Abstract
Nonalcoholic steatohepatitis (NASH) has emerged as one of the important causes of cirrhosis and hepatocellular carcinoma, and over 50 therapeutic agents are in various Phases of clinical development. Recently, obeticholic acid has achieved the interim histological endpoint of fibrosis improvement with no worsening of NASH in the phase 3 REGENERATE study, and now patients are being followed for long-term clinical outcomes. Several drugs are in Phase 3 trials with a goal to achieve conditional registration under the subpart H pathway by the United States Food and Drug Administration (FDA). It is thus timely to consider the current situation and the way ahead in the management of NASH. In this article, we review the natural history of nonalcoholic fatty liver disease, upcoming treatments for NASH and various assessments. Based on the current knowledge, we discuss what should be the target treatment population and whether non-invasive tests are ready to guide NASH treatments, both for patient selection and evaluation of treatment response.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is now the most common chronic liver disease, affecting at least a quarter of the global adult population.1, 2 Nonalcoholic steatohepatitis (NASH) is the progressive form of NAFLD associated with persistent liver cell injury leading to fibrosis and in a subset may progress to cirrhosis and end-stage-liver-disease.3 Among patients listed for liver transplantation, NASH is the fastest growing cause of hepatocellular carcinoma (HCC).4 Importantly, NAFLD/NASH is not confined to the western world. Recent meta-analyses highlighted the high prevalence of NAFLD in Asia and Latin America.5, 6
With this growing epidemic and the identification of a number of potential treatment targets, it is hoped that registered drugs for NASH will become available in the next few years.7 However, as in other medical disciplines, clinical trials for NASH have included highly selected patient populations; the external validity of the findings is uncertain. In particular, most studies target patients with NASH (defined as the presence of hepatic steatosis, lobular inflammation and hepatocyte ballooning) with various degrees of liver fibrosis. In real life, physicians face patients with a wider histologic spectrum. Furthermore, clinical trials often exclude patients with severe comorbidities such as coronary artery disease and chronic kidney disease in spite of their high prevalence in the NASH population.8 In other words, when NASH drugs become available, physicians will have to make judgements on the likely efficacy and safety of treatment for patients not represented in the pivotal studies.
In addition, patients and physicians are not the only stakeholders in deciding how to use NASH drugs. Additionally, depending on local arrangements, regulators and payers will also play a major role in shaping the prescription pattern, especially when a treatment is expected to have a major impact on healthcare expenses.9 The case of NASH is further complicated by the fact that the pivotal trials all select and evaluate patients by serial liver biopsies. Although most studies perform non-invasive tests of NASH and liver fibrosis in parallel, it is uncertain if the regulators and payers would mandate a liver biopsy before prescription.
It is therefore timely to consider the current situation and the way ahead. In this article, we review the natural history of NAFLD, upcoming treatments for NASH and various assessments. Based on the current knowledge, we discuss what should be the target treatment population and whether non-invasive tests are ready to guide NASH treatments.
NATURAL HISTORY OF NAFLD
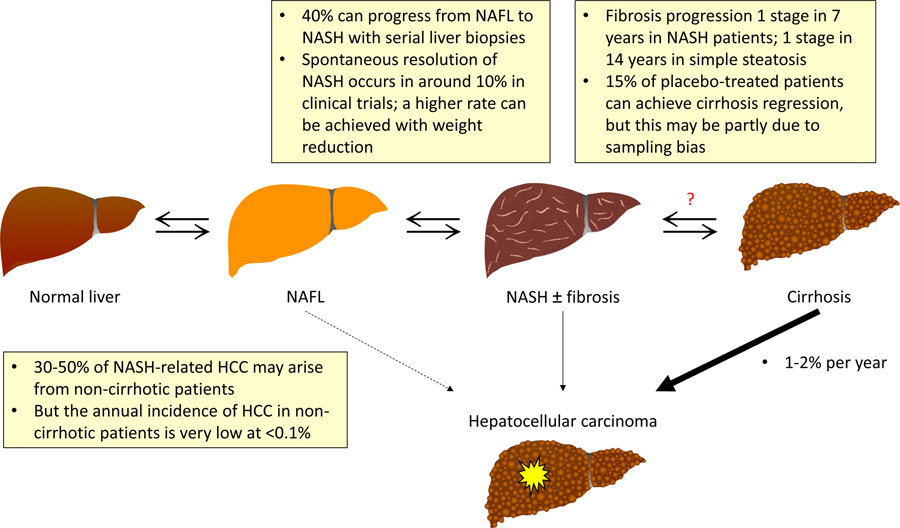
NAFLD is the umbrella term covering the entire spectrum of disease (Figure 1). Based on histology, nonalcoholic fatty liver (NAFL) or simple steatosis refers to the presence of hepatic steatosis but no significant necroinflammation. NASH is characterized by the presence of hepatic steatosis, lobular inflammation and hepatocyte ballooning. In longitudinal studies, NASH is associated with faster fibrosis progression and increased liver-related morbidity and mortality.3, 10,11 The two disease states are dynamic. Progression from NAFL to NASH has been well reported in studies using serial liver biopsies,12, 13 and weight reduction through lifestyle intervention can result in resolution of NASH.14
Figure 1.
