Abstract
Green mold (Penicillium digitatum) and blue mold (Penicillium italicum) are among the most economically impactful post-harvest diseases of citrus fruit worldwide. Post-harvest citrus diseases are largely controlled with synthetic fungicides such as pyrimethanil, imazalil, fludioxonil, and thiabendazole. Due to their toxic effects, prolonged and excessive application of these fungicides is gradually restricted in favor of safe and more eco-friendly alternatives. This review comprehensively describes alternative methods for the control of P. digitatum and P. italicum: (a) antagonistic micro-organisms, (b) plant extracts and essential oils, (c) biofungicides, (d) chitosan and chitosan-based citrus coatings, (e) heat treatments, (f) ionizing and non-ionizing irradiations, (g) food additives, and (h) synthetic elicitors. Integrating multiple approaches such as the application of biocontrol agents with food additives or heat treatments have overcome some drawbacks to single treatments. In addition, integrating treatment approaches could produce an additive or synergistic effect on controlling both molds for a satisfactory level of disease reduction in post-harvest citrus. Further research is warranted on plant resistance and fruit-pathogen interactions to develop safer strategies for the sustainable control of P. digitatum and P. italicum in citrus.
Keywords: green mold, blue mold, citrus, post-harvest diseases, alternative control methods
Introduction
The citrus family Rutaceae primarily comprises sweet oranges, mandarins (tangerines), grapefruit, kumquats, lemons, limes, and pummelos. These important fruit crops are cultivated throughout the tropical and subtropical regions and hold an important economic position in the global fruit industry, with global production exceeding 98 million tons (United States Department of Agriculture, 2021). Oranges represent half of the production, followed by mandarins/tangerines, lemons/limes, and grapefruits. China, Brazil, the European Union, and the United States are the top citrus-producing countries (Liu et al., 2017; United States Department of Agriculture, 2021). Global citrus export in 2021 is estimated at 11 million tons, with oranges accounting for over 40% and mandarins/tangerines around 30%. South Africa is the largest exporter, followed by Turkey and Egypt, and the United States is the seventh-largest exporter (United States Department of Agriculture, 2021). The United States' share in global citrus trade is dropping primarily due to lower oranges exports. The citrus trade consists of two main markets: fresh and processed, in which oranges account for the most production. In addition to huge quantities of fruit juice, byproducts like essential oils, pectin, molasses, blend syrup, and dried pulp are essential components. Citrus flavonoids exhibit anti-cancer and anti-inflammatory properties and are widely used for medicinal purposes (Benavente-Garcia and Castillo, 2008; Talibi et al., 2014; Al-Snafi, 2016).
Several biotic and abiotic stressors in the post-harvest handling and citrus agroindustry such as picking, packaging, storage, transportation, and stocking have predisposed citrus fruits to mechanical wounds, resulting in the invasion of fruit-decaying microorganisms that cause spoilage, reduction in shelf life and value, and economic losses (Talibi et al., 2014). In general, the citrus fruit rot rate is around 10–30%, though it can increase to 50% in severe conditions (Ladaniya, 2011; Li et al., 2019; Youssef and Hussien, 2020). Losses from fungal decay of untreated fruits have been estimated as high as 90% during post-harvest handling and marketing (Smoot et al., 1971).
Of the many post-harvest diseases reported in citrus, two major challenges for the industry are green and blue mold (Kanetis et al., 2007; Talibi et al., 2014). Green mold, caused by Penicillium digitatum Sacc, and blue mold, caused by Penicillium italicum Wehmer, result in substantial economic losses around the globe (Kavanagh and Wood, 1971; Zamani et al., 2009). The P. digitatum and P. italicum fungi have a short disease cycle ranging from 3 to 5 days at 25°C and reproduce one to two billion conidial spores (Holmes and Eckert, 1999; Zhu H. et al., 2019).
P. digitatum, a necrotrophic fungus infecting citrus species via mechanical wounds and environmental factors such as cold, wind, insects, and hail, accounts for ~90% of total post-harvest losses alone (Perez et al., 2017; Zhu et al., 2017; Lin et al., 2019). The pathogen reproduces quickly on fruit surfaces, and its spores are ubiquitous in the atmosphere (Kanetis et al., 2007). The fungus penetrates fruit pericarp cells, spreads to the mesocarp, and invades nearby cells via germ tube (Han et al., 2013). The infected fruit then produces white mycelia and greenish conidia, a characteristic symptoms of green mold (Lin et al., 2019). The mycelium produces enzymes that break down fruit cell walls and initiate shrinkage, resulting in a sunken mummified form (Papoutsis et al., 2019). The infected pericarp and mesocarp cells plasmolyze, causing a soft watery spot, and the fruit is rotted (Han et al., 2013).
In the case of green mold, infection of the adjacent fruit is rare; however, spores may soil fruits. Ruptured oil glands in the wounded tissue emit volatiles (limonene, myrcene, alphapinene, and betapinene), organic acids, and sugar that stimulate conidial germination (Pelser and Eckert, 1977; Droby et al., 2008). P. digitatum produces thermogenic alkaloids, including tryptoquialanine A and tryptoquialanine C, which are harmful mycotoxins with potential risk to public health (Ariza et al., 2002; de Vilhena Araújo et al., 2019; Costa et al., 2021).
Spores of P. italicum are encapsulated in narrow white mycelium bounded by fluffy, water-soaked rind (Holmes and Eckert, 1999; Palou et al., 2002a,b, 2008; Talibi et al., 2014; Papoutsis et al., 2019). The white mycelium grows and digs into the infected tissue, sporulating blue conidia (Louw and Korsten, 2015). P. italicum is a nesting-type pathogen that spreads rapidly in packed containers to infect adjacent fruits (Ladaniya, 2008) even at lower temperatures in cold storage (Whiteside et al., 1993; Palou et al., 2002a; Iqbal et al., 2012, 2017) with reduced water availability (Plaza et al., 2003). Although initial lesions caused by green mold resemble blue mold disease, a thick non-sporulating mycelium limited by decaying peel surrounds green mold spores (Palou et al., 2008; Talibi et al., 2014).
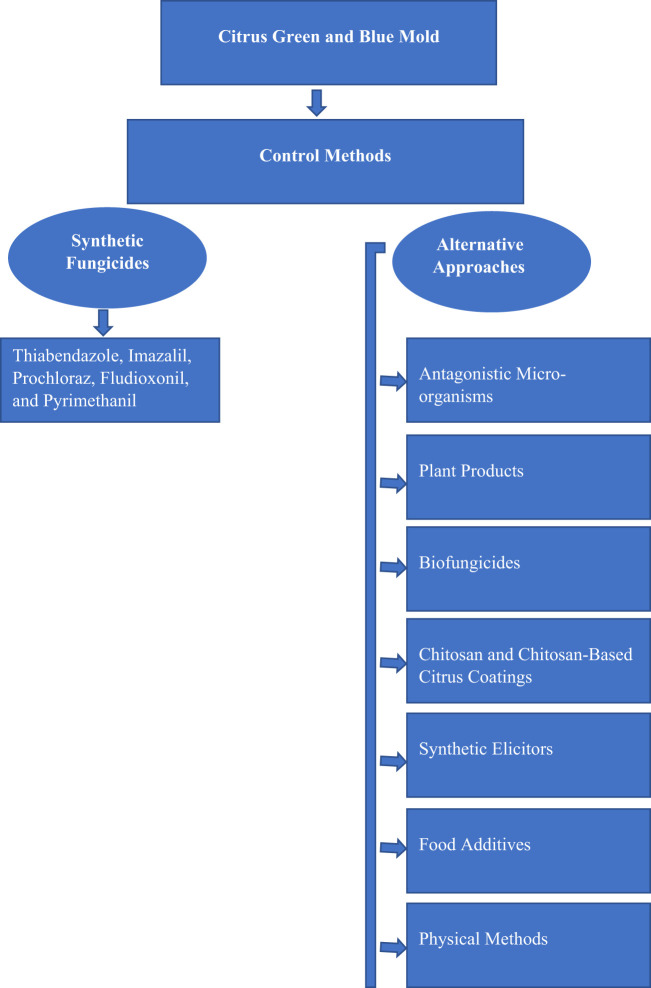
The economic losses caused by green and blue mold in citriculture are minimized using synthetic fungicides such as thiabendazole, imazalil, prochloraz, fludioxonil, and pyrimethanil, which are primarily used as control agents (Chen et al., 2019). The extensive use of synthetic fungicides has caused the proliferation of resistant strains of these phytopathogens and compromised the effectiveness of chemical treatments (Zhang X. et al., 2018; Chen et al., 2020a). Additionally, concerns about soil quality, environmental pollution, risks associated with human health, and accumulation of chemical residues in food have increased (Palou et al., 2008). Therefore, reducing harmful pressures on the environment is a key to creating a sustainable and healthy food system. Thus, an urgent search for safe and effective methods to replace and reduce the use of harmful chemicals to control P. digitatum and P. italicum is warranted. Alternative approaches and compounds from non-chemical sources are usually much less toxic to humans, safe, and eco-friendly when compared to chemical fungicides. These alternative methods include biological control with antagonistic microbes such as yeast, bacteria, and fungi, bio-fungicides, plant extracts and essential oils, chitosan, food additives and generally regarded as safe (GRAS) salts, synthetic elicitors, and physical methods such as heat or irradiation (Palou et al., 2016; Liu et al., 2017; Palou, 2018; Papoutsis et al., 2019). These approaches alone or combined with two or more methods represent a promising alternative to the existing chemical pesticides and offer a balanced solution for the control of citrus molds and sustainable production (Moraes Bazioli et al., 2019; Hulot and Hiller, 2021). The flow diagram for citrus green and blue mold management practices is shown in Figure 1.
Figure 1.
PRISMA flow diagram of the citrus green and blue mold management practices.
Although several reviews on post-harvest diseases of citrus have been performed to date (Palou, 2009; Talibi et al., 2014; Chen et al., 2019; Moraes Bazioli et al., 2019; Papoutsis et al., 2019), no studies have reviewed all alternatives in a single paper. Further, the effectiveness and performance of these alternatives have not been discussed in detail. In order to fill this gap in the literature, this review paper aims to comprehensively review past and current research on alternative control methods and their successful application, implications for fruit quality, challenges, and future prospects. This review contributes to the literature on choosing appropriate alternative approaches to control post-harvest citrus disease. A wide range of scientific databases (Google Scholar, Scopus, PubMed, and SciELO) was utilized to search for articles on citrus post-harvest disease management. Based on the literature reviewed, a single treatment has been found less effective than two or more alternatives combined. I conclude that two or more alternatives can be combined to reduce disease incidence and extend shelf life of citrus fruits.
Antagonistic Micro-Organisms
Biological control based on the application of antagonistic microorganisms has emerged as one of the most effective methods for controlling P. digitatum and P. italicum in citrus fruit. These microorganisms provide an effective eco-friendly choice to chemical fungicides and are easily accepted by consumers (Wilson and Eggemeier, 1991; Droby and Chalutez, 1999; Janisiewicz and Korsten, 2002; Droby et al., 2009). Microbial antagonists display protective and curative action comparable to synthetic fungicides. Antagonistic microorganisms such as yeast, bacteria, and some fungi have been widely used in the biological control of green and blue mold in citrus. An ideal microorganism should be genetically stable, effective at low concentrations, capable of surviving under adverse environmental conditions, inexpensive to formulate and produce, shelf-stable, resistant to common pesticides, compatible with commercial processing practices, and non-pathogenic to human health and the host (Wisniewski and Wilson, 1992). To suppress post-harvest pathogens, these microorganisms provide more than one control mechanisms, such as competition for nutrients and space (Droby et al., 1989, 2002; Panebianco et al., 2015), induction of resistance (Droby et al., 2002), secretion of specific enzymes and toxins and stimulation of secondary metabolism (Bar-Shimon et al., 2004; Luo et al., 2012), biofilm formation (Benhamou, 2004), antibiosis (Nunes et al., 2009), and mycoparasitism (Droby et al., 2002). Factors such as pH of growth media, type of strain used for the control, and time of application, such as pre- or post-harvest affect the efficacy of microbial antagonist (Droby et al., 2002).
Past studies have investigated the use of yeasts to control post-harvest fungal diseases (Platania et al., 2012; Kupper et al., 2013; Moretto et al., 2014; Ferraz et al., 2016; Liu et al., 2017). Yeasts have promising characteristics of pathogenic biocontrol because they infrequently produce antibiotic or mycotoxins substances that could leave residues on fruits (Droby et al., 2002; Gamagae et al., 2004; Zhang et al., 2005). Moreover, yeasts have been studied as antagonists for their inhibitory capacity to colonize surfaces for a long period. Some yeast strains such as Wickerhamomyces anomalus, Saccharomyces cerevisiae, Rhodotorula minuta, and Aureobasidium pullulans have been reported as “killer yeasts” with a killer phenotype: they produce killer proteins for suppressing pathogen development and deforming fungal hyphae (Comitini et al., 2009; Platania et al., 2012; Aloui et al., 2015; Ferraz et al., 2016). In mandarin oranges, the yeasts W. anomalus, Metschnikowia pulcherrima, and A. pullulans increase the activity of peroxidase and superoxide dismutase, thereby reducing the incidence and severity of P. italicum (Parafati et al., 2016). Other promising biocontrol agents against P. digitatum and P. italicum in citrus are bacteria such as Bacillus subtilis and Streptomyces sp. Bacterial pathogens such as Bacillus can act as antagonists or produce volatile organic compounds (VOCs) that increase plant resistance (Leelasuphakul et al., 2008). Maldonado et al. (2010) reported lemon fruit treated with Streptomyces RO3 metabolites showed fungicidal action and reduced the incidence of P. digitatum. Moreover, antifungal compounds such as 3-phenyllactic acid and benzeneacetic acid, 2-propenyl ester isolated from Lactobacillus plantarum IMAU10014 have exhibited antifungal activity against P. digitatum (Wang et al., 2012).
A bacterial strain SG-6 (identified as Paenibacillus polymyxa) was isolated as an endophyte from the root tissue of Sophora tonkinensis, which was highly efficient in reducing P. digitatum in citrus (Lai et al., 2012). Similarly, the application of endophytic P. polymyxa strain SG-6 in an in-vitro assay inhibited the growth of P. digitatum conidia and reduced decay in storage. This bacterium did not impair fruit quality parameters such as total soluble solids, ascorbic acid, titratable acidity, or firmness (Lai et al., 2012). In addition, a strain of Bacillus amyloliquefaciens HF-01, isolated from citrus fruit surfaces, was screened for in vitro antagonism toward P. digitatum. The isolate was further evaluated alone on artificially inoculated “Wuzishatangju” mandarin fruit. The isolate was found to perform significantly better than the water control in reducing the incidence of green and blue mold. Moreover, combination of tea saponin and B. amyloliquefaciens Hf-01 has been found to significantly improve the biocontrol activity of B. amyloliquefaciens HF-01. HF-01 combined with 50 μg mL−1 tea saponin was found to provide 90% control of green and blue mold without impairing any fruit quality parameters (Hao et al., 2011).
In a study by Wang et al. (2021b), citrus fruits with blue mold were treated with VOCs produced by Pseudomonas fluorescens ZX incubated on nutrient agar (NA) and in nutrient broth (NB). The study revealed that the VOCs from P. fluorescens ZX inhibited mycelial growth and conidial germination of P. italicum by 42.14% and 77.86%, respectively. Also, in vivo experiments showed that blue mold disease incidence and lesion size on citrus fruits were significantly suppressed by VOCs from P. fluorescens ZX incubated on NA, in NB, and on healthy fruits. Similarly, in vitro testing suggested organic acids and sulfur compounds were the active components of VOCs, with dimethyl disulfide and dimethyl trisulfide exhibiting the highest antifungal activity.
Studies have also examined the combined effect of multiple biocontrol agents: either different bacterial strains or bacteria with yeast/fungi, resulting in effective inhibition of citrus molds. For instance, the combined application of Pseudomonas and Trichoderma resulted in significant inhibition of P. digitatum on oranges and lemons (Panebianco et al., 2015). In addition, Meziane et al. (2006) investigated Serratia plymuthica, strains IC1270 and IC14 separately and in combination for inhibiting P. digitatum or P. italicum on oranges. A higher disease suppression and efficacy were observed when combined two bacterial strains (1 × 108 cells/mL). Nutrient competition is considered the primary mode of action in strain IC1270, while antagonism requires a direct cell-to-cell interaction between IC14 and the pathogen.
Similarly, with the aim to inhibit mold and extend storage life in Citrus reticulata Blanco (“Xinyu” tangerine), one study immersed a biocontrol bacterium Paenibacillus brasilensis YS-1 into Xinyu tangerines. Xinyus soaked with P. brasilensis YS-1 for 10 min showed increased activity of peroxidase, superoxide dismutase (SOD), phenylalanine ammonia-lyase (PAL), and polyphenol oxidase (PPO) in comparison to the control water. This result shows that the post-harvest application of P. brasilensis YS-1 can control post-harvest decay, increase defensive enzyme activity, and maintain fruit quality (Chen et al., 2020c).
A few well-known fungal antagonists prove as effective as yeast and bacteria in controlling citrus mold diseases. Antagonistic fungi such as Muscodor albus and Verticillium lecanii produce volatile antimicrobial compounds that control P. digitatum decay (Benhamou, 2004; Mercier and Smilanick, 2005). Biofumigant M. albus has been used to fumigate lemons in storage to inhibit green mold (Mercier and Smilanick, 2005). Another study explored biocontrol activity of an entomopathogenic fungus V. lecanii on the pathogen P. digitatum at the cellular level (Benhamou, 2004). Treatment with V. lecanii in infected exocarp tissue had dramatic cellular changes characterized by rapid necrotization of the host exocarp cells with severely collapsed hyphae. This finding led to the hypothesis that molds exhibit fungus protection thanks to their direct antimicrobial properties and fruit-induced resistance (Benhamou and Brodeur, 2001). Some endophytic fungi produce volatile antifungal substances (Dennis and Webster, 1971; Strobel et al., 2001; Ezra et al., 2004). For instance, a fungus identified as Nodulisporium sp. CMU-UPE34 produced volatile antifungal compounds, namely alcohols, acids, esters, and monoterpene, with eucalyptol in the greatest abundance. In vitro tests showed the fungus killed a dozen different plant pathogens. In vivo mycofumigation with jasmine rice cultures of Nodulisporium spp. CMU-UPE34 was found to control decay by P. digitatum on Citrus limon and by P. italicum on Citrus aurantifolia (Suwannarach et al., 2013).
Combination with other treatment methods such as physical treatments, salts, and elicitors can boost the competency of biological control agents against citrus molds: Candida membranifaciens combined with ultraviolet irradiation and hot-water brushing (Terao et al., 2017); Cryptococcus laurentii with methyl jasmonate (MeJA) (Guo et al., 2014); Kluyveromyces marxianus with sodium bicarbonate (Geng et al., 2011); Saccharomycopsis crataegensis with sodium bicarbonate (Pimenta et al., 2010; see Table 1 for details). In recent work, Wang et al. (2021a) studied the joint application of Meyerozyma guilliermondii and an alginate oligosaccharide (bioactive compound from brown algae) as an effective method of controlling P. italicum on mandarin fruit. The study found that a combination of alginate oligosaccharide and M. guilliermondii provided better control than either treatment alone.
Table 1.
Antagonistic microorganisms for the control of P. digitatum and P. italicum.
