Abstract
Background
Many people recovering from coronavirus disease 2019 (COVID-19) experience prolonged symptoms, particularly breathlessness. We urgently need to identify safe and effective COVID-19 rehabilitative strategies. The aim of the current study was to investigate the potential rehabilitative role of inspiratory muscle training (IMT).
Methods
281 adults (age 46.6±12.2 years; 88% female) recovering from self-reported COVID-19 (9.0±4.2 months post-acute infection) were randomised 4:1 to an 8-week IMT or a “usual care” waitlist control arm. Health-related quality-of-life and breathlessness questionnaires (King's Brief Interstitial Lung Disease (K-BILD) and Transition Dyspnoea Index (TDI)), respiratory muscle strength, and fitness (Chester Step Test) were assessed pre- and post-intervention. The primary end-point was K-BILD total score, with the K-BILD domains and TDI being key secondary outcomes.
Results
According to intention to treat, there was no difference between groups in K-BILD total score post-intervention (control: 59.5±12.4; IMT: 58.2±12.3; p<0.05) but IMT elicited clinically meaningful improvements in the K-BILD domains for breathlessness (control: 59.8±12.6; IMT: 62.2±16.2; p<0.05) and chest symptoms (control: 59.2±18.7; IMT: 64.5±18.2; p<0.05), along with clinically meaningful improvements in breathlessness according to TDI (control: 0.9±1.7 versus 2.0±2.0; p<0.05). IMT also improved respiratory muscle strength and estimated aerobic fitness.
Conclusions
IMT may represent an important home-based rehabilitation strategy for wider implementation as part of COVID-19 rehabilitative strategies. Given the diverse nature of long COVID, further research is warranted on the individual responses to rehabilitation; the withdrawal rate herein highlights that no one strategy is likely to be appropriate for all.
Short abstract
IMT can significantly improve breathlessness and respiratory muscle function in people with long COVID, and represents an effective, home-based rehabilitation strategy that could be widely implemented as part of COVID-19 recovery strategies https://bit.ly/3HiEyz0
Introduction
As of 31 January 2022, coronavirus disease-2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has affected all but four countries globally, with over 364 million cases and 5.63 million deaths [1]. COVID-19 is a multisystem disease, with a nonlinear evolution and potential long-term implications [2]. The persistence of COVID-19-related symptoms was noted in May 2020, with long COVID now defined as ongoing or new symptoms ≥4 weeks post-infection [3]. 1.9% of the UK population alone are estimated to be living with long COVID [4], which will increase as infections continue. Nonetheless, no clear rehabilitative pathway has been identified.
Over 200 different symptoms are associated with long COVID [5], with people experiencing symptoms for >6 months still experiencing approximately 14 symptoms [5]. One of the top three most debilitating symptoms associated with a poor quality of life was dyspnoea (breathlessness) [5, 6]. While the aetiology of dyspnoea in long COVID is unknown, in other chronic respiratory conditions the association between breathlessness and vicious cycles of physical, cognitive/emotional and functional deterioration [7], often referred to as the spiral of disability [8], is well evidenced. With the potential deleterious consequences of this downward spiral in people living with long COVID, the need for safe and effective rehabilitation strategies to help combat this global health and economic crisis cannot be overstated.
Numerous nonpharmacological interventions have been highly effective at managing breathlessness in other chronic respiratory conditions [8]. Although pulmonary rehabilitation is effective at improving physical performance and quality of life following hospitalisation [9, 10], current worldwide availability of pulmonary rehabilitation services is alarmingly low [11]. Furthermore, these conventional approaches, almost without exception, are limited in the context of COVID-19 by their intensive, predominantly in-person, hospital- and/or group-based delivery formats, which present significant transmission risks and time and resource burdens on overstretched healthcare systems. Inspiratory muscle training (IMT) utilises restricted airflow breathing to challenge the respiratory muscles, eliciting a hypertrophic response equivalent to that observed in the peripheral musculature following a strength training programme [12], and can be carried out independently at home. IMT has elicited clinically meaningful improvements in dyspnoea and quality of life in chronic obstructive pulmonary disease (COPD) [13], and has been well tolerated and perceived as beneficial in bronchiectasis [14]. As respiratory muscle weakness predicts poor outcomes following COVID-19 infection [15], IMT could represent a feasible initial step towards a whole-body rehabilitation programme. We hypothesised that IMT may be an effective home-based, unsupervised rehabilitation strategy for adults with long COVID, eliciting clinically meaningful reductions in breathlessness and improvements in quality of life and functional capacity.
Methods
Participants
281 adults (age 46.6±12.2 years; 88% female; 9.0±4.2 months post-acute COVID-19) (table 1) were recruited through social media, online COVID-19 support groups or following hospital discharge. Inclusion criteria comprised: prior self-reported COVID-19 infection, primary symptom of breathlessness and age ≥18 years. Standard pulmonary rehabilitation exclusion criteria were applied, excluding individuals with: 1) dementia meaning they could not follow commands/training, 2) unstable cardiac disease, myocardial infarction or non-ST-elevation myocardial infarction within 6 weeks and/or 3) high risk of falls.
TABLE 1.
