Highlights
-
•
Sucrose treatment is more effective than water treatment in postharvest baby mustard.
-
•
Visual quality of stored baby mustard was well maintained by sucrose treatment.
-
•
Antioxidant properties of stored baby mustard were improved by sucrose treatment.
-
•
Sucrose treatment performed well in glucosinolate preservation in baby mustard.
Keywords: Sucrose, Baby mustard (Brassica juncea var. gemmifera), Lateral bud, Sensory, Antioxidant, Glucosinolate
Abstract
The effect of water and sucrose treatments on the sensory quality and content of health-promoting compounds in the lateral buds of baby mustard stored at 20 °C was investigated in this study. Although water treatment maintained the content of various nutrients, the decay of baby mustard was greater under water treatment. Sucrose treatment delayed the weight loss and the decline in sensory parameter scores, chlorophyll and sucrose content; slowed the decline in antioxidant capacity by maintaining the content of carotenoids and ascorbic acid; suppressed the increase in total phenolics; and maintained and even increased the content of several individual glucosinolates in the lateral buds of baby mustard. These findings indicate that sucrose application can maintain the sensory and nutritional qualities of the lateral buds of postharvest baby mustard.
1. Introduction
Sugars are vital components in various aspects of plant development. Sucrose not only provides material and energy for plant growth and development but also serves as a signal molecule that regulates source–sink metabolism (Ho, Chao, Tong, & Yu, 2001). Sucrose is synthesized in source leaves and transported to sink tissue, such as the lateral buds of baby mustard, under normal conditions (Griffiths et al., 2016, Zanon et al., 2015). However, after the import of sucrose to the lateral buds of baby mustard is terminated after harvest, the concentration of sugars rapidly declines (Sun et al., 2021). Sucrose upregulates the transcript levels of cell wall-related genes and modulates the cell wall components in asparagus, which preserves its firmness and tenderness (Park, 2016). The exogenous application of sucrose affects sucrose transport in phloem tissue (Yoon, Cho, Tun, Jeon, & An, 2021). Supplying exogenous sucrose delays the decline in the sucrose content and increases the longevity of broccoli (Coupe, Sinclair, Watson, Heyes, & Eason, 2003). Sucrose feeding at the cut surface of the stem has been previously shown to suppress the yellowing of sepals and the loss of ascorbic acid in harvested broccoli florets (Nishikawa et al., 2005, Smirnoff and Pallanca, 1996). Another study of postharvest broccoli has shown that sucrose retards yellowing in harvested broccoli, suggesting that the sucrose supply could be a discrete senescence factor with an effect independent of its role as a respiratory substrate (Irving & Joyce, 1995). Sucrose has also been shown to regulate the accumulation of anthocyanins and glucosinolates in broccoli sprouts and increase the antioxidant level (Guo, Yuan, & Wang, 2011b).
Baby mustard (Brassica juncea var. gemmifera) is a vegetable native to China that occurs widely in the moist climate of Southwest China. It contains a considerable amount of soluble sugars, ascorbic acid, and various phenolics (Sun et al., 2018). As a member of the Brassica family, it is also a rich source of glucosinolates, which have anti-carcinogenic activity (Wang et al., 2021). The heads of baby mustard, including the lateral buds and swollen stem, are often harvested and can be conveniently transported and stored. Although the lateral buds and swollen stem are both edible, the lateral buds are more often consumed because of their aesthetically pleasing appearance, better taste, and higher nutritional value compared with the swollen stem. However, baby mustard deteriorates rapidly after harvest, and water loss and shrinkage are the most serious problems (Sun et al., 2021); therefore, retailers sometimes use water treatment to maintain its freshness and quality. Although several methods have been used to preserve the quality of postharvest baby mustard, such as long-term freezing treatment (Zhang et al., 2021), modified atmosphere packaging (Lin et al., 2021), and light exposure (Sun et al., 2021), the effect of water treatment on the postharvest quality of baby mustard has yet to be explored. There is also a need to develop techniques that could be applied to the heads of baby mustard after harvest.
No studies have examined whether immersion in sucrose could prolong the shelf life of baby mustard by stimulating sucrose uptake. Here, we characterized changes in the sensory quality, pigments, sucrose, glucose, fructose, ascorbic acid, total phenolics, antioxidant capacity, and glucosinolates of lateral buds of baby mustard treated with sucrose solution.
2. Materials and methods
2.1. Plant materials and sucrose treatments
Baby mustard (Brassica juncea var. gemmifera cv. Linjiang-Ercai) was harvested early in the morning from a local farm in Chengdu City, China, and immediately transported to the laboratory. Baby mustard heads used in experiments were uniform in size (0.45 ± 0.05 kg), free of insects, disease, and mechanical damage. The whole heads of baby mustard were randomly divided into three groups (sucrose treatment, water treatment, and air treatment (the control)), and there were four replicates for each group in the experiments. Each group of baby mustard was placed in wide-mouth glass bottles. The treatment stem ends were immersed in a 15 g L−1 sucrose solution with 0.05% sodium hypochlorite. In water-treated samples, 0.05% sodium hypochlorite was administered in the same manner. Sucrose solution or water was taken up continuously from the cut surface of the swollen stem tissue. Solutions were replaced daily; the amount of solution provided was sufficient to submerge the bottom of the swollen stem. Baby mustard in the non-solution-treated group was kept in the air. Afterwards, each replicate was transferred to a temperature-controlled chamber at 20 °C under 75% relative humidity in the dark.
Analyses of sensory quality and weight loss were conducted on samples taken at 0, 1, 3, and 5 d. Following the procedures of a previous study of edible parts (Sun et al., 2018), the lateral buds were removed from the swollen stems before sucrose treatment (0 d) and at the end of storage (5 d); they were then lyophilized and stored at − 20 °C for analyses of phytochemicals and antioxidant capacity.
2.2. Shelf life and sensory quality evaluation
Shelf life and sensory quality of baby mustard were assessed daily and on sampling day, respectively. Baby mustard was considered to have reached the end of their shelf life when they became soft, shrank, and exhibited browning (Sun et al., 2021). Sensory attributes include color, form, odor, decay, and acceptance. They were quantified on a scale from 5 (best) to 1 (worst), in which 3 represents “unsaleable”.
2.3. Weight loss
Weight loss (%) was calculated by the formula (Wx − W0)/W0 × 100, where W0 is the weight at 0 d, and Wx is the weight at a certain day after storage (Sun et al., 2021).
2.4. Chlorophyll and carotenoids content
Two hundred mg powder of lateral bud were ground and extracted with 25 mL acetone. The samples were sonicated for 20 min, and centrifuged at 4,000g at room temperature (20 ± 2 °C) for 5 min. The supernatant was filtered through 0.22 µm nylon syringe filters and analyzed by high performance liquid chromatography (HPLC). HPLC analysis of chlorophyll and carotenoids were carried out using an Agilent 1260 instrument with a VWD detector (Agilent Technologies, Inc., Palo Alto, USA). Samples (10 µL) were separated at 30 °C on a Waters Nova-Pak C18 column (150 mm × 3.9 mm i.d.; 4 µm particle size) using isopropanol and 80% acetonitrile–water at a flow rate of 0.5 mL min−1. Absorbances were detected at 448 and 428 nm. Chlorophyll (a and b) and carotenoids (neoxanthin, violaxanthin, lutein, and β-carotene) were quantified according to the respective standard calibration curves, and their standards were obtained from Solarbio Science and Technology Co., Ltd. (Beijing, China). Result of chlorophyll and carotenoids content was expressed as g kg−1 of dry weight (Sun et al., 2021).
