Abstract
Chemogenetics enables precise, non-invasive, and reversible modulation of neural activity via the activation of engineered receptors that are pharmacologically selective to endogenous or exogenous ligands. With recent advances in therapeutic gene delivery, chemogenetics is poised to support novel interventions against neuropsychiatric diseases and disorders. To evaluate its translational potential, we performed a scoping review of applications of chemogenetics that led to the reversal of molecular and behavioral deficits in studies relevant to neuropsychiatric diseases and disorders. In this review, we present these findings and discuss the potential and challenges for using chemogenetics as a precision medicine-based neuromodulation strategy.
Keywords: chemogenetics, DREADDs, neuromodulation, neurotherapeutics, gene delivery, gene therapy
Graphical abstract
Recent advances in neurological gene therapy enable delivery of chemogenetic receptors for pharmacologically controlled neuromodulation. Song et al. perform a scoping review of all existing applications of chemogenetic systems in models of neuropsychiatric diseases and disorders to reveal major areas of consideration for future translation.
Introduction
Continuous advances in genetic targeting, protein engineering, and the ever-expanding knowledge of the relationship between neural circuits and behavior hold considerable promise for the development of novel precision medicine-based therapeutics. Many existing gene therapy applications focus on the targeted overexpression of a specific gene with known mechanistic involvement in disease. However, the current mechanistic understanding of many classes of neuropsychiatric diseases and disorders is not sufficient for rationalizing therapeutic strategies based on functional gene transfer, and an alternative approach may involve circuit modulation-based approaches via the direct stimulation of engineered receptors targeted to the cell surface.
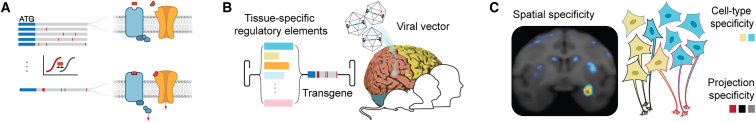
Chemogenetic technologies modulate targeted cell populations through engineered receptors or channels that are pharmacologically selective for specific ligands.1 The selectivity between the engineered receptor/channel-ligand pair enables their reversible and titratable activation and the subsequent modulation of cells expressing such receptors in the local or systemic presence of the ligand. Using viral vectors to deliver genetic cargo that encodes such engineered receptors or channels restricts the direct stimulation to a discrete molecular and/or anatomically defined neural population by introducing unique regulatory elements to the gene expression cassette. These properties have rendered chemogenetics powerful tools to causally examine neural correlates of behavior and disease in basic or preclinical experimental disease models. Importantly, the pharmacological requirement for neuromodulation via chemogenetics has generated interest in the engineering of such receptor/channel-ligand systems into therapeutic tools for precision medicine-based therapeutics (Figure 1).
Figure 1.
Principles of precision neuromodulation through chemogenetics
(A) Using either site-directed mutagenesis or directed evolution, candidate signaling receptors are modified to exhibit elevated or diminished affinities for cognate ligands. Ligands are either exogenous, pharmacologically inert small molecules or endogenous signaling molecules. (B) Chemogenetic receptors are delivered to the brain using viral vectors. Receptor expression is restricted by the localized infusion of the viral vector and tissue-specific regulatory elements of gene expression. (C) Therapeutic neuromodulation is achieved through reversible and titratable signaling events mediated by the chemogenetic receptors in the presence of cognate chemogenetic ligands, with therapeutic efficacy and safety contingent upon neuromodulation of specific brain regions, cell types, and/or circuits. Receptor expression is non-invasively and longitudinally monitored by positron emission tomography.
To better understand the current level of evidence for the therapeutic potential of chemogenetics and its promise for potential therapeutic applications, we performed a scoping review of basic and preclinical studies that applied chemogenetic technologies and demonstrated therapeutic benefit in various models of neuropsychiatric diseases and disorders. Given the versatility of chemogenetics, we chose to focus on clinical abnormalities associated with the nervous system, which spans diseases for which causality is established and disorders for which there exist defined clinical presentations (e.g., neuropathic pain). In this review we provide a primer to chemogenetics, present findings from the scoping review, and discuss the concept of chemogenetics as a clinical method and the challenges to such an end.
Primer to chemogenetics
Chemogenetic technologies developed to date modulate cellular activity through metabotropic and ionotropic signaling. The metabotropic approach employs engineered G-protein-coupled receptors (GPCRs) that activate effector proteins to initiate downstream signaling cascades, whereas the ionotropic approach employs engineered ligand-gated ion channels to directly modulate membrane ion conductance. While these chemogenetic technologies have been extensively reviewed elsewhere,2 recent technological developments in chemogenetics, especially in relation to the translational potential of the technology, necessitate a revised and updated primer. Chemogenetics has been traditionally defined to utilize exogenous small molecules for receptor activation. Given recent engineering of receptors that utilize endogenous ligands and, separately, genetically encoded peptides for neuromodulation,3,4 we define chemogenetics to include any engineered receptor-ligand system that modulates cell function via altered ligand specificity and ligand affinity. This definition is agnostic to the identity and source of the ligand and reconciles differences among existing chemogenetic systems.
Metabotropic systems
The earliest metabotropic chemogenetic systems originated from efforts to study GPCR signaling in mammalian neurons. The expression of a Gi-coupled allatostatin receptor from Drosophila melanogaster in mammalian neurons led to complete and reversible neuronal silencing via allatostatin administration.5,6 Alternatively, site-directed mutagenesis of human GPCRs has led to a family of receptors activated solely by synthetic ligands (RASSLs).7 Due to issues with blood-brain barrier (BBB) penetrance by allatostatin and off-targeted interactions by both ligands and receptors observed in RASSL systems, these systems were insufficiently generalizable for neuromodulatory functions beyond their original purposes.6,8,9
To overcome such a challenge, some members of the RASSLs family were engineered into designer receptors exclusively activated by designer drugs (DREADDs).7 Despite divergent nomenclature, “synthetic ligand” in RASSLs and “designer drugs” in DREADDs both refer to cognate small-molecule actuators of these systems. Systems derived from human muscarinic (hM) receptors, hM DREADDs, are the most ubiquitously applied tools to date. hM DREADDs are products of directed evolution of hM receptors, whereby large libraries of mutant receptors were screened for selective activation by clozapine (CZP) and clozapine N-oxide (CNO).10,11 The Gq-coupled hM3 DREADD (hM3D) and the Gi/o-coupled hM4 DREADD (hM4D), two most common systems, leverage the associated G-protein signaling pathway to promote and suppress neuronal excitability, respectively.10 Both hM3D and hM4D exhibit well-tolerated expression by tissues of the nervous system across rodent and non-human primate (NHP) models.12 Furthermore, dopaminergic neurons induced from human pluripotent stem cells that express hM3D or hM4D exhibit CNO-dependent depolarization or hyperpolarization, respectively, with no changes to resting membrane potential in the absence of CNO.13
The translational potential of hM DREADDs has been challenged by findings of metabolic conversion from CNO to CZP and the off-targeted pharmacological actions by both molecules in vivo. Extensive studies now support the notion that (1) CZP much more effectively penetrates the BBB and accumulates in brain tissue than does CNO, (2) CZP has greater potency for hM DREADDs than does CNO, and (3) the reverse metabolism of CNO into CZP following peripheral administration of CNO is primarily responsible for hM DREADD activation.14, 15, 16, 17 Both CNO and CZP have displayed affinity for a wide class of endogenous receptors,15,18,19 and both drugs can induce significant behavioral effects, e.g., locomotion, anxiety, and cognitive changes, in the absence of hM DREADD expression in rats.17,20
Given the requirement of high receptor-ligand selectivity, new DREADD agonists were recently developed. Novel CZP analogs such as JHU37160 (J60) have been shown to display higher brain penetrance and to selectively drive hM DREADD-modulated behaviors in mice at the same or lower effective dosages than that of CZP.21 More recently, deschloroclozapine (DCZ) was characterized as a potent and selective DREADD activator in mice and NHP with reduced affinity for off-target receptors.22,23 Olanzapine (OZP) was also identified as a Food and Drug Administration (FDA)/European Medicines Agency (EMA)-approved drug with structural and electrochemical similarities to CZP.24 Notably, its potency at hM4D in vitro was reportedly 20 times greater than that of CZP. Collectively, these new generations of DREADD actuators may necessitate lower effective dosages than those of CZP and CNO to enable precise neuromodulation without off-targeted effects. However, in terms of translational potential, use of FDA-approved medications such as CZP and OZP would hold the greatest promise, as DCZ and J60 would require extensive safety testing and FDA approval. Regardless of the ligand used, ligand-dependent off-targeted effects would require careful assessment in future preclinical and clinical applications.
Beyond hM DREADDs, rationally site-directed mutagenesis of the Gi-coupled human κ opioid receptor (hKOR) has generated KOR DREADDs (KORD). These mutations abolish the affinity for the endogenous ligand, dynorphin A (DynA), and increase the affinity for salvinorin B (SalB).25 The SalB-KORD system has been validated in mice to demonstrate dose-dependent behavioral effects in locomotion and food intake after peripheral SalB administration.25,26 However, despite pharmacokinetic and pharmacodynamic characterizations of SalB, which support its use for acute chemogenetic studies,27 evaluations of its off-targeted effects are required to evaluate SalB's potential for clinical applications. Beyond KORD, modifications of other human GPCRs have generated several other DREADDs systems, although they have much fewer applications in animal models and all lack pharmacological characterization to the extent similar to those on the panel of hM DREADD agonists.28,29 Nonetheless, these examples demonstrate that mutagenic and chimera-based engineering can expand the repertoire of metabotropic receptors to modulate not only neuronal excitability but also other signaling pathways.
Ionotropic systems
The early generation of ionotropic chemogenetic receptors combined ligand-binding domains (LBDs) and ion pore domains (IPDs) across members of the Cys-loop receptor family.30 This comprises the largest family of ionotropic channels in the nervous system whose LBDs and IPDs are coupled by flexible loops, which allow structural and functional chimeras of various LBDs and IPDs.31 Traditionally, the LBD of the nicotinic acetylcholine receptor (nAchR) was fused with excitatory (cation-specific) and inhibitory (chloride-specific) IPDs. However, these simple modular combinations still retained sensitivity to endogenous acetylcholine.
Current ionotropic chemogenetic systems overcome the challenge of acetylcholine sensitivity through site-directed mutagenesis of the nAchR LBD, yielding pharmacologically selective actuator modules (PSAMs) that are activated by pharmacologically selective effector molecules (PSEMs).30 Fusion of PSAMs with various functional IPDs generates chimeric ion channels capable of potent, reversible, and titratable excitation and inhibition of targeted neurons via direct electrochemical changes to the membrane potential. To date, four PSAMs have been developed (PSAM-A/B/C/4) (Table 1).30,32 Each PSAM is exclusively activated by a panel of PSEMs, permitting orthogonal combinations of PSAM/PSEM systems for multiplexed control of targeted cells.33 The current repertoire of functional IPDs fused with PSAMs includes (1) an excitatory IPD from a serotonergic receptor, (2) an inhibitory IPD from a glycinergic receptor, and (3) a calcium-specific IPD from a cholinergic receptor. Further mutagenesis of IPDs has generated derivatives that exhibit elevated single-channel conductance or attenuated sensitization for prolonged activation, both of which are features that enable more robust modulation of targeted cells.30
Table 1.
Overview of chemogenetic systems
| Type | Receptor | Genesis | Signaling mechanism | Agonist |
|---|---|---|---|---|
| Metabotropic | hM3D | directed evolution of hM3 for affinity for CNO/CZP | Gq cascade | DREADD activators |
| hM4D | directed evolution of hM4 for affinity for CNO/CZP | Gi/o cascade | DREADD activators | |
| rM3D | chimeric product of rM3Dq and β1-adrenergic receptor | Gs cascade | DREADD activators | |
| KORD | modified hKOR (D138N) for increased affinity for SalB | Gi cascade | SalB | |
| Beta arrrestin DREADD | modified hM3Dq that switches Gq with β-arrestin | β-arrestin cascade | DREADD activators | |
| Ionotropic | PSAM-A-5HT | chimeric product of a modified α7 LBD (L141F,Y115F) and the 5-HT3 IPD | increased membrane cation conductance | PSEM89S, PSEM308 |
| PSAM-A-GlyR | chimeric product of a modified α7 LBD (L141F,Y115F) and the GlyR chloride-selective IPD | increased membrane chloride conductance | PSEM89S, PSEM308 | |
| PSAM-B-5HT | chimeric product of a modified α7 LBD (Q79G,Q139G) and the 5-HT3 cation-selective IPD | increased membrane cation conductance | PSEM22S | |
| PSAM-C-ɑ7 | chimeric product of a modified α7 LBD (Q79G,L141S) and the α7 calcium-selective IPD | increased membrane calcium conductance | PSEM9S | |
| PSAM4-GlyR | chimeric product of a modified α7 LBD (L131G,Q139L,Y217F) and the GlyR chloride-selective IPD | increased membrane chloride conductance | varenicline, uPSEMs | |
| GluCl | chloride-specific ion channel sourced from C. elegans | increased membrane chloride conductance | ivermectin, glutamate | |
| eGluCl | modified eGluCl (L9′F) for increased glutamate sensitivity | increased membrane chloride conductance | glutamate |
Descriptions of currently available chemogenetic systems, which include any engineered receptor-ligand system that modulates cell function via altered ligand specificity and affinity. DREADD, designer receptors exclusively activated by designer drugs; hM3D, human muscarinic 3 DREADD; hM4D, human muscarinic 4 DREADD; rM3D, rat muscarinic 3 DREADD; KORD, κ-opioid receptor DREADD; hKOR, human κ-opioid receptor; PSAM, pharmacologically selective activator modules; LBD, ligand-binding domain; IPD, ion pore domain; PSEM, pharmacologically selective effector modules; GlyR, glycine receptor; uPSEMs, ultrapotent PSEMs; GluCl, glutamate-gated chloride channels; eGluCl, enhanced GluCl.
