Abstract
Background:
Altered gut integrity is central to HIV-related immune activation. Opioids may promote similar changes in gut permeability and/or increase systemic inflammation, potentially augmenting processes already occurring in people with HIV (PWH).
Setting:
Urban hospital systems in Cleveland, Ohio, and surrounding communities.
Methods:
This is a prospectively enrolled, cross-sectional study including people with and without HIV using heroin and people with and without HIV who have never used heroin, matched by age, sex, and CD4+ T-cell count (PWH only) to compare markers of gut integrity, microbial translocation, systemic inflammation, and immune activation.
Results:
A total of 100 participants were enrolled. Active heroin use was associated with higher concentrations of lipopolysaccharide-binding protein (LBP), beta-D-glucan (BDG), high-sensitivity C-reactive protein (hsCRP), soluble tumor necrosis factor-α-receptors I and II, soluble CD163, inflammatory monocytes, and activated CD4+ lymphocytes in adjusted models. HIV status tended to modify the effect between heroin use and LBP, BDG, hsCRP, patrolling monocytes, and activated CD4+ lymphocytes (P < 0.15 for interactions); however, it was not as expected. The effect of heroin on these markers (except patrolling monocytes) was greatest among those without HIV rather than among those with HIV.
Conclusions:
Heroin use is associated with heightened microbial translocation, systemic inflammation, and immune activation. Concurrent HIV infection in virologically suppressed individuals does not seem to substantially worsen the effects heroin has on these markers.
Key Words: heroin, HIV, gut integrity, immune activation, systemic inflammation
INTRODUCTION
People with HIV (PWH) whose mode of HIV acquisition was intravenous drug use are at higher risk of progression to AIDS, death due to AIDS, and all-cause mortality even when controlling for access to care and antiretroviral therapy (ART) duration.1–4 Although high-risk lifestyle or death from comorbid conditions, such as bacterial infection or hepatitis C, certainly contribute, chronic immune activation may play an important role. In an observational study of people with a history of injecting drugs, an inflammatory index constructed from serum interleukin (IL)-6 and soluble tumor necrosis factor-α-receptor I (sTNF-α-RI) was associated with frailty and increased mortality in PWH and people without HIV who inject drugs.5 Systemic inflammation associated with intravenous drug use is particularly relevant for PWH where chronic immune activation is already apparent and the added contribution of drug use is unclear.
It is plausible that HIV and heroin use together result in a synergistic effect on immune activation. Peripheral blood mononuclear cells (PBMCs) from healthy donors cocultured with PBMCs from PWH in the presence of morphine have significantly greater p24 antigen release than PBMCs from controls or PMBCs from PWH in the presence of interleukin-2, which promotes HIV proliferation.6 Heroin use is associated with lower gene expression and concentrations of antiviral restriction factors and type 1 interferon, in effect, limiting antiviral activity.7 Morphine also downregulates β-chemokine production and upregulates CCR5 receptor expression, resulting in enhanced R5-tropic HIV infection of macrophages, which is not seen with X4 strains.8 Finally, altered gut integrity and resultant microbial translocation to the systemic circulation seem to be a central factor in HIV-related immune activation9,10; heroin use may further exacerbate this pathology.11
In this study, our aim was to evaluate associations between heroin use and gut integrity, microbial translocation, systemic and vascular inflammation, and immune activation and to understand how HIV infection modifies the effects of heroin on these factors. Our hypothesis was that heroin use would be associated with worse markers of gut integrity, microbial translocation, inflammation, and immune activation; and the effects would be greatest in PWH.
METHODS
Study Participants
The Impact of Heroin on Immune Activation in HIV study (or ACTIVATE study) is a prospective, observational cohort study designed to understand the effect of heroin use on innate and acquired immune activation and to assess the factors, such as gut integrity, which may mediate these associations in PWH who actively use heroin (HIV+ heroin+ individuals) and age-matched, sex-matched, and CD4+ T-cell count–matched PWH who have never used heroin (HIV+ heroin− individuals) in Cleveland, Ohio. People without HIV who actively use heroin (HIV− heroin+ individuals) and those who have never used heroin (HIV− heroin− individuals) were enrolled for a single visit designed for cross-sectional analyses. Eligibility criteria included age 18 years or older, self-report of current injecting or snorting heroin for at least 1 month with cumulative use of at least 12 months in the past for the active heroin use groups, or no heroin use ever for groups that never use heroin. Participants with HIV were required to have HIV-1 RNA ≤ 400 copies/mL if they were on ART. Presence of an inflammatory condition, active malignancy, infection in the past 2 weeks, pregnancy, uncontrolled diabetes, known cardiovascular disease, liver function tests greater than 2.5 times the upper limit of normal, hemoglobin <9 g/dL, and creatinine clearance by the Cockcroft–Gault formula < 30 mL/min were exclusionary for all groups. Potential participants were recruited from the MetroHealth System (MHS), University Hospitals Cleveland Medical Center (UHCMC), and drug treatment facilities across Cleveland, Ohio, and surrounding communities. All participants provided written informed consent. The study protocol and informed consent were approved by the MHS and UHCMC Institutional Review Boards. The analysis presented in this article was performed after the first 100 participants were enrolled.
Study Evaluations
Demographics, medical history, and substance use history were obtained by self-report, and a targeted physical examination, an 8-hour fasting blood draw, and urine collection were performed. Plasma, urine, and PBMCs were cryopreserved until thawed once and analyzed in batches.
