Abstract
Three-dimensional (3D) in vitro culture systems using human induced pluripotent stem cells (hiPSCs) are useful tools to model neurodegenerative disease biology in physiologically relevant microenvironments. Though many successful biomaterials-based 3D model systems have been established for other neurogenerative diseases, such as Alzheimer’s disease, relatively few exist for Parkinson’s disease (PD) research. We employed tissue engineering approaches to construct a 3D silk scaffold-based platform for the culture of hiPSC-dopaminergic (DA) neurons derived from healthy individuals and PD patients harboring LRRK2 G2019S or GBA N370S mutations. We then compared results from protein, gene expression, and metabolic analyses obtained from two-dimensional (2D) and 3D culture systems. The 3D platform enabled the formation of dense dopamine neuronal network architectures and developed biological profiles both similar and distinct from 2D culture systems in healthy and PD disease lines. PD cultures developed in 3D platforms showed elevated levels of α-synuclein and alterations in purine metabolite profiles. Furthermore, computational network analysis of transcriptomic networks nominated several novel molecular interactions occurring in neurons from patients with mutations in LRRK2 and GBA. We conclude that the brain-like 3D system presented here is a realistic platform to interrogate molecular mechanisms underlying PD biology.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-021-04047-7.
Keywords: 3D bioengineered disease model, Dopaminergic neurons, Metabolomics, Transcriptomics, Network biology, Link prediction analysis
Introduction
Parkinson’s disease is the second most common neurodegenerative disorder in the world, the prevalence of which is projected to rapidly increase over the next decade [1–3]. Current therapeutic options, such as l-3,4-dihydroxyphenylalaninedopa, only manage symptoms in PD, which offers temporary relief but typically leads to severe side effects [4, 5]. The paucity of effective disease modifying treatments [4] can be partly attributed to the limited clinical translatability of animal [6–8] and in vitro culture models of the disease [9–17].
Although two-dimensional (2D) cultures utilizing hiPSC-derived neurons have proven to be useful for our understanding of the human brain [18, 19], the stiff properties of culture substrates do not reproduce the physiological characteristics of native organs [20]. Moreover, the behavior of neurons grown in a 2D microenvironment differs from that observed in vivo because of the unnatural transport kinetics of secreted factors, oxygen, and nutrients [21, 22], as well as unrealistic neuronal network density, architecture, connectivity, and functionality of the cells [10, 21]. To address these limitations, biomimetic 3D culture systems and organoids have been developed to better replicate healthy [21, 23, 24] and pathological features of the brain [25, 26]. This has led to new insights into cytoskeletal dynamics and protein deposition in Alzheimer’s disease [22, 25, 27, 28], as well as aberrant electrophysiology and synaptic transmission in Parkinson’s disease [18, 29–34].
The use of biomaterials offers several advantages for engineering 3D human brain models, such as the flexibility of adding multiple brain cell types with different functions (e.g., neurons, astrocytes, endothelial cells) and from different germ layers (e.g., ectoderm derived neurons, mesoderm derived microglia) to concisely study cell–cell interactions in healthy and disease states. Additionally, the manipulation of extracellular cues makes it possible to examine contributions of extracellular matrix components on neural development and function [24]. Bioengineered tools can also be used to replicate the neural circuit geometry found in vivo to examine, for instance, neural activity in multi-layered cerebral cortical constructs [35, 36]. While organoid cultures accurately represent in vivo development, the absence of a necrotic core makes biofabricated 3D tissues powerful tools to sustain long-term cultures and model the inherently slow development and progression of neurodegenerative disorders [10, 18].
Previously, we developed a biomaterials-based system to create experimental models of traumatic brain injury, Alzheimer’s disease, and viral infection utilizing rodent or human brain cell types [37–40]. The 3D culture is assembled from a biocompatible silk protein scaffold and casted with soft collagen hydrogel to enable neuronal network formation in 3D space. Silk-hydrogel composites have been previously demonstrated to outperform collagen-only systems by promoting neuronal viability and neurite outgrowth [36]. Additionally, the utilization of a solid microporous structure provides adsorption sites for functional brain-relevant peptides/proteins to facilitate cell attachment, migration, and neuronal network formation [10, 41]. We employed collagen hydrogels for its biocompatibility [42], capacity to support multiple brain cell types [43], and ease of incorporation of other native brain extracellular matrix components [44–46]. Here, we implemented this system to model PD phenotypes in human DA neurons derived from patient hiPSCs (Table 1) with mutations in LRRK2 (G2019S) or GBA (N370S), the two most prevalent mutations in PD [47]. Mutations in LRRK2 account for significant fractions of familial (5–13%) and sporadic (1–5%) forms of PD cases, and affect synaptic kinase signaling, dopamine transport, and other neuronal processes [48–50]. In particular, the G2019S mutation in the protein’s kinase domain of LRRK2 results in high levels of autophosphorylation and has been associated with α-synuclein pathophysiology and dysfunctional autophagic processes [51–54]. Mutations in GBA render the glucosylceramidase enzyme partially defective, consequently affecting lipid metabolism, lysosome-mediated protein degradation (e.g., α-synuclein), and autophagic processes [53, 55–57].
Table 1.
Human induced pluripotent stem cell (hiPSC) donors employed in this study
| Donor identification | Donor abbreviation | Genotype | Age at sample collection | Age at PD diagnosis | Sex |
|---|---|---|---|---|---|
| WT050598 | CTRL_1 | Wild Type | 58 | N/A | Male |
| WT050176 | CTRL_2 | Wild Type | 53 | N/A | Male |
| GBA010197 | GBA_CTRL* | GBA N370S | 65 | N/A | Male |
| GBA010198 | GBA_PD* | GBA N370S | 65 | 63 | Male |
| LRRK050401-01 | LRRK2_1 | LRRK2 G2019S | 72 | 72 | Female |
| LRRK050772 | LRRK2_2 | LRRK2 G2019S | 72 | 71 | Female |
All lines were derived from independent donors
An asterisk (*) indicates that hiPSC donors were monozygotic twins but clinically discordant for Parkinson’s disease (i.e., GBA_PD: patient diagnosed with Parkinson’s disease; GBA_CTRL the identical twin brother, unaffected by PD at the time of donation)
By comparing hiPSC-derived DA neurons from PD patients to healthy subjects grown in 2D or 3D culture condition, we showed that the microenvironment distinctly influenced metabolomic and transcriptomic profiles in diseased states, including elevated α-synuclein protein levels. Candidate disease gene prioritization and link prediction analyses identified novel molecular interactions specific to LRRK2 and GBA genotypes that are shared between 2D and 3D culture systems. These results highlight the power of the bioengineered 3D model system as a physiological platform to interrogate disease mechanisms underlying PD in hiPSC-derived DA neurons.
Methods
Silk scaffold processing
Silk scaffolds were generated as previously described [37, 58, 59]. Bombyx mori cocoons were cut into small pieces and boiled in 0.02 M sodium bicarbonate solution for 30 min to remove unwanted sericin protein. Purified silk fibroin was excessively rinsed with deionized water followed by overnight drying in a chemical hood. Silk fibers were then dissolved in 9.3 M lithium bromide solution for 4 h at 60 °C, followed by 72 h of dialysis to remove lithium bromide. Aqueous silk solution was centrifuged twice to remove insoluable debris, adjusted to 6% w/v, and added to a 10 cm petri dish. Sodium chloride particles 415–500 μm in diameter were poured over the silk solution to create pores in the silk, and allowed to sit for 48 h at room temperature followed by 1 h at 60 °C to facilitate β-sheet formation. The 3D scaffolding was washed in deionized water for 72 h to remove sodium chloride, and then cut into cylinders of 3 mm radius with biopsy punches (Integra 33–32, Plainsboro, NJ, USA). Scaffolds were adjusted to 1.5 mm height with a custom 3D printed trimmer guide and razor blades, sterilized in ultrapure water using an autoclave, and stored in at 4 °C for no longer than 2 weeks before use.
Generation of hiPSCs
All hiPSC lines (Table 1) were derived from skin biopsies. Participants were consented into a study approved by the Western Institutional Review Board (WIRB). This protocol included the collection, research use, and biobanking of biological samples. Skin fibroblasts were reprogrammed using the NYSCF Global Stem Cell Array®, a fully automated reprogramming process used to minimize line-to-line variability [60]. In brief, fibroblasts were reprogrammed using modified mRNA (Reprocell 000076, Beltsville, MD, USA) and reprogrammed cells isolated using anti-fibroblast beads (Miltentyi Biotec 130-050-601, Auburn, CA, USA). HiPSCs were expanded using PSC Feeder Free Media (ThermoFisher Scientific A14577SA, Waltham, MA, USA) and grown on Cultrex coated plates (R&D Systems 3434-010-02, Minneapolis, MN, USA). Cells were routinely passaged using our automated platform with Accutase (ThermoFisher A11105-01) and media supplemented with 1 µM thiazovivin (MilliporeSigma SML1045, Burlington, MA, USA). Master hiPSC stocks were frozen in Synth-a-Freeze (ThermoFisher A1254201). All samples were tested for Mycoplasma (Lonza LT07-710, Basel, Switzerland) and sterility (Hardy Diagnostics K82, Santa Maria, CA, USA), had identity testing using the Fluidigm SNPTrace Panel, and karyotypic stability confirmed using SNP arrays (Illumina 20030770, San Diego, CA, USA). Gene expression of both hiPSCs and differentiated embryoid bodies were assessed using custom Nanostring panels as previously described [61]. Working stocks of hiPSC lines were subsequently cultured on Matrigel-coated dishes (Corning, Corning, NY, USA) in mTeSR1 medium (STEMCELL Technologies, 85850, Vancouver, Canada) or StemFlex medium (ThermoFisher A3349401, Waltham, MA, USA). Lines were passaged every 3–4 days using enzymatic detachment with Accutase for 5 min and re-plated in mTeSR1 or StemFlex medium with 10 μM Y-27632 (Reprocell 04-0012) for 24 h.
Dopaminergic neuron differentiation
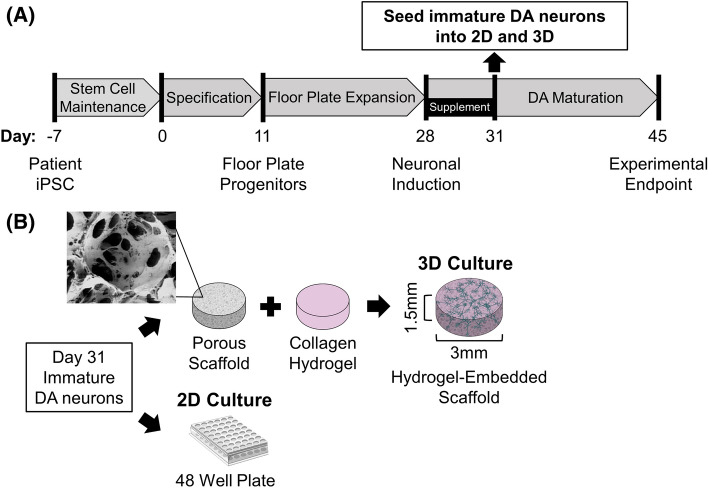
On day 0 (Fig. 1), a single cell suspension of hiPSCs was seeded in 50:50 (v/v) mTeSR1:floor plate specification medium (neurobasal medium (ThermoFisher 21103049) with 1X floor plate specification supplement (ThermoFisher A3146801)) supplemented with 10 μM Y-27632 (STEMCELL Technologies 72302) at a density of 2 × 105 cells/cm2 on 6-well plates coated with Matrigel. The next day, cells were washed with Dulbecco’s phosphate-buffered saline (DPBS, ThermoFisher) and fresh specification medium was added. Medium was carefully changed every 48 h. On day 11, floor plate progenitors (FPPs) were split 1:2 using Accutase (Innovative Cell Technologies AT104, San Diego, CA, USA) onto new Matrigel-coated 6-well plates with FPP expansion medium (ThermoFisher A3165801) + 10 μm Y-27642. FPPs were expanded (replacing FPP expansion medium every 48 h) until cryopreservation on day 22 with Synth-a-Freeze reagent to establish batches of cells for experimental consistency. For each individual experiment, FPPs were thawed and plated at a density of 6.25 × 105 cells/cm2 in Matrigel coated 6-well plates supplemented with 10 μM Y-27642. On day 28, FPPs were differentiated to DA neurons starting with a 72-h treatment of maturation medium (DMEM/F12 (ThermoFisher 10565018) with 1X maturation supplement (ThermoFisher A3147401)) containing 1 μM PD0325901 (MilliporeSigma PZ0162) and 5 μM SU5402 (MilliporeSigma SML0443). PD0325901 and SU5402 were employed to inhibit progenitor cell proliferation and promote neurogenesis [62]. On day 31, cells were harvested with Accutase treatment and seeded into 2D and 3D culture formats (see following methods section “2D and 3D Cell Seeding and Culture Conditions”), replacing maturation media every 48 h thereafter.
