Abstract
Time-restricted feeding (TRF, animal-based studies) and time-restricted eating (TRE, humans) are an emerging behavioral intervention approach based on the understanding of the role of circadian rhythms in physiology and metabolism. In this approach, all calorie intake is restricted within a consistent interval of less than 12 hours without overtly attempting to reduce calories. This article will summarize the origin of TRF/TRE starting with concept of circadian rhythms and the role of chronic circadian rhythm disruption in increasing the risk for chronic metabolic diseases. Circadian rhythms are usually perceived as the sleep-wake cycle and dependent rhythms arising from the central nervous system. However, the recent discovery of circadian rhythms in peripheral organs and the plasticity of these rhythms in response to changes in nutrition availability raised the possibility that adopting a consistent daily short window of feeding can sustain robust circadian rhythm. Preclinical animal studies have demonstrated proof of concept and identified potential mechanisms driving TRF-related benefits. Pilot human intervention studies have reported promising results in reducing the risk for obesity, diabetes, and cardiovascular diseases. Epidemiological studies have indicated that maintaining a consistent long overnight fast, which is similar to TRE, can significantly reduce risks for chronic diseases. Despite these early successes, more clinical and mechanistic studies are needed to implement TRE alone or as adjuvant lifestyle intervention for the prevention and management of chronic metabolic diseases.
Keywords: Circadian rhythm, Time-restricted eating, Time-restricted feeding, Intermittent fasting, Metabolic disease Metabolism
Graphical Abstract
Graphical Abstract.
ESSENTIAL POINTS.
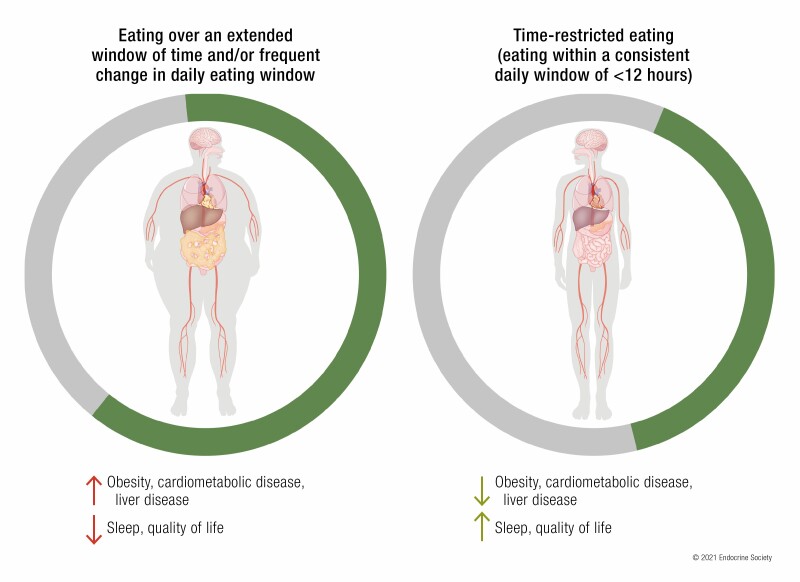
Time-restricted eating or feeding (TRE or TRF) is a nutrition intervention approach in which daily caloric intake is restricted to a consistent window of approximately 8 to 10 hours.
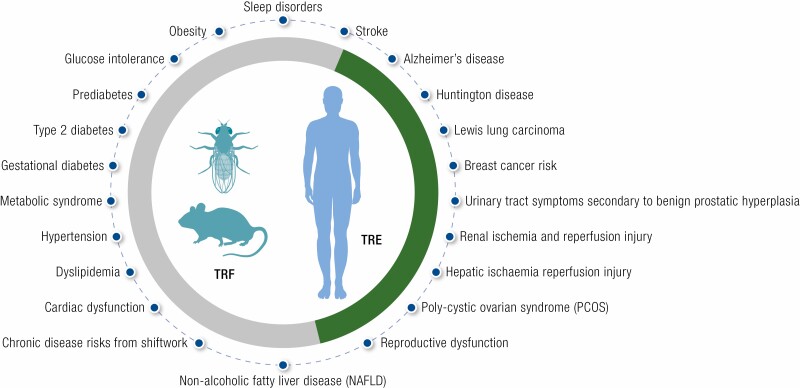
In preclinical animal models, TRF without reducing caloric intake has been shown to prevent or attenuate severity of several metabolic diseases, including obesity, glucose intolerance, hepatic steatosis, dyslipidemia, and age-related decline in cardiac function.
In pilot human studies, TRE with or without explicit calorie reduction can reduce body weight, glucose intolerance, hypertension, and dyslipidemia.
TRF is based on the concepts of circadian rhythm and in animal models is shown to improve metabolism by at least partly acting through the molecular circadian clock.
Molecular studies in preclinical animal models show TRF exerts pleiotropic effects on multiple pathways in different organs and on gut microbiome composition.
Better methods to monitor and promote compliance to a daily eating pattern in humans is necessary to accurately assess TRE benefits.
The evolution of metazoans accompanied the division of cellular functions along the spatial axis with different tissue systems and organs establishing specialized functions. Molecules and mechanisms including paracrine and endocrine functions facilitated intercellular or interorgan communications. These tissue system functions improved organismal functions and survival. In parallel, functional refinement along the time axis was also necessary to adapt to the predictable changes in the earth’s environment: light, nutrients, temperature, humidity, and associated factors changing with the 24-hour rotation of the earth.
Accordingly, most organisms including humans evolved to have intrinsic circadian (near 24-hour) rhythms whose major task is to promote preparatory changes in the metabolic functions in anticipation of predictable changes in light, temperature, and nutrient availability. Circadian rhythms modulate cell-autonomous processes and also influence the production and secretion of endocrine factors and their functions in target cells. An outcome of such a regulatory mechanism is the emergence of optimum windows of time within the 24-hour day for sleep, activity, and nutrient intake. When sleep, activity, and nutrition intake occur within the respective optimal circadian windows, they reinforce the circadian rhythms, optimizing organ functions and health. Conversely, when these events occur outside the preferred window, they dampen or disrupt circadian rhythms, compromise organ functions, and raise risks both for infectious and noninfectious diseases.
Humans in the postindustrial era often experience circadian rhythm disruptions (CRDs) due to suboptimal light exposure, sleep, and random eating patterns. Such CRD is correlated with the increased risk for numerous chronic metabolic diseases (1-6). Controlled animal and human studies have shown correcting the CRD by controlled lighting or optimal sleep can prevent or lessen the respective disease risks (7-10). Recently, the timing of food intake has emerged as a powerful human behavior that influences the circadian rhythm and physiology. Accordingly, restricting the timing of food intake in alignment with the circadian opportune eating window is being shown to improve several health conditions both in preclinical animal models and some human studies (11).
This article will introduce the concept of circadian rhythms, CRD, and time-restricted eating (TRE). We will review the literature on potential mechanisms underlying TRE, recent human studies, and offer some future perspective of its translation to practice for the prevention and management of metabolic diseases.
Circadian Rhythms Disruption and How it Can Be Addressed With Time-restricted Eating
Molecules and mechanisms of circadian clock
The temporal organization of behavior and physiology that repeats every 24 hours comprises the circadian regulatory system. The central feature of this regulatory system is approximately 24-hour rhythm in the expression and function of numerous cellular and endocrine factors that are directly or indirectly regulated by circadian clocks. At the molecular level, the circadian clock is based on cell-autonomous feedback circuit driven by the basic helix–loop–helix-PER-ARNT-SIM (bHLH-PAS) transcription factors BMAL1 (also known as ARNTL and MOP3) and CLOCK, or the CLOCK homolog NPAS2 (for simplicity, this proposal will use “CLOCK/BMAL1” when referring to all heterodimer forms). In this circuit, CLOCK/BMAL1 heterodimers bind to cis-acting E-box (CACGTG) elements present in the promoter regions of the Period (Per1 and Per2) and Cryptochrome (Cry1 and Cry2) genes to activate their transcription. In turn, the CRY and PER proteins heterodimerize to inhibit CLOCK/BMAL1 activity, thus producing approximately 24-hour rhythms in Per and Cry transcription. In addition, CLOCK/BMAL1 and PER/CRY generate rhythmic transcription of Ror and Rev-erb classes of nuclear hormone receptors, whose opposing actions on the RRE (Rev-ErbA/ROR response element) site in the Bmal1 promoter results in an approximately 24-hour rhythm in Bmal1 transcription (reviewed in [12]). This self-sustained oscillation persists in the absence of any external timing cues, such as food or light, thus offering the organism an intrinsic timing system.
The circadian clock is present in almost all tissues and cells. The circadian clock directly or indirectly drives daily rhythms in the messenger RNA (mRNA) levels of a large number of genes across all organ systems (13-16). First, clock components are recruited to thousands of promoters in the mouse liver and pancreas that temporally correlated with the transcriptional activation or repression marks and pre-mRNA levels (17-23). The clock components also regulate the expression of downstream transcription factors such as D-Box binding protein (DBP), TEF, NFIL3, etc, which themselves and their targets show rhythmic transcription (24-26). Second, the clock components interact with tissue-specific transcription factors to drive tissue-specific rhythmic transcription (20). Third, some of the clock repressor components also act as repressors of other transcription factors. For example, Cry proteins act as repressors of the glucocorticoid receptor and PPARδ (27, 28), and PER2 represses PPARγ transcription, thereby imposing rhythmic expression of their respective targets (29).
The circadian system also changes with the seasonal change in daylength. The master circadian clock in the hypothalamic suprachiasmatic nucleus (SCN) responds to light signals received and transmitted through blue light–sensitive and melanopsin expressing retinal ganglion cells (30, 31). The SCN, in turn, uses synaptic and diffusible signals to synchronize circadian clocks in other organs. Some of these signals include melatonin from the pituitary, major endocrine regulators of the hypothalamic-pituitary-adrenal axis, hypothalamic-pituitary-gonadal axis, and even rhythm in body temperature (32, 33). The SCN also modulates the autonomic system to produce circadian rhythms in organ functions including a critical role in glucose homeostasis (34, 35). The peripheral circadian clocks respond to these intercellular or interorgan signals and synchronize the phase of oscillation of clock components. The peripheral circadian clocks reciprocally interact with nutrient signals through numerous mechanisms (reviewed in (36, 37)). The principal pancreatic hormones, glucagon and insulin, also reciprocally interact with the clock components in the liver (38-40). The extensive network of the circadian clock components and their interacting molecules result in mRNA rhythms in more than 80% of protein-coding genes in a tissue-specific manner in primates (16), which is presumed to optimize behavior, physiology, and metabolism.
Circadian Rhythm Disruption
CRDs directly affect the temporal molecular regulation of metabolism and indirectly facilitate overnutrition and sedentary behaviors, further accelerating progression to metabolic diseases. There are 5 primary modes of CRD: genetic, age-related, disease-related, environmental, and anthropogenic.
Human genetic mutations have been detected in the clock components that disrupt the sleep-wake cycle by changing the pace and thereby phase of the clock. The overt manifestations of these diseases are familial advanced sleep–phase syndrome, familial delayed sleep–phase syndrome, and short-sleep and non–24-hour sleep-wake syndrome (41, 42). However, the allele frequencies of these core clock components are relatively rare. Although not a clock component, melatonin is an important output mediator of circadian rhythms. Mutations in the melatonin receptor 1B, which is relatively prevalent in as many as approximately 30% of the population in the Western world, have been recognized as closely linked to obesity and diabetes (43-48). Other genetic diseases such as Smith-Magenis syndrome also disrupt circadian rhythm by nearly inverting the sleep-wake cycle, and Smith-Magenis syndrome patients often suffer from obesity and metabolic diseases (49).
Age-related CRD can be of many types. There is a general age-related dampening of the molecular circadian rhythm, which can result in pervasive pleiotropic effects on multiple organ systems and the brain (50, 51). However, age-related decline in other facets of biological systems has made it difficult to establish a clear causal role of age in circadian disruption. Nevertheless, arousal threshold declines at old age, and animal studies have shown there is a deficit in registering sleep deprivation and the countermeasure of sufficient restorative sleep in old age. As a result, in old age, insufficient sleep and lack of sufficient rebound sleep can exacerbate CRD.
Disease-related CRD has been documented in many diseases, including various dementia-related disorders (52). In addition, there is accumulating evidence that some chronic diseases such as type 2 diabetes may also have some molecular circadian disruption as the daily cycle of insulin sensitivity and markers of glucose tolerance in patients with diabetes is different from healthy individuals (53). As researchers and clinicians assess circadian rhythm and disease, the list of diseases in which overt circadian rhythms and/or endocrine agents are disrupted is expected to grow. Irrespective of whether CRD is a cause or consequence of the disease, unchecked CRD can further exacerbate the disease condition.
Environmental CRD includes the extremely long and short days in extreme latitudes. While winter depression and spring mania-like behavior are often associated with such environmental circadian disruption, controlled animal studies have shown continuous light can disrupt the molecular circadian clock, overt behavioral rhythms, and can increase adiposity (54, 55).
Finally, the anthropogenic CRD is the most common and widespread. Shift workers, who account for approximately 20% of the workforce in developed or developing countries, experience chronic CRD. According to the International Labor Organization’s definition of shift work, anyone who stays awake for work for 2 to 3 hours between 10 pm and 5 am for approximately 50 days in a year qualifies as a person with shift work–like lifestyle (56). Hence, a large proportion of the general population who stay awake late into the night an average of one day in a week to study, complete work assignments, socialize, or care for family members may also be living the circadian disruptive life of a shift worker. Such CRD, termed social jetlag, afflicts nearly 40% of the general population. Chronic CRD under experimental conditions that mimic shift work or social jetlag can increase risks for noninfectious chronic diseases, including glucose intolerance, body weight gain, adiposity, liver diseases, various forms of cancer, depression, and cardiovascular diseases, among others (6). Chronic CRD also compromises the immune system so that the animals become more susceptible to elevated inflammation and septic shock (57).