Soluble Markers of Gut Integrity, Microbial Translocation, Inflammation, and Monocyte Activation
Soluble markers of gut integrity (intestinal fatty acid–binding protein or I-FABP), intestinal permeability (zonulin), bacterial translocation (LPS-binding protein or LBP), fungal translocation (beta-D-glucan or BDG), systemic inflammation (high-sensitivity C-reactive protein or hsCRP, IL-6, sTNF-α-RI and sTNF-α-RII, and D-dimer), vascular inflammation (soluble vascular cell adhesion molecule-1 or sVCAM-1), and monocyte activation (soluble CD14 or sCD14 and soluble CD163 or sCD163) were quantified using ELISA as per manufacturers' instructions (R&D Systems, Minneapolis, MN, for all; except PromoCell GmbH, Heidelberg, Germany, for zonulin; Hycult Biotech Inc., Wayne, PA, for LBP; MyBioSource, San Diego, CA, for BDG; and Diagnostic Stago Inc., Parsippany, NJ, for D-dimer).
Cellular Markers of Monocyte and T-cell Activation
Cellular markers of immune activation were phenotyped from PBMCs by flow cytometry, as previously described, using a Miltenyi MACSQuant flow cytometer (Miltenyi Biotec, Bergisch Gladbach, Germany).12 Monocyte subsets, including CD14+ CD16+ (inflammatory) and CD14 dim CD16+ (patrolling), were quantified as a percentage of the overall monocyte population. CD4+ and CD8+ lymphocytes expressing CD38 and HLA-DR (activated) were quantified as a percentage of the overall CD4+ and CD8+ lymphocyte population, respectively.
Statistical Analysis
Demographics, clinical characteristics, and HIV-related characteristics were compared among group using the Kruskal–Wallis test for continuous variables and the χ2, Fisher exact, or Pearson exact χ2 test for categorical variables, as appropriate. Outcomes including markers of gut integrity, microbial translocation, systemic and vascular inflammation, and monocyte and T-cell activation were compared between groups in a pairwise fashion. Comparisons planned a priori were between (1) HIV+, heroin+ and HIV+, heroin−; (2) HIV−, heroin+ and HIV−, heroin−; and (3) HIV+, heroin+ and HIV−, heroin+. Unadjusted followed by adjusted pairwise comparisons of the least square means for each outcome variable were made using ANOVA (GLM procedure). Additional variables included were age, sex, race, trunk fat, smoking, and hepatitis C status. To evaluate for effect modification by HIV status, ANOVA models included heroin use status, HIV status, and a heroin use-by-HIV status interaction term. A conservative P value of <0.15 was considered a potentially important interaction effect. Finally, markers were compared between heroin users with and without HIV after adjusting for route of heroin administration and intensity of heroin use, that is, self-reported grams of heroin used per week and the average number of days per week heroin was used, separately. Outcome variables were transformed before analyses by using the natural logarithm. All analyses were performed using SAS software, version 9.4 (Carey, NC). Statistical significance was defined by P < 0.05 except where previously described.
RESULTS
Demographics and Clinical Characteristics
From July 2017 to August 2018, 100 participants were enrolled (19 HIV+ heroin+, 19 HIV+ heroin−, 38 HIV− heroin+, and 24 HIV− heroin− participants). Overall, the median (IQR) age was 42 years (33–51 years), and 75% were men. There were differences in race/ethnicity distribution among the groups (Table 1), with most of the participants in each group being non-Hispanic White, except in the HIV+ heroin− group where most were non-Hispanic Black. A high proportion of HIV+ heroin+ participants were Hispanic (37%), reflecting the demographics of heroin use among PWH in Cleveland, Ohio. The body mass index was lowest in the HIV+ heroin+ participants (P = 0.02 among all groups). Participants using heroin were more likely to experience chronic hepatitis C (56% vs 0%; P < 0.0001), to be current smokers (98% vs 42%; P < 0.0001), with a greater number of pack-years (17 vs 6; P < 0.01), and to currently use cocaine or amphetamines (49% vs 7%, P < 0.0001; or 12% vs 0%, P = 0.03, respectively) when compared with participants not using heroin. Current cannabis use among all participants was high and similar between groups using heroin [53% vs 58% for HIV+ heroin+ vs HIV− heroin+ groups (P = 0.71 between heroin+ groups), 73% for HIV+ heroin− group, and 25% for HIV− heroin− group (P = 0.01 among all groups)]. Among those who self-reported heroin use, 49% were positive for fentanyl, 35% for morphine, 18% for methadone, 16% for heroin metabolite, and 5% for oxycodone by urine toxicology. Multiple opioids were present in 39% of the participants. The distribution of opioids was similar between participants with and without HIV. More participants in the HIV+ heroin+ group reported using heroin exclusively through the intranasal route than the HIV− heroin+ group (26% vs 0%; P < 0.0001), but self-reported average grams of heroin used per week and the number of days heroin used per week were similar (P = 0.92 and 0.72, respectively).
TABLE 1.