2.5. Sucrose, glucose, and fructose content
Sucrose, glucose, and fructose were extracted and analyzed as previously described (Sun et al., 2021). Freeze-dried samples (100 mg) were added to distilled water. The mixture was then extracted in a water bath at 80 °C for 30 min. The supernatant was collected after centrifugation at 8000g at room temperature (20 ± 2 °C) for 5 min, and filtered through 0.45 µm cellulose acetate filter, and then analyzed by HPLC using an Agilent 1260 instrument equipped with a refractive index detector (Agilent Technologies, Inc., Palo Alto, USA). Samples were separated at 35 °C on an Agilent ZORBAX carbohydrate column (250 mm × 4.6 mm i.d.; 5 µm particle size) using 80% acetonitrile at a flow rate of 1.0 mL min−1. Content of sucrose, glucose, and fructose were determined using the standard curves for each sugar. Result of sucrose, glucose, and fructose content was expressed as g kg−1 of dry weight.
2.6. Ascorbic acid content
Fifty mg of sample powder was extracted with 5 mL 1.0% oxalic acid, subsequently centrifuged 5 min at 4000g. Each sample was filtered through a 0.45 µm cellulose acetate filter. HPLC analysis of ascorbic acid was carried out using an Agilent 1260 instrument with a VWD detector (Agilent Technologies, Inc., Palo Alto, USA). Samples were separated on a Waters Spherisorb C18 column (150 mm × 4.6 mm i.d.; 5 µm particle size), using a solvent of 0.1% oxalic acid at a flow rate of 1.0 mL min−1. The amount of ascorbic acid was calculated from absorbance values at 243 nm, using authentic ascorbic acid (Sangon Biotech Co., Ltd., shanghai, China) as a standard. Result of ascorbic acid content was expressed as g kg−1 of dry weight (Sun et al., 2018).
2.7. Total phenolics content
Total phenolics were homogenized for 1 min and extracted with 10 mL of 50% ethanol, and then incubated at room temperature (20 ± 2 °C) for 24 h in the dark. The suspension was centrifuged at 4000g for 5 min at room temperature. The supernatant was used for the measurements of total phenolics content and antioxidant activity. The supernatant was mixed with Folin-Ciocalteu reagent, after 3 min, saturated sodium carbonate was added. The absorbance was measured at 760 nm with a UV-1800 spectrophotometer (Mapada Instruments Co., Ltd., Shanghai, China). Gallic acid (Sangon Biotech Co., Ltd., shanghai, China) was used as a standard and the results were expressed as g gallic acid equivalent kg−1 dry weight (Sun et al., 2018).
2.8. Ferric reducing antioxidant power (FRAP)
The extracted samples were added to the FRAP working solution incubated at 37 °C. The absorbance was then recorded at 593 nm using a spectrophotometer after the mixture had been incubated in at 37 °C for 10 min. FRAP values were calculated based on FeSO4·7H2O standard curves and expressed as mmol kg−1 dry weight (Sun et al., 2018).
2.9. 2,2-azinobis (3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS) assay
An aliquot of 300 μL of each extracted sample was added to 3 mL of ABTS+ solution. The absorbance was measured spectrophotometrically at 734 nm after exactly 2 h. The percentage inhibition was calculated according to the formula: % inhibition = [(Acontrol-Asample)/Acontrol] × 100% (Sun et al., 2018).
2.10. Glucosinolate composition and content
Freeze-dried samples (100 mg) were boiled in 5 mL water for 10 min. The supernatant was collected and applied to a DEAE-Sephadex A-25 column (Sigma Chemical Co., Saint Louis, USA). The glucosinolates were converted into their desulpho analogues by treated with aryl sulphatase, and the desulphoglucosinolates were eluted. HPLC analysis was carried out using an Agilent 1260 HPLC instrument equipped with a variable wavelength detector (VWD) detector (Agilent Technologies, Inc., Palo Alto, USA). Samples were separated at 30 °C on a Waters Spherisorb C18 column (250 mm × 4.6 mm i.d.; 5 µm particle size) using acetonitrile and water at a flow rate of 1.0 mL min−1. Absorbance was detected at 226 nm. Glucosinolates were quantified by using ortho-Nitrophenyl β-d-galactopyranoside (Sigma Chemical Co., Saint Louis, USA) as the internal standard and considering the response factor of each glucosinolate. Result of glucosinolate content was expressed as mmol kg−1 of dry weight (Sun et al., 2018).
2.11. Statistical analysis
The design was completely random. Statistical analysis was performed using the SPSS package program version 18 (SPSS Inc., Chicago, IL, USA). Data were analyzed using one-way ANOVAs. The means were compared through the least significant difference (LSD) test at a significance level of 0.05. A principal components analysis (PCA) was performed using SIMCA 13.0 software (Umetrics, Malmö, Sweden) with unit variance scaling to determine the relationships among the samples. The correlation result was visualized using Cytoscape v. 3.5.1 (The Cytoscape Consortium, New York, USA) (Sun et al., 2021), and the data are shown in Supplementary Table 1.
3. Results and discussion
3.1. Sensory quality
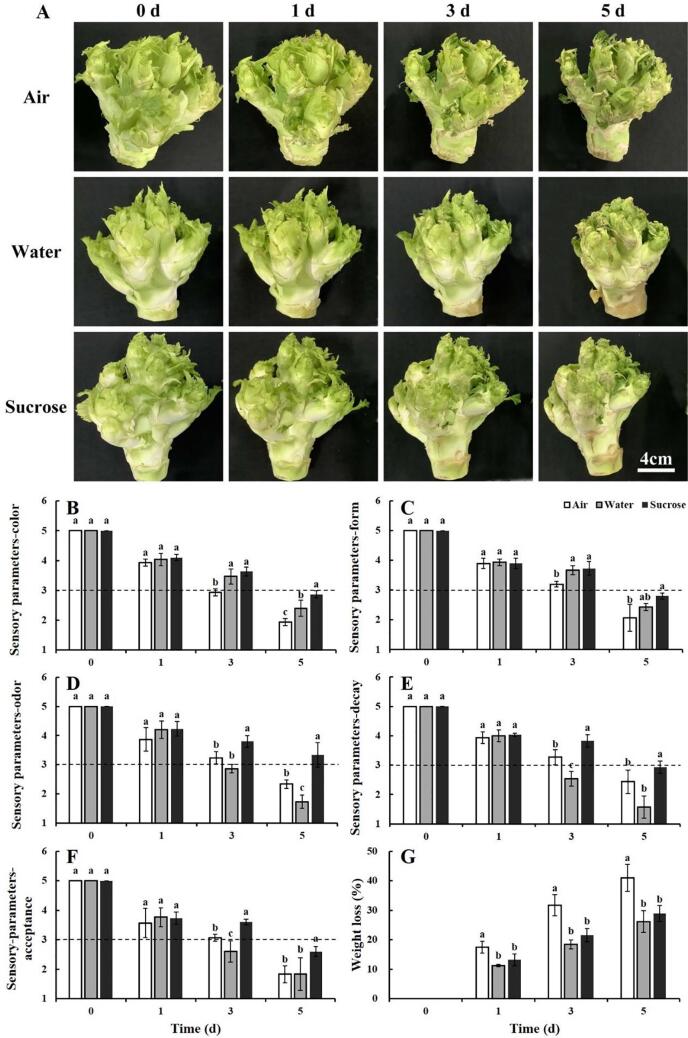
Baby mustard in the control gradually deteriorated, and the lateral buds showed more severe shriveling and browning of the peel during storage (Fig. 1A). Water treatment reduced the shrinkage and browning of baby mustard; however, it also caused the decay of the swollen stem, which shortened its shelf life compared with the control. The samples under sucrose treatment had the best visual quality among the control and treatments (Fig. 1A).