The impact of nutrition timing on circadian rhythms
The concept of time-restricted feeding in animals (TRF), or time-restricted eating in humans (TRE), arose from studies to examine the effect of nutrition timing on the circadian system in rodents. To test how food affects the circadian clock, mice were given access to food for a short period during the day when they do not anticipate food (58). Reducing the timing of food access to less than 8 hours also reduced caloric intake by mice. These experiments showed that rodents would wake up a few hours before the arrival of food and initiate ambulatory activity as if they were anticipating food. Such food-anticipatory activity would also occur when calories are reduced and presented at night and the magnitude of activity would increase with the reduction in calories (59). These daytime TRF experiments also revealed that daytime access to food changed the phase of the circadian clock components in the liver of mice, so that clock components whose expression typically rises at night would rise during the day and vice versa (60). However, experiments with liver-specific circadian mutant mice with ad libitum access to food still had several cycling transcripts in the liver, which was interpreted as the SCN using systemic signals to drive rhythmic transcription in the liver (61). Finally, well-controlled daytime TRF along with comprehensive gene expression analyses established that timing of food access is the dominant driver of all rhythmic transcripts in the liver (62). Subsequent experiments have also confirmed this outcome for several other organs and even some brain regions outside the SCN (63, 64). Therefore, it was concluded that the timing of food intake is by far the most powerful cue that determines the phase and rhythmicity of circadian transcriptional programs in almost all peripheral organs and the majority of the brain, while the SCN clock is tied to the light:dark cycle. This discovery offered a new opportunity to use nutritional intake to alleviate CRD and lessen the burden of associated diseases.
Mouse studies on time-restricted feeding
Metabolic benefits of TRF were first demonstrated in the mouse model of diet-induced obesity (DIO) (65). In this model, when mice are given ad libitum access to a high-fat diet, the daily feeding rhythm dampens. While mice fed a standard diet consume approximately 85% of calories during the night, the DIO mice consume less than 70% food during the night and more than 30% calories during the day (66). This eating pattern also dampens the liver circadian clock and systemically affects a large number of circadian metabolites both in metabolic organs and the brain (67). To test the relative contribution of an obesogenic diet and the disrupted eating pattern to obesity and metabolic diseases in these mice, we and others have subjected mice to an isocaloric obesogenic diet within an 8-hour window. These TRF rodents that consume the same calories as an ad libitum DIO cohort from the same food source exhibit improved molecular rhythms in circadian clock components and are largely protected from high-fat diet-induced obesity and related metabolic illnesses (65, 68). It is noteworthy that all these mice cohorts irrespective of diet type and eating patterns (ad lib or TRF) were housed under an identical light:dark cycle that presumably had the same entraining effects on the SCN clock.
The initial rodent experiments were performed with 8-hour access to food (65) and hence a popular diet called the 8-hour diet or 8:16 diet arose. In popular media it is also grouped under the umbrella of intermittent fasting, which broadly encompasses all types of fasting from a few hours to a few days without any explicit reference to circadian rhythms.
What is the optimum time and length of time-restricted feeding in animals?
In rodents, TRF of 8 to 12 hours at night without reducing caloric intake prevented obesity and diseases in a dose-dependent manner with greater restriction of the eating window associated with greater benefits (68). Daytime TRF also shows some benefit for weight loss and liver health relative to ad libitum controls (69). Daytime TRF for rodents directly disturbs their sleep and also temporally decouples their physical activity from feeding time. Therefore, it is not clear whether it is the direct effect of day TRF that introduces a mismatch between the clocks in the SCN and peripheral organs, or the indirect effect of sleep loss and lack of physical activity after consuming a meal that contributes to the slightly attenuated benefits of daytime TRF in mice.
Time restriction and calorie restriction in rodents
TRF of less than 8 hours inadvertently reduces calorie consumption in rodents, making it difficult to ascribe any benefits to TRF or calorie restriction (CR). However, most CR studies in rodents are conducted in a manner that inadvertently imposes some form of time restriction. In CR studies, control mice often receive food ad libitum, whereas the CR mice are given a meal that contains 20% to 30% fewer calories at a fixed time every day. The mice typically consume the CR meal within 2 to 3 hours, leaving a prolonged 20- to 22-hour fasting interval (70). Irrespective of the time of day when the CR diet was given to mice consistently, in the morning, afternoon, or evening, CR led to an extended lifespan (71). In a short-term CR study, however, CR mice receiving food in the morning (during their circadian sleep time), experienced less weight loss than mice that received the same calories at night (70). In an experiment to test the effect of time of meal in CR experiments, when the control mice were also given the daily ration of standard chow at a fixed time (meal fed), the rodents finished the food within 10 to 12 hours. These meal-fed mice also lived longer than the control ad libitum–fed mice, but shorter than the CR mice (72). Similar results were also reported when the meal-fed mice received a high-fat diet (73). These observations raise the possibility that some benefits of CR or paired-feeding experiments also arise from time restriction (TR). Along the same line, experiments in which mice were given 2 meals within a 12-hour interval (isocaloric twice-a-day) also showed better health benefits compared to ad lib–fed control mice (74). In summary, these rodent experiments concluded that (a) a TRF of 8 to 12 hours without calorie reduction imparts health benefits, (b) some of the CR benefits may arise from inadvertent TR, and (c) some feeding protocols, such as paired feeding, once-daily meal feeding, or feeding mice twice or 3 times a day within an approximately 12-hour window, may also inadvertently have a TR component.
Mechanisms of Time-Restricted Feeding
Mechanistic studies of the effects of TRE in humans remain extremely sparse (75-77). In contrast, the mechanism driving the effects of TRF are more developed in rodent models, particularly regarding its effects on the liver, as the liver is intimately involved in the fasting/fed states. The following discussions are largely based on time-series analyses of tissue or plasma samples that, unlike the single time point analyses conducted in a vast majority of metabolic studies, reveal molecular changes both in the fasted and fed state. Hence, most discussions of changes in the expression or function of genes, proteins, and metabolites mostly refer to changes in the fasted or fed state. In the following sections we will discuss the intertwined mechanisms by which TRF acts on circadian clock components, nutrient-sensing pathways, gut function, and how through these actions TRF improves the metabolism of glucose and fatty acids.
Time-restricted feeding activates pathways in the liver known to mediate the benefits of fasting
TRF imposes a relatively longer period of daily fast, which in turn activates pathways implicated in mediating the benefits of CR. These include the increased expression and functional states of liver-based adenosine monophosphate–activated protein kinase C (AMPK), FoxO, ATF, Sirtuins, and a modest increase in ketone bodies (ketogenesis) (62, 65, 68). In some dietary patterns similar to TRF, daily activation of mediators or markers of autophagy in the liver is also documented (74). Furthermore, under TRF, these mediators of fasting or feeding are likely to be active for only a few hours toward the end of the daily fasting period or after feeding begins. Direct comparison between TRF and CR in the magnitude of changes in these molecular pathways is currently lacking. But it is likely that TRF modestly increases the activities of these mediators, while CR may have a larger effect.
Time-restricted feeding improves circadian rhythms
TRF changes the temporal pattern activity of the key regulator of gluconeogenesis 3′,5′-cyclic adenosine 5′-monophosphate response element binding protein (CREB) and insulin signaling pathway within the liver (65). These 2 pathways reciprocally interact with several clock components. As a result, TRF improves daily rhythms in CREB, insulin signaling, and clock components.
Glucagon acts through its cognate receptor in the liver to trigger CREB phosphorylation, which in turn acts on its downstream target genes to promote gluconeogenesis to maintain blood glucose specifically during the fasted state. For some unexplained reason, in ad lib–fed obese mice CREB remains phosphorylated during both the fasted and fed state. CREB phosphorylation during the fed state supports unnecessary gluconeogenesis, which likely contributes to elevated blood glucose levels in diet-induced obese mice. Under TRF, liver phosphorylated-CREB level remains elevated during the fasted phase and declines during the fed state (65). Such desirable rhythms in CREB phosphorylation likely arises because of at least 2 mechanisms. During the fed state, the α cells of the pancreas may reduce the expression or release of glucagon (78) and increased CRY expression in the liver can further suppress glucagon signaling downstream of its receptor (39). The reduced phosphorylated-CREB levels during the fed state correlate with reduced expression of its target genes pyruvate carboxylase (Pcx) and glucose-6-phosphatase (G6pc), which encode enzymes that mediate 2 committing steps in gluconeogenesis. This can redirect the carbons destined for gluconeogenesis and release from the liver to 2 intracellular processes. Reduced gluconeogenesis can redirect at least a portion of pyruvate to the tricarboxylic acid (TCA) cycle. Consequently, TCA cycle intermediates (eg, malate, fumarate, and citrate) are elevated in the TRF liver (65). Reduced expression of G6pc reduces the flux of glucose-6-phosphate (G6P) that can be dephosphorylated to glucose and released from the liver. Instead, the increased G6P in the liver of TRF mice can act as a substrate level activator of G6P dehydrogenase, whose expression is also elevated in the TRF liver by an unknown mechanism. G6p dehydrogenase mediates the first committing step for the pentose phosphate pathway (PPP) and likely diverts some G6P toward PPP. The PPP is a major source of NADPH (nicotinamide adenine dinucleotide phosphate) used to reduce glutathione. The levels of reduced glutathione and several intermediate products of the PPP are elevated in the TRF liver (65).
The pentose sugars from the PPP and intermediates of the TCA cycle are substrates for nucleotide biosynthesis. The rate-limiting steps for these pathways are mediated by Tk1 and Umps (thymidine kinase 1 [Tk1] and uridine monophosphate synthase [Umps]), whose expression are circadian and activated by clock component Bmal1 (79). TRF increases the expression of Bmal1 as well as its targets Tk1 and Umps. Accordingly, increased levels of several nucleotides in the liver of TRF mice are also observed.
TRF reduces insulin resistance by mechanisms yet to be fully understood and may involve multiple organs. Fasting and postprandial levels of serum insulin levels both are relatively high in ad lib–fed mice, whereas TRF reverses this trend. Insulin activates mechanistic target of rapamycin (mTOR), which in turn phosphorylates S6. Because mice habitually feed during the night, phospho-S6 levels are typically higher at night. However, mice on ad libitum feeding of a high-fat diet show an inverted pS6 rhythm, with higher levels during the day. TRF corrects this temporal activation of pS6 and aligns the higher levels with the fed state (65), implying normal postprandial insulin signaling. During the fed state, improved insulin signaling can direct glucose for glycogen synthesis. TRF mouse liver accumulates increased levels of glycogen. Overall, these coordinated changes in gene expression and metabolites imply TRF reprograms glucose metabolism away from gluconeogenesis toward anabolic pathways (65).
The nutrient-sensing pathways such as AMPK, CREB, mTOR, and Sirtuins directly or indirectly affect the expression or function of clock components (reviewed in [80]). Accordingly, there are parallels between daily rhythms in these pathways and circadian clock components. It is known that ad libitum access to an obesogenic diet dampens the feeding rhythm, disrupting the normal expression or function of nutrient-sensing pathways that contributes to dampening the rhythmic expression of many clock components by primarily reducing peak levels with little effects on the trough levels of the respective mRNA or protein. TRF, conversely, increases the peak expression levels both of activators and repressors of core clock components, including that of the repressors Cry1, Rev-erbα, Per2, and of the activator Bmal1 (65).
The complementary function of nutrient-sensing pathways and circadian clock components is thought to improve hepatic lipid homeostasis in the TRF liver. The increased level of AMP in the TRF liver can activate AMP kinase, which can phosphorylate acetyl CoA carboxylase (ACC or ACACA). ACC catalyzes the carboxylation of acetyl-CoA to malonyl-CoA, the first and rate-limiting step in fatty acid synthesis. As phosphorylated ACC is enzymatically inactive, TRF may reduce de novo lipogenesis. Additionally, an increased peak expression level of circadian clock repressor Rev-erb alpha acts as a repressor of several genes implicated in lipid synthesis (22), such as fatty acid synthase (Fasn). Reduced Fasn mRNA levels and increased relative phospho-ACC levels act synergistically to reduce fatty acid synthesis, leading to reduced levels of several long-chain free fatty acids, including myristate and palmitate. Another clock repressor, Per2, may indirectly contribute to reduced lipogenesis in the TRF liver. PER2 protein is an inhibitor of the lipogenic transcriptional regulator PPAR-gamma (29). Pparg levels are typically elevated in the liver of high-fat ad libitum–fed mice (81), whereas in TRF mice, their levels reduce to what is found in mice fed a standard diet (68). The reduced level of PPAR-γ expression along with increased levels of its inhibitor PER2 parallels reduced expression of PPAR-γ target genes, including stearoyl CoA desaturase1 (Scd1), which codes an enzyme mediating fatty acid desaturation, and the unsaturated fatty acid elongase Elovl5. Expression of other Elovl genes is also reduced in the TRF liver. Such reduced expression or rate-determining steps in fatty acid desaturation and elongation is concomitant, with a greater than 50% decline in several unsaturated fatty acids, including oleate (18:1n9), palmitoleate (16:1n7), vaccenate (18:1n7), and eicosenoate (20:1n9 or 11) in the TRF liver (65).
Time-restricted feeding effects on adipose tissue
TRF also affects the adipose tissues. Specifically, TRF increases lipolysis and β-oxidation. Increased expression of hepatic lipase (Lipc) and reduced expression of the lipid droplet-associated and lipolysis inhibitor gene Cidec (82) in the TRF liver support more lipolysis and reduced size of lipid droplets in hepatocytes. Further lipid oxidation via the β-oxidation pathway is negatively regulated by fatty acid synthesis. Malonyl-CoA, a product of ACC activity in the first step of fatty acid synthesis, allosterically inhibits mitochondrial carnitine palmitoyl transferase (CPT). CPT is essential for the transit of long-chain fatty acids and acylcarnitine esters into the mitochondria for β-oxidation. In ad libitum–fed liver, elevated levels of malonylcarnitine imply increased inhibition of CPT and reduced β-oxidation caused by the impaired entry of fatty acids into the mitochondria. In TRF liver, reduced ACC activity reduces malonylcarnitine levels, which can promote fatty acid oxidation leading to an increased level of β-hydroxybutyrate, one of the end products of β-oxidation. Additionally, fatty acid-binding protein 1 (Fabp1), which binds to and clears potentially toxic unesterified long-chain fatty acids from the cytoplasm (83), exhibits a moderate increase in expression in the liver of TRF mice (65).
