinase inhibitor GSK3357679 rescued mitophagy defects in LRRK2 G2019S mice322. It has been reported that LRRK2 kinase inhibitors LRRK2-IN-1 or PF-06447475 rescue RAB10 accumulation on depolarized mitochondria in LRRK2 mutant fibroblasts, which may be the mechanism allowing RAB1–OPTN interaction and rescue mitophagy and mitochondrial function323. Whether enhancement of mitophagy through inhibition of LRRK2 kinase activity is beneficial for metabolism and bioenergetics and for alleviating AD phenotypes is still unclear.
Using mito-SRAI, which localize to mitochondria, as a mitophagy reporter, chemical inducers of mitophagy have been identified, including those that spare mitochondrial respiration in a Seahorse XF stress test assay, although the molecular targets of the inducers are currently unclear324. Using mt-Keima as a reporter protein, a pharmacological compound UMI-77 has been identified, which induces mitophagy in an ATG5 dependent, NBR1, TAX1BP1, P62, NDP52, FUNDC1, BNIP3, NIX, BAX, BECN, and PARKIN independent manner, by targeting MCL-1 that bind to LC3A on mitochondria325. In the APP/PS1 mouse model of AD, UMI-77 mitigates cognitive deficits and pathology325. Whether mitochondrial function is improved by UMI-77 is unclear.
3.3. Neuroinflammation and DAMPs
Neuroinflammation is a common theme among neurodegenerative disorders, including AD. Similarly, mitochondrial dysfunction and oxidative stress have been associated with pathologies of these diseases. High levels of pro-inflammatory mediators can be found in the brain and CSF of mild cognitive impairment (MCI) and AD patients326, 327, 328, 329, 330, 331, 332. In this respect, damage-associated molecular patterns (DAMPs) have been found in AD and other diseases326,333. DAMPs are intra- and extra-cellular molecules released during periods of cellular stress that activate innate immune response via interactions with specific receptors and signaling pathways. Whereas DAMPs can be released from multiple cellular compartments and organelles following stress, mitochondria can release various molecules that intra- and extra-cellularly initiate innate immune response. It is perhaps not surprising, therefore, that mitochondrial dysfunction and mtDNA alterations have been noted in AD334, 335, 336, 337, 338, 339, 340, 341, 342, 343, 344, 345, 346, 347.
Mitochondria are ancient α-proteobacterial symbionts; hence, they have many bacterial-like constituents, including N-formyl peptides, cardiolipin, and DNA (exhibiting low methylation at CpG sites), that when released from the organelle act as mitochondrial DAMPs (mtDAMPs; Fig. 7)348, 349, 350. N-Formyl peptides stimulate the secretion of cytokines by activating the formyl peptide receptor 1 (FPR-1) receptor351. Mitochondrial reactive oxygen species (ROS) production can activate mitochondrial anti-viral signaling (MAVS) proteins on the outer mitochondrial membrane (MAVS are also activated by proteins that detect viral RNA) that can induce immune and inflammatory gene expression through the regulation of NF-κB and interferon regulatory factor352, 353, 354, independent of cytosolic viral sensors350. MAVS also promote the activation of the nucleotide-binding oligomerization domain (NOD), leucine-rich repeat (LRR)-containing protein (NLR)-like receptor family pyrin domain containing 3 (collectively referred to as NLRP3) inflammasome, while cardiolipin recruits NLRP3 to the outer membrane during mitochondrial membrane depolarization when it translocates from the inner to the outer membrane355. Fragments of the mitochondrial DNA (mtDNA) interact with TLR9 (toll-like receptor 9), activating the NF-κB signaling pathway and transcription of proinflammatory cytokines such as TNFα and IL-6351,356,357. Both mtDNA DAMPs and mitochondrial-generated ROS can activate the NLRP3 inflammasome, triggering inflammation (production of IL-1β and IL-18) and cell death358, 359, 360. Finally, in the cytosol, mtDNA can induce conformational changes in cyclic GMP/AMP synthase (cGAS) that facilitate interactions with stimulator of interferon genes (STING) that activate interferon regulatory factor 3 (IRF-3), which triggers the expression of interferon-stimulated genes and potentiates interferon responses361.
Figure 7.
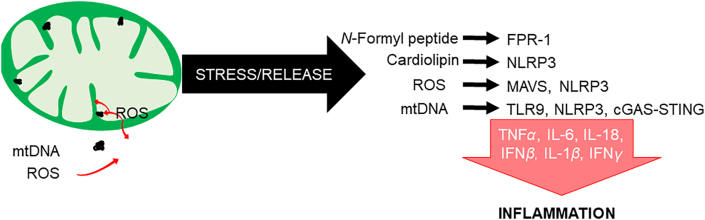
Mitochondrial-mediated immune functions and cell signaling. Under conditions of stress, mitochondria can release molecules such as N-formyl peptides, ROS, mtDNA, and cardiolipin. These components can initiate an immune response and related signaling pathways in the cytosol or extracellular space. N-Formyl peptides are released and act as chemoattractants for neutrophils, binding to the formyl peptide receptor 1 (FPR-1) and promoting their activation and release of pro-inflammatory cytokines including TNFα, IL-1β, and IFNγ. Cardiolipin translocates from the inner to the outer mitochondrial membrane, where it interacts with the nucleotide-binding oligomerization domain (NOD), leucine-rich repeat (LRR)-containing protein (NLR)-like receptor family pyrin domain containing 3 (NLRP3) inflammasome. ROS activates mitochondrial anti-viral signaling (MAVS) protein located on the outer membrane, triggering activation of the NF-κB pathway and the release of interferon-regulating factors (IRFs). Additionally, MAVS can promote the oligomerization and activation of the NLRP3 inflammasome. MtDNA fragments (or components containing mtDNA, e.g., mtDNA nucleoids) released from the organelle bind TLR9 receptors that activate NF-κB signaling or, in the cytosol, initiate cyclic GMP/AMP synthase—stimulator of interferon genes (cGAS–STING) pathways that potentiate interferon responses. Individually or collectively, these mitochondrial-initiated processes mediate a cascade of pro-inflammatory pathways and cytokines that contribute to innate immune response.
MtDNA copy number decreases and increased heteroplasmy have been noted in neurons from postmortem brains of AD patients362,363, and it has been noted that mitochondrial proteins and mtDNA sustain oxidative damage early in AD, suggesting mitochondrial dysfunction as an early phase in disease progression364,365. Interestingly, amyloid β (Aβ) peptide and neurofibrillary tangles have been implicated in mitochondrial dysfunction, as these proteins have been known to impact mitochondrial import machinery and to detrimentally affect organelle bioenergetics and increase ROS production366, 367, 368. The combination of increased oxidant response and declining ATP production not only increases the possibility of mitochondrial-mediated apoptosis, but also the risk of mtDAMP release349. Injection of mitochondrial lysates or mtDNA into mouse hippocampus has also been shown to have pro-inflammatory effects consistent with the alteration of AD-related pathways369. It is also noteworthy that fragments of APOE4, the E4 variant of apolipoprotein E (which is a major susceptibility gene for sporadic AD) is associated with mitochondrial dysfunction and oxidative stress when localized to the mitochondrion in hippocampal neurons370,371. Finally, observations that mtDAMPs are components of circulating extracellular vesicles with aging and neurodegeneration372,373 are consistent with a mito-centric role/etiology in AD development.
Because innate immunity appears to play a role in many forms of neurodegenerative diseases including AD, studies examining how mtDAMP release influences disease development are of interest. It is likely that release of such molecules initially is protective temporally; for example, it has been noted that TLR9 activation can lead to behavioral improvements in transgenic AD mice and decreased extracellular Aβ and associated Tau pathology, suggesting that TLR9 stimulation may be beneficial for microglial function in mouse models of AD374,375. However, chronic induction of these pathways results in inflammatory processes that contribute to neurodegeneration. Hence, therapies targeting mtDAMPs and/or their downstream pathways may be efficacious in treating AD. For example, while defects in mitophagy can contribute to organelle dysfunction, it also contributes to the release of mtDAMPs as part of the quality-control process—in this respect, the finding that pharmacologic restoration of mitophagy improves cognitive deficits and decreases Aβ accumulation in mouse models supports this notion376. Similarly, reduced tau phosphorylation and microglia-induced inflammation have been observed subsequent to pro-mitophagic pharmacological treatments35.
4. Conclusions and future directions
Metabolic perturbations, both at the systemic level and at the intracellular mitochondria, are linked to both aging and AD. Based on that diabetes may be a risk factor for AD, and that abnormal glucose metabolism and insulin signaling occur in AD brains, targeting whole-body and brain insulin pathways has been considered as an intervention strategy for AD. Recent studies have also highlighted the contributions of sex, gut microbiome, and circadian regulation in both whole-body and mitochondrial-mediated metabolism, which present additional potential targets for development of AD therapeutics. Compounds that affect mitochondrial bioenergetics and mitochondrial quality control, as well as mitochondria-linked inflammatory responses, have been tested and refined in current preclinical models and considered for clinical trials.
Based on our current understanding of metabolism and mitochondrial bioenergetics, major barriers to developing AD therapy with metabolism and bioenergetics as targeted pathways are as follows: 1) Few preclinical studies have measured parameters of mitochondrial bioenergetics in the blood or the brain after therapeutic interventions targeted at metabolism in AD. 2) A coordinated effort in assessing the extent and aspects of corresponding whole-body metabolism versus mitochondrial bioenergetics in blood or brain samples is needed in the context of AD. 3) Although there is more awareness of gut microbiome, sex, and circadian time as biological variables, scarcely any studies test mitochondrial bioenergetics with these in mind. 4) There are still limited system biology studies linking whole-body and mitochondrial energetics to transcripts, proteins, and post-translationally modified proteins.
These major barriers can be overcome with recent development of new method that enables measurement of mitochondrial bioenergetics in cells, blood and tissues377,378, with integrated views of whole-body metabolism and mitochondria-mediated metabolism, with consideration of gut–brain axis, sex and circadian regulation, and with system biology-omics analyses of connectomes and networks379, 380, 381, 382, 383. Over the next 10 years, we will witness more studies in these exciting areas that will impact how we view AD therapy. Major breakthroughs may involve a better understanding of the interconnection of whole-body and brain metabolism.
Acknowledgments
We thank the UAB NSC P30 AG05886 (SA, SB, TB, CC, DLS, VDU, JZ) for partial support. We would like to thank Rebecca Lipscomb for assistance with language editing and proofreading.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Author contributions
The following authors all contribute to drafting part of the manuscript and proofreading of the entire manuscript: Steven N. Austad, Scott Ballinger, Thomas W. Buford, Christy S. Carter, Daniel L. Smith Jr., Victor Darley-Usmar, and Jianhua Zhang.
Conflicts of interest
We have no competing interests to declare.
