Abstract
Interactions between microbes can both constrain and enhance their adaptation to the environment. However, most studies to date have employed simplified microbial communities and environmental conditions. We determined how the presence of a commercial potting compost microbial community affected adaptation of the soil bacterium Pseudomonas fluorescens SBW25 in potting compost. Pseudomonas fluorescens clones isolated from populations evolved in both the presence and absence of the community showed similar fitness increases when measured in the absence of the community. This suggests the presence of the community did not constrain adaptation. By contrast, fitness measured in the presence of the community increased for community-evolved populations, but decreased below the ancestral state for populations evolved in the absence of the community. This suggests some, but not all, mutations that were beneficial with respect to the abiotic environment were costly in the presence of the community, with the former selected against in the presence of the community. Whole-genome sequencing supports this interpretation: most mutations underpinning fitness changes were clone-specific, suggesting multiple genetic pathways to adaptation. Such extreme mutational effects have not been observed in comparable in vitro studies, suggesting that caution is needed when extrapolating results from simplified in vitro systems to natural contexts.
Keywords: bacteria fitness, cost of adaptation, microbial community, Pseudomonas fluorescens, potting soil
1. Introduction
Understanding how the presence of interacting species affects adaptation to other (abiotic and biotic) components of the environment is a fundamental aspect of evolutionary ecology [1]. Adaptation may be constrained by other species through a range of processes, including reductions in population size (in the case of negative interactions) [2], trade-offs between adaptation to different components of the environment [3] and species filling ecological niches faster than evolution occurs [4,5]. Conversely, adaptation may be enhanced if interactions increase population size, open up new ecological niches [6,7] or buffer population sizes in the face of environmental perturbations [8]. Thus, examining the extent to which community affects species interactions is crucial to understand how species adapt and shape ecosystem functioning.
Experimental work in this area has primarily used microbes, because of the speed at which they evolve. Studies frequently report constrained adaptation to other components of the environment as a consequence of species interactions, primarily through reductions in population size and trade-offs [3,9–13] (but see [14]). However, the majority of studies focus on short-term adaptation to greatly simplified communities grown under nutrient-rich in vitro conditions. The novelty of the abiotic environments and the high densities of other organisms will invariably impose very strong selection on the focal organism, potentially leading to findings that may not be observed under more natural conditions. Even in conditions where the abiotic environment more closely resembles a natural environment (such as the use of beech leaf ‘tea’, to emulate a beech tree hole environment [10], or wheat grass [14]), the interacting community is invariably greatly simplified.
In this study, we examine the interplay between adaptation to the biotic and abiotic environment within a managed natural system: potting compost. We previously evolved the soil and plant-associated bacterium Pseudomonas fluorescens SBW25 in commercial potting compost, in the presence or absence of the naturally associated community [15,16]. We found that the community imposed selection on the population (density was reduced) and inhibited metabolic diversification [16]. Here, we measure the fitness of evolved clones in the presence and absence of the resident microbial community by competing each against an isogenic marked ancestral strain. Additionally, we sequence whole genomes of the clones in an attempt to provide insight into mechanisms underpinning fitness differences.
2. Material and methods
(a) . Selection experiment
From a previous study [15], we randomly isolated single bacterial clones of a gentamicin-resistant strain of P. fluorescens SBW25 [17] that had been evolving in 12 independent populations in commercial potting compost (John Innes no. 2) microcosms for 48 days; six in the presence and six in the absence of the natural potting compost community. Briefly, 5 ml of a P. fluorescens suspension (at 2 × 108 CFU ml−1 in M9 buffer) was inoculated into 12 polypropylene trays (10 × 10 cm) with lids containing 100 g of twice-autoclaved potting compost. The potting compost microbial community from a potting compost wash (20 g of potting compost in 100 ml M9 buffer [15,16,18,19]) was inoculated into half of the microcosms. Microcosms were placed in an environmental chamber at 26°C and 80% relative humidity. After 48 days, a soil suspension wash from each of the 12 microcosms was plated onto gentamicin (15 µg ml−1) KB agar plates, and individual clones isolated. Note that the previous experiment focused on bacteria–phage coevolution, but here we only focus on the phage-free control populations.