The latest PSAM/PSEM system features PSAM4 along with a panel of cognate ligands that includes ultrapotent PSEMs (uPSEMs) and the FDA/EMA-approved anti-smoking drug varenicline.32 While varenicline is clinically approved for smoking cessation as an nAchR agonist, it displays ∼630-fold greater affinity for PSAM4 than for the nAchR LBD, which allows a dosage of varenicline for effective PSAM4 activation far lower than that required for nAchR agonism. Intraperitoneal and oral delivery of varenicline effectively induces behaviors driven by an inhibitory PSAM4 receptor in mice, and subcutaneous delivery of varenicline induces neurophysiological changes in adult rhesus monkeys. Due to varenicline's off-targeted effects on food intake in mice at elevated doses, likely due to activation of endogenous 5-HT3 receptors,34 synthetic varenicline analogs have been generated and display sub-nanomolar potency at PSAM4 and no off-targeted effects on food intake analogous to those induced by varenicline.32 Applications of the uPSEMs/PSAM4-GlyR systems in mice show sustained inhibition of CA1 hippocampal neurons and substantia nigral neurons via intraperitoneal administration of uPSEMs.32
Beyond PSAM-based systems, two ivermectin-sensitive chloride channels, GluCl, a chloride channel from Caenorhabditis elegans, and the engineered human α1 glycine receptor can inhibit mammalian neurons in vivo.3,35, 36, 37, 38 Interestingly, GluCl is also further engineered to acquire higher affinity for its endogenous ligand, glutamate, as a strategy to autoregulate elevated extrasynaptic glutamate in rodent models for focal-onset epilepsy.3
Similar to DREADDs, ionotropic chemogenetic systems are designed to be modular and generalizable across the nervous system. However, ionotropic systems are dependent on the local electrochemical gradient, which may drastically differ from canonical homeostatic levels. Particularly, the chloride electrochemical gradient is known to reverse during neural development,39 and PSAM4-GlyR was shown to be excitatory rather than inhibitory in the mouse striatum.40 Nonetheless, the engineering of ion channels to directly target pathological electrochemical mechanisms, such as neuronal hyperexcitability in epilepsy, has been a promising approach for chemogenetic design.
Scoping review
To identify the current level of evidence for the therapeutic potential of chemogenetics and inform the design of future therapeutic approaches, we performed a scoping review for studies up until May 1, 2021, which applied chemogenetic tools in models of neuropsychiatric disease and disorders and observed reduction of relevant phenotypes (Figure 2).
Figure 2.
PRISMA diagram of the scoping review
Results from an initial search of PubMed with keywords—("gene therapy" OR "gene transfer" OR viral-vector OR Chemogenetics OR Pharmaco-genetic OR DREADD OR hM3D OR hM4D OR PSAM OR PSEM) AND (model)—for original articles published before May 1, 2021, were combined with author contributions. Two authors independently screened study titles and abstracts to select relevant articles. The full texts of relevant articles were assessed to determine article eligibility based on three criteria, which required (i) application(s) of chemogenetic tools in (ii) model(s) of neuropsychiatric diseases and disorders that (iii) reduced disease-/disorder-associated phenotypes. Differences between the two authors were arbitrated by a third author.
Analysis of 171 included studies identified 239 individual experiments that demonstrated chemogenetic-dependent attenuation of disease-associated phenotypes across models of epilepsy, neurodegeneration, pain, substance use disorders, developmental disorders, psychiatric disorders, homeostatic disorders, and trauma (Figure 2; Tables 2 and S1). Included experiments were broadly distributed between two types: (1) preclinical investigations that primarily aim to assess therapeutic efficacy and (2) basic neuroscience studies that apply chemogenetics as a line of evidence for the identification of neural correlates of disease-associated behaviors. Given the heterogeneity of the experiments and the early stage of chemogenetics as a therapeutic approach, we do not distinguish between these types, with interest for the latter drawing from the perception that chemogenetics-dependent reduction of disease phenotypes simultaneously implicates the role of the targeted cells in such phenotypes as well as the potential of the chemogenetic tool itself as a therapeutic approach.
Table 2.
Summary of included experiments
| Category | Count (%) |
|---|---|
| Modelled disease category | |
| Pain | 54 (22.6) |
| Psychiatric Disorders | 44 (18.4) |
| Neurodegeneration | 35 (14.6) |
| Epilepsy | 34 (14.2) |
| Substance Use Abuse | 32 (13.4) |
| Developmental Disorders | 20 (8.4) |
| Homeostatic disorders | 11 (4.6) |
| Trauma | 9 (3.8) |
| Model type | |
| Mouse (In vivo) | 170 (71.1) |
| Rat (In vivo) | 59 (24.7) |
| Mouse (Ex vivo) | 4 (1.7) |
| Mouse (In vitro) | 2 (0.8) |
| Rats (Ex vivo) | 1 (0.4) |
| Rat (In vitro) | 1 (0.4) |
| Human (In vitro) | 1 (0.4) |
| NHP (In vivo) | 1 (0.4) |
| Receptors/ligand system | |
| hM3Dq / CNO | 111 (46.4) |
| hM4Di / CNO | 103 (43.1) |
| PSAM-A-5HT / PSEM308 | 6 (2.6) |
| hM4Di / CZP | 4 (1.7) |
| KORD / SalB | 4 (1.7) |
| GluCl / Ivermectin | 2 (0.8) |
| eGlucl / Endogenous glutamate | 2 (0.8) |
| PSAM-A-GlyR / PSEM308 | 1 (0.4) |
| hM3Dq / CZP | 1 (0.4) |
| hM4Di / OZP | 1 (0.4) |
| hM3Dq / J60 | 1 (0.4) |
| PSAM-A-5HT / PSAM308 | 1 (0.4) |
| PSAM-A-5HT / PSAM89S | 1 (0.4) |
| hM4Di / C21 | 1 (0.4) |
| Receptor delivery | |
| AAV vector | 211 (88.3) |
| Animal transgenic knock-in | 18 (7.5) |
| Lentiviral vector | 4 (1.7) |
| Rabies viral vector | 3 (1.3) |
| Adenoviral vector | 3 (1.3) |
| Ligand delivery | |
| Intraperitoneal | 187 (78.2) |
| Intracranial infusion | 13 (5.4) |
| Subcutaneous | 12 (5.0) |
| Media bath (ex vivo) | 10 (4.2) |
| Osmotic pump | 5 (2.1) |
| Intramuscular | 3 (1.3) |
| Drinking water, ad libitum | 3 (1.3) |
| Intrathecal | 2 (0.8) |
| None (endogenous ligand) | 2 (0.8) |
| Intravenous | 1 (0.4) |
| Microinjection | 1 (0.4) |
| Ligand off-targeted effects investigated | |
| Not studied | 134 (56.1) |
| Studied, no off-target effects | 103 (43.1) |
| Studied, off-target effects reported | 2 (0.8) |
| Cell-type specificity | |
| Cell-type specific | 195 (81.6) |
| Pan-neuronal | 36 (15.1) |
| Non-specific | 8 (3.3) |
| Cre-dependent | 133 (55.6) |
| Cre-independent | 106 (44.4) |
| Publications by year | |
| 2021 (up to May 2021) | 23 (9.6) |
| 2020 | 52 (21.8) |
| 2019 | 51 (21.3) |
| 2018 | 45 (18.8) |
| 2017 | 28 (11.7) |
| 2016 | 19 (7.9) |
| 2015 | 11 (4.6) |
| 2014 | 8 (3.3) |
| 2013 | 2 (0.8) |
To capture the diversity of existing demonstrations of chemogenetics in disease models, we briefly summarize experimental approaches and outcomes for each category of neurological disorders (unless specified, all named regions of the nervous system refer to those in rodent models). We then discuss prospects and challenges to the future translation of chemogenetic tools.
Focal-onset epilepsy
Given its ability to modulate neuronal excitability, chemogenetics has supported a diverse set of strategies in rodent models of focal-onset epilepsy. Focal-onset epilepsy arises from specific brain regions, such as the frontal and temporal lobes, that can be identified by imaging and electrophysiological techniques.41 The mechanism behind ictogenesis is attributed to pathological hyperexcitability of epileptogenic zones that propagates across local and global neural circuits.42 Chemogenetic inhibition of excitatory neurons and excitation of inhibitory neurons, therefore, become compelling approaches at both ictogenic and propagated zones. Indeed, targeting both strategies to cortical, hippocampal, and thalamic neurons in epileptic rodent models reduces chronic and acute convulsive behaviors and electrographic markers.43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53 The ictogenic mechanism has further rationalized autoregulatory approaches to attenuating neuronal excitability. In one study, engineering a glutamate-gated chloride channel for sensitivity against elevated extrasynaptic glutamate, a molecular signature of hyperexcitability, generated an inhibitory ion channel that was preferentially active during ictogenesis.3
Neurodegeneration
Chemogenetic modulation at cortical, subcortical, and spinal levels also reduced molecular and behavioral deficits of neurodegenerative disorders. In a mouse model for amyotrophic lateral sclerosis (ALS), DREADD-driven excitation of motor neurons and inhibition of interneurons in the lumbar spinal cord reduced biochemical markers of ALS,54 while excitation of interneurons in the motor cortex improved motor functions.55 Modulation of astrocytes has also shown benefit in ALS models.56 In models of tau- and amyloid β-associated pathologies, inhibition of neuronal activity led to reduced amyloid and tau deposition,57 and DREADD-mediated inhibition of calcium-specific signaling was sufficient to decrease intracellular and extracellular misfolded tau in induced pluripotent stem cells from patients with frontotemporal lobar dementia.58 DREADD-mediated inhibition of excitatory neurons in the spinal cord attenuated motor deficits and decreased demyelination in a multiple sclerosis model.59 In rodent models of Parkinson's disease (PD), DREADD-mediated modulations of striatal neurons differentially engaged diverse circuits to restore motor function and potentiate effects of existing pharmacological interventions for PD.60, 61, 62, 63, 64, 65, 66, 67 Bidirectional DREADD modulation of dopaminergic neurons grafted to the striatum enabled regulation of dopaminergic tone.13,68,69
Neuropathic pain
Neuropathic pain is a highly heterogeneous condition that represents either discrete disorders or comorbidities with inflammatory and psychiatric disorders. Neural representations of pain have been characterized across multiple levels of the neuraxis in a hierarchical manner.70 Current demonstrations of chemogenetic modulation target several regulatory nodes, from peripheral sensory neurons to higher-order cognitive nuclei. PSAM- and DREADD-mediated modulation of the locus coeruleus,71 paraventricular thalamus,72 and the periaqueductal gray has been shown to reduce hyperalgesia in rodent models.73 DREADD-mediated excitation of specific circuits including the medial prefrontal cortex,74 medial septum,75 hippocampus,75 and the paraventricular thalamus attenuated visceral pain.74 Both neuronal and astrocytic populations have been targeted for reduction of allodynia,76, 77, 78 Modulation of neurons in the ventral medial prefrontal cortex (vmPFC), ventrolateral orbital cortex, and cingulate cortices reduced pain-induced anxiety.79, 80, 81 Beyond cortical and subcortical manipulations, peripheral chemogenetic modulation at the level of the spinal cord and other areas posed interesting possibilities for pain-management strategies. DREADD-mediated inhibition of spinal microglia reduced allodynia.82,83 In addition to inhibition of spinal sensory neurons that showed analgesic effects in mice,84 inhibition of specific subtypes of dorsal root ganglion neurons reduced hyperalgesia and allodynia, and increased the threshold for pain across six independent studies—with subtypes defined by either molecularly defined cell types or neurons innervating targeted peripheral regions.85, 86, 87, 88, 89, 90
Neurodevelopmental disorders
Neurodevelopmental disorders in rodent models are largely generated by genetic mutations that partially recapitulate behavioral and molecular deficits observed in human patients. Chemogenetic manipulations across such models primarily aim to rescue deficits observed through experimental tasks that interrogate cognitive performance. In models of autism spectrum disorder (ASD), activation of specific neuronal populations in the striatum,91 cerebellum,92 nucleus accumbens,93 medial preoptic area,94 and, particularly, oxytocin neurons in the hypothalamus rescued social deficits.95,96 Additional studies in models of Down syndrome,97 Fragile X syndrome,98 Rett syndrome,99 fetal alcohol syndrome,100 and 22q11.2 deletion syndrome further suggested that cognitive and behavioral deficits are reversible upon targeted neuromodulation.101 Interestingly, chemogenetic manipulations of local circuits via excitation of inhibitory interneurons were sufficient to rescue deficits associated with multiple models of schizophrenia, with targeted brain regions including the hippocampus, hypothalamus, ventral tegmental area, and medial prefrontal cortex (mPFC).102, 103, 104, 105, 106, 107
Substance use disorders
The study of substance use disorders via rodent models focuses on specific circuits known to be impacted by drug exposure within the central nervous system (CNS), and various chemogenetic modulations of distinct nodes and pathways within these circuits were sufficient to reduce pathological reward-seeking behavior. In rodent models of alcohol overconsumption, chemogenetic inhibition of the mPFC,108 amygdala,109 nucleus accumbens,110,111 and dorsal striatum reduced alcohol-seeking behavior,112 while modulation of both cortical and subcortical regions reduced behavioral comorbidities.113, 114, 115 DREADD-mediated modulation of the NAc and associated circuits with the dorsal raphe,116,117 vmPFC,118 ventral subiculum,119 and paraventricular thalamus reduced reinstatement of drug seeking.120 Furthermore, chemogenetic modulation of non-neuronal cell types has been shown to rescue pathologic reward-seeking behavior. Excitation of astrocytes within the core of the nucleus accumbens inhibited cue-reinstate cocaine seeking via a defined molecular mechanism,117 and excitation of astrocytes in the amygdala reduced alcohol consumption.121
Psychiatric disorders
Chemogenetic manipulations have revealed important circuits associated with psychiatric disorders. In rodent models of major depressive disorder, chemogenetic modulation of newborn neurons in the dentate gyrus of the hippocampus established a direct relationship between neuronal activity and depression-like behavior, and activation of such neurons was sufficient to alleviate depression-like behaviors.122 Modulation of the lateral habenula,123 amygdala,124,125 the shell of the nucleus accumbens,126 and the arcuate nucleus also demonstrated reversal of depression-like behaviors.127 Interestingly, a study activated hM3D to specifically initiate a Gq signaling cascade to reverse depression-like behavior.128 Further applications of chemogenetic tools demonstrate attenuation of depression-like and anxious behaviors in models of obsessive-compulsive disorder, eating disorders, and stress-related disorders.129, 130, 131, 132, 133, 134, 135, 136 Finally, DREADD-mediated modulation of the amygdala has been shown to reduce anxiety-related behaviors in non-human primates for the first time, illustrating the high translational potential of this neuromodulation approach.137
Trauma
In models of traumatic brain injury and stroke, DREADD-mediated activation of glutamatergic neurons and inhibition of interneurons at the cortex demonstrated neuroprotective effects, including recovery of global neurological status, cognitive performance, and motor function.138, 139, 140 Furthermore, excitation of ganglionic populations in the spinal cord after peripheral nerve injury has been shown to promote axonal regeneration.141, 142, 143 Inhibition of spinal interneurons was shown to rescue lymphocytes after spinal cord injury.144
Homeostatic maintenance
Chemogenetics has been applied to modulate sympathetic and parasympathetic tone for the control of deficits in models of heart failure.145, 146, 147 Modulation of sleep-regulating pathways implicated by oxytocin signaling and the ventrolateral preoptic nucleus has demonstrated the reduction of cataplexy-like episodes.148 Finally, recent reports applied several viral delivery strategies to enable DREADD-mediated excitation of hypoglossal motor neurons to dilate the airway in obstructive sleep apnea.149,150
Translational potential
In summary, this review identified applications of chemogenetic neuromodulation across diverse brain regions, cell types, and disease models. These applications led to the reversal of associated molecular and behavioral deficits and have increased the knowledge of discrete brain circuit involvement across several diseases and disorders. Chemogenetic systems were primarily applied in a modular manner, whereby the chemogenetic receptors were generalized as excitatory and inhibitory tools despite differences in their downstream signaling mechanisms. While neuronal excitability is indeed an attribute of some pathophysiological processes, such as those in epilepsy and Alzheimer's disease, many neurological diseases and psychiatric disorders have highly diverse and elusive pathophysiological mechanisms. Given only a few identified studies that leveraged the effector functions of chemogenetic receptors to modulate specific molecular pathways,3,128 and given limitations in existing rodent models for diseases and disorders for which the underlying pathological mechanisms are poorly understood, we conclude that the potential of current chemogenetic receptors as a method for precision neuromodulation, whereby the mechanism of action is directly rationalized from molecular pathophysiology rather than neuronal excitation, remains largely conceptual.
