Keywords: bariatric, bariatric surgery, bile acids, biliary limb, bypass, common limb, one anastomosis gastric bypass
Abstract
The alimentary limb has been proposed to be a key driver of the weight-loss-independent metabolic improvements that occur upon bariatric surgery. However, the one anastomosis gastric bypass (OAGB) procedure, consisting of one long biliary limb and a short common limb, induces similar beneficial metabolic effects compared to Roux-en-Y Gastric Bypass (RYGB) in humans, despite the lack of an alimentary limb. The aim of this study was to assess the role of the length of biliary and common limbs in the weight loss and metabolic effects that occur upon OAGB. OAGB and sham surgery, with or without modifications of the length of either the biliary limb or the common limb, were performed in Gottingen minipigs. Weight loss, metabolic changes, and the effects on plasma and intestinal bile acids (BAs) were assessed 15 days after surgery. OAGB significantly decreased body weight, improved glucose homeostasis, increased postprandial GLP-1 and fasting plasma BAs, and qualitatively changed the intestinal BA species composition. Resection of the biliary limb prevented the body weight loss effects of OAGB and attenuated the postprandial GLP-1 increase. Improvements in glucose homeostasis along with changes in plasma and intestinal BAs occurred after OAGB regardless of the biliary limb length. Resection of only the common limb reproduced the glucose homeostasis effects and the changes in intestinal BAs. Our results suggest that the changes in glucose metabolism and BAs after OAGB are mainly mediated by the length of the common limb, whereas the length of the biliary limb contributes to body weight loss.
NEW & NOTEWORTHY Common limb mediates postprandial glucose metabolism change after gastric bypass whereas biliary limb contributes to weight loss.
INTRODUCTION
The rapidly rising global prevalence of obesity and its complications represents a major public health problem. Bariatric surgery induces substantial and sustained body weight loss and is considered the most effective treatment for morbid obesity, as well as its comorbidities, such as type 2 diabetes and nonalcoholic fatty liver disease (1). Several bariatric surgery techniques have been developed over time, but the most studied and, until recently, most performed, is the Roux-en-Y Gastric Bypass (RYGB) (2). Surprisingly, RYGB, which excludes a portion of the stomach and the proximal intestine from the alimentary circuit, lowers glycemia more rapidly and to a greater extent than would be expected from the body weight loss alone. Numerous mechanisms of action have been proposed to explain this striking weight-loss-independent metabolic effect in preclinical models, but their clinical relevance remains unclear (3–5).
The bypass of the proximal intestine results in altered duodenal sensing of the nutrients and brain signaling in the RYGB (6). The exclusion of the pylorus accelerates gastrointestinal nutrient transit, leading to early systemic glucose appearance, and an enhanced postprandial release of incretins (e.g., glucagon-like peptide 1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP)), and satiety peptides (e.g., peptide YY (PYY) and oxyntomodulin (OXY)) (7–9). RYGB shortens the length of the intact intestinal segment, also called the common limb, where food meets bile and other sodium-rich digestive fluids (10) (Fig. 1A). Thus, a shorter common limb results in a decrease of intestinal sodium-glucose active transport (11) and reduces the overall postprandial glucose response after a mixed meal (12). Other mechanisms have been proposed, such as changes in the gut microbiota (13), or in the bile acid (BA) pool size and composition, (14–16), which could modulate metabolism through activation of the Farnesoid X receptor (FXR) and the Takeda G protein-coupled receptor 5 (TGR5). Finally, hypertrophy of the alimentary limb, which results in increased splanchnic glucose utilization, has also been incriminated (17, 18). This latter mechanism is, however, difficult to reconcile with the metabolic improvements that occur with the one anastomosis gastric bypass (OAGB) (Fig. 1B), a more recent procedure resulting in a longer biliary limb, but the lack of an alimentary limb (19). Since biliopancreatic secretions travel alone within the biliary limb before coming into contact with the ingested nutrients, and since BA signaling in the intestine regulates metabolic homeostasis, it is conceivable that changes in the intestinal or circulating BAs could contribute to the metabolic improvements observed after OAGB.
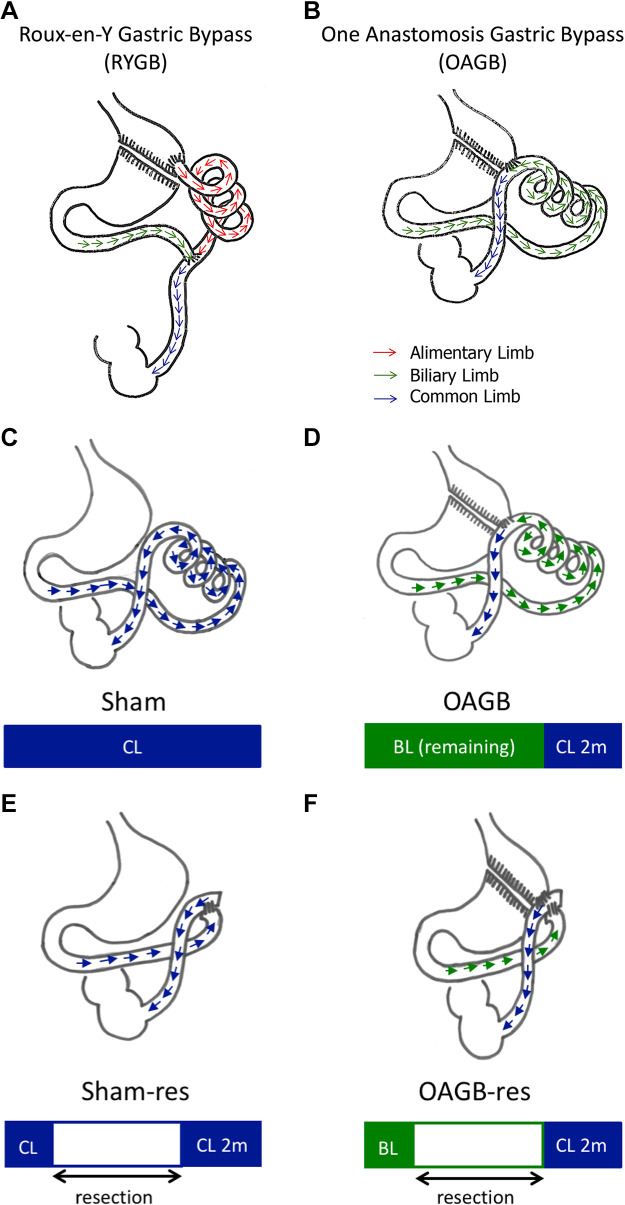
Figure 1.
Bypass procedures. A and B: Roux-en-Y gastric bypass (RYGB) and one anastomosis gastric bypass (OAGB). Red arrow: alimentary limb, green arrow: biliary limb, and blue arrow: common limb. Surgical procedures. C–F: sham procedure (Sham), and OAGB procedure (OAGB), sham with resection of the common limb procedure (Sham-res), and OAGB with resection of the biliary limb procedure (OAGB-res). Blue arrows represent transit in the common limb (CL) and green arrows represent transit in the biliary limb (BL).
Although OAGB was reported to induce similar or even greater improvements in body weight and type 2 diabetes (T2D) remission (20, 21), the safety of this procedure is a current matter of debate, as many cases of severe protein deficiency have been reported (22), some of them lethal (23). In consequence, OAGB technical guidelines have recommended decreasing the length of the biliary limb to prevent protein deficiency (24). Paradoxically, an increase in the length of the biliary limb in RYGB is favored by some authors to foster weight loss (25) and/or diabetes resolution (26). Clearly, the length of the biliary limb requires further study to determine its contribution to the metabolic effects of OAGB.
The aim of the present study was to metabolically characterize OAGB in a validated (27) preclinical minipig model, to investigate the respective role of the biliary and common limbs in OAGB.
MATERIALS AND METHODS
Animal Care
All animal procedures were performed in accordance with French regulations for animal experimentation [approval code: CEEA 152012]. In total, 24 healthy adult female Göttingen-like minipigs (mean weight: 51.4 ± 1.4 kg) (Pannier’s breeding) were used. The experiments were performed on female minipigs to reduce the risk of postoperative infection (favored by the position of the urethra in males upon lower laparotomy), and because bariatric surgery is performed predominantly in women in the clinic (28). The pigs were fed a standard diet (Uneal cooperative, Aire sur la Lys, France, corresponding to 1.5% of their body weight/day, in two totally consumed meals).
After overnight fasting, surgical procedures were performed under general anesthesia by midline laparotomy after ketamine/xylazine premedication and isoflurane inhalation (2%). Central venous catheter was placed for repeated blood sampling.
After surgery, minipigs were closely monitored and treated with fentanyl transdermal and a 5-day antibiotic prophylaxis (lincomycine/spectinomycine). Drinking water was allowed from postoperative day one, and oral feeding was allowed 72 h after surgery.