Natural history of NAFLD.
Footnote: HCC, hepatocellular carcinoma; NAFL, nonalcoholic fatty liver; NASH, nonalcoholic steatohepatitis
Among patients with NASH-related cirrhosis, the reported annual incidence of HCC is 1–2%.15–17 Over 20% may develop hepatic decompensation in 2–5 years18, 19. Similar to other chronic liver diseases, cirrhosis is the most important risk factor of HCC development in NASH patients19. Nevertheless, 30–50% of NASH-related HCC may develop in non-cirrhotic patients.20–22
Due to the close association with metabolic syndrome, patients with NAFLD have increased risk of cardiovascular disease and other comorbidities.23, 24 However, the relatively importance of different causes of death depends on the severity of liver disease. In a multicenter study of 458 patients with biopsy-proven NAFLD, liver-related complications accounted for half of the deaths in those with bridging fibrosis and all of the deaths in cirrhotic patients.17 Thus, patients with advanced fibrosis have the greatest unmet need for treatment.
CURRENT AND INVESTIGATIONAL THERAPIES
NASH therapeutics is a rapidly evolving field. Readers may refer to recent reviews for further details.25, 26 Regardless of the disease severity, NAFLD patients should be encouraged to have a healthy diet and regular exercise. A 10% weight reduction is often cited as the target to improve NASH and reverse liver fibrosis in those with overweight and obesity.14 However, many patients can still have improvements in NAFLD with a lesser degree of weight reduction.27 That said, not everyone can adopt lifestyle changes, and long-term adherence is difficult28 and some patients will need pharmacological treatment for NASH.
American Association for the Study of Liver Diseases (AASLD) NAFLD practice guidance statement recommends off label use of vitamin E in non-cirrhotic, non-diabetic patients with biopsy-proven NASH and pioglitazone for the management of diabetic patients with biopsy-proven NASH29. These are based upon data from published randomized controlled trials in NASH30 Whilst there are some retrospective data supporting the use of the former in terms of reductions in clinical events31, there are no prospective randomized trials to confirm their impact on liver-related morbidity or mortality. Similarly, the GLP-1 receptor agonist liraglutide was tested in the LEAN trial and led to resolution of NASH with no worsening of fibrosis in 39% of patients, compared with 9% in the placebo group.32 Semaglutide is a new GLP-1 receptor agonist with more profound weight reduction than liraglutide33. Oral formulations are under development and may be better accepted by patients.34 Recently, Newsome and colleagues conducted a phase 2 trial in patients with NASH related fibrosis and showed that 59% of patients treated with semaglutide 0.4mg daily achieved NASH resolution at 72 weeks, compared with 17% in the placebo arm.35
Among agents in phase 3 development, three have completed the interim analysis of the primary histologic endpoints. Obeticholic acid, a potent farnesoid X receptor (FXR) agonist, increased the rate of fibrosis improvement with no worsening of NASH at 18 months in the REGENERATE study (23.1% in patients receiving obeticholic acid 25 mg daily, 17.6% in 10 mg daily, and 11.9% in placebo group).36 There was no significant difference in the rate of resolution of NASH in the three groups. Nonetheless, around half of the patients in the 25 mg daily group developed pruritus, and the treatment was associated with increased level of low-density lipoprotein-cholesterol. Thus, further clarifications and/or clinical outcome data are needed before final adjudication can be made by the Food and Drug Administration.
In contrast, selonsertib, an apoptosis signal-regulating kinase-1 (ASK1) antagonist, failed to demonstrate any effect on NASH or fibrosis in the phase 3 STELLAR studies.37 ASK1 is a key molecule in an inflammatory pathway. Inhibition of this pathway has been shown to ameliorate NASH in multiple preclinical studies.38, 39 In a small phase 2 study, selonsertib treatment for 6 months improved fibrosis in 40% of NASH patients.40 The failure of selonsertib in the subsequent phase 3 study suggests the effect of redundant pathways, particularly when the underlying metabolic dysfunction is left unchecked.
Other drugs in phase 3 development include elafibranor (PPARα/δ dual agonist; with recent announcement of negative topline results),41 cenicriviroc (inhibitor of CC chemokine receptors 2 and 5),42 armachol (a bile acid and fatty acid analogue)43, and resmetirom (MGL-3196, a thyroid hormone receptor-beta agonist).44 Many agents are in phase 2 development (Table 1) and it is reasonable to expect that one or more drugs may become available for the treatment of NASH in the near future. Since the response rate to individual drugs has so far been modest, it is anticipated that combination treatment will likely be required and the field needs robust methods to determine whether a patient is responding to treatment45.
Table 1.