Fig. 1.
Sensory quality and weight loss of baby mustard that had been exposed to air, water, or sucrose solution during the storage. A: Appearance of the whole heads of baby mustard at 0, 1, 3, and 5 d after harvest under different treatments. Scale bar = 4 cm; B–F: Sensory parameters including color, form, odor, decay, and acceptance of samples; G: weight loss. Different letters in the figure indicate statistically significant differences among treatments for each storage day (P < 0.05).
During the entire storage period, the sensory parameter scores of the three groups of baby mustard gradually decreased (Fig. 1B–F). Compared with the water and sucrose treatments, the color and form scores in the control declined most rapidly with a sensory score of approximately 3 at 3 d. The color and form scores were higher for baby mustard under water and sucrose treatments compared with the control (Fig. 1B and C). There existed similar tendancy in the scores of odor, decay, and acceptance (Fig. 1D–F). The off-odor related to decay has been shown to be the factor limiting the commercial acceptance of baby mustard (Lin et al., 2021). A visible area of decay could be observed on water-treated baby mustard heads, and the odor score was only 1.7 at the end of the storage period; the odor score of the control was 2.3 (Fig. 1D). Sucrose treatment maintained the fragrant flavor of baby mustard (Fig. 1D) and suppressed decay. The acceptance score of baby mustard was 2.6 at 5 d under sucrose treatment, which prolonged the shelf life of baby mustard by approximately 2 d compared with the control (Fig. 1F). The application of sucrose has been shown to significantly extend the shelf life of broccoli (Xu et al., 2016). Similarly, the overall appearance of asparagus treated with sucrose and stored at 2 ℃ was rated as good and excellent for 18 d (Park, 2016). Sucrose treatment has also been shown to prolong the vase life of many types of cut flowers (Abdulla and Çelikel, 2019, Norikoshi et al., 2016, Rabiza-Świder et al., 2020).
3.2. Weight loss
The freshness and tenderness of postharvest vegetables mainly depend on the water pressure in the body: if water is lost, cell turgor pressure decreases, and baby mustard heads begin to shrink. Therefore, weight loss is important for evaluating the quality of postharvest baby mustard during storage (Sun et al., 2021). A progressive decline in weight was observed in all three treatments. Weight loss in the control was the most dramatic, reaching 41% at 5 d (Fig. 1G). The heads of baby mustard experienced less weight loss compared with the lateral buds, which exceeded 60% under similar storage conditions (Sun et al., 2021). This may be ascribed to the smaller degree of damage and better skin strength in the whole heads than the seperated lateral buds. The supply of water and sucrose solution significantly suppressed the weight loss. At 5 d of storage, the weight loss of water- and sucrose-treated baby mustard was 26% and 29%, respectively (Fig. 1G). A similar result was observed in broccoli: the fresh weight of branchlets without solution rapidly decreased, whereas little change was observed when branchlets were treated with water and sucrose solution (Irving & Joyce, 1995). Weight loss is mainly caused by water transpiration and carbohydrate loss during respiration (Jiang et al., 2012, Mastropasqua et al., 2016). The water pressure gradient between baby mustard tissue and the surrounding atmosphere is an important factor affecting the rate of water transpiration (Jiang et al., 2012). Water and sucrose solution not only provide water to baby mustard but also increase the humidity of the microenvironment, which reduces the water pressure gradient and suppresses transpiration. There was no significant difference between sucrose and water treatments, indicating that sucrose provided no additional benefit in preventing weight loss.
3.3. Chlorophyll and carotenoids
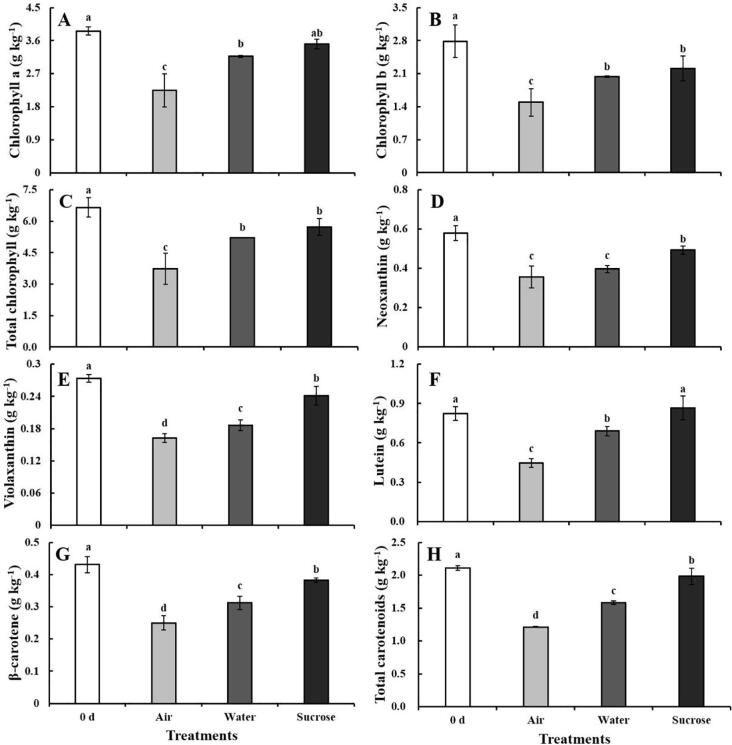
The content of pigments is directly correlated with the senescence status of green tissues and consumer preference for vegetables (Hu, Zhao, Zhang, Zhou, & Li, 2021). The individual and total pigment content generally decreased during storage, which was consistent with the senescence phenotype (Fig. 2A–H). The total chlorophyll and carotenoids content in the control at 5 d was 56% and 58% of that at 0 d, respectively. Water treatment had a certain benefit on the maintenance of pigments content. The total chlorophyll and carotenoids content in samples at 5 d under water treatment was 78% and 75% of that at 0 d, respectively, and 1.4- and 1.3-fold higher compared with the control (Fig. 2C, H). The total chlorophyll and carotenoids content was significantly higher under sucrose treatment compared with the other treatments and 1.5- and 1.6-fold higher under sucrose treatment compared with the control. This might be explained by the fact that both water and sucrose solution maintained cell turgor and the integrity of the cell structure to a certain extent; consequently, the pigments do not permeate out of the chloroplasts and vacuoles. The total carotenoids content was significantly higher in samples under sucrose treatment than under water treatment at 5 d (Fig. 2H), suggesting that the mechanisms protecting pigments from degradation under sucrose solution were distinct. A previous study showed that sucrose treatment can preserve the cell wall of asparagus spears by slowing the decomposition rate of protopectin and inhibiting the increase in the water-soluble pectin content (Park, 2016). Sucrose can also inhibit the activity of chlorophyll catabolic enzymes and the expression of genes associated with chlorophyll degradation in broccoli (Xu et al., 2016). Thus, the supply of solution was effective in maintaining the chlorophyll and carotenoids content of baby mustard, especially under sucrose treatment.