Fig. 1.
Differentiation of hiPSC-DA neurons for two-dimensional (2D) cultures and three-dimensional (3D) in vitro brain tissue construction. A Floor plate progenitors (FPPs) were differentiated from healthy and diseased patient hiPSC and then used to generate immature DA neurons by day 31. The cells where then harvested and seeded into 2D and 3D culture formats and matured for 14 days until experimental endpoint on day 45. ‘Supplement’ indicates application of small molecules PD0325901 and SU5402 to inhibit progenitor cell proliferation and promote neurogenesis. B Day 31 DA neurons are first seeded onto laminin-coated silk scaffolding before subsequent encapsulation with 3 mg/mL collagen I hydrogel. For comparison, DA neurons were grown in 2D cultures in 48-well tissue culture plates. The scanning electron microscope image of the silk scaffold microstructure was
adapted from Kim et al. [59]
2D and 3D cell seeding and culture conditions
Prior to cell seeding, multi-well plates and scaffolds were coated for 24 h with undiluted poly-l-ornithine solution (MilliporeSigma P4957), followed by 5 washes of DPBS, and coated with 20 μg/mL laminin (ThermoFisher 23017015) for another 24 h at 4 °C. Cells 31 days after the start of differentiation (see previous methods section “Dopaminergic Neuron Differentiation” and Fig. 1) were used for 2D and 3D seeding. For 2D culture, 2.5 × 105 cells were seeded onto 48-well plates with 500 μL/well maturation media supplemented with 10 μM Y-27642. For 3D culture, scaffolds were placed in an empty 48-well plate (1 scaffold/well) and aspirated immediately before the addition of 7.5 × 105 cells suspended in 5 μL of maturation media with 10 μM Y-27642. For each culture condition, the number of cells seeded was used to achieve a dense, homogenously distributed neuronal network based on nuclear (DAPI), TH, and TUJ1 imaging (Fig. 2 and Supplementary File S8 for examples of 2D and 3D systems, respectively). After 30 min, 500 μL of media was gently added for 4 h of incubation to allow for cells to attach to the scaffold. Afterwards, seeded scaffolds were moved to a new well where 10 μL of a collagen 1 solution (Corning 354,236; concentration adjusted to 3 mg/mL and pH neutralized with 1 N sodium hydroxide) was added to envelope the cell containing scaffold then incubated at 37 °C for 30 min. Maturation medium (500 μL) without Y-27642 was added to each well. Culture medium for 2D cultures were also replaced at this time to align feeding schedules. A full replacement of maturation media occurred every 48 h until experimental endpoint on day 45 (Fig. 1).
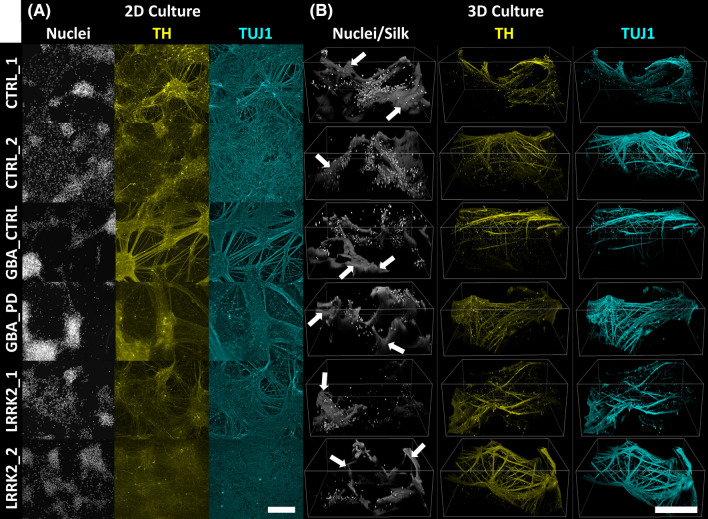
Fig. 2.
Characterization of dopamine neuron differentiation and neural network formation in 2D and 3D cultures. All hiPSC lines generated cells expressing the neuronal marker β3-tubulin (cyan) and dopamine (DA) marker tyrosine hydroxylase (yellow) after 14 days of culture in A 2D and B 3D microenvironments. Cell nuclei were stained with DAPI (bright white punctate signal). B Neuronal networks grew along the 3D silk scaffolding (white arrows, non-punctate gray signal) and through the embedded hydrogel. Similar distribution of TH+ /β3-tubulin+ signal at experimental endpoint indicated comparable DA neuron identity across all cell donors used in 2D and 3D. Scale bar = 250 μm
Immunofluorescence analysis
Forty-five days after differentiation, 2D cultures and tissue constructs were fixed in DPBS containing 4% paraformaldehyde (Electron Microscopy Sciences 15713S Hatfield, PA, USA) and 4% w/v sucrose (MilliporeSigma S0389). Cell membrane permeabilization and blocking of non-specific antibody binding was achieved with incubation of PBS containing 10% normal donkey serum (MilliporeSigma D9663), 1% w/v bovine serum albumin (Jackson Immunoresearch 001-000-162, West Grave, PA, USA), and 0.18% Triton-X (MilliporeSigma T8787). Primary antibodies recognizing tyrosine hydroxylase (TH, Pel-Freez Biologicals P40101, Rodgers, AR, USA) and β3-tubulin (TUJ1, Abcam ab78078, Cambridge, UK) were applied in blocking buffer for 16 h at 4 °C. Following multiple washes with DPBS, fluorescently conjugated secondary antibodies (ThermoFisher A-21206 and A-31571) were applied in blocking buffer for 1 h at room temperature. Nuclei were stained with 4′, 6-diamidino-2-phenylindole (DAPI, ThermoFisher D1306). 3D z-stack and 2D fluorescence images were acquired on a SP2 confocal microscope (Leica, Wetzlar, Germany) and a Ti2 Eclipse inverted fluorescent microscope (Nikon, Tokyo, Japan), respectively. Confocal microscopy utilized a Fluotar VISIR 25 × /0.95 water-immersion objective. 2D imaging (with the Ti2 Eclipse) utilized a 4X objective (Nikon MRH20041, CFI Plan Fluor DL, 4x, na 0.13, wd 16.5 mm) and 20X objective (Nikon MRH48230, CFI Plan Fluor ELWD, 20x, na 0.45, wd 8.2–6.9 mm). TH+ and TUJ1+ signal ratios were quantified from immunolabeled 3D z-stacks carried out in MATLAB using a customized code, as previously described [37]. For the 2D culture condition, this signal was quantified using ImageJ’s default thresholding and measure functions [63].
DNA quantification and α-synuclein ELISA
Scaffolds (3D) or cell pellets (2D) were flash frozen in liquid nitrogen and stored at −80 °C until analysis. Samples were lysed in 150 μL volume of 1X RIPA buffer (MilliporeSigma 20-188) containing protease inhibitors (ThermoFisher A32953) and left on ice for the remaining steps. Cells in pellets and scaffolds were mechanically disrupted with gentle probe sonication (ThermoFisher model no. FB120, 20% power, 1 s on followed by 1 s off for 10 pulses total) followed by 15 min of incubation on ice and subsequent vortexing for 15 s. α-synuclein protein levels in the lysate were measured by ELISA (ThermoFisher KHB0061) according to manufacturer’s instructions. α-synuclein protein levels were normalized to DNA (protein concentration / DNA mass), measured from the same lysate using the PicoGreen double stranded DNA (dsDNA) assay kit (ThermoFisher P7589) according to manufacturer’s instructions.
Untargeted liquid chromatography tandem mass spectrometry (LC–MS) and metabolite annotation
Conditioned media (CM) from three independent 2D or 3D cultures were pooled every 48 h and frozen at −80 °C until the day of metabolite extraction. First, 750 μL of ice-cold 100% HPLC-grade methanol (ThermoFisher A4521) was added to 250 μL of CM and vortexed for 15 s. The protein precipitates were pelleted by 15,000g centrifugation at 4 °C for 15 min. The supernatant was moved to a new tube, evaporated using a vacuum concentrator (Eppendorf Vacufuge 5301, Hamburg, Germany), and subsequently stored at −80 °C. On the day of LC–MS analysis, samples were reconstituted in 85 μL HPLC-grade 50:50 methanol:water (v/v) (MilliporeSigma 270,733), vortexed for 15 s and centrifuged at 15,000 g at 4 °C for 15 min. The resulting supernatant was used for untargeted metabolite profiling experiments using information-dependent acquisition (IDA) settings on a triple-quadrupole time-of-flight mass spectrometer (TripleTOF 5600 + , AB Sciex, Framingham, MA, USA). A LunaNH2 aminopropyl column (Phenomenex 00G-4378-B0, Torrance, CA, USA) was employed to separate water-soluble and polar compounds with hydrophilic interaction chromatography methods adapted from Bajad et al. [64]. Solvent A was 95:5 water:acetonitrile (v/v) (MilliporeSigma 34851) with 20 mM ammonium acetate (MilliporeSigma 73,594), adding ammonium hydroxide (MilliporeSigma 221228) to a pH of 9.45. Solvent B was 100% acetonitrile. The column was maintained at 25 °C using a mobile phase flow rate of 0.3 mL/min starting at 85% B, then followed by the gradients 85 0% B from 0–15 min, 0% B from 15 to 28 min, 0 85% B from 28–30 min, and 85% B from 30 to 60 min. An injection volume of 10 μL was used with a collision energy spread of 20–30 eV. Samples were processed on both positive and negative ionization modes to broaden compound coverage and ion peaks were determined using the XCMS R package, as previously described [65, 66]. Putative metabolites were annotated using R to match fragmentation spectra (MS2) between sample features and online spectral databases of purified standards (METLIN [67], HMDB [68], NIST [69]), as previously described [66]. Only the metabolites consistently annotated across independent experiments underwent further analysis. Validation of metabolite identity was achieved by comparing MS2 spectral data from the experimental samples and pure chemical standards acquired under identical conditions (Supplemental file S1).
Bulk mRNA sequencing
Total RNA was isolated from independent cultures using the RNeasy mini kit (Qiagen 74106, Hilden, Germany) utilizing the on-column DNA-digestion procedure according to manufacturer’s instructions. To improve extraction efficiency, the 2D cultures and 3D constructs were gently sonicated in the kit’s lysis buffer prior to extraction (identical settings as protein and DNA isolation). Sample purity and concentration was measured with a Nanodrop (ThermoFisher) spectrophotometer and 1 μg RNA per sample with a 260/280 nm reading greater than 2 were sent to Novogene for additional quality control tests, library preparation, and sequencing. First, RNA degradation and contamination were assessed on 1% agarose gels and RNA purity was checked using a NanoPhotometer spectrophotometer (Implen, Munich, Germany). RNA integrity and quantitation were assessed using the RNA Nano 6000 assay kit of the Bioanalyzer 2100 system (Agilent Technologies, Santa Clara, CA, USA). Library preparation was achieved using the NEBNext Ultra RNA library prep kit for Illumina systems following manufacturer’s recommendations (New England Biolabs, Ipswich, MA, USA) and index codes were added to attribute sequences to each sample. Briefly, mRNA was purified from total RNA using poly-T oligo-attached magnetic beads. Fragmentation was carried out using divalent cations under elevated temperature in NEBNext First Strand Synthesis Reaction Buffer (5X). First strand complementary DNA (cDNA) was synthesized using random hexamer primer and M-MuLV reverse transcriptase (RNase H-). Second strand cDNA synthesis was subsequently performed using DNA polymerase I and RNase H. Remaining overhangs were converted into blunt ends via exonuclease/polymerase activities. After adenylation of 3’ ends of DNA fragments, NEBNext Adaptors with a hairpin loop structure were ligated to prepare for hybridization. To select cDNA fragments of 150–200 bp in length, the library fragments were purified with AMPure XP system (Beckman Coulter, Brea, CA, USA). Then 3 μL USER Enzyme (New England Biolabs) was used with size-selected, adaptor-ligated cDNA at 37 °C for 15 min followed by 5 min at 95 °C before PCR. Then PCR was performed with Phusion High-Fidelity DNA polymerase, Universal PCR primers and Index (X) Primer. At last, PCR products were purified (AMPure XP system, Beckman Coulter) and library quality was assessed on the Agilent Bioanalyzer 2100 system. The clustering of the index-coded samples was performed on an Illumina Novaseq sequencer according to the manufacturer’s instructions. After cluster generation, the libraries were sequenced on the same machine and paired-end reads were generated at a read depth of 20 million reads.
