SUMMARY
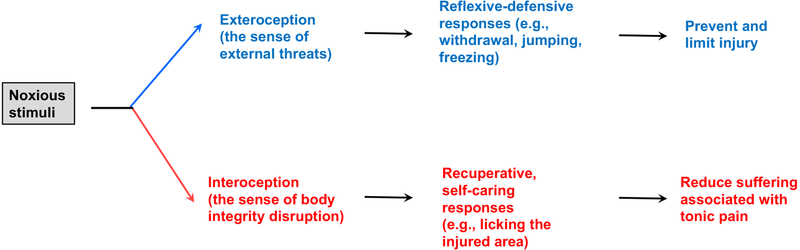
Somatosensory afferents are traditionally classified by soma size, myelination and their response specificity to external and internal stimuli. Here we propose the functional subdivision of the nociceptive somatosensory system into two branches. The exteroceptive branch detects external threats and drives reflexive-defensive reactions to prevent or limit injury. The interoceptive branch senses the disruption of body integrity, produces tonic pain with strong aversive emotional components, and drives self-caring responses towards to the injured region to reduce suffering. The central thesis behind this functional subdivision comes from a reflection on the dilemma faced by the pain research field, namely the use of reflexive-defensive behaviors as surrogate assays for interoceptive tonic pain. The interpretation of these assays is now being challenged by the discovery of distinct but interwoven circuits that drive exteroceptive versus interoceptive types of behaviors, with the conflation of these two components contributing partially to the poor translation of therapies from preclinical studies.
In Brief
Ma proposes a functional subdivision of the somatosensory system into two branches that respond to external threats and internal body injuries, respectively. The conflation of these behavioral responses may contribute to the poor translation of therapies from preclinical pain studies.
INTRODUCTION
The International Association for the Study of Pain (IASP) recently re-defined pain as “an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage” (Raja et al., 2020). Meanwhile, in early 2019 the National Institute of Health (NIH) sponsored a pain workshop discussing animal models and behavioral assays, which was motivated by the deadly opioid epidemics across the United States and the poor track record of translating preclinical “successes” to new pain medicines. Among the challenges discussed were how well animals model clinically relevant pain in humans, and how reliably behavioral assays reflect the sensory and emotional experience of pain, although poor translation could be caused by many other factors, ranging from data reproducibility due to poor practice of statistical analyses to the progressive increase of placebo effects seen in more recent clinical trials that could mask real drug effects (Borsook et al., 2014; Mogil, 2018; Tuttle et al., 2015). In this review, I try to argue that the somatosensory system can be functionally divided into two branches that drive exteroceptive and interoceptive types of behaviors, respectively (Figure 1), whose conflation in preclinical studies may have contributed to the poor translation of therapies. Exteroception is referred here to as the sensing (consciously or subconsciously) of external threats that drives behaviors aimed to prevent or limit body injury. If these first-line defensive behaviors fail to prevent the occurrence of body injury, the mental state will now be switched from exteroception to interoception-a sense of body integrity disruption, whose associated tonic pain perception will drive a new set of self-caring behaviors towards to the injured region that aim to soothe suffering (see below for detailed discussion on behaviors). Note that use of interoception here is not narrowly defined as visceral sensation per se, but rather encompasses the sensing of any disruption to body integrity, including injuries to the skin, echoing a recent discussion (Chen et al., 2021). For this review, I will first summarize evidence for the presence of distinct circuits driving these two types of behaviors under acute conditions and then discuss how these two branches interact dynamically under physiological and pathological conditions, particularly on concurrent driving of chronic pain and the associated comorbidities. Lastly, the implication of this functional subdivision on pain research and treatment will be discussed.
Figure 1.
Noxious stimuli evoke exteroceptive and interoceptive perceptions and associated behaviors.
1. Insights from human subjects with brain lesions
Many of the insights in this review have come from studies on how brain lesions and/or neuronal ablations impact on somatosensory perception in humans and behaviors in animals. I particularly pay attention to those lesions with differential impacts on two distinct sets of behaviors, as a way to understand the organizational rule of the nervous system. Take an example from the fear research field. The famous S.M. patient with a lesion covering the amygdala exhibited a loss of fear evoked by the presence of normally fearful subjects such as spiders and snakes, but retained fear evoked by inhaling air with elevated carbon dioxide; such studies offer a great insight: there are distinct neural substrates processing exteroceptive versus interoceptive forms of fear (Adolphs et al., 2005; Adolphs et al., 1994; Barrett, 2018; Feinstein et al., 2013).
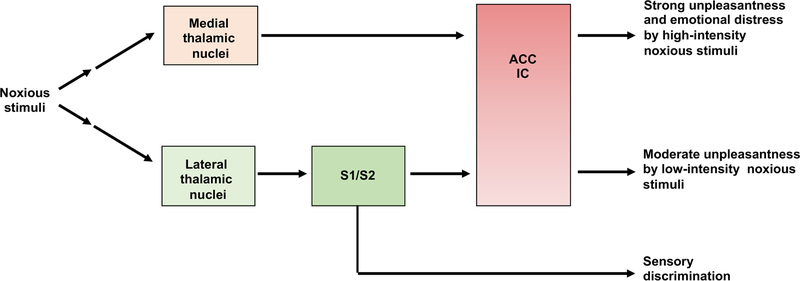
Analogous human brain lesion studies have provided crucial insights into the functional subdivision of the somatosensory system. In 1911, Head and Holmes revealed a lateral versus medial subdivision at the thalamic level, based on analyses of “thalamic syndrome patients” with lesions in the lateral thalamus (Head and Holmes, 1911). The lateral thalamus encompasses multiple nuclei, including the ventral posterior lateral, medial and inferior nuclei that relay somatosensory information to the somatosensory cortex (S1/S2) (Bushnell et al., 2013; Fields, 1999; Kuner and Kuner, 2021; Price, 2002) (Figure 2). Patients with verified lateral thalamic lesions (Head and Holmes, 1911), as well as a more recently reported case with a lesion in a postcentral gyrus region that includes the somatosensory cortex (Ploner et al., 1999), showed a loss of capacity to perceive i) the type, intensity and location of external stimuli applied to their bodies, ii) the sensory quality of pain (such as pricking, sharp, burning perceptions), and iii) the moderate unpleasantness evoked by short-lasting, low-intensity noxious stimuli. However, upon applying intense or prolonged noxious stimuli that should cause tissue damage, the patients felt excessive unpleasantness and emotional distress, despite their inability to perceive the nature or location of the stimuli (Head and Holmes, 1911). Conversely, patients with lesions in medial thalamic regions produced opposite phenotypes, with a selective failure to experience the strong unpleasantness and emotional distress evoked by intense noxious stimuli (Mark et al., 1960; Mark et al., 1963; Young et al., 1995b). These observations suggest a functional subdivision of the lateral versus medial thalamic systems in driving distinct components of noxious sensory perception (Figure 2).
Figure 2. Human studies reveal the segregation and convergence of the lateral versus medial thalamic pathways.
S1/S2: somatosensory cortex 1 and 2. ACC: anterior cingulate cortex. IC: insular cortex.
The major targets of the medial thalamic region include the anterior cingulate cortex (ACC) and the insular cortex (IC) (Devinsky et al., 1995; Jones and Leavitt, 1974; Krettek and Price, 1977; Liang et al., 2013; Price, 2002; Vogt and Pandya, 1987; Vogt et al., 1987; Wang and Shyu, 2004). The IC and ACC also receive direct and indirect inputs from the lateral thalamic pathways, via the somatosensory cortex, indicating a certain degree of convergence by the medial and lateral pathways at the IC/ACC levels (Price, 2002) (Figure 2). IC is crucial for conscious feeling of body states (Craig, 2009; Livneh and Andermann, 2021), and ACC is pivotal to the processing of the affective-emotional-motivational-autonomic contents of external and internal stimuli (Devinsky et al., 1995; Fuchs et al., 2014; Phelps et al., 2021; Price, 2002; Xiao and Zhang, 2018). Human patients with frontal lobotomies or lesions covering IC retained sensory discrimination but experienced a form of pseudo “pain” percept without any threat value or distress attached, referred to pain asymbolia (Berthier et al., 1988). Patients with lesions covering ACC also retained sensory discrimination and can recognize external threats if they were brought to patients’ attention, but they did not display emotional distress or motivation to escape from such threats, a deficit referred to as pain indifference (Ballantine et al., 1967; Foltz and White, 1962; Freeman and Watts, 1948). A role of the ACC in driving the affective component of pain is also supported by functional imaging studies in humans (Rainville et al., 1997), as well as in animal studies (see below).
The segregation of the lateral versus medial thalamic pathways and their partial convergence at IC/ACC allow Melzack, Price and others to propose a parallel but serially connected model in processing different types of noxious sensory information (Bushnell et al., 2013; Melzack and Casey, 1968; Price, 2002; Xiao and Zhang, 2018). These two pathways apparently play distinct roles in exteroception versus interoception, at least under acute conditions. The lateral-somatosensory cortical pathways appears to dominate with exteroception, including sensory perception and discriminating the types, intensities and locations of external stimuli. The medial pathway contains a strong interoceptive component, in terms of emotional responses to the disruption of body integrity, although it may contain an exteroceptive component as well. Fields argued that initial unpleasantness evoked by intense noxious stimuli, which is dependent on the medial pathways, does reflect an appreciation of threat levels (Fields, 1999); as such, activation of the medial thalamic pathways can still elicit strong motivation to escape from noxious stimuli for patients with lateral thalamic lesions (Head and Holmes, 1911). We will come back to discuss how the medial pathways may drive purer interoceptive responses to persistent, inescapable body injury. As discussed below, the insights gained from these human brain lesion studies will help to understand the meaning of distinct sets of animal behaviors, after assessing how those behaviors are impacted by analogous brain lesions.
2. Exteroceptive and interoceptive responses to external threats and body injury in animals
Unlike human subjects that can report sensory and emotional experience of pain, animal studies rely on behavioral observations and interpretations. Here I will discuss a set of behaviors that may have differential implications on exteroception versus interoception under acute conditions.
Threshold-level reflexive responses to external threats
For decades, acute thermal and mechanical pain types are commonly measured via withdrawal thresholds or latencies in response to minimally aversive mechanical force, heat or cold (Brenner et al., 2012; Deuis et al., 2017; Hargreaves et al., 1988; Le Bars et al., 2001; Mogil, 2009, 2018). However, these absolute threshold responses are preserved following decerebration and cervical spinal transection, as well as in animals with lesions in brain regions crucial for processing the affective and emotional components of pain, such as ACC and the basolateral amygdala (Barik et al., 2018; Corder et al., 2019; Gao et al., 2004; Johansen et al., 2001; Matthies and Franklin, 1992; Woolf, 1984; Yeomans et al., 1996). In other words, basic reflexive responses to low-intensity noxious stimuli can be sufficiently mediated via local spinal circuits. Human studies also show that withdrawal reflexes can be evoked before stimulation reaches the pain threshold (Bromm and Treede, 1980), and Apkarian and his colleagues have been arguing that much of nociceptive information might be processed subconsciously, without evoking conscious pain percepts in humans (Baliki and Apkarian, 2015). Notably, recent high-speed camera has captured the involvement of supraspinal circuits that coordinate nociceptive and proprioceptive information to optimize sensorimotor responses (Abdus-Saboor et al., 2019; Blivis et al., 2017; Browne et al., 2017). However, forebrain lesion studies are warranted to assess if such whole-body coordinated motor responses indicate any unpleasant sensory and/or emotional experience of pain.
Supraspinal defensive reactions to external threats
Supraspinal circuits are recruited to produce defensive reactions when external stimuli imminently impact body homeostasis or threaten an animals’ survival. Because of the involvement of supraspinal circuits, these defensive reactions have been used to measure the “affective-emotional-motivational dimension of pain”. However, as discussed below, some of these assays need a revisit.
First, the temperature-chamber selection assay or the temperature gradient assay, developed for measuring thermal responses (Bautista et al., 2007; Dhaka et al., 2007; Knowlton et al., 2013; Pogorzala et al., 2013; Shimizu et al., 2005), has also been used for thermal pain measurement (Corder et al., 2019), with animals choosing to avoid the place with more aversive temperatures. Such avoidance responses, while requiring supraspinal circuits such as the parabrachial and amygdaloid nuclei (Corder et al., 2019; Yahiro et al., 2017), may represent a form of innate behavioral thermoregulation to reduce the cost of maintaining body temperature homeostasis (Bokiniec et al., 2018; Tan and Knight, 2018). It is questionable if these assays can really measure cold/heat-evoked pain. When one chamber was set at the innocuous temperature (e.g., 30 °C) and another one set at aversive cold (0 °C) or heat (50 °C), the mouse quickly retreated to the preferred 30 °C chamber even before the whole body entered the cold or heat chamber (Bautista et al., 2007). It is unlikely such short-lasting exposure caused tissue injury and evoked “cold or heat pain” perception per se. Consistently, exteroceptive responses to avoid aversive environment temperatures were preserved in mice with a lesion in the lateral thalamus that caused a loss of cold-evoked responses in the somatosensory cortex (Yahiro et al., 2017).
Responses to electric shock have also been used to measure “affective pain” (Barik et al., 2018; Han et al., 2015). However, when the whole body was subjected to electric shock in an inescapable chamber, animals initially displayed panic escape responses, followed by freezing behaviors that mimic the response to the presence of a natural predator (Misslin, 2003). In other words, the primary emotional state during and right after electric shocks may reflect a form of fear toward extreme external danger, and such brain states could suppress pain (Bolles and Fanselow, 1982; Helmstetter, 1992; Rhudy et al., 2004; Rhudy and Meagher, 2000; Veinante et al., 2013). In fact, whenever survival consideration is the priority, pain is often suppressed, as is seen in injured soldiers fighting in the battlefield or a fleeing deer injured by a hunter (Beecher, 1946; Wall, 1979).
Jumping is another exteroceptive response requiring supraspinal circuits, when mice are trapped in a hot plate with extreme high temperature (56–58 oC) (Barik et al., 2018; Han et al., 2015; Huang et al., 2019). However, jumping evoked by noxious stimuli is preserved in decerebrated animals (Barik et al., 2018; Keay and Bandler, 2001; Lovick, 1972), and might therefore not necessarily measure conscious sensory and emotional experience of pain that requires cortical structures (Ballantine et al., 1967; Foltz and White, 1962; Freeman and Watts, 1948; Ploner et al., 1999).
Exteroceptive responses that might reflect phasic pain
In response to superthreshold noxious stimuli that cause or imminently cause injury and that should evoke pain perception in humans, animals will display a set of post-stimulation responses, including paw guarding (Abdus-Saboor et al., 2019; Beaulieu-Laroche et al., 2020; Corder et al., 2019; Corder et al., 2017). Notably, guarding responses are dependent on ACC (Ren et al., 2008) and the lateral amygdala (Corder et al., 2019), two brain structures crucial for processing the affective and emotional component of pain (Devinsky et al., 1995; Phelps et al., 2021; Price, 2002; Xiao et al., 2021; Xiao and Zhang, 2018), and might therefore represent a way to measure short-lasting phasic pain in response to escapable noxious stimuli. Phasic pain experience may also be measured via real-time operant escape assays, with animals learning to avoid the chamber with the ongoing presence of noxious stimuli (Fuchs and McNabb, 2012; Fuchs et al., 2014; LaBuda and Fuchs, 2000; LaGraize et al., 2004; Vierck and Yezierski, 2015), since such responses are dependent on both ACC (Fuchs et al., 2014; LaGraize et al., 2004; Vierck and Yezierski, 2015) and secondary somatosensory cortex (Dong et al., 1996). From the functionality point of view, both guarding and operant escape serve to avoid a re-exposure to the ongoing presence of pain-evoking noxious stimuli (or as a learned hypervigilant response to avoid pain-evoking events under pathological conditions, see below), thereby representing exteroceptive responses to external threats, although the brain state may contain an interoceptive component, such as a sense of body having just been injured.
Interoceptive responses to inescapable body injury that produces tonic pain
When all above exteroceptive defensive reactions fail to prevent the occurrence of body injury or when injury-causing noxious stimuli cannot be escaped, animals develop a new set of interoceptive responses towards to injured area, such as persistent licking. Such behaviors can be evoked in response to prolonged hot plate exposure (Corder et al., 2019; Corder et al., 2017; Huang et al., 2019; Pastoriza et al., 1996), severe skin burn injury (Huang et al., 2019), prolonged pinch (Huang et al., 2019), or intradermal injection of chemical irritants (Dubuisson and Dennis, 1977; Huang et al., 2019; Le Bars et al., 2001; Ren et al., 2008). Persistent licking appears to correlate with tonic pain perception in humans. For example, licking response caused by prolonged skin pinching in mice matches with the latency and duration of pain rating by humans evoked by the same alligator clip (Huang et al., 2019). This view is further supported by brain lesion studies. Although decerebrate animals can display a licking motor behavior per se, they fail to develop persistent licking to burn injury or to persistent heat exposure (Woolf, 1984). In humans, the ACC is crucial for negative emotional experiences evoked by intense and/or sustained noxious stimuli (Devinsky et al., 1995; Mercer Lindsay et al., 2021; Phelps et al., 2021; Price, 2002; Xiao et al., 2021; Xiao and Zhang, 2018). Consistently, mice with ACC lesions exhibit a marked reduction in persistent licking to prolonged noxious stimuli without affecting lifting/flinching reflexive responses (Donahue et al., 2001; Pastoriza et al., 1996; Ren et al., 2008).