Fig. 2.
Chlorophyll and carotenoids content in lateral buds after the cut ends of baby mustard had been exposed to air, water, or sucrose solution at 0 d and 5 d. A: chlorophyll a; B: chlorophyll b; C: total chlorophyll; D: neoxanthin; E: violaxanthin; F: lutein; G: β-carotene; H: Total carotenoids.
3.4. Sucrose, glucose, and fructose
Three types of soluble sugars (sucrose, glucose, and fructose) were detected in baby mustard, and glucose was the most abundant (Fig. 3). The protective effects of soluble sugars against oxidative stress have mostly been attributed to their signaling effects, which trigger the production of specific ROS scavengers (Couée et al., 2006, Ramel et al., 2009). Among a range of small sugars, sucrose showed the strongest antioxidant capacity in vitro, which suggests that sucrose might have similar antioxidant functions in vivo (Bolouri-Moghaddam et al., 2010, Morelli et al., 2003, Nishizawa et al., 2008). Sucrose not only serves as a substrate or signal for stress-induced modifications but also interacts with classic, cytoplasmic antioxidant systems to function as a protective agent in vacuoles of sugar beet and sugar cane plants (Van den Ende & Valluru, 2008).
Fig. 3.
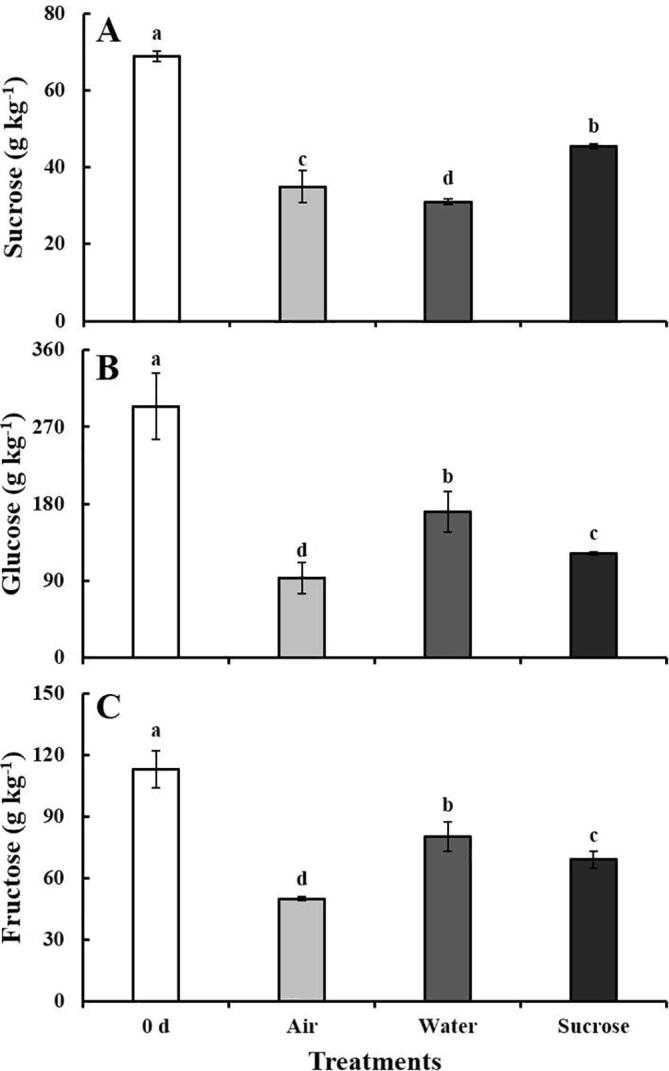
Soluble sugars content in lateral buds after the cut ends of baby mustard had been exposed to air, water or sucrose solutions at 0 d and 5 d. A: sucrose; B: glucose; C: fructose.
The sucrose content in the lateral buds decreased during storage (Fig. 3A). In the control, the sucrose content of lateral buds decreased by 49% at 5 d. The sucrose content in samples under water treatment decreased to 55%. Sucrose treatment significantly decreased the decline in the sucrose content of the lateral buds, which was 1.3- and 1.5-fold higher than that in the control and water-treated samples, respectively (Fig. 3A). The maintenance of the sucrose content might stem in part to the synthesis of glucose and fructose but is likely mostly associated with the supply of exogenous sucrose. Exogenous sucrose provides a sufficient supply source for sink tissues (e.g., lateral buds and swollen stem), and a concentration gradient of sucrose is formed between the source and the sink, thereby facilitating the transportation of sucrose via the phloem (Yoon et al., 2021). The lateral buds have a greater demand for assimilation partitioning and greater sink strength compared with the swollen stem (Sun et al., 2018). Therefore, the transportation and partitioning of baby mustard under sucrose treatment exhibited “sink priority”, which describes the preferential supply of available photosynthate between competing sinks (Minchin & Lacointe, 2004).
The content of glucose and fructose varied among the different treatments. The glucose content was highest in water-treated samples (170.64 g kg−1), which was reduced by 42% at the end of the storage period relative to 0 d, followed by sucrose treatment and the control (122.10 and 93.34 g kg−1; reductions of 58% and 68%, respectively). The content of fructose was highest in the water treatment, followed by sucrose treatment and the control (80.5, 69.2, and 50.1 g kg−1, respectively).
