Abstract
Background
Data suggest lower coronavirus disease-2019 (COVID-19) vaccination coverage among minority and disadvantaged groups. We aimed to identify interactions between sociodemographic factors associated with vaccination gaps.
Methods
This population study used Israeli National COVID-19 data (extracted: 10 May 2021). The analysis comprised 6 478 999 individuals age ≥15 years with aggregated area-level data on sex and age distribution and no COVID-19 history. We estimated vaccination hazard and cumulative incidence using the Fine and Gray competing risk model.
Results
Older age and higher socioeconomic status (SES) were associated, with stepwise higher cumulative vaccination rates (age 20–24: 67%, age ≥ 75: 96%; SES 1–3: 61%, 4–5: 74.2%, 6–7: 82%, 8–10: 87%). We found the lowest vaccination rates in Arab (65%) and Ultra-Orthodox Jewish (54%) areas. SES modified the association in Arab neighbourhoods, with higher coverage than in the non-Orthodox Jewish reference group in SES 1–3 [adjusted hazard ratio (HR) = 1.06; 95% confidence interval (CI): 1.02–1.11], and gradually lower coverage in higher SES classes (SES 6–7: HR = 0.83; 95% CI: 0.79–0.87). Vaccination rates were also higher among younger Arabs (≤45 years) compared with age counterparts in the reference population group (age 25–34: HR = 1.18; 95% CI: 1.12–1.28) and lower than the reference group among Arabs age ≥45 years. Among Ultra-Orthodox Jews, vaccination HRs remained below one across age and SES classes.
Conclusions
Age and SES modified the association between population group and vaccination coverage. Identifying the interplay between sociodemographic characteristics and the underlying explanations may improve targeted efforts, aimed at closing vaccination coverage gaps and mitigating COVID-19.
Keywords: COVID-19, vaccine, equity
Key Messages.
Lower vaccination coverage was reported among minority and disadvantaged groups.
In this population study among nearly 6.5 million Israelis aged 15 years and older, age, socioeconomic rank and minority status were key determinants of vaccination, with generally lower rates in low-socioeconomic status (SES) areas and areas with minority predominance.
Nevertheless, the associations between age and SES with vaccine coverage differed by population group.
Compared with the reference majority group, vaccine coverage was consistently lower in Ultra-Orthodox Jewish areas and paradoxically higher among young and low-SES Arab community members.
Our study demonstrates the importance of identifying interactions as the basis for targeted efforts to maximize vaccination coverage and increase equity. Further research aimed at identifying the underlying barriers to vaccination will help implement better-designed and -focused interventions.
Introduction
Minority ethnicity/race and socioeconomic deprivation are associated with higher coronavirus disease-2019 (COVID-19) burden and worse prognosis.1,2 These disproportionate effects on disadvantaged groups accentuate preexisting social and structural inequalities.3,4 Drawing from experience,5,6 concerns regarding vaccination disparities, particularly during early deployment of limited supplies, arose while vaccines were still under study.7 Advisory boards such as the World Health Organization Strategic Advisory Group of Experts on Immunization and the US Committee on Equitable Allocation of Vaccine for the Novel Coronavirus recommended prioritizing vaccine allocation based on biological and social vulnerability.3,8
The messenger RNA BNT162b (Pfizer-BioNTech) vaccine was the first to receive a Food and Drug Administration (FDA) emergency use authorization on 11 December 2020. On 19 December 2020, Israel launched its national immunization campaign with the BNT162b2 vaccine administered in two doses 21 days apart.9 By that time, 373 509 COVID-19 cases out of a population of more than 9 million were detected.10
Persons aged 60 years or older, people at risk due to preexisting morbidity and health care workers were among the first vaccinated. By mid-January 2021, the eligibility age was lowered to 45 and by 4 February, the vaccine was offered to all from age 16. The contributors to Israel’s successful initial vaccine rollout were discussed by Rosen et al. and included country-, health system- and vaccine-specific factors.11
Early data suggested lower vaccination rates among the Israeli Arab minority and Ultra-Orthodox Jewish population groups and variation in vaccine coverage across socioeconomic status (SES) among the 60+ population segment.12,13 Both above-mentioned studies relied on municipal aggregated data without examining further factors that might explain these findings. Disparities in COVID-19 vaccination rates were recently reported in the USA.14 The factors driving these gaps may differ between populations. The latest infection surges, driven by the severe acute respiratory syndrome corona virus-2 (SARS-Cov-2) variants highlight the importance of vaccination for reducing transmission rates and the burden of hospitalizations and severe disease. The identification of specific sociodemographic characteristics and their interplay related to COVID-19 vaccination is important for informed decision making to maximize equity alongside efficiency in reaching this goal. We, therefore, aimed to identify interactions between sociodemographic characteristics associated with lower vaccination coverage.
