Abstract
Ticks transmit various pathogens, including parasites, bacteria and viruses to humans and animals. To investigate the ticks and the potentially zoonotic pathogens that they may carry, questing ticks were collected in 2017 from 7 sites in Tokachi District, eastern Hokkaido, Japan. A total of 1563 ticks including adults (male and female), nymphs and larvae were collected. Four species of ticks were identified: Ixodes ovatus, Ixodes persulcatus, Haemaphysalis japonica and Haemaphysalis megaspinosa. Of the 1563 ticks, 1155 were used for DNA extraction. In total, 527 individual tick DNA samples prepared from adults (n = 484), nymphs (n = 41) and larvae (n = 2); and 67 pooled tick DNA samples prepared from larval stages (n = 628) were examined using PCR methods and sequencing to detect Borrelia burgdorferi (sensu lato) and Rickettsia spp. The phylogenetic analysis of Borrelia spp. flaB gene sequences showed the presence of the human pathogenic B. burgdorferi (s.l.) species (Borrelia garinii, Borrelia bavariensis and Borrelia afzelii) in I. persulcatus, whereas the non-pathogenic species Borrelia japonica was found only in I. ovatus. In I. persulcatus, B. garinii and/or its closely related species B. bavariensis was detected in both adults and nymphs at a prevalence of 21.9% whereas B. afzelii was found only in adults (1.8%). The prevalence of B. japonica in adult I. ovatus was 21.8%. Rickettsia species were identified through phylogenetic analysis based on gltA, 16S rRNA, ompB and sca4 genes. Four genotypes were detected in the samples which were classified into three species. The prevalence of human pathogenic Rickettsia helvetica was 26.0% in I. persulcatus adults and nymphs, 55.6% in I. persulcatus larval pools, and 1.7% in H. megaspinosa larval pools. The prevalence of “Candidatus R. tarasevichiae” was 15.4% in I. persulcatus adults and nymphs and 33.3% in I. persulcatus larval pools. The prevalence of “Candidatus R. principis” in H. megaspinosa adults and nymphs was 11.1% whereas it was detected in 3.4% of the H. megaspinosa larval pools. These results indicate that most of the risks of Lyme borreliosis and spotted fever group rickettsiosis infection in eastern Hokkaido, Japan, are restricted to I. persulcatus.
Keywords: Ixodes ovatus, Ixodes persulcatus, Haemaphysalis japonica, Haemaphysalis megaspinosa, Borrelia, Rickettsia
Graphical abstract
Highlights
-
•
Four species of ticks were collected in Tokachi District, eastern Hokkaido, Japan.
-
•
Tick DNA samples were subjected to PCR to detect Borrelia spp. and Rickettsia spp.
-
•
Borrelia garinii/Borrelia bavariensis and Borrelia afzelii were detected in Ixodes persulcatus.
-
•
Rickettsia helvetica and “Candidatus R. tarasevichiae” were detected in I. persulcatus.
-
•
First molecular detection of “Candidatus R. principis” in larvae of Haemaphysalis megaspinosa.
1. Introduction
In Japan located in East Asia, the dynamics of tick-borne pathogens is changing as the incidence of related human disease cases is increasing (Yamaji et al., 2018). Mapping of the distribution of ticks and the zoonotic pathogens they carry, in each locality of the country is one of the constant key efforts to assess the risk of the occurrence of infectious diseases. Tokachi District in eastern Hokkaido, Japan, is famous for its agriculture and dairy farming that utilize the areaʼs vast plains. Because dairy cows live in meadows shared with wild animals such as sika deer, they frequently suffer tick infestations while grazing, are at great risk of pathogenic infections (Ota et al., 2009; Shibata et al., 2018) and may serve as a source of infected ticks. Likewise, not only dairy farmers but also farmworkers and hunters are at high risk for tick bites which potentially transmit various tick-borne zoonotic diseases (Kubo et al., 1992; Chaligiannis et al., 2018). Moreover, outdoor nature-based recreational activities such as hiking, fishing and barbecuing are popular in the natural environment of Tokachi District surrounded by mountains and might also be associated with risks of tick bites.
Lyme borreliosis and rickettsioses are important tick-borne zoonotic diseases. Despite substantial efforts to improve surveillance and control of Lyme borreliosis, it remains prevalent in the northern hemisphere (Rizzoli et al., 2011). Most cases of Lyme borreliosis in Japan have been confirmed in Hokkaido and are caused by Borrelia garinii and Borrelia afzelii infections (IDSC, 2011). Previous research indicated that Borrelia burgdorferi (sensu stricto) has a greater inflammatory potential than B. afzelii or B. garinii (Strle et al., 2009), but B. burgdorferi (s.s.) has not yet been found in Japan (Masuzawa, 2004). As the geographical distribution of pathogens and the number of patients are ever changing in Japan, molecular epidemiological surveys for Borrelia spp. are required to assess the risk of infection in different geographical areas of the country. Tick-borne rickettsioses are globally distributed and one of the oldest known zoonoses (Parola et al., 2005). Multiple distinct tick-borne spotted fever group (SFG) rickettsioses have been recognized by the development of molecular methods to identify rickettsiae (Parola et al., 2005). From 1984 through 2005, 11 additional rickettsiae were described as causative agents of tick-borne rickettsioses (Raoult & Roux, 1997; Parola et al., 2005). Among them, seven were initially isolated from ticks, before their association with human disease was confirmed (Parola et al., 2005). At present, there are 25 pathogenic Rickettsia species in the world (Piotrowski & Rymaszewska, 2020). In Japan, notification of Japanese spotted fever (JSF) caused by Rickettsia japonica increases year by year, especially from 2007. In total, 2726 JSF patients were reported in 2007–2019 (IDSC, 2020), with the largest annual number of notifications (337 cases) being recorded in 2017. Several other SFG rickettsiae, namely Rickettsia heilongjiangensis, Rickettsia helvetica, Rickettsia tamurae and “Candidatus R. tarasevichiae”, have also been recognized as etiological agents of human diseases (IDSC, 2006; Ando et al., 2010; Jia et al., 2013; Liu et al., 2016). Rickettsia asiatica, has also been reported in Japan but its pathogenicity is currently unknown (Fujita et al., 2006). Molecular epidemiological surveys for Borrelia spp. and Rickettsia spp. in ticks provide useful data for the diagnosis and prevention of these tick-borne diseases (Eisen & Paddock, 2020).
In the present study, to investigate the zoonotic pathogens carried by ticks in Tokachi District, eastern Hokkaido, Japan, we collected questing ticks from seven sites of the district in 2017. The tick samples were identified to the species level, then selected specimens were submitted to DNA extraction, PCR and sequencing to detect B. burgdorferi (s.l.) and Rickettsia spp.
2. Materials and methods
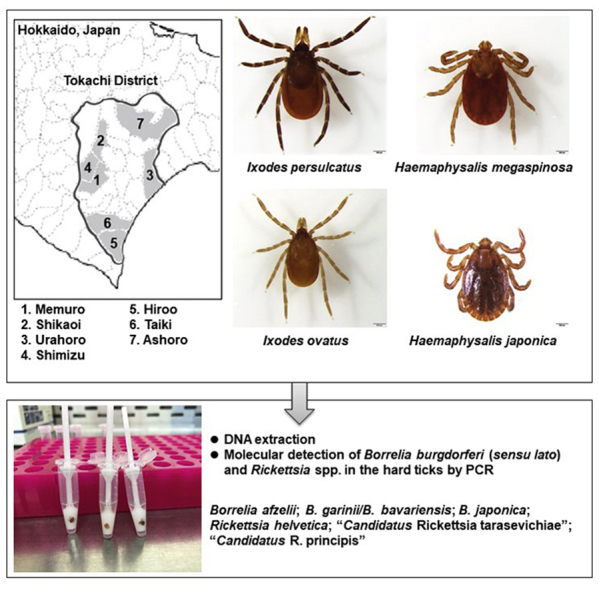
2.1. Tick collection and identification
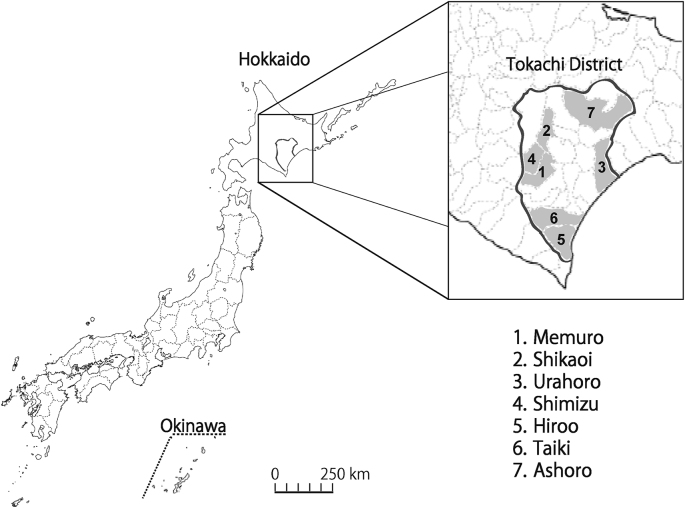
The fieldwork was conducted in 7 municipalities in the Tokachi District, located in the eastern part of Hokkaido in northern Japan. We collected questing ticks throughout the district: two northern sites (Shikaoi (43°11′N, 142°59′E) and Ashoro (43°20′N, 143°36′E)), three central sites (Memuro (42°49′N, 142°58′E), Urahoro (42°46′N, 143°44′E) and Shimizu (42°59′N, 142°50′E)) and two southern sites (Hiroo (42°17′N, 143°16′E) and Taiki (42°31′N, 143°11′E)) (Fig. 1). Detailed geographical and climate features of the Tokachi District are described by Yazaki et al. (2013) and Fukushima et al. (2019). Between May and November 2017, field expeditions were performed once per month to collect questing ticks by the flagging method (Dantas-Torres et al., 2013). Microscopic observation was performed to determine the species and developmental stages (larva, nymph and adult) in accordance with the morphology of ticks. Ticks were identified using recognized morphological keys (Kitaoka, 1985; Yamaguti et al., 1971). Identified ticks were stored in 70% ethanol prior to DNA extraction.
