Abstract
Patients with type 2 diabetes can have several neuropathologies, such as memory deficits. Recent studies have focused on the association between metabolic imbalance and neuropathological problems, and the associated molecular pathology. Diabetes triggers neuroinflammation, impaired synaptic plasticity, mitochondrial dysfunction, and insulin resistance in the brain. Glucose is a main energy substrate for neurons, but under certain conditions, such as fasting and starvation, ketone bodies can be used as an energy fuel for these cells. Recent evidence has shed new light on the role of ketone bodies in regulating several anti-inflammation cellular pathways and improving glucose metabolism, insulin action, and synaptic plasticity, thereby being neuroprotective. However, very high amount of ketone bodies can be toxic for the brain, such as in ketoacidosis, a dangerous complication that may occur in type 1 diabetes mellitus or alcoholism. Recent findings regarding the relationship between ketone bodies and neuropathogenesis in dementia are reviewed in this article. They suggest that the adequately low amount of ketone bodies can be a potential energy source for the treatment of diabetes-induced dementia neuropathology, considering the multifaceted effects of the ketone bodies in the central nervous system. This review can provide useful information for establishing the therapeutic guidelines of a ketogenic diet for diabetes-induced dementia.
Keywords: diabetes-induced dementia, insulin resistance, ketone bodies, mitochondrial dysfunction, neuroinflammation, sirtuins
INTRODUCTION
The prevalence of type 2 diabetes is markedly increasing all over the world; subsequently, many researchers have investigated various pathological problems caused by type 2 diabetes.1 Among diverse pathological changes, recent studies have highlighted the strong association between severe neuropathology and the onset of diabetes.2,3 Results of several epidemiologic studies suggested a positive correlation between the progression of diabetes and severity of memory loss, such as mild cognitive impairment and dementia.2–4 Elevated glucose levels and insulin resistance in blood serum are reported to be critical risk factors for dementia onset.5–7 Furthermore, researchers have reported that the brains of patients with type 2 diabetes have more brain atrophy, less brain volume, and impaired synaptic connectivity compared with normal brains.8,9 Given these data, many researchers are focusing on various pathological mechanisms in diabetes-induced dementia.
Glucose is well known as a main fuel in the brain; it supports the survival of various brain cells.10,11 However, during nutrient deprivation, after exercise, and during low-carbohydrate states, ketone bodies are used as an alternative energy source in the brain as well as in the rest of the body.12–14 In systemic metabolism processes, ketone bodies are associated with major metabolic pathways, including fatty acid β-oxidation, the tricarboxylic acid cycle, and gluconeogenesis.15 In the body, fatty acids undergo fatty β-oxidation after food ingestion and subsequently are converted to a acetyl coenzyme A in liver and also ketone bodies.14
Ketone bodies contribute to the elevation of antioxidant responses by regulating NAD and NAD+/NADH–coupled reactions and glutathione activity through complex pathways.16 Ketosis—the increased but low levels of ketone bodies in blood, which is naturally produced in the fasted state or the low-carbohydrate state—is emerging as a therapeutic approach in various diseases.17 In particular, many researchers have suggested that ketosis could treat several neurologic disorders, including epilepsy, Parkinson’s disease, stroke, and dementia.17–20 Several studies have demonstrated that ketone bodies, ketone esters, and β-hydroxybutyrate administration provide neuroprotective effects in diverse neurologic disorders.16,21–23 However, increased levels of ketone bodies are not always beneficial for neuronal metabolism, because very high levels of ketone bodies, such as in ketoacidosis, a dangerous complication that may occur in type 1 diabetes mellitus and alcoholism, can be toxic for the brain.24,25
In this review, we summarize recently reported evidence, from many viewpoints, on the beneficial roles of ketone bodies in diabetes-induced dementia. More studies are needed into the role of ketone bodies in various neurologic pathologies.
CURRENT STATUS OF KNOWLEDGE ON BRAIN ENERGY METABOLISM
Brain energy metabolism is different from systemic body energy metabolism and is affected by the endocrine regulation of appetite and reward.26 The brain requires continuous provision of energy in the form of adenosine triphosphate (ATP), made by glucose oxidative reactions in mitochondria.26 Glucose is used as the main fuel in the brain, and glucose uptake supports the survival of various brain cells, including brain endothelial cells, neuron, astrocytes, and microglia.10,11 ATP, the main form of energy in brain metabolism, is used by ion channel kinases such as Na+/K+–ATPase and Ca2+-ATPase,27,28 and is the neurotransmitter produced from the mitochondria of neurons and glia through the tricarboxylic acid cycle.29,30 Briefly, excitatory neurons consume approximately 85% of ATP in the brain, whereas inhibitory neurons consume approximately 15% of remaining ATP.31,32 ATP produced from glucose metabolism contributes to synaptic transmission in neurons and glia, leading to a stable neural network.32,33
Recent studies demonstrated that impaired brain glucose metabolism leads to neurologic disorders, including Alzheimer’s disease (AD), and Parkinson’s disease.31,34,35 Authors of previous studies have mentioned that γ-aminobutyric acid (GABA)ergic inhibitory interneurons are more sensitive to energy deprivation than are other neurons in brain regions related to memory function.27,36 In 1 study, authors reported that the increase of glucose uptake in neurons by glucose transporter 1 promotes energy metabolism in cortical and hippocampal neurons and enhances memory function.37 In the brain, poor glucose metabolism results in impaired ion transport, impaired vesicle recycling, and impaired synaptic plasticity.36,38 Considering these previous findings, brain energy metabolism supplies nutrients and oxygen to neurons and glia and so contributes to brain function. Therefore, the regulation of brain energy metabolism may be a therapeutic issue to cure neurological pathology.
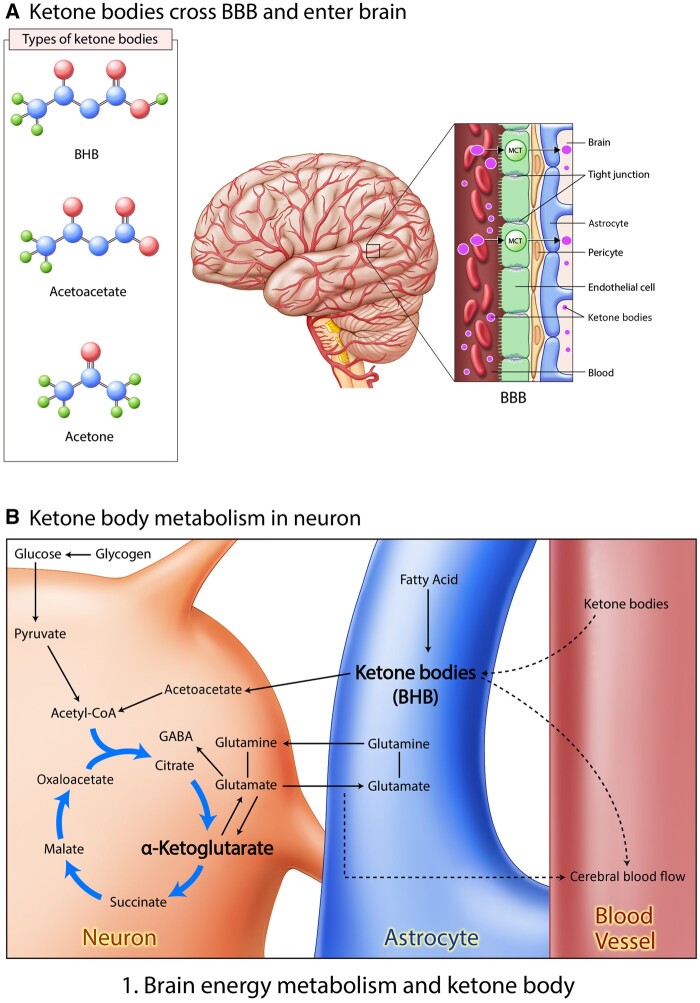
In the 1920s, the ketogenic diet (KD), a low-carbohydrate diet consisting initially of <20% of daily energy intake derived from carbohydrates, was developed to cure patients with epilepsy, and many researchers are continuing to gain pharmacological insights from it.19 Ketone bodies generated in the liver provide an alternative energy source during a fasting and starving state.39 In a starving state, fatty acid is converted to ketone bodies in astrocytes; subsequently, these ketone bodies enter a neuron to be used as a fuel (Figure 124 Achanta et al 2017).
Figure 1.
Brain energy metabolism and ketone bodies. (A) Ketone bodies cross the BBB and enter the brain. There are several types of ketone bodies, including BHB, acetoacetate, and acetone. These ketone bodies could cross the BBB and subsequently enter the brain. (B) Ketone body metabolism in a neuron. After food ingestion, glucose changes into pyruvate, which, in turn, is metabolized to acetyl-coenzyme A, which circulates in the citric acid cycle. During fasting, fatty acids change into ketone bodies through β-oxidation. α-Ketoglutarate could change into glutamate, converting to glutamine and GABA neurotransmitters.78Abbreviations: BBB, blood-brain barrier; BHB, β-hydroxybutyrate; MCT, monocarboxylate transporter.