References
- 1.2020 Alzheimer’s disease facts and figures. Alzheimers Dement. 2020;16:391–460. [Google Scholar]
- 2.2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 2021;17:327–406. doi: 10.1002/alz.12328. [DOI] [PubMed] [Google Scholar]
- 3.Murphy S.L., Xu J., Kochanek K.D., Arias E., Tejada-Vera B. Deaths: final data for 2018. Natl Vital Stat Rep. 2021;69:1–83. [PubMed] [Google Scholar]
- 4.Hoyer S. Glucose metabolism and insulin receptor signal transduction in Alzheimer disease. Eur J Pharmacol. 2004;490:115–125. doi: 10.1016/j.ejphar.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 5.Cheng G., Huang C., Deng H., Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J. 2012;42:484–491. doi: 10.1111/j.1445-5994.2012.02758.x. [DOI] [PubMed] [Google Scholar]
- 6.Vieira M.N.N., Lima-Filho R.A.S., De Felice F.G. Connecting Alzheimer's disease to diabetes: underlying mechanisms and potential therapeutic targets. Neuropharmacology. 2018;136:160–171. doi: 10.1016/j.neuropharm.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 7.Holscher C. Diabetes as a risk factor for Alzheimer's disease: insulin signalling impairment in the brain as an alternative model of alzheimer's disease. Biochem Soc Trans. 2011;39:891–897. doi: 10.1042/BST0390891. [DOI] [PubMed] [Google Scholar]
- 8.Scheltens P., Blennow K., Breteler M.M., de Strooper B., Frisoni G.B., Salloway S., et al. Alzheimer's disease. Lancet. 2016;388:505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 9.Szablewski L. Glucose transporters in brain: in health and in Alzheimer's disease. J Alzheimers Dis. 2017;55:1307–1320. doi: 10.3233/JAD-160841. [DOI] [PubMed] [Google Scholar]
- 10.Goedert M., Spillantini M.G. A century of Alzheimer's disease. Science. 2006;314:777–781. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- 11.Kandimalla R., Thirumala V., Reddy P.H. Is Alzheimer's disease a type 3 diabetes? A critical appraisal. Biochim Biophys Acta Mol Basis Dis. 2017;1863:1078–1089. doi: 10.1016/j.bbadis.2016.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pugazhenthi S., Qin L., Reddy P.H. Common neurodegenerative pathways in obesity, diabetes, and Alzheimer's disease. Biochim Biophys Acta Mol Basis Dis. 2017;1863:1037–1045. doi: 10.1016/j.bbadis.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kubis-Kubiak A.M., Rorbach-Dolata A., Piwowar A. Crucial players in Alzheimer's disease and diabetes mellitus: friends or foes?. Mech Ageing Dev. 2019;181:7–21. doi: 10.1016/j.mad.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Macklin L., Griffith C.M., Cai Y., Rose G.M., Yan X.X., Patrylo P.R. Glucose tolerance and insulin sensitivity are impaired in APP/PS1 transgenic mice prior to amyloid plaque pathogenesis and cognitive decline. Exp Gerontol. 2017;88:9–18. doi: 10.1016/j.exger.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 15.Biessels G.J., Kamal A., Ramakers G.M., Urban I.J., Spruijt B.M., Erkelens D.W., et al. Place learning and hippocampal synaptic plasticity in streptozotocin-induced diabetic rats. Diabetes. 1996;45:1259–1266. doi: 10.2337/diab.45.9.1259. [DOI] [PubMed] [Google Scholar]
- 16.Huang H.J., Liang K.C., Chen C.P., Chen C.M., Hsieh-Li H.M. Intrahippocampal administration of A β1–40 impairs spatial learning and memory in hyperglycemic mice. Neurobiol Learn Mem. 2007;87:483–494. doi: 10.1016/j.nlm.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Salas I.H., De Strooper B. Diabetes and Alzheimer's disease: a link not as simple as it seems. Neurochem Res. 2019;44:1271–1278. doi: 10.1007/s11064-018-2690-9. [DOI] [PubMed] [Google Scholar]
- 18.Holscher C. Brain insulin resistance: role in neurodegenerative disease and potential for targeting. Expet Opin Invest Drugs. 2020;29:333–348. doi: 10.1080/13543784.2020.1738383. [DOI] [PubMed] [Google Scholar]
- 19.Boccardi V., Murasecco I., Mecocci P. Diabetes drugs in the fight against Alzheimer's disease. Ageing Res Rev. 2019;54:100936. doi: 10.1016/j.arr.2019.100936. [DOI] [PubMed] [Google Scholar]
- 20.Meng L., Li X.Y., Shen L., Ji H.F. Type 2 diabetes mellitus drugs for Alzheimer's disease: current evidence and therapeutic opportunities. Trends Mol Med. 2020;26:597–614. doi: 10.1016/j.molmed.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Li R., Zhang Y., Rasool S., Geetha T., Babu J.R. Effects and underlying mechanisms of bioactive compounds on type 2 diabetes mellitus and Alzheimer's disease. Oxid Med Cell Longev. 2019;2019:8165707. doi: 10.1155/2019/8165707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X., Li W., Marcus J., Pearson M., Song L., Smith K., et al. Mk-8719, a novel and selective O-GlcNacase inhibitor that reduces the formation of pathological tau and ameliorates neurodegeneration in a mouse model of tauopathy. J Pharmacol Exp Therapeut. 2020;374:252–263. doi: 10.1124/jpet.120.266122. [DOI] [PubMed] [Google Scholar]
- 23.Pinho T.S., Correia S.C., Perry G., Ambrosio A.F., Moreira P.I. Diminished O-GlcNAcylation in Alzheimer's disease is strongly correlated with mitochondrial anomalies. Biochim Biophys Acta Mol Basis Dis. 2019;1865:2048–2059. doi: 10.1016/j.bbadis.2018.10.037. [DOI] [PubMed] [Google Scholar]
- 24.Wheatley E.G., Albarran E., White C.W., 3rd, Bieri G., Sanchez-Diaz C., Pratt K., et al. Neuronal O-GlcNAcylation improves cognitive function in the aged mouse brain. Curr Biol. 2019;29:3359–33569. doi: 10.1016/j.cub.2019.08.003. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akan I., Olivier-Van Stichelen S., Bond M.R., Hanover J.A. Nutrient-driven O-GlcNac in proteostasis and neurodegeneration. J Neurochem. 2018;144:7–34. doi: 10.1111/jnc.14242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wani W.Y., Chatham J.C., Darley-Usmar V., McMahon L.L., Zhang J. O-GlcNAcylation and neurodegeneration. Brain Res Bull. 2017;133:80–87. doi: 10.1016/j.brainresbull.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rigotto G., Basso E. Mitochondrial dysfunctions: a thread sewing together Alzheimer's disease, diabetes, and obesity. Oxid Med Cell Longev. 2019;2019:7210892. doi: 10.1155/2019/7210892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swerdlow R.H., Koppel S., Weidling I., Hayley C., Ji Y., Wilkins H.M. Mitochondria, cybrids, aging, and Alzheimer's disease. Prog Mol Biol Transl Sci. 2017;146:259–302. doi: 10.1016/bs.pmbts.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swerdlow R.H., Burns J.M., Khan S.M. The Alzheimer's disease mitochondrial cascade hypothesis: progress and perspectives. Biochim Biophys Acta. 2014;1842:1219–1231. doi: 10.1016/j.bbadis.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butterfield D.A., Halliwell B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci. 2019;20:148–160. doi: 10.1038/s41583-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kunkle B.W., Grenier-Boley B., Sims R., Bis J.C., Damotte V., Naj A.C., et al. Genetic meta-analysis of diagnosed Alzheimer's disease identifies new risk loci and implicates abeta, tau, immunity and lipid processing. Nat Genet. 2019;51:414–430. doi: 10.1038/s41588-019-0358-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bloom G.S., Norambuena A. Alzheimer's disease as a metabolic disorder. OCL. 2018;25:D403. [Google Scholar]
- 33.Holper L., Ben-Shachar D., Mann J.J. Multivariate meta-analyses of mitochondrial complex I and IV in major depressive disorder, bipolar disorder, schizophrenia, alzheimer disease, and Parkinson disease. Neuropsychopharmacology. 2019;44:837–849. doi: 10.1038/s41386-018-0090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swerdlow R.H., Burns J.M., Khan S.M. The Alzheimer's disease mitochondrial cascade hypothesis. J Alzheimers Dis. 2010;20 Suppl 2:S265–S279. doi: 10.3233/JAD-2010-100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fang E.F., Hou Y., Palikaras K., Adriaanse B.A., Kerr J.S., Yang B., et al. Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer's disease. Nat Neurosci. 2019;22:401–412. doi: 10.1038/s41593-018-0332-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swerdlow R.H., Khan S.M. A "mitochondrial cascade hypothesis" for sporadic Alzheimer's disease. Med Hypotheses. 2004;63:8–20. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 37.Weidling I.W., Swerdlow R.H. Mitochondria in Alzheimer's disease and their potential role in Alzheimer's proteostasis. Exp Neurol. 2020;330:113321. doi: 10.1016/j.expneurol.2020.113321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swerdlow R.H. The mitochondrial hypothesis: dysfunction, bioenergetic defects, and the metabolic link to Alzheimer's disease. Int Rev Neurobiol. 2020;154:207–233. doi: 10.1016/bs.irn.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cunnane S.C., Trushina E., Morland C., Prigione A., Casadesus G., Andrews Z.B., et al. Brain energy rescue: an emerging therapeutic concept for neurodegenerative disorders of ageing. Nat Rev Drug Discov. 2020;19:609–633. doi: 10.1038/s41573-020-0072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hill B.G., Shiva S., Ballinger S., Zhang J., Darley-Usmar V.M. Bioenergetics and translational metabolism: implications for genetics, physiology and precision medicine. Biol Chem. 2019;401:3–29. doi: 10.1515/hsz-2019-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalyanaraman B. Teaching the basics of redox biology to medical and graduate students: oxidants, antioxidants and disease mechanisms. Redox Biol. 2013;1:244–257. doi: 10.1016/j.redox.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson E.C.B., Dammer E.B., Duong D.M., Ping L., Zhou M., Yin L., et al. Large-scale proteomic analysis of Alzheimer's disease brain and cerebrospinal fluid reveals early changes in energy metabolism associated with microglia and astrocyte activation. Nat Med. 2020;26:769–780. doi: 10.1038/s41591-020-0815-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Habib N., McCabe C., Medina S., Varshavsky M., Kitsberg D., Dvir-Szternfeld R., et al. Disease-associated astrocytes in Alzheimer's disease and aging. Nat Neurosci. 2020;23:701–706. doi: 10.1038/s41593-020-0624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Webers A., Heneka M.T., Gleeson P.A. The role of innate immune responses and neuroinflammation in amyloid accumulation and progression of Alzheimer's disease. Immunol Cell Biol. 2020;98:28–41. doi: 10.1111/imcb.12301. [DOI] [PubMed] [Google Scholar]
- 46.Tarafdar A., Pula G. The role of nadph oxidases and oxidative stress in neurodegenerative disorders. Int J Mol Sci. 2018;19:3824. doi: 10.3390/ijms19123824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Afridi R., Kim J.H., Rahman M.H., Suk K. Metabolic regulation of glial phenotypes: implications in neuron-glia interactions and neurological disorders. Front Cell Neurosci. 2020;14:20. doi: 10.3389/fncel.2020.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ibrahim A.M., Pottoo F.H., Dahiya E.S., Khan F.A., Kumar J.B.S. Neuron-glia interactions: molecular basis of Alzheimer's disease and applications of neuroproteomics. Eur J Neurosci. 2020;52:2931–2943. doi: 10.1111/ejn.14838. [DOI] [PubMed] [Google Scholar]
- 49.Bernier L.P., York E.M., MacVicar B.A. Immunometabolism in the brain: how metabolism shapes microglial function. Trends Neurosci. 2020;43:854–869. doi: 10.1016/j.tins.2020.08.008. [DOI] [PubMed] [Google Scholar]
- 50.Henstridge C.M., Hyman B.T., Spires-Jones T.L. Beyond the neuron-cellular interactions early in Alzheimer disease pathogenesis. Nat Rev Neurosci. 2019;20:94–108. doi: 10.1038/s41583-018-0113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gonzalez-Reyes R.E., Nava-Mesa M.O., Vargas-Sanchez K., Ariza-Salamanca D., Mora-Munoz L. Involvement of astrocytes in Alzheimer's disease from a neuroinflammatory and oxidative stress perspective. Front Mol Neurosci. 2017;10:427. doi: 10.3389/fnmol.2017.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Welikovitch L.A., Do Carmo S., Magloczky Z., Malcolm J.C., Loke J., Klein W.L., et al. Early intraneuronal amyloid triggers neuron-derived inflammatory signaling in APP transgenic rats and human brain. Proc Natl Acad Sci U S A. 2020;117:6844–6854. doi: 10.1073/pnas.1914593117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anwar S., Rivest S. Alzheimer's disease: microglia targets and their modulation to promote amyloid phagocytosis and mitigate neuroinflammation. Expert Opin Ther Targets. 2020;24:331–344. doi: 10.1080/14728222.2020.1738391. [DOI] [PubMed] [Google Scholar]
- 54.Hall K.D., Heymsfield S.B., Kemnitz J.W., Klein S., Schoeller D.A., Speakman J.R. Energy balance and its components: implications for body weight regulation. Am J Clin Nutr. 2012;95:989–994. doi: 10.3945/ajcn.112.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alford S., Patel D., Perakakis N., Mantzoros C.S. Obesity as a risk factor for Alzheimer's disease: weighing the evidence. Obes Rev. 2018;19:269–280. doi: 10.1111/obr.12629. [DOI] [PubMed] [Google Scholar]