Surgical Procedures
The minipigs were randomly assigned to one of the four groups (n = 6 minipigs/group), Sham surgery, OAGB, sham surgery with resection of the common limb (Sham-res), or OAGB with resection of the biliary limb (OAGB-res) (Figs. 1, C–F). The different groups presented no significant differences in body weight (P = 0.392) or glucose parameters (P = 0.774) at baseline (Supplemental Table S1, see https://doi.org/10.6084/m9.figshare.13640735).
OAGB and OAGB-res.
After dissection of the oesogastric junction, a 100-mL gastric pouch was constructed using linear staplers (Proximate, green cartridges, Ethicon, Issy-les-Moulineaux, France). As the intestine of a minipig is approximately twice the size of that of humans, a distal OAGB was performed to amplify the observed effects. The distal portion of the ileum (200 cm before the ileocecal junction) was taken up and anastomosed with the gastric pouch (Proximate, blue cartridges, Ethicon, Issy-les-Moulineaux, France) to form an omega loop. In the OAGB-res procedure, the proximal portion of the jejunum (50 cm from the duodenojejunal junction) was taken up and anastomosed to the gastric pouch. The intestine between the gastrojejunal anastomosis and the distal portion of the ileum (200 cm before the ileocecal junction) was then resected and an entero-enteral anastomosis was performed.
Sham and Sham-res.
The Sham procedure consisted in esogastric junction dissection, gastrotomy, enterotomy, and repair. The Sham-res procedure consisted in resection of the intestine, starting 50 cm from the duodenojejunal junction and down to 200 cm before the ileocecal junction followed by entero-enteral anastomosis.
Mixed-Meal Test
Fifteen days after surgery, after overnight fasting, mixed-meal test (MMT; 200 mL nutrition shake with crushed solid energy bar (Ovomaltine, Switzerland) and 30 g of d-xylose) was administered via a nasogastric tube for 10 min. Blood was sampled before and at 15, 30, 60, 90, 120, and 180 min after completion of the meal. Blood samples were immediately kept on ice until centrifugation (5,000 rpm, 10 min). Plasma or serum aliquots were stored at −80°C.
Tissue and Intestinal Liquid Sampling
Under general anesthesia, the livers and intestines were sampled during and 20 days after surgery. A 50 cm-long intestinal segment was clamped and 40 mL of distilled water was injected and then reaspired from the intestinal lumen (20 cm-long segment and 20 mL for the short biliary limb). Bile was sampled by gall bladder puncture and the cecal content was collected during the tissue harvest. Stools were also sampled. All samples were immediately frozen at −80°C.
Biochemical Analysis
Blood glucose was measured in duplicate immediately after sampling using the amperometric glucose oxidase method (glucose meter, FreeStyle Optium, Abbott, Rungis, France). Plasma d-xylose concentration was measured by a colorimetric micromethod with phloroglucinol as previously described (29). Insulin was measured by ELISA (Access Ultrasensitive Insulin, Beckman Coulter, Brea), and total GLP-1 by RIA (GLP-1T-36HK, Millipore-IDS, France). Plasma concentrations of the BAs and 7-α-hydroxy-4-cholesten-3-one (C4) were determined after extraction by protein precipitation as previously published (16). Intestinal intraluminal BA concentrations were performed similarly. BAs from feces and the cecal content were quantified after extraction on samples lyophilized at −80°C to avoid bacterial BA transformation as previously described (14). The 21 BA species in the different biological samples were quantified by phase liquid chromatography associated with tandem mass spectrometry (LC-MS/MS). Due to the nonquantitative intraluminal liquid sampling, the intraluminal BA species concentrations are expressed herein as the percentage or ratios of the total BAs.
RNA Extraction and Quantification by qPCR
Total RNA was extracted from liver and intestinal biopsies using NucleoZOL according to the manufacturer’s protocol. After DNAse treatment (Fermentas, St Rémy Les Chevreuse, France), the total RNA (0.5–1 mg) was reverse transcribed using High-Capacity Multiscribe Reverse Transcriptase (Applied Biosystems, St Aubin, France) according to the manufacturer’s protocol. qPCR with cDNA from the reverse transcription was performed using Master MIX SYBR Green Brilliant Fast II (Agilent, Santa Clara, CA) on MX4000 apparatus (Stratagene). Forward and reverse primers were for cyclophiline: GCATACGGGTCCTGGCATCTTGTCC and ATGGTGATCTTCTTGCTGGTCTTGC, for ASBT (apical sodium dependent bile transporter): AGACTAGCTGGTCAGTCCTGGG and GAAGGAGAGCTGGACGATGGTG, for IBABP (intestinal bile acid binding protein): CATCGGCAAGGAGTGCGACATA and GGTAGTTGGGGCTGTTCACCAC, for OSTa (organic solute transporter): ATTTTCCGTCAAGCCAAGCTGC and AGGGAGGCGAACAAGCAATCTG, for OSTb: TGGTGGTCGTGGTGAGCTTTG and CCAGGTAGAGGGCTTCTGGATG, for CYP8B1: AAGGCAGGCAAGAAGATCCACC and GTCCACCAGCTCCAGGTCAAAG, for NTCP: GATGGGACCCTGAAGGACAAGG and CCACGTTGAGGACAGAGAGCAC, for BSEP (bile salt export pump): GCTGGACAACGAGAGTGAAGCC and TCTGACCGTGGATAGGCGATGC, for BACS (bile acid-coA synthase): ATCCAGGACACCTTGGAGACCA and CAACAACGCCCACGTTGAAGC, and for OATP1B3 (organic anion transporting polypeptide 1B3): TCCGATCCTTGGCTTTTCACTGG and AAGCACCAACCCAACGAGAGTC, for OAT2 (organic anion transporter 2): GCTACTTGATACGGGACTGGCG and TACCTATGGGCGTCCTCCACAC. The results are presented using the DDCt method and normalized to a reference gene (cyclophilin). Controls were set at 1 and all the conditions were expressed comparatively to the controls.
Statistical Analyses
The results are expressed as the means ± standard error of the mean (SEM). Continuous variables were analyzed by the unpaired or paired one-way ANOVA or Kruskal–Wallis test according to the data distribution. Variables measured during the MMT are expressed as an incremental value from baseline: blood glucose excursion (i.e., incremental value of blood glucose), incremental d-xylose, incremental GLP-1, or incremental insulin. The areas under the curves (incremental AUC, iAUC) during MMT were calculated using the trapezoidal method: postprandial glucose response (PPGR, i.e., iAUC of blood glucose), iAUC D-xylose, iAUC GLP-1, or iAUC insulin. One-way repeated-measures ANOVA and post hoc Tukey’s multiple comparisons test were used to compare the differences of AUC. A P-value <0.05 was considered as significant. All the statistical analyses were performed using Prism 7 for Mac OS X (GraphPad La Jolla, CA).
RESULTS
OAGB Induces Body Weight Loss and Improves Glucose Metabolism
The first aim of the present study was to characterize the effect of OAGB surgery on body weight and glucose homeostasis control during a mixed-meal test in a minipig model. During the 20-day follow-up, OAGB steadily decreased the body weight compared to the Sham group (Fig. 2A). The mixed-meal test performed at the 15th postoperative day demonstrated a significantly decreased glucose excursion (Fig. 2B) and postprandial glucose response (Fig. 2C). These results could be explained at least in part by decreased intestinal absorption, as the excursion of d-xylose was also significantly lower in the OAGB group (Figs. 2, D and E). In addition, postprandial GLP-1 concentrations were higher in the OAGB group (Figs. 2, F and G). Postprandial insulin concentrations remained identical despite a lower glucose response (Figs. 2, H and I).
Figure 2.