| Yeasts | Citrus species | Pathogens | Control mechanisms | References |
|---|---|---|---|---|
| Candida membranifaciens combined with hot water brushing and ultraviolet irradiation | Oranges | P. digitatum | Induced systemic resistance on fruit peel. Provided additive effect, higher disease control efficacy, and extend fruit's shelf-life | Terao et al., 2017 |
| Yeast isolates B13 and grape | Navel oranges, lemons and Valencia oranges | P. digitatum | Excellent control of P. digitatum, when applied to citrus fruit 48 h prior to artificial inoculation. Reduced decay of lemons and navel oranges, and <5% incidence on Valencia oranges, compared to >50% incidence in untreated fruits | Abraham et al., 2010 |
| Rhodotorula glutinis | Oranges | P. digitatum | The higher efficacy of R. glutinis and increased competition for nutrients and space with pathogens | Zheng et al., 2005 |
| Cryptococcus laurentii with methyl jasmonate (MeJA) | Mandarins (Citrus reticulata Blanco cv. Ponkan) | P. digitatum | Cryptococcus laurentii at 1 × 108 cells/ml combined with 100 μmol/L, MeJA induced more natural resistance in fruit inoculated with P. digitatum. Greater activity of peroxidase, catalase, polyphenol oxidase, and rise in the mRNA expression level of PR5 (pathogenesis-related protein family 5) compared to the control | Guo et al., 2014 |
| Kluyveromyces marxianus with sodium bicarbonate | Citrus reticulata Blanco cv. Wuzishatangju | P. digitatum | Compete with pathogens for nutrients and space. Salt stimulates K. marxianus growth and inhibits the germination of fungal spores | Geng et al., 2011 |
| Rhodosporidium paludigenum (possess broad-spectrum antifungal effects) with ammonium molybdate | Satsuma mandarins | P. digitatum | Ammonium molybdate significantly increased the biological movement of R. paludigenum against green mold and reduced disease incidence by 89.3%. It reduced infection by suppressing proton-pump activity and spore germination of P. digitatum | Lu et al., 2018 |
| Pichia membranefaciens | Citrus sinensis Osbeck cv. Jincheng | P. digitatum and P. italicum | Higher competition for nutrients and space with pathogen and induced host defenses | Luo et al., 2012 |
| Saccharomycopsis crataegensis + sodium bicarbonate | Oranges | P. digitatum | Yeast and sodium bicarbonate alone lowered the disease severity by 41.7% and 19.8%, respectively. The combined applications impeded symptoms development from 2 to 10 days | Pimenta et al., 2010 |
| Pichia galeiformis (BAF03) | Oranges, lemons | P. digitatum | Compete for space and nutrients Production of volatile organic compounds |
Chen et al., 2020f, 2021 |
|
Saccharomyces cerevisiae ACBL-82 strain ACBL-76 strain, Candida stellimalicola ACBL-84 strain ACBL-87 strain Meyerozyma caribbica ACBL-86 strain |
Orange fruits (Citrus sinensis cv. Lima) | P. digitatum | S. cerevisiae ACBL-82 strain, C. stellimalicola ACBL-84 strain, ACBL-87, and S. cerevisiae ACBL-76 inhibited more than 80% of pathogen mycelial growth in vitro. M. caribbica ACBL-86 strain, S. cerevisiae ACBL-82 strain decreased disease severity, and blocked green mold incidence in Lima sweet oranges | da Cunha et al., 2020 |
| Pichia membranaefaciens combined with salicylic acid (SA) | Citrus sinensis L. Osbeck cv. Jincheng 447 | P. digitatum and P. italicum | SA increased the yeast population in fruit wounds, facilitated P. membranaefaciens growth, and promoted nutrient and space competition. P. membranaefaciens in combination with SA effectively enhanced the synthesis of phenylalanine ammonia-lyase, chitinase, peroxidase, β-1,3-glucanase, polyphenoloxidase, and phenolics | Zhou et al., 2014; Spadaro and Droby, 2016 |
| Candida famata | Oranges | P. digitatum | Induced resistance and stimulated the fruit to produce phytoalexins (scoparone and scopoletin). Enhanced rapid colonization of the fungal mycelium and the wounds | Arras, 1996 |
| Candida saitoana with 0.2% glycolchitosan | Oranges, lemons | P. digitatum | Increased competition for nutrients and space. Glycolchitosan has antifungal and film-forming properties | El-Ghaouth et al., 2000 |
| Kloeckera apiculata | Guoqing 1, Owari, Ponkan, and Newhall Navel Orange | P. digitatum and P. italicum | Competition for nutrients and space | Long et al., 2007 |
| Wickerhamomyces anomalus, Metschnikowia pulcherrima, and Aureobasidium pullulans species with locust bean gum (LGB) | Mandarin fruit | P. digitatum and P. italicum | Competition for nutrients, antibiosis, fruit resistance induction, and killer activity. In combination with edible LGB coatings, yeast strains are effective for the long-term maintenance of biocontrol efficacy and yeast cell viability |
Parafati et al., 2016 |
| Yarrowia lipolytica | Mandarin oranges | P. digitatum and P. italicum | Induced higher activities of peroxidase, catalase, polyphenol oxidase, flavonoid, phenylalanine ammonia lyase compounds, and total phenols, which activates the defense mechanisms and improves resistance in mandarins | Zhu H. et al., 2019 |
| Candida oleophila | “Marsh Seedless” grapefruit, Citrus sinensis (L.) Osbeck cv. Jincheng 447 | P. digitatum and P. italicum | Nutrient competition, site exclusion, fungal cell wall degradation, resistance induction including accumulation of phytoalexins (scoparone, scopoletin, and umbelliferone), and direct mycoparasitism | Droby et al., 2002; Liu et al., 2019 |
| Metschnikowia citriensis | Citrus sinensis (L.) Osbeck cv. Jincheng 447 | P. digitatum and P. italicum | Deplete iron, adhesion, biofilm formation, and induce host resistance | Liu et al., 2019 |
| Saccharomyces cerevisiae with calcium chloride (CaCl2) | Tarocco and Valencia oranges | P. digitatum | Competition for nutrients, space, and “killer” activity. Synergistic activity when applied along with CaCl2 | Strano et al., 2002 |
| Cryptococcus laurentii (1 × 107 cells/mL) with cinnamic acid (1.5 mM) | “Orah” mandarins | P. italicum | Inhibits spore germination, loss of membrane integrity, and mycelial growth of P. italicum. Competition for nutrient and space | Li et al., 2019 |
| Pseudozyma antarctica | Citrus sinensis (L.) Osbeck cv. Jincheng 447 | P. digitatum and P. italicum | Fungal cell wall degradation, high lytic enzyme activity, and direct parasitism | Liu et al., 2019 |
| Candida stellimalicola | Valencia sweet orange | P. italicum | The killer activity is a common biocontrol mechanism | da Cunha et al., 2018 |
| Debaryomyces hansenii | Grapefruit, Mexican lime | P. digitatum and P. italicum | Competition for nutrients and space | Droby et al., 1989; Chalutz and Wilson, 1990; Hernández-Montiel et al., 2010 |
| Bacteria | ||||
| Bacillus amyloliquefaciens HF-01 in combination with tea saponin | “Wuzishatangju” mandarin | P. digitatum and P. italicum | Colonization and secretion of antifungal peptides or other antibiotics or proteins by B. amyloliquefaciens. Plant growth promotion and systemic resistance | Kim and Chung, 2004; Wong et al., 2008; Hao et al., 2011 |
| Bacillus subtilis | Citrus reticulata Blanco | P. digitatum | Antibiotics, proteins, secondary metabolites, enzymes, and volatile organic compounds have an inhibitory effect on mycelial growth and spore germination | Leelasuphakul et al., 2008 |
| Streptomyces sp. | Oranges | P. digitatum | In vitro assays revealed competition for nutrients and space. Production of lytic enzymes acting on the fungus cell wall by altering growth and presence of antifungal metabolites | Najmeh et al., 2014 |
| Enterobacter cloacae | “Gold Seal” orange (Citrus sinensis (L.) Osbeck) | P. digitatum | Competition for nutrients and space. The E. cloacae strain produces three volatile organic compounds: butyl acetate, 4,5-dimethyl-1-hexene, and phenylethyl alcohol, which inhibit conidial germination, and hyphal elongation | Chen et al., 2016 |
| Pseudomonas cepacia, Pseudomonas fluorescens, Pseudomonas corrugata, and Pseudomonas syringae | Lemon fruit (Citrus limon) | P. digitatum | Antibiosis and competition for nutrients and space | Smilanick and Denis-Arrue, 1992 |
| Pantoea agglomerans in combination with sodium bicarbonate | Oranges | P. digitatum and P. italicum | The exact mechanism of control is not clear. Colonization and parasitizing the pathogen through nutrient competition could be possible mechanisms | Riggle and Klos, 1972; Teixidó et al., 2001 |
| Fungi | ||||
| Muscodor albus | Lemons | P. digitatum | Production of volatile compounds | Mercier and Smilanick, 2005 |
| Verticillium lecanii | Lemons | P. digitatum | Fruit-induced resistance and antimicrobial activity | Benhamou, 2004 |
Citrus fruits can be infected either prior to harvest or during harvesting and processing; however, most studies on biocontrol agents have examined the post-harvest period, and only a few have studied the pre-harvest period. More work is needed on the application of these biocontrol agents before fruit harvest, as the timing of application impacts the effectiveness of the biocontrol agent. Application time is crucial as the biocontrol agent can utilize available nutrients otherwise consumed by the pathogen in the pre-harvest period (Luo et al., 2012; Panebianco et al., 2015; Papoutsis et al., 2019). So far, most studies have examined the antifungal activities of microbial antagonists as a stand-alone product. Results have shown inconsistent performance and control of previously established infections compared to many commercial fungicides (Ippolito and Nigro, 2000; Zheng et al., 2005). Integrated approaches combining biological control with other methods such as salts or food additives, physical treatments, and non-chemical elicitors or plant growth regulators are one of the most promising means of disease management (Huang et al., 1995; Droby et al., 1998; El-Ghaouth et al., 2000; Arras et al., 2002; Janisiewicz and Korsten, 2002; Porat et al., 2002; Zhang et al., 2004; Papoutsis et al., 2019). Overall, an integrated approach facilitates additive, synergistic, complementary, preventive, curative effects on citrus molds while minimizing fungicidal residues (Palou et al., 2008; Smilanick et al., 2008).
Natural Plant Products
In recent years, plant extracts, essential oils, and natural compounds have been evaluated as an alternative chemical means for controlling citrus post-harvest decay (Table 2). More than 1,340 plant species are documented sources of antimicrobial compounds and novel botanical fungicides (Cowan, 1999; Tripathi and Dubey, 2004). Plant extracts are biodegradable, non-phytotoxic, generally safe for human health and the environment, inexpensive, and equally effective as chemical fungicides. Some phytochemicals of plant origin have been successfully formulated as botanical pesticides in integrated pest management programs (Tripathi et al., 2004). Plant extracts and essential oils obtained from medicinal and aromatic plants have effectively controlled agar plates (Ameziane et al., 2007) and wounded citrus fruits (Wilson et al., 1997; Mari and Guizzardi, 1998; Talibi et al., 2012). Plants contain secondary compounds such as acetaldehyde, ethanol, ethyl formate, ethyl benzoate, benzaldehyde, methyl salicylate, eugenol, jasmonates, glucosinolates, hexanal, thymol, allicin, isothiocyanates, citral, limonene, and a variety of phenolic compounds (for example, flavanones, polymethoxylated flavones, and coumarins) that possess antifungal property. These compounds have been derived from plants such as mint, cinnamon, thyme, clove, garlic, oregano, pomegranate, Acacia sp., Aloe sp., and citrus fruits (Utama et al., 2002; Tripathi and Dubey, 2004; Palou et al., 2008).
Table 2.
Plant extracts, essential oils, and natural compounds for the control P. digitatum and P. italicum.
| Pathogens | Plants | Plant extracts | References |
|---|---|---|---|
| P. digitatum | Citrus reticulata (mandarin) | Waxy components, hexane extract, tangeritin, citral, and nobiletin | Johann et al., 2007 |
| Glycine max | β-conglycinin and glycinin | Villalobos et al., 2016 | |
| Citrus japonica (kumquat) | Scoparone and scopoletin | Rodov et al., 1992 | |
| Rosmarinus officinalis L. (rosemary) | Essential oils, flavonoids, polyphenols, and methanol extracts |
Hendel et al., 2016 | |
| Aloe vera | Aloe saponins and anthraquinones | Sitara et al., 2011 | |
| Saliva fruticosa Mill. | Carnosic acid, carnosol, and hispidulin | Exarchou et al., 2015 | |
| Coriandrum sativum, Cuminum cyminum, Cinnamomum zeylanicum, Thymbra spicata, Anethum graveolens (dill), Syzygium aromaticum, Cymbopogon citratus, Pelargonium graveolens, Mentha spicata (spearmint), Mentha piperita (peppermint), Foeniculum vulgare, Artemisia annua, Lavandula stoechas, Origanum syriacum, Lippia scaberrima, and Laurus nobilis (laurel) | Volatile oil | Yigit et al., 2000; Hall and Fernandez, 2004; Soylu et al., 2005; Du Plooy et al., 2009; De Corato et al., 2010; Tyagi and Malik, 2011 | |
| Origanum dictamnus, Origanum vulgare, Origanum majorana, and Eugenia caryophyllata | Essential oils, thymol, carvacrol, and eugenol | Daferera et al., 2000; Yahyazadeh et al., 2008 | |
| Parastrephia lepidophylla, Parastrephia phyliciformis, and Chuquiraga atacamensis | Aqueous extracts and total phenolics | Sayago et al., 2012 | |
| Solanum nigrum, Sisymbrium irio, Ranunculus asiaticus, Crepis aspera, Chenopodium murale, and Ceratonia siliqua | Aqueous extracts, and flavonoids alkaloids | Qasem and Abu-Blan, 1995; Qasem, 1996; Ameziane et al., 2007; Askarne et al., 2012; Musto et al., 2014 | |
| Astilbe myriantha Diels, Breonadia salicina | Triterpenoid | Eloff and Mahlo, 2011; Song et al., 2011 | |
| Withania somnifera | Caffeic acid, salicylic acid, and 3,4-dihydroxybenzoic acid | Mekbib et al., 2007, 2009 | |
| Acacia seyal | Methanol extracts and gallic acid | Mekbib et al., 2007 | |
| Lippia rehmannii | Verbascoside | Shikanga et al., 2009 | |
| Lippia graveolens | Ethanolic and hexanic extracts | De Rodríguez et al., 2011 | |
| Sapium baccatum | Tannic acid | Zhu H. et al., 2019 | |
| P. italicum | Citrus aurantium (sour orange) | Essential oils, α-terpineol, terpinen-4-ol, linalool, and limonene | Trabelsi et al., 2016 |
| Ageratum conyzoides | Essential oils | Dixit et al., 1995 | |
| Citrus sinensis (sweet orange), Citrus clementina (clementine), and Mentha arvensis (wild mint) | Volatile oil, citronellal limonene, linalool | Del Río et al., 1998; Tripathi et al., 2004; Droby et al., 2008 | |
| Arenaria rubra and Acacia nilotica | Aqueous extracts | Tripathi et al., 2002; Ameziane et al., 2007; Askarne et al., 2012 | |
| Ocimum canum and Zingiber officinale | Volatile oil | Tripathi et al., 2004 | |
| Anvillea radiata, Asteriscus graveolens, Bubonium odorum, and Inula viscosa | Petroleum ether, chloroform, and ethyl acetate extracts | Ameziane et al., 2007; Askarne et al., 2012, 2013 | |
| Halimium umbellatum | Methanol extract | Ameziane et al., 2007; Askarne et al., 2012, 2013 | |
| Lantana camara | Verbascoside | Oyourou et al., 2013 | |
| Populus × euramericana cv. “Neva” (poplar buds) | Flavonoids of chrysin, pinocembrin, and galangin | Yang et al., 2016 | |
| Ficus hirta Vahl's | Pinocembrin-7-o-beta-D-glucoside | Wan et al., 2017a | |
| Simmondsia chinensis | Oil emulsion | Ahmed et al., 2007 | |
| P. digitatum and P. italicum | Citrus paradisi (grapefruit) | Coumarins including scoparone, umbelliferone, osthol, seselin, auraptene 7-geranoxycoumarin; essential oils, citral, myrcene, α-terpineol, linalool, α-pinene, limonene, sabinene, and nootkatone | Afek et al., 1999; Droby et al., 2008 |
| Citrus limon (Lemon) | Waxy components, scoparone, hexane extract, and xanthyletin; limettin, 5-geranoxy-7-methoxycoumarin, isopimpinellin, and scoparone; citral, and volatile oil | Rodov et al., 1995; Johann et al., 2007 | |
| Aloe ferox, Aloe saponaria, and Aloe mitriformis | Aloin | Zapata et al., 2013 | |
| Camellia sinensis | Tea saponins | Hao et al., 2010 | |
| Plantago lanceolata and Sanguisorba minor | Caffeic acid derivatives, flavonoids, and (iso) verbascoside | Gatto et al., 2011 | |
| Ramulus cinnamomi | Cinnamaldehyde and cinnamic acid | Wan et al., 2017b | |
| Allium sativum (garlic) | Aqueous extracts, ethanolic extracts, allicin | Obagwu and Korsten, 2003 | |
| Melaleuca alternifolia, Zataria multiflora, and Thymus vulgaris (thyme) | Essential oils | Ramezani et al., 2009; Fatemi et al., 2012; Zhang X. et al., 2018 | |
| Thymus capitatus, Thymus leptobotrys, and Thymus riatarum | Volatile oil, carvacrol, and thymol | Arras and Usai, 2001; Boubaker et al., 2016 | |
| Cistus villosus | Aqueous extracts | Ameziane et al., 2007; Askarne et al., 2012 | |
| Punica granatum | Ethanol, methanol and water extracts, and phenolic compounds | Nicosia et al., 2016 | |
| Lippia javanica | Verbascoside | Shikanga et al., 2009 | |
| Thymus broussonnetii subsp. hannonis | Camphor and α-terpineol | Boubaker et al., 2016 | |
| Thymus satureioides subsp. pseudomastichina | Borneol and thymol | Boubaker et al., 2016 | |
| Peganum harmala | Harmine, tetrahydroharmine, and harmaline | Kanan and Al-Najar, 2009 | |
| Chinese propolis | Ethyl acetate extract, methanol, and pinocembrin | Yang et al., 2010
Peng et al., 2012 |
|
| Sonchus oleraceus, Borago officinalis, and Sanguisorba minor | Methanol extract | Gatto et al., 2011 |
Plant extracts are known for their preservative, antimicrobial, and antifungal properties. Several studies have investigated aqueous plant extracts and organic solvent extracts against citrus post-harvest pathogens. Plant extracts from species Withania somnifera and Acacia seyal have resulted in the inhibition of P. digitatum in citrus by up to 70% under storage conditions (Samson, 1984). Similarly, flavonone pinocembroside compounds obtained from the fruit of Ficus hirta Vahl. have shown antifungal activity against P. italicum in “Navel” oranges by direct inhibition of mycelial growth via membrane targeting (Chen et al., 2020d). In addition, secondary compounds such as acetaldehyde, benzaldehyde, cinnamaldehyde, ethanol, benzyl alcohol, nerolidol, and 2-nonanone have shown effectiveness against P. digitatum (Utama et al., 2002). Citral, an active compound produced in the flavedo of citrus induced a strong defense mechanism against P. digitatum inhibiting mycelial growth and spore germination (Rodov et al., 1995; Klieber et al., 2002; Fisher and Phillips, 2008). Methanol extracts from Sanguisorba minor and Cistus villosus showed satisfactory control of P. digitatum (Ameziane et al., 2007; Gatto et al., 2011).
Likewise, in vitro and in vivo assays have demonstrated the capacity of 7-geranoxy coumarin, a natural compound of grapefruit, to act against P. digitatum and P. italicum (Agnioni et al., 1998). A recent study examined the antifungal potential of pinocembrin-7-glucoside (P7G) isolated from Ficus hirta Vahl. against P. italicum. The in vivo test showed P7G significantly inhibited mycelial growth in artificially inoculated “Newhall Navel” oranges. Further, P7G triggered a marked decline in both chitin and glucanase contents of P. italicum mycelia and destroyed the cell wall structure (Chen et al., 2020b). Ethyl extracts such as Chinese propolis ethyl acetate extract (PEAE) have been assessed to control P. digitatum and P. italicum on post-harvest citrus fruits. Studies show that PEAE strongly inhibited spore germination and mycelium growth, induced unusual morphological alterations, and reduced decay caused by P. digitatum and P. italicum on “Zhongqiu” mandarins (Yang et al., 2010). Recently, Zhu H. et al. (2019) investigated the antifungal activity of tannins, a natural polyphenolic compound on P. digitatum. In vivo tests showed a significant reduction of P. digitatum symptoms in artificially inoculated citrus fruit in storage conditions. The study demonstrated that the antifungal mechanism of tannic acid resulted in the disruption of the cell walls and the plasmatic membrane, causing leakage of intracellular contents such as sugars.
The role of plant extracts in combination with alternative treatments such as wax, oil, biocontrol agents, and thermotherapy has been examined against P. digitatum and P. italicum. For instance, Obagwu and Korsten (2003) tested the water and ethanol extracts of garlic clove alone or in combination with sunflower oil or fruit wax (Obagwu and Korsten, 2003). The authors found all treatment combinations effective; however, a 1% extract in sunflower oil being the most effective against P. digitatum and P. italicum in “Valencia” oranges and as effective as fungicides. Sukorini et al. (2013) investigated biofungicidal yeast Candida utilis TISTR 5001 combined with Eugenia caryophyllata crude extract to control P. digitatum in tangerines and suggested the application of these extracts as a potential disease management approach. In addition, Koltz et al. (2020) studied the effects of canola and mustard extracts and sachets alone and in combination with thermotherapy to control P. digitatum. The study found that the fungitoxic volatile compounds produced with canola and mustard extracts and sachets significantly reduced P. digitatum in vitro and in inoculated oranges.
Use of essential oils to control citrus green and blue mold is gaining importance, thanks to antimicrobial and antioxidant properties, synergistic effects, presence of active compounds, and low residue levels (Tripathi and Dubey, 2004; Bakkali et al., 2008). In a study by Chen et al. (2019), the efficacy of clove essential oil (CEO) against P. italicum was investigated. Application of CEO was found to inhibit P. italicum growth when used at 0.05%–0.8% (v/v). In addition to its direct antifungal activity, the CEO has demonstrably enhanced the activity of defense-related enzymes such as chitinase and peroxidase to induce host defense responses. Similarly, the influence of numerous essential oils such as lemon grass, eucalyptus, clove, and neem was investigated on “Kinnow” mandarin inoculated with P. digitatum and P. italicum. The results indicated that these essential oils inhibited both pathogens' growth/colony diameter over untreated PDA plates and reduced decay loss in storage; however, lemon grass oil produced the most potent effect (Jhalegar et al., 2015). Among essential oils, p-anisaldehyde is a naturally occurring and fragrant phenolic compound primarily isolated from anise, cumin, fennel, and garlic. A study was conducted to investigate the antifungal efficiency of p-anisaldehyde on the mycelial growth of P. digitatum and P. italicum on “Satsuma” mandarin. P-anisaldehyde exhibited a robust inhibitory effect on P. digitatum and P. italicum leading to altered mycelia morphology, cell wall integrity, and membrane permeability, with minimum inhibitory and fungicidal concentrations of 2.00 μl/ml (Che et al., 2020).
Applying essential oil-amended coatings to citrus is one of several approaches for the control of post-harvest pathogens while maintaining fruit quality. Commercial coatings modified with Lippia scaberrima essential oil achieved 100% efficacy against P. digitatum in “Tomango” oranges (Du Plooy et al., 2009). Yahyazadeh et al. (2009) stated that applying thyme or clove oil to the surface of oranges in polyethylene films lowered P. digitatum in vapor-phase experiments at 25°C. In other research, supplementing carnauba wax with Cinnamomum zeylanicum essential oil (0.5%, v/v) achieved 90% disease control against post-harvest P. digitatum and P. italicum in citrus (Kouassi et al., 2012). In addition, citral, a naturally occurring isoprenoid compound with two isomers (geranial and neral), provided antifungal activity against P. digitatum in “Navel” oranges (Wolken et al., 2002; Wuryatmo et al., 2003; Droby et al., 2008). Fan et al. (2014) found that citral combined with wax in vitro and in vivo decreased the incidence of P. digitatum in “Ponkan” mandarins with significant increases in Vc content and antioxidant enzymes (catalase, SOD, and peroxidase).
Generally, plant products provide antifungal activity and trigger defense mechanisms resulting in cell wall synthesis and elevation of the total soluble phenolic compound concentration of orange peels. These products serve as physical and biological barriers to invading pathogens by altering their membrane functionality (Lanciotti et al., 2004; Mekbib et al., 2007). However, the use of natural plant products to inhibit P. digitatum and P. italicum is in its initial phase as not much is known about their mechanisms of action against post-harvest pathogens. In addition, important limitations with regard to essential oils include potential phytotoxicity, low stability, and induction of strong odors or flavors in treated fruits (Palou et al., 2008; Talibi et al., 2014). More investigations into the mechanisms of such plant products are warranted to control citrus post-harvest diseases.
Biofungicides
Biofungicides are formulations of biocontrol microorganisms used against plant diseases (Roger and Keinath, 2010). These fungicides are manufactured in different forms such as wettable powder, emulsion concentrate, suspension concentrate, and tablets, usually for soil drench and application on leaves, seeds, and roots. Biofungicides possess advantages such as low toxicity, low concentrations of active substances, enhanced plant resistance, effective colonization of wounds, nutrient competition, and cost-effectiveness. In 2020, the biofungicides global market was valued at $1.6 billion and estimated to grow at a compound annual growth rate of 16.1% to $3.4 billion by 2025 (Matrose et al., 2021).
Some biofungicides used in agricultural production are Binab-T (Trichoderma harzianum and Trichoderma polysporum), Serenada, Baktofit, Kodiak, Rhizo-plus, and Phytosporin (Bacillus subtilis), Phytolavin (Streptomyces griseus), and Planriz (Pseudomonas fluorescens) (Khakimov et al., 2020). Commercial biofungicides based on antagonistic yeasts available on the market to control P. digitatum and P. italicum include “Shemer” (Metschnikowia fructicola) (Kurtzman and Droby, 2001), “Aspire” (Candida oleophila) (Liu et al., 2013), and “Pantovital” (Pantoea agglomerans) (Vinas et al., 1998). Likewise, two biofungicidal products based on antagonistic bacteria are “Biosave” (Pseudomonas syringae) (Fravel and Larkin, 1996) and “Natamycin” (Streptomyces sp.) (Chen et al., 2020e). The biofungicide “Shemer” is reported to be as effective against molds in oranges as the chemical fungicide imazalil (Wisniewski et al., 2009; Piombo et al., 2018). The biofungicide “Aspire” has been used commercially but was later reported to have low and inconsistent efficacy (Liu et al., 2013; Spadaro and Droby, 2016).
