Abstract
Significance: Acute kidney injury (AKI) is a common and life-threatening complication in hospitalized and critically ill patients. It is defined by an abrupt deterioration in renal function, clinically manifested by increased serum creatinine levels, decreased urine output, or both. To execute all its functions, namely excretion of waste products, fluid/electrolyte balance, and hormone synthesis, the kidney requires incredible amounts of energy in the form of adenosine triphosphate.
Recent Advances: Adequate mitochondrial functioning and nicotinamide adenine dinucleotide (NAD+) homeostasis are essential to meet these high energetic demands. NAD+ is a ubiquitous essential coenzyme to many cellular functions. NAD+ as an electron acceptor mediates metabolic pathways such as oxidative phosphorylation (OXPHOS) and glycolysis, serves as a cosubstrate of aging molecules (i.e., sirtuins), participates in DNA repair mechanisms, and mediates mitochondrial biogenesis.
Critical Issues: In many forms of AKI and chronic kidney disease, renal function deterioration has been associated with mitochondrial dysfunction and NAD+ depletion. Based on this, therapies aiming to restore mitochondrial function and increase NAD+ availability have gained special attention in the last two decades.
Future Directions: Experimental and clinical studies have shown that by restoring mitochondrial homeostasis and increasing renal tubulo-epithelial cells, NAD+ availability, AKI incidence, and chronic long-term complications are significantly decreased. This review covers some general epidemiological and pathophysiological concepts; describes the role of mitochondrial homeostasis and NAD+ metabolism; and analyzes the underlying rationale and role of NAD+ aiming therapies as promising preventive and therapeutic strategies for AKI. Antioxid. Redox Signal. 35, 1449–1466.
Keywords: NAD, nicotinamide, acute kidney injury, mitochondrial dysfunction
Introduction
Acute kidney injury (AKI) is a loose collection of syndromes whose defining characteristic is an abrupt loss of glomerular function (57). It can be caused by diverse mechanisms from urinary tract obstruction to sepsis, to nephrotoxin exposure—frequently multiple etiologies are involved (91). AKI is common and life-threatening, complicating more than 10% of hospital admissions and up to 50% of those who require critical care (1, 46). It increases the risk of death by three- to sevenfold when compared with those who never develop AKI (1). In addition, about 50% of patients developing moderate to severe AKI [i.e., Kidney Disease: Improving Global Outcomes (KDIGO) (58) stage 2 or 3] do not recover renal function by 30 days (86, 97, 98). Despite the high morbidity and mortality associated with AKI, therapies remain reactive and nonspecific and little impact has been made in decreasing AKI incidence and its associated long-term complications. Thus, there is a critical unmet need to find therapeutics that can predict and mitigate kidney damage, and/or improve recovery across a range of different AKI etiologies.
The pathogenesis of AKI is complex and multifactorial (40). Among the multiple pathogenic mechanisms that may lead to AKI, energy metabolism pathway alterations seem to play a key role in the development of many forms of AKI. Energy metabolic alterations can be attributed primarily to mitochondrial dysfunction and nicotinamide adenine dinucleotide (NAD+) deficiency. NAD+ is a ubiquitous coenzyme that participates in different mitochondrial and nonmitochondrial energy producing pathways, serves as a cosubstrate of aging mediating enzymes, and participates in DNA repair mechanisms. NAD+ is crucial to maintain mitochondrial homeostasis and adenosine triphosphate (ATP) production either through oxidative and nonoxidative mechanisms. Thus, its importance in renal physiology as it is essential to preserve an adequate kidney functioning and prevent cellular injury. Indeed, renal tubular epithelial cells (TECs) deficient in NAD+ cannot defend themselves against AKI and cannot repair damage once injury has occurred. Moreover, studies in which therapies aiming to improve availability of NAD+ have been used, and have shown that the replenishment of NAD+ in TECs results in decreased AKI incidence and the less probability of development of long-term complications such as chronic kidney disease (CKD). This review covers some general concepts of AKI emphasizing on its relevance as a continuously growing public health problem; it covers some pathophysiologic concepts focusing on the role of mitochondrial dysfunction and NAD+ deficiency in the development of AKI; and finally, it analyzes the existing evidence of NAD+ as a rising and promising therapeutic target in AKI.
AKI: A Public Health Problem
The modern definition of AKI was based on the RIFLE (Risk, Injury, Failure, Loss, and End-stage renal disease) criteria (6). These criteria are centered on alterations in the glomerular filtration rate (GFR), estimated imperfectly by changes in serum creatinine and, more imperfectly still, by changes in urine volume over time. The inclusion of urine output, however, provides considerable discrimination to the severity of kidney dysfunction and often is more time-sensitive compared with serum creatinine alone (53, 59). Serum creatinine is very insensitive to kidney damage and while urine output is less specific, it is more sensitive to many forms of AKI. In 2012, the KDIGO AKI Clinical Practice Guidelines (58) harmonized the adult and pediatric criteria for AKI and have become the standard definition (Table 1). The discovery of specific biomarkers for kidney damage will likely enrich the definition and diagnosis of AKI in the near future (79, 91).
Table 1.
Kidney Disease: Improving Global Outcomes Criteria for Acute Kidney Injury
| Stage | sCr | UO |
|---|---|---|
| 1 | 1.5–1.9 × baseline | <0.5 mL/kg/h for 6–12 h |
| OR | ||
| ≥0.3 mg/dL (>26.5 μM) increase | ||
| 2 | 2.0–2.9 × baseline | <0.5 mL/kg/h for 12 h |
| 3 | 3 × baseline | <0.3 mL/kg/h for ≥24 h |
| OR | OR | |
| Increase in sCr ≥4.0 mg/dL (353.6 μM) | Anuria for ≥12 h | |
| OR | ||
| Initiation of RRT | ||
| OR | ||
| In patients <18 years, decrease in eGFR to <35 mL/min per 1.73 m2 |
AKI, acute kidney injury; GFR, glomerular filtration rate; KDIGO, Kidney Disease: Improving Global Outcomes; RRT, renal replacement therapy; sCr, serum creatinine; UO, urine output.
The incidence of AKI in Europe is estimated to be as high as 39 per 1000 population with similar rates in Australia (91). In the United States, estimates have been lower, but still quite high—about 18 per 1000. Thus, rates of AKI are approaching 6 million cases a year in the United States and more than four times this in all of Europe. As for the rest of the world, rates among hospitalized patients are quite similar to those reported in Europe meaning that there could be as many as 300 million new cases of AKI in the world in 2020! These numbers underscore the massive public health crisis that AKI represents and the urgent need to find effective treatment.
Early attempts to address AKI pharmacologically focused almost exclusively on altering renal hemodynamics. This was due, in part, to a mistaken notion that the pathogenesis of AKI was mainly ischemic and by the equally tragic approach ignoring that AKI is a collection of syndromes with multiple etiologies rather than a single disease with a unique cause and a singular therapeutic approach. Therefore, limited progress has been made and therapies still remain reactive and nonspecific with a shallow impact in morbidity and mortality. Understanding AKI as a global entity, in which multiple and different pathogenic mechanisms take part simultaneously, is crucial for the better comprehension of this syndrome, and furthermore, will allow broadening the perspective in the development of newer effective preventive and therapeutic strategies. Common causes of AKI are listed in Table 2. Moreover, understanding that although clinical manifestations of AKI might be similar among patients, the underlying pathophysiology may widely differ between them. Thus, interventions developed for one particular type/etiology of AKI may fail when studied in heterogenous groups of patients.
Table 2.
Common Causes of Acute Kidney Injury
| Sepsisa |
| Drugs |
| Cardiac surgery |
| Acute decompensated heart failure |
| Noncardiac surgery |
| Trauma/burns |
| Oncologic disease |
| Hepatorenal syndrome |
| Environmental toxins, plants, insects, animals |
AKI is often the sepsis-defining organ injury in severe infections (e.g., pneumonia)—thus, patients do not need to have sepsis first.
Pathogenesis of AKI
Understanding of AKI has advanced greatly since the beginning of the 21st century. We now understand that AKI is not a single disease, but a collection of syndromes that can be provoked by diverse pathogenic mechanisms, which often coincide (83). Nevertheless, most of the current knowledge has been extrapolated from animal models, in vitro studies, and postmortem observations in humans (83). Therefore, these observations should be interpreted with caution since the response to various kidney insults (e.g., sepsis, nephrotoxins, and ischemia) or therapies may vary widely from that in humans.
AKI is a “silent disease” in which clinical manifestations may not become evident until severe stages of the disease are reached. Because of this, prevention, early diagnosis, and intervention are almost impossible. Moreover, establishing the exact time of injury and main cause is hindered by the silent course of AKI, unlike in preclinical experimental models in which these variables are mostly controlled, and time and cause of injury are primarily determined by the characteristic features of the implemented AKI model. Conversely, AKI in the clinical setting is a multifactorial entity in which multiple pathogenic mechanisms coincide, increasing the complexity to understand and build a framework that encompasses the different elements that play a role during the pathogenesis of acute renal injury. These and many other limitations frustrate the translation of promising effective diagnostic and therapeutic strategies to the bedside.
The leading causes of AKI, namely sepsis, major surgery, heart failure, and hypovolemia, are associated to greater or lesser extent with decreased renal perfusion as a consequence of shock and extensive tubule-epithelial cell death (113). Therefore, it was compelling to explain most forms of AKI as a manifestation of decreased global renal blood flow and acute tubular necrosis (94). However, in the last two decades, the paradigm that encompasses these elements as main pathogenic events of AKI has been challenged. It is now clear that several mechanisms other than renal hypoperfusion play a role in AKI, and the extension of TEC death is not as vast as initially contemplated (62, 67, 92, 105). In multiple preclinical and clinical studies, it has been demonstrated that renal injury and AKI might occur in the absence of overt signs of hypoperfusion and/or clinical signs of hemodynamic instability (63, 74, 86). Murugan et al. (74) demonstrated in a large clinical cohort study that one-third of patients admitted to the hospital with a diagnosis of community-acquired pneumonia developed AKI without presenting clinical signs of hypoperfusion, hemodynamic instability, or requiring admission to an intensive care unit. These clinical observations have been supported and documented in preclinical models of AKI too. For instance, in animal models in which sepsis was induced to provoke AKI, animals that had normal or even increased global renal blood flow also developed AKI (10, 25–27, 61, 63, 90). In addition, histopathologic studies made in postmortem human biopsies of patients who suffered AKI have also rebutted the presumption that rapid renal function deterioration is associated with extensive acute tubular necrosis (62, 67, 105). Conversely to what was initially considered, heterogenous, focal, and patchy tubular injuries, with minimal tubule-epithelial cell death (<5%), apical vacuolization, and minor focal mesangial expansion, are the consistent findings in these specimens (62, 67, 105).
These observations clearly challenge the traditional notion of ischemic injury and significant cell death underlying AKI. Moreover, the evident dissociation that exists between the described structural alterations and the renal functional decline strongly suggests that other factors must be at play. Several heterogenous theories and mechanisms have been proposed to explain the still puzzling pathogenesis of AKI such as energy metabolism impairment, cellular metabolic reprogramming, impaired inflammatory response, and microcirculatory dysfunction (40). Although some of these seem to be disparate, many forms of AKI seem to share many of these mechanisms of injury such as energy metabolism impairment caused by mitochondrial dysfunction and NAD+ depletion and cellular metabolic reprogramming (41, 83).
Mitochondria and AKI
Interest in mitochondrial dysfunction, once limited to cancer, heart, and brain pathology, has exploded in the field of AKI. The kidney is one of the highest energy-demanding organs in the body, second only to the heart in abundance of mitochondria (8, 80, 120). Mitochondria are a complex network of organelles, which functions include energy synthesis, nucleotide and heme synthesis, regulation of reactive oxygen species (ROS), calcium homeostasis, and thermogenesis and regulation of mechanisms of programmed cell death (i.e., apoptosis) among others. In the kidney, mitochondria play a crucial role in terms of providing and maintaining adequate pools of energy allowing the kidney to execute all its functions, namely waste product excretion, secretion and reabsorption of nutrients, hormone synthesis, and maintenance of fluid/electrolyte equilibrium and acid-base balance. Therefore, the high susceptibility of the kidney to any sort of energy metabolic impairment and mitochondrial dysfunction. Mitochondrial dysfunction may lead to TEC injury and organ function deterioration in several ways either due to ATP deficiency or dysregulation in cell death mechanisms. ATP deficiency can occur either due to decreased synthesis due to impaired energy metabolic pathways or impaired utilization of substrates that fuel these pathways. Therefore, an adequate mitochondrial integrity, bioenergetics, and recycling (mitophagy) are essential to meet the high-energy demands of the kidney. On the contrary, healthy mitochondria are not only crucial for adequate cell functioning and survival but also to initiate repair and healing mechanisms once injury has been caused (18, 19, 23, 40, 41, 51, 67, 100, 119).
