Abstract
Introduction:
Little is known about how antecedent vascular risk factor (VRF) profiles impact late-life brain health.
Methods:
We examined baseline VRFs, and cognitive testing and neuroimaging measures (β-amyloid [Aβ] PET, MRI) in a diverse longitudinal cohort (N = 159; 50% African-American, 50% White) from Wake Forest’s Multi-Ethnic Study of Atherosclerosis Core.
Results:
African-Americans exhibited greater baseline Cardiovascular Risk Factors, Aging, and Incidence of Dementia (CAIDE), Framingham stroke risk profile (FSRP), and atherosclerotic cardiovascular disease risk estimate (ASCVD) scores than Whites. We observed no significant racial differences in Aβ positivity, cortical thickness, or white matter hyperintensity (WMH) volume. Higher baseline VRF scores were associated with lower cortical thickness and greater WMH volume, and FSRP and CAIDE were associated with Aβ. Aβ was cross-sectionally associated with cognition, and all imaging biomarkers were associated with greater 6-year cognitive decline.
Discussion:
Results suggest the convergence of multiple vascular and Alzheimer’s processes underlying neurodegeneration and cognitive decline.
Keywords: aging, cognition, magnetic resonance imaging, positron emission tomography, vascular risk factors
1 |. BACKGROUND
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized by brain β-amyloid (Aβ) and tau pathology and cognitive decline, and culminating in dementia.1,2 Brain positron emission tomography (PET) measures of Aβ pathology precede early cognitive symptoms of AD.3–5 AD pathology occurs close in time to the development of cerebral small vessel disease (SVD) in the mid- to late-life transition period. Magnetic resonance imaging (MRI)6–8 has shown that SVD impacts white matter (WM) health and brain networks including elevations in WM hyperintensities (WMH),9,10 an MRI biomarker of SVD. Further, measures of cortical atrophy serve as a biomarker of neurodegeneration related to numerous disease pathologies, including both SVD and AD.11
While AD and SVD are thought to be independent processes, vascular risk factors (VRFs) are associated with both forms of disease. Growing evidence suggests that VRFs (eg, hyperlipidemia, systolic blood pressure [BP], type 2 diabetes, arterial stiffness) relate to not only SVD-linked imaging markers, but also AD-linked pathological measures such as atrophy and Aβ accumulation.12–14 In addition, while late-life VRFs are not associated with dementia and its related pathology, midlife VRFs tend to be associated with pathology (particularly imaging biomarkers) and cognition; one study found elevated midlife VRFs were significantly associated with elevated Aβ on PET, consistent with a role of vascular disease in the development of AD.15
VRFs are also being used to stratify risk in dementia prevention trials. The FINGER16 study, which recruited older individuals with a high Cardiovascular Risk Factors, Aging, and Incidence of Dementia (CAIDE) risk score,17 found a multidomain intervention targeting VRFs (diet, physical activity, VRF management) helped to protect against cognitive decline. In the small FINGER imaging substudy, CAIDE was associated with more deep WMH and lower cortical thickness, but not with Aβ or periventricular WMH.18 These studies often lack sufficient diversity to explore relationships between VRFs, neuropathology, and cognition in older adults from different racial groups.
The Multi-Ethnic Study of Atherosclerosis (MESA) is a unique diverse study with over 20 years of extensive longitudinal vascular phenotyping, as well as cognitive testing 10 and 15 years later.19,20 At the Wake Forest University (WFU) site, a diverse (White and African-American [AA]) cohort additionally underwent brain MRI and Aβ PET. The examination of imaging biomarkers in this deeply phenotyped older adult sample is important to understanding the role of VRFs in neuropathology and cognitive decline.
In this study, we examined relationships among (1) various composite VRF measures, (2) imaging biomarkers of SVD and AD-related pathology, and (3) cognitive performance and change in cognition, in a diverse sample of community-dwelling older adults. Multiple commonly used VRF scores were evaluated in order to better understand contributions of vascular risk to neuropathology and cognitive decline. We hypothesized elevated antecedent VRF scores (assessed by clinical vascular composites 10–16 years prior to neuroimaging and cognitive testing) would be differentially associated with imaging biomarkers of Aβ (global Pittsburgh compound B [PiB] PET3,21), SVD (global WMH volume22), and neurodegeneration (temporal lobe MRI cortical thickness11), and these baseline VRF scores and imaging biomarkers would predict cognitive performance. We additionally examined whether there would be differences by race in the relationship between VRF scores and brain imaging biomarkers.
2 |. METHODS
2.1 |. Participants
Participants were recruited from the parent MESA cohort study into the WFU Alzheimer’s Disease Research Center affiliated MESA Core study. MESA Core participants (N = 159; 49.7% AA and 50.3% White) were included in this analysis. As previously described,19,20 MESA participants were free from clinical cardiovascular disease (including stroke) at baseline (2000–2002, when participants were 55.8 ± 6.7 years old) and underwent VRF assessment over the course of six exams and annual follow-up calls; baseline VRF values were analyzed in this study. Participants underwent cognitive testing with the Cognitive Abilities Screening Instrument (CASI)23 at two timepoints, first in 2010–12 (MESA Exam 5) and again in 2016–2018 (Exam 6). At the WFU site, participants also received more detailed cognitive testing, brain MRI and PET as part of MESA Core (2016–2018). DNA was analyzed for APOE genotypes as previously described;24 APOE-ε4 carriage was defined as the presence of one or more ε4 allele(s). Participants self-reported their sex, racial group, and years of education. All participants were free from clinical stroke. The research protocol was approved by the local Institutional Review Board, and informed consent was obtained for all participants; all research was carried out in accordance with the Declaration of Helsinki.