However, we still identified many studies that successfully addressed pathological phenotypes in animal models by simply modulating neuronal excitability at specific nodes of the associated neural circuits. This demonstrated ability to tune neuronal excitability poses interesting possibilities for focal and programmable neuromodulation. Therefore, we discuss the translational potential of chemogenetics not in the context of specific molecular mechanisms of disease but rather in the context of its potential as a generalized technique for neuromodulation, such as deep brain stimulation (DBS).
The development of DBS, whereby intracranial electrodes deliver electrical impulses, added a powerful and safer option than its predecessor (focal lesions) to the surgical toolkit for treating behavioral and motor disorders.151 DBS has advantages over surgical lesioning for its lack of irreversible tissue ablation and the ability to control stimulation to influence circuit behavior.152 Programming the frequency, intensity, and pattern of DBS to achieve symptom reduction can be challenging clinically. However, enhancements in DBS configurability have made it the standard of care for patients with highly refractory disorders—most commonly PD, dystonia, and essential tremor. Many DBS-based strategies to treat diseases such as depression, Alzheimer's disease, and neuropathic pain have entered clinical trials. Further, DBS has been coupled with electrocorticography for real-time detection and prevention of focal-onset epilepsy.153 Nevertheless, despite its efficacy in treating neurological diseases, its effectiveness in treating psychiatric disorders and its general mechanism of action remain unclear. Indeed, high-frequency stimulation was originally believed to be inhibitory, given the similar clinical effects observed as in lesioning.154 However, it is evident that the mechanism is more complex, with hypotheses proposed such as informational lesioning and synaptic filtering, which reject the notion of simple inhibition or excitation.152 It is also unknown to what extent DBS can differentiate specific cell types or discrete neuronal processes (e.g., axons, fiber tracts, and cell bodies).
In contrast to DBS, targeting chemogenetic applications to the nervous system entails a much more versatile and precise approach, as targeted delivery of engineered receptors to discrete cell populations can enable cell-type-specific neuromodulation. Selective chemogenetic receptor targeting could maximize the modulation of neurons critical for symptom reduction and avoid affecting proximal regions that lead to side effects, a common challenge encountered with DBS stimulator programming. The duration and intensity of the effect from chemogenetic receptors is also pharmacologically controlled by agonists, a potential advantage in facilitating symptom reduction while enhancing patient independence—specifically in obviating the need for and complications associated with electrical devices in DBS. Chemogenetics further introduces the possibility of precision network control. Various classes of receptors could be introduced in different nuclei for multiplexed network control, with the ability to modulate distinct neuronal populations in different connectomes simultaneously. The possibilities of a system that can work inclusively on multiple levels of a circuit are profound, as it may enable optimization of the therapeutic effect for patients. Recently, a review favorably discussed the therapeutic potential of chemogenetic modulation for pain, epilepsy, and movement disorders,155 and our systematic analysis further demonstrates the versatility of chemogenetics for nearly all classes of neuropsychiatric diseases and disorders.
Another widely used neuromodulation approach with higher temporal precision than chemogenetics is optogenetics, which relies on the use of light to activate excitatory or inhibitory light-sensitive genetically encoded ion channels. However, its need for an external light source has limited translational developments in terms of targeting only superficial organs or tissues.156,157 Until there is a non-invasive solution for light delivery to deep brain structures, optogenetics shares similar challenges with DBS, whereby the need for invasive surgical procedures introduces safety risks and financial costs of a lifelong implant. Lastly, preclinical applications of optogenetic systems have relied on engineered ion channels. Similar to PSAM-based chemogenetic systems, the neuromodulatory effects of optogenetic receptors are dependent on the local electrochemical environment. Indeed, optogenetic chloride channels have displayed both inhibitory and excitatory effects in different cellular spaces.158 Metabotropic receptors, namely DREADDs, are a more reliable counterpart in producing desired neuromodulatory effects. Accordingly, DREADD-based systems represented over 90% of the identified experiments in our scoping review (Table 2).
Furthermore, clinical applications of chemogenetics would necessitate non-invasive, longitudinal monitoring of the spatial and temporal expression of chemogenetic receptors. To this end, chemogenetic ligands have been engineered to permit receptor detection via positron emission tomography (PET). Radiolabeled tracers, including [11C]DCZ, [11C]CZP, and the fluorinated analog [18F]JHU37107, have been shown to display specific binding to hM DREADD receptors in mice and NHPs.21,23,137,159,160 Namely, [11C]DCZ was shown to exhibit considerable improvement in signal-to-background ratio over [11C]CZP in NHPs.159 Administration of these radiotracers in live animals led to visualization of the location and the levels of DREADD expression in the CNS and simultaneously enabled high-resolution identification of relevant neuronal projections across the brain. Pluripotent stem cells expressing DREADDs grafted to the mouse brain have also been visualized by PET to track differentiated neurons using [11C]CNO.161 Beyond monitoring receptor expression, combining DREADD activation with PET and functional magnetic resonance imaging (fMRI) has revealed global metabolic and functional networks involving the cell-specific mesocorticolimbic pathways and serotoninergic signaling circuitries.162, 163, 164, 165 These developments will be critical to the non-invasive validation and longitudinal monitoring of successful delivery of DREADD receptors to the human patient to enable precision medicine-based therapies.
Finally, beyond the lines of evidence identified by this review that present current chemogenetic systems as a therapeutic method for circuit manipulation, the concept of chemogenetics poses vast potential for precise molecular and cellular modulation. First, given that the delivery of chemogenetic receptors can be restricted to specific cell types, divergent functions of distinct types of cells within a given anatomical circuit can be decoupled and specifically modulated. This is especially important given recent advances in neuroscience that implicate non-neuronal cell types, such as astrocytes and microglia, as critical mediators of neuronal function.166 Glia engage neural circuitry in a diverse set of ways, including modulating local synaptic transmission by maintaining the extrasynaptic environment. Indeed, our scoping review identified several studies that specifically targeted glia (Table 2).56,76,117,121 For example, chemogenetic inhibition of astrocytes was shown to reduce neuropathic pain by modulating astrocytic cytokine secretion.76 Coupling transcriptional expression of chemogenetic receptors with cell states, rather than cell types, would also introduce temporal control to chemogenetic neuromodulation. Second, latest generations of chemogenetic systems are entirely derived from engineering of the hM3, hM4, and nAchRs. The process by which these systems are created is a proof of concept that can drive the expansion of the chemogenetic repertoire to encompass derivatives of many more endogenous metabotropic and ionotropic receptors. For example, an engineered voltage-gated potassium channel designed to reduce neuronal excitability effectively reduced seizure frequency in focal-onset epileptic models.44 As such, this would allow chemogenetic manipulations not only through modulation of neuronal excitability but also through tuning of more defined intercellular and intracellular signaling pathways.
Translational challenge
From our identified studies, analysis of the experimental approaches revealed that the diverse therapeutic potential of chemogenetics is contingent upon the ability to express chemogenetic receptors in discrete cell types. All brain regions comprise intermixed cell populations with multiple neuronal and non-neuronal cell types whereby each mediates specific functions by participating in local cell-to-cell and/or distal, long-range, whole-brain interactions. Therefore, effective neuromodulation is expected to require the spatio-, cell-type-, and projection-specific identification and targeting of discrete cells in order to induce desired outcomes and avoid off-target effects. Here, we discuss current strategies to overcome these technical challenges and their shortcomings.
Receptor delivery
Chemogenetic systems' therapeutic efficacy necessitates restricted expression of chemogenetic receptors in spatially defined brain regions. To this end, recombinant adeno-associated viral vectors (rAAVs) are the predominant viral-mediated gene delivery vehicle to the CNS given their low immunogenicity, high transduction efficiencies, and well-validated applications.167 Neurosurgical delivery of rAAVs primarily relies on convection enhanced delivery (CED). CED is an intraparenchymal injection method that has been validated in clinical trials to efficiently diffuse rAAV vectors in brain regions around the surgically placed infusion cannula.168 Developments in MRI-assisted CED and cannula design also enhance the accuracy, efficiency, and safety of intraparenchymal injections.169, 170, 171, 172, 173 Alternative to CED, a recent proof-of-concept study applied intravenous infusion of rAAV vectors and microbubbles accompanied by magnetic resonance-guided focused ultrasound (MRgFUS) to temporarily open the BBB at the target brain region and permit rAAV entry to the parenchyma,174 permitting targeted expression of hM DREADDs in mice.175 However, both delivery methods face the challenge of delocalized rAAV transduction due to infusion leakage or axonal transport. Intravenous infusion of rAAV vectors in MRgFUS may result in peripheral transduction and even peripheral transgene expression if driven by the appropriate promoters.170,174 Indeed, localized intraparenchymal injections have been shown to result in transgene expression in distant brain regions due to the distribution of intact viral particles through perivascular space and bidirectional axonal transport of intact rAAV particles.168,176, 177, 178, 179 To our knowledge, no studies that examine these off-targeted consequences specifically within the application of chemogenetic systems are yet available.
Whereas non-invasive delivery would enable fully non-invasive chemogenetic neuromodulation, local infusion of rAAVs still possesses translational feasibility. Preclinical and clinical developments that rely on local AAV infusions may be less prohibited by the cost of clinical-grade AAV production. Furthermore, existing interventions that target deep brain structures (e.g., DBS and lesioning) or spinal structures (e.g., dorsal root entry zone and ganglionectomy) already demonstrate the translational feasibility of focal delivery of chemogenetic receptors through invasive procedures.
Cell-type specificity
Cell-type-specific expression of chemogenetic receptors is not only advantageous but also sometimes necessary due to the presence of heterogeneous and antagonistic cell types at a given brain region. For example, cortical regions are characterized by the functional integration between excitatory pyramidal neurons and inhibitory interneurons. Pan-neuronal chemogenetic manipulations of such regions, therefore, may yield neutralized effects from simultaneous activation of both cell types. Indeed, in a study that sought to inhibit the anterior insular cortex (aIC) to increase the threshold for pain in a rat model of visceral pain, pan-neuronal inhibition of the aIC produced a statistically insignificant behavioral trend that was later confirmed by specific inhibition of pyramidal neurons in the same region.180 In a mouse model for obsessive-compulsive disorder, inhibition of inhibitory neurons receiving projections from the amygdala reversed behavioral deficits, and activation of excitatory neurons receiving the same projection interestingly recapitulated the same phenotype.129 Antagonistic or cooperative cell types and pathways are also found beyond the cortex. D1 and D2 dopaminergic neurons are implicated in the direct and indirect pathways in the basal ganglia that regulate motor behavior.181 A recent study found opposite analgesic effects by chemogenetic modulation of D1 and D2 neurons in the brainstem of a mouse model for trigeminal neuralgia.182 Beyond antagonistic cell types, heterogeneous cell populations across the nervous system mediate highly specific functions. Chemogenetic applications at subcortical and spinal regions necessitated targeting of, for example, somatostatin neurons,62 orexin neurons,132,140 and a molecularly defined population of dorsal root ganglion neurons.84 As an example of the importance of cell type-specific modulation, over 80% of all experiments within our included studies targeted specific neuronal and glial subtypes (Table 2).
Current strategies to achieve specific expression in rodent models either employ cell-type-specific promoters or use recombinases to conditionally enable functional expression. Conceptually, an ideal promoter would fit within the rAAV packaging limit, strongly drive transgene expression, and show no leaky expression in other cell types. However, there is currently a limited repertoire of suitable promoters that satisfies these requirements. Given the rAAV genome size, only a small subset of established promoters for CNS cell types are small enough to be packaged in cis with coding sequences. A common strategy in basic neuroscience to achieve cell-type-specific expression of transgenes is cell-type-dependent Cre-driven recombination, whereby Cre recombinase recognizes two pairs of Lox sequences flanking an inverted transgene sequence to irreversibly establish the correct coding orientation and drive functional gene expression.183 However, despite the ubiquitous application of Cre-mediated recombination in chemogenetic studies (Table 2), Cre has been shown to cause DNA damage in mammalian genomes in both the presence and absence of pseudo-LoxP sites and to induce brain lesions within rodent models.184,185 It is worth noting that studies that specifically investigate Cre-associated toxicity usually induce hyperexpression of Cre beyond levels seen in typical chemogenetic applications,186,187 and self-excising Cre constructs have been shown to reduce toxicity.188,189 However, the self-inactivating technique to drive transgene expression levels in neuronal systems sufficient for chemogenetic applications has not yet been validated.
