Summary
DNA ligases act in the final step of many DNA repair pathways and are commonly regulated by the DNA sliding clamp PCNA, but there are limited insights into the physical basis for this regulation. Here we use single particle cryo-EM to analyze an archaeal DNA ligase and heterotrimeric PCNA in complex with a single-strand DNA break. The cryo-EM structures highlight a continuous DNA binding surface formed between DNA ligase and PCNA that supports the distorted conformation of the DNA break undergoing repair, and contributes to PCNA stimulation of DNA ligation. DNA ligase is conformationally flexible within the complex, with its domains fully ordered only when encircling the repaired DNA to form a stacked ring structure with PCNA. The structures highlight DNA ligase structural transitions while docked on PCNA, changes in DNA conformation during ligation, and the potential for DNA ligase domains to regulate PCNA accessibility to other repair factors.
Graphical Abstract

eTOC Blurb
Sverzhinsky et al. use cryoelectron microscopy to investigate the complex formed by DNA ligase and the sliding clamp PCNA as they bind a nicked DNA molecule. They obtain snapshots of DNA ligation reaction intermediate states and identify the missing factor in PCNA stimulation of DNA ligation.
Introduction
The final step of most DNA repair pathways involves joining of one or both strands of the DNA duplex by one of the three human ligases (hLigI, hLigIII and hLigIV). hLigI is the principal enzyme that carries out DNA ligation during DNA replication, where the lagging strand is synthesized as Okazaki fragments. Okazaki fragment processing is completed by at least three enzymes working in a coordinated fashion (Beattie & Bell, 2012). The enzymes are topologically linked to DNA via the trimeric sliding clamp Proliferating Cell Nuclear Antigen (PCNA) (Figure 1A). Client enzymes are primarily linked to the inter-domain connecting loop (IDCL) of PCNA using a canonical PCNA-interacting peptide (PIP) motif (Warbrick, 2000; Chapados et al., 2004). The PIP motif of hLigI is found at the amino terminus of a flexible N-terminal region (Jónsson et al., 1998). DNA is most strongly bound by the DNA-binding domain (DBD), which cooperates with the Adenylation (AdD) and OB-fold (OBD) domains (Figure 1B) to encircle DNA (Pascal et al., 2004).
Figure 1. The Lig PIP motif is necessary for PCNA interaction but only partially involved in PCNA stimulation.

Heterotrimeric PCNA (A) contacts Lig through its PIP motif (B) to stimulate DNA end-joining (C). (D) A fluorescently labeled loop peptide was used to measure the affinity to PCNA1–2 heterodimer, PCNA3 subunit, PCNA1–2-3 heterotrimer, and hPCNA homotrimer. The graph shows model fits (solid lines) to the data (circles). (E) A DNA end-joining assay at 50°C with 10 nM DNA, 15 nM Lig, and 15, 45, and 135 nM PCNA. PCNA3 and the heterotrimer stimulated Lig activity. Quantification of the ligated and non-ligated DNA yielded a percentage of ligated product that is listed at the bottom of each reaction lane. (F, G) Gel filtration chromatograms (F) and the resulting fractions as visualized by SDS-PAGE (G) for samples of Lig/PCNA/DNA alone or in complexes as indicated. The region representing complex formation is shown by a black rectangle in both panels F and G. Lig PIP mutants show decreased co-elution with PCNA and DNA. A dimer of PCNA trimers co-elutes with DNA in the absence of complexation with Lig ΔLoop (lowest panel in G). (H) DNA end-joining assay as in panel E using the indicated Lig proteins and PCNA heterotrimer (15 nM and 45 nM). Despite progressively decreasing co-migration on gel filtration, PCNA stimulates the indicated Lig PIP mutants, even when lacking the PIP motif loop (ΔLoop). See also Figure S1.
DNA ligation is a dynamic process involving efficient conformational coordination between the three domains of the catalytic region: DBD, AdD, and OBD. Whereas bacterial DNA ligases use energy from NAD+, eukaryotic and archaeal homologs typically use ATP (Tomkinson et al., 2006). The self-adenylation of the AdD (step 1 of ligation) is accelerated by motif VI residues of the OBD (Sriskanda & Shuman, 1998). The OBD then rotates so that residues on the opposite side of the β-barrel can interact with DNA during steps 2 and 3 of ligation (Doherty & Wigley, 1999), where the AdD transfers its AMP adduct to the 5’-phosphate of nicked DNA (step 2), and the nick is resolved with the release of AMP and formation of a phosphodiester bond with the 3’OH of an adjacent DNA strand (step 3) (Sriskanda & Shuman, 1998).
Archaeal and eukaryotic DNA ligases have provided insights into the structural conformations that occur during the three-step ligation reaction. Crystal structures of hLigI (Pascal et al., 2004), hLigIII (Cotner-Gohara et al., 2010) and hLigIV (Kaminski et al., 2018) bound to nicked DNA demonstrate a conserved conformational state in which the ligase catalytic core domains encircle the DNA. A “closed” ligase conformation, representing step 1 of ligation, was observed in the thermophilic archaea Pyrococcus furiosus (Nishida et al., 2006) and Archaeoglobus fulgidus (Kim et al., 2009), whereas an ”open” conformation was observed in the hyperthermophilic archaeon Saccharolobus solfataricus (Pascal et al., 2006) (Figure 1B). The principal difference between the open, closed and DNA-bound conformations is the position of the OBD, whereas the DBD and AdD have a fixed relative orientation. Archaeal DNA ligases lack the flexible N-terminal extension of hLigI that bears a PIP motif. The PIP motifs of DNA ligases from S. solfataricus (henceforth referred to as Lig) (Pascal et al., 2006) and P. furiosus (Kiyonari et al., 2006) are found on a loop within the DBD (Figure 1B).
There are conflicting reports regarding stimulation of DNA ligation by human PCNA (hPCNA). Depending on the experimental conditions, groups have found no effect (Levin et al., 1997), an inhibitory effect (Jónsson et al., 1998) and a stimulating effect (Tom et al., 2001) by hPCNA on hLigI-mediated DNA end-joining. Stimulation has been observed in the P. furiosus (Kiyonari et al., 2006) and S. solfataricus (Dionne et al., 2003) archaeal systems. In the latter case, heterotrimeric S. solfataricus PCNA was necessary to stimulate Lig (Dionne et al., 2003). PCNA may stimulate ligation by increasing ligase presence at nicked DNA (Tom et al., 2001) and/or by facilitating ligase encirclement of the DNA nick. Evidence for PCNA serving a templating function was found in a negative stain electron microscopy (NS-EM) structure of P. furiosus ligase in complex with homotrimeric PCNA and a nicked, non-ligatable DNA (Mayanagi et al., 2009). Along with the predicted PIP-IDCL linkage with one PCNA subunit, a second contact was observed between the ligase AdD and a second PCNA subunit. Due to the limited resolution of NS-EM, it is unclear if the second site involves the PCNA IDCL or its central loop. Owing to their homotrimeric nature, PCNA from humans, P. furiosus, and A. fulgidus can potentially bind various stoichiometries of client enzymes. In contrast, each subunit of the heterotrimeric S. solfataricus PCNA binds a specific client enzyme (Dionne et al., 2003). PCNA1 and PCNA2 link to Flap Endonuclease (FEN) and DNA Polymerase (Pol) B1, respectively. The PCNA3 client enzyme is Lig (Dionne et al., 2003).
The S. solfataricus system allows a more direct investigation into PCNA stimulation of DNA ligation by means of its heterotrimeric PCNA. In the absence of DNA, these proteins form a 1:1 complex where Lig in its open conformation is tethered to PCNA3 through a link between its PIP motif and the IDCL of PCNA3 (Pascal et al., 2006) (Figure 1C). Upon encountering nicked DNA during Okazaki fragment processing, Lig presumably encircles DNA as was observed for hLigI (Pascal et al., 2004). Here we present the currently highest-resolution molecular details of Lig-PCNA interactions in cryo-EM structures of the DNA-bound Lig-PCNA complex before and after DNA end-joining. Along with our biochemical analysis, we provide insights into PCNA regulation of DNA Ligase, Lig domain dynamics while docked on the PCNA surface, DNA conformational changes associated with ligation, and the potential for Lig regulation of PCNA accessibility to other repair factors.
Results
The Lig PIP Motif is Necessary for PCNA Interaction but Only Partially Involved in PCNA Stimulation
We took advantage of the heterotrimeric nature of S. solfataricus PCNA by separately purifying the PCNA3 subunit, the PCNA1–2 heterodimer, and the full PCNA1–2-3 heterotrimer (Figure S1A). Dionne et al. reported that PCNA3 can weakly pull-down the PCNA2 client enzyme DNA Pol B through interaction with its PIP motif (Dionne et al., 2003). Therefore, we considered if there might be additional cross-talk between the Lig PIP motif and other PCNA subunits using fluorescence polarization with a fluorescently labeled Lig loop peptide containing 17 residues (KSKQQSTGILGFLGTTS; PIP motif residues in bold). The loop peptide bound PCNA1–2-3 with a Kd of 13.1 ± 2.5 μM (Figure 1D). As expected, most binding was mediated by PCNA3, contributing a Kd of 32.5 ± 6.6 μM. PCNA1–2 bound the peptide with much weaker affinity (Kd 176.7 ± 74.4 μM), but still above the background level observed with hPCNA. Once bound to PCNA3, the fluorescent peptide was outcompeted in a similar manner by Lig or an unlabelled loop peptide (Figure S1B). Notably, the PCNA3 subunit alone stimulated DNA end-joining at 50°C (Figure 1E); yet, the greatest stimulation was seen in context of the full PCNA heterotrimer, which is probably due to the increased DNA binding affinity of three PCNA subunits over just one (Figure S1C).
The medium micromolar affinity between PCNA and the Lig PIP loop corresponded to results from gel filtration analysis. Co-injection of Lig and PCNA at 60 μM showed incomplete complexation (Figure 1F, purple trace), whereas in the presence of DNA, Lig and PCNA co-eluted at 5 μM (Figure 1F, green trace and Figure 1G, black rectangle). We sought to determine which elements of the Lig PIP motif mediate PCNA interaction through mutagenesis. Mutating the residues F110 and L111 to Alanine in the Lig PIP motif (Lig FL-AA) disrupted co-elution with PCNA on gel filtration but did not ablate stimulation by PCNA (Pascal et al., 2006) (Figures 1G and 1H). We further mutated the Q103 and I107 residues alone (Lig QI-AA), or in addition to the FL residues (Lig QIFL-AAAA), and we deleted the entire PIP motif-containing loop (Lig ΔLoop). The mutations targeting Q103 and I107 residues showed marginal disruptions in co-elution with PCNA alone or with PCNA and DNA (Figures S1D and S1E). While the F110 and L111 residues are responsible for most of the interaction with PCNA, Lig ΔLoop showed a further decrease in co-elution with PCNA (Figure 1G). It is noteworthy that although Lig ΔLoop did not co-elute with PCNA, PCNA nonetheless co-eluted with DNA at the volume of the ternary complex (Figure 1F, blue trace and Figure S1E). Lig and nicked DNA did not co-migrate on gel filtration at the above concentrations.