(b) . Competition assays
Competition experiments between all bacterial clones and a lacZ-marked SBW25 ancestor were carried out as in previous studies [20,21] to estimate the fitness of evolved bacteria in both the presence and absence of the soil microbial community. Briefly, bacterial clones were independently grown in Lysogeny Broth (LB) liquid medium overnight, and 5 ml M9 buffer (minimal salts solution) containing approximately 108 CFU of each clone was inoculated into two microcosms each, along with the same density and volume of the ancestral competitor. The soil microbial community, or M9 buffer only, was then added to one of the microcosms per clone. Prior to inoculation and after 5 days growth, bacterial population densities were determined by plating on LB agar supplemented with X-gal (40 µg ml−1), in order to distinguish lacZ-marked P. fluorescens SBW25 strain and evolved SBW25 populations [20,22]. Selection rate constants (S = mevolved − mancestor, where m = ln (density after 5 days/starting density)) [23] were calculated for each clone; positive values of S indicate higher fitness of the evolved bacteria as compared with the ancestor. Competition experiments were replicated three times per clone.
(c) . Genome re-sequencing
The whole genomes of the 12 bacterial clones were sequenced by HiSeq-Illumina technology at the Centre for Genomic Research (University of Liverpool). First, each bacterial clone was incubated at 28°C and shaking at 140 r.p.m. overnight, reaching densities of approximately 109 CFU ml−1. Then bacterial cultures were aliquoted to carry out the total genomic DNA extraction, which was performed using the Qiagen DNeasy Blood and Tissue kit according to the manufacturer's instructions. DNA libraries were prepared with the Illumina-TrueSeq kit and sequenced by 2 × 100 bp paired-end reads on an Illumina-HiSeq2000 platform. Casava v. 1.8.2, Cutadapt v. 1.2.2 and Sickle v. 1.200 were used to perform the basecalling, de-multiplexing and trimming of the indexed reads, with a minimum window quality score of 20, and reads with more than 3 bp of adapter or shorter than 10 bp were removed. Per sample, an average of 12.4 million filtered read pairs (range 7.3–18.7 million) were mapped to the SBW25 reference genome (GenBank NC_012660.1) using BWA (v. 0.5.9-r16), with local realignment and variant calling (relative to the ancestral SBW25 genome sequenced at the same time) achieved using GATK Unified Genotyper (v. 2.1-13-g1706365) followed by snpEff (v. 4.1) to assign effects on coding genes. Only non-synonymous SNPs with high impact effect were considered. The data for this study have been deposited in the European Nucleotide Archive (ENA) at EMBL-EBI under accession number PRJEB38430.
(d) . Data analyses
Relative fitness analysis for each individual bacterial clone was performed with a linear mixed effects model fitted with REML, where the selective environment (presence and absence of the community) was fitted as a main factor, and nested replicates (n = 3) as a random effect. This was carried out in JMP software. To test whether different sets of genes were mutated in clones evolved in the absence/presence of the community, we used permutational analysis of variance, PERMANOVA [24,25], using the adonis function of the vegan package in R v. 3.3.3 and Euclidean distance as the measure of dissimilarity (distance was measured at the level of mutated genes, so the distance between two clones decreases if they have mutations in the same genes, even if the nucleotide changes involved are different). Dataset files are available from the Dryad repository linked to https://doi.org/10.5061/dryad.vdncjs [26].