In summary, acute noxious stimulation can evoke a distinct set of behaviors that serve different purposes. First-line reflexive and defensive responses to external threats, mediated via subcortical circuits, appear to represent a form of subconscious exteroception to prevent or limit body injury. Even if animals and humans have conscious motivation to do such withdrawal-defensive responses, measurement of such motivation is masked by the presence of subcortical circuits sufficiently driving these responses. Post-stimulation guarding responses and real-time operant escape responses, which require cortical structures, might correlate with conscious sensory and emotional experience of phasic pain; these behaviors by nature are still exteroceptive, serving to prevent re-exposure to escapable noxious stimuli. Tonic pain-associated persistent licking may represent a form of interoceptive responses to body injury or to inescapable noxious stimuli. Beecher referred to the short-lasting phasic pain as early experimental pain in human testing, with phasic pain intensity reflecting the levels of external threats (Beecher, 1966; Smith et al., 1966). Beecher and others then noted that tonic pain associated with body injury or irritation has stronger emotional distress and higher sensitivity to opioids in comparison with phasic pain (Beecher, 1957, 1966; Cobos and Portillo-Salido, 2013; Cooper et al., 1986; Handwerker and Kobal, 1993; Kleggetveit et al., 2012; Namer et al., 2015; Price, 2002; Rainville et al., 1992; Smith et al., 1966). Paradoxically, for many decades, first-line reflexive and defensive reactions to transient, low-intensity stimuli have been widely used as surrogate assays to measure the clinically more relevant tonic pain. To make such surrogate assays valid, at least in terms of studying primary sensory afferents and spinal/pontine relay neurons mainly discussed in this article (see below), we would have to assume that the same neurons engage different downstream circuits to produce both exteroceptive and interoceptive behaviors, such that a loss of exteroceptive responses can indicate a concurrent loss of interoceptive, tonic pain experience. However, in the next few sections, this assumption has been challenged.
3. Primary sensory neurons that drive distinct types of behaviors under acute conditions
Here I will first summarize neuronal subtypes in dorsal root ganglia (DRG) that are defined by recent single cell RNA sequencing (scRNA-seq), and then discuss a developmental, anatomical and functional segregation of somatosensory neurons that drive exteroceptive versus interoceptive behaviors.
Molecular classification and developmental segregation of DRG neurons in mice
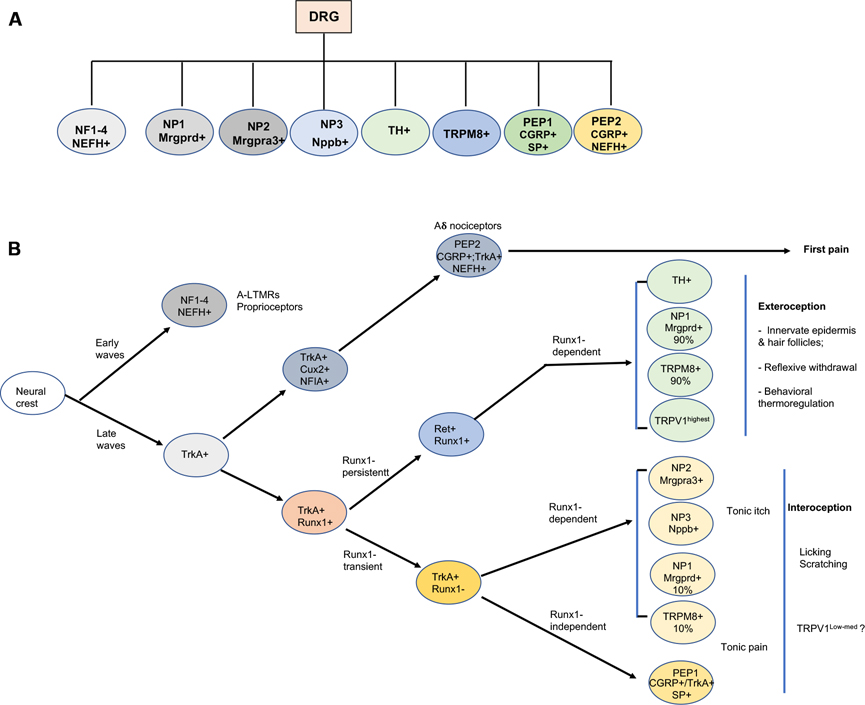
scRNA-seq studies in mice have classified DRG neurons into 11 molecularly defined cell types and their associated subtypes, as summarized in Figure 3A (Emery and Ernfors, 2018; Kupari et al., 2021; Li et al., 2018; Li et al., 2016; Sharma et al., 2020; Usoskin et al., 2015; Wang et al., 2021; Zeisel et al., 2018). Briefly, the NF1–3 subtypes represent myelinated low threshold mechanoreceptors (LTMRs) transmitting tactile information, and NF4 neurons are proprioceptors sensing the movement and position of the body (Abraira and Ginty, 2013; Emery and Ernfors, 2018). TH cells that express the tyrosine hydroxylase (TH) are unmyelinated C fiber LTMRs, although they also encode the noxious range of sensory information (Delfini et al., 2013; Li et al., 2011; Seal et al., 2009). TRPM8 cells are marked by their expression of the cold-sensitive transient receptor potential channel TRPM8. PEP1 cells are unmyelinated peptidergic neurons that coexpress genes encoding neuropeptides such as CGRP (calcitonin gene-related peptide) and substance P. PEP2 neurons are myelinated peptidergic neurons that express CGRP and the neurofilament heavy chain protein NEFH, but not SP, including recently identified Aδ nociceptors (Ghitani et al., 2017; Sharma et al., 2020). NP1 neurons, marked by the expression of Mrgprd, are unmyelinated polymodal nociceptors that respond to noxious mechanical and thermal stimuli (Cavanaugh et al., 2009; Olson et al., 2017; Rau et al., 2009), and to pruritic stimuli (Liu et al., 2012). Mrgpra3+ NP2 neurons and Nppb+ NP3 neurons are crucial for itch sensation (Lay and Dong, 2020), although these neurons may transmit pain-related information as well (Kupari et al., 2021; Sharif et al., 2020).
Figure 3: Molecular, developmental and functional classification of DRG neurons.
Molecular classification (A) and developmental-functional segregation (B) of the sensory neurons in mouse dorsal root ganglia.
These molecularly defined DRG neurons can be regrouped according to their developmental ontogeny (Figure 3B), with medium to large sized NF1–4 neurons (myelinated LTMRs and proprioceptors) being generated during the early wave of neurogenesis and small nociceptors developing later (Bachy et al., 2011; Carr and Simpson, 1978; Lawson and Biscoe, 1979; Ma et al., 1999; Venteo et al., 2019). This article focuses on neurons formed during the late wave of neurogenesis (Bachy et al., 2011; Ma et al., 1999; Venteo et al., 2019), which include the TH, TRMP8, NP1–3 and PEP1–2 subtypes. These late-born neurons are initially marked by the expression of the nerve growth factor (NGF) receptor (TrkA) and depend on NGF for their survival (Cranfill and Luo, 2021; Lallemend and Ernfors, 2012; Liu and Ma, 2011; Meltzer et al., 2021). Among TrkA lineage neurons, thinly myelinated peptidergic neurons (PEP2) are formed first and require the transcription factor (TF) Cux2 for their development (Bachy et al., 2011). By contrast, unmyelinated TrkA lineages are formed later and initially show high level expression of the runt domain TF Runx1 (Chen et al., 2006; Lallemend and Ernfors, 2012; Liu and Ma, 2011) (Figure 3). These Runx1+;TrkA+ unmyelinated neurons are then segregated into two heterogeneous populations based on their dynamic expression of Runx1 and TrkA (Figure 3). Runx1-persistent neurons, which switch off TrkA and activate the Ret receptor tyrosine kinase (Chen et al., 2006; Kramer et al., 2006; Liu and Ma, 2011; Luo et al., 2007), account for a majority (~90%) of Mrgprd+ NP1 and TRPM8+ neurons, TH+ neurons, and a small subset of neurons with highest level of expression of the heat/capsaicin receptor TRPV1 (TRPV1highest) (Abdel Samad et al., 2010; Lou et al., 2013; Yang et al., 2013). Runx1-transient neurons retain TrkA and switch off Runx1, which include PEP1, NP2 and NP3, as well as a small subset (~10%) of Mrgprd+ and TRPM8+ neurons (Abdel Samad et al., 2010; Gascon et al., 2010; Lallemend and Ernfors, 2012; Liu and Ma, 2011; Qi et al., 2017; Yang et al., 2013). Interestingly, Runx1 acts as a genetic switch to control the expression of all known genes specific to Runx1-persistent non-peptidergic neurons, and at the same time prevents them from expressing the molecular program normally associated with Runx1-transient TrkA+ neurons (Abdel Samad et al., 2010; Chen et al., 2006; Yang et al., 2013). While downregulation of Runx1 expression is necessary for the terminal differentiation of PEP1, NP2, and NP3 neurons, these Runx1-transient neurons further segregate into two groups. The first group includes NP2 and NP3 crucial for itch sensation, whose development needs early transient Runx1 activity before Runx1 undergoes a switch to become a repressor and its expression has to be switched off (Chen et al., 2006; Liu et al., 2008; Lou et al., 2015). The second group includes Runx1-independent PEP1 neurons, and together with Runx1-negative PEP2 neurons, these PEP1 neurons are thought to play a crucial role in pain sensation (see below).
Exteroceptive behaviors driven by Runx1-persistent DRG neurons
Anatomical studies showed that Runx1-persistent neurons, including 90% of Mrgprd+ NP1 neurons, 90% of TRPM8+ neurons, TRPV1highest neurons that represent 10% of total TRPV1+ neurons, and TH neurons all innervate exclusively or predominantly in the skin epidermis and/or hair follicles (Dhaka et al., 2008; Kiasalari et al., 2010; Li et al., 2011; Takashima et al., 2007; Zylka et al., 2005), thereby placing them in a position for detecting the presence of, and driving exteroceptive reflexive-defensive reactions to, external threats. Consistently, Mrgprd+ NP1 neurons are necessary for driving threshold-level spinal reflexes evoked by light punctate mechanical force delivered by von Frey filaments (Cavanaugh et al., 2009; Huang et al., 2019), but dispensable for tonic mechanical pain evoked by inescapable pinching (Huang et al., 2019). Optogenetic studies further showed that activation of Mrgprd+ neurons was insufficient to produce aversive experience under naïve conditions, as indicated by the lack of induction of real-time operant escape or conditioned place avoidance (CPA) (Abdus-Saboor et al., 2019; Beaudry et al., 2017; Vrontou et al., 2013; Warwick et al., 2021), although as discussed later, these neurons can gain the ability to drive affective pain under pathological conditions. This is consistent with human psychophysical studies, showing that during 2-min skin pinching stimulation, the first 20 seconds saw massive firing by polymodal nociceptors that may correspond to rodent Mrgprd+-like neurons capable of responding to pinch (Liu et al., 2012; Vrontou et al., 2013); such firing in humans can occur without eliciting mechanical pain sensation (Adriaensen et al., 1984; Schmidt et al., 2000). Similarly, neurons expressing the cold-sensitive TRPM8 channel drive cold-evoked exteroceptive responses, such as reflexive withdrawal from a glass plate cooled by dry ice and behavioral thermoregulation in terms of avoiding aversive cold temperatures when the choice for a warmer place is available (Knowlton et al., 2013; McKemy, 2018; Pogorzala et al., 2013), although a subset of TRPM8+ neurons could also contribute to interoceptive responses to injury-causing noxious cold (see below). Among neurons expressing the heat-sensitive channel TRPV1 (Caterina et al., 1997), Runx1 is required selectively for the development of Runx1-persistent TRPV1highest neurons, which innervate exclusively the skin epidermis and start to be activated when the temperature reaches 38 oC (Kiasalari et al., 2010), below the threshold that evokes pain in humans (Price et al., 1980). The development of TRPV1highest neurons, but not those TRPV1low-mediium neurons with low-medium levels of TRPV1 expression, was selectively impaired in Runx1 knockout mice, and these mice showed marked deficits in reflexive responses to moderate noxious heat (Chen et al., 2006). Thus, Runx1-persistent TRPV1highest neurons likely involve with threshold-level spinal reflexes evoked by heat, although it remains unknown if these neurons additionally involve in behavioral thermoregulation such as avoidance of aversive heat. Runx1-persistent TH+ C-LTMRs innervate hair follicles and can respond to innocuous stroking across the skin (Li et al., 2011; Seal et al., 2009). Unmyelinated C-LTMRs in humans have been implicated in coding pleasant touch (Bohic and Abraira, 2021; McGlone et al., 2014), and a recent study suggests a similar role for mouse TH+ C-LTMRs, although the evidence is not yet unequivocal since the viral approach infected TH-negative cells as well (Bourinet et al., 2021). These neurons in fact can additionally encode the intensity of noxious punctate mechanical stimuli (Li et al., 2011; Seal et al., 2009), and accordingly, a role for these neurons for driving reflexive responses in the hairy skin has not yet been ruled out. Moreover, C-LTMRs marked by the expression of Runx1-transient MrgprB4 neurons (Liu et al., 2008) have been implicated for the transmission of pleasant mechanical information (Middleton et al., 2021; Vrontou et al., 2013). Thus, with exclusive or predominant projections to the skin, Runx1-persistent DRG neurons apparently evolve to drive first-line exteroceptive defensive responses to noxious mechanical, cold and heat stimuli under naïve conditions.
Interoceptive behaviors driven by Runx1-transient DRG neurons
Unlike predominant innervation to the skin by Runx1-persistent neurons, Runx1-transient TrkA+;CGRP+ peptidergic nociceptors innervate the whole body (Jimenez-Andrade et al., 2010; McCoy et al., 2012; Yang et al., 2013), and are therefore in a position to play prominent interoceptive roles by responding to tissue disruption or irritation. Many of these Runx1-transient neurons in mice express TRPV1 at low-medium levels (TRPV1Low-medium), which account for 90% of total TRPV1+ neurons (Abdel Samad et al., 2010; Chen et al., 2006). Consistently, persistent licking evoked by prolonged hot plate exposure or following severe skin burn injury were markedly reduced in mice with ablation of TRPV1+ neurons (Huang et al., 2019). As discussed below, TRPV1+ neurons are also crucial for driving tonic pain and interoceptive responses evoked by mechanical and cold stimuli.
An involvement of TRPV1+ neurons for tonic mechanical pain was first suggested from human psychophysical studies, showing that pinch-evoked pain is correlated with delayed-firing of chemo-nociceptors that progressively gain mechanical sensitivity (Adriaensen et al., 1984; Schmelz et al., 2000; Schmidt et al., 2000), and these nociceptors respond to capsaicin (Ringkamp et al., 2001; Schmidt et al., 1995; Wooten et al., 2014). Consistently, mice with ablation of capsaicin-sensitive TRPV1 neurons showed a marked reduction in licking responses evoked by inescapable/prolonged noxious pinch, without affecting threshold-level withdrawal responses to von Frey filament stimulation (Cavanaugh et al., 2009; Huang et al., 2019). As discussed above, opposite impacts were seen upon ablation of Runx1-persistent Mrgprd+ neurons that caused a loss of withdrawal responses to von Frey filament stimulation, without affecting pinch-evoked persistent licking (Cavanaugh et al., 2009; Huang et al., 2019). Moreover, activation of TRPV1 neurons in mice produced a negative emotional experience, as indicated by the driving of conditioned place avoidance (CPA), whereas activation of Mrgprd+ neurons failed to do so under naïve conditions (Abdus-Saboor et al., 2019; Beaudry et al., 2017; Vrontou et al., 2013; Warwick et al., 2021). These observations support the presence of separate primary neuron subtypes driving exteroceptive reflexive responses to the presence of escapable noxious mechanical stimuli versus interoceptive self-caring responses to body injury or irritation evoked by inescapable noxious mechanical stimuli.
Similar functional subdivisions are seen in sensory neurons that process noxious cold. Ablation of TRPV1 neurons did not affect threshold-level cold-evoked reflexes and behavioral thermoregulation in terms of avoiding aversive cold temperature but did cause a marked reduction in licking responses evoked by sustained exposure to 0 °C cold plate, which delivers a moderate noxious cold stimulation (Huang et al., 2019; Pogorzala et al., 2013). In contrast, all these behaviors were impacted upon ablation of TRPM8+ neurons (Knowlton et al., 2013; McKemy, 2018; Pogorzala et al., 2013). TRPM8+ neurons are subdivided into low-threshold and high threshold subsets, with the latter one responding to capsaicin as well as to mustard oil (that can activate TRPA1), indicating that they coexpress TRPM8, TRPV1 and TRPA1 (Bautista et al., 2007; McKemy, 2018; Xing et al., 2006). Note that TRPA1 is an injury sensor (Arenas et al., 2017; del Camino et al., 2010; Viana, 2016). Thus, while TRPV1-negative TRPM8+ neurons that are spared in TRPV1 neuron-ablated mice are sufficient to drive reflexive and defensive reactions to external cold stimulation, neurons expressing TRPM8 and TRPV1 (and possibly TRPA1 as well) are required for interoceptive licking responses caused by sustained moderate noxious cold exposure evoked by 0 °C cold plate. Notably, in response to more extreme noxious cold stimulation that should cause severe injury (e.g., after exposure to temperature as low as −20 °C), many TRPM8-negative, initially cold-insensitive nociceptors could respond secondarily to mediators from injured tissues, creating pseudo-cold sensitivity (Foulkes and Wood, 2007; Luiz et al., 2019; Simone and Kajander, 1997; Zimmermann et al., 2007). It is warranted to determine if cold-induced severe injury will cause robust licking responses, and if such responses depend on Runx1-transient neurons, such as TRPV1Low-medium neurons. Taken together, these findings suggest that the developmental segregation of unmyelinated TrkA lineage neurons prefaces the separation of DRG neurons into two lineages, one that drives rapid first-line exteroceptive defensive reactions to external threats and another that generates second-line interoceptive self-caring behaviors to bodily injury or irritation (Figure 3).