Baseline Demographics and HIV-Related Characteristics of Group
| HIV+ Heroin+, n = 19 |
HIV+ Heroin−, n = 19 |
HIV− Heroin+, n = 38 |
HIV− Heroin−, n = 24 |
P, Overall* |
P, HIV+ vs HIV−* |
P, Heroin+ vs Heroin−* |
|
| Age, yrs | 47 (32.9, 54.9) | 46.5 (32.4, 53.8) | 37.5 (34.1, 50.5) | 44.3 (32.6, 52.3) | 0.61 | 0.28 | 0.56 |
| Male | 14 (74) | 15 (79) | 27 (71) | 19 (79) | 0.88 | 0.81 | 0.41 |
| Race/ethnicity | <0.0001 | <0.001 | <0.001 | ||||
| White, non-Hispanic | 10 (53) | 2 (11) | 26 (68) | 17 (71) | |||
| Black, non-Hispanic | 2 (11) | 15 (79) | 5 (13) | 5 (21) | |||
| Asian, non-Hispanic | 0 (0) | 1 (5) | 2 (5) | 1 (4) | |||
| Hispanic | 7 (37) | 1 (5) | 5 (13) | 1 (4) | |||
| Current smoker | 19 (100) | 11 (58) | 37 (97) | 7 (29) | <0.0001 | 0.38 | <0.0001 |
| BMI, kg/m2 | 23.6 (21.6, 25.2) | 26 (23.5, 29.8) | 25.8 (22.9, 28.3) | 26.6 (22.9, 29.7) | 0.02 | 0.12 | 0.06 |
| Trunk fat, g | 6839 (5561, 9452) | 11,576 (8460, 15,969) | 9126 (6632, 14,819) | 12,136 (7233, 14,623) | <0.01 | 0.17 | <0.01 |
| Hepatitis C | 11 (58) | 0 (0) | 21 (55) | 0 (0) | <0.0001 | 0.61 | <0.0001 |
| HIV duration, yrs | 11.8 (2.3, 18.9) | 13.2 (8.1, 16.7) | — | — | — | — | 0.82 |
| ART duration, yrs | 4.5 (1.8, 9.4) | 8.7 (6.8, 13.8) | — | — | — | — | 0.02 |
| On ISTI | 10 (53) | 13 (68) | — | — | — | — | 0.32 |
| On PI | 6 (32) | 3 (16) | — | — | — | — | 0.25 |
| On NNRTI | 1 (5) | 7 (37) | — | — | — | — | 0.02 |
| CD4+, cells/mm3 | 562 (276, 799) | 767 (623, 1035) | — | — | — | — | 0.02 |
| Nadir CD4+, cells/mm3 | 201 (42, 301) | 212 (110, 280) | — | — | — | — | 0.83 |
| HIV-1 RNA <200 copies/mL | 16 (84) | 19 (100) | — | — | — | — | 0.07 |
| Heroin route | — | <0.0001 | — | ||||
| Intravenous | 14 (74) | — | 38 (100) | — | |||
| Intranasal | 5 (26) | — | 0 (0) | — | |||
| Amount of heroin per week, in grams | 7 (2, 7) | 6 (3.5, 7) | 0.92 | ||||
| Current substance use | |||||||
| Cannabis | 10 (53) | 14 (73) | 22 (58) | 6 (25) | 0.01 | 0.08 | 0.34 |
| Cocaine | 9 (47) | 1 (5) | 19 (50) | 2 (8) | <0.001 | 0.43 | <0.0001 |
| Methamphetamine | 3 (16) | 0 (0) | 4 (11) | 0 (0) | 0.16 | >0.99 | 0.04 |
Values shown are median (interquartile range) and frequency (column percentage).
The Kruskal–Wallis or Pearson exact and χ2 tests for comparisons of more than 2 groups and the Wilcoxon rank sum or χ2 test for comparison between 2 groups.
BMI, body mass index; ISTI, integrase strand transfer inhibitor; PI, protease inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor.
In participants with HIV, the median (IQR) known duration of HIV was 13 years (8–18 years). PWH using heroin had a shorter ART duration [4 (2–9) vs 9 (7–14) years, P = 0.02] and lower current but similar nadir CD4+ T-cell counts than those who did not use [562 (276–799) vs 767 (623–1035) cells/mm3; P = 0.02, and 201 (42–301) vs 212 (110–280) cells/mm3; P = 0.82, respectively]. All but 2 participants were on ART, and all participants on ART had HIV-1 RNA < 400 copies/mL.
Gut Integrity and Microbial Translocation Markers
Figure 1 shows the adjusted least square means for each outcome by group. Concentrations of I-FABP were similar among groups (P = 0.21), and neither heroin use nor HIV were independently associated with I-FABP in unadjusted analyses. However, with adjustment, there was some evidence that among participants without HIV, I-FABP concentration was higher in those who use heroin (HIV− heroin+ vs HIV− heroin−; P = 0.06). HIV status, but not heroin use, was independently associated with zonulin (P = 0.02), and there was some evidence that among heroin users, those with HIV had higher zonulin in adjusted models (HIV+ heroin+ vs HIV− heroin+; P = 0.07). When adjusting for the route of heroin use, among participants who use heroin, those with HIV had higher zonulin concentrations than those without HIV (P = 0.04). HIV status did not modify the effect of heroin on I-FABP or zonulin.
FIGURE 1.
