Abstract
Introduction:
In China, Huaier granule (HG) is widely applied to tumor adjuvant therapy. However, systematic reviews (SRs) or meta-analyses (MAs) published continuously failed to reach a consensus, without convincing evidence. An overview should be conducted to summarize the evidence-based progress and try to provide some value references for relative research and clinical practice in the future.
Methods:
From inception to October 2021, 8 databases in English and Chinese were searched. SRs/MAs meeting the inclusion and exclusion criteria were included. Relevant criteria were used to evaluate SRs/MAs including methodological quality, reporting quality, risk of bias, and evidence quality of effect and safety.
Results:
The short-term effect, long-term effect, and safety in 6 included SRs/MAs were assessed in this overview according to quantitative synthesis. Results assessed by AMSTAR-2, PRISMA, and ROBIS were generally unsatisfactory with the main problems on registration or protocol, a search of grey literature, a list of excluded studies, bias of each synthetic result, and inadequate report of search strategy and synthesis methods. Additionally, 28 items were assessed as moderate quality while 12 items were low-quality and 6 items were very low-quality in GRADE. Risk of bias was the main downgrading factor.
Conclusion:
HG may be a promising adjuvant therapy for cancer. However, high-quality SRs/MAs and RCTs should be conducted to provide sufficient evidence so as to draw a definitive conclusion.
Keywords: Huaier granule, cancer, overview, TCM, adjuvant therapy
Introduction
Cancer is still one of the most fatal threats to human health. According to the estimation of Global Cancer Observatory (GCO), there were more than 19 million new cases in 2020 worldwide. 1 Although the efficacy of modern anticancer therapy has been improved in recent years, it still cannot reverse the present status of anticancer treatment. What’s more, the occurrence of side effects is a worrisome factor which may enhance socioeconomic burden and health care system expense. 2 Thus, finding an adjuvant therapy to enhance efficacy or decrease adverse reactions is necessary for present anticancer therapy.
It is well known that Chinese medicine plays an important role in enhancing effects and reducing adverse reactions for modern cancer treatment. 3 Huaier (Trametes robiniophila Murr.), a commonly known Chinese medicine, has been applied to anticancer therapy in different kinds of cancers for many years. 4 The experimental results showed that Huaier had effects including anti-proliferation, anti-metastasis, anti-angiogenesis, inducing apoptosis, inhibiting cancer stem cells and modulating tumor-specific immunity. 5 In addition, a high-quality clinical trial has been conducted to verify the efficacy of Huaier granule, which is the aqueous product of Huaier extract, 6 supporting that Huaier granule has the potential to be an adjuvant therapy for cancer.
However, evidence-based results of efficacy and safety for Huaier were still debatable due to the uneven quality of SMs/MAs. An overview is a novel way for integrating several SRs/MAs by assessing their quality and results in order to provide comprehensive evidence for clinical practice. 7 This research is intended to reveal the deficiencies and improvements of current SRs/MAs about this topic by evaluating them objectively so as to provide a reasonable choice of potential adjuvant therapy for cancer patients.
Methods
Registration and Instructions
The protocol of this overview was registered in PROSPERO (ID: CRD42021284967). The process of this overview follows Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement 8 and the Cochrane Handbook 9 for systematic reviews. The third reviewer could step in and handle any inconsistencies caused by 2 reviewers during searching and selecting SRs/MAs, extracting data, and evaluating quality separately.
Literature Search
From inception to October 2021, 8 databases in English and Chinese including CNKI, EMBASE, Web of Science, PubMed, SinoMed, the Cochrane Library, VIP, and Wanfang were searched. After much practice, we decided to use the following search strategy: (Huaier OR Trametes robiniophila Murr.) AND (meta-analysis OR systematic review) as subject word and random word for all fields. Besides, references of included studies and other gray literature also should necessarily be searched.
Study Selection
After removing duplicate literature, we reviewed title and abstract of all article and looked up full texts of possibly eligible papers for further selection. The inclusion criteria were as follows: (a) study design: SRs/MAs based on patients with cancer diagnosed by pathology or cytology in controlled clinical trials (CCTs) or randomized controlled trials (RCTs); (b) intervention: HG plus conventional treatment (CT) versus CT alone; (c) SRs/MAs with quantitative analysis; (d) language limited by Chinese and English. However, repeated publications, protocol studies, studies with unavailable full text, and studies whose data couldn’t be extracted were excluded.
Assessment of Quality of Included Reviews
First, methodological quality of each included study was evaluated by Assessing the Methodological Quality of Systematic Reviews 2 (AMSTAR-2) 10 which is based on 16 items, 7 of which (Q2, Q4, Q7, Q9, Q11, Q13, Q15) are critical domains. The rules of assessing total quality of a study were as follows: (a) A study with less than 2 non-critical items that didn’t match should be assessed as high quality; (b) A study with more than 1 non-critical item that didn’t match should be assessed as moderate quality; (c) A study with 1 critical item that didn’t match should be assessed as low quality; (d) A study with more than 1 critical item that didn’t match should be assessed as critically low quality. Second, reporting quality of each included study was evaluated by the PRISMA 2020 version checklist 8 with 27 items. Third, risk of bias of each included study was evaluated as “low risk,” “high risk,” or “unclear” by Risk of Bias in Systematic reviews (ROBIS) 11 including 3 phases. Finally, evidence quality of short-term effect, long-term effect, and safety extracted from each included study would be evaluated by the Grades of Recommendations, Assessment, Development and Evaluation (GRADE). 12
Data Extraction and Analysis
The designed items were extracted from each eligible review using a standardized form and they were as follows: author and publication year (country), cancer types, number of trials (subjects), quality assessment method for trials, interventions, main results, and conclusions. Especially, main outcomes were focused on 3 aspects including short-term effect (ORR or DCR), long-term effect (OS or DFS), and safety (the incidence of different adverse reactions). A narrative integration of included studies was applied to this overview. Tabulation and figures were utilized to summarize the characteristics of each included study and the results of literature search and selection, quality assessment, and evidence quality. Especially, GRADE profiler 3.6.1 version software was utilized to generate and summarize the evidence quality.
