Abstract
Evaluation of the acidic characteristics of a jet fuel, especially for the total acid number (TAN), is of great significance to ensure flight safety. Methylbenzene is commonly used as the titration solvent; however, it is poisonous and harmful to the environment. It is highly desirable to develop an alternative solvent for methylbenzene to extract the acidic compounds from the jet fuel during the determination of the TAN. Here, we develop a desirable alternative solvent of a mixed ethanol–water solution with the volume ratio of ethanol to water of 99:1, which exhibits a value of TAN similar to that of the solvent of methylbenzene in potentiometric titration and acid–base titration methods. The TAN value derived from the different titration solvents was in the order of 2.96 μg KOH g–1 (Vcyclohexane/Visopropanol/Vwater = 100:99:1) > 2.68 μg KOH g–1 (Vmethylbenzene/Visopropanol/Vwater = 100:99:1) ≈ 2.6 μg KOH g–1 (Vabsolute ethanol/Vwater = 99:1) > 2.34 μg KOH g–1 (Visopropanol/Vwater = 99:1). The current report presents a nontoxic and eco-friendly alternative solvent for methylbenzene, which may open up an avenue for evaluating the TAN of jet fuels.
1. Introduction
Jet fuel, refined from petroleum, consists primarily of hydrocarbons in different structural families and their derivatives formed by hydrocarbons with heteroatoms such as oxygen, nitrogen, sulfur, and metals (iron, sodium, etc.).1−4 Generally, hydrocarbons are non-polar and do not react with metals at operating temperatures. However, some heteroatom-containing compounds are acidic and corrosive, such as naphthenic acids, thiols, and so on, which were generated during the refining progress, easily leading to the corrosion and swelling of the equipment and materials to which they are exposed.4,5 The possible chemical reactions are listed in equations as 2RCOOH + Fe → Fe(RCOO)2 + H2 and 2RSH + Fe → Fe(RS)2 + H2.6 Such a corrosion and swelling effect not only affects the service life of the storage and transport equipment but also has an impact on the cleanliness and oxidation stability of jet fuels themselves. More seriously, some severe cases may pose a threat to flight safety directly, for example, the crash of Aloha airline flight 243 in 1988 because of the corrosion. Therefore, evaluation of the acidic characteristics of jet fuel accurately, especially for the total acid number (TAN, the weight of KOH required to neutralize a gram of acidic jet fuel, determined according to the ASTM D664 protocol), is of great significance to ensure the flight safety.7
Until now, two main types of measurement methods have been used to evaluate the TAN of jet fuels, that is, color indicator titration (acid–base titration) and potentiometric titration methods according to the ASTM D3242 (similar to GB/T 12574) protocol8−11 and ASTM D664 (similar to GB/T 7304) protocol,12−14 respectively. The above methods both use the mixed solution of water, isopropanol, and methylbenzene (Vmethylbenzene/Visopropanol/Vwater = 100:99:1) as the titration solvent to extract the acidic compounds from the jet fuel and then titrated with the standard solution of KOH (RCOOH + KOH → RCOOK + H2O and RSH + KOH → RSK + H2O). However, the titration solvent contains a large amount of methylbenzene, which is poisonous and harms the health of the operator seriously.15−17 Meanwhile, methylbenzene is greatly harmful to the environment and is difficult to deal with because it could pollute water and air as well as it is not easily decomposed and can exist in the environment for a long time. Furthermore, methylbenzene is heavily regulated by the law-enforcing department in China as it is employed as an indispensable solvent in the manufacture of drugs, making it difficult to buy freely on the market. Therefore, it is highly desirable to develop a green titration solvent to extract the acidic compounds from the jet fuel during the determination of the TAN.
In this study, we develop a desirable alternative solvent of the mixed solution of water, isopropanol, and methylbenzene, that is, ethanol–water solution with the volume ratio of ethanol to water of 99:1, which can effectively extract the acidic compounds from the jet fuel and exhibits a value of TAN similar to that of the solvent of the mixed solution of water, isopropanol, and methylbenzene. Such titration results are verified by the potentiometric titration and acid–base titration methods.
