Abstract
Research suggests social connectedness may help older adults with dementia maintain cognitive functionality and quality of life. However, little is known about its specific social and biological mechanisms. This paper proposes two pathways through social bridging (i.e., cognitive enrichment through expansive social networks) and bonding (i.e., neuroendocrine benefits of integration in cohesive social networks). We provide preliminary evidence for these pathways using neuroimaging, cognitive, and egocentric social network data from the Social Networks and Alzheimer’s Disease (SNAD) study (N=280). We found that network size, density, and presence of weak ties (i.e., social bridging) moderated the association between brain atrophy and cognitive function, while marriage/cohabitation (i.e., social bonding) moderated the association between perceived stress and cognitive function. We argue that social connectedness may have downstream implications for multiple pathophysiological processes in cognitive aging, even negating existing structural damage to the brain, making it a strong candidate for clinical or policy intervention.
(1). NARRATIVE
Decades of intensive research on a relatively narrow set of pathogenic processes occurring in the brain and body have produced few effective new drugs or other significant advances in the prevention and treatment of Alzheimer’s disease and related dementias (ADRD).1–3 Moreover, the disproportionate focus on biological pathogenesis has diverted empirical attention and resources away from upstream social environmental factors like social connectedness that have a larger effect on older adults’ morbidity and mortality than proximate risk factors, pound for pound.4,5 This is because upstream social factors determine people’s “risk for risk factors” of premature cognitive aging, such as neuroendocrine function and cardiovascular health.6,7 Consequently, intervening at the level of the social environment can simultaneously disrupt multiple cascading pathogenic processes occurring downstream in a chain of causation.
In the current study, we focus on social connectedness as one upstream social determinant that holds great promise for reducing morbidity and maintaining cognitive functionality and quality of life among older adults with ADRD. While it has long been known that the incidence and progression of cognitive decline are associated with the extent to which individuals are socially active and connected to others,8–12 the specific underlying social and biological mechanisms are less clear and rarely explicitly tested.9,11 Drawing linkages between adjacent social and neurological theories, we propose two major hypothesis-driving mechanisms – social bridging and bonding – through which social connectedness determines or disrupts biological pathways in cognitive aging. We then present preliminary evidence for these mechanisms using a personal social network approach that precisely measures bridging and bonding by collecting detailed information about the set of family members, friends, coworkers, neighbors and others with whom older adults interact regularly, as well as the ties between them.13
Mechanism 1:
Social bridging.
The mechanism of social bridging refers to social enrichment occurring in the context of expansive networks with casual relationships that cut across or link different social groups.14,15 The older adult with social bridging potential has an active social life and engages in various work and/or leisure activities with a heterogenous set of friends and acquaintances. Imagine the older adult who volunteers for a community organization or participates in a supper club, and occasionally gets together with friends or colleagues of different ages, races, and political affiliations. Social scientists have shown that access to “weak” ties such as these provides opportunities for exposure to novel social stimuli, including a diversity of ideas, information, activities, verbal and nonverbal social cues, faces, and speech patterns.16–18 These kinds of interactions are likely to be more cognitively enriching than familiar, repetitive, and comfortable exchanges with immediate family members and other close confidantes.
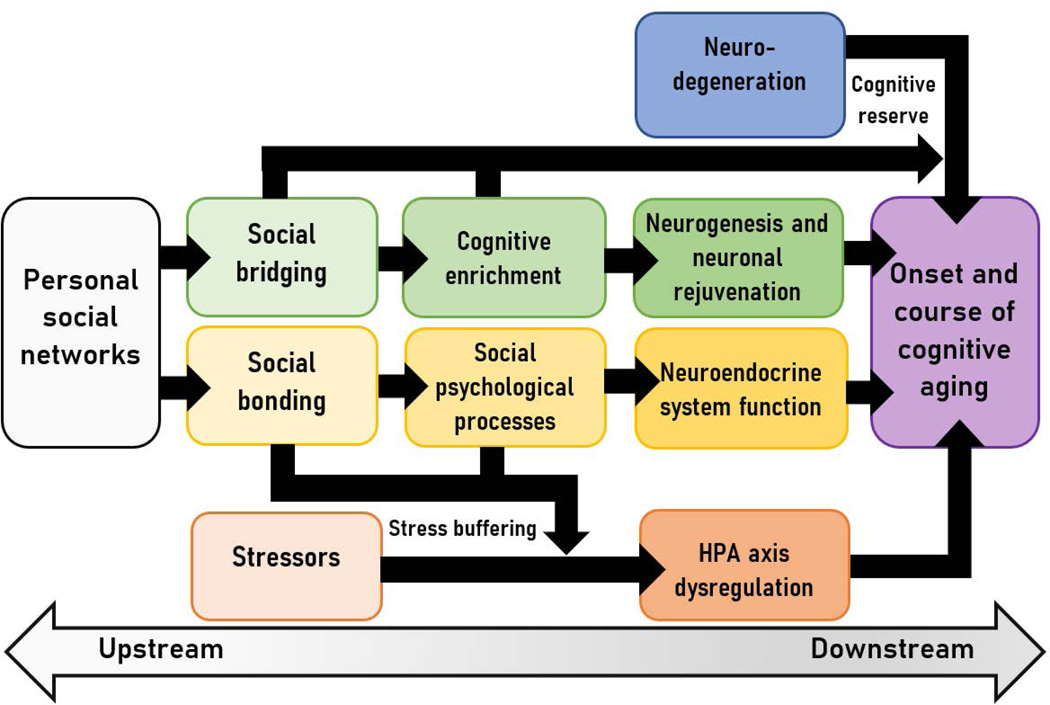
The cognitive enrichment provided by social bridging may shape brain health directly, as shown in Fig. 1, by triggering gene expression and catalyzing functional connectivity and neuronal growth, including brain repair.19–22 These effects of social bridging on the brain are consistent with recent studies indicating that greater network complexity, measured as irregular contact with a diversity of social roles, frequent interactions with friends (but not family members), and less densely connected networks are associated with healthy brain volume and connectivity, and better cognitive functioning in older adults.23–28
Figure 1.