3. Results
(a) . Fitness of P. fluorescens clones in the presence and absence of the community
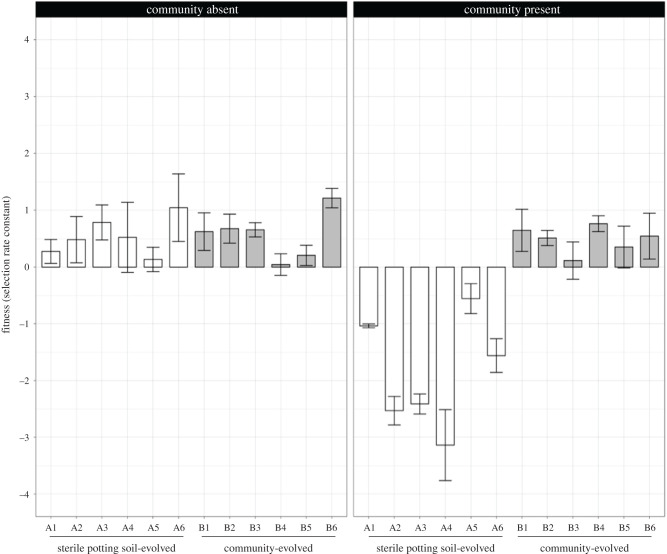
We measured the fitness of each bacterial clone in both selective environments (i.e. in the presence and absence of the microbial community), and found that there was a significant interaction between the selection environment and the environment in which fitness was measured (F1,58 = 82.32, p < 0.001). Figure 1 shows that bacterial clones that were evolved in sterile potting compost or in the presence of the community had similar fitness in the absence of community (F1,10 = 0.018, p = 0.896), with fitness greater than the ancestor in both treatments (t5 = 4.83 and t5 = 5.64; p < 0.002). However, community-evolved populations had much greater fitness in the presence of the community than populations evolved in sterile potting compost only (F1,10 = 32.64, p < 0.002), with the former showing significantly higher fitness than the ancestor (t5 = 6.12, p < 0.001), and the latter significantly lower (t5 = 8.22, p < 0.001). The community-evolved populations had approximately equal fitness in both environments (t11 = 0.63, p < 0.533). These data suggest no cost to adaptation to the community, while populations rapidly became maladapted to the community when evolved in its absence.
Figure 1.
Fitness (selection rate constant) of P. fluorescens SBW25 in different selective environments: in sterile potting soil and in potting soil with the microbial community. Each bar represents the bacterial growth rate of the different evolved clones (A: in the absence, and B: in the presence of the microbial community) related to the ancestral after 5 days competition calculated by the difference in the estimated Malthusian parameter (m). Each competition assay was performed with three replicates. Positive values indicate higher relative fitness of the evolved bacteria as compared with the ancestor.
(b) . Genetic changes in P. fluorescens bacteria clones
We re-sequenced the 12 evolved clones to attempt to identify mutations underpinning the phenotypic differences between treatments. The number of non-synonymous single nucleotide polymorphisms (SNPs) ranged between 0 and 17 per clone (table 1), and between 1 and 6 INDELs (table 2), with mean numbers of each not differing between treatments (Welch's t-test: p > 0.05 for SNPs and INDELs). The majority of mutations were unique to individual clones. There were seven cases (4 SNPs and 3 INDELs) where the same gene was mutated in two out of six clones in one treatment and zero out of six in another; six of these seven genes were mutated only in clones evolved without the community. Despite this, clones evolved in the same treatment group (with/without community) did not have significantly smaller genetic distances than those from different treatment groups (PERMANOVA at level of SNPs: F1,10 = 1.15, p = 0.18; INDELs: F1,10 = 1.17, p = 0.33; SNPs and INDELS combined: F1,10 = 1.14, p = 0.21). Linking mutated genes to specific biological process (tables 1 and 2) did not reveal any pattern between treatments in the functional consequence of mutations. For example, PFLU2423 and PFLU3233 are both components of type II secretion and both only mutated in absence-evolved, but PFLU3230 is also linked to type II SS and mutated in presence. One gene (PFLU1668—a putative epimerase) was mutated in three populations evolved with the community and two without. In particular, the A2 and B1 clones displayed the similar insertion type at the same location for this PFLU1668 gene, possibly generating the same alternative sequence, but it should be noted that the A2 clone additionally exhibited a non-synonymous mutation in the PFLU2493 hypothetical gene predicted to be of moderate impact. Taken together, these results suggest there are many mutations that can lead to adaptation and maladaptation to the complex potting soil environment.
Table 1.