Results
Literature Search and Selection
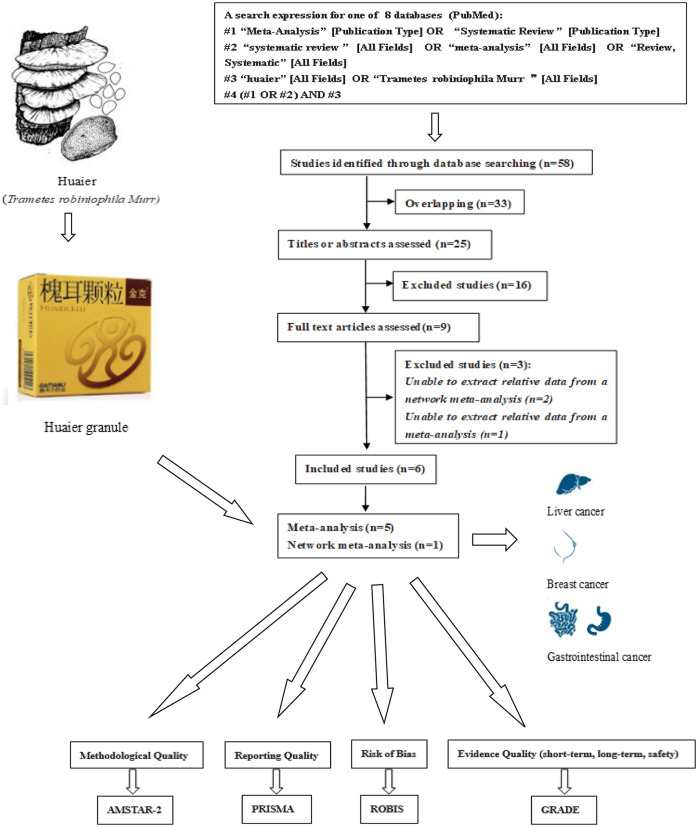
Based on the established search strategy, a total of 58 records was identified. After 33 overlapping records were deleted, 25 remaining records were assessed by the titles or abstracts. After that, 16 studies are excluded and 9 studies were further assessed by accessing the full text. Finally, 3 studies were excluded because they were unable to extract data about Huaier granule and 6 SRs/MAs13-18 were included for further comprehensive analysis (Figure 1).
Figure 1.
Flow diagram of overview and literature selection process.
Characteristics of Included Systematic Reviews
Six included SRs/MAs, including 5 meta-analyses and 1 network meta-analysis, were published between 2018 and 2021. Four of them were published in Chinese while 2 other SRs/MAs were in English but all SRs/MAs were conducted in China. Including from 13 to 33 trials, individual SR/MA sample sizes ranged from 919 to 2884. The quality assessment criteria of the original trials were as follows: Cochrane risk of bias criteria was used in 3 studies,15,16,18 Jadad scale was adopted in 1 study, 17 the Physiotherapy Evidence Database (PEDro) scale score and the Newcastle-Ottawa Scale (NOS) was used in 1 study, 14 both Cochrane risk of bias criteria and Methodological Index for Nonrandomized Studies (MINRRS) was used in 1 study. 13 For the clinical information, first, HG was mainly researched in liver cancer. Breast cancer and other gastrointestinal cancers (except liver cancer) were less researched relatively. Second, HG was the major adjuvant therapy for transcatheter arterial chemoembolization (TACE) in liver cancer while HG was the major adjuvant therapy for chemotherapy in other gastrointestinal cancers. It is unfortunate that conventional therapy wasn’t fully described in breast cancer. Last but not least, main clinical results including short-term effect, long-term effect, and safety in these SRs/MAs have not reached a statistical consistency, respectively. More details can be found in Table 1.
Table 1.
The Characteristics of the Included SRs/MAs.
