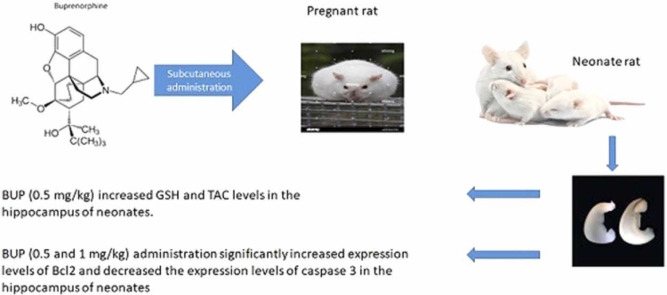
Abstract
The study investigated the effect of buprenorphine (BUP) on oxidative indices and gene expression of apoptotic molecules in the hippocampus of neonates during the fetal stage. BUP (1 or 0.5 mg/kg) was subcutaneously administrated to pregnant rat dams. After parturition, the pups were maintained to the end of breastfeeding period, then hippocampi were assessed for oxidative stress indices [glutathione (GSH), thiobarbituric acid reactive substances (TBARS), superoxide dismutase (SOD), total antioxidant capacity (TAC)] and mRNA expression of apoptotic markers (Bax, Bcl2 and caspase 3). Our data indicated that BUP (0.5 mg/kg) administration during gestation significantly increased GSH and TAC concentrations in the hippocampus of pups versus control group (p < 0.05). BUP (0.5 and 1 mg/kg) administration significantly elevated the expression levels of Bcl2 in the hippocampus of neonates compared with controls. BUP injection (0.5 and 1 mg/kg) to pregnant rats markedly reduced the expression levels of caspase 3 in the hippocampus of neonates in BUP 0.5 group (p < 0.01) and BUP 1 group (p < 0.05) versus the controls. Our study indicated that BUP may potentiate antioxidant system and inhibit apoptosis and oxidative stress in the hippocampus of neonates received this drug during the fetal stage.
Keywords: Buprenorphine, Apoptosis, Oxidative stress, Brain, Rat, Pup
Graphical Abstract
Highlights
-
•
BUP at low doses may potentiate antioxidant system.
-
•
BUP at low dose may inhibit oxidative stress.
-
•
BUP at low dose may act as an anti-apoptotic agent.
1. Introduction
Opioid maintenance therapy with methadone, a µ-opioid receptor agonist or buprenorphine (BUP), has been approved by WHO (2014) during pregnancy. Although, patients under opioid maintenance therapy have improved health conditions than those receiving illicit opioids [1]; yet, opioid maintenance therapy in pregnant women may pose a health risk to the fetus [2]. The opioid receptors expression is elevated in the neuronal cells of developing brain of human and animal, and endogenous opioids, as inhibitory growth factors, can regulate the growth of dendritic cells and spine formation [3]. Fetus is likely susceptible to opioids due to the effect of endogenous opioid on the brain development.
Several clinical studies have reported that the risk of cognitive and behavioral dysfunction in children born to mothers under opioid therapy is increased compared to non-exposed children [4], [5], [6].
Experimental studies also confirmed that BUP and methadone caused cognitive impairment in rat offspring prenatally exposed to these drugs [7], [8], [9]. Several studies have also indicated that opioids may affect neurogenesis, cell proliferation, and myelination during brain development [10], [11], [12]. Thus, it is needed to find the mechanisms underlying opiate-induced cognitive impairment in the fetus. The high expression of the µ-opioid receptors was seen on the hippocampus [13]. It has been suggested that altered expression of molecular signaling pathways involved in learning and memory function via activation of µ receptors mediated over generation of reactive oxygen species (ROS), apoptosis and inflammation in the hippocampus [13], [14].
However, knowledge on the neurological impact of prenatal exposure to BUP is incomplete. Therefore, this study aimed to evaluate gestational exposure to BUP and its effects on the oxidative stress indices [thiobarbituric acid reactive substances (TBARS), glutathione (GSH), total antioxidant capacity (TAC), superoxide dismutase (SOD)] and mRNA expression of apoptotic markers (Bax, Bcl2 and caspase 3) in the pups’ hippocampus exposed to this drug during the fetal stage.
