Key Points
Question
What are the risks and risk factor characteristics for nonkeratinocyte skin cancers in solid organ transplant recipients (SOTRs)?
Findings
In this US cohort study of 444 497 SOTRs, melanoma and Merkel cell carcinoma were the most common nonkeratinocyte skin cancers. Risks were elevated for cancers associated with viruses; important risk factors were time since transplant and factors related to UV radiation exposure; and treatment with mammalian target of rapamycin inhibitors was associated with reduced risk of melanoma.
Meaning
Some SOTRs may benefit from enhanced surveillance, and mammalian target of rapamycin inhibitors may be effective for melanoma chemoprevention in SOTRs.
This cohort study examines the spectrum of disease and risk factors for nonkeratinocyte skin cancers in solid organ transplant recipients.
Abstract
Importance
Nonkeratinocyte skin cancers are an important cause of morbidity and mortality for immunosuppressed solid organ transplant recipients (SOTRs), but the spectrum of disease and risk factor characteristics are unknown.
Objective
To characterize the spectrum of disease and risk factors for common and rare nonkeratinocyte skin cancers in SOTRs.
Design, Setting, and Participants
This population-based cohort study included 444 497 SOTRs who underwent a transplant in the US between January 1, 1987, and December 31, 2017, using linked data from the national transplant registry and 32 cancer registries. Data analysis was conducted from April 1, 2021, to September 30, 2021.
Main Outcomes and Measures
Standardized incidence ratios (SIRs) were used to assess risk relative to the general population, and Poisson regression was used to evaluate risk factors.
Results
A total of 2380 nonkeratinocyte skin cancers were identified among 444 497 SOTRs (median age at transplant, 50 years; range, 0-96 years; 274 276 [61.7%] male; 272 241 [61.2%] non-Hispanic White). Melanoma was the most common cancer (1471 [61.8%]), followed by Merkel cell carcinoma (334 [14.0%]), Kaposi sarcoma (186 [7.8%]), sebaceous carcinoma (170 [7.1%]), and cutaneous lymphomas (108 [4.5%]). Risks were most strongly elevated for cancers associated with viruses, including Kaposi sarcoma (SIR, 20.5; 95% CI, 17.7-23.7), Merkel cell carcinoma (SIR, 16.2; 95% CI, 14.5-18.1), and extranodal natural killer/T-cell lymphoma (SIR, 44.3; 95% CI, 5.37-160). Risks were also significantly elevated for sebaceous carcinoma (SIR, 15.2; 95% CI, 13.0-17.7), anaplastic large cell lymphoma (SIR, 6.82; 95% CI, 4.53-9.85), and diffuse large B-cell lymphoma (SIR, 5.17; 95% CI, 3.28-7.76). Several characteristics were independently associated with greater risk for multiple skin cancer types, including male sex, older age at transplant, factors associated with UV radiation exposure (non-Hispanic White race and ethnicity, living in an area with higher UV radiation exposure, and posttransplant diagnosis of keratinocyte carcinoma), and increasing time since transplantation. Treatment with mammalian target of rapamycin inhibitors was associated with reduced melanoma incidence (incidence rate ratio, 0.75; 95% CI, 0.57-0.98). A total of 847 skin cancers (39.4%) occurred on the head and neck.
Conclusions and Relevance
The findings of this cohort study suggest that viruses, UV radiation exposure, and immunosuppression are associated with the development of skin cancer in SOTRs. Certain high-risk subgroups may benefit from increased skin surveillance, and treatment with mammalian target of rapamycin inhibitors could be effective for melanoma chemoprevention in the transplant population.
Introduction
Solid organ transplantation provides life-saving treatment for patients with end-stage organ disease. Solid organ transplant recipients (SOTRs) are therapeutically immunosuppressed to prevent rejection of their transplanted organ, but this long-term immunosuppression increases the risk of cancers caused by viruses (eg, non-Hodgkin lymphoma caused by Epstein-Barr virus [EBV]) and some cancers unrelated to infections (eg, melanoma).1
Skin is a common site for cancers in SOTRs, but risk factors for skin cancer have not been systematically evaluated for this immunosuppressed group.2 In the general population, UV radiation (UVR) exposure is a major etiologic factor for keratinocyte carcinomas (ie, squamous cell carcinoma [SCC] and basal cell carcinoma [BCC]), melanoma, Merkel cell carcinoma (MCC), and sebaceous carcinoma, and these cancers frequently occur on sun-exposed parts of the body.3,4,5 Photosensitizing medications, such as azathioprine, may amplify the carcinogenic effect of UVR exposure for cancers, such as SCC.6 Viruses have also been causally linked to MCC and Kaposi sarcoma (KS).5,7
For SOTRs, the most common skin cancers are keratinocyte carcinomas, followed by melanoma.8,9 Risks are greatly elevated for SCC and rarer skin cancers, including MCC, sebaceous carcinoma, and KS, with reported increases of more than 20-fold compared with the general population.10,11,12,13,14 Other rare skin cancers (eg, adnexal cancers and cutaneous sarcomas) and cutaneous lymphomas have not been comprehensively studied using population data.
Transplant-associated skin cancers often behave aggressively (eg, with melanoma-specific mortality increased 3-fold compared with patients in the general population).8,15 Immune checkpoint inhibitors, which provide improved survival for patients with advanced melanoma and MCC,5,16 are not a viable treatment option for most SOTRs because of the high risk of allograft rejection, which occurs in 40% to 50% of patients.17,18 Given limited therapeutic options, primary prevention and screening strategies are critically important for early cancer detection and improving survival.19,20
In this study, we systematically evaluated the risk for 25 skin cancer types in SOTRs. We also assessed body site distribution patterns for posttransplant cutaneous cancers and their associations with measures of exposure to UVR and immunosuppression. Precise risk estimates along with detailed body mapping analyses of tumor occurrence can help elucidate the cause of these cancers, guide prevention and early detection, and reduce skin cancer morbidity and mortality for SOTRs.
Methods
The Transplant Cancer Match Study links the Scientific Registry of Transplant Recipients (SRTR) with 32 US cancer registries (Table 1). Additional details about the Transplant Cancer Match Study are available online21 and were described previously.1,12,13 Informed consent was not required by the participating cancer registries and the SRTR because data were deidentified. This cohort study was approved by human participants’ review committees at participating cancer registries and follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Table 1. Clinical Characteristics of Solid Organ Transplant Recipients in the US.
| Characteristic | No. (%)a |
|---|---|
| Transplants | |
| First | 402 229 (90.5) |
| Second or later | 42 268 (9.5) |
| Total | 444 497 (100) |
| Sex | |
| Female | 170 221 (38.3) |
| Male | 274 276 (61.7) |
| Race and ethnicity | |
| Asian or Pacific Islander | 23 818 (5.4) |
| Hispanic | 66 336 (14.9) |
| Non-Hispanic Black | 82 102 (18.5) |
| Non-Hispanic White | 272 241 (61.2) |
| Age at transplant, y | |
| 0-17 | 30 665 (6.9) |
| 18-34 | 63 187 (14.2) |
| 35-49 | 126 422 (28.4) |
| 50-64 | 173 212 (39.0) |
| ≥65 | 51 011 (11.5) |
| Transplanted organ | |
| Kidney | 259 845 (58.5) |
| Liver | 93 200 (21.0) |
| Heart | 41 852 (9.4) |
| Lung | 22 225 (5.0) |
| Other or multiple | 27 375 (6.2) |
| Calendar year of transplant | |
| 1987-1994 | 41 366 (9.3) |
| 1995-2002 | 127 875 (28.8) |
| 2003-2010 | 160 466 (36.1) |
| 2011-2017 | 114 790 (25.8) |
A total of 444 497 transplant procedures were performed for 410 176 individuals during the study period. Transplant data were available for Alaska (calendar years of cancer data: 1996-2017), California (1995-2017), Colorado (1995-2016), Connecticut (1995-2017), Florida (1995-2009), Georgia (1995-2017), Hawaii (1995-2017), Iowa (1995-2017), Idaho (1995-2017), Illinois (1995-2013), Kentucky (1995-2017), Louisiana (1995-2017), Michigan (1995-2009), Montana (1995-2017), Nebraska (1995-2017), Nevada (1995-2015), New Jersey (1995-2016), New Mexico (1995-2016), New York (1995-2017), North Carolina (1995-2010), North Dakota (1995-2016), Ohio (1995-2015), Oklahoma (1995-2017), Oregon (1995-2016), Pennsylvania (1995-2017), Puerto Rico (1995-2016), Rhode Island (1995-2015), Seattle/Puget Sound (1995-2017), South Carolina (1995-2016), Texas (1995-2016), Utah (1995-2017), and Virginia (1995-2016).