2. Results and Discussion
2.1. Color Indicator Titration (Acid–Base Titration)
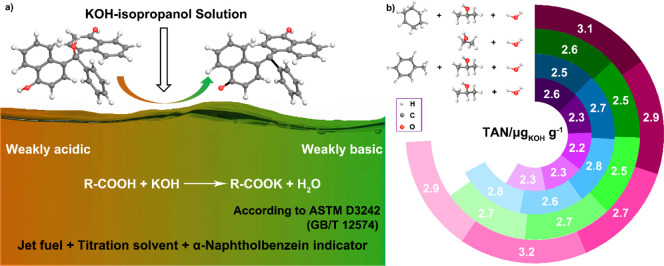
In the acid–base titration process, α-naphtholbenzein (pH: 8.5–9.8) usually serves as an indicator to determine the titration end point, whose color changes from yellow to bright green during titration,18,19 as shown in Figure 1a. To eliminate the influence of carbon dioxide in the environment on titration results, N2 is used as a protective gas to fill the entire titration flask until the end of the titration.20 The whole titration process is carried out according to ASTM D3242 (GB/T 12574). Before the titration, the volume of the KOH solution consumed by the titration solvents was measured. As shown in Tables S1 and S2, 0.23, 0.14, 0.28, and 0.32 mL of KOH solution are consumed by the titration solvents of methylbenzene, isopropanol, and water (Vmethylbenzene/Visopropanol/Vwater = 100:99:1), absolute ethanol and water (Vabsolute ethanol/Vwater = 99:1), isopropanol and water (Visopropanol/Vwater = 99:1), and cyclohexane, isopropanol, and water (Vcyclohexane/Visopropanol/Vwater = 100:99:1), respectively. Note that in the titration solvents, the organic solvents are used to extract the organic acids in the jet fuel, such as naphthenic acids, and the water is used to extract the water-soluble acids.21−23 Meanwhile, the organic solvents can also improve the solubility of water in the jet fuel because the hydrocarbon chains of organic solvents interact with the fuel physicochemically, and the hydroxyl group forms hydrogen bonds with water molecules.1,24
Figure 1.
(a) Illustrations of the titration process and the structure transformation of the α-naphtholbenzein indicator during titration. (b) TAN titration results of jet fuels with different titration solvents (Visopropanol/Vwater = 99:1, Vabsolute ethanol/Vwater = 99:1, Vmethylbenzene/Visopropanol/Vwater = 100:99:1, and Vcyclohexane/Visopropanol/Vwater = 100:99:1).
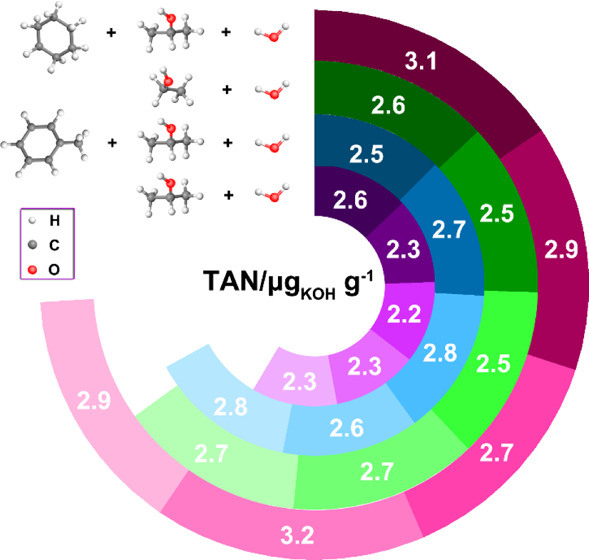
Figure 1b shows the TAN titration results of jet fuels with different titration solvents, and the experimental details are listed in Tables S1 and S2. With the mixed solution of methylbenzene, isopropanol, and water (Vmethylbenzene/Visopropanol/Vwater = 100:99:1) as the titration solvent, the TAN value of five times titration are 2.5, 2.7, 2.8, 2.6, and 2.8 μg KOH g–1 respectively. The repeatability of the test results (0.3 μg KOH g–1) is in line with the required accuracy of 0.6 μg KOH g–1, specified by GB/T 12574.21,25 The mean of the five titration results is about 2.7 μg KOH g–1. If methylbenzene is removed from the titration solvent, namely, the mixed solution of isopropanol and water (Visopropanol/Vwater = 99:1) as the titration solvent, the mean of the five titration results is about 2.3 μg KOH g–1, which is slightly lower than the titration value of methylbenzene, isopropanol, and water as the titration solvent. Based on the above data, we speculate that the ability to extract acids from the jet fuel decreases when methylbenzene is removed from the titration solvent.