Bioinformatic analysis of RNAseq data
Raw sequencing data were uploaded to the Tufts University Galaxy Server [70] for quality and adapter trimming with Trim Galore, read alignment with RNA STAR [71] (reference human genome build 38), and read quantification with FeatureCounts using default settings [72]. Principle component analysis (PCA) was performed on transformed data using variance stabilization with the DESeq2 R package [73], correcting for batch effects with the limma R package [74] (Supplemental file S2). Normal confidence ellipses were calculated for each PCA group (Supplemental file S2) assuming a normal multivariate distribution with the ggplot2 R package [75, 76]. DESeq2 was further used to create six differentially expressed gene (DEG) lists by comparing neurons derived from PD patients to healthy controls (LRRK2 versus CTRL, GBA_PD versus CTRL, and GBA_PD versus GBA_CTRL) in 2D and 3D culture conditions (Supplemental file S3). Shrunken fold change values (ashr method) were used to reduce the impact of genes with high dispersion and low read counts in downstream analyses [77]. For comparison of healthy DA neurons grown in 3D and 2D culture conditions, GO enrichment using biological process (BP) terms was performed with clusterProfiler [78] (Supplemental file 4). REVIGO was used to summarize redundant GO terms (terms under the biological process sub-ontology that shared overlap in their respective gene sets) under a single GO term, the results of which are semantically plotted in Fig. 5 [79]. For comparisons between neurons derived from PD patients and healthy controls (Supplemental file S3), Venn diagrams were generated with InteractiVenn [80] and formatted with InkScape (http://www.inkscape.org). The STRING database [81] identified DEGs that had molecular interaction scores with α-synuclein and subsequently visualized using CytoScape software [82].
Fig. 5.
Enriched biological processes (BPs) identified from transcriptomic comparisons of healthy DA neurons cultured in 3D versus 2D systems reveal microenvironment dependent effects. First two principle components of significantly enriched (q < 0.01) Gene Ontology (GO) terms for upregulated and downregulated BPs clustered by semantic similarity; similar sets of genes were condensed under a parent term using a semantic similarity algorithm (REVIGO). Upregulated BPs included glycolytic energy metabolism, synaptic transmission, and action potential propagation. Notable processes downregulated as a result of 3D culture condition include cell stress, apoptosis, DNA replication, and cellular migration. Differentially expressed genes with q < 0.01 and either positive (upregulated) or downregulated (negative) log2(fold change) were used for GO over representation analyses using the clusterProfiler R package. A semantic similarity index of 0.7 was used for upregulated pathways. For downregulated pathways, a semantic similarity index was adjusted to 0.4 since the number of downregulated GO terms considerably outnumbered those upregulated. Only the most significant downregulated pathways (in the 50th percentile) were labeled due to space constraints
Link prediction analysis
We used computational network approaches to leverage literature-curated molecular interaction data in combination with seed networks composed of DEGs derived from PD vs healthy transcriptomic comparisons (Supplemental file S3). We selected the human high-quality molecular interaction network called DREAM3, a signaling network derived from OmniPath which integrates literature-curated human signaling pathways from 27 different sources [83, 84]. DREAM3 was presented as a directed network, but for this work we considered an undirected version where all directed edges were assumed to be bidirectional.
We first selected the top 100 DEGs (Supplemental file S5) from each PD comparison (Supplemental file S3) that also appeared as nodes in the largest connected component of DREAM3 (5009 nodes (i.e., genes) and 18,269 edges (i.e., molecular interactions)), including any molecular interactions that connected between the DEGs. We limited the number of starting nodes to provide an appropriate network size (relative to DREAM3) for downstream network augmentation algorithms to perform optimally. In order to nominate additional genes that were most common in the neighborhoods of the DEGs, we applied Kohler’s Random Walk with Restart to each network (with default restart parameter of 0.5) to return the top 200 candidate disease genes (CDGs) [85]. We added these genes to each network, but the known interconnections between genes were very sparse, making it desirable to also computationally predict how the DEGs and CDGs might interconnect. For this purpose, we applied global and local integrated diffusion embedding (GLIDE) [86] to augment each network with new predicted molecular interactions (PMIs), performed with a two-step process. First, we added 9135 top-ranked interactions the GLIDE algorithm returned for the entire DREAM3 network, referred to as global GLIDE interactions. In the second step, we added additional edges on a gene-by-gene basis, referred to as local GLIDE rankings. For each DEG or CDG, we ranked all GLIDE edges that were incident to that gene within the entire DREAM3 network and added the top 20 highest scoring predicted edges to each node. A composite link prediction network was then constructed for each disease genotype (Fig. 7) by retaining only those DEGs and CDGs present across all individual 2D and 3D disease state networks (Supplemental file S6), where molecular interaction edges appeared only if both endpoint genes were present (i.e., a DEG or CDG). The final product is a consensus link prediction network for the LRRK2 and GBA disease genotype comparisons (Fig. 7), derived from the common overlap across their respective networks generated from individual 2D and 3D differential expression datasets (Supplemental file S6).
Fig. 7.
Novel PD-associated molecular interactions for LRRK2 and GBA disease genotypes were predicted by application of global and local integrated diffusion embedding (GLIDE) analysis. Six individual link prediction networks (Supplemental file S6) were separately created for all disease comparisons made (LRRK2 vs CTRL, GBA_PD vs CTRL, and GBA_PD vs GBA_CTRL in 2D and 3D systems) by utilizing their top 100 differentially expressed genes (DEGs, Supplemental file S5). Only the consensus subnetwork of genes and links that were independent of culture condition (shared across 2D and 3D data) were retained, using the individual network overlap generated from A LRRK2 and B GBA genotype comparisons. Nodes (genes) were shaded to distinguish their identity in pre-combined networks, either a candidate disease gene (CDG, white) from application of Kohler’s Random Walk with Restart or a DEG (black) found in the respective expression datasets. Genes are shaded gray if the genes existed as both a CDG and DEG in the pre-combined networks. Molecular interactions between the nodes were either previously known from the DREAM3 molecular interaction network (solid lines) or computationally predicted (dashed and wavy lines) to exist in the local neighborhoods containing DEGs and CDGs
Statistical analyses
Results from the two healthy controls or two LRRK2 PD patient lines were grouped to generalize observations across diagnosis or genotype. This study reports on data collected from n = 2 independent experiments with ≥ 3 replicate cultures, depending on the analysis (exact sample sizes are shown in their respective figures or figure captions). Fluorescent neuronal network quantifications (TH+ and TUJ1+) were compared across donor lines and culture conditions using a two-way ANOVA with a significance cutoff of p = 0.05. Comparisons of α-synuclein protein levels within each culture condition were tested by Brown-Forsythe and Welch ANOVA tests followed by Dunnett’s T3 multiple comparison tests, where a false discovery rate (q value) less than 0.05 was significant (GraphPad Prism v8.2.0, San Diego, CA). Comparing α-synuclein fold changes (diseased relative to healthy lines) across culture conditions was achieved with multiple unpaired t-tests with the two-stage set up procedure of Benjamini, Krieger, and Yekutieli, where q < 0.05 indicated significance (Prism v8.2.0). Results from α-synuclein protein analysis from all six individual lines are provided in Supplemental file S7. Annotated metabolite data were log transformed and pareto scaled before statistical testing by ANOVA and Fisher’s LSD post-hoc tests using the MetaboAnalyst R package [87, 88]. All transcriptomic data were obtained from 40 independent mRNA samples over two experiments (n ≥ 3 per line per culture condition) and analyzed with DESeq2 [73].
Results
Establishment of a 3D bioengineered model of Parkinson’s disease using hiPSC-derived DA neurons
Here we utilized our bioengineered 3D culture platform to create a new model of Parkinson’s disease and use it to address how the culture microenvironment may shape human DA neuron biology. HiPSC populations derived from healthy subjects and PD patients with LRRK2 G2019S and GBA N370S mutations (Table 1) were differentiated into DA neurons via a floor plate progenitor intermediate cell population (Fig. 1A). Thirty-one days after the start of differentiation from hiPSCs, immature DA neurons were seeded in 2D well plates and in the 3D scaffold-hydrogel biocomposite system (Fig. 1B). The cells adapted to their respective microenvironments for 14 days before analyses. We then used immunofluorescent staining to detect tyrosine hydroxylase (TH) and β3-tubulin (TUJ1) for the evaluation of dopaminergic neuron differentiation and neuronal network formation, respectively. A two-way ANOVA on ratios of TH+ and TUJ1+ fluorescent network signal indicated no significant changes in the expression of these markers as a result of donor line or culture condition (Supplemental File S8). This suggested similar DA neuron content across all six donor lines in 2D (Fig. 2A) and 3D (Fig. 2B) culture conditions. Wide-field imaging of the 3D system at millimeter scale (Supplemental file S9) showed that cell nuclei (DAPI) and neuronal networks (β3-tubulin) were homogeneously spread across the tissue construct. Confocal image acquisition showed that TH and β3-tubulin positive neuronal processes grew along the scaffolding and penetrated the hydrogel structure (Fig. 2B). We additionally utilized mRNA sequencing to support similar differentiation efficiencies across donor lines. The expression levels of markers for astrocytes, microglia, oligodendrocytes, and hiPSCs were several orders of magnitude lower than pan-neuronal markers, indicating that all donor cultures were predominantly neuronal across 2D and 3D culture conditions (Supplemental file S10). Despite reduced mRNA expression for DA neuron markers in the GBA_PD line, the TH and TUJ1 protein ratios suggested similar DA neuron content, allowing for subsequent analyses to be made. These observations indicated that the 3D scaffold-based system provides a robust platform for generating dopamine neuronal networks derived from multiple hiPSC lines donated from patients with diseased and healthy backgrounds.
3D dopamine neuron cultures with familial PD mutations accumulated more α-synuclein compared to 2D cultures and healthy subject control lines
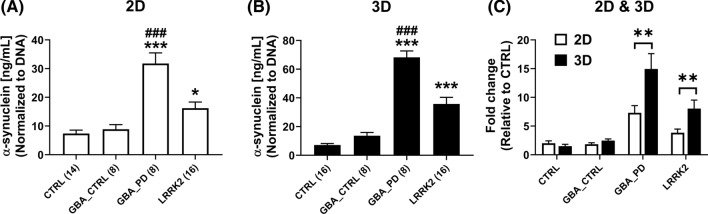
Increased levels and aggregations of α-synuclein protein are seen in many, but not all patients with Parkinson’s disease [89, 90]. Accordingly, we used α-synuclein protein measurements as an initial assessment of disease state in the culture systems. Quantities of protein relative to cell number were achieved by normalizing protein measurements to total dsDNA content in the culture. DA neurons from the affected GBA patient (GBA_PD) and LRRK2 patients showed significantly higher amounts of α-synuclein when compared to healthy controls in 2D and 3D culture (Fig. 3A, B). Additionally, cells generated from the affected GBA patient contained elevated α-synuclein compared to the asymptomatic GBA twin brother (GBA_CTRL) in 2D and 3D cultures (Fig. 3A, B). Consistent with the healthy diagnosis of the unaffected brother carrying the familial PD GBA N370S mutation, the amounts of α-synuclein protein are similar to cultures generated from healthy donors in 2D and 3D culture systems (Fig. 3A, B). Interestingly, cells with LRRK2 and GBA mutations express elevated levels of α-synuclein in 3D cultures compared to 2D cultures (Fig. 3C), suggesting the 3D environment facilitates α-synuclein accumulation. We obtained similar outcomes when individual donor lines are treated as independent groups and statistically tested (Supplemental file S7).
Fig. 3.
α-synuclein protein is elevated in PD cultures compared to healthy controls and accumulated more in 3D DA neuronal systems. α-synuclein protein levels were measured by ELISA and were found significantly increased in A 2D and B 3D LRRK2 and GBA_PD groups, relative to healthy controls. C When normalized to healthy cultures (CTRL), α-synuclein protein levels were found to be significantly increased in 3D diseased groups relative to 2D. Data displayed as the mean ± SEM of 8–16 replicate cultures across 2 independent experiments. For (A, B), group-wide significance were determined with One-way ANOVA followed by Tukey’s multiple comparison tests. Compared to CTRL: *p.adj < 0.05, ***p.adj < 0.0001. Compared to GBA_CTRL: ###p.adj < 0.0001. For (C), significance of fold change comparisons was assessed using multiple t-tests with the two-stage step-up procedure of Benjamini, Krieger, and Yekutieli, where **q < 0.01. Sample sizes for each group are numbered next to each x-axis label and represent replicate cultures
Untargeted LC–MS profiling identified metabolic profiles altered in PD cultures dependent on culture condition and disease genotype
The metabolite profiles of biological systems are the end product of all upstream interactions between genomic, transcriptomic, and proteomic activity and are thus considered to be a direct representation of physiological state [91, 92]. Accordingly, untargeted characterization of metabolite profiles have provided valuable insights into pathological mechanisms and identified biomarkers of disease [92]. The majority of biological metabolites are hydrophilic and polar in nature. Characterization of these compounds from the conditioned medium identified alterations in three purine pathway metabolites in hiPSC-DA neurons from Parkinson’s disease patients (Fig. 4, see “Methods”). Levels of adenosine in 2D and 3D DA cultures from the LRRK2 G2019S patients and unaffected GBA brother were decreased relative to healthy controls (Fig. 4A). In contrast, adenosine levels were increased in the GBA_PD line compared to the unaffected twin brother and healthy controls (Fig. 4A). Levels of the adenine metabolite are uniquely changed in 2D cultures in LRRK2 and GBA_CTRL DA neurons, with a trend similar to adenosine. (Fig. 4B). Conversely, reduction in cyclic AMP levels was unique to 3D DA systems generated from the affected GBA N370S patient. These results show that 2D and 3D culture conditions and PD genotype have impact on the metabolite profiles of dopamine neurons.
