Abstract
Weight losses >10% favorably modulate biomarkers of breast cancer risk but are not typically achieved by comprehensive weight loss programs, including the Diabetes Prevention Program (DPP). Combining the DPP with Hunger Training (HT), an evidence-based self-regulation strategy that uses self-monitored glucose levels to guide meal timing, has potential to enhance weight losses and cancer-related biomarkers, if proven feasible. This 2-arm RCT examined the feasibility of adding HT to the DPP and explored effects on weight and metabolic and breast cancer risk biomarkers. Fifty postmenopausal women (BMI > 27 kg/m2) at risk of breast cancer were randomized to the DPP+HT or DPP-only arm. Both arms followed a 16-week version of the DPP delivered weekly by a trained registered dietitian. Those in the DPP+HT also wore a continuous glucose monitor during weeks 4–6 of the program. Feasibility criteria were accrual rates > 50%, retention rates > 80%, and adherence to the HT protocol >75%. All a priori feasibility criteria were achieved. The accrual rate was 67%; retention rate was 81%; and adherence to HT was 90%. Weight losses and BMI reductions were significant over time as were changes in metabolic and breast cancer risk biomarkers but did not vary by group. This trial demonstrated that HT was feasible to add to comprehensive weight management program targeted towards postmenopausal women at high risk of breast cancer, though upon preliminary examination it does not appear to enhance weight loss or metabolic changes.
Keywords: weight loss, postmenopause, breast neoplasms, feasibility studies, energy intake
Introduction
Recent estimates indicate that 40% of U.S. women are obese (1). Despite widespread health promotion efforts, the prevalence of obesity and obesity-related cancers, including postmenopausal breast cancer, continues to rise (2). Breast cancer is the leading cause of obesity-related cancer death among women in the U.S. (3). The link between postmenopausal breast cancer and obesity is particularly strong in women at high risk for the disease (4). Obesity and maladaptive eating patterns unfavorably impact insulin resistance, a key, modifiable risk factor for postmenopausal breast cancer that has downstream effects on insulin-signaling (e.g., IGF-1), adipokines (5), and circulating proinflammatory cytokines (6) that promote tumorigenesis (7). Diet-induced weight loss modulates these biomarkers, supporting the underlying biology linking obesity to breast cancer (8). Improving weight management in the context of breast cancer prevention is needed, given continued increases in the prevalence of obesity, particularly in older adults (1), which will undoubtedly increase the incidence of postmenopausal breast cancer in the U.S.
Weight loss programs have had limited effectiveness in producing sufficient weight losses to reduce breast cancer risk. In a clinical trial, Fabian and colleagues (9) showed that women who lost >10% of their initial body weight during a comprehensive weight loss intervention had significant reductions in breast cancer risk biomarkers including adiponectin, leptin, insulin, and CRP. Similar reductions in biomarkers have been observed after bariatric surgery (10). Intensive behavioral interventions largely avoid these dangers and are the better option for safe weight management but typically achieve weight losses of only 4–7% (11). Novel weight loss and cancer prevention strategies with clinically-meaningful outcomes are essential to reduce the prevalence of breast cancer.
Eating only in response to hunger facilitates energy homeostasis as an intermediary step in weight regulation (12). Yet, in today’s permissive food environment, many eating events are unrelated to energy depletion (13). Rather, they are predicted by non-physiological determinants of food intake, such as the hedonic properties of palatable foods (14); individual differences in hedonic eating behavior (e.g., emotional eating) (15); or sensitivity to food rewards (16), and impulsivity and self-control (17). Eating behaviors not regulated by physiological signals of hunger and satiety have been empirically linked to weight gain (18). Self-regulation skills predict beneficial weight outcomes (19). Teaching people to self-regulate their energy intake by differentiating between physiological hunger and their hedonic desire to eat when not hungry is an empirically supported weight-control strategy (20). Furthermore, research shows that obese people are less sensitive to elevated glucose levels, an indicator of available short-term energy (21), and thus may be less likely to distinguish physiological hunger from their hedonic desire to eat without help (22–26). Learning to eat in response to physiological hunger (Hunger Training) may be the key to improved weight loss outcomes.
Hunger Training (HT) has been validated as a standalone intervention in young and middle-aged women and men to enhance the self-regulation of energy intake and promote weight loss in a manner that has favorable metabolic effects (22,24–28). Because it uses glucose monitoring as real-time biological feedback (biofeedback) of short-term energy status to guide decisions about when to eat without additional dietary restrictions or physical activity (29–32), HT is a strategy that has independent effects on weight regulation (29–32) that, when combined with traditional behavioral approaches, could enhance weight loss outcomes (23). The scientific premise of HT does not require glucose to be a valid proxy for hunger. Rather, it is that energy intake should occur in a deprived state to promote energy balance. HT induces rapid, substantial, and lasting weight losses that exceed the 4–7% expected from lifestyle changes. In overweight individuals, HT alone—without any additional diet or physical activity guidance—has been shown to induce weight losses of as much as 1.7% in as little as 2 weeks (22) and 7.4% in 5 months (27). Previous studies have shown that individuals learn to associate feelings of hunger and glucose levels after as little as 1 week, and the vast majority can accurate predict their glucose within 2 weeks of training (33). HT has advantages over conventional dieting in that it teaches a sustainable self-regulatory skill and uses real-time biofeedback to guide meal timing (27).
Beyond improving weight control, HT has positive effects on glycemic control and insulin sensitivity in both diabetic and non-diabetic populations even in the absence of weight loss (34–38). What remains to be determined is whether adding HT to a comprehensive weight loss intervention is: [1] feasible and [2] has the potential to enhance weight loss outcomes above the 4–7% by producing synergistic improvements in biomarkers of postmenopausal breast cancer risk.
