Abstract
Obstructive sleep apnea (OSA) is the most prevalent sleep-disordered breathing in the adult population and if untreated remains a significant cause of morbidity and mortality. Continuous positive airway pressure (CPAP) therapy is still the gold standard treatment for OSA, but patient acceptance and adherence are often poor due to a multitude of factors, thereby compromising treatment success. Mandibular advancement devices (MADs) have been proposed not only as a first line therapy for symptomatic snoring patients, but also for those suffering from mild to moderate OSA, or those who refuse or do not tolerate CPAP. Yet, improved understanding of MAD regarding design, construction, and mechanisms of action is an important requirement to successfully implement MAD as a therapeutic tool. Therefore, the main focus of this paper is to focus on the general concepts and mechanisms of action of MAD, while highlighting important characteristics in the context of their use as a viable and effective treatment option for OSA patients.
Keywords: Sleep Apnea, Obstructive, Mandibular Advancement, Occlusal Splints
INTRODUCTION
Obstructive sleep apnea (OSA), the most prevalent clinical entity in the spectrum of sleep-disordered breathing among adults and children, is defined by the presence of repeated episodes of complete or partial obstruction of the upper airways during sleep, despite ongoing respiratory efforts1. Its high prevalence, currently estimated as affecting nearly 1 billion people worldwide, imposes significant morbidity and mortality, such that OSA has clearly emerged as a major public health issue, while leading to major financial and social burden on both healthcare systems and society in general2.
The daytime consequences of OSA include a variety of symptoms, the most insidious being excessive daytime sleepiness (EDS), neurocognitive and behavioral impairments, and mood disorders3. In addition, OSA has clearly emerged as an independent risk factor for cardiovascular and metabolic diseases, such as hypertension, coronary artery disease, and endothelial dysfunction leading to myocardial ischemia, cardiac arrhythmias, stroke, as well as promoting or exacerbating dyslipidemias, insulin resistance, and type 2 diabetes mellitus4-12.
The pathophysiology of OSA is complex, involving a multitude of genetic, craniofacial - anatomical, neuromuscular, and inflammatory factors whose contributions differ from patient to patient13. Furthermore, OSA and insomnia may frequently co-occur challenging the usual standards of care14. In an effort to preserve the patency of the upper airway during sleep, various treatment modalities have been proposed13. Continuous positive airway pressure therapy (CPAP), initially developed by Sullivan et al.15, in the early 1980s, is still considered the most efficient therapeutic approach for OSA, since it stents the upper airway open throughout the respiratory cycle. Accordingly, CPAP has not only established itself as the gold standard and first line of treatment but has further shown its efficacy in a multitude of trials in which improvements in EDS, quality of life, and reductions in systemic blood pressure have clearly emerged16. Despite these benefits, patient acceptance, and adherence of CPAP is often poor, with attendant consequences17.
Oral sleep medicine field and mandibular advancement devices
Oral sleep medicine (OSM) is an area of dental medicine that investigates and diagnoses sleep-disordered breathing (SDB) and its oral and maxillofacial determinants, while also exploring its consequences on general health and sleep18. Increased recognition of SDB has resulted in major expansion of maxillofacial surgical techniques and approaches aimed at correcting the underlying respiratory disturbance during sleep. In parallel, OSM has led to the development and to an incremental use of oral appliances (OA)19,20. Among the many types of OA, mandibular advancement devices (MAD), and tongue retainers (TR), have been incorporated as the most commonly used types of intraoral devices. These types of appliances underwent substantial developments over time and have been adopted as a standard therapeutic option in the clinical practice of sleep medicine. However, TR have not been extensively evaluated, are considered less effective than MAD and have been associated with lower patient adherence when compared to MAD19-32. Notwithstanding, TR are still indicated for partial or total edentulous patients who use removable prosthetic dentures, which restrict MAD use, and may be also of benefit among those patients who have limited mandibular protrusion, with a large tongue, or those suffering from acute temporomandibular disorders, or from advanced periodontal disease21,22,31,33.
The primary focus of this paper is to discuss the general concepts and mechanisms of action that govern development and application of MAD as a treatment option for patients suffering from OSA.
MAD: concepts and mechanisms of action
MAD developed for treatment of sleep-disordered breathing are devices used in the oral cavity during sleep with the purpose of preventing the collapse between oropharyngeal tissues and the base of the tongue21. In general, MAD are geared to generate mandibular advancement and stabilization during sleep19 by promoting anterior traction displacement of the mandible with subsequent increases in the tension of the genioglossus muscle and the supra and infrahyoid muscles, expanding the air space in the pharyngeal region22.
A recent study evaluated skeletal/dental changes in a three-dimensional form using cone beam tomography during the use of MAD. It has been shown that mandibular protrusion promotes a linear vertical increase between the mandible and the maxilla and an anterosuperior displacement and rotation of the hyoid bone. Both of these features can assist professionals in deciding on the best candidates for this type of treatment23. Treatment with MAD aims to maintain the upper airways open during sleep by decreasing its resistance as well as the frequency and/or duration of the apneas and hypopneas, arousals related to respiratory effort, and snoring events24. In addition, MAD improve nighttime oxygenation25 at all levels of disease severity in adult patients26 with benefits upon the social and adverse health consequences of OSA and snoring (e.g., decreasing daytime sleepiness and improving quality of life)25,26.
Treatment of OSA with MAD improves both subjective and objective measures of excessive daytime sleepiness. Yet, the obtained subjective improvement may be also attributable to a placebo effect34,35.
A meta-analysis of seven randomized controlled studies confirms a modest, but rather significant benefit of MAD treatment on blood pressure26. The impact of treatment on other cardiovascular endpoints, such as cardiovascular events and mortality, remains unresolved36.
MAD types
Since the first commercially available oral devices were introduced in the 1980s36 there was a proliferation in the development of several models of MAD but the lack of standardization for device design often hampered the interpretation regarding comparisons in clinical use and research results22,25. Table 1 characterizes the main types of FDA approved MAD for use in adult OSA treatment. MAD can be made of distinct materials and may have different designs, some of them with the ability of a progressive mandibular advancement and lateral movements. MAD are classified as prefabricated and customized37-39 in a single block (monoblock) or two blocks, which can be adjustable with some freedom in lateral movements39. MAD customization also involve material choice, which must be adapted to the oral structure and physical needs for each patient38.