The Lig PIP motif mutants were evaluated in a DNA ligation assay to correlate co-elution on gel filtration and stimulation of ligation by PCNA (Figure 1H). PCNA stimulation of Lig QI-AA was only slightly attenuated compared to Lig WT. Following the trend from gel filtration, PCNA showed decreased stimulation of Lig FL-AA and the addition of the QI-AA mutations did not appreciably change the level of stimulation. Surprisingly, PCNA was able to stimulate Lig ΔLoop, despite a lack of co-elution by gel filtration, suggesting at least one other contact between the proteins outside of the PIP loop. Therefore, we turned to investigating the structural interactions between Lig and the PCNA heterotrimer to identify additional contacts.
Equimolar Stoichiometry and Conformations of Lig-PCNA-DNA by NS-EM
The ternary Lig-PCNA-DNA complex was analyzed by size exclusion chromatography coupled to multi-angle light scattering (SEC-MALS). Lig was incubated with PCNA and ligatable nicked DNA at room temperature for 15 min prior to injection (Figure 2A). The major peak (12.5 mL elution) resulted in a scattering-derived mass of 170 ± 1.4 kDa, closely matching the expected mass of ~175 kDa of a 1:1:1 complex. The minor peak (red bracket) represented a mixture of the non-complexed components: excess Lig, a nicked version of Lig with a disrupted PIP motif that is unable to bind PCNA3 (Pascal et al., 2006), excess PCNA1–2 heterodimer, and DNA. Visualization of the protein and DNA components supported a stoichiometric ternary complex (Figure 2B). Contrary to the activity assays carried out for a few minutes at 50°C (Figures 1E and 1H), thermophilic Lig and PCNA act more slowly at room temperature (Figure S1F). Therefore, it was expected that the stoichiometric complex analyzed by SEC-MALS represented the proteins in progress of sealing nicked DNA.
Figure 2. Equimolar stoichiometry and conformations of Lig-PCNA-DNA by negative stain-EM.

(A, B) A complex of Lig and PCNA with ligatable DNA was measured to be ~170 kDa by SEC-MALS (A) and contained equimolar protein (SDS-PAGE) and DNA (agarose) (B). (C–E) Lig and PCNA were mixed with ligatable DNA and used for NS-EM, resulting in three conformers (“OBD-front”, “OBD-up”, “OBD-out”) each showing two contacts between the upper (Lig) and lower (PCNA) portions of the 3D reconstructions, but differing in the distal extremity representing the OBD. (F, G) The crystallized Lig DBD and AdD domains match the upper density (F) and the crystal structure of PCNA fits the toroidal density (G). (H) A control complex of PCNA with DNA resulted in a NS-EM reconstruction of two PCNA rings on DNA. See also Figure S2.
To visualize Lig and PCNA in action, the complex was reconstituted with ligatable nicked DNA and imaged by NS-EM. Due to the relatively low affinity between the proteins for each other and for DNA, the complex dissociated following gel filtration (Figure S2A), but not when a 10 μM mixture of the proteins was diluted onto a grid (Figure S2B). Interestingly, upon dissociation of Lig from DNA, two PCNA rings could bind to the linear DNA, forming two stacked rings (Figure S2A). Indeed, the same stacked rings on DNA were observed using control grids (Figure S2C). Particles representing the ternary complex were retained for 3D classification and refined to ~20–25 Å resolution (Figure S2D).
Three predominant classes of the Lig-PCNA-3’OH DNA structure were identified and yielded 3D maps (Figures 2C–E). The upper portion of the maps was interpreted as Lig, and indeed the three NS-EM maps accommodated well the DBD and AdD domains (Figure 2F) without altering the relative domain orientations seen in the Lig crystal structure (Pascal et al., 2006). In contrast, the OBD appeared to adopt a different orientation in each of the three classes and required re-positioning to fit within the maps (Figures S2E–H). The toroidal portion of each structure was interpreted as the PCNA ring (Figure 2G), and the PCNA heterotrimer was positioned within the maps with the PCNA3 subunit adjacent to the DBD of Lig, where the proteins appeared to form an extensive interface (Figure 2F). Although DNA can be challenging to observe in NS-EM, the upstream extremity of the DNA could be visualized emerging from the PCNA ring (Figure 2G) and the density directly above the DBD (Figure 2F) was interpreted as the downstream extremity of the 47 bp nicked DNA (Figure S2I). Furthermore, DNA was also clearly observed in a 3D reconstruction of the two stacked rings of PCNA that formed when Lig dissociated from the complex (Figure 2H). In addition to the DBD-PCNA3 interface, the Lig AdD invariably contacted the PCNA2 subunit (Figures 2F and S2E–G). We turned to cryo-EM to obtain detailed insights into the nature of the Lig-PCNA interactions and their contributions to PCNA stimulation of ligation.
Architecture of Lig-PCNA-DNA Complexes by Cryo-EM
For cryo-EM analysis, we initially focused on a reconstituted complex of Lig and PCNA with non-ligatable (3’dideoxy-terminated) DNA to try to limit the multiple Lig conformations observed with NS-EM. The resulting map with a global resolution of ~4.1 Å (Figures 3A and S3A) confirmed and extended our observations from NS-EM. However, the OBD was not visible within our map, except for partial density extending from the AdD (Figure S4F), likely reflecting that the OBD still adopted multiple conformations. For visualization, we placed the OBD in the “open” orientation relative to the AdD and DBD (Figure 3A).
Figure 3. Reconstruction and modeling of the Lig-PCNA complex with non-ligatable DNA.

(A) The 4.16 Å cryo-EM map and associated model (B) viewed from two opposite sides of the complex: Lig (dark red), PCNA1 (pink), PCNA2 (magenta), PCNA3 (blue). The Lig OBD was not completely resolved; it was placed approximately in the apo orientation observed in the crystal structure without DNA. The upstream DNA traverses the PCNA ring and contains the template strand (salmon) and the 3’dideoxy strand (olive). The downstream DNA contains the 5’PO4 strand (cyan). (C) The Lig DBD and its PIP motif loop interface closely with PCNA3. (D) The upstream DNA duplex engages the DNA binding surface of PCNA3. (E) A minor site of interaction is formed between the Lig AdD and the PCNA2 central loop. See also Figures S3, S4 and S5.
The map quality allowed us to model the DNA, the three PCNA subunits, and the Lig DBD and AdD domains (Figure 3B). The map was discontinuous over certain regions of the Lig PIP motif loop (Figure 3C), indicating that parts of the loop remain flexible even when complexed with PCNA on DNA. The PIP loop extends from the Lig DBD and binds PCNA3 at its IDCL through the motif residues I107, F110, and L111, whereas Q103 was notably not involved in the interaction (Figure 3C). However, the majority of the PCNA3-DBD interaction arises from the core structure of the DBD, rather than the PIP loop, with a total buried surface area of roughly 1000 Å2. The DNA-binding surface of the PCNA3 subunit is adjacent to the DNA-binding surface of the DBD, thus forming an extended interface with DNA (Figure 3B) that spans more than two helical turns of DNA and encompasses 854 Å2 of buried surface area. The extended PCNA3-DBD interface supports an arched conformation of the DNA that positions the nick site within the AdD (see section below on DNA conformation). The AdD orientation pivots slightly relative to the DBD when compared to the apo Lig structure (Figure S5A). Within the AdD, Lig Y337 contacts residue S42 of the PCNA2 central loop (Figure 3E).
We next assembled Lig and PCNA with ligatable DNA and allowed the proteins to bind and repair DNA at room temperature for about 1 hour before vitrification for cryo-EM. This time-point allowed approximately half of the DNA molecules to be repaired (Figure S1F). An initial consensus map was obtained with partial density for the OBD in both the “open” and the DNA-encircled positions (Figure S3B). We subjected this map to the 3D Variability Analysis algorithm (Punjani & Fleet, 2021) and observed large movements near the terminus of the downstream DNA as the principal mode of variability. The second mode of variability showed the OBD transitioning between the “open” and DNA-encircled positions, and this transition was coupled with flexing of the DNA duplex at the Lig active site (Video S1). We partitioned the particles into two classes representing the two Lig OBD positions (Figures 4A, 5A and S3B). Separating the particles into two classes improved the quality of the maps and allowed more confident modeling of Lig and DNA conformations.
Figure 4. Reconstruction and modeling of Lig-PCNA with ligatable DNA after DNA adenylation.

(A) The 4.38 Å cryo-EM map and associated model (B) from one subset of the cryo-EM dataset of Lig and PCNA with ligatable DNA, in which AMP was transferred to the DNA. The map and model are viewed from two opposite sides of the complex. The colors are the same as in Figure 3, except that Lig is colored orange. The reconstruction lacks coherent density for the Lig OBD and parts of the AdD. See also Figures S3, S4 and S5.
Figure 5. Reconstruction and modeling of Lig-PCNA with ligatable DNA after DNA end-joining.

(A) The 4.20 Å cryo-EM map and associated model (B) from the second subset of the cryo-EM dataset of Lig and PCNA with ligatable DNA, in which the DNA has been end-joined. The map and model are viewed from two opposite sides of the complex. The Lig OBD was docked as a rigid body into the incomplete density above PCNA1. The colors are the same as in Figures 3 and 4, except Lig is colored green. See also Figures S3, S4 and S5.
One of the 3D reconstructions of Lig and PCNA with ligatable DNA (Figure 4A) lacked adequate density to position the OBD, and parts of the Lig AdD were unresolved, including residues 311–346 and 376–412. The second 3D reconstruction was more complete and showed Lig fully encircling the DNA with its OBD (Figure 5A). Whereas the DBD and AdD were well represented in this map and largely unchanged from the complex containing 3’dideoxy DNA, the OBD region of the map was not highly detailed and the OBD was thus positioned as a rigid body (Figure 5B). Notably, all three cryo-EM maps contained density on the backbone of the DNA that we modeled as Mn2+ ions (Figure 6A), and one of these binding sites corresponds to the “high fidelity” Mg2+ binding site recently described for hLigI (Tumbale et al., 2019). Two of the maps (see below) contained density in the active that we interpreted as catalytic Mn2+ based on comparison to high-resolution structures (Figures 6B and C).
Figure 6. Lig and PCNA arch the DNA to widen the active site where Lig adenylates and repairs the DNA break.

