Abstract
Background: Previous findings on using Glucagon-like peptide-1 receptor agonist (GLP1-RA) as an antidepressant were conflicting, lacking large-scale studies. We used population-based data to investigate depression and anxiety risk in diabetic patients receiving the medication.
Methods: From claims records of the National Health Insurance Research Database (NHIRD) of Taiwan, we identified cohorts of 10,690 GLP1-RA users and 42,766 propensity score-matched patients without GLP1-RA use from patients with diabetes mellitus (DM) diagnosed in 2011–2017, matched by age, gender, index year, occupation, urbanization, comorbidities, and medications. Incidence, hazard ratios (HR) and 95% confidence interval (CI) of depression and/or anxiety were estimated by the end of 2017.
Results: The overall combined incidence of anxiety and/or depression was lower in GLP1-RA users than in non-users (6.80 versus 9.36 per 1,000 person-years), with an adjusted HR adjusted hazard ratio (aHR) of 0.8 (95% CI: 0.67–0.95) after controlling for covariates. The absolute incidence reduction was greater in anxiety (2.13 per 1,000 person-years) than in depression (0.41 per 1,000 person-years). The treatment effectiveness was significant for women. Patients taking GLP1-RA for longer than 180 days had the incidence of anxiety reduced to 2.93 per 1,000 person-years, with an aHR of 0.41 (95%CI: 0.27–0.61), compared to non-users. Dulaglutide could significantly decrease risks of both anxiety and depression.
Conclusion: Patients with DM receiving GLP1-RA therapy have a greater reduction of the risk of anxiety than that of depression. Our findings strengthen previous research that advocated possible anti-depressant or anxiolytic effects of GLP1-RA and may lead to improved treatment adherence among patients with DM.
Keywords: depression, anxiety, glucagon-like peptide-1 receptor agonist, diabetes, neuroendocrine
Introduction
Diabetes mellitus (DM) is a group of endocrinological and metabolic disorders affecting approximately 422 million patients worldwide (Saeedi et al., 2019). Patients with DM are at an elevated risk of developing acute and serious long-term complications (Fowler, 2008; Gregg et al., 2016; Harding et al., 2019). Considerable evidence has also associated DM with increased risk of incident or recurrent depression (Gavard et al., 1993; Lustman et al., 2000; Anderson et al., 2001; Snoek et al., 2015; Chima et al., 2017; Khaledi et al., 2019). An earlier meta-analysis found that the risk of depression was 2-fold higher for patients with DM than individuals without DM (Anderson et al., 2001). Another meta-analysis review reported that poor glycemic control is associated with the occurrence of depression in patients with both type 1 and type 2 DM (Lustman et al., 2000).
Metformin, thiazolinediones (TZDs), glucagon-like peptide-1 receptor agonists (GLP1-RA), and dipeptidyl peptidase 4 (DPP-4) inhibitors are medications commonly prescribed for glycemic and weight control (Pozzi et al., 2019). A meta-analysis of clinical trials found that the use of pioglitazone, a TZD, was associated with significant improvements in depressive symptoms. The effect was more marked in women. The treatment effectiveness of metformin for diabetic patients was not consistent among studies (Moulton et al., 2018). However, a recent study in Saudi Arabia found that metformin could lower the probability of major depressive symptoms by 70% among patients with polycystic ovary syndrome (PCOS), but did not influence the occurrence of anxiety (AlHussain et al., 2020).
GLP1-RAs can cross the blood brain barrier, exerting function at both peripheral and central systems (Pozzi et al., 2019). A meta-analysis advocated that GLP1-RAs could exert antidepressant or anxiolytic effects on reducing the depression rating score of −2.09 (95% CI −2.28 – −1.91, p < 0.001) for diabetic patients (Pozzi et al., 2019). A United Kingdom study evaluating changes of quality of life for patients with GLP1-RA demonstrated that the therapy significantly reduced the Hospital Anxiety and Depression Scale (HADS) scores, compared with insulin-treated patients. (Grant et al., 2011). A placebo-controlled single-blind study, investigating the effect of GLP1-RA on excessive daytime sleepiness with 16 male DM patients, demonstrated a significant reduction in depression scores after the treatment compared to the baseline scores. Nonetheless, the reduction was not significant compared to placebo (Idris et al., 2013). A 26-weeks randomized controlled trial in the Netherlands found that GLP1-RA treatment in 26 patients significantly improved the quality of life measured by the Problem Areas in Diabetes Scale (PAID) questionnaire, compared to 24 patients using standard treatment (de Wit et al., 2014). Further follow-up study revealed that the effectiveness was sustained to 52 weeks, but there was insignificant improvement in scores of Beck Depression Index (BDI) (de Wit et al., 2016). Another 6-month treatment with liraglutide for 19 women with polycystic ovary syndrome without DM revealed no significant association with reduced depression, compared to 17 controls matched by age (Kahal et al., 2019). A pooled analysis on 5,325 individuals with higher BMI without diabetes evaluating the effectiveness of trials using GLP1-RA for 32–160 weeks also found no significant differences in depression (2.1 versus 2.1 events/100 person-years) and anxiety (1.9 versus 1.7 events/100 person-years) (O'Neil et al., 2017).
Most previous studies on GLP1-RA were undertaken with small sample sizes and reported inconsistent findings on the treatment effectiveness related to risks of depression and anxiety. These studies ascertained conditions of depression or anxiety mainly based on screening instruments rather than clinical diagnoses. A large long-term cohort study with sufficient sample size focusing on DM patients is lacking. Therefore, we used the insurance claims data of Taiwan to conduct this study to evaluate risks of anxiety and/or depression between DM patients with and without GLP1-RA, with sufficient large population-based cohorts and follow-up time.
Methods and Materials
Data Source
The National Health Insurance (NHI) of Taiwan is a compulsory health insurance system, launched in 1995 for all residents. For this study, we used the National Health Insurance Research Database (NHIRD) issued by NHI containing claims data of all insured individuals. The claims data provided records of birth date, gender, places of residence, and enrollee category, as well as diagnoses, drug prescriptions, and treatments for emergency and outpatient visits, and hospitalizations from 2000 to 2017. All identifications of insured people in the NHIRD were replaced with surrogate numbers and analyzed anonymously, hence consents were waived. This study was approved by the Institutional Review Board of Mackay Memorial Hospital (20MMHIS431e).
Study Cohorts
GLP1-RA was one of the available treatment options for DM patients, with approval from the NHI given in 2011. We therefore first identified 4,079,299 patients with the diagnosis of DM during the period of 2011–2017 (Figure 1). Patients who were less than 20 years old or had been diagnosed with depression and/or anxiety at baseline were excluded. Patients who were newly diagnosed in 2011–2017 with DM (ICD-10-CM code E08, E09, E10, E11, and E13; ICD-9-CM codes: 250) at least twice were considered as the potential study population. The date of the initial diagnosis with DM was designated as the index date. Patients with DM prescribed with GLP1-RA (Liraglutide, Dulaglutide, Exenatide) in 2011–2017 were included in the GLP1-RA group. For the comparison group, propensity score-matched DM patients without GLP1-RA use were selected and matched at a 1:4 ratio. The propensity score was calculated by logistic regression using covariates of age, gender, index year, occupation, urbanization, comorbidities, and medications.
FIGURE 1.
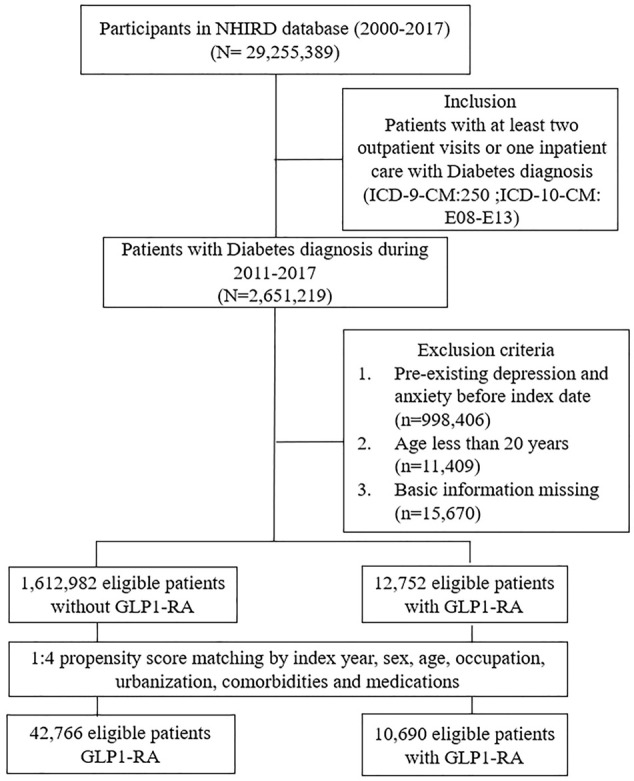
Selection scheme for study population.
Outcome
Both cohorts were followed to estimate follow-up person-years for each individual in both groups until incident depression (ICD-10-CM codes: F32.0, F32.1, F32.2, F32.3, F32.4, F32.5, F32.9, F33.0, F33.1, F33.2, F33.3, F33.40, F33.41, F33.42, F34.1, and F43.21; ICD-9-CM codes: 296.20, 296.21, 296.22, 296.23, 296.24, 296.25, 296.26, 296.30, 296.31, 296.32, 296.33, 296.34, 296.35, 296.36, 311, 300.4, 309.0, and 309.1) and/or anxiety (ICD-10-CM code: F41; ICD-9-CM codes: 300) were identified, or the individual withdrew from the insurance, or the end of 2017. Depression and anxiety were defined as having the diagnoses from at least two outpatient visits or one hospitalization to ensure the validity (Chien et al., 2007). The incidence rate of depression or anxiety was estimated by per 1,000 person-years.
