Abstract
Background
Regdanvimab (CT-P59) is a monoclonal antibody with neutralizing activity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). We report on part 1 of a 2-part randomized, placebo-controlled, double-blind study for patients with mild-to-moderate coronavirus disease 2019 (COVID-19).
Methods
Outpatients with mild-to-moderate COVID-19 received a single dose of regdanvimab 40 mg/kg (n = 100), regdanvimab 80 mg/kg (n = 103), or placebo (n = 104). The primary end points were time to negative conversion of SARS-CoV-2 from nasopharyngeal swab based on quantitative reverse transcription polymerase chain reaction (RT-qPCR) up to day 28 and time to clinical recovery up to day 14. Secondary end points included the proportion of patients requiring hospitalization, oxygen therapy, or mortality due to COVID-19.
Results
Median (95% CI) time to negative conversion of RT-qPCR was 12.8 (9.0–12.9) days with regdanvimab 40 mg/kg, 11.9 (8.9–12.9) days with regdanvimab 80 mg/kg, and 12.9 (12.7–13.9) days with placebo. Median (95% CI) time to clinical recovery was 5.3 (4.0–6.8) days with regdanvimab 40 mg/kg, 6.2 (5.5–7.9) days with regdanvimab 80 mg/kg, and 8.8 (6.8–11.6) days with placebo. The proportion (95% CI) of patients requiring hospitalization or oxygen therapy was lower with regdanvimab 40 mg/kg (4.0% [1.6%–9.8%]) and regdanvimab 80 mg/kg (4.9% [2.1%–10.9%]) vs placebo (8.7% [4.6%–15.6%]). No serious treatment-emergent adverse events or deaths occurred.
Conclusions
Regdanvimab showed a trend toward a minor decrease in time to negative conversion of RT-qPCR results compared with placebo and reduced the need for hospitalization and oxygen therapy in patients with mild-to-moderate COVID-19.
Clinical trial registration.
NCT04602000 and EudraCT 2020-003369-20.
Keywords: COVID, 19, CT, P59, regdanvimab, SARS-CoV-2
The coronavirus disease 19 (COVID-19) pandemic that began in early 2020 is still ongoing, with >200 million confirmed cases and at least 4.4 million deaths as of August 2021 [1]. Several vaccines for COVID-19 have been developed, and programs are ongoing in many countries [2–5]. In some countries, the incidence of COVID-19 was reduced temporarily after the start of the vaccination program; however, the Delta variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has opened a new phase, and COVID-19 cases are increasing again in many countries [6].
Effective treatments are urgently needed to decrease morbidity and mortality related to COVID-19 and to reduce the associated burden on public health services [7]. Therapies applied early in the course of clinical disease can speed up clinical recovery, shorten the duration of viral shedding, and limit the need for hospitalization, thereby lessening the pressure on the overall health care system. Monoclonal antibodies to spike (S) protein of SARS-CoV-2, which target cellular entry of SARS-CoV-2 through interaction with the angiotensin-converting enzyme 2 receptor [8–12], have been shown to reduce hospitalizations, viral titers, and clinical symptoms in patients with COVID-19 [13–15], as well as lower the risk of COVID-19 in nursing home residents [16, 17].
Regdanvimab (CT-P59) is a neutralizing antibody that is active against various SARS-CoV-2 isolates, including the D614G S protein variant [18]. Regdanvimab has been shown to bind to the receptor-binding motif within the SARS-CoV-2 receptor-binding domain, which sterically inhibits interaction with ACE2 [18]. Administration of regdanvimab reduced the viral load and reduced mortality in animal models of SARS-CoV-2 infection [18]. The South Korean Ministry of Food and Drug Safety granted marketing authorization (September 2021) for regdanvimab in patients with mild-to-moderate COVID-19 (aged >50 years or with certain predefined medical conditions) [19]. The European Medicines Agency approved regdanvimab (November 2021) for use in adult patients with COVID-19 who do not require supplemental oxygen and are at high risk of progressing to severe COVID-19 [20].
We report 28-day results from part 1 of a 2-part phase 2/3 study of regdanvimab in outpatients with mild-to-moderate COVID-19.
METHODS
Trial Oversight
This was a 2-part, phase 2/3, randomized, parallel-group, placebo-controlled, double-blind study (NCT04602000; EudraCT 2020-003369-20). Part 1, a phase 2 study, assessed the SARS-CoV-2 suppression effect of regdanvimab and followed patients for 180 days after study drug administration; part 2, a phase 3 study, examines the clinical effect of regdanvimab on COVID-19 symptoms, hospitalization and oxygen therapy, and mortality. Part 2 will be reported separately. The study was conducted according to the ethical principles of the Declaration of Helsinki and in compliance with the International Council for Harmonisation’s Good Clinical Practice guidelines and applicable regulatory requirements.
Patient Consent
Written informed consent was obtained from each participant before participation in the study. The design of the study was approved by the appropriate local institutional review boards and/or independent ethics committees, and the study was monitored by an independent data safety monitoring board.
Participants
COVID-19 infection status was diagnosed at screening using the sponsor-supplied rapid SARS-CoV-2 diagnostic test or reverse transcription polymerase chain reaction (RT-PCR). Patients with mild-to-moderate COVID-19 according to the World Health Organization (WHO) criteria were enrolled [21]. According to the WHO guidelines, having clinical signs and symptoms of nonsevere pneumonia is categorized as having moderate disease. Therefore, chest imaging was performed at screening to assist with diagnosis of pneumonia and correct disease staging. Eligible participants were aged ≥18 years, had oxygen saturation of >94% on room air, and did not require supplemental oxygen. Onset of symptoms (feverishness, cough, shortness of breath, sore throat, body/muscle pain, fatigue, headache, chills, nasal congestion, loss of taste/smell, or diarrhea) was required to be within 7 days before study drug administration, and patients were required to present with fever, cough, shortness of breath, sore throat, body pain, fatigue, or headache within 48 hours before study drug administration. Patients with a current serious health condition, or with ongoing or history of active or severe infections, were excluded.