The objective of the current 16-week feasibility RCT was to determine the feasibility and preliminary efficacy of adding HT, facilitated by continuous glucose monitoring, to the Diabetes Prevention Program (DPP), on weight loss outcomes and metabolic and breast cancer risk biomarkers in a sample of postmenopausal women at high risk of breast cancer. We hypothesized that adding HT to the DPP (DPP + HT) would be feasible as reflected by accrual rates >50%, attrition rates <20%, and adherence to the HT protocol >75%. We also explored whether HT had the potential to enhance weight losses and metabolic and breast cancer risk biomarkers achieved by the DPP alone. To our knowledge, this study is the first to modify the highly disseminated, evidence-based DPP by incorporating biological feedback as a behavior change technique.
Materials and Methods
This feasibility study was a 16-week RCT examining the feasibility of adding HT to the DPP with secondary aims to compare changes in body weight, metabolic, and breast cancer risk biomarkers between groups. Study protocols were approved by The University of Texas MD Anderson Cancer Center Institutional Review Board and registered with ClinicalTrials.gov (NCT03546972). The study was conducted in accordance with the International Conference on Harmonization - Good Clinical Practice (ICH)-GCP.
A total of 50 postmenopausal women with a body mass index (BMI) >27 kg/m2 were recruited between 2016 and 2018 from Houston, Texas. Women were included if they had a high risk of developing breast cancer, defined as Gail model lifetime risk >20% or 5-year risk >1.66%, a history of deleterious BRCA1/2 mutation or Mantle radiation, a history of ductal cancer in situ, or a history of high-risk premalignant breast lesion. Additional inclusion criteria were: being 30–70 years of age; having daily internet access and the ability to take digital time-stamped photographs; and reporting proficiency in the English language. Women were excluded if they reported being treated for cancer other than non-melanoma skin cancer; being unwilling to use (or calibrate by finger stick) a continuous glucose monitor; a diagnosis of type 1 or type 2 diabetes, use of oral antidiabetic agents (except metformin), or current treatment with any insulin regimen or GLP-1 receptor agonists (e.g., Exnatide, Liraglutide); or having a measured fasting blood glucose level >126 mg/dL or HbA1c >7%. Women on any active hormone therapy (n=5) or metformin (n=1) were not excluded. Written informed consent was obtained from all participants, and they were compensated $50 at each of the three assessment points.
Women were recruited from the High-Risk Breast Cohort at a Cancer Center in Houston, Texas via targeted letters and from a radio advertisement aired over a two-week period during project year. Women who expressed interest in participating by telephone or email were initially assessed for eligibility by telephone or by electronic screener using research electronic data capture (REDCap) (39). If women were screened as eligible and remained interested in participating, they were scheduled for an in-person study visit to provide informed consent and have their eligibility confirmed (BMI, fasting glucose levels, glycated hemoglobin levels, and medication use). A letter confirming initial eligibility and instructions for their in-person study visit (e.g., minimum of an 8-hour pre-visit fast) was sent following the initial screening procedures. Eligibility was confirmed by assessing fasting glucose levels (<126 mg/dl) by a commercially-available glucometer (OneTouch, UltraMini Blood Glucose Meter) and glycated hemoglobin (Hb1Ac) levels (<7%) by a commercially-available test kit (A1CNow® Test Systems), and by measured weight and height. Fasting was confirmed by self-report and study visits were scheduled during early-to-mid morning hours. Finally, a medication confirmation form was completed by potentially-eligible women and reviewed by the study staff. Women who met the enrollment criteria were deemed eligible for study participation and invited to provide informed consent.
Randomization
Participants were randomized to one of two intervention arms in a 1:1 ratio using a minimization approach based on baseline BMI and age. Neither those delivering or receiving the interventions were blinded; however, the investigative team was blinded to group assignment.
Interventions
Diabetes Prevention Program (DPP)
The DPP is a lifestyle intervention that serves as the standard of care in weight-loss and diabetes prevention (40). Participants met weekly with a Registered Dietitian, trained in the DPP requirements, to discuss session topics in the order listed in Figure 1. Participants’ goals during the intervention were to lose 7% of their body weight, decrease their fat consumption to 25% of calories, and increase their energy expenditure by 700 kcals (equivalent to 2.5 hours of moderate-intensity walking) per week. The DPP sessions were delivered to study participants through 16 weekly in-person or telephone sessions. Each participant received publicly available DPP print materials and worksheets. During the sessions, strategies to encourage weight loss, including self-monitoring of weight and reduction of calorie and fat intake, were addressed. Opportunities for supervised exercise (e.g., group exercise class or walks) were also offered at least twice per week.
Figure 1.
Diabetes Prevention Program sessions and integration of Hunger Training. This figure includes the title of each of 16 sessions included in the Diabetes Prevention Program, and the addition of Hunger Training at weeks 4, 5, and 6.
Diabetes Prevention Program plus Hunger Training (DPP+HT)
With the exception of incorporating the HT protocols during intervention, the procedures for the DPP+HT intervention matched those of the DPP-only intervention. In the DPP+HT group, HT was introduced during DPP session 4 and continued through session 6 (Figure 1). Between these sessions, women in the DPP+HT group followed the HT protocol. Specifically, women wore a continuous glucose monitor (CGM; FreeStyle Libre Flash Glucose Monitoring system, Abbott Laboratories) and recorded glucose, hunger levels (1=Not hungry at all, 10=Extremely hungry), and related symptoms of hunger (e.g., stomach pangs) on a paper log when the desire to eat arose, in a manner consistent with previously published protocols (22,27). Participants were instructed to follow the training protocol for two consecutive rounds of wearing the CGM sensors, equivalent to 20 days. Participants were encouraged to eat only when glucose levels were at or below a personalized threshold (27). Personalized glucose thresholds were established for each DPP+HT participant as the average of two consecutive morning glucose levels after fasting overnight (8 hours or more). The personalized threshold was described to participants as reflecting ‘true’ or physiological hunger (often used in research to confirm a state of energy deprivation) (41,42). A glucose level above the personalized threshold indicated that a meal (or snack; any calorie-containing food or beverage) should be skipped for at least 30 minutes, at which time glucose levels could be reassessed. In the meantime, non-calorie containing beverages (e.g., black coffee, tea, water) could be consumed.