Distinct DRG neurons drive phasic pain and tonic pain with differential implications on exteroception and interoception
Noxious stimulation in humans can drive two to three forms of pain, referred to as first, second and tonic pain, respectively (Ackerley and Watkins, 2018; Lewis, 1938; Lewis and Pochin, 1937). In response to pinpricking stimulation, activation of fast-conducting thinly myelinated Aδ nociceptors mediates the first pain response which has a sharp, pricking, stinging, and precisely localized quality (Handwerker and Kobal, 1993). In mice, Aδ nociceptors are derived from early-born Runx1-negative sensory neurons (Figure 3). A subset of Aδ mechanonociceptors are marked by persistent coexpression of CGRP and Bmpr1R and transient expression of NPY2R (Arcourt et al., 2017; Ghitani et al., 2017; Qi et al., 2020; Sharma et al., 2020), and they did not express TRPV1, thereby lacking sensitivity to capsaicin (Ringkamp et al., 2001; Szolcsanyi et al., 1988), although capsaicin-sensitive Aδ nociceptors do exist (Lin et al., 2000). If slow-conducting unmyelinated nociceptors are simultaneously activated, a delayed second pain with a burning, dull, and diffuse quality is evoked (Bishop, 1946; Handwerker and Kobal, 1993; Head et al., 1905; Lewis, 1938; Lewis and Pochin, 1937). In humans, this pinprick-evoked second pain is mediated via capsaicin-sensitive mechanonociceptors (Magerl et al., 2001). In contrast, pinch-evoked tonic pain in humans appears to be mediated by capsaicin-sensitive silent mechanonociceptors that gradually gain mechanical sensitivity (Ackerley and Watkins, 2018; Adriaensen et al., 1984; Kleggetveit et al., 2012; Ringkamp et al., 2001; Schmelz et al., 2000; Schmidt et al., 1995; Schmidt et al., 2000; Serra et al., 2014; Wooten et al., 2014). Thus, there must have some degree of cellular segregation of sensory afferents for driving different phases of pain.
In summary, from a developmental, anatomical, and functional point of view, we can re-classify TrkA lineage DRG neurons into three categories that play distinct roles. Runx1-persistent nociceptors innervating the skin epidermis and hair follicles are the first-line defense system. They drive threshold-level withdrawal responses via spinal reflex pathways and behavioral thermoregulation via supraspinal circuits at pre-pain stages; such exteroceptive behaviors serve to prevent body injury. Early-onset, mechanically/thermally sensitive nociceptors that drive short-lasting phasic pain (first and second pain), with the first pain being mediated by Runx1-negative Aδ nociceptors and the second pain potentially by Runx1-transient C fiber nociceptors. Despite the aversive sensory and emotional experience associated with phasic pain, these nociceptors might still predominantly play an exteroceptive role by encoding threat levels and by eliciting behaviors serving to prevent re-exposure to the ongoing presence of noxious stimuli, such as guarding and operant escape. The third-class nociceptors, including Runx1-transient TRPV1low-medium chemosensitive neurons that respond to mediators from injured tissues, are well positioned to signal body integrity disruption and drive interoceptive self-caring responses to reduce suffering. Candidates include silent mechanonociceptors expressing the cholinergic receptor CHRNA3 (Prato et al., 2017). Prospective markers for unmyelinated nociceptors that are associated with rapid-onset second pain and that drive the guarding and operant escape responses in animals are yet to be characterized, though they include capsaicin-sensitive cells in humans (Magerl et al., 2001).
4. PB subnuclei that drive distinct types of behaviors
Before discussing functional segregation at spinal levels, we need first to discuss the lateral parabrachial nuclei (lPBNs), which are located in the rostral pons and serve as a major relay station for transmitting somatosensory information (Barik et al., 2021; Barik et al., 2018; Campos et al., 2018; Chiang et al., 2020; Choi et al., 2020; Gauriau and Bernard, 2002; Han et al., 2015; Huang et al., 2019; Mu et al., 2017; Saper, 2000). The lPBNs are divided into several subnuclei (Fulwiler and Saper, 1984), including the external lPBN (eLPBN, the most ventral part), the dorsal LPBN (dlPBN), the central lPBN (clPBN), and the superior-internal lPBNs (for simplicity, jointly referred to as slPBNs, according to the Allen Brain Atlas nomenclature) (Figure 4). All four major subnuclei receive direct inputs from the spinal cord (Gauriau and Bernard, 2002; Todd, 2010) (see below).
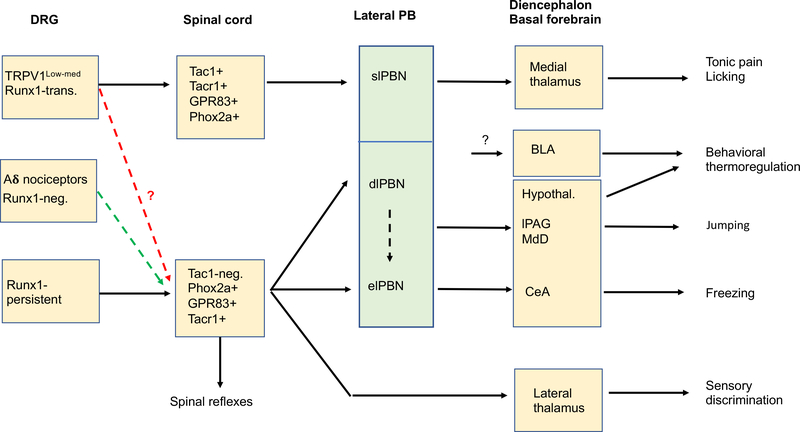
Figure 4. Partially segregated neural pathways driving different types of behaviors under acute conditions.
Adult Runx1-transient (“Runx1-trans.”; for example, TRPV1low-med) neurons (see Figure 3) may be connected to the spinal-slPBN-medial thalamic pathways that drive acute tonic pain and interoceptive self-caring behaviors such as persistent licking around the injured area. Adult Runx1-persistent neurons (see Figure 3) drive spinal reflexes vial local circuits and behavioral thermoregulation via the spinal-dlPBN-hypothalamic pathways plus the BLA pathway, although the route leading to BLA activation remains unknown (black “?”). Runx1-negative Aδ nociceptors also contribute to reflexive behaviors, such as reflexes in response to pinpricking stimulation by a thin needle (Arcourt et al., 2017; Qi et al., 2020). it remains unclear if defensive reactions such as freezing and jumping are driven by Runx1-persistent, Runx1-negative Aδ nociceptors (green dashed arrow) and/or Runx1-transient TRPV1low-medium neurons (red dashed arrow). PB: parabrachial nuclei. slPBN, dlPBN and elPBN: the superior lateral, the dorsolateral, and exterior lateral PB nuclei, respectively. BLA: the basal lateral nucleus of the amygdala. Hypothal.: hypothalamic nuclei. lPAG: the lateral periaqueductal gray nuclei. MdD: the dorsal reticular formation of the medullar (MdD). CeA: the central nuclei of the amygdala. Tac1-neg.: spinal Tac1-negative neurons.
elPBN and dlPBN neurons drive defensive reactions and behavioral thermoregulation
Recent anatomical and functional studies showed that molecularly or genetically defined PB neurons may drive distinct types of behaviors. elPBN neurons marked by the expression of CGRP serve as a general alarm for the presence of external (and internal) dangers that threaten animal survival (Campos et al., 2018; Han et al., 2015). These CGRP+ neurons project to the central nucleus of the amygdala (CeA), the bed nucleus of the stria terminalis (BNST, an extended nucleus of the amygdala), the insular cortex and a variety of targets in the forebrain and hindbrain (Campos et al., 2018; Chiang et al., 2020; Han et al., 2015; Huang et al., 2021a; Huang et al., 2021b; Rodriguez et al., 2017). Activation of elPBN neurons or activation of trigeminal afferents projecting directly to elPBN is clearly aversive, sufficient to produce conditioned aversive learning and memory (Chiang et al., 2020; Han et al., 2015; Rodriguez et al., 2017). Behaviorally, they are required to drive a cohort of defensive reactions, such as freezing and conditioned fear evoked by electric shocks and jumping evoked by sustained hot plate exposure (Han et al., 2015; Sato et al., 2015). Tac1-expressing PB neurons, which are located mainly in elPBN and include neurons that project to the dorsal reticular formation of the medulla (MdD), can sensitize jumping evoked by hot plate exposure (Barik et al., 2018). Activation of undefined dlPBN neurons that project to the ventromedial hypothalamus and/or to the lateral periaqueductal gray (lPAG) can also induce jumping (Barik et al., 2018; Chiang et al., 2020), but it remains unknown how neurons in elPBN and dlPBN jointly generate this jumping response.
For neurons located in dlPBN, functional studies reveal an essential role in behavioral thermoregulation as measured by two-chamber temperature selection assays (Yahiro et al., 2017). Thermo-sensitive neurons in dlPBN express the preprodynorphin (Pdyn) peptide and other peptide markers (Geerling et al., 2016; Norris et al., 2021; Yang et al., 2021). Warm-sensitive neurons, including Pdyn+ neurons project to the preoptic area of the hypothalamus (Geerling et al., 2016; Huang et al., 2021a; Huang et al., 2021b; Nakamura and Morrison, 2008, 2010) that is crucial for body temperature control (Norris et al., 2021; Tan et al., 2016; Tan and Knight, 2018). Neurons in the lateral nuclei of the amygdala are also required for avoiding aversive temperatures (Corder et al., 2019), although it remains unclear if and how Pdyn neurons in the dlPBN interact with the lateral amygdala to drive behavioral thermoregulation, since Pdyn neurons do not send direct projections to the amygdala (Huang et al., 2021a; Huang et al., 2021b).
slPBN neurons drive interoceptive self-caring responses
The function of slPBNs is apparently different. These neurons, partly marked by the expression of Tacr1, respond to high threshold noxious stimuli but not to low-moderate noxious mechanical or thermal stimuli (Barik et al., 2021; Bourgeais et al., 2001; Deng et al., 2020; Gauriau and Bernard, 2002; Hermanson and Blomqvist, 1997). They have large bilateral receptive fields (Bourgeais et al., 2001; Gauriau and Bernard, 2002), and project to medial thalamic and other brain nuclei (Barik et al., 2021; Bester et al., 1999; Bourgeais et al., 2001; Deng et al., 2020; Fulwiler and Saper, 1984; Huang et al., 2019). Unlike CGRP+ neurons in elPBN (Huang et al., 2021a; Huang et al., 2021b; Huang et al., 2019), they do not send direct projections to the amygdala (Barik et al., 2021; Deng et al., 2020; Huang et al., 2019). Functionally, these neurons are associated with interoceptive self-caring responses such as sustained licking responses to intradermal formalin injection, sustained noxious heat stimulation, or to prolonged skin pinch (Deng et al., 2020), all of which produce tonic pain in humans (Dubuisson and Dennis, 1977; Huang et al., 2019). These neurons are dispensable for formalin-induced lifting/flinching responses (Deng et al., 2020), in line with the selective loss of formalin-evoked licking seen in mice with ACC lesions (Donahue et al., 2001) and the prominent projections of slPBN neurons to the medial thalamic nuclei that in turn transmit aversive sensory information to ACC (Devinsky et al., 1995; Krettek and Price, 1977; Price, 2002; Wang and Shyu, 2004).
Thus, there is at least partial functional segregation among PB subnuclei (Figure 4). The most ventral (elPBN) and intermediate (dlPBN) portions of lPBNs are required to generate a set of exteroceptive defensive reactions to external threats, whereas neurons in the most dorsal PBNs (slPBNs) are essential for driving interoceptive self-caring responses towards to injured body regions (Figure 4). However, with recently reported additional projections of CGRP+ elPBN neurons to the medial thalamic nuclei and to the insular cortex (Huang et al., 2021b), and possible interconnections among PB subnuclei (Chiang et al., 2020), we may expect to see some synergistic interactions by these PB nuclei in driving certain exteroceptive and interoceptive behaviors.
5. Segregated spinal substrates that drive exteroceptive versus interoceptive behaviors
The dorsal horn of the spinal cord is the first integrative center involved in transmitting somatosensory information. It receives inputs from primary sensory afferents and descending facilitating or inhibitory inputs from the brain. Most dorsal horn neurons are local excitatory and inhibitory interneurons that transmit or gate sensory information (see below). The output neurons (spinal ascending projection neurons) represent a small subset of dorsal horn neurons and send information to various brain structures, including thalamic subnuclei (referred to as the spinothalamic tract or STT neurons) and LPB subnuclei (Gauriau and Bernard, 2002; Peirs et al., 2020; Todd and Wang, 2018; Todd, 2010). Here we will discuss how distinct spinal local interneurons and ascending projection neurons drive exteroceptive versus interoceptive types of behaviors.
Anatomical segregation of STT and spino-parabrachial neurons
Early electrophysiological studies by Giesler et al. revealed three populations of STT neurons that project to the central lateral (CL) thalamic nuclei (CL-STT), the lateral VPL nuclei (VPL-STT), or both (Giesler et al., 1981). Yet other studies identify additional STT neurons that project to the medial thalamic nuclei (M-STT) (Ammons et al., 1985; Choi et al., 2020; Huang et al., 2019; Iwata et al., 2011; Roome et al., 2020; Willis et al., 2002). Interestingly, a majority of M-STT and CL-STT neurons respond selectively to high threshold noxious stimuli with large bilateral receptive fields (Dong et al., 1978; Giesler et al., 1981; Willis et al., 2002), whereas most VPL-STT neurons are wide dynamic range neurons, whose firing rates correlate with stimulation intensities (Groh et al., 2018; Price, 2002; Willis et al., 2002). Consistently, recent genetic studies have shown that spinal Tac1Cre neurons marked by the persistent or developmental expression of the Tac1 gene include M-STT but not VPL-STT neurons (Huang et al., 2019). In contrast, spinal Phox2aCre and Robo3Cre neurons, marked by developmental expression of Phox2a and Robo3, respectively, project to both the lateral and medial thalamic nuclei (Choi et al., 2020; Roome et al., 2020); these spinal neurons could contain a mixture of VPL-STT and M-STT neurons, as supported by a partial overlapping with spinal Tac1 neurons, and/or contain neurons with dual projections to lateral and medial thalamic nuclei.
Other molecular and genetic studies have characterized spinal neurons that project to distinct parabrachial subnuclei (Barik et al., 2021; Choi et al., 2020; Gauriau and Bernard, 2002; Huang et al., 2019; Roome et al., 2020; Todd, 2010). Spinal Tac1Cre neurons project specifically to slPBN (Gutierrez-Mecinas et al., 2017; Huang et al., 2019), that then send connections to the medial but not lateral thalamic nuclei. As such, there are direct and indirect connections from spinal Tac1Cre neurons to the medial thalamus. Neurons in slPBN also receive inputs from spinal Tacr1Cre neurons, as well as spinal GPR83Cre neurons marked by the expression of the G-protein coupled receptor protein GPR83, both of which include a subset of Tac1+ neurons (Barik et al., 2021; Choi et al., 2020; Deng et al., 2020; Gauriau and Bernard, 2002; Huang et al., 2019; Roome et al., 2020; Todd, 2010). Spinal Tacr1Cre neurons also show dense projections to clPBN, moderate projections to dlPBN and minimal projections to elPBN, whereas spinal GPR83Cre neurons send prominent projections to dlPBN with few projections to clPBN (Choi et al., 2020). In contrast, Phox2aCre labels a much larger fraction of spinal ascending projection neurons that send axons to both the medial and lateral thalamic nuclei and most LPB subnuclei (Roome et al., 2020).
Spinal substrates driving acute exteroceptive reflexive-defensive reactions
As discussed above, threshold-level withdrawal responses can be mediated via local spinal circuits (preserved in spinalized animals), even though supraspinal circuits are involved in more elaborate coordinated motor responses. In other words, they can be mediated via local interneurons. Consistently, basic withdrawal responses to noxious stimuli under acute conditions were preserved in mice with silencing, ablation or developmental defect of a set of spinal neurons that include ascending projection neurons, including spinal Tac1+, GPR83+, Tacr1+ and Phox2a+ neurons (Choi et al., 2020; Huang et al., 2019; Mantyh et al., 1997; Roome et al., 2020). Meanwhile, reflexes evoked by noxious mechanical stimuli (von Frey filament and pinprick) were eliminated upon ablation of spinal neurons expressing somatostatin (SOM), which are enriched in inner lamina II (Duan et al., 2014), a region known to contain interneurons (Todd, 2010). Acute nociceptive reflexes evoked by light punctate mechanical force was also attenuated upon ablation of spinal interneurons that partially overlap with SOM+ neurons, including those marked by developmental expression of VGLUT3, Calb1, Calb2, or CCK (Cheng et al., 2017; Duan et al., 2014; Gatto et al., 2021; Liu et al., 2018).
Spinal neurons associated with supraspinal defensive reactions, such as jumping, behavioral thermoregulation and fear-indicating freezing, are only beginning to be characterized. In addition to their roles in interoceptive licking responses (see below), GPR83+ and Phox2a+ neurons would be attractive candidates with prominent projections to the dlPBN and elPBN (Choi et al., 2020; Roome et al., 2020), two nuclei crucial for driving defensive reactions (see above). Although spinal Tacr1+ neurons show limited innervation of the dlPBN, their activation can robustly activate neurons in the elPBN (Choi et al., 2020), possibly via intra-PB projections (Chiang et al., 2020). This activation induced jumping and post-stimulation freezing responses, suggesting a possible role of Tacr1+ neurons in driving defensive reactions to threats (Choi et al., 2020). Since Tac1-negative neurons are sufficient to mediate all known forms of reflexive and defensive reactions (see below), future studies are needed to determine if there are Tac1-negative subsets of Tacr1+, GPR83+, and/or Phox2a+ neurons that are dedicated to exteroceptive responses to threats.