Fig. 1.
Tick collection sites in the Tokachi District (Hokkaido, Japan). We collected questing ticks throughout the district: two northern sites (Shikaoi and Ashoro), three central sites (Urahoro, Shimizu and Memuro) and two southern sites (Hiroo and Taiki).
2.2. DNA extraction
To avoid contamination, all steps were carried out on a clean bench. Throughout all manipulations, sterile filter tips were used. DNA extraction was processed individually or in pools for larvae, whereas nymph and adult ticks were processed individually. Identified larvae were pooled according to collection month, collection site, and species (2–10 individuals per pool). For Ixodes ovatus, one larva was tested. The ethanol-preserved ticks were rinsed twice with 70% ethanol and then immersed in distilled water (Yu et al., 2016). Ticks were homogenized in 75 μl of PBS using a PowerMasher II homogenizer (Nippi, Tokyo, Japan) and sterilized homogenization tube BioMasher II (Nippi). DNA was extracted using NucleoSpin Tissue (Macherey–Nagel, Düren, Germany) according to the manufacturerʼs instructions. DNA was eluted from columns with 50 μl of elution buffer. To confirm the purity of the eluates, the concentration of each DNA extract was assessed by Nanodrop spectrophotometers (Thermo Fisher Scientific, MA, USA). The extracted samples were stored at −30 °C until use.
2.3. PCR detection of B. burgdorferi (s.l.)
A nested PCR reaction was run on all DNA samples to detect B. burgdorferi (s.l.). Specific primers for B. burgdorferi (s.l.) (Table 1) were used to amplify the flaB gene encoding the flagellin protein (Yu et al., 2016). A 0.75 μl of DNA sample was added to 9.25 μl of reaction mixture that contained 1 μl of 10× PCR buffer, 1 μl of dNTPs, 0.2 μl of each primer (primers flaB outer primer F and flaB outer primer R, Table 1), 0.1 μl of Taq DNA polymerase (Blend Taq-Plus-; Toyobo, Osaka, Japan) and 6.75 μl of sterile Milli-Q water. The reaction conditions for the first PCR involved 4 min of pre-denaturation at 94 °C followed by 35 cycles of 30 s of denaturation at 94 °C, 30 s of annealing at 55 °C and extension at 72 °C for 1 min, and a final extension step at 72 °C for 10 min. The second PCR was performed in the same buffer using 0.5 μl of the first PCR products as a template and primers flaB nested primer F and flaB nested primer R (Table 1). The protocol for the second PCR was initial denaturation (94 °C, 4 min); followed by 35 cycles at 94 °C for 30 s, 58 °C for 30 s and 72 °C for 45 s, with the final extension step at 72 °C for 10 min. Amplicons of the second PCR were electrophoresed and stained with ethidium bromide as described previously (Yu et al., 2016). Detection of a band close to 411 bp under UV was considered positive.
Table 1.
Oligonucleotide primers used in this study
| Organism | Target gene | Oligonucleotide primer | Primer sequence (5′-3′) | Amplicon size (bp) | Reference |
|---|---|---|---|---|---|
| B. burgdorferi (s.l.) | flaB | Outer primer F | CTGCTGGCATGGGAGTTTCT | 725 | Yu et al. (2016) |
| Outer primer R | TCAATTGCATACTCAGTACT | ||||
| Nested primer F | GCAGTTCAATCAGGTAACGGC | 411 | Yu et al. (2016) | ||
| Nested primer R | AGAAGGTGCTGTAGCAGGTG | ||||
| rrf (5s)-rrl (23s) | 23SN1 | ACCATAGACTCTTATTACTTTGAC | 373 | Choi et al. (2007) | |
| 23SC1 | TAAGCTGACTAATACTAATTACCC | ||||
| 23SN2 | ACCATAGACTCTTATTACTTTGACCA | 227 | Choi et al. (2007) | ||
| 5SCB | GAGAGTAGGTTATTGCCAGGG | ||||
| Rickettsia spp. | gltA | CS-78 | GCAAGTATCGGTGAGGATGTAAT | 401 | Labruna et al. (2004) |
| CS-323 | GCTTCCTTAAAATTCAATAAATCAGGAT | ||||
| 16S rRNA | Rick_16S_F3 | ATCAGTACGGAATAACTTTTA | 1328 | Anstead & Chilton (2013) | |
| Rick_16S_F4 | TGCCTCTTGCGTTAGCTCAC | ||||
| rrs2_seq_1 | AGGCCTTCATCACTCACTCGa | Thu et al. (2019) | |||
| rrs2_seq_2 | CTACACGCGTGCTACAATGGa | ||||
| R16S_Fw1 | AGAAAAAGCCCCGGCTAACTCa | This study | |||
| R16S_Rv1 | CCATGCAACACCTGTGTGTGa | ||||
| ompB | 120_2788 | AAACAATAATCAAGGTACTGT | 816 | Roux & Raoult (2000) | |
| 120_3599 | TACTTCCGGTTACAGCAAAGT | ||||
| sca4 | D1f | ATGAGTAAAGACGGTAACCT | 928 | Sekeyova et al. (2001) | |
| D928r | AAGCTATTGCGTCATCTCCG |
Primers that were used only for the sequencing of amplicons.
The species of the B. burgdorferi (s.l.) complex detected in the samples were later identified through sequencing of the amplicons of the second PCR. When the flaB sequence did not allow a precise identification, the rrf (5S)-rrl (23S) ribosomal RNA (5S–23S) gene was amplified and sequenced for confirmation of the B. burgdorferi (s.l.) species. The primer sets used are shown in Table 1. PCR conditions were the same as previously reported (Choi et al., 2007).
2.4. PCR detection of Rickettsia spp.
PCR preparation was carried out in sterile conditions as described above. All of the tick DNA samples were subjected to PCR targeting gltA as a screening test for Rickettsia spp. DNA. Primers and PCR conditions have been described previously (de Sousa et al., 2018, Table 1). One microliter of DNA sample was added to a 9-μl reaction mixture that contained 1 μl of 10× PCR buffer, 1 μl of dNTPs, 0.2 μl of each primer set (primers CS-78 and CS-323, Table 1), 0.1 μl of Taq DNA polymerase (Blend Taq-Plus-; Toyobo) and 6.5 μl of sterile Milli-Q water. Detection of a band around 401 bp under UV was considered positive. Additional PCR assays were performed based on three genes: 16S ribosomal RNA gene (16S rRNA), outer membrane protein B gene (ompB), and surface cell antigen-4 (sca4) gene. The primer sets used for each reaction are shown in Table 1. PCR conditions were the same as previously reported by Thu et al. (2019) except for the annealing temperatures for ompB and sca4 PCR (54 °C for ompB PCR and 56 °C for sca4 PCR).
The Rickettsia species detected in the samples were identified in two steps. First, gltA genotyping was performed through sequencing of the amplicons of gltA PCR. Then, selected-sample representatives of the gltA genotype were submitted to further characterization of Rickettsia spp. through sequencing of 16S rRNA, ompB and sca4 genes.
2.5. Sequencing
The amplified PCR products were purified using a NucleoSpin Gel and PCR Clean-up kit (Macherey-Nagel). Each PCR product was subjected to DNA sequencing (Fasmac, Kanagawa, Japan), and ClustalW was used to align the sequences. The newly generated DNA sequences were submitted to the DNA Data Bank of Japan (DDBJ) (http://www.ddbj.nig.ac.jp) under the accession numbers: LC496815-LC496832 (Borrelia flaB); LC496811-LC496814 (Rickettsia gltA); MT378425-MT378437 (Rickettsia 16S rRNA); LC544128-LC544135 (Rickettsia ompB); and LC544136-LC544138 (Rickettsia sca4). Inter-species comparison of sequences based on BLASTn was performed using NCBI software Megablast (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The nucleotide collection (nr/nt) database, which links GenBank, EMBL, DDBJ, PDB and RefSeq sequences was used.
2.6. Phylogenetic analysis
DNA sequences obtained were aligned with sequences of representative Borrelia spp. or Rickettsia spp. using ClustalW 1.6 as implemented in MEGA 7 (Kumar et al., 2016). After manual confirmation, phylogenetic trees were constructed using the maximum likelihood method according to the Tamura 3-parameter (Tamura, 1992) model with bootstrap tests of 1000 replicates using MEGA 7.
2.7. Statistical tests
Microorganism prevalence in the individual tick DNA samples and their 95% confidence intervals (95% CI) were calculated using the GraphPad Prism software (GraphPad Software, San Diego, CA, USA). Microorganism prevalence in larvae was estimated by taking into account the number of pools and the number of larvae per pool. We assumed a maximum positive rate if all larvae of a positive pool were infected and a minimum positive rate if only one larva in a positive pool was infected.
3. Results
3.1. Tick collection
In total, 1563 ticks (599 adults, 191 nymphs and 773 larvae) were collected between May and November 2017 from 7 sites in Tokachi District. The number of ticks, tick species, and developmental stages collected varied based on sampling month and site. Four species of ticks were identified based on morphological criteria: I. ovatus (n = 364), Ixodes persulcatus (n = 296), Haemaphysalis japonica (n = 143) and Haemaphysalis megaspinosa (n = 760). Ixodes persulcatus was found in all sites examined across Tokachi District. The detailed features of the tick samples (sampling month, sampling sites, species and developmental stage) are presented in Supplementary Table S1. The 1563 live ticks that were collected by flagging, was divided into two sets. One set was allocated to laboratory rearing in other experiments (Umemiya-Shirafuji et al., unpublished data) and the remaining samples were submitted to DNA extraction: a total of 1155 ticks were used for DNA extraction and microorganism detection in the present study. Overall, 594 DNA samples including 527 individual tick DNA samples and 67 pooled larval tick DNA samples were prepared. The individual tick DNA samples were prepared from 295 I. ovatus (123 males; 171 females; and 1 larva), 169 I. persulcatus (72 males; 77 females; and 20 nymphs), 18 H. japonica (6 males; 1 female; 10 nymphs; and 1 larva), and 45 H. megaspinosa (26 males; 8 females; and 11 nymphs). The pooled tick DNA samples were prepared from larval stages of I. persulcatus (61; 9 pools) and H. megaspinosa (567; 58 pools) (Supplementary Table S1).
