Abstract
Overexpression of TRIM24 is observed in several human cancers and is correlated with an increase in the progression and metastasis of tumors. In this study, we investigated the changes in activity and biochemical events that occur after overexpression of TRIM24 in a colorectal cancer (CRC) mouse model. We observed upregulated TRIM24 expression in CRC tissues compared to that in nonneoplastic adjacent tissues. Enhanced expression of TRIM24 was significantly associated with the status of lymph nodes and poor recurrence-free survival of patients with CRC. The role of TRIM24 in CRC tumor growth was investigated using an orthotopic model of MC38 mouse colon cancer cells overexpressing TRIM24, and CRC tumor growth was found to increase dramatically by TRIM24 overexpression. Moreover, angiogenesis was stimulated by TRIM24 overexpression via the upregulation of vascular endothelial growth factor (VEGF) expression. Overexpression of TRIM24 in MC38 cells led to an increase in the protein levels of ALDH1 and other stem cell markers. In addition, we observed that Wnt/β-catenin signaling is required for the function of TRIM24 in CRC cells. Tumor-associated macrophages (TAMs) were found to be recruited by tumor cells overexpressing TRIM24 via the increased expression of CCL2/5, CSF-1, and VEGF, further enhancing CRC tumor growth. In conclusion, overexpression of TRIM24 facilitates the growth of CRC and the remodeling of the tumor stroma via angiogenesis stimulation and TAM recruitment. The Wnt/β-catenin pathway is a possible crucial link in the TRIM24-associated progression of tumors, which may provide opportunities for pharmacological intervention.
Keywords: TRIM24, angiogenesis, TAM, CRC, stem-like cells
Introduction
Colorectal cancer (CRC) is a highly devastating cancer of the digestive system; it is the third most commonly diagnosed cancer and the second most deadly cancer [1,2]. Although there has been an increase in screening for CRC, a large number of patients are diagnosed in the advanced stage [3]. The prognosis of CRC is poor due to the absence of molecular biomarkers and early specific symptoms as well as chemotherapy resistance, distant metastasis, and tumor recurrence [4,5]. Therefore, the identification and development of more efficient biomarkers and targets to promote CRC diagnosis and treatment are urgent.
TRIM24, which is also called TIF1α, harbors a motif called TRIM (N-terminal tripartite motif), consists of a B1B2 (B-box type 1 and 2) domain, a RING (E3 ubiquitin ligase) domain, a coiled-coil (BBC) region, and a PHD-Bromo dual epigenetic reader at the C-terminus [6,7]. Unlike other TRIM proteins, the central domain of TRIM24 contains an LxxLL motif that is evolutionarily conserved, in addition to the PHD-Bromo domain, and this motif associates with various ligand-dependent nuclear transcription factors, including AR, at their AF-2 domain [8]. The chromatin localization of TRIM24 is considered to be partly mediated by PHDBROMO, a tandem plant homeodomain finger-bromodomain that can sense H3K23ac and H3K4me0 histone modifications and act as an chromatin-associated epigenetic reader protein [9]. A previous study indicated that TRIM24 is abnormally expressed in numerous tumors [10,11]. Upregulation of TRIM24 expression contributes to prostate cancer progression and is inversely related to the survival of patients with breast cancer [11,12]. Furthermore, TRIM24 promotes the degradation of P53 ubiquitin; therefore, TRIM24 can be used as a therapeutic target to restore the tumor suppressor function of P53 to treat tumors [13]. Additionally, TRIM24 was identified as a novel crucial factor in acute leukemia [14]. Recent research demonstrated that TRIM24 enhances tumor progression by inducing EMT in renal cell carcinoma [15]. However, the functions of TRIM24 in patients with CRC are not well understood.
The two intracellular signaling pathways associated with the Wnt signaling pathway are the noncanonical Wnt pathway, which is a β-catenin-independent pathway, and the canonical Wnt pathway which is the β-catenin-dependent pathway [16,17]. The canonical Wnt/β-catenin pathway, in contrast to other signaling pathways, is highly conserved and primarily involves β-catenin [18]. In the absence of Wnt ligands, cytoplasmic β-catenin is recruited to a complex harboring adenomatous polyposis coli (APC), axin, glycogen synthase kinase 3β, and casein kinase (CK) 1α and is then phosphorylated by GSK3β and CK1α [19,20]. Phosphorylated β-catenin is directly ubiquitinated by an E3 ligase containing a β-transducin repeat, and a low level of β-catenie and phosphorylate LRP5/6 (low-density lipoprotein receptor-related proteins 5/6), which in turn dismantles the destruction complex to enable phosphorylated LRP5/6 to provide a GSK3β- and axin-binding site [22]. After stabilization, β-catenin accumulates in the cytoplasm, which is accompanied by its translocation to the nucleus, where it activates the transcription of downstream genes, including c-Myc, cyclin D1, and matrix metalloproteinase (MMP)-1 and MMP-7, by binding to lymphocyte enhancer factor/T-cell factor (LEF/TCF) of the transcriptional complex [23,24]. A previous study reported that TRIM24 promotes the aggressiveness of gastric cancer via the Wnt/β-catenin signaling pathway [25].
In the current study, the functions of TRIM24 in the progression of mouse and human CRC tumors were examined. We observed that TRIM24 overexpression facilitated the in vitro and in vivo growth of CRC tumors, enhanced cancer stem cell (CSC) characteristics, and upregulated VEGF (vascular endothelial growth factor) expression to consequently stimulate angiogenesis. Furthermore, TRIM24 overexpression caused TAM (tumor-associated macrophage)-recruitment by increasing the expression of VEGF, macrophage-specific CSF-1 (colony stimulating factor), and chemokine CCL2/5 (C-C motif ligand 2/5), thus promoting the growth of CRC tumors.
Materials and methods
Patient samples
This study was approved by the Ethics Committee of The People’s Hospital of China Medical University (The People’s Hospital of Liaoning Province) (20190426). Patients with CRC who underwent surgical resection at The People’s Hospital of China Medical University (The People’s Hospital of Liaoning Province) were enrolled in this study, and written informed consent was obtained from all the patients. During surgery, CRC tissue and matched non-cancerous colorectal tissue samples were obtained. Clinical characteristics, including gender, age, and tumor stage were retrieved from the medical records (Table 2).
Table 2.
Clinical parameters and their correlation with TRIM24 level
| Variables | All cases (n=81; %) | TRIM24 high expression (40) | TRIM24 low expression (41) | P values |
|---|---|---|---|---|
| Gender | ||||
| Male | 53 (65.4) | 25 (62.5) | 28 (68.3) | 0.256 |
| Female | 28 (34.6) | 15 (37.5) | 13 (31.7) | |
| Age (Year) | ||||
| <65 | 32 (39.5) | 14 (35) | 18 (43.9) | 0.134 |
| ≥65 | 49 (60.5) | 26 (65) | 23 (56.1) | |
| Liver metastasis | ||||
| Yes | 16 (19.8) | 14 (35) | 2 (4.9) | 0.035* |
| No | 65 (80.2) | 26 (65) | 39 (95.1) | |
| Depth of invasion | ||||
| T1/T2 | 20 (24.7) | 5 (12.5) | 15 (36.6) | 0.007** |
| T3/T4 | 61 (75.3) | 35 (87.5) | 26 (63.4) | |
| Lymph node status | ||||
| Absent | 38 (47) | 12 (30) | 26 (63.4) | 0.002** |
| Present | 43 (53) | 28 (70) | 15 (36.6) | |
| Location | ||||
| Rectum | 30 (37) | 14 (35) | 16 (39) | 0.15 |
| Colon | 51 (63) | 26 (65) | 25 (61) | |
| TNM stage | ||||
| I-II | 35 (43.2) | 11 (27.5) | 24 (58.5) | 0.022* |
| III-IV | 46 (56.8) | 29 (72.5) | 17 (41.5) |
n (%).
P<0.05;
P<0.01.
Cell culture
Human colorectal cancer cell lines (LS180, HCT-8, Lim1215, DiFi, Lim2405, and DLD1 cells) and murine colon adenocarcinoma MC38 cells were obtained from ATCC (Manassas, USA). All the cell lines were grown in DMEM (MD, USA) containing 10% FBS (fetal bovine serum, Sigma). The normal colonic epithelial cell line NCM356 was obtained from INCELL Corporation LLC (San Antonio, USA) and grown in M3 media with 5% FBS. Each cell was grown in an appropriately humidified atmosphere with 5% CO2 at 37°C. IWP-2 (Selleckchem, Houston, USA) was diluted in DMSO (Sigma-Aldrich, St. Louis, USA).