Table 1.
Some of the FDA-approved MAD that can be applied to improve mild to moderate obstructive sleep apnea in patients 18 years and older.
| Technical data of the available MADs | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MAD | EMA | Narval | Pantera | NOA | Somnodent avant | Somnodent dorsal wings | Micro2 | Herbst | Dream TAP | Orthoapnea classic |
| Coupling mechanism | Lateral superior-anterior to inferior-posterior | Lateral superior-anterior to inferior-posterior | Lateral superior-anterior to inferior-posterior | Lateral superior-posterior to inferior-posterior | Lateral superior-posterior to inferior-posterior | Lateral superior-posterior to inferior-posterior | Lateral superior-posterior to inferior-anterior | Anterior superior and inferior | Anterior superior and inferior | |
| Coupling element | Lateral Straps | Lateral Straps | Lateral Straps | Cam/Follower | Anterior strap | Dorsal wings | Dorsal wings | Fixed rod | Anterior hook | Anterior rod |
| Titration mechanism | Diferent lenths of strips | Diferent lenths of strips | Diferent lenths of strips | Diferent lenths of anterior strip | Screw on lateral wings | Different lower splints | Screw on rod | Different lower splints | Screw on anterior rod | |
| Titration protocol | "9 different lengths allow for 1 mm advancement of the mandible" |
Different lengths | Different lengths | Customized titration | Initial set of 10 straps (+1mm) provided | One 360º turn of the screw is 0.4 mm / 6.0mm protrusion range | Customized titration | Range of advancement (8mm | One 360° turn of the srew is 0.5 mm / 15mm protrusion range | From-3mm to +7mm total range of screw 10mm |
| Material | Thermoformed splints | 3D printed nylon appliance | 3D printed nylon appliance | 3D printed nylon appliance | Acrylic splint | Crystal-clear acrylic | Acrylic splint | Thermoformed splints | Thermoformed dual Laminate Splints | |
| Fabrication | Tradicional laboratories | CAD/CAM-printed 3D | CAD/CAM-printed 3D | CAD/CAM-printed 3D | CAD/CAM-printed 3D | Tradicional laboratories | CAD/CAM-milled | Tradicional laboratories | Tradicional laboratories | Tradicional laboratories |
Prefabricated MAD
Prefabricated MAD tend to be bulky with some challenges regarding its retaining capacity on a stable mandibular protrusive position during sleep. Therefore, this type of MAD are more prone to loss efficacy and lead to patient’s discomfort40,41. Nevertheless, a recent study demonstrated the efficacy of a titratable thermoplastic MAD in reducing OSA and related symptoms in patients with mild to severe disease presentation, but more studies are still needed to evaluate their efficacy in OSA treatment42 (Figure 1).
Figure 1.
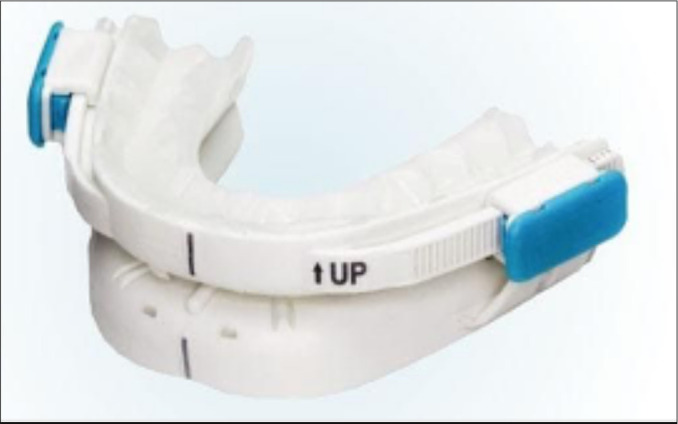
Prefabricated Intraoral mandibular advancement devices (MAD); titratable thermoplastic MAD BluePro® (France).
Custom-made MAD
The use of a custom-made MAD (MADc) has been associated with increased patient-reported comfort, greater range of protrusive movement, and higher therapeutic effectiveness25,26.
MADc are tailored to the patient’s dentition in a laboratory-controlled advancement. This kind of appliance can be adjustable (bi-block) or non adjustable (monoblock). Clinically, non-adjustable MAD are made in a fixed protrusive position remaining unchanged during treatment, while the protrusive position of the adjustable MAD allows progressive advances (e.g, titration process) aiming to increase the treatment efficacy and the patient’s comfort and quality of life (Figures 2 to 5)26,41,43.
Figure 2.

Intraoral mandibular advancement devices (MAD); a customized MAD‘s DIA Apnia®, Spain.
Figure 5.

Intra oral mandibular advancement device (MAD) NOA, Spain.
Coupling mechanism
In the design of a non-adjustable MAD there are a variety of coupling mechanisms such as elastic straps, lateral fins, bars, telescopic rods, springs, and tube connectors among others44. The differences in the designs affect effectiveness and comfort. Devices with lateral coupling mechanism offer greater comfort than other systems because they allow mandibular lateral and protrusive movement while maximizing the space available for the tongue. Yet, the coupling mechanisms that allow a certain degree of opening and prevent the jaw from moving backwards at any time while opening the mouth are more effective45-47. Mouth opening can be limited by the integration of elastic bands in the design.
Although the range of protrusive movement differ between individuals, studies indicates that the ability to advance the jaw is a key feature in MAD treatment success. This mostly depends on the amount of progression needed to reduce apnea-hypopnea index (AHI) and snoring38,40,43,48,49. Nevertheless, a precise linear relationship between mandibular advancement and treatment success has not yet been shown50.
The mandibular protrusion mechanism must allow advancement in increments of 1mm or less, with a minimal range of 5mm38,49. Small increments allow the assessment of important parameters, pursuing to minimize, for example, the temporomandibular discomfort and to improve comfort48,49. Current data support an initial position of, at least, 50% of the patient’s protrusive range, but there is no consensus as to whether this should be measured from an initial position of maximum intercuspation or maximum retrusive mandibular position49,51. The mechanism should be reversible in order to adapt to the patient’s health related dynamics and to adequately manage side effects48.
