Abstract
With the dramatic increase in cancer incidence all over the world in the last decades, studies on identifying novel efficient anti-cancer agents have been intensified. Historically, natural products have represented one of the most important sources of new lead compounds with a wide range of biological activities. In this article, the multifaceted anti-cancer action of propolis-derived flavonoid, galangin, is presented, discussing its antioxidant, anti-inflammatory, antiproliferative, pro-apoptotic, anti-angiogenic, and anti-metastatic effects in various cancer cells. In addition, co-effects with standard chemotherapeutic drugs as well as other natural compounds are also under discussion, besides highlighting modern nanotechnological advancements for overcoming the low bioavailability issue characteristic of galangin. Although further studies are needed for confirming the anti-cancer potential of galangin in vivo malignant systems, exploring this natural compound might open new perspectives in molecular oncology.
Keywords: Galangin, absorption, anti-cancer, epigenetics, synergistic, nano-delivery
Impact statement
Galangin is known to have therapeutic potential against various cancers. It interacts with a variety of recognized cellular targets and inhibits cancer cell proliferation. Current review highlights the apoptotic, cell cycle arrest, anti-metastasis, and anti-angiogenic effect of galangin. In addition, synergistic and nanotherapeutic actions of galangin have also been discussed. The uniqueness of the review is to present all the possible anti-cancer interactions of galangin at a single platform.
Introduction
Although our understanding about the etiology and molecular mechanisms of carcinogenesis has dramatically improved in the recent years, cancer is still considered as an incurable disease in many cases. Moreover, the incidence of this dreadful public health concern has rapidly increased during the last decades and is unfortunately estimated to further rise, reaching 28.4 million new cases in 2040.1,2 This rapid increase in incidence has intensified investigations into novel more potent and safe anticancer agents. 3 As natural products have historically provided an important source for identifying several chemotherapeutic drugs that are currently approved for the use in clinical settings, 4 studies on the potential anticancer effects of diverse plant-derived compounds have become more and more popular all over the world.
Galangin (3,5,7-trihydroxyflavone) is a flavonol-type naturally active polyphenol found primarily in propolis and the roots of Alpinia officinarum Hance, but also in Plantago major L., Alnus pendula Matsum. and Scutellaria galericulata L. Numerous studies have reported the diverse biological activities of galangin, exerting antioxidant, anti-inflammatory, antiviral, and antimicrobial effects. 5 In addition, this flavonol has been demonstrated to exhibit also anticancer potential against a variety of malignant neoplasms, including lung cancer, 6 breast cancer, 7 ovarian cancer, 8 gastric cancer, 9 colon cancer, 10 hepatocellular carcinoma, 11 glioblastoma,12,13 and osteosarcoma. 14 Such anticancer action of galangin comprises antiproliferative, pro-apoptotic, antiangiogenic, and antimetastatic effects, through interacting with different molecular targets and affecting activity of various intracellular signaling pathways. 15 Furthermore, galangin can modulate also the action of conventional cancer treatment modalities, leading to augmentation of therapeutic responses in diverse tumor cells and malignant tissues,16,17 thereby ameliorating adverse side effects induced by anticancer drugs. 18
Although the current in vitro data suggest galangin as a very promising anticancer agent, this compound, like other natural flavonoids, undergoes extensive metabolic conversion in in vivo systems, resulting in the formation of several glucuronidated, sulfated, and methylated derivatives. 19 Several modes have been proposed to overcome the issue of such low bioavailability, including preparation of different nanoparticles encapsulating galangin. 20
In this review article, the current knowledge about anticancer activities of galangin in diverse in vitro cancer cell lines as well as in vivo tumor models is compiled, with the aim to highlight the strong potential of galangin as a novel anticancer agent and emphasize the necessity for further studies with this natural compound.
Absorption and metabolism studies
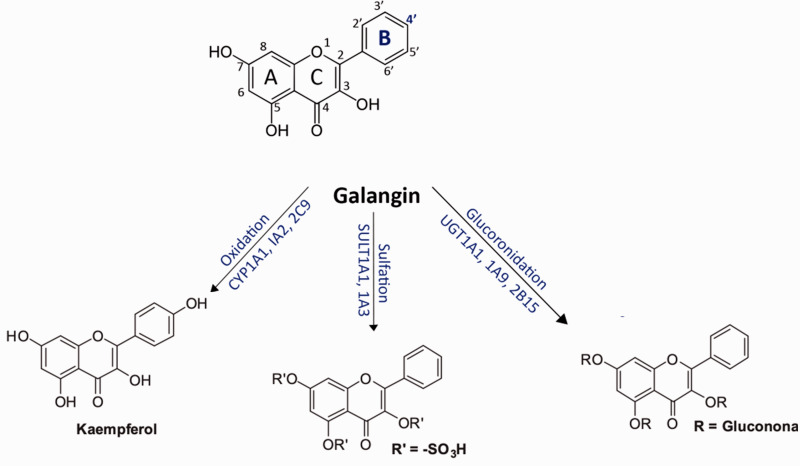
The chemical moiety present in galangin is 4H-1-benzopyran-4-one,3,5,7-trihydroxy-2-phenyl or 3,5,7-trihydroxyflavone which can be synthesized by refluxing 2-methoxy-1–(2,4,6-trihydroxyphenyl) ethanone with benzoic acid and benzoyl chloride. Studies have proven that galangin is converted in the liver to kaempferol and quercetin by cytochrome P450, both of which have anti-oxidant properties. Galangin has three hydroxyl groups on its carbon rings, acts as an enzyme modulator, and can reduce chemical genotoxicity. Galangin has been shown to be a strong inhibitor of the aryl hydrocarbon receptor in earlier studies. It has also been found to exert a range of biological functions in organisms at non-toxic doses. From the 4'-position, human liver microsomes metabolize galangin to kaempferol (Figure 1). The cytochrome enzymes CYP1A1, CYP1A2, and CYP2C9 catalyze this reaction. 21 Galangin is seen to be glucuronidated and sulfated seven times more frequently than it is oxidized. 22 The UDP-glucuronosyltransferase (UGT) 1A9 isoform in the liver appears to catalyze glucuronidation efficiently.21,23 Interestingly, UGT1A9, as well as UGT1A1 and UGT2B15, were shown to glucuronidize the 7-position of galangin. 24 Galangin and its metabolites are usually excreted in the feces. 25
Figure 1.
Principle metabolic pathways of galangin.
According to the study done by Goran Benković et al., CYP2C19 and CYP2D6 enzymes were found to be responsible for galangin metabolism. Incubations of CYP2C19 and CYP2D6 revealed the production of one metabolite—kaempferol—similar to the studies using human liver microsomes, suggesting that this enzyme catalyzes the aromatic hydroxylation of galangin at position 4' of ring B. 26
Liu et al. identified two galangin metabolites in rat plasma using ultra-fast liquid chromatography coupled with electrospray ionization triple quadrupole tandem mass spectrometry (UFLC-MS/MS). After oral treatment, two galangin metabolites, galangin-3-O—D-glucuronic acid (GG-1) and galangin-7-O—D-glucuronic acid (GG-2), were identified, reiterating the result that galangin was glucuronidated. 19
Chen et al. investigated galangin metabolites in rat plasma after oral (p.o.) and intravenous (i.v.) administration. Female Sprague-Dawley (SD) rats were chosen. Each group received 10 mg/kg oral galangin and 2 mg/kg intravenous galangin. The routes of administration, according to the findings, have a substantial influence on the systemic exposure level of galangin and its metabolites. They also reported that after p.o. and i.v. administration, galangin was glucuronidated and sulfated, and that the parent galangin's oral bioavailability was extremely poor. 27
When Curti et al. investigated a mouse animal model, they observed a similar result. They used 29 mature male C57BL/6 mice in their study (8 weeks old, 20 g average weight). Brown propolis preparations were found to have approximately identical quantities of two compounds, galangin and chrysin. However, only glucuronated galangin was identified in the blood, indicating that galangin is likely adsorbed and promptly glucuronidated after oral administration, suggesting that it has weak bioavailability in mice. 28
Given the myriad of biological applications of galangin, such as anti-cancer,10,29,30 anti-inflammatory,31,32 lipid lowering effects33,34 and anti-oxidative effects,35,36 with certain studies showing that galangin glucuronidated metabolites have better bioactivities than parent galangin, biological activity of galangin metabolites must be evaluated more to be more efficient or safe for biological use.