The respiration rate of lateral buds increased gradually in the control, which resulted in an increase in glucose consumption. Acid invertase was found to cleave vacuolar sucrose and played a pivotal role in postharvest primary metabolism. However, because aging is also accompanied by the breakdown of proteins (Cui et al., 2020), the activity of invertase might also decrease; consequently, the cleavage of endogenous sucrose may not be able to make up for the decline in the glucose content. The fact that the glucose content was highest in samples under water treatment suggests that the acid invertase activity was higher under water treatment than in the control. The turgor pressure of the cell wall is retained under water treatment, which preserves the integrity of the cell structure. Therefore, endogenous sucrose can be cleaved in time to maintain the higher respiration rate associated with aging. As a result, the sucrose content was lower in water-treated samples than in control samples, and the content of glucose and fructose was higher in water-treated samples than in control samples. The findings regarding the effects of sucrose treatment are more complex. Sucrose treatment is more effective for maintaining cell integrity than water treatment (Park, 2016). Sucrose can prevent damage to proteins; in addition to being a nutrition source, it can improve the water balance in cut flowers by promoting the closure of stomata (Rabiza-Świder et al., 2020). Sucrose has also been found to alleviate the excessive metabolism stemming from aging, mediate the consumption of mobilized vacuolar sugars, and retard the increase in plasma membrane permeability (McKenzie, Greer, Heyes, & Hurst, 2004). Therefore, the sucrose content in the sucrose-treated samples was the highest, and the content of glucose and fructose in sucrose-treated samples was intermediate between control and water-treated samples.
3.5. Ascorbic acid
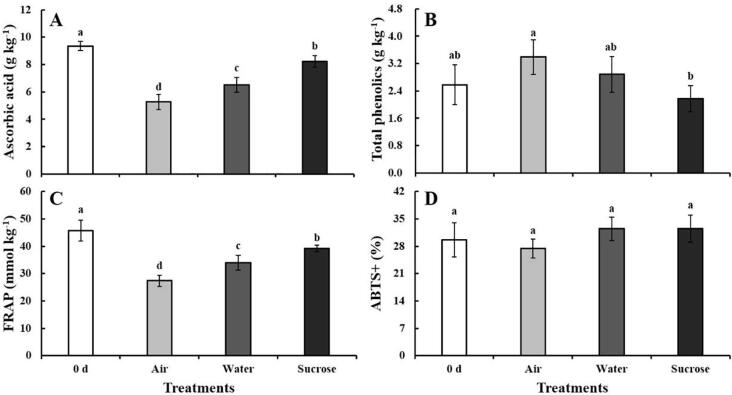
At 5 d, the ascorbic acid content in the control decreased, and the content in the lateral buds decreased by 44%. As expected, the ascorbic acid content decreased the decline in the water and sucrose treatments. The content of ascorbic acid under water treatment decreased by 30%. The highest ascorbic acid content (8.2 g kg−1) was observed in sucrose-treated samples, which was 1.6-fold higher compared with the control (Fig. 4A). Water loss can promote ascorbic acid degradation (Sun et al., 2021), and exogenous sucrose significantly delayed the weight loss in baby mustard. Ascorbic acid occurs in chloroplasts and is synthesized from a hexose precursor (Sun et al., 2021). Sucrose maintains a high ascorbic acid level by protecting the structure of chloroplasts and acting as a hexose reserve. Exogenous sucrose treatment can improve the postharvest quality of broccoli by increasing the ascorbic acid content (Smirnoff & Pallanca, 1996).
Fig. 4.
Main antioxidants content and antioxidant capacity levels in lateral buds after the cut ends of baby mustard had been exposed to air, water or sucrose solutions at 0 d and 5 d. A: ascorbic acid; B: total phenolics; C: FRAP; D: ABTS+.
3.6. Total phenolics
The total phenolics content in the control increased during storage and was 1.3-fold higher at 5 d compared with 0 d. The content of total phenolics in samples under water treatment was approximately equivalent to that at 0 d. Compared with the control at 5 d, sucrose treatment suppressed the increase in total phenolics, which was 36% lower in treated samples (Fig. 4B). The increase in the total phenolics content might be related to the defense mechanisms associated with the stress induced by senescence (Huang, Yang, Sridhar, & Tsai, 2021). The phenolics content has also been shown to increase in fresh-cut cauliflower curds (Mashabela, Mahajanb, & Sivakumar, 2019). Our results showed that sucrose treatment alleviated senescence stress and delayed the induction of total phenolics.
3.7. Antioxidant capacity
The antioxidant capacity was investigated using both FRAP and ABTS (Fig. 4C and D). The FRAP level at 5 d in the control decreased by 40% compared with that at 0 d. Water treatment delayed the decline in the FRAP level. The FRAP level in the lateral buds was 1.4-fold higher under sucrose treatment than in the control (Fig. 4C). Changes in the FRAP level were consistent with observed changes in the content of ascorbic acid. Ascorbic acid, anthocyanins, and total phenolics are considered important factors contributing to the antioxidant activity of plants (Mashabela et al., 2019). Glucose treatment has been shown to increase the anthocyanin and ascorbic acid content in radish sprouts, but the antioxidant activity estimated by the FRAP assay was reduced (Wei, Miao, & Wang, 2011). Wei et al. (2011) speculated that the antioxidant activity in radish sprouts was associated with phenolic compounds and glucoraphasatin. The antioxidant activity estimated by FRAP might be mainly associated with ascorbic acid, which is consistent with the results of our previous research (Zhang et al., 2021).
ABTS levels at 5 d were similar to those at 0 d in both water and sucrose treatments. The ABTS levels of the lateral buds in the two treatments was 1.2-fold higher relative to those in the control at 5 d; however, no significant difference was observed among the control and treatments (Fig. 4D). Changes in the ABTS levels may reflect the combined effect of ascorbic acid, total phenolics, and other antioxidant compounds.
3.8. Glucosinolates
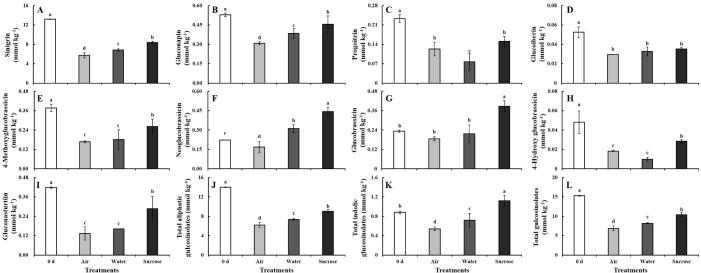
Four aliphatic glucosinolates, four indolic glucosinolates, and one aromatic glucosinolate were detected in baby mustard (Fig. 5). Among aliphatic glucosinolates, the content of sinigrin in the lateral buds in the control decreased significantly during storage (by 56%) at 5 d. In contrast, the content of sinigrin in the water-treated samples decreased by 48% at 5 d. The highest sinigrin content of the lateral buds was observed in sucrose-treated samples, which was 1.2- and 1.5-fold higher in sucrose-treated samples than in the control and under water treatment, respectively (Fig. 5A). Because of the large proportion of sinigrin, changes in the total aliphatic and total glucosinolate content were similar to observed changes in sinigrin: the total aliphatic and total glucosinolate content was 1.5-fold higher in sucrose-treated lateral buds than in control buds at 5 d (Fig. 5J and L). The content of gluconapin, the second most abundant glucosinolate after sinigrin, was reduced by 41% in the control and was 1.5-fold higher in sucrose-treated samples than in the control (Fig. 5B). Sucrose treatment had no pronounced positive effect on the progoitrin and glucoiberin content (Fig. 5C and D).
Fig. 5.
