Abstract
Fertilization is a central event during the life cycle of most eukaryotic organisms and involves gamete recognition and fusion, ultimately resulting in zygote formation. Gamete fertilization in the malaria-causing Plasmodium parasites occurs inside the mosquito midgut and represents a major bottleneck in the life cycle. Cysteine Rich Secretory Proteins (CRISPs) are key molecules involved in fertilization in vertebrates and the presence of a CRISP ortholog in human malaria infective Plasmodium falciparum suggested a possible role in fertilization. Strikingly, P. falciparum CRISP exhibited a unique terminal localization in the male microgamete. Parasites with a CRISP gene deletion (P. falciparum crisp−) proliferated asexually similar to wildtype NF54 parasites and differentiated into gametocytes. Further analysis showed that Plasmodium falciparum crisp− gametocytes underwent exflagellation to form male gametes and no apparent defect in transmission to the mosquito vector was observed. These data show that P. falciparum CRISP is a marker for the apical end of the microgamete and that it might only have an ancillary or redundant function in the male sexual stages.
Keywords: Malaria, signaling, exflagellation, fertilization, mosquito, transmission
Graphical Abstract
1. Introduction
Plasmodium falciparum (Pf) is a digenetic parasite whose life cycle alternates between the human host and an Anopheles mosquito host. Pf proliferates asexually inside the human host, and the sexual phase of the life cycle is completed in the mosquito vector (Cowman et al., 2016). During human infection, a small fraction of asexually replicating parasites commit to sexual development and differentiate into sexual stages called gametocytes, which are taken up by the mosquito vector during a blood meal (Meibalan and Marti, 2017). Within the mosquito midgut, mature gametocytes are activated to form male and female gametes. These gametes fuse to form a zygote that subsequently differentiates into an elongated ookinete. This motile stage passes through the midgut epithelium and forms an oocyst. Oocysts form sporozoites, which invade the salivary glands and are eventually injected into a new human host.
Gamete fusion and fertilization involve the interaction between species-specific gamete surface recognition molecules but in Plasmodium, the fertilization process is not well studied. However, 6-cysteine family proteins have been implicated in gamete recognition and fertilization (van Dijk et al., 2010) and in addition, a major facilitator family protein (MFS) Pb115, and a HAP2/GCS1 family protein have been shown to play roles in gamete fertilization in a rodent malaria model (Liu et al., 2019; Liu et al., 2008).
Proteins belonging to the CAP superfamily (cysteine-rich secretory proteins, antigen 5, and pathogenesis related 1 proteins) (PFAM ID PF00188) are found in all the kingdoms of life and have been implicated in diverse physiological processes, including capacitation and fertilization (Abraham and Chandler, 2017; Fry et al., 2009). They include three subfamilies, the cysteine-rich secretory proteins (CRISPs) in vertebrates, antigen 5 (Ag 5) related proteins in insects and pathogenesis-related 1 (PR-1) proteins in plants. Members of this CAP superfamily possess significant sequence homology defined by a conserved CAP domain (Gibbs et al., 2008). CAP proteins are also known as sperm coating glycoproteins (SCP). The SCP family of proteins contain a C-terminal CRISP domain, which consists of an ion channel regulator (ICR) domain and a ‘hinge’ which links the ICR and CAP domains. The mouse genome encodes four members of the CRISP family (CRISP 1–4) while humans only have three CRISP proteins, which are mainly expressed in the male reproductive tract (Gaikwad et al., 2020). Human CRISP1 is considered homologous to both mouse CRISP1 and CRISP 4 based on sequence similarity and has roles in sperm zona pellucida binding and gamete fusion (Busso et al., 2007; Cohen et al., 2001; Maldera et al., 2014). CRISP proteins are further characterized by the presence of 16 well conserved cysteines; the N-terminal SCP domain contains 6 cysteines, and the C-terminal CRISP domain contains 10 cysteine residues (Roberts et al., 2007). Biochemical studies have demonstrated that the 16 cysteine residues form disulfide bonds (Gibbs et al., 2008; Roberts et al., 2007).
Our search for CRISP family proteins in Plasmodium led to identification of PfCRISP (PlasmoDB ID PF3D7_0705800). Since CRISPs have a major role in fertilization in other species, we sought to determine its role in parasite sexual stage biology and fertilization.
2. Material and methods
2.1. Reagents and primary antibodies.
Unless stated otherwise, all reagents were purchased from Millipore Sigma, USA. All oligonucleotides were sourced from Integrated DNA Technologies, USA. Generation of polyclonal mouse antisera against PfCRISP was undertaken as described below. Generation of anti-PfCDPK4 (calcium dependent protein kinase 4) rabbit antisera is described elsewhere (Kumar et al., 2021). The pAVA-421 plasmid was kindly provided by Prof. Peter J. Myler, CGIDR, Seattle Children’s.
2.2. Pf culture and transfection.
In accordance with standard procedures P. falciparum NF54 and Pfcrisp− parasites were cultured at 37°C and supplemented with gas containing 5% O2/5% CO2/90% N2. Asexual and gametocyte cultures were set up with 5% hematocrit using O+ human red blood cells (Valley Biomedical, VA, US) and maintained with 24 hr media changes. All cultures were sustained with complete RPMI media supplemented with either 0.5% AlbuMAX™ (Thermo Scientific) medium or 10% (v/v) O+ human serum (Interstate Blood Bank, TN, US). Gametocyte cultures were grown in six well plates with a final volume of 5 mL and a 1% starting parasitemia.
Oligonucleotide primers used in the creation and analysis of P. falciparum Pfcrisp−parasites are specified in Table 1. Utilizing the previously reported CRISPR/Cas9 strategy, the PfCRISP locus (PlasmoDB identifier Gene-PF3D7_0705800) was deleted by double crossover homologous recombination (Goswami et al., 2020; Zhang et al., 2014). The pFCL3_CRISP_KO plasmids were generated through the ligation of a 20-nucleotide guide RNA sequence along with the complementary regions of Pf CRISP flanking both ends of the open reading frame. Transfection of the generated plasmid into P. falciparum NF54 ring stage parasites was accomplished through Bio-Rad Gene Pulser II (Bio-Rad Laboratories, Hercules, CA) mediated electroporation at 310V and 950 μF using 100 μg of pFCL3_CRISP_KO DNA. Parasites were selected using 8 nM WR99210 (graciously donated by Jacobus Pharmaceuticals). Verification of the gene deletion was accomplished via genotyping PCR (Figure 3).
Table 1.
PCR Primers used in the studies
| Oligo | Forward (5’−3’) |
|---|---|
| PfCRISP_F | ATGGTACCATAGGAATCATGAATATTTTTTTACTAT |
| PfCRISP_R | TAATCTCGAGTTATGACTGTGTTCTCTTATCTGAATG |
| PfCRISP 5’Homo For | TGCGGCCGCGAACAAAAGGTTATCATATATGATAGC |
| PfCRISP 5’Homo Rev | GCTTTATAAATCTCCTTATGCCTATCATAGTATAAATATAATAATTATCTC |
| PfCRISP 3’Homo For | TATATTTATACTATGATAGGCATAAGGAGATTTATAAAGCTTAT |
| PfCRISP 3’Homo For | TAAGTCGACGTTCCATTTATTATTTTTTTTTATATATAA |
| PfCRISP Guide 1 For | TATTGATAAAGCCAAACCACTTCAA |
| PfCRISP Guide 1 Rev | AAACTTGAAGTGGTTTGGCTTTATC |
| PfCRISP Guide 2 For | TATTGAACATGCTGTAGCACATCCA |
| PfCRISP Guide 2 Rev | AAACTGGATGTGCTACAGCATGTTC |
| PfCRISP Geno5 For | GCGAAAATTCCACTATATGAAAAATTATAGATC |
| PfCRISP Geno5 Rev | CAAATTTGCTTCGTATGTTGCTATCTCTTC |
| PfCRISP Geno3 For | CAGGTTATTTTGCTGACAATGTTGGG |
| PfCRISP Geno3 For | CCATTGCCTAGATCCTTGACCATATTtc |
Figure 3. Disruption of the PfCRISP locus via CRISPR/Cas9.
