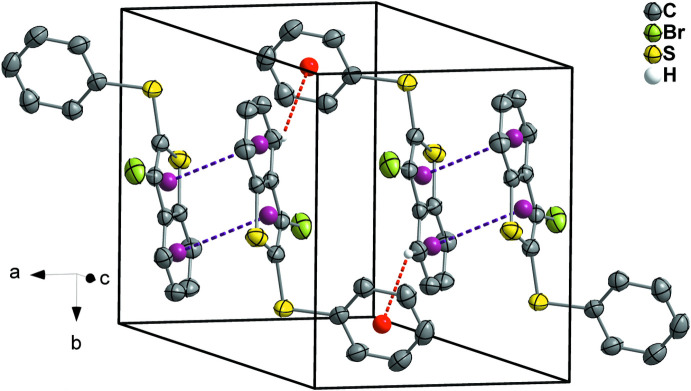
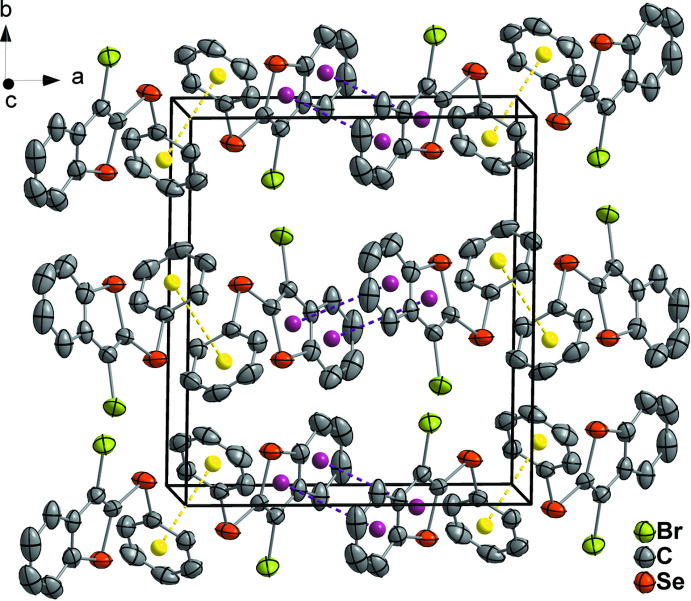
The structure of four benzo[b]chalcogenophenes are described. The presence of a phenylselanyl group at a vicinal position of bromide or iodine triggers a stabilizing intramolecular orbital interaction between a lone pair of electrons of a halogen atom and the antibonding σ*(Se–C) orbital (n halogen–σ*(Se–C), resulting in the almost linear alignment of the halogen–selenium–carbon atoms that changes the conformation and also the three-dimensional packing.
Keywords: crystal structure, benzo[b]chalcogenophenes, intramolecular orbital interaction
Abstract
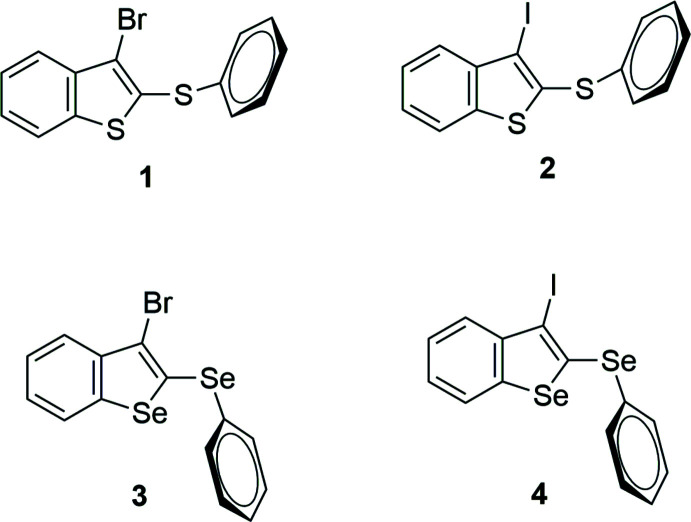
The structure of the title compounds 3-bromo-2-(phenylsulfanyl)benzo[b]thiophene (C14H9BrS2; 1), 3-iodo-2-(phenylsulfanyl)benzo[b]thiophene (C14H9IS2; 2), 3-bromo-2-(phenylselanyl)benzo[b]selenophene (C14H9BrSe2; 3), and 3-iodo-2-(phenylselanyl)benzo[b]selenophene (C14H9ISe2; 4) were determined by single-crystal X-ray diffraction; all structures presented monoclinic (P21/c) symmetry. The phenyl group is distant from the halogen atom to minimize the steric hindrance repulsion for all structures. Moreover, the structures of 3 and 4 show an almost linear alignment of halogen–selenium–carbon atoms arising from the intramolecular orbital interaction between a lone pair of electrons on the halogen atom and the antibonding σ*Se–C orbital (n halogen→σ*Se–C). This interaction leads to significant differences in the three-dimensional packing of the molecules, which are assembled through π–π and C—H⋯π interactions. These data provide a better comprehension of the intermolecular packing in benzo[b]chalcogenophenes, which is relevant for optoelectronic applications.
Chemical context
Chalcogenophenes derivatives are an attractive synthetic class of compounds with a wide range of relevant applications in medicinal chemistry (Keri et al., 2017 ▸; Mahmoud et al., 2017 ▸; Paegle et al., 2016 ▸), electrochemistry (Wei et al., 2017 ▸; Shahjad et al., 2017 ▸), agrochemistry (Zani et al., 2004 ▸) and as organic semiconductors (Yang et al., 2018 ▸; Ostroverkhova, 2016 ▸). π-extended benzo[b]chalcogenophenes derivatives have been widely studied as improved materials for optoelectronic devices such as organic photovoltaic cells (OPVs) (Ashraf et al., 2015 ▸; An et al., 2018 ▸; Chen et al., 2017 ▸), liquid-crystal displays (LCD) (Ghosh & Lehmann, 2017 ▸; Mei et al., 2013 ▸), organic light-emitting diodes (OLEDs) (Grimsdale et al., 2009 ▸; Zampetti et al., 2017 ▸; Arsenyan et al., 2019 ▸), and in organic field-effect transistors (OFETs) (Lee et al., 2019 ▸; Tisovský et al., 2019 ▸). Benzo[b]chalcogenophenes derivatives also show relevant biological activities as anti-tumor (Arsenyan et al., 2011 ▸) and anti-inflammatory agents (Shah et al., 2018 ▸). As part of our continuing work on benzo[b]chalcogenophenes (Luz et al., 2021 ▸), we report herein the crystallographic structural comparison of four 3-halo-2-(organochalcogenyl)benzo[b]chalcogenophene derivatives.
Structural commentary
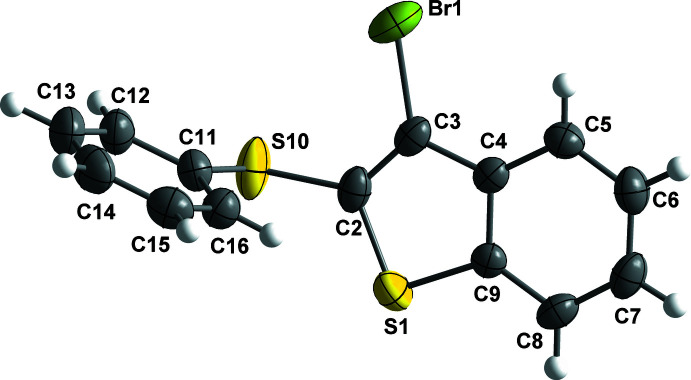
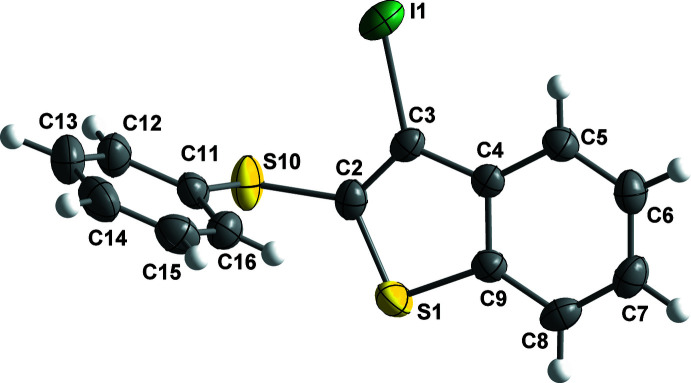
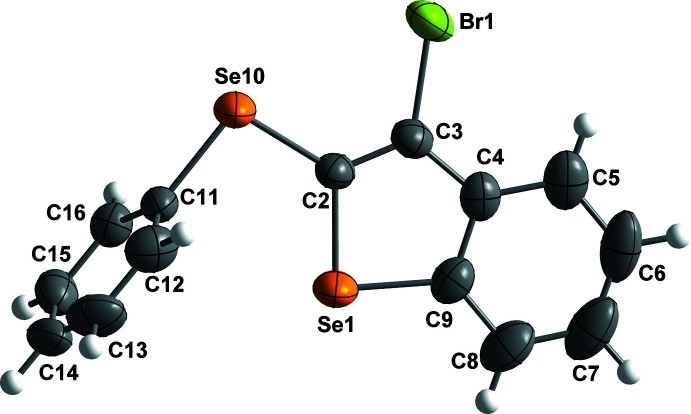
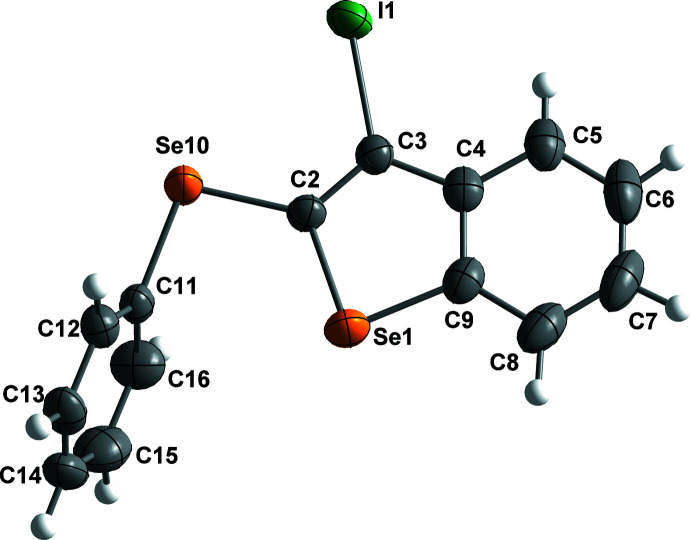
The four organic compounds crystallize in the monoclinic P21/c space group, and all atoms occupy unique positions. Compounds 1 and 2 are isostructural containing an identical 3-halo-2-(phenysulfanyl)benzo[b]thiophene unit with bromine (1) or iodine (2) at the C3 position of the benzo[b]thiophene ring (Figs. 1 ▸ and 2 ▸). The isostructural compounds 3 and 4 also contain identical 3-halo-2-(phenylselanyl)benzo[b]selenophene units with bromine (3) or iodine (4) at the C3 position of the benzo[b]selenophene ring (Figs. 3 ▸ and 4 ▸). The respective benzo[b]chalcogenophene rings and the phenylsulfanyl and phenyselanyl groups are planar. As expected, the carbon–selenium bonds in molecules 3 and 4 are longer than the respective carbon–sulfur bonds in molecules 1 and 2.
Figure 1.
The molecular structure of 3-bromo-2-(phenylsulfanyl)benzo[b]thiophene (1), with displacement ellipsoids drawn at the 50% probability level.
Figure 2.
The molecular structure of 3-iodo-2-(phenylsulfanyl)benzo[b]thiophene (2), with displacement ellipsoids drawn at the 50% probability level.
Figure 3.