2. Methods
2.1. Animals
This study was approved by the ethic committee of National Institutes for Medical Research Development (NIMAD). In the present study, 12 female Wistar rats were housed in polypropylene cages in the standard animal house in Birjand University of Medical Sciences under normal condition. Female and male rats were mated for 6 days and the presence of vaginal plugs was examined each morning to ascertain pregnancy. Upon confirmation of pregnancy, male rats were removed from the cage.
3. Animal grouping
Pregnant animals were randomly allocated to control which received olive oil (0.2cc/rat) and two BUP (0.5 and 1 mg/kg, subcutaneously) groups during pregnancy once a day. After delivery the neonates were maintained for 28 days. At the end of the treatment period, the rats were anesthetized with ketamine/xylazine (50/10 mg /kg), and their hippocampi were obtained for biochemical and molecular analysis.
4. Thiobarbituric acid reactive substances measurement
A Nalondi™ assay kit (Navand Salamat, Iran) containing thiobarbituric acid reactive substances (TBARS) was used to measure lipid peroxidation. In this method malondialdehyde (MDA) levels were used to determine lipid peroxidation [15]. MDA is a lipid peroxidation product that reacts with thiobarbituric acid (TBA) under acidic and high temperature conditions with a purplish result whose color intensity is measured at 532 nm by using Epoch Microplate Reader (USA / Bio-Tek).
5. Determination of reduced glutathione level
The reduced glutathione in the homogeneous tissue was measured using a standard NarGul™ kit (Navand Salamat, Iran). In this method, GSSG converts into GSH (reduced glutathione) by glutathione reductase in the presence of NADPH, which then produces GSTNB in the presence of NADPH and DTNB. This cycle is activated by the glutathione reductase enzyme. Light absorption was assessed at 412 nm by using Epoch Microplate Reader (USA / Bio-Tek.
6. Superoxide dismutase measurement
A Nasdox™ assay kit (Navand Salamat, Iran) was used to measure the superoxide dismutase enzyme activity in homogenous tissue samples. The kit was designed according to the inhibition of the oxidation of pyrogallol [16]. Pyrogallol is normally oxidized in air and whose oxidation half-life is determined at a certain concentration; For a sample containing SOD at an unknown concentration, the inhibition of pyrogallol oxidation at a given time was determined and compared with the concentration of SOD of control at a wavelength of 405 nm by using Epoch Microplate Reader (USA / Bio-Tek.
7. Total antioxidant capacity measurement
A Naxifer™ assay kit (Navand Salamat, Iran) was used to evaluate total antioxidant capacity (TAC) based on the ferric reducing antioxidant power (FRAP) method. This kit was designed based on the ability to reduce ferric ions and convert them into ferrous ions by antioxidants in biological samples and to form a ferrous ion complex with the TPTZ reagent and a bluish complex [17]. The intensity of the blue color is measured at a wavelength of 593 nm by using Epoch Microplate Reader (USA / Bio-Tek.
8. Real-time PCR to detect mRNA expression
8.1. RNA extraction
RNA extraction was performed using the Parstous Nucleic acid extraction. In brief, approximately 5 g of tissue sample was transferred into a 1.5 ml microtube containing 750 μl of RL solution and incubated at room temperature for 5 min. Chloroform (150 μl) was then added to the mix, the tube is capped securely and vortexed vigorously for 15 s and incubated for 3 min at room temperature. The tube was centrifuged at 13000 rpm for 12 min at 4 °C. after that, 400 μl of the aqueous phase was transferred into a new tube and an equal amount of absolute ethanol was added. The mixture was then transferred into a filter tube and centrifuged for one minute at 13000 rpm. The filter tube was washed with PW solution for one minute at 13000 rpm. Finally, the RNA was eluted in 50 μl of DEPS treated water and stored at − 80 °C.
Both quantity and purity of the extracted RNAs were determined using UV absorbance measurement at 260 and 280 nm by NanoDrop spectrophotometer. OD260/OD280 ratio of ~2 was considered as an acceptable purity for the RNA samples. The integrity and quality of the purified RNAs were further checked using 2% agarose gel electrophoresis.
cDNA synthesis:
The cDNAs were synthesized using Yekta Tajhiz Azma cDNA synthesis kit, Iran according to the manufacturer's procedure:
-
1.Total RNA and random hexamer primer were mixed in a nuclease-free 0.2 ml microtube and the volume was adjusted to 13.4 μl using nuclease-free water:
Total RNA 0.1 ng–5 μl Random hexamer 1 μl nuclease-free H2O variable Total Volume -
4.μl
-
4.