Ketones known as β-hydroxybutyrate and acetoacetate (AcAc) are the major alternative fuels in the brain if blood glucose levels decrease due to food starvation15,40 (Figures 124 Achanta et al 2017 and 214,41). β-Hydroxybutyrate metabolism boosts ATP generation in comparison with glucose metabolism in the brain.42
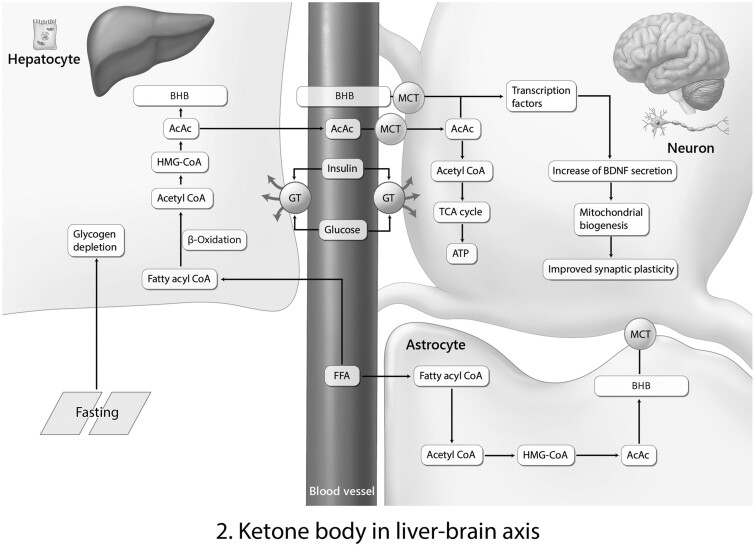
Figure 2.
Ketone bodies in liver-brain axis. Ketone body BHB made by the liver could cross the blood-brain barrier (BBB) and contribute to the brain function. This liver-brain axis is the typical pathway of BHB and acetoacetate (AcAc) from liver and brain. During fasting, fatty acid changes into BHB through acetyl coenzyme-A. BHB and AcAc cross the BBB and enter neurons through an MCT channel. Next, BHB and AcAc contribute to the secretion of BDNF and the improvement of synapse plasticity.14,41Abbreviations: ATP, adenosine triphosphate; BHB, β-hydroxybutyrate; BDNF, brain-derived neurotrophic factor; CoA, coenzyme A; FFA, free fatty acid; GT, glucose transporter; HMG, 3-hydroxy-3-methylglutaryl; MCT, monocarboxylate transporter; TCA, the citric acid [cycle].
Ketone bodies can cross the blood-brain barrier, and administration of a KD leads to increased concentration of the amino acid leucine in the brain43 (Figure 1A24 Achanta et al 2017). A KD also increases AcAc levels in the blood44 and induces various cellular responses.24 Ketone bodies are associated with diverse metabolic pathways, such as fatty acid β-oxidation, the tricarboxylic acid cycle, and gluconeogenesis,45 and tend to be the critical energy fuel for extrahepatic tissues, such as brain, under several physiological conditions, including fasting, low-carbohydrate diets, hypoglycemia, and pregnancy.14
Ketone bodies in blood vessels enter neurons and are converted to AcAc, and they lead to GABA secretion in neurons through the citrate cycle (Figure 1B24 Achanta et al 2017).
In the central nervous system (CNS), neurons can use ketone bodies as a fuel source, and this ketogenic metabolism in the brain is linked to cognitive function46 as well as imbalanced glucose metabolism.47 The ketone bodies AcAc and β-hydroxybutyrate are used as the main energy source in the brain during ketosis48 (Figure 214,41). Another study demonstrated that a KD has similar effects as calorie restriction in improving the metabolic state in humans.49 And in another study, researchers found that a KD enhanced neurovascular function against metabolic imbalance and promoted specific intestinal microbiota patterns.50
Authors of a positron emission tomography study reported that the absence of ketogenic intervention resulted in normal brain ketone uptake in mild AD.51 Furthermore, KD results in a reduction in brain glucose uptake because ketone bodies are the favored fuel in the brain, compared with glucose.52
Ketone bodies from liver and blood could enter neurons and subsequently activate an increase in brain-derived neurotrophic factor production, which is involved in the improvement of mitochondrial biogenesis and synaptic plasticity (Figure 214,41). Several studies have indicated that ketosis caused by a low-calorie diet enhances synaptic plasticity53 and improves cognitive function.54,55 Recent studies have also demonstrated that the administration of ketone bodies reduced neuronal cell damage, improved cognitive dysfunction and anxiety in an AD mouse model,56 and protected against memory loss in patients with mild cognitive impairment57,58 and AD.44 Furthermore, in a recent clinical study, authors reported that brain ketone metabolism is involved in the severity of mild cognitive impairment and dementia, compared with healthy study subjects.51 Ketone bodies have therapeutic effects in the treatment of neuropathology in diverse neurologic diseases, including epilepsy, AD,59 Parkinson’s disease,60 autism,40 brain tumor,61 and stroke.62
Reviewing previous studies, we have identified the effects of ketone bodies in diabetes-induced dementia. In this review, we provide evidence on the potency of the KD for diabetes-induced dementia.
DIABETES-INDUCED DEMENTIA AND KETONE BODIES
Ketone bodies activate sirtuins in diabetes-induced dementia
Results of recent studies indicate impaired metabolic status, such as type 2 diabetes, aggravates learning and memory function63 and is accompanied by severe neuroinflammation,64 impaired synaptic plasticity,65 increased toxic amyloid β (Aβ) aggregation, increased τ phosphorylation,65 and abnormal mitochondrial function.66 These poor metabolic statuses damage neuronal cell function and boost rapid memory loss, ultimately triggering the onset of neurodegenerative diseases.67
Sirtuins (SIRTs) are NAD+-dependent enzymes that affect multiple cellular responses such as energy metabolism, mitochondrial function, and the antioxidant redox pathway.68 SIRTs mediate calorie restriction effects such as cell survival activation by regulating NAD+ enzymes and through the 5′ AMP–activated protein kinase and mammalian target of rapamycin (mTOR) pathways.69,70 SIRTs also could promote oxidative phosphorylation, deacetylation of transcription factors, anti-inflammatory responses, cell survival, and DNA repair, and inhibit glycolysis against oxidative stress.71,72
In particular, SIRT1 is required for cognition and the maintenance of neuronal plasticity,73 and has been used for epilepsy.74 SIRT6 and SIRT7 are strongly involved in DNA repair and antiaging responses.75 SIRT3 and SIRT5 are major controllers of energy metabolism in mitochondria and regulate the acetylation and succinylation states of various energy enzymes.76
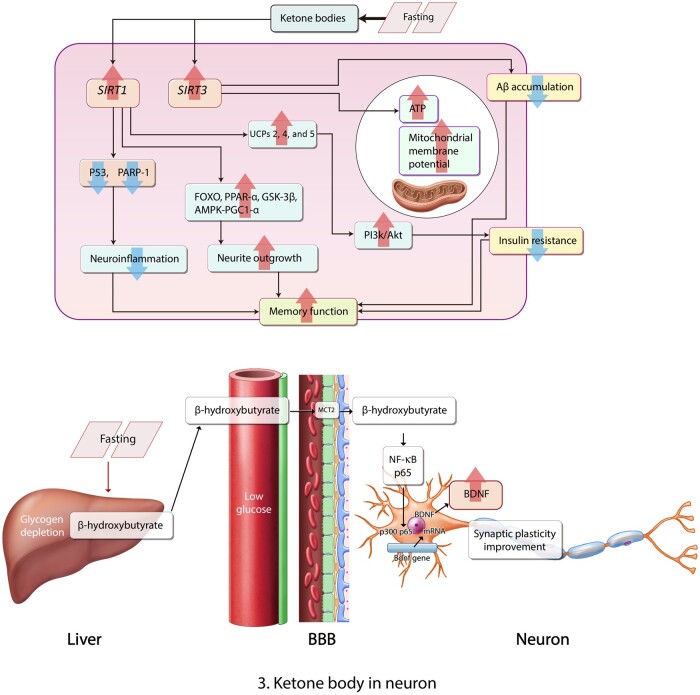
Ketones control the acetylation of proteins and increase histone deacetylation of the SIRT1 gene in neurons77 (Figure 3A78). The activation of the SIRT1 gene caused by ketone bodies in the brain leads to the activation of uncoupling protein (UCP)2, UCP4, and UCP5 expression in the hippocampus79,80 (Figure 3A78). Ultimately, the induced expression of UCPs by ketone bodies is linked to the activation of SIRT1 in the brain.81 SIRT1 results in increased insulin secretion through the repression of UCP2.82 In addition, ketosis promotes macroautophagy through SIRT1 activation in the cells.83 Furthermore, the elevation of SIRT protein concentration by a KD reduces the production of reactive oxygen species (ROS) and DNA damage84,85 caused by fasting, and activates mitochondrial function, and inhibits the activation of poly[ADP-ribose] polymerase 1 as a DNA damage index86,87 caused by fasting (Figure 3A78). In the AD brain, SIRT3 attenuates the accumulation of Aβ and contributes to the improvement of neuronal hypometabolism.88 SIRT3 is an important activator for oxidative phosphorylation89 and promotes energy metabolism by increasing mitochondrial biogenesis.90 The KD was found to exert neuroprotective effects by mediating SIRT3 in mouse model of stroke.91 The KD could enhance SIRT3 activation and subsequently lead to the increase of ATP production in mitochondria92 (Figure 3A78). Several studies have reported that a KD could induce SIRT3 gene activation and subsequently protect neurons against brain damage.91,93 Notably, SIRT1 is strongly associated with cognitive performance94 in metabolic-imbalance conditions such as diabetes.95 SIRT1 binds with deacetylated p53, inhibits the activation of the p53 pathway, and reduces the downstream target genes of p5396 in the hippocampus97 and cortex,98 leading to memory dysfunction.
