Abstract
Metabolic reprogramming, as a key hallmark of cancers, leads to the malignant behavior of pancreatic cancer, which is closely related to tumor development and progression, as well as the supportive tumor microenvironments. Although cells produce adenosine triphosphate (ATP) from glucose by glycolysis when lacking oxygen, pancreatic cancer cells elicit metabolic conversion from oxide phosphorylation to glycolysis, which is well-known as “Warburg effect”. Glycolysis is critical for cancer cells to maintain their robust biosynthesis and energy requirement, and it could promote tumor initiation, invasion, angiogenesis, and metastasis to distant organs. Multiple pathways are involved in the alternation of glycolysis for pancreatic cancer cells, including UHRF1/SIRT4 axis, PRMT5/FBW7/cMyc axis, JWA/AMPK/FOXO3a/FAK axis, KRAS/TP53/TIGAR axis, etc. These signaling pathways play an important role in glycolysis and are potential targets for the treatment of pancreatic cancer. Mutations in glycolytic enzymes (such as LDH, PKM2, and PGK1) also contribute to the early diagnosis and monitoring of pancreatic cancer. In this review, we summarized the recent advances on the mechanisms for glycolysis in pancreatic cancer and the function of glycolysis in the progression of pancreatic cancer, which suggested new targets for cancer diagnosis and treatment.
Keywords: Pancreatic cancer, metabolism, glycolysis, tumor progression, tumor microenvironment
Introduction
Known as the “King of cancers”, pancreatic cancer is a malignant and invasive digestive tumor with a 5-year survival rate of only below 6% [1,2]. Uncontrolled continuous proliferation of tumor cells requires large amounts of energy and related molecules, and thus exhibits metabolic characteristics different from normal tissues, the more common of which is the enhanced aerobic glycolysis, known as the “Warburg effect” [3]. The effect, a significant feature of cancer, is characterized by active glycolysis and increased lactate production even with sufficient oxygen supply. Although glucose is less efficient at producing ATP through glycolysis, it yields much faster than oxidized phosphorylation and produces a large number of carbon skeletons and NADPH [4]. During recent years, more researches have revealed that glycolysis played critical roles in the pathogenesis of pancreatic cancer. This review summarized the recent advances on the relationship between aerobic glycolysis and pancreatic cancer, explored the underlying molecular basis, and provided the new directions for targeted therapies for pancreatic cancer.
Reprogramming of glycolysis in pancreatic cancer
Cancer cells rewire the metabolism to meet their increased needs for glucose and ferment glucose to lactate, thus promoting their initiation, development, proliferation, and even metastasis. This phenomenon, named as the “Warburg effect” [5], is observed in the presence of sufficient and complete functional mitochondria. Aerobic glycolysis, a pivotal metabolic feature of the Warburg effect, is caused by active metabolic reprogramming required for the continued cell proliferation. The metabolic conversion is achieved by changing the growth factor signal, hypoxia or normal oxygen, and oncogene activation, as well as the tumor microenvironment. Metabolic reprogramming, including the overexpression of glucose transporter proteins, key glycolytic enzymes and the accelerated glycolysis flux, results in the accumulation and transfer of glycolysis intermediates, thus promoting the biosynthesis of cancer. The high-rate production of ATP meets the energy requirements, and the massive accumulation of lactic acid promotes tumorigenesis. To a large extent, tumor acidosis, which in turn synergistically promotes tumor growth, enhances resistance to some anti-tumor treatments and impairs the anti-tumor immunity [6]. In conclusion, Warburg effect is an important contributor for cancer development and progression.
Mechanisms for glycolysis in pancreatic cancer
Dysfunction of mitochondrial function
Oxidative metabolism of eukaryotes, energy release by final oxidation of sugars, fats and amino acids, and synthesis of hemoglobin and phospholipids all occur in mitochondria. Mitochondrial matrix also contains enzymes involved in tricarboxylic acid cycle, fatty acid oxidation and amino acid degradation. When mitochondrial dysfunction occurs, complexes I, III and IV in mitochondrial electron transfer chain are inhibited, which enhances the glucose consumption and lactic acid production, thus promoting glycolysis [7]. Recent studies have proved that Paris saponin D could promote pancreatic cancer cell apoptosis through mitochondrial apoptosis, thus resulting in the inhibition of cancer cell proliferation [8]. The inhibitory role of microtubule-associated protein JWA on pancreatic cancer cell migration might be achieved through its regulation of mitochondrial energy metabolism in pancreatic cancer cells to suppress focal adhesion kinase (FAK) expression levels [9]. It was reported that chloroquine (CQ) could modulate glycolysis and improve gemcitabine (GEM) sensitivity to pancreatic cancer. Meanwhile, CQ can promote the production of reactive oxygen species (ROS) by inhibiting autophagy and reducing mitochondrial membrane potential, and finally promote apoptosis induced by gemcitabine [10].
Various carcinogenic signaling pathways involved in glycolysis
UHRF1/SIRT4 axis
UHRF1 (ubiquitin 1 similar to plant homologous domain and ring finger domain) serves as an epigenetic modulator to silence tumor suppressor genes, and it is overexpressed in pancreatic cancer. Recent research displayed that inhibiting UHRF1 significantly suppresses aerobic glycolysis of cancer cells and the knockout of UHRF1 reduces the level of hypoxia inducible factor 1α (HIF1α) and downstream glycolysis genes. The in vitro experiments illustrated that UHRF1 has positive effects on aerobic glycolysis and cell proliferation. In addition, as a tumor suppressor of pancreatic cancer cells, SIRT4 negatively regulates aerobic glycolysis as a target for UHRF1. UHRF1 inhibits SIRT4, and then promotes aerobic glycolysis, thereby enhancing the proliferation of tumor cells (Figure 1). UHRF1/SIRT4 axis can be used as a potential target for prediction and therapy of pancreatic cancer, and further researches are needed to determine a new strategy by targeting this axis to improve the overall survival rate of pancreatic cancer [11].
Figure 1.
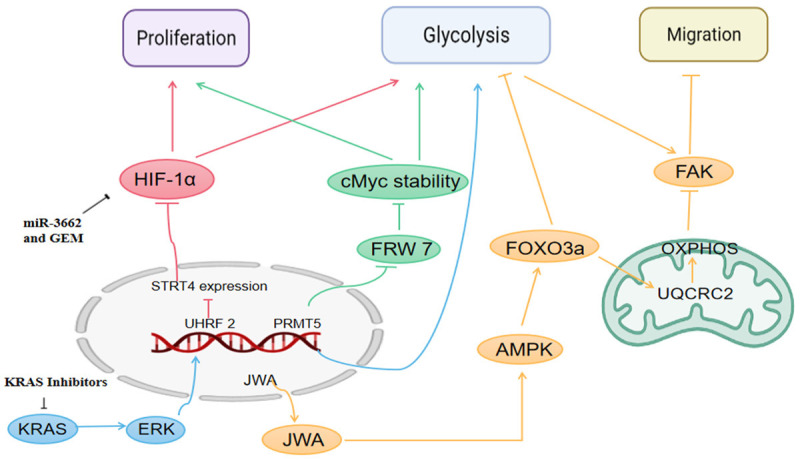
Four signaling pathways mediating glycolysis in pancreatic cancer. Green line: PRMT5 can epigenetically inhibit FBW7 expression and stabilize cMyc, and subsequently increase the proliferation and glycolysis of pancreatic cancer cells. Red line: Up-regulated UHRF2 silences SIRT4 expression, and SIRT4 negatively regulates aerobic glycolysis and suppresses HIF1α. MiRNA-3662 inhibits aerobic glycolysis through HIF-1α, and the codelivery of miR-3662 and gemcitabine can be used as a promising therapeutic option. Orange line: JWA gene expression levels significantly enhance mitochondrial aerobic respiration, along with inhibition of glycolysis. The role of JWA was achieved through the AMPK/FOXO3a/FAK signaling pathway, an inhibition on both glycolysis and cancer migration. Blue line: KRAS can activate the protein kinase ERK signaling pathway, and in turn mediate intranuclear expression and promote glycolysis. Small-molecule KRAS inhibitors can act on KRAS.