Anti-cancer potential of galangin
Apoptosis and cell cycle arrest effect
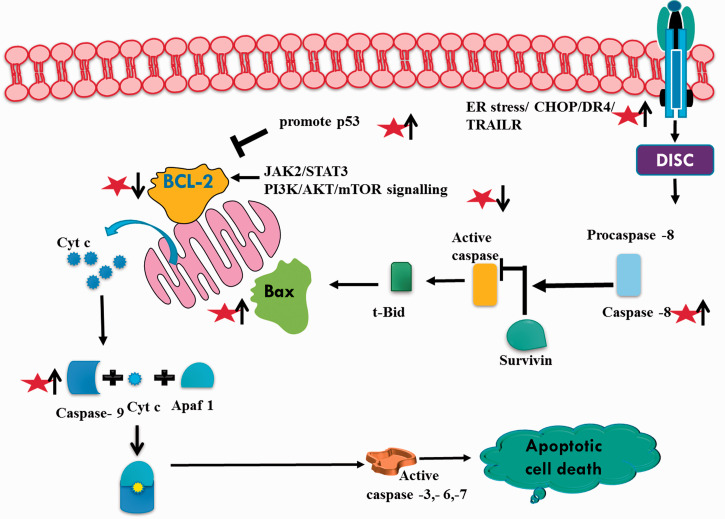
Natural compounds potentiate their anti-cancer effects through modulating multiple signaling pathways including apoptosis and cell cycle arrest. In line with apoptotic inducing potential of natural compounds (Figure 2), galangin has been shown to induce apoptosis-related decreased cell viability at 43.45 μg/mL and 168 μg/mL for 48 h in MCF-7 and LNCaP cells, respectively. However, no such effect was observed in primary fibroblast cells. 37 Similarly, in gastric cancer, galangin reduced cell viability in MGC803 cells but not normal gastric mucosal epithelial GES-1 cells. 9 Apoptotic promotion by galangin in MGC803 cells was supported by decreased Bcl-2 and inactivated JAK2/STAT3 pathway, cleaved caspase-3 and PARP. These findings were further corroborated by the authors in nude mice xenografted with MGC 803 cells suggesting the involvement of STAT3/ROS axis in galangin-induced apoptosis. 9 It has also been reported that galangin may exert its apoptotic effect through inhibition of glyoxalase-1 thereby inducing oxidative and carbonyl stress. 38 It has been reported that galangin promotes apoptosis in cholangiocarcinoma cells by downregulating mir-21 which targets PTEN. Probable mechanism suggests that the downregulation of mir-21 allows galangin to maintain PTEN levels, 39 which leads to reduced phosphorylation of Akt controlling downstream survival and apoptosis of cholangiocarcinoma cells. 40 In another report, it has been shown that galangin induced down regulation of H19 and mir 675, promotes p53 expression and apoptosis in hepatocellular carcinoma cells MHCC97H cells. 11 As stated before, preferential anticancer effects of galangin against cancer cells only have also been reported in ovarian cancer. It has been shown that galangin induced apoptosis in A2780/CP70 and OVCAR-3 cells more effectively as compared to normal IOSE 364 cell line. Galangin acted through both p53 dependent intrinsic and extrinsic pathways possibly mediated through Akt/p70S6K pathway. 8 Galangin also induced apoptosis in U251, U87MG, and A172 glioblastoma cells in a dose-dependent manner as compared to normal human astrocytes. 13 In kidney cancer cell line A498, galangin promotes apoptosis through suppressive targeting of PI3K/AKT/mTOR signaling pathway supported with decreased bcl-2 and increased Bax,Cyt-c.41,42 Similarly, in breast cancer MCF-7 cells, galangin promotes apoptosis through mitochondrial pathway and PI3K/Akt inhibition. 7 Galangin induces endoplasmic reticulum stress by promoting the expression of CHOP and DR4 gene. The ER stress leads to TRAIL sensitization, Caspases activation and AMPK phosphorylation-mediated apoptosis in human breast cancer cells. 43 Galangin-induced TRAIL-mediated apoptosis was also reported in renal carcinoma Caki, ACHN, and A498 cell lines. NF-kB pathway was inactivated which mediated down regulation of bcl-2, cFLIP, Mcl-1, and survivin associated apoptosis. 44 In human nasopharyngeal carcinoma, PI3K/Akt pathway-mediated apoptosis was p53 independent and therefore, effect of galangin treatment may exhibit apoptotic potential independent of p53.45
Figure 2.
Apoptotic effect of galangin (represented as star) in cancer. It up-regulates and down-regulates apoptotic (p53, caspases, Bax) and anti-apoptotic (Bcl-2, survivin) proteins respectively. (A color version of this figure is available in the online journal.)
Potential anti-cancer effect of galangin also includes its ability to arrest cancer cells. It has been reported that galangin arrests MCF-7 breast cancer cells by regulating proteins involved in cell cycle regulation. In particular, galangin treatment leads to downregulation of cyclin D3, cyclin B1, cyclin-dependent kinases CDK1, CDK2, CDK4, and upregulation of p21, p27 and p53.7 Galangin has been reported to arrest nasopharyngeal carcinoma cells in S phase independent of p53 status. 45 Inhibition of p38 by galangin in laryngeal carcinoma is reported to be the cause for cell cycle arrest, 42 whereas galangin-induced GO/G1 cell cycle arrest in human head and neck squamous cell carcinoma cells accompanied with reduced expression of cyclin D1, CDK4, and CDK6. 46 Similar observation of cell cycle arrest at GO/G1 stage by galangin treatment was also reported in leukemic Bcr-Abl+ cells and human mammary tumor cell line Hs578T.17,47
Anti-angiogenic and anti-metastatic effect
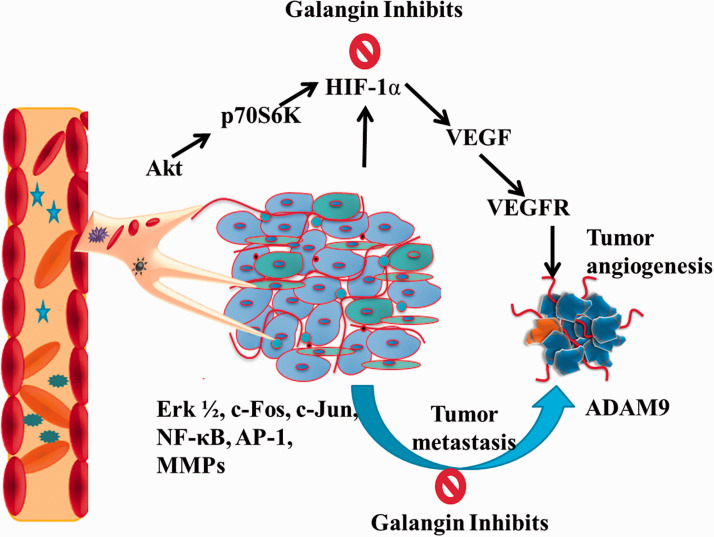
Angiogenesis or neo-vasculature is considered as an important characteristic of developing tumor. Growing blood vessels not only provide nutrition and oxygen to the cancer cells but also facilitates tumor migration.48–51 Therefore, a diverse range of synthetic and natural compounds are being investigated to inhibit tumor angiogenesis and metastasis.52–57 Huang et al. investigated the anti-angiogenic potential of galangin in HUVECS, CAM, and ovarian cancer cell culture models. 58 Galangin was found to inhibit the secretion of vascular endothelial growth factor (VEGF) via downregulating the expression of p-Akt, p-70S6K and hypoxia-inducible factor-1α (HIF-1α). In tumor microenvironment, high expression of HIF-1 considered to upregulate the expression of VEGF which is known to be a primary cytokine for angiogenesis. Angiogenic environment further supports tumor invasion and metastasis. In a study, the effects of galangin in human glioma cancer (A172) cells revealed downregulation of ADAM9 via extracellular signal regulated kinase (Erk)1/2 activation to inhibit tumor invasion. 59 In another study using human glioblastoma cells, Xiong et al. found galangin inhibited Skp2 (an oncogene) expression which is accountable for epithelial-to-mesenchymal transition (EMT). Similarly, using renal cell carcinoma (786 0 and Caki 1) galangin inhibited EMT through downregulation of E-cadherin. 12 Upregulated CD44, another important characteristic hall mark in several tumors was found to be suppressed by galangin treatment. Consequently, promising anti-angiogenic and anti-invasive effects were seen in glioma (U87 and U251) cells. 15 In 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced invasive HepG2 liver cancer cell culture model Chien et al. investigated the anti-metastatic effect of galangin. Results of the study revealed that galangin reduced enzymatic activity of MMPs 2, 9 via ERK1/2, c-Fos, c-Jun, nuclear factor kappa B (NF-κB), and activator protein 1 (AP-1). The incidences of biliary tumors including cholangiocarcinoma (CCA) are increasing globally. In CCA cancer, galangin treatment was found to upregulate the expression of PTEN and downregulates the phosphorylation of AKT via miR-21inhibition. Another important oncogene, i.e., H19 is known to associate with tumor progression, proliferation, and migration. Using hepatocellular carcinoma (MHCC97H) cells, galangin was found to inhibit the expression of H19 and miR 675 via up-regulating p53 levels. Figure 3 depicts the anti-angiogenic and anti-metastatic mechanisms of action of galangin.
Figure 3.
Anti-angiogenic and anti-metastatic actions of galangin by inhibiting p-Akt, p-70S6K, HIF-1, VEGF/R and Erk1/2, c-Fos, c-Jun, NF-κB and AP-1 pathways respectively. (A color version of this figure is available in the online journal.)
Antioxidant and anti-inflammatory effect
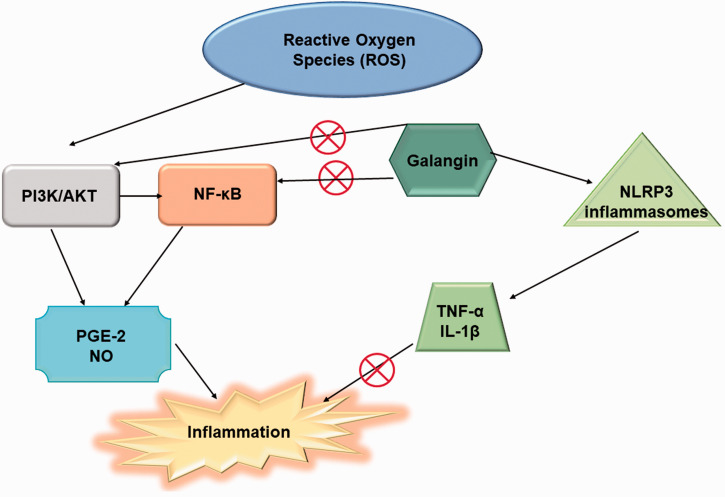
The body in its normal physiological state can maintain a dynamic balance between antioxidation and oxidation by eliminating the smaller proportions of ROS generated. However, when this balance is disturbed, i.e., ROS accumulates at a higher speed than their elimination from body, it leads to the propagation of various diseases like Alzheimer’s disease, pulmonary fibrosis, cerebral ischemia, and Parkinson’s disease. These diseases can be prevented by the antioxidants such as galangin. Galangin (3,5,7-trihydroxyflavone) serves various medicinal properties such as anti-viral, anti-tumor, anti-microbial, anti-mutagenic, antioxidant, and anti-inflammatory.60–62 The anti-inflammatory properties of galangin were observed in different diseases such as asthma, acute lung injury, arthritis, acute kidney injury, and paw edema. It also helps in reducing renal inflammation18,63 by the activation of NLRP3 inflammasomes and inhibition of PI3K/AKT and NF-κB pathways (Figure 4). It reduces the inflammation caused by uric acid by inhibiting the caspase-1 activity and ASC adaptors as well as decreasing the levels of IL-18 and IL-1β. 63
Figure 4.
Antioxidant mechanisms of galangin to prevent the inflammation. It modulates the expression of PI3K/AKT, NF-κB, PGE-2, IL-1β and TNF-α during oxidative stress. (A color version of this figure is available in the online journal.)