Randomization and Masking
Randomization was performed using an interactive web response system, and a randomization schedule was prepared by unblinded biostatisticians. Randomization was stratified by age (≥60 vs <60 years), region (United States vs Asia vs Europe vs other), baseline comorbidities (yes vs no for having at least 1 of cardiovascular disease, chronic respiratory disease, hypertension, diabetes mellitus, or pneumonia), and participation in the pharmacokinetic substudy (yes vs no). Participants, personnel, and outcome assessors were blinded to treatment allocation for the duration of the study. Regdanvimab and placebo were supplied in identical vials identified by a study drug number, and infusions were prepared by designated unblinded personnel.
Trial Procedures
Participants were assigned randomly (1:1:1 ratio) to receive a single dose of regdanvimab 40 mg/kg, regdanvimab 80 mg/kg, or placebo on study day 1. Regdanvimab and placebo were reconstituted in 250 mL of 0.9% sodium chloride and administered via intravenous infusion over 90 ± 15 minutes. Patients could receive standard-of-care treatment, excluding antiviral drugs and/or possible SARS-CoV-2 active drugs (only to be administered as rescue therapies).
Nasopharyngeal swabs for assessment of viral shedding (based on quantitative reverse transcription polymerase chain reaction [RT-qPCR]) were taken predose on day 1 and at 24, 48, 72, 96, 120, 144, 216, 312 (day 14), 384 (day 17), 480 (day 21), and 648 (day 28) hours after study drug administration. To quantitate viral loads, 1 real-time RT-qPCR assessment (Sarbeco E-gene assay) was employed, which is specific to the E gene of sarbecoviruses including SARS-CoV and SARS-CoV-2.
Participants were required to complete a patient diary at screening and twice daily on days 1–28, which included a checklist of 7 symptoms of COVID-19 (fever, cough, shortness of breath or difficulty breathing, sore throat, body pain or muscle pain, fatigue, and headache).
Outcomes
The primary study end points were time to conversion to negative nasopharyngeal swab specimen based on RT-qPCR (negative titer threshold of 2.33 log10 copies/mL) up to day 28, and time to clinical recovery up to day 14.
Conversion to negative RT-qPCR result was defined as a negative nasopharyngeal swab specimen based on RT-qPCR result at 2 or more consecutive time points (the first time point was taken as the time to conversion to negative RT-qPCR result). Clinical recovery was defined as all symptoms scored as “absent” or “mild” for ≥24 hours, based on checklist results; symptoms scored as “moderate” or “severe” at baseline were required to be scored as “mild” or “absent” at recovery, whereas symptoms rated as “mild” or “absent” at baseline were required to be rated as “absent” at recovery.
Secondary efficacy end points included (1) the proportion of patients with clinical symptoms requiring hospitalization (≥24 hours of acute care), oxygen therapy (≥24 hours of supplemental oxygen care, with oxygen saturation of ≤94% on room air before administration), or death due to COVID-19 up to day 28; (2) the proportion of patients with conversion to negative RT-qPCR result; (3) the proportion of patients with hospital admission; (4) the proportion of patients requiring supplemental oxygen; (5) the proportion of patients with mechanical ventilation use; (6) the proportion of patients requiring rescue therapy; (7) the proportion of patients with intensive care unit admission (each due to COVID-19); or (8) the proportion of patients with all-cause mortality.
Safety evaluations were based on the monitoring of treatment-emergent adverse events (TEAEs) and treatment-emergent serious adverse events (TESAEs), vital sign measurements, physical examination, electrocardiograms, and clinical laboratory analyses. TEAEs were coded according to the Medical Dictionary for Regulatory Activities, version 23.1, and graded for severity according to the Common Terminology Criteria for Adverse Events, version 5.0. Hypersensitivity monitoring was performed before and after administration of study drug on day 1.
Statistical Analyses
It was estimated that a sample size of 100 individuals per group would provide at least 80% power, at a 2-sided significance level of .05, to detect an increase in the improvement rate ratio between regdanvimab groups and placebo for the primary end points.
Efficacy was evaluated in the intent-to-treat infected (ITTI) population, which comprised all randomly assigned patients with confirmed COVID-19 assessed by pre-infusion RT-qPCR on day 1 and receiving a partial or full dose of the study drug. The safety population included all randomized participants who had received a partial or full dose of the study drug.
Primary efficacy analyses were performed using a stratified log-rank test for time-to-event end points. Confidence intervals for median time to event were constructed using the method of Brookmeyer and Crowley [22] with log–log transformation. Improvement/clinical recovery rate ratios (with 95% CIs) were estimated using a stratified Cox proportional hazards model. The Wald test was applied to construct CIs for hazard ratios. Adjustments for multiple testing were not performed; therefore, 95% CIs and P values should not be used to infer definitive treatment effects. Secondary efficacy end points were summarized using descriptive statistics, frequency tables, or Kaplan-Meier (time-to-event) methods. The Wilson score interval test [23] was used to construct the 95% CIs for proportions.
Subgroup analyses were conducted according to COVID-19 severity (mild vs moderate; prespecified) and age (≥50 years; exploratory). Post hoc analyses of the primary efficacy outcome were conducted based on negative titer thresholds of 3.0 and 4.0 log10 copies/mL.
