Abstract
β-Lactamases continue to be the leading cause of resistance to β-lactam antibiotics among gram-negative bacteria. In recent years there has been an increased incidence and prevalence of extended-spectrum β-lactamases (ESBLs), enzymes that hydrolyze and cause resistance to oxyimino-cephalosporins and aztreonam. The majority of ESBLs are derived from the widespread broad-spectrum β-lactamases TEM-1 and SHV-1. There are also new families of ESBLs, including the CTX-M and OXA-type enzymes as well as novel, unrelated β-lactamases. Several different methods for the detection of ESBLs in clinical isolates have been suggested. While each of the tests has merit, none of the tests is able to detect all of the ESBLs encountered. ESBLs have become widespread throughout the world and are now found in a significant percentage of Escherichia coli and Klebsiella pneumoniae strains in certain countries. They have also been found in other Enterobacteriaceae strains and Pseudomonas aeruginosa. Strains expressing these β-lactamases will present a host of therapeutic challenges as we head into the 21st century.
INTRODUCTION AND HISTORY
Emergence of resistance to β-lactam antibiotics began even before the first β-lactam, penicillin, was developed. The first β-lactamase was identified in Escherichia coli prior to the release of penicillin for use in medical practice (1). The age of penicillin saw the rapid emergence of resistance in Staphylococcus aureus due to a plasmid-encoded penicillinase. This β-lactamase quickly spread to most clinical isolates of S. aureus as well as other species of staphylococci.
Many genera of gram-negative bacteria possess a naturally occurring, chromosomally mediated β-lactamase. These enzymes are thought to have evolved from penicillin-binding proteins, with which they show some sequence homology. This development was likely due to the selective pressure exerted by β-lactam-producing soil organisms found in the environment (61). The first plasmid-mediated β-lactamase in gram-negatives, TEM-1, was described in the early 1960s (48). The TEM-1 enzyme was originally found in a single strain of E. coli isolated from a blood culture from a patient named Temoniera in Greece, hence the designation TEM (96). Being plasmid and transposon mediated has facilitated the spread of TEM-1 to other species of bacteria. Within a few years after its first isolation, the TEM-1 β-lactamase spread worldwide and is now found in many different species of members of the family Enterobacteriaceae, Pseudomonas aeruginosa, Haemophilus influenzae, and Neisseria gonorrhoeae. Another common plasmid-mediated β-lactamase found in Klebsiella pneumoniae and E. coli is SHV-1 (for sulphydryl variable). The SHV-1 β-lactamase is chromosomally encoded in the majority of isolates of K. pneumoniae but is usually plasmid mediated in E. coli.
Over the last 20 years, many new β-lactam antibiotics have been developed that were specifically designed to be resistant to the hydrolytic action of β-lactamases. However, with each new class that has been used to treat patients, new β-lactamases emerged that caused resistance to that class of drug. Presumably, the selective pressure of the use and overuse of new antibiotics in the treatment of patients has selected for new variants of β-lactamase. One of these new classes was the oxyimino-cephalosporins, which became widely used for the treatment of serious infections due to gram-negative bacteria in the 1980s.
Not surprisingly, resistance to these expanded-spectrum β-lactam antibiotics due to β-lactamases emerged quickly. The first of these enzymes capable of hydrolyzing the newer β-lactams, SHV-2, was found in a single strain of Klebsiella ozaenae isolated in Germany (81). Because of their increased spectrum of activity, especially against the oxyimino-cephalosporins, these enzymes were called extended-spectrum β-lactamases (ESBLs). Today, over 150 different ESBLs have been described. These β-lactamases have been found worldwide in many different genera of Enterobacteriaceae and P. aeruginosa. This review will focus on the characterization of ESBLs, the importance of detection of these enzymes, and their epidemiology.
CHARACTERIZATION OF ESBLS
Functional and Molecular Grouping
The majority of ESBLs contain a serine at the active site and belong to Ambler's molecular class A (4). Class A enzymes are characterized by an active-site serine, a molecular mass of approximately 29,000 Da, and the preferential hydrolysis of penicillins (95). Class A β-lactamases include such enzymes as TEM-1, SHV-1, and the penicillinase found in S. aureus. The molecular classification scheme is still used to characterize β-lactamases; however, it does not sufficiently differentiate the many different types of class A enzymes. The classification scheme of Richmond and Sykes was based on the substrate profile and the location of the gene encoding the β-lactamase (145). This classification scheme was developed before ESBLs arose, and it did not allow for the differentiation between the original TEM and SHV enzymes and their ESBL derivatives. More recently, a classification scheme was devised by Bush, Jacoby, and Medeiros that uses the biochemical properties of the enzyme plus the molecular structure and nucleotide sequence of the genes to place β-lactamases into functional groups (32). Using this scheme, ESBLs are defined as β-lactamases capable of hydrolyzing oximino-cephalosporins that are inhibited by clavulanic acid and are placed into functional group 2be (32).
Susceptibility and Biochemical Characteristics
ESBLs contain a number of mutations that allow them to hydrolyze expanded-spectrum β-lactam antibiotics. While TEM- and SHV-type ESBLs retain their ability to hydrolyze penicillins, they are not catalytically as efficient as the parent enzymes (33). In addition, the expansion of the active site that allows the increased activity against expanded-spectrum cephalosporins may also result in the increased susceptibility of ESBLs to β-lactamase inhibitors (74). ESBLs are not active against cephamycins, and most strains expressing ESBLs are susceptible to cefoxitin and cefotetan. However, it has been reported that ESBL-producing strains can become resistant to cephamycins due to the loss of an outer membrane porin protein (92, 121, 181).
TYPES OF ESBLS
Most ESBLs are derivatives of TEM or SHV enzymes (32, 74). There are now >90 TEM-type β-lactamases and >25 SHV-type enzymes (for amino acid sequences for TEM, SHV, and OXA extended-spectrum and inhibitor-resistant β-lactamases, see http://www.lahey.org/studies/webt.htm). With both of these groups of enzymes, a few point mutations at selected loci within the gene give rise to the extended-spectrum phenotype. TEM- and SHV-type ESBLs are most often found in E. coli and K. pneumoniae; however, they have also been found in Proteus spp., Providencia spp., and other genera of Enterobacteriaceae.
TEM
TEM-1 is the most commonly encountered β-lactamase in gram-negative bacteria. Up to 90% of ampicillin resistance in E. coli is due to the production of TEM-1 (85). This enzyme is also responsible for the ampicillin and penicillin resistance that is seen in H. influenzae and N. gonorrhoeae in increasing numbers. TEM-1 is able to hydrolyze penicillins and early cephalosporins such as cephalothin and cephaloridine. TEM-2, the first derivative of TEM-1, had a single amino acid substitution from the original β-lactamase (10). This caused a shift in the isoelectric point from a pI of 5.4 to 5.6, but it did not change the substrate profile. TEM-3, originally reported in 1989, was the first TEM-type β-lactamase that displayed the ESBL phenotype (157). In the years since that first report, over 90 additional TEM derivatives have been described (for amino acid sequences for TEM, SHV, and OXA extended-spectrum and inhibitor-resistant β-lactamases, see http://www.lahey.org /studies/webt.htm). Some of these β-lactamases are inhibitor-resistant enzymes, but the majority of the new derivatives are ESBLs.
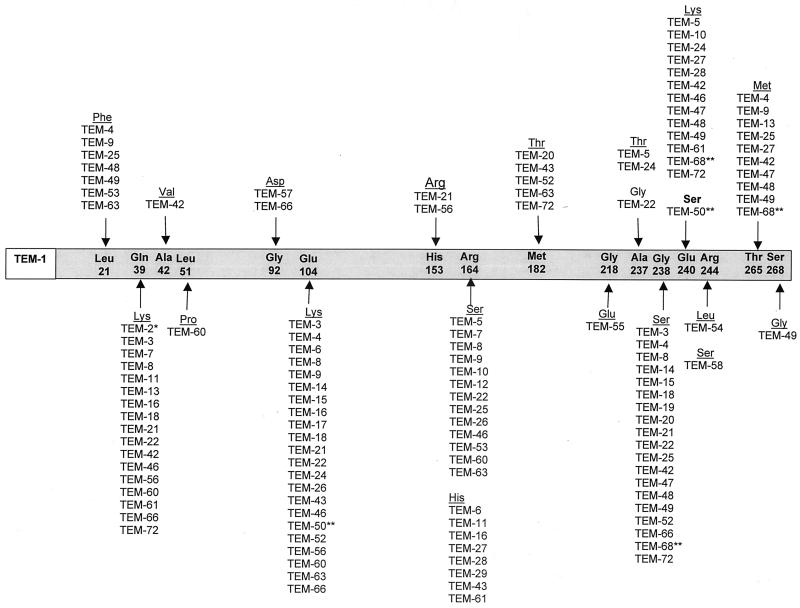
As shown in Fig. 1, the amino acid substitutions that occur within the TEM enzyme occur at a limited number of positions. The combinations of these amino acid changes result in various subtle alterations in the ESBL phenotypes, such as the ability to hydrolyze specific oxyimino-cephalosporins such as ceftazidime and cefotaxime, or a change in their isoelectric points, which can range from a pI of 5.2 to 6.5 (Table 1). A number of amino acid residues are especially important for producing the ESBL phenotype when substitutions occur at that position. They include glutamate to lysine at position 104, arginine to either serine or histidine at position 164, glycine to serine at position 238, and glutamate to lysine at position 240 (Fig. 1). In addition to β-lactamases TEM-1 through TEM-92 shown in Fig. 1 and Table 1, there has been a report of a naturally occurring TEM-like enzyme, TEM-AQ, that contained a number of amino acid substitutions and one amino acid deletion that have not been noted in other TEM enzymes (127).
FIG. 1.
Amino acid substitutions in TEM ESBL derivatives. The amino acids listed within the grey bar are those found in the structural gene of the TEM-1 β-lactamase (162). The amino acid numbering is according to the scheme of Ambler et al. (5). Substitutions found in TEM-type ESBL derivatives are shown under the amino acids of TEM-1. TEM-type variants may contain more than one amino acid substitution. ∗, TEM-2 is not an ESBL but is included in the figure as a derivative of TEM-1. The Gln39Lys substitution does not contribute to the ESBL phenotype, but a number of ESBLs are derived from TEM-2. ∗∗, TEM-50 and TEM-68 contain amino acid substitutions that are common to both the ESBL and the IRT phenotypes. Only the amino acid substitutions that are common to TEM-type ESBLs are shown in this figure.
TABLE 1.
Characteristics of TEM-type β-lactamasesa
| pI | Enzymes | Enzyme type
|
||
|---|---|---|---|---|
| Broad spectrum | ESBL | IRT | ||
| 5.2 | TEM-12, TEM-55, TEM-57, TEM-58 | X | ||
| TEM-30, TEM-31, TEM-35, TEM-36, TEM-37, TEM-38, TEM-41, TEM-45, TEM-51, TEM-73, TEM-74 | X | |||
| 5.3 | TEM-25 | X | ||
| 5.4 | TEM-1 | X | ||
| TEM-7, TEM-19, TEM-20, TEM-65 | X | |||
| TEM-32, TEM-33, TEM-34, TEM-39, TEM-40, TEM-44 | X | |||
| 5.42 | TEM-29 | X | ||
| 5.55 | TEM-5, TEM-17 | X | ||
| 5.59 | TEM-9 | X | ||
| 5.6 | TEM-2 | X | ||
| TEM-10, TEM-11, TEM-13, TEM-26, TEM-63 | X | |||
| TEM-50 | X | X | ||
| TEM-59 | X | |||
| 5.7 | TEM-68 | X | X | |
| 5.8 | TEM-42 | X | ||
| 5.9 | TEM-4, TEM-6, TEM-8, TEM-27, TEM-72 | X | ||
| 6.0 | TEM-15, TEM-47, TEM-48, TEM-49, TEM-52, TEM-66, TEM-92 | X | ||
| 6.1 | TEM-28, TEM-43 | X | ||
| 6.3 | TEM-3, TEM-16, TEM-21, TEM-22 | X | ||
| 6.4 | TEM-56, TEM-60 | X | ||
| 6.5 | TEM-24, TEM-46, TEM-61 | X | ||
| Not determined | TEM-14, TEM-53, TEM-54 | X | ||
| TEM-76, TEM-77, TEM-78, TEM-79, TEM-81, TEM-82, TEM-83, TEM-84 | X | |||
Amino acid sequences for TEM, SHV, and OXA extended-spectrum and inhibitor-resistant β-lactamases may be found at http://www.lahey.org/studies/webt.htm. All enzymes listed are naturally occurring mutants.
It is interesting that laboratory mutants of TEM-1 that contain mutations at positions other than the ones described in nature have been constructed (18, 130, 180, 182). It has been suggested that the naturally occurring TEM-type ESBLs are the result of fluctuating selective pressure from several β-lactams within a given institution rather than selection with a single agent (18). Although TEM-type β-lactamases are most often found in E. coli and K. pneumoniae, they are also found in other species of gram-negative bacteria with increasing frequency. TEM-type ESBLs have been reported in genera of Enterobacteriaceae such as Enterobacter aerogenes, Morganella morganii, Proteus mirabilis, Proteus rettgeri, and Salmonella spp. (19, 91, 101, 120, 128, 166). Furthermore, TEM-type ESBLs have been found in non-Enterobacteriaceae gram-negative bacteria. The TEM-42 β-lactamase was found in a strain of P. aeruginosa (103). Additionally, a recent report found the TEM-17 β-lactamase being expressed from a plasmid in a blood culture isolate of Capnocytophaga ochracea (146).
Inhibitor-Resistant β-Lactamases
Although the inhibitor-resistant β-lactamases are not ESBLs, they are often discussed with ESBLs because they are also derivatives of the classical TEM- or SHV-type enzymes. In the early 1990s β-lactamases that were resistant to inhibition by clavulanic acid were discovered. Nucleotide sequencing revealed that these enzymes were variants of the TEM-1 or TEM-2 β-lactamase. These enzymes were at first given the designation IRT for inhibitor-resistant TEM β-lactamase; however, all have subsequently been renamed with numerical TEM designations. There are at least 19 distinct inhibitor-resistant TEM β-lactamases (for amino acid sequences for TEM, SHV and OXA extended-spectrum and inhibitor resistant β-lactamases, see http://www.lahey.org/studies/webt.htm). Inhibitor-resistant TEM β-lactamases have been found mainly in clinical isolates of E. coli, but also some strains of K. pneumoniae, Klebsiella oxytoca, P. mirabilis, and Citrobacter freundii (31, 83). Although the inhibitor-resistant TEM variants are resistant to inhibition by clavulanic acid and sulbactam, thereby showing clinical resistance to the β-lactam–β-lactamase inhibitor combinations of amoxicillin-clavulanate, ticarcillin-clavulanate, and ampicillin-sulbactam, they remain susceptible to inhibition by tazobactam and subsequently the combination of piperacillin and tazobactam (23, 37). To date, these β-lactamases have primarily been detected in France and a few other locations within Europe (37). In a recent survey of amoxicillin-clavulanate-resistant E. coli in a hospital in France, Leflon-Guibout et al. found that up to 41% of these isolates produced inhibitor-resistant TEM variants (82). Although these enzymes have not yet been reported in isolates originating in the United States, it is likely that they will eventually be detected here as well.