A beneficial byproduct of the overall reduction in the free fatty acid pool in the TRF liver is reduced inflammation. Free fatty acids are substrates for increased production of proinflammatory long-chain n-6 fatty acids dihomo-linoleate (20:2n6) and arachidonate (20:4n6). The ad libitum–fed liver is prone to more oxidative stress because it contains relatively less reduced-glutathione, a major cellular antioxidant. Oxidation of arachidonate and linoleic acid in the livers of the ad libitum–fed mice further increases the levels of proinflammatory eicosanoids: 15-hydroxyeicosatetraenoic acid (HETE), 5-HETE, and 13-HODE. In contrast, reduced hepatic free fatty acids along with glutathione-enriched cellular environment in the livers of the TRF mice attenuated the levels of proinflammatory lipids and oxidative damage to hepatocytes. Such reduced hepatic stress may result in reduced levels of plasma alanine aminotransferase, a biomarker of fatty liver disease, found in TRF mice. TRF liver histology also implies a healthier liver. TRF mice had significantly less hepatic steatosis compared to those from the ad libitum–fed mice. In addition, volume analyses of serial block-face scanning electron microscope images of the liver samples revealed they have increased volume of mitochondria and endoplasmic reticulum relative to the liver of ad libitum–fed mice (65).
Time-restricted feeding effects on gut function
Humans exhibit a daily rhythm in the production of saliva, gastric acids, digestive enzymes, and bile salts, all of which decline late at night. Intestinal peristalsis also shows a daily rhythm with reduced contractions at night and increased colonic movement in the early morning driving a daily rhythm in excretion. With these rhythms in ingestion, secretion, digestion, absorption, and excretion, there is a daily rhythm in the gut chemical environment. Accordingly, the gut microbiome composition and function also vary throughout the day (84). Nightly increase in mucus secretion and cellular repair are activated to maintain gut integrity. The maintenance of the gut intestinal lining integrity is critical in maintaining intestinal barrier function that is essential to prevent potential food allergens and bacterial lipopolysaccharide breaching through the gut and causing systemic inflammation. Although it is too early to measure how TRF affects these gut functions, these daily rhythms in gut physiology offer a framework to understand the impact of TRF on gut health.
A survey of gut microbiome composition across 24 hours in mice revealed that TRF decreases the relative amounts of presumed obesogenic microflora and increases relative amounts of presumed obesity-protective microflora (85). Specifically, the abundance of Lactococcus species directly correlates with body fat percentage in mice consuming an obesogenic diet. TRF significantly reduces the abundance of Lactococcus species specifically during the inactive or rest phase of mice. Similarly, the abundance of Lactobacillus species is correlated with metabolic disorders; a decrease in Lactobacillus is protective against metabolic diseases in obesity. TRF mice had significantly reduced levels of Lactobacillus species. Conversely, TRF mice also had relatively high levels of Oscillobacter and Ruminococcaceae species that are protective against obesity and nonalcoholic fatty liver disease. Although it is generally accepted that having a diverse group of species in the gut microflora is protective against obesity and metabolic diseases, there was no significant difference in the overall diversity or species richness between mice fed an obesogenic diet ad libitum or TRF (85). However, we cannot rule out that the gene expression pattern of these species is affected by ad libitum or an TRF eating pattern, which might affect microbiome-host interaction leading to changes in luminal metabolite content.
Metabolomics analyses of fecal matter can explain some of the improvements in TRF mice. Hemicellulose in the diet is typically broken down by the gut microbes to xylose and galactose, some of which is absorbed by the host. Relative to ad libitum–fed mice, the TRF mice excreted significantly more xylose and galactose in their stool, which implies that TRF reduced host absorption of these simple sugars. The stool of TRF mice was also rich both in primary and secondary bile acids (85). Usually, a significant portion of bile acids is reabsorbed from the gut. Elevated levels of bile acids in the stool of TRF mice indicates some of the reduction in hepatic and serum cholesterol in TRF mice may be due to net elimination of bile acids in the stool. Furthermore, the elevated luminal concentration of bile acids in these mice might also affect bile acid signaling in the gut as well as the generation of secondary bile acids by the microbiome.
An important role of the gut is in gut-brain communication to optimize the timing of hunger and meal size. Mechanosensitive pathways sensing gut distention and chemical pathways sensing meal composition participate in this gut-brain communication. Mechanosensitive gastric vagal afferents exhibit diurnal rhythmicity in their response to food-related stimuli, allowing time of day–specific satiety signaling (86). When an obesogenic diet is given ad libitum to rodents, this diurnal rhythmicity is ablated, which may enable animals to consume food at a nonpreferred time. The loss of diurnal rhythm in the gastric vagal afferents axis can increase hyperphagia and obesity. TRF of the same obesogenic diet has been reported to restore this daily rhythm in gastric vagal afferents responsiveness to meal size (87). This observation in rats, if conserved in humans, might explain the reduced hunger at bedtime among human volunteers practicing TRE (75, 88).
Time-restricted feeding effects on the integrated stress response
Transcriptome and metabolomics studies in the liver of mice that have an intact or dysfunctional circadian clock have revealed integrated stress response (ISR) pathways may contribute to the TRF benefits (11). These studies showed a diurnal rhythm in nutrient-sensing pathway activation, in particular activation of the mTORC1 pathway and in amino acids known to engage this pathway (phenylalanine, tryptophan, and leucine). mTORC1 also plays a major role in activating the ISR, a physiological cellular response that allows restoration of homeostasis following exposure to a variety of stressors. One of the hallmarks of ISR activation is inhibition of general protein translation while increasing the quality control of mRNAs and proteins. These include hundreds of transcripts involved in the processing of pre-mRNA, RNA, protein folding (chaperonins), unfolded protein response, response to endoplasmic reticulum stress, protein targeting to organelles, and vesicle-mediated transport (89). Many chaperones specifically expressed in TRF are integral to ISR (eg, the TCP protein family). Remarkably, TCP chaperones are also induced in response to TRF in the Drosophila heart. Flies carrying deletion or knockdown of TCP components do not show beneficial effects of TRF, thus supporting an essential role of the TCP class of chaperonins in TRF (90). Induction of ISR pathways is increasingly recognized as prosurvival and sustenance of cellular homeostasis in the face of various stresses (89). If TRF induces ISR in multiple tissues, then it can also have preventive or therapeutic benefits against many diseases linked to disrupted protein folding or proteostasis.
Time-Restricted Feeding Effects on the Endocrine Regulators of Physiology and Metabolism
There is much interest in the effects of restricting the eating window on endocrine regulators, such as glycemic (insulin) and appetite (leptin, ghrelin, glucagon-like peptide-1 [GLP-1]) regulators. Yet, there are several facets to consider, namely isocaloric vs ad libitum intake and eating window timing. As restricting the eating window in humans despite ad libitum intake frequently results in reduced caloric intake and weight loss (88, 91), this section will focus on the effects of isocaloric TRE on endocrine regulators.
In mice, isocaloric TRF during the dark cycle improves glucose tolerance and insulin resistance (65, 68, 69, 85, 92, 93) as well as increasing ghrelin (92) and adiponectin (68) and reducing leptin (65, 68, 69, 92). In humans, restricting the eating window improves glucose tolerance in the setting of prediabetes (75) and normal glucose tolerance (77, 94). A 24-hour inpatient study examining the effects of isocaloric TRE (5 days, 10 am to 5 pm) vs extended eating (7 am to 9 pm) found that TRE was associated with lower 24-hour glucose levels, primarily due to lower nocturnal glucose levels. However, 24-hour insulin, nonesterified fatty acids, and cortisol levels were not different between groups (94).
As TRE was initially derived from matching food intake relative to the active, dark phase in animals (65), there is much interest in the metabolic effect of early TRE in humans, both relative to an extended eating window (~ 12 hours) (75, 94-97) or late TRE (77). Generally speaking, in humans, eating earlier during the day is associated with improved glucose tolerance (98), higher thermic effect of food (99), and reduced overall energy intake (100). In men with prediabetes, a 6-hour TRE (dinner before 3 pm, 5-week intervention) with matched and supervised energy intake improved postprandial insulin, insulin sensitivity, and β‐cell function while reducing the subjective desire to eat and perceived capacity to eating in the evening relative to an extended eating window (12-hour eating window, 5-week intervention). Interestingly, fasting levels of ghrelin, GLP-1, leptin, and adiponectin remained unchanged with early TRE despite the subjective appetite improvement (75). In overweight adults without diabetes, 4 days of early eating (8 am to 2 pm for 4 days) reduced 24-hour glucose levels and glycemic excursions relative to an extended eating window (8 am to 8 pm) (95) while reducing mean ghrelin levels, appetite, and increasing fat oxidation (76). Although TRE improves glucose tolerance (~ 36% reduction in glycemic response to a test meal) compared to baseline, the effects of early TRE (8-5 pm for 7 days) vs late TRE (noon-9 pm for 7 days) remain extremely modest (77). Early TRE reduced mean fasting glucose relative to late TRE, whereas the effects on glucose tolerance, fasting ghrelin, ghrelin response to oral glucose tolerance testing, fasting GLP-1, gastric emptying, or hunger assessment (perceived hunger, fullness, desire to eat) were not significantly different (77). In contrast, a significantly delayed TRE may be associated with adverse effects. Eating only one meal every day (4-8 pm for 8 weeks) was associated with higher fasting glucose, impaired glucose tolerance, increased ghrelin (101), and increased hunger measures (97) relative to an extended eating window (3 meals per day for 8 weeks).
Given the setting of isocaloric intake, TRE particularly earlier in the day is associated with the most marked improvements in endocrine regulators and appetite reduction. Yet, many barriers exist to implementing early eating window restriction in humans, notably the social and societal influences that encourage late eating (102) that will need to be addressed in future studies.
Timing of food intake and human health
Timing of diet and human health outcomes have a relatively long history. For more than 30 years it has been well known that an evening or late-night meal produces higher postprandial glucose than in response to the same meal ingested in the morning (103, 104). This difference in postprandial glucose response may arise from at least 2 different mechanisms linked to the circadian clock. There is a circadian rhythm in insulin release that in healthy humans is higher during the first half of the day (105, 106). At night, the rise in melatonin before bedtime can inhibit glucose-induced insulin release from the pancreatic β cells (48). Hence, consuming a bigger portion of daily caloric intake in the first half of wakeful hours may be preferred for better blood glucose regulation, body weight control, and related health outcomes. In light of this new finding from circadian rhythm, it is worth revisiting the belief that breakfast skipping is detrimental to health. The reports of breakfast skipping having detrimental health effects often failed to highlight that those skipping breakfast might have had late-night eating events (107, 108). Therefore the causal link between breakfast skipping and health outcomes remains inconclusive.
Some human dietary studies have also found the timing of food intake can modulate health benefits. A caloric reduction intervention study found greater weight loss if lunch is consumed earlier rather than later (109). Another study found earlier dinner consumption was associated with reduced risks for certain types of cancer both in males and females (110, 111). Earlier dinner may inadvertently impose a longer overnight fast and a shorter eating window during the day. Accordingly, retrospective analyses have found prolonged overnight fast is associated with a better prognosis of breast cancer and reduced risk for breast cancer relapse (112, 113).
Time-restricted Eating in Humans
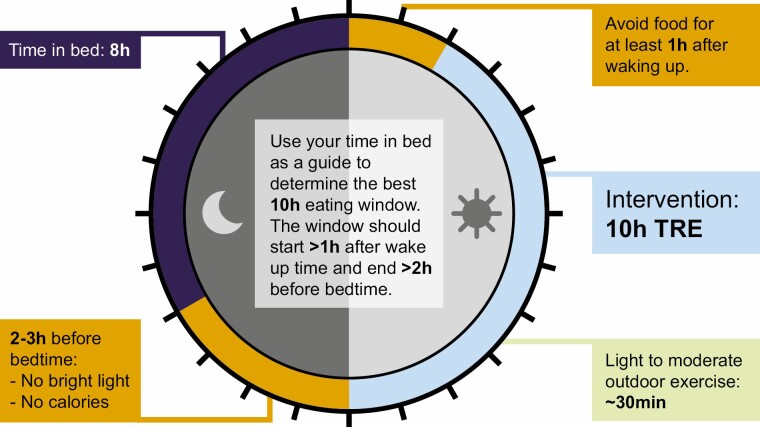
A basic tenet of any dietary intervention is to define the intervention, measure the relevant dietary factor before delivering the intervention, and measure it during the intervention. It is also desirable to collect other behavioral data for accurate interpretation of outcome. For example, calorie restriction studies often measure the calorie intake of study participants from food diaries or through 24-hour recalls delivered by trained professionals at baseline, set a target of calorie reduction, and measure actual calorie intake during the intervention. Similarly, it is expected that TRE studies should measure baseline eating pattern or eating window, and monitor it for at least 1 to 2 weeks during the intervention to measure compliance and interpret results. Because a shorter window of TRE can inadvertently reduce calorie intake, affect sleep, and may potentially change physical activity, it is also desirable to measure sleep, and physical activity at baseline and during intervention. Furthermore, some benefits of TRE arise from the period of abstinence from food or caloric intake that can influence endocrine or physiological response to fasting. Hence, it is also important to note whether study participants are allowed to consume low-calorie food/beverages during the fasting period. The choice of the TRE window relative to wake up and sleep time would also inadvertently interact with daily variation in endocrine factors (eg, melatonin or insulin production). Hence, it is desirable to pay attention to several factors (Fig. 1) for carrying out a successful TRE intervention study.
Figure 1.
A schematic of ideal time-restricted eating (TRE) intervention for long-term adherence by incorporating the optimal sleep time and duration relative to TRE interval. Because bright light is known to inhibit the production and secretion of sleep promoting hormone melatonin, it is desirable to avoid bright light for 2 to 3 hours before habitual bedtime to promote better sleep. Adequate sleep is known to reduce food craving, which can support adherence to TRE. Since melatonin can potentially attenuate glucose-induced insulin release from the pancreas, avoiding food before bedtime immediately after wake up may further accentuate the TRE benefit on glucose regulation. The eating interval of 10 hours is illustrated as an example.
When do humans eat?
To understand when people eat and its relevance to TRE, we have to first define what constitutes eating. In this context, eating will be defined as caloric or any other ingestion event that may affect a neuroendocrine, endocrine, or metabolic factor that is associated with the state of fasting. Calorie consumption of as little as 5 Kcalories, artificial sweeteners (114), and caffeine (115, 116) can each influence these factors.
What is an eating pattern in relation to time-restricted eating?
