Abstract
This study updates the mycobiota resident in groundnut seeds, their phenology during storage with the view to ascertain their occurrence, potential toxigenic species, and pathologically important species in the stored samples. The moisture content of the seeds ranged from 5.7% to 6.5% within the stipulated safe moisture content of 8% for extension of shelf life. Culturing the seeds on mycological media (Sabouraud's Dextrose Agar SDA; Oxytetracycline Glucose Yeast Extract OGYE, Potato Dextrose Agar, PDA) caused a de novo growth of the quiescent spores at 28–30°C for 7–14 days. Fungal population counts on the three media ranged from 2.01 to 2.16 log10 CFU/g samples to a final 6‐month count of 1.67–2.60 log10 CFU/g. Eighteen different fungal species belonging to ten genera were encountered on the media, namely Aspergillus, Cladosporium, Curvularia, Fusarium, Penicillium, Trichoderma, Rhizopus, Rhodotorula, Sporendonema, and Paecilomyces. Aspergillus spp. (A. niger, A. flavus, A. fumigatus, and A. terreus) were the most frequently isolated, followed by Fusarium species (F. oxysporum, F. solani, and F. verticillioides), Trichoderma (T. harzianum and T. viride), Rhizopus spp (R. oligosporus and R. stolonifer), and Penicillium verrucosum. The species which were seed borne (A. niger, A. flavus, A. terreus, A. fumigatus, F. solani, F. verticillioides, T. viride, C. herbarum, and Curvularia lunata) were isolated on both surface sterilized and non‐surface sterilized seeds. The phenology of the encountered fungal species generally followed five patterns. The most frequently isolated Aspergillus niger, A. flavus, and A. fumigatus predominated throughout the 6 months sampling period, while A. ustus and A. terreus appeared sporadically and disappeared. The early colonizers (R. oligosporus, R. stolonifer, and Paecilomyces) could not be isolated after 2–3 months owing presumably to stronger antibiosis competition from the Aspergillus species. The most predominant Aspergillus species initially constituted 36%–48% of the total population but declined to 10%–36% in 6 months. Mycobiota encountered with mycotoxigenic potential and human health importance were A. niger, A. flavus, A. fumigatus, F. verticillioides, and Penicillium verrucosum. Other species of pathological importance to plants were Curvularia lunata and Fusarium oxysporum. The practical implications of these findings are discussed.
Keywords: Fusarium and Curvularia species, Groundnut, mycobiota phenology, mycotoxicogenic and pathogenic Aspergillus , Penicillium
Mycobiota phenology of toxicogenic fungi that contaminate groundnuts that are an excellent source of nutrients and used extensively to curb malnutrition.

1. INTRODUCTION
Groundnut (Arachis hypogaea) is an annual leguminous crop, which provides food for humans and serves as feed ingredients for animals. It belongs to the family Fabaceae (Leguminosae) and it is known by many local names such as earthnuts, ground nuts, goober peas, monkey nuts, pygmy nuts, and pig nuts (wikipedia.org). Although its origin is in South America, it is cultivated in many countries including: China, India, Mali, Gambia, Senegal, Chad, Ghana, and Nigeria (Yussif, 2016). Groundnut is the 4th most important oilseed crop and the 13th most important crop worldwide (Reddy et al., 2011). In the sub‐Saharan region of Africa, groundnut is considered a valuable crop as it comprises 40% of the world's production area, but contributes about 26% to the groundnut production worldwide (Angelucci and Bazzucchi, 2019). In Ghana, it is grown in almost all the 16 regions but predominates in the Northern, Upper East, Upper West, Oti, and Volta Regions where it is cultivated as a cash crop (Oteng‐Frimpong et al., 2015). It is estimated that the total production nationwide was about 193,200 metric tons from these regions according to Chakuri (2018). In 2015, farmers in Ghana produced 417,000 metric tons from the pulse from 336,000 hectares of land (MoFA‐SRID, 2016). Groundnut is grown throughout tropical Africa as a cash crop particularly in Senegal, Gambia, Nigeria, Sudan, and Ghana (Senghor et al., 2020).
Apart from deriving income from cultivating groundnut, it provides an inexpensive source of high‐quality dietary protein, oil, and mineral elements (Asibuo et al., 2008). The chemical composition of Ghanaian varieties of groundnut is different from what is obtained elsewhere and the vast food preparations incorporating groundnut to improve the protein level has helped in no small measure, in reducing malnutrition in developing countries (Asibuo, Akromah, Adu‐Dapaah, et al., 2008; Asibuo, Akromah, Safo‐Kantanka, et al., 2008).
There are about 21 cultivars of groundnut in Ghana divided into subspecies hypoaea and fastigita. This division is based on their branch patterns, presence or absence of flowers on the main stem, flower arrangement on leaf axils, etc. (Asibuo, Akromah, Safo‐Kantanka, et al., 2008). Cultivars with flowers on the main stem, sequential branching, and flowering were grouped into the subspecies fastigita Waldon and those without flowers on the main stem, alternative branching patterns, and alternate flowering were grouped into subspecies hypogaea category (Stalker et al., 2016). Those varieties belonging to the Arachis hypogaea Dagomba, F‐mix, Nkatepa, Manipinta, Sinkazie, Kumawu red/early, and Nkate kokoo; and those belonging to Arachis fastigita are Baasare, Broni nkatee, Afu, Nkoranza local, Atebubu local, Aprewa, Kintampo local, Shitaochi, Broni, Kamaloo, Kofi Nsarko, Koweka, Broni fufuo, and Florispan runner (Asibuo, Akromah, Safo‐Kantanka, et al., 2008). Oil content of the listed varieties ranged from 33.6% to 54.95% (Asibuo, Akromah, Safo‐Kantanka, et al., 2008). Crude protein ranges from 18.92% to 30.53%; carbohydrates ranges between 6.0% and 24.9%; and potassium, K values range between 1180 and 1693 mg/100 g, Na (19.0–40.0 mg/100 g), Ca (44–134 mg/100 g), and Mg (308–456 mg/100 g). The range of K, Na, and Mg on the 20 cultivars in Ghana was generally higher than the results from other workers (Galvao et al., 1976; Khalil & Chughtai, 1983; Oerise et al., 1974; Oke, 1967). Zinc, Zn, content ranged from 0 to 6.5 mg/100 g with a mean of 5.2 mg/100 g; Cu differed from 0 to 27 mg/100 g with a mean of 1.9 mg/100 g; Fe (0.2–3.7 mg/100 g; mean of 2.8 mg/100 g); and Mn (1.7–2.9 mg/100 g with a mean of 2.1/100 g). All the varieties had appreciable amounts of Zn, Cu, Fe, and Mn except Kintampo local which had no zinc and copper. Same range of values were obtained in findings of Galvao et al. (1976), Khalil and Chughtai (1983), Oerise et al. (1974), and Oke (1967). The amounts of micronutrients in the cultivars reported by Asibuo, Akromah, Safo‐Kantanka, et al. (2008) were nutritionally significant because small quantities are needed by the body (Toomer, 2018).
Matured groundnut seed contains per 100 g edible portion, 6.5 g water, energy 237 KJ (567 kcal); protein 25.8 g; fat 49.2 g, dietary fiber 8.5 g, thiamine 0.64 mg, riboflavin 0.14 mg, niacin 12.1 mg, vitamin B6 0.35 mg, folic acid 240 µg, and vitamin C 0 mg (Burkill, 1995). Essential amino acids in groundnut per 100 g of edible portion are tryptophan (250 mg), lysine (926), methionine (317 mg), phenylalanine (13,337 mg), threonine (883 mg), valine (1082 mg), leucine (1672 mg), and isoleucine (907 mg) (PROTA, 2015; Burkill, 1995). Fatty acids per 100 g edible portion are oleic (27.7 g), linoleic (15.6 g), and palmitic acids (5.2 g) (Ntare et al., 2008). Clearly, groundnut is a rich source of nutrition and does play a significant role in food and nutrition security as a valuable source of protein, fat, energy, minerals, and income‐generating commodity for many poor farmers in Sub‐Saharan Africa and Asia (Diop et al., 2004). However, its rich nutrient source is also the reason for utilization by microorganisms, especially fungi, for further growth and survival both in the field and in storage, thus reducing the market and nutritional values.
There are other human benefits to the consumption of groundnut. For example, consumption of groundnut reduces the risk of cardiovascular diseases, increases serum magnesium, and provides protection against certain human cancers (Alasalvar et al., 2020; Arya et al., 2016; Ros, 2010). Studies by Lokko et al. (2007) have demonstrated that groundnut lowers the total cholesterol and triacylglycerol concentration (Brostow et al., 2011). Lilly et al. (2018) showed that groundnut can be used to control type 2 diabetes (groundnut butter has low glycemic index and glycemic load). It contains high levels of vitamin E which helps in protecting one against Alzheimer's disease and age‐related cognitive decline (Morris, 2004). Recently, Mupunga et al. (2017) showed that groundnut butter is used as a main ingredient in the ready‐to‐use therapeutic food (RUTF),—an energy and protein—dense paste which meets the nutritional needs of population at risk of malnutrition.
Groundnut production is faced with a plethora of constraints, namely drought, fungal (rusts and leaf spots), bacterial, viral diseases, as well as insect pests (leaf miners and aphids) (Okello et al., 2010). Prominent among these diseases is the infection of groundnut seeds by species of Aspergillus and Penicillium producing mycotoxins. Mycotoxigenic fungi can invade and produce potent mycotoxins in seeds of groundnut prior to harvest, during harvest, and in storage after harvest. Recently, Bediako, Ofori, et al. (2019) reviewed the predisposing factors and management of aflatoxin contamination of groundnut. The genus Aspergillus is subdivided into seven (7) subgenera, which are further divided into sections (Klich, 2002). Aspergillus section flavi commonly referred to as Aspergillus flavus group (Amaike & Keller, 2011) belonging to the subgenus Circumdati has gained popularity due to its ability to produce toxins. Aspergillus oryzae, A. sojae, and A. tamarii form the non‐aflatoxigenic group while A. flavus, A. parasiticus, and A. nomius constitute the aflatoxigenic group of the section Flavi (Rodrigues et al., 2007). A. nomius is considered a minor pathogen and not of practical importance (Pitt, 2000). A. flavus and A. parasiticus are, on the other hand, economically and agronomically important species which infect and produce aflatoxin (B1, B2, G1, and G2) in agricultural crops prior to harvest, or during storage (Yu et al., 2004), under suitable environment during pre‐ or post‐harvest operations.