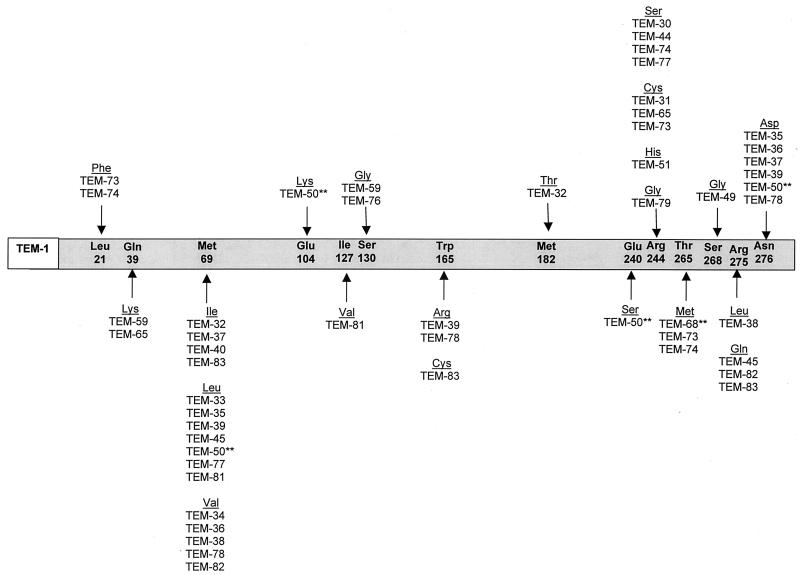
As shown in Fig. 2, point mutations that lead to the inhibitor-resistant phenotype occur at a few specific amino acid residues within the structural gene for the TEM enzyme, Met-69, Arg-244, Arg-275, and Asn-276 (16, 66, 191). The sites of these amino acid substitutions are distinct from those that lead to the ESBL phenotype. Laboratory mutants that contain amino acid substitutions which are common to both the IRT and the ESBL phenotype have been constructed (159). These strains were found to possess either the ESBL or IRT phenotype, but not both. However, the TEM-50 enzyme, which had amino acid substitutions common to both the ESBL and inhibitor-resistant TEMs, was recently identified. This enzyme was resistant to inhibition by clavulanate, but it also conferred a slight resistance to the expanded-spectrum cephalosporins (154). This could indicate the possibility of a new group of β-lactamases with a complex phenotype sharing some characteristics of ESBLs and inhibitor-resistant enzymes. In addition to the variants of TEM, inhibitor-resistant variants of SHV-1 and the related enzyme OHIO-1 have been detected (22, 137).
FIG. 2.
Amino acid substitutions in TEM IRT derivatives. The amino acids listed within the grey bar are those found in the structural gene of the TEM-1 β-lactamase (162). The amino acid numbering is according to the scheme of Ambler et al. (5). Substitutions found in TEM-type IRT derivatives are shown under the amino acids of TEM-1. TEM-type variants may contain more than one amino acid substitution. ∗∗, TEM-50 and TEM-68 contain amino acid substitutions that are common to both the ESBL and the IRT phenotypes. Only the amino acid substitutions that are common to TEM-type IRTs are shown in this figure.
SHV
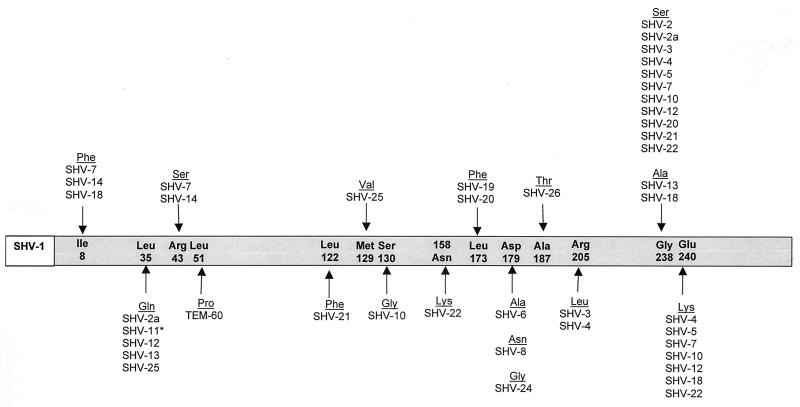
The SHV-1 β-lactamase is most commonly found in K. pneumoniae and is responsible for up to 20% of the plasmid-mediated ampicillin resistance in this species (172). In many strains of K. pneumoniae, blaSHV-1 or a related gene is integrated into the bacterial chromosome (85). Although it has been hypothesized that the gene encoding SHV-1 may exist as part of a transposable element, it has never been proven (75). Unlike the TEM-type β-lactamases, there are relatively few derivatives of SHV-1 (Table 2). Furthermore, the changes that have been observed in blaSHV to give rise to the SHV variants occur in fewer positions within the structural gene (Fig. 3). The majority of SHV variants possessing an ESBL phenotype are characterized by the substitution of a serine for glycine at position 238. A number of variants related to SHV-5 also have a substitution of lysine for glutamate at position 240. It is interesting that both the Gly238Ser and Glu240Lys amino acid substitutions mirror those seen in TEM-type ESBLs. The serine residue at position 238 is critical for the efficient hydrolysis of ceftazidime, and the lysine residue is critical for the efficient hydrolysis of cefotaxime (69).
TABLE 2.
Characteristics of SHV-type β-lactamasesa
| pI | Enzymes | Enzyme type
|
||
|---|---|---|---|---|
| Broad spectrum | ESBL | Inhibitor resistant | ||
| 7.0 | OHIO-1, LEN-1 | X | ||
| SHV-3, SHV-14 | X | |||
| 7.5 | SHV-24 | X | ||
| 7.6 | SHV-1, SHV-11 | X | ||
| SHV-2, SHV-2a, SHV-6, SHV-8, SHV-13, SHV-19, SHV-20, SHV-21, SHV-22 | X | |||
| 7.8 | SHV-4, SHV-7b, SHV-18 | X | ||
| 8.2 | SHV-5, SHV-9, SHV-12 | X | ||
| SHV-10 | X | |||
Amino acid sequences for TEM, SHV, and OXA extended-spectrum and inhibitor-resistant β-lactamases may be found at http://www.lahey.org/studies/webt.htm. All enzymes listed are naturally occurring mutants.
SHV-7 was reported to have a pI of 7.6 (27), but further examination of this enzyme indicates that the pI is most likely to be 7.8. This enzyme shows an interesting phenomenon in that the isoelectric point varies depending on the day of testing and the purity of the enzyme (unpublished observations).
FIG. 3.
Amino acid substitutions in SHV ESBL derivatives. The amino acids listed within the grey bar are those found in the structural gene of the SHV-1 β-lactamase (25). The amino acid numbering is according to the scheme of Ambler et al. (5). Substitutions found in SHV-type ESBL derivatives are shown under the amino acids of SHV-1. SHV-type variants may contain more than one amino acid substitution. ∗, SHV-11 is not an ESBL but is included in the figure as a derivative of SHV-1.
To date, the majority of SHV-type derivatives possess the ESBL phenotype. However, one variant, SHV-10, is reported to have an inhibitor-resistant phenotype. This enzyme appears to be derived from SHV-5 and contains one additional amino acid substitution of glycine for serine 130 (137). It is interesting that the inhibitor-resistant phenotype conferred by the Ser140Gly mutation seems to override the strong ESBL phenotype usually seen in enzymes containing the Gly238Ser and the Glu240Lys mutations seen in other SHV-5-type enzymes. The majority of SHV-type ESBLs are found in strains of K. pneumoniae. However, these enzymes have also been found in Citrobacter diversus, E. coli, and P. aeruginosa (27, 51, 108, 139).
CTX-M
In recent years a new family of plasmid-mediated ESBLs, called CTX-M, that preferentially hydrolyze cefotaxime has arisen. They have mainly been found in strains of Salmonella enterica serovar Typhimurium and E. coli, but have also been described in other species of Enterobacteriaceae (Table 3). They include the CTX-M-type enzymes CTX-M-1 (formerly called MEN-1), CTX-M-2 through CTX-M-10 (9, 11, 12, 13, 21, 29, 58, 59, 64, 148; A. Oliver, J. C. Pérez-Díaz, T. M. Coque, F. Baquero, and R. Cantón, 40th Intersci. Conf. Antmicrob. Agents Chemother., abstr. 1480, 2000) as well as Toho enzymes 1 and 2 (72, 88).
TABLE 3.
Characteristics of CTX-M-type ESBLs
| β-Lactamase | Alternative name | pI | Country of origin | Bacterial species | Reference(s) |
|---|---|---|---|---|---|
| CTX-M-1 | MEN-1 | 8.9 | Germany, Italy | E. coli | 12, 13 |
| CTX-M-2 | 7.9 | Argentina | S. entericaa | 11, 13 | |
| CTX-M-3 | 8.4 | Poland | C. freundii, E. coli | 64 | |
| CTX-M-4 | 8.4 | Russia | S. enterica | 57, 59 | |
| CTX-M-5 | CTX-M-3 | 8.8 | Latvia | S. enterica | 29 |
| CTX-M-6 | 8.4 | Greece | S. enterica | 58, 173 | |
| CTX-M-7 | CTX-M-5 | 8.4 | Greece | S. enterica | 58, 173 |
| CTX-M-8 | 7.6 | Brazil | P. mirabilis, E. cloacae, E. aerogenes, C. amalonaticus | 21 | |
| CTX-M-9 | 8.0 | Spain | E. coli | 148 | |
| CTX-M-10 | 8.1 | Spain | E. coli | Oliver et al.b | |
| Toho-1 | 7.8 | Japan | E. coli | 72 | |
| Toho-2 | 7.7 | Japan | E. coli | 88 |
All strains of S. enterica were serovar Typhimurium.
A. Oliver, J. C. Pérez-Díaz, T. M. Coque, F. Baquero, and R. Cantón, 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1480, 2000.
These enzymes are not very closely related to TEM or SHV β-lactamases in that they show only approximately 40% identity with these two commonly isolated β-lactamases (174). Previously, the most closely related enzymes outside this family were thought to be the chromosomally encoded class A cephalosporinases found in K. oxytoca, C. diversus, Proteus vulgaris, and Serratia fonticola (73 to 77% homology) (13, 19). However, it was recently reported by Humeniuk et al. that there is a high degree of homology between the chromosomal AmpC enzyme of Kluyvera ascorbata (designated Klu-1 and Klu-2) and the CTX-M-type enzymes, suggesting that the latter probably originated from this species (G. Humeniuk, G. Arlet, R. Labia, P. Grimont, and A. Philippon, Abstr. Reunion Interdis. Chimiother. Anti-infect., abstr. 20/C4, 2000) A phylogenetic study of the CTX-M family of β-lactamases showed four major types: the CTX-M-1 type, including CTX-M-1, and CTX-M-3; the CTX-M-2 type, including CTX-M-2, CTX-M-4, CTX-M-5, CTX-M-6, CTX-M-7, and Toho-1; Toho-2; and CTX-M-8, the latter two groups containing only one member to date (21). The evolutionary distances between each of these groupings suggest an early divergence from a common ancestor (21).
Kinetic studies have shown that the CTX-M-type β-lactamases hydrolyze cephalothin or cephaloridine better than benzylpenicillin and they preferentially hydrolyze cefotaxime over ceftazidime (29, 174). Although there is some hydrolysis of ceftazidime by these enzymes, it is usually not enough to provide clinical resistance to organisms in which they reside. It has been suggested that the serine residue at position 237, which is present in all of the CTX-M enzymes, plays an important role in the extended-spectrum activity of the CTX-M-type β-lactamases (174). Although it has been shown not to be essential, the Arg-276 residue lies in a position equivalent to Arg-244 in TEM- or SHV-type ESBLs, as suggested by molecular modeling, and may also play a role in the hydrolysis of oxyimino-cephalosporins (56). Recent crystallographic data for the Toho-1 enzyme suggested that there was increased flexibility of the interacting β3 strand and Ω loop of this enzyme in comparison to other class A β-lactamases. Furthermore, the lack of hydrogen bonds in the vicinity of the Ω loop could account for the extended-spectrum phenotype (71). In addition to the rapid hydrolysis of cefotaxime, another unique feature of these enzymes is that they are inhibited better by the β-lactamase inhibitor tazobactam than by sulbactam and clavulanate (29, 88, 148, 174).
Strains expressing CTX-M-type β-lactamases have been isolated from many parts of the world, but have most often been associated with focal outbreaks in eastern Europe (29, 57, 64), South America, and Japan (88). There have been a few reports of these enzymes in isolates from patients in western Europe, mostly in isolates from immigrants from the outbreak areas (173). However, Sabeté et al. recently reported that 23 strains of E. coli and Salmonella isolated in Spain expressed the CTX-M-9 β-lactamase, suggesting that there may be an endemic focus of this enzyme in western Europe as well (148). Moreover, a CTX-M-3-producing strain of Enterobacter cloacae was recently isolated in France (50). Several institutions in the areas where outbreaks have occurred reported that the CTX-M-type enzyme is the most frequently isolated ESBL among clinical isolates in their laboratories (148).
Interestingly, a number of these enzymes have been found among isolates of Salmonella enterica serovar Typhimurium (11, 29, 57, 58, 173). Large outbreaks of isolates of S. enterica serovar Typhimurium expressing CTX-M β-lactamases have occurred in both South America and eastern Europe. These isolates have also been found to express a variety of CTX-M-type variants. Therefore, it is unlikely that a single origin for the occurrence and propensity of this type of β-lactamase among S. enterica serovar Typhimurium can be found.
OXA
The OXA-type enzymes are another growing family of ESBLs. These β-lactamases differ from the TEM and SHV enzymes in that they belong to molecular class D and functional group 2d (32). The OXA-type β-lactamases confer resistance to ampicillin and cephalothin and are characterized by their high hydrolytic activity against oxacillin and cloxacillin and the fact that they are poorly inhibited by clavulanic acid (32). The OXA β-lactamase family was originally created as a phenotypic rather than a genotypic group for a few β-lactamases that had a specific hydrolysis profile. Therefore, there is as little as 20% sequence homology among some of the members of this family. However, recent additions to this family show some degree of homology to one or more of the existing members of the OXA β-lactamase family.
While most ESBLs have been found in E. coli, K. pneumoniae, and other Enterobacteriaceae, the OXA-type ESBLs have been found mainly in P. aeruginosa (Table 4). Several of the OXA-type ESBLs have been derived from OXA-10 (OXA-11, -14, -16, and -17) (44, 45, 65, 104). OXA-14 differs from OXA-10 by only one amino acid residue, OXA-11 and OXA-16 differ by two, and OXA-13 and OXA-19 differ by nine (Table 4). Among the enzymes related to OXA-10, the ESBL variants have one of two amino acid substitutions: an asparagine for serine at position 73, or an aspartate for glycine at position 157. In particular, the Gly157Asp substitution may be necessary for high-level resistance to ceftazidime (44). It appears that either of these mutations may be required to confer the ESBL phenotype on the OXA-type variant. In addition to the OXA-10 group, OXA-15 is a derivative of OXA-2, and OXA-18 is not directly derived from other OXA-type enzymes (closest relative is OXA-9, with 42% homology) (Table 4) (131).
TABLE 4.
Characteristics of OXA-type ESBLs
| β-Lactamase | Derivation | pI | Amino acid substitutions vs. OXA-10 | Country of origin | Bacterial species | Reference |
|---|---|---|---|---|---|---|
| OXA-11 | OXA-10 | 6.4 | Asn143Ser, Gly157Asp | Turkey | P. aeruginosa | 65 |
| OXA-13 | OXA-10 | 8.0 | Ile10Thr, Gly20Ser, Asp55N, Asn73Ser, Thr107Ser, Tyr174Phe, Glu229Gly, Ser245Asn, Glu259Ala | France | P. aeruginosa | 104 |
| OXA-14 | OXA-10 | 6.2 | Gly157Asp | Turkey | P. aeruginosa | 45 |
| OXA-15 | OXA-2 | 8.7, 8.9 doublet | NAa | Turkey | P. aeruginosa | 46 |
| OXA-16 | OXA-10 | 6.2 | Ala124Thr, Gly157Asp | Turkey | P. aeruginosa | 47 |
| OXA-17 | OXA-10 | 6.1 | Asn73Ser | Turkey | P. aeruginosa | 44 |
| OXA-18 | OXA-9, OXA-12 | 5.5 | NA | France | P. aeruginosa | 131 |
| OXA-19 | OXA-10 | 7.6 | Ile10Thr, Gly20Ser, Asp55Asn, Thr107Ser, Gly157Asp, Tyr174Phe, Glu229Gly, Ser245Asn, Glu259Ala | France | P. aeruginosa | 102 |
| OXA-28 | OXA-10 | 7.6 | Ile10Thr, Gly20Ser, Thr107Ser, Trp154Gly, Gly157Asp, Tyr174Phe, Glu229Gly, Ser245Asn, Glu259Ala | France | P. aeruginosa | 134 |
NA, not applicable; these enzymes do not originate from OXA-10.