Vertical adjustment has been another controversial subject in the design of oral appliances25. Although with a lack of consensus, some studies have suggested that increased vertical dimension (inter-incisal distance) resulted in reduced patient acceptance38,52. Indeed, an increase in inter-incisal distance during mandibular protrusion with MAD was shown to result in a slightly more retrusive position caused by posterior rotation of the mandible. This finding lead to the standard of avoiding vertical increase as much as possible53.
MAD indications and effectiveness
MAD are indicated for patients with mild to moderate OSA and primary snoring. However, MAD are also an accepted therapy for patients with severe OSA who do not respond to or do not tolerate positive airway pressure (PAP) therapies24,26. Snoring treatment should be recommended for patients who fail to take conservative measures (e.g., weight loss, positional therapy, and avoidance of alcohol) and request additional treatment taking into account the risk/benefit ratio of the therapeutic action in view of the individual condition26. Contraindications such as periodontal damage and the presence of caries or poor oral hygiene should be prior evaluated by the dentist who should take care of the required dental treatments before MAD insertion. The risk of cclusal changes or exacerbation of temporomandibular joint disorders should also be considered and discussed with the patient29.
Although MAD are typically used as a single therapy, they can also be combined with CPAP and/or other therapeutic modalities for better OSA control. This is a relevant issue since patients report higher preference and higher adherence rates to treatment with MAD compared with CPAP devices24,25,28.
According to the American Academy of Sleep Medicine, one of the primary indications for MAD treatment is persistent snoring26. Such treatment indication occurs without the presence of a standardized classification in terms of severity or frequency of primary snoring, despite studies showing its possible relationship with factors associated with cardio-metabolic risk (e.g., atherosclerosis and endothelial dysfunction)54-56. Numerous questions related to this topic still remain unanswered. For example, questions like when treatment is needed and in which situations snoring is considered to be harmful are still controversial and would benefit of further debate54.
MAD have been shown to improve polysomnographic based outcomes, reduce AHI, arousals and the rate of oxygen desaturation26, also with significant improvements in daily function and quality of life. However, treatment with MAD failed to demonstrate a significant effect on sleep architecture and efficiency. Despite being less effective than CPAP in AHI improvement in moderate to severe OSA, several recent studies have found that oral appliances and CPAP were almost equally effective in tackling daytime sleepiness, hypertension, neurocognitive function, quality of life, and cardiovascular mortality25. A study from Hoekema et al. (2008)57 compared polysomnographic data between MAD and CPAP at 8-12 weeks with MAD being adjusted until the AHI<5 was obtained or until they caused discomfort. Results showed that 76.5% of the MAD patients’ group was effectively treated (with 69.2% critically ill patients being considered effectively treated and 84.0% of non-severe patients were considered effectively treated) versus 82.7% of the CPAP group57.
Although MAD are more effective than other types of OA in OSA treatment, their available design features (e.g., used materials, method of manufacture, and device adjustability) together with the disease severity might have a different impact on treatment effectiveness38,58,59. In fact, several studies have reported that for a higher efficacy, an advancement greater than 50% of a patient’s maximum protrusion will be required48,51,57,60,61.
Anatomical features resulting in increased nasal resistance seem to have a negative impact in the treatment with MAD17. Hence, studies evaluating the importance of nasal breathing in candidates for MAD found that those who had increased nasal resistance responded less effectively and had less adherence to treatment when compared to patients without nasal changes62.
Studies have also tried to prove the efficacy of treatment with MAD by imaging tests. Convincing evidence of improvement in airway permeability with MAD use was obtained by drug-induced sedation endoscopy (DISE), an objective method for visualizing upper airway obstruction25,63.
Numerous imaging studies have been performed in awake patients to elucidate tissue changes after mandibular advancement that could predict improved breathing during sleep. The use of computed tomography (CT-scan) and magnetic resonance imaging (MRI) in awake patients revealed that the greatest changes in the airways after MAD insertion were frequently observed in the transversal dimension, limiting the interpretation of the two-dimensional cephalometric images of the sagittal plane, and therefore failing to provide a reliable way of predicting treatment results25. Three-dimensional examinations at wakefulness and sleep conditions with and without MAD revealed that MAD increase the retropalatal and retro-lingual spaces and decrease the length of the soft palate. The increase in the retropalatal space is a crucial indicator for a positive treatment outcome64,65.
Treatment adherence and follow-up
In general, studies show that adherence to MAD is higher when compared to CPAP in adult patients with OSA. Indeed, the larger adherence to MAD seems to compensate for its lower efficacy in relation to CPAP, resulting in significant improvements in most clinical and polysomnographic outcomes, the so-called average disease relief factors66. However, we must consider that, unlike CPAP, there is little objective data to assess adherence to MAD26. In an interesting study, Vanderveken et al. (2013)28 showed an average use of MAD per night of ~6.6h in 82% of the evaluated patients.
Among the distinct MAD available types, the most effective one meets both the therapeutic success criteria and the higher patient acceptance. This highlights the role of a trained dentist in the treatment of OSA and the need of a personalized treatment using MAD.
Due to its lifelong nature, MAD treatment for OSA requires a careful and prolonged monitoring of patients through sleep tests to confirm treatment effectiveness as subjective feedback is not sufficient to follow-up MAD therapeutic effects in OSA management67. In fact, without objective data, the patient may remain suboptimally treated. Follow-up of adherence, MAD deterioration/maladjustment, health of oral/craniofacial structures, and integrity of the occlusion should be performed. Sleep monitoring test might also be considered for the search of worsening OSA signs and in treated patients who develop recurrent symptoms, present weight gain or receive diagnoses of comorbidities relevant to OSA.
MAD therapy side-effects
The side effects resulting from MAD treatment are of short- and long-term nature. Short-term effects are generally mild, transient, and occur within 6 months of MAD application. Such effects, usually controllable by a sleep-trained dental professional, include excessive salivation, dry mouth, discomfort in the teeth, irritation in the gums, headaches, and discomfort in the temporomandibular joint and in the masticatory muscles68. The long-term side effects occurring beyond the 6 months after the initiation of treatment have a poor prognosis and are most often related occlusal changes67,69. Among these effects are the decreased overbite and overjet, lingual inclination of the upper incisors, the vestibular inclination of the lower incisors, the mesialization of the lower molars and distalization of the upper molars, changes in dental arches crowding, appearance of posterior open bite, and decreased occlusal contacts69-72. Dental changes develop as a result of the MAD exerted forces on the upper and lower dental arches in order to maintain protrusion, and jaw resistant counter forces to persist in its initial position72. A 21-year follow-up study on the monitoring of MAD side-effects confirmed that there are significant and progressive dental changes with the prolonged use of MAD but skeletal or postural changes were insignificant. In addition, the duration of treatment was the most consistent predictor associated with the magnitude of the observed side effects67.