NASH drugs in phase 2 and 3 development
| Drug | Mode of action | ClinicalTrials.gov number |
|---|---|---|
| Phase 3 | ||
| Obeticholic acid | Farnesoid X receptor agonist |
NCT02548351 (REGENERATE) NCT03439254 (REVERSE) |
| Elafibranor | Peroxisome proliferator-activated receptor-alpha and -delta agonist | NCT02704403 (RESOLVE-IT) |
| Cenicriviroc | Inhibitor of CC chemokine receptors 2 and 5 | NCT03028740 (AURORA) |
| Resmetirom | Thyroid hormone receptor-beta agonist | NCT03900429 (MAESTRO-NASH) |
| Phase 2 | ||
| Aramchol | Fatty acid bile acid conjugate | NCT02279524 |
| Cilofexor | Farnesoid X receptor agonist | NCT02854605 |
| Tropifexor | Farnesoid X receptor agonist | NCT02855164 |
| EDP-305 | Farnesoid X receptor agonist | NCT03421431 |
| Pegbelfermin | Fibroblast growth factor 21 analogue | NCT03486899 |
| NGM282 | Fibroblast growth factor 19 analogue | NCT03912532 |
| Firsocostat | Acetyl-CoA carboxylase inhibitor | NCT02856555 |
| PF-05221304 | Acetyl-CoA carboxylase inhibitor | NCT03248882 |
| Liraglutide | Glucagon-like peptide-1 agonist | NCT01237119 |
| Semaglutide | Glucagon-like peptide-1 agonist | NCT02970942 |
| Lanifibranor | Peroxisome proliferator-activated receptor-alpha agonist | NCT03008070 |
| Seladelpar | Peroxisome proliferator-activated receptor-delta agonist | NCT03551522 |
| Saroglitazar | Peroxisome proliferator-activated receptor-alpha and -gamma agonist | NCT03061721 |
| MSDC-0602K | Mitochondrial pyruvate carrier inhibitor | NCT02784444 |
| VK2809 | Thyroid hormone receptor-beta agonist | NCT02927184 |
THERAPEUTIC ENDPOINTS
The primary purpose of treatments for NASH are to reduce morbidity and mortality from liver disease. In terms of defining the efficacy of treatment therefore the “hard” clinical endpoints in NASH consist of all-cause mortality, liver-related mortality, and liver decompensation events. It is notable that the first successful trials of medical therapy to reduce cardiovascular disease mortality were in secondary prevention whereas the majority of the efforts in therapeutic trials in NASH are in the non-cirrhotic population as primary prevention of liver related morbidity and mortality.46, 47 In that context it is anticipated that the proportion of patients with non-cirrhotic NASH who will develop a hard clinical endpoint in the short term is low. Consequently the regulatory agencies (the Food and Drug Administration (FDA) of the Unites States, and the European Medicines Agency (EMA) have agreed histological surrogate endpoints that will allow both early and late assessments of efficacy.48–50
The histological endpoints endorsed by the FDA for subpart H approval pathway consist of either resolution of steatohepatitis without worsening of fibrosis or improvement in ≥ 1 stage fibrosis without worsening of steatohepatitis. The full definitions of these endpoints are provided in Box 1. In a current discussion document, the EMA consider these to be co-primary endpoints and for a drug to be given a conditional license both would need to be met. If the mechanism of action of a drug is to target fibrosis only, then the expectation is that a greater degree of fibrosis improvement is required, meaning in practice that fibrosis regression of at least two stages would be needed. The later histological endpoint in the non-cirrhotic population is progression to cirrhosis. Whilst the development of cirrhosis is clearly an important landmark in the natural history of liver disease, it is considered as part of the composite events for full approval of a drug along with clinical hepatic decompensation and mortality.51, 52
BOX 1. Early histological endpoints considered reasonably likely to predict clinical benefit in phase 3 trials in non-cirrhotic NASH.
Food and Drug Administration
Resolution of steatohepatitis on overall histopathological reading and no worsening of liver fibrosis on NASH CRN Histologic Scoring System. Resolution of steatohepatitis is defined as absent fatty liver disease or isolated simple steatosis without steatohepatitis and a score of 0–1 for inflammation, 0 for ballooning and any value for steatosis.
OR
Improvement in liver fibrosis greater than or equal to one stage (NASH CRN Histologic Scoring System) and no worsening of steatohepatitis (defined as no increase in NAS for ballooning, inflammation, or steatosis).
OR
Both resolution of steatohepatitis and improvement in fibrosis.
European Medicines Agency
The resolution of NASH – with the presence of any grade of steatosis, no ballooning, and only minimal (grade 1) lobular inflammation and – at the same time – no worsening of the stage of fibrosis.
AND
The improvement of fibrosis of at least 1 stage without any worsening of NASH (no worsening of ballooning and lobular inflammation).
For those patients with NASH who develop cirrhosis the latest guidance from the regulatory authorities is for trials that examine hard clinical endpoints, including the development of the hepatic decompensation, the need for liver transplantation, and progression of synthetic dysfunction as assessed by the model for end-stage liver disease (MELD) score ≥ 15. This is an important change in the assessment of patients in clinical trials with cirrhosis where previously regression of fibrosis was accepted as an appropriate interim endpoint.
In all phase 3 trials in NASH, regardless of whether patients with or without cirrhosis are included, all cause mortality data are to be collected. This is an important consideration in patients who are at substantial risk of death from causes other than liver disease. In particular, reporting of major adverse cardiac events (MACE) is advisable during these studies to assess the collateral safety of treatment. A favorable reduction in MACE, as has been observed with several classes of therapies such as GLP-1 receptor agonists and SGLT-2 inhibitors that are also under investigation for treatment of NASH-related fibrosis,53, 54 is potentially of immense interest as a therapeutic strategy in patients with NASH.