- 56.Profenno L.A., Porsteinsson A.P., Faraone S.V. Meta-analysis of Alzheimer's disease risk with obesity, diabetes, and related disorders. Biol Psychiatr. 2010;67:505–512. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 57.Jimenez A., Pegueroles J., Carmona-Iragui M., Vilaplana E., Montal V., Alcolea D., et al. Weight loss in the healthy elderly might be a non-cognitive sign of preclinical Alzheimer's disease. Oncotarget. 2017;8:104706–104716. doi: 10.18632/oncotarget.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whitmer R.A., Gustafson D.R., Barrett-Connor E., Haan M.N., Gunderson E.P., Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71:1057–1064. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- 59.Chuang Y.F., An Y., Bilgel M., Wong D.F., Troncoso J.C., O'Brien R.J., et al. Midlife adiposity predicts earlier onset of Alzheimer's dementia, neuropathology and presymptomatic cerebral amyloid accumulation. Mol Psychiatr. 2016;21:910–915. doi: 10.1038/mp.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singh-Manoux A., Dugravot A., Shipley M., Brunner E.J., Elbaz A., Sabia S., et al. Obesity trajectories and risk of dementia: 28 years of follow-up in the Whitehall II study. Alzheimers Dement. 2018;14:178–186. doi: 10.1016/j.jalz.2017.06.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beydoun M.A., Lhotsky A., Wang Y., Dal Forno G., An Y., Metter E.J., et al. Association of adiposity status and changes in early to mid-adulthood with incidence of Alzheimer's disease. Am J Epidemiol. 2008;168:1179–1189. doi: 10.1093/aje/kwn229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qizilbash N., Gregson J., Johnson M.E., Pearce N., Douglas I., Wing K., et al. BMI and risk of dementia in two million people over two decades: a retrospective cohort study. Lancet Diabetes Endocrinol. 2015;3:431–436. doi: 10.1016/S2213-8587(15)00033-9. [DOI] [PubMed] [Google Scholar]
- 63.Emmerzaal T.L., Kiliaan A.J., Gustafson D.R. 2003–2013: a decade of body mass index, Alzheimer's disease, and dementia. J Alzheimers Dis. 2015;43:739–755. doi: 10.3233/JAD-141086. [DOI] [PubMed] [Google Scholar]
- 64.Vidoni E.D., Townley R.A., Honea R.A., Burns J.M. Alzheimer's disease neuroimaging I. Alzheimer disease biomarkers are associated with body mass index. Neurology. 2011;77:1913–1920. doi: 10.1212/WNL.0b013e318238eec1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ewers M., Schmitz S., Hansson O., Walsh C., Fitzpatrick A., Bennett D., et al. Body mass index is associated with biological CSF markers of core brain pathology of Alzheimer's disease. Neurobiol Aging. 2012;33:1599–1608. doi: 10.1016/j.neurobiolaging.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Poehlman E.T., Toth M.J., Goran M.I., Carpenter W.H., Newhouse P., Rosen C.J. Daily energy expenditure in free-living non-institutionalized Alzheimer's patients: a doubly labeled water study. Neurology. 1997;48:997–1002. doi: 10.1212/wnl.48.4.997. [DOI] [PubMed] [Google Scholar]
- 67.Poehlman E.T., Dvorak R.V. Energy expenditure in Alzheimer's disease. J Nutr Health Aging. 1998;2:115–118. [PubMed] [Google Scholar]
- 68.Donaldson K.E., Carpenter W.H., Toth M.J., Goran M.I., Newhouse P., Poehlman E.T. No evidence for a higher resting metabolic rate in noninstitutionalized Alzheimer's disease patients. J Am Geriatr Soc. 1996;44:1232–1234. doi: 10.1111/j.1532-5415.1996.tb01375.x. [DOI] [PubMed] [Google Scholar]
- 69.Doorduijn A.S., de van der Schueren M.A.E., van de Rest O., de Leeuw F.A., Hendriksen H.M.A., Teunissen C.E., et al. Energy intake and expenditure in patients with Alzheimer's disease and mild cognitive impairment: the nudad project. Alzheimer's Res Ther. 2020;12:116. doi: 10.1186/s13195-020-00687-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vloeberghs E., Van Dam D., Franck F., Serroyen J., Geert M., Staufenbiel M., et al. Altered ingestive behavior, weight changes, and intact olfactory sense in an app overexpression model. Behav Neurosci. 2008;122:491–497. doi: 10.1037/0735-7044.122.3.491. [DOI] [PubMed] [Google Scholar]
- 71.Knight E.M., Brown T.M., Gumusgoz S., Smith J.C., Waters E.J., Allan S.M., et al. Age-related changes in core body temperature and activity in triple-transgenic Alzheimer’s disease (3xTgAD) mice. Dis Model Mech. 2013;6:160–170. doi: 10.1242/dmm.010173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.James D., Kang S., Park S. Injection of beta-amyloid into the hippocampus induces metabolic disturbances and involuntary weight loss which may be early indicators of Alzheimer's disease. Aging Clin Exp Res. 2014;26:93–98. doi: 10.1007/s40520-013-0181-z. [DOI] [PubMed] [Google Scholar]
- 73.Redjems-Bennani N., Jeandel C., Lefebvre E., Blain H., Vidailhet M., Gueant J.L. Abnormal substrate levels that depend upon mitochondrial function in cerebrospinal fluid from Alzheimer patients. Gerontology. 1998;44:300–304. doi: 10.1159/000022031. [DOI] [PubMed] [Google Scholar]
- 74.Liguori C., Chiaravalloti A., Sancesario G., Stefani A., Sancesario G.M., Mercuri N.B., et al. Cerebrospinal fluid lactate levels and brain [18F]FDG PET hypometabolism within the default mode network in Alzheimer's disease. Eur J Nucl Med Mol Imag. 2016;43:2040–2049. doi: 10.1007/s00259-016-3417-2. [DOI] [PubMed] [Google Scholar]
- 75.Parnetti L., Gaiti A., Polidori M.C., Brunetti M., Palumbo B., Chionne F., et al. Increased cerebrospinal fluid pyruvate levels in Alzheimer's disease. Neurosci Lett. 1995;199:231–233. doi: 10.1016/0304-3940(95)12058-c. [DOI] [PubMed] [Google Scholar]
- 76.Chen Y., Wang J., Cui C., Su Y., Jing D., Wu L., et al. Evaluating the association between brain atrophy, hypometabolism, and cognitive decline in Alzheimer's disease: a PET/MRI study. Aging (Albany NY) 2021;13:7228–7246. doi: 10.18632/aging.202580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marchitelli R., Aiello M., Cachia A., Quarantelli M., Cavaliere C., Postiglione A., et al. Simultaneous resting-state FDG-PET/FMRI in Alzheimer disease: relationship between glucose metabolism and intrinsic activity. Neuroimage. 2018;176:246–258. doi: 10.1016/j.neuroimage.2018.04.048. [DOI] [PubMed] [Google Scholar]
- 78.La Joie R., Perrotin A., Barre L., Hommet C., Mezenge F., Ibazizene M., et al. Region-specific hierarchy between atrophy, hypometabolism, and beta-amyloid (Aβ) load in Alzheimer's disease dementia. J Neurosci. 2012;32:16265–16273. doi: 10.1523/JNEUROSCI.2170-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Buckner R.L., Snyder A.Z., Shannon B.J., LaRossa G., Sachs R., Fotenos A.F., et al. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Edison P., Archer H.A., Hinz R., Hammers A., Pavese N., Tai Y.F., et al. Amyloid, hypometabolism, and cognition in Alzheimer disease: an [11C]PIB and [18F]FDG pet study. Neurology. 2007;68:501–508. doi: 10.1212/01.wnl.0000244749.20056.d4. [DOI] [PubMed] [Google Scholar]
- 81.Schroeter M.L., Stein T., Maslowski N., Neumann J. Neural correlates of Alzheimer's disease and mild cognitive impairment: a systematic and quantitative meta-analysis involving 1351 patients. Neuroimage. 2009;47:1196–1206. doi: 10.1016/j.neuroimage.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mielke R., Schopphoff H.H., Kugel H., Pietrzyk U., Heindel W., Kessler J., et al. Relation between 1H MR spectroscopic imaging and regional cerebral glucose metabolism in Alzheimer's disease. Int J Neurosci. 2001;107:233–245. doi: 10.3109/00207450109150687. [DOI] [PubMed] [Google Scholar]
- 83.Mullins R., Reiter D., Kapogiannis D. Magnetic resonance spectroscopy reveals abnormalities of glucose metabolism in the Alzheimer's brain. Ann Clin Transl Neurol. 2018;5:262–272. doi: 10.1002/acn3.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Craft S., Watson G.S. Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol. 2004;3:169–178. doi: 10.1016/S1474-4422(04)00681-7. [DOI] [PubMed] [Google Scholar]
- 85.Ferreira S.T. Brain insulin, insulin-like growth factor 1 and glucagon-like peptide 1 signalling in Alzheimer's disease. J Neuroendocrinol. 2021;33 doi: 10.1111/jne.12959. [DOI] [PubMed] [Google Scholar]
- 86.Willette A.A., Johnson S.C., Birdsill A.C., Sager M.A., Christian B., Baker L.D., et al. Insulin resistance predicts brain amyloid deposition in late middle-aged adults. Alzheimers Dement. 2015;11:504–510. doi: 10.1016/j.jalz.2014.03.011. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ishibashi K., Sakurai K., Shimoji K., Tokumaru A.M., Ishii K. Altered functional connectivity of the default mode network by glucose loading in young, healthy participants. BMC Neurosci. 2018;19:33. doi: 10.1186/s12868-018-0433-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim B., Elzinga S.E., Henn R.E., McGinley L.M., Feldman E.L. The effects of insulin and insulin-like growth factor I on amyloid precursor protein phosphorylation in in vitro and in vivo models of Alzheimer's disease. Neurobiol Dis. 2019;132:104541. doi: 10.1016/j.nbd.2019.104541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bomfim T.R., Forny-Germano L., Sathler L.B., Brito-Moreira J., Houzel J.C., Decker H., et al. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer's disease-associated Aβ oligomers. J Clin Invest. 2012;122:1339–1353. doi: 10.1172/JCI57256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao W.Q., Lacor P.N., Chen H., Lambert M.P., Quon M.J., Krafft G.A., et al. Insulin receptor dysfunction impairs cellular clearance of neurotoxic oligomeric Aβ. J Biol Chem. 2009;284:18742–18753. doi: 10.1074/jbc.M109.011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bailey C.J., Turner R.C. Metformin. N Engl J Med. 1996;334:574–579. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- 92.Foretz M., Guigas B., Bertrand L., Pollak M., Viollet B. Metformin: from mechanisms of action to therapies. Cell Metabol. 2014;20:953–966. doi: 10.1016/j.cmet.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 93.Martin-Montalvo A., Mercken E.M., Mitchell S.J., Palacios H.H., Mote P.L., Scheibye-Knudsen M., et al. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Owen M.R., Doran E., Halestrap A.P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348 Pt 3:607–614. [PMC free article] [PubMed] [Google Scholar]
- 95.El-Mir M.Y., Nogueira V., Fontaine E., Averet N., Rigoulet M., Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275:223–228. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- 96.He L., Wondisford F.E. Metformin action: concentrations matter. Cell Metabol. 2015;21:159–162. doi: 10.1016/j.cmet.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 97.Arvanitakis Z., Wang H.Y., Capuano A.W., Khan A., Taib B., Anokye-Danso F., et al. Brain insulin signaling, Alzheimer disease pathology, and cognitive function. Ann Neurol. 2020;88:513–525. doi: 10.1002/ana.25826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Benedict C., Hallschmid M., Hatke A., Schultes B., Fehm H.L., Born J., et al. Intranasal insulin improves memory in humans. Psychoneuroendocrinology. 2004;29:1326–1334. doi: 10.1016/j.psyneuen.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 99.Reger M.A., Watson G.S., Frey W.H., 2nd, Baker L.D., Cholerton B., Keeling M.L., et al. Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by apoe genotype. Neurobiol Aging. 2006;27:451–458. doi: 10.1016/j.neurobiolaging.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 100.Reger M.A., Watson G.S., Green P.S., Baker L.D., Cholerton B., Fishel M.A., et al. Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-β in memory-impaired older adults. J Alzheimers Dis. 2008;13:323–331. doi: 10.3233/jad-2008-13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reger M.A., Watson G.S., Green P.S., Wilkinson C.W., Baker L.D., Cholerton B., et al. Intranasal insulin improves cognition and modulates β-amyloid in early AD. Neurology. 2008;70:440–448. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- 102.Craft S., Baker L.D., Montine T.J., Minoshima S., Watson G.S., Claxton A., et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012;69:29–38. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Craft S., Raman R., Chow T.W., Rafii M.S., Sun C.K., Rissman R.A., et al. Safety, efficacy, and feasibility of intranasal insulin for the treatment of mild cognitive impairment and Alzheimer disease dementia: a randomized clinical trial. JAMA Neurol. 2020;77:1099–1109. doi: 10.1001/jamaneurol.2020.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hallschmid M. Intranasal insulin for Alzheimer's disease. CNS Drugs. 2021;35:21–37. doi: 10.1007/s40263-020-00781-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McClean P.L., Parthsarathy V., Faivre E., Holscher C. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer's disease. J Neurosci. 2011;31:6587–6594. doi: 10.1523/JNEUROSCI.0529-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Femminella G.D., Frangou E., Love S.B., Busza G., Holmes C., Ritchie C., et al. Correction to: evaluating the effects of the novel GLP-1 analogue liraglutide in Alzheimer's disease: study protocol for a randomised controlled trial (elad study) Trials. 2020;21:660. doi: 10.1186/s13063-020-04608-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maskery M., Goulding E.M., Gengler S., Melchiorsen J.U., Rosenkilde M.M., Holscher C. The dual GLP-1/GIP receptor agonist DA4-JC shows superior protective properties compared to the GLP-1 analogue liraglutide in the APP/PS1 mouse model of Alzheimer's disease. Am J Alzheimers Dis Other Demen. 2020;35 doi: 10.1177/1533317520953041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Grizzanti J., Corrigan R., Servizi S., Casadesus G. Amylin signaling in diabetes and Alzheimer's disease: therapy or pathology?. J Neurol Neuromedicine. 2019;4:12–16. doi: 10.29245/2572.942X/2019/1.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen X.Y., Du Y.F., Chen L. Neuropeptides exert neuroprotective effects in Alzheimer's disease. Front Mol Neurosci. 2018;11:493. doi: 10.3389/fnmol.2018.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mueller T.O.X., Johnson M., Qian W.J., Chatham J.C., Darley-Usmar V., Zhang J. New insights into the biology of protein O-GlcNAcylation: approaches and observations. Front Aging. 2021;1:5. doi: 10.3389/fragi.2020.620382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wani W.Y., Boyer-Guittaut M., Dodson M., Chatham J., Darley-Usmar V., Zhang J. Regulation of autophagy by protein post-translational modification. Lab Invest. 