The inflammatory response in the body is regulated by various pathways such as NLRP3 inflammasome, PI3K/AKT, and NF-κB. PI3K/AKT is closely associated with inflammation and helps in the activation of NF-κB (Chien et al. 2015). NF-κB mediates the expression of mediators (PGE-2 and NO) and cytokines (IL-1β and TNF-α) to prevent inflammation and aids in host defense. The NLRP3 inflammasomes help in inhibiting the inflammation to various agents by activation of cysteinyl aspartate-specific proteinase-1 (caspase-1) and secretion of mediators or cytokines.
Despite possessing different medicinal properties, galangin has a limited number of applications due to its insoluble nature, semi-permeability to gastrointestinal barriers, and sensitive nature to environmental parameters like pH, temperature, and light. 64 Its bioavailability can be enhanced by immobilizing into various carriers such as β-cyclodextrin (βCD), hydro xypropyl-β-cyclodextrin (HPβCD). 65
Epigenetic effects
Galangin triggered autophagy and apoptosis in cancer cells through epigenetic modifications, such as histone acetylation and DNA methylation. Numerous studies reported that epigenetic alterations are responsible for gene dysregulation in several human cancers.66–68 Galangin possesses tremendous potential to treat triple-negative breast cancer by epigenetically restoring estrogen levels via reduction of hypermethylation level of the BRCA1 gene in the HCC38 tripe-negative breast cancer cell. Thus, in the future, it may be used as anti-hormonal therapy for triple-negative breast cancer. 69 For example, Li et al. demonstrated that galangin induced autophagy in hepatocellular carcinoma cell HepG2 by deacetylation of LC3 (Light Chain 3) by SIRT1 (Sirtuin 1). 70 Mechanistically, galangin promoted the conversion of LC3 I to LC3 II and decreased the acetylation of LC3. However, in SIRT1 knockdown cells, galangin failed to reduce the acetylation of LC3 in vector-infected cells. These results suggest that deacetylation of endogenous LC3 by SIRT1 is essential for galangin-induced autophagy in HepG2 cells. 70 Overall, galangin induces autophagy via the deacetylation of endogenous LC3 by SIRT1 in HepG2 cells. Moreover, Lei et al. showed reduced A172 cell migration and invasion upon treatment with various concentration of galangin 0, 5, 10, 25 μM for 24 hours compared to non-treated cells. 59 Mechanistically, the authors analyzed the invasion associated protein ADAM9 inside the cell and observed that galangin reduced the ADAM9 protein expression via activation of ERK1/2 following treatment with different doses of galangin. 59 Taken together, in vitro and in vivo studies suggested that galangin played a widespread role in cancer management through blocking the vital oncogenic pathways, activated during cancer manifestation.
Recently, galangin also acts as an effective molecule for treating Alzheimer’s disease (AD). Generally, the aberrant acetylation pattern in the BACE1 (Beta-Secretase 1) gene leads to the pathogenesis of AD. It was shown that galangin decreases the acetylation level of the BACE1 promoter region by increasing the deacetylation pattern of HDAC1 (Histone Deacetylase 1). 71
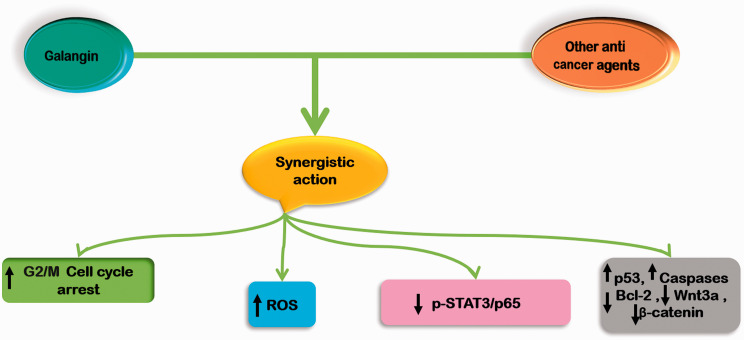
Synergistic effects of galangin
Naturally occurring compounds like flavonols play remarkable roles in cancer therapy due to their multi-targeted actions and lack of considerable toxicity. In order to have maximum therapeutic efficacy and minimum side-effects with little or no drug resistance, various synergistic chemo-preventive strategies in combination with established chemotherapeutic drugs, had gained a lot attention in the recent years.72–75 Galangin (GA, 3,5,7-trihydroxyflavone), one of the most important and naturally active flavonoids, is a polyphenolic compound extracted primarily from the roots of Alpinia officinarum Hance, Alnus pendula Matsum., Plantago major L., and Scutellaria galericulata L (S. scrodifolia Fisch.), have been applied as herbal medicines for various ailments in Asian cultures for centuries. 5 The activity of natural compound is associated with the ability of flavonols to influence membrane-dependent processes and a broad range of biological properties, including prohibitive effects on bacteria, fungi, viruses, the control of hypertension and diabetes, and chemoprevention of several cancers. 5 In addition, galangin has also been found to enhance the chemo preventive potentiality (Figure 5) of various other therapeutic drugs. Studies have demonstrated that the combination of galangin with berberine synergistically resulted in cell growth inhibition, apoptosis, and cell cycle arrest at G2/M phase with the increased intracellular reactive oxygen species (ROS) levels in esophageal carcinoma cells. 76 Furthermore, this bioactive compound has been documented to exhibit potent synergism with fisetin (FTN) and quercetin (QTN) and thus correlated the biological activity of these molecules with their interaction and localization in dipalmitoyl phosphatidyl choline (DPPC) bilayers, using differential scanning calorimetry (DSC) and nuclear magnetic resonance (NMR) methods. Results indicated that galangin interacts to the alkyl chains of the lipid bilayer involving hydrophobic interactions and play an important role in membrane binding and thereby in biological activity. 77 The compound has also been known to synergize with other cytotoxic natural flavonoids quercetin and chrysin to show effects on cisplatin (cis-Pt)-induced apoptosis of human promyelocytic leukemia HL-60 cells and murine leukemia L1210 cells. 78 Recently, it was investigated that the combination of galangin with cisplatin at a low concentration induced the apoptosis of lung cancer cells, even in cells with resistance to cisplatin through inactivation of p-STAT3/p65 and Bcl-2 pathways. 16 Additionally, this natural flavonoid has also been reported to simultaneously induce apoptosis, pytoptosis, and protective autophagy in human glioblastoma multiforme (GBM) cells, indicating that combination treatment of galangin with autophagy inhibitors may also be an effective therapeutic approach for GBM i.e. one of the most common and deadly primary malignant tumor of the central nervous system in humans. 13 Likewise, studies also revealed that the galangin therapeutic development as synergistic with gold nanoparticles against human breast cancer cell line (SKOV-3) through induction apoptosis instantiated biological mechanism increasing expression of p53, caspase-8 respect therapeutic targets via mitochondria pathway. 79
Figure 5.
This illustration represents synergistic mechanisms of action of galangin in cancer. (A color version of this figure is available in the online journal.)
Role of nanotechnology in galangin delivery
Galangin is a flavonoid with poor pharmacokinetic profile, low water solubility, and incompatibility in physiological media, which limits its therapeutic application.19,80 Among flavonoid molecules like quercetin, kaempherol, morin, and myricetin, it is the most lyophilic. The development of new drug delivery methods might not only overcome solubility barriers, but also enhance effective drug concentration in tumors while reducing drug accumulation in normal tissues, reducing undesirable side effects.5,81–85 Nanoparticular delivery methods might therefore be created to enhance galangin's therapeutic benefits, as an anticancer medication.
Ahmed M Al-Shammari et al. investigated the safety of AuNPs nanoparticles in conjunction with galangin in the treatment of ovarian cancer. Galangin therapeutic development as a synergistic with gold nanoparticles against human breast cancer cell line (SKOV-3) by induction apoptosis indicated a biological mechanism boosting p53, caspase-8, and therapeutic targets via mitochondria pathway, according to the findings. 79 Hui Yao et al. developed galangin-loaded PEG-modified liposomes and tested their targeting to liver cancer cells in vitro, as well as the pharmacokinetic characteristics in rats (in vivo). Under experimental storage circumstances, Gal entrapment efficiency was 80%, with minimum leakage. PEG-modified liposomes have enhanced solubility, antitumor effectiveness, and pharmacokinetics when compared to free Gal. 86 To improve galangin’s low permeability and poor water solubility, Yinghua Li et al. designed Ga-functionalized SeNPs (selenium nanoparticles) and enhanced the anti-cancer potential against hepatocellular carcinoma. It induced caspase-3-mediated HepG2 cell death via ROS production, according to the underlying molecular processes. 20 Jing Zhu et al. effectively produced galangin-liposomes utilizing a thin-film hydration technique to test its oral bioavailability. When compared to free galangin, the galangin-liposomes demonstrated higher hepatoprotective benefits in ameliorating CCl4 toxicity in mice, according to biochemical and histological analyses. 87 A study done by Hamed Hajipour confirmed that the GA loaded-Arginyl-glycyl-aspartic acid nanostructured lipid carrier could significantly improve the anticancer potential of doxorubicin. Arginyl-glycyl-aspartic acid-nanostructured lipid carrier, increases galangin accumulation in malignant cells via integrin-mediated endocytosis, according to uptake tests. In comparison to doxorubicin and galangin loaded-Arginyl-glycyl-aspartic acid nanostructured lipid carrier, doxorubicin and galangin loaded-Arginyl-glycyl-aspartic acid nanostructured lipid carrier demonstrated greater cytotoxicity and apoptotic effects. 88 Table 1 summarizes the galangin's anticancer effects in combination with nanoformulation.
Table 1.