3.2. Detection of B. burgdorferi (s.l.) in ticks
Borrelia burgdorferi (s.l.)-positive ticks were detected in 6 (Memuro, Shikaoi, Urahoro, Shimizu, Taiki and Ashoro) of the 7 sites surveyed (Supplementary Table S1). DNA fragments of Borrelia spp. were detected in I. ovatus and I. persulcatus in the present study. The overall prevalence of B. burgdorferi (s.l.) was 21.8% (64/294; 95% CI: 17.2–26.9%) in I. ovatus (8/123 males; 56/171 females). One larva of I. ovatus tested was negative. The prevalence in I. persulcatus was 23.7% (40/169; 95% CI: 17.5–30.8%) and positive ticks included adults (12/72 males; 26/77 females,) and nymphs (2/20). All larval pools were negative (0/9 pools).
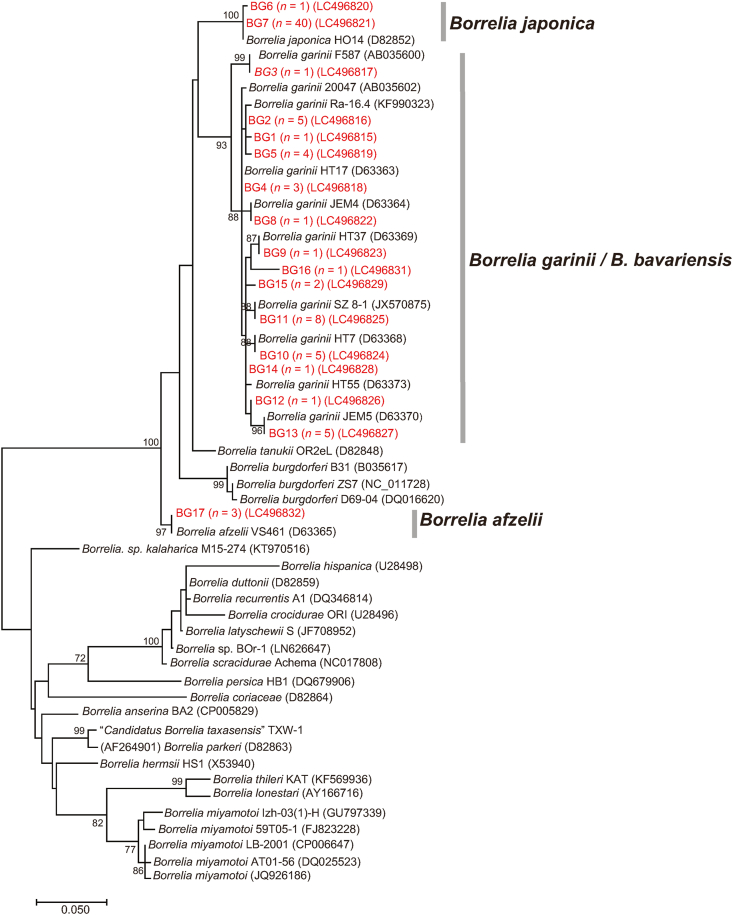
3.3. flaB genotyping and species classification of B. burgdorferi (s.l.)
Of the 104 samples that tested positive for B. burgdorferi (s.l.), 83 flaB PCR amplicons were successfully sequenced. The species identity of the remaining 21 samples was confirmed by sequencing the 5S–23S gene. From analyzing the 83 flaB sequences, 17 different flaB genotypes (referred to as BG1 to BG17) were identified. Fifteen genotypes were detected in I. persulcatus (BG1, BG2, BG3, BG4, BG5, BG8, BG9, BG10, BG11, BG12, BG13 BG14, BG15, BG16 and BG17) whereas the remaining two (BG6 and BG7) were only found in I. ovatus. The flaB sequences obtained in this study shared 99 or 100% identity with the closest Borrelia species sequences available in the GenBank database (Table 2). In the phylogenetic tree inferred from flaB analysis, 14 genotypes (BG1, BG2, BG3, BG4, BG5, BG8, BG9, BG10, BG11, BG12, BG13 BG14, BG15 and BG16) were found in the same clade with B. garinii and the closely related Borrelia bavariensis. The BLASTn analysis of the 5S–23S gene sequences obtained from the corresponding samples (data not shown) identified some of these genotypes as B. garinii and others as B. bavariensis. The 14 genotypes were therefore classified as B. garinii/B. bavariensis (Fig. 2). BG6 and BG7 formed a distinct cluster with Borrelia japonica and were identified as B. japonica. The 5S–23S gene sequences (data not shown) also confirmed the presence of B. japonica in the samples from which BG6 and BG7 were amplified. BG17 clustered with B. afzelii (Fig. 2).
Table 2.
Percentage of identity of flaB sequences of this study with sequences of the closest Borrelia species by BLAST analysis
| BG No. | Tick ID | Tick species | Developmental stage | % Identity of flaB with the closest Borrelia spp. (GenBank ID) |
|---|---|---|---|---|
| BG1 (n = 1) | Ipers20170036 | I. persulcatus | Female | 99% with B. garinii (MK604262) |
| BG2 (n = 5) | Iper20170048 | I. persulcatus | Female | 99% with B. garinii (MN193533) |
| BG3 (n = 1) | Iper20170143 | I. persulcatus | Nymph | 100% with B. garinii (AB035600) |
| BG4 (n = 3) | Iper20170022 | I. persulcatus | Female | 100% with B. garinii (D63363) |
| BG5 (n = 4) | Iper20170037 | I. persulcatus | Female | 99% with B. garinii (MK604263) |
| BG6 (n = 1) | Iovat20170110 | I. ovatus | Female | 99% with B. japonica (D82852) |
| BG7 (n = 40) | Iovat20170021 | I. ovatus | Female | 100% with B. japonica (D82852) |
| BG8 (n = 1) | Iper20170112 | I. persulcatus | Male | 100% with B. garinii (D63364) |
| BG9 (n = 1) | Iper20170102 | I. persulcatus | Male | 100% with B. garinii (D63369) |
| BG10 (n = 5) | Iper20170054 | I. persulcatus | Female | 100% with B. garinii (D63368) |
| BG11 (n = 8) | Iper20170057 | I. persulcatus | Female | 100% with B. garinii (JX570875a) |
| BG12 (n = 1) | Iper20170040 | I. persulcatus | Female | 100% with B. garinii (KU672560) |
| BG13 (n = 5) | Iper20170071 | I. persulcatus | Female | 100% with B. garinii (D63370) |
| BG14 (n = 1) | Iper20170073 | I. persulcatus | Female | 100% with B. garinii (KU672560) |
| BG15 (n = 2) | Iper20170072 | I. persulcatus | Female | 100% with B. garinii (AB001714) |
| BG16 (n = 1) | Iper20170101 | I. persulcatus | Male | 99% with B. garinii (KT356614) |
| BG17 (n = 3) | Iper20170039 | I. persulcatus | Female | 100% with B. afzelii (MT007941) |
Abbreviation: BG, flaB genotype of Borrelia spp.
JX570875 is registered in the GenBank as Borrelia garinii strain SZ 8-1. However, recent findings (see http://borreliabase.org/) indicate that Borrelia garinii strain SZ is rather B. bavariensis, a species closely related to Borrelia garinii.
Fig. 2.
Phylogenetic tree based on the sequences of the flaB gene (321 bp) for Borrelia spp. Seventeen different flaB genotypes (BG1 to BG17) were identified in the present study (red-colored letters). Numbers in parentheses represent GenBank accession numbers. The unit of branch length is nucleotide substitutions per site.
3.4. Detection of Rickettsia spp. in ticks
Ticks positive for Rickettsia spp. were detected in 6 (Memuro, Shikaoi, Urahoro, Hiroo, Taiki and Ashoro) out of the 7 provinces (Supplementary Table S1). Tick samples that showed positivity for Rickettsia spp. gltA included adults, nymphs and larvae of I. persulcatus and H. megaspinosa. All of the I. ovatus and H. japonica samples were negative. The overall prevalence of Rickettsia spp. was 41.4% (70/169; 95% CI: 34.5–49.8%) in I. persulcatus adults and nymphs (21/72 males; 44/77 females; 5/20 nymphs). Most of the I. persulcatus larval pools were positive (8/9), and the maximum and minimum positive rates among the larvae were estimated at 90.2% (55/61) and 13% (8/61), respectively. Among H. megaspinosa ticks the prevalence was 11.1% (5/45; 95% CI: 3.7–24.1%) for adults and nymphs (3/26 males; 2/8 females; 0/11 nymphs). Three out of the 58 larval pools were positive, suggesting 5.29% (30/567) and .53% (3/567) for the maximum and minimum positive rates among the H. megaspinosa larvae.