Eating pattern takes into account the timing of eating including the time of day/phase, frequency, and variation. In the context of TRE, it is important to understand the window of time during which individuals are eating and when they are fasting. As the circadian system is anticipatory, and change in eating time, especially breakfast time, can reset the circadian clock in peripheral organs, the consistency of the eating times is crucial and should be incorporated into calculating the eating window. To do this, the eating window is calculated as the 95% interval of all eating events logged over 7 to 14 days or more. This excludes a few outlier calorie ingestive events, while still taking variability into account (88, 91, 117). Another advantage of this approach is that this method is relatively resilient to a person’s inability to log every meal during the observation period.
How to measure eating patterns.
Capturing the timing of all ingestion events (except for water and medication) in a free-living population is rare and has been documented only recently. Even within most TRE clinical trials, the timing of eating and the eating window are not assessed (Table 1). When temporal food intake is captured, it can be done via food recalls, written or online food journals, or smartphone applications created for tracking energy intake and/or circadian lifestyle. Food recall and written food journals are typically for only 1 to 7 days and are frequently inaccurate (155, 156). Assessing solely the first and last calorie intake can be informative in describing the eating window but fails to fully capture eating pattern. Smartphone applications, such as the myCircadianClock app, have simplified continuous logging of all intake events for extended durations of time (≥ 1-16 weeks) (88, 91, 117). This continuous collection of data allows for a full description of the eating pattern and the simplicity of logging (taking a time-stamped picture and making a quick annotation) provides a large amount of information while easing participant burden.
Table 1.
Time restricted studies in humans: experiment design and outcomes
| Study | Design | Participants | Baseline BMI (mean (SD)a | Age, y (mean (SD)) | TRE intervention; duration of intervention | Method of tracking temporal eating pattern/adherence | Adherence to TRE intervention (mean (SD)) | Baseline eating duration, h | TRE eating duration, h | Major findings, short version |
|---|---|---|---|---|---|---|---|---|---|---|
| LeCheminant et al, 2013 (118) | RXT | 27 Healthy M | 24.4 (2.5) | 20.9 (2.5) | 13-h TRE (6 am-7 pm, eliminated night time eating); 2 wk in each are w/ 1-wk washout | Self-reported and computerized multiple-pass 24 h recall (4 across 2-wk lead-in period) | 93.6% (5.6 %) (13/14 days). Self-reported | Not reported | Not reported | ↓ energy intake, body weight, and BMI, ↑ hunger on waking, no change in mood |
| Gill and Panda, 2015 (88) | Observation/pre-post | 156 (91 F) adults; intervention: 8 (3 F) adults with BMI > 25 and ≥ 14-h eating window | Observation: 24.74 (95% CI, 23.94-25.53); TRE: male: 31.77 (2.05); F: 34.91 (3.84) | 27.6 (26.4-28.8); intervention M: 34.4 (2.9); F: 36.3 (4.3) | 10-h TRE, self-selected; 16-wk w/1-y follow-up | myCircadianClock App: daily logging | Not reported | 95% eating window; Median 14.75 h | 10-12 h, (deceased by 4.32 h), actual eating duration not reported | ↓ body weight, BMI, hunger at bedtime, and energy intake, ↑ sleep satisfaction and energy level; No change in BMI in observation only (3 wk) |
| Moro et al, 2016 (96) | RCT | 34 M resistance-trained | a TRE: 26.5; Normal Diet: 27.2 | 29.21 (3.8) | 8-h TRE in 3 meals (1 pm, 4 pm, and 8 pm); Normal diet group had 3 meals (8 am, 1 pm, and 8 pm), both with diet and resistance training program; 8wks. | 7-d food diary validated by weekly structured interview with dietician to assess adherence to mealtime and diet composition | Not reported | Not reported | Not reported | ↓ fat-mass, IGF-1, and testosterone. No change in fat-free mass, muscle area of the arm and thigh, and maximal strength. |
| Tinsley et al, 2017 (119) | RCT | 18 M healthy young adult active | a 24.3 | 22 (2.4) | 4-hTRE(6pm-10pm) 4 d/wk (resistance training on nonrestricted eating d (3 d/wk); 8 wks | Dietary assessments at baseline, 4 wk, and 8 wk. Daily checklists completed on TRF days. | 80% compliance to TRE required to be included in analysis. Actual not reported | Not reported | Not reported | ↓ energy intake. No effect on lean mass retention or muscular improvement with training. |
| Antoni et al, 2018 (120) | NRCT | 13 (12 F) healthy adults; TRE = 7 (6 F), control = 6 (6 F) | a TRE: 29.0 (1.7); Control: 28.6 (2.8) | 29-57 (mean, SD not reported) | Shorten eating window by 3-h (delay first and advance last meal by 1.5 h each);10 wk | 4-day food diaries at baseline and wk 5 and 10 of intervention | 62.5% of d (2.5/4 d) | 12.4 h | 8.6 h | ↓ eating time and energy intake |
| Gabel et al., 2018; Gabel et al, 2019 (121, 122) | Pre-post with matched historical control with weight loss trial | TRE: 23 (20 F) adults with BMI 30-45; historical control 23(21 F) | TRE: 35.0 (1.0); control: 34.0 (1.0) | TRE: 50.0 (2.0); control: 48.0 (2.0) | 8-h TRE (10 am-6 pm); 12 wk | Daily adherence log (first and last calorie). And 7-d food journal during baseline and wk 12 of intervention | 80% of d, 5.6 (0.3) d/wk | 11 (1) h at baseline, self-reported | 8 (1) h | ↓ body weight and systolic blood pressure. No change in fat mass or fasting glucose. No adverse events |
| Gabel et al, 2020 (secondary analysis of (121)) (123) | Pre-post | 14 Adults with BMI 30-45 (sex not specified) | Weight: 94.85 (3.77) kg | 25-60 (mean, SD not reported) | 8-h TRE (10 am-6 pm); 12 wk | Daily adherence log (first and last calorie). And 7-d food journal during baseline and wk 12 of intervention | 80% of d, 5.6 (1.1) d/wk | Not reported | Not reported | ↓ body weight, fat mass, and systolic blood pressure, ↑ heart rate. No change in lean mass or gut microbiotic phylogenetic diversity. |
| Sutton et al, 2018 (75) | RXT | 8 (0 F) adults with prediabetes | 32.2 (4.4) | 56.0 (9.0) | 6-h early-TRE (3 meals starting 6:30-8:30 am); 12-h control (3 meals); 5 wks in each arm w/ 7-wk washout | All meals provided and consumed under supervision | 100% to TRE and 98.9% (1.8%) to control | Not reported | ≤ 6 h based on adherence (actual not reported) | ↑ insulin sensitivity and β-cell function, ↓ blood pressure, oxidative stress, evening appetite, and postprandial insulin. No change in body weight |
| Hutchison et al, 2019 (77) | RXT | 15 M (aged 30-70) with waist circumference ≥ 102 cm | 33.9 (0.8) | 55.0 (3.0) | 9-h TRE early and delayed (eTRE: 8 am to 5 pm; dTRE: noon-9 pm); 4 wk in each arm w/ 2-wk washout | Daily food diaries. | Not reported | ~ 9-15 h | 9 h assigned (actual not reported) | ↓ Glucose AUC (eTRE and dTRE, ↓ fasting glucose by CGM (eTRE) |
| Tinsley et al, 2019 (124) | Randomized, placebo-controlled-reduced factorial design | 40 (ITT)/24 (PP) resistance-trained women | TRE: 23.8; TREHMB: 22.9; Control: 22.5 | TRE: 22.1 (2.1); TREHMB: 22.3 (3.4); control: 22 (2.4) | 8-h TRE (noon-8 pm); 8 wk | Diet records at baseline and 2 follow-up assessments | Not reported. 80% compliance to TRE required to be included in PP analysis | Not reported | TRF: 7.5 (1.6) h; TRFHMB: 7.6 (0.7) h | ↓ fat mass in TRE and TREHMB in PP analysis, but not ITT. ↑ muscular performance in all groups |
| Ravussin et al, 2019, Jamshet et al, 2019 (76, 95) | RXT | 11 (4 F) adults with BMI 25-35 | 30.1 (2.7) | 32.0 (7.0) | 6-h eTRE: 8 am-2 pm (meals at 8 am, 11 am, and 2 pm), Control: 8 am-8 pm (meals at 8 am, 2 pm, and 8 pm); 4 d in each arm, 4- to 5-d washout | Not reported for the first 2 d. D 3 and 4 meals provided and consumption supervised | Not reported | Not reported | 6 h (in-lab d) Not reported for outpatient d | eTRE: ↓ mean 24-h glucose levels and glycemic excursions, ↑ MTOR expression, and altered lipid metabolism and circadian clock gene expression |
| Kesztyus et al, 2019 (125) | Pre-post | 40 (31 F) adults with ≥ 1 component of metabolic syndrome (63% on daily medication) | 31.3 (5.9) | 49.1 (12.4) | 8- to 9-h TRE; 12 wk | Daily food journal of first and last calorie. | 85% (15.2%) of d | 12.23 (2.27) h | 7.73 (0.82) h | ↓ weight, waist circumference, and HbA1c |
| Kesztyus et al, 2020 (secondary analysis of 2 studies) (126) | Secondary analysis of 2 pre-post studies (1 at university and 1 through general practitioner) | 99 (83 F) adults | 28.0 (5.7) | 48.9 (1.1) | 8- to 9-h TRE; 12 wk | Daily food journal of first and last calorie | 77.2% (18.7%) of d | University: 12.4 (1.8) h n = 61; general practitioner: 12.3 (1.2) h n = 38 | University: 8.4 (0.9) h n = 61; general practitioner: 7.7 (0.8) h n = 38 | ↑ quality of life and sleep quality |
| McAllister et al, 2019 (127) | Pre-post | 22 healthy M | 28.5 (8.3) | 22 (2.5) | 8-h TRE, self-selected (randomized to ad lib calorie or decreased by 300/d); 4 wk | MyFitnessPal and daily logging of time of first and last calorie | Not reported | Not reported | 7.2 (0.7) h | ↓ body fat, blood pressure, and ↑ adiponectin and HDL-c |
| Wilkinson and Manoogian et al, 2020 (91) | Pre-post | 19 (6 F) adults with metabolic syndrome and eating window ≥ 14 h | 33.06 (4.76) | 59 (11.14) | 10-h TRE, self-selected; 12 wk | myCircadian Clock App: daily logging | Participants outside their eating window by > 1 h on 7.13% (7.55) of days. | 95% eating window: 15.13 (1.13) h | 95% eating window: 10.78 (1.18) h | ↓ body weight, blood pressure, waist circumference, LDL cholesterol, non-HDL cholesterol, HbA1c, and ↑sleep restfulness in participants already on medication |
| Chow et al, 2020; Malaeb et al, 2020, Lobene et al, 2021; Crose et al, 2021 (117, 128-130) | RCT | 20 (17 F) adults with BMI ≥ 25 and eating window ≥ 14 h | 34.1 (7.5) | 45.5 (12.1) | 8-h TRE, self-selected; 12 wk | myCircadian Clock App: daily logging | 60% (23%) of d ± 30 min of eating window | 95% eating window: TRE (n = 11): 15.2 (0.7) h non-TRE (n = 9): 15.5 (1.1) h | 95% eating window: TRE (n = 11): 9.9 (2.0) h non-TRE (n = 9): 15.1 (1.1) h | ↓ body weight, lean mass, visceral fat; ↓ eating events and caffeinated beverages; TRE does not adversely alter bond turnover; ↑ quality of life |
| Zeb et al, 2020 (131) | RCT (2:1) | 80 young healthy M | TRE: 24.14 (3.5): control: 26.13 (5.2) | Not reported | 8-h TRE (19:30-03:30); 25 d | Not reported | Not reported | Not reported | Not reported | ↑ enrichment of microbiome, and circadian gene expression, improved lipid and liver profiles |
| Lowe et al, 2020 (132) | RCT (66 remote/50 local) | 116 (46 F) adults with BMI 27-43 | 32.7 (4.2) | 46.5 (10.5) | 8-h TRE (noon-8 pm); 12 wk | Smartphone App: asked participants daily if they were compliant with TRE | 22.8% (1128/4956 d) d reported in TRE and 22.7% (1088/4788 d) in control. Of reported d, 92.1% (1002/1088) in control; 83.5% (1128/1351) in TRE | Not reported | Not reported | ↓ body weight in TRE and control groups |
| Cienfuegos et al, 2020 (133) | RCT (3-arm) | 49 (5 F) adults with BMI 30-49.9 | b 4-h TRE: 36.0 (1.0); 6-h TRE: 37.0 (1.0); Control: 36.0 (1.0) | b 4-h: 49.0 (2.0 y); 6-h: 36.0 (3.0) y; 45 (2.0) y | 4-h TRE (3-7 pm), 6-h TRE (1 pm and 7 pm); 8 wk | Daily adherence log (first and last calorie) w/ weekly review with study coordinator | 89% for both groups, b4-h: 6.2 (0.02) d/wk; 6-h: 6.2 (0.1) d/wk | b 4-h: 13.2 (0.5) h (8-14h); 6-h: 13.4 (0.6) h (5-15 h) | Assumed intervention given high adherence | Similar results for 4- and 6-h TRE: ↓ body weight (–3.2% for both), insulin resistance, and oxidative stress for both TRE regimens produced compared to controls |
| Anton et al, 2019; Lee et al, 2020 (134, 135) | Pre-post | 10 (6 F) adults ≥ 65 and with BMI 25-45 | 34.1 (3.3) | 77.1 (SD not reported) | 8-h TRE (consistent timing not required), 12-h eating interval allowed first wk; 4 wks | Daily food journal of first and last calorie. | 84% of d | Not reported | Not Reported | ↓ body weight and BMI; TRE was safe and feasible for older adults with no change in energy levels while fasting |
| Parr et al, 2020; Lundell et al, 2020 (94, 136) | RXT | 11 M with BMI 27-35 | 32.2 (2.0) | 38.5 (5.0) | 8-h TRE (10 am, 1 pm, and 5 pm) and 15-h control (7 am, 2 pm, and 9 pm); 5 d each arm, w/ 10-d washout | Written food diary (3 d in each condition). | 6.7/10 self-reported score of ability to adhere to TRE (1 being not possible). Adherence to 5-d intervention not reported | Not reported | Not reported | TRE improved nocturnal glycemic control and was positively perceived. TRE affects rhythmicity of serum and muscle metabolites and regulates rhythmicity of genes controlling amino acid transport without disrupting clock gene expression |