Methods
This population study combined anonymized individual-level data from the Israeli COVID-19 National surveillance Database (extracted: 10 May 2021), and sociodemographic data published by the Israeli Central Bureau of Statistics (ICBS; updated throughout 2019). ICBS published data were aggregated by the smallest published geospatial units (referred to as ‘area’ hereafter) representing relatively homogeneous neighbourhoods, comprising 3000–5000 inhabitants or the entire population for small localities (<10 000 inhabitants).15 The Sheba Medical Center Institutional Review Board approved the study.
Study population
Data on area, sex, and age distribution existed for 2895 areas (99% of areas in localities permanently inhabited by at least 40 adults). Data on age were available in 5-year groups, 15–19 being the youngest group relevant for vaccination from the age of 16. The analysis population included 6 779 750 residents, 15 years and older, with complete age and sex distribution data. We deducted the number of COVID-19 cases before 19 December 2020 from each area population (300 751 individuals in total). Compared with the general population, pre-vaccination COVID-19 cases were younger and more likely to reside in Arab and Jewish Ultra-Orthodox areas and to belong to lower SES classes (Supplementary Table S1, available as Supplementary data at IJE online).
Variable definition
We used ICBS indexes for periphery (2015 version) based on standardized distance between residence locality and a central economic centre,16 and for SES based on demography, education, occupation and standard of living.17 Population group (Arab minority, Ultra-Orthodox Jews, Orthodox Jews, other) was determined at area level according to population group predominance in the area. The proportion of Arab inhabitants in areas labelled ‘Arab’ was particularly high [median = 98.4%; interquartile range (IQR): 79.5–99.6%].
Statistical analysis
Time to vaccination was the primary endpoint. We used the Fine and Gray model18 for estimating vaccine cumulative incidence and sub-distribution hazard while accounting for competing SARS-CoV-2 infection, precluding subsequent vaccination. Age-adjusted cumulative vaccination incidence rates are presented for the 60–64 age group included in the first vaccination priority group. Time was defined as days between 19 December 2020 and first dose administration (event of interest), or positive COVID-19 polymerase chain reaction (PCR) test (competing event) or censoring for end of follow-up at the data extraction date (10 May 2021). Data preparation included augmentation by event type (vaccination, infection or censoring) within time, area, sex and age-group strata (see Supplementary material for method details, available as Supplementary data at IJE online). We also examined cause-specific hazard censoring on a competing event. The proportional hazard assumption was tested based on weighted Schoenfeld residuals in cause-specific models. Whereas the hypothesis of zero correlation with time rank was rejected for all predictors due to large numbers, a visual inspection of graphs satisfied the assumption with correlations ranging between −0.07 and 0.01 (Supplementary Methods, available as Supplementary data at IJE online). Age groups above 44 years were outliers, with correlations between −0.2 to −0.1, reflecting higher vaccination priority earlier in the campaign. We computed age-adjusted cumulative infection rates (the competing event) by sociodemographic characteristics, with Cox proportional hazard models as 1-infection-free survival. We first present the result of an analysis without interactions (main effect model) and then repeat the analyses adding each interaction, separately, to the main effect model. Effect modifiers considered included SES, age, sex, population density and pre-vaccination infection rate. Data were analysed with SAS 9.4 (SAS Institute, Cary, NC).
Results
During the follow-up period, 5 001 535 individuals received at least one COVID-19 vaccine dose and 4 709 981 (94%) received two doses. Cumulative vaccination rates among vaccine-eligible men (73.8%) and women (73.6%) were similar (Supplementary Figure S1, available as Supplementary data at IJE online).
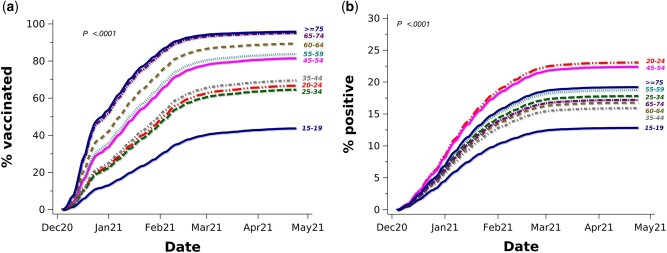
The age distribution of the analysis population varied by sociodemographic characteristics (Supplementary Table S2, available as Supplementary data at IJE online). Accounting for the competing SARS-Cov-2 infection risk, older age was associated with higher cumulative vaccination rates, by 10 May 2021 reaching 96% for age ≥75 and 67% for age 20–24 (Figure 1a). The multivariable-adjusted hazard ratio (HR) for age ≥75 years was 3.24 [95% confidence interval (CI): 3.14–3.34; reference = 20–24; Table 1). The corresponding cumulative SARS-Cov-2 infection rates are presented in Figure 1b.
