Abstract
Significance: The vasculature responds to the respiratory needs of tissue by modulating luminal diameter through smooth muscle constriction or relaxation. Coronary perfusion, diastolic function, and coronary flow reserve are drastically reduced with aging. This loss of blood flow contributes to and exacerbates pathological processes such as angina pectoris, atherosclerosis, and coronary artery and microvascular disease.
Recent Advances: Increased attention has recently been given to defining mechanisms behind aging-mediated loss of vascular function and development of therapeutic strategies to restore youthful vascular responsiveness. The ultimate goal aims at providing new avenues for symptom management, reversal of tissue damage, and preventing or delaying of aging-induced vascular damage and dysfunction in the first place.
Critical Issues: Our major objective is to describe how aging-associated mitochondrial dysfunction contributes to endothelial and smooth muscle dysfunction via dysregulated reactive oxygen species production, the clinical impact of this phenomenon, and to discuss emerging therapeutic strategies. Pathological changes in regulation of mitochondrial oxidative and nitrosative balance (Section 1) and mitochondrial dynamics of fission/fusion (Section 2) have widespread effects on the mechanisms underlying the ability of the vasculature to relax, leading to hyperconstriction with aging. We will focus on flow-mediated dilation, endothelial hyperpolarizing factors (Sections 3 and 4), and adrenergic receptors (Section 5), as outlined in Figure 1. The clinical implications of these changes on major adverse cardiac events and mortality are described (Section 6).
Future Directions: We discuss antioxidative therapeutic strategies currently in development to restore mitochondrial redox homeostasis and subsequently vascular function and evaluate their potential clinical impact (Section 7). Antioxid. Redox Signal. 35, 974–1015.
Keywords: aging, endothelial dysfunction, mitochondrial dysfunction, reactive oxygen species, oxidative stress, vasodilation
1. Historically Explored Mechanisms of Mitochondrial Oxidative Changes in Aging
1.1. Causes of reactive oxygen species production in aging
Increased oxidative stress coincides and contributes to the aging process. The oxidative stress theory of aging postulates that age-associated functional loss (such as vascular function to maintain adequate perfusion) is the result of oxidative damage of proteins, DNA (nuclear and mitochondrial), and lipids, leading to cellular senescence. Cellular senescence is characterized by the senescence-associated secretory phenotype (SASP), whereby cells exhibit increased secretion of interleukins, chemokines, growth factors, matrix metalloproteinases, insoluble proteins, and extracellular matrix (132). This leads to a proinflammatory state with feedforward mechanisms that further increase harmful reactive oxygen and nitrogen species (ROS and RNS), affecting many pathological processes linked to chronic diseases such as cardiovascular disease, respiratory diseases, chronic kidney disease, cancer, neurodegenerative diseases, and muscular disorders (132). Indeed, chronic inflammation may be induced in the elderly population by genetic inflammasome involvement, leading to increased oxidative stress, hypertension, and arterial stiffness (144). In addition, increased apoptosis has been demonstrated in senescent endothelial cells in humans, and vascular senescence can lead to irreversible pulmonary arterial hypertension in both rats and humans (462). The occurrence of SASP and proinflammatory state is variable between individuals depending on genetic, environmental, and behavioral influences, leading to the Geroscience hypothesis, which proposes that the “rate of aging” can be influenced by modifiable factors (132). Specific to this review, we will focus on the role and mechanisms by which oxidative stress hastens vascular aging, resulting in endothelial dysfunction.
The mitochondria are responsible for not only cellular energy production but also the majority of ROS generation (90, 185). During oxidative phosphorylation, the electron transport chain (ETC) utilizes the transfer of electrons between complexes to establish the proton gradient responsible for powering ATP-synthase generating ATP (438). The electrons traversing the ETC end up at complex IV, where they are ultimately utilized in the production of water from oxygen and hydrogen. One to three percent of these electrons do not make it to this step; instead, they leak to prematurely react with oxygen to form superoxide (O2•−) (113). In senescent endothelial cells (via passaging), the catalytic function of complex IV is reduced by up to 84%, allowing for exacerbated electron leakage with aging (521). Superoxide generation occurs predominantly at complex I (39, 302) during conditions of high proton motive force, NADH/NAD+ ratio, and CoQH2/CoQ ratio (sensor of respiratory chain efficiency), with the latter two occurring more frequently with aging (165, 309, 326).
A significant amount of superoxide from complex I is generated during reverse electron transport, where electrons are shunted back from CoQH2 back to complex I and reduce NAD+ to NADH. This process is highly sensitive to membrane potential (protonmotive force) changes, with a 10 mV decrease leading to a 70% reduction in superoxide production within the mitochondrial matrix, although not affecting cytosolic production (292). Mitochondrial membrane potential decreases with aging, which would theoretically lead to a decrease in superoxide production in aging. This is due to an inherent increase in leakage of protons, reducing the membrane potential with age, as a probable adaptive mechanism to reduce oxidative stress in aging (320, 388, 426). Despite this adaptation, accumulation of ROS dominates aging-associated mitochondrial alterations.
There is an increase in pro-oxidation processes in the aging vasculature (121). With aging, there is a known increase in circulating angiotensin II (Ang II), angiotensin converting enzyme (ACE), and angiotensin receptor 1 (AT1) expression (206, 231, 482). Ang II induces endothelial dysfunction through activation of inflammatory signaling (e.g., VCAM-1, NFκB), activation of COX2-producing vasoactive prostaglandins when bound to AT1 receptors, and activation of NADPH oxidase (NOX) (79, 94, 111, 206, 277, 448, 449, 451, 490, 494, 504). These transmembrane proteins transfer electrons from NADPH to molecular oxygen, leading to the generation of superoxide and hydrogen peroxide (H2O2) (2, 101, 111, 171, 180, 234, 434). NOX 1 and 2 contribute to superoxide production in endothelial cells and endothelial and smooth muscle cells (SMCs), respectively (101, 180, 210, 450). AT1 and NOX activation also lead to endothelial nitric oxide synthase (eNOS) uncoupling, shifting from nitric oxide (NO) to superoxide production and attenuating endothelium-dependent dilation (EDD) (93, 103, 371, 527). In aging, NOX subunits are overexpressed, including the p67 subunit in mouse aorta (119, 135), p22phox subunit in rat mesenteric arteries (199), and p47phox subunit in aging men (107). Collectively, these signaling cascades result in enhanced ROS generation and endothelial dysfunction, whereas inhibition of NOX improves overall EDD in vessels from aging humans and rats (88, 306, 367, 456).
Of the NOX isoforms, NOX4 is considered one of the primary vascular NOXs responsible for endothelial hydrogen peroxide production. NOX4 is further upregulated by hypertrophic signaling and β-arrestin mediated signaling, and along with the other NOXs are increased with age (2, 3, 41, 163, 284, 334). There is current lack of clarity and consensus on the vascular effects of NOX4, with studies showing both deleterious and protective roles (296). The discrepancy in these studies, likely stemming from differences in knockout/overexpression strategies, showcases the tight redox balance required for homeostatic vascular function. Low chronic hydrogen peroxide accumulation can be vasculoprotective, lowering blood pressure if produced acutely as an endothelium-dependent hyperpolarizing factor in response to shear stress.
In NOX4 knockout mice, aortas develop increased inflammation, increased media hypertrophy, reduced heme oxygenase-1 and nrf-2 gene expression, and endothelial dysfunction, including reduced eNOS function and nitric oxide (391, 392). On the other hand, overabundance of hydrogen peroxide chronically can induce autophagy, apoptosis, and reduced vascular function, and it is classically known as a pathogenic mediator of atherosclerosis. In aging, NOX4 may err on the side of promoting endothelial dysfunction, rather than protection (247, 248). Potential positive effects of NOX4 are likely mitigated due to eNOS uncoupling-mediated superoxide production, whereby any effect of NOX4 to active eNOS through protein kinase B (Akt) signaling is potentially counterproductive. Evidence suggests upregulated NOX4 and subsequent chronic hydrogen peroxide exposure in aging causes eNOS uncoupling, reduced nitric oxide production and acetylcholine (Ach)-mediated vasodilation, endoplasmic reticulum (ER) stress via IRE1α-oxidation, and mitochondrial dysfunction (189, 247, 248, 472). Further details on the vascular protective and deleterious effects of NOX4 are reviewed by Salazar (380).
Xanthine oxidase has increased expression and activity with aging and is associated with oxidative stress in several tissues, including the aorta, coronary, and mesenteric arteries (13, 241, 317, 422, 476, 477, 517), and it contributes to superoxide generation in response to increased pressure (204). Although inhibition of xanthine oxidase reverses endothelial dysfunction in hypoxia, hypercholesterolemia, hyperuricemia, and heart failure patients (54, 92, 105, 110), inhibition seems unable to reverse aging-mediated endothelial dysfunction in humans, at least while evaluating peripheral vasculature (122).
The adaptor protein p66shc has been considered one of the master regulators of superoxide production. Its expression, increased in advancing age, is mediated by Ang II signaling, hypertrophy, and alpha-adrenergic receptor (αADR) agonism (78, 150, 166, 237, 409, 438). In addition, p66shc is imported into the intermembrane space of the mitochondria, where it steals an electron from cytochrome c, facilitating its transfer to molecular oxygen to produce superoxide (454), while also inhibiting the transcription of manganese superoxide dismutase (SOD2) through α1ADR/FOXO3A-mediated signaling (166). Genetic deletion of p66shc leads to a 30% increase in lifespan in mice (289). Genetic knockout of p66shc in old murine aortic rings preserves, whereas overexpression reduces EDD to acetylcholine (ACh) and nitric oxide bioavailability (52, 78, 138, 506).
1.2. Reduction of anti-oxidative processes in aging
During youth and into healthy middle age (roughly ages <55), antioxidants quench ROS to maintain redox homeostasis. Superoxide is converted into hydrogen peroxide by SOD2 in the mitochondrial matrix and copper/zinc superoxide dismutase (SOD1) in the intermitochondrial space and cytoplasm. Hydrogen peroxide can further react via the Fenton reaction to form the hydroxyl ion. This is prevented by the conversion of hydrogen peroxide to water and molecular oxygen by catalase and glutathione peroxidase (predominant). Glutathione peroxidase oxidizes glutathione in concert with glutathione reductase to reduce GSH, occurring in a cyclic manner (303, 438). Superoxide can also react with NO to form peroxynitrite (ONOO−), a potent oxidizing and nitrosylating agent.
Vascular aging is associated with a decrease in density and function of SOD1/2 (64, 100, 291, 491, 526), glutathione, glutathione reductase, and glutathione peroxidase (32, 58, 95, 136, 177, 349, 352, 368, 497), which are associated with reduced EDD and aortic stiffening in mice and rats. Positive correlations of aortic catalase concentration and activity with aging have been observed as a potential compensatory mechanism (although insufficient) for increased hydrogen peroxide concentration and vascular sensitivity to hydrogen peroxide (200, 439). In contrast, in aged pulmonary arteries and patients with untreated essential hypertension catalase concentration is decreased (356, 379). Overexpression of catalase, glutathione peroxidase, or SOD in animal models of aging is demonstrated to restore EDD and protect against vascular pathologies such as aneurisms, atherosclerosis, with the added benefit of increased lifespan (42, 82, 84, 127, 276, 343, 489).
Additional antioxidative contributors to maintaining vascular redox homeostasis are sirtuin deacetylases (Sirt), which have been demonstrated to be decreased in aging mice and humans (109, 225, 400). Sustaining eNOS deacetylation status preserves its ability to produce nitric oxide, and shifting to acetylated status with aging-mediated loss of Sirt1 contributes to loss of nitric oxide bioavailability and increased superoxide (255, 281, 329, 366). This loss of nitric oxide is associated with diminished capacity for endothelium- or shear stress-induced vasodilation in aorta, femoral, and middle cerebral arteries that are subject to Sirt1 inhibition (109, 281, 433). Conversely, overexpression of endothelial Sirt1 mediates vasoprotection in aging through nitric oxide-independent effects via enhancing soluble guanylyl cyclase in smooth muscle and reducing AT1 receptor expression (14, 168, 294, 425).
Sirt1 and mitochondrial Sirt3 regulate transcription factors for antioxidant proteins, including FOXO, the transcription factor for SOD2, to suppress the effects of p66shc and reduce ROS (43, 153, 359, 526). Childhood Sirt1 expression is a predictor of adulthood microvascular function, as reduced Sirt1 in young participants in a longitudinal cohort was associated with premature cutaneous microvascular dysfunction in adulthood. This was assessed via maximal response to local thermal hyperemia, post-occlusive reactive hyperemia, and iontophoresis with ACh (369). Many of the beneficial effects of caloric restriction on vascular function are induced via sirtuins as they suppress senescence by delaying telomere attrition, instigating DNA repair and genomic stability, and preserving proteostasis by regulating heat shock proteins (233, 249, 359, 360, 522).
1.3. Effect of estrogen loss on oxidative stress during aging
Aging-associated changes in hormones contribute to increased oxidative stress. Loss of estrogen during the menopausal transition to postmenopause is associated with elevated risk for cardiovascular disease. Postmenopausal females may exhibit coronary microvascular dysfunction, defined by endothelial dysfunction, pathologic smooth muscle tone, and increased oxidative stress (9). The loss of estrogen is one mediator of this disease due to the loss of its antioxidative properties. Estrogen modulates NOX to reduce superoxide production in rat in vivo mesenteric microvessels (91, 505).
Loss of estrogen diminishes nitric oxide-mediated flow-mediated dilation (FMD) in young ovariectomized rats while exhibiting elevated nitrotyrosine (oxidative stress marker) and reduced SOD protein (213). Ovariectomized rats given hormone replacement therapy (HRT) show restored SOD and nitric oxide-mediated FMD. Estrogen also activates telomerase, limiting mitochondrial ROS (mtROS) production via the subunit telomerase reverse transcriptase (TERT) (239). The activation of mitochondrial estrogen receptor-β leads to increased S-nitrosylation (SNO) of proteins, such as proteins that have a role in the homeostatic balance of β-adrenergic function.
The mitochondrial prooxidant effects on EDD in aging promote pathologic cardiovascular remodeling and hypertension, whereas reduced perfusion and chronic ischemia have an impact on morbidity and mortality. Therefore, understanding these intricate interactions is vital for understanding proper therapeutic management and development. How these aging-dependent vascular changes impact mechanisms for vasorelaxation, including flow-mediated, potassium channel-mediated, and βADR-mediated relaxation as well as parallel processes such as mitochondrial fission and fusion, are the focus of the remainder of this review.
2. Vascular Mitochondrial Fission/Fusion in Relation to Mitochondrial Redox Homeostasis with Aging
2.1. Mitochondrial dynamics and dysfunction with aging-induced oxidative stress and hyperglycemia
Mitochondria are dynamic organelles, changing shape and function as a direct result of the surrounding molecular environment. Within other tissues and organs, the mitochondria are traditionally known as energy hubs, producing ATP through oxidative phosphorylation. However, within the vasculature, mitochondria act as a signaling hub rather than for energy as endothelial cells are highly glycolytic (229, 438). The mitochondria are integral in maintaining a balance between production of ROS and signaling actions of nitric oxide, which can affect vasodilative balance as alluded to in later sections. In this capacity, the mitochondria act as a signaling hub, with structure and function playing an important role in vascular health and disease (438).
Mitochondrial dynamics are delineated by biogenesis and mitophagy. On the one hand, biogenesis results in production of new mitochondria characterized by a highly filamentous and networked structure that is viewed as “fused.” Conversely, damaged mitochondria tend to exhibit a more diffuse, punctuated structure, caused by a process termed fission, which is mediated by dynamin-related protein-1 (DRP-1) and mitochondrial fission protein-1 (Fis-1). Damaged mitochondria either fuse with healthy mitochondria—mediated by mitofusin 1 or 2 (Mfn-1 or 2) or optic atrophy 1 (Opa-1)—or are encapsulated and degraded via mitophagy. Oxidative stress alters cultured endothelial mitochondrial structure, transitioning from a more structured, tubular, networked state (e.g., fusion), to a more fragmented, disrupted, or punctate state (e.g., fission). Aging-related changes to antioxidant versus prooxidant expression and function and their relation to mitochondrial dynamics, mitophagy, FMD, and adrenergic-mediated dilation are illustrated in Figure 1.
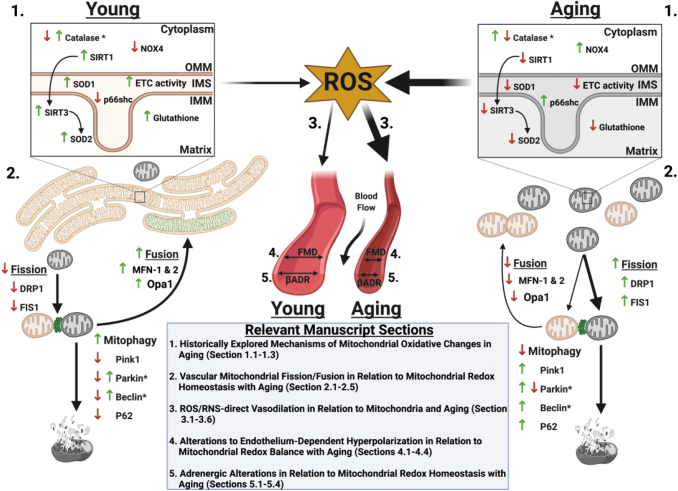
FIG. 1.
Aging-related changes in mitochondrial dynamics, pro- and antioxidant enzymes, and effect of subsequent ROS on flow and adrenergic-medicated dilation. 1. During youth, homeostatic redox balance occurs due to adequate antioxidant and limited prooxidant enzyme expression, function, and/or signaling (up or down arrows) that is reversed with aging to increase prooxidant and limit antioxidant enzyme expression, function, and signaling. *Note for catalase, expression is up- or downregulated with aging depending on vascular location (up in aging in aorta and coronary, down with aging in pulmonary arteries). Arrows between Sirt1 to Sirt3 to SOD2 refer to deacetylation that activates SOD2. 2. In youth, redox homeostasis allows for elongated and networked mitochondrial morphology. When mitochondrial damage occurs in youth, fusion and mitophagy are favored over fission with lower expression of fission proteins DRP-1 and FIS-1 in youth relative to aging. Aging mitochondria are characterized morphologically as fragmented and more likely to undergo fission than fusion or mitophagy. *Note that beclin and parkin expression can be increased or decreased with age depending on source. 3–5. Greater ROS generation in aging from dysfunctional mitochondria contributes to attenuation of effectiveness of flow- and β-adrenergic mediated dilation. DRP-1, dynamin-related protein-1; FIS-1, mitochondrial fission protein-1; ROS, reactive oxygen species; SIRT, sirtuin deacetylase; SOD2, manganese superoxide dismutase. Figure created with BioRender.com
Mitochondrial dynamics during health exist in a homeostatic balance between fission and fusion, the disruption of which is implicated in cell senescence, quiescence, and aging processes (423). Indeed, Jendrach et al. demonstrated in human vascular endothelial cells serially cultured to induce senescence, thereby mimicking aging, that mitochondrial structure is altered in aging via reduced fusion and fission events; aging was associated with mitochondrial DNA (mtDNA) damage and loss of mitochondrial membrane potential opening of the mitochondrial permeability transition pore (mPTP), resulting in the release of cytochrome C and enhancing further oxidative stress (205).
Hyperfusion is a protective response to boost mitochondrial respiration and counteract cell stress. A more connected (fused) network of mitochondria allows for a more closely linked network of mitochondrial respiratory complexes, maximizing energy production and signaling (351). However, excessive hyperfusion via Mfn-1 overactivation may be associated with induction of senescence, and DRP-1 repression may induce ATR-mediated DNA damage response.
Some level of fission appears necessary, as senescent endothelial progenitor cells exhibit reduced Fis-1 with restoration of expression, thereby reducing ROS and restoring youthful morphology, bioenergetics, and angiogenic potential (480). Overly reduced DRP-1 function, while inducing hyperfusion, also reduces Mfn-1 and 2 and Opa-1 expression and processing (295). On the other hand, disruption of this interconnected mitochondrial network with hyperfission is linked to aberrant respiratory complexes and also excessive ROS (197, 419).
In endothelial cell senescence, hyperfission dominates. In senescent rat aortic endothelial cells (induced by Ang II), mitochondrial fission is upregulated alongside the pro-inflammatory phenotype (293). One mechanism for increased senescent endothelial fission is enhanced activity of DRP-1 due to loss of protein disulfide isomerase A1 (PDIA1). PDIA1 acts as a thiol reductase for DRP-1, without which DRP-1 becomes sulfenylated at cysteine 644, leading to activation with increased mitochondrial fragmentation and ROS production, further induction of senescence with reduced angiogenesis, and reduced endothelium-dependent vasodilation to ACh (223). DRP-1 may also be redox regulated by SNO-mediated activation, although this notion is controversial (34, 71). DRP-1 is negatively whereas Opa-1 is positively regulated by Sirt 3 deacetylation, which is downregulated in aging (300, 382).
Reductions in rat aortic NO bioavailability (which occurs in aging) also reduce the amount of fused mitochondria, while increasing mitochondrial fission (290). These aging-induced balance shifts toward hyperfission have been observed in our lab, with RNA sequencing showing reduced Mfn-1 and enhanced DRP-1 in aging rat coronary microvessels (unpublished observations).
Changes in mitochondrial structure and function are impacted by their surrounding environment. Exposure to ROS imparts damage to mtDNA, with excessive oxidative stress impairing oxidative phosphorylation and subsequently increasing the amount of ROS produced in a feedforward manner (16). As it relates to fission/fusion balance, these alterations have consequences for dilative ability of the vasculature to maintain proper patency for oxygen and metabolic and demand and for processes of aging-associated cardiovascular pathologies.
2.2. What are the functional ramifications of age-associated changes in mitochondrial dynamics?
Reductions in vasodilation in aging have been attributed to excessive mtROS production as a result of dysfunctional mitochondria, including dynamics of fission/fusion balance. As described in subsequent major sections of this review, aging is associated with skewed balance of ROS and vasoprotective nitric oxide bioavailability (108, 460, 461), in part due to altered mitochondrial dynamics, with reductions in vasodilation to shear stress (e.g., exercise or FMD) and adrenergic agonism in older adults (298, 303, 314, 347, 455, 460). For now, we describe associations of altered fission/fusion balance and their functional consequences on vasodilative capacity and classic pathologies.
Evidence from animal models indicate that aging is associated with a general decrease in mitochondria content within endothelial cells, coinciding with decreased mitochondrial respiration and increased mitochondria superoxide generation and hydrogen peroxide (438). These maladaptive alterations with aging are associated with decreased messenger RNA (mRNA) expression of mitochondrial biogenesis, and reductions in Mfn-1 and 2 (401, 459, 460). Functionally, aging-induced increased DRP-1-induced mitochondrial fragmentation increases mtROS generation and reduces EDD to ACh (223, 458).
Exposure to high glucose, mimicking insulin resistance with aging (independent of adiposity) (120, 378), is associated with increased ROS, thus associating exposure of ROS to changes in mitochondria shape. Using a photo-activatable mitochondrial reporter within the endothelium in mice both in vivo and ex vivo, Durand et al. demonstrated that acute exposure to high glucose (via tail vein injection or cultured primary cells) resulted in fragmented, disrupted mitochondrial networks relative to normal glucose conditions (117). Type 2 diabetes mellitus (T2DM) and manipulation of glucose concentrations (mimicking aging-increased insulin resistance) induces mitochondrial fission and reduces microvascular endothelial function (226, 408, 440). Within the brachial artery and in resistance arterioles, the presence of T2DM is associated with increased ROS, increased mitochondrial membrane potential, and increased protein markers of mitochondrial fission. These hyperfission changes in mitochondrial dynamics are associated with reduced vasodilation to ACh within resistance arterioles (223). Interestingly, administration of mitochondrial-targeted antioxidants improved FMD and ACh-induced vasodilation and decreased ROS (152, 375). Although T2DM and exposure to high glucose induced mitochondrial fission, Tanner et al. (440) demonstrated that exposure to low glucose also is associated with increased mitochondrial fission in cultured endothelial cells, whereas both pharmacological and genetic disruption of mitochondrial fission in low-glucose exposed resistance arterioles improved microvascular endothelial function (ACh-induced vasodilation). Although the specific role for aging in-it-of-itself (vs. aging-associated hyperglycemic stress) within this vasodilative context has yet to be fully elucidated, particularly within the microvasculature, it does provide evidence of mitochondrial dynamics driving both conduit artery and microvascular endothelial function. Therefore, further studies to directly link aging-mediated mitochondrial dynamic dysfunction to vasodilative function are highly warranted.
Similar to endothelial cells, mitochondrial dynamics drastically impact vascular smooth muscle (VSM) function, which is reviewed elsewhere (70, 283). Liu et al. demonstrated in rat mesenteric arteries and thoracic aorta that administration of phenylephrine (α-adrenergic agonist) and potassium resulted in mitochondrial fission within VSM cells, whereas inhibition of mitochondrial fission reduced vasoconstrictor responses to phenylephrine and potassium (263). Interestingly, aging favors α-adrenergic constriction in coronary vessels as described in Section 5 (19). In a similar light, Chen et al. (62) demonstrated that inhibition of mitochondrial fission reduced the vasoconstrictor response of mesenteric arteries and thoracic aorta in rats to endothelin-1, which is also increased in aging (155, 424).
There are implications for a role for mitochondrial hyperfission in aging-associated cardiovascular pathologies. Wang et al. (481) demonstrated that reduction of mitochondrial fission protein DRP-1 decreased VSM migration and reduced ROS, implicating a role for mitochondrial hyperfission in atherosclerosis. Interestingly, Mfn-2 has been demonstrated to inhibit VSM proliferation. Mfn-2 mediates apoptosis independent of its role within mitochondrial dynamics, and it is critical for the apoptotic response to hydrogen peroxide in VSM cells (hydrogen peroxide being an important pathologic mediator of atherosclerosis) (167, 401). However, Mfn-2 expression has been shown to be reduced in mouse and rat atherosclerosis and hypertension (66). Cardiac ischemia–reperfusion injury is associated with downregulation of Opa-1 in a DRP-1 hyperfission-dependent manner (67, 328), and Opa-1 and Mfn-1 and 2 have been shown to be protective against the development of heart failure from pressure overload (280, 355).
2.3. Vascular mitochondrial mitophagy
Over the past decade, the role of mitophagy in vascular structure and function in health and disease has become a popular area of research. The most well-characterized mitophagy signaling cascade is the PTEN-induced kinase 1 (Pink1)-Parkin-mediated pathway. Emerging evidence has demonstrated that mitophagy may be activated independent of Parkin-mediated mitophagy. More recent work has shown that these distinct signaling pathways are unique to the environmental stressors that mitochondria are exposed to, as well as distinct to the tissue studied (285).
2.4. Receptor-mediated mitophagy
The outer mitochondrial membrane expresses receptors that are specific for mitophagy, described as containing LC3-interacting regions, which function to dock with LC3 for canonical autophagy degradation. Currently, three receptors have been described as such: NIX, BNIP3 (BCL2/adenovirus e1B 19 kDa interacting protein 3), and FUNDC1 (FUN14 domain-containing protein 1). De-phosphorylation of threonine and serine residues allows for FUNDC1 to link with LC3B and undergo canonical autophagy (65, 262). Hypoxia appears to be the primary driving factor that activates FUNDC1-mediated mitophagy, and this mitophagy pathway may be particularly important within the vascular responses to hypoxia (e.g., ischemia–reperfusion). Zhang et al. demonstrated that hypoxia elicits FUNDC1-mediated mitophagy, evidenced by elevated mitochondrial proteins and the reciprocal marker of autophagy, p62, in FUNDC1 knockout mice, and no change in LC3-I expression (524). Further, platelets (which are exposed to varying levels of oxygen throughout the vasculature) from FUNDC1 knockout (KO) mice do not demonstrate formation of autophagosomes containing mitochondria. These responses are autophagy-dependent, as deletion of Atg5 resulted in no mitophagy activation in response to hypoxia in platelets.
Functionally, exposure to coronary ischemia–reperfusion increased mitophagy in wild-type (Cre negative) but not FUNDC1 platelet-specific KO (524). Although the specific role of FUNDC1-mediated mitophagy has been described in other organs and tissues, the specific role within the vasculature and whether this is altered with aging is unclear, representing a ripe area for future investigation. Fundamental questions regarding the role of FUNDC1 need to be addressed. Specifically, is FUNDC1-mediated mitophagy altered with disease or aging? In vascular-specific diseases such as peripheral arterial disease (PAD), the primary culprit of symptoms is a mismatching of oxygen delivery to oxygen demand, rendering tissues hypoxic. Do aberrant deviations in FUNDC1 and mitochondrial function play a role in the development and progression of PAD?
The aforementioned mitophagy-related receptors BNIP3 and NIX are also oxygen sensitive. Both BNIP3 and NIX express LC3 interacting region and interact with LC3 via phosphorylation of various serine sites to degrade mitochondria via canonical autophagy. In old mouse aortas (aged 27–28 months), no BNIP3 protein expression differences were found relative to young mice (242); however, protein expression of NIX was increased in older mice (18–19 months) basally and in response to serum starvation relative to young mice (457).
Interestingly, administration of trehalose (2% in drinking water) increased protein expression of BNIP3 (242). Within these studies, mitochondrial morphology of the older mice aortas was not examined. From a cell perspective, BNIP3-mediated mitophagy is important for maintenance of homeostasis in response to experimental hyperglycemia in the cerebral vasculature (207). Together, these findings indicate that receptor-mediated-mitophagy within the vasculature may be impaired with advancing age and in the presence of cardiovascular risk factors. Future investigations into the specific roles of receptor-mediated mitophagy are warranted.
2.5. Non-receptor mediated mitophagy
The most widely described mitophagy pathway is Pink1-Parkin mitophagy. Broadly, under non-stressed conditions, Pink1 is shuttled into the mitochondria, where it is subsequently degraded. Pink1 and Parkin act in concert with mitochondrial fission to encapsulate and degrade damaged mitochondria. On mitochondrial outer membrane depolarization, Pink1 accumulates on the outer membrane and recruits the E3 ubiquitin ligase Parkin. Parkin ubiquinates the damaged, depolarized mitochondria, which are then encapsulated by an autophagosome that fuses with an autolysosome containing acidic hydrolases, thereby degrading the mitochondria. Loss of Parkin function or aberrant actions of regulated (S-nitrosylated) Parkin results in the inadequate clearance of damaged mitochondria and may trigger further mitochondrial fission or fragmentation (74, 269, 479). Parkin plays a crucial role in mitochondrial dynamics. The role of mitophagy within premature hypertension and vascular aging has recently been succinctly reviewed elsewhere (390).
In relation to vascular aging, LaRocca et al. (242) demonstrated that within the aorta of old mice, protein expression of Parkin is reduced relative to young mice. The reduction in Parkin was paralleled with an increase in superoxide production and an increase in aortic stiffness (aortic pulse-wave velocity). Interestingly, Tyrrell et al. (457) demonstrated that aged mice demonstrated increased levels of Parkin within the aorta, coinciding with reduced mitochondrial function and increased mitophagy.
The differences in Parkin expression in these two studies may be due to the selected age range, as LaRocca et al. (242) studied mice that can be considered very old (27–28 months) whereas Tyrrell et al. (457) were interested in mice transitioning from middle age to old (18–19 months). Activation of autophagy with trehalose (2% drinking water) normalized Parkin expression, whereas spermidine did not influence levels of Pink-1 or Parkin in older mice fed a low-fat diet. Interestingly however, when mice were administered a western style diet (high fat, high sugar), spermidine offset the hypercholesteremic-induced increases in Parkin, and it preserved mitochondrial respiration within the aorta (457). Future investigations should examine the association between changes in mitochondrial proteins such as Pink1, Parkin, BNIP3, FUNDC1, and NIX and alterations in mitochondrial structure as they relate to functional outcomes.
3. ROS/RNS-Direct Vasodilation in Relation to Mitochondria and Aging
3.1. Age-related decline in nitric oxide with increased mitochondrial hydrogen peroxide signaling
In healthy adult individuals, nitric oxide plays a pivotal role in endothelium-dependent regulation of coronary vasodilation and blood flow (213). In the aging population, the endothelium has diminished control over vascular tone (496). This loss of endothelial function is induced by a reduction in nitric oxide availability via diminished flow-induced endothelial nitric oxide production (99, 213, 299) and/or amplified nitric oxide scavenging due to oxidative stress (45, 267, 299). In addition, nitric oxide generated in response to local hypoxia from red blood cell nitric oxide synthase (RBC-NOS) and RBC ATP-purinergic receptor agonist signaling-induced nitric oxide are reduced with aging due to reduced red blood cell deformability and oxidative stress (236, 335, 364, 365).