In terms of energy consumption, renal functions could be assorted into active or passive processes. For instance, glomerular blood ultrafiltration is one of the most important energy passive processes that occur in the kidney. In the glomeruli, the plasma gets filtered through the glomerular basement membrane into the tubular collecting system to produce urine. The energy requirements of this process are very low, as it is mainly regulated by endocrine/hemodynamic changes of the afferent and efferent arterioles. Conversely, functions such as tubular reabsorption and secretion of nutrients require higher amounts of energy constituting active processes. Approximately 80% of solute transport, nutrient reabsorption, and ultrafiltrate osmotic gradient regulation occur in the proximal TECs and the medullary thick ascending limb of Henle's loop. Therefore, the major pool of renal mitochondria and the highest oxygen consumption rates and oxidative phosphorylation (OXPHOS) are registered in these two segments of the renal tubular system (11, 121).
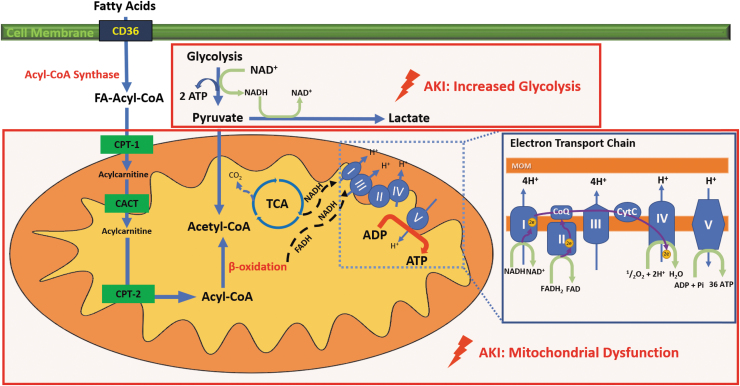
TECs primarily rely on oxidative metabolic pathways to keep an adequate flow of energy supply to be able to meet the high energetic demands (11, 49, 103, 121). These oxygen-dependent pathways mostly take place in the mitochondria, where oxygen will act as the last electron acceptor of the electron transport chain (ETC). Mitochondria transform energy in the form of ATP, through oxidation of nutrients, including glucose, fatty acids, and amino acids, in a two-prong process known as OXPHOS. First, the sequential cycling of nutrient substrates through the tricarboxylic acid cycle in the mitochondrial matrix delivers reducing equivalents (i.e., electrons) to the mitochondrial ETC in the inner mitochondrial membrane. Then, nicotinamide adenine dinucleotide hydrogen (NADH) and flavin adenine dinucleotide (FADH2) hand over electrons to complex I and II, respectively. This delivery of electrons to the ETC constitutes the rate-limiting step of fatty acid oxidation (FAO) (111). Then, cycling of electrons in the ETC promotes the extrusion of hydrogen ions (H+) out of the matrix into the intermembrane space, generating an electrochemical gradient of H+ across the mitochondrial membrane, which holds the energy derived from nutrients into potential energy. Finally, H+ re-enters the mitochondrial matrix through complex V in the ETC in favor of the electrochemical gradient, releasing the potential energy that ATP synthetase then uses to assemble ATP molecules (Fig. 1).
FIG. 1.
FAO, ETC, and glycolysis. The kidney has a high-energy demand. After cardiac myocytes, TECs contain the second-most abundant mitochondria population. For an adequate functioning, TECs require high amounts of ATP. Fatty acid oxidation (FAO) is the preferred energy source of the kidney. Long-chain FA enter the TECs with the help of CD36. Once in the cytoplasm, acyl-CoA synthetase activates FA into FA-acyl-CoA. As the outer mitochondrial membrane is impermeable to FA-acyl-CoA, CPT-1 is necessary for the transesterification of FA-acyl-CoA into acylcarnitine allowing it to cross the outer membrane to get into the inter membrane space. Acylcarnitine diffuses into the inner matrix of the mitochondria using the CACT transporter. Inside the mitochondrial matrix, the acylcarnitine is transformed back to FA-Acyl-CoA by CPT-2. β-oxidation of FA-Acyl-CoA will fuel the TCA cycle, which then will result in the production of high-energy electrons stored in NADH and FADH2 cofactors to be carried and transferred to the ETC to act as electron donors (44). Pyruvate, a breakdown product of glycolysis can also be converted to acetyl-coA via pyruvate dehydrogenase present in the mitochondrial matrix. In the ETC, NADH and FADH2 transfer electrons to complex I and complex II, respectively. Then a flux of electrons occurs along the ETC traveling the complex IV where they bind O2, the ultimate electron acceptor, via the NADPH oxidase. As a final step, protons generated by this process flow back into the mitochondrial matrix through ATP synthase converting ADP into ATP. ADP, adenosine-diphosphate; ATP, adenosine-triphosphate; CACT, carnitine-acylcarnitine translocase; CPT-1, carnitine palmytoiltransferase-1; CPT-2, carnitine palmytoiltransferase-2; ETC, electron transport chain; FA, fatty acids; FADH2, flavin adenine dinucleotide; FAO, fatty acid oxidation; NADH, nicotinamide adenine dinucleotide hydrogen; TECs, tubular epithelial cells; TCA, tricarboxylic acid.
Oxidative pathways are very productive when it comes to producing energy compared with glycolytic and anaerobic mechanisms. The high amounts of ATP that are produced through the oxidation of fuel substrates are mostly intended to be used by Na+/K+ ATPase pumps along the basolateral membrane of TECs. Na+/K+ ATPase pumps constitute the main ion transporters in the tubular collecting system. Among the several functions of the Na+/K+ ATPase pumps, the most important include creating electrochemical gradients between the extracellular and intracellular matrix to facilitate diffusion of ions and reabsorption of nutrients into the blood and secretion of solutes and waste products into the ultrafiltrate that will result in the formation of urine. To generate an osmotic gradient across the cellular membrane, the Na+/K+ ATPase pump fueled by a molecule of ATP pushes 3 Na+ ions out and pulls 2 K+ ions in (103). In addition, by regulating Na+ and water concentrations, it adjusts the osmolarity of the urine according to the physiological needs of the organisms to maintain an adequate fluid and electrolyte balance (8).
As aforementioned, oxidative pathways are not the only source that TECs could appeal to synthesize ATP. TECs have also the ability to synthesize ATP in the absence of oxygen. In anaerobic glycolysis, pyruvate is converted to lactate instead of feeding the tricarboxylic acid cycle, inducing NAD+ regeneration (19). From these anaerobic pathways, only two molecules of ATP result, a small profit when compared with the more than 30 units that can be produced in the presence of oxygen (41, 49, 55). Although less efficient in terms of units of ATP generated, these anaerobic pathways play a key role during several renal physiologic and pathologic mechanisms. These anaerobic pathways allow renal cells, especially TECs, to maintain a minimum of vital energetic requirements in case of stress and injury even when this low-energy state might manifest with a downregulation of cellular functions. For instance, in sepsis-associated AKI where tissue perfusion and oxygen utilization are impaired, TECs switch to a nonoxidative metabolism in an attempt to promote cell survival at the expense of TEC function (41). However, the kidney does not only appeal to glycolysis during pathologic conditions. The renal medulla, unlike the cortex, constantly bears with low concentrations of oxygen. Most of the oxygen delivered to the kidney is utilized before reaching the medullary vasculature, making the medulla highly susceptible to hypoxic injury. Because of this constant hypoxemic state, glycolysis becomes the most suitable alternative to synthesize ATP (28, 32, 69, 77, 109, 120).
When mitochondrial function is impaired, many essential cellular mechanisms fail. This is what occurs in most forms of AKI, where mitochondrial homeostasis is busted and renal function deteriorates. A decreased mitochondria population, structural alterations (e.g., mitochondrial swelling structural disruption of the cristae), and impaired mitochondrial biogenesis are commonly observed in AKI (81, 112). Tran et al. (109) found that in sepsis-induced AKI, TEC mitochondria presented structural alterations characterized by the accumulation of acylglycerols. Accumulation of acylglycerols has a direct effect on prostaglandin E2 (PGE2) synthesis and production. During sepsis, systemic vasodilation and decreased vascular resistance are seen. As a response to these mechanisms, the sympathetic autonomic nervous system, activation of the renin/angiotensin/aldosterone system, and secretion of vasopressin cause intrarenal vasoconstriction to compensate for the decrease in renal perfusion pressure. Concomitantly, the absence of PGE2 and vasodilator counteracting effects due to mitochondrial dysfunction will exacerbate medullary ischemia by promoting renal vasculature vasoconstriction (109). Some studies have also shown that metabolic impairment contributes to the endothelial injury and microcirculatory dysfunction seen in sepsis-AKI (5, 111). Despite the increasing understanding, therapies targeting mitochondrial injury in AKI are still limited. Guo, et al. (43) found that in cisplatin-induced AKI in the mouse and in biopsy samples of human AKI kidney tissue, micro-RNA (miR)-709 was significantly upregulated in proximal tubular cells (PTCs). The expression of miR-709 in PTCs also correlated with the severity of AKI. In cultured mouse PTCs, overexpression of miR-709 induced mitochondrial dysfunction, whereas inhibition of miR-709 ameliorated cisplatin-induced mitochondrial dysfunction and cell injury. The investigators also showed that the mitochondrial transcriptional factor A (TFAM) is a target gene of miR-709, and genetic restoration of TFAM attenuated mitochondrial dysfunction and cell injury in vitro. These findings reveal a pathogenic role of miR-709 in acute tubular injury and suggest a novel target for the treatment of AKI. These findings support the concept that mitochondrial homeostasis is fundamental for the proper functioning of the kidney (19, 32). Moreover, in cases where injury has already occurred, re-establishing and preserving a healthy and functional mitochondrial population are needed to achieve adequate regeneration and healing outcomes.
Mitochondrial biogenesis
Mitochondrial biogenesis is a tightly regulated adaptive process by which the cell is able to produce new and functional mitochondria (4, 65, 104). Mitochondrial biogenesis is primarily regulated by peroxisome proliferator-activated receptor γ -coactivator-1α (PGC-1α). PGC-1α is a transcription factor that, in response to mitochondrial dysfunction, upregulates mitochondrial gene expression (8). Therefore, in cells with high mitochondrial populations, such as renal TECs, PGC-1α activity is more abundant (110). PGC-1α activity is regulated by the mammalian target of rapamycin complex 1 (mTORC1), adenosine monophosphate-activated protein kinase (AMPK), and sirtuins, more specifically sirtuin 1 (Sirt 1) and sirtuin 6 (Sirt 6). mTORC1 is a serine/threonine kinase complex that mediates glycolytic metabolism. It induces PGC-1α activity and mitochondrial biogenesis through the activation of the transcriptional repressor yin and yang 1 (YY1) (21). On the contrary, AMPK and sirtuins, key regulators of oxidative metabolism, also have the ability to upregulate the activity of PGC-1α (41).
In some forms of AKI, especially in those associated with ischemic injury and sepsis, it has been observed that PGC-1α expression and activity are downregulated. It seems that this decrease in function may be attributed to the increased inflammatory cytokines (38, 111). Although it has not been demonstrated that PGC-1α deficiency causes damage, it seems that when associated with other metabolic factors it may induce some predisposition to cell injury (109, 110). Conversely, increased expression and activity of PGC-1α seem to be protective. For instance, in TECs in which PGC-1α activity was augmented, AKI severity was markedly decreased. Furthermore, these cells had a higher capacity of regeneration after injury (109). PGC-1α also participates in the production of NAD+ by mediating the de novo synthesis pathway. Although under normal circumstances PGC-1α plays an important role in this pathway, its absence may be overcome by repletion of NAD by administering nicotinamide. When NAD+ is repleted, risk and severity of AKI are decreased, even with low levels of PGC-1α (85). Based on this, AKI might be the result of direct or indirect correlations among PGC-1α shortfall, impaired adaptive mitochondrial biogenesis, and NAD+ metabolic alterations. However, it seems important to highlight that NAD+ seems to play a more significant role in AKI, especially when all the other mechanisms are compromised.