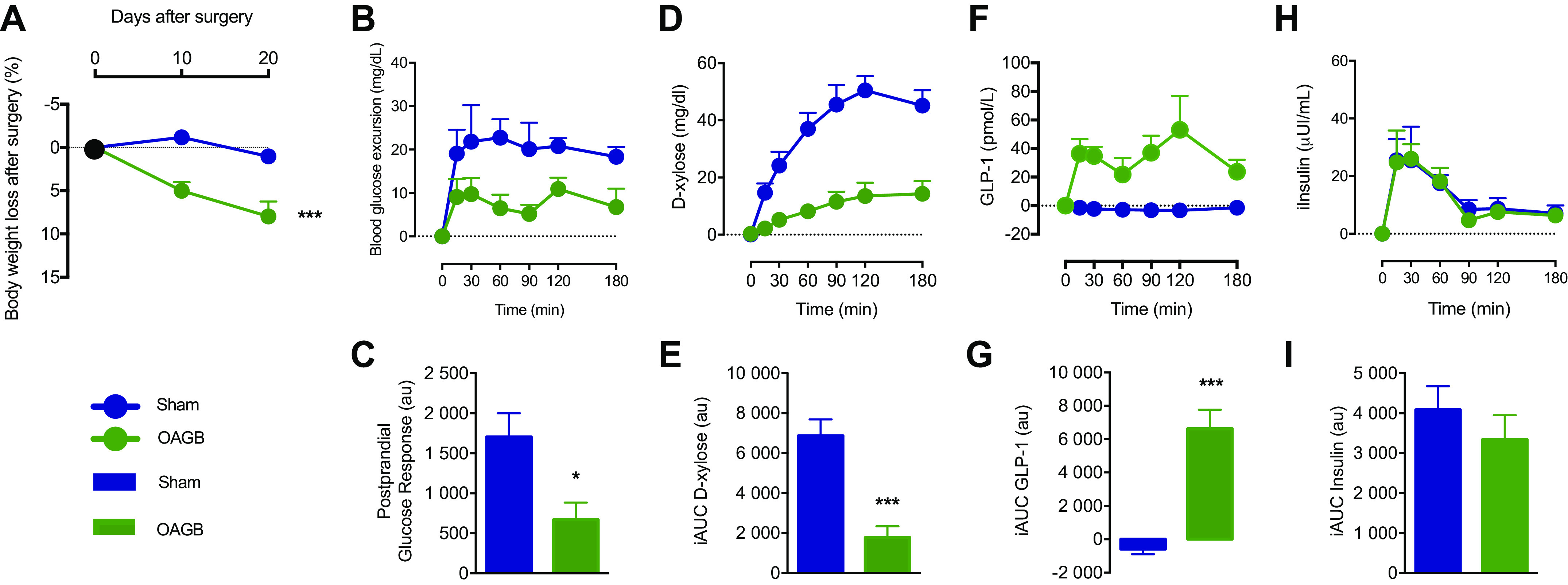
Weight loss and metabolic response to the mixed-meal test. A: weight loss at 10 and 20 days after surgery; B: blood glucose excursion (incremental blood glucose) during the mixed-meal test (MMT); C: postprandial glucose response (incremental AUC for blood glucose); D: d-xylose plasmatic profile during the MMT; E: incremental area under the curve for d-xylose; F: incremental GLP-1 excursion during the MMT; G: incremental area under the curve for GLP-1; H: incremental insulin excursion during the MMT; I: incremental area under the curve for insulin. Blue dot: Sham group, green dot: OAGB group, blue bar: Sham group, and green bar: OAGB group. Statistical analysis: two-way ANOVA (A), one-way ANOVA (C, E, G, and I). *P < 0.05; ***P < 0.001. AUC, area under the curve; GLP-1, glucagon-like peptide 1; OAGB, one anastomosis gastric bypass.
OAGB Modifies Fasting Plasma BA Concentrations and Intestinal BA Species Composition
Since BAs have been implicated with the metabolic improvements of RYGB, and since OAGB strongly impacts the enterohepatic circulation of BAs by the formation of a biliary limb moving the site of BAs–ingested nutrients encounter further down to the common limb, we next studied intestinal and plasma BA changes upon OAGB.
In plasma, OAGB increased fasting total BA concentrations to a similar extent as observed in other gastric bypass techniques (Fig. 3A, OAGB: 17,249 ± 2,236 nM vs. Sham: 9,028 ± 1,801 nM, P < 0.05) (30). However, unlike in RYGB (14), no significant qualitative change was induced by OAGB as both primary (i.e., liver-derived), and secondary (i.e., intestinal microbiota-generated) BAs uniformly increased [Fig. 3B, OAGB vs. Sham, cholic acid (CA): P = 0.008, chenodeoxycholic acid (CDCA): P = 0.008, deoxycholic acid (DCA): P = 0.005, lithocholic acid (LCA): P = 0.014, ursodeoxycholic acid (UDCA): P = 0.001, hyocholic acid (HCA): P = 0.035, and hyodeoxycholic acid (HDCA): P = 0.138)].
Figure 3.

Plasma BA signatures. A: total BA concentration (nM); B: BA species concentration (nM). Blue bar: Sham group and green bar: OAGB group. CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; LCA, lithocholic acid; UDCA, ursodeoxycholic acid; HCA, hyocholic acid; HDCA, hyodeoxycholic acid. Statistical analysis: (A) t test and (B) Mann–Whitney test. *P < 0.05; **P < 0.01. BA, bile acid; OAGB, one anastomosis gastric bypass.
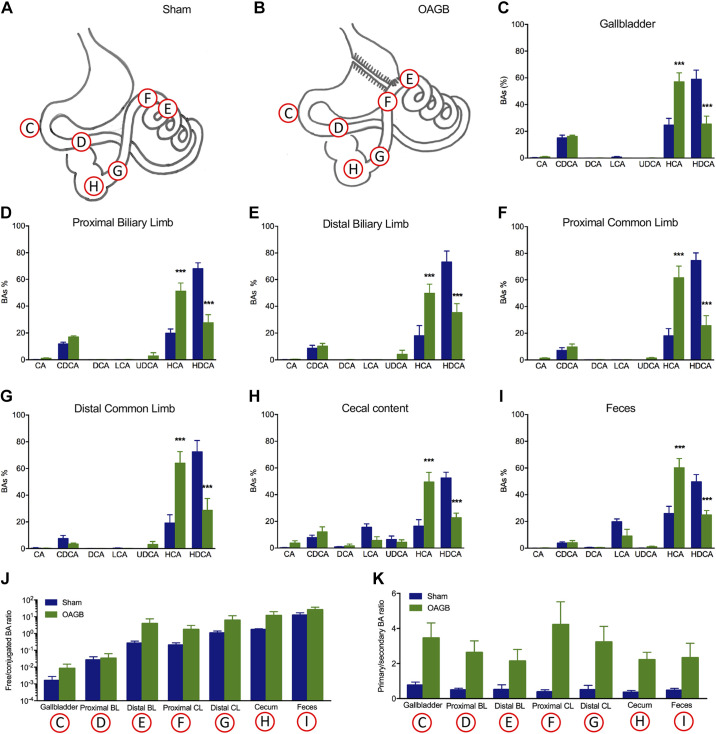
Qualitative BA analysis in the different parts of the intestine (Figs. 4, A–I) revealed the expected BA bacterial deconjugation, as the free/conjugated BA ratio progressively increased from proximal to distal in the sham model (Fig. 4J). It is worth noting that the primary/secondary BA ratio did not change along the intestinal compartments in the sham model (Fig. 4K). The OAGB did not alter the progressive changes in free/conjugated BA ratio throughout the different sampling sites along the gastrointestinal tract (Figs. 4J), but significantly increased the ratio of primary/secondary BAs in comparison with the Sham group in bile, in all the intestinal compartments and in the feces (Fig. 4K). This was due to the increase in HCA and the decrease in HDCA, which are major BAs in pigs (Figs. 4, C–H). To determine the mechanism driving the OAGB-induced BA changes, intestinal BA transporter expression was assessed along the intestinal tract. The Sham group presented the expected increasing expression gradient, from proximal to distal, of intestinal BA transporters (ASBT, IBABP, OSTα, and OSTβ), but there were no differences between the OAGB and Sham groups (Figs. 5, A–D). Since the changes were not explained by the intestinal BA transporter expression, genes encoding hepatic BA synthesis enzymes and transporters were analyzed next. Only the expression of CYP7A1 (the rate-limiting enzyme of the classical pathway) was increased upon OAGB compared to the Sham group (Fig. 5E), but its overall activity was likely unaltered, as illustrated by the similar plasma concentrations of C4 (biomarker of the classical pathway synthesis rate) between the groups (Fig. 5F). Altogether, these results demonstrate that the OAGB induced increases in plasma BAs and changes in the intestinal BA composition, independent of changes in the intestinal transporter expression or hepatic synthesis.
Figure 4.
Signatures of the intestinal BAs. Localization of sampling along the GI for BA signature determination in Sham group (A) and OAGB group (B). Intestinal BA signature in gall bladder (C), proximal biliary limb (D), distal biliary limb (E), proximal common limb (F), distal common limb (G); cecal content (H), and feces (I). J: intestinal free/conjugated BA ratio; K: intestinal primary/secondary BA ratio (C–K). Blue bar: sham group and green bar: OAGB group. Statistical analysis: linear trend and two-way ANOVA (A), two-way ANOVA (B), and one-way ANOVA (E–K). ***P < 0.001. BA, bile acid; GI, gastrointestinal; OAGB, one anastomosis gastric bypass.
Figure 5.