Fig. 4.
Parkinson’s disease genotype and culture condition dependent changes in metabolic profiles. Metabolites were profiled from hiPSC-DA neuron conditioned media using untargeted liquid chromatography tandem mass spectrometry and information-dependent acquisition. A Adenosine levels are consistently reduced in 2D and 3D cultures of LRRK2 and unaffected GBA DA neurons. It is increased in the GBA N370S line derived from the PD patient. B Extracellular adenine levels are increased in the GBA PD line and decreased in LRRK2 G2019S cells relative to control, but only in 2D cultures. C Cyclic 3´,5´-cyclic adenosine monophosphate (cyclic AMP) levels are reduced in the affected GBA group, but only in 3D culture. Peak area corresponds to relative concentration. Data is displayed as Tukey box-and-whisker plots with 75th and 25th percentiles for the upper and lower bounds of the boxes, respectively. Data points that were beyond the range of the whiskers were individually plotted as black circles. Statistical analysis was performed on untargeted datasets by ANOVA with Fisher’s LSD post-hoc tests, where a false discovery rate (q-value) of less than 0.05 was considered significant (denoted by an asterisk). Sample sizes are numbered next to each x-axis label and represent conditioned media samples from replicate cultures, collected from two independent experiments
Transcriptomic comparison of healthy DA neurons revealed the 3D microenvironment’s influence on biological processes
The differences in α-synuclein and metabolite levels observed between 2D and 3D culture systems prompted further investigation into other potential effects of the microenvironment on the biological processes (BPs) driving neuronal function. We first performed unsupervised principle component analysis (PCA) on transcriptomic data. This showed that the 3D culture condition shifted the global gene expression profiles of DA neurons when compared to 2D culture (Supplemental file S2). The direction of the shift was consistent across all healthy donors and PD patient lines, suggesting a fundamental change in DA neuron biology caused by the different culture microenvironments.
To gain a better understanding of the processes influenced by the culture environments, we first compared gene expression profiles of the healthy groups cultured in 2D and 3D formats (CTRL in 3D versus CTRL in 2D). The resulting differential expression data were plotted for three gene sets (hDA0, hDA1, and hDA2) that represent dopaminergic neurons during human development in vivo [93]. In all three gene sets, the 3D culture condition upregulated a larger number of DA genes relative to the 2D system, indicative of a more in vivo-like phenotype (Supplemental file S11). Additional GO pathway over-representation analysis of transcriptomic data indicated several BPs were affected by the culture condition (Supplemental file S4) [78]. To reveal broader categories of affected BPs, we condensed similar GO terms that passed a statistical significance threshold of q < 0.01 with the REVIGO semantic similarity algorithm [79]. This analysis revealed that 3D culture condition downregulated processes related to cell stress (e.g., response to endoplasmic reticulum stress, GO:0034976) and apoptosis (intrinsic apoptotic signaling pathway, GO:0097193), while energy metabolism (glycolysis, GO:0006096), synaptic transmission (synaptic vesicle cycle, GO:0099504; modulation of synaptic transmission, GO:0050804), and electrophysiological function (regulation of membrane potential, GO:0042391; membrane depolarization during action potential, GO: 0086010) were all upregulated (Fig. 5). Downregulated BPs associated with cell division (e.g., DNA replication, GO:0006260; negative regulation of mitotic cell cycle, GO:0045930) and cell movement (e.g., positive regulation of cell migration, GO:0030335) may suggest proper differentiation of progenitor populations in to mature, post-mitotic neurons. Altogether, these results may indicate improved health, differentiation, development, and physiological function of DA neurons when grown in the 3D model system.
Comparisons between DA neurons from PD patients and healthy controls revealed the influence of culture condition on transcriptomic profiles
We next sought to identify the gene expression profiles in Parkinson’s disease that are dependent on the microenvironment. This analysis revealed shared and unique differential expression profiles between 2D and 3D cultures for each PD genotype comparison (Fig. 6A, B, Supplemental file S12).
Fig. 6.
Gene expression profiles from disease comparisons suggest the effects of culture condition on molecular interactions with α-synuclein (SNCA). Differentially expressed genes (DEGs) were found (abs(Log2(Fold Change)) > 0.585 and q < 0.01) in A LRRK2 and B GBA diseased genotypes comparisons. Supplemental file S12 provides the list of DEGs (bolded) unique or shared between 2D and 3D cultures for each of the LRRK2 and GBA genotypes. DEGs that had an interaction score with SNCA in the STRING database were used to build relationship networks. Accordingly, unique profiles for each culture condition and C LRRK2 and D GBA genotype comparisons were identified. The blue box represents DEGs consistently identified across all genotype comparisons and culture conditions. Darker lines (interaction) and nodes (gene) indicate a higher STRING interaction score, corresponding to higher confidence in the proposed molecular interactions with α-synuclein. Molecular interaction relationships below a STRING interaction score less than 0.4 are not shown
The observed elevation in α-synuclein (SNCA) protein in PD patient-derived cultures (Fig. 3A, B) prompted us to understand if culture conditions influenced the expression of genes with a known molecular interaction to α-synuclein. Exploring the STRING database [81], we nominated sets of DEGs that are distinct for each genotype and culture condition that may link to α-synuclein accumulation (Fig. 6C, D, Supplemental file S13). Additionally, several genes were shared across all genotype comparisons and culture conditions (blue box in Fig. 6C, D), including LRRK2 and genes related to dopamine biology (MAOB, TH, DRD1, PITX3). Overall, these analyses suggest the contribution of culture conditions on the expression of gene networks interacting with α-synuclein in LRRK2 and GBA diseased genotypes.
Link prediction analysis nominated novel molecular interactions in PD
Thus far our analyses of gene expression profiles relied on prior knowledge from GO processes (Fig. 5) and STRING molecular interaction (Fig. 6) databases. To nominate novel molecular interactions in PD biology, we employed computational approaches to expand upon a published high-quality molecular signaling network (called DREAM3; see “Methods”) using our differential expression data sets. In a first step, individual networks were constructed for each of the six PD genotype comparisons, using the top 100 DEGs (Supplemental file S5) as an input that were also present in DREAM3, along with their respective molecular interactions. These networks were expanded using the ‘Kohler's Random Walk with Restart’ method, adding additional DREAM3 genes that were most common in the neighborhoods of the DEGs (hereafter called candidate disease genes, or CDG) [85]. This was followed by the addition of predicted molecular interactions (PMIs) between the DEGs and CDGs nominated by the GLIDE algorithm (see “Methods”, Supplemental file S6) [86]. To construct consensus disease gene networks for each genotype, we retained only those genes and molecular interactions in LRRK2 or GBA link prediction networks that were shared across the respective 2D and 3D cultures (Fig. 7). Finding the common overlap between 2D and 3D culture conditions yielded link prediction networks specific to the LRRK2 and GBA diseased states containing novel gene clusters with several PMIs of interest (Fig. 7). The DEGs in the consensus disease gene networks are plotted in Supplemental file S14 to indicate the fold change direction relative to control groups. In contrast to the overlap, the disease gene networks unique to a particular culture condition and disease genotype are displayed in Supplemental file S15.
Discussion
3D culture systems are powerful tools to identify molecular, cellular, and neural network-related mechanisms underlying normal brain function and disease onset and progression [21–25, 27–33]. Here, we used our well-established tissue engineering approaches [38, 39, 45, 94] to build a 3D culture system of PD based on silk biomaterial technology, comparing the behavior of DA neurons from two healthy subjects, two patients carrying the LRRK2 G2019S mutation and two identical twin siblings with a genetic lesion in GBA (N370S), one of whom was diagnosed with the disease. Comparing identical twin donors discordant for PD diagnosis may help reduce potential noise from donor-to-donor variability. Overall, the similarities in elevated α-synuclein protein levels, partial overlap of differentially expressed genes with molecular interaction with SNCA, and alterations in purine metabolism in DA neurons generated from the LRRK2 and affected GBA patients highlights the utility of the bioengineered 3D culture system as an experimental model suitable for the study of PD.
The 3D culture system supported the development of dense DA neural networks (TH+ and TUJ1+) spanning across the millimeter-scale tissue, consistent for multiple healthy and PD donor lines. Inconsistent with the TH+ and TUJ1+ results, we observed lower gene expression of DA neuron markers for the GBA_PD line compared to the other groups. This may be the result of the GBA mutation or high levels of α-synuclein, as both of these factors have been shown to interfere with DA neuron gene expression [95, 96].
A larger number of DA neuron genes were upregulated by the 3D condition, which indicated that this culture environment may promote an in vivo-like phenotype. Additionally, gene regulatory processes related to energy metabolism, synaptic formation, and electrophysiological function were upregulated relative to cells grown in conventional culture dishes. Furthermore, the 3D culture condition downregulated processes associated with cell death and stress. As expected, DA neurons derived from diseased patients accumulated larger amounts of α-synuclein protein than lines from healthy subjects or the unaffected GBA N370S carrier. Comparing our results to another study that utilized the same GBA donor lines [97], we report a statistically insignificant elevation of α-synuclein protein from the GBA_CTRL group relative to healthy controls (increased 19% and 89% in 2D and 3D culture conditions, respectively). Additionally, Woodard et al. [97] observed non-significant elevations of α-synuclein mRNA in the GBA_PD group, whereas our analysis indicated a statistical increase in only half of the GBA comparisons made (2D GBA_PD vs CTRL and 3D GBA_PD vs GBA_CTRL, but not 2D GBA_PD vs GBA_CTRL and 3D GBA_PD vs CTRL). It is worth noting that multiple studies have found increases in α-synuclein mRNA from post mortem PD brains, which is not often observed from in vitro studies [98, 99]. We suspect differences in differentiation protocols and cell isolation methods may significantly contribute to these discrepancies. Strikingly, the α-synuclein protein levels were significantly higher in our 3D diseased cultures relative to 2D (Fig. 3C). This finding may be an important advantage of modeling PD in this 3D system since elevated α-synuclein have been shown to negatively impact other disease related processes such as dopamine neurotransmission, autophagy, synaptic vesicle transport, axonal degeneration, and mitochondrial function [89, 90, 100]. Consistent with this, we identified differentially expressed genes in 3D cultures associated with some of these pathways that differ from 2D (see below for further discussion). Altogether, these results demonstrate that our bioengineered 3D platform may facilitate the emergence of Parkinson’s disease phenotypes in a physiologically relevant microenvironment for hiPSC-DA neural networks.
We used untargeted metabolomic profiling to gain new insights into molecular pathways associated with Parkinson’s disease and the influence of culture environment. Levels of adenosine and its precursors adenine and cyclic AMP [101, 102], three metabolites in the purine pathway, were significantly changed in diseased groups (KEGG pathway hsa00230) [102, 103]. While the trend in the adenosine profile is similar across culture conditions, changes in adenine and cyclic AMP are unique to the 2D and 3D microenvironments, respectively, supporting the notion that culture condition may affect the underlying metabolic pathway. Adenosine metabolism and dopamine signaling pathways are reported to be coupled, which has supported approaches that modulate adenosine signaling processes to develop anti-Parkinsonian compounds [104]. Although purine metabolism dysregulation has been previously reported in PD patient brain and plasma samples [105–107], these results are, to our knowledge, the first to provide evidence of purine metabolism dysregulation in patient derived DA neurons.
We additionally explored RNA sequencing datasets to identify differential expression profiles associated with α-synuclein protein levels using the STRING molecular interaction database [81]. Salient examples of high-scoring DEGs related to α-synuclein aggregation, phosphorylation, and degradation include SNCAIP and USP9X in 2D LRRK2 cultures [108–110], LYN in 3D LRRK2 cultures [111], KLK6 in 3D GBA cultures [112], and GRK5 and PLK1 in 2D GBA cultures [113, 114]. Unique to 3D systems, a second category related to diacylglycerol and phosphatidic acid metabolism emerges through the differential expression of PLCB2 and DGKG [115–119] (in GBA) and PLD1 [120] (in LRRK2), suggesting that LRRK2 and GBA disease models in 3D culture systems may share dysfunctional cell signaling through lipid messengers involved with α-synuclein regulation. Interestingly, PLD1 is a regulator of autophagic processes and α-synuclein accumulation through other means [121]. Additionally, the five DEGs associated with dopamine biology (LRRK2, MAOB, TH, PITX3, DRD1) that are shared across genotype and culture condition have been previously implicated in DA neuron dysfunction in PD [97, 122–124]. In contrast to other in vitro studies that report MAOB mRNA upregulation [97, 125], we present evidence of downregulation in 2D and 3D PD cultures (Supplemental file S13), mirroring observations made in PD patient brains [126, 127]. One possible explanation to these discrepancies between the in vitro models is the use of different dopaminergic differentiation protocols or cell selection methods. However, since α-synuclein binding to MAOB can enhance enzymatic activity [128], we speculate that downregulated gene expression is a compensatory mechanism to re-establish physiological MAOB activity in the context of elevated α-synuclein protein levels in our PD cultures.
