Abstract
Background
Hydrogen sulfide (H2S) has been proposed to exert anti-oxidative effect under many environmental stresses; however, how it influences oxidative stress remains largely unclear.
Results
Here, we assessed the effects of H2S on oxidative stress responses such as salicylic acid (SA)-dependent cell death, which triggered by increased H2O2 availability in Arabidopsis thaliana catalase-deficient mutants cat2 displaying around 20% wild-type catalase activity. H2S generation and its producing enzyme L-cysteine desulfhydrase (LCD/DES) were found to transient increase in response to intracellular oxidative stress. Although introducing the mutation of des1, an important LCD, into the cat2 background produced little effect, H2S fumigation not only rescued the cell death phenotype of cat2 plant, but also attenuated SA accumulation and oxidation of the glutathione pool. Unexpectedly, the activities of major components of ascorbate–glutathione pathway were less affected by the presence of H2S treatment, but decreased glycolate oxidase (GOX) in combination with accumulation of glycolate implied H2S treatment impacts the cellular redox homeostasis by repressing the GOX-catalyzed reaction likely via altering the major GOX transcript levels.
Conclusions
Our findings reveal a link between H2S and peroxisomal H2O2 production that has implications for the understanding of the multifaceted roles of H2S in the regulation of oxidative stress responses.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-022-03490-3.
Keywords: Cell death, Hydrogen sulfide, Glycolate oxidase, H2O2, Oxidative stress, Salicylic acid
Background
Abiotic stresses, such as drought, salinity, or heavy metal, are major constraint to productivity of all major crops worldwide. These environmental stresses influence plant yield and quality by affecting various cellular and whole-plant processes particularly through either reducing photosynthesis or the availability of water for basic cellular functions [1]. Moreover, these adverse factors can trigger rapid changes in the production and scavenging of reactive oxygen species (ROS) as its-associated oxidative stress has been assumed to be involved in these processes. For instance, hydrogen peroxide (H2O2) could affect several primary metabolic pathways such as carbon metabolism, glycolysis/gluconeogenesis, and amino acid biosynthesis presumably through the cysteine oxidation at specific proteins [2]. Stress-triggered ROS accumulation is mainly from mitochondrial respiration, photosynthesis in chloroplasts, peroxisome-localized photorespiration, and by apoplastic NADPH oxidases [3].
Other than its toxicity, H2S has been gradually accepted as a key messenger involved in a vast number of physiologically important processes in photosynthetic organisms, such as stress resistance, autophagy, stomatal movement [4–7]. The plant itself can generate H2S in different subcellular sites including cytosol, chloroplasts, mitochondria, and nuclei [8–13]. When plants are exposed to these adverse environmental cues, the production of H2S increases through enzymatic reactions mainly including the activation of sulfur assimilation pathway or the cysteine-degrading enzymes such as D-cysteine desulfhydrase (DCD) and L-cysteine desulfhydrase (LCD/DES) [6], Other factors and/or pathways such as β-cyanoalanine synthase and 3-mercaptopyruvate sulfurtransferases are also involved in H2S biogenesis [13–15]. Furthermore, due to its nucleophilic properties, H2S is capable of reacting with different ROS, thereby potentially severing as an antioxidant involving stress regulation [16]. However, other metabolites such as ascorbate and glutathione that are present within mM range at much higher concentrations than H2S [17], might be expected to be more effective in the removal of excess ROS. Instead of this, H2S could activate the transcriptional and/or translational levels of antioxidant enzymes and key biosynthetic enzymes of their substrates, to enhance ROS-processing capacity under many stress conditions. Indeed, numerous studies found that the major components including ascorbate peroxidase (APX), dehydroascorbate reductase (DHAR), glutathione reductase (GR) in ascorbate–glutathione pathway could be key regulatory nodes by H2S [18–20]. Treatment with H2S into several plant species under certain stress conditions was also observed to activate the expressions of L-galactose dehydrogenase, glutamate cysteine ligase and glutathione synthetase for ascorbate and glutathione synthesis, respectively [20–23]. As well as the effects on transcriptional regulation, H2S is proposed to be involved in generating cysteine persulfides on proteins, a process termed persulfidation which may lead to the alteration of protein functions. For example, H2S can drive persulfidation of certain antioxidant enzymes such as APX and the other peroxidase to potentiate their scavenging activities of ROS [19, 24]. Although it has been established that H2S-controlled redox balance could operate via up-regulation of antioxidant defense enzymes at the multiple levels, its effects on ROS biosynthesis in higher plants is much less understood except for the case studies that DES1-derived H2S participates in guard cell ABA signaling through modulating NADPH oxidase activity [25, 26].
In photosynthetic organisms, oxidative stress triggered by enhanced accumulation of ROS is common to environmental stress responses. Till now, studies on investigating the interactions between H2S and oxidative stress was only based on the analyses of effects of H2S on abiotic stress-triggered indirectly ROS accumulation and redox imbalance. In view of its importance in the context of oxidative stress, the aim of this work was to study the role of H2S in the regulation of oxidative responses by utilizing the catalase2 (cat2) knockout mutants to directly induce stress responses such as salicylic acid (SA)-dependent cell death [27, 28], where H2O2 is produced in peroxisomes from O2 by the reaction of glycolate oxidase (GOX)-catalyzed glycolate oxidation during photorespiration.
Results
Effects of intracellular oxidative stress on H2S production and LCD activity
Because studies of T-DNA insertion knockouts confirm CAT2 encodes the major leaf catalase isoform [29]. Increased H2O2 availability due to catalase deficiency in cat2 knockout mutants led to the activation of intracellular oxidative stress responses mainly including the induction of SA-dependent cell death [27, 28]. Thus, we first assess the temporal profile of H2S production in response to increased H2O2 availability in cat2 knockouts. H2S generation showed a marked transient increase in cat2 mutants (Fig. 1a), with the initial increase correlating with the onset of lesions, which begin to be visible after about 14–15 days of growth [30]. During the same period of growth, isochorismate synthase-dependent SA accumulation gradually increased [28, 31]. Moreover, the extractable activities of LCD, a major H2S-generating component in Arabidopsis, gradually increased alongside H2S production in cat2 mutants (Fig. 1b). These results demonstrate that the dynamic changes in cat2-impacted H2S levels are similar as cat2-triggered SA responses. A specific question we sought to answer was: Whether H2S is related to the H2O2-triggered SA pathway?
Fig. 1.