Aqueous “Natamycin” treatments followed by fruit coating reduced the incidence of decay from P. digitatum to <10% in grapefruit and lemons compared to the Natamycin untreated control at 81.9%. Moreover, experimental and commercial packing line studies have demonstrated that “Natamycin” mixed with fludioxonil or propiconazole applied as a storage fruit coating or aqueous flooder treatment typically resulted in >85% reduction of P. digitatum (Chen et al., 2020e). El Guilli et al. (2020) observed the effects of a Pichia guilliermondii strain Z1 granular-formulated product on “Valencia-late” oranges with a suspension of 105 conidia/ml of P. digitatum and P. italicum at temperatures 4 and 20°C. Results showed the control achieved with strain Z1 on “Valencia-late” oranges was comparable to that of thiabendazole, indicating the promise of Pichia guilliermondii strain Z1 as a biofungicide against P. digitatum and P. italicum. Moreover, the strain Z1 is compatible with various waxes used in citrus packinghouses (Lahlali et al., 2014).
The major drawback in the commercialization of bioproducts based on biological control agents is the failure to provide consistent and reliable disease control in field experiments. Despite the growing demand for bioproducts, this failure hinders adoption and has slowed dissemination (Spadaro and Droby, 2016; Abbey et al., 2019). While biocontrol products alone do not provide complete control over green and blue molds, enhancements with mixtures of beneficial organisms, integration with low doses of fungicides, and adjustment of storage atmosphere can increase their protective powers (Spotts et al., 2002).
Chitosan-Based Citrus Coatings
One of the most abundant polysaccharides in nature, chitosan is a biodegradable biopolymer obtained from deacetylation of the chitin present in the exoskeleton of crustaceans (e.g., crabs, lobster, shrimp; Hafdani and Sadeghinia, 2011; Talibi et al., 2014; Zhang et al., 2014). It exhibits direct antimicrobial properties against various microorganisms, including fungi, yeasts, and bacteria, and specifically against the post-harvest diseases P. digitatum and P. italicum in citrus (Palou et al., 2015). Citrus fruits in packing lines are often coated with wax-based compounds amended with conventional fungicides such as imazalil or thiabendazole to reduce weight loss, improve appearance, and control the post-harvest disease. Chitosan and its derivatives are the most-tested antifungal edible coatings to replace commercial waxes against P. digitatum and P. italicum formulated either alone or with other antifungal ingredients (Palou et al., 2015). The progress of edible antifungal coatings as a safe technology can address post-harvest physiological and pathological problems in citrus.
Past studies have investigated the effectiveness of chitosan to control green mold and blue mold diseases. Panebianco et al. (2014) conducted in vitro and in vivo assays with chitosan applications at 0.02–0.5% concentration levels against P. digitatum. In vitro tests showed that concentrations higher than 0.1% completely inhibited the pathogen growth. Moreover, in vivo assays demonstrated chitosan application at a concentration of 0.5% significantly reduced green mold on “Washington Navel”, “Valencia” oranges, “Femminello” lemons, and “Marsh Seedless” grapefruits (Panebianco et al., 2014). In another study, El Guilli et al. (2016) investigated the effects of chitosan on green mold and citrus fruit quality by wounding treated fruits with different concentrations of chitosan 24 h before inoculation with P. digitatum. Both in vitro and in vivo results revealed improved antifungal activity against P. digitatum with increased chitosan concentrations as well as enhanced chitinase and glucanase activities and elicitation of biochemical defense responses without impairing fruit quality. Chitosan not only reduced disease incidence and severity but also enhanced the activities of several enzymes such as SOD, peroxidase, hydrogen peroxide (H2O2), and the levels of glutathione in “Navel” oranges inoculated with P. digitatum and P. italicum (Zeng et al., 2010).
Researchers have explored the antifungal activity of chitosan coatings in combination with other antifungal treatments such as plant extracts, biological control antagonists, salts, and essential oils before or after the application of chitosan-based coatings. El-Mohamedy et al. (2015) observed the effects of coatings amended with chitosan and essential oil on citrus fruits as fungicide alternatives to control post-harvest diseases. A combination of chitosan, lemongrass essential oil, citral essential oil, and chitosan-essential oil mixtures significantly reduced the growth and spore germination of P. digitatum and P. italicum in vitro (El-Mohamedy et al., 2015). Similarly, Shao et al. (2015) observed that chitosan combined with clove oil inhibited P. digitatum mycelial growth in Satsuma mandarins in vivo and exhibited high antifungal activity in vitro with stimulation of fruit defense enzymes. Tayel et al. (2016) evaluated fungal chitosan from Mucor rouxii and plant extracts from cress seeds, pomegranate peels, olive leaves, and senna pods. In vitro qualitative and quantitative assays found that all of these agents exhibited antifungal activity against P. digitatum and P. italicum, resulting in inhibition of fungal growth and viability; however, P. digitatum was more resistant than P. italicum towards the examined agents (Tayel et al., 2016). In general, chitosan-amended coatings gradually release preservatives and provide additional properties for fruit quality maintenance and fungal growth inhibition (Galed et al., 2004). According to Cháfer et al. (2012), thymol, oil of thyme, has greater antifungal activity against P. italicum when incorporated with the chitosan coating on “Powell Navel” oranges without affecting fruit quality attributes.
In recent work, the antifungal activities of carboxymethyl chitosan (CMCS) combined with Cryptococcus laurentii controlled the spore germination of P. italicum in grapefruit. Combined treatments of CMCS and C. laurentii exerted a significant synergistic effect resulting in smaller lesion diameter, reduced decay incidence, and stimulation of defense enzyme activities with no impairment of fruit quality parameters (Wang et al., 2019). Likewise, Waewthongrak et al. (2015) evaluated the effect of Bacillus subtilis ABS-S14 endospores, a crude extract from its culture medium, cyclic lipopeptide antibiotics, and chitosan on the suppression of P. digitatum in mandarin fruit. Efficacy tests showed a significant reduction of fruit decay and induction of defense-related enzymes such as peroxidase and phenylalanine ammonia-lyase in the infected flavedo tissues. Furthermore, the combination of Candida saitoana with glycolchitosan was more effective in reducing P. digitatum infection in lemons and oranges as compared to stand-alone treatments matching the fungicide imazalil in effectiveness (El-Ghaouth et al., 2000). Pretreatment with sodium carbonate salts followed with a combination of C. saitoana and glycolchitosan most effectively controlled green mold in light green and yellow lemons (El-Ghaouth et al., 2000).
Research into the combined effects of salicylic acid (SA) and chitosan on the control of P. digitatum in grapefruits showed significantly reduced lesion diameter and disease incidence in comparison to applications of chitosan or SA individually. It also enhanced the β-1,3-glucanase, chitinase, PAL, peroxidase, and PPO activities and stimulated the synthesis of total phenolic-compound content without impairing post-harvest quality (Shi et al., 2018). Besides the use of chitosan and its derivative, several other edible antifungal coatings applied alone or in combination with other treatments were effective against citrus green and blue mold. For instance, Velásquez et al. (2014) observed the effect of pectin-based edible coatings made with essential oil against Penicillium sp. on “Valenica” oranges and found a concentration 1.5% essential oil reduced decay by 83% with improved shelf life. Essential oils from Mentha spicata and Lippia scaberrima integrated into commercial citrus waxes also stemmed in protective activity against green mold on “Valencia” and “Tomango” oranges (Du Plooy et al., 2009). Wax coatings not only reduce weight loss in oranges but also maintain overall fruit quality. Formulation of wax coatings with the essential oils carvacrol and thymol reduce green mold incidence and ethylene production in lemons artificially inoculated with P. digitatum (Pérez-Alfonso et al., 2012). Moreover, citral incorporated into commercial carnauba wax and applied to citrus fruit showed a concentration 10 times the minimum fungicidal concentration was required to decrease the incidence of P. digitatum (Fan et al., 2014). Valencia-Chamorro et al. (2008) developed and optimized hydroxypropyl methylcellulose -lipid edible composite films formulated with food additives or GRAS salts to inhibit the in vitro growth of P. digitatum and P. italicum. Subsequently, the authors also tested the curative activity of selected coatings in vivo on oranges and mandarins and found that coatings containing the GRAS salts potassium sorbate, sodium benzoate, sodium propionate, and their mixtures most effectively reduced green and blue mold (Valencia-Chamorro et al., 2009).
The quality maintenance and extended shelf life of chitosan-coated fruits suggest that chitosan application can partly substitute for synthetic fungicides in commercial storage and marketing (El Guilli et al., 2016). Limited studies exist on combining chitosan with GRAS salts, microbial antagonists, and physical treatment methods to enhance control of citrus decay, and further research is warranted.
Synthetic Elicitors
Nowadays, industry favors synthetic chemicals that induce plant immunity and natural disease resistance to activate, bolster, or prime plant defense machinery over biocidal agrochemicals (Droby et al., 2002; Liu et al., 2010; Nantawanit et al., 2010; Zhou and Wang, 2018). The induction of natural resistance to pathogens in harvested fruit using non-toxic chemical elicitors in place of chemical fungicides is a promising and ecologically friendly approach for controlling post-harvest diseases (Zhou and Wang, 2018). Advantages to inducing disease resistance with elicitors include the ability to fight pathogens, lower costs than specific biological antagonists, environmental safety and friendliness, and effectiveness at all stages of fruit development, including preharvest and post-harvest (van Hulten et al., 2006; Conrath et al., 2015).
The SA has antifungal properties against specific pathogens in citrus, mango, and pear (Joyce et al., 2001; Shaat and Galal, 2004; Cao et al., 2006). Increasing SA concentrations through the exogenous application or endogenous synthesis stimulates systemic acquired resistance in plants (Verberne et al., 2000). It also delays senescence, retards fruit decay, and facilitates plant growth regulation and interaction with other organisms in response to biotic or abiotic stresses (Yalpani et al., 1994; Senaratna et al., 2000).
The plant hormone MeJA, formed via the octadecanoid pathway together with jasmonic acid (JA) (Holopainen et al., 2009), has been found to mediate diverse developmental processes and defense responses (Cheong and Do Choi, 2003) and enhance the disease resistance (Meng et al., 2009). Similarly, JA is a natural inducer of disease resistance that stimulates antifungal activity in crops such as mango, pear, and citrus fruits and regulates plant growth and development (Shaat and Galal, 2004; Yao and Tian, 2005).
The chemical elicitor β-aminobutyric acid (BABA) is a non-proteinogenic amino acid that behaves as a safe priming molecule of systemic resistance induction in several crops such as apple, citrus, and strawberry (Conrath et al., 2015; Baccelli et al., 2017; Guolin et al., 2019; Aghdam et al., 2020). In plants, BABA acts on various post-harvest fungi (Wang J. et al., 2018; Cheng et al., 2019) with multiple biochemical and physical defense mechanisms, including the creation of physical barriers (callose, lignin, and papillae), hypersensitivity reaction, accumulation of phytoalexins, induction of pathogenesis-related (PR) proteins, biosynthesis of terpenoids, generation of reactive oxygen species (ROS) with H2O2 and activation of defense pathways mediated by abscisic acid, SA, and JA (Cohen, 2002; Walters et al., 2013).
Iqbal et al. (2012) studied the effect of two organic elicitors, SA and MeJA, in “Lane Late” sweet orange, including pre- and post-harvest application for resistance induction. In vitro experiments with post-harvest treatment showed that SA ≥6 mM substantially inhibited the sporulation, radial growth, and spore germination of Penicillium sp. compared to MeJA and control. However, MeJA showed only a suppressive effect on fungal propagules at concentrations ≥4 mM. In contrast, preharvest spray application of 8 mM SA and 3 mM MeJA to “Lane Late” orange effectively reduced wound rotting, colony/lesion diameter, and spore mass density of P. digitatum. Overall, pre- and post-harvest treatments of fruit with SA proved more effective in lessening mold severity than MeJA.
Similarly, Shaat and Galal (2004) assessed the preharvest spray application of SA on the incidence of P. digitatum in grapefruit, lime, mandarin, and six orange cultivars. In general, preharvest application of elicitors proved more effective as it allowed the host to develop more induced resistance and fruit protection than in post-harvest application. In vitro growth inhibition of P. digitatum was highest with 400 mg L−1 SA treatment. A recent study examined the capacity of SA and JA to suppresses P. digitatum and P. italicum in post-harvest infection on Citrus reticulata “Kinnow,” Citrus limon “Meyer Lemon,” and Citrus limetta “Mosambi.” SA and JA significantly reduced the severity of P. digitatum and P. italicum on all tested citrus species compared to the non-treated control. The efficacy of both SA and JA in reducing disease severity depended on concentration; higher concentrations resulted in a greater degree of suppression. Results also showed that SA and JA increase the activity of PPO and peroxidase, which suppressed the development of green and blue mold most effectively in C. reticulata and least so in C. limon (Moosa et al., 2019).
In addition, INA (2,6-dichloroisonicotinic acid), a synthetic analog of SA, was investigated for controlling the post-harvest incidence of P. digitatum and P. italicum. Treatments of 1.0 mmol L−1 INA significantly reduced green and blue molds on both wound-inoculated and naturally infected fruit compared with the control. Moreover, β-1,3-glucanase, chitinase, PAL, peroxidase, and PPO can be used to control diseases (Jing et al., 2020).
Elsherbiny et al. (2021) studied the mechanisms and effects of BABA treatment on the inhibition of P. digitatum both in orange fruit and in vitro. BABA at 125 mM was found to significantly inhibit spore germination, mycelial growth, and germ tube elongation of P. digitatum and suppress disease incidence and disease severity compared to untreated fruit. In accordance with this study, Porat et al. (2003) found that treatments with BABA at 20 mM reduced the incidence of P. digitatum in infected wounds on grapefruit. Panebianco et al. (2014) likewise reported that very high concentrations of BABA reduce P. digitatum decay on oranges cvs. “Tarocco” and “Valencia” and on grapefruit cv. “Marsh Seedless” by 70–90%. The fungistatic properties of BABA affect the fungal cell membrane by inhibiting P. digitatum growth, increasing cell membrane permeability and malondialdehyde content, and decreasing the ergosterol and the total lipid contents. Similar structural defects have been observed in P. digitatum treated with cecropin A-melittin hybrid peptide BP21 (Wang W. et al., 2018), the essential oil of C. reticulata (Tao et al., 2014), and pinocembroside isolated from Ficus hirta Vahl. fruit (Chen et al., 2020d).
As biological control does function across a spectrum as broad as chemical fungicides, combining biocontrol agents with synthetic elicitors can enhance their performance. Zhou et al. (2014) investigated the effects of Pichia membranaefaciens and SA for the control of P. digitatum and P. italicum in citrus fruit. Combining the yeast with SA effectively enhanced the phenylalanine ammonialyase, peroxidase, polyphenoloxidase, chitinase, and β-1,3-glucanase activities and synthesis of phenolic compounds without ruining fruit quality parameters. In line with Zhang et al. (2010), these results suggested that SA enhanced the biocontrol efficacy of this yeast in post-harvest diseases by facilitating its growth and promoting nutrient and space competition.
Similarly, Guo et al. (2014) examined the preventive activity of MeJA alone and in combination with the antagonistic yeast C. laurentii for preventing green mold in citrus fruit. MeJA alone with a concentration of 100 μmol/L inhibited disease incidence and lesion diameter of mold decay compared with the control or the application of 100 μmol/L combined with C. laurentii at 1 × 108 cells/mL. Relative to single-treatment groups and the control, MeJA and C. laurentii induced higher peroxidase, polyphenol oxidase, and catalase activity and a rise in the mRNA expression of PR5 (pathogenesis-related protein family 5); it likewise induced natural resistance and stimulated the proliferation of antagonistic yeast on the fruit surface. Additionally, when Zhou et al. (2018) applied SA (2.5 mmol L−1), P. membranaefaciens (1 × 108 cells mL−1), or oligochitosan (15 g L−1), these exogenous elicitors led to effective inhibition of P. digitatum and P. italicum in pathogen-inoculated citrus fruit. Results indicated that the activation of the phenylpropanoid biosynthesis pathway led to the induction of resistance in citrus fruit.
These studies suggest potential strategies by which combinations of two to three different methods complement each other to suppress post-harvest disease in citrus; however, the results do not fully predict control efficacy under commercial practice. Therefore, additional research under commercial practice is warranted. Because little is known about the combined effects of elicitors on the metabolic pathway and regulatory network of phenylpropanoids in citrus fruit, further study into the interaction of citrus species with Penicillium sp. is needed to understand how different hosts respond to various treatments during storage and commercial application (Louw and Korsten, 2015).
Food Additives
Food additives, particularly preservatives or substances classified as GRAS by the U.S. Food and Drug Administration are alternatives to conventional fungicides for post-harvest disease control in citrus fruits. The food industry commonly adds organic and inorganic salts to food for the purposes of leavening, pH control, taste, and texture modification (Smilanick et al., 1999). These compounds also exhibit a broad spectrum of activity against bacteria and fungi. The inorganic salts most widely used to control post-harvest disease in citrus fruit are sodium carbonate, sodium bicarbonate, and potassium sorbate, all classified as GRAS compounds (Table 3). Several other food-grade preservatives that have proven effective against citrus green and blue mold diseases include sodium paraben salts, sodium benzoate, and potassium silicate (Montesinos and Palou, 2016). Major advantages of using these salts for post-harvest treatment include antimicrobial properties, low toxicity, relatively low cost, ready availability, safety for humans and the environment, and its unrestricted use (El-Mougy et al., 2008; Deliopoulos et al., 2010; Palou, 2016).
Table 3.
Salts and food additives for the control of P. digitatum and P. italicum.
| Pathogens | Salts | Fruits | References |
|---|---|---|---|
| P. digitatum | Acetic acid, formic acid, and propionic acid | Grapefruit and oranges | Sholberg, 1998 |
| Sodium propionate | “Valencia” oranges | Hall, 1988 | |
| Calcium chloride | Grapefruit | Droby et al., 1997 | |
| Calcium polysulfide | Oranges and lemons | Smilanick and Sorenson, 2001 | |
| Potassium sorbate | “Valencia” oranges | Smilanick et al., 2008 | |
| P. italicum | Sodium salicylate, sodium sulfite, boric acid, copper sulfate, and sodium ethylenediaminetetraacetic acid | Mandarin (Citrus reticulata Blanco) cv. clementine | Askarne et al., 2013 |
| Ammonium carbonate | Oranges, lemons | Askarne et al., 2011; Montesinos-Herrero et al., 2011 | |
| Sodium ethylparaben | “Valencia” oranges | Moscoso-Ramírez et al., 2013 | |
| Sodium hydrosulfide | Mandarins and oranges | Fu et al., 2014 | |
| P. digitatum and P. italicum | Sodium carbonate Sodium bicarbonate |
Oranges, mandarins, and lemons | Plaza et al., 2004b; Youssef et al., 2012b; Askarne et al., 2013 |
| Sodium benzoate | “Valencia,” “Lane Late” oranges, lemons, and “Ortanique” mandarins | Montesinos-Herrero and Palou, 2016; Montesinos-Herrero et al., 2016 | |
| Benzoic acid | Lemons | El-Mougy et al., 2008 | |
| Potassium sorbate | “Valencia” oranges | Smilanick et al., 2008; D'Aquino et al., 2013 | |
| Sodium metabisulfite and potassium metabisulfite | Oranges | Martínez-Blay et al., 2020 | |
| Sodium dehydroacetate | “Ponkan” tangerines (Citrus reticulata Blanco) | Duan et al., 2016 | |
| Calcium chelate Sodium silicate Potassium carbonate |
“Comune” clementine and “Valencia late” oranges | Youssef et al., 2012b
Palou et al., 2001 |
In vivo and in vitro studies have demonstrated the high effectiveness of salts such as potassium sorbate and sodium benzoate against P. digitatum and P. italicum decay in oranges and lemons (Palou et al., 2002a; Montesinos-Herrero et al., 2016). Non-toxic and tasteless sodium benzoate is specifically known for its bactericidal and bacteriostatic properties (El-Mougy et al., 2008). Likewise, in-vivo assays with sodium carbonate, ammonium carbonate, boric acid, copper sulfate, sodium ethylenediaminetetraacetic acid, sodium salicylate, sodium sulfite, and sodium metabisulfite are known to inhibit mycelial growth of P. italicum in citrus (Askarne et al., 2013). These salts act against molds through membrane disruption, stresses on pH homeostasis through anion accumulation within the cell, inhibition of essential metabolic functions, and activation of defense mechanisms in fruits (Smilanick et al., 2005; Youssef et al., 2014). Although these salts provide good control of citrus molds, application time is crucial because salts applied before the harvest period have more time to interact with mold pathogens than after harvest, resulting in greater efficacy (Youssef et al., 2012b).
Post-harvest P. digitatum and P. italicum incidence on lemons and oranges was effectively controlled by fumigation with ammonia gas not exceeding 6,000 μL/L (Montesinos-Herrero et al., 2011). Oranges treated with ammonium molybdate and sodium molybdate have shown a significant decrease in the incidence of P. digitatum and P. italicum (Palou et al., 2002a). Ammonium molybdate can inhibit acid phosphatase, which interferes with phosphorylation and dephosphorylation and affects metabolic processes in several organisms (Mukhopadhyay et al., 1988; Bodart et al., 1999). Fumigation of mandarins and oranges by hydrogen sulfide salts decreases the growth of P. italicum on tested fruit surfaces, inhibiting spore germination through ROS related mechanisms (Fu et al., 2014). Moreover, food preservatives are as successful as salts in controlling citrus molds. Sodium dehydroacetate, a common food preservative, inhibited mycelial growths of P. digitatum and P. italicum in vivo and in vitro experiments (Duan et al., 2016).
Several salts are known to induce host resistance via stimulation of defense-related genes. Electrolyzed sodium bicarbonate induced oxidative stress in the P. digitatum conidia via accumulation of ROS, the collapse of mitochondrial membrane, disrupted adenosine triphosphate production, and upregulated defense-related gene coding for peroxidase and PAL (Fallanaj et al., 2016). Furthermore, the electrolytes sodium metabisulfite, potassium sorbate, potassium carbonate, and sodium chloride were used to generate alkaline- (alEW), and acidic- (acEW) electrolyzed water for inhibition of P. digitatum and P. italicum on “Valencia” sweet orange (Youssef and Hussien, 2020).