Last, we utilized the differential gene expression profiles to nominate candidate disease genes (CDGs) and novel molecular interactions specific to PD biology. First, PD genotype comparisons analyses confirmed the predicted molecular interactions (PMIs) between genes previously implicated in pathology, such as MAPT (tau) [129] in LRRK2 cultures. Some of the observed PMIs that are connected to MAPT relate to other features of Parkinson’s, such as hallucinations (CCK [130] and its receptor CCKBR [131]), biomarkers of LRRK2 cases (ENPEP [132]), and mitochondrial processes (PDK4 [133]). Other genes of interest associated with the LRRK2 genotype are indirectly implicated in PD-related processes, such as protein degradation and autophagy (PSMD4 [134–136]). In the GBA genotype, synaptic remodeling (HES1 [137, 138]), apoptosis (TCF12 [139]), DA neuron cell stress (ID3 [140]), heparin/heparin sulfate metabolism (EXTL1 [141, 142]), and hippo signaling-mediated apoptosis and autophagy (YAP1, TEAD1, TEAD2, TEAD3, and LATS2 [143, 144]) were found in the link prediction network. In addition, a number of transcription factor genes (Fig. 7) with function in DA neurodevelopment were identified and their dysregulation is thought to predispose the neurons to environmental stressors [145, 146]. The specific function of these predicted genes in LRRK2- and GBA-related Parkinson’s pathobiology may be further explored in future experiments.
In conclusion, we bioengineered a hiPSC-DA neuron-based human 3D brain tissue model that recapitulates α-synuclein accumulation, gene expression signatures, and metabolic profiles reminiscent of PD pathology. These results highlight the power of the 3D brain-like tissue model to identify mechanisms and biomarkers in Parkinson’s disease. Future experimentation with the 3D hiPSC-DA neuronal model could include other brain-relevant cell types (e.g., microglia, astrocytes) differentiated from hiPSCs to parse cell–cell interactions in development and disease. Additionally, the development of a physiologically mature and functional tissue model for the study of age-related neurogenerative phenotypes may be achieved by culturing for years, which has previously been demonstrated utilizing this culture platform with other brain cell types [38, 45, 94]. In conclusion, our 3D platform presents as a novel tool to study the complex multifactorial interaction between genetics, environmental stress, cell type crosstalk, and the aging process in neurodegenerative disease states.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplemental File S1 Representative fragmentation spectra generated from untargeted liquid chromatography tandem mass spectrometry analysis. Mass/charge (m/z) peaks were compared between experimental samples (top panels, representative samples shown) and purified standards (bottom panels), which confirmed the identities of (A) Adenosine, (B) adenine, and (C) adenosine 3',5'-cyclic monophosphate metabolites. All results were acquired in positive ionization mode
Supplemental File S2 Principle component analysis of transcriptomic data of control, GBA and LRRK2 DA neurons. (A) 3D culture is consistently shifted along the PC1 and PC2 axes compared to 2D, indicating a culture condition dependent shift in global gene expression. Panel (B) highlights the results for the individual groups
Supplemental File S3 Differential expression results using the DESeq2 R package, sorted by PD comparison in 2D and 3D culture format. A differential expression cutoff of abs(Log2(Fold change) > 0.585 in combination with q < 0.01 was used to determine significance
Supplemental File S4 Over-representation analysis of biological processes when comparing heathy hiPSC-DA neurons cultured in 3D versus 2D conditions (3D CTRL versus 2D CTRL). Upregulated and downregulated GO terms were determined using differentially expressed genes (q < 0.01) with positive and negative fold change, respectively
Supplemental File S5 Lists of the top 100 differentially expressed genes (HGNC symbols) from each Parkinson’s disease comparison that also existed in the DREAM3 molecular interaction network. Each list was utilized as the initial seed network for candidate disease gene prioritization and subsequent link prediction analysis
Supplemental File S6 List of genes and molecular interactions for each link prediction network generated from PD genotype comparisons. The top 100 differentially expressed genes (DEGs), that also existed in the molecular interaction network DREAM3 (Supplemental file S5), were utilized to generate individual link prediction networks from all disease comparisons made in 2D and 3D culture formats: LRRK2 vs CTRL, GBA_PD vs CTRL, and GBA_PD vs GBA_CTRL. Application of Kohler’s random walk with restart nominated candidate disease genes (CDGs) within the neighborhood of the DEGs. Likely molecular interactions between DEGs and CDGs were computationally predicted by using global and local integrated diffusion embedding (GLIDE)
Supplemental File S7 α-synuclein protein measurements in 2D and 3D cultures (with reference to Fig. 3). α-synuclein protein levels (ELISA) in (A) 2D and (B) 3D culture conditions were determined from individual cell donor lines. (C) Fold change of α-synuclein protein levels was normalized to results from healthy individual cultures indicating increased expression in 3D cultures relative to the 2D culture. Statistical significance for (D) 2D and (E) 3D cultures were determined with one-way ANOVA followed by Tukey’s multiple comparison testing. (F) Fold change of α-synuclein protein levels (C) were compared across 2D and 3D culture systems by multiple t-tests with the two-stage step-up procedure of Benjamini, Krieger, and Yekutieli. Six or eight individual cultures were collected from two independent experiments, depending on the sample. A false discovery rate (q value or adjusted p value) of less than 0.05 was considered significant
Supplemental File S8 Neuronal network quantifications of TH+ and TUJ1+ signal ratios from immunofluorescent images suggested similar dopamine neuron content across individual donor lines and culture conditions. Statistical testing was performed using a two-way ANOVA with a significance cutoff of p = 0.05. Data displayed as the mean ± SEM from n = 4 replicate cultures per group
Supplemental File S9 Representative immunofluorescent images of 3D construct seeded with patient derived dopaminergic neurons. Wide-field images show homogeneously attached cells stained with DAPI (nuclei, blue) onto the scaffolding and neuronal processes (β3-tubulin, green) extending along the scaffolding and into the surrounding collagen hydrogel. To account for the autofluorescence of the silk scaffolding [147], digitally expanded images provide a clarified view of individual nuclei and neurites. Scale bars represent 500 μm
Supplemental File S10 Gene expression levels for neuronal, glial, and pluripotency markers. The low expression levels of markers for astrocytes, microglia, oligodendrocytes, and hiPSCs suggested that each groups’ culture composition were primarily neuronal
Supplemental File S11 3D CTRL versus 2D CTRL differential expression datasets for human dopamine neuron gene sets (hDA0, hDA1, and hDA2, characterized from developing human brains [93]). The 3D system upregulated a higher number of dopamine neuron genes, suggesting a more in vivo-like phenotype compared to 2D culture. Genes were highlighted blue if upregulated in 3D systems (log2(Fold Change) > 0.585 and p.adj < 0.05) or red if upregulated in 2D systems (log2(Fold Change) < 0.585 and p.adj < 0.05). Genes unaffected by culture condition were colored gray
Supplemental File S12 Differentially expressed genes that are unique or shared between 2D and 3D cultures for each of the LRRK2 and GBA genotype comparisons (with reference to Fig. 6A, 6B). Differential expression analysis was performed with the DESeq2 R package and statistical significance was determined with a cutoff of abs(Log2(Fold change) > 0.585 in combination with q < 0.01
Supplemental File S13 Log2(Fold Change) of differentially expressed genes with a STRING association score to α-synuclein (with reference to Fig. 6C, Fig. 6D). Differential expression analysis was performed with the DESeq2 R package and statistical significance was determined with a cutoff of abs(Log2(Fold change) > 0.585 in combination with q < 0.01
Supplemental File S14 Log2(Fold Change) of differentially expressed genes in link prediction networks (with reference to Fig. 7). Differential expression analysis was performed with the DESeq2 R package and statistical significance was determined with a cutoff of abs(Log2(Fold change) > 0.585 in combination with q < 0.01
Supplemental File S15 Link prediction networks unique to 2D or 3D culture systems generated using differential expression data. The top 100 differentially expressed genes (DEGs), that also existed in the molecular interaction network DREAM3 (Supplemental file S5), were utilized to generate individual link prediction networks from all disease comparisons made in 2D and 3D culture formats: LRRK2 vs CTRL, GBA_PD vs CTRL, and GBA_PD vs GBA_CTRL. Application of Kohler’s random walk with restart nominated candidate disease genes (CDGs) within the neighborhood of the DEGs. Molecular interactions between DEGs and CDGs were computationally predicted using global and local integrated diffusion embedding (GLIDE). Networks unique to each culture condition were extracted for GBA or LRRK2 genotype comparisons. Genes with a green frame were commonly observed across GBA and LRRK2 genotype comparisons for the given culture condition
Acknowledgements
We thank the National Institutes of Health and the National Science Foundation for financial support of this project, and the New York Stem Cell Foundation’s Global Stem Cell Array® team for generating and providing the hiPSC lines. The authors acknowledge the Tufts University High Performance Compute Cluster (https://it.tufts.edu/high-performance-computing) and the confocal microscopy lab core (National Institutes of Health S10 OD021624) which were utilized for the research reported in this paper. Additional support from the National Science Foundation came through the Tufts T-Tripods Institute from the Harnessing the Data Revolution “Big Idea” effort. We also thank Dr. Volha Liaudanskaya and Dr. Mattia Bonzanni for experimental and intellectual feedback, as well as Sydney Peters and Emily Kim for technical support.
Abbreviations
- 2D
Two dimensional
- 3D
Three dimensional
- ANOVA
Analysis of variance
- BP
Biological processes
- CCK
Cholecystokinin
- CCKBR
Cholecystokinin B receptor
- CDG
Candidate disease gene
- CM
Conditioned media
- Cyclic AMP
Cyclic 3´,5´-cyclic adenosine monophosphate
- DA
Dopaminergic
- DAPI
4′, 6-Diamidino-2-phenylindole
- DEG
Differentially expressed gene
- DGKG
Diacylglycerol Kinase Gamma
- DPBS
Dulbecco’s phosphate buffered saline
- DRD1
Dopamine receptor D1
- dsDNA
Double-stranded Deoxyribonucleic acid
- ENPEP
Glutamyl Aminopeptidase
- EXTL1
Exostosin Like Glycosyltransferase 1
- FPPs
Floor plate progenitors
- GBA
Glucocerebrosidase
- GLIDE
Global and local integrated diffusion embedding
- GO
Gene ontology
- GRK5
G Protein-Coupled Receptor Kinase 5
- HES1
Hes Family BHLH Transcription Factor 1
- hiPSCs
Human induced pluripotent stem cells
- IDA
Information-dependent acquisition
- IGFBP5
Insulin Like Growth Factor Binding Protein 5
- KLK6
Kallikrein Related Peptidase 6
- LATS2
Large Tumor Suppressor Kinase 2
- LC–MS
Liquid chromatography tandem mass spectrometry
- LRRK2
Leucine-rich repeat kinase 2
- LYN
LYN Proto-Oncogene, Src Family Tyrosine Kinase
- MAOB
Monoamine oxidase B
- MAPT
Microtubule associated protein tau
- miRNA
Micro ribonucleic acid
- mRNA
Messenger ribonucleic acid
- MS2
Fragmentation spectra
- PCA
Principle component analysis
- PD
Parkinson’s Disease
- PDK4
Pyruvate Dehydrogenase Kinase 4
- PITX3
Paired Like Homeodomain 3
- PLCB2
Phospholipase C Beta 2
- PLD1
Phospholipase D1
- PLK1
Polo like kinase 1
- PSMD4
Proteasome 26S Subunit, Non-ATPase 4
- PTER
Phosphotriesterase Related
- SNCA
α-Synuclein (gene)
- SNCAIP
α-Synuclein interacting protein
- SPON1
Spondin 1
- TCF12
Transcription Factor 12
- TEAD1
TEA domain transcription factor 1
- TEAD2
TEA domain transcription factor 2
- TEAD3
TEA domain transcription factor 3
- TH
Tyrosine hydroxylase
- TUJ1
β3-Tubulin
- USP9X
Ubiquitin specific peptidase 9 X-linked
- YAS1
Yes1 associated transcriptional regulator
Author contributions
Conceptualization: NJF, TJFN, DLK; Methodology: NJF, YMG, YG, LJC; Formal analysis: NJF, RB, KD, LJC; Investigation: NJF, TJFN, DLK; Resources: ML, KL, YMG, GC, SN, DLK; Data curation: NJF, KD, RB, ML, KL; Original manuscript writing: NJF, DLK, TJFN; Supervision: TJFN, DLK; Funding acquisition: DLK, LJC. All authors read and approved the final manuscript.
Funding
NIH (P41EB027062) to DLK, NSF (1934553) to LJC, NSF (1337760) to KL.