During the first week of hunger training, each participant had a one-on-one phone call with the study dietitian to answer any questions about the training period or address any concerns about following the protocol. Participants were encouraged to contact the study staff or study dietitian anytime during the study using provided contact information.
Outcome Measures
Feasibility of adding HT to the DPP was assessed using the following criteria: 1. accrual goal > 50% defined as the ratio of consenting participants to total initially-eligible women; 2. retention goal > 80% defined as the ratio of participants attending post-intervention laboratory assessments to participants attending baseline laboratory assessments; and 3. average protocol adherence > 75% defined as the ratio of days logging pre-meal glucose and hunger levels to the total number of valid CGM days during the training period (DPP+HT group only). Previous work has shown that the most predictive measure of weight loss with hunger training was the frequency of recording on a paper log (43). A valid day of logging had ≥ 1 instance where both glucose and hunger were recorded. A valid CGM day was one that allowed most participant to have at least two opportunities (meals or snacks) to record glucose and hunger levels based on NHANES data (44). Service satisfaction was assessed in all participants with an 8-item questionnaire with responses rates on a 4-point scale (from ‘poor’ to ‘excellent’). Acceptability of HT was assessed as the difficulty of eating according to glucose levels and the helpfulness of wearing the CGM in only the DPP+HT group. They were also asked about their preferred CGM dose over a year.
Anthropometrics.
Weight (light clothing) and height (without shoes) were measured in duplicate using calibrated equipment to within 0.2 kg and 0.3 cm by trained study staff at baseline, 8 weeks, and 16 weeks.
Biomarker assessments.
Biomarkers were assessed at baseline and 16 weeks. Blood draws were conducted (10-ml samples) into 10-ml speckled red tubes. Samples were allowed to clot and then spun for 20 minutes at 3200 RPM. Serum was aliquoted into 10 tubes (1.0–2.0 ml each) and analyzed according to standardized laboratory protocols at The University of Texas MD Anderson Cancer Center or nearby Labcorp location. Metabolic biomarkers included: total cholesterol, HDL, LDL, VLDL, triglycerides, HbA1c, fasting glucose, fasting insulin, and insulin resistance. Insulin resistance was assessed as HOMA-IR using fasting glucose and insulin levels by the following equation: (Fasting Glucose X Fasting Insulin)/405 (45). Other breast cancer risk biomarkers included: CRP, adiponectin, IGF-1, IGF-2, IGFBP-2.
Statistical Analysis
Statistical analysis was performed using RStudio (2016) and based on intention to treat. As this was a feasibility study, power calculations were not required (46). It was estimated that >20 people per group would provide sufficient information on feasibility of each intervention. The analysis was based on available data, with no modelling of missing data. We considered imputing missing values in a sensitivity analysis of our analysis, which assumes data are missing at random; however, we observed large uncertainty in the estimated treatment effects, each of which was not statistically significant. Modest to moderate deviations from missing at random, for example by constructing a tipping point analysis (47) where imputations based on missing at random are modified to exhibit a larger or smaller effect are not likely to produce an alternative conclusion. We compared the baseline characteristics between those who participated the study and those who declined to participate or dropped out as a sensitivity analysis.
Estimates of the differences between the groups were assessed using ANCOVA, adjusted for baseline. Mean change and standard deviations are reported along with mean differences between groups and 95% CI.
Data Availability
The data generated in this study are available upon request from the corresponding author.
Results
Feasibility
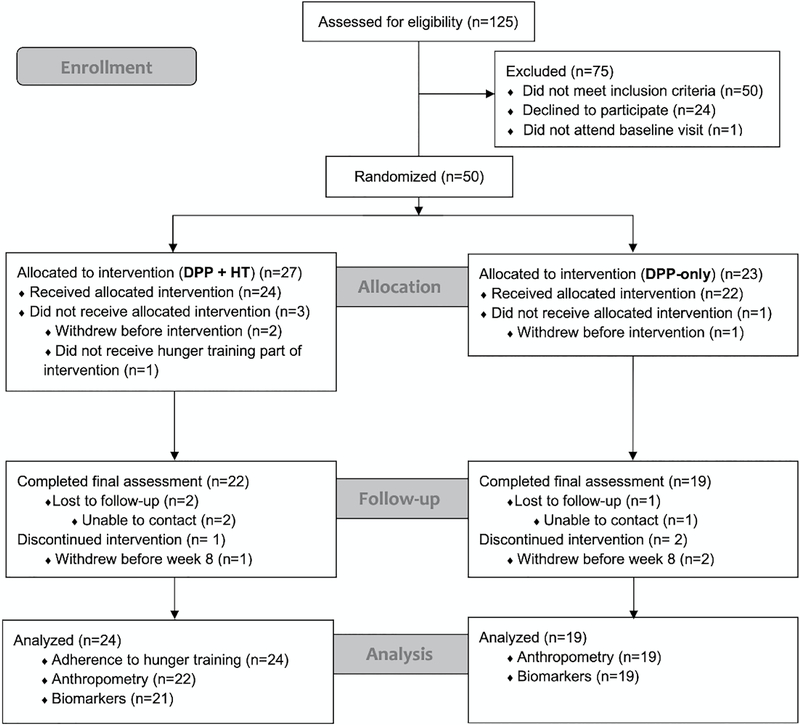
Participant flow is shown in Figure 2. We enrolled and randomized 50 women to the DPP-only (n=23) or DPP+HT (n=27) interventions. Of 125 women assessed for eligibility, 50 did not meet inclusion criteria and another 25 were determined to be initially eligible but declined further participation (n=24) or did not attend the baseline visit at which eligibility would have been verified (n=1). The achieved accrual rate was 67% (50 consented/75 total initially-eligible), which exceeded the 50% feasibility target.