| Author, year, country, cancer types | Trials, subjects, quality assessment | Intervention | Main results and conclusion | |
|---|---|---|---|---|
| TG | CG | |||
| Hou et al, 2021, China, Liver cancer | 22, 2676, Cochrane | HG+CT | CT: TACE: 11 trials TACE + RT: 2 trials RT: 2 trials Chemo: 2 trials MTT: 2 trials RFA: 1 trial PMCT: 1 trial SR: 1 trial |
Main results: ① Short-term effect: ORR: RR = 1.39, 95% CI (1.24, 1.55), P < .001. ② Long-term effect: 1-y OS: RR = 1.43, 95% CI (1.23, 1.66), P < .001. ③ Safety: Incidence of gastrointestinal reactions: RR = 0.61, 95% CI (0.46, 0.81), P < .001; Incidence of myelosuppression: RR = 0.44, 95% CI (0.30, 0.65), P < .001. Conclusion: HG combined with CT can improve clinical efficacy and safety for primary liver cancer. |
| Zhang et al, 2020, China, Liver cancer | 15, 1781, Jadad | HG+CT | CT: TACE: 15 trials |
Main results: ① Short-term effect: ORR: OR = 2.00, 95% CI (1.51, 2.67), P < .001; DCR: OR = 1.94, 95% CI (1.47, 2.55), P < .001. ② Long-term effect: 6-mo OS: OR = 1.83, 95% CI (1.16, 2.87), P = .009; 1-y OS: OR = 2.19, 95% CI (1.45, 3.30), P < .001. ③ Safety: Incidence of nausea and vomiting: OR = 0.62, 95% CI (0.32, 1.20), P = .16. Conclusion: HG combined with CT could increase survival rate of primary liver cancer. |
| Zhang et al, 2021, China, Liver cancer | 24, 2664, Cochrane | HG+CT | CT: TACE: 11 trials TACE + RT: 2 trials TACE + Others: 2 trials Chemo: 2 trials RT: 2 trials MTT: 2 trials PMCT: 1 trial RFA: 1 trial SR: 1 trial |
Main results: ① Short-term effect: ORR: RR = 1.38, 95% CI (1.26, 1.51), P < .001; DCR: RR = 1.29, 95% CI (1.10, 1.52), P = .002. ② Long-term effect: 6-mo OS: RR = 1.2, 95% CI (1.1, 1.32), P < .001; 1-y OS: RR = 1.39, 95% CI (1.23, 1.58), P < .001; 2-y OS: RR = 1.95, 95% CI (1.28, 2.96), P = .002. ③ Safety: incidence of adverse reactions: RR = 0.60, 95% CI (0.41, 0.89), P = .01. Conclusion: HG have certain efficacy in adjuvant treatment of primary liver cancer, but its effect in reducing adverse reactions remains to be verified. |
| Li et al, 2020, China, Liver cancer | 13, 919, Cochrane | HG+CT | CT: TACE: 8 trials Chemo: 2 trials RT: 1 trial RFA: 1 trial TACE + RT: 1 trial |
Main results: ① Short-term effect: ORR: RR = 1.4, 95% CI (1.22, 1.92), P < .001. ② Long-term effect: 1-y OS: RR = 1.52, 95% CI (1.21, 1.92), P < .001. ③ Safety: Incidence of gastrointestinal reactions: RR = 0.68, 95% CI (0.39, 1.19), P = .18; Incidence of myelosuppression: RR = 0.44, 95% CI (0.31, 0.61), P < .001; Incidence of hepatotoxicity: RR = 0.44, 95% CI (0.33, 0.58), P < .001. Conclusion: HG assisted with CT show better efficacy and safety in the treatment of primary hepatocellular carcinoma than the control group. |
| Yao et al, 2020, China, Breast cancer | 27, 2562, Cochrane + MINRRS | HG+CT | CT: SR + RT + Chemo |
Main results: ① Short-term effect: ORR: RR = 1.46, 95% CI (1.06, 2.01), P = .02; DCR: RR = 1.06, 95% CI (0.97, 1.15), P = .19. ② Long-term effect: 1-y OS: RR = 1.04, 95% CI (0.98, 1.10), P = .16; 1-y DFS: RR = 1.05, 95% CI (1.02, 1.08), P = .003; 2-year OS: RR = 1.21, 95% CI (1.03, 1.43), P = .02; 2-y DFS: RR = 1.15, 95% CI (1.09, 1.21), P < .001; 3-year OS: RR = 1.16, 95% CI (1.08, 1.24), P < .001; 3-y DFS: RR = 1.14, 95% CI (1.08, 1.21), P < .001; 5-y OS: RR = 1.13, 95% CI (1.04, 1.23), P = .004; 5-y DFS: RR = 1.16, 95% CI (1.01, 1.32), P = .03. ③ Safety: Incidence of gastrointestinal reactions: RR = 0.70, 95% CI (0.43, 1.13), P = .14; Incidence of myelosuppression: RR = 0.66, 95% CI (0.51, 0.85), P = .001; Incidence of hepatotoxicity: RR = 0.36, 95% CI (0.13, 0.98), P = .05; Incidence of leukopenia: RR = 0.50, 95% CI (0.24, 1.02), P = .06; Incidence of nausea and vomiting: RR = 0.83, 95% CI (0.48, 1.45), P = .52; Incidence of alopecia: RR = 0.58, 95% CI (0.26, 1.33), P = .20. Conclusion: the combination of CT and HG are more effective for the treatment of breast cancer than CT alone. |
| Ma et al, 2018, China, Gastrointestinal cancer | 33, 2884, PEDro + NOS | HG+CT | CT: ① Hepatocellular Cancer (n = 22): TACE: 13 trials Others: 4 trials TACE + RT: 2 trials Chemo: 2 trials RT: 1 trial ② Other gastrointestinal Cancers (n = 11): Chemo: 10 trials Others: 2 trials (a multi-arm trial repeated counting) |
Main results: ① Short-term effect: Treatment response: OR = 2.48, 95% CI (1.83, 3.35), P = .027. ② Long-term effect: 6-mo OS: OR = 2.28, 95% CI (1.48, 3.45), P = .926; 1-y OS: OR = 1.76, 95% CI (1.36, 2.29), P = .860; 2-y OS: OR = 2.24, 95% CI (1.23, 4.09), P = .302. ③ Safety: Incidence of leukocyte decrease: OR = 0.35, 95% CI (0.25, 0.51), P = .986; Incidence of platelet decrease: OR = 0.66, 95% CI (0.29, 1.49), P = .976; Incidence of nausea and vomiting: OR = 0.64, 95% CI (0.33, 1.25), P = .009; Incidence of diarrhea: OR = 0.70, 95% CI (0.19, 2.60), P = .018; Incidence of stomachache: OR = 0.47, 95% CI (0.23, 0.97), P = .458; Incidence of debilitation: OR = 0.36, 95% CI (0.19, 0.69), P = .901. Conclusion: HG might improve clinical therapeutic effects and immune functions without increasing side effects. |
Abbreviations: TG, treatment group; CG, control group; HG, Huaier granule; CT, conventional therapy; TACE, transcatheter arterial chemoembolization; Chemo, chemotherapy; RT, radiotherapy; RFA, radiofrequency ablation; PMCT, percutaneous microwave coagulation therapy; MTT, molecular targeted therapy; SR, surgical resection; Others, normal treatment (antiviral, liver protection, and so on); ORR, overall response rate; DCR, disease control rate; OS, overall survival; DFS, disease free survival; OR, odds ratio; RR, risk ratio.