2.2 |. Vascular risk factor composite scores
Multiple composite baseline (2000–2002) mid- to late-life clinical VRF scores were evaluated: CAIDE,17 Framingham stroke risk profile (FSRP) score,25 and the atherosclerotic cardiovascular disease risk estimate from the pooled cohort equation (ASCVD).26 CAIDE is a composite of traditional VRFs (eg, systolic BP, cholesterol) and AD risk factors (eg, education, APOE-ε4). It is a useful predictor of dementia risk, with a higher score indicating greater risk.17 FSRP estimates the risk of stroke over 10 years and includes a combination of traditional VRFs with prevalent cardiovascular disease (CVD) and atrial fibrillation. FSRP is associated with lower brain volume, higher WMH, and cognitive decrements.27–29 ASCVD is a predictor of 10-year risk of a first ASCVD event such as nonfatal myocardial infarction or coronary heart disease. Both FSRP and ASCVD are represented as a percent risk with higher percentages indicating increased risk. Components of each composite VRF score are summarized in Supplementary Table 1 (in the Supporting Information). All VRF scores include age, sex, and systolic BP; ASCVD and FSRP share components (type 2 diabetes, use of anti-hypertensive medications, current smoking status), as do ASCVD and CAIDE (total cholesterol).
2.3 |. Cognitive testing and adjudication
At the MESA Exam 5 (2010–2012), participants had a brief cognitive examination using the CASI.23 This testing was repeated at MESA Exam 6 (2016–2018). Cross-sectional cognitive performance (Exam 5, Exam 6) as well as longitudinal cognitive change (Exam 5 CASI score subtracted from Exam 6 CASI) were calculated and entered into the analyses. The CASI is a measure of global cognitive function developed for cross-cultural use; however, known differences exist in CASI scores between racial/ethnic groups.30,31 CASI scores were missing or deemed invalid by the test administrator for three AA participants at Exam 5 and 1 White participant at Exam 6; we did not evaluate cognitive performance in these four participants.
Also at Exam 6, MESA Core participants were administered the UDSv3 cognitive assessment protocol.32 The National Alzheimer’s Coordinating Centers UDSv3 cognitive assessment protocol includes detailed cognitive testing; information about family history of AD, medications, and health history; clinician-assessed medical conditions and judgment of symptoms; and a neurological examination. All of these data were used in the adjudication process. The consensus panel consisted of neuropsychologists, geriatricians, neurologists, and other aging experts. Consensus adjudicated cognitive status, according to published criteria,33,34 included cognitively normal, mild cognitive impairment (MCI), and dementia subtypes (including AD).
2.4 |. MRI acquisition and processing
Brain MRI data were acquired for all participants at WFU on a 3T Siemens Skyra scanner using a high-resolution 32-channel head coil. MRI sequences included T1-weighted 3D volumetric MPRAGE (to quantify cortical thickness) and T2-weighted FLAIR (to quantify WMH). Sequence details are provided in the supplementary information S1. Cortical thickness was calculated on T1 MRI using FreeSurfer v5.3 (https://surfer.nmr.mgh.harvard.edu). We examined brain structure using a temporal lobe region of interest; this was calculated by averaging surface area-weighted cortical thickness of bilateral entorhinal, inferior/middle temporal, and fusiform regions.11 This cortical thickness measure has been shown to be a useful measure of neurodegeneration in regions characteristic of AD. Lesions were segmented by the lesion growth algorithm (LGA)35 implemented in the LST toolbox v2.0.15 (www.statistical-modelling.de/lst.html), running in Matlab SPM12 (www.fil.ion.ucl.ac.uk/spm) using FLAIR images with T1 images as reference. WMH masks were manually edited by trained observers. Total WMH lesion volume was divided by FreeSurfer total intracranial volume (to correct for head size) and log-normalized to generate a global measure of WMH volume, which served as an SVD biomarker.
2.5 |. PET acquisition and processing
[11C]PiB36 was used for assessing fibrillar Aβ brain deposition on PET. Following a computed tomography (CT) scan for attenuation correction, participants were injected with approximately 370 MBq [11C]PiB and scanned from 40–70 minutes (6 × 5-min frames) post-injection in the WFU PET research center on a 64-slice GE Discovery MI DR PET/CT scanner. Each participant’s CT image was coregistered to their structural MRI, and PET frames were coregistered to MRI space using the affine matrix from the CT-MRI coregistration. A voxelwise 40–70 minutes standardized uptake volume ratio (SUVR) image was then generated. Global brain PiB uptake was calculated as PiB SUVR (40–70 minutes, cerebellar grey reference) averaged from a cortical region of interest sensitive to early AD, using FreeSurfer-segmented regions.3,37 This global PiB SUVR measure served as a biomarker of Aβ burden. Participants were classified using a previously defined threshold (≥1.21 PiB SUVR21) to define Aβ− and Aβ+ groups.