Potential Risk Covariates
Variables that might have an association with the development of depression or anxiety included comorbidities, other medications and demographic variables of occupation and urbanization level of residential area. We classified residential areas into four levels, with level 1 as the most urbanized, and level 4 as the least urbanized. We were interested in baseline comorbidities (Read et al., 2017), including hyperlipidemia (ICD-10-CM code: E78; ICD-9-CM codes: 272), hypertension (ICD-10-CM codes: I10, I11, I12, I13, and I15; ICD-9-CM codes: 401, 402, 403, 404, and 405), coronary artery disease (ICD-10-CM codes: I20, I21, I22, I23, I24, and I25; ICD-9-CM codes: 410, 411, 412, 413, 414, and 429), chronic kidney disease (ICD-10-CM codes: N18 and N19; ICD-9-CM codes: 585, 586, and 593), stroke (ICD-10-CM codes: I60, I61, I62, and I63; ICD-9-CM codes: 430, 431, 432, and 433), heart failure (ICD-10-CM code I50; ICD-9-CM codes: 428), chronic obstructive pulmonary disease (COPD) (ICD-10-CM codes: J44, J45, and J47; ICD-9-CM codes: 491, 493, 494, and 496), connective tissue disease (ICD-10-CM codes L93, L94; ICD-9-CM codes: 695 and 701), obesity (ICD-10-CM code E66; ICD-9-CM codes: 278), malignancy (ICD-10-CM codes D49; ICD-9-CM codes: 239), liver cirrhosis (ICD-10-CM code: K74; ICD-9-CM codes: 571), alcoholic liver disease (ICD-10-CM code: K70; ICD-9-CM codes: 571) and adapted Diabetes Complications Severity Index (aDCSI). The aDCSI is a good measure of diabetes severity that uniquely incorporates a wide range of diabetes complications (Young et al., 2008; Chang et al., 2012). The other anti-glycemic agents, including metformin, thiazolinedione (TZD), sodium-glucose co-transporters 2 (SGLT2) inhibitors, acarbose, sulfonylurea, dipeptidyl peptidase 4 (DPP-4) inhibitors, insulin, and compound agents, were also possible confounders to consider in the analyses.
Statistical Analysis
Demographic characteristics and prevalence of comorbidities in the study and the comparison groups were compared and examined with Chi-square tests for categorical variables and t-tests for continuous variables. The Kaplan-Meier method was used to graphically describe the cumulative incidence of depression and anxiety during the 7-years follow-up period. Cox proportional hazards regression analysis was used to determine the GLP1-RA group to compare group hazard ratio (HR) and 95% confidence interval (CI) of depression or anxiety. Demographic variables, comorbidities, and medications were included in multivariable models to estimate the adjusted hazard ratio (aHR): Model 1 adjusted for demographic factors, Model 2 adjusted for Model 1 variables and comorbidities, and Model 3 adjusted for Model 2 variables and medications. We further evaluated the treatment effectiveness by the length of treatment, stratifying the medication courses into 3 periods, 30–90 days, 91–180 days, and >180 days. All statistical analyses were performed using STATA version 14.0 (StataCorp) and results with p values less than 0.05 considered as significant.
Results
A total of 10,690 DM patients prescribed GLP1-RA and 42,766 comparisons with non-users were identified from the NHIRD. Both groups had similar distributions of age, gender, occupation, urbanization and comorbidities, with a mean age of 53.33 years (SD 13.04) and 45.06% women (Table 1). Although, the percentage of using metformin was higher in non-GLP1-RA users.
TABLE 2.
Incidence of depression and anxiety in diabetes patients with/without GLP1-RA use.
| Outcome variables | Non-GLP1-RA vs. GLP1-RA user | |
|---|---|---|
| Non-GLP1-RA user | GLP1-RA user | |
| Any depression or anxiety | ||
| Follow-up years | 1.97 ± 1.53 | 2.62 ± 1.51 |
| Event | 857 | 157 |
| Person-year | 91546 | 23090 |
| Incidence Rate | 9.36 | 6.80 |
| Crude HR (95% CI) | ref. | 0.72 (0.60, 0.85)*** |
| Model 1 | ref. | 0.73 (0.61, 0.86)*** |
| Model 2 | ref. | 0.73 (0.62, 0.87)*** |
| Model 3 | ref. | 0.80 (0.67, 0.95)** |
| Anxiety | ||
| Follow-up years | 1.93 ± 1.52 | 2.39 ± 1.48 |
| Event | 656 | 116 |
| Person-year | 91838 | 23158 |
| Incidence Rate | 7.14 | 5.01 |
| Crude HR (95% CI) | ref. | 0.70 (0.57, 0.85)*** |
| Model 1 | ref. | 0.70 (0.58, 0.86)*** |
| Model 2 | ref. | 0.71 (0.58, 0.86)*** |
| Model 3 | ref. | 0.78 (0.64, 0.95)* |
| Depression | ||
| Follow-up years | 2.08 ± 1.57 | 2.92 ± 1.60 |
| Event | 308 | 68 |
| Person-year | 92594.49 | 23304.4 |
| Incidence Rate | 3.32 | 2.91 |
| Crude HR (95% CI) | ref. | 0.85 (0.66, 1.11) |
| Model 1 | ref. | 0.85 (0.66, 1.11) |
| Model 2 | ref. | 0.87 (0.67, 1.14) |
| Model 3 | ref. | 0.94 (0.72, 1.23) |
*p < 0.05, **p < 0.01, ***p < 0.001.
Incidence rate, per 1,000 person-years; HR, hazard ratio; CI, confidence interval.
Demographic factors include age, gender, urbanization level and enrollee category.
1:4 propensity score matching.
Model 1: Adjust for demographic factors.
Model 2: Adjust for model 1 + comorbidity.
Model 3: Adjust for model 2 + medication.
TABLE 1.
Baseline demographic factors and comorbidities of study population according to GLP1-RA use.
| Characteristics | Non-GLP1-RA user N = 42766 | GLP1-RA user N = 10690 | p-value | SMD | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Gender | ||||||
| female | 18851 | 44.08 | 4,817 | 45.06 | 0.068 | 0.020 |
| male | 23915 | 55.92 | 5,873 | 54.94 | 0.068 | 0.020 |
| Age, year | ||||||
| 20-40 | 6,240 | 14.59 | 1,693 | 15.84 | 0 | 0.035 |
| 40-60 | 21561 | 50.42 | 5,442 | 50.91 | 0 | 0.010 |
| >60 | 14965 | 34.99 | 3,555 | 33.26 | 0 | 0.037 |
| Mean Age (SD) | 54 | 12.91 | 53.33 | 13.04 | 0 | 0.052 |
| Urbanization | ||||||
| 1 (high) | 23609 | 55.21 | 6,018 | 56.30 | 0.240 | 0.022 |
| 2 | 16193 | 37.86 | 3,950 | 36.95 | 0.240 | 0.019 |
| 3 | 2,420 | 5.66 | 586 | 5.48 | 0.240 | 0.008 |
| 4 (low) | 544 | 1.27 | 136 | 1.27 | 0.240 | 0 |
| Enrollee category | ||||||
| White collar | 21956 | 51.34 | 5,573 | 52.13 | 0.336 | 0.016 |
| Blue collar | 12734 | 29.78 | 3,138 | 29.35 | 0.336 | 0.009 |
| Others | 8,076 | 18.88 | 1979 | 18.51 | 0.336 | 0.010 |
| Comorbidities | ||||||
| Hyperlipidemia | 17170 | 40.15 | 4,310 | 40.32 | 0.749 | 0.004 |
| Hypertension | 20908 | 48.89 | 5,247 | 49.08 | 0.720 | 0.004 |
| Coronary artery disease | 11576 | 27.07 | 2,896 | 27.09 | 0.963 | 0.001 |
| Chronic kidney disease | 7,922 | 18.52 | 1972 | 18.45 | 0.855 | 0.002 |
| Stroke | 8,687 | 20.31 | 2,158 | 20.19 | 0.772 | 0.003 |
| COPD | 10129 | 23.68 | 2,535 | 23.71 | 0.950 | 0.001 |
| Connective tissue disease | 4,230 | 9.89 | 1,033 | 9.66 | 0.480 | 0.008 |
| Heart failure | 3,233 | 7.56 | 781 | 7.31 | 0.373 | 0.010 |
| Malignancy | 4,956 | 11.59 | 1,232 | 11.52 | 0.854 | 0.002 |
| Liver cirrhosis | 13898 | 32.5 | 3,476 | 32.52 | 0.971 | 0 |
| Alcoholic liver disease | 3,078 | 7.2 | 739 | 6.91 | 0.307 | 0.011 |
| Obesity | 1,215 | 2.84 | 284 | 2.66 | 0.302 | 0.011 |
| aDCSI score | ||||||
| 0 | 5,201 | 12.16 | 1,328 | 12.42 | 0.386 | 0.008 |
| 1 | 4,917 | 11.5 | 1,183 | 11.07 | 0.386 | 0.014 |
| ≥2 | 32648 | 76.34 | 8,179 | 76.51 | 0.386 | 0.004 |
| Medication | ||||||
| Metformin | 39194 | 91.65 | 9,412 | 88.04 | <0.0001 | 0.120 |
| TZD | 13600 | 31.8 | 3,366 | 31.49 | 0.533 | 0.007 |
| SGLT2 inhibitor | 7,907 | 18.49 | 2054 | 19.21 | 0.085 | 0.019 |
| acarbose | 9,799 | 22.91 | 2,461 | 23.02 | 0.811 | 0.003 |
| Sulfonylurea | 31181 | 72.91 | 7,541 | 70.54 | <0.0001 | 0.053 |
| DPP-4 inhibitor | 11938 | 27.91 | 2,783 | 26.03 | <0.0001 | 0.042 |
| Insulin | 20690 | 48.38 | 5,159 | 48.26 | 0.825 | 0.002 |
| Oral medication combination | 6,404 | 14.97 | 1,481 | 13.85 | 0.003 | 0.032 |
Data shown as n(%) or mean ± SD.
SMD: standardized mean difference. A standardized mean difference of 0.1 or less indicates a negligible difference.
GLP1-RA, glucagon-like peptide-1, receptor agonist; COPD, chronic obstructive pulmonary disease; TZD, thiazolidinedione; SGLT2, Sodium-glucose co-transporter-2; DPP-4, inhibitor, dipeptidyl peptidase-4 inhibitor.
The urbanization level was categorized by the population density of the residential area into four levels, with level 1 as the most urbanized and level 4 as the least urbanized.
1:4 propensity score matching.