Knockdown and overexpression of TRIM24
Mouse TRIM24 cDNA was cloned into the pcDNA3.1 plasmid (V79020, ThermoFisher Scientific, USA) to generate pcDNA3.1-mTRIM24. Stable transfection of MC38 cells with pcDNA3.1 vector or pcDNA3.1-mTRIM24 was performed with Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s instructions. Stable MC38-V and MC38-TRIM24 cell lines were selected in medium supplemented with 300 µg/mL Zeocin (Invitrogen, Carlsbad, USA). Transfection of the HCT-8 cell line with pcDNA3.1-TRIM24 or pcDNA3.1 was performed using FuGENE 6 (Promega, Madison, WI). The LS180 cell line was transfected with green fluorescent protein-tagged constructs of lentivirus (pGIPZ) carrying scrambled shRNA (sh con, 5’-CTCGCTTGGGCGAGAGTAAG-3’) or TRIM24-targeting shRNA (sh TRIM24, 5’-ATTGTTTAGAGAGTCCAGC-3’) (Dharmacon, Lafayette, USA) and the packaging plasmids pCMV-VSV-G (#8454) and pCMV-Δ8.2 (#8455, Addgene, Cambridge, USA) with Lipofectamine 2000. Sixty hours post-transfection, viral supernatants were collected, and CRC cells were immediately infected in the presence of polybrene (10 μg/mL), Sigma-Aldrich (St. Louis, USA). Scrambled RNA- or TRIM24 shRNA-expressing cells were selected with puromycin (5 μg/mL). Then the cells were sorted and the top 10-20% GFP-positive cells were collected.
MTT assay
For cell proliferation, the MTT assay was conducted as previously described [26,27]. In brief, 5×103 cells were seeded in each well of 96-well plates and maintained in medium with 10% FBS. The viability of the cells was assessed by adding MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) solution. The color intensity in each plate was measured at 560 nm on a microplate reader. To assess anchorage-dependent colony formation, the indicated cells were seeded in 6-well plates and then incubated for 14 days. The colonies were fixed in methanol and stained with 0.1% crystal violet (Sigma, St. Louis, USA) before counting.
Western blotting
Western blotting was carried out as previously described [28,29]. In brief, 30 μg of cell lysate or tumor tissue homogenate was mixed with Laemmli buffer from BioRad (Hercules, USA) and loaded into Precast Mini-PRTOEAN® TGX™ Gels from BioRad (Hercules, USA). After electrophoresis, the proteins were transferred to PVDF membranes, and immunoblotting analysis was conducted using primary antibodies against ALDH1, TRIM24, active β-catenin, β-catenin, KLF4, Nanog, ABCG2, c-Myc, cyclin D1, Sox2, E-cadherin, vimentin, LOX, and ZO-1 (all from Cell Signaling Technology, MA, USA), VEGF, MMP2, MMP9, and MMP7 (Abcam, Cambridge, USA), CSF-1, CCL2, β-actin, and CCL5 (Santa Cruz Biotechnology, Dallas, USA). Then, the membranes were incubated with peroxidase-conjugated anti-rabbit/mouse IgG (Cell Signaling Technology, Danvers, USA) for 60 min at room temperature, and the protein bands were detected using ECL. The uncropped western blots were provided in Figures S3 and S4.
Assay for the formation of in vitro tumorsphere
The indicated cells (1×104/well) were plated in ultra-low attachment 6-well plates (Corning, USA) in 2 mL of serum-free DMEM/F12 medium supplemented with EGF (20 ng/mL), bFGF (20 ng/mL), and B27 (2%). The cells were grown at 37°C in 5% CO2 for 10 days. Then, the number of tumorspheres in each well was manually counted.
Cell invasion and migration assays
For the cell migration assay, a 24-well plate containing 8-μm (pore size) polycarbonate membrane inserts (BD Biosciences) was used. A total of 4×104 cells were added to the upper chamber of each well and the lower chamber contained medium supplemented with 2.5% fetal calf serum; the plates were incubated at 37°C for 24 hours. Cell invasion was analyzed by a Transwell chamber invasion assay (BD Biosciences). A total of 1×104 cells were seeded into the upper chamber in serum-free medium and allowed to invade the lower chamber, which contained 15% fetal calf serum as a chemical attractant. After 24 hours, the cells that invaded through the Matrigel matrix and adhered to the underside of the membrane were fixed in 4% paraformaldehyde for 15 min and stained with crystal violet for 30 min. All the cells were counted under a microscope at 200× magnification. This experiment was repeated 3 times, each with duplicate samples.
Gene expression analysis by real-time quantitative PCR (RT-qPCR)
Total RNA was isolated from cells using an Agilent’s Total RNA Isolation Mini Kit (Wilmington, USA). One microgram of total RNA was used for reverse transcription with an iScript cDNA Synthesis Kit (Bio-Rad), and RT-qPCR was conducted using iQ SYBR Green Supermix (Bio-Rad) in a final reaction volume of 20 μL, which included gene-specific primer/probe sets. The reaction was conducted with a standard thermal cycling procedure (35 cycles) in a Bio-Rad CFX96TM real-time PCR system. The conditions for the PCRs were as follows: 5 min at 95°C followed by 35 cycles of 30 s at 95°C, 1 min at 60°C and 1 min at 72°C. The results are expressed as the threshold cycles (Ct). The relative quantification of the target transcripts was determined by the comparative Ct method (ΔΔCt) according to the manufacturer’s protocol. The 2-ΔΔCt method was used to analyze the relative changes in gene expression. The internal control was β-actin. Each sample was examined in triplicate. Primers used in this study are list in Table 3.
Table 3.
Primers sequences
| Genes | Sequences | |
|---|---|---|
| VEGF | Forward | 5’-GTACCTCCACCATGCCAAGT-3’ |
| Reverse | 5’-AATAGCTGCGCTGGTAGACG-3’ | |
| TRIM24 | Forward | 5’-CATATGCAGCAACAGCAACCG-3’ |
| Reverse | 5’-GAAAGCCATCTGTAGGGGGT-3’ | |
| β-catenin | Forward | 5’-GGATCAAACCTGACAGCCA-3’ |
| Reverse | 5’-GAAAACGCCATCACCACGTC-3’ | |
| cyclin D1 | Forward | 5’-GATGCCAACCTCCTCAACGA-3’ |
| Reverse | 5’-ACTTCTGTTCCTCGCAGACC-3’ | |
| c-Myc | Forward | 5’-CCCTCCACTCGGAAGGACTA-3’ |
| Reverse | 5’-GCGGTGCATTTTCGGTTGT-3’ | |
| ALDH1 | Forward | 5’-AGCCTTCACAGGATCAACAGA-3’ |
| Reverse | 5’-GTCGGCATCAGCTAACACAA-3’ | |
| Sox2 | Forward | 5’-CGAGTGGAAACTTTGTCGGA-3’ |
| Reverse | 5’-TGTGCAGCGCTCGCAG-3’ | |
| ABCG2 | Forward | 5’-ACCTGAAGGCATTTACTGAA-3’ |
| Reverse | 5’-TCTTTCCTTGCAGCTAAGAC-3’ | |
| Nanog | Forward | 5’-AAAGGATGAAGTGCAAGCGGTGG-3’ |
| Reverse | 5’-CTGGCTTTGCCCTGACTTTT-3’ | |
| KLF4 | Forward | 5’-TACCAAGAGCTCATGCCACC-3’ |
| Reverse | 5’-GCGAATTTCCATCCACAGCC-3’ | |
| MMP7 | Forward | 5’-CAGGAAGCCGGAGAAGTGAC-3’ |
| Reverse | 5’-TCTCCGGCAAACCGAAGAAC-3’ | |
| CXCL1 | Forward | 5’-ACTGCACCCAAACCGAAGTC-3’ |
| Reverse | 5’-TGGGGACACCTTTTAGCATCTT-3’ | |
| CSF-1 | Forward | 5’-ACCCCTCCACCCTCTCTG-3’ |
| Reverse | 5’-CTGCCCCTTCACTTGCTG-3’ | |
| CSF-2 | Forward | 5’-TCGTCTCTAACGAGTTCTCCTT-3’ |
| Reverse | 5’-CGTAGACCCTGCTCGAATATCT-3’ | |
| CSF-3 | Forward | 5’-GCTGCTTGAGCCAACTCCATA-3’ |
| Reverse | 5’-GAACGCGGTACGACACCTC-3’ | |
| CCL2 | Forward | 5’-AGGTCCCTGTCATGCTTCTG-3’ |
| Reverse | 5’-TCTGGACCCGTTCCTTCTTG-3’ | |
| CCL5 | Forward | 5’-ACTCCCTGCTGCTTTGCCTAC-3’ |
| Reverse | 5’-GAGGTTCCTTCGAGTGACA-3’ | |
| β-actin | Forward | 5’-GCCGCCAGCTCACCATGGAT-3’ |
| Reverse | 5’-TGGGCCTCGTCGCCCACATA-3’ | |
Mouse model
All the animal experiments were approved by and performed following the instructions of the committee of China Medical University (20190426). An orthotopic CRC mouse model was established as previously described [30,31]. In brief, C57BL/6 mice were anesthetized by inhaling oxygen containing 2% isoflurane. MC38-TRIM24 cells or MC38-V cells were injected into the sub-serosa of the cecum of the C57BL/6 mice (8-week-old) to establish the mouse orthotopic model. The mice were sacrificed by CO2 at the end of the study, and the tumor tissues were incubated in paraformaldehyde (4%) before being embedded in paraffin for immunohistochemical and histological analyses.