The OXA-type ESBLs provide weak resistance to oxyimino-cephalosporins when cloned into E. coli, but provide fairly high-level resistance in P. aeruginosa transconjugants (65). In contrast to the majority of the OXA-type ESBLs, which confer resistance to ceftazidime, the OXA-17 β-lactamase confers resistance to cefotaxime and ceftriaxone but provides only marginal protection against ceftazidime (44). With respect to β-lactamase inhibitors, the original OXA enzymes were characterized by their lack of inhibition by clavulanic acid; however, the OXA-18 β-lactamase was reported to be inhibited by this compound (131). One additional OXA-type enzyme has been identified, OXA-21 (184). This enzyme was found in a strain of Acinetobacter baumannii and is the first incidence of an OXA-type enzyme's originating in this organism. Because the clinical isolate of A. baumannii also expressed two other β-lactamases, it is unclear whether OXA-21 is an ESBL or an original-spectrum enzyme (184).
In addition to the OXA-type ESBLs, a number of recent OXA derivatives that are not ESBLs have also been described. These include OXA-20 (110), OXA-22 (115), OXA-24 (24), OXA-25, -26, and -27 (2), and OXA-30 (155). Many of the newer members of the OXA β-lactamase family have been found in bacterial isolates originating in Turkey and in France. It is not certain whether these two countries represent foci of strains harboring these enzymes or if they represent the locale of the investigators studying these β-lactamases.
Other ESBLs
While the majority of ESBLs are derived from TEM or SHV β-lactamases and others can be categorized with one of the newer families of ESBLs, a few ESBLs have been reported that are not closely related to any of the established families of β-lactamases (Table 5). The PER-1 β-lactamase was first discovered in strains of P. aeruginosa isolated from patients in Turkey (113). Later, it was also found among isolates of S. enterica serovar Typhimurium and A. baumanii (176, 177, 179). The PER-1 β-lactamase is widespread across Turkey and is found in up to 60% of ceftazidime-resistant strains of A. baumanii, which represent 46% of total isolates (179). A common plasmid encoding PER-1 was found in multiple nosocomial isolates of S. enterica serovar Typhimurium, suggesting that the strains acquired the resistance plasmids in the hospital setting (176). A related enzyme, PER-2, which has 86% amino acid homology with PER-1, was found among S. enterica serovar Typhimurium strains in Argentina. (14). It is interesting that PER-1 is found almost exclusively in Turkey, while PER-2 has been found almost exclusively in South America.
TABLE 5.
Characteristics of novel, unrelated ESBLs
| β-Lactamase | Closest relative | pI | Preferred substratea | Country of origin | Bacterial species | Reference |
|---|---|---|---|---|---|---|
| BES-1 | Penicllinase from Yersinia enterocolitica | 7.5 | CTX, CAZ, ATM | Brazil | S. marcescens | 20 |
| FEC-1 | 8.2 | CTX | Japan | E. coli | 93 | |
| GES-1 | Penicillinase from P. mirabilis | 5.8 | CAZ | French Guiana | K. pneumoniae | 136 |
| CME-1 | VEB-1 | >9.0 | CAZ | Isolated from reference strain | Chryseobacterium meningosepticum | 147 |
| PER-1 | PER-2 | 5.4 | CAZ | France | P. aeruginosa | 113 |
| PER-2 | PER-1 | 5.4 | CAZ | Argentina | S. enterica serovar Typhimurium | 14 |
| SFO-1 | AmpA from S. fonticola | 7.3 | CTX | Japan | E. cloacae | 94 |
| TLA-1 | CME-1 | 9.0 | CAZ, CTX, ATM | Mexico | E. coli | 153 |
| VEB-1 | PER-1, PER-2 | 5.35 | CAZ, ATM | Vietnam/Thailand | E. coli | 135 |
CTX, cefotaxime; CAZ, ceftazidime; ATM, aztreonam.
Another enzyme that is somewhat related to PER-1 is the VEB-1 β-lactamase (135). VEB-1 was first found in a single isolate of E. coli in a patient from Vietnam, but was subsequently also found in a P. aeruginosa isolate from a patient from Thailand (109). A third related enzyme is CME-1, which was isolated from Chryseobacterium meningosepticum (147). A fourth enzyme in this group is TLA-1, which was identified in an E. coli isolate from a patient in Mexico (153). The PER-1, PER-2, VEB-1, CME-1, and TLA-1 β-lactamases are related but show only 40 to 50% homology. These enzymes all confer resistance to oxyimino-cephalosporins, especially ceftazidime, and aztreonam. They also show some homology to the chromosomal cephalosporinases in Bacteroides spp. and may have originated from this genus (147).
An unusual feature of SFO-1, which is highly related to a class A β-lactamase from Serratia fonticola, is that it is a transferable β-lactamase that can be induced to high-level production of β-lactamase by imipenem (94). The plasmid carrying the gene encoding the SFO-1 β-lactamase also carries the ampR regulatory gene that is necessary for the induction of class C β-lactamases. However, unlike class C β-lactamases, SFO-1 cannot hydrolyze cephamycins and is inhibited well by clavulanic acid (94). GES-1 is another uncommon ESBL enzyme that is not closely related to any other plasmid-mediated β-lactamase but does show 36% homology to a carbenicillinase from Proteus mirabilis (136).
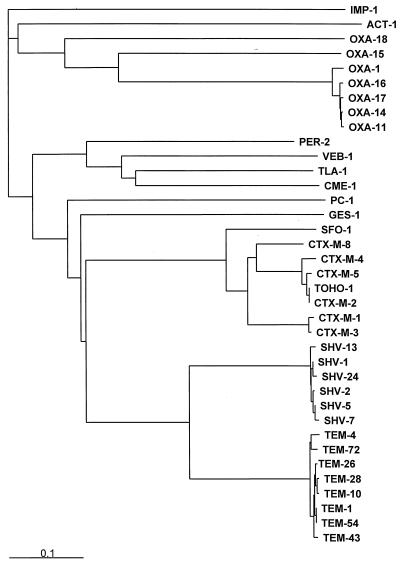
A dendrogram of the phylogeny of ESBL sequences is shown in Fig. 4. The TEM and SHV families are tightly clustered and are related to each other. All of the class A ESBLs are more closely related to each other than they are to any of the class D OXA-type enzymes.
FIG. 4.
Phylogeny of ESBLs. Representative sequences of various ESBLs were obtained from GenBank. The PC1 (class A, S. aureus enzyme), IMP-1 (class B, metallo-enzyme), and ACT-1 (class C, AmpC-type enzyme) β-lactamases were included for comparison. Signal peptides were identified with SPSScan and removed prior to alignment. Sequences were aligned using Clustal X (168). Trees were constructed with Clustal X, which uses the neighbor-joining method, with a bootstrap value of 1,000. The IMP-1 sequence was used to root the tree. Trees were visualized with TREEVIEW (118).
ESBL DETECTION METHODS
The increased prevalence of Enterobacteriaceae producing ESBLs creates a great need for laboratory testing methods that will accurately identify the presence of these enzymes in clinical isolates. Although most ESBLs confer resistance to one or more of the oxyimino-β-lactam antibiotics, the β-lactamase does not always increase the MICs to high enough levels to be called resistant by the National Committee for Clinical Laboratory Standards (NCCLS) interpretive guidelines (78, 111). The sensitivity and specificity of a susceptibility test to detect ESBLs vary with the cephalosporin tested. A number of investigators have suggested that either dilution tests or disk diffusion susceptibility tests performed with cefpodoxime detected more ESBLs than other cephalosporins such as ceftazidime, cefotaxime, and ceftriaxone (52, 100). However, more recent data suggest that susceptibility testing with cefpodoxime can lead to a high number of false-positives if the current NCCLS interpretive criteria are applied (F. C. Tenover, P. Raney, P. P. Williams, K. L. Brittan, C. D. Steward, S. K. Fridkin, R. P. Gaynes, and J. E. McGowan, Jr., 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1606, 2000). The NCCLS is currently reevaluating the testing procedures and interpretive criteria that should be used for the detection of ESBLs.
The failure of either MIC or disk tests alone to accurately detect the presence of an ESBL in all strains of E. coli and K. pneumoniae has been well documented (52, 73). In a recent survey conducted through the World Health Organization, 5.4% of laboratories using disk diffusion tests found an ESBL-producing challenge strain to be susceptible to all cephalosporins (165). In that study, Tenover et al. reported that only 2 of the 130 laboratories surveyed specifically reported the isolate as an ESBL producer (165). It also appears that there is a difference in the ability of various susceptibility-testing methods used for detecting cephalosporin resistance in an ESBL-producing strain. Steward et al. reported the results of a proficiency test assessing the ability of hospital laboratories participating in Project ICARE (Intensive Care Antimicrobial Resistance Epidemiology) to detect specific types of antimicrobial resistance (160). Only 35% of laboratories using the Vitek system reported an ESBL challenge strain of K. pneumoniae as being resistant to ceftazidime and ceftriaxone. In contrast, 100% of the laboratories using the MicroScan system reported the same strain as being resistant. However, only 29% of the laboratories using MicroScan reported the strain as being resistant to ceftriaxone (160).
This lack of sensitivity and specificity in traditional susceptibility tests to detect ESBLs has prompted the search for an accurate test to detect the presence of ESBLs in clinical isolates. In the years since ESBLs were first described, a number of different testing methods have been suggested.
Clinical Microbiology Techniques
Clinical microbiology tests employ a β-lactamase inhibitor, usually clavulanate, in combination with an oxyimino-cephalosporin such as ceftazidime or cefotaxime. In these tests, the clavulanate inhibits the ESBL, thereby reducing the level of resistance to the cephalosporin.
Several ESBL detection tests that have been proposed are based on the Kirby-Bauer disk diffusion test methodology. One of the first detection tests to be described was the double-disk approximation test described by Jarlier et al. (76). In this test, the organism is swabbed onto a Mueller-Hinton agar plate. A susceptibility disk containing amoxicillin-clavulanate is placed in the center of the plate, and disks containing one of the oxyimino-β-lactam antibiotics are placed 30 mm (center to center) from the amoxicillin-clavulanate disk. As shown in Fig. 5, enhancement of the zone of inhibition of the oxyimino-β-lactam caused by the synergy of the clavulanate in the amoxicillin-clavulanate disk is a positive result (76). This test remains a reliable method for the detection of ESBLs. However, it has been suggested that the sensitivity of this test can be increased by reducing the distance between the disks to 20 mm (169, 171). The use of cefpodoxime as the expanded-spectrum cephalosporin of choice for use in double-disk tests for ESBL detection has been suggested (41). Alternatively, the addition of clavulanate (4 μg/ml) to the Mueller-Hinton agar can be used to potentiate the zone of inhibition of one or more disks containing expanded-spectrum cephalosporins (67).
FIG. 5.
Double-disk diffusion and Etest ESBL detection tests. (A) The double-disk diffusion ESBL detection test as suggested by Jarlier et al. is shown (76). A disk containing amoxicillin-clavulanate (AMC) is placed in proximity to a disk containing ceftazidime (CAZ) or another oxyimino-cephalosporin. The clavulanate in the amoxicillin-clavulanate disk diffuses through the agar and inhibits the β-lactamase surrounding the ceftazidime disk. Enhancement of the zone of the ceftazidime disk on the side facing the amoxicillin-clavulanate disk is interpreted as a positive test. (B) Etest ESBL strip (AB Biodisk, Solna, Sweden). The zone of inhibition is read from two halves of the strip containing ceftazidime alone (TZ) or ceftazidime plus clavulanate (TZL). A reduction in the MIC of ceftazidime of ≥3 dilutions in the presence of clavulanate is interpreted as a positive test. (C) The Etest ESBL strip is sometimes difficult to interpret with weak enzyme producers such as the strain expressing TEM-12 shown in this panel. The clavulanate from the ceftazidime plus clavulanate half of the strip diffuses into the agar and interferes with the reading of the MICs for the half of the strip containing ceftazidime alone.
A similar test was designed by Jacoby and Han, in which 20 μg of sulbactam was added to susceptibility disks containing one of the oxyimino-β-lactam antibiotics (73). An increase of 5 mm in the zone of inhibition in a disk containing sulbactam compared to the drug alone was considered a positive test. Although many ESBL-producing strains were detected with this method, a significant number of strains were not. In addition, a number of AmpC-producing strains also showed an enhancement of the zone diameter with the addition of sulbactam (73). Recently, several commercial manufacturers have developed disks that contain an expanded-spectrum cephalosporin plus clavulanate. A differential between results obtained with 10-μg disks containing cefpodoxime, ceftazidime, or cefotaxime with or without the addition of 1 μg of clavulanate was shown to accurately detect the presence of an ESBL (35, 105).
Another method suggested for the detection of ESBLs is the three-dimensional test described by Thomson and Sanders (169). In this test, following inoculation of the test organism onto the surface of a Mueller-Hinton agar plate, a slit is cut into the agar, into which a broth suspension of the test organism is introduced. Subsequently, antibiotic disks are placed on the surface of the plate 3 mm from the slit. Distortion or discontinuity in the expected circular zone of inhibition is considered a positive test (169). This test was determined to be very sensitive in detecting ESBLs, but it is more technically challenging and labor intensive than other methods. All of the tests utilizing one of the variations of a disk diffusion technique require some interpretation and therefore should be performed by clinical microbiologists experienced in reading these tests.
It has also been suggested that dilution tests performed with an expanded-spectrum cephalosporin with and without the addition of clavulanic acid or another β-lactamase inhibitor be used for the detection of ESBLs in a clinical isolate. In general, these tests look for a reduction in the MIC of the cephalosporin in the presence of a β-lactamase inhibitor. However, the question of which cephalosporin to use has not been definitively resolved (170).
Currently, the NCCLS recommends an initial screening by testing for growth in a broth medium containing 1 μg/ml of one of five expanded-spectrum β-lactam antibiotics. A positive result is to be reported as suspicious for the presence of an ESBL (111). This screen is then followed by a phenotypic confirmatory test that consists of determining MICs of either ceftazidime or cefotaxime with and without the presence of clavulanic acid (4 μg/ml). A decrease in the MIC of ≥3 twofold dilutions in the presence of clavulanate is indicative of the presence of an ESBL. If an ESBL is detected, the strain should be reported as nonsusceptible to all expanded-spectrum cephalosporins and aztreonam regardless of the susceptibility testing result (111).
Several commercial manufacturers have developed ESBL detection tests that can be used along with MIC test methods already in place in the clinical laboratory. Etest ESBL strips (AB Biodisk, Solna, Sweden) are two-sided strips that contain a gradient of ceftazidime on one end and ceftazidime plus clavulanate on the other end. As shown in Fig. 5, a positive test for an ESBL is a >3-dilution reduction in the MIC of ceftazidime in the presence of clavulanic acid. This test was shown to be more sensitive than the double-disk approximation test in detecting ESBLs in clinical isolates (39). This method is convenient and easy to use, but it is sometimes difficult to read the test when the MICs of ceftazidime are low because the clavulanate sometimes diffuses over to the side that contains ceftazidime alone (Fig. 5) (183).
The automated microbial susceptibility test system Vitek (Biomerieux, Hazlewood, Mo.) has also produced an ESBL test that utilizes either ceftazidime or cefotaxime alone and in combination with clavulanic acid (4 μg/ml). A predetermined reduction in growth in wells containing clavulanate compared to those containing drug alone indicates the presence of an ESBL. In a study of Klebsiella spp. and E. coli expressing well-characterized β-lactamases, Sanders et al. showed that the Vitek ESBL test was 99% sensitive and specific for the detection of ESBLs (149). Furthermore, updated computer algorithms in the new Vitek system have also been shown to categorize the β-lactamases present in many gram-negative clinical isolates based on the phenotype of susceptibility patterns with various β-lactam antibiotics (150).