Anti-cancer activity of galangin nanoformulation in different models.
| Sr.no | Nanoformulation | Experimental model | Dose | Results | Ref. |
|---|---|---|---|---|---|
| 1 | Galangin - gold nanoparticles(18) | SKOV3,ovarian cancer cell line, and HBL, human epithelial breast tissue | 5,10, 25, 50, 100 μg/ml | Synergistic cytotoxic effect against ovarian cancer cells | 30 |
| 2 | Galangin loaded PEG- modified liposomes(19) | Hep G2 cells, Male Wistar rats | 5 μM5 mg/kg | PEG-modified liposomes have a greater cytotoxic impact. Hep G2 cells had a 1.34-fold increase in overall apoptosis when compared to Gal-loaded unmodified liposomes. | 31 |
| 3 | Galangin loaded selenium nanoparticle(20) | HepG2 cells(normal human liver cell line) | 10 µM | Cytotoxic effect, Induced apoptosis of cancer cells | 32 |
| 4 | Galangin-loaded liposomes(21) | ICR mice | 300 mg/kg | Increase in oral bioavailability by 470.12% in encapsulated galanginBetter hepatoprotective effects observed compared with free galangin | 33 |
| 5 | Galangin loaded-NLC-RGD (loaded-Arginyl-glycyl-aspartic acid nanostructured lipid carrier) (22) | Human lung carcinoma (A549) cell line | 3 mg/mL | When galangin was used in the form of GA loaded-NLC-RGD, its effects on the expression of the ABC transporter were amplified. Increased Apoptosis and Cytotoxic Effect (123.4 µM GA -NLC-RGD). | 34 |
Toxicity studies using galangin
Galangin as a natural compound is generally considered to be safe. However, the experimental evidence confirming this statement is still rather scarce, pointing to the necessity for further thorough studies on safety profile of galangin. Based on the recently reported data, orally administered galangin displayed no signs of toxicity in male Wistar rats at doses up to 320 mg/kg. 89 In addition, no significant histological changes were observed in the liver, renal, heart, and lung tissues of male nude mice injected intraperitoneally with galangin at 30 mg/kg, along with no alterations in the serum ALT and AST levels. 39 Tables 2 and 3 represent a bird eye view of various in vitro and in vivo anticancer applications of galangin to support its promising chemopreventive candidature in near future, respectively.
Table 2.
Anti-cancer effects of galangin based on In vitro studies.
| Type of Cancer | Cell lines | Effects | Mechanisms | Concentration | References |
|---|---|---|---|---|---|
| Glioblastoma | U87, U251 and U87-luciferase | Induces apoptosis | ↓ GBM cell growth, migration, and invasion, ↓ Skp2, ↓ Zeb1, ↓ N-cadherin, ↓ snail, ↓ vimentin , ↑ Skp2 gegradation through the Ubiquitin-Proteasome-Dependent pathway | 0, 10, 20, 40 and 80 µM | 12 |
| U87 and U251 | Inhibited angiogenesis, | ↓ proliferation, migration and invasion of cancer cells , ↓ mRNA and protein levels of CD44, ↓ Snail, ↓ Vimentin ↓ ZEB1, ↓VEGF | 0, 5, 10, 20 and 40 μM | 15 | |
| U251, U87MG, and A172 | Induces apoptosis, pytoptosis, and protective autophagy | ↓ viability and proliferation of GBM cells, induces G0/G1 Cell Cycle arrest, ↓ CCND1, ↓ CDK4, ↓ PCNA, ↓ cyclin-dependent kinase inhibitor p21, ↓ Bcl-2, ↑ BAX, ↑ cleaved PARP-1, ↑ nuclear DNA damage in GBM cells, ↑ formation of autophagic vesicles, ↑ MAP1LC3B-II, ↓ SQSTM1, ↑ AMPK activity, ↓ mTOR , ↓ P-AMPKα (Thr172) | 0, 50, 100, 200, 400 μM | 13 | |
| A172 | Induces apoptosis | ↓ A172 cell migration and invasion, ↓ ADAM9 expression, ↑ p-ERK1/2, ↓ total expression of ERK1/2 | 0,5,10 and 25 μM | 7 | |
| Nasopharyngeal | NPC-TW 039 and NPC-TW 076 | Induces p53-independent S-phase arrest and apoptosis | ↑ numbers of apoptotic bodies and ↑ condensed/fragmented nuclei, ↑ DNA fragmentation, ↓ PI3K-AKT Signaling Pathway, ↑ Cleavage of procaspase-3 and PARP, induced caspase-3 activation, ↑ apoptotic cells (sub-G1-phase population), ↓ p-AKT (Ser 473), ↓ PI3K, ↓ AKT, ↑ cleavage of pro-caspase-9, ↑ p21, ↑ BAX, ↑ BAD, ↑ BAK protein expression, ↓ BCL-2, ↓ BCL-xL protein levels | 0–100 μM | 45 |
| Laryngeal | TU212 and M4e | Activating apoptosis and autophagy | ↓ carcinoma cell viability, migration, invasion and proliferation, ↑ Bax, ↓ Bcl-2, ↑ caspase-3, ↑ caspase-9, ↑ PARP cleavage, ↑ LC3I, ↑ LC3II, ↑ Beclin 1, ↓ Raf, ↓ Ras. ↓ p-p38, ↓ PI3K/AKT, ↓ PI3K-Akt-mTOR signaling pathway, ↑ TSC1(inhibitor of mTOR activation), ↓ p- mTOR | 0, 2.5, 5.0, 10.0, 20.0, 30.0 and 40.0 µM | 42 |
| Esophageal(galangin and berberine) | Eca9706,TE-1, and EC109 | Induces apoptosis | ↓ survival and growth cancer cells, cell cycle arrest at G2/M phase, ↑ ROS levels, ↓ Wnt3a, ↓ β-catenin, ↓ Cyclin B, ↓ Cyclin D, ↓ Cyclin E, ↓ CDK1, ↓ CDK2, ↓ CDK4, ↓ CDK6, ↓ transition of G2/M phase, ↑ P21, ↑ P27, ↑ P53, ↑ cleaved PARP, ↑ Caspase-3, ↓ Bcl-2, ↓ Mcl-, ↓ XIAP, ↑ Bax, ↑ PI3K, ↑ Rac, ↑ p-JAK2, ↑ p-STAT3 | Galangin 0,2.5,5.0, 10.0,20.0,30.0,40.0 and 50 µM + Berberine 0,10,30,60, 90,120,160 and 200 µM | 76 |
| Retinoblastoma | Y-79, C-33A, and WERI-Rb-1 | Induced apoptosis | ↓ Human retinoblastoma cell proliferation and migration. ↑ PTEN, ↓ protein kinase B (Akt) phosphorylation, ↑ PIP3, ↑ PIP2, ↑ caspase-3, ↓ KI-67 positive levels, ↓ p-Akt (S473 and T308 sites), ↓ PIP2 , ↑ active Caspase-9, ↑ Caspase-3 expression levels | 0, 5, 10, 20, 40, 80, and 100 uM | 39 |
| Osteosarcoma | MG-63 and U2-OS | Induced apoptosis | ↓ cell proliferation, ↑ mRNA levels of Col I, ALP, OPN, and OC (osteoblastic differentiation markers), ↑ protein level of Runx2, ↑ TGF-b1 production, ↑ phosphorylation of Smad2 and Smad3 | 0, 25, 50 and 100 μM | 90 |
| MG63 and U20S | Induces apoptosis | ↓ proliferation, migration and invasion osteosarcoma cells, ↓ PI3K and Aktp (Thr308), ↓ cyclin D1, ↓ MMP 2/9, ↑ p27Kip1, ↑ caspase-3, ↑ caspase-8 | 0, 5, 10, 25, 50, 100, 200 and 300 µM | 14 | |
| Fibrosarcoma | HT-1080 | Inhibited metastasis | ↓ MMP-9 secretion, ↓ MMP-9 mRNA, ↓ p-JNK, ↓ activation of NF-κB and AP-1, ↑ p-IκBα, ↓ IκBα | 0,10, 30 and 100 µM | 91 |
| Breast | MCF-7 | Induces apoptosis | ↑ Bax and decreased the expression of Bcl-2, ↑ cleavage of caspase-9, ↑ caspase-8, ↑ caspase-3, ↑ Bid, ↑ Bad, ↓ p-PI3K, ↓ pAkt, ↓ cyclin D3, ↓ cyclin B1, ↓ CDK1, ↓ CDK2, ↓ CDK4, ↑ p21,↑ p27, ↑ p53 | 10, 20, 40, 80, and 160 µM | 7 |
| MCF-7 and T47D | Induce apoptosis | ↓ cancer cell lines viability, proliferation, ↓ BCl-2, ↑ ROS production, ↑ NADPH, ↑ caspase-3 activity, ↑ Caspase-9 activity, ↑ p-PERK, ↑ GRP78, ↑ CHOP, ↑ p-eIF2a, ↑ ATF4, ↑ p-AMPK, ↑ DR4, ↑ Caspase-9, ↑ Caspase-3 cleavage, ↑ Bax | Galangin 20 and 40 µM + TRAIL 100 and 200 ng/ml | 43 | |
| Lung (galangin and cisplatin) | A549, DDP-resistant variant A549/DDP cells | Induce apoptosis | ↓ cell proliferation, viability, migration and colony formation, ↓ p65 in nucleus, ↓ p-IκBα in whole cells, ↑ IκBα, ↓ p-STAT3, ↑ cleaved Caspase-3, ↑ PARP, ↓ Bcl-2, ↑ Bax, ↑ Bid | 0, 2, 5 and 10 µM galangin + 2 µM DDP (cisplatin) | 16 |
| Colon | HCT-15 and HT-29 | Induced apoptosis and DNA condensation | ↓ cancer cell viability, ↑ nuclear rounding and shrinkage, ↓ caspase-3, ↓ caspase-9, ↑ release of apoptosis inducing factor from the mitochondria into the cytoplasm, alteration of mitochondria membrane potential and dysfunction | 0, 5, 25, 50, 100 and 200 μM | 10 |
| Hepatocellular | MHCC97H | Promoted cell apoptosis | ↓ H19, ↓ cell migration and invasion, ↓ S phase cells, mRNA of TP53- and p53-related genes (CDIP1, FOS, and CREB3L3) were significantly differentially expressed | 0, 20, 50, 100 and 150 μM | 11 |
| HepG2 | Induces apoptosis | ↓ HepG2 cell proliferation and viability, ↑cytoplasm shrinkage, disappearance of microvilli, ↑ shrinkage cytoplasm, distorted organelles and condensed chromatin ↓ mitochondrial membrane potential ↑ mitochondrial dysfunction, ↑ caspase-3 ↑ ROS production | Selenium nanoparticles with galangin (11 µM) | 20 | |
| HepG2, Hep3B and PLC/PRF/5 | -- | ↓ proliferation of HCC cells, ↓ glucose absorption, ↓ lactate production, ↑ pyruvate kinase, ↓ Warburg effect, ↑ aerobic metabolism, ↓ glycolysis, ↓ glucose absorption, ↓ lactate production, ↑ glycolytic rate-limiting enzyme pyruvate kinase activity, ↓ Glut1, ↓ PKM2, ↓ LDHA, ↓ PDHK, ↑ HKII, ↑ PKM1, ↑ PDH, ↑ CS | 0, 65, 130 and 260 µM | 92 | |
| HepG2 | Induces autophagy | ↑ binding of SIRT1-LC3, ↓ acetylation of endogenous LC3, ↑ LC3 II, ↑ Beclin1, ↑ ratio of LC3 II to LC3 I, ↓ p62, activating the TGF-β receptor/Smad pathway, ↑ AMP/TAN ratio, ↑ p53, ↑ glucose starvation | 130 µM | 70 | |
| HepG2 | Induced autophagy | ↑ TGF-β receptor/Smad pathway activity, ↑ TGF-β receptor I (RI), ↑ TGF-β RII, ↑ Smad1, ↑ Smad2, ↑ Smad3, ↑ Smad4 levels, ↓ Smad6, ↓ Smad7, ↑ Beclin1, ↑ ATG16L, ↑ ATG12, ↑ ATG3 and ↑ LC3-II, ↑ number of cells with LC3 foci, ↑ TGF-β RI, ↑ TGF-β RII, ↑ phosphorylation of Smad1, Smad2 and Smad3, ↓ subG1 ratio | 0, 37, 74 and 148 µM | 93 | |