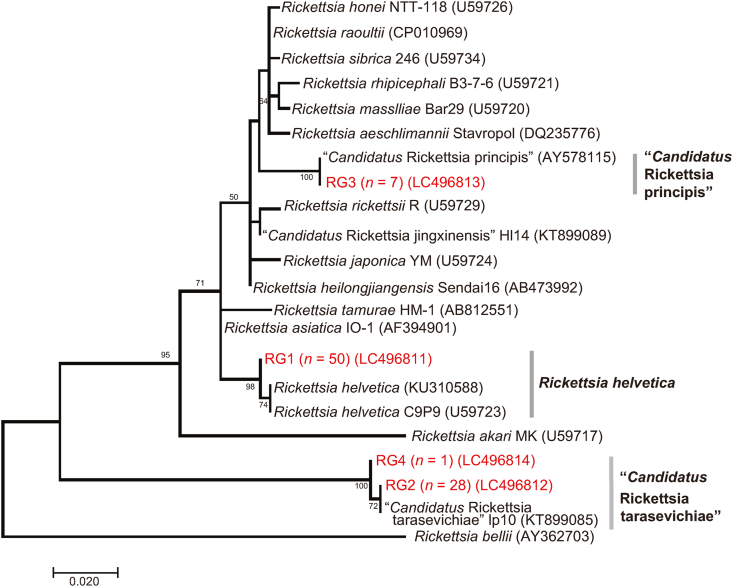
3.5. gltA genotyping and species classification of Rickettsia spp.
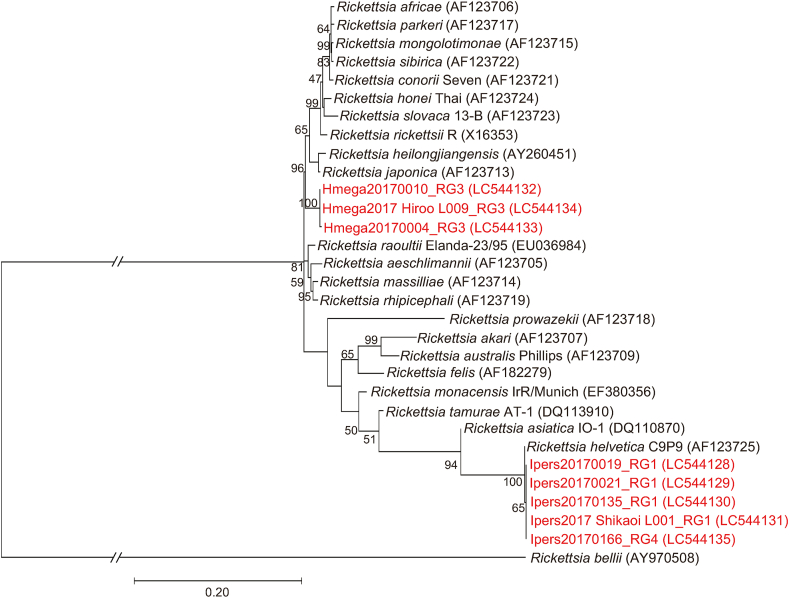
PCR amplicons of 86 samples found positive for Rickettsia spp. were sequenced and differentiated into 4 gltA genotypes (RG1, RG2, RG3 and RG4). RG1 was obtained from I. persulcatus (adult, nymph and larva) and a larval pool of H. megaspinosa. RG2 and RG4 were recovered from I. persulcatus (adult, nymph and larva) whereas RG3 was detected in H. megaspinosa (adult and larva). In the multiple gene sequencing of samples representing the gltA genotypes, the 16S rRNA gene was successfully amplified for all genotypes. The ompB gene was amplified in all samples of 3 genotypes (RG1, RG3 and RG4) except for H. megaspinosa (Hmega2017 Hiroo L007; RG1). The sca4 gene, however, was successfully amplified only for RG3. The gltA sequences obtained in this study shared 98–100% identity with the closest published Rickettsia species sequence (Table 3).
Table 3.
Results of the BLAST analysis of the Rickettsia spp. gltA, 16S rRNA, ompB, and sca4 sequences obtained in this study
| RG No. | Tick ID | Tick species and developmental stage | % Identity with the closest Rickettsia species (GenBank ID) |
|||
|---|---|---|---|---|---|---|
| gltA | 16S rRNA | ompB | sca4 | |||
| RG1 (n = 50) | Ipers20170019 | I. persulcatus (male) | 100% with R. helvetica (KU310588) | 100% with R. helvetica (MH618376) | 99% with R. helvetica (MF163037) | na |
| Ipers20170021 | I. persulcatus (female) | 100% with R. helvetica (KU310588) | 100% with R. helvetica (MH618376) | 99% with R. helvetica (MF163037) | na | |
| Ipers20170135 | I. persulcatus (nymph) | 100% with R. helvetica (KU310588) | 100% with R. helvetica (MH618376) | 99% with R. helvetica (MF163037) | na | |
| Ipers2017 Shikaoi L001 | I. persulcatus (larva) | 100% with R. helvetica (KU310588) | 100% with R. helvetica (MH618376) | 99% with R. helvetica (MF163037) | na | |
| Hmega2017 Hiroo L007 | H. megaspinosa (larva) | 100% with R. helvetica (KU310588) | 98% with R. raoultii (MK304546) 98% with R. conorii (NR_074480) 98% with R. helvetica (MH618376) |
na | na | |
| RG2 (n = 28) | Ipers20170103 | I. persulcatus (male) | 100% with “Ca. R. tarasevichiae” (MN450397) | 100% with “Ca. R. tarasevichiae” (MN446745) | na | na |
| Ipers20170025 | I. persulcatus (female) | 100% with “Ca. R. tarasevichiae” (MN450397) | 100% with “Ca. R. tarasevichiae” (MN446745) | na | na | |
| Ipers20170184 | I. persulcatus (nymph) | 100% with “Ca. R. tarasevichiae” (MN450397) | 100% with “Ca. R. tarasevichiae” (MN446745) | na | na | |
| Ipers2017 Taiki L001 | I. persulcatus (larva) | 100% with “Ca. R. tarasevichiae” (MN450397) | 100% with “Ca. R. tarasevichiae” (MN446745) | na | na | |
| RG3 (n = 7) | Hmega20170010a | H. megaspinosa (male) | 100% with “Ca. R. principis” (AY578115) | 99% with R. raoultii (MK304546) 99% R. conorii (NR_074480) |
100% with “Ca. R. principis” (MG544987)b | 99% with R. heilongjiangensis (AP019865) |
| Hmega20170004a | H. megaspinosa (female) | 100% with “Ca. R. principis” (AY578115) | 99% with R. raoultii (MK304546) 99% R. conorii (NR_074480) |
100% with “Ca. R. principis” (MG544987)b | 98% with R. heilongjiangensis (AP019865) | |
| Hmega2017 Hiroo L009a | H. megaspinosa (larva) | 100% with “Ca. R. principis” (AY578115) | 99% with R. raoultii (MK304546) 99% with R. conorii (NR_074480) |
100% with “Ca. R. principis” (MG544987)b | 98% with R. heilongjiangensis (AP019865) | |
| RG4 (n = 1) | Ipers20170166 | I. persulcatus (male) | 99% with “Ca. R. tarasevichiae” (MN450397) 89% with R. helvetica (KU310588) |
100% with R. helvetica (MH618376) | 99% with R. helvetica (MF163037) | na |
Abbreviations: Ca., Candidatus; na, not amplified; RG, gltA genotype of Rickettsia spp.
Identities with “Ca. R. principis” 16S rRNA and sca4 are not shown due to absence of reference sequences in the database.
Query coverage was 95%.
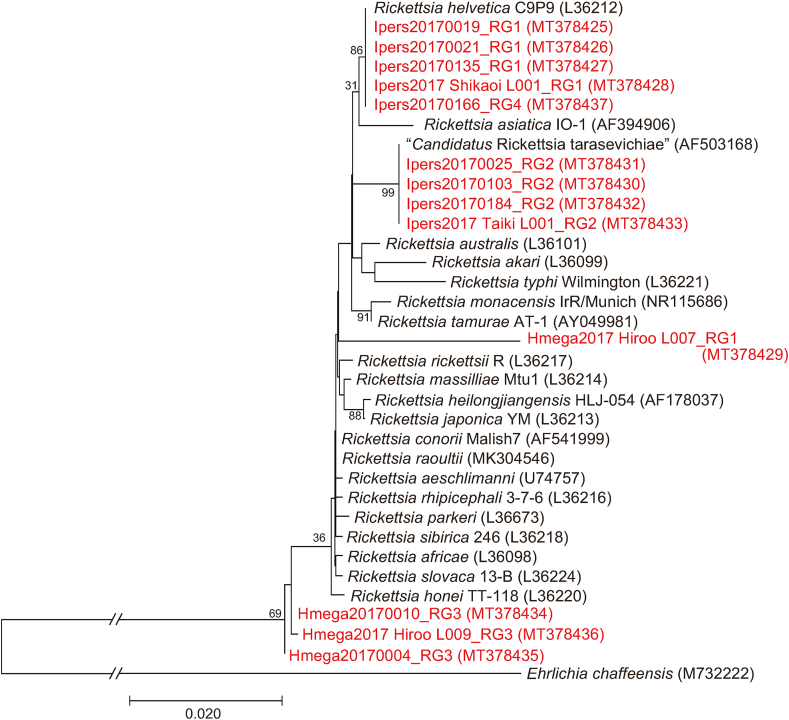
Phylogenetic trees inferred from gltA (Fig. 3), 16S rRNA (Fig. 4), ompB (Fig. 5) and sca4 (Fig. 6) sequences were constructed using the data from our study and public sequences of validated Rickettsia species. Four out of the five representatives of the RG1 genotype are close to R. helvetica and formed a distinct cluster with R. helvetica in the gltA and ompB phylogenetic trees. One RG1 obtained from a larval pool of H. megaspinosa was located in the R. helvetica gltA cluster but formed a divergent branch in the 16S rRNA phylogenetic tree (ompB and sca4 could not be amplified for that sample; Table 3). RG2 species identity was assessed using gltA and 16S rRNA phylogenetic trees, both of which located the genotype in a cluster with “Ca. R. tarasevichiae”. The RG3 genotype was classified as “Ca. R. principis” in the gltA phylogenetic tree. However, in the 16S rRNA, ompB and sca4 phylogenetic trees, RG3 sequences formed a cluster distinct from other validated Rickettsia species. RG4 was identified in the “Ca. R. tarasevichiae” cluster of the gltA phylogenetic tree, whereas in the 16S rRNA and ompB trees, it was located within the cluster of R. helvetica.
Fig. 3.