Figure 3.
Ketone bodies in a neuron. (A) In a fasting state, ketone bodies increase SIRT1 activation, which reduces neuroinflammation through P53 signaling and poly[ADP-ribose] polymerase 1 (PARP-1); increases FOXO, PPAR-α, GSK-2β, and AMPK-PGC1-α signaling; and UCP2, UCP4, and UCP5 activation, leading to the improvement of memory function. Also, ketone bodies activate SIRT3, which increases ATP production in mitochondria and reduces the accumulation of Aβ in the neuron, leading to the improvement of memory function. (B) β-Hydroxybutrate in hepatocyte cross the BBB and enter a neuron through MCT2. Subsequently, β-hydroxybutrate regulates NF-κB signaling and boosts the production of brain-derived neurotrophic factor in neuron, involving synaptic plasticity improvement.78Abbreviations: Aβ, amyloid β; ATP, adenosine triphosphate; BBB, blood-brain barrier; BDNF, brain-derived neurotrophic factor; MCT2, human monocarboxylate transporter 2; UCP, uncoupling protein.
Moreover, SIRT1 regulates inflammatory pathway nuclear factor-κβ99 and τ phosphorylation-related extracellular signal-regulated kinase signaling100; neuronal survival related to the forkhead box subgroup O pathway,101; energy metabolism–related peroxisome proliferator–activated receptor γ and its transcriptional coactivator PPARγ coactivator 1-α102; and neurite outgrowth involved in the mTOR/p70S6 kinase pathway103 under normal and metabolic-imbalance conditions (Figure 3A78). These modulations of SIRT1 prove that it can ameliorate cognitive decline under metabolic-imbalance conditions.104,105
Several studies have demonstrated that SIRT1 increases the activation of glycogen synthesis kinase 3 β98 and increases memory function, such as long-term potentiation, through glycogen synthesis kinase 3 β signaling.106 In previous studies, researchers have mentioned that the inhibition of SIRT1 aggravates the progress of type 2 diabetes and insulin resistance.107,108 Furthermore, SIRT1 promotes neurite outgrowth and improves insulin sensitivity in neurons through the PI3K/Akt signaling pathway.109 In recent studies, authors have reported that SIRT1 and SIRT3 enhance mitochondrial dysfunction through the 5′ AMP–activated protein kinase–PPARγ coactivator 1-α pathway,110,111 and ultimately improve cognitive dysfunction in a high-fat diet–induced metabolically imbalanced brain66 (Figure 3A78). Some researchers have suggested that a KD reduces the activation of mTORC1 in the hippocampus by attenuating the phosphorylation of ribosomal protein S6.112 The activation of SIRT1 by ketone bodies in neurons leads to the reduction of mTOR expression and reduces the activation of mTORC1 complex in neurons.103
To sum up, a KD could ameliorate cognitive decline in diabetes-induced dementia by activating SIRT genes, given that diabetes induces learning dysfunction and memory loss.63
Ketone bodies attenuate insulin resistance and oxidative stress in diabetes-induced dementia
In a recent study, researchers demonstrated that brain insulin resistance could damage hippocampal synaptic plasticity and decrease cognitive decline.113 Insulin plays complex roles in the CNS, including feeding control, neurogenesis, neuronal survival, brain aging, and memory function.114,115 Insulin receptors are expressed in memory function–related brain regions including the cortex and hippocampus.116
Insulin resistance is the impairment of insulin action and is found in diverse neurodegenerative diseases such as AD and Parkinson’s disease and contributes to abnormal neural function, synaptic dysfunction, and cell death.34,117,118 Many researchers have highlighted the importance of insulin resistance, because insulin resistance leads to metabolic syndromes such as diabetes and also triggers neurodegenerative diseases such as dementia.119 Brain insulin resistance aggravated toxic Aβ production and τ hyperphosphorylation in several studies.120–123 Some authors reported that insulin administration could improve memory deficits in normal and AD mouse models.124,125
Ketogenesis is inhibited by insulin and stimulated by insulin deficiency.126 Low levels of ketone bodies are linked to obesity and insulin-resistance status.127,128 Some researchers demonstrated that ketone bodies could improve insulin sensitivity and so attenuate insulin resistance.127–129 However, other in vitro studies showed that prolonged exposure to ketone bodies altered insulin action, and thus ketone bodies could play a role in the development of insulin resistance.130,131
Clinically, the KD has already been used to treat insulin resistance in patients with type 2 diabetes.132 Given that improvement of brain insulin resistance enhances memory function in the diabetic brain,133 the modulation of ketone bodies may a good option for the treatment of diabetes-induced dementia by increasing brain insulin sensitivity. In addition, results of some studies suggested a KD reduces the generation of ROS79,134 against oxidative stress and attenuates DNA methylation135 involving antioxidant responses. Ketone bodies cross the blood-brain barrier by binding monocarboxylic acid transporters and enter neurons to reduce oxidative stress.134,136 In 1 study, a KD decreased the secretion of proapoptotic mediators in an animal model of neurodegenerative disease.137 Furthermore, previous studies have provided significant evidence that ketone bodies have neuroprotective effects against oxidative stress and metabolic-imbalance stress in brain cells.134,138
Collectively, ketone bodies could protect neuronal damage by inhibiting DNA breakdown, the apoptotic signal pathway, and the production of ROS in the brain of patients with diabetes, suggesting that diabetes-induced dementia is associated with neuronal damage, neuroinflammation, and excessive ROS production under oxidative stress conditions.139,140
Ketone bodies improve synaptic dysfunction and the imbalance of secretion of neurotransmitters in diabetes-induced dementia
Diabetes-related conditions such as hyperglycemia lead to a reduction in neurotransmitters, including GABA and glutamate, in the brain of animals with diabetes.141 The abnormal reduction of cholinergic transmission was observed in STZ-induced diabetes in animal brain hippocampus in 1 study142 as was a decrease in N-methyl d-aspartate receptors143; subsequently, the reduction of N-methyl d-aspartate receptors results in the decrease of long-term potentiation and postsynaptic density proteins.143
Dopamine is associated with cognition, feeding behavior, and emotion,144 and dopamine receptors are decreased in the type 2 diabetes brain.145,146 Serotonin, known as the 5-HT neurotransmitter, is associated with feeding behavior, sleep, and learning, and could regulate the secretion of insulin, neuronal cell regeneration, and synaptic plasticity.147,148 In the brains of patients with type 2 diabetes and obesity, the activity of 5-HT signaling is commonly observed and triggers insulin resistance.149 Glucagon-like peptide 1, a hormone associated with glucose metabolism, improves neuronal cell survival, synaptic plasticity, and neurogenesis, and inhibits insulin resistance and neuroinflammation in the brain under oxidative stress.150–154
The KD invokes acidosis and ultimately contributes to changes in neurotransmitter receptors and ion channel opening.155 Previous research has shown that a KD could increase the levels of the inhibitory neurotransmitter GABA156 and the excitatory neurotransmitter glutamate157 (Figure 1B78 Marosi et al. 2016). In addition, a KD could result in a change in the level of monoamine neurotransmitters such as serotonin and dopamine,158 which are related to depression and anxiety symptoms.159 β-Hydroxybutyrate, a ketone body, could increase the level of brain-derived neurotrophic factor, which is involved in neuronal cell survival and antiapoptosis pathways160 (Figures 214,41 and 3B78). Furthermore, a KD could boost the level of ATP and the activation of synaptic receptors.161
On the basis of this evidence, ketone bodies could regulate the secretion of neurotransmitters such as GABA, glutamate, serotonin, dopamine, and brain-derived neurotrophic factor involved in neurologic pathology.
Thus, a KD may be beneficial for improving the progress of neurologic pathology in diabetes-induced dementia through the modulation of neurotransmitter production.
Ketone bodies ameliorate mitochondrial dysfunction in diabetes-induced dementia
The mitochondrion is a central organelle for neurotransmission, synaptic plasticity, and energy homeostasis of neurons and glia.162 Mitochondria take charge of the maintenance of cellular Ca2+ homeostasis, a required response in normal neuronal functioning.163
Excessive Ca2+ uptake into mitochondria occurs with the increase of ROS production and suppresses the synthesis of ATP, boosting the release of cytochrome c and increasing mitochondrial membrane potential.164 Inappropriate mitochondrial permeability transition results in mitochondrial swelling and apoptosis of related proteins in mitochondria.165 Mitochondrial dysfunction leads to impaired energy homeostasis and is highly correlated with the onset of neuronal degeneration.166,167 Poor mitochondrial function is linked to neuronal death and neurodegenerative disease onset.168 Metabolic imbalance conditions such as diabetes aggravate glucose use and lead to mitochondrial dysfunction, ultimately contributing to neuropathologic problems.167,169 Suppression of energy production caused by mitochondrial dysfunction also damages insulin action in cells.170 Cells of the CNS require a high amount of ATP for neuronal transmission of electrical impulses; therefore, impaired mitochondrial function results in neurodegeneration and loss.169 Previous studies have demonstrated that diabetes conditions lead to abnormal mitochondrial structure and elevated levels of oxidative phosphorylation, which are associated with high production of ROS.171–173 Authors of other studies mentioned that mitochondrial dysfunction could result in a high level of interaction between Aβ and Aβ-binding dehydrogenase,174,175 ultimately leading to cognitive impairment.176 Impaired mitochondrial function was found both in diabetes and dementia in 1 study, and it also acted as a bridge regulator between diabetic pathology and neuropathology.177 Considering these findings, diabetic conditions appear to lead to mitochondrial dysfunction in CNS cells and subsequently influence neuropathology, such as memory loss, in dementia.