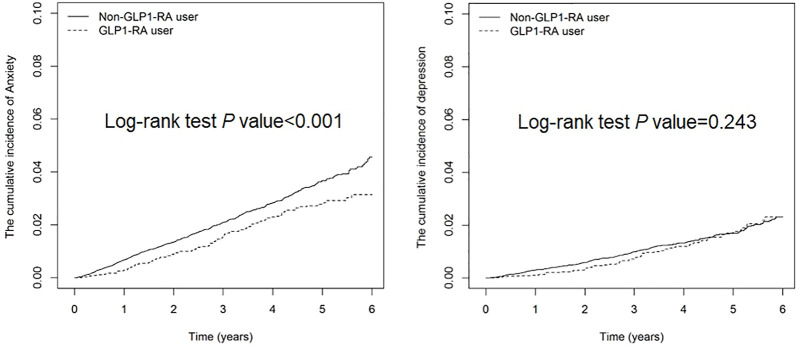
The cumulative incidence of anxiety was 2.13% lower in GLP1-RA users than non-users (Log-rank test p < 0.001), whereas that of depression was not significantly different between the 2 groups (Figure 2). The overall incidence of depression and/or anxiety was lower in GLP1-RA users than non-users (6.80 versus 9.36 per 1,000 person-years), with an aHR of 0.8 (95% CI: 0.67–0.95) for users, after controlling for demographic factors, comorbidities and medications (Table 2). The difference of incidence rates between the two groups was greater for anxiety than depression. The aHRs of developing anxiety and depression for the GLP1-RA group, compared to non-users, were 0.78 (95% CI: 0.64–0.95) and 0.94 (95% CI: 0.72–1.23), respectively.
FIGURE 2.
Cumulative incidence of depression and anxiety in diabetes patients with/without GLP1-RA.
The beneficial effect on anxiety (Table 3) was specific to patients between 40 and 60 years old, with aHR of 0.73 (95% CI: 0.55–0.96). Compared with men, women in both groups exhibited greater incidences of depression and anxiety (Table 4). However, for women, risks of anxiety were significantly lower in GLP1-RA users than non-users. GLP1-RA users with either kind of comorbidity or that used other hypoglycemia agents showed no significant difference on the risk of depression (Table 4). On the other hand, GLP1-RA users without comorbidities except for hypertension showed significant reductions of anxiety (Table 3). GLP1-RA users with hypertension tended to have lower risk of anxiety, with aHR of 0.73 (95% CI: 0.54–0.97). GLP1-RA users that took metformin, sulfonylurea and oral medication combination had lower risk of anxiety, so as to those that did not use TZD, acarbose, SGLT2 inhibitors, DPP4 inhibitors, or insulin (Table 3).
TABLE 3.
Factors associated with anxiety incidence in diabetes patients with/without GLP1-RA: subgroup analysis.
| Variables | Non-GLP1-RA user | GLP1-RA user | cHR | (95% CI) | p-value | aHR | (95% CI) | p-value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Event | PY | IR | Event | PY | IR | |||||||
| Anxiety | ||||||||||||
| Gender | ||||||||||||
| Female | 348 | 42,211 | 8.24 | 63 | 11,093 | 5.68 | 0.68 | (0.52, 0.89)** | 0.005 | 0.75 | (0.57, 0.98)* | 0.034 |
| Male | 308 | 49,627 | 6.21 | 53 | 12,065 | 4.39 | 0.71 | (0.53, 0.94)* | 0.019 | 0.80 | (0.60, 1.08) | 0.145 |
| Age group | ||||||||||||
| 20-40 | 82 | 12,988 | 6.31 | 24 | 4,519 | 5.31 | 0.87 | (0.55, 1.37) | 0.538 | 0.92 | (0.57, 1.46) | 0.713 |
| 40-60 | 328 | 47,119 | 6.96 | 59 | 12,642 | 4.67 | 0.66 | (0.50, 0.87)** | 0.003 | 0.73 | (0.55, 0.96)* | 0.027 |
| >60 | 246 | 31,731 | 7.75 | 33 | 5,997 | 5.50 | 0.71 | (0.50, 1.03) | 0.071 | 0.75 | (0.52, 1.09) | 0.130 |
| Comorbidities | ||||||||||||
| Hyperlipidemia | ||||||||||||
| No | 381 | 54,417 | 7.00 | 67 | 14,265 | 4.70 | 0.66 | (0.51, 0.85)** | 0.002 | 0.72 | (0.56, 0.94)* | 0.014 |
| Yes | 275 | 37,422 | 7.35 | 49 | 8,893 | 5.51 | 0.76 | (0.56, 1.03) | 0.072 | 0.86 | (0.63, 1.17) | 0.328 |
| Hypertension | ||||||||||||
| No | 311 | 46,371 | 6.71 | 63 | 12,092 | 5.21 | 0.76 | (0.58, 1.00)* | 0.048 | 0.82 | (0.62, 1.08) | 0.157 |
| Yes | 345 | 45,467 | 7.59 | 53 | 11,066 | 4.79 | 0.63 | (0.47, 0.85)** | 0.002 | 0.73 | (0.54, 0.97)* | 0.033 |
| Coronary artery disease | ||||||||||||
| No | 469 | 66,416 | 7.06 | 77 | 17,076 | 4.51 | 0.63 | (0.50, 0.80)*** | <0.001 | 0.69 | (0.54, 0.88)** | 0.003 |
| Yes | 187 | 25,422 | 7.36 | 39 | 6,083 | 6.41 | 0.87 | (0.62, 1.23) | 0.441 | 1.01 | (0.71, 1.44) | 0.953 |
| Chronic kidney disease | ||||||||||||
| No | 519 | 75,604 | 6.86 | 94 | 19,379 | 4.85 | 0.7 | (0.56, 0.87)** | 0.001 | 0.77 | (0.61, 0.96)* | 0.018 |
| Yes | 137 | 16,234 | 8.44 | 22 | 3,779 | 5.82 | 0.69 | (0.44, 1.09) | 0.111 | 0.79 | (0.50, 1.24) | 0.303 |
| Stroke | ||||||||||||
| No | 510 | 73,191 | 6.97 | 87 | 18,674 | 4.66 | 0.66 | (0.53, 0.83)*** | <0.001 | 0.73 | (0.58, 0.92)** | 0.008 |
| Yes | 146 | 18,647 | 7.83 | 29 | 4,484 | 6.47 | 0.82 | (0.55, 1.23) | 0.343 | 0.93 | (0.62, 1.39) | 0.717 |
| COPD | ||||||||||||
| No | 488 | 69,597 | 7.01 | 81 | 17,840 | 4.54 | 0.64 | (0.51, 0.81)*** | <0.001 | 0.70 | (0.55, 0.89)** | 0.003 |
| Yes | 168 | 22,241 | 7.55 | 35 | 5,319 | 6.58 | 0.86 | (0.60, 1.24) | 0.429 | 0.99 | (0.69, 1.44) | 0.978 |
| Connective tissue disease | ||||||||||||
| No | 576 | 82,793 | 6.96 | 100 | 21,190 | 4.72 | 0.67 | (0.54, 0.83)*** | <0.001 | 0.74 | (0.60, 0.92)** | 0.006 |
| Yes | 80 | 9,045 | 8.84 | 16 | 1,969 | 8.13 | 0.91 | (0.53, 1.57) | 0.746 | 1.03 | (0.59, 1.78) | 0.921 |
| Heart failure | ||||||||||||
| No | 594 | 85,201 | 6.97 | 103 | 21,739 | 4.74 | 0.67 | (0.55, 0.83)*** | <0.001 | 0.75 | (0.60, 0.92)** | 0.007 |
| Yes | 62 | 6,637 | 9.34 | 13 | 1,420 | 9.16 | 0.98 | (0.54, 1.78) | 0.943 | 1.11 | (0.60, 2.05) | 0.744 |
| Malignancy | ||||||||||||
| No | 575 | 81,517 | 7.05 | 98 | 20,816 | 4.71 | 0.66 | (0.53, 0.82)*** | <0.001 | 0.73 | (0.59, 0.90)** | 0.004 |
| Yes | 81 | 10,321 | 7.85 | 18 | 2,343 | 7.68 | 0.97 | (0.58, 1.62) | 0.907 | 1.11 | (0.66, 1.86) | 0.703 |
| Liver cirrhosis | ||||||||||||
| No | 412 | 60,426 | 6.82 | 72 | 15,490 | 4.65 | 0.67 | (0.52, 0.87)** | 0.002 | 0.72 | (0.56, 0.93)* | 0.012 |
| Yes | 244 | 31,413 | 7.77 | 44 | 7,668 | 5.74 | 0.74 | (0.54, 1.02) | 0.066 | 0.85 | (0.62, 1.18) | 0.339 |
| Alcoholic liver disease | ||||||||||||
| No | 606 | 85,550 | 7.08 | 103 | 21,819 | 4.72 | 0.66 | (0.54, 0.81)*** | <0.001 | 0.73 | (0.59, 0.90)** | 0.003 |
| Yes | 50 | 6,288 | 7.95 | 13 | 1,340 | 9.70 | 1.21 | (0.65, 2.22) | 0.549 | 1.30 | (0.69, 2.45) | 0.411 |
| Obesity | ||||||||||||
| No | 632 | 89,423 | 7.07 | 111 | 22,657 | 4.90 | 0.69 | (0.56, 0.84)*** | <0.001 | 0.76 | (0.62, 0.93)** | 0.007 |
| Yes | 24 | 2,416 | 9.94 | 5 | 501 | 9.97 | 1 | (0.38, 2.62) | 0.997 | 1.28 | (0.45, 3.66) | 0.640 |
| Medication | ||||||||||||
| Metformin | ||||||||||||
| No | 65 | 5,843 | 11.12 | 16 | 1,567 | 10.21 | 0.9 | (0.52, 1.55) | 0.694 | 1.11 | (0.62, 1.98) | 0.721 |
| Yes | 591 | 85,995 | 6.87 | 100 | 21,591 | 4.63 | 0.67 | (0.54, 0.82)*** | <0.001 | 0.74 | (0.60, 0.92)** | 0.007 |
| TZD | ||||||||||||
| No | 463 | 56,264 | 8.23 | 82 | 14,733 | 5.57 | 0.68 | (0.54, 0.86)** | 0.001 | 0.77 | (0.60, 0.97)* | 0.027 |
| Yes | 193 | 35,575 | 5.43 | 34 | 8,425 | 4.04 | 0.72 | (0.50, 1.04) | 0.079 | 0.78 | (0.54, 1.13) | 0.189 |
| Acarbose | ||||||||||||
| No | 480 | 63,351 | 7.58 | 78 | 15,938 | 4.89 | 0.64 | (0.50, 0.81)*** | <0.001 | 0.72 | (0.56, 0.91)** | 0.007 |
| Yes | 176 | 28,487 | 6.18 | 38 | 7,221 | 5.26 | 0.85 | (0.60, 1.20) | 0.357 | 0.87 | (0.61, 1.24) | 0.443 |
| SGLT2 inhibitor | ||||||||||||
| No | 613 | 73,197 | 8.37 | 103 | 15,888 | 6.48 | 0.78 | (0.63, 0.96)* | 0.019 | 0.78 | (0.63, 0.96)* | 0.018 |
| Yes | 43 | 18,641 | 2.31 | 13 | 7,270 | 1.79 | 0.7 | (0.37, 1.31) | 0.263 | 0.66 | (0.34, 1.26) | 0.207 |
| Sulfonylurea | ||||||||||||
| No | 135 | 17,895 | 7.54 | 25 | 4,376 | 5.71 | 0.76 | (0.49, 1.16) | 0.207 | 0.84 | (0.55, 1.31) | 0.446 |
| Yes | 521 | 73,943 | 7.05 | 91 | 18,782 | 4.85 | 0.68 | (0.54, 0.85)*** | <0.001 | 0.75 | (0.60, 0.95)* | 0.014 |
| DPP-4 inhibitor | ||||||||||||
| No | 480 | 56,240 | 8.53 | 67 | 13,045 | 5.14 | 0.6 | (0.47, 0.78)*** | <0.001 | 0.65 | (0.50, 0.84)** | 0.001 |
| Yes | 176 | 35,598 | 4.94 | 49 | 10,114 | 4.85 | 0.91 | (0.66, 1.25) | 0.575 | 1.01 | (0.73, 1.41) | 0.939 |
| Insulin | ||||||||||||
| No | 283 | 37,285 | 7.59 | 45 | 8,647 | 5.20 | 0.69 | (0.5, 0.94)* | 0.019 | 0.68 | (0.49, 0.93)* | 0.015 |
| Yes | 373 | 54,554 | 6.84 | 71 | 14,511 | 4.89 | 0.7 | (0.54, 0.90)** | 0.005 | 0.82 | (0.63, 1.06) | 0.127 |
| Oral medication combination | ||||||||||||
| No | 580 | 74,490 | 7.79 | 100 | 18,767 | 5.33 | 0.68 | (0.55, 0.85)*** | <0.001 | 0.76 | (0.62, 0.94)* | 0.013 |
| Yes | 76 | 17,348 | 4.38 | 16 | 4,392 | 3.64 | 0.76 | (0.44, 1.31) | 0.321 | 0.88 | (0.51, 1.53) | 0.652 |
*p < 0.05, **p < 0.01, ***p < 0.001.