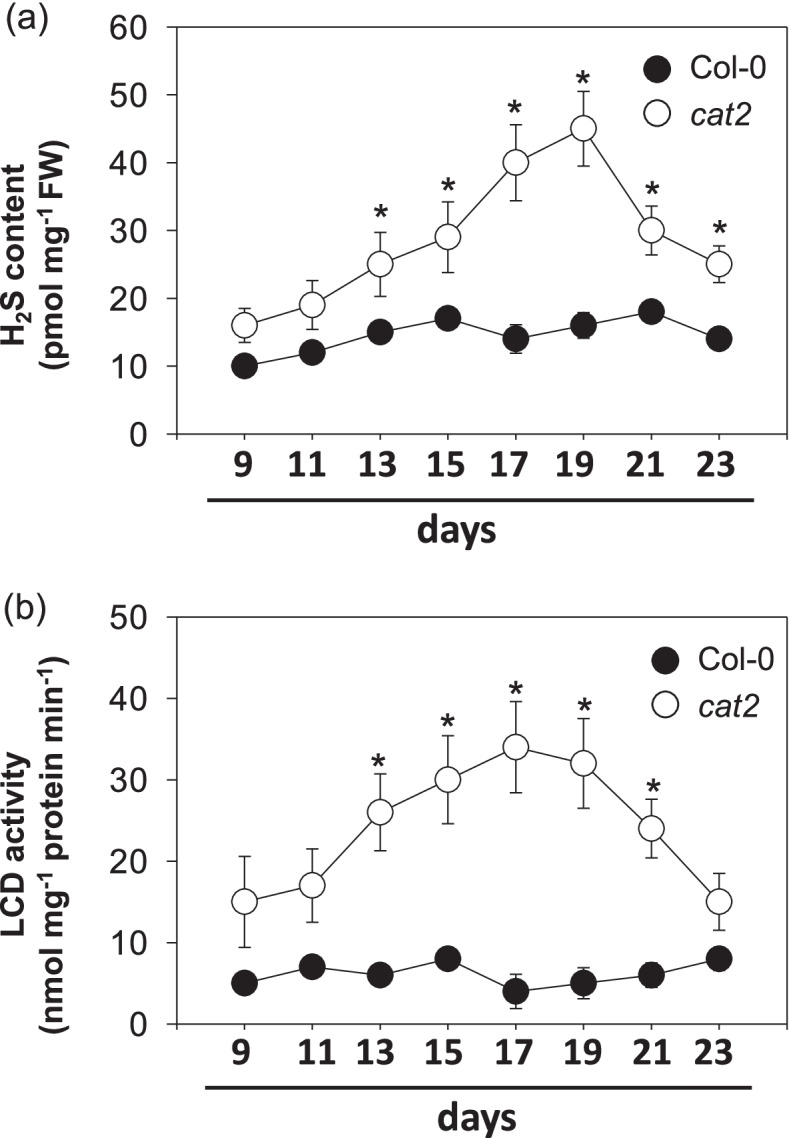
Time-course analysis of (a) H2S production and (b) LCD activity in Col-0 and cat2 seedlings. Time indicates days after seeds sowing. All values are means ± SE of three biological replicates. Asterisks indicate significant differences between cat2 and Col-0 at P < 0.05 at the same time point
DES1-derived H2S appears to be dispensable for oxidative stress responses
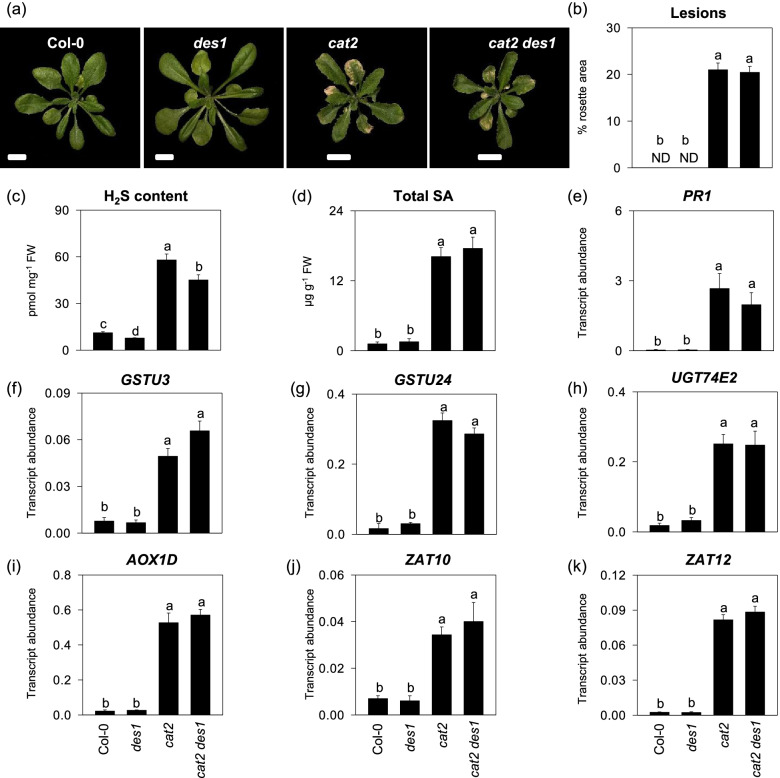
The function of DES1 has been proposed in stress responses and disruption of DES1 provokes a 30% decrease in the amount of endogenous H2S concentration [25, 32]. We next examined if DES1-produced H2S is involved in modulating stress responses by genetically crossing cat2 with des1 knockout mutants. Phenotypic analysis showed that the des1 mutation failed to alter spontaneous cell death triggered by cat2 (Fig. 2a and b), although disrupted DES1 function significantly reduced cat2-indcued H2S production (Fig. 2c). Consistent with cell death phenotypes, SA levels and PATHOGENESIS-RELATED GENE 1 (PR1) transcripts, indicators of SA-dependent pathways, in cat2 des1 and cat2 mutants were indistinguishable after twenty days of growth (Fig. 2d and e). These observations indicate that DES1-sourced H2S likely had little effect on oxidative stress responses. To further examine above, six H2O2-responsive genes including GLUTATHIONE S-TRANSFERASE TAU 3 (GSTU3), GLUTATHIONE S-TRANSFERASE TAU 24 (GSTU24), URIDINE DIPHOSPHATE GLYCOSYLTRANSFERASE 74E2 (UGT74E2), ALTERNATIVE OXIDASE 1D (AOX1D), ZINC-FINGER OF ARABIDOPSIS THALIANAA 10 (ZAT10) and ZINC-FINGER OF ARABIDOPSIS THALIANA 12 (ZAT12) were selected [33]. All of them were markedly induced in cat2 mutants [34], but their transcript levels from cat2 des1 leaves were comparable to those from single cat2 mutants (Fig. 2f to k). Nevertheless, increased H2O2 in the leaves of cat2 is difficult to detect. As measured by several approaches in our previous studies, no difference in leaf H2O2 levels was observed between cat2 and Col-0 [31, 34]. This observation is consistent with previous studies of catalase-deficient arabidopsis, tobacco and barley lines [28, 35, 36]. Engagement of other H2O2-metabolizing pathways in catalase deficient plants is evidenced by adjustments in antioxidant systems [27, 31]. To assess the impact of the des1 mutation on the H2O2-antioxidant interaction. The extractable activities of major antioxidant enzymes in the ascorbate–glutathione pathway as well as CAT, were measured. effects on activities of ascorbate peroxidase (APX), dehydroascorbate reductase (DHAR) and glutathione reductase (GR) in cat2 des1 were comparable to those observed in cat2 (Supplemental Figure S1).
Fig. 2.