The molecular structure of 3-bromo-2-(phenylselanyl)benzo[b]selenophene (3), with displacement ellipsoids drawn at the 50% probability level.
Figure 4.
The molecular structure of 3-iodo-2-(phenylselanyl)benzo[b]selenophene (4), with displacement ellipsoids drawn at the 50% probability level.
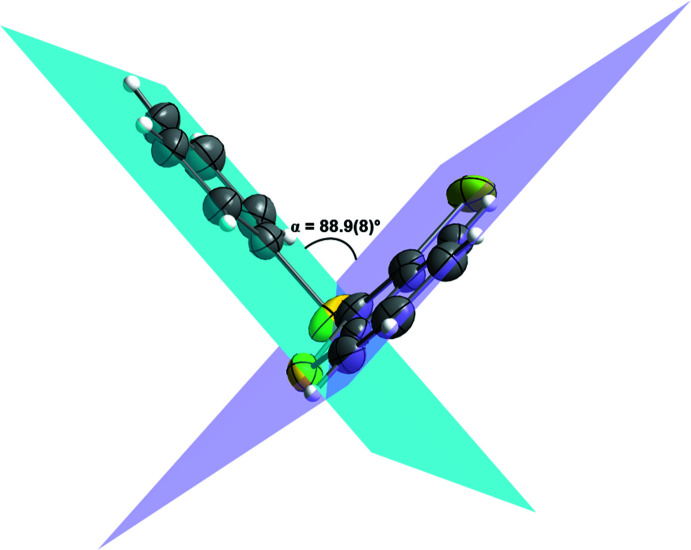
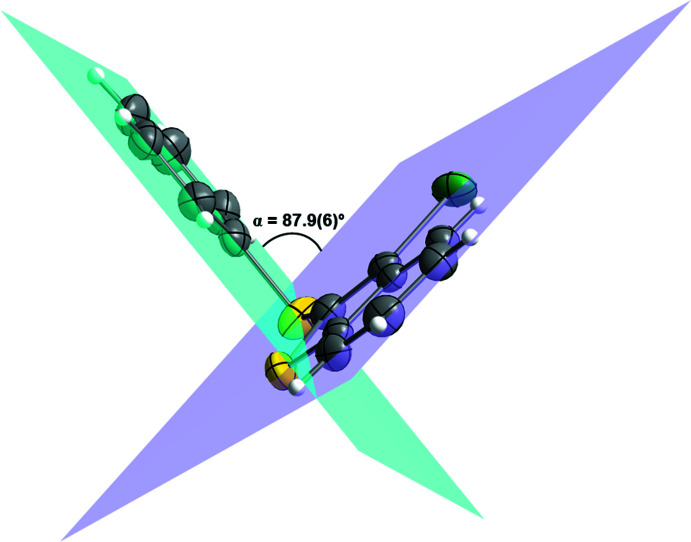
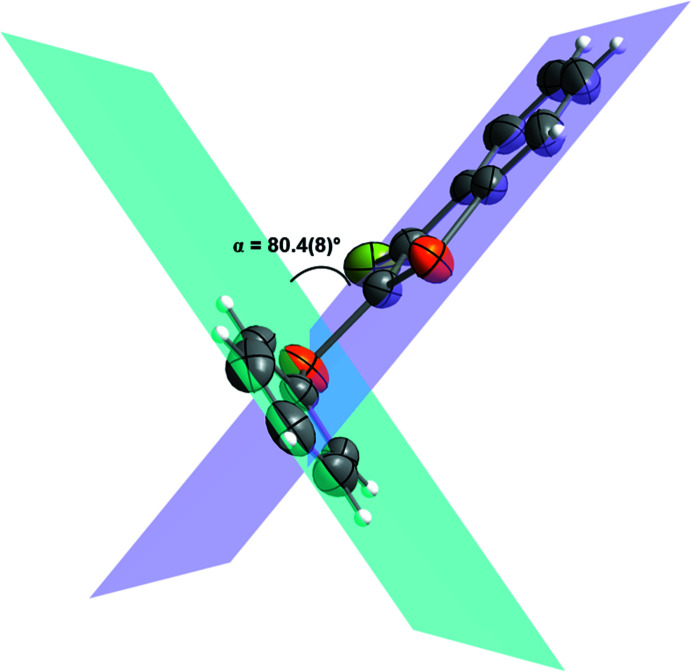
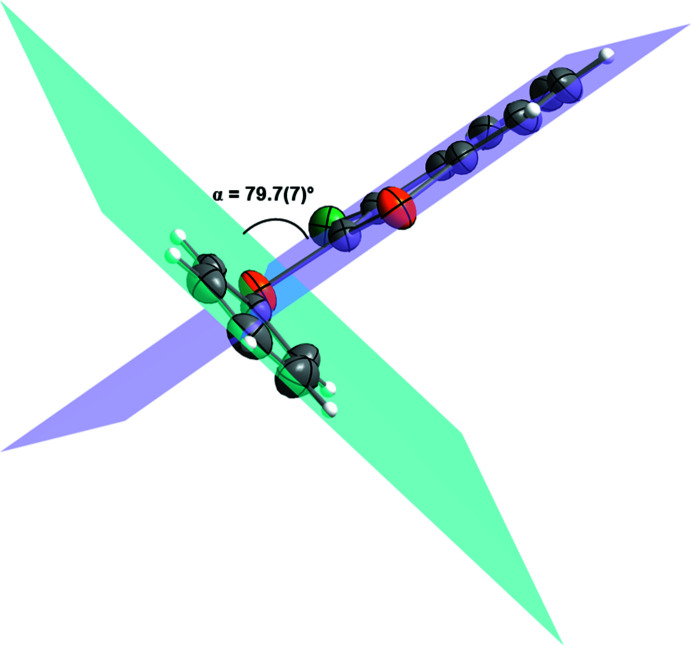
Conformational changes are observed when we compare molecules 1 and 2 containing sulfur atoms with molecules 3 and 4 containing selenium atoms, as described below. In molecules 1 and 2, the benzo[b]thiophene ring is twisted away from the plane of phenylsulfanyl group showing interplanar angles of 88.9 (8) and 87.9 (6)°, respectively (Figs. 5 ▸ and 6 ▸). Additionally, for 1 and 2 the S1—C2—S10—C11 torsion angles are −97.56 (14) and 98.17 (15)°, respectively. Molecules 3 and 4 also show the benzo[b]selenophene ring twisted away from the plane of the phenylselanyl group with interplanar angles of 80.4 (8) and 79.7 (7)°, respectively (Figs. 7 ▸ and 8 ▸). Conversely, the torsion angles (Se1—C2—Se10—C11) in molecules 3 and 4 are 1.9 (3) and −4.0 (3)°, respectively, quite different than the S1—C2—S10—C11 torsion angles in molecules 1 and 2. It is clear that the coplanarity between the phenyl and benzo[b]chalcogenophene rings is avoided in both pairs of molecules to minimize steric hindrance. This structural arrangement is reinforced by the presence of the halogen atom at the C3 position of the benzo[b]chalcogenophene ring (Figs. 1 ▸, 2 ▸, 3 ▸ and 4 ▸). Nevertheless, there is an almost linear alignment between the atoms Br1—Se10—C11 (3) and I1—Se10—C11 (4), which cannot be explained by steric factors alone. For instance, if we consider merely the higher steric hindrance between the phenyl and benzo[b]selenophene rings arising from the lower intrinsic C11—Se10–C2 angle directing the conformation of molecules 3 and 4, the almost linear alignment between the atoms Br1—Se10—C11 (3) and I1—Se10—C11 (4) is still not fully understood. We have observed that the interatomic distances between the chalcogen and the halogen atoms [S10⋯Br1 (1) = 3.5061 (8) Å, S10⋯I1 (2) = 3.6310 (7) Å, Se10⋯Br (3) = 3.4196 (7) Å, Se10⋯-I (4) = 3.5260 (7) Å] are 0.14, 0.15, 0.33 and 0.35 Å shorter than the sum of the van der Waals radii of the respective two atoms in molecules 1, 2, 3, and 4, respectively (Bondi, 1964 ▸). The shorter interatomic distances Se10⋯Br and Se10⋯I and the remarkably almost linear alignment of the atoms in 3 [C11—Se10⋯Br1 = 152.95 (9)°] and in 4 [C11—Se10⋯I1 = 156.52 (1)°] when compared to molecules 1 [C11—S10⋯Br1 = 93.01 (7)°] and 2 [C11—S10⋯I1 = 91.35 (7)°] indicate a stabilizing intramolecular orbital interaction (3-center-4-electrons, 3c–4e) between a lone pair of electrons of the halogen atom and the antibonding σ*Se–C11 orbital (n halogen→σ*Se–C11) (Mukherjee, 2010 ▸). The lower energy of the antibonding σ*Se–C11 orbital makes it a better acceptor when compared to the higher energy antibonding σ*S–C11 orbital, therefore making the intramolecular n halogen→σ*Se–C11 orbital interaction in molecules 3 and 4 strong enough to change their molecular conformation.
Figure 5.
Representation of the interplanar angle (α) between the planes containing the phenylsulfanyl, blue plane, and the benzo[b]thiophene, purple plane, groups for 3-bromo-2-(phenylsulfanyl)benzo[b]thiophene (1). Displacement ellipsoids are drawn at the 50% probability level. Gray: carbon; yellow: sulfur; light green: bromine; white: hydrogen.
Figure 6.
Representation of the interplanar angle (α) between the planes containing the phenylsulfanyl, blue plane, and the benzo[b]thiophene, purple plane, groups for 3-iodo-2-(phenylsulfanyl)benzo[b]thiophene (2). Displacement ellipsoids are drawn at the 50% probability level. Gray: carbon; yellow: sulfur; bluish green: iodine; white: hydrogen.
Figure 7.
Representation of the interplanar angle (α) between the planes containing the phenylselanyl, blue plane, and the benzo[b]selenophene, purple plane, groups for 3-bromo-2-(phenylselanyl)benzo[b]selenophene (3). Displacement ellipsoids are drawn at the 50% probability level. Gray: carbon; orange: selenium; light green: bromine; white: hydrogen.
Figure 8.
Representation of the interplanar angle (α) between the planes containing the phenylselanyl, blue plane, and the benzo[b]selenophene, purple plane, groups for 3-iodo-2-(phenylselanyl)benzo[b]selenophene (4). Displacement ellipsoids are drawn at the 50% probability level. Gray: carbon; orange: selenium; bluish green: iodine; white: hydrogen.
Supramolecular features
The crystals of organic compounds 1 and 2 are related by an inversion center and assembled through C—H⋯π intermolecular interactions along the b-axis direction (Fig. 9 ▸). The weak C—H⋯π interactions are between the H5 atom and the centroid formed by atoms C11–C16 of the phenylsulfanyl group. The distances and angles comprising these contacts are 2.97 (2) Å, 137.1 (2)° for 1 and 2.93 (3) Å, 138.4 (2)° for 2. The structures 1 and 2 also show π–π stacking interactions between adjacent benzo[b]thiophene rings along the c-axis direction with centroid–centroid distances of 3.7166 (2) and 3.7602 (4) Å for 1 and 2, respectively (Fig. 9 ▸, for 1). On the other hand, in compounds 3 and 4 C—H⋯π interactions are not present. However, π–π stacking interactions involving adjacent benzo[b]thiophene rings are present along the a-axis direction, with centroid–centroid distances of 3.8139 (3) Å and 3.8772 (1) Å, respectively. Furthermore, π–π stacking interactions are observed along the b-axis direction between phenylsulfanyl groups related by an inversion center, with centroid–centroid distances of 3.6644 (2) and 3.7351 (1) Å for 3 and 4, respectively (Fig. 10 ▸, for 3).
Figure 9.