Participant characteristics at pre-intervention
| Control (n=37) | IMT (n=111) | p-value | |
| Age (years) | 46.13±12.73 | 46.76±12.03 | 0.765 |
| BMI (kg·m−2) | 27.81±5.83 | 27.64±6.80 | 0.881 |
| Male/female (%) | 5/95 | 14/86 | |
| Time since COVID-19 (months) | 9.00±3.67 | 9.04±4.29 | 0.791 |
| K-BILD | |||
| Breathlessness and activities | 59.7±15.8 | 55.0±12.6 | 0.081 |
| Psychological | 40.6±23.3 | 36.8±14.9 | 0.302 |
| Chest symptoms | 56.9±18.7 | 57.8±17.2 | 0.479 |
| Total | 56.6±13.5 | 53.2±8.9 | 0.105 |
Data are presented as mean±sd, unless otherwise stated. BMI: body mass index; K-BILD: King's Brief Interstitial Lung Disease questionnaire.
Study design
This two-arm, randomised control trial compared 8 weeks of IMT with a “usual care” waitlist control to which, following informed consent, participants were randomised using a computer-generated allocation on a 4:1 basis, respectively. The study was conducted entirely remotely via video-conferencing software (Zoom, San Jose, CA, USA), with pre- and post-intervention measures collected during one-to-one calls. Institutional (2020-037) and NHS Research Ethics Committees (20/HRA/3536) approved the study, which was conducted in accordance with the Declaration of Helsinki and registered on the Health and Care Research Wales Research Directory (48075).
IMT intervention
The PrO2 (PrO2Fit Health, Smithfield, RI, USA), a handheld inspiratory flow resistive device that wirelessly syncs to a computer, smartphone or tablet via an app to provide users with graphical biofeedback during and following each inspiratory effort, was used. Participants were trained on its set-up and use during the first session.
Intervention participants were asked to perform three unsupervised IMT sessions per week, on nonconsecutive days, for 8 weeks, as in previous studies [14]. Before each session, participants performed a maximal inspiratory effort from residual volume to determine sustained maximal inspiratory pressure (SMIP; in pressure time units (PTU)), with training subsequently requiring >80% SMIP to be maintained. This reassessment prior to each session ensured individually optimised training loads, accounting for the relapsing and remitting nature of long COVID. Each session involved up to six blocks of six inspirations, with the rest periods interspersing each inspiration progressively decreasing from 40 to 10 s with each block, producing maximum session durations of 20 min. Participants completed as many inspirations as they could prior to failure, defined as not achieving 80% SMIP on three consecutive breaths. Data from all sessions were automatically uploaded to a secure cloud server, enabling remote adherence monitoring.
Primary outcomes
The primary outcome was health-related quality of life, as estimated by the 15-item King's Brief Interstitial Lung Disease (K-BILD) questionnaire, with a seven-point Likert scale [16], and three domains: psychological, breathlessness and activities, and chest symptoms. The domain raw scores and total score were converted to logit scores using Rasch analysis, and then transformed to a 0–100 scale, with 100 representing the best health status [17]. The minimal clinically important difference (MCID) for the logit-transformed total score is 5, with 6, 7 and 11 representing the MCID for the psychological, breathlessness and activities, and chest symptoms domains, respectively [17].
Secondary outcomes
Perceived breathlessness was assessed by the Baseline Dyspnoea Index (BDI) and Transition Dyspnoea Index (TDI). These clinically validated questionnaires assess three domains (functional impairment, magnitude of task and magnitude of effort) that quantify dyspnoea-related limitations [18]. A 1-unit change in the TDI is considered the MCID [19].
Inspiratory muscle strength was assessed using the PrO2 device. Following device familiarisation, participants performed a maximal sustained inspiratory effort following a full expiration to residual volume to provide measures of maximal inspiratory pressure (MIP), SMIP, inspiratory duration and the Fatigue Index Time (FIT).
Exploratory outcomes
The Chester Step Test, with standardised instructions and a demonstration of the initial step rate, was used to evaluate fitness [20]. Participants stepped on and off a 15–30-cm step at a metronome-dictated rate that progressively increased every 2 min until they reached 80% maximum predicted heart rate or withdrew. Estimated maximal oxygen uptake (V′O2max) was calculated from participant-measured heart rate and ratings of perceived exertion at the end of each stage.
To assess changes in daily function, habitual physical activity and sleep, a nondominant wrist-worn GT9X accelerometer was used (ActiGraph, Pensacola, FL, USA), measuring at 30 Hz for 7 consecutive days. Physical activity, sedentary time and sleep analyses were performed in R (www.r-project.org) using the GGIR package (version 2.3-0). To be included in the analyses, a minimum of 12 h per day of wake wear-time on 3 week days and 1 weekend day [21] and daily sleep time of ≥160 min per night with >90% estimated wear-time [22] were required, resulting in 132 and 113 pre- and post-intervention participants, respectively, in the analyses. To provide insights into the full 24-h activity profile, average acceleration, intensity gradient, and the acceleration associated with each participant's most active 30 and 60 min were obtained, together with time spent in each activity intensity, according to age- and accelerometer-specific raw acceleration cut-points [23].
Mental health and wellbeing were assessed using validated questionnaires underpinned by self-determination theory [24] that measured treatment self-regulation, perceived competence and needs satisfaction in relation to IMT [25–27]. The 15-item Treatment Self-Regulation Questionnaire assessed reasons for completing IMT regularly via different forms of motivation. Responses to each subscale were averaged to provide a reflection of each form of motivation. The Perceived Competence Scale includes four items with a seven-point Likert scale to assess participants’ context-specific competence perceptions. A total score was calculated, with a higher score representing higher perceived competence. The 21-item Basic Needs Satisfaction Scale assessed the degree to which participants felt their basic psychological needs for autonomy, competence and relatedness were satisfied [25, 28].