| Parr et al, 2020 (137) | Pre-post | 19 (10 F) adults with T2DM (HbA1c > 6.5 and < 9%) and eating window > 12 h | 34.0 (5.0) | 50.0 (9.0) | 9-h TRE (10 am-7 pm); 4 wks | Written food diary or smartphone app (EasyDietDiary) to record all food entries. Photos of each food/beverage also taken using phone | 72% (24%), 5 d/wk | > 12 h, exact not reported | 72% adherent to 9-h eating window, exact not reported | TRE feasible and did not alter dietary intake, psychological well-being, or cognitive function. Focus should be on overcoming barriers. |
| de Oliveira Maranhão Pureza et al, 2020 (138) | RCT | 27 F (age 19-44 y) with low-income and BMI 30-45 | TRE + HD: 33.53 (4.53); HD: 33.12 (3.63) | TRE + HD: 31.03 (7.16); HD: 31.80 (6.96) | 12-h TRE with hypoenergetic diet (TRE + HD) or hypoenergetic diet only (HD); 12 mo | Three 24 h food records (2 work days, one weekend). Monthly meetings. | Not reported | Not reported | Not reported | Nonsignificant ↓ in % body fat, and waist circumference. Small ↑ in body temperature in TRE compared to control. No change in body weight |
| Martens et al, 2020 (139) | RXT | 22 (12 F) healthy midlife to older adults | 24.7 (0.6) | 67.0 (1.0) | 8-h TRE (self-selected w/ start 10-11 am); 6 wk | Daily electronic survey administered via email sent at 7 pm daily—participants reported first and last eating event | 84% to 8-h eating window, 95% were adherent to 8.5-h eating window | ~ 12 h, exact not reported | ~ 8 h, exact not reported | TRE safe, well-tolerated, and associated with high adherence and decreased hunger. No change in lean mass, bone density, nutrient intake, or cardiovascular function. Endurance and glucose tolerance modestly improved. |
| Kim et al, 2020 (140) | Pre-post | 15 (6 F) adults with BMI ≥ 25 | 29.3 (4.6) | 36.8 (8.44) | 8-h TRE (noon-8 pm), 2 provided meals/d; 4 wk | Called participants every morning to confirm compliance | Not reported | Not reported | Not reported | ↓ body weight; improvement of sleep apnea |
| Moro et al, 2020 (141) | RCT | 16 M elite cyclists | 21.85 (1.65) | 19.38 (2.39) | 8-h TRE (meals: 10-11 am, noon-1 pm, and 6-7 pm); control 7 am-9 pm (meals: 7-9 am, noon-1 pm, and 7-9 pm) and 1 snack w/ exercise for both groups; 4 wk | Dietician supervised most meals and ensured participants followed intervention | Not reported | Eating window not reported. Participants reported breakfast 7 am-9 am and dinner 7 pm-9 pm at baseline | Not reported | ↓ body weight, % fat mass, free testosterone, IGF-1, neutrophils to lymphocyte ratio, and ↑ peak power/body weight, ratio |
| Schroder et al, 2021 (142) | NRCT | 32 F with BMI ≥ 30 | TRE: 32.53 (1.13); control: 34.55 (1.20) | TRE: 36.6 (1.6); control: 42.3 (3.5) | 8-h TRE (noon-8 pm); 12 wk | Not assessed | Not reported | Not reported | Not reported | ↓ body weight, BMI, % body fat, waist circumference, and 30-y cardiovascular disease risk |
| Peeke et al, 2021 (143) | Virtual RCT | 60 (53 F) adults (18–65) with BMI ≥ 30 | 38.9 (7.7) | 44.0 (11.0) | TRE: 10-h TRE w/ snack at 12-h of fasting; active control: 12-h TRE. All participants on Jenny Craig diet (3 provided meals and 1 fruit snack/daily); 8 wk | Weekly phone visits and provided food | Not reported | Not reported | Not reported | TRE (14:10) ↓ body weight (within group and compared to 12:12 control) and ↓ fasting blood glucose (within group) |
| Przulj et al, 2021 (144) | Pre-post | 50 (37 F) adults with obesity with BMI ≥ 30 or >28 with comorbidities | 35.1 (4.0) | 50.1 (12.0) | 8-h TRE, self-selected; 12 wk | Clinic visits 1, 6, and 12 wk. Phone calls wk 2, 3, 4, and 5 | ~ 5 d/wk (wk 1-6, n = 32-47) and 5.1 (2.4) d/wk in wk 12 (n = 39) | Not reported | Not reported | ↓ body weight and hunger over intervention |
| Phillips et al, 2021 (145) | Observation and RCT | Observation: 213 adults (151 F) with BMI ≥ 20; RCT: 54. w />14-h eating window and ≥ 1 component of metabolic syndrome | Observation: 24.9 (22.6-29.1), RCT: 28.3 (24.6-30.5) | Observation: 40.1 (13.3), RCT: 43.4 (13.3) | Observation: 4 wk; RCT: 12-hTRE or standard dietary advice (SDA); 6 mo | myCircadianClock app: daily logging; phone or email reminders at 2 wk of observation phase and at 3 and 4 mo of intervention | Not reported | TRE: 15.48 (1.14) h, SDA: 15.17 (0.96) h | TRE: 12.50 (1.29) h, SDA: 14.89 (1.21) h | ↓ body weight (1.6%) |
| Domaszewski et al, 2020 (146) | RCT | 45 F age > 60 | All: 27.9 TRE: 28.99 (5.18); control: 26.99 (4.20) | 65 (5) | TRE: 8-h (noon-8 pm); 8 wk | Weekly diet reports from participants | 22/25 participants completed 6-wk program; excluded if not adherence for > 10% of intervention d | Not reported | Not reported | ↓ body weight, BMI, and fat mass, TRE better accepted than other forms of intermittent fasting |
| Jones et al, 2020 (147) | RCT | 16 healthy M | 24.0 (0.6) | 23 (1) | TRE: 8-h eTRE (8 am-4 pm), control/caloric restriction: matched to consumption of eTRE group; 2 wk | Food diaries throughout baseline and intervention | 1 event outside eating window, supported by CGM | TRE: 12.32 (0.25) h, control/CR: 11.33 (0.45) h | TRE: 6.87 (0.27) h, Control/CR: 11.68 (0.37) h | TRE improved whole-body insulin sensitivity and ↑ skeletal muscle glucose and BCAA uptake; ↓ energy intake and body weight matched in control/CR |
| Kesztyus et al, 2021 (148) | Pre-post | 63 adults (54 F), employed and without metabolic conditions (61 completed) | 26.1 (4.6) | 47.8 (10.5) | 8-9 h-TRE; 12 wk | Daily food journal of first and last calorie. | Fasting target reached 72.2% (18.9%) of recorded d | 12.42 (1.88) h | 8.38 (0.91) h | ↓ body weight and waist circumference, ↑ health-related quality of life, TRE was feasible and well accepted in working adults |
| McAllister et al, 2020 (149) | Pre-post | 16 M, resistance-trained firefighters | 21.3 (4.4) (n = 15) | 37 (6) (n = 15) | 10-h TRE; 6 wk | Zero smartphone app to document first and last food intake daily, or written food log and MyFitnessPal or food diary to track food intake. For 3-d pre- and post-TRF testing sessions | Not reported | 14.9 (0.2) h | 9.1 h (0.2 h) | ↓ advanced oxidation protein products and advanced glycated end products in blood |
| Pureza et al, 2020 (150) | RCT | 58 obese F living in social vulnerability | TRE (n = 31): 31.80 (29.25-34.36); control (n = 27): 31.03 (95% CI, 28.20-33.87) | TRE (n = 31): 33.53 (32.00-35.50); control (n = 27): 33.12 (95% CI, 31.68-34.56) | TRE: 12-h daily fasting (times could change) with hypoenergetic diet, control: hypoenergetic diet, 21 d, follow-up at 81 d | TRE: analog scale of difficulty. Eating pattern not monitored. For diet: 24-h dietary recalls (2 on weekdays and 1 on a weekend day), weekly consultations with dietician for 21 d, then every 2 wk until end of 81-d intervention | Not reported | Not reported | Not reported | ↓ body fat at 21 d and waist circumference at 81 d |
| Li et al, 2021 (151) | Pre-post | 15 F with PCOS | 29.75 (4.31) | 18-31 (mean and SD not provided) | 8-h TRE (8 am-4 pm); 5 wk | Food diary and record daily food intake in the Boohee software (diet and fitness app in China to calculate calories). Weekly phone calls | Not reported | Not reported | Not reported | ↓ body weight, BMI, body fat mass, body fat %, visceral fat area, total testosterone, sex hormone-binding globulin, free androgen index, fasting glucose, fasting insulin, HOMA-IR, AUCInsulin, AUCInsulin/AUCGlucose ratio, lipids, uric acid, ALT, hs-CRP, and IFG-1 |
| Prasad et al, 2021 (152) | Pre-post | 50 (41 F) adults (observation, 16 (TRE intervention) with BMI 25-50 and ≥ 14-h eating duration | Observation: 31.0 (10.8) | 51 (12) | 10-h TRE self-selected; 90 days. | myCircadianClock app: daily logging | 47% (19%) of d in intervention (daily logging and within eating window) | 95% eating window: observation (n = 50): 14.53 (2.60) h; TRE (n = 16): 16.07 (1.40) h | 95% eating window: TRE, n = 16: 11.90 (2.10) h | ↓ body weight, ↓ waist circumference, ↓ systolic blood pressure |
| Brady et al, 2021 (153) | RCT | 23 M middle- and long-distance runners | 23.6a | 36.4 (7.4) | 8-h TRE; 8 wk | Daily food log of eating windows. Full food diaries at baseline, 4 wk, and 8 wk | 94.5% (5.1) of d | Not reported | TRE: 7.9 (0.2) h, control: 12.9 (1.0) h | ↓ body weight and energy intake. No change in running performance or endurance |
| Bjerre et al, 2021 (154) | Pre-post interviews from TRE group of ongoing RCT (RESET study) | 17 (11 F) adults w/ BMI ≥ 30 and eating window of ≥ 12 h and at ≥ d/wk ≥ 14 | 32.9, range: 27.7-47.1 | 46-68 (11 > 60) | 10 h TRE, self-selected 6 am-8 pm; 12 wk | Food diary, 3 d (2 weekdays and 1 weekend) at baseline, 6 wk, and 12-wk | Self-reported: 100% for 2 participants, noncompliant 1-5 d/12 wk for 7 participants, noncompliant ≥ 1 d/wk for 8 participants | Not reported | Not reported | Participants had to rearrange daily activities to adhere to TRE. Easy for some and barrier for others. Participants delayed breakfast and advanced dinner by 1-2 h |
All TRE interventions are consistent eating windows unless noted.
Abbreviations: ALT, alanine aminotransferase; AUC, area under the curve; dTRE, delayed time-restricted eating; F, female; HbA1c, glycated hemoglobin A1c; HOMA-IR, homeostatic model assessment of insulin resistance; Hs-CRP, high-sensitivity C-reactive protein; IFG-1, insulin-like growth factor-1; ITT, intention to treat analysis; LDL, low-density lipoprotein; NRCT, nonrandomized control trial; M, male; PCOS, polycystic ovarian syndrome; PP, per protocol analysis; RCT, randomized controlled trial; RXT, randomized crossover trial; SOC, standard of care; TRE, time-restricted eating; TRF, time-restricted feeding; TRFHMB, TRF with 3 g/d of leucine metabolite b-hydroxy b-methylbutyrate. German Register for Clinical Trials (DRKS). NIH Clinicaltrials.gov Number (NCT). TREe/eTRE, early time-restricted eating; T2DM, type 2 diabetes mellitus.
a If BMI was not reported, BMI was calculated from reported height/weight. If height was not reported, weight was provided. CI is provided if that was reported in place of SD.
b Mean (SEM) reported in place of mean (SD). Eating duration is reported as daily average unless otherwise noted.
Various approaches to time-restricted eating
TRF models in rodents and flies have used 8- to 12-hour eating windows and found similar benefits, with greater results at 8- to 9-hour eating windows (65, 68, 85, 93). Animal models of TRF have not used eating intervals shorter than 8 hours to ensure that both TRE and ad lib groups can consume the same amount of calories. When food is restricted to less than 8 hours, there is a calorie deficit that can confound results. In humans, clinical trials on TRE typically choose 8-to 10-hour intervals, with some studies using an eating interval as short as 4 or 6 hours and others as long as 12 hours (see Table 1). Interestingly, a 12-hour eating window has also been used as a control arm in TRE intervention (75, 143). A recently completed 12-hour TRE intervention failed to find significant improvement in factors for metabolic syndrome (145). However, it is unclear if patients who habitually eat over 16 hours or longer will benefit from a 12-hour TRE. Although most TRE trials in humans do not overtly restrict calorie intake and/or recommend any dietary recommendations, participants on TRE with ad libitum intake still commonly reduce their calories by 7% to 22% (88, 91, 118-120).
How to choose an eating window
Duration.
There is not yet a consensus on the duration of a daily eating window that is needed to achieve the benefits of TRE. In rodents, cardiometabolic benefits were still observed for 9- and 12-hour eating windows. However, additional improvements in endurance were observed with a 9-hour eating window, but not 12-hour (68). In clinical trials, a 12-hour eating window has been used as an active control compared to a 6-hour and 10-hour eating window (75, 143). In both cases, there were significant metabolic benefits in comparison to the 12-hour eating window. Multiple trials with 10-hour eating windows have shown comparable health benefits to short eating windows of 6 to 8 hours. Moreover, eating windows of 6 hours or less have reported mild adverse side effects such as headaches. A study comparing 4-hour TRE to 6-hour TRE found no significant differences between the groups (133). Thus, an eating window of 8 to 10 hours is an eating window that has shown promising results and is long enough to work with most schedules to allow for proper compliance. More studies are needed to determine the optimal eating window for a variety of individuals.
Phase.
