Abstract
When epithelial cells are exposed to potentially threatening external stimuli such as allergens, bacteria, viruses, and helminths, they instantly produce “alarmin” cytokines, namely, IL-33, IL-25, and TSLP. These alarmins alert the immune system about these threats, thereby mobilizing host immune defense mechanisms. Specifically, the alarmins strongly stimulate type-2 immune cells, including eosinophils, mast cells, dendritic cells, type-2 helper T cells, and type-2 innate lymphoid cells. Given that the alarm-raising role of IL-33, IL-25, and TSLP was first detected in allergic and infectious diseases, most studies on alarmins focus on their role in these diseases. However, recent studies suggest that alarmins also have a broad range of effector functions in other pathological conditions, including psoriasis, multiple sclerosis, and cancer. Therefore, this review provides an update on the epithelium-derived cytokines in both allergic and non-allergic diseases. We also review the progress of clinical trials on biological agents that target the alarmins and discuss the therapeutic potential of these agents in non-allergic diseases.
Keywords: Alarmins, Hypersensitivity, Autoimmune disease, Biological therapy
INTRODUCTION
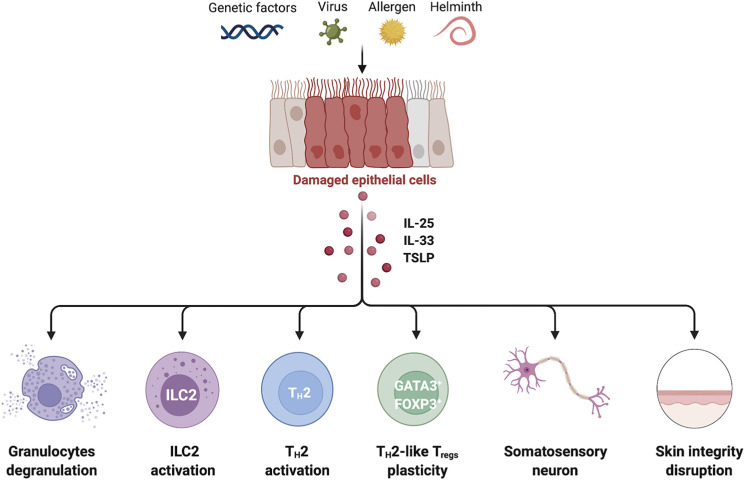
The body’s first line of defense against allergens, viruses, bacteria, and helminths consists of the epithelial lining of the skin, intestines, and lungs, which acts as physical barriers. However, recent studies have shown that epithelial cells also play a hitherto poorly understood but apparently vital role in immunological host defense by sensing potential physical/chemical or infectious threats and then initiating immune responses (1). This crucial early role is largely mediated by the epithelial-cell release of IL-33, IL-25, and TSLP, which have been designated “alarmins” (2,3). Once released in response to allergen or infection, these cytokines prime the immune system to produce type-2 immune responses characterized by upregulated eosinophils, mast cells, dendritic cells (DCs), type-2 helper T (TH2) cells, and type-2 innate lymphoid cells (ILC2s) (Fig. 1). It is now well known that this pathway plays a critical role in the initiation of allergic diseases such as asthma and atopic dermatitis (AD) (2). However, recent studies have discovered that alarmins can also directly regulate many innate and adaptive immune cells and several non-immune cells (Fig. 1) and in fact are important in various inflammatory diseases that do not involve type-2 responses, including psoriasis, multiple sclerosis (MS), and cancer (4,5,6,7,8,9,10). Therefore, here we will discuss these findings. We will also present the current status of the clinical trials on biologics that target the epithelium-derived alarmins.
Figure 1. Cellular targets of IL-25, IL-33, and TSLP in allergic diseases. Genetic defects and environmental stimuli stimulate epithelial cells to secret alarmins. These cytokines exert pro-inflammatory effects by acting on a wide range of cell populations. Specifically, they can 1) enhance the survival, recruitment, and degranulation of granulocytes such as eosinophils and mast cells; 2) immediately cause ILC2s to secrete IL-5 and IL-13; 3) act directly on TH2 cells since they induce their terminal differentiation in peripheral inflamed tissues; 4) convert ST2-expressing Tregs into TH2-like Tregs that produce IL-5 and IL-13; and 5) act on somatosensory neurons and keratinocytes, thereby promoting itching and disrupting the skin barrier, respectively.
MATURATION OF EPITHELIUM-DERIVED CYTOKINES
IL-33
IL-33 is a member of the IL-1 family and binds to heterodimeric receptors composed of IL-1 receptor accessory protein and the transmembrane isoform of ST-2 (Fig. 2). Once IL-33 binds to its receptor ST2, the cytoplasmic domain of ST2 recruits the adaptor protein MyD88, which ignites NF-κB and AP-1 signaling pathways and induce the expression of pro-inflammatory molecules (11). In homeostatic conditions, the 270-amino acid IL-33 protein is constitutively expressed by epithelial and endothelial cells as a full-length immature form that is sequestered in the nucleus (12). However, once epithelial cells are exposed to an allergen, bacterium, or virus, IL-33 is cleaved into mature forms by either an exogenous or endogenous proteolytic mechanism. The exogenous proteolysis cleaves the central domain of IL-33 and results in the IL-3395–270, IL-33107–270, and/or IL-33109–270 fragments. This cleavage is mediated by serine and cysteine proteases from either neutrophils and mast cells or allergens (e.g. proteases in fungi, house dust mite, pollen, bacteria, and cockroach) (13,14,15). By contrast, the endogenous proteolysis is triggered in epithelial cells when they directly detect an allergen: this activates the ripoptosome, an intracellular signaling platform that consists of pro-caspase 8, FADD, and RIP1. Once triggered, caspase 8 activates caspase 3 and 7. These caspases then cleave full-length IL-33 in the C-terminal domain into the IL-331–175/178 fragments (16). These exogenous and endogenous cleavage events all result in mature IL-33 fragments in the extracellular environment that bind to and activate a plethora of immune cells, including macrophages, eosinophils, mast cells, TH2 cells, Tregs, and ILC2s (17,18,19,20).
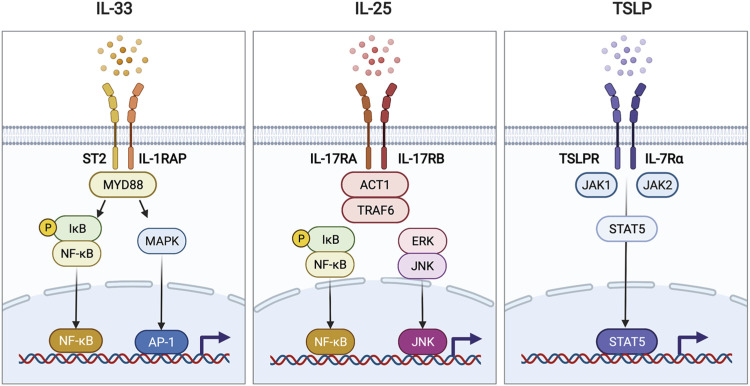
Figure 2. The receptors and downstream signaling of each alarmin. IL-33 binds to a heterodimeric receptor composed of ST2 and IL-1RAP. Ligation of IL-33 recruits and activates the adaptor protein MyD88. MyD88 activates the transcription factors NF-κB and AP-1, which are then delivered to the nucleus and bind to specific DNA motifs. IL-25 exerts its pro-inflammatory effects by binding to a heterodimeric receptor composed of IL-17RA and IL-17RB. The intracellular domain of the IL-25 receptor recruits ACT1 and TRAF6 and subsequently promotes the activation of the NF-κB or ERK-JNK signaling axis. TSLP binds to TSLPR paired with IL-7Rα. This ligation event activates TSLP receptor-associated JAK1 and JAK2, which activate the transcription factor STAT5, thereby causing it to translocate into the nucleus. All of these alarmin signaling pathways lead to the production of type-2 cytokines and chemokines by the target cells.
IL-1RAP, IL-1 receptor accessory protein.
The exogenous cleavage event significantly increases the biological activity of IL-33: for example, it is 10–30-fold more potent than full-length IL-33 in terms of inducing ILC2s to produce type-2 cytokines (13,14,21). Travers et al. (22) suggested that this is because the mature IL-33 is released in a complex with histones; they observed that the histones synergized with IL-33 when activating the pro-inflammatory cytokine production of mast cells. However, the relative role and significance of cleaved IL-33 endogenously and exogenously remain unclear. Nonetheless, these findings suggest that therapies for diseases that are triggered by IL-33 should target the mature form of IL-33.
IL-25
IL-25 is also known as IL-17E and belongs to the 6-member (A–F) IL-17 family. All members bind to a homodimeric or heterodimeric receptor composed of 2 subunits of the 5-member (A–E) IL-17 receptor family (23). IL-25 binds to a heterodimeric receptor (IL-25R) composed of IL-17 receptor A (IL-17RA) and IL-17 receptor B (IL-17RB) (Fig. 2). Ligation of IL-25R recruits the adaptor proteins, such as ACT1 and TRAF6, and then activates NF-κB, MAPK/ERK, and JNK signaling to upregulate genes involved in proliferation and differentiation (24). Unlike the other IL-17 family members, which promote type-3 inflammation, IL-25 amplifies type-2 immunity in multiple tissues (23,25). Given this unique function, IL-25 has been designated a distinct IL nomenclature.
IL-25 can be secreted from a variety of cells, including lung epithelial cells, brain capillary endothelial cells, and synovial fibroblasts in rheumatoid arthritis patients. This diversity of sources suggests that IL-25 may participate in many diseases (26,27,28). When IL-25-expressing cells are exposed to external stimuli such as helminths or allergens, IL-25 is secreted as a disulfide-linked homodimer (29,30). Although very little is known about early IL-25 processing and its effect on IL-25 bioactivity, Goswami et al. (31) have shown that IL-25 is cleaved by matrix metalloproteinase (MMP) 7. They also showed that this extracellular cleavage event is important for IL-25 function: the MMP7-cleaved form of IL-25 elevated anti-CD3/28-induced I splenocyte expression of IL-4, IL-5, and IL-13 significantly better than full-length IL-25. Moreover, when MMP7 was knocked out, Aspergillus-induced asthma model mice developed less airway hyperresponsiveness (AHR), lower type-2 cytokine levels, and lower eosinophil counts in the bronchoalveolar lavage (31). Thus, IL-25 may resemble IL-33 in the sense that post-translational maturation is needed for its bioactivity. This supports the notion that therapies should target mature alarmins.
TSLP
TLSP is a member of the IL-2 cytokine family and is mainly produced by epithelial and stromal cells. It exerts its effector functions by binding to the heterodimeric receptor composed of the TSLP receptor (TSLPR) and IL-7Rα (32,33) (Fig. 2). When TLSP binds to its receptor, JAK1 and 2 are recruited and phosphorylated, and downstream STAT5 is activated to initiate expression of pro-inflammatory genes (34).
TSLP has 2 isoforms with opposing immunomodulatory effects, namely, the anti-inflammatory short-form TSLP (sfTSLP) and the pro-inflammatory long-form TSLP (lfTSLP). sfTLSP is constitutively expressed in the skin, intestine, and lung under homeostatic conditions whereas lfTSLP is upregulated in inflammatory environments such as the asthmatic lung and the intestine in ulcerative colitis patients (35,36). Dong et al. (35) reported that treatment with synthetic sfTSLP improved house dust mite-induced asthma and airway epithelial cell damage in mice whereas treatment with lfTSLP impaired and worsened barrier function. However, to date, the sfTSLP receptor has not been fully identified and the regulatory mechanism by which it acts has not been reported in non-human species (37). For this reason, most studies on TSLP have been conducted on lfTSLP. Therefore, we will not discuss sfTSLP further in this review. All further references to TSLP allude to the lfTSLP isoform.
The primary sources of TSLP are the epithelial cells in the lungs, skin, and gastrointestinal tract, although immune cells, such as DCs, mast cells and mucosal T cells can also express it (35,38,39). Like the other alarmins, TSLP also appears to be activated by extracellular proteolytic activity. Thus, Nagarkar et al. (40) reported that proteases in nasal polyp extract degrade full-length TSLP and that the resulting cleaved TSLP enhances the IL-5 produced by IL-1β-stimulated mast cells. Furthermore, Poposki et al. (41) showed that proprotein convertases in nasal polyp extracts generate truncated TSLP products that potently upregulate the ability of DCs and ILC2s to produce CCL17 and IL-5, respectively. However, the exact cleavage sites of TSLP have not yet been identified. Notably, TSLP cleavage has not been observed in non-primates, which suggests that the murine model is inappropriate for examining the effects of truncated TSLP (41).