Immunohistochemical (IHC) staining
Tumor sections were processed as previously described [32,33]. Non-specific epitopes were blocked with normal horse serum for 60 min. The samples were incubated overnight with antibodies at 4°C. For IHC staining, tumor sections were incubated with specific secondary antibodies conjugated to HRP (Bio-Rad, CA, USA) for 60 min at room temperature. The antigen signals were detected using the DAB (2-Solution Diaminobenzidine) Kit (Invitrogen, Carlsbad, USA), and the samples were counterstained using hematoxylin and mounted on Acrymount from StatLab (TX, USA). The samples were visualized with a fluorescence microscope (Olympus×81).
Statistical analysis
The statistical analyses of the data were carried out with GraphPad Prism. The data are presented as the means ± standard deviations. Student’s t test was used to identify the significance of differences between two groups, and one-way analysis of variance (ANOVA) with Fisher’s LSD post-hoc tests was used for multiple groups. The Kaplan-Meier method was used to analyze survival data, and the significance was examined using the log-rank test. Data collected from a minimum of three independent experiments were used for statistical analyses, and P<0.05 indicated statistically significant differences.
Results
Increased expression of TRIM24 in CRC tissues and its correlation with clinicopathological features
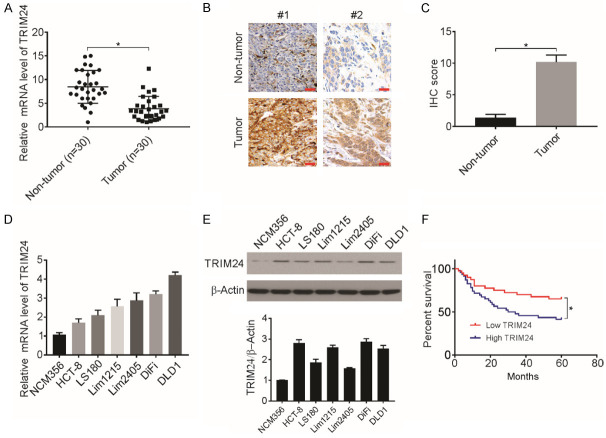
Previous studies have suggested an important role of TRIM24 in the progression of multiple cancers [10,34,35]; however, little information is available on the function of TRIM24 in CRC cells. To address this question, RT-qPCR analysis was carried out to assess TRIM24 mRNA expression in 30 pairs of CRC tissues and adjacent healthy tissues. The CRC tissues exhibited significantly upregulated TRIM24 expression (Figure 1A). Then, TRIM24 expression in 25 CRC tissues and 25 adjacent healthy tissues was detected using IHC staining, and significant upregulation of the TRIM24 protein levels was observed in the CRC tissues (Figure 1B and 1C; Table 1). Western blotting and RT-qPCR assays revealed upregulated expression of TRIM24 in the CRC cell lines compared to the NCM356 cell line, a normal colonic epithelial cell line (Figure 1D and 1E).
Figure 1.
TRIM24 expression in CRC tissues predicts poor prognosis. A. Relative TRIM24 mRNA expression level in 30 paired CRC tissues measured by RT-qPCR. *P<0.05. B and C. Representative images and score of IHC staining of TRIM24 in CRC and adjacent normal mucosal tissues. *P<0.05, n=25. Scale bar: 50 μm. D. Relative mRNA level of TRIM24 was analyzed by RT-qPCR. E. Protein level of TRIM24 in indicated cell lines was analyzed by western blotting and normalized to β-actin. F. Kaplan-Meier analysis of overall survival for patients with CRC (*P<0.05).
Table 1.
IHC staining of TRIM24 in colorectal cancer and adjacent normal tissues
| Low expression | High expression | P | |
|---|---|---|---|
| Tumor tissues (n=25) | 3 (12%) | 22 (88%) | P<0.001 |
| Normal tissues (n=25) | 21 (84%) | 4 (16%) |
Then, the association of TRIM24 expression with CRC tissues clinicalpathological factors was analyzed. Based on the average TRIM24 levels, the patients were categorized into two groups, and TRIM24 expression and its association with clinicopathological features were analyzed. Increased levels of TRIM24 were associated with increased lymph node metastasis, deeper invasion, advanced tumor stage, and increased liver metastasis (Table 2). However, there was no significant association of TRIM24 expression with age, sex, or tumor location. The Kaplan-Meier curves analyzed the impact of TRIM24 expression on CRC patient OS (overall survival) over a median follow-up time of five years, and enhanced TRIM24 mRNA expression was associated with poorer CRC patient prognosis (Figure 1F). Therefore, these results strongly indicate the important function of TRIM24 in CRC development and progression.
Overexpression of TRIM24 promotes the growth, migration, and invasion of CRC cells in vitro
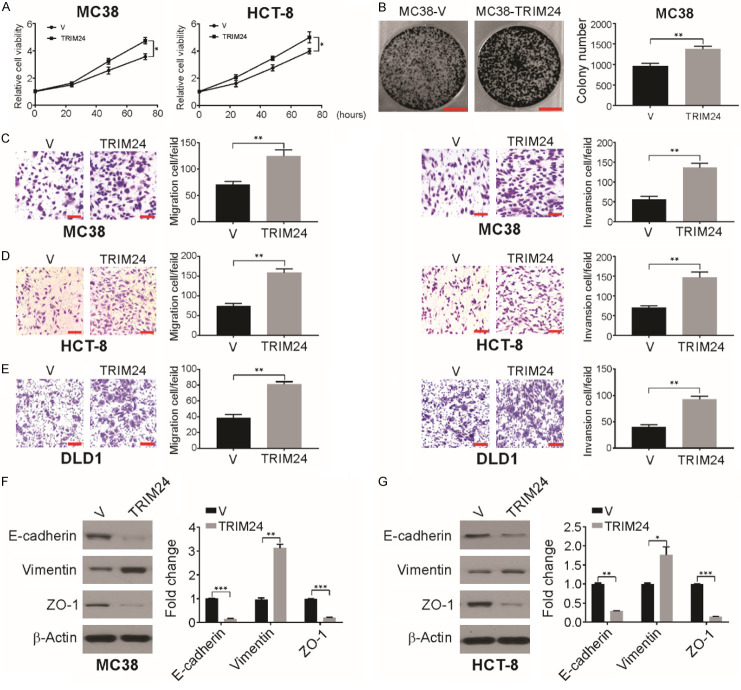
We then examined CRC progression when TRIM24 was overexpressed and observed slightly enhanced growth of HCT-8 and MC38 cells in vitro (Figure 2A). Further colony formation assays confirmed that TRIM24 overexpression promoted tumor growth (Figures 2B and S1A). Moreover, MC38-TRIM24 cells exhibited enhanced cell migration and invasion through Matrigel compared to MC38-V cells (Figure 2C), and TRIM24 significantly enhanced the migration and invasion of HCT-8 cells (Figure 2D) and DLD1 cells (Figure 2E). In contrast, TRIM24 knockdown in LS180 and HCT-8 cells caused a decrease in cell invasion and migration (Figure S1B and S1C). Thus, in vitro overexpression of TRIM24 increases CRC cell invasion and migration.
Figure 2.
TRIM24 promotes migration and invasion in CRC cells. A. Relative cell viability was analyzed by MTT in the indicated cells transfected with vector or TRIM24. B. Colony forming abilities of MC38 cells were analyzed following TRIM24 overxepression. Scale bar: 1 cm. C. Cell migration and invasion were determined in MC38-V and MC38-TRIM24 using Matrigel transwell assay. Scale bar: 50 μm. D. Cell migration and invasion were determined in HCT-8 cells transited with vector or TRIM24 using Matrigel transwell assay. Scale bar: 50 μm. E. Cell migration and invasion were determined in DLD1 cells transfected with vector or TRIM24 using Matrigel transwell assay. Scale bar: 50 μm. F. The indicated protein level in MC38-V and MC38-TRIM24 cells was detected by western blotting and normalized to β-actin. G. The indicated protein level in HCT-8 cells transfected with vector or TRIM24 was detected by western blotting and normalized to β-actin. Data are presented as the mean ± SD from three independent experiments. *P<0.05; **P<0.01; ***P<0.001.
Epithelial-mesenchymal transition (EMT) enhances the migratory and invasive capacities of epithelial cells and is considered a crucial step in the tumor progression [36,37]. We observed reduced expression of ZO-1 and E-cadherin and enhanced expression of vimentin due to TRIM24 overexpression in MC38 cells (Figure 2F). Similarly, TRIM24 overexpression in human CRC HCT-8 cells significantly reduced the expression of ZO-1 and E-cadherin (Figure 2G). shRNA-mediated TRIM24 knockdown in LS180 and HCT-8 cells notably increased the expression of E-cadherin (Figure S1D and S1E). Taken together, the above data indicate that TRIM24 promotes EMT in CRC cells.
TRIM24 overexpression in vivo causes an increase in CRC tumor growth and stimulates angiogenesis
We then used a surgical orthotopic mouse model to assess the effect of TRIM24 on tumor characteristics. MC38-V cells (MC38 vector control cells) and MC38-TRIM24 cells (MC38 cells with high TRIM24 expression) were injected into C57BL/6 mice. As shown in Figure 3A and 3B, the MC38-V group had tumors of higher weight than the MC38-TRIM24 group 21 days after injection. Therefore, a high level of TRIM24 expression in MC38 cells causes a significant increase in tumor growth.