Sample size considerations
A target sample size of 250 participants was calculated to detect a post-intervention between-group MCID of 5 in K-BILD total score. A 1:1 allocation ratio and α of 0.05 yields a power of 0.90. However, to allow more participants to receive the intervention, an allocation ratio of 4:1 was chosen, resulting in a power of 0.75. We deemed this acceptable because 1) the study was conducted during an ongoing pandemic with no available rehabilitation strategies, 2) the intervention included implementation of an entirely new technology in home-based rehabilitation and 3) a higher dropout rate was expected in the intervention group, thus enhancing the power of the per-protocol-based analysis [29].
Statistical analysis
Following confirmation of a normal distribution, linear mixed models with a random intercept at the individual level were used to determine the influence of time, group and their interaction, along with time post-acute COVID-19 infection and the number of IMT sessions completed. Planned contrasts were used to explore significant interaction effects. All analyses were conducted according to an intention-to-treat (ITT; last one carried forward) and per-protocol approach, for which participants were required to adhere to the IMT intervention by completing at least 16 sessions (equivalent to two sessions per week for 8 weeks). All analyses were conducted in Stata version 13 (StataCorp, College Station, TX, USA) and significance was accepted as p<0.05, with data presented as mean±sd, unless otherwise stated.
Results
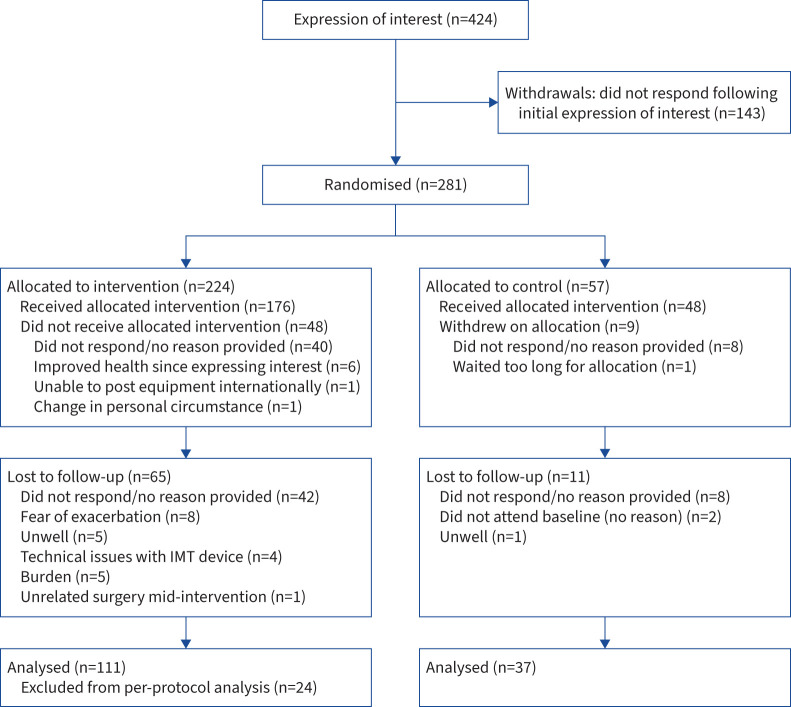
148 (IMT: n=111; controls: n=37) participants completed the post-intervention testing and were included in the ITT analysis (figure 1 and table 2). Withdrawal between recruitment and enrolment (n=57) was predominately associated with enrolment delays due to COVID-19-related IMT device manufacturing issues. On average, participants completed two IMT sessions per week, with 87 participants meeting the adherence criterion and thus being included in the per-protocol analysis, along with 37 controls (table 3).
FIGURE 1.
CONSORT (Consolidated Standards of Reporting Trials) flow diagram. IMT: inspiratory muscle training.
TABLE 2.