Glucosinolate content in lateral buds after the cut ends of baby mustard had been exposed to air, water or sucrose solutions at 0 d and 5 d. A: sinigrin; B: gluconapin; C: progoitrin; D: glucoiberin; E: 4-methoxyglucobrassicin; F: neoglucobrassicin; G: glucobrassicin; H: 4-hydroxy glucobrassicin; I: gluconasturtiin; J: total aliphatic gulcosinolates; K: total indolic gulcosinolates; L: total gulcosinolates.
In the control, the content of neoglucobrassicin decreased and the content of glucobrassicin did not change at 5 d; in the sucrose treatment, the content of neoglucobrassicin and glucobrassicin significantly was 2.1- and 1.8-fold higher compared with 0 d, respectively (Fig. 5F and G). The content of 4-methoxyglucobrassicin and 4-hydroxyglucobrassicin was significantly higher in sucrose-treated lateral buds than in control lateral buds, which were both 1.6-fold higher compared with the control (Fig. 5E and H). The total indolic glucosinolate content markedly increased in samples under sucrose treatment (Fig. 5K). In addition, gluconasturtiin was the only aromatic glucosinolate detected in baby mustard. The gluconasturtiin content in the lateral buds was 2.1- higher under sucrose treatment than in the control (Fig. 5I).
The levels of glucosinolates were preserved in other Brassica vegetables under various treatments, including 1-methylcyclopropene (Sun, Yan, Liu, Wei, & Wang, 2012) and light irradiation (Wang et al., 2021), among other treatments (Chiu, Matak, & Ku, 2020). The content of glucosinolates in these vegetables during postharvest life was determined by hydrolysis and biosynthesis (Sun et al., 2012). Sucrose is crucial for maintaining cellular turgor (Park, 2016). The cell structures protected by sucrose delayed the contact between glucosinolates and myrosinase, which mitigated the decrease in the glucosinolate content. This might also be the main reason why water treatment delayed the decrease in the content of glucosinolates and the deterioration in the quality of baby mustard. As sugars are the main substrates of primary metabolism, higher sucrose content may induce higher production of essential precursors as substrates for the synthesis of secondary metabolites (Wu, Tu, Yang, Xu, & Yu, 2020). The content of indolic and aliphatic glucosinolates has been shown to increase in broccoli sprouts (Guo et al., 2011b, Guo et al., 2011a). Transcription factors regulating glucosinolate biosynthesis are significantly induced by glucose, and the biosynthesis of glucosinolates is enhanced by glucose in Arabidopsis thaliana (Miao et al., 2013, Miao et al., 2016). It is possible that the regulation of glucosinolate by sucrose in postharvest baby mustard is also at the level of transcription.
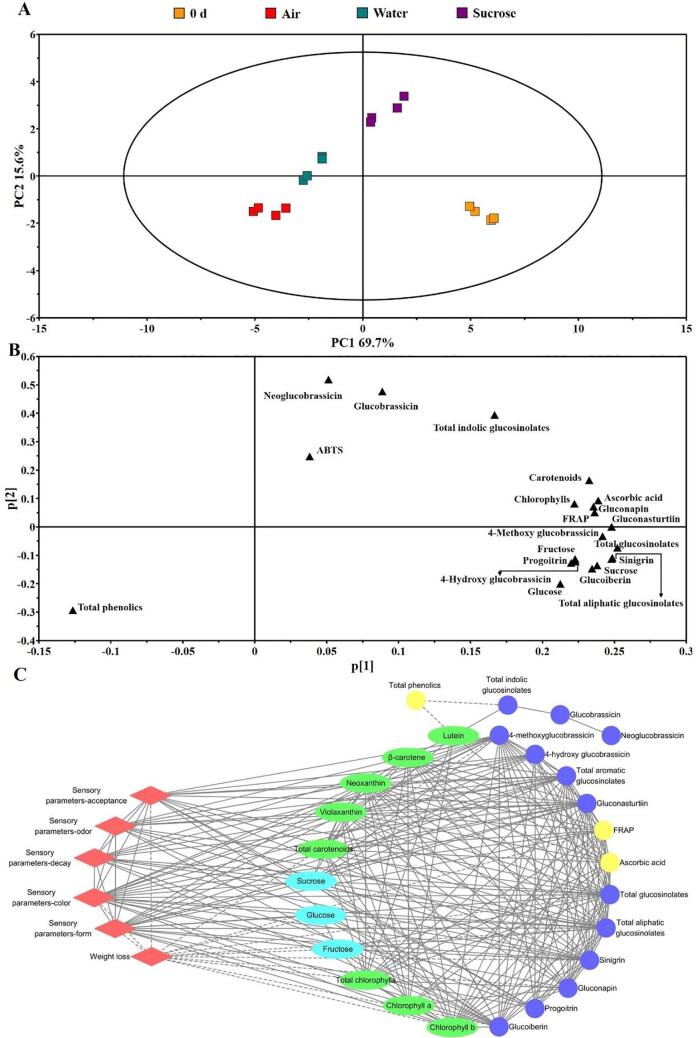
3.9. PCA
PCA was performed to characterize variation in the quality of the lateral buds of baby mustard among the different postharvest treatments. Samples from the different treatments of baby mustard were clearly separated by PCA; the distances between treatments were correlated with differences in the nutritional quality of postharvest baby mustard (Fig. 6A). The first component (PC1) and second component (PC2) explained 69.7% and 15.6% of the variance, respectively. The lateral buds at 0 d and 5 d under sucrose treatment could be distinguished by PC1 from the samples in the control and under water treatment at 5 d. PC1 was positively correlated with most of the measured indicators and negatively correlated with total phenolics (Fig. 6B). This suggests that both water and sucrose affected the changes in the aforementioned indicators. The maximum nutritional quality was retained under the sucrose treatment. Neoglucobrassicin, glucobrassicin, and total indolic glucosinolates loaded heavily on PC2, suggesting that sucrose had a considerable effect on the content of indolic glucosinolates.
Fig. 6.
PCA and correlation analysis of lateral buds. A: PCA score plot; B: PCA loading plot; C: Correlation plot between the visual and nutritional qualities. The dashed lines between indices represent negative correlations, whereas solid lines represent positive correlations. All correlations in the figure reflect Pearson correlation coefficient values above the threshold (|ρ| > 0.9).
3.10. Correlation analysis
The correlation analysis of visual quality evaluation and various phytochemicals revealed much more information. In the present study, Cytoscape was used to visualize that visual quality (red diamonds in left side of Fig. 6C) and nutritional qualities (green, blue, and yellow circles in right side of Fig. 6C) were highly correlated (|ρ| > 0.9). Here we showed that a network analysis was conducted with six sensory quality indices, 28 nutritional quality indices, and 249 edges (Fig. 6C). Consistent with previous research (Sun et al., 2021), the visual quality parameters were positively correlated with chlorophyll, carotenoids, soluble sugars, ascorbic acid, antioxidant capacity and most glucosinolates. Futhermore, there were significant positive correlations between sucrose and neoxanthin, violaxanthin as well as six individual glucosinolates. The vast majority of negative correlations occurred between weight loss and visual or nutritional indicators.