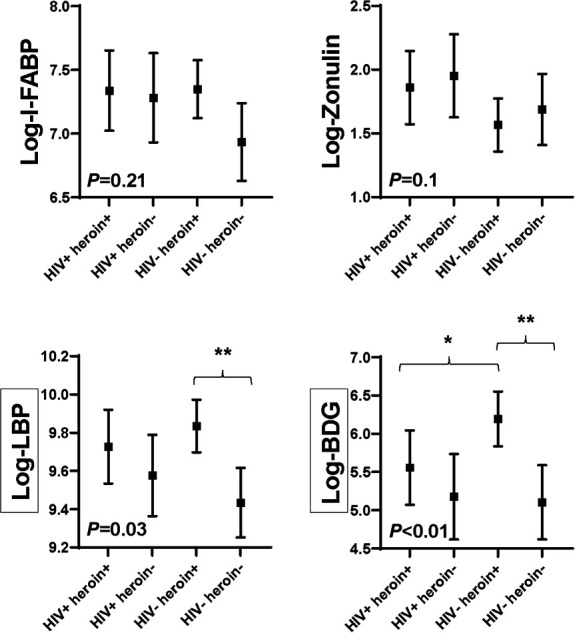
Gut integrity and microbial translocation markers by groups. Symbols represent adjusted least square means for each marker by group. The mean values are adjusted for age, sex, race, trunk fat, smoking, and hepatitis C status. Error bars show 95% confidence interval around the mean values. P value < 0.05 indicates that there are differences between the mean values among the groups. **P < 0.01 between groups; *P < 0.05 between groups. Black boxes surrounding a y axis variable indicate that there is effect modification by HIV status for this marker, that is, P < 0.15 for heroin use-by-HIV status interaction term. BDG, beta-D-glucan; I-FABP, intestinal fatty acid–binding protein; LBP, lipopolysaccharide-binding protein.
Heroin use was independently associated with higher concentrations of LBP and BDG (P < 0.01 and P = 0.01 in the fully adjusted models, respectively), and these effects were modified by HIV status (P = 0.07 and P = 0.10 for heroin use by HIV status interactions, respectively). Concentrations of LBP and BDG were higher in heroin users than nonusers among those without HIV in adjusted models (HIV− heroin+ vs HIV− heroin−; P < 0.01 for both), although concentrations were more similar between heroin users and nonusers among those with HIV (HIV+ heroin+ vs HIV+ heroin−; P = 0.36 and P = 0.38, respectively). Of interest, BDG was higher in heroin users without HIV than in heroin users with HIV (HIV+ heroin+ vs HIV− heroin+; P = 0.02). The results described earlier were similar after excluding 3 participants on antibiotics (2 HIV+ heroin+ participants on cephalexin and on doxycycline, respectively, and 1 HIV− heroin− participant on penicillin); there were no participants on antifungal medications. Furthermore, adjusting for the route of heroin use and intensity of use did not change the results.
Systemic and Vascular Inflammation Markers
Heroin use was independently associated with higher hsCRP (P = 0.01), sTNF-α-RI (P < 0.01), sTNF-α-RII (P < 0.001), and D-dimer (P < 0.01) concentrations in adjusted models. Associations between heroin use and IL-6 and sVCAM-1 attenuated with adjustment (P = 0.29 and 0.57, respectively). HIV status was not independently associated with inflammation marker concentrations. In fact, all inflammation markers were similar among those using heroin, regardless of HIV status (HIV+ heroin+ vs HIV− heroin−). HIV status did modify the effect between heroin use and hsCRP (P = 0.09 for heroin use by HIV status interaction). Concentrations of hsCRP were higher in heroin users than in nonusers among those without HIV (HIV− heroin+ vs HIV− heroin−; P < 0.01 in adjusted model), whereas differences among participants with HIV were smaller and did not meet statistical significance (HIV+ heroin+ vs HIV+ heroin−; P = 0.34). See Figure 2.
FIGURE 2.
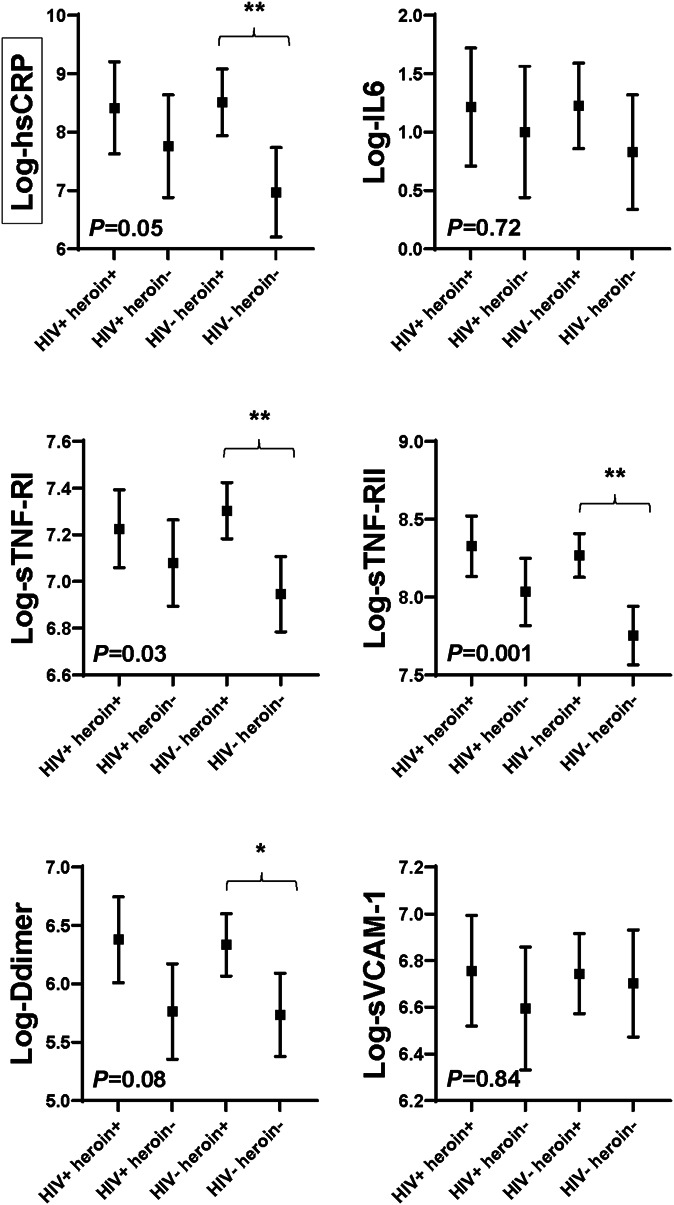
Systemic and vascular inflammation markers by group. Symbols represent adjusted least square means for each marker by group. Means are adjusted for age, sex, race, trunk fat, smoking, and hepatitis C status. Error bars show 95% confidence interval around the mean values. P < 0.05 indicates there are differences between the mean values among groups. **P < 0.01 between groups; *P < 0.05 between groups. Black boxes surrounding a y axis variable indicate that there is effect modification by HIV status for this marker, that is, P < 0.15 for heroin use-by-HIV status interaction term. hsCRP, high-sensitivity C-reactive protein; IL-6, interleukin-6; sTNF-α-RI and sTNF-α-RII, soluble tumor necrosis factor-α receptor I and receptor II; sVCAM-1, soluble vascular cell adhesion molecule-1.