All statistical analyses were conducted using Statistical Analysis System (SAS) software, version 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Patient Disposition and Baseline Characteristics
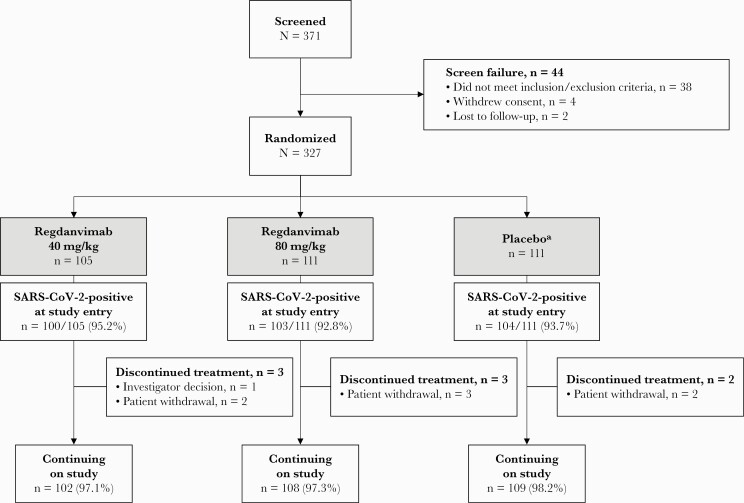
Patient enrollment began on October 7, 2020, and the last patient’s day 28 visit was on December 18, 2020. Screening was conducted at 23 centers across South Korea, Romania, Spain, and the United States (details of participating centers are listed in the Supplementary Data). A total of 371 patients were screened, and 327 were randomized and included in the intent-to-treat (ITT) population (Figure 1). One patient randomized to the placebo group was incorrectly administered a study drug that partially contained regdanvimab; this patient was analyzed per their original treatment assignment (placebo) for ITT and ITTI analyses, included in the regdanvimab 40 mg/kg group for safety analyses, and excluded from the pharmacokinetic analyses.
Figure 1.
Patient disposition. aIn the placebo group, 1 patient was randomized to the placebo group but was administered study drug partially containing regdanvimab; the individual was excluded from the pharmacokinetic population. Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Baseline demographic and disease characteristics were generally well balanced between participants in the ITT population (Table 1). Concomitant medications are recorded in Supplementary Table 1.
Table 1.
Baseline Demographics and Characteristics (ITT Population)
| Characteristics | Regdanvimab 40 mg/kg | Regdanvimab 80 mg/kg | Regdanvimab Combined | Placebo |
|---|---|---|---|---|
| n = 105 | n = 111 | n = 216 | n = 111 | |
| Age | ||||
| Median (IQR), y | 51.0 (42–60) | 51.0 (40–60) | 51.0 (40–60) | 52.0 (41–61) |
| ≥60 y, n (%) | 27 (25.7) | 28 (25.2) | 55 (25.5) | 30 (27.0) |
| <60 y, n (%) | 78 (74.3) | 83 (74.8) | 161 (74.5) | 81 (73.0) |
| Male sex, n (%) | 59 (56.2) | 59 (53.2) | 118 (54.6) | 48 (43.2) |
| Race, n (%) | ||||
| White | 94 (89.5) | 96 (86.5) | 190 (88.0) | 96 (86.5) |
| Asian | 11 (10.5) | 15 (13.5) | 26 (12.0) | 15 (13.5) |
| Ethnicity, n (%) | ||||
| Hispanic or Latino | 6 (5.7) | 11 (9.9) | 17 (7.9) | 10 (9.0) |
| Non-Hispanic or non-Latino | 99 (94.3) | 100 (90.1) | 199 (92.1) | 101 (91.0) |
| Region, n (%) | ||||
| USA | 1 (1.0) | 4 (3.6) | 5 (2.3) | 3 (2.7) |
| Asia | 11 (10.5) | 15 (13.5) | 26 (12.0) | 14 (12.6) |
| Europe | 93 (88.6) | 92 (82.9) | 185 (85.6) | 94 (84.7) |
| BMI | ||||
| Mean (SD), kg/m2 | 27.1 (4.8) | 27.1 (4.1) | 27.1 (4.5) | 26.8 (4.2) |
| Baseline comorbidities, n (%) | ||||
| Yes | 78 (74.3) | 80 (72.1) | 158 (73.1) | 82 (73.9) |
| Confirmed SARS-CoV-2 infection,a n (%) | 101 (96.2) | 103 (92.8) | 204 (94.4) | 103 (92.8) |
| Moderate disease,b n (%) | 64 (61.0) | 65 (58.6) | 129 (59.7) | 60 (54.1) |
| High-risk disease,c n (%) | 73 (69.5) | 82 (73.9) | 155 (71.8) | 76 (68.5) |
| Time since symptom onset | ||||
| All patients, median (IQR), d | 3.0 (2–4) | 3.0 (2–4) | 3.0 (2–4) | 3.0 (2–4) |
| Moderate disease, median (IQR), d | 3.0 (2–4) | 3.0 (2–4) | 3.0 (2–4) | 3.0 (2–4) |
| Received ≥1 prior medication,d n (%) | 19 (18.1) | 23 (20.7) | 42 (19.4) | 26 (23.4) |
Abbreviations: BMI, body mass index; IQR, interquartile range; ITT, intent-to-treat; RT-PCR, reverse transcription polymerase chain reaction; RT-qPCR, quantitative reverse transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
All patients were enrolled based on a local rapid SARS-CoV-2 diagnostic test or RT-PCR-positive result; following enrollment, SARS-CoV-2 infection was confirmed centrally by RT-qPCR.
Based on the presence of x-ray-confirmed or computed tomography–confirmed pneumonia at screening.