Review Quality Assessment
Methodological quality
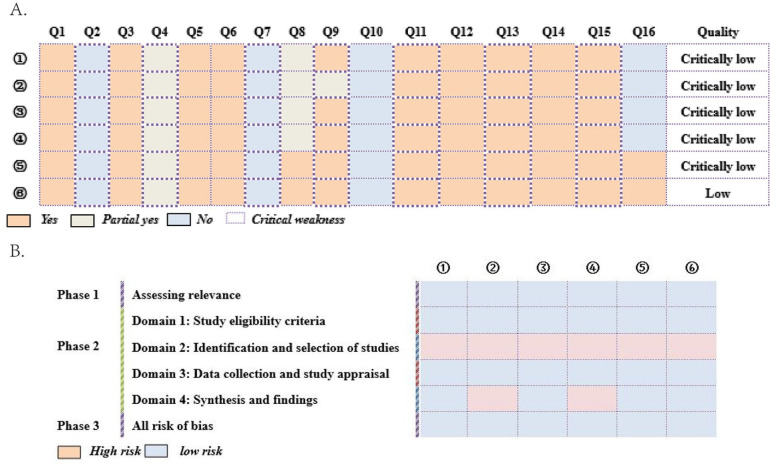
After being evaluated by AMSTAR-2, 5 studies14-18 were rated as critically low quality and 1 study 13 was rated as low quality (Figure 2). Disadvantages for those studies assessed by the AMSTAR-2 were the following: partial SRs/MAs didn’t explicitly state that a protocol was established before conducting the SR/MA; none of SRs/MAs stated that the search strategy had searched for gray literatures; none of SRs/MAs provided the list of excluded studies and described the reasons for exclusions.
Figure 2.
The assessment of AMSTAR-2 (A) and ROBIS (B). ① Hou et al; ② Zhang et al; ③ Zhang et al; ④ Li et al; ⑤ Yao et al; ⑥ Ma et al.
Reporting quality
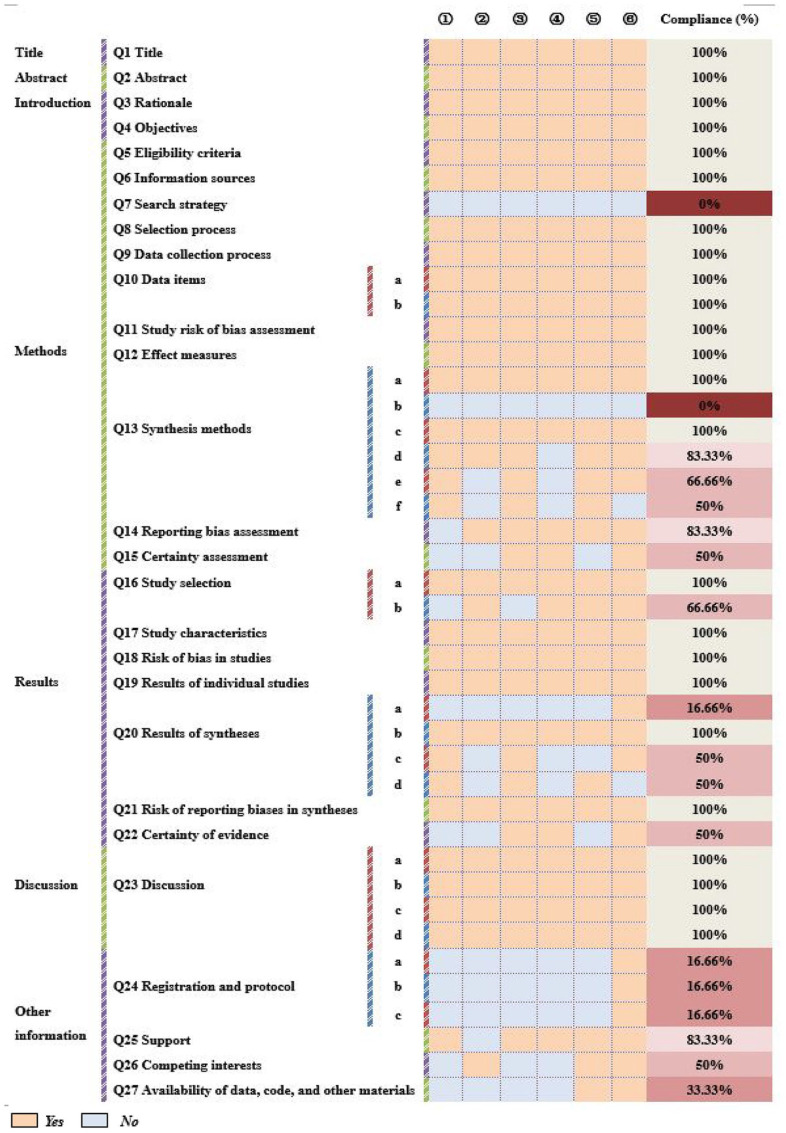
As shown in Figure 3, although title, abstract, introduction, and discussion were completely reported, some reporting defects still were found in other sections. In the section of methods, Q7 (Search strategy) and Q13b (Synthesis methods) were reported deficiently (0%) while Q13f (Synthesis methods) and Q15 (Certainty assessment) were reported inadequately (50%). Besides, Q20a, Q20c, and Q20d in Results of syntheses were reported inadequately (<66.66%). Furthermore, in other sections, the assessment of Q24 (Registration and protocol) and Q27 (Availability of data, code, and other materials) was unsatisfactory (16.66% and 33.33%).