Availability of data and material
The datasets used and analyzed for this study can be obtained from corresponding authors upon reasonable request. Raw sequencing data (consented hiPSC donors only) and normalized read counts can be freely accessed through the Gene Expression Omnibus (GSE172409).
Declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
All work was approved by the Tufts Institutional Biosafety Committee.
Consent to participate
The skin biopsies used for the generation of hiPSCs were donated from consenting participants under a protocol covering use in research, approved by the Western Institutional Review Board (WIRB).
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Thomas J. F. Nieland, Email: thomas.nieland@tufts.edu
David L. Kaplan, Email: david.kaplan@tufts.edu
References
- 1.Dorsey ER, Constantinescu R, Thompson JP, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68:384–386. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- 2.Marras C, Beck JC, Bower JH, et al. Prevalence of Parkinson’s disease across North America. npj Parkinson's Dis. 2018;4:21. doi: 10.1038/s41531-018-0058-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cacabelos R. Parkinson’s disease: from pathogenesis to pharmacogenomics. Int J Mol Sci. 2017;18:551. doi: 10.3390/ijms18030551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Obeso JA, Stamelou M, Goetz CG, et al. Past, present, and future of Parkinson's disease: a special essay on the 200th Anniversary of the Shaking Palsy. Mov Disord. 2017;32:1264–1310. doi: 10.1002/mds.27115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katzenschlager R, Lees AJ. Treatment of Parkinson's disease: levodopa as the first choice. J Neurol. 2002;249:1–1. doi: 10.1007/s00415-002-1204-4. [DOI] [PubMed] [Google Scholar]
- 6.Ekstrand MI, Terzioglu M, Galter D, et al. Progressive Parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proc Natl Acad Sci U S A. 2007;104:1325–1330. doi: 10.1073/pnas.0605208103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konnova EA, Swanberg M. Animal models of Parkinson’s disease. In: Stoker TB, Greenland JC, editors. Parkinson’s disease: pathogenesis and clinical aspects. 1. Brisbane, AU: Codon Publications; 2018. pp. 83–106. [Google Scholar]
- 8.Kin K, Yasuhara T, Kameda M, Date I. Animal models for Parkinson’s disease research: Trends in the 2000s. Int J Mol Sci. 2019;20:5402–5402. doi: 10.3390/ijms20215402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckner RL, Krienen FM. The evolution of distributed association networks in the human brain. Trends Cogn Sci. 2013;17:648–665. doi: 10.1016/j.tics.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 10.Lovett ML, Nieland TJF, Dingle YTL, Kaplan DL. Innovations in 3D tissue models of human brain physiology and diseases. Adv Func Mater. 2020 doi: 10.1002/adfm.201909146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Defelipe J. The evolution of the brain, the human nature of cortical circuits, and intellectual creativity. Front Neuroanat. 2011;5:29. doi: 10.3389/fnana.2011.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laubach M, Amarante LM, Swanson K, White SR. What, if anything, is rodent prefrontal cortex? eNeuro. 2018 doi: 10.1523/ENEURO.0315-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bayes A, Collins MO, Croning MD, et al. Comparative study of human and mouse postsynaptic proteomes finds high compositional conservation and abundance differences for key synaptic proteins. PLoS ONE. 2012;7:e46683. doi: 10.1371/journal.pone.0046683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bozek K, Wei Y, Yan Z, et al. Organization and evolution of brain lipidome revealed by large-scale analysis of human, chimpanzee, macaque, and mouse tissues. Neuron. 2015;85:695–702. doi: 10.1016/j.neuron.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Pinson A, Namba T, Huttner WB. Malformations of human neocortex in development—their progenitor cell basis and experimental model systems. Front Cell Neurosci. 2019;13:305. doi: 10.3389/fncel.2019.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ransohoff RM. All (animal) models (of neurodegeneration) are wrong. Are they also useful? J Exp Med. 2018;215:2955–2958. doi: 10.1084/jem.20182042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simmnacher K, Lanfer J, Rizo T, et al. Modeling cell-cell interactions in Parkinson's disease using human stem cell-based models. Front Cell Neurosci. 2019;13:571. doi: 10.3389/fncel.2019.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang C, Campbell KR, Ryan BJ, et al. Single-cell sequencing of iPSC-dopamine neurons reconstructs disease progression and identifies HDAC4 as a regulator of parkinson cell phenotypes. Cell Stem Cell. 2019;24(93–106):e6. doi: 10.1016/j.stem.2018.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teixeira AI, Ilkhanizadeh S, Wigenius JA, et al. The promotion of neuronal maturation on soft substrates. Biomaterials. 2009;30:4567–4572. doi: 10.1016/j.biomaterials.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Baker BM, Chen CS. Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J Cell Sci. 2012;125:3015–3024. doi: 10.1242/jcs.079509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centeno EGZ, Cimarosti H, Bithell A. 2D versus 3D human induced pluripotent stem cell-derived cultures for neurodegenerative disease modelling. Mol Neurodegener. 2018;13:27. doi: 10.1186/s13024-018-0258-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birgersdotter A, Sandberg R, Ernberg I. Gene expression perturbation in vitro–a growing case for three-dimensional (3D) culture systems. Semin Cancer Biol. 2005;15:405–412. doi: 10.1016/j.semcancer.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 24.Tekin H, Simmons S, Cummings B, et al. Effects of 3D culturing conditions on the transcriptomic profile of stem-cell-derived neurons. Nat Biomed Eng. 2018;2:540–554. doi: 10.1038/s41551-018-0219-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D'Avanzo C, Aronson J, Kim YH, et al. Alzheimer's in 3D culture: challenges and perspectives. BioEssays. 2015;37:1139–1148. doi: 10.1002/bies.201500063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watson PMD, Kavanagh E, Allenby G, Vassey M. Bioengineered 3D glial cell culture systems and applications for neurodegeneration and neuroinflammation. SLAS Discov. 2017;22:583–601. doi: 10.1177/2472555217691450. [DOI] [PubMed] [Google Scholar]
- 27.Choi SH, Kim YH, Hebisch M, et al. A three-dimensional human neural cell culture model of Alzheimer's disease. Nature. 2014;515:274–278. doi: 10.1038/nature13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang D, Pekkanen-Mattila M, Shahsavani M, et al. A 3D Alzheimer's disease culture model and the induction of P21-activated kinase mediated sensing in iPSC derived neurons. Biomaterials. 2014;35:1420–1428. doi: 10.1016/j.biomaterials.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 29.Jo J, Xiao Y, Sun AX, et al. Midbrain-like organoids from human pluripotent stem cells contain functional dopaminergic and neuromelanin-producing neurons. Cell Stem Cell. 2016;19:248–257. doi: 10.1016/j.stem.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bolognin S, Fossepre M, Qing X, et al. 3D Cultures of Parkinson's disease-specific dopaminergic neurons for high content phenotyping and drug testing. Adv Sci (Weinh) 2018;6:1800927. doi: 10.1002/advs.201800927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kane KIW, Moreno EL, Hachi S, et al. Automated microfluidic cell culture of stem cell derived dopaminergic neurons. Sci Rep. 2019;9:1796. doi: 10.1038/s41598-018-34828-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Son MY, Sim H, Son YS, et al. Distinctive genomic signature of neural and intestinal organoids from familial Parkinson's disease patient-derived induced pluripotent stem cells. Neuropathol Appl Neurobiol. 2017;43:584–603. doi: 10.1111/nan.12396. [DOI] [PubMed] [Google Scholar]
- 33.Monzel AS, Smits LM, Hemmer K, et al. Derivation of human midbrain-specific organoids from neuroepithelial stem cells. Stem Cell Rep. 2017;8:1144–1154. doi: 10.1016/j.stemcr.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caiazza MC, Lang C, Wade-Martins R. What we can learn from iPSC-derived cellular models of Parkinson's disease. In: Björklund A, Cenci MA, editors. Recent advances in Parkinson's disease. 1. Amsterdam, NL: Elsevier; 2020. pp. 3–25. [DOI] [PubMed] [Google Scholar]
- 35.Bouyer C, Chen P, Güven S, et al. A Bio-acoustic levitational (BAL) assembly method for engineering of multilayered, 3D brain-like constructs, using human embryonic stem cell derived neuro-progenitors. Adv Mater. 2016;28:161–167. doi: 10.1002/adma.201503916. [DOI] [PubMed] [Google Scholar]
- 36.Tang-Schomer MD, White JD, Tien LW, et al. Bioengineered functional brain-like cortical tissue. Proc Natl Acad Sci. 2014;111:13811–13816. doi: 10.1073/pnas.1324214111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liaudanskaya V, Chung JY, Mizzoni C, et al. Modeling controlled cortical impact injury in 3D brain-like tissue cultures. Adv Healthc Mater. 2020;9:e2000122. doi: 10.1002/adhm.202000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rouleau N, Cantley WL, Liaudanskaya V, et al. A long-living bioengineered neural tissue platform to study neurodegeneration. Macromol Biosci. 2020;20:e2000004. doi: 10.1002/mabi.202000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sood D, Cairns DM, Dabbi JM, et al. Functional maturation of human neural stem cells in a 3D bioengineered brain model enriched with fetal brain-derived matrix. Sci Rep. 2019;9:17874. doi: 10.1038/s41598-019-54248-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cairns DM, Rouleau N, Parker RN, et al. A 3D human brain-like tissue model of herpes-induced Alzheimer's disease. Sci Adv. 2020;6:eaay8828. doi: 10.1126/sciadv.aay8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhuang P, Sun AX, An J, et al. 3D neural tissue models: from spheroids to bioprinting. Biomaterials. 2018;154:113–133. doi: 10.1016/j.biomaterials.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Naahidi S, Jafari M, Logan M, et al. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol Adv. 2017;35:530–544. doi: 10.1016/j.biotechadv.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 43.Watanabe K, Nakamura M, Okano H, Toyama Y. Establishment of three-dimensional culture of neural stem/progenitor cells in collagen Type-1 Gel. Restor Neurol Neurosci. 2007;25:109–117. [PubMed] [Google Scholar]
- 44.Wang X, He J, Wang Y, Cui F-Z. Hyaluronic acid-based scaffold for central neural tissue engineering. Interface Focus. 2012;2:278–291. doi: 10.1098/rsfs.2012.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sood D, Chwalek K, Stuntz E, et al. Fetal brain extracellular matrix boosts neuronal network formation in 3D bioengineered model of cortical brain tissue. ACS Biomater Sci Eng. 2016;2:131–140. doi: 10.1021/acsbiomaterials.5b00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moxon SR, Corbett NJ, Fisher K, et al. Blended alginate/collagen hydrogels promote neurogenesis and neuronal maturation. Mater Sci Eng, C. 2019;104:109904. doi: 10.1016/j.msec.2019.109904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klein C, Westenberger A. Genetics of Parkinson's disease. Cold Spring Harb Perspect Med. 2012;2:a008888–a008888. doi: 10.1101/cshperspect.a008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumari U, Tan EK. LRRK2 in Parkinson’s disease: genetic and clinical studies from patients. FEBS J. 2009;276:6455–6463. doi: 10.1111/j.1742-4658.2009.07344.x. [DOI] [PubMed] [Google Scholar]
- 49.Harvey K, Outeiro TF. The role of LRRK2 in cell signalling. Biochem Soc Trans. 2019;47:197–207. doi: 10.1042/bst20180464. [DOI] [PubMed] [Google Scholar]
- 50.Martin I, Kim JW, Dawson VL, Dawson TM. LRRK2 pathobiology in Parkinson's disease. J Neurochem. 2014;131:554–565. doi: 10.1111/jnc.12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Hara DM, Pawar G, Kalia SK, Kalia LV. LRRK2 and α-synuclein: distinct or synergistic players in Parkinson’s disease? Front Neurosci. 2020 doi: 10.3389/fnins.2020.00577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bieri G, Brahic M, Bousset L, et al. LRRK2 modifies α-syn pathology and spread in mouse models and human neurons. Acta Neuropathol. 2019;137:961–980. doi: 10.1007/s00401-019-01995-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu J, Wu M, Yue Z. Autophagy and Parkinson’s disease. In: Le W, editor. Autophagy: biology and diseases: clinical science. Singapore: Springer Singapore; 2020. pp. 21–51. [Google Scholar]
- 54.Schapira AHV. The importance of LRRK2 mutations in Parkinson disease. Arch Neurol. 2006;63:1225–1228. doi: 10.1001/archneur.63.9.1225. [DOI] [PubMed] [Google Scholar]