Considering the similar molecular structure of cyclohexane with naphthenic acids, cyclohexane may be a good alternative for toluene in the titration solvent.26−28 As shown in Figure 1b, with the mixed solution of cyclohexane, isopropanol, and water (Vcyclohexane/Visopropanol/Vwater) as the titration solvent, the TAN values of five times titration are 3.1, 2.9, 2.7, 3.2, and 2.9 μg KOH g–1, which are slightly higher than that of methylbenzene, isopropanol, and water as the titration solvent. This is mainly because the main organic acid compounds in petroleum products are naphthenic acid compounds; the TAN value should be higher with the solution of cyclohexane, isopropanol, and water as the titration solvent according to the compatibility principle of the dissolution in the similar structure. Compared with the titration results of the mixed solution of cyclohexane, isopropanol, and water, the titration values are closer to 2.7 μg KOH g–1 with the mixed solution of absolute ethanol and water as the titration solvent. The TAN value of five times titration is 2.6, 2.5, 2.5, 2.7, and 2.7 μg KOH g–1, and the repeatability of the test results (0.2 μg KOH g–1) is also within the standard range.
2.2. Potentiometric Titration Method
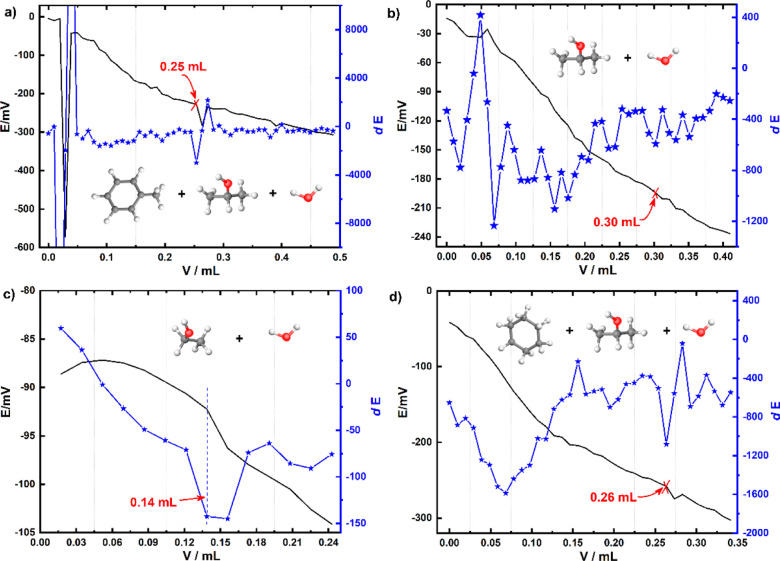
It is well known that the potentiometric titration method possesses the characteristics of higher accuracy and less influence of human factors than the acid–base titration method.29,30 The above titration results measured by the acid–base titration method are further verified by the potentiometric titration method, which determines the end-point of titration by the sudden potential change of the indicatory electrode. As shown in Figure 2, the sudden potential changes appear when the consumptions of KOH solution are 0.25, 0.30, 0.14, and 0.26 mL for the titration solvents of methylbenzene, isopropanol, and water (Vmethylbenzene/Visopropanol/Vwater = 100:99:1), isopropanol and water (Visopropanol/Vwater = 99:1), absolute ethanol and water (Vabsolute ethanol/Vwater = 99:1), and cyclohexane, isopropanol, and water (Vcyclohexane/Visopropanol/Vwater = 100:99:1), respectively, which are consistent with the acid–base titration results. It should be noted that multiple sudden potential changes appear in the potentiometric titration curve of the isopropanol and water titration solvent, which may be caused because the minimum addition volume of potassium hydroxide solution is too small, resulting in small potential changes but the signal drifts generated by the electrode are relatively large.