Several studies conducted to control Penicillium molds suggested the performance of these salts could be enhanced by combining them with other treatments such as antagonistic microorganisms, hot water, low-dose chemical fungicides, and wax coatings (Smilanick et al., 2008; Youssef et al., 2012a). Teixidó et al. (2001) evaluated the potential of Pantoea agglomerans (strain CPA-2) in combination with sodium carbonate or bicarbonate solutions under ambient (20°C) and cold storage (3°C) conditions for the control of P. digitatum and P. italicum. The study detected a 97.6% reduction of decay incidence with sodium bicarbonate. In addition, Smilanick et al. (1999) observed a significant improvement in the effectiveness of sodium bicarbonate and carbonate when treatments were followed with Pseudomonas syringae ESC-10. The residues of biological control antagonists were found to persist long after treatment, thereby protecting fruit from reinfection.
Moreover, the combined application of marine yeast Rhodosporidium paludigenum and sodium bicarbonate proved as effective as a fungicide, eliminating the decay incidence of green mold in citrus fruit (Zhu et al., 2013). Also, Lu et al. (2018) assessed the effectiveness of the combined treatment of ammonium molybdate and R. paludigenum to control green mold disease in satsuma mandarin. The addition of 0.1 mmol L−1 ammonium molybdate significantly enhanced the biological activity of R. paludigenum against P. digitatum, reduced disease incidence by 89.3%, and discontinued mold development within 0–12 h of infection. Ammonium molybdate depresses the ecto-phosphate activity of P. digitatum, disturbs the environmental acidification of the pathogen, and suppresses spore germination.
Sorbic acid salts (also used as food additives) such as potassium sorbate are classified as minimal-risk active ingredients, similar to chemical fungicides in effectiveness, and appropriate for aqueous application (Smilanick et al., 2008). Heated aqueous solutions were found to enhance the performance of potassium sorbate against green mold. Inhibitory effects of sorbic acid on P. digitatum and P. italicum include inhibition of enzymes and protein synthesis, alteration of cell-membrane and cell-transport function, and uncoupling of oxidative phosphorylation in mitochondria (El-Mougy et al., 2008). Also, short dip treatments of citrus fruits in salt solutions of 2–3% have significantly reduced the incidence of P. digitatum and P. italicum without causing rind phytotoxicities (Palou, 2016). For instance, dips in sodium metabisulfite and potassium metabisulfite at 20 and 50 mM for 60 or 120 s at room temperature (20°C) significantly reduced the incidence and severity of P. digitatum and P. italicum on “Valencia” oranges (Martínez-Blay et al., 2020). Dip treatments of 60 s with 3% sodium benzoate heated above 50°C resulted in 90% reduction of P. digitatum and P. italicum on “Valencia” oranges, “Lanelate” oranges, “Fino” lemons, and “Ortanique” mandarins, offering an important disease control alternative for the commercialization of citrus without fungicidal residues (Montesinos-Herrero et al., 2016).
In addition, Cerioni et al. (2013) investigated the use of potassium sorbate, sodium bicarbonate, and potassium phosphite in combination with heat and H2O2 in the presence of copper sulfate to control Penicillium molds in lemons. The authors reported phosphite solutions controlled P. digitatum only when heated or combined with fungicides. Moreover, combining wax with potassium sorbate salts provided antifungal activity against citrus molds but impaired the film-forming capacity of the wax, eventually resulting in fruit weight loss (Youssef et al., 2012a; Parra et al., 2014). In a study by Youssef et al. (2012a), ammonium bicarbonate was found not to interfere with the capacity of wax to retard weight loss.
Although these treatments offer alternatives to fungicides for post-harvest disease control, concerns regarding dietary safety, disposal, fruit quality parameters, worker safety, and other regulatory issues must be addressed before these compounds gain approval for post-harvest use. For instance, the disposal of sodium bicarbonate raises regulatory issues in some locations because of its high pH, electrical conductivity, and sodium content (Smilanick et al., 2008). Further research from an integrated approach should seek a suitable combination of salts with non-chemical treatments such as wax to improve fruit quality parameters.
Physical Control Methods
Physical treatments are gaining popularity in the control of citrus post-harvest diseases as it leaves no residue and has the least environmental impact than other options (Palou, 2009; Usall et al., 2016). Of the physical technologies so far investigated for reducing P. digitatum and P. italicum in citrus fruit and prolonging its storage life, the most promising include heat, ultraviolet light (UV-C and UV-B), blue light, x-rays, and gamma irradiation; complementary methods include controlled and modified atmospheres and cold storage (Droby et al., 1993; Nafussi et al., 2001; Kader, 2002; Smilanick et al., 2003; Palou et al., 2007; Gündüz and Pazir, 2013; Lafuente and Alférez, 2015; Jeong et al., 2016; Yamaga et al., 2016; Table 4).
Table 4.
Physical methods for the control of P. digitatum and P. italicum.
| Treatments | Treatment intensity | Cultivars | Pathogens | References |
|---|---|---|---|---|
| Hot water treatment | Dipping for 5 min at 50°C | Oranges and lemons | P. digitatum | Smoot and Melvin, 1963 |
| Brief immersions for 2–5 min at 45–55°C | Citrus spp. | P. digitatum and P. italicum | Spalding and Reeder, 1986; Couey, 1989; Rodov et al., 1995 | |
| Dipping at 50–55°C for 150 s, incubated at 20°C for 7 days | Oranges and lemons | P. digitatum and P. italicum | Palou et al., 2001 | |
| 2–3 min hot water dipping at 50–53°C; 56°C for 20 s; Hot water rinse brushing (HWRB) at 56°C for 20 s; 63°C for 15 s; 62.8°C for 30 s | Oranges | P. digitatum and P. italicum | Schirra et al., 1997; Porat et al., 2000; Smilanick et al., 2003; Strano et al., 2014 | |
| 3 min hot water dipping at 53°C | Pummelo grapefruit hybrid “Oroblanco” | P. digitatum and P. italicum | Rodov et al., 1995 | |
| HWRB at 56°C for 20 s | Tangerines, oranges, and red grapefruits | P. digitatum | Porat et al., 2000 | |
| Hot water dipping at 52–53°C for 2 min. HWRB at 63°C for 15 s | Lemons | P. digitatum | Nafussi et al., 2001; Smilanick et al., 2003 | |
| HWRB at 62°C for 20 s | Oranges and lemons | P. digitatum | Lanza et al., 2002 | |
| HWRB at 56°C for 20 s | Tangerine, oranges, and red grapefruits | P. digitatum | Porat et al., 2000 | |
| Hot water treatment at 56–60°C for 10 s | Pummelo grapefruit hybrid “Oroblanco” | P. digitatum and P. italicum | Rodov et al., 2000 | |
| HWRB at 55°C for 20 s | Kumquats | P. digitatum and P. italicum | Ben-Yehoshua and Porat, 2005 | |
| 3 min hot water dipping at 56°C | Citrus reticulata Blanco × Citrus sinensis (L.) Osbeck | P. digitatum | Kyriacou, 2011 | |
| Curing | 35°C for 72 h | Washington Navel oranges | P. digitatum and P. italicum | Tuset, 1987 |
| 33°C for 65 h | Oranges and lemons | P. digitatum and P. italicum | Plaza et al., 2003, 2004a | |
| 32°C in a saturated water atmosphere for 48 h | Lemons | P. digitatum | Stange and Eckert, 1994 | |
| Two cycles intermittent curing for 18 h at 38°C | Mandarins | P. italicum | Pérez et al., 2005 | |
| Curing at 40°C for 18 h | Oranges | P. digitatum and P. italicum | Nunes et al., 2007 | |
| 32°C for 3 days | “Femminello” lemons and “Valencia” oranges | P. digitatum | Lanza and Di Martino Aleppo, 1996 | |
| 36°C and longer exposure | Tarocco oranges | P. digitatum | Lanza and Di Martino Aleppo, 1996 | |
| “Valencia,” “Pineapple” oranges, and “Flame” grapefruit at 30–35°C for 24 h or longer optimum condition for “Valencia” oranges and “Flame” grapefruit at 35°C with 95–100% humidity for 48 h | “Valencia” oranges, “Flame” grapefruit, and “Pineapple” oranges | P. digitatum | Zhang and Swingle, 2005 | |
| 30°C with high humidity (90–95%) for 72 h | Satsuma mandarins | P. digitatum and P. italicum | Kinay et al., 2005 | |
| Non-ionizing irradiation and UV-C | Fruit exposed to low doses of UV-C irradiation | Marsh seedless grapefruit | P. digitatum | Droby et al., 1993 |
| UV-C application at doses of 0.5 KJ m−2 | Star Ruby grapefruit | P. digitatum | D'Hallewin et al., 2000 | |
| Low UV-C irradiation (7.92 kJ m−2) inactivates spores on the surface of the fruit | Oranges | P. digitatum and P. italicum | Gündüz and Pazir, 2013 | |
| The antifungal activity of lemon peel extracts was improved by short-time UV-B irradiation | Lemons | P. digitatum | Ruiz et al., 2017 | |
| 210 and 630 μmol m−2 s−1 LED blue light quantum fluxes of boosted scoparone in the flavedo | Oranges | P. digitatum | Ballester and Lafuente, 2017 | |
| Low-intensity LED blue light irradiation lowered blue mold symptom development and suppressed fungal sporulation in satsuma mandarins | Mandarins | P. italicum | Yamaga et al., 2015 | |
| Ionizing irradiation | 510 and 875 Gy X-ray irradiations decreased the sporulation of P. digitatum and P. italicum on mandarins that were treated with sodium carbonate | Mandarins | P. digitatum and P. italicum | Palou et al., 2007 |
| 1.0 kGy gamma-irradiation demonstrated germ tube elongation, total inhibition of spore germination, and mycelial growth of P. digitatum | Mandarins | P. digitatum | Jeong et al., 2016 | |
| Cold storage | Temperatures of 3–5°C and relative humidity of 90–95% | Oranges and mandarins | P. digitatum and P. italicum | Kader, 2002 |
| Temperatures of 10–14°C | Lemons, limes, and grapefruit | P. digitatum and P. italicum | Kader, 2002 | |
| Temperatures of 0–3°C | Citrus spp | P. digitatum and P. italicum | Tuset, 1987; Snowdon, 1990; Brown and Eckert, 2000a,b | |
| Storage in a controlled atmosphere | 5–10% O2 + 0–5% CO2 | Oranges and mandarins | P. digitatum and P. italicum | Kader, 2002 |
| 5–10% O2 + 0–10% CO2 | Lemons, limes, and grapefruit | P. digitatum and P. italicum | Kader, 2002 | |
| Storage in ozonated atmosphere | 200 μl/L ozone gas in humid air with 95% relative humidity at 5°C for 1 h | Citrus spp | P. digitatum and P. italicum | Margosan and Smilanick, 1998 |
Heat treatments can be applied to citrus fruit via hot water dips and sprays, hot vapor or curing (hot air application), and hot water rinsing and brushing. When infection structures are present on fruit surfaces, heat applied for a short period can easily affect these tissues, inducing several physiochemical changes to achieve a significant degree of control. Factors that determine the effectiveness of heat treatments include the product's condition prior to treatment, type of commodity, temperature, duration of treatment, and mode of heat application. Studies on the performance of water temperatures ranging from 40 to 65°C have been conducted (Palou et al., 2002a; García et al., 2016). Hot water treatment (HWT) was found to interrupt fungal spore growth for 24–48 h by the accumulation of secondary metabolites such as PR proteins, phytoalexins, accumulation of lignins in fruit infected by fungus, and production of ROS contributing to resistance against P. digitatum and P. italicum in citrus fruit (Nafussi et al., 2001; Yun et al., 2013; Perotti et al., 2015; Sui et al., 2016). Curing is another HWT post-harvest decay control method whereby citrus fruits are exposed for 2–3 days to air atmospheres heated to temperatures higher than 30°C at high relative humidity (RH >90%) (Palou, 2009). Improper heat treatments such as excessive temperatures and long durations have damaged fruit. For example, 53–55°C for 2–3 min. and 60°C for 20 s. have led to surface injury and rind browning in oranges (Schirra et al., 1997; Porat et al., 2000; Palou et al., 2001).
Both UV-C and UV-B are non-ionizing irradiations that have been widely studied for the prevention of green and blue mold in citrus. UV-B irradiation has been reported to harm the surface of citrus fruit less than UV-C treatment (Kaewsuksaeng et al., 2011) and at intensities higher than 30 kJ m−2 inactivated P. digitatum and P. italicum conidia in vitro (Yamaga et al., 2016). Factors determining the effectiveness of UV irradiation include type and intensity, harvesting period, stage of fruit development, and storage temperature (Droby et al., 1993; Yamaga et al., 2016). Overall, UV treatment causes metabolic and anatomical changes and accumulation in citrus flavedo of secondary metabolites such as polyphenols and phytoalexins, which are involved in fruit resistance (Droby et al., 1993; Ruiz et al., 2017). However, high intensities of UV-C irradiation might damage the flavedo of citrus and warrant precautions (Kim et al., 1991). Large-scale studies on UV treatment for citrus decay are needed to make any recommendations for the commercial use of irradiation in post-harvest citrus handling.
Blue light has the potential to reduce green and blue mold disease during post-harvest storage of citrus. It has been reported that blue light enhances fruit resistance against P. digitatum and P. italicum by stimulating the production of secondary metabolites and impairing fungal growth (Lafuente and Alférez, 2015; Ballester and Lafuente, 2017). For instance, Liao et al. (2013) found that blue light at a photon fluence rate of 40 μmol m−2 s−1 decreased the symptomatic development of green and blue mold in “Fallglo” tangerine and in vitro fungal growth of P. italicum, leading to the induction of defensive responses in the host. In vitro experiments also revealed that the efficacy of blue light increases with the duration of the application and light quantum flux by affecting fungal morphology and sporulation and increasing the phytoalexin scoparone and production of ROS in fungal cell walls (Lafuente and Alférez, 2015; El-Esawi et al., 2017).
Similarly, X-ray and gamma irradiation have been recognized as sustainable methods for extending the post-harvest life of citrus from decay (Rojas-Argudo et al., 2012; Guerreiro et al., 2016). X-ray differs from gamma irradiations in that X-rays are concentrated in the same direction as the electron beam while gamma rays are emitted uniformly in all directions (Palou, 2009). Rojas-Argudo et al. (2012) found that X-ray irradiation stimulates rind biosynthesis and synthesis of the phytoalexins scoparone and scopoletin in “Clemenules” mandarins at a storage temperature of 20°C. X-rays have increased scoparone levels after fruit inoculation in combination with sodium carbonate. The ionizing characteristics of X-ray irradiation was found to cause oxidative stress in fruits, which can influence the bioactive compounds located in fruit tissues and potentially improve the resistance of those fruits to pathogens (Oufedjikh et al., 2000).
Gamma radiation has likewise proven detrimental to fungal physiology by disrupting fungal cell membranes, retarding fruit ripening and respiration rate, and regulating the activity of enzymes (Cia et al., 2007; Schweiggert et al., 2007; Wang et al., 2017). However, Jeong et al. (2016) reported that higher doses may cause severe damage to the surface of citrus. Operating gamma-irradiation at lower doses can exclude this issue with other treatments such as sodium dichloro-s-triazinetrione (Jeong et al., 2016). Overall, if not applied correctly (in terms of dose, intensity, and duration), irradiation can adversely affect fruit quality parameters, stimulate phytotoxicities, and even impact human health. Combining irradiation treatment with other environmentally friendly techniques would help to minimize detrimental effects and increase efficacy.
Cold storage and storage in controlled or modified atmospheres are complementary tools that provide fungistatic activity by inhibiting or delaying the growth and development of pathogens. They also help reduce host metabolic activity, delay senescence, and maintain fruit resistance to fungal infection (Usall et al., 2016).
Outlook and Prospects
Green and blue mold disease in citrus poses a major threat worldwide. The conventional disease management approaches involving synthetic fungicides are increasingly being questioned because of their potential deleterious impacts on human and environmental health, and the growing problem of fungi developing resistance to the synthetic fungicides. Non-toxic alternatives to synthetic fungicides include biological control, bio-fungicides, plant extracts and essential oils, chitosan, salts, hot water treatments and UV-radiation, and synthetic elicitors (Janisiewicz and Korsten, 2002; Droby et al., 2009). Although producers and consumers accept these new approaches, limited research has investigated the effectiveness of these methods on large-scale production. Not much is known about mechanisms of action of these alternative control approaches against green and blue mold disease, while the pathogen continues to cause huge economic losses. To manage these diseases, researchers might explore the molecular mechanisms of plant/fruit-pathogen interactions, including pathogenicity and plant resistance. Identification and functional analysis of citrus genes that regulate citrus fruit via transgenesis or genome editing may lead to the development of novel and durable control strategies against P. digitatum and P. italicum. Recent advances in the study of disease resistance in citrus are made possible, thanks to the availability of complete genome sequence of citrus, optimization of genetic transformation systems, and establishment of CRISPR/Cas9 gene-editing approaches. Thus, identification of resistant genes and developing resistant citrus varieties via genome editing might provide a promising pathway to control P. digitatum and P. italicum pathogens.
Author Contributions
UB conceptualized the review, wrote the original draft, investigated, revised, and edited the draft manuscript.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
I sincerely thank Chandra Dhakal and Genevieve Guzman for their comments and English language editing. The author also thank the editor, Zhongwei Zou, and two referees for their comments and suggestions.
