Abstract
Diabetic kidney disease (DKD) is one of the most frequent causes of chronic kidney disease (CKD) in the United States. Chronic hyperglycemic conditions are thought to be the primary cause of DKD. However, it is clinically difficult to achieve glycemic control in individuals with diabetes. Recent advances in mitochondrial biology have provided a new understanding of mitochondrial dysfunction in DKD. Studies have revealed impaired mitochondrial function in a variety of diabetic complications, including DKD; moreover, abnormal mitochondrial fission may be involved in the progression of DKD. It has been reported that metformin or sodium-glucose cotransporter 2 (SGLT2) inhibitors may provide renal protection by improving mitochondrial dynamics and reducing oxidative stress. Thus, drugs that target the restoration of mitochondrial function may become novel therapeutic agents for DKD. Imeglimin is the first in a new class of oral antidiabetic drugs that can reduce reactive oxygen species production and increase mitochondrial DNA synthesis. This review outlines the potential therapeutic interventions that affect mitochondrial function and prevent DKD.
Keywords: Diabetic kidney disease (DKD), Imeglimin, Metformin, Mitochondrial function, NFE-2 related factor 2 (Nrf2), Reactive oxygen species (ROS), Sodium-glucose cotransporter 2 (SGLT2)
Diabetic kidney disease (DKD), Imeglimin, Metformin, Mitochondrial function, NFE-2 related factor 2 (Nrf2), Reactive oxygen species (ROS), Sodium-glucose cotransporter 2 (SGLT2).
1. Introduction
Diabetes is the most common cause of chronic kidney disease (CKD) and chronic hyperglycemia is a major cause of diabetic kidney disease (DKD). Several studies have shown that there is a causal relationship between the degree of blood glucose control and the development of complications in patients with diabetes (Diabetes et al., 1993). Long-term follow-up of The Diabetic Control and Complications Trial (DCCT) showed that patients who underwent intensive blood glucose control had a reduced incidence of cardiovascular disease with atherosclerosis (Nathan, 1993). Furthermore, multiple factors, such as insulin resistance and the levels of fatty acids and lipids are known to increase the risk of DKD (Banba et al., 2000; Mima et al., 2018; Okada et al., 2003; Sassy-Prigent et al., 2000). Despite this evidence, conventional therapies used for maintaining blood glucose levels in patients with diabetes do not always prevent the progression of DKD (Mima, 2013). Therefore, there is a need for new drugs that can help achieve glycemic control and improve DKD. Prolonged hyperglycemia is thought to inhibit the catabolic pathway and cause excessive production of reactive oxygen species (ROS) by mitochondria, which may contribute to DKD development (Lachaux et al., 2020). Mitochondrial homeostasis is modulated by several mechanisms, such as the mitochondrial dynamics known as fission and fusion (Alexander et al., 2000; Bhargava and Schnellmann, 2017; Delettre et al., 2000). Mitophagy suppression reportedly impairs mitochondria in the proximal tubules in DKD cases (Bhargava and Schnellmann, 2017). Thus, DKD therapy may help the restoration of mitochondrial function. It has been reported that metformin can reduce the production of ROS at mitochondrial respiratory-chain complex 1 and prevent mitochondrial-mediated apoptosis, suggesting that it may protect against oxidative stress-induced renal cell death (Birsoy et al., 2014; El-Mir et al., 2000).
Furthermore, empagliflozin, a sodium-glucose cotransporter 2 (SGLT2) inhibitor, regulates mitochondrial biogenesis and the balance of proteins responsible for mitochondrial fission and fusion, thus reducing ROS in cultured proximal tubular cells (Lee et al., 2019).
Imeglimin is a novel oral hypoglycemic agent that is being clinically tested as a monotherapy or an add-on therapy to reduce fasting blood glucose levels or hemoglobin A1c (Fouqueray et al., 2013, Fouqueray et al., 2014, Pirags et al., 2012). Improved glucose tolerance has been reported in humans and rodents treated with imeglimin due to a variety of mechanisms, including decreased hepatic lipids, improved insulin signaling in the liver and muscle, and ameliorated β-cell function (Birsoy et al., 2014). More recent data indicate that imeglimin prevents endothelial death by decreasing the size of the mitochondrial permeability transition pore, which plays a pivotal role in cell death, without inhibiting mitochondrial respiration (Detaille et al., 2016).
These data suggest that this series of oral antidiabetic drugs exerts a renoprotective effect by improving mitochondrial function in the kidneys. In this review, we further discuss the potential benefits of using imeglimin, as well as other agents, in treating DKD.
2. Diabetes and oxidative stress
Several previous studies have reported oxidant production in both type 1 and type 2 diabetes. Oxidative stress occurs when the rate of oxidant production exceeds that of scavengers. Furthermore, it can also be caused by changes in the ratio of nicotinamide adenine dinucleotide phosphate (NADPH) to nicotinamide adenine dinucleotide (NADP) (Bedard and Krause, 2007). Abnormal metabolism of glucose and free fatty acids (FFAs) in the mitochondrial pathway and the activation of NADPH oxidase via protein kinase C (PKC) were previously identified as factors contributing to oxidant production (King and Loeken, 2004) (Mima, 2013). Our previous studies revealed that reactive oxygen species (ROS) levels were elevated in the kidney and retina under diabetic conditions or insulin resistance (Mima et al., 2011a) (Mima et al., 2012c). The levels of plasma 8-hydroxydeoxyguanosine, isoprostanes, and lipid peroxides are significantly increased in both diabetic rodents and patients (Mima et al., 2012c) (Wu et al., 2004). Thus, increased ROS production in diabetes mellitus is attributed to abnormal metabolism of glucose and FFAs. This phenomenon could explain the increased oxidative stress in insulin-resistant patients without diabetes (Mezzetti et al., 2000) (Mima, 2013).