PRMT5/FBW7/cMyc axis
Protein arginine methyltransferase 5 (PRMT5) is an epigenetic enzyme with methyltransferase activity to modulate multiple cell processes by specifically catalyzing the methylation of arginine residues of histones and non-histones. Moreover, it mainly inhibits the transcription of target genes by symmetrically dimethyl arginine residues, including H4R3, H3R8 and H2AR3. More and more evidences indicated that PRMT5 may be used as a carcinogen, and epigenetic silencing of some tumor suppressor genes drove the proliferation and metastasis of tumor cells. Recent research suggested that in pancreatic cancer, PRMT5 can promote the expression of cMyc by inhibiting tumor suppressor FBW7, thus enhancing the aerobic glycolysis and tumor proliferation (Figure 1). Therefore, PRMT5/FBW7/cMyc axis plays important roles in the modulation of the glycolysis of pancreatic cancer [12].
JWA/AMPK/FOXO3a/FAK axis
As a new regulator, JWA can inhibit the adhesion, invasion and metastasis of melanoma cells, and have prognostic and predictive effects on gastric cancer [13]. Some researchers have proved that JWA positively regulates the expression of FOXO3a but inhibits the expression of FAK through AMPK signaling pathway [9]. FOXO3 belongs to the O subclass of the forked transcription factor family, participates in the signal transduction of metabolic reprogramming, and inhibits the growth and metastasis of pancreatic adenocarcinoma (PDAC) by inducing apoptosis (Figure 1) [14].
KRAS/TP53/TIGAR axis
Once the oncogene KRAS is activated, the expression of glucose transporter 1 (GLUT1) is enhanced to promote the increase of glucose uptake and lactic acid production (Figure 1).
KRAS mutant results in increased glycolytic enzyme expression, such as phosphofructokinase 1 (PFK1), hexokinase 2 (HK2) and lactate dehydrogenase A (LDHA) [15], thus leading to the inhibition of TP53 pathway [16]. Afterwards, the TP53 inactivation supports cell glycolysis by disrupting the translocation of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) from nuclear to cytosol [17]. Due to the higher activity of anabolic pathway, tumor invasiveness is increased. Autophagy is involved in the activation of these pathways. Therefore, autophagy inhibitor hydroxychloroquine increased PDAC cell growth and TP53 loss in KRAS mutant mice [18]. As a glycolytic enzyme, different roles of TP53-induced glycolysis and apoptosis regulator (TIGAR) were reported in tumor metabolism [19]. TIGAR suppresses glycolysis by its bisphosphatase activity and transferring metabolic intermediates to replace pentosephosphate pathway (PPP) under oxidative stress. The process is critical in regulating redox balance, and suppressing ROS production in cells by increasing NADPH production. TIGAR is highly expressed in many cancers and PDAC patient-derived xenografts (PDX) with wide type TP53 rather than mutant TP53 [19]. Besides, the modulation of ROS production for TIGAR was also observed in a mouse model of PDAC development [20,21]. Given mutant-activated KRAS expression in more than 90% of pancreatic cancers and its role in the onset and development of the disease, it is an attractive but “untreatable” therapeutic target. However, this limitation may be overcomed by new and innovative approaches, such as siRNA delivery technology (phase II trial NCT01676259) and small-molecule KRAS inhibitors [22].
Changes of glycolytic enzyme activity
Lactate dehydrogenase (LDH)
LDHA, one of the key enzymes in glycolysis and subgroups of LDH, oxidizes reduced NADH to NAD+ and converts pyruvate to lactic acid. Furthermore, the expression of LDHA is significantly upregulated in various malignant tumors such as ovarian cancer [23], breast cancer [24], osteoma [25], pancreatic gland cancer [26], melanoma [27] and prostate cancer [28]. In the carcinogenesis process from pancreatitis to cancer, LDHA was over-expressed at a very early stage, and increased particularly in the highly aggressive tumors [29]. Recent study found that FX11, an inhibitor of LDHA, could inhibit tumor growth and promote the apoptosis of tumor cells in the xenotransplantation model of mouse pancreatic cancer with mutant TP53. However, the tumor containing wild-type TP53 had complete drug resistance to FX11 [30], which may be due to the fact that the high expression of TIGAR was beneficial to PPP instead of glycolysis [31]. Novel N-hydroxylindindex (NHI) inhibitor targeting LDH-A impairs the proliferation, growth and migration of PDAC. Furthermore, when the NHI inhibitor was used together with gemcitabine, its anticancer activity against PDAC blasts was enhanced [32].
Pyruvate kinase 2 (PKM2)
In the glycolytic metabolism of tumor cells, PKM2, one of its rate-limiting enzymes, catalyzes phosphoenolpyruvate to synthesize pyruvic acid, and then enters tricarboxylic acid cycle to produce lactic acid to form ATP (Figure 2). This irreversible reaction is a very important regulatory step in glycolytic pathway [33]. The latest research exhibited that the gene expression of PKM2 is mainly to maintain the steady state of glycolytic intermediates instead of controlling glycolysis flow [34]. PKM2 expression was highly up-regulated in gastric cancer [35], cervical cancer [36] and prostate cancer [37]. The expression of PKM2 gradually increased in the process of transforming into pancreatic cancer with a low expression in cyst, the middle in pancreatic intraepithelial neoplasia (PanIN) and the highest in cancer [28]. So far, it was proved the detection of serum PKM2 is useful in the diagnosis of pancreatic cancer, and its sensitivity and specificity are comparable to those of CA19-9 [38-40]. The silencing of PMK2 gene in human or mouse PDAC cell line indicated that it participated in the tumor initiation, growth, invasion and metastasis [41-45]. Mechanistically, PKM2 may be regulated by serine/threonine protein kinase 2 (PAK2). In fact, PKM2 knockout reduced the half-life of PAK2 protein by increasing the ubiquitin-dependent proteasome degradation [45]. The expression of PKM2 and PAK2 in PDAC was highly associated with metastasis, which indicated that the complex was a possible target [45]. In addition, the deletion of PKM2 negatively affects the secretion of HIF-1α and VEGF through Akt/c-Myc, NF-kB, and mTOR pathways, thus impairing proliferation, increasing apoptosis and reducing the growth of PDAC tumor [42,46-48]. PKM2 also participates in the regulation of MAPK pathway [49,50], and may target and regulate STAT3 and TWIST1, especially in EMT, which increases the invasion and metastasis [43,51]. Furthermore, MIR210HG affects the phenotype of pancreatic cancer cells by regulating the miR-125b-5p/HK2/PKM2 axis, thus affecting the tumor glycolysis and progression [52]. Recent studies indicated that the combination of ethanol extract of Hedyotis diffusa (EEHD) and Scutellaria barbata (EESB) can synergistically suppress the in vivo and in vitro growth of pancreatic cancer. Its inhibitory effects on aerobic glycolysis may be achieved by affecting PKM subtype transformation.