Indeed, oxidative stress is a primary player in declining endothelial function in aging; excessive production of superoxide in the aged vasculature leads to an increase in nitric oxide scavenging as superoxide and nitric oxide combine to produce the cytotoxic free radical peroxynitrite in mice (31), rats (130, 172, 463), and humans (367). Combination of superoxide and nitric oxide to produce peroxynitrite involves a diffusion-limited reaction that occurs approximately three times faster than the dismutation of superoxide to hydrogen peroxide by SOD (178, 308).
Flow-induced hydrogen peroxide generation is not diminished in advanced age (213), but rather is excessively enhanced in an aging rat model (322). Beyer et al. utilized a series of inhibitors/scavengers of vasodilative precursors (indomethacin for COX mediated prostacyclin synthesis, L-NAME for eNOS, and PEG-Catalase for hydrogen peroxide) and evaluated vasodilative function in human coronary and adipose microvessels across the human lifespan (30). The mechanism of FMD evolves throughout life. In children (0–18 years old) and young adults (19–55 years old), prostacyclin PGI2 and nitric oxide are the main vasodilative mediators, respectively. With onset of coronary artery disease (CAD) in older adults (>55 years old), the FMD mediator switches to hydrogen peroxide independent of age, and total vasodilative effect is diminished irrespective of aging, effects also seen in rodents (213). Supplementation of exogenous low-dose DETANONOate (nitric oxide donor, mimicking youth) in CAD vessels reduces the mtROS and hydrogen peroxide-FMD response, whereas eNOS blockade in young vessels (mimicking age/CAD) induces compensatory hydrogen peroxide-FMD (30). Collectively, this evidence demonstrates a plasticity in vasodilator mechanisms that changes throughout the human lifespan.
Hydrogen peroxide (as well as superoxide and peroxynitrite) can serve as an endothelium-derived hyperpolarizing factor that acts through calcium-activated potassium channels to elicit FMD (256, 267, 303, 324, 487). It is thought that eNOS-generated superoxide may be a primary contributor to hydrogen peroxide generated for FMD (414). Against the dogma that ROS are intrinsically harmful, it appears that they function as a compensatory mechanism in aging (and CAD), at least initially, leading to reduced nitric oxide bioavailability although with less efficacious vasodilation (303). Hydrogen peroxide may be the least damaging of the ROS, as it cannot quench nitric oxide and may confer some benefit. In some cases, hydrogen peroxide has been shown to improve nitric oxide production, upregulate eNOS expression and function, and enhance nitric oxide-FMD by stabilizing sGCβ1 mRNA to increase cGMF production (50, 51, 114, 279, 446).
Peroxynitrite may have a beneficial function, as it is a nitric oxide donor, acting as a reserve pool for nitric oxide (303). However, these potential beneficial effects are limited or reversed when concentrations exceed buffering capacity due to antioxidant protein dysfunction and pro-oxidant signaling, as discussed in the first section, as well as by enhanced toxic hydroxyl radical formation. In some cases, hydrogen peroxide can exhibit vasoconstriction (303). Overall, these lessons in the complexity of redox balance for vasodilative function and perfusion capacity teach us that the aim for future therapeutics should be to restore youthful nitric oxide-mediated FMD as opposed to merely inhibiting ROS, as ROS serve as a beneficial compensatory mechanism (although insufficient).
3.2. ACh and sodium nitroprusside-mediated vasodilation reduced in age
Impairment of EDD in aging is further evidenced by reduced relaxation to the eNOS agonist ACh in mice (97, 157, 252, 511), rats (72, 299, 463, 496), pigs (353), and humans (318, 431, 432). Proper dose-dependent dilation to ACh indicates an intact, healthy endothelium, whereas vasoconstriction to ACh may point to endothelial dysfunction (447). In contrast, endothelium-independent relaxation to sodium nitroprusside (SNP) is not commonly compromised (244, 431, 432, 463), which suggests that SMC-mediated dilation to nitric oxide is preserved in advancing age. Oxidative stress appears to trigger this age-related loss in endothelial function as well, as the simultaneous decreasing nitric oxide and increasing superoxide levels result in deteriorating nitric oxide-mediated signaling. Scavenging of superoxide via SOD transiently reestablished normal endothelial function in a transgenic mouse model of aging (97).
3.3. mtROS uncouple eNOS in age, shift to superoxide instead of nitric oxide production
The mitochondria play an incredibly important role in vascular stasis, regulating ROS levels via a complex interplay of enzymes as well as non-enzymatic antioxidants (12). When this mitochondrial regulatory network is imbalanced, vascular deficits follow. For example, the uncoupling of eNOS by mtROS plays a pivotal role in the shift from nitric oxide to superoxide in advancing age (307). In addition to direct mtROS release and NOX, uncoupled eNOS is one of the primary sources of endothelium-derived superoxide (99, 172). Tetrahydrobiopterin (or BH4), an essential cofactor in the catalysis of nitric oxide from l-arginine, superoxide, and NAPDH, is oxidized by high concentrations of peroxynitrite, high levels of which have been associated with atherosclerosis, hypertension, inflammation, cancer, and other deleterious effects of aging (339, 362, 429). Then, in the absence of BH4, eNOS becomes uncoupled and leads to the production of superoxide as well as additional peroxynitrite in a detrimental positive feedback loop (156, 238, 393). Peroxynitrite nitrosylates and further uncouples eNOS, and it also nitrosylates and inactivates SOD2, allowing further superoxide accumulation (463).
These are example of ROS-induced ROS release (RIRR), which can also trigger dysfunctional mitochondria and/or NOX to produce additional superoxide (85, 87, 531). In RIRR events, a slow accumulation of ROS serves as the trigger for the ensuing burst of ROS released on depolarization of the mitochondrial membrane potential (530, 531) and opening of mPTP (86). This phenomenon can be adaptive, as in instances of culling injurious organelles or cells, or maladaptive, leading to the death of otherwise healthy mitochondria and cells (531). The opening of mPTP can also directly stimulate the release of superoxide from the mitochondrial matrix; this efflux of superoxide from a subset of dysfunctional and/or activated mitochondria can prompt nearby mitochondria to do the same, leading to even higher levels of circulating ROS (85).
Uncoupled eNOS can also lead to endothelial dysfunction in both coronary and peripheral vessels (87). SOD2 plays a vital role in the dismutation of superoxide to hydrogen peroxide and the regulation of cellular redox homeostasis (387). In a transgenic mouse model of aging involving SOD2, amplified levels of mtROS and mtDNA damage were found in SOD2+/− mice compared with wild-type littermates; the resulting eNOS uncoupling in these mice was evidenced by severely impaired vasorelaxation (491). Transgenic SOD2−/− mice only survive roughly 10 days to 3 weeks due to a number of physiological ailments, including extensive mitochondrial injury within cardiomyocytes (245, 257). Similar studies have shown that endothelial dysfunction associated with eNOS uncoupling by mtROS can be prevented by the mitochondrial-targeted antioxidant mitoTEMPO (102). In addition, SOD2-overexpressing mice were moderately spared from eNOS uncoupling-induced vascular dysfunction (394).
3.4. FMD by nitric oxide or hydrogen peroxide influenced by mitochondria signaling (ROS)-dependent exocytosis of endothelium-derived extracellular vesicles
Mitochondrial generation of both superoxide (267) and hydrogen peroxide (48) directly contribute to flow-induced vasodilation and are upregulated in response to shear stress. In human coronary and other resistance arterioles, mitochondrial-derived superoxide and hydrogen peroxide are required for the formation of endothelium-derived extracellular vesicles (eEV), which bind with the cell membrane and release their contents into the circulation (140). These eEV can be beneficial, assisting with protein, lipid, and microRNA transport. However, the presence of high levels of eEV in blood serum has been linked to endothelial dysfunction (36), likely through the contents contained within the eEV, including ceramide and plasminogen activator inhibitor 1, which can cause arterioles to shift from nitric oxide to hydrogen peroxide production, inducing further endothelial dysfunction (140). Indeed, exogenous introduction of ceramide to healthy human arterioles induces mtROS production, leading to a diseased phenotype (139).
3.5. Mitochondrial respiratory dysfunction correlates to endothelium-dependent (ACh) but not independent (SNP) vasodilation in aging, likely through free radical-linked mechanism
Mitochondrial respiration also plays a role in vasodilatory responses. In experiments involving human skeletal muscle feed arteries, vasodilation via endothelium-dependent agonist ACh is drastically reduced in vessels from older patients and directly correlated via linear regression to reduced mitochondrial respiratory function within the vessels in an age-dependent fashion (346). Vascular mitochondrial oxidative respiratory capacity, as measured by state 3 mitochondrial respiratory complex I as well as complex I + II, is significantly higher in vessels from young when compared with elderly subjects (345, 346). Similar to the EDD deficits, this mitochondrial respiratory dysfunction in older patients is significantly correlated with endothelium-dependent (ACh) but not endothelium-independent (SNP) vasodilation, again suggesting normal smooth muscle control of dilation to nitric oxide even in the elderly population. Concomitantly, mitochondrial levels of superoxide are highest in the older patients.
3.6. FMD attenuation in aging correlates with increased S-nitrotyrosine
The attenuation of FMD observed in advanced aging is correlated with increased nitrotyrosine, an oxidatively modified amino acid and marker of oxidative stress usually linked to peroxynitrite. In humans, excessive levels of nitrotyrosine were found in brachial artery endothelial cells of older men; deficits in flow-mediated vasodilation were strongly correlated with increased levels of nitrotyrosine (107). Similar age-linked increases in nitrotyrosine have been described in rat arteries (81, 427, 463). In aortas isolated from aged rats, decreased nitric oxide, increased eNOS activity, superoxide overproduction, and subsequent peroxynitrite formation all combined to increase nitrosylation of tyrosine within mitochondrial SOD2 in a dynamic example of the complex interplay of ROS within the aging vasculature (463). In addition, overexpression of nitrotyrosine and flow-induced deficits have also been linked to estrogen levels, as ovariectomized rats show similar levels of peroxynitrite-induced nitrosylation when compared with aged rats (213). Scavenging of peroxynitrite via FeTMPyP, a synthetic porphyrin complexed with iron, significantly improved endothelium-dependent relaxation in aging rats (427). Antibiotic treatment has also shown beneficial antioxidant effects in aortic oxidative stress of old mice, as shown by decreased pro-inflammatory cytokines, decreased nitrotyrosine, and lower levels of superoxide (46).
4. Alterations to Endothelium-Dependent Hyperpolarization in Relation to Mitochondrial Redox Balance with Aging
4.1. Description of normal vasodilative endothelium-dependent hyperpolarization
Various factors mediate endothelium-dependent hyperpolarization (EDH), starting in the endothelial membrane and conducting through to the VSM cells to facilitate vasodilation. When endothelial intracellular [Ca2+] is increased due to shear stress activation of mechanosensitive transient receptor potential vanilloid type 4 channel (TRPV4), calcium-dependent potassium channels (SKCa, calcium-sensitive small conductance potassium channels [SKCa] and calcium-sensitive intermediate conductance potassium channels [IKCa]) cause hyperpolarization and K+ efflux, thereby stimulating VSM Na+, K+-ATPase and inward rectifying potassium channels (KIR) (147). The calcium increase to initiate this pathway can also activate eNOS to produce nitric oxide; however, this is diminished with aging and instead is instigated by ROS such as hydrogen peroxide to activate the endothelial KCa channels (361).
Hyperpolarization and calcium signaling are propagated through connexin gap junctions to the VSM, which together with hyperpolarization from other VSM potassium channels (voltage-gated potassium channels [Kv], ATP-sensitive potassium channels [KATP], and calcium-dependent large conductance potassium channels [BKCa]) lead to closing of voltage-gated calcium channels (VGCCs), reducing intracellular calcium and leading to vasodilation (147–149, 203). ACh- and FMD-mediated vasodilation can be blocked in part by inhibiting endothelial potassium channels with apamin (SKCa) and charybdotoxin (IKCa and BKCa), highlighting the critical role of potassium channels in the signaling of vasodilation. These pathways are of unique importance, as they allow axial conductive signaling for coordinated modulation of tone and blood flow of a large area of vascular network. Therefore, alterations due to pathology may be especially consequential. More details on EDH mechanisms can be read in the comprehensive reviews by Feletou and Garland and Dora and depicted along with influences of aging and oxidative stress in Figures 2 and 3 (126, 147).
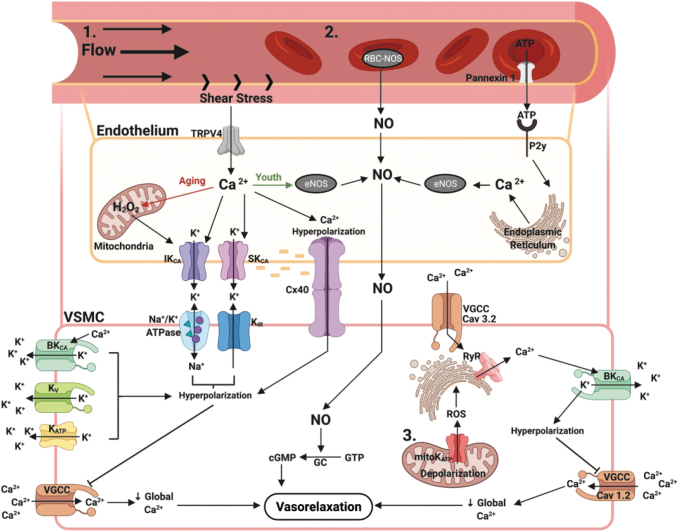
FIG. 2.
Flow-mediated dilation pathway with mitochondrial contributions and effects of aging and ROS/RNS. 1. Flow-induced shear stress activates mechanosensitive endothelial TRPV4 channels, allowing for calcium entry, which activates basal membrane SKCa and IKCa, causing potassium efflux and hyperpolarization that along with calcium can spread to the VSM cell via connexin 40 gap junctions. Potassium in the intercellular space activates the Na+/K+-ATPase and KIR, causing smooth muscle hyperpolarization. Hyperpolarization is amplified by contributions from BKCa, KV, and KATP. Taken together, the hyperpolarization signal inhibits the VGCC from transporting calcium into the cell, reducing intracellular calcium and inducing vasodilation. In youth, the initial calcium signal activates eNOS to produce nitric oxide, which causes VSM cell relaxation via production of cGMP, activation of potassium channels, and stimulation of cGMP-dependent protein kinases that activate myosin light chain kinase phosphatase. In aging, eNOS is downregulated and dysfunctional and instead shear stress induces production of hydrogen peroxide that activates potassium channels to induce hyperpolarization-mediated vasodilation. 2. RBCs contribute to hypoxic vasodilatory response by production of nitric oxide from RBC-NOS and ATP traversal through pannexin 1 to activate endothelial purinergic (P2y) receptors, increasing intracellular calcium and activation of eNOS to produce nitric oxide. In aging, reduced deformability of RBCs leads to reduced contribution of this pathway toward vasodilation. 3. Mitochondrial depolarization caused by activation of mitochondrial KATP channels induces ROS mediated alterations of ryanodine receptors on the nearby endoplasmic reticulum, which leads to the release of calcium sparks. Calcium sparks activate the BKCa channel, causing hyperpolarization, inhibition of VGCC, reduced intracellular calcium, and vasodilation. Overall, sedentary aging and oxidative stress reduce FMD efficacy. BKCa, calcium-dependent large conductance potassium channels; eNOS, endothelial nitric oxide synthase; FMD, flow-mediated dilation; IKCa, calcium sensitive intermediate conductance potassium channels; KATP, ATP sensitive potassium channels; KIR, inward rectifying potassium channels; Kv, voltage-gated potassium channels; RBC, red blood cell; RBC-NOS, red blood cell nitric oxide synthase; RNS, reactive nitrogen species; SKCa, calcium sensitive small conductance potassium channels; TRPV4, transient receptor potential vanilloid type 4 channel; VGCC, voltage-gated calcium channels; VSM, vascular smooth muscle. Figure created with BioRender.com
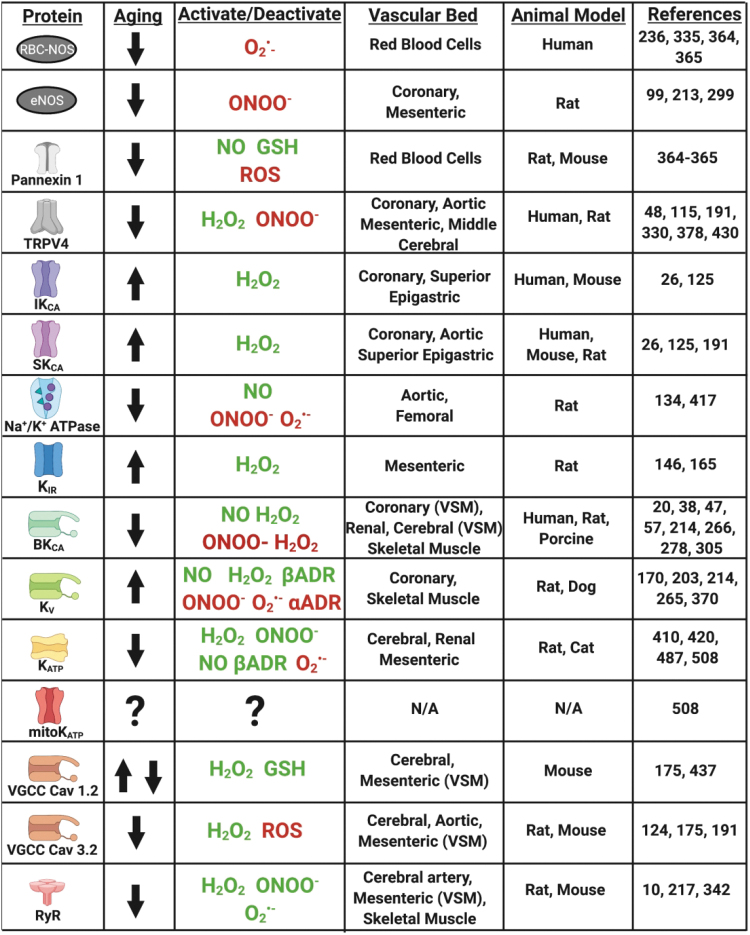
FIG. 3.
The effects of aging on the various channel expression and/or function in the FMD pathways with activating (green) or inhibitory (red) effects of ROS/RNS/adrenergic signaling. Figure created with BioRender.com
4.2. Effect of ROS and RNS on EDH
Mitochondrial superoxide, hydrogen peroxide, and peroxynitrite demonstrate vasodilator actions through activation of hyperpolarization pathways, and therefore there is a reliance on this in aging. This effect is partly mediated by TRPV4, which can be activated by hydrogen peroxide but is inhibited by peroxynitrite (48, 330, 428, 430).
Superoxide generated through xanthine oxidase and peroxynitrite has been shown to inhibit Kv channel activity, which can be restored by SOD whereas hydrogen peroxide is able to activate Kv channels (170, 265, 370). In rat cerebral arterioles, peroxynitrite inhibits BKCa channels (47, 266). In porcine coronary arteries and rat renal microvessels, nitric oxide and hydrogen peroxide were shown to activate BKCa (20, 305, 306). On the contrary, hydrogen peroxide has also been shown to close BKCa channels in porcine renal artery patch clamp experiments (38). This discrepancy likely indicates a direct inactivating and indirect activating effect of hydrogen peroxide on BKCa channels.
Superoxide decreases whereas hydrogen peroxide, peroxynitrite, and nitric oxide enhance KATP function in renal, cerebral, mesenteric, and coronary vasculature (420, 487). The KIR channel is activated by superoxide in rat mesenteric artery (146). These complex effects are likely due to thiol modification of key cysteines, including glutathionylation (510). Further details on how oxidative stress affects potassium channel function are available in various reviews (59, 69, 170, 179).
4.3. Mitochondrial depolarization-mediated vasodilation
Mitochondrial depolarization induced by ROS generates calcium sparks that have vascular effects (501). In a study by Katakam et al., ROS-dependent (diazoxide) and -independent (BMS-191095) activators of mitochondrial depolarization were utilized in wild-type, lean, and insulin resistant obese rats to elucidate a pathway for mitochondrial depolarization-mediated vasodilation in cerebral artery VSM (217). In the VSM, diazoxide and BMS-191095 lead to activation of mitochondrial KATP channel activation and depolarization. In mitochondrial microdomains adjacent to the sarcoplasmic reticulum, ryanodine-sensitive calcium channels become activated, releasing calcium sparks that activate plasma membrane BKCa channels, leading to K+ efflux and hyperpolarization to close VGCCs to decrease overall intracellular [Ca2+] and facilitate vasodilation (217). Activation of the ryanodine receptor occurs due to ROS-mediated post-translational modification (hydrogen peroxide-, superoxide-, or peroxynitrite-mediated) (10, 217, 342). However, since BMS-191905 also activated this pathway, the authors conclude that there is a secondary ROS-independent mechanism for mitochondrial depolarization-mediated vasodilation.
In obese insulin resistant rats, there is impairment of calcium spark release due to ER stress, impaired activation of BKCa, and plasma membrane ATP-sensitive K+ channels. In aging, the functional release of calcium sparks from ryanodine receptor activation with caffeine is reduced, owing to reduced coupling of the ryanodine receptor with the T-type calcium channel CaV3.2 in mouse mesenteric arteries (124). It is unknown how aging affects mitochondrial KATP function, although there are indications of dysfunction since the potassium cycle is impaired with decreased intramitochondrial potassium in aging rats (508).
4.4. Effect of aging on EDH
During certain phases of pathology or aging with active lifestyle (i.e., “healthy aging”), hyperpolarization-mediated vasodilative mechanisms can increase in function to partially compensate for lack of nitric oxide-mediated signaling (25, 313, 403, 404). However, in diabetes and studies of sedentary aging, it appears that elevated oxidative stress including increased superoxide, hydrogen peroxide, and peroxynitrite overall culminates in reduced EDH function (125, 126, 142, 265, 525). For instance, in aged rats, EDH-mediated relaxation from ACh or TRPV4 activator GSK1016790A was reduced compared with young rats; the response in young rats is reduced to old levels by inhibiting TRPV4 and SKCa (191). In old age, Feher et al. found that although local responses to bradykinin remain intact with age, the conductive axial response (spread hyperpolarization and dilation at distant sites) was diminished in coronary arteries of humans, and local conductive responses were due to enhanced SKCa and IKCa activity, with similar findings observed in mouse models (26, 125). Similarly, KIR function is increased with aging, with no change in KIR mRNA (176).
On the other hand, endothelial TRPV4 expression and calcium signaling, an initiator of the hyperpolarization vasodilation response, are reduced in aging but can be restored by lentiviral induced overexpression in aging rat mesenteric arteries (115). Expression and function of the BKCa channel is diminished in aging mesenteric, skeletal muscle, and coronary (but not cerebral) vasculature, leading to inhibited vasorelaxation (57, 214, 278). However, KV1.5 channel protein expression does not change with age, although inhibition leads to increased myogenic tone in aging versus youth, indicating increased KV1.5 function with aging in skeletal muscle arterioles (214). Direct inhibition of VSM cell Na+/K+ ATPase leads to constriction in young, but not old rats, indicating potentially reduced contribution for the Na/K ATPase in EDH-mediated vasodilation in aging (417). It is known that ROS-mediated glutathionylation of the β1 subunit inhibits Na+/K+ ATPase function and is reversible by nitric oxide (134).
In addition, KATP channels are dysfunctional during aging, as the necessary subunits Kir6.1 and SUR2B are downregulated and protein kinase A (PKA)-mediated activation is attenuated (although activation with nicorandil and direct PKA activation leads to no age-related differences) (508). The Cav3.2 T-type VGCC, involved in the ryanodine receptor/BKCa-mediated hyperpolarization and dilation response, is also downregulated in aging, including in coronary arteries, and can be inhibited by hydrogen peroxide and ROS (175, 190). Alternatively, the L-Type Cav1.2 channel expression and function in aging varies greatly from study to study, being either over- or under-expressed depending on the arterial bed being studied. In coronary artery VSM cells, Cav1.2 expression and current is not affected by aging in rats but is decreased in posterior and middle cerebral arteries and increased in mesenteric arteries in mice (175). The Cav1.2 channel can be glutathionylated and activated by hydrogen peroxide and oxidized glutathione (437).
Considering ROS are important factors in EDH, yet excessive oxidative stress tends to have inhibitory effects on EDH, this implies that a fine homeostatic balance is required for ideal vasodilative performance. The EDH is also a minor contributor to relaxation during β-adrenergic-mediated vasodilation through simultaneous activation of Kv and KATP channels (170, 203, 410), and α-adrenergic agonism can close Kv channels (203). Further, βADR activation leads to a transient increase of ROS, which can activate L-type calcium channels and induce alterations in calcium signaling (11, 37). Therefore, EDH dysfunction in aging may also contribute to βADR dysfunction. In addition, the fine redox balance necessary for functional EDH extends to intrinsic βADR function. In all, mitochondrial dysfunction plays a critical role in the signaling of vasodilation, and alterations of mitochondrial function with aging have significant implications for vasodilative function.
5. Adrenergic Alterations in Relation to Mitochondrial Redox Homeostasis with Aging
5.1. Description of adrenergic receptor homeostatic shift with aging
The expression and function of adrenergic receptors varies with tissue location and branching order, and they are influenced by redox status of endothelial cells causing adrenergic dysfunction in aging. The major role of these receptors on vascular tissue, specifically, is to mediate vasorelaxation or vasoconstriction via agonism typically in response to circulating catecholamines. The β-adrenergic receptors β1, β2, and β3 activate G-stimulatory (Gs) protein to activate adenylate cyclase, which, in turn, converts ATP into cyclic AMP (cAMP), leading to activation of PKA to phosphorylate the myosin light chain kinase, resulting in inactivation of the myosin light chain, and finally inducing vasodilation (96, 466).
On the other hand, α1ADR activates Gq alpha subunit and phospholipase C to hydrolyze phosphatidylinositol 4,5-bisphosphate (PIP2) into diacylglycerol (DAG) and inositol triphosphate (IP3), which, in turn, activates its receptor in the ER to release calcium and induce vasoconstriction. The α2 receptors induce vasoconstriction via activation of G inhibitory (Gi) protein, thereby inhibiting adenylate cyclase.
In the coronary vasculature, endothelial cells express predominantly β1 whereas the SMCs express predominantly β1 and α1 receptors (19). As branching order increases, relative expression of β1ADR and α1ADR decreases whereas β2ADR, β3ADR, and α2ADR increase. In healthy vessels, the balance favors β vasodilation to adrenergic agonism; however, with aging or disease, this balance shifts to favor αADR-mediated vasoconstriction (19, 376). This is, in part, due to the decline in function and expression of βADRs with age in many tissues, including the aortic (270), mesenteric (142, 143), and coronary (376) vasculature, but increased in aging cerebral vasculature (211, 389, 395).
Coronary and aortic α1ADR, on the other hand, do not display changes in expression with age, promoting a propensity for hyperconstriction (19, 104, 169), whereas α1ADR expression or function with aging decreases in skeletal muscle and renal artery, although skeletal muscle is paradoxically hyperconstricted with aging (258, 348, 377, 500). The changes in vascular adrenergic function with age are irrespective of alterations of downstream signaling. βADR dysfunction with age leads to insufficient cAMP synthesis, whereas exogenous stimulation of elements downstream from the receptor stimulates normal cAMP production (209, 218, 271, 272, 398). Age-related βADR function is not due to G-protein switching from stimulatory to inhibitory (218, 272, 397), suggesting dysfunction of the βADR itself or with coupling, rather than downstream signaling messengers.
5.2. Effect of aging on adrenergic receptor regulation through desensitization, internalization, and recycling
Current consensus is that functional adrenergic changes in aging are mediated by regulatory proteins and post-translational modifications at the level of the βADR, which can be influenced by aging-induced redox changes. On agonism in young vessels, a protein complex including either G-protein receptor kinase (GRK) or β-Adrenergic Receptor Kinase, and β-arrestin associates with the βADR to cause desensitization, blunting the ability of agonists to increase cAMP. This regulatory system is upregulated in aging, alongside increased circulating catecholamines (61, 341). Interestingly, GRK2 (the most commonly implicated GRK) increases 3.6- and 1.5-fold in the cytosolic and membrane fraction, respectively, with β-arrestin increasing 1.6-fold in aged (24 months old) Fischer 344 rat aortas (145, 396).
On receptor-agonist binding, GRKs associate with the β receptor, leading to receptor phosphorylation, thereby sterically hindering the G protein coupling and causing G stimulatory protein dissociation (28). The β receptor can also be phosphorylated independent of agonist (or at very low agonist concentration) binding by PKA and PKC. Both of these mechanisms are negative feedback regulators since their activation is cAMP-dependent (251). In addition, GRK-mediated phosphorylation recruits β-arrestin to the receptor-GRK complex, further causing steric hindrance and desensitization (251). β-arrestin primes the β-receptor for dynamin-mediated internalization into clathrin-coated endosomes for storage, trafficking to lysosomes for degradation, or eventual recycling to the plasma membrane (112, 128, 250, 273).
These pro-desensitization and internalization processes exist in a homeostatic balance with the process of resensitization, where βADR receptors are dephosphorylated at the plasma membrane or predominantly at the endosome, then trafficked back to the plasma membrane ready to receive agonistic signals and induce vasodilation (411, 466, 516, 519, 520). Resensitization is mediated by phosphatases, namely protein phosphatase 2A (PP2A) (235). This homeostatic balance between desensitization/internalization and resensitization favors the former in aging, not only because of increased GRK2 and β-arrestin expression, but also due to inhibition of resensitization. The dephosphorylation action by PP2A is inhibited by the endogenous inhibitor of PP2A, I2PP2A. I2PP2A is activated by PI3Kγ-mediated phosphorylation (357, 466). The Ang II activation of the AT1 receptor activates PI3Kγ-mediated pathways and increases with aging (181, 240, 332, 333).
Oxidative stress has been shown to activate PI3Kγ signaling in the diabetic rat cardiomyocyte model (high glucose co-culture) (418). Together, aging-induced Ang II and ROS signaling could, in part, explain the eschewed balance toward βADR phosphorylation, desensitization, and, ultimately, hyperconstriction with diminished agonist-relaxation. Inhibiting PI3Kγ in peripheral vessels rescues endothelium-independent vasodilation through induced L-type calcium channel dysfunction and also preserves β-adrenergic receptor function in cardiomyocytes from heart failure rats, although the ability to preserve βADR function in the endothelium by PI3Kγ inhibition still needs confirmation (55, 56, 311, 321, 331, 350, 412, 467, 468).
5.3. Effect of redox status on adrenergic receptor regulation
The processes mentioned earlier can be influenced by redox status to affect βADR function and location. The βADR and its regulatory proteins can be oxidized/nitrosylated by ROS/RNS. A major known regulator of desensitization/internalization is SNO post-translational modification (83, 466); SNO of β-arrestin and dynamin favor desensitization/internalization and inhibit αADR mediated vasoconstriction (4, 323, 337, 464, 478). In contrast, SNO of GRK2 prevents desensitization/internalization and is the major post-translational regulator against desensitization/internalization (275, 493). Studies utilizing exogenous SNO agents find βADR localization to the plasma membrane, with decreased β-arrestin localization, and enhanced vasodilation suggesting that SNO of GRK2 supersedes pro-desensitization/internalization SNO processes (493). In addition, PKA is constitutively activated by SNO and facilitates cross-talk by increasing cAMP to induce vasodilation in “β-adrenergic-like” signaling (49).
Of equal importance is the process for denitrosylation of these proteins by nitrosoglutathione reductase. Knockout of nitrosoglutathione reductase reduces vascular tone, whereas inhibition improves β-adrenergic function, suggesting that a fine balance of SNO is necessary for ideal βADR function (27, 68, 129). These proteins are nitrosylated, in part, via nitric oxide produced by eNOS, which is dysfunctional in aging (511). Therefore, it is possible that aging-mediated reduction of eNOS function, increased superoxide (which siphons nitric oxide to form peroxynitrite), and reduced nitric oxide bioavailability reduce the ability to maintain the nitrosylated status of these proteins, causing a shift favoring adrenergic receptor desensitization/internalization.