PY, person-years; IR, incidence rate, per 1,000 person-years; HR, hazard ratio; CI, confidence interval.
Demographic factors include age, gender, urbanization level and enrollee category.
1:4 propensity score matching.
Fully adjusted model: Adjusted for demographic factors, comorbidities and medication.
TABLE 4.
Factors associated with depression incidence in diabetes patients with/without GLP1-RA: subgroup analysis.
| Variables | Non-GLP1-RA user | GLP1-RA user | cHR | (95% CI) | p-value | aHR | (95% CI) | p-value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Event | PY | IR | Event | PY | IR | |||||||
| Depression | ||||||||||||
| Gender | ||||||||||||
| Female | 169 | 42,634 | 3.96 | 39 | 11,180 | 3.49 | 0.85 | (0.60, 1.21) | 0.367 | 0.91 | (0.64, 1.29) | 0.594 |
| Male | 139 | 49,961 | 2.78 | 29 | 12,125 | 2.39 | 0.85 | (0.57, 1.26) | 0.414 | 0.95 | (0.64, 1.43) | 0.812 |
| Age group | ||||||||||||
| 20-40 | 48 | 13,071 | 3.67 | 20 | 4,538 | 4.41 | 1.13 | (0.67, 1.91) | 0.654 | 1.32 | (0.77, 2.26) | 0.316 |
| 40-60 | 141 | 47,504 | 2.97 | 30 | 12,723 | 2.36 | 0.77 | (0.52, 1.15) | 0.202 | 0.86 | (0.58, 1.28) | 0.461 |
| >60 | 119 | 32,020 | 3.72 | 18 | 6,043 | 2.98 | 0.8 | (0.49, 1.32) | 0.392 | 0.8 | (0.49, 1.32) | 0.388 |
| Comorbidities | ||||||||||||
| Hyperlipidemia | ||||||||||||
| No | 162 | 54,908 | 2.95 | 40 | 14,355 | 2.79 | 0.9 | (0.64, 1.28) | 0.567 | 0.96 | (0.67, 1.36) | 0.798 |
| Yes | 146 | 37,687 | 3.87 | 28 | 8,949 | 3.13 | 0.81 | (0.54, 1.21) | 0.296 | 0.93 | (0.62, 1.40) | 0.726 |
| Hypertension | ||||||||||||
| No | 139 | 46,700 | 2.98 | 32 | 12,182 | 2.63 | 0.85 | (0.58, 1.25) | 0.416 | 0.87 | (0.59, 1.28) | 0.479 |
| Yes | 169 | 45,894 | 3.68 | 36 | 11,123 | 3.24 | 0.86 | (0.60, 1.24) | 0.428 | 1.01 | (0.70, 1.45) | 0.967 |
| Coronary artery disease | ||||||||||||
| No | 198 | 67,040 | 2.95 | 47 | 17,161 | 2.74 | 0.89 | (0.64, 1.22) | 0.457 | 0.93 | (0.68, 1.29) | 0.681 |
| Yes | 110 | 25,555 | 4.30 | 21 | 6,143 | 3.42 | 0.81 | (0.50, 1.29) | 0.366 | 0.94 | (0.59, 1.51) | 0.802 |
| Chronic kidney disease | ||||||||||||
| No | 228 | 76,269 | 2.99 | 54 | 19,496 | 2.77 | 0.89 | (0.66, 1.20) | 0.456 | 0.94 | (0.70, 1.27) | 0.689 |
| Yes | 80 | 16,325 | 4.90 | 14 | 3,808 | 3.68 | 0.76 | (0.43, 1.34) | 0.339 | 0.92 | (0.52, 1.63) | 0.773 |
| Stroke | ||||||||||||
| No | 225 | 73,841 | 3.05 | 51 | 18,776 | 2.72 | 0.86 | (0.64, 1.17) | 0.346 | 0.93 | (0.69, 1.27) | 0.668 |
| Yes | 83 | 18,754 | 4.43 | 17 | 4,528 | 3.75 | 0.84 | (0.50, 1.42) | 0.512 | 0.97 | (0.57, 1.66) | 0.925 |
| COPD | ||||||||||||
| No | 205 | 70,232 | 2.92 | 51 | 17,928 | 2.85 | 0.94 | (0.69, 1.28) | 0.689 | 1 | (0.73, 1.36) | 0.988 |
| Yes | 103 | 22,362 | 4.61 | 17 | 5,376 | 3.16 | 0.69 | (0.41, 1.15) | 0.151 | 0.83 | (0.50, 1.4) | 0.493 |
| Connective tissue disease | ||||||||||||
| No | 256 | 83,497 | 3.07 | 59 | 21,319 | 2.77 | 0.87 | (0.66, 1.16) | 0.349 | 0.95 | (0.71, 1.27) | 0.740 |
| Yes | 52 | 9,098 | 5.72 | 9 | 1986 | 4.53 | 0.8 | (0.39, 1.63) | 0.545 | 0.91 | (0.44, 1.87) | 0.792 |
| Heart failure | ||||||||||||
| No | 269 | 85,902 | 3.13 | 62 | 21,875 | 2.83 | 0.87 | (0.66, 1.15) | 0.343 | 0.95 | (0.72, 1.25) | 0.697 |
| Yes | 39 | 6,692 | 5.83 | 6 | 1,429 | 4.20 | 0.75 | (0.32, 1.78) | 0.516 | 0.9 | (0.37, 2.17) | 0.817 |
| Malignancy | ||||||||||||
| No | 251 | 82,226 | 3.05 | 57 | 20,935 | 2.72 | 0.87 | (0.65, 1.15) | 0.325 | 0.93 | (0.70, 1.25) | 0.636 |
| Yes | 57 | 10,369 | 5.50 | 11 | 2,369 | 4.64 | 0.84 | (0.44, 1.60) | 0.590 | 1 | (0.52, 1.92) | 0.996 |
| Liver cirrhosis | ||||||||||||
| No | 171 | 60,946 | 2.81 | 44 | 15,577 | 2.83 | 0.97 | (0.70, 1.35) | 0.854 | 1 | (0.72, 1.40) | 0.995 |
| Yes | 137 | 31,649 | 4.33 | 24 | 7,728 | 3.11 | 0.71 | (0.46, 1.10) | 0.126 | 0.85 | (0.54, 1.32) | 0.459 |
| Alcoholic liver disease | ||||||||||||
| No | 271 | 86,283 | 3.14 | 65 | 21,946 | 2.96 | 0.91 | (0.69, 1.20) | 0.503 | 0.98 | (0.74, 1.29) | 0.887 |
| Yes | 37 | 6,311 | 5.86 | 3 | 1,358 | 2.21 | 0.4 | (0.12, 1.28) | 0.122 | 0.47 | (0.14, 1.57) | 0.221 |
| Obesity (numbers of samples in these cell were less than 3 and therefore cannot be compared) | ||||||||||||
| No | 297 | 90,162 | 3.29 | 68 | 22,796 | 2.98 | 0.88 | (0.68, 1.15) | 0.343 | 0.96 | (0.74, 1.25) | 0.768 |
| Yes | 11 | 2,433 | 4.52 | 0 | NA | NA | NA | NA | NA | NA | NA | NA |
| Medication | ||||||||||||
| Metformin | ||||||||||||
| No | 37 | 5,911 | 6.26 | 5 | 1,600 | 3.13 | 0.51 | (0.20, 1.29) | 0.155 | 0.53 | (0.19, 1.42) | 0.206 |
| Yes | 271 | 86,684 | 3.13 | 63 | 21,704 | 2.90 | 0.9 | (0.68, 1.18) | 0.441 | 0.98 | (0.74, 1.30) | 0.908 |
| TZD | ||||||||||||
| No | 221 | 56,829 | 3.89 | 46 | 14,832 | 3.10 | 0.78 | (0.57, 1.07) | 0.124 | 0.89 | (0.65, 1.23) | 0.479 |
| Yes | 87 | 35,765 | 2.43 | 22 | 8,472 | 2.60 | 1.03 | (0.65, 1.65) | 0.891 | 1.06 | (0.65, 1.71) | 0.821 |
| Acarbose | ||||||||||||
| No | 225 | 63906 | 3.52 | 45 | 16,044 | 2.81 | 0.77 | (0.56, 1.06) | 0.112 | 0.88 | (0.63, 1.21) | 0.427 |
| Yes | 83 | 28689 | 2.89 | 23 | 7,260 | 3.17 | 1.08 | (0.68, 1.71) | 0.749 | 1.08 | (0.67, 1.74) | 0.737 |
| SGLT2 inhibitor | ||||||||||||
| No | 285 | 73,937 | 3.85 | 54 | 16,036 | 3.37 | 0.88 | (0.66, 1.18) | 0.387 | 0.87 | (0.65, 1.17) | 0.362 |
| Yes | 23 | 18,657 | 1.23 | 14 | 7,268 | 1.93 | 1.21 | (0.62, 2.37) | 0.579 | 1.1 | (0.55, 2.22) | 0.780 |
| Sulfonylurea | ||||||||||||
| No | 69 | 18,015 | 3.83 | 17 | 4,402 | 3.86 | 1.02 | (0.60, 1.73) | 0.950 | 1.24 | (0.72, 2.14) | 0.440 |
| Yes | 239 | 74,579 | 3.20 | 51 | 18,903 | 2.70 | 0.81 | (0.60, 1.10) | 0.177 | 0.86 | (0.64, 1.18) | 0.353 |
| DPP-4 inhibitor | ||||||||||||
| No | 211 | 56,832 | 3.71 | 33 | 13,138 | 2.51 | 0.68 | (0.47, 0.98)* | 0.040 | 0.75 | (0.52, 1.09) | 0.132 |
| Yes | 97 | 35,762 | 2.71 | 35 | 10,167 | 3.44 | 1.17 | (0.79, 1.73) | 0.424 | 1.23 | (0.82, 1.84) | 0.310 |
| Insulin | ||||||||||||
| No | 112 | 37,656 | 2.97 | 23 | 8,708 | 2.64 | 0.91 | (0.58, 1.43) | 0.682 | 0.93 | (0.59, 1.47) | 0.765 |
| Yes | 196 | 54,938 | 3.57 | 45 | 14,597 | 3.08 | 0.82 | (0.59, 1.14) | 0.237 | 0.93 | (0.67, 1.30) | 0.685 |
| Oral medication combination | ||||||||||||
| No | 267 | 75,194 | 3.55 | 61 | 18,888 | 3.23 | 0.89 | (0.68, 1.18) | 0.428 | 0.98 | (0.74, 1.30) | 0.908 |
| Yes | 41 | 17,400 | 2.36 | 7 | 4,416 | 1.59 | 0.61 | (0.27, 1.37) | 0.233 | 0.66 | (0.29, 1.50) | 0.319 |
*p < 0.05, **p < 0.01, ***p < 0.001.