Social bridging and bonding pathways linking personal social networks and ADRD risk and resilience
Social bridging may also indirectly affect trajectories of cognitive decline through an accumulation of cognitive reserve (See Fig. 1). Up to one-third of older adults meeting full neuropathological criteria for ADRD exhibit no significant cognitive impairment, suggesting that even relatively advanced neurodegeneration may be masked (or moderated) by compensatory processes, including social enrichment.29–31 For example, individuals with higher levels of social engagement, more friends, and a diverse set of associates are more resilient than similar peers, on average, to the cognitive effects of neurodegeneration.32,33 Likewise, social network size and complexity are associated with functional connectivity between the amygdala and other brain regions and with neural response to social information,34,35,35 suggesting that neural networks may be a mechanism of cognitive reserve linked to social bridging.
Mechanism 2:
Social bonding.
The mechanism of social bonding refers to health-enhancing resources conferred through integration in a cohesive network of close and homogenous ties.16,36 The older adult experiencing social bonding has a relatively small and tight-knit group of very close family members and friends who interact regularly with him or her, providing companionship, emotional support, and help with day-to-day tasks when needed. Bonding ties are intimate, trusting, cooperative, and associated with meaningful social roles like spouse/partner or parent.37 People with high levels of social bonding seldom feel lonely or isolated, and express a sense of purpose in life, mattering, and belonging – perceptions which foster behavioral guidance and security, as well as self-esteem and a sense of control or mastery.16,37
As seen in Figure 1, social bonding likely contributes to brain health through primary neuroendocrine pathways that affect numerous brain and bodily functions.6,38 For example, perceived social support and loneliness have been associated with variation in basal cortisol, which influences immune system, metabolic, and cardiovascular outcomes implicated in ADRD risk in older adults.39,40 Levels of oxytocin, produced during courtship, intimate touch, and other bonding interactions, have similar beneficial downstream effects41–44
Social bonding may also affect brain health indirectly by buffering the negative impact of stress (See Fig. 1). Experiences of acute and chronic stress lead to hypersecretion of stress hormones in the hypothalamic-pituitary-adrenal (HPA) axis, and have adverse consequences for brain health.45,46 In humans, stress is associated with memory impairment and faster rates of cognitive decline among those with early stage ADRD.38,46,47 Research provides evidence that social bonding can moderate these biological effects of stress on the brain by reducing negative appraisal of stressors, promoting active and effective coping, and downregulating the HPA response to stress.16,48–51
Implications and Future Research.
To this point, we have argued that social bridging and bonding are two key mechanisms linking older adults’ social networks to cognitive health, and that these reduce risk and promote resilience both directly and indirectly through downstream biological pathways. In the following section, we present preliminary evidence for these mechanisms. Remarkably, among older adults with expansive and loosely-connected social networks, we find no observable relationship between structural neurodegeneration and cognitive function, suggesting that social bridging confers cognitive reserve. Likewise, among older adults who are married or cohabitating – arguably the most important bonding relationship – we find no association between perceived stress and impairment in cognitive function, in contrast to their single, divorced, or widowed peers.
Why should we care about characterizing the specific social and biological mechanisms of social connectedness in cognitive and brain health? First and foremost, our findings indicate that in some circumstances, social bridging and bonding may negate damage already done to the brain and body, including neurodegeneration.32,50 Better understanding these processes can inform sustainable and scalable translational activities that reduce morbidity and allow older adults with ADRD to maintain cognitive functionality and quality of life. For example, social policies and programs that facilitate engagement across the life course, such as intergenerational social service or volunteering programs,52,53 accessible public spaces engineered to encourage socialization,54,55 and paratransit programs that provide free transportation for older adults,56 are likely to enhance social bridging potential. Likewise, initiatives like universal broadband and economic and social support for caregivers can help older adults prevent social isolation and maintain strong bonding ties to family and other loved ones.57,58
Second, intervening on social connectedness may help solve the “whack-a-mole” problem created by the multifactorial etiology of ADRD. Neurologists have argued that any successful intervention for ADRD must target multiple pathophysiological processes simultaneously.2 As an upstream social determinant, social connectedness is an outstanding candidate for basic and translational research because it has downstream implications for nearly every biological pathway in ADRD etiology. However, testing the mechanisms proposed here and designing social interventions that account for the complexity of underlying biological pathways will require strategic investment in interdisciplinary team science, longitudinal cohort studies, and integration of social and biological data.6
Third, these preliminary findings underscore the multidimensional functionality of social networks. That is, not all social ties or forms of social engagement work via the same mechanisms or have equal outcomes. Remaining active in a broader and more diverse network of friends and associates may be necessary for building cognitive reserve. At the same time, being embedded in strong, integrating relationships is likely beneficial for buffering the inflammatory effects of stress on the brain. The ideal network, from a cognitive aging perspective, might have a robust core-periphery structure – that is, a small and highly supportive set of core bonding ties, including a spouse or romantic partner, complemented by an extensive and diverse network of casual friends and acquaintances. Together, these preliminary findings suggest that it is critical to expand hypothesis testing about the role of social connectedness in ADRD to include additional social and biological mechanisms (i.e., not whether social networks matter, but how and why), and to be more precise in our approach to measuring and analyzing them.