Monocyte and T-lymphocyte Activation
Heroin use was independently associated with higher sCD163 (P = 0.04 in adjusted model) concentrations, and there was some evidence of association with higher sCD14 (P = 0.05) concentrations. In addition, heroin use was independently associated with a higher proportion of inflammatory monocytes (CD14+ CD16+) (P < 0.05), and there was some evidence of association with a lower proportion of patrolling monocytes (CD14+ CD16 dim) (P = 0.09). HIV infection was associated only with higher sCD163 (P = 0.02). HIV status modified the effect of heroin use on proportion of patrolling monocytes (P = 0.13 for heroin use by HIV status interaction). The proportion of patrolling monocytes were lower in heroin users than in nonusers among those with HIV (P < 0.05 in adjusted model), whereas proportions were more similar among those without HIV (P = 0.32). See Figure 3.
FIGURE 3.
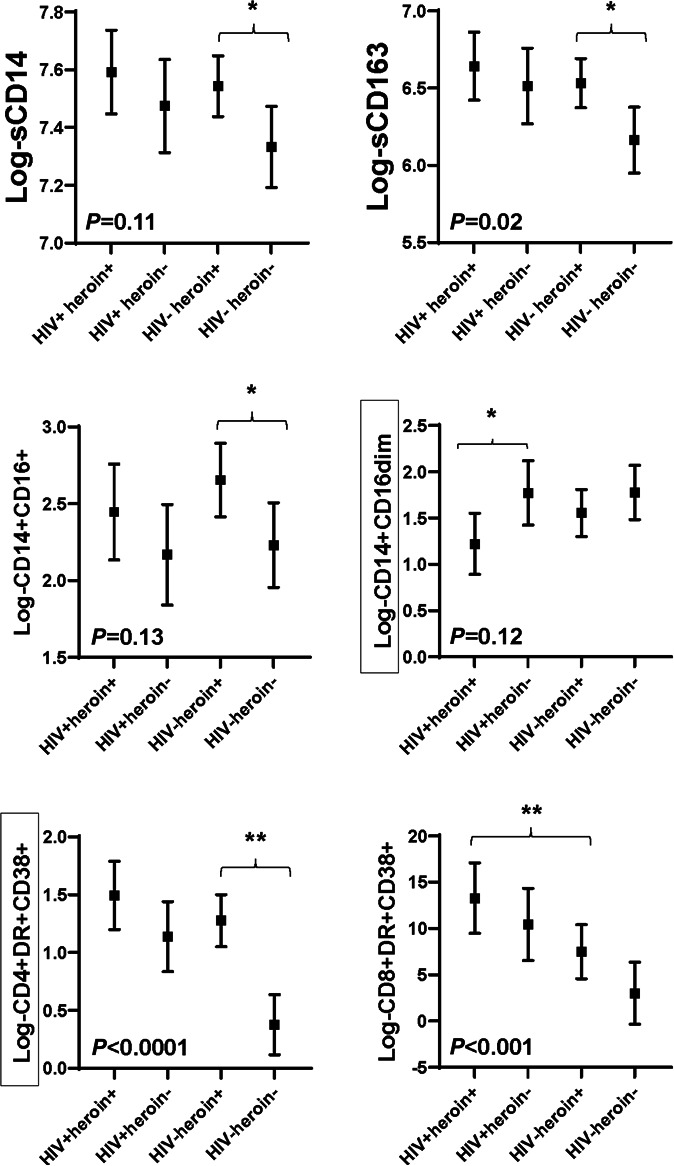
Soluble and cellular markers of monocyte and T-cell activation by group. Symbols represent adjusted least square means for each marker by group. The mean values are adjusted for age, sex, race, trunk fat, smoking, and hepatitis C status. Error bars show 95% confidence interval around the mean values. P < 0.05 indicates there are differences between the mean values among groups. **P < 0.01 between groups; *P < 0.05 between groups. Black boxes surrounding a y axis variable indicate that there is effect modification by HIV status for this marker, that is, P < 0.15 for heroin use-by-HIV status interaction term. sCD14, soluble CD14; sCD163, soluble CD163.