-
2.
The components were mixed gently and incubated at 70 °C for 5 min. The microtube was then incubated on ice to cool the mixture.
-
3.The following components were added to the above mixture and the volume was adjusted to 20 μl:
5x first strand buffer 4 μl dNTPs (10 mM each) 1 μl RNasin (40 U/μl) 0.5 μl M-MLV)200 U/μl) 1 μl -
4.
The microtube was incubated for 5 min at 37 °C and the reaction was terminated by a 5-minute incubation at 70 °C.
-
5.
Finally, the synthesized cDNA was stored at − 20°C.
9. Quantitative PCR (qRT-PCR)
qRT-PCR test were performed by using the synthesized cDNAs as a template. To quantify the relative mRNA level of each gene, SYBR green-based real-time PCR was carried out using Real-time PCR Master Mix (SYBR Green), on Stratagene mx3000p Real-Time PCR machine. About 50 ng cDNA sample 1 μl of each forward and reverse primer (10 µM) and 10 μl 2X Real-time PCR Master Mix (SYBR Green), at a final volume of 20 μl were used.
A reaction without cDNA and gapdh housekeeping gene was used as a negative control and internal control, respectively. Melting curve analysis confirmed the specificity of the primer pairs and the purity of real-time PCR products.
Actin f: AAGATCCTGACCGAGCGTGG.
Actin R: CAGCACTGTGTTGGCATAGAGG.
| Sequence | Gene |
|---|---|
| F: AGGGCTGCTTTTAACTCTGG R:CCCCACTTGATTTTGGAGGG |
GAPDH |
| F:TGCTTCAGGGTTTCATCCA R:GACACTCGCTCAGCTTCTTG |
Bax |
| F:GATAACGGAGGCTGGGTAGG R: CCAGAGGAGGAGGTAGGGAC |
Bcl2 |
| F:GTTTGAGCCTGAGCAGAGACAT R:GGCAGCATCATCCACACATACC |
Caspase 3 |
10. Statistical method
Data analysis was done with the SPSS 22 software (SPSS Inc., Chicago, IL) using Analysis of Variance (ANOVA) and Tukey’s post hoc tests Data was indicated as mean ± SD. P value below 0.05 was considered as the significance level.
11. Result
11.1. Oxidative stress parameters
BUP (0.5 mg/kg) significantly increased neonatal hippocampal GSH and TAC levels versus the controls (p = 0.018, p = 0.033, respectively). There was no significant difference in the GSH and TAC levels in the hippocampus of neonates receiving BUP 1 mg/kg in fetal stage versus control animals (Table 1).
Table 1.
Oxidative stress parameters in hippocampus of the control and buprenorphine-exposed pups during the fetal stage.
| Groups | SOD | MDA | GSH | TAC |
|---|---|---|---|---|
| Control | 7.85 ± 1.08 | 16.06 ± 1.56 | 18.55 ± 0.71 | 6.08 ± 1.29 |
| Buprenorphine 0.5 mg/kg | 8.23 ± 1.70 | 12.12 ± 0.80 | 23.89 ± 1.55 | |
| * | 10.23 ± 1.08 | |||
| * | ||||
| Buprenorphine 1 mg/kg | 6.93 ± 0.97 | 15.23 ± 1.96 | 20.74 ± 1.03 | 6.90 ± 1.16 |
| F = 0.26 | ||||
| P Value = 0.77 | F = 1.86 | |||
| P Value = 0.19 | F = 5.28 | |||
| P Value = 0.018 | F = 4.89 | |||
| P Value = 0.03 |
12. Gene expression of Bax, Bcl2, and caspase 3
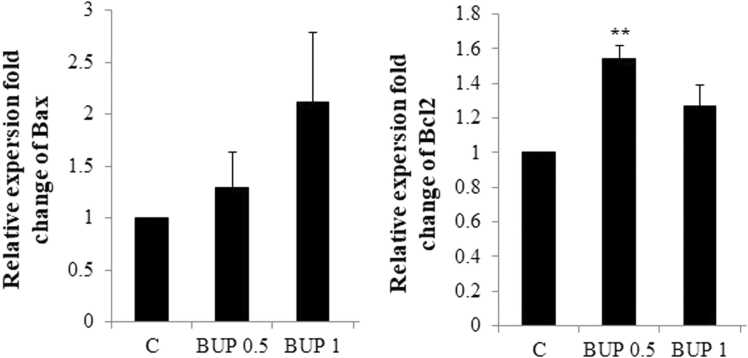
BUP (0.5 and 1 mg/kg) could not change the Bax expression in the hippocampus of neonate versus the controls. The significant difference was not observed in the Bax expression levels in the hippocampus of neonates between BUP 0.5 and BUP 1 groups (Fig. 1).