(A) Orthogonal views of the three DNA models overlayed on the map from Figure 5A (surface map is clipped to focus on the DNA conformations). The DNA bends toward the DBD. Density that was modeled as Mn2+ ions along the DNA backbone is indicated. (B) A comparison of the active sites shows that Lig has retained its AMP moiety on K260 (post-step 1) when complexed with dideoxy DNA. (C) The map from Figure 4 shows the formation of a phosphoanhydride bond between AMP and the 5’PO4 (AppDNA; post-step 2). (D) The reconstruction from Figure 5 does not contain AMP and represents end-joined DNA (post-step 3). (E) Increased DNA arching is demonstrated by alignment of Lig-AMP with dideoxy DNA (red protein with colored DNA) and hLigIV-AMP with dideoxy DNA (grey; PDB 6BKF). The 3’ terminus of Lig-AMP is pulled farther from the 5’ terminus by the combined DNA binding of PCNA3 and the DBD. (F–H) Transparent cryo-EM maps and the corresponding models of Lig-PCNA in complex with dideoxy DNA (F), adenylated DNA (G), and end-joined DNA (H). The top and bottom rows represent opposing views of the same maps and structures. Density interpreted as Mn2+ ions along the DNA backbone, or as catalytic Mn2+ ions (MnCAT) in maps containing AMP are indicated. See also Figure S5.
The principal difference between the three maps (Figures 3A, 4A, and 5A) lies in the Lig active site and the interaction with the DNA nick. Whereas hLigI carried out step 2 of ligation by transferring AMP to non-ligatable DNA prior to crystallization (Pascal et al., 2004), Lig with non-ligatable DNA retained the AMP moiety on its active site residue K260 (Figures 6B and S5D). AMP appears poised for transfer to the downstream DNA strand 5’PO4. Indeed, an activity assay indicated that the non-ligatable 3’dideoxy DNA did not proceed to step 2 of ligation (Figure S1F), probably due to the dideoxyribose residue perturbing the local structure around the DNA break. In the presence of ligatable DNA, one of the reconstructions contained DNA adenylated at the 5’PO4 following step 2 of DNA ligation (Figure 6C). In the second reconstruction of Lig-PCNA with ligatable DNA, there was no longer an AMP moiety in the active site and the DNA was best modeled as a continuous duplex. Hence the second reconstruction represents post-step 3 of DNA ligation and the new DNA phosphodiester bond was formed (Figure 6D). A comparison between hLigI encircling DNA and Lig encircling DNA with PCNA showed some conformational differences, with the greatest difference in the OBD position (Figure S5B). This observation may be due to the different DNA substrates (end-joined DNA versus nicked DNA).
Our structure shows that Lig remains in complex with PCNA and duplex DNA after completing the final step of DNA ligation (Figure 5A). Indeed, we observed the proteins co-eluting with DNA on gel filtration while in the process of repairing DNA (Figures S6A and S6B). Under the same conditions as those used for cryo-EM grid preparation, the proteins alone or with DNA were injected and the fractions analyzed by protein and DNA gels. Compared to Lig-PCNA alone or duplex DNA alone, their combination led to an earlier-eluting peak containing all the constituents. As the proteins repaired DNA at room temperature, they eluted with the ligatable DNA earlier than they did with duplex DNA, most likely representing dynamic and extended movements of Lig relative to PCNA that are reflected by flexible regions in the post-step 2 map (Figure 4A). Importantly, they remained bound even after 2 hours, when most DNA has been repaired (Figures S1F, S6A and S6B).
Alterations in DNA Conformation
In addition to the structural changes at the DNA break ends, there were also changes in the overall conformation of the DNA when the three structures were compared. Despite modest local resolutions of the DNAs (Figures S4F–H), the data were of sufficient quality to overcome base pairing restraints included in model refinement (Figure 6F–H). DNA structure analysis by DSSR (Lu & Olson, 2008) found perturbations in DNA base pairing and helical parameters relative to B-form DNA. While the upstream duplex traversing PCNA and the Lig DBD is straight and contains a typical helical twist, base pairs beginning at three positions upstream of the nick begin to incline towards the DBD, leading to a change of the DNA path (Figure 6A). Whereas DNA ligases alone distort the DNA double helix opposite the nick, our structures show more pronounced arching when PCNA contributes to binding upstream DNA. This difference in the extent of DNA arching is best appreciated by comparing the structure of Lig(-AMP)-PCNA-dideoxy DNA to the structure of hLigIV(-AMP) with dideoxy DNA (Kaminski et al. 2018), which represents the equivalent “open” position of DNA ligase (Figures 6E and S5C). The increased DNA arching results in a longer distance between the DNA nick termini. Whereas the upstream DNA is pulled towards PCNA3 and the DBD, the 5’PO4 of the downstream strand is coordinated by AdD residues R280 and R427 (Figures S5D–F), and this results in the third nucleotide downstream of the nick exhibiting much longer hydrogen bonding distances with the template strand (Figure S5G). The weakened base pairing is restored, and the downstream strand moves closer to the upstream strand in the context of adenylated DNA (Figure S5H). Following DNA end-joining, there is an opening between the base pairs at the former 3’OH terminus of DNA (Figures 6H and S5I). Taken together, the downstream strand moves closer to the upstream strand as it is processed by Lig until DNA end-joining (Figure S5F). Finally, the post-step 1 (Figure 3B) and post-step 2 (Figure 4B) models show that the DNA helical twist stops at the nick, and is restored with end-joined DNA in the post-step 3 model (Figures S5J–L).
PCNA Stimulates Lig Through Contacts at the Major and not the Minor Site of Interaction
The NS and cryo-EM structures invariably show two points of contact between the PCNA ring and Lig. We evaluated the role that each of the Lig-PCNA interfaces plays in PCNA stimulation of ligation. We hypothesized that the Lig AdD-PCNA2 interaction (Figure 3E) contributes to stimulation by templating Lig around DNA, whereas the extensive non-PIP motif interface between Lig DBD and PCNA3 would solidify Lig presence at the PCNA-DNA junction. Central to the latter site is Lig R145 that contacts PCNA3 Y200 (Figure 3C). We mutated R145 and purified Lig R145L and Lig R145D in the context of the WT PIP motif and the PIP loop deletion. The R145L and R145D mutants retained co-elution with PCNA on gel filtration at 60 μM (Figure S6C). However, an activity assay showed that while Lig R145D partially lost stimulation by PCNA at a level similar to Lig ΔLoop, both R145L and R145D mutants became insensitive to PCNA stimulation when combined with the deletion of the PIP loop (Figures 7A and 7B).
Figure 7. PCNA stimulates Lig through contacts at the major and not the minor site of interaction.
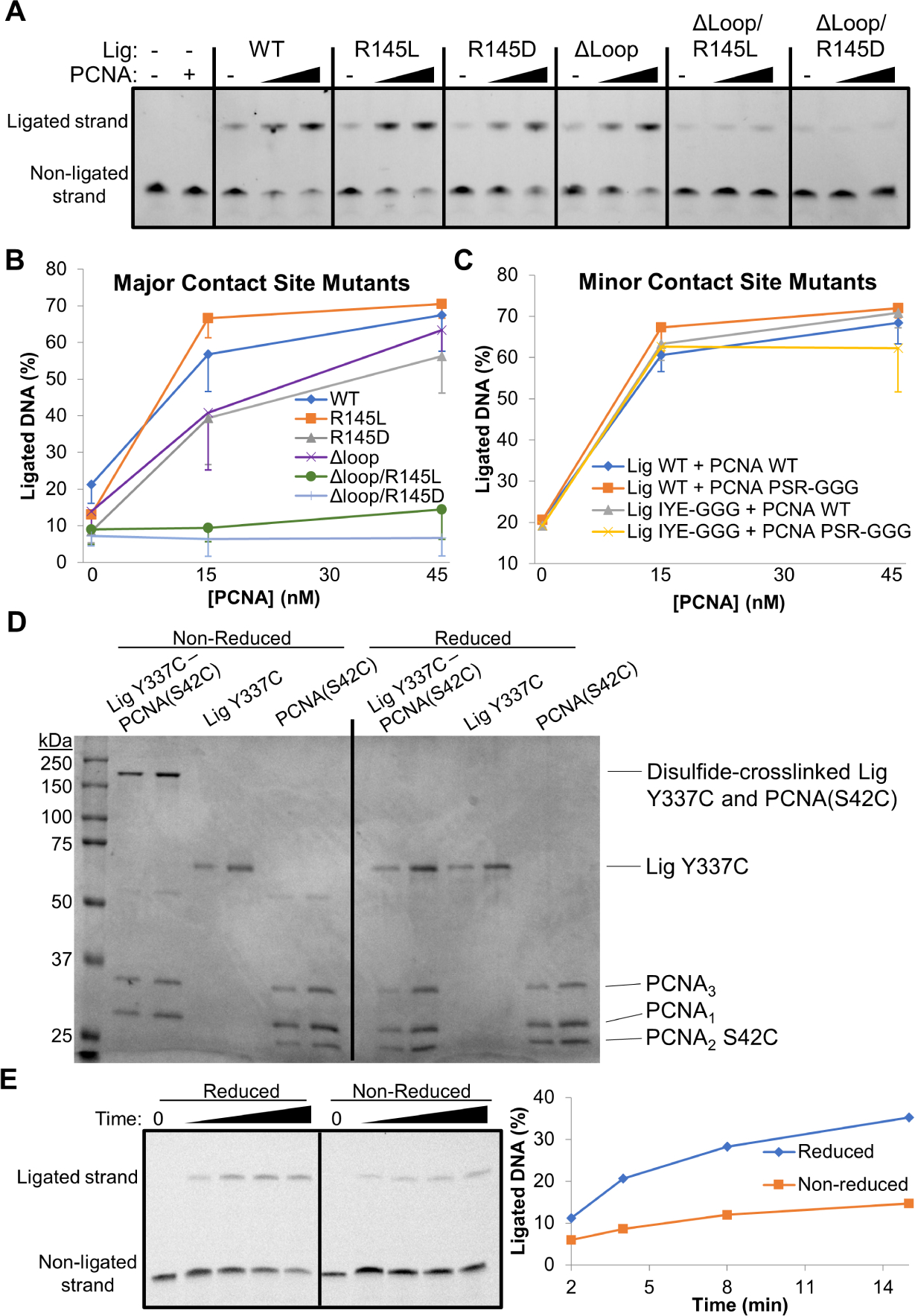
Lig non-PIP mutants in the DBD and the AdD were generated and assayed for PCNA stimulation of DNA end-joining. (A) DNA end-joining assay at 50°C with 10 nM DNA, 15 nM Lig (WT or the indicated mutants), and 15 or 45 nM PCNA. Lig DBD mutants showed insensitivity to PCNA when R145 was combined with a PIP loop deletion. (B) Six replicates of the assay in panel A were quantified and plotted. (C) Quantification of DNA end-joining assays as in panel A. The averages and standard deviations of three replicates are shown. A representative DNA end-joining assay is shown in Figure S7H. A triple-Glycine mutant of PCNA2 in combination with a triple-Glycine mutant of the Lig AdD retained PCNA-mediated stimulation of DNA ligation. (D) A complex of Lig and PCNA disulfide-crosslinked at the AdD-PCNA2 interface was purified and analyzed on SDS-PAGE under reducing and non-reducing conditions in comparison to the individual proteins prior to complex formation. Two amounts (2 and 4 pmols) were loaded for each sample. (E) A DNA end-joining assay of the AdD-PCNA2 crosslinked complex under reducing and non-reducing conditions, with a quantification of the fraction ligated. The proteins repaired DNA more efficiently when the disulfide crosslink was reduced. See also Figures S6 and S7.
