Structured Abstract:
Background:
In response to concerns about opioid addiction following surgery, many states have implemented laws capping the days supplied for initial postoperative prescriptions. However, few studies have examined changes in risk of prolonged opioid use associated with the initial amount prescribed.
Objective:
To estimate the risk of prolonged opioid use associated with length of initial opioid prescribed and the potential impact of prescribing limits.
Research Design:
Using Medicare insurance claims (2007 – 2017) we identified opioid-naïve adults undergoing surgery. Using g-computation methods with logistic regression models, we estimated the risk of prolonged opioid use (≥1 opioid prescription dispensed in 3 consecutive 30-day windows following surgery) associated with varying initial number of days supplied. We then estimate the potential reduction in cases of prolonged opioid use associated with varying prescribing limits.
Results:
We identified 1,060,596 opioid-naïve surgical patients. Among the 70.0% who received an opioid for postoperative pain, 1.9% had prolonged opioid use. The risk of prolonged use increased from 0.7% (1 day supply) to 4.4% (15+ days). We estimated that a prescribing limit of 4 days would be associated with a risk reduction of 4.84 (3.59,6.09) per thousand patients and would be associated with 2,255 cases of prolonged use potentially avoided. The commonly used day supply limit of 7 would be associated with a smaller reduction in risk (aRD=2.04 [−0.17,4.25] per thousand).
Conclusions:
Risk of prolonged opioid use following surgery increased monotonically with increasing prescription duration. Common prescribing maximums based on days supplied may impact many patients but are associated with relatively low numbers of reduced cases of prolonged use. Any prescribing limits need to be weighed against the need for adequate pain management.
Introduction
Chronic opioid use is reported to be the most common postsurgical complication among opioid-naïve patients.1 One recent study of opioid-naïve adults found that the amount of opioids prescribed for postoperative pain increased between 2005 and 2015, and longer prescriptions were associated with an increased risk of opioid use in the 90–180 days following surgery.2 Persistent postoperative opioid use is associated with increased healthcare spending attributable to readmissions and ambulatory care visits.3
Management of pain in older adults is further complicated by the relatively higher prevalence of comorbidity, polypharmacy, cognitive impairment, and physiologic changes affecting pharmacokinetic processes.4–7 One study found that older adults receiving a prescription within the week following low-risk surgery were 44% more likely to go on to use opioids a year from surgery.8 As the demographic composition of the United States shifts towards older individuals and advances in surgical techniques continue to develop, the number of older adults receiving surgical therapy continues to rise.7,9–12
In response to the concerns of excessive opioid prescribing, 36 states (as of October 2019) have implemented laws placing limits on opioid prescriptions.13 While specific limits vary, 23 states use a 7 day supply maximum for initial prescriptions for acute or post-surgical pain. Despite the popularity of this limit in legislation, little data exists to inform and support the effectiveness of prescribing limits in general or the 7 day limit in particular in reducing risk of overuse.14 Thus far, studies have found that effectiveness of these laws vary widely between states and implementation challenges may further reduce the ability of these limits to reduce risks as intended.15,16 While one study conducted among Medicaid enrollees found that initial prescribing limits reduced the volume of prescriptions reimbursed by Medicaid, other early findings suggest that legislative limits have had little impact on in reducing the amounts of opioids dispensed for acute pain.17,18 In a recent policy analysis, Kertesz et.al. discuss the shortcomings of prescription limits in addressing the opioid crisis (e.g., failure of policy makers to engage stakeholders, understanding complex needs of patients), and potential harm induced by these policies.19
The objectives of this study were to examine the association between the risk of prolonged opioid use following surgery and the initial number of days of opioids prescribed, and to use historical data to estimate the potential impact of day supply-based prescribing limits on prolonged opioid use.
Methods
Data Source
A 20% random sample of the Medicare Parts A (inpatient), B (outpatient), and D (prescription drug) claims data from 2007 – 2017 was used for this study. The data contain insurance billing records for Medicare beneficiaries which enables linkage across enrollment data, inpatient claims, outpatient claims, and outpatient prescription medications, producing a comprehensive longitudinal health history across providers and hospital systems. The outpatient prescription medication claims include all prescriptions reimbursed through Medicare Part D, and include National Drug Codes, a universal product identifier for drugs, and number of days supplied, as entered by the pharmacy.
Study Population
Patients undergoing invasive surgery between July 2007 and December 2017 were identified using Current Procedural Terminology codes according to the Healthcare Cost and Utilization Project Surgery Flag Software classification.20 Ocular surgeries (cataracts, revision of eyelid, etc.) were removed.21 Both outpatient and inpatient surgeries were included. Surgical procedures conducted during inpatient stays were included if the procedure was conducted within the first 2 days of admission (e.g., pre-operative work-up phase), and the total length of stay was 4 nights or less (in order to exclude more complex surgical cases and those with complications). If patients had more than one invasive surgery code in a single day or inpatient stay, the surgery was classified according to the procedure with the highest associated insurance charge.
All patients were required to have at least 182 days of prior continuous enrollment in Medicare Parts A, B, and D to allow for complete capture of baseline medical history and screen for opioid exposure prior to initiation (Figure 1). Patients with surgery in the prior 182 days were excluded, as were those receiving hospice care during baseline.
Figure 1.