(2). CONSOLIDATED RESULTS AND STUDY DESIGN
The bridging and bonding mechanisms outlined above provide a blueprint for hypothesis testing about the social and biological mechanisms that affect the onset and course of ADRD. Here, we examined whether social bridging moderated the association between brain atrophy and cognitive function, consistent with a cognitive reserve hypothesis. This constitutes one of only a handful of studies that leverage neuroimaging biomarkers to concretely link connectedness to downstream biological processes. Second, we assessed whether social bonding moderated the association between stress and cognitive function, consistent with a stress buffering hypothesis. We were unable to find similar published results, despite considerable evidence for its plausibility, as described above.
We employed data from the ongoing Social Networks in Alzheimer’s Disease (SNAD) study, a cohort study of older adults with normal cognition, mild cognitive impairment (MCI), and early-stage ADRD (N=280). We used personal social network methods, described in detail below, to facilitate disaggregation of distinct dimensions of social connectedness. A hallmark of this methodology is the collection of data about the structure, function, and composition of individuals’ social ties across a range of different social contexts (e.g., family, friends, coworkers, neighbors). In a true personal network approach, individuals to whom respondents are connected are individually distinguishable, and characteristics of each network member are collected to provide information about the nature of relationships and interactions occurring within the network.13
We used three personal social network measures to operationalize social bridging – number of people in the network (i.e., network size), the connectedness of people in the network to one another (i.e., network density), and the presence of weak ties in the network (i.e., strength of weakest tie). To operationalize social bonding, we used marital status (i.e., currently married or cohabitating as married). Our outcome was global cognitive function, and we also modeled the effects of amygdalar atrophy and perceived stress to approximate pathological processes.
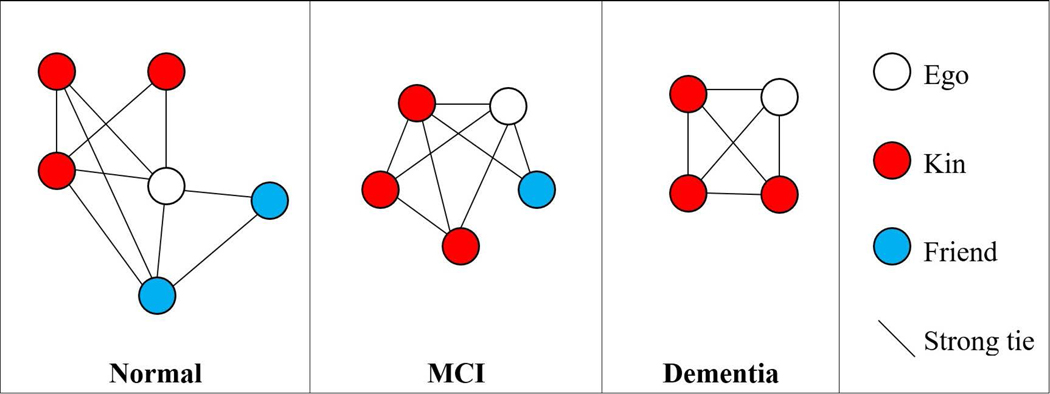
We first examined correlations between social network characteristics and consensus clinical diagnosis. Figure 2 depicts average network characteristics of participants across diagnostic categories. Cognitively normal older adults had personal networks that were significantly larger, less densely connected, and contained weaker ties, on average, than those with a diagnosis of MCI or ADRD.
Figure 2.
Typical network structure and composition by diagnostic group (SNAD; N=280)
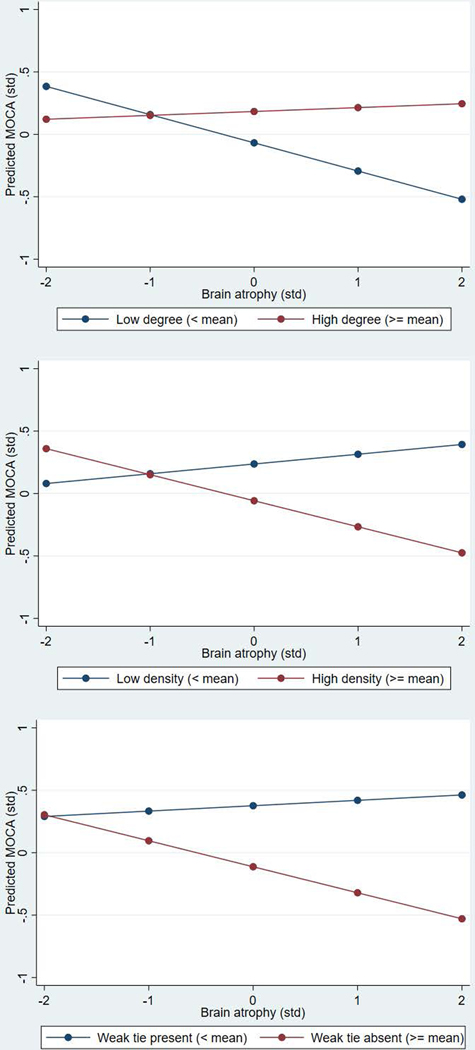
Next, we examined social bridging pathways. Results indicated that older adults with larger and less densely connected networks that contained weaker ties had better global cognitive function, controlling for gender, education, age, marital status, and depression symptoms. Additionally, network size, density, and minimum tie strength significantly moderated the association between amygdalar atrophy and standardized cognitive function, as depicted in Figure 3. That is, tere was no significant association between brain atrophy and cognitive function among subjects with high social bridging, but the expected relationships were observed for those with low social bridging.