4. Conclusions
Supplying sucrose through the cut surface of the swollen stem can improve the postharvest quality of the lateral buds of baby mustard. The visual quality and health-promoting compounds in the lateral buds of baby mustard were more effectively maintained under sucrose treatment than under water treatment. Water treatment accelerated the decay of baby mustard after harvest. The application of sucrose solution delayed sensory quality deterioration by inhibiting the degradation of chlorophyll; mitigated the decrease in the sucrose content; sustained the antioxidant potential of postharvest baby mustard by maintaining higher levels of ascorbic acid and antioxidant capacity; and maintained and even increased the content of glucosinolates. In sum, sucrose treatment is effective for preserving the postharvest quality of baby mustard.
Funding
This work was supported by National Natural Science Foundation of China (32072586, 31500247), Project of New Varieties Breeding of Sichuan Vegetable Innovation Team (sccxtd-2020-05).
CRediT authorship contribution statement
Hongmei Di: Investigation, Writing – original draft. Yi Zhang: Investigation, Writing – original draft. Jie Ma: Data curation, Writing – original draft. Jia Wei: Data curation. Yating Wang: Investigation. Zhiqing Li: Data curation. Cexian Cui: Investigation. Pengcheng Fang: Data curation. Wei Ma: Investigation. Huanxiu Li: Investigation, Funding acquisition. Bo Sun: Funding acquisition, Writing – review & editing, Conceptualization. Fen Zhang: Conceptualization, Funding acquisition, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100272.
Contributor Information
Bo Sun, Email: bsun@sicau.edu.cn.
Fen Zhang, Email: zhangf@sicau.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abdulla M.F., Çelikel F.G. Postharvest quality and extending vase life of Helleborus orientalis flowers by sucrose pulsing. Acta Horticulturae. 2019;1263:449–454. doi: 10.17660/ActaHortic.2019.1263.58. [DOI] [Google Scholar]
- Bolouri-Moghaddam M.R., Roy K.L., Xiang L., Rolland F., Ende W.V.D. Sugar signalling and antioxidant network connections in plant cells. The FEBS Journal. 2010;277(9):2022–2037. doi: 10.1111/j.1742-4658.2010.07633.x. [DOI] [PubMed] [Google Scholar]
- Chiu Y.C., Matak K., Ku K.M. Methyl jasmonate treatment of broccoli enhanced glucosinolate concentration, which was retained after boiling, steaming, or microwaving. Foods. 2020;9(6):758. doi: 10.3390/foods9060758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couée I., Sulmon C., Gouesbet G., El-Amrani A. An involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. Journal of Experimental Botany. 2006;3:449–459. doi: 10.1093/jxb/erj027. [DOI] [PubMed] [Google Scholar]
- Coupe S.A., Sinclair B.K., Watson L.M., Heyes J.A., Eason J.R. Identification of dehydration-responsive cysteine proteases during post-harvest senescence of broccoli florets. Journal of Experimental Botany. 2003;54(384):1045–1056. doi: 10.1093/jxb/erg105. [DOI] [PubMed] [Google Scholar]
- Cui X., Zhao P.Y., Liang W.W., Cheng Q., Mu B.B., Niu F.F.…Yang B. A rapeseed WRKY transcription factor phosphorylated by CPK modulates cell death and leaf senescence by regulating the expression of ROS and SA-synthesis-related genes. Journal of Agricultural and Food Chemistry. 2020;68(28):7348–7359. doi: 10.1021/acs.jafc.0c02500. [DOI] [PubMed] [Google Scholar]
- Griffiths C.A., Paul M.J., Foyer C.H. Metabolite transport and associated sugar signalling systems underpinning source/sink interactions. Biochimica et Biophysica Acta. 2016;1857:1715–1725. doi: 10.1016/j.bbabio.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R.F., Yuan G.F., Wang Q.M. Effect of sucrose and mannitol on the accumulation of health-promoting compounds and the activity of metabolic enzymes in broccoli sprouts. Scientia Horticulturae. 2011;128(3):159–165. doi: 10.1016/j.scienta.2011.01.014. [DOI] [Google Scholar]
- Guo R.F., Yuan G.F., Wang Q.M. Sucrose enhances the accumulation of anthocyanins and glucosinolates in broccoli sprouts. Food Chemistry. 2011;129(3):1080–1087. doi: 10.1016/j.foodchem.2011.05.078. [DOI] [PubMed] [Google Scholar]
- Ho S.L., Chao Y.C., Tong W.F., Yu S.M. Sugar coordinately and differentially regulates growth- and stress-related gene expression via a complex signal transduction network and multiple control mechanisms. Plant Physiology. 2001;2:2. doi: 10.1104/pp.125.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H.L., Zhao H.H., Zhang L.G., Zhou H.S., Li P.X. The application of 1-methylcyclopropene preserves the postharvest quality of cabbage by inhibiting ethylene production, delaying chlorophyll breakdown and increasing antioxidant capacity. Scientia Horticulturae. 2021;281 doi: 10.1016/j.scienta.2021.109986. [DOI] [Google Scholar]
- Huang Y.C., Yang Y.H., Sridhar K., Tsai P.J. Synergies of modified atmosphere packaging and high-voltage electrostatic field to extend the shelf-life of fresh-cut cabbage and baby corn. LWT. 2021;138 doi: 10.1016/j.lwt.2020.110559. [DOI] [Google Scholar]
- Irving D.E., Joyce D.C. Sucrose supply can increase longevity of broccoli (Brassica oleracea) branchlets kept at 22 °C. Plant Growth Regulation. 1995;17(3):251–256. doi: 10.1007/BF00024733. [DOI] [Google Scholar]
- Jiang T.J., Feng L.F., Li J.R. Changes in microbial and postharvest quality of shiitake mushroom (Lentinus edodes) treated with chitosan-glucose complex coating under cold storage. Food Chemistry. 2012;131(3):780–786. doi: 10.1016/j.foodchem.2011.08.087. [DOI] [Google Scholar]
- Lin P.X., Di H.M., Wang G.Y., Li Z.Q., Li H.X., Zhang F., Sun B. Modified atmosphere packaging maintains the sensory and nutritional qualities of post-harvest baby mustard during low-temperature storage. Frontiers in Nutrition. 2021;8 doi: 10.3389/fnut.2021.730253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashabela M., Mahajanb P.V., Sivakumar D. Influence of different types of modified atmosphere packaging films and storage time on quality and bioactive compounds in fresh-cut cauliflower. Food Packaging and Shelf Life. 2019;22 doi: 10.1016/j.fpsl.2019.100374. [DOI] [Google Scholar]