Fig. 1.
Bax and Bcl2 mRNA expressions in neonatal rat hippocampus.
Abbreviations: C: control, BUP 0.5: Buprenorphine 05 mg/kg, BUP 1: Buprenorphine 1 mg/kg.
Significant difference between the data of the C group vs other control groups: * *; p < 0.01.
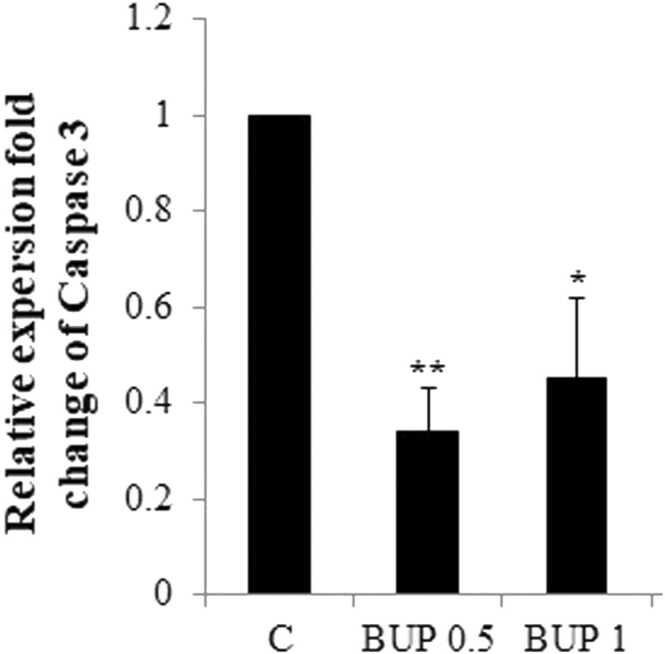
BUP (0.5 mg/kg) significantly increased neonatal hippocampal expression levels of Bcl2 compared with controls (p = 0.003). The significant difference was not observed in the Bcl2 expression levels in neonatal hippocampus of neonates born to mothers exposed to BUP at 1 mg/kg during gestation and control animals (Fig. 1). BUP)0.5 and 1 mg/k( significantly decreased neonatal hippocampal expression levels of Caspase 3 compared with controls (p = 0.003, p = 0.01, respectively). There was no significant difference in the Caspase 3 expression levels in the hippocampus of neonates in BUP 0.5 and BUP 1 groups (Fig. 2).
Fig. 2.
Caspase 3 mRNA expressions in neonatal rat hippocampus.
Abbreviations: C: control, BUP 0.5: Buprenorphine 05 mg/kg, BUP 1: Buprenorphine 1 mg/kg.
Significant difference between the data of the C group vs other control groups: * ; p < 0.05, * *; p < 0.01.
13. Discussion
In this study we show increased GSH and TAC levels as well the Bcl2 expression levels in neonatal hippocampus of pus born to dams treated during gestation with BUP (0.5 mg/kg). In addition, BUP at 0.5 and 1 mg/kg decreased the expression levels of Caspase 3 in the hippocampus of these animals.
The developing human and animal brain displays high expression levels of µ-OPIOID receptors. The prenatal period is exquisitely critical for development.
Numerous studies have shown that opioids, including BUP, are readily transported across the placental barrier to the fetus and accumulate in the brain, especially in developing animals which lack an intact blood-brain barrier [18], [19]. Our study assessed the toxic effect of BUP on oxidative stress and apoptosis in the developing hippocampus.
A mechanism implicated in opioids-induced neurotoxicity is oxidative stress, as evidenced by increased lipid peroxidation products [20], [21] of polyunsaturated fatty acid (PUFA) chains, leading to the neurotoxicity [22].