A KD containing a high amount of fat and a low amount of carbohydrates has benefit in the treatment of neurologic diseases, including epilepsy.178 β-Hydroxybutyrate can pass the blood-brain barrier through specific monocarboxylate transporters and move from the systemic circulation into the brain.179,180 The KD and β-hydroxybutyrate boost mitochondrial density and mitochondrial function in neuronal processes in the hippocampus, the cognition-related region of the brain17 (Figure 3A78). UCPs regulate the production of ROS and mitochondrial membrane potential in mitochondria, and some isoforms have a neuroprotective effect in CNS diseases.181,182 The KD could increase the level of UCPs and ultimately attenuate secretion of ROS in hippocampal neurons79 (Figure 3A78).
Furthermore, a KD regulates the deacetylation of various mitochondrial proteins, increases mitochondrial mass, and promotes mitochondrial function76,91,183 (Figure 3A78). Mitochondrial biogenesis is affected by mitochondrial SIRTs111 and PGC-1α.110 A KD could ameliorate mitochondrial function under normal conditions as well as metabolically imbalanced conditions, such as increased free fatty acid levels and insulin resistance.184–186
In summary, on the basis of data from previous studies, a KD could enhance mitochondrial function in CNS cells, including the improvement of mitochondrial mass, density, and biogenesis, and the appropriate regulation of mitochondrial membrane potentiation. Thus, a KD may lead to improvements in cognitive function in diabetes-induced dementia.
Ketone bodies attenuate amyloid β aggregation in diabetes-induced dementia
Studies have focused on the relationship between the high risk of AD and diabetes pathologies.119,187 Findings from 1 study suggested that diabetes leads to neuropathologic problems including severe Aβ toxicity, as well as brain insulin resistance, mitochondrial dysfunction, and neuroinflammation.188 In the brain, Aβ oligomers promote insulin resistance in hippocampal neurons in the diabetic brain, ultimately leading to memory loss.189 Another study demonstrated that metabolic imbalance exacerbated AD pathogenesis, including memory deficit and neuroinflammation.190 Administration of antidiabetic drugs resulted in the improvement of memory function in patients with AD and in a mouse model of AD.191,192 In another study, high-fat diet–induced diabetic conditions increased Aβ deposition in APP/PS1xdb/db mice, compared with APP/PS1 knockout mice.193 β-Hydroxybutyrate attenuates Aβ toxicity in SH-SY5Y neuronal cells through the inhibition of histone deacetylase.194 In 1 study, researchers suggested that β-hydroxybutyrate could be considered an endogenous histone deacetylase inhibitor.195 Researchers identified that ketone body administration could reduce toxic Aβ deposition and improve memory function in an APP swe/PS1dE9 transgenic AD mouse model.20 In addition, β-hydroxybutyrate could upregulate the expression of tropomyosin receptor kinase A, affecting neuroprotective pathways and cholinergic neuronal function.194,196 In some studies, authors reported that a KD inhibited the accumulation and total level of Aβ197 and also inhibited the entrance of Aβ from the peripheral circulation system into the brain, resulting in improved cognitive function20 (Figure 3A78).
Considering these findings, ketone bodies could attenuate toxic Aβ deposition and reduce the entrance of Aβ into the brain, ultimately contributing to the improvement of cognitive function.
CONCLUSIONS
Herein, we summarized recently reported evidence regarding the neuronal protective effects of ketone bodies in diabetes-induced dementia. Positive roles of ketone bodies include activating SIRTs in neurons associated with neuronal cell survival signaling, promoting insulin sensitivity, and protecting neuronal cells against oxidative stress damage. Moreover, ketone bodies ameliorate normal neurotransmitter secretion and enhance synaptic function. Ketone bodies also promote mitochondrial biogenesis and membrane potential. Furthermore, ketone bodies suppress toxic Aβ deposition in the brain. Given these findings, ketone bodies could improve neuropathogenesis such as memory decline in diabetes-induced dementia.
Taken together, this review on the neuroprotective properties of ketone bodies at low levels may provide useful information for establishing the appropriate clinical approach for use of a KD for diabetes-induced dementia. More studies are needed to elucidate the exact mechanisms of the neuroprotective role of ketone bodies at low levels, which reverse to neurotoxic at high levels, and to understand the roles and specific modulation pathway of ketone bodies for the treatment of diabetes-induced dementia.
Acknowledgments
Funding. This research was supported by a grant from the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (grant NRF-2019R1F1A1054111 awarded to J.S., and grant NRF-2019R1I1A3A01058861 awarded to O.Y.K.).
Author contributions. All authors contributed to the writing of this manuscript. J.Y.C. provided the figures, and J.S. finalized the revised manuscript. All authors read and approved the final manuscript.
Declaration of interest. The authors have no relevant interests to declare.
References
- 1. Kahn SE, Cooper ME, Del Prato S.. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet. 2014;383:1068–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koekkoek PS, Kappelle LJ, van den Berg E, et al. Cognitive function in patients with diabetes mellitus: guidance for daily care. Lancet Neurol. 2015;14:329–340. [DOI] [PubMed] [Google Scholar]
- 3. Zhang J, Chen C, Hua S, et al. An updated meta-analysis of cohort studies: diabetes and risk of Alzheimer's disease. Diabetes Res Clin Pract. 2017;124:41–47. [DOI] [PubMed] [Google Scholar]
- 4. Biessels GJ, Staekenborg S, Brunner E, et al. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74. [DOI] [PubMed] [Google Scholar]
- 5. Crane PK, Walker R, Larson EB.. Glucose levels and risk of dementia. N Engl J Med. 2013;1863–1864. [DOI] [PubMed] [Google Scholar]
- 6. Rawlings AM, Sharrett AR, Mosley TH, et al. Glucose peaks and the risk of dementia and 20-year cognitive decline. Diabetes Care. 2017;40:879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zuloaga KL, Johnson LA, Roese NE, et al. High fat diet-induced diabetes in mice exacerbates cognitive deficit due to chronic hypoperfusion. J Cereb Blood Flow Metab. 2016;36:1257–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Biessels GJ, Reijmer YD.. Brain changes underlying cognitive dysfunction in diabetes: what can we learn from MRI? Diabetes. Jul 2014;63:2244–2252. [DOI] [PubMed] [Google Scholar]
- 9. Moran C, Beare R, Phan T, et al. Neuroimaging and its relevance to understanding pathways linking diabetes and cognitive dysfunction. J Alzheimers Dis. 2017;59:405–419. [DOI] [PubMed] [Google Scholar]
- 10. Cheng J, Korte N, Nortley R, et al. Targeting pericytes for therapeutic approaches to neurological disorders. Acta Neuropathol. 2018;136:507–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lecrux C, Bourourou M, Hamel E.. How reliable is cerebral blood flow to map changes in neuronal activity? Auton Neurosci. 2019;217:71–79. [DOI] [PubMed] [Google Scholar]
- 12. Aneja P, Dziak R, Cai GQ, et al. Identification of an acetoacetyl coenzyme A synthetase-dependent pathway for utilization of L-(+)-3-hydroxybutyrate in Sinorhizobium meliloti. J Bacteriol. 2002;184:1571–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krishnakumar AM, Sliwa D, Endrizzi JA, et al. Getting a handle on the role of coenzyme M in alkene metabolism. Microbiol Mol Biol Rev. 2008;72:445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Puchalska P, Crawford PA.. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 2017;25:262–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cahill GF Jr. Fuel metabolism in starvation. Annu Rev Nutr. 2006;26:1–22. [DOI] [PubMed] [Google Scholar]
- 16. Ziegler DR, Ribeiro LC, Hagenn M, et al. Ketogenic diet increases glutathione peroxidase activity in rat hippocampus. Neurochem Res. 2003;28:1793–1797. [DOI] [PubMed] [Google Scholar]
- 17. Stafstrom CE, Rho JM.. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol. 2012;3:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kessler SK, Neal EG, Camfield CS, Kossoff EH.. Dietary therapies for epilepsy: future research. Epilepsy Behav. 2011;22:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wheless JW. History of the ketogenic diet. Epilepsia 2008;49(suppl 8):3–5. [DOI] [PubMed] [Google Scholar]
- 20. Yin JX, Maalouf M, Han P, et al. Ketones block amyloid entry and improve cognition in an Alzheimer's model. Neurobiol Aging. 2016;39:25–37. [DOI] [PubMed] [Google Scholar]
- 21. Rahman M, Muhammad S, Khan MA, et al. The β-hydroxybutyrate receptor HCA2 activates a neuroprotective subset of macrophages. Nat Commun. 2014;5:3944. [DOI] [PubMed] [Google Scholar]