Figure 2.
Flow of participants through the study. This figure shows the randomization, allocation, follow-up and analysis of the 50 participants in the study.
The resulting retention rates, reflecting the ratio of women who were assessed at baseline and post-intervention and received either the DPP-only or the DPP+HT intervention were 83% and 81%, respectively, which exceeded the 80% feasibility targets.
Baseline demographics and characteristics of the enrolled participants are presented in Table 1. Participants overall well-educated, with a BMI in the obese range. Any small differences between groups were due to chance, and not significant. Compared to the 50 women who enrolled in the study, those who declined further participation or chose to not have their eligibility verified (n=25) were less likely to be married or widowed vs. single or divorced (χ2 = 8.16, p = .017), less likely to have college-level education (χ2 = 6.32, p = .042), and more likely to be employed full- or part-time (χ2 = 8.52, p = .014). Additionally, women recruited from the community (n = 3) had Gail scores that met our recruitment criteria (5-year risk = 1.87 ± .06). One of the three women recruited from the community was assigned to the DPP+HT intervention.
Table 1.
Baseline characteristics by randomized group1 (N=50)
| DPP-only | DPP+HT | ||
|---|---|---|---|
|
| |||
| N | 23 | 27 | |
| Age (years), mean (SD) | 61.3 (6.9) | 59.5 (5.1) | |
| BMI (kg/m2), mean (SD) | 36.1 (5.6) | 33.8 (4.7) | |
| BMI category | Overweight (BMI=27–29.9 kg/m2) | 2 (8.7%) | 3 (11.1%) |
| Obese class I (BMI=30–34.9 kg/m2) | 11 (47.8%) | 16 (59.3%) | |
| Obese class II and III (BMI≥35 kg/m2) | 10 (43.5%) | 8 (29.6%) | |
| Taking hormone therapy | 1 (4.3%) | 5 (18.5%) | |
| Taking metformin | 0 (0%) | 1 (3.7%) | |
| Ethnicity | Non-Hispanic | 21 (91.3%) | 26 (96.3%) |
| Hispanic | 0 (0%) | 1 (3.7%) | |
| Not reported | 2 (8.6%) | 0 (0%) | |
| Race | White | 16 (69.6%) | 25 (92.6%) |
| Black | 6 (26.1%) | 2 (7.4%) | |
| Other | 1 (4.3%) | 0 (0%) | |
| Education | Any college | 22 (95.7%) | 22 (81.5%) |
| No college | 1 (4.3%) | 5 (19.2) | |
| Employed | 10 (43.5%) | 16 (59.3%) | |
| Partnered | 17 (73.9%) | 22 (81.5%) | |
| Household income | <$75,000 | 5 (21.7%) | 7 (25.9%) |
| ≥$75,000 | 13 (56.5%) | 14 (51.9%) | |
| Not reported | 5 (21.7%) | 6 (22.2%) | |
Values are number (%) unless otherwise indicated
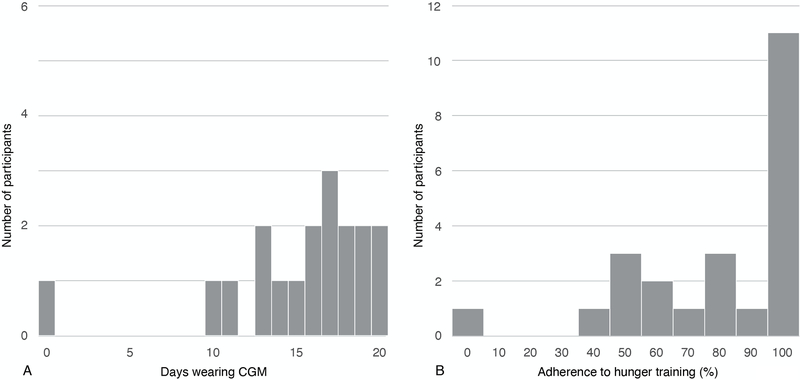
Twenty-three of 24 participants in the DPP+HT group wore a CGM; one participant was not provided with a CGM due to study error and is included in the intent-to-treat analysis. A sensitivity analysis showed that her inclusion did not significantly change the results of days wearing CGM (p=.589); however, she could not be included in the adherence analysis.
For the first two CGM wear periods, CGM were worn for a mean of 14.4 (SD 4.3) days (range 0–20). Out of these CGM wear days, the DPP+HT participants logged a median of 89.5% of days (25th percentile: 57.3%, 75th percentile: 100%) (Figure 3), which met the feasibility criterion of 75%. Eleven women (45.8%) achieved 100% adherence. The average glucose threshold was 90 mg/dL (SD 8.6, range 71–106).
Figure 3.
Adherence to Hunger Training. A. Days wearing continuous glucose monitor (CGM) (N=24). This depicts the duration (in days) that the participants wore the CGM; and B. Adherence to logging glucose and hunger on CGM days (N=23). This shows the number of participants that adhered at different levels. The percentage adherence was calculated as the ratio of days logging pre-meal glucose and hunger levels to the total number of valid CGM days during the training period worn. N=1 participant was not included in B since she did not have any days wearing CGM.