Pre- and post-intervention respiratory muscle strength, estimated physical fitness, physical activity, sleep levels, and mental health and wellbeing in the intention-to-treat population (n=148)
| Pre-intervention | Post-intervention | |||
| Control (n=37) | IMT (n=111) | Control (n=37) | IMT (n=111) | |
| Respiratory muscle strength | ||||
| MIP (cmH2O) | 82.8±38.5 | 76.6±33.9 | 90.5±42.1 | 104.0±52.8# |
| MIP (% pred) | 84.2±41.6 | 92.3±46.6 | 79.8±37.2 | 108.9±60.0# |
| FIT (AU) | 20.5±13.7 | 19.7±17.2 | 22.3±25.9 | 23.0±17.3# |
| SMIP (PTU) | 475.1±218.3 | 442.7±215.3 | 478.0±225.0 | 539.1±251.7# |
| Physical fitness | ||||
| Estimated V′O2max (mL·kg−1·min−1) | 37.9±12.4 | 38.3±15.1 | 36.8±4.8 | 42.0±16.4# |
| Device-based physical activity | ||||
| Sedentary time (min) | 757.8±105.0 | 737.5±95.4 | 774.6±72.9 | 734.3±94.8¶ |
| LPA (min) | 125.2±62.05 | 122.8±48.8 | 120.2±44.7 | 122.3±41.7 |
| MPA (min) | 64.8±36.6 | 83.7±39.2 | 69.8±32.3 | 86.8±41.4¶ |
| VPA (min) | 2.6±5.40 | 1.9±3.2 | 2.5±6.3 | 2.5±5.3 |
| Most active 60 min (mg) | 107.8±60.3 | 111.1±30.4 | 107.1±42.5 | 114.5±33.0 |
| Most active 30 min (mg) | 150.8±90.7 | 153.3±55.0 | 156.2±105.5 | 159.1±63.3 |
| Intensity gradient | −3.2±0.4 | −3.4±0.4 | −3.3±0.4 | −3.3±0.4# |
| Device-based sleep | ||||
| Sleep duration (min) | 413.3±81.6 | 422.1±73.3 | 399.8±62.5 | 419.9±77.7 |
| Sleep efficiency (%) | 85±11 | 86±9 | 85±9 | 85±11 |
| Perceived Competence Scale | 14.1±9.3 | 14.3±8.0 | 16.7±8.9# | 15.4±8.2# |
| Basic Needs Satisfaction Scale | ||||
| Autonomy | 4.6±0.9 | 4.6±0.8 | 4.8±0.7 | 4.6±0.8 |
| Competence | 4.8±1.0 | 4.8±1.0 | 4.9±0.9 | 4.9±1.0 |
| Relatedness | 5.8±0.8 | 5.7±0.9 | 5.8±0.7 | 5.7±0.9 |
| Treatment Self-Regulation Questionnaire | ||||
| Amotivation | 5.4±3.0 | 5.3±2.5 | 5.4±2.2 | 5.6±2.8 |
| External regulation | 7.4±3.5 | 8.4±4.7 | 8.0±3.8 | 8.7±4.3 |
| Introjected regulation | 7.4±3.1 | 7.4±3.4 | 6.9±3.7 | 7.3±3.3 |
| Autonomous motivation | 36.6±6.2 | 35.7±6.1 | 36.6±5.2 | 35.3±6.3 |
Data are presented as mean±sd. IMT: inspiratory muscle training; MIP: maximal inspiratory pressure; FIT: Fatigue Index Test; AU: arbitrary units; SMIP: sustained maximal inspiratory pressure; PTU: pressure time units; V′O2max: maximal oxygen uptake estimated from the Chester Step Test; LPA: light physical activity; MPA: moderate physical activity; VPA: vigorous physical activity. #: significant (p<0.05) difference between time-points within group; ¶: significant (p<0.05) difference between groups within time-point.
TABLE 3.
Pre- and post-intervention health-related quality of life, respiratory muscle strength, estimated physical fitness, physical activity, sleep levels, and mental health and wellbeing in the per-protocol population (n=124)
| Pre-intervention | Post-intervention | |||
| Control (n=37) | IMT (n=87) | Control (n=37) | IMT (n=37) | |
| K-BILD | ||||
| Total | 59.7±15.8 | 56.8±12.4 | 59.8±12.6 | 67.8±14.4#,¶ |
| Breathlessness and activities | 40.6±23.3 | 36.7±13.1 | 41.9±20.7 | 49.7±19.5# |
| Psychological | 56.9±18.7 | 60.4±17.4 | 59.2±18.7# | 70.1±16.9#,¶ |
| Chest symptoms | 56.6±13.5 | 54.0±7.8 | 59.5±12.4 | 60.7±10.8# |
| Respiratory muscle strength | ||||
| MIP (cmH2O) | 82.8±38.5 | 73.3±28.7 | 90.5±42.1 | 112.6±42.9#,¶ |
| FIT (AU) | 20.5±13.7 | 19.7±14.7 | 22.0±27.6 | 24.9±14.0# |
| SMIP (PTU) | 475.1±218.3 | 445.3±183.9 | 464.2±226.8 | 587.7±215.0#,¶ |
| Physical fitness | ||||
| Estimated V′O2max (mL·kg−1·min−1) | 37.9±12.4 | 37.5±15.6 | 36.8±4.8 | 43.4±17.5# |
| Device-based physical activity | ||||
| Sedentary time (min) | 757.8±105.0 | 766.2±89.9 | 774.6±72.9 | 742.8±95.5 |
| LPA (min) | 125.2±62.05 | 117.7±58.9 | 120.2±44.7 | 124.2±42.3 |
| MPA (min) | 64.8±36.6 | 70.9±36.2 | 69.8±32.3 | 87.0±40.0# |
| VPA (min) | 2.6±5.4 | 0.96±1.6 | 2.5±6.3 | 2.5±6.3 |
| Most active 60 min (mg) | 107.8±60.3 | 99.6±29.6 | 107.1±42.5 | 115.6±33.7# |
| Most active 30 min (mg) | 150.8±90.7 | 132.8±37.3 | 156.2±105.5 | 158.1±59.4# |
| Intensity gradient | −3.2±0.4 | −3.4±0.4 | −3.3±0.4 | −3.3±0.4 |
| Device-based sleep | ||||
| Sleep duration (min) | 413.3±81.6 | 398.3±84.9 | 399.8±62.5 | 412.5±73.9 |
| Sleep efficiency (%) | 85±11 | 84±14 | 85±9 | 85±10 |
| Perceived Competence Scale | 14.1±9.3 | 15.1±7.7 | 16.7±8.9# | 16.5±7.8 |
| Basic Needs Satisfaction Scale | ||||
| Autonomy | 4.6±0.9 | 4.6±0.9 | 4.8±0.7 | 4.7±0.8 |
| Competence | 4.8±1.0 | 4.9±1.1 | 4.9±0.9 | 5.0±1.0 |
| Relatedness | 5.8±0.8 | 5.7±0.9 | 5.8±0.7 | 5.7±1.0 |
| Treatment Self-Regulation Questionnaire | ||||
| Amotivation | 5.4±3.0 | 5.1±2.2 | 5.4±2.2 | 5.7±2.6 |
| External regulation | 7.4±3.5 | 8.0±4.5 | 8.0±3.8 | 8.2±3.7 |
| Introjected regulation | 7.4±3.1 | 7.0±3.5 | 6.9±3.7 | 6.9±3.3 |
| Autonomous motivation | 36.6±6.2 | 35.2±6.6 | 36.6±5.2 | 33.8±6.9 |
Data are presented as mean±sd. IMT: inspiratory muscle training; K-BILD: King's Brief Interstitial Lung Disease questionnaire; MIP: maximal inspiratory pressure; FIT: Fatigue Index Test; AU: arbitrary units; SMIP: sustained maximal inspiratory pressure; PTU: pressure time units; V′O2max: maximal oxygen uptake estimated from the Chester Step Test; LPA: light physical activity; MPA: moderate physical activity; VPA: vigorous physical activity. #: significant (p<0.05) difference between time-points within group; ¶: significant (p<0.05) difference between groups within time-point.