Although it has been known that oxidative stress negatively impacts functional vasodilation, its role in βADR physiology has only recently being investigated. The β1–3ADRs contain cysteine residues that are susceptible to oxidation, whereas only β1–2ADRs have extracellular tyrosine residues susceptible to nitration (254). Unpublished data from our lab in female Fischer-344 rats suggest that percent vasorelaxation to β1 agonist norepinephrine directly and positively correlates with nitric oxide concentration and inversely correlates with superoxide and hydrogen peroxide, but not peroxynitrite concentration, in an aging-dependent manner (unpublished observations). Exogenous superoxide/hydrogen peroxide incubation in young rat vessels completely abrogates β1ADR receptor agonist (dobutamine and norepinephrine) mediated vasodilation (116).
Exogenous peroxynitrite also blunts vasodilation to S-nitrosocysteine (nitrosylating agent) by oxidizing cysteine or nitrosylating tyrosine recognition sites (253). Peroxynitrite forms S-nitrosoglutathione with glutathione presence, providing a potential pathway for SNO of GRK2 and inhibition of desensitization/internalization (or dynamin/β-arrestin promoting desensitization/internalization). This pathway is likely reduced with aging as is GSH (379). Of interest, S-nitrosoglutathione also inhibits NOX and could, therefore, also reduce oxidative stress (379). Phosphorylated STAT3 is a positive transcriptional regulator of cardiomyocyte β1ADR that also mediates transcription of antioxidant (SOD2), proangiogenic (VEGF), and antiapoptotic proteins, and it exerts noncanonical actions to reduce ROS production, regulate mitochondrial complex 1 function and mPTP opening (523, 532). In addition, STAT3 can become activated on agonism of the β1ADR (523). This provides another regulatory link between β1ADR and ROS, and of interest STAT3 is known to be reduced with aging and therefore contributing to endothelial dysfunction, although many of these listed actions of STAT3 need to be confirmed in the vasculature (532).
Although oxidation tends to attenuate βADR vasodilatory function, nitrosylation has a protective effect. Exogenous S-nitrosoglutathione (a nitrosylating agent) is able to mediate nitrosylation of GRK2, inhibiting phosphorylation and subsequent desensitization and internalization of the β2ADR even during isoproterenol agonism, with subsequent cAMP production (275). Frame et al. showed that pre-incubation with SNP (a nitrosylating agent and nitric oxide donor) improves β2ADR function to vasodilate in vivo arcade and terminal arteries of the hamster cheek pouch tissue (137). Specifically, SNP pre-incubation “uncovers” distinct pools of βADR receptors to allow for increased potency at the picomolar range (although with reduced efficacy at the micromolar range).
In addition, in the study by Frame et al., a culture system and fluorescence imaging (fluorescence resonance energy transfer analysis) were used to show the influence of nitrosative and oxidative stress on internalization of the β2-adrenergic receptor. Exogenous superoxide production increased internalization of the β2ADR. Desensitization/internalization was blocked with SNP and dynasore (dynamin inhibitor of endocytosis) incubation; GRK2 was seemingly nitrosylated by nitric oxide, whereas dynamin-mediated endosome formation was blocked by dynasore. The concentration response and fluorescence data suggest that with this treatment, some of the β2ADR were protected from internalization (i.e., not internalized) and remain functional at the plasma membrane. It was hypothesized that preventing desensitization and endosome formation would improve βADR function in the classic dose ranges (10−9–10−4 M). Instead, SNP/dynasore pretreatment altered the pharmacodynamics by improving isoproterenol potency but reducing efficacy. The explanation is that increased cellular RNS state also facilitates dynamin and β-arrestin nitrosylation, which are pro-internalization processes. The balance between GRK2 nitrosylation/dynamin inhibition and dynamin/β-arrestin nitrosative activation leads to the functional changes seen. It is speculated that SNP shortens the time for β2ADR dephosphorylation with increased cycling of phosphorylation state, uncovering dilation potential at lower doses (10−14–10−11 M).
To summarize, βADR density and function are reduced in aging vasculature, leading to diminished vasodilatory capacity and hyperconstricted tone. Adrenergic regulatory proteins are subject to regulation by ROS/RNS. Therefore, aging-mediated alterations of redox balance may influence the homeostatic balance of desensitization/internalization and resensitization. This is supported since changes in ROS/RNS with age correlate with β1ADR function and the results of Frame et al. show that exogenously supplying ROS (mimicking aging) or RNS (mimicking youth) influences desensitization/internalization of the β2-adrenergic receptors (although in HeLa cells, not endothelial cells) (137). These complex interactions are illustrated in Figure 4, including pathways for how ROS/RNS may influence βADR function in what we name for the first time the ROS/RNS-βADR Desensitization and Internalization Axis. Further experimentation in young versus aged endothelium, mimicking studies such as by Frame et al., are needed to establish the validity of this axis, which could represent several novel opportunities for therapeutic targeting for diseases with adrenergic pathologies (137).
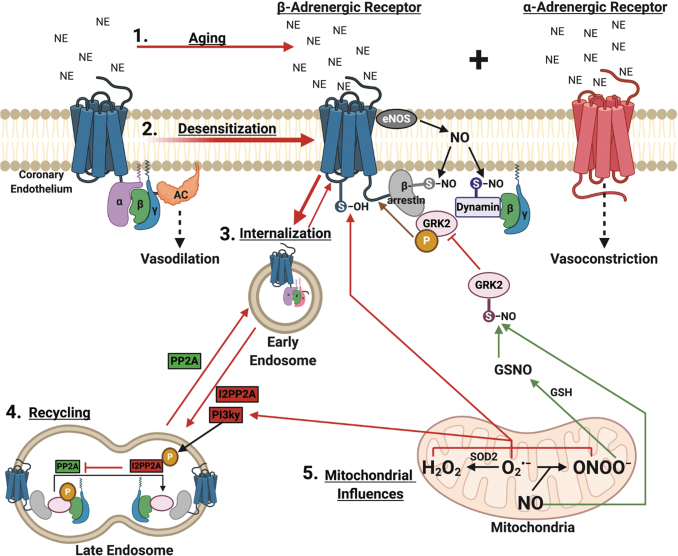
FIG. 4.
Influence of mitochondrial ROS/RNS on β-adrenergic receptor function with aging. 1. With aging, there is a sympathetic overdrive of systemic catecholamines that saturate the adrenergic receptors. 2. The βADR becomes desensitized due to GRK2 translocation to the βγ subunits and subsequent receptor phosphorylation. 3. Receptor phosphorylation recruits β-arrestin and dynamin to facilitate receptor internalization into clatherin-coated endosomes, the effect of which is enhanced by eNOS-produced NO-mediated nitrosylation of β-arrestin and dynamin. The internalized receptor can be degraded by further trafficking to lysosomes. 4. Alternatively, receptors can be recycled back to the plasma membrane on dephosphorylation mediated by PP2A. However, recycling can be inhibited by I2PP2A, activated by PI3kγ-mediated phosphorylation. 5. Mitochondrial ROS potentially encourages further desensitization and internalization by thiol-oxidation of the βADR itself and by inhibiting recycling by activating PI3kγ signaling. On the other hand, nitric oxide, plentiful in youth, provides nitrosylation of GRK2, sterically inhibiting recruitment of β-arrestin and dynamin, blocking internalization. Peroxynitrite can potentially nitrosylate GRK2 as well, as it can serve as either an oxidizing or nitrosylating agent, or it can combine with glutathione-forming nitrosoglutathione, another nitrosylating agent. These mitochondrial influences on βADR function can be described as an ROS/RNS-βADR Desensitization and Internalization Axis. AC, adenylate cyclase; ADR, adrenergic receptor; GRK2, G-protein receptor kinase 2; GSH, glutathione; GSNO, S-nitrosoglutathione; I2PP2A, endogenous inhibitor of PP2A; NE, norepinephrine; NO, nitric oxide; S-NO, thiol nitrosylation; S-OH, thiol oxidation; PP2A, protein phosphatase 2A. Figure created with BioRender.com
5.4. Clinical consequences of aging-induced adrenergic alterations
These alterations in adrenergic homeostasis with aging that favor αADR hyperconstriction manifest in clinical pathologies such as CAD and coronary microvascular disease (CMD), also known as cardiac syndrome X (19, 376). CMD presents in a majority of aging postmenopausal women with chronic angina due to microvascular hyperconstriction, as opposed to atherosclerotic blockage seen typically in men. Diagnostically, CMD is suspected when a female patient has a chief complaint of chest pain but there is no obvious obstruction on a coronary angiogram. Clinically, CMD is defined as having coronary flow reserve (CFR) ≤2.5, endothelial dysfunction with constriction to ACh, or <20% coronary dilation to nitroglycerin. The CFR may be calculated via dobutamine (β1ADR agonist) or adenosine-induced stress echocardiography, highlighting the adrenergic ties in CMD (348), although other imaging modalities (positron emission tomography, computerized tomography) are more often employed clinically with various merits and cons to each (348).
The CMD has limited guidelines and treatment effectiveness. The European Society of Cardiology recommends symptomatic relief with nitrate, β-blocker, or calcium channel blocker (443). However, such a strategy does not address the root cause of the pathology, that is, hyperconstriction; rather, it focuses on anti-ischemic protection via reduced inotropism and oxygen conservation. Further, β-blockers may actually potentiate dysfunction by blocking βADR-mediated dilation. The information in this section as well as the proposed ROS/RNS-βADR Desensitization and Internalization Axis (Figs. 4 and 5) provides numerous potential targets to restore functional βADR such as attenuating aging-induced mitochondrial-derived oxidative stress, inhibiting proteins involved in the βADR desensitization and internalization process, or activation of βADR recycling.
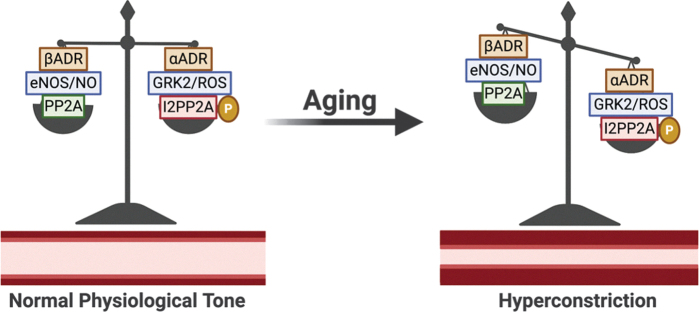
FIG. 5.
Effect of aging on vascular adrenergic signaling homeostatic balance. These effects culminate in reduced vascular βADR with aging, although αADR functional expression is unchanged, favoring hyperconstriction. Figure created with BioRender.com
In CMD, such a strategy is of significant clinical interest considering that up to 75% of women and 15% of men with angina have no obstructive coronary pathology, representing 90,000 cases per year with a greater chance of major adverse cardiac events (MACEs) than asymptomatic women (3.0%–8.2% 5-year mortality for normal to nonobstructive disease) (75). The CMD represents significant societal economic cost burden, similar to that of obstructive disease (∼$750,000 vs. ∼$1,000,000 lifetime cost burden), driven by repeat angiography, increased anti-ischemic therapy costs, and increased hospitalization, thus showcasing the significance of this pathology and the need for comprehensive management strategies (407). More details on CMD can be found in the Women's Ischemia Syndrome Evaluation study (9).
6. Aging-Mediated Mitochondrial/Endothelial Dysfunction and Current Therapies: Effect on Major Adverse Cardiac Events
mtDNA4977 deletion, also referred to as the “common deletion,” is responsible for key subunits of mitochondria respiratory complexes that, if damaged, result in impaired respiration and production of ROS (488). mtDNA damage is associated with the presence and progression of atherosclerosis, resulting in impaired mitochondrial structure and function to ultimately produce excess ROS, which, if left unchecked, further produces ROS (RIRR) (514, 515). The relation of mtDNA damage and atherosclerosis has been recently reviewed elsewhere (469, 513), and higher mtDNA damage is associated with impaired vascular function in peripheral blood mononuclear cells (133).
In relation to aging, accumulation of mtDNA4977 deletions within cardiac tissue (e.g., left ventricle) over time can result in pathological cardiovascular consequences (6, 77, 133, 286). In patients with CAD, increased levels of mtDNA4977 deletion in peripheral blood are associated with MACEs and all-cause mortality, suggesting that accumulation rather than the presence of the common deletion alone is a crucial factor in predicting adverse outcomes (35, 470, 471). Further, mtDNA copy number may also decrease with pathologies. Indeed, for every one standard deviation reduction in mtDNA copy number, Wang et al. (484) demonstrated that the risk of CAD increases 1.14-fold in peripheral blood leukocytes. Further, Koller et al. demonstrated that lower mtDNA copy number in peripheral blood leukocytes was associated with an almost twofold increase in risk for peripheral artery disease and all-cause mortality (230).
This evidence suggests that mtDNA copy number is an independent risk factor for future CAD (484), vascular dysfunction, and all-cause mortality. On the contrary, Vecoli et al. (470) demonstrated that changes in mtDNA copy number in peripheral blood alone did not predict MACE or all-cause mortality; rather, changes in mtDNA copy number coupled with increased mtDNA4977 deletion increase the risk for MACE. In further support of this notion, data from the VA Normative Aging Study demonstrated that peripheral blood mtDNA copy number is associated with increased mtDNA damage, independent of chronological age (106). The link between mitochondrial dysfunction and MACE is further propagated by the finding that in patients with mitochondrial diseases, there is a 2.4-fold greater risk for MACE, and 14-fold increased risk of all-cause mortality (319). Taken together, mtDNA damage accumulation and decreased mtDNA copy number significantly increase the risk for future MACEs.
Current pharmacologic strategies to alleviate symptoms of angina include the use of nitrates, a strategy that has been employed since 1876 when nitroglycerine was first used to treat stable angina (441). Nitrates work by activating NO-cGMP signaling pathways. Rapid relief of acute angina symptoms is accomplished by peripheral venous dilation-mediated reduction of preload and left ventricular wall stress, lowering afterload and systemic blood pressure, reduced vasospasm, and epicardial coronary artery dilation. Overall, this leads to decreased myocardial oxygen demand and improved myocardial flow. Long-acting nitrates that may be taken for angina prophylaxis are second-line therapy to β blockers and calcium channel antagonists.
The major limitation to nitrate therapy is tolerance with rebound angina, necessitating carefully planned dosing regimens (441, 442). Tolerance occurs within 12–24 h of use; therefore, nitrates are currently indicated for symptomatic relief only. The causes of tolerance are due to exhaustion of cofactors, downregulation and inactivation of enzymes, and, most importantly, paradoxical induction of oxidative stress (161, 441). Superoxide formation in response to chronic nitrate therapy uncouples eNOS, decreasing NO availability and eventually causing further endothelial dysfunction. Another pharmacologic, nicorandil, is without the problem of tolerance and has the additional benefit of activating KATP channel-mediated dilation (441). Nicorandil has been shown in the Japanese Coronary Artery Disease (JCAD) Study to significantly reduce deaths from all causes, cardiac deaths, and fatal (but not nonfatal) myocardial infarction events (186). However, there is no conclusive evidence for improved clinical outcomes over currently indicated therapies utilizing either nitrates or nicorandil alone or in combination, and combination therapy may actually increase MACE (232, 435, 441, 442). Therefore, although these therapies are useful for providing symptomatic relief, it is incumbent to develop alternative strategies without these limitations that are superior to current standards and to encourage healthy preventative evidence-based habits.
7. Emerging Therapeutic Strategies to Improve Relaxation of Aging Vessels
Therapeutic targeting to dissuade desensitization/internalization and/or bolster resensitization of the β-adrenergic receptor would have impactful implications on the treatment of adrenergic-related pathologies, such as asthma, chronic obstructive pulmonary disease, muscle wasting, heart failure, and endothelial dysfunction seen in CMD. Such a strategy may have a benefit over conventional treatments, such as direct agonism (salbutamol effects on β2ADR for asthma, isoproterenol effects on β1 and β2ADRs for CMD, etc.), as these treatments can further potentiate desensitization/internalization by facilitating recruitment of GRK2, β-arrestin, and PI3Kγ to the receptor. Further, therapeutic approaches to encourage boosting nitric oxide availability and signaling, such as for FMD, is of benefit while considering the cardio and vasculo-protective effects of nitric oxide and that hydrogen peroxide signaling coincides with atherosclerotic development and aging pathogenesis.
Several approaches to address mitochondrial dysfunction-mediated oxidative stress and vascular pathology are currently being investigated with the philosophy that since arterial endothelial dysfunction is a precursor to cardiovascular disease, early therapeutic intervention may stave off premature aging-induced cardiovascular disease. These interventions are presented in Table 1 as a theoretical construct for which current therapeutic investigation and development in the field have been recently focused. Table 1 does not necessarily indicate current medically indicated treatments. The most effective method to ameliorate aging-associated vascular pathology is to focus on preventative measures, of which simple exercise and dietary considerations (such as the Mediterranean diet) may be most easily achieved and effective (1, 227, 228).
Table 1.
Therapies to Reduce Reactive Oxygen Species and Restore Cardiovascular Function in Aging
| Therapy | Molecular and cellular changes | Study model | Tissue model | Subject age/weight | References | |
|---|---|---|---|---|---|---|
| Mediterranean diet and supplemental vitamins | ||||||
| Vitamin E | ↓ Superoxide | Wistar rats | Aortic rings | 11–14 Weeks old | (20) | |
| Vitamin B12 | ↑ Blood flow in skeletal muscles | Human subjects | Brachial and superficial femoral arteries | 60–80 Years old | (372) | |
| Omega 3 (fish oil) | ↑ Mfn-2 fusion ↑ Opa-1 |
↓ Oxidative stress | Wistar rats (M) | Liver | 2 Months old | (358) |
| Blueberries, apples, green tea, and nuts (polyphenols, flavonoids, catechins, selenium) | ↑ NO ↑ eNOS ↑ SOD ↑ Mfn-2 fusion |
↓ Blood glucose ↓ ACE ↓ NOX4 ↓ Xanthine oxidase-1 ↓ LDL ↓ DRP-1 fission |
C57 mice (M) In vitro In vitro In vitro In vitro Human subjects |
Aorta HUVEC HRCEC HUVEC HUVEC Mortality |
200 ± 25 g 8 Weeks old 55–75 Years old |
(63, 193–195, 201, 222, 354) |
| Mushrooms, yeast, and seaweed (trehalose) | ↑ NO ↑ EDD ↑ Mitophagy |
↓ Oxidative stress ↓ Inflammation |
Human subjects Human subjects (M) and C57BL/6 mice (M) |
Forearm arteries and veins HUVECs and brachial artery and isolated coronaries and aortic samples |
50–77 Years old 20–31 and 61–71 Years old 4–6 and 27–28 Months old |
(216, 243) |
| Red wine (resveratrol) | ↑ NO ↑ Peroxynitrite ↑ eNOS ↑ Sirt1 ↑ EDD |
↓ Oxidative stress ↓ NOX1 ↓ p22phox ↓ Arginase 1 ↓ AT1 |
Mice and in vitro Wistar rats (M) Fisher 344xBrown Norway rats (M) C57BL/6JJcl mice (M) Pigs Sprague-Dawley rats (M) Sprague-Dawley rats (M) Sprague-Dawley rats (M) Sprague-Dawley rats (M) Human subjects (R) Human subjects Human subjects Human subjects Human subjects (F) Human subjects (M) Human subjects (R) Human subjects (F) Human subjects (R) Mice, rats, and human subjects (R) |
Thoracic aorta, mesenteric arteries, and HUVEC Mesenteric arteries Mesenteric arteries and aorta Retina and retinal glial cells Retinal arteries Renal artery Renal artery Renal function Hindlimb muscle blood flow Epidemiology and cardiac function Platelets Cerebral blood flow Brachial artery and cardiac function Brachial artery Saphenous vein and mammary artery Blood biomarkers and brachial artery Cerebral blood flow Brachial artery and blood biomarkers Coronary arterioles, aortic rings, and cardiac function |
3 Months old 16–40 Weeks old 6 and 24 Months old adult 8–12 Weeks old 300–400 g 225–250 g 200–225 g 300 g 32–51 Years old 21–29 Years old 57–75 Years old 52–63 Years old 47–56 Years old 45–85 Years old |
(17, 53, 60, 88, 154, 158–160, 164, 221, 274, 312, 338, 363, 416, 445, 483, 498, 529) |
| Aerobic Exercise | ↑ EDD ↑ SOD ↑ Blood flow in skeletal muscles |
↓ Oxidative stress ↓ NAPDH oxidase ↓ Endothelin-mediated vasoconstriction |
B6D2F1 mice (M) Sprague-Dawley rats (M) Human subjects (R) Fisher 344 rats (M) Fisher 344 rats (M) Human subjects (M) Human subjects (M) Human subjects Human subjects Mice and human subjects (R) Human subjects (M) Human subjects (M) Human subjects Human subjects (R) |
Telemetry and carotid arteries Aortic rings Meta-analysis, coronary arteries, and blood biomarkers Coronary arterioles and cardiac function Coronary arterioles Brachial artery Brachial artery Brachial artery Radial artery and cardiac function Aorta, brachial, carotid, and leg arteries Brachial artery Popliteal artery Brachial artery Cerebral, brachial, skeletal arteries, and aorta |
4–7 and 29–32 Months old 19–21 Months old 3–4 and 20–21 Months old 3–4 and 20–21 Months old 22–35 and 50–76 Years old 18–30 and 60–70 Years old 18–75 Years old ≤55 and ≥65 Years old 21–34 and 52–70 Years old 62–72 Years old 64–70 Years old |
(21, 44, 98, 119, 123, 187, 191, 198, 212, 304, 384, 386, 399, 436, 465) |
| MitoTEMPO and MitoTEMPOL | ↑ Phenylephrine-induced contraction ↑ Acetylcholine-induced dilation ↑ Mitochondrial fission/fusion balance |
↓ Superoxide ↓ Oxidative stress |
C57BL/6 strain mice (F and M) Human subjects (F and M) Human subjects (F and M) Human subjects (F and M) Wistar rat (M) |
Brain Brain Skin Brachial artery Aorta |
3–30 Months old 78–85 Years old 57–63 Years old 35–70 Years old 6–24 Months old |
(8, 40, 224, 225, 325) |
| mitoQ | ↑ NO ↑ eNOS ↑ SOD2 ↑ COV-IV ↑ PGC-1α ↑ EDD ↑ LV diastolic function |
↓ Superoxide ↓ Oxidative stress ↓ p66shc ↓ Aortic stiffness ↓ Oxidized LDL |
C57BL/6 mice and human tissues Human subjects Wistar rats (M) In vitro C57BL/6 mice (M) C57BL/6 mice (M) Human subjects and mice (R) Human subjects Wistar rats (M) Human subjects |
Brain Kidney Cardiac function and aorta LLC Aorta Aorta Coronary, brachial artery, and aorta Brachial artery Cardiac function and myocardium Skeletal muscle arteries |
3–30 Months old and 30–35 years old 56–63 Years old 6 and 24 Months old 6 and 25 Months old 4 and 25 Months old 60–79 Years old 275–300 g 26–38 and 68–82 Years old |
(8, 40, 151, 152, 224, 325, 344, 347, 374, 375, 473) |
| TA-65 | ↑ Telomerase ↑ Glutathione |
↓ Oxidants ↓ Inflammation ↓ Blood glucose ↓ Blood lipids ↓ Blood pressure |
Human subjects Human subjects and in vitro Human subjects wt and TERT KO mice Fisher344 (F) Human subjects Rat (R) Human subjects in vitro Human subjects C57/B16 TERT−/− mice (M) |
Blood, bone density Small coronary artery, small adipose artery, and HUVEC Small coronary and adipose arteries Small mesenteric and septal arteries Coronary microvessels Blood biomarkers Myocardium Blood biomarkers HASMC and HMVEC-L Adipose and atrial coronaries Aortic samples |
63 ± 12 Years old 46–68 Years old 4–70 Years old 3–4 Months old 4 and 24 Months old 51–75 Years old 53–87 Years old 60–67 Years old 12 Weeks old |
(7, 29, 30, 118, 173, 174, 192, 264, 373, 381, 453, 492) |
| SVF | ↑ Peroxynitrite mediated dilation ↑ β1 ADR mediated dilation ↑ β1 ADR density ↑ Antioxidants ↑ EDD ↑ LV diastolic function ↑ Connexin-43 |
↓ Oxidants | Fisher344 rats (F) Fisher344 rats (F) Sprague-Dawley rats (M) and in vitro In vitro (R) In vitro In vitro (R) In vitro BALB/c mice Mice (M) C57BL/6 mice (M) Wistar rats (M) In vitro Sprague-Dawley rats (M) In vitro Sprague-Dawley rats (M) BALB/c-nu/nu mice (F) Sprague-Dawley rats (M) Rat and mice (R) Sprague-Dawley rats (F) Fisher344 rats (F) Fisher344 (F) |
Coronary vasculature and function Coronary vasculature and function Myocardium, cardiac function, and RVSMC MSC Human BM-MSC and HUVEC BM- and adipose-MSC lung MSC Lung and human iPSC-MSC saphenous artery Coronary function, myocardium, and MSC Liver and BM-MSC Human BM-MSC and PASMC Kidney and adipose-MSC ADSC BM-MSC, myocardium, and cardiac function Liver and human umbilical MSC Colon and BM-MSCs Meta-analysis and cardiac function Kidney and human umbilical MSC Coronary microvascular function In vitro and subcutaneous |
3 and 24 Months old 3 and 22 Months old 4–6 Weeks old 10–23 Weeks old 10–12 Weeks old 250–350 g 320–350 g 250–300 g 4–5 Weeks old 80–100 g 200–240 g 4 and 24 Months old 4 and 24 Months old |
(5, 15, 89, 184, 188, 220, 260, 261, 282, 340, 376, 413, 444, 452, 502, 507, 509, 512, 518, 528) |
ACE, angiotensin converting enzyme; ADR, adrenergic receptor; ADSC, adipose-derived stem cells; AT1, angiotensin receptor 1; BM, bone marrow; DRP-1, dynamin-related protein-1; EDD, endothelium-dependent dilation; eNOS, endothelial nitric oxide synthase; F, female subjects only; HASMC, human aortic smooth muscle cell; HMVEC-L, human lung microvascular endothelial cells; HRCEC, human retinal capillary endothelial cell; HUVEC, human vascular endothelial cell; iPSC, induced pluripotent stem cell; KO, knock out; LDL, low-density lipoprotein; LLC, Lewis lung carcinoma; LV, left ventricular; M, male subjects only; Mfn, mitofusin; mitoQ, mitochondrial-targeted ubiquinone; MSC, mesenchymal stem cell; NO, nitric oxide; NOX, NADPH oxidase; Opa-1, optic atrophy 1; PASMC, human pulmonary artery smooth muscle cell; R, review; RVSMC, rat vascular smooth muscle cell; Sirt1, sirtuin deacetylase 1; SOD, superoxide dismutase; SOD2, manganese superoxide dismutase; SVF, adipose-derived stromal vascular fractions; TERT, telomerase reverse transcriptase; wt, wild type.
7.1. Diet modification to preserve vascular function in aging
Diet supplementation with antioxidant Vitamins (B6, B12, C, D, E) shows promising results in aging rodents to restore EDD, although studies in humans are inconsistent (421). Vitamin E supplementation reduces superoxide in heart failure to restore rat aortic ACh-mediated relaxation; this effect was also seen in postmenopausal women brachial artery FMD (22). Vitamin B12 (folic acid) improved skeletal muscle blood flow in aged patients (372). In contrast, the Women's Angiographic Vitamin and Estrogen trial showed that vitamins (E and C) do not rescue postmenopausal EDD (219). Metanalysis has since found that vitamin supplementation alone cannot reduce MACE (310). Deficiency, on the other hand, is associated with endothelial dysfunction, and inclusion of a “healthy diet” (as opposed to mere vitamin supplementation) including vitamins (fruits, vegetables, nuts) shows favorable outcomes (76, 202, 287, 372, 415).
The most effectively studied “healthy diet” is the Mediterranean diet. In a recent review, Shannon et al. describe the ways in which the Mediterranean diet has been shown to positively impact hallmarks of aging, such as genomic instability, senescence, telomere attrition, and mitochondrial dysfunction (406). Omega 3 polyunsaturated fatty acids (fish oil) has been shown to decrease mitochondrial fission proteins DRP-1 and Fis-1, increase fusion proteins Mfn-2 and Opa-1, promote uncoupling via uncoupling protein 2, and induce mitochondrial tubular morphology, culminating in reduced mtROS production (358). The mitophagy activator trehalose, found in Mediterranean diet foods such as mushrooms, crustaceans, baker's yeast (bread), and seaweed restores nitric oxide bioavailability, reduces oxidative stress and aortic inflammatory markers, and it restores EDD and endothelial-independent dilation in aging mice with similar results in human (216, 243). As mitophagy eliminates damaged organelles as sources of ROS, redox homeostasis can be restored. From a clinical standpoint, large-scale meta-analysis of randomized control and observational trials shows that the Mediterranean diet reduces diastolic and systolic blood pressure, decreases odds of developing hypertension, reduces polypharmacy, and lowers risk of coronary/cerebrovascular events and all-cause mortality, stratified based on the level of adherence and length of the diet (80, 405, 475).
Polyphenols (blueberries, strawberries, red grapes, murtilla berry, blackberries, garlic, tea, etc.), flavonoids (apples, spinach, etc.), theanine and catechins (green and black tea), cyanidine 3-glucoside (cardamom), and selenium (nuts) confer antioxidative properties that protect endothelial function and restore EDD and FMD in aging mice (33, 141, 182, 183, 194, 196, 208, 215, 259, 315, 336, 385, 474, 485, 495, 499, 503). As reviewed by Kicinska and Jarmuszkiewicz, flavonoids are capable of targeting mitochondria to promote fission over fusion, modulate antioxidant enzyme response to ROS (including SOD2, catalase, glutathione peroxidase), and reduce complex 1 and 3 ROS generation, thereby activating mitochondrial biogenesis, skewing mitochondrial dynamics toward fusion, and modulating Pink1/PARKIN-mediated mitophagy (222). In settings of aortic calcification and murine endothelium, the flavonoid quercetin can inhibit DRP-1 activation by blocking phosphorylation, likely by inhibiting PKCδ (63).
Blueberry anthrocyanins increase vasodilator nitric oxide, promote and rescue eNOS and SOD function, protect against high-glucose mediated vascular damage, and decrease pro-oxidant and vasoconstrictor functions of ACE, NOX4, xanthine oxidase-1, and low-density lipoprotein levels (193–195, 354). Meta-analysis of 39 prospective cohort studies by Micek et al. spanning ∼1.5 million individuals found that a flavonoid-rich diet (anthrocyanins, catechin, flavan-3-ols) significantly lowers the relative risk of cardiovascular disease in a dose-dependent manner (288). Further, high flavonoid intake reduced cardiovascular, cancer-related, and all-cause mortality in a cohort (1063) of older (>75 years) women (201).
Red grape skin and red wine contain the anti-aging polyphenol compound resveratrol, which is hypothetically responsible for the “French Paradox.” French citizens have been found to have a lower risk of cardiovascular disease than Americans, despite having similar smoking tendencies, low exercise, and Western diets high in saturated fats (131). Resveratrol rescues mesenteric endothelial function in diabetes mellitus, obesity, and prevents aging-induced vascular pathology if consumed starting during youth in mice (60). These effects are mediated by increases in Sirt1-dependent on peroxisome proliferator-activated receptor δ (PPARδ). Drinking water supplemented with red wine polyphenols (RWP) given to rats at age 16 weeks improves EDD of mesenteric arteries to ACh at week 40 (88, 154). The RWP treatment also reduced oxidative stress, normalized peroxynitrite and eNOS levels, normalized aging-dependent increases in pro-oxidant NOX subunit p22phox, nox1, arginase 1 (competitive inhibitor of eNOS), and AT1 receptor expression similar to that of young levels.
Resveratrol can itself elicit vasodilation response via nitric oxide signaling from ERK signaling and guanylyl cyclase activation and smooth muscle relaxation from BKCa channel activation in porcine retinal arteries, and it even confers retinal ganglion protection from ocular hypertension (53, 312). The benefits of resveratrol also extend to the renal system; resveratrol dilates renal arteries via increased eNOS and nitric oxide synthesis, improving naturesis and chronically limiting angiotensin pressor effects on the renal artery (158–160). Resveratrol also has potential implications for sarcopenia, as injection improved muscular microvascular perfusion and blood volume, also through eNOS and nitric oxide (483). These findings in animal models have been shown to translate in humans, including evidence for increased cerebrovascular and cognitive function, improved nitric oxide-mediated FMD in postmenopausal women, and cardioprotection during CAD (17, 164, 221, 274, 338, 363, 416, 445, 498, 529).