ROLE OF ePITHELIUM-DERIVED CYTOKINES IN ALLERGIC DISEASES
Asthma
Asthma is an obstructive airway disease that is characterized by sporadically recurring symptoms, including dyspnea, coughing, and wheezing (42). It is underpinned by both genetic and environmental risk factors (Fig. 1), many of which associate strongly with epithelium-derived cytokines (26,43,44,45,46,47). Thus, several genome-wide association studies have shown that single nucleotide polymorphisms in IL33, IL1RL1 (the gene expressing the IL-33 receptor ST2), and TSLP associate with asthma severity and blood eosinophil counts (45,46,47). While associations between IL25 polymorphisms and asthma have not been found to date, an analysis of bronchial biopsies from asthma patients has shown that allergen inhalation increases IL-25-and IL-25R-expressing cell frequencies and that these frequencies correlate negatively with maximum change in forced expiratory volume in 1 second (FEV1) % (48). Moreover, bronchial epithelial biopsies from asthma patients have higher IL-33, IL-25, and TSLP protein levels than healthy controls (49,50,51). When murine models of allergic asthma are treated with anti-IL-33, anti-IL-25, or anti-TSLP mAbs, they reduce AHR and the type-2 cytokine levels and eosinophil counts in the lung (52,53,54).
It appears that these alarmins can act indirectly and directly on asthma-associated immune cells, including eosinophils, mast cells ILC2s, effector TH2 cells, and Tregs (Fig. 1). Regarding eosinophils, Cherry et al. (18) showed with in vitro experiments that IL-33 promotes eosinophil superoxide production, degranulation, and survival as potently as the key eosinophil regulator IL-5. This prosurvival effect of IL-33 appears to relate to its ability to induce the autocrine secretion of GM-CSF in eosinophils (55). Moreover, Salter et al. (56) found that the eosinophilogenic potential of supernatants from cultured bronchial epithelial cells (as measured by adding the supernatants to non-adherent mononuclear cell cultures) was much higher when the epithelial cells were from asthma patients rather than from healthy controls; in addition, this eosinophilogenic potential was extinguished by antibodies against TSLP. Thus, IL-33 and TSLP can directly influence eosinophil formation and activity. For mast cells, IL-33 induces mast-cell maturation and degranulation and enhances their adherence to fibronectin in vitro (57,58). TSLP induces mast-cell production of cytokines (IL-5, IL-6, and IL-13) and chemokines (CXCL8 and CCL1) in vitro but only when IL-1β and TNF are also present (43).
Not only granulocytes but also tissue-resident innate lymphocytes, particularly ILCs, can mediate type 2 inflammation in an immediate response to alarmins (59,60,61). Kim et al. (62) found with in vitro experiments that ILCs are directly activated by IL-33 produced by alveolar macrophages, DCs, and type-2 pneumocytes. Their in vivo experiments using ST2-deficient mice then showed that activated ILCs are needed to induce the AHR and airway inflammation in glycolipid-induced asthma (62). Two studies have also shown that when ovalbumin-induced asthma model mice are treated with neutralizing IL-25 Ab during the antigen challenge period, it markedly abrogates the development of AHR and the IL-5 and IL-13 levels and ILC2 counts in the lung (52,63). In addition, TSLP receptor-deficient mice showed reduced BAL eosinophilia and type 2 cytokine production from ILC2 after Alternaria alternata challenge (64). Interestingly, Liu et al. (65) found that the ILC2s from the TSLP-rich bronchoalveolar lavage of asthma patients are more resistant to steroids than the circulating ILC2s and that this resistance can be reversed in vitro by clinically available inhibitors of MEK and STAT5. Kabata et al. (66) also found that TSLP, but not IL-25 or IL-33, induces the steroid resistance of ILC2s in the lung of asthma model mice, and that this is mediated via STAT5 phosphorylation and anti-apoptotic Bcl-xL expression. Thus, targeting TSLP could be an alternative therapeutic strategy for uncontrolled steroid-resistant asthma.
Although receiving less attention than innate lymphocytes, T lymphocytes are also affected by epithelium-derived alarmins at sites of inflammation. It has been shown that while mice that are deficient in all 3 alarmins have normal T-cell priming in the lymph node, effector TH2 cells cannot undergo terminal differentiation in peripheral tissues (67). In an in vitro T cell differentiation system, each alarmin directly enhances TH2 differentiation (68,69,70). Besides T helper cells, Tregs, which are pivotal in maintaining immune tolerance against innocuous external agents (71,72), are also affected by alarmins in allergic conditions: Chen et al. (73) showed that lung-resident Tregs express ST-2 and respond to intranasal IL-33 administration by upregulating GATA3, IL5, and IL13 expression; consequently, these cells come to resemble “TH2-like” Tregs and associate with impaired airway tolerance and increased airway inflammation. Thus, the epithelial-derived alarmins may play multiple roles in the development of asthma. Further studies on these roles are warranted.
AD
AD is an allergic type of eczema characterized by epidermal hyperplasia and crusted, inflamed, highly pruritic, erythematous, and oozing vesicular lesions (74). AD shares many of the pathological features of asthma, including TH2-dominant immune responses and elevated plasma IgE levels (75). Several clinical and pre-clinical studies suggest that targeting alarmins in AD may have therapeutic potential because they appear to promote the disruption of the barrier epithelium, as follows (76,77,78,79,80,81). First, several studies show that AD associates with overexpression of IL-33 and TSLP by local keratinocytes and IL-25 overexpression by dermal DCs (38,76,77,78,79). Second, studies have found that 1) IL-33, IL-25, or TSLP treatment directly reduces the AD keratinocyte expression of skin barrier proteins (claudin-1 and/or filaggrin) in vitro (82,83,84,85); 2) IL-25R-deficient mice have better barrier integrity and thinner dermal epithelium than wild-type mice after AD induction (86); 3) keratinocyte-specific overexpression of TSLP and IL-33 elicit type-2 inflammation and AD-like phenotypes in mice (87,88); and 4) disruption of the barrier epithelium can promote AD by inducing trans-epithelial water loss, allergen penetration, and bacterial colonization in the skin (89). These findings together suggest that alarmins may act directly on keratinocytes in an autocrine manner and that targeting alarmins therapeutically could ameliorate AD formation and progression. The latter notion is supported by several genome-wide association studies that suggest the susceptibility loci for AD-associated with epithelial barrier integrity (90,91,92,93).
Several studies also show that ILC2s are key cellular players in AD pathogenesis. First, ILC2s appeared to be enriched in skin lesions from AD patients (80,81,94). Second, depletion of skin ILC2s reduces the skin thickness and type-2 cytokine production that is caused by topical application of MC903 (calcipotriol, a vitamin D analog), which induces AD-like inflammation (81). Thus, alarmins may contribute to AD via their well-known ability to stimulate ILCs. This is supported by Kim et al., (80) who reported that knocking out TSLP (but not IL-33 or IL-25) blocks MC903-induced AD. Interestingly, a very similar study showed that knocking out IL-25 or IL-33 attenuates the skin inflammation and ILC2 infiltration in MC903-induced AD better than knocking out TSLP (81). The reason for this discrepancy between studies is unclear. Nonetheless, these studies suggest that targeting alarmins may improve AD.
Two studies have also suggested that blocking alarmins could improve pruritis in AD. Pruritus is mediated by somatosensory neurons whose bodies are located in the dorsal root ganglia. Liu et al. (8) and Wilson et al. (95) showed that dorsal root ganglion neurons can express the alarmin receptor molecules ST-2 and TSLPR, and that IL-33 and TSLP bind directly to these cells. Thus, alarmins may promote pruritus by acting as signaling mediators between the epithelium and cutaneous sensory neurons. Given that scratching itself can exacerbate skin inflammation (96), these studies suggest that targeting alarmins may improve not only pruritis but also AD inflammation.
ROLE OF EPITHELIUM-DERIVED CYTOKINES IN AUTOIMMUNE DISEASES
Psoriasis
Psoriasis is a chronic autoimmune skin disease that is characterized by epidermal hyperplasia, dermal infiltration with immune cells, and increased dermal capillary density; this leads to scaly red skin plaques (97). Unlike asthma and AD, psoriasis is considered to be a type-17 disease, meaning that it is mediated by IL-17, IFN-γ, and IL-23 produced by TH17 cells and neutrophils (10,98,99,100,101,102,103). Thus, these molecular and cellular entities are the key therapeutic targets in psoriasis. Nonetheless, multiple clinical studies have hinted that alarmins may also participate in the pathophysiology of psoriasis. We will discuss the evidence for each alarmin in turn.
Mitsui et al. (102) showed that psoriasis patients have higher serum levels of IL-33 than healthy controls. Moreover, Zeng et al. (10) found with a murine model of imiquimod (IMQ)-induced psoriasis that keratinocytes in the inflamed skin predominantly produce IL-33 and also express the IL-33 receptor ST-2. This study then showed that mice with IL-33-deficient keratinocytes are less susceptible to IMQ-induced psoriasis (10). In addition, Duan et al. (104) reported that treating HaCaT human epidermal cells with IL-33 increases their proliferation. Besides, IL-33 administration on IMQ-induced psoriasis mice inhibits the expression of autophagy-related proteins from skin inflamed lesions (104): the latter finding is relevant because downregulation of autophagy associates with the progression of several inflammatory and autoimmune diseases, including psoriasis and AD. Thus, similar to the findings in AD, these studies suggest that IL-33 from keratinocytes may induce or exacerbate the epithelial hyperplasia in psoriasis (and AD) in an autocrine fashion. Immune cells may also participate in the IL-33-mediated exacerbation of psoriasis. Hueber et al. (105) showed that intradermal injection of IL-33 in murine ears not only induced an inflammatory psoriasis-like lesion that increased ear thickness, it also elevated the local levels of the neutrophil chemokine CXCL1 and neutrophil counts. Local mast cell counts also rose and were at least partly responsible for the inflammation since mast-cell deficient mice (KitW-sh/W-sh mice) showed a significant delay in the evolution of the psoriatic phenotype (105).
Like the IL-33, both IL-25 and TSLP were also involved in the pathogenesis of psoriasis. Papp et al. (9) showed that treating psoriasis patients with an IL-17RA (1 of the 2 IL-25 receptor subunits) blockade had strong therapeutic effects on psoriasis. Moreover, Suto et al. (106) showed that IL-25 stimulates DCs to produce IL-1β, and this is essential for TH17 cell-mediated contact hypersensitivity since Il25−/− mice are protected from this inflammatory response. Thus, the IL-25/IL-17R axis may be an effective target for psoriasis therapeutics.
Regarding TSLP, Suwarsa et al. (103) found that psoriasis patients have higher serum levels of TSLP than healthy controls while Volpe et al. (107) reported that the keratinocytes in untreated skin lesions from psoriasis patients strongly expressed TSLP. Notably, the latter study also found that TSLP works synergistically with CD40L to activate skin and blood DCs and that once activated in this manner, these DCs express high levels of IL-23 in vitro (107). This is significant because IL-23 (a heterodimeric cytokine composed of IL-12p40 and IL-23p19) induces epidermal acanthosis in mice when injected intradermally. Moreover, blocking IL-23 with a mAb against IL-12p40 reduces psoriasis symptoms in patients. Since IL-23 induces and activates both type-17 T cells and ILC3s, these findings suggest that although TSLP is known to promote type-2 immune responses, it can also aid type-17 inflammation. Additional studies suggest that keratinocytes are not only a key source of skin TSLP (38), they also express TSLPR (108). This suggests that keratinocyte-produced TSLP not only has paracrine immune effects, it also affects keratinocyte behavior in an autocrine manner. This is supported by an in vitro study that showed that treatment of primary keratinocytes with recombinant TSLP induces their proliferation in a dose-dependent fashion (109). Thus, these studies together suggest that IL-33, IL-25, and TSLP also contribute to psoriasis pathogenesis and that blocking alarmin signaling may be a therapeutic target in psoriasis.
MS
MS is a neurodegenerative disease that is characterized by demyelination and neurodegeneration in the central nerve system (CNS) (110). Analyses of IL-33 reporter mice showed that IL-33 is constitutively expressed in the corpus callosum, hippocampus, thalamus, and cerebellum (111); moreover, the astrocytes in both the brain and spinal cord express high levels of this alarmin (112). The involvement of IL-33 in the pathogenesis of MS is supported by increased IL-33 and/or ST2 expression in CNS lesions of MS patients (113,114) and experimental autoimmune encephalomyelitis (EAE), a mouse model for human MS (7,115). IL-33 was elevated in plasma (116,117), cerebrospinal fluid (117), and brain tissue (113,114) from MS patients. Several studies suggest that blocking IL-33 signalings can protect against MS. Li et al. (7) reported that IL-33 blockade reduces the development of EAE and that this effect is mediated by inhibition of TH1- and/or TH17-related immune responses. Similarly, when myelinating rat neurospheres are treated in vitro with recombinant IL-33, their axon myelination is inhibited (113). As the defect on axon myelination is the major feature of the MS, accumulated IL-33 may be involved in the initiation and aggravation of MS. However, other studies have found the opposite. Jiang et al. (115) showed that in vivo administration of IL-33 attenuates the development of EAE by promoting TH2 cell and M2 macrophage polarization. Moreover, genetic deletion of IL33 in oligodendrocyte precursor cells impairs oligodendrocyte maturation and differentiation (118). IL-33 receptor-deficient (St2−/−) mice also develop more severe EAE and exhibit enhanced inflammatory T cell accumulation in the brain and spinal cord (119). Thus, the therapeutic potential of targeting the IL-33/ST-2 axis in MS remains unclear.