An eating window should be a consistent daily interval that optimally aligns endocrine mediators of sleep and alertness with eating and fasting. To achieve this, it is ideal for individuals to consider their sleep patterns and any required meal times. First, an eating window should be concurrent with the active phase to coordinate with circadian rhythms in metabolism and sleep. Melatonin is secreted at night, and in the absence of light, to aid sleep. Melatonin also inhibits insulin secretion. Thus, eating when melatonin levels are high (late at night, or in the early morning), can inhibit a proper glucose response to food. As a general guide, to avoid eating when melatonin levels are high, choose an eating window that does not start for at least an hour after waking and at least 3 hours before sleep onset. Assuming there is an 8-hour window allotted for sleep, that leaves only a 12-hour window for possible eating times. Second, an eating window should be consistent and thus needs to be appropriate both for work and off days. If any meal times cannot be changed, such as a family dinner, it should be taken into consideration during the eating window selection. Finally, some research suggests eating in the earlier phase of the day is better than delayed eating (77). Thus, it may be beneficial to select an earlier eating window when possible.
Challenges of time-restricted eating in humans
Unlike in animal studies where time-restricted access to food is easy to impose in a vivarium, implementing TRE in humans faces challenges inherent in lifestyle intervention programs. Successful lifestyle interventions involve (a) evaluating the current lifestyle, (b) educational and informational program enabling desired behavioral change, (c) assessing compliance to the new lifestyle, and (d) measuring the impact of the lifestyle change both on clinical and psychosomatic outcomes. Because TRE is a relatively new intervention, there are a vast majority of published studies on TRE that do not report on the existing pattern of participants before the intervention, monitor eating patterns during the intervention, or report compliance other than self-reported compliance from participants (see Table 1). As seen with the Diabetes Prevention Program and numerous follow-up studies, the success of lifestyle intervention on obesity and diabetes prevention is closely associated with self-monitoring, education, and compliance (157-159). Nevertheless, the vast majority of TRE studies have reported positive health outcomes; which suggest that attention to the stages of TRE lifestyle intervention, self-monitoring, and compliance monitoring can further improve outcomes, identify potential hurdles, and develop cognitive behavioral therapy to adopt TRE.
Time-restricted eating clinical trials
Clinical trials on TRE started in 2013 to 2015 and have undergone a huge increase in the past 2 years alone (160). Similarly, there are a large number of ongoing clinical trials assessing TRE that will greatly improve our understanding of a TRE intervention in the coming years. Published studies have varied greatly in the duration and timing of fasting, duration of intervention, participant population, assessment of eating window, and outcomes (see Table 1). Here we will quickly review what has been published and note a few ongoing clinical trials.
There are 2 key factors needed to define a TRE intervention. First is the duration of the eating/fasting window. TRE clinical trials range from a 4- to 12-hour daily eating window (20-12 hours fasting) with most (30/39 studies reported) using an 8- to 10-hour daily eating window (16-14 hours fasting) (77, 88, 91, 94, 96, 117, 121-128, 131, 132, 134-137, 139-143). Others focused on the Δ change in the eating window, delaying the time of the first energy intake and advancing the last intake by 1.5 hour each, rather than a set eating window duration (120, 144, 146-149, 151-154) (see Table 1). Another study simply aimed to eliminate nighttime eating (fasting 7 pm-6 am), which allowed for up to a 13-hour eating window (118). Second is the phase or timing of the daily eating window. Some studies allow participants to choose the timing of the eating window that works for them (sometimes with restrictions relative to sleep) and others require a set time. In studies that set eating times, they may be early in the day or later in the day or even set the timing and number of meals and or snacks (see Table 1). This introduces meal frequency as another variable between studies.
The duration of the TRE intervention has ranged from 4 days to 1 year, with most (31/39 studies) between 4 and 12 weeks (see Table 1). Study outcomes have largely depended on the participant population being tested. TRE has been assessed only in adults to date, with individuals having overweight or obesity as the most common participant (18/39 studies). In these studies, body weight and associated measures (percentage of body fat, waist circumference, and body mass index) were common outcomes, with other metabolic health assessments such as glucose regulation and cardiovascular health also included in some studies. Other participant populations included healthy adults (7/39 studies) and active or professional athletes (5/39 studies). These studies assessed feasibility, performance, hunger, lean mass, physical fitness and body weight, and other cardiometabolic characteristics. Older populations were the focus of 2 studies, assessing feasibility/safety and body weight. Four studies studied the effects of TRE in participants who had a cardiometabolic disease or disease risk including one aspect of metabolic syndrome (125), metabolic syndrome and on medication (91), prediabetes (75), and type 2 diabetes (137). In these, as well as some studies in participants with obesity, a deeper assessment of glucose regulation and cardiovascular health was conducted. Surprisingly, only 6 studies had a long eating window at baseline (≥ 14 hours) as an inclusion criterion (88, 91, 117, 145, 152, 154). In fact, most studies did not assess eating windows at baseline or during the intervention. This speaks to the larger issues that most studies did not measure the timing of food intake throughout the study. Because the eating window and change in the eating window are key components of TRE, this lack of knowledge compromises the ability to conclude whether the results speak to the intervention or the potential lack of adherence. This is not an issue for some of the more controlled studies, but others require more participant engagement and/or assessment of energy intake to properly assess the effects of TRE.
The most common finding was a decrease in body weight and associated factors (24 studies, see Table 1). Associated factors such as percentage of body fat, body mass index, and waist circumference, were also decreased in many trials. Decreased energy intake was also observed in 6 studies without overt instruction to change diet (88, 91, 118-120, 153). One study found that changes in energy intake were not sufficient to explain the changes in weight and cardiometabolic health factors (91), yet this could contribute to some effects of TRE. Improvement in one or more aspects of glucose regulation (decreased fasting glucose and glucose area under the curve assessed via continuous glucose monitoring [CGM], improved insulin sensitivity, and increased β-cell function) was observed in 12 studies, 2 of which noted larger improvements in glucose regulation in participants with baseline fasting glucose greater than or equal to 100 mg/dL (91, 143). Other studies saw benefits to cardiovascular health with decreases in blood pressure as the most common factor (see Table 1). It is unclear if decreased blood pressure is a common result of TRE because it was not assessed in most studies. One pilot study assessed TRE in women with polycystic ovarian syndrome and reported weight loss, improved glycemic control, and decreased lipids and inflammation (151). Importantly, TRE was found to be feasible and safe for all studies. Larger randomized, controlled trials (RCTs) are needed because many of the studies to date are smaller pre-post or crossover trials. Yet, the replication of findings, even in diverse patient populations, speak to the potential impact of TRE as a health intervention.
Ongoing time-restricted eating clinical trials
There are now many clinical trials ongoing internationally as can be seen on clinicaltrials.gov. Some to note are larger RCTs evaluating TRE as an intervention for participants with prediabetes (344 participants, NCT03504683), diabetes (144 participants NCT04155619), metabolic syndrome (118 participants, NCT04057339), and firefighters on 24-hour shifts (150 participants, NCT03533023). There are also many smaller pilot studies evaluating the effects of TRE in participants with specific diseases such as cancer (NCT04243512) and polycystic ovarian syndrome (NCT03792282). Over the coming years, the results of these studies will greatly improve our understanding of the implementation of TRE (eg, eating window, time of day) and of the potential of TRE to be used as a preventive and cotreatment for a variety of diseases.
Potential Impacts of Time-restricted Eating on Chronic Diseases and Future Perspective in Treating Human Diseases
Preclinical studies clearly point to the importance of the timing of feeding. TRF results in well-demonstrated amelioration in metabolism and obesity in various animal models. Results from human studies with TRE are encouraging (Fig. 2). TRE reduces body weight and improves metabolism. The decrease in insulin resistance, oxidative stress, inflammation, and blood pressure is seen even when body weight is maintained constant (75). However, many of these studies involve small sample size (10-20 participants), short term, (4-12 weeks), or single sex. Only a few TRE-based studies have been RCTs (96, 117, 119, 131-133, 138, 141, 143, 145-147, 150, 153) or have rigorously assessed eating duration before the TRE intervention (88, 91, 117, 125, 133, 145, 147-149, 152) and/or have reliable methods to assess adherence (88, 91, 117, 145, 152). The focus has been on metabolism and weight, and other important clinical and physiological outcomes, such as body composition, gut motility, microbiome, liver steatosis, adipose tissue inflammation, to name a few, have remained incompletely investigated.
Figure 2.
Summary of various metabolic and other chronic diseases or risk factors that respond favorably to time-restricted feeding (TRF) or time-restricted eating (TRE) in animal models (rodents and Drosophila) or in humans. For simplicity, the green portion of the wheel represents the eating window and the gray portion represents the fasting window.
Human trials are essential for clinical translation. We need RCTs with rigorous design and methods to assess the short and long-term efficacy of TRE, and the mechanisms by which it might exert its effects in humans. Large-scale effectiveness trials in a population of various ages, sexes, ethnicities, and disease states should follow. These TRE interventions should target people who may benefit the most from it, that is, individuals who spread their calorie intake over prolonged daily windows. While more challenging in humans than in rodents, the dose-response effect of TRE needs to be investigated. A short duration trial demonstrated the efficacy of a drastic 6-hour TRE intervention; could a more manageable 8-hour, 10-hour, or even 12-hour TRE also be effective in improving metabolism? Does the restriction of the duration of the eating window need to be proportional to the duration of the baseline eating window? Will TRE 5 of 7 days, or every other week, be sufficient to obtain the desired effect, as shown with intermittent energy restriction (161)? Is TRE sustainable? Calorie restriction is usually not beyond a few months. TRE should be tested in combination with other behavioral changes, such as increased physical activity, better sleep hygiene, and amelioration of diet composition.
Should the proven success of TRF in resolving metabolic consequences of obesity in animals be replicated in humans, TRE would become a significant lifestyle tool. Its modest effect on weight loss coupled with possible weight loss–independent effects could result in improved metabolic health, public health, and decreased health care cost. Health care providers should encourage self-monitoring techniques of meal and sleep timing in high-risk patients and suggest easy-to-implement behavior changes, such as decreased after-dinner snacking, and increased regularity of bedtime.
In summary, TRE represents new avenues to assess the effects of the timing of eating on metabolism. While the mechanisms of TRE are not fully elucidated, animal experiments have generated impressive data in preventing or reversing metabolic diseases associated with obesity. More rigorous human studies are needed to assess the efficacy, mechanism, and sustainability of TRE in a wide range of populations and diseases.
Acknowledgments
Financial Support: Relevant research in S.P.’s laboratory is funded by the US National Institutes of Health (NIH) (grant Nos. DK115214, AG068550, CA236352, and AG065992), the Department of Defense (grant No. W81XWH1810645), the Department of Homeland Security (grant No. EMW-2016-FP-00788), the Robert Wood Johnson Foundation (grant No. 76014), and the Wu Tsai Human Performance Alliance. P.T. is supported by NIH grant DK118278; L.C. is supported by NIH grants DK124484 and DK098203; and B.L. is supported by NIH grants DK048404 and AG065569. E.N.C.M. is supported by the Hillblom Foundation. We thank I-Hsun Wu for creating illustrations for the graphical abstract.
Glossary
Abbreviations
- ACC
acetyl CoA carboxylase
- AMPK
adenosine monophosphate–activated protein kinase C
- CPT
carnitine palmitoyl transferase
- CR
calorie restriction
- CRD
circadian rhythm disruption
- CREB
5′-cyclic adenosine 5′-monophosphate response element binding protein
- DIO
diet-induced obesity
- G6P
glucose-6-phosphate
- GLP-1
glucagon-like peptide-1
- ISR
integrated stress response
- mRNA
messenger RNA
- mTOR
mechanistic target of rapamycin
- PPP
pentose phosphate pathway
- SCN
suprachiasmatic nucleus
- TCA
tricarboxylic acid
- TR
time restriction
- TRE
time-restricted eating
- TRF
time-restricted feeding
Additional Information
Disclosures: P.R.T. is a consultant for Sanofi/Regeneron, Novo-Nordisk, Boehringer-Ingleheim, Janssen, Pfizer, Amarin, and Amgen. She is a stockholder of Cardero Therapeutics. S.P. is the author of the book “The Circadian Code”.