Phylogenetic tree based on the sequences of the gltA gene (332 bp) for Rickettsia spp. Amplicons by PCR for Rickettsia gltA were sequenced and classified into 4 different gltA genotypes (RG1 to RG4). DNA sequences obtained in the present study are indicated in red. Numbers in parentheses represent GenBank accession numbers. The unit of branch length is nucleotide substitutions per site.
Fig. 4.
Phylogenetic tree based on the Rickettsia 16S rRNA gene (1179 bp). The tree was constructed using the maximum likelihood method with the Kimura 2-parameter model. The analysis was performed with bootstrap tests of 1000 replicates. DNA sequences obtained in the present study are indicated in red-highlighted-tick ID. Numbers in parentheses are GenBank accession numbers. The units of branch length are nucleotide substitutions per site. The long branches are shortened and presented as interrupted branches (see full-size image in Supplementary Fig. S1).
Fig. 5.
Phylogenetic tree based on the Rickettsia ompB gene (815 bp). The tree was constructed using the maximum likelihood method with the Tamura 3-parameter model. The analysis was performed with bootstrap tests of 1000 replicates. DNA sequences obtained in the present study are indicated in red-highlighted-tick ID. Numbers in parentheses represent GenBank accession numbers. The units of branch length are nucleotide substitutions per site. The long branches are shortened and presented as interrupted branches (see full-size image in Supplementary Fig. S2).
Fig. 6.
Phylogenetic tree based on the Rickettsia sca4 (847 bp) gene. The tree was constructed using the maximum likelihood method with the Time reversible model. The analysis was performed with bootstrap tests of 1000 replicates. DNA sequences obtained in the present study are indicated in red-highlighted-tick ID. Numbers in parentheses are GenBank accession numbers. The units of branch length are nucleotide substitutions per site.
Altogether, the BLAST and phylogenetic analyses based on the gltA, 16S rRNA, ompB and sca4 sequences, showed that the Rickettsia spp. detected in the samples include species related to R. helvetica, “Ca. R. principis”, and “Ca. R. tarasevichiae”.
3.6. Distribution of B. burgdorferi (s.l.) and Rickettsia species
The presence of Borrelia spp. and Rickettsia spp. in tick samples collected from Tokachi District in 2017 is summarized in Table 4. Borrelia japonica was only detected in adult I. ovatus samples with a prevalence of 21.8% (64/294; 95% CI: 17.2–26.9%). Borrelia garinii/B. bavariensis was detected in both adult and nymphal I. persulcatus at a prevalence of 21.9% (37/169; 95% CI: 15.9–28.9%). Borrelia afzelii was found only in adult I. persulcatus at a prevalence of 1.8% (3/169; 95% CI: 0.4–5.1%). Concerning Rickettsia species, R. helvetica was detected at a prevalence of 26.0% (44/169; 95% CI: 19.6–33.3%) in adult and nymphal I. persulcatus and in 55.6% (5/9) of the larval pools. Among the H. megaspinosa larval pools, one (1.7%; 1/58) was positive for R. helvetica. The prevalence of “Ca. R. tarasevichiae” was 15.4% (26/169; 95% CI: 10.3–21.7%) in I. persulcatus adults and nymphs and 33.3% (3/9) in the larval pools. “Candidatus R. principis” was related to H. megaspinosa with a prevalence of 11.1% (5/45; 95% CI: 3.7–24.1%) and 3.4% (2/58) for adults and nymphs, and larval pools, respectively. The prevalence of these microorganisms in female ticks appeared to be higher than the values recorded in male ticks (Table 4). However, larger sample sizes will be needed to compare statistically the prevalence of the microorganisms between female and male ticks. The distribution of Borrelia and Rickettsia species varied across the sampling sites (Supplementary Table S1). Rickettsia helvetica and “Ca. R. tarasevichiae” found in 6 sites (Memuro, Shikaoi, Urahoro, Hiroo, Taiki and Ashoro) were the most widely distributed, followed by B. japonica (Memuro, Urahoro, Shimizu, Taiki and Ashoro) and B. garinii/B. bavariensis (Memuro, Shikaoi and Urahoro). “Candidatus R. principis” and B. afzelii were found in 2 (Hiroo, Taiki) and 1 (Memuro) site, respectively.
Table 4.
Molecular detection of Borrelia burgdorferi (s.l.) and Rickettsia spp. among tick species collected in Tokachi district, Japan
| Tick species | Stagea |
Borrelia burgdorferi (s.l.) (%) |
Rickettsia spp. (%) |
||||
|---|---|---|---|---|---|---|---|
| B. j. | B. g./B. b. | B. a. | R. h. | “Ca. R. t.” | “Ca. R. p.” | ||
| I. ovatus | Male | 8/123 (6.5) | 0/123 (0) | 0/123 (0) | 0/123 (0) | 0/123 (0) | 0/123 (0) |
| Female | 56/171 (32.7) | 0/171 (0) | 0/171 (0) | 0/171 (0) | 0/171 (0) | 0/171 (0) | |
| Larva | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | |
| I. persulcatus | Male | 0/72 (0) | 11/72 (15.3) | 1/72 (1.4) | 10/72 (13.9) | 11/72 (15.3) | 0/72 (0) |
| Female | 0/77 (0) | 24/77 (31.2) | 2/77 (2.6) | 30/77 (39.0) | 14/77 (18.2) | 0/77 (0) | |
| Nymph | 0/20 (0) | 2/20 (10.0) | 0/20 (0) | 4/20 (20.0) | 1/20 (5.0) | 0/20 (0) | |
| Larvab | 0/9 (0) | 0/9 (0) | 0/9 (0) | 5/9 (56.0) | 3/9 (33.0) | 0/9 (0) | |
| H. megaspinosa | Male | 0/26 (0) | 0/26 (0) | 0/26 (0) | 0/26 (0) | 0/26 (0) | 3/26 (12.0) |
| Female | 0/8 (0) | 0/8 (0) | 0/8 (0) | 0/8 (0) | 0/8 (0) | 2/8 (25.0) | |
| Nymph | 0/11 (0) | 0/11 (0) | 0/11 (0) | 0/11 (0) | 0/11 (0) | 0/11 (0) | |
| Larvab | 0/58 (0) | 0/58 (0) | 0/58 (0) | 1/58 (1.7) | 0/58 (0) | 2/58 (3.4) | |
| H. japonica | Male | 0/6 (0) | 0/6 (0) | 0/6 (0) | 0/6 (0) | 0/6 (0) | 0/6 (0) |
| Female | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | |
| Nymph | 0/10 (0) | 0/10 (0) | 0/10 (0) | 0/10 (0) | 0/10 (0) | 0/10 (0) | |
| Larva | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | |
Abbreviations: B. a., Borrelia afzelii; B. b., B. bavariensis; B. g., Borrelia garinii; B. j., Borrelia japonica; “Ca. R. p.”, “Candidatus Rickettsia principis”; “Ca. R. t. ”, “Candidatus Rickettsia tarasevichiae”; R. h., Rickettsia helvetica.
Developmental stages of ticks collected.
Larvae of I. persulcatus and H. megaspinosa were pooled according to collection month and collection site (2–10 individuals per pool).
4. Discussion
In this study, four hard tick species namely I. ovatus, I. persulcatus, H. japonica and H. megaspinosa were collected in 2017 from Tokachi District, Japan. The identified tick species are consistent with previous surveys on tick species in Tokachi (Inokuma et al., 2007), suggesting that these species are still dominant hard ticks in this area. Ixodes ovatus and I. persulcatus are the main causative species of human tick bites in Hokkaido to Honshu, northern to central Japan (Natsuaki, 2021). Some human cases with H. japonica bites were found in Hokkaido and Honshu (Yamauchi et al., 2010; Yamauchi & Nakatani, 2016; Sasaki et al., 2021). Although the primary hosts of H. megaspinosa are large mammals (e.g. deer, boar and Japanese serow) (Yamaguti et al., 1971), human cases of tick bites by H. megaspinosa were reported in Honshu (Seishima et al., 2000; Tsunoda, 2004; Hasegawa et al., 2016). The present PCR assays using DNA samples of questing ticks revealed that I. persulcatus carried B. garinii/B. bavariensis, B. afzelii, R. helvetica and “Ca. R. tarasevichiae”, and that I. ovatus was associated with B. japonica. Haemaphysalis megaspinosa carried R. helvetica and “Ca. R. principis”, while none of the H. japonica ticks collected in the present study was positive for the microorganisms that were targeted.
Currently, the B. burgdorferi (s.l.) species complex consists of more than 20 pathogenic, potentially pathogenic, non-pathogenic and unknown pathogenic bacteria that utilize Ixodes ticks as vectors (Stanek & Strle, 2018; Margos et al., 2019). Borrelia garinii, B. bavariensis and B. afzelii are human pathogens and cause Lyme borreliosis, whereas the pathogenicity of B. japonica is unknown. Borrelia garinii and B. bavariensis, previously referred to as B. garinii genospecies, are closely related species sharing the same geographical distribution and tick vectors (Margos et al., 2019). Discrimination of these Borrelia species requires a multilocus genotyping approach including eight genes. In our study, a two-gene approach confirming the occurrence of both species was selected and the samples of B. burgdorferi (s.l.) showing sequence identity with any of these two species were classified as B. garinii/B. bavariensis. Ixodes persulcatus is a suspected vector for B. garinii, B. bavariensis and B. afzelii (Masuzawa et al., 2005; Margos et al., 2019; Eisen, 2020). These pathogens have previously been reported in I. persulcatus collected from Japan (Fukunaga et al., 1996; Masuzawa, 2004; Murase et al., 2012). Furthermore, I. persulcatus was considered as a primary tick vector for Lyme borreliosis in Hokkaido (Fukunaga et al., 1993; Murase et al., 2012). The presence of B. garinii/B. bavariensis and B. afzelii in I. persulcatus from Tokachi area relates therefore to the vector role of this tick and its importance in Lyme borreliosis transmission.