A qualified MAD provider should be able to individually assess each patient, choose the best MAD and adapt it in order to evaluate and minimize its side effects. Adequate evaluation in accordance with the individual’s health condition is critical, as some of the observed side effects such as destabilization of the occlusion may not revert26,67,73,74. In this context, patients should be aware of such probable effects before starting the treatment.
Patients’ phenotypes and prediction of outcomes
There is a growing recognition that OSA is a heterogeneous disorder either in terms of risk factors, clinical presentation, pathophysiology, risk of comorbidity, or response to treatment. A better characterization of this heterogeneity is an essential step for personalized approaches to therapy, ensuring more effectiveness and adherence to the proposed treatment75.
Recent studies on patients’ phenotypes responding to MAD treatment have shown that about one third of the included patients have little or no reduction of OSA severity76. These data display the critical need of identifying which patients are most likely to have a positive treatment outcome eventually leading to an optimization regarding the choice of the treatment modality, search for adjuvant measures and also to avoid wasting medical resources77. Drug Induced Sleep Endoscopy DISE titration can be used as a predictor of MAD treatment outcomes due to a direct assessment of upper airways obstruction sites as well as a way to prospectively determine which patients will have the best response to treatment and which mandibular protrusion should be targeted78-80. Recently, anatomical and physiological phenotypes have been identified as critical variables for the choice and the prediction of OSA treatment success and they include both anatomical and functional factors such as critical pressure to close the upper airways (Pcrit), arousal threshold and muscle response capacity, all of them playing an important role in OSA pathophysiology81-87. OSA-related additional comorbidities such as hypertension and/or other sleep disturbances (e.g., insomnia) can negatively influence the response to MAD treatment83,88.
Nevertheless, updated knowledge on the extent to which these factors interfere in OSA treatment with MAD is still needed.
Phenotypic characteristics prone to MAD treatment response (level of evidence)
Table 2.
Major phenotypic characteristics prone to MAD treatment according to levels of evidence.
| Clinical characteristics | Lower age, female gender, lower BMI, lower neck circumference (strong) |
| Craniofacial profile | Mandibular an maxillary retrognathism, smaller airway, shorter soft palate (strong) |
| PSG parameters | Mild OSA (moderate) (strong), Positional OSA |
| Physiological parameters | Primary oropharyngeal collapse, low CPAP therapeutic pressure (weak) |
Adapted from Chen et al. (2020)77.
Sleep dentist as a qualified MAD provider
There are currently many specializations and post graduated programs in sleep medicine directed towars dentists. Such an achievement is important for screening, diagnosis, optimized clinical approaches, and technical adequacy of therapeutic tools provided by dental professionals. Although the dentist’s role in sleep medicine should depend on their specific knowledge and expertise, sleep apnea is a core issue in any dental sleep medicine program89. It is well accepted that MAD treatment require qualified knowledge and expertise from a dentist trained in sleep medicine, therefore allowing for an optimal therapeutic achievement particularly related to proper MAD selection and treatment decision algorithms aiming to prevent occlusal and pain related issues as well as adequate referral when indicated Standards of care were provided by American Academy of Dental Sleep Medicine for this purpose90. These standards will be probably associated with higher rates of success as well as lower rates of complications and/or adverse issues.
Conclusions and future perspectives
Current literature provides robust evidence that adjustable and personalized double-arch oral mandibular advancement devices are highly effective for the treatment of snoring and mild to moderate OSA. Although less effective than CPAP for improving AHI in moderate to severe OSA, several recent studies have found that oral appliances and CPAP were equally effective in improving daytime sleepiness, hypertension, neurocognitive function, quality of life, and cardiovascular mortality. Further studies are needed to establish the impact of different models of devices available on therapeutic success and patient compliance as well as related side effects.
A qualified MAD provider needs to have the necessary skills to choose, adjust and manage the side effects of the most appropriate device. MAD treatment protocol should only start after a medical evaluation based on standard clinical, physical and polysomnographic parameters. A careful candidate selection must be carried out by both the sleep specialist and qualified MAD provider in order to achieve a higher therapeutic success rate. Still, the development of tools to identify individual phenotypes and the combination of two or more therapies to obtain synergistic additive effects will be required for the adoption of an optimal personalized OSA treatment.
Figure 3.
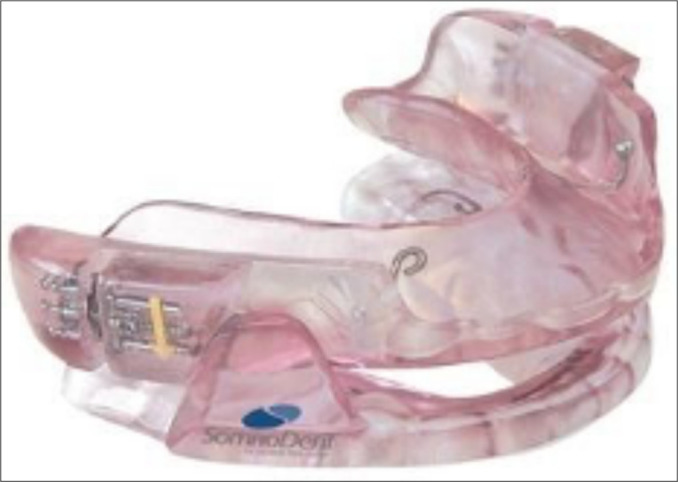
Intraoral mandibular advancement devices (MAD)a SomnoDent® (France)42.
Figure 4.