PY, person-years; IR, incidence rate, per 1,000 person-years; HR, hazard ratio; CI, confidence interval.
Demographic factors include age, gender, urbanization level and enrollee category.
1:4 propensity score matching.
Fully adjusted model: Adjusted for demographic factors, comorbidities and medication.
The incidence of depression or anxiety decreased with increasing duration of treatment after the initiation of GLP1-RA medication (Table 5). The reduction trends were significant after controlling for all covariates. After taking the medicine for 180 days or longer, rates of incidence of depression or anxiety reduced to 2.19 and 2.93 per 1,000 person-years, respectively. The risk of having any depression or anxiety fell to an aHR of 0.5 (95% CI: 0.36–0.69) in GLP1-RA users, compared to non-users. We also evaluated the effect of different GLP1-RA on anxiety or depression in Tables 6 and 7. Our results revealed that dulaglutide could significantly reduce risks of anxiety and depression, while liraglutide and exenatide showed no significant reductions on risks of either anxiety or depression.
TABLE 5.
Risk of depression and anxiety associated with duration of GLP1-RA use.
| Variables | Any depression or anxiety (n = 1,014) | cHR | (95% CI) | aHR | (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Event | PY | IR | |||||
| non-use GLP1-RA | 857 | 91,546 | 9.36 | 1.00 | (Reference) | 1.00 | (Reference) |
| GLP1-RA drug days | |||||||
| 30–90 days | 72 | 7,542 | 9.55 | 1.01 | (0.79, 1.29) | 1.22 | (0.96, 1.56) |
| 91–180 days | 47 | 7,380 | 6.37 | 0.68 | (0.5, 0.91)** | 0.76 | (0.57, 1.02) |
| >180 | 38 | 8,169 | 4.65 | 0.49 | (0.35, 0.67)*** | 0.5 | (0.36, 0.69)*** |
| Variables | Anxiety (n = 772) | cHR | (95% CI) | aHR | (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Event | PY | IR | |||||
| non-use GLP1-RA | 656 | 91,838 | 7.14 | 1.00 | (Reference) | 1.00 | (Reference) |
| GLP1-RA drug days | |||||||
| 30–90 days | 56 | 7,570 | 7.40 | 1.03 | (0.79, 1.36) | 1.3 | (0.98, 1.71) |
| 91–180 days | 36 | 7,403 | 4.86 | 0.68 | (0.48, 0.95)* | 0.77 | (0.55, 1.07) |
| >180 days | 24 | 8,185 | 2.93 | 0.4 | (0.27, 0.61)*** | 0.41 | (0.27, 0.61)*** |
| Variables | Depression (n = 376) | cHR | (95% CI) | aHR | (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Event | PY | IR | |||||
| non-use GLP1-RA | 308 | 92,594 | 3.33 | 1.00 | (Reference) | 1.00 | (Reference) |
| GLP1-RA drug days | |||||||
| 30–90 days | 32 | 7,629 | 4.19 | 1.24 | (0.86, 1.78) | 1.4 | (0.97, 2.03) |
| 91–180 days | 18 | 7,460 | 2.41 | 0.71 | (0.44, 1.15) | 0.79 | (0.49, 1.27) |
| >180 days | 18 | 8,215 | 2.19 | 0.63 | (0.39, 1.02) | 0.68 | (0.42, 1.10) |
PY, person-years; IR, incidence rate, per 1,000 person-years; HR, hazard ratio; CI, confidence interval.
Demographic factors include age, gender, urbanization level and enrollee category1:4 propensity score matching.
TABLE 6.
Cox proportional hazard model estimated GLP1-RA hazard ratio of anxiety among different GLP1-RA types.
| Variables | Anxiety | cHR | (95% CI) | aHR # | (95% CI) | ||
|---|---|---|---|---|---|---|---|
| n | PY | IR | |||||
| Non-use GLP1-RA | 688 | 102,030 | 6.74 | 1.00 | (Reference) | 1.00 | (Reference) |
| Liraglutide | 84 | 129,66 | 6.48 | 0.91 | (0.72, 1.14) | 1.07 | (0.85, 1.35) |
| Non-use GLP1-RA | 741 | 110,339 | 6.72 | 1.00 | (Reference) | 1.00 | (Reference) |
| Exenatide | 31 | 4,658 | 6.66 | 0.92 | (0.64, 1.33) | 1.3 | (0.90, 1.89) |
| Non-use GLP1-RA | 750 | 105,355 | 7.12 | 1.00 | (Reference) | 1.00 | (Reference) |
| Dulaglutide | 22 | 9,642 | 2.28 | 0.34 | (0.22, 0.52)*** | 0.32 | (0.21, 0.49)*** |
PY: person-years; IR: incidence rate per 1,000 person-years; cHR: crude hazard ratio; aHR: adjusted hazard ratio.
1:4 propensity score matching.
: Adjusted by sex, age, urbanization level, enrollee category, comorbidities and medication.
*p < 0.05, **p < 0.01, ***p < 0.001.
TABLE 7.
Cox proportional hazard model estimated GLP1-RA hazard ratio of depression among different GLP1-RA types.
| Variables | Depression | cHR | (95% CI) | aHR # | (95% CI) | ||
|---|---|---|---|---|---|---|---|
| n | PY | IR | |||||
| Non-use GLP1-RA | 329 | 102,822 | 3.20 | 1.00 | (Reference) | 1.00 | (Reference) |
| Liraglutide | 47 | 13,077 | 3.59 | 1 | (0.73, 1.36) | 1.13 | (0.82, 1.54) |
| Non-use GLP1-RA | 356 | 111,188 | 3.20 | 1.00 | (Reference) | 1.00 | (Reference) |
| Exenatide | 20 | 4,711 | 4.25 | 1.13 | (0.71, 1.78) | 1.43 | (0.90, 2.28) |
| Non-use GLP1-RA | 363 | 106,247 | 3.42 | 1.00 | (Reference) | 1.00 | (Reference) |
| Dulaglutide | 13 | 9,652 | 1.35 | 0.44 | (0.25, 0.78)** | 0.45 | (0.26, 0.79)** |
PY: person-years; IR: incidence rate per 1,000 person-years; cHR: crude hazard ratio; aHR: adjusted hazard ratio.
1:4 propensity score matching.
: adjusted by sex, age, urbanization level, enrollee category, comorbidities and medication; *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
To our knowledge, this study represents the largest population-based analyses investigating whether GLP1-RA medication is associated with reduced risks of depression or anxiety in DM patients. GLP1-RA users exhibited a significant risk reduction for anxiety, and a moderate reduction for depression, compared with non-users. This treatment effectiveness on anxiety was observed in female users but not in male users. The effectiveness increased with the duration of medication and the significant risk reduction was observed after 6-months or longer therapy. Further age-specific stratified analyses revealed significant reduction of anxiety in patients between 40 and 60 years. GLP1-RA users taking metformin or sulfonylurea at the same time had decreased risk of anxiety, which was also noted in patients with hypertension. As for the specific effectiveness of each GLP1-RA, dulaglutide use could significantly decrease risks of both anxiety and depression, while liraglutide and exenatide showed no significant effect on reductions of either anxiety or depression.
Our findings on reduced risks of anxiety or depression associated with GLP1-RA use were consistent with some previous findings (Bode et al., 2010; Grant et al., 2011; Idris et al., 2013; de Wit et al., 2014), but contrast with others that showed no significant association between GLP1-RA medication and depression (de Wit et al., 2016; O'Neil et al., 2017; Kahal et al., 2019). However, studies with non-significant findings were either based on small sample sizes or were not focused on DM patients. Although Eren-Yazicioglu et al. (2021) reported that exenatide was associated with higher depressive scores indirectly through its effect on perceived stress, their study participants already had lifetime or current psychiatric diagnosis at baseline, which was different from our study (we excluded those with prior anxiety or depression diagnosis at baseline).