2015;95:14–25. doi: 10.1038/labinvest.2014.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wende A.R., Young M.E., Chatham J., Zhang J., Rajasekaran N.S., Darley-Usmar V.M. Redox biology and the interface between bioenergetics, autophagy and circadian control of metabolism. Free Radic Biol Med. 2016;100:94–107. doi: 10.1016/j.freeradbiomed.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wani W.Y., Ouyang X., Benavides G.A., Redmann M., Cofield S.S., Shacka J.J., et al. O-GlcNac regulation of autophagy and α-synuclein homeostasis; implications for Parkinson's disease. Mol Brain. 2017;10:32. doi: 10.1186/s13041-017-0311-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wright J.N., Benavides G.A., Johnson M.S., Wani W., Ouyang X., Zou L., et al. Acute increases in O-GlcNac indirectly impair mitochondrial bioenergetics through dysregulation of LonP1-mediated mitochondrial protein complex turnover. Am J Physiol Cell Physiol. 2019;316:C862–C875. doi: 10.1152/ajpcell.00491.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang J., Chatham J.C., Young M.E. Circadian regulation of cardiac physiology: rhythms that keep the heart beating. Annu Rev Physiol. 2020;82:79–101. doi: 10.1146/annurev-physiol-020518-114349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chatham J.C., Young M.E., Zhang J. Role of O-linked n-acetylglucosamine (O-GlcNac) modification of proteins in diabetic cardiovascular complications. Curr Opin Pharmacol. 2020;57:1–12. doi: 10.1016/j.coph.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chatham J.C., Zhang J., Wende A.R. Role of O-linked n-acetylglucosamine (O-GlcNac) protein modification in cellular (patho)physiology. Physiol Rev. 2021;101:427–493. doi: 10.1152/physrev.00043.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yuzwa S.A., Macauley M.S., Heinonen J.E., Shan X., Dennis R.J., He Y., et al. A potent mechanism-inspired O-GlcNacase inhibitor that blocks phosphorylation of tau in vivo. Nat Chem Biol. 2008;4:483–490. doi: 10.1038/nchembio.96. [DOI] [PubMed] [Google Scholar]
- 119.Yuzwa S.A., Shan X., Macauley M.S., Clark T., Skorobogatko Y., Vosseller K., et al. Increasing O-GlcNac slows neurodegeneration and stabilizes tau against aggregation. Nat Chem Biol. 2012;8:393–399. doi: 10.1038/nchembio.797. [DOI] [PubMed] [Google Scholar]
- 120.Graham D.L., Gray A.J., Joyce J.A., Yu D., O'Moore J., Carlson G.A., et al. Increased O-GlcNAcylation reduces pathological tau without affecting its normal phosphorylation in a mouse model of tauopathy. Neuropharmacology. 2014;79:307–313. doi: 10.1016/j.neuropharm.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 121.Hastings N.B., Wang X., Song L., Butts B.D., Grotz D., Hargreaves R., et al. Inhibition of O-GlcNacase leads to elevation of O-GlcNac tau and reduction of tauopathy and cerebrospinal fluid tau in rtg4510 mice. Mol Neurodegener. 2017;12:39. doi: 10.1186/s13024-017-0181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Guo B., Liang Q., Li L., Hu Z., Wu F., Zhang P., et al. O-GlcNac-modification of SNAP-29 regulates autophagosome maturation. Nat Cell Biol. 2014;16:1215–1226. doi: 10.1038/ncb3066. [DOI] [PubMed] [Google Scholar]
- 123.Zhang X., Wang L., Lak B., Li J., Jokitalo E., Wang Y. GRASP55 senses glucose deprivation through O-GlcNAcylation to promote autophagosome–lysosome fusion. Dev Cell. 2018;45:245–261. doi: 10.1016/j.devcel.2018.03.023. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Taylor E.W., Wang K., Nelson A.R., Bredemann T.M., Fraser K.B., Clinton S.M., et al. O-GlcNAcylation of ampa receptor GLUA2 is associated with a novel form of long-term depression at hippocampal synapses. J Neurosci. 2014;34:10–21. doi: 10.1523/JNEUROSCI.4761-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ott A., Stolk R.P., van Harskamp F., Pols H.A., Hofman A., Breteler M.M. Diabetes mellitus and the risk of dementia: the rotterdam study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 126.Silverstein J., Maclaren N., Riley W., Spillar R., Radjenovic D., Johnson S. Immunosuppression with azathioprine and prednisone in recent-onset insulin-dependent diabetes mellitus. N Engl J Med. 1988;319:599–604. doi: 10.1056/NEJM198809083191002. [DOI] [PubMed] [Google Scholar]
- 127.Goodpaster B.H., Krishnaswami S., Harris T.B., Katsiaras A., Kritchevsky S.B., Simonsick E.M., et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med. 2005;165:777–783. doi: 10.1001/archinte.165.7.777. [DOI] [PubMed] [Google Scholar]
- 128.Gustafson D., Rothenberg E., Blennow K., Steen B., Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med. 2003;163:1524–1528. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- 129.Han W., Li C. Linking type 2 diabetes and Alzheimer's disease. Proc Natl Acad Sci U S A. 2010;107:6557–6558. doi: 10.1073/pnas.1002555107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kivipelto M., Ngandu T., Fratiglioni L., Viitanen M., Kareholt I., Winblad B., et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62:1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 131.Kroner Z. The relationship between Alzheimer’s disease and diabetes: type 3 diabetes?. Altern Med Rev. 2009;14:373–379. [PubMed] [Google Scholar]
- 132.Razay G., Vreugdenhil A., Wilcock G. The metabolic syndrome and Alzheimer disease. Arch Neurol. 2007;64:93–96. doi: 10.1001/archneur.64.1.93. [DOI] [PubMed] [Google Scholar]
- 133.Vilalta-Franch J., Lopez-Pousa S., Garre-Olmo J., Turon-Estrada A., Pericot-Nierga I. Metabolic syndrome in Alzheimer's disease: clinical and developmental influences. Rev Neurol. 2008;46:13–17. [PubMed] [Google Scholar]
- 134.O'Hara A.M., Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ley R.E., Peterson D.A., Gordon J.I. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 136.Cox L.M., Blaser M.J. Antibiotics in early life and obesity. Nat Rev Endocrinol. 2015;11:182–190. doi: 10.1038/nrendo.2014.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Metchnikoff E. Putnam; New York: 1908. The prolongation of life. [Google Scholar]
- 138.Hornef M. Pathogens, commensal symbionts, and pathobionts: discovery and functional effects on the host. ILAR J. 2015;56:159–162. doi: 10.1093/ilar/ilv007. [DOI] [PubMed] [Google Scholar]
- 139.Egert M., de Graaf A.A., Smidt H., de Vos W.M., Venema K. Beyond diversity: functional microbiomics of the human colon. Trends Microbiol. 2006;14:86–91. doi: 10.1016/j.tim.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 140.Thaiss C.A., Zeevi D., Levy M., Zilberman-Schapira G., Suez J., Tengeler A.C., et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell. 2014;159:514–529. doi: 10.1016/j.cell.2014.09.048. [DOI] [PubMed] [Google Scholar]
- 141.Barlow G.M., Yu A., Mathur R. Role of the gut microbiome in obesity and diabetes mellitus. Nutr Clin Pract. 2015;30:787–797. doi: 10.1177/0884533615609896. [DOI] [PubMed] [Google Scholar]
- 142.Qin J., Li R., Raes J., Arumugam M., Burgdorf K.S., Manichanh C., et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Komaroff A.L. The microbiome and risk for obesity and diabetes. J Am Med Assoc. 2017;317:355–356. doi: 10.1001/jama.2016.20099. [DOI] [PubMed] [Google Scholar]
- 144.Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 145.Lippert K., Kedenko L., Antonielli L., Kedenko I., Gemeier C., Leitner M., et al. Gut microbiota dysbiosis associated with glucose metabolism disorders and the metabolic syndrome in older adults. Benef Microbes. 2017;8:545–556. doi: 10.3920/BM2016.0184. [DOI] [PubMed] [Google Scholar]
- 146.Ley R.E., Backhed F., Turnbaugh P., Lozupone C.A., Knight R.D., Gordon J.I. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hu X., Wang T., Jin F. Alzheimer's disease and gut microbiota. Sci China Life Sci. 2016;59:1006–1023. doi: 10.1007/s11427-016-5083-9. [DOI] [PubMed] [Google Scholar]
- 148.Seo D.O., Holtzman D.M. Gut microbiota: from the forgotten organ to a potential key player in the pathology of Alzheimer's disease. J Gerontol A Biol Sci Med Sci. 2020;75:1232–1241. doi: 10.1093/gerona/glz262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Quigley E.M.M. Microbiota–brain–gut axis and neurodegenerative diseases. Curr Neurol Neurosci Rep. 2017;17:94. doi: 10.1007/s11910-017-0802-6. [DOI] [PubMed] [Google Scholar]
- 150.Zhuang Z.Q., Shen L.L., Li W.W., Fu X., Zeng F., Gui L., et al. Gut microbiota is altered in patients with Alzheimer's disease. J Alzheimers Dis. 2018;63:1337–1346. doi: 10.3233/JAD-180176. [DOI] [PubMed] [Google Scholar]
- 151.Vogt N.M., Kerby R.L., Dill-McFarland K.A., Harding S.J., Merluzzi A.P., Johnson S.C., et al. Gut microbiome alterations in Alzheimer's disease. Sci Rep. 2017;7:13537. doi: 10.1038/s41598-017-13601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chen S., Henderson A., Petriello M.C., Romano K.A., Gearing M., Miao J., et al. Trimethylamine N-oxide binds and activates perk to promote metabolic dysfunction. Cell Metabol. 2019;30:1141–11451. doi: 10.1016/j.cmet.2019.08.021. e5. [DOI] [PubMed] [Google Scholar]
- 153.Vogt N.M., Romano K.A., Darst B.F., Engelman C.D., Johnson S.C., Carlsson C.M., et al. The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer's disease. Alzheimer's Res Ther. 2018;10:124. doi: 10.1186/s13195-018-0451-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Perino A., Demagny H., Velazquez-Villegas L.A., Schoonjans K. Molecular physiology of bile acid signaling in health, disease and aging. Physiol Rev. 2021;101:683–731. doi: 10.1152/physrev.00049.2019. [DOI] [PubMed] [Google Scholar]
- 155.Baloni P., Funk C.C., Yan J., Yurkovich J.T., Kueider-Paisley A., Nho K., et al. Metabolic network analysis reveals altered bile acid synthesis and metabolism in Alzheimer's disease. Cell Rep Med. 2020;1:100138. doi: 10.1016/j.xcrm.2020.100138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li P., Killinger B.A., Ensink E., Beddows I., Yilmaz A., Lubben N., et al. Gut microbiota dysbiosis is associated with elevated bile acids in Parkinson's disease. Metabolites. 2021;11:29. doi: 10.3390/metabo11010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.MahmoudianDehkordi S., Arnold M., Nho K., Ahmad S., Jia W., Xie G., et al. Altered bile acid profile associates with cognitive impairment in Alzheimer's disease-an emerging role for gut microbiome. Alzheimers Dement. 2019;15:76–92. doi: 10.1016/j.jalz.2018.07.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ojo O., Feng Q.Q., Ojo O.O., Wang X.H. The role of dietary fibre in modulating gut microbiota dysbiosis in patients with type 2 diabetes: a systematic review and meta-analysis of randomised controlled trials. Nutrients. 2020;12:3239. doi: 10.3390/nu12113239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Erny D., Hrabe de Angelis A.L., Jaitin D., Wieghofer P., Staszewski O., David E., et al. Host microbiota constantly control maturation and function of microglia in the cns. Nat Neurosci. 2015;18:965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Taleb S. Tryptophan dietary impacts gut barrier and metabolic diseases. Front Immunol. 2019;10:2113. doi: 10.3389/fimmu.2019.02113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Agudelo L.Z., Femenia T., Orhan F., Porsmyr-Palmertz M., Goiny M., Martinez-Redondo V., et al. Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell. 2014;159:33–45. doi: 10.1016/j.cell.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 162.Curto M., Lionetto L., Negro A., Capi M., Perugino F., Fazio F., et al. Altered serum levels of kynurenine metabolites in patients affected by cluster headache. J Headache Pain. 2015;17:27. doi: 10.1186/s10194-016-0620-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gulaj E., Pawlak K., Bien B., Pawlak D. Kynurenine and its metabolites in Alzheimer's disease patients. Adv Med Sci. 2010;55:204–211. doi: 10.2478/v10039-010-0023-6. [DOI] [PubMed] [Google Scholar]
- 164.Lovelace M.D., Varney B., Sundaram G., Lennon M.J., Lim C.K., Jacobs K., et al. Recent evidence for an expanded role of the kynurenine pathway of tryptophan metabolism in neurological diseases. Neuropharmacology. 2017;112:373–388. doi: 10.1016/j.neuropharm.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 165.Parrott J.M., Redus L., Santana-Coelho D., Morales J., Gao X., O'Connor J.C. Neurotoxic kynurenine metabolism is increased in the dorsal hippocampus and drives distinct depressive behaviors during inflammation. Transl Psychiatry. 2016;6:e918. doi: 10.1038/tp.2016.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Widner B., Leblhuber F., Walli J., Tilz G.P., Demel U., Fuchs D. Tryptophan degradation and immune activation in Alzheimer's disease. J Neural Transm. 2000;107:343–353. doi: 10.1007/s007020050029. [DOI] [PubMed] [Google Scholar]
- 167.Zhang L.S., Davies S.S. Microbial metabolism of dietary components to bioactive metabolites: opportunities for new therapeutic interventions. Genome Med. 2016;8:46. doi: 10.1186/s13073-016-0296-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hashimoto T., Perlot T., Rehman A., Trichereau J., Ishiguro H., Paolino M., et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Singer D., Camargo S.M., Ramadan T., Schafer M., Mariotta L., Herzog B., et al. Defective intestinal amino acid absorption in Ace2 null mice. Am J Physiol Gastrointest Liver Physiol. 2012;303:G686–G695. doi: 10.1152/ajpgi.00140.2012. [DOI] [PubMed] [Google Scholar]