Solid organ transplant recipients were considered at risk of cancer starting at transplantation or January 1, 1995 (whichever was later), and followed up until the earliest of death, graft failure or subsequent transplantation, loss to follow-up, or end of cancer registry coverage (the latest follow-up was December 31, 2017). For SOTRs who received their transplant during 1987 to 1994, before availability of cancer registry data, cancer risk was assessed starting on January 1, 1995 (exclusion of recipients with delayed entry did not affect skin cancer risk estimates). Follow-up for patients with subsequent transplants (9.5% of transplants) was continued as additional person-time at risk. Solid organ transplant recipients were not censored when they developed their first malignant tumor and could develop multiple cancers.
We identified invasive skin cancers from the linked cancer registry data using International Classification of Diseases for Oncology (Third Edition) site codes (C44.0-C44.9) and histology codes (eTable 1 in the Supplement). Keratinocyte carcinomas are not captured by cancer registries and consequently were not included as outcomes, although we considered diagnoses recorded in the SRTR as risk factors for other skin cancers.
We calculated standardized incidence ratios (SIRs) to compare skin cancer risk in SOTRs with risk in the general population. The SIR is the number of observed cancers among SOTRs divided by the number of expected cancers, estimated from incidence rates in Surveillance, Epidemiology, and End Results (SEER) 13 cancer registries stratified by sex, age, race and ethnicity, and calendar year.22 For KS, we used SEER 9 rates from 1973 to 1979 because general-population KS rates since 1980 largely reflect cases in people with HIV infection.23
We used Poisson regression to calculate incidence rate ratios (IRRs) to identify risk factors for skin cancers within the transplant population. We evaluated the following risk factors: demographic characteristics (sex, race and ethnicity, age at transplant, and calendar year of transplant); measures of immunosuppression (time since transplant, transplant number, and intensity as captured by transplanted organ [least intense for liver, intermediate for kidney, and most intense for heart and/or lung]); and baseline induction and maintenance immunosuppressive medications. Recipient and donor EBV serologic status at baseline was assessed as a risk factor for lymphoma. As a measure of UVR exposure, we used annual mean daily UVR levels (cloud adjusted, 305 nm, averaged over years 1982-1992) in the residential zip code of SOTRs at the time of transplantation. The UVR data were derived from the Total Ozone Mapping Spectrometer database maintained by the National Aeronautics and Space Administration.24 The SRTR collects information on posttransplant SCC and BCC diagnoses from transplant centers. A large proportion of keratinocyte carcinomas are missed by the SRTR (sensitivity, 14%-41%), but keratinocyte carcinoma reporting has high positive predictive value (71%-88%).25,26 We assessed the occurrence of these UVR-related skin cancers as time-dependent risk factors for other skin cancers in our Poisson models. Unadjusted IRRs are presented in eTables 2 and 3 in the Supplement, and we report IRRs from the full multivariate models as our primary results.
We mapped the distribution of skin cancers by body site (head and neck, trunk, upper extremities, and lower extremities) and report observed to expected (O:E) ratios for all cases combined and for the most common cancers. The expected number of cancers was determined by multiplying the total number of cases at all sites (excluding overlapping and unspecified sites) by the proportional body surface area (head and neck, 9%; trunk, 37%; upper extremities, 18%; and lower extremities, 36%). Body surface area estimates were derived from Wallace’s Rule of Nines.27
All statistical tests were 2-sided. The SIR estimates were considered significant at P < .002 based on a Bonferroni correction for multiple comparisons of 25 cancer types. For IRR estimates, P < .05 was the cutoff for statistical significance.
Results
A total of 2380 nonkeratinocyte skin cancers were identified among 444 497 SOTRs (median age at transplant, 50 years; range, 0-96 years; 274 276 [61.7%] male and 170 221 [38.3%] female; 23 818 [5.4%] Asian or Pacific Islander, 66 336 [14.9%] Hispanic, 82 102 [18.5%] non-Hispanic Black, and 272 241 [61.2%] non-Hispanic White) (Table 1), comprising 66% of all US transplants during 1987 to 2017. The most commonly transplanted organ was the kidney (259 845 [58.5%]) followed by the liver (93 200 [21.0%]), heart (41 852 [9.4%]), and lung (22 225 [5.0%]). Solid organ transplant recipients were followed up for 2 561 537 person-years (median follow-up, 4.6 years; IQR, 1.8-8.6 years; 88 697 SOTRs [20.0%] were followed up for at least 10 years).
There were 2380 nonkeratinocyte skin cancers diagnosed, corresponding to a 2-fold increased risk compared with the general population (SIR, 2.18; 95% CI, 2.09-2.27; P < .001) and an excess absolute risk of 50.3 additional skin cancers per 100 000 person-years (Table 2). The most common skin cancers were melanoma (1471 [61.8%]), MCC (334 [14.0%]), KS (186 [7.8%]), sebaceous carcinoma (170 [7.1%]), and lymphomas (108 [4.5%]). The greatest elevations in risk were observed for KS (SIR, 20.5; 95% CI, 17.7-23.7), MCC (SIR, 16.2; 95% CI, 14.5-18.1), and sebaceous carcinoma (SIR, 15.2; 95% CI, 13.0-17.7). For melanoma, risk was increased overall (SIR, 1.52; 95% CI, 1.44-1.60), with the greatest elevation observed for amelanotic (SIR, 3.14; 95% CI, 1.62-5.49) and nodular (SIR, 1.60; 95% CI, 1.31-1.94) subtypes. Risk was also increased for lymphomas (SIR, 2.25; 95% CI, 1.84-2.71), with the greatest increase observed for anaplastic large cell lymphoma (SIR, 6.82; 95% CI, 4.53-9.85) and diffuse large B-cell lymphoma (SIR, 5.17; 95% CI, 3.28-7.76). Risk was highly elevated for extranodal natural killer/T-cell lymphoma, nasal type (SIR, 44.3; 95% CI, 5.37-160), but this finding was not statistically significant after Bonferroni correction (P = .002). Recipient and donor EBV status was not associated with lymphoma risk for SOTRs, although there were substantial missing data (eTable 4 in the Supplement). Risk for sarcomas was not increased (Table 2).
Table 2. Standardized Incidence Ratios (SIRs) for Cutaneous Cancers.