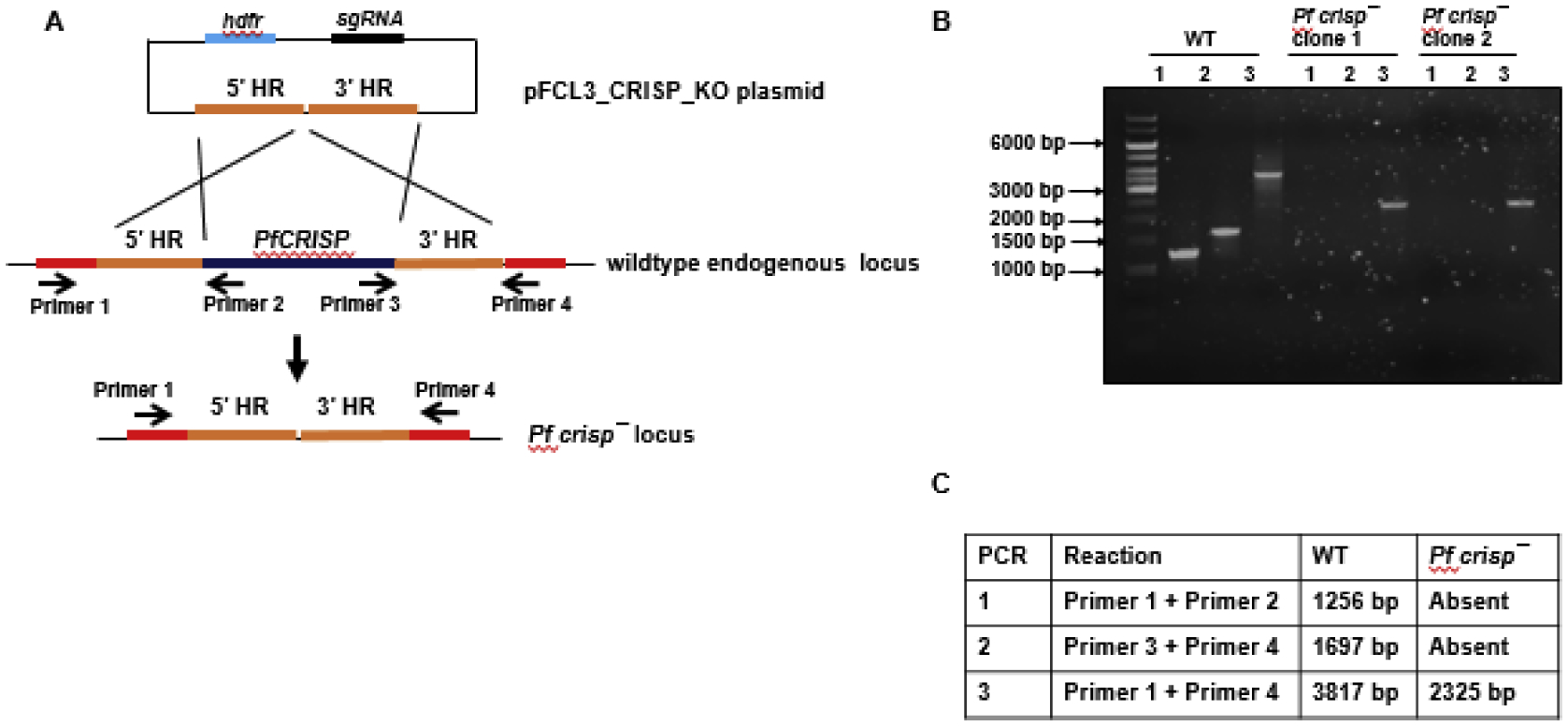
(A) The schematic shows the strategy for disrupting the PfCRISP gene. pFC_CRISP_KO plasmids has homology regions from 5’ (5’HR) and 3’ (3’HR) of PfCRISP locus, single guide RNA sequence (sgRNA) and human dihydrofolate reductase (hDHFR) locus cloned. (B) Diagnostic PCR for the confirmation of PfCRISP deletion. The oligonucleotides were designed from outside 5’HR and 3’HR and PfCRISP locus and positions are indicated by arrows in (A). The expected sizes for different set of PCRs are indicated in (C).
2.3. Expression of recombinant protein.
The CRISP open reading frame was cloned into the pAVA-421 vector using PCR primers PfCRISP_F and PfCRISP_R (Table 1). 6xHis-tagged protein was expressed in E. coli BL31 (RIL) DE3 cells and purified using Ni-NTA affinity matrices using standard methods.
2.4. Sequence analysis and cholesterol binding motif (CBM).
Plasmodium DNA and protein sequences were retrieved from PlasmoDB (http://plasmodb.org/plasmo/) and aligned using Multalign (http://multalin.toulouse.inra.fr/multalin/) and Espript 3 (http://espript.ibcp.fr/ESPript/ESPript/) programs. Signal peptide, and secondary structures were predicted using the SignalP-5.0 Server, and Expasy tool available at Swiss Institute of Bioinformatics respectively.
2.5. Generation of antisera.
Peptide corresponding to amino acids 157–175 of PfCRISP was synthesized and was conjugated to carrier protein Keyhole limpet hemocyanin (KLH) (KLH-CKYDNNTNKPGYFADNVGT) and was used for immunization of mice. Prescribed institute guidelines for animal handling and minimization of pain to the mouse were followed.
2.6. Measurement of asexual blood stage growth and gametocyte development.
Synchronized parasite cultures of WT Pf NF54 and Pfcrisp− with a 1% ring stage starting parasitemia were established and maintained in 6-well plates as described above for comparative analysis of asexual blood stage development and replication as well as gametocyte formation. Asexual parasitemia was scored per 1000 erythrocytes after 48 and 96 hrs through Giemsa-stained thin blood smear microscopy. Gametocytemia per 1000 erythrocytes was scored on day 15 of in vitro culture, likewise through Giemsa-stained thin blood smear microscopy.
2.7. Indirect immunofluorescence (IFA).
IFAs were performed by fixing asexual stages (ring, trophozoite and schizont) and sexual stages (gametocytes) using 4% paraformaldehyde/0.0025% glutaraldehyde in PBS in solution in microcentrifuge tubes for 30 min. Fixed parasites were washed twice with PBS and permeabilized using 0.1% Triton X-100/PBS solution for 10 min. Parasites were washed with PBS and blocked with 3%BSA/PBS for 45 min. Primary antisera were prepared in 3% BSA/PBS and was added to the slides and incubated at 4°C. Using a 100 × 1.4 NA objective 90 (Olympus) on a Delta Vision Elite High-Resolution Microscope (GE Healthcare Life Sciences) for imaging, antigens were visualized using anti-species antibodies.