Influence of des1 mutation on cat2-induced phenotype, SA accumulation and associated gene expression, and ROSdependent genes. a Representative phenotypic image of twenty-day-old given plant genotypes. Scale Bars indicate 1 cm. b Lesion quantification in the different genotypes as a percentage of the total rosette area. ND, not detected. c H2S production. d and e, total SA and PR1 transcript level. In (f) to (k), selected ROS-related genes in Col-0, des1, cat2, and cat2 des1 by qPCR. Rosette samples of each line were taken after 20 days of growth. Values are means ± SE of three biological replicates. Different letters indicate significant differences at P < 0.05 by multiple pairwise t-test comparisons at P < 0.05
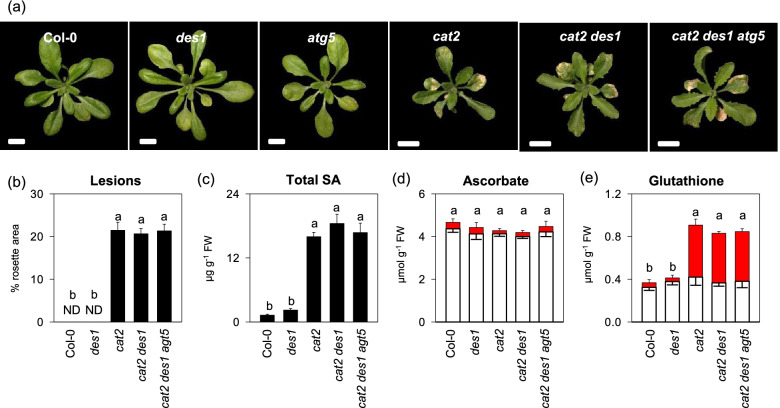
While autophagy plays crucial roles against oxidative stress, its pathway can be induced in the presence of des1 mutation or oxidative stress conditions such as H2O2 treatment and nitrogen deprivation [32, 37, 38]. We therefore assess if autophagy contributes to oxidative stress responses by complementing the function of DES1. Because AUTOPHAGY 5 (ATG5) is a core component in autophagy [39]. We used atg5 knockout mutants as a representative of autophagy-defective mutants and generated cat2 des1 atg5 triple mutants for further experiments. As we unexpected, cell death and SA content were comparable in cat2, cat2 des1 and cat2 des1 atg5 mutants (Fig. 3a and b). Ascorbate and glutathione, both of which are key redox antioxidant buffers, were also determined. As reported previously, ascorbate is less affected in cat2 than is glutathione [30]. No difference in ascorbate contents was observed in any of the mutants relative to Col-0 (Fig. 3d). By contrast, compared with wide-type Col-0 plants, total glutathione and its oxidized form (GSSG) substantially accumulated in cat2, However, des1 and des1 atg5 combined mutations in the cat2 background produced little effect on glutathione state in comparison with the cat2 single mutation (Fig. 3e).
Fig. 3.
Analysis of phenotype, SA content and redox buffer in the given double and triple mutants. a representative image of twenty-day-old indicated genotypes. Scale Bars indicate 1 cm. b Lesion quantification in the different genotypes as a percentage of the total rosette area. ND, not detected. c Leaf total SA. d and e Leaf ascorbate and glutathione content. White bars, reduced form. Red bars, oxidized form. Samples of each line were taken after 20 days of growth. Values are means ± SE of three biological replicates. Different letters indicate significant differences by multiple pairwise t-test comparisons at P < 0.05
Effects of H2S exposure on SA-dependent cell death and redox status
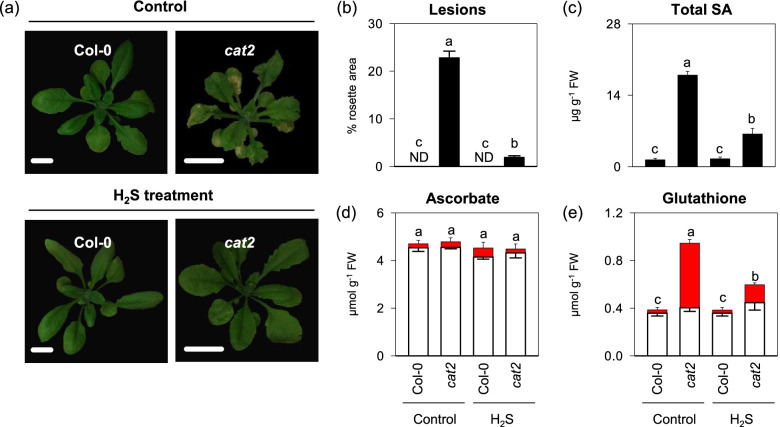
To further investigate the effect of the H2S on the H2O2-triggerecd oxidative responses, a H2S donor NaHS was employed for H2S fumigation treatment (See Materials and methods for details). As shown in Fig. 4a and b, H2S exposure did not impact leaf phenotype of wild-type background Col-0, but apparently attenuated H2O2-elcited lesion formation and SA accumulation in the cat2 background (Fig. 4a to c), suggesting that this could be related to effects on enhanced antioxidant capacity or decreased H2O2 generation. As a result, the ascorbate status of H2S-treated Col-0 and cat2 was similar to those of H2S-free control lines. No effect on ascorbate was observed (Fig. 4d)., The glutathione reduction state, which was above 90% in Col-0, was only 43% in cat2. Strikingly, glutathione in cat2 was significantly less oxidized in the presence of H2S treatment, and this was accompanied by decreased total glutathione (Fig. 4e).
Fig. 4.
Effects of H2S exposure on phenotype, SA content and redox buffer in cat2 mutants. a representative image of twenty-day-old indicated genotypes. Scale Bars indicate 1 cm. b Lesion quantification in the different genotypes as a percentage of the total rosette area. ND, not detected. c Leaf total SA. d and e Leaf ascorbate and glutathione content. White bars, reduced form. Red bars, oxidized form. 16-day-old seedlings of Col-0 and cat2 were treated with gaseous H2S released from 0.5 mM NaHS solution under standard growth conditions for another 4 d. Values are means ± SE of three biological replicates. Different letters indicate significant differences at P < 0.05 by multiple pairwise t-test comparisons at P < 0.05
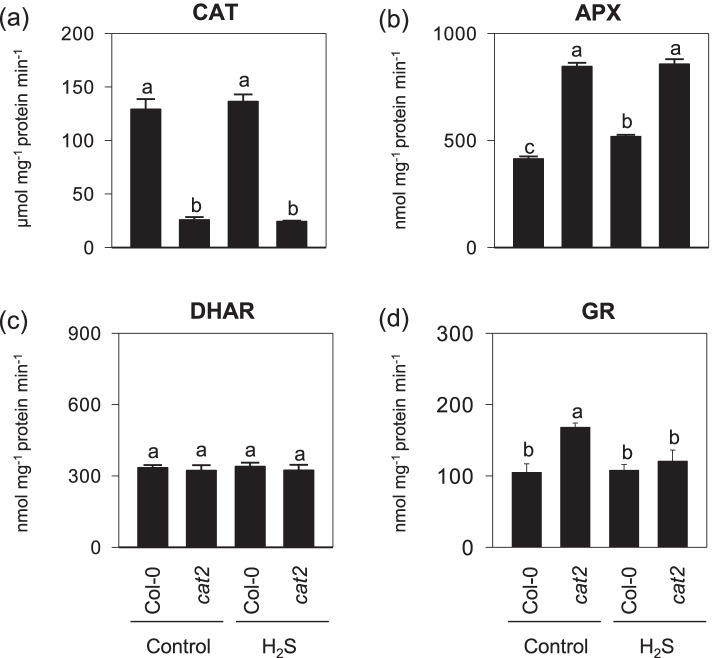
Furthermore, the activities of CAT, APX, GR, and DHAR, the enzymes linking glutathione pools [3], were measured (Fig. 5). As reported previously, the cat2 mutant showed only about 20% of wild-type leaf catalase activity [27], and this was not affected by the H2S treatment (Fig. 5a). The activities of two other enzymes of the ascorbate–glutathione pathway, APX and GR, were increased in cat2, but H2S exposure did not promote or had little effect on either (Fig. 5b and d). Compared with Col-0, no increase in extractable DHAR activity was detected in all tested lines and treatments (Fig. 5c). Taken together, these results provide little evidence that exogenously applied H2S alleviated H2O2-triggered oxidative stress responses by enhancing the scavenging capacities of H2O2 related with ascorbate–glutathione pathway. Rather, the data indicate that H2S-fumiagted cat2 plants showed a more reduced cellular redox environment.