K-BILD
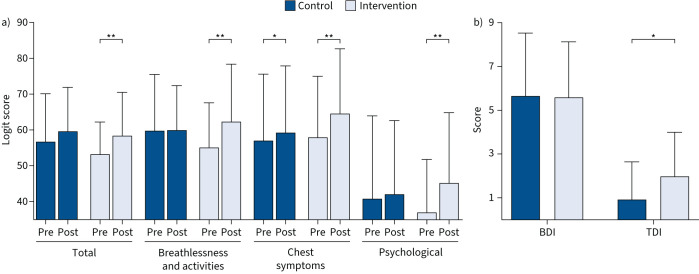
There was no between-group difference post-intervention for K-BILD total score in the ITT population, even though there was a main effect of time (figure 2a), with a significant improvement in the IMT group. In the per-protocol population, a higher K-BILD total score was present in the IMT than control group post-intervention (table 3). Furthermore, significant improvements were found in only the intervention group for the K-BILD breathlessness and activities and psychological domains, while both groups demonstrated improvements in the chest symptoms domain in both the ITT (figure 2a) and per-protocol (table 3) populations. The improvements in the K-BILD total and domain scores were positively associated with adherence in the ITT population (β=0.33–0.49; p≤0.001–0.03). Increased time since COVID-19 was associated with poorer psychological quality of life (β= −0.80, 95% CI −1.38– −0.22; p=0.007).
FIGURE 2.
Pre- and post-intervention self-reported health and breathlessness according to a) King's Brief Interstitial Lung Disease (K-BILD) questionnaire, and b) Baseline Dyspnoea Index (BDI) and Transition Dyspnoea Index (TDI). *: p<0.05; **: p<0.01.
BDI and TDI
While there were no differences between groups pre-intervention in dyspnoea (BDI: 5.60±2.54 versus 5.65±2.89), IMT was associated with a greater reduction in dyspnoea post-intervention in the ITT (TDI: 2.0±2.0 versus 0.9±1.7; p=0.005) (figure 2b) and per-protocol populations (TDI: 2.1±0.2 versus 0.9±0.3; p=0.005).
Inspiratory muscle strength
IMT significantly improved inspiratory muscle strength, with MIP, SMIP and FIT significantly increased post-intervention in the IMT group only, both in the ITT and per-protocol populations (tables 2 and 3, respectively). Furthermore, in the per-protocol population, a greater time since COVID-19 acute infection was associated with lower MIP (β= −1.7, 95% CI −3.3– −0.07; p=0.04).
Physical fitness and functional capacity
IMT led to improvements in functional capacity, with increased estimated V′O2max irrespective of the analysis approach (tables 2 and 3) and greater time spent in moderate physical activity in the IMT group post-intervention in the per-protocol analysis (table 3). Furthermore, IMT elicited a significantly less steep intensity gradient (greater distribution of activity intensities) and increased physical activity during the most active 30 and 60 min post-intervention in the per-protocol population only. These changes in physical activity were accompanied by less sedentary time post-intervention in the IMT group compared with the control group. Functional capacity was not affected by time since acute COVID-19 infection.
Perceived competence and satisfaction
Neither perceived competence nor the domains of the Basic Needs Satisfaction Scale or Treatment Self-Regulation Scale were influenced by IMT, with perceived competence improving over 8 weeks in both groups and populations (ITT and per-protocol; tables 2 and 3). However, perceived competence and autonomous motivation were both associated with time since COVID-19 infection, with greater time periods associated with a lower perceived competence (β= −0.37, 95% CI −0.62– −0.11; p=0.005) and greater autonomous motivation (β=0.23, 95% CI 0.04–0.43; p=0.020). Time since COVID-19 infection was also associated with decreased amotivation in the per-protocol population (β= −0.12, 95% CI −0.23– −0.02; p=0.02).