| Cancer type | No. of cases (%) | SIR (95% CI) | P valuea |
|---|---|---|---|
| All skin cancer types, excluding keratinocyte carcinomas | 2380 (100) | 2.18 (2.09-2.27) | <.001 |
| Melanoma | 1471 (61.8) | 1.52 (1.44-1.60) | <.001 |
| Superficial spreading melanoma | 428 (18.0) | 1.30 (1.18-1.42) | <.001 |
| Lentigo maligna melanoma | 114 (4.8) | 1.31 (1.08-1.58) | .006 |
| Nodular melanoma | 105 (4.4) | 1.60 (1.31-1.94) | <.001 |
| Acral lentiginous melanoma | 13 (0.5) | 1.32 (0.70-2.26) | .39 |
| Desmoplastic melanoma | 18 (0.8) | 1.56 (0.92-2.46) | .10 |
| Amelanotic melanoma | 12 (0.5) | 3.14 (1.62-5.49) | .001 |
| Other melanoma subtypes or not specified | 781 (32.8) | 1.69 (1.57-1.81) | <.001 |
| Merkel cell carcinoma | 334 (14.0) | 16.2 (14.5-18.1) | <.001 |
| Adnexal cancersb | 254 (10.7) | 10.1 (8.88-11.4) | <.001 |
| Sebaceous carcinoma | 170 (7.1) | 15.2 (13.0-17.7) | <.001 |
| Eccrine carcinoma | 51 (2.1) | 5.59 (4.16-7.35) | <.001 |
| Sweat gland adenocarcinoma | 8 (0.3) | 8.04 (3.47-15.8) | <.001 |
| Nodular hidradenoma, malignant | 7 (0.3) | 6.48 (2.60-13.3) | <.001 |
| Sclerosing sweat duct carcinoma | 10 (0.4) | 4.93 (2.36-9.06) | <.001 |
| Eccrine | |||
| Papillary adenocarcinoma | 1 (0.04) | 2.42 (0.06-13.5) | .68 |
| Poroma, malignant | 11 (0.5) | 6.51 (3.25-11.6) | <.001 |
| Adenocarcinoma | 8 (0.3) | 5.66 (2.44-11.1) | <.001 |
| Mucinous adenocarcinoma | 6 (0.3) | 3.99 (1.47-8.69) | .009 |
| Apocrine adenocarcinoma | 6 (0.3) | 7.56 (2.78-16.5) | <.001 |
| Adenoid cystic carcinoma | 3 (0.1) | 3.73 (0.77-10.9) | .10 |
| Skin appendage carcinoma | 24 (1.0) | 7.25 (4.65-10.8) | <.001 |
| Sarcomasc | 27 (1.1) | 1.43 (0.94-2.08) | .09 |
| Dermatofibrosarcoma protuberans | 11 (0.5) | 0.82 (0.41-1.46) | .62 |
| Cutaneous | |||
| Leiomyosarcoma | 8 (0.3) | 2.32 (1.00-4.56) | .05 |
| Angiosarcoma | 7 (0.3) | 3.62 (1.46-7.46) | .008 |
| Glomangiosarcoma | 1 (0.04) | 56.2 (1.42-313) | .04 |
| Kaposi sarcoma | 186 (7.8) | 20.5 (17.7-23.7) | <.001 |
| Lymphomad | 108 (4.5) | 2.25 (1.84-2.71) | <.001 |
| T-cell lymphoma | 85 (3.6) | 1.95 (1.56-2.41) | <.001 |
| Mycosis fungoides | 29 (1.2) | 1.05 (0.71-1.51) | .83 |
| Sézary syndrome | 2 (0.1) | 2.25 (0.27-8.13) | .45 |
| Anaplastic large cell lymphoma | 28 (1.2) | 6.82 (4.53-9.85) | <.001 |
| Subcutaneous panniculitis-like T-cell lymphoma | 2 (0.1) | 13.2 (1.59-47.5) | .02 |
| Extranodal natural killer/T-cell lymphoma, nasal type | 2 (0.1) | 44.3 (5.37-160) | .002 |
| Peripheral T-cell lymphoma, not otherwise specified | 22 (0.9) | 2.01 (1.26-3.05) | .004 |
| Diffuse large B-cell lymphoma | 23 (1.0) | 5.17 (3.28-7.76) | <.001 |
The SIR estimates were considered significant at P < .002 based on a Bonferroni correction for multiple comparisons of 25 cancer types.
There were no cases of the following cutaneous adnexal cancers: malignant eccrine spiradenoma, ceruminous adenocarcinoma, trichilemmocarcinoma, malignant pilar tumor, and pilomatrix carcinoma.
There were no cases of the following cutaneous sarcomas: clear cell sarcoma, malignant peripheral nerve sheath tumor, liposarcoma, and angiomyoliposarcoma.
There were no cases of the following cutaneous lymphomas or histiocytic disorders: adult T-cell leukemia-lymphoma, follicle center lymphoma, and Langerhans cell histiocytosis.
Risk factors are reported for melanoma (Table 3), MCC (Table 3), sebaceous carcinoma (Table 3), nonsebaceous adnexal cancers (NSACs) (Table 3), KS (Table 4), and lymphomas (Table 4). Older age (≥65 years compared with 0-34 years) at transplant was associated with increased risk for each cancer(IRR, 9.03; 95% CI, 7.01-11.6 for melanoma; IRR, 22.7; 95% CI, 11.3-45.8 for MCC; IRR, 21.7; 95% CI, 7.62-62.0 for sebaceous carcinoma: IRR, 10.2; 95% CI, 3.35-30.9 for NSACs; IRR, 13.9; 95% CI, 7.18-27.0 for KS; and IRR, 2.52; 95% CI, 1.28-4.99 for lymphomas). Risks of melanoma and MCC were decreased for non-Hispanic Black (IRR, 0.05; 95% CI, 0.03-0.08 for melanoma and IRR, 0.09; 95% CI, 0.04-0.22 for MCC), Hispanic or Latino (IRR, 0.11; 95% CI, 0.08-0.16 for melanoma and IRR, 0.40; 95% CI, 0.25-0.63 for MCC), and Asian or Pacific Islander (IRR, 0.05; 95% CI, 0.02-0.11 for melanoma and IRR, 0.23; 95% CI, 0.10-0.56 for MCC) SOTRs compared with non-Hispanic White SOTRs. Similarly, risks of sebaceous carcinoma and NSACs were also lower for non-Hispanic Black SOTRs (IRR, 0.41; 95% CI, 0.21-0.82 for sebaceous carcinoma and IRR, 0.13; 95% CI, 0.03-0.54 for NSACS) (vs non-Hispanic White SOTRs). Male sex was associated with increased risk of melanoma (IRR, 1.86; 95% CI, 1.65-2.11), MCC (IRR, 1.90; 95% CI, 1.46-2.48), sebaceous carcinoma (IRR, 2.01; 95% CI, 1.38-2.91), and KS (IRR, 2.37; 95% CI, 1.65-3.39).
Table 3. Risk of Melanoma, Merkel Cell Carcinoma, and Adnexal Cancers in Transplant Recipients.
| Characteristic | Melanoma | Merkel cell carcinoma | Sebaceous carcinoma | Nonsebaceous adnexal cancers | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. (n = 1471) | IRR (95% CI)a | P value | No. (n = 334) | IRR (95% CI)a | P value | No. (n = 170) | IRR (95% CI)a | P value | No. (n = 84) | IRR (95% CI)a | P value | |
| Sex | ||||||||||||
| Female | 334 | 1 [Reference] | NA | 72 | 1 [Reference] | NA | 36 | 1 [Reference] | NA | 24 | 1 [Reference] | NA |
| Male | 1137 | 1.86 (1.65-2.11) | <.001 | 262 | 1.90 (1.46-2.48) | <.001 | 134 | 2.01 (1.38-2.91) | <.001 | 60 | 1.30 (0.81-2.11) | .28 |
| Race and ethnicity | ||||||||||||
| Asian or Pacific Islander | 6 | 0.05 (0.02-0.11) | <.001 | 5 | 0.23 (0.10-0.56) | .001 | 11 | 1.34 (0.71-2.52) | .37 | 2 | 0.33 (0.08-1.34) | .12 |
| Hispanic or Latino | 31 | 0.11 (0.08-0.16) | <.001 | 21 | 0.40 (0.25-0.63) | <.001 | 23 | 1.29 (0.80-2.08) | .30 | 4 | 0.25 (0.09-0.70) | .008 |
| Non-Hispanic Black | 15 | 0.05 (0.03-0.08) | <.001 | 5 | 0.09 (0.04-0.22) | <.001 | 9 | 0.41 (0.21-0.82) | .01 | 2 | 0.13 (0.03-0.54) | .005 |
| Non-Hispanic White | 1419 | 1 [Reference] | NA | 303 | 1 [Reference] | NA | 127 | 1 [Reference] | NA | 76 | 1 [Reference] | NA |
| Age at transplant, y | ||||||||||||
| 0-34 | 77 | 1 [Reference] | NA | 9 | 1 [Reference] | NA | 4 | 1 [Reference] | NA | 4 | 1 [Reference] | NA |
| 35-49 | 292 | 2.52 (1.96-3.25) | <.001 | 69 | 5.21 (2.60-10.4) | <.001 | 36 | 6.31 (2.24-17.7) | <.001 | 17 | 2.92 (0.98-8.71) | .05 |
| 50-64 | 776 | 5.31 (4.19-6.73) | <.001 | 177 | 11.4 (5.79-22.3) | <.001 | 95 | 13.2 (4.82-36.1) | <.001 | 45 | 6.13 (2.17-17.3) | .001 |
| ≥65 | 326 | 9.03 (7.01-11.6) | <.001 | 79 | 22.7 (11.3-45.8) | <.001 | 35 | 21.7 (7.62-62.0) | <.001 | 18 | 10.2 (3.35-30.9) | <.001 |
| P for trend | NA | NA | <.001 | NA | NA | <.001 | NA | NA | <.001 | NA | NA | <.001 |
| Transplanted organ | ||||||||||||
| Kidney | 814 | 1 [Reference] | NA | 206 | 1 [Reference] | NA | 97 | 1 [Reference] | 54 | 1 [Reference] | NA | |