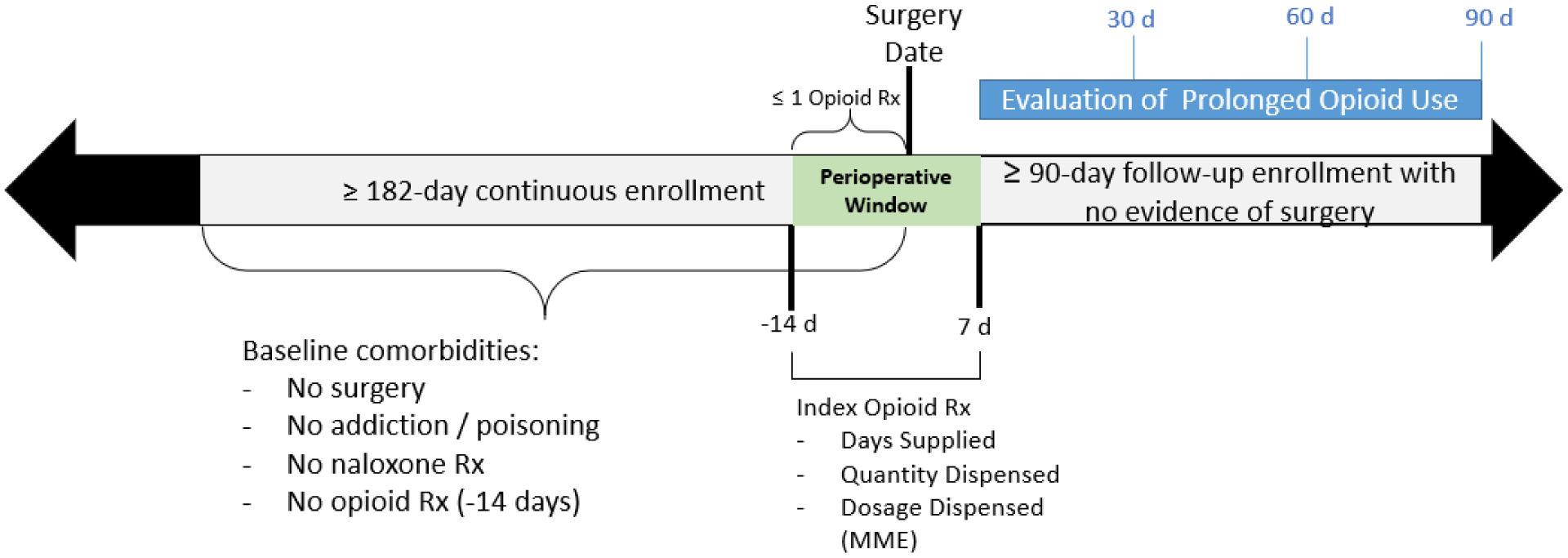
Study timeline schematic.
Patients who were discharged from surgery to another facility were excluded because postsurgical medications may not be captured in outpatient prescription claims. Patients with opioid use during the 182 days to 14 days prior to surgery were excluded to align with clinical practice concerns of initiating opioid use in naïve patients and subsequent development of opioid-related problems. Patients with two or more prescriptions in the 14 days prior to surgery were also excluded, however those with one prescription in this window were assumed to be receiving this prescription to have on hand for postoperative pain. Lastly, patients with any evidence of opioid poisoning, abuse, or addiction (See table, Supplemental Digital Content 1) during baseline were excluded.
To assess prolonged opioid use, patients were required to have at least 90 days of continuous enrollment (Medicare Parts A, B, and D) after discharge from surgery, with no invasive surgery procedure or hospice care during that time (See Supplemental Figure 1, Supplemental Digital Content 2).
Exposure
The perioperative surgical window began 14 days prior to the date of surgery and extended 7 days following surgery discharge. The number of days supplied (main analysis) and the total dosage dispensed in morphine milligram equivalents (MME) (secondary analysis) for the first opioid prescription during this time was identified. If multiple prescriptions were filled during this window, subsequent prescriptions were counted as follow-up prescriptions. The list of opioids considered in this analysis is listed in Supplemental Digital Content 2 (Appendix Table 2).
Outcome
Prolonged opioid use was defined as at least one active opioid prescription (defined using the fill date and day supply) in each of three consecutive 30-day windows immediately following the perioperative period. Each opioid prescription could only contribute towards one 30-day window. The proportion exposed to opioids in all three months is reported.
Statistical Analyses
Risk of Prolonged Opioid Use
We present the observed risk of prolonged opioid use at varying day supply values, as well as the expected risk in the overall study population at each day supply value. The expected risk of prolonged use was estimated using g-computation methods (see text, Supplemental Digital Content 1). This approach uses predicted outcomes (based on a statistical model) combined with the observed outcomes to estimate the causal effects assuming no unmeasured confounding and correct model specification.22–25 Logistic regression models were used to predict the probability of prolonged opioid use at each level of initial number of day supply in the entire study population as a function of patient demographics (age, sex, region), calendar year (categorical), surgical procedure, surgery characteristics (surgical setting, procedure cost, anesthesiology cost), recent inpatient hospitalization, baseline medication use, and baseline comorbidities (See Supplemental Figures 3a and 3b, Supplemental Digital Content 2). The normal approximation method using 200 bootstrap samples was used to estimate 95% confidence intervals.24,25
Impact of Prescribing Limits
The potential impact of varying prescribing limits was estimated using the nine most commonly prescribed day supply values as hypothetical limits. For each potential limit analyzed, we report the observed risk of prolonged use among patients who received a prescription above that limit. We then use g-computation to estimate the risk of prolonged use had these patients received a prescription equal to the limit (instead of a prescription exceeding the limit) based on their characteristics and the estimated model parameters for those characteristics.
The risk difference comparing the observed risk of prolonged use above each hypothetical limit and the estimated risk had these patients instead received the limit is reported, with 95% confidence intervals estimated as described above. For example, the impact of a hypothetical 7-day prescribing limit was estimated by calculating the difference between a) the observed risk of prolonged use among patients receiving 8 or more days, and b) the estimated risk had those patients instead received 7 days. Causal risk differences, the number of patients above each limit, the “number needed to treat” (NNT)26, interpreted as the estimated number of patients needed to be impacted by the limit (eg. receive a prescription equal to the limit instead of a longer prescription) in order to avoid one case of prolonged use, and the estimated number of potentially avoided cases of prolonged use at each hypothetical limit are reported.