Genetic characterization for the evolved clones of P. fluorescens SBW25 (A: in the absence, and B: in the presence of the microbial community). The occurrence of non-synonymous single nucleotide polymorphisms (SNPs) is marked in binary format (0: absence, 1: presence) after filtering with a cut-off of 95% frequency. The reference of each gene, in addition to the subcellular localization and the biological processes are provided along with the end-product affected by functional categories.
|
P. fluorescens SBW25 clone |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A |
B |
|||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 | gene | product name | subcelullar localization | biological process (GO term) | functional category |
| 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU0988 | putative alginate biosynthesis-like protein | periplasmic | efflux pump membrane protein (multidrug resistance protein A) | drug transport |
| 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU1511 | putative transporter-like membrane protein | cytoplasmic membrane | response to drug | drug binding |
| 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU3233 | putative general secretory pathway protein | unknown | type II secretory pathway, component PulM | transport |
| 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ureC | urease subunit alpha | cytoplasmic | urea metabolic process | resource utilization |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | gltP | glutamate/aspartate:proton symporter | cytoplasmic membrane | Na+/H+-dicarboxylate symporters | transport |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | lepB | signal peptidase I | cytoplasmic membrane | protein secretion | resource utilization |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU0808 | putative transporter-like membrane protein | cytoplasmic membrane | sulfite reductase complex (NADPH) | cellular component |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU1694 | putative ABC transporter ATP-binding protein | cytoplasmic membrane | ABC-type multidrug transport system, ATPase component | resource utilization |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU1829 | hypothetical protein | unknown | PaaI_thioesterase | catalytic activity |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU2096 | putative transporter-like membrane protein | cytoplasmic membrane | arabinose efflux permease | resource utilization |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU2490 | putative chloride transport channel membrane protein | cytoplasmic membrane | chloride channel protein EriC | membrane component |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU2809 | UDP pyrophosphate phosphatase | cytoplasmic membrane | undecaprenyl-diphosphatase activity | unknown |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU2923 | AraC family transcriptional regulator | cytoplasmic | AraC-type DNA-binding domain-containing proteins | regulation biological process |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU2961 | arsenical pump membrane protein | cytoplasmic membrane | response to arsenic-containing substance | response to stimulus |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU4512 | putative transmembrane transport protein | cytoplasmic membrane | purine-cytosine permease and related proteins | catalytic activity |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU4789 | putative glycerophosphoryl diester phosphodiesterase | periplasmic | glycerophosphoryl diester phosphodiesterase | catalytic activity |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | rpoN | RNA polymerase factor sigma-54 | cytoplasmic | sigma factor activity | resource utilization |
| 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU0584 | putative amino acid ABC transporter ATP-binding protein | cytoplasmic membrane | ATP-binding cassette (ABC) transporter complex, substrate-binding subunit-containing | membrane component |
| 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU1686 | putative RHS repeat-like protein | outer membrane | Rhs family protein | cellular component |
| 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU3083 | putative dioxygenase | cytoplasmic | catechol 2,3-dioxygenase activity | catalytic activity |
| 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU5328 | hypothetical protein | cytoplasmic membrane | putative threonine efflux protein | transport |
| 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU5329 | putative sensory box GGDEF/EAL domain-containing protein | cytoplasmic membrane | signal transduction | regulation biological process |
| 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | potF1 | putrescine ABC transporter substrate-binding periplasmic protein | periplasmic | ATP-binding cassette (ABC) transporter complex, substrate-binding subunit-containing | membrane component |
| 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | recG | ATP-dependent DNA helicase RecG | cytoplasmic | DNA recombination | DNA recombination |
| 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU0052 | putative dehydrogenase | unknown | choline dehydrogenase and related flavoproteins | catalytic activity |
| 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU0310 | hypothetical protein | cytoplasmic | unknown | unknown |
| 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU0458 | hypothetical protein | cytoplasmic membrane | cyclase activity | catalytic activity |
| 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU1581 | hypothetical protein | outer membrane | surface lipoprotein | transport |
| 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU2489 | hypothetical protein | cytoplasmic | methylisocitrate lyase activity | catalytic activity |