Figure 3.
The assessment of PRISMA. ① Hou et al; ② Zhang et al; ③ Zhang et al; ④ Li et al; ⑤ Yao et al; ⑥ Ma et al.
Risk of bias
Evaluated by ROBIS, items of all SRs/MAs in Phase 1, Domain 1, Domain 3, and Phase 3 were assessed as low risk. On the contrary, items of all SRs/MAs in Domain 2 were assessed as high risk. In Domain 4, items of 4 SRs/MAs13-15,18 were assessed as low risk while items of 2 SRs/MAs16,17 were high risk. More details are shown in Figure 2.
Efficacy and Safety Evaluation With Evidence Quality
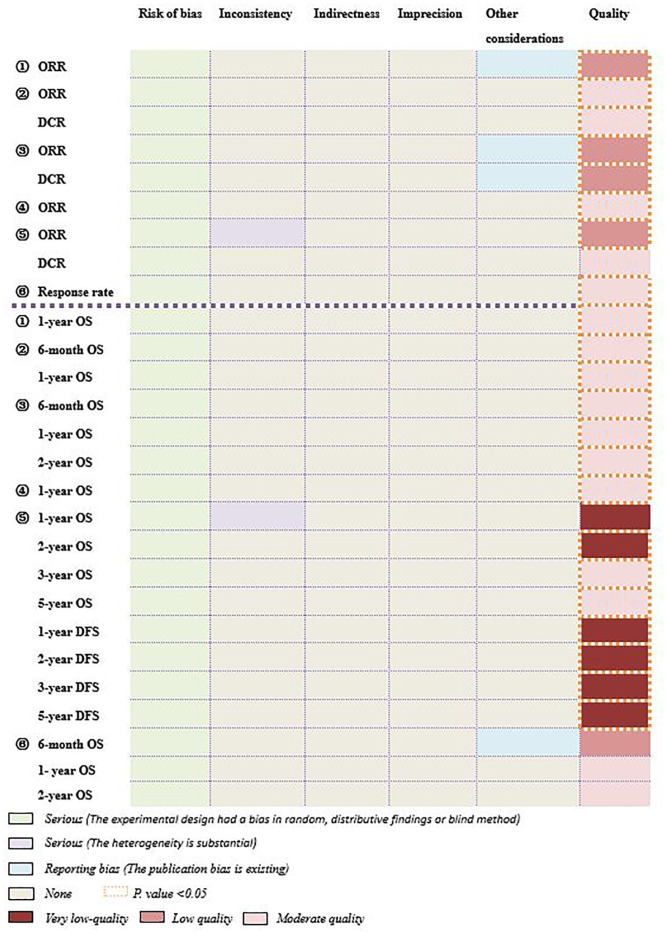
A narrative synthesis was conducted for short-term effect, long-term effect and safety; each outcome measure of these should be assessed by at least 2 SRs/MAs. Summarized in Supplemental Table 1, 46 items were related to the efficacy and safety of HG combined with CT for cancer patients in 6 SRs/MAs. Among these items, 28 items were assessed as moderate quality while 12 items were low-quality and 6 items were very low-quality. Additionally, risk of bias (n = 46) played an important role in downgrading factors and inconsistency (n = 5), publication bias (n = 4), and imprecision (n = 3) were secondary.
Short-term effect evaluation
As shown in Figure 4, short-term effect was mainly reported as ORR and DCR. For ORR, 5 SRs/MAs14-18 shown that HG combined with CT was superior to CT alone (P < .05). For DCR, 2 SRs/MAs15,17suggested that HG combined with CT was significantly better than CT alone (P < .05). Above all, the positive short-term effect was assessed as “moderate quality” in 3 items and “low quality” in 4 items. According to the GRADE statement, 19 although short-term effect of HG plus CT had a favorable trend, further research may change its estimate or reduce our confidence in its estimate.
Figure 4.
The assessment of efficacy by GRADE. ① Hou et al; ② Zhang et al; ③ Zhang et al; ④ Li et al; ⑤ Yao et al; ⑥ Ma et al.
Long-term effect evaluation
As shown in Figure 4, long-term effect was mainly reported as OS. Especially, compared with control group, it was shown that 6-month OS (2 SRs/MAs15,17), 1-year OS (4 SRs/MAs15-18), and 2-year OS (2 SRs/MAs14,15) could be prolonged respectively (P < .05). Besides, the positive long-term effect was assessed as “moderate quality” in 9 items, “low quality” in 1 item, and “very low quality” in 2 items. According to the GRADE statement, 19 although long-term effect of HG plus CT had a favourable trend, further research may influence its estimate or our confidence in its estimate.