Despite these promising results, further epidemiologic scrutiny through meta-analysis concludes that the health-promoting effects of resveratrol have not yet been definitively confirmed (73). Omidian et al. found 16 clinical trials that reported that resveratrol mediated increases in glutathione peroxidase concentration, but it neither increased SOD nor significantly reduced the markers of oxidative stress (327). Although in human studies it was determined that resveratrol improves systolic blood pressure, in a meta-analysis by Weaver et al., resveratrol did not improve diastolic pressure or FMD as seen in animal studies, and improvement is greater in pure resveratrol compound studies versus wine drinking (486). These conflicting meta-studies suggest that additional large, prospective, randomized, double-blind, and placebo-controlled studies with standardization of protocol are necessary to properly delineate the effect of resveratrol in aging.
An important confounding phenomenon to note is the metabolism of these phenolic compounds. Resveratrol is quickly metabolized and its bioavailability is only <1%. Further, it has been calculated that the red wine necessary to obtain sufficient resveratrol for cardiovascular benefit would be 50 L per day (73). Therefore, it is no surprise that in a prospective cohort study by Semba et al. of older adults (>65 years) there was no predictive association between urine resveratrol concentration and cardiovascular events or all-cause mortality (402). For pure pharmacologic resveratrol compound, the proper dosing regimen is unclear and based on animal-to-human dose conversions (73).
7.2. Exercise to preserve vascular function in aging
Aerobic exercise has been shown to be vasculoprotective in aging. In aged mice, routine wheel running is associated with restored endothelium-dependent and -independent vasodilation in carotid arteries, reduced aortic nitrotyrosine oxidative stress, increased SOD activity, and reduced NOX activity and expression (119). In rat ex vivo arterial tension assays, Huang et al. found that endothelium-derived hyperpolarizing factor-mediated relaxation in aging is restored on regular aerobic exercise (191). Even if initiated late in life, aerobic exercise is able to improve coronary microvascular function, prefusion, and EDD, and it reverses diastolic dysfunction in aged rats; these benefits are translated in humans (44, 187, 304). In humans, consistent aerobic but not resistance exercise is found to enhance EDD and reduce endothelin-1-mediated vasoconstriction in both middle-aged and elderly adults (98, 123, 212, 384, 399, 465). This enhanced vasodilation allows increased skeletal muscle microvascular blood flow in active versus sedentary older adults (198, 386). It is thought that exercise-induced recovery of vascular dilative function extends to cerebral arteries and has positive impacts on reducing cognitive decline during aging (21). Related to mitochondrial function, exercise has been shown to reduce fission via reduced DRP-1 activation and promote fusion via enhanced Mnf-2 and Opa-1 in various tissues and pathological states (436), but these studies have not, to our knowledge, explored the setting of aging vasculature.
The positive effects of exercise on preserving endothelial function seem to provide less benefit to postmenopausal women, possibly due to estrogen deficiency (men with low testosterone also exhibit greater pronounced endothelial dysfunction) (297). With estrogen (and testosterone) deficiencies, oxidative stress increases, whereas BH4, an essential eNOS cofactor, is depleted. Exercise training in postmenopausal women can confer restoration of endothelial function only coinciding with estradiol (but not placebo) therapy, indicating its necessity for exercise-induced vascular adaptation. Therapies to activate estrogen receptors without the risks associated with estradiol, such as resveratrol, have so far come up short in providing similar restoration of function with exercise as seen in men (although resveratrol improves basal FMD) (338). These sex-specific disparities, difficulty in patient adherence regarding advice for diet and exercise, and patient populations unable to exercise, warrant investigation into other restorative strategies.
7.3. Pharmacologic mitochondrial targeted antioxidation
MitoTEMPO and related compound MitoTEMPOL are scavengers of mitochondrial-specific superoxide. They have been shown to reduce endothelial oxidative stress, improve phenylephrine-induced contraction and ACh-induced dilation in rat aortic rings, and can restore mitochondrial fission/fusion balance in mice with endothelial dysfunction and oxidative stress (8, 40, 224, 226, 325, 344). Mitochondrial-targeted ubiquinone (mitoQ) is a compound that includes a conjugation between the antioxidant ubiquinone and the lipophilic cation decyl-triphenylphosphonium and associates with the mitochondrial inner membrane. After serving in its antioxidant capacity, respiratory complex II serves to restore mitoQ's potential for reduction, therefore allowing for cyclic antioxidant activity, a limitation of previous antioxidants studies such as vitamins E and C.
A study of old mice given oral mitoQ by Gioscia-Ryan et al. demonstrated the protective effects of mitoQ on age-associated endothelial dysfunction in mice (152). In this study, mitoQ facilitated a reduction of aortic mitochondrial superoxide production and oxidative stress as evidenced by electron paramagnetic resonance spectroscopy and reduced nitrotyrosine levels, respectively. This was accompanied by a restoration of NO, as treatment with L-NAME (eNOS inhibitor) yielded less reduction in ACh-mediated vasodilation in the old group than the young or old+mitoQ group (although aging-mediated reduction in eNOS activity itself was not restored by mitoQ). The EDD assessed with ACh revealed significantly increased vasorelaxation in the old + mitoQ group to near youthful levels. Exogenously producing acute mitochondrial oxidative stress with rotenone led to ∼25% reduction in EDD in the old but not young or old+mitoQ mouse carotid artery, indicating mitoQ's ability to confer resistance to acute oxidative stress. The protein profile showed an aging-associated increase in p66SHC (prooxidant) with decreased SOD2 (antioxidant), COV-IV (mitochondrial mass marker), and PGC-1α (mitochondrial biogenesis marker), all restored to youthful levels with mitoQ, which indicates that the maintenance of mitochondrial homeostasis is crucial to maintain vasodilative capacity.
Age-associated aortic stiffness with aging, as evaluated with Pulse-Wave Velocity, was attenuated with mitoQ supplementation in association with restored elastin expression (relative to youth) without affecting aging-related increases in collagen or proinflammatory cytokine expression (151). The authors speculate that this reduction in aortic stiffness was mediated by attenuation of ROS-mediated stimulation of αADR hyperconstriction (374). In addition, mitoQ also reverses diastolic dysfunction as assessed by reduced left ventricular relaxation constant Tau, left ventricular end diastolic (LVED) pressure, and LVED-volume relation (473).
Additional studies with mitoQ have been replicated in human skeletal muscle feed arteries, showing similar capacity to restore mitochondrial homeostasis with corresponding improvement in EDD. Unlike the Gioscia-Ryan et al. study (152), mitoQ was able to restore eNOS (increased phosphorylated eNOS to eNOS ratio) in human skeletal arteries with mitoQ-mediated EDD improvement ablated by the eNOS blockade (347). In a randomized triple-blinded clinical trial of 55 participants, mitoQ (20 mg/day for 6 weeks) or placebo was supplemented in patients 60–79 years of age with impaired (brachial artery) endothelial function. At the clinical endpoint, brachial artery FMD was 42% higher in the mitoQ group compared with the placebo with concomitant reduction of aortic stiffness and oxidized low-density lipoprotein (global oxidative stress marker) (375). More details on mitochondrial and vascular dysfunction, mitoQ, future directions, and detailed current gaps in research can be found in the recent review by Rossman et al. (374).
7.4. Modulation of TERT
The importance of telomerase in aging is well known, as its role is to prevent telomere shortening during DNA replication, the function of which is diminished with aging and is a cause of senescence phenotype. TERT, the catalytic subunit of telomerase, limits ROS production in both the cytosol and mitochondria. It is trafficked from the nucleus to the mitochondria during times of peak ROS production by heat shock protein 90 and Akt, whereby it inhibits ROS production through a currently unknown mechanism. The trafficking fate of TERT is dependent on phosphorylation of different amino acid residues; nuclear import via phosphorylation of serine 227 mediated by Akt, nuclear export via tyrosine 707 mediated by Src, and overall activation via serine 823 are also mediated by Akt. With this knowledge, strategies to influence the trafficking of TERT, as well as TERT overexpression and loss of function, have been used experimentally to study its effects on vascular function.
The non-canonical functions of TERT have recently been summarized in a review by Rosen et al., including resistance to apoptosis in somatic cells, mtDNA protection, antioxidation, and improvement of mitochondrial function (373). Although TERT cannot function to preserve mtDNA by preserving telomeres, which mtDNA does not have, reduction of ROS in the mitochondria can reduce the damaging “hits” that the mtDNA takes. Therefore, TERT can indirectly protect mtDNA, which has been confirmed in overexpression models of TERT. Overexpression or targeting the shunting of TERT to the mitochondria also leads to increased glutathione (reduced form), non-oxidized peroxiredoxin, SOD2, and diminished ROS (373). On the other hand, TERT knockout or excluding TERT from the mitochondria leads to increased ROS with increased susceptibility to apoptosis through caspase and Bax signaling, reduced SOD2, and reduced ATP production (373).
The effects of TERT on redox balance have direct implications for vascular function. Inhibition of TERT with BIBR-1532 in vessels from healthy rats leads to a switch in the flow-mediated vasodilator agent nitric oxide to hydrogen peroxide, mimicking the CAD and aging phenotype (29, 30). Interestingly, vascular TERT is downregulated in both CAD and aging. As described earlier, Ang II-mediated AT1 receptor agonism leads to increased ROS and endothelial dysfunction with reduced vasodilator capacity (7). However, mice overexpressing TERT are protected from reductions in vasodilator capacity from Ang II infusions seen in wild-type, and TERT knockout significantly reduces FMD in response to ANG II. In addition, loss of TERT function is associated with reduced peak dilation to flow and ACh in mice, with worsening FMD in subsequent generations.
Exogenous peptides incubated with aging rat coronary microvessels that block nuclear import of TERT (increasing mitochondrial TERT), as well as incubation with TERT activator, AGS 499, improve ACh as well as β1ADR (dobutamine and norepinephrine) concentration responses compared with the scrambled peptide control (453). On the other hand, young rat vessels incubated with trafficking peptides that shunt TERT away from the mitochondria by blocking trafficking to the mitochondria abrogate vasodilation from β1ADR agonists and ACh (453).
The TERT expression and function can be influenced therapeutically. Cycloastragenol (CAG) is a molecule purified from the root of Astragalus membranaceous indigenous to China and used in traditional Chinese medicine for nearly 2000 years. The CAG increases telomerase activity, is an antioxidant, has anti-inflammatory properties (reversing aging-associated immune system changes), and has been used in a variety of other ways: as a diuretic to reduce blood pressure, as an expectorant, to improve bone density, to reverse hyperglycemia, for hepatoprotection, and to reduce hyperlipidemia (173, 174, 264, 381).
The TERT can also be activated in human aortic SMCs and pulmonary microvascular endothelial cells by supplementation with resveratrol found in red wine or red grape skin (192). Interestingly, TERT activation by resveratrol seems to be dependent on an axis, including the activation of nicotinamide phosphoribosyltransferase and Sirt4. Healthy human atrial and adipose arterioles normally vasodilate during incubation with Angiotensin 1–7, but this does not occur in vessels from CAD patients. However, if incubated overnight with angiotensin 1–7, nitric oxide-dependent FMD is restored in CAD-derived vessels through the activation of telomerase (118). In addition, PPAR-γ inhibition with pioglitazone improves telomerase activation by 2 × in mouse aorta and confers protection against hydrogen peroxide-induced oxidative stress and apoptosis (492). Perhaps the most potent known activator of telomerase is estrogen, with loss of telomerase as a potential factor contributing to aging-associated CMD exhibited preferentially in women (9, 23, 24, 162, 239, 383). Although HRT can restore telomerase activity, risks of oncogenesis and stroke are typically thought to be too much of a limitation (246). Although alternative strategies discussed earlier have shown to be safe, since TERT inhibits apoptosis and has a role in tumor development, it is important to weigh the risks and benefits when utilizing these therapeutic options (18).
7.5. Cellular therapy to preserve and recover vascular function in aging
Adipose-derived stromal vascular fraction (SVF) has antioxidant properties that, when injected systemically (via tail vein) in aged female rats, restores β1ADR density and agonist-driven vasodilation (via dobutamine and norepinephrine) in coronary microvasculature (376). Markers of diastolic function (Tau, E/A, E/e′, and Isovolumic Relaxation Time) are restored to youthful levels by SVF alongside an improved CFR, a measure of microvascular function (220, 376). Once cleared of adipocytes, isolated adipose-derived SVF represents a heterogenous population of cells, including microvascular endothelial cells, perivascular cells, fibroblasts, mesenchymal stem cells, regulatory and natural killer T-lymphocytes, B-lymphocytes, dendritic cells, and macrophages. Injected SVF cells are able to incorporate into the vasculature (and myocardium to a minimal extent), where they are thought to exert their effects through paracrine and perhaps some direct cell–cell interactions (282). Although unknown in SVF, antioxidative benefits are conferred through direct mitochondrial transfer to endothelial cells, often mediated by connexin 43 gap junctional connections in mesenchymal cells from other cell sources (188, 261, 340, 413, 512).
The SVF cells in vascular tissue express CD11b; therefore, the macrophage population is postulated to have a role in these observed effects (301). However, injected SVF-CD11b+ cells alone are insufficient to rescue function, indicating that the potential requirement of synchrony from other cells or paracrine mechanisms is required. Exosomes from mesenchymal stem cell sources, including bone marrow, umbilical cord, and adipose SVF-derived stem cells, have antioxidant capabilities, possibly due to the delivery of antioxidant proteins such as glutathione and glutathione peroxidase (15, 89, 184, 260, 268, 444, 502, 507, 509, 518, 528). Therefore, one possible mechanism for SVF-mediated restoration of adrenergic function is that SVF-derived exosomes deliver antioxidant proteins to recipient endothelial cells, whereby the restored youth-like homeostatic redox balance dissuades thiol-oxidation and protective nitrosylation, thus preventing βADR desensitization and internalization as happens with aging (Figs. 4 and 5). Therapy with SVF also alters FMD in aging from hydrogen peroxide-mediated to peroxynitrite-mediated, indicating minimal increase in nitric oxide production with SVF treatment (452). This effect is too minimal to directly influence dilation, but enough to combine with superoxide to form peroxynitrite to then influence FMD.
It is the hope that SVF is an immunologically safe, autologous therapeutic option that can holistically address vascular pathology by targeting oxidative stress, endothelial and adrenergic dysfunction, and pathologic smooth muscle tone and hyperconstriction. Importantly, SVF from old donors seems to be less effective than from young donors at rescuing vascular and cardiac function (5). Since patients with these cardiovascular pathologies are usually aged, this hurdle may need to be overcome using strategies to invigorate the cells before systemic injection. Interestingly, transfection of elderly donor SVF with TERT nucleoside-modified mRNA to increase SVF TERT has the effect to improve SVF secretory function, thereby improving regenerative capacity (316). This finding is typical in the field of stem cell therapeutics, where therapies that are promising in rodent models are not as efficient in clinical translation, leading researchers to investigate the potential of “priming” cell populations before therapeutic delivery.
Despite this hurdle, SVF or SVF-derived subcellular populations or exosomes may still be of advantage over other strategies due to its ability to preferentially target the vasculature. If antioxidation provides a pathway to increase βADR functionality, this is, of course, a benefit in the vasculature to improve cardiac perfusion (which is reduced by ∼43% in aging) to prevent chronic ischemia culminating in the angina of CMD, for example. However, if βADR receptors are similarly increased in the myocardium by therapies that do not preferentially target vasculature, this could be a detriment to patients with heart failure, where a subsequent increase in ionotropic potential could increase oxygen demand outpacing supply, worsening function. This outcome is only a postulation and should be considered as these mitochondrial antioxidative therapeutic strategies are developed.
8. Conclusion
Homeostatic regulation of perfusion is essential to properly match oxygen and nutrient delivery to tissue demand and waste removal needs. Chronic imbalance in the heart vasculature leads to chest pain as transient ischemic angina pectoris, thereby elevating risk for eventual severe acute imbalance and leading to myocardial infarction. The pathological emergence and progression of these imbalances, including coronary artery and microvascular disease, are driven by the aging process. In this review, our major objective was to discuss the pathological alterations of the mitochondria during aging, namely a trend toward fission/fusion imbalance and reduction of mitophagy that is both caused by and contributes to reduction of antioxidative regulation of oxidative stress. These mitochondrial alterations lead to dysfunctional vasodilation and ability to maintain appropriate patency, often leading to hyperconstricted tone. A common theme is the aging-dependent accumulation and reliance of mitochondrial-derived ROS signaling in the place of vasoprotective signaling by nitric oxide.
In Figure 1, we summarize the aging-associated changes to mitochondrial and cellular antioxidant and pro-oxidant enzymes as well as the ETC that lead to (and/or are a consequence of) increasing mtROS. The damage caused by this accumulation leads to mitochondrial dysfunction and a favor toward fission rather than protective fusion or mitophagy processes, all of which is associated with reduced flow and βADR-mediated vasodilative efficacy. In Figures 2 and 3, the signaling cascades initiated by intraluminal flow are shown to alter with aging by favoring mitochondrial-derived hydrogen peroxide as opposed to nitric oxide or prostaglandin-mediated signaling in youth. In addition, receptors and potassium channel expression and function in the endothelial hyperpolarization pathway are altered with aging, due in part to post-translational modifications by mitochondrial-derived ROS, which further affect dilation capacity. In Figures 4 and 5, we illustrate how aging affects the balance between βADR and αADR-mediated dilation and constriction, with emphasis on desensitization, internalization, and recycling with their mitochondrial influences, that is, how mitochondrial-derived ROS or nitric oxide can directly affect receptor trafficking through βADR thiol oxidation, PI3Kγ activation, or GRK2 nitrosylation.
Traditionally, aging has been referred to as an unmodifiable risk factor of chronological aging. However, the Geroscience hypothesis suggests that biological aging and the so called “rate of aging” can vary between individuals owing to genetic, environmental, and pathological influences. The central question is whether this “rate of aging” can be manipulated for therapeutic targeting to protect against aging-mediated pathologies such as atherosclerosis and coronary vascular and microvascular disease. Current therapies such as β blockers may not take into consideration vascular adrenergic trafficking changes that occur in advancing age. Nitrates can offer acute symptom relief but are unable to provide meaningful long-term mortality and MACE benefits due to tolerance and paradoxical induction of oxidative stress and endothelial dysfunction.
Therefore, we discussed emerging therapeutic strategies and targets such as drugs to attenuate mitochondrial dysfunction-induced oxidative stress such as mitoQ and mitoTEMPOL, CAG to increase TERT function, SVF, and recent data explaining the anti-oxidative and vasculoprotective effects of diet and exercise supplementation, all summarized in Table 1. Future directions should continue to elucidate aging-mediated vascular changes to find additional therapeutic targets, including the role and potential manipulation of nitrosative and oxidative modifications. Studies should also provide more extensive and higher-powered clinical trials with standardized protocols for the discussed therapies with emphasis on symptom management, mortality, MACE, and superiority over current clinical standards. When these antioxidative and aging-reversal strategies should begin is a question of importance scientifically and ethically. Should the emphasis be at the start of disease presentation? Beforehand, and if so, when? In all, the current direction of regenerative medicine described is providing exciting avenues to achieve a meaningful benchmark to bedside translational care.
Abbreviations Used
- ACE
angiotensin converting enzyme
- ACh
acetylcholine
- ADR
adrenergic receptor
- ADSC
adipose-derived stem cell
- Akt
protein kinase B
- Ang II
angiotensin II
- AT1
angiotensin receptor 1
- BH4
tetrahydrobiopterin
- BKCa
calcium dependent large conductance potassium channels
- BM
bone marrow
- BNIP3
BCL2/adenovirus e1B 19 kDa interacting protein 3
- CAD
coronary artery disease
- CAG
cycloastragenol
- cAMP
cyclic AMP
- CFR
coronary flow reserve
- CMD
coronary microvascular disease
- DRP-1
dynamin-related protein-1
- EDD
endothelium-dependent dilation
- EDH
endothelial-dependent hyperpolarization
- eEV
endothelium-derived extracellular vesicles
- eNOS
endothelial nitric oxide synthase
- ER
endoplasmic reticulum
- ETC
electron transport chain
- F
female subjects only
- Fis-1
mitochondrial fission protein-1
- FMD
flow-mediated dilation
- FUNDC1
FUN14 domain-containing protein 1
- GRK
G-protein receptor kinase
- GSH
glutathione
- GSNO
S-nitrosoglutathione
- HASMC
human aortic smooth muscle cell
- HMVEC-L
human vascular endothelial cell-lung
- HRCEC
human retinal capillary endothelial cell
- HRT
hormone replacement therapy
- HUVEC
human vascular endothelial cell
- I2PP2A
endogenous inhibitor of PP2A
- IKCa
calcium sensitive intermediate conductance potassium channels
- iPSC
induced pluripotent stem cell
- KATP
ATP sensitive potassium channels
- KIR
inward rectifying potassium channels
- KO
knock out
- Kv
voltage-gated potassium channels
- LDL
low-density lipoprotein
- LLC
Lewis lung carcinoma cell
- LV
left ventricular
- LVED
left ventricular end diastolic pressure
- M
male subjects only
- MACEs
major adverse cardiac events
- Mfn
mitofusin
- mitQ
mitochondrial-targeted ubiquinone
- mPTP
mitochondrial permeability transition pores
- mRNA
messenger RNA
- MSC
mesenchymal stem cell
- mtDNA
mitochondrial DNA
- mtROS
mitochondrial ROS
- NE
norepinephrine
- NO
nitric oxide
- NOX
NADPH oxidase
- Opa-1
optic atrophy 1
- PAD
peripheral arterial disease
- PASMC
human pulmonary artery smooth muscle cell
- PDIA1
protein disulfide isomerase A1
- Pink1
PTEN-induced kinase 1
- PKA
protein kinase A
- PP2A
protein phosphatase 2A
- PPAR
peroxisome proliferator-activated receptor
- R
review
- RBC
red blood cell
- RBC-NOS
red blood cell nitric oxide synthase
- RIRR
ROS-induced ROS release
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- RVSMC
rat vascular smooth muscle cell
- RWP
red wine polyphenols
- SASP
senescence-associated secretory phenotype
- Sirt
sirtuin deacetylase
- SKCa
calcium-sensitive small conductance potassium channels
- SMC
smooth muscle cell
- SNO
S-nitrosylation
- S-NO
thiol nitrosylation
- SNP
sodium nitroprusside
- SOD
superoxide dismutase
- SOD1
copper/zinc superoxide dismutase
- SOD2
manganese superoxide dismutase
- S-OH
thiol oxidation
- SVF
adipose-derived stromal vascular fractions
- T2DM
type 2 diabetes mellitus
- TERT
telomerase reverse transcriptase
- TRPV4
transient receptor potential vanilloid type 4 channel
- VGCCs
voltage-gated calcium channels
- VSM
vascular smooth muscle
- wt
wild type
Authors' Contributions
E.P.T., W.H., A.B., and A.J.L. created a conceptual outline; E.P.T., W.H., and J.B. wrote the article; E.P.T. and G.R. made all figures/tables; E.P.T., W.H., J.B., G.R., A.B., and A.J.L. edited and approved the article text, figures, and tables.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
The authors acknowledge funding from National Institutes of Health (AG053585 to A.J.L., T32GM089586 to A.B., R01HL133029 to A.B.), Department of Defense (W81XWH-19-RTRP-IDA and W81XWH-13-2-0057 to A.J.L.), the Gheen's Foundation (A.J.L.), and AHA 20POST35050017 (W.H.).