Figure 2.
Potentiometric titration curve and the corresponding first-order differential curve of the total acid value of the titration solvent: (a) Vmethylbenzene/Visopropanol/Vwater = 100:99:1, (b) Visopropanol/Vwater = 99:1, (c) Vabsolute ethanol/Vwater = 99:1, and (d) Vcyclohexane/Visopropanol/Vwater = 100:99:1.
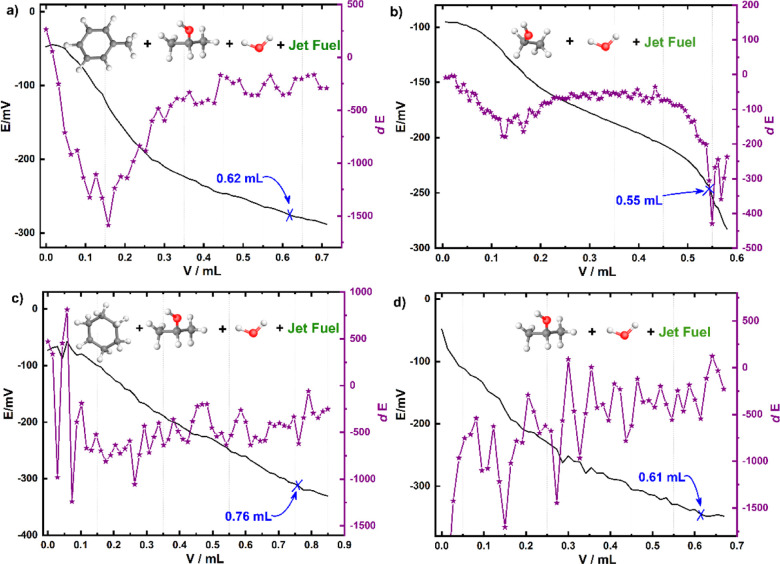
Figure 3 shows the potentiometric titration curve and the corresponding first-order differential curve of the total acid value of jet fuels with different titration solvents. 0.37, 0.41, 0.31, and 0.50 mL of KOH solution are consumed by the jet fuel with the mixed solutions of methylbenzene, isopropanol, and water (Vmethylbenzene/Visopropanol/Vwater = 100:99:1), absolute ethanol and water (Vabsolute ethanol/Vwater = 99:1), isopropanol and water (Visopropanol/Vwater = 99:1), and cyclohexane, isopropanol, and water (Vcyclohexane/Visopropanol/Vwater = 100:99:1) as the titration solvent, and the corresponding TAN values are 2.5, 2.7, 2.1, and 3.3 μg KOH g–1, respectively. The TAN value derived using the potentiometric titration method is slightly smaller than that derived using the acid–base titration method, indicating the measuring accuracy of the potentiometric titration method.31
Figure 3.
Potentiometric titration curve and the corresponding first-order differential curve of the total acid value of jet fuels with different titration solvents: (a) Vmethylbenzene/Visopropanol/Vwater = 100:99:1, (b) Vabsolute ethanol/Vwater = 99:1, (c) Vcyclohexane/Visopropanol/Vwater = 100:99:1, and (d) Visopropanol/Vwater = 99:1.