Figure 3.
Association between amygdalar atrophy and predicted cognitive function at low and high network degree, density, and minimum tie strength (SNAD; n=135)
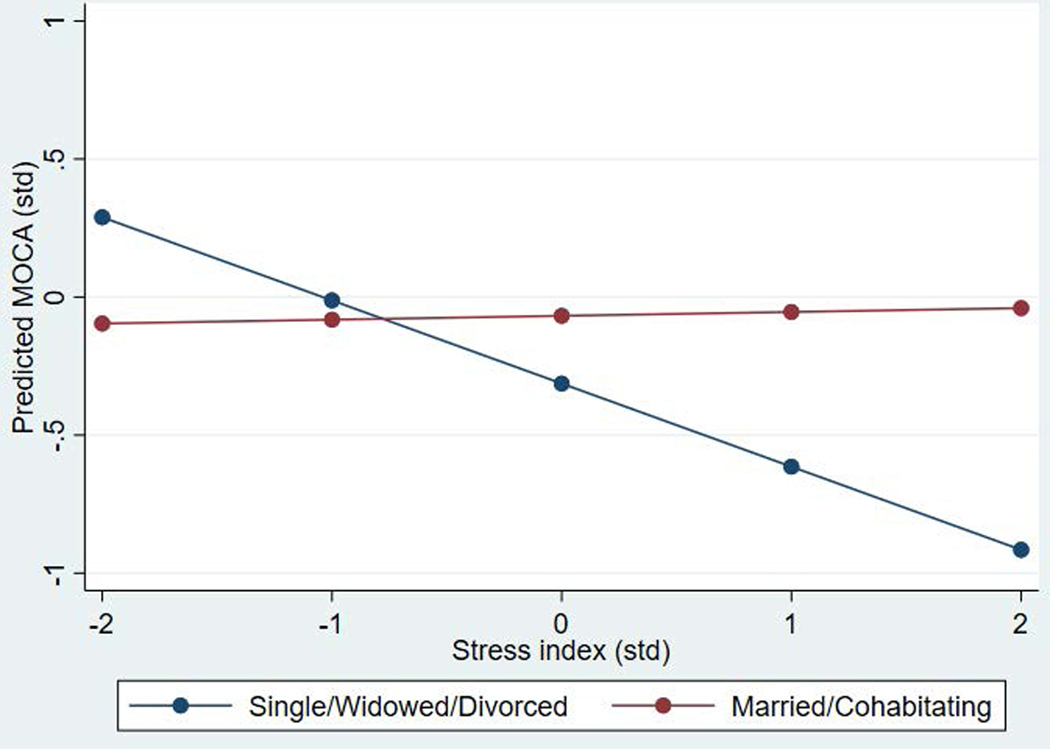
Analyses examining social bonding pathways also provided preliminary evidence of moderation consistent with stress-buffering. As depicted in Figure 4, there was no relationship between the stress index and cognitive function among those who were married or cohabitating, but there was a strong association among those who were widowed, divorced, or never married.
Figure 4.
Association between a stress index and predicted cognitive function by marital/cohabitating status (SNAD; n=271)
(3). DETAILED METHODS AND RESULTS
SNAD is an ongoing longitudinal study that leverages neuroimaging research being conducted at the Indiana Alzheimer Disease Research Center (IADRC). Three groups of subjects from the IADC cohort were recruited (89% response rate): 1) cognitively normal (CN) older adults, 2) mild cognitive impairment (MCI), and 3) early-stage AD. Subjects with advanced AD (MoCA<10) or other types of dementia were excluded (diagnoses made by consensus panel). The social bonding analysis included 271 subjects without missing data who were enrolled in the baseline SNAD study (N=280), while the social bridging analysis included a subset of 135 subjects who completed neuroimaging. We limited analyses to the baseline sample because data collection is ongoing and there were insufficient observations over time to conduct longitudinal analysis. See Appendix A (Tables A1 and A2) for full and restricted sample descriptive statistics.
Measures.
SNAD personal network data were collected using an expanded PhenX Social Network Battery tailored to ADRD, which took about 25 minutes to administer. Participants were first asked a series of “name generator” questions designed to elicit particular types of social ties. These targeted discussants (people participants actively sought out for advice and discussion) and regulators (people who encouraged healthy behavior change) to maximize measurement of potential social mechanisms (e.g., social support and social control). There was no limit on the number of alters named. Subsequently, we administered a set of questions about each named alter, including gender, years of education, frequency of contact, relationship strength, and duration of relationship. In addition, we elicited basic information about ties between alters. For analysis, data were aggregated to construct independent variables that measure three basic characteristics of social networks: degree (i.e., network size, or number of alters), density (i.e., mean strength of ties between alters, ranging from 1–4), and minimum tie strength (i.e., strength of weakest tie, ranging from 1–10). Social bridging was operationalized using high degree, low density, and low minimum tie strength. Additionally, we used a variable indicating that a subject was currently married or cohabitating to operationalize social bonding. See Appendix A for measurement details.