Secondary Analysis
We analyzed the risk of prolonged opioid use at varying levels of initial dosage dispensed, using morphine milligram equivalents (MME) for the index prescription. We also estimate the impact of hypothetical prescribing limits based on the nine most common values of MME dispensed.
Sensitivity Analyses
We conducted stratified analyses using three distinct calendar periods: (2007–2010, 2011–2014, 2015–2017). We also conducted an analysis in the overall population controlling for calendar time using 6-month categorical indicators instead of 1-year indicators.
To understand how confounding due to state legislation may impact the results, we conducted a sensitivity analysis that excluded all patients that underwent surgery after any state-specific legislation had been enacted. Two additional sensitivity analyses focused on the parameterization of age in the analyses. While the main analysis included age as a linear term, we conducted one analysis using age categories (65–74, 75–84, 85+), and one including a quadratic term for age.
All analyses were conducted using SAS 9.4, Cary, NC. This project was approved by the University of North Carolina at Chapel Hill Institutional Review Board (IRB #18–1248).
Results
A total of 1,060,596 patients meeting eligibility criteria underwent invasive surgery during the study period (Table 1, Supplemental Figure 1, Supplemental Digital Content 2). The mean age was 74 years, 55% of patients were female, and 91% where White (Supplemental Table 1, Supplemental Digital Content 2). Almost one-third (32%) of surgeries were conducted in the inpatient setting, with a median length of stay of 2 nights. The three most common surgeries were knee arthroplasty, inguinal and femoral hernia repair, and laparoscopic cholecystectomy (Supplemental Table 2, Supplemental Digital Content 2). Overall, 70% of patients (745,109) received a perioperative opioid prescription. The median days supplied for the initial perioperative prescription was 5 (IQR: 4,7) and the median dosage dispensed was 240 MME (IQR: 180,375) (Supplemental Figures 2a–2f, Supplemental Digital Content 2). The most frequently prescribed opioids were hydrocodone (51%) and oxycodone (31%) (Table 2).
Table 1.
Baseline characteristics for Medicare patients undergoing surgery, stratified by perioperative opioid receipt.
| Characteristic | No Opioid Rx | Any Opioid Rx |
|---|---|---|
| N=315,487 | N=745,109 | |
| Age, mean(SD) | 75.9 (6.98) | 73.5 (5.97) |
| Sex, Male | 147,936 (46.9%) | 325,825 (43.7%) |
| Race | ||
| White | 284,286 (90.1%) | 676,000 (90.7%) |
| Black | 13,603 (4.3%) | 31,718 (4.3%) |
| Asian | 4,948 (1.6%) | 9,300 (1.2%) |
| Hispanic | 5,409 (1.7%) | 10,304 (1.4%) |
| Other | 4,412 (1.4%) | 9,461 (1.3%) |
| Total Cost (USD) of Procedures, mean(SD) | 4603.4 (6365.25) | 6088.3 (7698.98) |
| Total Cost (USD) of Anesthesia, mean(SD) | 1698.5 (1321.41) | 1721.0 (1299.95) |
| Place of Service | ||
| Ambulatory Surgical Center | 47,556 (15.1%) | 139,520 (18.7%) |
| Outpatient Hospital | 158,786 (50.3%) | 362,199 (48.6%) |
| Inpatient Hospital | 104,324 (33.1%) | 235,753 (31.6%) |
| Other | 4,821 (1.5%) | 7,637 (1.0%) |
| Days Inpatient, mean(SD) | 2.2 (1.07) | 2.2 (1.01) |
| Baseline Diagnoses | ||
| Cancer | 124,912 (39.6%) | 259,021 (34.8%) |
| Acute Pain | 14,386 (4.6%) | 95,470 (12.8%) |
| Chronic Back Pain | 84,434 (26.8%) | 216,262 (29.0%) |
| COPD | 51,271 (16.3%) | 95,144 (12.8%) |
| Depression | 33,811 (10.7%) | 81,508 (10.9%) |
| Drug Dependence | 16,306 (5.2%) | 39,007 (5.2%) |
| Neuralgia | 35,671 (11.3%) | 69,345 (9.3%) |
| Rheumatoid / Osteoarthritis | 116,607 (37.0%) | 364,315 (48.9%) |
| Surgical Procedure | ||
| Knee Arthroplasty | 6703 (2.1%) | 71,393 (9.6%) |
| Inguinal / Femoral Hernia Repair | 12008 (3.8%) | 49,102 (6.6%) |
| Laparoscopic Cholecystectomy | 15,191 (4.8%) | 40,049 (5.4%) |
| Prostate Surgery | 24,277 (7.7%) | 28,134 (3.8%) |
| Knee Arthroscopy | 9,199 (2.9%) | 43,032 (5.8%) |
| Cardiac Pacemaker | 35,363 (11.2%) | 9,983 (1.3%) |
| Skin Graft | 17,123 (5.4%) | 22,998 (3.1%) |
| Lumpectomy | 12,360 (3.9%) | 24,576 (3.3%) |
| Other Hernia Repair | 8,424 (2.7%) | 26,340 (3.5%) |
| Hip Replacement | 4,643 (1.5%) | 26,808 (3.6%) |
| Carpal Tunnel Surgery | 8,609 (2.7%) | 21,081 (2.8%) |
| Other | 161,587 (51.2%) | 381,613 (51.2%) |
Table 2.