2.6 |. Statistical analysis
We tested differences between racial groups and Aβ positivity groups using t tests and chi-square tests; racial group differences in Aβ were also assessed using multivariable linear regression with covariates of age, sex, education, APOE-ε4, WMH volume, and intracranial volume, in line with previous studies.38 We assessed bivariate associations between VRF scores and imaging variables using the Pearson correlation. This analysis focused on three types of brain imaging: temporal lobe cortical thickness, WMH volume, and brain Aβ burden (methods described above). We developed multivariable general linear models assessing relationships of (1) VRF scores with brain imaging variables, (2) VRF scores with cognition (CASI performance), and (3) brain imaging variables with cognition. Results from general linear models were reported as t values and P values. Linear models were adjusted for a basic set of covariates: age, sex, race, education, and APOE-ε4. Covariates were not included in models when they were included in VRF scores (Supplementary Table 1). Because we were interested in differences by racial groups in VRF scores and their impacts on brain health and cognition, we examined interactions by race. It is important to note that in this analysis, we interpret race as a social, and not biological, construct. We additionally examined interactions by baseline median age (54 years) to assess potential effect modifications by age, as well as interactions by cognitive impairment status (comparing cognitively normal vs MCI and dementia combined). Participants with VRF scores ≥4 standard deviations above the sample mean were removed from analyses. One participant was removed from analyses that included FSRP; results were similar when this participant was included in the analyses.
3 |. RESULTS
Demographics by Aβ status for 159 participants (102 Aβ−, 57 Aβ+) in the present study are presented in Table 1. Aβ+ participants (35.8%) were more likely to be older, APOE-ε4 carriers, and have higher WMH volume and lower temporal lobe cortical thickness. Further, the Aβ+ group exhibited lower Exam 6 CASI scores and significantly greater decline in CASI from Exams 5 to 6; there was no difference in the Exam 5 CASI scores. A significantly greater proportion of Aβ+ participants had cognitive impairment (P < .001), and higher CAIDE (P < .001) and FSRP scores (P = .004).
TABLE 1.
Participant measures by Aβ positivity
| Aβ− | Aβ+ | P | |
|---|---|---|---|
| n (%) | 102 (64.1) | 57 (35.8) | |
| Age, Exam 1, M (SD) | 54.3 (6.3) | 58.4 (6.5) | <.001* |
| Age, Exam 6, M (SD) | 70.1 (6.2) | 74.5 (6.5) | <.001* |
| Sex (M/F) | 47/55 | 24/33 | .629 |
| Race (White/AA) | 48/54 | 32/25 | .272 |
| Years of education, M (SD) | 15.0 (2.6) | 15.1 (2.9) | .857 |
| aCognitive status, n (%) | <.001* | ||
| Normal | 79 (78.2) | 27 (48.2) | |
| MCI | 19 (18.8) | 23 (41.1) | |
| Dementia | 1 (1.0) | 5 (8.9) | |
| APOE-ε4, (+/−/missing) | 26/75/1 | 27/25/5 | <.001* |
| %ASCVD, M (SD) | 6.59 (5.7) | 8.01 (6.4) | .152 |
| b% FSRP, M (SD) | 1.69 (1.6) | 2.69 (2.7) | .004* |
| CAIDE, M (SD) | 6.48 (2.2) | 7.65 (2.2) | .002* |
| SBP, M (SD) | 127.0 (21.7) | 126.9 (18.6) | .965 |
| DBP, M (SD) | 73.9 (10.4) | 73.5 (10.1) | .828 |
| cCASI Exam 5, M (SD) | 92.3 (5.9) | 92.1 (5.3) | .865 |
| dCASI Exam 6, M (SD) | 93.6 (4.5) | 90.3 (8.4) | .002* |
| eCASI change (5 to 6), M (SD) | 1.4 (5.0) | −1.8 (6.8) | .001* |
| PiB SUVR, M (SD) | 1.11 (0.05) | 1.71 (0.27) | <.001* |
| fWMH vol. (% ICV), median (IQR) | 0.19 (0.10, 0.42) | 0.33 (0.14, 0.72) | .025* |
| dCort. thick., mm, M (SD) | 2.69 (0.12) | 2.65 (0.13) | .037* |
Abbreviations: Aβ, β-amyloid; ASCVD, atherosclerotic cardiovascular disease risk estimate from the pooled cohort equation; FSRP, Framingham stroke risk profile; CAIDE, cardiovascular risk factors, aging and incidence of dementia risk score; SBP, systolic blood pressure; DBP, diastolic blood pressure; CASI, Cognitive Abilities Screening Instrument; PiB SUVR, Pittsburgh compound B Standardized Uptake Value Ratio; Cort. thick., cortical thickness; WMH vol., white matter hyperintensity volume; ICV, intracranial volume.
Statistically significant at P < .05.
Note: 5 participants (3 Aβ−, 2 Aβ+) did not have cognitive status available
Aβ− n = 99; Aβ+ n = 55.
Aβ− n = 101; Aβ+ n = 57.
Aβ− n = 99; Aβ+ n = 57.
Aβ− n = 102; Aβ+ n = 56.
Aβ− n = 99; Aβ+ n = 56.
Aβ− n = 101; Aβ+ n = 56.
When examining self-reported racial group membership (Table 2; 80 White, 79 AA), we found an expected significant difference in ASCVD score (race is a component of the ASCVD score), and a marginal difference in CAIDE (P = .051), such that AA participants had higher mid-to late-life VRF scores. Systolic and diastolic blood pressure (SBP and DBP) were significantly elevated in the AA compared to the White group. AA participants were more likely to be male (P = .045) and had lower overall PiB SUVR (P = .029), yet notably there were no racial differences in Aβ positivity (P = .272, Table 2) even after controlling for other covariates (Supplementary Table 2). We found no significant group differences by race in other demographic or imaging measures.
TABLE 2.