Metabolic Reprogramming in AKI
In various forms of illness, such as cancer, cells undergo metabolic reprogramming as an adaptive response to stress or injury. Metabolic reprogramming is a process in which the cell switches from using a high-effective oxidative metabolism (i.e., OXPHOS) to less-effective glycolytic metabolism pathways. Furthermore, this metabolic reprogramming is tightly correlated with the inflammatory response. This metabolic switch is also known as the “Warburg” effect (41). Experimental data suggest that a similar reprioritization of energy metabolism pathways may occur in AKI especially when associated with sepsis or ischemia (102, 117). A decrease in fuel substrates and oxygen utilization primarily caused by macro- and microvascular dysfunction in AKI “obliges” TECs to appeal to less-efficient energy production mechanisms in an attempt to survive. Although functionally speaking, the cell enters a state of “hibernation,” where only life-essential functions are being executed, and thus, a decline in organ function will be seen, one of the mechanisms the cell possesses to bear with stress and injury.
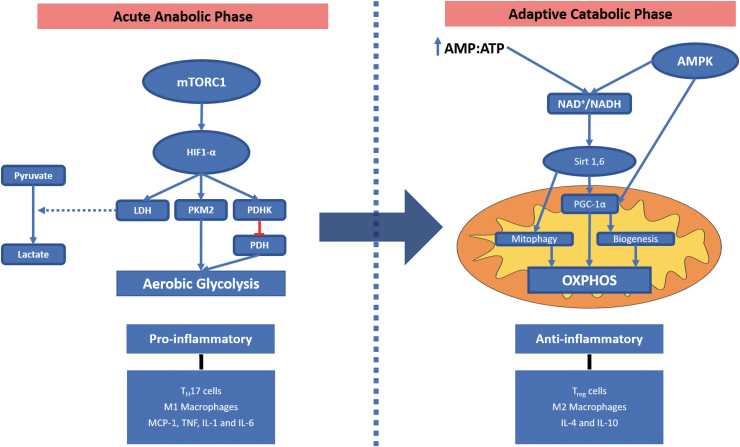
Metabolic reprogramming in TECs occurs in a biphasic manner: an early proinflammatory response phase, primarily mediated by aerobic glycolysis, followed by a late anti-inflammatory phase, in which OXPHOS predominance is re-established (Fig. 2) (36). Although for sake of understanding and clarity these two phases are often described as individual and independent processes, it is important to state that these two phases share significant overlap (41). In the early phase, TECs, which are normally in an oxidative metabolic state (i.e., OXPHOS), reprogram their metabolic line and deviate it to glycolysis (41). The Akt/mTORC1/hypoxia-inducible factor-1 alpha (HIF-1α) complex drives the induction of aerobic glycolysis by upregulating the gene expression of glycolytic enzymes such as lactate dehydrogenase, pyruvate kinase M2, and pyruvate dehydrogenase kinase (Fig. 2). The limited amounts of ATP produced through glycolysis are intended to maintain TEC viability by preserving critical cellular components (e.g., Na+/K+ ATPase pump) that will allow the cell to survive (114). In addition, decreased usage of oxidative metabolism is also accompanied by diminished production of ROS and less oxidative stress. However, with limited ATP supplies, “nonvital” functions such as protein synthesis and solute and ion transportation (e.g., decreased synthesis and function of ion transporters) are downregulated (8, 42, 50, 68, 83, 93). The usage of glycolysis also results in increased lactate levels due to the upregulated conversion of pyruvate into lactate promoted by HIF-1α (Fig. 2) (16, 17). When the AMP:ATP ratio starts to increase, the activity of AMPK is upregulated resulting in TEC metabolic switch back to oxidative metabolism. AMPK is an essential mediator for cellular metabolism by sensing energy catabolism playing a key role in multiple functions in the cell. AMPK promotes PGC-1α activity therefore stimulating mitochondrial biogenesis. Furthermore, it stimulates the synthesis of antioxidant reagents, activates autophagy and mitophagy, and more important re-establishes the utilization of OXPHOS and finally inhibits mTORC limiting glycolysis.
FIG. 2.
Metabolic reprogramming. In the early metabolic response to AKI, renal tubular epithelial cells undergo a proinflammatory phase (acute anabolic phase) metabolism in which the Akt/mTORC1/HIF-1α complex drives the induction of aerobic glycolysis by increasing the expression of glycolytic enzymes (e.g., LDH, PKM2, and PDHK). HIF-1α promotes the conversion of pyruvate to lactate and along with PDHK inhibits the conversion of lactate into acetyl-CoA hindering the induction of the Krebs cycle and decreasing OXPHOS. In the late anti-inflammatory (adaptive catabolic phase), OXPHOS metabolic pathways are re-established. This is driven by AMPK activation, Sirt1, and Sirt6. AMPK activates Sirt1 and Sirt6. Sirt6 will block the activity of HIF-1α switching back from aerobic glycolysis to OXPHOS and is induced by the decrease in ATP levels. AMPK activates PGC-1α and with CPT-1 will stimulate FA oxidation and oxidative metabolism. Furthermore, PGC-1α along with AMPK will induce mitochondrial biogenesis (31, 32, 34). AKI, acute kidney injury; AMPK, adenosine monophosphate-activated protein kinase; HIF-1α, hypoxia-inducible factor-1 alpha; LDH, lactate dehydrogenase; mTORC1, mammalian target of rapamycin complex 1; OXPHOS, oxidative phosphorylation; PDHK, pyruvate dehydrogenase kinase; PGC-1α, PPAR γ coactivator-1α; PKM2, pyruvate kinase M2; PPAR, peroxisome proliferator-activated receptor.
This metabolic reprogramming results in energy expenditure optimization (41, 83, 117) , refinement of fuel substrate utilization, and counteraction of proapoptotic triggers (41, 48, 100, 105). These changes explain to a certain extent the cellular structural and functional dissociation observed in AKI, in which organ function decline is not directly associated with cell death, supporting the premise that TECs prioritize energy expenditure and cell survival at the expense of cell function (41, 83, 117).
In preclinical and clinical studies of sepsis-induced AKI, it has been demonstrated that the switch from OXPHOS to glycolysis may be associated with deleterious effects on mortality. Also, it has been demonstrated that in sepsis-induced AKI, AMPK activation and stimulation of OXPHOS at early time points are associated with improved overall rates of survival (33, 52). In addition, Gomez et al. have shown some preliminary data in septic diabetic patients, in whom previous exposure to metformin, an AMPK activator, had lower mortality rates at 90 days when compared with other treatments (unpublished data). However, a dissociation between renal structure and function is constantly observed. In many of these studies although survival rates improve with the activation of AMPK, this is not directly associated with an improvement in renal function, suggesting that other mechanisms must be at play (33, 78, 123).
NAD+ and AKI
In the last two decades, NAD+ has gained special attention, as increased body of evidence has demonstrated that NAD+ deficiency is associated with increased risk to develop acute and CKDs (3, 14, 118, 122). NAD+ is a ubiquitous enzyme that plays a key role in several cellular metabolic functions, aging mechanisms, and adaptive responses to cellular stress or injury among others (35). NAD+ along with its reduced form (i.e., NADH) serves as an electron acceptor and as a donor in the ETC in the mitochondria, and constitutes the ATP synthesis rate-limiting substrates of FAO and glycolysis. In addition, NAD+ also participates as a substrate in many other nonredox essential cellular functions (e.g., sirtuins, poly-adenosine diphosphate-ribose polymerases [PARPs], and ectonucleotidases), mediates oxidative stress response, and regulates calcium signaling (34, 35, 85). Therefore, it is not surprising that any type of NAD+ deficiency, either due to decreased synthesis or increased consumption, may lead to mitochondrial function deterioration resulting in subsequent renal injury due to impaired ATP synthesis (82, 110).
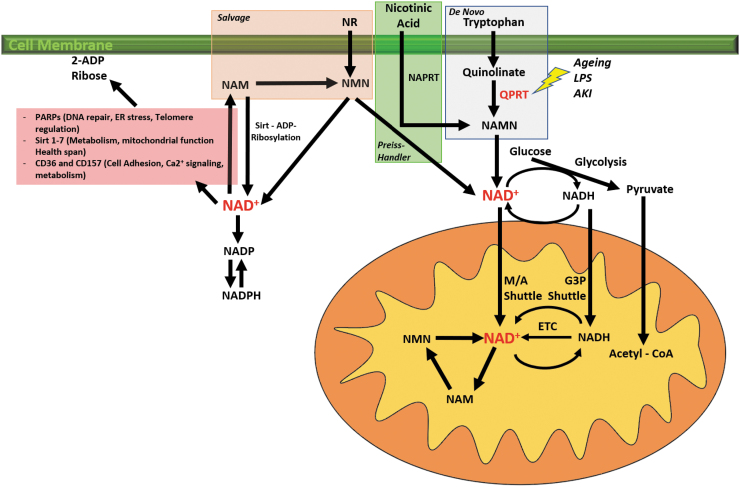
NAD+ intracellular concentrations, and therefore availability, are determined by a tightly regulated balance between consumption, salvage, and de novo synthesis. Alterations at any of these levels may lead to NAD+ deficiency, which has been associated with the development of a wide range of renal diseases (44). NAD+ can be synthesized de novo or recycled through several pathways in which a sort of host intrinsic and/or dietary precursors are necessary to execute these processes. Tryptophan, one of the essential amino acids, is the main precursor in the de novo NAD+ synthesis pathway. Tryptophan enters the cell and is converted to quinolinate, which is then converted into nicotinic acid mononucleotide (NAMN) by the action of quinolinate phosphoribosyl transferase (QPRT), the bottleneck enzyme of this process. Finally, NAMN is going to produce NAD+. QPRT deficiency results in NAD+ defective synthesis and depletion, which as discussed later in this chapter are associated with increased susceptibility to develop AKI (56, 85). Another important regulator in the de novo pathway is the mitochondrial biogenesis regulator PGC-1α. Tran and his group have described the PGC-1α ability to induce the de novo pathway, and how PGC-1α malfunctioning may result in impaired synthesis of NAD+ (82, 110).
NAD+ deficiency may result from different mechanisms such as dietary deficiency, genetic alterations of precursor's transporters, or cellular injury (44, 60). NAD+ depletion and impaired homeostasis may also manifest as diseases, especially affecting high-energy-consuming organs such as the brain, heart, and kidney (33, 35). In ischemic models of renal injury, it has been shown that ischemia induces the activation of NAD+ consuming enzymes such as PARPs and CD38 (30, 82). Also, in AKI, an accelerated consumption of NAD+ results due to the increased gene expression PARP (71). Furthermore, these same studies have demonstrated that impaired activity of the PAPR either by pharmacologically inhibition or in PARP1-knockout mice resulted in less severe findings of AKI (71, 89, 125).
Vitamin B3 and its different associated forms play a key role in the regulation of NAD+. For instance, nicotinamide one of the forms of vitamin B3 serves as a precursor of NAD+ in the salvage pathway (Fig. 3) (85). In experimental and clinical studies, administration of high concentrations of oral nicotinamide, much higher than the recommended nutritional dose, induces the salvage pathway resulting in increased levels of NAD+ regardless if other alternative pathways are compromised. Furthermore, in a recent study, Simic et al. (99) demonstrated that administration of nicotinamide riboside (NR) and pterostilbene to AKI patients resulted in increased total NAD+ concentrations, with no significant adverse events secondary to the administrations of the vitamin B3 precursors. Furthermore, some studies described it as the “most potent” therapy when it comes to replenishing NAD+ reserves (13, 108), making NR a safe and viable therapy with high feasibility of implementation (34).
FIG. 3.
NAD+ metabolism. NAD+ and its reduced form (NADH) are essential cofactors for most metabolic pathways, especially the oxidative metabolism. NAD+ and NADH redox cycling are necessary to produce energy in the form of ATP from fuel substrates such as glucose and FA through glycolysis and OXPHOS, respectively. NAD+ and NADH gain access to the mitochondria through the malate and aspartate shuttle and the glycerol-3-phosphate shuttle. NAD+ needs to be in constant regeneration and production to supply the energetic needs of the cell. Several NAD+ synthesis pathways have been described. De novo pathway: Dietary tryptophan is converted in quinolinate and then in NAMN by the QPRT. Preiss/Handler pathway: Nicotinic acid is converted to NM by NAPRT. Salvage pathway is the most important pathway by which NAD+ is produced. NR and NAM are converted into NMN that will be converted into NAD+ (94). 2-ADP ribose, 2-adenosine diphosphate ribose; ATP, adenosine triphosphate; ER, endoplasmic reticulum; G-3-P, glycerol 3 phosphate shuttle; LPS, lipopolysaccharide; M/A shuttle, malate/aspartate shuttle; NAD+, nicotinamide adenine dinucleotide; NAM, nicotinamide; NAMN, nicotinic acid mononucleotide; NAPRT, nicotinate phosphoribosyltransferase; NMN, nicotinamide mononucleotide; NR, nicotinamide riboside; QPRT, quinolinate phosphoribosyl transferase.