| 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU2753 | putative EAL/GGDEF domain-containing signalling protein | cytoplasmic membrane | cyclase activity | catalytic activity |
| 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU3370 | hypothetical protein | cytoplasmic membrane | oxidoreductase activity | metabolic process |
| 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU3508 | hypothetical protein | cytoplasmic membrane | unknown | unknown |
| 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU3940 | allantoate amidohydrolase | cytoplasmic | acetylornithine deacetylase/succinyl-diaminopimelate desuccinylase and related deacylases | catalytic activity |
| 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU3948 | putative family S43 non-peptidase protein | outer membrane | unknown | unknown |
| 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU4304 | LysR family transcriptional regulator | cytoplasmic | transcriptional regulator | regulation biological process |
| 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU5618 | hypothetical protein | unknown | predicted phosphatase | resource utilization |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | cyaA | adenylate cyclase | cytoplasmic membrane | adenylate cyclase | resource utilization |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | PFLU0916 | putative methyl-accepting chemotaxis protein | cytoplasmic membrane | methyl-accepting chemotaxis protein | catalytic activity |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | PFLU2428 | hypothetical protein | extracellular | unknown | unknown |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | PFLU2600 | cyn operon positive regulator | cytoplasmic | transcriptional regulator | regulation biological process |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | PFLU2693 | putative haloacid dehalogenase-like hydrolase | unknown | hydrolase activity | catalytic activity |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | PFLU3596 | hypothetical protein | outer membrane | unknown | unknown |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | phnH | carbon-phosphorus lyase complex subunit | cytoplasmic | uncharacterized enzyme of phosphonate metabolism | unknown |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | pobA | 4-hydroxybenzoate 3-monooxygenase | cytoplasmic | benzoate metabolic process | resource utilization |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | PFLU0850 | putative aldehyde dehydrogenase | cytoplasmic | betaine-aldehyde dehydrogenase activity | catalytic activity |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | PFLU3230 | general secretion pathway protein F/S | cytoplasmic membrane | protein secretion by the type II secretion system | resource utilization |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | PFLU3585 | putative FAD-dependent oxidoreductase | cytoplasmic | glycine/d-amino acid oxidases (deamination) | catalytic activity |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | PFLU3747 | putative sigma-54-activated regulatory protein | cytoplasmic | transcriptional activator of acetoin/glycerol metabolism | resource utilization |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | PFLU3920 | 2-oxoacid dehydrogenase subunit E1 | cytoplasmic | cytosolic pyruvate dehydrogenase complex | cellular component |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | PFLU4306 | putative GGDEF/GAF domain sensory box protein | cytoplasmic | cyclase activity | catalytic activity |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | PFLU4516 | LysR family transcriptional regulator | cytoplasmic | transcriptional regulator | regulation biological process |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | ddl | d-alanyl-alanine synthetase A | cytoplasmic | peptidoglycan biosynthetic process | resource utilization |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | PFLU0087 | putative two-component system sensor kinase | cytoplasmic membrane | phosphate ion transport | transport |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | PFLU0478 | putative glycosyl transferase | cytoplasmic | glycosyltransferase | catalytic activity |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | PFLU1394 | putative hydrolase | unknown | beta-lactamase class C and other penicillin-binding proteins | drug binding |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | PFLU3262 | putative amidase | cytoplasmic | glutaminyl-tRNA synthase (glutamine-hydrolysing) activity | catalytic activity |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | PFLU3345 | hypothetical protein | unknown | unknown | unknown |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | PFLU3969 | putative acetyltransferase | unknown | ribosome biogenesis | cellular component |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | PFLU6085 | putative cobalamin biosynthesis-like protein | cytoplasmic | cobalamin biosynthetic process | catalytic activity |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | prfA | peptide chain release factor 1 | cytoplasmic | translational termination | resource utilization |
Table 2.
Genetic characterization for the evolved clones of P. fluorescens SBW25 (A: in the absence, and B: in the presence of the microbial community). The occurrence of indels (insertion or deletion) is marked in binary format (0: absence, 1: presence) after filtering with a cut-off of 95% frequency. The reference of each gene, in addition to the subcellular localization and the biological processes are provided along with the end-product affected by functional categories.