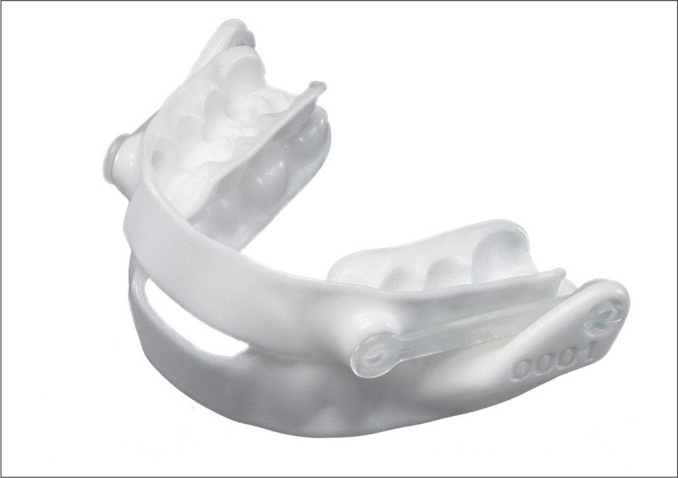
Intra oral mandibular advancement device (MAD) Narval CC, France.
REFERENCES
- 1.Benjafield AV, Najib T Ayas NT, Eastwood PR, Heizer R, Ip MSM, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019 Aug;7(8):687–98. doi: 10.1016/S2213-2600(19)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jennum P, Kjellberg J. Health, social and economical consequences of sleep-disordered breathing: a controlled national study. Thorax. 2011 Jul;66(7):560–6. doi: 10.1136/thx.2010.143958. [DOI] [PubMed] [Google Scholar]
- 3.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002 May;165(9):1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 4.Kohler M, Stradling JR. Mechanisms of vascular damage in obstructive sleep apnea. Nat Rev Cardiol. 2010 Dec;7(12):677–85. doi: 10.1038/nrcardio.2010.145. [DOI] [PubMed] [Google Scholar]
- 5.Mehra R, Benjamin EJ, Shahar E, Gottlieb DJ, Nawabit R, Kirchner HL, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2006 Apr;173(8):910–6. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodson BL, Wung SF, Archbold KH. Obstructive sleep apnea hypopnea syndrome and metabolic syndrome: a synergistic cardiovascular risk factor. J Am Acad Nurse Pract. 2012 Dec;24(12):695–703. doi: 10.1111/j.1745-7599.2012.00771.x. [DOI] [PubMed] [Google Scholar]
- 7.Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008 Aug;31(8):1079–85. [PMC free article] [PubMed] [Google Scholar]
- 8.Punjabi NM, Caffo BS, Goodwin JL, Newman AB, O’Connor GT, Rapoport DM, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009 Aug;6(8):e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O’Connor GT, Resnick HE, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Critical Care Med. 2010 Jul;182(2):269–77. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Nieto FJ, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Critical Care Med. 2001 Jan;163(1):19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 11.Shamsuzzaman ASM, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003 Oct;290(14):1906–14. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- 12.Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008 Aug;31(8):1071–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Rundo JV. Obstructive sleep apnea basics. Cleve Clin J Med. 2019 Sep;86(9 Suppl 1):2–9. doi: 10.3949/ccjm.86.s1.02. [DOI] [PubMed] [Google Scholar]
- 14.Meira E Cruz M, Salles C, Gozal D. A reappraisal on the associations between sleep-disordered breathing, insomnia and cardiometabolic risk. Am J Respir Crit Care Med. 2021 Mar; doi: 10.1164/rccm.202102-0337LE. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1(8225):862–5. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 16.McDaid C, Durée KH, Griffin SC, Weatherly HLA, Stradling JR, Davies RJO, et al. A systematic review of continuous positive airway pressure for obstructive sleep apnoea-hypopnoea syndrome. Sleep Med Rev. 2009 Dec;13(6):427–36. doi: 10.1016/j.smrv.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008 Feb;5(2):173–8. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lobezzo F, Aarab G, Wetselaar P, Hoekema A, Lange J, Vries N. A new definition of dental sleep medicine. J Dent Sleep Med. 2018 Oct;5(4):109–12. [Google Scholar]
- 19.Haddad FLM, Gregório LC. Manual do residente: medicina do sono. Barueri: Manole; 2017. [Google Scholar]
- 20.American Academy of sleep Medicine (AASM) Diagnostic and coding manual. 3. Westchester: AASP; 2014. International classification of sleep disorders. [Google Scholar]
- 21.Alencar Junior FGP, Dal-Fabbro C, Sanitá PV. Oclusão, dores orofaciais crônicas e sono. 2. São Paulo: Quintessence; 2020. [Google Scholar]
- 22.Ferguson KA, Cartwright R, Rogers R, Schmidt-Nowara W. Oral appliances for snoring and obstructive sleep apnea: a review. Sleep. 2006;29(2):244–62. doi: 10.1093/sleep/29.2.244. [DOI] [PubMed] [Google Scholar]
- 23.Kim DI, Vich ML, Mayoral P, Miguez M. Three-dimensional changes in skeletal/ dental landmarks with use of mandibular advancement devices. J Dent Sleep Med. 2020;7(2):1–18. [Google Scholar]
- 24.Mogell K, Blumenstock N, Mason E, Rohatgi R, Shah S, Schwartz D. Definition of an effective oral appliance for the treatment of obstructive sleep apnea and snoring: an update for 2019. J Dent Sleep Med. 2019;6(3):1–4. [Google Scholar]
- 25.Scherr SC, Dort LC, Almeida FR, Bennett KM, Bumenstock NT, Demko BG, et al. Definition of an effective oral appliance for the treatment of obstructive sleep apnea and snoring: a report of the American Academy of Dental Sleep Medicine. J Dent Sleep Med. 2014;1(1):39–50. [Google Scholar]
- 26.Ramar K, Dort LC, Katz SG, Lettieri CJ, Harrod CG, Thomas SM, et al. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015. J Clin Sleep Med. 2015 Jul;11(7):773–827. doi: 10.5664/jcsm.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sutherland K, Vanderveken MD, Tsuda H, Marklund M, Gagnadoux F, Kushida CA, et al. Oral appliance treatment for obstructive sleep apnea: an update. J Clin Sleep Med. 2014 Feb;10(2):215–27. doi: 10.5664/jcsm.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanderveken OM, Dieltjens M, Wouters K, DeBacker WA, Van de Heyning PH, Braem MJ. Objective measurement of compliance during oral appliance therapy for sleep-disordered breathing. Thorax. 2013 Jan;68(1):91–6. doi: 10.1136/thoraxjnl-2012-201900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marklund M. Subjective versus objective dental side effects from oral sleep apnea appliances. Sleep Breath. 2020;24:111–7. doi: 10.1007/s11325-019-01852-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cartwright RD, Samuelson CF. The effects of a nonsurgical treatment for obstructive sleep apnea: the tongue-retaining device. JAMA. 1982 Aug;248(6):705–9. [PubMed] [Google Scholar]