Figure 2.
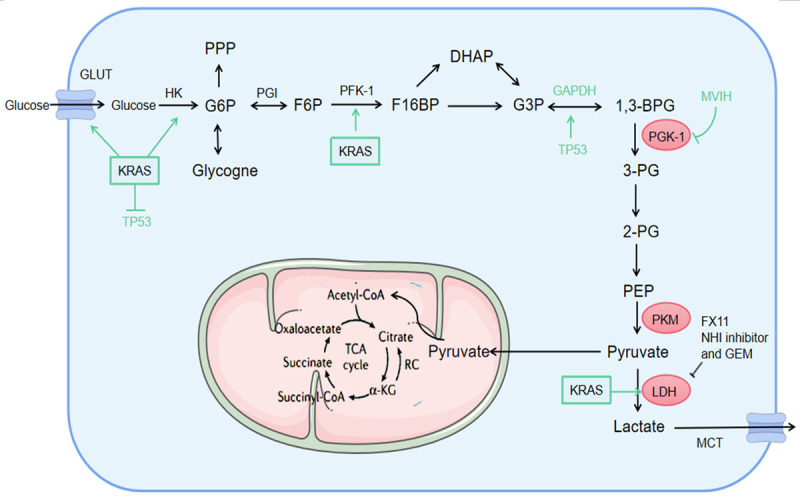
Glycolytic enzymes in pancreatic cancer. The regulation of key enzymes is particularly important. The activation of KRAS enhances the transcription of GLUT1, promotes the glucose uptake and lactic acid production, and also promotes the activation of glycolytic enzymes (such as HK2, PFK1, and LDHA). Upregulated KRAS leads to the inhibition of TP53 pathway. The TP53 inactivation supports cell glycolysis by disrupting the translocation of GAPDH from nuclear to cytosol. MVIH inhibits the activity of PGK-1, and FX11. And NHI inhibitor and gemcitabine inhibit the LDH activity. PPP, pentose phosphate pathway; TCA, tricarboxylic acid; HK2, hexokinase 2; PFK1, Phosphofructokinase 1; LDHA, lactate dehydrogenase A; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MVIH, microvascular invasion in hepatocellular carcinoma.
Phosphoglycerate kinase 1 (PGK1)
PGK1 produces an ATP by glycolysis, thereby catalyzing the conversion of 1,3-diphosphoglyceric acid to 3-phosphoglyceric acid (Figure 2). PGK1 is also involved in DNA repairmen and autophagy, and it has been overexpressed in most cancers, and its overexpression in some specific cancer types indicates poor prognosis [53]. However, extracellular PGK1 plays an opposite role to intracellular PGK1. Under certain conditions, extracellular PGK1 can inhibit tumor growth and metastasis. For example, the intraperitoneal injection of recombinant human PGK in fibrosarcoma mice and pancreatic tumor mice will result in an increase in plasma angiostatin level and a decrease in tumor angiogenesis and tumor growth [54]. In addition, PGK1 is secreted into cell matrix, which reduces VEGF level and promotes the production of angiostatin, thereby inhibiting the angiogenesis of prostate cancer (PCA) [55]. In another recent study, the expression of PGK1 was inhibited by MVIH, a long non-coding RNA (LncRNA) that activated tumor-induced angiogenesis in hepatocellular carcinoma (HCC) [56]. Apart from producing angiogenesis inhibitor angiostatin as tumor suppressor, PGK1 can also inhibit the tumor growth of Lewis lung cancer by down-regulating the COX-2 expression and promoting anti-tumor immunity in vivo [57]. The latest data implied that PGK1 can play a role as an oncogene in patients with SMAD4 negative PDAC, thus enhancing glycolysis and making tumors more invasive [58]. The existence of PGK1 can predict the fate of tumor metastasis and become a potential therapeutic target.
Influence of tumor microenvironment
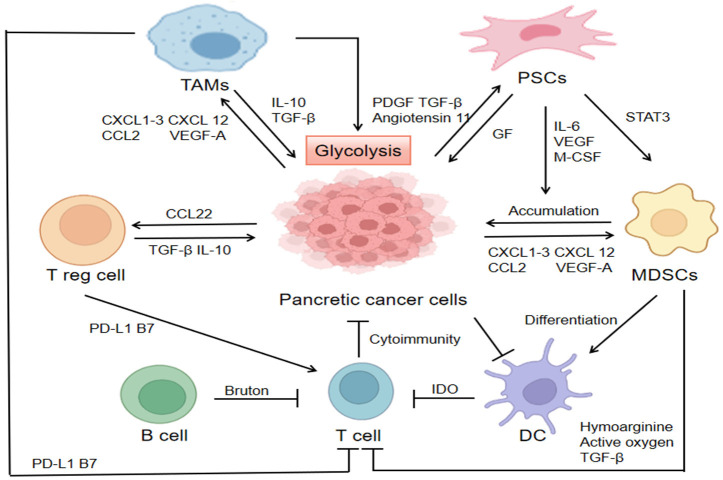
Tumor microenvironment refers to the local internal environment composed of tumor cells, immune cells, interstitial cells and secreted active media. Recent researches have confirmed that the tumor microenvironment plays a key role in PDAC progression [59], which revealed the relationship between microenvironment and metastasis. The microenvironment of pancreatic cancer has two main characteristics, as dense fibrous hyperplasia and extensive immunosuppression [60]. The two characteristics can promote the proliferation of pancreatic cancer cells, and evade immune surveillance and metastasis by directly inhibiting anti-tumor immunity or inducing the proliferation of immunosuppressive cells (Figure 3).
Figure 3.
Tumor microenvironment in pancreatic cancer. Pancreatic cancer cells secrete multiple cytokines (such as IL-10, TGF-β, and IL-23) and chemokines (such as CXCL1-3, CXCL5, CXCL12, CCL2, and VEGFα) to activate surrounding stromal cells and attract immunosuppressive cells such as Treg, MDSCs, and TAMs to cluster at tumor sites. High expression of glycolytic enzymes (such as HK2) in TAMs enhances its glycolysis, but also promotes the glycolytic levels of pancreatic cancer cells, which impacts on the growth of pancreatic cancer cells. MDSCs, myeloid-derived suppressor cells; TAMs, tumor-associated macrophages; PSCs, pancreatic stellate cells; HK2, hexokinase 2.
Hypoxic microenvironment
There are two reasons that account for the hypoxic microenvironment in tumor [61]. One is the rapid proliferation of tumor cells that leads to a continuous decrease of oxygen in tumor tissue [62], and another is the construction of vascular networks with abnormal growth within the tumor [63], especially vascular dysfunction [64]. PDAC is characterized by large areas of hypoxic regions, which may be due to poor perfusion of blood and the dense matrix [65]. Tumor cells in hypoxic regions tend to undergo epithelial-stromal transformation (EMT) and exhibit high glycolysis [66].
Hypoxia-inducible factor (HIF1α) is an important transcription factor in tumor microenvironment, and plays a key role in regulation. HIF-1α, a nuclear protein with transcriptional activity as the regulator for aerobic glycolysis, can make cell survive in hypoxic microenvironment by metabolism reprogramming for glycolysis. Besides, HIF1α can induce angiogenesis, pH balance and cell proliferation. Mechanistically, HIF1α can regulate the transcription of its target genes, such as GLUT1, HK2 and LDHA, which is critical in the regulation of aerobic glycolysis. Hu et al. discovered that SIRT4, a mitochondrial tumor suppressor, can inhibit the expression of HIF1α and negatively regulate the pancreatic cancer glycolysis [11]. UHFR1, an epigenetic modifier, is upregulated in pancreatic cancer patients and associated with poor prognosis of patients. On the other hand, UHFR1 can positively regulate the HIF1α transcription in a dose-dependent manner by inhibiting SIRT4, while HIF1α regulates aerobic glycolysis through regulating of glycolytic gene transcription and promoting the development of pancreatic cancer. Transcription analysis further indicated that UHFR1 inhibited SIRT4 transcription in pancreatic cancer.