IFAs were also performed by fixing smears for exflagellating gametocytes and free microgametes. Smears were fixed on Teflon coated slides for 2 min followed by permeabilization using 0.005% saponin for 1 min and kept in a humidity chamber throughout processing. Following 1xPBS wash, parasites were blocked through 45 minutes of 3%BSA/PBS contact. Primary antisera were prepared in 3% BSA/PBS and was added to the slides and incubated at 4°C. Using a 100 × 1.4 NA objective 90 (Olympus) on a Delta Vision Elite High-Resolution Microscope (GE Healthcare Life Sciences) for imaging, antigens were visualized using anti-species antibodies.
2.8. Statistical analysis.
Data collected was expressed as average ± SD. Using unpaired two-tailed Student’s t test or Nested one-way ANNOVAtest with one-way ANOVA, the statistical differences in data were determined. GraphPad Prism 8 was used to calculate significances, with values of p < 0.05 being considered as statistically significant. Significance is represented in the figures as either ns- not significant, p > 0.05; *p < 0.05; **p < 0.01; or ***p < 0.001.).
3. Results
3.1. Identification of CRISP in P. falciparum.
To identify CRISP orthologs in Plasmodium species, the SCP domain (SM00198) amino acid sequence was used to search the Plasmodium genome database PlasmoDB (https://plasmodb.org/plasmo/app) which retrieved a gene with the gene identifier PF3D7_0705800. The gene is 1492 bp and has eight exons, leading to a coding DNA sequence of 582 bp (Figure 1A and B). Pf CRISP is atypical and lacks a canonical CRISP domain and contains only N-terminal signal peptide (SP) and a SCP domain (Figure 1B). Since, this gene has been annotated as CRISP in PlasmoDB, we herein retain the name CRISP. The lack of a CRISP domain would suggest a role for PfCRISP in lipid binding as shown for PRY (pathogenesis-related in yeast) proteins PRY1 and PRY2 (Choudhary and Schneiter, 2012) but not in ion channel regulation (Harper et al., 2004; Koppers et al., 2011) (Gibbs et al., 2006).
Figure 1. PfCRISP shows structural features of SCP family proteins.
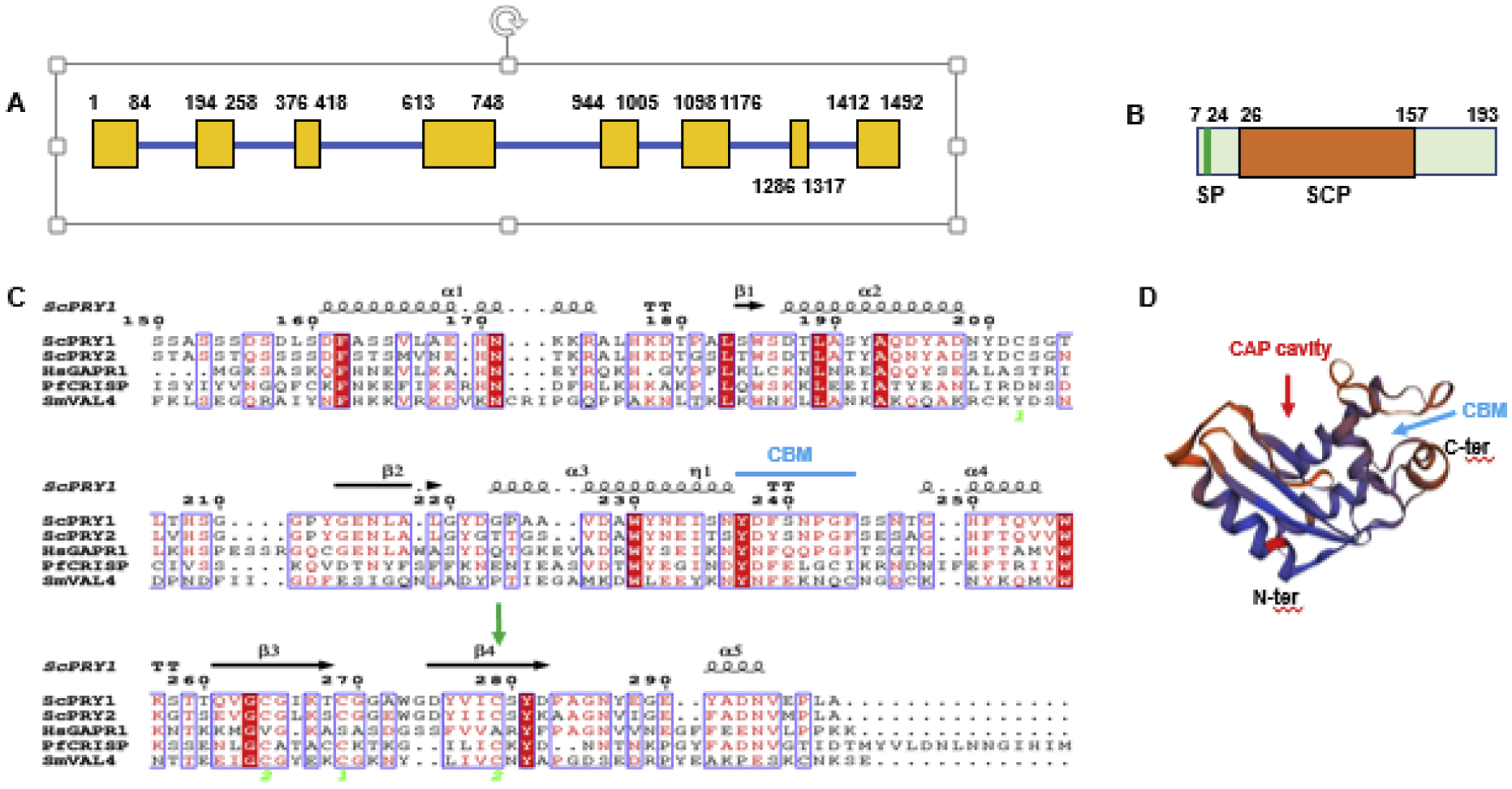
(A) Schematic for the PfCRISP gene showing exons (yellow boxes) and introns (blue bar). (B) PfCRISP domains: SP (signal peptide), in green; SCP domain in orange. (C) Sequence alignment for ScPRY1, ScPRY2, HsGAPR1, PfCRISP and SmVAL4. Conserved residues are in white font on a red background. α-helices and ß sheets are indicated based on structural features of ScPRY1 structure (PDB ID: 5JYS). Cyan arrow indicates CBM (Caveolin Binding motif, FxFxxxxF). Green arrow indicates C147 and its conservation across all the proteins. Sc-Saccharomyces cerevisiae, Hs-Homo sapiens, Pf-Plasmodium falciparum, Sm-Schistosoma mansoni. (D) Predicted three-dimensional structure of PfCRISP. PDB template used 5JYS (ScPRY1). The CAP cavity is marked by red arrow and cyan arrow indicates CBM. See also supplementary Figure S1.
A comparison of CRISP among different Pf strains and isolates showed that its amino acid sequence is nearly completely conserved with CRISP of only one isolate from Cambodia showing few amino acid differences (data not shown). Interestingly, Pf CRISP is present in human malaria parasites and in parasite species infecting nonhuman primates but absent in rodent malaria parasites. A sequence alignment of Plasmodium CRISPs showed a high degree of conservation among species, but more divergence compared to within Pf conservation (Figure S1).