References
- Abbey J. A., Percival D., Abbey L., Asiedu S. K., Prithiviraj B., Schilder A. (2019). Biofungicides as alternative to synthetic fungicide control of grey mould (Botrytis cinerea)–prospects and challenges. Biocontrol Sci. Technol. 29, 207–228. 10.1080/09583157.2018.1548574 [DOI] [Google Scholar]
- Abraham A. O., Laing M. D., Bower J. P. (2010). Isolation and in vivo screening of yeast and Bacillus antagonists for the control of Penicillium digitatum of citrus fruit. Biol. Control 53, 32–38. 10.1016/j.biocontrol.2009.12.009 [DOI] [Google Scholar]
- Afek U., Orenstein J., Carmeli S., Rodov V., Joseph M. B. (1999). Umbelliferone, a phytoalexin associated with resistance of immature Marsh grapefruit to Penicillium digitatum. Phytochemistry 50, 1129–1132. 10.1016/S0031-9422(98)00671-2 [DOI] [Google Scholar]
- Aghdam M. S., Luo Z., Aminian-Dehkordi R., Jannatizadeh A., Farmani B., Younessi-Hamzekhanlu M., et al. (2020). Exogenous β-aminobutyric acid application attenuates Aspergillus decay, minimizes aflatoxin B1 accumulation, and maintains nutritional quality in fresh-in-hull pistachio kernels. J. Sci. Food Agric. 100, 2130–2135. 10.1002/jsfa.10236 [DOI] [PubMed] [Google Scholar]
- Agnioni A., Cabras P., Dhallewin G., Pirisi F. M., Reniero F., Schirra M. (1998). Synthesis and inhibitory activity of 7-geranoxy coumarin against Penicillium species in citrus fruits. Phytochemistry 47, 1521–1525. 10.1016/S0031-9422(97)00771-1 [DOI] [Google Scholar]
- Ahmed D. M., El-Shami S. M., El-Mallah M. H. (2007). Jojoba oil as a novel coating for exported Valencia orange fruit. Part 1. The use of trans (isomerized) jojoba oil. Am. Eurasian. J. Agric. Environ. Sci. 2, 173–181. [Google Scholar]
- Aloui H., Licciardello F., Khwaldia K., Hamdi M., Restuccia C. (2015). Physical properties and antifungal activity of bioactive films containing Wickerhamomyces anomalus killer yeast and their application for preservation of oranges and control of postharvest green mold caused by Penicillium digitatum. Int. J. Food Microbiol. 200, 22–30. 10.1016/j.ijfoodmicro.2015.01.015 [DOI] [PubMed] [Google Scholar]
- Al-Snafi A. E. (2016). Nutritional value and pharmacological importance of citrus species grown in Iraq. IOSR J. Pharm. 6, 76–108. 10.9790/3013-0680176108 [DOI] [Google Scholar]
- Ameziane N., Boubaker H., Boudyach H., Msanda F., Jilal A., Benaoumar A. A. (2007). Antifungal activity of Moroccan plants against citrus fruit pathogens. Agron. Sustain. Dev. 27, 273–277. 10.1051/agro:2007022 [DOI] [Google Scholar]
- Ariza M. R., Larsen T. O., Petersen B. O., Duus J. Ø., Barrero A. F. (2002). Penicillium digitatum metabolites on synthetic media and citrus fruits. J. Agric. Food Chem. 50, 6361–6365. 10.1021/jf020398d [DOI] [PubMed] [Google Scholar]
- Arras G. (1996). Mode of action of an isolate of Candida famata in biological control of Penicillium digitatum in orange fruits. Postharvest Biol. Technol. 8, 191–198. 10.1016/0925-5214(95)00071-2 [DOI] [Google Scholar]
- Arras G., Scherm B., Migheli Q. (2002). Improving biocontrol activity of Pichia guillermondii against post-harvest decay of oranges in commercial packing-houses by reduced concentrations of fungicides. Biocontrol Sci. Technol. 12, 547–553. 10.1080/0958315021000016216 [DOI] [Google Scholar]
- Arras G., Usai M. (2001). Fungitoxic activity of 12 essential oils against four postharvest citrus pathogens: chemical analysis of Thymus capitatus oil and its effect in subatmospheric pressure conditions. J. Food Prot. 64, 1025–1029. 10.4315/0362-028X-64.7.1025 [DOI] [PubMed] [Google Scholar]
- Askarne L., Boubaker H., Boudyach E. H., Aoumar A. A. B. (2013). Use of food additives to control postharvest citrus blue mold disease. Atlas J. Biol. 2, 147–153. 10.5147/ajb.v2i3.25 [DOI] [Google Scholar]
- Askarne L., Talibi I., Boubaker H., Boudyach E. H., Msanda F., Saadi B., et al. (2012). In vitro and in vivo antifungal activity of several Moroccan plants against Penicillium italicum, the causal agent of citrus blue mold. Crop Prot. 40, 53–58. 10.1016/j.cropro.2012.04.023 [DOI] [Google Scholar]
- Askarne L., Talibi I., Serghini M. A., Boubaker H., Msanda F., Aoumar A. A. B., Boudyach E. H. (2011). Antifungal activity of Moroccan plants against Penicillium italicum, the causal agent of blue mold of citrus. IOBC/wprs Bull. 62, 51–54. [Google Scholar]
- Baccelli I., Glauser G., Mauch-Mani B. (2017). The accumulation of β-aminobutyric acid is controlled by the plant's immune system. Planta 246, 791–796. 10.1007/s00425-017-2751-3 [DOI] [PubMed] [Google Scholar]
- Bakkali F., Averbeck S., Averbeck D., Idaomar M. (2008). Biological effects of essential oils–a review. Food Chem. Toxicol. 46, 446–475. 10.1016/j.fct.2007.09.106 [DOI] [PubMed] [Google Scholar]
- Ballester A. R., Lafuente M. T. (2017). LED blue light-induced changes in phenolics and ethylene in citrus fruit: implication in elicited resistance against Penicillium digitatum infection. Food Chem. 218, 575–583. 10.1016/j.foodchem.2016.09.089 [DOI] [PubMed] [Google Scholar]
- Bar-Shimon M., Yehuda H., Cohen L., Weiss B., Kobeshnikov A., Daus A., et al. (2004). Characterization of extracellular lytic enzymes produced by the yeast biocontrol agent Candida oleophila. Curr. Genet. 45, 140–148. 10.1007/s00294-003-0471-7 [DOI] [PubMed] [Google Scholar]
- Benavente-Garcia O., Castillo J. (2008). Update on uses and properties of citrus flavonoids: new findings in anticancer, cardiovascular, and anti-inflammatory activity. J. Agric. Food Chem. 56, 6185–6205. 10.1021/jf8006568 [DOI] [PubMed] [Google Scholar]
- Benhamou N. (2004). Potential of the mycoparasite, Verticillium lecanii, to protect citrus fruit against Penicillium digitatum, the causal agent of green mold: a comparison with the effect of chitosan. Phytopathology 94, 693–705. 10.1094/PHYTO.2004.94.7.693 [DOI] [PubMed] [Google Scholar]
- Benhamou N., Brodeur J. (2001). Pre-inoculation of Ri T-DNA transformed cucumber roots with the mycoparasite, Verticillium lecanii, induces host defense reactions against Pythium ultimum infection. Physiol. Mol. Plant Pathol. 58, 133–146. 10.1006/pmpp.2001.0322 [DOI] [Google Scholar]
- Ben-Yehoshua S., Porat R. (2005). Heat treatments to reduce decay, in Environmentally Friendly Technologies of Agricultural Produce Quality, ed Yeoshua S. B. (Boca Raton, FL: Taylor & Francis Group; ), 11–42. 10.1201/9780203500361 [DOI] [Google Scholar]
- Bodart J. F., Béchard D., Bertout M., Rousseau A., Gannon J., Vilain J. P., Flament S. (1999). Inhibition of protein tyrosine phosphatases blocks calcium-induced activation of metaphase II-arrested oocytes of Xenopus laevis. FEBS Lett. 457, 175–178. 10.1016/S0014-5793(99)00986-2 [DOI] [PubMed] [Google Scholar]
- Boubaker H., Karim H., El Hamdaoui A., Msanda F., Leach D., Bombarda I., et al. (2016). Chemical characterization and antifungal activities of four Thymus species essential oils against postharvest fungal pathogens of citrus. Ind. Crops Prod. 86, 95–101. 10.1016/j.indcrop.2016.03.036 [DOI] [Google Scholar]
- Brown G. E., Eckert J. W. (2000a). Blue mold, in Compendium of Citrus Diseases, 2nd Edn, eds Timmer L. W., Garnsey S. M., Graham J. H. (St. Paul, MN: American Phytopathological Society; ), 41. [Google Scholar]
- Brown G. E., Eckert J. W. (2000b). Green mold, in Compendium of Citrus Diseases, 2nd Edn, eds Timmer L. W., Garnsey S. M., Graham J. H. (St. Paul, MN: American Phytopathological Society; ), 41–42. [Google Scholar]
- Cao J., Zeng K., Jiang W. (2006). Enhancement of postharvest disease resistance in Ya Li pear (Pyrus bretschneideri) fruit by salicylic acid sprays on the trees during fruit growth. Eur. J. Plant Pathol. 114, 363–370. 10.1007/s10658-005-5401-8 [DOI] [Google Scholar]
- Cerioni L., Sepulveda M., Rubio-Ames Z., Volentini S. I., Rodríguez-Montelongo L., Smilanick J. L., et al. (2013). Control of lemon postharvest diseases by low-toxicity salts combined with hydrogen peroxide and heat. Postharvest Biol. Technol. 83, 17–21. 10.1016/j.postharvbio.2013.03.002 [DOI] [Google Scholar]
- Cháfer M., Sánchez-González L., González-Martínez C., Chiralt A. (2012). Fungal decay and shelf life of oranges coated with chitosan and bergamot, thyme, and tea tree essential oils. J. Food Sci. 77, E182–E187. 10.1111/j.1750-3841.2012.02827.x [DOI] [PubMed] [Google Scholar]
- Chalutz E., Wilson C. L. (1990). Postharvest biocontrol of green and blue mold and sour rot of citrus fruit by Debaryomyces hansenii. Plant Dis. 74, 134–137. 10.1094/PD-74-013430812563 [DOI] [Google Scholar]
- Che J., Chen X., Ouyang Q., Tao N. (2020). p-Anisaldehyde exerts its antifungal activity against Penicillium digitatum and Penicillium italicum by disrupting the cell wall integrity and membrane permeability. J. Microbiol. Biotechnol. 30, 878–884. 10.4014/jmb.1911.11032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Cai N., Chen J., Wan C. (2019). Clove essential oil as an alternative approach to control postharvest blue mold caused by Penicillium italicum in citrus fruit. Biomolecules 9:197. 10.3390/biom9050197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Cai N., Chen J., Wan C. (2020a). UHPLC-Q-TOF/MS-based metabolomics approach reveals the antifungal potential of pinocembroside against citrus green mold phytopathogen. Plants 9:17. 10.3390/plants9010017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Chen J., Wan C. (2020b). Pinocembrin-7-glucoside (P7G) reduced postharvest blue mold of navel orange by suppressing Penicillium italicum growth. Microorganisms 8:536. 10.3390/microorganisms8040536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Wan C., Guo J., Chen J. (2020c). Paenibacillus brasilensis YS-1: a potential biocontrol agent to retard Xinyu tangerine senescence. Agriculture 10:330. 10.3390/agriculture10080330 [DOI] [Google Scholar]
- Chen C., Wan C., Peng X., Chen J. (2020d). A flavonone pinocembroside inhibits Penicillium italicum growth and blue mold development in ‘Newhall’ navel oranges by targeting membrane damage mechanism. Pestic. Biochem. Physiol. 165:104505. 10.1016/j.pestbp.2019.11.025 [DOI] [PubMed] [Google Scholar]
- Chen D., Forster H., Adaskaveg J. (2020e). Natamycin, a biofungicide for managing major postharvest fruit decays of citrus. Plant Dis. 105, 1408–1414. 10.1094/PDIS-08-20-1650-RE [DOI] [PubMed] [Google Scholar]
- Chen O., Deng L., Ruan C., Yi L., Zeng K. (2021). Pichia galeiformis induces resistance in postharvest citrus by activating the phenylpropanoid biosynthesis pathway. J. Agric. Food Chem. 69, 2619–2631. 10.1021/acs.jafc.0c06283 [DOI] [PubMed] [Google Scholar]
- Chen O., Yi L., Deng L., Ruan C., Zeng K. (2020f). Screening antagonistic yeasts against citrus green mold and the possible biocontrol mechanisms of Pichia galeiformis (BAF03). J. Sci. Food Agric. 100, 3812–3821. 10.1002/jsfa.10407 [DOI] [PubMed] [Google Scholar]
- Chen P., Peng Y., Chung W., Chung K., Huang H., Huang J. (2016). Inhibition of Penicillium digitatum and citrus green mold by volatile compounds produced by Enterobacter cloacae. J. Plant Pathol. Microbiol. 7:1000339. 10.4172/2157-7471.1000339 [DOI] [Google Scholar]
- Cheng L., Nie X., Jiang C., Li S. (2019). The combined use of the antagonistic yeast Hanseniaspora uvarum with β-aminobutyric acid for the management of postharvest diseases of kiwifruit. Biol. Control 137:104019. 10.1016/j.biocontrol.2019.104019 [DOI] [Google Scholar]
- Cheong J. J., Do Choi Y. (2003). Methyl jasmonate as a vital substance in plants. Trends Genet. 19, 409–413. 10.1016/S0168-9525(03)00138-0 [DOI] [PubMed] [Google Scholar]
- Cia P., Pascholati S. F., Benato E. A., Camili E. C., Santos C. A. (2007). Effects of gamma and UV-C irradiation on the postharvest control of papaya anthracnose. Postharvest Biol. Technol. 43, 366–373. 10.1016/j.postharvbio.2006.10.004 [DOI] [Google Scholar]
- Cohen Y. R. (2002). β-aminobutyric acid-induced resistance against plant pathogens. Plant Dis. 86, 448–457. 10.1094/PDIS.2002.86.5.448 [DOI] [PubMed] [Google Scholar]
- Comitini F., Mannazzu I., Ciani M. (2009). Tetrapisispora phaffii killer toxin is a highly specific β-glucanase that disrupts the integrity of the yeast cell wall. Microb. Cell Fact. 8:55. 10.1186/1475-2859-8-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrath U., Beckers G. J., Langenbach C. J., Jaskiewicz M. R. (2015). Priming for enhanced defense. Annu. Rev. Phytopathol. 53, 97–119. 10.1146/annurev-phyto-080614-120132 [DOI] [PubMed] [Google Scholar]
- Costa J. H., Bazioli J. M., Barbosa L. D., dos Santos Júnior P. L. T., Reis F. C., Klimeck T., et al. (2021). Phytotoxic tryptoquialanines produced in vivo by Penicillium digitatum are exported in extracellular vesicles. MBio 12:e03393-20. 10.1128/mBio.03393-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couey H. M. (1989). Heat treatment for control of postharvest diseases and insect pests of fruits. HortScience 24, 198–202. [Google Scholar]
- Cowan M. M. (1999). Plant products as antimicrobial agents. Clin. Microbiol. Rev. 12, 564–582. 10.1128/CMR.12.4.564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Cunha T., Ferraz L. P., de Sousa-Júnior G. D. S., Kupper K. C. (2020). The action of yeast strains as biocontrol agents against Penicillium digitatum in Lima sweet oranges. Citrus Res. Technol. 41:e1054. 10.4322/crt.18819 [DOI] [Google Scholar]
- da Cunha T., Ferraz L. P., Wehr P. P., Kupper K. C. (2018). Antifungal activity and action mechanisms of yeasts isolates from citrus against Penicillium italicum. Int. J. Food Microbiol. 276, 20–27. 10.1016/j.ijfoodmicro.2018.03.019 [DOI] [PubMed] [Google Scholar]
- Daferera D. J., Ziogas B. N., Polissiou M. G. (2000). GC-MS analysis of essential oils from some Greek aromatic plants and their fungitoxicity on Penicillium digitatum. J. Agric. Food Chem. 48, 2576–2581. 10.1021/jf990835x [DOI] [PubMed] [Google Scholar]
- D'Aquino S., Fadda A., Barberis A., Palma A., Angioni A., Schirra M. (2013). Combined effects of potassium sorbate, hot water and thiabendazole against green mould of citrus fruit and residue levels. Food Chem. 141, 858–864. 10.1016/j.foodchem.2013.03.083 [DOI] [PubMed] [Google Scholar]
- De Corato U., Maccioni O., Trupo M., Di Sanzo G. (2010). Use of essential oil of Laurus nobilis obtained by means of a supercritical carbon dioxide technique against post-harvest spoilage fungi. Crop Prot. 29, 142–147. 10.1016/j.cropro.2009.10.012 [DOI] [Google Scholar]
- De Rodríguez D. J., García R. R., Castillo F. H., González C. A., Galindo A. S., Quintanilla J. V., Zuccolotto L. M. (2011). In vitro antifungal activity of extracts of Mexican Chihuahuan Desert plants against postharvest fruit fungi. Ind. Crops Prod. 34, 960–966. 10.1016/j.indcrop.2011.03.001 [DOI] [Google Scholar]
- de Vilhena Araújo É., Vendramini P. H., Costa J. H., Eberlin M. N., Montagner C. C., Fill T. P. (2019). Determination of tryptoquialanines A and C produced by Penicillium digitatum in oranges: are we safe? Food Chem. 301:125285. 10.1016/j.foodchem.2019.125285 [DOI] [PubMed] [Google Scholar]
- Del Río J. A., Arcas M. C., Benavente-García O., Ortuño A. (1998). Citrus polymethoxylated flavones can confer resistance against Phytophthora citrophthora, Penicillium digitatum, and Geotrichum species. J. Agric. Food Chem. 46, 4423–4428. 10.1021/jf980229m [DOI] [Google Scholar]
- Deliopoulos T., Kettlewell P. S., Hare M. C. (2010). Fungal disease suppression by inorganic salts: a review. Crop Prot. 29, 1059–1075. 10.1016/j.cropro.2010.05.011 [DOI] [Google Scholar]
- Dennis C., Webster J. (1971). Antagonistic properties of species-groups of Trichoderma: II. Production of volatile antibiotics. Trans. Br. Mycol. Soc. 57, 25–39. 10.1016/S0007-1536(71)80077-3 [DOI] [Google Scholar]
- D'Hallewin G., Schirra M., Pala M., Ben-Yehoshua S. (2000). Ultraviolet C irradiation at 0.5 kJ.m(-)(2) reduces decay without causing damage or affecting postharvest quality of star ruby grapefruit (C. paradisi Macf.). J. Agric. Food Chem. 48, 4571–4575. 10.1021/jf000559i [DOI] [PubMed] [Google Scholar]
- Dixit S. N., Chandra H., Tiwari R., Dixit V. (1995). Development of a botanical fungicide against blue mould of mandarins. J. Stored Prod. Res. 31, 165–172. 10.1016/0022-474X(94)00041-Q [DOI] [Google Scholar]
- Droby S., Chalutez E. (1999). Biological control of postharvest decay in citrus fruit. Advances in postharvest diseases and disorders control of citrus fruit. Research Signpost, Trivandrum, India. Eur. J. Plant Pathol. 123, 37–45. [Google Scholar]
- Droby S., Chalutz E., Horev B., Cohen L., Gaba V., Wilson C. L., Wisniewski M. (1993). Factors affecting UV-induced resistance in grapefruit against the green mould decay caused by Penicillium digitatum. Plant Pathol. 42, 418–424. 10.1111/j.1365-3059.1993.tb01520.x [DOI] [Google Scholar]
- Droby S., Chalutz E., Wilson C. L., Wisniewski M. (1989). Characterization of the biocontrol activity of Debaryomyces hansenii in the control of Penicillium digitatum on grapefruit. Can. J. Microbiol. 35, 794–800. 10.1139/m89-132 [DOI] [Google Scholar]
- Droby S., Cohen L., Daus A., Weiss B., Horev B., Chalutz E., et al. (1998). Commercial testing of Aspire: a yeast preparation for the biological control of postharvest decay of citrus. Biol. Control 12, 97–101. 10.1006/bcon.1998.0615 [DOI] [Google Scholar]
- Droby S., Eick A., Macarisin D., Cohen L., Rafael G., Stange R., et al. (2008). Role of citrus volatiles in host recognition, germination and growth of Penicillium digitatum and Penicillium italicum. Postharvest Biol. Technol. 49, 386–396. 10.1016/j.postharvbio.2008.01.016 [DOI] [Google Scholar]
- Droby S., Vinokur V., Weiss B., Cohen L., Daus A., Goldschmidt E. E., Porat R. (2002). Induction of resistance to Penicillium digitatum in grapefruit by the yeast biocontrol agent Candida oleophila. Phytopathology 92, 393–399. 10.1094/PHYTO.2002.92.4.393 [DOI] [PubMed] [Google Scholar]
- Droby S., Wisniewski M., Macarisin D., Wilson C. (2009). Twenty years of postharvest biocontrol research: is it time for a new paradigm? Postharvest Biol. Technol. 52, 137–145. 10.1016/j.postharvbio.2008.11.009 [DOI] [Google Scholar]
- Droby S., Wisniewski M. E., Cohen L., Weiss B., Touitou D., Eilam Y., Chalutz E. (1997). Influence of CaCl2 on Penicillium digitatum, grapefruit peel tissue, and biocontrol activity of Pichia guilliermondii. Phytopathology 87, 310–315. 10.1094/PHYTO.1997.87.3.310 [DOI] [PubMed] [Google Scholar]
- Du Plooy W., Regnier T., Combrinck S. (2009). Essential oil amended coatings as alternatives to synthetic fungicides in citrus postharvest management. Postharvest Biol. Technol. 53, 117–122. 10.1016/j.postharvbio.2009.04.005 [DOI] [Google Scholar]
- Duan X., Jing G., Fan F., Tao N. (2016). Control of postharvest green and blue molds of citrus fruit by application of sodium dehydroacetate. Postharvest Biol. Technol. 113, 17–19. 10.1016/j.postharvbio.2015.10.015 [DOI] [Google Scholar]
- El Guilli M., Hamza A., Clément C., Ibriz M., Ait Barka E. (2016). Effectiveness of postharvest treatment with chitosan to control citrus green mold. Agriculture 6:12. 10.3390/agriculture6020012 [DOI] [Google Scholar]
- El Guilli M., Jijakli M. H., Lahlali R. (2020). Pilot testing of two biofungicide formulations for the control of citrus blue and green mold in two Moroccan packinghouses. Moroccan J. Agric. Sci. 1, 215–220. [Google Scholar]
- El-Esawi M., Arthaut L. D., Jourdan N., d'Harlingue A., Link J., Martino C. F., Ahmad M. (2017). Blue-light induced biosynthesis of ROS contributes to the signaling mechanism of Arabidopsis cryptochrome. Sci. Rep. 7:13875. 10.1038/s41598-017-13832-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ghaouth A., Smilanick J. L., Wilson C. L. (2000). Enhancement of the performance of Candida saitoana by the addition of glycolchitosan for the control of postharvest decay of apple and citrus fruit. Postharvest Biol. Technol. 19, 103–110. 10.1016/S0925-5214(00)00076-4 [DOI] [Google Scholar]
- El-Mohamedy R. S., El-Gamal N. G., Bakeer A. R. T. (2015). Application of chitosan and essential oils as alternatives fungicides to control green and blue moulds of citrus fruits. Int. J. Curr. Microbiol. Appl. Sci 4, 629–643. [Google Scholar]
- El-Mougy N. S., El-Gamal N. G., Abd-El-Kareem F. (2008). Use of organic acids and salts to control postharvest diseases of lemon fruits in Egypt. Archiv. Phytopathol. Plant Protect. 41, 467–476. 10.1080/03235400600813532 [DOI] [Google Scholar]
- Eloff J. N., Mahlo S. N. (2011). Breonadia salicina (Rubiaceae) extracts are as effective as a commercial fungicide in post-harvest protection of oranges against Penicillium infections. Planta Med. 77:PM219. 10.1055/s-0031-128297726606158 [DOI] [Google Scholar]
- Elsherbiny E. A., Dawood D. H., Safwat N. A. (2021). Antifungal action and induction of resistance by β-aminobutyric acid against Penicillium digitatum to control green mold in orange fruit. Pestic. Biochem. Physiol. 171:104721. 10.1016/j.pestbp.2020.104721 [DOI] [PubMed] [Google Scholar]