Unlike the controversial roles of IL-33, IL-25 may play a protective role in MS since MS patients have very low serum IL-25 levels (120). Moreover, IL-25-deficient (Il25−/−) mice are highly susceptible to EAE, and this susceptibility is blocked when the IL-25 receptor IL-17A is neutralized (6). In addition, when T cells are activated in vitro by anti-CD3/28 in the presence of IL-25, they lose their ability to kill fetal neurons. This effect is mediated by IL-25-induced inhibition of LFA-1 on the T cells, which generates the cytotoxic immune synapse between T cells and their target cells (121). In addition, lentiviral-mediated administration of IL-25 into the CNS reduces the neuroinflammation in both EAE and the entorhinal cortex lesion model of neuroinflammation; this effect is due to IL-25-induced shifting of the microglia from the pro-inflammatory M1 to the anti-inflammatory M2 phenotype (122). Thus, IL-25 could potentially be a therapeutic target for MS.
There is some limited evidence that suggests TSLP signaling pathways contribute to MS pathogenesis. Eckhardt et al. (123) showed that when EAE is induced in TSLP-deficient (Tslp−/−) mice by myelin oligodendrocyte glycoprotein injections, the disease is both delayed and attenuated. Moreover, these mice display impaired differentiation of T cells into myelin oligodendrocyte glycoprotein-specific effector and memory T cells (123). Yu et al. (124) showed that TSLP receptor-deficient (Tslpr−/−) mice also exhibit resistance to EAE. Moreover, they found that TSLPR signaling induces neuroinflammation by activating Janus kinase, which then hyperactivates the NLR family pyrin domain containing 3 inflammasome (124), which in turn drives systemic TH1 and TH17 inflammation and chemotaxis of immune cells to the CNS. Thus, blocking TSLP signaling may be a prospective target for MS.
These studies suggest that epithelial-derived alarmins could participate in MS. However, the limited research on IL-25 and TSLP and the discrepant research on IL-33 mean that further studies are needed to determine the therapeutic potential of these cytokines in MS.
Cancer
Cancer is characterized by the development of abnormal cells that divide uncontrollably and can penetrate and destroy normal body tissues. These features of cancer cells are intimately controlled by the tumor microenvironment (TME), which participates in all stages of tumorigenesis from initiation to metastasis (125). Cytokines that mediate cell-cell communications, including the alarmins, are central to this role of the TME.
IL-33 levels are elevated in the serum and tumor tissues of patients with a wide range of cancers, including glioma (126), head and neck squamous cell carcinoma (127), gastric cancer (128), colorectal cancer (129), hepatocellular carcinoma (130), uterine leiomyoma (131), non-small cell lung cancer (132), and breast cancer (133). Additional studies on IL-33 have shown that this alarmin can both promote and suppress tumorigenesis. Both effects are mediated by the ability of IL-33 to shape innate and adaptive immunity, thereby converting TME to a pro-tumoral or anti-tumoral environment. Below, we will first discuss the studies on the pro-tumoral activities of IL-33.
Yang et al. (133) showed that the serum IL-33 levels in breast cancer patients correlate positively with their serum concentrations of molecules that are well known to associate with tumor-related angiogenesis (vascular endothelial growth factor), matrix remodeling (MMP-11), and growth and survival (platelet-derived growth factor-C). Similarly, Jovanovic et al. (134) showed that treating IL-33 on a murine breast cancer model accelerates tumor growth and metastasis and that this is mediated by increased intratumoral accumulation of myeloid-derived suppressor cells and Treg cells. Moreover, Liu et al. (129) reported that colorectal cancer cells express higher IL-33 and ST-2 levels when they are derived from patients with a poor tumor, nodes, metastasis stage, which signifies the extent to which the tumor has spread. In addition, Fang et al. (4) showed that when IL-33 transgenic mice are inoculated with murine colon cancer cells, they exhibit increased tumor growth, which is induced by macrophage recruitment to the TME and stimulating prostaglandin E2 production from recruited macrophages.
Contrary to the pro-tumor effects of IL-33, this cytokine can also have a tumor-suppressive function. The studies on the tumor-suppressor functions of IL-33 show that this suppression is due to IL-33-modulated activation of either cytotoxic immune cells, the pro-inflammatory TH9 cell subset, or ILC2s. With regard to the studies on IL-33-induced cytotoxicity, Gao et al. (135) showed that artificially upregulating IL-33 expression in cancer cells inhibits tumor growth by increasing CD8+ T and NK cell numbers in tumor tissues. Similarly, IL-33-transgenic mice display significantly increased tumor infiltration of CD8+ T and NK cells, and recombinant IL-33 treatment increases CD8+ T- and NK-cell cytotoxicity in vitro (136). Several studies also show that IL-33 can increase anti-tumor immune activity indirectly by increasing the ability of DCs to either cross-present leukemia or melanoma Ags to CD8+ T cells (137,138) or express OX40L, which induces TH9 cells that promote anti-tumor immune responses (139). Moreover, with regard to the studies showing that IL-33 fosters the anti-tumoral functions of ILC2s, Moral et al. (140) found that 1) human and mouse pancreatic ductal adenocarcinomas bear increased intratumoral ILC2 frequencies, 2) IL-33 deletion increases the growth of the tumors in vivo, and 3) IL-33 acts by activating ILC2s, which recruit CD103+ DCs, which in turn prime tumor-specific CD8+ T cells and recruit them into the tumor. In addition, Ikutani et al. (141) reported that ILC2s promote tumor recruitment of eosinophils, which have anti-tumoral activity. Notably, a recent study that used the B16.F10 melanoma model showed that the acidification of the TME by lactic acid produced by tumor cells facilitates cancer growth by impairing ILC2 survival and function. This mechanism explains why the expression of lactate dehydrogenase A (the enzyme that produces lactic acid) in human cutaneous melanoma samples correlates negatively with ILC2 marker expression (142).
In line with IL-33, IL-25 also has a Janus face in tumor immunity. Its pro-tumoral role is mainly mediated by inducing CD4+ T cells to differentiate into TH2 cells, which can promote tumor progression. Thus, Jiang et al. (143) showed first that IL-25 is produced in the TME of breast cancer by CD4+ T cells and F4/80+ macrophages. Subsequently, they found that IL-25 neutralization reduces these cell frequencies, TH2 responses and M2 polarization in the TME. These changes enhance tumor progression (143). Nakajima et al. (144) observed a similar relationship between IL-25, TH2 responses, and cancer progression: they found that the keratinocytes in cutaneous T-cell lymphoma (CTCL) skin lesions express high levels of IL-25 and that PBMCs in patients with an aggressive form of this cancer express IL-25 receptors and produce high levels of the type-2 cytokine IL-13.
In contrast, the tumor-suppressor functions of IL-25 are demonstrated by Ferretti et al., (145) who showed that human germinal center-derived non-Hodgkin B cell lymphomas express the IL-25 receptor, IL-17RB, and that treating model mice with these tumors with IL-25 inhibits tumor growth by suppressing pro-angiogenic molecules. Moreover, Furuta et al. (146) showed that IL-25 treatment of breast cancer cells can induce their apoptosis in vitro, and that this is mediated by IL-25 binding to IL-25 receptor. Interestingly, they found that the intracellular region of the IL-25 receptor bears a death domain-like segment that is recognized by death domain adaptor proteins, which subsequently activate apoptosis (146).
Like the other 2 alarmins, TSLP both promotes and suppresses tumors. Ragonnaud et al. (147) demonstrated that TSLP is secreted from breast cancer cell lines and supports pre-B cell proliferation in the bone marrow, ultimately leading to cancer metastasis. Xie et al. (148) reported that cervical cancer cells also secrete TSLP and that stimulates cancer cells’ in vitro growth in an autocrine manner. The same group also showed with in vitro experiments that cervical cancer-derived TSLP may induce the recruitment of eosinophils which produces anti-inflammatory cytokines, further promotes cervical cancer cell proliferation, and reduces their apoptosis (149). Moreover, Takahashi et al. (150) reported that TSLP is expressed by CTCL lesional skin TSLP enhances CTCL progression by directly stimulating cancer cell growth and by inducing a TH2-dominant TME that is hostile to TH1 anti-tumor immune responses. This was supported by a study on human pancreatic cancer cells, which found that the source of TSLP in the cancer lesions is cancer-associated fibroblasts; these cells were shown to activate TSLPR+ DCs, which then induce TH2 differentiation (151).
The studies showing that TSLP can also have anti-tumor effects include that by Demehri et al., (152) who observed that the keratinocyte-specific overexpression of TSLP in K14-TSLP-transgenic mice leads to TH2 cell-mediated inflammation that blocks the spontaneous development of metastatic breast cancer in MMVT-pyMT mice. This suggests that the TSLP can prevent the early stages of tumorigenesis (152). Moreover, an analysis of NCBI GEO datasets by Yue et al. (153) showed that human colon adenomas express lower levels of TSLP than normal mucosa. This was confirmed by analysis of 40 additional colon adenomas, which also showed that the clinical severity of the cases correlated with lower TSLP expression. They then reported that TSLP treatment induces primary human colonic cancer cells to undergo caspase-8-initiated apoptosis in vitro and that peritumoral injection of TSLP suppresses colon cancer growth in vivo (153).
Given these mixed pro- and anti-tumoral roles of all 3 epithelial cell-derived cytokines, it remains unclear whether targeting these molecules could have therapeutic value. Nonetheless, the research in this area has greatly extended our view of alarmins: they are no longer seen as mere inducers of an immune response, rather, it is clear that they can modulate cancer cell growth both directly via receptor ligation and indirectly by shaping the immune status in the TME. Therefore, further research that untangles these roles is warranted.
DEVELOPMENT OF BIOLOGICS AND ON-GOING CLINICAL TRIALS TARGETING EPITHELIUM-DERIVED CYTOKINES
Although the roles of epithelium-derived cytokines in some diseases remain controversial, many mouse studies and clinical findings in allergic diseases have confirmed their therapeutic possibilities. In particular, these studies have shown that the alarmins can induce more extensive and potent allergic responses than downstream mediators such as IgE or type-2 cytokines. This suggests that targeting the alarmins may result in better immunomodulatory effects than the existing therapeutics. This has led to multiple clinical trials on alarmins. Most have been or are being conducted on allergic diseases due to the well-established roles of IL-33, IL-25, and TSLP in these diseases. The trial drugs are Astegolimab (154,155,156,157,158,159), Etokimab (160,161,162,163), GSK3772847 (164,165), Itepekimab (166,167,168,169,170,171,172,173), and MEDI3506 (174,175,176,177), which target the IL-33/ST-2 pathway (Table 1), and Tezepelumab (178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194) and CSJ117 (195,196,197,198), which target the TSLP pathway (Table 2). Although blocking the IL-25 pathway is also a possible target, there are no clinical trials on agents against IL-25 at present. While biologics that target IL-33 and TSLP have not yet received FDA approval, some have promising results, as detailed below, and are now in phase-3 clinical trials.
Table 1. Clinical trials that target IL-33/ST-2.
| Study title | Reference | Identifier | Stage* | Drug | Disease | Status |
|---|---|---|---|---|---|---|
| A first-in-human, double blind, single dose study in healthy subjects and subjects with mild atopic asthma | (154) | NCT01928368 | I | Astegolimab | Asthma | Completed |
| A study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of AMG 282 in healthy subjects and subjects with chronic rhinosinusitis with nasal polyps | (155) | NCT02170337 | I | Astegolimab | CRS with NP | Completed |
| A study to assess the efficacy and safety of MSTT1041A in participants with uncontrolled severe asthma | (156) | NCT02918019 | II | Astegolimab | Asthma | Completed |
| A study to assess the efficacy and safety of MSTT1041A in participants with moderate to severe atopic dermatitis | (157) | NCT03747575 | II | Astegolimab | AD | Completed |
| Anti-ST2 (MSTT1041A) in COPD (COPD-ST2OP) | (158) | NCT03615040 | II | Astegolimab | COPD | Completed |
| A study to evaluate the efficacy and safety of astegolimab in participants with chronic obstructive pulmonary disease | (159) | NCT05037929 | II | Astegolimab | COPD | Recruiting |
| A study investigating the efficacy, safety, and PK profile of ANB020 administered to adult subjects with moderate-to-severe AD (ATLAS) | (160) | NCT03533751 | II | Etokimab | AD | Recruiting |
| Proof of concept study to investigate ANB020 activity in adult patients with severe eosinophilic asthma | (161) | NCT03469934 | II | Etokimab | Asthma | Completed |
| Etokimab in adult patients with chronic rhinosinusitis with nasal polyps (CRSwNP) | (162) | NCT03614923 | II | Etokimab | CRS with NP | Completed |
| Placebo-controlled study to investigate ANB020 activity in adult patients with peanut allergy | (163) | NCT02920021 | II | Etokimab | Peanut allergy | Completed |
| A study to evaluate the safety and tolerability, pharmacokinetics (PK) and pharmacodynamics (PD) of Melrilimab (GSK3772847) in healthy participants | (164) | NCT04366349 | I | GSK3772847 | Healthy/asthma | Completed |
| Efficacy and safety study of GSK3772847 in subjects with moderately severe asthma | (165) | NCT03207243 | II | GSK3772847 | Asthma | Completed |
| Study of REGN3500 and dupilumab in patients with asthma | (166) | NCT03112577 | I | Itepekimab | Asthma | Completed |
| Study of safety, tolerability, and pharmacokinetics of multiple ascending doses of REGN3500 in adults with moderate asthma | (167) | NCT02999711 | I | Itepekimab | Asthma | Completed |
| Evaluation of SAR440340 and as combination therapy with dupilumab in moderate-to-severe asthma participants | (168) | NCT03387852 | II | Itepekimab | Asthma | Completed |
| Efficacy, safety, and pharmacokinetic profiles of REGN3500 administered to adult patients with moderate-to-severe atopic dermatitis | (169) | NCT03738423 | II | Itepekimab | AD | Completed |
| Efficacy and safety of REGN3500 monotherapy and combination of REGN3500 plus dupilumab in adult patients with moderate-to-severe atopic dermatitis | (170) | NCT03736967 | II | Itepekimab | AD | Terminated (Lack of efficacy) |
| Proof-of-concept study to assess the efficacy, safety and tolerability of SAR440340 (anti-IL-33 mAb) in patients with moderate-to-severe chronic obstructive pulmonary disease (COPD) | (171) | NCT03546907 | II | Itepekimab | COPD | Completed |
| Study to assess the efficacy, safety, and tolerability of SAR440340/REGN3500/Itepekimab in chronic obstructive pulmonary disease (COPD) (AERIFY-1,2) | (172,173) | NCT04701983 | III | Itepekimab | COPD | Recruiting |
| NCT04751487 | ||||||
| Safety and tolerability of MEDI3506 in healthy participants, in participants with COPD and healthy Japanese participants | (174) | NCT03096795 | I | MEDI3506 | Healthy/COPD | Completed |
| Study to assess the efficacy and safety of MEDI3506 in adults with uncontrolled moderate-to-severe asthma (FRONTIER-3) | (175) | NCT04570657 | II | MEDI3506 | Asthma | Recruiting |
| Efficacy and safety of MEDI3506 in adult subjects with atopic dermatitis | (176) | NCT04212169 | II | MEDI3506 | AD | Recruiting |
| A phase II, randomized, double-blind, placebo-controlled study to assess MEDI3506 in participants with COPD and chronic bronchitis (FRONTIER-4) | (177) | NCT04631016 | II | MEDI3506 | COPD/chronic bronchitis | Recruiting |
NP, nasal polyp.