Representation of some molecules of 3-bromo-2-(phenylsulfanyl)benzo[b]selenophene (1) viewed approximately down the c axis of the unit cell. The red dashed lines represent C—H⋯π interactions involving the H5 atom of the benzo[b]thiopehene ring with an adjacent phenylsulfanyl group; the purple dashed lines represent π–π stacking interactions between adjacent benzo[b]thiopehene rings. Displacement ellipsoids are drawn at the 50% probability level. The hydrogen atoms, except for H4, are omitted for clarity. Red and purple spheres represent the centroids of the respective organic groups.
Figure 10.
Representation of the molecules of 3-bromo-2-(phenylselanyl)benzo[b]selenophene (3) viewed down the c axis of the unit cell. The purple and yellow dashed lines represent π–π stacking interactions between adjacent benzo[b]thiopehene rings and between adjacent phenylsulfanyl groups, respectively. Displacement ellipsoids are drawn at the 50% probability level. The hydrogen atoms were omitted to clarity. Purple and yellow spheres represent the centroids of the respective organic groups.
Database survey
Several crystal structures of benzo[b]chalcogenophenes derivatives have been published. To the best of our knowledge, there are no studies about chalcogen atoms attached directly at position 2 of the benzo[b]chalcogenophene ring. With regard to benzo[b]thiophenes, Xu et al. (2017 ▸) described the structure of 3-(arylsulfonyl)benzo[b]thiophene obtained by single-crystal X-ray diffraction. Additionally, Ramesh et al. (2016 ▸) reported the structures of 6-fluoro-2,2-(diphenyl)benzo[b]thiophene and 6-isopropyl-2,2-(diphenyl)benzo[b]thiophene obtained by single-crystal X-ray diffraction studies.
Synthesis and crystallization
The structures reported here were obtained by the one-pot synthesis of 3-halo-2-organochalcogenylbenzo[b]chalcogenophenes from 1-(2,2-dibromovinyl)-2-organochalcogenylbenzenes. By this method, a series of 2,3-disubstituted benzo[b]chalcogenophenes were prepared in yields of ca 80% (Luz et al., 2021 ▸). The title compounds were prepared as follows:
3-Bromo-2-(phenylsulfanyl)benzo[ b ]thiophene (1)
To a Schlenk tube containing 1-(2,2-dibromovinyl)-2-butylsulfanylbenzene (0.25 mmol, 1.0 equiv.), diphenyl disulfide (0.125 mmol, 1.0 equiv.) was added in dry dimethyl sulfoxide (2.0 mL) followed by the addition of cesium carbonate (0.244 g, 0.75 mmol, 3.0 equiv.). The reaction system was heated to 383 K and stirred for 1.5 h. Then, the reaction mixture was cooled to room temperature and 2.5 equivalents of NBS (N-bromosuccinimide) in 2 mL of dichloromethane were slowly added (2.0 min) into the system. The reaction mixture was stirred at room temperature for 2 h. After this, the reaction solution was diluted in saturated thiosulfate solution (20 mL) and washed with ethyl acetate (3 × 10 mL). The organic phase was dried over magnesium sulfate and concentrated under reduced pressure. The product was further purified by flash chromatography using hexane as eluent. Colorless needle-shaped single crystals of 1 suitable for X-ray analysis were grown by slow evaporation of a concentrated ethyl acetate solution over several days at room temperature. Yield: 0.066 g (82%); withe solid, m.p. 337–340 K. 1H NMR (CDCl3, 400 MHz) δ (ppm) = 7.77–7.75 (m, 1 H); 7.70–7.68 (m, 1H); 7.58–7.55 (m, 2H); 7.44–7.40 (m, 1H); 7.36–7.30 (m, 4H). 13C{1H} NMR (CDCl3, 100 MHz) δ (ppm) = 141.1, 138.5, 135.9, 133.1, 129.5, 128.3, 126.4, 125.4, 125.2, 123.3, 121.9, 114.4. MS (Rel. Int.) m/z: 321 (84.0), 241 (100), 210 (63.4), 77 (54.8) HRMS: Calculated mass for C14H10BrS2 [M]+: 321.9302, found: 321.9310.
3-Iodo-2-(phenylsulfanyl)benzo[ b ]thiophene (2)
The first step for obtaining 2 was analogous to that described for 1. The reaction mixture was cooled to room temperature and 1.5 equivalents of I2 in 2 mL of dichloromethane were slowly added (2.0 min) into the system. The reaction mixture was stirred at room temperature for 3.5 h. After this, the reaction solution was diluted in saturated sodium thiosulfate solution (20 mL) and washed with ethyl acetate (3 × 10 mL). The organic phase was dried over magnesium sulfate and concentrated under reduced pressure. The product was further purified by flash chromatography using hexane as eluent. Colorless needle-shaped single crystals of 2 were obtained in the same way of 1. Yield: 0.073 g (79%); yellow solid, m.p. 325–327K. 1H NMR (CDCl3, 400 MHz) δ (ppm) = 7.72 (d, J = 8.0 Hz, 1 H); 7.66 (d, J = 7.6 Hz, 1 H); 7.44–7.40 (m, 2H); 7.34–7.21 (m, 5H). 13C{1H} NMR (CDCl3, 100 MHz) δ (ppm) = 141.5, 141.2, 136.5, 134.7, 130.3, 129.3, 127.7, 126.2, 126.0, 125.5, 122.1, 90.3. MS (Rel. Int.) m/z: 368 (94.3), 240 (100), 120 (50.3), 77 (10.5). HRMS: Calculated mass for C14H9IS2 [M]+: 367.9185, found: 367.9188.
3-Bromo-2-(phenylselnyl)benzo[ b ]selenophene (3)
To a Schlenk tube containing 1-(2,2-dibromovinyl)-2-butylselanylbenzene (0.25 mmol, 1.0 equiv.), diphenyl diselenide (0.125 mmol, 1.0 equiv.) was added in dry dimethyl sulfoxide (2.0 mL) followed by the addition of cesium carbonate (0.244 g, 0.75 mmol, 3.0 equiv.). The reaction system was heated to 384 K and stirred for 0.5 h. Then, the reaction mixture was cooled to room temperature and 2.5 equivalents of NBS (N-bromosuccinimide) in 2 mL of dichloromethane were slowly added (2.0 min) into the system. The reaction mixture was stirred at room temperature for 1 h. After this, the reaction solution was diluted in saturated sodium thiosulfate solution (20 mL) and washed with ethyl acetate (3 × 10 mL). The organic phase was dried over magnesium sulfate and concentrated under reduced pressure. The product were further purified by flash chromatography using hexane as eluent. Colorless needle-shaped single crystals of 3 were obtained in the same way as 1. Yield: 0.081 g (79%); yellow oil. 1H NMR (CDCl3, 400 MHz) δ (ppm) = 7.76 (dd, J = 8.1 and 1.0 Hz, 1H); 7.69–7.66 (m, 3H); 7.41–7.32 (m, 4H); 7.24 (ddd, J = 8.2, 7.3 and 1.3 Hz, 1H). 13C{1H} NMR (CDCl3, 100 MHz) δ (ppm) = 141.1, 140.6, 134.4, 129.6, 129.5, 129.4, 129.0, 125.4, 125.1, 125.0, 124.9, 112.9. MS (Rel. Int.) m/z: 416 (96.8), 336 (100), 256 (42.4), 77 (62.0).
3-Iodo-2-(phenylselanyl)benzo[ b ]selenophene (4)
The first step for obtaining 4 was similar to that described for 3. The reaction mixture was cooled to room temperature and 1.5 equivalents of I2 in 2 mL of dichloromethane were slowly added (2.0 min) into the system. The reaction was stirred at room temperature for 1 h. After this, the reaction solution was diluted in saturated sodium thiosulfate solution (20 mL) and washed with ethyl acetate (3 × 10 mL). The organic phase was dried over magnesium sulfate and concentrated under reduced pressure. The product was further purified by flash chromatography using hexane as eluent. Colorless needle single crystals of 4 were obtained in the same way of (1). Yield: 0.090 g (78%); Orange solid, m.p. 329–331 K. 1H NMR (CDCl3, 400 MHz) δ (ppm) = 7.73–7.64 (m, 4H); 7.41–7.33 (m, 4H); 7.23–7.20 (m, 1H). 13C{1H} NMR (CDCl3, 100 MHz) δ (ppm) = 143.9, 142.2, 134.9, 134.7, 129.8, 129.7, 129.1, 127.6, 125.7, 125.1, 88.9. MS (Rel. Int.) m/z: 464 (48.2), 334 (47.0), 256 (51.4), 77 (53.2), 51 (100).
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 1 ▸. Hydrogen atoms of 1, 2 and 4 were located in difference-Fourier maps and were refined freely; the hydrogen atoms of 3 were included in idealized positions with aromatic C—H distances set to 0.93 Å and refined using a riding model U iso(H) = 1.2U eq(C).
Table 1. Experimental details.
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Crystal data | ||||
| Chemical formula | C14H9BrS2 | C14H9IS2 | C14H9BrSe2 | C14H9ISe2 |
| M r | 321.24 | 368.23 | 415.04 | 462.03 |
| Crystal system, space group | Monoclinic, P21/c | Monoclinic, P21/c | Monoclinic, P21/c | Monoclinic, P21/c |
| Temperature (K) | 296 | 294 | 297 | 292 |
| a, b, c (Å) | 8.2471 (8), 9.9562 (8), 15.7601 (14) | 8.4872 (3), 9.9629 (4), 15.6485 (7) | 12.3864 (11), 13.6816 (11), 8.0982 (6) | 12.9606 (6), 13.5999 (7), 8.0448 (4) |
| β (°) | 98.967 (3) | 97.052 (1) | 96.398 (3) | 95.585 (2) |
| V (Å3) | 1278.2 (2) | 1313.18 (9) | 1363.82 (19) | 1411.27 (12) |
| Z | 4 | 4 | 4 | 4 |
| Radiation type | Mo Kα | Mo Kα | Mo Kα | Mo Kα |
| μ (mm−1) | 3.51 | 2.73 | 8.33 | 7.40 |
| Crystal size (mm) | 0.28 × 0.21 × 0.14 | 0.16 × 0.13 × 0.05 | 0.17 × 0.17 × 0.12 | 0.51 × 0.47 × 0.24 |
| Data collection | ||||
| Diffractometer | Bruker D8 Venture/Photon 100 CMOS | Bruker D8 Venture/Photon 100 CMOS | Bruker D8 Venture/Photon 100 CMOS | Bruker D8 Venture/Photon 100 CMOS |
| Absorption correction | Multi-scan (SADABS; Krause et al., 2015 ▸) | Multi-scan (SADABS; Krause et al., 2015 ▸) | Multi-scan (SADABS; Krause et al., 2015 ▸) | Multi-scan (SADABS; Krause et al., 2015 ▸) |
| T min, T max | 0.628, 0.746 | 0.690, 0.746 | 0.543, 0.746 | 0.390, 0.746 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 55020, 3078, 2559 | 56046, 3001, 2585 | 43875, 2976, 2208 | 54241, 3391, 2702 |
| R int | 0.041 | 0.045 | 0.060 | 0.054 |
| (sin θ/λ)max (Å−1) | 0.660 | 0.650 | 0.639 | 0.660 |
| Refinement | ||||
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.029, 0.073, 1.04 | 0.022, 0.051, 1.08 | 0.035, 0.069, 1.03 | 0.037, 0.083, 1.04 |
| No. of reflections | 3078 | 3001 | 2976 | 3391 |
| No. of parameters | 190 | 190 | 154 | 190 |
| H-atom treatment | All H-atom parameters refined | All H-atom parameters refined | H-atom parameters constrained | All H-atom parameters refined |
| Δρmax, Δρmin (e Å−3) | 0.41, −0.76 | 0.45, −0.86 | 0.93, −0.90 | 1.14, −1.82 |
Supplementary Material
Crystal structure: contains datablock(s) 1, 2, 3, 4, shelx. DOI: 10.1107/S2056989022000962/jy2015sup1.cif
Structure factors: contains datablock(s) 1. DOI: 10.1107/S2056989022000962/jy20151sup2.hkl
Supporting information file. DOI: 10.1107/S2056989022000962/jy20151sup6.cml
Structure factors: contains datablock(s) 2. DOI: 10.1107/S2056989022000962/jy20152sup3.hkl
Supporting information file. DOI: 10.1107/S2056989022000962/jy20152sup7.cml
Structure factors: contains datablock(s) 3. DOI: 10.1107/S2056989022000962/jy20153sup4.hkl
Supporting information file. DOI: 10.1107/S2056989022000962/jy20153sup8.cml
Structure factors: contains datablock(s) 4. DOI: 10.1107/S2056989022000962/jy20154sup5.hkl
Supporting information file. DOI: 10.1107/S2056989022000962/jy20154sup9.cml
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
ELQ and DSR thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for fellowships.