|
P. fluorescens SBW25 clone |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A |
B |
|||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 | gene | product name | subcelullar localization | biological process (GO term) | functional category |
| 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU2423 | putative type II secretion pathway protein D | outer membrane | type II secretory pathway, component PulD | cell motility |
| 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU3208 | amidase | cytoplasmic | Asp-tRNAAsn/Glu-tRNAGln amidotransferase A subunit and related amidases | translation, ribosomal structure and biogenesis |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU3658 | putative extracellular polysaccharide biosynthesis protein | cytoplasmic membrane | sugar transferases involved in lipopolysaccharide synthesis | cell wall/membrane/envelope biogenesis |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU0788 | zinc-binding protein | unknown | uncharacterized protein conserved | unknown |
| 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU1420 | LysR family transcriptional regulator | cytoplasmic | transcriptional regulator | transcription |
| 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU5282A | putative DNA invertase | cytoplasmic | site-specific recombinases, DNA invertase Pin homologues | replication, recombination and repair |
| 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | codA | cytosine deaminase | cytoplasmic | cytosine deaminase and related metal-dependent hydrolases | resource utilization |
| 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | PFLU2169 | putative LuxR family regulatory protein | cytoplasmic | response regulator containing a CheY-like receiver domain and an HTH DNA-binding domain | transcription |
| 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | PFLU1668 | putative polysaccharide biosynthesis-related membrane protein | cytoplasmic membrane | predicted nucleoside-diphosphate sugar epimerases | resource utilization |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | PFLU1693 | putative ABC transporter membrane protein | cytoplasmic membrane | unknown | unknown |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | PFLU0066 | protohaem IX farnesyltransferase | cytoplasmic membrane | polyprenyltransferase | post-translational modification |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | PFLU3067 | 3-oxoacyl-ACP reductase | cytoplasmic | dehydrogenases | resource utilization |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | PFLU3323 | putative amino acid permease membrane protein | cytoplasmic membrane | amino acid transporters | resource utilization |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | PFLU3510 | putative transmembrane protein | cytoplasmic membrane | unknown | unknown |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | PFLU2381 | hypothetical protein | cytoplasmic membrane | arabinose efflux permease | resource utilization |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | mutL | DNA mismatch repair protein | cytoplasmic | DNA mismatch repair enzyme | replication, recombination and repair |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | PFLU5103 | hypothetical protein | unknown | unknown | unknown |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | PFLU0808 | putative transporter-like membrane protein | cytoplasmic membrane | sulfite reductase complex (NADPH) | resource utilization |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | PFLU4038 | putative tartrate dehydrogenase | cytoplasmic | isocitrate/isopropylmalate dehydrogenase | resource utilization |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | PFLU2281 | putative ABC transporter membrane protein | cytoplasmic membrane | ABC-type dipeptide/permease | resource utilization |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | PFLU1849 | putative two-component system sensor kinase | cytoplasmic membrane | signal transduction histidine kinase | signal transduction mechanisms |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | PFLU5027 | hypothetical protein | cytoplasmic membrane | unknown | unknown |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | PFLU4348 | hypothetical protein | cytoplasmic membrane | unknown | unknown |
4. Discussion
We investigated how the presence of the microbial community affected the rate of adaptation of a focal bacterium in a commercial potting compost. Populations that had been evolved for 48 days in both the presence and absence of a potting soil community showed equal increases in fitness when measured in the absence of the community. This suggests no major community-imposed constraint on adaptation, despite reductions in population size reported in our previous study [16]. This contrasts with many in vitro studies using highly simplified communities in nutrient media, where biotic interactions typically constrain adaptation. It is notable that in another recent study using a more natural environment, the community interactions increased abiotic adaptation of one of the species [14]. It is possible that the extreme selection pressures associated with laboratory environments may greatly exaggerate inhibitory effects of community interactions. Fitness increases of the community-evolved populations were comparable in both the presence and absence of the community, suggesting that most adaptation is to the abiotic environment. We previously reported increased metabolic diversity evolved in the absence of the community [16], but this clearly had no major effect on mean fitness of individual clones.
Our most striking finding, and not observed in comparable in vitro studies, is the large reduction in fitness in the presence of the community, following evolution in the absence. This suggests that some mutations (or epistatic combinations) confer advantages in the absence of the community, but are costly in the presence, i.e. they are antagonistically pleiotropic [27]. However, the absence of any obvious cost to adaptation of the community-evolved populations suggests that other equally accessible mutations are not antagonistically pleiotropic in these contexts. Our genomics analyses are consistent with this interpretation. Total mutations, including SNPs and INDELs, varied between 1 and 21 per clone, and the vast majority were unique. This suggests there are many ways in which populations could adapt to the complex potting soil environment. However, we note that by phenotyping and sequencing only a single clone per population, this between-population variation may be exaggerated given within-population variation. Mutations that were beneficial in the absence but costly in the presence of the community would be selected against when the community was present, and selected for in the absence (mutation accumulation [28]). Our results highlight the need to be cautious about extrapolating results from simplified in vitro systems to real-world contexts, particularly without clear-cut theoretical expectations.