| Liver | 273 | 0.65 (0.56-0.74) | <.001 | 46 | 0.46 (0.33-0.63) | <.001 | 21 | 0.44 (0.27-0.72) | .001 | 10 | 0.39 (0.19-0.77) | .007 |
| Heart and/or lung | 315 | 0.85 (0.74-0.98) | .02 | 72 | 0.71 (0.53-0.95) | .02 | 47 | 1.39 (0.95-2.04) | .09 | 19 | 0.75 (0.43-1.31) | .31 |
| Other or multiple | 69 | 0.93 (0.73-1.20) | .58 | 10 | 0.58 (0.30-1.10) | .09 | 5 | 0.61 (0.25-1.53) | .30 | 1 | 0.22 (0.03-1.59) | .13 |
| Maintenance immunosuppressive therapy | ||||||||||||
| Tacrolimus or mycophenolate mofetil without cyclosporine or azathioprine | 808 | 1 [Reference] | NA | 164 | 1 [Reference] | NA | 101 | 1 [Reference] | NA | 41 | 1 [Reference] | NA |
| Cyclosporine or azathioprine without tacrolimus or mycophenolate mofetil | 343 | 1.13 (0.95-1.36) | .17 | 103 | 0.94 (0.65-1.36) | .74 | 38 | 0.83 (0.50-1.39) | .48 | 27 | 1.22 (0.59-2.53) | .59 |
| Other medication combination | 320 | 1.10 (0.95-1.27) | .21 | 67 | 1.00 (0.72-1.38) | .99 | 31 | 0.66 (0.42-1.05) | .08 | 16 | 1.02 (0.53-1.96) | .95 |
| Exposure to mammalian target of rapamycin inhibitor | ||||||||||||
| No | 1413 | 1 [Reference] | NA | 307 | 1 [Reference] | NA | 160 | 1 [Reference] | NA | 77 | 1 [Reference] | NA |
| Yes | 58 | 0.75 (0.57-0.98) | .03 | 27 | 1.87 (1.24-2.81) | .003 | 10 | 1.14 (0.59-2.18) | .70 | 7 | 1.75 (0.78-3.93) | .17 |
| Posttransplant basal cell carcinoma | ||||||||||||
| No | 1378 | 1 [Reference] | NA | 294 | 1 [Reference] | NA | 156 | 1 [Reference] | NA | 74 | 1 [Reference] | NA |
| Yes | 93 | 1.81 (1.45-2.26) | <.001 | 40 | 2.30 (1.60-3.29) | <.001 | 14 | 1.59 (0.89-2.86) | .12 | 10 | 2.53 (1.23-5.20) | .01 |
| Posttransplant squamous cell carcinoma | ||||||||||||
| No | 1362 | 1 [Reference] | NA | 270 | 1 [Reference] | NA | 144 | 1 [Reference] | NA | 69 | 1 [Reference] | NA |
| Yes | 109 | 1.54 (1.25-1.90) | <.001 | 64 | 3.67 (2.70-4.99) | <.001 | 26 | 2.86 (1.80-4.54) | <.001 | 15 | 3.61 (1.92-6.76) | <.001 |
| Annual mean daily UVR exposureb | ||||||||||||
| Quartile | ||||||||||||
| 1 | 389 | 1 [Reference] | NA | 84 | 1 [Reference] | NA | 56 | 1 [Reference] | NA | 18 | 1 [Reference] | NA |
| 2 | 390 | 1.02 (0.88-1.17) | .81 | 75 | 0.90 (0.66-1.23) | .52 | 35 | 0.58 (0.38-0.88) | .01 | 18 | 1.00 (0.52-1.93) | >.99 |
| 3 | 382 | 1.19 (1.03-1.37) | .02 | 88 | 1.21 (0.90-1.64) | .21 | 45 | 0.79 (0.53-1.18) | .25 | 21 | 1.35 (0.72-2.54) | .36 |
| 4 | 310 | 1.21 (1.04-1.41) | .01 | 87 | 1.46 (1.08-1.99) | .02 | 34 | 0.65 (0.41-1.02) | .06 | 27 | 2.19 (1.19-4.02) | .01 |
| P for trend | NA | NA | .002 | NA | NA | .005 | NA | NA | .13 | NA | NA | .007 |
| Time since transplant, y | ||||||||||||
| Within 1 y | 140 | 1 [Reference] | NA | 15 | 1 [Reference] | NA | 3 | 1 [Reference] | NA | 4 | 1 [Reference] | NA |
| 1-4.9 | 595 | 1.32 (1.10-1.57) | .002 | 89 | 1.72 (1.04-2.84) | .04 | 51 | 6.40 (2.00-20.5) | .002 | 23 | 2.05 (0.71-5.88) | .18 |
| 5-9.9 | 439 | 1.43 (1.18-1.73) | <.001 | 123 | 2.73 (1.63-4.56) | <.001 | 58 | 10.7 (3.30-34.6) | <.001 | 34 | 3.64 (1.25-10.6) | .02 |
| ≥10 | 297 | 1.55 (1.24-1.93) | <.001 | 107 | 3.32 (1.92-5.76) | <.001 | 58 | 19.4 (5.87-64.5) | <.001 | 23 | 3.28 (1.02-10.5) | .046 |
| P for trend | NA | NA | <.001 | NA | NA | <.001 | NA | NA | <.001 | NA | NA | .02 |
Abbreviations: IRR, incidence rate ratio; NA, not applicable; UVR, UV radiation.
Results are from multivariate Poisson regression models that included the variables listed in the table as well as transplant number (first and second or later) and calendar year of transplant (1987-1994, 1995-2002, 2003-2010, and 2011-2017).
Quartile cutoffs for ambient UVR (4.6-26.3, 26.4-34.2, 34.3-45.5, and 45.6-84.1 mW/m2) were based on the UVR (UV-B, 305 nm) distribution among the transplant population.
Table 4. Risk of Kaposi Sarcoma and Lymphoma in Transplant Recipients.
| Characteristic | Kaposi sarcoma | B- or T-cell lymphoma | ||||
|---|---|---|---|---|---|---|
| No. (n = 186) | IRR (95% CI)a | P value | No. (n = 108) | IRR (95% CI)a | P value | |
| Sex | ||||||
| Female | 38 | 1 [Reference] | NA | 40 | 1 [Reference] | NA |
| Male | 148 | 2.37 (1.65-3.39) | <.001 | 68 | 1.04 (0.70-1.54) | .85 |
| Race and ethnicity | ||||||
| Asian or Pacific Islander | 7 | 1.04 (0.48-2.27) | .92 | 4 | 0.72 (0.26-1.99) | .52 |
| Hispanic or Latino | 52 | 3.10 (2.13-4.50) | <.001 | 17 | 1.20 (0.68-2.11) | .53 |
| Non-Hispanic Black | 43 | 2.16 (1.48-3.17) | <.001 | 13 | 0.83 (0.45-1.51) | .53 |
| Non-Hispanic White | 84 | 1 [Reference] | NA | 74 | 1 [Reference] | NA |
| Age, y | ||||||
| 0-34 | 11 | 1 [Reference] | NA | 21 | 1 [Reference] | NA |
| 35-49 | 26 | 1.81 (0.89-3.67) | .10 | 30 | 1.13 (0.65-1.98) | .67 |
| 50-64 | 96 | 5.87 (3.13-11.0) | <.001 | 41 | 1.35 (0.79-2.32) | .28 |
| ≥65 | 53 | 13.9 (7.18-27.0) | <.001 | 16 | 2.52 (1.28-4.99) | .008 |
| P for trend | NA | NA | <.001 | NA | NA | .02 |
| Transplanted organ | ||||||
| Kidney | 123 | 1 [Reference] | NA | 63 | 1 [Reference] | NA |
| Liver | 33 | 0.83 (0.56-1.24) | .37 | 18 | 0.71 (0.42-1.22) | .21 |
| Heart and/or lung | 26 | 0.77 (0.50-1.20) | .25 | 24 | 1.29 (0.78-2.12) | .32 |
| Other or multiple | 4 | 0.66 (0.24-1.81) | .42 | 3 | 0.59 (0.18-1.90) | .38 |
| Maintenance immunosuppressive therapy | ||||||
| Tacrolimus or mycophenolate mofetil without cyclosporine or azathioprine | 94 | 1 [Reference] | NA | 48 | 1 [Reference] | NA |
| Cyclosporine or azathioprine without tacrolimus or mycophenolate mofetil | 35 | 1.20 (0.71-2.04) | .50 | 39 | 1.49 (0.78-2.86) | .23 |
| Other medication combination | 57 | 1.74 (1.18-2.57) | .005 | 21 | 1.16 (0.64-2.08) | .63 |
| Exposure to mammalian target of rapamycin inhibitor | ||||||
| No | 180 | 1 [Reference] | NA | 104 | 1 [Reference] | NA |
| Yes | 6 | 0.57 (0.25-1.30) | .18 | 4 | 0.80 (0.29-2.22) | .67 |
| Posttransplant basal cell carcinoma | ||||||
| No | 184 | 1 [Reference] | NA | 106 | 1 [Reference] | NA |
| Yes | 2 | 1.76 (0.43-7.24) | .43 | 2 | 0.81 (0.19-3.39) | .77 |
| Posttransplant squamous cell carcinoma | ||||||
| No | 186 | 1 [Reference] | NA | 104 | 1 [Reference] | NA |
| Yes | 0 | No estimate | NA | 4 | 1.29 (0.45-3.66) | .64 |
| Annual mean daily UVRb | ||||||
| Quartile | ||||||
| 1 | 27 | 1 [Reference] | NA | 31 | 1 [Reference] | NA |
| 2 | 64 | 1.78 (1.13-2.81) | .01 | 22 | 0.62 (0.36-1.07) | .09 |
| 3 | 33 | 1.02 (0.61-1.71) | .94 | 26 | 0.81 (0.48-1.37) | .42 |
| 4 | 62 | 1.63 (1.02-2.62) | .04 | 29 | 0.94 (0.55-1.60) | .81 |
| P for trend | NA | NA | .32 | NA | NA | .97 |
| Time since transplant, y | ||||||
| Within 1 y | 70 | 1 [Reference] | NA | 13 | 1 [Reference] | NA |
| 1-4.9 | 94 | 0.41 (0.30-0.56) | <.001 | 34 | 0.87 (0.46-1.65) | .67 |
| 5-9.9 | 16 | 0.09 (0.05-0.16) | <.001 | 35 | 1.30 (0.66-2.55) | .45 |
| ≥10 | 6 | 0.06 (0.02-0.16) | <.001 | 26 | 1.31 (0.61-2.80) | .49 |
| P for trend | NA | NA | <.001 | NA | NA | .21 |
Abbreviations: IRR, incidence rate ratio; NA, not applicable; UVR, UV radiation.