- 22. D'Agostino DP, Pilla R, Held HE, et al. Therapeutic ketosis with ketone ester delays central nervous system oxygen toxicity seizures in rats. Am J Physiol Regul Integr Comp Physiol. 2013;304:R829–36. [DOI] [PubMed] [Google Scholar]
- 23. Yum MS, Lee M, Woo DC, et al. β-Hydroxybutyrate attenuates NMDA-induced spasms in rats with evidence of neuronal stabilization on MR spectroscopy. Epilepsy Res. 2015;117:125–132. [DOI] [PubMed] [Google Scholar]
- 24. Achanta LB, Rae CD.. β-Hydroxybutyrate in the brain: one molecule, multiple mechanisms. Neurochem Res. 2017;42:35–49. [DOI] [PubMed] [Google Scholar]
- 25. McGuire LC, Cruickshank AM, Munro PT.. Alcoholic ketoacidosis. Emerg Med J. 2006;23:417–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tups A, Benzler J, Sergi D, et al. Central regulation of glucose homeostasis. Compr Physiol. 2017;7:741–764. [DOI] [PubMed] [Google Scholar]
- 27. Oyarzabal A, Marin-Valencia I.. Synaptic energy metabolism and neuronal excitability, in sickness and health. J Inherit Metab Dis. 2019;42:220–236. [DOI] [PubMed] [Google Scholar]
- 28. Magistretti PJ, Allaman I.. Lactate in the brain: from metabolic end-product to signalling molecule. Nat Rev Neurosci. 2018;19:235–249. [DOI] [PubMed] [Google Scholar]
- 29. Gundersen V, Storm-Mathisen J, Bergersen LH.. Neuroglial transmission. Physiol Rev. 2015;95:695–726. [DOI] [PubMed] [Google Scholar]
- 30. Bordone MP, Salman MM, Titus HE, et al. The energetic brain - a review from students to students. J Neurochem. 2019;151:139–165. [DOI] [PubMed] [Google Scholar]
- 31. Dienel GA. Brain glucose metabolism: integration of energetics with function. Physiol Rev. 2019;99:949–1045. [DOI] [PubMed] [Google Scholar]
- 32. Ashrafi G, Wu Z, Farrell RJ, et al. GLUT4 mobilization supports energetic demands of active synapses. Neuron. 2017;93:606–615.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nave KA, Werner HB.. Myelination of the nervous system: mechanisms and functions. Annu Rev Cell Dev Biol. 2014;30:503–533. [DOI] [PubMed] [Google Scholar]
- 34. Duarte AI, Santos MS, Oliveira CR, et al. Brain insulin signalling, glucose metabolism and females' reproductive aging: a dangerous triad in Alzheimer's disease. Neuropharmacology. 2018;136:223–242. [DOI] [PubMed] [Google Scholar]
- 35. Craft S, Claxton A, Baker LD, et al. Effects of regular and long-acting insulin on cognition and Alzheimer's disease biomarkers: a pilot clinical trial. J Alzheimers Dis. 2017;57:1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kann O. The interneuron energy hypothesis: implications for brain disease. Neurobiol Dis. 2016;90:75–85. [DOI] [PubMed] [Google Scholar]
- 37. Pearson-Leary J, Jahagirdar V, Sage J, et al. Insulin modulates hippocampally-mediated spatial working memory via glucose transporter-4. Behav Brain Res. 2018;338:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Frere S, Slutsky I.. Alzheimer's disease: from firing instability to homeostasis network collapse. Neuron. 2018;97:32–58. [DOI] [PubMed] [Google Scholar]
- 39. Hartman AL, Vining EP.. Clinical aspects of the ketogenic diet. Epilepsia. 2007;48:31–42. [DOI] [PubMed] [Google Scholar]
- 40. Ruskin DN, Murphy MI, Slade SL, et al. Ketogenic diet improves behaviors in a maternal immune activation model of autism spectrum disorder. PLoS One. 2017;12:e0171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jensen NJ, Wodschow HZ, Nilsson M, et al. Effects of ketone bodies on brain metabolism and function in neurodegenerative diseases. IJMS. 2020;21:8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Veech RL. The therapeutic implications of ketone bodies: The effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fatty Acids. 2004;70:309–319. [DOI] [PubMed] [Google Scholar]
- 43. Yudkoff M, Daikhin Y, Nissim I, et al. Brain amino acid metabolism and ketosis. J Neurosci Res. 2001;66:272–281. [DOI] [PubMed] [Google Scholar]
- 44. Cunnane SC, Courchesne-Loyer A, St-Pierre V, et al. Can ketones compensate for deteriorating brain glucose uptake during aging? Implications for the risk and treatment of Alzheimer's disease. Ann N Y Acad Sci. 2016;1367:12–20. [DOI] [PubMed] [Google Scholar]
- 45. Yang H, Shan W, Zhu F, et al. Ketone bodies in neurological diseases: focus on neuroprotection and underlying mechanisms. Front Neurol. 2019;10:585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hernandez AR, Hernandez CM, Campos K, et al. A ketogenic diet improves cognition and has biochemical effects in prefrontal cortex that are dissociable from hippocampus. Front Aging Neurosci. 2018;10:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Choi YJ, Jeon SM, Shin S.. Impact of a ketogenic diet on metabolic parameters in patients with obesity or overweight and with or without type 2 diabetes: a meta-analysis of randomized controlled trials. Nutrients. 2020;12:2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cotter DG, Schugar RC, Crawford PA.. Ketone body metabolism and cardiovascular disease. Am J Physiol Heart Circ Physiol. 2013;304:H1060–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cunnane SC, Courchesne-Loyer A, Vandenberghe C, et al. Can ketones help rescue brain fuel supply in later life? Implications for cognitive health during aging and the treatment of Alzheimer's disease. Front Mol Neurosci 2016;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ma D, Wang AC, Parikh I, et al. Ketogenic diet enhances neurovascular function with altered gut microbiome in young healthy mice. Sci Rep. 2018;8:6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Croteau E, Castellano CA, Fortier M, et al. A cross-sectional comparison of brain glucose and ketone metabolism in cognitively healthy older adults, mild cognitive impairment and early Alzheimer's disease. Exp Gerontol. 2018;107:18–26. [DOI] [PubMed] [Google Scholar]
- 52. Courchesne-Loyer A, Croteau E, Castellano CA, et al. Inverse relationship between brain glucose and ketone metabolism in adults during short-term moderate dietary ketosis: a dual tracer quantitative positron emission tomography study. J Cereb Blood Flow Metab. 2017;37:2485–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Eckles-Smith K, Clayton D, Bickford P, et al. Caloric restriction prevents age-related deficits in LTP and in NMDA receptor expression. Brain Res Mol Brain Res. 2000;78:154–162. [DOI] [PubMed] [Google Scholar]
- 54. Pitsikas N, Carli M, Fidecka S, et al. Effect of life-long hypocaloric diet on age-related changes in motor and cognitive behavior in a rat population. Neurobiol Aging. 1990;11:417–423. [DOI] [PubMed] [Google Scholar]
- 55. Dutta S, Sengupta P.. Men and mice: relating their ages. Life Sci. 2016;152:244–248. [DOI] [PubMed] [Google Scholar]
- 56. Pawlosky RJ, Kemper MF, Kashiwaya Y, et al. Effects of a dietary ketone ester on hippocampal glycolytic and tricarboxylic acid cycle intermediates and amino acids in a 3xTgAD mouse model of Alzheimer's disease. J Neurochem. 2017;141:195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Krikorian R, Shidler MD, Dangelo K, et al. Dietary ketosis enhances memory in mild cognitive impairment. Neurobiol Aging. 2012;33:425.e19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Neth BJ, Mintz A, Whitlow C, et al. Modified ketogenic diet is associated with improved cerebrospinal fluid biomarker profile, cerebral perfusion, and cerebral ketone body uptake in older adults at risk for Alzheimer's disease: a pilot study. Neurobiol Aging. 2020;86:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ota M, Matsuo J, Ishida I, et al. Effects of a medium-chain triglyceride-based ketogenic formula on cognitive function in patients with mild-to-moderate Alzheimer's disease. Neurosci Lett. 2019;690:232–236. [DOI] [PubMed] [Google Scholar]
- 60. Phillips MCL, Murtagh DKJ, Gilbertson LJ, et al. Low-fat versus ketogenic diet in Parkinson's disease: a pilot randomized controlled trial. Mov Disord. 2018;33:1306–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Woolf EC, Syed N, Scheck AC.. Tumor metabolism, the ketogenic diet and β-hydroxybutyrate: novel approaches to adjuvant brain tumor therapy. Front Mol Neurosci. 2016;9:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gibson CL, Murphy AN, Murphy SP.. Stroke outcome in the ketogenic state–a systematic review of the animal data. J Neurochem. 2012;123(suppl 2):52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Qi D, Wang A, Chen Y, et al. Default mode network connectivity and related white matter disruption in type 2 diabetes mellitus patients concurrent with amnestic mild cognitive impairment. Curr Alzheimer Res. 2017;14:1238–1246. [DOI] [PubMed] [Google Scholar]
- 64. Chung CC, Pimentel D, Jor'dan AJ, et al. Inflammation-associated declines in cerebral vasoreactivity and cognition in type 2 diabetes. Neurology. 2015;85:450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mehla J, Chauhan BC, Chauhan NB.. Experimental induction of type 2 diabetes in aging-accelerated mice triggered Alzheimer-like pathology and memory deficits. J Alzheimers Dis. 2014;39:145–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Palomera-Avalos V, Grinan-Ferre C, Izquierdo V, et al. Metabolic stress induces cognitive disturbances and inflammation in aged mice: protective role of resveratrol. Rejuvenation Res. 2017;20:202–217. [DOI] [PubMed] [Google Scholar]