3. The effects of oxidative stress on DKD
Several studies have shown that diabetic state and insulin resistance can lead to oxidative stress. This occurs when free glucose activates aldose reductase activity and the polyol pathway, decreasing the NADPH/NADP+ ratio. Elevated intracellular glucose activates PKC via de novo synthesis of diacylglucose (DAG) (King and Loeken, 2004). The DAG-PKC signal transduction pathway has been associated with DKD, with increases in PKC activity known to induce extracellular matrix (ECM) accumulation in the glomeruli (Mima et al., 2011a) (Mima et al., 2012a) (Koya et al., 2000). Increased oxidative stress may be involved in the development of DKD. However, inhibition of the polyol pathway with aldose reductase inhibitors may reduce the effect of hyperglycemia on DKD. Furthermore, the administration of vitamins C and E could effectively improve DKD (Bursell and King, 1999). High doses of vitamin E can normalize ROS and ameliorate vascular abnormalities caused by the activation of the DAG-PKC pathway in the kidneys (Lee et al., 1999).
4. The effects of inflammatory processes on DKD
Metabolism or hemodynamics are the main causes of developing DKD, but recent studies have shown that DKD is an inflammatory processes and immune cells may be involved. Hyperglycemia could increase interleukin (IL)-1β, IL-6, or IL-12 which are involved in the progression of DKD (Chen et al., 2004, Ha et al., 2002, Mima, 2013, Myint et al., 2006, Wu et al., 2010). Further, recent study showed that hyperglycemia activated IL8-CXCR1/2 axis, inducing podocyte damage in DKD (Loretelli et al., 2021). Interestingly, podocytes have shown to express Toll-like receptor 4 (TLR4), and stimulation with TLR4-specific ligands induces the costimulatory molecule B7-1. It is reported that the upregulation of B7-1 could be a novel mechanism of developing DKD. CTLA4-Ig, which targets B7-1, has been clinically applied to the treatment of inflammatory and immunological diseases. Thus, it is expected to have beneficial effects on DKD (Bassi et al., 2016).
5. DKD and mitochondrial dysfunction
In diabetic nephropathy, mitochondrial energy is altered by increased ROS and hyperglycemia. ROS production is directly related to insulin resistance (Rovira-Llopis et al., 2018). However, the mechanism by which a mitochondrion produces more ROS in diabetes patients is still unclear. Hyperglycemia and dyslipidemia promote glucose and lipid catabolism, thus increasing the production of nicotinamide adenine dinucleotide (NADH) and dihydroflavine-adenine dinucleotide (FADH2), which are used to generate ATP in the mitochondrial electron transport chain (ETC) (Bonnefont-Rousselot, 2002). Decreases in podocyte number, which contributes to the breakdown of the glomerular filtration barrier, are recognized in the development of DKD and are related to ROS (Mima et al., 2012b). It has been reported that diabetes-induced changes in the ETC may increase apoptosis (Jiang et al., 2016). We have reported that inhibition of insulin receptor substrate-1 (IRS1) signaling induces DKD (Mima et al., 2011a). Mitochondria are one of the various sources of ROS that can cause serine phosphorylation of IRS, which results in impaired IRS1 signaling (Evans et al., 2003). Consistent with these observations, increased fission, fusion, mitophagy, and decreased levels of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) are all observed in the early phase of diabetes (Mootha et al., 2003).
It has been reported that PGC-1α expression was decreased in diabetic rat kidneys. Furthermore, overexpression of PGC-1α in cultured mesangial cells suppressed the pathophysiological changes induced by hyperglycemia (Guo et al., 2015). Both mRNA and protein levels of PGC-1α were decreased in cultured podocytes when incubated with high glucose medium, indicating reduced mitochondrial biogenesis (Imasawa et al., 2017).
Hyperglycemia increases advanced glycation end products (AGEs), which can activate the transformation of growth factor-beta (TGF-β)/Smad1 pathway and thus increase the extracellular matrix in mesangial cells (Mima et al., 2006, 2008). AGEs also activate the PKC and hexosamine pathways, thus contributing to mitochondrial dysfunction (Giacco and Brownlee, 2010; Hallan and Sharma, 2016).
A recent clinical study, called the Joslin 50-year Medalist Study, examined patients with a long duration (more than 50 years) of type 1 diabetes and revealed that the expression and activation of pyruvate kinase M2 (PKM2) are increased in the glomeruli of diabetic patients who did not show clinically significant DKD, as confirmed by renal pathologic examinations. Pathologically, the expression levels of ECM proteins, inflammatory cytokines, TGF-β, and connective tissue growth factor (CTGF) play a significant role in the development of CKD and were decreased with PKM2 deletion in podocytes in vitro and in diabetic mice in vivo. When the diabetic mice were treated for 3 months with TEPP-46, PKM2 activation increased and pathological glomerular changes ceased or were reversed, even after 3 months of diabetes onset. Furthermore, the activation of PKM2 in mice suppressed the diabetes-induced decrease in PPARGC1A mRNA, increased OPA1 expression, and promoted mitochondrial fusion (Qi et al., 2017).
Mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) are heterogeneous clinical syndromes related to defects in mitochondrial function. Most patients with MELAS have a heteroplasmic A to G transition at nucleotide 3243 (3243A > G) in the transfer RNA (tRNA) leucin (UUR) gene of the mitochondrial DNA (Goto et al., 1990). CKD is associated with MELAS syndrome, and renal involvement associated with this condition has been reported in clinical manifestations and molecular genetic studies (Mima et al., 2011b). Thus, mitochondrial dysfunction itself is thought to be a cause of CKD, and the accumulation of abnormal mitochondria in podocytes is observed in MELAS patients with renal impairment.
6. DKD therapy targeting oxidative stress and mitochondrial function
6.1. Vitamin C and E
Treatment of DKD with vitamin C—alone or in combination with vitamin E—has been suggested to decrease microalbuminuria. In addition, high doses (1,800 IU/day) of vitamin E administered to patients with type 1 diabetes for less than 10 years resulted in renal function recovery (Dunlop, 2000). However, the duration of these studies was very short, and the studies had small sample sizes. On the other hand, the 4-year Heart Outcomes Prevention Evaluation (HOPE) study, which enrolled more than 3,600 patients with diabetes, some of whom already had microalbuminuria, found that vitamin E supplementation (400 IU/day) did not significantly reduce the cardiovascular outcomes (Heart Outcomes Prevention Evaluation Study et al., 2000). Therefore, these results do not clarify the efficacy of vitamins C and E on DKD.