While each of these tests has its merit, none of these methods can accurately detect all strains producing ESBLs. Vercauteren et al. showed that the Etest ESBL test with ceftazidime only detected 81% of ESBLs tested in their laboratory, compared to 97 and 91% for the double-disk test and the three-dimensional test, respectively (183). Tzelepi et al. have reported that the Vitek ESBL detection test failed to detect the majority of ESBL-producing strains of Enterobacter spp. (171). In a recent survey of detection of ESBLs in clinical isolates, Tenover et al. found that only 18% of laboratories correctly identified challenge organisms as potential ESBL producers using susceptibility to one or more expanded-spectrum β-lactam antibiotics as the method of detection (164). Furthermore, a survey in Europe found that 37% of ESBL-producing organisms were mistakenly reported as being susceptible to expanded-spectrum cephalosporins (86).
The merits and shortcomings of each of the detection tests are outlined in Table 6. Of the tests that have been developed to date, the double-disk approximation test recommended by Jarlier et al. (76), and the broth-dilution MIC reduction method (NCCLS confirmatory test) (111) are the easiest and most cost-effective methods for use by many clinical laboratories. However, none of the detection tests that are based on the phenotype of the β-lactamase produced are 100% sensitive or specific for the accurate detection of ESBLs among clinical isolates of gram-negative bacteria. The need for improved detection of ESBLs in clinical isolates is well recognized (123).
TABLE 6.
ESBL detection techniques
| Technique type | Test | Advantages | Disadvantages | Reference(s) |
|---|---|---|---|---|
| Clinical microbiology | Standard NCCLS interpretive criteria | Easy to use, performed in every lab | ESBLs not always “resistant” | 78, 111 |
| NCCLS ESBL confirmatory test | Easy to use and interpret | Sensitivity depends on choice of oxyimino-cephalosporin | 111 | |
| Double-disk approximation test | Easy to use, easy to interpret | Distance of disk placement for optimal sensitivity not standardized | 76, 169, 71 | |
| Three-dimensional test | Sensitive, easy to interpret | Not specific for ESBLS, labor intensive | 169 | |
| Etest ESBL strips | Easy to use | Not always easy to interpret, not as sensitive as double-disk test | 183 | |
| Vitek ESBL test | Easy to use, easy to interpret | Reduced sensitivity | 149, 164 | |
| Molecular detection | DNA probes | Specific for gene family (e.g., TEM or SHV) | Labor intensive, cannot distinguish between ESBLs and non-ESBLs, cannot distinguish between variants of TEM or SHV | 7, 55, 70 |
| PCR | Easy to perform, specific for gene family (e.g., TEM or SHV) | Cannot distinguish between ESBLs and non-ESBLs, cannot distinguish between variants of TEM or SHV | 42, 90, 116 | |
| Oligotyping | Detects specific TEM variants | Requires specific oligonucleotide probes, labor intensive, cannot detect new variants | 117 | |
| PCR-RFLP | Easy to perform, can detect specific nucleotide changes | Nucleotide changes must result in altered restriction site for detection | 116 | |
| PCR-SSCP | Can distinguish between a number of SHV variants | Requires special electrophoresis conditions | 106, 107 | |
| LCR | Can distinguish between a number of SHV variants | Requires a large number of oligonucleotide primers | 80 | |
| Nucleotide sequencing | The gold standard, can detect all variants | Labor intensive, can be technically challenging, can be difficult to interpret manual methods | 25 |
It should also be noted that caution must be employed when interpreting ESBL detection tests because there have been reports of false-positive results for ESBL phenotypic screening tests that can occur with strains that do not possess an ESBL. Several groups have reported that the high-level expression of SHV-1 in K. pneumoniae can cause the MIC of ceftazidime to rise to levels at which an ESBL would be suspected (99, 129, 141). In addition, Rasheed et al. reported that the production of SHV-1 in a strain of K. pneumoniae that was also lacking an outer membrane porin protein caused a false-positive in ESBL detection tests that looked at the differential between MICs of oxyimino-β-lactam antibiotics with and without clavulanate (139). The presence of an ESBL can also be masked by the expression of an AmpC-type enzyme in the same strain (28).
Molecular Detection Methods
The tests described above only presumptively identify the presence of an ESBL. The task of identifying which specific ESBL is present in a clinical isolate is more complicated. In the early days of studying ESBLs, determination of the isoelectric point was usually sufficient to identify the ESBL that was present. However, with >90 TEM-type β-lactamases, many of which possess identical isoelectric points, determination of the ESBL by isoelectric point is no longer possible. A similar situation is found in the SHV, CTX-M, and OXA families of ESBLs.
Early detection of β-lactamase genes was performed using DNA probes that were specific for TEM and SHV enzymes (7, 55, 70). However, using DNA probes can sometimes be rather labor intensive. The easiest and most common molecular method used to detect the presence of a β-lactamase belonging to a family of enzymes is PCR with oligonucleotide primers that are specific for a β-lactamase gene. Oligonucleotide primers can be chosen from sequences available in public databases such as Genbank (GenBank, National Center for Biotechnology Information, http://www.ncbi.nlm.nih.gov/Genbank/index.html). These primers are usually chosen to anneal to regions where various point mutations are not known to occur. However, PCR will not discriminate among different variants of TEM or SHV. Several molecular methods that will aid in the detection and differentiation of ESBLs without sequencing have been suggested.
The first molecular method for the identification of β-lactamase was the oligotyping method developed by Ouellette et al., which was used to discriminate between TEM-1 and TEM-2 (117). This method used oligonucleotide probes that are designed to detect point mutations under stringent hybridization conditions. Subsequently, Mabilat and Courvalin developed additional oligonucleotide probes to detect mutations at six positions within the blaTEM gene (89). Using this method, several new TEM variants were identified within a set of clinical isolates. The probes used in oligotyping tests for TEM β-lactamases have been labeled either with a radioisotope or with biotin (89, 167). Another approach for molecular characterization of the TEM β-lactamase gene was to add restriction fragment length polymorphism analysis to PCR (PCR-RFLP) (6). In this test, amplified PCR products were subjected to digestion with several restriction endonucleases, and the subsequent fragments were separated by electrophoresis. The sizes of the fragments generated by each restriction enzyme indicate point mutations within the blaTEM structural gene.
A number of different tests have been proposed for the detection and identification of SHV derivatives. The simplest of these was suggested by Nüesch-Inderbinen et al. and employs PCR-RFLP (116). Following PCR, the amplified DNA is digested with restriction enzyme NheI, which detects the G-to-A nucleotide change that gives rise to the glycine-to-serine substitution at position 238 that is common to many of the early SHV-type ESBLs. Although this method cannot determine which SHV-type ESBL is present, it can detect the specific mutation at position 238 (116). Another method used to characterize SHV-type ESBLs is PCR single-strand conformational polymorphism (PCR-SSCP) analysis. This method has been used to detect a single base mutation at specific locations within the blaSHV gene (106, 107). In this test, a 475-bp amplimer is generated using oligonucleotide primers that are internal to the coding sequence of the blaSHV gene, digested with restriction enzyme PstI. The fragments are then denatured and separated on a 20% polyacrylamide gel. Genes for SHV-1, -2, -3, -4, -5, and -7 β-lactamases can be identified by the electrophoretic pattern of the digested amplimer (106, 107). With the identification of a number of additional SHV-type β-lactamase genes, PCR-RFLP was developed to help with the identification of some of the newer SHV variants (38). Following PCR, Chanawong et al. used a variety of restriction endonucleases to detect 12 mutations at 11 positions within the blaSHV structural gene. The combination of PCR-SSCP with PCR-RFLP allows the identification of 17 different SHV genes (38).
Another method proposed for the identification of SHV genes is the use of ligase chain reaction (LCR) (80). LCR allows the discrimination of DNA sequences that differ by a single base pair by the use of a thermostable ligase with four oligonucleotide primers that are complimentary to the target sequence and hybridize adjacent to each other. A single base mismatch in the oligonucleotide junction will not be ligated and subsequently amplified. In this LCR test, the target DNA containing the blaSHV gene is denatured in a thermocycler and annealed with biotinylated oligonucleotide primers that detect mutations at four positions. The LCR product is detected by an enzymatic reaction using NADPH-alkaline phosphatase. This method was able to detect seven of the SHV variants.
For OXA-10-derived ESBLs, the presence of an OXA-type gene in clinical isolates of P. aeruginosa was first detected using a colony hybridization technique (178). Subsequently, positive isolates were subjected to PCR with specific OXA primers and then digested with restriction endonucleases that would distinguish several groups of related OXA enzymes based on the sizes of the restriction fragments. While this technique does not completely identify which OXA gene is present in a strain, it can distinguish the ESBL OXA-type β-lactamases from non-ESBLs that are also related to OXA-10 (178).
Nucleotide sequencing remains the standard for determination of the specific β-lactamase gene present in a strain. However, this too can give variable results depending on the method used (25). It is possible that some of the variability seen in the sequences for some of the SHV β-lactamases was due to compressions and difficulty in reading traditional sequencing autoradiographs, rather than actual differences in the sequence (25).
Medical Significance of Detection of ESBLs
It is generally thought that patients having infections caused by an ESBL-producing organism are at an increased risk of treatment failure with an expanded-spectrum β-lactam antibiotic. Therefore, it is recommended that any organism that is confirmed for ESBL production according to NCCLS criteria be reported as resistant to all expanded-spectrum β-lactam antibiotics, regardless of the susceptibility test result (111). While some ESBL-producing strains have overt resistance to expanded-spectrum β-lactam antibiotics, many isolates will not be phenotypically “resistant” according to guidelines such as those previously used by the NCCLS. Therefore, it is important for the clinical microbiology lab to be aware of isolates that may show increased MICs of oxyimino-cephalosporins even though they may not be reported as resistant, as this might suggest the presence of an ESBL. It is also important for the clinical microbiology lab to then implement one or more methods to detect ESBLs. In contrast, the susceptibility test results of the β-lactam–β-lactamase inhibitor combinations can be reported as is. There have been several reports that these inhibitor combinations may provide a viable alternative for the treatment of infections caused by ESBL-producing organisms (124, 125).
The concern for the accurate detection of ESBLs is twofold. First, there is an increasing prevalence of ESBLs worldwide (see below). Second, many strains producing ESBLs demonstrate an inoculum effect in that the MICs of expanded-spectrum cephalosporins rise as the inoculum increases (36, 74, 158). Medeiros and Crellin found that the MICs of most cephalosporins rose dramatically when the inoculum of susceptibility tests was raised from 105 to 107 CFU/ml (97). This in vivo inoculum effect has also been demonstrated in animal models of endocarditis and intra-abdominal abscesses (34, 53, 144). There are many types of infections in which the bacterial load could reach these levels. Therefore, it is imperative that the detection of ESBLs accurately reflect the level of resistance that would be achieved by strains expressing these enzymes in vivo.
EPIDEMIOLOGY
ESBLs are now a problem in hospitalized patients worldwide. The ESBL phenomenon began in western Europe, most likely because expanded-spectrum β-lactam antibiotics were first used there clinically. However, it did not take long before ESBLs had been detected in the United States and Asia. The prevalence of ESBLs among clinical isolates varies from country to country and from institution to institution. In the United States, occurrence of ESBL production in Enterobacteriaceae ranges from 0 to 25%, depending on the institution, with the national average being around 3% (CDC National Nosocomial Infections Surveillance, http://www.cdc.gov/ncidod/hip/SURVEILL/NNIS.HTM) Among isolates of K. pneumonia, the percentage of ceftazidime resistance ranges from 5 to 10% for non-intensive care unit (non-ICU) and ICU isolates, respectively (D. Mathai, R. N. Jones, M. Stilwell, and M. A. Pfaller, 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1027, 2000). Some hospitals with low levels of ESBLs may not find it cost-effective to test for ESBLs on a routine basis (52). However, these institutions should monitor the rates of resistance in their own hospitals and be aware of an increase in resistance.
In Europe, the prevalence of ESBL production among isolates of Enterobacteriaceae varies greatly from country to country. In the Netherlands, a survey of 11 hospital laboratories showed that <1% of E. coli and K. pneumoniae strains possessed an ESBL (161). However, in France, as many as 40% of K. pneumoniae isolates were found to be ceftazidime resistant (30). Across Europe, the incidence of ceftazidime resistance among K. pneumoniae strains was 20% for non-ICU isolates and 42% for isolates from patients in the ICU (Mathai et al., abstr. 1027). In Japan, the percentage of β-lactam resistance due to ESBL production in E. coli and K pneumoniae remains very low. In a recent survey of 196 institutions across the country, <0.1% of E. coli and 0.3% of K. pneumoniae strains possessed an ESBL (187). Elsewhere in Asia, the percentage of ESBL production in E. coli and K. pneumoniae varies, from 4.8% in Korea (119) to 8.5% in Taiwan (188) and up to 12% in Hong Kong (68).
It is interesting that specific ESBLs appear to be unique to a certain country or region. For example, TEM-10 has been responsible for several unrelated outbreaks of ESBL-producing organisms in the United States for a number of years (26, 112, 143, 175). However, TEM-10 has only recently been reported in Europe with the same frequency (8, 84). Similarly, TEM-3 is common in France, but has not been detected in the United States (114, 156). In recent years, there have been reports of outbreaks of TEM-47-producing organisms in Poland (62), and the prevalence of TEM-52 in Korea is unique to that country (119). Another recent survey of Korea revealed that the SHV-12 and SHV-2a β-lactamases are the most common ESBLs found in Korea (79). In contrast, the SHV-5 β-lactamase is commonly encountered worldwide and has been reported in Croatia, France, Greece, Hungary, Poland, South Africa, the United Kingdom, and the United States (15, 43, 54, 63, 133, 152, 163, 181).
A common theme among hospitals plagued by organisms that produce ESBLs is the high volume and indiscriminate administration of expanded-spectrum cephalosporins (140, 142). Specific risk factors include length of hospital stay, severity of illness, time in the ICU, intubation and mechanical ventilation, urinary or arterial catheterization, and previous exposure to antibiotics (126, 140). Many of the patients infected with ESBLs are found in ICUs, but they can occur in surgical wards as well as most other areas of the hospital. ESBLs are also being isolated with increasing frequency from patients in extended-care facilities (27, 143, 186). In addition, whereas early outbreaks of ESBL-producing strains were caused by isolates that produced only a single β-lactamase, more recently outbreaks have been caused by organisms with multiple β-lactamases (26, 27, 189). This combination of non-ESBL class A enzymes and AmpC-type enzymes along with ESBLs often compounds the resistance, so that many of these strains are now resistant to β-lactam–β-lactamase inhibitor combinations, cephamycins, and even carbapenems in addition to the oxyimino-cephalosporins and aztreonam (28). In addition, there is a high association with ciprofloxacin resistance in strains that produce ESBLs (122).
Many hospitals have experienced outbreaks of ESBL-producing organisms. These outbreaks are often fueled by the large number of patient transfers between units and between hospitals (87). It was found that barrier precautions were often difficult to enforce with a mobile patient population. Eventually, many of the reported outbreaks were successfully managed using infection control methods (87), restriction of the use of oxyimino-cephalosporins (125, 138), and antibiotic cycling (49, 77). A successful approach to the control of the spread of ESBL-producing organisms involved switching to different classes of broad-spectrum antibiotics for the treatment of serious infections (140). The two most successful replacement antibiotics have been imipenem and piperacillin-tazobactam (98, 124, 125, 142, 143).
In the mid-1990s, Rice et al. reported that an outbreak of TEM-6-producing ceftazidime-resistant K. pneumoniae in a Veterans Administration hospital was successfully controlled after the institution switched from ceftazidime to piperacillin-tazobactam for empiric therapy for gram-negative infections (142). Although the ceftazidime-resistant strains causing the outbreak were originally resistant to piperacillin-tazobactam, they saw a rapid decrease in the isolation of K. pneumoniae strains resistant to both ceftazidime and piperacillin-tazobactam. The incidence of ESBL-producing K. pneumoniae has remained low since that time in that institution (142). This phenomenon of a reduction in the resistance rate to piperacillin-tazobactam following the switch from expanded-spectrum cephalosporin use to piperacillin-tazobactam has been confirmed by several other investigators (124, 125). Moreover, it has been reported that the use of β-lactam–β-lactam inhibitor combinations results in a protective effect, in that they are associated with a lower incidence of colonization with an ESBL-producing isolate (132).