We found that 0.5 mg/kg of BUP increased TAC and GSH levels in the hippocampus of animals. Furthermore, we observed that lower dose of BUP (0.5 mg /kg) potentiated the antioxidant system which may cause the stimulatory impact of BUP on delta receptors [23]. In contrast, our previous study indicated that BUP (1 mg/kg) markedly elevated the hippocampal concentration of MDA as well as reduced the GSH concentration [24]. Activation of delta opioid receptors increases the tolerance of the tissue to oxidative stress by low doses of opioids [23]. Activation of delta opioid receptors caused an increase in the expression and translocation of Nrf2, resulted in the cytoprotection [25].
It was found that the high doses of opioids stimulates dopamine receptors, leading to a decrease in the antioxidant content in rat brains [26].
Apoptotic changes are inherent to drug-induced cytotoxicity in the hippocampus [27]. Apoptosis has a critical role in CNS development, and it is regulated both by stimulatory (Bax and Bad), and several inhibitory apoptotic genes [28], [29], [30].
Bax protein is located in the mitochondrial membrane and apoptosis ensues following Bax over-expression [31]. Bax is induced by several apoptotic signaling molecules, leading to the cytochrome c release from the mitochondria and apoptotic induction. Bcl-2 can inhibit Bax activity and cytochrome c release [32], [33].
We observed that Bcl-2 expression in 0.5 mg/kg BUP exposure was increased in the hippocampus of animals. In contrast, previous studies suggested that ROS over-production following opioid administration might reduce the antioxidant levels in cells, leading to increased mitochondrial permeability, thereby inducing the release of mitochondrial proteins, such as Bax, and altering the function of survival-regulating factors such as Bcl-2 [34]. The seemingly discordant findings between the present and previous studies may be related to the low bioavailability of BUP in the fetus after transfer through the placenta. It has yet to be determined whether low dose opioids can potentiate the antioxidant system by stimulating delta receptors, thereby inhibiting apoptotic pathways.
In conclusion, we found that neonatal rat hippocampal TAC and GSH levels and BCl2 expression increased upon 0.5 mg/kg BUP exposure. In addition, a decrease in the expression of caspase 3 was found in neonatal rat hippocampus of pups born to mothers exposed to BUP at doses 0.5 and 1 mg/kg. These findings suggest that BUP at doses 0.5 and 1 mg/kg fails to elicit oxidative and apoptotic damage in the hippocampus of these pups. However, more investigations are required to find the mechanisms of BUP’s effect in the brain during the sensitive stage of brain development and its related toxic mechanisms.
Funding
Research reported in this publication was supported by Young Researcher Grant Committee under award number [978651] from the National Institutes for Medical Research Development (NIMAD), Tehran, Iran.
Ethics approval and consent to participate
This study was approved by the ethic committee of National Institutes for Medical Research Development (NIMAD).
Human and animal rights
All animal research procedures followed were in accordance with the standards of Guide for the US National Research Council's and Guide for the Care and Use of Laboratory Animals.
Consent for publication
Not applicable.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author, [TF], upon reasonable request.
References
- 1.Minozzi S., Amato L., Davoli M. Development of dependence following treatment with opioid analgesics for pain relief: a systematic review. Addiction. 2013;108(4):688–698. doi: 10.1111/j.1360-0443.2012.04005.x. [DOI] [PubMed] [Google Scholar]
- 2.Kongstorp M., Bogen I.L., Stiris T., Andersen J.M. High accumulation of methadone compared with buprenorphine in fetal rat brain after maternal exposure. J. Pharmacol. Exp. Ther. 2019;371(1):130–137. doi: 10.1124/jpet.119.259531. [DOI] [PubMed] [Google Scholar]