- Exarchou V., Kanetis L., Charalambous Z., Apers S., Pieters L., Gekas V., Goulas V. (2015). HPLC-SPE-NMR characterization of major metabolites in Salvia fruticosa Mill. Extract with antifungal potential: relevance of carnosic acid, carnosol, and hispidulin. J. Agric. Food Chem. 63, 457–463. 10.1021/jf5050734 [DOI] [PubMed] [Google Scholar]
- Ezra D., Hess W. M., Strobel G. A. (2004). New endophytic isolates of Muscodor albus, a volatile-antibiotic-producing fungus. Microbiology 150, 4023–4031. 10.1099/mic.0.27334-0 [DOI] [PubMed] [Google Scholar]
- Fallanaj F., Ippolito A., Ligorio A., Garganese F., Zavanella C., Sanzani S. M. (2016). Electrolyzed sodium bicarbonate inhibits Penicillium digitatum and induces defence responses against green mould in citrus fruit. Postharvest Biol. Technol. 115, 18–29. 10.1016/j.postharvbio.2015.12.009 [DOI] [Google Scholar]
- Fan F., Tao N., Jia L., He X. (2014). Use of citral incorporated in postharvest wax of citrus fruit as a botanical fungicide against Penicillium digitatum. Postharvest Biol. Technol. 90, 52–55. 10.1016/j.postharvbio.2013.12.005 [DOI] [Google Scholar]
- Fatemi S., Jafarpour M., Eghbalsaied S. (2012). Study of the effect of Thymus vulgaris and hot water treatment on storage life of orange (Citrus sinensis CV. Valencia). J. Med. Plants Res. 6, 968–971. 10.5897/JMPR11.475 [DOI] [Google Scholar]
- Ferraz L. P., da Cunha T., da Silva A. C., Kupper K. C. (2016). Biocontrol ability and putative mode of action of yeasts against Geotrichum citri-aurantii in citrus fruit. Microbiol. Res. 188, 72–79. 10.1016/j.micres.2016.04.012 [DOI] [PubMed] [Google Scholar]
- Fisher K., Phillips C. (2008). Potential antimicrobial uses of essential oils in food: is citrus the answer? Trends Food Sci. Technol. 19, 156–164. 10.1016/j.tifs.2007.11.006 [DOI] [Google Scholar]
- Fravel D. R., Larkin R. P. (1996). Availability and application of biocontrol products. Biol. Cult. Tests Control Plant Dis. 11, 1–7. [Google Scholar]
- Fu L. H., Hu K. D., Hu L. Y., Li Y. H., Hu L. B., Yan H., et al. (2014). An antifungal role of hydrogen sulfide on the postharvest pathogens Aspergillus niger and Penicillium italicum. PLoS ONE 9:e104206. 10.1371/journal.pone.0104206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galed G., Fernández-Valle M. E., Martinez A., Heras A. (2004). Application of MRI to monitor the process of ripening and decay in citrus treated with chitosan solutions. Magn. Reson. Imaging 22, 127–137. 10.1016/j.mri.2003.05.006 [DOI] [PubMed] [Google Scholar]
- Gamagae S. U., Sivakumar D., Wijesundera R. L. C. (2004). Evaluation of post-harvest application of sodium bicarbonate-incorporated wax formulation and Candida oleophila for the control of anthracnose of papaya. Crop Prot. 23, 575–579. 10.1016/j.cropro.2003.11.003 [DOI] [Google Scholar]
- García J. F., Olmo M., García J. M. (2016). Decay incidence and quality of different citrus varieties after postharvest heat treatment at laboratory and industrial scale. Postharvest Biol. Technol. 118, 96–102. 10.1016/j.postharvbio.2016.03.019 [DOI] [Google Scholar]
- Gatto M. A., Ippolito A., Linsalata V., Cascarano N. A., Nigro F., Vanadia S., Di Venere D. (2011). Activity of extracts from wild edible herbs against postharvest fungal diseases of fruit and vegetables. Postharvest Biol. Technol. 61, 72–82. 10.1016/j.postharvbio.2011.02.005 [DOI] [Google Scholar]
- Geng P., Chen S., Hu M., Rizwan-ul-Haq M., Lai K., Qu F., Zhang Y. (2011). Combination of Kluyveromyces marxianus and sodium bicarbonate for controlling green mold of citrus fruit. Int. J. Food Microbiol. 151, 190–194. 10.1016/j.ijfoodmicro.2011.08.023 [DOI] [PubMed] [Google Scholar]
- Guerreiro D., Madureira J., Silva T., Melo R., Santos P. M., Ferreira A., et al. (2016). Post-harvest treatment of cherry tomatoes by gamma radiation: microbial and physicochemical parameters evaluation. Innovat. Food Sci. Emerg. Technol. 36, 1–342. 10.1016/j.ifset.2016.05.008 [DOI] [Google Scholar]
- Gündüz G. T., Pazir F. (2013). Inactivation of Penicillium digitatum and Penicillium italicum under in vitro and in vivo conditions by using UV-C light. J. Food Prot. 76, 1761–1766. 10.4315/0362-028X.JFP-12-511 [DOI] [PubMed] [Google Scholar]
- Guo J., Fang W., Lu H., Zhu R., Lu L., Zheng X., Yu T. (2014). Inhibition of green mold disease in mandarins by preventive applications of methyl jasmonate and antagonistic yeast Cryptococcus laurentii. Postharvest Biol. Technol. 88, 72–78. 10.1016/j.postharvbio.2013.09.008 [DOI] [Google Scholar]
- Guolin L., Meng F., Wei X., Lin M. (2019). Postharvest dipping treatment with BABA induced resistance against rot caused by Gilbertella persicaria in red pitaya fruit. Sci. Hortic. 257:108713. 10.1016/j.scienta.2019.108713 [DOI] [Google Scholar]
- Hafdani F. N., Sadeghinia N. (2011). A review on application of chitosan as a natural antimicrobial. World Acad. Sci. Eng. Technol. 50, 252–256. 26396358 [Google Scholar]
- Hall D. J. (1988). Comparative activity of selected food preservatives as citrus postharvest fungicides, in Proceedings of the Annual Meeting of the Florida State Horticulture Society (USA) (Orlando, FL: ). [Google Scholar]
- Hall D. J., Fernandez Y. J. (2004). In vitro evaluation of selected essential oils as fungicides against Penicillium digitatum sacc, in Proceedings of the Florida State Horticultural Society, Vol. 117 (Mascotte, FL: ), 377–379. [Google Scholar]
- Han Q., Zhao H., Cheng Y., Yao J., Huang L., Kang Z. (2013). A cytological study on infection of citrus fruits by Penicillium digitatum. Mycosystema 32, 967–977. [Google Scholar]
- Hao W., Li H., Hu M., Yang L., Rizwan-ul-Haq M. (2011). Integrated control of citrus green and blue mold and sour rot by Bacillus amyloliquefaciens in combination with tea saponin. Postharvest Biol. Technol. 59, 316–323. 10.1016/j.postharvbio.2010.10.00234223648 [DOI] [Google Scholar]
- Hao W., Zhong G., Hu M., Luo J., Weng Q., Rizwan-ul-Haq M. (2010). Control of citrus postharvest green and blue mold and sour rot by tea saponin combined with imazalil and prochloraz. Postharvest Biol. Technol. 56, 39–43. 10.1016/j.postharvbio.2009.10.003 [DOI] [Google Scholar]
- Hendel N., Larous L., Belbey L. (2016). Antioxidant activity of rosemary (Rosmarinus officinalis L.) and its in vitro inhibitory effect on Penicillium digitatum. Int. Food Res. J. 23, 1725–1732. [Google Scholar]
- Hernández-Montiel L. G., Ochoa J. L., Troyo-Diéguez E., Larralde-Corona C. P. (2010). Biocontrol of postharvest blue mold (Penicillium italicum Wehmer) on Mexican lime by marine and citrus Debaryomyces hansenii isolates. Postharvest Biol. Technol. 56, 181–187. 10.1016/j.postharvbio.2009.12.010 [DOI] [Google Scholar]
- Holmes G. J., Eckert J. W. (1999). Sensitivity of Penicillium digitatum and Penicillium italicum to postharvest citrus fungicides in California. Phytopathology 89, 716–721. 10.1094/PHYTO.1999.89.9.716 [DOI] [PubMed] [Google Scholar]
- Holopainen J. K., Heijari J., Nerg A. M., Vuorinen M., Kainulainen P. (2009). Potential for the use of exogenous chemical elicitors in disease and insect pest management of conifer seedling production. Open For. Sci. J. 2, 17–24. 10.2174/1874398600902010017 [DOI] [Google Scholar]
- Huang Y., Deverall B. J., Morris S. C. (1995). Postharvest control of green mould on oranges by a strain of Pseudomonas glathei and enhancement of its biocontrol by heat treatment. Postharvest Biol. Technol. 5, 129–137. 10.1016/0925-5214(94)00016-L [DOI] [Google Scholar]
- Hulot J. F., Hiller N. (2021). Exploring the Benefits of Biocontrol for Sustainable Agriculture – A Literature Review on Biocontrol in Light of the European Green Deal. Brussels: Institute for European Environmental Policy. [Google Scholar]
- Ippolito A., Nigro F. (2000). Impact of preharvest application of biological control agents on postharvest diseases of fresh fruits and vegetables. Crop Prot. 19, 715–723. 10.1016/S0261-2194(00)00095-8 [DOI] [Google Scholar]
- Iqbal Z., Iqbal J., Abbas I., Kamran M. (2017). Innovative strategies for eco-friendly management of citrus blue mold disease caused by Penicillium italicum Wehmer. J. Int. Sci. Publ. Agric. Food 5, 361–365. [Google Scholar]
- Iqbal Z., Singh Z., Khangura R., Ahmad S. (2012). Management of citrus blue and green moulds through application of organic elicitors. Austral. Plant Pathol. 41, 69–77. 10.1007/s13313-011-0091-5 [DOI] [Google Scholar]
- Janisiewicz W. J., Korsten L. (2002). Biological control of postharvest diseases of fruits. Annu. Rev. Phytopathol. 40, 411–441. 10.1146/annurev.phyto.40.120401.130158 [DOI] [PubMed] [Google Scholar]
- Jeong R. D., Chu E. H., Lee G. W., Cho C., Park H. J. (2016). Inhibitory effect of gamma irradiation and its application for control of postharvest green mold decay of Satsuma mandarins. Int. J. Food Microbiol. 234, 1–8. 10.1016/j.ijfoodmicro.2016.06.026 [DOI] [PubMed] [Google Scholar]
- Jhalegar M. J., Sharma R. R., Singh D. (2015). In vitro and in vivo activity of essential oils against major postharvest pathogens of Kinnow (Citrus nobilis × C. deliciosa) mandarin. J. Food Sci. Technol. 52, 2229–2237. 10.1007/s13197-014-1281-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J. Y., Zhang H. Y., Xue Y. B., Zeng K. F. (2020). Effects of INA on postharvest blue and green molds and anthracnose decay in citrus fruit. J. Integr. Agric. 19, 1396–1406. 10.1016/S2095-3119(20)63169-0 [DOI] [Google Scholar]
- Johann S., Oliveira V. L. D., Pizzolatti M. G., Schripsema J., Braz-Filho R., Branco A., Smânia A., Jr. (2007). Antimicrobial activity of wax and hexane extracts from Citrus spp. peels. Memórias do Instituto Oswaldo Cruz 102, 681–685. 10.1590/S0074-02762007000600004 [DOI] [PubMed] [Google Scholar]
- Joyce D. C., Wearing H., Coates L., Terry L. (2001). Effects of phosphonate and salicylic acid treatments on anthracnose disease development and ripening of 'Kensington Pride' mango fruit. Aust. J. Exp. Agric. 41, 805–813. 10.1071/EA9910428948418 [DOI] [Google Scholar]
- Kader A. A. (2002). Postharvest Technology of Horticultural Crops, Vol. 3311. Davis, CA: University of California Agriculture and Natural Resources. [Google Scholar]
- Kaewsuksaeng S., Urano Y., Aiamla-or S., Shigyo M., Yamauchi N. (2011). Effect of UV-B irradiation on chlorophyll-degrading enzyme activities and postharvest quality in stored lime (Citrus latifolia Tan.) fruit. Postharvest Biol. Technol. 61, 124–130. 10.1016/j.postharvbio.2011.02.014 [DOI] [Google Scholar]
- Kanan G., Al-Najar R. (2009). In vitro and in vivo activity of selected plant crude extracts and fractions against Penicillium italicum. J. Plant Protect. Res. 49, 341–352. 10.2478/v10045-009-0054-9 [DOI] [Google Scholar]
- Kanetis L., Förster H., Adaskaveg J. E. (2007). Comparative efficacy of the new postharvest fungicides azoxystrobin, fludioxonil, and pyrimethanil for managing citrus green mold. Plant Dis. 91, 1502–1511. 10.1094/PDIS-91-11-1502 [DOI] [PubMed] [Google Scholar]
- Kavanagh J. A., Wood R. K. S. (1971). Green mould of oranges caused by Penicillium digitatum Sacc.; effect of additives on spore germination and infection. Ann. Appl. Biol. 67, 35–44. 10.1111/j.1744-7348.1971.tb02906.x [DOI] [Google Scholar]
- Khakimov A. A., Omonlikov A. U., Utaganov S. B. U. (2020). Current status and prospects of the use of biofungicides against plant diseases. GSC Biol. Pharmac. Sci. 13, 119–126. 10.30574/gscbps.2020.13.3.0403 [DOI] [Google Scholar]
- Kim J. J., Ben-Yehoshua S., Shapiro B., Henis Y., Carmeli S. (1991). Accumulation of scoparone in heat-treated lemon fruit inoculated with Penicillium digitatum Sacc. Plant Physiol. 97, 880–885. 10.1104/pp.97.3.880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P. I., Chung K. C. (2004). Production of an antifungal protein for control Colletotrichum lagenarium by Bacillus amyloliquefaciens MET0908. FEMS Microbiol. Lett. 234, 177–183. 10.1016/j.femsle.2004.03.032 [DOI] [PubMed] [Google Scholar]
- Kinay P., Yildiz F., Sen F., Yildiz M., Karacali I. (2005). Integration of pre- and postharvest treatments to minimize Penicillium decay of Satsuma mandarins. Postharvest Biol. Technol. 37, 31–36. 10.1016/j.postharvbio.2005.02.008 [DOI] [Google Scholar]
- Klieber A., Scott E., Wuryatmo E. (2002). Effect of method of application on antifungal efficacy of citral against postharvest spoilage fungi of citrus in culture. Austral. Plant Pathol. 31, 329–332. 10.1071/AP0203428948418 [DOI] [Google Scholar]
- Koltz E. A., dos Santos I., Dallemole-Giaretta R., Pazolini K., Leite C. D., Steilmann P. (2020). Combining Brassica sachets and extracts with thermotherapy against postharvest green mold of orange. Sci. Hortic. 268:109389. 10.1016/j.scienta.2020.109389 [DOI] [Google Scholar]
- Kouassi K. H. S., Bajji M., Jijakli H. (2012). The control of postharvest blue and green molds of citrus in relation with essential oil–wax formulations, adherence and viscosity. Postharvest Biol. Technol. 73, 122–128. 10.1016/j.postharvbio.2012.06.008 [DOI] [Google Scholar]
- Kupper K. C., Cervantes A. L. L., Klein M. N., Da Silva A. C. (2013). Assessment of antagonistic micro-organisms Saccharomyces cerevisiae and Bacillus subtilis for controlling Penicillium digitatum. Rev. Bras. Frutic. 35, 425–436. 10.1590/S0100-29452013000200011 [DOI] [Google Scholar]
- Kurtzman C. P., Droby S. (2001). Metschnikowia fructicola, a new ascosporic yeast with potential for biocontrol of postharvest fruit rots. Syst. Appl. Microbiol. 24, 395–399. 10.1078/0723-2020-00045 [DOI] [PubMed] [Google Scholar]
- Kyriacou M. C. (2011). Influence of a post-harvest hot water treatment on the development of green mould [Penicillium digitatum (Pers.: Fr.) Sacc.] and on the quality of ‘Mandora’fruit [Citrus reticulata Blanco Citrus sinensis (L.) Osbeck]. J. Hortic. Sci. Biotechnol. 86, 359–365. 10.1080/14620316.2011.11512774 [DOI] [Google Scholar]
- Ladaniya M. S. (2008). Commercial fresh citrus cultivars and producing countries, in Citrus Fruit: Biology, Technology and Evaluation, ed Ladaniya M. S. (San Diego, CA: Academic Press; ), 13–65. 10.1016/B978-012374130-1.50004-8 [DOI] [Google Scholar]
- Ladaniya M. S. (2011). Physico-chemical, respiratory and fungicide residue changes in wax coated mandarin fruit stored at chilling temperature with intermittent warming. J. Food Sci. Technol. 48, 150–158. 10.1007/s13197-010-0160-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafuente M. T., Alférez F. (2015). Effect of LED blue light on Penicillium digitatum and Penicillium italicum strains. Photochem. Photobiol. 91, 1412–1421. 10.1111/php.12519 [DOI] [PubMed] [Google Scholar]
- Lahlali R., Hamadi Y., Drider R., Misson C., El Guilli M., Jijakli M. H. (2014). Control of citrus blue mold by the antagonist yeast Pichia guilliermondii Z1: compatibility with commercial fruit waxes and putative mechanisms of action. Food Control 45, 8–15. 10.1016/j.foodcont.2014.04.014 [DOI] [Google Scholar]
- Lai K., Chen S., Hu M., Hu Q., Geng P., Weng Q., Jia J. (2012). Control of postharvest green mold of citrus fruit by application of endophytic Paenibacillus polymyxa strain SG-6. Postharvest Biol. Technol. 69, 40–48. 10.1016/j.postharvbio.2012.03.001 [DOI] [Google Scholar]
- Lanciotti R., Gianotti A., Patrignani F., Belletti N., Guerzoni M. E., Gardini F. (2004). Use of natural aroma compounds to improve shelf-life and safety of minimally processed fruits. Trends Food Sci. Technol. 15, 201–208. 10.1016/j.tifs.2003.10.004 [DOI] [Google Scholar]
- Lanza G., Di Martino Aleppo E. (1996). Control of green mould of oranges and lemons by curing at high temperatures, in Proceedings of the International Society of Citriculture : VIII International Citrus Congress (Sun City Resort: ), 12–17. [Google Scholar]
- Lanza G., Di Martino Aleppo E., Strano M. C. (2002). Evaluation of alternative treatments to control green mold in citrus fruit, in XXVI International Horticultural Congress: Citrus and Other Subtropical and Tropical Fruit Crops: Issues, Advances and Opportunities, Vol. 632 (Toronto, ON: ), 343–349. 10.17660/ActaHortic.2004.632.4511997972 [DOI] [Google Scholar]
- Leelasuphakul W., Hemmanee P., Chuenchitt S. (2008). Growth inhibitory properties of Bacillus subtilis strains and their metabolites against the green mold pathogen (Penicillium digitatum Sacc.) of citrus fruit. Postharvest Biol. Technol. 48, 113–121. 10.1016/j.postharvbio.2007.09.024 [DOI] [Google Scholar]
- Li J., Li H., Ji S., Chen T., Tian S., Qin G. (2019). Enhancement of biocontrol efficacy of Cryptococcus laurentii by cinnamic acid against Penicillium italicum in citrus fruit. Postharvest Biol. Technol. 149, 42–49. 10.1016/j.postharvbio.2018.11.018 [DOI] [Google Scholar]
- Liao H. L., Alferez F., Burns J. K. (2013). Assessment of blue light treatments on citrus postharvest diseases. Postharvest Biol. Technol. 81, 81–88. 10.1016/j.postharvbio.2013.02.019 [DOI] [Google Scholar]
- Lin Y., Fan L., Xia X., Wang Z., Yin Y., Cheng Y., Li Z. (2019). Melatonin decreases resistance to postharvest green mold on citrus fruit by scavenging defense-related reactive oxygen species. Postharvest Biol. Technol. 153, 21–30. 10.1016/j.postharvbio.2019.03.016 [DOI] [Google Scholar]
- Liu J., Sui Y., Wisniewski M., Droby S., Liu Y. (2013). Utilization of antagonistic yeasts to manage postharvest fungal diseases of fruit. Int. J. Food Microbiol. 167, 153–160. 10.1016/j.ijfoodmicro.2013.09.004 [DOI] [PubMed] [Google Scholar]
- Liu X., Fang W., Liu L., Yu T., Lou B., Zheng X. (2010). Biological control of postharvest sour rot of citrus by two antagonistic yeasts. Lett. Appl. Microbiol. 51, 30–35. 10.1111/j.1472-765X.2010.02851.x [DOI] [PubMed] [Google Scholar]
- Liu Y., Wang W., Zhou Y., Yao S., Deng L., Zeng K. (2017). Isolation, identification and in vitro screening of Chongqing orangery yeasts for the biocontrol of Penicillium digitatum on citrus fruit. Biol. Control 110, 18–24. 10.1016/j.biocontrol.2017.04.002 [DOI] [Google Scholar]
- Liu Y., Yao S., Deng L., Ming J., Zeng K. (2019). Different mechanisms of action of isolated epiphytic yeasts against Penicillium digitatum and Penicillium italicum on citrus fruit. Postharvest Biol. Technol. 152, 100–110. 10.1016/j.postharvbio.2019.03.002 [DOI] [Google Scholar]
- Long C. A., Deng B. X., Deng X. X. (2007). Commercial testing of Kloeckera apiculata, isolate 34-9, for biological control of postharvest diseases of citrus fruit. Ann. Microbiol. 57, 203–207. 10.1007/BF03175208 [DOI] [Google Scholar]
- Louw J. P., Korsten L. (2015). Pathogenicity and host susceptibility of Penicillium spp. on citrus. Plant Dis. 99, 21–30. 10.1094/PDIS-02-14-0122-RE [DOI] [PubMed] [Google Scholar]
- Lu L., Ji L., Qiao L., Zhang Y., Chen M., Wang C., et al. (2018). Combined treatment with Rhodosporidium paludigenum and ammonium molybdate for the management of green mold in satsuma mandarin (Citrus unshiu Marc.). Postharvest Biol. Technol. 140, 93–99. 10.1016/j.postharvbio.2018.01.005 [DOI] [Google Scholar]
- Luo Y., Zeng K., Ming J. (2012). Control of blue and green mold decay of citrus fruit by Pichia membranefaciens and induction of defense responses. Sci. Hortic. 135, 120–127. 10.1016/j.scienta.2011.11.031 [DOI] [Google Scholar]
- Maldonado M. C., Orosco C. E. F., Gordillo M., Navarro A. R. (2010). In vivo and in vitro antagonism of Streptomyces sp. RO3 against Penicillium digitatum and Geotrichum candidum. Afr. J. Microbiol. Res. 4, 2451–2456. [Google Scholar]
- Margosan D. A., Smilanick J. L. (1998). Mortality of spores of Botrytis cinerea, Monilinia fructicola, Penicillium digitatum, and Rhizopus stolonifer after exposure to ozone under humid conditions. Phytopathology 88:S58. [Google Scholar]
- Mari M., Guizzardi M. (1998). The postharvest phase: emerging technologies for the control of fungal diseases. Phytoparasitica 26, 59–66. 10.1007/BF02981267 [DOI] [Google Scholar]
- Martínez-Blay V., Taberner V., Pérez-Gago M. B., Palou L. (2020). Control of major citrus postharvest diseases by sulfur-containing food additives. Int. J. Food Microbiol. 330:108713. 10.1016/j.ijfoodmicro.2020.108713 [DOI] [PubMed] [Google Scholar]
- Matrose N. A., Obikeze K., Belay Z. A., Caleb O. J. (2021). Plant extracts and other natural compounds as alternatives for postharvest management of fruit fungal pathogens: a review. Food Bioscience 41:100840. 10.1016/j.fbio.2020.100840 [DOI] [Google Scholar]
- Mekbib S. B., Regnier T. J., Korsten L. (2007). Control of Penicillium digitatum on citrus fruit using two plant extracts and study of their mode of action. Phytoparasitica 35, 264–276. 10.1007/BF02981160 [DOI] [Google Scholar]
- Mekbib S. B., Regnier T. J., Sivakumar D., Korsten L. (2009). Evaluation of Ethiopian plant extracts, Acacia seyal and Withania somnifera, to control green mould and ensure quality maintenance of citrus (Citrus sinensis L.). Fruits 64, 285–294. 10.1051/fruits/2009023 [DOI] [Google Scholar]