Currently, the most predominant effort to bypass cell-type-dependent recombination entails the discovery or synthesis of minimal promoters. However, minimal promoters often display leaky gene expression. Within the included studies, one experiment targeted Purkinje neurons via the minimal Purkinje cell-specific PCP2/L7 promoter to rescue social impairment in a mouse model for ASD.190 In another study that sought to delay motor neuron degeneration, expressing Cre-conditional hM3D in the motor cortex of parvalbumin-Cre transgenic mice generated a neuroprotective effect, and the same effect was observed when hM3D was driven by hDlx, the human form of Dlx5/6 enhancer.55 However, while 79% of transduced cells in the local region were parvalbumin neurons, 19% were somatostatin interneurons.55
In this regard, chemogenetic-mediated neuromodulation shares the same challenge with the larger field of neurological gene therapy: the need to establish programmable, cell-type-specific transgene expression. It is important to note, however, that in comparison with current neuromodulatory interventions such as lesioning and DBS, the current ability to utilize cell-type-specific promoters to restrict the expression of chemogenetic receptors is already a paradigmatic leap in precision. Furthermore, there exist diverse regulating elements of mammalian transcription and translation beyond promoters. For example, RNA folding has been leveraged to design translation activators and repressors that are sensitive to the presence of sequence-specific RNA.191,192 Cell-type-specific enhancers have also been harnessed to drive cell-type-specific gene expression while occupying smaller portions of the rAAV cassette.193 Lastly, efforts in expanding the rAAV genome and in developing alternative vectors further supports the possibility of incorporating large promoters in the future.194
Projection specificity
Beyond targeting molecularly defined cell types, effective chemogenetic neuromodulation has been repeatedly demonstrated to rely on exclusive targeting of cells that engage in a specific neural circuit. The conventional orientation of the CNS defines regions of interest (ROIs) first by spatial location. The circuit connectivity of an ROI is then identified to include heterogeneous axonal projections to and from the ROI, thereby implicating multiple functions based on the associated circuits. Furthermore, neurons within an ROI that participate in distinct circuits are often indistinguishable via molecular markers. Projection specificity is a critical dimension in the design of chemogenetic applications. In a mouse model for binge drinking, activation of NPY1+ neurons in the mPFC decreased ethanol consumption but did not significantly reduce blood ethanol concentration, while exclusive activation of NPY1+ neurons that project from the mPFC to the basolateral amygdala led to a more robust reduction of ethanol consumption and significantly reduced blood ethanol concentration levels.108 In a mouse model for cocaine relapse, inhibition of neurons that project from the ventral tegmental area (VTA) to the prelimbic cortex reduced context-dependent reinstatement of cocaine seeking, and such an effect was not recapitulated in the mere stimulation of VTA neurons.195
Achieving projection-specific manipulations faces separate challenges in the identification of circuits and the selective expression of chemogenetic receptors in projection-specific neurons. Retrograde and anterograde tracers are common tools to identify neuronal projections and have been demonstrated to enable effective rationalization of chemogenetic manipulations.119 However, there exist few non-invasive alternatives for circuit identification in humans, despite recent attempts to computationally characterize neuronal connectivity using fMRI.196 Projection-specific transgene expression is often achieved via retrograde AAVs, which package a recombinase, such as Cre, that leads to transgene expression in transduced neurons whose axons terminate exclusively in a specific brain region. For example, retro-DREADDs, an adaptation of the dual vector approach for cell-type specificity described above, delivers a retrograde rAAV packaging Cre to the projected destination of the targeted neurons, restricting DREADD expression to specific neurons that have the corresponding axonal projections. This technique has been validated in vivo for hM3D systems.136 However, while these strategies technically permit projection-specific and cell-type-specific receptor expression in future preclinical and clinical applications, they are nonetheless limited by the fact that recombinase-conditional transgene expression poses a risk for cell toxicity.
Interestingly, chemogenetic manipulation at the central/peripheral nervous system (CNS/PNS) interface has been shown to be a unique category that is exempt from such challenges. Retrograde AAVs can be delivered to specific tissues innervated by the PNS for the specific targeting of cognate CNS components. This approach was demonstrated to allow chemogenetic control of motor neurons innervating hypoglossal muscles in a mouse model for obstructive sleep apnea.149,150 Similarly, intra-articular delivery of engineered AAVs allowed for targeted delivery of hM4D to dorsal root ganglion neurons innervating the knee and the successful reduction of pain in a mouse model of joint pain.89 Beyond the CNS/PNS interface, however, future projection-specific chemogenetic applications will need to rely on advancements in brain connectome studies and synthetic regulation of gene expression.
Receptor-ligand interactions
Beyond challenges in multidimensionally targeted expression of chemogenetic receptors, therapeutic applications from chemogenetics must also establish specific receptor-ligand interactions and minimize off-targeted effects. Thus far, pharmacodynamic characterizations of DREADD activators in rodents and NHPs demonstrate rapid onset of neuromodulatory effects within minutes after systemic delivery of activators. However, off-targeted effects are often observed in these studies, and dosage thresholds for minimal off-targeted actions for CZP, OZP, and DCZ appear to be dependent on the cellular and molecular characteristics of target brain regions.23,24,197,198 Our identified studies did not display a clear standard of experimental design that controls for ligand-associated off-target behavioral effects (Table 2). More than half of the identified experiments did not establish such a control, and experiments that did control for ligand interactions nearly always report that the ligands did not induce any off-target effects. Moving forward, applications of chemogenetics in translational and clinical investigations would need to stringently establish the safety of future chemogenetic ligands in the context not only of the intended neuromodulatory effects but also that of the ligand's own pharmacological profile.
Conclusion
In summary, over the past decade we have observed the development of chemogenetic systems mainly from engineering of the hM3, hM4, and nAchRs that exhibit significant potential in the therapy of pharmacoresistant neuropsychiatric diseases and disorders. Chemogenetics is still well within its infancy. Its ability to specifically target cellular functions via the native functions of the receptors themselves implies a paradigmatic shift toward circuit-based, cellular, and molecular neuromodulation. Pairing this capability with other therapeutic options or monitoring imaging modalities could provide dramatic relief for refractory patients struggling with the consequences of neurological disorders and encompass a precision medicine-based approach to treatment. Achieving safe chemogenetic interventions will inevitably necessitate a strong fundamental understanding of neural circuitry, controlled transgene expression, and agonist interactions. Yet the personalized modulation that can come from potential chemogenetic platforms holds the promise of being a powerful tool, one that can elucidate the mysteries of pathological brain circuitry and fit the therapeutic needs of each individual.
Acknowledgments
We thank H. Adwanikar, P.D. Judge, and D.A. Harris for helpful discussions. M.M. is supported by the National Institute on Drug Abuse Intramural Research Program (ZIA000069).
Author contributions
J.S. conceived the work. J.S. and R.V.P. designed the scoping review. J.S., M.S., and A.A. performed the scoping review and analyzed the results with support from M.M. J.S., R.V.P., and M.M. wrote the manuscript with input from all authors.
Declaration of interests
M.M. is a cofounder of Metis Laboratories, Inc., and has received research funding in the form of Cooperative Research and Development Agreements from AstraZeneca, Attune, Inc., and Redpin Therapeutics, Inc., a private biotechnology company developing chemogenetic therapeutics. M.M. is listed as an inventor on a US Patent (62/627,527) regarding the novel DREADD compounds described herein. The remaining authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2021.11.019.
Contributor Information
Jingwei Song, Email: jsong64@jh.edu.
Michael Michaelides, Email: mike.michaelides@nih.gov.
Supplemental information
References
- 1.English J.G., Roth B.L. Chemogenetics—a transformational and translational platform. JAMA Neurol. 2015;72:1361–1366. doi: 10.1001/jamaneurol.2015.1921. [DOI] [PubMed] [Google Scholar]
- 2.Roth B.L. DREADDs for neuroscientists. Neuron. 2016;89:683–694. doi: 10.1016/j.neuron.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lieb A., Qiu Y., Dixon C.L., Heller J.P., Walker M.C., Schorge S., Kullmann D.M. Biochemical autoregulatory gene therapy for focal epilepsy. Nat. Med. 2018;24:1324–1329. doi: 10.1038/s41591-018-0103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ngo H.B., Melo M.R., Layfield S., Connelly A.A., Bassi J.K., Xie L., Menuet C., McDougall S.J., Bathgate R.A.D., Allen A.M. A chemogenetic tool that enables functional neural circuit analysis. Cell Rep. 2020;32:108139. doi: 10.1016/j.celrep.2020.108139. [DOI] [PubMed] [Google Scholar]
- 5.Tan E.M., Yamaguchi Y., Horwitz G.D., Gosgnach S., Lein E.S., Goulding M., Albright T.D., Callaway E.M. Selective and quickly reversible inactivation of mammalian neurons in vivo using the Drosophila allatostatin receptor. Neuron. 2006;51:157–170. doi: 10.1016/j.neuron.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 6.Ikrar T., Shi Y., Velasquez T., Goulding M., Xu X. Cell-type specific regulation of cortical excitability through the allatostatin receptor system. Front. Neural Circuits. 2012;6:2. doi: 10.3389/fncir.2012.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conklin B.R., Hsiao E.C., Claeysen S., Dumuis A., Srinivasan S., Forsayeth J.R., Guettier J.M., Chang W., Pei Y., McCarthy K.D., et al. Engineering GPCR signaling pathways with RASSLs. Nat. Methods. 2008;5:673–678. doi: 10.1038/nmeth.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsiao E.C., Boudignon B.M., Chang W.C., Bencsik M., Peng J., Nguyen T.D., Manalac C., Halloran B.P., Conklin B.R., Nissenson R.A. Osteoblast expression of an engineered Gs-coupled receptor dramatically increases bone mass. Proc. Natl. Acad. Sci. U S A. 2008;105:1209–1214. doi: 10.1073/pnas.0707457105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sweger E.J., Casper K.B., Scearce-Levie K., Conklin B.R., McCarthy K.D. Development of hydrocephalus in mice expressing the G(i)-coupled GPCR Ro1 RASSL receptor in astrocytes. J. Neurosci. 2007;27:2309–2317. doi: 10.1523/JNEUROSCI.4565-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armbruster B.N., Li X., Pausch M.H., Herlitze S., Roth B.L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl. Acad. Sci. U S A. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexander G.M., Rogan S.C., Abbas A.I., Armbruster B.N., Pei Y., Allen J.A., Nonneman R.J., Hartmann J., Moy S.S., Nicolelis M.A., et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galvan A., Raper J., Hu X., Paré J.-F., Bonaventura J., Richie C.T., Michaelides M., Mueller S.A.L., Roseboom P.H., Oler J.A., et al. Ultrastructural localization of DREADDs in monkeys. Eur. J. Neurosci. 2019;50:2801–2813. doi: 10.1111/ejn.14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y., Xiong M., Dong Y., Haberman A., Cao J., Liu H., Zhou W., Zhang S.C. Chemical control of grafted human PSC-derived neurons in a mouse model of Parkinson’s disease. Cell Stem Cell. 2016;18:817–826. doi: 10.1016/j.stem.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jendryka M., Palchaudhuri M., Ursu D., van der Veen B., Liss B., Katzel D., Nissen W., Pekcec A. Pharmacokinetic and pharmacodynamic actions of clozapine-N-oxide, clozapine, and compound 21 in DREADD-based chemogenetics in mice. Sci. Rep. 2019;9:4522. doi: 10.1038/s41598-019-41088-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomez J.L., Bonaventura J., Lesniak W., Mathews W.B., Sysa-Shah P., Rodriguez L.A., Ellis R.J., Richie C.T., Harvey B.K., Dannals R.F., et al. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science. 2017;357:503–507. doi: 10.1126/science.aan2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manvich D.F., Webster K.A., Foster S.L., Farrell M.S., Ritchie J.C., Porter J.H., Weinshenker D. The DREADD agonist clozapine N-oxide (CNO) is reverse-metabolized to clozapine and produces clozapine-like interoceptive stimulus effects in rats and mice. Sci. Rep. 2018;8:3840. doi: 10.1038/s41598-018-22116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacLaren D.A., Browne R.W., Shaw J.K., Krishnan Radhakrishnan S., Khare P., Espana R.A., Clark S.D. Clozapine N-oxide administration produces behavioral effects in long-evans rats: implications for designing DREADD experiments. eNeuro. 2016;3 doi: 10.1523/ENEURO.0219-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baerentzen S., Casado-Sainz A., Lange D., Shalgunov V., Tejada I.M., Xiong M., L’Estrade E.T., Edgar F.G., Lee H., Herth M.M., et al. The chemogenetic receptor ligand clozapine N-oxide induces in vivo neuroreceptor occupancy and reduces striatal glutamate levels. Front. Neurosci. 2019;13:187. doi: 10.3389/fnins.2019.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang X.F., Tan Y.Y., Huang X., Wang Q. Effect of chronic treatment with clozapine and haloperidol on 5-HT(2A and 2C) receptor mRNA expression in the rat brain. Neurosci. Res. 2007;59:314–321. doi: 10.1016/j.neures.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Ilg A.K., Enkel T., Bartsch D., Bahner F. Behavioral effects of acute systemic low-dose clozapine in wild-type rats: implications for the use of DREADDs in behavioral neuroscience. Front. Behav. Neurosci. 2018;12:173. doi: 10.3389/fnbeh.2018.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonaventura J., Eldridge M.A.G., Hu F., Gomez J.L., Sanchez-Soto M., Abramyan A.M., Lam S., Boehm M.A., Ruiz C., Farrell M.R., et al. High-potency ligands for DREADD imaging and activation in rodents and monkeys. Nat. Commun. 2019;10:4627. doi: 10.1038/s41467-019-12236-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stroup T.S., Gerhard T., Crystal S., Huang C., Olfson M. Comparative effectiveness of clozapine and standard antipsychotic treatment in adults with schizophrenia. Am. J. Psychiatry. 2016;173:166–173. doi: 10.1176/appi.ajp.2015.15030332. [DOI] [PubMed] [Google Scholar]