Figure 3.
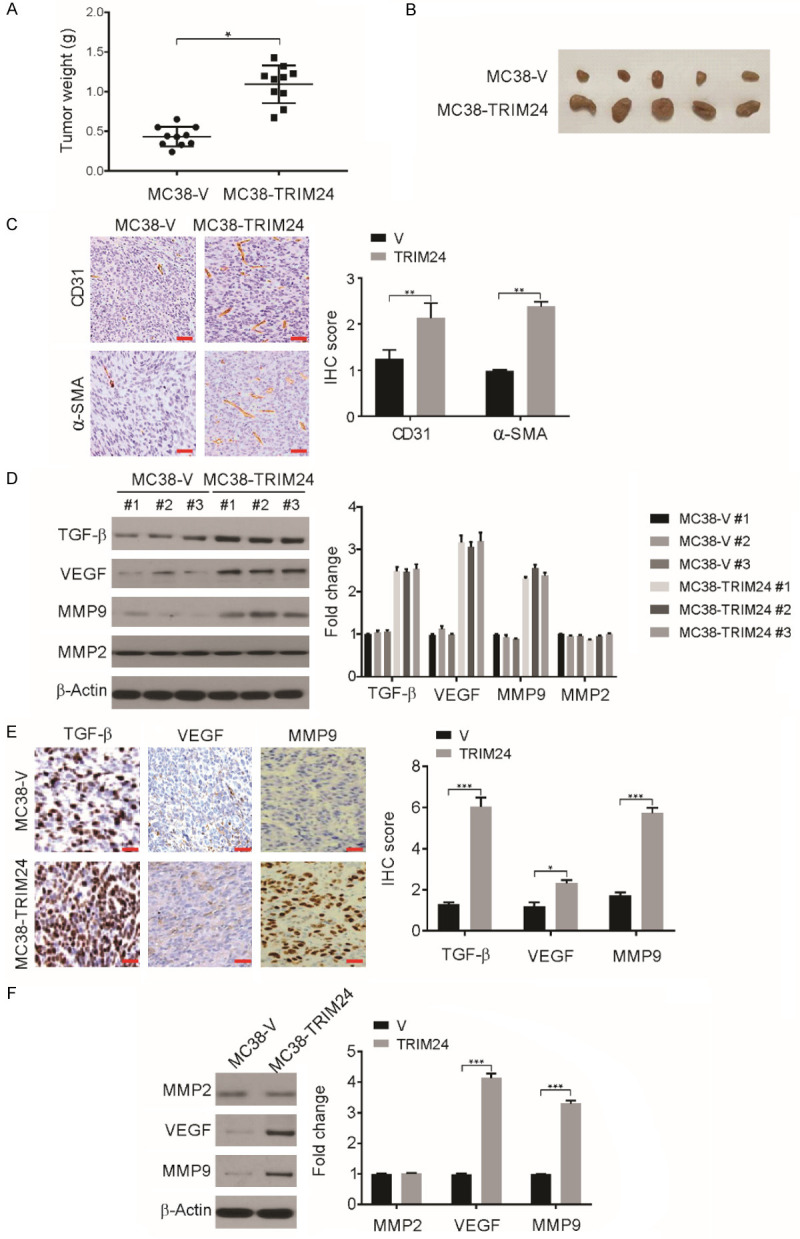
TRIM24 overexpression increases CRC tumor growth in vivo. A. MC38-TRIM24 or MC38-V was orthotopically injected into the cecum subserosa of mice and mice were killed after 6 weeks. The weight of tumors in mice was shown. *P<0.05. B. Representative tumors at the end of the experiment. C. Representative images of CD31 and α-SMA staining in MC38-V and MC38-TRIM24 tumors. Scale bar: 50 μm. n=6. D. The expression of VEGF, MMP9, MMP2 and TGF-β in MC38-V and MC38-TRIM24 tumors was analyzed by western blotting and normalized to β-actin. E. The expression of MMP9, VEGF and TGF-β in MC38-TRIM24 and MC38-V tumors was analyzed by immunohistochemistry. Scale bar: 50 μm. n=6. F. The expression of VEGF, MMP9 and MMP2 in MC38-V and MC38-TRIM24 cells was analyzed by western blotting and normalized to β-actin. Data are presented as the mean ± SD from three independent experiments. *P<0.05; **P<0.01; ***P<0.001.
Angiogenesis is a crucial factor in the progression of cancer [38]. The presence of pericytes in blood vessels and vascular endothelial cells was demonstrated by α-SMA and CD31 staining. Tumors derived from MC38-TRIM24 cells had a greater number of α-SMA- and CD31-positive cells than tumors derived from MC38-V cells (Figure 3C), clearly indicating that high expression of TRIM24 enhances angiogenesis in tumors. Various proteins have been indicated to be angiogenic activators, including VEGF, angiogenin, basic fibroblast growth factor, transforming growth factor-α, TGF-β, tumor necrosis factor-α, granulocyte colony-stimulating factor, platelet-derived endothelial growth factor, interleukin-8, placental growth factor, epidermal growth factor, and hepatocyte growth factor [39-41]. We then examined the levels of these proteins in MC38-V and MC38-TRIM24 tumors. The levels of MMP9, TGF-β, and VEGF were increased in MC38-TRIM24 tumors relative to those of MC38-V tumors while the level of MMP2 remained unchanged (Figure 3D). A similar result was observed by IHC analysis of MC38-TRIM24 cell-derived tumors compared to MC38-V cell-derived tumors (Figure 3E). Consistent with these findings, the in vitro examination revealed that compared with MC38-V cells, MC38-TRIM24 cells had increased levels of VEGF and MMP9 but not MMP2 (Figure 3F). These findings indicate that the stimulation of angiogenesis was mediated by the high expression of TRIM24 in CRC tumors via increased levels of MMP9, TGF-β, and VEGF.
TRIM24 enhances cancer stem cell characteristics in CRC
We examined the characteristics of the MC38-TRIM24 cell-derived or MC38-V cell-derived tumors from the cecum. We found a dramatic increase in the protein and mRNA levels of KLF4, ALDH1, ABCG2, c-Myc, and Nanog in MC38-TRIM24 cell-derived tumors (Figure 4A and 4B). Then, the tumorsphere-forming ability of MC38-TRIM24 and MC38-V cells was examined. The number of tumorspheres was higher in the MC38-TRIM24 cells than that in MC38-V cells (Figure 4C). Furthermore, tumorsphere formation and cell proliferation were examined in TRIM24 knockdown or TRIM24 overexpressing human CRC cells, and a slight increase in tumorsphere formation was observed in TRIM24 overexpressing HCT-8 cells (Figure 4C). However, shRNA-mediated TRIM24 knockdown in LS180 cells reduced the formation of tumorspheres (Figure 4C). These findings suggest an increase in the CSC-like population due to TRIM24 overexpression in CRC cells.
Figure 4.
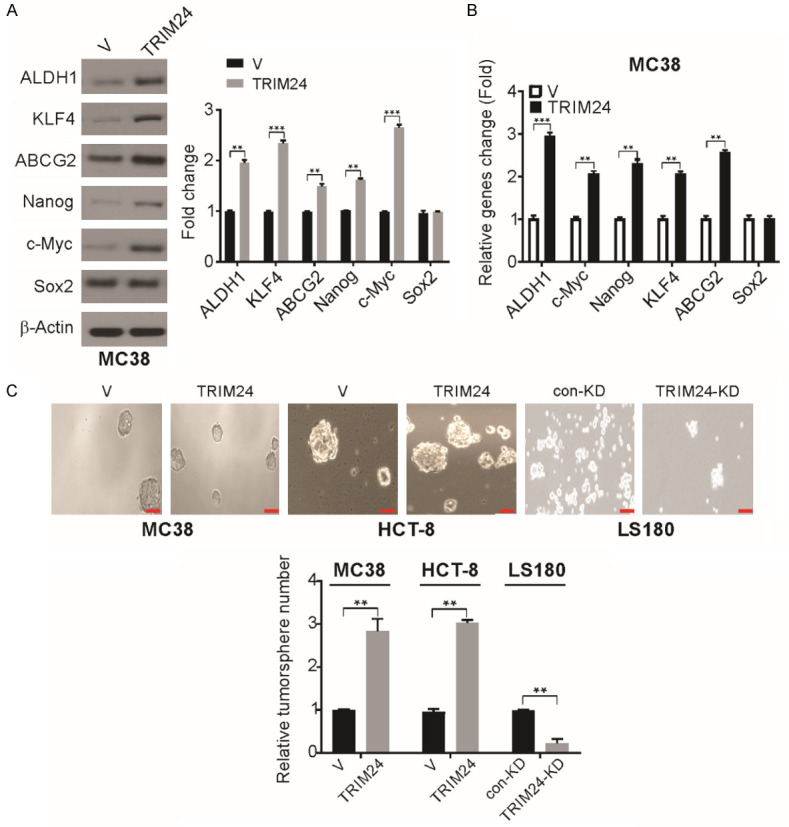
TRIM24 overexpression increases CSC characteristics in CRC cells. A. The indicated protein level in MC38-V and MC38-TRIM24 tumors was detected by Western blotting and normalized to β-actin. B. The indicated mRNA level was detected by real-time PCR in MC38-V and MC38-TRIM24 tumors. C. Tumorsphere formation ability in indicated cells. **P<0.01; ***P<0.001. Scale bar: 50 μm.