To determine the structural similarity between PfCRISP and other SCP family proteins, a sequence alignment of PfCRISP, Saccharomyces cerevisiae pathogen related in yeast 1 (ScPRY1), ScPRY2, Human Golgi-associated PR-1 protein (HsGAPR1) and Schistosoma mansoni venom allergen-like protein 4 (SmVAL4) was performed. This revealed sequence conservation between all these SCP family proteins and identification of the caveolin binding motif (CBM, FxFxxxxF) (Figure 1C), which is present in SCP family proteins. PfCRISP showed 43% amino acid sequence similarity to ScPRY1. A conserved cysteine residue in PfCRISP (C147) was identified and is conserved with C279 of ScPRY1, which has previously been implicated in cholesterol binding (Choudhary and Schneiter, 2012). CAP proteins across prokaryotes and eukaryotes contain a CAP domain with a large ‘central cavity’ known as the CAP cavity (Wilbers et al., 2018). However, sequence alignment did not identify features of a CAP cavity in PfCRISP. Three-dimensional protein structural prediction for PfCRISP was performed with ScPRY1 (PDB ID: 5JYS) as a template using the Expasy tool available at Swiss Institute of Bioinformatics. This led to identification of a CAP cavity in PfCRISP in addition to the CBM (Figure 1D), indicating PfCRISP may have a cholesterol and/or lipid binding function. CRISP proteins are known to form dimers for their lipid/sterol binding function (Asojo et al., 2005; Darwiche et al., 2016; Gibbs et al., 2008; Hickox et al., 2001). To analyze if PfCRISP also forms dimers or multimers, recombinant 6xHis tagged PfCRISP protein was overexpressed in E. coli BL21 RIL cells. Recombinant PfCRISP migrated on a reducing Coomassie stained SDS PAGE gel at ~26 kDa and ~ 70 kDa, revealing that it is expressed as monomer and also forms a dimer or trimer (Figure. 2A). This reveals an important biochemical conservation of a CRISP property in PfCRISP.
Figure 2. Protein expression and localization of PfCRISP in sexual stages.
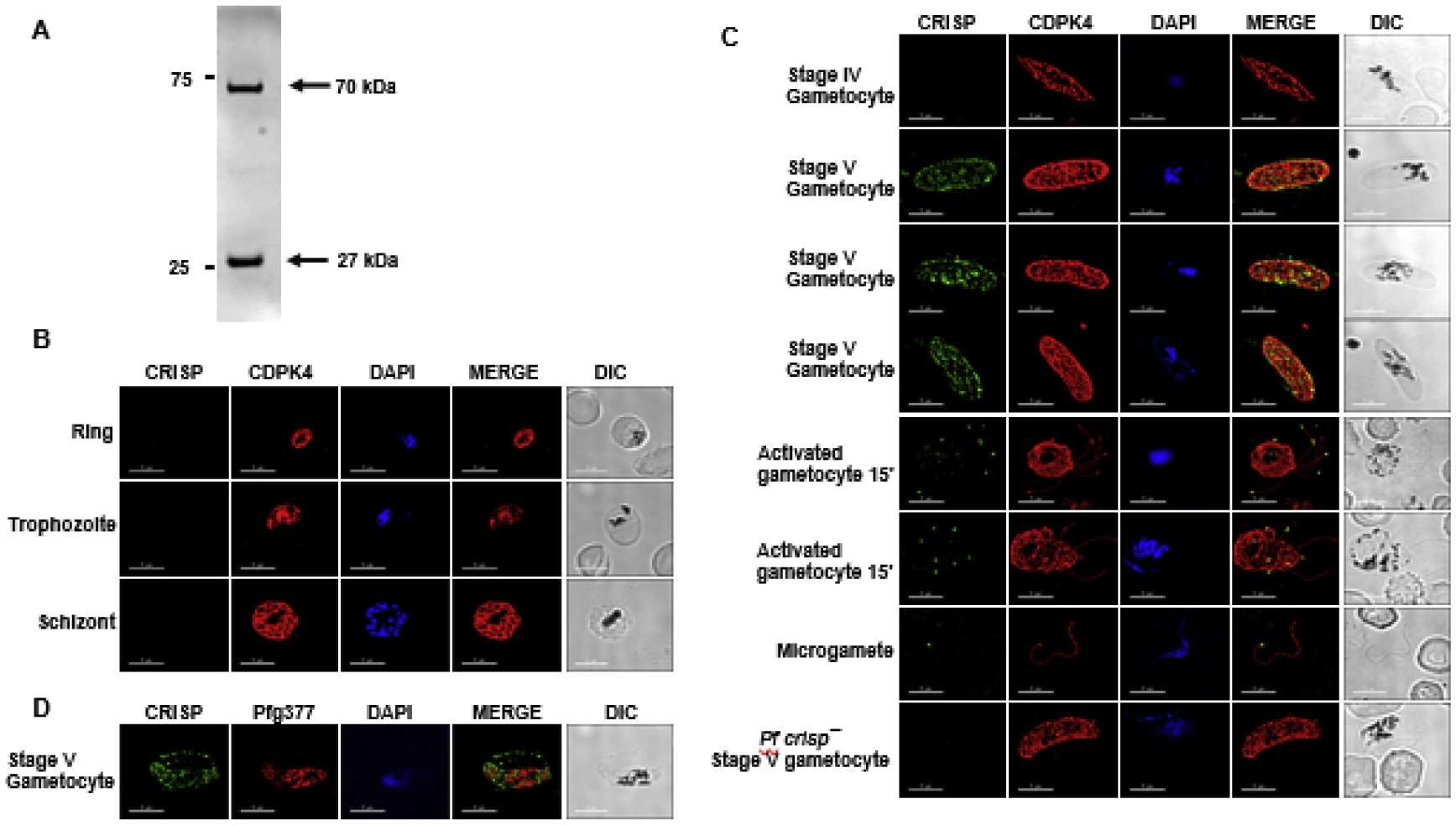
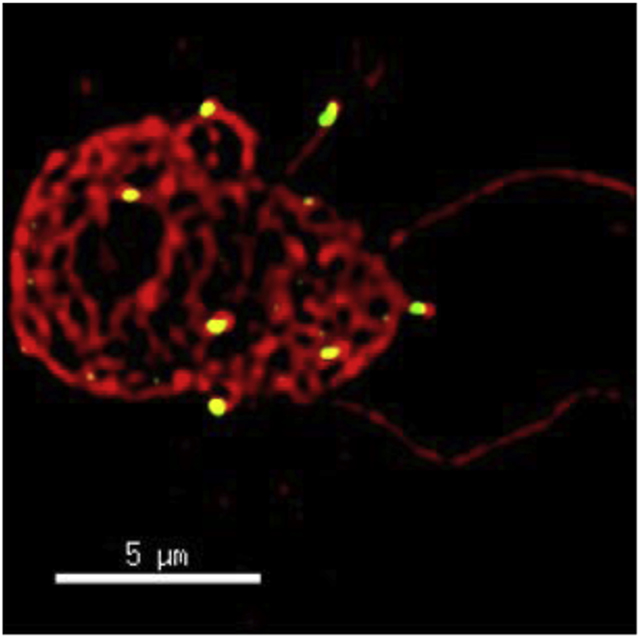
(A) Bacterially expressed recombinant 6×His tagged PfCRISP electrophoresed on SDS-PAGE gel revealed that PfCRISP exists as a monomer which runs around ~ 27 kDa and an oligomer around ~75 kDa. (B) IFAs were performed on WT NF54 asexual blood stages (ring, trophozoite, schizont) to localize PfCRISP (green) in combination with PfCDPK4 (red) in solution in microcentrifuge tubes. The parasite nucleus was localized with 4′,6-diamidino-2-phenylindole (DAPI) (in blue). Scale bar = 5 μm. (C) IFAs were performed on gametocytes in solution in microcentrifuge tubes using anti-PfCRISP antisera (in green) in combination with PfCDPK4 (in red). IFAs were performed on gametocytes 10 min post activation and free microgametes on thin smears using anti-PfCRISP antisera (in green) in combination with PfCDPK4 (in red). The parasite nucleus was stained with DAPI (blue). White arrow marks one end of the microgamete. Scale bar = 5 μm. (D) IFAs were performed on gametocytes on thin smears using anti-PfCRISP antisera (in green) in combination with Pfg377 (in red). The parasite nucleus was stained with DAPI (blue). Scale bar = 5 μm.