supplementary crystallographic information
3-Bromo-2-(phenylsulfanyl)benzo[b]thiophene (1) . Crystal data
| C14H9BrS2 | F(000) = 640 |
| Mr = 321.24 | Dx = 1.669 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| a = 8.2471 (8) Å | Cell parameters from 9946 reflections |
| b = 9.9562 (8) Å | θ = 2.6–28.0° |
| c = 15.7601 (14) Å | µ = 3.51 mm−1 |
| β = 98.967 (3)° | T = 296 K |
| V = 1278.2 (2) Å3 | Parallelepiped, colourless |
| Z = 4 | 0.28 × 0.21 × 0.14 mm |
3-Bromo-2-(phenylsulfanyl)benzo[b]thiophene (1) . Data collection
| Bruker D8 Venture/Photon 100 CMOS diffractometer | 3078 independent reflections |
| Radiation source: fine-focus sealed tube | 2559 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.041 |
| Detector resolution: 10.4167 pixels mm-1 | θmax = 28.0°, θmin = 3.2° |
| φ and ω scans | h = −10→10 |
| Absorption correction: multi-scan (SADABS; Krause et al., 2015) | k = −13→13 |
| Tmin = 0.628, Tmax = 0.746 | l = −20→20 |
| 55020 measured reflections |
3-Bromo-2-(phenylsulfanyl)benzo[b]thiophene (1) . Refinement
| Refinement on F2 | Primary atom site location: dual |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.029 | Hydrogen site location: difference Fourier map |
| wR(F2) = 0.073 | All H-atom parameters refined |
| S = 1.04 | w = 1/[σ2(Fo2) + (0.0347P)2 + 0.7059P] where P = (Fo2 + 2Fc2)/3 |
| 3078 reflections | (Δ/σ)max < 0.001 |
| 190 parameters | Δρmax = 0.41 e Å−3 |
| 0 restraints | Δρmin = −0.76 e Å−3 |
3-Bromo-2-(phenylsulfanyl)benzo[b]thiophene (1) . Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
3-Bromo-2-(phenylsulfanyl)benzo[b]thiophene (1) . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C2 | 0.1651 (3) | 0.3373 (2) | 0.41693 (14) | 0.0405 (5) | |
| C3 | 0.2308 (2) | 0.4295 (2) | 0.47529 (13) | 0.0361 (4) | |
| C4 | 0.1731 (2) | 0.56405 (19) | 0.45645 (12) | 0.0315 (4) | |
| C5 | 0.2140 (3) | 0.6832 (2) | 0.50152 (14) | 0.0399 (4) | |
| C6 | 0.1421 (3) | 0.8012 (2) | 0.47034 (16) | 0.0460 (5) | |
| C7 | 0.0310 (3) | 0.8041 (2) | 0.39437 (16) | 0.0468 (5) | |
| C8 | −0.0120 (3) | 0.6888 (2) | 0.34898 (14) | 0.0426 (5) | |
| C9 | 0.0599 (2) | 0.56820 (19) | 0.38079 (12) | 0.0325 (4) | |
| C11 | 0.3783 (3) | 0.1481 (2) | 0.36842 (14) | 0.0392 (4) | |
| C12 | 0.4614 (3) | 0.0261 (2) | 0.38205 (16) | 0.0487 (5) | |
| C13 | 0.6017 (3) | 0.0046 (3) | 0.34629 (17) | 0.0562 (6) | |
| C14 | 0.6591 (3) | 0.1024 (3) | 0.29691 (18) | 0.0578 (6) | |
| C15 | 0.5762 (3) | 0.2225 (3) | 0.28289 (16) | 0.0512 (6) | |
| C16 | 0.4356 (3) | 0.2460 (2) | 0.31852 (14) | 0.0435 (5) | |
| Br1 | 0.38366 (3) | 0.38960 (3) | 0.57285 (2) | 0.05735 (10) | |
| S1 | 0.02548 (7) | 0.41026 (6) | 0.33534 (4) | 0.04409 (13) | |
| S10 | 0.19752 (8) | 0.16429 (6) | 0.41634 (5) | 0.05842 (19) | |
| H5 | 0.289 (3) | 0.680 (2) | 0.5496 (15) | 0.042 (6)* | |
| H6 | 0.172 (3) | 0.880 (3) | 0.4980 (17) | 0.053 (7)* | |
| H7 | −0.009 (4) | 0.887 (3) | 0.3766 (18) | 0.060 (8)* | |
| H8 | −0.089 (3) | 0.691 (3) | 0.2997 (17) | 0.055 (7)* | |
| H12 | 0.429 (3) | −0.039 (3) | 0.4186 (17) | 0.056 (7)* | |
| H13 | 0.660 (4) | −0.081 (3) | 0.3556 (18) | 0.065 (8)* | |
| H14 | 0.751 (4) | 0.092 (3) | 0.278 (2) | 0.073 (9)* | |
| H15 | 0.613 (3) | 0.286 (3) | 0.2530 (18) | 0.058 (8)* | |
| H16 | 0.379 (3) | 0.325 (3) | 0.3084 (17) | 0.058 (8)* |
3-Bromo-2-(phenylsulfanyl)benzo[b]thiophene (1) . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C2 | 0.0426 (11) | 0.0318 (9) | 0.0515 (12) | 0.0038 (8) | 0.0207 (9) | 0.0049 (9) |
| C3 | 0.0300 (9) | 0.0371 (10) | 0.0432 (10) | 0.0039 (7) | 0.0116 (8) | 0.0087 (8) |
| C4 | 0.0281 (8) | 0.0343 (9) | 0.0337 (9) | 0.0004 (7) | 0.0100 (7) | 0.0047 (7) |
| C5 | 0.0368 (10) | 0.0437 (11) | 0.0389 (11) | −0.0035 (8) | 0.0049 (8) | −0.0012 (9) |
| C6 | 0.0514 (13) | 0.0329 (10) | 0.0562 (13) | −0.0040 (9) | 0.0160 (10) | −0.0046 (10) |
| C7 | 0.0511 (13) | 0.0332 (10) | 0.0583 (14) | 0.0064 (9) | 0.0157 (10) | 0.0122 (10) |
| C8 | 0.0433 (11) | 0.0431 (11) | 0.0405 (11) | 0.0038 (9) | 0.0038 (9) | 0.0130 (9) |
| C9 | 0.0339 (9) | 0.0331 (9) | 0.0321 (9) | −0.0002 (7) | 0.0097 (7) | 0.0025 (7) |
| C11 | 0.0416 (11) | 0.0342 (10) | 0.0429 (11) | −0.0005 (8) | 0.0103 (8) | −0.0054 (8) |
| C12 | 0.0587 (14) | 0.0356 (11) | 0.0536 (13) | 0.0060 (10) | 0.0147 (11) | −0.0019 (10) |
| C13 | 0.0590 (15) | 0.0477 (13) | 0.0632 (15) | 0.0141 (12) | 0.0134 (12) | −0.0077 (12) |
| C14 | 0.0489 (14) | 0.0706 (17) | 0.0573 (15) | 0.0079 (12) | 0.0186 (11) | −0.0125 (13) |
| C15 | 0.0492 (13) | 0.0611 (15) | 0.0452 (12) | −0.0055 (11) | 0.0135 (10) | 0.0010 (11) |
| C16 | 0.0439 (11) | 0.0415 (11) | 0.0455 (12) | 0.0020 (9) | 0.0083 (9) | 0.0024 (9) |
| Br1 | 0.03881 (13) | 0.06510 (17) | 0.06559 (17) | 0.00695 (10) | 0.00017 (10) | 0.02733 (12) |
| S1 | 0.0519 (3) | 0.0402 (3) | 0.0404 (3) | −0.0055 (2) | 0.0080 (2) | −0.0061 (2) |
| S10 | 0.0637 (4) | 0.0289 (3) | 0.0928 (5) | 0.0022 (2) | 0.0440 (4) | 0.0058 (3) |
3-Bromo-2-(phenylsulfanyl)benzo[b]thiophene (1) . Geometric parameters (Å, º)
| C2—C3 | 1.352 (3) | C8—H8 | 0.92 (3) |
| C2—S10 | 1.744 (2) | C9—S1 | 1.733 (2) |
| C2—S1 | 1.745 (2) | C11—C16 | 1.381 (3) |
| C3—C4 | 1.437 (3) | C11—C12 | 1.395 (3) |
| C3—Br1 | 1.873 (2) | C11—S10 | 1.781 (2) |
| C4—C9 | 1.396 (3) | C12—C13 | 1.380 (3) |
| C4—C5 | 1.397 (3) | C12—H12 | 0.93 (3) |
| C5—C6 | 1.373 (3) | C13—C14 | 1.376 (4) |
| C5—H5 | 0.90 (2) | C13—H13 | 0.98 (3) |
| C6—C7 | 1.390 (4) | C14—C15 | 1.377 (4) |
| C6—H6 | 0.91 (3) | C14—H14 | 0.87 (3) |
| C7—C8 | 1.369 (3) | C15—C16 | 1.385 (3) |
| C7—H7 | 0.92 (3) | C15—H15 | 0.87 (3) |
| C8—C9 | 1.397 (3) | C16—H16 | 0.91 (3) |
| C3—C2—S10 | 128.98 (18) | C4—C9—S1 | 111.78 (14) |
| C3—C2—S1 | 111.54 (15) | C8—C9—S1 | 126.76 (16) |
| S10—C2—S1 | 119.44 (14) | C16—C11—C12 | 119.9 (2) |
| C2—C3—C4 | 114.06 (18) | C16—C11—S10 | 124.14 (17) |
| C2—C3—Br1 | 124.17 (16) | C12—C11—S10 | 115.92 (17) |
| C4—C3—Br1 | 121.76 (15) | C13—C12—C11 | 119.7 (2) |
| C9—C4—C5 | 119.07 (18) | C13—C12—H12 | 118.7 (16) |
| C9—C4—C3 | 111.10 (17) | C11—C12—H12 | 121.4 (17) |
| C5—C4—C3 | 129.82 (18) | C14—C13—C12 | 120.3 (2) |
| C6—C5—C4 | 119.2 (2) | C14—C13—H13 | 120.0 (17) |
| C6—C5—H5 | 122.1 (16) | C12—C13—H13 | 119.7 (17) |
| C4—C5—H5 | 118.6 (16) | C13—C14—C15 | 119.9 (2) |
| C5—C6—C7 | 121.1 (2) | C13—C14—H14 | 121 (2) |
| C5—C6—H6 | 119.9 (17) | C15—C14—H14 | 119 (2) |
| C7—C6—H6 | 118.9 (17) | C14—C15—C16 | 120.5 (2) |
| C8—C7—C6 | 121.0 (2) | C14—C15—H15 | 120.8 (18) |
| C8—C7—H7 | 123.0 (18) | C16—C15—H15 | 118.7 (18) |
| C6—C7—H7 | 116.0 (18) | C11—C16—C15 | 119.6 (2) |
| C7—C8—C9 | 118.2 (2) | C11—C16—H16 | 119.7 (18) |
| C7—C8—H8 | 120.6 (18) | C15—C16—H16 | 120.7 (18) |
| C9—C8—H8 | 121.2 (18) | C9—S1—C2 | 91.51 (10) |
| C4—C9—C8 | 121.46 (19) | C2—S10—C11 | 103.35 (10) |
| S10—C2—C3—C4 | −178.52 (15) | C7—C8—C9—S1 | 179.94 (17) |
| S1—C2—C3—C4 | −0.7 (2) | C16—C11—C12—C13 | 0.7 (4) |
| S10—C2—C3—Br1 | 1.5 (3) | S10—C11—C12—C13 | 179.2 (2) |