Results are from multivariate Poisson regression models that included the variables shown in the table as well as transplant number (first and second or later) and calendar year of transplant (1987-1994, 1995-2002, 2003-2010, and 2011-2017).
Quartile cutoffs for ambient UVR (4.6-26.3, 26.4-34.2, 34.3-45.5, and 45.6-84.1 mW/m2) were based on the UVR (UV-B, 305 nm) distribution among the transplant population.
After a diagnosis of posttransplant SCC, risk was increased for melanoma (IRR, 1.54; 95% CI, 1.25-1.90), MCC (IRR, 3.67; 95% CI, 2.70-4.99), sebaceous carcinoma (IRR, 2.86; 95% CI, 1.80-4.54), and NSACs (IRR, 3.61; 95% CI, 1.92-6.76). Similarly, posttransplant BCC was a risk factor for each of these malignant tumors (IRR, 1.81; 95% CI, 1.45-2.26 for melanoma; IRR, 2.30; 95% CI, 1.60-3.29 for MCC; IRR, 2.53; 95% CI, 1.23-5.20 for NSACs), except for sebaceous carcinoma (IRR, 1.59; 95% CI, 0.89-2.86). Higher mean daily UVR levels (quartile 4 vs quartile 1) were also associated with increased risk for melanoma (IRR, 1.21; 95% CI, 1.04-1.41), MCC (IRR, 1.46; 95% CI, 1.08-1.99), and NSACs (IRR, 2.19; 95% CI, 1.19-4.02).
Prolonged time since transplantation (≥10 years vs <1 year) was associated with increased risk for several cancers (IRR, 1.55; 95% CI, 1.24-1.93 for melanoma; IRR, 3.32; 95% CI, 1.92-5.76 for MCC; IRR, 19.4; 95% CI, 5.87-64.5 for sebaceous carcinoma; IRR, 3.28; 95% CI, 1.02-10.5 for NSACs) (Table 3). In contrast, risk of KS was greatest in the first year after transplantation and decreased thereafter (1-4.9 years: IRR, 0.41; 95% CI, 0.30-0.56; 5-9.9 years: IRR, 0.09; 95% CI, 0.05-0.16; ≥10 years: IRR, 0.06; 95% CI, 0.02-0.16) (vs <1 year for all comparisons). Transplanted organ was associated with risk for some skin cancers. Specifically, compared with kidney recipients, liver recipients had decreased risk of melanoma (IRR, 0.65; 95% CI, 0.56-0.74), MCC (IRR, 0.46; 95% CI, 0.33-0.63), sebaceous carcinoma (IRR, 0.44; 95% CI, 0.27-0.72), and NSACs (IRR, 0.39; 95% CI, 0.19-0.77). Heart and/or lung recipients had decreased risk of melanoma (IRR, 0.85; 95% CI, 0.74-0.98) and MCC (IRR, 0.71; 95% CI, 0.53-0.95) compared with kidney recipients, although they exhibited increased risk for these cancers in unadjusted models (eTable 2 in the Supplement).
With respect to maintenance immunosuppressive medications, use of a mammalian target of rapamycin (mTOR) inhibitor was associated with decreased risk of melanoma (IRR, 0.75; 95% CI, 0.57-0.98) but increased risk of MCC (IRR, 1.87; 95% CI, 1.24-2.81). Frequently prescribed combinations of calcineurin inhibitors and antimetabolites (tacrolimus and/or mycophenolate mofetil as well as cyclosporine and/or azathioprine) were not associated with risk. Unadjusted estimates for individual induction and maintenance medications and combination therapy are provided in eTables 5 and 6 in the Supplement.
Tumor location was available for 2149 of the 2380 cutaneous cancers in SOTRs. The most common site was the head and neck (n = 847 [39.4%]). The O:E ratio for head and neck was greatly increased for NSACs (7.63), lentigo maligna melanoma (7.47), sebaceous carcinoma (7.34), and MCC (5.96) and was also elevated (based on a global test for heterogeneity) for all skin cancers (combined), other forms of melanoma, and lymphomas (Figure). For melanoma, MCC, sebaceous carcinoma, and NSACs as a group, 46.1% of diagnoses occurred on the head and neck. In contrast, KS preferentially occurred on the lower extremity (96 of 118 cases; O:E = 2.26, global P < .001).
Figure. Ratios of Observed to Expected (O:E) Number of Skin Cancers by Body Site.