Despite sharing the same geographical range and vector, B. garinii, B. bavariensis and B. afzelii seem to have different relative frequency. Fukunaga & Hamase (1995) indicated that B. garinii was predominant among isolates obtained from Lyme borreliosis patients and ticks in Japan. Meanwhile, Murase et al. (2012) showed that B. garinii infection rate was higher than that of B. afzelii in I. persulcatus from Hokkaido. In accordance, in our study, 92.5% (37/40) of the pathogens found in the B. burgdorferi (s.l.)-positive I. persulcatus samples were identified as B. garinii/B. bavariensis, and the remaining three positive samples were identical to B. afzelii. However, the infection rate (21.9%; 95% CI: 15.9–28.9%) of B. garinii/B. bavariensis in I. persulcatus from Tokachi area was lower than the B. garinii infection rate (33.8%) obtained by Murase et al. (2012) in the same tick species but in another site in Hokkaido Prefecture. The infection rate of B. afzelii (1.8%; 95% CI: 0.4–5.1%) showed in our study was similar to the value (4.9%) obtained by Murase et al. (2012). These authors investigated a ranching farm where a confirmed case of Lyme borreliosis has been reported, whereas we did not collect ticks in such farm in our study. It might suggest that the positive rates of pathogens vary depending on the characteristics of the study areas. While B. garinii, B. bavariensis and B. afzelii are found in Europe and Asia, B. japonica seems to be restricted to Japan (Kawabata et al., 1993; Li et al., 1998). Previous studies indicated that I. ovatus carries only B. japonica (Kawabata et al., 1993; Masuzawa, 2004; Murase et al., 2012) and Nakao et al. (1994) indicated that the tick and Borrelia species are not related to Lyme borreliosis. The presence of B. japonica and not the other B. burgdorferi (s.l.) genospecies in I. ovatus collected in this study, therefore relates to this tick species not being a vector of Lyme borreliosis. Borrelia japonica prevalence in I. ovatus (21.8%; 64/294, 95% CI: 17.2–26.9%) was also similar to the values (19.5%; 8/41) obtained by Murase et al. (2012).
We did not find I. persulcatus larvae carrying B. garinii/B. bavariensis or B. afzelii. In the absence of transovarial transmission of B. burgdorferi (s.l.) in I. persulcatus (Nefedova et al., 2004), acquisition of B. garinii, B. bavariensis and B. afzelii by its vector obviously results from feeding on the blood of host animals. The principal reservoir host for the species of Borrelia transmitted by I. persulcatus immatures in Hokkaido is the wood mouse, Apodemus speciosus ainu (Nakao & Miyamoto, 1993a). The Borrelia isolated from A. speciosus ainu was formerly identified as B. garinii (currently probably B. bavariensis) (Kawabata et al., 1993; Margos et al., 2019). In contrast, the long-tailed shrew Sorex unguiculatus is a reservoir for B. japonica and also a host animal for I. ovatus immatures in Hokkaido (Nakao & Miyamoto, 1993b). Tokachi area is a sympatric region for I. ovatus and I. persulcatus. Although the host ranges of I. ovatus and I. persulcatus partially overlap for their immature stages (Nakao & Miyamoto, 1993b), our results and previous studies suggest that Borrelia species detected from questing adults of both tick species are clearly distinguishable (Table 4). Further experimental investigations such as xenodiagnosis are needed to test the vector competence of the two tick species for different Borrelia species and observe the effect of these Borrelia infections on tick physiology.
In the present study, Rickettsia species were detected not only in adults and nymphs, but also in larvae. Of the three Rickettsia spp. detected, R. helvetica and “Ca. R. tarasevichiae” are pathogenic to humans, whereas “Ca. R. principis”, pathogenicity remains unknown. R. helvetica was first reported in Europe (Parola et al., 1998). Its prevalence in Europe has been evidenced in France (Fournier et al., 2000) and then in Japan (Fournier et al., 2002; Noji et al., 2005). It is known that vectors of R. helvetica in Japan are I. persulcatus, I. ovatus and Ixodes monospinosus (Fournier et al., 2002; Ishiguro et al., 2008). In the present study, we detected R. helvetica gltA genotype in I. persulcatus, corresponding to the finding of Inokuma et al. (2007) and suggesting that I. persulcatus could be a vector of R. helvetica in Tokachi District. Furthermore, the detection of R. helvetica in questing I. persulcatus larvae suggests transovarial transmission, although contaminations or partial feeding could not be excluded. Rickettsia helvetica gltA genotype (RG1) was also found in one pool of H. megaspinosa larvae (Hmega2017 Hiroo L007) that included 10 individual larvae; however, the sequence of the 16S rRNA gene from this pool formed a different clade with R. helvetica (Fig. 4; Hmega2017 Hiroo L007 RG1 (MT378429)). Although such finding indicates that H. megaspinosa might play a role as a vector for R. helvetica, further research is needed to fully characterize the Rickettsia species/strain carried by these larvae.
“Candidatus R. tarasevichiae” was regarded as non-pathogenic until 2012, when five patients with recent tick bites sought treatment in a hospital of northeastern China and were found to be infected with this pathogen (Jia et al., 2013). More recently, a retrospective investigation in eastern central China found that 56 patients who showed severe fever with thrombocytopenia syndrome-like illnesses were infected with “Ca. R. tarasevichiae” (Liu et al., 2016). The identification of “Ca. R. tarasevichiae” in I. persulcatus in our study is in agreement with a previous study (Inokuma et al., 2007) in the same district. “Candidatus R. tarasevichiae” was detected not only in adults and nymphs but also in larvae, suggesting that transovarial transmission occurred under natural conditions. Among the samples in which “Ca. R. tarasevichiae” gltA was identified, one (RG4 (n = 1); Ipers20170166) showed 16S rRNA and ompB sequences that clustered with R. helvetica sequences (Fig. 4, Fig. 5). This may be explained by the tick being infected with both “Ca. R. tarasevichiae” and R. helvetica. On the other hand, the life-cycle and pathogenicity for humans of “Ca. R. principis” remain unknown (Mediannikov et al., 2006). This rickettsial agent has previously been detected in the ticks Haemaphysalis flava and H. japonica in Japan (Gaowa et al., 2013) and in Haemaphysalis danieli in China (Chahan et al., 2007). A recent survey of SFG rickettsiae in questing ticks in Japan identified a Rickettsia genotype that was obtained from adult H. megaspinosa and clustered in the same clade with “Ca. R. principis” (Thu et al., 2019). In our study, Rickettsia gltA genotype RG3 was detected in H. megaspinosa adults and larvae and located in “Ca. R. principis” clade. In the BLASTn search, the ompB sequences of RG3 showed 100% identity with previously published “Ca. R. principis” sequences, although the query coverage was 95%. Because published “Ca. R. principis” sequences were shorter (ompB) or not available (16S rRNA and sca4), RG3 sequences formed a cluster distinct from other validated Rickettsia species in the ompB, 16S rRNA and sca4 phylogenetic trees. Based on BLASTn search (gltA and ompB) and gltA phylogenetic tree, RG3 was identified as genetically related to “Ca. R. principis”. These results support previous reports and also provide, to our knowledge, the first evidence of the occurrence of “Ca. R. principis” in larvae of H. megaspinosa. The detection of “Ca. R. principis” in questing H. megaspinosa larvae indicates transovarial transmission of “Ca. R. principis” in the tick species.
5. Conclusions
Our findings suggest that major risks of Lyme borreliosis and SFG rickettsiosis agents in the Tokachi District are currently restricted to I. persulcatus. It would be informative for farmworkers, hunters, local residents as well as tourists to make aware of infection risks associated with tick bites. Furthermore, the sampling design (sampling season and frequency) as well as tick species life-cycle patterns might explain the differences in tick species and tick life stage between sampling sites. In the future, annual or yearly surveys of ticks and their carrying microorganisms will be needed to reveal their seasonal occurrence in the region to constantly avoid the risk for tick-borne diseases.
Funding
This study was supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan as a project for Joint Usage/Research Center.
Ethical approval
Not applicable.
CRediT author statement
Kiyoshi Okado: methodology, validation, formal analysis, investigation, writing - original draft, visualization. Paul Franck Adjou Moumouni: methodology, validation, formal analysis, investigation, resources, writing - review and editing. Seung-Hun Lee: methodology, validation, formal analysis, investigation, writing - review and editing. Thillaiampalam Sivakumar: formal analysis, writing - review and editing. Naoaki Yokoyama: conceptualization, investigation, writing - review and editing, project administration. Kozo Fujisaki: conceptualization, writing - review and editing. Hiroshi Suzuki: conceptualization, investigation, writing - review and editing, project administration. Xuenan Xuan: conceptualization, investigation, resources, project administration, funding acquisition. Rika Umemiya-Shirafuji: conceptualization, investigation, resources, writing - review and editing, visualization, supervision, funding acquisition. All authors read and approved the final manuscript.
Data availability
The newly generated DNA sequences were submitted to the DNA Data Bank of Japan (DDBJ) (http://www.ddbj.nig.ac.jp) under the accession numbers: LC496815-LC496832 (Borrelia flaB); LC496811-LC496814 (Rickettsia gltA); MT378425-MT378437 (Rickettsia 16S rRNA); LC544128-LC544135 (Rickettsia ompB); and LC544136-LC544138 (Rickettsia sca4).
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank to all the staff who participated in the collection of ticks in Tokachi District. We thank Hirono Suganuma and Hiroyuki Sugawara (National Research Center for Protozoan Diseases, Obihiro University of Agriculture and Veterinary Medicine) for their excellent technical assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crpvbd.2021.100059.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Figure S1. Full size image of Fig. 4.
Supplementary Figure S2. Full size image of Fig. 5.
Supplementary Table S1. Tick species distribution and prevalence of Borrelia burgdorferi (s.l.) and Rickettsia spp. among questing ticks collected in Tokachi District, eastern Hokkaido, Japan.