Both heroin use and HIV infection were independently associated with heightened CD4+ (P < 0.001 for both) and CD8+ (P = 0.09 and <0.0001, respectively) T-cell activation. HIV status modified the effect of heroin use on CD4+ T-cell activation (P = 0.12 for heroin use by HIV status interaction). The difference in CD4+ T-cell activation between heroin users and nonusers was significant and much greater among those without HIV (HIV− heroin+ vs HIV− heroin−; P < 0.0001 in adjusted model) than among those with HIV (HIV+ heroin+ vs HIV+ heroin−; P = 0.14). CD8+ T-cell activation was higher in participants with HIV than in those without HIV among heroin users (HIV+ heroin+ vs HIV− heroin+; P < 0.01). For CD4+ and CD8+ T-cell activation, adjusting for current CD4+ T-cell count did not change the results among participants with HIV.
DISCUSSION
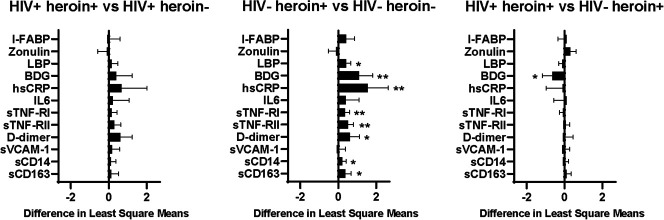
In this study of 100 participants with and without HIV who use and do not use heroin, we have shown that heroin use is independently associated with higher microbial translocation (LBP and BDG), systemic inflammation (hsCRP, sTNF-α-RI, sTNF-α-RII, and D-dimer), monocyte activation (sCD163 and a proportion of inflammatory monocytes), and CD4+ T-cell activation; HIV infection is independently associated with higher gut permeability (zonulin), monocyte activation (sCD163), and CD4+ and CD8+ T-cell activation; and HIV status modifies the effect of heroin on concentrations of microbial translocation markers (LBP and BDG), hsCRP, proportion of patrolling monocytes, and CD4+ T-cell activation. Counter to our hypothesis that heroin use would worsen these markers to a greater degree in people with HIV, differences in these markers between heroin and nonheroin users were greatest in those without HIV for all markers except in the proportion of patrolling monocytes (Fig. 4).
FIGURE 4.The.
mean difference between groups for soluble markers of gut integrity, microbial translocation, systemic and vascular inflammation, and monocyte activation. Bars represent difference in adjusted least square means between groups. The mean values are adjusted for age, sex, race, trunk fat, smoking, and hepatitis C status. Error bars show 95% confidence interval around difference in the mean values. **P < 0.01; *P < 0.05. As an example, in the first graph, values to the right of zero indicate that marker values are higher in the HIV+ heroin+ group compared with those in the HIV+ heroin-group.
Of importance, heightened concentrations of both bacterial and fungal translocation markers were associated with heroin use in this study. In a cross-sectional study (N = 119), circulating LPS was higher in PWH who use intravenous drugs compared with people who do not use intravenous drugs.11 In that study, despite lower HIV-1 RNA levels and higher CD4+ T-cell counts, those who used intravenous heroin, but not intravenous cocaine, had higher plasma LPS levels compared with those without substance use. Our study extends these results because higher BDG was also associated with heroin use, which has high significance, given this fungal translocation marker has links to HIV-associated immune activation and downstream metabolic effects.13–18 Of interest, in this study, although there was some evidence that HIV infection was associated with higher zonulin, presence of HIV did not seem to substantially worsen either gut integrity or microbial translocation markers among participants using heroin. It is possible that greater differences were not apparent because most of the participants with HIV in this study were on ART and virologically suppressed. The chronic immune activation that characterizes HIV infection is driven largely by the depletion of intestinal CD4+ T cells, leading to altered gut integrity and microbial translocation. Although not to preinfection levels, ART initiation can improve gastrointestinal-associated lymphoid tissue function, especially if ART is initiated early.19,20 In addition, we are unaware of the additives in the heroin preparations used by participants. Additives, such as cellulose, may have resulted in higher concentrations of BDG in plasma. Although we did further adjust models for how heroin was used (injection vs intranasal) and results were unchanged, we cannot exclude this as a possible explanation for why BDG concentrations were higher in heroin users without HIV compared with those with HIV.
Often aligned with heightened microbial translocation, markers of monocyte activation, sCD14 and sCD163, and the proportion of inflammatory monocytes were also associated with heroin use in adjusted analyses. In a matched, cross-sectional study (N = 50) of viremic PWH and people without HIV who inject drugs and viremic PWH and people without HIV who never injected drugs, sCD14 concentrations were highest in PWH regardless of injection drug use.21 In our study, differences in soluble measures of monocyte activation were not significantly different among people who use heroin with and without HIV possibly because most of the PWH were virologically suppressed on ART. Our results extend the literature by showing a heightened proportion of inflammatory monocytes similar among people who use heroin regardless of HIV status, but a lower proportion of patrolling monocytes that were lowest in PWH who use heroin. This is of particular relevance to PWH who are at heightened risk of cardiovascular disease22 because patrolling monocytes function to remove damaged cells and debris from vasculature, promoting the resolution of inflammation.23 In the aforementioned study by Mehandru et al,21 CD4+ T cells were depleted in peripheral blood and colonic tissue and CD8+ T cells increased in peripheral blood and colonic tissue, without regard for injection drug use in viremic PWH. Both CD4+ and CD8+ T-cell activation were significantly increased in people who inject drugs regardless of HIV status, although the magnitude of increase in T-cell activation was greater in PWH who inject drugs. In our study, CD8+ T-cell activation was highest in the heroin users with HIV. However, heightened CD4+ T-cell activation in heroin users was independent of HIV status.