Withdrawals
In general, no reason was provided for subsequent withdrawals following enrolment (figure 1). Those who withdrew following the pre-intervention testing were significantly younger (42.1±13.0 versus 48.4±11.4 years; p<0.001) and had a higher MIP (86.2±40.2 versus 74.3±31.8 cmH2O; p=0.02), introjected regulation (8.0±3.2 versus 7.1±3.4; p<0.05) and autonomous motivation (37.0±35.4; p=0.04). There was a similar distribution of males (14%) and females (11%) and time since COVID-19 (8.5±5.2 versus 9.3±3.5 months), irrespective of attrition status. While not significant, those who withdrew were more active (moderate physical activity: 85.1±39.2 versus 75.5±39.1 min; p=0.09), less sedentary (732.7±84.7 versus 749.9±108.5 min; p=0.16), and had a higher SMIP (474.2±254.6 versus 438.7±196.5 PTU; p=0.14) and FIT (21.3±21.3 versus 19.2±14.0 AU; p=0.20).
Discussion
In this first randomised controlled trial focusing on a home-based, unsupervised rehabilitation strategy for people recovering from COVID-19, the impact of 8 weeks of IMT on health-related quality of life, breathlessness, respiratory muscle strength and functional capacity was investigated. While there was no clinically meaningful effect of IMT compared with standard care on the primary outcome (K-BILD total score) in the ITT population, such an effect was evident in the per-protocol analyses for those that adhered to the prescribed intervention. Importantly, IMT elicited significant and clinically meaningful improvements in markers of breathlessness (K-BILD domain and TDI) in both the ITT and per-protocol populations, along with concomitant benefits in estimated aerobic fitness and physical activity levels. However, IMT was not associated with significant changes in habitual physical activity or mental health and wellbeing.
The discrepancy in the effect of IMT on the primary outcome in the ITT and per-protocol populations likely reflects the lower, albeit not significantly, total score pre-intervention in those randomly allocated to the IMT arm; the total score significantly and clinically meaningfully increased from pre- to post-intervention in the IMT arm but did not increase significantly above that of the control arm. However, the relatively high withdrawal rate from the study and the nonadherence to the protocol reducing the power associated with our ITT analyses must be noted. Caution is therefore required in interpreting the results, although they are suggestive that IMT is an efficacious home-based rehabilitation strategy when used as prescribed in the recovery after COVID-19.
In accord with the clinical effects of IMT reported here, a recent meta-analysis of the effect of IMT in COPD concluded that IMT was associated with reductions in breathlessness and improvements in quality of life, exercise capacity and MIP [13]. A recent systematic scoping review suggested that IMT exerted largely similar effects in interstitial lung disease [30]. These beneficial effects may be elicited through reductions in the neural respiratory drive and improvements in breathing patterns, which equalise previous imbalances between respiratory muscle loading and capacity [31]. Furthermore, improved respiratory muscle strength and dyspnoea have been associated with reduced diaphragm activation during maximal exercise, compatible with a decreased motor unit recruitment to generate a given force as a result of respiratory muscle hypertrophy [32]. Neural drive impairment and respiratory muscle weakness reported in patients with COVID-19 [33] are supported by the present novel finding of MIP percentage predicted values of 90% pre-intervention. Although the mechanisms underpinning the improvements observed in the current study are hard to conclusively elucidate, given the remote and indirect measures available during COVID-19 lockdowns, the ability of 8 weeks of IMT to elicit improvements in breathlessness indices that are of at least minimal clinically important magnitude is significant on individual and public health levels.
Current estimates suggest over 1.2 million people in the UK are living with long COVID [4], presenting an unprecedented and, as of yet, unmet need for rehabilitation. Following numerous reports of the most persistent symptoms of breathlessness and respiratory muscle weakness [34], pulmonary rehabilitation has been advocated as a rehabilitation strategy [35]. While pulmonary rehabilitation is highly effective in a range of chronic respiratory conditions [36], including COVID-19 [9, 10], pulmonary rehabilitation provision does not, and cannot, meet the COVID-19-related demand due to the time and resource burden it places on an overstretched healthcare system. There is therefore an urgent need to identify home-based, self-guided rehabilitation strategies. Our findings suggest that IMT represents such a strategy that has a notable impact on several critical aspects of long COVID. Furthermore, the remote nature of IMT, requiring minimal supervision or monitoring, meets the recommendations of the Stanford Hall consensus statement for post-COVID-19 rehabilitation [35].
While the present study is highly novel and impactful, it has limitations. Many of the participants did not have confirmed SARS-CoV-2 according to PCR/lateral flow due to lack of available testing when many experienced COVID-19 symptoms. However, in the current definitions of long COVID, a microbiological confirmation is not always required. We believe our population is representative of the target population describing long COVID. Our higher proportion of females also reflects the suggested distribution of long COVID in the general population [37], but it precludes gender comparisons. While we had an 8-week waitlist control group, a learning effect may have been evident in the IMT group as no sham IMT protocol was utilised, converse to other ongoing trials [38]. It is, however, pertinent to note that even low-intensity IMT may elicit a beneficial response, thereby confounding interpretation of the intervention effect. Finally, we were unable to contact many of those who withdrew from the study to ascertain why. This limits our interpretation as to the generalisability of IMT as we cannot preclude the possibility of completion or self-selection bias (including, but not limited to, technological competency, high treatment self-regulation and participant choice) in our findings. While the withdrawal rate from this study may be higher than typically reported in interventions prior to the pandemic, the unique situation in which this intervention was remotely delivered (i.e. national lockdown, rapidly evolving disease and understanding of its aetiology, treatment and recovery) precludes comparisons to pre-pandemic interventions. Furthermore, we sought to minimise the impact of such withdrawals on the statistical power of the study by using a 4:1 allocation ratio, but the findings must nonetheless be interpreted with caution, notably in the ITT population. Despite the withdrawals, the sample size of the present study remains considerably larger than previous IMT trials.