*Stage I, II, and III represent phase 1, phase 2, and phase 3 respectively.
Table 2. Clinical trials that target TSLP.
| Study title | Reference | Identifier | Stage* | Drug | Disease | Status |
|---|---|---|---|---|---|---|
| A study to evaluate the pharmacokinetics of MEDI9929 (AMG 157) in adolescents with mild to moderate asthma | (178) | NCT02512900 | I | Tezepelumab | Asthma | Completed |
| Study to evaluate the pharmacokinetics of tezepelumab in children with asthma (TRAILHEAD) | (179) | NCT04673630 | I | Tezepelumab | Asthma | Recruiting |
| Double-blind, multiple dose study in subjects with mild atopic asthma | (180) | NCT01405963 | I | Tezepelumab | Asthma | Completed |
| Safety study of AMG 157 in healthy subjects and subjects with atopic dermatitis | (181) | NCT00757042 | I | Tezepelumab | Healthy/AD | Completed |
| Study to evaluate the efficacy and safety of MEDI9929 (AMG 157) in adult subjects with inadequately controlled, severe asthma | (182) | NCT02054130 | II | Tezepelumab | Asthma | Completed |
| Effects of anti-TSLP in patients with asthma (UPSTREAM) | (183) | NCT02698501 | II | Tezepelumab | Asthma | Completed |
| Study to evaluate tezepelumab on airway inflammation in adults with uncontrolled asthma (CASCADE) (CASCADE) | (184) | NCT03688074 | II | Tezepelumab | Asthma | Completed |
| Phase 2a study to evaluate the efficacy and safety of MEDI9929 in adults with atopic dermatitis (ALLEVIAD) | (185) | NCT02525094 | II | Tezepelumab | AD | Completed |
| Anti-TSLP (AMG 157) plus antigen-specific immunotherapy for induction of tolerance in individuals with cat allergy | (186) | NCT02237196 | I/II | Tezepelumab | Cat allergy/cat hypersensitivity | Completed |
| Study to evaluate tezepelumab in adults with chronic spontaneous urticaria (INCEPTION) | (187) | NCT04833855 | II | Tezepelumab | Chronic spontaneous urticaria | Recruiting |
| Tezepelumab COPD exacerbation study (COURSE) | (188) | NCT04039113 | II | Tezepelumab | COPD | Recruiting |
| Study to evaluate tezepelumab in adults & adolescents with severe uncontrolled asthma (NAVIGATOR) | (189) | NCT03347279 | III | Tezepelumab | Asthma | Completed |
| Study to evaluate the efficacy and safety of tezepelumab in reducing oral corticosteroid use in adults with oral corticosteroid dependent asthma (SOURCE) | (190) | NCT03406078 | III | Tezepelumab | Asthma | Completed |
| Long-term safety of tezepelumab in japanese subjects with inadequately controlled severe asthma (NOZOMI) | (191) | NCT04048343 | III | Tezepelumab | Asthma | Completed |
| Extension study to evaluate the safety and tolerability of tezepelumab in adults and adolescents with severe, uncontrolled asthma (DESTINATION) | (192) | NCT03706079 | III | Tezepelumab | Asthma | On going (active) |
| Study to evaluate tezepelumab in adults with severe uncontrolled asthma (DIRECTION) | (193) | NCT03927157 | III | Tezepelumab | Asthma | Recruiting |
| Efficacy and safety of tezepelumab in participants with severe chronic rhinosinusitis with nasal polyposis (WAYPOINT) | (194) | NCT04851964 | III | Tezepelumab | CRS with NP | Recruiting |
| A bronchoprovocation study to assess the safety, tolerability, pharmacokinetics and pharmacodynamics of CSJ117 in adult subjects with mild atopic asthma | (195) | NCT03138811 | I | CSJ117 | Asthma | Completed |
| Study of efficacy and safety of CSJ117 in patients with severe uncontrolled asthma | (196) | NCT04410523 | II | CSJ117 | Asthma | Recruiting |
| Study of safety of CSJ117 in participants with moderate to severe uncontrolled asthma | (197) | NCT04946318 | II | CSJ117 | Asthma | Recruiting |
| Study of effect of CSJ117 on symptoms, pharmacodynamics and safety in patients with COPD | (198) | NCT04882124 | II | CSJ117 | COPD | Recruiting |
NP, nasal polyp.
*Stage I, II, and III represent phase 1, phase 2, and phase 3 respectively.
IL-33/ST-2 signaling pathway blockade
Astegolimab (MSTT1041A/RG 6149) is a human IgG2 mAb that blocks IL-33 signaling by targeting its receptor ST-2. Of the 6 trials on Astegolimab, 2 are in phase-1 studies for healthy subjects, patients with mild asthmatics (154), or chronic rhinosinusitis (CRS) with nasal polyps (155). The remainders are phase-2 studies on patients with AD (157), chronic obstructive pulmonary disease (COPD) (158,159), or asthma (156). The latter study has shown that Astegolimab significantly reduces the asthma exacerbation rates in a broad range of adult patients with severe asthma, including those with low eosinophil counts and those with inadequately controlled severe asthma (156,199).
Etokimab (ANB020) is a human IgG1 mAb that neutralizes IL-33. It is manufactured by AnaptysBio and has been subjected to 4 completed phase-2 trials on AD (160), asthma (161), CRS with nasal polyps (162), and peanut allergy (163). The results are still pending. An interim analysis of the phase-2 study on asthma showed that Etokimab improved the FEV1 of adults with severe eosinophilic asthma compared to placebo-treated patients (161).
GSK3772847, like Astegolimab, also blocks the IL-33 receptor. It is currently being tested for safety in a phase-1 trial in healthy participants and a phase-2 trial in patients with asthma (164,165). Itepekimab (SAR440340/REGN3500) is a human IgG4 mAb against IL-33. It is the subject of 2 phase-1 trials in asthma patients (166,167), 4 phase-2 trials in asthma (168), AD (169,170), and COPD patients (171), and 2 phase-3 trials in COPD patients (172,173). The results of the phase-2 trial in COPD patients have been reported: although Itepekimab failed to meet the primary endpoint in the overall population, it did reduce the exacerbation rate in former smokers with COPD. These patients also demonstrated improved lung function (171,200). MEDI3506 is a newly developed IL-33-targeting mAb. It is currently being studied in 1 phase-1 trial in healthy subjects and patients with COPD (174) and 3 phase-2 trials in asthma (175), AD (176), and COPD patients (177).
TSLP signaling pathway blockade
Tezepelumab (MEDI9929/AMG 157) is a human IgG2 mAb that binds TSLP, thereby blocking the TSLP/TSLPR signaling pathway. It is the subject of 17 trials: 5 phase-1 trials in asthma (178,179,180), AD patients (181), or cat allergy (186); 7 phase-2 trials in patients with asthma (182,183,184), AD (185), cat hypersensitivity (186), chronic spontaneous urticaria (187), or COPD (188); and 6 phase-3 trials in asthma patients (189,190,191,192,193) or patients with CRS and nasal polyps (194). The phase-2 PATHWAY study showed that Tezepelumab markedly reduced the blood eosinophil counts and serum IL-5 and IL-13 levels in patients with severe and uncontrolled asthma. This suggests that it decreased asthma severity (182,201). In addition, the phase-3 NAVIGATOR trial suggested that Tezeplumab effectively reduced asthma exacerbations and improved lung function in adult patients with severe asthma (189,202). Moreover, the phase-2 ALLEVIAD trial in adults with AD showed that while Tezepelumab did not achieve significant levels in the primary endpoint, the Tezepelumab-treated patients were significantly more likely to achieve >50% improvement in the eczema site severity index (185,203).
CSJ117 is a potent neutralizing Ab fragment that targets human TSLP. It was developed as an inhaled dry powder formulation for targeted delivery to the lungs. It is the subject of 1 phase-1 trial in asthma patients (195) and 3 phase-2 trials in asthma (196,197) or COPD patients (198). An abstract recently reported that CSJ117 attenuated allergen-induced bronchoconstriction and sputum eosinophil counts in patients with mild atopic asthma (204).
CONCLUSIONS
This review shows that the epithelium-derived cytokines IL-33, IL-25, and TSLP play roles in a spectrum of diseases that range broadly from allergic disorders to tumors and autoimmune diseases. The roles of these alarmins not only include their well-known ability to initiate type-2 responses, but they also include participation in type-1 and type-17 immune responses. They have a direct impact against ILCs as well as many immune cells (DC, T cells, Treg cells, brain and liver macrophages, pre-B cells, eosinophils, mast cells) and non-immune cells (keratinocytes, oligodendrocytes, and tumors). These diverse targets and functions suggest that controlling alarmin signaling may be a novel therapeutic target. Indeed, numerous clinical trials have been or are being conducted on these alarmins. All focus on IL-33 or TSLP, most are on allergic diseases, and most are still ongoing, but the existing data are promising and suggest that biologics that target the alarmins can safely and effectively treat allergy. Moreover, although the research on the roles of alarmins in non-allergic diseases such as cancer and autoimmune diseases is still in its infancy, the preliminary pre-clinical and clinical data suggest that anti-alarmin biologics could be useful in these diseases as well.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (2019R1A2C2087574 and SRC 2017R1A5A1014560).
Abbreviations
- AD
atopic dermatitis
- AHR
airway hyperresponsiveness
- CNS
central nerve system
- COPD
chronic obstructive pulmonary disease
- CRS
chronic rhinosinusitis
- CTCL
cutaneous T-cell lymphoma
- DC
dendritic cell
- EAE
experimental autoimmune encephalomyelitis
- IL-17RA
IL-17 receptor A
- IL-17RB
IL-17 receptor B
- IL-25R
IL-25 receptor
- ILC
innate lymphoid cell
- IMQ
imiquimod
- lfTSLP
long-form TSLP
- MMP
metalloproteinase
- MS
multiple sclerosis
- sfTSLP
short-form TSLP
- TH2
type 2 helper T
- TME
tumor microenvironment
- TSLPR
TSLR receptor
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Kim HY.
- Investigation: Ham J, Shin JW, Ko BC.
- Writing - original draft: Ham J, Shin JW, Kim HY.
- Writing - review & editing: Ham J, Shin JW, Kim HY.