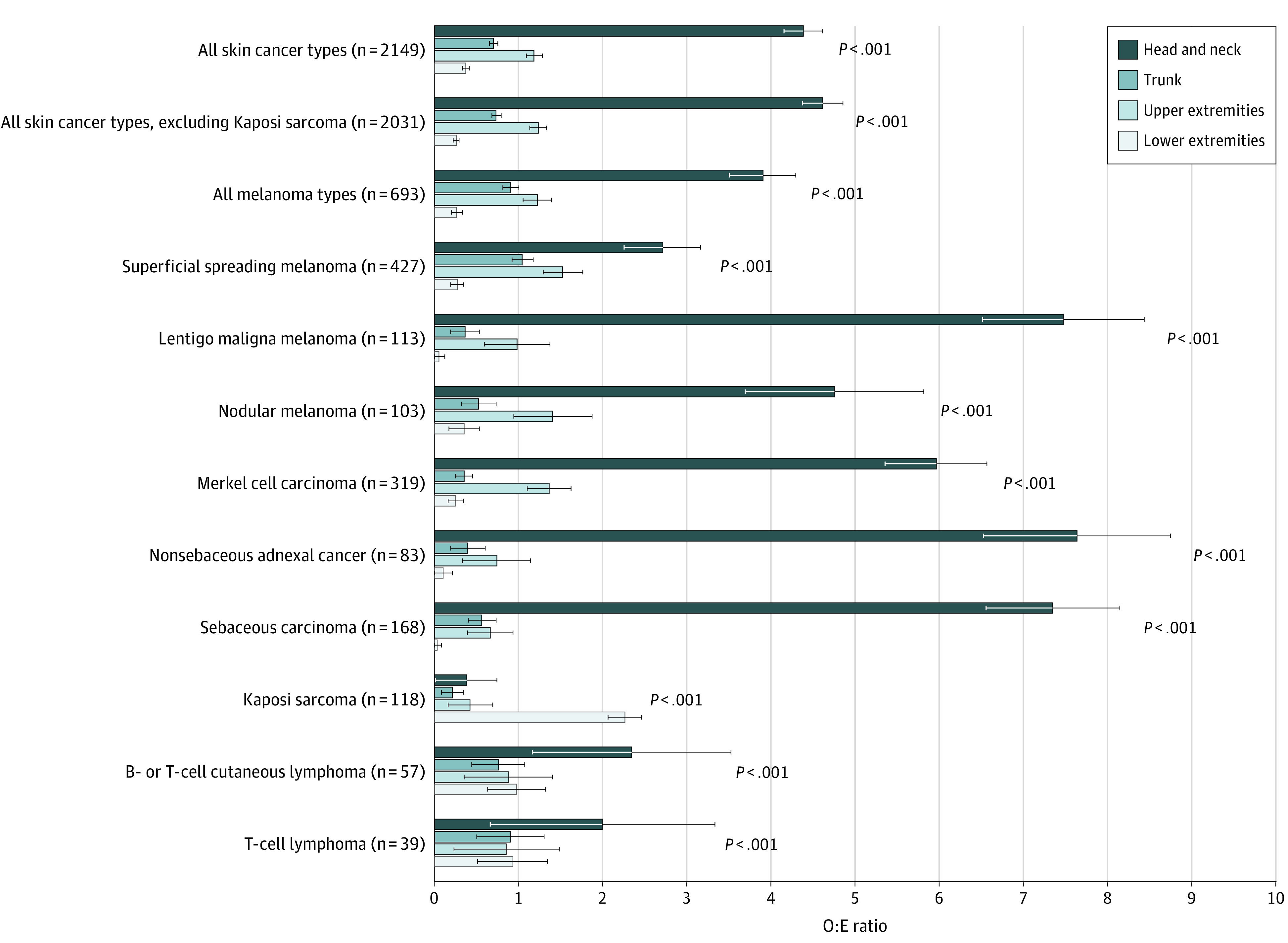
The O:E ratios with 95% CIs are reported for all cases combined and for the most common cancers by multiplying the total number of cases at all sites (excluding overlapping and unspecified sites) by the proportional body surface area (head and neck, 9%; trunk, 37%; upper extremities, 18%; and lower extremities, 36%). Body surface area estimates were derived from Wallace’s Rule of Nines.27 We report P values from a χ2 test assessing whether there is a global difference in the observed and expected counts across the 4 body sites.
Discussion
In this cohort study, we characterized the spectrum of nonkeratinocyte skin cancer risk in SOTRs. We observed a greatly elevated incidence of cancers known to be caused by oncogenic viruses and found epidemiologic evidence that UVR exposure and immunosuppression are associated with skin carcinogenesis for this population. These findings provide insights into the causes of skin cancer among SOTRs and guidance for screening and prevention strategies in this population.
Our results are consistent with the known contribution of oncogenic viruses to the origin of certain skin cancers diagnosed in SOTRs. We observed the greatest elevations in risk for cancers with well-established viral causes, including KS (caused by KS-associated herpesvirus [KSHV], with an SIR of 20.5), MCC (Merkel cell polyomavirus, with an SIR of 16.2), and extranodal natural killer/T-cell lymphoma, nasal type (EBV, with an SIR of 44.3). Epstein-Barr virus has been detected in tumor cells of diffuse large B-cell lymphoma and anaplastic large cell lymphoma,28,29 and we saw strong increases in these cancers as well (SIRs, 5.17-6.82). Indeed, elevated incidence among immunosuppressed individuals, such as people living with HIV or SOTRs, is an important hallmark of cancers caused by viruses.12,30 Sebaceous carcinoma risk was also highly increased in SOTRs in our study, as reported previously,12,13 and investigators have hypothesized a viral cause for this cancer.31,32 A viral cause has been postulated for dermatofibrosarcoma protuberans because of its increased incidence in individuals with adenosine deaminase–deficient severe combined immune deficiency,33 although the risk of dermatofibrosarcoma protuberans and other sarcomas was not increased among SOTRs in our study.
Our results also suggest an important association of UVR exposure with the development of several cancers in SOTRs. Risks for melanoma, MCC, and NSACs were increased up to 3- to 4-fold for SOTRs after a diagnosis of UVR-related keratinocyte carcinomas (BCC and SCC). Furthermore, risk of these cancers was often elevated for SOTRs living in areas with higher ambient UVR and was decreased among non-Hispanic Black, Hispanic or Latino, and Asian or Pacific Islander individuals with greater amounts of photoprotective melanin pigment in their skin. A similar pattern was observed for sebaceous carcinoma (increased risk after a posttransplant SCC and decreased risk for non-Hispanic Black individuals). For these cancers as a group (melanoma, MCC, sebaceous carcinoma, and NSACs), 46.1% of diagnoses occurred on chronically sun-exposed skin of the head and neck, further supporting a role for UVR in tumor development. For melanoma, the highest elevation in risk was observed for the nodular and amelanotic subtypes, both of which are associated with UVR exposure and worse overall survival.34,35
Several results implicate duration and intensity of immunosuppression in the development of cutaneous cancers. Risk for melanoma, MCC, sebaceous carcinoma, and NSACs increased with greater time since transplantation. This pattern may reflect the loss of immune control of premalignant and malignant cells as they gradually accumulate UVR-induced mutations.36,37 In contrast, KS risk was highest immediately after transplantation. Kaposi sarcoma can develop as a result of primary KSHV infection or reactivation after intensive immunosuppressive therapy at the time of transplantation,38,39 and KS risk is associated with immunosuppression among HIV-infected individuals.40
Liver recipients, who are typically the least immunosuppressed among SOTRs with different organ types,41,42 had decreased risk of melanoma, MCC, sebaceous carcinoma, and NSACs compared with kidney recipients. Of interest, heart and/or lung recipients, who are the most immunosuppressed, exhibited increased risk of melanoma and MCC in unadjusted models, but the direction of association reversed after adjustment for potential confounders (Table 3). The change in direction was the result of adding race and ethnicity to the multivariate model. Three-quarters of heart and/or lung recipients were non-Hispanic White, and this racial and ethnic group is more likely to receive education about photoprotection and undergo skin surveillance than other racial and ethnic groups based on current screening recommendations.43 Adherence to photoprotection measures could explain the reduced risk of these cancers in multivariate models, whereas increased surveillance and removal of precancerous dysplastic nevi or in situ melanomas could have led to a reduction in invasive melanoma diagnoses in this group.43
Mammalian target of rapamycin inhibitor use was associated with a 25% decrease in melanoma risk in our analyses of baseline immunosuppressive medications. Both mitogen-activated protein kinase/extracellular signal-regulated kinase and phosphatidylinositol 3-kinase signaling are critically important for melanoma development and progression, and both pathways activate mTOR,44,45 which may explain this observation. In contrast, as reported previously based on a smaller cohort,11 mTOR inhibitors were associated with an almost 90% increase in the risk of MCC, a much less common cancer. Increased risk of MCC appears to be related to mTOR inhibitors directly activating polyomavirus replication through a Skp2-dependent mechanism.46 Mammalian target of rapamycin inhibitors interfere with KSHV lytic replication, block KS progression, and may be an effective therapy for KS,47,48 but they were not associated with reduced KS risk in our study. We also did not see associations between skin cancer risk and other immunosuppressive medications, including agents used for induction or common combinations of calcineurin inhibitors and antimetabolites.
Several of our findings have important clinical implications. Most expert groups recommend annual dermatologic screening for SOTRs. Our study supports this recommendation by highlighting the broad spectrum of skin cancer risk in the transplant population.49,50 Moreover, SOTRs with multiple risk factors for skin carcinogenesis may benefit from more frequent screening than annual surveillance, which has been associated with decreased keratinocyte cancer morbidity and mortality.51
Lastly, mTOR inhibitors have proven efficacy for prevention of cutaneous SCCs in the transplant setting. Our study provides a clinical rationale for future studies to investigate whether treatment with mTOR inhibitors would likewise be effective in preventing melanoma, which accounts for approximately 60% of all nonkeratinocyte skin cancers in the transplant population.52 This approach may be especially beneficial for SOTRs with melanoma risk factors (eg, dysplastic nevi, personal or family history of melanoma, and significant sun exposure history). Any chemoprevention benefit from mTOR inhibitors should also be weighed against the potential increased risk of MCC. Mammalian target of rapamycin inhibitors may also allow for safe treatment with immune checkpoint inhibitors among SOTRs with cancer, with a recent case report53 showing that treatment with sirolimus resulted in allograft tolerance in a patient with organ rejection caused by programmed death 1 blockade.