3. Conclusions
In summary, three nontoxic and eco-friendly solutions are investigated as substitutes for the methylbenzene-containing titration solvent in evaluating the TAN of jet fuels. Potentiometric titration and acid–base titration results show that with the mixed solution of isopropanol and water as the titration solvent, the TAN value of the jet fuel is lower than that of the TAN value derived from the standard-specified titration solvent, that is, the mixed solution of methylbenzene, isopropanol, and water (Vmethylbenzene/Visopropanol/Vwater = 100:99:1). With cyclohexane, isopropanol, and water (Vcyclohexane/Visopropanol/Vwater = 100:99:1) as the titration solvent, the derived TAN value is higher, while the TAN value obtained from absolute ethanol and water (Vabsolute ethanol/Vwater = 99:1) as the titration solvent is very close to the TAN value derived from the standard-specified titration solvent.
4. Experimental Section
4.1. Materials
The jet fuel sample used in this study was produced by Sinopec Shanghai Gao Qiao Petrochemical Corp, whose composition and physicochemical properties are listed in Table 1. Methylbenzene, cyclohexane, isopropanol, and absolute ethanol were purchased from Sinopharm Chemical Reagent Co. Ltd. of China. Alpha (α)-naphtholbenzein from Sigma-Aldrich (USA) was used as received. Potassium hydroxide (KOH) powder was purchased from Aladdin Industrial Corporation (Shanghai, China). All chemicals were of analytical grade and used without further purification. Ultrapure water (18.2 MΩ cm) was used throughout all the experiments.
Table 1. Jet Fuel Properties in Specifications.
| parameter | value | parameter | value |
|---|---|---|---|
| aromatics, % v/v | 12.0 | olefins, % v/v | 1.0 |
| naphthalenes, % v/v | 0.40 | total acid value, mg KOH/g | 0.003 |
| density @ 20 °C, kg m–3 | 795.3 | freezing point, °C | –67.0 |
| smoke point, mm | 24.6 | initial boiling point, °C | 153.0 |
| 10% v/v recovered, °C | 172.0 | 50%v/v recovered, °C | 192.0 |
| 90%v/v recovered, °C | 223.0 | final boiling point, °C | 248.0 |
| flash point, °C | 45.0 | sulfur, % m/m | 0.0176 |
| kinematic viscosity @ −20 °C, mm2 s–1 | 3.971 | kinematic viscosity @ 20 °C, mm2 s–1 | 1.631 |
4.2. Test Methods
The TAN of the current jet fuel was evaluated using the color indicator titration and potentiometric titration methods, respectively. The color indicator titration was operated according to the GB/T 12574 protocol. Specifically, 100 ± 5 g of the jet fuel, 100 mL of the titration solvent, and 0.1 mL of the α-naphtholbenzein indicator were mixed thoroughly in a titration bottle under the protection of N2. The mixed solution was bubbled with a nitrogen flow of 600–800 mL·min–1 for 3 min under the condition of ventilation to extrude the dissolved CO2. Then, the pre-treated solution was titrated by a standard solution of KOH–isopropanol under stirring and bubbling with N2 until the solution turned bright green and held this color for 15 s. The TAN of the sample was determined following eq 1
| 1 |
where V and V0 represent the volumes of the KOH–isopropanol standard solution consumed by the titrated sample and blank sample (i.e., the samples with and without the jet fuel), respectively, and c and m are the actual concentration of the KOH–isopropanol standard solution and quality of the jet fuel, respectively. Note that the actual concentration of the standard solution of KOH–isopropanol was calibrated by potassium biphthalate. For the accuracy of this research, the value of TAN was always averaged with five results.