Aflatoxigenic fungi invade and produce aflatoxin in pods and seeds of groundnut prior to harvest, during harvest, and after harvest. The inoculation and colonization of A. flavus/A. parasiticus with initial inoculation from the soil bank of resident spores depend on the fungal population, temperature, relative humidity, insect infestation, and moisture content of the soil (Bediako, Ofori, et al., 2019; Gnonlonfin et al., 2013; Guchi, 2015). Complex interactions among the listed factors occur which could result in significant invasion of groundnut seeds (Gnonlonfin et al., 2013; Pandey et al., 2019). Furthermore, high soil temperature and late‐season drought stress are two important factors which occur concurrently to promote pre‐harvest infestation and aflatoxin contamination of groundnut (Bediako, Ofori, et al., 2019). Post‐harvest aflatoxin contamination of groundnut is, however, influenced by high humidity and high temperature during storage (Guchi, 2015). Aflatoxin contamination results in the damaging of grains and oilseed and depletion of their nutritional value (Jolly et al., 2009). Prolonged consumption of aflatoxins has been associated with impaired immune function (immunosuppressive effect), malnutrition and stunted growth in children, disabilities, and death (Achaglinkame et al., 2017; Bbosa et al., 2013). Other adverse health effects of intake of aflatoxins include liver cirrhosis, hepatitis B and C infection, and liver cancer (Bbosa et al., 2013).
Fumonisin, another type of fungal toxin, is produced by several species of the genus Fusarium such as Fusarium verticillioides and Fusarium moniliforme, which can infect many crops, especially cereals such as maize, wheat, and barley (Zeng et al., 2020). Cinar and Onbaşı (2019) also stated that 28 types of fumonisin have been identified in foods including groundnuts and have been divided into four groups: Fumonisin A (A1, A2, and A3), Fumonisin B (B1, B2, and B3), Fumonisin C (C4, C3, and C1), and Fumonisin P (P1, P2, and P3). Frequently ingested food or feed containing fumonisin cause diseases such as equine leukoencephalomalacia, porcine pulmonary edema, and possibly kidney and liver cancer (Riley and Merill, 2019). The co‐occurrence of fumonisin and aflatoxins in foods or feed is common.
Generally, there is a safe moisture content for all grains, pulses, and legumes which can prolong the shelf life of the seeds. Moisture content of 10% or higher after harvest predisposes groundnut to aflatoxin contamination (Bediako, Ofori, et al., 2019; Waliyar et al., 2015). Therefore, timely drying and maintenance of safe moisture level would achieve effective content of post‐harvest aflatoxin contamination (Torres et al., 2014). A positive correlation between kernel moisture content of groundnut and aflatoxin production was found by Kaaya and Kyamuhangire, (2006), cowpea, (Houssou et al., 2009) and maize (Hell et al., 2000). The water activity (aw) is a measure of the amount of free humidity in a product and the water vapor pressure of a substance divided by the vapor pressure of pure water at the same temperature. Water activity beyond 0.85aw at 25°C provides a conducive environment for fungal growth and spore germination (Hassane et al., 2017; Lasram et al., 2010). The optimum water activity (aw) for growth of A. flavus is 0.996 aw and the minimum is 0.80–0.82 aw (Giorni et al., 2012; Northolt et al., 1977). To prevent aflatoxin formation in groundnut, the seeds must be dried to or below aw 0.83 after harvest.
Proper storage of groundnut is one maintained under clean, dry conditions with low kernel moisture content about 8% and low temperature with protection from insect infestation to avoid contamination and aflatoxin formation (Torres et al., 2014). In Ghana, Awuah and Ellis (2002) observed that groundnuts dried to 6.6% were devoid of fungal infection for 6 months irrespective of the storage method, while at 12% and stored in only jute bags with 3% (w/w) Syzygium aromaticum powder as a protectant, effectively inhibited A. parasiticus infection. They recommended that for post‐harvest management of aflatoxin contamination, maximum moisture contents 9% mc for unshelled groundnuts and 7% for shelled groundnuts in Ghana should be maintained during storage at 70% ERH and 25–27°C. The calculated moisture content to maintain 90% germination for shelled groundnut seeds storage for 6 months at 15°C was 8.09%mc for variety Hanoch and 7.9% for Congo variety (Navarro et al., 1989). There is no information on the Ghanaian groundnut seed varieties in this respect.
Bediako, Dzidzienyo, et al. (2019) recently reported the incidence of fungi on unspecified varieties of groundnut in four growing regions (Ashanti, Upper East, Upper West, and Northern) of Ghana. Aspergillus niger (39.9%) and A. flavus (26.3%) were predominant species recovered, respectively, from 73.3% to 83.3% of 60 groundnut samples. A. flavus was found in equal proportion in the four regions studied. Other fungal species identified were Collectotrichum (13.3%), Rhizopus (14.8%), Penicillium (5.4%), Curvularia (0.2%), and A. ochraceus (=A. alutaceus). Awuah and Kpodo (1996) found A. flavus (12.8%–31.7%), A. parasiticus (0.24%), A. niger (34.0%), A. candidus (1.45%), A. tamarii (3.93%), A. alutaceus (=A. ochraceus; 5.26%), Fusarium spp (1.7%), Penicillium spp. (5.19%), Mucor sp. ( 2.3%), Trichoderma sp. (0.2%), Rhizopus stolonifer (12%), and unidentifiable fungi (11.72%) on groundnut from 21 selected markets in the then 10 regions of Ghana. According to Waliyar et al. (2007), Fusarium, Chaetomium, Alternaria, Basidiomycota, and Sordaria affect yield and quality of groundnut worldwide. The environmental conditions where the different groundnut cultivators grow may also influence the structure of the fungal community (Li et al., 2018).
In one of the first detailed study of mycobiota associated with groundnuts in Ghana, Markwei (1976) used two varieties of groundnuts (Kumawu Red and Florispan Runner) cultivated in the Volta Region in Ghana. The following results were obtained:
1.1. Florispan runner (Dabala)
Aspergillus spp., Alternaria sp., Botryodiplodia theobromae, Cladosporium herbarum, Chaetomium sp., Curvularia sp., Fusarium sp., Helminthosporium sp., Macrophomina, Mucor sp., Neurospora crassa, Nigrospora, Papulospora, 12 Penicillium species, Piptocephalis sp., Rhizopus oryzae, Trichothecium roseum, Trichoderma viride, and sterile mycelium (Mycelia sterilia), Syncephalastrum racemosum, and Verticillium.
1.2. Florispan runner (Sogakope)
Aspergillus species, Arthrobotrys anoidea, Chaetomium, Cladosporium, Curvularia, Fusarium, Gliocladium roseum, Helminthosporium sp., Macrophomina, Mucor, Neurospora crassa, Nigrospora, Papulospora, 12Penicillium spp., Piptocephalis, Rhizopus oryzae, Sclerotium rolfsii, Verticillium, and Mycelia sterilia.
1.3. Kumawu red (Dabala)
Aspergillus sp., Botryodiplodia theobromae, Chaetomium, Cladosporium, Collectotrichum, Curvularia, Fusarium, Macrophomina, Mucor sp., Penicillium, Periconia, Pestalotia, Phoma, Piptocephalis, Rhizopuz oryzae, Trichoderma viride, and Mycelia sterilia.
The predominant genera of the florispan runner seeds were Aspergillus and Penicillin followed by Chaetomium, Fusarium, Macrophomina, Mucor, Papulospora, and Rhizopus. Aspergillus and Penicllium were again the outstanding genera of fungi isolated from the Kumawu red seeds followed by Chaetomium, Macrophomina, and Mucor (Markwei, 1976). In most instances, three pattern of phenology were shown. A. flavus and A. niger increased with storage time; on the other hand, Macrophomina and Penicillium declined with prolonged storage (Markwei, 1976). Finally, Chaetomium population increased for 3–4 months and then declined. The same was true for Mucor sp. Many species did not show any trend and there was marked fluctuations. Presumably, the unique environmental conditions in the farms where the different cultivars (Florispan runner and Kumawu Red) were cultivated influenced the structure of the fungal community (Li et al., 2018).
The objective of this present study was to update the mycobiota of the Kumawu red varieties cultivated in the Volta Region of Ghana with the view to updating the mycobiota profile and their phenology and to identify potential mycotoxigenic and pathogenic species associated with stored groundnuts cultivated in the Kpetoe District of the Volta Region of Ghana.
2. MATERIALS AND METHODS
2.1. Sample collection
Twenty groundnut samples (variety: Kumawu red; mass 0.5 kg; deshelled) were purchased from a farm at Kpetoe (6° 33 N 0° 43E) in the Volta Region of Ghana, 23 km Southeast of Ho. The samples were transported in standard jute bags to the laboratories of the University of Health and Allied Sciences, Dave, Ho, in the Volta Region and the Department of Plant and Environmental Biology, University of Ghana, Legon, where the experiments were carried out. The sample of groundnut was stored in the laboratory under normal environmental regime of 75%–80% ERH and 28–32°C. The seeds (500 g) were sampled monthly from the top (0–20 cm), middle (20–40 cm), and bottom (40–60 cm) of the jute bags and then pooled together.
2.2. Mycobiota determination
2.2.1. Blotter method
A modified method of de Tempe (1967) and Limonard (1967) was employed. Ten seeds, surface sterilized with 1% sodium hypochlorite for 15–20 min and 10 non‐surface sterilized seeds were placed on Whatman's filter paper in 9 cm sterile Petri dishes and then moistened with about 10 ml sterile distilled water. There were 25 replicates (250 seeds) for each treatment. The plates were incubated for up to 14 days at 28–30°C. The following quantitative assessments were made:
Percentage germination of seed initially and after 6 months in the jute bag
Percentage of seeds infected with fungi
Percentage of seeds infected with a particular fungus species
The total number of fungal colonies on the grains
2.2.2. Solid medium method
Surface sterilized seeds (with 1% sodium hypochlorite) and non‐surface sterilized seeds were placed directly on each Petri plate containing Solid Potato Dextrose Agar (PDA, Oxoid), Oxytetracycline Glucose Yeast Extract Agar (OGYE, Oxoid), and Sabouraud's Agar (SA, Oxoid). Each medium was amended with chloramphenicol (500 mg/L). There were 20 replicates for each treatment and medium used. Sodium hypochlorite treatment was used with the view to reducing or removing completely, external saprophytes, which compete with resident pathogens within the seed. Each treatment and plates were incubated at 28–32°C for a period of 7 days until fungi grew.
2.2.3. Estimation of viable fungal colonies
Exactly 50 g of sample was weighed into 100‐ml 0.1% peptone water in 250‐ml Erlenmeyer flasks using electronic weighing balance (OHAUS®) with a readability of 0.01 g. The samples were shaken in a Gallenkamp Orbital Shaker (140 rev/min) for 30 min. From the stock suspension, decimal serial dilutions up to 1:103 were prepared. Exactly 1 ml aliquots of each dilution level were dispensed into 20 ml of media Potato Dextrose Agar (PDA), Sabouraud's Dextrose Agar (SDA), and Oxytetracycline Glucose Yeast Extract Agar (OGYE) as previously outlined by Kortei et al. (2018) and Odamtten et al. (2018). There were triplicate samples for each media and dilution level. The plates were incubated at 28–30°C for up to 7 days.