Satisfaction and Acceptability
Women in both arms of the trial rated their satisfaction with the program highly. Over 80% of participants reported the interventions were “excellent” in every respect, including the quality of the service, satisfaction with the program, and the program helping them to deal more effectively with their eating behaviors. Similarly, 91% of those in DPP-only and 80% in DPP+HT reported being “highly likely” to recommend the program to a friend. Additionally, those in the DPP+HT group felt positive about wearing CGM to guide eating (Table 2). Participants were asked whether they would wear the CGM again, and if so, how many times per year and for how long each time. Most (n=13/15) reported that they were willing to wear CGM again, n=9/13 wanted to wear the CGM 4 times per year (range = 3–28 times), and n=10/13 wanted to wear it for 1–2 weeks each time (range = 1–4 weeks).
Table 2.
Acceptability of the hunger training components of intervention
| Question | Responses | ||||
|---|---|---|---|---|---|
|
| |||||
| How difficult was it to eat based on your glucose levels (n=14) | Very difficult | Not very difficult | |||
| 2 | 0 | 6 | 3 | 3 | |
|
| |||||
| How helpful was wearing the continuous glucose monitor in teaching you to eat according to your hunger? (n=15) | It did not help at all | It helped a lot | |||
| 0 | 2 | 5 | 1 | 7 | |
Five women in the DPP+HT arm had contacted study staff regarding concerns. The most common concern was related to extended periods of time between meals (6 hours or more). Women with this concern were advised to adjust their glucose threshold based on a review by the study dietitian and research team. The glucose thresholds of these women were raised (range = 4–10 mg/dL; median = 5 mg/dL).
Weight, metabolic, and breast cancer risk biomarkers
Table 3 summarizes changes in weight, and metabolic and breast cancer risk biomarkers by group over the 16-week intervention period.
Table 3.
Differences in weight and biomarker outcomes between intervention groups
| DPP-only Mean (SD) | DPP + HT Mean (SD) | Difference in change between groupsa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| n | Baseline | n | Post-Intervention | Change | n | Baseline | n | Post-intervention | Change | Mean (95% CI) | |
| Weight (kg) | 23 | 95.8 (17.0) | 19 | 87.7 (13.2) | −5.0 (4.7) | 27 | 89.1 (15.6) | 22 | 82.9 (16.5) | −4.9 (3.7) | −0.3 (−2.9, 2.3) |
| BMI (kg/m2) | 23 | 36.1 (5.6) | 19 | 33.6 (5.2) | −1.9 (1.7) | 27 | 33.8 (4.7) | 22 | 31.4 (5.1) | −1.9 (1.3) | −0.1 (−1.1, 0.9) |
| Triglycerides (mg/dL) | 23 | 114.6 (32.9) | 19 | 120.7 (55.4) | 3.3 (50.3) | 27 | 121.1 (67.1) | 21 | 125.3 (65.8) | 1.0 (38.8) | −0.7 (−28.5, 27.1) |
| Total cholesterol (mg/dL) | 23 | 197.2 (40.0) | 19 | 196.4 (29.9) | 3.4 (16.5) | 27 | 209.4 (44.1) | 21 | 205.5 (41.4) | −4.2 (24.4) | −3.5 (−15.7, 8.7) |
| HDL (mg/dL) | 23 | 62.3 (12.1) | 19 | 63.4 (10.9) | 0.0 (8.4) | 27 | 62.5 (12.1) | 21 | 58.8 (10.2) | −3.3 (7.4) | −3.7 (−8.3, 0.9) |
| LDL (mg/dL) | 23 | 112.0 (38.2) | 19 | 108.8 (25.7) | 2.7 (17.4) | 27 | 122.6 (37.9) | 21 | 121.6 (35.0) | −1.1 (18.3) | 0.7 (−9.1, 10.5) |
| VLDL (mg/dL) | 23 | 22.9 (6.6) | 19 | 24.2 (11.1) | 0.7 (10.1) | 27 | 24.3 (13.4) | 21 | 25.1 (13.2) | 0.2 (7.8) | −0.2 (−5.7, 5.4) |
| HbA1c (%) | 23 | 5.6 (0.4) | 18 | 5.5 (0.4) | −0.1 (0.2) | 27 | 5.5 (0.3) | 21 | 5.5 (0.2) | −0.1 (0.2) | 0.0 (−0.1, 0.1) |
| Fasting glucose (mg/dL) | 23 | 97.0 (10.3) | 19 | 93.2 (13.6) | −3.6 (16.2) | 27 | 97.1 (6.4) | 21 | 97.2 (5.9) | 0.6 (7.0) | 4.5 (−2.1, 11.2) |
| Fasting insulin (mIU/L) | 23 | 16.8 (7.8) | 19 | 14.5 (7.8) | −2.5 (6.7) | 27 | 16.7 (8.2) | 21 | 13.1 (6.5) | −4.5 (5.3) | −1.8 (−5.1, 1.5) |
| HOMA-IR | 23 | 1.6 (0.8) | 19 | 1.4 (0.9) | −0.3 (0.7) | 27 | 1.6 (0.8) | 21 | 1.3 (0.6) | −0.4 ( 0.7) | −0.1 (−0.5, 0.3) |
| Adiponectin (μg/ml) | 23 | 9.4 (4.9) | 18 | 10.8 (5.8) | 1.0 (2.7) | 27 | 11.2 (5.7) | 21 | 11.4 (4.9) | 0.2 (2.6) | −0.6 (−2.2, 1.1) |
| CRP (mg/L) | 23 | 7.6 (6.4) | 19 | 6.0 (6.2) | −1.3 (5.6) | 27 | 4.6 (4.4) | 21 | 3.8 (2.7) | −0.9 (3.1) | −0.9 (−3.2, 1.5) |
| IGF-1 (ng/ml) | 23 | 104.6 (22.7) | 19 | 110.4 (28.4) | 2.9 (18.5) | 27 | 134.1 (42.4) | 20 | 143.1 (45.7) | 10.7 (16.9) | 7.6 (−5.0, 20.3) |
| IGF-2 (ng/ml) | 23 | 652.5 (162.8) | 17 | 630.8 (116.5) | −24.8 (158.3) | 27 | 803.5 (370.9) | 19 | 675.7 (203.8) | −124.9 (303.3) | −1.7 (−100.0, 96.7) |
| IGFBP-2 (ng/ml) | 23 | 193.9 (84.5) | 18 | 225.4 (131.0) | 33.3 (90.2) | 27 | 202.5 (136.4) | 19 | 257.9 (163.8) | 65.8 (91.2) | 32.5 (−29.0, 93.9) |
Calculated using ANCOVA, adjusted for baseline. DPP = Diabetes Prevention Plan, HT = Hunger Training
Weight:
Weight losses and BMI reductions were significant over time (p < .001) but did not vary by intervention group. Weight loss was greater in women who reported use of hormone therapy or metformin at baseline compared to those who did not (−9.8 (3.2) kg vs. −4.9 (4.2) kg, p = .017); however, this group of women was small (n = 5). In the DPP-only group, 9 women lost a clinically significant amount of their initial body weight (≥5%), 8 women achieved the DPP weight loss goal (≥7%), and 3 reached weight loss goals associated with reduced breast cancer risk (≥10%). In the DPP+HT group, 12 women lost ≥5%, 8 women lost ≥7%, and 4 lost ≥10% initial body weight.