- 170.Camargo S.M., Singer D., Makrides V., Huggel K., Pos K.M., Wagner C.A., et al. Tissue-specific amino acid transporter partners ACE2 and collectrin differentially interact with hartnup mutations. Gastroenterology. 2009;136:872–882. doi: 10.1053/j.gastro.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kowalczuk S., Broer A., Tietze N., Vanslambrouck J.M., Rasko J.E., Broer S. A protein complex in the brush-border membrane explains a hartnup disorder allele. FASEB J. 2008;22:2880–2887. doi: 10.1096/fj.08-107300. [DOI] [PubMed] [Google Scholar]
- 172.Syed Y.Y. Correction to: sodium oligomannate: first approval. Drugs. 2020;80:445–446. doi: 10.1007/s40265-020-01274-3. [DOI] [PubMed] [Google Scholar]
- 173.Syed Y.Y. Sodium oligomannate: first approval. Drugs. 2020;80:441–444. doi: 10.1007/s40265-020-01268-1. [DOI] [PubMed] [Google Scholar]
- 174.Wang T., Kuang W., Chen W., Xu W., Zhang L., Li Y., et al. A phase II randomized trial of sodium oligomannate in Alzheimer's dementia. Alzheimer's Res Ther. 2020;12:110. doi: 10.1186/s13195-020-00678-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang X., Sun G., Feng T., Zhang J., Huang X., Wang T., et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer's disease progression. Cell Res. 2019;29:787–803. doi: 10.1038/s41422-019-0216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Xiao S., Chan P., Wang T., Hong Z., Wang S., Kuang W., et al. A 36-week multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase 3 clinical trial of sodium oligomannate for mild-to-moderate Alzheimer's dementia. Alzheimer's Res Ther. 2021;13:62. doi: 10.1186/s13195-021-00795-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Leng Y., Musiek E.S., Hu K., Cappuccio F.P., Yaffe K. Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol. 2019;18:307–318. doi: 10.1016/S1474-4422(18)30461-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang C., Holtzman D.M. Bidirectional relationship between sleep and Alzheimer's disease: role of amyloid, tau, and other factors. Neuropsychopharmacology. 2020;45:104–120. doi: 10.1038/s41386-019-0478-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Holth J., Patel T., Holtzman D.M. Sleep in Alzheimer's disease—beyond amyloid. Neurobiol Sleep Circadian Rhythms. 2017;2:4–14. doi: 10.1016/j.nbscr.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Musiek E.S., Holtzman D.M. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science. 2016;354:1004–1008. doi: 10.1126/science.aah4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Musiek E.S., Xiong D.D., Holtzman D.M. Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Exp Mol Med. 2015;47:e148. doi: 10.1038/emm.2014.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Asher G., Schibler U. Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metabol. 2011;13:125–137. doi: 10.1016/j.cmet.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 183.Pastore N., Vainshtein A., Herz N.J., Huynh T., Brunetti L., Klisch T.J., et al. Nutrient-sensitive transcription factors TFEB and TFE3 couple autophagy and metabolism to the peripheral clock. EMBO J. 2019;38 doi: 10.15252/embj.2018101347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Loehfelm A., Boucsein A., Pretz D., Tups A. Timing matters: circadian effects on energy homeostasis and Alzheimer's disease. Trends Endocrinol Metab. 2019;30:132–143. doi: 10.1016/j.tem.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 185.Reinke H., Asher G. Crosstalk between metabolism and circadian clocks. Nat Rev Mol Cell Biol. 2019;20:227–241. doi: 10.1038/s41580-018-0096-9. [DOI] [PubMed] [Google Scholar]
- 186.Garcia J.A., Zhang D., Estill S.J., Michnoff C., Rutter J., Reick M., et al. Impaired cued and contextual memory in NPAS2-deficient mice. Science. 2000;288:2226–2230. doi: 10.1126/science.288.5474.2226. [DOI] [PubMed] [Google Scholar]
- 187.Van der Zee E.A., Havekes R., Barf R.P., Hut R.A., Nijholt I.M., Jacobs E.H., et al. Circadian time-place learning in mice depends on cry genes. Curr Biol. 2008;18:844–848. doi: 10.1016/j.cub.2008.04.077. [DOI] [PubMed] [Google Scholar]
- 188.Kondratova A.A., Dubrovsky Y.V., Antoch M.P., Kondratov R.V. Circadian clock proteins control adaptation to novel environment and memory formation. Aging. 2010;2:285–297. doi: 10.18632/aging.100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Jilg A., Lesny S., Peruzki N., Schwegler H., Selbach O., Dehghani F., et al. Temporal dynamics of mouse hippocampal clock gene expression support memory processing. Hippocampus. 2010;20:377–388. doi: 10.1002/hipo.20637. [DOI] [PubMed] [Google Scholar]
- 190.Wardlaw S.M., Phan T.X., Saraf A., Chen X., Storm D.R. Genetic disruption of the core circadian clock impairs hippocampus-dependent memory. Learn Mem. 2014;21:417–423. doi: 10.1101/lm.035451.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Rawashdeh O., Jilg A., Jedlicka P., Slawska J., Thomas L., Saade A., et al. PERIOd1 coordinates hippocampal rhythms and memory processing with daytime. Hippocampus. 2014;24:712–723. doi: 10.1002/hipo.22262. [DOI] [PubMed] [Google Scholar]
- 192.Snider K.H., Obrietan K. Modulation of learning and memory by the genetic disruption of circadian oscillator populations. Physiol Behav. 2018;194:387–393. doi: 10.1016/j.physbeh.2018.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Schnell A., Chappuis S., Schmutz I., Brai E., Ripperger J.A., Schaad O., et al. The nuclear receptor REV-ERBα regulates Fabp7 and modulates adult hippocampal neurogenesis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0099883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jager J., O'Brien W.T., Manlove J., Krizman E.N., Fang B., Gerhart-Hines Z., et al. Behavioral changes and dopaminergic dysregulation in mice lacking the nuclear receptor REV-ERBα. Mol Endocrinol. 2014;28:490–498. doi: 10.1210/me.2013-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Riemersma-van der Lek R.F., Swaab D.F., Twisk J., Hol E.M., Hoogendijk W.J., Van Someren E.J. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. J Am Med Assoc. 2008;299:2642–2655. doi: 10.1001/jama.299.22.2642. [DOI] [PubMed] [Google Scholar]
- 196.Wade A.G., Farmer M., Harari G., Fund N., Laudon M., Nir T., et al. Add-on prolonged-release melatonin for cognitive function and sleep in mild to moderate Alzheimer's disease: a 6-month, randomized, placebo-controlled, multicenter trial. Clin Interv Aging. 2014;9:947–961. doi: 10.2147/CIA.S65625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Rubino J.A., Gamundi A., Akaarir M., Canellas F., Rial R., Nicolau M.C. Bright light therapy and circadian cycles in institutionalized elders. Front Neurosci. 2020;14:359. doi: 10.3389/fnins.2020.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kolahdouzan M., Hamadeh M.J. The neuroprotective effects of caffeine in neurodegenerative diseases. CNS Neurosci Ther. 2017;23:272–290. doi: 10.1111/cns.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Larsson S.C., Orsini N. Coffee consumption and risk of dementia and Alzheimer's disease: a dose-response meta-analysis of prospective studies. Nutrients. 2018;10:1501. doi: 10.3390/nu10101501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lundell L.S., Parr E.B., Devlin B.L., Ingerslev L.R., Altintas A., Sato S., et al. Time-restricted feeding alters lipid and amino acid metabolite rhythmicity without perturbing clock gene expression. Nat Commun. 2020;11:4643. doi: 10.1038/s41467-020-18412-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Parr E.B., Devlin B.L., Radford B.E., Hawley J.A. A delayed morning and earlier evening time-restricted feeding protocol for improving glycemic control and dietary adherence in men with overweight/obesity: a randomized controlled trial. Nutrients. 2020;12:505. doi: 10.3390/nu12020505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ravussin E., Beyl R.A., Poggiogalle E., Hsia D.S., Peterson C.M. Early time-restricted feeding reduces appetite and increases fat oxidation but does not affect energy expenditure in humans. Obesity (Silver Spring) 2019;27:1244–1254. doi: 10.1002/oby.22518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Jamshed H., Beyl R.A., Della Manna D.L., Yang E.S., Ravussin E., Peterson C.M. Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients. 2019;11:1234. doi: 10.3390/nu11061234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hatori M., Vollmers C., Zarrinpar A., DiTacchio L., Bushong E.A., Gill S., et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metabol. 2012;15:848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Martens C.R., Rossman M.J., Mazzo M.R., Jankowski L.R., Nagy E.E., Denman B.A., et al. Short-term time-restricted feeding is safe and feasible in non-obese healthy midlife and older adults. Geroscience. 2020;42:667–686. doi: 10.1007/s11357-020-00156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Parr E.B., Devlin B.L., Lim K.H.C., Moresi L.N.Z., Geils C., Brennan L., et al. Time-restricted eating as a nutrition strategy for individuals with type 2 diabetes: a feasibility study. Nutrients. 2020;12:3228. doi: 10.3390/nu12113228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Raghuram S., Stayrook K.R., Huang P., Rogers P.M., Nosie A.K., McClure D.B., et al. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBα and REV-ERBβ. Nat Struct Mol Biol. 2007;14:1207–1213. doi: 10.1038/nsmb1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kojetin D., Wang Y., Kamenecka T.M., Burris T.P. Identification of SR8278, a synthetic antagonist of the nuclear heme receptor REV-ERB. ACS Chem Biol. 2011;6:131–134. doi: 10.1021/cb1002575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Solt L.A., Wang Y., Banerjee S., Hughes T., Kojetin D.J., Lundasen T., et al. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature. 2012;485:62–68. doi: 10.1038/nature11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kojetin D.J., Burris T.P. REV-ERB and ror nuclear receptors as drug targets. Nat Rev Drug Discov. 2014;13:197–216. doi: 10.1038/nrd4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Griffin P., Dimitry J.M., Sheehan P.W., Lananna B.V., Guo C., Robinette M.L., et al. Circadian clock protein REV-ERBα regulates neuroinflammation. Proc Natl Acad Sci U S A. 2019;116:5102–5107. doi: 10.1073/pnas.1812405116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Roby D.A., Ruiz F., Kermath B.A., Voorhees J.R., Niehoff M., Zhang J., et al. Pharmacological activation of the nuclear receptor REV-ERB reverses cognitive deficits and reduces amyloid-β burden in a mouse model of Alzheimer's disease. PLoS One. 2019;14 doi: 10.1371/journal.pone.0215004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lee J., Kim D.E., Griffin P., Sheehan P.W., Kim D.H., Musiek E.S., et al. Inhibition of REV-ERBs stimulates microglial amyloid-β clearance and reduces amyloid plaque deposition in the 5xfad mouse model of Alzheimer's disease. Aging Cell. 2020;19 doi: 10.1111/acel.13078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Austad S.N. In: Hazard's geriatric medicine and gerontology. 7th ed. Halter J., Ouslander J., Studenski S., et al., editors. McGraw Hill; New York: 2017. Sex differences in health and longevity; pp. 133–147. [Google Scholar]
- 215.Cao Q., Tan C.C., Xu W., Hu H., Cao X.P., Dong Q., et al. The prevalence of dementia: a systematic review and meta-analysis. J Alzheimers Dis. 2020;73:1157–1166. doi: 10.3233/JAD-191092. [DOI] [PubMed] [Google Scholar]
- 216.Montgomery W., Ueda K., Jorgensen M., Stathis S., Cheng Y., Nakamura T. Epidemiology, associated burden, and current clinical practice for the diagnosis and management of Alzheimer's disease in Japan. Clinicoecon Outcomes Res. 2018;10:13–28. doi: 10.2147/CEOR.S146788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Lopez-Lee C., Kodama L., Gan L. Sex differences in neurodegeneration: the role of the immune system in humans. Biol Psychiatr. 2022;91:72–80. doi: 10.1016/j.biopsych.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Sattler C., Toro P., Schonknecht P., Schroder J. Cognitive activity, education and socioeconomic status as preventive factors for mild cognitive impairment and Alzheimer's disease. Psychiatr Res. 2012;196:90–95. doi: 10.1016/j.psychres.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 219.Demarest T.G., McCarthy M.M. Sex differences in mitochondrial (dys)function: implications for neuroprotection. J Bioenerg Biomembr. 2015;47:173–188. doi: 10.1007/s10863-014-9583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hall A.M., Roberson E.D. Mouse models of Alzheimer's disease. Brain Res Bull. 2012;88:3–12. doi: 10.1016/j.brainresbull.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Oddo S., Caccamo A., Shepherd J.D., Murphy M.P., Golde T.E., Kayed R., et al. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 222.Demarest T.G., Varma V.R., Estrada D., Babbar M., Basu S., Mahajan U.V., et al. Biological sex and DNA repair deficiency drive Alzheimer's disease via systemic metabolic remodeling and brain mitochondrial dysfunction. Acta Neuropathol. 2020;140:25–47. doi: 10.1007/s00401-020-02152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Arnold A.P. Conceptual frameworks and mouse models for studying sex differences in physiology and disease: why compensation changes the game. Exp Neurol. 2014;259:2–9. doi: 10.1016/j.expneurol.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kodama L., Gan L. Do microglial sex differences contribute to sex differences in neurodegenerative diseases?. Trends Mol Med. 2019;25:741–749. doi: 10.1016/j.molmed.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Cox K.H., Bonthuis P.J., Rissman E.F. Mouse model systems to study sex chromosome genes and behavior: relevance to humans. Front Neuroendocrinol. 2014;35:405–419. doi: 10.1016/j.yfrne.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Davis E.J., Broestl L., Abdulai-Saiku S., Worden K., Bonham L.W., Minones-Moyano E., et al. A second x chromosome contributes to resilience in a mouse model of Alzheimer's disease. Sci Transl Med. 2020;12:eaaz5677. doi: 10.1126/scitranslmed.aaz5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Austad S.N., Fischer K.E. Sex differences in lifespan. Cell Metabol. 2016;23:1022–1033. doi: 10.1016/j.cmet.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Cohen R.M., Rezai-Zadeh K., Weitz T.M., Rentsendorj A., Gate D., Spivak I., et al. A transgenic alzheimer rat with plaques, tau pathology, behavioral impairment, oligomeric Aβ, and frank neuronal loss. J Neurosci. 2013;33:6245–6256. doi: 10.1523/JNEUROSCI.3672-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wang W., Zhao F., Ma X., Perry G., Zhu X. Mitochondria dysfunction in the pathogenesis of Alzheimer's disease: recent advances. Mol Neurodegener. 2020;15:30. doi: 10.1186/s13024-020-00376-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Snow W.M., Cadonic C., Cortes-Perez C., Adlimoghaddam A., Roy Chowdhury S.K., Thomson E., et al. Sex-specific effects of chronic creatine supplementation on hippocampal-mediated spatial cognition in the 3xTg mouse model of Alzheimer’s disease. Nutrients. 2020;12:3589. doi: 10.3390/nu12113589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Djordjevic J., Roy Chowdhury S., Snow W.M., Perez C., Cadonic C., Fernyhough P., et al. Early onset of sex-dependent mitochondrial deficits in the cortex of 3xTg Alzheimer’s mice. Cells. 2020;9:1541. doi: 10.3390/cells9061541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Morais F.M., Ribeiro A.M., Moreira F.A., Silva P.V.G. Systematic review and meta-analysis on the role of mitochondrial cytochrome c oxidase in Alzheimer's disease. Acta Neuropsychiatr. 2020;33:55–64. doi: 10.1017/neu.2020.43. [DOI] [PubMed] [Google Scholar]