| S1—C2—C3—Br1 | 179.28 (10) | C11—C12—C13—C14 | −0.4 (4) |
| C2—C3—C4—C9 | 0.2 (2) | C12—C13—C14—C15 | −0.1 (4) |
| Br1—C3—C4—C9 | −179.83 (13) | C13—C14—C15—C16 | 0.3 (4) |
| C2—C3—C4—C5 | −179.69 (19) | C12—C11—C16—C15 | −0.5 (3) |
| Br1—C3—C4—C5 | 0.3 (3) | S10—C11—C16—C15 | −178.85 (18) |
| C9—C4—C5—C6 | 0.0 (3) | C14—C15—C16—C11 | 0.0 (4) |
| C3—C4—C5—C6 | 179.8 (2) | C4—C9—S1—C2 | −0.72 (15) |
| C4—C5—C6—C7 | −0.7 (3) | C8—C9—S1—C2 | 179.07 (19) |
| C5—C6—C7—C8 | 1.0 (4) | C3—C2—S1—C9 | 0.82 (16) |
| C6—C7—C8—C9 | −0.5 (3) | S10—C2—S1—C9 | 178.86 (13) |
| C5—C4—C9—C8 | 0.5 (3) | C3—C2—S10—C11 | −84.8 (2) |
| C3—C4—C9—C8 | −179.35 (18) | S1—C2—S10—C11 | 97.56 (14) |
| C5—C4—C9—S1 | −179.67 (14) | C16—C11—S10—C2 | −19.9 (2) |
| C3—C4—C9—S1 | 0.5 (2) | C12—C11—S10—C2 | 161.70 (18) |
| C7—C8—C9—C4 | −0.3 (3) |
3-Iodo-2-(phenylsulfanyl)benzo[b]thiophene (2) . Crystal data
| C14H9IS2 | F(000) = 712 |
| Mr = 368.23 | Dx = 1.863 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| a = 8.4872 (3) Å | Cell parameters from 9841 reflections |
| b = 9.9629 (4) Å | θ = 2.6–27.5° |
| c = 15.6485 (7) Å | µ = 2.73 mm−1 |
| β = 97.052 (1)° | T = 294 K |
| V = 1313.18 (9) Å3 | Parallelepiped, colourless |
| Z = 4 | 0.16 × 0.13 × 0.05 mm |
3-Iodo-2-(phenylsulfanyl)benzo[b]thiophene (2) . Data collection
| Bruker D8 Venture/Photon 100 CMOS diffractometer | 3001 independent reflections |
| Radiation source: fine-focus sealed tube | 2585 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.045 |
| Detector resolution: 10.4167 pixels mm-1 | θmax = 27.5°, θmin = 2.6° |
| φ and ω scans | h = −11→11 |
| Absorption correction: multi-scan (SADABS; Krause et al., 2015) | k = −12→12 |
| Tmin = 0.690, Tmax = 0.746 | l = −20→20 |
| 56046 measured reflections |
3-Iodo-2-(phenylsulfanyl)benzo[b]thiophene (2) . Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.022 | Hydrogen site location: difference Fourier map |
| wR(F2) = 0.051 | All H-atom parameters refined |
| S = 1.08 | w = 1/[σ2(Fo2) + (0.0218P)2 + 0.8146P] where P = (Fo2 + 2Fc2)/3 |
| 3001 reflections | (Δ/σ)max = 0.001 |
| 190 parameters | Δρmax = 0.45 e Å−3 |
| 0 restraints | Δρmin = −0.85 e Å−3 |
3-Iodo-2-(phenylsulfanyl)benzo[b]thiophene (2) . Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
3-Iodo-2-(phenylsulfanyl)benzo[b]thiophene (2) . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C2 | 0.6667 (3) | 0.8425 (2) | −0.08322 (15) | 0.0350 (5) | |
| C3 | 0.7271 (2) | 0.9337 (2) | −0.02410 (14) | 0.0308 (4) | |
| C4 | 0.6724 (2) | 1.0681 (2) | −0.04289 (13) | 0.0279 (4) | |
| C5 | 0.7097 (3) | 1.1876 (2) | 0.00262 (15) | 0.0351 (5) | |
| C6 | 0.6420 (3) | 1.3057 (2) | −0.02866 (17) | 0.0415 (5) | |
| C7 | 0.5388 (3) | 1.3091 (2) | −0.10526 (17) | 0.0434 (6) | |
| C8 | 0.5000 (3) | 1.1942 (2) | −0.15099 (15) | 0.0397 (5) | |
| C9 | 0.5672 (2) | 1.0732 (2) | −0.11926 (13) | 0.0306 (4) | |
| C11 | 0.8796 (3) | 0.6562 (2) | −0.13223 (14) | 0.0359 (5) | |
| C12 | 0.9622 (3) | 0.5360 (2) | −0.11839 (17) | 0.0459 (6) | |
| C13 | 1.1021 (3) | 0.5172 (3) | −0.15346 (19) | 0.0547 (7) | |
| C14 | 1.1602 (3) | 0.6167 (3) | −0.20217 (19) | 0.0539 (7) | |
| C15 | 1.0784 (3) | 0.7353 (3) | −0.21568 (17) | 0.0466 (6) | |
| C16 | 0.9382 (3) | 0.7558 (2) | −0.18094 (16) | 0.0408 (5) | |
| S1 | 0.53633 (8) | 0.91592 (6) | −0.16492 (4) | 0.04052 (14) | |
| S10 | 0.69891 (8) | 0.66946 (6) | −0.08560 (5) | 0.04924 (16) | |
| I1 | 0.88517 (2) | 0.88685 (2) | 0.08375 (2) | 0.04613 (7) | |
| H5 | 0.779 (3) | 1.183 (3) | 0.0538 (17) | 0.047 (7)* | |
| H6 | 0.667 (3) | 1.387 (3) | −0.0011 (18) | 0.048 (8)* | |
| H7 | 0.502 (3) | 1.394 (3) | −0.1290 (19) | 0.059 (9)* | |
| H8 | 0.434 (3) | 1.196 (3) | −0.2021 (17) | 0.049 (7)* | |
| H12 | 0.924 (3) | 0.471 (3) | −0.0845 (17) | 0.049 (7)* | |
| H13 | 1.162 (3) | 0.435 (3) | −0.1465 (19) | 0.061 (8)* | |
| H14 | 1.251 (4) | 0.604 (3) | −0.229 (2) | 0.071 (10)* | |
| H15 | 1.116 (3) | 0.797 (3) | −0.2450 (17) | 0.043 (7)* | |
| H16 | 0.882 (3) | 0.829 (3) | −0.1901 (18) | 0.053 (8)* |
3-Iodo-2-(phenylsulfanyl)benzo[b]thiophene (2) . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C2 | 0.0372 (11) | 0.0290 (11) | 0.0406 (12) | 0.0014 (9) | 0.0124 (9) | 0.0018 (9) |
| C3 | 0.0288 (10) | 0.0313 (10) | 0.0331 (11) | 0.0020 (8) | 0.0070 (8) | 0.0060 (8) |
| C4 | 0.0265 (9) | 0.0296 (10) | 0.0286 (10) | −0.0001 (8) | 0.0083 (8) | 0.0024 (8) |
| C5 | 0.0355 (11) | 0.0351 (12) | 0.0347 (12) | −0.0020 (9) | 0.0036 (9) | −0.0015 (9) |
| C6 | 0.0468 (13) | 0.0287 (12) | 0.0501 (14) | −0.0016 (10) | 0.0104 (11) | −0.0024 (10) |
| C7 | 0.0490 (14) | 0.0316 (12) | 0.0501 (14) | 0.0057 (10) | 0.0089 (11) | 0.0110 (10) |
| C8 | 0.0425 (12) | 0.0415 (13) | 0.0345 (12) | 0.0017 (10) | 0.0020 (10) | 0.0118 (10) |
| C9 | 0.0330 (10) | 0.0313 (10) | 0.0281 (10) | −0.0021 (8) | 0.0066 (8) | 0.0019 (8) |
| C11 | 0.0447 (12) | 0.0290 (10) | 0.0344 (11) | −0.0015 (9) | 0.0058 (9) | −0.0068 (9) |
| C12 | 0.0600 (16) | 0.0310 (13) | 0.0482 (15) | 0.0038 (11) | 0.0125 (12) | −0.0003 (11) |
| C13 | 0.0622 (17) | 0.0407 (14) | 0.0622 (18) | 0.0121 (13) | 0.0111 (14) | −0.0098 (13) |
| C14 | 0.0503 (15) | 0.0624 (18) | 0.0507 (16) | 0.0050 (13) | 0.0131 (12) | −0.0176 (14) |
| C15 | 0.0530 (15) | 0.0495 (15) | 0.0386 (13) | −0.0092 (12) | 0.0100 (11) | −0.0004 (12) |
| C16 | 0.0479 (13) | 0.0340 (13) | 0.0406 (13) | 0.0002 (11) | 0.0052 (10) | 0.0022 (10) |
| S1 | 0.0494 (3) | 0.0374 (3) | 0.0340 (3) | −0.0051 (2) | 0.0024 (2) | −0.0054 (2) |
| S10 | 0.0561 (4) | 0.0257 (3) | 0.0708 (4) | −0.0024 (3) | 0.0276 (3) | 0.0005 (3) |
| I1 | 0.03649 (9) | 0.04740 (10) | 0.05267 (11) | 0.00385 (7) | −0.00193 (6) | 0.01649 (7) |
3-Iodo-2-(phenylsulfanyl)benzo[b]thiophene (2) . Geometric parameters (Å, º)
| C2—C3 | 1.353 (3) | C8—H8 | 0.92 (3) |
| C2—S1 | 1.745 (2) | C9—S1 | 1.728 (2) |
| C2—S10 | 1.746 (2) | C11—C16 | 1.381 (3) |
| C3—C4 | 1.436 (3) | C11—C12 | 1.391 (3) |
| C3—I1 | 2.076 (2) | C11—S10 | 1.782 (2) |
| C4—C9 | 1.402 (3) | C12—C13 | 1.380 (4) |
| C4—C5 | 1.403 (3) | C12—H12 | 0.92 (3) |
| C5—C6 | 1.374 (3) | C13—C14 | 1.378 (4) |
| C5—H5 | 0.93 (3) | C13—H13 | 0.96 (3) |
| C6—C7 | 1.395 (4) | C14—C15 | 1.374 (4) |
| C6—H6 | 0.93 (3) | C14—H14 | 0.93 (3) |
| C7—C8 | 1.369 (4) | C15—C16 | 1.383 (4) |
| C7—H7 | 0.96 (3) | C15—H15 | 0.85 (3) |
| C8—C9 | 1.399 (3) | C16—H16 | 0.87 (3) |
| C3—C2—S1 | 111.84 (17) | C8—C9—S1 | 126.88 (17) |
| C3—C2—S10 | 129.12 (18) | C4—C9—S1 | 111.62 (15) |
| S1—C2—S10 | 119.02 (14) | C16—C11—C12 | 119.7 (2) |
| C2—C3—C4 | 113.64 (19) | C16—C11—S10 | 123.96 (19) |
| C2—C3—I1 | 123.89 (16) | C12—C11—S10 | 116.29 (19) |
| C4—C3—I1 | 122.46 (16) | C13—C12—C11 | 119.8 (3) |
| C9—C4—C5 | 118.87 (19) | C13—C12—H12 | 120.7 (17) |
| C9—C4—C3 | 111.34 (19) | C11—C12—H12 | 119.5 (17) |
| C5—C4—C3 | 129.8 (2) | C14—C13—C12 | 120.4 (3) |
| C6—C5—C4 | 119.2 (2) | C14—C13—H13 | 117.1 (18) |
| C6—C5—H5 | 122.5 (17) | C12—C13—H13 | 122.5 (18) |