References
- 1. Tahara Y, Shibata S. Circadian rhythms of liver physiology and disease: experimental and clinical evidence. Nat Rev Gastroenterol Hepatol. 2016;13(4):217-226. [DOI] [PubMed] [Google Scholar]
- 2. Firsov D, Bonny O. Circadian rhythms and the kidney. Nat Rev Nephrol. 2018;14(10):626-635. [DOI] [PubMed] [Google Scholar]
- 3. Logan RW, McClung CA. Rhythms of life: circadian disruption and brain disorders across the lifespan. Nat Rev Neurosci. 2019;20(1):49-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crnko S, Du Pré BC, Sluijter JPG, Van Laake LW. Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat Rev Cardiol. 2019;16(7):437-447. [DOI] [PubMed] [Google Scholar]
- 5. Sulli G, Lam MTY, Panda S. Interplay between circadian clock and cancer: new frontiers for cancer treatment. Trends Cancer. 2019;5(8):475-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sulli G, Manoogian ENC, Taub PR, Panda S. Training the circadian clock, clocking the drugs, and drugging the clock to prevent, manage, and treat chronic diseases. Trends Pharmacol Sci. 2018;39(9):812-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leproult R, Deliens G, Gilson M, Peigneux P. Beneficial impact of sleep extension on fasting insulin sensitivity in adults with habitual sleep restriction. Sleep. 2015;38(5):707-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Henst RHP, Pienaar PR, Roden LC, Rae DE. The effects of sleep extension on cardiometabolic risk factors: a systematic review. J Sleep Res. 2019;28(6):e12865. [DOI] [PubMed] [Google Scholar]
- 9. Figueiro MG, Plitnick BA, Lok A, et al. . Tailored lighting intervention improves measures of sleep, depression, and agitation in persons with Alzheimer’s disease and related dementia living in long-term care facilities. Clin Interv Aging. 2014;9:1527-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Figueiro MG. Light, sleep and circadian rhythms in older adults with Alzheimer’s disease and related dementias. Neurodegener Dis Manag. 2017;7(2):119-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chaix A, Manoogian ENC, Melkani GC, Panda S. Time-restricted eating to prevent and manage chronic metabolic diseases. Annu Rev Nutr. 2019;39:291-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet. 2017;18(3):164-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ceriani MF, Hogenesch JB, Yanovsky M, Panda S, Straume M, Kay SA. Genome-wide expression analysis in Drosophila reveals genes controlling circadian behavior. J Neurosci. 2002;22(21):9305-9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Panda S, Antoch MP, Miller BH, et al. . Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109(3):307-320. [DOI] [PubMed] [Google Scholar]
- 15. Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A. 2014;111(45):16219-16224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mure LS, Le HD, Benegiamo G, et al. . Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science. 2018;359(6381):eaao0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rey G, Cesbron F, Rougemont J, Reinke H, Brunner M, Naef F. Genome-wide and phase-specific DNA-binding rhythms of BMAL1 control circadian output functions in mouse liver. PLoS Biol. 2011;9(2):e1000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koike N, Yoo SH, Huang HC, et al. . Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338(6105):349-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang Y, Fang B, Emmett MJ, et al. . Gene regulation. Discrete functions of nuclear receptor Rev-erbα couple metabolism to the clock. Science. 2015;348(6242):1488-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang Y, Fang B, Damle M, et al. . HNF6 and Rev-erbα integrate hepatic lipid metabolism by overlapping and distinct transcriptional mechanisms. Genes Dev. 2016;30(14):1636-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vollmers C, Schmitz RJ, Nathanson J, Yeo G, Ecker JR, Panda S. Circadian oscillations of protein-coding and regulatory RNAs in a highly dynamic mammalian liver epigenome. Cell Metab. 2012;16(6):833-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cho H, Zhao X, Hatori M, et al. . Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature. 2012;485(7396):123-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Perelis M, Marcheva B, Ramsey KM, et al. . Pancreatic β cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science. 2015;350(6261):aac4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ueda HR, Hayashi S, Chen W, et al. . System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nat Genet. 2005;37(2):187-192. [DOI] [PubMed] [Google Scholar]
- 25. Ripperger JA, Schibler U. Rhythmic CLOCK-BMAL1 binding to multiple E-box motifs drives circadian Dbp transcription and chromatin transitions. Nat Genet. 2006;38(3):369-374. [DOI] [PubMed] [Google Scholar]
- 26. Wang Y, Kuang Z, Yu X, Ruhn KA, Kubo M, Hooper LV. The intestinal microbiota regulates body composition through NFIL3 and the circadian clock. Science. 2017;357(6354):912-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lamia KA, Papp SJ, Yu RT, et al. . Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature. 2011;480(7378):552-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jordan SD, Kriebs A, Vaughan M, et al. . CRY1/2 selectively repress PPARδ and limit exercise capacity. Cell Metab. 2017;26(1):243-255.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grimaldi B, Bellet MM, Katada S, et al. . PER2 controls lipid metabolism by direct regulation of PPARγ. Cell Metab. 2010;12(5):509-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hatori M, Le H, Vollmers C, et al. . Inducible ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. PLoS One. 2008;3(6):e2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hatori M, Panda S. The emerging roles of melanopsin in behavioral adaptation to light. Trends Mol Med. 2010;16(10):435-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schibler U. The 2008 Pittendrigh/Aschoff Lecture: peripheral phase coordination in the mammalian circadian timing system. J Biol Rhythms. 2009;24(1):3-15. [DOI] [PubMed] [Google Scholar]
- 34. Buijs RM, Escobar C, Swaab DF. The circadian system and the balance of the autonomic nervous system. Handb Clin Neurol. 2013;117:173-191. [DOI] [PubMed] [Google Scholar]
- 35. Ruiter M, Buijs RM, Kalsbeek A. Hormones and the autonomic nervous system are involved in suprachiasmatic nucleus modulation of glucose homeostasis. Curr Diabetes Rev. 2006;2(2):213-226. [DOI] [PubMed] [Google Scholar]
- 36. Asher G, Schibler U. Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metab. 2011;13(2):125-137. [DOI] [PubMed] [Google Scholar]
- 37. Asher G, Sassone-Corsi P. Time for food: the intimate interplay between nutrition, metabolism, and the circadian clock. Cell. 2015;161(1):84-92. [DOI] [PubMed] [Google Scholar]
- 38. Crosby P, Hamnett R, Putker M, et al. . Insulin/IGF-1 drives PERIOD synthesis to entrain circadian rhythms with feeding time. Cell. 2019;177(4):896-909.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang EE, Liu Y, Dentin R, et al. . Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med. 2010;16(10):1152-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jouffe C, Cretenet G, Symul L, et al. . The circadian clock coordinates ribosome biogenesis. PLoS Biol. 2013;11(1):e1001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ashbrook LH, Krystal AD, Fu YH, Ptáček LJ. Genetics of the human circadian clock and sleep homeostat. Neuropsychopharmacology. 2020;45(1):45-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jan M, O’Hara BF, Franken P. Recent advances in understanding the genetics of sleep. F1000Res. 2020;9:F1000 Faculty Rev-214. [Google Scholar]
- 43. Langenberg C, Pascoe L, Mari A, et al. ; RISC Consortium . Common genetic variation in the melatonin receptor 1B gene (MTNR1B) is associated with decreased early-phase insulin response. Diabetologia. 2009;52(8):1537-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rönn T, Wen J, Yang Z, et al. . A common variant in MTNR1B, encoding melatonin receptor 1B, is associated with type 2 diabetes and fasting plasma glucose in Han Chinese individuals. Diabetologia. 2009;52(5):830-833. [DOI] [PubMed] [Google Scholar]
- 45. Prokopenko I, Langenberg C, Florez JC, et al. . Variants in MTNR1B influence fasting glucose levels. Nat Genet. 2009;41(1):77-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bouatia-Naji N, Bonnefond A, Cavalcanti-Proença C, et al. . A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat Genet. 2009;41(1):89-94. [DOI] [PubMed] [Google Scholar]
- 47. Lyssenko V, Nagorny CL, Erdos MR, et al. . Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 2009;41(1):82-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tuomi T, Nagorny CL, Singh P, et al. . Increased melatonin signaling is a risk factor for type 2 diabetes. Cell Metab. 2016;23(6):1067-1077. [DOI] [PubMed] [Google Scholar]
- 49. Spruyt K, Braam W, Smits M, Curfs LM. Sleep complaints and the 24-h melatonin level in individuals with Smith-Magenis syndrome: assessment for effective intervention. CNS Neurosci Ther. 2016;22(11):928-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mattis J, Sehgal A. Circadian rhythms, sleep, and disorders of aging. Trends Endocrinol Metab. 2016;27(4):192-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kondratova AA, Kondratov RV. The circadian clock and pathology of the ageing brain. Nat Rev Neurosci. 2012;13(5):325-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Leng Y, Musiek ES, Hu K, Cappuccio FP, Yaffe K. Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol. 2019;18(3):307-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Poggiogalle E, Jamshed H, Peterson CM. Circadian regulation of glucose, lipid, and energy metabolism in humans. Metabolism. 2018;84:11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shi SQ, Ansari TS, McGuinness OP, Wasserman DH, Johnson CH. Circadian disruption leads to insulin resistance and obesity. Curr Biol. 2013;23(5):372-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Coomans CP, van den Berg SA, Houben T, et al. . Detrimental effects of constant light exposure and high-fat diet on circadian energy metabolism and insulin sensitivity. FASEB J. 2013;27(4):1721-1732. [DOI] [PubMed] [Google Scholar]
- 56. IARC. Shiftwork: Definition and Occurrence of Exposure. In: Painting, Firefighting, and Shiftwork: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 98. IARC;2010. https://www.ncbi.nlm.nih.gov/books/NBK326824/ [PMC free article] [PubMed] [Google Scholar]
- 57. Davidson AJ, Sellix MT, Daniel J, Yamazaki S, Menaker M, Block GD. Chronic jet-lag increases mortality in aged mice. Curr Biol. 2006;16(21):R914-R916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mistlberger RE. Circadian food-anticipatory activity: formal models and physiological mechanisms. Neurosci Biobehav Rev. 1994;18(2):171-195. [DOI] [PubMed] [Google Scholar]
- 59. Mitchell SE, Delville C, Konstantopedos P, et al. . The effects of graded levels of calorie restriction: V. Impact of short term calorie and protein restriction on physical activity in the C57BL/6 mouse. Oncotarget. 2016;7(15):19147-19170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14(23):2950-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kornmann B, Schaad O, Bujard H, Takahashi JS, Schibler U. System-driven and oscillator-dependent circadian transcription in mice with a conditionally active liver clock. PLoS Biol. 2007;5(2):e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vollmers C, Gill S, DiTacchio L, Pulivarthy SR, Le HD, Panda S. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc Natl Acad Sci U S A. 2009;106(50):21453-21458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mukherji A, Kobiita A, Chambon P. Shifting the feeding of mice to the rest phase creates metabolic alterations, which, on their own, shift the peripheral circadian clocks by 12 hours. Proc Natl Acad Sci U S A. 2015;112(48):E6683-E6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mukherji A, Kobiita A, Damara M, et al. . Shifting eating to the circadian rest phase misaligns the peripheral clocks with the master SCN clock and leads to a metabolic syndrome. Proc Natl Acad Sci U S A. 2015;112(48):E6691-E6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hatori M, Vollmers C, Zarrinpar A, et al. . Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15(6):848-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kohsaka A, Laposky AD, Ramsey KM, et al. . High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6(5):414-421. [DOI] [PubMed] [Google Scholar]
- 67. Dyar KA, Lutter D, Artati A, et al. . Atlas of circadian metabolism reveals system-wide coordination and communication between clocks. Cell. 2018;174(6):1571-1585.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014;20(6):991-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sherman H, Genzer Y, Cohen R, Chapnik N, Madar Z, Froy O. Timed high-fat diet resets circadian metabolism and prevents obesity. FASEB J. 2012;26(8):3493-3502. [DOI] [PubMed] [Google Scholar]
- 70. Acosta-Rodríguez VA, de Groot MHM, Rijo-Ferreira F, Green CB, Takahashi JS. Mice under caloric restriction self-impose a temporal restriction of food intake as revealed by an automated feeder system. Cell Metab. 2017;26(1):267-277.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nelson W, Halberg F. Meal-timing, circadian rhythms and life span of mice. J Nutr. 1986;116(11):2244-2253. [DOI] [PubMed] [Google Scholar]
- 72. Mitchell SJ, Bernier M, Mattison JA, et al. . Daily fasting improves health and survival in male mice independent of diet composition and calories. Cell Metab. 2019;29(1):221-228.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Shi D, Han T, Chu X, et al. . An isocaloric moderately high-fat diet extends lifespan in male rats and Drosophila. Cell Metab. 2021;33(3):581-597.e9. [DOI] [PubMed] [Google Scholar]
- 74. Martinez-Lopez N, Tarabra E, Toledo M, et al. . System-wide benefits of intermeal fasting by autophagy. Cell Metab. 2017;26(6):856-871.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018;27(6):1212-1221.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ravussin E, Beyl RA, Poggiogalle E, Hsia DS, Peterson CM. Early time-restricted feeding reduces appetite and increases fat oxidation but does not affect energy expenditure in humans. Obesity (Silver Spring). 2019;27(8):1244-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hutchison AT, Regmi P, Manoogian ENC, et al. . Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: a randomized crossover trial. Obesity (Silver Spring). 2019;27(5):724-732. [DOI] [PubMed] [Google Scholar]
- 78. Petrenko V, Dibner C. Circadian orchestration of insulin and glucagon release. Cell Cycle. 2017;16(12):1141-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Fustin JM, Doi M, Yamada H, Komatsu R, Shimba S, Okamura H. Rhythmic nucleotide synthesis in the liver: temporal segregation of metabolites. Cell Rep. 2012;1(4):341-349. [DOI] [PubMed] [Google Scholar]
- 80. Panda S. Circadian physiology of metabolism. Science. 2016;354(6315):1008-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Eckel-Mahan KL, Patel VR, de Mateo S, et al. . Reprogramming of the circadian clock by nutritional challenge. Cell. 2013;155(7):1464-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Puri V, Konda S, Ranjit S, et al. . Fat-specific protein 27, a novel lipid droplet protein that enhances triglyceride storage. J Biol Chem. 2007;282(47):34213-34218. [DOI] [PubMed] [Google Scholar]
- 83. Atshaves BP, Martin GG, Hostetler HA, McIntosh AL, Kier AB, Schroeder F. Liver fatty acid-binding protein and obesity. J Nutr Biochem. 2010;21(11):1015-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Thaiss CA, Zeevi D, Levy M, et al. . Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell. 2014;159(3):514-529. [DOI] [PubMed] [Google Scholar]
- 85. Zarrinpar A, Chaix A, Yooseph S, Panda S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. 2014;20(6):1006-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kentish SJ, Page AJ. Plasticity of gastro-intestinal vagal afferent endings. Physiol Behav. 2014;136:170-178. [DOI] [PubMed] [Google Scholar]
- 87. Kentish SJ, Hatzinikolas G, Li H, Frisby CL, Wittert GA, Page AJ. Time-restricted feeding prevents ablation of diurnal rhythms in gastric vagal afferent mechanosensitivity observed in high-fat diet-induced obese mice. J Neurosci. 2018;38(22):5088-5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gill S, Panda S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. 2015;22(5):789-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pakos-Zebrucka K, Koryga I, Mnich K, Ljujic M, Samali A, Gorman AM. The integrated stress response. EMBO Rep. 2016;17(10):1374-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gill S, Le HD, Melkani GC, Panda S. Time-restricted feeding attenuates age-related cardiac decline in Drosophila. Science. 2015;347(6227):1265-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wilkinson MJ, Manoogian ENC, Zadourian A, et al. . Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab. 2020;31(1):92-104.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sundaram S, Yan L. Time-restricted feeding reduces adiposity in mice fed a high-fat diet. Nutr Res. 2016;36(6):603-611. [DOI] [PubMed] [Google Scholar]