Figure 1.
Cumulative vaccination incidence (a) and competing SARS-CoV-2 infection (b) rates by age group. Percentages (%) of vaccinated refer to cumulative incidence/100 accounting for the competing COVID-19 infection risk. % positive refers to cumulative SARS-CoV-2 infection rate/100 based on Kaplan-Meier infection-free survival.
Table 1.
Multivariable-adjusted vaccination hazard ratios among 6 478 999 individuals aged 15 years and older
| Characteristic (reference category) | HR | (95% CI) | |
|---|---|---|---|
| Men (women) | 1.06 | (1.06–1.07) | |
| Age, years (20–24) | 15–19 | 0.52 | (0.50–0.54) |
| 25–34 | 0.93 | (0.91–0.95) | |
| 35–44 | 1.08 | (1.05–1.11) | |
| 45–54 | 1.51 | (1.47–1.55) | |
| 55–59 | 1.71 | (1.67–1.76) | |
| 60–64 | 2.18 | (2.12–2.24) | |
| 65–74 | 3.10 | (3.01–3.20) | |
| ≥75 | 3.24 | (3.14–3.34) | |
| Population group (other) | Arabs | 0.96 | (0.94–0.98) |
| Orthodox Jews | 0.95 | (0.90–1.00) | |
| Ultra-Orthodox Jews | 0.70 | (0.68–0.72) | |
| SES class (1–3) | 4–5 | 1.24 | (1.21–1.28) |
| 6–7 | 1.67 | (1.63–1.72) | |
| 8–10 | 2.05 | (1.98–2.11) | |
| Locality population (<2000) | 2000–99 900 | 0.84 | (0.82–0.87) |
| 100 000–499 900 | 0.80 | (0.77–0.82) | |
| ≥500 000 | 0.75 | (0.72–0.79) | |
| Population density population/km2 (<746) | 746–3064 | 1.02 | (0.99–1.04) |
| 3064–11 137 | 1.00 | (0.97–1.04) | |
| >11 137 | 0.95 | (0.92–0.99) | |
| Periphery (central) | Very central | 1.03 | (1.01–1.06) |
| Peripheral | 1.07 | (1.05–1.09) | |
| Very peripheral | 1.00 | (0.97–1.02) | |
| Past SARS-CoV-2 infection rates %a (<0.011) | 0.011–0.383 | 1.50 | (1.45–1.55) |
| >0.383 | 1.71 | (1.66–1.77) |
Model without interactions.
CI, confidence interval; HR, hazard ratio; SES, socioeconomic status.
P <0.0001 for all.
Past SARS-CoV-2 infection rates refer to the % of area population infected up to 19 December 2020, categorized by tertiles.
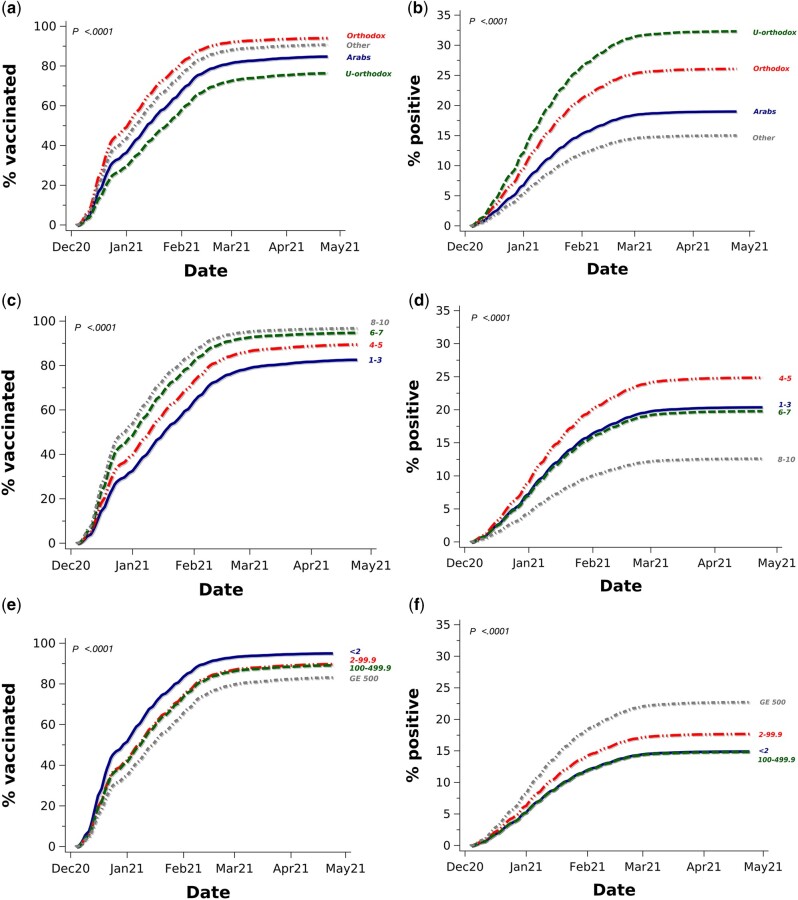
Comparing population groups, we found the lowest vaccination rates in areas predominantly populated by Ultra-Orthodox Jews or by the Arab minority (54% and 65%, respectively, vs 78% in the reference group comprising mostly non-Orthodox Jews; Supplementary Table S3, available as Supplementary data at IJE online). Comparable age-adjusted cumulative incidences are presented in Figure 2a. The adjusted HR for Ultra-Orthodox Jews was 0.7 (95% CI: 0.68–0.72) and was 0.96 (95% CI: 0.94–0.98) for Arab communities (Table 1). Areas populated by Ultra-Orthodox Jews also had the highest competing SARS-CoV-2 infection rates (30%), whereas the rates in areas predominantly inhabited by Arabs (17%) were only slightly higher than among the reference group (15%; see Figure 2b for age-adjusted comparable cumulative rates).