Since combined mutations of the DBD-PCNA3 interaction completely blocked PCNA stimulation of ligation, we aggressively mutated the minor site of interaction to evaluate any contribution to stimulation. Lig Y337 and flanking residues were mutated to Glycines (Lig IYE-GGG) and PCNA2 S42 and flanking residues were mutated to Glycines (PCNA2 PSR-GGG). The PCNA heterotrimer was purified using the PCNA2 PSR-GGG mutant and its effect on Lig WT and Lig IYE-GGG was evaluated with a DNA repair assay (Figures 7C and S7H). Despite the substantial amino acid changes on both sides of the interface, the mutations at this site of interaction did not disrupt stimulation of ligation by PCNA.
To verify the AdD-PCNA2 interaction surface and further explore its contribution to DNA ligation, we turned to disulfide crosslinking of residues at the AdD-PCNA2 interface. Cysteine point mutants were chosen in the Lig AdD and in PCNA2 based on our cryo-EM structure (Figure 3E) and sequence conservation (Figures S7B and S7D). PCNA heterotrimers incorporating either S42C or R43C in PCNA2 were purified and allowed to undergo disulfide crosslinking with Lig AdD Y337C or E338C mutants. Non-reducing SDS-PAGE gels confirmed the experimental observation of an interaction between PCNA2 S42 and Lig Y337 (Figure S7I). Lig E338C also underwent disulfide crosslinking, but to a lesser extent than Lig Y337C, especially in the presence of DNA. Furthermore, crosslinking efficiency at the AdD-PCNA2 interface depended in part on the strength of the DBD-PCNA3 interaction, as seen for Lig Y337C and DBD PIP motif double mutants (Figure S7J). The most prominent crosslinked complex of Lig Y337C and PCNA2 S42C was purified over gel filtration to remove non-crosslinked proteins (Figures 7D, S6D and S6E) and then assayed for DNA end-joining activity under reducing conditions that reverse the crosslink or non-reducing conditions that maintain the crosslink (Figures 7D and 7E). Lig Y337C crosslinked to PCNA2 S42C was impaired in DNA ligation relative to the same complex analyzed under reducing conditions. The crosslinking results suggest that the AdD and PCNA2 indeed form an interaction surface, as observed in the structures, but that a rigidly fixed interface is detrimental to ligation activity, perhaps by limiting the Lig conformational dynamics required for efficient ligation. The AdD-PCNA2 interaction could play a role in DNA repair dynamics, potentially in coordination with the other DNA repair enzymes.
Discussion
Here we present biochemical and structural insights into the final step of Okazaki fragment processing. Whereas the thermophilic proteins from S. solfataricus are more rigid at ambient temperatures than their human counterparts, they were nonetheless challenging for structural investigations. Initial attempts to co-purify the Lig-PCNA-DNA complex by gel filtration resulted in a second PCNA heterotrimer replacing Lig on nicked DNA. While we did not detect Lig and DNA co-migration by gel filtration, PCNA and DNA co-migrated readily as double trimers (Figures 1F, 2H and S1E). Interestingly, Naryzhny and co-authors observed double trimers of hPCNA mediated by residues on the back side of the ring (Naryzhny et al., 2005). Furthermore, we observed multiple conformations of Lig and especially its OBD, which did not attain the same resolution as the full complex (Figure 5A). However, the structures revealed the details of the contacts between the proteins and with DNA and led us to identify the extensive DBD-PCNA3 interface that had remained the missing factor in PCNA stimulation of DNA ligation.
The Lig DBD and PIP Motif Bind PCNA3
The PIP motif of S. solfataricus Lig is unusual in its location within a domain and not at the extreme amino or carboxy terminus, as is the case in most PCNA-interacting proteins (Warbrick, 2000). This is also seen for P. furiosus, another thermophilic archaeon (Nishida et al., 2005). However, unlike P. furiosus ligase, S. solfataricus Lig exhibits a typical intrinsically disordered PIP motif loop (Prestel et al., 2019) that is apparent in its crystal structure (Pascal et al., 2006). Here we describe the first visualizations of the S. solfataricus Lig PIP motif bound to PCNA.
Building on the initial biophysical analyses (Pascal et al., 2006), we show that simultaneous Alanine substitutions of Lig PIP motif residues Q103 and I107 only results in mild disruption of co-migration with and stimulation by PCNA (Figures 1G, 1H, S1D and S1E) and their addition only marginally exacerbates the defect in the more severely disrupted F110A-L111A double mutant. This result is explained by our cryo-EM structures (Figure 3C), where we observe that the PIP motif loop does not form a long, sprawling contact typical of other PIP motif-PCNA structures (Prestel et al., 2019). Although conserved in canonical PIP motifs, Lig Q103 does not engage PCNA3. Indeed, this Glutamine is not conserved in crenarchaeal ligases (Imamura et al., 2007; Sun et al., 2008), of which the genus Saccharolobus is a member. The Lig loop is just long enough to insert a 310-helix comprising residues I107, F110 and L111 into the hydrophobic pocket in PCNA3. This contact has the highest affinity for PCNA3 with, nonetheless, weak affinity for the PCNA1–2 heterodimer (Figure 1D). The slight cross-talk between the Lig PIP motif and the PCNA1–2 heterodimer is potentially the complement to the finding that PCNA3 weakly binds to the PCNA2-interacting enzyme Pol B (Dionne et al., 2003).
Once tethered by the PIP motif loop, the Lig DBD creates an extensive interface with PCNA3 (Figure 3C). This DBD-PCNA3 interaction alone results in stimulation of a PIP-containing Lig (Figure 1E), a result that contradicts the report of Dionne et al. (Dionne et al., 2003) where the full PCNA heterotrimer was necessary to stimulate DNA ligation. We expect that differences in the type of DNA used and experimental parameters may account for the discrepancy. PCNA3 most likely acts as a bridge for Lig to bind nicked DNA (Tom et al., 2001), presumably by positioning its DBD along the DNA. Indeed, an additional amino acid substitution in the center of this interface was necessary to completely ablate stimulation by PCNA (Figures 7A and 7B). Thus, the two PCNA binding sites within the DBD act as a robust mechanism to stimulate ligation by recruiting Lig and securing its position on DNA.
The Lig AdD Binds PCNA2
DNA polymerases from yeast, humans and archaea interact at two sites with PCNA (Lancey et al., 2020; Madru et al., 2020; Zheng et al., 2020), albeit on the same PCNA monomer. It is likely that ligases in general interact with multiple subunits of PCNA, as seen with NS-EM structures of archaeal P. furiosus DNA ligase (Mayanagi et al., 2009) and hLigI (Matsumoto et al., 2020) in complex with PCNA. This is the first report of a DNA ligase contacting two subunits of a heterotrimeric PCNA, and the asymmetric nature of this PCNA allowed us to dissect the contributions from each of the subunits.
We initially suspected that the AdD-PCNA2 interaction might contribute to stimulation of ligation by PCNA. However, triple-Glycine substitutions on both sides of the contact did not disrupt stimulation by PCNA. Crosslinking residues at this second site of interaction verified the proximity of these surfaces, but the crosslinked proteins impeded the ligation reaction. As seen in our cryo-EM structures, Lig undergoes conformational changes during ligation. We expect that crosslinking of the AdD-PCNA2 interface has restricted Lig dynamics. We note that this contact is more present in the cryo-EM map of Lig and PCNA with non-ligatable DNA (Figure 3A), and indeed, this secondary interaction between the proteins is diminished in our cryo-EM map of Lig undergoing step 2 of ligation (Figure 4A).
The second point of contact is mediated by the Lig AdD residue Y337 and the PCNA2 central loop residue S42, leaving its IDCL mostly accessible (Figure 3E). It was recently demonstrated that the T4 DNA ligase contacts the homotrimeric sliding clamp gp45 through an extended loop in its AdD (Shi et al., 2018). A structure alignment shows that this AdD insertion coincides with a Lig AdD loop having at its extremity the residue E317 (Figures 3E and S7G). An acidic residue is conserved at this position in PCNA-interacting ligases (Figure S7D). However, a Lig E317C mutant did not form disulfide crosslinks with the central loop Cysteine mutant of PCNA2 (data not shown). In contrast, we confirmed that the less-conserved Y337 contacts PCNA2 by disulfide crosslinking (Figure S7I). It is interesting that other polar residues substitute for Tyrosine in P. furiosus ligase and hLigI (Figure S7D). A structure alignment shows that adjacent acidic residues may occupy this position (Figure S7C). Indeed, Lig E338C also crosslinked efficiently to S42C of PCNA2, but to a lesser extent than Y337C (Figure S7I). It is not yet known if a second point of contact exists and its location within other PCNA-interacting DNA ligases. However, hLigIII and hLigIV do not interact with PCNA (Martin & MacNeill, 2002) and lack structural (Figure S7E) and sequence (Figure S7F) conservation at both sites.
We also observed little disulfide crosslinking between PCNA2 residue R43 and either Y337 or E338 of Lig (Figure S7I). This position is well conserved as a basic residue throughout PCNAs (Figure S7B). hPCNA H44 forms a salt bridge to an acidic non-consensus residue within the human FEN1 PIP (Sakurai et al., 2005), and PCNA1 K44 forms a salt bridge with D343 of S. solfataricus FEN (Doré et al., 2006). Indeed, archaeal, human and yeast FENs contain an acidic residue at this position that may be a general mechanism of FEN recruitment.
PCNA2 residue S42 is conserved in structure (Figure S7A) and in sequence (Figure S7B) in most PCNAs, but not in A. fulgidus and S. solfataricus PCNA1 and PCNA3. DNA ligase and PCNA of A. fulgidus both contain Alanine instead of Tyrosine and Serine, respectively, possibly a case of co-evolution. S. solfataricus PCNA1 and PCNA3 may have lost the conserved Serine because they are not involved in a non-PIP contact with Lig, arguing that this interaction is widespread in nature.