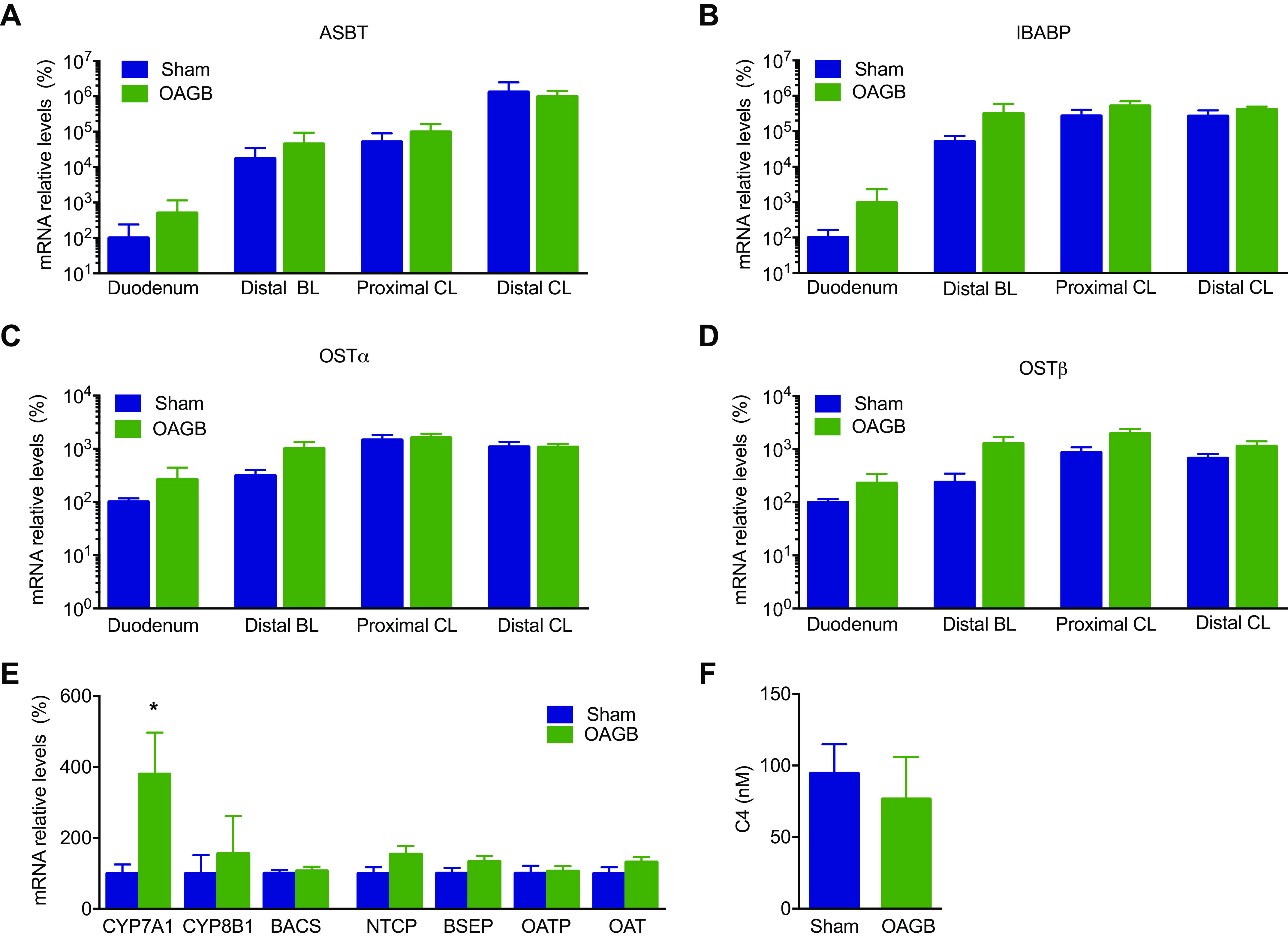
Intestinal bile transporters and markers of hepatic BA synthesis. A–E: mRNA relative levels of apical sodium bile acid transporter (ASBT) (A), ileal bile acid binding protein (IBABP) (B), organic solute transporter α (OST α) (C), organic solute transporter β (OSTβ) (D), hepatic enzyme and BA transporters (E), and C4 plasma concentration (nM) (F). Statistical analysis: (A–D) Kruskal–Wallis test; (E) Mann–Whitney test; and (F) one-way ANOVA. *P < 0.05. BA, bile acid.
A Long Biliary Limb Favors Body Weight Loss upon OAGB, whereas a Short Common Limb Suffices to Evoke Metabolic Improvements Regardless of the Biliary Limb
We hypothesized that the anatomical rearrangements generated by the OAGB could drive the body weight and metabolic changes. The main structures are a very short common limb, formed by anastomosing the gastric pouch to the distal intestine, and a long biliary limb, formed from the excluded upper intestine (Fig. 1, B and D). To study the role of the biliary limb length in the effects of OAGB, OAGB was performed along with resection of the majority of the excluded intestine (OAGB-res) (Fig. 1, B and F). Importantly, the decrease in body weight was reduced by partial resection of the biliary limb, in OAGB-res, as compared to in OAGB (respectively 4.54 ± 0.66% vs. 7.96 ± 1.70%, P = 0.0348) (Fig. 6A). Interestingly, the OAGB model with resection of the biliary limb presented similar metabolic responses (blood glucose excursion (Figs. 6, B and C), d-xylose absorption (Figs. 6, D and E), GLP-1 (Figs. 6, F and G), and insulin (Figs. 6, H and I)) during the mixed-meal test to the responses in the group that underwent the classical OAGB (OAGB-res vs. OAGB). This suggests that the biliary limb is not responsible for the postprandial metabolic improvements associated with OAGB. Therefore, the common limb could be responsible for those effects. The minipigs with a similarly short common limb to OAGB but without the gastric bypass (Sham-res) displayed the same body weight as the OAGB-res group at the 20th postoperative day (3.88 ± 0.53 vs. 4.54 ± 0.66, P = 0.95). Interestingly, the formation of a short common limb reproduced the postprandial response of OAGB and OAGB-res in terms of glucose excursion (Figs. 6, B and C), d-xylose absorption (Figs. 6, D and E), GLP-1 (Figs. 6, F and G), and insulin (Figs. 6, H and I), demonstrating that a sole short common limb suffices to evoke the postprandial responses of OAGB, regardless of the biliary limb or the bypass itself.
Figure 6.
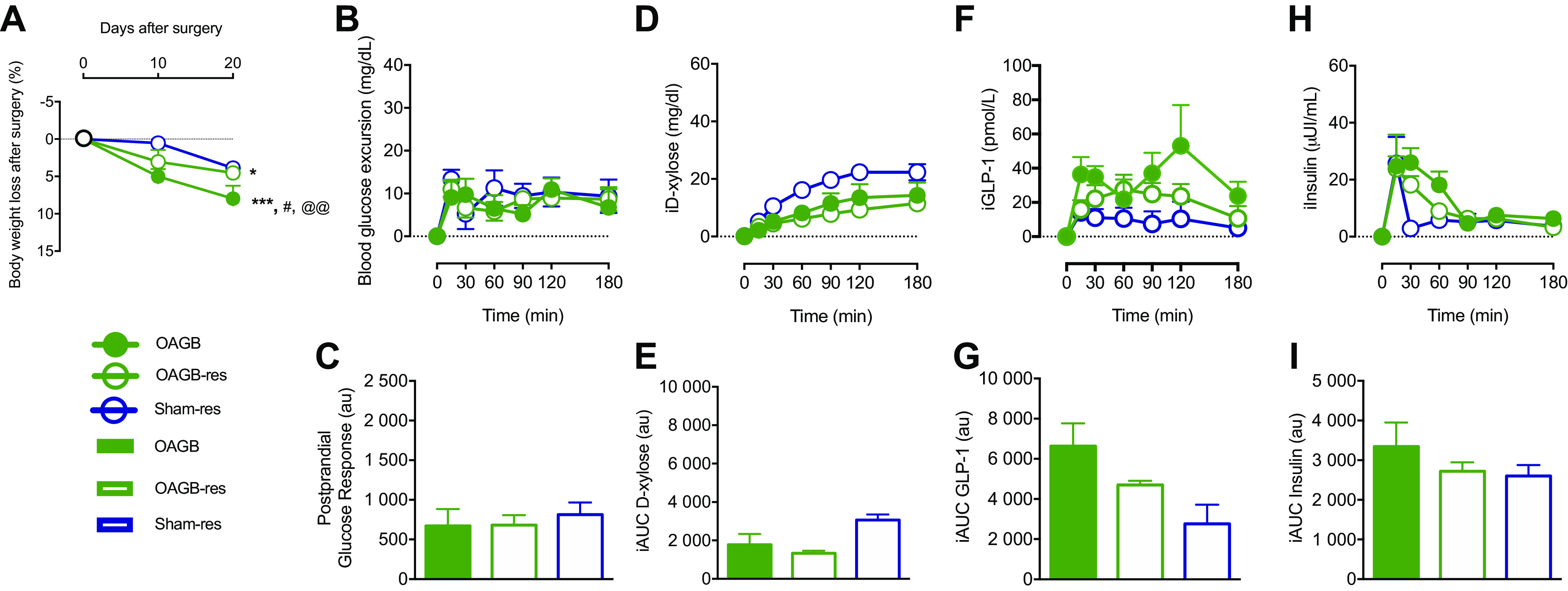
Metabolic phenotype after limb shortening. A: weight loss at 10 and 20 days after surgery; B: blood glucose excursion (incremental blood glucose) during the mixed-meal test (MMT); C: postprandial glucose response (incremental AUC for blood glucose); D: d-xylose plasmatic profile during the MMT; E: incremental area under the curve for d-xylose; F: incremental GLP-1 excursion during the MMT; G: incremental area under the curve for GLP-1; H: incremental insulin excursion during the MMT; and I: incremental area under the curve for insulin. Green dot: OAGB group, white dot with green lining: OAGB-res group, and white dot with blue lining: Sham-res group. Green bar: OAGB group, white bar with green lining: OAGB-res group, and white bar with blue lining: Sham-res group. Statistical analysis: (A) two-way ANOVA and (C, E, G, and I) one-way ANOVA. *P < 0.05 vs. Sham; ***P < 0.001 vs. Sham; #P < 0.05 vs. OAGB-res; @@P < 0.01 vs. Sham-res. AUC, area under the curve; GLP-1, glucagon-like peptide 1; OAGB, one anastomosis gastric bypass.