The key dependent variable was the Montreal Cognitive Assessment (MoCA), a brief screening tool that assesses global cognitive function.59 Bilateral atrophy in the amygdala (normed by intracranial volume) was measured with structural magnetic resonance imaging (MRI). The stress index was computed using a 4-item anxiety screener and 3 items (reverse coded) from the Quality of Life Alzheimer’s Disease scale that measured current circumstances (e.g., financial, living situation).60 These items factored together in an exploratory factor analysis, with a Cronbach’s alpha of 0.76 for the scale (See Table A5). Controls included sex, age, married/cohabitating, years of education, and the 15-item Geriatric Depression Scale.61
Analysis.
We examined bivariate relationships between diagnostic group and network characteristics at baseline. We then conducted regression models predicting standardized MoCA using the above variables (standardized where appropriate). To assess the cognitive reserve and stress buffering hypotheses, we used pooled models with interaction terms to determine whether 1) network degree, density, and strength of weakest tie moderated the relationship between brain atrophy and cognitive function and whether 2) being married or cohabitating moderated the association between the stress index and cognitive function. Figures of predicted values were used to depict interaction effects.
Detailed Results.
Bivariate correlations between social network characteristics and consensus clinical diagnosis are presented in Tables A1 and A2. Cognitively normal older adults had personal networks that were larger (Mean=5.34, p<.001), less densely connected (Mean=1.65; p<.01), and contained weaker ties (Mean=6.52; p<.001), on average, than those with a diagnosis of MCI (Mean=4.58; Mean=1.93; Mean=7.98, respectively) or ADRD (Mean=3.47; Mean=2.03; Mean=8.20, respectively). There were no significant differences in marital status across diagnostic groups.
Next, we examined social bridging pathways using multivariate analyses. Results from linear regression models (See Appendix A, Table A3) indicated that network size (B=0.16; p<.05), density (B=−0.23; p<.01), and minimum tie strength (B=−0.21; p<.001) were associated with standardized cognitive function, adjusting for controls. Additionally, network size (p<.01), density (p<.001), and minimum tie strength (p<.001) significantly moderated the association between amygdalar atrophy and standardized cognitive function (See Table A3).
Multivariate results examining social bonding pathways are presented in Appendix A, Table A4. We did not identify a direct significant relationship between either marital status or stress and cognitive function. However, there was evidence of a significant interaction between these variables (p<.05). There was no relationship between stress and cognitive function among those who were married or cohabitating, but there was a strong association among those who were widowed, divorced, or never married.
Limitations.
The cross-sectional nature of these preliminary data is a limitation of this analysis. Attention to issues of causation and selection is of paramount importance as the pathways presented here are empirically tested. Symptoms of cognitive decline may precede changes in social interaction.62 Loss of cognitive function may cause a person’s network to become smaller, more densely-knit, kin-centered, and increasingly supportive as caregiving needs intensify and relationships are harder to maintain. The presence of reverse causation does not negate the influence of social causation, but may reflect feedback loops between cognitive decline and network dynamics that represent critical points of intervention. Moreover, subtle changes in social connectedness may be a prodromal indicator of declining social cognitive abilities and MCI, making the reverse causation pathway a valuable subject for future study in its own right.
Another limitation of the personal social network methods used here is that they are inefficient to administer and can be cognitively burdensome, raising the possibility that findings are attributable to people with MCI or ADRD forgetting to name more peripheral members of their network. Our research comparing network accounts provided by older adults and their study partners supports the validity of the personal network approach and reduces concerns about forgetting.63 Moreover, there are significant advantages to this approach in a research setting. Namely, participants in studies of ADRD are typically asked proxy questions that summarize complex social phenomena using a single measure or scale, such as “How many friends do you have?” However, the social lives of older adults are multiplex and heterogeneous, and not suitably captured by summary indices.64 Consequently, measurement of this kind often does not permit testing of alternative hypotheses about social or biological mechanisms.8,11 For example, proxy measures cannot distinguish whether more social connectedness corresponds to a higher quantity of social support, engagement with a diverse set of weak ties, more frequent interactions, or some combination of the above. Nonetheless, the personal network approach is ill-suited for clinical settings and for older adults with advanced dementia, limiting its utility in some contexts.
Finally, given the space constraints of a single published article, we were unable to include a comprehensive set of social mechanisms through which social connectedness might affect cognitive and brain health. Specifically, although most research focuses on the beneficial effects of social connectedness, social network ties can themselves be sources of stress, including negative life events involving losses or exits from relationships and social roles (e.g., widowhood), or dysfunction and strain in ongoing relationships (e.g., caregiver burden).45,65 Spillover stress, a condition that results from close social or emotional proximity to others experiencing a negative life event or chronic strain, is also common.66 Likewise, a large literature points to the role of social influence, suggesting that the attitudes, beliefs, and behaviors of social network members shape the diet, physical activity, substance use, and sleep habits of adults – all of which are associated with ADRD risk and resilience through downstream pathophysiological mechanisms.67–72 We were unable to give these other important social mechanisms sufficient attention here.