- Meng X., Han J., Wang Q., Tian S. (2009). Changes in physiology and quality of peach fruits treated by methyl jasmonate under low temperature stress. Food Chem. 114, 1028–1035. 10.1016/j.foodchem.2008.09.109 [DOI] [Google Scholar]
- Mercier J., Smilanick J. L. (2005). Control of green mold and sour rot of stored lemon by biofumigation with Muscodor albus. Biol. Control 32, 401–407. 10.1016/j.biocontrol.2004.12.002 [DOI] [Google Scholar]
- Meziane H., Gavriel S., Ismailov Z., Chet I., Chernin L., Höfte M. (2006). Control of green and blue mould on orange fruit by Serratia plymuthica strains IC14 and IC1270 and putative modes of action. Postharvest Biol. Technol. 39, 125–133. 10.1016/j.postharvbio.2005.10.007 [DOI] [Google Scholar]
- Montesinos-Herrero C., Moscoso-Ramírez P. A., Palou L. (2016). Evaluation of sodium benzoate and other food additives for the control of citrus postharvest green and blue molds. Postharvest Biol. Technol. 115, 72–80. 10.1016/j.postharvbio.2015.12.022 [DOI] [Google Scholar]
- Montesinos-Herrero C., Palou L. (2016). Synergism between potassium sorbate dips and brief exposure to high CO2 or O2 at curing temperature for the control of citrus postharvest green and blue molds. Crop Prot. 81, 43–46. 10.1016/j.cropro.2015.12.00530727139 [DOI] [Google Scholar]
- Montesinos-Herrero C., Smilanick J. L., Tebbets J. S., Walse S., Palou L. (2011). Control of citrus postharvest decay by ammonia gas fumigation and its influence on the efficacy of the fungicide imazalil. Postharvest Biol. Technol. 59, 85–93. 10.1016/j.postharvbio.2010.07.010 [DOI] [Google Scholar]
- Moosa A., Sahi S. T., Khan S. A., Malik A. U. (2019). Salicylic acid and jasmonic acid can suppress green and blue moulds of citrus fruit and induce the activity of polyphenol oxidase and peroxidase. Folia Hortic. 31, 195–204. 10.2478/fhort-2019-0014 [DOI] [Google Scholar]
- Moraes Bazioli J., Belinato J. R., Costa J. H., Akiyama D. Y., Pontes J. G. D. M., Kupper K. C., et al. (2019). Biological control of citrus postharvest phytopathogens. Toxins 11:460. 10.3390/toxins11080460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretto C., Cervantes A. L. L., Batista Filho A., Kupper K. C. (2014). Integrated control of green mold to reduce chemical treatment in post-harvest citrus fruits. Sci. Hortic. 165, 433–438. 10.1016/j.scienta.2013.11.019 [DOI] [Google Scholar]
- Moscoso-Ramírez P. A., Montesinos-Herrero C., Palou L. (2013). Control of citrus postharvest Penicillium molds with sodium ethylparaben. Crop Prot. 46, 44–51. 10.1016/j.cropro.2012.12.007 [DOI] [Google Scholar]
- Mukhopadhyay N. K., Saha A. K., Lovelace J. K., Da silva R., Sacks D. l., Glew R. H. (1988). Comparison of the protein kinase and acid phosphatase activities of five species of Leishmania. J. Protozool. 35, 601–607. 10.1111/j.1550-7408.1988.tb04158.x [DOI] [PubMed] [Google Scholar]
- Musto M., Potenza G., Francesco C. (2014). Inhibition of Penicillium digitatum by a crude extract from Solanum nigrum leaves. BASE 18, 174–180. [Google Scholar]
- Nafussi B., Ben-Yehoshua S., Rodov V., Peretz J., Ozer B. K., D'Hallewin G. (2001). Mode of action of hot-water dip in reducing decay of lemon fruit. J. Agric. Food Chem. 49, 107–113. 10.1021/jf000700n [DOI] [PubMed] [Google Scholar]
- Najmeh S., Hosein S. B. G., Sareh S., Bonjar L. S. (2014). Biological control of citrus green mould, Penicillium digitatum, by antifungal activities of Streptomyces isolates from agricultural soils. Afr. J. Microbiol. Res. 8, 1501–1509. 10.5897/AJMR2013.6289 [DOI] [Google Scholar]
- Nantawanit N., Chanchaichaovivat A., Panijpan B., Ruenwongsa P. (2010). Induction of defense response against Colletotrichum capsici in chili fruit by the yeast Pichia guilliermondii strain R13. Biol. Control 52, 145–152. 10.1016/j.biocontrol.2009.10.011 [DOI] [Google Scholar]
- Nicosia M. G. L. D., Pangallo S., Raphael G., Romeo F. V., Strano M. C., Rapisarda P., et al. (2016). Control of postharvest fungal rots on citrus fruit and sweet cherries using a pomegranate peel extract. Postharvest Biol. Technol. 114, 54–61. 10.1016/j.postharvbio.2015.11.012 [DOI] [Google Scholar]
- Nunes C., Manso T., Lima-Costa M. E. (2009). Postharvest biological control of citrus fruit. Tree For. Sci. Biotechnol. 3, 116–126. [Google Scholar]
- Nunes C., Usall J., Manso T., Torres R., Olmo M., García J. M. (2007). Effect of high temperature treatments on growth of Penicillium spp. and their development on ‘Valencia’oranges. Food Sci. Technol. Int. 13, 63–68. 10.1177/1082013207075601 [DOI] [Google Scholar]
- Obagwu J., Korsten L. (2003). Control of citrus green and blue molds with garlic extracts. Eur. J. Plant Pathol. 109, 221–225. 10.1023/A:1022839921289 [DOI] [Google Scholar]
- Oufedjikh H., Mahrouz M., Amiot M. J., Lacroix M. (2000). Effect of γ-irradiation on phenolic compounds and phenylalanine ammonia-lyase activity during storage in relation to peel injury from peel of Citrus clementina hort. Ex. tanaka. J. Agric. Food Chem. 48, 559–565. 10.1021/jf9902402 [DOI] [PubMed] [Google Scholar]
- Oyourou J. N., Combrinck S., Regnier T., Marston A. (2013). Purification, stability and antifungal activity of verbascoside from Lippia javanica and Lantana camara leaf extracts. Ind. Crops Prod. 43, 820–826. 10.1016/j.indcrop.2012.08.028 [DOI] [Google Scholar]
- Palou L. (2009). Control of citrus postharvest diseases by physical means. Tree For. Sci. Biotechnol. 3, 127–142. [Google Scholar]
- Palou L. (2016). Non-polluting chemical approaches to control citrus postharvest diseases. J Bacteriol Mycol Open Access 2:00019. 10.15406/jbmoa.2016.02.00019 [DOI] [Google Scholar]
- Palou L. (2018). Postharvest treatments with GRAS salts to control fresh fruit decay. Horticulturae 4:46. 10.3390/horticulturae4040046 [DOI] [Google Scholar]
- Palou L., Ali A., Fallik E., Romanazzi G. (2016). GRAS, plant-and animal-derived compounds as alternatives to conventional fungicides for the control of postharvest diseases of fresh horticultural produce. Postharvest Biol. Technol. 122, 41–52. 10.1016/j.postharvbio.2016.04.017 [DOI] [Google Scholar]
- Palou L., Crisosto C. H., Garner D. (2007). Combination of postharvest antifungal chemical treatments and controlled atmosphere storage to control gray mold and improve storability of ‘Wonderful’ pomegranates. Postharvest Biol. Technol. 43, 133–142. 10.1016/j.postharvbio.2006.08.013 [DOI] [Google Scholar]
- Palou L., Smilanick J. L., Droby S. (2008). Alternatives to conventional fungicides for the control of citrus postharvest green and blue moulds. Stewart Postharvest Rev. 2, 1–16. 10.2212/spr.2008.2.2 [DOI] [Google Scholar]
- Palou L., Smilanick J. L., Usall J., Viñas I. (2001). Control of postharvest blue and green molds of oranges by hot water, sodium carbonate, and sodium bicarbonate. Plant Dis. 85, 371–376. 10.1094/PDIS.2001.85.4.371 [DOI] [PubMed] [Google Scholar]
- Palou L., Usall J., Munoz J. A., Smilanick J. L., Vinas I. (2002a). Hot water, sodium carbonate, and sodium bicarbonate for the control of postharvest green and blue molds of clementine mandarins. Postharvest Biol. Technol. 24, 93–96. 10.1016/S0925-5214(01)00178-8 [DOI] [Google Scholar]
- Palou L., Usall J., Smilanick J. L., Aguilar M. J., Vinas I. (2002b). Evaluation of food additives and low-toxicity compounds as alternative chemicals for the control of Penicillium digitatum and Penicillium italicum on citrus fruit. Pest Manag. Sci. 58, 459–466. 10.1002/ps.477 [DOI] [PubMed] [Google Scholar]
- Palou L., Valencia-Chamorro S. A., Pérez-Gago M. B. (2015). Antifungal edible coatings for fresh citrus fruit: a review. Coatings 5, 962–986. 10.3390/coatings5040962 [DOI] [Google Scholar]
- Panebianco S., Vitale A., Platania C., Restuccia C., Polizzi G., Cirvilleri G. (2014). Postharvest efficacy of resistance inducers for the control of green mold on important Sicilian citrus varieties. J. Plant Dis. Protect. 121, 177–183. 10.1007/BF03356507 [DOI] [Google Scholar]
- Panebianco S., Vitale A., Polizzi G., Scala F., Cirvilleri G. (2015). Enhanced control of postharvest citrus fruit decay by means of the combined use of compatible biocontrol agents. Biol. Control 84, 19–27. 10.1016/j.biocontrol.2015.02.001 [DOI] [Google Scholar]
- Papoutsis K., Mathioudakis M. M., Hasperué J. H., Ziogas V. (2019). Non-chemical treatments for preventing the postharvest fungal rotting of citrus caused by Penicillium digitatum (green mold) and Penicillium italicum (blue mold). Trends Food Sci. Technol. 86, 479–491. 10.1016/j.tifs.2019.02.053 [DOI] [Google Scholar]
- Parafati L., Vitale A., Restuccia C., Cirvilleri G. (2016). The effect of locust bean gum (LBG)-based edible coatings carrying biocontrol yeasts against Penicillium digitatum and Penicillium italicum causal agents of postharvest decay of mandarin fruit. Food Microbiol. 58, 87–94. 10.1016/j.fm.2016.03.014 [DOI] [PubMed] [Google Scholar]
- Parra J., Ripoll G., Orihuel-Iranzo B. (2014). Potassium sorbate effects on citrus weight loss and decay control. Postharvest Biol. Technol. 96, 7–13. 10.1016/j.postharvbio.2014.04.011 [DOI] [Google Scholar]
- Pelser P. D. T., Eckert J. W. (1977). Constituents of orange juice that stimulate the germination of conidia of Penicillium digitatum. Phytopathology 67, 747–754. 10.1094/Phyto-67-74730812563 [DOI] [Google Scholar]
- Peng L., Yang S., Cheng Y. J., Chen F., Pan S., Fan G. (2012). Antifungal activity and action mode of pinocembrin from propolis against Penicillium italicum. Food Sci. Biotechnol. 21, 1533–1539. 10.1007/s10068-012-0204-0 [DOI] [Google Scholar]
- Pérez A. G., Luaces P., Olmo M., Sanz C., García J. M. (2005). Effect of intermittent curing on mandarin quality. J. Food Sci. 70, M64–M68. 10.1111/j.1365-2621.2005.tb09048.x [DOI] [Google Scholar]
- Perez M. F., Ibarreche J. P., Isas A. S., Sepulveda M., Ramallo J., Dib J. R. (2017). Antagonistic yeasts for the biological control of Penicillium digitatum on lemons stored under export conditions. Biol. Control 115, 135–140. 10.1016/j.biocontrol.2017.10.006 [DOI] [Google Scholar]
- Pérez-Alfonso C. O., Martínez-Romero D., Zapata P. J., Serrano M., Valero D., Castillo S. (2012). The effects of essential oils carvacrol and thymol on growth of Penicillium digitatum and Penicillium italicum involved in lemon decay. Int. J. Food Microbiol. 158, 101–106. 10.1016/j.ijfoodmicro.2012.07.002 [DOI] [PubMed] [Google Scholar]
- Perotti V. E., Moreno A. S., Trípodi K., Del Vecchio H. A., Meier G., Bello F., et al. (2015). Biochemical characterization of the flavedo of heat-treated Valencia orange during postharvest cold storage. Postharvest Biol. Technol. 99, 80–87. 10.1016/j.postharvbio.2014.08.007 [DOI] [Google Scholar]
- Pimenta R. S., Silva J. F. M., Coelho C. M., Morais P. B., Rosa C. A., Corrêa A., Jr. (2010). Integrated control of Penicillium digitatum by the predacious yeast Saccharomycopsis crataegensis and sodium bicarbonate on oranges. Brazil. J. Microbiol. 41, 404–410. 10.1590/S1517-83822010000200022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piombo E., Sela N., Wisniewski M., Hoffmann M., Gullino M. L., Allard M. W., et al. (2018). Genome sequence, assembly and characterization of two Metschnikowia fructicola strains used as biocontrol agents of postharvest diseases. Front. Microbiol. 9:593. 10.3389/fmicb.2018.00593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platania C., Restuccia C., Muccilli S., Cirvilleri G. (2012). Efficacy of killer yeasts in the biological control of Penicillium digitatum on Tarocco orange fruits (Citrus sinensis). Food Microbiol. 30, 219–225. 10.1016/j.fm.2011.12.010 [DOI] [PubMed] [Google Scholar]
- Plaza P., Usall J., Smilanick J. L., Lamarca N., Viñas I. (2004a). Combining Pantoea agglomerans (CPA-2) and curing treatments to control established infections of Penicillium digitatum on lemons. J. Food Prot. 67, 781–786. 10.4315/0362-028X-67.4.781 [DOI] [PubMed] [Google Scholar]
- Plaza P., Usall J., Torres R., Abadias M., Smilanick J. L., Viñas I. (2004b). The use of sodium carbonate to improve curing treatments against green and blue moulds on citrus fruits. Pest Manag. Sci. For. Pestic. Sci. 60, 815–821. 10.1002/ps.880 [DOI] [PubMed] [Google Scholar]
- Plaza P., Usall J., Torres R., Lamarca N., Asensio A., Viñas I. (2003). Control of green and blue mould by curing on oranges during ambient and cold storage. Postharvest Biol. Technol. 28, 195–198. 10.1016/S0925-5214(02)00127-815307674 [DOI] [Google Scholar]
- Porat R., Daus A., Weiss B., Cohen L., Droby S. (2002). Effects of combining hot water, sodium bicarbonate and biocontrol on postharvest decay of citrus fruit. J. Hortic. Sci. Biotechnol. 77, 441–445. 10.1080/14620316.2002.11511519 [DOI] [Google Scholar]
- Porat R., Daus A., Weiss B., Cohen L., Fallik E., Droby S. (2000). Reduction of postharvest decay in organic citrus fruit by a short hot water brushing treatment. Postharvest Biol. Technol. 18, 151–157. 10.1016/S0925-5214(99)00065-4 [DOI] [Google Scholar]
- Porat R., Vinokur V., Weiss B., Cohen L., Daus A., Goldschmidt E. E., Droby S. (2003). Induction of resistance to Penicillium digitatum in grapefruit by β-aminobutyric acid. Eur. J. Plant Pathol. 109, 901–907. 10.1023/B:EJPP.0000003624.28975.45 [DOI] [PubMed] [Google Scholar]
- Qasem J. R. (1996). Aqueous extract effects of sorne common weed species against certain plant pathogenic fungi. Revue Marocaine des Sciences Agronomiques et Vétérinaires 16, 11–19. [Google Scholar]
- Qasem J. R., Abu-Blan H. A. (1995). Antifungal activity of aqueous extracts from some common weed species. Ann. Appl. Biol. 127, 215–219. 10.1111/j.1744-7348.1995.tb06666.x [DOI] [Google Scholar]
- Ramezani S., Rahemi M., Saharkhiz M. J. (2009). Biological control of postharvest disease caused by Penicillium digitutum and P. italicum on stored citrus fruits by Shiraz Thyme essential oil. Adv. Environ. Biol. 249–255. [Google Scholar]
- Riggle J. H., Klos E. J. (1972). Relationship of Erwinia herbicola to Erwinia amylovora. Can. J. Bot. 50, 1077–1083. 10.1139/b72-133 [DOI] [Google Scholar]
- Rodov V., Agar T., Peretz J., Nafussi B., Kim J. J., Ben-Yehoshua S. (2000). Effect of combined application of heat treatments and plastic packaging on keeping quality of ‘Oroblanco’ fruit (Citrus grandis L. × C. paradisi Macf.). Postharvest Biol. Technol. 20, 287–294. 10.1016/S0925-5214(00)00129-0 [DOI] [Google Scholar]
- Rodov V., Ben-Yehoshua S., Albagli R., Fang D. Q. (1995). Reducing chilling injury and decay of stored citrus fruit by hot water dips. Postharvest Biol. Technol. 5, 119–127. 10.1016/0925-5214(94)00011-G [DOI] [Google Scholar]
- Rodov V., Ben-Yehoshua S., Kim J. J., Shapiro B., Ittah Y. (1992). Ultraviolet illumination induces scoparone production in kumquat and orange fruit and improves decay resistance. J. Am. Soc. Hortic. Sci. 117, 788–792. 10.21273/JASHS.117.5.788 [DOI] [Google Scholar]
- Roger F., Keinath A. (2010). Biofungicides and Chemicals for Managing Diseases in Organic Vegetable Production. Clemson, SC: Clemson University Cooperative Ext. Information Leaflet, 88. [Google Scholar]
- Rojas-Argudo C., Palou L., Bermejo A., Cano A., del Río M. A., González-Mas M. C. (2012). Effect of X-ray irradiation on nutritional and antifungal bioactive compounds of ‘Clemenules’ clementine mandarins. Postharvest Biol. Technol. 68, 47–53. 10.1016/j.postharvbio.2012.02.004 [DOI] [Google Scholar]
- Ruiz V. E., Cerioni L., Zampini I. C., Cuello S., Isla M. I., Hilal M., Rapisarda V. A. (2017). UV-B radiation on lemons enhances antifungal activity of flavedo extracts against Penicillium digitatum. LWT Food Sci. Technol. 85, 96–103. 10.1016/j.lwt.2017.07.002 [DOI] [Google Scholar]
- Samson J. A. (1984). Tropical Fruits-Tropical Agricultural Series. New York, NY: Longman Inc. [Google Scholar]
- Sayago J. E., Ordoñez R. M., Kovacevich L. N., Torres S., Isla M. I. (2012). Antifungal activity of extracts of extremophile plants from the Argentine Puna to control citrus postharvest pathogens and green mold. Postharvest Biol. Technol. 67, 19–24. 10.1016/j.postharvbio.2011.12.011 [DOI] [Google Scholar]
- Schirra M., Agabbio M., D'Hallewin G., Pala M., Ruggiu R. (1997). Response of Tarocco oranges to picking date, postharvest hot water dips, and chilling storage temperature. J. Agric. Food Chem. 45, 3216–3220. 10.1021/jf970273m [DOI] [Google Scholar]
- Schweiggert U., Carle R., Schieber A. (2007). Conventional and alternative processes for spice production–a review. Trends Food Sci. Technol. 18, 260–268. 10.1016/j.tifs.2007.01.005 [DOI] [Google Scholar]
- Senaratna T., Touchell D., Bunn E., Dixon K. (2000). Acetyl salicylic acid (Aspirin) and salicylic acid induce multiple stress tolerance in bean and tomato plants. Plant Growth Regul. 30, 157–161. 10.1023/A:1006386800974 [DOI] [Google Scholar]
- Shaat M. N. M., Galal A. A. (2004). Response of citrus fruits to pre-harvest antioxidant spraying and infection with Alternaria fruit rot and green mould. Ann. Agr. Sci. 49, 747–758. [Google Scholar]
- Shao X., Cao B., Xu F., Xie S., Yu D., Wang H. (2015). Effect of postharvest application of chitosan combined with clove oil against citrus green mold. Postharvest Biol. Technol. 99, 37–43. 10.1016/j.postharvbio.2014.07.014 [DOI] [Google Scholar]
- Shi Z., Wang F., Lu Y., Deng J. (2018). Combination of chitosan and salicylic acid to control postharvest green mold caused by Penicillium digitatum in grapefruit fruit. Sci. Hortic. 233, 54–60. 10.1016/j.scienta.2018.01.039 [DOI] [Google Scholar]
- Shikanga E., Regnier T., Combrinck S., Botha B. (2009). Polar Lippia extracts as alternatives for the postharvest control of Guazatine®-resistant strains of Penicillium digitatum in citrus. Fruits 64, 75–82. 10.1051/fruits/2009002 [DOI] [Google Scholar]
- Sholberg P. L. (1998). Fumigation of fruit with short-chain organic acids to reduce the potential of postharvest decay. Plant Dis. 82, 689–693. 10.1094/PDIS.1998.82.6.689 [DOI] [PubMed] [Google Scholar]
- Sitara U., Hassan N., Naseem J. (2011). Antifungal activity of Aloe vera gel against plant pathogenic fungi. Pak. J. Bot. 43, 2231–2233. 33509168 [Google Scholar]
- Smilanick J. L., Denis-Arrue R. (1992). Control of green mold of lemons with Pseudomonas species. Plant Dis. 76, 481–485. 10.1094/PD-76-048130812563 [DOI] [Google Scholar]
- Smilanick J. L., Mansour M. F., Gabler F. M., Sorenson D. (2008). Control of citrus postharvest green mold and sour rot by potassium sorbate combined with heat and fungicides. Postharvest Biol. Technol. 47, 226–238. 10.1016/j.postharvbio.2007.06.020 [DOI] [Google Scholar]
- Smilanick J. L., Mansour M. F., Margosan D. A., Gabler F. M., Goodwine W. R. (2005). Influence of pH and NaHCO3 on effectiveness of imazalil to inhibit germination of Penicillium digitatum and to control postharvest green mold on citrus fruit. Plant Dis. 89, 640–648. 10.1094/PD-89-0640 [DOI] [PubMed] [Google Scholar]
- Smilanick J. L., Margosan D. A., Mlikota F., Usall J., Michael I. F. (1999). Control of citrus green mold by carbonate and bicarbonate salts and the influence of commercial postharvest practices on their efficacy. Plant Dis. 83, 139–145. 10.1094/PDIS.1999.83.2.139 [DOI] [PubMed] [Google Scholar]
- Smilanick J. L., Sorenson D. (2001). Control of postharvest decay of citrus fruit with calcium polysulfide. Postharvest Biol. Technol. 21, 157–168. 10.1016/S0925-5214(00)00142-3 [DOI] [Google Scholar]
- Smilanick J. L., Sorenson D., Mansour M., Aieyabei J., Plaza P. (2003). Impact of a brief postharvest hot water drench treatment on decay, fruit appearance, and microbe populations of California lemons and oranges. Horttechnology 13, 333–338. 10.21273/HORTTECH.13.2.0333 [DOI] [Google Scholar]
- Smoot J. J., Houck L. G., Johnson H. B. (1971). Market Diseases of Citrus and Other Subtropical Fruits (No. 398). US Agricultural Research Service. [Google Scholar]
- Smoot J. J., Melvin C. F. (1963). Hot water as a control for citrus decay, in Proceedings of the Florida State Horticultural Society, Vol. 76 (Orlando, FL: ), 322–327. [Google Scholar]
- Snowdon A. L. (1990). A Colour Atlas of Post-Harvest Diseases and Disorders of Fruits and Vegetables. Volume 1: General Introduction and Fruits. Boca Raton, FL: Wolfe Scientific Ltd. [Google Scholar]
- Song Q. Y., Qi W. Y., Li Z. M., Zhao J., Chen J. J., Gao K. (2011). Antifungal activities of triterpenoids from the roots of Astilbe myriantha Diels. Food Chem. 128, 495–499. 10.1016/j.foodchem.2011.03.059 [DOI] [PubMed] [Google Scholar]
- Soylu E. M., Tok F. M., Soylu S., Kaya A. D., Evrendilek G. A. (2005). Antifungal activities of the essential oils on post-harvest disease agent Penicillium digitatum. Pak. J. Biol. Sci. 8, 25–29. 10.3923/pjbs.2005.25.29 [DOI] [Google Scholar]