| C4—C5—H5 | 118.3 (17) | C15—C14—C13 | 119.7 (3) |
| C5—C6—C7 | 121.2 (2) | C15—C14—H14 | 118 (2) |
| C5—C6—H6 | 121.4 (17) | C13—C14—H14 | 122 (2) |
| C7—C6—H6 | 117.3 (17) | C14—C15—C16 | 120.7 (3) |
| C8—C7—C6 | 120.9 (2) | C14—C15—H15 | 119.1 (18) |
| C8—C7—H7 | 119.3 (18) | C16—C15—H15 | 120.2 (18) |
| C6—C7—H7 | 119.5 (18) | C11—C16—C15 | 119.7 (2) |
| C7—C8—C9 | 118.3 (2) | C11—C16—H16 | 117.6 (19) |
| C7—C8—H8 | 121.6 (18) | C15—C16—H16 | 122.6 (19) |
| C9—C8—H8 | 120.0 (18) | C9—S1—C2 | 91.54 (11) |
| C8—C9—C4 | 121.5 (2) | C2—S10—C11 | 103.12 (11) |
| S1—C2—C3—C4 | −0.9 (2) | C3—C4—C9—S1 | 0.7 (2) |
| S10—C2—C3—C4 | −179.35 (17) | C16—C11—C12—C13 | 0.0 (4) |
| S1—C2—C3—I1 | 179.47 (11) | S10—C11—C12—C13 | 179.1 (2) |
| S10—C2—C3—I1 | 1.0 (3) | C11—C12—C13—C14 | −0.1 (4) |
| C2—C3—C4—C9 | 0.1 (3) | C12—C13—C14—C15 | 0.1 (4) |
| I1—C3—C4—C9 | 179.77 (14) | C13—C14—C15—C16 | 0.0 (4) |
| C2—C3—C4—C5 | −179.4 (2) | C12—C11—C16—C15 | 0.1 (4) |
| I1—C3—C4—C5 | 0.2 (3) | S10—C11—C16—C15 | −178.93 (19) |
| C9—C4—C5—C6 | 0.2 (3) | C14—C15—C16—C11 | −0.1 (4) |
| C3—C4—C5—C6 | 179.7 (2) | C8—C9—S1—C2 | 178.9 (2) |
| C4—C5—C6—C7 | −0.9 (4) | C4—C9—S1—C2 | −1.01 (17) |
| C5—C6—C7—C8 | 0.9 (4) | C3—C2—S1—C9 | 1.09 (17) |
| C6—C7—C8—C9 | −0.3 (4) | S10—C2—S1—C9 | 179.73 (14) |
| C7—C8—C9—C4 | −0.3 (3) | C3—C2—S10—C11 | −83.5 (2) |
| C7—C8—C9—S1 | 179.78 (19) | S1—C2—S10—C11 | 98.17 (15) |
| C5—C4—C9—C8 | 0.4 (3) | C16—C11—S10—C2 | −20.5 (2) |
| C3—C4—C9—C8 | −179.2 (2) | C12—C11—S10—C2 | 160.48 (19) |
| C5—C4—C9—S1 | −179.70 (16) |
3-Bromo-2-(phenylselanyl)benzo[b]selenophene (3) . Crystal data
| C14H9BrSe2 | F(000) = 784 |
| Mr = 415.04 | Dx = 2.021 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| a = 12.3864 (11) Å | Cell parameters from 9947 reflections |
| b = 13.6816 (11) Å | θ = 2.9–27.1° |
| c = 8.0982 (6) Å | µ = 8.33 mm−1 |
| β = 96.398 (3)° | T = 297 K |
| V = 1363.82 (19) Å3 | Parallelepiped, colourless |
| Z = 4 | 0.17 × 0.17 × 0.12 mm |
3-Bromo-2-(phenylselanyl)benzo[b]selenophene (3) . Data collection
| Bruker D8 Venture/Photon 100 CMOS diffractometer | 2976 independent reflections |
| Radiation source: fine-focus sealed tube | 2208 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.060 |
| Detector resolution: 10.4167 pixels mm-1 | θmax = 27.0°, θmin = 3.0° |
| φ and ω scans | h = −15→15 |
| Absorption correction: multi-scan (SADABS; Krause et al., 2015) | k = −17→17 |
| Tmin = 0.543, Tmax = 0.746 | l = −10→10 |
| 43875 measured reflections |
3-Bromo-2-(phenylselanyl)benzo[b]selenophene (3) . Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.035 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.069 | H-atom parameters constrained |
| S = 1.03 | w = 1/[σ2(Fo2) + (0.0201P)2 + 2.3843P] where P = (Fo2 + 2Fc2)/3 |
| 2976 reflections | (Δ/σ)max = 0.001 |
| 154 parameters | Δρmax = 0.93 e Å−3 |
| 0 restraints | Δρmin = −0.90 e Å−3 |
3-Bromo-2-(phenylselanyl)benzo[b]selenophene (3) . Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
3-Bromo-2-(phenylselanyl)benzo[b]selenophene (3) . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Br1 | 0.27259 (4) | 0.68913 (3) | −0.08792 (6) | 0.06404 (14) | |
| C2 | 0.2358 (3) | 0.5151 (2) | 0.0834 (5) | 0.0433 (8) | |
| C3 | 0.2903 (3) | 0.5551 (3) | −0.0323 (5) | 0.0451 (8) | |
| C4 | 0.3618 (3) | 0.4929 (3) | −0.1127 (4) | 0.0465 (9) | |
| C5 | 0.4294 (3) | 0.5160 (4) | −0.2330 (5) | 0.0632 (12) | |
| H5 | 0.431618 | 0.579335 | −0.274038 | 0.076* | |
| C6 | 0.4933 (4) | 0.4437 (5) | −0.2911 (5) | 0.0795 (16) | |
| H6 | 0.540102 | 0.459228 | −0.369395 | 0.095* | |
| C7 | 0.4887 (4) | 0.3486 (5) | −0.2344 (6) | 0.0819 (16) | |
| H7 | 0.531156 | 0.300749 | −0.277171 | 0.098* | |
| C8 | 0.4225 (4) | 0.3237 (4) | −0.1160 (6) | 0.0700 (13) | |
| H8 | 0.419621 | 0.259777 | −0.077799 | 0.084* | |
| C9 | 0.3605 (3) | 0.3961 (3) | −0.0552 (5) | 0.0496 (9) | |
| Se1 | 0.26699 (4) | 0.38117 (3) | 0.11070 (6) | 0.05761 (13) | |
| Se10 | 0.13853 (4) | 0.58065 (3) | 0.21047 (6) | 0.06282 (15) | |
| C11 | 0.1055 (3) | 0.4715 (2) | 0.3458 (4) | 0.0422 (8) | |
| C12 | 0.1734 (4) | 0.4475 (3) | 0.4854 (5) | 0.0616 (11) | |
| H12 | 0.236377 | 0.483315 | 0.515093 | 0.074* | |
| C13 | 0.1472 (5) | 0.3698 (4) | 0.5807 (6) | 0.0723 (14) | |
| H13 | 0.193489 | 0.352550 | 0.674522 | 0.087* | |
| C14 | 0.0552 (4) | 0.3181 (3) | 0.5402 (6) | 0.0653 (13) | |
| H14 | 0.038533 | 0.265737 | 0.606089 | 0.078* | |
| C15 | −0.0132 (4) | 0.3424 (3) | 0.4031 (6) | 0.0611 (11) | |
| H15 | −0.076485 | 0.306615 | 0.375672 | 0.073* | |
| C16 | 0.0109 (3) | 0.4202 (3) | 0.3041 (5) | 0.0475 (9) | |
| H16 | −0.036003 | 0.437373 | 0.210910 | 0.057* |
3-Bromo-2-(phenylselanyl)benzo[b]selenophene (3) . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Br1 | 0.0617 (3) | 0.0504 (2) | 0.0805 (3) | −0.0051 (2) | 0.0103 (2) | 0.0238 (2) |
| C2 | 0.043 (2) | 0.0332 (17) | 0.054 (2) | −0.0027 (15) | 0.0087 (17) | −0.0002 (15) |
| C3 | 0.043 (2) | 0.0412 (19) | 0.051 (2) | −0.0057 (16) | 0.0034 (17) | 0.0038 (16) |
| C4 | 0.036 (2) | 0.063 (2) | 0.0385 (19) | −0.0047 (18) | −0.0016 (16) | −0.0023 (17) |
| C5 | 0.045 (2) | 0.098 (4) | 0.045 (2) | −0.002 (2) | −0.0015 (19) | 0.001 (2) |
| C6 | 0.052 (3) | 0.145 (5) | 0.042 (2) | 0.007 (3) | 0.008 (2) | −0.008 (3) |
| C7 | 0.070 (3) | 0.117 (5) | 0.057 (3) | 0.027 (3) | 0.001 (3) | −0.026 (3) |
| C8 | 0.070 (3) | 0.075 (3) | 0.063 (3) | 0.016 (2) | −0.001 (2) | −0.021 (2) |
| C9 | 0.045 (2) | 0.057 (2) | 0.046 (2) | 0.0023 (18) | 0.0009 (17) | −0.0117 (18) |
| Se1 | 0.0678 (3) | 0.0342 (2) | 0.0741 (3) | 0.00042 (18) | 0.0227 (2) | 0.00167 (18) |
| Se10 | 0.0762 (3) | 0.0350 (2) | 0.0840 (3) | 0.00590 (19) | 0.0385 (3) | 0.00663 (19) |
| C11 | 0.046 (2) | 0.0352 (18) | 0.048 (2) | 0.0015 (16) | 0.0148 (17) | −0.0002 (15) |
| C12 | 0.051 (3) | 0.061 (3) | 0.070 (3) | 0.000 (2) | −0.008 (2) | −0.002 (2) |
| C13 | 0.089 (4) | 0.066 (3) | 0.058 (3) | 0.023 (3) | −0.009 (3) | 0.012 (2) |
| C14 | 0.095 (4) | 0.045 (2) | 0.060 (3) | 0.017 (2) | 0.030 (3) | 0.015 (2) |
| C15 | 0.063 (3) | 0.049 (2) | 0.075 (3) | −0.012 (2) | 0.024 (2) | −0.006 (2) |
| C16 | 0.046 (2) | 0.051 (2) | 0.045 (2) | −0.0014 (18) | 0.0037 (17) | −0.0043 (17) |
3-Bromo-2-(phenylselanyl)benzo[b]selenophene (3) . Geometric parameters (Å, º)
| Br1—C3 | 1.895 (4) | C8—H8 | 0.9300 |
| C2—C3 | 1.333 (5) | C9—Se1 | 1.881 (4) |
| C2—Se1 | 1.881 (3) | Se10—C11 | 1.923 (3) |
| C2—Se10 | 1.894 (3) | C11—C12 | 1.372 (5) |
| C3—C4 | 1.435 (5) | C11—C16 | 1.375 (5) |
| C4—C5 | 1.391 (5) | C12—C13 | 1.374 (6) |
| C4—C9 | 1.404 (6) | C12—H12 | 0.9300 |
| C5—C6 | 1.381 (7) | C13—C14 | 1.350 (7) |
| C5—H5 | 0.9300 | C13—H13 | 0.9300 |
| C6—C7 | 1.383 (8) | C14—C15 | 1.361 (6) |
| C6—H6 | 0.9300 | C14—H14 | 0.9300 |
| C7—C8 | 1.372 (7) | C15—C16 | 1.385 (5) |
| C7—H7 | 0.9300 | C15—H15 | 0.9300 |
| C8—C9 | 1.378 (6) | C16—H16 | 0.9300 |
| C3—C2—Se1 | 111.6 (3) | C8—C9—Se1 | 126.1 (4) |