Immune microenvironment
Pancreatic cancer has significant immune cell infiltration in microenvironment, with immunosuppression as the prominent feature. On the one hand, the tumor educates the immune system so that it cannot be recognized by the immune system. On the other hand, it recruits and activates various immunosuppressive cells such as pancreatic stellate cells (PSCs), myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs) and regulatory T cells (Tregs), and then secretes immunosuppressive molecules and inhibits the host’s anti-tumor immune response, thus inducing the immune escape of tumor, and “domesticating” the body’s immune system to promote the growth, invasion and metastasis of tumor [67,68].
Pancreatic cancer cells secrete multiple cytokines (such as IL-10, TGF-β and IL-23) and chemokines (such as CXCL1-3, CXCL5, CXCL12, CCL2, and VEGF-α) to activate surrounding stromal cells and recruit Tregs, MDSCs and TAMs and other immunosuppressive cells to the tumor site. Tregs, MDSCs, and TAMs act on pancreatic T cancer cells by having secreted immunosuppressors (such as TGF-β, IL-10) and express immunosuppressive ligands (such as PD-L1, B7) to inhibit effector T cell function [69]. Meanwhile, pancreatic cancer cells secrete platelet-derived growth factor (PDGF), Angiotensin 11 and TGF-β to activate PSCs [70], which can also secrete multiple growth factors to drive the growth and proliferation of PDAC [71]. MDSCs decompose the arginine necessary for T cell protein synthesis by expressing high levels of arginine for protein synthesis, producing reactive oxygen species, and secreting TGF-β to inhibit the function of effector T cells [72]. Dendritic cells of patients with pancreatic cancer suppress the anti-tumor immunity of T cells by expressing indindomine 2, 3-dioxygenase, and inducing immune tolerance to tumor cells [73]. B cells can inhibit the response of antitumor T cells via Bruton tyrosine kinase pathway [74].
TAMs are highly plastic in tumor microenvironment, which can account for 50% of some solid tumors. The results showed that the decrease of glycolytic activity in TAMs was achieved through nutrition and immune circuit, thus promoting tumor progression. In addition, according to Liu et al. found that Glycolytic enzymes including hexokinase 2 (HK2) were up-regulated in TAMs of pancreatic cancer patients [75], which indicated that glycolysis ability was enhanced. Penny [76] held the view that this metabolic reprogramming was responsible for the increased metastasis of PDAC. Though competition for local glucose supply is intensified, the glycolysis of TAMs may still promote tumor growth. Recent research proved that there is a significant positive correlation between tumor glycolysis and tumor immunity in various cancer types. Tumor glycolysis can increase the expression of PD-L1 in tumor cells [77]. Therefore, the glycolytic activity of tumor can be used as a biomarker to predict the response to immunotherapy for various cancers.
The influence of glycolysis to pancreatic cancer
Glycolysis in proliferation of pancreatic cancer
Metabolic reprogramming of cancer cells is conducive to the initiation, proliferation, invasion and metastasis of tumors in the TME. Recent research revealed that chromobox protein homolog 3 (CBX3) can promote aerobic glycolysis of pancreatic cancer cells, inhibit mitochondrial respiration, and induce cell proliferation by inhibiting the expression of fructose diphosphatase 1 (FBP1). Interference with CBX3-FBP1 signal axis can suppress the cell proliferation, which effectively inhibits the pancreatic cancer growth by targeting aerobic glycolysis [78]. Meanwhile, the latest research displayed that long-chain non-coding RNA (lncRNA) HOXA antisense RNA2 (HOXA-AS2) is up-regulated in pancreatic cancers, and is related to clinical indicators of malignant progression. Silencing its expression can reduce cell proliferation and inhibit cell invasion [79].
Glycolysis in metastasis of pancreatic cancer
Transcription factors, including c-Myc, EST1 and FOXM1, can promote the glycolysis of pancreatic cancer and regulate the invasion and metastasis of PDAC by combining with the promoters of glycolytic genes (such as HK1, PFKFB3, LDHA and GLUTs) [80,81]. With the expression of glycolytic enzyme, PDAC can change its enzyme activity through post-translational modification, thus promoting glycolysis. For example, PFKFB3 can enhance the glycolysis of cancer cells by phosphorylation, dimethyl and acetylation under energy crisis [82,83]. Li et al. uncovered the mechanism that cisplatin induced the acetylation of lysine 472 (K472) of PFKFB3 and disrupted the nuclear localization, thus leading to the cytoplasm accumulation, which promoted the AMPK phosphorylation of PFKFB3 and caused its activation and glycolysis [82]. Besides, due to the degradation of LDHA acetylated by lysine 5 (K5), LDHA was overexpressed in PDAC cells, which reduced the level of LDHA and inhibited the migration of glycolysis and PDAC cells [84]. With the development of nanoparticles, nanoalbumin-bound paclitaxel (Abraxane) has been approved in combination with gemcitabine for first-line treatment with metastatic pancreatic cancer [85].
Glycolysis in drug resistance of pancreatic cancer
Based on previous studies, the metabolites of pancreatic cancer cells exposed to gemcitabine do not show obvious changes. However, the chemotherapy resistance to gemcitabine will induce metabolic reprogramming and participate in the regulation process of chemotherapy resistance to tumor cells. L-type amino acid transporter 2 (LAT2) is up-regulated in many tumor types, and can reduce the gemcitabine sensitivity of PDAC cells in vivo and in vitro. The research conducted by Feng et al. [86] indicated that higher expression of LAT2 and lactate dehydrogenase B (LDHB) was in PDAC tissues than those in adjacent tissues, which may predict poor prognosis of PDAC patients. LAT2 can regulate two glutamine-dependent positive feedback loops (LAT2/p-mTORSer2448 loop and glutamine/p-mTORSer2448/glutamine syntheses loop), promote the glycolytic pathway of pancreatic cancer cells and reduce the sensitivity to gemcitabine. The activation of glycolysis induced by LAT2 can be reversed by mTOR inhibitor (RAD001). RAD001 combined with gemcitabine can promote the sensitivity of chemotherapy in PDAC cells with LAT2 overexpression.
Recent research revealed that miRNA-3662 inhibited PDAC cell chemoresistance and aerobic glycolysis through a negative feedback loop with HIF-1α and argued that co-delivery of miR-3662 and gemcitabine could serve as a promising therapeutic regimen for PDAC patients [87]. Another research shed light on a reciprocal feedback of antisense RNA1 of HIF1α (HIF1A-AS1) and HIF-1α promoted gemcitabine resistance of pancreatic cancer, which provided an applicable therapeutic target [88]. Besides, human balanced nucleoside transport protein 1 (hENT1) reversed the gemcitabine resistance by inhibiting glycolysis and modifying HIF-1α mediated glucose transport in pancreatic cancer [89].