2.3. Fungal enumeration and identification
Fungal enumeration was done using a colony counter (STAR 8500 Funke Gerber) and then calculated as colony‐forming unit per gram sample (CFU/g). Data obtained in the standard units were transformed into the logarithmic form and presented as log10 CFU/g sample (Kortei et al., 2018; Odamtten et al., 2018).
Molds and yeast that appeared were identified by their cultural and morphological characteristics using standard identification manuals (Samson and Reenen‐Hoekstra, 1988; Samson et al., 1995).
2.4. Determination of moisture content (MC)
The moisture content (MC) of the groundnut seeds used at each sampling interval was determined using a modification of the oven dry weight method of Basoglu and Uylaser (2000). The seeds were milled using Waring Blender. Ten grams of flour was dried for 24 h in an oven (Gallen kamp) at 105°C and cooled in a desiccator. The sample was weighed using an Accu Lab ALC‐150.3 balance and MC determined using the following equation:
where W1 is weight of empty Petri dish
W2 is weight of sample + Petri dish before drying
W3 is weight of sample + Petri dish after drying
2.5. Data analysis
Data recorded and graphical representations were made using Microsoft Excel for Windows 9. Means were exported to Graphic Software System, STCC, Inc., USA, for analysis. Comparisons of means were performed using Duncan's multiple‐range test. A p‐value of <.05 was considered as significant at all points of analysis.
3. RESULTS
3.1. Mycobiota determination
Figure 1 summarizes results obtained using three media PDA, SDA, and OGYE for the isolation of fungal isolation of fungal spores. On the three media, the fungi behaved differently and increased marginally from a population of log10 0.9–2.6 log10 CFU/g in 6 months. There were no significant (p > .05) differences observed in the 6 months storage period (Figure 1). During this same period of sampling, the total number of fungal colonies decreased from 12 to 5 colonies except for a spike to 8 colonies/plate after 4 months (Figure 2a). Correspondingly, the mean moisture content of seeds in the storage bags varied marginally between 5.7 ± 0.21% and 6.5 ± 0.04% (Figure 2a) from top to bottom as shown in Table 1. There was a poor fit R 2 = −63.9 to the linear equation y = 0.7948x, as shown in Figure 2b.
FIGURE 1.
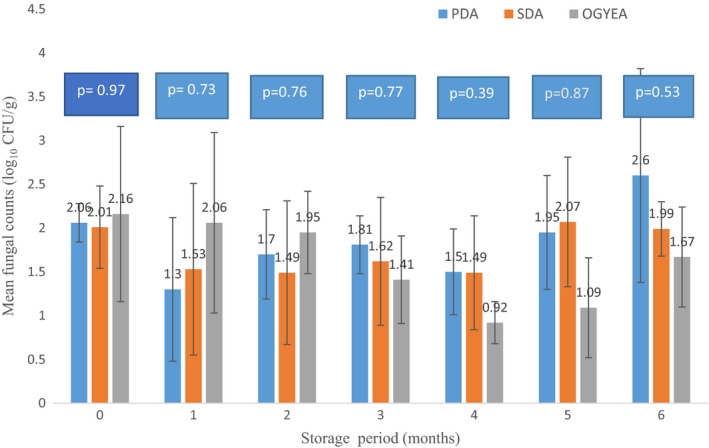
Comparative population of resident fungi (log10 CFU/g sample) in Arachis hypogaea L stored for up to 6 months and incubated on PDA, SDA, or OGYE after each sampling interval at 26–32°C. Abbreviations: PDA, Potato Dextrose Agar; SDA, Sabouraud Dextrose Agar; OGYE, Oxytetracycline Glucose Yeast Extract Agar
FIGURE 2.
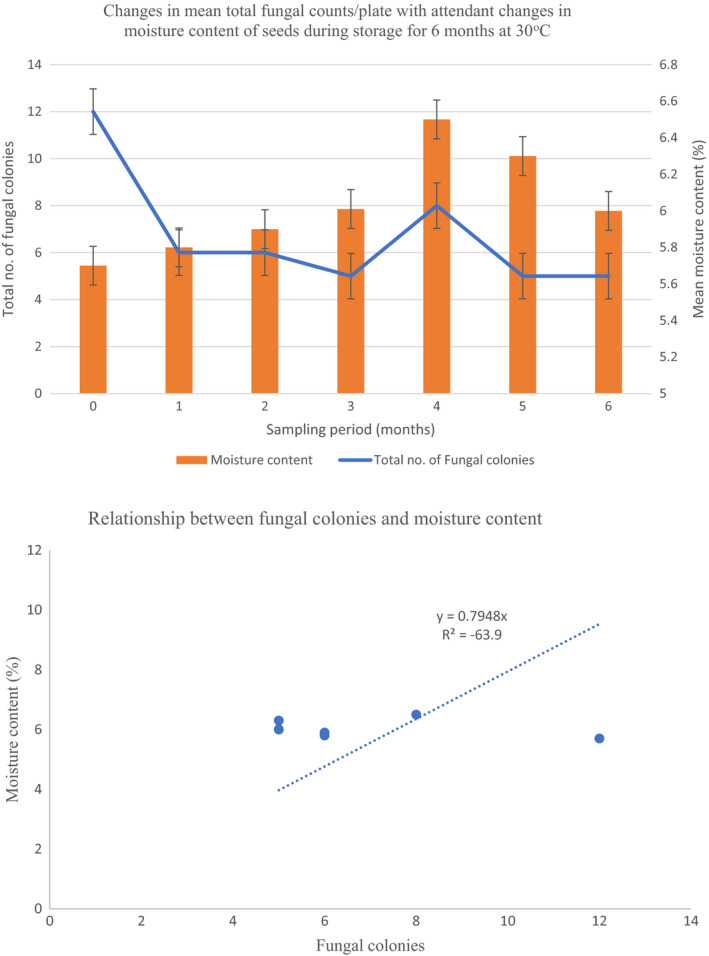
(a) Changes in mean total fungal counts/plate with attendant changes in moisture content of seeds during storage for 6 months at 30°C. (b) Relationship between fungal colonies and moisture content
TABLE 1.
Moisture content (%) of groundnuts stored over the entire 6 months storage period
| % Moisture content of seed in bag after (months) | |||||||
|---|---|---|---|---|---|---|---|
| Sampling position in the bag | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| Top (0–20 cm depth) | 5.9 | 6.1 | 5.8 | 6.0 | 6.4 | 6.3 | 5.8 |
| Middle (20–40 cm depth) | 5.8 | 5.7 | 6.2 | 6.8 | 6.5 | 6.5 | 6.3 |
| Bottom (40–60 cm depth) | 5.4 | 5.5 | 5.9 | 6.3 | 6.5 | 6.1 | 5.9 |
| Mean ± Std | 5.7 ± 0.21c | 5.8 ± 0.25c | 5.9 ± 0.17bc | 6.4 ± 0.33a | 6.5 ± 0.04a | 6.3 ± 0.16ab | 6.0 ± 0.22bc |
Means with same superscripts letters in a row are not significantly different (p < .05)
Eighteen different fungal species belonging to ten genera were encountered; namely Aspergillus, Cladosporium, Curvularia, Fusarium, Penicillium, Trichoderma, Rhizopus, Rhodotorula, Sporendonema, and Paecilomyces (Table 2). Aspergillus species (A. niger, A. flavus, and A. terreus) were most frequently encountered followed by Fusarium (F. oxysporum, F. solani, and F. verticillioides), Trichoderma (T. harzianum and T. viride), Rhizopus (R. oligosporus and R. stolonifer), and a single species of Penicillium (P. verrucossum) (Table 2). Surface sterilization removed some contaminants. However, species which appeared on the unsterilized seeds were almost same as recorded on the sterilized seeds (Table 2), indicating that they were seed borne from the field. These were A. niger, A. flavus, A. terreus, A. fumigatus, F. solani, T. viride, C. herbarum, F. verticillioides, and F. oxysporum.
TABLE 2.
Pooled data of total fungi isolated from shelled groundnut (Kumawu red variety) stored for 6 months under laboratory conditions (ERH 75%–85%; 28–32°C)
| Aspergillus niger Van TiegherSS, 0, 1, 2, 3, 4, 5, 6 |
| A. flavus LinkSS, 0, 1, 3, 4, 5, 6 |
| A. fumigatus FresenSS, 0, 2, 3, 4, 5, 6 |
| A. ustus (Banier) Thom and Church3 |
| A. terreus ThomSS |
| Cladosporuim herbarum (Pers.) LinkSS, 0, 1, 2 |
| Curvularia lunata Boedjin0, 2 |
| Fusarium oxysporum Sehldt.1, 3, 4, 5, 6 |
| F. verticillioides (Sacc.) Nirenberg0 |
| F. solani Von MartiusSS, 0 |
| Penicillium verrucosum Dierckx.0, 1, 2, 4, 5, 6 |
| Paecilomyces variotii Bainier0, 1, 6 |
| Trichoderma harzianum Rifai2 |
| T. viride Pers Ex Fr.SS, 4, 5 |
| Rhizopus oligosporus Saito0 |
| R. stolonifera (Ehrenb) Vuill.1, 4 |
| Rhodotorula mucilaginosa (A. Jorg) F.C. Harrison4 |
| Sporendonema casei Desm1, 4 |
Superscripts show treatments in which fungal species appeared in.
3.2. Phenology of the fungal species in the seeds
The phenology of the fungal species isolated from the seeds followed interesting trends (Figure 3):
The most frequently isolated Aspergillus species predominated throughout the sampling period. A niger was isolated at each sampling interval followed by A. flavus and A. fumigatus in all but one sampling time.
The occurrence of A. ustus and A. terreus can be described as sporadic then disappeared thereafter.
Cladosporium herbarum was isolated at the initial stages and persisted for 2 months and thereafter could not be isolated.
F. oxysporum was not initially isolated but subsequently could be detected from 3 to 6 months.
Penicillium verrucosum was isolated throughout the sampling period except the 3rd month akin to A. flavus and A. fumigatus.
The occurrence of the rest of the fungal species can be described as early colonizer (e.g., Rhizopus oligosporus, R. stolonifer, S. casei, and Paecilomyces), which disappeared with stronger competition.
FIGURE 3.