- 67. Roberts RO, Knopman DS, Geda YE, et al. Association of diabetes with amnestic and nonamnestic mild cognitive impairment. Alzheimers Dement. 2014;10:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kitada M, Ogura Y, Monno I, et al. Sirtuins and type 2 diabetes: role in inflammation, oxidative stress, and mitochondrial function. Front Endocrinol (Lausanne). 2019;10:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Giovannini L, Bianchi S.. Role of nutraceutical SIRT1 modulators in AMPK and mTOR pathway: Evidence of a synergistic effect. Nutrition. 2017;34:82–96. [DOI] [PubMed] [Google Scholar]
- 70. Zullo A, Simone E, Grimaldi M, et al. Sirtuins as mediator of the anti-ageing effects of calorie restriction in skeletal and cardiac muscle. IJMS. 2018;19:928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Singh CK, Chhabra G, Ndiaye MA, et al. The role of sirtuins in antioxidant and redox signaling. Antioxid Redox Signal. 2018;28:643–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Singh P, Hanson PS, Morris CM.. SIRT1 ameliorates oxidative stress induced neural cell death and is down-regulated in Parkinson's disease. BMC Neurosci. 2017;18:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Michan S, Li Y, Chou MM, et al. SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci. 2010;30:9695–9707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wang D, Li Z, Zhang Y, et al. Targeting of microRNA-199a-5p protects against pilocarpine-induced status epilepticus and seizure damage via SIRT1-p53 cascade. Epilepsia. 2016;57:706–716. [DOI] [PubMed] [Google Scholar]
- 75. Vazquez BN, Thackray JK, Simonet NG, et al. SIRT7 promotes genome integrity and modulates non-homologous end joining DNA repair. EMBO J. 2016;35:1488–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rardin MJ, He W, Nishida Y, et al. SIRT5 regulates the mitochondrial lysine succinylome and metabolic networks. Cell Metab. 2013;18:920–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kwon S, Seok S, Yau P, et al. Obesity and aging diminish sirtuin 1 (SIRT1)-mediated deacetylation of SIRT3, leading to hyperacetylation and decreased activity and stability of SIRT3. J Biol Chem. 2017;292:17312–17323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Marosi K, Kim SW, Moehl K, et al. 3-Hydroxybutyrate regulates energy metabolism and induces BDNF expression in cerebral cortical neurons. J Neurochem. 2016;139:769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sullivan PG, Rippy NA, Dorenbos K, et al. The ketogenic diet increases mitochondrial uncoupling protein levels and activity. Ann Neurol. 2004;55:576–580. [DOI] [PubMed] [Google Scholar]
- 80. Fisler JS, Warden CH.. Uncoupling proteins, dietary fat and the metabolic syndrome. Nutr Metab (Lond). 2006;3:3:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Morris G, Puri BK, Carvalho A, et al. Induced ketosis as a treatment for neuroprogressive disorders: food for thought? Int J Neuropsychopharmacol. 2020;23:366–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bordone L, Motta MC, Picard F, et al. Correction: sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 2015;13:e1002346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. McCarty MF, DiNicolantonio JJ, O'Keefe JH.. Ketosis may promote brain macroautophagy by activating Sirt1 and hypoxia-inducible factor-1. Med Hypotheses. 2015;85:631–639. [DOI] [PubMed] [Google Scholar]
- 84. Hou Y, Lautrup S, Cordonnier S, et al. NAD(+) supplementation normalizes key Alzheimer's features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. Proc Natl Acad Sci USA. 2018;115:E1876–E1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kussmaul L, Hirst J.. The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc Natl Acad Sci USA. 2006;103:7607–7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bai P, Canto C, Oudart H, et al. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 2011;13:461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Canto C, Sauve AA, Bai P.. Crosstalk between poly(ADP-ribose) polymerase and sirtuin enzymes. Mol Aspects Med. 2013;34:1168–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yin J, Li S, Nielsen M, et al. Sirtuin 3 attenuates amyloid-β induced neuronal hypometabolism. Aging (Albany NY). 2018;10:2874–2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Brenmoehl J, Hoeflich A.. Dual control of mitochondrial biogenesis by sirtuin 1 and sirtuin 3. Mitochondrion. 2013;13:755–761. [DOI] [PubMed] [Google Scholar]
- 90. Zhao Z, Lange DJ, Voustianiouk A, et al. A ketogenic diet as a potential novel therapeutic intervention in amyotrophic lateral sclerosis. BMC Neurosci. 2006;7:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yin J, Han P, Tang Z, et al. Sirtuin 3 mediates neuroprotection of ketones against ischemic stroke. J Cereb Blood Flow Metab. 2015;35:1783–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hasan-Olive MM, Lauritzen KH, Ali M, et al. A ketogenic diet improves mitochondrial biogenesis and bioenergetics via the PGC1α-SIRT3-UCP2 axis. Neurochem Res. 2019;44:22–37. [DOI] [PubMed] [Google Scholar]
- 93. Shimazu T, Hirschey MD, Hua L, et al. SIRT3 deacetylates mitochondrial 3-hydroxy-3-methylglutaryl CoA synthase 2 and regulates ketone body production. Cell Metab. 2010;12:654–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Chen YY, Zhang L, Shi DL, et al. Resveratrol attenuates subacute systemic inflammation-induced spatial memory impairment via inhibition of astrocyte activation and enhancement of synaptophysin expression in the hippocampus. Ann Clin Lab Sci. 2017;47:17–24. [PubMed] [Google Scholar]
- 95. Cao Y, Yan Z, Zhou T, et al. SIRT1 regulates cognitive performance and ability of learning and memory in diabetic and nondiabetic models. J Diabetes Res. 2017;2017:7121827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Luo J, Nikolaev AY, Imai S, et al. Negative control of p53 by Sir2α promotes cell survival under stress. Cell. 2001;107:137–148. [DOI] [PubMed] [Google Scholar]
- 97. Bayod S, Guzman-Brambila C, Sanchez-Roige S, et al. Voluntary exercise promotes beneficial anti-aging mechanisms in SAMP8 female brain. J Mol Neurosci. 2015;55:525–532. [DOI] [PubMed] [Google Scholar]
- 98. Porquet D, Casadesus G, Bayod S, et al. Dietary resveratrol prevents Alzheimer's markers and increases life span in SAMP8. Age (Dordr). 2013;35:1851–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Salminen A, Huuskonen J, Ojala J, et al. Activation of innate immunity system during aging: NF-κB signaling is the molecular culprit of inflamm-aging. Ageing Res Rev. 2008;7:83–105. [DOI] [PubMed] [Google Scholar]
- 100. Lan F, Cacicedo JM, Ruderman N, et al. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem. 2008;283:27628–27635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Cohen HY, Miller C, Bitterman KJ, et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. [DOI] [PubMed] [Google Scholar]
- 102. Rodgers JT, Lerin C, Haas W, et al. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature. 2005;434:113–118. [DOI] [PubMed] [Google Scholar]
- 103. Guo W, Qian L, Zhang J, et al. Sirt1 overexpression in neurons promotes neurite outgrowth and cell survival through inhibition of the mTOR signaling. J Neurosci Res. 2011;89:1723–1736. [DOI] [PubMed] [Google Scholar]
- 104. Du LL, Xie JZ, Cheng XS, et al. Activation of sirtuin 1 attenuates cerebral ventricular streptozotocin-induced tau hyperphosphorylation and cognitive injuries in rat hippocampi. Age (Dordr). 2014;36:613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Tajes M, Gutierrez-Cuesta J, Folch J, et al. Neuroprotective role of intermittent fasting in senescence-accelerated mice P8 (SAMP8). Exp Gerontol. 2010;45:702–710. [DOI] [PubMed] [Google Scholar]
- 106. Villemagne VL, Dore V, Bourgeat P, et al. Aβ-amyloid and tau imaging in dementia. Semin Nucl Med. 2017;47:75–88. [DOI] [PubMed] [Google Scholar]
- 107. Wang RH, Kim HS, Xiao C, Xu X, et al. Hepatic Sirt1 deficiency in mice impairs mTorc2/Akt signaling and results in hyperglycemia, oxidative damage, and insulin resistance. J Clin Invest. 2011;121:4477–4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zhou B, Li C, Qi W, et al. Downregulation of miR-181a upregulates sirtuin-1 (SIRT1) and improves hepatic insulin sensitivity. Diabetologia. 2012;55:2032–2043. [DOI] [PubMed] [Google Scholar]
- 109. Liu Y, Yao Z, Zhang L, et al. Insulin induces neurite outgrowth via SIRT1 in SH-SY5Y cells. Neuroscience. 2013;238:371–380. [DOI] [PubMed] [Google Scholar]