References
- 1.Larsen SB, Cowley CJ, Fuchs E. Epithelial cells: liaisons of immunity. Curr Opin Immunol. 2020;62:45–53. doi: 10.1016/j.coi.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hammad H, Lambrecht BN. Barrier epithelial cells and the control of type 2 immunity. Immunity. 2015;43:29–40. doi: 10.1016/j.immuni.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17:359–365. doi: 10.1016/j.coi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Fang M, Li Y, Huang K, Qi S, Zhang J, Zgodzinski W, Majewski M, Wallner G, Gozdz S, Macek P, et al. IL33 promotes colon cancer cell stemness via JNK activation and macrophage recruitment. Cancer Res. 2017;77:2735–2745. doi: 10.1158/0008-5472.CAN-16-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gamez C, Metcalfe J, Prescott SL, Palmer DJ. Lower cord blood IL-17 and IL-25, but not other epithelial cell-derived cytokines are associated with atopic dermatitis in infancy. Int Arch Allergy Immunol. 2021;182:474–478. doi: 10.1159/000512919. [DOI] [PubMed] [Google Scholar]
- 6.Kleinschek MA, Owyang AM, Joyce-Shaikh B, Langrish CL, Chen Y, Gorman DM, Blumenschein WM, McClanahan T, Brombacher F, Hurst SD, et al. IL-25 regulates Th17 function in autoimmune inflammation. J Exp Med. 2007;204:161–170. doi: 10.1084/jem.20061738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li M, Li Y, Liu X, Gao X, Wang Y. IL-33 blockade suppresses the development of experimental autoimmune encephalomyelitis in C57BL/6 mice. J Neuroimmunol. 2012;247:25–31. doi: 10.1016/j.jneuroim.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Liu B, Tai Y, Achanta S, Kaelberer MM, Caceres AI, Shao X, Fang J, Jordt SE. IL-33/ST2 signaling excites sensory neurons and mediates itch response in a mouse model of poison ivy contact allergy. Proc Natl Acad Sci U S A. 2016;113:E7572–E7579. doi: 10.1073/pnas.1606608113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papp KA, Leonardi C, Menter A, Ortonne JP, Krueger JG, Kricorian G, Aras G, Li J, Russell CB, Thompson EH, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181–1189. doi: 10.1056/NEJMoa1109017. [DOI] [PubMed] [Google Scholar]
- 10.Zeng F, Chen H, Chen L, Mao J, Cai S, Xiao Y, Li J, Shi J, Li B, Xu Y, et al. An autocrine circuit of IL-33 in keratinocytes is involved in the progression of psoriasis. J Invest Dermatol. 2021;141:596–606.e7. doi: 10.1016/j.jid.2020.07.027. [DOI] [PubMed] [Google Scholar]
- 11.Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008;7:827–840. doi: 10.1038/nrd2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS One. 2008;3:e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cayrol C, Duval A, Schmitt P, Roga S, Camus M, Stella A, Burlet-Schiltz O, Gonzalez-de-Peredo A, Girard JP. Environmental allergens induce allergic inflammation through proteolytic maturation of IL-33. Nat Immunol. 2018;19:375–385. doi: 10.1038/s41590-018-0067-5. [DOI] [PubMed] [Google Scholar]
- 14.Lefrançais E, Roga S, Gautier V, Gonzalez-de-Peredo A, Monsarrat B, Girard JP, Cayrol C. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc Natl Acad Sci U S A. 2012;109:1673–1678. doi: 10.1073/pnas.1115884109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy A, Ganesh G, Sippola H, Bolin S, Sawesi O, Dagälv A, Schlenner SM, Feyerabend T, Rodewald HR, Kjellén L, et al. Mast cell chymase degrades the alarmins heat shock protein 70, biglycan, HMGB1, and interleukin-33 (IL-33) and limits danger-induced inflammation. J Biol Chem. 2014;289:237–250. doi: 10.1074/jbc.M112.435156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brusilovsky M, Rochman M, Rochman Y, Caldwell JM, Mack LE, Felton JM, Habel JE, Porollo A, Pasare C, Rothenberg ME. Environmental allergens trigger type 2 inflammation through ripoptosome activation. Nat Immunol. 2021;22:1316–1326. doi: 10.1038/s41590-021-01011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cayrol C, Girard JP. Interleukin-33 (IL-33): a nuclear cytokine from the IL-1 family. Immunol Rev. 2018;281:154–168. doi: 10.1111/imr.12619. [DOI] [PubMed] [Google Scholar]
- 18.Cherry WB, Yoon J, Bartemes KR, Iijima K, Kita H. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J Allergy Clin Immunol. 2008;121:1484–1490. doi: 10.1016/j.jaci.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin NT, Martin MU. Interleukin 33 is a guardian of barriers and a local alarmin. Nat Immunol. 2016;17:122–131. doi: 10.1038/ni.3370. [DOI] [PubMed] [Google Scholar]
- 20.Tran VG, Cho HR, Kwon B. IL-33 priming enhances peritoneal macrophage activity in response to candida albicans. Immune Netw. 2014;14:201–206. doi: 10.4110/in.2014.14.4.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lefrançais E, Duval A, Mirey E, Roga S, Espinosa E, Cayrol C, Girard JP. Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells. Proc Natl Acad Sci U S A. 2014;111:15502–15507. doi: 10.1073/pnas.1410700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Travers J, Rochman M, Miracle CE, Habel JE, Brusilovsky M, Caldwell JM, Rymer JK, Rothenberg ME. Chromatin regulates IL-33 release and extracellular cytokine activity. Nat Commun. 2018;9:3244. doi: 10.1038/s41467-018-05485-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGeachy MJ, Cua DJ, Gaffen SL. The IL-17 family of cytokines in health and disease. Immunity. 2019;50:892–906. doi: 10.1016/j.immuni.2019.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borowczyk J, Shutova M, Brembilla NC, Boehncke WH. IL-25 (IL-17E) in epithelial immunology and pathophysiology. J Allergy Clin Immunol. 2021;148:40–52. doi: 10.1016/j.jaci.2020.12.628. [DOI] [PubMed] [Google Scholar]
- 25.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo . Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 26.Kouzaki H, Tojima I, Kita H, Shimizu T. Transcription of interleukin-25 and extracellular release of the protein is regulated by allergen proteases in airway epithelial cells. Am J Respir Cell Mol Biol. 2013;49:741–750. doi: 10.1165/rcmb.2012-0304OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Min HK, Won JY, Kim BM, Lee KA, Lee SJ, Lee SH, Kim HR, Kim KW. Interleukin (IL)-25 suppresses IL-22-induced osteoclastogenesis in rheumatoid arthritis via STAT3 and p38 MAPK/IκBα pathway. Arthritis Res Ther. 2020;22:222. doi: 10.1186/s13075-020-02315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sonobe Y, Takeuchi H, Kataoka K, Li H, Jin S, Mimuro M, Hashizume Y, Sano Y, Kanda T, Mizuno T, et al. Interleukin-25 expressed by brain capillary endothelial cells maintains blood-brain barrier function in a protein kinase Cepsilon-dependent manner. J Biol Chem. 2009;284:31834–31842. doi: 10.1074/jbc.M109.025940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Owyang AM, Zaph C, Wilson EH, Guild KJ, McClanahan T, Miller HR, Cua DJ, Goldschmidt M, Hunter CA, Kastelein RA, et al. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J Exp Med. 2006;203:843–849. doi: 10.1084/jem.20051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamachi T, Maezawa Y, Ikeda K, Kagami S, Hatano M, Seto Y, Suto A, Suzuki K, Watanabe N, Saito Y. IL-25 enhances allergic airway inflammation by amplifying a TH2 cell-dependent pathway in mice. J Allergy Clin Immunol. 2006;118:606–614. doi: 10.1016/j.jaci.2006.04.051. [DOI] [PubMed] [Google Scholar]
- 31.Goswami S, Angkasekwinai P, Shan M, Greenlee KJ, Barranco WT, Polikepahad S, Seryshev A, Song LZ, Redding D, Singh B, et al. Divergent functions for airway epithelial matrix metalloproteinase 7 and retinoic acid in experimental asthma. Nat Immunol. 2009;10:496–503. doi: 10.1038/ni.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park LS, Martin U, Garka K, Gliniak B, Di Santo JP, Muller W, Largaespada DA, Copeland NG, Jenkins NA, Farr AG, et al. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: formation of a functional heteromeric complex requires interleukin 7 receptor. J Exp Med. 2000;192:659–670. doi: 10.1084/jem.192.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quentmeier H, Drexler HG, Fleckenstein D, Zaborski M, Armstrong A, Sims JE, Lyman SD. Cloning of human thymic stromal lymphopoietin (TSLP) and signaling mechanisms leading to proliferation. Leukemia. 2001;15:1286–1292. doi: 10.1038/sj.leu.2402175. [DOI] [PubMed] [Google Scholar]
- 34.Rochman Y, Kashyap M, Robinson GW, Sakamoto K, Gomez-Rodriguez J, Wagner KU, Leonard WJ. Thymic stromal lymphopoietin-mediated STAT5 phosphorylation via kinases JAK1 and JAK2 reveals a key difference from IL-7-induced signaling. Proc Natl Acad Sci U S A. 2010;107:19455–19460. doi: 10.1073/pnas.1008271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong H, Hu Y, Liu L, Zou M, Huang C, Luo L, Yu C, Wan X, Zhao H, Chen J, et al. Distinct roles of short and long thymic stromal lymphopoietin isoforms in house dust mite-induced asthmatic airway epithelial barrier disruption. Sci Rep. 2016;6:39559. doi: 10.1038/srep39559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fornasa G, Tsilingiri K, Caprioli F, Botti F, Mapelli M, Meller S, Kislat A, Homey B, Di Sabatino A, Sonzogni A, et al. Dichotomy of short and long thymic stromal lymphopoietin isoforms in inflammatory disorders of the bowel and skin. J Allergy Clin Immunol. 2015;136:413–422. doi: 10.1016/j.jaci.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varricchi G, Pecoraro A, Marone G, Criscuolo G, Spadaro G, Genovese A, Marone G. Thymic stromal lymphopoietin isoforms, inflammatory disorders, and cancer. Front Immunol. 2018;9:1595. doi: 10.3389/fimmu.2018.01595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 39.Ying S, O’Connor B, Ratoff J, Meng Q, Fang C, Cousins D, Zhang G, Gu S, Gao Z, Shamji B, et al. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol. 2008;181:2790–2798. doi: 10.4049/jimmunol.181.4.2790. [DOI] [PubMed] [Google Scholar]
- 40.Nagarkar DR, Poposki JA, Tan BK, Comeau MR, Peters AT, Hulse KE, Suh LA, Norton J, Harris KE, Grammer LC, et al. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013;132:593–600.e12. doi: 10.1016/j.jaci.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poposki JA, Klingler AI, Stevens WW, Peters AT, Hulse KE, Grammer LC, Schleimer RP, Welch KC, Smith SS, Sidle DM, et al. Proprotein convertases generate a highly functional heterodimeric form of thymic stromal lymphopoietin in humans. J Allergy Clin Immunol. 2017;139:1559–1567.e8. doi: 10.1016/j.jaci.2016.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Busse WW, Lemanske RF., Jr Asthma. N Engl J Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 43.Allakhverdi Z, Comeau MR, Jessup HK, Yoon BR, Brewer A, Chartier S, Paquette N, Ziegler SF, Sarfati M, Delespesse G. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204:253–258. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kato A, Favoreto S, Jr, Avila PC, Schleimer RP. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol. 2007;179:1080–1087. doi: 10.4049/jimmunol.179.2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gudbjartsson DF, Bjornsdottir US, Halapi E, Helgadottir A, Sulem P, Jonsdottir GM, Thorleifsson G, Helgadottir H, Steinthorsdottir V, Stefansson H, et al. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat Genet. 2009;41:342–347. doi: 10.1038/ng.323. [DOI] [PubMed] [Google Scholar]
- 46.He JQ, Hallstrand TS, Knight D, Chan-Yeung M, Sandford A, Tripp B, Zamar D, Bossé Y, Kozyrskyj AL, James A, et al. A thymic stromal lymphopoietin gene variant is associated with asthma and airway hyperresponsiveness. J Allergy Clin Immunol. 2009;124:222–229. doi: 10.1016/j.jaci.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 47.Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, von Mutius E, Farrall M, Lathrop M, Cookson WOCM, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363:1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corrigan CJ, Wang W, Meng Q, Fang C, Eid G, Caballero MR, Lv Z, An Y, Wang YH, Liu YJ, et al. Allergen-induced expression of IL-25 and IL-25 receptor in atopic asthmatic airways and late-phase cutaneous responses. J Allergy Clin Immunol. 2011;128:116–124. doi: 10.1016/j.jaci.2011.03.043. [DOI] [PubMed] [Google Scholar]
- 49.Cheng D, Xue Z, Yi L, Shi H, Zhang K, Huo X, Bonser LR, Zhao J, Xu Y, Erle DJ, et al. Epithelial interleukin-25 is a key mediator in Th2-high, corticosteroid-responsive asthma. Am J Respir Crit Care Med. 2014;190:639–648. doi: 10.1164/rccm.201403-0505OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Y, Wang W, Lv Z, Li Y, Chen Y, Huang K, Corrigan CJ, Ying S. Elevated expression of IL-33 and TSLP in the airways of human asthmatics in vivo: a potential biomarker of severe refractory disease. J Immunol. 2018;200:2253–2262. doi: 10.4049/jimmunol.1701455. [DOI] [PubMed] [Google Scholar]