Supplementary Material
Acknowledgements:
We are grateful to Evan Finley, Hope Sheean, Tugce Duran, Shannon Risacher, Bernice Pescosolido, Andrew Saykin, and Fred Unverzagt for their contributions to data collection and their support of the Social Networks and Alzheimer Disease study.
Funding:
This work was funded by the National Institute on Aging (5R01AG057739; 5P30AG010133), an Indiana University Collaborative Research Grant through the Vice President of Research, and a Proposal Development Grant from the Indiana Clinical and Translational Sciences Institute (UL1TR002529). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Indiana University.
Brea L. Perry: This work was funded by the National Institute on Aging (R01 AG057739; P30 AG010133), an Indiana University Collaborative Research Grant through the Vice President of Research, and a Proposal Development Grant from the Indiana Clinical and Translational Sciences Institute (UL1TR002529). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Indiana University. Additional funding obtained but not directly related to this manuscript includes grants from NIH (R01 AG070931; MPIs Brea Perry and Anne Krendl) and a Trustee Grant from the Russell Sage Foundation. All grants were to the institution. In addition, Perry received consulting fees from Shatterproof LLC and an honorarium from the University of Illinois.
William R. McConnell: Obtained internal funding unrelated to this manuscript from Florida Atlantic University (Seed grant and faculty research account). McConnell also received consulting fees from Duke University and a travel stipend from Florida Atlantic University.
Max Coleman: Obtained funding for attending conferences from his PhD advisor through Indiana University.
Adam Roth: Obtained funding unrelated to this manuscript from the National Institutes of Health through a pilot grant (R24AG065159).
Siyun Peng: Received a travel stipend from his former PhD granting institution, Purdue University.
Liana Apostolova: Obtained funding for this research as MPI (R01 AG057739; P30 AG010133). Apostolova also received funding unrelated to this research from the National Institute on Aging (U01 AG057195; R01 AG057739; P30 AG010133), the Alzheimer Association (LEADS GENETICS 19–639372), and Roche Diagnostics (RD005665). All grants were to the institution. She received consulting fees from Eli Lilly, Biogen, and Two Labs, honoraria from the American Academy of Neurology, the Mayo Clinic, Purdue University, Biogen, MillerMed, APhA, and MJH Holdings LLC, and travel support from the Alzheimer Association. Apostolova served on the IQVIA Advisory Board and a National Institutes of Health Data Safety and Monitoring Board. She holds stock options in Cassava Sciences Inc. and Semiring.
Footnotes
Declarations of interest
References
- 1.Cummings J, Ritter A, Zhong K. Clinical trials for disease-modifying therapies in Alzheimer’s disease: a primer, lessons learned, and a blueprint for the future. Journal of Alzheimer’s disease. 2018;64(s1):S3–S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nehls M. Unified theory of Alzheimer’s disease (UTAD): implications for prevention and curative therapy. Journal of molecular psychiatry. 2016;4(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinstein JD. A new direction for Alzheimer’s research. Neural regeneration research. 2018;13(2):190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: a meta-analytic review. PLoS med. 2010;7(7):e1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holt-Lunstad J, Robles TF, Sbarra DA. Advancing social connection as a public health priority in the United States. American Psychologist. 2017;72(6):517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uchino BN. Social support and health: a review of physiological processes potentially underlying links to disease outcomes. Journal of behavioral medicine. 2006;29(4):377–387. [DOI] [PubMed] [Google Scholar]
- 7.Phelan JC, Link BG. Controlling disease and creating disparities: a fundamental cause perspective. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2005;60(Special_Issue_2):S27–S33. [DOI] [PubMed] [Google Scholar]
- 8.Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. The Lancet Neurology. 2004;3(6):343–353. [DOI] [PubMed] [Google Scholar]
- 9.Kuiper JS, Zuidersma M, Zuidema SU, et al. Social relationships and cognitive decline: a systematic review and meta-analysis of longitudinal cohort studies. International journal of epidemiology. 2016;45(4):1169–1206. [DOI] [PubMed] [Google Scholar]
- 10.Kuiper JS, Zuidersma M, Voshaar RCO, et al. Social relationships and risk of dementia: A systematic review and meta-analysis of longitudinal cohort studies. Ageing research reviews. 2015;22:39–57. [DOI] [PubMed] [Google Scholar]
- 11.Kelly ME, Duff H, Kelly S, et al. The impact of social activities, social networks, social support and social relationships on the cognitive functioning of healthy older adults: a systematic review. Systematic reviews. 2017;6(1):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans IE, Martyr A, Collins R, Brayne C, Clare L. Social isolation and cognitive function in later life: A systematic review and meta-analysis. Journal of Alzheimer’s disease. 2018;(Preprint):1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perry BL, Pescosolido BA, Borgatti SP. Egocentric Network Analysis: Foundations, Methods, and Models. Cambridge University Press; 2018. [Google Scholar]
- 14.Bonds Narayan D. and Bridges: Social Capital and Poverty. Vol 2167. Citeseer; 1999. [Google Scholar]
- 15.Coleman JS. Social capital in the creation of human capital. American Journal of Sociology. 1988;94:S95–S120. [Google Scholar]