- 110. Ansari A, Rahman MS, Saha SK, et al. Function of the SIRT3 mitochondrial deacetylase in cellular physiology, cancer, and neurodegenerative disease. Aging Cell. 2017;16:4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Su J, Liu J, Yan XY, et al. Cytoprotective effect of the UCP2-SIRT3 signaling pathway by decreasing mitochondrial oxidative stress on cerebral ischemia-reperfusion injury. IJMS. 2017;18:1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. McDaniel SS, Rensing NR, Thio LL, et al. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia. 2011;52:e7–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Spinelli M, Fusco S, Grassi C.. Brain insulin resistance and hippocampal plasticity: mechanisms and biomarkers of cognitive decline. Front Neurosci. 2019;13:788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Zhao WQ, Alkon DL.. Role of insulin and insulin receptor in learning and memory. Mol Cell Endocrinol. 2001;177:125–134. [DOI] [PubMed] [Google Scholar]
- 115. Kullmann S, Heni M, Hallschmid M, et al. Brain insulin resistance at the crossroads of metabolic and cognitive disorders in humans. Physiol Rev. 2016;96:1169–1209. [DOI] [PubMed] [Google Scholar]
- 116. Nistico R, Cavallucci V, Piccinin S, et al. Insulin receptor β-subunit haploinsufficiency impairs hippocampal late-phase LTP and recognition memory. Neuromolecular Med. 2012;14:262–269. [DOI] [PubMed] [Google Scholar]
- 117. Griffith CM, Eid T, Rose GM, et al. Evidence for altered insulin receptor signaling in Alzheimer's disease. Neuropharmacology. 2018;136:202–215. [DOI] [PubMed] [Google Scholar]
- 118. Yang L, Wang H, Liu L, et al. The role of insulin/IGF-1/PI3K/Akt/GSK3β signaling in Parkinson's disease dementia. Front Neurosci. 2018;12:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Arnold SE, Arvanitakis Z, Macauley-Rambach SL, et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol. 2018;14:168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Son SM, Song H, Byun J, et al. Accumulation of autophagosomes contributes to enhanced amyloidogenic APP processing under insulin-resistant conditions. Autophagy. 2012;8:1842–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Devi L, Alldred MJ, Ginsberg SD, et al. Mechanisms underlying insulin deficiency-induced acceleration of β-amyloidosis in a mouse model of Alzheimer's disease. PLoS One. 2012;7:e32792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kim B, Backus C, Oh S, et al. Increased tau phosphorylation and cleavage in mouse models of type 1 and type 2 diabetes. Endocrinology. 2009;150:5294–5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Puig KL, Floden AM, Adhikari R, et al. Amyloid precursor protein and proinflammatory changes are regulated in brain and adipose tissue in a murine model of high fat diet-induced obesity. PLoS One. 2012;7:e30378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Marks DR, Tucker K, Cavallin MA, et al. Awake intranasal insulin delivery modifies protein complexes and alters memory, anxiety, and olfactory behaviors. J Neurosci. 2009;29:6734–6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Biessels GJ, Reagan LP.. Hippocampal insulin resistance and cognitive dysfunction. Nat Rev Neurosci. 2015;16:660–671. [DOI] [PubMed] [Google Scholar]
- 126. Fukao T, Lopaschuk GD, Mitchell GA.. Pathways and control of ketone body metabolism: on the fringe of lipid biochemistry. Prostaglandins Leukot Essent Fatty Acids. 2004;70:243–251. [DOI] [PubMed] [Google Scholar]
- 127. Bergman BC, Cornier MA, Horton TJ, et al. Effects of fasting on insulin action and glucose kinetics in lean and obese men and women. Am J Physiol Endocrinol Metab. 2007;293:E1103–E1111. [DOI] [PubMed] [Google Scholar]
- 128. Soeters MR, Sauerwein HP, Faas L, et al. Effects of insulin on ketogenesis following fasting in lean and obese men. Obesity (Silver Spring).2009;17:1326–1331. [DOI] [PubMed] [Google Scholar]
- 129. Yamada T, Zhang SJ, Westerblad H, et al. β-Hydroxybutyrate inhibits insulin-mediated glucose transport in mouse oxidative muscle. Am J Physiol Endocrinol Metab. 2010;299:E364–E373. [DOI] [PubMed] [Google Scholar]
- 130. Tardif A, Julien N, Pelletier A, et al. Chronic exposure to β-hydroxybutyrate impairs insulin action in primary cultures of adult cardiomyocytes. Am J Physiol Endocrinol Metab. 2001;281:E1205–E1212. [DOI] [PubMed] [Google Scholar]
- 131. Wurtz P, Makinen VP, Soininen P, et al. Metabolic signatures of insulin resistance in 7,098 young adults. Diabetes 2012;61:1372–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Hussain TA, Mathew TC, Dashti AA, Asfar S, et al. Effect of low-calorie versus low-carbohydrate ketogenic diet in type 2 diabetes. Nutrition. 2012;28:1016–1021. [DOI] [PubMed] [Google Scholar]
- 133. Kellar D, Craft S.. Brain insulin resistance in Alzheimer's disease and related disorders: mechanisms and therapeutic approaches. Lancet Neurol. 2020;19:758–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Maalouf M, Rho JM, Mattson MP.. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev. 2009;59:293–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Lusardi TA, Akula KK, Coffman SQ, et al. Ketogenic diet prevents epileptogenesis and disease progression in adult mice and rats. Neuropharmacology. 2015;99:500–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Morris AA. Cerebral ketone body metabolism. J Inherit Metab Dis. 2005;28:109–121. [DOI] [PubMed] [Google Scholar]
- 137. Noh HS, Kang SS, Kim DW, et al. Ketogenic diet increases calbindin-D28k in the hippocampi of male ICR mice with kainic acid seizures. Epilepsy Res. 2005;65:153–159. [DOI] [PubMed] [Google Scholar]
- 138. Paoli A, Bosco G, Camporesi EM, et al. Ketosis, ketogenic diet and food intake control: a complex relationship. Front Psychol. 2015;6:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Ahmad W, Ijaz B, Shabbiri K, et al. Oxidative toxicity in diabetes and Alzheimer's disease: mechanisms behind ROS/RNS generation. J Biomed Sci. 2017;24:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Khan MSH, Hegde V.. Obesity and diabetes mediated chronic inflammation: a potential biomarker in Alzheimer's disease. JPM. 2020;10:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. van Bussel FC, Backes WH, Hofman PA, et al. Increased GABA concentrations in type 2 diabetes mellitus are related to lower cognitive functioning. Medicine (Baltimore). 2016;95:e4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Molina J, Rodriguez-Diaz R, Fachado A, et al. Control of insulin secretion by cholinergic signaling in the human pancreatic islet. Diabetes. 2014;63:2714–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. van der Heide LP, Kamal A, Artola A, et al. Insulin modulates hippocampal activity-dependent synaptic plasticity in a N-methyl-d-aspartate receptor and phosphatidyl-inositol-3-kinase-dependent manner. J Neurochem. 2005;94:1158–1166. [DOI] [PubMed] [Google Scholar]
- 144. Undieh AS. Pharmacology of signaling induced by dopamine D(1)-like receptor activation. Pharmacol Ther. 2010;128:37–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Grunberger G. Novel therapies for the management of type 2 diabetes mellitus: part 1. Pramlintide and bromocriptine-QR. J Diabetes. 2013;5:110–117. [DOI] [PubMed] [Google Scholar]
- 146. Pijl H, Edo AM.. Modulation of monoaminergic neural circuits: potential for the treatment of type 2 diabetes mellitus. Treat Endocrinol. 2002;1:71–78. [DOI] [PubMed] [Google Scholar]
- 147. Pucadyil TJ, Kalipatnapu S, Chattopadhyay A.. Membrane organization and dynamics of the G-protein-coupled serotonin1A receptor monitored using fluorescence-based approaches. J Fluoresc. 2005;15:785–796. [DOI] [PubMed] [Google Scholar]
- 148. Gaspar P, Cases O, Maroteaux L.. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–1012. [DOI] [PubMed] [Google Scholar]
- 149. Gingrich JA, Hen R.. Dissecting the role of the serotonin system in neuropsychiatric disorders using knockout mice. Psychopharmacology (Berl). 2001;155:1–10. [DOI] [PubMed] [Google Scholar]
- 150. Adamska E, Ostrowska L, Gorska M, et al. The role of gastrointestinal hormones in the pathogenesis of obesity and type 2 diabetes. Prz Gastroenterol. 2014;9:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Ravassa S, Beaumont J, Huerta A, et al. Association of low GLP-1 with oxidative stress is related to cardiac disease and outcome in patients with type 2 diabetes mellitus: a pilot study. Free Radic Biol Med. 2015;81:1–12. [DOI] [PubMed] [Google Scholar]
- 152. Hamilton A, Patterson S, Porter D, et al. Novel GLP-1 mimetics developed to treat type 2 diabetes promote progenitor cell proliferation in the brain. J Neurosci Res. 2011;89:481–489. [DOI] [PubMed] [Google Scholar]
- 153. Salcedo I, Tweedie D, Li Y, et al. Neuroprotective and neurotrophic actions of glucagon-like peptide-1: an emerging opportunity to treat neurodegenerative and cerebrovascular disorders. Br J Pharmacol. 2012;166:1586–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Toft-Nielsen MB, Damholt MB, Madsbad S, et al. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab. 2001;86:3717–3723. [DOI] [PubMed] [Google Scholar]