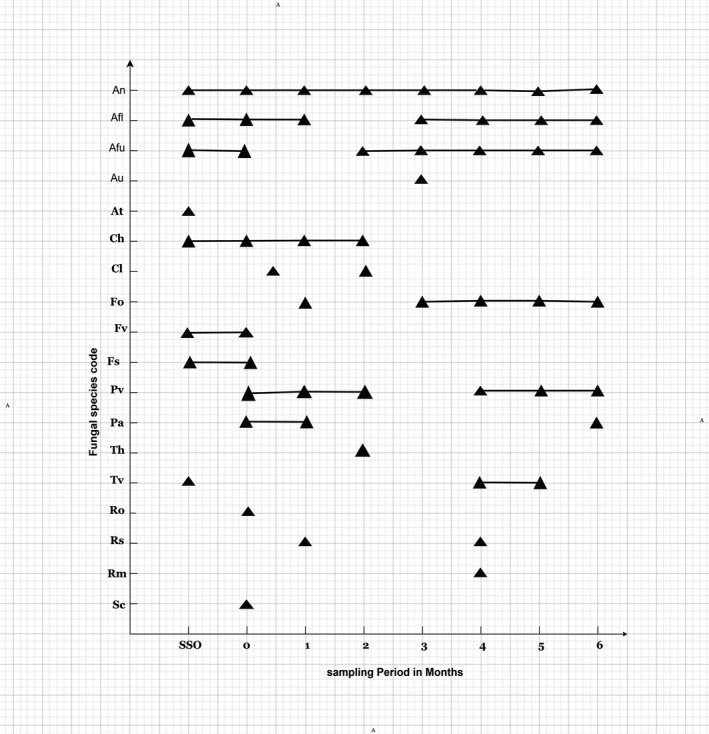
Phenology of resident fungi in groundnut (Arachis hypogaea) stored in the laboratory for up to 6 months (SSO—Surface Sterilized; 0—initial, non‐surface sterilized)
There was, therefore, generally five patterns of infection in the phenology of the contaminating fungal species. The corresponding percentage occurrence of the individual species contaminating the groundnut seeds is summarized in Table 3. The most predominant Aspergillus species (A. niger, A. flavus, and A. fumigatus) initially recorded 36%–48% occurrence declining with time to 10%–15% in 6 months; A. niger was the most dominant contributing 10%–48% followed by A. flavus (10%–36%) and A. fumigatus (10%–36%) (Table 3). Percentage occurrence of P. verrucosum ranged from 10% to 20% in 6 months. The remaining species (Rhizopus, Rhodotorula, and Sporendonema) showed sporadic occurrence ranging from 2% to 10%. There were more fungal species isolated from the non‐sterilized seeds (12) as compared to eight on the sterilized seeds and the fungal diversity oscillated between 5 and 8 during the observation period (Table 3).
TABLE 3.
Percentage occurrence of individual contaminating fungal species in groundnut (Kumawu Red) from Kpetoe, Volta Region, stored for 6 months
| Fungal species | Percentage (%) occurrence after (months) | |||||||
|---|---|---|---|---|---|---|---|---|
| SS* | 0 | 1 | 2 | 3 | 4 | 5 | 6 | |
| Aspergillus niger | 48.0 | 41.2 | 20.0 | 20.0 | 15.0 | 10.0 | 10.0 | 10.0 |
| A. flavus | 36.0 | 15.9 | 32.5 | ‐ | 10.0 | 25.0 | 25.0 | 20.0 |
| A. fumigatus | 36.0 | 38.2 | – | 25.0 | 30.0 | 10.0 | 10.0 | 15.0 |
| A. ustus | – | – | – | – | 15.0 | – | – | – |
| A. terreus | 4.0 | – | – | – | – | – | – | – |
| Cladosporium herbarum | 4.0 | 8.8 | 10.0 | 15.0 | – | – | – | – |
| Curvularia lunata | – | 10.0 | – | 5.0 | – | – | – | – |
| Fusarium verticillioides | 20.7 | 50.0 | – | – | – | – | – | – |
| Fusarium oxysporum | – | – | 10.0 | – | 30.0 | 20.0 | 25.0 | 35.0 |
| Fusarium solani | 2.7 | 2.9 | – | – | – | – | – | – |
| Paecilomyces variotii | – | 10.0 | 10.0 | – | – | – | – | 5.0 |
| Penicillium verrucosum | – | 20.0 | 10.0 | 20.0 | ‐ | 15.0 | 20.0 | 15.0 |
| Trichoderma harzianum | – | – | – | 15.0 | – | – | – | – |
| Trichoderma viride | 8.0 | – | – | – | – | 15.0 | 10.0 | ‐ |
| Rhizopus oligosporus | – | 2.9 | – | – | – | – | – | – |
| Rhizopus stolonifer | – | 7.5 | – | – | – | 3.0 | – | – |
| Rhodotorula mucilaginosa | – | ‐ | – | – | – | 2.0 | – | – |
| Sporendonema casei | – | 10.0 | – | – | – | – | – | – |
| Total | 8 | 12 | 6 | 6 | 5 | 8 | 6 | 6 |
SS*, Initial, Surface Sterilized; O, Initial non‐surface sterilized.
Clearly, the fungal species isolated in this study with toxigenic potential and human health and plant pathogenic importance were A. niger, A. flavus, A. fumigatus, Fusarium verticillioides and Penicillium verucosum, Curvularia lunata, and Fusarium oxysporum (Figure 3). Some of the mycobiota appearing on the non‐surface sterilized seed (Plate 1) and on the surface‐sterilized seeds (Plate 2).
PLATE 1.

Fungi appearing on non‐surface sterilized groundnut seeds (Kumawu red variety)
PLATE 2.
Fungi appearing on surface‐sterilized groundnut seeds (Kumawu red variety)
4. DISCUSSION
Significant constraints such as drought, fungal (rusts and leaf spots), bacterial, viral diseases, as well as insect pests (leaf miners and aphids) affect groundnut production (Okello et al., 2010, 2013). Proper storage of groundnut after harvest is one maintained under clean, dry conditions with low kernel moisture content of about 8% and a low temperature with protection from insects and infestation to avoid contamination and aflatoxin formation (Torres et al., 2014). Generally, there is a safe moisture for all grains, pulses, and legumes which can prolong their shelf‐life and preclude fungal invasion. Moisture content 10% or higher after harvest of groundnut predisposes the nuts to aflatoxins contamination. Therefore, timely drying and maintenance of safe moisture level would achieve effective control of post‐harvest mycotoxin contamination (Torres et al., 2014). Indeed, according to FAO (2010), mold growth is generally curtailed when moisture is low and fungal spores are unable to have free water to commence de novo growth in storage of groundnut.
In these present investigations, fungal populations recorded on the media PDA, SDA, and OGYE increased only marginally from 0.9 to 2.6 log10 CFU/g in 6 months but was attended by a decline in fungal colonies from 12 to 5, except for a spike to 8 colonies/plate after 4 months (Figures 1 and 2). Correspondingly, moisture content of the groundnut seeds increased to 6.5% in 6 months (Figure 2, Table 1). However, this moisture value falls within the recommended safe moisture content for groundnut as noted by Torres et al. (2014). Awuah and Ellis (2002) also observed that groundnuts in Ghana dried to a moisture content of 6.6% were devoid of fungal infection visually for 6 months irrespective of the storage method, while at 12% MC infection by Aspergillus parasiticus was visible in jute bags unless a powder of Syzygium aromaticum was used as a protectant biofungicide to inhibit infection by A. parasiticus. It is recommended that for post‐harvest management of mycotoxin contamination (especially aflatoxin), maximum moisture content of 9% for unshelled groundnut and 7% for shelled groundnuts in Ghana should be maintained during storage at 10% ERH and 25–27°C (Awuah & Ellis, 2002).
Fungi invade and produce mycotoxins in foods and seeds prior to harvest, during and after harvest (often referred to as field fungi), and also during storage (storage fungi). In this case of groundnut, the inoculation and colonization by fungi and moisture content of the soil could be favorable for spores in the soil bank to infect seeds accentuated by favorable temperatures, relative humidity, and presumably insect infestation (Bediako, Ofori, et al., 2019; Gnonlonfin et al., 2013; Guchi, 2015). It was therefore not surprising that despite the low moisture content of the groundnut seeds under storage, 18 different fungal species belonging to 10 genera were isolated on the two mycological media (Table 2).
Aspergillus species (A. niger, A. flavus, and A. terreus) were most frequently encountered followed by Fusarium (F. oxysporum, F. solani, and F. verticillioides), Trichoderma (T. harzianum and T. viride), and Rhizopus (R. oligosporus and R. stolonifer) (Table 2). Even though only a single species of Penicillium (P. verrucossum) was isolated, it is of pathological importance. Surface sterilization of seeds before plating was to remove external contamination. However, some species which appeared in the Petri plates with unsterilized seeds were same as on the surface‐sterilized seeds (Table 2, Plates 1 and 2), indicating that they were seed‐borne from the field. These were A. niger, A. flavus, A. terreus, F. solani, T. viride, C. herbarum, and F. verticillioides.
Antibiosis is a biological interaction between two or more organisms (especially microorganisms which is detrimental to at least one of them; it can also be an antagonistic association between an organism and the metabolite substance produced by another). This phenomenon of antibiosis is common among fungal species in a closed ecological niche and be responsible for the phenology of the fungal species resident in the groundnut seeds stored for 6 months. There were about five succession patterns of infection shown by the contaminating fungi (Figure 3) and this data corroborated by the preponderance of species and the disappearance of some shown in Table 3. Markwei (1976) found three patterns of infection as compared to five in this present study. Markwei (1976) also used two groundnut varieties, Florispan runner and Kumawu red from Volta Region (also used in this present study). According to Markwei (1976), the preponderance of A. flavus and A. niger on the samples increased with time. This is at variance with our findings with five patterns of infection where A. flavus and A. niger, although preponderant, decreased throughout the sampling period (Table 3).
Awuah and Kpodo (1996) sampled unspecific varieties of groundnuts from 21 markets in 10 Regions of Ghana. They found A. flavus (12.8%–31.7%), A. parasiticus (0%–0.24%), A. niger (34.0%), A. candidus (1.4%), A. tamarii (3.93%), A. alutaceus (=A. ochraceus, 5.26%), Fusarium spp. (1.45%), Penicillium (5.19%), Mucor (2.3%), Trichoderma spp. (0.2%), Rhizopus stolonifer (12.0%), and unidentified fungi (11.72%), some of which were encountered in our present study. In a recent study, Bediako, Dzidzienyo, et al. (2019); Bediako, Ofori, et al. (2019) reported the incidence of fungal flora in unspecified groundnut varieties growing in the Ashanti, Upper East, Upper West, and Northern Regions of Ghana. A. niger (39.9%) and A. flavus (26.3%) constituted the most predominant species isolated. This agrees with our findings. In this study, however, we report for the first time the incidence of A. fumigatus on groundnuts in Ghana. Bediako, Dzidzienyo, et al. (2019); Bediako, Ofori, et al. (2019) also recorded Collectotrichum (13.3%), Rhizopus (14.8%), Penicillium (5.4%), Curvularia (0.2%), and A. alutaceus (=A. ochraceus), some of which were also isolated. Clearly, the soil type, environmental conditions, as well as the variety of groundnut grown may influence the structure and phenology of the community (Li et al., 2018; Waliyar et al., 2007; Wang et al., 2017).