Many investigators are using molecular methods such as pulsed-field gel electrophoresis (PFGE) to examine epidemiology with the strains involved in outbreaks of infections caused by ESBLs (30, 40, 54). Other methods for studying the epidemiology of these strains include plasmid profiles, ribotyping, random amplified polymorphic DNA (RAPD), and arbitrarily primed PCR (17, 43, 151, 185, 190). These outbreaks often start in an ICU and then spread to other parts of the hospital by the usual transmission routes (17). Very often, the exact source of outbreaks caused by ESBL-producing organisms is never identified. However, some interesting epidemiology of these resistant bacteria has been reported. In one hospital in France, ceftazidime-resistant K. pneumoniae expressing SHV-5 was isolated from six peripartum women and two neonates. Plasmid and PFGE profiles of the strains revealed that all of the strains were identical to a strain that was cultured from contaminated ultrasonography coupling gel (54). Another study demonstrated that cockroaches infesting a neonatal ICU in South Africa carried the same PFGE strain types of ESBL-producing K. pneumoniae that were responsible for an outbreak of infections and high mortality rate among neonates in that institution (40).
ESBLs are most often encoded on plasmids, which can easily be transferred between isolates. In an outbreak of ESBL-producing K. pneumoniae and E. coli in Chicago, it was shown that a common plasmid expressing TEM-10 was found in isolates from numerous patients in several hospitals and nursing homes (26, 186). Because this plasmid was found in multiple different strain types, as demonstrated by PFGE, it was presumed that this promiscuous plasmid expressing TEM-10 was transferred to the normal flora of some of the patients. In another report from France, a 180-kb self-transmissible plasmid expressing TEM-24 was found in four different species of Enterobacteriaceae (E. coli, K. pneumoniae, E. aerogenes, and P. rettgeri) isolated from a single patient (91).
CONCLUSION
In the last 15 years, ESBLs have gone from being an interesting scientific observation to a reality of great medical importance. The introduction of the oxyimino-β-lactam antibiotics was met with the emergence of new β-lactamases. Some of these new β-lactamases, like the TEM- and SHV-type ESBLs, result from simple point mutations in existing β-lactamase genes that lead to a changed substrate profile. Other new β-lactamases, such as the CTX-M-type enzymes, have been borrowed from the chromosomally encoded β-lactamases that occur naturally in other species of Enterobacteriaceae. The development and spread of ESBLs have most likely been caused by the overuse of expanded-spectrum cephalosporins in the hospital setting.
Numerous methods have been proposed for the detection of ESBLs in clinical isolates. Regardless of the method used for detection, it is important to note that none of the methods that rely on phenotypic expression of the β-lactamase will detect every ESBL-producing isolate. Nevertheless, increased awareness of the ESBL problem among clinical microbiology laboratory and infection control personnel will help in the interpretation of these tests.
Current therapy for strains of Enterobacteriaceae that express ESBLs is limited to such broad-spectrum agents as imipenem. However, there have already been reports of therapeutic failures of this drug with strains that produce multiple β-lactamases (3). There are limited therapeutic options left for some of these organisms. Strains expressing extended-spectrum β-lactamases will present a host of challenges for clinical microbiologists and clinicians alike as we head into the 21st century.
ACKNOWLEDGMENTS
I thank Ellen Murphy for the sequence alignments and creating the dendrogram, Steven J. Projan for critical review of the manuscript, and Melissa Visalli and David Correa for help in gathering references.
REFERENCES
- 1.Abraham E P, Chain E. An enzyme from bacteria able to destroy penicillin. Nature. 1940;146:837. [PubMed] [Google Scholar]
- 2.Afazal-Shah M, Woodford N, Livermore D M. Characterization of OXA-25, OXA-26, and OXA-27, molecular class D β-lactamases associated with carbapenem resistance in clinical isolates of Acinetobacter baumanii. Antimicrob Agents Chemother. 2001;45:583–588. doi: 10.1128/AAC.45.2.583-588.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad M, Urban C, Mariano N, Bradford P A, Calcagni E, Projan S J, Bush K, Rahal J J. Clinical characteristics and molecular epidemiology associated with imipenem-resistant Klebsiella pneumoniae. Clin Infect Dis. 1999;29:352–355. doi: 10.1086/520214. [DOI] [PubMed] [Google Scholar]
- 4.Ambler R P. The structure of β-lactamases. Phil Trans R Soc Lond Biol. 1980;289:321–331. doi: 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- 5.Ambler R P, Coulson A F W, Frére J-M, Ghuysen J-M, Joris B, Forsman M, Levesque R C, Tiraby G, Waley S G. A standard numbering scheme for the class A β-lactamases. Biochem J. 1991;276:269–270. doi: 10.1042/bj2760269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arlet G, Brami G, Dècrère D, Flippo A, Galtolot O, Lagrange P H, Philippon A. Molecular characterization by PCR-restriction fragment length polymorphism of TEM β-lactamases. FEMS Microbiol Lett. 1995;134:1498–1500. doi: 10.1111/j.1574-6968.1995.tb07938.x. [DOI] [PubMed] [Google Scholar]
- 7.Arlet G, Philippon A. Construction by polymerase chain reaction and intragenic DNA probes for three main types of transferable β-lactamases (TEM, SHV, CARB) FEMS Microbiol Lett. 1991;82:19–26. doi: 10.1016/0378-1097(91)90414-6. [DOI] [PubMed] [Google Scholar]
- 8.Barroso H, Freitas-Vieira A, Lito L M, Cristino J M, Salgado M J, Neto H F, Sousa J C, Soveral G, Moura T, Duarte A. Survey of Klebsiella pneumoniae producing extended-spectrum β-lactamases at a Portuguese hospital: TEM-10 as the endemic enzyme. J Antimicrob Chemother. 2000;45:611–616. doi: 10.1093/jac/45.5.611. [DOI] [PubMed] [Google Scholar]
- 9.Barthélémy M, Péduzzi J, Bernard H, Tancrède C, Labia R. Close amino acid sequence relationship between the new plasmid-mediated extended-spectrum β-lactamase MEN-1 and chromosomally encoded enzymes of Klebsiella oxytoca. Biochim Biophys Acta. 1992;1122:15–22. doi: 10.1016/0167-4838(92)90121-s. [DOI] [PubMed] [Google Scholar]
- 10.Barthélémy M, Péduzzi J, Labia R. Distinction entre les structures primaires des β-lactamases TEM-1 et TEM-2. Ann Inst Pasteur Microbiol. 1985;136A:311–321. doi: 10.1016/s0769-2609(85)80093-4. [DOI] [PubMed] [Google Scholar]
- 11.Bauernfeind A, Casellas J M, Goldberg M, Holley M, Jungwirth R, Mangold P, Röhnisch T, Schweighart S, Wilhelm R. A new plasmidic cefotaximase from patients infected with Salmonella typhimurium. Infection. 1992;20:158–163. doi: 10.1007/BF01704610. [DOI] [PubMed] [Google Scholar]
- 12.Bauernfeind A, Grimm H, Schweighart S. A new plasmidic cefotaximase in a clinical isolate of Escherichia coli. Infection. 1990;18:294–298. doi: 10.1007/BF01647010. [DOI] [PubMed] [Google Scholar]
- 13.Bauernfeind A, Stemplinger I, Jungwirth R, Ernst S, Casellas J M. Sequences of β-lactamase genes encoding CTX-M-1 (MEN-1) and CTX-M-2 and relationship of their amino acid sequences with those of other β-lactamases. Antimicrob Agents Chemother. 1996;40:509–513. doi: 10.1128/aac.40.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bauernfeind A, Stemplinger I, Jungwirth R, Mangold P, Amann S, Akalin E, Ang Ö, Bal C, Casellas J M. Characterization of β-lactamase gene blaPER-2, which encodes an extended-spectrum class A β-lactamase. Antimicrob Agents Chemother. 1996;40:616–620. doi: 10.1128/aac.40.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bedenic B, Zagar Z. Extended-spectrum beta-lactamases in clinical isolates of Klebsiella pneumoniae from Zagreb, Croatia. J Chemother. 1998;10:449–459. doi: 10.1179/joc.1998.10.6.449. [DOI] [PubMed] [Google Scholar]
- 16.Belaaouaj A, Lapoumeroulie C, Caniça M M, Vedel G, Névot P, Krishnamoorthy R, Paul G. Nucleotide sequences of the genes coding for the TEM-like β-lactamases IRT-1 and IRT-2 (formerly called TRI-1 and TRI-2) FEMS Microbiol Lett. 1994;120:75–80. doi: 10.1111/j.1574-6968.1994.tb07010.x. [DOI] [PubMed] [Google Scholar]
- 17.Bermudes H, Arpin C, Jude F, El-Harrif Z, Bébéar C, Quentin C. Molecular epidemiology of an outbreak due to extended-spectrum beta-lactamase-producing enterobacteria in a French hospital. Eur J Clin Microbiol Infect Dis. 1997;16:523–529. doi: 10.1007/BF01708236. [DOI] [PubMed] [Google Scholar]
- 18.Blazquez J, Morosini M-I, Negri M-C, Baquero F. Selection of naturally occurring extended-spectrum TEM β-lactamase variants by fluctuating β-lactam pressure. Antimicrob Agents Chemother. 2000;44:2182–2184. doi: 10.1128/aac.44.8.2182-2184.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonnet R, Champs C D, Sirot D, Chanal C, Labia R, Sirot J. Diversity of TEM mutants in Proteus mirabilis. Antimicrob Agents Chemother. 1999;43:2671–2677. doi: 10.1128/aac.43.11.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonnet R, Sampaio J L M, Chanal C, Sirot D, Champs C D, Viallard J L, Labia R, Sirot J. A novel class A extended-spectrum β-lactamase (BES-1) in Serratia marcescens isolated in Brazil. Antimicrob Agents Chemother. 2000;44:3061–3068. doi: 10.1128/aac.44.11.3061-3068.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonnet R, Sampaio J L M, Labia R, Champs C D, Sirot D, Chanel C, Sirot J. A novel CTX-M β-lactamase (CTX-M-8) in cefotaxime-resistant Enterobacteriaceae isolated in Brazil. Antimicrob Agents Chemother. 2000;44:1936–1942. doi: 10.1128/aac.44.7.1936-1942.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonomo R A, Currie-McCumber C, Shlaes D M. OHIO-1 β-lactamase resistant to mechanism-based inactivators. FEMS Microbiol Lett. 1992;71:79–82. doi: 10.1016/0378-1097(92)90545-y. [DOI] [PubMed] [Google Scholar]
- 23.Bonomo R A, Rudin S A, Shlaes D M. Tazobactam is a potent inactivator of selected inhibitor-resistant class A β-lactamases. FEMS Microbiol Lett. 1997;148:59–62. doi: 10.1111/j.1574-6968.1997.tb10267.x. [DOI] [PubMed] [Google Scholar]
- 24.Bou G, Oliver A, Martínez-Beltrán J. OXA-24, a novel class D β-lactmase with carbapenemase activity in an Acinetobacter baumannii clinical strain. Antimicrob Agents Chemother. 2000;44:1556–1561. doi: 10.1128/aac.44.6.1556-1561.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradford P A. Automated thermal cycling is superior to traditional methods for nucleotide sequencing of blaSHV genes. Antimicrob Agents Chemother. 1999;43:2960–2963. doi: 10.1128/aac.43.12.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradford P A, Cherubin C E, Idemyor V, Rasmussen B A, Bush K. Multiply resistant Klebsiella pneumoniae strains from two Chicago Hospitals: identification of the extended-spectrum TEM-12 and TEM-10 ceftazidime-hydrolyzing β-lactamases in a single isolate. Antimicrob Agents Chemother. 1994;38:761–766. doi: 10.1128/aac.38.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradford P A, Urban C, Jaiswal A, Mariano N, Rasmussen B A, Projan S J, Rahal J J, Bush K. SHV-7, a novel cefotaxime-hydrolyzing β-lactamase, identified in Escherichia coli isolates from hospitalized nursing home patients. Antimicrob Agents Chemother. 1995;39:899–905. doi: 10.1128/aac.39.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradford P A, Urban C, Mariano N, Projan S J, Rahal J J, Bush K. Imipenem resistance in Klebsiella pneumoniae is associated with the combination of ACT-1, a plasmid-mediated AmpC β-lactamase and the loss of an outer membrane protein. Antimicrob Agents Chemother. 1997;41:563–569. doi: 10.1128/aac.41.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bradford P A, Yang Y, Sahm D, Grope I, Gardovska D, Storch G. CTX-M-5, a novel cefotaxime-hydrolyzing β-lactamase from an outbreak of Salmonella typhimurium in Latvia. Antimicrob Agents Chemother. 1998;42:1980–1894. doi: 10.1128/aac.42.8.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Branger C, Lesimple A L, Bruneu B, Berry P, Lambert-Zechovsky N. Long-term investigation of the clonal dissemination of Klebsiella pneumoniae isolates producing extended-spectrum β-lactamases in a university hospital. J Med Microbiol. 1998;47:210–209. doi: 10.1099/00222615-47-3-201. [DOI] [PubMed] [Google Scholar]
- 31.Bret L, Chanel C, Sirot D, Labia R, Sirot J. Characterization of an inhibitor-resistant enzyme IRT-2 derived from TEM-2 β-lactamase produced by Proteus mirabilis strains. J Antimicrob Chemother. 1996;38:183–191. doi: 10.1093/jac/38.2.183. [DOI] [PubMed] [Google Scholar]
- 32.Bush K, Jacoby G A, Medeiros A A. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bush K, Singer S B. Biochemical characteristics of extended broad spectrum β-lactamases. Infection. 1989;17:429–433. doi: 10.1007/BF01645566. [DOI] [PubMed] [Google Scholar]
- 34.Caron R, Gutmann L, Bure A, Pangon B, Vallois J-M, Pechinot A, Carbon C. Ceftriaxone-sulbactam combination in a rabbit endocarditis caused by a strain of Klebsiella pneumoniae producing extended broad-spectrum TEM-3 β-lactamase. Antimicrob Agents Chemother. 1990;39:1211–1233. doi: 10.1128/aac.34.11.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carter M W, Oakton K J, Warner M, Livermore D M. Detection of extended-spectrum β-lactamases in klebsiellae with the Oxoid combination disk method. J Clin Microbiol. 2000;38:4228–4232. doi: 10.1128/jcm.38.11.4228-4232.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casellas J M, Goldberg M. Incidence of strains producing extended-spectrum β-lactamase in Argentina. Infection. 1989;17:434–436. doi: 10.1007/BF01645567. [DOI] [PubMed] [Google Scholar]
- 37.Chaibi E B, Sirot D, Paul G, Labia R. Inhibitor-resistant TEM-β-lactamases: phenotypic, genetic and biochemical characteristics. J Antimicrob Chemother. 1999;43:447–458. doi: 10.1093/jac/43.4.447. [DOI] [PubMed] [Google Scholar]
- 38.Chanawong A, M'Zali F H, Heritage J, Lulitanond A, Hawkey P M. Characterization of extended-spectrum β-lactamases of the SHV family using a combination of PCR-single strand conformational polymorphism (PCR-SSCP) and PCR-restriction fragment length polymorphism (PCR-RFLP) FEMS Microbiol Lett. 2000;184:85–89. doi: 10.1111/j.1574-6968.2000.tb08995.x. [DOI] [PubMed] [Google Scholar]
- 39.Cormican M G, Marshall S A, Jones R N. Detection of extended-spectrum β-lactamase (ESBL)-producing strains by the Etest ESBL screen. J Clin Microbiol. 1996;34:1880–1884. doi: 10.1128/jcm.34.8.1880-1884.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cotton M F, Wasserman E, Pieper C H, Theron D C, van Tubbergh D, Campbell G, Fang F C, Barnes J. Invasive disease due to extended-spectrum beta-lactamase-producing Klebsiella pneumoniae in a neonatal unit: the possible role of cockroaches. J Hosp Infect. 2000;44:13–17. doi: 10.1053/jhin.1999.0650. [DOI] [PubMed] [Google Scholar]