Changes in the Bile and Cecal BA Profiles are Independent of the Biliary Limb Length, but Vary with the Common Limb Length
The metabolic improvements of OAGB were accompanied by increased plasma BAs and a change in the intestinal BA signature that was present all along the intestine from the bile to the feces, suggesting that BAs might be implicated in the OAGB-induced postprandial changes. Therefore, we studied whether resection of the biliary limb in OAGB-res, or the sole formation of a short common limb in Sham-res, could reproduce the BA changes of OAGB, as it reproduced the metabolic improvements. The intestinal BA signatures of the OAGB-res and Sham-res groups were analyzed at both ends of the intestinal tract (bile and cecal content, as the BA intestinal overall signature was similar across the intestinal segments). Interestingly, shortening of the biliary limb (OAGB-res) or shortening of the common limb (Sham-res) resulted in changes in the primary/secondary BA ratio similar to the ones observed after OAGB (Table 1). Similar to OAGB, these changes were mainly due to the increase in HCA and decrease in HDCA in OAGB-res and SHAM-res in comparison with the Sham group. Hence, intestinal and plasma BA changes were related to a shortened common limb, which determines the postprandial metabolic improvements, and not to the biliary limb, which drives the body weight loss.
Table 1.
Intestinal BA profile in biliary limb and common limb resection groups
| Bile |
Cecal Content |
|||||||
|---|---|---|---|---|---|---|---|---|
| Sham | OAGB | Sham-Res | OAGB-Res | Sham | OAGB | Sham-Res | OAGB-Res | |
| Primary/secondary BA ratio | 0.77 ± 0.42 | 3.00 ± 1.79* | 3.38 ± 0.53** | 2.58 ± 1.03* | 0.36 ± 0.24 | 2.21 ± 1.04*** | 1.67 ± 0.64* | 1.86 ± 0.58** |
| Free/conjugated BA ratio | 0.002 ± 0.003 | 0.009 ± 0.015 | 0.003 ± 0.002 | 0.13 ± 0.19** | 1.72 ± 0.53 | 11.82 ± 20.28 | 1.74 ± 0.88 | 5.25 ± 7.38 |
| CA (%) | 0.290 ± 0.39 | 2.50 ± 3.73 | 0.68 ± 0.83 | 1.38 ± 1.05 | 0.35 ± 0.26 | 3.74 ± 4.26 | 0.17 ± 0.12 | 3.06 ± 4@ |
| CDCA (%) | 15.17 ± 4.92 | 17.96 ± 3.98 | 26.41 ± 2.29* | 27.58 ± 3.01**$ | 7.88 ± 4.40 | 12.22 ± 8.90 | 12.3 ± 3.13 | 18.67 ± 5.31* |
| DCA (%) | 0.11 ± 0.12 | 2.68 ± 5.70 | 0.04 ± 0.046 | 0.40 ± 0.18@ | 0.85 ± 0.79 | 1.67 ± 3.23 | 0.18 ± 0.14 | 1.57 ± 1.59 |
| LCA (%) | 0.70 ± 1.01 | 0.45 ± 0.80 | 0.15 ± 0.048 | 0.36 ± 0.23 | 15.61 ± 6.13 | 5.79 ± 6.59 | 8.01 ± 2.90 | 4.02 ± 3.75* |
| UDCA (%) | 0 ± 0 | 0.93 ± 1.73 | 0.19 ± 0.10 | 5.35 ± 4.83*** | 6.36 ± 6.47 | 4.39 ± 4.45 | 0.51 ± 0.77 | 10.57 ± 6.55@@ |
| HCA (%) | 24.77 ± 12.05 | 49.79 ± 20 | 49.84 ± 2.71* | 40.61 ± 9.13 | 16.45 ± 11.76 | 49.46 ± 17.83* | 48.38 ± 8.46** | 42.32 ± 6.72 |
| HDCA (%) | 58.96 ± 16.79 | 25.68 ± 10.36 | 22.71 ± 2.79* | 24.33 ± 12.18* | 52.5 ± 10.73 | 22.73 ± 8.21* | 30.46 ± 6.65 | 19.8 ± 6.64** |
Primary/secondary intestinal BA ratio, free/conjugated intestinal BA ratio, and intestinal BAs species composition in bile from the gall bladder and in the cecum luminal content. BA, bile acid; CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; HCA, hyocholic acid; HDCA, hyodeoxycholic acid; LCA, lithocholic acid; UDCA, ursodeoxycholic acid. Statistical analysis: A–D: Kruskal–Wallis test. *P < 0.05 vs. Sham; **P < 0.01 vs. Sham; ***P < 0.001 vs. Sham; @P < 0.05 vs. Sham-res; @@P < 0.01 vs. Sham-res; $P < 0.05 vs. OAGB.
DISCUSSION
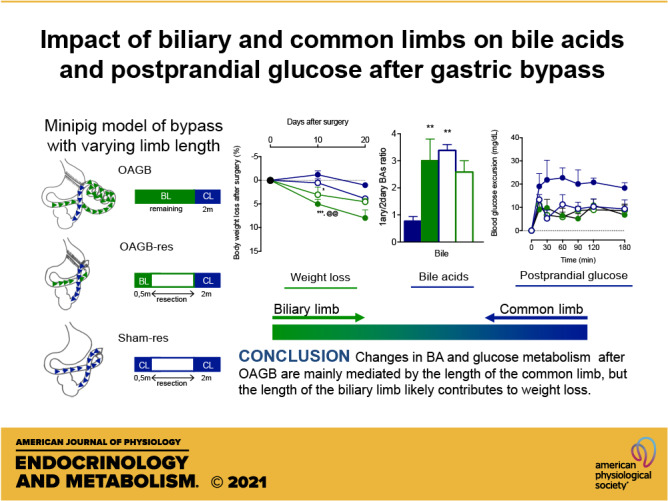
This study characterized the effects of the poorly studied OAGB procedure on body weight, postprandial glucose, GLP-1 response, and BA profiles in a minipig model (27), and the role of the biliary and common limbs therein. In clinical OAGB, one limb can only be increased at the expense of the other. The present experiments allowed us to disentangle the respective role of each limb in a relevant large animal model. Our data demonstrated that glucose metabolism and BA changes with the OAGB were not controlled by the length of the biliary limb, but rather by the shortening of the common limb, whereas the body weight loss was determined by the biliary limb length.
Under our experimental conditions, OAGB decreased postprandial glucose excursion and intestinal carbohydrate absorption in comparison with the Sham group. In addition, postprandial GLP-1 secretion was increased, without enhancing glucose-mediated insulin secretion.
It is hypothesized that the accelerated intestinal transit of poorly digested nutrients is responsible for the increased release of incretins in RYGB (31). Anastomosis of the gastric pouch with the ileum reproduces this phenomenon in our OAGB model, suggesting that enhanced postprandial incretin release due to early exposure of the distal gut to undigested nutrients might be a mechanism shared by OAGB and RYGB. In contrast, an increased insulinotropic effect of GLP-1 was demonstrated to play a key role in the increase of the post-prandial insulin response after RYGB (32), but this effect was not observed in our experimental model, suggesting that other mechanisms might underlie the glucose metabolism improvements.