- Spadaro D., Droby S. (2016). Development of biocontrol products for postharvest diseases of fruit: the importance of elucidating the mechanisms of action of yeast antagonists. Trends Food Sci. Technol. 47, 39–49. 10.1016/j.tifs.2015.11.003 [DOI] [Google Scholar]
- Spalding D. H., Reeder W. F. (1986). Effect of hot water and gamma radiation on postharvest decay of grapefruit, in Proceedings of the Annual Meeting of the Florida State Horticultural Society, Vol. 98 (Orlando, FL: ), 207–208. [Google Scholar]
- Spotts R. A., Cervantes L. A., Facteau T. J. (2002). Integrated control of brown rot of sweet cherry fruit with a preharvest fungicide, a postharvest yeast, modified atmosphere packaging, and cold storage temperature. Postharvest Biol. Technol. 24, 251–257. 10.1016/S0925-5214(01)00155-7 [DOI] [Google Scholar]
- Stange R. R., Eckert J. W. (1994). Influence of postharvest handling and surfactants on control of green mold of lemons by curing. Phytopathology 84, 612–616. 10.1094/Phyto-84-61230812563 [DOI] [Google Scholar]
- Strano L., Campisano A., Coco V., Grimaldi V., Catara A. (2002). Effectiveness of CaCl2 and Tween 80 in enhancing yeast biocontrol activity against Penicillium digitatum on tarocco orange. Plant Prot. Sci 38, 626–628. [Google Scholar]
- Strano M. C., Calandra M., Aloisi V., Rapisarda P., Strano T., Ruberto G. (2014). Hot water dipping treatments on Tarocco orange fruit and their effects on peel essential oil. Postharvest Biol. Technol. 94, 26–34. 10.1016/j.postharvbio.2014.01.026 [DOI] [Google Scholar]
- Strobel G. A., Dirkse E., Sears J., Markworth C. (2001). Volatile antimicrobials from Muscodor albus, a novel endophytic fungus. Microbiology 147, 2943–2950. 10.1099/00221287-147-11-2943 [DOI] [PubMed] [Google Scholar]
- Sui Y., Wisniewski M., Droby S., Norelli J., Liu J. (2016). Recent advances and current status of the use of heat treatments in postharvest disease management systems: is it time to turn up the heat? Trends Food Sci. Technol. 51, 34–40. 10.1016/j.tifs.2016.03.004 [DOI] [Google Scholar]
- Sukorini H., Sangchote S., Khewkhom N. (2013). Control of postharvest green mold of citrus fruit with yeasts, medicinal plants, and their combination. Postharvest Biol. Technol. 79, 24–31. 10.1016/j.postharvbio.2013.01.001 [DOI] [Google Scholar]
- Suwannarach N., Kumla J., Bussaban B., Nuangmek W., Matsui K., Lumyong S. (2013). Biofumigation with the endophytic fungus Nodulisporium spp. CMU-UPE34 to control postharvest decay of citrus fruit. Crop Protect. 45, 63–70. 10.1016/j.cropro.2012.11.015 [DOI] [Google Scholar]
- Talibi I., Askarne L., Boubaker H., Boudyach E. H., Msanda F., Saadi B., Ait Ben Aoumar A. (2012). Antifungal activity of Moroccan medicinal plants against citrus sour rot agent Geotrichum candidum. Lett. Appl. Microbiol. 55, 155–161. 10.1111/j.1472-765X.2012.03273.x [DOI] [PubMed] [Google Scholar]
- Talibi I., Boubaker H., Boudyach E. H., Ait Ben Aoumar A. (2014). Alternative methods for the control of postharvest citrus diseases. J. Appl. Microbiol. 117, 1–17. 10.1111/jam.12495 [DOI] [PubMed] [Google Scholar]
- Tao N., Jia L., Zhou H. (2014). Anti-fungal activity of Citrus reticulata Blanco essential oil against Penicillium italicum and Penicillium digitatum. Food Chem. 153, 265–271 10.1016/j.foodchem.2013.12.070 [DOI] [PubMed] [Google Scholar]
- Tayel A. A., Moussa S. H., Salem M. F., Mazrou K. E., El-Tras W. F. (2016). Control of citrus molds using bioactive coatings incorporated with fungal chitosan/plant extracts composite. J. Sci. Food Agric. 96, 1306–1312. 10.1002/jsfa.7223 [DOI] [PubMed] [Google Scholar]
- Teixidó N., Usall J., Palou L., Asensio A., Nunes C., Viñas I. (2001). Improving control of green and blue molds of oranges by combining Pantoea agglomerans (CPA-2) and sodium bicarbonate. Eur. J. Plant Pathol. 107, 685–694. 10.1023/A:1011962121067 [DOI] [Google Scholar]
- Terao D., de Lima Nechet K., Ponte M. S., Maia A. D. H. N., de Almeida Anjos V. D., de Almeida Halfeld-Vieira B. (2017). Physical postharvest treatments combined with antagonistic yeast on the control of orange green mold. Sci. Hortic. 224, 317–323. 10.1016/j.scienta.2017.06.038 [DOI] [Google Scholar]
- Trabelsi D., Hamdane A. M., Said M. B., Abdrrabba M. (2016). Chemical composition and antifungal activity of essential oils from flowers, leaves and peels of Tunisian Citrus aurantium against Penicillium digitatum and Penicillium italicum. J. Essential Oil Bearing Plants 19, 1660–1674. 10.1080/0972060X.2016.1141069 [DOI] [Google Scholar]
- Tripathi P., Dubey N. K. (2004). Exploitation of natural products as an alternative strategy to control postharvest fungal rotting of fruit and vegetables. Postharvest Biol. Technol. 32, 235–245. 10.1016/j.postharvbio.2003.11.005 [DOI] [Google Scholar]
- Tripathi P., Dubey N. K., Banerji R., Chansouria J. P. N. (2004). Evaluation of some essential oils as botanical fungitoxicants in management of post-harvest rotting of citrus fruits. World J. Microbiol. Biotechnol. 20, 317–321. 10.1023/B:WIBI.0000023844.80464.59 [DOI] [Google Scholar]
- Tripathi P., Dubey N. K., Pandey V. B. (2002). Kaempferol: the antifungal principle of Acacia nilotica Linn. Del J. Indian Bot. Soc. 81, 51–54. [Google Scholar]
- Tuset J. J. (1987). Podedumbres de los frutos cítricos. Valencia: Generalitat Valenciano, Chancillería de Agricultura y Pesca. Generalitat Valenciana. [Google Scholar]
- Tyagi A. K., Malik A. (2011). Antimicrobial potential and chemical composition of Mentha piperita oil in liquid and vapour phase against food spoiling microorganisms. Food Control 22, 1707–1714. 10.1016/j.foodcont.2011.04.002 [DOI] [Google Scholar]
- United States Department of Agriculture (2021). Citrus: World Markets and Trade. U.S. Production and Exports Forecast Down Despite Global Gains. United States Department of Agriculture. Foreing Agricultural Service. Available online at: https://apps.fas.usda.gov/psdonline/circulars/citrus.pdf (accessed May 30, 2021). [Google Scholar]
- Usall J., Ippolito A., Sisquella M., Neri F. (2016). Physical treatments to control postharvest diseases of fresh fruits and vegetables. Postharvest Biol. Technol. 122, 30–40. 10.1016/j.postharvbio.2016.05.002 [DOI] [Google Scholar]
- Utama I. M. S., Wills R. B., Ben-Yehoshua S., Kuek C. (2002). In vitro efficacy of plant volatiles for inhibiting the growth of fruit and vegetable decay microorganisms. J. Agric. Food Chem. 50, 6371–6377. 10.1021/jf020484d [DOI] [PubMed] [Google Scholar]
- Valencia-Chamorro S. A., Palou L., Del Rio M. A., Perez-Gago M. B. (2008). Inhibition of Penicillium digitatum and Penicillium italicum by hydroxypropyl methylcellulose–lipid edible composite films containing food additives with antifungal properties. J. Agric. Food Chem. 56, 11270–11278. 10.1021/jf802384m [DOI] [PubMed] [Google Scholar]
- Valencia-Chamorro S. A., Perez-Gago M. B., Del Rio M. A., Palou L. (2009). Curative and preventive activity of hydroxypropyl methylcellulose-lipid edible composite coatings containing antifungal food additives to control citrus postharvest green and blue molds. J. Agric. Food Chem. 57, 2770–2777. 10.1021/jf803534a [DOI] [PubMed] [Google Scholar]
- van Hulten M., Pelser M., Van Loon L. C., Pieterse C. M., Ton J. (2006). Costs and benefits of priming for defense in Arabidopsis. Proc. Nat. Acad. Sci. U.S.A. 103, 5602–5607. 10.1073/pnas.0510213103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velásquez M. A., Passaro C. P., Lara-Guzmán O. J., Álvarez R., Londono J. (2014). Effect of an edible, fungistatic coating on the quality of the 'Valencia'orange during storage and marketing. Acta Hortic. 163–169. 10.17660/ActaHortic.2014.1016.23 [DOI] [Google Scholar]
- Verberne M. C., Verpoorte R., Bol J. F., Mercado-Blanco J., Linthorst H. J. (2000). Overproduction of salicylic acid in plants by bacterial transgenes enhances pathogen resistance. Nat. Biotechnol. 18, 779–783. 10.1038/77347 [DOI] [PubMed] [Google Scholar]
- Villalobos M. D. C., Serradilla M. J., Martín A., Ordiales E., Ruiz-Moyano S., Córdoba M. D. G. (2016). Antioxidant and antimicrobial activity of natural phenolic extract from defatted soybean flour by-product for stone fruit postharvest application. J. Sci. Food Agric. 96, 2116–2124. 10.1002/jsfa.7327 [DOI] [PubMed] [Google Scholar]
- Vinas I., Usall J., Teixidó N., Sanchis V. (1998). Biological control of major postharvest pathogens on apple with Candida sake. Int. J. Food Microbiol. 40, 9–16. 10.1016/S0168-1605(98)00009-9 [DOI] [PubMed] [Google Scholar]
- Waewthongrak W., Pisuchpen S., Leelasuphakul W. (2015). Effect of Bacillus subtilis and chitosan applications on green mold (Penicilium digitatum Sacc.) decay in citrus fruit. Postharvest Biol. Technol. 99, 44–49. 10.1016/j.postharvbio.2014.07.016 [DOI] [Google Scholar]
- Walters D. R., Ratsep J., Havis N. D. (2013). Controlling crop diseases using induced resistance: challenges for the future. J. Exp. Bot. 64, 1263–1280. 10.1093/jxb/ert026 [DOI] [PubMed] [Google Scholar]
- Wan C., Chen C., Li M., Yang Y., Chen M., Chen J. (2017a). Chemical constituents and antifungal activity of Ficus hirta Vahl. fruits. Plants 6:44. 10.3390/plants6040044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan C., Li P., Chen C., Peng X., Li M., Chen M., et al. (2017b). Antifungal activity of Ramulus cinnamomi explored by 1H-NMR based metabolomics approach. Molecules 22:2237. 10.3390/molecules22122237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Gao Y., Tao Y., Wu X., Zhibo C. (2017). Influence of γ-irradiation on the reactive-oxygen metabolism of blueberry fruit during cold storage. Innovative Food Sci. Emerg. Technolo. 41, 397–403. 10.1016/j.ifset.2017.04.007 [DOI] [Google Scholar]
- Wang F., Deng J., Jiao J., Lu Y., Yang L., Shi Z. (2019). The combined effects of Carboxymethyl chitosan and Cryptococcus laurentii treatment on postharvest blue mold caused by Penicillium italicum in grapefruit fruit. Sci. Hortic. 253, 35–41. 10.1016/j.scienta.2019.04.031 [DOI] [Google Scholar]
- Wang H., Yan Y., Wang J., Zhang H., Qi W. (2012). Production and characterization of antifungal compounds produced by Lactobacillus plantarum IMAU10014. PLoS ONE 7:e29452. 10.1371/journal.pone.0029452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Cao S., Wang L., Wang X., Jin P., Zheng Y. (2018). Effect of β-aminobutyric acid on disease resistance against Rhizopus rot in harvested peaches. Front. Microbiol. 9:1505. 10.3389/fmicb.2018.01505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Liu S., Deng L., Ming J., Yao S., Zeng K. (2018). Control of citrus post-harvest green molds, blue molds, and sour rot by the Cecropin A-melittin hybrid peptide BP21. Front. Microbiol. 9:2455. 10.3389/fmicb.2018.02455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Li J., Liu J., Tian X., Zhang D., Wang Q. (2021a). Management of blue mold (Penicillium italicum) on mandarin fruit with a combination of the yeast, Meyerozyma guilliermondii and an alginate oligosaccharide. Biol. Control 152:104451. 10.1016/j.biocontrol.2020.104451 [DOI] [Google Scholar]
- Wang Z., Zhong T., Chen K., Du M., Chen G., Chen X., et al. (2021b). Antifungal activity of volatile organic compounds produced by Pseudomonas fluorescens ZX and potential biocontrol of blue mold decay on postharvest citrus. Food Control 120:107499. 10.1016/j.foodcont.2020.10749931637724 [DOI] [Google Scholar]
- Whiteside J. O., Garnsey S. M., Timmer L. W. (1993). Compendium of Citrus Disease. 2nd Edn. Saint Paul, MN: APS Press. [Google Scholar]
- Wilson C. L., Solar J. M., El Ghaouth A., Wisniewski M. E. (1997). Rapid evaluation of plant extracts and essential oils for antifungal activity against Botrytis cinerea. Plant Dis. 81, 204–210. 10.1094/PDIS.1997.81.2.204 [DOI] [PubMed] [Google Scholar]
- Wilson G. F., Eggemeier F. T. (1991). Psychophysiological assessment of workload in multi-task environments, in Multiple-Task Performance, eds Wilson G. F., Thomas Eggemeier F. (Bristol, PA: Taylor & Francis Inc.), 329–360. [Google Scholar]
- Wisniewski M., Macarisin D., Droby S. (2009). Challenges and opportunities for the commercialization of postharvest biocontrol, in VI International Postharvest Symposium, Vol. 877 (Antalya: ), 1577–1582. 10.17660/ActaHortic.2010.877.217 [DOI] [Google Scholar]
- Wisniewski M. E., Wilson C. L. (1992). Biological control of postharvest diseases of fruits and vegetables: recent advances. HortScience 27, 94–98. 10.21273/HORTSCI.27.2.94 [DOI] [Google Scholar]
- Wolken W. A., Van Loo W. J., Tramper J., Van Der Werf M. J. (2002). A novel, inducible, citral lyase purified from spores of Penicillium digitatum. Eur. J. Biochem. 269, 5903–5910. 10.1046/j.1432-1033.2002.03312.x [DOI] [PubMed] [Google Scholar]
- Wong J. H., Hao J., Cao Z., Qiao M., Xu H., Bai Y., Ng T. B. (2008). An antifungal protein from Bacillus amyloliquefaciens. J. Appl. Microbiol. 105, 1888–1898. 10.1111/j.1365-2672.2008.03917.x [DOI] [PubMed] [Google Scholar]
- Wuryatmo E., Klieber A., Scott E. S. (2003). Inhibition of citrus postharvest pathogens by vapor of citral and related compounds in culture. J. Agric. Food Chem. 51, 2637–2640. 10.1021/jf026183l [DOI] [PubMed] [Google Scholar]
- Yahyazadeh M., Omidbaigi R., Zare R., Taheri H. (2008). Effect of some essential oils on mycelial growth of Penicillium digitatum Sacc. World J. Microbiol. Biotechnol. 24, 1445–1450. 10.1007/s11274-007-9636-8 [DOI] [Google Scholar]
- Yahyazadeh M., Zare R., Omidbaigi R., Faghih-Nasiri M., Abbasi M. (2009). Control of Penicillium decay on citrus fruit using essential oil vapours of thyme or clove inside polyethylene and nano-clay polyethylene films. J. Hortic. Sci. Biotechnol. 84, 403–409. 10.1080/14620316.2009.11512540 [DOI] [Google Scholar]
- Yalpani N., Enyedi A. J., León J., Raskin I. (1994). Ultraviolet light and ozone stimulate accumulation of salicylic acid, pathogenesis-related proteins and virus resistance in tobacco. Planta 193, 372–376. 10.1007/BF00201815 [DOI] [Google Scholar]
- Yamaga I., Kuniga T., Aoki S. (2016). Effect of ultraviolet-B irradiation on control of postharvest decay and scoparone production in non-inoculated satsuma mandarin fruit. Trop. Agric. Dev. 60, 283–285. 10.11248/jsta.60.283 [DOI] [Google Scholar]
- Yamaga I., Takahashi T., Ishii K., Kato M., Kobayashi Y. (2015). Suppression of blue mold symptom development in satsuma mandarin fruits treated by low-intensity blue LED irradiation. Food Sci. Technol. Res. 21, 347–351. 10.3136/fstr.21.347 [DOI] [Google Scholar]
- Yang S., Liu L., Li D., Xia H., Su X., Peng L., Pan S. (2016). Use of active extracts of poplar buds against Penicillium italicum and possible modes of action. Food Chem. 196, 610–618. 10.1016/j.foodchem.2015.09.101 [DOI] [PubMed] [Google Scholar]
- Yang S., Peng L., Cheng Y., Chen F., Pan S. (2010). Control of citrus green and blue molds by Chinese propolis. Food Sci. Biotechnol. 19, 1303–1308. 10.1007/s10068-010-0186-8 [DOI] [Google Scholar]
- Yao H., Tian S. (2005). Effects of pre- and post-harvest application of salicylic acid or methyl jasmonate on inducing disease resistance of sweet cherry fruit in storage. Postharvest Biol. Technol. 35, 253–262. 10.1016/j.postharvbio.2004.09.001 [DOI] [Google Scholar]
- Yigit F., Özcan M., Akgül A. (2000). Inhibitory effect of some spice essential oils on Penicillium digitatum causing postharvest rot in citrus. Grasas y Aceites 51, 237–240. 10.3989/gya.2000.v51.i4.417 [DOI] [Google Scholar]
- Youssef K., Hussien A. (2020). Electrolysed water and salt solutions can reduce green and blue molds while maintain the quality properties of ‘Valencia’ late oranges. Postharvest Biol. Technol. 159:111025. 10.1016/j.postharvbio.2019.111025 [DOI] [Google Scholar]
- Youssef K., Ligorio A., Nigro F., Ippolito A. (2012a). Activity of salts incorporated in wax in controlling postharvest diseases of citrus fruit. Postharvest Biol. Technol. 65, 39–43. 10.1016/j.postharvbio.2011.10.006 [DOI] [Google Scholar]
- Youssef K., Ligorio A., Sanzani S. M., Nigro F., Ippolito A. (2012b). Control of storage diseases of citrus by pre- and postharvest application of salts. Postharvest Biol. Technol. 72, 57–63. 10.1016/j.postharvbio.2012.05.004 [DOI] [Google Scholar]
- Youssef K., Sanzani S. M., Ligorio A., Ippolito A., Terry L. A. (2014). Sodium carbonate and bicarbonate treatments induce resistance to postharvest green mould on citrus fruit. Postharvest Biol. Technol. 87, 61–69. 10.1016/j.postharvbio.2013.08.006 [DOI] [Google Scholar]
- Yun Z., Gao H., Liu P., Liu S., Luo T., Jin S., et al. (2013). Comparative proteomic and metabolomic profiling of citrus fruit with enhancement of disease resistance by postharvest heat treatment. BMC Plant Biol. 13:44. 10.1186/1471-2229-13-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamani M., Tehrani A. S., Ahmadzadeh M., Hosseininaveh V., Mostofy Y. (2009). Control of Penicillium digitatum on orange fruit combining Pantoea agglomerans with hot sodium bicarbonate dipping. J. Plant Pathol. 437–442. [Google Scholar]
- Zapata P. J., Navarro D., Guillén F., Castillo S., Martínez-Romero D., Valero D., Serrano M. (2013). Characterisation of gels from different Aloe spp. as antifungal treatment: potential crops for industrial applications. Industr. Crops Products 42, 223–230. 10.1016/j.indcrop.2012.06.002 [DOI] [Google Scholar]
- Zeng K., Deng Y., Ming J., Deng L. (2010). Induction of disease resistance and ROS metabolism in navel oranges by chitosan. Sci. Hortic. 126, 223–228. 10.1016/j.scienta.2010.07.017 [DOI] [Google Scholar]
- Zhang H., Ma L., Turner M., Xu H., Zheng X., Dong Y., Jiang S. (2010). Salicylic acid enhances biocontrol efficacy of Rhodotorula glutinis against postharvest Rhizopus rot of strawberries and the possible mechanisms involved. Food Chem. 122, 577–583. 10.1016/j.foodchem.2010.03.013 [DOI] [Google Scholar]
- Zhang H. Y., Fu C. X., Zheng X. D., He D., Shan L. J., Zhan X. (2004). Effects of Cryptococcus laurentii (Kufferath) Skinner in combination with sodium bicarbonate on biocontrol of postharvest green mold decay of citrus fruit. Bot. Bull. Acad. Sin. Taipei 45, 159–164. [Google Scholar]
- Zhang H. Y., Zheng X. D., Xi Y. F. (2005). Biological control of postharvest blue mold of oranges by Cryptococcus laurentii (Kufferath) Skinner. Biocontrol 50, 331–342. 10.1007/s10526-004-0452-x [DOI] [Google Scholar]
- Zhang J., Swingle P. P. (2005). Effects of curing on green mold and stem-end rot of citrus fruit and its potential application under Florida packing system. Plant Dis. 89, 834–840. 10.1094/PD-89-0834 [DOI] [PubMed] [Google Scholar]
- Zhang X., Guo Y., Guo L., Jiang H., Ji Q. (2018). In vitro evaluation of antioxidant and antimicrobial activities of Melaleuca alternifolia essential oil. Biomed Res. Int. 2018:2396109. 10.1155/2018/2396109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Niu Y., Luo Y., Ge M., Yang T., Yu L. L., Wang Q. (2014). Fabrication, characterization and antimicrobial activities of thymol-loaded zein nanoparticles stabilized by sodium caseinate–chitosan hydrochloride double layers. Food Chem. 142, 269–275. 10.1016/j.foodchem.2013.07.058 [DOI] [PubMed] [Google Scholar]
- Zheng X. D., Zhang H. Y., Sun P. (2005). Biological control of postharvest green mold decay of oranges by Rhodotorula glutinis. Eur. Food Res. Technol. 220, 353–357. 10.1007/s00217-004-1056-5 [DOI] [Google Scholar]
- Zhou M., Wang W. (2018). Recent advances in synthetic chemical inducers of plant immunity. Front. Plant Sci. 9:1613. 10.3389/fpls.2018.01613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Ma J., Xie J., Deng L., Yao S., Zeng K. (2018). Transcriptomic and biochemical analysis of highlighted induction of phenylpropanoid pathway metabolism of citrus fruit in response to salicylic acid, Pichia membranaefaciens and oligochitosan. Postharvest Biol. Technol. 142, 81–92. 10.1016/j.postharvbio.2018.01.021 [DOI] [Google Scholar]
- Zhou Y., Ming J., Deng L., Zeng K. (2014). Effect of Pichia membranaefaciens in combination with salicylic acid on postharvest blue and green mold decay in citrus fruits. Biol. Control 74, 21–29. 10.1016/j.biocontrol.2014.03.007 [DOI] [Google Scholar]
- Zhu C., Sheng D., Wu X., Wang M., Hu X., Li H., Yu D. (2017). Identification of secondary metabolite biosynthetic gene clusters associated with the infection of citrus fruit by Penicillium digitatum. Postharvest Biol. Technol. 134, 17–21. 10.1016/j.postharvbio.2017.07.011 [DOI] [Google Scholar]
- Zhu H., Zhao L., Zhang X., Foku J. M., Li J., Hu W., Zhang H. (2019). Efficacy of Yarrowia lipolytica in the biocontrol of green mold and blue mold in Citrus reticulata and the mechanisms involved. Biol. Control 139:104096. 10.1016/j.biocontrol.2019.104096 [DOI] [Google Scholar]
- Zhu R., Lu L., Guo J., Lu H., Abudureheman N., Yu T., Zheng X. (2013). Postharvest control of green mold decay of citrus fruit using combined treatment with sodium bicarbonate and Rhodosporidium paludigenum. Food Bioproc. Technol. 6, 2925–2930. 10.1007/s11947-012-0863-0 [DOI] [Google Scholar]