References
- 1. Accardi G, Aiello A, Gambino CM, Virruso C, Caruso C, and Candore G. Mediterranean nutraceutical foods: strategy to improve vascular ageing. Mech Ageing Dev 159: 63–70, 2016. [DOI] [PubMed] [Google Scholar]
- 2. Ago T, Kitazono T, Ooboshi H, Iyama T, Han YH, Takada J, Wakisaka M, Ibayashi S, Utsumi H, and Iida M. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation 109: 227–233, 2004. [DOI] [PubMed] [Google Scholar]
- 3. Ago T, Kuroda J, Pain J, Fu C, Li H, and Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res 106: 1253–1264, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ahn S, Maudsley S, Luttrell LM, Lefkowitz RJ, and Daaka Y. Src-mediated tyrosine phosphorylation of dynamin is required for beta2-adrenergic receptor internalization and mitogen-activated protein kinase signaling. J Biol Chem 274: 1185–1188, 1999. [DOI] [PubMed] [Google Scholar]
- 5. Aird AL, Nevitt CD, Christian K, Williams SK, Hoying JB, and LeBlanc AJ. Adipose-derived stromal vascular fraction cells isolated from old animals exhibit reduced capacity to support the formation of microvascular networks. Exp Gerontol 63: 18–26, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ait-Aissa K, Blaszak SC, Beutner G, Tsaih SW, Morgan G, Santos JH, Flister MJ, Joyce DL, Camara AKS, Gutterman DD, Donato AJ, Porter GA Jr., and Beyer AM. Mitochondrial oxidative phosphorylation defect in the heart of subjects with coronary artery disease. Sci Rep 9: 7623, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ait-Aissa K, Kadlec AO, Hockenberry J, Gutterman DD, and Beyer AM. Telomerase reverse transcriptase protects against angiotensin II-induced microvascular endothelial dysfunction. Am J Physiol Heart Circ Physiol 314: H1053–H1060, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Akhter F, Chen D, Akhter A, Yan SF, and Yan SS. Age-dependent accumulation of dicarbonyls and advanced glycation endproducts (AGEs) associates with mitochondrial stress. Free Radic Biol Med 164: 429–438, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anderson RD, Petersen JW, Mehta PK, Wei J, Johnson BD, Handberg EM, Kar S, Samuels B, Azarbal B, Kothawade K, Kelsey SF, Sharaf B, Shaw LJ, Sopko G, Bairey Merz CN, and Pepine CJ. Prevalence of coronary endothelial and microvascular dysfunction in women with symptoms of ischemia and no obstructive coronary artery disease is confirmed by a new cohort: the NHLBI-sponsored Women's Ischemia Syndrome Evaluation-Coronary Vascular Dysfunction (WISE-CVD). J Interv Cardiol 2019: 7169275, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Andersson DC, Betzenhauser MJ, Reiken S, Meli AC, Umanskaya A, Xie W, Shiomi T, Zalk R, Lacampagne A, and Marks AR. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab 14: 196–207, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Andersson DC, Fauconnier J, Yamada T, Lacampagne A, Zhang SJ, Katz A, and Westerblad H. Mitochondrial production of reactive oxygen species contributes to the beta-adrenergic stimulation of mouse cardiomycytes. J Physiol 589: 1791–1801, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Andreyev AY, Kushnareva YE, and Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 70: 200–214, 2005. [DOI] [PubMed] [Google Scholar]
- 13. Aranda R, Domenech E, Rus AD, Real JT, Sastre J, Vina J, and Pallardo FV. Age-related increase in xanthine oxidase activity in human plasma and rat tissues. Free Radic Res 41: 1195–1200, 2007. [DOI] [PubMed] [Google Scholar]
- 14. Arrick DM, Sharpe GM, Sun H, and Mayhan WG. Losartan improves impaired nitric oxide synthase-dependent dilatation of cerebral arterioles in type 1 diabetic rats. Brain Res 1209: 128–135, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G, Lim SK, and de Kleijn DP. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res 10: 301–312, 2013. [DOI] [PubMed] [Google Scholar]
- 16. Ballinger SW, Patterson C, Yan CN, Doan R, Burow DL, Young CG, Yakes FM, Van Houten B, Ballinger CA, Freeman BA, and Runge MS. Hydrogen peroxide- and peroxynitrite-induced mitochondrial DNA damage and dysfunction in vascular endothelial and smooth muscle cells. Circ Res 86: 960–966, 2000. [DOI] [PubMed] [Google Scholar]
- 17. Banez MJ, Geluz MI, Chandra A, Hamdan T, Biswas OS, Bryan NS, and Von Schwarz ER. A systemic review on the antioxidant and anti-inflammatory effects of resveratrol, curcumin, and dietary nitric oxide supplementation on human cardiovascular health. Nutr Res 78: 11–26, 2020. [DOI] [PubMed] [Google Scholar]
- 18. Bar C and Thum T. Changing direction: from therapeutic telomerase inhibition to activation? Circ Res 120: 1393–1395, 2017. [DOI] [PubMed] [Google Scholar]
- 19. Barbato E. Role of adrenergic receptors in human coronary vasomotion. Heart 95: 603–608, 2009. [DOI] [PubMed] [Google Scholar]
- 20. Barlow RS and White RE. Hydrogen peroxide relaxes porcine coronary arteries by stimulating BKCa channel activity. Am J Physiol 275: H1283–H1289, 1998. [DOI] [PubMed] [Google Scholar]
- 21. Barnes JN and Corkery AT. Exercise improves vascular function, but does this translate to the brain? Brain Plast 4: 65–79, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bauersachs J, Fleming I, Fraccarollo D, Busse R, and Ertl G. Prevention of endothelial dysfunction in heart failure by vitamin E: attenuation of vascular superoxide anion formation and increase in soluble guanylyl cyclase expression. Cardiovasc Res 51: 344–350, 2001. [DOI] [PubMed] [Google Scholar]
- 23. Bayne S, Jones ME, Li H, Pinto AR, Simpson ER, and Liu JP. Estrogen deficiency leads to telomerase inhibition, telomere shortening and reduced cell proliferation in the adrenal gland of mice. Cell Res 18: 1141–1150, 2008. [DOI] [PubMed] [Google Scholar]
- 24. Bayne S, Li H, Jones ME, Pinto AR, van Sinderen M, Drummond A, Simpson ER, and Liu JP. Estrogen deficiency reversibly induces telomere shortening in mouse granulosa cells and ovarian aging in vivo. Protein Cell 2: 333–346, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Behringer EJ and Segal SS. Impact of aging on calcium signaling and membrane potential in endothelium of resistance arteries: a role for mitochondria. J Gerontol A Biol Sci Med Sci 72: 1627–1637, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Behringer EJ, Shaw RL, Westcott EB, Socha MJ, and Segal SS. Aging impairs electrical conduction along endothelium of resistance arteries through enhanced Ca2+-activated K+ channel activation. Arterioscler Thromb Vasc Biol 33: 1892–1901, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beigi F, Gonzalez DR, Minhas KM, Sun QA, Foster MW, Khan SA, Treuer AV, Dulce RA, Harrison RW, Saraiva RM, Premer C, Schulman IH, Stamler JS, and Hare JM. Dynamic denitrosylation via S-nitrosoglutathione reductase regulates cardiovascular function. Proc Natl Acad Sci U S A 109: 4314–4319, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Benovic JL, Pike LJ, Cerione RA, Staniszewski C, Yoshimasa T, Codina J, Caron MG, and Lefkowitz RJ. Phosphorylation of the mammalian beta-adrenergic receptor by cyclic AMP-dependent protein kinase. Regulation of the rate of receptor phosphorylation and dephosphorylation by agonist occupancy and effects on coupling of the receptor to the stimulatory guanine nucleotide regulatory protein. J Biol Chem 260: 7094–7101, 1985. [PubMed] [Google Scholar]
- 29. Beyer AM, Freed JK, Durand MJ, Riedel M, Ait-Aissa K, Green P, Hockenberry JC, Morgan RG, Donato AJ, Peleg R, Gasparri M, Rokkas CK, Santos JH, Priel E, and Gutterman DD. Critical role for telomerase in the mechanism of flow-mediated dilation in the human microcirculation. Circ Res 118: 856–866, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beyer AM, Zinkevich N, Miller B, Liu Y, Wittenburg AL, Mitchell M, Galdieri R, Sorokin A, and Gutterman DD. Transition in the mechanism of flow-mediated dilation with aging and development of coronary artery disease. Basic Res Cardiol 112: 5, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Blackwell KA, Sorenson JP, Richardson DM, Smith LA, Suda O, Nath K, and Katusic ZS. Mechanisms of aging-induced impairment of endothelium-dependent relaxation: role of tetrahydrobiopterin. Am J Physiol Heart Circ Physiol 287: H2448–H2453, 2004. [DOI] [PubMed] [Google Scholar]
- 32. Blankenberg S, Rupprecht HJ, Bickel C, Torzewski M, Hafner G, Tiret L, Smieja M, Cambien F, Meyer J, and Lackner KJ; AtheroGene Investigators. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N Engl J Med 349: 1605–1613, 2003. [DOI] [PubMed] [Google Scholar]
- 33. Bondonno CP, Yang X, Croft KD, Considine MJ, Ward NC, Rich L, Puddey IB, Swinny E, Mubarak A, and Hodgson JM. Flavonoid-rich apples and nitrate-rich spinach augment nitric oxide status and improve endothelial function in healthy men and women: a randomized controlled trial. Free Radic Biol Med 52: 95–102, 2012. [DOI] [PubMed] [Google Scholar]
- 34. Bossy B, Petrilli A, Klinglmayr E, Chen J, Lutz-Meindl U, Knott AB, Masliah E, Schwarzenbacher R, and Bossy-Wetzel E. S-Nitrosylation of DRP1 does not affect enzymatic activity and is not specific to Alzheimer's disease. J Alzheimers Dis 20(Suppl 2): S513–S526, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Botto N, Berti S, Manfredi S, Al-Jabri A, Federici C, Clerico A, Ciofini E, Biagini A, and Andreassi MG. Detection of mtDNA with 4977 bp deletion in blood cells and atherosclerotic lesions of patients with coronary artery disease. Mutat Res 570: 81–88, 2005. [DOI] [PubMed] [Google Scholar]
- 36. Boulanger CM, Scoazec A, Ebrahimian T, Henry P, Mathieu E, Tedgui A, and Mallat Z. Circulating microparticles from patients with myocardial infarction cause endothelial dysfunction. Circulation 104: 2649–2652, 2001. [DOI] [PubMed] [Google Scholar]
- 37. Bovo E, Lipsius SL, and Zima AV. Reactive oxygen species contribute to the development of arrhythmogenic Ca(2)(+) waves during beta-adrenergic receptor stimulation in rabbit cardiomyocytes. J Physiol 590: 3291–3304, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brakemeier S, Eichler I, Knorr A, Fassheber T, Kohler R, and Hoyer J. Modulation of Ca2+-activated K+ channel in renal artery endothelium in situ by nitric oxide and reactive oxygen species. Kidney Int 64: 199–207, 2003. [DOI] [PubMed] [Google Scholar]
- 39. Brand MD. The sites and topology of mitochondrial superoxide production. Exp Gerontol 45: 466–472, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brand MD. Riding the tiger—physiological and pathological effects of superoxide and hydrogen peroxide generated in the mitochondrial matrix. Crit Rev Biochem Mol Biol 55: 592–661, 2020. [DOI] [PubMed] [Google Scholar]
- 41. Briones AM, Montoya N, Giraldo J, and Vila E. Ageing affects nitric oxide synthase, cyclooxygenase and oxidative stress enzymes expression differently in mesenteric resistance arteries. Auton Autacoid Pharmacol 25: 155–162, 2005. [DOI] [PubMed] [Google Scholar]
- 42. Brown KA, Chu Y, Lund DD, Heistad DD, and Faraci FM. Gene transfer of extracellular superoxide dismutase protects against vascular dysfunction with aging. Am J Physiol Heart Circ Physiol 290: H2600–H2605, 2006. [DOI] [PubMed] [Google Scholar]
- 43. Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, and Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303: 2011–2015, 2004. [DOI] [PubMed] [Google Scholar]
- 44. Bruning RS and Sturek M. Benefits of exercise training on coronary blood flow in coronary artery disease patients. Prog Cardiovasc Dis 57: 443–453, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bruno RM, Duranti E, Ippolito C, Segnani C, Bernardini N, Di Candio G, Chiarugi M, Taddei S, and Virdis A. Different impact of essential hypertension on structural and functional age-related vascular changes. Hypertension 69: 71–78, 2017. [DOI] [PubMed] [Google Scholar]
- 46. Brunt VE, Gioscia-Ryan RA, Richey JJ, Zigler MC, Cuevas LM, Gonzalez A, Vazquez-Baeza Y, Battson ML, Smithson AT, Gilley AD, Ackermann G, Neilson AP, Weir T, Davy KP, Knight R, and Seals DR. Suppression of the gut microbiome ameliorates age-related arterial dysfunction and oxidative stress in mice. J Physiol 597: 2361–2378, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brzezinska AK, Gebremedhin D, Chilian WM, Kalyanaraman B, and Elliott SJ. Peroxynitrite reversibly inhibits Ca(2+)-activated K(+) channels in rat cerebral artery smooth muscle cells. Am J Physiol Heart Circ Physiol 278: H1883–H1890, 2000. [DOI] [PubMed] [Google Scholar]
- 48. Bubolz AH, Mendoza SA, Zheng X, Zinkevich NS, Li R, Gutterman DD, and Zhang DX. Activation of endothelial TRPV4 channels mediates flow-induced dilation in human coronary arterioles: role of Ca2+ entry and mitochondrial ROS signaling. Am J Physiol Heart Circ Physiol 302: H634–H642, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Burgoyne JR and Eaton P. Transnitrosylating nitric oxide species directly activate type I protein kinase A, providing a novel adenylate cyclase-independent cross-talk to beta-adrenergic-like signaling. J Biol Chem 284: 29260–29268, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cai H, Davis ME, Drummond GR, and Harrison DG. Induction of endothelial NO synthase by hydrogen peroxide via a Ca(2+)/calmodulin-dependent protein kinase II/janus kinase 2-dependent pathway. Arterioscler Thromb Vasc Biol 21: 1571–1576, 2001. [DOI] [PubMed] [Google Scholar]
- 51. Cai H, Li Z, Davis ME, Kanner W, Harrison DG, and Dudley SC Jr. Akt-dependent phosphorylation of serine 1179 and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase 1/2 cooperatively mediate activation of the endothelial nitric-oxide synthase by hydrogen peroxide. Mol Pharmacol 63: 325–331, 2003. [DOI] [PubMed] [Google Scholar]
- 52. Camici GG, Cosentino F, Tanner FC, and Luscher TF. The role of p66Shc deletion in age-associated arterial dysfunction and disease states. J Appl Physiol (1985) 105: 1628–1631, 2008. [DOI] [PubMed] [Google Scholar]
- 53. Cao K, Ishida T, Fang Y, Shinohara K, Li X, Nagaoka N, Ohno-Matsui K, and Yoshida T. Protection of the retinal ganglion cells: intravitreal injection of resveratrol in mouse model of ocular hypertension. Invest Ophthalmol Vis Sci 61: 13, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cardillo C, Kilcoyne CM, Cannon RO, 3rd, Quyyumi AA, and Panza JA. Xanthine oxidase inhibition with oxypurinol improves endothelial vasodilator function in hypercholesterolemic but not in hypertensive patients. Hypertension 30: 57–63, 1997. [DOI] [PubMed] [Google Scholar]
- 55. Carnevale D and Lembo G. PI3Kgamma in hypertension: a novel therapeutic target controlling vascular myogenic tone and target organ damage. Cardiovasc Res 95: 403–408, 2012. [DOI] [PubMed] [Google Scholar]
- 56. Carnevale D, Vecchione C, Mascio G, Esposito G, Cifelli G, Martinello K, Landolfi A, Selvetella G, Grieco P, Damato A, Franco E, Haase H, Maffei A, Ciraolo E, Fucile S, Frati G, Mazzoni O, Hirsch E, and Lembo G. PI3Kgamma inhibition reduces blood pressure by a vasorelaxant Akt/L-type calcium channel mechanism. Cardiovasc Res 93: 200–209, 2012. [DOI] [PubMed] [Google Scholar]
- 57. Carvalho-de-Souza JL, Varanda WA, Tostes RC, and Chignalia AZ. BK channels in cardiovascular diseases and aging. Aging Dis 4: 38–49, 2013. [PMC free article] [PubMed] [Google Scholar]
- 58. Ceballos-Picot I, Trivier JM, Nicole A, Sinet PM, and Thevenin M. Age-correlated modifications of copper-zinc superoxide dismutase and glutathione-related enzyme activities in human erythrocytes. Clin Chem 38: 66–70, 1992. [PubMed] [Google Scholar]
- 59. Chatterjee S and Fisher AB. Mechanotransduction in the endothelium: role of membrane proteins and reactive oxygen species in sensing, transduction, and transmission of the signal with altered blood flow. Antioxid Redox Signal 20: 899–913, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cheang WS, Wong WT, Wang L, Cheng CK, Lau CW, Ma RCW, Xu A, Wang N, Huang Y, and Tian XY. Resveratrol ameliorates endothelial dysfunction in diabetic and obese mice through sirtuin 1 and peroxisome proliferator-activated receptor delta. Pharmacol Res 139: 384–394, 2019. [DOI] [PubMed] [Google Scholar]
- 61. Cheitlin MD. Cardiovascular physiology-changes with aging. Am J Geriatr Cardiol 12: 9–13, 2003. [DOI] [PubMed] [Google Scholar]
- 62. Chen C, Gao JL, Liu MY, Li SL, Xuan XC, Zhang XZ, Zhang XY, Wei YY, Zhen CL, Jin J, Shen X, and Dong DL. Mitochondrial fission inhibitors suppress endothelin-1-induced artery constriction. Cell Physiol Biochem 42: 1802–1811, 2017. [DOI] [PubMed] [Google Scholar]
- 63. Chen C, Huang J, Shen J, and Bai Q. Quercetin improves endothelial insulin sensitivity in obese mice by inhibiting Drp1 phosphorylation at serine 616 and mitochondrial fragmentation. Acta Biochim Biophys Sin (Shanghai) 51: 1250–1257, 2019. [DOI] [PubMed] [Google Scholar]
- 64. Chen DD and Chen AF. CuZn superoxide dismutase deficiency: culprit of accelerated vascular aging process. Hypertension 48: 1026–1028, 2006. [DOI] [PubMed] [Google Scholar]
- 65. Chen G, Han Z, Feng D, Chen Y, Chen L, Wu H, Huang L, Zhou C, Cai X, Fu C, Duan L, Wang X, Liu L, Liu X, Shen Y, Zhu Y, and Chen Q. A regulatory signaling loop comprising the PGAM5 phosphatase and CK2 controls receptor-mediated mitophagy. Mol Cell 54: 362–377, 2014. [DOI] [PubMed] [Google Scholar]
- 66. Chen KH, Guo X, Ma D, Guo Y, Li Q, Yang D, Li P, Qiu X, Wen S, Xiao RP, and Tang J. Dysregulation of HSG triggers vascular proliferative disorders. Nat Cell Biol 6: 872–883, 2004. [DOI] [PubMed] [Google Scholar]
- 67. Chen L, Gong Q, Stice JP, and Knowlton AA. Mitochondrial OPA1, apoptosis, and heart failure. Cardiovasc Res 84: 91–99, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chen Q, Sievers RE, Varga M, Kharait S, Haddad DJ, Patton AK, Delany CS, Mutka SC, Blonder JP, Dube GP, Rosenthal GJ, and Springer ML. Pharmacological inhibition of S-nitrosoglutathione reductase improves endothelial vasodilatory function in rats in vivo. J Appl Physiol (1985) 114: 752–760, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chen YR and Zweier JL. Cardiac mitochondria and reactive oxygen species generation. Circ Res 114: 524–537, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chiong M, Cartes-Saavedra B, Norambuena-Soto I, Mondaca-Ruff D, Morales PE, Garcia-Miguel M, and Mellado R. Mitochondrial metabolism and the control of vascular smooth muscle cell proliferation. Front Cell Dev Biol 2: 72, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, and Lipton SA. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science 324: 102–105, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chou TC, Yen MH, Li CY, and Ding YA. Alterations of nitric oxide synthase expression with aging and hypertension in rats. Hypertension 31: 643–648, 1998. [DOI] [PubMed] [Google Scholar]
- 73. Chudzinska M, Rogowicz D, Wolowiec L, Banach J, Sielski S, Bujak R, Sinkiewicz A, and Grzesk G. Resveratrol and cardiovascular system-the unfulfilled hopes. Ir J Med Sci 190: 981–986, 2021. [DOI] [PubMed] [Google Scholar]
- 74. Chung KK, Thomas B, Li X, Pletnikova O, Troncoso JC, Marsh L, Dawson VL, and Dawson TM. S-nitrosylation of parkin regulates ubiquitination and compromises parkin's protective function. Science 304: 1328–1331, 2004. [DOI] [PubMed] [Google Scholar]
- 75. Cocco G and Jerie P. Angina pectoris in patients without flow-limiting coronary artery disease (cardiac syndrome X). A forest of a variety of trees. Cardiol J 22: 605–612, 2015. [DOI] [PubMed] [Google Scholar]
- 76. Corina A, Rangel-Zuniga OA, Jimenez-Lucena R, Alcala-Diaz JF, Quintana-Navarro G, Yubero-Serrano EM, Lopez-Moreno J, Delgado-Lista J, Tinahones F, Ordovas JM, Lopez-Miranda J, and Perez-Martinez P. Low intake of vitamin E accelerates cellular aging in patients with established cardiovascular disease: the CORDIOPREV study. J Gerontol A Biol Sci Med Sci 74: 770–777, 2019. [DOI] [PubMed] [Google Scholar]
- 77. Corral-Debrinski M, Shoffner JM, Lott MT, and Wallace DC. Association of mitochondrial DNA damage with aging and coronary atherosclerotic heart disease. Mutat Res 275: 169–180, 1992. [DOI] [PubMed] [Google Scholar]
- 78. Cosentino F, Francia P, Camici GG, Pelicci PG, Luscher TF, and Volpe M. Final common molecular pathways of aging and cardiovascular disease: role of the p66Shc protein. Arterioscler Thromb Vasc Biol 28: 622–628, 2008. [DOI] [PubMed] [Google Scholar]
- 79. Costanzo A, Moretti F, Burgio VL, Bravi C, Guido F, Levrero M, and Puri PL. Endothelial activation by angiotensin II through NFkappaB and p38 pathways: involvement of NFkappaB-inducible kinase (NIK), free oxygen radicals, and selective inhibition by aspirin. J Cell Physiol 195: 402–410, 2003. [DOI] [PubMed] [Google Scholar]
- 80. Cowell OR, Mistry N, Deighton K, Matu J, Griffiths A, Minihane AM, Mathers JC, Shannon OM, and Siervo M. Effects of a Mediterranean diet on blood pressure: a systematic review and meta-analysis of randomized controlled trials and observational studies. J Hypertens 39: 729–739, 2021. [DOI] [PubMed] [Google Scholar]
- 81. Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, and Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res 90: 1159–1166, 2002. [DOI] [PubMed] [Google Scholar]
- 82. Csiszar A, Yabluchanskiy A, Ungvari A, Ungvari Z, and Tarantini S. Overexpression of catalase targeted to mitochondria improves neurovascular coupling responses in aged mice. Geroscience 41: 609–617, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Daaka Y. S-nitrosylation-regulated GPCR signaling. Biochim Biophys Acta 1820: 743–751, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Dai DF, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, Gollahon K, Martin GM, Loeb LA, Ladiges WC, and Rabinovitch PS. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation 119: 2789–2797, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Daiber A, Di Lisa F, Oelze M, Kroller-Schon S, Steven S, Schulz E, and Munzel T. Crosstalk of mitochondria with NADPH oxidase via reactive oxygen and nitrogen species signalling and its role for vascular function. Br J Pharmacol 174: 1670–1689, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Daiber A, Steven S, Vujacic-Mirski K, Kalinovic S, Oelze M, Di Lisa F, and Munzel T. Regulation of vascular function and inflammation via cross talk of reactive oxygen and nitrogen species from mitochondria or NADPH oxidase-implications for diabetes progression. Int J Mol Sci 21: 3405, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Daiber A, Xia N, Steven S, Oelze M, Hanf A, Kroller-Schon S, Munzel T, and Li H. New therapeutic implications of endothelial nitric oxide synthase (eNOS) function/dysfunction in cardiovascular disease. Int J Mol Sci 20: 187, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Dal-Ros S, Zoll J, Lang AL, Auger C, Keller N, Bronner C, Geny B, and Schini-Kerth VB. Chronic intake of red wine polyphenols by young rats prevents aging-induced endothelial dysfunction and decline in physical performance: role of NADPH oxidase. Biochem Biophys Res Commun 404: 743–749, 2011. [DOI] [PubMed] [Google Scholar]
- 89. Damania A, Jaiman D, Teotia AK, and Kumar A. Mesenchymal stromal cell-derived exosome-rich fractionated secretome confers a hepatoprotective effect in liver injury. Stem Cell Res Ther 9: 31, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Dan Dunn J, Alvarez LA, Zhang X, and Soldati T. Reactive oxygen species and mitochondria: a nexus of cellular homeostasis. Redox Biol 6: 472–485, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Dantas AP, Tostes RC, Fortes ZB, Costa SG, Nigro D, and Carvalho MH. In vivo evidence for antioxidant potential of estrogen in microvessels of female spontaneously hypertensive rats. Hypertension 39: 405–411, 2002. [DOI] [PubMed] [Google Scholar]
- 92. De Becker B, Coremans C, Chaumont M, Delporte C, Van Antwerpen P, Franck T, Rousseau A, Zouaoui Boudjeltia K, Cullus P, and van de Borne P. Severe hypouricemia impairs endothelium-dependent vasodilatation and reduces blood pressure in healthy young men: a randomized, placebo-controlled, and crossover study. J Am Heart Assoc 8: e013130, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. de Cavanagh EM, Inserra F, and Ferder L. Angiotensin II blockade: a strategy to slow ageing by protecting mitochondria? Cardiovasc Res 89: 31–40, 2011. [DOI] [PubMed] [Google Scholar]
- 94. de Cavanagh EM, Inserra F, Ferder M, and Ferder L. From mitochondria to disease: role of the renin-angiotensin system. Am J Nephrol 27: 545–553, 2007. [DOI] [PubMed] [Google Scholar]
- 95. de la Sierra A and Larrousse M.. Endothelial dysfunction is associated with increased levels of biomarkers in essential hypertension. J Hum Hypertens 24: 373–379, 2010. [DOI] [PubMed] [Google Scholar]
- 96. de Lucia C, Eguchi A, and Koch WJ. New insights in cardiac beta-adrenergic signaling during heart failure and aging. Front Pharmacol 9: 904, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. De Silva TM, Li Y, Kinzenbaw DA, Sigmund CD, and Faraci FM. Endothelial PPARgamma (peroxisome proliferator-activated receptor-gamma) is essential for preventing endothelial dysfunction with aging. Hypertension 72: 227–234, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, and Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102: 1351–1357, 2000. [DOI] [PubMed] [Google Scholar]
- 99. Diaz M, Degens H, Vanhees L, Austin C, and Azzawi M. The effects of resveratrol on aging vessels. Exp Gerontol 85: 41–47, 2016. [DOI] [PubMed] [Google Scholar]
- 100. Didion SP, Kinzenbaw DA, Schrader LI, and Faraci FM. Heterozygous CuZn superoxide dismutase deficiency produces a vascular phenotype with aging. Hypertension 48: 1072–1079, 2006. [DOI] [PubMed] [Google Scholar]
- 101. Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, and Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med 45: 1340–1351, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, and Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res 107: 106–116, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Dikalova AE, Gongora MC, Harrison DG, Lambeth JD, Dikalov S, and Griendling KK. Upregulation of Nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via eNOS uncoupling. Am J Physiol Heart Circ Physiol 299: H673–H679, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Dinenno FA and Joyner MJ. Alpha-adrenergic control of skeletal muscle circulation at rest and during exercise in aging humans. Microcirculation 13: 329–341, 2006. [DOI] [PubMed] [Google Scholar]
- 105. Doehner W, Schoene N, Rauchhaus M, Leyva-Leon F, Pavitt DV, Reaveley DA, Schuler G, Coats AJ, Anker SD, and Hambrecht R. Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: results from 2 placebo-controlled studies. Circulation 105: 2619–2624, 2002. [DOI] [PubMed] [Google Scholar]
- 106. Dolcini J, Wu H, Nwanaji-Enwerem JC, Kiomourtozlogu MA, Cayir A, Sanchez-Guerra M, Vokonas P, Schwarz J, and Baccarelli AA. Mitochondria and aging in older individuals: an analysis of DNA methylation age metrics, leukocyte telomere length, and mitochondrial DNA copy number in the VA normative aging study. Aging (Albany NY) 12: 2070–2083, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, and Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res 100: 1659–1666, 2007. [DOI] [PubMed] [Google Scholar]
- 108. Donato AJ, Machin DR, and Lesniewski LA. Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circ Res 123: 825–848, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Donato AJ, Magerko KA, Lawson BR, Durrant JR, Lesniewski LA, and Seals DR. SIRT-1 and vascular endothelial dysfunction with ageing in mice and humans. J Physiol 589: 4545–4554, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Dopp JM, Philippi NR, Marcus NJ, Olson EB, Bird CE, Moran JJ, Mueller SW, and Morgan BJ. Xanthine oxidase inhibition attenuates endothelial dysfunction caused by chronic intermittent hypoxia in rats. Respiration 82: 458–467, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Doughan AK, Harrison DG, and Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res 102: 488–496, 2008. [DOI] [PubMed] [Google Scholar]
- 112. Drake MT, Shenoy SK, and Lefkowitz RJ. Trafficking of G protein-coupled receptors. Circ Res 99: 570–582, 2006. [DOI] [PubMed] [Google Scholar]
- 113. Dromparis P and Michelakis ED. Mitochondria in vascular health and disease. Annu Rev Physiol 75: 95–126, 2013. [DOI] [PubMed] [Google Scholar]
- 114. Drummond GR, Cai H, Davis ME, Ramasamy S, and Harrison DG. Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression by hydrogen peroxide. Circ Res 86: 347–354, 2000. [DOI] [PubMed] [Google Scholar]
- 115. Du J, Wang X, Li J, Guo J, Liu L, Yan D, Yang Y, Li Z, Zhu J, and Shen B. Increasing TRPV4 expression restores flow-induced dilation impaired in mesenteric arteries with aging. Sci Rep 6: 22780, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Dukes M, LeBlanc A, and Tracy E. Inhibition of GRK2, but not HSP90 reduces mitochondrial superoxide and improves vasodilation capacity of coronary arterioles from aged female rats. University of Louisville Undergraduate Arts and Research Showcase 15, 2020. [Google Scholar]
- 117. Durand MJ, Ait-Aissa K, Levchenko V, Staruschenko A, Gutterman DD, and Beyer AM. Visualization and quantification of mitochondrial structure in the endothelium of intact arteries. Cardiovasc Res 115: 1546–1556, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Durand MJ, Zinkevich NS, Riedel M, Gutterman DD, Nasci VL, Salato VK, Hijjawi JB, Reuben CF, North PE, and Beyer AM. Vascular actions of angiotensin 1–7 in the human microcirculation: novel role for telomerase. Arterioscler Thromb Vasc Biol 36: 1254–1262, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, and Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol 587: 3271–3285, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ehrhardt N, Cui J, Dagdeviren S, Saengnipanthkul S, Goodridge HS, Kim JK, Lantier L, Guo X, Chen YI, Raffel LJ, Buchanan TA, Hsueh WA, Rotter JI, Goodarzi MO, and Peterfy M. Adiposity-independent effects of aging on insulin sensitivity and clearance in mice and humans. Obesity (Silver Spring) 27: 434–443, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. El Assar M, Angulo J, and Rodriguez-Manas L. Oxidative stress and vascular inflammation in aging. Free Radic Biol Med 65: 380–401, 2013. [DOI] [PubMed] [Google Scholar]
- 122. Eskurza I, Kahn ZD, and Seals DR. Xanthine oxidase does not contribute to impaired peripheral conduit artery endothelium-dependent dilatation with ageing. J Physiol 571: 661–668, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Eskurza I, Monahan KD, Robinson JA, and Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol 556: 315–324, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Fan G, Kassmann M, Cui Y, Matthaeus C, Kunz S, Zhong C, Zhu S, Xie Y, Tsvetkov D, Daumke O, Huang Y, and Gollasch M. Age attenuates the T-type CaV 3.2-RyR axis in vascular smooth muscle. Aging Cell 19: e13134, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Feher A, Broskova Z, and Bagi Z. Age-related impairment of conducted dilation in human coronary arterioles. Am J Physiol Heart Circ Physiol 306: H1595–H1601, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Feletou M. Endothelium-dependent hyperpolarization and endothelial dysfunction. J Cardiovasc Pharmacol 67: 373–387, 2016. [DOI] [PubMed] [Google Scholar]
- 127. Fennell JP, Brosnan MJ, Frater AJ, Hamilton CA, Alexander MY, Nicklin SA, Heistad DD, Baker AH, and Dominiczak AF. Adenovirus-mediated overexpression of extracellular superoxide dismutase improves endothelial dysfunction in a rat model of hypertension. Gene Ther 9: 110–117, 2002. [DOI] [PubMed] [Google Scholar]
- 128. Ferguson SS, Menard L, Barak LS, Koch WJ, Colapietro AM, and Caron MG. Role of phosphorylation in agonist-promoted beta 2-adrenergic receptor sequestration. Rescue of a sequestration-defective mutant receptor by beta ARK1. J Biol Chem 270: 24782–24789, 1995. [DOI] [PubMed] [Google Scholar]
- 129. Ferrer M, Meyer M, and Osol G. Estrogen replacement increases beta-adrenoceptor-mediated relaxation of rat mesenteric arteries. J Vasc Res 33: 124–131, 1996. [DOI] [PubMed] [Google Scholar]
- 130. Ferrer M, Sanchez M, Minoves N, Salaices M, and Balfagon G. Aging increases neuronal nitric oxide release and superoxide anion generation in mesenteric arteries from spontaneously hypertensive rats. J Vasc Res 40: 509–519, 2003. [DOI] [PubMed] [Google Scholar]
- 131. Ferrieres J. The French paradox: lessons for other countries. Heart 90: 107–111, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ferrucci L and Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol 15: 505–522, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Fetterman JL, Holbrook M, Westbrook DG, Brown JA, Feeley KP, Breton-Romero R, Linder EA, Berk BD, Weisbrod RM, Widlansky ME, Gokce N, Ballinger SW, and Hamburg NM. Mitochondrial DNA damage and vascular function in patients with diabetes mellitus and atherosclerotic cardiovascular disease. Cardiovasc Diabetol 15: 53, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Figtree GA, Keyvan Karimi G, Liu CC, and Rasmussen HH. Oxidative regulation of the Na(+)−K(+) pump in the cardiovascular system. Free Radic Biol Med 53: 2263–2268, 2012. [DOI] [PubMed] [Google Scholar]