Neuroinflammation might be a critical factor for the onset, deterioration, relapse, and maintenance of depression or anxiety (Kopschina Feltes et al., 2017; Paudel et al., 2018; Woelfer et al., 2019). GLP-1 has been reported to promote productions of anti-inflammatory cytokines in various organs, including the adipose tissue, the pancreas, and the brain (Dobrian et al., 2011; Lee et al., 2012; Darsalia et al., 2014; Augestad et al., 2020; Reed et al., 2020). Kim et al. (2020) summarized that GLP1-RA may ameliorate depression by reducing neuroinflammation, balancing neurotransmitter homeostasis, promoting neuronal differentiation and neural stem cell proliferation and improving synaptic function. Albeit we failed to find significant reductions in the overall incidence of depression in GLP1-RA users, our subgroup analysis showed that patients using dulaglutide were at a lower risk of depression than non-users. GLP1-RA treatment-associated decrease in the risk of depression or anxiety in DM patients may be driven by combinations of anti-inflammation, better glycemic control, greater weight loss, and reduced concern about weight gain (Bode et al., 2010).
Women are generally more likely than men to develop an anxiety disorder (Leach et al., 2008; McLean and Anderson, 2009). The present study demonstrated that women were also at a higher incidence of anxiety risk than men even after using GLP1-RA. However, female users had a greater benefit from GLP1-RA therapy, than male users. The treatment effectiveness of GLP1-RA might be due to interactions between GLP-1R, the central and peripheral nervous system, estrogen, and the anorexic actions. GLP-1 is secreted from gut enteroendocrine cells and brain preproglucagon (PPG) neurons, which are known as the peripheral and central GLP-1 systems (Brierley et al., 2021). GLP-1 secreted by the intestine releases into the hepatoportal vein. This activates the vagus nerve to generate a neural signal towards the brain stem, such as the nucleus of the solitary tract nucleus (NTS) and the area postrema (AP), which send axons to the hypothalamus to release GLP-1 and activate the receptors. A new signal is then sent towards peripheral tissues through the autonomic nervous system (ANS) to regulate numerous functions (Cabou and Burcelin, 2011). However, research indicated that the intake-inhibitory effects of the GLP-1 RA, exendin-4 and liraglutide, were mediated by activation of GLP-1R expressed on sub-diaphragmatic vagal afferents as well as in the brain (Kanoski et al., 2011). That is, central and peripheral GLP-1 systems suppress eating via independent gut-brain circuits (Brierley et al., 2021). Central injections of GLP1-RA strongly decrease food intake, due to GLP-1R expression targeting the hypothalamic and brainstem inducing anorexic action. (Larsen et al., 1997; McMahon and Wellman, 1998; Hayes et al., 2008; Hayes et al., 2010; Kanoski et al., 2011). The same CNS regions are implicated for the anorexic action of estrogens (Palmer and Gray, 1986; Osterlund et al., 1998; Merchenthaler et al., 2004; Musatov et al., 2007), and may provide neuroanatomical grounds for the interaction between GLP-1 receptor and gender (Richard et al., 2016) in our results. Such a mechanism was also supported by Richard et al. (2016), who reported that women are more sensitive than men to the food reward impact of central GLP-1 receptor activation. In addition, individuals with obesity and overweight are more likely to have anxiety than non-obese persons (Amiri and Behnezhad, 2019). Since GLP1-RA is well known for its effect on weight loss, this might lead to the elimination of anxiety in women. Furthermore, younger DM patients with fewer comorbidities may be more concerned about body weight. They were probably more willing to adhere to the prescription of GLP1-RA and thus the risk of anxiety might be decreased.
Our study demonstrated that the combination of GLP1-RA with metformin exerted an anxiolytic effect, which was consistent with previous preclinical studies (Fan et al., 2019; Ji et al., 2019; Zemdegs et al., 2019; Turan et al., 2021). We also found that GLP1-RA users taking sulfonylurea had lower risk of anxiety, which may be due to the lower dose of sulfonylurea needed after using GLP1-RA, which could decrease the hypoglycemic risk of sulfonylurea. In our study, GLP1-RA users not using TZD also had a reduced risk of anxiety. This might be owing to the lowered side effect of weight gain from not using TZD. The finding that GLP1-RA users not using DPP-4 inhibitors had better reduction of anxiety was somewhat surprising. Numerous studies have reported that patients with mood and anxiety-related disorders are characterized by increased circulating inflammatory cytokines, including interleukin (IL)-1, IL-6, tumor necrosis factor (TNF), their soluble receptors, and acute phase reactants such as C-reactive protein (CRP) (Maes et al., 1992; Maes, 1999; Sluzewska, 1999; Michopoulos et al., 2017). DPP-4 is a novel adipokine secreted from adipose tissue (Lamers et al., 2011) with a pro-inflammatory role (Cordero et al., 1997). Obese patients may have higher DPP-4 levels from adipose tissue, and DPP-4 might induce depression by activating the immune system and promoting proinflammatory cytokine secretion. Previous studies revealed that although very low levels of the DPP-4 inhibitors were found in the brain (Fuchs et al., 2009; Fura et al., 2009), its neuroprotective effects might be more attributed to peripheral functions rather than directly in the central nervous system (Darsalia et al., 2013; Lin and Huang, 2016). On the other hand, GLP-1 RA crossed the BBB successfully (Hunter and Hölscher, 2012; Athauda and Foltynie, 2016). In Taiwan, combination usage of GLP1-RA and DPP-4 inhibitor is not reimbursed by health insurance. That is, either kind of medication has to be paid by the patient himself/herself. This may be another reason why GLP1-RA users not using DPP-4 inhibitors had better reduction of anxiety, since when the patient needs to pay for the other hypoglycemic agent, it may indicate that his or her glucose control is poorer, and may have more anxiety regarding health conditions compared to those who did not combine DPP-4 inhibitor with GLP1-RA.
Our study found different effects from each subtype of GLP1-RA on anxiety or depression, and dulaglutide was the only subtype associated with significant reduction in such risks. The distinct result may be due to differences in molecular structure that affects the potency, duration, and frequency of use (Monami et al., 2009; Buse et al., 2011). Dulaglutide may have better reductions of anxiety or depression due to its weekly injection formulation, when compared with liraglutide (daily injection). Exenatide and liraglutide also showed neuroprotective effect in few clinical studies among patients with Parkinson’s disease, Alzheimer’s disease, ischemia, traumatic brain injury, neuropathies, neurogenesis, or epilepsy, but not in patients with anxiety disorder as in our study. Erbil et al. (2019) suggested that not all GLP1-RAs have the same neuroprotective effect and the effect of GLP1-RAs on reversing neurodegenerative or neuro-destructive processes might be time and dose-dependent. Behavioral assessments in these preclinical studies are limited to the lesion model and may not cover all aspects of cortical function and all cortical layers. Hence, more studies investigating cortical effects of different subtypes of GLP-1 on depression or anxiety at the whole brain level, with longer assessment periods, and complete neuropsychological evaluations or behavioral observations are still needed both for preclinical and clinical studies.
The strength of this study lies in the very large number of patients used, the largest to-date, that focused on anxiety or depression associated with using GLP1-RA for the treatment for DM. We were able to conduct a longer duration follow-up than previous studies. The stratified data analysis was able to evaluate factors associated with treatment effectiveness. The identification of anxiety and depression were based on at least two clinical diagnoses rather than by screening instruments. However, several limitations should be acknowledged for this study. First, the study was observational, rather than a randomized control design, for the population of Taiwan. The generalizability of findings to other population may be restricted. Second, our efforts to match and adjust for possible confounding factors might be biased by unmeasured or unknown confounders not available in the NHIRD. Information on some potential confounders, including body weight, smoking, diet, exercise, stress, or family history, was not available in the NHIRD, the bias associated with unmeasured factors was unclear, despite our efforts to match study groups and controlling for available variables. Third, information on changes in body weight was unavailable in the NHIRD, we were unable to evaluate risks of anxiety or depression related to body weight changes.
Conclusion
This population-based study demonstrated that recipients of GLP1-RA treatment for DM were at a significantly decreased risk of anxiety. However, the effectiveness was significant only for female users and patients using the medicine for longer than 180 days. The treatment effectiveness was moderate in reducing depression risk, especially for patients that used dulaglutide. These findings strengthen previous research advocating the antidepressant or anxiolytic effects of GLP1-RA.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by The Mackay Memorial Hospital Committee Review Board approved the study protocol (IRB 20MMHIS431e approved with exempt review). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
Conceived and designed the analysis: W-HT, S-IW, M-CT, and F-CS. Contributed data or analysis tools: L-TC and Y-HS. Wrote the paper: W-HT, S-IW, and F-CS.