Day supply distribution of the initial opioid prescription by active ingredient.
| Initial Opioid | % of Total | Initial Day Supply | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1st Percentile | 5th Percentile | 25th Percentile | 50th Percentile | 75th Percentile | 90th Percentile | 95th Percentile | 99th Percentile | ||
| Overall | 100% | 2 | 2 | 4 | 5 | 7 | 10 | 15 | 30 |
| Hydrocodone | 51% | 2 | 2 | 3 | 5 | 7 | 10 | 11 | 20 |
| Oxycodone | 31% | 2 | 2 | 4 | 5 | 7 | 10 | 14 | 24 |
| Tramadol | 6% | 2 | 3 | 5 | 7 | 10 | 15 | 20 | 30 |
| Codeine | 5% | 1 | 2 | 3 | 4 | 5 | 8 | 10 | 20 |
| Other | 7% | 2 | 2 | 5 | 7 | 13 | 21 | 28 | 40 |
Risk of Prolonged Use
The observed risk of prolonged opioid use was 1.45% (95%CI: 1.43,1.47). Among those who received a perioperative prescription, the 90-day risk was 1.90% (95% CI: 1.87,1.93). Among those who did not receive a perioperative prescription, the 90-day risk was 0.37% (95%CI: 0.35,0.39).
The observed risk of prolonged use increased steadily with increasing day supply. After taking into account differences in the distributions of covariates between patients who were observed to receive different numbers of days supplied, the increasing trend was substantially attenuated (Figure 2, Supplemental Table 3). For patients with no evidence of a perioperative opioid prescription, the expected risk of prolonged use was 0.52% (95%CI: 0.49, 0.56). Among patients with a perioperative opioid prescription, the expected risk of prolonged use increased from 1.26% (95% CI: 0.71, 1.81) among patients receiving a 1-day supply to 3.02% (2.80, 3.23) among patients receiving 15 or more days.
Figure 2.
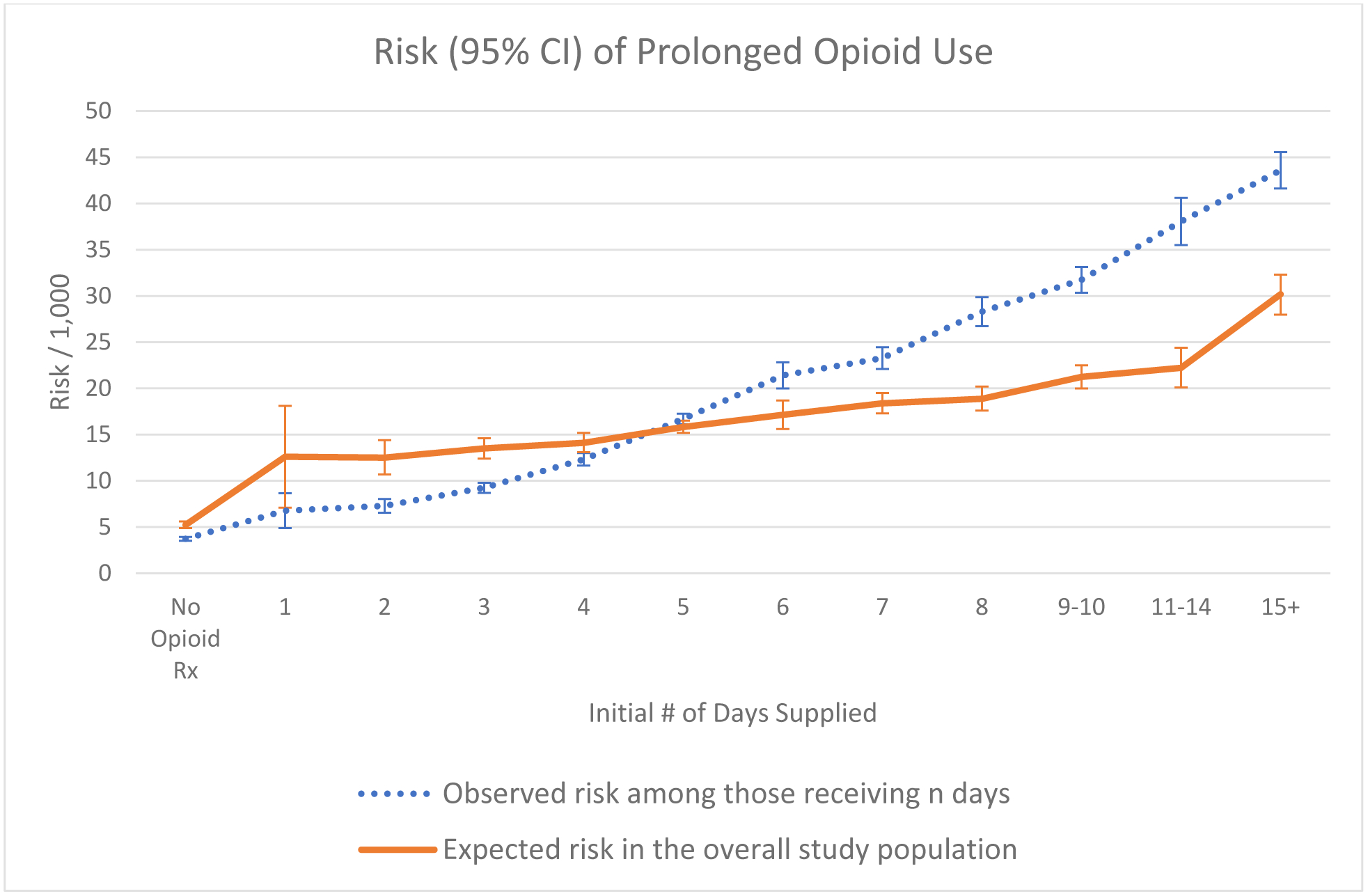
Observed and expected risk and 95% confidence intervals of prolonged opioid use following surgery by initial number of days supplied.
Expected risk in the overall population was estimated using g-computation with 200 bootstraps. Confidence intervals were estimated using the normal approximation.