- 55.Macías-García D, Periñán MT, Muñoz-Delgado L, et al. Serum lipid profile among sporadic and familial forms of Parkinson’s disease. npj Parkinson's Dis. 2021;7:59. doi: 10.1038/s41531-021-00206-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dehay B, Martinez-Vicente M, Caldwell GA, et al. Lysosomal impairment in Parkinson's disease. Mov Disord. 2013;28:725–732. doi: 10.1002/mds.25462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barkhuizen M, Anderson DG, Grobler AF. Advances in GBA-associated Parkinson's disease—pathology, presentation and therapies. Neurochem Int. 2016;93:6–25. doi: 10.1016/j.neuint.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 58.Rockwood DN, Preda RC, Yucel T, et al. Materials fabrication from Bombyx mori silk fibroin. Nat Protoc. 2011;6:1612–1631. doi: 10.1038/nprot.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim UJ, Park J, Kim HJ, et al. Three-dimensional aqueous-derived biomaterial scaffolds from silk fibroin. Biomaterials. 2005;26:2775–2785. doi: 10.1016/j.biomaterials.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 60.Paull D, Sevilla A, Zhou H, et al. Automated, high-throughput derivation, characterization and differentiation of induced pluripotent stem cells. Nat Methods. 2015;12:885–892. doi: 10.1038/nmeth.3507. [DOI] [PubMed] [Google Scholar]
- 61.Kahler DJ, Ahmad FS, Ritz A, et al. Improved methods for reprogramming human dermal fibroblasts using fluorescence activated cell sorting. PLoS ONE. 2013;8:e59867. doi: 10.1371/journal.pone.0059867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qi Y, Zhang XJ, Renier N, et al. Combined small-molecule inhibition accelerates the derivation of functional cortical neurons from human pluripotent stem cells. Nat Biotechnol. 2017;35:154–163. doi: 10.1038/nbt.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bajad SU, Lu W, Kimball EH, et al. Separation and quantitation of water soluble cellular metabolites by hydrophilic interaction chromatography-tandem mass spectrometry. J Chromatogr A. 2006;1125:76–88. doi: 10.1016/j.chroma.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 65.Smith CA, Want EJ, O'Maille G, et al. XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal Chem. 2006;78:779–787. doi: 10.1021/ac051437y. [DOI] [PubMed] [Google Scholar]
- 66.Alden N, Krishnan S, Porokhin V, et al. Biologically consistent annotation of metabolomics data. Anal Chem. 2017;89:13097–13104. doi: 10.1021/acs.analchem.7b02162. [DOI] [PubMed] [Google Scholar]
- 67.Smith CA, O'Maille G, Want EJ, et al. METLIN: a metabolite mass spectral database. Ther Drug Monit. 2005;27:747–751. doi: 10.1097/01.ftd.0000179845.53213.39. [DOI] [PubMed] [Google Scholar]
- 68.Wishart DS, Feunang YD, Marcu A, et al. HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res. 2018;46:D608–D617. doi: 10.1093/nar/gkx1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johnson SG. NIST standard reference database 1A v17. Gaithersburg: National Institute of Standards and Technology; 2018. [Google Scholar]
- 70.Afgan E, Baker D, Batut B, et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 2018;46:W537–W544. doi: 10.1093/nar/gky379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 73.Love M, Anders S, Huber W. Differential analysis of count data–the DESeq2 package. Genome Biol. 2014;15(10):1186. [Google Scholar]
- 74.Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47–e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wickham H (2016) ggplot2: elegant graphics for data analysis. New York
- 76.Fox J, Weisberg S (2011) Multivariate linear models in R. Thousand Oaks, CA, USA
- 77.Stephens M. False discovery rates: a new deal. Biostatistics. 2017;18:275–294. doi: 10.1093/biostatistics/kxw041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Supek F, Bosnjak M, Skunca N, Smuc T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS ONE. 2011;6:e21800. doi: 10.1371/journal.pone.0021800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heberle H, Meirelles GV, da Silva FR, et al. InteractiVenn: a web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015;16:169. doi: 10.1186/s12859-015-0611-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cv M, Huynen M, Jaeggi D, et al. STRING: a database of predicted functional associations between proteins. Nucleic Acids Res. 2003;31:258–261. doi: 10.1093/nar/gkg034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Turei D, Korcsmaros T, Saez-Rodriguez J. OmniPath: guidelines and gateway for literature-curated signaling pathway resources. Nat Methods. 2016;13:966–967. doi: 10.1038/nmeth.4077. [DOI] [PubMed] [Google Scholar]
- 84.Choobdar S, Ahsen ME, Crawford J, et al. Assessment of network module identification across complex diseases. Nat Methods. 2019;16:843–852. doi: 10.1038/s41592-019-0509-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kohler S, Bauer S, Horn D, Robinson PN. Walking the interactome for prioritization of candidate disease genes. Am J Hum Genet. 2008;82:949–958. doi: 10.1016/j.ajhg.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Devkota K, Murphy JM, Cowen LJ. GLIDE: combining local methods and diffusion state embeddings to predict missing interactions in biological networks. Bioinformatics. 2020;36:i464–i473. doi: 10.1093/bioinformatics/btaa459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chong J, Xia J. MetaboAnalystR: an R package for flexible and reproducible analysis of metabolomics data. Bioinformatics. 2018;34:4313–4314. doi: 10.1093/bioinformatics/bty528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barnes S, Benton HP, Casazza K, et al. Training in metabolomics research. II. Processing and statistical analysis of metabolomics data, metabolite identification, pathway analysis, applications of metabolomics and its future. J Mass Spectrom. 2016;51:535–548. doi: 10.1002/jms.3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koch JC, Bitow F, Haack J, et al. Alpha-Synuclein affects neurite morphology, autophagy, vesicle transport and axonal degeneration in CNS neurons. Cell Death Dis. 2015;6:e1811. doi: 10.1038/cddis.2015.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cheng F, Vivacqua G, Yu S. The role of alpha-synuclein in neurotransmission and synaptic plasticity. J Chem Neuroanat. 2011;42:242–248. doi: 10.1016/j.jchemneu.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 91.Nicholson JK, Connelly J, Lindon JC, Holmes E. Metabonomics: a platform for studying drug toxicity and gene function. Nat Rev Drug Discov. 2002;1:153–161. doi: 10.1038/nrd728. [DOI] [PubMed] [Google Scholar]
- 92.Shao Y, Le W. Recent advances and perspectives of metabolomics-based investigations in Parkinson’s disease. Mol Neurodegener. 2019;14:3. doi: 10.1186/s13024-018-0304-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.La Manno G, Gyllborg D, Codeluppi S, et al. Molecular diversity of midbrain development in mouse, human, and stem cells. Cell. 2016;167:566–580.e19. doi: 10.1016/j.cell.2016.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Abbott RD, Kimmerling EP, Cairns DM, Kaplan DL. Silk as a biomaterial to support long-term three-dimensional tissue cultures. ACS Appl Mater Interfaces. 2016;8:21861–21868. doi: 10.1021/acsami.5b12114. [DOI] [PubMed] [Google Scholar]
- 95.Jia C, Qi H, Cheng C, et al. α-Synuclein negatively regulates Nurr1 expression through NF-κB-related mechanism. Front Mol Neurosci. 2020 doi: 10.3389/fnmol.2020.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Awad O, Panicker LM, Deranieh RM, et al. Altered differentiation potential of Gaucher’s disease iPSC neuronal progenitors due to Wnt/β-catenin downregulation. Stem Cell Reports. 2017;9:1853–1867. doi: 10.1016/j.stemcr.2017.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Woodard CM, Campos BA, Kuo SH, et al. iPSC-derived dopamine neurons reveal differences between monozygotic twins discordant for Parkinson's disease. Cell Rep. 2014;9:1173–1182. doi: 10.1016/j.celrep.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Elkouris M, Kouroupi G, Vourvoukelis A, et al. Long non-coding RNAs associated with neurodegeneration-linked genes are reduced in Parkinson’s disease patients. Front Cell Neurosci. 2019 doi: 10.3389/fncel.2019.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chiba-Falek O, Lopez GJ, Nussbaum RL. Levels of alpha-synuclein mRNA in sporadic Parkinson disease patients. Mov Disord. 2006;21:1703–1708. doi: 10.1002/mds.21007. [DOI] [PubMed] [Google Scholar]
- 100.Rocha EM, De Miranda B, Sanders LH. Alpha-synuclein: pathology, mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurobiol Dis. 2018;109:249–257. doi: 10.1016/j.nbd.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 101.Dale N, Frenguelli B. Release of adenosine and ATP during ischemia and epilepsy. Curr Neuropharmacol. 2009;7:160–179. doi: 10.2174/157015909789152146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Frolkis A, Knox C, Lim E, et al. SMPDB: the small molecule pathway database. Nucleic Acids Res. 2010;38:D480–D487. doi: 10.1093/nar/gkp1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nazario LR, da Silva RS, Bonan CD. Targeting adenosine signaling in Parkinson's disease: from pharmacological to non-pharmacological approaches. Front Neurosci. 2017 doi: 10.3389/fnins.2017.00658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Huang W, Xu Y, Zhang Y, et al. Metabolomics-driven identification of adenosine deaminase as therapeutic target in a mouse model of Parkinson's disease. J Neurochem. 2019;150:282–295. doi: 10.1111/jnc.14774. [DOI] [PubMed] [Google Scholar]
- 106.Johansen KK, Wang L, Aasly JO, et al. Metabolomic profiling in LRRK2-related Parkinson's disease. PLoS ONE. 2009;4:e7551. doi: 10.1371/journal.pone.0007551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Garcia-Esparcia P, Hernandez-Ortega K, Ansoleaga B, et al. Purine metabolism gene deregulation in Parkinson's disease. Neuropathol Appl Neurobiol. 2015;41:926–940. doi: 10.1111/nan.12221. [DOI] [PubMed] [Google Scholar]
- 108.Engelender S, Kaminsky Z, Guo X, et al. Synphilin-1 associates with α-synuclein and promotes the formation of cytosolic inclusions. Nat Genet. 1999;22:110–114. doi: 10.1038/8820. [DOI] [PubMed] [Google Scholar]
- 109.Alvarez-Castelao B, Castaño JG. Synphilin-1 inhibits alpha-synuclein degradation by the proteasome. Cell Mol Life Sci. 2011;68:2643–2654. doi: 10.1007/s00018-010-0592-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rott R, Szargel R, Haskin J, et al. α-Synuclein fate is determined by USP9X-regulated monoubiquitination. Proc Natl Acad Sci. 2011;108:18666–18671. doi: 10.1073/pnas.1105725108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nakamura T, Yamashita H, Takahashi T, Nakamura S. Activated fyn phosphorylates α-synuclein at tyrosine residue 125. Biochem Biophys Res Commun. 2001;280:1085–1092. doi: 10.1006/bbrc.2000.4253. [DOI] [PubMed] [Google Scholar]
- 112.Pampalakis G, Sykioti V-S, Ximerakis M, et al. KLK6 proteolysis is implicated in the turnover and uptake of extracellular alpha-synuclein species. Oncotarget. 2017;8:14502–14515. doi: 10.18632/oncotarget.13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pronin AN, Morris AJ, Surguchov A, Benovic JL. Synucleins are a novel class of substrates for G protein-coupled receptor kinases. J Biol Chem. 2000;275:26515–26522. doi: 10.1074/jbc.M003542200. [DOI] [PubMed] [Google Scholar]
- 114.Waxman EA, Giasson BI. Characterization of kinases involved in the phosphorylation of aggregated α-synuclein. J Neurosci Res. 2011;89:231–247. doi: 10.1002/jnr.22537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu Y, Su Y, Wang X. Phosphatidic acid-mediated signaling. In: Capelluto D, editor. Lipid-mediated protein signaling. 1. Dordrecht: Springer; 2013. pp. 159–176. [DOI] [PubMed] [Google Scholar]
- 116.Ishisaka M, Hara H. The roles of diacylglycerol kinases in the central nervous system: review of genetic studies in mice. J Pharmacol Sci. 2014;124:336–343. doi: 10.1254/jphs.13R07CR. [DOI] [PubMed] [Google Scholar]
- 117.Wood PL, Tippireddy S, Feriante J, Woltjer RL. Augmented frontal cortex diacylglycerol levels in Parkinson’s disease and Lewy Body Disease. PLoS ONE. 2018;13:e0191815. doi: 10.1371/journal.pone.0191815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Raben DM, Barber CN. Phosphatidic acid and neurotransmission. Adv Biol Regul. 2017;63:15–21. doi: 10.1016/j.jbior.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang X, Devaiah SP, Zhang W, Welti R. Signaling functions of phosphatidic acid. Prog Lipid Res. 2006;45:250–278. doi: 10.1016/j.plipres.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 120.Conde MA, Alza NP, Iglesias González PA, et al. Phospholipase D1 downregulation by α-synuclein: Implications for neurodegeneration in Parkinson's disease. Biochim Biophys Acta. 2018;1863:639–650. doi: 10.1016/j.bbalip.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 121.Bae EJ, Lee HJ, Jang YH, et al. Phospholipase D1 regulates autophagic flux and clearance of α-synuclein aggregates. Cell Death Differ. 2014;21:1132–1141. doi: 10.1038/cdd.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Javoy-Agid F, Hirsch EC, Dumas S, et al. Decreased tyrosine hydroxylase messenger RNA in the surviving dopamine neurons of the substantia nigra in Parkinson's disease: An in situ hybridization study. Neuroscience. 1990;38:245–253. doi: 10.1016/0306-4522(90)90389-l. [DOI] [PubMed] [Google Scholar]