Funding
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial Center (No. MOHW109-TDU-B-212-114004), MOST Clinical Trial Consortium for Stroke (No. MOST 109-2321-B-039-002), China Medical University Hospital (No. DMR-108-154), Tseng-Lien Lin Foundation of Taichung, Taiwan.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Alhussain F., Alruthia Y., Al-Mandeel H., Bellahwal A., Alharbi F., Almogbel Y., et al. (2020). Metformin Improves the Depression Symptoms of Women with Polycystic Ovary Syndrome in a Lifestyle Modification Program. Patient Prefer Adherence 14, 737–746. 10.2147/PPA.S244273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri S., Behnezhad S. (2019). Obesity and Anxiety Symptoms: a Systematic Review and Meta-Analysis. Neuropsychiatr 33, 72–89. 10.1007/s40211-019-0302-9 [DOI] [PubMed] [Google Scholar]
- Anderson R. J., Freedland K. E., Clouse R. E., Lustman P. J. (2001). The Prevalence of Comorbid Depression in Adults with Diabetes: a Meta-Analysis. Diabetes Care 24, 1069–1078. 10.2337/diacare.24.6.1069 [DOI] [PubMed] [Google Scholar]
- Athauda D., Foltynie T. (2016). The Glucagon-like Peptide 1 (GLP) Receptor as a Therapeutic Target in Parkinson's Disease: Mechanisms of Action. Drug Discov. Today 21, 802–818. 10.1016/j.drudis.2016.01.013 [DOI] [PubMed] [Google Scholar]
- Augestad I. L., Pintana H., Larsson M., Krizhanovskii C., Nyström T., Klein T., et al. (2020). Regulation of Glycemia in the Recovery Phase after Stroke Counteracts the Detrimental Effect of Obesity-Induced Type 2 Diabetes on Neurological Recovery. Diabetes 69, 1961–1973. 10.2337/db20-0095 [DOI] [PubMed] [Google Scholar]
- Bode B. W., Testa M. A., Magwire M., Hale P. M., Hammer M., Blonde L., et al. (2010). Patient-reported Outcomes Following Treatment with the Human GLP-1 Analogue Liraglutide or Glimepiride in Monotherapy: Results from a Randomized Controlled Trial in Patients with Type 2 Diabetes. Diabetes Obes. Metab. 12, 604–612. 10.1111/j.1463-1326.2010.01196.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley D. I., Holt M. K., Singh A., De Araujo A., Mcdougle M., Vergara M., et al. (2021). Central and Peripheral GLP-1 Systems Independently Suppress Eating. Nat. Metab. 3, 258–273. 10.1038/s42255-021-00344-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse J. B., Garber A., Rosenstock J., Schmidt W. E., Brett J. H., Videbæk N., et al. (2011). Liraglutide Treatment Is Associated with a Low Frequency and Magnitude of Antibody Formation with No Apparent Impact on Glycemic Response or Increased Frequency of Adverse Events: Results from the Liraglutide Effect and Action in Diabetes (LEAD) Trials. J. Clin. Endocrinol. Metab. 96, 1695–1702. 10.1210/jc.2010-2822 [DOI] [PubMed] [Google Scholar]
- Cabou C., Burcelin R. (2011). GLP-1, the Gut-Brain, and Brain-Periphery Axes. Rev. Diabet Stud. 8, 418–431. 10.1900/RDS.2011.8.418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H. Y., Weiner J. P., Richards T. M., Bleich S. N., Segal J. B. (2012). Validating the Adapted Diabetes Complications Severity Index in Claims Data. Am. J. Manag. Care 18, 721–726. [PubMed] [Google Scholar]
- Chien I. C., Kuo C. C., Bih S. H., Chou Y. J., Lin C. H., Lee C. H., et al. (2007). The Prevalence and Incidence of Treated Major Depressive Disorder Among National Health Insurance Enrollees in Taiwan, 1996 to 2003. Can. J. Psychiatry 52, 28–36. 10.1177/070674370705200106 [DOI] [PubMed] [Google Scholar]
- Chima C. C., Salemi J. L., Wang M., Mejia De Grubb M. C., Gonzalez S. J., Zoorob R. J. (2017). Multimorbidity Is Associated with Increased Rates of Depression in Patients Hospitalized with Diabetes Mellitus in the United States. J. Diabetes Complications 31, 1571–1579. 10.1016/j.jdiacomp.2017.08.001 [DOI] [PubMed] [Google Scholar]
- Cordero O. J., Salgado F. J., Viñuela J. E., Nogueira M. (1997). Interleukin-12 Enhances CD26 Expression and Dipeptidyl Peptidase IV Function on Human Activated Lymphocytes. Immunobiology 197, 522–533. 10.1016/s0171-2985(97)80084-8 [DOI] [PubMed] [Google Scholar]
- Darsalia V., Hua S., Larsson M., Mallard C., Nathanson D., Nyström T., et al. (2014). Exendin-4 Reduces Ischemic Brain Injury in normal and Aged Type 2 Diabetic Mice and Promotes Microglial M2 Polarization. PLoS One 9, e103114. 10.1371/journal.pone.0103114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsalia V., Ortsäter H., Olverling A., Darlöf E., Wolbert P., Nyström T., et al. (2013). The DPP-4 Inhibitor Linagliptin Counteracts Stroke in the normal and Diabetic Mouse Brain: a Comparison with Glimepiride. Diabetes 62, 1289–1296. 10.2337/db12-0988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit H. M., Vervoort G. M., Jansen H. J., De Galan B. E., Tack C. J. (2016). Durable Efficacy of Liraglutide in Patients with Type 2 Diabetes and Pronounced Insulin-Associated Weight Gain: 52-week Results from the Effect of Liraglutide on Insulin-Associated wEight GAiN in Patients with Type 2 Diabetes' (ELEGANT) Randomized Controlled Trial. J. Intern. Med. 279, 283–292. 10.1111/joim.12447 [DOI] [PubMed] [Google Scholar]
- De Wit H. M., Vervoort G. M., Jansen H. J., De Grauw W. J., De Galan B. E., Tack C. J. (2014). Liraglutide Reverses Pronounced Insulin-Associated Weight Gain, Improves Glycaemic Control and Decreases Insulin Dose in Patients with Type 2 Diabetes: a 26 Week, Randomised Clinical Trial (ELEGANT). Diabetologia 57, 1812–1819. 10.1007/s00125-014-3302-0 [DOI] [PubMed] [Google Scholar]
- Dobrian A. D., Ma Q., Lindsay J. W., Leone K. A., Ma K., Coben J., et al. (2011). Dipeptidyl Peptidase IV Inhibitor Sitagliptin Reduces Local Inflammation in Adipose Tissue and in Pancreatic Islets of Obese Mice. Am. J. Physiol. Endocrinol. Metab. 300, E410–E421. 10.1152/ajpendo.00463.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbil D., Eren C. Y., Demirel C., Küçüker M. U., Solaroğlu I., Eser H. Y. (2019). GLP-1's Role in Neuroprotection: a Systematic Review. Brain Inj. 33, 734–819. 10.1080/02699052.2019.1587000 [DOI] [PubMed] [Google Scholar]
- Eren-Yazicioglu C. Y., Kara B., Sancak S., Uysal S. P., Yazici D., Okuroglu N., et al. (2021). Effect of Exenatide Use on Cognitive and Affective Functioning in Obese Patients with Type 2 Diabetes Mellitus: Exenatide Use Mediates Depressive Scores through Increased Perceived Stress Levels. J. Clin. Psychopharmacol. 41, 428–435. 10.1097/JCP.0000000000001409 [DOI] [PubMed] [Google Scholar]
- Fan J., Li D., Chen H. S., Huang J. G., Xu J. F., Zhu W. W., et al. (2019). Metformin Produces Anxiolytic-like Effects in Rats by Facilitating GABAA Receptor Trafficking to Membrane. Br. J. Pharmacol. 176, 297–316. 10.1111/bph.14519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler M. J. (2008). Microvascular and Macrovascular Complications of Diabetes. Clin. Diabetes 26, 77–82. 10.2337/diaclin.26.2.77 [DOI] [Google Scholar]
- Fuchs H., Binder R., Greischel A. (2009). Tissue Distribution of the Novel DPP-4 Inhibitor BI 1356 Is Dominated by Saturable Binding to its Target in Rats. Biopharm. Drug Dispos 30, 229–240. 10.1002/bdd.662 [DOI] [PubMed] [Google Scholar]
- Fura A., Khanna A., Vyas V., Koplowitz B., Chang S. Y., Caporuscio C., et al. (2009). Pharmacokinetics of the Dipeptidyl Peptidase 4 Inhibitor Saxagliptin in Rats, Dogs, and Monkeys and Clinical Projections. Drug Metab. Dispos 37, 1164–1171. 10.1124/dmd.108.026088 [DOI] [PubMed] [Google Scholar]
- Gavard J. A., Lustman P. J., Clouse R. E. (1993). Prevalence of Depression in Adults with Diabetes. An Epidemiological Evaluation. Diabetes Care 16, 1167–1178. 10.2337/diacare.16.8.1167 [DOI] [PubMed] [Google Scholar]
- Grant P., Lipscomb D., Quin J. (2011). Psychological and Quality of Life Changes in Patients Using GLP-1 Analogues. J. Diabetes Complications 25, 244–246. 10.1016/j.jdiacomp.2011.03.002 [DOI] [PubMed] [Google Scholar]
- Gregg E. W., Sattar N., Ali M. K. (2016). The Changing Face of Diabetes Complications. Lancet Diabetes Endocrinol. 4, 537–547. 10.1016/S2213-8587(16)30010-9 [DOI] [PubMed] [Google Scholar]
- Harding J. L., Pavkov M. E., Magliano D. J., Shaw J. E., Gregg E. W. (2019). Global Trends in Diabetes Complications: a Review of Current Evidence. Diabetologia 62, 3–16. 10.1007/s00125-018-4711-2 [DOI] [PubMed] [Google Scholar]
- Hayes M. R., De Jonghe B. C., Kanoski S. E. (2010). Role of the Glucagon-Like-Peptide-1 Receptor in the Control of Energy Balance. Physiol. Behav. 100, 503–510. 10.1016/j.physbeh.2010.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes M. R., Skibicka K. P., Grill H. J. (2008). Caudal Brainstem Processing Is Sufficient for Behavioral, Sympathetic, and Parasympathetic Responses Driven by Peripheral and Hindbrain Glucagon-Like-Peptide-1 Receptor Stimulation. Endocrinology 149, 4059–4068. 10.1210/en.2007-1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter K., Hölscher C. (2012). Drugs Developed to Treat Diabetes, Liraglutide and Lixisenatide, Cross the Blood Brain Barrier and Enhance Neurogenesis. BMC Neurosci. 13, 33. 10.1186/1471-2202-13-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idris I., Abdulla H., Tilbrook S., Dean R., Ali N. (2013). Exenatide Improves Excessive Daytime Sleepiness and Wakefulness in Obese Patients with Type 2 Diabetes without Obstructive Sleep Apnoea. J. Sleep Res. 22, 70–75. 10.1111/j.1365-2869.2012.01030.x [DOI] [PubMed] [Google Scholar]
- Ji S., Wang L., Li L. (2019). Effect of Metformin on Short-Term High-Fat Diet-Induced Weight Gain and Anxiety-like Behavior and the Gut Microbiota. Front. Endocrinol. (Lausanne) 10, 704. 10.3389/fendo.2019.00704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahal H., Kilpatrick E., Rigby A., Coady A., Atkin S. (2019). The Effects of Treatment with Liraglutide on Quality of Life and Depression in Young Obese Women with PCOS and Controls. Gynecol. Endocrinol. 35, 142–145. 10.1080/09513590.2018.1505848 [DOI] [PubMed] [Google Scholar]