Factors Associated with Prolonged Opioid Use
While we did not formally examine risk factors for prolonged opioid use, results from our models found that patients whose surgeries took place in earlier years (prior to 2015), were conducted in inpatient settings, and were knee arthroplasties were more likely to have prolonged opioid use. We also found that diagnosis of tobacco use disorder, acute pain, chronic back pain, arthritis, COPD, and depression diagnoses during baseline, as well as anticonvulsants, benzodiazepines, NSAID, and SSRI prescriptions during baseline were positively associated with prolonged opioid use (Supplemental Figures 3a and 3b, Supplemental Digital Content 2).
Impact of Prescribing Limits
We evaluated the potential impacts of various prescribing maximums based on the nine most common values of days supplied by calculating the difference in risk of prolonged opioid use between patients receiving a prescription above a stated limit to the estimated risk had they instead received a prescription exactly equal to that limit. Overall, the largest risk differences were observed at the lower end of hypothetical day supply limits (Table 3).
Table 3.
Risk difference for prolonged opioid use per 1000 patients and projected number of reduced cases of prolonged opioid use associated with varying day supply limits.
| Day Supply Limit | Observed Risk/1,000 Above Limit | Estimated Risk/1,000 At Limita | Risk Difference (95% CI) | NNTb | No. (%) of Surgeries above Cutoff | # of Reduced Prolonged Opioid Use Casesc |
|---|---|---|---|---|---|---|
| 2 | 20.0 | 15.4 | 4.61 (2.12,7.10) | 217 | 687,941 (92.3%) | 3,170 |
| 3 | 22.2 | 17.4 | 4.81 (3.36,6.26) | 208 | 570,079 (76.5%) | 2,740 |
| 4 | 24.4 | 19.6 | 4.84 (3.59,6.09) | 207 | 466,960 (62.7%) | 2,255 |
| 5 | 30.0 | 25.8 | 4.19 (3.01,5.38) | 239 | 271,288 (36.4%) | 1,135 |
| 6 | 31.5 | 27.2 | 4.28 (2.06,6.51) | 234 | 230,795 (31.0%) | 986 |
| 7 | 34.6 | 32.6 | 2.04 (−0.17,4.25) | 491 | 167,548 (22.5%) | 341 |
| 8 | 36.8 | 33.0 | 3.78 (1.57,5.99) | 265 | 124,513 (16.7%) | 469 |
| 10 | 41.7 | 39.8 | 1.93 (−0.97,4.83) | 519 | 63,302 (8.5%) | 121 |
| 15 | 46.7 | 45.2 | 1.51 (−2.85,5.87) | 663 | 25,662 (3.4%) | 38 |
Risk calculated using g-computation methods with 95% confidence intervals based on the standard deviation of 200 bootstrapped resamples, estimating risk of prolonged use if all patients above the limit had instead received a prescription equal to that limit.
NNT: Number needed to treat = RD−1, interpreted as the number of patients needed to be impacted by the limit to reduce one case of prolonged opioid use.
# of Reduced Cases = (# of surgeries above cutoff / NNT).
A limit of 4 days was associated with the largest reduction in absolute risk from 24.4 to 19.6 per thousand patients. If patients who had received a prescription exceeding 4 days had instead received a prescription for 4 days, risk of prolonged use was estimated to decrease by 4.84 (3.59, 6.09) per 1,000 patients (Table 2). A 4-day limit would have impacted an estimated 466,960 (62.7%) surgical patients and would have resulted in an estimated 2,255 fewer cases of prolonged use. The most common limit across states continues to be a 7-day supply. This limit would have impacted 167,548 (22.5%) surgeries and was associated with a risk difference of 2.04 (−0.17, 4.25) / 1,000 patients, for an estimated 341 fewer cases of prolonged opioid use.
Secondary Analysis
We found that risk of prolonged use generally increased as the initial dosage measured in MME increased, ranging from 13.7 / 1,000 in patients receiving less than 90 MME to 30.5/1,000 in patients receiving 800 MME or more (Supplemental Table 4, Supplemental Digital Content 2). The limit associated with the largest absolute reduction in risk was 240 MME (Supplemental Table 5, Supplemental Digital Content 2), which on average is consistent with a 5-day supply (Supplemental Table 6, Supplemental Digital Content 2). This limit would impact 47% of surgeries, and result in an estimated 2,986 fewer cases of prolonged opioid use.
Sensitivity Analyses
When stratifying by calendar year of surgery, we found that the largest absolute reductions in risk were associated with a 10- and 5-day supply in the earliest period (2007–2010). Between 2011 and 2014, the largest absolute reduction in risk was associated with a 6-day supply, and in the latest period (2015–2017) the 2- and 4-day supply limits were associated with the largest absolute reduction in risk (Supplemental Table 7, Supplemental Digital Content 2).
Results across all other sensitivity analyses (excluding patients undergoing surgery in states after opioid prescribing limits had been placed [Supplemental Table 8, Supplemental Digital Content 2], controlling for 6-month intervals instead of 1-year intervals [Supplemental Table 9, Supplemental Digital Content 2], including age categories [Supplemental Table 10, Supplemental Digital Content 2], and including a quadratic term for age [Supplemental Table 11, Supplemental Digital Content 2]) were consistent with main findings.
Discussion
In an opioid-naive Medicare population undergoing invasive surgery, we found that 70% of surgical patients received perioperative opioids, and 1.9% of those receiving perioperative opioids had prolonged use. A recent study conducted in a younger population found that patients who received initial postoperative opioid prescriptions for 7 days supplied or more were at higher risk of long-term postoperative opioid use.2 In this study conducted among an older Medicare population, the results were consistent showing that risk of prolonged use increased with increasing number of days supplied. Importantly, after adjusting for baseline characteristics, the increased risk was much attenuated, suggesting that much of the increase in the observed risk across day supply is related to the types of patients receiving longer duration prescriptions, rather than the prescription duration itself.