- 93. Chaix A, Lin T, Le HD, Chang MW, Panda S. Time-restricted feeding prevents obesity and metabolic syndrome in mice lacking a circadian clock. Cell Metab. 2019;29(2):303-319.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Parr EB, Devlin BL, Radford BE, Hawley JA. A delayed morning and earlier evening time-restricted feeding protocol for improving glycemic control and dietary adherence in men with overweight/obesity: a randomized controlled trial. Nutrients. 2020;12(2):505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, Peterson CM. Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients. 2019;11(6):1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Moro T, Tinsley G, Bianco A, et al. . Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med. 2016;14(1):290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Stote KS, Baer DJ, Spears K, et al. . A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am J Clin Nutr. 2007;85(4):981-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Takahashi M, Ozaki M, Kang MI, et al. . Effects of meal timing on postprandial glucose metabolism and blood metabolites in healthy adults. Nutrients. 2018;10(11):1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Bo S, Fadda M, Castiglione A, et al. . Is the timing of caloric intake associated with variation in diet-induced thermogenesis and in the metabolic pattern? A randomized cross-over study. Int J Obes (Lond). 2015;39(12):1689-1695. [DOI] [PubMed] [Google Scholar]
- 100. de Castro JM. The time of day of food intake influences overall intake in humans. J Nutr. 2004;134(1):104-111. [DOI] [PubMed] [Google Scholar]
- 101. Carlson O, Martin B, Stote KS, et al. . Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal-weight middle-aged men and women. Metabolism. 2007;56(12):1729-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Regmi P, Heilbronn LK. Time-restricted eating: benefits, mechanisms, and challenges in translation. iScience. 2020;23(6):101161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Van Cauter E, Désir D, Decoster C, Féry F, Balasse EO. Nocturnal decrease in glucose tolerance during constant glucose infusion. J Clin Endocrinol Metab. 1989;69(3):604-611. [DOI] [PubMed] [Google Scholar]
- 104. Reutrakul S, Hood MM, Crowley SJ, et al. . Chronotype is independently associated with glycemic control in type 2 diabetes. Diabetes Care. 2013;36(9):2523-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Boden G, Ruiz J, Urbain JL, Chen X. Evidence for a circadian rhythm of insulin secretion. Am J Physiol. 1996;271(2 Pt 1):E246-E252. [DOI] [PubMed] [Google Scholar]
- 106. Kessler K, Hornemann S, Petzke KJ, et al. . The effect of diurnal distribution of carbohydrates and fat on glycaemic control in humans: a randomized controlled trial. Sci Rep. 2017;7:44170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Cahill LE, Chiuve SE, Mekary RA, et al. . Prospective study of breakfast eating and incident coronary heart disease in a cohort of male US health professionals. Circulation. 2013;128(4):337-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. St-Onge MP, Ard J, Baskin ML, et al. ; American Heart Association Obesity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; and Stroke Council . Meal timing and frequency: implications for cardiovascular disease prevention: a scientific statement from the American Heart Association. Circulation. 2017;135(9):e96-e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Garaulet M, Gómez-Abellán P, Alburquerque-Béjar JJ, Lee YC, Ordovás JM, Scheer FA. Timing of food intake predicts weight loss effectiveness. Int J Obes (Lond). 2013;37(4):604-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kogevinas M, Espinosa A, Castelló A, et al. . Effect of mistimed eating patterns on breast and prostate cancer risk (MCC-Spain Study). Int J Cancer. 2018;143(10):2380-2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Srour B, Plancoulaine S, Andreeva VA, et al. . Circadian nutritional behaviours and cancer risk: new insights from the NutriNet-santé prospective cohort study: disclaimers. Int J Cancer. 2018;143(10):2369-2379. [DOI] [PubMed] [Google Scholar]
- 112. Marinac CR, Nelson SH, Breen CI, et al. . Prolonged nightly fasting and breast cancer prognosis. JAMA Oncol. 2016;2(8):1049-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Marinac CR, Natarajan L, Sears DD, et al. . Prolonged nightly fasting and breast cancer risk: findings from NHANES (2009-2010). Cancer Epidemiol Biomarkers Prev. 2015;24(5):783-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Pearlman M, Obert J, Casey L. The association between artificial sweeteners and obesity. Curr Gastroenterol Rep. 2017;19(12):64. [DOI] [PubMed] [Google Scholar]
- 115. Battram DS, Arthur R, Weekes A, Graham TE. The glucose intolerance induced by caffeinated coffee ingestion is less pronounced than that due to alkaloid caffeine in men. J Nutr. 2006;136(5):1276-1280. [DOI] [PubMed] [Google Scholar]
- 116. Smith HA, Hengist A, Thomas J, et al. . Glucose control upon waking is unaffected by hourly sleep fragmentation during the night, but is impaired by morning caffeinated coffee. Br J Nutr. 2020;124(10):1114-1120. [DOI] [PubMed] [Google Scholar]
- 117. Chow LS, Manoogian ENC, Alvear A, et al. . Time-restricted eating effects on body composition and metabolic measures in humans who are overweight: a feasibility study. Obesity (Silver Spring). 2020;28(5):860-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. LeCheminant JD, Christenson E, Bailey BW, Tucker LA. Restricting night-time eating reduces daily energy intake in healthy young men: a short-term cross-over study. Br J Nutr. 2013;110(11):2108-2113. [DOI] [PubMed] [Google Scholar]
- 119. Tinsley GM, Forsse JS, Butler NK, et al. . Time-restricted feeding in young men performing resistance training: a randomized controlled trial. Eur J Sport Sci. 2017;17(2):200-207. [DOI] [PubMed] [Google Scholar]
- 120. Antoni R, Robertson TM, Robertson MD, Johnston JD. A pilot feasibility study exploring the effects of a moderate time-restricted feeding intervention on energy intake, adiposity and metabolic physiology in free-living human subjects. J Nutr Sci. 2018;7:E22. [Google Scholar]
- 121. Gabel K, Hoddy KK, Haggerty N, et al. . Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: a pilot study. Nutr Healthy Aging. 2018;4(4):345-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Gabel K, Hoddy KK, Varady KA. Safety of 8-h time restricted feeding in adults with obesity. Appl Physiol Nutr Metab. 2019;44(1):107-109. [DOI] [PubMed] [Google Scholar]
- 123. Gabel K, Marcell J, Cares K, et al. . Effect of time restricted feeding on the gut microbiome in adults with obesity: a pilot study. Nutr Health. 2020;26(2):79-85. [DOI] [PubMed] [Google Scholar]
- 124. Tinsley GM, Moore ML, Graybeal AJ, et al. . Time-restricted feeding plus resistance training in active females: a randomized trial. Am J Clin Nutr. 2019;110(3):628-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kesztyüs D, Cermak P, Gulich M, Kesztyüs T. Adherence to time-restricted feeding and impact on abdominal obesity in primary care patients: results of a pilot study in a pre-post design. Nutrients. 2019;11(12):2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kesztyüs D, Fuchs M, Cermak P, Kesztyüs T. Associations of time-restricted eating with health-related quality of life and sleep in adults: a secondary analysis of two pre-post pilot studies. Bmc Nutr. 2020;6(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. McAllister MJ, Pigg BL, Renteria LI, Waldman HS. Time-restricted feeding improves markers of cardiometabolic health in physically active college-age men: a 4-week randomized pre-post pilot study. Nutr Res. 2020;75:32-43. [DOI] [PubMed] [Google Scholar]
- 128. Malaeb S, Harindhanavudhi T, Dietsche K, et al. . Time-restricted eating alters food intake patterns, as prospectively documented by a smartphone application. Nutrients. 2020;12(11):3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lobene AJ, Panda S, Mashek DG, Manoogian ENC, Hill Gallant KM, Chow LS. Time-restricted eating for 12 weeks does not adversely alter bone turnover in overweight adults. Nutrients. 2021;13(4):1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Crose A, Alvear A, Singroy S, et al. . Time-restricted eating improves quality of life measures in overweight humans. Nutrients. 2021;13(5):1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Zeb F, Wu X, Chen L, et al. . Effect of time-restricted feeding on metabolic risk and circadian rhythm associated with gut microbiome in healthy males. Br J Nutr. 2020;123(11):1216-1226. [DOI] [PubMed] [Google Scholar]
- 132. Lowe DA, Wu N, Rohdin-Bibby L, et al. . Effects of time-restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity: the TREAT randomized clinical trial. JAMA Intern Med. 2020;180(11):1491-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Cienfuegos S, Gabel K, Kalam F, et al. . Effects of 4- and 6-h time-restricted feeding on weight and cardiometabolic health: a randomized controlled trial in adults with obesity. Cell Metab. 2020;32(3):366-378.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Lee SA, Sypniewski C, Bensadon BA, et al. . Determinants of adherence in time-restricted feeding in older adults: lessons from a pilot study. Nutrients. 2020;12(3):874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Anton SD, Lee SA, Donahoo WT, et al. . The effects of time restricted feeding on overweight, older adults: a pilot study. Nutrients. 2019;11(7):1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Lundell LS, Parr EB, Devlin BL, et al. . Author correction: time-restricted feeding alters lipid and amino acid metabolite rhythmicity without perturbing clock gene expression. Nat Commun. 2020;11(1):5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Parr EB, Devlin BL, Lim KHC, et al. . Time-restricted eating as a nutrition strategy for individuals with type 2 diabetes: a feasibility study. Nutrients. 2020;12(11):3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. de Oliveira Maranhão Pureza IR, da Silva Junior AE, Silva Praxedes DR, et al. . Effects of time-restricted feeding on body weight, body composition and vital signs in low-income women with obesity: a 12-month randomized clinical trial. Clin Nutr. 2021;40(3):759-766. [DOI] [PubMed] [Google Scholar]
- 139. Martens CR, Rossman MJ, Mazzo MR, et al. . Short-term time-restricted feeding is safe and feasible in non-obese healthy midlife and older adults. Geroscience. 2020;42(2):667-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Kim H, Jang BJ, Jung AR, Kim J, Ju HJ, Kim YI. The impact of time-restricted diet on sleep and metabolism in obese volunteers. Medicina (Kaunas). 2020;56(10):540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Moro T, Tinsley G, Longo G, et al. . Time-restricted eating effects on performance, immune function, and body composition in elite cyclists: a randomized controlled trial. J Int Soc Sports Nutr. 2020;17(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Schroder JD, Falqueto H, Mânica A, et al. . Effects of time-restricted feeding in weight loss, metabolic syndrome and cardiovascular risk in obese women. J Transl Med. 2021;19(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Peeke PM, Greenway FL, Billes SK, Zhang D, Fujioka K. Effect of time restricted eating on body weight and fasting glucose in participants with obesity: results of a randomized, controlled, virtual clinical trial. Nutr Diabetes. 2021;11(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Przulj D, Ladmore D, Smith KM, Phillips-Waller A, Hajek P. Time restricted eating as a weight loss intervention in adults with obesity. PLoS One. 2021;16(1):e0246186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Phillips NE, Mareschal J, Schwab N, et al. . The effects of time-restricted eating versus standard dietary advice on weight, metabolic health and the consumption of processed food: a pragmatic randomised controlled trial in community-based adults. Nutrients 2021;13(3):1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Domaszewski P, Konieczny M, Pakosz P, Bączkowicz D, Sadowska-Krępa E. Effect of a six-week intermittent fasting intervention program on the composition of the human body in women over 60 years of age. Int J Environ Res Public Health. 2020;17(11):4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Jones R, Pabla P, Mallinson J, et al. . Two weeks of early time-restricted feeding (eTRF) improves skeletal muscle insulin and anabolic sensitivity in healthy men. Am J Clin Nutr. 2020;112(4):1015-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Kesztyüs D, Vorwieger E, Schönsteiner D, Gulich M, Kesztyüs T. Applicability of time-restricted eating for the prevention of lifestyle-dependent diseases in a working population: results of a pilot study in a pre-post design . Ger Med Sci. 2021;19:Doc04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. McAllister MJ, Gonzalez AE, Waldman HS. Impact of time restricted feeding on markers of cardiometabolic health and oxidative stress in resistance-trained firefighters. J Strength Cond Res. Published online October 30, 2020. doi:10.1519/JSC.0000000000003860 [DOI] [PubMed] [Google Scholar]
- 150. Pureza IROM, Melo ISV, Macena ML, et al. . Acute effects of time-restricted feeding in low-income women with obesity placed on hypoenergetic diets: randomized trial. Nutrition. 2020;77:110796. [DOI] [PubMed] [Google Scholar]
- 151. Li C, Xing C, Zhang J, Zhao H, Shi W, He B. Eight-hour time-restricted feeding improves endocrine and metabolic profiles in women with anovulatory polycystic ovary syndrome. J Transl Med. 2021;19(1):148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Prasad M, Fine K, Gee A, et al. . A smartphone intervention to promote time restricted eating reduces body weight and blood pressure in adults with overweight and obesity: a pilot study. Nutrients. 2021;13(7):2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Brady AJ, Langton HM, Mulligan M, Egan B. Effects of 8 wk of 16:8 time-restricted eating in male middle- and long-distance runners. Med Sci Sports Exerc. 2021;53(3):633-642. [DOI] [PubMed] [Google Scholar]
- 154. Bjerre N, Holm L, Quist JS, Færch K, Hempler NF. Watching, keeping and squeezing time to lose weight: implications of time-restricted eating in daily life. Appetite. 2021;161:105138. [DOI] [PubMed] [Google Scholar]
- 155. Kipnis V, Midthune D, Freedman L, et al. . Bias in dietary-report instruments and its implications for nutritional epidemiology. Public Health Nutr. 2002;5(6A):915-923. [DOI] [PubMed] [Google Scholar]
- 156. Schatzkin A, Kipnis V, Carroll RJ, et al. . A comparison of a food frequency questionnaire with a 24-hour recall for use in an epidemiological cohort study: results from the biomarker-based Observing Protein and Energy Nutrition (OPEN) study. Int J Epidemiol. 2003;32(6):1054-1062. [DOI] [PubMed] [Google Scholar]
- 157. Hoskin MA, Bray GA, Hattaway K, et al. ; for the Diabetes Prevention Program Research Group . Prevention of diabetes through the lifestyle intervention: lessons learned from the Diabetes Prevention Program and Outcomes Study and its translation to practice. Curr Nutr Rep. 2014;3(4):364-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Kim C, Williamson DF, Mangione CM, et al. ; Translating Research Into Action for Diabetes (TRIAD) Study . Managed care organization and the quality of diabetes care: the Translating Research Into Action for Diabetes (TRIAD) study. Diabetes Care. 2004;27(7):1529-1534. [DOI] [PubMed] [Google Scholar]
- 159. Marrero DG, Ackermann RT. Providing long-term support for lifestyle changes: a key to success in diabetes prevention. Diabetes Spectr. 2007;20(4):205-209. [Google Scholar]
- 160. Adafer R, Messaadi W, Meddahi M, et al. . Food timing, circadian rhythm and chrononutrition: a systematic review of time-restricted eating’s effects on human health. Nutrients. 2020;12(12):3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Cioffi I, Evangelista A, Ponzo V, et al. . Intermittent versus continuous energy restriction on weight loss and cardiometabolic outcomes: a systematic review and meta-analysis of randomized controlled trials. J Transl Med. 2018;16(1):371. [DOI] [PMC free article] [PubMed] [Google Scholar]