Fig. 5.
Effects of H2S exposure on major antioxidative enzyme in cat2 mutants. a CAT. b APX. c, DHAR. d GR. 16- day-old seedlings of Col-0 and cat2 were treated with gaseous H2S released from 0.5 mM NaHS solution under standard growth conditions for another 4 d. Values are means ± SE of three biological replicates. Different letters indicate significant differences at P < 0.05 by multiple pairwise t-test comparisons at P < 0.05
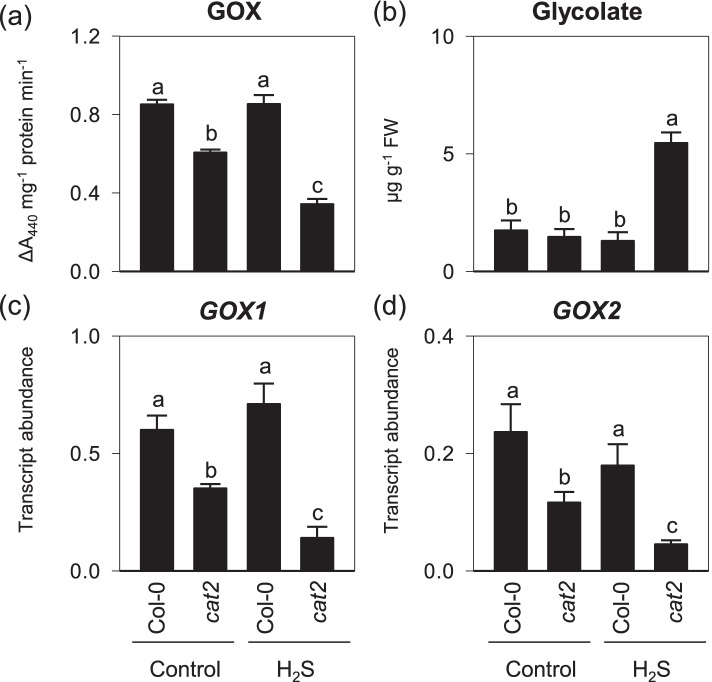
Because H2O2 generated from the reaction of glycolate oxidase (GOX)-catalyzed glycolate oxidation during photorespiration is metabolized by CAT. Decreased GOX activity thus might limit H2O2 accumulation. To examine whether the more reduced redox environment in the presence of H2S is due to the alteration of GOX activity, we compared the activities of GOX and the contents of its substrate glycolate between Col-0 and cat2 in the absence or presence of H2S treatments. In agreement with our previous results and others [33, 34, 40], decreased GOX activity was observed in the control treatment of cat2 leaves, but glycolate remained at Col-0 levels. H2S-fumigated cat2 mutants displayed significantly lower GOX than did the control treatment of cat2 mutants, accompanying by elevated levels of glycolate (Fig. 6a and b). Furthermore, the impaired GOX activity in H2S-treated cat2 plants correlates with reduced transcript abundancies of the two major GOX isoforms GOX1 and GOX2 (Fig. 6c and d). These observations infer that H2S exposure regulated cat2-triggered oxidative stress responses likely via repressing the GOX-catalyzed reaction.
Fig. 6.
Effects of H2S exposure on photorespiratory H2O2 -producing enzyme and associated substrate in cat2 mutants. a and b, Extractable leaf GOX activity and glycolate content. c and d, GOX1 and GOX2 transcript levels. 16-day-old seedlings of Col-0 and cat2 were treated with gaseous H2S released from 0.5 mM NaHS solution under standard growth conditions for another 4 d. Values are means ± SE of three biological replicates. Different letters indicate significant differences by multiple pairwise t-test comparisons at P < 0.05
Discussion
Our previous work demonstrated that increased H2O2 availability in the cat2 mutants could increase the metabolism of cysteine and glutathione [31], both of which are essential for redox homeostasis and signaling [17]. Glutathione-dependent pathways were reported to be involved in oxidative stress responses such as the activation of SA and JA pathways downstream of H2O2 [30, 31, 34]. However, the functional actions of cysteine-related in the regulation of oxidative stress remains to be determined. H2S generation via cysteine-degrading reactions has been shown to be induced in response to various environmental stresses [6]. Hence, uncovering if and how H2S participates in stress responses has had more paid attention in recent years. In this study, H2S production, and the activities of LCD, an enzyme involved in cysteine degradation, were observed to transient increase in response to intracellular oxidative stress. DES1 is by far the best-characterized cysteine desulfhydrase, which confers plant stress acclimation including nitrogen starvation responses, senescence, stomatal closure, and immunity [32, 37, 41, 42]. Nevertheless, the des1 mutation seems to have less apparent effect on oxidative stress responses in cat2 (Fig. 2). Moreover, autophagy, which was regulated by DES1-derived H2S signaling [32], failed to affect above process (Fig. 3). It should be noted that the des1 mutation led to only 30% and 22% reduction in endogenous sulfide in wild type and cat2 background respectively, and identified very few proteins (47 proteins) with decreased levels of persulfidation relative to wild-type plants [32, 43], indicating a limited role of DES1 in H2S signaling. Alternatively, this could raise another possibility that other and/or unidentified factors/pathways promoting H2S generation may compensate for the loss of function of DES1 if they have redundant roles in the regulation of oxidative stress. Indeed, several candidate genes, encoding the cysteine desulfhydrase, have been implicated in enhancing resistance to many stresses such as heavy metal stress, salt, drought and cold stress, pathogen attack, and preventing ROS accumulation [44–48]. For instance, cytosolic O-acetylserine-(thiol) lyase A (OASTLA) and chloroplastic OASTLB have substantially higher LCD activities than the cytosolic DES1, and likely act as major cysteine desulfhydrases in Arabidopsis [44].
Regardless of which specific isoforms of cysteine desulfhydrases are involved, the exposure to gaseous H2S led to the alteration in oxidative stress. Unlike other studies that reported that H2S modulated oxidative stress through elevating antioxidant capacities especially for the components in the ascorbate–glutathione pathway [18–20], our results presented here revealed an additional role of H2S in regulating stress responses. The photorespiratory GOX-generating H2O2 accounts for about 70% of the total pool and is therefore the most crucial H2O2 source in photosynthesizing C3 leaves [49]. Glycolate accumulation in H2S-treated cat2, jointly with decreased GOX activities and increased reduction status of glutathione (Fig. 4), implies that the peroxisomal H2O2 production via the photorespiratory pathway is reduced. Therefore, this partially restricted photorespiratory activity conferred by H2S treatment might be sufficient to attenuate the SA-associated cell death of cat2 mutants. Because H2S has been considered to regulate oxidative stress at the multiple levels [6]. Thus, such inhibitory effect could be likely linked to decreased transcript levels of the major forms of GOX (Fig. 6) [40], but we cannot exclude the possibility that the H2S-based post-translational modifications exert an effect on GOX activities. Both GOX1 and GOX2 have a single, and highly conserved cysteine residue that potentially could serve as a site for multiple redox modifications governing protein functionality under oxidative conditions. In line with above speculation, GOX1 has been implicated in undergoing glutathionylation [50]. Several lines of evidence showed that GOX from Kalanchoe pinnata and Pisum sativum were susceptible to nitrosation, which displayed decreased catalytic activity [51, 52]. Intriguingly, Both GOX isoforms were found to be targets of persulfidation observed in Arabidopsis [43], but whether such modification could repress GOX activity is, at least in vitro, difficulty to determine. Because the S in H2S is in the same reduced state as thiols and cannot react with protein cysteine residues to form persulfides unless an oxidant is added. However, the direct addition of H2S and H2O2, even at similar concentrations is less efficient and uncontrollable. Very recently, Ni et al. [53] developed an unique method to induce efficient persulfidation on proteins under physiologically relevant H2O2/H2S concentrations. This system can simultaneously produce H2S and H2O2 in a slow and controllable manner as this could be extensively applied to assess the effects of persulfidation in the future of H2S biological research.