| HepG2, Hep3B and PLC/PRF/5 | Induce apoptosis | ↓ proliferation and viability of carcinoma cells, ↑ endoplasmic reticulum stress, ↑ Ca2+ levels, ↑ GRP94, ↑ GRP78, ↑ CHOP, ↑ p38 MAPK, ↑ JNK, ↑ ERK | 134.0, 87.3 and 79.8 µM | 29 | |
| HepG2, Hep3B, and PLC/PRF/5 | Induced autophagy | ↑ AMP/TAN, ↑p-AMPK, ↑ p- LKB1, ↓ p-AKT, ↓ p-mTOR, ↑ PARP, ↑ LC3-II, ↑ formation of autophagic vacuoles, ↑ cellular relative AMP level | 0, 65,130 and 260 µM | 30 | |
| Liver | Chang liver, AGS, Hep3B, and HepG2 | Inhibited metastasis | ↓ viability of cancer cells, ↓ TPA-induced enzyme activity, ↓ MMP-2 and MMP-9, ↓PKCα, PKCδ, ↓ p-ERK1/2, phospho-IκBα, ↓c-Fos, ↓ c-Jun, ↓ NF-κB | 0, 1, 2.5, 5, 10, 15, 20, 25, and 30 μM | 62 |
| Cholangiocarcinoma | HCCC9810 and CCA cell line TFK-1 | Induces cell apoptosis | ↓ proliferation, migration, and invasion of cancer cells, ↓ microRNA-21 (miR-21) expression, ↓ p-AKT, ↓ MMP9, ↓ Vimentin, ↑ PTEN, ↑ cleaved caspase 3 protein expression, ↑ ratio of Bax to Bcl-2 | 0, 50, 100, 150, or 200 μM | 40 |
| Gastric | MGC 803 | Promoted apoptosis | ↓ cancer cell proliferation, ↓ Ki67, ↓ PCNA, ↓ Bcl-2, ↑ cleaved caspase- 3, ↑ cleaved PARP, inactivated JAK2/STAT3 pathway, ↑ ROS, ↓ Nrf2, ↓ NQO-1, ↑ HO-1, ↓ caspase-3, ↓ p-JAK2, ↓ p-STAT3 | 0, 5, 10, 20, 40, 80, 120, 160 and 200 μM | 9 |
| SNU-484 | Induces apoptosis | ↓ viability of SNU-484 cells, ↑ chromatin condensation and DNA damage, ↑ Bax, ↓ Bcl-2, ↓ Bcl-xl, ↑ caspase-3, -9, and PARP, protein ↓ levels of glutathione S-transferase P, ↓ peroxiredoxin 5, ↓ cytochrome c oxidase subunit 5 A (mitochondrial), ↓ Bfl-1 in complex with Noxa Bh3 peptide, ↑ carboxylterminal hydrolase isozyme L1,↑ nucleoside diphosphate kinase A, ↑ eukaryotic translation initiation factor 5 A-1, ↑ galectin-1 | 0, 25, 50,7 5, 100, 125, 150, 175 and 200 μM | 94 | |
| Pancreatic | PANC-1 | Induced apoptosis | ↓ cell proliferation viability, ↓p-Thr-179 site at Smad3 linker region, ↓ p-CDK4, ↑ p21 (TGF-b1-induced tumor suppressor), ↑ PARP, ↑ caspase-3 | 0, 25, 50 and 100 μM | 95 |
| Renal | A498 | Induction of Mitochondrial mediated apoptosis | ↑ protein expression of Bax and Cyt-c ↓ Bcl-2, ↓ motility, Invasion and migration of the A498 cells, ↓ p-PI3K, ↓ pAKT, ↓ p-mTOR proteins, ↓ PI3K/AKT/mTOR signaling pathway, | 0, 10, 20 and 40 μM | 41 |
| Caki, ACHN and A498 | Induce apoptosis | ↑ sub-G1 population, ↑ PARP cleavage, caused chromatin damaged in the nuclei, ↓ Bcl-2 ↓ NF-κB activation, ↓ cFLIP, ↓ Mcl-1, ↓ survivin expression (at the post-translational levels), ↑ proteasome activity | 0. 50. 100, 50. 200 and 250 µM + TRAIL 100, 200, 300 and 400 ng/ml | 44 | |
| Prostate | PC3M and DU145, | Induced apoptosis | ↓ cell proliferation viability, ↓p-Thr-179 site at Smad3 linker region, ↓ p-CDK4, ↑ p21 (TGF-b1-induced tumor suppressor), ↑ PARP, ↑ caspase-3 | 0, 25,50, and 100 μM | 95 |
| Cervical | HeLa | Induction of apoptosis | ↓ proliferation and migration of HeLa cells, ↑ ROS production, ↓ cytotoxic metabolite methy glyoxal, ↓ Nrf-2 (a trascription factor), ↓ glyoxalase-1, ↑ oxidative and carbonyl stress, ↑ total carbonyl content (an indicator of oxidation damage) | 0, 25, 50,100 and 150 μM | 38 |
| Ovarian | A2780/CP70 and OVCAR-3 | Induces apoptosis | ↓ proliferation of ovarian cancer cells, ↑ cleaved caspase-3, caspase-7 and PARP-1, ↓ procaspase-3, ↓ procaspase-7, ↑ DR5, ↑ cleaved caspase-8 and ↓ procaspase-8 , ↑ Bax protein, ↓ Bcl-2, ↓ procaspase-9, ↑ p53, ↑ p21 protein expressions , ↓ p-Akt, ↓ p-p70S6K, ↓ cmyc protein levels | 0,10, 20, 40, 80 and 160 µM | 8 |
| OVCAR-3 and A2780/CP70 | Anti-angiogenic | ↓ VEGF, ↓ p-Akt,↓ p-70S6K, ↓ HIF-1α proteins, ↓ secretion of VEGF by the Akt/p70S6K/ HIF-1α pathway, | 0, 10, 20, 40, 80 and 160 µM | 58 |
Table 3.
Anti-cancer effects of galangin based on in vivo studies.
| Type of cancer | Animal models | Effects | Mechanisms | Dosage | Duration | References |
|---|---|---|---|---|---|---|
| Glioblastoma | Male BALB/C Nude mice administered with 1 × 10 6 U87-luciferase cells | Inhibited tumor proliferation | ↓ Skp2, ↓ EMT, ↑ survival galangin treated mice, ↑ ubiquitination of Skp2 in the intracranial | 100 mg/kg | 21 days | 12 |
| Female BALB/C Nude Mice Injected Intracranially With U87-Luciferase Cells (5 × 10 5 Cells) | Inhibited tumor growth | ↓ CD44 levels, ↓ vessel density, ↓ CD44, ↓ N-cadherin, ↓ Vimentin, ↓ Snail, ↓ VEGF | 200mg/kg/ | 28 days | 15 | |
| Male BALB/C Athymic mice xenografted with 3 × 10 5 U87MG cells | Suppressed tumor growth | ↓ Ki67, ↓ cyclin-dependent kinase inhibitor p21, ↓ Bcl-2, ↑ BAX, ↑ cleaved PARP-1, | 100 mg/kg | 21 days | 13 | |
| Retinoblastoma | Male, Nude mice subcutaneously injected with HXO-RB44/Y-79 suspensions (2 × 10 6 cells) | Suppressed tumor growth | ↓ KI-67, ↑ PTEN, ↑ Caspase-3, ↓ p-Akt (S473 and T308), ↓ PIP2, ↓ PIP3 | 15 and 30 mg/kg | 21 days | 39 |
| Laryngeal | SPF MaleBALB/C Nude Mice Subcutaneously Injected With U212 Cell Suspension (2 × 10 7 Cells) | Tumorsuppression | ↓ Ki-67, ↑ TUNEL | 10, 20 and 30 mg/kg | 42 days | 42 |
| Esophageal | SPF male BALB/C Nude mice injected subcutaneously with ECA 9706 (2 × 107 cells) | Inhibited the tumor growth | ↑ TUNEL, ↑ P53, ↓ Ki-67, ↓ Wnt3a, no significanteffect on AST, ALT and ALB compared to the control group | galangin group (25 mg/kg), berberine group (20 mg/kg) | 35 days | 76 |
| Osteosarcoma (galangin and berberine) | Male BALB/C Nude mice subcutaneously injected with 1 × 107 MG-63 | Tumorvolume was obviously decreased | ↑ Col I, ALP, OPN, and OC (osteoblastic differentiation markers), ↑ protein level Runx2, ↑ TGF-b1 production, ↑ phosphorylation of Smad2 and Smad3 | 50 or 100 mg/kg | 28 days | 90 |
| Breast | Athymic nude male mice subcutaneously injected with 5 × 105 MCF-7 cells | Inhibited tumor growth | ↓ toxicity to mice, ↑ CHOP, ↑ p-AMPK, ↑ DR4, ↑ cleavage Caspase 3,9 | 20 mg/kg and TRAIL (100 µg/mouse) | 28 days | 43 |
| Lung (galangin and cisplatin) | Athymic Nude Male, mice injected subcutaneously with A549/DDP (5 × 106 cells) | Suppressed tumor growth | ↓ p-STAT3-, ↓ p-NFκB and ↓ Bcl-2-, ↑ Cleaved Caspase-3 and PARP levels | 10 mg/kg + DDP (cisplatin) 5 mg/kg | 28 days | 16 |
| Male swiss albino mice administered with B(a)P (50 mg/kg body weight dissolved in corn oil, orally) | Inhibits tumor initiation | ↓ Cytochrome P450, ↓ Cytochrome b5, ↓ NADPH Cytochrome P450 redcutase ↓ NADPH Cytochrome b5 reductase (phase I), ↑ GST, ↑ UDP-GT, ↑ DTD | 20 mg/kg body | 14 days | 6 | |
| Hepatocellular | Female nude mice xenografted with PCDNA3.1-H19, and H19-KO cells (3 × 105cells) | Inhibited tumor growth | ↓ H19, ↓ cell migration and invasion, ↓ S phase cells, mRNA of TP53- and p53-related genes (CDIP1, FOS, and CREB3L3) were significantly differentially expressed | 20 mg/kg | 14 days | 11 |
| BALB/C Athymic nude mice injected subcutaneously with HepG 2 cells (2 × 106 cells) | Suppressed tumor volume | ↓p- AKT, ↓ mTOR, ↑ p-AMPK | 70 mg/kg, 35 mg/kg or 17.5 mg/kg | 28 days | 30 | |
| Gastric | Male Nude mice inoculated subcutaneously with MGC 803 cells (5 × 106 cells | Inhibited tumor growth | ↓ p-JAK2/JAK2, ↓ p-STAT3/STAT3, ↓ Bcl-2, ↓ caspase-3, ↓ Ki67, ↑ cleaved caspase-3, ↑cleaved PARP | 120 mg/kg | 21 days | 9 |
| Renal | Male albino wistar rats | Ameliorates cisplatin induced nephrotoxicity | ↑ Bax, ↓ Bcl-2, ↓ DNA fragmentation, ↓ NFκB↓ p38, ↓ JNK, ↓ ERK1/2 | 25, 50 and 100 mg/kg (Administered orally ) | 10 days | 96 |
| Ovarian | Ovcar-3 cells (1.2 × 106 cells) implanted into the chorioallantoic membrane (cam) of the 9-day-old chicken embryo | Inhibit in vivo angiogenesis | ↓ blood vessels, ↓ HIF-1α, ↓ phosphorylation of Akt and p70S6K, no effecton the expression of PTEN and NFκB (p50) | 40 µM | 9 days | 8 |
Conclusions
With the ever-increasing global cancer incidence, there is a rising need for the identification of new efficient and safe compounds as molecular leads for novel cancer drugs. In this article, the natural compound galangin is represented as a potential anticancer agent. Despite the strong in vitro basis of chemopreventive and chemotherapeutic properties of this compound, more in vivo studies with different types of tumor models are definitely needed to be performed in the future. Also, the safety issue of galangin must be clarified before moving on with the clinical trials. Last but not least, it is hoped that the use of modern nanotechnological methods will reveal the best carrier systems for delivering enough amounts of parent bioactive galangin to the target malignant tissues.