Metabolic biomarkers:
Changes in LDL, VLDL, HbA1c, fasting insulin, and HOMA-IR were significant over time (p ≤ .005) but did not vary by intervention group. No other metabolic biomarkers changed significantly over time.
Other breast cancer risk biomarkers:
Changes in IGF-1 and IGFBP-2 were significant over time (ps ≤ .026) but did not vary by intervention group. No other cancer risk biomarkers changed significantly over time.
Discussion
The primary aim of this 16-week feasibility RCT was to determine the feasibility of adding Hunger Training to the Diabetes Prevention Program in a sample of postmenopausal women at high risk of breast cancer. The accrual, retention, and adherence rates all surpassed our a priori criteria, supporting the feasibility of adding HT to the DPP. These results are consistent with previous findings showing HT to be feasible as a standalone intervention (22,28), however this is the first study to demonstrate its viability as an adjunct to a comprehensive weight loss program.
The secondary aims were to explore the effect of adding HT to the DPP on changes in body weight, and metabolic and breast cancer risk biomarkers. We hypothesized that HT and DPP might work synergistically to enhance weight loss and metabolic effects. Results from this trial provide little evidence of an enhanced effect but the small sample size of this feasibility trial limits the accurate estimation of the treatment effect. A similar finding was reported from the SWIFT study, which added HT as a form of self-monitoring to a standard weight loss program consisting of nutrition and physical activity information provision (48). Comparatively, the present study added HT to a considerably more intensive and comprehensive program than that used in SWIFT. In contrast to the present results, prior trials of HT as a standalone intervention have demonstrated clinically-significant weight losses and improved metabolic outcomes (28,43).
Strengths of this study include setting a priori feasibility criteria, which is important in objectively assessing the viability of an intervention (46). This study clarified issues with effectiveness before proceeding to a larger trial – specifically, that there is little capacity for increased effectiveness with the already intensive DPP. Additionally, we were able to isolate the effect of one behavior change technique – biofeedback, which has seldom been examined but is becoming increasingly available to consumers (49). Lastly, this trial focused on a specific but common population, woman with BMI >27 kg/m2 who are at high risk of postmenopausal breast cancer and could experience great benefits from weight loss. Limitations of the study include the relatively small sample size and short duration, which, while befitting a feasibility study, limits our ability to draw conclusions from the findings. Additionally, one participant did not receive the HT component of the intervention due to staff error. However, she was included in the DPP+HT group in accordance with intention-to-treat analysis, and sensitivity analysis showed that her exclusion did not alter the results of the feasibility or other outcomes. Lastly, it is unclear from this study whether the HT period of up to 20 days during the 16-week intervention was sufficient to produce meaningful effects. Future studies could examine the feasibility and enhanced effectiveness of longer training periods, as well as the feasibility and effectiveness of providing booster sessions at regular intervals during extended intervention periods (e.g., quarterly during a 12-month intervention).
This study demonstrated that HT can be feasibly added to comprehensive weight management program targeted towards postmenopausal women at high risk of breast cancer. In contrast to standalone HT interventions, which effectively produce clinically-relevant weight changes when combined with an intensive lifestyle intervention, up to 20 days of HT does not appear to enhance weight loss or metabolic changes.
Prevention Relevance:
This study found that it was feasible to add a short glucose-guided eating intervention to a comprehensive weight management program targeting postmenopausal women at high risk of breast cancer. However, further development of this novel intervention as a cancer prevention strategy is needed.
Acknowledgements:
The University of Texas MD Anderson Cancer, Center Duncan Family Institute for Cancer Prevention and Risk Assessment; The University of Texas MD Anderson Cancer Center, Center for Energy Balance in Cancer Prevention and Survivorship, and The University of Texas MD Anderson Cancer, Center Research-Related Patient Care Charges Fund.
Financial support:
Supported in part by R21CA215415 (S.M. Schembre and K.M. Basen-Engquist) and P30CA016672 (S.M. Schembre, L. Li, A.M. Brewster, E. Levy, D. D. Dirba, M. Campbell, K.M. Basen-Engquist).