3.2. Analysis of PfCRISP expression in Plasmodium falciparum sexual stages.
To study PfCRISP expression and localization, antisera were generated using a synthetic KLH-conjugated peptide based on the SCP domain (Figure. 1A). Immunofluorescence assays (IFAs) carried out on asexual stage parasites in solution (see methods), revealed that PfCRISP is not expressed in ring-, trophozoite- and schizont stages (Figure 2B). Further IFAs carried out on gametocyte stages in solution, revealed that PfCRISP is expressed in stage V gametocytes (Figure 2C). Interestingly, PfCRISP was also detected in activated male gametocytes and microgametes, appearing in a discrete dot like structure at one end of the emerging and liberated microgametes (Figure 2C). While Pf gametocytes export several proteins called PfGEXPs (P. falciparum gametocyte-exported proteins) during gametocyte maturation (Silvestrini et al., 2010), no proteins have been described that localize to one end of microgametes. IFA performed with dual labelling using antibodies against Pfg377 (a female gametocyte marker protein) along with PfCRISP revealed that PfCRISP is also expressed by female stage V gametocytes and appeared to also be exported into the infected red blood cell (RBC) (Figure 2D and Supplementary Fig. S1B).
3.3. Production and activation of gametocytes occurs normally in Pfcrisp− parasites.
Previous studies using a PiggyBac mutagenesis system indicated that PfCRISP might be essential for asexual stages (Zhang et al., 2018). Since targeted technologies are available for creating gene deletion in Pf, the endogenous PfCRISP gene was disrupted using a CRISPR/Cas9 strategy. Genomic regions from upstream and downstream of PfCRISP were PCR-amplified and fused through an overlapping linker region and cloned into a modified pYC vector (Goswami et al., 2020; Zhang et al., 2014) (Figure 3A). A 20-nucleotide guide sequence was also cloned into the same vector (Figure 3A). Parasites were transfected using standard electroporation methods and parasite cloning was carried out by limiting dilution. Gene deletion parasites were confirmed by a set of diagnostic PCRs with oligonucleotides specific for the PfCRISP locus and its upstream and downstream regions (Figure 3A, 3B and 3C).
To analyze the role of PfCRISP in asexual stages, parasite growth rates were measured using two clones of Pf crisp−(clone 1 and 2) alongside wildtype (WT) Pf NF54 parasites and the growth was monitored over two replication cycles. Giemsa-stained thin smears prepared every 48-hr indicated that the growth rate of Pf crisp− was similar to NF54 (Figure 4A), suggesting no significant defect in asexual parasite replication.
Figure 4. Pfcrisp− parasites grow normally as asexuals and undergo gametocytogenesis.
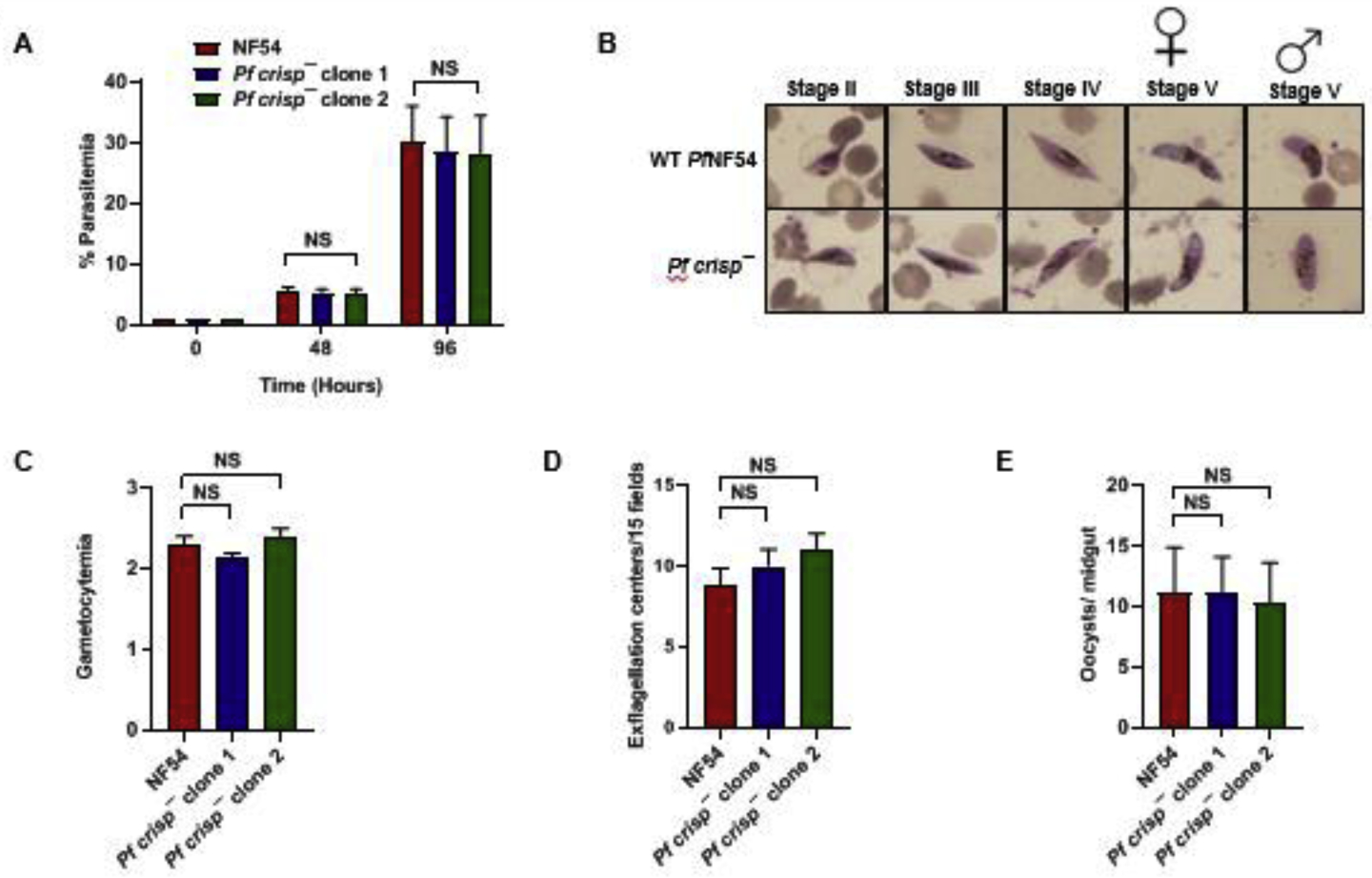
(A) Ring stage synchronous cultures for WT PfNF54 and Pfcrisp− (clone 1 and 2) were set up at 1% parasitemia and parasite growth was measured over the course of two erythrocytic cycles using Giemsa-stained smears. Data were averaged from three biological replicates and presented as the mean ± standard deviation (SD). (B) Day 15 gametocytemia for WT PfNF54 and Pfcrisp− (clone 1 and 2) was measured using Giemsa-stained smears. Data were averaged from three biological replicates and presented as the mean ± standard deviation (SD). (C) Number of exflagellation centers per field at 15 min post-activation WT PfNF54 and Pfcrisp− (clone 1 and 2). Data were averaged from three biological replicates and presented as the mean ± standard deviation (SD). (D) Oocysts per mosquito midgut were enumerated on day 7 post feed for WT PfNF54 and Pfcrisp− (clone 1 and 2) fed mosquitoes. Data were averaged from three biological replicates with a minimum of 50 mosquito guts and presented as the mean ± standard deviation (SD). NS-Not significant.