Other NAD+ roles have been extensively described especially those associated with aging. NAD+ serves as a cofactor to the sirtuin pathway and its deficiency is associated with shorter longevity and increased risk of degenerative diseases (9, 15, 35, 85). In addition, NAD+-dependent activation of Sirt-1 leads to Sirt1-mediated deacetylation and activation of PGC-1α stimulating mitochondrial biogenesis. NAD+ also plays a key role during the regenerative and repair mechanisms after injury, and cumulating evidence is showing that preserved NAD+ homeostasis is protective against damage and injury (89).
NAD+ and NADPH oxidases
ROS play critical roles in cellular physiology by regulating multiple biological processes and are involved in the pathological process of different types of AKI. As one of the major sources of ROS, NADPH oxidases (NOXs) produce many kinds of free radicals. ROS levels increase early in the pathological process and are predominantly localized in tubules with no capillary blood flow. NOX4 is the major renal isoform and highly expressed in the kidney, mostly in the highly metabolic proximal tubular compartment with lower levels in glomerulus (39). Furthermore, NOX4 expression is increased in a renal ischemic/reperfusion (I/R) injury model, a classical model mimicking human ischemic acute tubular necrosis that causes kidney injury and leads to programmed cell death (7, 73, 75, 124). Although evidence is clear that oxidative stress is the primary factor in AKI, NOX-derived superoxide is central to I/R- and cisplatin-induced AKI (72, 95), the functional role of NOX4 in AKI is still controversial.
Other Pathogenic Mechanisms in AKI
Inflammatory response
The inflammatory response is the host's main defense mechanism against infection. Without a coordinated and regulated inflammatory response, the host would not be able to fight and survive the invasion of external pathogens. However, in some cases, the host response to infection can be excessive or dysregulated causing more harm instead of benefit. In AKI, the inflammatory response plays a key role. It is the main mechanism by which the host eliminates the insult and initiates regeneration and healing processes. From a metabolic and phenotypical standpoint, the inflammatory response in sepsis-induced AKI can be described as biphasic: a proinflammatory phase, primarily mediated by glycolysis, followed by an anti-inflammatory phase mediated by oxidative metabolism.
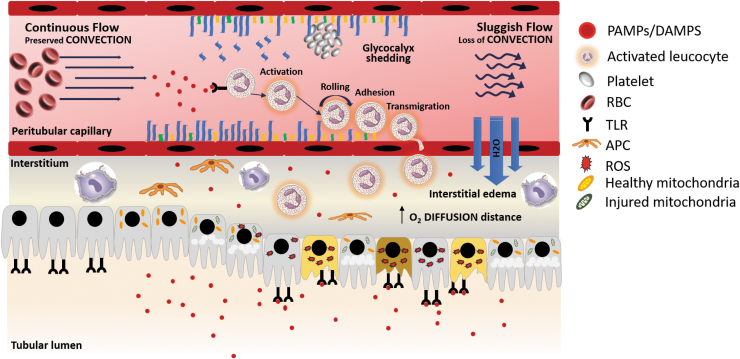
The inflammatory response is initiated when an infectious organism penetrates the physical barriers and invades the host. A series of molecules, present in most external pathogens, known as pathogen-associated molecular patterns (PAMPs) are recognized by immune cells (31, 51). These inflammatory cells express in their membranes and cytoplasm a series of receptors that bind in a nonspecific manner these patterns. These receptors are known as pattern recognition receptors and can be classified as membrane toll-like receptors (TLRs) or intracytoplasmic NOD-like receptors (31). Binding of PAMPs to these pattern recognition receptors triggers a downstream cascade of signals that will end activating the nuclear factor-kappa B (NF-κB), a transcription factor which main function is to upregulate the gene expression of inflammatory cytokines and recruit immune cells to the site of injury (76). However, these pattern recognition receptors are not unique to inflammatory cells. Pattern recognition receptors are also expressed by TECs (i.e., TLR-2 and TLR-4). The expression of these inflammatory mediator receptors outside the immune system might contribute and exacerbate the tubular inflammatory damage observed in AKI (40). Furthermore, tubular expression of TLRs increases the susceptibility of TECs to inflammatory injury. During sepsis-induced AKI, TECs are exposed to PAMPs on the apical and basolateral poles, suffering a “double hit” insult resulting in increased synthesis inflammatory mediators, production of ROS, oxidative stress, and cell injury (Fig. 4) (40, 83).
FIG. 4.
Inflammatory response and microcirculatory dysfunction in AKI. PAMPs and DAMPs are inflammatory mediators derived from bacteria and host immune cells, respectively. These inflammatory mediators bind to PRRs expressed on the surface of innate immune cells, endothelial cells, and renal TECs initiating a downstream cascade of signals that will increase the synthesis of proinflammatory cytokines, ROS, oxidative stress, and endothelial activation by nitric oxide and iNOS upregulation. During inflammation, DAMPs and PAMPs are filtered in the glomeruli. Once in the tubule these are going to bind the TLRs present in the apical membrane of the TECs. In addition, some evidence suggests that TECs are also exposed to the inflammatory mediators that are present in the peritubular circulation, although the mechanisms of how this occurs are not still clear. Moreover, the inflammatory response can also injure the TECs by increasing the oxidative stress and producing ROS. Microcirculatory dysfunction is the result of a series of events that lead to an impaired delivery of oxygen and nutrients to the tissue. Endothelial activation provoked by the inflammatory response results in a cascade of events that lead to shedding of the glycocalyx, increased leukocyte migration, and endothelial permeability. In addition, microcirculatory dysfunction is characterized by a heterogeneous flow, reduced number of capillaries with continuous flow with an associated increase of capillaries with sluggish flow, or no flow. Sluggish flow and no flow, result of the increased expression of adhesion molecules on the inflammatory and endothelial cells, facilitate the migration of neutrophils and macrophage to the interstitial space. Furthermore, the areas with sluggish flow have increased production of ROS and oxidative stress. Manifested by TEC apical vacuolization (31, 32, 34). DAMP, damage-associated molecular patterns; iNOS, inducible nitric oxide synthase; PAMP, pathogen-associated molecular pattern; PRRs, pattern recognition receptors; ROS, reactive oxygen species; TLRs, toll-like receptors.
Once the insult has been eliminated and the cell is switching back to oxidative metabolism and mitochondrial homeostasis is being restored, the anti-inflammatory phase of the immune response is initiated. This anti-inflammatory phase is characterized by the activation of regeneration and healing processes. However, persistent injury and organ dysfunction are associated when mitochondrial dysfunction persists (24, 37, 47, 54).
Microcirculatory dysfunction
Although the “global-ischemia” hypothesis of AKI has been discredited, many investigators have found evidence that alterations at the microcirculatory level might play a role in the development of organ injury (84). Evidence has shown that conditions such as sepsis, trauma, and major surgery are characterized by regional changes in microcirculatory flow. Importantly, in many instances, microcirculatory alterations are not correlated with changes at a macrohemodynamic level (22, 23, 45, 96, 107, 115). Multiple mechanisms have been proposed that may lead to microcirculatory dysfunction and explain somehow the dissociation of macro- and microcirculation. Increased renal blood flow with associated decline in renal function has been attributed to a simultaneous afferent arteriolar vasoconstriction, efferent arteriolar vasodilation, and intrarenal shunting (12, 64, 66). Moreover, endothelial injury and sympathetic autonomic nervous system response to the systemic vasodilation and decreased vascular resistance that occurs in sepsis-AKI may also explain these changes. The secretion of catecholamines, activation of the renin/angiotensin system, and vasopressin may lead to a redistribution of the renal blood flow from the already hypoperfused medulla to the cortex (61, 84). This ischemic response is worsened by the decreased synthesis of prostaglandins associated with mitochondrial dysfunction due to ATP deficiency (20, 104, 110). Furthermore, renal endothelial injury impairs the activity of nitric oxide synthase, impairing the vasoconstriction counteracting effect of this molecule (106). Other mechanisms such as shedding of the glycocalyx and activation of the coagulation cascade also participate in this process resulting in increased leukocyte and platelet rolling and adhesion, reduction in blood flow velocity, and microthrombus formation (Fig. 4) (23, 83, 116). As a consequence of these pathophysiologic changes, TECs may be exposed to an increased inflammatory burden as slow moving leukocytes and platelets release damage-associated molecular patterns and PAMPs in the vicinity of TECs. More importantly, microcirculatory dysfunction can lead to altered regional blood flow distribution potentially resulting in patchy areas of ischemia and loss of autoregulation, aggravating TEC injury and dysfunction (2, 29, 84, 88). Loss of glomerular and tubular function mainly manifests as an increase in creatinine levels and decrease in urine output. These changes are usually attributed to macrohemodynamic alterations, but several studies have also demonstrated that these can also be a consequence of impaired microcirculatory hemodynamics (84). Under normal physiologic states, the GFR is tightly controlled by a series of renal regulatory mechanisms, whose main objective is to maintain a constant GFR over a wide range of blood pressures. This regulation is possible by dilation and contraction of the renal afferent and efferent arterioles. However, in AKI, these regulatory mechanisms fail and GFR control is impaired. Vasoconstriction of the afferent arteriole with associated dilation of the efferent arteriole decreases the glomerular hydrostatic pressure and decreases urine output. In addition, constriction of the afferent arteriole results in glomerular shunting and a heterogeneous redistribution of flow (12, 70, 84, 86, 101).
Cellular Regeneration Versus Fibrosis
A more in-depth and a better understanding of AKI recovery and repair is now available (43, 122–125). This increased understanding of the pathophysiological mechanisms of AKI has allowed researchers to propose novel therapeutic targets for renal diseases, especially AKI. Furthermore, with the available evidence it is clearer, that the regeneration and repair processes play a major role during AKI, and that a coordinated and regulated response of the elements that participate in these mechanisms is crucial to achieve complete recovery of renal function following injury. AKI is associated with an increased risk of progression to CKD. Following removal of the insult, renal injury can progress to either complete and adequate cellular regeneration and functional recovery, or development of fibrosis and subsequent evolution to CKD. Progression to any of these two pathways is determined by several factors such as type and severity of injury, cellular signaling mechanisms, age, previous episodes of AKI, and renal reserve among others. For example, patients with lower renal reserve (e.g., CKD, congenital heart diseases, or previous episodes of AKI) are more prone to progress to fibrosis and ESRD. However, despite improved understanding of this biology and advances in identifying novel biomarkers that better forecast the development of AKI, predicting whether repair programs after injury will result in renal recovery or fibrosis remains challenging in the absence of suitable biomarkers.
The mechanisms that mediate kidney repair and regeneration are very complex and tightly regulated, and in many ways differ from that of other organs. Mitochondrial homeostasis, monocytes/macrophages, and regulators of the cell cycle play a major role in this process. Coordination among these elements is required to achieve a successful recovery and restoration of renal function. Much of our current understanding of the pathobiology of AKI has been extrapolated from studies done in nonhuman model systems such as the zebrafish model of AKI. Zebrafish renal development, physiology, and pathophysiologic mechanisms of AKI resemble the ones observed in mammalian models of AKI, making them a good model to study AKI (28a, 122a). From these studies it is known that under normal conditions, TECs are in the G0 phase of the cell cycle (stable cells). Following injury, TECs undergo metabolic reprogramming by which various embryologic pathways are reactivated inducing a process of dedifferentiation into a less differentiated progenitor state that will allow them to re-enter the cell cycle and give rise to newer functional cells. Through this mechanism, TECs are able to restore the tubulo-epithelial cell population (50a, 101a). Enhancing of TEC proliferation has been proposed as a possible target for therapy, and studies have shown promising results that could eventually be translated to the clinical setting. For example, Brilli Skvarca et al. (11a) demonstrated that treatment with 4-(phenylthio) butanoic acid (PTBA), a histone deacetylase inhibitor, an enhancer of renal recovery, resulted in increased TEC proliferation and reduced renal fibrosis by inducing reactivation of the Pax2a gene (renal progenitor gene). Furthermore, this resulted in less expression of renal tubular injury biomarkers such as kidney injury molecule-1 (KIM-1), and less leukocyte infiltration especially macrophages. Evidence has also demonstrated that this process is regulated by inflammatory mediators such as TLR-4 and IL-22 (3a, 60a). In preclinical models, downregulation of IL-22 activity is associated with impaired regeneration and progression to fibrosis.