Acknowledgements
We thank three reviewers and the handling editor for their detailed and helpful comments.
Data accessibility
Dataset files are available from the Dryad repository, linked to https://doi.org/10.5061/dryad.vdncjs [26]. Sequencing data for this study have been deposited in the European Nucleotide Archive (ENA) at EMBL-EBI under accession number PRJEB38430.
Authors' contributions
P.G.: conceptualization, data curation, formal analysis, investigation, methodology, writing—original draft, writing—review and editing; A.R.H.: data curation, formal analysis, methodology, writing—original draft, writing—review and editing; S.P.: data curation, formal analysis, methodology, writing—original draft, writing—review and editing; A.B.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, visualization, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed herein.
Competing interests
We declare we have no competing interests.
Funding
The work was supported by funding from NERC.
References
- 1.Barraclough TG. 2015. How do species interactions affect evolutionary dynamics across whole communities? Annu. Rev. Ecol. Evol. Syst. 46, 25-48. ( 10.1146/annurev-ecolsys-112414-054030) [DOI] [Google Scholar]
- 2.Johansson J. 2008. Evolutionary responses to environmental changes: how does competition affect adaptation? Evolution 62, 421-435. ( 10.1111/j.1558-5646.2007.00301.x) [DOI] [PubMed] [Google Scholar]
- 3.Zhang Q, Lambert G, Liao D, Kim H, Robin K, Tung CK, Pourmand N, Austin RH. 2011. Acceleration of emergence of bacterial antibiotic resistance in connected microenvironments. Science 333, 1764-1767. ( 10.1126/science.1208747) [DOI] [PubMed] [Google Scholar]
- 4.Urban MC, et al. 2008. The evolutionary ecology of metacommunities. Trends Ecol. Evol. 23, 311-317. ( 10.1016/j.tree.2008.02.007) [DOI] [PubMed] [Google Scholar]
- 5.Bolnick DI, et al. 2011. Why intraspecific trait variation matters in community ecology. Trends Ecol. Evol. 26, 183-192. ( 10.1016/j.tree.2011.01.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Livingston G, Matias M, Calcagno V, Barbera C, Combe M, Leibold MA, Mouquet N. 2012. Competition–colonization dynamics in experimental bacterial metacommunities. Nat. Commun. 3, 1234. ( 10.1038/ncomms2239) [DOI] [PubMed] [Google Scholar]
- 7.Calcagno V, Jarne P, Loreau M, Mouquet N, David P. 2017. Diversity spurs diversification in ecological communities. Nat. Commun. 8, 15810. ( 10.1038/ncomms15810) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Northfield TD, Ives AR. 2013. Coevolution and the effects of climate change on interacting species. PLoS Biol. 11, e1001685. ( 10.1371/journal.pbio.1001685) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee HH, Molla MN, Cantor CR, Collins JJ. 2010. Bacterial charity work leads to population-wide resistance. Nature 467, 82-85. ( 10.1038/nature09354) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawrence D, Fiegna F, Behrends V, Bundy JG, Phillimore AB, Bell T, Barraclough TG. 2012. Species interactions alter evolutionary responses to a novel environment. PLoS Biol. 10, e1001330. ( 10.1371/journal.pbio.1001330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friman VP, Buckling A. 2013. Effects of predation on real-time host–parasite coevolutionary dynamics. Ecol. Lett. 16, 39-46. ( 10.1111/ele.12010) [DOI] [PubMed] [Google Scholar]
- 12.Scheuerl T, Hopkins M, Nowell RW, Rivett DW, Barraclough TG, Bell T. 2020. Bacterial adaptation is constrained in complex communities. Nat. Commun. 11, 754. ( 10.1038/s41467-020-14570-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall JPJ, Harrison E, Brockhurst MA. 2018. Competitive species interactions constrain abiotic adaptation in a bacterial soil community. Evol. Lett. 2, 580-589. ( 10.1002/evl3.83) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans SE, Bell-Dereske LP, Dougherty KM, Kittredge HA. 2020. Dispersal alters soil microbial community response to drought. Environ. Microbiol. 22, 905-916. ( 10.1111/1462-2920.14707) [DOI] [PubMed] [Google Scholar]