3.4. Pfcrisp− parasites are transmissible to the mosquito vector and produce viable oocysts.
We next analyzed the ability of Pfcrisp− parasites to generate gametocytes. For this, WT and Pfcrisp− (clone 1 and 2) gametocytes were induced as described elsewhere (Tripathi et al., 2020). Percent gametocytemia was scored for all the cultures on day 15 of in vitro culture using Giemsa-stained smears. Pfcrisp− parasites were able to develop into mature stage V gametocytes and had similar gametocytemia as the WT parasites (Figure 4B). We further tested the ability of Pf crisp− gametocytes to undergo gametogenesis. Day 15 gametocyte cultures for WT and Pf crisp− parasites were activated by addition of O+ human serum and a temperature drop from 37°C to room temperature (RT). Activated gametocytes were used to prepare wet mounts and the number of exflagellation centers were measured in ten fields of view. We observed a similar number of exflagellation centers for Pfcrisp− and WT PfNF54 (Figure 4C), indicating male gamete formation was normal in Pf crisp−. We next examined the transmissibility of Pfcrisp− gametocytes to Anopheles stephensi mosquitoes. Infectious blood meals were prepared using standard methods for WT PfNF54 and Pfcrisp− stage V gametocytes and were fed to mosquitoes. Mosquitoes were dissected on Day 7 post blood meal for scoring midgut oocysts numbers. This showed that both Pfcrisp− clones and WT PfNF54 produced similar average oocyst numbers in mosquitoes (Figure 4D). Taken together, these results indicate that PfCRISP is dispensable for gametocytogenesis, gametogenesis and transmission to the mosquito vector.
4. Discussion
The ingestion of Plasmodium gametocytes by the mosquito vector during a blood meal is critical for the completion of the Plasmodium life cycle. Inside the mosquito midgut, male and female gametocytes are activated to release micro (male) and macro (female) gametes. A male gametocyte undergoes three rapid rounds of mitosis to form eight motile microgametes (Sinden et al., 1978). Male gamete development is often defective in nuclear division and therefore some of the male gametes lack a nucleus and are not viable (Sinden et al., 1978). Since few gametocytes are taken up by the mosquito vector during a natural infectious blood meal, gamete fusion represents a bottleneck in the parasite life cycle (Kappe et al., 2010). In Plasmodium, very few proteins are implicated in this process (Liu et al., 2008; van Dijk et al., 2010). Our results identify PfCRISP to be expressed at one terminus of the microgamete, which might indicate a function in fertilization.
CRISP proteins are secreted glycoproteins which belong to SCP/CAP superfamily proteins and have a role in gamete attachment and fusion (Gaikwad et al., 2020). Since they are highly abundant in the male reproductive tract, CRISPs have gained special attention in the field of reproductive biology. In mammals, sperm released from the testis are functionally immature and lack the capacity for progressive motility and fertilization and need to undergo a maturation process called “capacitation”. Only during their transition through the various segments of the epididymis do sperm gain their fertilization competence (Aitken et al., 2007). Both humans and rats express CRISP1 and CRISP 4 respectively in the epididymis (Roberts et al., 2007). In mice, there are two epididymal CRISPs, CRISP1 and CRISP4 (Arévalo et al., 2020; Cohen et al., 2011; Da Ros et al., 2015; Nolan et al., 2006). CRISP2 is the only CRISP protein expressed during spermatogenesis (Lim et al., 2019). Sperm of crisp2− mice have a severely decreased ability to manifest the acrosome reaction in response to progesterone, have a stiff mid-piece and very few sperm cells are able to penetrate oocytes, and are therefore sub fertile (Lim et al., 2019). Crisp1− mice are fertile (Da Ros et al., 2008). Crisp4− mice show a reduced ability during sperm-zona pellucida interaction and are sub fertile (Turunen et al., 2012). Crisp1−4− mice exhibit clear fertility defects with defects in epididymal epithelium differentiation and male fertility (Carvajal et al., 2018).
In the present study, we show that human and nonhuman primate-infective Plasmodium species encode a CRISP ortholog in their respective genomes, but it is not found in rodent malaria parasites. Identification of a CRISP ortholog, which have been implicated in animal reproduction (Koppers et al., 2011), in addition to proteins with roles in plant reproduction such as HAP2 (Liu et al., 2008) and GEX1 (Ning et al., 2013), renders Plasmodium with a unique combination of fertility related proteins from the kingdoms. PRY proteins which belong to the SCP/CRISP superfamily, have been shown to play a role in lipid/cholesterol binding and lipid export (Choudhary and Schneiter, 2012) and mating (Cottier et al., 2020) and harbor a CAP cavity and CBM motif in their secondary structure (Gaikwad et al., 2020). Dimerization of both the CAP cavity and the CBM motif are required for Mg2+-dependent sterol binding (Darwiche et al., 2016). Typical CAP domain secondary structures have a cavity characterized by the cavity tetrad, formed by four key residues from the four CAP motifs: His from CAP1, Glu from CAP2, His from CAP3 and Glu from CAP4 and these tetrad residues bind divalent cations including zinc and magnesium (Gaikwad et al., 2020). Our initial sequence alignment of PfCRISP with SCP/CAP family proteins did not identify these conserved features but a secondary structure prediction did reveal a CAP cavity in PfCRISP. Since PfCRISP has a CMB motif and a CAP cavity in its predicted structure and a conserved cysteine residue at 147 aa position, which corresponds to cholesterol-binding cysteine residue C279 of ScPRY1 (Choudhary and Schneiter, 2012), it might play a role in lipid binding. Interestingly, we show that PfCRISP exists as monomeric form and an oligomeric form in vitro, which is indicative of conserved features of CRISP family proteins (Darwiche et al., 2016). Dimerization of ScPry1CAP has been proposed to bring both CAP and CBM together to form a large cleft, which may be relevant for the Mg2+ -dependent sterol binding by the Pry1CAP protein (Darwiche et al., 2016). It is possible that the PfCRISP’s oligomerization, which we show here, might be relevant for sterol-binding.
While PfCRISP is not expressed in asexual blood stages, it is expressed in stage V gametocytes and appears to also be exported to the RBC cytoplasm at this stage of development. PfCRISP contains an N-terminal signal peptide, which might mediate its localization, but no known export motif was evident. Thus, it remains unknown which sequence elements might mediate export into the infected erythrocyte. Since, CRISP proteins have been shown to have a role in export of lipids and cholesterol (Choudhary and Schneiter, 2012; Koppers et al., 2011), it is possible that PfCRISP performs a lipid export function during the gametocyte maturation process. Interestingly, PfCRISP primarily displays a terminal, one-sided localization in the emerging microgametes and free microgametes. This localization at one end of the microgamete is unique and raises the question whether microgamete termini are functionally distinct. Previous studies have shown that Plasmodium microgametes can move in both directions with the presumed active end exhibiting ‘more curves than the other end’ (Wilson et al., 2013). It is possible that PfCRISP marks the curvy “active” end of the microgamete, which engages with the macrogamete in preparation for membrane fusion. PfCRISP is also expressed by the stage V female gametocytes where it is also exported into the RBCs. Lipid regulatory proteins are present in female gametocytes (Tran et al., 2014). It is possible that PfCRISP interacts with lipids inside the female gametocytes and exports some of these into the infected RBCs.