The biliary limb length is a good candidate to explain the antidiabetic effects of OAGB (33). However, the postprandial responses on glucose excursion and absorption were similar after OAGB, OAGB-res, and Sham-res, where the biliary limb was either long (OAGB), short (OAGB-res), or absent (Sham-res), suggesting that the biliary limb length was not responsible for the glucose homeostasis changes in our OAGB model. In contrast, the common limb was equally short in the three procedures, suggesting that it could be involved in glucose homeostasis ameliorations, but that it might not be essential for the differential effects on GLP-1 and body weight loss. The present results are in line with the outcome of a randomized clinical trial in RYGB. In that study, increasing the length of the biliary limb at the expense of the alimentary limb, without changing the length of the common limb induced more weight loss but no difference in diabetes resolution (34). Similar to our model, recently published data in rats (35) demonstrated that the intestinal resection decreased the postprandial glucose response and increased GLP-1 secretion, supporting the importance of the common limb in the metabolic changes after OAGB (10).
Interestingly, the body weight loss induced by OAGB was dissociated from the metabolic changes associated with the common limb, and the body weight lowering mechanisms remain unclear. Our results suggest that the body weight-lowering effects of OAGB are modulated by the length of the biliary limb, irrespective of the common limb, supporting the hypothesis that intestinal metabolism is a significant contributor to weight loss after surgery (17). Our observations are in line with an observational clinical study in which the random length of the biliary limb in RYGB was positively associated with the level of body weight loss. The selective resection of the biliary limb in our minipig model has never been done in a clinical setting due to potential complications of the surgery and because the biliary limb is always lengthened at the expense of other limbs. We hypothesized that the body weight-lowering mechanism of OAGB takes place in the biliary limb, and its resection in the OAGB-res group attenuated the weight-lowering effects of OAGB. A potential mechanism explaining the role of the biliary limb on the body weight-lowering effects of OAGB is the absence of nutrient absorption from the lumen of the biliary limb as a consequence of the bypass, which renders the biliary limb metabolically dependent from the uptake of nutrients from the bloodstream to sustain its own metabolism. This hypothesis is supported by the observation that glucose uptake from the bloodstream is increased upon RYGB (17, 18, 36). In our model, upon resection of the long biliary limb, the amount of energy necessary to sustain that intestinal segment would no longer be consumed, thus decreasing the intestinal energy expenditure, and attenuating the body weight loss. In line with this hypothesis, resection of the biliary limb in a rat model of a duodenal–jejunal bypass prevented the weight-loss effect induced by the surgery (37). Our data support this concept as in the Sham-res group, a short common limb was formed by resecting the intestinal segment corresponding to the biliary limb in OAGB, and the jejunum was connected to the distal ileum without bypass or biliary limb formation. This model resembles the ileo–jejunal bypass procedure (also known as Payne’s shunt) (38), proven to induce major weight loss and glucose homeostasis improvements. Since the body weight loss in the Sham-res group was modest, in contrast with the data reported on Payne’s shunt, we hypothesize that the lack of body weight loss in the Sham-res group may have been due to the resection of the small bowel tissue, decreasing intestinal glucose utilization and thus attenuating the body weight loss. Because pair feeding was not enforced between groups, differences in food intake could have contributed to the body weight loss differences, which is a limitation of our study.
OAGB induces nutrient malabsorption more frequently than RYGB (39). Since the main difference between OAGB and RYGB is the long biliary limb driving the biliopancreatic secretions from the duodenum to the anastomosis site, we hypothesized that a long biliary limb could contribute to malabsorption in the common limb in OAGB through modifying components of the biliopancreatic secretions, such as the BAs or pancreatic zymogens. Therefore, shortening the biliary limb could attenuate the malabsorption induced by the OAGB to reduce the body weight-loss effects.
The changes in BAs observed in other bariatric surgery techniques modulate metabolic homeostasis and are possible mediators of the weight-loss-independent improvements of bariatric surgery. Thus, we analyzed plasma and intestinal BAs upon OAGB with and without resection of the biliary limb. We observed increased fasting plasma BAs upon OAGB, similar to what has been described after other bariatric surgery procedures (15, 40). In minipigs, the increase in fasting plasma BAs after RYGB is mainly due to conjugated BA species (16). In contrast, in OAGB (and also in OAGB-res), the ratio of free/conjugated BAs is unaltered, and this qualitative discrepancy between surgical techniques suggests that the mechanism driving the increase in BAs may be different. The increased gene expression of CYP7A1, the rate-limiting enzyme of the classical BA synthesis pathway, suggested that the increased fasting plasma BAs upon OAGB could be due to increased hepatic BA synthesis. However, the lack of increase in C4 plasma concentrations, which is a biomarker of the BA synthesis rate via the classical pathway, indicated that the increased expression of CYP7A1 was not associated with the increase of its activity in our experimental model of OAGB, which contrasts with the increase in the BA synthesis rate through the classical pathway reported in RYGB (41).
Under our experimental conditions, OAGB compellingly changed the intestinal BA signature, shifting the proportions of HDCA toward HCA in all the intestinal compartments. HCA and HDCA are 6α-hydroxylated BA species, formed upon hepatic 6α-hydroxylation of CDCA via CYP3A4 in humans (42). These BA species have been poorly studied and are often neglected in human BA analyses due to their minor concentrations in plasma, notwithstanding their high concentrations in human fetal bile (43). HCA is of interest in studies of metabolic disorders since it was recently reported to be negatively associated with BMI and insulin resistance (44–46). Moreover, previous studies reported an association between a polymorphism in CYP3A4 and the risk of T2D in the Japanese population (47) and the reduced expression and activity of CYP3A4 in livers from diabetic donors (48, 49). Although the underlying mechanisms remain to be elucidated, these data strongly support a link between HCA, CYP3A4 activity, and metabolic homeostasis. In contrast to humans, the 6α-hydroxylated BAs HCA and HDCA constitute the main BA species of the pig BA pool (16), raising the hypothesis that the high concentrations of these species render pigs more resistant to obesity-induced insulin resistance and diabetes. We hypothesize that this effect could be induced by the shortening of the common limb length, inducing microbiota changes leading to reduced 7α-dehydroxylation of HCA into HDCA, thus changing the 1ary/2dary BA ratio in the entero-hepatic circulation. This hypothesis needs to be further explored and opens a new research question in this field. A second possibility is that the malabsorption associated with a short common limb, lead to increased fecal loss of the secondary BA HDCA, thus decreasing its proportion versus HCA in the bile acid pool. Our hypothesis that the short common limb is a driver of these changes is supported by the observation that the SHAM-res group presented similar changes in the primary/secondary ratio in the presence of a similarly short common limb, regardless of the length of the biliary limb. Changes in intestinal BAs upon bariatric surgery are important, since preclinical data in rodents suggested that the BA receptors FXR and TGR5 (50) could mediate their metabolic improvements, and since bile diversion to the ileum reproduced the metabolic effects of bariatric surgery in TGR5 knockout mice, but not in intestine-specific FXR knockout mice. Recent evidence suggests that the GLP-1–FXR axis (51) could be implicated in such improvements (52). These data suggest that the role of BA signaling in the metabolic improvements of bariatric surgery has not been fully elucidated. Further studies are necessary to confirm whether the reported results are also present in humans.
CONCLUSIONS
Our results suggest that in minipigs, the changes in glucose metabolism and BAs after OAGB are mainly mediated by the length of the common limb, whereas increasing the length of the biliary limb contributes to the body weight loss. Similar to other bile-diverting procedures, OAGB increases the circulating BAs under fasting conditions. Finally, a short common limb is enough to induce metabolic changes regardless of the OAGB, suggesting that it is a crucial player in the metabolic improvements following bariatric surgery.
GRANTS
This work was supported by the European Genomic Institute for Diabetes (ANR-10-LABX-46) and the Fondation Francophone de Recherche sur le Diabète (FFRD-2015-1). O. Chàvez-Talavera received a PhD fellowship from the French Ministry of Research. B. Staels is a recipient of an Advanced ERC Grant (694717).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.M., O.C-T., R.C., T.H.V., G.B., J.F.G., V.G., V.R., S.L., B.L., B.S., A.T., and F.P., conceived and designed research; C.M., O.C-T., L.Z., A.Q., A.D., E.V., V.V., M.G., M.D., and B.D. performed experiments; C.M., O.C-T, and P.P. analyzed data; C.M., O.C-T., R.C., T.H.V., L.Z., M.K., A.K., V.G., S.L., B.L., and A.T. interpreted results of experiments; C.M. and O.C-T. prepared figures; C.M. and O.C-T. drafted manuscript; C.M., O.C-T., R.C., B.L., B.S., A.T., and F.P. edited and revised manuscript; C.M., O.C-T., B.L., B.S., A.T., and F.P. approved final version of manuscript.