6.2. Nrf2
The transcriptional factor, NFE-2 related factor 2 (Nrf2), is a master regulator of cellular detoxification reactions and redox status. Kelch-like ECH-associated protein 1 (Keap1) is an adaptor protein for the ubiquitin E3 ligase that is involved in the ubiquitination of Nrf2. However, during oxidative stress exposure, the binding of Keap1 to Nrf2 is inhibited and the Keap1-Cullin 3 complex loses its E3 ligase activity, resulting in a decrease in the degradation rate of Nrf2 (Zheng et al., 2011). The stabilized Nrf2 translocates into the nucleus and forms a heterodimer with small musculoaponeurotic fibrosarcoma (sMAF), and binds to the antioxidant response element (ARE), activating cytoprotective genes and suppressing inflammatory cytokines (Hirotsu et al., 2012). Bardoxolone methyl is a Nrf2 activator and a direct inhibitor of nuclear factor-kappa B (NF-κB), which may induce albuminuria by blocking the inhibitor of nuclear factor kappa Β kinase subunit β (IKKβ) activity (Rushworth et al., 2012). In clinical trials, bardoxolone methyl appears to have beneficial effects on DKD. One study, called The Bardoxolone Methyl Evaluation in Patients with Chronic Kidney Disease and Type 2 Diabetes Mellitus: The Occurrence of Renal Events (BEACON) trial, showed a significant reduction in eGFR decline in the treatment group. However, the phase 3 trial was discontinued in October 2012 because of high mortality rates (de Zeeuw et al., 2013). To dispel this concern, The Phase 2 study of Bardoxolone Methyl in Patients with CKD and Type 2 Diabetes (TSUBAKI) study was conducted, and no serious side effects have been reported so far (Nangaku et al., 2020).
The Phase 2/3 trial of the Efficacy and Safety of Bardoxolone Methyl in Patients with Alport Syndrome (CARDINAL) is a multicenter, open-label, randomized controlled trial that enrolled patients with Alport syndrome. These patients showed a significant increase in eGFR following bardoxolone methyl treatment (Chertow et al., 2021). A Phase 2 Trial of the Safety and Efficacy of Bardoxolone Methyl in Patients with Rare Chronic Kidney Diseases, PHOENIX, is also a multicenter, open-label trial, in which bardoxolone methyl increased eGFR in 31 patients (Toto, 2018). Taken together, these clinical results suggest that bardoxolone methyl may be a promising drug for treating DKD and CKD.
6.3. DPP-4 inhibitors
Inhibition of dipeptidyl peptidase-4 (DPP-4) increases circulating glucagon-like peptide-1 (GLP-1) and has been shown to be effective in treating type 2 diabetes (Drucker, 2006). DPP-4 inhibitors can reduce inflammation and decrease MCP-1 levels (Hung et al., 2020). Both DPP-4 and GLP-1 elicit vasotropic effects and decrease diabetes-induced oxidative stress in the glomerulus, ameliorating DKD (Mima et al., 2020) (Mima et al., 2012a). Recent studies have shown that sitagliptin treatment activates the transcription factor cAMP response element-binding protein (CREB), which increases β cell mass or islet angiogenesis (Samikannu et al., 2013). Furthermore, CREB plays a pivotal role in regulating PGC-1α (Yao et al., 2016).
Sitagliptin increases the expression of mitochondrial DNA and genes encoding not only COX-1 and COX-4 but also mitochondria-specific proteins (Weng et al., 2019). We have reported that GLP-1 increased by DPP-4 inhibitors can activate adenylyl cyclase, promote cAMP production, and foster PKA activation, which subsequently induces serine phosphorylation of CREB (Mima et al., 2012a). Interestingly, linagliptin was shown to increase anti-oxidative stress effects in podocytes by increasing Nrf2 (Mima et al., 2020).
6.4. SGLT2 inhibitors
Mitochondrial fusion depends on mitofusion (Mfn)1 or Mfn2 and dynamin family GTPase optic atrophy factor 1 (Opa1) (Chan, 2006). A recent study showed that mice that were fed a high-fat diet developed a metabolic abnormality, suppressing Mfn2 and Opa1 in the renal tubules (Takagi et al., 2018). Ipragliflozin inhibited this vicious cycle by restoring Mfn2 and Opa1 to normal levels without causing changes in blood glucose and body weight (Takagi et al., 2018). Furthermore, empagliflozin decreased the expression of TGF-β, which plays a significant role in the development of DKD and is an essential cytokine in renal apoptosis (Lee et al., 2019). In cultured renal proximal tubular cells, empagliflozin was shown to reduce diabetes-induced mitochondrial ROS, which is related to abnormal mitophagy (Pirklbauer et al., 2019).
6.5. Metformin
Metformin's interference with mitochondria seems to occur in addition to its ability to inhibit the electron transfer chain in respiratory complex (ETC) I, and it is thought that metformin may exert its metabolic effects by directly binding to copper ions in mitochondria (Logie et al., 2012). In purified enzymes of different species, metformin has been shown to act as a non-competitive inhibitor of mitochondrial glycerol 3-phosphate dehydrogenase (mGPDH). Therefore, when the glycerol-phosphate shuttle is inhibited, respiration is impaired, and cytoplasmic NAD+ is reduced. Consequently, the cytoplasmic NAD+/NADH ratio is reduced and gluconeogenesis from both glycerol and lactate is inhibited (Madiraju et al., 2014). Downregulation of GPD2 (the gene encoding mGPDH) mimics the antihyperglycemic effects of metformin (Alshawi and Agius, 2019, Madiraju et al., 2018). A recent study showed that metformin at therapeutic concentrations decreases glucogenesis by inhibiting mGPDH action in a redox-dependent manner (Thakur et al., 2018). Activation of NF-κB is associated with the development of DKD (Mima et al., 2018). Metformin can act on NF-κB signaling, inhibit monocyte to macrophage differentiation, and suppress several pro-inflammatory cytokines in the plasma of non-diabetic patients (Isoda et al., 2006). Furthermore, metformin ameliorated renal oxidative stress and tubulointerstitial fibrosis in DKD mice by activating mitophagy through Pink1 (PTEN-induced putative kinase 1) and Parkin (Han et al., 2021).