High risk of progression to severe disease was defined as patients aged >50 years, with BMI >30 kg/m², cardiovascular disease (including hypertension), chronic lung disease (including asthma), type 1 or type 2 diabetes mellitus, chronic kidney disease (including those on dialysis), chronic liver disease, or immunosuppressed at baseline.
The most commonly reported prior medication by drug class in all groups was analgesics (9 [8.6%], 14 [12.6%], and 15 [13.5%] patients in the regdanvimab 40 mg/kg, regdanvimab 80 mg/kg, and placebo groups, respectively).
Twenty patients in the ITT population did not have confirmed COVID-19 and were excluded from the ITTI population (N = 307 [regdanvimab 40 mg/kg: n = 100; regdanvimab 80 mg/kg: n = 103; placebo: n = 104]) (Supplementary Table 2).
Efficacy (ITTI Population)
The estimated median (95% CI) time to conversion to negative RT-qPCR result with a threshold <2.33 log10 copies/mL up to day 28 was 12.8 days in the regdanvimab 40 mg/kg group, 11.9 days in the regdanvimab 80 mg/kg group, 12.7 days in the combined regdanvimab group, and 12.9 days in the placebo group (Table 2, Figure 2A). The corresponding improvement rate ratios (95% CI) relative to placebo were 1.3 (1.0–1.8; P = .06), 1.2 (0.9–1.6; P = .21), and 1.3 (1.0–1.6; P = .08), respectively (Table 2). When a threshold of <3.0 log10 copies/mL was applied in a post hoc analysis, the median time to conversion to negative RT-qPCR result was 6.0 days in the combined regdanvimab group vs 8.9 days with placebo (Supplementary Table 3, Supplementary Figure 1).
Table 2.
Efficacy End Points (ITTI Populationa)
| End Point | Regdanvimab 40 mg/kg | Regdanvimab 80 mg/kg | Regdanvimab Combined | Placebo |
|---|---|---|---|---|
| n = 100 | n = 103 | n = 203 | n = 104 | |
| Primary end points | ||||
| Conversion to negative RT-qPCR result to day 28 | ||||
| Negative titer threshold of 2.33 log10 copies/mL | ||||
| All patients, n | 100 | 103 | 203 | 104 |
| Time to negative RT-qPCR result, median (95% CI), d | 12.8 (9.0–12.9) | 11.9 (8.9–12.9) | 12.7 (9.0–12.8) | 12.9 (12.7–13.9) |
| Improvement rate ratio (95% CI) | 1.3 (1.0–1.8) | 1.2 (0.9–1.6) | 1.3 (1.0–1.6) | – |
| Log-rank test P valueb | .06 | .21 | .08 | – |
| Mild SARS-CoV-2 infection,c n | 38 | 40 | 78 | 46 |
| Time to negative RT-qPCR result, median (95% CI), d | 12.7 (8.9–12.9) | 9.1 (8.9–12.9) | 10.5 (8.9–12.8) | 13.0 (9.0–15.8) |
| Negative conversion ratio (95% CI) | 1.7 (1.1–2.8) | 1.1 (0.7–1.7) | 1.3 (0.9–2.0) | – |
| Log-rank test P valueb | .02 | .69 | .16 | – |
| Moderate SARS-CoV-2 infection,c n | 62 | 63 | 125 | 57 |
| Time to negative RT-qPCR result, median (95% CI), d | 12.8 (8.8–15.8) | 12.7 (8.9–13.8) | 12.8 (9.1–13.0) | 12.9 (10.8–15.8) |
| Negative conversion ratio (95% CI) | 1.4 (0.9–2.0) | 1.3 (0.9–2.0) | 1.4 (1.0–1.9) | – |
| Log-rank test P valueb | .13 | .17 | .10 | – |
| Clinical recovery to day 14 d | ||||
| All patients, n | 94 | 92 | 186 | 99 |
| Time to event, median (95% CI), d | 5.3 (4.0–6.8) | 6.2 (5.5–7.9) | 5.7 (5.2–6.8) | 8.8 (6.8–11.6) |
| Clinical recovery ratio (95% CI) | 1.6 (1.1–2.2) | 1.4 (1.0–2.0) | 1.5 (1.1–2.0) | – |