Strengths and Limitations
Major strengths of this study include its large size, representativeness of the US transplant population (including its racial and ethnic as well as geographic diversity), and availability of clinical data from the SRTR and population-based cancer registries. Our systematic assessment of multiple cancer types allowed us to identify common risk factors with biological and clinical relevance. Study limitations include the use of multiple comparisons, which could lead to false-positive associations. This issue was mitigated by use of a Bonferroni correction in the SIR calculations, and we provide P values for IRR estimates to facilitate assessment of the statistical evidence. There were also relatively few cases for some cancers, which limited our analyses. We evaluated baseline medication use as a risk factor for skin cancer but were unable to account for changes in medications over time, which could impact risk. Annual mean daily UVR levels were also measured at baseline and may not be representative of actual UVR exposure, depending on unmeasured mitigating factors (eg, adherence to sun protection measures and indoor vs outdoor occupation). Lastly, recipient and donor EBV serologic status at transplant was not associated with lymphoma risk, but our analysis was limited by the high proportion of cases with missing data.
Conclusions
To our knowledge, this cohort study provides the first comprehensive assessment of the spectrum of nonkeratinocyte skin cancer risk in the US transplant population. Risk of many of these cancers is strongly increased among SOTRs, and viruses, UVR exposure, and immunosuppression likely play important roles in their etiology. These results support modifications to current screening guidelines among SOTRs and suggest that wider use of mTOR inhibitors in high-risk patients could reduce the incidence of melanoma.
eTable 1. Cancer ICD-O-3 Histology/Behavior Codes
eTable 2. Unadjusted Risk Estimates for Melanoma, Merkel Cell Carcinoma, and Adnexal Cancers in Transplant Recipients
eTable 3. Unadjusted Risk Estimates for Kaposi Sarcoma and Lymphoma in Transplant Recipients
eTable 4. Transplant Donor and Recipient EBV Serologic Status at Time of Transplant and Lymphoma Risk
eTable 5. Baseline Induction and Maintenance Immunosuppressive Therapy and Risk for Melanoma, Merkel Cell Carcinoma, and Adnexal Cancers in Transplant Recipients
eTable 6. Baseline Induction and Maintenance Immunosuppressive Therapy and Risk for Kaposi Sarcoma and Lymphoma in Transplant Recipients
References
- 1.Engels EA, Pfeiffer RM, Fraumeni JF Jr, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306(17):1891-1901. doi: 10.1001/jama.2011.1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370(9581):59-67. doi: 10.1016/S0140-6736(07)61050-2 [DOI] [PubMed] [Google Scholar]
- 3.Sargen MR, Mai ZM, Engels EA, et al. Ambient ultraviolet radiation and sebaceous carcinoma incidence in the United States, 2000-2016. J Natl Cancer Inst Cancer Spectr. 2020;4(2):pkaa020. doi: 10.1093/jncics/pkaa020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63(1-3):8-18. doi: 10.1016/S1011-1344(01)00198-1 [DOI] [PubMed] [Google Scholar]
- 5.Paulson KG, Lahman MC, Chapuis AG, Brownell I. Immunotherapy for skin cancer. Int Immunol. 2019;31(7):465-475. doi: 10.1093/intimm/dxz012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiyad Z, Olsen CM, Burke MT, Isbel NM, Green AC. Azathioprine and risk of skin cancer in organ transplant recipients: systematic review and meta-analysis. Am J Transplant. 2016;16(12):3490-3503. doi: 10.1111/ajt.13863 [DOI] [PubMed] [Google Scholar]
- 7.Casper C, Fitzmaurice C. Infection-related cancers: prioritising an important and eliminable contributor to the global cancer burden. Lancet Glob Health. 2016;4(9):e580-e581. doi: 10.1016/S2214-109X(16)30169-3 [DOI] [PubMed] [Google Scholar]
- 8.Robbins HA, Clarke CA, Arron ST, et al. Melanoma risk and survival among organ transplant recipients. J Invest Dermatol. 2015;135(11):2657-2665. doi: 10.1038/jid.2015.312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Reilly Zwald F, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management: part II: management of skin cancer in solid organ transplant recipients. J Am Acad Dermatol. 2011;65(2):263-279. doi: 10.1016/j.jaad.2010.11.063 [DOI] [PubMed] [Google Scholar]
- 10.Krynitz B, Edgren G, Lindelöf B, et al. Risk of skin cancer and other malignancies in kidney, liver, heart and lung transplant recipients 1970 to 2008: a Swedish population-based study. Int J Cancer. 2013;132(6):1429-1438. doi: 10.1002/ijc.27765 [DOI] [PubMed] [Google Scholar]
- 11.Clarke CA, Robbins HA, Tatalovich Z, et al. Risk of Merkel cell carcinoma after solid organ transplantation. J Natl Cancer Inst. 2015;107(2):dju382. doi: 10.1093/jnci/dju382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Arcy ME, Castenson D, Lynch CF, et al. Risk of rare cancers among solid organ transplant recipients. J Natl Cancer Inst. 2021;113(2):199-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sargen MR, Cahoon EK, Lynch CF, Tucker MA, Goldstein AM, Engels EA. Sebaceous carcinoma incidence and survival among solid organ transplant recipients in the United States, 1987-2017. JAMA Dermatol. 2020;156(12):1307-1314. doi: 10.1001/jamadermatol.2020.3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cahoon EK, Linet MS, Clarke CA, Pawlish KS, Engels EA, Pfeiffer RM. Risk of Kaposi sarcoma after solid organ transplantation in the United States. Int J Cancer. 2018;143(11):2741-2748. doi: 10.1002/ijc.31735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garrett GL, Lowenstein SE, Singer JP, He SY, Arron ST. Trends of skin cancer mortality after transplantation in the United States: 1987 to 2013. J Am Acad Dermatol. 2016;75(1):106-112. doi: 10.1016/j.jaad.2016.02.1155 [DOI] [PubMed] [Google Scholar]
- 16.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350-1355. doi: 10.1126/science.aar4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.d’Izarny-Gargas T, Durrbach A, Zaidan M. Efficacy and tolerance of immune checkpoint inhibitors in transplant patients with cancer: a systematic review. Am J Transplant. 2020;20(9):2457-2465. doi: 10.1111/ajt.15811 [DOI] [PubMed] [Google Scholar]
- 18.Abdel-Wahab N, Safa H, Abudayyeh A, et al. Checkpoint inhibitor therapy for cancer in solid organ transplantation recipients: an institutional experience and a systematic review of the literature. J Immunother Cancer. 2019;7(1):106. doi: 10.1186/s40425-019-0585-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benoni H, Eloranta S, Ekbom A, Wilczek H, Smedby KE. Survival among solid organ transplant recipients diagnosed with cancer compared to nontransplanted cancer patients: a nationwide study. Int J Cancer. 2020;146(3):682-691. doi: 10.1002/ijc.32299 [DOI] [PubMed] [Google Scholar]
- 20.Noone AM, Pfeiffer RM, Dorgan JF, et al. Cancer-attributable mortality among solid organ transplant recipients in the United States: 1987 through 2014. Cancer. 2019;125(15):2647-2655. doi: 10.1002/cncr.32136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Cancer Institute. Transplant Cancer Match Study. Accessed January 28, 2022. https://transplantmatch.cancer.gov/index.html
- 22.Surveillance, Epidemiology, and End Results Program . Registry Groupings in SEER Data and Statistics. 2021. Accessed September 15, 2021. https://seer.cancer.gov/registries/terms.html
- 23.Chaturvedi AK, Mbulaiteye SM, Engels EA. Underestimation of relative risks by standardized incidence ratios for AIDS-related cancers. Ann Epidemiol. 2008;18(3):230-234. doi: 10.1016/j.annepidem.2007.10.005 [DOI] [PubMed] [Google Scholar]
- 24.National Aeronautics and Space Administration . TOMS Nimbus-7 Total Ozone Aerosol Index UV-Reflectivity UV-B Erythemal Irradiances Daily L3 Global 1 deg x 1.25 deg V008. Accessed January 28, 2022. https://disc.gsfc.nasa.gov/datasets/TOMSN7L3_008/summary