Safety evaluation
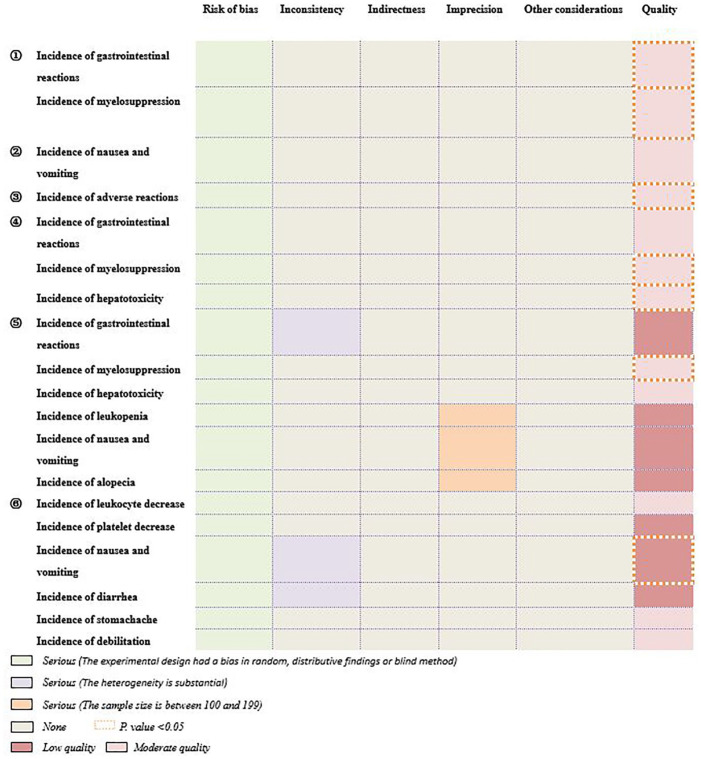
As shown in Figure 5, various adverse reactions were mainly reported to describe the safety, including gastrointestinal reactions, myelosuppression, hepatotoxicity, and nausea and vomiting. When it comes to safety, compared with control group, incidences of gastrointestinal reactions (1 SR/MA 18 ), myelosuppression (3 SRs/MAs14,16,18), hepatotoxicity (1 SR/MA 16 ), and nausea and vomiting (1 SR/MA 13 ) were reported as decreasing respectively (P < .05). In addition, the safety was assessed as “moderate quality” in 5 items and “low quality” in 1 item. According to the GRADE statement, 19 although HG could decrease adverse reactions of CT, further research may influence its estimate or our confidence in its estimate.
Figure 5.
The assessment of safety by GRADE. ① Hou et al; ② Zhang et al; ③ Zhang et al; ④ Li et al; ⑤ Yao et al; ⑥ Ma et al.
Discussion
Research Significance
In 1992, huaier granule was certified for use in cancer treatment by the Chinese State Food and Drug Administration (SFDA). Different from other Chinese patent medicines, Huaier granule, with simple ingredients, is made from Trametes robiniophila Murr. A polysaccharide is the main active anti-tumor and immunomodulatory component. 20 However, non-standardized trials hindered its clinical popularization. Based on the synthesis of RCTs with low quality, SRs/MAs are difficult to provide consistent findings and sound conclusions. Overview is an integrated research strategy for giving clinicians with higher-quality data by reorganizing relevant SRs/MAs. 21 Despite the fact that publication of SRs/MAs about this topic is increasing, there is no published overview to take them together and assess their quality so far. Therefore, an overview about this topic is necessary.
Key Findings From This Overview
6 SRs/MAs were identified from 58 records and their methodological quality, reporting quality and risk of bias were assessed respectively. Efficacy and safety of HG in those SRs/MAs were summarized by narrative synthesis in order to provide some value references for relative research and clinical practice in the future.
Huaier granule has broad-spectrum anticancer effects reflected in cancer types and enhancing efficacy of anticancer therapies. Except for above cancer types, many experimental studies have also shown its anticancer effect in other solid tumors including lung cancer, 22 cervical cancer, 23 renal cancer, 24 prostate cancer, 25 and cholangiocarcinoma 26 by various classic anticancer signaling pathways. 27 In addition, from this overview, we know that HG can enhance the efficacy of many anticancer therapies such as TACE, chemotherapy, radiotherapy, molecular targeted therapy, surgical resection, radiofrequency ablation to varying degrees.
Huaier granule as an adjuvant therapy mainly functions in prolonging survival and reducing recurrence and adverse reaction. In this overview, it was reported that HG mainly prolonged 1-year OS and reduced adverse reactions caused by CT in many SRs/MAs (Supplemental Table). However, recurrence rate has been less reported in those SRs/Mas, so we have not paid more attention to it in this overview. At present, the development of HG clinical research mainly focuses on liver cancer and breast cancer. A multicenter, randomized, controlled, phase IV trial was conducted to demonstrate that prolonging recurrence-free survival and reducing extrahepatic recurrence were the advantages of HG as an adjuvant therapy for patients accepting radical resection of liver cancer. 6 Besides, HG also played an important adjuvant role in thermal ablation 28 and transarterial chemoembolization 29 of liver cancer. For triple-negative breast cancer, a refractory type, HG was shown to prolong disease-free survival and overall survival. 30 When it comes to adverse reactions, it was reported that cisplatin nephrotoxicity could be ameliorated by huaier polysaccharide which was able to decrease oxidative stress and apoptosis. 31 In addition, uncontrolled infection, autoimmune diseases, and metabolic disorders caused by NLRP3 inflammasome unregulated activation for many factors could be inhibited by huaier aqueous extract.32-34
At present, HG as a systemic therapy for advanced hepatocellular carcinoma was written into guidelines with level 1 evidence. 35 However, as an adjuvant therapy, HG has not been included in any guidelines. From this overview, we should perhaps pay more attention to the combination of HG and TACE in liver cancer and take this as a breakthrough point to conduct more high-quality trials and promote the development of HG in clinical adjuvant therapy evidence. Besides, although 6 SRs/MAs included in this overview have reported positive conclusions of HG as an adjuvant therapy for cancer, inadequate evidence grade greatly affects their clinical reliability. Besides, statistical synthesis results of some outcomes were inconsistent, which possibly were ascribed to the differences of cancer type, trial type, method of data synthesis and sample size. From this overview, we also found some deficiencies in trials and SR/MA about HG. On the one hand, the problems of trials mainly focused on registration, allocation sequence concealment, blinding, and normative reporting. On the other hand, registration or protocol, search of gray literature, the list of excluded studies, bias of each synthetic result, and inadequate report of search strategy and synthesis methods seriously affected the quality of SRs/MAs.