- 16.Thoits PA. Mechanisms linking social ties and support to physical and mental health. Journal of health and social behavior. 2011;52(2):145–161. [DOI] [PubMed] [Google Scholar]
- 17.Granovetter MS. The strength of weak ties. American Journal of Sociology. 1973;78(6):1360–1380. [Google Scholar]
- 18.Pan X, Chee KH. The power of weak ties in preserving cognitive function: a longitudinal study of older Chinese adults. Aging & mental health. 2020;24(7):1046–1053. [DOI] [PubMed] [Google Scholar]
- 19.Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nature Reviews Neuroscience. 2009;10(9):647–658. [DOI] [PubMed] [Google Scholar]
- 20.Sale A, Berardi N, Maffei L. Enrich the environment to empower the brain. Trends in neurosciences. 2009;32(4):233–239. [DOI] [PubMed] [Google Scholar]
- 21.Sallet J, Mars RB, Noonan MP, et al. Social network size affects neural circuits in macaques. Science. 2011;334(6056):697–700. doi: 10.1126/science.1210027 [DOI] [PubMed] [Google Scholar]
- 22.Ainsworth M, Sallet J, Joly O, et al. Viewing ambiguous social interactions increases functional connectivity between frontal and temporal nodes of the social brain. Journal of Neuroscience. Published online 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellwardt L, Van Tilburg TG, Aartsen MJ. The mix matters: Complex personal networks relate to higher cognitive functioning in old age. Social science & medicine. 2015;125:107–115. [DOI] [PubMed] [Google Scholar]
- 24.Kanai R, Bahrami B, Roylance R, Rees G. Online social network size is reflected in human brain structure. Proc Biol Sci. 2012;279(1732):1327–1334. doi: 10.1098/rspb.2011.1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noonan MP, Mars RB, Sallet J, Dunbar RIM, Fellows LK. The structural and functional brain networks that support human social networks. Behav Brain Res. 2018;355:12–23. doi: 10.1016/j.bbr.2018.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharifian N, Kraal AZ, Zaheed AB, Sol K, Zahodne LB. Longitudinal Associations Between Contact Frequency with Friends and with Family, Activity Engagement, and Cognitive Functioning. Journal of the International Neuropsychological Society. Published online undefined/ed:1–10. doi: 10.1017/S1355617720000259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sommerlad A, Sabia S, Singh-Manoux A, Lewis G, Livingston G. Association of social contact with dementia and cognition: 28-year follow-up of the Whitehall II cohort study. PLoS medicine. 2019;16(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharifian N, Kraal AZ, Zaheed AB, Sol K, Zahodne LB. The longitudinal association between social network composition and episodic memory in older adulthood: the importance of contact frequency with friends. Aging & Mental Health. 2020;24(11):1789–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esiri MM, Chance SA. Cognitive reserve, cortical plasticity and resistance to Alzheimer’s disease. Alzheimer’s research & therapy. 2012;4(2):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Esiri MM, Matthews F, Brayne C, et al. Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Lancet. 2001;357(9251). [DOI] [PubMed] [Google Scholar]
- 31.Whalley LJ, Deary IJ, Appleton CL, Starr JM. Cognitive reserve and the neurobiology of cognitive aging. Ageing research reviews. 2004;3(4):369–382. [DOI] [PubMed] [Google Scholar]
- 32.Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS. The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: a longitudinal cohort study. The Lancet Neurology. 2006;5(5):406–412. [DOI] [PubMed] [Google Scholar]
- 33.Sharifian N, Zaheed AB, Morris EP, et al. Social network characteristics moderate associations between cortical thickness and cognitive functioning in older adults. Alzheimer’s & Dementia. Published online 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dziura SL, Thompson JC. Social-network complexity in humans is associated with the neural response to social information. Psychological science. 2014;25(11):2095–2101. [DOI] [PubMed] [Google Scholar]
- 35.Bickart KC, Hollenbeck MC, Barrett LF, Dickerson BC. Intrinsic amygdala-cortical functional connectivity predicts social network size in humans. J Neurosci. 2012;32(42):14729–14741. doi: 10.1523/JNEUROSCI.1599-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berkman LF, Glass T. Social integration, social networks, social support, and health. In: Social Epidemiology. Oxford University Press; 2000:137–173. [Google Scholar]
- 37.Cohen S. Social relationships and health. American psychologist. 2004;59(8):676. [DOI] [PubMed] [Google Scholar]
- 38.Kiecolt-Glaser JK, Gouin J-P, Hantsoo L. Close relationships, inflammation, and health. Neuroscience & Biobehavioral Reviews. 2010;35(1):33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cacioppo JT, Cacioppo S. Social relationships and health: The toxic effects of perceived social isolation. Social and personality psychology compass. 2014;8(2):58–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doane LD, Adam EK. Loneliness and cortisol: Momentary, day-to-day, and trait associations. Psychoneuroendocrinology. 2010;35(3):430–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karelina K, Stuller KA, Jarrett B, et al. Oxytocin mediates social neuroprotection after cerebral ischemia. Stroke. 2011;42(12):3606–3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Onaka T, Takayanagi Y, Yoshida M. Roles of oxytocin neurones in the control of stress, energy metabolism, and social behaviour. Journal of neuroendocrinology. 2012;24(4):587–598. [DOI] [PubMed] [Google Scholar]
- 43.Carter CS, Kenkel WM, MacLean EL, et al. Is Oxytocin “Nature’s Medicine”? Pharmacological Reviews. 2020;72(4):829–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jankowski M, Broderick TL, Gutkowska J. The role of oxytocin in cardiovascular protection. Frontiers in psychology. 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pearlin LI, Schieman S, Fazio EM, Meersman SC. Stress, health, and the life course: Some conceptual perspectives. Journal of health and social behavior. 2005;46(2):205–219. [DOI] [PubMed] [Google Scholar]