The potentiometric titration was tested according to the GB/T 7304 protocol on a ZDJ-5B potentiometric titrator (Shanghai Precision Scientific Instrument Co., Ltd.). Briefly, 100 ± 5 g of the jet fuel and 100 mL of the titration solvent were mixed thoroughly in a titration vessel under the protection of a 600–800 mL·min–1 N2 flow. Subsequently, the pre-treated solution was titrated using the KOH–isopropanol solution under stirring. A saturated calomel electrode and a glass electrode were used as the reference and working electrodes, respectively. The fixed value mode was used during titrating, and 0.02 mL of the KOH–isopropanol standard solution was injected each time. Note that the concentration of the KOH–isopropanol standard solution was measured by potassium acid phthalate before use.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (51705530), the Natural Science Foundation of Jiangsu Province (BK20180180), the Air Force Equipment Military Scientific Research Project (KJ2020C018-4), and the Air Force Logistics Academy Fund (KQQNJ21-007).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c07015.
Detailed titration results with different titration solvents: mixed solution of methylbenzene–isopropanol–water (Vmethylbenzene/Visopropanol/Vwater = 100:99:1) and absolute ethanol–water (Vabsolute ethanol/Vwater = 99:1) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Chen T.; Xu X.; Hu J.; Guo L.; Yang S.; Zhao T.; Ma J. Water behavior of current jet fuel versus operating conditions: Storage time, temperature, relative humidity and anti-icing agent. Fuel 2022, 309, 122088. 10.1016/j.fuel.2021.122088. [DOI] [Google Scholar]
- Zeng P.; Wang B.-Y.; He R.; Liang J.; Yang Z.-Y.; Xia Z.-X.; Wang Q.-D. Single-Pulse Shock Tube Pyrolysis Study of RP-3 Jet Fuel and Kinetic Modeling. ACS Omega 2021, 6, 11039–11047. 10.1021/acsomega.1c00972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Barrera D.; Rueda-Chacón H.; Molina V D. Prediction of the total acid number (TAN) of colombian crude oils via ATR-FTIR spectroscopy and chemometric methods. Talanta 2020, 206, 120186. 10.1016/j.talanta.2019.120186. [DOI] [PubMed] [Google Scholar]
- Mao J.-X.; Zheng Q.-X.; Xu X.; Guo L.; Yang S.-Z.; Chen B.-H.; Hu J.-Q. Research on the Influence of Cosolvent on the Determination of the Contamination Degree of Jet Fuel. ACS Omega 2020, 5, 12184–12190. 10.1021/acsomega.0c00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B.; Li X.; Zhu J.; Huang Y.; Cheng H. Identification of acidic species extracted from heavy crude oil by fourier transform ion cyclotron resonance mass spectrometry. Korean J. Chem. Eng. 2014, 31, 1082–1087. 10.1007/s11814-014-0002-x. [DOI] [Google Scholar]
- Vrtiška D.; Vozka P.; Váchová V.; Šimáček P.; Kilaz G. Prediction of HEFA content in jet fuel using FTIR and chemometric methods. Fuel 2019, 236, 1458–1464. 10.1016/j.fuel.2018.09.102. [DOI] [Google Scholar]
- Cho K.; Rana B. S.; Cho D.-W.; Beum H. T.; Kim C.-H.; Kim J.-N. Catalytic removal of naphthenic acids over Co-Mo/γ-Al2O3 catalyst to reduce total acid number (TAN) of highly acidic crude oil. Appl. Catal., A 2020, 606, 117835. 10.1016/j.apcata.2020.117835. [DOI] [Google Scholar]