- 31.Ono T, Lowe AA, Fergusson KA, Fleethan JA. A tongue retaining device and sleep-state genioglossus muscle activity in patients with obstructive sleep apnea. Angle Orthod. 1996;66(4):273–80. doi: 10.1043/0003-3219(1996)066<0273:ATRDAS>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 32.Deane SA, Cistulli PA, Ng AT, Zeng B, Petocz P, Darendeliler MA. Comparison of mandibular advancement splint and tongue stabilizing device in obstructive sleep apnea: a randomized controlled trial. Sleep. 2009 May;32(5):648–53. doi: 10.1093/sleep/32.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okuno K, Hamoda MM, Alshhrani WM, Fleetham JA, Ayas NT, Comey R, et al. The efficacy of a titrated tongue stabilizing device on obstructive sleep apnea and the quality of life: a clinical trial study protocol. J Dent Sleep Med. 2017 Jul;4(3):65–9. doi: 10.5664/jcsm.9260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gotsopoulos H, Chen C, Qian J, Cistulli PA. Oral appliance therapy improves symptoms in obstructive sleep apnea: a randomized, controlled trial. Am J Respir Crit Care Med. 2002 Sep;166(5):743–8. doi: 10.1164/rccm.200203-208OC. [DOI] [PubMed] [Google Scholar]
- 35.Sutherland K, Lee RWW, Chan TO, Ng S, Hui DS, Cistulli PA. Craniofacial phenotyping in Chinese and Caucasian patients with sleep apnea: influence of ethnicity and sex. J Clin Sleep Med. 2018 Jul;14(7):1143–51. doi: 10.5664/jcsm.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan ASL, Sutherland K, Cistulli PA. Mandibular advancement splints for the treatment of obstructive sleep apnea. Expert Rev Respir Med. 2020 Jan;14(1):81–8. doi: 10.1080/17476348.2020.1686978. [DOI] [PubMed] [Google Scholar]
- 37.Hoffstein V. Review of oral appliances for treatment of sleep-disordered breathing. Sleep Breath. 2007 Mar;11(1):1–22. doi: 10.1007/s11325-006-0084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahrens A, McGrath C, Hägg U. A systematic review of the efficacy of oral appliance design in the management of obstructive sleep apnoea. Eur J Orthod. 2011 Jun;33(3):318–24. doi: 10.1093/ejo/cjq079. [DOI] [PubMed] [Google Scholar]
- 39.Dal Fabbro C, Chaves Júnior CM, Tufik S. A odontologia na medicina do sono. Maringá: Dental Press; 2012. [Google Scholar]
- 40.Cooke ME, Battagel JM. A thermoplastic mandibular advancement device for the management of non-apnoeic snoring: a randomized controlled trial. Eur J Orthod. 2006 Aug;28(4):327–38. doi: 10.1093/ejo/cji122. [DOI] [PubMed] [Google Scholar]
- 41.Vanderveken OM, Devolder A, Marklund M, Boudewyns AN, Braem MJ, Okkerse W, et al. Comparison of a custom-made and a thermoplastic oral appliance for the treatment of mild sleep apnea. Am J Respir Crit Care Med. 2008 Jul;178(2):197–202. doi: 10.1164/rccm.200701-114OC. [DOI] [PubMed] [Google Scholar]
- 42.Gagnadoux F, Nguyen XL, LeVaillant M, Priou P, Meslier N, Eberlein A, et al. Comparison of titrable thermoplastic versus custom-made mandibular advancement device for the treatment of obstructive sleep apnoea. Respir Med. 2017 Oct;131:35–42. doi: 10.1016/j.rmed.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 43.Lettieri CJ, Paolino N, Eliasson AH, Shah AA, Holley AB. Comparison of adjustable and fixed oral appliances for the treatment of obstructive sleep apnea. J Clin Sleep Med. 2011 Oct;7(5):439–45. doi: 10.5664/JCSM.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dieltjens M, Vanderveken OM, Van de Heyning PH, Braem MJ. Current opinions and clinical practice in the titration of oral appliances in the treatment of sleep-disordered breathing. Sleep Med Rev. 2012 Apr;16(2):177–85. doi: 10.1016/j.smrv.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 45.Norrhem N, Marklund M. An oral appliance with or without elastic bands to control mouth opening during sleep-a randomized pilot study. Sleep Breath. 2016 Sep;20(3):929–38. doi: 10.1007/s11325-016-1312-5. [DOI] [PubMed] [Google Scholar]
- 46.Milano F, Mutinelli S, Sutherland K, Millioli G, Scaramuzzino G, Cortesi AB, et al. Influence of vertical mouth opening on oral appliance treatment outcome in positional obstructive sleep apnea. J Dent Sleep Med. 2018;5(1):17–23. [Google Scholar]
- 47.Bataller A, Cabrera JA, Garcia M, Castillo JJ, Mayoral P. Cam synthesis applied to the design of a customized mandibular advancement device for the treatment of obstructive sleep apnea. Mech Mach Theory. 2018 May;123:153–65. [Google Scholar]
- 48.Campbell AJ, Reynolds G, Trengrove H, Neill AM. Mandibular advancement splint titration in obstructive sleep apnoea. Sleep Breath. 2009 May;13(2):157–62. doi: 10.1007/s11325-008-0230-6. [DOI] [PubMed] [Google Scholar]
- 49.Marklund M, Verbraecken J, Randerath W. Non-CPAP therapies in obstructive sleep apnoea: mandibular advancement device therapy. Eur Respir J. 2012 May;39(5):1241–7. doi: 10.1183/09031936.00144711. [DOI] [PubMed] [Google Scholar]