- 23.Nagai Y., Miyakawa N., Takuwa H., Hori Y., Oyama K., Ji B., Takahashi M., Huang X.-P., Slocum S.T., DiBerto J.F., et al. Deschloroclozapine, a potent and selective chemogenetic actuator enables rapid neuronal and behavioral modulations in mice and monkeys. Nat. Neurosci. 2020;23:1157–1167. doi: 10.1038/s41593-020-0661-3. [DOI] [PubMed] [Google Scholar]
- 24.Weston M., Kaserer T., Wu A., Mouravlev A., Carpenter J.C., Snowball A., Knauss S., von Schimmelmann M., During M.J., Lignani G., et al. Olanzapine: a potent agonist at the hM4D(Gi) DREADD amenable to clinical translation of chemogenetics. Sci. Adv. 2019;5:eaaw1567. doi: 10.1126/sciadv.aaw1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vardy E., Robinson J.E., Li C., Olsen R.H.J., DiBerto J.F., Giguere P.M., Sassano F.M., Huang X.P., Zhu H., Urban D.J., et al. A new DREADD facilitates the multiplexed chemogenetic interrogation of behavior. Neuron. 2015;86:936–946. doi: 10.1016/j.neuron.2015.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marchant N.J., Whitaker L.R., Bossert J.M., Harvey B.K., Hope B.T., Kaganovsky K., Adhikary S., Prisinzano T.E., Vardy E., Roth B.L., et al. Behavioral and physiological effects of a novel kappa-opioid receptor-based DREADD in rats. Neuropsychopharmacology. 2016;41:402–409. doi: 10.1038/npp.2015.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hooker J.M., Munro T.A., Béguin C., Alexoff D., Shea C., Xu Y., Cohen B.M. Salvinorin A and derivatives: protection from metabolism does not prolong short-term, whole-brain residence. Neuropharmacology. 2009;57:386–391. doi: 10.1016/j.neuropharm.2009.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakajima K.-I., Wess J. Design and functional characterization of a novel, arrestin-biased designer G protein-coupled receptor. Mol. Pharmacol. 2012;82:575–582. doi: 10.1124/mol.112.080358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guettier J.M., Gautam D., Scarselli M., Ruiz de Azua I., Li J.H., Rosemond E., Ma X., Gonzalez F.J., Armbruster B.N., Lu H., et al. A chemical-genetic approach to study G protein regulation of beta cell function in vivo. Proc. Natl. Acad. Sci. U S A. 2009;106:19197–19202. doi: 10.1073/pnas.0906593106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magnus C.J., Lee P.H., Atasoy D., Su H.H., Looger L.L., Sternson S.M. Chemical and genetic engineering of selective ion channel-ligand interactions. Science. 2011;333:1292–1296. doi: 10.1126/science.1206606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grutter T., de Carvalho L.P., Dufresne V., Taly A., Edelstein S.J., Changeux J.P. Molecular tuning of fast gating in pentameric ligand-gated ion channels. Proc. Natl. Acad. Sci. U S A. 2005;102:18207–18212. doi: 10.1073/pnas.0509024102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magnus C.J., Lee P.H., Bonaventura J., Zemla R., Gomez J.L., Ramirez M.H., Hu X., Galvan A., Basu J., Michaelides M., et al. Ultrapotent chemogenetics for research and potential clinical applications. Science. 2019;364 doi: 10.1126/science.aav5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donato F., Rompani S.B., Caroni P. Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature. 2013;504:272–276. doi: 10.1038/nature12866. [DOI] [PubMed] [Google Scholar]
- 34.Lummis S.C.R., Thompson A.J., Bencherif M., Lester H.A. Varenicline is a potent agonist of the human 5-hydroxytryptamine3 receptor. J. Pharmacol. Exp. Ther. 2011;339:125–131. doi: 10.1124/jpet.111.185306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lynagh T., Lynch J.W. An improved ivermectin-activated chloride channel receptor for inhibiting electrical activity in defined neuronal populations. J. Biol. Chem. 2010;285:14890–14897. doi: 10.1074/jbc.M110.107789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lynagh T., Lynch J.W. A glycine residue essential for high ivermectin sensitivity in Cys-loop ion channel receptors. Int. J. Parasitol. 2010;40:1477–1481. doi: 10.1016/j.ijpara.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Lynagh T., Webb T.I., Dixon C.L., Cromer B.A., Lynch J.W. Molecular determinants of ivermectin sensitivity at the glycine receptor chloride channel. J. Biol. Chem. 2011;286:43913–43924. doi: 10.1074/jbc.M111.262634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haubensak W., Kunwar P.S., Cai H., Ciocchi S., Wall N.R., Ponnusamy R., Biag J., Dong H.W., Deisseroth K., Callaway E.M., et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rivera C., Voipio J., Payne J.A., Ruusuvuori E., Lahtinen H., Lamsa K., Pirvola U., Saarma M., Kaila K. The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- 40.Gantz S.C., Ortiz M.M., Belilos A.J., Moussawi K. Excitation of medium spiny neurons by “inhibitory” ultrapotent chemogenetics via shifts in chloride reversal potential. eLife. 2021;10:e64241. doi: 10.7554/eLife.64241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stafstrom C.E., Carmant L. Seizures and epilepsy: an overview for neuroscientists. Cold Spring Harb. Perspect. Med. 2015;5:a022426. doi: 10.1101/cshperspect.a022426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Staley K. Molecular mechanisms of epilepsy. Nat. Neurosci. 2015;18:367–372. doi: 10.1038/nn.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee D., Krishnan B., Zhang H., Park H.R., Ro E.J., Jung Y.-N., Suh H. Activity of hippocampal adult-born neurons regulates alcohol withdrawal seizures. JCI Insight. 2019;4:e128770. doi: 10.1172/jci.insight.128770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Snowball A., Chabrol E., Wykes R.C., Shekh-Ahmad T., Cornford J.H., Lieb A., Hughes M.P., Massaro G., Rahim A.A., Hashemi K.S., et al. Epilepsy gene therapy using an engineered potassium channel. J. Neurosci. 2019;39:3159–3169. doi: 10.1523/JNEUROSCI.1143-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Avaliani N., Andersson M., Runegaard A.H., Woldbye D., Kokaia M. DREADDs suppress seizure-like activity in a mouse model of pharmacoresistant epileptic brain tissue. Gene Ther. 2016;23:760–766. doi: 10.1038/gt.2016.56. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y., Liang J., Chen L., Shen Y., Zhao J., Xu C., Wu X., Cheng H., Ying X., Guo Y., et al. Pharmaco-genetic therapeutics targeting parvalbumin neurons attenuate temporal lobe epilepsy. Neurobiol. Dis. 2018;117:149–160. doi: 10.1016/j.nbd.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 47.Zhou Q.-G., Nemes A.D., Lee D., Ro E.J., Zhang J., Nowacki A.S., Dymecki S.M., Najm I.M., Suh H. Chemogenetic silencing of hippocampal neurons suppresses epileptic neural circuits. J. Clin. Invest. 2019;129:310–323. doi: 10.1172/JCI95731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berglind F., Andersson M., Kokaia M. Dynamic interaction of local and transhemispheric networks is necessary for progressive intensification of hippocampal seizures. Sci. Rep. 2018;8:5669. doi: 10.1038/s41598-018-23659-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kahn J.B., Port R.G., Yue C., Takano H., Coulter D.A. Circuit-based interventions in the dentate gyrus rescue epilepsy-associated cognitive dysfunction. Brain. 2019;142:2705–2721. doi: 10.1093/brain/awz209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goossens M.-G., Boon P., Wadman W., Van den Haute C., Baekelandt V., Verstraete A.G., Vonck K., Larsen L.E., Sprengers M., Carrette E., et al. Long-term chemogenetic suppression of seizures in a multifocal rat model of temporal lobe epilepsy. Epilepsia. 2021;62:659–670. doi: 10.1111/epi.16840. [DOI] [PubMed] [Google Scholar]
- 51.Kätzel D., Nicholson E., Schorge S., Walker M.C., Kullmann D.M. Chemical-genetic attenuation of focal neocortical seizures. Nat. Commun. 2014;5:3847. doi: 10.1038/ncomms4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wicker E., Forcelli P.A. Chemogenetic silencing of the midline and intralaminar thalamus blocks amygdala-kindled seizures. Exp. Neurol. 2016;283:404–412. doi: 10.1016/j.expneurol.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Desloovere J., Boon P., Larsen L., Merckx C., Goossens M.-G., Van Den Haute C., Baekelandt V., Carrette E., Delbeke J., Meurs A., et al. Long term chemogenetic suppression of spontaneous seizures in a mouse model for temporal lobe epilepsy. Front. Neurosci. 2019;13:2314. doi: 10.1111/epi.16368. [DOI] [PubMed] [Google Scholar]
- 54.Saxena S., Roselli F., Singh K., Leptien K., Julien J.-P., Gros-Louis F., Caroni P. Neuroprotection through excitability and mTOR required in ALS motoneurons to delay disease and extend survival. Neuron. 2013;80:80–96. doi: 10.1016/j.neuron.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 55.Khademullah C.S., Aqrabawi A.J., Place K.M., Dargaei Z., Liang X., Pressey J.C., Bedard S., Yang J.W., Garand D., Keramidis I., et al. Cortical interneuron-mediated inhibition delays the onset of amyotrophic lateral sclerosis. Brain. 2020;143:800–810. doi: 10.1093/brain/awaa034. [DOI] [PubMed] [Google Scholar]
- 56.Ouali Alami N., Tang L., Wiesner D., Commisso B., Bayer D., Weishaupt J., Dupuis L., Wong P., Baumann B., Wirth T., et al. Multiplexed chemogenetics in astrocytes and motoneurons restore blood-spinal cord barrier in ALS. Life Sci. Alliance. 2020;3 doi: 10.26508/lsa.201900571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodriguez G.A., Barrett G.M., Duff K.E., Hussaini S.A. Chemogenetic attenuation of neuronal activity in the entorhinal cortex reduces Aβ and tau pathology in the hippocampus. PLoS Biol. 2020;18:e3000851. doi: 10.1371/journal.pbio.3000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Imamura K., Sahara N., Kanaan N.M., Tsukita K., Kondo T., Kutoku Y., Ohsawa Y., Sunada Y., Kawakami K., Hotta A., et al. Calcium dysregulation contributes to neurodegeneration in FTLD patient iPSC-derived neurons. Sci. Rep. 2016;6:34904. doi: 10.1038/srep34904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakazato Y., Fujita Y., Nakazato M., Yamashita T. Neurons promote encephalitogenic CD4+ lymphocyte infiltration in experimental autoimmune encephalomyelitis. Sci. Rep. 2020;10:7354. doi: 10.1038/s41598-020-64363-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alcacer C., Andreoli L., Sebastianutto I., Jakobsson J., Fieblinger T., Cenci M.A. Chemogenetic stimulation of striatal projection neurons modulates responses to Parkinson’s disease therapy. J. Clin. Invest. 2017;127:720–734. doi: 10.1172/JCI90132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tanimura A., Du Y., Kondapalli J., Wokosin D.L., Surmeier D.J. Cholinergic interneurons amplify thalamostriatal excitation of striatal indirect pathway neurons in Parkinson’s disease models. Neuron. 2019;101:444–458.e6. doi: 10.1016/j.neuron.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 62.Chen K., Yang G., So K.-F., Zhang L. Activation of cortical somatostatin interneurons rescues synapse loss and motor deficits after acute MPTP infusion. iScience. 2019;17:230–241. doi: 10.1016/j.isci.2019.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McIver E.L., Atherton J.F., Chu H.-Y., Cosgrove K.E., Kondapalli J., Wokosin D., Surmeier D.J., Bevan M.D. Maladaptive downregulation of autonomous subthalamic nucleus activity following the loss of midbrain dopamine neurons. Cell Rep. 2019;28:992–1002.e4. doi: 10.1016/j.celrep.2019.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parker P.R.L., Lalive A.L., Kreitzer A.C. Pathway-specific remodeling of thalamostriatal synapses in parkinsonian mice. Neuron. 2016;89:734–740. doi: 10.1016/j.neuron.2015.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lemos J.C., Friend D.M., Kaplan A.R., Shin J.H., Rubinstein M., Kravitz A.V., Alvarez V.A. Enhanced GABA transmission drives bradykinesia following loss of dopamine D2 receptor signaling. Neuron. 2016;90:824–838. doi: 10.1016/j.neuron.2016.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sharma P.K., Wells L., Rizzo G., Elson J.L., Passchier J., Rabiner E.A., Gunn R.N., Dexter D.T., Pienaar I.S. DREADD activation of pedunculopontine cholinergic neurons reverses motor deficits and restores striatal dopamine signaling in parkinsonian rats. Neurotherapeutics. 2020;17:1120–1141. doi: 10.1007/s13311-019-00830-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Avila C., Kucinski A., Sarter M. Complex movement control in a rat model of parkinsonian falls: bidirectional control by striatal cholinergic interneurons. J. Neurosci. 2020;40:6049–6067. doi: 10.1523/JNEUROSCI.0220-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aldrin-Kirk P., Heuer A., Wang G., Mattsson B., Lundblad M., Parmar M., Björklund T. DREADD modulation of transplanted DA neurons reveals a novel parkinsonian dyskinesia mechanism mediated by the serotonin 5-HT6 receptor. Neuron. 2016;90:955–968. doi: 10.1016/j.neuron.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dell’Anno M.T., Caiazzo M., Leo D., Dvoretskova E., Medrihan L., Colasante G., Giannelli S., Theka I., Russo G., Mus L., et al. Remote control of induced dopaminergic neurons in parkinsonian rats. J. Clin. Invest. 2014;124:3215–3229. doi: 10.1172/JCI74664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tan L.L., Kuner R. Neocortical circuits in pain and pain relief. Nat. Rev. Neurosci. 2021;22:458–471. doi: 10.1038/s41583-021-00468-2. [DOI] [PubMed] [Google Scholar]