- 135. Fleenor BS, Seals DR, Zigler ML, and Sindler AL. Superoxide-lowering therapy with TEMPOL reverses arterial dysfunction with aging in mice. Aging Cell 11: 269–276, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Forgione MA, Weiss N, Heydrick S, Cap A, Klings ES, Bierl C, Eberhardt RT, Farber HW, and Loscalzo J. Cellular glutathione peroxidase deficiency and endothelial dysfunction. Am J Physiol Heart Circ Physiol 282: H1255–H1261, 2002. [DOI] [PubMed] [Google Scholar]
- 137. Frame MD, Dewar AM, Calizo RC, Qifti A, and Scarlata SF. Nitrosative stress uncovers potent beta2-adrenergic receptor-linked vasodilation further enhanced by blockade of clathrin endosome formation. Am J Physiol Heart Circ Physiol 314: H1298–H1308, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Francia P, delli Gatti C, Bachschmid M, Martin-Padura I, Savoia C, Migliaccio E, Pelicci PG, Schiavoni M, Luscher TF, Volpe M, and Cosentino F. Deletion of p66shc gene protects against age-related endothelial dysfunction. Circulation 110: 2889–2895, 2004. [DOI] [PubMed] [Google Scholar]
- 139. Freed JK, Beyer AM, LoGiudice JA, Hockenberry JC, and Gutterman DD. Ceramide changes the mediator of flow-induced vasodilation from nitric oxide to hydrogen peroxide in the human microcirculation. Circ Res 115: 525–532, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Freed JK, Durand MJ, Hoffmann BR, Densmore JC, Greene AS, and Gutterman DD. Mitochondria-regulated formation of endothelium-derived extracellular vesicles shifts the mediator of flow-induced vasodilation. Am J Physiol Heart Circ Physiol 312: H1096–H1104, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Fuchs D, Nyakayiru J, Draijer R, Mulder TP, Hopman MT, Eijsvogels TM, and Thijssen DH. Impact of flavonoid-rich black tea and beetroot juice on postprandial peripheral vascular resistance and glucose homeostasis in obese, insulin-resistant men: a randomized controlled trial. Nutr Metab (Lond) 13: 34, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Fujii K, Ohmori S, Tominaga M, Abe I, Takata Y, Ohya Y, Kobayashi K, and Fujishima M. Age-related changes in endothelium-dependent hyperpolarization in the rat mesenteric artery. Am J Physiol 265: H509–H516, 1993. [DOI] [PubMed] [Google Scholar]
- 143. Fujii K, Onaka U, Goto K, Abe I, and Fujishima M. Impaired isoproterenol-induced hyperpolarization in isolated mesenteric arteries of aged rats. Hypertension 34: 222–228, 1999. [DOI] [PubMed] [Google Scholar]
- 144. Furman D, Chang J, Lartigue L, Bolen CR, Haddad F, Gaudilliere B, Ganio EA, Fragiadakis GK, Spitzer MH, Douchet I, Daburon S, Moreau JF, Nolan GP, Blanco P, Dechanet-Merville J, Dekker CL, Jojic V, Kuo CJ, Davis MM, and Faustin B. Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states. Nat Med 23: 174–184, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Gaballa MA, Eckhart AD, Koch WJ, and Goldman S. Vascular beta-adrenergic receptor adenylyl cyclase system in maturation and aging. J Mol Cell Cardiol 32: 1745–1755, 2000. [DOI] [PubMed] [Google Scholar]
- 146. Gao YJ, Hirota S, Zhang DW, Janssen LJ, and Lee RM. Mechanisms of hydrogen-peroxide-induced biphasic response in rat mesenteric artery. Br J Pharmacol 138: 1085–1092, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Garland CJ and Dora KA. EDH: endothelium-dependent hyperpolarization and microvascular signalling. Acta Physiol (Oxf) 219: 152–161, 2017. [DOI] [PubMed] [Google Scholar]
- 148. Garland CJ, Hiley CR, and Dora KA. EDHF: spreading the influence of the endothelium. Br J Pharmacol 164: 839–852, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Garland CJ, Plane F, Kemp BK, and Cocks TM. Endothelium-dependent hyperpolarization: a role in the control of vascular tone. Trends Pharmacol Sci 16: 23–30, 1995. [DOI] [PubMed] [Google Scholar]
- 150. Gierhardt M, Sommer N, Schreckenberg R, Schlueter LD, Ghofrani A, Schermuly R, Rainer S, and Nobert W. Right heart hypertrophy in mice deficient of the mitochondrial regulator protein p66shc is decreased after chronic exposure by cyclophilin D dependent mechanism. Eur Repir J 44: P2301, 2014. [Google Scholar]
- 151. Gioscia-Ryan RA, Battson ML, Cuevas LM, Eng JS, Murphy MP, and Seals DR. Mitochondria-targeted antioxidant therapy with MitoQ ameliorates aortic stiffening in old mice. J Appl Physiol (1985) 124: 1194–1202, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Gioscia-Ryan RA, LaRocca TJ, Sindler AL, Zigler MC, Murphy MP, and Seals DR. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J Physiol 592: 2549–2561, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Giralt A and Villarroya F. SIRT3, a pivotal actor in mitochondrial functions: metabolism, cell death and aging. Biochem J 444: 1–10, 2012. [DOI] [PubMed] [Google Scholar]
- 154. Gocmez SS, Scarpace PJ, Whidden MA, Erdos B, Kirichenko N, Sakarya Y, Utkan T, and Tumer N. Age impaired endothelium-dependent vasodilation is improved by resveratrol in rat mesenteric arteries. J Exerc Nutrition Biochem 20: 41–48, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Goettsch W, Lattmann T, Amann K, Szibor M, Morawietz H, Munter K, Muller SP, Shaw S, and Barton M. Increased expression of endothelin-1 and inducible nitric oxide synthase isoform II in aging arteries in vivo: implications for atherosclerosis. Biochem Biophys Res Commun 280: 908–913, 2001. [DOI] [PubMed] [Google Scholar]
- 156. Golbidi S and Laher I. Exercise and the aging endothelium. J Diabetes Res 2013: 789607, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Golshiri K, Ataei Ataabadi E, Brandt R, van der Pluijm I, de Vries R, Danser J, and Roks A. Chronic sildenafil treatment improves vasomotor function in a mouse model of accelerated aging. Int J Mol Sci 21: 4667, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Gordish KL and Beierwaltes WH. Resveratrol induces acute endothelium-dependent renal vasodilation mediated through nitric oxide and reactive oxygen species scavenging. Am J Physiol Renal Physiol 306: F542–F550, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Gordish KL and Beierwaltes WH. Sustained resveratrol infusion increases natriuresis independent of renal vasodilation. Physiol Rep 2: e12144, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Gordish KL and Beierwaltes WH. Chronic resveratrol reverses a mild angiotensin II-induced pressor effect in a rat model. Integr Blood Press Control 9: 23–31, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Gori T and Parker JD. Long-term therapy with organic nitrates: the pros and cons of nitric oxide replacement therapy. J Am Coll Cardiol 44: 632–634, 2004. [DOI] [PubMed] [Google Scholar]
- 162. Grasselli A, Nanni S, Colussi C, Aiello A, Benvenuti V, Ragone G, Moretti F, Sacchi A, Bacchetti S, Gaetano C, Capogrossi MC, Pontecorvi A, and Farsetti A. Estrogen receptor-alpha and endothelial nitric oxide synthase nuclear complex regulates transcription of human telomerase. Circ Res 103: 34–42, 2008. [DOI] [PubMed] [Google Scholar]
- 163. Gray SP, Shah A, and Smyrnias I. NADPH oxidase 4 and its role in the cardiovascular system. Vasc Biol 1: H59–H66, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Gresele P, Pignatelli P, Guglielmini G, Carnevale R, Mezzasoma AM, Ghiselli A, Momi S, and Violi F. Resveratrol, at concentrations attainable with moderate wine consumption, stimulates human platelet nitric oxide production. J Nutr 138: 1602–1608, 2008. [DOI] [PubMed] [Google Scholar]
- 165. Guaras A, Perales-Clemente E, Calvo E, Acin-Perez R, Loureiro-Lopez M, Pujol C, Martinez-Carrascoso I, Nunez E, Garcia-Marques F, Rodriguez-Hernandez MA, Cortes A, Diaz F, Perez-Martos A, Moraes CT, Fernandez-Silva P, Trifunovic A, Navas P, Vazquez J, and Enriquez JA. The CoQH2/CoQ ratio serves as a sensor of respiratory chain efficiency. Cell Rep 15: 197–209, 2016. [DOI] [PubMed] [Google Scholar]
- 166. Guo J, Gertsberg Z, Ozgen N, and Steinberg SF. p66Shc links alpha1-adrenergic receptors to a reactive oxygen species-dependent AKT-FOXO3A phosphorylation pathway in cardiomyocytes. Circ Res 104: 660–669, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Guo X, Chen KH, Guo Y, Liao H, Tang J, and Xiao RP. Mitofusin 2 triggers vascular smooth muscle cell apoptosis via mitochondrial death pathway. Circ Res 101: 1113–1122, 2007. [DOI] [PubMed] [Google Scholar]
- 168. Guo Y, Xu C, Man AWC, Bai B, Luo C, Huang Y, Xu A, Vanhoutte PM, and Wang Y. Endothelial SIRT1 prevents age-induced impairment of vasodilator responses by enhancing the expression and activity of soluble guanylyl cyclase in smooth muscle cells. Cardiovasc Res 115: 678–690, 2019. [DOI] [PubMed] [Google Scholar]
- 169. Gurdal H, Tilakaratne N, Brown RD, Fonseca M, Friedman E, and Johnson MD. The expression of alpha 1 adrenoceptor subtypes changes with age in the rat aorta. J Pharmacol Exp Ther 275: 1656–1662, 1995. [PubMed] [Google Scholar]
- 170. Gutterman DD, Miura H, and Liu Y. Redox modulation of vascular tone: focus of potassium channel mechanisms of dilation. Arterioscler Thromb Vasc Biol 25: 671–678, 2005. [DOI] [PubMed] [Google Scholar]
- 171. Guzik TJ, Chen W, Gongora MC, Guzik B, Lob HE, Mangalat D, Hoch N, Dikalov S, Rudzinski P, Kapelak B, Sadowski J, and Harrison DG. Calcium-dependent NOX5 nicotinamide adenine dinucleotide phosphate oxidase contributes to vascular oxidative stress in human coronary artery disease. J Am Coll Cardiol 52: 1803–1809, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Hamilton CA, Brosnan MJ, McIntyre M, Graham D, and Dominiczak AF. Superoxide excess in hypertension and aging: a common cause of endothelial dysfunction. Hypertension 37: 529–534, 2001. [DOI] [PubMed] [Google Scholar]
- 173. Harley CB, Liu W, Blasco M, Vera E, Andrews WH, Briggs LA, and Raffaele JM. A natural product telomerase activator as part of a health maintenance program. Rejuvenation Res 14: 45–56, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Harley CB, Liu W, Flom PL, and Raffaele JM. A natural product telomerase activator as part of a health maintenance program: metabolic and cardiovascular response. Rejuvenation Res 16: 386–395, 2013. [DOI] [PubMed] [Google Scholar]
- 175. Harraz OF and Jensen LJ. Aging, calcium channel signaling and vascular tone. Mech Ageing Dev 191: 111336, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Hayoz S, Pettis J, Bradley V, Segal SS, and Jackson WF. Increased amplitude of inward rectifier K(+) currents with advanced age in smooth muscle cells of murine superior epigastric arteries. Am J Physiol Heart Circ Physiol 312: H1203–H1214, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. He T, Joyner MJ, and Katusic ZS. Aging decreases expression and activity of glutathione peroxidase-1 in human endothelial progenitor cells. Microvasc Res 78: 447–452, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Heitzer T, Schlinzig T, Krohn K, Meinertz T, and Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 104: 2673–2678, 2001. [DOI] [PubMed] [Google Scholar]
- 179. Hermann A, Sitdikova GF, and Weiger TM. Oxidative stress and maxi calcium-activated potassium (BK) channels. Biomolecules 5: 1870–1911, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, and Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 24: 677–683, 2004. [DOI] [PubMed] [Google Scholar]
- 181. Hirsch E, Lembo G, Montrucchio G, Rommel C, Costa C, and Barberis L. Signaling through PI3Kgamma: a common platform for leukocyte, platelet and cardiovascular stress sensing. Thromb Haemost 95: 29–35, 2006. [PubMed] [Google Scholar]
- 182. Hodgson JM, Burke V, and Puddey IB. Acute effects of tea on fasting and postprandial vascular function and blood pressure in humans. J Hypertens 23: 47–54, 2005. [DOI] [PubMed] [Google Scholar]
- 183. Hodgson JM, Puddey IB, Croft KD, Burke V, Mori TA, Caccetta RA, and Beilin LJ. Acute effects of ingestion of black and green tea on lipoprotein oxidation. Am J Clin Nutr 71: 1103–1107, 2000. [DOI] [PubMed] [Google Scholar]
- 184. Hogan SE, Rodriguez Salazar MP, Cheadle J, Glenn R, Medrano C, Petersen TH, and Ilagan RM. Mesenchymal stromal cell-derived exosomes improve mitochondrial health in pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 316: L723–L737, 2019. [DOI] [PubMed] [Google Scholar]
- 185. Holzerova E and Prokisch H. Mitochondria: much ado about nothing? How dangerous is reactive oxygen species production? Int J Biochem Cell Biol 63: 16–20, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Horinaka S, Yabe A, Yagi H, Ishimitsu T, Yamazaki T, Suzuki S, Kohro T, and Nagai R; JCAD Study Investigators. Effects of nicorandil on cardiovascular events in patients with coronary artery disease in the Japanese Coronary Artery Disease (JCAD) study. Circ J 74: 503–509, 2010. [DOI] [PubMed] [Google Scholar]
- 187. Hotta K, Chen B, Behnke BJ, Ghosh P, Stabley JN, Bramy JA, Sepulveda JL, Delp MD, and Muller-Delp JM. Exercise training reverses age-induced diastolic dysfunction and restores coronary microvascular function. J Physiol 595: 3703–3719, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Hsu YC, Wu YT, Yu TH, and Wei YH. Mitochondria in mesenchymal stem cell biology and cell therapy: from cellular differentiation to mitochondrial transfer. Semin Cell Dev Biol 52: 119–131, 2016. [DOI] [PubMed] [Google Scholar]
- 189. Hu Z, Chen J, Wei Q, and Xia Y. Bidirectional actions of hydrogen peroxide on endothelial nitric-oxide synthase phosphorylation and function: co-commitment and interplay of Akt and AMPK. J Biol Chem 283: 25256–25263, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Huang D, Shi S, Liang C, Zhang X, Du X, An H, Peers C, Zhang H, and Gamper N. Delineating an extracellular redox-sensitive module in T-type Ca(2+) channels. J Biol Chem 295: 6177–6186, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Huang J, Zhang H, Tan X, Hu M, and Shen B. Exercise restores impaired endothelium-derived hyperpolarizing factor-mediated vasodilation in aged rat aortic arteries via the TRPV4-KCa2.3 signaling complex. Clin Interv Aging 14: 1579–1587, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Huang P, Riordan SM, Heruth DP, Grigoryev DN, Zhang LQ, and Ye SQ. A critical role of nicotinamide phosphoribosyltransferase in human telomerase reverse transcriptase induction by resveratrol in aortic smooth muscle cells. Oncotarget 6: 10812–10824, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Huang W, Hutabarat RP, Chai Z, Zheng T, Zhang W, and Li D. Antioxidant blueberry anthocyanins induce vasodilation via PI3K/Akt signaling pathway in high-glucose-induced human umbilical vein endothelial cells. Int J Mol Sci 21: 1575, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Huang W, Yan Z, Li D, Ma Y, Zhou J, and Sui Z. Antioxidant and anti-inflammatory effects of blueberry anthocyanins on high glucose-induced human retinal capillary endothelial cells. Oxid Med Cell Longev 2018: 1862462, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Huang W, Zhu Y, Li C, Sui Z, and Min W. Effect of blueberry anthocyanins malvidin and glycosides on the antioxidant properties in endothelial cells. Oxid Med Cell Longev 2016: 1591803, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Huang WY, Zhang HC, Liu WX, and Li CY. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J Zhejiang Univ Sci B 13: 94–102, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Hung CH, Cheng SS, Cheung YT, Wuwongse S, Zhang NQ, Ho YS, Lee SM, and Chang RC. A reciprocal relationship between reactive oxygen species and mitochondrial dynamics in neurodegeneration. Redox Biol 14: 7–19, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Hurley DM, Williams ER, Cross JM, Riedinger BR, Meyer RA, Abela GS, and Slade JM. Aerobic exercise improves microvascular function in older adults. Med Sci Sports Exerc 51: 773–781, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Idris Khodja N, Chataigneau T, Auger C, and Schini-Kerth VB. Grape-derived polyphenols improve aging-related endothelial dysfunction in rat mesenteric artery: role of oxidative stress and the angiotensin system. PLoS One 7: e32039, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Inal ME, Kanbak G, and Sunal E. Antioxidant enzyme activities and malondialdehyde levels related to aging. Clin Chim Acta 305: 75–80, 2001. [DOI] [PubMed] [Google Scholar]
- 201. Ivey KL, Jensen MK, Hodgson JM, Eliassen AH, Cassidy A, and Rimm EB. Association of flavonoid-rich foods and flavonoids with risk of all-cause mortality. Br J Nutr 117: 1470–1477, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Jablonski KL, Chonchol M, Pierce GL, Walker AE, and Seals DR. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension 57: 63–69, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Jackson WF. Kv channels and the regulation of vascular smooth muscle tone. Microcirculation 25: e12421(1–25), 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Jacobson A, Yan C, Gao Q, Rincon-Skinner T, Rivera A, Edwards J, Huang A, Kaley G, and Sun D. Aging enhances pressure-induced arterial superoxide formation. Am J Physiol Heart Circ Physiol 293: H1344–H1350, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Jendrach M, Pohl S, Voth M, Kowald A, Hammerstein P, and Bereiter-Hahn J. Morpho-dynamic changes of mitochondria during ageing of human endothelial cells. Mech Ageing Dev 126: 813–821, 2005. [DOI] [PubMed] [Google Scholar]
- 206. Jiang L, Wang M, Zhang J, Monticone RE, Telljohann R, Spinetti G, Pintus G, and Lakatta EG. Increased aortic calpain-1 activity mediates age-associated angiotensin II signaling of vascular smooth muscle cells. PLoS One 3: e2231, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Jin H, Zhu Y, Li Y, Ding X, Ma W, Han X, and Wang B. BDNF-mediated mitophagy alleviates high-glucose-induced brain microvascular endothelial cell injury. Apoptosis 24: 511–528, 2019. [DOI] [PubMed] [Google Scholar]
- 208. Jofre I, Pezoa C, Cuevas M, Scheuermann E, Freires IA, Rosalen PL, de Alencar SM, and Romero F. Antioxidant and vasodilator activity of Ugni molinae Turcz. (Murtilla) and its modulatory mechanism in hypotensive response. Oxid Med Cell Longev 2016: 6513416, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Johnson MD, Zhou Y, Friedman E, and Roberts J. Expression of G protein alpha subunits in the aging cardiovascular system. J Gerontol A Biol Sci Med Sci 50A: B14–B19, 1995. [DOI] [PubMed] [Google Scholar]
- 210. Jones SA, O'Donnell VB, Wood JD, Broughton JP, Hughes EJ, and Jones OT. Expression of phagocyte NADPH oxidase components in human endothelial cells. Am J Physiol 271: H1626–H1634, 1996. [DOI] [PubMed] [Google Scholar]
- 211. Kalaria RN and Harik SI. Increased alpha 2- and beta 2-adrenergic receptors in cerebral microvessels in Alzheimer disease. Neurosci Lett 106: 233–238, 1989. [DOI] [PubMed] [Google Scholar]
- 212. Kambic T, Novakovic M, Tomazin K, Strojnik V, and Jug B. Blood flow restriction resistance exercise improves muscle strength and hemodynamics, but not vascular function in coronary artery disease patients: a pilot randomized controlled trial. Front Physiol 10: 656, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Kang LS, Chen B, Reyes RA, Leblanc AJ, Teng B, Mustafa SJ, and Muller-Delp JM. Aging and estrogen alter endothelial reactivity to reactive oxygen species in coronary arterioles. Am J Physiol Heart Circ Physiol 300: H2105–H2115, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Kang LS, Kim S, Dominguez JM, 2nd, Sindler AL, Dick GM, and Muller-Delp JM. Aging and muscle fiber type alter K+ channel contributions to the myogenic response in skeletal muscle arterioles. J Appl Physiol (1985) 107: 389–398, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Kanthlal SK, Joseph J, Paul B, Vijayakumar M, and Uma Devi P. Antioxidant and vasorelaxant effects of aqueous extract of large cardamom in L-NAME induced hypertensive rats. Clin Exp Hypertens 42: 581–589, 2020. [DOI] [PubMed] [Google Scholar]
- 216. Kaplon RE, Hill SD, Bispham NZ, Santos-Parker JR, Nowlan MJ, Snyder LL, Chonchol M, LaRocca TJ, McQueen MB, and Seals DR. Oral trehalose supplementation improves resistance artery endothelial function in healthy middle-aged and older adults. Aging (Albany NY) 8: 1167–1183, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Katakam PV, Gordon AO, Sure VN, Rutkai I, and Busija DW. Diversity of mitochondria-dependent dilator mechanisms in vascular smooth muscle of cerebral arteries from normal and insulin-resistant rats. Am J Physiol Heart Circ Physiol 307: H493–H503, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Kazanietz MG and Enero MA. Decreased beta-adrenoceptor-mediated vasodilation in aorta from aged rats: possible involvement of a stimulatory GTP-binding protein. Eur J Pharmacol 198: 177–181, 1991. [DOI] [PubMed] [Google Scholar]
- 219. Kelemen M, Vaidya D, Waters DD, Howard BV, Cobb F, Younes N, Tripputti M, and Ouyang P. Hormone therapy and antioxidant vitamins do not improve endothelial vasodilator function in postmenopausal women with established coronary artery disease: a substudy of the Women's Angiographic Vitamin and Estrogen (WAVE) trial. Atherosclerosis 179: 193–200, 2005. [DOI] [PubMed] [Google Scholar]
- 220. Kelm NQ, Beare JE, Yuan F, George M, Shofner CM, Keller BB, Hoying JB, and LeBlanc AJ. Adipose-derived cells improve left ventricular diastolic function and increase microvascular perfusion in advanced age. PLoS One 13: e0202934, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Kennedy DO, Wightman EL, Reay JL, Lietz G, Okello EJ, Wilde A, and Haskell CF. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: a double-blind, placebo-controlled, crossover investigation. Am J Clin Nutr 91: 1590–1597, 2010. [DOI] [PubMed] [Google Scholar]
- 222. Kicinska A and Jarmuszkiewicz W. Flavonoids and mitochondria: activation of cytoprotective pathways? Molecules 25: 3060, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Kim YM, Youn SW, Sudhahar V, Das A, Chandhri R, Cuervo Grajal H, Kweon J, Leanhart S, He L, Toth PT, Kitajewski J, Rehman J, Yoon Y, Cho J, Fukai T, and Ushio-Fukai M. Redox regulation of mitochondrial fission protein Drp1 by protein disulfide isomerase limits endothelial senescence. Cell Rep 23: 3565–3578, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Kirkman DL, Muth BJ, Ramick MG, Townsend RR, and Edwards DG. Role of mitochondria-derived reactive oxygen species in microvascular dysfunction in chronic kidney disease. Am J Physiol Renal Physiol 314: F423–F429, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Kitada M, Kume S, Takeda-Watanabe A, Kanasaki K, and Koya D. Sirtuins and renal diseases: relationship with aging and diabetic nephropathy. Clin Sci (Lond) 124: 153–164, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Kizhakekuttu TJ, Wang J, Dharmashankar K, Ying R, Gutterman DD, Vita JA, and Widlansky ME. Adverse alterations in mitochondrial function contribute to type 2 diabetes mellitus-related endothelial dysfunction in humans. Arterioscler Thromb Vasc Biol 32: 2531–2539, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Klonizakis M, Alkhatib A, Middleton G, and Smith MF. Mediterranean diet- and exercise-induced improvement in age-dependent vascular activity. Clin Sci (Lond) 124: 579–587, 2013. [DOI] [PubMed] [Google Scholar]
- 228. Klonizakis M, Grammatikopoulou MG, Theodoridis X, Milner M, Liu Y, and Chourdakis M. Effects of long-versus short-term exposure to the Mediterranean diet on skin microvascular function and quality of life of healthy adults in Greece and the UK. Nutrients 11: 2487, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Kluge MA, Fetterman JL, and Vita JA. Mitochondria and endothelial function. Circ Res 112: 1171–1188, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Koller A, Fazzini F, Lamina C, Rantner B, Kollerits B, Stadler M, Klein-Weigel P, Fraedrich G, and Kronenberg F. Mitochondrial DNA copy number is associated with all-cause mortality and cardiovascular events in patients with peripheral arterial disease. J Intern Med 287: 569–579, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Korystova AF, Emel'yanov MO, Kublik LN, Levitman M, Shaposhnikova VV, Kim YA, and Korystov YN. Distribution of the activity of the angiotensin-converting enzyme in the rat aorta and changes in the activity with aging and by the action of L-NAME. Age (Dordr) 34: 821–830, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Kosugi M, Nakagomi A, Shibui T, Kato K, Kusama Y, Atarashi H, and Mizuno K. Effect of long-term nitrate treatment on cardiac events in patients with vasospastic angina. Circ J 75: 2196–2205, 2011. [DOI] [PubMed] [Google Scholar]
- 233. Kourtis N and Tavernarakis N. Cellular stress response pathways and ageing: intricate molecular relationships. EMBO J 30: 2520–2531, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Krause KH. Aging: a revisited theory based on free radicals generated by NOX family NADPH oxidases. Exp Gerontol 42: 256–262, 2007. [DOI] [PubMed] [Google Scholar]
- 235. Krueger KM, Daaka Y, Pitcher JA, and Lefkowitz RJ. The role of sequestration in G protein-coupled receptor resensitization. Regulation of beta2-adrenergic receptor dephosphorylation by vesicular acidification. J Biol Chem 272: 5–8, 1997. [DOI] [PubMed] [Google Scholar]
- 236. Kuck L, Grau M, Bloch W, and Simmonds MJ. Shear stress ameliorates superoxide impairment to erythrocyte deformability with concurrent nitric oxide synthase activation. Front Physiol 10: 36, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Kumar S. P66Shc and vascular endothelial function. Biosci Rep 39: BSR20182134 (1–11), 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Kuzkaya N, Weissmann N, Harrison DG, and Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem 278: 22546–22554, 2003. [DOI] [PubMed] [Google Scholar]
- 239. Kyo S, Takakura M, Kanaya T, Zhuo W, Fujimoto K, Nishio Y, Orimo A, and Inoue M. Estrogen activates telomerase. Cancer Res 59: 5917–5921, 1999. [PubMed] [Google Scholar]
- 240. Lambert DW, Hooper NM, and Turner AJ. Angiotensin-converting enzyme 2 and new insights into the renin-angiotensin system. Biochem Pharmacol 75: 781–786, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Landmesser U, Spiekermann S, Dikalov S, Tatge H, Wilke R, Kohler C, Harrison DG, Hornig B, and Drexler H. Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: role of xanthine-oxidase and extracellular superoxide dismutase. Circulation 106: 3073–3078, 2002. [DOI] [PubMed] [Google Scholar]
- 242. LaRocca TJ, Hearon CM Jr., Henson GD, and Seals DR.. Mitochondrial quality control and age-associated arterial stiffening. Exp Gerontol 58: 78–82, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. LaRocca TJ, Henson GD, Thorburn A, Sindler AL, Pierce GL, and Seals DR. Translational evidence that impaired autophagy contributes to arterial ageing. J Physiol 590: 3305–3316, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. LeBlanc AJ, Shipley RD, Kang LS, and Muller-Delp JM. Age impairs Flk-1 signaling and NO-mediated vasodilation in coronary arterioles. Am J Physiol Heart Circ Physiol 295: H2280–H2288, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Lebovitz RM, Zhang H, Vogel H, Cartwright J Jr., Dionne L, Lu N, Huang S, and Matzuk MM.. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc Natl Acad Sci U S A 93: 9782–9787, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Lee DC, Im JA, Kim JH, Lee HR, and Shim JY. Effect of long-term hormone therapy on telomere length in postmenopausal women. Yonsei Med J 46: 471–479, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Lee HY, Kim HK, Hoang TH, Yang S, Kim HR, and Chae HJ. The correlation of IRE1alpha oxidation with Nox4 activation in aging-associated vascular dysfunction. Redox Biol 37: 101727, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Lee HY, Zeeshan HMA, Kim HR, and Chae HJ. Nox4 regulates the eNOS uncoupling process in aging endothelial cells. Free Radic Biol Med 113: 26–35, 2017. [DOI] [PubMed] [Google Scholar]
- 249. Lee SH, Lee JH, Lee HY, and Min KJ. Sirtuin signaling in cellular senescence and aging. BMB Rep 52: 24–34, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Lefkowitz RJ. G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. J Biol Chem 273: 18677–18680, 1998. [DOI] [PubMed] [Google Scholar]
- 251. Lefkowitz RJ, Pitcher J, Krueger K, and Daaka Y. Mechanisms of beta-adrenergic receptor desensitization and resensitization. Adv Pharmacol 42: 416–420, 1998. [DOI] [PubMed] [Google Scholar]
- 252. Lesniewski LA, Connell ML, Durrant JR, Folian BJ, Anderson MC, Donato AJ, and Seals DR. B6D2F1 Mice are a suitable model of oxidative stress-mediated impaired endothelium-dependent dilation with aging. J Gerontol A Biol Sci Med Sci 64: 9–20, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Lewis SJ, Graves JE, Bates JN, and Kooy NW. Peroxynitrite elicits dysfunction of stereoselective s-nitrosocysteine recognition sites. J Cardiovasc Pharmacol 46: 637–645, 2005. [DOI] [PubMed] [Google Scholar]
- 254. Lewis SJ, Hoque A, Walton TM, and Kooy NW. Potential role of nitration and oxidation reactions in the effects of peroxynitrite on the function of beta-adrenoceptor sub-types in the rat. Eur J Pharmacol 518: 187–194, 2005. [DOI] [PubMed] [Google Scholar]
- 255. Li D, Wang X, Huang Q, Li S, Zhou Y, and Li Z. Cardioprotection of CAPE-oNO2 against myocardial ischemia/reperfusion induced ROS generation via regulating the SIRT1/eNOS/NF-kappaB pathway in vivo and in vitro. Redox Biol 15: 62–73, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Li J, Li W, Altura BT, and Altura BM. Peroxynitrite-induced relaxation in isolated rat aortic rings and mechanisms of action. Toxicol Appl Pharmacol 209: 269–276, 2005. [DOI] [PubMed] [Google Scholar]
- 257. Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, Wallace DC, and Epstein CJ. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet 11: 376–381, 1995. [DOI] [PubMed] [Google Scholar]
- 258. Li YF, Cao XJ, Bai XY, Lin SP, and Shi ST. Change of expression of renal alpha1-adrenergic receptor and angiotensin II receptor subtypes with aging in rats. Aging Clin Exp Res 22: 123–128, 2010. [DOI] [PubMed] [Google Scholar]
- 259. Liang Y-R, Liu C, Xiang L-P, and Zheng X-Q. Health benefits of theanine in green tea: a review. Trop J Pharm Res 14: 1943–1949, 2015. [Google Scholar]
- 260. Lin KC, Yip HK, Shao PL, Wu SC, Chen KH, Chen YT, Yang CC, Sun CK, Kao GS, Chen SY, Chai HT, Chang CL, Chen CH, and Lee MS. Combination of adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes for protecting kidney from acute ischemia-reperfusion injury. Int J Cardiol 216: 173–185, 2016. [DOI] [PubMed] [Google Scholar]
- 261. Liu K, Ji K, Guo L, Wu W, Lu H, Shan P, and Yan C. Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia-reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer. Microvasc Res 92: 10–18, 2014. [DOI] [PubMed] [Google Scholar]
- 262. Liu L, Feng D, Chen G, Chen M, Zheng Q, Song P, Ma Q, Zhu C, Wang R, Qi W, Huang L, Xue P, Li B, Wang X, Jin H, Wang J, Yang F, Liu P, Zhu Y, Sui S, and Chen Q. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat Cell Biol 14: 177–185, 2012. [DOI] [PubMed] [Google Scholar]
- 263. Liu MY, Jin J, Li SL, Yan J, Zhen CL, Gao JL, Zhang YH, Zhang YQ, Shen X, Zhang LS, Wei YY, Zhao Y, Wang CG, Bai YL, and Dong DL. Mitochondrial fission of smooth muscle cells is involved in artery constriction. Hypertension 68: 1245–1254, 2016. [DOI] [PubMed] [Google Scholar]
- 264. Liu P, Zhao H, and Luo Y. Anti-Aging implications of astragalus membranaceus (Huangqi): a well-known Chinese tonic. Aging Dis 8: 868–886, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Liu Y and Gutterman DD. Oxidative stress and potassium channel function. Clin Exp Pharmacol Physiol 29: 305–311, 2002. [DOI] [PubMed] [Google Scholar]
- 266. Liu Y, Terata K, Chai Q, Li H, Kleinman LH, and Gutterman DD. Peroxynitrite inhibits Ca2+-activated K+ channel activity in smooth muscle of human coronary arterioles. Circ Res 91: 1070–1076, 2002. [DOI] [PubMed] [Google Scholar]
- 267. Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, and Gutterman DD. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res 93: 573–580, 2003. [DOI] [PubMed] [Google Scholar]
- 268. Liu Z, Xu Y, Wan Y, Gao J, Chu Y, and Li J. Exosomes from adipose-derived mesenchymal stem cells prevent cardiomyocyte apoptosis induced by oxidative stress. Cell Death Discov 5: 79, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Lutz AK, Exner N, Fett ME, Schlehe JS, Kloos K, Lammermann K, Brunner B, Kurz-Drexler A, Vogel F, Reichert AS, Bouman L, Vogt-Weisenhorn D, Wurst W, Tatzelt J, Haass C, and Winklhofer KF. Loss of parkin or PINK1 function increases Drp1-dependent mitochondrial fragmentation. J Biol Chem 284: 22938–22951, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Mader SL. Influence of animal age on the beta-adrenergic system in cultured rat aortic and mesenteric artery smooth muscle cells. J Gerontol 47: B32–B36, 1992. [DOI] [PubMed] [Google Scholar]