- 50.Bartolucci ML, Bortolotti F, Raffaelli E, D’Antò V, Michelotti A, Bonetti GA. The effectiveness of different mandibular advancement amounts in OSA patients: a systematic review and meta-regression analysis. Sleep Breath. 2016 Sep;20(3):911–9. doi: 10.1007/s11325-015-1307-7. [DOI] [PubMed] [Google Scholar]
- 51.Aarab G, Lobbezoo F, Hamburger HL, Naeije M. Effects of an oral appliance with different mandibular protrusion positions at a constant vertical dimension on obstructive sleep apnea. Clin Oral Investig. 2010 Jun;14(3):339–45. doi: 10.1007/s00784-009-0298-9. [DOI] [PubMed] [Google Scholar]
- 52.Pitsis AJ, Darendeliler MA, Gotsopoulos H, Petocz P, Cistulli PA. Effect of vertical dimension on efficacy of oral appliance therapy in obstructive sleep apnea. Am J Respir Crit Care Med. 2002 Sep;166(6):860–4. doi: 10.1164/rccm.200204-342OC. [DOI] [PubMed] [Google Scholar]
- 53.Mayoral P, Lagravère MO, Míguez-Contreras M, Garcia M. Antero-posterior mandibular position at different vertical levels for mandibular advancing device design. BMC Oral Health. 2019 May;19(1):85. doi: 10.1186/s12903-019-0783-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meira e Cruz M, Soca R, Kryger M. How much is too much after all? Primary snoring as a remaining unsolved issue. J Clin Sleep Med. 2020 Jun;16(6):991. doi: 10.5664/jcsm.8442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deeb R, Judge P, Peterson E, Lin JC, Yaremchuk K. Snoring and carotid artery intima-media thickness. Laryngoscope. 2014 Jun;124(6):1486–91. doi: 10.1002/lary.24527. [DOI] [PubMed] [Google Scholar]
- 56.Lee SA, Amis TC, Byth K, Larcos G, Kairaitis K, Robinson TD, et al. Heavy snoring as a cause of carotid artery atherosclerosis. Sleep. 2008 Sep;31(9):1207–13. [PMC free article] [PubMed] [Google Scholar]
- 57.Hoekema A, Stegenga B, Wijkstra PJ, Van Der Hoeven JH, Meinesz AF, Bont LG. Obstructive sleep apnea therapy. J Dent Res. 2008 Sep;87(9):882–7. doi: 10.1177/154405910808700917. [DOI] [PubMed] [Google Scholar]
- 58.Hoekema A, Stegenga B, Bont LGM. Efficacy and co-morbidity of oral appliances in the treatment of obstructive sleep apnea-hypopnea: a systematic review. Crit Rev Oral Biol Med. 2004 Jun;15(3):137–55. doi: 10.1177/154411130401500303. [DOI] [PubMed] [Google Scholar]
- 59.Chan ASL, Lee RWW, Cistulli PA. Dental appliance treatment for obstructive sleep apnea. Chest. 2007 Aug;132(2):693–9. doi: 10.1378/chest.06-2038. [DOI] [PubMed] [Google Scholar]
- 60.Aarab G, Lobbezoo F, Hamburger HL, Naeije M. Oral appliance therapy versus nasal continuous positive airway pressure in obstructive sleep apnea: a randomized, placebo-controlled trial. Respiration. 2011;81(5):411–9. doi: 10.1159/000319595. [DOI] [PubMed] [Google Scholar]
- 61.Mehta A, Qian J, Petocz P, Darendeliler MA, Cistulli PA. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Crit Care Med. 2001 May;163(6):1457–61. doi: 10.1164/ajrccm.163.6.2004213. [DOI] [PubMed] [Google Scholar]
- 62.Prescinotto R, Haddad FL, Fukuchi I, Gregório LC, Cunali PA, Tufik S, et al. Impact of upper airway abnormalities on the success and adherence to mandibular advancement device treatment in patients with obstructive sleep apnea syndrome. Braz J Otorhinolaringol. 2015 Nov/Dec;81(6):663–70. doi: 10.1016/j.bjorl.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morrison DL, Launois SH, Isono S, Feroah TR, Whitelaw WA, Remmers JE. Pharyngeal narrowing and closing pressures in patients with obstructive sleep apnea. Am Rev Respir Dis. 1993 Sep;148(3):606–11. doi: 10.1164/ajrccm/148.3.606. [DOI] [PubMed] [Google Scholar]
- 64.Lee CH, Kim JW, Lee HJ, Seo BS, Yun PY, Kim DY, et al. Determinants of treatment outcome after use of the mandibular advancement device in patients with obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 2010 Jul;136(7):677–81. doi: 10.1001/archoto.2010.106. [DOI] [PubMed] [Google Scholar]
- 65.Lee CH, Kim JW, Lee HJ, Yun PY, Kim DY, Seo BS, et al. An investigation of upper airway changes associated with mandibular advancement device using sleep videofluoroscopy in patients with obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 2009 Sep;135(9):910–4. doi: 10.1001/archoto.2009.112. [DOI] [PubMed] [Google Scholar]
- 66.Sutherland K, Phillips C, Cistulli P. Efficacy versus effectiveness in the treatment of obstructive sleep apnea: CPAP and oral appliance. J Clin Sleep Med. 2015;2(4):175–81. [Google Scholar]
- 67.Hamoda MM, Almeida FR, Pliska BT. Long-term side effects of sleep apnea treatment with oral appliances: nature, magnitude and predictors of long-term changes. Sleep Med. 2019 Apr;56:184–91. doi: 10.1016/j.sleep.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 68.Almeida FR, Lowe AA, Tsuiki S, Otsuka R, Wong M, Fastlicht S, et al. Long-term compliance and side effects of oral appliances used for the treatment of snoring and obstructive sleep apnea syndrome. J Clin Sleep Med. 2005;1(2):143–52. [PubMed] [Google Scholar]
- 69.Almeida FR, Lowe AA, Otsuka R, Fastlicht S, Farbood M, Tsuiki S. Long- term sequellae of oral appliance therapy in obstructive sleep apnea patients: part 2. Study-model analysis. Am J Orthod Dentofacial Orthop. 2006 Feb;129(2):205–13. doi: 10.1016/j.ajodo.2005.04.034. [DOI] [PubMed] [Google Scholar]
- 70.Alessandri-Bonetti G, D’Anto V, Stipa C, Rongo R, Incerti-Parenti S, Michelotti A. Dentoskeletal effects of oral appliance wear in obstructive sleep apnoea and snoring patients. Eur J Orthod. 2017 Oct;39(5):482–8. doi: 10.1093/ejo/cjw078. [DOI] [PubMed] [Google Scholar]