Methods
Plant material and growth conditions
Arabidopsis thaliana wild-type Columbia-0 (Col-0) and homozygous knockout lines including cat2 (SALK076998) [27], atg5 (SAIL_129B07) [54] and des1 (SALK_103855) [32]used in this study, were obtained from the Arabidopsis Biological Research Center (ABRC), Columbus, Ohio, USA. A triple mutant cat2 des1 atg5 and a double mutant cat2 des1 were generated for this study. Above mutant lines used in this study were verified by PCR. All specific PCR primers used for genotyping are listed in Supplemental Table S1. Seeds were incubated for 2 d at 4ºC and then sown in soil. Plants were grown in a controlled-environment growth chamber at a day/night regime of 16 h/8 h (light/dark), an irradiance of 150 μmol m−2 s−1 at leaf level, temperatures of 22 ºC day/20 ºC night, 65% humidity. Our study complied with relevant institutional, national, and international guidelines and legislation, and no specific permits were required to collect the plant samples. Samples were rapidly frozen in liquid nitrogen and stored at -80ºC until analysis. Unless otherwise stated, data are means SE of at least three independent samples from different plants.
H2S fumigation treatment
The procedure of H2S fumigation was carried out as described in Wei et al. [42]. Solutions of sodium hydrosulfide (NaHS•3H2O) were used as a donor of H2S. In a sealed glass desiccator (volume 3 L), 16-day-old seedling lines were treated with gaseous H2S for 4 h per day, which was released from the 0.5 mM NaHS aqueous solution (200 mL). The aqueous solution of NaHS at 0 mM was set as the control. The NaHS solution and its control treatment were renewed each day and treated leaves were collected at designated time intervals for analyses.
Determinations of H2S content and LCD activity
H2S quantification was performed as described by Singh et al. [55]. 0.5 g plant leaves were ground into fine powder with a mortar and pestle under liquid nitrogen and then homogenized in 1 ml of the following extraction buffer: 20 mM Tris–HCl buffer (pH 8.0), 10 mM EDTA, 20 mM Zn(OAc)2. The homogenate was centrifuged at 15,000 g for 15 min at 4ºC and 0.1 mL of the supernatant was used for the H2S quantification, in an assay mixture containing 1.88 mL extraction buffer and 0.02 mL of 20 mM 5,5’-dithiobis(2-nitrobenzoic acid). The reaction mixture was incubated at room temperature for 2 min and the absorbance was read at 412 nm. H2S was quantified based on a standard curve prepared with NaHS.
The activity of LCD was measured as described previously [11]. Freshly sampled or stored at -80 °C leaves (200 mg) were ground to a fine powder in liquid nitrogen, and the soluble proteins were extracted by adding 1 mL of 20 mM Tris–HCl (pH 8.0), and centrifuged at 15,000 g for 15 min at 4ºC. LCD activity was detected by monitoring the release of H2S from L-cysteine in the presence of dithiothreitol (DTT). The assay was performed in a total volume of 1 mL comprising 2.5 mM dithiothreitol (DTT), 0.8 mM L-cysteine, 100 mM Tris–HCl (pH 9.0), and 10 μg of protein solution. The reaction was initiated by the addition of L-cysteine after incubation for 15 min at 37 °C. and was terminated by the addition of 0.1 mL of 30 mM FeCl3 dissolved in 1.2 N HCl and 0.1 mL 20 mM N,N-dimethyl-p-phenylenediaminedihy drochloride dissolved in 7.2 N HCl. The formation of methylene blue was determined at 670 nm. LCD enzymatic activity was calculated using a standard curve of known concentrations of NaHS.
Lesion quantification and SA assay
Percentage lesion area in cat2 in the absence or presence of H2S, cat2 des1 or cat2 des1 atg5, was quantified using IQmaterials software. Total SA was extracted as described in Langlois-Meurinne et al. [56]. SA was determined by HPLC-fluorescence according to the protocol of [31]. Identification and quantification was performed by comparison of peaks with SA standards.
qPCR analysis
Total RNA was extracted with TRIzol reagent (Takara) following the manufacturer’s instructions. To avoid potential genomic DNA contamination, RNA was treated with RNase-free DNase I (Takara). RNA quality and concentration were determined by gel electrophoresis and estimated using a nanodrop spectrophotometer at 260 nm respectively. Reverse transcription and first-strand cDNA synthesis were performed using the All-in-One cDNA Synthesis SuperMix (Bimake). qPCR was performed according to Zhang et al. [34]. In all experiments, three biological replicates of each sample and three technical (PCR) replicates were performed. Primer sequences are listed in Supplemental Table S1.
Ascorbate and glutathione assays
Oxidized and reduced forms of ascorbate and glutathione were quantified using the plate-reader method described by Queval and Noctor [57]. Leaf samples (100 mg) were ground in liquid nitrogen and then extracted into 1 mL of 0.2 N HCl. Before ascorbate and glutathione assays, the final pH of all samples was neutralized between 5 and 6. Ascorbate was measured by the absorbance at 265 nm that is specifically removed by ascorbate oxidase (AO). The reduced form was measured in untreated acid extract aliquots, while total ascorbate (dehydroascorbate + ascorbate) was measured following pre-incubation of aliquots with DTT to reduce dehydroascorbate to ascorbate. To assay ascorbate, the buffer contained 100 μL 0.2 M NaH2PO4 (pH 5.6), 40 μL extract and 55 μL H2O. The absorbance at 265 nm was read before and 5 min after adding 0.2 U of AO. Total ascorbate was measured after incubation of 100 μL neutralized extract with 140 μL 0.12 M NaH2PO4 (pH 7.5), 10 μL 25 mM DTT for 30 min at room temperature. Both of above assays were done in triplicate for each extract. According to a standard extinction coefficient of 14 mM−1 cm−1, absorbance changes in A265 were converted to quantities of ascorbate.