SHOULD WE RESTRICT PHARMACOLOIGIC THERAPIES TO SELECTED PATIENTS?
Defining the optimal patient population with NASH for treatment is challenging due to lack of a complete understanding of the pathogenesis and natural history of disease of NASH. Ultimately the decision to treat a patient with NASH for the prevention of liver-related morbidity and mortality will rest on the underlying probability of an adverse liver-related event and the ability of these new therapies to reduce the future risk of these events. In a prevalent condition, where treatment is likely to be given long-term to slow the progression of disease, the cost-effectiveness of treatment will also be a major driver in decision-making in many health systems.
Patient selection
All patients with NAFLD who are overweight or obese will benefit from lifestyle interventions that are focused to induce caloric deficit by dietary restriction and increasing exercise. Therefore, lifestyle interventions are the cornerstone of the management of NAFLD across all stages of disease.
It is evident that the risk of liver-related mortality is highest in those patients with cirrhosis and these individuals are at greatest need of treatment. In non-cirrhotic NASH, the current paradigm identifies patients with NASH and significant liver fibrosis (stage 2–3) as being those at greatest risk of developing cirrhosis and future liver morbidity and mortality and this is where there is the majority of late phase clinical trial activity. This is supported by recent data from the phase 2b trials of simtuzumab. These data have shown that patients with NASH and stage 3 fibrosis have a 20% risk of progression to cirrhosis over a 2 year period18. Identification of this population currently requires liver biopsy and the utility of non-invasive assessments for the identification of NASH is reviewed below.
When considering the role of treatment, the absolute risk of liver-related morbidity and mortality in these patients must be considered. For instance, the estimated 10-year risk of liver decompensation for a 50-year old woman with F2 or F3 is estimated to be 1% and 4%, respectively.55 Therefore, there is broad acceptance that patients with NASH and stage 3 fibrosis are the more attractive group of patients who might benefit from a pharmacologic therapy if it is effective in slowing the progression of disease to cirrhosis and reduces liver related morbidity and mortality.
Clinical and cost-effectiveness of treatment
There are three fundamental principles that should be met before establishing routine clinical use of a pharmacologic treatment in NASH: clinical efficacy of the drug in improving liver-related outcomes, the overall safety profile of the drug to have a favorable risk-benefit ratio and established cost-effectiveness of the therapeutic approach compared to the current standard of care. It is forseable that the cost-effectiveness and societal benefit would be higher in those with NASH with ≥ stage 2 fibrosis particularly in those with bridging fibrosis and cirrhosis given the higher risk of liver-related events in patients with advanced fibrosis. This may be an area where real-world studies might provide important insights through robust identification of so-called “fast progressors” at early stages of fibrosis who may have more to gain from treatment than those with slower disease trajectories.
While NAFLD is highly prevalent, NASH with significant fibrosis is less so, and liver biopsy to identify and characterise the severity of NASH in line with trial entry criteria may be required for treatment further limiting the applicability of treatment.56 Therefore, development and validation of non-invasive tests will be required for identification of patients who need to be treated by pharmacologic therapies without needing a liver biopsy as these therapies become available for clinical use.
PATIENT SELECTION FOR INCLUSION INTO A CLINICAL TRIAL BY NON-INVASIVE TESTS
In routine clinical practice, ultrasound of the liver is typically used to assess presence of hepatic steatosis. However, despite its widespread use and availability, it lacks sensitivity, accuracy and precision to be used as a tool for inclusion into a clinical trial.57 Therefore, more quantitative tests have been utilized in this setting. The utility of such testing and its applicability for the selection of patients for treatment in clinical practice is described hereafter.
Early phase trials
Magnetic resonance imaging based proton-density-fat-fraction (MRI-PDFF) has emerged as the leading imaging based quantitative, accurate, reproducible and precise biomarker for the quantification of liver fat in the setting of NASH clinical trials.57, 58 To improve efficiency and reduce costs, most trials apply a pre-screening strategy with controlled attenuation parameter (CAP), a liver fat quantification method, which is currently available on the FibroScan. A threshold in the CAP of ≥300 db/min is used to maximize the likelihood that patients will mee the MRI-PDFF ≥8% as an inclusion criteria employed in most early phase trials.59