Furthermore, it is intriguing that S42 lines the PIP-binding cleft of PCNA without directly binding the PIP (Prestel et al., 2019). To date there are no structural data on S. solfataricus Pol B in complex with PCNA2. Further studies are required to investigate if PCNA2 can simultaneously bind the PIP motif of Pol B and the AdD of Lig. One possibility is that during DNA synthesis Lig remains tethered by its DBD to PCNA3 without binding PCNA2 due to steric hindrance by Pol B. Once the complex reaches the previously completed Okazaki fragment, Pol B would swing out and allow Lig to engage PCNA2 with its AdD, thereby beginning its encirclement around DNA. It is also possible that Lig engages Pol B prior to binding PCNA2, as seen between hLigI and Pol δ during Okazaki fragment repair (Matsumoto et al., 2020).
The Lig OBD Completes DNA Encirclement
Our NS and cryo-EM structures show that the Lig OBD is positionally heterogenous (Figures 2–5). This domain carries out step 1 (self-adenylation) and step 3 (nick sealing) with opposite faces (Sriskanda & Shuman, 1998), and swings and swivels for step 2 (Kaminski et al., 2018). AMP transfer to the 5’PO4 of the downstream DNA strand (step 2) has been captured in human (Kaminski et al., 2018; Pascal et al., 2004) and bacteriophage (Shi et al., 2018) ligases. Thermophilic Archaeal ligases may not perform this AMP transfer in the context of a dideoxy-terminated DNA nick (Figure S1F). Indeed, the NS-EM structure of P. furiosus ligase in complex with PCNA and non-ligatable DNA shows an incomplete OBD partially encircling DNA (Mayanagi et al., 2009). Likewise, the OBD in our corresponding cryo-EM structure is too dynamic for reconstruction using this form of DNA (Figure 3A).
With ligatable DNA and the appropriate cations, the thermophilic Lig-PCNA complex ligates DNA rather slowly at room temperature (Figure S1F), allowing us to vitrify and visualize their dynamics in action. Approximately 60% of the particles show the OBD and parts of the AdD in motion (Figure S3B). The rest of the particles show ligated DNA corresponding with the Lig OBD completing DNA encirclement and contacting the Lig DBD (Figure 5A). The local resolution of the OBD limited modelling to rigid body placement of the crystallized OBD (Pascal et al., 2006) within the secondary structure features in the cryo-EM map. Despite these modeling limitations, it is likely that OBD residue K457 forms a salt-bridge with D90 of the DBD, as this ring-stabilizing interaction is conserved structurally (Jurkiw et al., 2021) and in sequence among DNA ligases. The Lig OBD occupies a position above PCNA1, although there are no direct contacts observed between the OBD and PCNA1. Thus, it appears that this OBD conformation would still allow PCNA1 to bind FEN simultaneously, as observed by NS-EM with P. furiosus PCNA, ligase and FEN (Mayanagi et al., 2018). However, the “OBD-front” position (Figure 5A) would clash with FEN as observed in its complex with PCNA (Doré et al., 2006); thus the OBD could potentially regulate access to PCNA1.
Along with the major contact between the Lig DBD and PCNA3 and the minor contact between the Lig AdD and PCNA2, the proteins form a stacked ring structure that was predicted (Pascal et al., 2004) and visualized by NS-EM (Matsumoto et al., 2020) in the human system. Lig differs from hLigI primarily by the N-terminal region of the latter. It is well-established that the hLigI PIP motif, found at its extreme N-terminus (Jónsson et al., 1998), is the initial contact with hPCNA, followed by its DBD preferentially at a non-IDCL position (Song et al., 2009). These consecutive binding events would template hLigI onto the toroidal hPCNA and hence nicked DNA. Based on our data, we propose that hLigI binds residue S43 of an adjacent hPCNA subunit through S649 or E650 of its AdD to continue encircling DNA.
Our structural (Figure 5A) and biochemical (Figure S6A) data of stacked rings on ligated DNA is further evidence that this complex persists at the completion of processing of an Okazaki fragment, as observed for the human system (Matsumoto et al., 2020). Instead of Lig simply releasing the duplex DNA and PCNA sliding off, the stacked rings on bent DNA may signal to nuclear factors, such as Replication Factor C or Replication Factor C-Like Complexes, to unload and recycle the proteins to further process DNA replication and repair (Kupiec, 2016). Indeed, the Saccharomyces cerevisiae PCNA unloader Elg1 is dependent on successful ligation by the PCNA-bound DNA ligase homolog Cdc9 during Okazaki fragment maturation (Kubota et al., 2015). Inability to unload PCNA leads to genomic instability through DNA damage checkpoint activation (Kubota et al., 2013; Sau et al., 2019). Our structural analysis provides a framework for understanding how Lig conformations and PCNA engagement can actively participate in the coordination of repair processing.
STAR Methods
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, John M. Pascal (john.pascal@umontreal.ca).
Materials Availability
All unique/stable reagents generated in this study are available from the lead contact with a completed materials transfer agreement.
Data and Code Availability
All cryo-EM data have been deposited at the electron microscopy databank (https://www.ebi.ac.uk/emdb) and protein databank (https://www.rcsb.org) and are publicly available as of the date of publication (see the key resources table). Accession numbers are EMD-24618, 7RPO (Lig-PCNA with 3’dideoxy DNA); EMD-24624, 7RPW (Lig-PCNA-AppDNA); EMD-24625, 7RPX (Lig-PCNA-end-joined DNA). This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental Model and Subject Details
Cells of Rosetta 2 (DE3) strain of Escherichia coli were transformed with expression plasmids.
Method Details
Protein Purification and Mutagenesis
S. solfataricus Lig and PCNA were expressed from pET vectors in Rosetta 2 (DE3) E. coli. Cells were grown at 37°C in Luria Broth media supplemented with 50 μg/mL kanamycin and 35 μg/mL chloramphenicol until OD600 reached 0.8. Expression was induced with 200 μM IPTG for 2 hours at 37°C (PCNA subunits) or 16 hours at 20°C (Lig). Cells were pelleted, resuspended in 20 mM HEPES pH 8.0, 60 mM NaCl, 0.5 mM TCEP and frozen at −20°C until use. Thawed cells were supplemented with protease inhibitors (leupeptin, pepstatin, antipain, aprotinin, benzamidine, phenylmethylsulfonyl fluoride), and 0.1% Nonidet P40 and lysed using an EmulsiFlex (Avestin). Lysates were clarified by centrifugation, then boiled at 85°C for 25 min and then centrifuged again to remove denatured E. coli proteins.
Cell lysates were loaded onto Ni(II)NTA affinity chromatography (GE Healthcare) equilibrated in 20 mM HEPES pH 8.0, 400 mM NaCl, 0.5 mM TCEP and eluted with 500 mM imidazole. Eluted proteins were then injected onto a Sephacryl S200 gel filtration column (GE Healthcare) equilibrated in 20 mM HEPES pH 8.0, 150 mM NaCl, 0.1 mM TCEP, and 1 mM EDTA (gel filtration buffer, GFB) and operated on an ÄKTA Pure (GE Healthcare) at 4°C. Fractions containing purified protein(s) of interest were pooled, concentrated with a 10 kDa MWCO Amicon Ultra spin concentrator (Millipore), aliquoted, flash frozen in liquid nitrogen, and stored at −80°C until use.
hPCNA was purified as above, with the following changes. Cells in media supplemented with 35 μg/mL chloramphenicol and 100 μg/mL ampicillin were induced for 16 hours at 16°C and lysate was not heated to 85°C following cell lysis. Proteins bound to Ni(II)NTA were washed with 1 M NaCl before elution in 500 mM imidazole and then loaded onto a HiTrap Q (GE Healthcare) column equilibrated in 50 mM Tris pH 7.0, 50 mM NaCl, 0.1 mM TCEP and eluted using increasing concentrations of NaCl. Fractions containing hPCNA were concentrated and injected onto a Sephacryl S200 gel filtration column, as described above.
Preparative gel filtration to isolate stoichiometric Lig-PCNA (alone or with DNA) complexes for EM used a 10/300 Superdex 200 Increase gel filtration column (GE Healthcare) at room temperature.
Site-directed mutagenesis was carried out using QuikChange (Agilent), except for Lig Y337C, which was created by subcloning a 905 nt insert containing a Lig WT PIP motif into a 6194 nt vector containing the Lig Y337C mutation. All mutants were verified by automated Sanger sequencing and were expressed and purified as above.
The disulfide-crosslinked complex of Lig Y337C and PCNA heterotrimer containing PCNA2 S42C was further purified over gel filtration after a 3 hour incubation of 10 μM complex at 37°C in 25 mM HEPES pH 7.5, 50 mM NaCl, and 4 mM MnCl2. A 10/300 Superdex 200 Increase gel filtration column (GE Healthcare) equilibrated in the above buffer was used to separate the disulfide-crosslinked complex from the non-crosslinked components. Fractions were boiled in non-reducing loading buffer and resolved on 11% SDS-PAGE.
Analytical Gel Filtration and SEC-MALS
Protein co-elution by analytical gel filtration alone or coupled to SEC-MALS was carried out using an ÄKTAmicro (GE Healthcare) FPLC at room temperature. Lig PIP mutants incubated with PCNA and DNA were passed over a 3.2/30 Superdex 200 Increase (GE Healthcare) gel filtration column equilibrated in 20 mM MES pH 6.5, 50 mM NaCl, 10 mM MnCl2 and 0.1 mM TCEP. The 10/300 Superdex 200 Increase gel filtration column was used to analyze complexes undergoing DNA ligation (buffer: 25 mM HEPES pH 7.5, 10 mM MnCl2, 50 mM NaCl, 1 mM ATP) and complexes of Lig DBD mutants with PCNA (buffer: 25 mM HEPES pH 7.5, 50 mM NaCl, 10 mM MnCl2, 0.1 mM TCEP).
The Lig-PCNA-DNA molecular mass was measured using the 10/300 Superdex 200 Increase gel filtration column coupled in-line to a UV detector, Dawn HELEOS II MALS flow cell (Wyatt Technology) and OptiLab T-rEX differential refractometer (Wyatt Technology). The buffer contained 20 mM MES pH 6.5, 50 mM NaCl, 10 mM MnCl2 and the detectors were equilibrated with BSA. Data were processed using ASTRA Version 6.1.6.5 (Wyatt Technology).
DNA Ligation Assay
Nicked linear DNAs were assembled by annealing a template strand to downstream (5’PO4) and upstream (3’OH or 3’dd) strands. All DNAs were purchased from Integrated DNA Technologies (IDT). 32 bp DNA (19 bp upstream: 5’-GCTTCTGTGCTGATGCGTC-3’, 13 bp downstream: 5’-GTCGGACTGAACC-3’, template strand with complementary sequence) was used to assay ligation stimulation by combinations of PCNA subunits, as well as the full PCNA heterotrimer stimulation of Lig PIP mutants. 47 bp DNA (24 bp upstream: 5’-GTATCCTCGTAGTGCAGATGCGTC-3’; 23 bp downstream: 5’-GTCGGACTGATTCGGTAGATCTG-3’, template with complementary sequence) was used in all other DNA ligation assays. The downstream strands contained a FAM moiety at their 3’ end. 15 nM Lig and PCNA heterotrimer (at the indicated concentrations ranging from 15 to 135 nM) were incubated with 10 nM DNA in 50 mM HEPES pH 7.5, 10 mM NaCl, 1 mM TCEP, 50 μg/ml BSA, 1 mM ATP and 4 mM MnCl2 at 50°C or at room temperature. Reactions were quenched with formamide and 25 mM EDTA, boiled and loaded onto a 15% acrylamide/7 M urea gel. Ligated DNA was quantified using ImageJ (Schneider et al., 2012).