In conclusion, in the first randomised controlled trial investigating the effects of an entirely home-based rehabilitation strategy for those recovering from COVID-19, despite the absence of an effect of IMT on K-BILD-based health-related quality of life, IMT elicited clinically meaningful reductions in the severity of dyspnoea and chest-related symptoms, as well as improved respiratory muscle strength and aerobic fitness. Our findings thus indicate IMT may be an efficacious home-based rehabilitation strategy during recovery from COVID-19.
Shareable PDF
Acknowledgements
Our sincere thanks are extended to Mark Williams (University of South Wales, Pontypridd, UK).
Footnotes
This study is registered in the Health and Care Research Wales Research Directory (reference 48075). De-identified data are available on reasonable request from the corresponding author.
Conflict of interest: The authors have no conflicts of interest to declare.
Support statement: This research was funded by the Welsh Government Sêr Cymru III Tackling COVID-19 grant scheme (reference MA/KW/1457/20) and The Higher Education Funding Council for Wales Research Wales Innovation Fund (Collaboration Booster Faculty Fund) (grant number FF4). The Centre for Physical Activity Research is supported by TrygFonden grants (ID 101390, ID 20045 and ID 125132). All researchers were independent of the funders, had full access to the data, and take responsibility for the integrity of the data and the accuracy of the analysis. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.World Health Organization . WHO Coronavirus (COVID-19) Dashboard. 2021. www.covid19.who.int Date last accessed: 31 January 2022.
- 2.Jimeno-Almazán A, Pallarés JG, Buendía-Romero Á, et al. Post-COVID-19 syndrome and the potential benefits of exercise. Int J Environ Res Public Health 2021; 18: 5329. doi: 10.3390/ijerph18105329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah W, Hillman T, Playford ED, et al. Managing the long term effects of Covid-19: summary of NICE, SIGN, and RCGP rapid guideline. BMJ 2021; 372: n136. doi: 10.1136/bmj.n136 [DOI] [PubMed] [Google Scholar]
- 4.Ayoubkhani D, Pawelek P, Bosworth M. Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK: 4 November 2021. 2021. www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases Date last accessed: 4 November 2021.
- 5.Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021; 38: 101019. doi: 10.1016/j.eclinm.2021.101019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis 2020; 34: 101623. doi: 10.1016/j.tmaid.2020.101623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hutchinson A, Barclay-Klingle N, Galvin K, et al. Living with breathlessness: a systematic literature review and qualitative synthesis. Eur Respir J 2018; 51: 1701477. doi: 10.1183/13993003.01477-2017 [DOI] [PubMed] [Google Scholar]
- 8.Booth S, Johnson MJ. Improving the quality of life of people with advanced respiratory disease and severe breathlessness. Breathe 2019; 15: 198–215. doi: 10.1183/20734735.0200-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu K, Zhang W, Yang Y, et al. Respiratory rehabilitation in elderly patients with COVID-19: a randomized controlled study. Complement Ther Clin Pract 2020; 39: 101166. doi: 10.1016/j.ctcp.2020.101166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zampogna E, Paneroni M, Belli S, et al. Pulmonary rehabilitation in patients recovering from COVID-19. Respiration 2021; 100: 416–422. doi: 10.1159/000514387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desveaux L, Janaudis-Ferreira T, Goldstein R, et al. An international comparison of pulmonary rehabilitation: a systematic review. COPD 2015; 12: 144–153. doi: 10.3109/15412555.2014.922066 [DOI] [PubMed] [Google Scholar]
- 12.Enright SJ, Unnithan VB, Heward C, et al. Effect of high-intensity inspiratory muscle training on lung volumes, diaphragm thickness, and exercise capacity in subjects who are healthy. Phys Ther 2006; 86: 345–354. doi: 10.1093/ptj/86.3.345 [DOI] [PubMed] [Google Scholar]
- 13.Beaumont M, Forget P, Couturaud F, et al. Effects of inspiratory muscle training in COPD patients: a systematic review and meta-analysis. Clin Respir J 2018; 12: 2178–2188. doi: 10.1111/crj.12905 [DOI] [PubMed] [Google Scholar]
- 14.McCreery JL, Mackintosh KA, Mills-Bennett R, et al. The effect of a high-intensity PrO2Fit inspiratory muscle training intervention on physiological and psychological health in adults with bronchiectasis: a mixed-methods study. Int J Environ Res Public Health 2021; 18: 3051. doi: 10.3390/ijerph18063051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Severin R, Arena R, Lavie CJ, et al. Respiratory muscle performance screening for infectious disease management following COVID-19: a highly pressurized situation. Am J Med 2020; 133: 1025–1032. doi: 10.1016/j.amjmed.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel AS, Siegert RJ, Brignall K, et al. The development and validation of the King's Brief Interstitial Lung Disease (K-BILD) health status questionnaire. Thorax 2012; 67: 804–810. doi: 10.1136/thoraxjnl-2012-201581 [DOI] [PubMed] [Google Scholar]