REFERENCES
- 1.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet 384: 766–781, 2014. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchwald H, Buchwald JN, McGlennon TW. Systematic review and meta-analysis of medium-term outcomes after banded Roux-en-Y gastric bypass. Obes Surg 24: 1536–1551, 2014. doi: 10.1007/s11695-014-1311-1. [DOI] [PubMed] [Google Scholar]
- 3.Carbajo MA, Luque-de-León E, Jiménez JM, Ortiz-de-Solórzano J, Pérez-Miranda M, Castro-Alija MJ. Laparoscopic one-anastomosis gastric bypass: technique, results, and long-term follow-up in 1200 patients. Obes Surg 27: 1153–1167, 2017. doi: 10.1007/s11695-016-2428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laferrère B, Pattou F. Weight-independent mechanisms of glucose control after Roux-en-Y gastric bypass. Front Endocrinol (Lausanne) 9: 530, 2018. doi: 10.3389/fendo.2018.00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sweeney TE, Morton JM. Metabolic surgery: action via hormonal milieu changes, changes in bile acids or gut microbiota? A summary of the literature. Best Pract Res Clin Gastroenterol 28: 727–740, 2014. doi: 10.1016/j.bpg.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breen DM, Rasmussen BA, Kokorovic A, Wang R, Cheung GWC, Lam TKT. Jejunal nutrient sensing is required for duodenal-jejunal bypass surgery to rapidly lower glucose concentrations in uncontrolled diabetes. Nat Med 18: 950–955, 2012. doi: 10.1038/nm.2745. [DOI] [PubMed] [Google Scholar]
- 7.Laferrère B, Swerdlow N, Bawa B, Arias S, Bose M, Oliván B, Teixeira J, McGinty J, Rother KI. Rise of oxyntomodulin in response to oral glucose after gastric bypass surgery in patients with type 2 diabetes. J Clin Endocrinol Metab 95: 4072–4076, 2010. doi: 10.1210/jc.2009-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laferrère B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, Kovack B, Bawa B, Koshy N, Lee H, Yapp K, Olivan B. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab 93: 2479–2485, 2008. doi: 10.1210/jc.2007-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oliván B, Teixeira J, Bose M, Bawa B, Chang T, Summe H, Lee H, Laferrère B. Effect of weight loss by diet or gastric bypass surgery on peptide YY3–36 levels. Ann Surg 249: 948–953, 2009. doi: 10.1097/SLA.0b013e3181a6cdb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laferrère B, Pattou F. A Gut check explains improved glucose metabolism after surgery. Cell Metab 30: 852–854, 2019. doi: 10.1016/j.cmet.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baud G, Daoudi M, Hubert T, Raverdy V, Pigeyre M, Hervieux E, Devienne M, Ghunaim M, Bonner C, Quenon A, Pigny P, Klein A, Kerr-Conte J, Gmyr V, Caiazzo R, Pattou F. Bile diversion in Roux-en-Y gastric bypass modulates sodium-dependent glucose intestinal uptake. Cell Metab 23: 547–553, 2016. doi: 10.1016/j.cmet.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 12.Harris L-A, Kayser BD, Cefalo C, Marini L, Watrous JD, Ding J, Jain M, McDonald JG, Thompson BM, Fabbrini E, Eagon JC, Patterson BW, Mittendorfer B, Mingrone G, Klein S. Biliopancreatic diversion induces greater metabolic improvement than Roux-en-Y gastric bypass. Cell Metab 30: 855–864.e3, 2019. doi: 10.1016/j.cmet.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anhê FF, Varin TV, Schertzer JD, Marette A. The gut microbiota as a mediator of metabolic benefits after bariatric surgery. Can J Diabetes 41: 439–447, 2017. doi: 10.1016/j.jcjd.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Chávez-Talavera O, Baud G, Spinelli V, Daoudi M, Kouach M, Goossens J-F, Vallez E, Caiazzo R, Ghunaim M, Hubert T, Lestavel S, Tailleux A, Staels B, Pattou F. Roux-en-Y gastric bypass increases systemic but not portal bile acid concentrations by decreasing hepatic bile acid uptake in minipigs. Int J Obes (Lond) 41: 664–668, 2017. doi: 10.1038/ijo.2017.7. [DOI] [PubMed] [Google Scholar]
- 15.Dutia R, Embrey M, O'Brien CS, O'Brien S, Haeusler RA, Agénor KK, Homel P, McGinty J, Vincent RP, Alaghband-Zadeh J, Staels B, Le Roux CW, Yu J, Laferrère B. Temporal changes in bile acid levels and 12α-hydroxylation after Roux-en-Y gastric bypass surgery in type 2 diabetes. Int J Obes (Lond) 39: 806–813, 2015. [Erratum in Int J Obes (Lond) 40: 554, 2016]. doi: 10.1038/ijo.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spinelli V, Lalloyer F, Baud G, Osto E, Kouach M, Daoudi M, Vallez E, Raverdy V, Goossens J-F, Descat A, Doytcheva P, Hubert T, Lutz TA, Lestavel S, Staels B, Pattou F, Tailleux A. Influence of Roux-en-Y gastric bypass on plasma bile acid profiles: a comparative study between rats, pigs and humans. Int J Obes (Lond) 40: 1260–1267, 2016. doi: 10.1038/ijo.2016.46. [DOI] [PubMed] [Google Scholar]
- 17.Cavin J-B, Couvelard A, Lebtahi R, Ducroc R, Arapis K, Voitellier E, Cluzeaud F, Gillard L, Hourseau M, Mikail N, Ribeiro-Parenti L, Kapel N, Marmuse J-P, Bado A, Le Gall M. Differences in alimentary glucose absorption and intestinal disposal of blood glucose after Roux-en-Y gastric bypass vs sleeve gastrectomy. Gastroenterology 150: 454–464.e9, 2016. doi: 10.1053/j.gastro.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Saeidi N, Meoli L, Nestoridi E, Gupta NK, Kvas S, Kucharczyk J, Bonab AA, Fischman AJ, Yarmush ML, Stylopoulos N. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science 341: 406–410, 2013. doi: 10.1126/science.1235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rutledge R. The mini-gastric bypass: experience with the first 1,274 cases. Obes Surg 11: 276–280, 2001. doi: 10.1381/096089201321336584. [DOI] [PubMed] [Google Scholar]
- 20.Lee W-J, Yu P-J, Wang W, Chen T-C, Wei P-L, Huang M-T. Laparoscopic Roux-en-Y versus mini-gastric bypass for the treatment of morbid obesity: a prospective randomized controlled clinical trial. Ann Surg 242: 20–28, 2005. doi: 10.1097/01.sla.0000167762.46568.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruiz-Tovar J, Carbajo MA, Jimenez JM, Castro MJ, Gonzalez G, Ortiz-de-Solorzano J, Zubiaga L. Long-term follow-up after sleeve gastrectomy versus Roux-en-Y gastric bypass versus one-anastomosis gastric bypass: a prospective randomized comparative study of weight loss and remission of comorbidities. Surg Endosc 33: 401–410, 2019. doi: 10.1007/s00464-018-6307-9. [DOI] [PubMed] [Google Scholar]
- 22.Khalaj A, Kalantar Motamedi MA, Mousapour P, Valizadeh M, Barzin M. Protein-calorie malnutrition requiring revisional surgery after one-anastomosis-mini-gastric bypass (OAGB-MGB): case series from the Tehran Obesity Treatment Study (TOTS). Obes Surg 29: 1714–1720, 2019. doi: 10.1007/s11695-019-03741-7. [DOI] [PubMed] [Google Scholar]
- 23.Motamedi MAK, Barzin M, Ebrahimi M, Ebrahimi R, Khalaj A. Severe fatal protein malnutrition and liver failure in a morbidly obese patient after mini-gastric bypass surgery: Case report. Int J Surg Case Rep 33: 71–74, 2017. doi: 10.1016/j.ijscr.2017.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahawar KK, Kumar P, Parmar C, Graham Y, Carr WRJ, Jennings N, Schroeder N, Balupuri S, Small PK. Small bowel limb lengths and Roux-en-Y gastric bypass: a systematic review. Obes Surg 26: 660–671, 2016. doi: 10.1007/s11695-016-2050-2. [DOI] [PubMed] [Google Scholar]
- 25.Buchwald H, Oien DM. Revision Roux-en-Y gastric bypass to biliopancreatic long-limb gastric bypass for inadequate weight response: case series and analysis. Obes Surg 27: 2293–2302, 2017. doi: 10.1007/s11695-017-2658-x. [DOI] [PubMed] [Google Scholar]