- 46.Scott SB, Graham-Engeland JE, Engeland CG, et al. The effects of stress on cognitive aging, physiology and emotion (ESCAPE) project. BMC psychiatry. 2015;15(1):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Magri F, Cravello L, Barilli L, et al. Stress and dementia: the role of the hypothalamic-pituitary-adrenal axis. Aging clinical and experimental research. 2006;18(2):167–170. [DOI] [PubMed] [Google Scholar]
- 48.Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological psychiatry. 2003;54(12):1389–1398. [DOI] [PubMed] [Google Scholar]
- 49.Smith AS, Wang Z. Hypothalamic oxytocin mediates social buffering of the stress response. Biological psychiatry. 2014;76(4):281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee S-Y, Park S-H, Chung C, Kim JJ, Choi S-Y, Han J-S. Oxytocin protects hippocampal memory and plasticity from uncontrollable stress. Scientific reports. 2015;5(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith AS, Wang Z. Salubrious effects of oxytocin on social stress-induced deficits. Hormones and Behavior. 2012;61(3):320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knight T, Skouteris H, Townsend M, Hooley M. The act of giving: A systematic review of nonfamilial intergenerational interaction. Journal of Intergenerational Relationships. 2014;12(3):257–278. [Google Scholar]
- 53.Weaver AJF, Hutter A, Almeida B. Intergenerational Programs: The Missing Link in Today’s Aging Initiatives. Published online 2018. [Google Scholar]
- 54.Leavell MA, Leiferman JA, Gascon M, Braddick F, Gonzalez JC, Litt JS. Nature-based social prescribing in urban settings to improve social connectedness and mental well-being: a review. Current environmental health reports. 2019;6(4):297–308. [DOI] [PubMed] [Google Scholar]
- 55.Ageing Toepoel V., leisure, and social connectedness: how could leisure help reduce social isolation of older people? Social indicators research. 2013;113(1):355–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levasseur M, Généreux M, Bruneau J-F, et al. Importance of proximity to resources, social support, transportation and neighborhood security for mobility and social participation in older adults: results from a scoping study. BMC public health. 2015;15(1):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Czaja SJ. The role of technology in supporting social engagement among older adults. Public Policy & Aging Report. 2017;27(4):145–148. [Google Scholar]
- 58.Ablitt A, Jones GV, Muers J. Living with dementia: a systematic review of the influence of relationship factors. Aging & mental health. 2009;13(4):497–511. [DOI] [PubMed] [Google Scholar]
- 59.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society. 2005;53(4):695–699. [DOI] [PubMed] [Google Scholar]
- 60.Thorgrimsen L, Selwood A, Spector A, et al. Whose quality of life is it anyway?: The validity and reliability of the Quality of Life-Alzheimer’s Disease (QoL-AD) scale. Alzheimer Disease & Associated Disorders. 2003;17(4):201–208. [DOI] [PubMed] [Google Scholar]
- 61.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clinical Gerontologist: The Journal of Aging and Mental Health. Published online 1986. [Google Scholar]
- 62.Aartsen MJ, Smits CH, Van Tilburg T, Knipscheer KC, Deeg DJ. Activity in older adults: cause or consequence of cognitive functioning? A longitudinal study on everyday activities and cognitive performance in older adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2002;57(2):P153–P162. [DOI] [PubMed] [Google Scholar]
- 63.Roth AR, Peng S, Coleman ME, Finley E, Perry BL. Network recall among older adults with cognitive impairments. Social Networks. 2021;64:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fiori KL, Smith J, Antonucci TC. Social network types among older adults: A multidimensional approach. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2007;62(6):P322–P330. [DOI] [PubMed] [Google Scholar]
- 65.Adelman RD, Tmanova LL, Delgado D, Dion S, Lachs MS. Caregiver burden: a clinical review. Jama. 2014;311(10):1052–1060. [DOI] [PubMed] [Google Scholar]
- 66.Bakker AB, Demerouti E. The spillover-crossover model. New frontiers in work and family research. Published online 2013:54–70. [Google Scholar]
- 67.Reid AE, Cialdini RB, Aiken LS. Social norms and health behavior. In: Handbook of Behavioral Medicine. Springer; 2010:263–274. [Google Scholar]
- 68.Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer disease. Jama. 2009;302(6):627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maesako M, Uemura K, Kubota M, et al. Exercise is more effective than diet control in preventing high fat diet-induced β-amyloid deposition and memory deficit in amyloid precursor protein transgenic mice. Journal of Biological Chemistry. 2012;287(27):23024–23033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Camiletti-Moirón D, Aparicio VA, Aranda P, Radák Z. Does exercise reduce brain oxidative stress? A systematic review. Scandinavian journal of medicine & science in sports. 2013;23(4):e202–e212. [DOI] [PubMed] [Google Scholar]
- 71.Dias GP, Cavegn N, Nix A, et al. The role of dietary polyphenols on adult hippocampal neurogenesis: molecular mechanisms and behavioural effects on depression and anxiety. Oxidative medicine and cellular longevity. 2012;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith KP, Christakis NA. Social networks and health. Annual Review of Sociology. 2008;34(1):405–429. doi: 10.1146/annurev.soc.34.040507.134601 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.