- 41.Coudron P E, Moland E S, Sanders C C. Occurrence and detection of extended-spectrum β-lactamases in members of the family Enterobacteriaceae at a Veterans Medical Center: seek and you may find. J Clin Microbiol. 1997;35:2593–2597. doi: 10.1128/jcm.35.10.2593-2597.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Courvalin P. Genotypic approach to the study of bacterial resistance to antibiotics. Antimicrob Agents Chemother. 1991;35:1019–1023. doi: 10.1128/aac.35.6.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D'Agata E, Venkataraman L, DeGirolami P, Weigel L, Samore M, Tenover F. The molecular and clinical epidemiology of enterobacteriaceae-producing extended-spectrum beta-lactamase in a tertiary care hospital. J Infect. 1998;36:279–285. doi: 10.1016/s0163-4453(98)94171-8. [DOI] [PubMed] [Google Scholar]
- 44.Danel F, Hall L M C, Duke B, Gur D, Livermore D M. OXA-17, a further extended-spectrum variant of OXA-10 β-lactamase, isolated from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1999;43:1362–1366. doi: 10.1128/aac.43.6.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Danel F, Hall L M C, Gur D, Livermore D M. OXA-14, another extended-spectrum variant of OXA-10 (PSE-2) β-lactamase from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:1881–1884. doi: 10.1128/aac.39.8.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Danel F, Hall L M C, Gur D, Livermore D M. OXA-15, an extended-spectrum variant of OXA-2 β-lactamase, isolated from a Pseudomonas aeruginosa strain. Antimicrob Agents Chemother. 1997;41:785–790. doi: 10.1128/aac.41.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Danel F, Hall L M C, Gur D, Livermore D M. OXA-16, a further extended-spectrum variant of OXA-10 β-lactamase, from two Pseudomonas aeruginosa isolates. Antimicrob Agente Chemother. 1998;42:3117–3122. doi: 10.1128/aac.42.12.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Datta N, Kontomichalou P. Penicillinase synthesis controlled by infectious R Factors in Enterobacteriaceae. Nature. 1965;208:239–244. doi: 10.1038/208239a0. [DOI] [PubMed] [Google Scholar]
- 49.Dominguez E A, Smith T L, Reed E, Sanders C C, Sanders W E., Jr A pilot study of antibiotic cycling in a hematology-oncology unit. Infect Control Hosp Epidemiol. 2000;21:S4–S8. doi: 10.1086/503166. [DOI] [PubMed] [Google Scholar]
- 50.Doucet-Populaire F, Ghnassia J C, Bonnet R, Sirot J. First isolation of a CTX-M-3-producing Enterobacter cloacae in France. Antimicrob Agents Chemother. 2000;44:3239–3240. doi: 10.1128/aac.44.11.3239-3240.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.El Harrif-Heraud Z, Arpin C, Benliman S, Quentin C. Molecular epidemiology of a nosocomial outbreak due to SHV-4 producing strains of Citrobacter diversus. J Clin Microbiol. 1997;35:2561–2567. doi: 10.1128/jcm.35.10.2561-2567.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Emery C L, Weymouth L A. Detection and clinical significance of extended-spectrum β-lactamases in a tertiary-care medical center. J Clin Microbiol. 1997;35:2061–2067. doi: 10.1128/jcm.35.8.2061-2067.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fantin B, Pangon B, Potel G, Caron F, Valee E, Vallois J-M, Mohler J, Bure A, Philippon A, Carbon C. Activity of sulbactam in combination with ceftriaxone in vitro and in experimental endocarditis caused by Escherichia coli producing SHV-2-like β-lactamase. Antimicrob Agents Chemother. 1990;34:581–586. doi: 10.1128/aac.34.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gaillot O, Maruéjouls C, Abachin É, Lecuru F, Arlet G, Simonet M, Berche P. Nosocomial outbreak of Klebsiella pneumoniae producing SHV-5 extended-spectrum β-lactamase, originating from a contaminated ultrasonography coupling gel. J Clin Microbiol. 1998;36:1357–1360. doi: 10.1128/jcm.36.5.1357-1360.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gallego L, Umaran A, Garaizar J, Colom K, Cisterna R. Digoxigenin-labeled DNA probe to detect TEM type β-lactamases. J Microbiol Methods. 1990;11:261–267. [Google Scholar]
- 56.Gazouli M, Legakis N J, Tzouvelekis L S. Effect of substitution of Asn for Arg-276 in the cefotaxime-hydrolysing class A β-lactamase CTX-M-4. FEMS Microbiol Lett. 1998;169:289–293. doi: 10.1111/j.1574-6968.1998.tb13331.x. [DOI] [PubMed] [Google Scholar]
- 57.Gazouli M, Sidorenko S V, Tzelepi E, Kozlova N S, Gladin D P, Tzouvelekis L S. A plasmid-mediated β-lactamase conferring resistance to cefotaxime in a Salmonella typhimurium clone found in St. Petersburg, Russia. J Antimicrob Chemother. 1998;41:119–121. doi: 10.1093/jac/41.1.119. [DOI] [PubMed] [Google Scholar]
- 58.Gazouli M, Tzelepi E, Markogiannakis A, Legakis N J, Tzouvelekis L S. Two novel plasmid-mediated cefotaxime-hydrolyzing β-lactamases (CTX-M-5 and CTX-M-6) from Salmonella typhimurium. FEMS Microbiol Lett. 1998;165:289–293. doi: 10.1111/j.1574-6968.1998.tb13159.x. [DOI] [PubMed] [Google Scholar]
- 59.Gazouli M, Tzelepi E, Sidorenko S V, Tzouvelekis L S. Sequence of the gene encoding a plasmid-mediated cefotaxime-hydrolyzing class A β-lactamase (CTX-M-4): involvement of serine 237 in cephalosporin hydrolysis. Antimicrob Agents Chemother. 1998;42:1259–1262. doi: 10.1128/aac.42.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reference deleted.
- 61.Ghuysen J M. Serine β-lactamases and penicillin-binding proteins. Annu Rev Microbiol. 1991;45:37–67. doi: 10.1146/annurev.mi.45.100191.000345. [DOI] [PubMed] [Google Scholar]
- 62.Gniadkowski M, Palucha A, Grzesioski P, Hryniewicz W. Outbreak of ceftazidime-resistant Klebsiella pneumoniae in a pediatric hospital in Warsaw, Poland: clonal spread of the TEM-47 extended-spectrum β-lactamase (ESBL)-producing strain and transfer of a plasmid carrying the SHV-5-like ESBL-encoding gene. Antimicrob Agents Chemother. 1998;42:3079–3085. doi: 10.1128/aac.42.12.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gniadkowski M, Schneider I, Jungwirth R, Hryniewicz W, Baurnfeind A. Ceftazidime-resistant Enterobacteriaceae isolates from three polish hospitals: identification of three novel TEM- and SHV-5-type extended-spectrum β-lactamases. Antimicrob Agents Chemother. 1998;42:514–520. doi: 10.1128/aac.42.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gniadkowski M, Schneider I, Palucha A, Jungwirth R, Mikiewicz B, Bauernfeind A. Cefotaxime-resistant Enterobacteriaceae isolates from a hospital in Warsaw, Poland: Identification of a new CTX-M-3 cefotaxime-hydrolyzing β-lactamase that is closely related to the CTX-M-1/MEN-1 enzyme. Antimicrob Agents Chemother. 1998;42:827–832. doi: 10.1128/aac.42.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hall L M C, Livermore D M, Gur D, Akova M, Akalin H E. OXA-11, an extended-spectrum variant of OXA-10 (PSE-2) β-lactamase from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1993;37:1637–1644. doi: 10.1128/aac.37.8.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Henquell C, Chanal C, Sirot D, Labia R, Sirot J. Molecular characterization of nine different types of mutants among 107 inhibitor-resistant TEM β-lactamases from clinical isolates of Escherichia coli. Antimicrob Agents Chemother. 1995;39:427–430. doi: 10.1128/aac.39.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ho P L, Chow K H, Yuen K Y, Ng W S, Chau P Y. Comparison of a novel, inhibitor-potentiated disc-diffusion test with other methods for the detection of extended-spectrum β-lactamases in Escherichia coli and Klebsiella pneumoniae. J Antimicrob Chemother. 1998;42:49–54. doi: 10.1093/jac/42.1.49. [DOI] [PubMed] [Google Scholar]
- 68.Ho P L, Tsang D N C, Que T L, Ho M, Yuen K Y. Comparison of screening methods for detection of extended-spectrum β-lactamases and their prevalence among Escherichia coli and Klebsiella species in Hong Kong. APMIS. 2000;108:237–240. doi: 10.1034/j.1600-0463.2000.d01-50.x. [DOI] [PubMed] [Google Scholar]
- 69.Huletsky A, Knox J R, Levesque R C. Role of Ser-238 and Lys-240 in the hydrolysis of 3rd-generation cephalosporins by SHV-type beta-lactamases probed by site-directed mutagenesis and 3-dimensional modeling. J Biol Chem. 1993;268:3690–3697. [PubMed] [Google Scholar]
- 70.Huovinen S, Huovinen P, Jacoby G A. Detection of plasmid-mediated β-lactamases with DNA probes. Antimicrob Agents Chemother. 1988;32:175–179. doi: 10.1128/aac.32.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ibuka A, Taguchi A, Ishiguro M, Fushinobu S, Ishii Y, Kamitori S, Okuyama K, Yamaguchi K, Konno M, Matsuzawa H. Crystal structure of the E166A mutatant of extended-spectrum β-lactamase Toho-1 at 1.8 Å resolution. J Mol Biol. 1999;285:2079–2087. doi: 10.1006/jmbi.1998.2432. [DOI] [PubMed] [Google Scholar]
- 72.Ishii Y, Ohno A, Taguchi H, Imajo S, Ishiguro M, Matsuzawa H. Cloning and sequence of the gene encoding a cefotaxime-hydrolyzing class A β-lactamase isolated from Escherichia coli. Antimicrob Agents Chemother. 1995;39:2269–2275. doi: 10.1128/aac.39.10.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jacoby G A, Han P. Detection of extended-spectrum β-lactamases in clinical isolates of Klebsiella pneumoniae and Escherichia coli. J Clin Microbiol. 1996;34:908–911. doi: 10.1128/jcm.34.4.908-911.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jacoby G A, Medeiros A A. More extended-spectrum β-lactamases. Antimicrob Agents Chemother. 1991;35:1697–1704. doi: 10.1128/aac.35.9.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jacoby G A, Sutton L. Properties of plasmids responsible for production of extended-spectrum β-lactamases. Antimicrob Agents Chemother. 1991;35:164–169. doi: 10.1128/aac.35.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jarlier V, Nicolas M, Fournier G, Philippon A. Extended broad-spectrum β-lactamases conferring transferable resistance to newer β-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev Infect Dis. 1988;10:867–878. doi: 10.1093/clinids/10.4.867. [DOI] [PubMed] [Google Scholar]
- 77.John J F J, Rice L B. The microbial genetics of antibiotic cycling. Infect Control Hosp Epidemiol. 2000;21:S22–S31. doi: 10.1086/503170. [DOI] [PubMed] [Google Scholar]
- 78.Katsanis G P, Spargo J, Ferraro M J, Sutton L, Jacoby G A. Detection of Klebsiella pneumoniae and Escherichia coli strains producing extended-spectrum β-lactamases. J Clin Microbiol. 1994;32:691–696. doi: 10.1128/jcm.32.3.691-696.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim J, Kwon Y, Pai H, Kim J-W, Cho D-T. Survey of Klebsiella pneumoniae strains producing extended-spectrum β-lactamases: prevalence of SHV-12 and SHV-2a in Korea. J Clin Microbiol. 1998;36:1446–1449. doi: 10.1128/jcm.36.5.1446-1449.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim J, Lee H-J. Rapid discriminatory detection of genes coding for SHV β-lactamases by ligase chain reaction. Antimicrob Agents Chemother. 2000;44:1860–1864. doi: 10.1128/aac.44.7.1860-1864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kliebe C, Nies B A, Meyer J F, Tolxdorff-Neutzling R M, Wiedemann B. Evolution of plasmid-coded resistance to broad-spectrum cephalosporins. Antimicrob Agents Chemother. 1985;28:302–307. doi: 10.1128/aac.28.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lefon-Guibout V, Speldooren V, Heym B, Nicolas-Chanoine M-H. Epidemiological survey of amoxicillin-clavulanate resistance and corresponding molecular mechanisms in Escherichia coli isolates in France: new genetic features of blaTEM genes. Antimicrob Agents Chemother. 2000;44:2709–2714. doi: 10.1128/aac.44.10.2709-2714.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lemozy J, Sirot D, Chanal C, Hue C, Labia R, Dabernat H, Sirot J. First characterization of inhibitor-resistant TEM (IRT) β-lactamases in Klebsiella pneumoniae strains. Antimicrob Agents Chemother. 1995;33:2580–2582. doi: 10.1128/aac.39.11.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu P Y F, Gur D, Hall L M C, Livermore D M. Survey of the prevalence of β-lactamases amongst 1000 gram-negative bacilli isolated consecutively at the Royal London Hospital. J Antimicrob Chemother. 1992;30:429–447. doi: 10.1093/jac/30.4.429. [DOI] [PubMed] [Google Scholar]
- 85.Livermore D M. β-Lactamases in laboratory and clinical resistance. Clin Microbiol Rev. 1995;8:557–584. doi: 10.1128/cmr.8.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Livermore D M, Yuan M. Antibiotic resistance and production of extended-spectrum β-lactamases amongst Klebsiella spp. from intensive care units in Europe. J Antimicrob Chemother. 1996;38:409–424. doi: 10.1093/jac/38.3.409. [DOI] [PubMed] [Google Scholar]
- 87.Lucet J-C, Decré D, Fichelle A, Joly-Guillou M-L, Pernet M, Deblangy C, Kosmann M-J, Régnier B. Control of a prolonged outbreak of extended-spectrum β-lactamase-producing Enterobacteriaceae in a university hospital. Clin Infect Dis. 1999;20:1411–1418. doi: 10.1086/313511. [DOI] [PubMed] [Google Scholar]
- 88.Ma L, Ishii Y, Ishiguro M, Matsuzawa H, Yamaguchi K. Cloning and sequencing of the gene encoding Toho-2, a class A β-lactamase preferentially inhibited by tazobactam. Antimicrob Agents Chemother. 1998;42:1181–1186. doi: 10.1128/aac.42.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mabilat C, Courvalin P. Development of “oligotyping” for characterization and molecular epidemiology of TEM β-lactamases in members of the family Enterobacteriaceae. Antimicrob Agents Chemother. 1990;34:2210–2216. doi: 10.1128/aac.34.11.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mabilat C, Goussard S. PCR detection and identification of genes for extended-spectrum β-lactamases. In: Persing D H, Smith T F, Tenover T C, White T J, editors. Diagnostic molecular microbiology. Washington, D.C.: American Society for Microbiology; 1993. pp. 553–559. [Google Scholar]
- 91.Marchandin H, Carriere C, Sirot D, Jean-Pierre H, Darbas H. TEM-24 produced by four different species of Enterobacteriaceae, including Providencia rettgeri, in a single patient. Antimicrob Agents Chemother. 1999;43:2069–2073. doi: 10.1128/aac.43.8.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martinéz-Martinéz L, Hernández-Allés S, Albertí S, Tomás J, Benedi V, Jacoby G. In vivo selection of porin-deficient mutants of Klebsiella pneumoniae with increased resistance to cefoxitin and expanded-spectrum cephalosporins. Antimicrob Agents Chemother. 1996;40:342–348. doi: 10.1128/aac.40.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Matsumoto Y, Ikeda F, Kamimura T, Yokota Y, Mine Y. Novel plasmid-mediated β-lactamase from Escherichia coli that inactivates oxyimino-cephalosporins. Antmicrob Agents Chemother. 1988;32:1243–1246. doi: 10.1128/aac.32.8.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Matsumoto Y, Inoue M. Characterization of SFO-1, a plasmid-mediated inducible class A β-lactamase from Enterobacter cloacae. Antimicrob Agents Chemother. 1999;43:307–313. doi: 10.1128/aac.43.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Medeiros A, Mayer K H, Opal S M. Plasmid-mediated Beta-lactamases. Antimicrob Newsl. 1988;5:61–65. [Google Scholar]