In the analysis of prescribing limits, our main analysis found that a day supply limit of 7 days, which is most common in state legislation, was associated with the third-smallest estimated risk difference. Meanwhile a 4-day supply limit was associated with the largest risk difference (4.8/1,000). In a secondary analysis of dosage limits, a 240 MME limit was associated with the largest risk difference (8.6/1,000). It is important to note that for many surgeries, pain will persist longer than 4 days, and that alternative methods (pharmacologic and non-pharmacologic) will need to be in place for continued pain management. Even with incorporation of other methods of pain management, some surgeries may require more than 4 days of opioid management, and procedure specific guidelines may be more useful than all-encompassing prescribing limits and allow for autonomy in prescribing27. While we included the 2-day supply limit in the suite of analyses to explore trends with changing day supply, it is important to note that a 2-day supply limit has not been enacted in any states and is likely to be too extreme for legislation. Analytically, this is also the limit most likely to challenge analytic assumptions of no unmeasured confounding, as the patients observed to receive a 2-day supply was small and are more likely to differ in ways that were not measurable in our data from patients receiving prescriptions with a longer day supply.
When considering prescribing maximums, there are necessary trade-offs between the number of patients being impacted adequate pain management, and the potential reduction of prolonged use. We found that many of the proposed prescribing limits may impact large proportions of patients putting certain patients at risk of inadequately managed pain, while resulting in relatively few instances of averted prolonged opioid use. The current results illustrate the need to balance the number of patients impacted by legislation with the potential reduction in risk of prolonged opioid use.
We also found that among patients with no evidence of a perioperative opioid prescription, risk of prolonged use was 0.4%. Prolonged opioid use in this population could be a general marker of prolonged opioid use for pain which may not be directly related to surgery or could reflect prolonged pain resulting in inadequate pain management upon surgical discharge. It is also possible patients had opioid prescriptions in the perioperative period that were not captured in our data, (e.g. paid by cash, had opioids on hand from prior prescriptions or family members), and did not fill new prescription opioids for surgical pain until after the perioperative window.
This study included a broad range of surgeries in a vulnerable population of older adults. We used G-computation methods which are well suited to estimate population level effects of policies or interventions.23,25 Using these methods we described changes in risk of prolonged opioid use at varying levels of day supply while not imposing any structural linearity. We additionally examine the potential impacts of varying prescribing limits, integrating the number of patients impacted by each limit and the change in risk at each limit to estimate the potential number of reduced cases of prolonged opioid use.
We defined prolonged opioid use as at least one prescription in 3 consecutive months following surgery. This is a more stringent definition and we found a lower risk of prolonged opioid use compared to many prior studies, which defined prolonged opioid use as any prescription occurring between 90–180 days following surgery.1,28 However this definition is more representative of prolonged opioid use that may be related to the index surgery.29 The current study design leverages large preexisting observational data to identify a treatment decision point comparing patients receiving varying levels of opioids. These analyses are anchored to a specific clinical event, producing estimates directly applicable to informing safe prescribing thresholds for opioid medications, and evaluating prescribing maximums.30,31
We used nationwide Medicare claims data that capture opioids prescribed across multiple health systems and provider, however any prescriptions that were paid for out of pocket or received through other means (diversion, stockpiling) would not be captured in our data. Innovative statistical methods were implemented to account for confounding to the degree possible, but there are factors (preoperative pain severity, body mass index, smoking and alcohol use, other underlying health conditions) that we were not able to measure. While we accounted for year of surgery in the analysis, and 6- month intervals in the sensitivity analyses, we were unable to include more granular time periods. Given changes in prescribing behavior and other attitudes around opioid prescribing, it is possible that changes, such as efforts to shift opioid use to other analgesia, implementation of enhanced recovery after surgery protocols, and other initiatives to reduce opioid prescribing may influence results. Our analyses stratified by time periods found that risk of prolonged opioid use decreased throughout the study period, and that patients receiving a given amount of opioids in more recent years may be at a lower risk of prolonged opioid use compared to earlier years. As vigilance surrounding opioid prescribing and prolonged use increases in the medical community, risk differences associated with prescribing limits may continue to decrease with time.
We excluded patients who had opioid use in the 182 days prior to surgery. The risk of prolonged postsurgical opioid use is likely higher among patients with opioid use prior to surgery. Surgical patients with existing opioid use are very important population that is clinically different than the current study focus, warranting further research. Our definition of prolonged opioid use required that all patients had at least 90 days of follow-up. This excluded 12.1% of patients. The majority (55%) of the excluded patients had another surgery, 39% were administratively censored, and 3% died within the 90-day follow-up. Overall follow-up was similar between those who received a perioperative opioid prescription and those who did not suggesting that potential bias on the reported estimates due to censoring is minimal.
We focused on the day supply of the initial opioid prescribed in the perioperative period because most legislation use limits based on day supply. However, while legislative efforts have focused on number of days supplied, this may not be the most proximal measure for prescribing physicians, who often prescribe in terms of quantity of pills or dosage. Days supplied values are typically calculated by the pharmacists, using frequency instructions and the total number of pills dispensed. We have presented the median dosage and quantity dispensed at each day supply category, and conducted a secondary analysis focusing on dosage dispensed. Our secondary analysis found that limits based on dosage may result in higher absolute differences in risk of prolonged use. Closer examination of risks associated with dosage and quantity of pills dispensed may be informative in future work.
The analysis of prescribing limits assumed that patients below the limit would not be affected, and all patients above the limit would instead receive the proposed limit. It is possible that patients would receive an amount lower than the limit. It is also likely that implementation of these limits may face challenges, uptake by clinicians may be slow, and work arounds (altering dosing instructions) may occur.16 However, this method of describing the potential impact under the assumption of 100% compliance is an important illustration when considering the clinical utility of these limits.