Clinical Strategies for AKI
Translating this new understanding of AKI pathobiology into the clinical realm will require careful characterization of the AKI phenotype both in terms of etiology and also time-course. In many of the most common forms of AKI (e.g., sepsis, nephrotoxic), patients present with AKI already present. Therefore, prophylactic strategies are not possible. Furthermore, the course of injury may be protracted limiting the chances to stimulate repair. Interventions that, for example, force cells into cell cycle will need to be carefully timed so that they do not force cells that are injured since such therapy would be detrimental. Whether the increasing use of biomarkers (55a, 59a) to characterize the clinical course of AKI will allow for the rationale use of therapies remains to be seen. However, there is strong evidence that even in conditions such as sepsis, recovery of the kidney can happen, and when it does prognosis improves dramatically.
NAD+ As a Drug Target for AKI
The bulk of renal NAD+ research reported to date has used models of cellular tubule biology. Cellular stress associated with different AKI syndromes triggers accelerated consumption of NAD+. Emerging evidence suggests that interindividual differences, both at risk of AKI and further disease outcomes following AKI, may relate to their differing ability to compensate the stress-associated consumption of tubular NAD+ with increased NAD+ biosynthesis (92, 94).
A shortfall of NAD+ has been identified in the pathobiology of several diseases, including AKI. In mice with AKI, the bottleneck enzyme in the de novo biosynthesis pathway, QPRT, was shown to maintain renal NAD+ and mediate resistance to AKI (Fig. 2) (7). When QPRT is deficient or depleted, an increased risk to develop AKI is observed (7). However, the deleterious effects of QPRT and subsequent NAD+ can be restrained by the stimulation of other NAD+ synthesis pathways by administering nicotinamide, and NAD+ precursor (7, 88). Mehr and colleagues also conducted a small clinical trial with oral nicotinamide, one of the physiological precursors of NAD+ biosynthesis, in patients undergoing cardiac surgery and found a dose-related increase in NAD+ metabolites and lower rates of AKI (7, 85). Metabolomics suggested an elevated urinary quinolinate/tryptophan ratio (uQ/T) as an indicator of reduced QPRT. Furthermore, this same group showed that increased uQ/T may work as a biomarker to predict AKI in patients. This is important as it directly identifies patients who are most likely to respond to this specific treatment—that is, enrichment and subclassification. Niacinamide, a precursor of vitamin B3, has also been proposed as potential therapy to replenish NAD+. In a recent study published by Parikh and colleagues (87), patients who developed COVID-19 AKI who received therapy supplementation with niacinamide presented lower rates of renal replacement therapy and death. Furthermore, an improvement in recovery was also observed.
Different approaches to replenishing NAD+ levels in the aged kidney or after experimental injuries mimicking AKI have been tested in preclinical rodent models (Fig. 5). For example, supplementation with nicotinamide mononucleotide (NMN), another NAD+ precursor, restored renal Sirt1 activity and NAD+ content in 20-month-old mice and further increased both in 3-month-old mice. Moreover, supplementation with NMN significantly protected mice in both age groups from cisplatin-induced AKI (42a). This study shows that the intraperitoneal injection of NMN boosts NAD+ levels in both young and aged kidneys, which was also associated with increased Sirt1 activity, and protection of the kidneys from both cisplatin- and ischemia/reperfusion-induced AKI, indicating that NMN rescued AKI by activating Sirt1. Importantly, the protective effect of NMN supplementation was substantially blunted in Sirt1-deficient mice, indicating that the protective effect of NMN occurs via Sirt1 activity (42a).
FIG. 5.
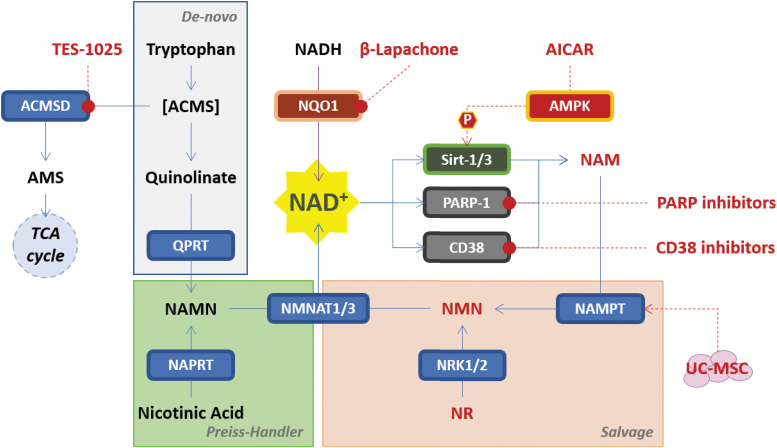
NAD+ as a drug target for AKI. Shown are different NAD+ replenishment approaches reported in preclinical models of AKI. ACMS, α-amino-β-carboxymuconate-6-semialdehyde; ACMSD, α-amino-β-carboxymuconate-6-semialdehyde decarboxylase; AICAR, 5-aminoimidazole-4-carboxamide ribonucleotide; AMS, α-amino-muconate-6-semialdehyde; CD38, ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1; NADH, nicotinamide adenine dinucleotide hydrogen; NAMPT, nicotinamide phosphoribosyl transferase; NAPRT, nicotinate phosphoribosyl transferase; NMNAT1/3, nicotinamide/nicotinic acid mononucleotide adenylyl transferase 1, 2 and 3; NQO1, NAD(P)H dehydrogenase quinone 1; NRK1/2, nicotinamide riboside kinase 1 and 2; P, phosphate; PARP-1, poly (ADP-ribose) polymerase 1; Sirt-1/3, sirtuin-1 and 3; UC-MSC, umbilical cord-derived mesenchymal stromal cells.
In the framework of the NAD+ biosynthesis, a novel approach to increase NAD+ level has been the inhibition of α-amino-β-carboxymuconate-6-semialdehyde decarboxylase (ACMSD). ACMSD is an enzyme synthesized in the kidney and in the liver that belongs to the de-novo NAD+ biosynthesis pathway. ACMSD inhibition resulted in increased cellular NAD+ levels and promoted mitochondrial homeostasis and function, enhancing sirtuins activity and protecting against AKI (and liver injury) (88). Katsyuba and colleagues have also characterized several novel inhibitors of ACMSD, including TES-1025, which shows preferential distribution to the kidney in mouse PK studies and demonstrates protective efficacy in the preventive treatment of ischemia/reperfusion- and cisplatin-induced AKI murine models (56). Physiological expression of ACMSD is largely restricted to the kidney and liver, and the recent study of Liu L. et al. (65b) identified the liver as the main site at which NAD+ is converted from tryptophan, by the de-novo NAD+ biosynthesis pathway, with the NAD+ produced released into the bloodstream to reach other tissues. These ACMSD inhibitors may thus be a promising therapeutic potential to prevent AKI (88).
The pharmacological inhibition of poly (adenosine-diphosphate-ribose) polymerase 1 (PARP-1), one of the principal NAD+ consuming enzymes, was reported to protect the kidney in various preclinical models of AKI, ischemia/reperfusion-, cisplatin-induced, or septic-induced AKI rodent models, rescuing NAD+ levels and improving mitochondrial fitness (15a, 65a, 73, 93). Another consuming NAD+ enzyme, CD38, was also reported to protect rodents from septic-induced AKI. In a mouse model with lipopolysaccharide (LPS)-induced AKI, blocking CD38 with quercetin could significantly relieve kidney dysfunction, kidney pathological changes, as well as inflammatory cell accumulation. Similar to those in the cultured cells, quercetin could inhibit macrophage M1 polarization and NF-κB signaling activation in macrophages from kidneys and spleens in mice after LPS injection (98a).
An alternative approach to increase the NAD+ level reported to protect the kidney from cisplatin-induced injury in a mouse model was treatment with a NAD(P)H dehydrogenase quinone 1 (NQO1) activator, β-lapachone. The intracellular NAD+/NADH ratio in renal tissues was significantly increased in wild-type mice cotreated with cisplatin and β-lapachone compared with the ratio in mice treated with cisplatin alone. Inflammatory cytokines and biochemical markers for renal damage were significantly attenuated by β-lapachone cotreatment compared with those in the cisplatin-alone group.
Finally, other “indirect” approaches have been reported to improve mitochondrial homeostasis and sirtuin activity by regulation of expression of nicotinamide phosphoribosyl transferase (NAMPT), one of the enzymes belonging to the NAD+ “salvage pathway,” rescuing the metabolite nicotinamide important in NMN for the regeneration of NAD+. One approach is based on AICAR, an AMPK activator, demonstrating protective effect in ischemia/reperfusion- and cisplatin-induced rat models of AKI (33, 64a, 77). The second is a cell-based approach exploiting the paracrine effect of human umbilical cord-derived mesenchymal stromal cells (UC-MSC), able to remodulate the transcription induction of several genes involved in mitochondrial functions and energy supply of tubular renal cells. The transplantation of UC-MSC in a cisplatin-induced AKI mouse model induced a global metabolic reprogramming of damaged tubular cells, with an increase of NAD+ content driven by NAMPT expression and Sirt-3 activity (83a).
Future Directions and Conclusions
The development of newly targeted protective and therapeutic strategies for AKI is urgently needed. It is now clear that targeting healing and regenerative processes by restoring mitochondrial homeostasis and NAD+ is a viable strategy to prevent and/or reduce severity of AKI, and furthermore to avoid maladaptive repair processes and progression to fibrosis and CKD. Although important advances have been made in translating experimental findings into the clinical setting, larger studies are needed to confirm the viability of these therapies. In addition, the exact mechanisms by which mitochondrial dysfunction and NAD+ deficiency provoke AKI are still unclear, and more experimental studies are needed to better understand the pathogenesis, and also to propose alternative therapeutic targets.
Abbreviations Used
- 2-ADP ribose
2-adenosine diphosphate ribose
- ACMS
α-amino-β-carboxymuconate-6-semialdehyde
- ACMSD
α-amino-β-carboxymuconate-6-semialdehyde decarboxylase
- ADP
adenosine-diphosphate
- AICAR
5-aminoimidazole-4-carboxamide ribonucleotide
- AKI
acute kidney injury
- AMPK
adenosine monophosphate-activated protein kinase
- AMS
α-amino-muconate-6-semialdehyde
- ATP
adenosine triphosphate
- CACT
carnitine-acylcarnitine translocase
- CKD
chronic kidney disease
- CPT-1
carnitine palmytoiltransferase-1
- CPT-2
carnitine palmytoiltransferase-2
- DAMP
damage-associated molecular pattern
- ER
endoplasmic reticulum
- ETC
electron transport chain
- FA
fatty acids
- FADH2
flavin adenine dinucleotide
- FAO
fatty acid oxidation
- GFR
glomerular filtration rate
- HIF-1α
hypoxia-inducible factor-1 alpha
- I/R
ischemic/reperfusion
- iNOS
inducible nitric oxide synthase
- KDIGO
Kidney Disease: Improving Global Outcomes
- KIM-1
kidney injury molecule-1
- LDH
lactate dehydrogenase
- LPS
lipopolysaccharide
- M/A shuttle
malate/aspartate shuttle
- miR
micro-RNA
- mTORC1
mammalian target of rapamycin complex 1
- NAD+
nicotinamide adenine dinucleotide
- NADH
nicotinamide adenine dinucleotide hydrogen
- NAM
nicotinamide
- NAMN
nicotinic acid mononucleotide
- NAMPT
nicotinamide phosphoribosyl transferase
- NAPRT
nicotinate phosphoribosyl transferase
- NF-κB
nuclear factor-kappa B
- NMN
nicotinamide mononucleotide
- NMNAT1/3
nicotinamide/nicotinic acid mononucleotide adenylyl transferase 1, 2 and 3
- NOXs
NADPH oxidases
- NQO1
NAD(P)H dehydrogenase quinone 1
- NR
nicotinamide riboside
- NRK1/2
nicotinamide riboside kinase 1 and 2
- OXPHOS
oxidative phosphorylation
- PAMP
pathogen-associated molecular pattern
- PARP-1
poly (ADP-ribose) polymerase 1
- PDHK
pyruvate dehydrogenase kinase
- PGC-1α
peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- PGE2
prostaglandin E2
- PKM2
pyruvate kinase M2
- PPAR
peroxisome proliferator-activated receptor
- PRRs
pattern recognition receptors
- PTBA
4-(phenylthio) butanoic acid
- PTCs
proximal tubular cells
- QPRT
quinolinate phosphoribosyl transferase
- ROS
reactive oxygen species
- Sirt 1
sirtuin 1
- Sirt 6
sirtuin 6
- TCA
tricarboxylic acid cycle
- TECs
tubular epithelial cells
- TFAM
transcriptional factor A, mitochondrial
- TLR
toll-like receptor
- UC-MSC
umbilical cord-derived mesenchymal stromal cells
- uQ/T
urinary quinolinate/tryptophan ratio
- YY1
yin and yang 1
Authors' Contributions
All authors contributed to the work. The concept for the article was developed by J.A.K., and C.L.M-C. drafted the text with revisions from the remaining authors.