Finally, heroin use was associated with heightened systemic inflammation independent of HIV infection. It is clear that injection drug use leads to an increase in systemic inflammation,24,25 possibly through direct injection-related injury or infection,26,27 type of heroin injected,28,29 filler agents, injection practices such as reusing cotton filters,30,31 other high-risk behaviors,32,33 or environmental factors, such as low individual and community socioeconomic status.34 From the Study to Assess Hepatitis C Risk (N = 541), nearly half of the people who inject drugs reported ever having an abscess.35 Of importance, participants had a greater number of injection partners were more likely to inject daily and to share cookers and less likely to use new syringes with each injection. In another survey of people who inject heroin (N = 145), two-thirds reported having at least 1 abscess ever and 20% reported having >2 abscesses per year.36 In this study, 39% of participants waited at least 2 weeks to seek care. It follows that injection drug use likely contributes to at least intermittent increases in systemic inflammation chronically. In our study, participants in the heroin use groups did not have active abscesses despite having higher levels of systemic inflammation, although we cannot rule out that previous or intermittent skin and soft tissue infections could have contributed to the heightened inflammatory response seen in these groups.
As with any cross-sectional evaluation, causality cannot be determined. A longitudinal follow-up for the ACTIVATE study is currently underway. In addition, although LBP and BDG are established markers of microbial translocation, it is possible that concentrations of these markers were elevated in this study population because of injection practices rather than altered gut integrity. Future study using colonic tissue is needed. Further limitations of this study include inadequate power to detect all possible interactions or to dissect the effect of different opioids on the markers assessed. Furthermore, it is possible that differences among the groups with HIV (HIV+ heroin+ and HIV+ heroin−) may not have met significance because of the small sample size.
CONCLUSIONS
Heroin use is associated with heightened microbial translocation, systemic inflammation, and monocyte and T-cell activation, and this is independent of HIV status. Participants with HIV who did not use heroin demonstrated heightened measures of nearly all markers tested when compared with those without HIV, demonstrating that HIV infection results in disruption of gut integrity, microbial translocation, systemic inflammation, and immune activation. This likely explains why differences in markers assessed among heroin users and nonusers with HIV were smaller than differences between heroin users and nonusers without HIV. Indeed, HIV infection did not seem to substantially worsen the multiple effects that heroin has on microbial translocation, systemic inflammation, and immune activation. Future studies should assess the effects of heroin in the context of HIV infection over time and potential reversal of these effects with cessation of heroin with and without medication-assisted treatment for opioid use disorder.
Footnotes
Supported by the National Institutes of Drug Abuse R01DA044576 to C.O.H. and G.A.M. and the Clinical and Translational Science Collaborative of Cleveland (UL1TR002548) from the National Center for Advancing Translational Sciences component of the NIH and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
C.O.H. has served as a consultant for Theratechnologies and Gilead and has received research grant support from Gilead. N.F. has served as a consultant for Gilead. G.A.M. has served as a consultant for Gilead, Merck, Theratechnologies, Jannsen, and GSK/ViiV and has received research grants from Roche, Genentech, Vanda, Astellas, Tetraphase, Gilead, Merck, and ViiV. The remaining authors have no conflicts of interest to disclose.
Preliminary results were presented at the Conference on Retroviruses and Opportunistic Infections, held virtually; during March 7–10, 2020; Boston, MA.
REFERENCES
- 1.An Q, Song R, Hernandez A, et al. Trends and differences among three new indicators of HIV infection progression. Public Health Rep. 2015;130:468–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ingle SM, May MT, Gill MJ, et al. Impact of risk factors for specific causes of death in the first and subsequent years of antiretroviral therapy among HIV-infected patients. Clin Infect Dis. 2014;59:287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore RD, Keruly JC, Chaisson RE. Differences in HIV disease progression by injecting drug use in HIV-infected persons in care. J Acquir Immune Defic Syndr. 2004;35:46–51. [DOI] [PubMed] [Google Scholar]
- 4.Trickey A, May MT, Vehreschild J, et al. Cause-Specific mortality in HIV-positive patients who survived ten years after starting antiretroviral therapy. PLoS One. 2016;11:11e0160460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piggott DA, Varadhan R, Mehta SH, et al. Frailty, inflammation, and mortality among persons aging with HIV infection and injection drug use. J Gerontol A Biol Sci Med Sci. 2015;70:1542–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peterson PK, Sharp BM, Gekker G, et al. Opiates, human peripheral blood mononuclear cells, and HIV. Adv Exp Med Biol. 1991;288:171–178. [DOI] [PubMed] [Google Scholar]
- 7.Zhu JW, Liu FL, Mu D, et al. Heroin use is associated with lower levels of restriction factors and type I interferon expression and facilitates HIV-1 replication. Microbes Infect. 2017;19:288–294. [DOI] [PubMed] [Google Scholar]
- 8.Guo CJ, Li Y, Tian S, et al. Morphine enhances HIV infection of human blood mononuclear phagocytes through modulation of beta-chemokines and CCR5 receptor. J Investig Med. 2002;50:435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. [DOI] [PubMed] [Google Scholar]