- 271. Mader SL and Alley PA. Age-related changes in adenylyl cyclase activity in rat aorta membranes. Mech Ageing Dev 101: 111–118, 1998. [DOI] [PubMed] [Google Scholar]
- 272. Mader SL, Downing CL, Amos-Landgraf J, and Swebjka P. Age-related changes in G proteins in rat aorta. J Gerontol A Biol Sci Med Sci 51: B111–B116, 1996. [DOI] [PubMed] [Google Scholar]
- 273. Magalhaes AC, Dunn H, and Ferguson SS. Regulation of GPCR activity, trafficking and localization by GPCR-interacting proteins. Br J Pharmacol 165: 1717–1736, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Magyar K, Halmosi R, Palfi A, Feher G, Czopf L, Fulop A, Battyany I, Sumegi B, Toth K, and Szabados E. Cardioprotection by resveratrol: a human clinical trial in patients with stable coronary artery disease. Clin Hemorheol Microcirc 50: 179–187, 2012. [DOI] [PubMed] [Google Scholar]
- 275. Makita N, Kabasawa Y, Otani Y, Firman, Sato J, Hashimoto M, Nakaya M, Nishihara H, Nangaku M, Kurose H, Ohwada T, and Iiri T. Attenuated desensitization of beta-adrenergic receptor by water-soluble N-nitrosamines that induce S-nitrosylation without NO release. Circ Res 112: 327–334, 2013. [DOI] [PubMed] [Google Scholar]
- 276. Maksimenko AV. Experimental antioxidant biotherapy for protection of the vascular wall by modified forms of superoxide dismutase and catalase. Curr Pharm Des 11: 2007–2016, 2005. [DOI] [PubMed] [Google Scholar]
- 277. Marchesi C, Paradis P, and Schiffrin EL. Role of the renin-angiotensin system in vascular inflammation. Trends Pharmacol Sci 29: 367–374, 2008. [DOI] [PubMed] [Google Scholar]
- 278. Marijic J, Li Q, Song M, Nishimaru K, Stefani E, and Toro L. Decreased expression of voltage- and Ca(2+)-activated K(+) channels in coronary smooth muscle during aging. Circ Res 88: 210–216, 2001. [DOI] [PubMed] [Google Scholar]
- 279. Martin-Garrido A, Gonzalez-Ramos M, Griera M, Guijarro B, Cannata-Andia J, Rodriguez-Puyol D, Rodriguez-Puyol M, and Saura M. H2O2 regulation of vascular function through sGC mRNA stabilization by HuR. Arterioscler Thromb Vasc Biol 31: 567–573, 2011. [DOI] [PubMed] [Google Scholar]
- 280. Marzetti E, Csiszar A, Dutta D, Balagopal G, Calvani R, and Leeuwenburgh C. Role of mitochondrial dysfunction and altered autophagy in cardiovascular aging and disease: from mechanisms to therapeutics. Am J Physiol Heart Circ Physiol 305: H459–H476, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, and Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A 104: 14855–14860, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Mazo M, Cemborain A, Gavira JJ, Abizanda G, Arana M, Casado M, Soriano M, Hernandez S, Moreno C, Ecay M, Albiasu E, Belzunce M, Orbe J, Paramo JA, Merino J, Penuelas I, Verdugo JM, Pelacho B, and Prosper F. Adipose stromal vascular fraction improves cardiac function in chronic myocardial infarction through differentiation and paracrine activity. Cell Transplant 21: 1023–1037, 2012. [DOI] [PubMed] [Google Scholar]
- 283. McCarron JG, Wilson C, Sandison ME, Olson ML, Girkin JM, Saunter C, and Chalmers S. From structure to function: mitochondrial morphology, motion and shaping in vascular smooth muscle. J Vasc Res 50: 357–371, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. McCrann DJ, Yang D, Chen H, Carroll S, and Ravid K. Upregulation of Nox4 in the aging vasculature and its association with smooth muscle cell polyploidy. Cell Cycle 8: 902–908, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. McWilliams TG, Prescott AR, Montava-Garriga L, Ball G, Singh F, Barini E, Muqit MMK, Brooks SP, and Ganley IG. Basal mitophagy occurs independently of PINK1 in mouse tissues of high metabolic demand. Cell Metab 27: 439.e5–449.e5, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Meissner C, Bruse P, Mohamed SA, Schulz A, Warnk H, Storm T, and Oehmichen M. The 4977 bp deletion of mitochondrial DNA in human skeletal muscle, heart and different areas of the brain: a useful biomarker or more? Exp Gerontol 43: 645–652, 2008. [DOI] [PubMed] [Google Scholar]
- 287. Meydani M. Vitamin E modulation of cardiovascular disease. Ann N Y Acad Sci 1031: 271–279, 2004. [DOI] [PubMed] [Google Scholar]
- 288. Micek A, Godos J, Del Rio D, Galvano F, and Grosso G. Dietary flavonoids and cardiovascular disease: a comprehensive dose-response meta-analysis. Mol Nutr Food Res 65: e2001019, 2021. [DOI] [PubMed] [Google Scholar]
- 289. Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, and Pelicci PG. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature 402: 309–313, 1999. [DOI] [PubMed] [Google Scholar]
- 290. Miller MW, Knaub LA, Olivera-Fragoso LF, Keller AC, Balasubramaniam V, Watson PA, and Reusch JE. Nitric oxide regulates vascular adaptive mitochondrial dynamics. Am J Physiol Heart Circ Physiol 304: H1624–H1633, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Minamino T and Komuro I. Vascular cell senescence: contribution to atherosclerosis. Circ Res 100: 15–26, 2007. [DOI] [PubMed] [Google Scholar]
- 292. Miwa S and Brand MD. Mitochondrial matrix reactive oxygen species production is very sensitive to mild uncoupling. Biochem Soc Trans 31: 1300–1301, 2003. [DOI] [PubMed] [Google Scholar]
- 293. Miyao M, Cicalese S, Kawai T, Cooper HA, Boyer MJ, Elliott KJ, Forrester SJ, Kuroda R, Rizzo V, Hashimoto T, Scalia R, and Eguchi S. Involvement of senescence and mitochondrial fission in endothelial cell pro-inflammatory phenotype induced by angiotensin II. Int J Mol Sci 21: 3112, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Miyazaki R, Ichiki T, Hashimoto T, Inanaga K, Imayama I, Sadoshima J, and Sunagawa K. SIRT1, a longevity gene, downregulates angiotensin II type 1 receptor expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 28: 1263–1269, 2008. [DOI] [PubMed] [Google Scholar]
- 295. Mopert K, Hajek P, Frank S, Chen C, Kaufmann J, and Santel A. Loss of Drp1 function alters OPA1 processing and changes mitochondrial membrane organization. Exp Cell Res 315: 2165–2180, 2009. [DOI] [PubMed] [Google Scholar]
- 296. Morawietz H. Cardiovascular protection by Nox4. Cardiovasc Res 114: 353–355, 2018. [DOI] [PubMed] [Google Scholar]
- 297. Moreau KL. Modulatory influence of sex hormones on vascular aging. Am J Physiol Heart Circ Physiol 316: H522–H526, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Moreau KL and Hildreth KL. Vascular aging across the menopause transition in healthy women. Adv Vasc Med 2014: 204390, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Moreira HS, Lima-Leal GA, Santos-Rocha J, Gomes-Pereira L, Duarte GP, and Xavier FE. Phosphodiesterase-3 inhibitor cilostazol reverses endothelial dysfunction with ageing in rat mesenteric resistance arteries. Eur J Pharmacol 822: 59–68, 2018. [DOI] [PubMed] [Google Scholar]
- 300. Morigi M, Perico L, Rota C, Longaretti L, Conti S, Rottoli D, Novelli R, Remuzzi G, and Benigni A. Sirtuin 3-dependent mitochondrial dynamic improvements protect against acute kidney injury. J Clin Invest 125: 715–726, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Morris ME, Beare JE, Reed RM, Dale JR, LeBlanc AJ, Kaufman CL, Zheng H, Ng CK, Williams SK, and Hoying JB. Systemically delivered adipose stromal vascular fraction cells disseminate to peripheral artery walls and reduce vasomotor tone through a CD11b+ cell-dependent mechanism. Stem Cells Transl Med 4: 369–380, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Mracek T, Drahota Z, and Houstek J. The function and the role of the mitochondrial glycerol-3-phosphate dehydrogenase in mammalian tissues. Biochim Biophys Acta 1827: 401–410, 2013. [DOI] [PubMed] [Google Scholar]
- 303. Muller-Delp JM, Gurovich AN, Christou DD, and Leeuwenburgh C. Redox balance in the aging microcirculation: new friends, new foes, and new clinical directions. Microcirculation 19: 19–28, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Muller-Delp JM, Hotta K, Chen B, Behnke BJ, Maraj JJ, Delp MD, Lucero TR, Bramy JA, Alarcon DB, Morgan HE, Cowan MR, and Haynes AD. Effects of age and exercise training on coronary microvascular smooth muscle phenotype and function. J Appl Physiol (1985) 124: 140–149, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Munoz M, Lopez-Oliva ME, Pinilla E, Martinez MP, Sanchez A, Rodriguez C, Garcia-Sacristan A, Hernandez M, Rivera L, and Prieto D. CYP epoxygenase-derived H2O2 is involved in the endothelium-derived hyperpolarization (EDH) and relaxation of intrarenal arteries. Free Radic Biol Med 106: 168–183, 2017. [DOI] [PubMed] [Google Scholar]
- 306. Munoz M, Martinez MP, Lopez-Oliva ME, Rodriguez C, Corbacho C, Carballido J, Garcia-Sacristan A, Hernandez M, Rivera L, Saenz-Medina J, and Prieto D. Hydrogen peroxide derived from NADPH oxidase 4- and 2 contributes to the endothelium-dependent vasodilatation of intrarenal arteries. Redox Biol 19: 92–104, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Munzel T, Camici GG, Maack C, Bonetti NR, Fuster V, and Kovacic JC. Impact of oxidative stress on the heart and vasculature: part 2 of a 3-part series. J Am Coll Cardiol 70: 212–229, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Munzel T, Daiber A, Ullrich V, and Mulsch A. Vascular consequences of endothelial nitric oxide synthase uncoupling for the activity and expression of the soluble guanylyl cyclase and the cGMP-dependent protein kinase. Arterioscler Thromb Vasc Biol 25: 1551–1557, 2005. [DOI] [PubMed] [Google Scholar]
- 309. Murphy MP. How mitochondria produce reactive oxygen species. Biochem J 417: 1–13, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Myung SK, Ju W, Cho B, Oh SW, Park SM, Koo BK, and Park BJ; Korean Meta-Analysis Study Group. Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: systematic review and meta-analysis of randomised controlled trials. BMJ 346: f10, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Naga Prasad SV, Barak LS, Rapacciuolo A, Caron MG, and Rockman HA. Agonist-dependent recruitment of phosphoinositide 3-kinase to the membrane by beta-adrenergic receptor kinase 1. A role in receptor sequestration. J Biol Chem 276: 18953–18959, 2001. [DOI] [PubMed] [Google Scholar]
- 312. Nagaoka T, Hein TW, Yoshida A, and Kuo L. Resveratrol, a component of red wine, elicits dilation of isolated porcine retinal arterioles: role of nitric oxide and potassium channels. Invest Ophthalmol Vis Sci 48: 4232–4239, 2007. [DOI] [PubMed] [Google Scholar]
- 313. Najibi S, Cowan CL, Palacino JJ, and Cohen RA. Enhanced role of potassium channels in relaxations to acetylcholine in hypercholesterolemic rabbit carotid artery. Am J Physiol 266: H2061–H2067, 1994. [DOI] [PubMed] [Google Scholar]
- 314. Nakamura A, Kajitani S, Sato K, Kanazawa M, Kondo M, Endo H, and Nozaki E. Decline of popliteal artery flow-mediated dilation with aging and possible involvement of asymmetric dimethylarginine in healthy men. J Med Ultrason (2001) 46: 503–511, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Naumovski N, Foscolou A, D'Cunha NM, Tyrovolas S, Chrysohoou C, Sidossis LS, Rallidis L, Matalas AL, Polychronopoulos E, Pitsavos C, and Panagiotakos D. The association between green and black tea consumption on successful aging: a combined analysis of the ATTICA and MEDiterranean ISlands (MEDIS) epidemiological studies. Molecules 24:1862, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Nazari-Shafti TZ and Cooke JP. Telomerase therapy to reverse cardiovascular senescence. Methodist Debakey Cardiovasc J 11: 172–175, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Newaz MA, Yousefipour Z, and Oyekan A. Oxidative stress-associated vascular aging is xanthine oxidase-dependent but not NAD(P)H oxidase-dependent. J Cardiovasc Pharmacol 48: 88–94, 2006. [DOI] [PubMed] [Google Scholar]
- 318. Newcomer SC, Leuenberger UA, Hogeman CS, and Proctor DN. Heterogeneous vasodilator responses of human limbs: influence of age and habitual endurance training. Am J Physiol Heart Circ Physiol 289: H308–H315, 2005. [DOI] [PubMed] [Google Scholar]
- 319. Nguygen T and Alzahrani J. Cardiovascular outcomes in patients with mitochondrial disease in the United States: a propensity score analysis. Circulation 140: A14168, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Nicholls DG. Mitochondrial membrane potential and aging. Aging Cell 3: 35–40, 2004. [DOI] [PubMed] [Google Scholar]
- 321. Nienaber JJ, Tachibana H, Naga Prasad SV, Esposito G, Wu D, Mao L, and Rockman HA. Inhibition of receptor-localized PI3K preserves cardiac beta-adrenergic receptor function and ameliorates pressure overload heart failure. J Clin Invest 112: 1067–1079, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. No MH, Heo JW, Yoo SZ, Jo HS, Park DH, Kang JH, Seo DY, Han J, and Kwak HB. Effects of aging on mitochondrial hydrogen peroxide emission and calcium retention capacity in rat heart. J Exerc Rehabil 14: 920–926, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Nozik-Grayck E, Whalen EJ, Stamler JS, McMahon TJ, Chitano P, and Piantadosi CA. S-nitrosoglutathione inhibits alpha1-adrenergic receptor-mediated vasoconstriction and ligand binding in pulmonary artery. Am J Physiol Lung Cell Mol Physiol 290: L136–L143, 2006. [DOI] [PubMed] [Google Scholar]
- 324. Ohashi M, Faraci F, and Heistad D. Peroxynitrite hyperpolarizes smooth muscle and relaxes internal carotid artery in rabbit via ATP-sensitive K+ channels. Am J Physiol Heart Circ Physiol 289: H2244–H2250, 2005. [DOI] [PubMed] [Google Scholar]
- 325. Olgar Y, Degirmenci S, Durak A, Billur D, Can B, Kayki-Mutlu G, Arioglu-Inan EE, and Turan B. Aging related functional and structural changes in the heart and aorta: mitoTEMPO improves aged-cardiovascular performance. Exp Gerontol 110: 172–181, 2018. [DOI] [PubMed] [Google Scholar]
- 326. Olgun A. Converting NADH to NAD+ by nicotinamide nucleotide transhydrogenase as a novel strategy against mitochondrial pathologies during aging. Biogerontology 10: 531–534, 2009. [DOI] [PubMed] [Google Scholar]
- 327. Omidian M, Abdolahi M, Daneshzad E, Sedighiyan M, Aghasi M, Abdollahi H, Omidian P, Dabiri S, and Mahmoudi M. The effects of resveratrol on oxidative stress markers: a systematic review and meta-analysis of randomized clinical trials. Endocr Metab Immune Disord Drug Targets 20: 718–727, 2020. [DOI] [PubMed] [Google Scholar]
- 328. Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, and Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation 121: 2012–2022, 2010. [DOI] [PubMed] [Google Scholar]
- 329. Ota H, Eto M, Ogawa S, Iijima K, Akishita M, and Ouchi Y. SIRT1/eNOS axis as a potential target against vascular senescence, dysfunction and atherosclerosis. J Atheroscler Thromb 17: 431–435, 2010. [DOI] [PubMed] [Google Scholar]
- 330. Ottolini M, Hong K, Cope EL, Daneva Z, DeLalio LJ, Sokolowski JD, Marziano C, Nguyen NY, Altschmied J, Haendeler J, Johnstone SR, Kalani MY, Park MS, Patel RP, Liedtke W, Isakson BE, and Sonkusare SK. Local peroxynitrite impairs endothelial transient receptor potential vanilloid 4 channels and elevates blood pressure in obesity. Circulation 141: 1318–1333, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Oudit GY, Crackower MA, Eriksson U, Sarao R, Kozieradzki I, Sasaki T, Irie-Sasaki J, Gidrewicz D, Rybin VO, Wada T, Steinberg SF, Backx PH, and Penninger JM. Phosphoinositide 3-kinase gamma-deficient mice are protected from isoproterenol-induced heart failure. Circulation 108: 2147–2152, 2003. [DOI] [PubMed] [Google Scholar]
- 332. Oudit GY, Herzenberg AM, Kassiri Z, Wong D, Reich H, Khokha R, Crackower MA, Backx PH, Penninger JM, and Scholey JW. Loss of angiotensin-converting enzyme-2 leads to the late development of angiotensin II-dependent glomerulosclerosis. Am J Pathol 168: 1808–1820, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Oudit GY, Kassiri Z, Patel MP, Chappell M, Butany J, Backx PH, Tsushima RG, Scholey JW, Khokha R, and Penninger JM. Angiotensin II-mediated oxidative stress and inflammation mediate the age-dependent cardiomyopathy in ACE2 null mice. Cardiovasc Res 75: 29–39, 2007. [DOI] [PubMed] [Google Scholar]
- 334. Oudot A, Martin C, Busseuil D, Vergely C, Demaison L, and Rochette L. NADPH oxidases are in part responsible for increased cardiovascular superoxide production during aging. Free Radic Biol Med 40: 2214–2222, 2006. [DOI] [PubMed] [Google Scholar]
- 335. Owusu BY, Stapley R, Honavar J, and Patel RP. Effects of erythrocyte aging on nitric oxide and nitrite metabolism. Antioxid Redox Signal 19: 1198–1208, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Oyama JI, Shiraki A, Nishikido T, Maeda T, Komoda H, Shimizu T, Makino N, and Node K. EGCG, a green tea catechin, attenuates the progression of heart failure induced by the heart/muscle-specific deletion of MnSOD in mice. J Cardiol 69: 417–427, 2017. [DOI] [PubMed] [Google Scholar]
- 337. Ozawa K, Whalen EJ, Nelson CD, Mu Y, Hess DT, Lefkowitz RJ, and Stamler JS. S-nitrosylation of beta-arrestin regulates beta-adrenergic receptor trafficking. Mol Cell 31: 395–405, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Ozemek C, Hildreth KL, Blatchford PJ, Hurt KJ, Bok R, Seals DR, Kohrt WM, and Moreau KL. Effects of resveratrol or estradiol on postexercise endothelial function in estrogen-deficient postmenopausal women. J Appl Physiol (1985) 128: 739–747, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Pacher P, Beckman JS, and Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87: 315–424, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Paliwal S, Chaudhuri R, Agrawal A, and Mohanty S. Regenerative abilities of mesenchymal stem cells through mitochondrial transfer. J Biomed Sci 25: 31, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Palmer GJ, Ziegler MG, and Lake CR. Response of norepinephrine and blood pressure to stress increases with age. J Gerontol 33: 482–487, 1978. [DOI] [PubMed] [Google Scholar]
- 342. Pan BX, Zhao GL, Huang XL, and Zhao KS. Calcium mobilization is required for peroxynitrite-mediated enhancement of spontaneous transient outward currents in arteriolar smooth muscle cells. Free Radic Biol Med 37: 823–838, 2004. [DOI] [PubMed] [Google Scholar]
- 343. Parastatidis I, Weiss D, Joseph G, and Taylor WR. Overexpression of catalase in vascular smooth muscle cells prevents the formation of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 33: 2389–2396, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Park JH, Ku HJ, Lee JH, and Park JW. Idh2 deficiency exacerbates acrolein-induced lung injury through mitochondrial redox environment deterioration. Oxid Med Cell Longev 2017: 1595103, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Park SH, Kwon OS, Park SY, Weavil JC, Andtbacka RHI, Hyngstrom JR, Reese V, and Richardson RS. Vascular mitochondrial respiratory function: the impact of advancing age. Am J Physiol Heart Circ Physiol 315: H1660–H1669, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Park SH, Kwon OS, Park SY, Weavil JC, Hydren JR, Reese V, Andtbacka RHI, Hyngstrom JR, and Richardson RS. Vasodilatory and vascular mitochondrial respiratory function with advancing age: evidence of a free radically mediated link in the human vasculature. Am J Physiol Regul Integr Comp Physiol 318: R701–R711, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Park SY, Kwon OS, Andtbacka RHI, Hyngstrom JR, Reese V, Murphy MP, and Richardson RS. Age-related endothelial dysfunction in human skeletal muscle feed arteries: the role of free radicals derived from mitochondria in the vasculature. Acta Physiol (Oxf) 222, 2018. [Epub ahead of print]; DOI: 10.1111/apha.12893. [DOI] [PubMed] [Google Scholar]
- 348. Passmore JC, Joshua IG, Rowell PP, Tyagi SC, and Falcone JC. Reduced alpha adrenergic mediated contraction of renal preglomerular blood vessels as a function of gender and aging. J Cell Biochem 96: 672–681, 2005. [DOI] [PubMed] [Google Scholar]
- 349. Pastori D, Pignatelli P, Farcomeni A, Menichelli D, Nocella C, Carnevale R, and Violi F. Aging-related decline of glutathione peroxidase 3 and risk of cardiovascular events in patients with atrial fibrillation. J Am Heart Assoc 5: e003682, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Patrucco E, Notte A, Barberis L, Selvetella G, Maffei A, Brancaccio M, Marengo S, Russo G, Azzolino O, Rybalkin SD, Silengo L, Altruda F, Wetzker R, Wymann MP, Lembo G, and Hirsch E. PI3Kgamma modulates the cardiac response to chronic pressure overload by distinct kinase-dependent and -independent effects. Cell 118: 375–387, 2004. [DOI] [PubMed] [Google Scholar]
- 351. Patten DA, Wong J, Khacho M, Soubannier V, Mailloux RJ, Pilon-Larose K, MacLaurin JG, Park DS, McBride HM, Trinkle-Mulcahy L, Harper ME, Germain M, and Slack RS. OPA1-dependent cristae modulation is essential for cellular adaptation to metabolic demand. EMBO J 33: 2676–2691, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Pedro-Botet J, Covas MI, Martin S, and Rubies-Prat J. Decreased endogenous antioxidant enzymatic status in essential hypertension. J Hum Hypertens 14: 343–345, 2000. [DOI] [PubMed] [Google Scholar]
- 353. Perrier E, Fournet-Bourguignon MP, Royere E, Molez S, Reure H, Lesage L, Gosgnach W, Frapart Y, Boucher JL, Villeneuve N, and Vilaine JP. Effect of uncoupling endothelial nitric oxide synthase on calcium homeostasis in aged porcine endothelial cells. Cardiovasc Res 82: 133–142, 2009. [DOI] [PubMed] [Google Scholar]
- 354. Persson IA, Persson K, and Andersson RG. Effect of Vaccinium myrtillus and its polyphenols on angiotensin-converting enzyme activity in human endothelial cells. J Agric Food Chem 57: 4626–4629, 2009. [DOI] [PubMed] [Google Scholar]
- 355. Piquereau J, Caffin F, Novotova M, Prola A, Garnier A, Mateo P, Fortin D, Huynh le H, Nicolas V, Alavi MV, Brenner C, Ventura-Clapier R, Veksler V, and Joubert F. Down-regulation of OPA1 alters mouse mitochondrial morphology, PTP function, and cardiac adaptation to pressure overload. Cardiovasc Res 94: 408–417, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Podlutsky A, Ballabh P, and Csiszar A. Oxidative stress and endothelial dysfunction in pulmonary arteries of aged rats. Am J Physiol Heart Circ Physiol 298: H346–H351, 2010. [DOI] [PubMed] [Google Scholar]
- 357. Prasad SN, Mohan M, Martelli E, Gupta M, and Vasudevan N. PI3Ky regulates age-dependent cardiac hypertrophy through kinase-independent GASK-3-PP2A axis. Circ Res 111, 2018. [Google Scholar]
- 358. Putti R, Sica R, Migliaccio V, and Lionetti L. Diet impact on mitochondrial bioenergetics and dynamics. Front Physiol 6: 109, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Qiu X, Brown K, Hirschey MD, Verdin E, and Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab 12: 662–667, 2010. [DOI] [PubMed] [Google Scholar]
- 360. Qiu X, Brown KV, Moran Y, and Chen D. Sirtuin regulation in calorie restriction. Biochim Biophys Acta 1804: 1576–1583, 2010. [DOI] [PubMed] [Google Scholar]
- 361. Quyyumi AA and Ozkor M. Vasodilation by hyperpolarization: beyond NO. Hypertension 48: 1023–1025, 2006. [DOI] [PubMed] [Google Scholar]
- 362. Radi R. Oxygen radicals, nitric oxide, and peroxynitrite: redox pathways in molecular medicine. Proc Natl Acad Sci U S A 115: 5839–5848, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Rakici O, Kiziltepe U, Coskun B, Aslamaci S, and Akar F. Effects of resveratrol on vascular tone and endothelial function of human saphenous vein and internal mammary artery. Int J Cardiol 105: 209–215, 2005. [DOI] [PubMed] [Google Scholar]
- 364. Retamal MA. Connexin and Pannexin hemichannels are regulated by redox potential. Front Physiol 5: 80, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Richardson KJ, Kuck L, and Simmonds MJ. Beyond oxygen transport: active role of erythrocytes in the regulation of blood flow. Am J Physiol Heart Circ Physiol 319: H866–H872, 2020. [DOI] [PubMed] [Google Scholar]
- 366. Rippe C, Lesniewski L, Connell M, LaRocca T, Donato A, and Seals D. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell 9: 304–312, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367. Rodriguez-Manas L, El-Assar M, Vallejo S, Lopez-Doriga P, Solis J, Petidier R, Montes M, Nevado J, Castro M, Gomez-Guerrero C, Peiro C, and Sanchez-Ferrer CF. Endothelial dysfunction in aged humans is related with oxidative stress and vascular inflammation. Aging Cell 8: 226–238, 2009. [DOI] [PubMed] [Google Scholar]
- 368. Rodriguez-Martinez MA, Alonso MJ, Redondo J, Salaices M, and Marin J. Role of lipid peroxidation and the glutathione-dependent antioxidant system in the impairment of endothelium-dependent relaxations with age. Br J Pharmacol 123: 113–121, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369. Rodriguez-Miguelez P, Looney J, Thomas J, Harshfield G, Pollock JS, and Harris RA. Sirt1 during childhood is associated with microvascular function later in life. Am J Physiol Heart Circ Physiol 318: H1371–H1378, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370. Rogers PA, Chilian WM, Bratz IN, Bryan RM Jr., and Dick GM. H2O2 activates redox- and 4-aminopyridine-sensitive Kv channels in coronary vascular smooth muscle. Am J Physiol Heart Circ Physiol 292: H1404–H1411, 2007. [DOI] [PubMed] [Google Scholar]
- 371. Romero M, Jimenez R, Sanchez M, Lopez-Sepulveda R, Zarzuelo MJ, O'Valle F, Zarzuelo A, Perez-Vizcaino F, and Duarte J. Quercetin inhibits vascular superoxide production induced by endothelin-1: role of NADPH oxidase, uncoupled eNOS and PKC. Atherosclerosis 202: 58–67, 2009. [DOI] [PubMed] [Google Scholar]
- 372. Romero SA, Gagnon D, Adams AN, Moralez G, Kouda K, Jaffery MF, Cramer MN, and Crandall CG. Folic acid ingestion improves skeletal muscle blood flow during graded handgrip and plantar flexion exercise in aged humans. Am J Physiol Heart Circ Physiol 313: H658–H666, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373. Rosen J, Jakobs P, Ale-Agha N, Altschmied J, and Haendeler J. Non-canonical functions of telomerase reverse transcriptase—impact on redox homeostasis. Redox Biol 34: 101543, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Rossman MJ, Gioscia-Ryan RA, Clayton ZS, Murphy MP, and Seals DR. Targeting mitochondrial fitness as a strategy for healthy vascular aging. Clin Sci (Lond) 134: 1491–1519, 2020. [DOI] [PubMed] [Google Scholar]
- 375. Rossman MJ, Santos-Parker JR, Steward CAC, Bispham NZ, Cuevas LM, Rosenberg HL, Woodward KA, Chonchol M, Gioscia-Ryan RA, Murphy MP, and Seals DR. Chronic supplementation with a mitochondrial antioxidant (MitoQ) improves vascular function in healthy older adults. Hypertension 71: 1056–1063, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376. Rowe G, Kelm NQ, Beare JE, Tracy E, Yuan F, and LeBlanc AJ. Enhanced beta-1 adrenergic receptor responsiveness in coronary arterioles following intravenous stromal vascular fraction therapy in aged rats. Aging (Albany NY) 11: 4561–4578, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377. Rudner XL, Berkowitz DE, Booth JV, Funk BL, Cozart KL, D'Amico EB, El-Moalem H, Page SO, Richardson CD, Winters B, Marucci L, and Schwinn DA. Subtype specific regulation of human vascular alpha(1)-adrenergic receptors by vessel bed and age. Circulation 100: 2336–2343, 1999. [DOI] [PubMed] [Google Scholar]
- 378. Ryan AS. Insulin resistance with aging: effects of diet and exercise. Sports Med 30: 327–346, 2000. [DOI] [PubMed] [Google Scholar]
- 379. Rybka J, Kupczyk D, Kedziora-Kornatowska K, Pawluk H, Czuczejko J, Szewczyk-Golec K, Kozakiewicz M, Antonioli M, Carvalho LA, and Kedziora J. Age-related changes in an antioxidant defense system in elderly patients with essential hypertension compared with healthy controls. Redox Rep 16: 71–77, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Salazar G. NADPH oxidases and mitochondria in vascular senescence. Int J Mol Sci 19: 1327, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Salvador L, Singaravelu G, Harley CB, Flom P, Suram A, and Raffaele JM. A natural product telomerase activator lengthens telomeres in humans: a randomized, double blind, and placebo controlled study. Rejuvenation Res 19: 478–484, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382. Samant SA, Zhang HJ, Hong Z, Pillai VB, Sundaresan NR, Wolfgeher D, Archer SL, Chan DC, and Gupta MP. SIRT3 deacetylates and activates OPA1 to regulate mitochondrial dynamics during stress. Mol Cell Biol 34: 807–819, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383. Sameer AS, Nissar S, and Aziz R. Telomeres and estrogens: the unholy nexus in pathogenesis of atherosclerosis. Cardiol Res 5: 85–90, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384. Sandri M, Viehmann M, Adams V, Rabald K, Mangner N, Hollriegel R, Lurz P, Erbs S, Linke A, Kirsch K, Mobius-Winkler S, Thiery J, Teupser D, Hambrecht R, Schuler G, and Gielen S. Chronic heart failure and aging—effects of exercise training on endothelial function and mechanisms of endothelial regeneration: results from the Leipzig Exercise Intervention in Chronic heart failure and Aging (LEICA) study. Eur J Prev Cardiol 23: 349–358, 2016. [DOI] [PubMed] [Google Scholar]
- 385. Sapper TN, Mah E, Ahn-Jarvis J, McDonald JD, Chitchumroonchokchai C, Reverri EJ, Vodovotz Y, and Bruno RS. A green tea-containing starch confection increases plasma catechins without protecting against postprandial impairments in vascular function in normoglycemic adults. Food Funct 7: 3843–3853, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. Sarmento AO, Santos ADC, Trombetta IC, Dantas MM, Oliveira Marques AC, do Nascimento LS, Barbosa BT, Dos Santos MR, Andrade MDA, Jaguaribe-Lima AM, and Brasileiro-Santos MDS. Regular physical exercise improves cardiac autonomic and muscle vasodilatory responses to isometric exercise in healthy elderly. Clin Interv Aging 12: 1021–1028, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387. Sarsour EH, Kalen AL, and Goswami PC. Manganese superoxide dismutase regulates a redox cycle within the cell cycle. Antioxid Redox Signal 20: 1618–1627, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Savitha S and Panneerselvam C. Mitochondrial membrane damage during aging process in rat heart: potential efficacy of L-carnitine and DL alpha lipoic acid. Mech Ageing Dev 127: 349–355, 2006. [DOI] [PubMed] [Google Scholar]
- 389. Schocken DD and Roth GS. Reduced beta-adrenergic receptor concentrations in ageing man. Nature 267: 856–858, 1977. [DOI] [PubMed] [Google Scholar]
- 390. Schreckenberger ZJ, Wenceslau CF, Joe B, and McCarthy CG. Mitophagy in hypertension-associated premature vascular aging. Am J Hypertens 33: 804–812, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391. Schroder K. Exercise: benefit more with Nox4! Cardiovasc Res 116: 1658. –1660, 2020. [DOI] [PubMed] [Google Scholar]
- 392. Schroder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, Kosowski J, Kruse C, Luedike P, Michaelis UR, Weissmann N, Dimmeler S, Shah AM, and Brandes RP. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ Res 110: 1217–1225, 2012. [DOI] [PubMed] [Google Scholar]
- 393. Schulz E, Jansen T, Wenzel P, Daiber A, and Munzel T. Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid Redox Signal 10: 1115–1126, 2008. [DOI] [PubMed] [Google Scholar]
- 394. Schulz E, Wenzel P, Munzel T, and Daiber A. Mitochondrial redox signaling: interaction of mitochondrial reactive oxygen species with other sources of oxidative stress. Antioxid Redox Signal 20: 308–324, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395. Schutzer WE and Mader SL. Age-related changes in vascular adrenergic signaling: clinical and mechanistic implications. Ageing Res Rev 2: 169–190, 2003. [DOI] [PubMed] [Google Scholar]
- 396. Schutzer WE, Reed JF, Bliziotes M, and Mader SL. Upregulation of G protein-linked receptor kinases with advancing age in rat aorta. Am J Physiol Regul Integr Comp Physiol 280: R897–R903, 2001. [DOI] [PubMed] [Google Scholar]
- 397. Schutzer WE, Xue H, Reed JF, and Mader SL. Effect of age on vascular beta2-adrenergic receptor desensitization is not mediated by the receptor coupling to Galphai proteins. J Gerontol A Biol Sci Med Sci 61: 899–906, 2006. [DOI] [PubMed] [Google Scholar]
- 398. Schutzer WE, Xue H, Reed JF, Roullet JB, Anderson S, and Mader SL. Angiotensin II enhances beta-adrenergic receptor-mediated vasorelaxation in aortas from young but not old rats. Am J Physiol Heart Circ Physiol 279: H2807–H2814, 2000. [DOI] [PubMed] [Google Scholar]
- 399. Seals DR, Desouza CA, Donato AJ, and Tanaka H. Habitual exercise and arterial aging. J Appl Physiol (1985) 105: 1323–1332, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400. Sebastian C, Satterstrom FK, Haigis MC, and Mostoslavsky R. From sirtuin biology to human diseases: an update. J Biol Chem 287: 42444–42452, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401. Sebastian D, Palacin M, and Zorzano A. Mitochondrial dynamics: coupling mitochondrial fitness with healthy aging. Trends Mol Med 23: 201–215, 2017. [DOI] [PubMed] [Google Scholar]