Fluorescence Polarization Assay
DNA binding affinity measurements were performed using 5 nM of 32 bp FAM-labeled ligatable nicked DNA, and PIP motif binding affinity measurements were performed using 25 nM FAM-labeled PIP motif peptide (Bio Basic Inc.). We used GFB containing 75 mM NaCl for PCNA and DNA incubations, whereas regular GFB was used for proteins incubated with the PIP motif peptide, and all samples contained 75 μg/ml BSA. Increasing concentrations of proteins were incubated with the fluorescent probes for 30 min at room temperature prior to taking fluorescence polarization measurements on a VictorV plate reader (Perkin Elmer). A 1:1 binding model was fit to the data using Solver in Microsoft Excel.
Negative Stain Electron Microscopy
All samples used for NS-EM were in 20 mM MES pH 6.5, 50 mM NaCl, 10 mM MnCl2 buffer. PCNA was incubated with 32 bp ligatable nicked DNA at 10 μM prior to injection onto analytical gel filtration. A single fraction eluting at 12.2 mL was used to prepare a NS-EM grid without dilution. Lig was incubated with PCNA and 32 bp ligatable nicked DNA (10 μM) for 15 min at room temperature and then analyzed over gel filtration. A NS-EM grid was prepared using a fraction eluting at 12 mL without dilution. Lig was also mixed with PCNA and 47 bp ligatable nicked DNA at 10 μM for 15 min prior to dilution to 50 nM. A NS-EM grid was prepared immediately using the diluted sample.
We used carbon-coated copper grids (Electron Microscopy Sciences) that were negatively glow-discharged (Leica Microsystems) immediately before adsorption of samples for 1 min, followed by freshly-prepared 1.5% uranyl formate (Electron Microscopy Sciences) for 1 min. Samples were imaged at room temperature with a FEI Tecnai T12 transmission electron microscope located at the Electron Imaging Facility at the Université de Montréal. The microscope was operated at 120 keV with a LaB6 filament. Micrographs were recorded on an FEI Eagle 4k × 4k CCD camera with defocus values ranging from −0.5 to −1.5 μm using SerialEM (Mastronarde, 2005). The PCNA-32 bp DNA and Lig-PCNA-32 bp DNA samples were acquired at 110,000x (0.986 Å pixel size), whereas the Lig-PCNA-47 bp DNA sample was acquired at 67,000x magnification (1.65 Å pixel size).
Negative stain datasets were processed in Relion 2.1 (Scheres, 2012) without CTF correction. Particles were selected automatically (Kimanius et al., 2017), extracted with 2-fold downsampling, curated through successive 2D classifications (160 Å mask) and an initial model was generated with a stochastic gradient descent algorithm (Punjani et al., 2017). The initial model low-pass filtered to 60 Å and 3D classification (160 Å mask) was used for the Lig-PCNA-47 bp DNA dataset to separate particle subsets. Each subset was refined separately, and resolutions were calculated using the gold-standard Fourier shell correlation at the 0.5 criterion (Scheres & Chen, 2012).
The published crystal structure of PCNA (PDB 2HII) (Pascal et al., 2006) was docked into the lower density of the three NS-EM maps oriented with PCNA3 adjacent to the Lig DBD. The numbering of PCNA2 in the PDB was offset by one amino acid to match the current protein sequence found in the UniProt database (Q97Z84). The Lig OB-fold domain was separated from the DBD and AdD domains from PDB 2HIX and the DBD-AdD model was fit into the upper NS-EM density using the “Fit to Map” command in UCSF Chimera (Pettersen et al., 2004). The OB-fold domain was manually placed into each NS-EM map. Nicked DNA (20 bp) from the co-crystal with hLigI (PDB 1X9N) was extended linearly on both sides of the nick to 47 bp to match the experimental DNA used. This DNA was docked manually into the density observed above and below PCNA-Lig. Images were produced with UCSF Chimera (Pettersen et al., 2004).
Cryo-EM
Lig and PCNA were incubated at 486 μM and injected onto analytical gel filtration (20 mM HEPES pH 7.5, 50 mM NaCl, 10 mM MnCl2) to isolate a stoichiometric complex. For the dideoxy dataset, the proteins (9 μM) were mixed with 47 bp non-ligatable nicked DNA (11 μM) and allowed to incubate at room temperature for 30 min before vitrification. The second dataset consisted of stoichiometric Lig and PCNA (9 μM) incubated with 47 bp ligatable nicked DNA (11 μM) for approximately 1 hour at room temperature prior to vitrification. The 3’dideoxy and 3’hydroxy DNA-containing samples were diluted to 1 and 2 μM, respectively, in the above buffer supplemented with 0.01% octyl glucoside.
UltrAufoil R1.2/1.3 grids (Electron Microscopy Sciences) were negatively glow-discharged for 15 sec at 15 mA (PELCO easiGlow, Ted Pella Inc) and mounted into a Vitrobot Mark IV (Thermo Fisher Scientific) at 4°C and 100% humidity. The protein-DNA samples were pipetted onto the grids and blotted with the following parameters: wait time 0, blot force 1, blot time 1, drain time 0. Cryo-grids were plunged into liquid ethane and stored in liquid nitrogen.
Cryo-EM data were collected at the Facility for Electron Microscopy Research at McGill University on a Titan Krios (Thermo Fisher Scientific) equipped with a K3 direct electron detector (Gatan) operated at 300 keV. Movies were acquired in super-resolution mode (physical pixel size of 1.09 Å) using SerialEM (Mastronarde, 2005). A total dose of 100 electrons/Å2 was fractionated over 37 and 35 frames for the dideoxy and hydroxy DNA samples, respectively. Defocus ranged from −0.5 to −2.5 μm with 0.25 μm steps. A total of 3014 and 8060 movies were collected for the dideoxy and hydroxy DNA samples, respectively. See Table S1 for details of cryo-EM data collection.
The dideoxy DNA dataset was processed in Relion 3.1 (Zivanov et al., 2018). MotionCor2 as implemented in Relion was used to create micrographs with a 1.09 Å pixel size. CTF parameters were estimated using Gctf (Zhang, 2016) and 2,745 high-resolution micrographs were retained. Approximately 3 million particles were picked automatically using 2D references that were created from 348 manually picked particles. Particles were extracted in 256-pixel boxes (279 Å) and re-scaled to 64 pixels, resulting in 4.36 Å/pix. Two rounds of 2D classification conserved 993,300 particles of intact Lig-PCNA-DNA complexes. An initial model was created by stochastic gradient descent (Punjani et al., 2017) and used for 3D classification with 4 classes. One class showed the OB-fold domain (202,843 particles). Particles were re-extracted at 1.09 Å/pix in 256-pixel boxes and another round of 3D classification was carried out with 4 classes. Particles from 3 classes were pooled (155,733) and refined to 7.75 Å. This map was used as a reference for a two-class 3D classification of the original dataset of 3.1 million particles. The better class was kept and its 1.4 million particles were re-extracted in boxes of 164 pixels re-scaled to 82 pixels (2.18 Å/pix) and refined to 5.76 Å. 3D classification after CTF refinement and Bayesian polishing resulted in one good class containing 306,507 particles that was refined to 5.25 Å. Particles underwent another round of CTF refinement/Bayesian polishing, 3D classification (removal of 4 particles) and refinement to 4.7 Å. Following re-extraction at 1.5 Å/pix (186-pixel boxsize), the particles underwent two more rounds of CTF refinement, Baysesian polishing and 3D refinement, resulting in resolutions of 4.29 and 4.16 Å, respectively.
The movies of Lig and PCNA with ligatable nicked 47 bp DNA were processed in cryoSPARC version 3.1 (Punjani et al., 2017). Patch motion correction produced micrographs with two-fold downsampling (1.09 Å/pix) and the CTF was estimated using the patch CTF estimation within cryoSPARC. Particles were picked automatically using dimensions 100 × 120 Å and 2,773,829 particles were extracted in 184-pixel boxes (200 Å) downsampled to 66 pixels, resulting in a pixel size of 3 Å. Particles underwent 2D classification to remove bad particle picks, resulting in 742,043 particles that were re-extracted in 366-pixel boxes (400 Å) and downsampled to 266 pixels (1.5 Å/pix). Three initial ab-initio reconstructions were created, resulting in the rejection of 196,416 particles belonging to a class of broken particles. The particles belonging to the remaining two ab-initio reconstructions (545,627) were subjected to Non-uniform Refinement (Punjani et al., 2020) with tilt and trefoil CTF correction. Particles then underwent 3D Variability Analysis (Punjani & Fleet, 2021) to identify the principal motions. The particle subsets (“OBD-front” vs “OBD-out”) were separated with 3D Heterogenous Refinement using 3 classes. One class (192,490 particles) containing the most complete OB-fold domain encircling DNA was further refined using several rounds of Non-uniform Refinement with CTF parameter optimization (4.20 Å). The remaining two classes from Heterogenous Refinement were pooled (353,137 particles) and further underwent 3D heterogenous refinement using as initial references previous Heterogenous Refinement output maps. Particles belonging to two classes (283,122 particles) showing the OB-fold domain in the outward position were retained and underwent Non-uniform Refinement to obtain a 4 Å map. These particles were further subdivided using Heterogenous Refinement into a less-resolved 4.7 Å map (130,353 particles) and a better-resolved 4.38 Å map (152,769 particles) showing an adenylated downstream 5’PO4 strand. These “OBD-out” maps were refined with Non-uniform Refinement. Images containing cryo-EM maps were created with ChimeraX (Goddard et al., 2018).
Model building was carried out using reconstructions of Lig-PCNA with 3’dideoxy DNA and with duplex DNA sharpened using DeepEMhancer (Sanchez-Garcia et al., 2021), and Lig-PCNA-AppDNA sharpened within cryoSPARC (Punjani et al., 2017). The assembled Lig-PCNA-DNA model lacking the OB-fold domain from NS-EM was docked into the Lig-PCNA map containing dideoxy DNA and adjusted manually in Coot (Emsley et al., 2010). The Lig PIP loop was modelled after S. solfataricus FEN co-crystallized with PCNA1-PCNA2 (PDB 2IZO) (Doré et al., 2006), and the residues were modified to the Lig sequence. The model was subsequently real space refined in Phenix (Liebschner et al., 2019) with secondary structure and Ramachandran restraints. Standard restraints were also supplied for DNA base pairing and stacking, as well as bond lengths and angles for the Lig Y260-AMP linkage. Rotamers were optimized manually. The DNA extremities were trimmed to 20 bp of upstream and 11 bp of downstream and the model was refined once more.