| C3—C2—Se10 | 126.2 (3) | C4—C9—Se1 | 111.7 (3) |
| Se1—C2—Se10 | 122.15 (18) | C9—Se1—C2 | 86.85 (16) |
| C2—C3—C4 | 117.5 (3) | C2—Se10—C11 | 97.51 (14) |
| C2—C3—Br1 | 120.7 (3) | C12—C11—C16 | 120.5 (4) |
| C4—C3—Br1 | 121.8 (3) | C12—C11—Se10 | 120.4 (3) |
| C5—C4—C9 | 118.4 (4) | C16—C11—Se10 | 119.1 (3) |
| C5—C4—C3 | 129.3 (4) | C11—C12—C13 | 119.2 (4) |
| C9—C4—C3 | 112.3 (3) | C11—C12—H12 | 120.4 |
| C6—C5—C4 | 119.3 (5) | C13—C12—H12 | 120.4 |
| C6—C5—H5 | 120.3 | C14—C13—C12 | 121.0 (4) |
| C4—C5—H5 | 120.3 | C14—C13—H13 | 119.5 |
| C5—C6—C7 | 120.9 (5) | C12—C13—H13 | 119.5 |
| C5—C6—H6 | 119.5 | C13—C14—C15 | 120.1 (4) |
| C7—C6—H6 | 119.5 | C13—C14—H14 | 119.9 |
| C8—C7—C6 | 121.0 (5) | C15—C14—H14 | 119.9 |
| C8—C7—H7 | 119.5 | C14—C15—C16 | 120.4 (4) |
| C6—C7—H7 | 119.5 | C14—C15—H15 | 119.8 |
| C7—C8—C9 | 118.1 (5) | C16—C15—H15 | 119.8 |
| C7—C8—H8 | 120.9 | C11—C16—C15 | 118.9 (4) |
| C9—C8—H8 | 120.9 | C11—C16—H16 | 120.5 |
| C8—C9—C4 | 122.2 (4) | C15—C16—H16 | 120.5 |
| Se1—C2—C3—C4 | −0.7 (5) | C5—C4—C9—Se1 | 178.4 (3) |
| Se10—C2—C3—C4 | 178.9 (3) | C3—C4—C9—Se1 | −0.9 (4) |
| Se1—C2—C3—Br1 | 179.03 (19) | C8—C9—Se1—C2 | 179.9 (4) |
| Se10—C2—C3—Br1 | −1.4 (5) | C4—C9—Se1—C2 | 0.5 (3) |
| C2—C3—C4—C5 | −178.2 (4) | C3—C2—Se1—C9 | 0.1 (3) |
| Br1—C3—C4—C5 | 2.1 (6) | Se10—C2—Se1—C9 | −179.5 (2) |
| C2—C3—C4—C9 | 1.1 (5) | C3—C2—Se10—C11 | −177.6 (4) |
| Br1—C3—C4—C9 | −178.6 (3) | Se1—C2—Se10—C11 | 1.9 (3) |
| C9—C4—C5—C6 | −0.4 (6) | C16—C11—C12—C13 | 1.7 (6) |
| C3—C4—C5—C6 | 178.8 (4) | Se10—C11—C12—C13 | 179.3 (3) |
| C4—C5—C6—C7 | 1.7 (7) | C11—C12—C13—C14 | −1.0 (7) |
| C5—C6—C7—C8 | −1.6 (8) | C12—C13—C14—C15 | 0.1 (7) |
| C6—C7—C8—C9 | 0.1 (7) | C13—C14—C15—C16 | 0.0 (7) |
| C7—C8—C9—C4 | 1.2 (6) | C12—C11—C16—C15 | −1.5 (6) |
| C7—C8—C9—Se1 | −178.2 (3) | Se10—C11—C16—C15 | −179.2 (3) |
| C5—C4—C9—C8 | −1.0 (6) | C14—C15—C16—C11 | 0.7 (6) |
| C3—C4—C9—C8 | 179.6 (4) |
3-Iodo-2-(phenylselanyl)benzo[b]selenophene (4) . Crystal data
| C14H9ISe2 | F(000) = 856 |
| Mr = 462.03 | Dx = 2.175 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| a = 12.9606 (6) Å | Cell parameters from 9830 reflections |
| b = 13.5999 (7) Å | θ = 3.0–27.9° |
| c = 8.0448 (4) Å | µ = 7.40 mm−1 |
| β = 95.585 (2)° | T = 292 K |
| V = 1411.27 (12) Å3 | Parallelepiped, colourless |
| Z = 4 | 0.51 × 0.47 × 0.24 mm |
3-Iodo-2-(phenylselanyl)benzo[b]selenophene (4) . Data collection
| Bruker D8 Venture/Photon 100 CMOS diffractometer | 3391 independent reflections |
| Radiation source: fine-focus sealed tube | 2702 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.054 |
| Detector resolution: 10.4167 pixels mm-1 | θmax = 28.0°, θmin = 3.0° |
| φ and ω scans | h = −17→17 |
| Absorption correction: multi-scan (SADABS; Krause et al., 2015) | k = −17→17 |
| Tmin = 0.390, Tmax = 0.746 | l = −10→10 |
| 54241 measured reflections |
3-Iodo-2-(phenylselanyl)benzo[b]selenophene (4) . Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.037 | Hydrogen site location: difference Fourier map |
| wR(F2) = 0.083 | All H-atom parameters refined |
| S = 1.04 | w = 1/[σ2(Fo2) + (0.030P)2 + 4.781P] where P = (Fo2 + 2Fc2)/3 |
| 3391 reflections | (Δ/σ)max = 0.001 |
| 190 parameters | Δρmax = 1.14 e Å−3 |
| 0 restraints | Δρmin = −1.82 e Å−3 |
3-Iodo-2-(phenylselanyl)benzo[b]selenophene (4) . Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
3-Iodo-2-(phenylselanyl)benzo[b]selenophene (4) . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C2 | 0.7614 (3) | 0.4907 (3) | 0.4123 (5) | 0.0348 (9) | |
| C3 | 0.7104 (3) | 0.4513 (3) | 0.5349 (5) | 0.0353 (9) | |
| C4 | 0.6366 (3) | 0.5137 (4) | 0.6061 (5) | 0.0395 (10) | |
| C5 | 0.5726 (4) | 0.4915 (5) | 0.7306 (6) | 0.0511 (13) | |
| C6 | 0.5065 (4) | 0.5634 (6) | 0.7810 (7) | 0.0655 (18) | |
| C7 | 0.5049 (5) | 0.6563 (6) | 0.7130 (8) | 0.0693 (19) | |
| C8 | 0.5669 (5) | 0.6814 (5) | 0.5885 (7) | 0.0587 (15) | |
| C9 | 0.6321 (4) | 0.6082 (4) | 0.5366 (6) | 0.0447 (11) | |
| C11 | 0.8883 (3) | 0.5295 (3) | 0.1453 (5) | 0.0354 (9) | |
| C12 | 0.9754 (4) | 0.5864 (4) | 0.1749 (6) | 0.0407 (10) | |
| C13 | 0.9950 (4) | 0.6605 (4) | 0.0638 (7) | 0.0512 (13) | |
| C14 | 0.9290 (5) | 0.6751 (4) | −0.0757 (7) | 0.0569 (15) | |
| C15 | 0.8411 (6) | 0.6181 (5) | −0.1068 (8) | 0.0673 (17) | |
| C16 | 0.8210 (5) | 0.5442 (5) | 0.0037 (8) | 0.0566 (14) | |
| I1 | 0.74197 (2) | 0.30752 (2) | 0.61759 (4) | 0.04627 (11) | |
| Se1 | 0.72215 (4) | 0.62182 (4) | 0.36770 (7) | 0.05061 (15) | |
| Se10 | 0.86334 (4) | 0.42591 (4) | 0.29872 (7) | 0.04875 (15) | |
| H5 | 0.575 (4) | 0.430 (4) | 0.777 (6) | 0.033 (12)* | |
| H6 | 0.465 (5) | 0.541 (5) | 0.859 (8) | 0.08 (2)* | |
| H8 | 0.571 (5) | 0.750 (5) | 0.540 (8) | 0.069 (19)* | |
| H7 | 0.459 (5) | 0.703 (5) | 0.757 (8) | 0.08 (2)* | |
| H12 | 1.022 (4) | 0.576 (4) | 0.263 (7) | 0.052 (16)* | |
| H13 | 1.057 (5) | 0.701 (5) | 0.085 (8) | 0.068 (18)* | |
| H14 | 0.941 (6) | 0.724 (6) | −0.147 (9) | 0.09 (2)* | |
| H15 | 0.793 (6) | 0.631 (5) | −0.196 (9) | 0.09 (2)* | |
| H16 | 0.768 (5) | 0.507 (5) | −0.015 (8) | 0.08 (2)* |
3-Iodo-2-(phenylselanyl)benzo[b]selenophene (4) . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C2 | 0.035 (2) | 0.028 (2) | 0.041 (2) | −0.0025 (17) | 0.0056 (18) | −0.0003 (17) |
| C3 | 0.033 (2) | 0.036 (2) | 0.037 (2) | −0.0010 (17) | 0.0029 (17) | −0.0001 (18) |
| C4 | 0.034 (2) | 0.050 (3) | 0.034 (2) | −0.0012 (19) | −0.0009 (17) | −0.0065 (19) |
| C5 | 0.041 (3) | 0.072 (4) | 0.039 (3) | 0.004 (2) | 0.003 (2) | −0.007 (3) |
| C6 | 0.044 (3) | 0.111 (6) | 0.042 (3) | 0.007 (3) | 0.008 (2) | −0.015 (3) |
| C7 | 0.062 (4) | 0.090 (5) | 0.054 (3) | 0.033 (3) | 0.000 (3) | −0.024 (3) |
| C8 | 0.064 (3) | 0.059 (4) | 0.051 (3) | 0.023 (3) | −0.006 (3) | −0.014 (3) |
| C9 | 0.040 (2) | 0.050 (3) | 0.043 (3) | 0.007 (2) | 0.000 (2) | −0.009 (2) |
| C11 | 0.037 (2) | 0.032 (2) | 0.039 (2) | 0.0015 (17) | 0.0112 (18) | −0.0031 (17) |
| C12 | 0.040 (2) | 0.042 (3) | 0.040 (2) | −0.004 (2) | 0.008 (2) | −0.003 (2) |
| C13 | 0.054 (3) | 0.039 (3) | 0.064 (3) | −0.012 (2) | 0.021 (3) | −0.004 (2) |
| C14 | 0.079 (4) | 0.038 (3) | 0.057 (3) | 0.008 (3) | 0.025 (3) | 0.014 (2) |
| C15 | 0.076 (4) | 0.070 (4) | 0.054 (3) | 0.013 (3) | −0.008 (3) | 0.007 (3) |
| C16 | 0.052 (3) | 0.055 (3) | 0.061 (3) | −0.009 (3) | −0.006 (3) | 0.001 (3) |
| I1 | 0.04859 (18) | 0.04025 (18) | 0.05068 (19) | −0.00477 (13) | 0.00843 (13) | 0.01099 (13) |
| Se1 | 0.0609 (3) | 0.0314 (3) | 0.0616 (3) | 0.0050 (2) | 0.0163 (2) | 0.0066 (2) |
| Se10 | 0.0558 (3) | 0.0320 (3) | 0.0628 (3) | 0.0051 (2) | 0.0282 (2) | 0.0073 (2) |
3-Iodo-2-(phenylselanyl)benzo[b]selenophene (4) . Geometric parameters (Å, º)
| C2—C3 | 1.351 (6) | C8—H8 | 1.01 (7) |
| C2—Se1 | 1.880 (4) | C9—Se1 | 1.885 (5) |
| C2—Se10 | 1.895 (4) | C11—C12 | 1.370 (6) |
| C3—C4 | 1.438 (6) | C11—C16 | 1.380 (7) |
| C3—I1 | 2.093 (4) | C11—Se10 | 1.921 (4) |