- 17.Sinha A, Patel AS, Siegert RJ, et al. The King's Brief Interstitial Lung Disease (KBILD) questionnaire: an updated minimal clinically important difference. BMJ Open Respir Res 2019; 6: e000363. doi: 10.1136/bmjresp-2018-000363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahler DA, Tomlinson D, Olmstead EM, et al. Changes in dyspnea, health status, and lung function in chronic airway disease. Am J Respir Crit Care Med 1995; 151: 61–65. doi: 10.1164/ajrccm.151.1.7812573 [DOI] [PubMed] [Google Scholar]
- 19.Witek TJ Jr, Mahler DA. Minimal important difference of the transition dyspnoea index in a multinational clinical trial. Eur Respir J 2003; 21: 267–272. doi: 10.1183/09031936.03.00068503a [DOI] [PubMed] [Google Scholar]
- 20.Bennett H, Parfitt G, Davison K, et al. Validity of submaximal step tests to estimate maximal oxygen uptake in healthy adults. Sports Med 2016; 46: 737–750. doi: 10.1007/s40279-015-0445-1 [DOI] [PubMed] [Google Scholar]
- 21.Ricardo LIC, Wendt A, Galliano LM, et al. Number of days required to estimate physical activity constructs objectively measured in different age groups: findings from three Brazilian (Pelotas) population-based birth cohorts. PLoS One 2020; 15: e0216017. doi: 10.1371/journal.pone.0216017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin Y, Tremblay MS, Katzmarzyk PT, et al. Temporal and bi-directional associations between sleep duration and physical activity/sedentary time in children: an international comparison. Prev Med 2018; 111: 436–441. doi: 10.1016/j.ypmed.2017.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hildebrand M, van Hees VT, Hansen BH, et al. Age group comparability of raw accelerometer output from wrist- and hip-worn monitors. Med Sci Sports Exerc 2014; 46: 1816–1824. doi: 10.1249/MSS.0000000000000289 [DOI] [PubMed] [Google Scholar]
- 24.Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am Psychol 2000; 55: 68–78. doi: 10.1037/0003-066X.55.1.68 [DOI] [PubMed] [Google Scholar]
- 25.Deci EL, Ryan RM. The “what” and “why” of goal pursuits: human needs and the self-determination of behavior. Psychological Inquiry 2000; 11: 227–268. doi: 10.1207/S15327965PLI1104_01 [DOI] [Google Scholar]
- 26.Williams GC, Deci EL. Internalization of biopsychosocial values by medical students: a test of self-determination theory. J Pers Soc Psychol 1996; 70: 767–779. doi: 10.1037/0022-3514.70.4.767 [DOI] [PubMed] [Google Scholar]
- 27.Ryan RM, Connell JP. Perceived locus of causality and internalization: examining reasons for acting in two domains. J Pers Soc Psychol 1989; 57: 749–761. doi: 10.1037/0022-3514.57.5.749 [DOI] [PubMed] [Google Scholar]
- 28.Gagné M. The role of autonomy support and autonomy orientation in prosocial behavior engagement. Motiv Emotion 2003; 27: 199–223. doi: 10.1023/A:1025007614869 [DOI] [Google Scholar]
- 29.Dumville JC, Hahn S, Miles JNV, et al. The use of unequal randomisation ratios in clinical trials: a review. Contemp Clin Trials 2006; 27: 1–12. doi: 10.1016/j.cct.2005.08.003 [DOI] [PubMed] [Google Scholar]
- 30.Hoffman M. Inspiratory muscle training in interstitial lung disease: a systematic scoping review. J Bras Pneumol 2021; 47: e20210089. doi: 10.36416/1806-3756/e20210089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charususin N, Dacha S, Gosselink R, et al. Respiratory muscle function and exercise limitation in patients with chronic obstructive pulmonary disease: a review. Expert Rev Respir Med 2018; 12: 67–79. doi: 10.1080/17476348.2018.1398084 [DOI] [PubMed] [Google Scholar]
- 32.Langer D, Ciavaglia C, Faisal A, et al. Inspiratory muscle training reduces diaphragm activation and dyspnea during exercise in COPD. J Appl Physiol 2018; 125: 381–392. doi: 10.1152/japplphysiol.01078.2017 [DOI] [PubMed] [Google Scholar]
- 33.Anastasio F, Barbuto S, Scarnecchia E, et al. Medium-term impact of COVID-19 on pulmonary function, functional capacity and quality of life. Eur Respir J 2021; 58: 2004015. doi: 10.1183/13993003.04015-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carfì A, Bernabei R, Landi F, et al. Persistent symptoms in patients after acute COVID-19. JAMA 2020; 324: 603–605. doi: 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barker-Davies RM, O'Sullivan O, Senaratne KPP, et al. The Stanford Hall consensus statement for post-COVID-19 rehabilitation. Br J Sports Med 2020; 54: 949–959. doi: 10.1136/bjsports-2020-102596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCarthy B, Casey D, Devane D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015; 2: CD003793. doi: 10.1002/14651858.CD003793.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med 2021; 27: 626–631. doi: 10.1038/s41591-021-01292-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maldaner V, Coutinho J, Santana A, et al. Adjunctive inspiratory muscle training for patients with COVID-19 (COVIDIMT): protocol for randomised controlled double-blind trial. BMJ Open 2021; 11: e049545. doi: 10.1136/bmjopen-2021-049545 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This one-page PDF can be shared freely online.
Shareable PDF ERJ-03101-2021.Shareable (385.9KB, pdf)