The AppDNA-containing map from the 3’OH dataset underwent refinement in Phenix using the model of Lig (DBD-AdD), PCNA and nicked DNA, but with a covalent bond between the AMP phosphate and the 5’PO4 of the downstream DNA strand. The 3’dideoxy cytidine was changed to a 3’hydroxy cytidine. Standard restraints were provided for DNA base pairing and stacking, in addition to the phosphoanhydride bond length and angles. Real space refinement used secondary structure and Ramachandran restraints.
Starting with the model containing the 3’dideoxy DNA, the nicked DNA strand was manually modified to create a continuous backbone. The OB-fold domain (PDB 2HIX) was docked into the density using Chimera and combined with the rest of the Lig molecule. The model was adjusted manually in Coot and then underwent real space refinement in Phenix with secondary structure and Ramachandran restraints. Standard restraints were also supplied for DNA base pairing and stacking. Rotamers were optimized manually. See Table S1 for details of refinement statistics.
Quantification and Statistical Analysis
Data from fluorescence polarization binding experiments were modelled using the GRG Nonlinear method within the Solver add-in of Microsoft Excel. DNA band intensities in DNA end-joining assays were quantified with ImageJ (Schneider et al., 2012) and shown in Figures 1E, 1H, 7E, and S1F. Replicate DNA end-joining experiments were performed on different days and the means and standard deviations are shown in Figures 7B and 7C. The number of replicates is indicated in the figure legend.
Supplementary Material
Video S1. Movement of the Lig OBD corresponds with DNA flexing at the Lig active site, Related to Figures 4 and 5.
Key resources table.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| E. coli strain BL21(DE3) Rosetta 2 | Millipore | Cat#71400 |
| Chemicals, peptides, and recombinant proteins | ||
| MnCl2 | Bio Basic Inc. | Cat#MB0331 |
| Synthetic peptide | Bio Basic Inc. | N/A |
| Synthetic peptide with 6-FAM | Bio Basic Inc. | N/A |
| Uranyl formate | Electron Microscopy Sciences | Cat#22450 |
| Octyl glucoside | Anatrace | Cat#0311 |
| Deposited data | ||
| Lig-PCNA-3’dideoxy DNA map | This paper | EMDB: EMD-24618 |
| Lig-PCNA-3’dideoxy DNA model | This paper | PDB: 7RPO |
| Lig-PCNA-adenylated DNA map | This paper | EMDB: EMD-24624 |
| Lig-PCNA-adenylated DNA model | This paper | PDB: 7RPW |
| Lig-PCNA-end-joined DNA map | This paper | EMDB: EMD-24625 |
| Lig-PCNA-end-joined DNA model | This paper | PDB: 7RPX |
| PCNA heterotrimer for modelling | (Pascal et al., 2006) | PDB: 2HII |
| Lig for modelling | (Pascal et al., 2006) | PDB: 2HIX |
| Nicked DNA from complex with hLigI | (Pascal et al., 2004) | PDB: 1X9N |
| FEN PIP motif loop from complex with PCNA1-PCNA2 | (Doré et al., 2006) | PDB: 2IZO |
| Oligonucleotides | ||
| 32 nt template DNA | IDT | N/A |
| 19 nt upstream strand with 3’hydroxy terminus DNA | IDT | N/A |
| 19 nt upstream strand with 3’dideoxy terminus DNA | IDT | N/A |
| 13 nt downstream strand with 5’PO4 DNA | IDT | N/A |
| 13 nt downstream strand with 5’PO4 and 6-FAM DNA | IDT | N/A |
| 47 nt template DNA | IDT | N/A |
| 24 nt upstream strand with 3’hydroxy terminus DNA | IDT | N/A |
| 24 nt upstream strand with 3’dideoxy terminus DNA | IDT | N/A |
| 23 nt downstream strand with 5’PO4 DNA | IDT | N/A |
| 23 nt downstream strand with 5’PO4 and 6-FAM DNA | IDT | N/A |
| Recombinant DNA | ||
| Lig 110/111 FL-AA mutant | (Pascal et al., 2006) | N/A |
| Lig 103/107 QI-AA mutant | This paper | N/A |
| Lig 103/107/110/111 QIFL-AAAA mutant | This paper | N/A |
| Lig 102–112 QQSTGILGFLG-QSSG (ΔLoop) mutant | This paper | N/A |
| Lig 337 Y-C mutant | This paper | N/A |
| Lig 338 E-C mutant | This paper | N/A |
| Lig 145 R-L mutant | This paper | N/A |
| Lig 145 R-D mutant | This paper | N/A |
| Lig ΔLoop + 145 R-L mutant | This paper | N/A |
| Lig ΔLoop + 145 R-D mutant | This paper | N/A |
| Lig 336–338 IYE-GGG mutant | This paper | N/A |
| PCNA2 43 R-C mutant | This paper | N/A |
| PCNA2 42 S-C mutant | This paper | N/A |
| PCNA2 41–43 PSR-GGG mutant | This paper | N/A |
| Software and algorithms | ||
| ASTRA | Wyatt Technology | https://www.wyatt.com/ |
| ImageJ | (Schneider et al., 2012) | https://imagej.nih.gov/ij/ |
| SerialEM | (Mastronarde, 2005) | https://bio3d.colorado.edu/SerialEM/ |
| Relion 2.1 | (Scheres, 2012) | https://www3.mrc-lmb.cam.ac.uk/relion//index.php?title=Main_Page |
| Relion 3.1 | (Zivanov et al., 2018) | https://www3.mrc-lmb.cam.ac.uk/relion//index.php?title=Main_Page |
| cryoSPARC | (Punjani et al., 2017) | www.cryosparc.com |
| Gctf | (Zhang, 2016) | www2.mrc-lmb.cam.ac.uk/research/locally-developed-software/zhang-software/ |
| DeepEMhancer | (Sanchez-Garcia et al., 2021) | https://github.com/rsanchezgarc/deepEMhancer |
| Phenix | (Liebschner et al., 2019) | http://www.phenix-online.org/ |
| Coot | (Emsley et al., 2010) | www.ccp4.ac.uk |
| UCSF Chimera | (Pettersen et al., 2004) | https://www.cgl.ucsf.edu/chimera/ |
| UCSF ChimeraX | (Goddard et al., 2018) | https://www.rbvi.ucsf.edu/chimerax/ |
Highlights.
Cryo-EM structures of archaeal DNA ligase with heterotrimeric PCNA and nicked DNA
DNA ligase contacts two subunits of the PCNA sliding clamp ring
PCNA and DNA ligase form a continuous DNA binding surface to support an arched DNA shape
Cryo-EM structures and biochemistry provide insight into PCNA stimulation of ligation
Acknowledgements
We thank Kaustuv Basu and Mike Strauss at the Facility for Electron Microscopy Research of McGill University for help in microscope operation and cryo-EM data collection. The NS-EM work was prepared and collected at the Electron Imaging Facility, Faculty of Dental Medicine, Université de Montréal. This work was supported by a Discovery grant from the National Science and Engineering Research Council of Canada (RGPIN-2015-05776 to J.M.P) and National Cancer Institute grant Structural Biology of DNA Repair (SBDR, CA92584).
Footnotes
Declaration of Interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beattie TR, & Bell SD (2012). Coordination of multiple enzyme activities by a single PCNA in archaeal Okazaki fragment maturation. The EMBO Journal, 31(6), 1556–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapados BR, Hosfield DJ, Han S, Qiu J, Yelent B, Shen B, & Tainer JA (2004). Structural Basis for FEN-1 Substrate Specificity and PCNA-Mediated Activation in DNA Replication and Repair. Cell, 116(1), 39–50. [DOI] [PubMed] [Google Scholar]
- Cotner-Gohara E, Kim I-K, Hammel M, Tainer JA, Tomkinson AE, & Ellenberger T (2010). Human DNA Ligase III Recognizes DNA Ends by Dynamic Switching between Two DNA-Bound States. Biochemistry, 49(29). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne I, Nookala RK, Jackson SP, Doherty AJ, & Bell SD (2003). A Heterotrimeric PCNA in the Hyperthermophilic Archaeon Sulfolobus solfataricus. Molecular Cell, 11(1), 275–282. [DOI] [PubMed] [Google Scholar]
- Doherty AJ, & Wigley DB (1999). Functional domains of an ATP-dependent DNA ligase 1 1 Edited by A. R. Fersht. Journal of Molecular Biology, 285(1), 63–71. [DOI] [PubMed] [Google Scholar]
- Doré AS, Kilkenny ML, Jones SA, Oliver AW, Roe SM, Bell SD, & Pearl LH (2006). Structure of an archaeal PCNA1-PCNA2-FEN1 complex: elucidating PCNA subunit and client enzyme specificity. Nucleic Acids Research, 34(16), 4515–4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, & Cowtan K (2010). Features and development of Coot. Acta Crystallographica Section D: Biological Crystallography, 66(4), 486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard TD, Huang CC, Meng EC, Pettersen EF, Couch GS, Morris JH, & Ferrin TE (2018). UCSF ChimeraX: Meeting modern challenges in visualization and analysis. Protein Science, 27(1), 14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura K, Fukunaga K, Kawarabayasi Y, & Ishino Y (2007). Specific interactions of three proliferating cell nuclear antigens with replication‐related proteins in Aeropyrum pernix. Molecular Microbiology, 64(2), 308–318. [DOI] [PubMed] [Google Scholar]
- Jónsson Z, Hindges R, & Journal H-U (1998). Regulation of DNA replication and repair proteins through interaction with the front side of proliferating cell nuclear antigen. The EMBO Journal, 17(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkiw TJ, Tumbale PP, Schellenberg MJ, Cunningham-Rundles C, Williams RS, & O’Brien PJ (2021). LIG1 syndrome mutations remodel a cooperative network of ligand binding interactions to compromise ligation efficiency. Nucleic Acids Research, 49(3), gkaa1297-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski AM, Tumbale PP, Schellenberg MJ, Williams RS, Williams JG, Kunkel TA, Pedersen LC, & Bebenek K (2018). Structures of DNA-bound human ligase IV catalytic core reveal insights into substrate binding and catalysis. Nature Communications, 9(1), 2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DJ, Kim O, Kim H-W, Kim HS, Lee SJ, & Suh SW (2009). ATP-dependent DNA ligase from Archaeoglobus fulgidus displays a tightly closed conformation. Acta Crystallographica Section F: Structural Biology and Crystallization Communications, 65(6), 544–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimanius D, Forsberg B, & Lindahl E (2017). Accelerated Cryo-EM Structure Determination with Parallelisation using GPUs in Relion-2. Biophysical Journal, 112(3), 575a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyonari S, Takayama K, Nishida H, & Ishino Y (2006). Identification of a Novel Binding Motif in Pyrococcus furiosus DNA Ligase for the Functional Interaction with Proliferating Cell Nuclear Antigen*. Journal of Biological Chemistry, 281(38), 28023–28032. [DOI] [PubMed] [Google Scholar]