Abbreviations:
- DPP
Diabetes Prevention Program
- HT
Hunger Training
- CGM
continuous glucose monitor
- BMI
body mass index
- HDL
high density lipoproteins
- LDL
low density lipoproteins
- VLDL
very low-density lipoproteins
- HbA1c
glycated hemoglobin
- CRP
C-reactive protein
- IGF
insulin-like growth factor
- IGFBP
insulin-like growth factor binding protein
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest
References
- 1.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA 2016;315(21):2284–91 doi 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Cancer Research Fund / American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. In: The American Institute for Cancer Research, editor. Washington, DC: 2007. [Google Scholar]
- 3.American Cancer Society. Breast Cancer Facts & Figures 2019–2020. Atlanta: American Cancer Society; 2019. [Google Scholar]
- 4.Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE. Adult weight change and risk of postmenopausal breast cancer. JAMA 2006;296(2):193–201 doi 10.1001/jama.296.2.193. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Lam KS, Xu A. Adiponectin as a negative regulator in obesity-related mammary carcinogenesis. Cell Res 2007;17(4):280–2 doi 10.1038/cr.2007.14. [DOI] [PubMed] [Google Scholar]
- 6.Renehan AG, Zwahlen M, Egger M. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat Rev Cancer 2015;15(8):484–98 doi 10.1038/nrc3967. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez AV, Guarnizo M, Miranda Y, Pasupuleti V, Deshpande A, Paico S, et al. Association between insulin resistance and breast carcinoma: a systematic review and meta-analysis. PLoS One 2014;9(6):e99317 doi 10.1371/journal.pone.0099317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reeves MM, Terranova CO, Eakin EG, Demark-Wahnefried W. Weight loss intervention trials in women with breast cancer: a systematic review. Obes Rev 2014;15(9):749–68 doi 10.1111/obr.12190. [DOI] [PubMed] [Google Scholar]
- 9.Fabian CJ, Kimler BF, Donnelly JE, Sullivan DK, Klemp JR, Petroff BK, et al. Favorable modulation of benign breast tissue and serum risk biomarkers is associated with > 10 % weight loss in postmenopausal women. Breast Cancer Res Treat 2013;142(1):119–32 doi 10.1007/s10549-013-2730-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ostlund MP, Lu Y, Lagergren J. Risk of obesity-related cancer after obesity surgery in a population-based cohort study. Ann Surg 2010;252(6):972–6 doi 10.1097/SLA.0b013e3181e33778. [DOI] [PubMed] [Google Scholar]
- 11.Stephens SK, Cobiac LJ, Veerman JL. Improving diet and physical activity to reduce population prevalence of overweight and obesity: an overview of current evidence. Prev Med 2014;62:167–78 doi 10.1016/j.ypmed.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Keesey RE, Powley TL. Body energy homeostasis. Appetite 2008;51(3):442–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wansink B. Environmental factors that increase the food intake and consumption volume of unknowing consumers. Annu Rev Nutr 2004;24:455–79 doi 10.1146/annurev.nutr.24.012003.132140. [DOI] [PubMed] [Google Scholar]
- 14.Lowe MR, Butryn ML. Hedonic hunger: a new dimension of appetite? Physiol Behav 2007;91(4):432–9 doi 10.1016/j.physbeh.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Schembre S, Greene G, Melanson K. Development and validation of a weight-related eating questionnaire. Eat Behav 2009;10(2):119–24 doi 10.1016/j.eatbeh.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Versace F, Kypriotakis G, Basen-Engquist K, Schembre SM. Heterogeneity in brain reactivity to pleasant and food cues: evidence of sign-tracking in humans. Soc Cogn Affect Neurosci 2015. doi 10.1093/scan/nsv143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.French SA, Epstein LH, Jeffery RW, Blundell JE, Wardle J. Eating behavior dimensions. Associations with energy intake and body weight. A review. Appetite 2012;59(2):541–9 doi 10.1016/j.appet.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bryant EJ, King NA, Blundell JE. Disinhibition: its effects on appetite and weight regulation. Obes Rev 2008;9(5):409–19. [DOI] [PubMed] [Google Scholar]
- 19.Teixeira PJ, Carraca EV, Marques MM, Rutter H, Oppert JM, De Bourdeaudhuij I, et al. Successful behavior change in obesity interventions in adults: a systematic review of self-regulation mediators. BMC Med 2015;13:84 doi 10.1186/s12916-015-0323-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warren JM, Smith N, Ashwell M. A structured literature review on the role of mindfulness, mindful eating and intuitive eating in changing eating behaviours: effectiveness and associated potential mechanisms. Nutrition Research Reviews 2017;30(2):272–83 doi 10.1017/S0954422417000154. [DOI] [PubMed] [Google Scholar]
- 21.Schembre SM, Huh J. Using pre-prandial blood glucose data as a marker of maladaptive eating events. Annals of Behavioral Medicine. Volume 472014. p s160. [Google Scholar]