6.6. Imeglimin
Imeglimin is a novel oral antidiabetic drug, the first of a new class of tetrahydrotriazine-containing molecules called “glimins”, for which the structure and mechanism of action have been studied (Pirags et al., 2012). The Trials of Imeglimin for Efficacy and Safety 1 (TIMES 1) study was designed to confirm the efficacy, safety, and tolerability of imeglimin monotherapy in Japanese patients with type 2 diabetes (Dubourg et al., 2021). In this study, imeglimin significantly improved HbA1c compared to placebo; changes from baseline HbA1c at week 24 were -0.87% (95% CI -1.04 to -0.69, p < 0.0001) (Dubourg et al., 2021). Based on these results, imeglimin was launched in Japan in 2021, ahead of the rest of the world. Imeglimin reduces oxidative stress by acting on the liver, muscle, and pancreas, which are involved in the pathogenesis of type 2 diabetes, through a mechanism that targets the mitochondria. Imeglimin decreases glucose production in the liver and increases glucose uptake in the muscles (Vial et al., 2015). A recent study using a hyperglycemic clamp in patients with diabetes showed that imeglimin increased insulin secretion in response to glucose {Perry, 2016 #936}. In addition, imeglimin has been reported to improve mitochondrial density and function (Hallakou-Bozec et al., 2021, Vial et al., 2015). Imeglimin also altered oxidative phosphorylation chain activity, thus reducing the activity of complex II, and decreasing the ROS generated from this complex when mitochondria oxidize succinate (Vial et al., 2015). Furthermore, imeglimin completely restored the activity of complex III by restoring the expression of one of its subunits (Vial et al., 2015). Cultured human vascular endothelial cells were used to reproduce the inhibitory effect of imeglimin on ROS production by decreasing reverse electron transport through complex I; however, no decrease in intracellular oxygen consumption was observed (Detaille et al., 2016).
Lachaux et al. showed that administration of imeglimin ameliorated cardiac dysfunction and increased coronary artery endothelium-dependent relaxation in insulin-resistant rodents; imeglimin decreased left ventricular (LV) end-diastolic pressure and increased LV tissue perfusion. Furthermore, these potential benefits were associated with a decrease in LV ROS production. More importantly, imeglimin decreased albuminuria and interstitial fibrosis in a rodent model (Lachaux et al., 2020). We have shown the relationship between nitric oxide (NO) and DKD via renal insulin signaling (Mima et al., 2011a), and the aforementioned results imply that DKD may have a negative effect on NO. Further studies are required to clarify this point (Figure 1, Table 1).
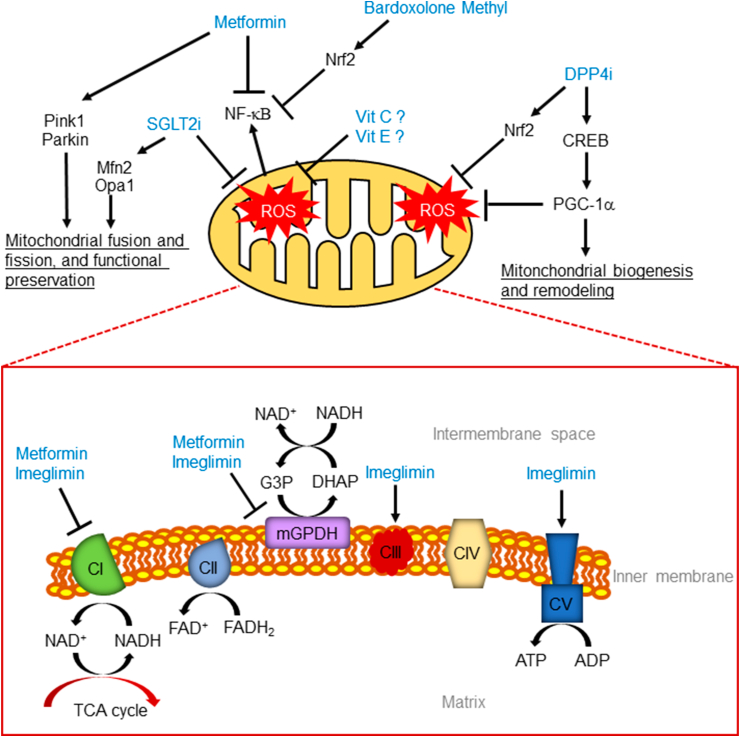
Figure 1.
Proposed mechanisms of mitochondria-mediated renoprotection. SGLT2i, sodium-glucose cotransporter 2 inhibitors; NF-κB, nuclear factor-kappa Β; Nrf2, NFE-2 related factor 2; Vit C, vitamin C; Vit E, vitamin E; DPP4i, dipeptidyl peptidase-4 inhibitors; CREB, cAMP response element-binding protein; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; CI, complex I; CII, complex II; CIII, complex III; CIV, complex IV; CV, complex V; G3P, glycerol-3-phosphate; DHAP, dihydroxyacetone phosphate; NAD, nicotinamide adenine dinucleotide; FAD, flavine-adenine dinucleotide; FADH2, dihydroflavine-adenine dinucleotide; mGPDH, mitochondrial glycerol 3-phosphate dehydrogenase; ATP, adenosine triphosphate; ADP, adenosine diphosphate.
Table 1.
Approaches to ameliorate abnormal mitochondrial function and to decrease ROS production in DKD.
| Agent | Mechanism of action | In vivo studies |
|---|---|---|
| Bardoxolone methyl | Suppressing inflammation Inhibition of NF-κB |
Reduction of eGFR decline |
| DPP-4 inhibitors | Increasing CREB and PGC-1α Increasing Nrf2 |
Decreases in albuminuria and mesangial expansion |
| SGLT2 inhibitors | Correcting abnormal Mfn and Opa1 Decreasing ROS |
Decreases in albuminuria and renal fibrosis |
| Metformin | Inhibition of complex I and mGPDH Inhibition of NF-kB Activation of Pink1 and Parkin |
Amelioration of renal oxidative stress and tubulointerstitial fibrosis |
| Imeglimin | Decreasing reverse electron transport through complex I Reducing the activity of complex II, decreasing ROS Restoring the expression of a subunit of complex III |
Decreases in albuminuria and interstitial fibrosis |
NF-κB, nuclear factor-kappa B; GFR, glomerular filtration rate; DPP-4, dipeptidyl peptidase-4; CREB, cAMP response element-binding protein; PGC-1a, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; Nrf2, NFE-2 related factor 2; ROS, reactive oxygen species; mGPDH, mitochondrial glycerol 3-phosphate dehydrogenase.