Spinal substrates driving interoceptive responses
Consistent with direct and indirect (via slPBNs) connections with medial thalamic nuclei, which are crucial for processing affective and emotional components of pain in humans (discussed above), spinal Tac1 neurons are required to drive interoceptive behaviors such as persistent licking (Huang et al., 2019). Skin pinch-evoked negative emotions, measured via CPA, are also abolished in mice after ablating the spinal Tac1Cre neurons, whereas activation of their central terminals within medial thalamic nuclei or the slPBNs is capable of producing CPA (Huang et al., 2019). In contrast, spinal Tac1 neurons are dispensable for a range of reflexive-defensive reactions evoked by noxious stimuli (Huang et al., 2019). Although spinal Tac1 neurons may include interneurons that synapse onto Tac1-negative projection neurons, these studies clearly suggest the existence of separate spinal substrates for driving exteroceptive versus interoceptive types of behaviors. Spinal Tacr1Cre, GPR83Cre and Phox2aCre neurons partially overlap with Tac1 neurons, and these neurons accordingly contribute to interoceptive licking responses as well (Choi et al., 2020; Mantyh et al., 1997; Roome et al., 2020). In view of their potential involvement in both exteroceptive and interoceptive behaviors, future studies will be needed to determine subtypes of Tacr1Cre, GPR83Cre and Phox2aCre neurons that drive more specific behaviors. It should also be noted that spinal neurons driving phasic pain-suggestive guarding and operant escape responses have not yet been characterized.
In summary, there is growing evidence for segregated pathways that drive exteroceptive versus interoceptive behavioral responses, from primary sensory neurons in DRG to ascending spinal projection neurons that innervate the lPBN (Figure 4).
6. Pain inhibition by exteroceptive circuits under acute conditions
The above discussion highlights a functional subdivision of the somatosensory system that drives exteroceptive versus interoceptive behaviors. However, these neural pathways are not independent of each other, but instead engage extensive crosstalk (see also next section). Such crosstalk is not confined to nociceptor subgroups, but also includes interactions between nociceptors and other exteroceptive circuits that respond to innocuous tactile or thermal stimuli.
Antagonistic interactions among different branches of the somatosensory system was first suggested from human studies. In 1905, Head reported the classic nerve division experiment done in his own hand, revealing a subdivision of the cutaneous somatosensory system into two branches that displayed differential rates of regeneration: i) the “epicritic” one associated with tactile and innocuous thermal discrimination (thereby a part of the exteroceptive system) and ii) the “protopathic” branch for driving diffuse, dull pain in response to intense noxious stimuli, which may contain both exteroceptive and interoceptive components (see above on “second pain” and “tonic pain”). Interestingly, he reported that before epicritic afferents for innocuous tactile and thermal sensations were regenerated, noxious stimulation produced stronger aversive feeling, leading him to propose pain suppression by epicritic inputs (Head et al., 1905). Furthermore, in 1911 Head and Holmes reported that for patients with lesions of lateral thalamic nuclei, noxious and warm stimuli caused exaggerated unpleasantness and pleasantness, respectively, allowing him to predict the presence of a lateral inhibitory gate that suppresses the affective-emotional-motivational component of somatosensory information that is transmitted through the medial pathway (Head and Holmes, 1911), long before the identification of thalamic reticular nuclei that send inhibitory outputs to medial thalamic nuclei (Bay and Cavdar, 2013; Guillery and Harting, 2003; Wang et al., 2005). 50 years later, Melzack and Wall proposed a more concrete gate control theory of pain, focusing more on sensory integration within the spinal cord (Melzack and Wall, 1965). One of key tenets of this theory is that sensory afferent interactions, locally or via spinal-supra-spinal loops, can reshape the flow of sensory information (Melzack and Wall, 1965).
During the past decade, great efforts have been made in mapping local spinal circuits that are associated with gate control (Duan et al., 2018; Koch et al., 2018; Peirs et al., 2020; Takazawa and MacDermott, 2010). Spinal transmission (T) neurons that receive c-fiber nociceptor inputs also receive monosynaptic or polysynaptic excitatory inputs from Aβ low-threshold mechanoreceptors (Aβ-LTMRs) that normally transmit exteroceptive tactile information. Under normal conditions, such Aβ inputs to pain transmission neurons could be electrically filtered by the presence of voltage-gated potassium channels (Hu et al., 2006; Zhang et al., 2018) or are gated via feedforward activation of a cohort of inhibitory neurons (Abraira et al., 2017; Baba et al., 2003; Bourane et al., 2015; Cui et al., 2016; Duan et al., 2014; Foster et al., 2015; Hughes and Todd, 2020; Lu et al., 2013; Petitjean et al., 2015; Takazawa and MacDermott, 2010). As such, the outputs in T neurons in response to Aβ inputs depend on the relative strengths of excitatory inputs, inhibitory inputs, and T cell excitability. Under naive conditions, inhibitory inputs are apparently dominant, sufficient to prevent innocuous mechanical stimuli from evoking pain. For example, concurrent activation of Aβ-LTMRs can attenuate behaviors evoked by Aδ nociceptor inputs (Arcourt et al., 2017). Similarly, activation of cool-sensitive Aδ fibers can prevent cold-sensitive (or cold/heat-sensitive) C fibers from evoking burning pain (Ma, 2010; McKemy, 2018; Prescott et al., 2014). These inhibitory roles by exteroceptive Aβ-LTMRs and Aδ-cool fibers might explain why humans and animals engage certain recuperative responses that may activate Aβ-LTMRs and/or Aδ-cool fibers, including licking and blowing fingers with skin burn injury to soothe suffering.
Pain could also be inhibited by activation of nociceptive circuits that drive defensive reactions under acute naïve conditions. As mentioned above, extreme external threats, such as electric shocks or the presence of a predator that could threat animal survival, may drive an emotional state dominant with fear that could suppress pain, a survival-promoting mechanism that allows animals to focus on imminent threats (Amit and Galina, 1986; Bolles and Fanselow, 1982; Butler and Finn, 2009; Helmstetter, 1992; Rhudy et al., 2004; Rhudy and Meagher, 2000; Veinante et al., 2013), although stress associated with future threats could sensitize nociception and pain (Rhudy and Meagher, 2000). CGRP+ neurons in elPBNs project to the central amygdala (CeA) and are crucial for driving fear-suggesting freezing in response to extreme threats (Han et al., 2015). More recent studies show that inhibitory neurons in the CeA are functionally heterogeneous, some of which could produce anti-pain and/or anti-nociception effects (Hua et al., 2020; Raver et al., 2020; Wilson et al., 2019). These CeA inhibitory neurons project to many brain regions associated with affective pain processing, including the lPBN, intralaminar thalamic nuclei, and the insular cortex, and their activation was able to suppress interoceptive responses associated with tonic pain, such as licking evoked by formalin and capsaicin (Hua et al., 2020). Future studies will be needed to determine if these anti-pain inhibitory neurons, which can be activated by low doses of anesthetic compounds (Hua et al., 2020), involve in pain inhibition induced by imminent fear-evoking external threats.
7. Pain and comorbidities driven by both exteroceptive and interoceptive circuits under pathological conditions
Unlike antagonistic interactions under acute naïve conditions, exteroceptive and interoceptive circuits can jointly drive pain and comorbidities under pathologic conditions, as discussed below. For patients suffering chronic pain, one of most prevalent symptoms is allodynia or pain evoked by innocuous tactile stimuli (Duan et al., 2018; Hill and Bautista, 2020; Koch et al., 2018; Moehring et al., 2018; Peirs et al., 2020). As aforementioned, Aβ-LTMR inputs into spinal pain transmission T neurons are normally gated via feedforward inhibition or via intrinsic electric filtering. Following inflammation, nerve lesions and traumatic injury, central sensitization in T cells and/or reduction of inhibitory inputs can allow Aβ inputs to activate T neurons, via monosynaptic or polysynaptic pathways, thereby leading to the manifestation of allodynia (Duan et al., 2018; Hill and Bautista, 2020; Ji et al., 2018; Koch et al., 2018; Moehring et al., 2018; Peirs et al., 2020; Price et al., 2009; Takazawa and MacDermott, 2010; Woolf, 2011). To date, a number of molecularly defined spinal excitatory neurons have been identified, which mediate mechanical hypersensitivity and/or allodynia developed under different pathological conditions, including those marked by the expression of Tacr1, Pkc-γ, SOM-Cre, VGLUT3-Cre, Calretinin-Cre, NPY1R, or CCK-Cre (Cheng et al., 2017; Christensen et al., 2016; Duan et al., 2014; Liu et al., 2018; Lu et al., 2013; Maiaru et al., 2018; Malmberg et al., 1997; Nelson et al., 2019; Neumann et al., 2008; Nichols et al., 1999; Peirs et al., 2021; Peirs et al., 2015; Petitjean et al., 2015).
C fiber nociceptors associated with reflexive responses can also promote pain under pathological conditions (Abdus-Saboor et al., 2019; Cheng et al., 2017; Warwick et al., 2021). For example, Mrgprd+ nociceptors innervate inner lamina II, and their activation normally drives exteroceptive reflexive response but fails to produce aversive emotions (Abdus-Saboor et al., 2019; Beaudry et al., 2017; Vrontou et al., 2013; Warwick et al., 2021). Nonetheless, these nociceptors do form polysynaptic connections to pain transmission neurons located in superficial laminae, although such inputs are normally gated via feedforward inhibition (Warwick et al., 2021). Following nerve lesions or inflammation, feedforward inhibition is apparently attenuated and/or pain transmission neurons are sensitized, allowing Mrgprd+ nociceptors to drive pain and aversive learning (Abdus-Saboor et al., 2019; Beaudry et al., 2017; Warwick et al., 2021).
Disruption of functional segregation could also occur in the forebrain regions. As discussed above, while the medial thalamic nuclei send direct projections to ACC, the lateral thalamic pathways send convergent inputs to ACC, either directly (Fillinger et al., 2017; Singh et al., 2020) or indirectly via insular cortex (Figure 2) (Price, 2002; Xiao and Zhang, 2018). This convergence suggests that under pathological conditions, sensitization of either lateral, medial, or both pathways could lead to increased excitatory inputs to ACC neurons that then drive strong affective pain (Singh et al., 2020), which is in contrast to low-moderate unpleasantness mediated normally by the lateral pathway under naïve conditions (Head and Holmes, 1911; Ploner et al., 1999; Price, 2002). Indeed, in some human chronic pain patients, skin stroking can evoke burning pain, suggesting that low threshold mechanoreceptors may connect to the lateral thalamic pathways to produce the discriminative sensory quality of pain, with or without concurrent connections to the medial thalamic pathways (Torebjörk et al., 1992). In other neuropathic pain patients, moving tactile stimulation across the skin can produce intense unpleasantness, without concrete sensory quality of pain (Fields, 1999), potentially via selective driving of the medial thalamic pathways that might include spinal Tac1-like neurons, although a role of Tac1 neurons in driving chronic pain has not yet been tested (Huang et al., 2019). Consistently, animal studies reveal the presence of multiple spinal pathways that allow innocuous mechanical stimulation to drive mechanical allodynia (Cheng et al., 2017; Peirs et al., 2021), although it remains unknown if these spinal interneurons are connected to distinct ascending pathways. Conceivably, different patients may have differential sensitization of the medial versus lateral pathways, explaining why surgical lesions in either medial or lateral thalamic regions only led to pain relief in a subset of patients (Mark et al., 1963; Spielgel et al., 1966; Steiner et al., 1980; Tasker, 1990; Young et al., 1995a; Young et al., 1995b).
Furthermore, sensitized exteroceptive and interoceptive circuits might jointly drive comorbidities seen in chronic pain patients, such as anxiety, fear and depression. One example for such interactions occurs in dopaminergic (DA) neurons located in the midbrain. Pain is long considered as the opposite of pleasure, and pain-evoking signals could lead to DA inactivation, whereas pain relief is considered as a reward, leading to DA activation (Borsook et al., 2016; Lumley et al., 2011; Navratilova et al., 2015). Dopaminergic neurons can, however, be inactivated via any aversive signals, including exterior threats and internal body injury. It has been long-known that electric shocks, which evoke unconditioned fear and drive exteroceptive post-stimulation freezing responses (Han et al., 2015), can inactivate DA neurons (Gao et al., 1990; Jhou et al., 2009; Yang et al., 2021). Meanwhile, intense noxious stimuli, including pinching that produces tonic pain in humans and interoceptive licking in mice, can activate neurons located in the lateral habenula (LHB) (Benabid and Jeaugey, 1989; Huang et al., 2019), an anti-reward center that can inactivate DA neurons via descending activation of the inhibitory neurons located in the rostromedial tegmental nucleus or RMTg (Herkenham and Nauta, 1979; Hu et al., 2020; Jhou et al., 2009; Metzger et al., 2017; Omelchenko et al., 2009). Notably, spinal Tac1 neurons, which drive pinch-evoked interoceptive licking responses, send direct projections to LHB and are necessary for pinch-evoked activation of LHB neurons and aversive learning (Huang et al., 2019). Conceivably, concurrent sensitization of exteroceptive and interoceptive pathways under pathological conditions could synergistically inactivate DA neurons, and subsequently cause reward deficits and depression. Moreover, DA neurons normally project to ACC and provide inhibitory inputs (Borsook et al., 2016; Bushnell et al., 2013; Fillinger et al., 2017; Navratilova et al., 2015; Williams and Goldman-Rakic, 1998), and their inactivation could in principle contribute to ACC neuron sensitization and increase pain rating in response to a pain-evoking event. Thus, exteroceptive and interoceptive pathways could jointly drive chronic pain and comorbidities under pathological conditions.
8. Implications for pain research
The subdivision of the somatosensory circuits along exteroception versus interoception and their dynamic interactions under physiological and pathological conditions may bear important implications for pain research.
Firstly, we need to revisit surrogate assays to measure acute pain, as already argued by other investigators (Beecher, 1957; Cobos and Portillo-Salido, 2013; Mao, 2012; Mogil, 2009, 2018; Tappe-Theodor et al., 2019). The exteroceptive pathways drive reflexive and defensive reactions to external threats, which have long been used to measure acute pain. However, as discussed above, primitive threshold-level withdrawal responses to noxious stimuli can be carried out by local spinal circuits. Moreover, supraspinal defensive reactions, including jumping and behavioral thermoregulation, can be carried out in decerebrated mice as well. In other words, under acute conditions, all these exteroceptive behaviors might not necessarily measure the sensory and emotional experience of pain that requires cortical structures, since in humans, lesions in lateral thalamus and sensory cortex lead to a loss of sensory discrimination perceptions (Head and Holmes, 1911; Ploner et al., 1999) and lesions in ACC lead to emotional indifference to a stimulus normally causing injury and pain (Ballantine et al., 1967; Foltz and White, 1962; Freeman and Watts, 1948). Moreover, with the involvement of segregated neuronal substrates at the primary sensory, spinal, and PB levels, reflexive-defensive assays fail to detect the selective loss of acute affective tonic pain (Deng et al., 2020; Huang et al., 2019). Nonetheless, it should be noted that some primary sensory neurons such as Mrgprd+ neurons, whose activation can drive reflexive responses but fail to produce aversion under naïve conditions, can gain ability to access affective pain pathways under pathological conditions. As such, we do not need to immediately throw away drugs screened from reflex assays, such as a drug capable of silencing Mrgprd+ neurons, and we instead need to carry out additional affective pain assays to re-determine if such drugs can block certain affective pain.
Secondly, pain is recently re-defined to as “an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage”. It seems that this definition does not concretely address different phases of acute pain (the first/second pain versus tonic pain) that have different means or purposes. As discussed above, the rapid-onset short-lasting phasic pain, including first sharp-pricking pain mediated via Aδ nociceptors and the second burning pain mediated via ready-to-act mechanically/thermally sensitive C-fiber nociceptors, may encode threat levels of external stimuli (Fields, 1999). In animal studies, phasic pain may drive a set of exteroceptive behaviors requiring the involvement of cortical structures and amygdala, such as operant escape and guarding responses (Corder et al., 2019; Fuchs et al., 2014; Vierck and Yezierski, 2015). From the functionality point of view, these behaviors serve to avoid a re-exposure to the ongoing presence of harmful stimuli, thereby still dominant with exteroception. In contrast, after completion of reflexive-defensive responses, tonic pain associated with actual body injury (for example, after skin burn injury) will drive recuperative responses (such as sustained licking) to soothe suffering, which is partially mediated via chemo-nociceptors that respond to mediators released from injured tissues, thereby representing an interoceptive sense of body integrity disruption. Beecher long recognized that tonic pain has a larger role in encoding the unpleasant emotional experience of pain in comparison with phasic pain (Beecher, 1957, 1966). Echoing this argument, recent brain imaging studies show that acute tonic pain and clinical chronic pain display similar network-level representations, distinct from the representation associated with phasic pain (Lee et al., 2021). With this differential clinical relevance, it is important to use existing assays and develop new assays that can distinguish different phases of acute pain, such as using post-stimulation guarding for phasic pain and persistent licking for tonic pain. It should be noted that both guarding and persistent licking represent motor outputs, whose generation may involve intermediate circuits; thus, despite cortical dependence for these behaviors, their loss alone following a treatment may not necessarily indicate a loss of phasic or tonic pain per se. Other studies are needed to consolidate the conclusion. For example, when we studied the role of spinal Tac1 neurons in driving tonic pain, we needed to i) use stimuli producing tonic pain in humans (such as skin burn injury and persistent skin pinching), ii) show a direct and/or indirection connection of these neurons to the medial thalamic-ACC pathways that drive the affective and emotional component of pain, iii) demonstrate the necessity of these neurons for painful stimuli to drive conditioned aversive learning and to activate brain centers crucial for affective pain processing (Huang et al., 2019).