- 71.Hirschberg S., Li Y., Randall A., Kremer E.J., Pickering A.E. Functional dichotomy in spinal- vs prefrontal-projecting locus coeruleus modules splits descending noradrenergic analgesia from ascending aversion and anxiety in rats. eLife. 2017;6:e29808. doi: 10.7554/eLife.29808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chang Y.-T., Chen W.-H., Shih H.-C., Min M.-Y., Shyu B.-C., Chen C.-C. Anterior nucleus of paraventricular thalamus mediates chronic mechanical hyperalgesia. Pain. 2019;160:1208–1223. doi: 10.1097/j.pain.0000000000001497. [DOI] [PubMed] [Google Scholar]
- 73.Zhu H., Xiang H.-C., Li H.-P., Lin L.-X., Hu X.-F., Zhang H., Meng W.-Y., Liu L., Chen C., Shu Y., et al. Inhibition of GABAergic neurons and excitation of glutamatergic neurons in the ventrolateral periaqueductal gray participate in electroacupuncture analgesia mediated by cannabinoid receptor. Front. Neurosci. 2019;13:484. doi: 10.3389/fnins.2019.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jurik A., Auffenberg E., Klein S., Deussing J.M., Schmid R.M., Wotjak C.T., Thoeringer C.K. Roles of prefrontal cortex and paraventricular thalamus in affective and mechanical components of visceral nociception. Pain. 2015;156:2479–2491. doi: 10.1097/j.pain.0000000000000318. [DOI] [PubMed] [Google Scholar]
- 75.Jiang Y.-Y., Shao S., Zhang Y., Zheng J., Chen X., Cui S., Liu F.-Y., Wan Y., Yi M. Neural pathways in medial septal cholinergic modulation of chronic pain: distinct contribution of the anterior cingulate cortex and ventral hippocampus. Pain. 2018;159:1550–1561. doi: 10.1097/j.pain.0000000000001240. [DOI] [PubMed] [Google Scholar]
- 76.Lu J., Yang L., Xu Y., Ai L., Chen J., Xiong F., Hu L., Chen H., Liu J., Yan X., et al. The modulatory effect of motor cortex astrocytes on diabetic neuropathic pain. J. Neurosci. 2021;41:5287–5302. doi: 10.1523/JNEUROSCI.2566-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sugimoto M., Takahashi Y., Sugimura Y.K., Tokunaga R., Yajima M., Kato F. Active role of the central amygdala in widespread mechanical sensitization in rats with facial inflammatory pain. Pain. 2021;162:2273–2286. doi: 10.1097/j.pain.0000000000002224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang S.-T., Song Z.-J., Liu Y., Luo W.-C., Yin Q., Zhang Y.-M. BNSTAV GABA-PVNCRF circuit regulates visceral hypersensitivity induced by maternal separation in vgat-cre mice. Front. Pharmacol. 2021;12:615202. doi: 10.3389/fphar.2021.615202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liang H.-Y., Chen Z.-J., Xiao H., Lin Y.-H., Hu Y.-Y., Chang L., Wu H.-Y., Wang P., Lu W., Zhu D.-Y., et al. nNOS-expressing neurons in the vmPFC transform pPVT-derived chronic pain signals into anxiety behaviors. Nat. Commun. 2020;11:2501. doi: 10.1038/s41467-020-16198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shen Z., Zhang H., Wu Z., He Q., Liu J., Xu Y., Yao S., He X., Chen Y., Liang Y., et al. Electroacupuncture alleviates chronic pain-induced anxiety disorders by regulating the rACC-thalamus circuitry. Front. Neurosci. 2020;14:615395. doi: 10.3389/fnins.2020.615395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brenner L., Zerlin L., Tan L.L. Functional disruption of cortical cingulate activity attenuates visceral hypersensitivity and anxiety induced by acute experimental colitis. Sci. Rep. 2021;11:2103. doi: 10.1038/s41598-021-81256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saika F., Matsuzaki S., Kobayashi D., Ideguchi Y., Nakamura T.Y., Kishioka S., Kiguchi N. Chemogenetic regulation of CX3CR1-expressing microglia using Gi-DREADD exerts sex-dependent anti-allodynic effects in mouse models of neuropathic pain. Front. Pharmacol. 2020;11:925. doi: 10.3389/fphar.2020.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grace P.M., Wang X., Strand K.A., Baratta M.V., Zhang Y., Galer E.L., Yin H., Maier S.F., Watkins L.R. DREADDed microglia in pain: implications for spinal inflammatory signaling in male rats. Exp. Neurol. 2018;304:125–131. doi: 10.1016/j.expneurol.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weir G.A., Middleton S.J., Clark A.J., Daniel T., Khovanov N., McMahon S.B., Bennett D.L. Using an engineered glutamate-gated chloride channel to silence sensory neurons and treat neuropathic pain at the source. Brain. 2017;140:2570–2585. doi: 10.1093/brain/awx201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jayaraj N.D., Bhattacharyya B.J., Belmadani A.A., Ren D., Rathwell C.A., Hackelberg S., Hopkins B.E., Gupta H.R., Miller R.J., Menichella D.M. Reducing CXCR4-mediated nociceptor hyperexcitability reverses painful diabetic neuropathy. J. Clin. Invest. 2018;128:2205–2225. doi: 10.1172/JCI92117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miller R.E., Ishihara S., Bhattacharyya B., Delaney A., Menichella D.M., Miller R.J., Malfait A.-M. Chemogenetic inhibition of pain neurons in a mouse model of osteoarthritis. Arthritis Rheumatol. 2017;69:1429–1439. doi: 10.1002/art.40118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Iyer S.M., Vesuna S., Ramakrishnan C., Huynh K., Young S., Berndt A., Lee S.Y., Gorini C.J., Deisseroth K., Delp S.L. Optogenetic and chemogenetic strategies for sustained inhibition of pain. Sci. Rep. 2016;6:30570. doi: 10.1038/srep30570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tashima R., Koga K., Yoshikawa Y., Sekine M., Watanabe M., Tozaki-Saitoh H., Furue H., Yasaka T., Tsuda M. A subset of spinal dorsal horn interneurons crucial for gating touch-evoked pain-like behavior. Proc. Natl. Acad. Sci. U S A. 2021;118 doi: 10.1073/pnas.2021220118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chakrabarti S., Pattison L.A., Doleschall B., Rickman R.H., Blake H., Callejo G., Heppenstall P.A., Smith E.S.J. Intraarticular adeno-associated virus serotype AAV-PHP.S-mediated chemogenetic targeting of knee-innervating dorsal root ganglion neurons alleviates inflammatory pain in mice. Arthritis Rheumatol. 2020;72:1749–1758. doi: 10.1002/art.41314. [DOI] [PubMed] [Google Scholar]
- 90.Kiguchi N., Saika F., Fukazawa Y., Matsuzaki S., Kishioka S. Critical role of GRP receptor-expressing neurons in the spinal transmission of imiquimod-induced psoriatic itch. Neuropsychopharmacol. Rep. 2020;40:287–290. doi: 10.1002/npr2.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang W., Li C., Chen Q., van der Goes M.-S., Hawrot J., Yao A.Y., Gao X., Lu C., Zang Y., Zhang Q., et al. Striatopallidal dysfunction underlies repetitive behavior in Shank3-deficient model of autism. J. Clin. Invest. 2017;127:1978–1990. doi: 10.1172/JCI87997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stoodley C.J., D’Mello A.M., Ellegood J., Jakkamsetti V., Liu P., Nebel M.B., Gibson J.M., Kelly E., Meng F., Cano C.A., et al. Altered cerebellar connectivity in autism and cerebellar-mediated rescue of autism-related behaviors in mice. Nat. Neurosci. 2017;20:1744–1751. doi: 10.1038/s41593-017-0004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang Y., Zhu Y., Cao S.-X., Sun P., Yang J.-M., Xia Y.-F., Xie S.-Z., Yu X.-D., Fu J.-Y., Shen C.-J., et al. MeCP2 in cholinergic interneurons of nucleus accumbens regulates fear learning. Elife. 2020;9:e55342. doi: 10.7554/eLife.55342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Grabrucker S., Pagano J., Schweizer J., Urrutia-Ruiz C., Schön M., Thome K., Ehret G., Grabrucker A.M., Zhang R., Hengerer B., et al. Activation of the medial preoptic area (MPOA) ameliorates loss of maternal behavior in a Shank2 mouse model for autism. EMBO J. 2021;40:e104267. doi: 10.15252/embj.2019104267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Horiai M., Otsuka A., Hidema S., Hiraoka Y., Hayashi R., Miyazaki S., Furuse T., Mizukami H., Teruyama R., Tamura M., et al. Targeting oxytocin receptor (Oxtr)-expressing neurons in the lateral septum to restore social novelty in autism spectrum disorder mouse models. Sci. Rep. 2020;10:22173. doi: 10.1038/s41598-020-79109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peñagarikano O., Lázaro M.T., Lu X.-H., Gordon A., Dong H., Lam H.A., Peles E., Maidment N.T., Murphy N.P., Yang X.W., et al. Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Sci. Transl. Med. 2015;7:271ra8. doi: 10.1126/scitranslmed.3010257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fortress A.M., Hamlett E.D., Vazey E.M., Aston-Jones G., Cass W.A., Boger H.A., Granholm A.-C.E. Designer receptors enhance memory in a mouse model of Down syndrome. J. Neurosci. 2015;35:1343–1353. doi: 10.1523/JNEUROSCI.2658-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Goel A., Cantu D.A., Guilfoyle J., Chaudhari G.R., Newadkar A., Todisco B., de Alba D., Kourdougli N., Schmitt L.M., Pedapati E., et al. Impaired perceptual learning in a mouse model of Fragile X syndrome is mediated by parvalbumin neuron dysfunction and is reversible. Nat. Neurosci. 2018;21:1404–1411. doi: 10.1038/s41593-018-0231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Howell C.J., Sceniak M.P., Lang M., Krakowiecki W., Abouelsoud F.E., Lad S.U., Yu H., Katz D.M. Activation of the medial prefrontal cortex reverses cognitive and respiratory symptoms in a mouse model of rett syndrome. eNeuro. 2017;4 doi: 10.1523/ENEURO.0277-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cuzon Carlson V.C., Gremel C.M., Lovinger D.M. Gestational alcohol exposure disrupts cognitive function and striatal circuits in adult offspring. Nat. Commun. 2020;11:2555. doi: 10.1038/s41467-020-16385-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kahn J.B., Port R.G., Anderson S.A., Coulter D.A. Modular, circuit-based interventions rescue hippocampal-dependent social and spatial memory in a 22q11.2 deletion syndrome mouse model. Biol. Psychiatry. 2020;88:710–718. doi: 10.1016/j.biopsych.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mukherjee A., Carvalho F., Eliez S., Caroni P. Long-lasting rescue of network and cognitive dysfunction in a genetic schizophrenia model. Cell. 2019;178:1387–1402.e14. doi: 10.1016/j.cell.2019.07.023. [DOI] [PubMed] [Google Scholar]
- 103.Yi Y., Song Y., Lu Y. Parvalbumin interneuron activation-dependent adult hippocampal neurogenesis is required for treadmill running to reverse schizophrenia-like phenotypes. Front. Cell Dev. Biol. 2020;8:24. doi: 10.3389/fcell.2020.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Marissal T., Salazar R.F., Bertollini C., Mutel S., De Roo M., Rodriguez I., Müller D., Carleton A. Restoring wild-type-like CA1 network dynamics and behavior during adulthood in a mouse model of schizophrenia. Nat. Neurosci. 2018;21:1412–1420. doi: 10.1038/s41593-018-0225-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Donegan J.J., Boley A.M., Yamaguchi J., Toney G.M., Lodge D.J. Modulation of extrasynaptic GABAA alpha 5 receptors in the ventral hippocampus normalizes physiological and behavioral deficits in a circuit specific manner. Nat. Commun. 2019;10:2819. doi: 10.1038/s41467-019-10800-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huang Y., Jiang H., Zheng Q., Fok A.H.K., Li X., Lau C.G., Lai C.S.W. Environmental enrichment or selective activation of parvalbumin-expressing interneurons ameliorates synaptic and behavioral deficits in animal models with schizophrenia-like behaviors during adolescence. Mol. Psychiatry. 2021;26:2533–2552. doi: 10.1038/s41380-020-01005-w. [DOI] [PubMed] [Google Scholar]