The method for glutathione measurement relies on GR-dependent reduction of DNTB by GSH monitored at 412 nm and is used to measure either total glutathione (GSH + GSSG) or GSSG. Specific assay of GSSG was done by pre-treatment of extract aliquots with 2 μL of 2-vinylpyridine (VPD), which efficiently complexes GSH. To measure total glutathione, the mixture contained 100 μL of 0.2 M NaH2PO4, 10 mM EDTA (pH 7.5), 10 μL 10 mM NADPH, 10 μL 12 mM DTNB, 10 μL of neutralized extract and 60 μL water. After shaking, the reaction was started by addition of 10 μL GR. GSSG measurement was based on the same principle except that extract aliquots were incubated with VPD for 30 min at room temperature and were centrifuged twice for 15 min at 4ºC before assay. The increase in absorbance at 412 nm was monitored for 5 min and all assays were done in triplicate for each extract. Rates were converted to quantities of glutathione using standard curves generated by assay of known concentration in the same plate.
Measurements of glycolate oxidase activity and glycolate content
The glycolate oxidase activity was performed by monitoring the formation of colored O-dianisidine radical cation in the presence of sodium glycolate at 440 nm according to protocol detailed by Waszczak et al. [33]. Glycolate content was measured by liquid chromatography-tandem mass spectrometry (LC–MS/MS, Agilent), using 5% acetic acid extracts from leaves as described in Saji et al. [58].
Measurements of antioxidant enzyme activities
Spectrophotometric assay to measure enzyme activities in the glutathione-ascorbate pathway was performed as described previously [59]. Briefly, freshly sampled or stored at -80 °C leaves or (150 mg) were homogenized with 1.5 mL extraction buffer containing 50 mg insoluble polyvinylpyrrolidine, 0.1 M phosphate buffer, 1 mM EDTA (pH7.5) and 1 mM ascorbate. Catalase was measured by the removal of H2O2 monitored at 240 nm, DHAR as GSH-dependent formation of ascorbate from DHA at 265 nm, APX as H2O2-dependent ascorbate oxidation at 290 nm, and GR as GSSG-dependent NADPH oxidation at 340 nm. Bradford’s method was adopted for measuring the protein concentration, and bovine serum albumin was used as standard [60].
Statistical analysis
The significance of differences was determined by Student’s t-test. Calculations were performed on a minimum of three independent datasets, assuming two samples equal variance and a two-tailed distribution. For comparing multiple treatments, different letters indicate significant differences using multiple pairwise t-test comparisons at P < 0.05.
Supplementary Information
Additional file 1: Figure S1. Effects of des1 mutation on major antioxidative enzyme in cat2 mutants. (a) CAT. (b), APX. (c), DHAR. (d), GR. Samples of each line were taken after 20 days of growth. Values are means ± SE of three biological replicates. Different letters indicate significant differences by multiple pairwise t-test comparisons at P < 0.05.
Additional file 2: Table S1. List of primers and restriction enzymes used in this study.
Acknowledgements
We would like to thank Dr. Wei Zhang and Miss Mingyue Ma for the initial efforts for this work.
Authors’ contributions
Y.H. planned and designed the research. Y.H., L.W., and X.M. performed experiments and analyzed data. Y.H., and X.C. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was mainly supported by the Natural Science Foundation of China (31300225), and start-up funding of Anhui Agricultural University.
Availability of data and materials
The data presented in this study are available in the graphs provided in the manuscript. Plant materials used during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lijuan Wang and Xiujie Mu contributed equally to this work.
References
- 1.de Zelicourt A, Colcombet J, Hirt H. The role of MAPK modules and ABA during abiotic stress signaling. Trends Plant Sci. 2016;21:677–685. doi: 10.1016/j.tplants.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Huang J, Willems P, Wei B, Tian C, Ferreira RB, Bodra N, et al. Mining for protein S-sulfenylation in Arabidopsis uncovers redox-sensitive sites. Proc Natl Acad Sci U S A. 2019;116:21256–21261. doi: 10.1073/pnas.1906768116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mhamdi A, Van Breusegem F. Reactive oxygen species in plant development. Development. 2018;145(15):dev164376. doi: 10.1242/dev.164376. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, Zhou M, Zhou H, Zhao D, Gotor C, Romero LC, et al. Hydrogen sulfide, a signaling molecule in plant stress responses. J Integr Plant Biol. 2021;63(1):146–160. doi: 10.1111/jipb.13022. [DOI] [PubMed] [Google Scholar]
- 5.Gu Q, Wang CY, Xiao QQ, Chen ZP, Han Y. Melatonin confers plant cadmium tolerance: an update. Int J Mol Sci. 2021;22(21):11704. doi: 10.3390/ijms222111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen T, Tian M, Han Y. Hydrogen sulfide: a multi-tasking signal molecule in the regulation of oxidative stress responses. J Exp Bot. 2020;71(10):2862–2869. doi: 10.1093/jxb/eraa093. [DOI] [PubMed] [Google Scholar]
- 7.Corpas FJ, González-Gordo S, Cañas A, Palma JM. Nitric oxide and hydrogen sulfide in plants: which comes first? J Exp Bot. 2019;70(17):4391–4404. doi: 10.1093/jxb/erz031. [DOI] [PubMed] [Google Scholar]
- 8.Hu KD, Zhang XY, Yao GF, Rong YL, Ding C, Tang J, et al. A nuclear-localized cysteine desulfhydrase plays a role in fruit ripening in tomato. Hortic Res. 2020;7(1):211. doi: 10.1038/s41438-020-00439-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi H, Kopriva S, Giordano M, Saito K, Hell R. Sulfur assimilation in photosynthetic organisms: molecular functions and regulations of transporters and assimilatory enzymes. Annu Rev Plant Biol. 2011;62:157–184. doi: 10.1146/annurev-arplant-042110-103921. [DOI] [PubMed] [Google Scholar]
- 10.Alvarez C, Calo L, Romero LC, García I, Gotor C. An O-acetylserine(thiol)lyase homolog with L-cysteine desulfhydrase activity regulates cysteine homeostasis in Arabidopsis. Plant Physiol. 2010;152(2):656–669. doi: 10.1104/pp.109.147975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riemenschneider A, Wegele R, Schmidt A, Papenbrock J. Isolation and characterization of a D-cysteine desulfhydrase protein from Arabidopsis thaliana. FEBS J. 2005;272(5):1291–1304. doi: 10.1111/j.1742-4658.2005.04567.x. [DOI] [PubMed] [Google Scholar]
- 12.Bauer M, Dietrich C, Nowak K, Sierralta WD, Papenbrock J. Intracellular localization of Arabidopsis sulfurtransferases. Plant Physiol. 2004;135(2):916–926. doi: 10.1104/pp.104.040121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatzfeld Y, Maruyama A, Schmidt A, Noji M, Ishizawa K, Saito K. β-cyanoalanine synthase is a mitochondrial cysteine synthase-like protein in spinach and Arabidopsis. Plant Physiol. 2000;123(3):1163–1171. doi: 10.1104/pp.123.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moseler A, Dhalleine T, Rouhier N, Couturier J. Arabidopsis thaliana 3-mercaptopyruvate sulfurtransferases interact with and are protected by reducing systems. J Biol Chem. 2021;296:100429. doi: 10.1016/j.jbc.2021.100429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamaguchi Y, Nakamura T, Kusano T, Sano H. Three Arabidopsis genes encoding proteins with differential activities for cysteine synthase and β-cyanoalanine synthase. Plant Cell Physiol. 2000;41(4):465–476. doi: 10.1093/pcp/41.4.465. [DOI] [PubMed] [Google Scholar]