- 96.Medeiros A A. β-Lactamases. Br Med Bull. 1984;40:18–27. doi: 10.1093/oxfordjournals.bmb.a071942. [DOI] [PubMed] [Google Scholar]
- 97.Medeiros A A, Crellin J. Comparative susceptibility of clinical isolates producing extended-spectrum beta-lactamases to ceftibuten: effect of a large inoculum. Pediatr Infect Dis J. 1997;16:S49–S55. doi: 10.1097/00006454-199703001-00005. [DOI] [PubMed] [Google Scholar]
- 98.Meyer K S, Urban C, Eagan J A, Berger B J, Rahal J J. Nosocomial outbreak of Klebsiella infection resistant to late generation cephalosporins. Ann Intern Med. 1993;119:353–358. doi: 10.7326/0003-4819-119-5-199309010-00001. [DOI] [PubMed] [Google Scholar]
- 99.Miró E, del Cuerpo M, Navarro F, Sabaté M, Mireleis B, Prats G. Emergence of clinical isolates with decreased susceptibility to ceftazidime and synergistic effect with co-amoxiclav due to SHV-1 hyperproduction. J Antimicrob Chemother. 1998;42:535–538. doi: 10.1093/jac/42.4.535. [DOI] [PubMed] [Google Scholar]
- 100.Moland E S, Sanders C C, Thomson K S. Can results obtained with commercially available MicroScan microdilution panels serve as an indicator of β-lactamase production among Escherichia coli and Klebsiella isolates with hidden resistance to expanded-spectrum cephalosporins and aztreonam? J Clin Microbiol. 1998;36:2575–2579. doi: 10.1128/jcm.36.9.2575-2579.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Morosini M I, Canton R, Martinez-Beltran J, Negri M C, Perez-Diaz J C, Baquero F, Blazquez J. New extended spectrum TEM-type β-lactamase from Salmonella enterica subsp. enterica isolated in a nosocomial outbreak. Antimicrob Agents Chemother. 1995;39:458–461. doi: 10.1128/aac.39.2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mugnier P, Casin I, Bouthors A T, Collatz E. Novel OXA-10-derived extended-spectrum β-lactamases selected in vivo or in vitro. Antimicrob Agents Chemother. 1998;42:3113–3116. doi: 10.1128/aac.42.12.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mugnier P, Dubrous P, Casin I, Arlet G, Collatz E. A TEM-derived extended-spectrum β-lactamase in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1996;40:2488–2493. doi: 10.1128/aac.40.11.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mugnier P, Podglajen I, Goldstein F W, Collatz E. Carbapenems as inhibitors of OXA-13, a novel integron-encoded β-lactamase in Pseudomonas aeruginosa. Microbiology. 1998;144:1021–1031. doi: 10.1099/00221287-144-4-1021. [DOI] [PubMed] [Google Scholar]
- 105.M'Zali F H, Chanawong A, Kerr K G, Birkenhead D, Hawkey P M. Detection of extended-spectrum β-lactamases in members of the family Enterobacteriaceae: comparison of the MAST DD test, the double disc and the Etest ESBL. J Antimicrob Chemother. 2000;45:881–885. doi: 10.1093/jac/45.6.881. [DOI] [PubMed] [Google Scholar]
- 106.M'Zali F H, Heritage J, Gascoyne-Binzi D M, Snelling A M, Hawkey P M. PCR single strand conformational polymorphism can be used to detect the gene encoding SHV-7 extended-spectrum β-lactamase and to identify different SHV genes within the same strain. J Antimicrob Chemother. 1998;41:123–125. doi: 10.1093/jac/41.1.123. [DOI] [PubMed] [Google Scholar]
- 107.M'Zali F-H, Gascoyne-Binzi D M, Heritage J, Hawkey P M. Detection of mutations conferring extended-spectrum activity on SHV β-lactamases using polymerase chain reaction single strand conformational polymorphism (PCR-SSCP) J Antimicrob Chemother. 1996;37:797–802. doi: 10.1093/jac/37.4.797. [DOI] [PubMed] [Google Scholar]
- 108.Naas T, Philippon L, Poirel L, Ronco E, Nordman P. An SHV-derived extended-spectrum β-lactamase in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1999;43:1281–1284. doi: 10.1128/aac.43.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Naas T, Poirel L, Karim A, Nordmann P. Molecular characterization of In50, a class1 integron encoding the gene for the extended-spectrum β-lactamase VEB-1 in Pseudomonas aeruginosa. FEMS Microbiol Lett. 1999;176:411–419. doi: 10.1111/j.1574-6968.1999.tb13691.x. [DOI] [PubMed] [Google Scholar]
- 110.Naas T, Sougakoff W, Casetta A, Nordman P. Molecular characterization of OXA-20, a novel class D β-lactamase, and its integron from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1998;42:2074–2083. doi: 10.1128/aac.42.8.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7–A5 and informational supplement M100–S10. Wayne, Pa: National Committee for Clinical Laboratory Standards; 2000. [Google Scholar]
- 112.Naumovski L, Quinn J P, Miyashiro D, Patel M, Bush K, Singer S B, Graves D, Palzkill T, Arvin A M. Outbreak of ceftazidime resistance due to a novel extended-spectrum β-lactamase in isolates from cancer patients. Antimicrob Agents Chemother. 1992;36:1991–1996. doi: 10.1128/aac.36.9.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nordman P, Ronco E, Naas T, Duport C, Michel-Briand Y, Labia R. Characterization of a novel extended-spectrum β-lactamase from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1993;37:962–969. doi: 10.1128/aac.37.5.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nordmann P. Trends in β-lactam resistance among Enterobacteriaceae. Clin Infect Dis. 1998;27:S100–S106. doi: 10.1086/514905. [DOI] [PubMed] [Google Scholar]
- 115.Nordmann P, Poirel L, Kubina M, Casetta A, Naas T. Biochemical-genetic characterization and distribution of OXA-22, a chromosomal and inducible class D β-lactamase from Ralstonia (Pseudomonas) pickettii. Antimicrob Agents Chemother. 2000;44:2201–2204. doi: 10.1128/aac.44.8.2201-2204.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nüesch-Inderbinen M T, Hächler F H K H. Detection of genes coding for extended-spectrum SHV beta-lactamases in clinical isolates by a molecular genetic method, and comparison with the Etest. Eur J Clin Microbiol Infect Dis. 1996;15:398–402. doi: 10.1007/BF01690097. [DOI] [PubMed] [Google Scholar]
- 117.Ouellette M, Paul G C, Philippon A M, Roy P H. Oligonucleotide probes (TEM-1, OXA-1) versus isoelectric focusing in β-lactamase characterization of 114 resistant strains. Antimicrob Agents Chemother. 1988;32:397–399. doi: 10.1128/aac.32.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Page R D M. TREEVIEW: an application to display phylogenetic trees on personal computers. Comput Appl Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- 119.Pai H, Lyu S, Lee J H, Kim J, Kwon Y, Kim J-W, Choe K W. Survey of extended-spectrum β-lactamases in clinical isolates of Escherichia coli and Klebsiella pneumoniae: prevalence of TEM-52 in Korea. J Clin Microbiol. 1999;37:1758–1763. doi: 10.1128/jcm.37.6.1758-1763.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Paltzkill T, Thomson K S, Sanders C C, Moland E S, Huang W, Milligan T W. New variant of TEM-10 β-lactamase gene produced by a clinical isolate of Proteus mirabilis. Antimicrob Agents Chemother. 1995;39:1199–1200. doi: 10.1128/aac.39.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pangon B, Bizet C, Buré A, Pichon F, Philippon A, Regnier B, Gutmann L. In vivo selection of a cephamycin-resistant, porin-deficient mutant of Klebsiella pneumoniae producing a TEM-3 β-lactamase. J Infect Dis. 1989;159:1005–1006. doi: 10.1093/infdis/159.5.1005. [DOI] [PubMed] [Google Scholar]
- 122.Paterson D L, Mulazimoglu L, Casellas J M, Ko W C, Goossens H, Von Gottberg A, Mohapatra S, Trenholme G M, Klugman K P, McCormack J G, Yu V L. Epidemiology of ciprofloxacin resistance and its relationship to extended-spectrum beta-lactamase production in Klebsiella pneumoniae isolates causing bacteremia. Clin Infect Dis. 2000;30:473–478. doi: 10.1086/313719. [DOI] [PubMed] [Google Scholar]
- 123.Paterson D L, Yu V L. Extended-spectrum β-lactamases: a call for improved detection and control. Clin Infect Dis. 1999;29:1419–1422. doi: 10.1086/313559. [DOI] [PubMed] [Google Scholar]
- 124.Patterson J E, Hardin T C, Kelly C A, Garcia R C, Jorgensen J H. Association of antibiotic utilization measures and control of multiple-drug resistance in Klebsiella pneumoniae. Infect Control Hosp Epidemiol. 2000;21:455–458. doi: 10.1086/501787. [DOI] [PubMed] [Google Scholar]
- 125.Peña C, Pujol M, Ardanuy C, Ricart A, Pallares R, Liñares J A J, Gudiol F. Epidemiology an successful control of a large outbreak due to Klebsiella pneumoniae producing extended-spectrum β-lactamases. Antimicrob Agents Chemother. 1998;42:53–58. doi: 10.1128/aac.42.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Peña C, Pujol M, Ricart A, Ardanuy C, Ayats J, Liñares J, Garrigosa F, Ariza J, Gudiol F. Risk factors for faecal carriage of Klebsiella pneumoniae producing extended-spectrum β-lactamase in the intensive care unit. J Hosp Infect. 1997;35:9–16. doi: 10.1016/s0195-6701(97)90163-8. [DOI] [PubMed] [Google Scholar]
- 127.Perilli M, Felici A, Franceschini N, Santis A D, Pagani L, Luzzaro F, Oratore A, Rossolini G M, Knox J R, Amicosante G. Characterization of a new TEM-derived β-lactamase produced in a Serratia marcescens strain. Antimicrob Agents Chemother. 1997;41:2374–2382. doi: 10.1128/aac.41.11.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Perilli M, Segatore B, Massis M R D, Riccio M L, Bianchi C, Zollo A, Rossolini G M, Amicosante G. TEM-72, a new extended-spectrum β-lactamase detected in Proteus mirabilis and Morganella morganii in Italy. Antimicrob Agents Chemother. 2000;44:2537–2539. doi: 10.1128/aac.44.9.2537-2539.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Petit A, Yaghlane-Bouslama H B, Sofer L, Labia R. Does high level production of SHV-type penicillinase confer resistance to ceftazidime in Enterobacteriaceae? FEMS Microbiol Lett. 1992;92:89–94. doi: 10.1016/0378-1097(92)90547-2. [DOI] [PubMed] [Google Scholar]
- 130.Petrosino J G, Palzkill T. Systematic mutagenesis of the active-site omega loop of TEM-1 beta-lactamase. J Bacteriol. 1996;178:1821–1828. doi: 10.1128/jb.178.7.1821-1828.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Philippon L N, Naas T, Bouthors A-T, Barakett V, Nordmann P. OXA-18, a class D clavulanic acid-inhibited extended-spectrum β-lactamase from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1997;41:2188–2195. doi: 10.1128/aac.41.10.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Piroth L, Aubé H, Doise J-M, Vincent-Martin M. Spread of extended-spectrum β-lactamase-producing Klebsiella pneumoniae: are β-lactamase inhibitors of therapeutic value? Clin Infect Dis. 1998;27:76–80. doi: 10.1086/514643. [DOI] [PubMed] [Google Scholar]
- 133.Pitout J D D, Thomson K S, Hanson N D, Ehrhardt A F, Moland E S, Sanders C C. β-Lactamases responsible for resistance to expanded-spectrum cephalosporins in Klebsiella pneumoniae, Escherichia coli, and Proteus mirabilis isolates recovered in South Africa. Antimicrob Agents Chemother. 1998;42:1350–1354. doi: 10.1128/aac.42.6.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Poirel L, Girlich D, Naas T, Nordmann P. OXA-28, an extended-spectrum variant of OXA-10, β-lactamase from Pseudomonas aeruginosa and its plasmid- and integron-located gene. Antimicrob Agents Chemother. 2001;45:447–453. doi: 10.1128/AAC.45.2.447-453.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Poirel L, Naas T, Guibert M, Chaibi E B, Labia R, Nordmann P. Molecular and biochemical characterization of VEB-1, a novel class A extended-spectrum β-lactamase encoded by an Escherichia coli integron gene. Antimicrob Agents Chemother. 1999;43:573–581. doi: 10.1128/aac.43.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Poirel L, Thomas I L, Naas T, Karim A, Nordman P. Biochemical sequence analyses of GES-1, a novel class A extended-spectrum β-lactamase, and the class 1 integron IN52 from Klebsiella pneumoniae. Antimicrob Agents Chemother. 2000;44:622–632. doi: 10.1128/aac.44.3.622-632.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Prinarakis E E, Miriagou V, Tzelepi E, Gazouli M, Tzouvelekis L S. Emergence of an inhibitor-resistant β-lactamase (SHV-10) derived from an SHV-5 variant. Antimicrob Agents Chemother. 1997;41:838–840. doi: 10.1128/aac.41.4.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rahal J J, Urban C, Horn D. Class restriction of cephalosporin use to control total cephalosporin resistance in nosocomial Klebsiella. JAMA. 1998;280:1233–1237. doi: 10.1001/jama.280.14.1233. [DOI] [PubMed] [Google Scholar]
- 139.Rasheed J K, Jay C, Metchock B, Berkowitz F, Weigel L, Crellin J, Steward C, Hill B, Medeiros A A, Tenover F C. Evolution of extended-spectrum β-lactam resistance (SHV-8) in a strain of Escherichia coli during multiple episodes of bacteremia. Antimicrob Agents Chemother. 1997;41:647–653. doi: 10.1128/aac.41.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rice L B. Successful interventions for gram-negative resistance to extended-spectrum β-lactam antibiotics. Pharmacotheropy. 1999;19:120S–128S. doi: 10.1592/phco.19.12.120s.31699. [DOI] [PubMed] [Google Scholar]
- 141.Rice L B, Carias L L, Hujer A M, Bonfede M, Hutton R, Hoyen C, Bonomo R A. High-level expression of chromosomally encoded SHV-1 β-lactamase and an outer membrane protein change confer resistance to ceftazidime and piperacillin-tazobactam in a clinical isolate of Klebsiella pneumoniae. Antimicrob Antimicrob Agents Chemother. 2000;44:362–367. doi: 10.1128/aac.44.2.362-367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rice L B, Eckstein E C, DeVente J, Shlaes D M. Ceftazidime-resistant Klebsiella pneumoniae isolates recovered at the Cleveland Department of Veterans Affairs Medical Center. Clin Infect Dis. 1996;23:118–124. doi: 10.1093/clinids/23.1.118. [DOI] [PubMed] [Google Scholar]
- 143.Rice L B, Willey S H, Papanicolaou G A, Medieros A A, Eliopoulos G M, Moellering J R C, Jacoby G A. Outbreak of ceftazidime resistance caused by extended-spectrum β-lactamases at a Massachusetts chronic-care facility. Antimicrob Agents Chemother. 1990;34:2193–2199. doi: 10.1128/aac.34.11.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rice L B, Yao J D C, Klimm K, Eliopoulous G M, Moellering R C. Efficacy of different β-lactams against an extended-spectrum β-lactamase-producing Klebsiella pneumoniae strain in the rat intra-abdominal abscess model. Antimicrob Agents Chemother. 1991;35:1243–1244. doi: 10.1128/aac.35.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Richmond M H, Sykes R B. The β-lactamases of gram-negative bacteria and their possible physiological role. Adv Microb Physiol. 1973;9:31–88. doi: 10.1016/s0065-2911(08)60376-8. [DOI] [PubMed] [Google Scholar]