Footnotes
AUTHORS’ CONTRIBUTIONS: HST: Literature analysis and concept; KS: Contributed in abstract and introduction section; SA and GK: Contributed in absorption and nanotechnology section; DA: Designed in vitro and in vivo tables; JK: contributed in anti-inflammation section; MK: contributed in structural description of galangin; NCP: Contributed in synergistic section; GP: contributed in apoptosis and cell cycle arrest; US and AJ: contributed in epigenetic section
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Hardeep Singh Tuli https://orcid.org/0000-0003-1155-0094
Shubham Adhikary https://orcid.org/0000-0003-2512-7510
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin 2021; 71:209–49 [DOI] [PubMed] [Google Scholar]
- 2.de Dios NR, Murcia-Mejia M. Current and future strategies in radiotherapy for small-cell lung cancer. J Clin Transl Res 2020; 6:97–108 [PMC free article] [PubMed] [Google Scholar]
- 3.Arellano EA, Diaz VD, Rodriguez JJC. Current status and future directions in unresectable stage III non-small cell lung cancer. J Clin Transl Res 2020; 6:109–20 [PMC free article] [PubMed] [Google Scholar]
- 4.Lichota A, Gwozdzinski K. Anticancer activity of natural compounds from plant and marine environment. Ijms 2018; 19: 3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang D, Xiong Z, Xu J, Yin J, Luo R. Chemopreventive mechanisms of galangin against hepatocellular carcinoma: a review. Biomed Pharmacother 2019; 109:2054–61 [DOI] [PubMed] [Google Scholar]
- 6.Devadoss D, Ramar M, Chinnasamy A. Galangin, a dietary flavonol inhibits tumor initiation during experimental pulmonary tumorigenesis by modulating xenobiotic enzymes and antioxidant status. Arch Pharm Res 2018; 41:265–75 [DOI] [PubMed] [Google Scholar]
- 7.Liu D, You P, Luo Y, Yang M, Liu Y. Galangin induces apoptosis in MCF-7 human breast cancer cells through mitochondrial pathway and phosphatidylinositol 3-Kinase/akt inhibition. Pharmacology 2018; 102:58–66 [DOI] [PubMed] [Google Scholar]
- 8.Huang H, Chen AY, Ye X, Guan R, Rankin GO, Chen YC. Galangin, a flavonoid from lesser galangal, induced apoptosis via p53-dependent pathway in ovarian cancer cells. Molecules 2020; 25:1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang X, Wang P, Yang C, Huang F, Wu H, Shi H, Wu X. Galangin inhibits gastric cancer growth through enhancing STAT3 mediated ROS production. Front Pharmacol 2021; 12:646628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ha TK, Kim ME, Yoon JH, Bae SJ, Yeom J, Lee JS. Galangin induces human colon cancer cell death via the mitochondrial dysfunction and caspase-dependent pathway. Exp Biol Med (Maywood) 2013; 238:1047–54 [DOI] [PubMed] [Google Scholar]
- 11.Zhong X, Huang S, Liu D, Jiang Z, Jin Q, Li C, Da L, Yao Q, Wang D. Galangin promotes cell apoptosis through suppression of H19 expression in hepatocellular carcinoma cells. Cancer Med 2020; 9:5546–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiong Y, Lai X, Xiang W, Zhou J, Han J, Li H, Deng H, Liu L, Peng J, Chen L. Galangin (GLN) suppresses proliferation, migration, and invasion of human glioblastoma cells by targeting Skp2-induced epithelial-mesenchymal transition (EMT). Onco Targets Ther 2020; 13:9235–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kong Y, Feng Z, Chen A, Qi Q, Han M, Wang S, Zhang Y, Zhang X, Yang N, Wang J, Huang B, Zhang Q, Xiang G, Li W, Zhang D, Wang J, Li X. The natural flavonoid galangin elicits apoptosis, pyroptosis, and autophagy in glioblastoma. Front Oncol 2019; 9:942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Z, Li X, Han W, Lu X, Jin S, Yang W, Li J, He W, Qian Y. Galangin suppresses human osteosarcoma cells: an exploration of its underlying mechanism. Oncol Rep 2017; 37:435–41 [DOI] [PubMed] [Google Scholar]
- 15.Chen D, Li D, Xu XB, Qiu S, Luo S, Qiu E, Rong Z, Zhang J, Zheng D. Galangin inhibits epithelial-mesenchymal transition and angiogenesis by downregulating CD44 in glioma. J Cancer 2019; 10:4499–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu S, Gong LS, Li NF, Pan YF, Zhang L. Galangin (GG) combined with cisplatin (DDP) to suppress human lung cancer by inhibition of STAT3-regulated NF-kappaB and bcl-2/bax signaling pathways. Biomed Pharmacother 2018; 97:213–24 [DOI] [PubMed] [Google Scholar]
- 17.Tolomeo M, Grimaudo S, Di Cristina A, Pipitone RM, Dusonchet L, Meli M, Crosta L, Gebbia N, Invidiata FP, Titone L, Simoni D. Galangin increases the cytotoxic activity of imatinib mesylate in imatinib-sensitive and imatinib-resistant Bcr-Abl expressing leukemia cells. Cancer Lett 2008; 265:289–97 [DOI] [PubMed] [Google Scholar]
- 18.Huang YC, Tsai MS, Hsieh PC, Shih JH, Wang TS, Wang YC, Lin TH, Wang SH. Galangin ameliorates cisplatin-induced nephrotoxicity by attenuating oxidative stress, inflammation and cell death in mice through inhibition of ERK and NF-kappaB signaling. Toxicol Appl Pharmacol 2017; 329:128–39 [DOI] [PubMed] [Google Scholar]
- 19.Liu R, Li H, Wei N, Tan Y. Simultaneous determination of two galangin metabolites from alpinia officinarum hance in rat plasma by UF LC-MS/MS and its application in pharmacokinetics study. PeerJ 2021; 9:e11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Guo M, Lin Z, Zhao M, Xia Y, Wang C, Xu T, Zhu B. Multifunctional selenium nanoparticles with galangin-induced HepG2 cell apoptosis through p38 and AKT signalling pathway. R Soc Open Sci 2018; 5:180509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Otake Y, Walle T. Oxidation of the flavonoids galangin and kaempferide by human liver microsomes and CYP1A1, CYP1A2, and CYP2C9. Drug Metab Dispos 2002; 30:103–5 [DOI] [PubMed] [Google Scholar]
- 22.Otake Y, Hsieh F, Walle T. Glucuronidation versus oxidation of the flavonoid galangin by human liver microsomes and hepatocytes. Drug Metab Dispos 2002; 30:576–81 [DOI] [PubMed] [Google Scholar]
- 23.Mak K-K, Tan J-J, Marappan P, Balijepalli MK, Choudhury H, Ramamurthy S, Pichika MR. Galangin’s potential as a functional food ingredient. Journal of Functional Foods 2018; 46:490–503 [Google Scholar]
- 24.Ma YL, Zhao F, Yin JT, Liang CJ, Niu XL, Qiu ZH, Zhang LT. Two approaches for evaluating the effects of galangin on the activities and mRNA expression of seven CYP450. Molecules 2019; 24:1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bloor SJ, Mitchell KA. Metabolic products of european-type propolis. Synthesis and analysis of glucuronides and sulfates. J Ethnopharmacol 2021; 274:114035. [DOI] [PubMed] [Google Scholar]
- 26.Benković G, Bojic M, Males Z, Tomic S. Screening of flavonoid aglycons' metabolism mediated by the human liver cytochromes P450. Acta Pharmaceut 2019; 69:541–62 [DOI] [PubMed] [Google Scholar]
- 27.Chen F, Tan YF, Li HL, Qin ZM, Cai HD, Lai WY, Zhang XP, Li YH, Guan WW, Li YB, Zhang JQ. Differential systemic exposure to galangin after oral and intravenous administration to rats. Chem Cent J 2015; 9:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curti V, Zaccaria V, Tsetegho Sokeng AJ, Dacrema M, Masiello I, Mascaro A, D'Antona G, Daglia M. Bioavailability and in vivo antioxidant activity of a standardized polyphenol mixture extracted from brown propolis. Ijms 2019; 20:1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su L, Chen X, Wu J, Lin B, Zhang H, Lan L, Luo H. Galangin inhibits proliferation of hepatocellular carcinoma cells by inducing endoplasmic reticulum stress. Food and chemical toxicology: an international journal published for the. Food Chem Toxicol 2013; 62:810–6 [DOI] [PubMed] [Google Scholar]
- 30.Zhang H, Li N, Wu J, Su L, Chen X, Lin B, Luo H. Galangin inhibits proliferation of HepG2 cells by activating AMPK via increasing the AMP/TAN ratio in a LKB1-independent manner. Eur J Pharmacol 2013; 718:235–44 [DOI] [PubMed] [Google Scholar]
- 31.Jung YC, Kim ME, Yoon JH, Park PR, Youn HY, Lee HW, Lee JS. Anti-inflammatory effects of galangin on lipopolysaccharide-activated macrophages via ERK and NF-kappaB pathway regulation. Immunopharmacol Immunotoxicol 2014; 36:426–32 [DOI] [PubMed] [Google Scholar]