For functional characterization of PfCRISP, we created gene deletion parasites using CRIPSR/Cas9 based gene editing. This work revealed that, PfCRISP is not required for asexual blood stage development or gametocyte development. We further showed that Pf crisp− parasites undergo normal exflagellation, indicating normal microgamete formation. Pf crisp− gametocytes also exhibited normal transmissibility to the mosquito vector, indicating an apparent lack of a role for CRISP in fertilization. The lack of an observable phenotype in Pf crisp− parasites is surprising, given the unique expression pattern of PfCRISP in microgametes and its high conservation among primate parasites. It is possible that the in vitro platform routinely used to create Pf gametocytes for mosquito transmission might not reveal an in vivo function for PfCRISP. Since, CRISP family proteins are known to perform role in the mechanobiology of sperm function in mammals (Gaikwad et al., 2021), it is possible that PfCRISP might provide a mechanical function to microgametes which will be interesting to explore in future studies. Furthermore, the observation that CRISP is present in human and nonhuman primate malaria parasite species but not in rodent malaria parasites, might indicate a unique role in the sexual stage biology of the former group of parasites. Despite the lack of an apparent critical role, PfCRISP can be used as a marker for studies involving protein export into the gametocyte-infected red blood cell as well as microgametes and fertilization and it might also be useful in studies of parasites exhibiting defects in the formation and emergence of microgametes.
Supplementary Material
Highlights.
Human infective and non-human primate infective Plasmodium species encode a CRISP homolog.
PfCRISP has conserved structural features such as CAP cavity and a caveolin binding motif.
PfCRISP is exported to RBCs in stage V gametocytes and also shows terminal microgametic localization.
Acknowledgements
This work was funded by the NIH P01 grant P01 AI27338 to A.M.V. and SHIK and seed funds from Seattle Children’s to S.H.I.K. Funders had no role in study design, data collection, and interpretation or the decision to submit this work for publication.
GRANTS
NIH NIAID P01 AI27338 (AMV, SHIK)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare no competing financial interests.
Ethical statement.
The animal experiments, which involved antisera generation in mice, were performed at CGIDR, SCRI and prescribed guidelines for minimization of pain and animal handling were followed.
Appendix A. Supplemental Information
References
- Abraham A, Chandler DE, 2017. Tracing the Evolutionary History of the CAP Superfamily of Proteins Using Amino Acid Sequence Homology and Conservation of Splice Sites. Journal of molecular evolution 85, 137–157. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Nixon B, Lin M, Koppers AJ, Lee YH, Baker MA, 2007. Proteomic changes in mammalian spermatozoa during epididymal maturation. Asian journal of andrology 9, 554–564. [DOI] [PubMed] [Google Scholar]
- Arévalo L, Brukman NG, Cuasnicú PS, Roldan ERS, 2020. Evolutionary analysis of genes coding for Cysteine-RIch Secretory Proteins (CRISPs) in mammals. BMC evolutionary biology 20, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asojo OA, Goud G, Dhar K, Loukas A, Zhan B, Deumic V, Liu S, Borgstahl GE, Hotez PJ, 2005. X-ray structure of Na-ASP-2, a pathogenesis-related-1 protein from the nematode parasite, Necator americanus, and a vaccine antigen for human hookworm infection. Journal of molecular biology 346, 801–814. [DOI] [PubMed] [Google Scholar]
- Busso D, Cohen DJ, Maldera JA, Dematteis A, Cuasnicu PS, 2007. A novel function for CRISP1 in rodent fertilization: involvement in sperm-zona pellucida interaction. Biology of reproduction 77, 848–854. [DOI] [PubMed] [Google Scholar]
- Carvajal G, Brukman NG, Weigel Muñoz M, Battistone MA, Guazzone VA, Ikawa M, Haruhiko M, Lustig L, Breton S, Cuasnicu PS, 2018. Impaired male fertility and abnormal epididymal epithelium differentiation in mice lacking CRISP1 and CRISP4. Scientific reports 8, 17531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary V, Schneiter R, 2012. Pathogen-Related Yeast (PRY) proteins and members of the CAP superfamily are secreted sterol-binding proteins. Proceedings of the National Academy of Sciences of the United States of America 109, 16882–16887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen DJ, Ellerman DA, Busso D, Morgenfeld MM, Piazza AD, Hayashi M, Young ET, Kasahara M, Cuasnicu PS, 2001. Evidence that human epididymal protein ARP plays a role in gamete fusion through complementary sites on the surface of the human egg. Biology of reproduction 65, 1000–1005. [DOI] [PubMed] [Google Scholar]
- Cohen DJ, Maldera JA, Vasen G, Ernesto JI, Muñoz MW, Battistone MA, Cuasnicú PS, 2011. Epididymal protein CRISP1 plays different roles during the fertilization process. Journal of andrology 32, 672–678. [DOI] [PubMed] [Google Scholar]
- Cottier S, Darwiche R, Meyenhofer F, Debelyy MO, Schneiter R, 2020. The yeast cell wall protein Pry3 inhibits mating through highly conserved residues within the CAP domain. Biology open 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowman AF, Healer J, Marapana D, Marsh K, 2016. Malaria: Biology and Disease. Cell 167, 610–624. [DOI] [PubMed] [Google Scholar]
- Da Ros VG, Maldera JA, Willis WD, Cohen DJ, Goulding EH, Gelman DM, Rubinstein M, Eddy EM, Cuasnicu PS, 2008. Impaired sperm fertilizing ability in mice lacking Cysteine-RIch Secretory Protein 1 (CRISP1). Developmental biology 320, 12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Ros VG, Muñoz MW, Battistone MA, Brukman NG, Carvajal G, Curci L, Gómez-ElIas MD, Cohen DB, Cuasnicu PS, 2015. From the epididymis to the egg: participation of CRISP proteins in mammalian fertilization. Asian journal of andrology 17, 711–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwiche R, Kelleher A, Hudspeth EM, Schneiter R, Asojo OA, 2016. Structural and functional characterization of the CAP domain of pathogen-related yeast 1 (Pry1) protein. Scientific reports 6, 28838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry BG, Roelants K, Champagne DE, Scheib H, Tyndall JD, King GF, Nevalainen TJ, Norman JA, Lewis RJ, Norton RS, Renjifo C, de la Vega RC, 2009. The toxicogenomic multiverse: convergent recruitment of proteins into animal venoms. Annual review of genomics and human genetics 10, 483–511. [DOI] [PubMed] [Google Scholar]
- Gaikwad AS, Hu J, Chapple DG, O’Bryan MK, 2020. The functions of CAP superfamily proteins in mammalian fertility and disease. Human reproduction update 26, 689–723. [DOI] [PubMed] [Google Scholar]
- Gaikwad AS, Nandagiri A, Potter DL, Nosrati R, O’Connor AE, Jadhav S, Soria J, Prabhakar R, O’Bryan MK, 2021. CRISPs Function to Boost Sperm Power Output and Motility. Frontiers in cell and developmental biology 9, 693258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs GM, Roelants K, O’Bryan MK, 2008. The CAP superfamily: cysteine-rich secretory proteins, antigen 5, and pathogenesis-related 1 proteins--roles in reproduction, cancer, and immune defense. Endocrine reviews 29, 865–897. [DOI] [PubMed] [Google Scholar]