We did not evaluate the impact of limits on reducing opioid diversion, or the benefits of opioids in treatment of surgical pain, such as functional improvements and improved quality of life. Understanding the benefits of opioids in managing surgical pain, as well as examining adequate pain management, and the rate of opioid refills needed will be an important avenue for research to balance prescribing recommendations. In the broader context of policy responses to the US opioid crisis, research targeting treatment of opioid use disorder and engagement of patients is needed.19
This study was subject to limitations and unable to measure certain factors. However, we used advanced analytic techniques and conducted multiple sensitivity analyses and found that final estimates were stable across scenarios. This study contributes to the current knowledge base, examining risk associated with granular changes in initial prescribing without imposing linearity among a large population of surgical patients.
While the factors contributing to the current opioid crisis are complicated and multifaceted, scrutiny has centered around opioid prescribing for post-surgical pain. In an effort to mitigate the risks of prescription opioids to the individual and society, many states have passed laws setting prescribing limits. These attempts to regulate opioid prescribing pose clinical challenges as prescribing maximums limit the opportunity to integrate individual assessment, pain severity, and other clinical factors that make a meaningful difference in the amount of pain medication a physician would typically prescribe. Our study finds that in an opioid-naïve Medicare population, while risk of prolonged postsurgical opioid use increases as patients receive larger initial opioid prescriptions, overall risk of prolonged opioid use is low. The flattening of the risk curve after adjusting for baseline patient and surgical characteristics further suggests that these characteristics, rather than the initial opioid duration, are likely driving much of the increased risk of prolonged opioid use. We additionally found that currently implemented prescribing limits may have limited impact on reducing the amount of prolonged use in this population. The findings from the Medicare population may differ from other populations that may have a higher risk of prolonged opioid use, in younger populations, or across specific surgical settings. Further work in different data sources, with potential linkage to electronic health records may be able to account for additional confounders such as pain score and evaluate how limits may impact different populations.
Supplementary Material
Funding for this project was supplied by the following sources:
JY received tuition and stipend support from NIH/NIDA R36 DA04588501(PI: Young). This research was partially supported by a National Research Service Award Pre-Doctoral/Post-Doctoral Traineeship from the Agency for Healthcare Research and Quality sponsored by The Cecil G. Sheps Center for Health Services Research, The University of North Carolina at Chapel Hill, Grant No. T32-HS000032.
ND is supported by the US Food and Drug Administration (HHSF223201810183C). Dr. Dasgupta does not accept personal compensation of any kind from any pharmaceutical company.
TS receives investigator-initiated research funding and support as Principal Investigator (R01 AG056479) from the National Institute on Aging (NIA), and as Co-Investigator (R01 HL118255, R01MD011680), National Institutes of Health (NIH). He also receives salary support as Director of Comparative Effectiveness Research (CER), NC TraCS Institute, UNC Clinical and Translational Science Award (UL1TR002489), the Center for Pharmacoepidemiology (current members: GlaxoSmithKline, UCB BioSciences, Takeda, AbbVie, Boehringer Ingelheim), from pharmaceutical companies (Novo Nordisk), and from a generous contribution from Dr. Nancy A. Dreyer to the Department of Epidemiology, University of North Carolina at Chapel Hill. Dr. Stürmer does not accept personal compensation of any kind from any pharmaceutical company.
MJF receives investigator-initiated research funding support as Principal Investigator from the U.S. Food and Drug Administration (FDA: 75F40119C10115) and the National Heart, Lung, and Blood Institute (NHLBI: R01 HL118255), and as Co-investigator from the FDA (CER-2017C3-9230), Centers for Disease Control and Prevention (CDC: 1U01DP006369-01), National Center for Advancing Translational Sciences (NCATS: 1 U54 TR002255), National Institute on Aging (NIA: 1R01AG056479), and Health Resources & Services Administration (HRSA: R40MC29455-01-00). She additionally receives salary support from the Center for Pharmacoepidemiology in the Department of Epidemiology, UNC (members: GlaxoSmithKline, UCB BioSciences, Merck (past member), Takeda, Abbvie, Boehringer Ingelheim), and consulting fees via UNC from GlaxoSmithKline.
The database infrastructure used for this project was funded by the Pharmacoepidemiology Gillings Innovation Lab (PEGIL) for the Population-Based Evaluation of Drug Benefits and Harms in Older US Adults (GIL200811.0010), the Center for Pharmacoepidemiology, Department of Epidemiology, UNC Gillings School of Global Public Health, the CER Strategic Initiative of UNC’s Clinical Translational Science Award (UL1TR002489), the Cecil G. Sheps Center for Health Services Research, UNC, and the UNC School of Medicine.
Footnotes
COI: JY receives consulting fees from CERobs Consulting, LLC. TS and MJF receive salary support via UNC from the Center for Pharmacoepidemiology (members: GlaxoSmithKline, UCB BioSciences, Merck (past member), Takeda, Abbvie, Boehringer Ingelheim). TS owns stock in Novartis, Roche, BASF, AstraZeneca, and Novo Nordisk. MJF receives consulting fees via UNC from GSK. ND is a consultant to the RADARS System of Denver Health and Hospital Authority, a political subdivision of the State of Colorado (USA). BAC, VP, and MH have no conflicts to disclose.
This work was presented as a virtual oral presentation at ICPE All Access, taking place September 16, 2020-September 17, 2020.