- 51.Préfontaine D, Nadigel J, Chouiali F, Audusseau S, Semlali A, Chakir J, Martin JG, Hamid Q. Increased IL-33 expression by epithelial cells in bronchial asthma. J Allergy Clin Immunol. 2010;125:752–754. doi: 10.1016/j.jaci.2009.12.935. [DOI] [PubMed] [Google Scholar]
- 52.Ballantyne SJ, Barlow JL, Jolin HE, Nath P, Williams AS, Chung KF, Sturton G, Wong SH, McKenzie AN. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J Allergy Clin Immunol. 2007;120:1324–1331. doi: 10.1016/j.jaci.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 53.Kabata H, Flamar AL, Mahlakõiv T, Moriyama S, Rodewald HR, Ziegler SF, Artis D. Targeted deletion of the TSLP receptor reveals cellular mechanisms that promote type 2 airway inflammation. Mucosal Immunol. 2020;13:626–636. doi: 10.1038/s41385-020-0266-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu X, Li M, Wu Y, Zhou Y, Zeng L, Huang T. Anti-IL-33 antibody treatment inhibits airway inflammation in a murine model of allergic asthma. Biochem Biophys Res Commun. 2009;386:181–185. doi: 10.1016/j.bbrc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 55.Willebrand R, Voehringer D. IL-33-induced cytokine secretion and survival of mouse eosinophils is promoted by autocrine GM-CSF. PLoS One. 2016;11:e0163751. doi: 10.1371/journal.pone.0163751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salter BMA, Smith SG, Mukherjee M, Plante S, Krisna S, Nusca G, Oliveria JP, Irshad A, Gauvreau GM, Chakir J, et al. Human bronchial epithelial cell-derived factors from severe asthmatic subjects stimulate eosinophil differentiation. Am J Respir Cell Mol Biol. 2018;58:99–106. doi: 10.1165/rcmb.2016-0262OC. [DOI] [PubMed] [Google Scholar]
- 57.Iikura M, Suto H, Kajiwara N, Oboki K, Ohno T, Okayama Y, Saito H, Galli SJ, Nakae S. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Lab Invest. 2007;87:971–978. doi: 10.1038/labinvest.3700663. [DOI] [PubMed] [Google Scholar]
- 58.Saluja R, Ketelaar ME, Hawro T, Church MK, Maurer M, Nawijn MC. The role of the IL-33/IL-1RL1 axis in mast cell and basophil activation in allergic disorders. Mol Immunol. 2015;63:80–85. doi: 10.1016/j.molimm.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 59.Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE, Dekruyff RH, Umetsu DT. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12:631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL-33-responsive lineage- CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol. 2012;188:1503–1513. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woo Y, Jeong D, Chung DH, Kim HY. The roles of innate lymphoid cells in the development of asthma. Immune Netw. 2014;14:171–181. doi: 10.4110/in.2014.14.4.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim HY, Chang YJ, Subramanian S, Lee HH, Albacker LA, Matangkasombut P, Savage PB, McKenzie AN, Smith DE, Rottman JB, et al. Innate lymphoid cells responding to IL-33 mediate airway hyperreactivity independently of adaptive immunity. J Allergy Clin Immunol. 2012;129:216–227.e1-6. doi: 10.1016/j.jaci.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang C, Liu Q, Chen F, Xu W, Zhang C, Xiao W. IL-25 promotes Th2 immunity responses in asthmatic mice via nuocytes activation. PLoS One. 2016;11:e0162393. doi: 10.1371/journal.pone.0162393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Toki S, Goleniewska K, Zhang J, Zhou W, Newcomb DC, Zhou B, Kita H, Boyd KL, Peebles RS., Jr TSLP and IL-33 reciprocally promote each other’s lung protein expression and ILC2 receptor expression to enhance innate type-2 airway inflammation. Allergy. 2020;75:1606–1617. doi: 10.1111/all.14196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu S, Verma M, Michalec L, Liu W, Sripada A, Rollins D, Good J, Ito Y, Chu H, Gorska MM, et al. Steroid resistance of airway type 2 innate lymphoid cells from patients with severe asthma: the role of thymic stromal lymphopoietin. J Allergy Clin Immunol. 2018;141:257–268.e6. doi: 10.1016/j.jaci.2017.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kabata H, Moro K, Fukunaga K, Suzuki Y, Miyata J, Masaki K, Betsuyaku T, Koyasu S, Asano K. Thymic stromal lymphopoietin induces corticosteroid resistance in natural helper cells during airway inflammation. Nat Commun. 2013;4:2675. doi: 10.1038/ncomms3675. [DOI] [PubMed] [Google Scholar]
- 67.Van Dyken SJ, Nussbaum JC, Lee J, Molofsky AB, Liang HE, Pollack JL, Gate RE, Haliburton GE, Ye CJ, Marson A, et al. A tissue checkpoint regulates type 2 immunity. Nat Immunol. 2016;17:1381–1387. doi: 10.1038/ni.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bredo G, Storie J, Shrestha Palikhe N, Davidson C, Adams A, Vliagoftis H, Cameron L. Interleukin-25 initiates Th2 differentiation of human CD4(+) T cells and influences expression of its own receptor. Immun Inflamm Dis. 2015;3:455–468. doi: 10.1002/iid3.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jang S, Morris S, Lukacs NW. TSLP promotes induction of Th2 differentiation but is not necessary during established allergen-induced pulmonary disease. PLoS One. 2013;8:e56433. doi: 10.1371/journal.pone.0056433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smithgall MD, Comeau MR, Yoon BR, Kaufman D, Armitage R, Smith DE. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol. 2008;20:1019–1030. doi: 10.1093/intimm/dxn060. [DOI] [PubMed] [Google Scholar]
- 71.Burzyn D, Benoist C, Mathis D. Regulatory T cells in nonlymphoid tissues. Nat Immunol. 2013;14:1007–1013. doi: 10.1038/ni.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sakaguchi S, Vignali DA, Rudensky AY, Niec RE, Waldmann H. The plasticity and stability of regulatory T cells. Nat Rev Immunol. 2013;13:461–467. doi: 10.1038/nri3464. [DOI] [PubMed] [Google Scholar]
- 73.Chen CC, Kobayashi T, Iijima K, Hsu FC, Kita H. IL-33 dysregulates regulatory T cells and impairs established immunologic tolerance in the lungs. J Allergy Clin Immunol. 2017;140:1351–1363.e7. doi: 10.1016/j.jaci.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ständer S. Atopic dermatitis. N Engl J Med. 2021;384:1136–1143. doi: 10.1056/NEJMra2023911. [DOI] [PubMed] [Google Scholar]
- 75.Eichenfield LF, Hanifin JM, Beck LA, Lemanske RF, Jr, Sampson HA, Weiss ST, Leung DY. Atopic dermatitis and asthma: parallels in the evolution of treatment. Pediatrics. 2003;111:608–616. doi: 10.1542/peds.111.3.608. [DOI] [PubMed] [Google Scholar]
- 76.Jariwala SP, Abrams E, Benson A, Fodeman J, Zheng T. The role of thymic stromal lymphopoietin in the immunopathogenesis of atopic dermatitis. Clin Exp Allergy. 2011;41:1515–1520. doi: 10.1111/j.1365-2222.2011.03797.x. [DOI] [PubMed] [Google Scholar]
- 77.Nakamura N, Tamagawa-Mineoka R, Yasuike R, Masuda K, Matsunaka H, Murakami Y, Yokosawa E, Katoh N. Stratum corneum interleukin-33 expressions correlate with the degree of lichenification and pruritus in atopic dermatitis lesions. Clin Immunol. 2019;201:1–3. doi: 10.1016/j.clim.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 78.Savinko T, Matikainen S, Saarialho-Kere U, Lehto M, Wang G, Lehtimäki S, Karisola P, Reunala T, Wolff H, Lauerma A, et al. IL-33 and ST2 in atopic dermatitis: expression profiles and modulation by triggering factors. J Invest Dermatol. 2012;132:1392–1400. doi: 10.1038/jid.2011.446. [DOI] [PubMed] [Google Scholar]
- 79.Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, Hippe A, Corrigan CJ, Dong C, Homey B, et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med. 2007;204:1837–1847. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, Hepworth MR, Van Voorhees AS, Comeau MR, Artis D. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013;5:170ra16. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, Huang LC, Johnson D, Scanlon ST, McKenzie AN, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013;210:2939–2950. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ryu WI, Lee H, Bae HC, Jeon J, Ryu HJ, Kim J, Kim JH, Son JW, Kim J, Imai Y, et al. IL-33 down-regulates CLDN1 expression through the ERK/STAT3 pathway in keratinocytes. J Dermatol Sci. 2018;90:313–322. doi: 10.1016/j.jdermsci.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 83.Seltmann J, Roesner LM, von Hesler FW, Wittmann M, Werfel T. IL-33 impacts on the skin barrier by downregulating the expression of filaggrin. J Allergy Clin Immunol. 2015;135:1659–1661.e4. doi: 10.1016/j.jaci.2015.01.048. [DOI] [PubMed] [Google Scholar]
- 84.Hvid M, Vestergaard C, Kemp K, Christensen GB, Deleuran B, Deleuran M. IL-25 in atopic dermatitis: a possible link between inflammation and skin barrier dysfunction? J Invest Dermatol. 2011;131:150–157. doi: 10.1038/jid.2010.277. [DOI] [PubMed] [Google Scholar]
- 85.Kim JH, Bae HC, Ko NY, Lee SH, Jeong SH, Lee H, Ryu WI, Kye YC, Son SW. Thymic stromal lymphopoietin downregulates filaggrin expression by signal transducer and activator of transcription 3 (STAT3) and extracellular signal-regulated kinase (ERK) phosphorylation in keratinocytes. J Allergy Clin Immunol. 2015;136:205–208.e9. doi: 10.1016/j.jaci.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 86.Leyva-Castillo JM, Galand C, Mashiko S, Bissonnette R, McGurk A, Ziegler SF, Dong C, McKenzie ANJ, Sarfati M, Geha RS. ILC2 activation by keratinocyte-derived IL-25 drives IL-13 production at sites of allergic skin inflammation. J Allergy Clin Immunol. 2020;145:1606–1614.e4. doi: 10.1016/j.jaci.2020.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Imai Y, Yasuda K, Sakaguchi Y, Haneda T, Mizutani H, Yoshimoto T, Nakanishi K, Yamanishi K. Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice. Proc Natl Acad Sci U S A. 2013;110:13921–13926. doi: 10.1073/pnas.1307321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yoo J, Omori M, Gyarmati D, Zhou B, Aye T, Brewer A, Comeau MR, Campbell DJ, Ziegler SF. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. 2005;202:541–549. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cork MJ, Danby SG, Vasilopoulos Y, Hadgraft J, Lane ME, Moustafa M, Guy RH, Macgowan AL, Tazi-Ahnini R, Ward SJ. Epidermal barrier dysfunction in atopic dermatitis. J Invest Dermatol. 2009;129:1892–1908. doi: 10.1038/jid.2009.133. [DOI] [PubMed] [Google Scholar]
- 90.Hirota T, Takahashi A, Kubo M, Tsunoda T, Tomita K, Sakashita M, Yamada T, Fujieda S, Tanaka S, Doi S, et al. Genome-wide association study identifies eight new susceptibility loci for atopic dermatitis in the Japanese population. Nat Genet. 2012;44:1222–1226. doi: 10.1038/ng.2438. [DOI] [PubMed] [Google Scholar]
- 91.Paternoster L, Standl M, Chen CM, Ramasamy A, Bønnelykke K, Duijts L, Ferreira MA, Alves AC, Thyssen JP, Albrecht E, et al. Meta-analysis of genome-wide association studies identifies three new risk loci for atopic dermatitis. Nat Genet. 2011;44:187–192. doi: 10.1038/ng.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Paternoster L, Standl M, Waage J, Baurecht H, Hotze M, Strachan DP, Curtin JA, Bønnelykke K, Tian C, Takahashi A, et al. Multi-ancestry genome-wide association study of 21,000 cases and 95,000 controls identifies new risk loci for atopic dermatitis. Nat Genet. 2015;47:1449–1456. doi: 10.1038/ng.3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schleimer RP, Berdnikovs S. Etiology of epithelial barrier dysfunction in patients with type 2 inflammatory diseases. J Allergy Clin Immunol. 2017;139:1752–1761. doi: 10.1016/j.jaci.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mashiko S, Mehta H, Bissonnette R, Sarfati M. Increased frequencies of basophils, type 2 innate lymphoid cells and Th2 cells in skin of patients with atopic dermatitis but not psoriasis. J Dermatol Sci. 2017;88:167–174. doi: 10.1016/j.jdermsci.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 95.Wilson SR, Thé L, Batia LM, Beattie K, Katibah GE, McClain SP, Pellegrino M, Estandian DM, Bautista DM. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. 2013;155:285–295. doi: 10.1016/j.cell.2013.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hashimoto Y, Arai I, Nakanishi Y, Sakurai T, Nakamura A, Nakaike S. Scratching of their skin by NC/Nga mice leads to development of dermatitis. Life Sci. 2004;76:783–794. doi: 10.1016/j.lfs.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 97.Krueger JG, Bowcock A. Psoriasis pathophysiology: current concepts of pathogenesis. Ann Rheum Dis. 2005;64(Suppl 2):ii30–ii36. doi: 10.1136/ard.2004.031120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chiang CC, Cheng WJ, Korinek M, Lin CY, Hwang TL. Neutrophils in Psoriasis. Front Immunol. 2019;10:2376. doi: 10.3389/fimmu.2019.02376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li B, Huang L, Lv P, Li X, Liu G, Chen Y, Wang Z, Qian X, Shen Y, Li Y, et al. The role of Th17 cells in psoriasis. Immunol Res. 2020;68:296–309. doi: 10.1007/s12026-020-09149-1. [DOI] [PubMed] [Google Scholar]