| Log-rank test P valueb | .01 | .04 | .01 | – |
| Mild SARS-CoV-2 infection,c n | 34 | 38 | 72 | 45 |
| Time to event, median (95% CI), d | 4.4 (2.2–7.7) | 5.5 (3.2–7.6) | 4.8 (3.0–5.9) | 6.9 (4.8–8.8) |
| Clinical recovery ratio (95% CI) | 1.5 (0.9–2.5) | 1.7 (1.1–2.9) | 1.6 (1.0–2.5) | – |
| Moderate SARS-CoV-2 infection,c n | 60 | 54 | 114 | 53 |
| Time to event, median (95% CI), d | 5.7 (4.1–7.3) | 7.3 (5.6–10.7) | 6.5 (5.5–7.7) | 10.8 (6.8–n.c.) |
| Clinical recovery ratio (95% CI) | 1.7 (1.1–2.7) | 1.3 (0.8–2.2) | 1.5 (1.0–2.3) | – |
| Secondary end points | ||||
| Patients with clinical symptoms requiring hospitalization or oxygen therapy due to COVID-19 to day 28, n/N (%), (95% CI) | 4/100 (4.0), (1.6–9.8) | 5/103 (4.9), (2.1–10.9) | 9/203 (4.4), (2.3–8.2) | 9/104 (8.7), (4.6–15.6) |
| Mild SARS-CoV-2 infection,c n/N | 0/38 | 0/40 | 0/78 | 0/46 |
| Moderate SARS-CoV-2 infection,c n/N (%), (95% CI) | 4/62 (6.5), (2.5–15.4) | 5/63 (7.9), (3.4–17.3) | 9/125 (7.2), (3.8–13.1) | 9/57 (15.8), (8.5–27.4) |
| Patients achieving conversion to negative RT-qPCR result (negative titer threshold of 2.33 log 10 copies/mL), n/N (%) | ||||
| Up to day 14 | 67/100 (67.0) | 68/103 (66.0) | 135/203 (66.5) | 63/104 (60.6) |
| Up to day 28 | 92/100 (92.0) | 90/103 (87.4) | 182/203 (89.7) | 87/104 (83.7) |
| Patients with ≥1 disease status to day 28, e n (%) | 7 (7.0) | 11 (10.7) | 18 (8.9) | 15 (14.4) |
| Patients requiring hospital admission, n (%) | 4 (4.0) | 5 (4.9) | 9 (4.4) | 9 (8.7) |
| Patients requiring supplemental oxygen, n (%) | 4 (4.0) | 4 (3.9) | 8 (3.9) | 9 (8.7) |
| Patients requiring mechanical ventilation, n (%) | 0 | 1 (1.0) | 1 (0.5) | 0 |
| Patients requiring rescue therapy, n (%) | 7 (7.0) | 11 (10.7) | 18 (8.9) | 15 (14.4) |
| Patients requiring ICU transfer, n (%) | 0 | 0 | 0 | 0 |
| All-cause mortality, n (%) | 0 | 0 | 0 | 0 |
| Patients achieving clinical recovery, n/N (%) | ||||
| Up to day 7 | 53/94 (56.4) | 46/92 (50.0) | 99/186 (53.2) | 37/99 (37.4) |
| Up to day 14 | 72/94 (76.6) | 72/92 (78.3) | 144/186 (77.4) | 63/99 (63.6) |
| Up to day 28 | 82/94 (87.2) | 79/92 (85.9) | 161/186 (86.6) | 71/99 (71.7) |
P values and CIs have not been adjusted for multiple comparisons.
Abbreviations: ICU, intensive care unit; ITTI, intent-to-treat infected; n.c., not calculated; RT-qPCR, quantitative reverse transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
One patient randomized to the placebo group mistakenly received a partial dose of regdanvimab; this patient was analyzed according to their original treatment assignment (placebo).
P value from log-rank test, stratified by age (≥60 vs <60 years) and baseline comorbidities (yes vs no).
Subgroup analyses (note: 1 patient was excluded from the subgroup analyses because their chest x-ray result was missing at screening).
Patients with at least 1 symptom record missing at baseline were excluded from this analysis.
Disease status included requirement for supplemental oxygen, hospital admission, ICU transfer, mechanical ventilation use, or rescue therapy due to COVID-19.
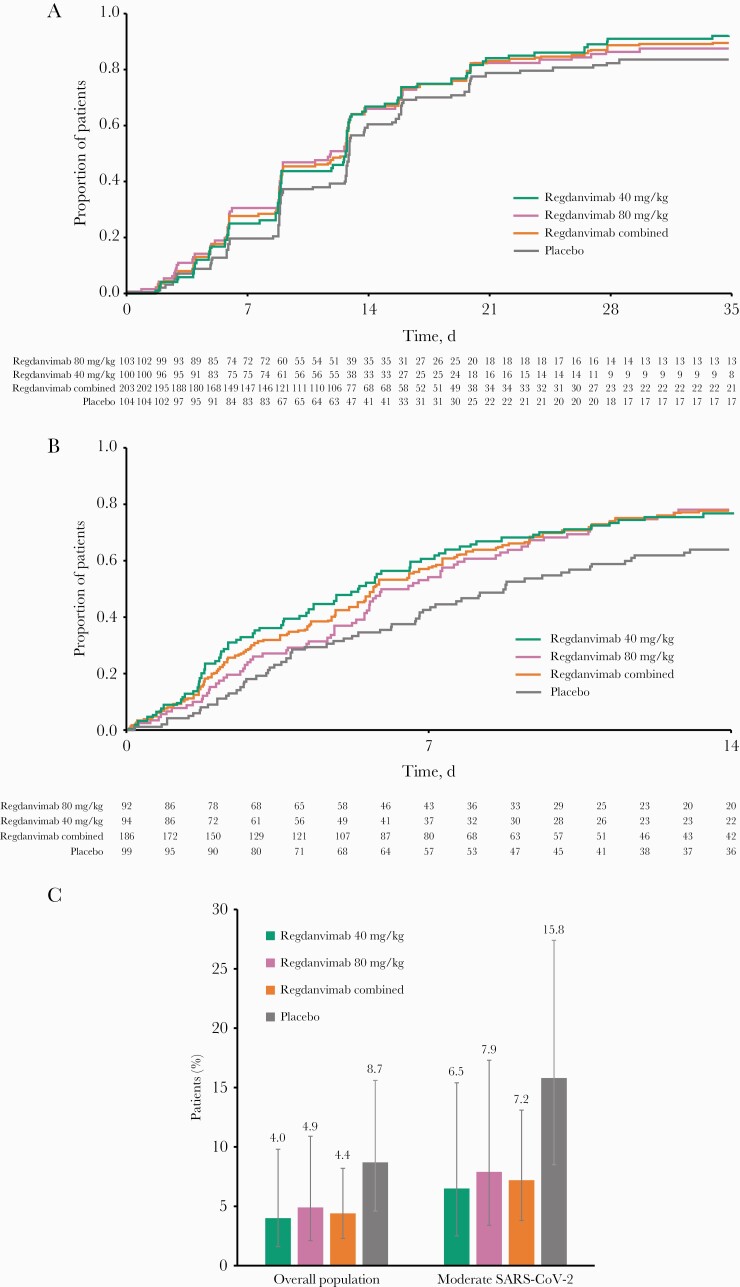
Figure 2.