Figure 2.
Age-adjusted cumulative vaccination incidence and age-adjusted competing SARS-CoV-2 infection rates by population group [(a), (b), respectively], socioeconomic status [(c), (d)] and locality population size in thousands [(e), (f)] in those aged 60–64 years. GE denotes greater than or equal to. Percentages (%) of vaccinated refer to cumulative incidence/100 accounting for the competing COVID-19 infection risk. % positive refers to cumulative SARS-CoV-2 infection rate/100 based on Kaplan-Meir infection-free survival. Results are presented for the 60–64 age slice for comparability.
Areas with Arab minority or Ultra-Orthodox Jews had lower median SES (1–3 for both compared with 6–7 for the reference population group) and were absent from the highest (8–10) SES classes. Lower SES was associated with stepwise lower age-adjusted cumulative vaccination rates (Figure 2c; see Figure 2d for the corresponding cumulative infection rates). Crude vaccination coverage ranged between 61% in SES classes 1–3 (82% in ages 60–64) to 82% in classes 6–7 (95% in ages 60–64) and 87% (97%) in classes 8–10. This vaccination rate gradient persisted, following adjustment, with an HR of 2.05 (95% CI: 1.98–2.11) for the highest SES (8–10) areas (reference = 1–3), which also had the lowest concurrent age-adjusted COVID-19 infection rate (Figure 2d). Vaccination coverage was higher in smaller localities (Figure 2e). The competing infection rates for the smallest (population < 2000: 15%) and largest (population ≥500 000: 23%) localities (see age-adjusted rates in Figure 2f) portray a reversed trend. Areas with larger households on average also had lower vaccination rates (for age-adjusted rates see Supplementary Figure S2, available as Supplementary data at IJE online).
Considering known SARS-CoV-2 infections prior to vaccination rollout, the multivariable-adjusted vaccination incidence rate was 71% higher (95% CI: 66–77%) in areas in the upper pre-campaign infection rate tertile than in the lowest tertile. Repeating the analysis with cause-specific models yielded similar results (Supplementary Table S4, available as Supplementary data at IJE online).