A high level of TRIM24 expression leads to Wnt/β-catenin signaling pathway activation
By investigating the signaling pathways that are activated in cells overexpressing TRIM24, we found a considerable increase in the level of β-catenin in MC38-TRIM24 cells compared to that in MC38-V cells (Figure 5A). The molecular changes related to TRIM24 overexpression in human CRC cell lines were then examined, and TRIM24 knockdown in LS180 cells clearly decreased the mRNA and protein levels of cyclin D1, β-catenin, MMP7, and c-Myc and increased the mRNA and protein levels of c-Myc, MMP7, β-catenin, and cyclin D1 in HCT-8 cells (Figure 5B and 5C). These observations indicate the possible regulation of the Wnt/β-catenin signaling pathway by TRIM24. To further confirm this regulation, we assessed the level of active β-catenin and observed a significant increase in HCT-8 cells with high TRIM24 expression and a decreased level in LS180 cells with no TRIM24 expression (Figure 5D). A TOP/FOP Flash assay, an established dual-luciferase reporter assay for TCF/β-catenin was used to assess the role of TRIM24 in β-catenin signaling [42]. TCF-responsive sites are present on the TOP Flash reporter, and mutant TCF binding sites are present on the FOP Flash reporter and serve as negative controls. We observed a significant reduction in TOP Flash luciferase activity after the knockdown of TRIM24 expression, while high expression of TRIM24 facilitated TCF reporter activation (Figure 5E-G). Therefore, TRIM24 positively mediates Wnt/β-catenin activity, which is a crucial function in MC38 cells and human CRC cells.
Figure 5.
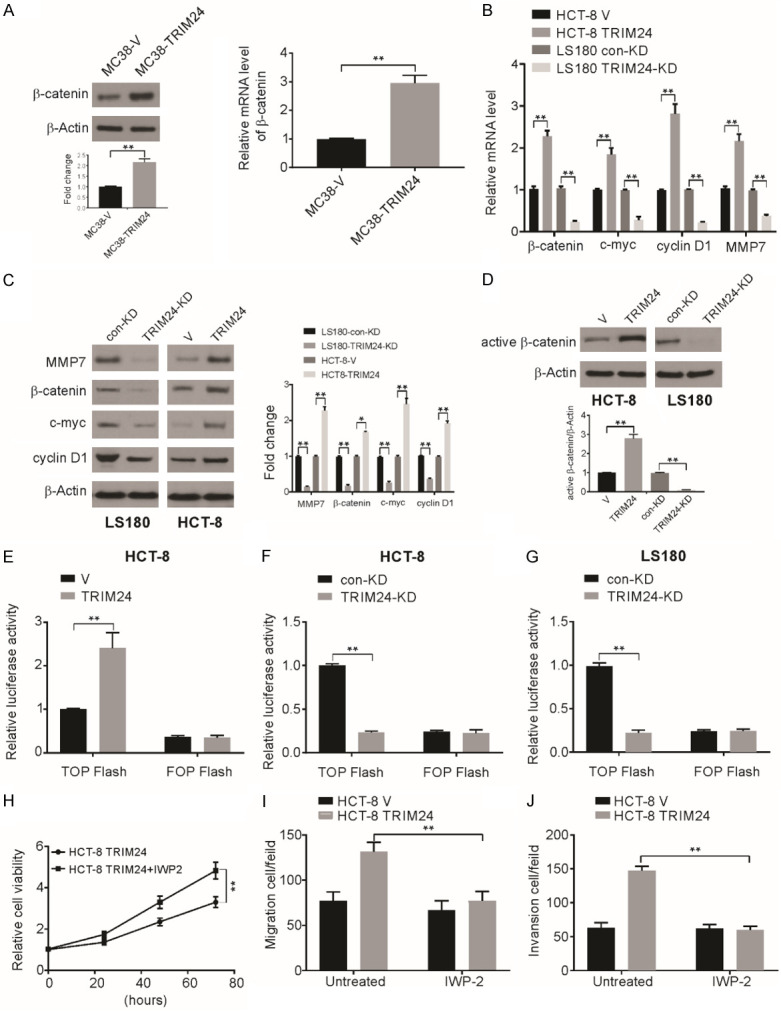
TRIM24 actives Wnt/β-catenin signaling pathway in CRC cell lines. (A) The indicated mRNA and protein levels were detected in MC38-V and MC38-TRIM24 cells. (B) The indicated mRNA level was detected in MC38-V, MC38-TRIM24, LS180 con-KD and TRIM24-KD cells. (C) The indicated protein level in MC38-V, MC38-TRIM24, LS180 con-KD and TRIM24-KD cells was detected by western blotting and normalized to β-actin. (D) The level of active β-catenin in MC38-V, MC38-TRIM24, LS180 con-KD and TRIM24-KD cells was detected by western blotting and normalized to β-actin. (E) The activity of TCF/β-catenin reporter (TOP/FOP Flash) in MC38-V and MC38-TRIM24 cells. (F) The activity of TCF/β-catenin reporter (TOP/FOP Flash) in HCT-8 con-KD and TRIM24-KD cells. (G) The activity of TCF/β-catenin reporter (TOP/FOP Flash) in LS180 con-KD and TRIM24-KD cells. (H-J) Effects of IWP-2 on TRIM24-enhanced cell proliferation (G), migration (H) and invasion (I) in HCT-8 cells. Data are presented as the mean ± SD from three independent experiments. *P<0.05; **P<0.01.
Then, TRIM24-overexpressing CRC cells were treated with an inhibitor of Wnt/β-catenin signaling, IWP-2. We observed significantly inhibited cell growth after treatment TRIM24-overexpressing cells with IWP-2 (Figure 5H). The Transwell assay also revealed significant reversal of the TRIM24-enhanced cell invasion and migration capacities due to Wnt/β-catenin pathway inhibition (Figure 5I and 5J).
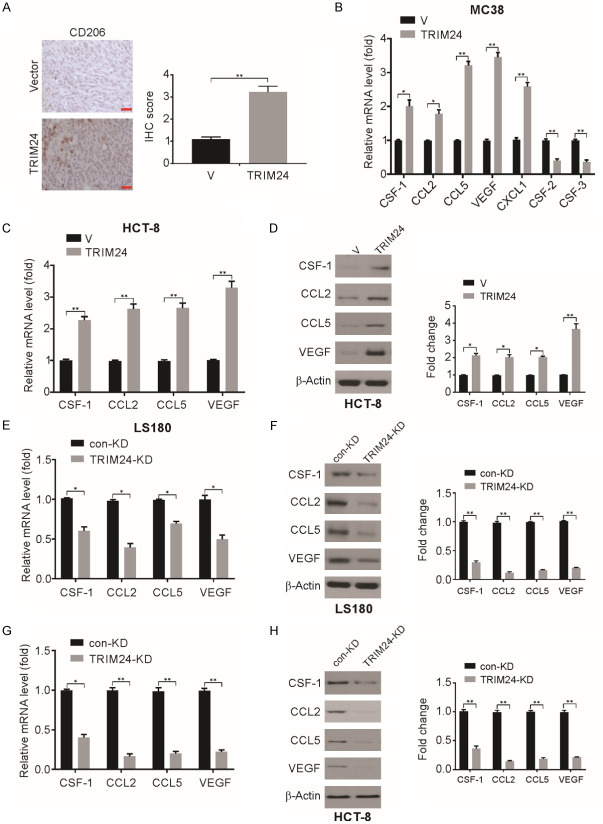
High expression of TRIM24 in MC38 cells increases TAM recruitment
Next, the tumor microenvironment and its effect on TRIM24-induced tumor progression were examined. The tumor microenvironment, which is also known as the tumor-associated stroma, includes cells that are non-neoplastic, including infiltrating immune cells, fibroblasts, structural components, and endothelial cells [43,44]. In CRC, cytokines, tumor-infiltrating immune cells, and other immune mediators participate in the progression of tumorigenesis in the colon, including initiation, promotion, progression, and metastasis [45]. Thus, the association between TRIM24 overexpression and systemic inflammation was investigated in an orthotopic model of CRC. Significantly higher infiltration of CD206+ cells was observed in MC38-TRIM24 cell-derived tumors than in MC38-V cell-derived tumors (Figure 6A). Therefore, the high expression of TRIM24 in MC38 cells recruits TAMs.
Figure 6.