| C4—C5 | 1.393 (7) | C12—C13 | 1.387 (7) |
| C4—C9 | 1.402 (7) | C12—H12 | 0.90 (6) |
| C5—C6 | 1.386 (8) | C13—C14 | 1.358 (9) |
| C5—H5 | 0.92 (5) | C13—H13 | 0.97 (6) |
| C6—C7 | 1.377 (10) | C14—C15 | 1.381 (9) |
| C6—H6 | 0.92 (7) | C14—H14 | 0.90 (7) |
| C7—C8 | 1.386 (10) | C15—C16 | 1.383 (9) |
| C7—H7 | 0.96 (7) | C15—H15 | 0.92 (7) |
| C8—C9 | 1.396 (7) | C16—H16 | 0.86 (7) |
| C3—C2—Se1 | 111.9 (3) | C8—C9—Se1 | 125.5 (5) |
| C3—C2—Se10 | 125.6 (3) | C4—C9—Se1 | 111.9 (3) |
| Se1—C2—Se10 | 122.5 (2) | C12—C11—C16 | 120.4 (5) |
| C2—C3—C4 | 116.7 (4) | C12—C11—Se10 | 119.3 (4) |
| C2—C3—I1 | 120.5 (3) | C16—C11—Se10 | 120.3 (4) |
| C4—C3—I1 | 122.8 (3) | C11—C12—C13 | 119.8 (5) |
| C5—C4—C9 | 118.7 (5) | C11—C12—H12 | 122 (4) |
| C5—C4—C3 | 128.6 (5) | C13—C12—H12 | 118 (4) |
| C9—C4—C3 | 112.7 (4) | C14—C13—C12 | 119.9 (5) |
| C6—C5—C4 | 119.1 (6) | C14—C13—H13 | 120 (4) |
| C6—C5—H5 | 122 (3) | C12—C13—H13 | 120 (4) |
| C4—C5—H5 | 119 (3) | C13—C14—C15 | 120.7 (5) |
| C7—C6—C5 | 121.1 (6) | C13—C14—H14 | 120 (5) |
| C7—C6—H6 | 127 (4) | C15—C14—H14 | 119 (5) |
| C5—C6—H6 | 112 (5) | C14—C15—C16 | 119.7 (6) |
| C6—C7—C8 | 121.8 (5) | C14—C15—H15 | 121 (5) |
| C6—C7—H7 | 117 (4) | C16—C15—H15 | 119 (5) |
| C8—C7—H7 | 122 (4) | C11—C16—C15 | 119.5 (5) |
| C7—C8—C9 | 116.8 (6) | C11—C16—H16 | 119 (5) |
| C7—C8—H8 | 124 (4) | C15—C16—H16 | 121 (5) |
| C9—C8—H8 | 119 (4) | C2—Se1—C9 | 86.8 (2) |
| C8—C9—C4 | 122.5 (5) | C2—Se10—C11 | 97.92 (18) |
| Se1—C2—C3—C4 | 0.8 (5) | C5—C4—C9—Se1 | −178.7 (4) |
| Se10—C2—C3—C4 | 179.3 (3) | C3—C4—C9—Se1 | 1.6 (5) |
| Se1—C2—C3—I1 | −177.8 (2) | C16—C11—C12—C13 | 1.4 (7) |
| Se10—C2—C3—I1 | 0.7 (6) | Se10—C11—C12—C13 | 179.1 (4) |
| C2—C3—C4—C5 | 178.7 (5) | C11—C12—C13—C14 | −1.3 (8) |
| I1—C3—C4—C5 | −2.7 (7) | C12—C13—C14—C15 | 1.2 (9) |
| C2—C3—C4—C9 | −1.6 (6) | C13—C14—C15—C16 | −1.1 (10) |
| I1—C3—C4—C9 | 177.0 (3) | C12—C11—C16—C15 | −1.3 (8) |
| C9—C4—C5—C6 | 0.3 (7) | Se10—C11—C16—C15 | −178.9 (5) |
| C3—C4—C5—C6 | 180.0 (5) | C14—C15—C16—C11 | 1.1 (10) |
| C4—C5—C6—C7 | −1.5 (8) | C3—C2—Se1—C9 | 0.1 (3) |
| C5—C6—C7—C8 | 1.8 (9) | Se10—C2—Se1—C9 | −178.5 (3) |
| C6—C7—C8—C9 | −0.8 (9) | C8—C9—Se1—C2 | 179.7 (5) |
| C7—C8—C9—C4 | −0.5 (8) | C4—C9—Se1—C2 | −1.0 (4) |
| C7—C8—C9—Se1 | 178.8 (4) | C3—C2—Se10—C11 | 177.6 (4) |
| C5—C4—C9—C8 | 0.7 (7) | Se1—C2—Se10—C11 | −4.0 (3) |
| C3—C4—C9—C8 | −179.1 (5) |
Funding Statement
This work was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior e Brasil (CAPES) - Finance code 001; CNPq Process: 400400/2016-2; Fundação Araucária; Universidade Federal do Paraná.
References
- An, Y., Oh, J., Chen, S., Lee, B., Lee, S. M., Han, D. & Yang, C. (2018). Polym. Chem. 9, 593–602.
- Arsenyan, P., Paegle, E., Belyakov, S., Shestakova, I., Jaschenko, E., Domracheva, I. & Popelis, J. (2011). Eur. J. Med. Chem. 46, 3434–3443. [DOI] [PubMed]
- Arsenyan, P., Petrenko, A., Leitonas, K., Volyniuk, D., Simokaitiene, J., Klinavičius, T., Skuodis, E., Lee, J.-H. & Gražulevičius, J. V. (2019). Inorg. Chem. 58, 10174–10183. [DOI] [PubMed]
- Ashraf, R. S., Meager, I., Nikolka, M., Kirkus, M., Planells, M., Schroeder, B. C., Holliday, S., Hurhangee, M., Nielsen, C. B., Sirringhaus, H. & McCulloch, L. (2015). J. Am. Chem. Soc. 137, 1314–1321. [DOI] [PubMed]
- Bondi, A. (1964). J. Phys. Chem. 68, 441–451.
- Brandenburg, K. & Putz, H. (2006). DIAMOND. Crystal Impact GbR, Bonn, Germany.
- Bruker (2002). SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (2015). APEX3. Bruker AXS Inc., Madison, Wisconsin, USA.
- Chen, G., Liu, S., Xu, J., He, R., He, Z., Wu, H.-B., Yang, W., Zhang, B. & Cao, Y. (2017). ACS Appl. Mater. Interfaces. 9, 4778–4787. [DOI] [PubMed]
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
- Ghosh, T. & Lehmann, M. (2017). J. Mater. Chem. C. 5, 12308–12337.
- Grimsdale, A. C., Leok Chan, K., Martin, R. E., Jokisz, P. G. & Holmes, A. B. (2009). Chem. Rev. 109, 897–1091. [DOI] [PubMed]
- Keri, R. S., Chand, K., Budagumpi, S., Balappa Somappa, S., Patil, S. A. & Nagaraja, B. M. (2017). Eur. J. Med. Chem. 138, 1002–1033. [DOI] [PubMed]
- Krause, L., Herbst-Irmer, R., Sheldrick, G. M. & Stalke, D. (2015). J. Appl. Cryst. 48, 3–10. [DOI] [PMC free article] [PubMed]
- Lee, S. M., Lee, H. R., Dutta, G., Lee, J., Oh, J. H. & Yang, G. (2019). Polym. Chem. 10, 2854–2862.
- Luz, E. Q., Silvério, G. L., Seckler, D., Lima, D. B., Santana, F. S., Barbosa, R. B., Montes D’Oca, C. R. & Rampon, D. S. (2021). Adv. Synth. Catal. 363, 2610–2618.
- Mahmoud, A. B. A., Kirsch, G. & Peagle, E. (2017). Curr. Org. Synth. 14, 1091–1101.
- Mei, J., Diao, Y., Appleton, A. L., Fang, L. & Bao, Z. (2013). J. Am. Chem. Soc. 135, 6724–6746. [DOI] [PubMed]
- Mukherjee, A., Zade, S., Singh, H. & Sunoj, R. (2010). Chem. Rev. 110, 4357–4416. [DOI] [PubMed]
- Ostroverkhova, O. (2016). Chem. Rev. 116, 13279–13412. [DOI] [PubMed]
- Paegle, E., Domracheva, I., Turovska, B., Petrova, M., Kanepe-Lapsa, I., Gulbe, A., Liepinsh, E. & Arsenyan, P. (2016). Chem. Asian J. 11, 1929–1938. [DOI] [PubMed]
- Ramesh, E., Shankar, M., Dana, S. & Sahoo, A. (2016). Org. Chem. Front. 3, 1126–1130.
- Shah, R. & Verma, P. K. (2018). Chem. Cent. J. 12, 1–22. [DOI] [PMC free article] [PubMed]
- Shahjad, A., Bhargav, R., Bhardwaj, D., Mishra, A. & Patra, A. (2017). Macromol. Chem. Phys. 218, 1700038–1700047.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Tisovský, P., Gáplovský, A., Gmucová, K., Novota, M., Pavúk, M. & Weis, M. (2019). Org. Electron. 68, 121–128.
- Wei, J., Meng, D., Zhang, L. & Wang, Z. (2017). Chem. Asian J. 12, 1879–1882. [DOI] [PubMed]
- Xu, J., Yu, X., Yan, J. & Song, Q. (2017). Org. Lett. 19, 6292–6295. [DOI] [PubMed]
- Yang, F., Cheng, S., Zhang, X., Ren, X., Li, R., Dong, H. & Hu, W. (2018). Adv. Mater. 30, 1702415–1702442. [DOI] [PubMed]
- Zampetti, A., Minotto, A., Squeo, B. M., Gregoriou, V. G., Allard, S., Scherf, U., Chochos, C. L. & Cacialli, F. (2017). Sci. Rep. 7, 1–7. [DOI] [PMC free article] [PubMed]
- Zani, F., Vicini, P. & Incerti, M. (2004). Eur. J. Med. Chem. 39, 135–140. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) 1, 2, 3, 4, shelx. DOI: 10.1107/S2056989022000962/jy2015sup1.cif
Structure factors: contains datablock(s) 1. DOI: 10.1107/S2056989022000962/jy20151sup2.hkl
Supporting information file. DOI: 10.1107/S2056989022000962/jy20151sup6.cml
Structure factors: contains datablock(s) 2. DOI: 10.1107/S2056989022000962/jy20152sup3.hkl
Supporting information file. DOI: 10.1107/S2056989022000962/jy20152sup7.cml
Structure factors: contains datablock(s) 3. DOI: 10.1107/S2056989022000962/jy20153sup4.hkl
Supporting information file. DOI: 10.1107/S2056989022000962/jy20153sup8.cml
Structure factors: contains datablock(s) 4. DOI: 10.1107/S2056989022000962/jy20154sup5.hkl
Supporting information file. DOI: 10.1107/S2056989022000962/jy20154sup9.cml
Additional supporting information: crystallographic information; 3D view; checkCIF report