References
- Ando S., Kurosawa M., Sakata A., Fujita H., Sakai K., Sekine M., et al. Human Rickettsia heilongjiangensis infection, Japan. Emerg. Infect. Dis. 2010;16:1306–1308. doi: 10.3201/eid.1608.100049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstead C.A., Chilton N.B. A novel Rickettsia species detected in vole ticks (Ixodes angustus) from western Canada. Appl. Environ. Microbiol. 2013;79:7583–7589. doi: 10.1128/AEM.02286-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahan B., Jian Z., Jilintai, Miyahara K., Tanabe S., Xuan X., et al. Detection of DNA closely related to “Candidatus Rickettsia principis” in Haemaphysalis danieli recovered from cattle in Xinjiang Uygur Autonomous Region area, China. Vet. Parasitol. 2007;144:184–187. doi: 10.1016/j.vetpar.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Chaligiannis Ι., Fernández de Mera I.G., Papa A., Sotiraki S., de la Fuente J. Molecular identification of tick-borne pathogens in ticks collected from dogs and small ruminants from Greece. Exp. Appl. Acarol. 2018;74:443–453. doi: 10.1007/s10493-018-0237-z. [DOI] [PubMed] [Google Scholar]
- Choi Y.J., Han S.H., Park J.M., Lee K.M., Lee E.M., Lee S.H., et al. First molecular detection of Borrelia afzelii in clinical samples in Korea. Microbiol. Immunol. 2007;51:1201–1207. doi: 10.1111/j.1348-0421.2007.tb04015.x. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F., Lia R.P., Capelli G., Otranto D. Efficiency of flagging and dragging for tick collection. Exp. Appl. Acarol. 2013;61:119–127. doi: 10.1007/s10493-013-9671-0. [DOI] [PubMed] [Google Scholar]
- de Sousa K.C.M., Herrera H.M., Rocha F.L., Costa F.B., Martins T.F., Labruna M.B., et al. Rickettsia spp. among wild mammals and their respective ectoparasites in Pantanal Wetland, Brazil. Ticks Tick Borne Dis. 2018;9:10–17. doi: 10.1016/j.ttbdis.2017.10.015. [DOI] [PubMed] [Google Scholar]
- Eisen L. Vector competence studies with hard ticks and Borrelia burgdorferi sensu lato spirochetes: A review. Ticks Tick Borne Dis. 2020;11 doi: 10.1016/j.ttbdis.2019.101359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen R.J., Paddock C.D. Tick and tickborne pathogen surveillance as a public health tool in the United States. J. Med. Entomol. 2020;58:1490–1502. doi: 10.1093/jme/tjaa087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier P.E., Fujita H., Takada N., Raoult D. Genetic identification of rickettsiae isolated from ticks in Japan. J. Clin. Microbiol. 2002;40:2176–2181. doi: 10.1128/JCM.40.6.2176-2181.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier P.E., Grunnenberger F., Jaulhac B., Gastinger G., Raoult D. Evidence of Rickettsia helvetica infection in humans, eastern France. Emerg. Infect. Dis. 2000;6:389–392. doi: 10.3201/eid.0604.000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H., Fournier P.E., Takada N., Saito T., Raoult D. Rickettsia asiatica sp. nov., isolated in Japan. Int. J. Syst. Evol. Microbiol. 2006;56:2365–2368. doi: 10.1099/ijs.0.64177-0. [DOI] [PubMed] [Google Scholar]
- Fukunaga M., Hamase A. Outer surface protein C gene sequence analysis of Borrelia burgdorferi sensu lato isolates from Japan. J. Clin. Microbiol. 1995;33:2415–2420. doi: 10.1128/jcm.33.9.2415-2420.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga M., Okada K., Nakao M., Konishi T., Sato Y. Phylogenetic analysis of Borrelia species based on flagellin gene sequences and its application for molecular typing of Lyme disease borreliae. Int. J. Syst. Bacteriol. 1996;46:898–905. doi: 10.1099/00207713-46-4-898. [DOI] [PubMed] [Google Scholar]
- Fukunaga M., Sohnaka M., Takahashi Y., Nakao M., Miyamoto K. Antigenic and genetic characterization of Borrelia species isolated from Ixodes persulcatus in Hokkaido, Japan. J. Clin. Microbiol. 1993;31:1388–1391. doi: 10.1128/jcm.31.5.1388-1391.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima H., Yazaki T., Hirota T., Iwata Y., Wajima A., Yokota A. Factors and mechanisms affecting the air temperature distribution on a clear winter night in a snow-covered mesoscale plain. J. Meteorol. Soc. Japan. 2019;97:105–121. doi: 10.2151/jmsj.2019-005. [DOI] [Google Scholar]
- Gaowa, Ohashi N., Aochi M., Wuritu, Wu D., Yoshikawa Y., Kawamori F., et al. Rickettsiae in ticks, Japan, 2007–2011. Emerg. Inf. Dis. 2013;19:338–340. doi: 10.3201/eid.1902.120856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa T., Takahashi T., Okano T., Kanoh H., Fujihiro M., Maeda M., et al. A clinical study of 94 patients with tick bite in 8 medical institutions in Gifu Prefecture. Jpn. J. Dermatol. 2016;126:2095–2102. (In Japanese) [Google Scholar]
- IDSC . Vol. 27. Infectious Disease Surveillance Center; Tokyo, Japan: 2006. The first case of Rickettsia helvetica infection diagnosed by serum antibody tests; pp. 40–41.http://idsc.nih.go.jp/iasr/27/312/dj312a.html (Infectious Agents Surveillance Report). (In Japanese) [Google Scholar]
- IDSC . Vol. 32. Infectious Disease Surveillance Center; Tokyo, Japan: 2011. Lyme disease in Japan, 2006–2010; pp. 216–217.https://idsc.niid.go.jp/iasr/32/378/tpc378.html (Infectious Agents Surveillance Report). [Google Scholar]
- IDSC . Vol. 41. Infectious Disease Surveillance Center; Tokyo, Japan: 2020. Japanese spotted fever 1999–2019; pp. 133–134.https://www.niid.go.jp/niid/images/idsc/iasr/41/486.pdf (Infectious Agents Surveillance Report). [Google Scholar]
- Inokuma H., Ohashi M., Jilintai, Tanabe S., Miyahara K. Prevalence of tick-borne Rickettsia and Ehrlichia in Ixodes persulcatus and Ixodes ovatus in Tokachi district, eastern Hokkaido, Japan. J. Vet. Med. Sci. 2007;69:661–664. doi: 10.1292/jvms.69.661. [DOI] [PubMed] [Google Scholar]
- Ishiguro F., Takada N., Fujita H., Noji Y., Yano Y., Iwasaki H. Survey of the vectorial competence of ticks in an endemic area of spotted fever group rickettsioses in Fukui prefecture, Japan. Microbiol. Immunol. 2008;52:305–309. doi: 10.1111/j.1348-0421.2008.00042.x. [DOI] [PubMed] [Google Scholar]
- Jia N., Zheng Y.C., Jiang J.F., Cao W.C. Human infection with “Candidatus Rickettsia tarasevichiae”. New Engl. J. Med. 2013;369:1178–1180. doi: 10.1056/NEJMc1303004. https://www.nejm.org/doi/full/10.1056/NEJMc1303004 [DOI] [PubMed] [Google Scholar]
- Kawabata H., Masuzawa T., Yanagihara Y. Genomic analysis of Borrelia japonica sp. nov. isolated from Ixodes ovatus in Japan. Microbiol. Immunol. 1993;37:843–848. doi: 10.1111/j.1348-0421.1993.tb01714.x. [DOI] [PubMed] [Google Scholar]
- Kitaoka S. Keys to the species in immature stages of the Japanese Haemaphysalis ticks (Ixodidae) Bull. Natl. Inst. Anim. Health. 1985;88:49–63. (In Japanese) [Google Scholar]
- Kubo N., Arashima Y., Yoshida M., Kawabata M., Nishinarita S., Hayama T., et al. Questionnaire surveys of cases of tick bite and Lyme borreliosis in hunters in Hokkaido with reference to detection of anti-Borrelia burgdorferi antibody. Intern. Med. 1992;31:1163–1168. doi: 10.2169/internalmedicine.31.1163. [DOI] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labruna M.B., Whitworth T., Bouyer D.H., McBride J., Camargo L.M., Camargo E.P., et al. Rickettsia bellii and Rickettsia amblyommii in Amblyomma ticks from the state of Rondônia, western Amazon, Brazil. J. Med. Entomol. 2004;41:1073–1081. doi: 10.1603/0022-2585-41.6.1073. [DOI] [PubMed] [Google Scholar]
- Li M., Masuzawa T., Takada N., Ishiguro F., Fujita H., Iwaki A., et al. Lyme disease Borrelia species in northeastern China resemble those isolated from far eastern Russia and Japan. Appl. Environ. Microbiol. 1998;64:2705–2709. doi: 10.1128/aem.64.7.2705-2709.1998. https://aem.asm.org/content/64/7/2705.short [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Li H., Lu Q.B., Cui N., Yang Z.D., Hu J.G., et al. “Candidatus Rickettsia tarasevichiae” infection in eastern central China. Ann. Intern. Med. 2016;164:641–654. doi: 10.7326/m15-2572. [DOI] [PubMed] [Google Scholar]
- Margos G., Fingerle V., Reynolds S. Borrelia bavariensis: Vector switch, niche invasion, and geographical spread of a tick-borne bacterial parasite. Front. Ecol. Evol. 2019;7:401. doi: 10.3389/fevo.2019.00401. [DOI] [Google Scholar]
- Masuzawa T. Terrestrial distribution of the Lyme borreliosis agent Borrelia burgdorferi sensu lato in East Asia. Jpn. J. Infect. Dis. 2004;57:229–235. [PubMed] [Google Scholar]