TRIM24 overexpression recruited macrophages to tumors. A. CD206 staining in MC38-V and MC38-TRIM24 tumors was analyzed by IHC. Scale bar: 50 μm. B. mRNA expression of indicated genes was determined by RT-qPCR in MC38-V and MC38-TRIM24 cells. C. mRNA expression of CCL2, CCL5 and CSF-1 in HCT-8-V and HCT-8-TRIM24 cells. D. Protein level of VEGF, CCL2, CCL5 and CSF-1 in HCT-8-V and HCT-8-TRIM24 cells was analyzed by western blotting and normalized to β-actin. E. mRNA expression of VEGF, CSF-1, CCL2 and CCL5 was determined by RT-qPCR in LS180 con-KD and TRIM24-KD cells. F. Protein level of CCL2, CCL5 and CSF-1 in LS180 con-KD and TRIM24-KD cells was analyzed by western blotting and normalized to β-actin. G. mRNA expression of VEGF, CSF-1, CCL2 and CCL5 was determined by RT-qPCR in HCT-8 con-KD and TRIM24-KD cells. H. Protein level of CCL2, CCL5 and CSF-1 in HCT-8 con-KD and TRIM24-KD cells was analyzed by western blotting and normalized to β-actin. Data are presented as the mean ± SD from three independent experiments. *P<0.05; **P<0.01.
TRIM24 enhances CCL2/5 and CSF-1 expression for TAM recruitment
TAMs exert pro-tumor functions by secreting chemokines, cytokines, and growth factors and promoting angiogenesis and tissue remodeling in lieu of cytotoxic activity [46,47]. Recruitment of TAMs to CRC tumor sites may occur via VEGF, CCL2, CCL5, and CSF-1. We observed an increase in the transcription of CSF-1, CCL2, VEGF, and CCL5 in MC38-TRIM24 cells compared to that in MC38-V cells (Figure 6B). We also examined the transcription of cytokines associated with neutrophil recruitment to tumors, including chemokine (C-X-C motif) ligand 1 (CXCL1), colony-stimulating factor-2 (CSF-2), and CSF-3. The levels of CSF-2 and CSF-3 were decreased and that of CXCL1 was increased in MC38-TRIM24 cells (Figure 6B). In addition, we also observed enhanced expressions of the mRNA and protein levels of CSF-1, CCL2, and CCL5 (Figure 6C and 6D) in HCT-8-TRIM24 cells compared to those in the corresponding controls. In LS180 cells, knockdown of TRIM24 expression significantly reduced the protein and mRNA levels of CSF-1, CCL2, and CCL5 (Figure 6E and 6F). In addition, knockdown of TRIM24 expression in HCT-8 cells significantly reduced the protein and mRNA levels of CSF-1, CCL2, and CCL5 (Figure 6G and 6H). Thus, TRIM24 causes an increase in the expression of cytokines that trigger the infiltration of macrophages to the tumor, leading to CRC growth and tumor progression.
Discussion
Recently, several studies have revealed an association of TRIM24 with the progression and survival of tumors in various types of cancers [48,49]. TRIM24 was found to be expressed at a very high level in non-small-cell lung cancer, malignant glioma, and breast cancer [10,50,51]. Data obtained from clinical studies reveal a correlation of TRIM24 expression with poor patient survival [51]. TRIM24 overexpression could also act as a prognostic factor in HNSCC, and the silencing of TRIM24 expression reduced the proliferation of cells [34]. Nevertheless, the mode of action of TRIM24 in the proliferation of CRC cells is still unknown. In this study, we demonstrated the upregulation of TRIM24 expression in CRC, which correlated with TNM stage; this was congruent with earlier reports, suggesting an association with malignancy. The oncogenic activity of TRIM24 was shown to be associated with proliferation and angiogenesis. In addition, high expression of TRIM24 stimulated angiogenesis and enhanced the tumorigenic potential of the MC38 mouse CRC cell line. TRIM24 was also observed to increase the CSC population and induce macrophage chemotaxis, possibly by causing an increase in the levels of VEGF, CSF-1, and CCL2/5, thereby promoting the growth of CRC tumors (Figure S2).
EMT, a crucial step in tumor metastasis, is characterized by the loss of E-cadherin expression [52]. We observed that TRIM24 overexpression promoted EMT, further suggesting that EMT may be required for the increases in CRC cell invasion and migration. We also observed increased MMP9 and VEGF levels in response to TRIM24 overexpression, which, as we expected, promoted angiogenesis in tumors, and may provide the biochemical basis for TRIM24-promoted CRC progression.
In tumors, an increase in Wnt/β-catenin signaling is common, and the association between cancer cell survival and angiogenesis, and Wnt/β-catenin signaling is well proven [53,54]. MMP9 expression is regulated by the Wnt/β-catenin pathway and is a vital step for the invasion and migration of several types of cancers, such as CRC [55]. Our results demonstrated that a very high level of TRIM24 causes an upregulation of β-catenin expression in human and mouse CRC cells, while TRIM24 knockdown results in reduced β-catenin levels. Therefore, we suggest the possibility of the Wnt/β-catenin signaling pathway as a common mechanism that causes progression of tumors overexpressing TRIM24. Thus, potential targets of the Wnt/β-catenin pathway may provide treatment options for CRC.
Stromal cells are a significant component of the tumor microenvironment, and these cells are affected by signals from the cancer cells with which they cooperate to support the progression of the tumor by facilitating its growth [56,57]. A powerful angiogenic factor, VEGF, is produced by tumor cells to induce the proliferation and migration of endothelial cells and to promote angiogenesis [58], which further fulfills the oxygen and other metabolic requirements of the tumor, ensures a pathway for cancer cell survival, and promotes the progression and metastasis of tumors [59-61]. In this study, we assessed the function of TRIM24 overexpression in the progression of CRC using an orthotopic CRC mouse model; this model was established by engrafting tumor cells into the cecum of immunocompetent mice. We also observed that high expression of TRIM24 not only influenced the malignancy of the tumor cells by enhancing CSC-like characteristics but also facilitated tumor stroma remodeling by recruiting TAMs and stimulating angiogenesis.
The function of TAMs in the promotion of tumorigenesis has been experimentally and clinically shown [62,63]. In the early tumor development stage, macrophages facilitate the development of a proinflammatory environment in the tumor, but in later stages, macrophages stimulate angiogenesis, promote tumor cell invasion and migration and regulate anti-tumor immunity [64-66]. For instance, CSF-1 is a major regulator of macrophages; its overexpression is related to poor prognosis in several cancers, including CRC [67]. The cells in a tumor stimulate MMP9 production by macrophages, which facilitates the invasion of cells and disrupts the extracellular matrix [68]. We observed that TRIM24 overexpression in CRC (grown as orthotopic tumors) induces the infiltration of TAMs by increasing the expression of VEGF, CCL2/5, and CSF-1. In turn, the recruited TAMs secrete molecules such as MMP9 and VEGF, which are beneficial for tumors and promote further angiogenesis in tumors.
In conclusion, our study demonstrates the upregulation of TRIM24 expression in CRC cells, which is associated with CRC aggressiveness. Furthermore, TRIM24 was observed to exert an important effect of regulating proliferation, migration, invasion, and stem-like characteristics. Finally, we observed that TRIM24 overexpression activates Wnt/β-catenin signaling in CRC cells of both humans and mice. Our results also suggest the necessity of Wnt/β-catenin activation for the progression of tumors that overexpress TRIM24. Therefore, the Wnt/β-catenin pathway can potentially provide therapeutic targets.