- 100.Elloso MM, Gomez-Angelats M, Fourie AM. Targeting the Th17 pathway in psoriasis. J Leukoc Biol. 2012;92:1187–1197. doi: 10.1189/jlb.0212101. [DOI] [PubMed] [Google Scholar]
- 101.Lowes MA, Suárez-Fariñas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol. 2014;32:227–255. doi: 10.1146/annurev-immunol-032713-120225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mitsui A, Tada Y, Takahashi T, Shibata S, Kamata M, Miyagaki T, Fujita H, Sugaya M, Kadono T, Sato S, et al. Serum IL-33 levels are increased in patients with psoriasis. Clin Exp Dermatol. 2016;41:183–189. doi: 10.1111/ced.12670. [DOI] [PubMed] [Google Scholar]
- 103.Suwarsa O, Dharmadji HP, Sutedja E, Herlina L, Sori PR, Hindritiani R, Dwiyana RF, Gunawan H. Skin tissue expression and serum level of thymic stromal lymphopoietin in patients with psoriasis vulgaris. Dermatol Rep. 2019;11:8006. doi: 10.4081/dr.2019.8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Duan Y, Dong Y, Hu H, Wang Q, Guo S, Fu D, Song X, Kalvakolanu DV, Tian Z. IL-33 contributes to disease severity in Psoriasis-like models of mouse. Cytokine. 2019;119:159–167. doi: 10.1016/j.cyto.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 105.Hueber AJ, Alves-Filho JC, Asquith DL, Michels C, Millar NL, Reilly JH, Graham GJ, Liew FY, Miller AM, McInnes IB. IL-33 induces skin inflammation with mast cell and neutrophil activation. Eur J Immunol. 2011;41:2229–2237. doi: 10.1002/eji.201041360. [DOI] [PubMed] [Google Scholar]
- 106.Suto H, Nambu A, Morita H, Yamaguchi S, Numata T, Yoshizaki T, Shimura E, Arae K, Asada Y, Motomura K, et al. IL-25 enhances TH17 cell-mediated contact dermatitis by promoting IL-1β production by dermal dendritic cells. J Allergy Clin Immunol. 2018;142:1500–1509.e10. doi: 10.1016/j.jaci.2017.12.1007. [DOI] [PubMed] [Google Scholar]
- 107.Volpe E, Pattarini L, Martinez-Cingolani C, Meller S, Donnadieu MH, Bogiatzi SI, Fernandez MI, Touzot M, Bichet JC, Reyal F, et al. Thymic stromal lymphopoietin links keratinocytes and dendritic cell-derived IL-23 in patients with psoriasis. J Allergy Clin Immunol. 2014;134:373–381. doi: 10.1016/j.jaci.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 108.Kubo T, Kamekura R, Kumagai A, Kawata K, Yamashita K, Mitsuhashi Y, Kojima T, Sugimoto K, Yoneta A, Sumikawa Y, et al. ΔNp63 controls a TLR3-mediated mechanism that abundantly provides thymic stromal lymphopoietin in atopic dermatitis. PLoS One. 2014;9:e105498. doi: 10.1371/journal.pone.0105498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gago-Lopez N, Mellor LF, Megías D, Martín-Serrano G, Izeta A, Jimenez F, Wagner EF. Role of bulge epidermal stem cells and TSLP signaling in psoriasis. EMBO Mol Med. 2019;11:e10697. doi: 10.15252/emmm.201910697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Trapp BD, Nave KA. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci. 2008;31:247–269. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- 111.Pichery M, Mirey E, Mercier P, Lefrancais E, Dujardin A, Ortega N, Girard JP. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33-LacZ gene trap reporter strain. J Immunol. 2012;188:3488–3495. doi: 10.4049/jimmunol.1101977. [DOI] [PubMed] [Google Scholar]
- 112.Fairlie-Clarke K, Barbour M, Wilson C, Hridi SU, Allan D, Jiang HR. Expression and function of IL-33/ST2 axis in the central nervous system under normal and diseased conditions. Front Immunol. 2018;9:2596. doi: 10.3389/fimmu.2018.02596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Allan D, Fairlie-Clarke KJ, Elliott C, Schuh C, Barnett SC, Lassmann H, Linnington C, Jiang HR. Role of IL-33 and ST2 signalling pathway in multiple sclerosis: expression by oligodendrocytes and inhibition of myelination in central nervous system. Acta Neuropathol Commun. 2016;4:75. doi: 10.1186/s40478-016-0344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Christophi GP, Gruber RC, Panos M, Christophi RL, Jubelt B, Massa PT. Interleukin-33 upregulation in peripheral leukocytes and CNS of multiple sclerosis patients. Clin Immunol. 2012;142:308–319. doi: 10.1016/j.clim.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jiang HR, Milovanović M, Allan D, Niedbala W, Besnard AG, Fukada SY, Alves-Filho JC, Togbe D, Goodyear CS, Linington C, et al. IL-33 attenuates EAE by suppressing IL-17 and IFN-γ production and inducing alternatively activated macrophages. Eur J Immunol. 2012;42:1804–1814. doi: 10.1002/eji.201141947. [DOI] [PubMed] [Google Scholar]
- 116.Mado H, Adamczyk-Sowa M, Bartman W, Wierzbicki K, Tadeusiak B, Sowa P. Plasma Interleukin-33 level in relapsing-remitting multiple sclerosis. Is it negatively correlated with central nervous system lesions in patients with mild disability? Clin Neurol Neurosurg. 2021;206:106700. doi: 10.1016/j.clineuro.2021.106700. [DOI] [PubMed] [Google Scholar]
- 117.Jafarzadeh A, Mahdavi R, Jamali M, Hajghani H, Nemati M, Ebrahimi HA. Increased concentrations of interleukin-33 in the serum and cerebrospinal fluid of patients with multiple sclerosis. Oman Med J. 2016;31:40–45. doi: 10.5001/omj.2016.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sung HY, Chen WY, Huang HT, Wang CY, Chang SB, Tzeng SF. Down-regulation of interleukin-33 expression in oligodendrocyte precursor cells impairs oligodendrocyte lineage progression. J Neurochem. 2019;150:691–708. doi: 10.1111/jnc.14788. [DOI] [PubMed] [Google Scholar]
- 119.Milovanovic M, Volarevic V, Ljujic B, Radosavljevic G, Jovanovic I, Arsenijevic N, Lukic ML. Deletion of IL-33R (ST2) abrogates resistance to EAE in BALB/C mice by enhancing polarization of APC to inflammatory phenotype. PLoS One. 2012;7:e45225. doi: 10.1371/journal.pone.0045225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Javan MR, Seyfizadeh N, Aslani S, Farhoodi M, Babaloo Z. Molecular analysis of interleukin-25 exons 1 and 2 and its serum levels in Iranian patients with multiple sclerosis. Am J Clin Exp Immunol. 2014;3:91–96. [PMC free article] [PubMed] [Google Scholar]
- 121.Turner DA, Haile Y, Giuliani F. IL-25 prevents T cell-mediated neurotoxicity by decreasing LFA-1 expression. J Neuroimmunol. 2013;265:11–19. doi: 10.1016/j.jneuroim.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 122.Maiorino C, Khorooshi R, Ruffini F, Løbner M, Bergami A, Garzetti L, Martino G, Owens T, Furlan R. Lentiviral-mediated administration of IL-25 in the CNS induces alternative activation of microglia. Gene Ther. 2013;20:487–496. doi: 10.1038/gt.2012.58. [DOI] [PubMed] [Google Scholar]
- 123.Eckhardt J, Döbbeler M, König C, Kuczera K, Kuhnt C, Ostalecki C, Zinser E, Mak TW, Steinkasserer A, Lechmann M. Thymic stromal lymphopoietin deficiency attenuates experimental autoimmune encephalomyelitis. Clin Exp Immunol. 2015;181:51–64. doi: 10.1111/cei.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yu X, Lv J, Wu J, Chen Y, Chen F, Wang L. The autoimmune encephalitis-related cytokine TSLP in the brain primes neuroinflammation by activating the JAK2-NLRP3 axis. Clin Exp Immunol. 2021;207:113–122. doi: 10.1093/cei/uxab023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 126.Zhang J, Wang P, Ji W, Ding Y, Lu X. Overexpression of interleukin-33 is associated with poor prognosis of patients with glioma. Int J Neurosci. 2017;127:210–217. doi: 10.1080/00207454.2016.1175441. [DOI] [PubMed] [Google Scholar]
- 127.Chen SF, Nieh S, Jao SW, Wu MZ, Liu CL, Chang YC, Lin YS. The paracrine effect of cancer-associated fibroblast-induced interleukin-33 regulates the invasiveness of head and neck squamous cell carcinoma. J Pathol. 2013;231:180–189. doi: 10.1002/path.4226. [DOI] [PubMed] [Google Scholar]
- 128.Sun P, Ben Q, Tu S, Dong W, Qi X, Wu Y. Serum interleukin-33 levels in patients with gastric cancer. Dig Dis Sci. 2011;56:3596–3601. doi: 10.1007/s10620-011-1760-5. [DOI] [PubMed] [Google Scholar]
- 129.Liu X, Zhu L, Lu X, Bian H, Wu X, Yang W, Qin Q. IL-33/ST2 pathway contributes to metastasis of human colorectal cancer. Biochem Biophys Res Commun. 2014;453:486–492. doi: 10.1016/j.bbrc.2014.09.106. [DOI] [PubMed] [Google Scholar]
- 130.Zhang P, Liu XK, Chu Z, Ye JC, Li KL, Zhuang WL, Yang DJ, Jiang YF. Detection of interleukin-33 in serum and carcinoma tissue from patients with hepatocellular carcinoma and its clinical implications. J Int Med Res. 2012;40:1654–1661. doi: 10.1177/030006051204000504. [DOI] [PubMed] [Google Scholar]
- 131.Santulli P, Even M, Chouzenoux S, Millischer AE, Borghese B, de Ziegler D, Batteux F, Chapron C. Profibrotic interleukin-33 is correlated with uterine leiomyoma tumour burden. Hum Reprod. 2013;28:2126–2133. doi: 10.1093/humrep/det238. [DOI] [PubMed] [Google Scholar]
- 132.Hu LA, Fu Y, Zhang DN, Zhang J. Serum IL-33 as a diagnostic and prognostic marker in non- small cell lung cancer. Asian Pac J Cancer Prev. 2013;14:2563–2566. doi: 10.7314/apjcp.2013.14.4.2563. [DOI] [PubMed] [Google Scholar]
- 133.Yang ZP, Ling DY, Xie YH, Wu WX, Li JR, Jiang J, Zheng JL, Fan YH, Zhang Y. The association of serum IL-33 and sST2 with breast cancer. Dis Markers. 2015;2015:516895. doi: 10.1155/2015/516895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jovanovic IP, Pejnovic NN, Radosavljevic GD, Pantic JM, Milovanovic MZ, Arsenijevic NN, Lukic ML. Interleukin-33/ST2 axis promotes breast cancer growth and metastases by facilitating intratumoral accumulation of immunosuppressive and innate lymphoid cells. Int J Cancer. 2014;134:1669–1682. doi: 10.1002/ijc.28481. [DOI] [PubMed] [Google Scholar]
- 135.Gao X, Wang X, Yang Q, Zhao X, Wen W, Li G, Lu J, Qin W, Qi Y, Xie F, et al. Tumoral expression of IL-33 inhibits tumor growth and modifies the tumor microenvironment through CD8+ T and NK cells. J Immunol. 2015;194:438–445. doi: 10.4049/jimmunol.1401344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gao K, Li X, Zhang L, Bai L, Dong W, Gao K, Shi G, Xia X, Wu L, Zhang L. Transgenic expression of IL-33 activates CD8(+) T cells and NK cells and inhibits tumor growth and metastasis in mice. Cancer Lett. 2013;335:463–471. doi: 10.1016/j.canlet.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 137.Qin L, Dominguez D, Chen S, Fan J, Long A, Zhang M, Fang D, Zhang Y, Kuzel TM, Zhang B. Exogenous IL-33 overcomes T cell tolerance in murine acute myeloid leukemia. Oncotarget. 2016;7:61069–61080. doi: 10.18632/oncotarget.11179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dominguez D, Ye C, Geng Z, Chen S, Fan J, Qin L, Long A, Wang L, Zhang Z, Zhang Y, et al. Exogenous IL-33 restores dendritic cell activation and maturation in established cancer. J Immunol. 2017;198:1365–1375. doi: 10.4049/jimmunol.1501399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen J, Zhao Y, Jiang Y, Gao S, Wang Y, Wang D, Wang A, Yi H, Gu R, Yi Q, et al. Interleukin-33 contributes to the induction of Th9 cells and antitumor efficacy by dectin-1-activated dendritic cells. Front Immunol. 2018;9:1787. doi: 10.3389/fimmu.2018.01787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Moral JA, Leung J, Rojas LA, Ruan J, Zhao J, Sethna Z, Ramnarain A, Gasmi B, Gururajan M, Redmond D, et al. ILC2s amplify PD-1 blockade by activating tissue-specific cancer immunity. Nature. 2020;579:130–135. doi: 10.1038/s41586-020-2015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ikutani M, Yanagibashi T, Ogasawara M, Tsuneyama K, Yamamoto S, Hattori Y, Kouro T, Itakura A, Nagai Y, Takaki S, et al. Identification of innate IL-5-producing cells and their role in lung eosinophil regulation and antitumor immunity. J Immunol. 2012;188:703–713. doi: 10.4049/jimmunol.1101270. [DOI] [PubMed] [Google Scholar]
- 142.Wagner M, Ealey KN, Tetsu H, Kiniwa T, Motomura Y, Moro K, Koyasu S, et al. Tumor-derived lactic acid contributes to the paucity of intratumoral ILC2s. Cell Rep. 2020;30:2743–2757.e5. doi: 10.1016/j.celrep.2020.01.103. [DOI] [PubMed] [Google Scholar]
- 143.Jiang Z, Chen J, Du X, Cheng H, Wang X, Dong C. IL-25 blockade inhibits metastasis in breast cancer. Protein Cell. 2017;8:191–201. doi: 10.1007/s13238-016-0345-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nakajima R, Miyagaki T, Hirakawa M, Oka T, Takahashi N, Suga H, Yoshizaki A, Fujita H, Asano Y, Sugaya M, et al. Interleukin-25 is involved in cutaneous T-cell lymphoma progression by establishing a T helper 2-dominant microenvironment. Br J Dermatol. 2018;178:1373–1382. doi: 10.1111/bjd.16237. [DOI] [PubMed] [Google Scholar]