Participant measures by race
| African-American | White | P | |
|---|---|---|---|
| n (%) | 79 (49.7) | 80 (50.3) | |
| Age, Exam 1, M (SD) | 55.8 (6.8) | 55.7 (6.5) | .945 |
| Age, Exam 6, M (SD) | 71.5 (6.7) | 71.8 (6.6) | .810 |
| Sex (M/F) | 50/29 | 38/42 | .045* |
| Years of education, M (SD) | 14.9 (2.7) | 15.1 (2.8) | .729 |
| aCognitive status | .618 | ||
| Normal, n (%) | 49 (62.8) | 57 (72.2) | |
| MCI, n (%) | 24 (30.8) | 18 (22.8) | |
| Dementia, n (%) | 3 (3.9) | 3 (3.8) | |
| APOE-ε4, (+/−/missing) | 31/45/3 | 22/55/3 | .283 |
| % ASCVD, M (SD) | 8.76 (6.8) | 5.45 (4.5) | <.001* |
| b% FSRP, M (SD) | 2.33 (2.4) | 1.78 (1.8) | .096 |
| CAIDE, M (SD) | 7.25 (2.3) | 6.55 (2.2) | .051 |
| SBP, M (SD) | 133.0 (21.9) | 121.0 (17.4) | <.001* |
| DBP, M (SD) | 75.7 (9.2) | 71.9 (11.0) | .020* |
| cCASI Exam 5, M (SD) | 89.7 (6.2) | 94.6 (3.7) | <.001* |
| dCASI Exam 6, M (SD) | 90.6 (7.2) | 94.3 (4.8) | <.001* |
| eCASI change (5 to 6), M (SD) | 0.8 (6.8) | −0.4 (4.9) | .224 |
| PiB SUVR, M (SD) | 1.27 (0.28) | 1.39 (0.37) | .029* |
| Aβ+, n (%) | 25 (31.7) | 32 (40.0) | .272 |
| fWMH vol. (% ICV), median (IQR) | 0.21 (0.11, 0.55) | 0.23 (0.10, 0.55) | .979 |
| dCort. Thick., mm, M (SD) | 2.66 (0.13) | 2.69 (0.12) | .143 |
Abbreviations: Aβ, β-amyloid; ASCVD, atherosclerotic cardiovascular disease risk estimate from the pooled cohort equation; FSRP, Framingham stroke risk profile; CAIDE, cardiovascular risk factors, aging, and incidence of dementia risk score; SBP, systolic blood pressure; DBP, diastolic blood pressure; CASI, Cognitive Abilities Screening Instrument; PiB SUVR, Pittsburgh compound B Standardized Uptake Value Ratio; Cort. thick., Cortical thickness; WMH vol., white matter hyperintensity volume; ICV, intracranial volume.
Statistically significant at P < .05
White n = 78; African-American n = 76.
White n = 80; African-American n = 78.
White n = 80; African-American n = 76.
White n = 79; African-American n = 79.
White n = 79; African-American n = 76.
White n = 79; African-American n = 78.
In bivariate analysis, higher baseline FSRP and CAIDE scores were significantly associated with global brain Aβ (Figure 1A). We observed that higher baseline VRF scores were significantly associated with both greater WMH volumes (Figure 1B) and reduced temporal lobe cortical thickness (Figure 1C).
FIGURE 1.
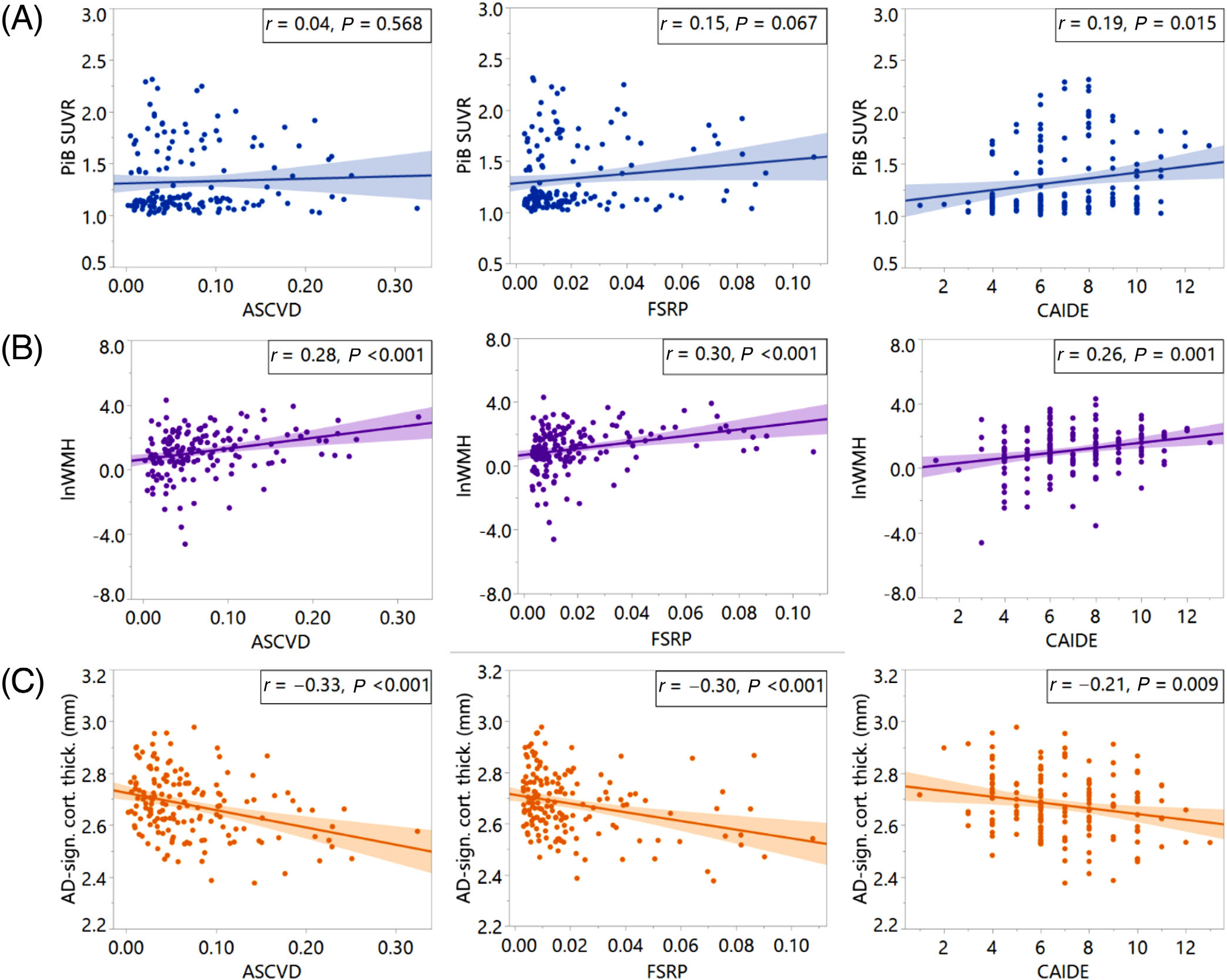
Vascular risk factor (VRF)-imaging scatterplots. VRFs (x-axes) versus (A) Global PiB SUVR, (B) log-norm. WMH volume, and (C) temporal lobe cortical thickness (y-axes). ASCVD, atherosclerotic cardiovascular disease risk estimate from the pooled cohort equation; FSRP, Framingham stroke risk profile; CAIDE, cardiovascular risk factors, aging and incidence of dementia risk score; PiB SUVR, Pittsburgh compound B Standardized Uptake Value Ratio; lnWMH, log-normalized white matter hyperintensity volume; AD-sign. cort. thick., temporal lobe cortical thickness