- 71.Araie T, Okuno K, Minagi HO, Sakai T. Dental and skeletal changes associated with long-term oral appliance use for obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev. 2018 Oct;41:161–72. doi: 10.1016/j.smrv.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 72.Rose EC, Staats R, Virchow Junior C, Jonas IE. Occlusal and skeletal effects of an oral appliance in the treatment of obstructive sleep apnea. Chest. 2002 Sep;122(3):871–7. doi: 10.1378/chest.122.3.871. [DOI] [PubMed] [Google Scholar]
- 73.Almeida FR, Lowe AA, Sung JO, Tsuik S, Otsuka R. Long-term sequellae of oral appliance therapy in obstructive sleep apnea patients: Part 1. Cephalometric analysis. Am J Orthod Dentofac Orthop. 2006 Feb;129(2):195–204. doi: 10.1016/j.ajodo.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 74.Pliska BT, Nam H, Chen H, Lowe AA, Almeida FR. Obstructive sleep apnea and mandibular advancement splints: occlusal effects and progression of changes associated with a decade of treatment. J Clin Sleep Med. 2014 Dec;10(12):1285–91. doi: 10.5664/jcsm.4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cistulli PA, Sutherland K. Phenotyping obstructive sleep apnoea - bringing precision to oral appliance therapy. J Oral Rehabil. 2019 Dec;46(12):1185–91. doi: 10.1111/joor.12857. [DOI] [PubMed] [Google Scholar]
- 76.Sutherland K, Takaya H, Qian J, Petocz P, Ng AT, Cistulli PA. Oral appliance treatment response and polysomnographic phenotypes of obstructive sleep apnea. J Clin Sleep Med. 2015 Aug;11(8):861–8. doi: 10.5664/jcsm.4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen H, Eckert DJ, Van Der Stelt PF, Guo S, Emami E, Almeida FR, et al. Phenotypes of responders to mandibular advancement device therapy in obstructive sleep apnea patients: a systematic review and meta-analysis. Sleep Med Rev. 2020 Feb;49:101229. doi: 10.1016/j.smrv.2019.101229. [DOI] [PubMed] [Google Scholar]
- 78.Croft CB, Pringle M. Sleep nasendoscopy: a technique of assessment in snoring and obstructive sleep apnoea. Clin Otolaryngol Allied Sci. 1991 Oct;16(5):504–9. doi: 10.1111/j.1365-2273.1991.tb01050.x. [DOI] [PubMed] [Google Scholar]
- 79.Kastoer C, Dieltjens M, Op de Beeck S, Braem MJ, Van de Heyning PH, Vanderveken OM. Remotely controlled mandibular positioning during drug- induced sleep endoscopy toward mandibular advancement device therapy: feasibility and protocol. J Clin Sleep Med. 2018 Aug;14(8):1409–13. doi: 10.5664/jcsm.7284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dieltjens M, Braem MJ, Op de Beeck S, Vroegop AVMT, Kazemeini E, Van de Perck E, et al. Remotely controlled mandibular positioning of oral appliance therapy during polysomnography and drug-induced sleep endoscopy compared with conventional subjective titration in patients with obstructive sleep apnea: protocol for a randomized crossover trial. Trials. 2019 Oct;20(1):615. doi: 10.1186/s13063-019-3698-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eckert DJ. Phenotypic approaches to obstructive sleep apnoea - new pathways for targeted therapy. Sleep Med Rev. 2018 Feb;37:45–59. doi: 10.1016/j.smrv.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 82.Jordan AS, Wellman A, Heinzer RC, Lo YL, Schory K, Dover L, et al. Mechanisms used to restore ventilation after partial upper airway collapse during sleep in humans. Thorax. 2007 Oct;62(10):861–7. doi: 10.1136/thx.2006.070300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Machado MAC, Carvalho LBC, Juliano ML, Taga M, Prado LBF, Prado GF. Clinical co-morbidities in obstructive sleep apnea syndrome treated with mandibular repositioning appliance. Respir Med. 2006;100(6):988–95. doi: 10.1016/j.rmed.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 84.Subramani Y, Singh M, Wong J, Kushida CA, Malhotra A, Chung F. Understanding phenotypes of obstructive sleep apnea: applications in anesthesia, surgery, and perioperative medicine. Anesth Analg. 2017 Jan;124(1):179–91. doi: 10.1213/ANE.0000000000001546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Edwards BA, Andara C, Landry S, Sands SA, Joosten SA, Owens RL, et al. Upper-airway collapsibility and loop gain predict the response to oral appliance therapy in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2016 Dec;194(11):1413–22. doi: 10.1164/rccm.201601-0099OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sutherland K, Chan ASL, Ngiam J, Dalci O, Darendeliler MA, Cistulli PA. Awake multimodal phenotyping for prediction of oral appliance treatment outcome. J Clin Sleep Med. 2018 Nov;14(11):1879–87. doi: 10.5664/jcsm.7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lai V, Carberry JC, Eckert DJ. Sleep apnea phenotyping: implications for dental sleep medicine. J Dent Sleep Med. 2019;6(2):1–12. [Google Scholar]
- 88.Lee GS, Kim HK, Kim ME. Risk factors for the efficacy of oral appliance for treating obstructive sleep apnea: a preliminary study. Cranio. 2018 Nov;36(6):352–9. doi: 10.1080/08869634.2017.1398285. [DOI] [PubMed] [Google Scholar]
- 89.Meira e Cruz M, Estevill E, Kryger MH. The dentist’s role in sleep medicine: why the hesitation? J Dent Sleep Med. 2020;7(2):1. [Google Scholar]
- 90.Levine M, Bennett K, Cantwell M, Postol K, Schwartz D. Dental sleep medicine standards for screening, treating, and managing adults with sleep- related breathing disorders. J Dent Sleep Med. 2018;5(3):61–8. [Google Scholar]