- Zhao L.; Zhang X.; Pan L.; Liu J. Storage period prediction and metal compatibility of endothermic hydrocarbon fuels. Fuel 2018, 233, 1–9. 10.1016/j.fuel.2018.06.034. [DOI] [Google Scholar]
- Long F.; Li F.; Zhai Q.; Wang F.; Xu J. Thermochemical conversion of waste acidic oil into hydrocarbon products over basic composite catalysts. J. Clean. Prod. 2019, 234, 105–112. 10.1016/j.jclepro.2019.06.109. [DOI] [Google Scholar]
- Mao J.; Chen T.; Guo L.; Yang S.; Xu X.; Ma J.; Hu J. Effect of additives on the foam behavior of aviation coolants: tendency, stability, and defoaming. ACS Omega 2020, 5, 17686–17691. 10.1021/acsomega.0c02238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Q.; Long G.; Zhao T.; Hu X. Catalyst-free selective N-formylation and N-methylation of amines using CO2 as a sustainable C1 source. Green Chem. 2020, 22, 1134–1138. 10.1039/c9gc03637g. [DOI] [Google Scholar]
- Moro M. K.; Neto Á. C.; Lacerda V. Jr.; Romão W.; Chinelatto L. S. Jr.; Castro E. V. R.; Filgueiras P. R. FTIR, 1H and 13C NMR data fusion to predict crude oils properties. Fuel 2020, 263, 116721. 10.1016/j.fuel.2019.116721. [DOI] [Google Scholar]
- Mao J.; Chen T.; Xu X.; Yang S.; Guo L.; Ma J.; Yao T.; Xin Y.; Hu J. Influence of Foam Characteristics on the Aviation Coolants’ Pollution Degree. ACS Omega 2020, 5, 30323–30328. 10.1021/acsomega.0c04943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long G.; Yang C.; Yang X.; Zhao T.; Liu F.; Cao J. Bisazole-Based Deep Eutectic Solvents for Efficient SO2 Absorption and Conversion without Any Additives. ACS Sustain. Chem. Eng. 2020, 8, 2608–2613. 10.1021/acssuschemeng.9b07735. [DOI] [Google Scholar]
- Deng C.; Cui Y.; Chen J.; Chen T.; Guo X.; Ji W.; Peng L.; Ding W. Enzyme-like mechanism of selective toluene oxidation to benzaldehyde over organophosphoric acid-bonded nano-oxides. Chin. J. Catal. 2021, 42, 1509–1518. 10.1016/s1872-2067(20)63758-5. [DOI] [Google Scholar]
- Long G.; Yang C.; Yang X.; Zhao T.; Xu M. Deep eutectic solvents consisting of 1-ethyl-3-methylimidazolium chloride and glycerol derivatives for highly efficient and reversible SO2 capture. J. Mol. Liq. 2020, 302, 112538. 10.1016/j.molliq.2020.112538. [DOI] [Google Scholar]
- Lan X.; Ma X.; Wang L.; Shi Y.; Gu Q.; Wu L.; Gu X.; Luo Z. Self-Assembly of Diblock Copolymers Containing Thermo-and Photoresponsive Lower Critical Solution Temperature Phase Behavior Polymer with Tunable Assembly Temperature in an Ionic Liquid Mixture. ACS Omega 2019, 4, 11229–11236. 10.1021/acsomega.9b01287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte M. W.; Liberatore M. W. Viscosity of biomass pyrolysis oils from various feedstocks. Energy Fuels 2010, 24, 6601–6608. 10.1021/ef101173r. [DOI] [Google Scholar]
- Huo J.; Luo B.; Chen Y. Crystalline Covalent Organic Frameworks from Triazine Nodes as Porous Adsorbents for Dye Pollutants. ACS Omega 2019, 4, 22504–22513. 10.1021/acsomega.9b03176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T.; Guo S.; Yang J.; Xu Y.; Sun J.; Wei D.; Chen Z.; Zhao B.; Ding W. Nitrogen-Doped Carbon Activated in Situ by Embedded Nickel through the Mott-Schottky Effect for the Oxygen Reduction Reaction. ChemPhysChem 2017, 18, 3454–3461. 10.1002/cphc.201700834. [DOI] [PubMed] [Google Scholar]