- 233.Emmerzaal T.L., Rodenburg R.J., Tanila H., Verweij V., Kiliaan A.J., Kozicz T. Age-dependent decrease of mitochondrial complex II activity in a familial mouse model for Alzheimer's disease. J Alzheimers Dis. 2018;66:75–82. doi: 10.3233/JAD-180337. [DOI] [PubMed] [Google Scholar]
- 234.Kayser E.B., Sedensky M.M., Morgan P.G., Hoppel C.L. Mitochondrial oxidative phosphorylation is defective in the long-lived mutant clk-1. J Biol Chem. 2004;279:54479–54486. doi: 10.1074/jbc.M403066200. [DOI] [PubMed] [Google Scholar]
- 235.Lapointe J., Hekimi S. Early mitochondrial dysfunction in long-lived Mclk1+/– mice. J Biol Chem. 2008;283:26217–26227. doi: 10.1074/jbc.M803287200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Lapointe J., Stepanyan Z., Bigras E., Hekimi S. Reversal of the mitochondrial phenotype and slow development of oxidative biomarkers of aging in long-lived Mclk1+/– mice. J Biol Chem. 2009;284:20364–20374. doi: 10.1074/jbc.M109.006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Van Raamsdonk J.M., Hekimi S. Reactive oxygen species and aging in caenorhabditis elegans: causal or casual relationship?. Antioxid Redox Signal. 2010;13:1911–1953. doi: 10.1089/ars.2010.3215. [DOI] [PubMed] [Google Scholar]
- 238.Yang W., Hekimi S. Two modes of mitochondrial dysfunction lead independently to lifespan extension in Caenorhabditis elegans. Aging Cell. 2010;9:433–447. doi: 10.1111/j.1474-9726.2010.00571.x. [DOI] [PubMed] [Google Scholar]
- 239.Lapointe J., Wang Y., Bigras E., Hekimi S. The submitochondrial distribution of ubiquinone affects respiration in long-lived Mclk1+/– mice. J Cell Biol. 2012;199:215–224. doi: 10.1083/jcb.201203090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Wang Y., Oxer D., Hekimi S. Mitochondrial function and lifespan of mice with controlled ubiquinone biosynthesis. Nat Commun. 2015;6:6393. doi: 10.1038/ncomms7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Schaar C.E., Dues D.J., Spielbauer K.K., Machiela E., Cooper J.F., Senchuk M., et al. Mitochondrial and cytoplasmic ros have opposing effects on lifespan. PLoS Genet. 2015;11 doi: 10.1371/journal.pgen.1004972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Copeland J.M., Cho J., Lo T., Jr., Hur J.H., Bahadorani S., Arabyan T., et al. Extension of drosophila life span by RNAi of the mitochondrial respiratory chain. Curr Biol. 2009;19:1591–1598. doi: 10.1016/j.cub.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 243.Ludovico P., Burhans W.C. Reactive oxygen species, ageing and the hormesis police. FEMS Yeast Res. 2014;14:33–39. doi: 10.1111/1567-1364.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Yun J., Finkel T. Mitohormesis. Cell Metabol. 2014;19:757–766. doi: 10.1016/j.cmet.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ristow M., Schmeisser K. Mitohormesis: promoting health and lifespan by increased levels of reactive oxygen species (ROS) Dose Response. 2014;12:288–341. doi: 10.2203/dose-response.13-035.Ristow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Chouchani E.T., Methner C., Nadtochiy S.M., Logan A., Pell V.R., Ding S., et al. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat Med. 2013;19:753–759. doi: 10.1038/nm.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zhang J. The promise of a golden era for exploring the frontiers of aging, metabolism and redox biology. Front Aging. 2020;1:4. doi: 10.3389/fragi.2020.610406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Baumgart M., Priebe S., Groth M., Hartmann N., Menzel U., Pandolfini L., et al. Longitudinal RNA-seq analysis of vertebrate aging identifies mitochondrial complex I as a small-molecule-sensitive modifier of lifespan. Cell Syst. 2016;2:122–132. doi: 10.1016/j.cels.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 249.Vial G., Detaille D., Guigas B. Role of mitochondria in the mechanism(s) of action of metformin. Front Endocrinol. 2019;10:294. doi: 10.3389/fendo.2019.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kickstein E., Krauss S., Thornhill P., Rutschow D., Zeller R., Sharkey J., et al. Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2a (PP2a) signaling. Proc Natl Acad Sci U S A. 2010;107:21830–21835. doi: 10.1073/pnas.0912793107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Chen Y., Zhou K., Wang R., Liu Y., Kwak Y.D., Ma T., et al. Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer's amyloid peptides via up-regulating BACE1 transcription. Proc Natl Acad Sci U S A. 2009;106:3907–3912. doi: 10.1073/pnas.0807991106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Barini E., Antico O., Zhao Y., Asta F., Tucci V., Catelani T., et al. Metformin promotes tau aggregation and exacerbates abnormal behavior in a mouse model of tauopathy. Mol Neurodegener. 2016;11:16. doi: 10.1186/s13024-016-0082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Kuhla A., Brichmann E., Ruhlmann C., Thiele R., Meuth L., Vollmar B. Metformin therapy aggravates neurodegenerative processes in ApoE–/– mice. J Alzheimers Dis. 2019;68:1415–1427. doi: 10.3233/JAD-181017. [DOI] [PubMed] [Google Scholar]
- 254.Li W., Chaudhari K., Shetty R., Winters A., Gao X., Hu Z., et al. Metformin alters locomotor and cognitive function and brain metabolism in normoglycemic mice. Aging Dis. 2019;10:949–963. doi: 10.14336/AD.2019.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Li J., Deng J., Sheng W., Zuo Z. Metformin attenuates Alzheimer's disease-like neuropathology in obese, leptin-resistant mice. Pharmacol Biochem Behav. 2012;101:564–574. doi: 10.1016/j.pbb.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Oliveira W.H., Nunes A.K., Franca M.E., Santos L.A., Los D.B., Rocha S.W., et al. Effects of metformin on inflammation and short-term memory in streptozotocin-induced diabetic mice. Brain Res. 2016;1644:149–160. doi: 10.1016/j.brainres.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 257.Ou Z., Kong X., Sun X., He X., Zhang L., Gong Z., et al. Metformin treatment prevents amyloid plaque deposition and memory impairment in APP/PS1 mice. Brain Behav Immun. 2018;69:351–363. doi: 10.1016/j.bbi.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 258.Lu X.Y., Huang S., Chen Q.B., Zhang D., Li W., Ao R., et al. Metformin ameliorates abeta pathology by insulin-degrading enzyme in a transgenic mouse model of Alzheimer's disease. Oxid Med Cell Longev. 2020;2020:2315106. doi: 10.1155/2020/2315106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Farr S.A., Roesler E., Niehoff M.L., Roby D.A., McKee A., Morley J.E. Metformin improves learning and memory in the SAMP8 mouse model of Alzheimer's disease. J Alzheimers Dis. 2019;68:1699–1710. doi: 10.3233/JAD-181240. [DOI] [PubMed] [Google Scholar]
- 260.Pilipenko V., Narbute K., Pupure J., Langrate I.K., Muceniece R., Klusa V. Neuroprotective potential of antihyperglycemic drug metformin in streptozocin-induced rat model of sporadic Alzheimer's disease. Eur J Pharmacol. 2020;881:173290. doi: 10.1016/j.ejphar.2020.173290. [DOI] [PubMed] [Google Scholar]
- 261.Saffari P.M., Alijanpour S., Takzaree N., Sahebgharani M., Etemad-Moghadam S., Noorbakhsh F., et al. Metformin loaded phosphatidylserine nanoliposomes improve memory deficit and reduce neuroinflammation in streptozotocin-induced Alzheimer's disease model. Life Sci. 2020;255:117861. doi: 10.1016/j.lfs.2020.117861. [DOI] [PubMed] [Google Scholar]
- 262.Syal C., Kosaraju J., Hamilton L., Aumont A., Chu A., Sarma S.N., et al. Dysregulated expression of monoacylglycerol lipase is a marker for anti-diabetic drug metformin-targeted therapy to correct impaired neurogenesis and spatial memory in Alzheimer's disease. Theranostics. 2020;10:6337–6360. doi: 10.7150/thno.44962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Rotermund C., Machetanz G., Fitzgerald J.C. The therapeutic potential of metformin in neurodegenerative diseases. Front Endocrinol. 2018;9:400. doi: 10.3389/fendo.2018.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Salvatore T., Pafundi P.C., Morgillo F., Di Liello R., Galiero R., Nevola R., et al. Metformin: an old drug against old age and associated morbidities. Diabetes Res Clin Pract. 2020;160:108025. doi: 10.1016/j.diabres.2020.108025. [DOI] [PubMed] [Google Scholar]
- 265.Chaudhari K., Reynolds C.D., Yang S.H. Metformin and cognition from the perspectives of sex, age, and disease. Geroscience. 2020;42:97–116. doi: 10.1007/s11357-019-00146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Zhang Q.Q., Li W.S., Liu Z., Zhang H.L., Ba Y.G., Zhang R.X. Metformin therapy and cognitive dysfunction in patients with type 2 diabetes: a meta-analysis and systematic review. Medicine (Baltim) 2020;99 doi: 10.1097/MD.0000000000019378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Sluggett J.K., Koponen M., Bell J.S., Taipale H., Tanskanen A., Tiihonen J., et al. Metformin and risk of Alzheimer's disease among community-dwelling people with diabetes: a national case-control study. J Clin Endocrinol Metab. 2020;105:dgz234. doi: 10.1210/clinem/dgz234. [DOI] [PubMed] [Google Scholar]
- 268.Bordt E.A., Clerc P., Roelofs B.A., Saladino A.J., Tretter L., Adam-Vizi V., et al. The putative Drp1 inhibitor mdivi-1 is a reversible mitochondrial complex I inhibitor that modulates reactive oxygen species. Dev Cell. 2017;40:583–594. doi: 10.1016/j.devcel.2017.02.020. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Baek S.H., Park S.J., Jeong J.I., Kim S.H., Han J., Kyung J.W., et al. Inhibition of Drp1 ameliorates synaptic depression, Aβ deposition, and cognitive impairment in an Alzheimer's disease model. J Neurosci. 2017;37:5099–5110. doi: 10.1523/JNEUROSCI.2385-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Wang W., Yin J., Ma X., Zhao F., Siedlak S.L., Wang Z., et al. Inhibition of mitochondrial fragmentation protects against Alzheimer's disease in rodent model. Hum Mol Genet. 2017;26:4118–4131. doi: 10.1093/hmg/ddx299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Maezawa I., Hong H.S., Wu H.C., Battina S.K., Rana S., Iwamoto T., et al. A novel tricyclic pyrone compound ameliorates cell death associated with intracellular amyloid-β oligomeric complexes. J Neurochem. 2006;98:57–67. doi: 10.1111/j.1471-4159.2006.03862.x. [DOI] [PubMed] [Google Scholar]