- 16.Filipovic MR, Zivanovic J, Alvarez B, Banerjee R. Chemical biology of H2S signaling through persulfidation. Chem Rev. 2018;118(3):1253–1337. doi: 10.1021/acs.chemrev.7b00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noctor G, Mhamdi A, Chaouch S, Han Y, Neukermans J, Marquez-Garcia B, et al. Glutathione in plants: an integrated overview. Plant Cell Environ. 2012;35(2):454–484. doi: 10.1111/j.1365-3040.2011.02400.x. [DOI] [PubMed] [Google Scholar]
- 18.Iqbal N, Umar S, Khan NA, Corpas FJ. Nitric oxide and hydrogen sulfide coordinately reduce glucose sensitivity and decrease oxidative stress via ascorbate-glutathione cycle in heat-stressed Wheat (Triticum aestivum L.) Plants. Antioxidants (Basel) 2021;10(1):108. doi: 10.3390/antiox10010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Shi C, Wang X, Liu C, Ding X, Ma P, et al. Hydrogen sulfide regulates the activity of antioxidant enzymes through persulfidation and improves the resistance of tomato seedling to Copper Oxide nanoparticles (CuO NPs)-induced oxidative stress. Plant Physiol Biochem. 2020;156:257–266. doi: 10.1016/j.plaphy.2020.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Christou A, Manganaris GA, Papadopoulos I, Fotopoulos V. Hydrogen sulfide induces systemic tolerance to salinity and non-ionic osmotic stress in strawberry plants through modification of reactive species biosynthesis and transcriptional regulation of multiple defence pathways. J Exp Bot. 2013;64(7):1953–1966. doi: 10.1093/jxb/ert055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui W, Chen H, Zhu K, Jin Q, Xie Y, Cui J, et al. Cadmium-induced hydrogen sulfide synthesis is involved in cadmium tolerance in Medicago sativa by reestablishment of reduced (homo)glutathione and reactive oxygen species homeostases. PLoS One. 2014;9(10):e109669. doi: 10.1371/journal.pone.0109669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shan C, Zhang S, Li D, Zhao Y, Tian X, Zhao X, et al. Effects of exogenous hydrogen sulfide on the ascorbate and glutathione metabolism in wheat seedlings leaves under water stress. Acta Physiol Plant. 2011;33:2533–2540. [Google Scholar]
- 23.Shan C, Dai H, Sun Y. Hydrogen sulfide protects wheat seedlings against copper stress by regulating the ascorbate and glutathione metabolism in leaves. Aust J Crop Sci. 2012;6(2):248–54.
- 24.Aroca Á, Serna A, Gotor C, Romero LC. S-sulfhydration: a cysteine posttranslational modification in plant systems. Plant Physiol. 2015;168(1):334–342. doi: 10.1104/pp.15.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen J, Zhang J, Zhou M, Zhou H, Cui B, Gotor C, et al. Persulfidation-based modification of cysteine desulfhydrase and the NADPH oxidase RBOHD controls guard cell abscisic acid signaling. Plant Cell. 2020;32(4):1000–1017. doi: 10.1105/tpc.19.00826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scuffi D, Nietzel T, Di Fino LM, Meyer AJ, Lamattina L, Schwarzländer M, et al. Hydrogen sulfide increases production of NADPH oxidase-dependent hydrogen peroxide and phospholipase D-derived phosphatidic acid in guard cell signaling. Plant Physiol. 2018;176(3):2532–2542. doi: 10.1104/pp.17.01636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Queval G, Issakidis-Bourguet E, Hoeberichts FA, Vandorpe M, Gakière B, Vanacker H, et al. Conditional oxidative stress responses in the Arabidopsis photorespiratory mutant cat2 demonstrate that redox state is a key modulator of daylength-dependent gene expression, and define photoperiod as a crucial factor in the regulation of H2O2-induced cell death. Plant J. 2007;52(4):640–657. doi: 10.1111/j.1365-313X.2007.03263.x. [DOI] [PubMed] [Google Scholar]
- 28.Chaouch S, Queval G, Vanderauwera S, Mhamdi A, Vandorpe M, Langlois-Meurinne M, et al. Peroxisomal hydrogen peroxide is coupled to biotic defense responses by ISOCHORISMATE SYNTHASE1 in a daylength-related manner. Plant Physiol. 2010;153(4):1692–1705. doi: 10.1104/pp.110.153957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mhamdi A, Queval G, Chaouch S, Vanderauwera S, Van Breusegem F, Noctor G. Catalase function in plants: a focus on Arabidopsis mutants as stress-mimic models. J Exp Bot. 2010;61(15):4197–4220. doi: 10.1093/jxb/erq282. [DOI] [PubMed] [Google Scholar]
- 30.Han Y, Mhamdi A, Chaouch S, Noctor G. Regulation of basal and oxidative stress-triggered jasmonic acid-related gene expression by glutathione. Plant Cell Environ. 2013;36(6):1135–1146. doi: 10.1111/pce.12048. [DOI] [PubMed] [Google Scholar]
- 31.Han Y, Chaouch S, Mhamdi A, Queval G, Zechmann B, Noctor G. Functional analysis of Arabidopsis mutants points to novel roles for glutathione in coupling H2O2 to activation of salicylic acid accumulation and signaling. Antioxid Redox Signal. 2013;18(16):2106–2121. doi: 10.1089/ars.2012.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Álvarez C, García I, Moreno I, Pérez-Pérez ME, Crespo JL, Romero LC, et al. Cysteine-generated sulfide in the cytosol negatively regulates autophagy and modulates the transcriptional profile in Arabidopsis. Plant Cell. 2012;24(11):4621–4634. doi: 10.1105/tpc.112.105403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waszczak C, Kerchev PI, Mühlenbock P, Hoeberichts FA, Van Der Kelen K, Mhamdi A, et al. SHORT-ROOT deficiency alleviates the cell death phenotype of the Arabidopsis catalase2 mutant under photorespiration-promoting conditions. Plant Cell. 2016;28(8):1844–1859. doi: 10.1105/tpc.16.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang T, Ma M, Chen T, Zhang L, Fan L, Zhang W, et al. Glutathione-dependent denitrosation of GSNOR1 promotes oxidative signalling downstream of H2O2. Plant Cell Environ. 2020;43:1175–1191. doi: 10.1111/pce.13727. [DOI] [PubMed] [Google Scholar]
- 35.Rizhsky L, Hallak-Herr E, Van Breusegem F, Rachmilevitch S, Barr JE, Rodermel S, et al. Double antisense plants lacking ascorbate peroxidase and catalase are less sensitive to oxidative stress than single antisense plants lacking ascorbate peroxidase or catalase. Plant J. 2002;32(3):329–342. doi: 10.1046/j.1365-313x.2002.01427.x. [DOI] [PubMed] [Google Scholar]