In multivariable models of VRF scores as predictors of imaging variables (Table 3), we found that elevated ASCVD, FSRP, and CAIDE significantly predicted lower temporal lobe cortical thickness and higher WMH volumes, independent of covariates. CAIDE and FSRP were significantly associated with brain Aβ burden. There were no interactions with baseline age or cognitive status and VRF scores with neuroimaging biomarkers (data not shown). There were significant interactions by race with respect to cortical thickness, such that associations with ASCVD and FSRP were stronger in White participants (Supplementary Table 3).
TABLE 3.
VRF-Imaging associations
| t | P | Race interaction P | |
|---|---|---|---|
| PiB SUVR (n = 159) | |||
| ASCVDa | 0.37 | .716 | .606 |
| FSRPb,d | 2.48 | .014* | .254 |
| CAIDEc | 2.89 | .004* | .666 |
| WMH Volume (n = 157) | |||
| ASCVDa | 3.71 | <.001* | .157 |
| FSRPb,d | 3.94 | <.001* | .183 |
| CAIDEc | 3.44 | <.001* | .945 |
| Cort. Thick. (n = 158) | |||
| ASCVDa | −4.28 | <.001* | .004* |
| FSRPb,d | −3.69 | <.001* | .013* |
| CAIDEc | −2.47 | .014* | .975 |
Note: all VRFs include age, sex, and systolic blood pressure. VRF components listed in Supplementary Table 1.
Abbreviations: VRF, vascular risk factor; ASCVD, atherosclerotic cardiovascular disease risk estimate from the pooled cohort equation; FSRP, Framingham stroke risk profile; CAIDE, cardiovascular risk factors, aging, and incidence of dementia risk score; PiB SUVR, Pittsburgh compound B Standardized Uptake Value Ratio; WMH, white matter hyperintensity; Cort. thick., cortical thickness.
Adjusted for education and APOE-ε4 carriage.
Adjusted for education, race, and APOE-ε4 carriage.
Adjusted for race.
One outlier excluded.
Significant at P < .05.
When examining relations among VRF scores and CASI (Table 4), we found no significant associations with Exam 5 CASI scores. Higher ASCVD, FSRP, and CAIDE scores were associated with worse Exam 6 CASI performance. The association of CAIDE and Exam 6 CASI was driven primarily by the AA group (Supplementary Table 4). Elevated FSRP and CAIDE were related to greater CASI decline. There were no interactions with baseline age (data not shown). We observed an interaction by cognitive status in the ASCVD-Exam 6 CASI association; the association was strongest in cognitively normal participants (data not shown).
TABLE 4.
VRF-CASI associations
| t | P | Race interaction P | |
|---|---|---|---|
| CASI Exam 5 (n = 156) | |||
| ASCVDa | −1.31 | .191 | .987 |
| FSRPb,d | 0.51 | .611 | .610 |
| CAIDEc | −1.73 | .085 | .176 |
| CASI Exam 6 (n = 158) | |||
| ASCVDa | −3.01 | .003* | .943 |
| FSRPb,d | −2.39 | .018* | .901 |
| CAIDEc | −4.16 | <.001* | .037* |
| CASI Change (n = 155) | |||
| ASCVDa | −1.70 | .091 | .984 |
| FSRPb,d | −2.74 | .007* | .789 |
| CAIDEc | −2.63 | .010* | .401 |
Note: all VRFs include age, sex, and systolic blood pressure. VRF components listed in Supplementary Table 1.
Abbreviations: VRF, vascular risk factor; ASCVD, atherosclerotic cardiovascular disease risk estimate from the pooled cohort equation; FSRP, Framingham stroke risk profile; CAIDE, cardiovascular risk factors, aging, and incidence of dementia risk score; CASI, Cognitive Abilities Screening Instrument.
Adjusted for education and APOE-ε4 carriage.