Author Disclosure Statement
J.A.K. has received consulting fees from TES Pharma and Astellas; H.G. received grant support from TES Pharma; and F.D.F., N.G., and R.P. are employees of TES Pharma.
Funding Information
This work was supported, in part, by the National Institutes of Health (NIH) Grant 1K08GM117310-01and the University of Pittsburgh School of Medicine Dean's Faculty Advancement Award.
References
- 1. Al-Jaghbeer M, Dealmeida D, Bilderback A, Ambrosino R, and Kellum JA. Clinical decision support for in-hospital AKI. J Am Soc Nephrol 29: 654–660, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Almac E, Siegemund M, Demirci C, and Ince C. Microcirculatory recruitment maneuvers correct tissue CO2 abnormalities in sepsis. Minerva Anestesiol 72: 507–519, 2006. [PubMed] [Google Scholar]
- 3. Anderson RM, Bitterman KJ, Wood JG, Medvedik O, and Sinclair DA. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature 423: 181–185, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a. Anders HJ and Schaefer L.. Beyond tissue injury-damage-associated molecular patterns, tolllike receptors, and inflammasomes also drive regeneration and fibrosis. J Am Soc Nephrol 25: 1387–1400, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arany Z, Novikov M, Chin S, Ma Y, Rosenzweig A, and Spiegelman BM. Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-gamma coactivator 1alpha. Proc Natl Acad Sci U S A 103: 10086–10091, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bartz RR, Fu P, Suliman HB, Crowley SD, MacGarvey NC, Welty-Wolf K, and Piantadosi CA. Staphylococcus aureus sepsis induces early renal mitochondrial DNA repair and mitochondrial biogenesis in mice. PLoS One 9: e100912, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, and Acute Dialysis Quality Initiative Workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8: R204–R212, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ben Mkaddem S, Pedruzzi E, Werts C, Coant N, Bens M, Cluzeaud F, Goujon JM, Ogier-Denis E, and Vandewalle A. Heat shock protein gp96 and NAD(P)H oxidase 4 play key roles in Toll-like receptor 4-activated apoptosis during renal ischemia/reperfusion injury. Cell Death Differ 17: 1474–1485, 2010. [DOI] [PubMed] [Google Scholar]
- 8. Bhargava P and Schnellmann RG. Mitochondrial energetics in the kidney. Nat Rev Nephrol 13: 629–646, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bonkowski MS and Sinclair DA. Slowing ageing by design: the rise of NAD(+) and sirtuin-activating compounds. Nat Rev Mol Cell Biol 17: 679–690, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brenner M, Schaer GL, Mallory DL, Suffredini AF, and Parrillo JE. Detection of renal blood flow abnormalities in septic and critically ill patients using a newly designed indwelling thermodilution renal vein catheter. Chest 98: 170–179, 1990. [DOI] [PubMed] [Google Scholar]
- 11. Brezis M and Rosen S. Hypoxia of the renal medulla—its implications for disease. N Engl J Med 332: 647–655, 1995. [DOI] [PubMed] [Google Scholar]
- 11a. Brilli Skvarca L, Han HI, Espiritu EB, Missinato MA, Rochon ER, McDaniels MD, Bais AS, Roman BL, Waxman JS, Watkins SC, Davidson AJ, Tsang M, and Hukriede NA. Enhancing regeneration after acute kidney injury by promoting cellular dedifferentiation in zebrafish. Dis Models Mech 12: dmm037390, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Calzavacca P, Evans RG, Bailey M, Bellomo R, and May CN. Cortical and medullary tissue perfusion and oxygenation in experimental septic acute kidney injury. Crit Care Med 43: e431–e439, 2015. [DOI] [PubMed] [Google Scholar]
- 13. Cantó C and Auwerx J. Targeting sirtuin 1 to improve metabolism: all you need is NAD(+)? Pharmacol Rev 64: 166–187, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cantó C, Houtkooper Riekelt H, Pirinen E, Youn Dou Y, Oosterveer Maaike H, Cen Y, Fernandez-Marcos Pablo J, Yamamoto H, Andreux Pénélope A, Cettour-Rose P, Gademann K, Rinsch C, Schoonjans K, Sauve Anthony A, and Auwerx J. The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab 15: 838–847, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cantó C, Menzies KJ, and Auwerx J. NAD(+) metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab 22: 31–53, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15a. Chatterjee PK, Zacharowski K, Cuzzocrea S, Otto M, and Thiemermann C. Inhibitors of poly (ADP-ribose) synthetase reduce renal ischemia-reperfusion injury in the anesthetized rat in vivo. Faseb j 14: 641–651, 2000. [DOI] [PubMed] [Google Scholar]
- 16. Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, Giamarellos-Bourboulis EJ, Martens JH, Rao NA, Aghajanirefah A, Manjeri GR, Li Y, Ifrim DC, Arts RJ, van der Veer BM, Deen PM, Logie C, O'Neill LA, Willems P, van de Veerdonk FL, van der Meer JW, Ng A, Joosten LA, Wijmenga C, Stunnenberg HG, Xavier RJ, and Netea MG. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345: 1250684, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cheng SC, Scicluna BP, Arts RJ, Gresnigt MS, Lachmandas E, Giamarellos-Bourboulis EJ, Kox M, Manjeri GR, Wagenaars JA, Cremer OL, Leentjens J, van der Meer AJ, van de Veerdonk FL, Bonten MJ, Schultz MJ, Willems PH, Pickkers P, Joosten LA, van der Poll T, and Netea MG. Broad defects in the energy metabolism of leukocytes underlie immunoparalysis in sepsis. Nat Immunol 17: 406–413, 2016. [DOI] [PubMed] [Google Scholar]
- 18. Chua HR, Glassford N, and Bellomo R. Acute kidney injury after cardiac arrest. Resuscitation 83: 721–727, 2012. [DOI] [PubMed] [Google Scholar]
- 19. Clark AJ and Parikh SM. Mitochondrial metabolism in acute kidney injury. Semin Nephrol 40: 101–113, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cumming AD, Driedger AA, McDonald JW, Lindsay RM, Solez K, and Linton AL. Vasoactive hormones in the renal response to systemic sepsis. Am J Kidney Dis 11: 23–32, 1988. [DOI] [PubMed] [Google Scholar]
- 21. Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, and Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature 450: 736–740, 2007. [DOI] [PubMed] [Google Scholar]
- 22. De Backer D, Creteur J, Preiser JC, Dubois MJ, and Vincent JL. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med 166: 98–104, 2002. [DOI] [PubMed] [Google Scholar]
- 23. De Backer D, Donadello K, Taccone FS, Ospina-Tascon G, Salgado D, and Vincent J-L. Microcirculatory alterations: potential mechanisms and implications for therapy. Ann Intensive Care 1: 27, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dellepiane S, Marengo M, and Cantaluppi V. Detrimental cross-talk between sepsis and acute kidney injury: new pathogenic mechanisms, early biomarkers and targeted therapies. Crit Care 20: 61, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Di Giantomasso D, Bellomo R, and May CN. The haemodynamic and metabolic effects of epinephrine in experimental hyperdynamic septic shock. Intensive Care Med 31: 454–462, 2005. [DOI] [PubMed] [Google Scholar]
- 26. Di Giantomasso D, May CN, and Bellomo R. Norepinephrine and vital organ blood flow during experimental hyperdynamic sepsis. Intensive Care Med 29: 1774–1781, 2003. [DOI] [PubMed] [Google Scholar]
- 27. Di Giantomasso D, May CN, and Bellomo R. Vital organ blood flow during hyperdynamic sepsis. Chest 124: 1053–1059, 2003. [DOI] [PubMed] [Google Scholar]
- 28. Dimmer KS and Scorrano L. (De)constructing mitochondria: what for? Physiology (Bethesda) 21: 233–241, 2006. [DOI] [PubMed] [Google Scholar]
- 28a. Drummond IA. Kidney development and disease in the zebrafish. J Am Soc Nephrol 16: 299–304, 2005. [DOI] [PubMed] [Google Scholar]
- 29. Dyson A, Bezemer R, Legrand M, Balestra G, Singer M, and Ince C. Microvascular and interstitial oxygen tension in the renal cortex and medulla studied in a 4-h rat model of LPS-induced endotoxemia. Shock 36: 83–89, 2011. [DOI] [PubMed] [Google Scholar]
- 30. Ebrahimkhani MR, Daneshmand A, Mazumder A, Allocca M, Calvo JA, Abolhassani N, Jhun I, Muthupalani S, Ayata C, and Samson LD. Aag-initiated base excision repair promotes ischemia reperfusion injury in liver, brain, and kidney. Proc Natl Acad Sci U S A 111: E4878–E4886, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Emlet DR, Gomez H, and Kellum JA. Chapter 21—Pathogen-associated molecular patterns, damage-associated molecular patterns, and their receptors in acute kidney injury. In: Critical Care Nephrology (Third Edition), edited by Ronco C, Bellomo R, Kellum JA, and Ricci Z. Philadelphia, PA: Content Repository Only!, 2019, pp. 121–127.e123. [Google Scholar]
- 32. Emma F, Montini G, Parikh SM, and Salviati L. Mitochondrial dysfunction in inherited renal disease and acute kidney injury. Nat Rev Nephrol 12: 267–280, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Escobar DA, Botero-Quintero AM, Kautza BC, Luciano J, Loughran P, Darwiche S, Rosengart MR, Zuckerbraun BS, and Gomez H. Adenosine monophosphate-activated protein kinase activation protects against sepsis-induced organ injury and inflammation. J Surg Res 194: 262–272, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Faivre A, Katsyuba E, Verissimo T, Lindenmeyer M, Rajaram RD, Naesens M, Heckenmeyer C, Mottis A, Feraille E, Cippà P, Cohen C, Longchamp A, Allagnat F, Rutkowski JM, Legouis D, Auwerx J, and de Seigneux S. Differential role of nicotinamide adenine dinucleotide deficiency in acute and chronic kidney disease. Nephrol Dial Transplant 36: 60–68, 2020. [DOI] [PubMed] [Google Scholar]
- 35. Fang EF, Lautrup S, Hou Y, Demarest TG, Croteau DL, Mattson MP, and Bohr VA. NAD(+) in aging: molecular mechanisms and translational implications. Trends Mol Med 23: 899–916, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, Elstrom RL, June CH, and Thompson CB. The CD28 signaling pathway regulates glucose metabolism. Immunity 16: 769–777, 2002. [DOI] [PubMed] [Google Scholar]
- 37. Fry DE. Sepsis, systemic inflammatory response, and multiple organ dysfunction: the mystery continues. Am Surg 78: 1–8, 2012. [PubMed] [Google Scholar]
- 38. Funk JA and Schnellmann RG. Persistent disruption of mitochondrial homeostasis after acute kidney injury. Am J Physiol Renal Physiol 302: F853–F864, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Geiszt M, Kopp JB, Várnai P, and Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci U S A 97: 8010–8014, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gomez H, Ince C, De Backer D, Pickkers P, Payen D, Hotchkiss J, and Kellum JA. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock 41: 3–11, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gomez H, Kellum JA, and Ronco C. Metabolic reprogramming and tolerance during sepsis-induced AKI. Nat Rev Nephrol 13: 143–151, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Good DW, George T, and Watts BA, 3rd. Lipopolysaccharide directly alters renal tubule transport through distinct TLR4-dependent pathways in basolateral and apical membranes. Am J Physiol Renal Physiol 297: F866–F874, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42a. Guan Y, Wang S-R, Huang X-Z, Xie Q-h, Xu Y-Y, Shang D, and Hao C-M. Nicotinamide mononucleotide, an NAD+ precursor, rescues age-associated susceptibility to AKI in a sirtuin 1–dependent manner. J Am Soc Nephrol 28: 2337–2352, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guo Y, Ni J, Chen S, Bai M, Lin J, Ding G, Zhang Y, Sun P, Jia Z, Huang S, Yang L, and Zhang A. MicroRNA-709 mediates acute tubular injury through effects on mitochondrial function. J Am Soc Nephrol 29: 449–461, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hershberger KA, Martin AS, and Hirschey MD. Role of NAD+ and mitochondrial sirtuins in cardiac and renal diseases. Nat Rev Nephrol 13: 213–225, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Holthoff JH, Wang Z, Seely KA, Gokden N, and Mayeux PR. Resveratrol improves renal microcirculation, protects the tubular epithelium, and prolongs survival in a mouse model of sepsis-induced acute kidney injury. Kidney Int 81: 370–378, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, Edipidis K, Forni LG, Gomersall CD, Govil D, Honore PM, Joannes-Boyau O, Joannidis M, Korhonen AM, Lavrentieva A, Mehta RL, Palevsky P, Roessler E, Ronco C, Uchino S, Vazquez JA, Vidal Andrade E, Webb S, and Kellum JA. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 41: 1411–1423, 2015. [DOI] [PubMed] [Google Scholar]