- 123.Simunovic F, Yi M, Wang Y, et al. Gene expression profiling of substantia nigra dopamine neurons: further insights into Parkinson's disease pathology. Brain. 2009;132:1795–1809. doi: 10.1093/brain/awn323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fuchs J, Mueller JC, Lichtner P, et al. The transcription factor PITX3 is associated with sporadic Parkinson's disease. Neurobiol Aging. 2009;30:731–738. doi: 10.1016/j.neurobiolaging.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 125.Nguyen Ha N, Byers B, Cord B, et al. LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell. 2011;8:267–280. doi: 10.1016/j.stem.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Donega V, Burm SM, van Strien ME, et al. Transcriptome and proteome profiling of neural stem cells from the human subventricular zone in Parkinson’s disease. Acta Neuropathol Commun. 2019;7:84. doi: 10.1186/s40478-019-0736-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Riley BE, Gardai SJ, Emig-Agius D, et al. Systems-based analyses of brain regions functionally impacted in Parkinson's disease reveals underlying causal mechanisms. PLoS ONE. 2014;9:e102909. doi: 10.1371/journal.pone.0102909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kang SS, Ahn EH, Zhang Z, et al. α-Synuclein stimulation of monoamine oxidase-B and legumain protease mediates the pathology of Parkinson's disease. EMBO J. 2018;37:e98878. doi: 10.15252/embj.201798878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Henderson MX, Sengupta M, Trojanowski JQ, Lee VMY. Alzheimer’s disease tau is a prominent pathology in LRRK2 Parkinson’s disease. Acta Neuropathol Commun. 2019 doi: 10.1186/s40478-019-0836-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fujii C, Harada S, Ohkoshi N, et al. Association between polymorphism of the cholecystokinin gene and idiopathic Parkinson's disease. Clin Genet. 1999;56:395–400. doi: 10.1034/j.1399-0004.1999.560508.x. [DOI] [PubMed] [Google Scholar]
- 131.Wang J, Si Y-M, Liu Z-L, Yu L. Cholecystokinin, cholecystokinin-A receptor and cholecystokinin-B receptor gene polymorphisms in Parkinson's disease. Pharmacogenet Genom. 2003 doi: 10.1097/00008571-200306000-00008. [DOI] [PubMed] [Google Scholar]
- 132.Virreira Winter S, Karayel O, Strauss MT, et al. Urinary proteome profiling for stratifying patients with familial Parkinson’s disease. EMBO Mol Med. 2021 doi: 10.15252/emmm.202013257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Duke DC, Moran LB, Kalaitzakis ME, et al. Transcriptome analysis reveals link between proteasomal and mitochondrial pathways in Parkinson’s disease. Neurogenetics. 2006;7:139–148. doi: 10.1007/s10048-006-0033-5. [DOI] [PubMed] [Google Scholar]
- 134.Demishtein A, Fraiberg M, Berko D, et al. SQSTM1/p62-mediated autophagy compensates for loss of proteasome polyubiquitin recruiting capacity. Autophagy. 2017;13:1697–1708. doi: 10.1080/15548627.2017.1356549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.B’Chir W, Maurin A-C, Carraro V, et al. The eIF2α/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res. 2013;41:7683–7699. doi: 10.1093/nar/gkt563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Husnjak K, Elsasser S, Zhang N, et al. Proteasome subunit Rpn13 is a novel ubiquitin receptor. Nature. 2008;453:481–488. doi: 10.1038/nature06926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Morato Torres CA, Wassouf Z, Zafar F, et al. The Role of Alpha-synuclein and other Parkinson’s genes in neurodevelopmental and neurodegenerative disorders. Int J Mol Sci. 2020;21:5724. doi: 10.3390/ijms21165724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang J-W, Ma Y-M, Jing L, et al. Synaptic remodeling and reduced expression of the transcription factors, HES1 and HES5, in the cortex neurons of cognitively impaired hyperhomocysteinemic mice. Pathology Res Pract. 2020;216:152953. doi: 10.1016/j.prp.2020.152953. [DOI] [PubMed] [Google Scholar]
- 139.Mesman S, Smidt MP. Tcf12 is involved in early cell-fate determination and subset specification of midbrain dopamine neurons. Front Mol Neurosci. 2017 doi: 10.3389/fnmol.2017.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Konishi H, Ogawa T, Nakagomi S, et al. Id1, Id2 and Id3 are induced in rat melanotrophs of the pituitary gland by dopamine suppression under continuous stress. Neuroscience. 2010;169:1527–1534. doi: 10.1016/j.neuroscience.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 141.Cohlberg JA, Li J, Uversky VN, Fink AL. Heparin and Other Glycosaminoglycans Stimulate the Formation of Amyloid Fibrils from α-Synuclein in Vitro†. Biochemistry. 2002;41:1502–1511. doi: 10.1021/bi011711s. [DOI] [PubMed] [Google Scholar]
- 142.Presto J, Thuveson M, Carlsson P, et al. Heparan sulfate biosynthesis enzymes EXT1 and EXT2 affect NDST1 expression and heparan sulfate sulfation. Proc Natl Acad Sci. 2008;105:4751–4756. doi: 10.1073/pnas.0705807105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang D, He J, Huang B, et al. Emerging role of the Hippo pathway in autophagy. Cell Death Dis. 2020 doi: 10.1038/s41419-020-03069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sahu MR, Mondal AC. The emerging role of Hippo signaling in neurodegeneration. J Neurosci Res. 2020;98:796–814. doi: 10.1002/jnr.24551. [DOI] [PubMed] [Google Scholar]
- 145.Jankovic J. Parkinson's disease and movement disorders: moving forward. Lancet Neurol. 2008;7:9–11. doi: 10.1016/S1474-4422(07)70302-2. [DOI] [PubMed] [Google Scholar]
- 146.Sulzer D. Multiple hit hypotheses for dopamine neuron loss in Parkinson's disease. Trends Neurosci. 2007;30:244–250. doi: 10.1016/j.tins.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 147.Amirikia M, Shariatzadeh SMA, Jorsaraei SGA, Mehranjani MS. Auto-fluorescence of a silk fibroin-based scaffold and its interference with fluorophores in labeled cells. Eur Biophys J. 2018;47:573–581. doi: 10.1007/s00249-018-1279-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental File S1 Representative fragmentation spectra generated from untargeted liquid chromatography tandem mass spectrometry analysis. Mass/charge (m/z) peaks were compared between experimental samples (top panels, representative samples shown) and purified standards (bottom panels), which confirmed the identities of (A) Adenosine, (B) adenine, and (C) adenosine 3',5'-cyclic monophosphate metabolites. All results were acquired in positive ionization mode
Supplemental File S2 Principle component analysis of transcriptomic data of control, GBA and LRRK2 DA neurons. (A) 3D culture is consistently shifted along the PC1 and PC2 axes compared to 2D, indicating a culture condition dependent shift in global gene expression. Panel (B) highlights the results for the individual groups
Supplemental File S3 Differential expression results using the DESeq2 R package, sorted by PD comparison in 2D and 3D culture format. A differential expression cutoff of abs(Log2(Fold change) > 0.585 in combination with q < 0.01 was used to determine significance
Supplemental File S4 Over-representation analysis of biological processes when comparing heathy hiPSC-DA neurons cultured in 3D versus 2D conditions (3D CTRL versus 2D CTRL). Upregulated and downregulated GO terms were determined using differentially expressed genes (q < 0.01) with positive and negative fold change, respectively
Supplemental File S5 Lists of the top 100 differentially expressed genes (HGNC symbols) from each Parkinson’s disease comparison that also existed in the DREAM3 molecular interaction network. Each list was utilized as the initial seed network for candidate disease gene prioritization and subsequent link prediction analysis
Supplemental File S6 List of genes and molecular interactions for each link prediction network generated from PD genotype comparisons. The top 100 differentially expressed genes (DEGs), that also existed in the molecular interaction network DREAM3 (Supplemental file S5), were utilized to generate individual link prediction networks from all disease comparisons made in 2D and 3D culture formats: LRRK2 vs CTRL, GBA_PD vs CTRL, and GBA_PD vs GBA_CTRL. Application of Kohler’s random walk with restart nominated candidate disease genes (CDGs) within the neighborhood of the DEGs. Likely molecular interactions between DEGs and CDGs were computationally predicted by using global and local integrated diffusion embedding (GLIDE)
Supplemental File S7 α-synuclein protein measurements in 2D and 3D cultures (with reference to Fig. 3). α-synuclein protein levels (ELISA) in (A) 2D and (B) 3D culture conditions were determined from individual cell donor lines. (C) Fold change of α-synuclein protein levels was normalized to results from healthy individual cultures indicating increased expression in 3D cultures relative to the 2D culture. Statistical significance for (D) 2D and (E) 3D cultures were determined with one-way ANOVA followed by Tukey’s multiple comparison testing. (F) Fold change of α-synuclein protein levels (C) were compared across 2D and 3D culture systems by multiple t-tests with the two-stage step-up procedure of Benjamini, Krieger, and Yekutieli. Six or eight individual cultures were collected from two independent experiments, depending on the sample. A false discovery rate (q value or adjusted p value) of less than 0.05 was considered significant
Supplemental File S8 Neuronal network quantifications of TH+ and TUJ1+ signal ratios from immunofluorescent images suggested similar dopamine neuron content across individual donor lines and culture conditions. Statistical testing was performed using a two-way ANOVA with a significance cutoff of p = 0.05. Data displayed as the mean ± SEM from n = 4 replicate cultures per group
Supplemental File S9 Representative immunofluorescent images of 3D construct seeded with patient derived dopaminergic neurons. Wide-field images show homogeneously attached cells stained with DAPI (nuclei, blue) onto the scaffolding and neuronal processes (β3-tubulin, green) extending along the scaffolding and into the surrounding collagen hydrogel. To account for the autofluorescence of the silk scaffolding [147], digitally expanded images provide a clarified view of individual nuclei and neurites. Scale bars represent 500 μm
Supplemental File S10 Gene expression levels for neuronal, glial, and pluripotency markers. The low expression levels of markers for astrocytes, microglia, oligodendrocytes, and hiPSCs suggested that each groups’ culture composition were primarily neuronal
Supplemental File S11 3D CTRL versus 2D CTRL differential expression datasets for human dopamine neuron gene sets (hDA0, hDA1, and hDA2, characterized from developing human brains [93]). The 3D system upregulated a higher number of dopamine neuron genes, suggesting a more in vivo-like phenotype compared to 2D culture. Genes were highlighted blue if upregulated in 3D systems (log2(Fold Change) > 0.585 and p.adj < 0.05) or red if upregulated in 2D systems (log2(Fold Change) < 0.585 and p.adj < 0.05). Genes unaffected by culture condition were colored gray
Supplemental File S12 Differentially expressed genes that are unique or shared between 2D and 3D cultures for each of the LRRK2 and GBA genotype comparisons (with reference to Fig. 6A, 6B). Differential expression analysis was performed with the DESeq2 R package and statistical significance was determined with a cutoff of abs(Log2(Fold change) > 0.585 in combination with q < 0.01
Supplemental File S13 Log2(Fold Change) of differentially expressed genes with a STRING association score to α-synuclein (with reference to Fig. 6C, Fig. 6D). Differential expression analysis was performed with the DESeq2 R package and statistical significance was determined with a cutoff of abs(Log2(Fold change) > 0.585 in combination with q < 0.01
Supplemental File S14 Log2(Fold Change) of differentially expressed genes in link prediction networks (with reference to Fig. 7). Differential expression analysis was performed with the DESeq2 R package and statistical significance was determined with a cutoff of abs(Log2(Fold change) > 0.585 in combination with q < 0.01
Supplemental File S15 Link prediction networks unique to 2D or 3D culture systems generated using differential expression data. The top 100 differentially expressed genes (DEGs), that also existed in the molecular interaction network DREAM3 (Supplemental file S5), were utilized to generate individual link prediction networks from all disease comparisons made in 2D and 3D culture formats: LRRK2 vs CTRL, GBA_PD vs CTRL, and GBA_PD vs GBA_CTRL. Application of Kohler’s random walk with restart nominated candidate disease genes (CDGs) within the neighborhood of the DEGs. Molecular interactions between DEGs and CDGs were computationally predicted using global and local integrated diffusion embedding (GLIDE). Networks unique to each culture condition were extracted for GBA or LRRK2 genotype comparisons. Genes with a green frame were commonly observed across GBA and LRRK2 genotype comparisons for the given culture condition
Data Availability Statement
The datasets used and analyzed for this study can be obtained from corresponding authors upon reasonable request. Raw sequencing data (consented hiPSC donors only) and normalized read counts can be freely accessed through the Gene Expression Omnibus (GSE172409).