The preponderant fungal species isolated in this study with toxigenic potential, human health, and pathological importance were A. niger, A. flavus, A. fumigatus, Fusarium verticillioides, F. oxysporum, Curvularia lunata, and Penicillium verrucosum. Basically, three major genera of fungi were identified to produce mycotoxins here. This includes Aspergillus, Fusarium, and Penicillium, although other genera also produce toxic compounds (Mohammed et al., 2013). Aspergilli and Penicilli are linked to agricultural commodities during post‐harvest storage (Agriopoulou et al., 2020; Balendres et al., 2019).
Groundnut is an annual leguminous crop and plays an important role in food and nutrition security as a valuable source of protein, fats, energy, and minerals. It is also an income‐generating commodity for sub‐Saharan Africa and Asia (Diop et al., 2004). It is the 13th most important food crop worldwide (Reddy et al., 2011). However, the nutritive value of groundnut is eroded by mycotoxin contamination. Oils from groundnuts are often downgraded of its seeds are contaminated with fungi and subsequently mycotoxins of health and pathological importance (Jolly et al., 2009).
Varietal differences in A. flavus infection and aflatoxin production in foods have been documented (Okello et al., 2010, 2013; Upadhyaya, 2005; Upadhyaya et al., 2002; Waliyar et al., 2016). This probably partly explains the differences in susceptibility of the Ghanaian groundnut varieties to infection by A. flavus, especially those obtained from the Volta and Oti Regions of Ghana. Future studies will focus on varietal differences in susceptibility to potential mycotoxigenic fungi from the different agro‐ecological zones of Ghana.
Although A. niger is not known to produce aflatoxins, it possesses the ability to produce other toxins such as ochratoxin A, malformin, and nigerone (Siddiquee, 2018; Wagacha et al., 2013; Wang et al., 2016). Ochratoxin A is also produced by A. alutaceus (=A. ochraceus) and A. carbonarius (Siddiquee, 2018). Ochratoxin A is lipid soluble and is not excreted efficiently and thus accumulate in meat which exposes humans to health risk after consuming contaminated meat (Denli & Perez, 2010).
Penicillium verrucosum was among the frequently isolated contaminants in stored groundnuts in this present study. Penicillium is in the Deuteromycetes made up of diverse fungal genera of the ascomycetous fungi and contains more than 350 species. More than 80 Penicillium species are documented toxin producers (Agriopoulou et al., 2020). Majority of these are of significance in food safety while the rest are not. Most important are ochratoxin A, citreoviridin, penitrem A, roquefortine, and secalonic acids. The genus has major importance in the natural environment as well as food and drug production industry. Citrinin is a mycotoxin initially isolated from P. citrinum. Nonetheless other species such as P. miczynski, P. hirsutum, P. verrucossum (also isolated in this study), P. westling, P. expansum, P. stechii, and P. cyclopium have been reported to produce citrinin (Kumar et al., 2018). P. verrucosum and P. viridicatum produce ochratoxins, cyclopiazonic acid, penicillic acid, and citrinin. In higher latitudes, for example, Canada, P. verrucosum is the primary producer of ochratoxin A (Amézqueta et al., 2009) and this toxin is comparatively 10 times more toxic than citrinin (Amézqueta et al., 2009).
Fusarium species isolated from groundnut seeds produce several mycotoxins such as biologically active trichothecenes, which when ingested in high concentrations cause vomiting and diarrhea in humans. Trichothecenes are also associated with reduced weight gain and immune dysfunction in animals (Huang et al., 2019). Zearalenone causes human uterotrophic (anti‐reproduction) effects in animals and pigs (Agriopoulou et al., 2020). Another Fusarium species, F. verticillioides (F. moniliforme), isolated from groundnuts in the study produces fumonisin, which have neurotoxic effect in animals and is associated with esophageal cancer in sub‐Saharan Africa (Bennett & Klich, 2003; Chain et al., 2018, 2020).
Aspergillus fumigatus was one of the predominant fungal contaminants in the Kumawu red groundnuts from the Volta Region of Ghana isolated throughout the 6‐month sampling period (Figure 3, Table 2). A. fumigatus produces a mycotoxin called fumagillin (Guruceaga et al., 2018, 2020; Raffa & Keller, 2019). Fumagillin has activity on its target, the methionine peptidase type 2 (met AP2 enzyme). The same fungus also produces several mycotoxins such as gliotoxin and pseudoritin (Fallon et al., 2010; Guruceaga et al., 2020). Fumagillin is able to inhibit the function of neutrophils in blood inducing cell death in erythrocytes and also plays a role in the damage of epithelial cells which open the way for fungal invasion (Gayathri et al., 2020; Guruceaga et al., 2020). Therefore, the presence of A. fumigatus in groundnut in Ghana cannot be taken lightly and discounted as it has serious health implications and toxic effects on human function such as metabolism (Guruceaga et al., 2018, 2020; Raffa & Keller, 2019). These findings open a new direction of study to ascertain the presence of fumagillin in samples of stored groundnuts infected with A. fumigatus in Ghana. Furthermore, there are records in pertinent literature that A. fumigatus also produces other mycotoxins such as fumitremorgans, verruculogen, and gliotoxin and also caused aspergillosis in both human and animals (Rhodes, 2006), pulmonary aspergillosis (lung), aspergilloma (fungal ball), skin and nail infection, as well as eye and ear infections (Pitt & Hocking, 2009).
There was another interesting observation of pathological importance. Fusarium oxysporum, which was initially visibly absent on the surface, was detected after 1 month and persisted in the seeds for 6 months (Figure 3). F. oxysporum is a well‐known plant pathogen causing severe damage in many agricultural crops, both in the field and during post‐harvest storage (Mondani et al., 2021; de Lamo and Takken, 2020). Interaction between plant and root‐colonizing F. oxysporum can be natural, beneficial, or detrimental to the host. F. oxysporum is famous for its ability to cause wilt, root, and fruit rot in many plant species including many agriculturally important crops (Dean et al., 2012). This fungus ranks among the 10 most devastating fungal plant pathogens worldwide (Dean et al., 2012) and wilts are a major threat for agricultural productivity (Fisher et al., 2012). The association of Fusarium oxysporum with stored groundnuts should give a cause for alarm and attract important attention especially if the seeds are meant to be used as seed bank for the next season's food cultivation. Future studies will examine the pathology of this fungus in the groundnut ecosystems with the view to assessing the pathogenicity of F. oxysporum in the groundnut varieties cultivated in Ghana.
Curvularia lunata (Ascomycota; order Pleosporales) found in soil was also isolated in this study (Tables 2 and 3; Figure 3). C. lunata has been identified causing brown leaf spots in Pakistan (Majeed et al., 2016); blight disease of rice in India (Kamaluddeen & Abhilasha, 2013); leaf spot of Brassica rapa ssp. pekinensis in Thailand (Wonglom et al., 2019); Curvularia leaf spot of corn (Zea mays) in USA (Anderson et al., 2019; Garcia‐Aroca et al., 2018); and a plant pathogen in China (Chang et al., 2020; Liu et al., 2014). This fungus has been reported as pathogenic to crops of economic importance in Africa including Burkina Faso, Egypt, Ethiopia, Democratic Republic of Congo, Gambia, Ghana, Guinea, Kenya, Nigeria, Senegal, Sierra Leone, South Africa, Sudan, and Tanzania (Lorrain et al., 2019). In Ghana, C. lunata has been reported to infect some cereal grains; sorghum (Nutsugah et al., 2007) and maize (Hackman, 1995).
The genus Curvularia is also a dematiaceous fungus in the environment worldwide, mainly in tropical regions (Revankar, 2007). The genus Curvularia comprises of several species, three of which are ubiquitous and cause several types of infection in both immunocompetent and immune‐compromised horses, namely C. lunata, C. pallescens, and C. geniculata. C. lunata is the most commonly reported in human infections (Brandt & Warnock, 2003; More et al., 2019). C. lunata causes allergenic nasal polyposis and occasionally eosinophilia. Building up population of C. lunata in storage bags may predispose workers to inhalation of spores during handling in storage rooms.
5. CONCLUSIONS AND RECOMMENDATIONS
Data from this present study have shown that Kumawu red groundnut seeds from the Volta and Oti Regions harbored 18 fungal species belonging to 10 genera. Aspergillus species (A. niger, A. flavus, A. fumigatus, A. ustus, and A. terreus) predominated over all the others encountered followed by Fusarium (F. oxysporum, F. verticillioides, F. solani), Trichoderma (T. harzianum and T. viride), Rhizopus (R. oligosporus, R. stolonifera), Cladosporium herbarum, Curvularia lunata, Penicillium verrucosum, Paecilomyces variotii, Rhodotorula mucilaginosa, and Sporendonema casei. Some of the species were seed borne (A. niger, A. flavus, A. terreus, A. fumigatus, F. solani, F. verticillioides, T. viride, C. herbarum, and Curvularia lunata) and were isolated from both surface sterilized and non‐surface sterilized groundnut seeds.
Mycotoxin analysis of groundnuts was concentrated on aflatoxin group and to a larger extent on other equally potent mycotoxin. Our results show that a wider range of mycotoxigenic fungi contaminate groundnuts grown in the study area. This suggests the need to widen the scope of mycotoxin analysis to cover possible mycotoxins such as fumagillin (A. fumigatus), ochratoxin A (P. verrucosum), and fumonisin (Fusarium spp.), which have demonstrated to have human health implications. Furthermore, F. oxysporum isolated in this study ranks among the 10 most destructive wilt fungal pathogens worldwide (Dean et al., 2012; Fisher et al., 2012). Its presence in groundnuts seed could decimate crop productivity in the field.
Curvularia lunata found in soil bank has been recorded as pathogenic to many crops of economic importance in Africa including Ghana (Kusai et al., 2016) and also is a human pathogen as well (Kiss et al., 2020). Future studies will address this aspect of the shelf‐life of the stored legumes like groundnut. Finally, there are varietal differences in susceptibility of groundnuts to fungal infection and mycotoxin formation in the field and in storage. This study only scratches at the surface of a wider crop productivity and seed storage pathology problem and it opens a relevant gate for future research on safety of stored groundnut, especially so far as toxin contamination and seed viability are concerned.
CONFLICT OF INTEREST
The authors declare that they do not have any conflict of interest.
AUTHOR CONTRIBUTIONS
Nii Korley Kortei: conceptualization (lead) ; methodology (supporting); writingOriginalDraft (equal); writingReviewEditing (equal). Rachel Adinorkie Tetteh: dataCuration (equal) ; investigation (equal); projectAdministration (equal). Michael Wiafe‐Kwagyan: conceptualization (equal) ; methodology (equal); writingOriginalDraft (equal); writingReviewEditing (equal). Denick Nii Kotey Amon: dataCuration (equal); methodology (equal); resources (equal). George Tawia Odamtten: conceptualization (equal); methodology (equal); supervision (lead); writingReviewEditing (equal).