List of SDC:
Supplemental Digital Content 1.docx
Supplemental Digital Content 2.docx
References
- 1.Brummett CM, Waljee JF, Goesling J, et al. New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA surgery. 2017;152(6):e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young JC, Dasgupta N, Chidgey BA, Jonsson Funk M. Postsurgical Opioid Prescriptions and Risk of Long-term Use: An Observational Cohort Study Across the United States. Annals of surgery. 2021;273(4):743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JS, Vu JV, Edelman AL, et al. Health Care Spending and New Persistent Opioid Use After Surgery. Annals of surgery. 2020;272(1):99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reid MC, Bennett DA, Chen WG, et al. Improving the pharmacologic management of pain in older adults: identifying the research gaps and methods to address them. Pain medicine (Malden, Mass). 2011;12(9):1336–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhong J, Si HB, Zeng Y, et al. Comparison of cortisol and inflammatory response between aged and middle-aged patients undergoing total hip arthroplasty: a prospective observational study. BMC musculoskeletal disorders. 2017;18(1):541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reeve E, Wiese MD, Mangoni AA. Alterations in drug disposition in older adults. Expert opinion on drug metabolism & toxicology. 2015;11(4):491–508. [DOI] [PubMed] [Google Scholar]
- 7.Bettelli G Preoperative evaluation in geriatric surgery: comorbidity, functional status and pharmacological history. Minerva anestesiologica. 2011;77(6):637–646. [PubMed] [Google Scholar]
- 8.Alam A, Gomes T, Zheng H, Mamdani MM, Juurlink DN, Bell CM. Long-term analgesic use after low-risk surgery: a retrospective cohort study. Archives of internal medicine. 2012;172(5):425–430. [DOI] [PubMed] [Google Scholar]
- 9.Brinson Z, Tang VL, Finlayson E. Postoperative Functional Outcomes in Older Adults. Current surgery reports. 2016;4(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W, Bagshaw SM, Norris CM, Zibdawi R, Zibdawi M, MacArthur R. Association between older age and outcome after cardiac surgery: a population-based cohort study. Journal of cardiothoracic surgery. 2014;9:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma NK, Olotu B, Mathew A, Waitman LR, Rasu R. Lumbar Spine Surgeries and Medication Usage During Hospital Stay: One-Center Perspective. Hospital pharmacy. 2017;52(11):774–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wunsch H, Wijeysundera DN, Passarella MA, Neuman MD. Opioids Prescribed After Low-Risk Surgical Procedures in the United States, 2004–2012. Jama. 2016;315(15):1654–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ballotpedia. Opioid prescription limits and policies by state. https://ballotpedia.org/Opioid_prescription_limits_and_policies_by_state. Published October 4, 2019. Accessed February 15, 2020.. Accessed.
- 14.Davis CS, Lieberman AJ, Hernandez-Delgado H, Suba C. Laws limiting the prescribing or dispensing of opioids for acute pain in the United States: A national systematic legal review. Drug and alcohol dependence. 2019;194:166–172. [DOI] [PubMed] [Google Scholar]
- 15.Chua KP, Brummett CM, Waljee JF. Opioid Prescribing Limits for Acute Pain: Potential Problems With Design and Implementation. Jama. 2019;321(7):643–644. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal S, Bryan JD, Hu HM, et al. Association of State Opioid Duration Limits With Postoperative Opioid Prescribing. JAMA Network Open. 2019;2(12):e1918361–e1918361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chua KP, Kimmel L, Brummett CM. Disappointing Early Results From Opioid Prescribing Limits for Acute Pain. JAMA surgery. 2020;155(5):375–376. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, Tallavajhala S, Kapadia SN, et al. State Opioid Limits and Volume of Opioid Prescriptions Received by Medicaid Patients. Medical care. 2020;58(12):1111–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kertesz SG, Gordon AJ. A crisis of opioids and the limits of prescription control: United States. Addiction (Abingdon, England). 2019;114(1):169–180. [DOI] [PubMed] [Google Scholar]
- 20.Surgery Flag Software. Healthcare Cost and Utilization Project (HCUP). August 2019. Agency for Healthcare Research and Quality, Rockville, MD. https://www.hcup-us.ahrq.gov/toolssoftware/surgeryflags_svcproc/surgeryflagssvc_proc.jsp. Accessed April 03, 202. [Google Scholar]
- 21.Criteria for CPT Cateogry II Codes. American Medical Association. https://www.ama-assn.org/practice-management/cpt/criteria-cpt-category-ii-codes. Accessed May 22, 2019, 2019.
- 22.Snowden JM, Rose S, Mortimer KM. Implementation of G-computation on a simulated data set: demonstration of a causal inference technique. Am J Epidemiol. 2011;173(7):731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller CJ, MacLehose RF. Estimating predicted probabilities from logistic regression: different methods correspond to different target populations. International journal of epidemiology. 2014;43(3):962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keil AP, Edwards JK, Richardson DB, Naimi AI, Cole SR. The parametric g-formula for time-to-event data: intuition and a worked example. Epidemiology (Cambridge, Mass). 2014;25(6):889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernán MA, Robins JM (2020). Causal Inference: What If. Boca Raton: Chapman & Hall/CRC. [Google Scholar]
- 26.Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. Bmj. 1995;310(6977):452–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heins SE, Castillo RC. The Impact of Morphine Equivalent Daily Dose Threshold Guidelines on Prescribed Dose in a Workers’ Compensation Population. Medical care. 2020;58(3):241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olds C, Spataro E, Li K, Kandathil C, Most SP. Assessment of Persistent and Prolonged Postoperative Opioid Use Among Patients Undergoing Plastic and Reconstructive Surgery. JAMA Facial Plastic Surgery. 2019;21(4):286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young JC, Wu JM, Willis-Gray M, Pate V, Jonsson Funk M. Persistent Opioid Use After Hysterectomy in the United States, 2005–2015. Obstetrics and gynecology. 2020;135(1):123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brookhart MA. Counterpoint: The Treatment Decision Design. American Journal of Epidemiology. 2015;182(10):840–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fralick M, Kesselheim AS, Avorn J, Schneeweiss S. Use of Health Care Databases to Support Supplemental Indications of Approved Medications. JAMA internal medicine. 2018;178(1):55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