- 25.D’Arcy ME, Pfeiffer RM, Rivera DR, et al. Voriconazole and the risk of keratinocyte carcinomas among lung transplant recipients in the United States. JAMA Dermatol. 2020;156(7):772-779. doi: 10.1001/jamadermatol.2020.1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zamoiski RD, Yanik E, Gibson TM, et al. Risk of second malignancies in solid organ transplant recipients who develop keratinocyte cancers. Cancer Res. 2017;77(15):4196-4203. doi: 10.1158/0008-5472.CAN-16-3291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sargen MR, Pfeiffer RM, Yang XR, Tucker MA, Goldstein AM. Variation in cutaneous patterns of melanomagenesis according to germline CDKN2A/CDK4 status in melanoma-prone families. J Invest Dermatol. 2020;140(1):174-181.e3. doi: 10.1016/j.jid.2019.06.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seçkin D, Barete S, Euvrard S, et al. Primary cutaneous posttransplant lymphoproliferative disorders in solid organ transplant recipients: a multicenter European case series. Am J Transplant. 2013;13(8):2146-2153. doi: 10.1111/ajt.12281 [DOI] [PubMed] [Google Scholar]
- 29.Swerdlow SH. T-cell and NK-cell posttransplantation lymphoproliferative disorders. Am J Clin Pathol. 2007;127(6):887-895. doi: 10.1309/LYXN3RGF7D7KPYG0 [DOI] [PubMed] [Google Scholar]
- 30.Moore PS, Chang Y. Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nat Rev Cancer. 2010;10(12):878-889. doi: 10.1038/nrc2961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sargen MR, Starrett GJ, Engels EA, Cahoon EK, Tucker MA, Goldstein AM. Sebaceous carcinoma epidemiology and genetics: emerging concepts and clinical implications for screening, prevention, and treatment. Clin Cancer Res. 2021;27(2):389-393. doi: 10.1158/1078-0432.CCR-20-2473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tetzlaff MT, Curry JL, Ning J, et al. Distinct biological types of ocular adnexal sebaceous carcinoma: HPV-driven and virus-negative tumors arise through nonoverlapping molecular-genetic alterations. Clin Cancer Res. 2019;25(4):1280-1290. doi: 10.1158/1078-0432.CCR-18-1688 [DOI] [PubMed] [Google Scholar]
- 33.Kesserwan C, Sokolic R, Cowen EW, et al. Multicentric dermatofibrosarcoma protuberans in patients with adenosine deaminase-deficient severe combined immune deficiency. J Allergy Clin Immunol. 2012;129(3):762-769.e1. doi: 10.1016/j.jaci.2011.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allais BS, Beatson M, Wang H, et al. Five-year survival in patients with nodular and superficial spreading melanomas in the US population. J Am Acad Dermatol. 2021;84(4):1015-1022. doi: 10.1016/j.jaad.2020.11.047 [DOI] [PubMed] [Google Scholar]
- 35.Thomas NE, Kricker A, Waxweiler WT, et al. ; Genes, Environment, and Melanoma (GEM) Study Group . Comparison of clinicopathologic features and survival of histopathologically amelanotic and pigmented melanomas: a population-based study. JAMA Dermatol. 2014;150(12):1306-1314. doi: 10.1001/jamadermatol.2014.1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med. 2017;377(25):2500-2501. doi: 10.1056/NEJMc1713444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hernando B, Dietzen M, Parra G, et al. The effect of age on the acquisition and selection of cancer driver mutations in sun-exposed normal skin. Ann Oncol. 2021;32(3):412-421. doi: 10.1016/j.annonc.2020.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riva G, Luppi M, Barozzi P, Forghieri F, Potenza L. How I treat HHV8/KSHV-related diseases in posttransplant patients. Blood. 2012;120(20):4150-4159. doi: 10.1182/blood-2012-04-421412 [DOI] [PubMed] [Google Scholar]
- 39.Copeland MMM, Trainor J, Cash WJ, Braniff C. Fatal donor-derived Kaposi sarcoma following liver transplantation. BMJ Case Rep. 2021;14(6):e236061. doi: 10.1136/bcr-2020-236061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guiguet M, Boué F, Cadranel J, Lang JM, Rosenthal E, Costagliola D; Clinical Epidemiology Group of the FHDH-ANRS CO4 cohort . Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol. 2009;10(12):1152-1159. doi: 10.1016/S1470-2045(09)70282-7 [DOI] [PubMed] [Google Scholar]
- 41.Charlton M, Levitsky J, Aqel B, et al. International liver transplantation society consensus statement on immunosuppression in liver transplant recipients. Transplantation. 2018;102(5):727-743. doi: 10.1097/TP.0000000000002147 [DOI] [PubMed] [Google Scholar]
- 42.Pilch NA, Bowman LJ, Taber DJ. Immunosuppression trends in solid organ transplantation: the future of individualization, monitoring, and management. Pharmacotherapy. 2021;41(1):119-131. doi: 10.1002/phar.2481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crow LD, Jambusaria-Pahlajani A, Chung CL, et al. Initial skin cancer screening for solid organ transplant recipients in the United States: Delphi method development of expert consensus guidelines. Transpl Int. 2019;32(12):1268-1276. doi: 10.1111/tri.13520 [DOI] [PubMed] [Google Scholar]
- 44.Wang B, Zhang W, Zhang G, et al. Targeting mTOR signaling overcomes acquired resistance to combined BRAF and MEK inhibition in BRAF-mutant melanoma. Oncogene. 2021;40(37):5590-5599. doi: 10.1038/s41388-021-01911-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kwong LN, Davies MA. Navigating the therapeutic complexity of PI3K pathway inhibition in melanoma. Clin Cancer Res. 2013;19(19):5310-5319. doi: 10.1158/1078-0432.CCR-13-0142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alvarez Orellana J, Kwun HJ, Artusi S, Chang Y, Moore PS. Sirolimus and other mechanistic target of rapamycin inhibitors directly activate latent pathogenic human polyomavirus replication. J Infect Dis. 2021;224(7):1160-1169. doi: 10.1093/infdis/jiaa071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nichols LA, Adang LA, Kedes DH. Rapamycin blocks production of KSHV/HHV8: insights into the anti-tumor activity of an immunosuppressant drug. PLoS One. 2011;6(1):e14535. doi: 10.1371/journal.pone.0014535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stallone G, Schena A, Infante B, et al. Sirolimus for Kaposi’s sarcoma in renal-transplant recipients. N Engl J Med. 2005;352(13):1317-1323. doi: 10.1056/NEJMoa042831 [DOI] [PubMed] [Google Scholar]
- 49.Acuna SA, Huang JW, Scott AL, et al. Cancer screening recommendations for solid organ transplant recipients: a systematic review of clinical practice guidelines. Am J Transplant. 2017;17(1):103-114. doi: 10.1111/ajt.13978 [DOI] [PubMed] [Google Scholar]
- 50.Acuna SA, Fernandes KA, Daly C, et al. Cancer mortality among recipients of solid-organ transplantation in Ontario, Canada. JAMA Oncol. 2016;2(4):463-469. doi: 10.1001/jamaoncol.2015.5137 [DOI] [PubMed] [Google Scholar]
- 51.Chan AW, Fung K, Austin PC, et al. Improved keratinocyte carcinoma outcomes with annual dermatology assessment after solid organ transplantation: Population-based cohort study. Am J Transplant. 2019;19(2):522-531. doi: 10.1111/ajt.14966 [DOI] [PubMed] [Google Scholar]
- 52.Euvrard S, Morelon E, Rostaing L, et al. ; TUMORAPA Study Group . Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med. 2012;367(4):329-339. doi: 10.1056/NEJMoa1204166 [DOI] [PubMed] [Google Scholar]
- 53.Esfahani K, Al-Aubodah TA, Thebault P, et al. Targeting the mTOR pathway uncouples the efficacy and toxicity of PD-1 blockade in renal transplantation. Nat Commun. 2019;10(1):4712. doi: 10.1038/s41467-019-12628-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Cancer ICD-O-3 Histology/Behavior Codes
eTable 2. Unadjusted Risk Estimates for Melanoma, Merkel Cell Carcinoma, and Adnexal Cancers in Transplant Recipients
eTable 3. Unadjusted Risk Estimates for Kaposi Sarcoma and Lymphoma in Transplant Recipients
eTable 4. Transplant Donor and Recipient EBV Serologic Status at Time of Transplant and Lymphoma Risk
eTable 5. Baseline Induction and Maintenance Immunosuppressive Therapy and Risk for Melanoma, Merkel Cell Carcinoma, and Adnexal Cancers in Transplant Recipients
eTable 6. Baseline Induction and Maintenance Immunosuppressive Therapy and Risk for Kaposi Sarcoma and Lymphoma in Transplant Recipients