Thirdly, chronic pain measurement may face various scenarios. First of all, threshold-level withdrawal responses to light von Fey filament stimulation no longer measure the sensitivity of mechanonociceptors, but rather serve as an indirect readout of central sensitization that allows Aβ-LTMRs to drive reflexes in response to innocuous stimuli. Since separate spinal neurons drive reflexes versus tonic pain under acute conditions, it is possible that Aβ-LTMRs may be connected to distinct spinal substrates in driving sensitized reflexes versus affective allodynia (pain evoked by innocuous stimuli). Evoked affective chronic pain could be measured via CPA assays, such as brush-evoked CPA for measuring one of most prevalent and bothersome forms of neuropathic mechanical pain-the morphine resistant allodynia evoked by dynamic tactile stimulation across the skin (Cheng et al., 2017; Truini et al., 2013), or via ACC-dependent operant escape assays (LaGraize et al., 2004). The presence of spontaneous chronic pain could be measured via the ACC-dependent conditioned place preference assay in response to an analgesic treatment, with a careful control showing that the drug does not directly produce a reward in naïve mice (King et al., 2009; Navratilova et al., 2015; Navratilova et al., 2012; Qu et al., 2011; Sufka, 1994). As discussed above, guarding behaviors, whose manifestation also requires ACC and amygdala, could potentially be used to measure phasic pain; for animals and humans with allodynia, spontaneous development of hypervigilant guarding may indicate the real-time occurrence of pain episodes or a form of conditioned fear learning evoked by unconditioned pain associated with daily life activity (Meulders, 2020; Vlaeyen and Crombez, 2020). However, animal models with robust hypervigilant guarding need to be developed, besides musculoskeletal pain models (see below). Facial grimace may represent another assay for measuring phasic or spontaneous pain evoked during daily life activity in some chronic pain models (Akintola et al., 2017; Dolensek et al., 2020; Langford et al., 2010; Mogil, 2018; Tuttle et al., 2018), although lesion studies need to be carried out to identify brain structures involved in this behavior. By using these affective pain assays, it was reported that loss of dynamic allodynia in mice with ablation of spinal VGLUT3 lineage neurons failed to be detected by sensitized reflexive assays (Cheng et al., 2017; Peirs et al., 2021). Forebrain studies also show that reflex assays fail to detect the loss of evoked or spontaneous affective pain (Corder et al., 2019; Gomtsian et al., 2018; Qu et al., 2011; Yan et al., 2012). Accordingly, a drug that selectively blocks sensitized reflexes might fail to relieve affective pain in human clinical trials, and most troublingly, an idea drug that selectively blocks affective pain would be discarded during preclinical studies if only reflexive responses are measured. Nonetheless, if a drug targets Aβ-LTMRs necessary for the expression of both sensitized reflexes and pain or targets peripheral nociceptors or central glia cells that drive central sensitization, a loss of sensitized reflex might indicate a loss of sensitized pain. As such, we again should not immediately throw away drugs previously screened from sensitized reflex assays, but instead we need to perform new assays to determine if they can relieve chronic affective pain.
Fourthly, most behaviors discussed above are evoked from the skin. There is a clear paradox. While clinical pain is often from deep tissues, such as muscles, bones, joints and visceral organs, preclinical studies have been predominantly measuring pain from the skin (Cervero, 2009; Gebhart and Bielefeldt, 2016; Queme et al., 2017). For visceral pain, recent studies have begun to suggest different neuronal substrates in driving sensitized reflexes versus affective pain. ACC lesions in rodents caused a loss of inflammatory affective pain measured through CPA evoked by colorectal distension, without affecting sensitized visceromotor reflexes measured by abdominal muscle contraction (Yan et al., 2012). Visceromotor reflexes might increase intraperitoneal pressure and help get rid of harmful objects within the gastrointestinal tract (a semi-external world), thereby representing a defensive reaction. Visceral affective pain can also be measured via recording poststimulation ultrasound vocalization (Ji et al., 2015) or the real-time passive avoidance assay (also called the step-down assay) (Ness and Gebhart, 1988; Ness et al., 1991). Using the step-down assay, unpublished studies from my lab show that there are distinct spinal substrates driving sensitized visceral pain versus sensitized visceromotor reflexes, mimicking the phenotype seen in mice with ACC lesions and suggesting that the commonly used visceromotor reflex assay fails to detect a selective loss of affective visceral pain. A notable feature of chronic visceral pain and deep muscle/bone pain is the manifestation of referred mechanical hypersensitivity in the skin, due to convergent inputs from cutaneous and deep tissue sensory afferents onto the same spinal substrates (Cervero, 2009; Gebhart and Bielefeldt, 2016). As discussed above, referred sensitized reflexes are, however, an indirect readout of central sensitization and may or may not measure the affective component of pain. As such, CRD-evoked CPA/step-down assays or poststimulation ultrasound vocalization assays may be included to measure affective visceral pain and emotional distress. Animals with muscle or bone injury do develop spontaneous guarding (Mantyh, 2014; Ross et al., 2014), which may help measure movement-caused pain or referred allodynia.
9. Conclusion and potential future directions
The above analyses argue for a functional subdivision of the somatosensory system into two branches: i) an exteroceptive branch driving reflexive-defensive responses to external threats and serving to prevent tissue injury and ii) an interoceptive branch driving recuperative responses to internal body injury and serving to reduce suffering associated with injury-evoked tonic pain. Under pathological conditions, the functional segregation of the exteroceptive and interoceptive branches is, however, disrupted, allowing both branches to drive pain and comorbidity. Against this backdrop, translational success could be improved by developing pain models and behavioral assays that measure clinically more relevant tonic pain and chronic affective pain, as discussed above. Towards this end, the presence of redundant chronic pain/comorbidity pathways driven by both exteroceptive and interoceptive branches, combined with mechanistic degeneracy in activating a given pathway (Latremoliere and Woolf, 2009; Matsuda et al., 2019; Ratte et al., 2014), might represent the biggest hurdle for translational success. Identification and targeting of neurons receiving convergent inputs from multiple pathways, such as ACC neurons, might be prioritized. Alternatively, activation of inhibitory neurons that can block multiple pathways could represent yet another strategy. For example, a small cluster of neurons in the CeA send inhibitory outputs to multiple sensory relay stations, whose activation by low dose anesthetic compounds can powerfully suppress acute tonic pain and chronic pain without causing sedation (Hua et al., 2020). Transplantation of precursor cells that can differentiate into inhibitory neurons may offer another solution, via restoring spinal inhibitory gate control that can broadly dampen somatosensory information transmission (Braz et al., 2017; Bráz et al., 2012; Manion et al., 2020). Finally, we may need to pay more attention to the root of diseases that drive redundant pain pathways, such as peripheral and central inflammation caused by complex interactions among nerves, glia, immune cells and target tissues (Jain et al., 2020; Ji et al., 2016; Kavelaars and Heijnen, 2021; Pinho-Ribeiro et al., 2017; Tao et al., 2020).
ACKNOWLEGMENTS
I thank Robert H. LaMotte, Martyn Goulding, Liqun Luo and three reviewers for critical comments on the manuscript, Charles D. Stiles for relentless pushing and encouragement, and my former and current lab members for stimulating discussions. The lab is currently supported by the National Institute of Health, United States (R01DK122833 and R01AT010629) and the Wellcome Trust (200183/Z/15/Z).
Footnotes
DECLARATION OF INTERESTS
The author declares no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abdel Samad O, Liu Y, Yang FC, Kramer I, Arber S, and Ma Q (2010). Characterization of two Runx1-dependent nociceptor differentiation programs necessary for inflammatory versus neuropathic pain. Mol Pain 6, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdus-Saboor I, Fried NT, Lay M, Burdge J, Swanson K, Fischer R, Jones J, Dong P, Cai W, Guo X, et al. (2019). Development of a Mouse Pain Scale Using Sub-second Behavioral Mapping and Statistical Modeling. Cell Rep 28, 1623–1634 e1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraira VE, and Ginty DD (2013). The sensory neurons of touch. Neuron 79, 618–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraira VE, Kuehn ED, Chirila AM, Springel MW, Toliver AA, Zimmerman AL, Orefice LL, Boyle KA, Bai L, Song BJ, et al. (2017). The Cellular and Synaptic Architecture of the Mechanosensory Dorsal Horn. Cell 168, 295–310 e219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerley R, and Watkins RH (2018). Microneurography as a tool to study the function of individual C-fiber afferents in humans: responses from nociceptors, thermoreceptors, and mechanoreceptors. J Neurophysiol 120, 2834–2846. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, and Damasio AR (2005). A mechanism for impaired fear recognition after amygdala damage. Nature 433, 68–72. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, and Damasio A (1994). Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 372, 669–672. [DOI] [PubMed] [Google Scholar]
- Adriaensen H, Gybels J, Handwerker HO, and Van Hees J (1984). Nociceptor discharges and sensations due to prolonged noxious mechanical stimulation--a paradox. Hum Neurobiol 3, 53–58. [PubMed] [Google Scholar]
- Akintola T, Raver C, Studlack P, Uddin O, Masri R, and Keller A (2017). The grimace scale reliably assesses chronic pain in a rodent model of trigeminal neuropathic pain. Neurobiol Pain 2, 13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amit Z, and Galina ZH (1986). Stress-induced analgesia: adaptive pain suppression. Physiol Rev 66, 1091–1120. [DOI] [PubMed] [Google Scholar]
- Ammons WS, Girardot MN, and Foreman RD (1985). T2-T5 spinothalamic neurons projecting to medial thalamus with viscerosomatic input. J Neurophysiol 54, 73–89. [DOI] [PubMed] [Google Scholar]
- Arcourt A, Gorham L, Dhandapani R, Prato V, Taberner FJ, Wende H, Gangadharan V, Birchmeier C, Heppenstall PA, and Lechner SG (2017). Touch Receptor-Derived Sensory Information Alleviates Acute Pain Signaling and Fine-Tunes Nociceptive Reflex Coordination. Neuron 93, 179–193. [DOI] [PubMed] [Google Scholar]
- Arenas OM, Zaharieva EE, Para A, Vasquez-Doorman C, Petersen CP, and Gallio M (2017). Activation of planarian TRPA1 by reactive oxygen species reveals a conserved mechanism for animal nociception. Nat Neurosci 20, 1686–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba H, Ji RR, Kohno T, Moore KA, Ataka T, Wakai A, Okamoto M, and Woolf CJ (2003). Removal of GABAergic inhibition facilitates polysynaptic A fiber-mediated excitatory transmission to the superficial spinal dorsal horn. Mol Cell Neurosci 24, 818–830. [DOI] [PubMed] [Google Scholar]
- Bachy I, Franck MC, Li L, Abdo H, Pattyn A, and Ernfors P (2011). The transcription factor Cux2 marks development of an A-delta sublineage of TrkA sensory neurons. Dev Biol 360, 77–86. [DOI] [PubMed] [Google Scholar]
- Baliki MN, and Apkarian AV (2015). Nociception, Pain, Negative Moods, and Behavior Selection. Neuron 87, 474–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantine HT Jr., Cassidy WL, Flanagan NB, and Marino R Jr. (1967). Stereotaxic anterior cingulotomy for neuropsychiatric illness and intractable pain. J Neurosurg 26, 488–495. [DOI] [PubMed] [Google Scholar]
- Barik A, Sathyamurthy A, Thompson J, Seltzer M, Levine A, and Chesler A (2021). A spinoparabrachial circuit defined by Tacr1 expression drives pain. Elife 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barik A, Thompson JH, Seltzer M, Ghitani N, and Chesler AT (2018). A Brainstem-Spinal Circuit Controlling Nocifensive Behavior. Neuron 100, 1491–1503 e1493. [DOI] [PubMed] [Google Scholar]
- Barrett LF (2018). Seeing Fear: It’s All in the Eyes? Trends Neurosci 41, 559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, and Julius D (2007). The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448, 204–208. [DOI] [PubMed] [Google Scholar]
- Bay HH, and Cavdar S (2013). Regional connections of the mediodorsal thalamic nucleus in the rat. J Integr Neurosci 12, 201–219. [DOI] [PubMed] [Google Scholar]
- Beaudry H, Daou I, Ase AR, Ribeiro-da-Silva A, and Seguela P (2017). Distinct behavioral responses evoked by selective optogenetic stimulation of the major TRPV1+ and MrgD+ subsets of C-fibers. Pain 158, 2329–2339. [DOI] [PubMed] [Google Scholar]
- Beaulieu-Laroche L, Christin M, Donoghue A, Agosti F, Yousefpour N, Petitjean H, Davidova A, Stanton C, Khan U, Dietz C, et al. (2020). TACAN Is an Ion Channel Involved in Sensing Mechanical Pain. Cell 180, 956–967 e917. [DOI] [PubMed] [Google Scholar]
- Beecher HK (1946). Pain in Men Wounded in Battle. Ann Surg 123, 96–105. [PMC free article] [PubMed] [Google Scholar]
- Beecher HK (1957). The measurement of pain; prototype for the quantitative study of subjective responses. Pharmacol Rev 9, 59–209. [PubMed] [Google Scholar]
- Beecher HK (1966). Pain: one mystery solved. Science 151, 840–841. [DOI] [PubMed] [Google Scholar]
- Benabid AL, and Jeaugey L (1989). Cells of the rat lateral habenula respond to high-threshold somatosensory inputs. Neurosci Lett 96, 289–294. [DOI] [PubMed] [Google Scholar]
- Berthier M, Starkstein S, and Leiguarda R (1988). Asymbolia for pain: a sensory-limbic disconnection syndrome. Ann Neurol 24, 41–49. [DOI] [PubMed] [Google Scholar]
- Bester H, Bourgeais L, Villanueva L, Besson JM, and Bernard JF (1999). Differential projections to the intralaminar and gustatory thalamus from the parabrachial area: a PHA-L study in the rat. J Comp Neurol 405, 421–449. [PubMed] [Google Scholar]
- Bishop GH (1946). Neural mechanisms of cutaneous sense. Physiol Rev 26, 77–102. [DOI] [PubMed] [Google Scholar]
- Blivis D, Haspel G, Mannes PZ, O’Donovan MJ, and Iadarola MJ (2017). Identification of a novel spinal nociceptive-motor gate control for Adelta pain stimuli in rats. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohic M, and Abraira VE (2021). Wired for social touch:the sense that binds us to others. Current Opinion in bBobehavioral Sciences 43, 207–215. [Google Scholar]
- Bokiniec P, Zampieri N, Lewin GR, and Poulet JF (2018). The neural circuits of thermal perception. Curr Opin Neurobiol 52, 98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolles RC, and Fanselow MS (1982). Endorphins and behavior. Annu Rev Psychol 33, 87–101. [DOI] [PubMed] [Google Scholar]
- Borsook D, Hargreaves R, Bountra C, and Porreca F (2014). Lost but making progress--Where will new analgesic drugs come from? Sci Transl Med 6, 249sr243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D, Linnman C, Faria V, Strassman AM, Becerra L, and Elman I (2016). Reward deficiency and anti-reward in pain chronification. Neurosci Biobehav Rev 68, 282–297. [DOI] [PubMed] [Google Scholar]
- Bourane S, Duan B, Koch SC, Dalet A, Britz O, Garcia-Campmany L, Kim E, Cheng L, Ghosh A, Ma Q, et al. (2015). Gate control of mechanical itch by a subpopulation of spinal cord interneurons. Science 350, 550–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeais L, Monconduit L, Villanueva L, and Bernard JF (2001). Parabrachial internal lateral neurons convey nociceptive messages from the deep laminas of the dorsal horn to the intralaminar thalamus. J Neurosci 21, 2159–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourinet E, Martin M, Huzard D, Jeanneteau F, Mery P-F, and Francois A (2021). The impact of C-tactile low threshold mechanoreceptors on affective touch and social interactions in mice. bioRxiv 10.1101/2021.01.13.426492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braz JM, Etlin A, Juarez-Salinas D, Llewellyn-Smith IJ, and Basbaum AI (2017). Rebuilding CNS inhibitory circuits to control chronic neuropathic pain and itch. Prog Brain Res 231, 87–105. [DOI] [PubMed] [Google Scholar]
- Bráz JM, Sharif-Naeini R, Vogt D, Kriegstein A, Alvarez-Buylla A, Rubenstein JL, and Basbaum AI (2012). Forebrain GABAergic neuron precursors integrate into adult spinal cord and reduce injury-induced neuropathic pain. Neuron 74, 663–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner DS, Golden JP, and Gereau R.W.t. (2012). A novel behavioral assay for measuring cold sensation in mice. PLoS One 7, e39765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromm B, and Treede DR (1980). Withdrawal reflex, skin resistance reaction and pain ratings due to electrical stimuli in man. Pain 9, 339–354. [DOI] [PubMed] [Google Scholar]