- Mastropasqua L., Tanzarella P., Paciolla C. Effects of postharvest light spectra on quality and health-related parameters in green Asparagus officinalis L. Postharvest Biology and Technology. 2016;112:143–151. doi: 10.1016/j.postharvbio.2015.10.010. [DOI] [Google Scholar]
- McKenzie M.J., Greer L.A., Heyes J.A., Hurst P.L. Sugar metabolism and compartmentation in asparagus and broccoli during controlled atmosphere storage. Postharvest Biology and Technology. 2004;32(1):45–56. doi: 10.1016/j.postharvbio.2003.09.015. [DOI] [Google Scholar]
- Miao H.Y., Cai C.X., Wei J., Huang J.R., Chang J., Qian H.M.…Wang Q.M. Glucose enhances indolic glucosinolate biosynthesis without reducing primary sulfur assimilation. Scientific Reports. 2016;6(1):31854. doi: 10.1038/srep31854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H.Y., Wei J., Zhao Y.T., Yan H.Z., Sun B., Huang J.R., Wang Q.M. Glucose signalling positively regulates aliphatic glucosinolate biosynthesis. Journal of Experimental Botany. 2013;64(4):1097–1109. doi: 10.1093/jxb/ers399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minchin P.E.H., Lacointe A. New understanding on phloem physiology and possible consequences for modelling long-distance carbon transport. New Phtologist. 2004;166(3):771–779. doi: 10.1111/j.1469-8137.2005.01323.x. [DOI] [PubMed] [Google Scholar]
- Morelli R., Russo-Volpe S., Bruno N., Scalzo R.L. Fenton dependent damage to carbohydrates: Free radical scavenging activity of some simple sugars. Journal of Agricultual and Food Chemistry. 2003;51(25):7418–7425. doi: 10.1021/jf030172q. [DOI] [PubMed] [Google Scholar]
- Nishikawa F., Kato M., Hyodo H., Ikoma Y., Sugiura M., Yano M. Effect of sucrose on ascorbate level and expression of genes involved in the ascorbate biosynthesis and recycling pathway in harvested broccoli florets. Journal of Experimental Botany. 2005;56(8):65–72. doi: 10.1093/jxb/eri007. [DOI] [PubMed] [Google Scholar]
- Nishizawa A., Yukinori Y., Shigeoka S. Galactinol and raffinose as a novel function to protect plants from oxidative damage. Plant Physiology. 2008;147(3):1251–1263. doi: 10.1104/pp.108.122465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norikoshi, R., Shibata, T., Niki, T., & Ichimura, K. (2016). Sucrose treatment enlarges petal cell size and increases vacuolar sugar concentrations in cut rose flowers. Postharvest Biology and Technology, 116, 59–65. /10.1016/j.postharvbio.2016.01.003.
- Park M.H. Sucrose delays senescence and preserves functional compounds in Asparagus officinalis L. Biochemical and Biophysical Research Communications. 2016;480:241–247. doi: 10.1016/j.bbrc.2016.10.036. [DOI] [PubMed] [Google Scholar]
- Rabiza-Świder J., Skutnik E., Jędrzejuk A., Rochala-Wojciechowska J. Nanosilver and sucrose delay the senescence of cut snapdragon flowers. Postharvest Biology and Technology. 2020;165 doi: 10.1016/j.postharvbio.2020.111165. [DOI] [Google Scholar]
- Ramel F., Sulmon C., Bogard M., Couée I., Gouesbet G. Differential patterns of reactive oxygen species and antioxidative mechanisms during atrazine injury and sucrose-induced tolerance in Arabidopsis thaliana plantlets. BMC Plant Biology. 2009;9:28. doi: 10.1186/1471-2229-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N., Pallanca J.E. Ascorbate metabolism in relation to oxidative stress. Biochemical Society Transactions. 1996;24(2):472–478. doi: 10.1042/bst0240472. [DOI] [PubMed] [Google Scholar]
- Sun B., Di H.M., Zhang J.Q., Xia P.X., Huang W.L., Jian Y.…Zhang F. Effect of light on sensory quality, health-promoting phytochemicals and antioxidant capacity in post-harvest baby mustard. Food Chemistry. 2021;339 doi: 10.1016/j.foodchem.2020.128057. [DOI] [PubMed] [Google Scholar]
- Sun B., Tian Y.X., Jiang M., Yuan Q., Chen Q., Zhang Y.…Tang H.R. Variation in the main health-promoting compounds and antioxidant activity of whole and individual edible parts of baby mustard (Brassica juncea var. gemmifera) RSC Advances. 2018;8:33845–33854. doi: 10.1039/c8ra05504a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B., Yan H.Z., Liu N., Wei J., Wang Q.M. Effect of 1-MCP treatment on postharvest quality characters, antioxidants and glucosinolates of Chinese kale. Food Chemistry. 2012;131(2):519–526. doi: 10.1016/j.foodchem.2011.09.016. [DOI] [Google Scholar]
- Van den Ende W., Valluru R. Sucrose, sucrosyl oligosaccharides, and oxidative stress: Scavenging and salvaging? Journal of Experimental Botany. 2008;60(1):9–18. doi: 10.1093/jxb/ern297. [DOI] [PubMed] [Google Scholar]
- Wang J.W., Mao S.X., Wu Q., Yuan Y.M., Liang M.T., Wang S.Z.…Wu Q.Y. Effects of LED illumination spectra on glucosinolate and sulforaphane accumulation in broccoli seedlings. Food Chemistry. 2021;356 doi: 10.1016/j.foodchem.2021.129550. [DOI] [PubMed] [Google Scholar]
- Wei J., Miao H.Y., Wang Q.M. Effect of glucose on glucosinolates, antioxidants and metabolic enzymes in Brassica sprouts. Scientia Horticulturae. 2011;129(4):535–540. doi: 10.1016/j.scienta.2011.04.026. [DOI] [Google Scholar]
- Wu Z.F., Tu M.M., Yang X.P., Xu J.H., Yu Z.F. Effect of cutting and storage temperature on sucrose and organic acids metabolism in postharvest melon fruit. Postharvest Biology and Technology. 2020;161 doi: 10.1016/j.postharvbio.2019.111081. [DOI] [Google Scholar]
- Xu F., Tang Y.C., Dong S.Q., Shao X.F., Wang H.F., Zheng Y.H., Yang Z.F. Reducing yellowing and enhancing antioxidant capacity of broccoli in storage by sucrose treatment. Postharvest Biology and Technology. 2016;112:39–45. doi: 10.1016/j.postharvbio.2015.09.038. [DOI] [Google Scholar]
- Yoon J., Cho L.H., Tun W., Jeon J.S., An G. Sucrose signaling in higher plants. Plant Science. 2021;302 doi: 10.1016/j.plantsci.2020.110703. [DOI] [PubMed] [Google Scholar]
- Zanon L., Falchi R., Santi S., Vizzotto G. Sucrose transport and phloem unloading in peach fruit: Potential role of two transporters localized in different cell types. Physiologia Plantarum. 2015;154(2):179–193. doi: 10.1111/ppl.12304. [DOI] [PubMed] [Google Scholar]
- Zhang F., Zhang J.Q., Di H.M., Xia P.X., Zhang C.L., Wang Z.H.…Bo S. Effect of long-term frozen storage on health-promoting compounds and antioxidant capacity in baby mustard. Frontiers in Nutrition. 2021;8 doi: 10.3389/fnut.2021.665482. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.