- 10.Marchetti G, Tincati C, Silvestri G. Microbial translocation in the pathogenesis of HIV infection and AIDS. Clin Microbiol Rev. 2013;26:2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ancuta P, Kamat A, Kunstman KJ, et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One. 2008;3:e2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Funderburg NT, Jiang Y, Debanne SM, et al. Rosuvastatin treatment reduces markers of monocyte activation in HIV-infected subjects on antiretroviral therapy. Clin Infect Dis. 2014;58:588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramendra R, Isnard S, Mehraj V, et al. Circulating LPS and (1-->3)-beta-D-glucan: a Folie a Deux contributing to HIV-associated immune activation. Front Immunol. 2019;10:465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dirajlal-Fargo S, Moser C, Rodriguez K, et al. Changes in the fungal marker beta-D-glucan after antiretroviral therapy and association with adiposity. Open Forum Infect Dis. 2019;6:ofz434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gianella S, Letendre SL, Iudicello J, et al. Plasma (1--> 3)-beta-D-glucan and suPAR levels correlate with neurocognitive performance in people living with HIV on antiretroviral therapy: a CHARTER analysis. J Neurovirol. 2019;25:837–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehraj V, Ramendra R, Isnard S, et al. Circulating (1-->3)-beta-D-glucan is associated with immune activation during human immunodeficiency virus infection. Clin Infect Dis. 2020;70:232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiner LD, Retuerto M, Hager CL, et al. Fungal translocation is associated with immune activation and systemic inflammation in treated HIV. AIDS Res Hum Retroviruses. 2019;35:461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El Kamari V, Moser C, Hileman CO, et al. Lower pretreatment gut integrity is independently associated with fat gain on antiretroviral therapy. Clin Infect Dis. 2019;68:1394–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allers K, Puyskens A, Epple HJ, et al. The effect of timing of antiretroviral therapy on CD4+ T-cell reconstitution in the intestine of HIV-infected patients. Mucosal Immunol. 2016;9:265–274. [DOI] [PubMed] [Google Scholar]
- 20.Costiniuk CT, Angel JB. Human immunodeficiency virus and the gastrointestinal immune system: does highly active antiretroviral therapy restore gut immunity? Mucosal Immunol. 2012;5:596–604. [DOI] [PubMed] [Google Scholar]
- 21.Mehandru S, Deren S, Kang SY, et al. Behavioural, mucosal and systemic immune parameters in HIV-infected and uninfected injection drug users. J Addict Res Ther. 2015;6:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah ASV, Stelzle D, Lee KK, et al. Global burden of atherosclerotic cardiovascular disease in people living with HIV: systematic review and meta-analysis. Circulation. 2018;138:1100–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas G, Tacke R, Hedrick CC, et al. Nonclassical patrolling monocyte function in the vasculature. Arterioscler Thromb Vasc Biol. 2015;35:1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salter ML, Lau B, Mehta SH, et al. Correlates of elevated interleukin-6 and C-reactive protein in persons with or at high risk for HCV and HIV infections. J Acquir Immune Defic Syndr. 2013;64:488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strickler HD, Blanchard JF, Vlahov D, et al. Elevated serum levels of neopterin but not beta 2-microglobulin in HIV-1-seronegative injecting drug users. AIDS. 1993;7:361–367. [DOI] [PubMed] [Google Scholar]
- 26.Phillips KT, Stein MD. Risk practices associated with bacterial infections among injection drug users in Denver, Colorado. Am J Drug Alcohol Abuse. 2010;36:92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith ME, Robinowitz N, Chaulk P, et al. High rates of abscesses and chronic wounds in community-recruited injection drug users and associated risk factors. J Addict Med. 2015;9:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mars SG, Bourgois P, Karandinos G, et al. The textures of heroin: user perspectives on “black tar” and powder heroin in two U.S. Cities. J Psychoactive Drugs. 2016;48:270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Summers PJ, Struve IA, Wilkes MS, et al. Injection-site vein loss and soft tissue abscesses associated with black tar heroin injection: a cross-sectional study of two distinct populations in USA. Int J Drug Pol. 2017;39:21–27. [DOI] [PubMed] [Google Scholar]
- 30.Torka P, Gill S. Cotton fever: an evanescent process mimicking sepsis in an intravenous drug abuser. J Emerg Med. 2013;44:e385–e387. [DOI] [PubMed] [Google Scholar]
- 31.Dwyer R, Topp L, Maher L, et al. Prevalences and correlates of non-viral injecting-related injuries and diseases in a convenience sample of Australian injecting drug users. Drug Alcohol Depend. 2009;100:9–16. [DOI] [PubMed] [Google Scholar]
- 32.Siegel AJ, Mendelson JH, Sholar MB, et al. Effect of cocaine usage on C-reactive protein, von Willebrand factor, and fibrinogen. Am J Cardiol. 2002;89:1133–1135. [DOI] [PubMed] [Google Scholar]
- 33.Kidd SE, Grey JA, Torrone EA, et al. Increased methamphetamine, injection drug, and heroin use among women and heterosexual men with primary and secondary syphilis—United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2019;68:144–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petersen KL, Marsland AL, Flory J, et al. Community socioeconomic status is associated with circulating interleukin-6 and C-reactive protein. Psychosom Med. 2008;70:646–652. [DOI] [PubMed] [Google Scholar]
- 35.Asher AK, Zhong Y, Garfein RS, et al. Association of self-reported abscess with high-risk injection-related behaviors among young persons who inject drugs. J Assoc Nurses AIDS Care. 2019;30:142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Summers PJ, Hellman JL, MacLean MR, et al. Negative experiences of pain and withdrawal create barriers to abscess care for people who inject heroin. A mixed methods analysis. Drug Alcohol Depend. 2018;190:200–208. [DOI] [PubMed] [Google Scholar]