- 107.Sotoyama H., Namba H., Kobayashi Y., Hasegawa T., Watanabe D., Nakatsukasa E., Sakimura K., Furuyashiki T., Nawa H. Resting-state dopaminergic cell firing in the ventral tegmental area negatively regulates affiliative social interactions in a developmental animal model of schizophrenia. Transl. Psychiatry. 2021;11:236. doi: 10.1038/s41398-021-01346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Robinson S.L., Marrero I.M., Perez-Heydrich C.A., Sepulveda-Orengo M.T., Reissner K.J., Thiele T.E. Medial prefrontal cortex neuropeptide Y modulates binge-like ethanol consumption in C57BL/6J mice. Neuropsychopharmacology. 2019;44:1132–1140. doi: 10.1038/s41386-018-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Anderson R.I., Lopez M.F., Griffin W.C., Haun H.L., Bloodgood D.W., Pati D., Boyt K.M., Kash T.L., Becker H.C. Dynorphin-kappa opioid receptor activity in the central amygdala modulates binge-like alcohol drinking in mice. Neuropsychopharmacology. 2019;44:1084–1092. doi: 10.1038/s41386-018-0294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cassataro D., Bergfeldt D., Malekian C., Van Snellenberg J.X., Thanos P.K., Fishell G., Sjulson L. Reverse pharmacogenetic modulation of the nucleus accumbens reduces ethanol consumption in a limited access paradigm. Neuropsychopharmacology. 2014;39:283–290. doi: 10.1038/npp.2013.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Valyear M.D., Glovaci I., Zaari A., Lahlou S., Trujillo-Pisanty I., Andrew Chapman C., Chaudhri N. Dissociable mesolimbic dopamine circuits control responding triggered by alcohol-predictive discrete cues and contexts. Nat. Commun. 2020;11:3764. doi: 10.1038/s41467-020-17543-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Robins M.T., Chiang T., Mores K.L., Alongkronrusmee D., van Rijn R.M. Critical role for Gi/o-protein activity in the dorsal striatum in the reduction of voluntary alcohol intake in C57Bl/6 mice. Front. Psychiatry. 2018;9:112. doi: 10.3389/fpsyt.2018.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Scarlata M.J., Lee S.H., Lee D., Kandigian S.E., Hiller A.J., Dishart J.G., Mintz G.E., Wang Z., Coste G.I., Mousley A.L., et al. Chemogenetic stimulation of the infralimbic cortex reverses alcohol-induced fear memory overgeneralization. Sci. Rep. 2019;9:6730. doi: 10.1038/s41598-019-43159-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Centanni S.W., Morris B.D., Luchsinger J.R., Bedse G., Fetterly T.L., Patel S., Winder D.G. Endocannabinoid control of the insular-bed nucleus of the stria terminalis circuit regulates negative affective behavior associated with alcohol abstinence. Neuropsychopharmacology. 2019;44:526–537. doi: 10.1038/s41386-018-0257-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pleil K.E., Rinker J.A., Lowery-Gionta E.G., Mazzone C.M., McCall N.M., Kendra A.M., Olson D.P., Lowell B.B., Grant K.A., Thiele T.E., et al. NPY signaling inhibits extended amygdala CRF neurons to suppress binge alcohol drinking. Nat. Neurosci. 2015;18:545–552. doi: 10.1038/nn.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.You I.-J., Wright S.R., Garcia-Garcia A.L., Tapper A.R., Gardner P.D., Koob G.F., David Leonardo E., Bohn L.M., Wee S. 5-HT1A autoreceptors in the dorsal raphe nucleus convey vulnerability to compulsive cocaine seeking. Neuropsychopharmacology. 2016;41:1210–1222. doi: 10.1038/npp.2015.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Scofield M.D., Boger H.A., Smith R.J., Li H., Haydon P.G., Kalivas P.W. Gq-DREADD selectively initiates glial glutamate release and inhibits cue-induced cocaine seeking. Biol. Psychiatry. 2015;78:441–451. doi: 10.1016/j.biopsych.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Augur I.F., Wyckoff A.R., Aston-Jones G., Kalivas P.W., Peters J. Chemogenetic activation of an extinction neural circuit reduces cue-induced reinstatement of cocaine seeking. J. Neurosci. 2016;36:10174–10180. doi: 10.1523/JNEUROSCI.0773-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Marchant N.J., Campbell E.J., Whitaker L.R., Harvey B.K., Kaganovsky K., Adhikary S., Hope B.T., Heins R.C., Prisinzano T.E., Vardy E., et al. Role of ventral subiculum in context-induced relapse to alcohol seeking after punishment-imposed abstinence. J. Neurosci. 2016;36:3281–3294. doi: 10.1523/JNEUROSCI.4299-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chisholm A., Rizzo D., Fortin É., Moman V., Quteishat N., Romano A., Capolicchio T., Shalev U. Assessing the role of corticothalamic and thalamo-accumbens projections in the augmentation of heroin seeking in chronically food-restricted rats. J. Neurosci. 2021;41:354–365. doi: 10.1523/JNEUROSCI.2103-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nwachukwu K.N., Evans W.A., Sides T.R., Trevisani C.P., Davis A., Marshall S.A. Chemogenetic manipulation of astrocytic signaling in the basolateral amygdala reduces binge-like alcohol consumption in male mice. J. Neurosci. Res. 2021;99:1957–1972. doi: 10.1002/jnr.24841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tunc-Ozcan E., Peng C.-Y., Zhu Y., Dunlop S.R., Contractor A., Kessler J.A. Activating newborn neurons suppresses depression and anxiety-like behaviors. Nat. Commun. 2019;10:3768. doi: 10.1038/s41467-019-11641-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sachs B.D., Ni J.R., Caron M.G. Brain 5-HT deficiency increases stress vulnerability and impairs antidepressant responses following psychosocial stress. Proc. Natl. Acad. Sci. U S A. 2015;112:2557–2562. doi: 10.1073/pnas.1416866112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Prakash N., Stark C.J., Keisler M.N., Luo L., Der-Avakian A., Dulcis D. Serotonergic plasticity in the dorsal raphe nucleus characterizes susceptibility and resilience to anhedonia. J. Neurosci. 2020;40:569–584. doi: 10.1523/JNEUROSCI.1802-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ma H., Li C., Wang J., Zhang X., Li M., Zhang R., Huang Z., Zhang Y. Amygdala-hippocampal innervation modulates stress-induced depressive-like behaviors through AMPA receptors. Proc. Natl. Acad. Sci. U S A. 2021;118 doi: 10.1073/pnas.2019409118. e2019409118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cheng J., Umschweif G., Leung J., Sagi Y., Greengard P. HCN2 channels in cholinergic interneurons of nucleus accumbens shell regulate depressive behaviors. Neuron. 2019;101:662–672.e5. doi: 10.1016/j.neuron.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 127.Fang X., Jiang S., Wang J., Bai Y., Kim C.S., Blake D., Weintraub N.L., Lei Y., Lu X.-Y. Chronic unpredictable stress induces depression-related behaviors by suppressing AgRP neuron activity. Mol. Psychiatry. 2021;26:2299–2315. doi: 10.1038/s41380-020-01004-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhong P., Liu X., Zhang Z., Hu Y., Liu S.J., Lezama-Ruiz M., Joksimovic M., Liu Q.-S. Cyclin-dependent kinase 5 in the ventral tegmental area regulates depression-related behaviors. J. Neurosci. 2014;34:6352–6366. doi: 10.1523/JNEUROSCI.3673-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sun T., Song Z., Tian Y., Tian W., Zhu C., Ji G., Luo Y., Chen S., Wang L., Mao Y., et al. Basolateral amygdala input to the medial prefrontal cortex controls obsessive-compulsive disorder-like checking behavior. Proc. Natl. Acad. Sci. U S A. 2019;116:3799–3804. doi: 10.1073/pnas.1814292116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bicks L.K., Yamamuro K., Flanigan M.E., Kim J.M., Kato D., Lucas E.K., Koike H., Peng M.S., Brady D.M., Chandrasekaran S., et al. Prefrontal parvalbumin interneurons require juvenile social experience to establish adult social behavior. Nat. Commun. 2020;11:1003. doi: 10.1038/s41467-020-14740-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hultman R., Mague S.D., Li Q., Katz B.M., Michel N., Lin L., Wang J., David L.K., Blount C., Chandy R., et al. Dysregulation of prefrontal cortex-mediated slow-evolving limbic dynamics drives stress-induced emotional pathology. Neuron. 2016;91:439–452. doi: 10.1016/j.neuron.2016.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Grafe L.A., Eacret D., Dobkin J., Bhatnagar S. Reduced orexin system function contributes to resilience to repeated social stress. eNeuro. 2018;5 doi: 10.1523/ENEURO.0273-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Milton L.K., Mirabella P.N., Greaves E., Spanswick D.C., van den Buuse M., Oldfield B.J., Foldi C.J. Suppression of corticostriatal circuit activity improves cognitive flexibility and prevents body weight loss in activity-based anorexia in rats. Biol. Psychiatry. 2020;90:819. doi: 10.1016/j.biopsych.2020.06.022. [DOI] [PubMed] [Google Scholar]
- 134.van Heukelum S., Tulva K., Geers F.E., van Dulm S., Ruisch I.H., Mill J., Viana J.F., Beckmann C.F., Buitelaar J.K., Poelmans G., et al. A central role for anterior cingulate cortex in the control of pathological aggression. Curr. Biol. 2021;31:2321–2333.e5. doi: 10.1016/j.cub.2021.03.062. [DOI] [PubMed] [Google Scholar]
- 135.Dieterich A., Floeder J., Stech K., Lee J., Srivastava P., Barker D.J., Samuels B.A. Activation of basolateral amygdala to nucleus accumbens projection neurons attenuates chronic corticosterone-induced behavioral deficits in male mice. Front. Behav. Neurosci. 2021;15:643272. doi: 10.3389/fnbeh.2021.643272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Weera M.M., Shackett R.S., Kramer H.M., Middleton J.W., Gilpin N.W. Central amygdala projections to lateral hypothalamus mediate avoidance behavior in rats. J. Neurosci. 2021;41:61–72. doi: 10.1523/JNEUROSCI.0236-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Roseboom P.H., Mueller S.A.L., Oler J.A., Fox A.S., Riedel M.K., Elam V.R., Olsen M.E., Gomez J.L., Boehm M.A., DiFilippo A.H., et al. Evidence in primates supporting the use of chemogenetics for the treatment of human refractory neuropsychiatric disorders. Mol. Ther. 2021 doi: 10.1016/j.ymthe.2021.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ling W.-Y., Cui Y., Gao J.-L., Jiang X.-H., Wang K.-J., Tian Y.-X., Sheng H.-X., Cui J.-Z. Long-term chemogenetic activation of M1 glutamatergic neurons attenuates the behavioral and cognitive deficits caused by intracerebral hemorrhage. Biochem. Biophys. Res. Commun. 2020;527:22–28. doi: 10.1016/j.bbrc.2020.04.083. [DOI] [PubMed] [Google Scholar]
- 139.Chandrasekar A., Heuvel F.O., Tar L., Hagenston A.M., Palmer A., Linkus B., Ludolph A.C., Huber-Lang M., Boeckers T., Bading H., et al. Parvalbumin interneurons shape neuronal vulnerability in blunt TBI. Cereb. Cortex. 2019;29:2701–2715. doi: 10.1093/cercor/bhy139. [DOI] [PubMed] [Google Scholar]
- 140.Hu K.-H., Li Y.-A., Jia W., Wu G.-Y., Sun L., Wang S.-R., Yu L.-H. Chemogenetic activation of glutamatergic neurons in the motor cortex promotes functional recovery after ischemic stroke in rats. Behav. Brain Res. 2019;359:81–88. doi: 10.1016/j.bbr.2018.10.029. [DOI] [PubMed] [Google Scholar]
- 141.Wu D., Jin Y., Shapiro T.M., Hinduja A., Baas P.W., Tom V.J. Chronic neuronal activation increases dynamic microtubules to enhance functional axon regeneration after dorsal root crush injury. Nat. Commun. 2020;11:6131. doi: 10.1038/s41467-020-19914-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jaiswal P.B., Mistretta O.C., Ward P.J., English A.W. Chemogenetic enhancement of axon regeneration following peripheral nerve injury in the slick-a mouse. Brain Sci. 2018;8:93. doi: 10.3390/brainsci8050093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gao Z., Yang Y., Feng Z., Li X., Min C., Zhu Z., Song H., Hu Y., Wang Y., He X. Chemogenetic stimulation of proprioceptors remodels lumbar interneuron excitability and promotes motor recovery after SCI. Mol. Ther. 2021;29:2483–2498. doi: 10.1016/j.ymthe.2021.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ueno M., Ueno-Nakamura Y., Niehaus J., Popovich P.G., Yoshida Y. Silencing spinal interneurons inhibits immune suppressive autonomic reflexes caused by spinal cord injury. Nat. Neurosci. 2016;19:784–787. doi: 10.1038/nn.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Garrott K., Dyavanapalli J., Cauley E., Dwyer M.K., Kuzmiak-Glancy S., Wang X., Mendelowitz D., Kay M.W. Chronic activation of hypothalamic oxytocin neurons improves cardiac function during left ventricular hypertrophy-induced heart failure. Cardiovasc. Res. 2017;113:1318–1328. doi: 10.1093/cvr/cvx084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Moreira T.S., Antunes V.R., Falquetto B., Marina N. Long-term stimulation of cardiac vagal preganglionic neurons reduces blood pressure in the spontaneously hypertensive rat. J. Hypertens. 2018;36:2444–2452. doi: 10.1097/HJH.0000000000001871. [DOI] [PubMed] [Google Scholar]
- 147.Wang Y., Jiang W., Chen H., Zhou H., Liu Z., Liu Z., Liu Z., Zhou Y., Zhou X., Yu L., et al. Sympathetic nervous system mediates cardiac remodeling after myocardial infarction in a circadian disruption model. Front Cardiovasc. Med. 2021;8:668387. doi: 10.3389/fcvm.2021.668387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hasegawa E., Maejima T., Yoshida T., Masseck O.A., Herlitze S., Yoshioka M., Sakurai T., Mieda M. Serotonin neurons in the dorsal raphe mediate the anticataplectic action of orexin neurons by reducing amygdala activity. Proc. Natl. Acad. Sci. U S A. 2017;114:E3526–E3535. doi: 10.1073/pnas.1614552114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fleury Curado T., Fishbein K., Pho H., Brennick M., Dergacheva O., Sennes L.U., Pham L.V., Ladenheim E.E., Spencer R., Mendelowitz D., et al. Chemogenetic stimulation of the hypoglossal neurons improves upper airway patency. Sci. Rep. 2017;7:44392. doi: 10.1038/srep44392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fleury Curado T., Pho H., Freire C., Amorim M.R., Bonaventura J., Kim L.J., Lee R., Cabassa M.E., Streeter S.R., Branco L.G., et al. Designer receptors exclusively activated by designer drugs approach to treatment of sleep-disordered breathing. Am. J. Respir. Crit. Care Med. 2021;203:102–110. doi: 10.1164/rccm.202002-0321OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sironi V.A. Origin and evolution of deep brain stimulation. Front. Integr. Neurosci. 2011;5:42. doi: 10.3389/fnint.2011.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lozano A.M., Lipsman N., Bergman H., Brown P., Chabardes S., Chang J.W., Matthews K., McIntyre C.C., Schlaepfer T.E., Schulder M., et al. Deep brain stimulation: current challenges and future directions. Nat. Rev. Neurol. 2019;15:148–160. doi: 10.1038/s41582-018-0128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nair D.R., Laxer K.D., Weber P.B., Murro A.M., Park Y.D., Barkley G.L., Smith B.J., Gwinn R.P., Doherty M.J., Noe K.H., et al. Nine-year prospective efficacy and safety of brain-responsive neurostimulation for focal epilepsy. Neurology. 2020;95:e1244–e1256. doi: 10.1212/WNL.0000000000010154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chiken S., Nambu A. Mechanism of deep brain stimulation: inhibition, excitation, or disruption? Neuroscientist. 2016;22:313–322. doi: 10.1177/1073858415581986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Poth K.M., Texakalidis P., Boulis N.M. Chemogenetics: beyond lesions and electrodes. Neurosurgery. 2021;89:185–195. doi: 10.1093/neuros/nyab147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sahel J.-A., Boulanger-Scemama E., Pagot C., Arleo A., Galluppi F., Martel J.N., Esposti S.D., Delaux A., de Saint Aubert J.-B., de Montleau C., et al. Partial recovery of visual function in a blind patient after optogenetic therapy. Nat. Med. 2021;27:1223–1229. doi: 10.1038/s41591-021-01351-4. [DOI] [PubMed] [Google Scholar]