- Browne LE, Latremoliere A, Lehnert BP, Grantham A, Ward C, Alexandre C, Costigan M, Michoud F, Roberson DP, Ginty DD, et al. (2017). Time-Resolved Fast Mammalian Behavior Reveals the Complexity of Protective Pain Responses. Cell Rep 20, 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell MC, Ceko M, and Low LA (2013). Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 14, 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler RK, and Finn DP (2009). Stress-induced analgesia. Prog Neurobiol 88, 184–202. [DOI] [PubMed] [Google Scholar]
- Campos CA, Bowen AJ, Roman CW, and Palmiter RD (2018). Encoding of danger by parabrachial CGRP neurons. Nature 555, 617–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr VM, and Simpson SB Jr. (1978). Proliferative and degenerative events in the early development of chick dorsal root ganglia. I. Normal development. J Comp Neurol 182, 727–739. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, and Julius D (1997). The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824 [DOI] [PubMed] [Google Scholar]
- Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, and Anderson DJ (2009). Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci U S A 106, 9075–9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervero F (2009). Visceral versus somatic pain: similarities and differences. Dig Dis 27 Suppl 1, 3–10. [DOI] [PubMed] [Google Scholar]
- Chen CL, Broom DC, Liu Y, de Nooij JC, Li Z, Cen C, Samad OA, Jessell TM, Woolf CJ, and Ma Q (2006). Runx1 determines nociceptive sensory neuron phenotype and is required for thermal and neuropathic pain. Neuron 49, 365–377. [DOI] [PubMed] [Google Scholar]
- Chen WG, Schloesser D, Arensdorf AM, Simmons JM, Cui C, Valentino R, Gnadt JW, Nielsen L, Hillaire-Clarke CS, Spruance V, et al. (2021). The Emerging Science of Interoception: Sensing, Integrating, Interpreting, and Regulating Signals within the Self. Trends Neurosci 44, 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Duan B, Huang T, Zhang Y, Chen Y, Britz O, Garcia-Campmany L, Ren X, Vong L, Lowell BB, et al. (2017). Identification of spinal circuits involved in touch-evoked dynamic mechanical pain. Nat Neurosci 20, 804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang MC, Nguyen EK, Canto-Bustos M, Papale AE, Oswald AM, and Ross SE (2020). Divergent Neural Pathways Emanating from the Lateral Parabrachial Nucleus Mediate Distinct Components of the Pain Response. Neuron 106, 927–939 e925. [DOI] [PubMed] [Google Scholar]
- Choi S, Hachisuka J, Brett MA, Magee AR, Omori Y, Iqbal NU, Zhang D, DeLisle MM, Wolfson RL, Bai L, et al. (2020). Parallel ascending spinal pathways for affective touch and pain. Nature 587, 258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen AJ, Iyer SM, Francois A, Vyas S, Ramakrishnan C, Vesuna S, Deisseroth K, Scherrer G, and Delp SL (2016). In Vivo Interrogation of Spinal Mechanosensory Circuits. Cell Rep 17, 1699–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobos EJ, and Portillo-Salido E (2013). “Bedside-to-Bench” Behavioral Outcomes in Animal Models of Pain: Beyond the Evaluation of Reflexes. Curr Neuropharmacol 11, 560–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper BY, Vierck CJ Jr., and Yeomans DC (1986). Selective reduction of second pain sensations by systemic morphine in humans. Pain 24, 93–116. [DOI] [PubMed] [Google Scholar]
- Corder G, Ahanonu B, Grewe BF, Wang D, Schnitzer MJ, and Scherrer G (2019). An amygdalar neural ensemble that encodes the unpleasantness of pain. Science 363, 276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder G, Tawfik VL, Wang D, Sypek EI, Low SA, Dickinson JR, Sotoudeh C, Clark JD, Barres BA, Bohlen CJ, et al. (2017). Loss of mu opioid receptor signaling in nociceptors, but not microglia, abrogates morphine tolerance without disrupting analgesia. Nat Med 23, 164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD (2009). How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci 10, 59–70. [DOI] [PubMed] [Google Scholar]
- Cranfill SL, and Luo W (2021). The development of somatosensory neurons: Insights into pain and itch. Curr Top Dev Biol 142, 443–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Miao X, Liang L, Abdus-Saboor I, Olson W, Fleming MS, Ma M, Tao YX, and Luo W (2016). Identification of Early RET+ Deep Dorsal Spinal Cord Interneurons in Gating Pain. Neuron 91, 1413. [DOI] [PubMed] [Google Scholar]
- del Camino D, Murphy S, Heiry M, Barrett LB, Earley TJ, Cook CA, Petrus MJ, Zhao M, D’Amours M, Deering N, et al. (2010). TRPA1 contributes to cold hypersensitivity. J Neurosci 30, 15165–15174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfini MC, Mantilleri A, Gaillard S, Hao J, Reynders A, Malapert P, Alonso S, François A, Barrere C, Seal R, et al. (2013). TAFA4, a chemokine-like protein, modulates injury-induced mechanical and chemical pain hypersensitivity in mice. Cell Rep 5, 378–388. [DOI] [PubMed] [Google Scholar]
- Deng J, Zhou H, Lin JK, Shen ZX, Chen WZ, Wang LH, Li Q, Mu D, Wei YC, Xu XH, et al. (2020). The Parabrachial Nucleus Directly Channels Spinal Nociceptive Signals to the Intralaminar Thalamic Nuclei, but Not the Amygdala. Neuron 107, 909–923 e906. [DOI] [PubMed] [Google Scholar]
- Deuis JR, Dvorakova LS, and Vetter I (2017). Methods Used to Evaluate Pain Behaviors in Rodents. Front Mol Neurosci 10, 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, and Vogt BA (1995). Contributions of anterior cingulate cortex to behaviour. Brain 118 ( Pt 1), 279–306. [DOI] [PubMed] [Google Scholar]
- Dhaka A, Earley TJ, Watson J, and Patapoutian A (2008). Visualizing cold spots: TRPM8-expressing sensory neurons and their projections. Journal of Neuroscience 28, 566–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, and Patapoutian A (2007). TRPM8 is required for cold sensation in mice. Neuron 54, 371–378. [DOI] [PubMed] [Google Scholar]
- Dolensek N, Gehrlach DA, Klein AS, and Gogolla N (2020). Facial expressions of emotion states and their neuronal correlates in mice. Science 368, 89–94. [DOI] [PubMed] [Google Scholar]
- Donahue RR, LaGraize SC, and Fuchs PN (2001). Electrolytic lesion of the anterior cingulate cortex decreases inflammatory, but not neuropathic nociceptive behavior in rats. Brain Res 897, 131–138. [DOI] [PubMed] [Google Scholar]
- Dong WK, Hayashi T, Roberts VJ, Fusco BM, and Chudler EH (1996). Behavioral outcome of posterior parietal cortex injury in the monkey. Pain 64, 579–587. [DOI] [PubMed] [Google Scholar]
- Dong WK, Ryu H, and Wagman IH (1978). Nociceptive responses of neurons in medial thalamus and their relationship to spinothalamic pathways. J Neurophysiol 41, 1592–1613. [DOI] [PubMed] [Google Scholar]
- Duan B, Cheng L, Bourane S, Britz O, Padilla C, Garcia-Campmany L, Krashes M, Knowlton W, Velasquez T, Ren X, et al. (2014). Identification of spinal circuits transmitting and gating mechanical pain. Cell 159, 1417–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan B, Cheng L, and Ma Q (2018). Spinal Circuits Transmitting Mechanical Pain and Itch. Neurosci Bull 34, 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuisson D, and Dennis SG (1977). The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain 4, 161–174. [DOI] [PubMed] [Google Scholar]
- Emery EC, and Ernfors P (2018). Dorsal root ganglion neuron types and their functional specialization. In: Wood JN (Ed.), The Oxford Handbook of the Neurobiology of Pain. Oxford University Press [Google Scholar]
- Feinstein JS, Buzza C, Hurlemann R, Follmer RL, Dahdaleh NS, Coryell WH, Welsh MJ, Tranel D, and Wemmie JA (2013). Fear and panic in humans with bilateral amygdala damage. Nat Neurosci 16, 270–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL (1999). Pain: an unpleasant topic. Pain Suppl 6, S61–69. [DOI] [PubMed] [Google Scholar]
- Fillinger C, Yalcin I, Barrot M, and Veinante P (2017). Afferents to anterior cingulate areas 24a and 24b and midcingulate areas 24a’ and 24b’ in the mouse. Brain Struct Funct 222, 1509–1532. [DOI] [PubMed] [Google Scholar]
- Foltz EL, and White LE Jr. (1962). Pain “relief” by frontal cingulumotomy. J Neurosurg 19, 89–100. [DOI] [PubMed] [Google Scholar]
- Foster E, Wildner H, Tudeau L, Haueter S, Ralvenius WT, Jegen M, Johannssen H, Hösli L, Haenraets K, Ghanem A, et al. (2015). Targeted ablation, silencing, and activation establish glycinergic dorsal horn neurons as key components of a spinal gate for pain and itch. Neuron 85, 1289–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes T, and Wood JN (2007). Mechanisms of cold pain. Channels (Austin) 1, 154–160. [DOI] [PubMed] [Google Scholar]
- Freeman W, and Watts JW (1948). Pain mechanisms and the frontal lobes; a study of prefrontal lobotomy for intractable pain. Ann Intern Med 28, 747–754. [DOI] [PubMed] [Google Scholar]
- Fuchs PN, and McNabb CT (2012). The place escape/avoidance paradigm: a novel method to assess nociceptive processing. J Integr Neurosci 11, 61–72. [DOI] [PubMed] [Google Scholar]
- Fuchs PN, Peng YB, Boyette-Davis JA, and Uhelski ML (2014). The anterior cingulate cortex and pain processing. Front Integr Neurosci 8, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulwiler CE, and Saper CB (1984). Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res 319, 229–259. [DOI] [PubMed] [Google Scholar]
- Gao DM, Jeaugey L, Pollak P, and Benabid AL (1990). Intensity-dependent nociceptive responses from presumed dopaminergic neurons of the substantia nigra, pars compacta in the rat and their modification by lateral habenula inputs. Brain Res 529, 315–319. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Ren WH, Zhang YQ, and Zhao ZQ (2004). Contributions of the anterior cingulate cortex and amygdala to pain- and fear-conditioned place avoidance in rats. Pain 110, 343–353. [DOI] [PubMed] [Google Scholar]
- Gascon E, Gailard S, Malpert P, Liu Y, Radat-DEspoix L, Samokhvalov IM, Delmas P, Helmbacher F, Maina F, and Moqrich A (2010). HGF-Met signaling is required for Runx1 extinction and peptidergic differentiation in primary nociceptive neurons. Journal of Neuroscience (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto G, Bourane S, Ren X, Di Costanzo S, Fenton PK, Halder P, Seal RP, and Goulding MD (2021). A Functional Topographic Map for Spinal Sensorimotor Reflexes. Neuron 109, 91–104 e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauriau C, and Bernard JF (2002). Pain pathways and parabrachial circuits in the rat. Exp Physiol 87, 251–258. [DOI] [PubMed] [Google Scholar]
- Gebhart GF, and Bielefeldt K (2016). Physiology of Visceral Pain. Compr Physiol 6, 1609–1633. [DOI] [PubMed] [Google Scholar]
- Geerling JC, Kim M, Mahoney CE, Abbott SB, Agostinelli LJ, Garfield AS, Krashes MJ, Lowell BB, and Scammell TE (2016). Genetic identity of thermosensory relay neurons in the lateral parabrachial nucleus. Am J Physiol Regul Integr Comp Physiol 310, R41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitani N, Barik A, Szczot M, Thompson JH, Li C, Le Pichon CE, Krashes MJ, and Chesler AT (2017). Specialized Mechanosensory Nociceptors Mediating Rapid Responses to Hair Pull. Neuron 95, 944–954 e944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesler GJ Jr., Yezierski RP, Gerhart KD, and Willis WD (1981). Spinothalamic tract neurons that project to medial and/or lateral thalamic nuclei: evidence for a physiologically novel population of spinal cord neurons. J Neurophysiol 46, 1285–1308. [DOI] [PubMed] [Google Scholar]
- Gomtsian L, Bannister K, Eyde N, Robles D, Dickenson AH, Porreca F, and Navratilova E (2018). Morphine effects within the rodent anterior cingulate cortex and rostral ventromedial medulla reveal separable modulation of affective and sensory qualities of acute or chronic pain. Pain 159, 2512–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh A, Krieger P, Mease RA, and Henderson L (2018). Acute and Chronic Pain Processing in the Thalamocortical System of Humans and Animal Models. Neuroscience 387, 58–71. [DOI] [PubMed] [Google Scholar]
- Guillery RW, and Harting JK (2003). Structure and connections of the thalamic reticular nucleus: Advancing views over half a century. J Comp Neurol 463, 360–371. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Mecinas M, Bell AM, Marin A, Taylor R, Boyle KA, Furuta T, Watanabe M, Polgar E, and Todd AJ (2017). Preprotachykinin A is expressed by a distinct population of excitatory neurons in the mouse superficial spinal dorsal horn including cells that respond to noxious and pruritic stimuli. Pain 158, 440–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Soleiman MT, Soden ME, Zweifel LS, and Palmiter RD (2015). Elucidating an Affective Pain Circuit that Creates a Threat Memory. Cell 162, 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerker HO, and Kobal G (1993). Psychophysiology of experimentally induced pain. Physiol Rev 73, 639–671. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, and Joris J (1988). A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32, 77–88. [DOI] [PubMed] [Google Scholar]
- Head H, and Holmes G (1911). Sensory disturbances from cerebral lesions. Brain 34, 102–154. [Google Scholar]
- Head H, Rivers WHR, and Sherren FRCS (1905). the afferent nervous system from a new aspect. Brain 28, 100–115. [Google Scholar]
- Helmstetter FJ (1992). The amygdala is essential for the expression of conditional hypoalgesia. Behav Neurosci 106, 518–528. [DOI] [PubMed] [Google Scholar]
- Herkenham M, and Nauta WJ (1979). Efferent connections of the habenular nuclei in the rat. J Comp Neurol 187, 19–47. [DOI] [PubMed] [Google Scholar]
- Hermanson O, and Blomqvist A (1997). Differential expression of the AP-1/CRE-binding proteins FOS and CREB in preproenkephalin mRNA-expressing neurons of the rat parabrachial nucleus after nociceptive stimulation. Brain Res Mol Brain Res 51, 188–196. [DOI] [PubMed] [Google Scholar]
- Hill RZ, and Bautista DM (2020). Getting in Touch with Mechanical Pain Mechanisms. Trends Neurosci 43, 311–325. [DOI] [PubMed] [Google Scholar]
- Hu H, Cui Y, and Yang Y (2020). Circuits and functions of the lateral habenula in health and in disease. Nat Rev Neurosci 21, 277–295. [DOI] [PubMed] [Google Scholar]
- Hu HJ, Carrasquillo Y, Karim F, Jung WE, Nerbonne JM, Schwarz TL, and Gereau R.W.t. (2006). The kv4.2 potassium channel subunit is required for pain plasticity. Neuron 50, 89–100. [DOI] [PubMed] [Google Scholar]
- Hua T, Chen B, Lu D, Sakurai K, Zhao S, Han BX, Kim J, Yin L, Chen Y, Lu J, et al. (2020). General anesthetics activate a potent central pain-suppression circuit in the amygdala. Nat Neurosci 23, 854–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Grady FS, Peltekian L, and Geerling JC (2021a). Efferent projections of Vglut2, Foxp2, and Pdyn parabrachial neurons in mice. J Comp Neurol 529, 657–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Grady FS, Peltekian L, Laing JJ, and Geerling JC (2021b). Efferent projections of CGRP/Calca-expressing parabrachial neurons in mice. J Comp Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T, Lin SH, Malewicz NM, Zhang Y, Zhang Y, Goulding M, LaMotte RH, and Ma Q (2019). Identifying the pathways required for coping behaviours associated with sustained pain. Nature 565, 86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes DI, and Todd AJ (2020). Central Nervous System Targets: Inhibitory Interneurons in the Spinal Cord. Neurotherapeutics 17, 874–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata K, Miyachi S, Imanishi M, Tsuboi Y, Kitagawa J, Teramoto K, Hitomi S, Shinoda M, Kondo M, and Takada M (2011). Ascending multisynaptic pathways from the trigeminal ganglion to the anterior cingulate cortex. Exp Neurol 227, 69–78. [DOI] [PubMed] [Google Scholar]
- Jain A, Hakim S, Woolf CJ (2020) Unraveling the Plastic Peripheral Neuroimmune Interactome. J Immunol 204, 257–263. [DOI] [PubMed] [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, Saper CB, and Holland PC (2009). The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron 61, 786–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Li Z, and Neugebauer V (2015). Reactive oxygen species mediate visceral pain-related amygdala plasticity and behaviors. Pain 156, 825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Chamessian A, and Zhang YQ (2016). Pain regulation by non-neuronal cells and inflammation. Science 354, 572–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Nackley A, Huh Y, Terrando N, and Maixner W (2018). Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 129, 343–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Andrade JM, Mantyh WG, Bloom AP, Xu H, Ferng AS, Dussor G, Vanderah TW, and Mantyh PW (2010). A phenotypically restricted set of primary afferent nerve fibers innervate the bone versus skin: therapeutic opportunity for treating skeletal pain. Bone 46, 306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Fields HL, and Manning BH (2001). The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc Natl Acad Sci U S A 98, 8077–8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG, and Leavitt RY (1974). Retrograde axonal transport and the demonstration of non-specific projections to the cerebral cortex and striatum from thalamic intralaminar nuclei in the rat, cat and monkey. J Comp Neurol 154, 349–377. [DOI] [PubMed] [Google Scholar]
- Kavelaars A, and Heijnen CJ (2021). Immune regulation of pain: Friend and foe. Sci Transl Med 13, eabj7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keay KA, and Bandler R (2001). Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev 25, 669–678. [DOI] [PubMed] [Google Scholar]
- Kiasalari Z, Salehi I, Zhong Y, McMahon SB, Michael-Titus AT, and Michael GJ (2010). Identification of perineal sensory neurons activated by innocuous heat. J Comp Neurol 518, 137–162. [DOI] [PubMed] [Google Scholar]