- 26.Pinheiro JS, Schiavon CA, Pereira PB, Correa JL, Noujaim P, Cohen R. Long-long limb Roux-en-Y gastric bypass is more efficacious in treatment of type 2 diabetes and lipid disorders in super-obese patients. Surg Obes Relat Dis 4: 521–525, 2008. doi: 10.1016/j.soard.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 27.Verhaeghe R, Zerrweck C, Hubert T, Tréchot B, Gmyr V, D'Herbomez M, Pigny P, Pattou F, Caiazzo R. Gastric bypass increases postprandial insulin and GLP-1 in nonobese minipigs. Eur Surg Res 52: 41–49, 2014. doi: 10.1159/000355678. [DOI] [PubMed] [Google Scholar]
- 28.Kochkodan J, Telem DA, Ghaferi AA. Physiologic and psychological gender differences in bariatric surgery. Surg Endosc 32: 1382–1388, 2018. doi: 10.1007/s00464-017-5819-z. [DOI] [PubMed] [Google Scholar]
- 29.Eberts TJ, Sample RH, Glick MR, Ellis GH. A simplified, colorimetric micromethod for xylose in serum or urine, with phloroglucinol. Clin Chem 25: 1440–1443, 1979. [PubMed] [Google Scholar]
- 30.Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature 489: 242–249, 2012. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 31.Santoro S. Is the metabolic syndrome a disease of the foregut? Yes, excessive foregut. Ann Surg 247: 1074–1075, 2008. doi: 10.1097/SLA.0b013e3181758ddb. [DOI] [PubMed] [Google Scholar]
- 32.Salehi M, Gastaldelli A, D'Alessio DA. Blockade of glucagon-like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass. Gastroenterology 146: 669–680.e2, 2014. doi: 10.1053/j.gastro.2013.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruiz-Tovar J, Vorwald P, Gonzalez-Ramirez G, Posada M, Salcedo G, Llavero C, Garcia-Olmo D. Impact of biliopancreatic limb length (70 cm vs 120 cm), with constant 150 cm alimentary limb, on long-term weight loss, remission of comorbidities and supplementation needs after Roux-En-Y gastric bypass: a prospective randomized clinical trial. Obes Surg 29: 2367–2372, 2019. doi: 10.1007/s11695-019-03717-7. [DOI] [PubMed] [Google Scholar]
- 34.Nergaard BJ, Leifsson BG, Hedenbro J, Gislason H. Gastric bypass with long alimentary limb or long pancreato-biliary limb—long-term results on weight loss, resolution of co-morbidities and metabolic parameters. Obes Surg 24: 1595–1602, 2014. doi: 10.1007/s11695-014-1245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prada-Oliveira JA, Camacho-Ramirez A, Salas-Alvarez J, Campos-Martinez FJ, Lechuga-Sancho AM, Almorza-Gomar D, Blandino-Rosano M, Perez-Arana GM. GLP-1 mediated improvement of the glucose tolerance in the T2DM GK rat model after massive jejunal resection. Ann Anat 223: 1–7, 2019. doi: 10.1016/j.aanat.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Franquet E, Watts G, Kolodny GM, Goldfine AB, Patti M-E. PET-CT reveals increased intestinal glucose uptake after gastric surgery. Surg Obes Relat Dis 15: 643–649, 2019. doi: 10.1016/j.soard.2019.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyachi T, Nagao M, Shibata C, Kitahara Y, Tanaka N, Watanabe K, Tsuchiya T, Motoi F, Naitoh T, Unno M. Biliopancreatic limb plays an important role in metabolic improvement after duodenal-jejunal bypass in a rat model of diabetes. Surgery 159: 1360–1371, 2016. doi: 10.1016/j.surg.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 38.DeWind LT, Payne JH. Intestinal bypass surgery for morbid obesity. Long-term results. JAMA 236: 2298–2301, 1976. [PubMed] [Google Scholar]
- 39.Robert M, Espalieu P, Pelascini E, Caiazzo R, Sterkers A, Khamphommala L, Poghosyan T, Chevallier J-M, Malherbe V, Chouillard E, Reche F, Torcivia A, Maucort-Boulch D, Bin-Dorel S, Langlois-Jacques C, Delaunay D, Pattou F, Disse E. Efficacy and safety of one anastomosis gastric bypass versus Roux-en-Y gastric bypass for obesity (YOMEGA): a multicentre, randomised, open-label, non-inferiority trial. The Lancet 393: 1299–1309, 2019. doi: 10.1016/S0140-6736(19)30475-1. [DOI] [PubMed] [Google Scholar]
- 40.Chávez-Talavera O, Tailleux A, Lefebvre P, Staels B. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology 152: 1679–1694.e3, 2017. doi: 10.1053/j.gastro.2017.01.055. [DOI] [PubMed] [Google Scholar]
- 41.Vincent R, Werling M, Cross G, Fädriks L, Lonroth H, Taylor D, Alaghband-Zadeh J, Olberts T, Le Roux C. The Ratio of 12 alpha-hydroxylated: non-12 alpha-hydroxylated bile acids is not altered after Roux-en-Y gastric bypass in humans . 2nd Diabetes Surgery Summit (DSS-II). London, UK, 2015. [Google Scholar]
- 42.Deo AK, Bandiera SM. Identification of human hepatic cytochrome p450 enzymes involved in the biotransformation of cholic and chenodeoxycholic acid. Drug Metab Dispos 36: 1983–1991, 2008. doi: 10.1124/dmd.108.022194. [DOI] [PubMed] [Google Scholar]
- 43.Setchell KD, Dumaswala R, Colombo C, Ronchi M. Hepatic bile acid metabolism during early development revealed from the analysis of human fetal gallbladder bile. J Biol Chem 263: 16637–16644, 1988. [PubMed] [Google Scholar]
- 44.Chávez-Talavera O, Wargny M, Pichelin M, Descat A, Vallez E, Kouach M, Bigot-Corbel E, Joliveau M, Goossens J-F, Le May C, Hadjadj S, Hanf R, Tailleux A, Staels B, Cariou B. Bile acids associate with glucose metabolism, but do not predict conversion from impaired fasting glucose to diabetes. Metabolism 103: 154042, 2020. doi: 10.1016/j.metabol.2019.154042. [DOI] [PubMed] [Google Scholar]
- 45.Suhre K, Meisinger C, Döring A, Altmaier E, Belcredi P, Gieger C, Chang D, Milburn MV, Gall WE, Weinberger KM, Mewes H-W, Hrabé de Angelis M, Wichmann H-E, Kronenberg F, Adamski J, Illig T. Metabolic footprint of diabetes: a multiplatform metabolomics study in an epidemiological setting. PLoS One 5: e13953, 2010. doi: 10.1371/journal.pone.0013953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wewalka M, Patti M-E, Barbato C, Houten SM, Goldfine AB. Fasting serum taurine-conjugated bile acids are elevated in type 2 diabetes and do not change with intensification of insulin. J Clin Endocrinol Metab 99: 1442–1451, 2014. doi: 10.1210/jc.2013-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamada Y, Matsuo H, Watanabe S, Kato K, Yajima K, Hibino T, Yokoi K, Ichihara S, Metoki N, Yoshida H, Satoh K, Nozawa Y. Association of a polymorphism of CYP3A4 with type 2 diabetes mellitus. Int J Mol Med 20: 703–707, 2007. [PubMed] [Google Scholar]
- 48.Dostalek M, Court MH, Yan B, Akhlaghi F. Significantly reduced cytochrome P450 3A4 expression and activity in liver from humans with diabetes mellitus. Br J Pharmacol 163: 937–947, 2011. doi: 10.1111/j.1476-5381.2011.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jamwal R, de la Monte SM, Ogasawara K, Adusumalli S, Barlock BB, Akhlaghi F. Nonalcoholic fatty liver disease and diabetes are associated with decreased CYP3A4 protein expression and activity in human liver. Mol Pharm 15: 2621–2632, 2018. doi: 10.1021/acs.molpharmaceut.8b00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McGavigan AK, Garibay D, Henseler ZM, Chen J, Bettaieb A, Haj FG, Ley RE, Chouinard ML, Cummings BP. TGR5 contributes to glucoregulatory improvements after vertical sleeve gastrectomy in mice. Gut 66: 226–234, 2017. doi: 10.1136/gutjnl-2015-309871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trabelsi M-S, Daoudi M, Prawitt J, Ducastel S, Touche V, Sayin SI, Perino A, Brighton CA, Sebti Y, Kluza J, Briand O, Dehondt H, Vallez E, Dorchies E, Baud G, Spinelli V, Hennuyer N, Caron S, Bantubungi K, Caiazzo R, Reimann F, Marchetti P, Lefebvre P, Bäckhed F, Gribble FM, Schoonjans K, Pattou F, Tailleux A, Staels B, Lestavel S. Farnesoid X receptor inhibits glucagon-like peptide-1 production by enteroendocrine L-cells. Nat Commun 6: 7629, 2015. doi: 10.1038/ncomms8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Albaugh VL, Banan B, Antoun J, Xiong Y, Guo Y, Ping J, Alikhan M, Clements BA, Abumrad NN, Flynn CR. Role of bile acids and GLP-1 in mediating the metabolic improvements of bariatric surgery. Gastroenterology 156: 1041–1051.e4, 2019. doi: 10.1053/j.gastro.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]