- 3.Hauser K.F., McLaughlin P.J., Zagon I.S. Endogenous opioids regulate dendritic growth and spine formation in developing rat brain. Brain Res. 1987;416(1):157–161. doi: 10.1016/0006-8993(87)91509-5. [DOI] [PubMed] [Google Scholar]
- 4.Lee S.J., Bora S., Austin N.C., Westerman A., Henderson J.M. Neurodevelopmental outcomes of children born to opioid-dependent mothers: a systematic review and meta-analysis. Acad. Pediatr. 2020;20(3) doi: 10.1016/j.acap.2019.11.005. 308-18. [DOI] [PubMed] [Google Scholar]
- 5.Lee S.J., Pritchard V.E., Austin N.C., Henderson J.M., Woodward L.J. Health and neurodevelopment of children born to opioid-dependent mothers at school entry. J. Develop. Behav. Pediatr. 2020;41(1):48–57. doi: 10.1097/DBP.0000000000000711. [DOI] [PubMed] [Google Scholar]
- 6.Monnelly V.J., Hamilton R., Chappell F.M., Mactier H., Boardman J.P. Childhood neurodevelopment after prescription of maintenance methadone for opioid dependency in pregnancy: a systematic review and meta‐analysis. Develop. Med. Child Neurol. 2019;61(7) doi: 10.1111/dmcn.14117. 750-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H.-H., Chiang Y.-C., Yuan Z.F., Kuo C.-C., Lai M.-D. Hung T-W. Buprenorphine, methadone, and morphine treatment during pregnancy: behavioral effects on the offspring in rats. Neuropsychiatr. Dis. Treat. 2015;11:609. doi: 10.2147/NDT.S70585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kongstorp M., Bogen I.L., Stiris T., Andersen J.M. Prenatal exposure to methadone or buprenorphine impairs cognitive performance in young adult rats. Drug Alcohol Depend. 2020;212 doi: 10.1016/j.drugalcdep.2020.108008. [DOI] [PubMed] [Google Scholar]
- 9.Kongstorp M., Bogen I.L., Steinsland S., Nerem E., Salih T.W., Stiris T., et al. Prenatal exposure to methadone or buprenorphine alters µ‐opioid receptor binding and downstream signaling in the rat brain. Intern. J. Develop. Neurosci. 2020;80(5):443–453. doi: 10.1002/jdn.10043. [DOI] [PubMed] [Google Scholar]
- 10.Liao D.-L., Huang C.-Y., Hu S., Fang S.-C., Wu C.-S., Chen W.-T., et al. Cognitive control in opioid dependence and methadone maintenance treatment. PloS one. 2014;9(4) doi: 10.1371/journal.pone.0094589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanchez E.S., Bigbee J.W., Fobbs W., Robinson S.E., Sato‐Bigbee C. Opioid addiction and pregnancy: perinatal exposure to buprenorphine affects myelination in the developing brain. Glia. 2008;56(9):1017–1027. doi: 10.1002/glia.20675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vestal-Laborde A.A., Eschenroeder A.C., Bigbee J.W., Robinson S.E., Sato-Bigbee C. The opioid system and brain development: effects of methadone on the oligodendrocyte lineage and the early stages of myelination. Develop. Neurosci. 2014;36(5) doi: 10.1159/000365074. 409-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi M.-M., Fan K.-M., Qiao Y.-N., Xu J.-H., Qiu L.-J., Li X., et al. Hippocampal µ-opioid receptors on GABAergic neurons mediate stress-induced impairment of memory retrieval. Mol. Psychiatr. 2020;25(5) doi: 10.1038/s41380-019-0435-z. 977-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meilandt W.J., Barea-Rodriguez E., Harvey S.A., Martinez J.L. Role of hippocampal CA3 μ-opioid receptors in spatial learning and memory. J. Neurosci. 2004;24(12):2953–2962. doi: 10.1523/JNEUROSCI.5569-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uchiyama M., Mihara M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978;86(1):271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 16.Roth E.F., Jr., Gilbert H.S. The pyrogallol assay for superoxide dismutase: absence of a glutathione artifact. Anal. Biochem. 1984;137(1):50–53. doi: 10.1016/0003-2697(84)90344-0. [DOI] [PubMed] [Google Scholar]
- 17.Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 18.Coles L.D., Lee I.J., Hassan H.E., Eddington N.D. Distribution of saquinavir, methadone, and buprenorphine in maternal brain, placenta, and fetus during two different gestational stages of pregnancy in mice. J. Pharmaceut. Sci. 2009;98(8):2832–2846. doi: 10.1002/jps.21644. [DOI] [PubMed] [Google Scholar]