Generally speaking, this overview provides a full view of clinical evidence for HG on treating cancer and it may be helpful to instruct clinical practice. Meanwhile, it not only reveals methodological deficiencies but also provides potential directions for the clinical development of HG.
However, considering the comprehensive assessment of those SRs/MAs, it is difficult to make a clear conclusion about HG for cancer, but results imply that it is a promising adjuvant therapy for cancer.
Strengths and Limitations
This is the first evaluation of the efficacy and safety of HG as an adjuvant therapy for cancer through conducting an overview. This overview may provide a certain reference values for future related SRs/MAs design and clinical practice by presenting the disadvantages of SRs/MAs and evidence quality of efficacy and safety clearly. However, unsatisfactory quality and quantity of SRs/MAs have limited us to draw a firm conclusion. Besides, original data of some SRs/MAs were not available, which prevented us from resynthesizing the data to meet the need for research.
Conclusions
Huaier granule may be a promising adjuvant therapy for cancer. However, based on the limited quality and quantity of included studies and unsatisfactory evidence rank of efficacy and safety, it is difficult to draw a definite conclusion. Therefore, it is still a long road to assess the efficacy and safety of Huaier granule as an adjuvant therapy for cancer.
Supplemental Material
Supplemental material, sj-docx-1-ict-10.1177_15347354221083910 for Efficacy and Safety of Huaier Granule as an Adjuvant Therapy for Cancer: An Overview of Systematic Reviews and Meta-Analyses by Jixin Chen, Shuqi Chen, Yushu Zhou, Sumei Wang and Wanyin Wu in Integrative Cancer Therapies
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is supported by grants from the Key project of State Key Laboratory of dampness syndrome of Chinese medicine jointly built by province and Ministry (SZ2021ZZ38), the Chinese Medicine Science and Technology Research Project of Guangdong Provincial Hospital of Chinese Medicine (YN2019MJ09), and the Science and Technology Planning Project of Guangdong Province (2017B030314166).
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2. Fitzmaurice C, Abate D, Abbasi N, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 Cancer Groups, 1990 to 2017: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2019;5:1749-1768. doi: 10.1001/jamaoncol.2019.2996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu J, Wang S, Zhang Y, Fan HT, Lin HS. Traditional Chinese medicine and cancer: history, present situation, and development. Thorac Cancer. 2015;6:561-569. doi: 10.1111/1759-7714.12270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pan J, Yang C, Jiang Z, Huang J. Trametes robiniophila murr: a traditional Chinese medicine with potent anti-tumor effects. Cancer Manag Res. 2019;11:1541-1549. doi: 10.2147/CMAR.S193174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Song X, Li Y, Zhang H, Yang Q. The anticancer effect of Huaier (Review). Oncol Rep. 2015;34:12-21. doi: 10.3892/or.2015.3950 [DOI] [PubMed] [Google Scholar]
- 6. Chen Q, Shu C, Laurence A, Chen XP. FRI-455-effect of Huaier granule on recurrence after curative resection of HCC: a multicenter, randomized clinical trial. Gut. 2018;67:2006-2016. doi: 10.1016/s0618-8278(19)31191-0 [DOI] [PubMed] [Google Scholar]
- 7. Pollock M, Fernandes RM, Becker LA, Featherstone R, Hartling L. What guidance is available for researchers conducting overviews of reviews of healthcare interventions? A scoping review and qualitative metasummary. Syst Rev. 2016;5:190. doi: 10.1186/s13643-016-0367-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1016/j.rec.2021.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Higgins JGS, Collaboration C. Cochrane Handbook for Systematic Reviews for Interventions. The Cochrane Collaboration; 2011. [Google Scholar]
- 10. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Whiting P, Savović J, Higgins JP, et al. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016;69:225-234. doi: 10.1016/j.jclinepi.2015.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pollock A, Farmer SE, Brady MC, et al. An algorithm was developed to assign GRADE levels of evidence to comparisons within systematic reviews. J Clin Epidemiol. 2016;70:106-110. doi: 10.1016/j.jclinepi.2015.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ma Y, Wang C, Zhang Q, Peng X, Feng Y, Meng X. The effects of polysaccharides from Auricularia auricula (Huaier) in adjuvant anti-gastrointestinal cancer therapy: a systematic review and network meta-analysis. Pharmacol Res. 2018;132:80-89. doi: 10.1016/j.phrs.2018.04.010 [DOI] [PubMed] [Google Scholar]
- 14. Yao X, Wu W, Qu K, Xi W. Traditional Chinese biomedical preparation (Huaier granule) for breast cancer: a PRISMA-compliant meta-analysis. Biosci Rep. 2020;40:BSR20202509. doi: 10.1042/BSR20202509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang RR, Shao MY, Fu Y, et al. [Systematic evaluation of Huaier granules adjuvant treatment of primary liver cancer]. Zhongguo Zhong Yao Za Zhi. 2021;46:478-487. doi: 10.19540/j.cnki.cjcmm.20200716.502 [DOI] [PubMed] [Google Scholar]