- 272.Zhang L., Zhang S., Maezawa I., Trushin S., Minhas P., Pinto M., et al. Modulation of mitochondrial complex I activity averts cognitive decline in multiple animal models of familial Alzheimer's disease. EBioMedicine. 2015;2:294–305. doi: 10.1016/j.ebiom.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Zhang L., Zhang S., Maezawa I., Trushin S., Minhas P., Pinto M., et al. Corrigendum to "modulation of mitochondrial complex i activity averts cognitive decline in multiple animal models of familial Alzheimer's disease" [ebiomedicine 2 (2015) 294-305] EBioMedicine. 2019;42:532. doi: 10.1016/j.ebiom.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Stojakovic A., Trushin S., Sheu A., Khalili L., Chang S.Y., Li X., et al. Partial inhibition of mitochondrial complex I ameliorates Alzheimer's disease pathology and cognition in APP/PS1 female mice. Commun Biol. 2021;4:61. doi: 10.1038/s42003-020-01584-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Stojakovic A., Chang S.Y., Nesbitt J., Pichurin N.P., Ostroot M.A., Aikawa T., et al. Partial inhibition of mitochondrial complex I reduces tau pathology and improves energy homeostasis and synaptic function in 3xTg-AD mice. J Alzheimers Dis. 2021;79:335–353. doi: 10.3233/JAD-201015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Chen Q., Prior M., Dargusch R., Roberts A., Riek R., Eichmann C., et al. A novel neurotrophic drug for cognitive enhancement and Alzheimer's disease. PLoS One. 2011;6 doi: 10.1371/journal.pone.0027865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Prior M., Dargusch R., Ehren J.L., Chiruta C., Schubert D. The neurotrophic compound J147 reverses cognitive impairment in aged Alzheimer's disease mice. Alzheimer's Res Ther. 2013;5:25. doi: 10.1186/alzrt179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Goldberg J., Currais A., Prior M., Fischer W., Chiruta C., Ratliff E., et al. The mitochondrial ATP synthase is a shared drug target for aging and dementia. Aging Cell. 2018;17 doi: 10.1111/acel.12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Wilkins H.M., Mahnken J.D., Welch P., Bothwell R., Koppel S., Jackson R.L., et al. A mitochondrial biomarker-based study of S-Equol in Alzheimer's disease subjects: results of a single-arm, pilot trial. J Alzheimers Dis. 2017;59:291–300. doi: 10.3233/JAD-170077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Qi X., Qvit N., Su Y.C., Mochly-Rosen D. A novel Drp1 inhibitor diminishes aberrant mitochondrial fission and neurotoxicity. J Cell Sci. 2013;126:789–802. doi: 10.1242/jcs.114439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Guo X., Disatnik M.H., Monbureau M., Shamloo M., Mochly-Rosen D., Qi X. Inhibition of mitochondrial fragmentation diminishes Huntington's disease-associated neurodegeneration. J Clin Invest. 2013;123:5371–5388. doi: 10.1172/JCI70911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Joshi A.U., Saw N.L., Shamloo M., Mochly-Rosen D. Drp1/Fis1 interaction mediates mitochondrial dysfunction, bioenergetic failure and cognitive decline in Alzheimer's disease. Oncotarget. 2018;9:6128–6143. doi: 10.18632/oncotarget.23640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Joshi A.U., Saw N.L., Vogel H., Cunnigham A.D., Shamloo M., Mochly-Rosen D. Inhibition of Drp1/Fis1 interaction slows progression of amyotrophic lateral sclerosis. EMBO Mol Med. 2018;10 doi: 10.15252/emmm.201708166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Disatnik M.H., Ferreira J.C., Campos J.C., Gomes K.S., Dourado P.M., Qi X., et al. Acute inhibition of excessive mitochondrial fission after myocardial infarction prevents long-term cardiac dysfunction. J Am Heart Assoc. 2013;2 doi: 10.1161/JAHA.113.000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Joshi A.U., Ebert A.E., Haileselassie B., Mochly-Rosen D. Drp1/Fis1-mediated mitochondrial fragmentation leads to lysosomal dysfunction in cardiac models of huntington's disease. J Mol Cell Cardiol. 2019;127:125–133. doi: 10.1016/j.yjmcc.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Schmitt K., Grimm A., Dallmann R., Oettinghaus B., Restelli L.M., Witzig M., et al. Circadian control of Drp1 activity regulates mitochondrial dynamics and bioenergetics. Cell Metabol. 2018;27:657–666. doi: 10.1016/j.cmet.2018.01.011. e5. [DOI] [PubMed] [Google Scholar]
- 287.Zhao Y., Sun X., Hu D., Prosdocimo D.A., Hoppel C., Jain M.K., et al. ATAD3A oligomerization causes neurodegeneration by coupling mitochondrial fragmentation and bioenergetics defects. Nat Commun. 2019;10:1371. doi: 10.1038/s41467-019-09291-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Toyama E.Q., Herzig S., Courchet J., Lewis T.L., Jr., Loson O.C., Hellberg K., et al. Metabolism. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science. 2016;351:275–281. doi: 10.1126/science.aab4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Quiros P.M., Langer T., Lopez-Otin C. New roles for mitochondrial proteases in health, ageing and disease. Nat Rev Mol Cell Biol. 2015;16:345–359. doi: 10.1038/nrm3984. [DOI] [PubMed] [Google Scholar]
- 290.Vangavaragu J.R., Valasani K.R., Gan X., Yan S.S. Identification of human presequence protease (hPreP) agonists for the treatment of Alzheimer's disease. Eur J Med Chem. 2014;76:506–516. doi: 10.1016/j.ejmech.2014.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Fang D., Wang Y., Zhang Z., Du H., Yan S., Sun Q., et al. Increased neuronal prep activity reduces Aβ accumulation, attenuates neuroinflammation and improves mitochondrial and synaptic function in Alzheimer disease's mouse model. Hum Mol Genet. 2015;24:5198–5210. doi: 10.1093/hmg/ddv241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Muraleva N.A., Stefanova N.A., Kolosova N.G. SkQ1 suppresses the P38 MAPK signaling pathway involved in Alzheimer's disease-like pathology in oxys rats. Antioxidants. 2020;9:676. doi: 10.3390/antiox9080676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Stefanova N.A., Ershov N.I., Kolosova N.G. Suppression of Alzheimer's disease-like pathology progression by mitochondria-targeted antioxidant SkQ1: a transcriptome profiling study. Oxid Med Cell Longev. 2019;2019:3984906. doi: 10.1155/2019/3984906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Kolosova N.G., Tyumentsev M.A., Muraleva N.A., Kiseleva E., Vitovtov A.O., Stefanova N.A. Antioxidant SkQ1 alleviates signs of Alzheimer's disease-like pathology in old oxys rats by reversing mitochondrial deterioration. Curr Alzheimer Res. 2017;14:1283–1292. doi: 10.2174/1567205014666170621111033. [DOI] [PubMed] [Google Scholar]
- 295.Stefanova N.A., Muraleva N.A., Maksimova K.Y., Rudnitskaya E.A., Kiseleva E., Telegina D.V., et al. An antioxidant specifically targeting mitochondria delays progression of Alzheimer's disease-like pathology. Aging. 2016;8:2713–2733. doi: 10.18632/aging.101054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.McManus M.J., Murphy M.P., Franklin J.L. The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer's disease. J Neurosci. 2011;31:15703–15715. doi: 10.1523/JNEUROSCI.0552-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Young M.L., Franklin J.L. The mitochondria-targeted antioxidant MitoQ inhibits memory loss, neuropathology, and extends lifespan in aged 3xTg-AD mice. Mol Cell Neurosci. 2019;101:103409. doi: 10.1016/j.mcn.2019.103409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Mitchell W., Ng E.A., Tamucci J.D., Boyd K.J., Sathappa M., Coscia A., et al. The mitochondria-targeted peptide SS-31 binds lipid bilayers and modulates surface electrostatics as a key component of its mechanism of action. J Biol Chem. 2020;295:7452–7469. doi: 10.1074/jbc.RA119.012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Manczak M., Mao P., Calkins M.J., Cornea A., Reddy A.P., Murphy M.P., et al. Mitochondria-targeted antioxidants protect against amyloid-β toxicity in Alzheimer’s disease neurons. J Alzheimers Dis. 2010;20 Suppl 2:S609–S631. doi: 10.3233/JAD-2010-100564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Reddy P.H., Manczak M., Kandimalla R. Mitochondria-targeted small molecule ss31: a potential candidate for the treatment of Alzheimer's disease. Hum Mol Genet. 2017;26:1597. doi: 10.1093/hmg/ddx129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Boylston J.A., Sun J., Chen Y., Gucek M., Sack M.N., Murphy E. Characterization of the cardiac succinylome and its role in ischemia–reperfusion injury. J Mol Cell Cardiol. 2015;88:73–81. doi: 10.1016/j.yjmcc.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Valls-Lacalle L., Barba I., Miro-Casas E., Alburquerque-Bejar J.J., Ruiz-Meana M., Fuertes-Agudo M., et al. Succinate dehydrogenase inhibition with malonate during reperfusion reduces infarct size by preventing mitochondrial permeability transition. Cardiovasc Res. 2016;109:374–384. doi: 10.1093/cvr/cvv279. [DOI] [PubMed] [Google Scholar]
- 303.Cordes T., Lucas A., Divakaruni A.S., Murphy A.N., Cabrales P., Metallo C.M. Itaconate modulates tricarboxylic acid and redox metabolism to mitigate reperfusion injury. Molecular metabolism. 2020;32:122–135. doi: 10.1016/j.molmet.2019.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Asadi Shahmirzadi A., Edgar D., Liao C.Y., Hsu Y.M., Lucanic M., Asadi Shahmirzadi A., et al. Alpha-ketoglutarate, an endogenous metabolite, extends lifespan and compresses morbidity in aging mice. Cell Metabol. 2020;32:447–456. doi: 10.1016/j.cmet.2020.08.004. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Ma X., McKeen T., Zhang J., Ding W.X. Role and mechanisms of mitophagy in liver diseases. Cells. 2020;9:837. doi: 10.3390/cells9040837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Redmann M., Benavides G.A., Wani W.Y., Berryhill T.F., Ouyang X., Johnson M.S., et al. Methods for assessing mitochondrial quality control mechanisms and cellular consequences in cell culture. Redox Biol. 2018;17:59–69. doi: 10.1016/j.redox.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Redmann M., Dodson M., Boyer-Guittaut M., Darley-Usmar V., Zhang J. Mitophagy mechanisms and role in human diseases. Int J Biochem Cell Biol. 2014;53:127–133. doi: 10.1016/j.biocel.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Zhang J. Autophagy and mitophagy in cellular damage control. Redox Biol. 2013;1:19–23. doi: 10.1016/j.redox.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Kim I., Rodriguez-Enriquez S., Lemasters J.J. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys. 2007;462:245–253. doi: 10.1016/j.abb.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Cai Q., Jeong Y.Y. Mitophagy in Alzheimer's disease and other age-related neurodegenerative diseases. Cells. 2020;9:150. doi: 10.3390/cells9010150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Collins H.E., Kane M.S., Litovsky S., Darley-Usmar V., Young M.E., Chatham J.C., Zhang J. Mitochondrial morphology and mitophagy in heart diseases: qualitative and quantitative analyses using transmission electron microscopy (TEM) Front Aging. 2021;1:9. doi: 10.3389/fragi.2021.670267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Clark E.H., Vazquez de la Torre A., Hoshikawa T., Briston T. Targeting mitophagy in Parkinson's disease. J Biol Chem. 2020;296:100209. doi: 10.1074/jbc.REV120.014294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Hertz N.T., Berthet A., Sos M.L., Thorn K.S., Burlingame A.L., Nakamura K., et al. A neo-substrate that amplifies catalytic activity of Parkinson's-disease-related kinase PINK1. Cell. 2013;154:737–747. doi: 10.1016/j.cell.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Osgerby L., Lai Y.C., Thornton P.J., Amalfitano J., Le Duff C.S., Jabeen I., et al. Kinetin riboside and its proteides activate the Parkinson's disease associated PTEN-induced putative kinase 1 (PINK1) independent of mitochondrial depolarization. J Med Chem. 2017;60:3518–3524. doi: 10.1021/acs.jmedchem.6b01897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Barini E., Miccoli A., Tinarelli F., Mulholland K., Kadri H., Khanim F., et al. The anthelmintic drug niclosamide and its analogues activate the Parkinson's disease associated protein kinase PINK1. Chembiochem. 2018;19:425–429. doi: 10.1002/cbic.201700500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Hsieh C.H., Li L., Vanhauwaert R., Nguyen K.T., Davis M.D., Bu G., et al. Miro1 marks Parkinson's disease subset and Miro1 reducer rescues neuron loss in Parkinson's models. Cell Metabol. 2019;30:1131–11340. doi: 10.1016/j.cmet.2019.08.023. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Rusilowicz-Jones E.V., Jardine J., Kallinos A., Pinto-Fernandez A., Guenther F., Giurrandino M., et al. USP30 sets a trigger threshold for PINK1-PARKIN amplification of mitochondrial ubiquitylation. Life Sci Alliance. 2020;3 doi: 10.26508/lsa.202000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Luo H., Krigman J., Zhang R., Yang M., Sun N. Pharmacological inhibition of USP30 activates tissue-specific mitophagy. Acta Physiol. 2021;232 doi: 10.1111/apha.13666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Liu Y., Lear T.B., Verma M., Wang K.Z., Otero P.A., McKelvey A.C., et al. Chemical inhibition of FBXO7 reduces inflammation and confers neuroprotection by stabilizing the mitochondrial kinase PINK1. JCI Insight. 2020;5 doi: 10.1172/jci.insight.131834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Takahashi D., Moriyama J., Nakamura T., Miki E., Takahashi E., Sato A., et al. Autacs: cargo-specific degraders using selective autophagy. Mol Cell. 2019;76:797–810. doi: 10.1016/j.molcel.2019.09.009. e10. [DOI] [PubMed] [Google Scholar]
- 321.Allen G.F., Toth R., James J., Ganley I.G. Loss of iron triggers PINK1/Parkin-independent mitophagy. EMBO Rep. 2013;14:1127–1135. doi: 10.1038/embor.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Singh F., Prescott A.R., Ball G., Reith A.D., Ganley I.G. Pharmacological rescue of impaired mitophagy in Parkinson’s disease-related LRRK2 G2019s knock-in mice. bioRxiv. 2020 doi: 10.1101/2020.12.07.414359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Wauters F., Cornelissen T., Imberechts D., Martin S., Koentjoro B., Sue C., et al. LRRK2 mutations impair depolarization-induced mitophagy through inhibition of mitochondrial accumulation of RAB10. Autophagy. 2020;16:203–222. doi: 10.1080/15548627.2019.1603548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Katayama H., Hama H., Nagasawa K., Kurokawa H., Sugiyama M., Ando R., et al. Visualizing and modulating mitophagy for therapeutic studies of neurodegeneration. Cell. 2020;181:1176–1187. doi: 10.1016/j.cell.2020.04.025. [DOI] [PubMed] [Google Scholar]
- 325.Cen X., Chen Y., Xu X., Wu R., He F., Zhao Q., et al. Pharmacological targeting of MCL-1 promotes mitophagy and improves disease pathologies in an Alzheimer's disease mouse model. Nat Commun. 2020;11:5731. doi: 10.1038/s41467-020-19547-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Venegas C., Heneka M.T. Danger-associated molecular patterns in Alzheimer's disease. J Leukoc Biol. 2017;101:87–98. doi: 10.1189/jlb.3MR0416-204R. [DOI] [PubMed] [Google Scholar]