7. Conclusion
Good glycemic control is the best preventive measure for DKD. However, despite the treatment of diabetes, DKD may still develop. It is well known that diabetes-induced mitochondrial dysfunction includes abnormal mitophagy, fission, fusion, and biosynthesis. Furthermore, if mitochondrial dysfunction is not restored, renal function may continue to decline, worsening DKD. The recovery of renal cells and renal function depends on the ATP-producing capacity of the mitochondria. Therefore, a DKD treatment that targets oxidative stress suppression or mitochondrial homeostasis improvement is promising.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by Osaka Kidney Foundation (OKF21-0003), Japan Health Foundation and Takeda Medical Research Foundation. Akira Mima was supported by research grants from Kyowa Kirin, Sumitomo Dainippon Pharma, Otsuka, Torii, Taisho-Toyama, Daiichi-Sankyo, Mitsubishi Tanabe, Boehringer Ingelheim, and Eli Lilly.
Data availability statement
No data was used for the research described in the article.
Declaration of interests statement
The authors declare the following conflict of interests: Akira Mima received a speaker honorarium from Otsuka, Kyowa Kirin, Mitsubishi Tanabe, Torii, Kowa, Bayer, Eli Lilly, Astellas, and Boehringer Ingelheim. Akira Mima received research grants from Kyowa Kirin, Sumitomo Dainippon Pharma, Otsuka, Torii, Taisho-Toyama, Daiichi-Sankyo, Mitsubishi Tanabe, Boehringer Ingelheim, and Eli Lilly.
Additional information
No additional information is available for this paper.
References
- Alexander C., Votruba M., Pesch U.E., Thiselton D.L., Mayer S., Moore A., Rodriguez M., Kellner U., Leo-Kottler B., Auburger G., et al. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat. Genet. 2000;26:211–215. doi: 10.1038/79944. [DOI] [PubMed] [Google Scholar]
- Alshawi A., Agius L. Low metformin causes a more oxidized mitochondrial NADH/NAD redox state in hepatocytes and inhibits gluconeogenesis by a redox-independent mechanism. J Biol Chem. 2019;294:2839–2853. doi: 10.1074/jbc.RA118.006670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banba N., Nakamura T., Matsumura M., Kuroda H., Hattori Y., Kasai K. Possible relationship of monocyte chemoattractant protein-1 with diabetic nephropathy. Kidney Int. 2000;58:684–690. doi: 10.1046/j.1523-1755.2000.00214.x. [DOI] [PubMed] [Google Scholar]
- Bassi R., Fornoni A., Doria A., Fiorina P. CTLA4-Ig in B7-1-positive diabetic and non-diabetic kidney disease. Diabetologia. 2016;59:21–29. doi: 10.1007/s00125-015-3766-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard K., Krause K.H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Bhargava P., Schnellmann R.G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 2017;13:629–646. doi: 10.1038/nrneph.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birsoy K., Possemato R., Lorbeer F.K., Bayraktar E.C., Thiru P., Yucel B., Wang T., Chen W.W., Clish C.B., Sabatini D.M. Metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides. Nature. 2014;508:108–112. doi: 10.1038/nature13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnefont-Rousselot D. Glucose and reactive oxygen species. Curr. Opin. Clin. Nutr. Metab. Care. 2002;5:561–568. doi: 10.1097/00075197-200209000-00016. [DOI] [PubMed] [Google Scholar]
- Bursell S.E., King G.L. Can protein kinase C inhibition and vitamin E prevent the development of diabetic vascular complications? Diabetes Res. Clin. Pract. 1999;45:169–182. doi: 10.1016/s0168-8227(99)00047-9. [DOI] [PubMed] [Google Scholar]
- Chan D.C. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Chen J.S., Lee H.S., Jin J.S., Chen A., Lin S.H., Ka S.M., Lin Y.F. Attenuation of mouse mesangial cell contractility by high glucose and mannitol: involvement of protein kinase C and focal adhesion kinase. J Biomed Sci. 2004;11:142–151. doi: 10.1007/BF02256557. [DOI] [PubMed] [Google Scholar]
- Chertow G.M., Appel G.B., Andreoli S., Bangalore S., Block G.A., Chapman A.B., Chin M.P., Gibson K.L., Goldsberry A., Iijima K., et al. Study Design and Baseline Characteristics of the CARDINAL Trial: A Phase 3 Study of Bardoxolone Methyl in Patients with Alport Syndrome. Am J Nephrol. 2021;52:180–189. doi: 10.1159/000513777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zeeuw D., Akizawa T., Audhya P., Bakris G.L., Chin M., Christ-Schmidt H., Goldsberry A., Houser M., Krauth M., Lambers Heerspink H.J., et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med. 2013;369:2492–2503. doi: 10.1056/NEJMoa1306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delettre C., Lenaers G., Griffoin J.M., Gigarel N., Lorenzo C., Belenguer P., Pelloquin L., Grosgeorge J., Turc-Carel C., Perret E., et al. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat. Genet. 2000;26:207–210. doi: 10.1038/79936. [DOI] [PubMed] [Google Scholar]
- Detaille D., Vial G., Borel A.L., Cottet-Rouselle C., Hallakou-Bozec S., Bolze S., Fouqueray P., Fontaine E. Imeglimin prevents human endothelial cell death by inhibiting mitochondrial permeability transition without inhibiting mitochondrial respiration. Cell Death Dis. 2016;2:15072. doi: 10.1038/cddiscovery.2015.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes C., Complications Trial Research G., Nathan D.M., Genuth S., Lachin J., Cleary P., Crofford O., Davis M., Rand L., Siebert C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- Drucker D.J. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Dubourg J., Fouqueray P., Thang C., Grouin J.M., Ueki K. Efficacy and Safety of Imeglimin Monotherapy Versus Placebo in Japanese Patients With Type 2 Diabetes (TIMES 1): A Double-Blind, Randomized, Placebo-Controlled, Parallel-Group, Multicenter Phase 3 Trial. Diabetes Care. 2021;44:952–959. doi: 10.2337/dc20-0763. [DOI] [PubMed] [Google Scholar]