- Gibbs GM, Scanlon MJ, Swarbrick J, Curtis S, Gallant E, Dulhunty AF, O’Bryan MK, 2006. The cysteine-rich secretory protein domain of Tpx-1 is related to ion channel toxins and regulates ryanodine receptor Ca2+ signaling. The Journal of biological chemistry 281, 4156–4163. [DOI] [PubMed] [Google Scholar]
- Goswami D, Betz W, Locham NK, Parthiban C, Brager C, Schäfer C, Camargo N, Nguyen T, Kennedy SY, Murphy SC, Vaughan AM, Kappe SH, 2020. A replication-competent late liver stage-attenuated human malaria parasite. JCI insight 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper CV, Barratt CL, Publicover SJ, 2004. Stimulation of human spermatozoa with progesterone gradients to simulate approach to the oocyte. Induction of [Ca(2+)](i) oscillations and cyclical transitions in flagellar beating. The Journal of biological chemistry 279, 46315–46325. [DOI] [PubMed] [Google Scholar]
- Hickox JR, Bi M, Hardy DM, 2001. Heterogeneous processing and zona pellucida binding activity of pig zonadhesin. The Journal of biological chemistry 276, 41502–41509. [DOI] [PubMed] [Google Scholar]
- Kappe SH, Vaughan AM, Boddey JA, Cowman AF, 2010. That was then but this is now: malaria research in the time of an eradication agenda. Science (New York, N.Y.) 328, 862–866. [DOI] [PubMed] [Google Scholar]
- Koppers AJ, Reddy T, O’Bryan MK, 2011. The role of cysteine-rich secretory proteins in male fertility. Asian journal of andrology 13, 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Haile MT, Hoopmann MR, Tran LT, Michaels SA, Morrone SR, Ojo KK, Reynolds LM, Kusebauch U, Vaughan AM, Moritz RL, Kappe SHI, Swearingen KE, 2021. Plasmodium falciparum Calcium-Dependent Protein Kinase 4 is Critical for Male Gametogenesis and Transmission to the Mosquito Vector. mBio 12, e0257521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S, Kierzek M, O’Connor AE, Brenker C, Merriner DJ, Okuda H, Volpert M, Gaikwad A, Bianco D, Potter D, Prabhakar R, Strünker T, O’Bryan MK, 2019. CRISP2 Is a Regulator of Multiple Aspects of Sperm Function and Male Fertility. Endocrinology 160, 915–924. [DOI] [PubMed] [Google Scholar]
- Liu F, Liu Q, Yu C, Zhao Y, Wu Y, Min H, Qiu Y, Jin Y, Miao J, Cui L, Cao Y, 2019. An MFS-Domain Protein Pb115 Plays a Critical Role in Gamete Fertilization of the Malaria Parasite Plasmodium berghei. Frontiers in microbiology 10, 2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Tewari R, Ning J, Blagborough AM, Garbom S, Pei J, Grishin NV, Steele RE, Sinden RE, Snell WJ, Billker O, 2008. The conserved plant sterility gene HAP2 functions after attachment of fusogenic membranes in Chlamydomonas and Plasmodium gametes. Genes & development 22, 1051–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldera JA, Weigel Muñoz M, Chirinos M, Busso D, F GER, Battistone MA, Blaquier JA, Larrea F, Cuasnicu PS, 2014. Human fertilization: epididymal hCRISP1 mediates sperm-zona pellucida binding through its interaction with ZP3. Molecular human reproduction 20, 341–349. [DOI] [PubMed] [Google Scholar]
- Meibalan E, Marti M, 2017. Biology of Malaria Transmission. Cold Spring Harbor perspectives in medicine 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning J, Otto TD, Pfander C, Schwach F, Brochet M, Bushell E, Goulding D, Sanders M, Lefebvre PA, Pei J, Grishin NV, Vanderlaan G, Billker O, Snell WJ, 2013. Comparative genomics in Chlamydomonas and Plasmodium identifies an ancient nuclear envelope protein family essential for sexual reproduction in protists, fungi, plants, and vertebrates. Genes & development 27, 1198–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan MA, Wu L, Bang HJ, Jelinsky SA, Roberts KP, Turner TT, Kopf GS, Johnston DS, 2006. Identification of rat cysteine-rich secretory protein 4 (Crisp4) as the ortholog to human CRISP1 and mouse Crisp4. Biology of reproduction 74, 984–991. [DOI] [PubMed] [Google Scholar]
- Roberts KP, Johnston DS, Nolan MA, Wooters JL, Waxmonsky NC, Piehl LB, Ensrud-Bowlin KM, Hamilton DW, 2007. Structure and function of epididymal protein cysteine-rich secretory protein-1. Asian journal of andrology 9, 508–514. [DOI] [PubMed] [Google Scholar]
- Silvestrini F, Lasonder E, Olivieri A, Camarda G, van Schaijk B, Sanchez M, Younis Younis S, Sauerwein R, Alano P, 2010. Protein export marks the early phase of gametocytogenesis of the human malaria parasite Plasmodium falciparum. Molecular & cellular proteomics : MCP 9, 1437–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinden RE, Canning EU, Bray RS, Smalley ME, 1978. Gametocyte and gamete development in Plasmodium falciparum. Proceedings of the Royal Society of London. Series B, Biological sciences 201, 375–399. [DOI] [PubMed] [Google Scholar]
- Tran PN, Brown SH, Mitchell TW, Matuschewski K, McMillan PJ, Kirk K, Dixon MW, Maier AG, 2014. A female gametocyte-specific ABC transporter plays a role in lipid metabolism in the malaria parasite. Nature communications 5, 4773. [DOI] [PubMed] [Google Scholar]
- Tripathi AK, Mlambo G, Kanatani S, Sinnis P, Dimopoulos G, 2020. Plasmodium falciparum Gametocyte Culture and Mosquito Infection Through Artificial Membrane Feeding. Journal of visualized experiments : JoVE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turunen HT, Sipilä P, Krutskikh A, Toivanen J, Mankonen H, Hämäläinen V, Björkgren I, Huhtaniemi I, Poutanen M, 2012. Loss of cysteine-rich secretory protein 4 (Crisp4) leads to deficiency in sperm-zona pellucida interaction in mice. Biology of reproduction 86, 1–8. [DOI] [PubMed] [Google Scholar]
- van Dijk MR, van Schaijk BC, Khan SM, van Dooren MW, Ramesar J, Kaczanowski S, van Gemert GJ, Kroeze H, Stunnenberg HG, Eling WM, Sauerwein RW, Waters AP, Janse CJ, 2010. Three members of the 6-cys protein family of Plasmodium play a role in gamete fertility. PLoS pathogens 6, e1000853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbers RHP, Schneiter R, Holterman MHM, Drurey C, Smant G, Asojo OA, Maizels RM, Lozano-Torres JL, 2018. Secreted venom allergen-like proteins of helminths: Conserved modulators of host responses in animals and plants. PLoS pathogens 14, e1007300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson LG, Carter LM, Reece SE, 2013. High-speed holographic microscopy of malaria parasites reveals ambidextrous flagellar waveforms. Proceedings of the National Academy of Sciences of the United States of America 110, 18769–18774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Xiao B, Jiang Y, Zhao Y, Li Z, Gao H, Ling Y, Wei J, Li S, Lu M, Su XZ, Cui H, Yuan J, 2014. Efficient editing of malaria parasite genome using the CRISPR/Cas9 system. mBio 5, e01414–01414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Wang C, Otto TD, Oberstaller J, Liao X, Adapa SR, Udenze K, Bronner IF, Casandra D, Mayho M, Brown J, Li S, Swanson J, Rayner JC, Jiang RHY, Adams JH, 2018. Uncovering the essential genes of the human malaria parasite Plasmodium falciparum by saturation mutagenesis. Science (New York, N.Y.) 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