- 19.Goasdoué K., Miller S.M., Colditz P.B., Björkman S.T. The blood-brain barrier; protecting the developing fetal brain. Placenta. 2017;54:111–116. doi: 10.1016/j.placenta.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Cunha-Oliveira T., Rego A.C., Oliveira C.R. Cellular and molecular mechanisms involved in the neurotoxicity of opioid and psychostimulant drugs. Brain Res. Rev. 2008;58(1):192–208. doi: 10.1016/j.brainresrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Shibani F., Sahamsizadeh A., Fatemi I., Allahtavakoli M., Hasanshahi J., Rahmani M., et al. Effect of oleuropein on morphine-induced hippocampus neurotoxicity and memory impairments in rats. Naunyn-Schmiedeberg's archives of pharmacology. 2019;392(11) doi: 10.1007/s00210-019-01678-3. 1383-91. [DOI] [PubMed] [Google Scholar]
- 22.Di Nunzio M., Valli V., Bordoni A. PUFA and oxidative stress. Differential modulation of the cell response by DHA. Intern. J. Food Sci. Nutrition. 2016;67(7):834–843. doi: 10.1080/09637486.2016.1201790. [DOI] [PubMed] [Google Scholar]
- 23.Rebrova T.Y., Maslov L., Lishmanov A.Y., Tam S. Stimulation of μ-and δ-opiate receptors and tolerance of isolated heart to oxidative stress: the role of NO-synthase. Biochemistry. 2001;66(4):422–428. doi: 10.1023/a:1010253530026. [DOI] [PubMed] [Google Scholar]
- 24.Samini M., Farkhondeh T., Azimi-Nezhad M., Samarghandian S. Impact of buprenorphine on learning and memory ability, oxidative status and inflammation in the hippocampus of rats. Curr. Mol. Pharmacol. 2021;14(4):597–603. doi: 10.2174/1874467213666200727123224. [DOI] [PubMed] [Google Scholar]
- 25.Cao S., Chao D., Zhou H., Balboni G., Xia Y. A novel mechanism for cytoprotection against hypoxic injury: δ‐opioid receptor‐mediated increase in N rf2 translocation. British J. Pharmacol. 2015;172(7):1869–1881. doi: 10.1111/bph.13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sadat-Shirazi M.-S., Zarrindast M.-R., Ashabi G. Oxidative stress enzymes are changed in opioid abusers and multidrug abusers. J. Clin. Neurosci. 2020;72:365–369. doi: 10.1016/j.jocn.2019.12.064. [DOI] [PubMed] [Google Scholar]
- 27.Choi H.J., Kang K.S., Fukui M., Zhu B.T. Critical role of the JNK‐p53–GADD45α apoptotic cascade in mediating oxidative cytotoxicity in hippocampal neurons. British J. Pharmacol. 2011;162(1):175–192. doi: 10.1111/j.1476-5381.2010.01041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng C.Y., Rise M.L. Characterization and expression analyses of anti-apoptotic Bcl-2-like genes NR-13, Mcl-1, Bcl-X1, and Bcl-X2 in Atlantic cod (Gadus morhua) Mol. Immunol. 2010;47(4):763–784. doi: 10.1016/j.molimm.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 29.Hsu Y.-T., Wolter K.G., Youle R.J. Cytosol-to-membrane redistribution of Bax and Bcl-XL during apoptosis. Proceed. Nat. Acad. Sci. 1997;94(8):3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lalier L., Cartron P.-F., Juin P., Nedelkina S., Manon S., Bechinger B., et al. Bax activation and mitochondrial insertion during apoptosis. Apoptosis. 2007;12(5):887–896. doi: 10.1007/s10495-007-0749-1. [DOI] [PubMed] [Google Scholar]
- 31.Ohtsuka T., Ryu H., Minamishima Y.A., Macip S., Sagara J., Nakayama K.I., et al. ASC is a Bax adaptor and regulates the p53–Bax mitochondrial apoptosis pathway. Nature Cell Biol. 2004;6(2):121–128. doi: 10.1038/ncb1087. [DOI] [PubMed] [Google Scholar]
- 32.Desagher S., Osen-Sand A., Nichols A., Eskes R., Montessuit S., Lauper S., et al. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J. Cell Biol. 1999;144(5):891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pastorino J.G., Shulga N., Hoek J.B. Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J. Biol. Chem. 2002;277(9):7610–7618. doi: 10.1074/jbc.M109950200. [DOI] [PubMed] [Google Scholar]
- 34.Saeed Samarghandian, Mohsen Azimi-Nezhad, Reza Afshari, Tahereh Farkhondeh, Fatemeh Karimnezhad. Effects of buprenorphine on balance of oxidant/antioxidant system in the different ages of male rat liver. J Biochem Mol Toxicol. 2015;29(6):249–253. doi: 10.1002/jbt.21691. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, [TF], upon reasonable request.