- 32.Kim HH, Bae Y, Kim SH. Galangin attenuates mast cell-mediated allergic inflammation. Food and chemical toxicology: an international journal published for the british industrial. Food Chem Toxicol 2013; 57:209–16 [DOI] [PubMed] [Google Scholar]
- 33.Jung CH, Jang SJ, Ahn J, Gwon SY, Jeon TI, Kim TW, Ha TY. Alpinia officinarum inhibits adipocyte differentiation and high-fat diet-induced obesity in mice through regulation of adipogenesis and lipogenesis. J Med Food 2012; 15:959–67 [DOI] [PubMed] [Google Scholar]
- 34.Kumar S, Alagawadi KR. Anti-obesity effects of galangin, a pancreatic lipase inhibitor in cafeteria diet fed female rats. Pharm Biol 2013; 51:607–13 [DOI] [PubMed] [Google Scholar]
- 35.Choi JK, Kim SH. Inhibitory effect of galangin on atopic dermatitis-like skin lesions. Food and chemical toxicology: an international journal published for the British industrial. Food Chem Toxicol 2014; 68:135–41 [DOI] [PubMed] [Google Scholar]
- 36.Shu YS, Tao W, Miao QB, Lu SC, Zhu YB. Galangin dampens mice lipopolysaccharide-induced acute lung injury. Inflammation 2014; 37:1661–8 [DOI] [PubMed] [Google Scholar]
- 37.Kazemi S, Asadi F, Barari L, Morakabati P, Jahani M, Kani SNM, Soorani F, Kolangi F, Memariani Z. Quantification of flavonoids in alpinia officinarum hance. via HPLC and evaluation of its cytotoxicity on human prostate carcinoma (LNCaP) and breast carcinoma (MCF-7) cells. Acamc 2021;doi: 10.2174/1871520621666210706142157. In press. [DOI] [PubMed] [Google Scholar]
- 38.Kumar R, Tiku AB. Galangin induces cell death by modulating the expression of glyoxalase-1 and nrf-2 in HeLa cells. Chem Biol Interact 2018; 279:1–9 [DOI] [PubMed] [Google Scholar]
- 39.Zou WW, Xu SP. Galangin inhibits the cell progression and induces cell apoptosis through activating PTEN and caspase-3 pathways in retinoblastoma. Biomed Pharmacother 2018; 97:851–63 [DOI] [PubMed] [Google Scholar]
- 40.Zou Y, Li R, Kuang D, Zuo M, Li W, Tong W, Jiang L, Zhou M, Chen Y, Gong W, Liu L, Tou F. Galangin inhibits cholangiocarcinoma cell growth and metastasis through downregulation of MicroRNA-21 expression. BioMed Res Int 2020;2020:5846938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu Y, Rao Q, Zhang X, Zhou X. Galangin induced antitumor effects in human kidney tumor cells mediated via mitochondrial mediated apoptosis, inhibition of cell migration and invasion and targeting PI3K/AKT/mTOR signalling pathway. Journal of BUON: official Journal of the Balkan Union of Oncology 2018; 23:795–9 [PubMed] [Google Scholar]
- 42.Wang HX, Tang C. Galangin suppresses human laryngeal carcinoma via modulation of caspase-3 and AKT signaling pathways. Oncol Rep 2017; 38:703–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song W, Yan CY, Zhou QQ, Zhen LL. Galangin potentiates human breast cancer to apoptosis induced by TRAIL through activating AMPK. Biomed Pharmacother 2017; 89:845–56 [DOI] [PubMed] [Google Scholar]
- 44.Han MA, Lee DH, Woo SM, Seo BR, Min KJ, Kim S, Park JW, Kim SH, Choi YH, Kwon TK. Galangin sensitizes TRAIL-induced apoptosis through down-regulation of anti-apoptotic proteins in renal carcinoma caki cells. Sci Rep 2016; 6:18642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee CC, Lin ML, Meng M, Chen SS. Galangin induces p53-independent S-phase arrest and apoptosis in human nasopharyngeal carcinoma cells through inhibiting PI3K-AKT signaling pathway. Anticancer Res 2018; 38:1377–89 [DOI] [PubMed] [Google Scholar]
- 46.Zhu L, Luo Q, Bi J, Ding J, Ge S, Chen F. Galangin inhibits growth of human head and neck squamous carcinoma cells in vitro and in vivo. Chem Biol Interact 2014; 224:149–56 [DOI] [PubMed] [Google Scholar]
- 47.Murray TJ, Yang X, Sherr DH. Growth of a human mammary tumor cell line is blocked by galangin, a naturally occurring bioflavonoid, and is accompanied by down-regulation of cyclins D3, E, and A. Breast Cancer Res 2006; 8:R17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kashyap D, Garg VK, Tuli HS, Yerer MB, Sak K, Sharma AK, Kumar M, Aggarwal V, Sandhu SS. Fisetin and quercetin: promising flavonoids with chemopreventive potential. Biomolecules 2019; 9:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kashyap D, Sharma A, Tuli HS, Sak K, Mukherjee T, Bishayee A. Molecular targets of celastrol in cancer: recent trends and advancements. Crit Rev Oncol Hematol 2018; 128:70–81 [DOI] [PubMed] [Google Scholar]
- 50.Tuli HS, Aggarwal V, Kaur J, Aggarwal D, Parashar G, Parashar NC, Tuorkey M, Kaur G, Savla R, Sak K, Kumar M. Baicalein: a metabolite with promising antineoplastic activity. Life Sci 2020; 259:118183. [DOI] [PubMed] [Google Scholar]
- 51.Kashyap D, Tuli HS, Yerer MB, Sharma A, Sak K, Srivastava S, Pandey A, Garg VK, Sethi G, Bishayee A. Natural product-based nanoformulations for cancer therapy: opportunities and challenges. Semin Cancer Biol 2021; 69:5–23 [DOI] [PubMed] [Google Scholar]
- 52.Tuli HS, Tuorkey MJ, Thakral F, Sak K, Kumar M, Sharma AK, Sharma U, Jain A, Aggarwal V, Bishayee A. Molecular mechanisms of action of genistein in cancer: recent advances. Front Pharmacol 2019; 10:1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yerer MB, Dayan S, Han MI, Sharma A, Tuli HS, Sak K. Nanoformulations of coumarins and the hybrid molecules of coumarins with potential anticancer effects. Anticancer Agents Med Chem 2020; 20:1797–816 [DOI] [PubMed] [Google Scholar]
- 54.Aggarwal V, Tuli HS, Thakral F, Singhal P, Aggarwal D, Srivastava S, Pandey A, Sak K, Varol M, Khan MA, Sethi G. Molecular mechanisms of action of hesperidin in cancer: recent trends and advancements. Exp Biol Med (Maywood) 2020; 245:486–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharma A, Ghani A, Sak K, Tuli HS, Sharma AK, Setzer WN, Sharma S, Das AK. Probing into therapeutic anti-cancer potential of apigenin: recent trends and future directions. Recent Pat Inflamm Allergy Drug Discov 2019; 13:124–33 [DOI] [PubMed] [Google Scholar]
- 56.Aggarwal V, Tuli HS, Tania M, Srivastava S, Ritzer EE, Pandey A, Aggarwal D, Barwal TS, Jain A, Kaur G, Sak K, Varol M, Bishayee A. Molecular mechanisms of action of epigallocatechin gallate in cancer: recent trends and advancement. Semin Cancer Biol 2020;579X–20 [DOI] [PubMed] [Google Scholar]
- 57.Yang Y, Ren L, Yang H, Ge B, Li W, Wang Y, Wang H, Du G, Tang B, Wang J. Research progress on anti-angiogenesis drugs in hepatocellular carcinoma. Cancer Plus 2021; 3: [Google Scholar]
- 58.Huang H, Chen AY, Rojanasakul Y, Ye X, Rankin GO, Chen YC. Dietary compounds galangin and myricetin suppress ovarian cancer cell angiogenesis. J Funct Foods 2015; 15:464–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lei D, Zhang F, Yao D, Xiong N, Jiang X, Zhao H. Galangin increases ERK1/2 phosphorylation to decrease ADA. M9 Expression and prevents invasion in A172 glioma cells. Molecular Medicine Reports 2018; 17:667–73 [DOI] [PubMed] [Google Scholar]
- 60.Tang X, Xu C, Yagiz Y, Simonne A, Marshall MR. Phytochemical profiles, and antimicrobial and antioxidant activities of greater galangal [alpinia galanga (linn.) swartz.] flowers. Food Chemistry 2018; 255:300–8 [DOI] [PubMed] [Google Scholar]
- 61.Meyer JJ, Afolayan AJ, Taylor MB, Erasmus D. Antiviral activity of galangin isolated from the aerial parts of helichrysum aureonitens. J Ethnopharmacol 1997; 56:165–9 [DOI] [PubMed] [Google Scholar]
- 62.Chien ST, Shi MD, Lee YC, Te CC, Shih YW. Galangin, a novel dietary flavonoid, attenuates metastatic feature via PKC/ERK signaling pathway in TPA-treated liver cancer HepG2 cells. Cancer Cell Int 2015; 15:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu H, Yao H, Zou R, Chen X, Xu H. Galangin suppresses renal inflammation via the inhibition of NF-kappaB, PI3K/AKT and NLRP3 in uric acid treated NRK-52E tubular epithelial cells. BioMed Res Int 2019;3018357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sulaiman GM. Molecular structure and anti-proliferative effect of galangin in HCT-116 cells: in vitro study. Food Sci Biotechnol 2016; 25:247–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jullian C. Improvement of galangin solubility using native and derivative cyclodextrins: an UV-Vis and NMR study. J Chil Chem Soc 2009; 54:201–3 [Google Scholar]