Adjusted for education, race, and APOE-ε4 carriage.
Adjusted for race.
One outlier excluded.
Significant at P < .05.
We finally examined multivariable models of imaging variables as predictors of CASI (Table 5). All imaging markers were significantly associated with CASI decline over 6 years. Measures of WMH and cortical thickness were not significantly associated with Exam 5 or Exam 6 CASI performance. While global PiB SUVR was not associated with the Exam 5 CASI, greater brain Aβ burden was significantly associated with lower Exam 6 CASI performance. We observed an interaction by cognitive status for associations of PiB SUVR with CASI Exam 6 and CASI Change, such that associations were strongest in cognitively impaired participants (data not shown).
TABLE 5.
Imaging-CASI associations
| n | t a | P | Race interaction P | |
|---|---|---|---|---|
| CASI Exam 5 | ||||
| PiB SUVR | 156 | −0.13 | .897 | .132 |
| WMH Volume | 154 | 1.15 | .252 | .695 |
| Cort. Thick. | 155 | −0.48 | .631 | .593 |
| CASI Exam 6 | ||||
| PiB SUVR | 158 | −2.47 | .015* | .779 |
| WMH Volume | 156 | −1.45 | .149 | .308 |
| Cort. Thick. | 157 | 1.66 | .099 | .859 |
| CASI Change | ||||
| PiB SUVR | 155 | −2.23 | .028* | .126 |
| WMH Volume | 153 | −2.35 | .020* | .221 |
| Cort. Thick. | 154 | 1.99 | .048* | .859 |
Abbreviations: PiB SUVR, Pittsburgh compound B Standardized Uptake Value Ratio; WMH, white matter hyperintensity; Cort. thick., cortical thickness; CASI, Cognitive Abilities Screening Instrument.
Adjusted forage, race, sex,education,and APOE-ε4 carriage.
Significant at P < .05.
4 |. DISCUSSION
In this diverse cohort, we assessed relationships among baseline VRF scores, brain imaging biomarkers of SVD and AD, and cognitive performance in a diverse older adult sample. The MESA cohort enabled the study of contributions of antecedent mid- to late-life VRFs to subsequent neurodegeneration, neuropathology, and cognitive decline; additionally, MESA allowed us to assess unique associations of each composite VRF measure with late-life imaging biomarkers, and to explore whether VRFs differentially predict Aβ deposition, WMH, and cortical thickness. It additionally enabled the study of differences in specific VRF pathways and imaging markers among a diverse group of individuals. Multiple VRF scores were evaluated (CAIDE,17 FSRP,25 and ASCVD26 scores) in order to better understand the contributions of vascular risk to neuropathology and cognitive decline in the context of other studies that have investigated these commonly-used VRFs.
We observed differences between AA and White participants in antecedent VRF scores, but these preliminary single-site results also suggest that AA group members were no more likely to be Aβ+ or show differences in WMH and cortical thickness in AD-prone temporal regions than White participants. Further, we found not only that all 3 VRF scores were strongly associated with MRI biomarkers of WMH and temporal lobe cortical thickness, but also that CAIDE and FSRP were associated with Aβ PET. VRFs showed a more complex pattern of associations with cognitive performance, described in more detail below. Finally, we found that VRF scores, WMH, cortical thickness, and Aβ deposition were significant predictors of cognitive performance and cognitive decline, even when controlling for covariates.
4.1 |. Aβ group differences
Aβ positivity in a diverse older adult sample was associated with expected predictors of age and APOE-ε4,38,39 with reduced cognitive performance at time of imaging and greater retrospective cognitive decline,40 and with greater WMH volume and lower temporal lobe cortical thickness.41 This set of regions is usually susceptible to age-related neurodegenerative disease, including typical amnestic AD and limbic-predominant age-related TDP-43-encephalopathy (LATE). These results in a diverse sample confirm and extend previous research (mostly conducted in White populations of European descent) that found similar results.39–41 Aβ positivity was associated with greater Prevalence of cognitive impairment and dementia, and higher FSRP and CAIDE as well.
4.2 |. Group differences by race
We found that AA participants had elevated ASVCD (expected, as race is a component of the ASCVD score), as well as a marginal difference in CAIDE. In contrast with the Atherosclerosis Risk in Communities (ARIC) study, which examined brain Aβ in AA and White cohorts from two different geographic regions, we did not find group differences by race in Aβ positivity in our single-site sample, suggesting that AA are not necessarily more likely to be Aβ+;38 indeed, we observed that AA had lower overall PiB SUVR, which warrants further investigation. These findings of group differences by race in vascular burden inimical toward the brain in a single-site cohort in the southeastern US, in the absence of neuroimaging differences, suggest complex late-life relationships among vascular, imaging, and cognitive measures in a diverse older adult sample. We again note that in this analysis, we interpret race as a social, and not biological, construct. We do not know (it was not tested) whether these group differences by race were due to social determinants of health or to intrinsic differences.42 A more extensive analysis of MESA VRF, neuroimaging, and cognitive testing data with respect to social determinants of health is planned for future work.
4.3 |. Associations among VRF scores, imaging biomarkers, and cognition
Higher baseline VRF scores were consistently associated with increased WMH volumes and reduced cortical thickness across groups; interestingly, elevated FSRP and CAIDE were associated with greater Aβ as well. The association between CAIDE and brain Aβ deposition may be related to the inclusion of education and APOE, both known dementia risk factors, in the CAIDE score. The association between Aβ and FSRP, which includes prevalent CVD and atrial fibrillation, suggests these factors may be important avenues to explore with respect to their relationship to Aβ. As expected, VRF scores were associated with WMH volume, a well-known biomarker of SVD.9,10 Further, baseline VRF scores were associated with lower cortical thickness, indicating elevated mid- to late-life VRFs can predict neurodegeneration approximately 16 years later. There were significant interactions by race with respect to cortical thickness as well, such that associations with ASCVD and FSRP were stronger in White than in AA participants.