- Dunlop M. Aldose reductase and the role of the polyol pathway in diabetic nephropathy. Kidney Int. Suppl. 2000;77:S3–12. doi: 10.1046/j.1523-1755.2000.07702.x. [DOI] [PubMed] [Google Scholar]
- El-Mir M.Y., Nogueira V., Fontaine E., Averet N., Rigoulet M., Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J. Biol. Chem. 2000;275:223–228. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- Evans J.L., Goldfine I.D., Maddux B.A., Grodsky G.M. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes. 2003;52:1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- Fouqueray P., Pirags V., Diamant M., Schernthaner G., Lebovitz H.E., Inzucchi S.E., Bailey C.J. The efficacy and safety of imeglimin as add-on therapy in patients with type 2 diabetes inadequately controlled with sitagliptin monotherapy. Diabetes Care. 2014;37:1924–1930. doi: 10.2337/dc13-2349. [DOI] [PubMed] [Google Scholar]
- Fouqueray P., Pirags V., Inzucchi S.E., Bailey C.J., Schernthaner G., Diamant M., Lebovitz H.E. The efficacy and safety of imeglimin as add-on therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy. Diabetes Care. 2013;36:565–568. doi: 10.2337/dc12-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacco F., Brownlee M. Oxidative stress and diabetic complications. Circ. Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y., Nonaka I., Horai S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990;348:651–653. doi: 10.1038/348651a0. [DOI] [PubMed] [Google Scholar]
- Guo K., Lu J., Huang Y., Wu M., Zhang L., Yu H., Zhang M., Bao Y., He J.C., Chen H., et al. Protective role of PGC-1alpha in diabetic nephropathy is associated with the inhibition of ROS through mitochondrial dynamic remodeling. PLoS One. 2015;10 doi: 10.1371/journal.pone.0125176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha H., Yu M.R., Choi Y.J., Kitamura M., Lee H.B. Role of high glucose-induced nuclear factor-kappaB activation in monocyte chemoattractant protein-1 expression by mesangial cells. J Am Soc Nephrol. 2002;13:894–902. doi: 10.1681/ASN.V134894. [DOI] [PubMed] [Google Scholar]
- Hallakou-Bozec S., Kergoat M., Fouqueray P., Bolze S., Moller D.E. Imeglimin amplifies glucose-stimulated insulin release from diabetic islets via a distinct mechanism of action. PLoS One. 2021;16:e0241651. doi: 10.1371/journal.pone.0241651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallan S., Sharma K. The role of mitochondria in diabetic kidney disease. Curr. Diabetes Rep. 2016;16:61. doi: 10.1007/s11892-016-0748-0. [DOI] [PubMed] [Google Scholar]
- Han Y.C., Tang S.Q., Liu Y.T., Li A.M., Zhan M., Yang M., Song N., Zhang W., Wu X.Q., Peng C.H. AMPK agonist alleviate renal tubulointerstitial fibrosis via activating mitophagy in high fat and streptozotocin induced diabetic mice. Cell Death Dis. 2021;12:925. doi: 10.1038/s41419-021-04184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heart Outcomes Prevention Evaluation Study I., Yusuf S., Dagenais G., Pogue J., Bosch J., Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. N. Engl. J. Med. 2000;342:154–160. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- Hirotsu Y., Katsuoka F., Funayama R., Nagashima T., Nishida Y., Nakayama K., Engel J.D., Yamamoto M. Nrf2-MafG heterodimers contribute globally to antioxidant and metabolic networks. Nucleic Acids Res. 2012;40:10228–10239. doi: 10.1093/nar/gks827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung Y.W., Wang Y., Lee S.L. DPP-4 inhibitor reduces striatal microglial deramification after sensorimotor cortex injury induced by external force impact. FASEB J. 2020;34:6950–6964. doi: 10.1096/fj.201902818R. [DOI] [PubMed] [Google Scholar]
- Imasawa T., Obre E., Bellance N., Lavie J., Imasawa T., Rigothier C., Delmas Y., Combe C., Lacombe D., Benard G., et al. High glucose repatterns human podocyte energy metabolism during differentiation and diabetic nephropathy. Faseb. J. 2017;31:294–307. doi: 10.1096/fj.201600293R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoda K., Young J.L., Zirlik A., MacFarlane L.A., Tsuboi N., Gerdes N., Schonbeck U., Libby P. Metformin inhibits proinflammatory responses and nuclear factor-kappaB in human vascular wall cells. Arterioscler Thromb Vasc Biol. 2006;26:611–617. doi: 10.1161/01.ATV.0000201938.78044.75. [DOI] [PubMed] [Google Scholar]
- Jiang X., Li L., Ying Z., Pan C., Huang S., Li L., Dai M., Yan B., Li M., Jiang H., et al. A small molecule that protects the integrity of the electron transfer chain blocks the mitochondrial apoptotic pathway. Mol. Cell. 2016;63:229–239. doi: 10.1016/j.molcel.2016.06.016. [DOI] [PubMed] [Google Scholar]
- King G.L., Loeken M.R. Hyperglycemia-induced oxidative stress in diabetic complications. Histochem. Cell Biol. 2004;122:333–338. doi: 10.1007/s00418-004-0678-9. [DOI] [PubMed] [Google Scholar]
- Koya D., Haneda M., Nakagawa H., Isshiki K., Sato H., Maeda S., Sugimoto T., Yasuda H., Kashiwagi A., Ways D.K., et al. Amelioration of accelerated diabetic mesangial expansion by treatment with a PKC beta inhibitor in diabetic db/db mice, a rodent model for type 2 diabetes. Faseb. J. 2000;14:439–447. doi: 10.1096/fasebj.14.3.439. [DOI] [PubMed] [Google Scholar]