- 66.Zhang HT, Luo H, Wu J, Lan LB, Fan DH, Zhu KD, Chen XY, Wen M, Liu HM. Galangin induces apoptosis of hepatocellular carcinoma cells via the mitochondrial pathway. World J Gastroenterol 2010; 16:3377–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang W, Lan Y, Huang Q, Hua Z. Galangin induces B16F10 melanoma cell apoptosis via mitochondrial pathway and sustained activation of p38 MAPK. Cytotechnology 2013; 65:447–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Szliszka E, Czuba ZP, Bronikowska J, Mertas A, Paradysz A, Krol W. Ethanolic extract of propolis augments TRAIL-Induced apoptotic death in prostate cancer cells. Evid Based Complement Alternat Med 2011; 2011:535172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Donovan MG, Selmin OI, Romagnolo DF. Galangin is an epigenetic modulator or BRCA1 and induces estrogen receptor alpha in triple negative breast cancer cells linked to antagonism toward aryl hydrocarbon receptor. Nutrients 11:2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li X, Wang Y, Xiong Y, Wu J, Ding H, Chen X, Lan L, Zhang H. Galangin induces autophagy via deacetylation of LC3 by SIRT1 in HepG2 cells. Sci Rep 2016; 6:30496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zeng H, Huang P, Wang X, Wu J, Wu M, Huang J. Galangin-induced down-regulation of BACE1 by epigenetic mechanisms in SH-SY5Y cells. Neuroscience 2015; 294:172–81 [DOI] [PubMed] [Google Scholar]
- 72.Tuli HS, Kashyap D, Sharma AK, Sandhu SS. Molecular aspects of melatonin (MLT)-mediated therapeutic effects. Life Sci 2015; 135:147–57 [DOI] [PubMed] [Google Scholar]
- 73.Maqueda LB, Falcon R, Tsai CY, Garcia-Perez A, Minasyan A, Gonzalez-Rivas D. Current role of uniportal video-assisted thoracic surgery for lung cancer treatment. J Clin Transl Res 2020; 6:135–44 [PMC free article] [PubMed] [Google Scholar]
- 74.Matilla JM, Zabaleta M, Martinez-Tellez E, Abal J, Rodriguez-Fuster A, Hernandez-Hernandez J. New TNM staging in lung cancer (8(th) edition) and future perspectives. Journal of Clinical and Translational Research 2020; 6:145–54 [PMC free article] [PubMed] [Google Scholar]
- 75.Wang Z, Dai H, Su H, Lai S, Dai L, Liu X, Wang Y, Shu G, Tang B, Li Y. Research progress on the role of enzymes involved in histone methylation in hepatocellular carcinoma. Cancer Plus 2021; 22:775 [Google Scholar]
- 76.Ren K, Zhang W, Wu G, Ren J, Lu H, Li Z, Han X. Synergistic anti-cancer effects of galangin and berberine through apoptosis induction and proliferation inhibition in oesophageal carcinoma cells. Biomed Pharmacother 2016; 84:1748–59 [DOI] [PubMed] [Google Scholar]
- 77.Sinha R, Srivastava S, Joshi A, Joshi UJ, Govil G. In-vitro anti-proliferative and anti-oxidant activity of galangin, fisetin and quercetin: role of localization and intermolecular interaction in model membrane. Eur J Med Chem 2014; 79:102–9 [DOI] [PubMed] [Google Scholar]
- 78.Cipak L, Rauko P, Miadokova E, Cipakova I, Novotny L. Effects of flavonoids on cisplatin-induced apoptosis of HL-60 and L1210 leukemia cells. Leuk Res 2003; 27:65–72 [DOI] [PubMed] [Google Scholar]
- 79.Al-Shammari AM, Al-Saadi H, Al-Shammari SM, Jabir MS. Galangin enhances gold nanoparticles as anti-tumor agents against ovarian cancer cells. AIP ConferenceProceedings 2020; 2213:020206 [Google Scholar]
- 80.Ye W, Sun W, Chen R, Wang Z, Cui X, Zhang H, Qian S, Zheng Q, Zhou Y, Wan J. Pharmacokinetics in rat plasma and tissue distribution in mice of galangin determined by UHPLC–MS/MS. Acta Chromatographica 2019; 31:120–5 [Google Scholar]
- 81.Jia X, Du Y, Xu J, Dong Y. Comparative pharmacokinetic study of five flavonoids in normal rats and rats with gastric ulcer following oral administration of mongolian medicine, shudage-4 by UPLC–ESI–MS/MS. Trop J Pharm Res 2020; 19:651–9 [Google Scholar]
- 82.de Lazaro I, Mooney DJ. Obstacles and opportunities in a forward vision for cancer nanomedicine. Nat Mater 2021; 20:1469–79 [DOI] [PubMed] [Google Scholar]
- 83.Cote B, Rao D, Alani AWG. Nanomedicine for drug delivery throughout the alimentary canal. Mol Pharmaceut 2021. [DOI] [PubMed] [Google Scholar]
- 84.Lakshmanan VK, Jindal S, Packirisamy G, Ojha S, Lian S, Kaushik A, Alzarooni A, Metwally YAF, Thyagarajan SP, Do Jung Y, Chouaib S. Nanomedicine-based cancer immunotherapy: recent trends and future perspectives. Cancer Gene Ther 2021; 28:911–23 [DOI] [PubMed] [Google Scholar]
- 85.Boix-Montesinos P, Soriano-Teruel PM, Arminan A, Orzaez M, Vicent MJ. The past, present, and future of breast cancer models for nanomedicine development. Adv Drug Deliv Rev 2021; 173:306–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yao H, Lu H, Zhang J, Xue X, Yin C, Hu J, Zou R, Wang L, Xu H. Preparation of prolonged-circulating galangin-loaded liposomes and evaluation of antitumor efficacy in vitro and pharmacokinetics in vivo. Journal of Nanomaterials 2019; 7236895: 10.1155/2019/7236895 [DOI] [Google Scholar]
- 87.Zhu J, Wang Q, Li H, Zhang H, Zhu Y, Omari-Siaw E, Sun C, Wei Q, Deng W, Yu J. Galangin-loaded, liver targeting liposomes: optimization and hepatoprotective efficacy. Journal of Drug Delivery Science and Technology 2018; 46:339–47 [Google Scholar]
- 88.Hajipour H, Nouri M, Ghorbani M, Bahramifar A, Emameh RZ, Taheri RA. Targeted nanostructured lipid carrier containing galangin as a promising adjuvant for improving anticancer effects of chemotherapeutic agents. Naunyn Schiedebergs Arch Pharmacol 2021;394:2353–62 [DOI] [PubMed]
- 89.Aloud AA, Veeramani C, Govindasamy C, Alsaif MA, El Newehy AS, Al-Numair KS. Galangin, a dietary flavonoid, improves antioxidant status and reduces hyperglycemia-mediated oxidative stress in streptozotocin-induced diabetic rats. Redox Rep 2017; 22:290–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu C, Ma M, Zhang J, Gui S, Zhang X, Xue S. Galangin inhibits human osteosarcoma cells growth by inducing transforming growth factor-beta1-dependent osteogenic differentiation. Biomed Pharmacother 2017; 89:1415–21 [DOI] [PubMed] [Google Scholar]
- 91.Choi YJ, Lee YH, Lee ST. Galangin and kaempferol suppress phorbol-12-myristate-13-acetate-induced matrix metalloproteinase-9 expression in human fibrosarcoma HT-1080 cells. Mol Cells 2015; 38:151–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Y, Lin B, Li H, Lan L, Yu H, Wu S, Wu J, Zhang H. Galangin suppresses hepatocellular carcinoma cell proliferation by reversing the warburg effect. Biomed Pharmacother 2017; 95:1295–300 [DOI] [PubMed] [Google Scholar]
- 93.Wang Y, Wu J, Lin B, Li X, Zhang H, Ding H, Chen X, Lan L, Luo H. Galangin suppresses HepG2 cell proliferation by activating the TGF-beta receptor/smad pathway. Toxicology 2014; 326:9–17 [DOI] [PubMed] [Google Scholar]
- 94.Kim DA, Jeon YK, Nam MJ. Galangin induces apoptosis in gastric cancer cells via regulation of ubiquitin carboxy-terminal hydrolase isozyme L1 and glutathione S-transferase P. Food Chem Toxicol 2012; 50:684–8 [DOI] [PubMed] [Google Scholar]
- 95.Kwak MK, Yang KM, Park J, Lee S, Park Y, Hong E, Sun EJ, An H, Park S, Pang K, Lee J, Kang JM, Kim P, Ooshima A, Kim SJ. Galangin enhances TGF-beta1-mediated growth inhibition by suppressing phosphorylation of threonine 179 residue in Smad3 linker region. Biochem Biophys Res Commun 2017; 494:706–13 [DOI] [PubMed] [Google Scholar]
- 96.Tomar A, Vasisth S, Khan SI, Malik S, Nag TC, Arya DS, Bhatia J. Galangin ameliorates cisplatin induced nephrotoxicity in vivo by modulation of oxidative stress, apoptosis and inflammation through interplay of MAPK signaling Cascade. Phytomedicine 2017; 34:154–61 [DOI] [PubMed] [Google Scholar]