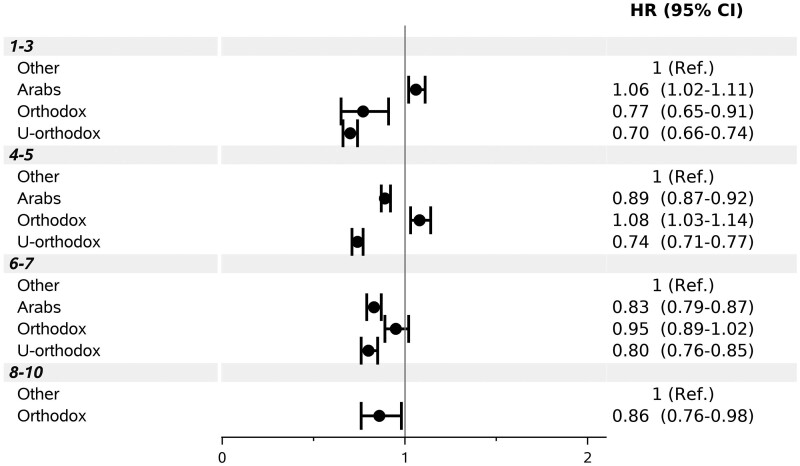
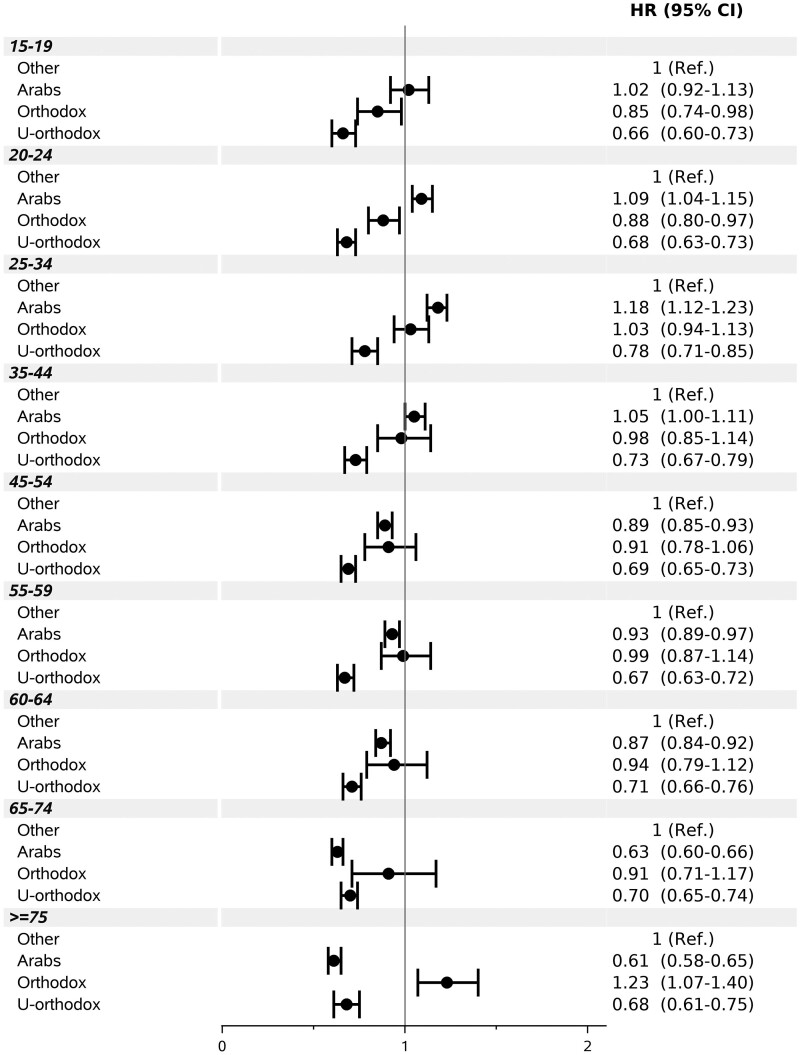
Examining interactions, male and female Ultra-Orthodox area residents had significantly lower adjusted vaccination rates than the reference population group (HR = 0.71; 95% CI: 0.68–0.74 and HR = 0.69; 95% CI: 0.66–0.72, respectively), whereas Arab area residents had only slightly lower (for women) or similar (for men) rates (Supplementary Figure S3, available as Supplementary data at IJE online). Vaccination rates in Arab areas varied by SES. The rate was higher in the lowest classes 1–3 (adjusted HR = 1.06; 95% CI: 1.02–1.11), and lower than in the same SES reference group, in higher classes (Figure 3). HRs for Arab neighbourhoods increased with increasing age up to age 25–34 (HR = 1.18; 95% CI: 1.12–1.23) and were lower than in the reference group from age 45 onwards (Figure 4). In areas populated mainly by Ultra-Orthodox Jews, HRs remained below 1.00 across SES classes and age groups. The interaction of population groups with population density was less consistent (Supplementary Figure S4, available as Supplementary data at IJE online).
Figure 3.
Multivariable-adjusted hazard ratio (HR) and 95% confidence interval (CI) under socioeconomic class and population group interaction. Ref. denotes reference. P for interaction <0.0001.
Figure 4.
Multivariable-adjusted hazard ratio (HR) and 95% confidence interval (CI) under age and population group interaction. Ref. denotes reference. P for interaction <0.0001.
No significant interaction was found between SES and sex (Supplementary Figure S5, available as Supplementary data at IJE online) but the SES gap widened with older age, (Supplementary Figure S6, available as Supplementary data at IJE online) and in areas with lower population density (Supplementary Figure S7, available as Supplementary data at IJE online). Examination of effect modification by pre-vaccination campaign infection rates showed adjusted HRs ranging from 0.55 (95% CI: 0.50–0.62) in the lowest tertile to 1.00 (0.98–1.03) in the upper infection rate tertile in Arab neighbourhoods, and from 0.29 (0.24–0.35) to 0.75 (0.73–0.78), respectively, in Ultra-Orthodox Jewish neighbourhoods. The gap between the lowest and higher SES classes was larger in areas with low pre-vaccine infection rates (Supplementary Figure S8, available as Supplementary data at IJE online).