- 22.Jospe MR, Brown RC, Roy M, Taylor RW. Adherence to hunger training using blood glucose monitoring: a feasibility study. Nutrition & Metabolism 2015;12:22 doi 10.1186/s12986-015-0017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor RW, Roy M, Jospe MR, Osborne HR, Meredith-Jones KJ, Williams SM, et al. Determining how best to support overweight adults to adhere to lifestyle change: protocol for the SWIFT study. BMC Public Health 2015;15:861 doi 10.1186/s12889-015-2205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ciampolini M, Bianchi R. Training to estimate blood glucose and to form associations with initial hunger. Nutr Metab (Lond) 2006;3:42 doi 10.1186/1743-7075-3-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ciampolini M, Lovell-Smith HD, Kenealy T, Bianchi R. Hunger can be taught: Hunger Recognition regulates eating and improves energy balance. Int J Gen Med 2013;6:465–78 doi 10.2147/IJGM.S40655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciampolini M, Sifone M. Differences in maintenance of mean blood glucose (BG) and their association with response to “recognizing hunger”. Int J Gen Med 2011;4:403–12 doi 10.2147/IJGM.S19035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciampolini M, Lovell-Smith D, Sifone M. Sustained self-regulation of energy intake. Loss of weight in overweight subjects. Maintenance of weight in normal-weight subjects. Nutr Metab (Lond) 2010;7:4 doi 10.1186/1743-7075-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jospe MR, de Bruin WE, Haszard JJ, Mann JI, Brunton M, Taylor RW. Teaching people to eat according to appetite - Does the method of glucose measurement matter? Appetite 2020;151:104691 doi 10.1016/j.appet.2020.104691. [DOI] [PubMed] [Google Scholar]
- 29.Campfield LA, Smith FJ. Transient declines in blood glucose signal meal initiation. Int J Obes 1990;14 Suppl 3:15–34. [PubMed] [Google Scholar]
- 30.Campfield LA, Smith FJ. Blood glucose dynamics and control of meal initiation: a pattern detection and recognition theory. Physiol Rev 2003;83(1):25–58 doi 10.1152/physrev.00019.2002. [DOI] [PubMed] [Google Scholar]
- 31.Campfield LA, Smith FJ, Rosenbaum M, Hirsch J. Human eating: evidence for a physiological basis using a modified paradigm. Neurosci Biobehav Rev 1996;20(1):133–7 doi 0149–7634(95)00043-E [pii]. [DOI] [PubMed] [Google Scholar]
- 32.Melanson KJ, Westerterp-Plantenga MS, Campfield LA, Saris WH. Short term regulation of food intake in humans. In: Westerterp-Plantenga MS, Steffens AB, Tremblay A, editors. Regulation of food intake and energy expenditure: EDRA Medical Publishing and New Media; 1999. p 37–58. [Google Scholar]
- 33.Ciampolini M, Bianchi R. Training to estimate blood glucose and to form associations with initial hunger. Nutrition & Metabolism 2006;3:42 doi 10.1186/1743-7075-3-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Virdi NS, Lefebvre P, Parise H, Duh MS, Pilon D, Laliberte F, et al. Association of self-monitoring of blood glucose use on glycated hemoglobin and weight in newly diagnosed, insulin-naive adult patients with type 2 diabetes. Journal of diabetes science and technology 2013;7(5):1229–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kempf K, Tankova T, Martin S. ROSSO-in-praxi-international: long-term effects of self-monitoring of blood glucose on glucometabolic control in patients with type 2 diabetes mellitus not treated with insulin. Diabetes Technol Ther 2013;15(1):89–96 doi 10.1089/dia.2012.0213. [DOI] [PubMed] [Google Scholar]
- 36.Yoo HJ, An HG, Park SY, Ryu OH, Kim HY, Seo JA, et al. Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res Clin Pract 2008;82(1):73–9 doi 10.1016/j.diabres.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 37.Ciampolini M, Lovell-Smith D, Bianchi R, de Pont B, Sifone M, van Weeren M, et al. Sustained self-regulation of energy intake: initial hunger improves insulin sensitivity. J Nutr Metab 2010;2010 doi 10.1155/2010/286952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allen N, Whittemore R, Melkus G. A continuous glucose monitoring and problem-solving intervention to change physical activity behavior in women with type 2 diabetes: a pilot study. Diabetes Technol Ther 2011;13(11):1091–9 doi 10.1089/dia.2011.0088. [DOI] [PubMed] [Google Scholar]
- 39.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics 2009;42(2):377–81 doi 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bray GA, Culbert IW, Champagne CM, Dawson L, Eberhardt B, Greenway FL, et al. The Diabetes Prevention Program - Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care 1999;22(4):623–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mattes R. Hunger ratings are not a valid proxy measure of reported food intake in humans. Appetite 1990;15(2):103–13 doi 0195–6663(90)90043–8 [pii]. [DOI] [PubMed] [Google Scholar]
- 42.Mattes R, Hollis J, Hayes D, Stunkard AJ. Appetite: measurement and manipulation misgivings. J Am Diet Assoc 2005;105(5 Suppl 1):S87–97 doi S0002822305002890 [pii] 10.1016/j.jada.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 43.Jospe MR, Taylor RW, Athens J, Roy M, Brown RC. Adherence to Hunger Training over 6 Months and the Effect on Weight and Eating Behaviour: Secondary Analysis of a Randomised Controlled Trial. Nutrients 2017;9(11):1260–13 doi 10.3390/nu9111260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kant AK, Graubard BI. 40-year trends in meal and snack eating behaviors of American adults. Journal of the Academy of Nutrition and Dietetics 2015;115(1):50–63 doi 10.1016/j.jand.2014.06.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28(7):412–9 doi 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 46.Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, et al. A tutorial on pilot studies: the what, why and how. BMC Medical Research Methodology 2010;10(1) doi 10.1186/1471-2288-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carpenter JR, Kenward MG. Multiple Imputation and its Application. Wiley; 2013. [Google Scholar]
- 48.Jospe MR, Roy M, Brown RC, Williams SM, Osborne HR, Meredith-Jones KA, et al. The effect of different types of monitoring strategies on weight loss: A randomized controlled trial. Obesity (Silver Spring, Md) 2017;25(9):1490–8 doi 10.1002/oby.21898. [DOI] [PubMed] [Google Scholar]
- 49.Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine 2013;46(1):81–95 doi 10.1007/s12160-013-9486-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated in this study are available upon request from the corresponding author.