- 47. Hotchkiss RS and Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 348: 138–150, 2003. [DOI] [PubMed] [Google Scholar]
- 48. Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, and Karl IE. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med 27: 1230–1251, 1999. [DOI] [PubMed] [Google Scholar]
- 49. Houten SM, Violante S, Ventura FV, and Wanders RJ. The biochemistry and physiology of mitochondrial fatty acid β-oxidation and its genetic disorders. Annu Rev Physiol 78: 23–44, 2016. [DOI] [PubMed] [Google Scholar]
- 50. Hsiao HW, Tsai KL, Wang LF, Chen YH, Chiang PC, Chuang SM, and Hsu C. The decline of autophagy contributes to proximal tubular dysfunction during sepsis. Shock 37: 289–296, 2012. [DOI] [PubMed] [Google Scholar]
- 50a. Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, and Bonventre JV. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2: 284–291, 2008. [DOI] [PubMed] [Google Scholar]
- 51. Jang HR and Rabb H. Immune cells in experimental acute kidney injury. Nat Rev Nephrol 11: 88–101, 2015. [DOI] [PubMed] [Google Scholar]
- 52. Jin K, Ma Y, Manrique-Caballero CL, Li H, Emlet DR, Li S, Baty CJ, Wen X, Kim-Campbell N, Frank A, Menchikova EV, Pastor-Soler NM, Hallows KR, Jackson EK, Shiva S, Pinsky MR, Zuckerbraun BS, Kellum JA, and Gomez H. Activation of AMP-activated protein kinase during sepsis/inflammation improves survival by preserving cellular metabolic fitness. FASEB J 34: 7036–7057, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL, and AWARE Investigators. Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med 376: 11–20, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kalakeche R, Hato T, Rhodes G, Dunn KW, El-Achkar TM, Plotkin Z, Sandoval RM, and Dagher PC. Endotoxin uptake by S1 proximal tubular segment causes oxidative stress in the downstream S2 segment. J Am Soc Nephrol 22: 1505–1516, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kang HM, Ahn SH, Choi P, Ko Y-A, Han SH, Chinga F, Park ASD, Tao J, Sharma K, Pullman J, Bottinger EP, Goldberg IJ, and Susztak K. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med 21: 37–46, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55a. Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, Bihorac A, Birkhahn R, Cely CM, Chawla LS, Davison DL, Feldkamp T, Forni LG, Gong MN, Gunnerson KJ, Haase M, Hackett J, Honore PM, Hoste EA, Joannes-Boyau O, Joannidis M, Kim P, Koyner JL, Laskowitz DT, Lissauer ME, Marx G, McCullough PA, Mullaney S, Ostermann M, Rimmele T, Shapiro NI, Shaw AD, Shi J, Sprague AM, Vincent JL, Vinsonneau C, Wagner L, Walker MG, Wilkerson RG, Zacharowski K, and Kellum JA. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 17: R25, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Katsyuba E, Mottis A, Zietak M, De Franco F, van der Velpen V, Gariani K, Ryu D, Cialabrini L, Matilainen O, Liscio P, Giacche N, Stokar-Regenscheit N, Legouis D, de Seigneux S, Ivanisevic J, Raffaelli N, Schoonjans K, Pellicciari R, and Auwerx J. De novo NAD(+) synthesis enhances mitochondrial function and improves health. Nature 563: 354–359, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kellum JA. Why are patients still getting and dying from acute kidney injury? Curr Opin Crit Care 22: 513–519, 2016. [DOI] [PubMed] [Google Scholar]
- 58. Kellum JA, Lameire N, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL, Herzog CA, Joannidis M, Kribben A, Levey AS, MacLeod AM, Mehta RL, Murray PT, Naicker S, Opal SM, Schaefer F, Schetz M, and Uchino S. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2: 1–138, 2012. [Google Scholar]
- 59. Kellum JA, Sileanu FE, Murugan R, Lucko N, Shaw AD, and Clermont G. Classifying AKI by urine output versus serum creatinine level. J Am Soc Nephrol 26: 2231–2238, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59a. Koyner JL, Shaw AD, Chawla LS, Hoste EA, Bihorac A, Kashani K, Haase M, Shi J, Kellum JA, and Sapphire I. Tissue inhibitor metalloproteinase-2 (TIMP-2) IGF-binding protein-7 (IGFBP7) levels are associated with adverse long-term outcomes in patients with AKI. J Am Soc Nephrol 26: 1747–1754, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Krehl WA, Teply LJ, Sarma PS, and Elvehjem CA. Growth-retarding effect of corn in nicotinic acid-low rations and its counteraction by tryptophane. Science 101: 489–490, 1945. [DOI] [PubMed] [Google Scholar]
- 60a. Kulkarni OP, Hartter I, Mulay SR, Hagemann J, Darisipudi MN, Kumar VR S, Romoli S, Thomasova D, Ryu M, Kobold S, and Anders H-J. Toll-like receptor 4–induced IL-22 accelerates kidney regeneration. J Am Soc Nephrol 25: 978–989, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Langenberg C, Bellomo R, May C, Wan L, Egi M, and Morgera S. Renal blood flow in sepsis. Crit Care 9: R363–R374, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Langenberg C, Gobe G, Hood S, May CN, and Bellomo R. Renal histopathology during experimental septic acute kidney injury and recovery. Crit Care Med 42: e58–e67, 2014. [DOI] [PubMed] [Google Scholar]
- 63. Langenberg C, Wan L, Egi M, May CN, and Bellomo R. Renal blood flow in experimental septic acute renal failure. Kidney Int 69: 1996–2002, 2006. [DOI] [PubMed] [Google Scholar]
- 64. Lankadeva YR, Kosaka J, Evans RG, Bailey SR, Bellomo R, and May CN. Intrarenal and urinary oxygenation during norepinephrine resuscitation in ovine septic acute kidney injury. Kidney Int 90: 100–108, 2016. [DOI] [PubMed] [Google Scholar]
- 64a. Lempiäinen J, Finckenberg P, Levijoki J, and Mervaala E. AMPK activator AICAR ameliorates ischaemia reperfusion injury in the rat kidney. Br J Pharmacol 166: 1905–1915, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, Mootha VK, Jäger S, Vianna CR, Reznick RM, Cui L, Manieri M, Donovan MX, Wu Z, Cooper MP, Fan MC, Rohas LM, Zavacki AM, Cinti S, Shulman GI, Lowell BB, Krainc D, and Spiegelman BM. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell 119: 121–135, 2004. [DOI] [PubMed] [Google Scholar]
- 65a. Liu SB, Liu J, Liu DW, Wang XT, and Yang RL. Inhibition of poly-(ADP-ribose) polymerase protects the kidney in a canine model of endotoxic shock. Nephron 130: 281–292, 2015. [DOI] [PubMed] [Google Scholar]
- 65b. Liu L, Su X, Quinn WJ, Hui S, Krukenberg K, Frederick DW, Redpath P, Zhan L, Chellappa K, White E, Migaud M, Mitchison TJ, Baur JA, and Rabinowitz JD. Quantitative analysis of NAD synthesis-breakdown fluxes. Cell Metab 27: 1067–1080.e5, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ma S, Evans RG, Iguchi N, Tare M, Parkington HC, Bellomo R, May CN, and Lankadeva YR. Sepsis-induced acute kidney injury: a disease of the microcirculation. Microcirculation 26: e12483, 2019. [DOI] [PubMed] [Google Scholar]
- 67. Maiden MJ, Otto S, Brealey JK, Finnis ME, Chapman MJ, Kuchel TR, Nash CH, Edwards J, and Bellomo R. Structure and function of the kidney in septic shock. A prospective controlled experimental study. Am J Respir Crit Care Med 194: 692–700, 2016. [DOI] [PubMed] [Google Scholar]
- 68. Mandel LJ and Balaban RS. Stoichiometry and coupling of active transport to oxidative metabolism in epithelial tissues. Am J Physiol 240: F357–F371, 1981. [DOI] [PubMed] [Google Scholar]
- 69. Manny J, Livni N, Schiller M, Guttman A, Boss J, and Rabinovici N. Structural changes in the perfused canine kidney exposed to the direct action of endotoxin. Isr J Med Sci 16: 153–161, 1980. [PubMed] [Google Scholar]
- 70. Martensson J and Bellomo R. Sepsis-induced acute kidney injury. Crit Care Clin 31: 649–660, 2015. [DOI] [PubMed] [Google Scholar]
- 71. Martin DR, Lewington AJ, Hammerman MR, and Padanilam BJ. Inhibition of poly(ADP-ribose) polymerase attenuates ischemic renal injury in rats. Am J Physiol Regul Integr Comp Physiol 279: R1834–R1840, 2000. [DOI] [PubMed] [Google Scholar]
- 72. Meng XM, Ren GL, Gao L, Yang Q, Li HD, Wu WF, Huang C, Zhang L, Lv XW, and Li J. NADPH oxidase 4 promotes cisplatin-induced acute kidney injury via ROS-mediated programmed cell death and inflammation. Lab Invest 98: 63–78, 2018. [DOI] [PubMed] [Google Scholar]
- 73. Mukhopadhyay P, Horváth B, Kechrid M, Tanchian G, Rajesh M, Naura AS, Boulares AH, and Pacher P. Poly(ADP-ribose) polymerase-1 is a key mediator of cisplatin-induced kidney inflammation and injury. Free Rad Biol Med 51: 1774–1788, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Murugan R, Karajala-Subramanyam V, Lee M, Yende S, Kong L, Carter M, Angus DC, Kellum JA, and Genetic and Inflammatory Markers of Sepsis (GenIMS) Investigators. Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int 77: 527–535, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nlandu-Khodo S, Dissard R, Hasler U, Schäfer M, Pircher H, Jansen-Durr P, Krause KH, Martin P-Y, and de Seigneux S. NADPH oxidase 4 deficiency increases tubular cell death during acute ischemic reperfusion injury. Sci Rep 6: 38598, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Novak ML and Koh TJ. Macrophage phenotypes during tissue repair. J Leukocyte Biol 93: 875–881, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. O'Toole JF. Renal manifestations of genetic mitochondrial disease. Int J Nephrol Renovasc Dis 7: 57–67, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Opal SM, Ellis JL, Suri V, Freudenberg JM, Vlasuk GP, Li Y, Chahin AB, Palardy JE, Parejo N, Yamamoto M, Chahin A, and Kessimian N. Pharmacological Sirt1 activation improves mortality and markedly alters transcriptional profiles that accompany experimental sepsis. Shock 45: 411–418, 2016. [DOI] [PubMed] [Google Scholar]
- 79. Ostermann M, Zarbock A, Goldstein S, Kashani K, Macedo E, Murugan R, Bell M, Forni L, Guzzi L, Joannidis M, Kane-Gill SL, Legrand M, Mehta R, Murray PT, Pickkers P, Plebani M, Prowle J, Ricci Z, Rimmelé T, Rosner M, Shaw AD, Kellum JA, and Ronco C. Recommendations on acute kidney injury biomarkers from the acute disease quality initiative consensus conference: a consensus statement. JAMA Network Open 3: e2019209, 2020. [DOI] [PubMed] [Google Scholar]
- 80. Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong SE, Walford GA, Sugiana C, Boneh A, Chen WK, Hill DE, Vidal M, Evans JG, Thorburn DR, Carr SA, and Mootha VK. A mitochondrial protein compendium elucidates complex I disease biology. Cell 134: 112–123, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Parekh DJ, Weinberg JM, Ercole B, Torkko KC, Hilton W, Bennett M, Devarajan P, and Venkatachalam MA. Tolerance of the human kidney to isolated controlled ischemia. J Am Soc Nephrol 24: 506–517, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Parikh SM. Metabolic stress resistance in acute kidney injury: evidence for a PPAR-gamma-coactivator-1 alpha-nicotinamide adenine dinucleotide pathway. Nephron 143: 184–187, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Peerapornratana S, Manrique-Caballero CL, Gómez H, and Kellum JA. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int 96: 1083–1099, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83a. Perico L, Morigi M, Rota C, Breno M, Mele C, Noris M, Introna M, Capelli C, Longaretti L, Rottoli D, Conti S, Corna D, Remuzzi G, and Benigni A. Human mesenchymal stromal cells transplanted into mice stimulate renal tubular cells and enhance mitochondrial function. Nat Commun 8: 983, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Post EH, Kellum JA, Bellomo R, and Vincent JL. Renal perfusion in sepsis: from macro- to microcirculation. Kidney Int 91: 45–60, 2017. [DOI] [PubMed] [Google Scholar]