ACKNOWLEDGEMENTS
Authors are very grateful to all the technical staff of the Mycology Unit, Department of Plant and Environmental Biology, University of Ghana, for conducting the seed test and also helped in the identification of some fungal species. Furthermore, to the technical staff of the Microbiology laboratory of the School of Allied Health Sciences, University of Health and Allied Sciences, for culturing and enumeration of fungi.
Kortei, N. K. , Tetteh, R. A. , Wiafe‐Kwagyan, M. , Amon, D. N. K. , & Odamtten, G. T. (2022). Mycobiota profile, phenology, and potential toxicogenic and pathogenic species associated with stored groundnuts (Arachis hypogaea L.) from the Volta Region, Ghana. Food Science & Nutrition, 10, 888–902. 10.1002/fsn3.2719
Funding information
No funding was received
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in this journal.
REFERENCES
- Achaglinkame, M. A. , Opoku, N. , & Amagloh, F. K. (2017). Aflatoxin contamination in cereals and legumes to reconsider usage as complementary food ingredients for Ghanaian infants: A review. Journal of Nutrition & Intermediary Metabolism, 10, 1–7. 10.1016/j.jnim.2017.09.001 [DOI] [Google Scholar]
- Agriopoulou, S. , Stamatelopoulou, E. , & Varzakas, T. (2020). Advances in occurrence, importance, and mycotoxin control strategies: Prevention and detoxification in foods. Foods, 9, 137. 10.3390/foods9020137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alasalvar, C. , Salvadó, J.‐S. , & Ros, E. (2020). Bioactives and health benefits of nuts and dried fruits. Food Chemistry, 314, 126192. 10.1016/j.foodchem.2020.126192 [DOI] [PubMed] [Google Scholar]
- Amaike, S. , & Keller, N. P. (2011). Aspergillus flavus . Annual Review of Phytopathology, 49, 107–133. [DOI] [PubMed] [Google Scholar]
- Amézqueta, S. , González‐Peñas, E. , Murillo‐Arbizu, M. , & de Cerain, A. L. (2009). Ochratoxin A decontamination: A review. Food Control, 20, 326–333. 10.1016/j.foodcont.2008.05.017 [DOI] [Google Scholar]
- Anderson, N. , Mehl, K. , Neves, D. , Bradley, C. , & Wise, K. (2019). First report of Curvularia leaf spot of corn, caused by Curvularia lunata, in Kentucky. Plant Disease, 103, 2692. [Google Scholar]
- Angelucci, F. , & Bazzucchi, A. (2019). Analysis of incentives and disincentives for groundnuts in Ghana. Technical notes series, MAFAP, FAO, Rome. [Google Scholar]
- Arya, S. S. , Salve, A. R. , & Chauhan, S. (2016). Peanuts as functional food: A review. Journal of Food Science and Technology, 53, 31–41. 10.1007/s13197-015-2007-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asibuo, J. , Akromah, R. , Adu‐Dapaah, H. , & Safo‐Kantanka, O. (2008). Evaluation of nutritional quality of groundnut (Arachis hypogaea L.) from Ghana. African Journal of Food, Agriculture, Nutrition and Development, 8, 133–150. [Google Scholar]
- Asibuo, J. Y. , Akromah, R. , Safo‐Kantanka, O. , Adu‐Dapaah, H. K. , Ohemeng‐Dapaah, S. , & Agyeman, A. (2008). Chemical composition of groundnut, Arachis hypogaea (L) landraces. African Journal of Biotechnology, 7, 2203–2208. [Google Scholar]
- Awuah, R. , & Ellis, W. (2002). Effects of some groundnut packaging methods and protection with Ocimum and Syzygium powders on kernel infection by fungi. Mycopathologia, 154, 29–36. [DOI] [PubMed] [Google Scholar]
- Awuah, R. T. , & Kpodo, K. A. (1996). High incidence of Aspergillus flavus and aflatoxins in stored groundnut in Ghana and the use of a microbial assay to assess the inhibitory effects of plant extracts on aflatoxin synthesis. Mycopathologia, 134, 109–114. 10.1007/BF00436873 [DOI] [PubMed] [Google Scholar]
- Balendres, M. A. O. , Karlovsky, P. , & Cumagun, C. J. R. (2019). Mycotoxigenic fungi and mycotoxins in agricultural crop commodities in the Philippines: A review. Foods, 8, 249. 10.3390/foods8070249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basoglu, F. , & Uylaser, V. (2000). Gıda Analizleri I‐II Uygulama Klavuzu (inTurkish). Uludag Universitesi Ziraat Fakültesi Gıda Müh. Bölümü, 9, 8–17. [Google Scholar]
- Bbosa, G. S. , Kitya, D. , Lubega, A. , Ogwal‐Okeng, J. , Anokbonggo, W. W. , & Kyegombe, D. B. (2013). Review of biological and health effects of aflatoxins on body organs and body systems. In Razzaghi‐Abyaneh M. (Ed.), Aflatoxins‐recent advances and future prospects (pp. 239–269). Intech. [Google Scholar]
- Bediako, K. A. , Dzidzienyo, D. , Ofori, K. , Offei, S. K. , Asibuo, J. Y. , Amoah, R. A. , & Obeng, J. (2019). Prevalence of fungi and aflatoxin contamination in stored groundnut in Ghana. Food Control, 104, 152–156. 10.1016/j.foodcont.2019.04.034 [DOI] [Google Scholar]
- Bediako, K. A. , Ofori, K. , Offei, S. K. , Dzidzienyo, D. , Asibuo, J. Y. , & Amoah, R. A. (2019). Aflatoxin contamination of groundnut (Arachis hypogaea L.): Predisposing factors and management interventions. Food Control, 98, 61–67. 10.1016/j.foodcont.2018.11.020 [DOI] [Google Scholar]
- Bennett, J. , & Klich, M. A. (2003). Mycotoxins. Clinical Microbiological Reviews, 16(3), 497–516. Article CAS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt, M. , & Warnock, D. (2003). Epidemiology, clinical manifestations, and therapy of infections caused by dematiaceous fungi. Journal of Chemotherapy, 15, 36–47. 10.1179/joc.2003.15.Supplement-2.36 [DOI] [PubMed] [Google Scholar]
- Brostow, D. P. , Odegaard, A. O. , Koh, W.‐P. , Duval, S. , Gross, M. D. , Yuan, J.‐M. , & Pereira, M. A. (2011). Omega‐3 fatty acids and incident type 2 diabetes: The Singapore Chinese Health Study. The American Journal of Clinical Nutrition, 94, 520–526. 10.3945/ajcn.110.009357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkill, H. M. (1995). The useful plants of west tropical Africa (Vol. 1–3). The useful plants of west tropical Africa, Vols. 1–3. [Google Scholar]
- Chain, E. P. o. C. i. t. F. , Knutsen, H. K. , Barregård, L. , Bignami, M. , Brüschweiler, B. , Ceccatelli, S. , Cottrill, B. , Dinovi, M. , Edler, L. , & Grasl‐Kraupp, B. (2018). Appropriateness to set a group health‐based guidance value for fumonisin and their modified forms. EFSA Journal, 16, e05172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chain, E. P. o. C. i. t. F. , Schrenk, D. , Bignami, M. , Bodin, L. , Chipman, J. K. , del Mazo, J. , Grasl‐Kraupp, B. , Hogstrand, C. , Hoogenboom, L. , & Leblanc, J. C. (2020). Risk assessment of aflatoxins in food. EFSA Journal, 18, e06040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakuri, D. (2018). Technical efficiency analysis of groundnut production in Ghana: A bayesian approach. University Of Ghana. [Google Scholar]
- Chang, J.‐Y. , Liu, S.‐S. , Shi, J. , Guo, N. , Zhang, H.‐J. , & Chen, J. (2020). A new Curvularia lunata variety discovered in Huanghuaihai Region in China. Journal of Integrative Agriculture, 19, 551–560. 10.1016/S2095-3119(19)62655-9 [DOI] [Google Scholar]
- Cinar, A. , & Onbaşı, E. (2019). Mycotoxins and Food Safety: The hidden danger in foods. Mycotoxins and Food Safety, 10.5772/intechopen.89001. https://www.intechopen.com/online‐first/mycotoxins‐thehidden‐danger‐in‐foods [DOI] [Google Scholar]
- de Lamo, FJ , & Takken, FLW (2020). Biocontrol by Fusarium oxysporum Using Endophyte‐Mediated Resistance. Front. Plant Sci, 11, 37. 10.3389/fpls.2020.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Tempe, J. (1967). Routine investigation of the Seed Health Condition in the Dutch Seed Testing Station at Wageningen. The Netherlands. Proceedings of the Institutional Seed Testing Association, 22, 1–12. [Google Scholar]
- Dean, R. , Van kan, J. A. L. , Pretorius, Z. A. , Hammond‐kosack, K. E. , Di pietro, A. , Spanu, P. D. , Rudd, J. J. , Dickman, M. , Kahmann, R. , Ellis, J. , & Foster, G. D. (2012). The Top 10 fungal pathogens in molecular plant pathology. Molecular Plant Pathology, 13, 414–430. 10.1111/j.1364-3703.2011.00783.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denli, M. , & Perez, J. F. (2010). Ochratoxins in feed, a risk for animal and human health: Control strategies. Toxins, 2, 1065–1077. 10.3390/toxins2051065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diop, N. , Beghin, J. , & Sewadeh, M. (2004). Groundnut policies, global trade dynamics, and the impact of trade liberalization. Global Trade Dynamics, and the Impact of Trade Liberalization (March 2004). World Bank Policy Research Working Paper. [Google Scholar]
- Fallon, J. P. , Reeves, E. P. , & Kavanagh, K. (2010). Inhibition of neutrophil function following exposure to the Aspergillus fumigatus toxin fumagillin. Journal of Medical Microbiology, 59, 625–633. 10.1099/jmm.0.018192-0 [DOI] [PubMed] [Google Scholar]
- Fisher, M. C. , Henk, D. A. , Briggs, C. J. , Brownstein, J. S. , Madoff, L. C. , McCraw, S. L. , & Gurr, S. J. (2012). Emerging fungal threats to animal, plant and ecosystem health. Nature, 484, 186–194. 10.1038/nature10947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Agricultural Organization of the United Nations (FAO) (2010). Reducing post‐harvest losses in grain supply chains in Africa: Lessons learned and practical guidelines. FAO/World Bank Work. 18–19 March 2010 [Google Scholar]
- Galvao, L. C. , Lopez, A. , & Williams, H. L. (1976). Essential mineral elements in peanuts and peanut butter. Journal of Food Science, 41, 1305–1307. 10.1111/j.1365-2621.1976.tb01158.x [DOI] [Google Scholar]