- Lachaux M., Soulie M., Hamzaoui M., Bailly A., Nicol L., Remy-Jouet I., Renet S., Vendeville C., Gluais-Dagorn P., Hallakou-Bozec S., et al. Short-and long-term administration of imeglimin counters cardiorenal dysfunction in a rat model of metabolic syndrome. Endocrinol. Diabetes Metab. 2020;3 doi: 10.1002/edm2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I.K., Koya D., Ishi H., Kanoh H., King G.L. d-Alpha-tocopherol prevents the hyperglycemia induced activation of diacylglycerol (DAG)-protein kinase C (PKC) pathway in vascular smooth muscle cell by an increase of DAG kinase activity. Diabetes Res. Clin. Pract. 1999;45:183–190. doi: 10.1016/s0168-8227(99)00048-0. [DOI] [PubMed] [Google Scholar]
- Lee Y.H., Kim S.H., Kang J.M., Heo J.H., Kim D.J., Park S.H., Sung M., Kim J., Oh J., Yang D.H., et al. Empagliflozin attenuates diabetic tubulopathy by improving mitochondrial fragmentation and autophagy. Am. J. Physiol. Ren. Physiol. 2019;317:F767–F780. doi: 10.1152/ajprenal.00565.2018. [DOI] [PubMed] [Google Scholar]
- Logie L., Harthill J., Patel K., Bacon S., Hamilton D.L., Macrae K., McDougall G., Wang H.H., Xue L., Jiang H., et al. Cellular responses to the metal-binding properties of metformin. Diabetes. 2012;61:1423–1433. doi: 10.2337/db11-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loretelli C., Rocchio F., D’Addio F., Ben Nasr M., Castillo-Leon E., Dellepiane S., Vergani A., Abdelsalam A., Assi E., Maestroni A. The IL-8-CXCR1/2 axis contributes to diabetic kidney disease. Metabolism. 2021;121:154804. doi: 10.1016/j.metabol.2021.154804. [DOI] [PubMed] [Google Scholar]
- Madiraju A.K., Erion D.M., Rahimi Y., Zhang X.M., Braddock D.T., Albright R.A., Prigaro B.J., Wood J.L., Bhanot S., MacDonald M.J., et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014;510:542–546. doi: 10.1038/nature13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madiraju A.K., Qiu Y., Perry R.J., Rahimi Y., Zhang X.M., Zhang D., Cline G.W., Butrico G.M., Kemp B.E., et al. Metformin inhibits gluconeogenesis via a redox-dependent mechanism in vivo. Nat Med. 2018;24:1384–1394. doi: 10.1038/s41591-018-0125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzetti A., Cipollone F., Cuccurullo F. Oxidative stress and cardiovascular complications in diabetes: isoprostanes as new markers on an old paradigm. Cardiovasc. Res. 2000;47:475–488. doi: 10.1016/s0008-6363(00)00118-8. [DOI] [PubMed] [Google Scholar]
- Mima A. Inflammation and oxidative stress in diabetic nephropathy: new insights on its inhibition as new therapeutic targets. J. Diabetes Res. 2013;2013:248563. doi: 10.1155/2013/248563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mima A., Arai H., Matsubara T., Abe H., Nagai K., Tamura Y., Torikoshi K., Araki M., Kanamori H., Takahashi T., et al. Urinary Smad1 is a novel marker to predict later onset of mesangial matrix expansion in diabetic nephropathy. Diabetes. 2008;57:1712–1722. doi: 10.2337/db07-1726. [DOI] [PubMed] [Google Scholar]
- Mima A., Hiraoka-Yamomoto J., Li Q., Kitada M., Li C., Geraldes P., Matsumoto M., Mizutani K., Park K., Cahill C., et al. Protective effects of GLP-1 on glomerular endothelium and its inhibition by PKCbeta activation in diabetes. Diabetes. 2012;61:2967–2979. doi: 10.2337/db11-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mima A., Kitada M., Geraldes P., Li Q., Matsumoto M., Mizutani K., Qi W., Li C., Leitges M., Rask-Madsen C., et al. Glomerular VEGF resistance induced by PKCdelta/SHP-1 activation and contribution to diabetic nephropathy. Faseb. J. 2012;26:2963–2974. doi: 10.1096/fj.11-202994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mima A., Matsubara T., Arai H., Abe H., Nagai K., Kanamori H., Sumi E., Takahashi T., Iehara N., Fukatsu A., et al. Angiotensin II-dependent Src and Smad1 signaling pathway is crucial for the development of diabetic nephropathy. Lab. Invest. 2006;86:927–939. doi: 10.1038/labinvest.3700445. [DOI] [PubMed] [Google Scholar]
- Mima A., Ohshiro Y., Kitada M., Matsumoto M., Geraldes P., Li C., Li Q., White G.S., Cahill C., Rask-Madsen C., et al. Glomerular-specific protein kinase C-beta-induced insulin receptor substrate-1 dysfunction and insulin resistance in rat models of diabetes and obesity. Kidney Int. 2011;79:883–896. doi: 10.1038/ki.2010.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mima A., Qi W., Hiraoka-Yamomoto J., Park K., Matsumoto M., Kitada M., Li Q., Mizutani K., Yu E., Shimada T., et al. Retinal not systemic oxidative and inflammatory stress correlated with VEGF expression in rodent models of insulin resistance and diabetes. Invest. Ophthalmol. Vis. Sci. 2012;53:8424–8432. doi: 10.1167/iovs.12-10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mima A., Shiota F., Matsubara T., Iehara N., Akagi T., Abe H., Nagai K., Matsuura M., Murakami T., Kishi S., et al. An autopsy case of mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) with intestinal bleeding in chronic renal failure. Ren. Fail. 2011;33:622–625. doi: 10.3109/0886022X.2011.585730. [DOI] [PubMed] [Google Scholar]
- Mima A., Yasuzawa T., King G.L., Ueshima S. Obesity-associated glomerular inflammation increases albuminuria without renal histological changes. FEBS Open Bio. 2018;8:664–670. doi: 10.1002/2211-5463.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mima A., Yasuzawa T., Nakamura T., Ueshima S. Linagliptin affects IRS1/Akt signaling and prevents high glucose-induced apoptosis in podocytes. Sci. Rep. 2020;10:5775. doi: 10.1038/s41598-020-62579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mootha V.K., Lindgren C.M., Eriksson K.F., Subramanian A., Sihag S., Lehar J., Puigserver P., Carlsson E., Ridderstrale M., Laurila E., et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- Myint K.M., Yamamoto Y., Doi T., Kato I., Harashima A., Yonekura H., Watanabe T., Shinohara H., Takeuchi M., Tsuneyama K., et al. RAGE control of diabetic nephropathy in a mouse model: effects of RAGE gene disruption and administration of low-molecular weight heparin. Diabetes. 2006;55:2510–2522. doi: 10.2337/db06-0221. [DOI] [PubMed] [Google Scholar]