- 157.Montgomery K.L., Iyer S.M., Christensen A.J., Deisseroth K., Delp S.L. Beyond the brain: optogenetic control in the spinal cord and peripheral nervous system. Sci. Transl. Med. 2016;8:337rv5. doi: 10.1126/scitranslmed.aad7577. [DOI] [PubMed] [Google Scholar]
- 158.Raimondo J.V., Kay L., Ellender T.J., Akerman C.J. Optogenetic silencing strategies differ in their effects on inhibitory synaptic transmission. Nat. Neurosci. 2012;15:1102–1104. doi: 10.1038/nn.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yan X., Telu S., Dick R.M., Liow J.-S., Zanotti-Fregonara P., Morse C.L., Manly L.S., Gladding R.L., Shrestha S., Lerchner W., et al. [11C]deschloroclozapine is an improved PET radioligand for quantifying a human muscarinic DREADD expressed in monkey brain. J. Cereb. Blood Flow Metab. 2021 doi: 10.1177/0271678X211007949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hu F., Morris P.J., Bonaventura J., Fan H., Mathews W.B., Holt D.P., Lam S., Boehm M., Dannals R.F., Pomper M.G., et al. 18F-labeled radiotracers for in vivo imaging of DREADD with positron emission tomography. Eur. J. Med. Chem. 2021;213:113047. doi: 10.1016/j.ejmech.2020.113047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ji B., Kaneko H., Minamimoto T., Inoue H., Takeuchi H., Kumata K., Zhang M.R., Aoki I., Seki C., Ono M., et al. Multimodal imaging for DREADD-expressing neurons in living brain and their application to implantation of iPSC-derived neural progenitors. J. Neurosci. 2016;36:11544–11558. doi: 10.1523/JNEUROSCI.1279-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Roelofs T.J.M., Verharen J.P.H., van Tilborg G.A.F., Boekhoudt L., van der Toorn A., de Jong J.W., Luijendijk M.C.M., Otte W.M., Adan R.A.H., Dijkhuizen R.M. A novel approach to map induced activation of neuronal networks using chemogenetics and functional neuroimaging in rats: a proof-of-concept study on the mesocorticolimbic system. Neuroimage. 2017;156:109–118. doi: 10.1016/j.neuroimage.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 163.Anderson S.A.R., Michaelides M., Zarnegar P., Ren Y., Fagergren P., Thanos P.K., Wang G.J., Bannon M., Neumaier J.F., Keller E., et al. Impaired periamygdaloid-cortex prodynorphin is characteristic of opiate addiction and depression. J. Clin. Invest. 2013;123:5334–5341. doi: 10.1172/JCI70395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Michaelides M., Anderson S.A., Ananth M., Smirnov D., Thanos P.K., Neumaier J.F., Wang G.J., Volkow N.D., Hurd Y.L. Whole-brain circuit dissection in free-moving animals reveals cell-specific mesocorticolimbic networks. J. Clin. Invest. 2013;123:5342–5350. doi: 10.1172/JCI72117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Giorgi A., Migliarini S., Galbusera A., Maddaloni G., Mereu M., Margiani G., Gritti M., Landi S., Trovato F., Bertozzi S.M., et al. Brain-wide mapping of endogenous serotonergic transmission via chemogenetic fMRI. Cell Rep. 2017;21:910–918. doi: 10.1016/j.celrep.2017.09.087. [DOI] [PubMed] [Google Scholar]
- 166.Allen N.J., Lyons D.A. Glia as architects of central nervous system formation and function. Science. 2018;362:181–185. doi: 10.1126/science.aat0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hudry E., Vandenberghe L.H. Therapeutic AAV gene transfer to the nervous system: a clinical reality. Neuron. 2019;101:839–862. doi: 10.1016/j.neuron.2019.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bankiewicz K.S., Sudhakar V., Samaranch L., San Sebastian W., Bringas J., Forsayeth J. AAV viral vector delivery to the brain by shape-conforming MR-guided infusions. J. Control Release. 2016;240:434–442. doi: 10.1016/j.jconrel.2016.02.034. [DOI] [PubMed] [Google Scholar]
- 169.Salegio E.A., Bringas J., Bankiewicz K.S. MRI-guided delivery of viral vectors. Methods Mol. Biol. 2016;1382:217–230. doi: 10.1007/978-1-4939-3271-9_15. [DOI] [PubMed] [Google Scholar]
- 170.Fiandaca M.S., Varenika V., Eberling J., McKnight T., Bringas J., Pivirotto P., Beyer J., Hadaczek P., Bowers W., Park J., et al. Real-time MR imaging of adeno-associated viral vector delivery to the primate brain. Neuroimage. 2009;47:T27–T35. doi: 10.1016/j.neuroimage.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nagahara A.H., Wilson B.R., Ivasyk I., Kovacs I., Rawalji S., Bringas J.R., Pivirotto P.J., Sebastian W.S., Samaranch L., Bankiewicz K.S., et al. MR-guided delivery of AAV2-BDNF into the entorhinal cortex of non-human primates. Gene Ther. 2018;25:104–114. doi: 10.1038/s41434-018-0010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.San Sebastian W., Kells A.P., Bringas J., Samaranch L., Hadaczek P., Ciesielska A., Macayan M., Pivirotto P.J., Forsayeth J., Osborne S., et al. Safety and tolerability of MRI-guided infusion of AAV2-HAADC into the mid-brain of non-human primate. Mol. Ther. Methods Clin. Dev. 2014;3:14049. doi: 10.1038/mtm.2014.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pearson, T., Gupta, N., Sebastian, W.S., Imamura-Ching, J., Viehoever, A., Grijalvo-Perez, A., Fay, A., Seth, N., Lundy, S., Seo, Y., et al. Gene therapy for aromatic L-amino acid decarboxylase deficiency by MR-guided direct delivery of AAV2-AADC to midbrain dopaminergic neurons.Nat. Commun. 12.4251 [DOI] [PMC free article] [PubMed]
- 174.Stavarache M.A., Petersen N., Jurgens E.M., Milstein E.R., Rosenfeld Z.B., Ballon D.J., Kaplitt M.G. Safe and stable noninvasive focal gene delivery to the mammalian brain following focused ultrasound. J. Neurosurg. 2018;130:989–998. doi: 10.3171/2017.8.JNS17790. [DOI] [PubMed] [Google Scholar]
- 175.Szablowski J.O., Lee-Gosselin A., Lue B., Malounda D., Shapiro M.G. Acoustically targeted chemogenetics for the non-invasive control of neural circuits. Nat. Biomed. Eng. 2018;2:475–484. doi: 10.1038/s41551-018-0258-2. [DOI] [PubMed] [Google Scholar]
- 176.Hadaczek P., Kohutnicka M., Krauze M.T., Bringas J., Pivirotto P., Cunningham J., Bankiewicz K. Convection-enhanced delivery of adeno-associated virus type 2 (AAV2) into the striatum and transport of AAV2 within monkey brain. Hum. Gene Ther. 2006;17:291–302. doi: 10.1089/hum.2006.17.291. [DOI] [PubMed] [Google Scholar]
- 177.Griffin J.M., Fackelmeier B., Fong D.M., Mouravlev A., Young D., O’Carroll S.J. Astrocyte-selective AAV gene therapy through the endogenous GFAP promoter results in robust transduction in the rat spinal cord following injury. Gene Ther. 2019;26:198–210. doi: 10.1038/s41434-019-0075-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fenno L.E., Mattis J., Ramakrishnan C., Hyun M., Lee S.Y., He M., Tucciarone J., Selimbeyoglu A., Berndt A., Grosenick L., et al. Targeting cells with single vectors using multiple-feature Boolean logic. Nat. Methods. 2014;11:763–772. doi: 10.1038/nmeth.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Samaranch L., Blits B., San Sebastian W., Hadaczek P., Bringas J., Sudhakar V., Macayan M., Pivirotto P.J., Petry H., Bankiewicz K.S. MR-guided parenchymal delivery of adeno-associated viral vector serotype 5 in non-human primate brain. Gene Ther. 2017;24:253–261. doi: 10.1038/gt.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bai Y., Ma L.-T., Chen Y.-B., Ren D., Chen Y.-B., Li Y.-Q., Sun H.-K., Qiu X.-T., Zhang T., Zhang M.-M., et al. Anterior insular cortex mediates hyperalgesia induced by chronic pancreatitis in rats. Mol. Brain. 2019;12:76. doi: 10.1186/s13041-019-0497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Upright N.A., Baxter M.G. Effect of chemogenetic actuator drugs on prefrontal cortex-dependent working memory in nonhuman primates. Neuropsychopharmacology. 2020;45:1793–1798. doi: 10.1038/s41386-020-0660-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Liu S., Tang Y., Shu H., Tatum D., Bai Q., Crawford J., Xing Y., Lobo M.K., Bellinger L., Kramer P., et al. Dopamine receptor D2, but not D1, mediates descending dopaminergic pathway-produced analgesic effect in a trigeminal neuropathic pain mouse model. Pain. 2019;160:334–344. doi: 10.1097/j.pain.0000000000001414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Atasoy D., Aponte Y., Su H.H., Sternson S.M. A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J. Neurosci. 2008;28:7025–7030. doi: 10.1523/JNEUROSCI.1954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rezai Amin S., Gruszczynski C., Guiard B.P., Callebert J., Launay J.-M., Louis F., Betancur C., Vialou V., Gautron S. Viral vector-mediated Cre recombinase expression in substantia nigra induces lesions of the nigrostriatal pathway associated with perturbations of dopamine-related behaviors and hallmarks of programmed cell death. J. Neurochem. 2019;150:330–340. doi: 10.1111/jnc.14684. [DOI] [PubMed] [Google Scholar]
- 185.Forni P.E., Scuoppo C., Imayoshi I., Taulli R., Dastrù W., Sala V., Betz U.A.K., Muzzi P., Martinuzzi D., Vercelli A.E., et al. High levels of Cre expression in neuronal progenitors cause defects in brain development leading to microencephaly and hydrocephaly. J. Neurosci. 2006;26:9593–9602. doi: 10.1523/JNEUROSCI.2815-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Loonstra A., Vooijs M., Beverloo H.B., Allak B.A., van Drunen E., Kanaar R., Berns A., Jonkers J. Growth inhibition and DNA damage induced by Cre recombinase in mammalian cells. Proc. Natl. Acad. Sci. U S A. 2001;98:9209–9214. doi: 10.1073/pnas.161269798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhu J., Nguyen M.-T., Nakamura E., Yang J., Mackem S. Cre-mediated recombination can induce apoptosis in vivo by activating the p53 DNA damage-induced pathway. Genesis. 2012;50:102–111. doi: 10.1002/dvg.20799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mähönen A.J., Airenne K.J., Lind M.M., Lesch H.P., Ylä-Herttuala S. Optimized self-excising Cre-expression cassette for mammalian cells. Biochem. Biophys. Res. Commun. 2004;320:366–371. doi: 10.1016/j.bbrc.2004.05.175. [DOI] [PubMed] [Google Scholar]
- 189.Silver D.P., Livingston D.M. Self-excising retroviral vectors encoding the Cre recombinase overcome Cre-mediated cellular toxicity. Mol. Cell. 2001;8:233–243. doi: 10.1016/s1097-2765(01)00295-7. [DOI] [PubMed] [Google Scholar]
- 190.Chao O.Y., Marron Fernandez de Velasco E., Pathak S.S., Maitra S., Zhang H., Duvick L., Wickman K., Orr H.T., Hirai H., Yang Y.-M. Targeting inhibitory cerebellar circuitry to alleviate behavioral deficits in a mouse model for studying idiopathic autism. Neuropsychopharmacology. 2020;45:1159–1170. doi: 10.1038/s41386-020-0656-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhao E.M., Mao A.S., de Puig H., Zhang K., Tippens N.D., Tan X., Ran F.A., Han I., Nguyen P.Q., Chory E.J., et al. RNA-responsive elements for eukaryotic translational control. Nat. Biotechnol. 2021 doi: 10.1038/s41587-021-01068-2. [DOI] [PubMed] [Google Scholar]
- 192.Green A.A., Silver P.A., Collins J.J., Yin P. Toehold switches: de-novo-designed regulators of gene expression. Cell. 2014;159:925–939. doi: 10.1016/j.cell.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Dimidschstein J., Chen Q., Tremblay R., Rogers S.L., Saldi G.-A., Guo L., Xu Q., Liu R., Lu C., Chu J., et al. A viral strategy for targeting and manipulating interneurons across vertebrate species. Nat. Neurosci. 2016;19:1743–1749. doi: 10.1038/nn.4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Chabrol E., Cornford J.H., Hashemi K.S., Hughes M.P., Kullmann D.M., Lieb A., Massaro G., Rahim A.A., Schorge S., Shekh-Ahmad T., et al. Epilepsy gene therapy using an engineered potassium channel. J. Neurosci. 2019;39:3159–3169. doi: 10.1523/JNEUROSCI.1143-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Vranjkovic O., Van Newenhizen E.C., Nordness M.E., Blacktop J.M., Urbanik L.A., Mathy J.C., McReynolds J.R., Miller A.M., Doncheck E.M., Kloehn T.M., et al. Enhanced CRFR1-dependent regulation of a ventral tegmental area to prelimbic cortex projection establishes susceptibility to stress-induced cocaine seeking. J. Neurosci. 2018;38:10657–10671. doi: 10.1523/JNEUROSCI.2080-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Grayson D.S., Bliss-Moreau E., Machado C.J., Bennett J., Shen K., Grant K.A., Fair D.A., Amaral D.G. The rhesus monkey connectome predicts disrupted functional networks resulting from pharmacogenetic inactivation of the amygdala. Neuron. 2016;91:453–466. doi: 10.1016/j.neuron.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Cho J., Ryu S., Lee S., Kim J., Kim H.-I. Optimizing clozapine for chemogenetic neuromodulation of somatosensory cortex. Sci. Rep. 2020;10:6001. doi: 10.1038/s41598-020-62923-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Surmeier D.J., James Surmeier D., Ding J., Day M., Wang Z., Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.