- Garcia‐Aroca, T. , Doyle, V. , Singh, R. , Price, T. , & Collins, K. (2018). First report of Curvularia leaf spot of corn, caused by Curvularia lunata, in the United States. Plant Health Progress, 19, 140–142. [Google Scholar]
- Gayathri, L. , Akbarsha, M. A. , & Ruckmani, K. (2020). In vitro study on aspects of molecular mechanisms underlying invasive aspergillosis caused by gliotoxin and fumagillin, alone and in combination. Scientific Reports, 10, 1–20. 10.1038/s41598-020-71367-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorni, P. , Leggieri, M. C. , Magan, N. , & Battilani, P. (2012). Comparison of temperature and moisture requirements for sporulation of Aspergillus flavus sclerotia on natural and artificial substrates. Fungal Biology, 116, 637–642. 10.1016/j.funbio.2012.03.003 [DOI] [PubMed] [Google Scholar]
- Gnonlonfin, G. , Hell, K. , Adjovi, Y. , Fandohan, P. , Koudande, D. , Mensah, G. , Sanni, A. , & Brimer, L. (2013). A review on aflatoxin contamination and its implications in the developing world: A sub‐Saharan African perspective. Critical Reviews in Food Science and Nutrition, 53, 349–365. 10.1080/10408398.2010.535718 [DOI] [PubMed] [Google Scholar]
- Guchi, E. (2015). Aflatoxin contamination in groundnut (Arachis hypogaea L.) caused by Aspergillus species in Ethiopia. Journal of Applied and Environmental Microbiology, 3, 11–19. [Google Scholar]
- Guruceaga, X. , Ezpeleta, G. , Mayayo, E. , Sueiro‐Olivares, M. , Abad‐Diaz‐De‐Cerio, A. , Aguirre Urízar, J. M. , Liu, H. G. , Wiemann, P. , Bok, J. W. , & Filler, S. G. (2018). A possible role for fumagillin in cellular damage during host infection by Aspergillus fumigatus . Virulence, 9, 1548–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guruceaga, X. , Perez‐Cuesta, U. , Abad‐Diaz de Cerio, A. , Gonzalez, O. , Alonso, R. M. , Hernando, F. L. , Ramirez‐Garcia, A. , & Rementeria, A. (2020). Fumagillin, a mycotoxin of Aspergillus fumigatus: Biosynthesis, biological activities, detection, and applications. Toxins, 12, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman, D. K. (1995). Studies on the Mycoflora and some Physical characteristics of Ghanaian Maize (Zea Mays L) Varieties and the Effect of Extracts of Zanthoxylum xanthoxyloides Lam and Kigelia africana Benth on some of the Contaminant Fungi. MPhil thesis submitted to University of Ghana. [Google Scholar]
- Hassane, A. M. A. , El‐Shanaway, A. A. , Abo‐Dahab, N. F. , Abdel‐Hadi, A. M. , Abdel‐Rauf, U. M. , & Mwanza, M. (2017). Influence of different moisture contents and temperature on growth and production of aflatoxins B1 by a toxigenic Aspergillus flavus isolate in wheat flour. Journal of Ecology of Health and Environment, 5(3), 77–83. [Google Scholar]
- Hell, K. , Cardwell, K. F. , Setamou, M. , & Poehling, H.‐M. (2000). The influence of storage practices on aflatoxin contamination in maize in four agroecological zones of Benin, West Africa. Journal of Stored Products Research, 36, 365–382. 10.1016/S0022-474X(99)00056-9 [DOI] [PubMed] [Google Scholar]
- Houssou, P. , Ahohuendo, B. , Fandohan, P. , Kpodo, K. , Hounhouigan, D. , & Jakobsen, M. (2009). Natural infection of cowpea (Vigna unguiculata (L.) Walp.) by toxigenic fungi and mycotoxin contamination in Benin, West Africa. Journal of Stored Products Research, 45, 40–44. [Google Scholar]
- Huang, D. , Cui, L. , Sajid, A. , Zainab, F. , Wu, Q. , Wang, X. , & Yuan, Z. (2019). The epigenetic mechanisms in Fusarium mycotoxins induced toxicities. Food and Chemical Toxicology, 123, 595–601. 10.1016/j.fct.2018.10.059 [DOI] [PubMed] [Google Scholar]
- Jolly, C. M. , Bayard, B. , Awuah, R. T. , Fialor, S. C. , & Williams, J. T. (2009). Examining the structure of awareness and perceptions of groundnut aflatoxin among Ghanaian health and agricultural professionals and its influence on their actions. The Journal of Socio‐Economics, 38, 280–287. 10.1016/j.socec.2008.05.013 [DOI] [Google Scholar]
- Kaaya, A. N. , & Kyamuhangire, W. (2006). The effect of storage time and agroecological zone on mould incidence and aflatoxin contamination of maize from traders in Uganda. International Journal of Food Microbiology, 110, 217–223. 10.1016/j.ijfoodmicro.2006.04.004 [DOI] [PubMed] [Google Scholar]
- Kamaluddeen, S. S. , & Abhilasha, A. (2013). A new blight disease of rice caused by Curvularia lunata from Uttar Pradesh. International Journal of Agricultural Science and Research, 3, 13–16. [Google Scholar]
- Khalil, J. , & Chughtai, M. (1983). Chemical composition and nutritional quality of five peanut cultivars grown in Pakistan. Plant Foods for Human Nutrition, 33, 63–70. 10.1007/BF01093738 [DOI] [Google Scholar]
- Kiss, N. , Homa, M. , Manikandan, P. , Mythili, A. , Krizsán, K. , Revathi, R. , Varga, M. , Papp, T. , Vágvölgyi, C. , & Kredics, L. (2020). New species of the genus Curvularia: C. tamilnaduensis and C. coimbatorensis from fungal Keratitis cases in South India. Pathogens, 9, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klich, M. A. (2002). Identification of common Aspergillus species. CBS. [Google Scholar]
- Kortei, N. K. , Odamtten, G. T. , Obodai, M. , & Wiafe‐Kwagyan, M. (2018). Mycofloral profile and the radiation sensitivity (D10 values) of solar dried and gamma irradiated Pleurotus ostreatus (Jacq. Ex. Fr.) Kummer fruitbodies stored in two different packaging materials. Food Science and Nutrition, 6, 180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, D. , Tannous, J. , Sionov, E. , Keller, N. , & Prusky, D. (2018). Apple intrinsic factors modulating the global regulator, LaeA, the patulin gene cluster and patulin accumulation during fruit colonization by Penicillium expansum . Frontiers in Plant Science, 9, 1094. 10.3389/fpls.2018.01094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusai, N. A. , Azmi, M. M. Z. , Zulkifly, S. , Yusof, M. T. , & Zainudin, N. A. I. M. (2016). Morphological and molecular characterization of Curvularia and related species associated with leaf spot disease of rice in Peninsular Malaysia. Rendiconti Lincei, 27, 205–214. 10.1007/s12210-015-0458-6 [DOI] [Google Scholar]
- Lasram, S. , Oueslati, S. , Valero, A. , Marin, S. , Ghorbel, A. , & Sanchis, V. (2010). Water activity and temperature effects on fungal growth and ochratoxin A production by ochratoxigenic Aspergillus carbonarius isolated from Tunisian grapes. Journal of Food Science, 75, M89–M97. [DOI] [PubMed] [Google Scholar]
- Li, X. , de Boer, W. , Zhang, Y. , Ding, C. , Zhang, T. , & Wang, X. (2018). Suppression of soil‐borne Fusarium pathogens of peanut by intercropping with the medicinal herb Atractylodes lancea . Soil Biology and Biochemistry, 116, 120–130. 10.1016/j.soilbio.2017.09.029 [DOI] [Google Scholar]
- Lilly, L. N. , Heiss, C. J. , Maragoudakis, S. F. , Braden, K. L. , & Smith, S. E. (2018). The effect of added peanut butter on the glycemic response to a high‐glycemic index meal: A pilot study. Journal of the American College of Nutrition, 38(4), 351–357. 10.1080/07315724.2018.1519404 [DOI] [PubMed] [Google Scholar]
- Limonard, T. (1967). Bacterial antagonism in seed health tests. Netherlands Journal of Plant Pathology, 73, 1–14. 10.1007/BF02023673 [DOI] [Google Scholar]
- Liu, T. , Ma, B. , Hou, J. , & Zuo, Y. (2014). Isolation and characterization of the PKAr gene from a plant pathogen, Curvularia lunata . Indian Journal of Microbiology, 54, 310–314. 10.1007/s12088-013-0439-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokko, P. , Lartey, A. , Armar‐Klemesu, M. , & Mattes, R. D. (2007). Regular peanut consumption improves plasma lipid levels in healthy Ghanaians. International Journal of Food Sciences and Nutrition, 58, 190–200. 10.1080/09637480701198067 [DOI] [PubMed] [Google Scholar]
- Lorrain, C. , Gonçalves dos Santos, K. C. , Germain, H. , Hecker, A. , & Duplessis, S. (2019). Advances in understanding obligate biotrophy in rust fungi. New Phytologist, 222, 1190–1206. 10.1111/nph.15641 [DOI] [PubMed] [Google Scholar]
- Majeed, R. , Shahid, A. , Ashfaq, M. , Saleem, M. , & Haider, M. (2016). First report of Curvularia lunata causing brown leaf spots of rice in Punjab, Pakistan. Plant Disease, 100, 219. [Google Scholar]
- Markwei, C. (1976). Studies on the mycoflora of freshly harvested and stored seeds of groundnut (Arachis hypogaea, L.) (p. 168). MSc. Thesis, Department of Botany, University of Ghana. [Google Scholar]
- MoFA‐SRID (2016). Agriculture in Ghana: Facts and figures (2015). Ministry of Food and Agriculture (MoFA), Statistics. Research and Information Directorate (SRID). [Google Scholar]
- Mohammed, A. , Chimbekujwo, I. , & Bristone, B. (2013). Identification and control of fungi associated with the post‐harvest rot of Solenostemon rotundifolius (Poir) JK Morton in Adamawa State of Nigeria. Journal of Biology, Agriculture and Healthcare, 3, 136–140. [Google Scholar]
- Mondani, L. , Chiusa, G. , & Battilani, P. (2021). Fungi Associated with Garlic During the Cropping Season, with Focus on Fusarium proliferatum and F. oxysporum. Plant Health Progress. [Google Scholar]
- More, S. N. , Hernandez, O. , & Castleman, W. L. (2019). Mycotic rhinitis and sinusitis in Florida horses. Veterinary Pathology, 56, 586–598. 10.1177/0300985818817046 [DOI] [PubMed] [Google Scholar]
- Morris, T. (2004). Nutrition of chicks and layers. World's Poultry Science Journal, 60, 5–18. 10.1079/WPS20031 [DOI] [Google Scholar]