Discussion
In this nationwide study among nearly 6.5 million Israeli residents aged 15 years and older, we found vaccination rates to be independently associated with age, SES, locality size, population group and prior area SARS-Cov-2 infection rates.
Older age, in the current study, was associated with higher vaccine coverage. This cannot be explained merely by longer accessibility, as the vaccine was offered from the age of 16 within less than 2 months, and the vaccination rate gradient persisted into age groups included in the first vaccination round. COVID-19 risk perception by older adults and targeted campaign efforts may provide additional explanations. Our finding of higher vaccination rates in areas experiencing higher past infection rates supports this assumption.
Arabs, the largest minority in Israel, constitute 21% of the population19 and Ultra-Orthodox Jews constitute 12.5%.20 These distinct social groups, comprising together more than a third of the Israeli population, suffered particularly high COVID-19 burden compared with other population groups. Lower COVID-19 vaccination rates among these groups are congruent with lower adult seasonal influenza vaccine rates and contrast with high rates of routine childhood vaccines among Arabs.12,13,21 The reasons for vaccination disparities are complex. Financial barriers, however, are less relevant for the COVID-19 vaccine, offered cost-free in Israel as in other countries.11
Another possible explanation is vaccine hesitancy. An international survey conducted in June 2020 found 71.5% of 13 426 people from 19 countries to be at least somewhat likely to get vaccinated.22 Factors associated with higher acceptance rates included older age, female sex and higher income, education, risk perception and trust in government and vaccine efficacy.22,23 Political leaning was a risk factor in the USA for reduced vaccine acceptance.24 Greater COVID-19 burden among disadvantaged groups may reinforce mistrust and vaccine hesitancy.25 Reduced uptake for past vaccines and hesitancy for COVID-19 vaccines were reported for ethnic minorities in the UK.23 In a survey conducted in October 2020, in a sample of Israelis aged 30 years or older, 41% of Arab women and 23% of Arab men rejected the COVID-19 vaccine altogether, compared with 17% and 8% of Jewish women and men, respectively.26 In the current study, lower personal risk perception in Ultra-Orthodox Jewish areas with the lowest pre-vaccine infection tertile may explain their particularly low vaccine coverage.
The links between social groups and SES are interlaced. Our findings suggest that there may be different explanations for lower vaccination coverage in areas with predominantly Arab minority or Ultra-Orthodox Jewish inhabitants. Among Ultra-Orthodox Jews, less likely to be vaccinated across the SES scale, higher SES had a mitigating effect. Conversely among Arab communities, lower SES was associated with higher vaccination rates, and lower rates, compared with the reference group, were limited to the highest area SES for this group (classes 6–7). Age was another effect modifier. Higher vaccine rates among younger and lower rates among older individuals compared with the reference group in Arab communities may stem from lower education and health literacy among older generations27 or work-related incentives among younger ones.
Disinformation may be more important where official information is restricted. Facebook as the main information source independently predicted vaccine hesitancy among health care workers in Italy.28 The Israeli Ultra-Orthodox Jewish society is diverse; their closed communities shun external influences. Internet use among them reached 66% during the COVID-19 pandemic, but 85% of the Ultra-Orthodox filter content inconsistent with strict religious observance.20 Alternative information sources used by these communities could channel unfounded concerns about the vaccine, particularly regarding fertility. Older and less Hebrew-proficient Arab community members may be more exposed to such sources, in spite of governmental campaigns providing reliable language and culturally congruent information through a variety of media.
The use of nationwide population data and accounting for competing infection risk are strengths of the current study. Several limitations however deserve noting. A previous study briefly reporting lower vaccination rates among Arab and Ultra-Orthodox Jewish population groups categorized mixed cities as Jewish.20 Our definition of sociodemographic characteristics, based on the smallest geospatial units and relatively uniform by definition, remains ecological and deserves consideration while interpreting results. This observational study could not determine the direct cause for lower uptake in any specific area, and residual confounding by factors not considered in the current study may exist. Targeted surveys on hesitancy and vaccination barriers employing in-person interviews may provide additional insight into possible barriers.
Missing information on non-COVID-19 related deaths resulted in censoring at data extraction date rather than at actual death. Assuming an average of 4500 deaths/month among the vaccine candidate population, we expect 22 500 deaths in total (0.3% of the candidate population) and the bias to be negligible.