- Kanoski S. E., Fortin S. M., Arnold M., Grill H. J., Hayes M. R. (2011). Peripheral and central GLP-1 Receptor Populations Mediate the Anorectic Effects of Peripherally Administered GLP-1 Receptor Agonists, Liraglutide and Exendin-4. Endocrinology 152, 3103–3112. 10.1210/en.2011-0174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaledi M., Haghighatdoost F., Feizi A., Aminorroaya A. (2019). The Prevalence of Comorbid Depression in Patients with Type 2 Diabetes: an Updated Systematic Review and Meta-Analysis on Huge Number of Observational Studies. Acta Diabetol. 56, 631–650. 10.1007/s00592-019-01295-9 [DOI] [PubMed] [Google Scholar]
- Kim Y. K., Kim O. Y., Song J. (2020). Alleviation of Depression by Glucagon-like Peptide 1 through the Regulation of Neuroinflammation, Neurotransmitters, Neurogenesis, and Synaptic Function. Front. Pharmacol. 11, 1270. 10.3389/fphar.2020.01270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopschina Feltes P., Doorduin J., Klein H. C., Juárez-Orozco L. E., Dierckx R. A., Moriguchi-Jeckel C. M., et al. (2017). Anti-inflammatory Treatment for Major Depressive Disorder: Implications for Patients with an Elevated Immune Profile and Non-responders to Standard Antidepressant Therapy. J. Psychopharmacol. 31, 1149–1165. 10.1177/0269881117711708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers D., Famulla S., Wronkowitz N., Hartwig S., Lehr S., Ouwens D. M., et al. (2011). Dipeptidyl Peptidase 4 Is a Novel Adipokine Potentially Linking Obesity to the Metabolic Syndrome. Diabetes 60, 1917–1925. 10.2337/db10-1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen P. J., Tang-Christensen M., Holst J. J., Orskov C. (1997). Distribution of Glucagon-like Peptide-1 and Other Preproglucagon-Derived Peptides in the Rat Hypothalamus and Brainstem. Neuroscience 77, 257–270. 10.1016/s0306-4522(96)00434-4 [DOI] [PubMed] [Google Scholar]
- Leach L. S., Christensen H., Mackinnon A. J., Windsor T. D., Butterworth P. (2008). Gender Differences in Depression and Anxiety across the Adult Lifespan: the Role of Psychosocial Mediators. Soc. Psychiatry Psychiatr. Epidemiol. 43, 983–998. 10.1007/s00127-008-0388-z [DOI] [PubMed] [Google Scholar]
- Lee Y. S., Park M. S., Choung J. S., Kim S. S., Oh H. H., Choi C. S., et al. (2012). Glucagon-like Peptide-1 Inhibits Adipose Tissue Macrophage Infiltration and Inflammation in an Obese Mouse Model of Diabetes. Diabetologia 55, 2456–2468. 10.1007/s00125-012-2592-3 [DOI] [PubMed] [Google Scholar]
- Lin C. L., Huang C. N. (2016). The Neuroprotective Effects of the Anti-diabetic Drug Linagliptin against Aβ-Induced Neurotoxicity. Neural Regen. Res. 11, 236–237. 10.4103/1673-5374.177724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustman P. J., Anderson R. J., Freedland K. E., De Groot M., Carney R. M., Clouse R. E. (2000). Depression and Poor Glycemic Control: a Meta-Analytic Review of the Literature. Diabetes Care 23, 934–942. 10.2337/diacare.23.7.934 [DOI] [PubMed] [Google Scholar]
- Maes M., Lambrechts J., Bosmans E., Jacobs J., Suy E., Vandervorst C., et al. (1992). Evidence for a Systemic Immune Activation during Depression: Results of Leukocyte Enumeration by Flow Cytometry in Conjunction with Monoclonal Antibody Staining. Psychol. Med. 22, 45–53. 10.1017/s0033291700032712 [DOI] [PubMed] [Google Scholar]
- Maes M. (1999). Major Depression and Activation of the Inflammatory Response System. Adv. Exp. Med. Biol. 461, 25–46. 10.1007/978-0-585-37970-8_2 [DOI] [PubMed] [Google Scholar]
- Mclean C. P., Anderson E. R. (2009). Brave Men and Timid Women? A Review of the Gender Differences in Fear and Anxiety. Clin. Psychol. Rev. 29, 496–505. 10.1016/j.cpr.2009.05.003 [DOI] [PubMed] [Google Scholar]
- Mcmahon L. R., Wellman P. J. (1998). PVN Infusion of GLP-1-(7-36) Amide Suppresses Feeding but Does Not Induce Aversion or Alter Locomotion in Rats. Am. J. Physiol. 274, R23–R29. 10.1152/ajpregu.1998.274.1.R23 [DOI] [PubMed] [Google Scholar]
- Merchenthaler I., Lane M. V., Numan S., Dellovade T. L. (2004). Distribution of Estrogen Receptor Alpha and Beta in the Mouse central Nervous System: In Vivo Autoradiographic and Immunocytochemical Analyses. J. Comp. Neurol. 473, 270–291. 10.1002/cne.20128 [DOI] [PubMed] [Google Scholar]
- Michopoulos V., Powers A., Gillespie C. F., Ressler K. J., Jovanovic T. (2017). Inflammation in Fear- and Anxiety-Based Disorders: PTSD, GAD, and beyond. Neuropsychopharmacology 42, 254–270. 10.1038/npp.2016.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monami M., Marchionni N., Mannucci E. (2009). Glucagon-like Peptide-1 Receptor Agonists in Type 2 Diabetes: a Meta-Analysis of Randomized Clinical Trials. Eur. J. Endocrinol. 160, 909–917. 10.1530/EJE-09-0101 [DOI] [PubMed] [Google Scholar]
- Moulton C. D., Hopkins C. W. P., Ismail K., Stahl D. (2018). Repositioning of Diabetes Treatments for Depressive Symptoms: A Systematic Review and Meta-Analysis of Clinical Trials. Psychoneuroendocrinology 94, 91–103. 10.1016/j.psyneuen.2018.05.010 [DOI] [PubMed] [Google Scholar]
- Musatov S., Chen W., Pfaff D. W., Mobbs C. V., Yang X. J., Clegg D. J., et al. (2007). Silencing of Estrogen Receptor Alpha in the Ventromedial Nucleus of Hypothalamus Leads to Metabolic Syndrome. Proc. Natl. Acad. Sci. U S A. 104, 2501–2506. 10.1073/pnas.0610787104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'neil P. M., Aroda V. R., Astrup A., Kushner R., Lau D. C. W., Wadden T. A., et al. (2017). Neuropsychiatric Safety with Liraglutide 3.0 Mg for Weight Management: Results from Randomized Controlled Phase 2 and 3a Trials. Diabetes Obes. Metab. 19, 1529–1536. 10.1111/dom.12963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund M., Kuiper G. G., Gustafsson J. A., Hurd Y. L. (1998). Differential Distribution and Regulation of Estrogen Receptor-Alpha and -beta mRNA within the Female Rat Brain. Brain Res. Mol. Brain Res. 54, 175–180. 10.1016/s0169-328x(97)00351-3 [DOI] [PubMed] [Google Scholar]
- Palmer K., Gray J. M. (1986). Central vs. Peripheral Effects of Estrogen on Food Intake and Lipoprotein Lipase Activity in Ovariectomized Rats. Physiol. Behav. 37, 187–189. 10.1016/0031-9384(86)90404-x [DOI] [PubMed] [Google Scholar]
- Paudel Y. N., Shaikh M. F., Shah S., Kumari Y., Othman I. (2018). Role of Inflammation in Epilepsy and Neurobehavioral Comorbidities: Implication for Therapy. Eur. J. Pharmacol. 837, 145–155. 10.1016/j.ejphar.2018.08.020 [DOI] [PubMed] [Google Scholar]
- Pozzi M., Mazhar F., Peeters G. G. A. M., Vantaggiato C., Nobile M., Clementi E., et al. (2019). A Systematic Review of the Antidepressant Effects of Glucagon-like Peptide 1 (GLP-1) Functional Agonists: Further Link between Metabolism and Psychopathology: Special Section on "Translational and Neuroscience Studies in Affective Disorders". Section Editor, Maria Nobile MD, PhD. This Section of JAD Focuses on the Relevance of Translational and Neuroscience Studies in Providing a Better Understanding of the Neural Basis of Affective Disorders. The Main Aim Is to Briefly Summaries Relevant Research Findings in Clinical Neuroscience with Particular Regards to Specific Innovative Topics in Mood and Anxiety Disorders. J. Affect Disord. 257. Section Editor, Maria Nobile MD, PhD. This Section of JAD focuses on the relevance of translational and neuroscience studies in providing a better understanding of the neural basis of affective disorders. The main aim is to briefly summaries relevant research findings in clinical neuroscience with particular regards to specific innovative topics in mood and anxiety disorders. 10.1016/j.jad.2019.05.044 [DOI] [PubMed] [Google Scholar]
- Read J. R., Sharpe L., Modini M., Dear B. F. (2017). Multimorbidity and Depression: A Systematic Review and Meta-Analysis. J. Affect Disord. 221, 36–46. 10.1016/j.jad.2017.06.009 [DOI] [PubMed] [Google Scholar]
- Reed J., Bain S., Kanamarlapudi V. (2020). Recent Advances in Understanding the Role of Glucagon-like Peptide 1. F1000Res 9, 239. 10.12688/f1000research.20602.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard J. E., Anderberg R. H., López-Ferreras L., Olandersson K., Skibicka K. P. (2016). Sex and Estrogens Alter the Action of Glucagon-like Peptide-1 on Reward. Biol. Sex. Differ. 7, 6. 10.1186/s13293-016-0059-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N., et al. (2019). Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 157, 107843. 9(th) edition. 10.1016/j.diabres.2019.107843 [DOI] [PubMed] [Google Scholar]
- Sluzewska A. (1999). Indicators of Immune Activation in Depressed Patients. Adv. Exp. Med. Biol. 461, 59–73. 10.1007/978-0-585-37970-8_4 [DOI] [PubMed] [Google Scholar]
- Snoek F. J., Bremmer M. A., Hermanns N. (2015). Constructs of Depression and Distress in Diabetes: Time for an Appraisal. Lancet Diabetes Endocrinol. 3, 450–460. 10.1016/S2213-8587(15)00135-7 [DOI] [PubMed] [Google Scholar]
- Turan I., Sayan Özaçmak H., Erdem S., Ergenc M., Ozacmak V. H. (2021). Protective Effect of Metformin against Ovariectomy Induced Depressive- and Anxiety-like Behaviours in Rats: Role of Oxidative Stress. Neuroreport 32, 666–671. 10.1097/WNR.0000000000001634 [DOI] [PubMed] [Google Scholar]
- Woelfer M., Kasties V., Kahlfuss S., Walter M. (2019). The Role of Depressive Subtypes within the Neuroinflammation Hypothesis of Major Depressive Disorder. Neuroscience 403, 93–110. 10.1016/j.neuroscience.2018.03.034 [DOI] [PubMed] [Google Scholar]
- Young B. A., Lin E., Von Korff M., Simon G., Ciechanowski P., Ludman E. J., et al. (2008). Diabetes Complications Severity index and Risk of Mortality, Hospitalization, and Healthcare Utilization. Am. J. Manag. Care 14, 15–23. [PMC free article] [PubMed] [Google Scholar]
- Zemdegs J., Martin H., Pintana H., Bullich S., Manta S., Marqués M. A., et al. (2019). Metformin Promotes Anxiolytic and Antidepressant-like Responses in Insulin-Resistant Mice by Decreasing Circulating Branched-Chain Amino Acids. J. Neurosci. 39, 5935–5948. 10.1523/JNEUROSCI.2904-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.