Acknowledgements
This study was supported by Natural Science Foundation of Liaoning (No. 2020-MS-054 to Shida Yang and No. 2019-ZD-0404 to Bo Qu).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.De Rosa M, Pace U, Rega D, Costabile V, Duraturo F, Izzo P, Delrio P. Genetics, diagnosis and management of colorectal cancer (review) Oncol Rep. 2015;34:1087–1096. doi: 10.3892/or.2015.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagner S, Mullins CS, Linnebacher M. Colorectal cancer vaccines: tumor-associated antigens vs neoantigens. World J Gastroenterol. 2018;24:5418–5432. doi: 10.3748/wjg.v24.i48.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng KT, Tsia AKV, Chong VYL. Robotic versus conventional laparoscopic surgery for colorectal cancer: a systematic review and meta-analysis with trial sequential analysis. World J Surg. 2019;43:1146–1161. doi: 10.1007/s00268-018-04896-7. [DOI] [PubMed] [Google Scholar]
- 4.Dhillon S. Regorafenib: a review in metastatic colorectal cancer. Drugs. 2018;78:1133–1144. doi: 10.1007/s40265-018-0938-y. [DOI] [PubMed] [Google Scholar]
- 5.Tong J, Zheng X, Tan X, Fletcher R, Nikolovska-Coleska Z, Yu J, Zhang L. Mcl-1 phosphorylation without degradation mediates sensitivity to HDAC inhibitors by liberating BH3-only proteins. Cancer Res. 2018;78:4704–4715. doi: 10.1158/0008-5472.CAN-18-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Appikonda S, Thakkar KN, Barton MC. Regulation of gene expression in human cancers by TRIM24. Drug Discov Today Technol. 2016;19:57–63. doi: 10.1016/j.ddtec.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Jain AK, Barton MC. Regulation of p53: TRIM24 enters the RING. Cell Cycle. 2009;8:3668–3674. doi: 10.4161/cc.8.22.9979. [DOI] [PubMed] [Google Scholar]
- 8.Hatakeyama S. TRIM proteins and cancer. Nat Rev Cancer. 2011;11:792–804. doi: 10.1038/nrc3139. [DOI] [PubMed] [Google Scholar]
- 9.Cambiaghi V, Giuliani V, Lombardi S, Marinelli C, Toffalorio F, Pelicci PG. TRIM proteins in cancer. Adv Exp Med Biol. 2012;770:77–91. doi: 10.1007/978-1-4614-5398-7_6. [DOI] [PubMed] [Google Scholar]
- 10.Lv D, Li Y, Zhang W, Alvarez AA, Song L, Tang J, Gao WQ, Hu B, Cheng SY, Feng H. TRIM24 is an oncogenic transcriptional co-activator of STAT3 in glioblastoma. Nat Commun. 2017;8:1454. doi: 10.1038/s41467-017-01731-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Groner AC, Cato L, de Tribolet-Hardy J, Bernasocchi T, Janouskova H, Melchers D, Houtman R, Cato ACB, Tschopp P, Gu L, Corsinotti A, Zhong Q, Fankhauser C, Fritz C, Poyet C, Wagner U, Guo T, Aebersold R, Garraway LA, Wild PJ, Theurillat JP, Brown M. TRIM24 is an oncogenic transcriptional activator in prostate cancer. Cancer Cell. 2016;29:846–858. doi: 10.1016/j.ccell.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kikuchi M, Okumura F, Tsukiyama T, Watanabe M, Miyajima N, Tanaka J, Imamura M, Hatakeyama S. TRIM24 mediates ligand-dependent activation of androgen receptor and is repressed by a bromodomain-containing protein, BRD7, in prostate cancer cells. Biochim Biophys Acta. 2009;1793:1828–1836. doi: 10.1016/j.bbamcr.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Allton K, Jain AK, Herz HM, Tsai WW, Jung SY, Qin J, Bergmann A, Johnson RL, Barton MC. Trim24 targets endogenous p53 for degradation. Proc Natl Acad Sci U S A. 2009;106:11612–11616. doi: 10.1073/pnas.0813177106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li C, Xin H, Shi Y, Mu J. Knockdown of TRIM24 suppresses growth and induces apoptosis in acute myeloid leukemia through downregulation of Wnt/GSK-3beta/beta-catenin signaling. Hum Exp Toxicol. 2020;39:1725–1736. doi: 10.1177/0960327120938845. [DOI] [PubMed] [Google Scholar]
- 15.Jiang T, Mao H, Chen Q, Cao L, He Y, Gao X, Chen W, Zhang H. Trim24 prompts tumor progression via inducing EMT in renal cell carcinoma. Open Med (Wars) 2020;15:1153–1162. doi: 10.1515/med-2020-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou L, Liu Y. Wnt/beta-catenin signalling and podocyte dysfunction in proteinuric kidney disease. Nat Rev Nephrol. 2015;11:535–545. doi: 10.1038/nrneph.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collu GM, Hidalgo-Sastre A, Brennan K. Wnt-notch signalling crosstalk in development and disease. Cell Mol Life Sci. 2014;71:3553–3567. doi: 10.1007/s00018-014-1644-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fagotto F. Looking beyond the Wnt pathway for the deep nature of beta-catenin. EMBO Rep. 2013;14:422–433. doi: 10.1038/embor.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark CE, Nourse CC, Cooper HM. The tangled web of non-canonical Wnt signalling in neural migration. Neurosignals. 2012;20:202–220. doi: 10.1159/000332153. [DOI] [PubMed] [Google Scholar]
- 20.Hussain M, Xu C, Lu M, Wu X, Tang L, Wu X. Wnt/beta-catenin signaling links embryonic lung development and asthmatic airway remodeling. Biochim Biophys Acta Mol Basis Dis. 2017;1863:3226–3242. doi: 10.1016/j.bbadis.2017.08.031. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H, Nie X, Shi X, Zhao J, Chen Y, Yao Q, Sun C, Yang J. Regulatory mechanisms of the Wnt/beta-catenin pathway in diabetic cutaneous ulcers. Front Pharmacol. 2018;9:1114. doi: 10.3389/fphar.2018.01114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krausova M, Korinek V. Wnt signaling in adult intestinal stem cells and cancer. Cell Signal. 2014;26:570–579. doi: 10.1016/j.cellsig.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 23.Zhang LS, Kang X, Lu J, Zhang Y, Wu X, Wu G, Zheng J, Tuladhar R, Shi H, Wang Q, Morlock L, Yao H, Huang LJ, Maire P, Kim J, Williams N, Xu J, Chen C, Zhang CC, Lum L. Installation of a cancer promoting Wnt/SIX1 signaling axis by the oncofusion protein MLL-AF9. EBioMedicine. 2018;39:145–158. doi: 10.1016/j.ebiom.2018.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doumpas N, Lampart F, Robinson MD, Lentini A, Nestor CE, Cantu C, Basler K. TCF/LEF dependent and independent transcriptional regulation of Wnt/beta-catenin target genes. EMBO J. 2018;38:e98873. doi: 10.15252/embj.201798873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang Z, Deng J, Zhang L, Xiang X, Yu F, Chen J, Feng M, Xiong J. TRIM24 promotes the aggression of gastric cancer via the Wnt/beta-catenin signaling pathway. Oncol Lett. 2017;13:1797–1806. doi: 10.3892/ol.2017.5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tong J, Wang P, Tan S, Chen D, Nikolovska-Coleska Z, Zou F, Yu J, Zhang L. Mcl-1 degradation is required for targeted therapeutics to eradicate colon cancer cells. Cancer Res. 2017;77:2512–2521. doi: 10.1158/0008-5472.CAN-16-3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tong J, Tan S, Zou F, Yu J, Zhang L. FBW7 mutations mediate resistance of colorectal cancer to targeted therapies by blocking Mcl-1 degradation. Oncogene. 2017;36:787–796. doi: 10.1038/onc.2016.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tong J, Tan S, Nikolovska-Coleska Z, Yu J, Zou F, Zhang L. FBW7-dependent Mcl-1 degradation mediates the anticancer effect of Hsp90 inhibitors. Mol Cancer Ther. 2017;16:1979–1988. doi: 10.1158/1535-7163.MCT-17-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen D, Tong J, Yang L, Wei L, Stolz DB, Yu J, Zhang J, Zhang L. PUMA amplifies necroptosis signaling by activating cytosolic DNA sensors. Proc Natl Acad Sci U S A. 2018;115:3930–3935. doi: 10.1073/pnas.1717190115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evans JP, Winiarski BK, Sutton PA, Jones RP, Ressel L, Duckworth CA, Pritchard DM, Lin ZX, Vicky FL, Tweedle EM, Costello E, Goldring CE, Copple IM, Park BK, Palmer DH, Kitteringham NR. The Nrf2 inhibitor brusatol is a potent antitumour agent in an orthotopic mouse model of colorectal cancer. Oncotarget. 2018;9:27104–27116. doi: 10.18632/oncotarget.25497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lannagan TRM, Lee YK, Wang T, Roper J, Bettington ML, Fennell L, Vrbanac L, Jonavicius L, Somashekar R, Gieniec K, Yang M, Ng JQ, Suzuki N, Ichinose M, Wright JA, Kobayashi H, Putoczki TL, Hayakawa Y, Leedham SJ, Abud HE, Yilmaz OH, Marker J, Klebe S, Wirapati P, Mukherjee S, Tejpar S, Leggett BA, Whitehall VLJ, Worthley DL, Woods SL. Genetic editing of colonic organoids provides a molecularly distinct and orthotopic preclinical model of serrated carcinogenesis. Gut. 2018;68:384–392. doi: 10.1136/gutjnl-2017-315920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knickelbein K, Tong J, Chen D, Wang YJ, Misale S, Bardelli A, Yu J, Zhang L. Restoring PUMA induction overcomes KRAS-mediated resistance to anti-EGFR antibodies in colorectal cancer. Oncogene. 2018;37:4599–4610. doi: 10.1038/s41388-018-0289-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan X, Zhang Z, Yao H, Shen L. Tim-4 promotes the growth of colorectal cancer by activating angiogenesis and recruiting tumor-associated macrophages via the PI3K/AKT/mTOR signaling pathway. Cancer Lett. 2018;436:119–128. doi: 10.1016/j.canlet.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Xue W, Jiang X. Overexpression of TRIM24 stimulates proliferation and glucose metabolism of head and neck squamous cell carcinoma. Biomed Res Int. 2018;2018:6142843. doi: 10.1155/2018/6142843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu Y, Zhao L, Shi K, Huang Z, Chen B. TRIM24 promotes hepatocellular carcinoma progression via AMPK signaling. Exp Cell Res. 2018;367:274–281. doi: 10.1016/j.yexcr.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Pastushenko I, Blanpain C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019;29:212–226. doi: 10.1016/j.tcb.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Weidenfeld K, Barkan D. EMT and stemness in tumor dormancy and outgrowth: are they intertwined processes? Front Oncol. 2018;8:381. doi: 10.3389/fonc.2018.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopez A, Harada K, Vasilakopoulou M, Shanbhag N, Ajani JA. Targeting angiogenesis in colorectal carcinoma. Drugs. 2019;79:63–74. doi: 10.1007/s40265-018-1037-9. [DOI] [PubMed] [Google Scholar]