Late phase trials
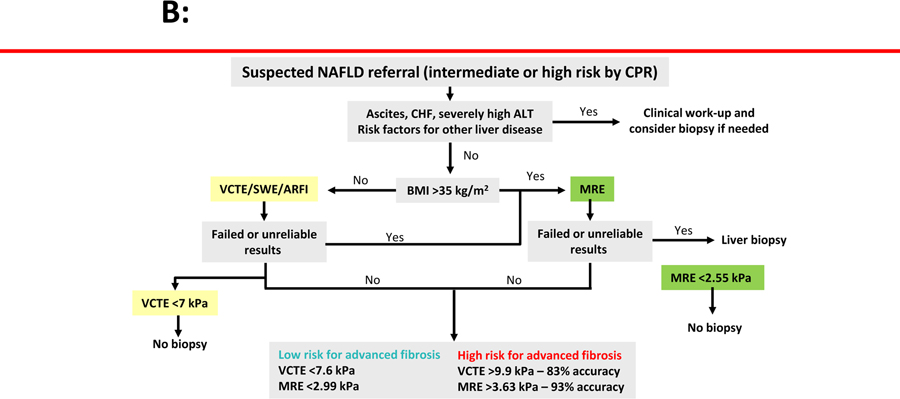
Phase 2b and phase 3 trials in NASH now typically enroll those patients most in need of treatment for NASH. These trials require a liver biopsy assessment at baseline showing the presence of NASH with liver fibrosis ranging from stage 1–3 fibrosis or patients with advanced fibrosis defined as stage 3 and stage 4 fibrosis. Various types of strategies have been employed in these two settings with different cut-points for various screening methods to enrich the cohort and reduce the screen failure rate largely based on non-invasive measures of fibrosis. Where the target patient population is those with stage 1–3 fibrosis, these approaches have used tools to exclude patients by liver stiffness measurement using either vibration controlled transient elastography (VCTE) <7.1 kPa or magnetic resonance elastography (MRE) <2.55 kPa.42, 59, 60 Combining liver stiffness values derived from VCTE with serum AST levels is a further approach that shows promise in this setting. 61,62 To identify patients with more advance fibrosis (stages 3 and 4) combinsations of non-invasive fibrosis tests have been used with some success. For example, the ATLAS trial utilized a non-invasive strategy by randomizing patients who had an Enhanced Liver Fibrosis Panel (ELF) ≥9.8 and a VCTE ≥14 kPa into the trial (NCT03449446). All patients underwent a liver biopsy who met the above-mentioned non-invasive criteria, and 83% of the patients met the liver biopsy criteria confirming stage 3 or stage 4 fibrosis due to NASH. In a recent study, Jung and colleagues demonstrated that a MRE ≥ 3.3 Kpa and FIB-4 ≥ 1.6 is associated with 97% positive predictive value for ≥ stage 2 fibrosis in NAFLD (EASL 2020). These data may have important clinical implications and further studies are needed to assess PPV of VCTE combined with FIB-4 for ruling in who needs to be treated.
These pre-screening methods are neither accurate nor precise but do help in reducing screen failure rate and improving efficiency by prioritizing higher risk patients as identified by the likely presence of advanced fibrosis (Figure 2a and 2b63, 64). Whether these approaches are sufficient to select patients for treatment in clinical practice where the registration clinical trials have included only patients identified with liver biopsy remains an open question.
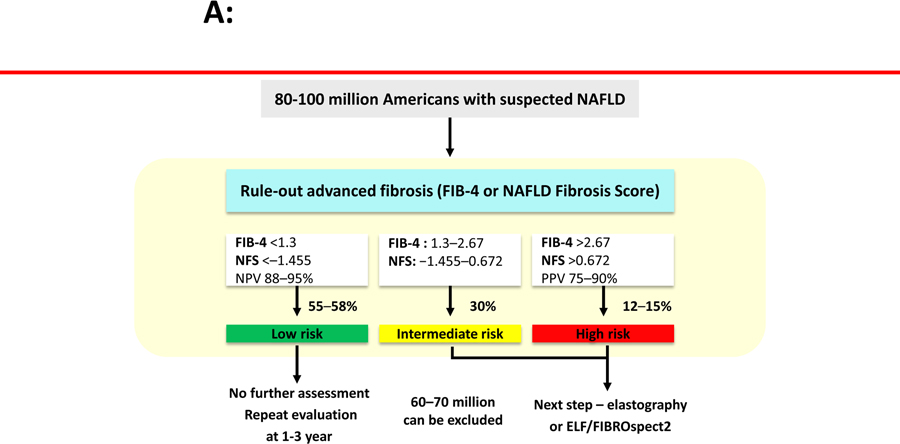
Figure 2.
Non-invasive tests of liver fibrosis for NAFLD. (A) Optimizing population management in NAFLD. (B) Elastography in assessing advanced fibrosis.
Footnote: ARFI, acoustic radiation force impulse; ALT, alanine aminotransferase; BMI, body mass index; CHF, congestive heart failure; ELF, Enhanced Liver Fibrosis score; MRE, magnetic resonance elastography; NFS, NAFLD fibrosis score; NPV, negative predictive value; SWE, shear-wave elastography; VCTE, vibration-controlled transient elastography.
NIT for identifying high-risk NASH patients in clinical practice
Patients with NASH with ≥ stage 2 fibrosis are candidates for pharmacologic therapies in registration trials as these individuals with “high-risk NASH” have significantly increased risk of liver-related mortality compared to those with stage 0–1 NAFLD. Recent studies have identified a sequential risk stratification that may be useful in clinical practice. Patients with FIB-4 ≤ 1.3 are considered to have low likelihood of having advanced fibrosis so those may be followed by serial testing with the same tool every 1–3 years. Those with a FIB-4 ≥ 2.67 are at significantly higher risk of having advanced fibrosis and may be considered for a liver biopsy assessment or elastography. Those patients with a FIB-4 between 1.3–2.67 may benefit from additional testing such as an imaging-based NIT e.g. VCTE or SWE or MRE or a serum-based fibrosis tests such as ELF or Fibrospect 264. More recently, the FAST score (a combination of CAP, AST and VCTE derived liver stiffness) has been shown to be useful for identification of at risk-NASH (lower cut-point 0.35 to rule out and 0.67 to rule in NASH with ≥ stage 2 fibrosis). The FAST score had an AUROC on 0.80 with a PPV that ranges between 65% and 83%61. Emerging data derived from the United States population and validated in a Japanese population suggest that a user-friendly metric of MRE ≥ 3.3 kPa and FIB-4 ≥ 1.6 (MEFIB index) rules in fibrosis stage ≥2 with an AUROC of 0.92 and PPV ranging between 90%−97%65. We refer the reader to recently written review articles and editorials that further expand on this risk stratification approach using currently available NITs66, 67. Further research is needed to prospectively assess the utility of application of these cut-points on liver-related outcomes.