- 16. Li Li WZ, Caijun L, Zhiyi H, Lisheng P. Meta-analysis on efficacy and safety of Huaier granules assisted with Western medicine in treatment of primary hepatocellular carcinoma. Chin J Inf TCM. 2020;27:103-109. [Google Scholar]
- 17. Zhang Shaohua TY, Yue H, Xingbo M, Shenqiao H, Guo W, Jingdong L. Efficacy of Huaier granule combined with transcatheter arterial chemoembolization in treatment of primary liver cancer: a meta analysis. Chin J Bases Clin Gen Surg. 2020;27:855-860. [Google Scholar]
- 18. Hou Jie SK, Xianbo W. Meta-analysis of the clinical efficacy of huaier granule combined with western medicine treatment in the treatment of primary liver cancer. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2021;31:440-448. [Google Scholar]
- 19. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924-926. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sun Y, Sun T, Wang F, et al. A polysaccharide from the fungi of Huaier exhibits anti-tumor potential and immunomodulatory effects. Carbohydr Polym. 2013;92:577-582. doi: 10.1016/j.carbpol.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 21. Huang J, Shen M, Qin X, Wu M, Liang S, Huang Y. Acupuncture for the treatment of Alzheimer’s Disease: an overview of systematic reviews. Front Aging Neurosci. 2020;12:574023. doi: 10.3389/fnagi.2020.574023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu X, Liu L, Chen K, Sun L, Li W, Zhang S. Huaier shows anti-cancer activities by inhibition of cell growth, migration and energy metabolism in lung cancer through PI3K/AKT/HIF-1α pathway. J Cell Mol Med. 2021;25:2228-2237. doi: 10.1111/jcmm.16215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yan L, Liu X, Yin A, Wei Y, Yang Q, Kong B. Huaier aqueous extract inhibits cervical cancer cell proliferation via JNK/p38 pathway. Int J Oncol. 2015;47:1054-1060. doi: 10.3892/ijo.2015.3094 [DOI] [PubMed] [Google Scholar]
- 24. Wei C, Liu Z, Li L, Zhang Y, Fang Z, Fan Y. The anticancer effect of Huaier extract in renal cancer 786-O Cells. Pharmacology. 2018;102:316-323. doi: 10.1159/000492935 [DOI] [PubMed] [Google Scholar]
- 25. Liu Z, Liu C, Yan K, Liu J, Fang Z, Fan Y. Huaier extract inhibits prostate cancer growth via targeting AR/AR-V7 pathway. Front Oncol. 2021;11:615568. doi: 10.3389/fonc.2021.615568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ji D, Zheng W, Huang P, et al. Huaier restrains cholangiocarcinoma progression in vitro and in vivo through modulating lncRNA TP73-AS1 and inducing oxidative stress. Onco Targets Ther. 2020;13:7819-7837. doi: 10.2147/OTT.S257738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qi T, Dong Y, Gao Z, Xu J. Research progress on the anti-cancer molecular mechanisms of Huaier. Onco Targets Ther. 2020;13:12587-12599. doi: 10.2147/OTT.S281328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang Z, Yu XL, Zhang J, et al. Huaier granule prevents the recurrence of early-stage hepatocellular carcinoma after thermal ablation: a cohort study. J Ethnopharmacol. 2021;281:114539. doi: 10.1016/j.jep.2021.114539 [DOI] [PubMed] [Google Scholar]
- 29. Zhou TY, Tao GF, Chen SQ, et al. Complete response of hepatocellular carcinoma with macroscopic vascular invasion and pulmonary metastasis to the combination of drug-eluting beads transarterial chemoembolization and Huaier granule: a case report. Onco Targets Ther. 2021;14:3873-3880. doi: 10.2147/ott.S309660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang M, Hu Y, Hou L, Pan Q, Tang P, Jiang J. A clinical study on the use of Huaier granules in post-surgical treatment of triple-negative breast cancer. Gland Surg. 2019;8:758-765. doi: 10.21037/gs.2019.12.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fang L, Zhang Y, Wang Q, et al. A polysaccharide from Huaier ameliorates cisplatin nephrotoxicity by decreasing oxidative stress and apoptosis via PI3K/AKT signaling. Int J Biol Macromol. 2019;139:932-943. doi: 10.1016/j.ijbiomac.2019.07.219 [DOI] [PubMed] [Google Scholar]
- 32. Lamkanfi M, Dixit VM. Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol. 2012;28:137-161. doi: 10.1146/annurev-cellbio-101011-155745 [DOI] [PubMed] [Google Scholar]
- 33. Wang L, Yu Z, Wei C, et al. Huaier aqueous extract protects against dextran sulfate sodium-induced experimental colitis in mice by inhibiting NLRP3 inflammasome activation. Oncotarget. 2017;8:32937-32945. doi: 10.18632/oncotarget.16513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16:407-420. doi: 10.1038/nri.2016.58 [DOI] [PubMed] [Google Scholar]
- 35. Zhou J, Sun H, Wang Z, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer. 2020;9:682-720. doi: 10.1159/000509424 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ict-10.1177_15347354221083910 for Efficacy and Safety of Huaier Granule as an Adjuvant Therapy for Cancer: An Overview of Systematic Reviews and Meta-Analyses by Jixin Chen, Shuqi Chen, Yushu Zhou, Sumei Wang and Wanyin Wu in Integrative Cancer Therapies