Conclusions and prospect
Our understanding of cancer metabolism has gone beyond the “Warburg” effect itself and has become increasingly aware of the enormous complexity behind. A slight loss leads to a thousand miles away, called “the whole body moves with one hair”, which requires us to pay attention to all the elements involved, including the “base” TME, the activity change of several key enzymes, and the abnormality of signal transduction pathway. At present, the survival rate of pancreatic cancer remains significantly low, and the molecular mechanism behind the pancreatic cancer and glycolysis needs to be further studied, so as to provide targeted therapy for regulating glycolysis and new targets and ideas for clinical treatment of pancreatic cancer. We hope to find a “control switch” for glycolysis as soon as possible, press the shortcut key for stopping cancer, build a bridge between basic research and clinical research, and truly realize the “precise” treatment of cancer.
Acknowledgements
This study was supported by the Shanghai Rising-Star Program (No.19QA1411300).
Disclosure of conflict of interest
None.
References
- 1.Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73–85. doi: 10.1016/S0140-6736(16)00141-0. [DOI] [PubMed] [Google Scholar]
- 2.Chen BB, Tien YW, Chang MC, Cheng MF, Chang YT, Wu CH, Chen XJ, Kuo TC, Yang SH, Shih IL, Lai HS, Shih TT. PET/MRI in pancreatic and periampullary cancer: correlating diffusion-weighted imaging, MR spectroscopy and glucose metabolic activity with clinical stage and prognosis. Eur J Nucl Med Mol Imaging. 2016;43:1753–1764. doi: 10.1007/s00259-016-3356-y. [DOI] [PubMed] [Google Scholar]
- 3.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 5.Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41:211–218. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaupel P, Schmidberger H, Mayer A. The Warburg effect: essential part of metabolic reprogramming and central contributor to cancer progression. Int J Radiat Biol. 2019;95:912–919. doi: 10.1080/09553002.2019.1589653. [DOI] [PubMed] [Google Scholar]
- 7.Reyes-Castellanos G, Masoud R, Carrier A. Mitochondrial metabolism in PDAC: from better knowledge to new targeting strategies. Biomedicines. 2020;8:270. doi: 10.3390/biomedicines8080270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao MF. Effect of polyphyllin D on proliferation and apoptosis of human pancreatic cancer cells. Zhongguo Zhong Yao Za Zhi. 2020;45:1418–1422. doi: 10.19540/j.cnki.cjcmm.20191230.401. [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Bai J, Ye J, Liu Z, Chen R, Mao W, Li A, Zhou J. JWA as a functional molecule to regulate cancer cells migration via MAPK cascades and F-actin cytoskeleton. Cell Signal. 2007;19:1315–1327. doi: 10.1016/j.cellsig.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Chen X, Tao Y, He M, Deng M, Guo R, Sheng Q, Wang X, Ren K, Li T, He X, Zang S, Zhang Z, Li M, He Q. Co-delivery of autophagy inhibitor and gemcitabine using a pH-activatable core-shell nanobomb inhibits pancreatic cancer progression and metastasis. Theranostics. 2021;11:8692–8705. doi: 10.7150/thno.60437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu Q, Qin Y, Ji S, Xu W, Liu W, Sun Q, Zhang Z, Liu M, Ni Q, Yu X, Xu X. UHRF1 promotes aerobic glycolysis and proliferation via suppression of SIRT4 in pancreatic cancer. Cancer Lett. 2019;452:226–236. doi: 10.1016/j.canlet.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 12.Qin Y, Hu Q, Xu J, Ji S, Dai W, Liu W, Xu W, Sun Q, Zhang Z, Ni Q, Zhang B, Yu X, Xu X. PRMT5 enhances tumorigenicity and glycolysis in pancreatic cancer via the FBW7/cMyc axis. Cell Commun Signal. 2019;17:30. doi: 10.1186/s12964-019-0344-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang S, Wu X, Chen Y, Zhang J, Ding J, Zhou Y, He S, Tan Y, Qiang F, Bai J, Zeng J, Gong Z, Li A, Li G, Roe OD, Zhou J. Prognostic and predictive role of JWA and XRCC1 expressions in gastric cancer. Clin Cancer Res. 2012;18:2987–2996. doi: 10.1158/1078-0432.CCR-11-2863. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Yang R, Dong Y, Chen M, Wang Y, Wang G. Knockdown of FOXO3a induces epithelial-mesenchymal transition and promotes metastasis of pancreatic ductal adenocarcinoma by activation of the beta-catenin/TCF4 pathway through SPRY2. J Exp Clin Cancer Res. 2019;38:38. doi: 10.1186/s13046-019-1046-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Liu W, Zhang B, Hu Q, Qin Y, Xu W, Shi S, Liang C, Meng Q, Xiang J, Liang D, Ji S, Liu J, Hu P, Liu L, Liu C, Long J, Ni Q, Yu X, Xu J. A new facet of NDRG1 in pancreatic ductal adenocarcinoma: suppression of glycolytic metabolism. Int J Oncol. 2017;50:1792–1800. doi: 10.3892/ijo.2017.3938. [DOI] [PubMed] [Google Scholar]
- 16.Buscail L, Bournet B, Cordelier P. Role of oncogenic KRAS in the diagnosis, prognosis and treatment of pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2020;17:153–168. doi: 10.1038/s41575-019-0245-4. [DOI] [PubMed] [Google Scholar]
- 17.Butera G, Pacchiana R, Mullappilly N, Margiotta M, Bruno S, Conti P, Riganti C, Donadelli M. Mutant p53 prevents GAPDH nuclear translocation in pancreatic cancer cells favoring glycolysis and 2-deoxyglucose sensitivity. Biochim Biophys Acta Mol Cell Res. 2018;1865:1914–1923. doi: 10.1016/j.bbamcr.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Rosenfeldt MT, O’Prey J, Morton JP, Nixon C, MacKay G, Mrowinska A, Au A, Rai TS, Zheng L, Ridgway R, Adams PD, Anderson KI, Gottlieb E, Sansom OJ, Ryan KM. p53 status determines the role of autophagy in pancreatic tumour development. Nature. 2013;504:296–300. doi: 10.1038/nature12865. [DOI] [PubMed] [Google Scholar]
- 19.Geng J, Yuan X, Wei M, Wu J, Qin ZH. The diverse role of TIGAR in cellular homeostasis and cancer. Free Radic Res. 2018;52:1240–1249. doi: 10.1080/10715762.2018.1489133. [DOI] [PubMed] [Google Scholar]
- 20.Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395:2008–2020. doi: 10.1016/S0140-6736(20)30974-0. [DOI] [PubMed] [Google Scholar]