Efficacy end points (ITTI population). A, Kaplan-Meier plot of primary end point “time to negative conversion” to day 28 (SARS-CoV-2-negative threshold: 2.33 log10 copies/mL). B, Kaplan-Meier plot of primary end point “time to clinical recovery” to day 14. C, Proportion of patients with clinical symptoms requiring hospitalization or oxygen therapy due to COVID-19 to day 28 in the overall population and subgroup by disease severity. Error bars are 95% CIs. Abbreviations: ITTI, intent-to-treat infected; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
The median time to conversion to negative RT-qPCR result up to day 28 in patients with mild COVID-19 is shown in Table 2. Post hoc analysis with a threshold of <3.0 log10 copies/mL is summarized in Supplementary Table 3.
The proportion of patients achieving conversion to negative RT-qPCR result was higher in the regdanvimab groups than the placebo group up to days 14 and 28 (Table 2). The regdanvimab groups also exhibited greater reduction from baseline in viral load in comparison with the placebo group. A reduction in viral load of 3.0 log10 copies/mL was achieved with regdanvimab between baseline and day 7; a similar reduction was not reached in the placebo group until day 10 (Supplementary Figure 2). Post hoc analysis of time-weighted average change from baseline in viral shedding is shown in Supplementary Table 4.
The median (95% CI) time to clinical recovery up to day 14 was shorter in the combined regdanvimab group vs the placebo group (5.7 [5.2–6.8] vs 8.8 [6.8–11.6] days) (Table 2, Figure 2B). Clinical recovery ratios (95% CI) were 1.6 (1.1–2.2; P = .01), 1.4 (1.0–2.0; P = .04), and 1.5 (1.1–2.0; P = .01) in the regdanvimab 40 mg/kg, regdanvimab 80 mg/kg, and combined regdanvimab groups, respectively (Table 2). Clinical recovery ratios also favored regdanvimab over placebo in prespecified subgroups by disease status (Table 2).
The proportion of patients (95% CI) with clinical symptoms requiring hospitalization or oxygen therapy due to COVID-19 was lower in the regdanvimab groups than the placebo group (regdanvimab 40 mg/kg: 4 [1.6–9.8]; 4.0%; regdanvimab 80 mg/kg: 5 [2.1–10.9]; 4.9%; placebo: 9 [4.6–15.6]; 8.7%) (Figure 2C, Table 2; Supplementary Table 3). Notably, only patients with moderate COVID-19 contributed events to this composite end point; the proportions of patients meeting this end point in the combined regdanvimab group was >50% lower than in the placebo group among patients with moderate infection (7.2% vs 15.8%). In post hoc analysis, this was also true for patients aged ≥50 years with moderate infection (8.8% vs 23.7%) and those at high risk of progressing to COVID-19 (5.5% vs 12.5%). In addition, regdanvimab effectively reduced the proportion of patients reporting requirement for supplemental oxygen, hospital admission, and rescue therapy, individually, and no all-cause mortality was reported up to day 28 (Table 2).
Safety
Overall, 182 TEAEs were reported in 92 patients (28.3%). A similar proportion of patients experienced ≥1 TEAE across treatment groups (Table 3), and the majority of TEAEs were grade 1 or 2 in intensity. The most frequently reported treatment-related TEAE was hypertriglyceridemia in patients receiving regdanvimab 40 mg/kg (3 [2.9%] patients) and infusion-related reaction or hypertriglyceridemia in patients receiving placebo (2 [1.8%] patients each). No treatment-related TEAEs considered to be related to the study drug were reported in >1 patient in the regdanvimab 80 mg/kg group. There were no TESAEs or TEAEs leading to permanent study discontinuation. Infusion-related reactions were reported in 3 patients (regdanvimab 40 mg/kg: 1; placebo: 2); the patient receiving regdanvimab developed grade 2 pyrexia and grade 1 dyspnea after drug administration but recovered on the same day after receiving paracetamol and oxygen therapy.
Table 3.
Safety and Tolerability (Safety Population)
| Adverse Event | Regdanvimab 40 mg/kg, n (%) | Regdanvimab 80 mg/kg, n (%) | Placebo, n (%) |
|---|---|---|---|
| n = 105 | n = 110 | n = 110 | |
| Any TEAE | 31 (29.5) | 27 (24.5) | 34 (30.9) |
| Related to study drug | 7 (6.7) | 5 (4.5) | 5 (4.5) |
| ≥1 grade 3 TEAEa | 5 (4.8) | 4 (3.6) | 2 (1.8) |
| Related to study drug | 1 (1.0) | 0 | 0 |
| Any TESAE | 0 | 0 | 0 |
| Any TEAE leading to discontinuation | 0 | 0 | 0 |
| Any TEAE of special interest | |||
| Infusion-related reactions | 1 (1.0) | 0 | 2 (1.8) |
| Death | 0 | 0 | 0 |
Abbreviations: TEAE, treatment-emergent adverse event; TESAE, treatment-emergent serious adverse event.
No patients experienced grade 4 or 5 TEAEs up to day 28.
There were no notable differences between groups in laboratory parameters, including liver and renal function, vital signs, or electrocardiogram results. At day 28, the proportion of patients with antidrug antibodies (ADAs) was low (regdanvimab 40 mg/kg: 0; regdanvimab 80 mg/kg: 3 [2.7%]; placebo: 5 [4.5%]), and no antibody-dependent enhancement events were reported.
Pharmacokinetics
In the pharmacokinetic population (n = 88), mean regdanvimab serum concentrations were higher in the regdanvimab 80 mg/kg group than the 40 mg/kg group at all time points following intravenous infusion (Supplementary Figure 3).