CONCLUSIONS
It is with great optimism we write this review describing the various advances in clinical trials in NASH a nd emerging therapies for reversal of NASH-related fibrosis. Defining groups with NASH for therapy is currently best done using a fibrosis assessment, with those patients with bridging fibrosis and cirrhosis most urgently in need of treatment. The critical determinant of success will be the demonstration of clinical efficacy and safety of these new therapies in this group of patients with advanced fibrosis over a long period of time given the chronic nature of this condition. How patients will ultimately be identified for treatment and how that treatment will be assessed remains an important challenge while liver biopsy remains the mainstay of assessment of patient selection and treatment response in clinical trials. Further studies are needed to develop a panel of serum and imaging based biomarkers for improved diagnosis and risk stratification of patients with NAFLD to enable non invasive selection of patients for treatment.
Grant support:
R.L. receives funding support from NIEHS (5P42ES010337), NCATS (5UL1TR001442), NIDDK (R01DK106419, 1R01DK121378, P30DK120515), and DOD PRCRP (CA170674P2).
Disclosures: I.A.R. has received personal fees from AbbVie, Bayer and Norgine. V.W-S.W. has served as a consultant or advisory board member for 3V-BIO, AbbVie, Allergan, Boehringer Ingelheim, Center for Outcomes Research in Liver Diseases, Echosens, Gilead Sciences, Intercept, Janssen, Novartis, Novo Nordisk, Perspectum Diagnostics, Pfizer, TARGET-NASH, and Terns; given lectures for AbbVie, Bristol-Myers Squibb, Echosens, Gilead Sciences, and Merck; and received an unrestricted grant from Gilead Sciences. R.L. serves as a consultant or advisory board member for 89bio, Alnylam, Arrowhead Pharmaceuticals, AstraZeneca, Boehringer Ingelheim, Bristol-Myer Squibb, Celgene, Cirius, CohBar, DiCerna, Galmed, Gemphire, Gilead, Glympse bio, Intercept, Ionis, Merck, Metacrine, NGM Biopharmaceuticals, Novo Nordisk, Pfizer, Sagimet and Viking Therapeutics. In addition, his institution has received grant support from Allergan, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly and Company, Galmed Pharmaceuticals, Genfit, Gilead, Intercept, Janssen, Madrigal Pharmaceuticals, NGM Biopharmaceuticals, Novartis, Pfizer, pH Pharma, and Siemens. He is also co-founder of Liponexus, Inc.
Abbreviations:
- ALT
alanine aminotransferase
- ASK-1
apoptosis signal-regulating kinase-1
- ELF
Enhanced Liver Fibrosis panel
- FXR
farnesoid X receptor
- GLP-1
glucagon-like peptide-1
- MRE
magnetic resonance elastography
- MRI-PDFF
magnetic resonance imaging proton-density-fat-fraction
- NAFL
nonalcoholic fatty live
- NAFLD
nonalcoholic fatty liver disease
- NAS
NAFLD activity score
- NASH
nonalcoholic steatohepatitis
- PPAR
peroxisome proliferator-activated receptor
REFERENCES
- 1.Younossi Z, Tacke F, Arrese M, et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2019;69:2672–2682. [DOI] [PubMed] [Google Scholar]
- 2.Eslam M, Sanyal AJ, George J, et al. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020;158:1999–2014 e1. [DOI] [PubMed] [Google Scholar]
- 3.Singh S, Allen AM, Wang Z, et al. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol 2015;13:643–54 e1–9; quiz e39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Younossi Z, Stepanova M, Ong JP, et al. Nonalcoholic Steatohepatitis Is the Fastest Growing Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clin Gastroenterol Hepatol 2019;17:748–755 e3. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Zou B, Yeo YH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2019;4:389–398. [DOI] [PubMed] [Google Scholar]
- 6.Zhou F, Zhou J, Wang W, et al. Unexpected Rapid Increase in the Burden of NAFLD in China From 2008 to 2018: A Systematic Review and Meta-Analysis. Hepatology 2019;70:1119–1133. [DOI] [PubMed] [Google Scholar]
- 7.Wong VW, Chitturi S, Wong GL, et al. Pathogenesis and novel treatment options for non-alcoholic steatohepatitis. Lancet Gastroenterol Hepatol 2016;1:56–67. [DOI] [PubMed] [Google Scholar]
- 8.Adams LA, Anstee QM, Tilg H, et al. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017;66:1138–1153. [DOI] [PubMed] [Google Scholar]
- 9.Marshall AD, Cunningham EB, Nielsen S, et al. Restrictions for reimbursement of interferon-free direct-acting antiviral drugs for HCV infection in Europe. Lancet Gastroenterol Hepatol 2018;3:125–133. [DOI] [PubMed] [Google Scholar]
- 10.Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006;44:865–73. [DOI] [PubMed] [Google Scholar]
- 11.Kleiner DE, Brunt EM, Wilson LA, et al. Association of Histologic Disease Activity With Progression of Nonalcoholic Fatty Liver Disease. JAMA Netw Open 2019;2:e1912565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong VW, Wong GL, Choi PC, et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut 2010;59:969–74. [DOI] [PubMed] [Google Scholar]
- 13.McPherson S, Hardy T, Henderson E, et al. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol 2015;62:1148–55. [DOI] [PubMed] [Google Scholar]
- 14.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015;149:367–78 e5; quiz e14–5. [DOI] [PubMed] [Google Scholar]