- 39.Montuori N, Ragno P. Role of uPA/uPAR in the modulation of angiogenesis. Chem Immunol Allergy. 2014;99:105–122. doi: 10.1159/000353310. [DOI] [PubMed] [Google Scholar]
- 40.Silvestre JS. Pro-angiogenic cell-based therapy for the treatment of ischemic cardiovascular diseases. Thromb Res. 2012;130(Suppl 1):S90–94. doi: 10.1016/j.thromres.2012.08.287. [DOI] [PubMed] [Google Scholar]
- 41.Prager GW, Poettler M, Unseld M, Zielinski CC. Angiogenesis in cancer: anti-VEGF escape mechanisms. Transl Lung Cancer Res. 2012;1:14–25. doi: 10.3978/j.issn.2218-6751.2011.11.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Du Z, Zhang H, Chen Q, Gao Y, Sun B. Intranasal calcitonin gene-related peptide protects against focal cerebral ischemic injury in rats through the Wnt/beta-catenin pathway. Med Sci Monit. 2018;24:8860–8869. doi: 10.12659/MSM.913777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Najafi M, Goradel NH, Farhood B, Salehi E, Solhjoo S, Toolee H, Kharazinejad E, Mortezaee K. Tumor microenvironment: interactions and therapy. J Cell Physiol. 2018;24:8860–8869. doi: 10.1002/jcp.27425. [DOI] [PubMed] [Google Scholar]
- 44.Lala PK, Nandi P, Majumder M. Roles of prostaglandins in tumor-associated lymphangiogenesis with special reference to breast cancer. Cancer Metastasis Rev. 2018;37:369–384. doi: 10.1007/s10555-018-9734-0. [DOI] [PubMed] [Google Scholar]
- 45.Zhou G, Noordam L, Sprengers D, Doukas M, Boor PPC, van Beek AA, Erkens R, Mancham S, Grunhagen D, Menon AG, Lange JF, Burger P, Brandt A, Galjart B, Verhoef C, Kwekkeboom J, Bruno MJ. Blockade of LAG3 enhances responses of tumor-infiltrating T cells in mismatch repair-proficient liver metastases of colorectal cancer. Oncoimmunology. 2018;7:e1448332. doi: 10.1080/2162402X.2018.1448332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao X, Li L, Starr TK, Subramanian S. Tumor location impacts immune response in mouse models of colon cancer. Oncotarget. 2017;8:54775–54787. doi: 10.18632/oncotarget.18423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krug S, Abbassi R, Griesmann H, Sipos B, Wiese D, Rexin P, Blank A, Perren A, Haybaeck J, Huttelmaier S, Rinke A, Gress TM, Michl P. Therapeutic targeting of tumor-associated macrophages in pancreatic neuroendocrine tumors. Int J Cancer. 2018;143:1806–1816. doi: 10.1002/ijc.31562. [DOI] [PubMed] [Google Scholar]
- 48.Wang P, Shen N, Liu D, Ning X, Wu D, Huang X. TRIM24 siRNA induced cell apoptosis and reduced cell viability in human nasopharyngeal carcinoma cells. Mol Med Rep. 2018;18:369–376. doi: 10.3892/mmr.2018.8946. [DOI] [PubMed] [Google Scholar]
- 49.Carrier M, Lutzing R, Gaouar S, Rochette-Egly C. TRIM24 mediates the interaction of the retinoic acid receptor alpha with the proteasome. FEBS Lett. 2018;592:1426–1433. doi: 10.1002/1873-3468.13033. [DOI] [PubMed] [Google Scholar]
- 50.Ma L, Yuan L, An J, Barton MC, Zhang Q, Liu Z. Histone H3 lysine 23 acetylation is associated with oncogene TRIM24 expression and a poor prognosis in breast cancer. Tumour Biol. 2016;37:14803–14812. doi: 10.1007/s13277-016-5344-z. [DOI] [PubMed] [Google Scholar]
- 51.Li H, Sun L, Tang Z, Fu L, Xu Y, Li Z, Luo W, Qiu X, Wang E. Overexpression of TRIM24 correlates with tumor progression in non-small cell lung cancer. PLoS One. 2012;7:e37657. doi: 10.1371/journal.pone.0037657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rout-Pitt N, Farrow N, Parsons D, Donnelley M. Epithelial mesenchymal transition (EMT): a universal process in lung diseases with implications for cystic fibrosis pathophysiology. Respir Res. 2018;19:136. doi: 10.1186/s12931-018-0834-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khalaf AM, Fuentes D, Morshid AI, Burke MR, Kaseb AO, Hassan M, Hazle JD, Elsayes KM. Role of Wnt/beta-catenin signaling in hepatocellular carcinoma, pathogenesis, and clinical significance. J Hepatocell Carcinoma. 2018;5:61–73. doi: 10.2147/JHC.S156701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang B, Tian T, Kalland KH, Ke X, Qu Y. Targeting Wnt/beta-catenin signaling for cancer immunotherapy. Trends Pharmacol Sci. 2018;39:648–658. doi: 10.1016/j.tips.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 55.Lee MA, Park JH, Rhyu SY, Oh ST, Kang WK, Kim HN. Wnt3a expression is associated with MMP-9 expression in primary tumor and metastatic site in recurrent or stage IV colorectal cancer. BMC Cancer. 2014;14:125. doi: 10.1186/1471-2407-14-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yin Z, Jiang K, Li R, Dong C, Wang L. Multipotent mesenchymal stromal cells play critical roles in hepatocellular carcinoma initiation, progression and therapy. Mol Cancer. 2018;17:178. doi: 10.1186/s12943-018-0926-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guo S, Deng CX. Effect of stromal cells in tumor microenvironment on metastasis Initiation. Int J Biol Sci. 2018;14:2083–2093. doi: 10.7150/ijbs.25720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shukla K, Sonowal H, Saxena A, Ramana KV. Didymin by suppressing NF-kappaB activation prevents VEGF-induced angiogenesis in vitro and in vivo. Vascul Pharmacol. 2019;115:18–25. doi: 10.1016/j.vph.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 59.Zanotelli MR, Reinhart-King CA. Mechanical forces in tumor angiogenesis. Adv Exp Med Biol. 2018;1092:91–112. doi: 10.1007/978-3-319-95294-9_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olsen JJ, Pohl SO, Deshmukh A, Visweswaran M, Ward NC, Arfuso F, Agostino M, Dharmarajan A. The role of Wnt signalling in angiogenesis. Clin Biochem Rev. 2017;38:131–142. [PMC free article] [PubMed] [Google Scholar]
- 61.Pinto MP, Owen GI, Retamal I, Garrido M. Angiogenesis inhibitors in early development for gastric cancer. Expert Opin Investig Drugs. 2017;26:1007–1017. doi: 10.1080/13543784.2017.1361926. [DOI] [PubMed] [Google Scholar]
- 62.Kovaleva OV, Samoilova DV, Shitova MS, Gratchev A. Tumor associated macrophages in kidney cancer. Anal Cell Pathol (Amst) 2016;2016:9307549. doi: 10.1155/2016/9307549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pierce AM, Keating AK. TAM receptor tyrosine kinases: expression, disease and oncogenesis in the central nervous system. Brain Res. 2014;1542:206–220. doi: 10.1016/j.brainres.2013.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Afferni C, Buccione C, Andreone S, Galdiero MR, Varricchi G, Marone G, Mattei F, Schiavoni G. The pleiotropic immunomodulatory functions of IL-33 and its implications in tumor immunity. Front Immunol. 2018;9:2601. doi: 10.3389/fimmu.2018.02601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Edgar LJ, Kawasaki N, Nycholat CM, Paulson JC. Targeted delivery of antigen to activated CD169(+) macrophages induces bias for expansion of CD8(+) T cells. Cell Chem Biol. 2018;26:131–136. e4. doi: 10.1016/j.chembiol.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Su S, Zhao J, Xing Y, Zhang X, Liu J, Ouyang Q, Chen J, Su F, Liu Q, Song E. Immune checkpoint inhibition overcomes ADCP-induced immunosuppression by macrophages. Cell. 2018;175:442–457. e423. doi: 10.1016/j.cell.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 67.Chen Y, Zhao Z, Chen Y, Lv Z, Ding X, Wang R, Xiao H, Hou C, Shen B, Feng J, Guo R, Li Y, Peng H, Han G, Chen G. An epithelial-to-mesenchymal transition-inducing potential of granulocyte macrophage colony-stimulating factor in colon cancer. Sci Rep. 2017;7:8265. doi: 10.1038/s41598-017-08047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Riabov V, Gudima A, Wang N, Mickley A, Orekhov A, Kzhyshkowska J. Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Front Physiol. 2014;5:75. doi: 10.3389/fphys.2014.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.