Serology
The proportions of patients with immunoglobulin M or G positivity increased over time and were similar across groups at all time points (Supplementary Table 5).
DISCUSSION
One of the most important factors for COVID-19 treatments is how effective they are in preventing patients from becoming critically ill. In the event of hypoxemia, hospitalization is required due to the need for oxygen therapy. It has been suggested that hyper-responsive immune responses against SARS-CoV-2 might be an underlying cause of hypoxemia in patients with COVID-19 [24], and high viral titer could be in part responsible for ongoing hypoxemia [13].
Thus, there is an increasing need to prevent COVID-19 patients from progressing to severe illness and to control the virus in the early stages of infection.
Regdanvimab has shown a promising safety profile in phase 1 studies in healthy subjects and patients with mild COVID-19 and exhibited potential antiviral and clinical efficacy in patients with mild COVID-19 [25]. In this phase 2 study in patients with mild-to-moderate COVID-19, regdanvimab showed a trend toward a minor decrease in time to conversion to a negative RT-qPCR result (0.3 days in the combined regdanvimab group compared with placebo at the 2.33-log10 copies/mL threshold). This trend was in line with other studies of monoclonal antibodies [13–15], suggesting low association between time to conversion to negative RT-qPCR result and clinical outcome. When the negative titer threshold was set to 3.0 log10 copies/mL in the post hoc analysis, the difference vs placebo was greater (3.0 days in the combined regdanvimab group vs placebo), in line with the trend observed in the results for time to clinical recovery. This could suggest that some further optimization of our understanding of the thresholds associated with progression to severe disease is needed as this pandemic unfolds.
Compared with placebo, the estimated median time to clinical recovery was shorter by 3 days in the combined regdanvimab group; the magnitude of this difference was greater in patients with moderate disease (4.3 days) and, as shown in the post hoc analysis, in patients aged ≥50 years with moderate disease (6.2 days). Consistent with other early therapies for COVID-19 [13–15], regdanvimab reduced the proportion of patients with clinical symptoms requiring hospitalization or oxygen therapy due to COVID-19. The magnitude of this reduction exceeded 50% in patients with moderate disease and, in the post hoc analysis, also in those with moderate disease who were aged ≥50 years.
At this early stage, direct comparisons between neutralizing antibody therapies for COVID-19 are difficult due to differences in key study end points. However, results presented for casirivimab/imdevimab, at a similar stage of development, showed similar differences compared with placebo (−0.3 log10 copies/mL) in time-weighted average change in viral load [13].
Regdanvimab was well tolerated, no clinically significant safety issues were identified, and there were no deaths up to day 28. Regdanvimab did not appear to interfere with the formation of antibodies (no antibody-dependent enhancement events were reported), no ADAs were measured in the regdanvimab 40 mg/kg group, and the proportion of patients with ADAs in the regdanvimab 80 mg/kg group was <3.0%.
Despite having dose-dependent pharmacokinetics, no dose–response relationship was observed for regdanvimab. Therefore, it was considered that 40 mg/kg represents a sufficient dose for virus neutralization, with no further advantages expected at higher doses. Our findings are in line with other monoclonal antibody products [13–15].
Based on these data, the second part of this study will compare the 40 mg/kg dose with placebo in a larger population of patients with mild-to-moderate COVID-19.
This study has some limitations; the current study enrolled no Black or Hispanic patients, 2 populations that are disproportionately affected by COVID-19 [26, 27]. Likewise, there was a relatively small proportion (20%–30%) of elderly patients enrolled, another group that is at high risk of complications and death from COVID-19 [28]. The impact of regdanvimab on infectious virus has not been studied, but it would be important to assess this to inform treatment decisions to reduce the risk of transmission. The pharmacokinetics of regdanvimab has not been evaluated in patients with renal and/or hepatic impairment. Although patients with abnormal liver function and/or renal impairment were not included in this study, metabolism via cytochrome P450 enzymes and elimination in the urine are not expected. Finally, patients enrolled in this study would not have been exposed to emerging SARS-CoV-2 variants that were identified after the data cutoff of December 18, 2020, in countries where the study had been performed. Further studies are ongoing to determine the effectiveness of regdanvimab against SARS-CoV-2 variants.
In conclusion, regdanvimab shortened the negative conversion time of SARS-CoV-2, reduced the median time to clinical recovery, and reduced the requirement for hospitalization and oxygen therapy in patients with mild-to-moderate COVID-19.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors thank all patients and investigators involved in the study. Medical writing support (including development of a draft outline and subsequent drafts in consultation with the authors, assembling tables and figures, collating author comments, copyediting, fact checking, and referencing) was provided by Duncan Campbell, PhD, CMPP, at Aspire Scientific (Bollington, UK), and was funded by Celltrion, Inc. (Incheon, Republic of Korea).
Financial support. This work was supported by Celltrion, Inc. (Incheon, Republic of Korea). This research was also supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HQ20C0045).
Disclaimer. Celltrion, Inc., contributed to the study design, data analysis, and interpretation. Celltrion authors contributed to the preparation of the manuscript, including critical review and revision of manuscript drafts, and approved the final version.