We also looked at predictors of cognitive performance and change over a 6-year period. We found Aβ predicted lower Exam 6 CASI performance and greater decline, and measures of WMH and cortical thickness were significantly related to CASI change. However, we note that cross-sectional associations between PiB SUVR and cognition appeared to be driven by those who were classified with MCI or dementia at Exam 6. Further, we found no significant associations of VRF scores with Exam 5 CASI scores, but increases in all three VRF scores were associated with worse Exam 6 CASI performance, with the effect of CAIDE driven primarily by the AA group. Elevated FSRP and CAIDE also related to greater CASI decline. These results suggest complex links between stroke risk and cognitive decline in this cohort free from stroke, and imply that controlling VRFs may be a potent mechanism for preventing cognitive decline in preclinical stages of AD.
4.4 |. Implications, limitations, and future directions
Our results were primarily in agreement with previous studies. We found FSRP, a known predictor of 10-year risk for vascular brain injury,43 to be associated with lower cortical thickness, higher WMH, and cognitive decrements, consistent with previous work.27–29,44 As initially demonstrated in the FINGER study, we found CAIDE to be associated with higher WMH and lower cortical thickness; however, in contrast, we also observed CAIDE to be significantly associated with elevated brain Aβ, in a diverse larger sample.18
There are some limitations in the present research. We chose to examine a series of global and composite measures in order to canvass the scope of late-life associations among VRFs, imaging biomarkers, and cognition; future efforts will examine more specific clinical and subclinical VRFs, and regional and voxelwise patterns of imaging measures. Repeated detailed cognitive testing and neuroimaging are ongoing in this study, and will enable more specific cognitive domains to be evaluated in future research. Finally, given the sample size, a proper examination of interactions by sex in multivariable models, as well as an analysis of individual VRFs examined independently, are beyond the scope of this analysis.
In this multimodal, longitudinal study, we found differential associations among mid- to late-life VRF scores, brain imaging biomarkers of Aβ pathology, SVD, and neurodegeneration, and cognitive performance and longitudinal cognitive decline. Limited previous research has found differential VRF-imaging associations for different imaging modalities,18 and growing evidence suggests that controlling VRFs may be beneficial to cognition.17 The examination of unique associations of VRFs with imaging biomarkers in a diverse study may illuminate targets for protecting brain health and cognition among diverse older adults.
Supplementary Material
RESEARCH IN CONTEXT.
Systematic review: The authors reviewed the literature using PubMed and meeting abstracts and presentations. It is poorly understood how antecedent vascular risk factor (VRF) profiles translate into late-life brain health, particularly among diverse older adults. Several recent studies have begun to investigate this question, using imaging biomarkers of disease and cognitive testing; these relevant citations are appropriately cited.
Interpretation: Our findings suggest that there are complex, long-acting interactions between multiple vascular and AD processes on late-life neurodegeneration, neuropathology, and cognitive decline.
Future directions: Future studies that incorporate multimodal variables (VRFs, imaging biomarkers, and cognitive performance) from mid- to late-life in diverse samples with longitudinal study designs are needed to clarify the complex and long-acting impacts of vascular and AD pathology on late-life brain and cognitive decline, and how they differ among racial groups.
ACKNOWLEDGMENTS
This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute; by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS); and by grants P30AG049638 and R01AG054069 from the National Institute on Aging (NIA). The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. The funding sources had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The authors declare that no competing interests or conflicts of interest exist.
Funding information
National Heart, Lung, and Blood Institute, Grant/Award Numbers: HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169; National Center for Advancing Translational Sciences, Grant/Award Numbers: UL1-TR-000040, UL1-TR-001079, UL1-TR-001420; National Institute on Aging, Grant/Award Numbers: P30AG049638, R01AG054069
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Feldman HH, Frisoni GB, et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87(5):539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011;7:280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mormino EC, Brandel MG, Madison CM, et al. Not quite PIB-positive, not quite PIB-negative: slight PIB elevations in elderly normal control subjects are biologically relevant. Neuroimage. 2012;59(2):1152–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kemppainen N, Johansson J, Teuho J, et al. Brain amyloid load and its associations with cognition and vascular risk factors in FINGER Study. Neurology 2018;90(3):e206–e213. [DOI] [PubMed] [Google Scholar]
- 5.Jack CR Jr, Bennett DA, Blennow K, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018;14(4):535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lockhart SN, Luck SJ, Geng J, et al. White matter hyperintensities among older adults are associated with futile increase in frontal activation and functional connectivity during spatial search. PLoS One. 2015;10(3):e0122445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rieckmann A, Van Dijk KR, Sperling RA, et al. Accelerated decline in white matter integrity in clinically normal individuals at risk for Alzheimer’s disease. Neurobiol Aging. 2016;42:177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuladhar AM, Lawrence A, Norris DG, et al. Disruption of rich club organisation in cerebral small vessel disease. Hum Brain Mapp. 2017;38(4):1751–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lockhart SN, DeCarli C. Structural imaging measures of brain aging. Neuropsychol Rev. 2014;24(3):271–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiol Aging. 2005;26(4):491–510. [DOI] [PubMed] [Google Scholar]