The Fine and Gray model used in this study is not without limitation. Whereas this model provides a useful way for predicting cumulative incidence and the association of covariates with the cumulative incidence in the presence of informative censoring by a competing risk, it may be less suitable for causal inference.29 It was also observed that the sum of cumulative incidence estimated by the model might exceed 1.00 under specific circumstances.30 The similar results obtained from a cause-specific Cox model, in which concurrent infections were treated as censoring events rather than as competing risks, support the validity of using the Fine and Gray model in this study.
In conclusion, our study demonstrates the importance of extending beyond a top-level view for identifying specific groups that might benefit from tailored interventions to increase vaccination rates. Revealing combinations of characteristics linked to reduced vaccination rates may also shed light on the underlying causes, and help funnel limited resources more efficiently.
The Israeli experience demonstrates that unrestricted vaccine availability cannot guarantee equity. As the totality of minority and disadvantaged groups may represent a non-negligible portion of the society, understanding and addressing disparities is not only a prerequisite for equity but also key to curtailing transmission by vaccination. Disparities between social groups may persist, as demonstrated by our study, despite a proactive approach including outreach by local clinics, language, culturally adapted vaccine information and support of religious leaders.11,31 Vaccination gaps are society dependent. Our results highlight the importance of identifying local factors associated with lower vaccine coverage and understanding the interplay between factors for improving targeted efforts, aimed at closing vaccination coverage gaps and mitigating COVID-19.
This study is based on data from the Israeli COVID-19 National Database and sociodemographic data published by the Israeli Central Bureau of Statistics [https://www.cbs.gov.il]. Aggregated COVID-19 data by area are available on the Israel Ministry of Health website [https://data.gov.il/dataset/covid-19]. SAS code of interest will be shared upon request.
Supplementary Data
Supplementary data are available at IJE online.
Funding
This work was not supported by external funding.
Supplementary Material
Contributor Information
Michal Benderly, Gertner Institute for Epidemiology and Health Policy Research, Sheba Medical Center, Ramat-Gan, Israel; Department of Epidemiology and Preventive Medicine, School of Public Health, Sackler Faculty of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Amit Huppert, Gertner Institute for Epidemiology and Health Policy Research, Sheba Medical Center, Ramat-Gan, Israel; Department of Epidemiology and Preventive Medicine, School of Public Health, Sackler Faculty of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Ilya Novikov, Gertner Institute for Epidemiology and Health Policy Research, Sheba Medical Center, Ramat-Gan, Israel.
Arnona Ziv, Gertner Institute for Epidemiology and Health Policy Research, Sheba Medical Center, Ramat-Gan, Israel.
Ofra Kalter-Leibovici, Gertner Institute for Epidemiology and Health Policy Research, Sheba Medical Center, Ramat-Gan, Israel; Department of Epidemiology and Preventive Medicine, School of Public Health, Sackler Faculty of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Author Contributions
All authors contributed to the study concept and design. A.Z. and M.B. were responsible for data acquisition and preparation. M.B. and I.N. performed the statistical analyses. M.B., A.Z., A.H. and O.K.L. contributed to data interpretation. The first draft was written by M.B., and all authors commented on previous versions of the manuscript and approved the final version.
Conflict of Interest
None declared.
References
- 1. Van Dorn A, Cooney RE, Sabin ML. Covid-19 exacerbating inequalities in the US. Lancet 2020;395:1243–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chiriboga D, Garay J, Buss P, Madrigal RS, Rispel LC. Health inequity during the COVID-19 pandemic: a cry for ethical global leadership. Lancet 2020;395:1690–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. National Academies of Sciences, Engineering, and Medicine. Framework for Equitable Allocation of COVID-19 Vaccine. Washington, DC: National Academies Press, 2020. [PubMed] [Google Scholar]
- 4. Riou J, Panczak R, Althaus CL et al. Socioeconomic position and the COVID-19 care cascade from testing to mortality in Switzerland: a population-based analysis. Lancet Public Health 2021;6:E683–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hart JT. The inverse care law. Lancet 1971;1:405–12. [DOI] [PubMed] [Google Scholar]
- 6. Vukovic V, Lillini R, Lupi S et al. Identifying people at risk for influenza with low vaccine uptake based on deprivation status: a systematic review. Eur J Public Health 2020;30:132–41. [DOI] [PubMed] [Google Scholar]
- 7. Todd A, Bambra C. Learning from past mistakes? The COVID-19 vaccine and the inverse equity hypothesis. Eur J Public Health 2021;31:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. WHO (World Health Organization). Values Framework for the Allocation and Prioritization of COVID-19 Vaccination. Geneva, Switzerland: WHO, 2020. [Google Scholar]
- 9. Leshem E, Wilder-Smith A. COVID-19 vaccine impact in Israel and a way out of the pandemic. Lancet 2021;397:1783–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Israel Ministry of Health. COVID-19 dashboard. https://datadashboard.health.gov.il/COVID-19/general?tileName=newVerifiedDaily (30 May 2021, date last accessed).