Potential conflicts of interest. An.S.-C., O.S., and Ad.S.-C. have been investigators in COVID-19 clinical trials by Algernon Pharmaceuticals, Atea Pharmaceuticals, Diffusion Pharmaceuticals, and Regeneron Pharmaceuticals, outside the scope of the submitted work, and by Celltrion, Inc., within the scope of the submitted work. L.-L.P., Y.-S.K., and S.H.C. declare no conflicts. J.Y.K. and Y.R.J. have been investigators in COVID-19 clinical trials by Daewoong Pharmaceuticals, Enzychem Lifesciences, and GC Pharma, outside the scope of the submitted work, and by Celltrion, Inc., within the scope of the submitted work. S.J.L. and S.H.K. are employees of and hold shares in Celltrion, Inc. I.C., J.H.S., S.G.L., M.R.K., D.R.C., and H.N.K. are employees of Celltrion, Inc. J.S.E. has been an investigator in COVID-19 clinical trials by Enzychem Lifesciences, Bukwang Pharm. Co., Ltd, and SK Chemicals, outside the scope of the submitted work, and by Celltrion, Inc., within the scope of the submitted work. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. S.J.L., S.H.K., I.C., J.H.S., S.G.L., M.R.K., D.R.C., and H.N.K. designed the study and contributed to data analysis/interpretation. J.Y.K. and Y.R.J. collected data and contributed to data analysis/interpretation. An.S.-C., O.S., L.-L.P., Y.-S.K., S.H.C., Ad.S.-C., and J.S.E. collected data. All authors contributed to the preparation of the report, including critical review and revision of manuscript drafts, and approved the final version.
Data availability. The study protocol and statistical analysis plan are available in the Supplementary Data. Individual participant data cannot be made available.
Clinical trial registration. NCT04602000 and EudraCT 2020-003369-20.
References
- 1. World Health Organization. WHO coronavirus disease (COVID-19) dashboard. Available at: https://covid19.who.int/. Accessed 17 May 2021.
- 2. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med 2021; 384:2187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stephenson KE, Le Gars M, Sadoff J, et al. Immunogenicity of the Ad26.COV2.S vaccine for COVID-19. JAMA 2021; 325:1535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. GISAID. Tracking of variants: VOC Delta, relative variant genome frequency per region. Available at: https://www.gisaid.org/hcov19-variants/. Accessed 9 September 2021.
- 7. World Health Organization. Maintaining essential health services: operational guidance for the COVID-19 context interim guidance. Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-essential-health-services-2020.1. Accessed 5 January 2021.
- 8. Liu L, Wang P, Nair MS, et al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature 2020; 584:450–6. [DOI] [PubMed] [Google Scholar]
- 9. Shi R, Shan C, Duan X, et al. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature 2020; 584:120–4. [DOI] [PubMed] [Google Scholar]
- 10. Wu Y, Wang F, Shen C, et al. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science 2020; 368:1274–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zost SJ, Gilchuk P, Case JB, et al. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature 2020; 584:443–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181:271–80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med 2021; 384:238–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen P, Nirula A, Heller B, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med 2021; 384:229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA 2021; 325:632–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. ClinicalTrials.gov. A study of LY3819253 (LY-CoV555) and LY3832479 (LY-CoV016) in preventing SARS-CoV-2 infection and COVID-19 in nursing home residents and staff (BLAZE-2) (NCT04497987). Available at: https://clinicaltrials.gov/ct2/show/NCT04497987. Accessed 16 July 2021.
- 17. Lilly. Lilly’s neutralizing antibody bamlanivimab (LY-CoV555) prevented COVID-19 at nursing homes in the BLAZE-2 trial, reducing risk by up to 80 percent for residents [press release]. Available at: https://investor.lilly.com/news-releases/news-release-details/lillys-neutralizing-antibody-bamlanivimab-ly-cov555-prevented. Accessed 16 July 2021.
- 18. Kim C, Ryu D-K, Lee J, et al. A therapeutic neutralizing antibody targeting receptor binding domain of SARS-CoV-2 spike protein. Nat Commun 2021; 12:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Business Wire. Celltrion’s monoclonal antibody treatment for COVID-19, regdanvimab (CT-P59) becomes the first authorized COVID-19 treatment approved from the Korean Ministry of Food and Drug Safety (MFDS). Available at: https://www.businesswire.com/news/home/20210918005026/en/%C2%A0Celltrion%E2%80%99s-monoclonal-antibody-treatment-for-COVID-19-regdanvimab-CT-P59-becomes-the-first-authorized-COVID-19-treatment-approved-from-the-Korean-Ministry-of-Food-and-Drug-Safety-MFDS. Accessed 23 September 2021.
- 20. European Medicines Agency. COVID-19: EMA recommends authorisation of two monoclonal antibody medicines. Available at: https://www.ema.europa.eu/en/news/covid-19-ema-recommends-authorisation-two-monoclonal-antibody-medicines. Accessed 13 January 2022.
- 21. World Health Organization. Clinical management of COVID-19. Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-2. Accessed 3 February 2021.
- 22. Brookmeyer R, Crowley J.. A confidence interval for the median survival time. Biometrics 1982; 38:29–41. [Google Scholar]
- 23. Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc 1927; 22:209–12. [Google Scholar]
- 24. Blanco-Melo D, Nilsson-Payant BE, Liu WC, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 2020; 181:1036–45.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim JY, Jang YR, Hong JH, et al. Safety, virologic efficacy, and pharmacokinetics of CT-P59, a monoclonal antibody against SARS-CoV-2 spike receptor binding protein: two randomised phase 1 studies in healthy subjects and patients with mild SARS-CoV-2 infection. Clin Ther 2021; 43:1706–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Perkin MR, Heap S, Crerar-Gilbert A, et al. Deaths in people from Black, Asian and minority ethnic communities from both COVID-19 and non-COVID causes in the first weeks of the pandemic in London: a hospital case note review. BMJ Open 2020; 10:e040638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wiley Z, Kubes JN, Cobb J, et al. Age, comorbid conditions, and racial disparities in COVID-19 outcomes. J Racial Ethn Health Disparities 2022; 9:117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Barek MA, Aziz MA, Islam MS.. Impact of age, sex, comorbidities and clinical symptoms on the severity of COVID-19 cases: a meta-analysis with 55 studies and 10014 cases. Heliyon 2020; 6:e05684. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.