- King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, and Porreca F (2009). Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci 12, 1364–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleggetveit IP, Namer B, Schmidt R, Helas T, Ruckel M, Orstavik K, Schmelz M, and Jorum E (2012). High spontaneous activity of C-nociceptors in painful polyneuropathy. Pain 153, 2040–2047. [DOI] [PubMed] [Google Scholar]
- Knowlton WM, Palkar R, Lippoldt EK, McCoy DD, Baluch F, Chen J, and McKemy DD (2013). A sensory-labeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia. J Neurosci 33, 2837–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch SC, Acton D, and Goulding M (2018). Spinal Circuits for Touch, Pain, and Itch. Annu Rev Physiol 80, 189–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer I, Sigrist M, de Nooij JC, Taniuchi I, Jessell TM, and Arber S (2006). A role for Runx transcription factor signaling in dorsal root ganglion sensory neuron diversification. Neuron 49, 379–393. [DOI] [PubMed] [Google Scholar]
- Krettek JE, and Price JL (1977). The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol 171, 157–191. [DOI] [PubMed] [Google Scholar]
- Kuner R, and Kuner T (2021). Cellular Circuits in the Brain and Their Modulation in Acute and Chronic Pain. Physiol Rev 101, 213–258. [DOI] [PubMed] [Google Scholar]
- Kupari J, Usoskin D, Parisien M, Lou D, Hu Y, Fatt M, Lonnerberg P, Spangberg M, Eriksson B, Barkas N, et al. (2021). Single cell transcriptomics of primate sensory neurons identifies cell types associated with chronic pain. Nat Commun 12, 1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBuda CJ, and Fuchs PN (2000). A behavioral test paradigm to measure the aversive quality of inflammatory and neuropathic pain in rats. Exp Neurol 163, 490–494. [DOI] [PubMed] [Google Scholar]
- LaGraize SC, Labuda CJ, Rutledge MA, Jackson RL, and Fuchs PN (2004). Differential effect of anterior cingulate cortex lesion on mechanical hypersensitivity and escape/avoidance behavior in an animal model of neuropathic pain. Exp Neurol 188, 139–148. [DOI] [PubMed] [Google Scholar]
- Lallemend F, and Ernfors P (2012). Molecular interactions underlying the specification of sensory neurons. Trends Neurosci Apr 17. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, Lacroix-Fralish ML, et al. (2010). Coding of facial expressions of pain in the laboratory mouse. Nat Methods 7, 447–449. [DOI] [PubMed] [Google Scholar]
- Latremoliere A, and Woolf CJ (2009). Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 10, 895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson SN, and Biscoe TJ (1979). Deveolpment of mouse dorsal root ganglia: An autoradiographic and quantitative study. J Neurocytol 8, 265–274. [DOI] [PubMed] [Google Scholar]
- Lay M, and Dong X (2020). Neural Mechanisms of Itch. Annu Rev Neurosci 43, 187–205. [DOI] [PubMed] [Google Scholar]
- Le Bars D, Gozariu M, and Cadden SW (2001). Animal models of nociception. Pharmacol Rev 53, 597–652. [PubMed] [Google Scholar]
- Lee JJ, Kim HJ, Ceko M, Park BY, Lee SA, Park H, Roy M, Kim SG, Wager TD, and Woo CW (2021). A neuroimaging biomarker for sustained experimental and clinical pain. Nat Med 27, 174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis T (1938). Study of Somatic Pain. Br Med J 1, 321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis T, and Pochin EE (1937). The double pain response of the human skin to a single stimulus. Clin Sci 3, 67. [Google Scholar]
- Li C, Wang S, Chen Y, and Zhang X (2018). Somatosensory Neuron Typing with High-Coverage Single-Cell RNA Sequencing and Functional Analysis. Neurosci Bull 34, 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CL, Li KC, Wu D, Chen Y, Luo H, Zhao JR, Wang SS, Sun MM, Lu YJ, Zhong YQ, et al. (2016). Somatosensory neuron types identified by high-coverage single-cell RNA-sequencing and functional heterogeneity. Cell Res 26, 83–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, Gong S, Jankowski MP, Luo W, Heintz N, Koerber HR, et al. (2011). The functional organization of cutaneous low-threshold mechanosensory neurons. Cell 147, 1615–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M, Mouraux A, and Iannetti GD (2013). Bypassing primary sensory cortices--a direct thalamocortical pathway for transmitting salient sensory information. Cereb Cortex 23, 1–11. [DOI] [PubMed] [Google Scholar]
- Lin Q, Zou X, and Willis WD (2000). Adelta and C primary afferents convey dorsal root reflexes after intradermal injection of capsaicin in rats. J Neurophysiol 84, 2695–2698. [DOI] [PubMed] [Google Scholar]
- Liu Q, Sikand P, Ma C, Tang Z, Han L, Li Z, Sun S, LaMotte RH, and Dong X (2012). Mechanisms of itch evoked by β-alanine. J Neurosci 32, 14532–14537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Latremoliere A, Li X, Zhang Z, Chen M, Wang X, Fang C, Zhu J, Alexandre C, Gao Z, et al. (2018). Touch and tactile neuropathic pain sensitivity are set by corticospinal projections. Nature 561, 547–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, and Ma Q (2011). Generation of somatic sensory neuron diversity and implications on sensory coding. Curr Opin Neurobiol 21, 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Yang FC, Okuda T, Dong X, Zylka MJ, Chen CL, Anderson DJ, Kuner R, and Ma Q (2008). Mechanisms of compartmentalized expression of Mrg class G-protein-coupled sensory receptors. J Neurosci 28, 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livneh Y, and Andermann ML (2021). Cellular activity in insular cortex across seconds to hours: Sensations and predictions of bodily states. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou S, Duan B, Vong L, Lowell BB, and Ma Q (2013). Runx1 controls terminal morphology and mechanosensitivity of VGLUT3-expressing C-mechanoreceptors. J Neurosci 33, 870–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou S, Pan X, Huang T, Duan B, Yang FC, Yang J, Xiong M, Liu Y, and Ma Q (2015). Incoherent feed-forward regulatory loops control segregation of C-mechanoreceptors, nociceptors, and pruriceptors. J Neurosci 35, 5317–5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovick TA (1972). The behavioural repertoire of precollicular decerebrate rats. J Physiol 226, 4P–6P. [PubMed] [Google Scholar]
- Lu Y, Dong H, Gao Y, Gong Y, Ren Y, Gu N, Zhou S, Xia N, Sun YY, Ji RR, et al. (2013). A feed-forward spinal cord glycinergic neural circuit gates mechanical allodynia. J Clin Invest 123, 4050–4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luiz AP, MacDonald DI, Santana-Varela S, Millet Q, Sikandar S, Wood JN, and Emery EC (2019). Cold sensing by NaV1.8-positive and NaV1.8-negative sensory neurons. Proc Natl Acad Sci U S A 116, 3811–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley MA, Cohen JL, Borszcz GS, Cano A, Radcliffe AM, Porter LS, Schubiner H, and Keefe FJ (2011). Pain and emotion: a biopsychosocial review of recent research. J Clin Psychol 67, 942–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Wickramasinghe SR, Savitt JM, Griffin JW, Dawson TM, and Ginty DD (2007). A Hierarchical NGF Signaling Cascade Controls Ret-Dependent and Ret-Independent Events during Development of Nonpeptidergic DRG Neurons. Neuron 54, 739–754. [DOI] [PubMed] [Google Scholar]
- Ma Q (2010). Labeled lines meet and talk: population coding of somatic sensations. Journal of Clinical Investigation 120, 3773–3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Fode C, Guillemot F, and Anderson DJ (1999). Neurogenin1 and neurogenin2 control two distinct waves of neurogenesis in developing dorsal root ganglia. Genes & Development 13, 1717–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magerl W, Fuchs PN, Meyer RA, and Treede RD (2001). Roles of capsaicin-insensitive nociceptors in cutaneous pain and secondary hyperalgesia. Brain 124, 1754–1764. [DOI] [PubMed] [Google Scholar]
- Maiaru M, Leese C, Certo M, Echeverria-Altuna I, Mangione AS, Arsenault J, Davletov B, and Hunt SP (2018). Selective neuronal silencing using synthetic botulinum molecules alleviates chronic pain in mice. Sci Transl Med 10. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Chen C, Tonegawa S, and Basbaum AI (1997). Preserved acute pain and reduced neuropathic pain in mice lacking PKCgamma. Science 278, 279–283. [DOI] [PubMed] [Google Scholar]
- Manion J, Khuong T, Harney D, Littleboy JB, Ruan T, Loo L, Costigan M, Larance M, Caron L, and Neely GG (2020). Human induced pluripotent stem cell-derived GABAergic interneuron transplants attenuate neuropathic pain. Pain 161, 379–387. [DOI] [PubMed] [Google Scholar]
- Mantyh PW (2014). The neurobiology of skeletal pain. Eur J Neurosci 39, 508–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh PW, Rogers SD, Honore P, Allen BJ, Ghilardi JR, Li J, Daughters RS, Lappi DA, Wiley RG, and Simone DA (1997. ). Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science 278, 275–279. [DOI] [PubMed] [Google Scholar]
- Mao J (2012). Current challenges in translational pain research. Trends Pharmacol Sci 33, 568–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark VH, Ervin FR, and Hackett TP (1960). Clinical aspects of stereotactic thalamotomy in the human. Part I. The treatment of chronic severe pain. Arch Neurol 3, 351–367. [DOI] [PubMed] [Google Scholar]
- Mark VH, Ervin FR, and Yakovlev PI (1963). Stereotactic thalamotomy III. The verification of anatomical lesion sites in the human thalamus. Archives of neurology 8, 528–538. [Google Scholar]
- Matsuda M, Huh Y, and Ji RR (2019). Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J Anesth 33, 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthies BK, and Franklin KBJ (1992). Formalin pain is expressed in decerebrate rats but not attenuated by morphine. Pain 51, 199–206. [DOI] [PubMed] [Google Scholar]
- McCoy ES, Taylor-Blake B, and Zylka MJ (2012). CGRPalpha-expressing sensory neurons respond to stimuli that evoke sensations of pain and itch. PLoS One 7, e36355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlone F, Wessberg J, and Olausson H (2014). Discriminative and Affective Touch: Sensing and Feeling. Neuron 82, 737–755. [DOI] [PubMed] [Google Scholar]
- McKemy DD (2018). Molecular basis of peripheral innocuous cold sensitivity. Handb Clin Neurol 156, 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer S, Santiago C, Sharma N, and Ginty DD (2021). The cellular and molecular basis of somatosensory neuron development. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack R, and Casey KL (1968). Sensory, motivational and central determinants of pain (Springfield, Thomas; ). [Google Scholar]
- Melzack R, and Wall PD (1965). Pain mechanisms: a new theory. Science 150, 971–979. [DOI] [PubMed] [Google Scholar]
- Mercer Lindsay N, Chen C, Gilam G, Mackey S, and Scherrer G (2021). Brain circuits for pain and its treatment. Sci Transl Med 13, eabj7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger M, Bueno D, and Lima LB (2017). The lateral habenula and the serotonergic system. Pharmacol Biochem Behav 162, 22–28. [DOI] [PubMed] [Google Scholar]
- Meulders A (2020). Fear in the context of pain: Lessons learned from 100 years of fear conditioning research. Behav Res Ther 131, 103635. [DOI] [PubMed] [Google Scholar]
- Middleton L, Schaffler M, Succi I, Foster W, Gradwell M, Bohic M, Ejoh L, Abraira V, and Abdus-Saboor I (2021). Identification of touch neurons underlying dopaminergic pleasurable touch and sexual receptivity. bioRxiv doi: 10.1101/2021.09.22.461355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misslin R (2003). The defense system of fear: behavior and neurocircuitry. Neurophysiol Clin 33, 55–66. [DOI] [PubMed] [Google Scholar]
- Moehring F, Halder P, Seal RP, and Stucky CL (2018). Uncovering the Cells and Circuits of Touch in Normal and Pathological Settings. Neuron 100, 349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS (2009). Animal models of pain: progress and challenges. Nat Rev Neurosci 10, 283–294. [DOI] [PubMed] [Google Scholar]
- Mogil JS (2018). The measurement of pain in the laboratory rodent. In: Wood JN (Ed.), The Oxford Handbook of the Neurobiology of Pain. Oxford University Press [Google Scholar]
- Mu D, Deng J, Liu KF, Wu ZY, Shi YF, Guo WM, Mao QQ, Liu XJ, Li H, and Sun YG (2017). A central neural circuit for itch sensation. Science 357, 695–699. [DOI] [PubMed] [Google Scholar]
- Nakamura K, and Morrison SF (2008). A thermosensory pathway that controls body temperature. Nat Neurosci 11, 62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, and Morrison SF (2010). A thermosensory pathway mediating heat-defense responses. Proc Natl Acad Sci U S A 107, 8848–8853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namer B, Schick M, Kleggetveit IP, Orstavik K, Schmidt R, Jorum E, Torebjork E, Handwerker H, and Schmelz M (2015). Differential sensitization of silent nociceptors to low pH stimulation by prostaglandin E2 in human volunteers. Eur J Pain 19, 159–166. [DOI] [PubMed] [Google Scholar]
- Navratilova E, Atcherley CW, and Porreca F (2015). Brain Circuits Encoding Reward from Pain Relief. Trends Neurosci 38, 741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova E, Xie JY, Okun A, Qu C, Eyde N, Ci S, Ossipov MH, King T, Fields HL, and Porreca F (2012). Pain relief produces negative reinforcement through activation of mesolimbic reward-valuation circuitry. Proc Natl Acad Sci U S A 109, 20709–20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson TS, Fu W, Donahue RR, Corder GF, Hokfelt T, Wiley RG, and Taylor BK (2019). Facilitation of neuropathic pain by the NPY Y1 receptor-expressing subpopulation of excitatory interneurons in the dorsal horn. Sci Rep 9, 7248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness TJ, and Gebhart GF (1988). Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Res 450, 153–169. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Randich A, and Gebhart GF (1991). Further behavioral evidence that colorectal distension is a ‘noxious’ visceral stimulus in rats. Neurosci Lett 131, 113–116. [DOI] [PubMed] [Google Scholar]
- Neumann S, Braz JM, Skinner K, Llewellyn-Smith IJ, and Basbaum AI (2008). Innocuous, not noxious, input activates PKCgamma interneurons of the spinal dorsal horn via myelinated afferent fibers. J Neurosci 28, 7936–7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols ML, Allen BJ, Rogers SD, Ghilardi JR, Honore P, Luger NM, Finke MP, Li J, Lappi DA, Simone DA, et al. (1999). Transmission of chronic nociception by spinal neurons expressing the substance P receptor. Science 286, 1558–1561. [DOI] [PubMed] [Google Scholar]
- Norris AJ, Shaker JR, Cone AL, Ndiokho IB, and Bruchas MR (2021). Parabrachial opioidergic projections to preoptic hypothalamus mediate behavioral and physiological thermal defenses. Elife 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson W, Abdus-Saboor I, Cui L, Burdge J, Raabe T, Ma M, and Luo W (2017). Sparse genetic tracing reveals regionally specific functional organization of mammalian nociceptors. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko N, Bell R, and Sesack SR (2009). Lateral habenula projections to dopamine and GABA neurons in the rat ventral tegmental area. Eur J Neurosci 30, 1239–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastoriza LN, Morrow TJ, and Casey KL (1996). Medial frontal cortex lesions selectively attenuate the hot plate response: possible nocifensive apraxia in the rat. Pain 64, 11–17. [DOI] [PubMed] [Google Scholar]
- Peirs C, Dallel R, and Todd AJ (2020). Recent advances in our understanding of the organization of dorsal horn neuron populations and their contribution to cutaneous mechanical allodynia. J Neural Transm (Vienna) 127, 505–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirs C, Williams SG, Zhao X, Arokiaraj CM, Ferreira DW, Noh MC, Smith KM, Halder P, Corrigan KA, Gedeon JY, et al. (2021). Mechanical Allodynia Circuitry in the Dorsal Horn Is Defined by the Nature of the Injury. Neuron 109, 73–90 e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirs C, Williams SP, Zhao X, Walsh CE, Gedeon JY, Cagle NE, Goldring AC, Hioki H, Liu Z, Marell PS, et al. (2015). Dorsal Horn Circuits for Persistent Mechanical Pain. Neuron 87, 797–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitjean H, Pawlowski SA, Fraine SL, Sharif B, Hamad D, Fatima T, Berg J, Brown CM, Jan LY, Ribeiro-da-Silva A, et al. (2015). Dorsal Horn Parvalbumin Neurons Are Gate-Keepers of Touch-Evoked Pain after Nerve Injury. Cell Rep pii: S2211–1247(15)01134–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps CE, Navratilova E, and Porreca F (2021). Cognition in the Chronic Pain Experience: Preclinical Insights. Trends Cogn Sci 25, 365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploner M, Freund HJ, and Schnitzler A (1999). Pain affect without pain sensation in a patient with a postcentral lesion. Pain 81, 211–214. [DOI] [PubMed] [Google Scholar]
- Pogorzala LA, Mishra SK, and Hoon MA (2013). The cellular code for Mammalian thermosensation. J Neurosci 33, 5533–5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prato V, Taberner FJ, Hockley JRF, Callejo G, Arcourt A, Tazir B, Hammer L, Schad P, Heppenstall PA, Smith ES, et al. (2017). Functional and Molecular Characterization of Mechanoinsensitive “Silent” Nociceptors. Cell Rep 21, 3102–3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott SA, Ma Q, and De Koninck Y (2014). Normal and abnormal coding of somatosensory stimuli causing pain. Nat Neurosci 17, 183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DD (2002). Central neural mechanisms that interrelate sensory and affective dimensions of pain. Mol Interv 2, 392–403, 339. [DOI] [PubMed] [Google Scholar]