- Nangaku M., Kanda H., Takama H., Ichikawa T., Hase H., Akizawa T. Randomized Clinical Trial on the Effect of Bardoxolone Methyl on GFR in Diabetic Kidney Disease Patients (TSUBAKI Study) Kidney Int Rep. 2020;5:879–890. doi: 10.1016/j.ekir.2020.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan D.M. Long-term complications of diabetes mellitus. N. Engl. J. Med. 1993;328:1676–1685. doi: 10.1056/NEJM199306103282306. [DOI] [PubMed] [Google Scholar]
- Okada S., Shikata K., Matsuda M., Ogawa D., Usui H., Kido Y., Nagase R., Wada J., Shikata Y., Makino H. Intercellular adhesion molecule-1-deficient mice are resistant against renal injury after induction of diabetes. Diabetes. 2003;52:2586–2593. doi: 10.2337/diabetes.52.10.2586. [DOI] [PubMed] [Google Scholar]
- Pirags V., Lebovitz H., Fouqueray P. Imeglimin, a novel glimin oral antidiabetic, exhibits a good efficacy and safety profile in type 2 diabetic patients. Diabetes Obes. Metabol. 2012;14:852–858. doi: 10.1111/j.1463-1326.2012.01611.x. [DOI] [PubMed] [Google Scholar]
- Pirklbauer M., Schupart R., Fuchs L., Staudinger P., Corazza U., Sallaberger S., Leierer J., Mayer G., Schramek H. Unraveling reno-protective effects of SGLT2 inhibition in human proximal tubular cells. Am J Physiol Renal Physiol. 2019;316:F449–F462. doi: 10.1152/ajprenal.00431.2018. [DOI] [PubMed] [Google Scholar]
- Qi W., Keenan H.A., Li Q., Ishikado A., Kannt A., Sadowski T., Yorek M.A., Wu I.H., Lockhart S., Coppey L.J., et al. Pyruvate kinase M2 activation may protect against the progression of diabetic glomerular pathology and mitochondrial dysfunction. Nat. Med. 2017;23:753–762. doi: 10.1038/nm.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovira-Llopis S., Apostolova N., Banuls C., Muntane J., Rocha M., Victor V.M. Mitochondria, the NLRP3 inflammasome, and sirtuins in type 2 diabetes: new therapeutic targets. Antioxidants Redox Signal. 2018;29:749–791. doi: 10.1089/ars.2017.7313. [DOI] [PubMed] [Google Scholar]
- Rushworth S.A., Zaitseva L., Murray M.Y., Shah N.M., Bowles K.M., MacEwan D.J. The high Nrf2 expression in human acute myeloid leukemia is driven by NF-kappaB and underlies its chemo-resistance. Blood. 2012;120:5188–5198. doi: 10.1182/blood-2012-04-422121. [DOI] [PubMed] [Google Scholar]
- Samikannu B., Chen C., Lingwal N., Padmasekar M., Engel F.B., Linn T. Dipeptidyl peptidase IV inhibition activates CREB and improves islet vascularization through VEGF-A/VEGFR-2 signaling pathway. PLoS One. 2013;8:e82639. doi: 10.1371/journal.pone.0082639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassy-Prigent C., Heudes D., Mandet C., Belair M.F., Michel O., Perdereau B., Bariety J., Bruneval P. Early glomerular macrophage recruitment in streptozotocin-induced diabetic rats. Diabetes. 2000;49:466–475. doi: 10.2337/diabetes.49.3.466. [DOI] [PubMed] [Google Scholar]
- Takagi S., Li J., Takagaki Y., Kitada M., Nitta K., Takasu T., Kanasaki K., Koya D. Ipragliflozin improves mitochondrial abnormalities in renal tubules induced by a high-fat diet. J Diabetes Investig. 2018;9:1025–1032. doi: 10.1111/jdi.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur S., Daley B., Gaskins K., Vasko V.V., Boufraqech M., Patel D., Sourbier C., Reece J., Cheng S.Y., Kebebew E., et al. Metformin Targets Mitochondrial Glycerophosphate Dehydrogenase to Control Rate of Oxidative Phosphorylation and Growth of Thyroid Cancer In Vitro and In Vivo. Clin Cancer Res. 2018;24:4030–4043. doi: 10.1158/1078-0432.CCR-17-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toto R.D. Bardoxolone-the Phoenix? J Am Soc Nephrol. 2018;29:360–361. doi: 10.1681/ASN.2017121317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vial G., Chauvin M.A., Bendridi N., Durand A., Meugnier E., Madec A.M., Bernoud-Hubac N., Pais de Barros J.P., Fontaine E., Acquaviva C., et al. Imeglimin normalizes glucose tolerance and insulin sensitivity and improves mitochondrial function in liver of a high-fat, high-sucrose diet mice model. Diabetes. 2015;64:2254–2264. doi: 10.2337/db14-1220. [DOI] [PubMed] [Google Scholar]
- Weng G., Zhou B., Liu T., Huang Z., Yang H. Sitagliptin promotes mitochondrial biogenesis in human SH-SY5Y cells by increasing the expression of PGC-1alpha/NRF1/TFAM. IUBMB Life. 2019;71:1515–1521. doi: 10.1002/iub.2076. [DOI] [PubMed] [Google Scholar]
- Wu C.C., Chen J.S., Lu K.C., Chen C.C., Lin S.H., Chu P., Sytwu H.K., Lin Y.F. Aberrant cytokines/chemokines production correlate with proteinuria in patients with overt diabetic nephropathy. Clin Chim Acta. 2010;411:700–704. doi: 10.1016/j.cca.2010.01.036. [DOI] [PubMed] [Google Scholar]
- Wu L.L., Chiou C.C., Chang P.Y., Wu J.T. Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin. Chim. Acta. 2004;339:1–9. doi: 10.1016/j.cccn.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Yao K., Zhang W.W., Yao L., Yang S., Nie W., Huang F. Carvedilol promotes mitochondrial biogenesis by regulating the PGC-1/TFAM pathway in human umbilical vein endothelial cells (HUVECs) Biochem Biophys Res Commun. 2016;470:961–966. doi: 10.1016/j.bbrc.2016.01.089. [DOI] [PubMed] [Google Scholar]
- Zheng H., Whitman S.A., Wu W., Wondrak G.T., Wong P.K., Fang D., Zhang D.D. Therapeutic potential of Nrf2 activators in streptozotocin-induced diabetic nephropathy. Diabetes. 2011;60:3055–3066. doi: 10.2337/db11-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.