- 146.Rosenau A, Cattier B, Gousset N, Harriau P, Philippon A, Quentin R. Capnocytophaga ochracea: characterization of a plasmid-encoded extended-spectrum TEM-17 β-lactamase in the phylum Flavobacter-Bacteroides. Antimicrob Agents Chemother. 2000;44:760–762. doi: 10.1128/aac.44.3.760-762.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rossolini G M, Franceschini N, Lauretti L, Caravelli B, Riccio M L, Galleni M, Frère J-M, Amicosante G. Cloning of a Chryseobacterium (Flavobacterium) menigiosepticum chromosomal gene (blaACME) encoding an extended-spectrum class A β-lactamase related to the Bacteroides cephalosporinases and the VEB-1 and PER β-lactamases. Antimicrob Agents Chemother. 1999;43:2193–2199. doi: 10.1128/aac.43.9.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sabaté M, Tarragó R, Navarro F, Miró E, Vergés C, Barbé J, Prats G. Cloning and sequence of the gene encoding a novel cefotaxime-hydrolyzing β-lactamase (CTX-M-9) from Escherichia coli in Spain. Antimicrob Agents Chemother. 2000;44:1970–1973. doi: 10.1128/aac.44.7.1970-1973.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sanders C C, Barry A L, Washington J A, Shubert C, Moland E S, Traczewski M M, Knapp C, Mulder R. Detection of extended-spectrum-β-lactamase-producing members of the family Enterobacteriaceae with the Vitek ESBL test. J Clin Microbiol. 1996;34:2997–3001. doi: 10.1128/jcm.34.12.2997-3001.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sanders C C, Peyret M, Moland E S, Shubert C, Thomson K S, Boufgras J-M, Sanders W E., Jr Ability of the VITEK 2 Advanced Expert system to identify β-lactam phenotypes in isolates of Enterobacteriaceae and Pseudomonas aeruginosa. J Clini Microbiol. 2000;38:570–574. doi: 10.1128/jcm.38.2.570-574.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shannon K, Fung K, Stapleton P, Anthony R, Power E, French G. A hospital outbreak of extended-spectrum β-lactamase-producing Klebsiella pneumoniae investigated by RAPD typing and analysis of the genetics and mechanisms of resistance. J Hosp Infect. 1998;39:291–300. doi: 10.1016/s0195-6701(98)90294-8. [DOI] [PubMed] [Google Scholar]
- 152.Shannon K, Stapleton P, Xiang X, Johnson A, Beattie H, Bakri F E, Cookson B, French G. Extended-spectrum β-lactamase-producing Klebsiella pneumoniae strains causing nosocomial outbreaks of infection in the United Kingdom. J Clin Microbiol. 1998;36:3105–3110. doi: 10.1128/jcm.36.10.3105-3110.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Silva J, Aguilar C, Ayala G, Estrada M A, Garza-Ramos U, Lara-Lemus R, Ledezma L. TLA-1: a new plasmid-mediated extended-spectrum β-lactamase from Escherichia coli. Antimicrob Agents Chemother. 2000;44:997–1003. doi: 10.1128/aac.44.4.997-1003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sirot D, Recule C, Chaibi E B, Bret L, Croize J, Chanal-Claris C, Labia R, Sirot J. A complex mutant of TEM-1 β-lactamase with mutations encountered in both IRT-4 and extended-spectrum TEM-15, produced by an Escherichia coli clinical isolate. Antimicrob Agents Chemother. 1997;41:1322–1325. doi: 10.1128/aac.41.6.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Siu L K, Lo J Y C, Yuen K Y, Chau P Y, Ng M H, Ho P L. β-Lactamases in Shigella flexneri isolates from Hong Kong and Shanghai and a novel OXA-1-like β-lactamase, OXA-30. Antimicrob Agents Chemother. 2000;44:2034–2038. doi: 10.1128/aac.44.8.2034-2038.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Soilleux M J, Morand A M, Arlet G J, Scavizzi M R, Labia R. Survey of Klebsiella pneumoniae producing extended-spectrum β-lactamases: prevalence of TEM-3 and first identification of TEM-26 in France. Antimicrob Agents Chemother. 1996;40:1027–1029. doi: 10.1128/aac.40.4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sougakoff W, Goussard S, Courvalin P. The TEM-3 β-lactamase, which hydrolyzes broad-spectrum cephalosporins, is derived from the TEM-2 penicillinase by two amino acid substitutions. FEMS Microbiol Lett. 1988;56:343–348. [Google Scholar]
- 158.Spencer R C, Wheat P F, Winstanley T G, Cox D M, Plested S J. Novel β-lactamase in a clinical isolate of Klebsiella pneumoniae conferring unusual resistance to β-lactam antibiotics. J Antimicrob Chemother. 1987;20:919–921. doi: 10.1093/jac/20.6.919. [DOI] [PubMed] [Google Scholar]
- 159.Stapleton P, Shannon K P, French G L. Construction and characterization of mutants of the TEM-1 β-lactamase containing amino acid substitutions associated with both extended-spectrum resistance and resistance to β-lactamase inhibitors. Antimicrob Agents Chemother. 1999;43:1881–1887. doi: 10.1128/aac.43.8.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Steward C D, Wallace D, Hubert S K, Lawton R, Fridkin S K, Gaynes R P, Jr J E M, Tenover F C. Ability of laboratories to detect emerging antimicrobial resistance in nosocomial pathogens: a survey of Project ICARE laboratories. Diagn Microbiol Infect Dis. 2000;38:59–67. doi: 10.1016/s0732-8893(00)00161-9. [DOI] [PubMed] [Google Scholar]
- 161.Stobberingh E E, Arends J, Hoogkamp-Korstanje J A A, Goessens W H F, Visser M R, Buiting A G M, Debets-Ossenkopp Y J, v. Ketel R J, v. Ogtrop M L, Sabbe L J M, Voorn G P, Winter H L J, van Zeijl J H. Occurrence of extended-spectrum beta-lactamases in Dutch hospitals. Infection. 1999;27:348–354. doi: 10.1007/s150100050041. [DOI] [PubMed] [Google Scholar]
- 162.Sutcliffe J G. Nucleotide sequence of the ampicillin resistance gene of Escherichia coli plasmid pBR322. Proc Natl Acad Sci USA. 1978;75:3737–3741. doi: 10.1073/pnas.75.8.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Szabó D, Filetóth Z, Szentandrássy J, Némedi M, Tóth E, Jeney C, Kispál G, Rozgonyi F. Molecular epidemiology of a cluster of cases due to Klebsiella pneumoniae producing SHV-5 extended-spectrum β-lactamase in the premature intensive care unit of a Hungarian hospital. J Clin Microbiol. 1999;37:4167–4169. doi: 10.1128/jcm.37.12.4167-4169.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tenover F C, Mohammed J M, Gorton T, Dembek Z F. Detection and reporting of organisms producing extended-spectrum β-lactamases: survey of laboratories in Connecticut. J Clin Microbiol. 1999;37:4065–4070. doi: 10.1128/jcm.37.12.4065-4070.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tenover F C, Mohammed M J, Stelling J, O'Brien T, Williams R. Ability of laboratories to detect emerging antimicrobial resistance: proficiency testing and quality control results from the World Health Organization's external quality assurance system for antimicrobial susceptibility testing. J Clin Microbiol. 2001;39:241–250. doi: 10.1128/JCM.39.1.241-250.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tessier F, Arpin C, Allery A, Quentin C. Molecular characterization of a TEM-21 β-lactamase in a clinical isolate of Morganella morganii. Antimicrob Agents Chemother. 1998;42:2125–2127. doi: 10.1128/aac.42.8.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tham T N, Mabilat C, Courvalin P, Guesdon J-L. Biotinylated oligonucleotide probes for the detection and characterization of TEM-type extended-spectrum β-lactamases in Enterobacteriaceae. FEMS Microbiol Lett. 1990;69:109–116. doi: 10.1016/0378-1097(90)90423-n. [DOI] [PubMed] [Google Scholar]
- 168.Thompson J D, Gibson T J, Plewniak F, Jeanmougin F, Higgins D G. The CLUSTAL_X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Thomson K S, Sanders C C. Detection of extended-spectrum β-lactamases in members of the family Enterobacteriaceae: Comparison of the double-disk and three-dimensional tests. Antimicrob Agents Chemother. 1992;36:1877–1882. doi: 10.1128/aac.36.9.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Thomson K S, Sanders C C, Moland E S. Use of microdilution panels with and without β-lactamase inhibitors as a phenotypic test for β-lactamase production among Escherichia coli, Klebsiella spp., Enterobacter spp., Citrobacter freundii, and Serratia marcescens. Antimicrob Agents Chemother. 1999;43:1393–1400. doi: 10.1128/aac.43.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tzelepi E, Giakkoupi P, Sofianou D, Loukova V, Kermeroglou A, Tsakris A. Detection of extended-spectrum β-lactamases in clinical isolates of Enterobacter cloacae and Enterobacter aerogenes. J Clin Microbiol. 2000;38:542–546. doi: 10.1128/jcm.38.2.542-546.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tzouvelekis L S, Bonomo R A. SHV-type β-lactamases. Curr Pharm Des. 1999;5:847–864. [PubMed] [Google Scholar]
- 173.Tzouvelekis L S, Gazouli M, Markogiannakis A, Paraskaki E, Legakis N J, Tzelepi E. Emergence of resistance to third-generation cephalosporins amongst Salmonella typhimurium isolates in Greece: report of the first three cases. J Antimicrob Chemother. 1998;42:273–275. doi: 10.1093/jac/42.2.273. [DOI] [PubMed] [Google Scholar]
- 174.Tzouvelekis L S, Tzelepi E, Tassios P T, Legakis N J. CTX-M-type β-lactamases: an emerging group of extended-spectrum enzymes. Int J Antimicrob Agents. 2000;14:137–143. doi: 10.1016/s0924-8579(99)00165-x. [DOI] [PubMed] [Google Scholar]
- 175.Urban C, Meyer K S, Mariano N, Rahal J J, Flamm R, Rasmussen B A, Bush K. Identification of TEM-26 β-lactamase responsible for a major outbreak of ceftazidime-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother. 1994;38:392–395. doi: 10.1128/aac.38.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Vahaboglu H, Dodanli S, Eroglu C, Öztürk R, Soyletir G, Yildirim I, Avkan V. Characterization of multiple-antibiotic-resistant Salmonella typhimurium strains: molecular epidemiology of PER-1-producing isolates and evidence for nosocomial plasmid exchange by a clone. J Clin Microbiol. 1996;34:2942–2946. doi: 10.1128/jcm.34.12.2942-2946.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Vahaboglu H, Hall L M C, Mulazimoglu L, Dodanli S, Yildirim I, Livermore D M. Resistance to extended-spectrum cephalosporins, caused by PER-1 β-lactamase, in Salmonella typhimurium from Istanbul, Turkey. J Med Microbiol. 1995;43:294–299. doi: 10.1099/00222615-43-4-294. [DOI] [PubMed] [Google Scholar]
- 178.Vahaboglu H, Ozturk R, Akbal H, Saribas S, Tansel O, Coskunkan F. Practical approach for detection and identification of OXA-10-derived ceftazidime-hydrolyzing extended-spectrum β-lactamases. J Clin Microbiol. 1998;36:827–829. doi: 10.1128/jcm.36.3.827-829.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Vahaboglu H, Öztürk R, Aygün G, Coskunkan F, Yaman A, Kaygusuz A, Leblebicioglu H, Balik I, Aydin K, Otkun M. Widespread detection of PER-1-type extended-spectrum β-lactamases among nosocomial Acinetobacter and Pseudomonas aeruginosa isolates in Turkey: a nationwide multicenter study. Antimicrob Agents Chemother. 1997;41:2265–2269. doi: 10.1128/aac.41.10.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Vakulenko S B, Toth M, Taibi P, Mobishary S, Lerner S A. Effects of Asp-179 mutations in TEMpUC19 β-lactamase on susceptibility to β-lactams. Antimicrob Agents Chemother. 1995;39:1878–1880. doi: 10.1128/aac.39.8.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Vatopoulos A C, Philippon A, Tzouvelekis L S, Komninou Z, Legakis N J. Prevalence of a transferable SHV-5 type β-lactamase in clinical isolates of Klebsiella pneumoniae and Escherichia coli in Greece. J Antimicrob Chemother. 1990;26:635–648. doi: 10.1093/jac/26.5.635. [DOI] [PubMed] [Google Scholar]
- 182.Venkatachalam K V, Huang W, LaRocco M, Palzkill T. Characterization of TEM-1 beta-lactamase mutants from positions 238–241 with increased catalytic efficiency for ceftazidime. J Biol Chem. 1994;269:23444–23450. [PubMed] [Google Scholar]
- 183.Vercauteren E, Descheemaeker P, Leven M, Sanders C C, Goossens H. Comparison of screening methods for the detection of extended-spectrum β-lactamases and their prevalence among blood isolates of Escherichia coli and Klebsiella spp. in a Belgian teaching hospital. J Clin Microbiol. 1997;35:2191–2197. doi: 10.1128/jcm.35.9.2191-2197.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Vila J, Navia M, Ruiz J, Casals C. Cloning and nucleotide sequence analysis of a gene encoding an OXA-derived β-lactamase in Acinetobacter baumannii. Antimicrob Agents Chemother. 1997;41:2757–2759. doi: 10.1128/aac.41.12.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Villari P, Iacuzio L, Torre I, Scarcella A. Molecular epidemiology as an effective tool in the surveillance of infections in the neonatal intensive care unit. J Infect. 1998;37:274–281. doi: 10.1016/s0163-4453(98)92107-7. [DOI] [PubMed] [Google Scholar]
- 186.Wiener J, Quinn J P, Bradford P A, Goering R V, Nathan C, Bush K, Weinstein R A. Multiple antibiotic-resistant Klebsiella and Escherichia coli in nursing homes. JAMA. 1999;281:517–523. doi: 10.1001/jama.281.6.517. [DOI] [PubMed] [Google Scholar]
- 187.Yagi T, Kruokawa H, Shibata N, Shibayama K, Arakawa Y. A preliminary survey of extended-spectrum β-lactamases (ESBLs) in clinical isolates of Klebsiella pneumoniae and Escherichia coli in Japan. FEMS Microbiol Lett. 2000;184:53–56. doi: 10.1111/j.1574-6968.2000.tb08989.x. [DOI] [PubMed] [Google Scholar]
- 188.Yan J-J, Wu S-M, Tsai S-H, Wu J-J, Su I-J. Prevalence of SHV-12 among clinical isolates of Klebsiella pneumoniae producing extended-spectrum β-lactamases and identification of a novel AmpC enzyme (CMY-8) in southern Taiwan. Antimicrob Agents Chemother. 2000;44:1438–1442. doi: 10.1128/aac.44.6.1438-1442.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yang Y, Bhachech N, Bradford P A, Jett B D, Sahm D F, Bush K. Ceftazidime-resistant Klebsiella pneumoniae and E. coli isolates producing TEM-10 and TEM-43 from St. Louis. Antimicrob Agents Chemother. 1998;42:1671–1676. doi: 10.1128/aac.42.7.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yuan M, Aucken H, Hall L M C, Pitt T L, Livermore D M. Epidemiological typing of kebsiellae with extended-spectrum β-lactamases from European intensive care units. J Antimicrob Chemother. 1998;41:527–539. doi: 10.1093/jac/41.5.527. [DOI] [PubMed] [Google Scholar]
- 191.Zhou X Y, Bordon F, Sirot D, Kitzis M-D, Gutman L. Emergence of clinical isolates of Escherichia coli producing TEM-1 derivatives or an OXA-1 β-lactamase conferring resistance to β-lactamase inhibitors. Antimicrob Agents Chemother. 1994;38:1085–1089. doi: 10.1128/aac.38.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]