- 85. Poyan Mehr A, Tran MT, Ralto KM, Leaf DE, Washco V, Messmer J, Lerner A, Kher A, Kim SH, Khoury CC, Herzig SJ, Trovato ME, Simon-Tillaux N, Lynch MR, Thadhani RI, Clish CB, Khabbaz KR, Rhee EP, Waikar SS, Berg AH, and Parikh SM. De novo NAD(+) biosynthetic impairment in acute kidney injury in humans. Nat Med 24: 1351–1359, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Prowle JR, Molan MP, Hornsey E, and Bellomo R. Measurement of renal blood flow by phase-contrast magnetic resonance imaging during septic acute kidney injury: a pilot investigation. Crit Care Med 40: 1768–1776, 2012. [DOI] [PubMed] [Google Scholar]
- 87. Raines NH, Ganatra S, Nissaisorakarn P, Pandit A, Morales A, Asnani A, Sadrolashrafi M, Maheshwari R, Patel R, Bang V, Shreyder K, Brar S, Singh A, Dani SS, Knapp S, Poyan Mehr A, Brown RS, Zeidel ML, Bhargava R, Schlondorff J, Steinman TI, Mukamal KJ, and Parikh SM. Niacinamide may be Associated with Improved Outcomes in COVID-19-Related Acute Kidney Injury: An Observational Study. Kidney360 2: 33, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Rajendram R and Prowle JR.. Venous congestion: are we adding insult to kidney injury in sepsis? Crit Care 18: 104, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ralto KM, Rhee EP, and Parikh SM. NAD(+) homeostasis in renal health and disease. Nat Rev Nephrol 16: 99–111, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ravikant T and Lucas CE. Renal blood flow distribution in septic hyperdynamic pigs. Surg Res 22: 294–298, 1977. [DOI] [PubMed] [Google Scholar]
- 91. Ronco C, Bellomo R, and Kellum JA. Acute kidney injury. Lancet 394: 1949–1964, 2019. [DOI] [PubMed] [Google Scholar]
- 92. Rosen S and Heyman SN. Difficulties in understanding human “acute tubular necrosis”: limited data and flawed animal models. Kidney Int 60: 1220–1224, 2001. [DOI] [PubMed] [Google Scholar]
- 93. Schmidt C, Hocherl K, Schweda F, and Bucher M. Proinflammatory cytokines cause down-regulation of renal chloride entry pathways during sepsis. Crit Care Med 35: 2110–2119, 2007. [DOI] [PubMed] [Google Scholar]
- 94. Schrier RW and Wang W. Acute renal failure and sepsis. N Engl J Med 351: 159–169, 2004. [DOI] [PubMed] [Google Scholar]
- 95. Sedeek M, Hébert RL, Kennedy CR, Burns KD, and Touyz RM. Molecular mechanisms of hypertension: role of Nox family NADPH oxidases. Curr Opin Nephrol Hypertens 18: 122–127, 2009. [DOI] [PubMed] [Google Scholar]
- 96. Seely KA, Holthoff JH, Burns ST, Wang Z, Thakali KM, Gokden N, Rhee SW, and Mayeux PR. Hemodynamic changes in the kidney in a pediatric rat model of sepsis-induced acute kidney injury. Am J Physiol Renal Physiol 301: F209–F217, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Self WH, Semler MW, Wanderer JP, Wang L, Byrne DW, Collins SP, Slovis CM, Lindsell CJ, Ehrenfeld JM, Siew ED, Shaw AD, Bernard GR, and Rice TW. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med 378: 819–828, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Semler MW, Self WH, Wanderer JP, Ehrenfeld JM, Wang L, Byrne DW, Stollings JL, Kumar AB, Hughes CG, Hernandez A, Guillamondegui OD, May AK, Weavind L, Casey JD, Siew ED, Shaw AD, Bernard GR, Rice TW, SMART Investigators, and the Pragmatic Critical Care Research Group. Balanced crystalloids versus saline in critically ill adults. N Engl J Med 378: 829–839, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98a. Shu B, Feng Y, Gui Y, Lu Q, Wei W, Xue X, Sun X, He W, Yang J, and Dai C. Blockade of CD38 diminishes lipopolysaccharide-induced macrophage classical activation and acute kidney injury involving NF-kappaB signaling suppression. Cell Signal 42: 249–258, 2018. [DOI] [PubMed] [Google Scholar]
- 99. Simic P, Vela Parada XF, Parikh SM, Dellinger R, Guarente LP, and Rhee EP. Nicotinamide riboside with pterostilbene (NRPT) increases NAD+ in patients with acute kidney injury (AKI): a randomized, double-blind, placebo-controlled, stepwise safety study of escalating doses of NRPT in patients with AKI. BMC Nephrol 21: 342, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Singer M, De Santis V, Vitale D, and Jeffcoate W. Multiorgan failure is an adaptive, endocrine-mediated, metabolic response to overwhelming systemic inflammation. Lancet 364: 545–548, 2004. [DOI] [PubMed] [Google Scholar]
- 101. Singh P and Okusa MD. The role of tubuloglomerular feedback in the pathogenesis of acute kidney injury. Contrib Nephrol 174: 12–21, 2011. [DOI] [PubMed] [Google Scholar]
- 101a. Smeets B, Boor P, Dijkman H, Sharma SV, Jirak P, Mooren F, Berger K, Bornemann J, Gelman IH, Floege J, van der Vlag J, Wetzels JF, and Moeller MJ. Proximal tubular cells contain a phenotypically distinct, scattered cell population involved in tubular regeneration. J Pathol 229: 645–659, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Smith JA, Stallons LJ, and Schnellmann RG. Renal cortical hexokinase and pentose phosphate pathway activation through the EGFR/Akt signaling pathway in endotoxin-induced acute kidney injury. Am J Physiol Renal Physiol 307: F435–F444, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Soltoff SP. ATP and the regulation of renal cell function. Annu Rev Physiol 48: 9–31, 1986. [DOI] [PubMed] [Google Scholar]
- 104. Sun J, Zhang J, Tian J, Virzi GM, Digvijay K, Cueto L, Yin Y, Rosner MH, and Ronco C. Mitochondria in sepsis-induced AKI. J Am Soc Nephrol 30: 1151–1161, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Takasu O, Gaut JP, Watanabe E, To K, Fagley RE, Sato B, Jarman S, Efimov IR, Janks DL, Srivastava A, Bhayani SB, Drewry A, Swanson PE, and Hotchkiss RS. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med 187: 509–517, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Titheradge MA. Nitric oxide in septic shock. Biochim Biophys Acta 1411: 437–455, 1999. [DOI] [PubMed] [Google Scholar]
- 107. Tiwari MM, Brock RW, Megyesi JK, Kaushal GP, and Mayeux PR. Disruption of renal peritubular blood flow in lipopolysaccharide-induced renal failure: role of nitric oxide and caspases. Am J Physiol Renal Physiol 289: F1324–F1332, 2005. [DOI] [PubMed] [Google Scholar]
- 108. Trammell SA, Schmidt MS, Weidemann BJ, Redpath P, Jaksch F, Dellinger RW, Li Z, Abel ED, Migaud ME, and Brenner C. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat Commun 7: 12948, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Tran M, Tam D, Bardia A, Bhasin M, Rowe GC, Kher A, Zsengeller ZK, Akhavan-Sharif MR, Khankin EV, Saintgeniez M, David S, Burstein D, Karumanchi SA, Stillman IE, Arany Z, and Parikh SM. PGC-1α promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Invest 121: 4003–4014, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Tran MT, Zsengeller ZK, Berg AH, Khankin EV, Bhasin MK, Kim W, Clish CB, Stillman IE, Karumanchi SA, Rhee EP, and Parikh SM. PGC1α drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature 531: 528–532, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Tran MT, Zsengeller ZK, Berg AH, Khankin EV, Bhasin MK, Kim W, Clish CB, Stillman IE, Karumanchi SA, Rhee EP, and Parikh SM. PGC1α drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature 531: 528–532, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Trump BF, Valigorsky JM, Jones RT, Mergner WJ, Garcia JH, and Cowley RA. The application of electron microscopy and cellular biochemistry to the autopsy. Observations on cellular changes in human shock. Hum Pathol 6: 499–516, 1975. [DOI] [PubMed] [Google Scholar]
- 113. Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C, Beginning, and Ending Supportive Therapy for the Kidney I. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294: 813–818, 2005. [DOI] [PubMed] [Google Scholar]
- 114. Vander Heiden MG, Cantley LC, and Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324: 1029–1033, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Verdant CL, De Backer D, Bruhn A, Clausi CM, Su F, Wang Z, Rodriguez H, Pries AR, and Vincent JL. Evaluation of sublingual and gut mucosal microcirculation in sepsis: a quantitative analysis. Crit Care Med 37: 2875–2881, 2009. [DOI] [PubMed] [Google Scholar]
- 116. Verma SK and Molitoris BA. Renal endothelial injury and microvascular dysfunction in acute kidney injury. Semin Nephrol 35: 96–107, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Waltz P, Carchman E, Gomez H, and Zuckerbraun B. Sepsis results in an altered renal metabolic and osmolyte profile. J Surg Res 202: 8–12, 2016. [DOI] [PubMed] [Google Scholar]
- 118. Wang G, Han T, Nijhawan D, Theodoropoulos P, Naidoo J, Yadavalli S, Mirzaei H, Pieper Andrew A, Ready Joseph M, and McKnight Steven L. P7C3 neuroprotective chemicals function by activating the rate-limiting enzyme in NAD salvage. Cell 158: 1324–1334, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wang Z, Holthoff JH, Seely KA, Pathak E, Spencer HJ, Gokden N, and Mayeux PR. Development of oxidative stress in the peritubular capillary microenvironment mediates sepsis-induced renal microcirculatory failure and acute kidney injury. Am J Pathol 180: 505–516, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wang Z, Ying Z, Bosy-Westphal A, Zhang J, Schautz B, Later W, Heymsfield SB, and Muller MJ. Specific metabolic rates of major organs and tissues across adulthood: evaluation by mechanistic model of resting energy expenditure. Am J Clin Nutr 92: 1369–1377, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Wilcox CS, Palm F, and Welch WJ. Renal oxygenation and function of the rat kidney: effects of inspired oxygen and preglomerular oxygen shunting. In: Oxygen Transport to Tissue XXXIV, edited by Welch WJ, Palm F, Bruley DF, and Harrison DK. New York, NY: Springer New York, 2013, pp. 329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Williams PA, Harder JM, Foxworth NE, Cochran KE, Philip VM, Porciatti V, Smithies O, and John SWM. Vitamin B3 modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science 355: 756–760, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122a. Wingert RA and Davidson AJ.. The zebrafish pronephros: a model to study nephron segmentation. Kidney Int 73: 1120–1127, 2008. [DOI] [PubMed] [Google Scholar]
- 123. Yang L, Xie M, Yang M, Yu Y, Zhu S, Hou W, Kang R, Lotze MT, Billiar TR, Wang H, Cao L, and Tang D. PKM2 regulates the Warburg effect and promotes HMGB1 release in sepsis. Nat Commun 5: 4436, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Yang L, Xing G, Wang L, Wu Y, Li S, Xu G, He Q, Chen J, Chen M, Liu X, Zhu Z, Yang L, Lian X, Ding F, Li Y, Wang H, Wang J, Wang R, Mei C, Xu J, Li R, Cao J, Zhang L, Wang Y, Xu J, Bao B, Liu B, Chen H, Li S, Zha Y, Luo Q, Chen D, Shen Y, Liao Y, Zhang Z, Wang X, Zhang K, Liu L, Mao P, Guo C, Li J, Wang Z, Bai S, Shi S, Wang Y, Wang J, Liu Z, Wang F, Huang D, Wang S, Ge S, Shen Q, Zhang P, Wu L, Pan M, Zou X, Zhu P, Zhao J, Zhou M, Yang L, Hu W, Wang J, Liu B, Zhang T, Han J, Wen T, Zhao M, and Wang H. Acute kidney injury in China: a cross-sectional survey. Lancet 386: 1465–1471, 2015. [DOI] [PubMed] [Google Scholar]
- 125. Zheng J, Devalaraja-Narashimha K, Singaravelu K, and Padanilam BJ. Poly(ADP-ribose) polymerase-1 gene ablation protects mice from ischemic renal injury. Am J Physiol Renal Physiol 288: F387–F398, 2005. [DOI] [PubMed] [Google Scholar]