- 145.Ferretti E, Di Carlo E, Ognio E, Fraternali-Orcioni G, Corcione A, Belmonte B, Ravetti JL, Tripodo C, Ribatti D, Pistoia V. IL-25 dampens the growth of human germinal center-derived B-cell non Hodgkin Lymphoma by curtailing neoangiogenesis. OncoImmunology. 2017;7:e1397249. doi: 10.1080/2162402X.2017.1397249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Furuta S, Jeng YM, Zhou L, Huang L, Kuhn I, Bissell MJ, Lee WH. IL-25 causes apoptosis of IL-25R-expressing breast cancer cells without toxicity to nonmalignant cells. Sci Transl Med. 2011;3:78ra31. doi: 10.1126/scitranslmed.3001374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ragonnaud E, Moritoh K, Bodogai M, Gusev F, Garaud S, Chen C, Wang X, Baljinnyam T, Becker KG, Maul RW, et al. Tumor-derived thymic stromal lymphopoietin expands bone marrow b-cell precursors in circulation to support metastasis. Cancer Res. 2019;79:5826–5838. doi: 10.1158/0008-5472.CAN-19-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xie F, Meng YH, Liu LB, Chang KK, Li H, Li MQ, Li DJ. Cervical carcinoma cells stimulate the angiogenesis through TSLP promoting growth and activation of vascular endothelial cells. Am J Reprod Immunol. 2013;70:69–79. doi: 10.1111/aji.12104. [DOI] [PubMed] [Google Scholar]
- 149.Xie F, Liu LB, Shang WQ, Chang KK, Meng YH, Mei J, Yu JJ, Li DJ, Li MQ. The infiltration and functional regulation of eosinophils induced by TSLP promote the proliferation of cervical cancer cell. Cancer Lett. 2015;364:106–117. doi: 10.1016/j.canlet.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 150.Takahashi N, Sugaya M, Suga H, Oka T, Kawaguchi M, Miyagaki T, Fujita H, Sato S. Thymic stromal chemokine TSLP acts through Th2 cytokine production to induce cutaneous T-cell lymphoma. Cancer Res. 2016;76:6241–6252. doi: 10.1158/0008-5472.CAN-16-0992. [DOI] [PubMed] [Google Scholar]
- 151.De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, Braga M, Di Carlo V, Doglioni C, Protti MP. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med. 2011;208:469–478. doi: 10.1084/jem.20101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Demehri S, Cunningham TJ, Manivasagam S, Ngo KH, Moradi Tuchayi S, Reddy R, Meyers MA, DeNardo DG, Yokoyama WM. Thymic stromal lymphopoietin blocks early stages of breast carcinogenesis. J Clin Invest. 2016;126:1458–1470. doi: 10.1172/JCI83724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yue W, Lin Y, Yang X, Li B, Liu J, He R. Thymic stromal lymphopoietin (TSLP) inhibits human colon tumor growth by promoting apoptosis of tumor cells. Oncotarget. 2016;7:16840–16854. doi: 10.18632/oncotarget.7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.A first-in-human, double blind, single dose study in healthy subjects and subjects with mild atopic asthma [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT01928368.
- 155.A study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of AMG 282 in healthy subjects and subjects with chronic rhinosinusitis with nasal polyps [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT02170337.
- 156.A study to assess the efficacy and safety of MSTT1041A in participants with uncontrolled severe asthma [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT02918019.
- 157.A study to assess the efficacy and safety of MSTT1041A in participants with moderate to severe atopic dermatitis [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT03747575.
- 158.Anti-ST2 (MSTT1041A) in COPD (COPD-ST2OP) [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT03615040.
- 159.A study to evaluate the efficacy and safety of astegolimab in participants with chronic obstructive pulmonary disease [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT05037929.
- 160.A study investigating the efficacy, safety, and PK profile of ANB020 administered to adult subjects with moderate-to-severe AD (ATLAS) [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT03533751.
- 161.Proof of concept study to investigate ANB020 activity in adult patients with severe eosinophilic asthma [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT03469934.
- 162.Etokimab in adult patients with chronic rhinosinusitis with nasal polyps (CRSwNP) [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT03614923.
- 163.Placebo-controlled study to investigate ANB020 activity in adult patients with peanut allergy [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT02920021.
- 164.A study to evaluate the safety and tolerability, pharmacokinetics (PK) and pharmacodynamics (PD) of Melrilimab (GSK3772847) in healthy participants [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT04366349.
- 165.Efficacy and safety study of GSK3772847 in subjects with moderately severe asthma [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT03207243.
- 166.Study of REGN3500 and dupilumab in patients with asthma [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT03112577.
- 167.Study of safety, tolerability, and pharmacokinetics of multiple ascending doses of REGN3500 in adults with moderate asthma [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT02999711.
- 168.Evaluation of SAR440340 and as combination therapy with dupilumab in moderate-to-severe asthma participants [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT03387852.
- 169.Efficacy, safety, and pharmacokinetic profiles of REGN3500 administered to adult patients with moderate-to-severe atopic dermatitis [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT03738423.
- 170.Efficacy and safety of REGN3500 monotherapy and combination of REGN3500 plus dupilumab in adult patients with moderate-to-severe atopic dermatitis [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT03736967.
- 171.Proof-of-concept study to assess the efficacy, safety and tolerability of SAR440340 (Anti-IL-33 mAb) in patients with moderate-to-severe chronic obstructive pulmonary disease (COPD) [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT03546907.
- 172.Study to assess the efficacy, safety, and tolerability of SAR440340/REGN3500/Itepekimab in chronic obstructive pulmonary disease (COPD) (AERIFY-1) [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT04701983.
- 173.Study to assess the efficacy, safety, and tolerability of SAR440340/REGN3500/Itepekimab in chronic obstructive pulmonary disease (COPD) (AERIFY-2) [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT04751487.
- 174.Safety and tolerability of MEDI3506 in healthy participants, in participants with COPD and healthy Japanese participants [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT03096795.
- 175.Study to assess the efficacy and safety of MEDI3506 in adults with uncontrolled moderate-to-severe asthma (FRONTIER-3) [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT04570657.
- 176.Efficacy and safety of MEDI3506 in adult subjects with atopic dermatitis [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT04212169.
- 177.A phase II, randomized, double-blind, placebo-controlled study to assess MEDI3506 in participants with COPD and chronic bronchitis (FRONTIER-4) [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT04631016.
- 178.A study to evaluate the pharmacokinetics of MEDI9929 (AMG 157) in adolescents with mild to moderate asthma [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT02512900.
- 179.Study to evaluate the pharmacokinetics of tezepelumab in children with asthma (TRAILHEAD) [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT04673630.
- 180.Double-blind, multiple dose study in subjects with mild atopic asthma [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT01405963.
- 181.Safety study of AMG 157 in healthy subjects and subjects with atopic dermatitis [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT00757042.
- 182.Study to evaluate the efficacy and safety of MEDI9929 (AMG 157) in adult subjects with inadequately controlled, severe asthma [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT02054130.
- 183.Effects of anti-TSLP in patients with asthma (UPSTREAM) [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT02698501.
- 184.Study to evaluate tezepelumab on airway inflammation in adults with uncontrolled asthma (CASCADE) (CASCADE) [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT03688074.
- 185.Phase 2a study to evaluate the efficacy and safety of MEDI9929 in adults with atopic dermatitis (ALLEVIAD) [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT02525094.
- 186.Anti-TSLP (AMG 157) plus antigen-specific immunotherapy for induction of tolerance in individuals with cat allergy [Internet] [accessed on 27 December 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT02237196.
- 187.Study to evaluate tezepelumab in adults with chronic spontaneous urticaria (INCEPTION) [Internet] [accessed on 27 December 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT04833855.
- 188.Tezepelumab COPD exacerbation study (COURSE) [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT04039113.
- 189.Study to evaluate tezepelumab in adults & adolescents with severe uncontrolled asthma (NAVIGATOR) [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT03347279.
- 190.Study to evaluate the efficacy and safety of tezepelumab in reducing oral corticosteroid use in adults with oral corticosteroid dependent asthma (SOURCE) [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT03406078.
- 191.Long-term safety of tezepelumab in japanese subjects with inadequately controlled severe asthma (NOZOMI) [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT04048343.
- 192.Extension study to evaluate the safety and tolerability of tezepelumab in adults and adolescents with severe, uncontrolled asthma (DESTINATION) [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT03706079.
- 193.Study to evaluate tezepelumab in adults with severe uncontrolled asthma (DIRECTION) [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT03927157.
- 194.Efficacy and safety of tezepelumab in participants with severe chronic rhinosinusitis with nasal polyposis (WAYPOINT) [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT04851964.
- 195.A bronchoprovocation study to assess the safety, tolerability, pharmacokinetics and pharmacodynamics of CSJ117 in adult subjects with mild atopic asthma [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT03138811.
- 196.Study of efficacy and safety of CSJ117 in patients with severe uncontrolled asthma [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT04410523.
- 197.Study of safety of CSJ117 in participants with moderate to severe uncontrolled asthma [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT04946318.
- 198.Study of effect of CSJ117 on symptoms, pharmacodynamics and safety in patients with COPD [Internet] [accessed on 27 December, 2021]. Available at https://clinicaltrials.gov/ct2/show/NCT04882124.
- 199.Kelsen SG, Agache IO, Soong W, Israel E, Chupp GL, Cheung DS, Theess W, Yang X, Staton TL, Choy DF, et al. Astegolimab (anti-ST2) efficacy and safety in adults with severe asthma: a randomized clinical trial. J Allergy Clin Immunol. 2021;148:790–798. doi: 10.1016/j.jaci.2021.03.044. [DOI] [PubMed] [Google Scholar]
- 200.Rabe KF, Celli BR, Wechsler ME, Abdulai RM, Luo X, Boomsma MM, Staudinger H, Horowitz JE, Baras A, Ferreira MA, et al. Safety and efficacy of itepekimab in patients with moderate-to-severe COPD: a genetic association study and randomised, double-blind, phase 2a trial. Lancet Respir Med. 2021;9:1288–1298. doi: 10.1016/S2213-2600(21)00167-3. [DOI] [PubMed] [Google Scholar]
- 201.Emson C, Corren J, Sałapa K, Hellqvist Å, Parnes JR, Colice G. Efficacy of tezepelumab in patients with severe, uncontrolled asthma with and without nasal polyposis: a post hoc analysis of the phase 2b PATHWAY study. J Asthma Allergy. 2021;14:91–99. doi: 10.2147/JAA.S288260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Menzies-Gow A, Corren J, Bourdin A, Chupp G, Israel E, Wechsler ME, Brightling CE, Griffiths JM, Hellqvist Å, Bowen K, et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl J Med. 2021;384:1800–1809. doi: 10.1056/NEJMoa2034975. [DOI] [PubMed] [Google Scholar]
- 203.Simpson EL, Parnes JR, She D, Crouch S, Rees W, Mo M, van der Merwe R. Tezepelumab, an anti-thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: a randomized phase 2a clinical trial. J Am Acad Dermatol. 2019;80:1013–1021. doi: 10.1016/j.jaad.2018.11.059. [DOI] [PubMed] [Google Scholar]
- 204.Gauvreau G, Hohlfeld J, Boulet LP, Cockcroft D, Davis B, Fitzgerald JM, Korn S, Kornmann O, Leigh R, Mayers I, et al. Late breaking abstract - efficacy of CSJ117 on allergen-induced asthmatic responses in mild atopic asthma patients. Eur Respir J. 2020;56:3690. [Google Scholar]