- Li F.; Jiang J.; Liu P.; Zhai Q.; Wang F.; Hse C.-y.; Xu J. Catalytic cracking of triglycerides with a base catalyst and modification of pyrolytic oils for production of aviation fuels. Energy Fuels 2018, 2, 1206–1215. 10.1039/c7se00505a. [DOI] [Google Scholar]
- Zhou J.; Xu S.; Kang Q.; Ni L.; Chen N.; Li X.; Lu C.; Wang X.; Peng L.; Guo X.; Ding W.; Hou W. Iron oxide encapsulated in nitrogen-rich carbon enabling high-performance lithium-ion capacitor. Sci. China Mater. 2020, 63, 2289–2302. 10.1007/s40843-020-1414-0. [DOI] [Google Scholar]
- Rana B. S.; Cho D.-W.; Cho K.; Kim J.-N. Total Acid Number (TAN) reduction of high acidic crude oil by catalytic esterification of naphthenic acids in fixed-bed continuous flow reactor. Fuel 2018, 231, 271–280. 10.1016/j.fuel.2018.05.074. [DOI] [Google Scholar]
- Li C.; Liu F.; Zhao T.; Gu J.; Chen P.; Chen T. Highly efficient CO2 fixation into cyclic carbonate by hydroxyl-functionalized protic ionic liquids at atmospheric pressure. Mol. Catal. 2021, 511, 111756. 10.1016/j.mcat.2021.111756. [DOI] [Google Scholar]
- Chen T.; Xu Y.; Guo S.; Wei D.; Peng L.; Guo X.; Xue N.; Zhu Y.; Chen Z.; Zhao B.; Ding W. Ternary heterostructural Pt/CN/Ni as a supercatalyst for oxygen reduction. iScience 2019, 11, 388–397. 10.1016/j.isci.2018.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J.; Ruan S.; Hu J.; Sun Y.; Fei Y.; Jiang X.; Dong S.; Chen T.; Wu N. The intrinsic relationship between color variation and performances of the deteriorated aviation lubrication oil. J. Ind. Eng. Chem. 2020, 92, 88–95. 10.1016/j.jiec.2020.08.023. [DOI] [Google Scholar]
- Chen T.; Ma J.; Chen S.; Wei Y.; Deng C.; Chen J.; Hu J.; Ding W. Construction of heterostructured CoP/CN/Ni: Electron redistribution towards effective hydrogen generation and oxygen reduction. Chem. Eng. J. 2021, 415, 129031. 10.1016/j.cej.2021.129031. [DOI] [Google Scholar]
- Hu J.-Q.; Zhang J.-J.; Guo L.; Miao C.-Q.; Yang S.-Z.; Ma J.; Xu X.; Xie F. Synthesis of Styrenated Sulfur- and Phosphorus-Free Organic Titanate and Evaluation of Its Tribological and Antioxidant Properties as an Additive in Poly-α-olefin. Ind. Eng. Chem. Res. 2019, 58, 1754–1759. 10.1021/acs.iecr.8b04652. [DOI] [Google Scholar]
- Meine N.; Benedito F.; Rinaldi R. Thermal stability of ionic liquids assessed by potentiometric titration. Green Chem. 2010, 12, 1711–1714. 10.1039/c0gc00091d. [DOI] [Google Scholar]
- Zhou J.; Kang Q.; Xu S.; Li X.; Liu C.; Ni L.; Chen N.; Lu C.; Wang X.; Peng L.; Guo X.; Ding W.; Hou W. Ultrahigh rate capability of 1D/2D polyaniline/titanium carbide (MXene) nanohybrid for advanced asymmetric supercapacitors. Nano Res. 2022, 15, 285–295. 10.1007/s12274-021-3472-2. [DOI] [Google Scholar]
- Black S.; Ferrell J. R. III Determination of carbonyl groups in pyrolysis bio-oils using potentiometric titration: review and comparison of methods. Energy Fuels 2016, 30, 1071–1077. 10.1021/acs.energyfuels.5b02511. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.