- 36.Willekens H, Chamnongpol S, Davey M, Schraudner M, Langebartels C, Van Montagu M, et al. Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. EMBO J. 1997;16(16):4806–4816. doi: 10.1093/emboj/16.16.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laureano-Marín AM, Moreno I, Romero LC, Gotor C. Negative regulation of autophagy by sulfide Is independent of reactive oxygen species. Plant Physiol. 2016;171(2):1378–1391. doi: 10.1104/pp.16.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiong Y, Contento AL, Nguyen PQ, Bassham DC. Degradation of oxidized proteins by autophagy during oxidative stress in Arabidopsis. Plant Physiol. 2007;143(1):291–299. doi: 10.1104/pp.106.092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshimoto K, Jikumaru Y, Kamiya Y, Kusano M, Consonni C, Panstruga R, et al. Autophagy negatively regulates cell death by controlling NPR1-dependent salicylic acid signaling during senescence and the innate immune response in Arabidopsis. Plant Cell. 2009;21(9):2914–2927. doi: 10.1105/tpc.109.068635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kerchev P, Waszczak C, Lewandowska A, Willems P, Shapiguzov A, Li Z, et al. Lack of GLYCOLATE OXIDASE1, but Not GLYCOLATE OXIDASE2, attenuates the photorespiratory phenotype of CATALASE2-deficient Arabidopsis. Plant Physiol. 2016;171(3):1704–1719. doi: 10.1104/pp.16.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Álvarez C, Ángeles Bermúdez M, Romero LC, Gotor C, García I. Cysteine homeostasis plays an essential role in plant immunity. New Phytol. 2012;193(1):165–177. doi: 10.1111/j.1469-8137.2011.03889.x. [DOI] [PubMed] [Google Scholar]
- 42.Wei B, Zhang W, Chao J, Zhang T, Zhao T, Noctor G, et al. Functional analysis of the role of hydrogen sulfide in the regulation of dark-induced leaf senescence in Arabidopsis. Sci Rep. 2017;7(1):2615. doi: 10.1038/s41598-017-02872-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aroca A, Benito JM, Gotor C, Romero LC. Persulfidation proteome reveals the regulation of protein function by hydrogen sulfide in diverse biological processes in Arabidopsis. J Exp Bot. 2017;68(17):4915–4927. doi: 10.1093/jxb/erx294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kurmanbayeva A, Bekturova A, Soltabayeva A, Oshanova D, Nurbekova Z, Srivastava S, et al. Active OASTLs confer improved Se resistance and degrade L-Cys and SeCys in Arabidopsis. J Exp Bot. 2022;27:erac021. doi: 10.1093/jxb/erac021. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Q, Cai W, Ji TT, Ye L, Lu YT, Yuan TT. WRKY13 enhances cadmium tolerance by promoting D-CYSTEINE DESULFHYDRASE and hydrogen sulfide production. Plant Physiol. 2020;183(1):345–357. doi: 10.1104/pp.19.01504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fang H, Liu Z, Long Y, Liang Y, Jin Z, Zhang L, et al. The Ca2+/calmodulin2-binding transcription factor TGA3 elevates LCD expression and H2 S production to bolster Cr6+ tolerance in Arabidopsis. Plant J. 2017;91(6):1038–1050. doi: 10.1111/tpj.13627. [DOI] [PubMed] [Google Scholar]
- 47.Shi H, Ye T, Han N, Bian H, Liu X, Chan Z. Hydrogen sulfide regulates abiotic stress tolerance and biotic stress resistance in Arabidopsis. J Integr Plant Biol. 2015;57(7):628–640. doi: 10.1111/jipb.12302. [DOI] [PubMed] [Google Scholar]
- 48.Jin Z, Shen J, Qiao Z, Yang G, Wang R, Pei Y. Hydrogen sulfide improves drought resistance in Arabidopsis thaliana. Biochem Biophys Res Commun. 2011;414(3):481–486. doi: 10.1016/j.bbrc.2011.09.090. [DOI] [PubMed] [Google Scholar]
- 49.Noctor G, Veljovic-Jovanovic S, Driscoll S, Novitskaya L, Foyer CH. Drought and oxidative load in the leaves of C3 plants: a predominant role for photorespiration? Ann Bot. 2002;89 Spec No(7):841–50. doi: 10.1093/aob/mcf096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rouhier N, Villarejo A, Srivastava M, Gelhaye E, Keech O, Droux M, et al. Identification of plant glutaredoxin targets. Antioxid Redox Signal. 2005;7(7–8):919–929. doi: 10.1089/ars.2005.7.919. [DOI] [PubMed] [Google Scholar]
- 51.Ortega-Galisteo AP, Rodríguez-Serrano M, Pazmiño DM, Gupta DK, Sandalio LM, Romero-Puertas MC. S-Nitrosylated proteins in pea (Pisum sativum L.) leaf peroxisomes: changes under abiotic stress. J Exp Bot. 2012;63(5):2089–103. doi: 10.1093/jxb/err414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abat JK, Mattoo AK, Deswal R. S-nitrosylated proteins of a medicinal CAM plant Kalanchoe pinnata- ribulose-1,5-bisphosphate carboxylase/oxygenase activity targeted for inhibition. FEBS J. 2008;275(11):2862–2872. doi: 10.1111/j.1742-4658.2008.06425.x. [DOI] [PubMed] [Google Scholar]
- 53.Ni X, Li X, Shen TL, Qian WJ, Xian M. A sweet H2S/H2O2 dual release system and specific protein S-persulfidation mediated by thioglucose/glucose oxidase. J Am Chem Soc. 2021;143(33):13325–13332. doi: 10.1021/jacs.1c06372. [DOI] [PubMed] [Google Scholar]
- 54.Inoue Y, Suzuki T, Hattori M, Yoshimoto K, Ohsumi Y, Moriyasu Y. AtATG genes, homologs of yeast autophagy genes, are involved in constitutive autophagy in Arabidopsis root tip cells. Plant Cell Physiol. 2006;47(12):1641–1652. doi: 10.1093/pcp/pcl031. [DOI] [PubMed] [Google Scholar]
- 55.Singh VP, Singh S, Kumar J, Prasad SM. Hydrogen sulfide alleviates toxic effects of arsenate in pea seedlings through up-regulation of the ascorbate-glutathione cycle: Possible involvement of nitric oxide. J Plant Physiol. 2015;181:20–29. doi: 10.1016/j.jplph.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 56.Langlois-Meurinne M, Gachon CM, Saindrenan P. Pathogen-responsive expression of glycosyltransferase genes UGT73B3 and UGT73B5 is necessary for resistance to Pseudomonas syringae pv tomato in Arabidopsis. Plant Physiol. 2005;139(4):1890–1901. doi: 10.1104/pp.105.067223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Queval G, Noctor G. A plate reader method for the measurement of NAD, NADP, glutathione, and ascorbate in tissue extracts: Application to redox profiling during Arabidopsis rosette development. Anal Biochem. 2007;363(1):58–69. doi: 10.1016/j.ab.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 58.Saji S, Bathula S, Kubo A, Tamaoki M, Aono M, Sano T, et al. Ozone-sensitive Arabidopsis mutants with deficiencies in photorespiratory enzymes. Plant Cell Physiol. 2017;58(5):914–924. doi: 10.1093/pcp/pcx027. [DOI] [PubMed] [Google Scholar]
- 59.Noctor G, Mhamdi A, Foyer CH. Oxidative stress and antioxidative systems: recipes for successful data collection and interpretation. Plant Cell Environ. 2016;39(5):1140–1160. doi: 10.1111/pce.12726. [DOI] [PubMed] [Google Scholar]
- 60.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Effects of des1 mutation on major antioxidative enzyme in cat2 mutants. (a) CAT. (b), APX. (c), DHAR. (d), GR. Samples of each line were taken after 20 days of growth. Values are means ± SE of three biological replicates. Different letters indicate significant differences by multiple pairwise t-test comparisons at P < 0.05.
Additional file 2: Table S1. List of primers and restriction enzymes used in this study.
Data Availability Statement
The data presented in this study are available in the graphs provided in the manuscript. Plant materials used during the current study are available from the corresponding author upon reasonable request.