- 155. Lima PA, Sampaio LP, Damasceno NR.. Neurobiochemical mechanisms of a ketogenic diet in refractory epilepsy. Clinics (Sao Paulo). 2014;69:699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Yudkoff M, Daikhin Y, Horyn O, et al. Ketosis and brain handling of glutamate, glutamine, and GABA. Epilepsia. 2008;49(suppl 8):73–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Bough KJ, Wetherington J, Hassel B, et al. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol. 2006;60:223–235. [DOI] [PubMed] [Google Scholar]
- 158. Dahlin M, Mansson JE, Amark P.. CSF levels of dopamine and serotonin, but not norepinephrine, metabolites are influenced by the ketogenic diet in children with epilepsy. Epilepsy. Res. 2012;99:132–138. [DOI] [PubMed] [Google Scholar]
- 159. Murphy P, Likhodii S, Nylen K, et al. The antidepressant properties of the ketogenic diet. Biol Psychiatry. 2004;56:981–983. [DOI] [PubMed] [Google Scholar]
- 160. Sleiman SF, Henry J, Al-Haddad R, et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. Elife. 2016;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Voronina PP, Adamovich KV, Adamovich TV, et al. High concentration of ketone body β-hydroxybutyrate modifies synaptic vesicle cycle and depolarizes plasma membrane of rat brain synaptosomes. J Mol Neurosci. 2020;70:112–119. [DOI] [PubMed] [Google Scholar]
- 162. Kann O, Kovacs R.. Mitochondria and neuronal activity. Am J Physiol Cell Physiol. 2007;292:C641–57. [DOI] [PubMed] [Google Scholar]
- 163. Rizzuto R, Bernardi P, Pozzan T.. Mitochondria as all-round players of the calcium game. J Physiol. 2000;529(pt 1):37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Brustovetsky N, Brustovetsky T, Jemmerson R, et al. Calcium-induced cytochrome c release from CNS mitochondria is associated with the permeability transition and rupture of the outer membrane. J Neurochem. 2002;80:207–218. [DOI] [PubMed] [Google Scholar]
- 165. Hansson MJ, Mansson R, Mattiasson G, et al. Brain-derived respiring mitochondria exhibit homogeneous, complete and cyclosporin-sensitive permeability transition. J Neurochem. 2004;89:715–729. [DOI] [PubMed] [Google Scholar]
- 166. van den Ouweland JM, Maechler P, Wollheim CB, et al. Functional and morphological abnormalities of mitochondria harbouring the tRNA(Leu)(UUR) mutation in mitochondrial DNA derived from patients with maternally inherited diabetes and deafness (MIDD) and progressive kidney disease. Diabetologia. 1999;42:485–492. [DOI] [PubMed] [Google Scholar]
- 167. Calabrese V, Scapagnini G, Giuffrida Stella AM, et al. Mitochondrial involvement in brain function and dysfunction: relevance to aging, neurodegenerative disorders and longevity. Neurochem Res. 2001;26:739–764. [DOI] [PubMed] [Google Scholar]
- 168. Wu Y, Chen M, Jiang J.. Mitochondrial dysfunction in neurodegenerative diseases and drug targets via apoptotic signaling. Mitochondrion. 2019;49:35–45. [DOI] [PubMed] [Google Scholar]
- 169. Orth M, Schapira AH.. Mitochondria and degenerative disorders. Am J Med Genet. 2001;106:27–36. [DOI] [PubMed] [Google Scholar]
- 170. Gerbitz KD, Gempel K, Brdiczka D.. Mitochondria and diabetes. Genetic, biochemical, and clinical implications of the cellular energy circuit. Diabetes. 1996;45:113–126. [DOI] [PubMed] [Google Scholar]
- 171. Kalichman MW, Powell HC, Mizisin AP.. Reactive, degenerative, and proliferative Schwann cell responses in experimental galactose and human diabetic neuropathy. Acta Neuropathol. 1998;95:47–56. [DOI] [PubMed] [Google Scholar]
- 172. Sasaki H, Schmelzer JD, Zollman PJ, et al. Neuropathology and blood flow of nerve, spinal roots and dorsal root ganglia in longstanding diabetic rats. Acta Neuropathol. 1997;93:118–128. [DOI] [PubMed] [Google Scholar]
- 173. Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. [DOI] [PubMed] [Google Scholar]
- 174. Lustbader JW, Cirilli M, Lin C, et al. ABAD directly links Aβ to mitochondrial toxicity in Alzheimer's disease. Science. 2004;304:448–452. [DOI] [PubMed] [Google Scholar]
- 175. Takuma K, Yao J, Huang J, et al. ABAD enhances Aβ-induced cell stress via mitochondrial dysfunction. FASEB J. 2005;19:597–598. [DOI] [PubMed] [Google Scholar]
- 176. Anandatheerthavarada HK, Biswas G, Robin MA, et al. Mitochondrial targeting and a novel transmembrane arrest of Alzheimer's amyloid precursor protein impairs mitochondrial function in neuronal cells. J Cell Biol. 2003;161:41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Moreira PI, Santos MS, Seica R, et al. Brain mitochondrial dysfunction as a link between Alzheimer's disease and diabetes. J Neurol Sci. 2007;257:206–214. [DOI] [PubMed] [Google Scholar]
- 178. Williams TJ, Cervenka MC.. The role for ketogenic diets in epilepsy and status epilepticus in adults. Clin Neurophysiol Pract. 2017;2:154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Halestrap AP, Meredith D.. The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch. 2004;447:619–628. [DOI] [PubMed] [Google Scholar]
- 180. Forero-Quintero LS, Deitmer JW, Becker HM.. Reduction of epileptiform activity in ketogenic mice: the role of monocarboxylate transporters. Sci Rep. 2017;7:4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Ho PW, Ho JW, Liu HF, et al. Mitochondrial neuronal uncoupling proteins: a target for potential disease-modification in Parkinson's disease. Transl Neurodegener. 2012;1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Ramsden DB, Ho PW, Ho JW, et al. Human neuronal uncoupling proteins 4 and 5 (UCP4 and UCP5): structural properties, regulation, and physiological role in protection against oxidative stress and mitochondrial dysfunction. Brain Behav. 2012;2:468–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Lauritzen KH, Hasan-Olive MM, Regnell CE, et al. A ketogenic diet accelerates neurodegeneration in mice with induced mitochondrial DNA toxicity in the forebrain. Neurobiol Aging. 2016;48:34–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Dhillon KK, Gupta S.. Biochemistry, Ketogenesis. Treasure Island, FL: StatPearls [Internet; }; 2020. [Google Scholar]
- 185. Qian N, Wang Y.. Ketone body metabolism in diabetic and non-diabetic heart failure. Heart Fail Rev. 2020;25:817–822. [DOI] [PubMed] [Google Scholar]
- 186. Mahendran Y, Vangipurapu J, Cederberg H, et al. Association of ketone body levels with hyperglycemia and type 2 diabetes in 9,398 Finnish men. Diabetes. 2013;62:3618–3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Biessels GJ, Despa F.. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol. 2018;14:591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Vieira MNN, Lima-Filho RAS, De Felice FG.. Connecting Alzheimer's disease to diabetes: underlying mechanisms and potential therapeutic targets. Neuropharmacology. 2018;136:160–171. [DOI] [PubMed] [Google Scholar]
- 189. Zhao WQ, Townsend M.. Insulin resistance and amyloidogenesis as common molecular foundation for type 2 diabetes and Alzheimer's disease. Biochim Biophys Acta. 2009;1792:482–496. [DOI] [PubMed] [Google Scholar]
- 190. Infante-Garcia C, Ramos-Rodriguez JJ, Galindo-Gonzalez L, et al. Long-term central pathology and cognitive impairment are exacerbated in a mixed model of Alzheimer's disease and type 2 diabetes. Psychoneuroendocrinology. 2016;65:15–25. [DOI] [PubMed] [Google Scholar]
- 191. Infante-Garcia C, Ramos-Rodriguez JJ, Hierro-Bujalance C, et al. Antidiabetic polypill improves central pathology and cognitive impairment in a mixed model of Alzheimer's disease and type 2 diabetes. Mol Neurobiol. 2018;55:6130–6144. [DOI] [PubMed] [Google Scholar]
- 192. Plastino M, Fava A, Pirritano D, et al. Effects of insulinic therapy on cognitive impairment in patients with Alzheimer disease and diabetes mellitus type-2. J Neurol Sci. 2010;288:112–116. [DOI] [PubMed] [Google Scholar]
- 193. Ramos-Rodriguez JJ, Jimenez-Palomares M, Murillo-Carretero MI, et al. Central vascular disease and exacerbated pathology in a mixed model of type 2 diabetes and Alzheimer's disease. Psychoneuroendocrinology. 2015;62:69–79. [DOI] [PubMed] [Google Scholar]
- 194. Li X, Zhan Z, Zhang J, et al. β-Hydroxybutyrate ameliorates Aβ-induced downregulation of TrkA expression by inhibiting HDAC1/3 in SH-SY5Y cells. Am J Alzheimers Dis Other Dement. 2020;35:1533317519883496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Huang C, Wang P, Xu X, et al. The ketone body metabolite β-hydroxybutyrate induces an antidepression-associated ramification of microglia via HDACs inhibition-triggered Akt-small RhoGTPase activation. Glia. 2018;66:256–278. [DOI] [PubMed] [Google Scholar]
- 196. Zhang Q, Descamps O, Hart MJ, et al. Paradoxical effect of TrkA inhibition in Alzheimer's disease models. J Alzheimers Dis. 2014;40:605–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Newport MT, VanItallie TB, Kashiwaya Y, et al. A new way to produce hyperketonemia: use of ketone ester in a case of Alzheimer's disease. Alzheimers Dement. 2015;11:99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]