- 21.Liu Z, Wu Y, Zhang Y, Yuan M, Li X, Gao J, Zhang S, Xing C, Qin H, Zhao H, Zhao Z. TIGAR promotes tumorigenesis and protects tumor cells from oxidative and metabolic stresses in gastric cancer. Front Oncol. 2019;9:1258. doi: 10.3389/fonc.2019.01258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zorde Khvalevsky E, Gabai R, Rachmut IH, Horwitz E, Brunschwig Z, Orbach A, Shemi A, Golan T, Domb AJ, Yavin E, Giladi H, Rivkin L, Simerzin A, Eliakim R, Khalaileh A, Hubert A, Lahav M, Kopelman Y, Goldin E, Dancour A, Hants Y, Arbel-Alon S, Abramovitch R, Shemi A, Galun E. Mutant KRAS is a druggable target for pancreatic cancer. Proc Natl Acad Sci U S A. 2013;110:20723–20728. doi: 10.1073/pnas.1314307110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Q, Xue L, Zhang X, Bu S, Zhu X, Lai D. Autophagy protects ovarian cancer-associated fibroblasts against oxidative stress. Cell Cycle. 2016;15:1376–1385. doi: 10.1080/15384101.2016.1170269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang X, Li X, Xie X, Ye F, Chen B, Song C, Tang H, Xie X. High expressions of LDHA and AMPK as prognostic biomarkers for breast cancer. Breast. 2016;30:39–46. doi: 10.1016/j.breast.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 25.Gao S, Tu DN, Li H, Jiang JX, Cao X, You JB, Zhou XQ. Pharmacological or genetic inhibition of LDHA reverses tumor progression of pediatric osteosarcoma. Biomed Pharmacother. 2016;81:388–393. doi: 10.1016/j.biopha.2016.04.029. [DOI] [PubMed] [Google Scholar]
- 26.He TL, Zhang YJ, Jiang H, Li XH, Zhu H, Zheng KL. The c-Myc-LDHA axis positively regulates aerobic glycolysis and promotes tumor progression in pancreatic cancer. Med Oncol. 2015;32:187. doi: 10.1007/s12032-015-0633-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang R, Su J, Xue SL, Yang H, Ju LL, Ji Y, Wu KH, Zhang YW, Zhang YX, Hu JF, Yu MM. HPV E6/p53 mediated down-regulation of miR-34a inhibits Warburg effect through targeting LDHA in cervical cancer. Am J Cancer Res. 2016;6:312–320. [PMC free article] [PubMed] [Google Scholar]
- 28.Xian ZY, Liu JM, Chen QK, Chen HZ, Ye CJ, Xue J, Yang HQ, Li JL, Liu XF, Kuang SJ. Inhibition of LDHA suppresses tumor progression in prostate cancer. Tumour Biol. 2015;36:8093–8100. doi: 10.1007/s13277-015-3540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohammad GH, Olde Damink SW, Malago M, Dhar DK, Pereira SP. Pyruvate kinase M2 and lactate dehydrogenase A are overexpressed in pancreatic cancer and correlate with poor outcome. PLoS One. 2016;11:e0151635. doi: 10.1371/journal.pone.0151635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajeshkumar NV, Dutta P, Yabuuchi S, de Wilde RF, Martinez GV, Le A, Kamphorst JJ, Rabinowitz JD, Jain SK, Hidalgo M, Dang CV, Gillies RJ, Maitra A. Therapeutic targeting of the Warburg effect in pancreatic cancer relies on an absence of p53 function. Cancer Res. 2015;75:3355–3364. doi: 10.1158/0008-5472.CAN-15-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheung EC, DeNicola GM, Nixon C, Blyth K, Labuschagne CF, Tuveson DA, Vousden KH. Dynamic ROS Control by TIGAR regulates the initiation and progression of pancreatic cancer. Cancer Cell. 2020;37:168–182. e164. doi: 10.1016/j.ccell.2019.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maftouh M, Avan A, Sciarrillo R, Granchi C, Leon LG, Rani R, Funel N, Smid K, Honeywell R, Boggi U, Minutolo F, Peters GJ, Giovannetti E. Synergistic interaction of novel lactate dehydrogenase inhibitors with gemcitabine against pancreatic cancer cells in hypoxia. Br J Cancer. 2014;110:172–182. doi: 10.1038/bjc.2013.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Z, Deng X, Liu Y, Liu Y, Sun L, Chen F. PKM2, function and expression and regulation. Cell Biosci. 2019;9:52. doi: 10.1186/s13578-019-0317-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rothman DL, Shulman RG. Two transition states of the glycogen shunt and two steady states of gene expression support metabolic flexibility and the Warburg effect in cancer. Neoplasia. 2021;23:879–886. doi: 10.1016/j.neo.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shiroki T, Yokoyama M, Tanuma N, Maejima R, Tamai K, Yamaguchi K, Oikawa T, Noguchi T, Miura K, Fujiya T, Shima H, Sato I, Murata-Kamiya N, Hatakeyama M, Iijima K, Shimosegawa T, Satoh K. Enhanced expression of the M2 isoform of pyruvate kinase is involved in gastric cancer development by regulating cancer-specific metabolism. Cancer Sci. 2017;108:931–940. doi: 10.1111/cas.13211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu H, Wu J, Zhang W, Luo H, Shen Z, Cheng H, Zhu X. PKM2 enhances chemosensitivity to cisplatin through interaction with the mTOR pathway in cervical cancer. Sci Rep. 2016;6:30788. doi: 10.1038/srep30788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Talesa VN, Ferri I, Bellezza G, Love HD, Sidoni A, Antognelli C. Glyoxalase 2 is involved in human prostate cancer progression as part of a mechanism driven by PTEN/PI3K/AKT/mTOR Signaling With Involvement of PKM2 and ERalpha. Prostate. 2017;77:196–210. doi: 10.1002/pros.23261. [DOI] [PubMed] [Google Scholar]
- 38.Joergensen MT, Heegaard NH, Schaffalitzky de Muckadell OB. Comparison of plasma Tu-M2-PK and CA19-9 in pancreatic cancer. Pancreas. 2010;39:243–247. doi: 10.1097/MPA.0b013e3181bae8ab. [DOI] [PubMed] [Google Scholar]
- 39.Goonetilleke KS, Mason JM, Siriwardana P, King NK, France MW, Siriwardena AK. Diagnostic and prognostic value of plasma tumor M2 pyruvate kinase in periampullary cancer: evidence for a novel biological marker of adverse prognosis. Pancreas. 2007;34:318–324. doi: 10.1097/MPA.0b013e31802ee9c7. [DOI] [PubMed] [Google Scholar]
- 40.Kumar Y, Gurusamy K, Pamecha V, Davidson BR. Tumor M2-pyruvate kinase as tumor marker in exocrine pancreatic cancer a meta-analysis. Pancreas. 2007;35:114–119. doi: 10.1097/mpa.0b013e3180537237. [DOI] [PubMed] [Google Scholar]
- 41.Yu L, Teoh ST, Ensink E, Ogrodzinski MP, Yang C, Vazquez AI, Lunt SY. Cysteine catabolism and the serine biosynthesis pathway support pyruvate production during pyruvate kinase knockdown in pancreatic cancer cells. Cancer Metab. 2019;7:13. doi: 10.1186/s40170-019-0205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Azoitei N, Becher A, Steinestel K, Rouhi A, Diepold K, Genze F, Simmet T, Seufferlein T. PKM2 promotes tumor angiogenesis by regulating HIF-1alpha through NF-kappaB activation. Mol Cancer. 2016;15:3. doi: 10.1186/s12943-015-0490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Masamune A, Hamada S, Yoshida N, Nabeshima T, Shimosegawa T. Pyruvate kinase isozyme M2 plays a critical role in the interactions between pancreatic stellate cells and cancer cells. Dig Dis Sci. 2018;63:1868–1877. doi: 10.1007/s10620-018-5051-2. [DOI] [PubMed] [Google Scholar]