- 11.Schwarz CG, Gunter JL, Wiste HJ, et al. A large-scale comparison of cortical thickness and volume methods for measuring Alzheimer’s disease severity. Neuroimage Clin. 2016;11:802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes TM, Kuller LH, Barinas-Mitchell EJM, et al. Pulse wave velocity is associated with -amyloid deposition in the brains of very elderly adults. Neurology 2013;81(19):1711–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes TM, Kuller LH, Barinas-Mitchell EJ, et al. Arterial stiffness and β-amyloid progression in nondemented elderly adults. JAMA Neurol. 2014;71(5):562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes TM, Wagenknecht LE, Craft S, et al. Arterial stiffness and dementia pathology: Atherosclerosis Risk in Communities (ARIC)-PET Study. Neurology. 2018;90(14):e1248–e1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gottesman RF, Schneider AL, Zhou Y, et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA. 2017;317(14):1443–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255–2263. [DOI] [PubMed] [Google Scholar]
- 17.Kivipelto M, Ngandu T, Laatikainen T, et al. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol. 2006;5(9):735–741. [DOI] [PubMed] [Google Scholar]
- 18.Stephen R, Liu Y, Ngandu T, et al. Associations of CAIDE Dementia Risk Score with MRI, PIB-PET measures, and cognition. J Alzheimers Dis. 2017;59(2):695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olson JL, Bild DE, Kronmal RA, Burke GL. Legacy of MESA. Glob Heart. 2016;11(3):269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. [DOI] [PubMed] [Google Scholar]
- 21.Villeneuve S, Rabinovici GD, Cohn-Sheehy BI, et al. Existing Pittsburgh Compound-B positron emission tomography thresholds are too high: statistical and pathological evaluation. Brain. 2015;138(Pt 7):2020–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lockhart SN, Mayda AB, Roach AE, et al. Episodic memory function is associated with multiple measures of white matter integrity in cognitive aging. Front Hum Neurosci. 2012;6:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teng EL, Hasegawa K, Homma A, et al. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr. 1994;6(1):45–62. [DOI] [PubMed] [Google Scholar]
- 24.Liang S, Steffen LM, Steffen BT, et al. APOE genotype modifies the association between plasma omega-3 fatty acids and plasma lipids in the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2013;228(1):181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flueckiger P, Longstreth W, Herrington D, Yeboah J. Revised Framingham Stroke Risk Score, nontraditional risk markers, and incident stroke in a multiethnic cohort. Stroke. 2018;49(2):363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Supp 2):S49–S73. [DOI] [PubMed] [Google Scholar]
- 27.Seshadri S, Wolf PA, Beiser A, et al. Stroke risk profile, brain volume, and cognitive function: the Framingham Offspring Study. Neurology. 2004;63(9):1591–1599. [DOI] [PubMed] [Google Scholar]
- 28.Jeerakathil T, Wolf PA, Beiser A, et al. Stroke risk profile predicts white matter hyperintensity volume: the Framingham Study. Stroke. 2004;35(8):1857–1861. [DOI] [PubMed] [Google Scholar]
- 29.Elias MF, Sullivan LM, D’Agostino RB, et al. Framingham stroke risk profile and lowered cognitive performance. Stroke. 2004;35(2):404–409. [DOI] [PubMed] [Google Scholar]
- 30.Hughes TM, Craft S, Baker LD, et al. Changes in metabolic risk factors over 10 years and their associations with late-life cognitive performance: the Multi-Ethnic Study of Atherosclerosis. Alzheimers Dement (Amst). 2017;8:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fitzpatrick AL, Rapp SR, Luchsinger J, et al. Sociodemographic correlates of cognition in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Geriatr Psychiatry. 2015;23(7):684–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weintraub S, Besser L, Dodge HH, et al. Version 3 of the Alzheimer Disease Centers’ Neuropsychological Test Battery in the Uniform Data Set (UDS). Alzheimer Dis Asso Disord. 2018;32(1):10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmidt P. Bayesian Inference for Structured Additive Regression Models for Large-scale Problems with Applications to Medical Imaging Dissertation. Ludwig Maximilian University of Munich; 2016. [Google Scholar]
- 36.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306–319. [DOI] [PubMed] [Google Scholar]
- 37.Maass A, Lockhart SN, Harrison TM, et al. Entorhinal Tau pathology, episodic memory decline, and neurodegeneration in aging. J Neurosci. 2018;38(3):530–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gottesman RF, Schneider ALC, Zhou Y, et al. The ARIC-PET amyloid imaging study: Brain amyloid differences by age, race, sex, and APOE. Neurology 2016;87(5):473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jack CR Jr, Wiste HJ, Weigand SD, et al. Age, sex, and APOEε4 effects on memory, brain structure, and β-amyloid across the adult life span. JAMA Neurology 2015;72(5):511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jansen WJ, Ossenkoppele R, Tijms BM, et al. Association of cerebral amyloid-β aggregation with cognitive functioning in persons without dementia. JAMA Psychiatry. 2018;75(1):84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whitwell JL, Tosakulwong N, Weigand SD, et al. Does amyloid deposition produce a specific atrophic signature in cognitively normal subjects? NeuroImage Clinical. 2013;2:249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hill CV, Pérez-Stable EJ, Anderson NA, Bernard MA. The National Institute on Aging Health Disparities Research Framework. Ethn Dis. 2015;25(3):245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dufouil C, Beiser A, McLure LA, et al. Revised Framingham Stroke Risk Profile to Reflect Temporal Trends. Circulation. 2017;135(12):1145–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaffashian S, Dugravot A, Elbaz A, et al. Predicting cognitive decline: a dementia risk score vs. the Framingham vascular risk scores. Neurology. 2013;80(14):1300–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