- 15.Ascha MS, Hanouneh IA, Lopez R, et al. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010;51:1972–8. [DOI] [PubMed] [Google Scholar]
- 16.Bhala N, Angulo P, van der Poorten D, et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology 2011;54:1208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, et al. Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients With Advanced Nonalcoholic Fatty Liver Disease: A Multi-National Cohort Study. Gastroenterology 2018;155:443–457 e17. [DOI] [PubMed] [Google Scholar]
- 18.Loomba R, Adams LA. The 20% Rule of NASH Progression: The Natural History of Advanced Fibrosis and Cirrhosis Caused by NASH. Hepatology 2019;70:1885–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loomba R, Lim JK, Patton H, et al. AGA Clinical Practice Update on Screening and Surveillance for Hepatocellular Carcinoma in Patients With Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology 2020;158:1822–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yasui K, Hashimoto E, Komorizono Y, et al. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin Gastroenterol Hepatol 2011;9:428–33; quiz e50. [DOI] [PubMed] [Google Scholar]
- 21.Mittal S, El-Serag HB, Sada YH, et al. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans is Associated With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol 2016;14:124–31 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stine JG, Wentworth BJ, Zimmet A, et al. Systematic review with meta-analysis: risk of hepatocellular carcinoma in non-alcoholic steatohepatitis without cirrhosis compared to other liver diseases. Aliment Pharmacol Ther 2018;48:696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sookoian S, Pirola CJ. Systematic review with meta-analysis: risk factors for non-alcoholic fatty liver disease suggest a shared altered metabolic and cardiovascular profile between lean and obese patients. Aliment Pharmacol Ther 2017;46:85–95. [DOI] [PubMed] [Google Scholar]
- 24.Arulanandan A, Ang B, Bettencourt R, et al. Association Between Quantity of Liver Fat and Cardiovascular Risk in Patients With Nonalcoholic Fatty Liver Disease Independent of Nonalcoholic Steatohepatitis. Clin Gastroenterol Hepatol 2015;13:1513–20 e1. [DOI] [PubMed] [Google Scholar]
- 25.Friedman SL, Neuschwander-Tetri BA, Rinella M, et al. Mechanisms of NAFLD development and therapeutic strategies. Nat Med 2018;24:908–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong VW, Singal AK. Emerging medical therapies for non-alcoholic fatty liver disease and for alcoholic hepatitis. Transl Gastroenterol Hepatol 2019;4:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong VW, Chan RS, Wong GL, et al. Community-based lifestyle modification programme for non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol 2013;59:536–42. [DOI] [PubMed] [Google Scholar]
- 28.Wong VW, Wong GL, Chan RS, et al. Beneficial effects of lifestyle intervention in non-obese patients with non-alcoholic fatty liver disease. J Hepatol 2018;69:1349–1356. [DOI] [PubMed] [Google Scholar]
- 29.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328–357. [DOI] [PubMed] [Google Scholar]
- 30.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010;362:1675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vilar-Gomez E, Vuppalanchi R, Gawrieh S, et al. Vitamin E Improves Transplant-Free Survival and Hepatic Decompensation Among Patients With Nonalcoholic Steatohepatitis and Advanced Fibrosis. Hepatology 2020;71:495–509. [DOI] [PubMed] [Google Scholar]
- 32.Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016;387:679–90. [DOI] [PubMed] [Google Scholar]
- 33.O’Neil PM, Birkenfeld AL, McGowan B, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet 2018;392:637–649. [DOI] [PubMed] [Google Scholar]
- 34.Davies M, Pieber TR, Hartoft-Nielsen ML, et al. Effect of Oral Semaglutide Compared With Placebo and Subcutaneous Semaglutide on Glycemic Control in Patients With Type 2 Diabetes: A Randomized Clinical Trial. JAMA 2017;318:1460–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newsome PN, Buchholtz K, Cusi K, et al. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N Engl J Med 2020. [DOI] [PubMed] [Google Scholar]
- 36.Younossi ZM, Ratziu V, Loomba R, et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2019;394:2184–2196. [DOI] [PubMed] [Google Scholar]
- 37.Harrison SA, Wong VW, Okanoue T, et al. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: Results from randomized phase III STELLAR trials. J Hepatol 2020. [DOI] [PubMed] [Google Scholar]
- 38.Zhang P, Wang PX, Zhao LP, et al. The deubiquitinating enzyme TNFAIP3 mediates inactivation of hepatic ASK1 and ameliorates nonalcoholic steatohepatitis. Nat Med 2018;24:84–94. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Wen H, Fu J, et al. Hepatocyte TNF Receptor-Associated Factor 6 Aggravates Hepatic Inflammation and Fibrosis by Promoting Lysine 6-Linked Polyubiquitination of Apoptosis Signal-Regulating Kinase 1. Hepatology 2020;71:93–111. [DOI] [PubMed] [Google Scholar]