- 327.Laske C., Stransky E., Hoffmann N., Maetzler W., Straten G., Eschweiler G.W., et al. Macrophage colony-stimulating factor (M-CSF) in plasma and CSF of patients with mild cognitive impairment and Alzheimer's disease. Curr Alzheimer Res. 2010;7:409–414. doi: 10.2174/156720510791383813. [DOI] [PubMed] [Google Scholar]
- 328.Sheng J.G., Mrak R.E., Griffin W.S. Microglial interleukin-1α expression in brain regions in Alzheimer's disease: correlation with neuritic plaque distribution. Neuropathol Appl Neurobiol. 1995;21:290–301. doi: 10.1111/j.1365-2990.1995.tb01063.x. [DOI] [PubMed] [Google Scholar]
- 329.Perlmutter L.S., Barron E., Chui H.C. Morphologic association between microglia and senile plaque amyloid in Alzheimer's disease. Neurosci Lett. 1990;119:32–36. doi: 10.1016/0304-3940(90)90748-x. [DOI] [PubMed] [Google Scholar]
- 330.Medeiros R., LaFerla F.M. Astrocytes: conductors of the alzheimer disease neuroinflammatory symphony. Exp Neurol. 2013;239:133–138. doi: 10.1016/j.expneurol.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 331.Griffin W.S., Stanley L.C., Ling C., White L., MacLeod V., Perrot L.J., et al. Brain interleukin 1 and S-100 immunoreactivity are elevated in down syndrome and Alzheimer disease. Proc Natl Acad Sci U S A. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Akiyama H., Barger S., Barnum S., Bradt B., Bauer J., Cole G.M., et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Wilkins H.M., Weidling I.W., Ji Y., Swerdlow R.H. Mitochondria-derived damage-associated molecular patterns in neurodegeneration. Front Immunol. 2017;8:508. doi: 10.3389/fimmu.2017.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Swerdlow R.H. Mitochondria and cell bioenergetics: increasingly recognized components and a possible etiologic cause of Alzheimer's disease. Antioxid Redox Signal. 2012;16:1434–1455. doi: 10.1089/ars.2011.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Hirai K., Aliev G., Nunomura A., Fujioka H., Russell R.L., Atwood C.S., et al. Mitochondrial abnormalities in Alzheimer's disease. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Parker W.D., Jr., Filley C.M., Parks J.K. Cytochrome oxidase deficiency in Alzheimer's disease. Neurology. 1990;40:1302–1303. doi: 10.1212/wnl.40.8.1302. [DOI] [PubMed] [Google Scholar]
- 337.Parker W.D., Jr., Parks J.K. Cytochrome c oxidase in Alzheimer's disease brain: purification and characterization. Neurology. 1995;45:482–486. doi: 10.1212/wnl.45.3.482. [DOI] [PubMed] [Google Scholar]
- 338.Kish S.J. Brain energy metabolizing enzymes in Alzheimer's disease: alpha-ketoglutarate dehydrogenase complex and cytochrome oxidase. Ann N Y Acad Sci. 1997;826:218–228. doi: 10.1111/j.1749-6632.1997.tb48473.x. [DOI] [PubMed] [Google Scholar]
- 339.Mutisya E.M., Bowling A.C., Beal M.F. Cortical cytochrome oxidase activity is reduced in Alzheimer's disease. J Neurochem. 1994;63:2179–2184. doi: 10.1046/j.1471-4159.1994.63062179.x. [DOI] [PubMed] [Google Scholar]
- 340.Cardoso S.M., Proenca M.T., Santos S., Santana I., Oliveira C.R. Cytochrome c oxidase is decreased in Alzheimer's disease platelets. Neurobiol Aging. 2004;25:105–110. doi: 10.1016/s0197-4580(03)00033-2. [DOI] [PubMed] [Google Scholar]
- 341.Curti D., Rognoni F., Gasparini L., Cattaneo A., Paolillo M., Racchi M., et al. Oxidative metabolism in cultured fibroblasts derived from sporadic Alzheimer's disease (AD) patients. Neurosci Lett. 1997;236:13–16. doi: 10.1016/s0304-3940(97)00741-6. [DOI] [PubMed] [Google Scholar]
- 342.Mancuso M., Filosto M., Bosetti F., Ceravolo R., Rocchi A., Tognoni G., et al. Decreased platelet cytochrome c oxidase activity is accompanied by increased blood lactate concentration during exercise in patients with Alzheimer disease. Exp Neurol. 2003;182:421–426. doi: 10.1016/s0014-4886(03)00092-x. [DOI] [PubMed] [Google Scholar]
- 343.Chang S.W., Zhang D., Chung H.D., Zassenhaus H.P. The frequency of point mutations in mitochondrial DNA is elevated in the Alzheimer's brain. Biochem Biophys Res Commun. 2000;273:203–208. doi: 10.1006/bbrc.2000.2885. [DOI] [PubMed] [Google Scholar]
- 344.Hamblet N.S., Castora F.J. Elevated levels of the kearns-sayre syndrome mitochondrial DNA deletion in temporal cortex of Alzheimer's patients. Mutat Res. 1997;379:253–262. doi: 10.1016/s0027-5107(97)00158-9. [DOI] [PubMed] [Google Scholar]
- 345.Krishnan K.J., Ratnaike T.E., De Gruyter H.L., Jaros E., Turnbull D.M. Mitochondrial DNA deletions cause the biochemical defect observed in Alzheimer's disease. Neurobiol Aging. 2012;33:2210–2214. doi: 10.1016/j.neurobiolaging.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 346.Lovell M.A., Markesbery W.R. Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer's disease. Nucleic Acids Res. 2007;35:7497–7504. doi: 10.1093/nar/gkm821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Mecocci P., MacGarvey M.S., Beal M.F. Oxidative damage to mitochondrial DNA is increased in Alzheimer's disease. Ann Neurol. 1994;36:747–751. doi: 10.1002/ana.410360510. [DOI] [PubMed] [Google Scholar]
- 348.Weinberg S.E., Sena L.A., Chandel N.S. Mitochondria in the regulation of innate and adaptive immunity. Immunity. 2015;42:406–417. doi: 10.1016/j.immuni.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Brown J.A., Sammy M.J., Ballinger S.W. An evolutionary, or "mitocentric" perspective on cellular function and disease. Redox Biol. 2020;36:101568. doi: 10.1016/j.redox.2020.101568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Mills E.L., Kelly B., O'Neill L.A.J. Mitochondria are the powerhouses of immunity. Nat Immunol. 2017;18:488–498. doi: 10.1038/ni.3704. [DOI] [PubMed] [Google Scholar]
- 351.Zhang Q., Raoof M., Chen Y., Sumi Y., Sursal T., Junger W., et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Seth R.B., Sun L.J., Ea C.K., Chen Z.J.J. Identification and characterization of MAVs, a mitochondrial antiviral signaling protein that activates NF-κB and IRF3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 353.Buskiewicz I.A., Montgomery T., Yasewicz E.C., Huber S.A., Murphy M.P., Hartley R.C., et al. Reactive oxygen species induce virus-independent MAVS oligomerization in systemic lupus erythematosus. Sci Signal. 2016;9:ra115. doi: 10.1126/scisignal.aaf1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Loo Y.M., Gale M., Jr. Immune signaling by RIG-I-like receptors. Immunity. 2011;34:680–692. doi: 10.1016/j.immuni.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Iyer S.S., He Q., Janczy J.R., Elliott E.I., Zhong Z., Olivier A.K., et al. Mitochondrial cardiolipin is required for NLRP3 inflammasome activation. Immunity. 2013;39:311–323. doi: 10.1016/j.immuni.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Wei X., Shao B., He Z., Ye T., Luo M., Sang Y., et al. Cationic nanocarriers induce cell necrosis through impairment of Na+/K+-ATPase and cause subsequent inflammatory response. Cell Res. 2015;25:237–253. doi: 10.1038/cr.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Zhang J.Z., Liu Z., Liu J., Ren J.X., Sun T.S. Mitochondrial DNA induces inflammation and increases TLR9/NF-κB expression in lung tissue. Int J Mol Med. 2014;33:817–824. doi: 10.3892/ijmm.2014.1650. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 358.Nakahira K., Haspel J.A., Rathinam V.A., Lee S.J., Dolinay T., Lam H.C., et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Zhou R., Yazdi A.S., Menu P., Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 360.Shimada K., Crother T.R., Karlin J., Dagvadorj J., Chiba N., Chen S., et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.West A.P., Khoury-Hanold W., Staron M., Tal M.C., Pineda C.M., Lang S.M., et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature. 2015;520:553–557. doi: 10.1038/nature14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Coskun P.E., Beal M.F., Wallace D.C. Alzheimer's brains harbor somatic mtDNA control-region mutations that suppress mitochondrial transcription and replication. Proc Natl Acad Sci U S A. 2004;101:10726–10731. doi: 10.1073/pnas.0403649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Wei W., Keogh M.J., Wilson I., Coxhead J., Ryan S., Rollinson S., et al. Mitochondrial DNA point mutations and relative copy number in 1363 disease and control human brains. Acta Neuropathol Commun. 2017;5:13. doi: 10.1186/s40478-016-0404-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.de la Monte S.M., Wands J.R. Molecular indices of oxidative stress and mitochondrial dysfunction occur early and often progress with severity of Alzheimer's disease. J Alzheimers Dis. 2006;9:167–181. doi: 10.3233/jad-2006-9209. [DOI] [PubMed] [Google Scholar]
- 365.Nunomura A., Perry G., Aliev G., Hirai K., Takeda A., Balraj E.K., et al. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 366.Devi L., Prabhu B.M., Galati D.F., Avadhani N.G., Anandatheerthavarada H.K. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer's disease brain is associated with mitochondrial dysfunction. J Neurosci. 2006;26:9057–9068. doi: 10.1523/JNEUROSCI.1469-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Eckert A., Schulz K.L., Rhein V., Gotz J. Convergence of amyloid-β and tau pathologies on mitochondria in vivo. Mol Neurobiol. 2010;41:107–114. doi: 10.1007/s12035-010-8109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Caspersen C., Wang N., Yao J., Sosunov A., Chen X., Lustbader J.W., et al. Mitochondrial Aβ: a potential focal point for neuronal metabolic dysfunction in Alzheimer’s disease. FASEB J. 2005;19:2040–2041. doi: 10.1096/fj.05-3735fje. [DOI] [PubMed] [Google Scholar]
- 369.Wilkins H.M., Koppel S.J., Weidling I.W., Roy N., Ryan L.N., Stanford J.A., et al. Extracellular mitochondria and mitochondrial components act as damage-associated molecular pattern molecules in the mouse brain. J Neuroimmune Pharmacol. 2016;11:622–628. doi: 10.1007/s11481-016-9704-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Montagne A., Nation D.A., Sagare A.P., Barisano G., Sweeney M.D., Chakhoyan A., et al. ApoE4 leads to blood–brain barrier dysfunction predicting cognitive decline. Nature. 2020;581:71–76. doi: 10.1038/s41586-020-2247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Mahley R.W., Weisgraber K.H., Huang Y. Apolipoprotein e4: a causative factor and therapeutic target in neuropathology, including Alzheimer's disease. Proc Natl Acad Sci U S A. 2006;103:5644–5651. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Picca A., Guerra F., Calvani R., Marini F., Biancolillo A., Landi G., et al. Mitochondrial signatures in circulating extracellular vesicles of older adults with Parkinson's disease: results from the exosomes in Parkinson's disease (expand) study. J Clin Med. 2020;9:504. doi: 10.3390/jcm9020504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Picca A., Guerra F., Calvani R., Bucci C., Lo Monaco M.R., Bentivoglio A.R., et al. Mitochondrial-derived vesicles as candidate biomarkers in Parkinson's disease: rationale, design and methods of the exosomes in Parkinson disease (expand) study. Int J Mol Sci. 2019;20:2373. doi: 10.3390/ijms20102373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Scholtzova H., Chianchiano P., Pan J., Sun Y., Goni F., Mehta P.D., et al. Amyloid β and tau Alzheimer's disease related pathology is reduced by toll-like receptor 9 stimulation. Acta Neuropathol Commun. 2014;2:101. doi: 10.1186/s40478-014-0101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Scholtzova H., Kascsak R.J., Bates K.A., Boutajangout A., Kerr D.J., Meeker H.C., et al. Induction of toll-like receptor 9 signaling as a method for ameliorating Alzheimer's disease-related pathology. J Neurosci. 2009;29:1846–1854. doi: 10.1523/JNEUROSCI.5715-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Fang E.F. Mitophagy and NAD+ inhibit Alzheimer disease. Autophagy. 2019;15:1112–1114. doi: 10.1080/15548627.2019.1596497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Chacko B.K., Zhi D., Darley-Usmar V.M., Mitchell T. The bioenergetic health index is a sensitive measure of oxidative stress in human monocytes. Redox Biol. 2016;8:43–50. doi: 10.1016/j.redox.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Acin-Perez R., Benador I.Y., Petcherski A., Veliova M., Benavides G.A., Lagarrigue S., et al. A novel approach to measure mitochondrial respiration in frozen biological samples. EMBO J. 2020;39 doi: 10.15252/embj.2019104073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Smith M.R., Chacko B.K., Johnson M.S., Benavides G.A., Uppal K., Go Y.M., et al. A precision medicine approach to defining the impact of doxorubicin on the bioenergetic-metabolite interactome in human platelets. Redox Biol. 2020;28:101311. doi: 10.1016/j.redox.2019.101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Chacko B.K., Smith M.R., Johnson M.S., Benavides G., Culp M.L., Pilli J., et al. Mitochondria in precision medicine: linking bioenergetics and metabolomics in platelets. Redox Biol. 2019;22:101165. doi: 10.1016/j.redox.2019.101165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Uppal K., Ma C., Go Y.M., Jones D.P., Wren J. xMWAS: a data-driven integration and differential network analysis tool. Bioinformatics. 2018;34:701–702. doi: 10.1093/bioinformatics/btx656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Go Y.M., Fernandes J., Hu X., Uppal K., Jones D.P. Mitochondrial network responses in oxidative physiology and disease. Free Radic Biol Med. 2018;116:31–40. doi: 10.1016/j.freeradbiomed.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Roede J.R., Uppal K., Park Y., Tran V., Jones D.P. Transcriptome-metabolome wide association study (TMWAS) of maneb and paraquat neurotoxicity reveals network level interactions in toxicologic mechanism. Toxicol Rep. 2014;1:435–444. doi: 10.1016/j.toxrep.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]