- Kubota T, Katou Y, Nakato R, Shirahige K, & Donaldson AD (2015). Replication-Coupled PCNA Unloading by the Elg1 Complex Occurs Genome-wide and Requires Okazaki Fragment Ligation. Cell Reports, 12(5), 774–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota T, Nishimura K, Kanemaki MT, & Donaldson AD (2013). The Elg1 Replication Factor C-like Complex Functions in PCNA Unloading during DNA Replication. Molecular Cell, 50(2), 273–280. [DOI] [PubMed] [Google Scholar]
- Kupiec M (2016). Alternative clamp loaders/unloaders. FEMS Yeast Research, 16(7), fow084. [DOI] [PubMed] [Google Scholar]
- Lancey C, Tehseen M, Raducanu V-S, Rashid F, Merino N, Ragan TJ, Savva CG, Zaher MS, Shirbini A, Blanco FJ, Hamdan SM, & Biasio AD (2020). Structure of the processive human Pol δ holoenzyme. Nature Communications, 11(1), 1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin DS, Bai W, Yao N, O’Donnell M, & Tomkinson AE (1997). An interaction between DNA ligase I and proliferating cell nuclear antigen: implications for Okazaki fragment synthesis and joining. Proceedings of the National Academy of Sciences of the United States of America, 94(24), 12863–12868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebschner D, Afonine PV, Baker ML, Bunkóczi G, Chen VB, Croll TI, Hintze B, Hung L-W, Jain S, McCoy AJ, Moriarty NW, Oeffner RD, Poon BK, Prisant MG, Read RJ, Richardson JS, Richardson DC, Sammito MD, Sobolev OV, … Adams PD (2019). Macromolecular structure determination using X‐rays, neutrons and electrons: recent developments in Phenix. Acta Crystallographica Section D, 75(10), 861–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X-J, & Olson WK (2008). 3DNA: a versatile, integrated software system for the analysis, rebuilding and visualization of three-dimensional nucleic-acid structures. Nature Protocols, 3(7), 1213–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madru C, Henneke G, Raia P, Hugonneau-Beaufet I, Pehau-Arnaudet G, England P, Lindahl E, Delarue M, Carroni M, & Sauguet L (2020). Structural basis for the increased processivity of D-family DNA polymerases in complex with PCNA. Nature Communications, 11(1), 1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin IV, & MacNeill SA (2002). ATP-dependent DNA ligases. Genome Biology, 3(4), reviews3005.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronarde DN (2005). Automated electron microscope tomography using robust prediction of specimen movements. Journal of Structural Biology, 152(1), 36–51. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Brooks RC, Sverzhinsky A, Pascal JM, & Tomkinson AE (2020). Dynamic DNA-bound PCNA complexes co-ordinate Okazaki fragment synthesis, processing and ligation. Journal of Molecular Biology, 432(24), 166698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayanagi K, Ishino S, Shirai T, Oyama T, Kiyonari S, Kohda D, Morikawa K, & Ishino Y (2018). Direct visualization of DNA baton pass between replication factors bound to PCNA. Scientific Reports, 8(1), 16209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayanagi K, Kiyonari S, Saito M, Shirai T, Ishino Y, & Morikawa K (2009). Mechanism of replication machinery assembly as revealed by the DNA ligase–PCNA–DNA complex architecture. Proceedings of the National Academy of Sciences, 106(12), 4647–4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naryzhny SN, Zhao H, & Lee H (2005). Proliferating Cell Nuclear Antigen (PCNA) May Function as a Double Homotrimer Complex in the Mammalian Cell*. Journal of Biological Chemistry, 280(14), 13888–13894. [DOI] [PubMed] [Google Scholar]
- Nishida H, Kiyonari S, Ishino Y, & Morikawa K (2006). The Closed Structure of an Archaeal DNA Ligase from Pyrococcus furiosus. Journal of Molecular Biology, 360(5), 956–967. [DOI] [PubMed] [Google Scholar]
- Nishida H, Tsuchiya D, Ishino Y, & Morikawa K (2005). Overexpression, purification and crystallization of an archaeal DNA ligase from Pyrococcus furiosus. Acta Crystallographica Section F: Structural Biology and Crystallization Communications, 61(12), 1100–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascal JM, O’Brien PJ, Tomkinson AE, & Ellenberger T (2004). Human DNA ligase I completely encircles and partially unwinds nicked DNA. Nature, 432(7016), 473–478. [DOI] [PubMed] [Google Scholar]
- Pascal JM, Tsodikov OV, Hura GL, Song W, Cotner EA, Classen S, Tomkinson AE, Tainer JA, & Ellenberger T (2006). A flexible interface between DNA ligase and PCNA supports conformational switching and efficient ligation of DNA. Molecular Cell, 24(2), 279–291. [DOI] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, & Ferrin TE (2004). UCSF Chimera - a visualization system for exploratory research and analysis. Journal of Computational Chemistry, 25(13), 1605–1612. [DOI] [PubMed] [Google Scholar]
- Prestel A, Wichmann N, Martins JM, Marabini R, Kassem N, Broendum SS, Otterlei M, Nielsen O, Willemoës M, Ploug M, Boomsma W, & Kragelund BB (2019). The PCNA interaction motifs revisited: thinking outside the PIP-box. Cellular and Molecular Life Sciences, 76(24), 4923–4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punjani A, & Fleet DJ (2021). 3D variability analysis: Resolving continuous flexibility and discrete heterogeneity from single particle cryo-EM. Journal of Structural Biology, 213(2), 107702. [DOI] [PubMed] [Google Scholar]
- Punjani A, Rubinstein JL, Fleet DJ, & Brubaker MA (2017). cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nature Methods, 14(3), 290–296. [DOI] [PubMed] [Google Scholar]
- Punjani A, Zhang H, & Fleet DJ (2020). Non-uniform refinement: adaptive regularization improves single-particle cryo-EM reconstruction. Nature Methods, 17(12), 1214–1221. [DOI] [PubMed] [Google Scholar]
- Sakurai S, Kitano K, Yamaguchi H, Hamada K, Okada K, Fukuda K, Uchida M, Ohtsuka E, Morioka H, & Hakoshima T (2005). Structural basis for recruitment of human flap endonuclease 1 to PCNA. The EMBO Journal, 24(4), 683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Garcia R, Gomez-Blanco J, Cuervo A, Carazo JM, Sorzano COS, & Vargas J (2021). DeepEMhancer: a deep learning solution for cryo-EM volume post-processing. Communications Biology, 4(1), 874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sau S, Liefshitz B, & Kupiec M (2019). The Yeast PCNA Unloader Elg1 RFC-Like Complex Plays a Role in Eliciting the DNA Damage Checkpoint. MBio, 10(3), e01159–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres SHW (2012). RELION: Implementation of a Bayesian approach to cryo-EM structure determination. Journal of Structural Biology, 180(3), 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres SHW, & Chen S (2012). Prevention of overfitting in cryo-EM structure determination. Nature Methods, 9(9), 853–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, & Eliceiri KW (2012). NIH Image to ImageJ: 25 years of image analysis. Nature Methods, 9(7), 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi K, Bohl TE, Park J, Zasada A, Malik S, Banerjee S, Tran V, Li N, Yin Z, Kurniawan F, Orellana K, & Aihara H (2018). T4 DNA ligase structure reveals a prototypical ATP-dependent ligase with a unique mode of sliding clamp interaction. Nucleic Acids Research, 46(19), 10474–10488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Pascal JM, Ellenberger T, & Tomkinson AE (2009). The DNA binding domain of human DNA ligase I interacts with both nicked DNA and the DNA sliding clamps, PCNA and hRad9-hRad1-hHus1. DNA Repair, 8(8), 912–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriskanda V, & Shuman S (1998). Mutational analysis of Chlorella virus DNA ligase: catalytic roles of domain I and motif VI. Nucleic Acids Research, 26(20), 4618–4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Seo MS, Kim JH, Kim YJ, Kim GA, Lee JI, Lee J, & Kwon S (2008). Novel DNA ligase with broad nucleotide cofactor specificity from the hyperthermophilic crenarchaeon Sulfophobococcus zilligii: influence of ancestral DNA ligase on cofactor utilization. Environmental Microbiology, 10(12), 3212–3224. [DOI] [PubMed] [Google Scholar]
- Tom S, Henricksen LA, Park MS, & Bambara RA (2001). DNA Ligase I and Proliferating Cell Nuclear Antigen Form a Functional Complex. Journal of Biological Chemistry, 276(27), 24817–24825. [DOI] [PubMed] [Google Scholar]
- Tomkinson AE, Vijayakumar S, Pascal JM, & Ellenberger T (2006). DNA ligases: structure, reaction mechanism, and function. Chemical Reviews, 106(2). [DOI] [PubMed] [Google Scholar]
- Tumbale PP, Jurkiw TJ, Schellenberg MJ, Riccio AA, O’Brien PJ, & Williams RS (2019). Two-tiered enforcement of high-fidelity DNA ligation. Nature Communications, 10(1), 5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warbrick E (2000). The puzzle of PCNA’s many partners. BioEssays, 22(11), 997–1006. [DOI] [PubMed] [Google Scholar]
- Zhang K (2016). Gctf: Real-time CTF determination and correction. Journal of Structural Biology, 193(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, Georgescu RE, Li H, & O’Donnell ME (2020). Structure of eukaryotic DNA polymerase δ bound to the PCNA clamp while encircling DNA. Proceedings of the National Academy of Sciences, 117(48), 30344–30353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivanov J, Nakane T, Forsberg BO, Kimanius D, Hagen WJ, Lindahl E, & Scheres SH (2018). New tools for automated high-resolution cryo-EM structure determination in RELION-3. ELife, 7, e42166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Movement of the Lig OBD corresponds with DNA flexing at the Lig active site, Related to Figures 4 and 5.
Data Availability Statement
All cryo-EM data have been deposited at the electron microscopy databank (https://www.ebi.ac.uk/emdb) and protein databank (https://www.rcsb.org) and are publicly available as of the date of publication (see the key resources table). Accession numbers are EMD-24618, 7RPO (Lig-PCNA with 3’dideoxy DNA); EMD-24624, 7RPW (Lig-PCNA-AppDNA); EMD-24625, 7RPX (Lig-PCNA-end-joined DNA). This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.