- 44.Zhu H, Luo H, Zhu X, Hu X, Zheng L, Zhu X. Pyruvate kinase M2 (PKM2) expression correlates with prognosis in solid cancers: a meta-analysis. Oncotarget. 2017;8:1628–1640. doi: 10.18632/oncotarget.13703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng TY, Yang YC, Wang HP, Tien YW, Shun CT, Huang HY, Hsiao M, Hua KT. Pyruvate kinase M2 promotes pancreatic ductal adenocarcinoma invasion and metastasis through phosphorylation and stabilization of PAK2 protein. Oncogene. 2018;37:1730–1742. doi: 10.1038/s41388-017-0086-y. [DOI] [PubMed] [Google Scholar]
- 46.Shen Y, Chen G, Zhuang L, Xu L, Lin J, Liu L. ARHGAP4 mediates the Warburg effect in pancreatic cancer through the mTOR and HIF-1alpha signaling pathways. Onco Targets Ther. 2019;12:5003–5012. doi: 10.2147/OTT.S207560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dando I, Donadelli M, Costanzo C, Dalla Pozza E, D’Alessandro A, Zolla L, Palmieri M. Cannabinoids inhibit energetic metabolism and induce AMPK-dependent autophagy in pancreatic cancer cells. Cell Death Dis. 2013;4:e664. doi: 10.1038/cddis.2013.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He G, Jiang Y, Zhang B, Wu G. The effect of HIF-1alpha on glucose metabolism, growth and apoptosis of pancreatic cancerous cells. Asia Pac J Clin Nutr. 2014;23:174–180. doi: 10.6133/apjcn.2014.23.1.14. [DOI] [PubMed] [Google Scholar]
- 49.Jia Y, Li HY, Wang J, Wang Y, Zhang P, Ma N, Mo SJ. Phosphorylation of 14-3-3zeta links YAP transcriptional activation to hypoxic glycolysis for tumorigenesis. Oncogenesis. 2019;8:31. doi: 10.1038/s41389-019-0143-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feng J, Ma T, Ge Z, Lin J, Ding W, Chen H, Zhu W, Zhou S, Tan Y. PKM2 gene regulates the behavior of pancreatic cancer cells via mitogen-activated protein kinase pathways. Mol Med Rep. 2015;11:2111–2117. doi: 10.3892/mmr.2014.2990. [DOI] [PubMed] [Google Scholar]
- 51.Wang XX, Yin GQ, Zhang ZH, Rong ZH, Wang ZY, Du DD, Wang YD, Gao RX, Xian GZ. TWIST1 transcriptionally regulates glycolytic genes to promote the Warburg metabolism in pancreatic cancer. Exp Cell Res. 2020;386:111713. doi: 10.1016/j.yexcr.2019.111713. [DOI] [PubMed] [Google Scholar]
- 52.Yu T, Li G, Wang C, Gong G, Wang L, Li C, Chen Y, Wang X. MIR210HG regulates glycolysis, cell proliferation, and metastasis of pancreatic cancer cells through miR-125b-5p/HK2/PKM2 axis. RNA Biol. 2021;18:2513–2530. doi: 10.1080/15476286.2021.1930755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He Y, Luo Y, Zhang D, Wang X, Zhang P, Li H, Ejaz S, Liang S. PGK1-mediated cancer progression and drug resistance. Am J Cancer Res. 2019;9:2280–2302. [PMC free article] [PubMed] [Google Scholar]
- 54.Lay AJ, Jiang XM, Kisker O, Flynn E, Underwood A, Condron R, Hogg PJ. Phosphoglycerate kinase acts in tumour angiogenesis as a disulphide reductase. Nature. 2000;408:869–873. doi: 10.1038/35048596. [DOI] [PubMed] [Google Scholar]
- 55.Wang J, Wang J, Dai J, Jung Y, Wei CL, Wang Y, Havens AM, Hogg PJ, Keller ET, Pienta KJ, Nor JE, Wang CY, Taichman RS. Retraction: a glycolytic mechanism regulating an angiogenic switch in prostate cancer. Cancer Res. 2021;81:1623. doi: 10.1158/0008-5472.CAN-21-0464. [DOI] [PubMed] [Google Scholar]
- 56.Yuan SX, Yang F, Yang Y, Tao QF, Zhang J, Huang G, Yang Y, Wang RY, Yang S, Huo XS, Zhang L, Wang F, Sun SH, Zhou WP. Long noncoding RNA associated with microvascular invasion in hepatocellular carcinoma promotes angiogenesis and serves as a predictor for hepatocellular carcinoma patients’ poor recurrence-free survival after hepatectomy. Hepatology. 2012;56:2231–2241. doi: 10.1002/hep.25895. [DOI] [PubMed] [Google Scholar]
- 57.Tang SJ, Ho MY, Cho HC, Lin YC, Sun GH, Chi KH, Wang YS, Jhou RS, Yang W, Sun KH. Phosphoglycerate kinase 1-overexpressing lung cancer cells reduce cyclooxygenase 2 expression and promote anti-tumor immunity in vivo. Int J Cancer. 2008;123:2840–2848. doi: 10.1002/ijc.23888. [DOI] [PubMed] [Google Scholar]
- 58.Liang C, Shi S, Qin Y, Meng Q, Hua J, Hu Q, Ji S, Zhang B, Xu J, Yu XJ. Localisation of PGK1 determines metabolic phenotype to balance metastasis and proliferation in patients with SMAD4-negative pancreatic cancer. Gut. 2020;69:888–900. doi: 10.1136/gutjnl-2018-317163. [DOI] [PubMed] [Google Scholar]
- 59.Hessmann E, Buchholz SM, Demir IE, Singh SK, Gress TM, Ellenrieder V, Neesse A. Microenvironmental determinants of pancreatic cancer. Physiol Rev. 2020;100:1707–1751. doi: 10.1152/physrev.00042.2019. [DOI] [PubMed] [Google Scholar]
- 60.Neesse A, Algul H, Tuveson DA, Gress TM. Stromal biology and therapy in pancreatic cancer: a changing paradigm. Gut. 2015;64:1476–1484. doi: 10.1136/gutjnl-2015-309304. [DOI] [PubMed] [Google Scholar]
- 61.Ou X, Lv W. Metabolic changes and interaction of tumor cell, myeloid-derived suppressor cell and T cell in hypoxic microenvironment. Future Oncol. 2020;16:383–393. doi: 10.2217/fon-2019-0692. [DOI] [PubMed] [Google Scholar]
- 62.Dewhirst MW, Secomb TW, Ong ET, Hsu R, Gross JF. Determination of local oxygen consumption rates in tumors. Cancer Res. 1994;54:3333–3336. [PubMed] [Google Scholar]
- 63.Arpino JM, Nong Z, Li F, Yin H, Ghonaim N, Milkovich S, Balint B, O’Neil C, Fraser GM, Goldman D, Ellis CG, Pickering JG. Four-dimensional microvascular analysis reveals that regenerative angiogenesis in ischemic muscle produces a flawed microcirculation. Circ Res. 2017;120:1453–1465. doi: 10.1161/CIRCRESAHA.116.310535. [DOI] [PubMed] [Google Scholar]
- 64.Gaustad JV, Simonsen TG, Andersen LMK, Rofstad EK. Vascular abnormalities and development of hypoxia in microscopic melanoma xenografts. J Transl Med. 2017;15:241. doi: 10.1186/s12967-017-1347-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koong AC, Mehta VK, Le QT, Fisher GA, Terris DJ, Brown JM, Bastidas AJ, Vierra M. Pancreatic tumors show high levels of hypoxia. Int J Radiat Oncol Biol Phys. 2000;48:919–922. doi: 10.1016/s0360-3016(00)00803-8. [DOI] [PubMed] [Google Scholar]