- Price DD, Barrell JJ, and Gracely RH (1980). A psychophysical analysis of experimential factors that selectively influence the affective dimension of pain. Pain 8, 137–149. [DOI] [PubMed] [Google Scholar]
- Price TJ, Cervero F, Gold MS, Hammond DL, and Prescott SA (2009). Chloride regulation in the pain pathway. Brain Res Rev 60, 149–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L, Huang C, Wu X, Tao Y, Yan J, Shi T, Cao C, Han L, Qiu M, Ma Q, et al. (2017). Hierarchical Specification of Pruriceptors by Runt-Domain Transcription Factor Runx1. J Neurosci 37, 5549–5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L, Yin G, Zhang Y, Tao Y, Wu X, Gronostajski RM, Qiu M, and Liu Y (2020). Nuclear Factor I/A Controls A-fiber Nociceptor Development. Neurosci Bull 36, 685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu C, King T, Okun A, Lai J, Fields HL, and Porreca F (2011). Lesion of the rostral anterior cingulate cortex eliminates the aversiveness of spontaneous neuropathic pain following partial or complete axotomy. Pain 152, 1641–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queme LF, Ross JL, and Jankowski MP (2017). Peripheral Mechanisms of Ischemic Myalgia. Front Cell Neurosci 11, 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, and Bushnell MC (1997). Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 277, 968–971. [DOI] [PubMed] [Google Scholar]
- Rainville P, Feine JS, Bushnell MC, and Duncan GH (1992). A psychophysical comparison of sensory and affective responses to four modalities of experimental pain. Somatosens Mot Res 9, 265–277. [DOI] [PubMed] [Google Scholar]
- Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, Keefe FJ, Mogil JS, Ringkamp M, Sluka KA, et al. (2020). The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain 161, 1976–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratte S, Zhu Y, Lee KY, and Prescott SA (2014). Criticality and degeneracy in injury-induced changes in primary afferent excitability and the implications for neuropathic pain. Elife 3, e02370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau KK, McIlwrath SL, Wang H, Lawson JJ, Jankowski MP, Zylka MJ, Anderson DJ, and Koerber HR (2009). Mrgprd enhances excitability in specific populations of cutaneous murine polymodal nociceptors. Journal of Neuroscience 29, 8612–8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raver C, Uddin O, Ji Y, Li Y, Cramer N, Jenne C, Morales M, Masri R, and Keller A (2020). An Amygdalo-Parabrachial Pathway Regulates Pain Perception and Chronic Pain. J Neurosci 40, 3424–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren LY, Lu ZM, Liu MG, Yu YQ, Li Z, Shang GW, and Chen J (2008). Distinct roles of the anterior cingulate cortex in spinal and supraspinal bee venom-induced pain behaviors. Neuroscience 153, 268–278. [DOI] [PubMed] [Google Scholar]
- Rhudy JL, Grimes JS, and Meagher MW (2004). Fear-induced hypoalgesia in humans: effects on low intensity thermal stimulation and finger temperature. J Pain 5, 458–468. [DOI] [PubMed] [Google Scholar]
- Rhudy JL, and Meagher MW (2000). Fear and anxiety: divergent effects on human pain thresholds. Pain 84, 65–75. [DOI] [PubMed] [Google Scholar]
- Ringkamp M, Peng YB, Wu G, Hartke TV, Campbell JN, and Meyer RA (2001). Capsaicin responses in heat-sensitive and heat-insensitive A-fiber nociceptors. J Neurosci 21, 4460–4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez E, Sakurai K, Xu J, Chen Y, Toda K, Zhao SH, Han BX, Ryu D, Yin H, Liedtke W, et al. (2017). A cranio-facial-specific monosynaptic circuit enables hieghtened affective pain. Nature Neuroscience 20, 1734–1743 (doi:1710.1038/s41593-41017-40012-41591). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roome RB, Bourojeni FB, Mona B, Rastegar-Pouyani S, Blain R, Dumouchel A, Salesse C, Thompson WS, Brookbank M, Gitton Y, et al. (2020). Phox2a Defines a Developmental Origin of the Anterolateral System in Mice and Humans. Cell Rep 33, 108425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JL, Queme LF, Shank AT, Hudgins RC, and Jankowski MP (2014). Sensitization of group III and IV muscle afferents in the mouse after ischemia and reperfusion injury. J Pain 15, 1257–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB (2000). Pain as a visceral sensation. Prog Brain Res 122, 237–243. [DOI] [PubMed] [Google Scholar]
- Sato M, Ito M, Nagase M, Sugimura YK, Takahashi Y, Watabe AM, and Kato F (2015). The lateral parabrachial nucleus is actively involved in the acquisition of fear memory in mice. Mol Brain 8, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz M, Schmid R, Handwerker HO, and Torebjork HE (2000). Encoding of burning pain from capsaicin-treated human skin in two categories of unmyelinated nerve fibres. Brain 123 Pt 3, 560–571. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Schmelz M, Forster C, Ringkamp M, Torebjork E, and Handwerker H (1995). Novel classes of responsive and unresponsive C nociceptors in human skin. J Neurosci 15, 333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Schmelz M, Torebjork HE, and Handwerker HO (2000). Mechano-insensitive nociceptors encode pain evoked by tonic pressure to human skin. Neuroscience 98, 793–800. [DOI] [PubMed] [Google Scholar]
- Seal RP, Wang X, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, and Edwards RH (2009). Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature 462, 651–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra J, Collado A, Sola R, Antonelli F, Torres X, Salgueiro M, Quiles C, and Bostock H (2014). Hyperexcitable C nociceptors in fibromyalgia. Ann Neurol 75, 196–208. [DOI] [PubMed] [Google Scholar]
- Sharif B, Ase AR, Ribeiro-da-Silva A, and Seguela P (2020). Differential Coding of Itch and Pain by a Subpopulation of Primary Afferent Neurons. Neuron 106, 940–951 e944. [DOI] [PubMed] [Google Scholar]
- Sharma N, Flaherty K, Lezgiyeva K, Wagner DE, Klein AM, and Ginty DD (2020). The emergence of transcriptional identity in somatosensory neurons. Nature 577, 392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu I, Iida T, Guan Y, Zhao C, Raja SN, Jarvis MF, Cockayne DA, and Caterina MJ (2005). Enhanced thermal avoidance in mice lacking the ATP receptor P2X3. Pain 116, 96–108. [DOI] [PubMed] [Google Scholar]
- Simone DA, and Kajander KC (1997). Responses of cutaneous A-fiber nociceptors to noxious cold. J Neurophysiol 77, 2049–2060. [DOI] [PubMed] [Google Scholar]
- Singh A, Patel D, Li A, Hu L, Zhang Q, Liu Y, Guo X, Robinson E, Martinez E, Doan L, et al. (2020). Mapping Cortical Integration of Sensory and Affective Pain Pathways. Curr Biol 30, 1703–1715 e1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GM, Egbert LD, Markowitz RA, Mosteller F, and Beecher HK (1966). An experimental pain method sensitive to morphine in man: the submaximum effort tourniquet technique. J Pharmacol Exp Ther 154, 324–332. [PubMed] [Google Scholar]
- Spielgel EA, Wycis HT, Szekely EG, and Gildenberg PL (1966). Pain, Henry Ford Hospital International Symposium (Boston: Little, Brown). [Google Scholar]
- Steiner L, Forster D, Leksell L, Meyerson BA, and Boethius J (1980). Gammathalamotomy in intractable pain. Acta Neurochir (Wien) 52, 173–184. [DOI] [PubMed] [Google Scholar]
- Sufka KJ (1994). Conditioned place preference paradigm: a novel approach for analgesic drug assessment against chronic pain. Pain 58, 355–366. [DOI] [PubMed] [Google Scholar]
- Szolcsanyi J, Anton F, Reeh PW, and Handwerker HO (1988). Selective excitation by capsaicin of mechano-heat sensitive nociceptors in rat skin. Brain Res 446, 262–268. [DOI] [PubMed] [Google Scholar]
- Takashima Y, Daniels RL, Knowlton W, Teng J, Liman ER, and McKemy DD (2007). Diversity in the neural circuitry of cold sensing revealed by genetic axonal labeling of transient receptor potential melastatin 8 neurons. Journal of Neuroscience 27, 14147–14157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takazawa T, and MacDermott AB (2010). Synaptic pathways and inhibitory gates in the spinal cord dorsal horn. Ann N Y Acad Sci 1198, 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CL, Cooke EK, Leib DE, Lin YC, Daly GE, Zimmerman CA, and Knight ZA (2016). Warm-Sensitive Neurons that Control Body Temperature. Cell 167, 47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CL, and Knight ZA (2018). Regulation of Body Temperature by the Nervous System. Neuron 98, 31–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X, Lee MS, Donnelly CR, and Ji RR (2020). Neuromodulation, Specialized Proresolving Mediators, and Resolution of Pain. Neurotherapeutics 17, 886–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappe-Theodor A, King T, and Morgan MM (2019). Pros and Cons of Clinically Relevant Methods to Assess Pain in Rodents. Neurosci Biobehav Rev 100, 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasker RR (1990). Thalamotomy. Neurosurg Clin N Am 1, 841–864. [PubMed] [Google Scholar]
- Todd A, and Wang F (2018). Central nervous system pain pathways.
- Todd AJ (2010). Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci 11, 823–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torebjörk HE, Lundberg LE, and LaMotte RH (1992). Central changes in processing of mechanoreceptive input in capsaicin-induced secondary hyperalgesia in humans. J Physiol 448, 765–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truini A, Garcia-Larrea L, and Cruccu G (2013). Reappraising neuropathic pain in humans--how symptoms help disclose mechanisms. Nat Rev Neurol 9, 572–582. [DOI] [PubMed] [Google Scholar]
- Tuttle AH, Molinaro MJ, Jethwa JF, Sotocinal SG, Prieto JC, Styner MA, Mogil JS, and Zylka MJ (2018). A deep neural network to assess spontaneous pain from mouse facial expressions. Mol Pain 14, 1744806918763658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle AH, Tohyama S, Ramsay T, Kimmelman J, Schweinhardt P, Bennett GJ, and Mogil JS (2015). Increasing placebo responses over time in U.S. clinical trials of neuropathic pain. Pain 156, 2616–2626. [DOI] [PubMed] [Google Scholar]
- Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, Hjerling-Leffler J, Haeggstrom J, Kharchenko O, Kharchenko PV, et al. (2015). Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci 18, 145–153. [DOI] [PubMed] [Google Scholar]
- Veinante P, Yalcin I, and Barrot M (2013). The amygdala between sensation and affect: a role in pain. J Mol Psychiatry 1, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venteo S, Desiderio S, Cabochette P, Deslys A, Carroll P, and Pattyn A (2019). Neurog2 Deficiency Uncovers a Critical Period of Cell Fate Plasticity and Vulnerability among Neural-Crest-Derived Somatosensory Progenitors. Cell Rep 29, 2953–2960 e2952. [DOI] [PubMed] [Google Scholar]
- Viana F (2016). TRPA1 channels: molecular sentinels of cellular stress and tissue damage. J Physiol 594, 4151–4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierck CJ, and Yezierski RP (2015). Comparison of operant escape and reflex tests of nociceptive sensitivity. Neurosci Biobehav Rev 51, 223–242. [DOI] [PubMed] [Google Scholar]
- Vlaeyen JWS, and Crombez G (2020). Behavioral Conceptualization and Treatment of Chronic Pain. Annu Rev Clin Psychol 16, 187–212. [DOI] [PubMed] [Google Scholar]
- Vogt BA, and Pandya DN (1987). Cingulate cortex of the rhesus monkey: II. Cortical afferents. J Comp Neurol 262, 271–289. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN, and Rosene DL (1987). Cingulate cortex of the rhesus monkey: I. Cytoarchitecture and thalamic afferents. J Comp Neurol 262, 256–270. [DOI] [PubMed] [Google Scholar]
- Vrontou S, Wong AM, Rau KK, Koerber HR, and Anderson DJ (2013). Genetic identification of C fibres that detect massage-like stroking of hairy skin in vivo. Nature 493, 669–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall PD (1979). On the relation of injury to pain. The John J. Bonica lecture. Pain 6, 253–264. [DOI] [PubMed] [Google Scholar]
- Wang CC, and Shyu BC (2004). Differential projections from the mediodorsal and centrolateral thalamic nuclei to the frontal cortex in rats. Brain Res 995, 226–235. [DOI] [PubMed] [Google Scholar]
- Wang J, Huo FQ, Li YQ, Chen T, Han F, and Tang JS (2005). Thalamic nucleus submedius receives GABAergic projection from thalamic reticular nucleus in the rat. Neuroscience 134, 515–523. [DOI] [PubMed] [Google Scholar]
- Wang K, Wang S, Chen Y, Wu D, Hu X, Lu Y, Wang L, Bao L, Li C, and Zhang X (2021). Single-cell transcriptomic analysis of somatosensory neurons uncovers temporal development of neuropathic pain. Cell Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warwick C, Cassidy C, Hachisuka J, Wright MC, Baubauer KM, Adelman PC, Lee KH, Smith KM, Sheahan TD, Ross SE, et al. (2021). MrgprdCre lineage neurons mediate optogenetic allodynia through an emergent polysynaptic circuit. Pain 10.1097/j.pain.0000000000002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SM, and Goldman-Rakic PS (1998). Widespread origin of the primate mesofrontal dopamine system. Cereb Cortex 8, 321–345. [DOI] [PubMed] [Google Scholar]
- Willis WD Jr., Zhang X, Honda CN, and Giesler GJ Jr. (2002). A critical review of the role of the proposed VMpo nucleus in pain. J Pain 3, 79–94. [DOI] [PubMed] [Google Scholar]
- Wilson TD, Valdivia S, Khan A, Ahn HS, Adke AP, Martinez Gonzalez S, Sugimura YK, and Carrasquillo Y (2019). Dual and Opposing Functions of the Central Amygdala in the Modulation of Pain. Cell Rep 29, 332–346 e335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ (1984). Long term alterations in the excitability of the flexion reflex produced by peripheral tissue injury in the chronic decerebrate rat. Pain 18, 325–343. [DOI] [PubMed] [Google Scholar]
- Woolf CJ (2011). Central sensitization: implications for the diagnosis and treatment of pain. Pain 152(3 Suppl), S2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooten M, Weng HJ, Hartke TV, Borzan J, Klein AH, Turnquist B, Dong X, Meyer RA, and Ringkamp M (2014). Three functionally distinct classes of C-fibre nociceptors in primates. Nat Commun 5, 4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Ding M, and Zhang YQ (2021). Role of the Anterior Cingulate Cortex in Translational Pain Research. Neurosci Bull. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, and Zhang YQ (2018). A new perspective on the anterior cingulate cortex and affective pain. Neurosci Biobehav Rev 90, 200–211. [DOI] [PubMed] [Google Scholar]
- Xing H, Ling J, Chen M, and Gu JG (2006). Chemical and cold sensitivity of two distinct populations of TRPM8-expressing somatosensory neurons. J Neurophysiol 95, 1221–1230. [DOI] [PubMed] [Google Scholar]
- Yahiro T, Kataoka N, Nakamura Y, and Nakamura K (2017). The lateral parabrachial nucleus, but not the thalamus, mediates thermosensory pathways for behavioural thermoregulation. Sci Rep 7, 5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan N, Cao B, Xu J, Hao C, Zhang X, and Li Y (2012). Glutamatergic activation of anterior cingulate cortex mediates the affective component of visceral pain memory in rats. Neurobiol Learn Mem 97, 156–164. [DOI] [PubMed] [Google Scholar]
- Yang H, de Jong JW, Cerniauskas I, Peck JR, Lim BK, Gong H, Fields HL, Lammel S (2021). Pain modulates dopamine neurons via a spinal-parabrachial-mesencephalic circuit. Nat Neurosci 1402–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang FC, Tan T, Huang T, Christianson J, Samad OA, Liu Y, Roberson D, Davis BM, and Ma Q (2013). Genetic control of the segregation of pain-related sensory neurons innervating the cutaneous versus deep tissues. Cell Rep 5, 1353–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WZ, Du X, Zhang W, Gao C, Xie H, Xiao Y, Jia X, Liu J, Xu J, Fu X, et al. (2021). Parabrachial neuron types categorically encode thermoregulation variables during heat defense. Sci Adv 6, eabb9414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans DC, Pirec V, and Proudfit HK (1996). Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: behavioral evidence. Pain 68, 133–140. [DOI] [PubMed] [Google Scholar]
- Young RF, Jacques DS, Rand RW, Copcutt BC, Vermeulen SS, and Posewitz AE (1995a). Technique of stereotactic medial thalamotomy with the Leksell Gamma Knife for treatment of chronic pain. Neurol Res 17, 59–65. [DOI] [PubMed] [Google Scholar]
- Young RF, Vermeulen SS, Grimm P, Posewitz AE, Jacques DB, Rand RW, and Copcutt BG (1995b). Gamma Knife thalamotomy for the treatment of persistent pain. Stereotact Funct Neurosurg 64 Suppl 1, 172–181. [DOI] [PubMed] [Google Scholar]
- Zeisel A, Hochgerner H, Lonnerberg P, Johnsson A, Memic F, van der Zwan J, Haring M, Braun E, Borm LE, La Manno G, et al. (2018). Molecular Architecture of the Mouse Nervous System. Cell 174, 999–1014 e1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu S, Zhang YQ, Goulding M, Wang YQ, and Ma Q (2018). Timing Mechanisms Underlying Gate Control by Feedforward Inhibition. Neuron 99, 941–955 e944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann K, Leffler A, Babes A, Cendan CM, Carr RW, Kobayashi J, Nau C, Wood JN, and Reeh PW (2007). Sensory neuron sodium channel Nav1.8 is essential for pain at low temperatures. Nature 447, 855–858. [DOI] [PubMed] [Google Scholar]
- Zylka MJ, Rice FL, and Anderson DJ (2005). Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron 2005 Jan 6;45(1):17–25 45, 17–25. [DOI] [PubMed] [Google Scholar]