- 11. Rosen B, Waitzberg R, Israeli A. Israel's rapid rollout of vaccinations for COVID-19. Isr J Health Policy Res 2021;10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Muhsen K, Na'aminh W, Lapidot Y et al. A nationwide analysis of population group differences in the COVID-19 epidemic in Israel, February 2020-February 2021. Lancet Reg Health Eur 2021;7:100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Caspi G, Dayan A, Eshal Y et al. Socioeconomic disparities and COVID-19 vaccination acceptance: experience from Israel. medRxiv 2021. doi: 10.1101/2021.01.28.21250716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Agarwal R, Dugas M, Ramaprasad J et al. Socioeconomic privilege and political ideology are associated with racial disparity in COVID-19 vaccination. Proc Natl Acad Sci U S A 2021;118:e2107873118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Central Bureau of Statistics. Characterization and Classification of Statistical Areas Within Municipalities and Local Councils by the Socio-Economic Level of the Population. 2015. https://www.cbs.gov.il/en/mediarelease/Pages/2019/Characterization-and-Classification-of-Statistical-Areas-Within-Municipalities-and-Local-Councils-by-the-Socio-Economic-Lev.aspx (30 June 2021, date last accessed)
- 16. Central Bureau of Statistics. Peripheriality Index of Localities and Local Authorities – 2015. https://www.cbs.gov.il/en/publications/Pages/2019/Peripheriality-Index-Of-Localities-And-Local-Authorities-2015.aspx (11 May 2021, date last accessed).
- 17. Central Bureau of Statistics. Characterization and Classification of Geographical Units by the Socio-Economic Level of the Population 2017. (S.P. 1832). https://www.cbs.gov.il/en/publications/Pages/2021/socio-2017-e.aspx (22 January 2022, date last accessed).
- 18. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94.446:496–509. [Google Scholar]
- 19. Central Bureau of Statistics. Population of Israel on the Eve of 2021. https://www.cbs.gov.il/en/mediarelease/Pages/2020/Population-of-Israel-on-the-Eve-of-2021.aspx (30 June 2021, date last accessed).
- 20. Malach G, Cahaner L. Statistical Report on Ultra-Orthodox Society in Israel. 2020. https://www.idi.org.il/media/15500/haredi-2020.pdf (in Hebrew. 30 June 2021, date last accessed).
- 21. Stein-Zamir C, Israeli A. Age-appropriate versus up-to-date coverage of routine childhood vaccinations among young children in Israel. Hum Vaccin Immunother 2017;13:2102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lazarus JV, Ratzan SC, Palayew A et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med 2021;27:225–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scientific Advisory Group for Emergencies (SAGE), ethnicity sub-group. Factors Influencing COVID-19 Vaccine Uptake among Minority Ethnic Groups. https://www.gov.uk/government/publications/factors-influencing-covid-19-vaccine-uptake-among-minority-ethnic-groups-17-december-2020 (9 June 2021, date last accessed).
- 24. Reiter PL, Pennell ML, Katz ML. Acceptability of a COVID-19 vaccine among adults in the United States: how many people would get vaccinated? Vaccine 2020;38:6500–07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nuti SV, Armstrong K. Lay epidemiology and vaccine acceptance. JAMA 2021;326:301. [DOI] [PubMed] [Google Scholar]
- 26. Green MS, Abdullah R, Vered S, Nitzan D. A study of ethnic, gender and educational differences in attitudes toward COVID-19 vaccines in Israel - implications for vaccination implementation policies. Isr J Health Policy Res 2021;10:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Okun BS, Friedlander D. Educational stratification among Arabs and Jews in Israel: historical disadvantage, discrimination, and opportunity. Popul Stud (Camb) 2005;59:163–80. [DOI] [PubMed] [Google Scholar]
- 28. Di Gennaro F, Murri R, Segala FV et al. Attitudes towards anti-SARS-CoV2 vaccination among healthcare workers: results from a national survey in Italy. Viruses 2021;13:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alison P. For Causal Analysis of Competing Risks, Don’t Use Fine & Gray’s Subdistribution Method. 2018. https://statisticalhorizons.com/for-causal-analysis-of-competing-risks (30 June 2021, date last accessed).
- 30. Austin PC, Steyerberg EW, Putter H. Fine-Gray subdistribution hazard models to simultaneously estimate the absolute risk of different event types: cumulative total failure probability may exceed 1. Stat Med 2021;40:4200–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Levin-Zamir D. Communication, health literacy and a systems approach for mitigating the COVID-19 pandemic: the case for massive vaccine roll-out in Israel. J Health Commun 2020;25:816–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.