- 15.Gómez P, Buckling A. 2011. Bacteria-phage antagonistic coevolution in soil. Science 332, 106-109. ( 10.1126/science.1198767) [DOI] [PubMed] [Google Scholar]
- 16.Gómez P, Buckling A. 2013. Real time microbial adaptive diversification in soil. Ecol. Lett. 16, 650-655. ( 10.1111/ele.12093) [DOI] [PubMed] [Google Scholar]
- 17.Pal C, Macia MD, Oliver A, Schachar I, Buckling A. 2007. Coevolution with viruses drives the evolution of bacterial mutation rates. Nature 450, 1079-1081. ( 10.1038/Nature06350) [DOI] [PubMed] [Google Scholar]
- 18.Gómez P, Ashby B, Buckling A. 2015. Population mixing promotes arms race host–parasite coevolution. Proc. R. Soc. B 282, 20142297. ( 10.1098/rspb.2014.2297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gómez P, Paterson S, De Meester L, Liu X, Lenzi L, Sharma MD, McElroy K, Buckling A.. 2016. Local adaptation of a bacterium is as important as its presence in structuring a natural microbial community. Nat. Commun. 7, 12453. ( 10.1038/ncomms12453) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luján AM, Gómez P, Buckling A. 2015. Siderophore cooperation of the bacterium Pseudomonas fluorescens in soil. Biol. Lett. 11, 20140934. ( 10.1098/rsbl.2014.0934) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Houte S, Padfield D, Gómez P, Lujan AM, Brockhurst MA, Paterson S, Buckling A. 2020. Compost spatial heterogeneity promotes evolutionary diversification of a bacterium. J. Evol. Biol. 34, 246-255. ( 10.1111/jeb.13722) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lennon JT, Khatana SAM, Marston MF, Martiny JBH. 2007. Is there a cost of virus resistance in marine cyanobacteria? ISME J. 1, 300-312. ( 10.1038/ismej.2007.37) [DOI] [PubMed] [Google Scholar]
- 23.Lenski RE, Rose MR, Simpson SC, Tadler SC. 1991. Long-term experimental evolution in Escherichia coli. 1. Adaptation and divergence during 2,000 generations. Am. Nat. 138, 1315-1341. ( 10.1086/285289) [DOI] [Google Scholar]
- 24.Zapala MA, Schork NJ. 2006. Multivariate regression analysis of distance matrices for testing associations between gene expression patterns and related variables. Proc. Natl Acad. Sci. USA 103, 19 430-19 435. ( 10.1073/pnas.0609333103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson MJ. 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 26, 32-46. ( 10.1111/j.1442-9993.2001.01070.pp.x) [DOI] [Google Scholar]
- 26.Gómez P, Hall AR, Paterson S, Buckling A. 2022. Data from: Rapid decline of adaptation of Pseudomonas fluorescens to soil biotic environment. ( 10.5061/dryad.vdncjs) [DOI] [PMC free article] [PubMed]
- 27.Futuyma DJ, Moreno G. 1988. The evolution of ecological specialization. Annu. Rev. Ecol. Syst. 19, 207-233. ( 10.1146/annurev.es.19.110188.001231) [DOI] [Google Scholar]
- 28.Kawecki TJ. 1994. Accumulation of deleterious mutations and the evolutionary cost of being a generalist. Am. Nat. 144, 833-838. ( 10.1086/285709) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Gómez P, Hall AR, Paterson S, Buckling A. 2022. Data from: Rapid decline of adaptation of Pseudomonas fluorescens to soil biotic environment. ( 10.5061/dryad.vdncjs) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Dataset files are available from the Dryad repository, linked to https://doi.org/10.5061/dryad.vdncjs [26]. Sequencing data for this study have been deposited in the European Nucleotide Archive (ENA) at EMBL-EBI under accession number PRJEB38430.