- Mupunga, I. , Mngqawa, P. , & Katerere, D. R. (2017). Peanuts, aflatoxins and undernutrition in children in Sub‐Saharan Africa. Nutrients, 9, 1287. 10.3390/nu9121287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro, S. , Donahaye, E. , Kleinerman, R. , & Haham, H. (1989). The influence of temperature and moisture content on the germination of peanut seeds. Peanut Science, 16, 6–9. [Google Scholar]
- Northolt, M. , Van Egmond, H. , & Paulsch, W. (1977). Differences between Aspergillus flavus strains in growth and aflatoxin B1 production in relation to water activity and temperature. Journal of Food Protection, 40, 778–781. 10.4315/0362-028X-40.11.778 [DOI] [PubMed] [Google Scholar]
- Ntare, B. , Diallo, A. , Ndjeunga, J. , & Waliyar, F. (2008). Groundnut seed production manual. Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi‐Arid Tropics (ICRISAT) (p. 20). [Google Scholar]
- Nutsugah, S. K. , Atokple, I. D. K. , & Leth, V. (2007). Sorghum Diseases prevalent in Ghana. Ghana Journal of Agricultural Science, 40, 119–126. [Google Scholar]
- Odamtten, G. T. , Nartey, L. , Wiafe‐Kwagyan, M. , Anyebuno, G. , & Kyei‐Baffour, V. (2018). Resident microbial load, toxigenic potential and possible quality control measures of six imported seasoning powders on the Ghanaian market. Journal of Nutritional Health and Food Engineering, 8, 24–35. [Google Scholar]
- Oerise, N. L. , Lau, H. A. , Ritchey, S. , & Murphy, E. W. (1974). Yield, proximate composition and mineral element content of three cultivars of raw and roasted peanuts. Journal of Food Science, 39, 264–266. 10.1111/j.1365-2621.1974.tb02871.x [DOI] [Google Scholar]
- Oke, O. (1967). Chemical studies on some Nigerian pulses. West African Journal of Biology and Applied Chemistry, 9, 52–55. [Google Scholar]
- Okello, D. , Biruma, M. , & Deom, C. (2010). Overview of groundnuts research in Uganda: Past, present and future. African Journal of Biotechnology, 9, 6448–6459. [Google Scholar]
- Okello, K. D. , Monyo, E. , Deom, C. M. , Ininda, J. , & Oloka, H. (2013). Groundnuts production guide for Uganda, recommended practices for farmers Entebbe, Uganda. National Agricultural Organization. [Google Scholar]
- Oteng‐Frimpong, R. , Sriswathi, M. , Ntare, B. R. , & Dakor, F. (2015). Assessing the genetic diversity of 48 groundnut (Arachis hypogaea L.) genotypes in the Guinea savanna agro‐ecology of Ghana, using microsatellite‐based markers. African Journal of Biotechnology, 14, 2485. [Google Scholar]
- Pandey, M. K. , Kumar, R. , Pandey, A. K. , Soni, P. , Gangurde, S. S. , Sudini, H. K. , Fountain, J. C. , Liao, B. , Desmae, H. , Okori, P. , Chen, X. , Jiang, H. , Mendu, V. , Falalou, H. , Njoroge, S. , Mwololo, J. , Guo, B. , Zhuang, W. , Wang, X. , … Varshney, R. K. (2019). Mitigating aflatoxin contamination in groundnut through a combination of genetic resistance and post‐harvest management practices. Toxins, 11, 315. 10.3390/toxins11060315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt, J. (2000). Toxigenic fungi and mycotoxins. British Medical Bulletin, 56, 184–192. 10.1258/0007142001902888 [DOI] [PubMed] [Google Scholar]
- Pitt, J. I. , & Hocking, A. D. (2009). Fungi and food spoilage. Springer Publishers. [Google Scholar]
- Raffa, N. , & Keller, N. P. (2019). A call to arms: Mustering secondary metabolites for success and survival of an opportunistic pathogen. PLoS Path, 15, e1007606. 10.1371/journal.ppat.1007606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, E. S. , Sudhakar, C. , & Reddy, N. E. (2011). Aflatoxin contamination in groundnut induced by Aspergillus flavus type fungi: A critical review. [Google Scholar]
- Revankar, S. G. (2007). Dematiaceous fungi. Mycoses, 50, 91–101. 10.1111/j.1439-0507.2006.01331.x [DOI] [PubMed] [Google Scholar]
- Rhodes, J. C. (2006). Aspergillus fumigatus: Growth and virulence. Medical Mycology, 44, S77–S81. [DOI] [PubMed] [Google Scholar]
- Riley, R. T. , & Merrill, A. H. (2019). Ceramide synthase inhibition by fumonisins: A perfect storm of perturbed sphingolipid metabolism, signaling, and disease. Journal of Lipid Research, 60, 1183–1189. 10.1194/jlr.S093815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues, P. , Soares, C. , Kozakiewicz, Z. , Paterson, R. , Lima, N. , & Venâncio, A. (2007). Identification and characterization of Aspergillus flavus and aflatoxins. In Mendez Vilas A. (Ed.), Communicating Current Research and Educational Topic and Trends in Applied Microbiology (pp. 527–534). Formatex. [Google Scholar]
- Ros, E. (2010). Health benefits of nut consumption. Nutrients, 2, 652–682. 10.3390/nu2070652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson, R. , Hoekstra, E. S. , & Frisvad, J. C. (1995). Introduction of Food‐Borne Fungi, 4th ed., (pp. 12–20). Netherlands: Pensen and Loogen. [Google Scholar]
- Samson, R. A. , & Van Reenen‐Hoekstra, E. S. (1988). Introduction to Food‐borne Fungi, Edit. 3, Centraalbureau voor Schimmelcultures, P. O Box 273, 3740 AG Baarn, The Netherlands (1988), p. 299, ISBN 90‐70351‐16‐1. Price Hfl. 42.50. Elsevier. [Google Scholar]
- Senghor, L. , Ortega‐Beltran, A. , Atehnkeng, J. , Callicott, K. , Cotty, P. , & Bandyopadhyay, R. (2020). The atoxigenic biocontrol product Aflasafe SN01 is a valuable tool to mitigate aflatoxin contamination of both maize and groundnut cultivated in Senegal. Plant Disease, 104, 510–520. 10.1094/PDIS-03-19-0575-RE [DOI] [PubMed] [Google Scholar]
- Siddiquee, S. (2018). Recent advancements on the role of biologically active secondary metabolites from Aspergillus, New and future developments in microbial biotechnology and bioengineering (pp. 69–94). Elsevier. [Google Scholar]
- Stalker, H. T. , Tallury, S. P. , Seijo, G. R. , & Leal‐Bertioli, S. C. (2016). Biology, speciation, and utilization of peanut species, Peanuts (pp. 27–66). Elsevier. [Google Scholar]
- Toomer, O. T. (2018). Nutritional chemistry of the peanut (Arachis hypogaea). Critical Reviews in Food Science and Nutrition, 58, 3042–3053. [DOI] [PubMed] [Google Scholar]
- Torres, A. M. , Barros, G. G. , Palacios, S. A. , Chulze, S. N. , & Battilani, P. (2014). Review on pre‐and post‐harvest management of peanuts to minimize aflatoxin contamination. Food Research International, 62, 11–19. 10.1016/j.foodres.2014.02.023 [DOI] [Google Scholar]
- Upadhyaya, H. D. (2005). Variability for drought resistance related traits in the mini core collection of peanut. Crop Science, 45, 1432–1440. 10.2135/cropsci2004.0389 [DOI] [Google Scholar]
- Upadhyaya, H. , Nigam, S. N. , & Thakur, R. P. (2002). Genetic Enhancement for Resistance to Aflatoxins contamination in groundnuts. Aflatoxins, 2, 29–36. http://oar.icrisat.org/3333/1/Genetic_enhancement_for_Resistacne_to_aflatoxin_contamination_in_Groundnut.pdf [Google Scholar]
- Wagacha, M. , Mutegi, C. , Christie, M. E. , Karanja, L. W. , & Kimani, J. (2013). Changes in fungal population and aflatoxin levels and assessment of major aflatoxin types in stored peanuts (Arachis hypogaea Linnaeus). Journal of Food Research, 2(5), 10. 10.5539/jfr.v2n5p10 [DOI] [Google Scholar]
- Waliyar, F. , Kumar, K. V. K. , Diallo, M. , Traore, A. , Mangala, U. , Upadhyaya, H. , & Sudini, H. (2016). Resistance to pre‐harvest aflatoxin contamination in ICRISAT's groundnut mini core collection. European Journal of Plant Pathology, 145, 901–913. 10.1007/s10658-016-0879-9 [DOI] [Google Scholar]
- Waliyar, F. , Ntare, B. , Diallo, A. , Kodio, O. , & Diarra, B. (2007). On‐farm management of aflatoxin contamination of groundnut in West Africa a synthesis report. [Google Scholar]
- Waliyar, F. , Osiru, M. , Ntare, B. , Kumar, K. , Sudini, H. , Traore, A. , & Diarra, B. (2015). Post‐harvest management of aflatoxin contamination in groundnut. World Mycotoxin Journal, 8, 245–252. 10.3920/WMJ2014.1766 [DOI] [Google Scholar]
- Wang, Y. , Wang, L. , Liu, F. , Wang, Q. , Selvaraj, J. N. , Xing, F. , Zhao, Y. , & Liu, Y. (2016). Ochratoxin A producing fungi, biosynthetic pathway and regulatory mechanisms. Toxins, 8, 83. 10.3390/toxins8030083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z. , Li, T. , Wen, X. , Liu, Y. , Han, J. , Liao, Y. , & DeBruyn, J. M. (2017). Fungal communities in rhizosphere soil under conservation tillage shift in response to plant growth. Frontiers in Microbiology, 8, 1301. 10.3389/fmicb.2017.01301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonglom, P. , Suwannarach, N. , Lumyong, S. , Ito, S.‐I. , Matsui, K. , & Sunpapao, A. (2019). Streptomyces angustmyceticus NR8‐2 as a potential microorganism for the biological control of leaf spots of Brassica rapa subsp. pekinensis caused by Colletotrichum sp. and Curvularia lunata . Biological Control, 138, 104046. [Google Scholar]
- Yu, J. , Whitelaw, C. A. , Nierman, W. C. , Bhatnagar, D. , & Cleveland, T. E. (2004). Aspergillus flavus expressed sequence tags for identification of genes with putative roles in aflatoxin contamination of crops. FEMS Microbiology Letters, 237, 333–340. 10.1111/j.1574-6968.2004.tb09715.x [DOI] [PubMed] [Google Scholar]
- Yussif, A. (2016). Evaluation of peanut paste in selected markets in Northern Ghana. [Google Scholar]
- Zeng, H.‐Y. , Li, C.‐Y. , & Yao, N. (2020). Fumonisin B1: A tool for exploring the multiple functions of sphingolipids in plants. Frontiers in Plant Science, 11, 600458. 10.3389/fpls.2020.600458 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in this journal.