- 66.Guillaumond F, Leca J, Olivares O, Lavaut MN, Vidal N, Berthezene P, Dusetti NJ, Loncle C, Calvo E, Turrini O, Iovanna JL, Tomasini R, Vasseur S. Strengthened glycolysis under hypoxia supports tumor symbiosis and hexosamine biosynthesis in pancreatic adenocarcinoma. Proc Natl Acad Sci U S A. 2013;110:3919–3924. doi: 10.1073/pnas.1219555110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morrison AH, Byrne KT, Vonderheide RH. Immunotherapy and prevention of pancreatic cancer. Trends Cancer. 2018;4:418–428. doi: 10.1016/j.trecan.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ren B, Cui M, Yang G, Wang H, Feng M, You L, Zhao Y. Tumor microenvironment participates in metastasis of pancreatic cancer. Mol Cancer. 2018;17:108. doi: 10.1186/s12943-018-0858-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Trac NT, Chung EJ. Peptide-based targeting of immunosuppressive cells in cancer. Bioact Mater. 2020;5:92–101. doi: 10.1016/j.bioactmat.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mews P, Phillips P, Fahmy R, Korsten M, Pirola R, Wilson J, Apte M. Pancreatic stellate cells respond to inflammatory cytokines: potential role in chronic pancreatitis. Gut. 2002;50:535–541. doi: 10.1136/gut.50.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mace TA, Ameen Z, Collins A, Wojcik S, Mair M, Young GS, Fuchs JR, Eubank TD, Frankel WL, Bekaii-Saab T, Bloomston M, Lesinski GB. Pancreatic cancer-associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner. Cancer Res. 2013;73:3007–3018. doi: 10.1158/0008-5472.CAN-12-4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011;32:19–25. doi: 10.1016/j.it.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jang JE, Hajdu CH, Liot C, Miller G, Dustin ML, Bar-Sagi D. Crosstalk between regulatory T cells and tumor-associated dendritic cells negates anti-tumor immunity in pancreatic cancer. Cell Rep. 2017;20:558–571. doi: 10.1016/j.celrep.2017.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gunderson AJ, Kaneda MM, Tsujikawa T, Nguyen AV, Affara NI, Ruffell B, Gorjestani S, Liudahl SM, Truitt M, Olson P, Kim G, Hanahan D, Tempero MA, Sheppard B, Irving B, Chang BY, Varner JA, Coussens LM. Bruton tyrosine kinase-dependent immune cell cross-talk drives pancreas cancer. Cancer Discov. 2016;6:270–285. doi: 10.1158/2159-8290.CD-15-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu PS, Wang H, Li X, Chao T, Teav T, Christen S, Di Conza G, Cheng WC, Chou CH, Vavakova M, Muret C, Debackere K, Mazzone M, Huang HD, Fendt SM, Ivanisevic J, Ho PC. alpha-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat Immunol. 2017;18:985–994. doi: 10.1038/ni.3796. [DOI] [PubMed] [Google Scholar]
- 76.Penny HL, Sieow JL, Adriani G, Yeap WH, See Chi Ee P, San Luis B, Lee B, Lee T, Mak SY, Ho YS, Lam KP, Ong CK, Huang RY, Ginhoux F, Rotzschke O, Kamm RD, Wong SC. Warburg metabolism in tumor-conditioned macrophages promotes metastasis in human pancreatic ductal adenocarcinoma. Oncoimmunology. 2016;5:e1191731. doi: 10.1080/2162402X.2016.1191731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vitale I, Manic G, Coussens LM, Kroemer G, Galluzzi L. Macrophages and metabolism in the tumor microenvironment. Cell Metab. 2019;30:36–50. doi: 10.1016/j.cmet.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 78.Chen LY, Cheng CS, Qu C, Wang P, Chen H, Meng ZQ, Chen Z. CBX3 promotes proliferation and regulates glycolysis via suppressing FBP1 in pancreatic cancer. Biochem Biophys Res Commun. 2018;500:691–697. doi: 10.1016/j.bbrc.2018.04.137. [DOI] [PubMed] [Google Scholar]
- 79.Lian Y, Li Z, Fan Y, Huang Q, Chen J, Liu W, Xiao C, Xu H. The lncRNA-HOXA-AS2/EZH2/LSD1 oncogene complex promotes cell proliferation in pancreatic cancer. Am J Transl Res. 2017;9:5496–5506. [PMC free article] [PubMed] [Google Scholar]
- 80.Cui J, Shi M, Xie D, Wei D, Jia Z, Zheng S, Gao Y, Huang S, Xie K. FOXM1 promotes the warburg effect and pancreatic cancer progression via transactivation of LDHA expression. Clin Cancer Res. 2014;20:2595–2606. doi: 10.1158/1078-0432.CCR-13-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hua S, Lei L, Deng L, Weng X, Liu C, Qi X, Wang S, Zhang D, Zou X, Cao C, Liu L, Wu D. miR-139-5p inhibits aerobic glycolysis, cell proliferation, migration, and invasion in hepatocellular carcinoma via a reciprocal regulatory interaction with ETS1. Oncogene. 2018;37:1624–1636. doi: 10.1038/s41388-017-0057-3. [DOI] [PubMed] [Google Scholar]
- 82.Li FL, Liu JP, Bao RX, Yan G, Feng X, Xu YP, Sun YP, Yan W, Ling ZQ, Xiong Y, Guan KL, Yuan HX. Acetylation accumulates PFKFB3 in cytoplasm to promote glycolysis and protects cells from cisplatin-induced apoptosis. Nat Commun. 2018;9:508. doi: 10.1038/s41467-018-02950-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yamamoto T, Takano N, Ishiwata K, Ohmura M, Nagahata Y, Matsuura T, Kamata A, Sakamoto K, Nakanishi T, Kubo A, Hishiki T, Suematsu M. Reduced methylation of PFKFB3 in cancer cells shunts glucose towards the pentose phosphate pathway. Nat Commun. 2014;5:3480. doi: 10.1038/ncomms4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhao D, Zou SW, Liu Y, Zhou X, Mo Y, Wang P, Xu YH, Dong B, Xiong Y, Lei QY, Guan KL. Lysine-5 acetylation negatively regulates lactate dehydrogenase A and is decreased in pancreatic cancer. Cancer Cell. 2013;23:464–476. doi: 10.1016/j.ccr.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Feng M, Xiong G, Cao Z, Yang G, Zheng S, Qiu J, You L, Zheng L, Zhang T, Zhao Y. LAT2 regulates glutamine-dependent mTOR activation to promote glycolysis and chemoresistance in pancreatic cancer. J Exp Clin Cancer Res. 2018;37:274. doi: 10.1186/s13046-018-0947-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu A, Zhou Y, Zhao T, Tang X, Zhou B, Xu J. MiRNA-3662 reverses the gemcitabine resistance in pancreatic cancer through regulating the tumor metabolism. Cancer Chemother Pharmacol. 2021;88:343–357. doi: 10.1007/s00280-021-04289-z. [DOI] [PubMed] [Google Scholar]
- 88.Xu F, Huang M, Chen Q, Niu Y, Hu Y, Hu P, Chen D, He C, Huang K, Zeng Z, Tang J, Wang F, Zhao Y, Wang C, Zhao G. LncRNA HIF1A-AS1 promotes gemcitabine resistance of pancreatic cancer by enhancing glycolysis through modulating the AKT/YB1/HIF1alpha pathway. Cancer Res. 2021;81:5678–5691. doi: 10.1158/0008-5472.CAN-21-0281. [DOI] [PubMed] [Google Scholar]
- 89.Xi Y, Yuan P, Li T, Zhang M, Liu MF, Li B. hENT1 reverses chemoresistance by regulating glycolysis in pancreatic cancer. Cancer Lett. 2020;479:112–122. doi: 10.1016/j.canlet.2020.03.015. [DOI] [PubMed] [Google Scholar]