- 402. Semba RD, Ferrucci L, Bartali B, Urpi-Sarda M, Zamora-Ros R, Sun K, Cherubini A, Bandinelli S, and Andres-Lacueva C. Resveratrol levels and all-cause mortality in older community-dwelling adults. JAMA Intern Med 174: 1077–1084, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403. Serviente C. The influence of healthy aging on K+ channel-dependent microvascular dilatory function. J Feder Am Soc Exp Biol 34: 1, 2020. [Google Scholar]
- 404. Serviente C, Berry CW, Kenney WL, and Alexander LM. Healthy active older adults have enhanced K(+) channel-dependent endothelial vasodilatory mechanisms. Am J Physiol Regul Integr Comp Physiol 319: R19–R25, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405. Sezaki A, Imai T, Miyamoto K, Kawase F, Shirai Y, Abe C, Sanada M, Inden A, Kato T, Suzuki N, and Shimokata H. Global relationship between Mediterranean diet and the incidence and mortality of ischaemic heart disease. Eur J Public Health 31: 608–612, 2021. [DOI] [PubMed] [Google Scholar]
- 406. Shannon OM, Ashor AW, Scialo F, Saretzki G, Martin-Ruiz C, Lara J, Matu J, Griffiths A, Robinson N, Lilla L, Stevenson E, Stephan BCM, Minihane AM, Siervo M, and Mathers JC. Mediterranean diet and the hallmarks of ageing. Eur J Clin Nutr 75: 1176–1192, 2021. [DOI] [PubMed] [Google Scholar]
- 407. Shaw LJ, Merz CN, Pepine CJ, Reis SE, Bittner V, Kip KE, Kelsey SF, Olson M, Johnson BD, Mankad S, Sharaf BL, Rogers WJ, Pohost GM, and Sopko G; Women's Ischemia Syndrome Evaluation (WISE) Investigators. The economic burden of angina in women with suspected ischemic heart disease: results from the National Institutes of Health—National Heart, Lung, and Blood Institute—sponsored Women's Ischemia Syndrome Evaluation. Circulation 114: 894–904, 2006. [DOI] [PubMed] [Google Scholar]
- 408. Shenouda SM, Widlansky ME, Chen K, Xu G, Holbrook M, Tabit CE, Hamburg NM, Frame AA, Caiano TL, Kluge MA, Duess MA, Levit A, Kim B, Hartman ML, Joseph L, Shirihai OS, and Vita JA. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation 124: 444–453, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409. Shi Y, Savarese G, Perrone-Filardi P, Luscher TF, and Camici GG. Enhanced age-dependent cerebrovascular dysfunction is mediated by adaptor protein p66Shc. Int J Cardiol 175: 446–450, 2014. [DOI] [PubMed] [Google Scholar]
- 410. Shi Y, Wu Z, Cui N, Shi W, Yang Y, Zhang X, Rojas A, Ha BT, and Jiang C. PKA phosphorylation of SUR2B subunit underscores vascular KATP channel activation by beta-adrenergic receptors. Am J Physiol Regul Integr Comp Physiol 293: R1205–R1214, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411. Sibley DR, Strasser RH, Benovic JL, Daniel K, and Lefkowitz RJ. Phosphorylation/dephosphorylation of the beta-adrenergic receptor regulates its functional coupling to adenylate cyclase and subcellular distribution. Proc Natl Acad Sci U S A 83: 9408–9412, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412. Sinagra T, Tamburella A, Urso V, Siarkos I, Drago F, Bucolo C, and Salomone S. Reversible inhibition of vasoconstriction by thiazolidinediones related to PI3K/Akt inhibition in vascular smooth muscle cells. Biochem Pharmacol 85: 551–559, 2013. [DOI] [PubMed] [Google Scholar]
- 413. Sinclair KA, Yerkovich ST, Hopkins PM, and Chambers DC. Characterization of intercellular communication and mitochondrial donation by mesenchymal stromal cells derived from the human lung. Stem Cell Res Ther 7: 91, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414. Sindler AL, Delp MD, Reyes R, Wu G, and Muller-Delp JM. Effects of ageing and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. J Physiol 587: 3885–3897, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415. Singh N, Graves J, Taylor PD, MacAllister RJ, and Singer DR. Effects of a ‘healthy’ diet and of acute and long-term vitamin C on vascular function in healthy older subjects. Cardiovasc Res 56: 118–125, 2002. [DOI] [PubMed] [Google Scholar]
- 416. Smoliga JM, Baur JA, and Hausenblas HA. Resveratrol and health—a comprehensive review of human clinical trials. Mol Nutr Food Res 55: 1129–1141, 2011. [DOI] [PubMed] [Google Scholar]
- 417. Soltis EE. Effect of age on blood pressure and membrane-dependent vascular responses in the rat. Circ Res 61: 889–897, 1987. [DOI] [PubMed] [Google Scholar]
- 418. Song J, Liu Q, Tang H, Tao A, Wang H, Kao R, and Rui T. Activation of PI3Kgamma/Akt pathway increases cardiomyocyte HMGB1 expression in diabetic environment. Oncotarget 7: 80803–80810, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419. Song SB and Hwang ES. A rise in ATP, ROS, and mitochondrial content upon glucose withdrawal correlates with a dysregulated mitochondria turnover mediated by the activation of the protein deacetylase SIRT1. Cells 8: 11, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420. Sorensen CM, Braunstein TH, Holstein-Rathlou NH, and Salomonsson M. Role of vascular potassium channels in the regulation of renal hemodynamics. Am J Physiol Renal Physiol 302: F505–F518, 2012. [DOI] [PubMed] [Google Scholar]
- 421. Spencer AP, Carson DS, and Crouch MA. Vitamin E and coronary artery disease. Arch Intern Med 159: 1313–1320, 1999. [DOI] [PubMed] [Google Scholar]
- 422. Spiekermann S, Landmesser U, Dikalov S, Bredt M, Gamez G, Tatge H, Reepschlager N, Hornig B, Drexler H, and Harrison DG. Electron spin resonance characterization of vascular xanthine and NAD(P)H oxidase activity in patients with coronary artery disease: relation to endothelium-dependent vasodilation. Circulation 107: 1383–1389, 2003. [DOI] [PubMed] [Google Scholar]
- 423. Spurlock B, Tullet J, Hartman JL, 4th, and Mitra K. Interplay of mitochondrial fission-fusion with cell cycle regulation: possible impacts on stem cell and organismal aging. Exp Gerontol 135: 110919, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424. Stauffer BL, Westby CM, and DeSouza CA. Endothelin-1, aging and hypertension. Curr Opin Cardiol 23: 350–355, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425. Suades R and Cosentino F. Sirtuin 1/soluble guanylyl cyclase: a nitric oxide-independent pathway to rescue ageing-induced vascular dysfunction. Cardiovasc Res 115: 485–487, 2019. [DOI] [PubMed] [Google Scholar]
- 426. Sugrue MM and Tatton WG. Mitochondrial membrane potential in aging cells. Biol Signals Recept 10: 176–188, 2001. [DOI] [PubMed] [Google Scholar]
- 427. Sun Q, Dong Y, Wang H, Jiao K, Xu J, Ma L, Huang H, Liu H, and Wang W. Antiperoxynitrite treatment ameliorates vasorelaxation of resistance arteries in aging rats: involvement with protection of circulating endothelial progenitor cells. J Cardiovasc Pharmacol 68: 334–341, 2016. [DOI] [PubMed] [Google Scholar]
- 428. Suresh K, Servinsky L, Reyes J, Baksh S, Undem C, Caterina M, Pearse DB, and Shimoda LA. Hydrogen peroxide-induced calcium influx in lung microvascular endothelial cells involves TRPV4. Am J Physiol Lung Cell Mol Physiol 309: L1467–L1477, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429. Szabo C, Ischiropoulos H, and Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov 6: 662–680, 2007. [DOI] [PubMed] [Google Scholar]
- 430. Szarka N, Pabbidi MR, Amrein K, Czeiter E, Berta G, Pohoczky K, Helyes Z, Ungvari Z, Koller A, Buki A, and Toth P. Traumatic brain injury impairs myogenic constriction of cerebral arteries: role of mitochondria-derived H2O2 and TRPV4-dependent activation of BKCa channels. J Neurotrauma 35: 930–939, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431. Taddei S, Virdis A, Ghiadoni L, Mattei P, Sudano I, Bernini G, Pinto S, and Salvetti A. Menopause is associated with endothelial dysfunction in women. Hypertension 28: 576–582, 1996. [DOI] [PubMed] [Google Scholar]
- 432. Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, and Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation 91: 1981–1987, 1995. [DOI] [PubMed] [Google Scholar]
- 433. Tajbakhsh N and Sokoya EM. Regulation of cerebral vascular function by sirtuin 1. Microcirculation 19: 336–342, 2012. [DOI] [PubMed] [Google Scholar]
- 434. Takac I, Schroder K, Zhang L, Lardy B, Anilkumar N, Lambeth JD, Shah AM, Morel F, and Brandes RP. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J Biol Chem 286: 13304–13313, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435. Takahashi J, Nihei T, Takagi Y, Miyata S, Odaka Y, Tsunoda R, Seki A, Sumiyoshi T, Matsui M, Goto T, Tanabe Y, Sueda S, Momomura S, Yasuda S, Ogawa H, and Shimokawa H; Japanese Coronary Spasm Association. Prognostic impact of chronic nitrate therapy in patients with vasospastic angina: multicentre registry study of the Japanese coronary spasm association. Eur Heart J 36: 228–237, 2015. [DOI] [PubMed] [Google Scholar]
- 436. Tanaka T, Nishimura A, Nishiyama K, Goto T, Numaga-Tomita T, and Nishida M. Mitochondrial dynamics in exercise physiology. Pflugers Arch 472: 137–153, 2020. [DOI] [PubMed] [Google Scholar]
- 437. Tang H, Viola HM, Filipovska A, and Hool LC. Ca(v)1.2 calcium channel is glutathionylated during oxidative stress in guinea pig and ischemic human heart. Free Radic Biol Med 51: 1501–1511, 2011. [DOI] [PubMed] [Google Scholar]
- 438. Tang X, Luo YX, Chen HZ, and Liu DP. Mitochondria, endothelial cell function, and vascular diseases. Front Physiol 5: 175, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439. Tanguy S, Boucher F, Toufektsian MC, Besse S, and de Leiris J. Aging exacerbates hydrogen peroxide-induced alteration of vascular reactivity in rats. Antioxid Redox Signal 2: 363–368, 2000. [DOI] [PubMed] [Google Scholar]
- 440. Tanner MJ, Wang J, Ying R, Suboc TB, Malik M, Couillard A, Branum A, Puppala V, and Widlansky ME. Dynamin-related protein 1 mediates low glucose-induced endothelial dysfunction in human arterioles. Am J Physiol Heart Circ Physiol 312: H515–H527, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441. Tarkin JM and Kaski JC. Vasodilator therapy: nitrates and nicorandil. Cardiovasc Drugs Ther 30: 367–378, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442. Tarkin JM and Kaski JC. Nicorandil and long-acting nitrates: vasodilator therapies for the management of chronic stable angina pectoris. Eur Cardiol 13: 23–28, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443. Task Force Members, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabate M, Senior R, Taggart DP, van der Wall EE, Vrints CJ, ESC Committee for Practice Guidelines, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Document R, Knuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner-Banzhoff N, Erol C, Frank H, Funck-Brentano C, Gaemperli O, Gonzalez-Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Ryden L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, and Zamorano JL. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 34: 2949–3003, 2013. [DOI] [PubMed] [Google Scholar]
- 444. Teng X, Chen L, Chen W, Yang J, Yang Z, and Shen Z. Mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and anti-inflammation. Cell Physiol Biochem 37: 2415–2424, 2015. [DOI] [PubMed] [Google Scholar]
- 445. Thaung Zaw JJ, Howe PRC, and Wong RHX. Sustained cerebrovascular and cognitive benefits of resveratrol in postmenopausal women. Nutrients 12: 828, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446. Thomas SR, Chen K, and Keaney JF Jr. Hydrogen peroxide activates endothelial nitric-oxide synthase through coordinated phosphorylation and dephosphorylation via a phosphoinositide 3-kinase-dependent signaling pathway. J Biol Chem 277: 6017–6024, 2002. [DOI] [PubMed] [Google Scholar]
- 447. Tousoulis D, Antoniades C, and Stefanadis C. Evaluating endothelial function in humans: a guide to invasive and non-invasive techniques. Heart 91: 553–558, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448. Touyz RM. Reactive oxygen species in vascular biology: role in arterial hypertension. Expert Rev Cardiovasc Ther 1: 91–106, 2003. [DOI] [PubMed] [Google Scholar]
- 449. Touyz RM and Briones AM. Reactive oxygen species and vascular biology: implications in human hypertension. Hypertens Res 34: 5–14, 2011. [DOI] [PubMed] [Google Scholar]
- 450. Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, Pagano PJ, and Schiffrin EL. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res 90: 1205–1213, 2002. [DOI] [PubMed] [Google Scholar]
- 451. Touyz RM and Schiffrin EL. Reactive oxygen species in vascular biology: implications in hypertension. Histochem Cell Biol 122: 339–352, 2004. [DOI] [PubMed] [Google Scholar]
- 452. Tracy E, Rowe G, Beare J, and LeBlanc A. Adipose stromal vascular fraction restores coronary microvascular flow-mediated dilation in aging female rats via enhanced peroxynitrite signaling. J Feder Am Soc Exp Biol 35(S1): 1, 2021. [Google Scholar]
- 453. Tracy E, Rowe G, Toro LN, Beyer A, and LeBlanc A. Telomerase reverse transcriptase mediates restoration of functional vasodilation in isolated coronary microvessels of aged female rats. Feder Am Soc Exp Biol 34(S1): 1, 2020. [Google Scholar]
- 454. Trinei M, Berniakovich I, Beltrami E, Migliaccio E, Fassina A, Pelicci P, and Giorgio M. P66Shc signals to age. Aging (Albany NY) 1: 503–510, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455. Trinity JD, Broxterman RM, and Richardson RS. Regulation of exercise blood flow: role of free radicals. Free Radic Biol Med 98: 90–102, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456. Trott DW, Seawright JW, Luttrell MJ, and Woodman CR. NAD(P)H oxidase-derived reactive oxygen species contribute to age-related impairments of endothelium-dependent dilation in rat soleus feed arteries. J Appl Physiol (1985) 110: 1171–1180, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457. Tyrrell DJ, Blin MG, Song J, Wood SC, Zhang M, Beard DA, and Goldstein DR. Age-associated mitochondrial dysfunction accelerates atherogenesis. Circ Res 126: 298–314, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458. Ungvari Z, Labinskyy N, Gupte S, Chander PN, Edwards JG, and Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol 294: H2121–H2128, 2008. [DOI] [PubMed] [Google Scholar]
- 459. Ungvari Z, Sonntag WE, and Csiszar A. Mitochondria and aging in the vascular system. J Mol Med (Berl) 88: 1021–1027, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460. Ungvari Z, Tarantini S, Donato AJ, Galvan V, and Csiszar A. Mechanisms of vascular aging. Circ Res 123: 849–867, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461. Ungvari Z, Tarantini S, Sorond F, Merkely B, and Csiszar A. Mechanisms of vascular aging, a geroscience perspective: JACC focus seminar. J Am Coll Cardiol 75: 931–941, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462. van der Feen DE, Bossers GPL, Hagdorn QAJ, Moonen JR, Kurakula K, Szulcek R, Chappell J, Vallania F, Donato M, Kok K, Kohli JS, Petersen AH, van Leusden T, Demaria M, Goumans MTH, De Boer RA, Khatri P, Rabinovitch M, Berger RMF, and Bartelds B. Cellular senescence impairs the reversibility of pulmonary arterial hypertension. Sci Transl Med 12: 1–14, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463. van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, and Luscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med 192: 1731–1744, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464. van Gastel J, Hendrickx JO, Leysen H, Santos-Otte P, Luttrell LM, Martin B, and Maudsley S. Beta-arrestin based receptor signaling paradigms: potential therapeutic targets for complex age-related disorders. Front Pharmacol 9: 1369, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465. Van Guilder GP, Westby CM, Greiner JJ, Stauffer BL, and DeSouza CA. Endothelin-1 vasoconstrictor tone increases with age in healthy men but can be reduced by regular aerobic exercise. Hypertension 50: 403–409, 2007. [DOI] [PubMed] [Google Scholar]
- 466. Vasudevan NT, Mohan ML, Goswami SK, and Naga Prasad SV. Regulation of beta-adrenergic receptor function: an emphasis on receptor resensitization. Cell Cycle 10: 3684–3691, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467. Vasudevan NT, Mohan ML, Gupta MK, Hussain AK, and Naga Prasad SV. Inhibition of protein phosphatase 2A activity by PI3Kgamma regulates beta-adrenergic receptor function. Mol Cell 41: 636–648, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468. Vecchione C, Patrucco E, Marino G, Barberis L, Poulet R, Aretini A, Maffei A, Gentile MT, Storto M, Azzolino O, Brancaccio M, Colussi GL, Bettarini U, Altruda F, Silengo L, Tarone G, Wymann MP, Hirsch E, and Lembo G. Protection from angiotensin II-mediated vasculotoxic and hypertensive response in mice lacking PI3Kgamma. J Exp Med 201: 1217–1228, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469. Vecoli C, Borghini A, and Andreassi MG. The molecular biomarkers of vascular aging and atherosclerosis: telomere length and mitochondrial DNA(4977) common deletion. Mutat Res 784: 108309, 2020. [DOI] [PubMed] [Google Scholar]
- 470. Vecoli C, Borghini A, Pulignani S, Mercuri A, Turchi S, Carpeggiani C, Picano E, and Andreassi MG. Prognostic value of mitochondrial DNA(4977) deletion and mitochondrial DNA copy number in patients with stable coronary artery disease. Atherosclerosis 276: 91–97, 2018. [DOI] [PubMed] [Google Scholar]
- 471. Vecoli C, Borghini A, Pulignani S, Mercuri A, Turchi S, Picano E, and Andreassi MG. Independent and combined effects of telomere shortening and mtDNA(4977) deletion on long-term outcomes of patients with coronary artery disease. Int J Mol Sci 20: 5508, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472. Vendrov AE, Vendrov KC, Smith A, Yuan J, Sumida A, Robidoux J, Runge MS, and Madamanchi NR. NOX4 NADPH oxidase-dependent mitochondrial oxidative stress in aging-associated cardiovascular disease. Antioxid Redox Signal 23: 1389–1409, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473. Vergeade A, Mulder P, Vendeville-Dehaudt C, Estour F, Fortin D, Ventura-Clapier R, Thuillez C, and Monteil C. Mitochondrial impairment contributes to cocaine-induced cardiac dysfunction: prevention by the targeted antioxidant MitoQ. Free Radic Biol Med 49: 748–756, 2010. [DOI] [PubMed] [Google Scholar]
- 474. Verma SK, Jain V, and Katewa SS. Blood pressure lowering, fibrinolysis enhancing and antioxidant activities of cardamom (Elettaria cardamomum). Indian J Biochem Biophys 46: 503–506, 2009. [PubMed] [Google Scholar]
- 475. Vicinanza R, Troisi G, Cangemi R, De Martino MU, Pastori D, Bernardini S, Crisciotti F, Di Violante F, Frizza A, Cacciafesta M, Pignatelli P, and Marigliano V. Aging and adherence to the mediterranean diet: relationship with cardiometabolic disorders and polypharmacy. J Nutr Health Aging 22: 73–81, 2018. [DOI] [PubMed] [Google Scholar]
- 476. Vida C, Corpas I, De la Fuente M, and Gonzalez EM. Age-related changes in xanthine oxidase activity and lipid peroxidation, as well as in the correlation between both parameters, in plasma and several organs from female mice. J Physiol Biochem 67: 551–558, 2011. [DOI] [PubMed] [Google Scholar]
- 477. Viel EC, Benkirane K, Javeshghani D, Touyz RM, and Schiffrin EL. Xanthine oxidase and mitochondria contribute to vascular superoxide anion generation in DOCA-salt hypertensive rats. Am J Physiol Heart Circ Physiol 295: H281–H288, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478. Wang G, Moniri NH, Ozawa K, Stamler JS, and Daaka Y. Nitric oxide regulates endocytosis by S-nitrosylation of dynamin. Proc Natl Acad Sci U S A 103: 1295–1300, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479. Wang H, Song P, Du L, Tian W, Yue W, Liu M, Li D, Wang B, Zhu Y, Cao C, Zhou J, and Chen Q. Parkin ubiquitinates Drp1 for proteasome-dependent degradation: implication of dysregulated mitochondrial dynamics in Parkinson disease. J Biol Chem 286: 11649–11658, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480. Wang HH, Wu YJ, Tseng YM, Su CH, Hsieh CL, and Yeh HI. Mitochondrial fission protein 1 up-regulation ameliorates senescence-related endothelial dysfunction of human endothelial progenitor cells. Angiogenesis 22: 569–582, 2019. [DOI] [PubMed] [Google Scholar]
- 481. Wang L, Yu T, Lee H, O'Brien DK, Sesaki H, and Yoon Y. Decreasing mitochondrial fission diminishes vascular smooth muscle cell migration and ameliorates intimal hyperplasia. Cardiovasc Res 106: 272–283, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482. Wang M, Zhang J, Jiang LQ, Spinetti G, Pintus G, Monticone R, Kolodgie FD, Virmani R, and Lakatta EG. Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension 50: 219–227, 2007. [DOI] [PubMed] [Google Scholar]
- 483. Wang N, Ko SH, Chai W, Li G, Barrett EJ, Tao L, Cao W, and Liu Z. Resveratrol recruits rat muscle microvasculature via a nitric oxide-dependent mechanism that is blocked by TNFalpha. Am J Physiol Endocrinol Metab 300: E195–E201, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484. Wang XB, Cui NH, Zhang S, Liu ZJ, Ma JF, and Ming L. Leukocyte telomere length, mitochondrial DNA copy number, and coronary artery disease risk and severity: a two-stage case-control study of 3064 Chinese subjects. Atherosclerosis 284: 165–172, 2019. [DOI] [PubMed] [Google Scholar]
- 485. Wasilewski R, Ubara EO, and Klonizakis M. Assessing the effects of a short-term green tea intervention in skin microvascular function and oxygen tension in older and younger adults. Microvasc Res 107: 65–71, 2016. [DOI] [PubMed] [Google Scholar]
- 486. Weaver SR, Rendeiro C, McGettrick HM, Philp A, and Lucas SJE. Fine wine or sour grapes? A systematic review and meta-analysis of the impact of red wine polyphenols on vascular health. Eur J Nutr 60: 1–28, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487. Wei EP, Kontos HA, and Beckman JS. Mechanisms of cerebral vasodilation by superoxide, hydrogen peroxide, and peroxynitrite. Am J Physiol 271: H1262–H1266, 1996. [DOI] [PubMed] [Google Scholar]
- 488. Wei YH and Lee HC. Oxidative stress, mitochondrial DNA mutation, and impairment of antioxidant enzymes in aging. Exp Biol Med (Maywood) 227: 671–682, 2002. [DOI] [PubMed] [Google Scholar]
- 489. Weiss N, Zhang YY, Heydrick S, Bierl C, and Loscalzo J. Overexpression of cellular glutathione peroxidase rescues homocyst(e)ine-induced endothelial dysfunction. Proc Natl Acad Sci U S A 98: 12503–12508, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490. Welch WJ. Angiotensin II-dependent superoxide: effects on hypertension and vascular dysfunction. Hypertension 52: 51–56, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491. Wenzel P, Schuhmacher S, Kienhofer J, Muller J, Hortmann M, Oelze M, Schulz E, Treiber N, Kawamoto T, Scharffetter-Kochanek K, Munzel T, Burkle A, Bachschmid MM, and Daiber A. Manganese superoxide dismutase and aldehyde dehydrogenase deficiency increase mitochondrial oxidative stress and aggravate age-dependent vascular dysfunction. Cardiovasc Res 80: 280–289, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492. Werner C, Gensch C, Poss J, Haendeler J, Bohm M, and Laufs U. Pioglitazone activates aortic telomerase and prevents stress-induced endothelial apoptosis. Atherosclerosis 216: 23–34, 2011. [DOI] [PubMed] [Google Scholar]
- 493. Whalen EJ, Foster MW, Matsumoto A, Ozawa K, Violin JD, Que LG, Nelson CD, Benhar M, Keys JR, Rockman HA, Koch WJ, Daaka Y, Lefkowitz RJ, and Stamler JS. Regulation of beta-adrenergic receptor signaling by S-nitrosylation of G-protein-coupled receptor kinase 2. Cell 129: 511–522, 2007. [DOI] [PubMed] [Google Scholar]
- 494. Widder JD, Fraccarollo D, Galuppo P, Hansen JM, Jones DP, Ertl G, and Bauersachs J. Attenuation of angiotensin II-induced vascular dysfunction and hypertension by overexpression of Thioredoxin 2. Hypertension 54: 338–344, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495. Williams MJ, Sutherland WH, McCormick MP, Yeoman DJ, and de Jong SA. Aged garlic extract improves endothelial function in men with coronary artery disease. Phytother Res 19: 314–319, 2005. [DOI] [PubMed] [Google Scholar]
- 496. Wilson C, Saunter CD, Girkin JM, and McCarron JG. Advancing age decreases pressure-sensitive modulation of calcium signaling in the endothelium of intact and pressurized arteries. J Vasc Res 53: 358–369, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497. Winter JP, Gong Y, Grant PJ, and Wild CP. Glutathione peroxidase 1 genotype is associated with an increased risk of coronary artery disease. Coron Artery Dis 14: 149–153, 2003. [DOI] [PubMed] [Google Scholar]
- 498. Wong RH, Coates AM, Buckley JD, and Howe PR. Evidence for circulatory benefits of resveratrol in humans. Ann N Y Acad Sci 1290: 52–58, 2013. [DOI] [PubMed] [Google Scholar]
- 499. Woodward KA, Hopkins ND, Draijer R, de Graaf Y, Low DA, and Thijssen DHJ. Acute black tea consumption improves cutaneous vascular function in healthy middle-aged humans. Clin Nutr 37: 242–249, 2018. [DOI] [PubMed] [Google Scholar]
- 500. Wray DW, Nishiyama SK, and Richardson RS. Role of {alpha}1-adrenergic vasoconstriction in the regulation of skeletal muscle blood flow with advancing age. Am J Physiol Heart Circ Physiol 296: H497–H504, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501. Xi Q, Cheranov SY, and Jaggar JH. Mitochondria-derived reactive oxygen species dilate cerebral arteries by activating Ca2+ sparks. Circ Res 97: 354–362, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502. Xu JY, Chen GH, and Yang YJ. Exosomes: a rising star in falling hearts. Front Physiol 8: 494, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503. Xu R, Yang K, Ding J, and Chen G. Effect of green tea supplementation on blood pressure: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 99: e19047, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504. Xu S, Zhi H, Hou X, and Jiang B. Angiotensin II modulates interleukin-1beta-induced inflammatory gene expression in vascular smooth muscle cells via interfering with ERK-NF-kappaB crosstalk. Biochem Biophys Res Commun 410: 543–548, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505. Xu Y, Armstrong SJ, Arenas IA, Pehowich DJ, and Davidge ST. Cardioprotection by chronic estrogen or superoxide dismutase mimetic treatment in the aged female rat. Am J Physiol Heart Circ Physiol 287: H165–H171, 2004. [DOI] [PubMed] [Google Scholar]
- 506. Yamamori T, White AR, Mattagajasingh I, Khanday FA, Haile A, Qi B, Jeon BH, Bugayenko A, Kasuno K, Berkowitz DE, and Irani K. P66shc regulates endothelial NO production and endothelium-dependent vasorelaxation: implications for age-associated vascular dysfunction. J Mol Cell Cardiol 39: 992–995, 2005. [DOI] [PubMed] [Google Scholar]
- 507. Yan Y, Jiang W, Tan Y, Zou S, Zhang H, Mao F, Gong A, Qian H, and Xu W. hucMSC exosome-derived GPX1 is required for the recovery of hepatic oxidant injury. Mol Ther 25: 465–479, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508. Yang HQ, Subbotina E, Ramasamy R, and Coetzee WA. Cardiovascular KATP channels and advanced aging. Pathobiol Aging Age Relat Dis 6: 32517, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509. Yang J, Liu XX, Fan H, Tang Q, Shou ZX, Zuo DM, Zou Z, Xu M, Chen QY, Peng Y, Deng SJ, and Liu YJ. Extracellular vesicles derived from bone marrow mesenchymal stem cells protect against experimental colitis via attenuating colon inflammation, oxidative stress and apoptosis. PLoS One 10: e0140551, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510. Yang Y, Shi W, Cui N, Wu Z, and Jiang C. Oxidative stress inhibits vascular K(ATP) channels by S-glutathionylation. J Biol Chem 285: 38641–38648, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511. Yang YM, Huang A, Kaley G, and Sun D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am J Physiol Heart Circ Physiol 297: H1829–H1836, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512. Yao Y, Fan XL, Jiang D, Zhang Y, Li X, Xu ZB, Fang SB, Chiu S, Tse HF, Lian Q, and Fu QL. Connexin 43-mediated mitochondrial transfer of iPSC-MSCs alleviates asthma inflammation. Stem Cell Reports 11: 1120–1135, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513. Yu EP and Bennett MR. Mitochondrial DNA damage and atherosclerosis. Trends Endocrinol Metab 25: 481–487, 2014. [DOI] [PubMed] [Google Scholar]
- 514. Yu EP and Bennett MR. The role of mitochondrial DNA damage in the development of atherosclerosis. Free Radic Biol Med 100: 223–230, 2016. [DOI] [PubMed] [Google Scholar]
- 515. Yu EPK, Reinhold J, Yu H, Starks L, Uryga AK, Foote K, Finigan A, Figg N, Pung YF, Logan A, Murphy MP, and Bennett M. Mitochondrial respiration is reduced in atherosclerosis, promoting necrotic core formation and reducing relative fibrous cap thickness. Arterioscler Thromb Vasc Biol 37: 2322–2332, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516. Yu SS, Lefkowitz RJ, and Hausdorff WP. Beta-adrenergic receptor sequestration. A potential mechanism of receptor resensitization. J Biol Chem 268: 337–341, 1993. [PubMed] [Google Scholar]
- 517. Zhang C, Hein TW, Wang W, Ren Y, Shipley RD, and Kuo L. Activation of JNK and xanthine oxidase by TNF-alpha impairs nitric oxide-mediated dilation of coronary arterioles. J Mol Cell Cardiol 40: 247–257, 2006. [DOI] [PubMed] [Google Scholar]
- 518. Zhang H, Xiang M, Meng D, Sun N, and Chen S. Inhibition of myocardial ischemia/reperfusion injury by exosomes secreted from mesenchymal stem cells. Stem Cells Int 2016: 4328362, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519. Zhang J, Barak LS, Anborgh PH, Laporte SA, Caron MG, and Ferguson SS. Cellular trafficking of G protein-coupled receptor/beta-arrestin endocytic complexes. J Biol Chem 274: 10999–11006, 1999. [DOI] [PubMed] [Google Scholar]
- 520. Zhang J, Barak LS, Winkler KE, Caron MG, and Ferguson SS. A central role for beta-arrestins and clathrin-coated vesicle-mediated endocytosis in beta2-adrenergic receptor resensitization. Differential regulation of receptor resensitization in two distinct cell types. J Biol Chem 272: 27005–27014, 1997. [DOI] [PubMed] [Google Scholar]
- 521. Zhang J, Block ER, and Patel JM. Down-regulation of mitochondrial cytochrome c oxidase in senescent porcine pulmonary artery endothelial cells. Mech Ageing Dev 123: 1363–1374, 2002. [DOI] [PubMed] [Google Scholar]
- 522. Zhang N, Li Z, Mu W, Li L, Liang Y, Lu M, Wang Z, Qiu Y, and Wang Z. Calorie restriction-induced SIRT6 activation delays aging by suppressing NF-kappaB signaling. Cell Cycle 15: 1009–1018, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523. Zhang W, Qu X, Chen B, Snyder M, Wang M, Li B, Tang Y, Chen H, Zhu W, Zhan L, Yin N, Li D, Xie L, Liu Y, Zhang JJ, Fu XY, Rubart M, Song LS, Huang XY, and Shou W. Critical roles of STAT3 in beta-adrenergic functions in the heart. Circulation 133: 48–61, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524. Zhang W, Ren H, Xu C, Zhu C, Wu H, Liu D, Wang J, Liu L, Li W, Ma Q, Du L, Zheng M, Zhang C, Liu J, and Chen Q. Hypoxic mitophagy regulates mitochondrial quality and platelet activation and determines severity of I/R heart injury. Elife 5: e21407, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525. Zhou E, Qing D, and Li J. Age-associated endothelial dysfunction in rat mesenteric arteries: roles of calcium-activated K(+) channels (K(ca)). Physiol Res 59: 499–508, 2010. [DOI] [PubMed] [Google Scholar]
- 526. Zhou S, Chen HZ, Wan YZ, Zhang QJ, Wei YS, Huang S, Liu JJ, Lu YB, Zhang ZQ, Yang RF, Zhang R, Cai H, Liu DP, and Liang CC. Repression of P66Shc expression by SIRT1 contributes to the prevention of hyperglycemia-induced endothelial dysfunction. Circ Res 109: 639–648, 2011. [DOI] [PubMed] [Google Scholar]
- 527. Zhou X, Bohlen HG, Unthank JL, and Miller SJ. Abnormal nitric oxide production in aged rat mesenteric arteries is mediated by NAD(P)H oxidase-derived peroxide. Am J Physiol Heart Circ Physiol 297: H2227–H2233, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528. Zhou Y, Xu H, Xu W, Wang B, Wu H, Tao Y, Zhang B, Wang M, Mao F, Yan Y, Gao S, Gu H, Zhu W, and Qian H. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther 4: 34, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529. Zordoky BN, Robertson IM, and Dyck JR. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim Biophys Acta 1852: 1155–1177, 2015. [DOI] [PubMed] [Google Scholar]
- 530. Zorov DB, Filburn CR, Klotz LO, Zweier JL, and Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med 192: 1001–1014, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531. Zorov DB, Juhaszova M, and Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 94: 909–950, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532. Zouein FA, Altara R, Chen Q, Lesnefsky EJ, Kurdi M, and Booz GW. Pivotal importance of STAT3 in protecting the heart from acute and chronic stress: new advancement and unresolved issues. Front Cardiovasc Med 2: 36, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]