- 40.Loomba R, Lawitz E, Mantry PS, et al. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: A randomized, phase 2 trial. Hepatology 2018;67:549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ratziu V, Harrison SA, Francque S, et al. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-alpha and -delta, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology 2016;150:1147–1159 e5. [DOI] [PubMed] [Google Scholar]
- 42.Friedman SL, Ratziu V, Harrison SA, et al. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology 2018;67:1754–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ajmera VH, Cachay E, Ramers C, et al. MRI Assessment of Treatment Response in HIV-associated NAFLD: A Randomized Trial of a Stearoyl-Coenzyme-A-Desaturase-1 Inhibitor (ARRIVE Trial). Hepatology 2019;70:1531–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harrison SA, Bashir MR, Guy CD, et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2019;394:2012–2024. [DOI] [PubMed] [Google Scholar]
- 45.Dufour JF, Caussy C, Loomba R. Combination therapy for non-alcoholic steatohepatitis: rationale, opportunities and challenges. Gut 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Randomised trial of intravenous atenolol among 16 027 cases of suspected acute myocardial infarction: ISIS-1. First International Study of Infarct Survival Collaborative Group. Lancet 1986;2:57–66. [PubMed] [Google Scholar]
- 47.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383–9. [PubMed] [Google Scholar]
- 48.Food and Health Administration. Noncirrhotic nonalcoholic steatohepatitis with liver fibrosis: developing drugs for treatment. https://www.fda.gov/media/119044/download (accessed on 23 November 2019).
- 49.Food and Health Administration. Nonalcoholic steatohepatitis with compensated cirrhosis: developing drugs for treatment. https://www.fda.gov/media/127738/download (accessed on 23 November 2019).
- 50.European Medicines Agency. Reflection paper on regulatory requirements for the development of medicinal products for chronic non-infectious liver diseases (PBC, PSC, NASH). https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-regulatory-requirements-development-medicinal-products-chronic-non-infectious-liver_en.pdf (accessed on 14 December 2019).
- 51.Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2015;149:389–97 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hagstrom H, Nasr P, Ekstedt M, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol 2017;67:1265–1273. [DOI] [PubMed] [Google Scholar]
- 53.Kristensen SL, Rorth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol 2019;7:776–785. [DOI] [PubMed] [Google Scholar]
- 54.Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019;393:31–39. [DOI] [PubMed] [Google Scholar]
- 55.Rafiq N, Bai C, Fang Y, et al. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol 2009;7:234–8. [DOI] [PubMed] [Google Scholar]
- 56.Roskilly A, Shearer J, Parker R, et al. High rates of ineligibility for participation in trials of new therapies in non-alcoholic steatohepatitis: a systematic review. Eur J Gastroenterol Hepatol 2020. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 57.Caussy C, Reeder SB, Sirlin CB, et al. Noninvasive, Quantitative Assessment of Liver Fat by MRI-PDFF as an Endpoint in NASH Trials. Hepatology 2018;68:763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Loomba R, Sirlin CB, Ang B, et al. Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial). Hepatology 2015;61:1239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Loomba R, Kayali Z, Noureddin M, et al. GS-0976 Reduces Hepatic Steatosis and Fibrosis Markers in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2018;155:1463–1473 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hsu C, Caussy C, Imajo K, et al. Magnetic Resonance vs Transient Elastography Analysis of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Pooled Analysis of Individual Participants. Clin Gastroenterol Hepatol 2019;17:630–637 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Newsome PN, Sasso M, Deeks JJ, et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol 2020;5:362–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harrison SA, Alkhouri N, Davison BA, et al. Insulin sensitizer MSDC-0602K in non-alcoholic steatohepatitis: A randomized, double-blind, placebo-controlled phase IIb study. J Hepatol 2020;72:613–626. [DOI] [PubMed] [Google Scholar]
- 63.Loomba R, Adams LA. Advances in non-invasive assessment of hepatic fibrosis. Gut 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Castera L, Friedrich-Rust M, Loomba R. Noninvasive Assessment of Liver Disease in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2019;156:1264–1281 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jung J, Loomba RR, Imajo K, et al. MRE combined with FIB-4 (MEFIB) index in detection of candidates for pharmacological treatment of NASH-related fibrosis. Gut 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ajmera V, Loomba R. Imaging biomarkers of NAFLD, NASH, and fibrosis. Mol Metab 2021:101167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vuppalanchi R, Loomba R. Non-invasive Tests to Phenotype Nonalcoholic Fatty Liver Disease - Sequence and Consequences of Arranging the Tools In the Tool Box. Hepatology 2021. [DOI] [PubMed] [Google Scholar]