- Masuzawa T., Kharitonenkov I.G., Kadosaka T., Hashimoto N., Kudeken M., Takada N., et al. Characterization of Borrelia burgdorferi sensu lato isolated in Moscow province - a sympatric region for Ixodes ricinus and Ixodes persulcatus. Int. J. Med. Microbiol. 2005;294:455–464. doi: 10.1016/j.ijmm.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Mediannikov O., Sidelnikov Y., Ivanov L., Fournier P.E., Tarasevich I., Raoult D. Far eastern tick-borne Rickettsiosis: Identification of two new cases and tick vector. Ann. N. Y. Acad. Sci. 2006;1078:80–88. doi: 10.1196/annals.1374.010. [DOI] [PubMed] [Google Scholar]
- Murase Y., Konnai S., Githaka N., Hidano A., Taylor K., Ito T., et al. Prevalence of Lyme borrelia in Ixodes persulcatus ticks from an area with a confirmed case of Lyme disease. J. Vet. Med. Sci. 2012;75:215–218. doi: 10.1292/jvms.12-0211. [DOI] [PubMed] [Google Scholar]
- Nakao M., Miyamoto K. Reservoir competence of the wood mouse, Apodemus speciosus Ainu, for the Lyme disease spirochete, Borrelia burgdorferi, in Hokkaido, Japan. Med. Entomol. Zool. 1993;44:69–84. doi: 10.7601/mez.44.69. [DOI] [Google Scholar]
- Nakao M., Miyamoto K. Long-tailed shrew, Sorex unguiculatus, as a potential reservoir of the spirochetes transmitted by Ixodes ovatus in Hokkaido, Japan. Med. Entomol. Zool. 1993;44:237–245. doi: 10.7601/mez.44.237. [DOI] [Google Scholar]
- Nakao M., Miyamoto K., Fukunaga M. Borrelia japonica in nature: Genotypic identification of spirochetes isolated from Japanese small mammals. Microbiol. Immunol. 1994;38:805–808. doi: 10.1111/j.1348-0421.1994.tb01861.x. [DOI] [PubMed] [Google Scholar]
- Natsuaki M. Tick bites in Japan. J. Dermatol. 2021;48:423–430. doi: 10.1111/1346-8138.15779. [DOI] [PubMed] [Google Scholar]
- Nefedova V.V., Korenberg E.I., Gorelova N.B., Kovalevskii Y.V. Studies on the transovarial transmission of Borrelia burgdorferi sensu lato in the taiga tick Ixodes persulcatus. Folia Parasitol. (Prague) 2004;51:67–71. doi: 10.14411/fp.2004.010. [DOI] [PubMed] [Google Scholar]
- Noji Y., Takada N., Ishiguro F., Fujino S., Aoyama T., Fujita H., et al. The first reported case of spotted fever in Fukui prefecture, the northern part of central Japan. Jpn. J. Infect. Dis. 2005;58:112–114. [PubMed] [Google Scholar]
- Ota N., Mizuno D., Kuboki N., Igarashi I., Nakamura Y., Yamashina H., et al. Epidemiological survey of Theileria orientalis infection in grazing cattle in the eastern part of Hokkaido, Japan . J. Vet. Med. Sci. 2009;71:937–944. doi: 10.1292/jvms.71.937. [DOI] [PubMed] [Google Scholar]
- Parola P., Beati L., Cambon M., Raoult D. First isolation of Rickettsia helvetica from Ixodes ricinus ticks in France. Eur. J. Clin. Microbiol. Infect. Dis. 1998;17:95–100. doi: 10.1007/BF01682163. [DOI] [PubMed] [Google Scholar]
- Parola P., Paddock C.D., Raoult D. Tick-borne rickettsioses around the world: Emerging diseases challenging old concepts. Clin. Microbiol. Rev. 2005;18:719–756. doi: 10.1128/CMR.18.4.719-756.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowski M., Rymaszewska A. Expansion of tick-borne rickettsioses in the world. Microorganisms. 2020;8:1906. doi: 10.3390/microorganisms8121906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raoult D., Roux V. Rickettsioses as paradigms of new or emerging infectious diseases. Clin. Microbiol. Rev. 1997;10:694–719. doi: 10.1128/CMR.10.4.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoli A., Hauffe H.C., Carpi G., Vourc’h G.I., Neteler M., Rosa R. Lyme borreliosis in Europe. Euro Surveill. 2011;16:1–8. https://www.eurosurveillance.org/content/10.2807/ese.16.27.19906-en [PubMed] [Google Scholar]
- Roux V., Raoult D. Phylogenetic analysis of members of the genus Rickettsia using the gene encoding the outer-membrane protein rOmpB (ompB) Int. J. Syst. Evol. Microbiol. 2000;50:1449–1455. doi: 10.1099/00207713-50-4-1449. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Honma M., Nakao M., Sasaki M., Hashimoto Y., Ishida-Yamamoto A., Yoshii K. Survey to detect tick-borne encephalitis virus from human-feeding ticks in Hokkaido, Japan. J. Dermatol. 2021;48:1094–1097. doi: 10.1111/1346-8138.15865. [DOI] [PubMed] [Google Scholar]
- Seishima M., Izumi T., Oyama Z., Kadosaka T. Tick bite by Haemaphysalis megaspinosa - first case. Jpn. J. Infect. Dis. 2000;10:389–391. [PubMed] [Google Scholar]
- Sekeyova Z., Roux V., Raoult D. Phylogeny of Rickettsia spp. inferred by comparing sequences of “gene D”, which encodes an intracytoplasmic protein. Int. J. Syst. Evol. Microbiol. 2001;51:1353–1360. doi: 10.1099/00207713-51-4-1353. [DOI] [PubMed] [Google Scholar]
- Shibata S., Sivakumar T., Igarashi I., Umemiya-Shirafuji R., Inokuma H., Fukumoto S., Yokoyama N. Epidemiological survey of a cervine Theileria in wild deer, questing ticks, and cattle in Hokkaido, Japan. Ticks Tick Borne Dis. 2018;9:1235–1240. doi: 10.1016/j.ttbdis.2018.05.006. [DOI] [PubMed] [Google Scholar]
- Stanek G., Strle F. Lyme borreliosis-from tick bite to diagnosis and treatment. FEMS Microbiol. Rev. 2018;42:233–258. doi: 10.1093/femsre/fux047. [DOI] [PubMed] [Google Scholar]
- Strle K., Drouin E.E., Shen S., El Khoury J., McHugh G., Ruzic-Sabljic E., et al. Borrelia burgdorferi stimulates macrophages to secrete higher levels of cytokines and chemokines than Borrelia afzelii or Borrelia garinii. J. Inf. Dis. 2009;200:1936–1943. doi: 10.1086/648091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G+C-content biases. Mol. Biol. Evol. 1992;9:678–687. doi: 10.1093/oxfordjournals.molbev.a040752. [DOI] [PubMed] [Google Scholar]
- Thu M.J., Qiu Y., Matsuno K., Kajihara M., Mori-Kajihara A., Omori R., et al. Diversity of spotted fever group rickettsiae and their association with host ticks in Japan. Sci. Rep. 2019;9 doi: 10.1038/s41598-018-37836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda T. Tick bite cases in researchers studying deer in Boso Peninsula, central Japan. Med. Entomol. Zool. 2004;55:243–245. doi: 10.7601/mez.55.243. [DOI] [Google Scholar]
- Yamaguti N., Tipton V.J., Keegan H.L., Toshioka S. Ticks of Japan, Korea, and the Ryukyu islands. Brigham Young Univ. Sci. Bull. Biol. Ser. 1971;15:1. https://scholarsarchive.byu.edu/byuscib/vol15/iss1/1/ [Google Scholar]
- Yamaji K., Aonuma H., Kanuka H. Distribution of tick-borne diseases in Japan: Past patterns and implications for the future. J. Infect. Chemother. 2018;24:499–504. doi: 10.1016/j.jiac.2018.03.012. [DOI] [PubMed] [Google Scholar]
- Yamauchi T., Fukui Y., Watanabe M., Nakagawa H., Kamimura K. Forty cases of human infestations with hard ticks (Acari: Ixodidae) in Toyama Prefecture, Japan. Med. Entomol. Zool. 2010;61:133–143. doi: 10.7601/mez.61.133. (In Japanese) [DOI] [Google Scholar]
- Yamauchi T., Nakatani T. Additional 10 cases of human infestation with hard ticks in Toyama Prefecture, Japan, with two cases by Amblyomma testudinarium (Acari: Ixodidae) Med. Entomol. Zool. 2016;67:239–242. doi: 10.7601/mez.67.239. [DOI] [Google Scholar]
- Yazaki T., Hirota T., Iwata Y., Inoue S., Usuki K., Suzuki T., et al. Effective killing of volunteer potato (Solanum tuberosum L.) tubers by soil frost control using agrometeorological information: An adaptive countermeasure to climate change in a cold region. Agricult. Forest Meteorol. 2013;182–183:91–100. doi: 10.1016/j.agrformet.2013.08.005. [DOI] [Google Scholar]
- Yu P.F., Niu Q.L., Liu Z.J., Yang J.F., Chen Z., Guan G.Q., et al. Molecular epidemiological surveillance to assess emergence and re-emergence of tick-borne infections in tick samples from China evaluated by nested PCRs. Acta Trop. 2016;158:181–188. doi: 10.1016/j.actatropica.2016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Full size image of Fig. 4.
Supplementary Figure S2. Full size image of Fig. 5.
Supplementary Table S1. Tick species distribution and prevalence of Borrelia burgdorferi (s.l.) and Rickettsia spp. among questing ticks collected in Tokachi District, eastern Hokkaido, Japan.
Data Availability Statement
The newly generated DNA sequences were submitted to the DNA Data Bank of Japan (DDBJ) (http://www.ddbj.nig.ac.jp) under the accession numbers: LC496815-LC496832 (Borrelia flaB); LC496811-LC496814 (Rickettsia gltA); MT378425-MT378437 (Rickettsia 16S rRNA); LC544128-LC544135 (Rickettsia ompB); and LC544136-LC544138 (Rickettsia sca4).